User login
Fall From Trail Leaves Woman in Pain
ANSWER
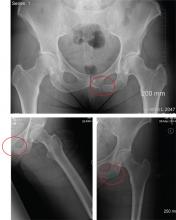
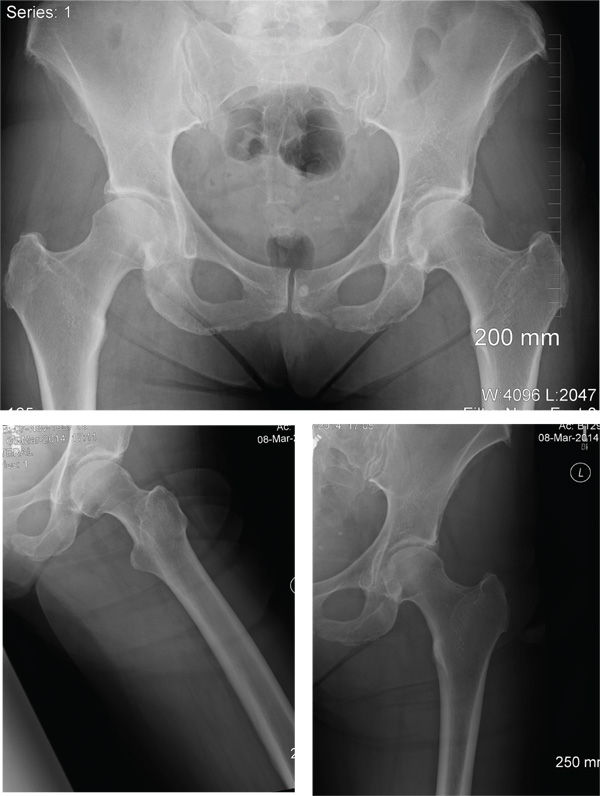
The radiographs demonstrate a left inferior pubic ramus fracture. The patient was referred to orthopedics for follow-up. She was given a walker and a series of home exercises for hip stretching and strengthening, as well as anti-inflammatories as needed for discomfort. She was scheduled for a four-week follow-up visit and repeat radiographs of the pelvis.
ANSWER
The radiographs demonstrate a left inferior pubic ramus fracture. The patient was referred to orthopedics for follow-up. She was given a walker and a series of home exercises for hip stretching and strengthening, as well as anti-inflammatories as needed for discomfort. She was scheduled for a four-week follow-up visit and repeat radiographs of the pelvis.
ANSWER
The radiographs demonstrate a left inferior pubic ramus fracture. The patient was referred to orthopedics for follow-up. She was given a walker and a series of home exercises for hip stretching and strengthening, as well as anti-inflammatories as needed for discomfort. She was scheduled for a four-week follow-up visit and repeat radiographs of the pelvis.
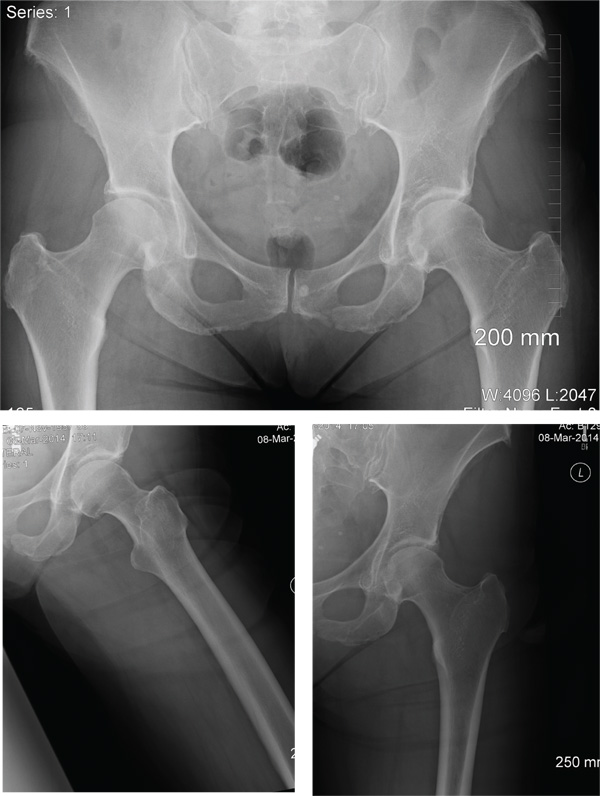
A 56-year old woman presents for evaluation of left hip pain. Several hours ago, she says, she was walking on a trail when she fell from an embankment. The 4-ft fall ended with her landing primarily on her left hip and elbow. Afterward, she was able to ambulate with assistance but noticed increased pain in her left hip and groin with movement. The elbow discomfort resolved shortly after the incident. She denies numbness or tingling in her extremities and loss of bowel or bladder function. Physical exam reveals a well-developed, well-nourished female without any extremity deformity or leg shortening. Palpation elicits left-sided groin pain, as well as left posterior hip and sacroiliac joint pain. Both active and passive range-of-motion of the hip elicit pain, but straight-leg raise does not. The patient can bear weight on the left leg with the assistance of a walker. There is no laxity in the knee joint, and the ankle mortise is stable. There are no signs of swelling or bruising, and the skin is intact. Dorsalis pedis and posterior tibial pulses are 2+, and sensation in the left foot is intact. Radiographs of the left hip and pelvis are obtained. What is your impression?
Case Studies in Toxicology: Managing Missed Methadone
A 53-year-old woman presented to the ED after experiencing a fall. Her medical history was significant for chronic obstructive pulmonary disease, hepatitis, and a remote history of intravenous drug use, for which she had been maintained on methadone for the past 20 years. She reported that she had suffered several “fainting episodes” over the past month, and the morning prior to arrival, had sustained what she thought was a mechanical fall outside of the methadone program she attended. She complained of tenderness on her head but denied any other injuries.
The methadone program had referred the patient to the ED for evaluation, noting to the ED staff that her daily methadone dose of 185 mg had not been dispensed prior to transfer. During evaluation, the patient requested that the emergency physician (EP) provide the methadone dose since the clinic would close prior to her discharge from the ED.
How can requests for methadone be managed in the ED?
Methadone is a long-acting oral opioid that is used for both opioid replacement therapy and pain management. When used to reduce craving in opioid-dependent patients, methadone is administered daily through federally sanctioned methadone maintenance treatment (MMT) programs. Patients who consistently adhere to the required guidelines are given “take home” doses. When used for pain management, methadone is typically administered several times daily and may be prescribed by any provider with an appropriate DEA registration.
When given for MMT, methadone saturates the µ-opioid receptors and hinders their binding and agonism by other opioids such as heroin or oxycodone. Patients in MMT programs are started on a low initial dose and slowly titrated upward as tolerance to the adverse effects (eg, sedation) develop.
How are symptomatic patients with methadone withdrawal treated?
Most methadone programs have limited hours and require that patients who miss a dose wait until the following day to return to the program. This is typically without medical consequence because the high dose dispensed by these programs maintains a therapeutic blood concentration for far longer than the expected delay. Although the half-life of methadone exhibits wide interindividual variability, it generally ranges from 12 hours to more than 40 hours.1 Regardless, patients may feel anxious about potential opioid withdrawal, and this often leads them to access the ED for a missed dose.
The neuropsychiatric symptoms attending withdrawal may precede the objective signs of opioid withdrawal. Patients with objective signs of opioid withdrawal (eg, piloerection, vomiting, diarrhea, dilated pupils) may be sufficiently treated with supportive care alone, using antiemetics, hydration, and sometimes clonidine.
Administration of substitute opioids is problematic due to the patient’s underlying tolerance necessitating careful dose titration. Therefore, direct replacement of methadone in the ED remains controversial, and some EDs have strict policies prohibiting the administration of methadone to patients who have missed an MMT dose. Such policies, which are intended to discourage patients from using the ED as a convenience, may be appropriate given the generally benign—though uncomfortable—course of opioid withdrawal due to abstinence.
Other EDs provide replacement methadone for asymptomatic, treat-and-release patients confirmed to be enrolled in an MMT program when the time to the next dose is likely to be 24 hours or greater from the missed dose. Typically, a dose of no more than 10 mg orally or 10 mg intramuscularly (IM) is recommended, and patients should be advised that they will be receiving only a low dose to sufficient to prevent withdrawal—one that may not have the equivalent effects of the outpatient dose.
Whenever possible, a patient’s MMT program should be contacted and informed of the ED visit. For patients who display objective signs of withdrawal and who cannot be confirmed or who do not participate in an MMT program, 10 mg of methadone IM will prevent uncertainty of drug absorption in the setting of nausea or vomiting. All patients receiving oral methadone should be observed for 1 hour, and those receiving IM methadone should be observed for at least 90 minutes to assess for unexpected sedation.2
Patients encountering circumstances that prevent opioid access (eg, incarceration) and who are not in withdrawal but have gone without opioids for more than 5 days may have a loss of tolerance to their usual doses—whether the medication was obtained through an MMT program or illicitly. Harm-reduction strategies aimed at educating patients on the potential vulnerability to their familiar dosing regimens are warranted to avert inadvertent overdoses in chronic opioid users who are likely to resume illicit opoiod use.
Does this patient need syncope evaluation?
Further complicating the decision regarding ED dispensing of methadone are the effects of the drug on myocardial repolarization. Methadone affects conduction across the hERG potassium rectifier current and can prolong the QTc interval on the surface electrocardiogram (ECG), predisposing a patient to torsade de pointes (TdP). Although there is controversy regarding the role of ECG screening during the enrollment of patients in methadone maintenance clinics, doses above 60 mg, underlying myocardial disease, female sex, and electrolyte disturbances may increase the risk of QT prolongation and TdP.3
Whether there is value in obtaining a screening ECG in a patient receiving an initial dose of methadone in the ED is unclear, and this practice is controversial even among methadone clinics. However, some of the excess death in patients taking methadone may be explained by the dysrhythmogenic potential of methadone.4 An ECG therefore may elucidate a correctable cause in methadone patients presenting with syncope.
Administering methadone to patients with documented QT prolongation must weigh the risk of methadone’s conduction effects against the substantial risks of illicit opioid self-administration. For some patients at-risk for TdP, it may be preferable to use buprenorphine if possible, since it does not carry the same cardiac effects as methadone.1,5 Such therapy requires referral to a physician licensed to prescribe this medication.
How should admitted patients be managed?
While administration of methadone for withdrawal or maintenance therapy in the ED is acceptable, outpatient prescribing of methadone for these reasons is not legal, and only federally regulated clinics may engage in this practice. Hospitalized patients who are enrolled in an MMT program should have their daily methadone dose confirmed and continued—as long as the patient has not lost tolerance. Patients not participating in an MMT program can receive up to 3 days of methadone in the hospital, even if the practitioner is not registered to provide methadone.6 For these patients, it is recommended that the physician order a low dose of methadone and also consult with an addiction specialist to determine whether the patient should continue on MMT maintenance or undergo detoxification.
It is important to note that methadone may be prescribed for pain, but its use in the ED for this purpose is strongly discouraged, especially in patients who have never received methadone previously. For admitted patients requiring such potent opioid analgesia, consultation with a pain service or, when indicated, a palliative care/hospice specialist is warranted as the dosing intervals are different in each setting, and the risk of respiratory depression is high.
Case Conclusion
As requested by the MMT clinic, the patient was administered methadone 185 mg orally in the ED, though a dose of 10 mg would have been sufficient to prevent withdrawal. Unfortunately, the EP did not appreciate the relationship of the markedly prolonged QTc and the methadone, which should have prompted a dose reduction.
Evaluation of the patient’s electrolyte levels, which included magnesium and potassium, were normal. An ECG was repeated 24 hours later and revealed a persistent, but improved, QT interval at 505 ms. The remainder of the syncope workup was negative. Because the patient had no additional symptoms or events during her stay, she was discharged. At discharge, the EP followed up with the MMT clinic to discuss lowering the patient’s daily methadone dose, as well as close cardiology follow-up.
Dr Rao is the chief of the division of medical toxicology at New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Chou R, Weimer MB, Dana T. Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain. 2014;15(4):338-365.
- National Highway Traffic Safety Administration Web site. Methadone. http://www.nhtsa.gov/people/injury/research/job185drugs/methadone.htm. Accessed August 3, 2015.
- Martin JA, Campbell A, Killip T, et al; Substance Abuse and Mental Health Services Administration. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306. Erratum in: J Addict Dis. 2012;31(1):91.
- Ray WA, Chung CP, Murray KT, Cooper WO, Hall K, Stein CM. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med. 2015;175(3):420-427.
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209-219.
- US Government Printing Office. Federal Digital System. Administering or dispensing of narcotic drugs. Code of Federal Regulations. Title 21 CFR §1306.07. http://www.gpo.gov/fdsys/pkg/CFR-1998-title21-vol9/pdf/CFR-1998-title21-vol9-sec1306-07.pdf. Accessed August 4, 2015.
A 53-year-old woman presented to the ED after experiencing a fall. Her medical history was significant for chronic obstructive pulmonary disease, hepatitis, and a remote history of intravenous drug use, for which she had been maintained on methadone for the past 20 years. She reported that she had suffered several “fainting episodes” over the past month, and the morning prior to arrival, had sustained what she thought was a mechanical fall outside of the methadone program she attended. She complained of tenderness on her head but denied any other injuries.
The methadone program had referred the patient to the ED for evaluation, noting to the ED staff that her daily methadone dose of 185 mg had not been dispensed prior to transfer. During evaluation, the patient requested that the emergency physician (EP) provide the methadone dose since the clinic would close prior to her discharge from the ED.
How can requests for methadone be managed in the ED?
Methadone is a long-acting oral opioid that is used for both opioid replacement therapy and pain management. When used to reduce craving in opioid-dependent patients, methadone is administered daily through federally sanctioned methadone maintenance treatment (MMT) programs. Patients who consistently adhere to the required guidelines are given “take home” doses. When used for pain management, methadone is typically administered several times daily and may be prescribed by any provider with an appropriate DEA registration.
When given for MMT, methadone saturates the µ-opioid receptors and hinders their binding and agonism by other opioids such as heroin or oxycodone. Patients in MMT programs are started on a low initial dose and slowly titrated upward as tolerance to the adverse effects (eg, sedation) develop.
How are symptomatic patients with methadone withdrawal treated?
Most methadone programs have limited hours and require that patients who miss a dose wait until the following day to return to the program. This is typically without medical consequence because the high dose dispensed by these programs maintains a therapeutic blood concentration for far longer than the expected delay. Although the half-life of methadone exhibits wide interindividual variability, it generally ranges from 12 hours to more than 40 hours.1 Regardless, patients may feel anxious about potential opioid withdrawal, and this often leads them to access the ED for a missed dose.
The neuropsychiatric symptoms attending withdrawal may precede the objective signs of opioid withdrawal. Patients with objective signs of opioid withdrawal (eg, piloerection, vomiting, diarrhea, dilated pupils) may be sufficiently treated with supportive care alone, using antiemetics, hydration, and sometimes clonidine.
Administration of substitute opioids is problematic due to the patient’s underlying tolerance necessitating careful dose titration. Therefore, direct replacement of methadone in the ED remains controversial, and some EDs have strict policies prohibiting the administration of methadone to patients who have missed an MMT dose. Such policies, which are intended to discourage patients from using the ED as a convenience, may be appropriate given the generally benign—though uncomfortable—course of opioid withdrawal due to abstinence.
Other EDs provide replacement methadone for asymptomatic, treat-and-release patients confirmed to be enrolled in an MMT program when the time to the next dose is likely to be 24 hours or greater from the missed dose. Typically, a dose of no more than 10 mg orally or 10 mg intramuscularly (IM) is recommended, and patients should be advised that they will be receiving only a low dose to sufficient to prevent withdrawal—one that may not have the equivalent effects of the outpatient dose.
Whenever possible, a patient’s MMT program should be contacted and informed of the ED visit. For patients who display objective signs of withdrawal and who cannot be confirmed or who do not participate in an MMT program, 10 mg of methadone IM will prevent uncertainty of drug absorption in the setting of nausea or vomiting. All patients receiving oral methadone should be observed for 1 hour, and those receiving IM methadone should be observed for at least 90 minutes to assess for unexpected sedation.2
Patients encountering circumstances that prevent opioid access (eg, incarceration) and who are not in withdrawal but have gone without opioids for more than 5 days may have a loss of tolerance to their usual doses—whether the medication was obtained through an MMT program or illicitly. Harm-reduction strategies aimed at educating patients on the potential vulnerability to their familiar dosing regimens are warranted to avert inadvertent overdoses in chronic opioid users who are likely to resume illicit opoiod use.
Does this patient need syncope evaluation?
Further complicating the decision regarding ED dispensing of methadone are the effects of the drug on myocardial repolarization. Methadone affects conduction across the hERG potassium rectifier current and can prolong the QTc interval on the surface electrocardiogram (ECG), predisposing a patient to torsade de pointes (TdP). Although there is controversy regarding the role of ECG screening during the enrollment of patients in methadone maintenance clinics, doses above 60 mg, underlying myocardial disease, female sex, and electrolyte disturbances may increase the risk of QT prolongation and TdP.3
Whether there is value in obtaining a screening ECG in a patient receiving an initial dose of methadone in the ED is unclear, and this practice is controversial even among methadone clinics. However, some of the excess death in patients taking methadone may be explained by the dysrhythmogenic potential of methadone.4 An ECG therefore may elucidate a correctable cause in methadone patients presenting with syncope.
Administering methadone to patients with documented QT prolongation must weigh the risk of methadone’s conduction effects against the substantial risks of illicit opioid self-administration. For some patients at-risk for TdP, it may be preferable to use buprenorphine if possible, since it does not carry the same cardiac effects as methadone.1,5 Such therapy requires referral to a physician licensed to prescribe this medication.
How should admitted patients be managed?
While administration of methadone for withdrawal or maintenance therapy in the ED is acceptable, outpatient prescribing of methadone for these reasons is not legal, and only federally regulated clinics may engage in this practice. Hospitalized patients who are enrolled in an MMT program should have their daily methadone dose confirmed and continued—as long as the patient has not lost tolerance. Patients not participating in an MMT program can receive up to 3 days of methadone in the hospital, even if the practitioner is not registered to provide methadone.6 For these patients, it is recommended that the physician order a low dose of methadone and also consult with an addiction specialist to determine whether the patient should continue on MMT maintenance or undergo detoxification.
It is important to note that methadone may be prescribed for pain, but its use in the ED for this purpose is strongly discouraged, especially in patients who have never received methadone previously. For admitted patients requiring such potent opioid analgesia, consultation with a pain service or, when indicated, a palliative care/hospice specialist is warranted as the dosing intervals are different in each setting, and the risk of respiratory depression is high.
Case Conclusion
As requested by the MMT clinic, the patient was administered methadone 185 mg orally in the ED, though a dose of 10 mg would have been sufficient to prevent withdrawal. Unfortunately, the EP did not appreciate the relationship of the markedly prolonged QTc and the methadone, which should have prompted a dose reduction.
Evaluation of the patient’s electrolyte levels, which included magnesium and potassium, were normal. An ECG was repeated 24 hours later and revealed a persistent, but improved, QT interval at 505 ms. The remainder of the syncope workup was negative. Because the patient had no additional symptoms or events during her stay, she was discharged. At discharge, the EP followed up with the MMT clinic to discuss lowering the patient’s daily methadone dose, as well as close cardiology follow-up.
Dr Rao is the chief of the division of medical toxicology at New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
A 53-year-old woman presented to the ED after experiencing a fall. Her medical history was significant for chronic obstructive pulmonary disease, hepatitis, and a remote history of intravenous drug use, for which she had been maintained on methadone for the past 20 years. She reported that she had suffered several “fainting episodes” over the past month, and the morning prior to arrival, had sustained what she thought was a mechanical fall outside of the methadone program she attended. She complained of tenderness on her head but denied any other injuries.
The methadone program had referred the patient to the ED for evaluation, noting to the ED staff that her daily methadone dose of 185 mg had not been dispensed prior to transfer. During evaluation, the patient requested that the emergency physician (EP) provide the methadone dose since the clinic would close prior to her discharge from the ED.
How can requests for methadone be managed in the ED?
Methadone is a long-acting oral opioid that is used for both opioid replacement therapy and pain management. When used to reduce craving in opioid-dependent patients, methadone is administered daily through federally sanctioned methadone maintenance treatment (MMT) programs. Patients who consistently adhere to the required guidelines are given “take home” doses. When used for pain management, methadone is typically administered several times daily and may be prescribed by any provider with an appropriate DEA registration.
When given for MMT, methadone saturates the µ-opioid receptors and hinders their binding and agonism by other opioids such as heroin or oxycodone. Patients in MMT programs are started on a low initial dose and slowly titrated upward as tolerance to the adverse effects (eg, sedation) develop.
How are symptomatic patients with methadone withdrawal treated?
Most methadone programs have limited hours and require that patients who miss a dose wait until the following day to return to the program. This is typically without medical consequence because the high dose dispensed by these programs maintains a therapeutic blood concentration for far longer than the expected delay. Although the half-life of methadone exhibits wide interindividual variability, it generally ranges from 12 hours to more than 40 hours.1 Regardless, patients may feel anxious about potential opioid withdrawal, and this often leads them to access the ED for a missed dose.
The neuropsychiatric symptoms attending withdrawal may precede the objective signs of opioid withdrawal. Patients with objective signs of opioid withdrawal (eg, piloerection, vomiting, diarrhea, dilated pupils) may be sufficiently treated with supportive care alone, using antiemetics, hydration, and sometimes clonidine.
Administration of substitute opioids is problematic due to the patient’s underlying tolerance necessitating careful dose titration. Therefore, direct replacement of methadone in the ED remains controversial, and some EDs have strict policies prohibiting the administration of methadone to patients who have missed an MMT dose. Such policies, which are intended to discourage patients from using the ED as a convenience, may be appropriate given the generally benign—though uncomfortable—course of opioid withdrawal due to abstinence.
Other EDs provide replacement methadone for asymptomatic, treat-and-release patients confirmed to be enrolled in an MMT program when the time to the next dose is likely to be 24 hours or greater from the missed dose. Typically, a dose of no more than 10 mg orally or 10 mg intramuscularly (IM) is recommended, and patients should be advised that they will be receiving only a low dose to sufficient to prevent withdrawal—one that may not have the equivalent effects of the outpatient dose.
Whenever possible, a patient’s MMT program should be contacted and informed of the ED visit. For patients who display objective signs of withdrawal and who cannot be confirmed or who do not participate in an MMT program, 10 mg of methadone IM will prevent uncertainty of drug absorption in the setting of nausea or vomiting. All patients receiving oral methadone should be observed for 1 hour, and those receiving IM methadone should be observed for at least 90 minutes to assess for unexpected sedation.2
Patients encountering circumstances that prevent opioid access (eg, incarceration) and who are not in withdrawal but have gone without opioids for more than 5 days may have a loss of tolerance to their usual doses—whether the medication was obtained through an MMT program or illicitly. Harm-reduction strategies aimed at educating patients on the potential vulnerability to their familiar dosing regimens are warranted to avert inadvertent overdoses in chronic opioid users who are likely to resume illicit opoiod use.
Does this patient need syncope evaluation?
Further complicating the decision regarding ED dispensing of methadone are the effects of the drug on myocardial repolarization. Methadone affects conduction across the hERG potassium rectifier current and can prolong the QTc interval on the surface electrocardiogram (ECG), predisposing a patient to torsade de pointes (TdP). Although there is controversy regarding the role of ECG screening during the enrollment of patients in methadone maintenance clinics, doses above 60 mg, underlying myocardial disease, female sex, and electrolyte disturbances may increase the risk of QT prolongation and TdP.3
Whether there is value in obtaining a screening ECG in a patient receiving an initial dose of methadone in the ED is unclear, and this practice is controversial even among methadone clinics. However, some of the excess death in patients taking methadone may be explained by the dysrhythmogenic potential of methadone.4 An ECG therefore may elucidate a correctable cause in methadone patients presenting with syncope.
Administering methadone to patients with documented QT prolongation must weigh the risk of methadone’s conduction effects against the substantial risks of illicit opioid self-administration. For some patients at-risk for TdP, it may be preferable to use buprenorphine if possible, since it does not carry the same cardiac effects as methadone.1,5 Such therapy requires referral to a physician licensed to prescribe this medication.
How should admitted patients be managed?
While administration of methadone for withdrawal or maintenance therapy in the ED is acceptable, outpatient prescribing of methadone for these reasons is not legal, and only federally regulated clinics may engage in this practice. Hospitalized patients who are enrolled in an MMT program should have their daily methadone dose confirmed and continued—as long as the patient has not lost tolerance. Patients not participating in an MMT program can receive up to 3 days of methadone in the hospital, even if the practitioner is not registered to provide methadone.6 For these patients, it is recommended that the physician order a low dose of methadone and also consult with an addiction specialist to determine whether the patient should continue on MMT maintenance or undergo detoxification.
It is important to note that methadone may be prescribed for pain, but its use in the ED for this purpose is strongly discouraged, especially in patients who have never received methadone previously. For admitted patients requiring such potent opioid analgesia, consultation with a pain service or, when indicated, a palliative care/hospice specialist is warranted as the dosing intervals are different in each setting, and the risk of respiratory depression is high.
Case Conclusion
As requested by the MMT clinic, the patient was administered methadone 185 mg orally in the ED, though a dose of 10 mg would have been sufficient to prevent withdrawal. Unfortunately, the EP did not appreciate the relationship of the markedly prolonged QTc and the methadone, which should have prompted a dose reduction.
Evaluation of the patient’s electrolyte levels, which included magnesium and potassium, were normal. An ECG was repeated 24 hours later and revealed a persistent, but improved, QT interval at 505 ms. The remainder of the syncope workup was negative. Because the patient had no additional symptoms or events during her stay, she was discharged. At discharge, the EP followed up with the MMT clinic to discuss lowering the patient’s daily methadone dose, as well as close cardiology follow-up.
Dr Rao is the chief of the division of medical toxicology at New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Chou R, Weimer MB, Dana T. Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain. 2014;15(4):338-365.
- National Highway Traffic Safety Administration Web site. Methadone. http://www.nhtsa.gov/people/injury/research/job185drugs/methadone.htm. Accessed August 3, 2015.
- Martin JA, Campbell A, Killip T, et al; Substance Abuse and Mental Health Services Administration. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306. Erratum in: J Addict Dis. 2012;31(1):91.
- Ray WA, Chung CP, Murray KT, Cooper WO, Hall K, Stein CM. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med. 2015;175(3):420-427.
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209-219.
- US Government Printing Office. Federal Digital System. Administering or dispensing of narcotic drugs. Code of Federal Regulations. Title 21 CFR §1306.07. http://www.gpo.gov/fdsys/pkg/CFR-1998-title21-vol9/pdf/CFR-1998-title21-vol9-sec1306-07.pdf. Accessed August 4, 2015.
- Chou R, Weimer MB, Dana T. Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline. J Pain. 2014;15(4):338-365.
- National Highway Traffic Safety Administration Web site. Methadone. http://www.nhtsa.gov/people/injury/research/job185drugs/methadone.htm. Accessed August 3, 2015.
- Martin JA, Campbell A, Killip T, et al; Substance Abuse and Mental Health Services Administration. QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30(4):283-306. Erratum in: J Addict Dis. 2012;31(1):91.
- Ray WA, Chung CP, Murray KT, Cooper WO, Hall K, Stein CM. Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Intern Med. 2015;175(3):420-427.
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol. 2012;10(6):209-219.
- US Government Printing Office. Federal Digital System. Administering or dispensing of narcotic drugs. Code of Federal Regulations. Title 21 CFR §1306.07. http://www.gpo.gov/fdsys/pkg/CFR-1998-title21-vol9/pdf/CFR-1998-title21-vol9-sec1306-07.pdf. Accessed August 4, 2015.
Respiratory artifact: A second vital sign on the electrocardiogram
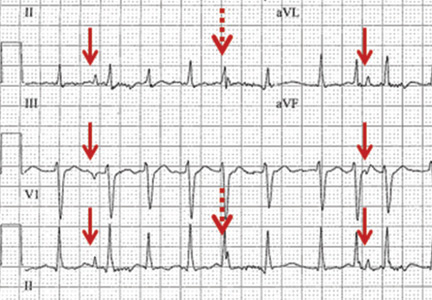
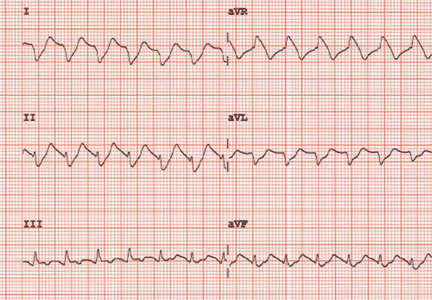
A 57-year-old man hospitalized for treatment of multilobar pneumonia was noted to have a rapid, irregular heart rate on telemetry. He was hypoxemic and appeared to be in respiratory distress. A 12-lead electrocardiogram (ECG) demonstrated atrial fibrillation with rapid ventricular response, as well as what looked like distinct and regular P waves dissociated from the QRS complexes at a rate of about 44/min (Figure 1). What is the explanation and clinical significance of this curious finding?
What appear to be dissociated P waves actually represent respiratory artifact.1–3 The sharp deflections mimicking P waves signify the tonic initiation of inspiratory effort; the subsequent brief periods of low-amplitude, high-frequency micro-oscillations represent surface electrical activity associated with the increased force of the accessory muscles of respiration.1–3
Surface electromyography noninvasively measures muscle activity using electrodes placed on the skin overlying the muscle.4 Using simultaneously recorded mechanical respiratory waveform tracings, we have previously demonstrated that the repetitive pseudo-P waves followed by micro-oscillations have a close temporal relationship with the inspiratory phase of respiration.3 The presence of respiratory artifact indicates a high-risk state frequently necessitating ventilation support.
In addition, when present, respiratory artifact can be viewed as the “second vital sign” on the ECG, the first vital sign being the heart rate. The respiratory rate can be approximated by counting the number of respiratory artifacts in a 10-second recording and multiplying it by 6. A more accurate rate assessment is achieved by measuring 1 or more respiratory artifact cycles in millimeters and then dividing that number into 1,500 or its multiples.3 Based on these calculations, the respiratory rate in this patient was 44/min.
The presence of two atrial rhythms on the same ECG, one not disturbing the other, is consistent with the diagnosis of atrial dissociation.5 Atrial dissociation is a common finding in cardiac transplant recipients in whom the transplantation was performed using atrio-atrial anastomosis.6 Most other cases of apparent atrial dissociation described in the old cardiology and critical care literature probably represented unrecognized respiratory artifact.7,8
An ECG from a different patient (Figure 2) demonstrates rapid respiratory artifact that raised awareness of severe respiratory failure. The respiratory rate calculated from spacing of the pseudo-P waves is 62/min, confirmed by simultaneous respirography.
A FREQUENT FINDING IN SICK HOSPITALIZED PATIENTS
Respiratory artifact is a frequent finding in sick hospitalized patients.3 Most commonly, it manifests as repetitive micro-oscillations.3 Pseudo-P waves, as in this 57-year-old patient, are less often observed; but if their origin is not recognized, the interpretation of the ECG can become puzzling.1–3,7,8
Respiratory artifact is a marker of increased work of breathing and a strong indicator of significant cardiopulmonary compromise. Improvement in the patient’s cardiac or respiratory condition is typically associated with a decrease in the rate or complete elimination of respiratory artifact.3
Recognition of rapid respiratory artifact is less important in critical care units, where patients’ vital signs and cardiorespiratory status are carefully observed. However, in hospital settings where respiratory rate and oxygen saturation are not continuously monitored, recognizing rapid respiratory artifact can help raise awareness of the possibility of severe respiratory distress.
- Higgins TG, Phillips JH Jr, Sumner RG. Atrial dissociation: an electrocardiographic artifact produced by the accessory muscles of respiration. Am J Cardiol 1966; 18:132–139.
- Cheriex EC, Brugada P, Wellens HJ. Pseudo-atrial dissociation: a respiratory artifact. Eur Heart J 1986; 7:357–359.
- Littmann L, Rennyson SL, Wall BP, Parker JM. Significance of respiratory artifact in the electrocardiogram. Am J Cardiol 2008; 102:1090–1096.
- Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000; 55:171–177.
- Chung EK. A reappraisal of atrial dissociation. Am J Cardiol 1971; 28:111–117.
- Stinson EB, Schroeder JS, Griepp RB, Shumway NE, Dong E Jr. Observations on the behavior of recipient atria after cardiac transplantation in man. Am J Cardiol 1972; 30:615–622.
- Cohen J, Scherf D. Complete interatrial and intra-atrial block (atrial dissociation). Am Heart J 1965; 70:23–34.
- Chung KY, Walsh TJ, Massie E. A review of atrial dissociation, with illustrative cases and critical discussion. Am J Med Sci 1965; 250:72–78.
A 57-year-old man hospitalized for treatment of multilobar pneumonia was noted to have a rapid, irregular heart rate on telemetry. He was hypoxemic and appeared to be in respiratory distress. A 12-lead electrocardiogram (ECG) demonstrated atrial fibrillation with rapid ventricular response, as well as what looked like distinct and regular P waves dissociated from the QRS complexes at a rate of about 44/min (Figure 1). What is the explanation and clinical significance of this curious finding?
What appear to be dissociated P waves actually represent respiratory artifact.1–3 The sharp deflections mimicking P waves signify the tonic initiation of inspiratory effort; the subsequent brief periods of low-amplitude, high-frequency micro-oscillations represent surface electrical activity associated with the increased force of the accessory muscles of respiration.1–3
Surface electromyography noninvasively measures muscle activity using electrodes placed on the skin overlying the muscle.4 Using simultaneously recorded mechanical respiratory waveform tracings, we have previously demonstrated that the repetitive pseudo-P waves followed by micro-oscillations have a close temporal relationship with the inspiratory phase of respiration.3 The presence of respiratory artifact indicates a high-risk state frequently necessitating ventilation support.
In addition, when present, respiratory artifact can be viewed as the “second vital sign” on the ECG, the first vital sign being the heart rate. The respiratory rate can be approximated by counting the number of respiratory artifacts in a 10-second recording and multiplying it by 6. A more accurate rate assessment is achieved by measuring 1 or more respiratory artifact cycles in millimeters and then dividing that number into 1,500 or its multiples.3 Based on these calculations, the respiratory rate in this patient was 44/min.
The presence of two atrial rhythms on the same ECG, one not disturbing the other, is consistent with the diagnosis of atrial dissociation.5 Atrial dissociation is a common finding in cardiac transplant recipients in whom the transplantation was performed using atrio-atrial anastomosis.6 Most other cases of apparent atrial dissociation described in the old cardiology and critical care literature probably represented unrecognized respiratory artifact.7,8
An ECG from a different patient (Figure 2) demonstrates rapid respiratory artifact that raised awareness of severe respiratory failure. The respiratory rate calculated from spacing of the pseudo-P waves is 62/min, confirmed by simultaneous respirography.
A FREQUENT FINDING IN SICK HOSPITALIZED PATIENTS
Respiratory artifact is a frequent finding in sick hospitalized patients.3 Most commonly, it manifests as repetitive micro-oscillations.3 Pseudo-P waves, as in this 57-year-old patient, are less often observed; but if their origin is not recognized, the interpretation of the ECG can become puzzling.1–3,7,8
Respiratory artifact is a marker of increased work of breathing and a strong indicator of significant cardiopulmonary compromise. Improvement in the patient’s cardiac or respiratory condition is typically associated with a decrease in the rate or complete elimination of respiratory artifact.3
Recognition of rapid respiratory artifact is less important in critical care units, where patients’ vital signs and cardiorespiratory status are carefully observed. However, in hospital settings where respiratory rate and oxygen saturation are not continuously monitored, recognizing rapid respiratory artifact can help raise awareness of the possibility of severe respiratory distress.
A 57-year-old man hospitalized for treatment of multilobar pneumonia was noted to have a rapid, irregular heart rate on telemetry. He was hypoxemic and appeared to be in respiratory distress. A 12-lead electrocardiogram (ECG) demonstrated atrial fibrillation with rapid ventricular response, as well as what looked like distinct and regular P waves dissociated from the QRS complexes at a rate of about 44/min (Figure 1). What is the explanation and clinical significance of this curious finding?
What appear to be dissociated P waves actually represent respiratory artifact.1–3 The sharp deflections mimicking P waves signify the tonic initiation of inspiratory effort; the subsequent brief periods of low-amplitude, high-frequency micro-oscillations represent surface electrical activity associated with the increased force of the accessory muscles of respiration.1–3
Surface electromyography noninvasively measures muscle activity using electrodes placed on the skin overlying the muscle.4 Using simultaneously recorded mechanical respiratory waveform tracings, we have previously demonstrated that the repetitive pseudo-P waves followed by micro-oscillations have a close temporal relationship with the inspiratory phase of respiration.3 The presence of respiratory artifact indicates a high-risk state frequently necessitating ventilation support.
In addition, when present, respiratory artifact can be viewed as the “second vital sign” on the ECG, the first vital sign being the heart rate. The respiratory rate can be approximated by counting the number of respiratory artifacts in a 10-second recording and multiplying it by 6. A more accurate rate assessment is achieved by measuring 1 or more respiratory artifact cycles in millimeters and then dividing that number into 1,500 or its multiples.3 Based on these calculations, the respiratory rate in this patient was 44/min.
The presence of two atrial rhythms on the same ECG, one not disturbing the other, is consistent with the diagnosis of atrial dissociation.5 Atrial dissociation is a common finding in cardiac transplant recipients in whom the transplantation was performed using atrio-atrial anastomosis.6 Most other cases of apparent atrial dissociation described in the old cardiology and critical care literature probably represented unrecognized respiratory artifact.7,8
An ECG from a different patient (Figure 2) demonstrates rapid respiratory artifact that raised awareness of severe respiratory failure. The respiratory rate calculated from spacing of the pseudo-P waves is 62/min, confirmed by simultaneous respirography.
A FREQUENT FINDING IN SICK HOSPITALIZED PATIENTS
Respiratory artifact is a frequent finding in sick hospitalized patients.3 Most commonly, it manifests as repetitive micro-oscillations.3 Pseudo-P waves, as in this 57-year-old patient, are less often observed; but if their origin is not recognized, the interpretation of the ECG can become puzzling.1–3,7,8
Respiratory artifact is a marker of increased work of breathing and a strong indicator of significant cardiopulmonary compromise. Improvement in the patient’s cardiac or respiratory condition is typically associated with a decrease in the rate or complete elimination of respiratory artifact.3
Recognition of rapid respiratory artifact is less important in critical care units, where patients’ vital signs and cardiorespiratory status are carefully observed. However, in hospital settings where respiratory rate and oxygen saturation are not continuously monitored, recognizing rapid respiratory artifact can help raise awareness of the possibility of severe respiratory distress.
- Higgins TG, Phillips JH Jr, Sumner RG. Atrial dissociation: an electrocardiographic artifact produced by the accessory muscles of respiration. Am J Cardiol 1966; 18:132–139.
- Cheriex EC, Brugada P, Wellens HJ. Pseudo-atrial dissociation: a respiratory artifact. Eur Heart J 1986; 7:357–359.
- Littmann L, Rennyson SL, Wall BP, Parker JM. Significance of respiratory artifact in the electrocardiogram. Am J Cardiol 2008; 102:1090–1096.
- Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000; 55:171–177.
- Chung EK. A reappraisal of atrial dissociation. Am J Cardiol 1971; 28:111–117.
- Stinson EB, Schroeder JS, Griepp RB, Shumway NE, Dong E Jr. Observations on the behavior of recipient atria after cardiac transplantation in man. Am J Cardiol 1972; 30:615–622.
- Cohen J, Scherf D. Complete interatrial and intra-atrial block (atrial dissociation). Am Heart J 1965; 70:23–34.
- Chung KY, Walsh TJ, Massie E. A review of atrial dissociation, with illustrative cases and critical discussion. Am J Med Sci 1965; 250:72–78.
- Higgins TG, Phillips JH Jr, Sumner RG. Atrial dissociation: an electrocardiographic artifact produced by the accessory muscles of respiration. Am J Cardiol 1966; 18:132–139.
- Cheriex EC, Brugada P, Wellens HJ. Pseudo-atrial dissociation: a respiratory artifact. Eur Heart J 1986; 7:357–359.
- Littmann L, Rennyson SL, Wall BP, Parker JM. Significance of respiratory artifact in the electrocardiogram. Am J Cardiol 2008; 102:1090–1096.
- Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000; 55:171–177.
- Chung EK. A reappraisal of atrial dissociation. Am J Cardiol 1971; 28:111–117.
- Stinson EB, Schroeder JS, Griepp RB, Shumway NE, Dong E Jr. Observations on the behavior of recipient atria after cardiac transplantation in man. Am J Cardiol 1972; 30:615–622.
- Cohen J, Scherf D. Complete interatrial and intra-atrial block (atrial dissociation). Am Heart J 1965; 70:23–34.
- Chung KY, Walsh TJ, Massie E. A review of atrial dissociation, with illustrative cases and critical discussion. Am J Med Sci 1965; 250:72–78.
Parvovirus mimicking acute HIV infection
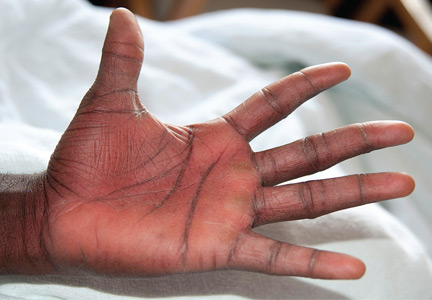
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
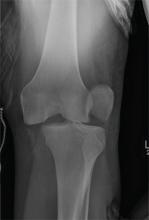
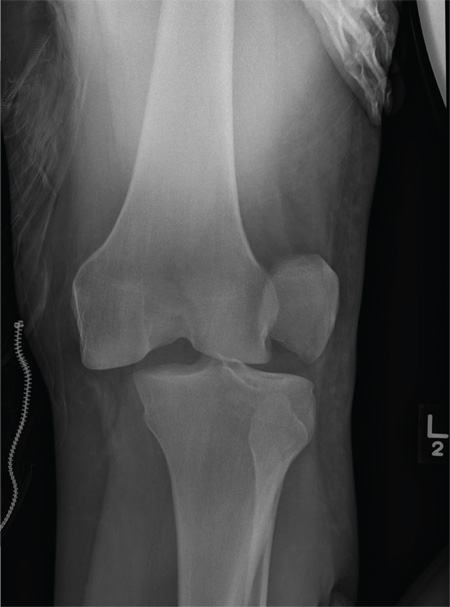
Driver Partially Ejected From Vehicle
ANSWER
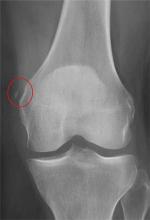
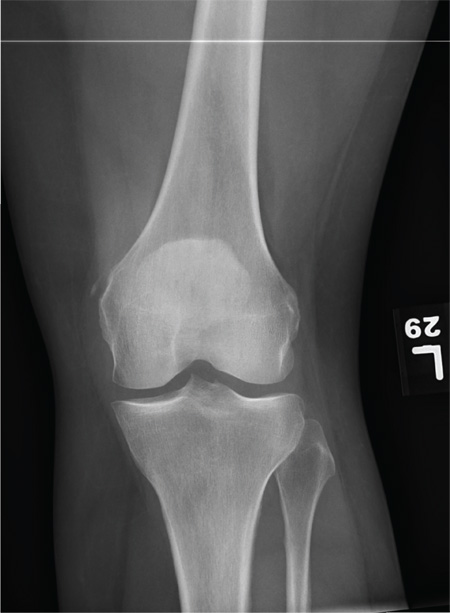
The radiograph shows that the distal femur is medially dislocated relative to the tibial plateau. In addition, the patella is laterally dislocated. No obvious fractures are evident.
Such injuries are typically associated with significant ligament injuries, especially of the medial collateral ligament (MCL), lateral collateral ligament (LCL), and anterior cruciate ligament (ACL). Orthopedics was consulted for reduction of the dislocation, as well as further workup (including MRI of the knee).
ANSWER
The radiograph shows that the distal femur is medially dislocated relative to the tibial plateau. In addition, the patella is laterally dislocated. No obvious fractures are evident.
Such injuries are typically associated with significant ligament injuries, especially of the medial collateral ligament (MCL), lateral collateral ligament (LCL), and anterior cruciate ligament (ACL). Orthopedics was consulted for reduction of the dislocation, as well as further workup (including MRI of the knee).
ANSWER
The radiograph shows that the distal femur is medially dislocated relative to the tibial plateau. In addition, the patella is laterally dislocated. No obvious fractures are evident.
Such injuries are typically associated with significant ligament injuries, especially of the medial collateral ligament (MCL), lateral collateral ligament (LCL), and anterior cruciate ligament (ACL). Orthopedics was consulted for reduction of the dislocation, as well as further workup (including MRI of the knee).
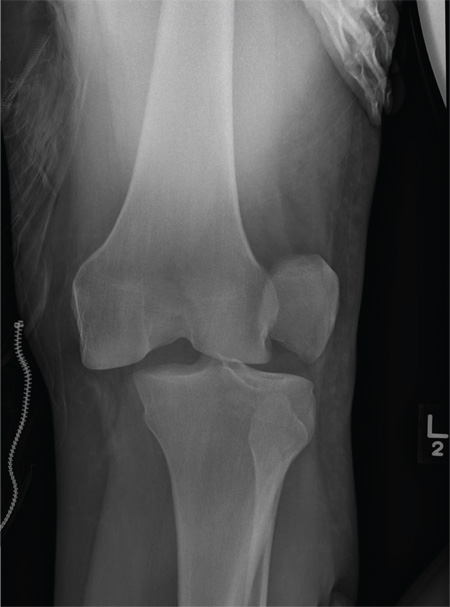
A 28-year-old man is brought to your facility by EMS for evaluation status post a motor vehicle accident. The patient was an unrestrained driver in a truck that went off the road into a ditch. The paramedics state that he was partially ejected, with his left leg caught in the window. There was brief loss of consciousness. Upon arrival, he is awake and alert, with a Glasgow Coma Scale score of 15. His primary complaints are of back and left leg pain. His medical history is unremarkable, and vital signs are stable. Primary survey shows no obvious injury. Secondary survey reveals moderate swelling and decreased range of motion in the left knee. Good distal pulses are present. As part of your orders, you request a portable radiograph of the left knee. What is your impression?
Case Studies in Toxicology: When Doing More for the Sake of Better Health Goes Wrong
Case
A 62-year-old man with a history of hypercholesterolemia and HIV infection presented to the ED for evaluation of diffuse myalgia and tea-colored urine. His medication history included lopinavir/ritonavir (Kaletra) and simvastatin. A week prior to presentation, the patient’s primary care physician had instructed him to increase his daily dose of simvastatin from 40 mg to 80 mg. The patient stated that he had taken simvastatin 80 mg daily for approximately 5 days and then, 2 days prior to presentation, had independently further increased the dose to 160 mg daily.
In the ED, the patient reported feeling fatigued. His initial vital signs were: blood pressure, 129/86 mm Hg; heart rate, 93 beats/minute; respiratory rate, 17 breaths/minute; and temperature, 98.5˚F. Oxygen saturation was 98% on room air. His physical examination was unremarkable. Initial laboratory testing revealed the following: creatine kinase (CK) 350,000 U/L; blood urea nitrogen, 27 mg/dL; creatinine, 0.7 mg/dL; aspartate aminotransferase (AST), 2,950 U/L; and alanine aminotransferase (ALT), 1,305 U/L.
What can cause tea-colored/cola-colored urine and myalgia?
Numerous medications can result in dark-colored urine. These include antimalarial drugs such as chloroquine and primaquine; antibiotics such as metronidazole or nitrofurantoin; and the muscle relaxant methocarbamol. Myalgia and tea-colored urine are the hallmarks of rhabdomyolysis. Rhabdomyolysis involves the destruction of myocytes, which can occur as a result of a long list of processes, including crush injuries, poor oxygenation or perfusion, hypermetabolic states, and direct (or indirect) toxin-mediated myocyte damage.1 The list of toxic substances that can cause rhabdomyolysis is extensive, and statins are one of the most common drug-induced causes (Table).
Simvastatin is one of seven currently available 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (ie, statins) that are commonly used to treat hypercholesterolemia. Because simvastatin is lipophilic, it can more readily cross cell membranes than nonlipophilic statins such as pravastatin. Simvastatin, therefore, has a propensity to disrupt the cellular integrity of myocytes and hepatocytes.What is the likely cause of this patient’s rhabdomyolysis?
At doses greater than 40 mg daily, simvastatin is associated with myalgia, myositis, and rhabdomyolysis. In December 2011, the US Food and Drug Administration (FDA) released a drug safety announcement recommending the originally approved maximum daily dose of simvastatin 80 mg be limited to patients who have already tolerated that dose for at least 12 months without evidence of muscular injury. The FDA further recommended no new patients be escalated to this dose. According to the FDA, patients taking 80 mg of simvastatin daily are also at increased risk of myopathy.
The metabolism of simvastatin, in addition to increased dosage of the drug, contributes to its potential for adverse effects. Of the seven available statins, only atorvastatin, lovastatin, and simvastatin are metabolized by the cytochrome P450 3A4 (CYP3A4). Lovastatin and simvastatin appear to have the highest potential for drug-drug interactions when coadministered with drugs that inhibit this enzyme (eg, ritonavir).2 The resulting elevation in blood concentration of simvastatin increases the risk of rhabdomyolysis. Other nonlipophilic statins, such as pravastatin, which are mostly eliminated unchanged in the urine and bile, would be preferable for patients taking CYP3A4 inhibitors.
How should patients with rhabdomyolysis be monitored?
Statins interfere with the myocyte’s ability to produce adenosine triphosphate, most likely by depleting coenzyme Q—one of the complexes found in the electron transport chain of the mitochondria. Under conditions of a high-energy requirement, myocytes incapable of producing sufficient energy ultimately fail and lyse, releasing cellular contents such as CK and myoglobin.1 The serum CK activity serves as a marker of muscle injury and should be monitored closely in patients with rhabdomyolysis. Although values above 5,000 U/L has been associated with renal injury,4 in healthy patients with access to hydration, renal injury is relatively uncommon with CK activities less than 50,000 U/L. Even though the prediction of renal failure is difficult, a validated nephrotoxicity prediction instrument using the patient’s age, gender, and initial laboratory data (serum creatinine, calcium, CK, phosphate, and bicarbonate) is available.5
Although the association between rhabdomyolysis and acute renal injury is well established, the mechanism remains unclear. Myoglobin from skeletal myocytes passes through the glomerulus without causing damage and is reabsorbed in the proximal renal tubular cell. Iron is subsequently released from the porphyrin ring and, in large concentrations, exceeds the binding capacity of the tissue ferritin. Because it is a transition metal, the free iron ion participates in oxidant stress reactions causing direct injury to the renal tubular cells.6 Furthermore, myoglobin also combines with renal tubular proteins, a process enhanced by an environment with lower pH, to form casts and cause renal tubular obstruction.
Patients with rhabdomyolysis may also be at risk for aminotransferase elevation, as occurred in the patient presented here. This elevation is most likely due to myocyte injury. In addition, potassium release due to myocyte destruction may cause life-threatening hyperkalemia, and phosphate liberation from these myocytes may cause hypocalcemia. Laboratory monitoring along with an electrocardiogram should be performed as required.
What is the treatment for rhabdomyolysis?
No adequate randomized controlled trials exist to guide the treatment of patients with rhabdomyolysis. As a result, recommendations for management come from retrospective observational studies, animal studies, case reports, and expert opinion.7
Once airway, breathing, and circulation have been addressed, patients with statin-induced rhabdomyolysis should be immediately treated with intravenous (IV) fluids to maintain renal perfusion, which helps to limit acute renal injury. Normal saline appears to be the most recommended fluid type, with a goal of maintaining a urine output of approximately 3 to 5 mL/kg/h.4,7
Some recommendations include the use of a sodium bicarbonate infusion to raise the urine pH, which may help limit the formation of renal casts from myoglobin. The data to support the benefit of sodium bicarbonate, however, is weak.3 A 2013 systematic review indicated that sodium bicarbonate should only be used to treat severe metabolic acidosis in patients with rhabdomyolysis.4
In addition to sodium bicarbonate, the use of diuretics is also discouraged by current recommendations. In patients with refractory electrolyte abnormalities or renal failure, hemodialysis may be required. Before disposition of a patient, his or her medication list should be reconciled to reflect statin discontinuation.
Case Conclusion
The patient received IV normal saline to maintain his urine output at 2 to 3 cc/kg/h. His repeat creatinine was 0.8 mg/dL and remained stable on repeat testing. His CK and AST concentrations trended down during his hospitalization. On hospital day 4, laboratory values were CK, less than 10,000 U/L; AST, 56 U/L; and ALT, 23 U/L. He had normal serum potassium levels and no dysrhythmia on electrocardiogram. His symptoms resolved on hospital day 2, and he was discharged on hospital day 4 with instructions to discontinue simvastatin.
Dr Fernandez is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815-831.
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196.
- Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90-105.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
- Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15(2):415-428, ix-x.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
Case
A 62-year-old man with a history of hypercholesterolemia and HIV infection presented to the ED for evaluation of diffuse myalgia and tea-colored urine. His medication history included lopinavir/ritonavir (Kaletra) and simvastatin. A week prior to presentation, the patient’s primary care physician had instructed him to increase his daily dose of simvastatin from 40 mg to 80 mg. The patient stated that he had taken simvastatin 80 mg daily for approximately 5 days and then, 2 days prior to presentation, had independently further increased the dose to 160 mg daily.
In the ED, the patient reported feeling fatigued. His initial vital signs were: blood pressure, 129/86 mm Hg; heart rate, 93 beats/minute; respiratory rate, 17 breaths/minute; and temperature, 98.5˚F. Oxygen saturation was 98% on room air. His physical examination was unremarkable. Initial laboratory testing revealed the following: creatine kinase (CK) 350,000 U/L; blood urea nitrogen, 27 mg/dL; creatinine, 0.7 mg/dL; aspartate aminotransferase (AST), 2,950 U/L; and alanine aminotransferase (ALT), 1,305 U/L.
What can cause tea-colored/cola-colored urine and myalgia?
Numerous medications can result in dark-colored urine. These include antimalarial drugs such as chloroquine and primaquine; antibiotics such as metronidazole or nitrofurantoin; and the muscle relaxant methocarbamol. Myalgia and tea-colored urine are the hallmarks of rhabdomyolysis. Rhabdomyolysis involves the destruction of myocytes, which can occur as a result of a long list of processes, including crush injuries, poor oxygenation or perfusion, hypermetabolic states, and direct (or indirect) toxin-mediated myocyte damage.1 The list of toxic substances that can cause rhabdomyolysis is extensive, and statins are one of the most common drug-induced causes (Table).
Simvastatin is one of seven currently available 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (ie, statins) that are commonly used to treat hypercholesterolemia. Because simvastatin is lipophilic, it can more readily cross cell membranes than nonlipophilic statins such as pravastatin. Simvastatin, therefore, has a propensity to disrupt the cellular integrity of myocytes and hepatocytes.What is the likely cause of this patient’s rhabdomyolysis?
At doses greater than 40 mg daily, simvastatin is associated with myalgia, myositis, and rhabdomyolysis. In December 2011, the US Food and Drug Administration (FDA) released a drug safety announcement recommending the originally approved maximum daily dose of simvastatin 80 mg be limited to patients who have already tolerated that dose for at least 12 months without evidence of muscular injury. The FDA further recommended no new patients be escalated to this dose. According to the FDA, patients taking 80 mg of simvastatin daily are also at increased risk of myopathy.
The metabolism of simvastatin, in addition to increased dosage of the drug, contributes to its potential for adverse effects. Of the seven available statins, only atorvastatin, lovastatin, and simvastatin are metabolized by the cytochrome P450 3A4 (CYP3A4). Lovastatin and simvastatin appear to have the highest potential for drug-drug interactions when coadministered with drugs that inhibit this enzyme (eg, ritonavir).2 The resulting elevation in blood concentration of simvastatin increases the risk of rhabdomyolysis. Other nonlipophilic statins, such as pravastatin, which are mostly eliminated unchanged in the urine and bile, would be preferable for patients taking CYP3A4 inhibitors.
How should patients with rhabdomyolysis be monitored?
Statins interfere with the myocyte’s ability to produce adenosine triphosphate, most likely by depleting coenzyme Q—one of the complexes found in the electron transport chain of the mitochondria. Under conditions of a high-energy requirement, myocytes incapable of producing sufficient energy ultimately fail and lyse, releasing cellular contents such as CK and myoglobin.1 The serum CK activity serves as a marker of muscle injury and should be monitored closely in patients with rhabdomyolysis. Although values above 5,000 U/L has been associated with renal injury,4 in healthy patients with access to hydration, renal injury is relatively uncommon with CK activities less than 50,000 U/L. Even though the prediction of renal failure is difficult, a validated nephrotoxicity prediction instrument using the patient’s age, gender, and initial laboratory data (serum creatinine, calcium, CK, phosphate, and bicarbonate) is available.5
Although the association between rhabdomyolysis and acute renal injury is well established, the mechanism remains unclear. Myoglobin from skeletal myocytes passes through the glomerulus without causing damage and is reabsorbed in the proximal renal tubular cell. Iron is subsequently released from the porphyrin ring and, in large concentrations, exceeds the binding capacity of the tissue ferritin. Because it is a transition metal, the free iron ion participates in oxidant stress reactions causing direct injury to the renal tubular cells.6 Furthermore, myoglobin also combines with renal tubular proteins, a process enhanced by an environment with lower pH, to form casts and cause renal tubular obstruction.
Patients with rhabdomyolysis may also be at risk for aminotransferase elevation, as occurred in the patient presented here. This elevation is most likely due to myocyte injury. In addition, potassium release due to myocyte destruction may cause life-threatening hyperkalemia, and phosphate liberation from these myocytes may cause hypocalcemia. Laboratory monitoring along with an electrocardiogram should be performed as required.
What is the treatment for rhabdomyolysis?
No adequate randomized controlled trials exist to guide the treatment of patients with rhabdomyolysis. As a result, recommendations for management come from retrospective observational studies, animal studies, case reports, and expert opinion.7
Once airway, breathing, and circulation have been addressed, patients with statin-induced rhabdomyolysis should be immediately treated with intravenous (IV) fluids to maintain renal perfusion, which helps to limit acute renal injury. Normal saline appears to be the most recommended fluid type, with a goal of maintaining a urine output of approximately 3 to 5 mL/kg/h.4,7
Some recommendations include the use of a sodium bicarbonate infusion to raise the urine pH, which may help limit the formation of renal casts from myoglobin. The data to support the benefit of sodium bicarbonate, however, is weak.3 A 2013 systematic review indicated that sodium bicarbonate should only be used to treat severe metabolic acidosis in patients with rhabdomyolysis.4
In addition to sodium bicarbonate, the use of diuretics is also discouraged by current recommendations. In patients with refractory electrolyte abnormalities or renal failure, hemodialysis may be required. Before disposition of a patient, his or her medication list should be reconciled to reflect statin discontinuation.
Case Conclusion
The patient received IV normal saline to maintain his urine output at 2 to 3 cc/kg/h. His repeat creatinine was 0.8 mg/dL and remained stable on repeat testing. His CK and AST concentrations trended down during his hospitalization. On hospital day 4, laboratory values were CK, less than 10,000 U/L; AST, 56 U/L; and ALT, 23 U/L. He had normal serum potassium levels and no dysrhythmia on electrocardiogram. His symptoms resolved on hospital day 2, and he was discharged on hospital day 4 with instructions to discontinue simvastatin.
Dr Fernandez is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 62-year-old man with a history of hypercholesterolemia and HIV infection presented to the ED for evaluation of diffuse myalgia and tea-colored urine. His medication history included lopinavir/ritonavir (Kaletra) and simvastatin. A week prior to presentation, the patient’s primary care physician had instructed him to increase his daily dose of simvastatin from 40 mg to 80 mg. The patient stated that he had taken simvastatin 80 mg daily for approximately 5 days and then, 2 days prior to presentation, had independently further increased the dose to 160 mg daily.
In the ED, the patient reported feeling fatigued. His initial vital signs were: blood pressure, 129/86 mm Hg; heart rate, 93 beats/minute; respiratory rate, 17 breaths/minute; and temperature, 98.5˚F. Oxygen saturation was 98% on room air. His physical examination was unremarkable. Initial laboratory testing revealed the following: creatine kinase (CK) 350,000 U/L; blood urea nitrogen, 27 mg/dL; creatinine, 0.7 mg/dL; aspartate aminotransferase (AST), 2,950 U/L; and alanine aminotransferase (ALT), 1,305 U/L.
What can cause tea-colored/cola-colored urine and myalgia?
Numerous medications can result in dark-colored urine. These include antimalarial drugs such as chloroquine and primaquine; antibiotics such as metronidazole or nitrofurantoin; and the muscle relaxant methocarbamol. Myalgia and tea-colored urine are the hallmarks of rhabdomyolysis. Rhabdomyolysis involves the destruction of myocytes, which can occur as a result of a long list of processes, including crush injuries, poor oxygenation or perfusion, hypermetabolic states, and direct (or indirect) toxin-mediated myocyte damage.1 The list of toxic substances that can cause rhabdomyolysis is extensive, and statins are one of the most common drug-induced causes (Table).
Simvastatin is one of seven currently available 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (ie, statins) that are commonly used to treat hypercholesterolemia. Because simvastatin is lipophilic, it can more readily cross cell membranes than nonlipophilic statins such as pravastatin. Simvastatin, therefore, has a propensity to disrupt the cellular integrity of myocytes and hepatocytes.What is the likely cause of this patient’s rhabdomyolysis?
At doses greater than 40 mg daily, simvastatin is associated with myalgia, myositis, and rhabdomyolysis. In December 2011, the US Food and Drug Administration (FDA) released a drug safety announcement recommending the originally approved maximum daily dose of simvastatin 80 mg be limited to patients who have already tolerated that dose for at least 12 months without evidence of muscular injury. The FDA further recommended no new patients be escalated to this dose. According to the FDA, patients taking 80 mg of simvastatin daily are also at increased risk of myopathy.
The metabolism of simvastatin, in addition to increased dosage of the drug, contributes to its potential for adverse effects. Of the seven available statins, only atorvastatin, lovastatin, and simvastatin are metabolized by the cytochrome P450 3A4 (CYP3A4). Lovastatin and simvastatin appear to have the highest potential for drug-drug interactions when coadministered with drugs that inhibit this enzyme (eg, ritonavir).2 The resulting elevation in blood concentration of simvastatin increases the risk of rhabdomyolysis. Other nonlipophilic statins, such as pravastatin, which are mostly eliminated unchanged in the urine and bile, would be preferable for patients taking CYP3A4 inhibitors.
How should patients with rhabdomyolysis be monitored?
Statins interfere with the myocyte’s ability to produce adenosine triphosphate, most likely by depleting coenzyme Q—one of the complexes found in the electron transport chain of the mitochondria. Under conditions of a high-energy requirement, myocytes incapable of producing sufficient energy ultimately fail and lyse, releasing cellular contents such as CK and myoglobin.1 The serum CK activity serves as a marker of muscle injury and should be monitored closely in patients with rhabdomyolysis. Although values above 5,000 U/L has been associated with renal injury,4 in healthy patients with access to hydration, renal injury is relatively uncommon with CK activities less than 50,000 U/L. Even though the prediction of renal failure is difficult, a validated nephrotoxicity prediction instrument using the patient’s age, gender, and initial laboratory data (serum creatinine, calcium, CK, phosphate, and bicarbonate) is available.5
Although the association between rhabdomyolysis and acute renal injury is well established, the mechanism remains unclear. Myoglobin from skeletal myocytes passes through the glomerulus without causing damage and is reabsorbed in the proximal renal tubular cell. Iron is subsequently released from the porphyrin ring and, in large concentrations, exceeds the binding capacity of the tissue ferritin. Because it is a transition metal, the free iron ion participates in oxidant stress reactions causing direct injury to the renal tubular cells.6 Furthermore, myoglobin also combines with renal tubular proteins, a process enhanced by an environment with lower pH, to form casts and cause renal tubular obstruction.
Patients with rhabdomyolysis may also be at risk for aminotransferase elevation, as occurred in the patient presented here. This elevation is most likely due to myocyte injury. In addition, potassium release due to myocyte destruction may cause life-threatening hyperkalemia, and phosphate liberation from these myocytes may cause hypocalcemia. Laboratory monitoring along with an electrocardiogram should be performed as required.
What is the treatment for rhabdomyolysis?
No adequate randomized controlled trials exist to guide the treatment of patients with rhabdomyolysis. As a result, recommendations for management come from retrospective observational studies, animal studies, case reports, and expert opinion.7
Once airway, breathing, and circulation have been addressed, patients with statin-induced rhabdomyolysis should be immediately treated with intravenous (IV) fluids to maintain renal perfusion, which helps to limit acute renal injury. Normal saline appears to be the most recommended fluid type, with a goal of maintaining a urine output of approximately 3 to 5 mL/kg/h.4,7
Some recommendations include the use of a sodium bicarbonate infusion to raise the urine pH, which may help limit the formation of renal casts from myoglobin. The data to support the benefit of sodium bicarbonate, however, is weak.3 A 2013 systematic review indicated that sodium bicarbonate should only be used to treat severe metabolic acidosis in patients with rhabdomyolysis.4
In addition to sodium bicarbonate, the use of diuretics is also discouraged by current recommendations. In patients with refractory electrolyte abnormalities or renal failure, hemodialysis may be required. Before disposition of a patient, his or her medication list should be reconciled to reflect statin discontinuation.
Case Conclusion
The patient received IV normal saline to maintain his urine output at 2 to 3 cc/kg/h. His repeat creatinine was 0.8 mg/dL and remained stable on repeat testing. His CK and AST concentrations trended down during his hospitalization. On hospital day 4, laboratory values were CK, less than 10,000 U/L; AST, 56 U/L; and ALT, 23 U/L. He had normal serum potassium levels and no dysrhythmia on electrocardiogram. His symptoms resolved on hospital day 2, and he was discharged on hospital day 4 with instructions to discontinue simvastatin.
Dr Fernandez is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815-831.
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196.
- Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90-105.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
- Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15(2):415-428, ix-x.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
- Bench-to-bedside review: Rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52(10):815-831.
- Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191-1196.
- Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90-105.
- McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
- Visweswaran P, Guntupalli J. Rhabdomyolysis. Crit Care Clin. 1999;15(2):415-428, ix-x.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
Electrocardiographic changes in amitriptyline overdose
A 49-year-old woman with a history of depression, bipolar disorder, and chronic back pain was brought to the emergency department unresponsive after having taken an unknown quantity of amitriptyline tablets.
On arrival, she was comatose, with a score of 3 (the lowest possible score) on the 15-point Glasgow Coma Scale. Her blood pressure was 65/22 mm Hg, heart rate 121 beats per minute, respiratory rate 14 per minute, and oxygen saturation 88% on room air. The rest of the initial physical examination was normal.
She was immediately intubated, put on mechanical ventilation, and given an infusion of a 1-L bolus of normal saline and 50 mmol (1 mmol/kg) of sodium bicarbonate. Norepinephrine infusion was started. Gastric lavage was not done.
Results of initial laboratory testing showed a serum potassium of 2.9 mmol/L (reference range 3.5–5.0) and a serum magnesium of 1.6 mmol/L (1.7–2.6), which were corrected with infusion of 60 mmol of potassium chloride and 2 g of magnesium sulfate. The serum amitriptyline measurement was ordered at the time of her presentation to the emergency department.
Arterial blood gas analysis showed:
- pH 7.15 (normal range 7.35–7.45)
- Paco2 66 mm Hg (34–46)
- Pao2 229 mm Hg (85–95)
- Bicarbonate 22 mmol/L (22–26).
The initial electrocardiogram (ECG) (Figure 1) showed regular wide-complex tachycardia with no definite right or left bundle branch block morphology, no discernible P waves, a QRS duration of 198 msec, right axis deviation, and no Brugada criteria to suggest ventricular tachycardia.
She remained hypotensive, with regular wide-complex tachycardia on the ECG. She was given an additional 1-L bolus of normal saline and 100 mmol (2 mmol/kg) of sodium bicarbonate, and within 1 minute the wide-complex tachycardia resolved to narrow-complex sinus tachycardia (Figure 2). At this point, an infusion of 150 mmol/L of sodium bicarbonate in dextrose 5% in water was started, with serial ECGs to monitor the QRS duration and serial arterial blood gas monitoring to maintain the pH between 7.45 and 7.55.
TRANSFER TO THE ICU
She was then transferred to the intensive care unit (ICU), where she remained for 2 weeks. While in the ICU, she had a single recurrence of wide-complex tachycardia that resolved immediately with an infusion of 100 mmol of sodium bicarbonate. A urine toxicology screen was negative, and the serum amitriptyline measurement, returned from the laboratory 48 hours after her initial presentation, was 594 ng/mL (reference range 100–250 ng/mL). She was eventually weaned off the norepinephrine infusion after 20 hours, the sodium bicarbonate infusion was discontinued after 4 days, and she was taken off mechanical ventilation after 10 days. Also during her ICU stay, she had seizures on day 3 and developed aspiration pneumonia.
From the ICU, she was transferred to a regular floor, where she stayed for another week and then was transferred to a rehabilitation center. This patient was known to have clinical depression and to have attempted suicide once before. She had recently been under additional psychosocial stresses, which likely prompted this second attempt.
She reportedly had no neurologic or cardiovascular sequelae after her discharge from the hospital.
AMITRIPTYLINE OVERDOSE
Amitriptyline causes a relatively high number of fatal overdoses, at 34 per 1 million prescriptions.1 Death is usually from hypotension and ventricular arrhythmia caused by blockage of cardiac fast sodium channels leading to disturbances of cardiac conduction such as wide-complex tachycardia.
Other manifestations of amitriptyline overdose include seizures, sedation, and anticholinergic toxicity from variable blockade of gamma-aminobutyric acid receptors, histamine 1 receptors, and alpha receptors.2
Of the various changes on ECG described with amitriptyline overdose, sinus tachycardia is the most common. A QRS duration greater than 100 msec, right to extreme-right axis deviation with negative QRS complexes in leads I and aVL, and an R-wave amplitude greater than 3 mm in lead aVR are indications for sodium bicarbonate infusion, especially in hemodynamically unstable patients.3 Sodium bicarbonate increases the serum concentration of sodium and thereby overcomes the sodium channel blockade. It also alkalinizes the serum, favoring an electrically neutral form of amitriptyline that binds less to receptors and binds more to alpha-1-acid glycoprotein, decreasing the fraction of free drug available for toxicity.4
In patients with amitriptyline overdose, wide-complex tachycardia and hypotension refractory to sodium bicarbonate infusion can be treated with lidocaine, magnesium sulfate, direct-current cardioversion, and lipid resuscitation.5,6 Treatment with class IA, IC, and III antiarrhythmics is contraindicated, as they block sodium channels and thus can worsen conduction disturbances.
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ 1995; 310:221–224.
- Shannon M, Merola J, Lovejoy FH Jr. Hypotension in severe tricyclic antidepressant overdose. Am J Emerg Med 1988; 6:439–442.
- Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med 1995; 26:195–201.
- Sayniuk BI, Jhamandas V. Mechanism of reversal of toxic effects of amitriptyline on cardiac Purkinje fibres by sodium bicarbonate. J Pharmacol Exp Ther 1984; 231:387.
- Kiberd MB, Minor SF. Lipid therapy for the treatment of a refractory amitriptyline overdose. CJEM 2012; 14:193–197.
- Harvey M, Cave G. Case report: successful lipid resuscitation in multidrug overdose with predominant tricyclic antidepressant toxidrome. Int J Emerg Med 2012; 5:8.
A 49-year-old woman with a history of depression, bipolar disorder, and chronic back pain was brought to the emergency department unresponsive after having taken an unknown quantity of amitriptyline tablets.
On arrival, she was comatose, with a score of 3 (the lowest possible score) on the 15-point Glasgow Coma Scale. Her blood pressure was 65/22 mm Hg, heart rate 121 beats per minute, respiratory rate 14 per minute, and oxygen saturation 88% on room air. The rest of the initial physical examination was normal.
She was immediately intubated, put on mechanical ventilation, and given an infusion of a 1-L bolus of normal saline and 50 mmol (1 mmol/kg) of sodium bicarbonate. Norepinephrine infusion was started. Gastric lavage was not done.
Results of initial laboratory testing showed a serum potassium of 2.9 mmol/L (reference range 3.5–5.0) and a serum magnesium of 1.6 mmol/L (1.7–2.6), which were corrected with infusion of 60 mmol of potassium chloride and 2 g of magnesium sulfate. The serum amitriptyline measurement was ordered at the time of her presentation to the emergency department.
Arterial blood gas analysis showed:
- pH 7.15 (normal range 7.35–7.45)
- Paco2 66 mm Hg (34–46)
- Pao2 229 mm Hg (85–95)
- Bicarbonate 22 mmol/L (22–26).
The initial electrocardiogram (ECG) (Figure 1) showed regular wide-complex tachycardia with no definite right or left bundle branch block morphology, no discernible P waves, a QRS duration of 198 msec, right axis deviation, and no Brugada criteria to suggest ventricular tachycardia.
She remained hypotensive, with regular wide-complex tachycardia on the ECG. She was given an additional 1-L bolus of normal saline and 100 mmol (2 mmol/kg) of sodium bicarbonate, and within 1 minute the wide-complex tachycardia resolved to narrow-complex sinus tachycardia (Figure 2). At this point, an infusion of 150 mmol/L of sodium bicarbonate in dextrose 5% in water was started, with serial ECGs to monitor the QRS duration and serial arterial blood gas monitoring to maintain the pH between 7.45 and 7.55.
TRANSFER TO THE ICU
She was then transferred to the intensive care unit (ICU), where she remained for 2 weeks. While in the ICU, she had a single recurrence of wide-complex tachycardia that resolved immediately with an infusion of 100 mmol of sodium bicarbonate. A urine toxicology screen was negative, and the serum amitriptyline measurement, returned from the laboratory 48 hours after her initial presentation, was 594 ng/mL (reference range 100–250 ng/mL). She was eventually weaned off the norepinephrine infusion after 20 hours, the sodium bicarbonate infusion was discontinued after 4 days, and she was taken off mechanical ventilation after 10 days. Also during her ICU stay, she had seizures on day 3 and developed aspiration pneumonia.
From the ICU, she was transferred to a regular floor, where she stayed for another week and then was transferred to a rehabilitation center. This patient was known to have clinical depression and to have attempted suicide once before. She had recently been under additional psychosocial stresses, which likely prompted this second attempt.
She reportedly had no neurologic or cardiovascular sequelae after her discharge from the hospital.
AMITRIPTYLINE OVERDOSE
Amitriptyline causes a relatively high number of fatal overdoses, at 34 per 1 million prescriptions.1 Death is usually from hypotension and ventricular arrhythmia caused by blockage of cardiac fast sodium channels leading to disturbances of cardiac conduction such as wide-complex tachycardia.
Other manifestations of amitriptyline overdose include seizures, sedation, and anticholinergic toxicity from variable blockade of gamma-aminobutyric acid receptors, histamine 1 receptors, and alpha receptors.2
Of the various changes on ECG described with amitriptyline overdose, sinus tachycardia is the most common. A QRS duration greater than 100 msec, right to extreme-right axis deviation with negative QRS complexes in leads I and aVL, and an R-wave amplitude greater than 3 mm in lead aVR are indications for sodium bicarbonate infusion, especially in hemodynamically unstable patients.3 Sodium bicarbonate increases the serum concentration of sodium and thereby overcomes the sodium channel blockade. It also alkalinizes the serum, favoring an electrically neutral form of amitriptyline that binds less to receptors and binds more to alpha-1-acid glycoprotein, decreasing the fraction of free drug available for toxicity.4
In patients with amitriptyline overdose, wide-complex tachycardia and hypotension refractory to sodium bicarbonate infusion can be treated with lidocaine, magnesium sulfate, direct-current cardioversion, and lipid resuscitation.5,6 Treatment with class IA, IC, and III antiarrhythmics is contraindicated, as they block sodium channels and thus can worsen conduction disturbances.
A 49-year-old woman with a history of depression, bipolar disorder, and chronic back pain was brought to the emergency department unresponsive after having taken an unknown quantity of amitriptyline tablets.
On arrival, she was comatose, with a score of 3 (the lowest possible score) on the 15-point Glasgow Coma Scale. Her blood pressure was 65/22 mm Hg, heart rate 121 beats per minute, respiratory rate 14 per minute, and oxygen saturation 88% on room air. The rest of the initial physical examination was normal.
She was immediately intubated, put on mechanical ventilation, and given an infusion of a 1-L bolus of normal saline and 50 mmol (1 mmol/kg) of sodium bicarbonate. Norepinephrine infusion was started. Gastric lavage was not done.
Results of initial laboratory testing showed a serum potassium of 2.9 mmol/L (reference range 3.5–5.0) and a serum magnesium of 1.6 mmol/L (1.7–2.6), which were corrected with infusion of 60 mmol of potassium chloride and 2 g of magnesium sulfate. The serum amitriptyline measurement was ordered at the time of her presentation to the emergency department.
Arterial blood gas analysis showed:
- pH 7.15 (normal range 7.35–7.45)
- Paco2 66 mm Hg (34–46)
- Pao2 229 mm Hg (85–95)
- Bicarbonate 22 mmol/L (22–26).
The initial electrocardiogram (ECG) (Figure 1) showed regular wide-complex tachycardia with no definite right or left bundle branch block morphology, no discernible P waves, a QRS duration of 198 msec, right axis deviation, and no Brugada criteria to suggest ventricular tachycardia.
She remained hypotensive, with regular wide-complex tachycardia on the ECG. She was given an additional 1-L bolus of normal saline and 100 mmol (2 mmol/kg) of sodium bicarbonate, and within 1 minute the wide-complex tachycardia resolved to narrow-complex sinus tachycardia (Figure 2). At this point, an infusion of 150 mmol/L of sodium bicarbonate in dextrose 5% in water was started, with serial ECGs to monitor the QRS duration and serial arterial blood gas monitoring to maintain the pH between 7.45 and 7.55.
TRANSFER TO THE ICU
She was then transferred to the intensive care unit (ICU), where she remained for 2 weeks. While in the ICU, she had a single recurrence of wide-complex tachycardia that resolved immediately with an infusion of 100 mmol of sodium bicarbonate. A urine toxicology screen was negative, and the serum amitriptyline measurement, returned from the laboratory 48 hours after her initial presentation, was 594 ng/mL (reference range 100–250 ng/mL). She was eventually weaned off the norepinephrine infusion after 20 hours, the sodium bicarbonate infusion was discontinued after 4 days, and she was taken off mechanical ventilation after 10 days. Also during her ICU stay, she had seizures on day 3 and developed aspiration pneumonia.
From the ICU, she was transferred to a regular floor, where she stayed for another week and then was transferred to a rehabilitation center. This patient was known to have clinical depression and to have attempted suicide once before. She had recently been under additional psychosocial stresses, which likely prompted this second attempt.
She reportedly had no neurologic or cardiovascular sequelae after her discharge from the hospital.
AMITRIPTYLINE OVERDOSE
Amitriptyline causes a relatively high number of fatal overdoses, at 34 per 1 million prescriptions.1 Death is usually from hypotension and ventricular arrhythmia caused by blockage of cardiac fast sodium channels leading to disturbances of cardiac conduction such as wide-complex tachycardia.
Other manifestations of amitriptyline overdose include seizures, sedation, and anticholinergic toxicity from variable blockade of gamma-aminobutyric acid receptors, histamine 1 receptors, and alpha receptors.2
Of the various changes on ECG described with amitriptyline overdose, sinus tachycardia is the most common. A QRS duration greater than 100 msec, right to extreme-right axis deviation with negative QRS complexes in leads I and aVL, and an R-wave amplitude greater than 3 mm in lead aVR are indications for sodium bicarbonate infusion, especially in hemodynamically unstable patients.3 Sodium bicarbonate increases the serum concentration of sodium and thereby overcomes the sodium channel blockade. It also alkalinizes the serum, favoring an electrically neutral form of amitriptyline that binds less to receptors and binds more to alpha-1-acid glycoprotein, decreasing the fraction of free drug available for toxicity.4
In patients with amitriptyline overdose, wide-complex tachycardia and hypotension refractory to sodium bicarbonate infusion can be treated with lidocaine, magnesium sulfate, direct-current cardioversion, and lipid resuscitation.5,6 Treatment with class IA, IC, and III antiarrhythmics is contraindicated, as they block sodium channels and thus can worsen conduction disturbances.
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ 1995; 310:221–224.
- Shannon M, Merola J, Lovejoy FH Jr. Hypotension in severe tricyclic antidepressant overdose. Am J Emerg Med 1988; 6:439–442.
- Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med 1995; 26:195–201.
- Sayniuk BI, Jhamandas V. Mechanism of reversal of toxic effects of amitriptyline on cardiac Purkinje fibres by sodium bicarbonate. J Pharmacol Exp Ther 1984; 231:387.
- Kiberd MB, Minor SF. Lipid therapy for the treatment of a refractory amitriptyline overdose. CJEM 2012; 14:193–197.
- Harvey M, Cave G. Case report: successful lipid resuscitation in multidrug overdose with predominant tricyclic antidepressant toxidrome. Int J Emerg Med 2012; 5:8.
- Henry JA, Alexander CA, Sener EK. Relative mortality from overdose of antidepressants. BMJ 1995; 310:221–224.
- Shannon M, Merola J, Lovejoy FH Jr. Hypotension in severe tricyclic antidepressant overdose. Am J Emerg Med 1988; 6:439–442.
- Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med 1995; 26:195–201.
- Sayniuk BI, Jhamandas V. Mechanism of reversal of toxic effects of amitriptyline on cardiac Purkinje fibres by sodium bicarbonate. J Pharmacol Exp Ther 1984; 231:387.
- Kiberd MB, Minor SF. Lipid therapy for the treatment of a refractory amitriptyline overdose. CJEM 2012; 14:193–197.
- Harvey M, Cave G. Case report: successful lipid resuscitation in multidrug overdose with predominant tricyclic antidepressant toxidrome. Int J Emerg Med 2012; 5:8.
Woman Complains of Knee Pain Following Fight
ANSWER
The radiograph shows a small calcification along the medial aspect of the medial collateral ligament. This finding is known as a Pellegrini-Stieda lesion. While it certainly could represent a small avulsion fracture, the lack of joint fluid and soft-tissue swelling makes this diagnosis less likely. The patient was treated symptomatically with anti-inflammatory medications.
ANSWER
The radiograph shows a small calcification along the medial aspect of the medial collateral ligament. This finding is known as a Pellegrini-Stieda lesion. While it certainly could represent a small avulsion fracture, the lack of joint fluid and soft-tissue swelling makes this diagnosis less likely. The patient was treated symptomatically with anti-inflammatory medications.
ANSWER
The radiograph shows a small calcification along the medial aspect of the medial collateral ligament. This finding is known as a Pellegrini-Stieda lesion. While it certainly could represent a small avulsion fracture, the lack of joint fluid and soft-tissue swelling makes this diagnosis less likely. The patient was treated symptomatically with anti-inflammatory medications.
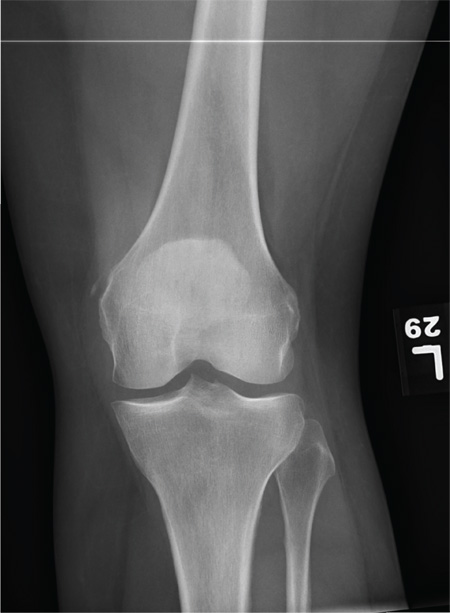
A 35-year-old woman presents for evaluation of left knee pain secondary to an assault. She says she was involved in a fight and was struck multiple times throughout her whole body. She states she is “sore all over,” but her knee bothers her the most, as it is difficult and painful to bear weight. The patient’s medical history is unremarkable. Physical exam shows a young female who is uncomfortable but in no obvious distress. Her vital signs are normal. You note bruises throughout her body. Inspection of her left knee shows no obvious deformity or swelling. There is some mild bruising and pain present to palpation. She has limited flexion and extension secondary to pain. However, the joint itself appears stable. Radiographs of the knee are obtained. What is your impression?
Girl, 5, With Fever and Hip Pain
A 5-year-old Filipino girl was brought to a pediatric clinic for follow up of an unresolved fever and for new-onset right hip pain, which occurred intermittently for the past week and was associated with a right-sided limp. She had been experiencing nightly fevers ranging from 101°F to 105°F for the past two weeks, for which her parents had been giving ibuprofen with mixed results; she remained afebrile during daytime hours.
Using the Wong-Baker FACES pain scale, the patient rated the pain as a 4/10 in severity (“Hurts a Little More” face).1 Standing and walking aggravated the pain but did not limit activity. Although ibuprofen decreased the fever, it did not alleviate the hip pain. Other symptoms included vomiting one to two times daily, without hematemesis, and four to five episodes of diarrhea daily, without abdominal pain, hematochezia, or melena. She also experienced decreased appetite, but her parents reported no change in her dietary or fluid intake. The patient and her parents denied additional symptoms.
Further investigation revealed that the patient had been seen a week earlier by two other clinicians in the office for complaints of fever, rash, nausea, hematemesis, and diarrhea. She had been diagnosed with a herpes simplex viral (HSV) lesion of the nose, epistaxis, and viral gastroenteritis. Her treatment plan consisted of acyclovir ointment for the HSV lesion and symptomatic support for the gastroenteritis associated diarrhea. The complaint of hematemesis was attributed to postnasal drip from the epistaxis, and reassurance was provided to the patient and family. In addition, six weeks earlier, the patient had been treated for otitis media with a full course of amoxicillin.
Medical history was negative for surgeries, trauma, injuries, and chronic medical conditions. She took no medications or supplements on a regular basis. Her parents denied any known drug allergies and stated that her immunizations were up to date.
The patient lived at home with her biological parents and two brothers, all of whom were healthy, without any recent infections or illnesses. Of significance, the family had travelled to the Philippines for vacation about four months earlier. Results of a tuberculin skin test done six weeks earlier (because the patient presented with respiratory symptoms shortly after traveling to the Philippines) were negative.
Physical examination revealed a well-developed, well-nourished 40-lb girl, in no acute distress, who was active and playful with her brother while in the exam room. Vital signs were significant for a fever of 101.9°F (last dose of ibuprofen was approximately six hours earlier) but were otherwise stable. Skin exam revealed that the prior HSV lesion of the nose had resolved. HEENT, cardiovascular, and pulmonary exam findings were noncontributory. Urine dipstick was negative.
Abdominal exam revealed normoactive bowel sounds in all four quadrants, and on palpation, the abdomen was soft, nontender, and without organomegaly. Specialized abdominal exams to assess for peritonitis, including those to elicit Rovsing, rebound tenderness, obturator, and psoas signs, were all negative. Bilateral extremity exams of the hips, knees, and ankles revealed full range of motion (active and passive), with normal muscle strength throughout. The only significant finding on the physical exam was mild pain of the right anterior hip at 15° of flexion, appreciated while the patient was supine on the exam table. The patient was also observed pushing off her right lower extremity when climbing onto the exam table, and she skipped down the hall when leaving the exam.
With fever of unknown origin (FUO) and a largely negative history and physical, the working list of differential diagnoses included
• Avascular necrosis
• Bacteremia
• Juvenile idiopathic arthritis
• Osteomyelitis
• Pyelonephritis
• Reiter syndrome
• Rheumatic fever
• Rheumatoid arthritis
• Septic joint
• Urinary tract infection
To begin the diagnostic process, a number of laboratory tests and imaging procedures were ordered. Table 1 presents the results of these studies. A tuberculin skin test was not repeated. While awaiting test results, the patient was started on naproxen oral suspension (125 mg/5 mL; 4 mL bid) for fever and pain control.
Based on findings consistent with an inflammatory pattern, the history of otitis media (of possible streptococcal origin) six weeks prior to this visit, and the elevated ASO titer, the patient was started on penicillin V (250 mg bid) and instructed to return for follow up in two days.
At the follow-up visit, no improvement was noted; the patient continued to experience nightly fevers and hip pain. Rovsing, rebound tenderness, obturator, and psoas signs continued to be negative. Physical examination did, however, reveal a mild abdominal tenderness in the right lower quadrant.
Due to this new finding, an abdominal ultrasound was ordered to screen for appendicitis. Despite the parents’ appropriate concern for the child, misunderstanding about the urgent need to obtain the abdominal ultrasound led to a two-day delay in scheduling the exam. Results of ultrasonography revealed psoas abscess, and the patient was promptly admitted to the pediatric floor of the local hospital.
Continue for discussion >>
DISCUSSION
Psoas abscess is a collection of pus in the iliopsoas compartment, an extraperitoneal space containing the psoas and iliacus muscles.2 It can be life-threatening if the infection progresses to septic shock. Historically, psoas abscesses were a frequent complication of tuberculosis (TB) of the spine; but with modern TB treatment, these abscesses have become rare.2 Paradoxically, increased utilization of CT to evaluate sepsis of unknown etiology has led to a recent increase in the frequency of psoas abscess diagnosis.3
Psoas abscesses are categorized as either primary or secondary, with primary infections originating in the psoas muscle and secondary infections spreading from adjacent organs.2 In 42% to 88% of cases (depending on the study), primary psoas abscesses are caused by the hematogenous spread of Staphylococcus aureus from distant infection sites.2,4,5 The psoas muscle is particularly susceptible to this mode of infection because of its rich vascular supply.6 Children, immunosuppressed adults (ie, patients with diabetes, HIV/AIDS, or renal failure), IV drug users, and patients with a history of trauma to the muscle are most susceptible to developing a primary psoas abscess.2,5
Secondary psoas abscesses are caused by infections involving adjacent structures of the gastrointestinal, urinary, and skeletal systems. They are most frequently associated with intra-abdominal inflammatory processes, with the most common etiology being Crohn disease.5 Secondary psoas abscesses, though more diverse in their bacterial flora, tend to follow certain microbiologic patterns based on the inoculating source; Escherichia coli is the most common pathogen in secondary abscesses caused by gastrointestinal (42%) and urinary (61%) sources, and S aureus the most common (35%) from skeletal origins (ie, osteomyelitis).4,5Mycobacterium tuberculosis is the more frequently found cause in developing countries but should be considered if the patient has recently travelled outside the United States.
Review of the literature suggests that the incidence of methicillin-resistant S aureus (MRSA) as the causative agent of psoas abscesses may be increasing. However, there is a wide variance in the incidence reported, ranging from 1.1% to 12% of confirmed microbial infections.5,7,8
The classic historical presentation of psoas abscess has been described as the triad of back pain, fever, and limp5,6; however, this triad has only been described in approximately 30% of cases.5 The typical presentation consists of flank or lower limb pain (91%), fever (75%), anorexia (46%), and/or weakness (43%).4 Laboratory abnormalities include leukocytosis (67%) and elevated markers of inflammation (eg, erythrocyte sedimentation rate, seen in 73% of cases).4
Imaging via abdominal ultrasound may be helpful to screen for psoas abscess; however, its utility is limited by a low diagnostic yield of 60% or less.2,4 Direct visualization of the retroperitoneal structures, for example, can be problematic due to the presence of bowel gas.9 Abdominal CT is considered the gold standard for the definitive diagnosis of psoas abscess due to its high sensitivity (100%) and specificity (77%); it can also be used simultaneously to guide percutaneous drainage to treat the abscess if needed.7 However, some clinicians prefer abdominal MRI because of its ability to enhance soft-tissue visualization without requiring use of IV contrast.2,4
The approach to treating psoas abscess varies from a strictly antibiotic regimen to percutaneous drainage, and in rare circumstances, open surgical drainage. Antibiotic therapy without drainage or surgical intervention is a sufficient starting point for treatment of abscesses less than 3 cm in size.3
The antibiotic regimen choice depends on the suspected pathogen. In cases of suspected S aureus, empiric antistaphylococcal antibiotics should be initiated while culture results are pending.2,4 Secondary psoas abscesses thought to be derived from a urinary or gastrointestinal source should prompt use of a broader spectrum antibiotic due to the higher probability of gram-negative, anaerobic, or polymicrobial involvement.2,4
Once final culture and sensitivity results are obtained, antibiotic therapy should be modified to target the isolated pathogen(s). Treatment duration is typically six weeks but may vary, depending on serial culture results and the inoculating source.4 Review of the literature reveals that abscesses resulting from skeletal sources have traditionally been treated longer, usually with antibiotics alone, than those from urinary or gastrointestinal sources, which are often treated with the combination of antibiotics and percutaneous drainage.4
In cases of psoas abscesses larger than 3 cm, management should include both appropriate antibiotics and percutaneous drainage of the abscess.2 Percutaneous drainage is preferred to open surgical drainage because outcomes are similar, it is less invasive, and there is less risk of spreading abscess contents.2-4 In a retrospective analysis by Dietrich et al, 50% of patients treated with antibiotics and percutaneous drainage responded after one drainage, but the success rate increased to 100% after a second drainage.7 In addition, percutaneous drainage was associated with a lower mortality rate and a shorter hospital stay when compared to open surgical drainage.7
Open surgical drainage is rarely performed and usually only considered if the patient is not responding to a combination of focused antibiotic treatment and percutaneous drainage or has associated comorbidities, such as Crohn ileocolitis.2-4 In a retrospective analysis by Tabrizian et al, percutaneous drainage served as a bridge to open surgical drainage in nearly all patients with a gastrointestinal origin, such as Crohn disease, diverticulitis, appendicitis, and/or pancreatitis.6
Treatment of psoas abscesses has an overall failure rate of 15.8%, with an associated mortality rate of less than 7%.4 Overall prognosis is good, but outcomes can be negatively affected by such factors as advanced age, delay in diagnosis, bacteremia, and other comorbidities.4
Next page: Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
The patient required an 11-day hospitalization; her day-by-day course is described briefly below.
Day 1. Upon admission, abdominal MRI was ordered (see Figure 1) and empiric piperacillin/tazobactam IV was initiated. C-reactive protein (CRP) level and white blood cell (WBC) counts were elevated (see Table 2). Infectious disease, surgery, and urology consults were obtained.
Day 2. Fine-needle aspiration of the abscess was performed for cultures, and 40 mL of purulent fluid was drained. Piperacillin/tazobactam administration was continued, but the patient experienced ongoing fever and vomiting.
Day 3. Preliminary aspirate culture results revealed S aureus infection. Piperacillin/tazobactam was discontinued, and vancomycin IV was started. CRP levels and WBC counts decreased, as did fever and vomiting.
Day 4. Final aspirate culture results identified MRSA infection, sensitive to clindamycin. Vancomycin was discontinued, and clindamycin IV was started. Although the patient’s condition improved somewhat, fever and vomiting persisted.
Day 5. Both CRP levels and WBC counts increased from day 3. A surgical consult was sought.
Day 6. Repeat abdominal MRI revealed a decrease in the size of the abscess (see Figure 2, page 30. CRP levels and WBC counts remained high, with persistent fever and vomiting.
Day 7. The clinical team, in consultation with the parents, determined that placement of a peripherally inserted central catheter (PICC) line for drainage of the abscess was necessary.
Day 8. A 10-French pigtail catheter was inserted into the abscess, 20 mL of purulent fluid was drained, and a PICC line was inserted. Clindamycin IV was continued and, eight hours after the catheter was placed, fever and vomiting resolved.
Day 9. Both CRP levels and WBC counts dropped by half (WBC count was normal), while 10 mL of clear fluid drained from the catheter. The patient remained afebrile, without nausea or vomiting, on clindamycin IV.
Day 10. After 36 hours of clear drainage, the catheter was removed. CRP level further decreased. Clindamycin IV was discontinued, and the patient, now asymptomatic, was started on oral clindamycin.
Day 11. The patient was discharged on a regimen of oral clindamycin for six weeks, with weekly abdominal ultrasounds. She completed her entire course of antibiotics and fully recovered from the infection.
Next page: Conclusion >>
CONCLUSION
Since children generally compensate well during times of increased stress on the body, it is vital that persistent FUOs continue to be evaluated until a definitive source is identified, especially in this population. Early diagnosis and treatment of psoas abscess is essential for better outcomes, since delay is associated with a greater risk for sepsis.
While the likelihood of developing psoas abscess is low, it is worth keeping the diagnosis in mind for cases of unexplained lower abdominal pain, flank pain, or hip pain when more common etiologies have been excluded. This is especially important in the setting of recent travel to a developing country due to the fact that a psoas abscess can be a complication of TB of the spine.
The authors would like to thank Jeff Brand, MD, for his assistance in the preparation of this manuscript.
REFERENCES
1. Wong-Baker Faces Corporation. Wong-Baker FACES Pain Rating Scale. www.wongbakerfaces.org. Accessed May 19, 2015.
2. Mallick IH, Thoufreeq MH, Rajendren TP. Iliopsoas abscesses. Postgrad Med J. 2004;80(946):459-462.
3. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223-227.
4. Lopez VN, Ramos JM, Meseguer V, et al; The Infectious Diseases Study Group of the Spanish Society of Internal Medicine. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine. 2009;88(2):120-130.
5. Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg. 2012;10(9):466-469.
6. Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg. 2009;144(10):946-949.
7. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23(1):45-48.
8. Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19(5):416-423.
9. Woo MY. Psoas abscess. J Emerg Med. 2014;47(5):e129-e130.
A 5-year-old Filipino girl was brought to a pediatric clinic for follow up of an unresolved fever and for new-onset right hip pain, which occurred intermittently for the past week and was associated with a right-sided limp. She had been experiencing nightly fevers ranging from 101°F to 105°F for the past two weeks, for which her parents had been giving ibuprofen with mixed results; she remained afebrile during daytime hours.
Using the Wong-Baker FACES pain scale, the patient rated the pain as a 4/10 in severity (“Hurts a Little More” face).1 Standing and walking aggravated the pain but did not limit activity. Although ibuprofen decreased the fever, it did not alleviate the hip pain. Other symptoms included vomiting one to two times daily, without hematemesis, and four to five episodes of diarrhea daily, without abdominal pain, hematochezia, or melena. She also experienced decreased appetite, but her parents reported no change in her dietary or fluid intake. The patient and her parents denied additional symptoms.
Further investigation revealed that the patient had been seen a week earlier by two other clinicians in the office for complaints of fever, rash, nausea, hematemesis, and diarrhea. She had been diagnosed with a herpes simplex viral (HSV) lesion of the nose, epistaxis, and viral gastroenteritis. Her treatment plan consisted of acyclovir ointment for the HSV lesion and symptomatic support for the gastroenteritis associated diarrhea. The complaint of hematemesis was attributed to postnasal drip from the epistaxis, and reassurance was provided to the patient and family. In addition, six weeks earlier, the patient had been treated for otitis media with a full course of amoxicillin.
Medical history was negative for surgeries, trauma, injuries, and chronic medical conditions. She took no medications or supplements on a regular basis. Her parents denied any known drug allergies and stated that her immunizations were up to date.
The patient lived at home with her biological parents and two brothers, all of whom were healthy, without any recent infections or illnesses. Of significance, the family had travelled to the Philippines for vacation about four months earlier. Results of a tuberculin skin test done six weeks earlier (because the patient presented with respiratory symptoms shortly after traveling to the Philippines) were negative.
Physical examination revealed a well-developed, well-nourished 40-lb girl, in no acute distress, who was active and playful with her brother while in the exam room. Vital signs were significant for a fever of 101.9°F (last dose of ibuprofen was approximately six hours earlier) but were otherwise stable. Skin exam revealed that the prior HSV lesion of the nose had resolved. HEENT, cardiovascular, and pulmonary exam findings were noncontributory. Urine dipstick was negative.
Abdominal exam revealed normoactive bowel sounds in all four quadrants, and on palpation, the abdomen was soft, nontender, and without organomegaly. Specialized abdominal exams to assess for peritonitis, including those to elicit Rovsing, rebound tenderness, obturator, and psoas signs, were all negative. Bilateral extremity exams of the hips, knees, and ankles revealed full range of motion (active and passive), with normal muscle strength throughout. The only significant finding on the physical exam was mild pain of the right anterior hip at 15° of flexion, appreciated while the patient was supine on the exam table. The patient was also observed pushing off her right lower extremity when climbing onto the exam table, and she skipped down the hall when leaving the exam.
With fever of unknown origin (FUO) and a largely negative history and physical, the working list of differential diagnoses included
• Avascular necrosis
• Bacteremia
• Juvenile idiopathic arthritis
• Osteomyelitis
• Pyelonephritis
• Reiter syndrome
• Rheumatic fever
• Rheumatoid arthritis
• Septic joint
• Urinary tract infection
To begin the diagnostic process, a number of laboratory tests and imaging procedures were ordered. Table 1 presents the results of these studies. A tuberculin skin test was not repeated. While awaiting test results, the patient was started on naproxen oral suspension (125 mg/5 mL; 4 mL bid) for fever and pain control.
Based on findings consistent with an inflammatory pattern, the history of otitis media (of possible streptococcal origin) six weeks prior to this visit, and the elevated ASO titer, the patient was started on penicillin V (250 mg bid) and instructed to return for follow up in two days.
At the follow-up visit, no improvement was noted; the patient continued to experience nightly fevers and hip pain. Rovsing, rebound tenderness, obturator, and psoas signs continued to be negative. Physical examination did, however, reveal a mild abdominal tenderness in the right lower quadrant.
Due to this new finding, an abdominal ultrasound was ordered to screen for appendicitis. Despite the parents’ appropriate concern for the child, misunderstanding about the urgent need to obtain the abdominal ultrasound led to a two-day delay in scheduling the exam. Results of ultrasonography revealed psoas abscess, and the patient was promptly admitted to the pediatric floor of the local hospital.
Continue for discussion >>
DISCUSSION
Psoas abscess is a collection of pus in the iliopsoas compartment, an extraperitoneal space containing the psoas and iliacus muscles.2 It can be life-threatening if the infection progresses to septic shock. Historically, psoas abscesses were a frequent complication of tuberculosis (TB) of the spine; but with modern TB treatment, these abscesses have become rare.2 Paradoxically, increased utilization of CT to evaluate sepsis of unknown etiology has led to a recent increase in the frequency of psoas abscess diagnosis.3
Psoas abscesses are categorized as either primary or secondary, with primary infections originating in the psoas muscle and secondary infections spreading from adjacent organs.2 In 42% to 88% of cases (depending on the study), primary psoas abscesses are caused by the hematogenous spread of Staphylococcus aureus from distant infection sites.2,4,5 The psoas muscle is particularly susceptible to this mode of infection because of its rich vascular supply.6 Children, immunosuppressed adults (ie, patients with diabetes, HIV/AIDS, or renal failure), IV drug users, and patients with a history of trauma to the muscle are most susceptible to developing a primary psoas abscess.2,5
Secondary psoas abscesses are caused by infections involving adjacent structures of the gastrointestinal, urinary, and skeletal systems. They are most frequently associated with intra-abdominal inflammatory processes, with the most common etiology being Crohn disease.5 Secondary psoas abscesses, though more diverse in their bacterial flora, tend to follow certain microbiologic patterns based on the inoculating source; Escherichia coli is the most common pathogen in secondary abscesses caused by gastrointestinal (42%) and urinary (61%) sources, and S aureus the most common (35%) from skeletal origins (ie, osteomyelitis).4,5Mycobacterium tuberculosis is the more frequently found cause in developing countries but should be considered if the patient has recently travelled outside the United States.
Review of the literature suggests that the incidence of methicillin-resistant S aureus (MRSA) as the causative agent of psoas abscesses may be increasing. However, there is a wide variance in the incidence reported, ranging from 1.1% to 12% of confirmed microbial infections.5,7,8
The classic historical presentation of psoas abscess has been described as the triad of back pain, fever, and limp5,6; however, this triad has only been described in approximately 30% of cases.5 The typical presentation consists of flank or lower limb pain (91%), fever (75%), anorexia (46%), and/or weakness (43%).4 Laboratory abnormalities include leukocytosis (67%) and elevated markers of inflammation (eg, erythrocyte sedimentation rate, seen in 73% of cases).4
Imaging via abdominal ultrasound may be helpful to screen for psoas abscess; however, its utility is limited by a low diagnostic yield of 60% or less.2,4 Direct visualization of the retroperitoneal structures, for example, can be problematic due to the presence of bowel gas.9 Abdominal CT is considered the gold standard for the definitive diagnosis of psoas abscess due to its high sensitivity (100%) and specificity (77%); it can also be used simultaneously to guide percutaneous drainage to treat the abscess if needed.7 However, some clinicians prefer abdominal MRI because of its ability to enhance soft-tissue visualization without requiring use of IV contrast.2,4
The approach to treating psoas abscess varies from a strictly antibiotic regimen to percutaneous drainage, and in rare circumstances, open surgical drainage. Antibiotic therapy without drainage or surgical intervention is a sufficient starting point for treatment of abscesses less than 3 cm in size.3
The antibiotic regimen choice depends on the suspected pathogen. In cases of suspected S aureus, empiric antistaphylococcal antibiotics should be initiated while culture results are pending.2,4 Secondary psoas abscesses thought to be derived from a urinary or gastrointestinal source should prompt use of a broader spectrum antibiotic due to the higher probability of gram-negative, anaerobic, or polymicrobial involvement.2,4
Once final culture and sensitivity results are obtained, antibiotic therapy should be modified to target the isolated pathogen(s). Treatment duration is typically six weeks but may vary, depending on serial culture results and the inoculating source.4 Review of the literature reveals that abscesses resulting from skeletal sources have traditionally been treated longer, usually with antibiotics alone, than those from urinary or gastrointestinal sources, which are often treated with the combination of antibiotics and percutaneous drainage.4
In cases of psoas abscesses larger than 3 cm, management should include both appropriate antibiotics and percutaneous drainage of the abscess.2 Percutaneous drainage is preferred to open surgical drainage because outcomes are similar, it is less invasive, and there is less risk of spreading abscess contents.2-4 In a retrospective analysis by Dietrich et al, 50% of patients treated with antibiotics and percutaneous drainage responded after one drainage, but the success rate increased to 100% after a second drainage.7 In addition, percutaneous drainage was associated with a lower mortality rate and a shorter hospital stay when compared to open surgical drainage.7
Open surgical drainage is rarely performed and usually only considered if the patient is not responding to a combination of focused antibiotic treatment and percutaneous drainage or has associated comorbidities, such as Crohn ileocolitis.2-4 In a retrospective analysis by Tabrizian et al, percutaneous drainage served as a bridge to open surgical drainage in nearly all patients with a gastrointestinal origin, such as Crohn disease, diverticulitis, appendicitis, and/or pancreatitis.6
Treatment of psoas abscesses has an overall failure rate of 15.8%, with an associated mortality rate of less than 7%.4 Overall prognosis is good, but outcomes can be negatively affected by such factors as advanced age, delay in diagnosis, bacteremia, and other comorbidities.4
Next page: Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
The patient required an 11-day hospitalization; her day-by-day course is described briefly below.
Day 1. Upon admission, abdominal MRI was ordered (see Figure 1) and empiric piperacillin/tazobactam IV was initiated. C-reactive protein (CRP) level and white blood cell (WBC) counts were elevated (see Table 2). Infectious disease, surgery, and urology consults were obtained.
Day 2. Fine-needle aspiration of the abscess was performed for cultures, and 40 mL of purulent fluid was drained. Piperacillin/tazobactam administration was continued, but the patient experienced ongoing fever and vomiting.
Day 3. Preliminary aspirate culture results revealed S aureus infection. Piperacillin/tazobactam was discontinued, and vancomycin IV was started. CRP levels and WBC counts decreased, as did fever and vomiting.
Day 4. Final aspirate culture results identified MRSA infection, sensitive to clindamycin. Vancomycin was discontinued, and clindamycin IV was started. Although the patient’s condition improved somewhat, fever and vomiting persisted.
Day 5. Both CRP levels and WBC counts increased from day 3. A surgical consult was sought.
Day 6. Repeat abdominal MRI revealed a decrease in the size of the abscess (see Figure 2, page 30. CRP levels and WBC counts remained high, with persistent fever and vomiting.
Day 7. The clinical team, in consultation with the parents, determined that placement of a peripherally inserted central catheter (PICC) line for drainage of the abscess was necessary.
Day 8. A 10-French pigtail catheter was inserted into the abscess, 20 mL of purulent fluid was drained, and a PICC line was inserted. Clindamycin IV was continued and, eight hours after the catheter was placed, fever and vomiting resolved.
Day 9. Both CRP levels and WBC counts dropped by half (WBC count was normal), while 10 mL of clear fluid drained from the catheter. The patient remained afebrile, without nausea or vomiting, on clindamycin IV.
Day 10. After 36 hours of clear drainage, the catheter was removed. CRP level further decreased. Clindamycin IV was discontinued, and the patient, now asymptomatic, was started on oral clindamycin.
Day 11. The patient was discharged on a regimen of oral clindamycin for six weeks, with weekly abdominal ultrasounds. She completed her entire course of antibiotics and fully recovered from the infection.
Next page: Conclusion >>
CONCLUSION
Since children generally compensate well during times of increased stress on the body, it is vital that persistent FUOs continue to be evaluated until a definitive source is identified, especially in this population. Early diagnosis and treatment of psoas abscess is essential for better outcomes, since delay is associated with a greater risk for sepsis.
While the likelihood of developing psoas abscess is low, it is worth keeping the diagnosis in mind for cases of unexplained lower abdominal pain, flank pain, or hip pain when more common etiologies have been excluded. This is especially important in the setting of recent travel to a developing country due to the fact that a psoas abscess can be a complication of TB of the spine.
The authors would like to thank Jeff Brand, MD, for his assistance in the preparation of this manuscript.
REFERENCES
1. Wong-Baker Faces Corporation. Wong-Baker FACES Pain Rating Scale. www.wongbakerfaces.org. Accessed May 19, 2015.
2. Mallick IH, Thoufreeq MH, Rajendren TP. Iliopsoas abscesses. Postgrad Med J. 2004;80(946):459-462.
3. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223-227.
4. Lopez VN, Ramos JM, Meseguer V, et al; The Infectious Diseases Study Group of the Spanish Society of Internal Medicine. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine. 2009;88(2):120-130.
5. Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg. 2012;10(9):466-469.
6. Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg. 2009;144(10):946-949.
7. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23(1):45-48.
8. Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19(5):416-423.
9. Woo MY. Psoas abscess. J Emerg Med. 2014;47(5):e129-e130.
A 5-year-old Filipino girl was brought to a pediatric clinic for follow up of an unresolved fever and for new-onset right hip pain, which occurred intermittently for the past week and was associated with a right-sided limp. She had been experiencing nightly fevers ranging from 101°F to 105°F for the past two weeks, for which her parents had been giving ibuprofen with mixed results; she remained afebrile during daytime hours.
Using the Wong-Baker FACES pain scale, the patient rated the pain as a 4/10 in severity (“Hurts a Little More” face).1 Standing and walking aggravated the pain but did not limit activity. Although ibuprofen decreased the fever, it did not alleviate the hip pain. Other symptoms included vomiting one to two times daily, without hematemesis, and four to five episodes of diarrhea daily, without abdominal pain, hematochezia, or melena. She also experienced decreased appetite, but her parents reported no change in her dietary or fluid intake. The patient and her parents denied additional symptoms.
Further investigation revealed that the patient had been seen a week earlier by two other clinicians in the office for complaints of fever, rash, nausea, hematemesis, and diarrhea. She had been diagnosed with a herpes simplex viral (HSV) lesion of the nose, epistaxis, and viral gastroenteritis. Her treatment plan consisted of acyclovir ointment for the HSV lesion and symptomatic support for the gastroenteritis associated diarrhea. The complaint of hematemesis was attributed to postnasal drip from the epistaxis, and reassurance was provided to the patient and family. In addition, six weeks earlier, the patient had been treated for otitis media with a full course of amoxicillin.
Medical history was negative for surgeries, trauma, injuries, and chronic medical conditions. She took no medications or supplements on a regular basis. Her parents denied any known drug allergies and stated that her immunizations were up to date.
The patient lived at home with her biological parents and two brothers, all of whom were healthy, without any recent infections or illnesses. Of significance, the family had travelled to the Philippines for vacation about four months earlier. Results of a tuberculin skin test done six weeks earlier (because the patient presented with respiratory symptoms shortly after traveling to the Philippines) were negative.
Physical examination revealed a well-developed, well-nourished 40-lb girl, in no acute distress, who was active and playful with her brother while in the exam room. Vital signs were significant for a fever of 101.9°F (last dose of ibuprofen was approximately six hours earlier) but were otherwise stable. Skin exam revealed that the prior HSV lesion of the nose had resolved. HEENT, cardiovascular, and pulmonary exam findings were noncontributory. Urine dipstick was negative.
Abdominal exam revealed normoactive bowel sounds in all four quadrants, and on palpation, the abdomen was soft, nontender, and without organomegaly. Specialized abdominal exams to assess for peritonitis, including those to elicit Rovsing, rebound tenderness, obturator, and psoas signs, were all negative. Bilateral extremity exams of the hips, knees, and ankles revealed full range of motion (active and passive), with normal muscle strength throughout. The only significant finding on the physical exam was mild pain of the right anterior hip at 15° of flexion, appreciated while the patient was supine on the exam table. The patient was also observed pushing off her right lower extremity when climbing onto the exam table, and she skipped down the hall when leaving the exam.
With fever of unknown origin (FUO) and a largely negative history and physical, the working list of differential diagnoses included
• Avascular necrosis
• Bacteremia
• Juvenile idiopathic arthritis
• Osteomyelitis
• Pyelonephritis
• Reiter syndrome
• Rheumatic fever
• Rheumatoid arthritis
• Septic joint
• Urinary tract infection
To begin the diagnostic process, a number of laboratory tests and imaging procedures were ordered. Table 1 presents the results of these studies. A tuberculin skin test was not repeated. While awaiting test results, the patient was started on naproxen oral suspension (125 mg/5 mL; 4 mL bid) for fever and pain control.
Based on findings consistent with an inflammatory pattern, the history of otitis media (of possible streptococcal origin) six weeks prior to this visit, and the elevated ASO titer, the patient was started on penicillin V (250 mg bid) and instructed to return for follow up in two days.
At the follow-up visit, no improvement was noted; the patient continued to experience nightly fevers and hip pain. Rovsing, rebound tenderness, obturator, and psoas signs continued to be negative. Physical examination did, however, reveal a mild abdominal tenderness in the right lower quadrant.
Due to this new finding, an abdominal ultrasound was ordered to screen for appendicitis. Despite the parents’ appropriate concern for the child, misunderstanding about the urgent need to obtain the abdominal ultrasound led to a two-day delay in scheduling the exam. Results of ultrasonography revealed psoas abscess, and the patient was promptly admitted to the pediatric floor of the local hospital.
Continue for discussion >>
DISCUSSION
Psoas abscess is a collection of pus in the iliopsoas compartment, an extraperitoneal space containing the psoas and iliacus muscles.2 It can be life-threatening if the infection progresses to septic shock. Historically, psoas abscesses were a frequent complication of tuberculosis (TB) of the spine; but with modern TB treatment, these abscesses have become rare.2 Paradoxically, increased utilization of CT to evaluate sepsis of unknown etiology has led to a recent increase in the frequency of psoas abscess diagnosis.3
Psoas abscesses are categorized as either primary or secondary, with primary infections originating in the psoas muscle and secondary infections spreading from adjacent organs.2 In 42% to 88% of cases (depending on the study), primary psoas abscesses are caused by the hematogenous spread of Staphylococcus aureus from distant infection sites.2,4,5 The psoas muscle is particularly susceptible to this mode of infection because of its rich vascular supply.6 Children, immunosuppressed adults (ie, patients with diabetes, HIV/AIDS, or renal failure), IV drug users, and patients with a history of trauma to the muscle are most susceptible to developing a primary psoas abscess.2,5
Secondary psoas abscesses are caused by infections involving adjacent structures of the gastrointestinal, urinary, and skeletal systems. They are most frequently associated with intra-abdominal inflammatory processes, with the most common etiology being Crohn disease.5 Secondary psoas abscesses, though more diverse in their bacterial flora, tend to follow certain microbiologic patterns based on the inoculating source; Escherichia coli is the most common pathogen in secondary abscesses caused by gastrointestinal (42%) and urinary (61%) sources, and S aureus the most common (35%) from skeletal origins (ie, osteomyelitis).4,5Mycobacterium tuberculosis is the more frequently found cause in developing countries but should be considered if the patient has recently travelled outside the United States.
Review of the literature suggests that the incidence of methicillin-resistant S aureus (MRSA) as the causative agent of psoas abscesses may be increasing. However, there is a wide variance in the incidence reported, ranging from 1.1% to 12% of confirmed microbial infections.5,7,8
The classic historical presentation of psoas abscess has been described as the triad of back pain, fever, and limp5,6; however, this triad has only been described in approximately 30% of cases.5 The typical presentation consists of flank or lower limb pain (91%), fever (75%), anorexia (46%), and/or weakness (43%).4 Laboratory abnormalities include leukocytosis (67%) and elevated markers of inflammation (eg, erythrocyte sedimentation rate, seen in 73% of cases).4
Imaging via abdominal ultrasound may be helpful to screen for psoas abscess; however, its utility is limited by a low diagnostic yield of 60% or less.2,4 Direct visualization of the retroperitoneal structures, for example, can be problematic due to the presence of bowel gas.9 Abdominal CT is considered the gold standard for the definitive diagnosis of psoas abscess due to its high sensitivity (100%) and specificity (77%); it can also be used simultaneously to guide percutaneous drainage to treat the abscess if needed.7 However, some clinicians prefer abdominal MRI because of its ability to enhance soft-tissue visualization without requiring use of IV contrast.2,4
The approach to treating psoas abscess varies from a strictly antibiotic regimen to percutaneous drainage, and in rare circumstances, open surgical drainage. Antibiotic therapy without drainage or surgical intervention is a sufficient starting point for treatment of abscesses less than 3 cm in size.3
The antibiotic regimen choice depends on the suspected pathogen. In cases of suspected S aureus, empiric antistaphylococcal antibiotics should be initiated while culture results are pending.2,4 Secondary psoas abscesses thought to be derived from a urinary or gastrointestinal source should prompt use of a broader spectrum antibiotic due to the higher probability of gram-negative, anaerobic, or polymicrobial involvement.2,4
Once final culture and sensitivity results are obtained, antibiotic therapy should be modified to target the isolated pathogen(s). Treatment duration is typically six weeks but may vary, depending on serial culture results and the inoculating source.4 Review of the literature reveals that abscesses resulting from skeletal sources have traditionally been treated longer, usually with antibiotics alone, than those from urinary or gastrointestinal sources, which are often treated with the combination of antibiotics and percutaneous drainage.4
In cases of psoas abscesses larger than 3 cm, management should include both appropriate antibiotics and percutaneous drainage of the abscess.2 Percutaneous drainage is preferred to open surgical drainage because outcomes are similar, it is less invasive, and there is less risk of spreading abscess contents.2-4 In a retrospective analysis by Dietrich et al, 50% of patients treated with antibiotics and percutaneous drainage responded after one drainage, but the success rate increased to 100% after a second drainage.7 In addition, percutaneous drainage was associated with a lower mortality rate and a shorter hospital stay when compared to open surgical drainage.7
Open surgical drainage is rarely performed and usually only considered if the patient is not responding to a combination of focused antibiotic treatment and percutaneous drainage or has associated comorbidities, such as Crohn ileocolitis.2-4 In a retrospective analysis by Tabrizian et al, percutaneous drainage served as a bridge to open surgical drainage in nearly all patients with a gastrointestinal origin, such as Crohn disease, diverticulitis, appendicitis, and/or pancreatitis.6
Treatment of psoas abscesses has an overall failure rate of 15.8%, with an associated mortality rate of less than 7%.4 Overall prognosis is good, but outcomes can be negatively affected by such factors as advanced age, delay in diagnosis, bacteremia, and other comorbidities.4
Next page: Outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
The patient required an 11-day hospitalization; her day-by-day course is described briefly below.
Day 1. Upon admission, abdominal MRI was ordered (see Figure 1) and empiric piperacillin/tazobactam IV was initiated. C-reactive protein (CRP) level and white blood cell (WBC) counts were elevated (see Table 2). Infectious disease, surgery, and urology consults were obtained.
Day 2. Fine-needle aspiration of the abscess was performed for cultures, and 40 mL of purulent fluid was drained. Piperacillin/tazobactam administration was continued, but the patient experienced ongoing fever and vomiting.
Day 3. Preliminary aspirate culture results revealed S aureus infection. Piperacillin/tazobactam was discontinued, and vancomycin IV was started. CRP levels and WBC counts decreased, as did fever and vomiting.
Day 4. Final aspirate culture results identified MRSA infection, sensitive to clindamycin. Vancomycin was discontinued, and clindamycin IV was started. Although the patient’s condition improved somewhat, fever and vomiting persisted.
Day 5. Both CRP levels and WBC counts increased from day 3. A surgical consult was sought.
Day 6. Repeat abdominal MRI revealed a decrease in the size of the abscess (see Figure 2, page 30. CRP levels and WBC counts remained high, with persistent fever and vomiting.
Day 7. The clinical team, in consultation with the parents, determined that placement of a peripherally inserted central catheter (PICC) line for drainage of the abscess was necessary.
Day 8. A 10-French pigtail catheter was inserted into the abscess, 20 mL of purulent fluid was drained, and a PICC line was inserted. Clindamycin IV was continued and, eight hours after the catheter was placed, fever and vomiting resolved.
Day 9. Both CRP levels and WBC counts dropped by half (WBC count was normal), while 10 mL of clear fluid drained from the catheter. The patient remained afebrile, without nausea or vomiting, on clindamycin IV.
Day 10. After 36 hours of clear drainage, the catheter was removed. CRP level further decreased. Clindamycin IV was discontinued, and the patient, now asymptomatic, was started on oral clindamycin.
Day 11. The patient was discharged on a regimen of oral clindamycin for six weeks, with weekly abdominal ultrasounds. She completed her entire course of antibiotics and fully recovered from the infection.
Next page: Conclusion >>
CONCLUSION
Since children generally compensate well during times of increased stress on the body, it is vital that persistent FUOs continue to be evaluated until a definitive source is identified, especially in this population. Early diagnosis and treatment of psoas abscess is essential for better outcomes, since delay is associated with a greater risk for sepsis.
While the likelihood of developing psoas abscess is low, it is worth keeping the diagnosis in mind for cases of unexplained lower abdominal pain, flank pain, or hip pain when more common etiologies have been excluded. This is especially important in the setting of recent travel to a developing country due to the fact that a psoas abscess can be a complication of TB of the spine.
The authors would like to thank Jeff Brand, MD, for his assistance in the preparation of this manuscript.
REFERENCES
1. Wong-Baker Faces Corporation. Wong-Baker FACES Pain Rating Scale. www.wongbakerfaces.org. Accessed May 19, 2015.
2. Mallick IH, Thoufreeq MH, Rajendren TP. Iliopsoas abscesses. Postgrad Med J. 2004;80(946):459-462.
3. Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg. 2008;196(2):223-227.
4. Lopez VN, Ramos JM, Meseguer V, et al; The Infectious Diseases Study Group of the Spanish Society of Internal Medicine. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine. 2009;88(2):120-130.
5. Shields D, Robinson P, Crowley TP. Iliopsoas abscess—a review and update on the literature. Int J Surg. 2012;10(9):466-469.
6. Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg. 2009;144(10):946-949.
7. Dietrich A, Vaccarezza H, Vaccaro CA. Iliopsoas abscess: presentation, management, and outcomes. Surg Laparosc Endosc Percutan Tech. 2013;23(1):45-48.
8. Wong OF, Ho PL, Lam SK. Retrospective review of clinical presentations, microbiology, and outcomes of patients with psoas abscess. Hong Kong Med J. 2013;19(5):416-423.
9. Woo MY. Psoas abscess. J Emerg Med. 2014;47(5):e129-e130.
Enterovirus D-68 presenting with acute pancreatitis
To the Editor: We read the review on enterovirus D681 (EV-D68) with great interest, and we thought it merited comment.
During the current influenza season, we have had several adult cases of EV-D68 presenting as an influenza-like illness. EV-D68 was diagnosed by nasal swab viral film array polymerase chain reaction (PCR) testing. We agree with the authors that the clinical spectrum of enteroviral infection includes a variety of extraintestinal manifestations, eg, acute pancreatitis. As more cases of EV-D68 are described, the range of clinical manifestations will be increased.2–5
We recently saw a 27-year-old woman who presented with an influenza-like illness, but with a main complaint of right-upper-quadrant abdominal pain. She denied recent travel or contacts with sick children or adults. Her past medical history was unremarkable, and she was not taking any medications. The physical examination was unremarkable except for moderately severe tenderness in the right upper quadrant, with no rebound or guarding.
Results of laboratory testing at hospital admission included a white blood cell count of 7.3 × 109/L (49% neutrophils, 41% lymphocytes, 7% monocytes, 3% eosinophils), a normal platelet count, serum lipase 73 U/L (reference range 5.6–51.3 U/L), and serum amylase 211 U/L (37–121 U/L). Serum aminotransferase and alkaline phosphatase levels were normal. Abdominal ultrasonography was unremarkable. Nasal swab for multiplex PCR testing for respiratory viruses was positive for human rhinovirus-enterovirus. Further PCR testing was positive for EV-D68 (New York State Department of Health, Wadsworth Laboratory). Her abdominal pain was treated symptomatically; she gradually improved and was discharged.
This instance of EV-D68 in a healthy 27-year-old woman presenting with influenza-like illness and acute pain in the right upper quadrant is the first we have seen of EV-D68 presenting as acute pancreatitis. Clinicians should be aware that EV-D68, like influenza, may present with gastrointestinal manifestations.
- Foster CB, Friedman N, Carl J, Piedimonte G. Enterovirus D68: a clinically important respiratory enterovirus. Cleve Clin J Med 2015; 82:26–31.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR 2014; 63:798–799.
To the Editor: We read the review on enterovirus D681 (EV-D68) with great interest, and we thought it merited comment.
During the current influenza season, we have had several adult cases of EV-D68 presenting as an influenza-like illness. EV-D68 was diagnosed by nasal swab viral film array polymerase chain reaction (PCR) testing. We agree with the authors that the clinical spectrum of enteroviral infection includes a variety of extraintestinal manifestations, eg, acute pancreatitis. As more cases of EV-D68 are described, the range of clinical manifestations will be increased.2–5
We recently saw a 27-year-old woman who presented with an influenza-like illness, but with a main complaint of right-upper-quadrant abdominal pain. She denied recent travel or contacts with sick children or adults. Her past medical history was unremarkable, and she was not taking any medications. The physical examination was unremarkable except for moderately severe tenderness in the right upper quadrant, with no rebound or guarding.
Results of laboratory testing at hospital admission included a white blood cell count of 7.3 × 109/L (49% neutrophils, 41% lymphocytes, 7% monocytes, 3% eosinophils), a normal platelet count, serum lipase 73 U/L (reference range 5.6–51.3 U/L), and serum amylase 211 U/L (37–121 U/L). Serum aminotransferase and alkaline phosphatase levels were normal. Abdominal ultrasonography was unremarkable. Nasal swab for multiplex PCR testing for respiratory viruses was positive for human rhinovirus-enterovirus. Further PCR testing was positive for EV-D68 (New York State Department of Health, Wadsworth Laboratory). Her abdominal pain was treated symptomatically; she gradually improved and was discharged.
This instance of EV-D68 in a healthy 27-year-old woman presenting with influenza-like illness and acute pain in the right upper quadrant is the first we have seen of EV-D68 presenting as acute pancreatitis. Clinicians should be aware that EV-D68, like influenza, may present with gastrointestinal manifestations.
To the Editor: We read the review on enterovirus D681 (EV-D68) with great interest, and we thought it merited comment.
During the current influenza season, we have had several adult cases of EV-D68 presenting as an influenza-like illness. EV-D68 was diagnosed by nasal swab viral film array polymerase chain reaction (PCR) testing. We agree with the authors that the clinical spectrum of enteroviral infection includes a variety of extraintestinal manifestations, eg, acute pancreatitis. As more cases of EV-D68 are described, the range of clinical manifestations will be increased.2–5
We recently saw a 27-year-old woman who presented with an influenza-like illness, but with a main complaint of right-upper-quadrant abdominal pain. She denied recent travel or contacts with sick children or adults. Her past medical history was unremarkable, and she was not taking any medications. The physical examination was unremarkable except for moderately severe tenderness in the right upper quadrant, with no rebound or guarding.
Results of laboratory testing at hospital admission included a white blood cell count of 7.3 × 109/L (49% neutrophils, 41% lymphocytes, 7% monocytes, 3% eosinophils), a normal platelet count, serum lipase 73 U/L (reference range 5.6–51.3 U/L), and serum amylase 211 U/L (37–121 U/L). Serum aminotransferase and alkaline phosphatase levels were normal. Abdominal ultrasonography was unremarkable. Nasal swab for multiplex PCR testing for respiratory viruses was positive for human rhinovirus-enterovirus. Further PCR testing was positive for EV-D68 (New York State Department of Health, Wadsworth Laboratory). Her abdominal pain was treated symptomatically; she gradually improved and was discharged.
This instance of EV-D68 in a healthy 27-year-old woman presenting with influenza-like illness and acute pain in the right upper quadrant is the first we have seen of EV-D68 presenting as acute pancreatitis. Clinicians should be aware that EV-D68, like influenza, may present with gastrointestinal manifestations.
- Foster CB, Friedman N, Carl J, Piedimonte G. Enterovirus D68: a clinically important respiratory enterovirus. Cleve Clin J Med 2015; 82:26–31.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR 2014; 63:798–799.
- Foster CB, Friedman N, Carl J, Piedimonte G. Enterovirus D68: a clinically important respiratory enterovirus. Cleve Clin J Med 2015; 82:26–31.
- Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 2012; 93:1952–1958.
- Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 2004; 85:2577–2584.
- Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol 2011; 52:103–106.
- Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR 2014; 63:798–799.