User login
Urgent surgery said to deserve separate classification
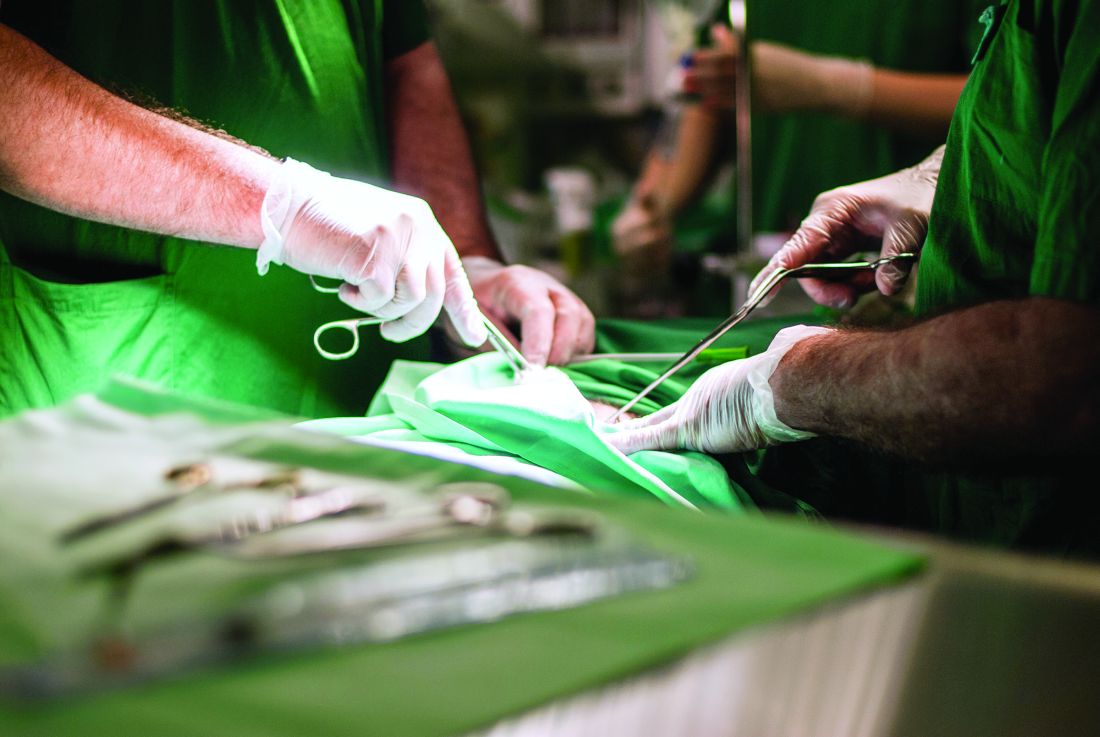
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Urgent surgery deserves a separate classification from elective surgery and emergency surgery for assessments of healthcare quality and performance, because the three types of surgery have distinct morbidity and mortality profiles, according to a report published in JAMA Surgery.
Current methods of assessing pay-for-performance reimbursement, surgical outcomes, and value-based care programs all classify surgeries as either elective or emergent procedures. They do not account for the many surgeries that are instead urgent – performed after a trial of nonoperative conservative management or after patients with acute disease processes undergo a brief period of medical optimization.
Common examples of surgeries that occupy this middle ‘urgent’ ground between elective and emergent procedures are those done for cholecystitis, adhesive small-bowel obstruction, and acute diverticulitis, said Matthew G. Mullen, MD, and his associates at the University of Virginia Health System, Charlottesville.
Such urgent surgeries should not be lumped together with elective surgeries, as they usually are at present, because they carry substantially higher complication rates and mortality. “At a time when reimbursement is contingent on value-based outcomes reporting and performance, it is imperative to ensure that appropriate risk adjustment is performed,” the researchers stated.
“Surgeons who commonly operate on an urgent basis, including many acute-care and emergency general surgeons, are at risk of being penalized” in Medicare’s value-based reimbursement for their services.
“These surgeons may even unfairly be labeled as poor performers by current outcome reporting guidelines,” the investigators noted.
Morbidity and mortality rates associated with the “substantial” population of patients undergoing urgent surgery have not been well-studied until now. Dr. Mullen and his associates examined the issue using information from a national database, the American College of Surgeons’ National Quality Improvement Program Participant Use File. They focused on 173,643 general surgeries performed at 435 hospitals during a 1-year period: 130,235 (75%) were categorized as elective, 20,816 (12%) as urgent (nonelective and nonemergency), and 22,592 (13%) as emergency procedures.
Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality. These rates are much greater than those of elective surgeries (6.7% and 0.4%, respectively), even though urgent surgeries typically fall into the category of “elective.” In fact, the morbidity and mortality rates for urgent surgeries closely approached those of emergency surgeries (13.8% and 3.7%, respectively), the investigators said (JAMA Surg. 2017 May 10 [doi:10.1001/jamasurg.2017.0918]).
In this cohort, patients in the “urgent” surgery category had the highest preoperative rates of congestive heart failure, chronic obstructive pulmonary disease, diabetes, hemodialysis, corticosteroid use, and disseminated cancer – all factors that markedly elevate mortality and morbidity risks.
“We have identified operative urgency as a key consideration for patient risk stratification. If this issue is not recognized, quality outcome reporting and value-based reimbursement will continue to incentivize operating on an elective basis and will make surgeons more reluctant to operate on patients who urgently require care,” Dr. Mullen and his associates said. And such delays in surgical intervention could further increase patient morbidity and mortality.
This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point: Urgent surgery deserves a separate classification from elective and emergency surgeries for assessments of healthcare quality and performance.
Major finding: Urgent general surgeries carried a 12.3% rate of morbidity and a 2.3% rate of mortality, approximately double the morbidity rate and 6 times the mortality rate of elective surgeries (6.7% and 0.4%, respectively).
Data source: A retrospective analysis of outcomes for 173,643 general surgeries performed during a 1-year period at 435 hospitals across U.S.
Disclosures: This study was supported by the National Institutes of Health. Dr. Mullen and his associates reported having no relevant financial disclosures.
Acute cholecystitis: Not always routine
The more we think we know, the less we actually may know. As new techniques develop and their use is closely examined and reported, details about the patient’s disease and the surgeon’s skill and judgment turn out to matter more and more in the decision-making process. So it is with acute cholecystitis.
I have recently been puzzled and intrigued by changing trends in the management of acute cholecystitis that are apparent in the medical literature, discussions in the ACS Communities, and practice in my local community.
When I was a resident, the debate about early cholecystectomy vs antibiotics with interval operation 6 weeks later was just being settled in the literature in favor of early cholecystectomy. The weight of evidence in the surgical literature found that delay made the eventual operation more difficult and costly.
In the following 2 decades, early cholecystectomy became the indicated treatment for acute cholecystitis. In that era, of course, these operations were open, as that was our only option and one which we all learned to perform with confidence during residency. Tube cholecystostomy was a rarity reserved only for the most severely ill and feeble, and done by surgeons, since interventional radiologists had not yet appeared on the scene.
In the rare instance of acute cholecystitis so severe, and anatomic landmarks so obscured, that the gallbladder could not be safely dissected away from the common bile duct, a remnant of the gallbladder might be left behind, the mucosa cauterized, and the right upper quadrant drained.
Fast forward 40 years, and we find a distinctly different landscape. As the Boomer generation reaches geriatric age in expanding numbers, surgeons encounter an increasingly older patient population, often with numerous comorbidities and high surgical risk. Our increased critical care capability to rescue patients from sepsis and organ failure also introduces new challenges in decision-making about whether immediate cholecystectomy or a temporizing option is better for the elderly septic ICU patient before us.
At the same time, our overwhelmingly most common elective biliary procedure has become a laparoscopic cholecystectomy (LC), with which our younger surgeons have become comfortable and facile. Multiple randomized studies also confirm the superiority of early LC for acute cholecystitis, although LC is associated with a higher rate of conversion to open cholecystectomy in acute cholecystitis than in the elective setting. Since it is acknowledged that the mortality and morbidity of an open cholecystectomy is greater than that of its laparoscopic counterpart, especially in the setting of severe inflammation, and the younger surgeons are less confident in performing open cholecystectomy, it is not surprising that they embrace a strategy that allows them to avoid surgical management of acute cholecystitis in the high-risk patient with severe disease.
The ready availability of interventional radiologists in the past 30 years also offers a less invasive option than surgery – the percutaneous tube cholecystostomy (PC). It is no wonder that PC has increasingly become the “go-to” early option when the patient is old and sick or the surgeon lacks confidence in his/her open surgical skills in a potentially hostile, inflamed right upper quadrant. If the increasing number of articles on PC appearing in the literature is any indication, its use has proliferated in the recent past. As yet, no randomized clinical trials or other high-quality evidence have emerged to support its increased use, but a consensus panel of experts has issued the Tokyo Guidelines, recommending PC as primary therapy for stage III acute cholecystitis, the form of disease associated with organ failure, but not citing evidence to support this recommendation (J Hepatobiliary Pancreat Surg. 2007;14[1]:91-7). Although the rate of PC use in Medicare patients with stage III acute cholecystitis has more than doubled in the past 20 years, Tokyo Guidelines have clearly not been uniformly adopted in the U.S., since PC use in patients with stage III acute cholecystitis is only 10% (J Am Coll Surg. 2017;224[4]: 502-14).
Whether the increase in PC use is appropriate or not remains undetermined. Other uncertainties about PC need clarification. When patients have a PC placed for acute cholecystitis, do they always need their gallbladders removed later? The rate of recurrent acute cholecystitis after PC is variable in the literature, although it appears to be more likely in patients with acute calculous than acalculous cholecystitis. The likelihood that the patient will later undergo a cholecystectomy varies from a low of 3% to a high of 57% in various studies.(Surgery. 2014;155[4]:615-22; J Am Coll Surg. 2012; 214[2]:196-201).
The exact rate may not even accurately be known, since some patients may be lost to follow-up or get subsequent care in another facility. The decision to perform cholecystectomy after PC involves assessment of patient risk for surgery and, ultimately, surgeon judgment. Other questions also remain unanswered: What is the role of surgeon experience in the decision to defer surgical therapy for acute cholecystitis? Is the surgeon even the one who is in charge of the decision in all cases, or is that decision being made by an internist, intensivist, or hospitalist, who may judge the patient’s risk differently than a surgeon would? Are we witnessing an evolution in management of severe cholecystitis in the high-risk, septic and elderly patient towards antibiotics and PC unless the patient fails that treatment? This strategy appears to be gaining in popularity, since several studies have shown that the minority of patients who have PC end up having their gallbladders removed. If symptoms recur and nonoperative treatment has clearly failed, should the decision be made to refer to a highly experienced surgeon (by virtue of laparoscopic skills or reputation as a hepatic-pancreatic biliary specialist)? Recent studies show that 46%-86% of elective interval cholecystectomies after successful PC can be performed laparoscopically with low complication rates, although those studies came from institutions with notable laparoscopic expertise (J Am Coll Surg. 2012;214[2]:196-201; J Gastrointest Surg. 2017; 21[5]:761-69).
One of my most revered senior surgical mentors recently opined that the safest strategy for the high-risk patient with severe acute cholecystitis was indeed PC and antibiotics followed by watchful waiting, and reserving cholecystectomy only for those who fail nonoperative therapy. I initially bristled at that concept as being antithetic to the surgical bias in favor of cholecystectomy as the answer to all gallbladder evils. But after reflecting further on the changing landscape of our therapeutic options and our changing surgical training, I’m thinking that his strategy may be reasonable.
After all, it’s about choosing the safest path for the patient. All cholecystectomies are not routine.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
The more we think we know, the less we actually may know. As new techniques develop and their use is closely examined and reported, details about the patient’s disease and the surgeon’s skill and judgment turn out to matter more and more in the decision-making process. So it is with acute cholecystitis.
I have recently been puzzled and intrigued by changing trends in the management of acute cholecystitis that are apparent in the medical literature, discussions in the ACS Communities, and practice in my local community.
When I was a resident, the debate about early cholecystectomy vs antibiotics with interval operation 6 weeks later was just being settled in the literature in favor of early cholecystectomy. The weight of evidence in the surgical literature found that delay made the eventual operation more difficult and costly.
In the following 2 decades, early cholecystectomy became the indicated treatment for acute cholecystitis. In that era, of course, these operations were open, as that was our only option and one which we all learned to perform with confidence during residency. Tube cholecystostomy was a rarity reserved only for the most severely ill and feeble, and done by surgeons, since interventional radiologists had not yet appeared on the scene.
In the rare instance of acute cholecystitis so severe, and anatomic landmarks so obscured, that the gallbladder could not be safely dissected away from the common bile duct, a remnant of the gallbladder might be left behind, the mucosa cauterized, and the right upper quadrant drained.
Fast forward 40 years, and we find a distinctly different landscape. As the Boomer generation reaches geriatric age in expanding numbers, surgeons encounter an increasingly older patient population, often with numerous comorbidities and high surgical risk. Our increased critical care capability to rescue patients from sepsis and organ failure also introduces new challenges in decision-making about whether immediate cholecystectomy or a temporizing option is better for the elderly septic ICU patient before us.
At the same time, our overwhelmingly most common elective biliary procedure has become a laparoscopic cholecystectomy (LC), with which our younger surgeons have become comfortable and facile. Multiple randomized studies also confirm the superiority of early LC for acute cholecystitis, although LC is associated with a higher rate of conversion to open cholecystectomy in acute cholecystitis than in the elective setting. Since it is acknowledged that the mortality and morbidity of an open cholecystectomy is greater than that of its laparoscopic counterpart, especially in the setting of severe inflammation, and the younger surgeons are less confident in performing open cholecystectomy, it is not surprising that they embrace a strategy that allows them to avoid surgical management of acute cholecystitis in the high-risk patient with severe disease.
The ready availability of interventional radiologists in the past 30 years also offers a less invasive option than surgery – the percutaneous tube cholecystostomy (PC). It is no wonder that PC has increasingly become the “go-to” early option when the patient is old and sick or the surgeon lacks confidence in his/her open surgical skills in a potentially hostile, inflamed right upper quadrant. If the increasing number of articles on PC appearing in the literature is any indication, its use has proliferated in the recent past. As yet, no randomized clinical trials or other high-quality evidence have emerged to support its increased use, but a consensus panel of experts has issued the Tokyo Guidelines, recommending PC as primary therapy for stage III acute cholecystitis, the form of disease associated with organ failure, but not citing evidence to support this recommendation (J Hepatobiliary Pancreat Surg. 2007;14[1]:91-7). Although the rate of PC use in Medicare patients with stage III acute cholecystitis has more than doubled in the past 20 years, Tokyo Guidelines have clearly not been uniformly adopted in the U.S., since PC use in patients with stage III acute cholecystitis is only 10% (J Am Coll Surg. 2017;224[4]: 502-14).
Whether the increase in PC use is appropriate or not remains undetermined. Other uncertainties about PC need clarification. When patients have a PC placed for acute cholecystitis, do they always need their gallbladders removed later? The rate of recurrent acute cholecystitis after PC is variable in the literature, although it appears to be more likely in patients with acute calculous than acalculous cholecystitis. The likelihood that the patient will later undergo a cholecystectomy varies from a low of 3% to a high of 57% in various studies.(Surgery. 2014;155[4]:615-22; J Am Coll Surg. 2012; 214[2]:196-201).
The exact rate may not even accurately be known, since some patients may be lost to follow-up or get subsequent care in another facility. The decision to perform cholecystectomy after PC involves assessment of patient risk for surgery and, ultimately, surgeon judgment. Other questions also remain unanswered: What is the role of surgeon experience in the decision to defer surgical therapy for acute cholecystitis? Is the surgeon even the one who is in charge of the decision in all cases, or is that decision being made by an internist, intensivist, or hospitalist, who may judge the patient’s risk differently than a surgeon would? Are we witnessing an evolution in management of severe cholecystitis in the high-risk, septic and elderly patient towards antibiotics and PC unless the patient fails that treatment? This strategy appears to be gaining in popularity, since several studies have shown that the minority of patients who have PC end up having their gallbladders removed. If symptoms recur and nonoperative treatment has clearly failed, should the decision be made to refer to a highly experienced surgeon (by virtue of laparoscopic skills or reputation as a hepatic-pancreatic biliary specialist)? Recent studies show that 46%-86% of elective interval cholecystectomies after successful PC can be performed laparoscopically with low complication rates, although those studies came from institutions with notable laparoscopic expertise (J Am Coll Surg. 2012;214[2]:196-201; J Gastrointest Surg. 2017; 21[5]:761-69).
One of my most revered senior surgical mentors recently opined that the safest strategy for the high-risk patient with severe acute cholecystitis was indeed PC and antibiotics followed by watchful waiting, and reserving cholecystectomy only for those who fail nonoperative therapy. I initially bristled at that concept as being antithetic to the surgical bias in favor of cholecystectomy as the answer to all gallbladder evils. But after reflecting further on the changing landscape of our therapeutic options and our changing surgical training, I’m thinking that his strategy may be reasonable.
After all, it’s about choosing the safest path for the patient. All cholecystectomies are not routine.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
The more we think we know, the less we actually may know. As new techniques develop and their use is closely examined and reported, details about the patient’s disease and the surgeon’s skill and judgment turn out to matter more and more in the decision-making process. So it is with acute cholecystitis.
I have recently been puzzled and intrigued by changing trends in the management of acute cholecystitis that are apparent in the medical literature, discussions in the ACS Communities, and practice in my local community.
When I was a resident, the debate about early cholecystectomy vs antibiotics with interval operation 6 weeks later was just being settled in the literature in favor of early cholecystectomy. The weight of evidence in the surgical literature found that delay made the eventual operation more difficult and costly.
In the following 2 decades, early cholecystectomy became the indicated treatment for acute cholecystitis. In that era, of course, these operations were open, as that was our only option and one which we all learned to perform with confidence during residency. Tube cholecystostomy was a rarity reserved only for the most severely ill and feeble, and done by surgeons, since interventional radiologists had not yet appeared on the scene.
In the rare instance of acute cholecystitis so severe, and anatomic landmarks so obscured, that the gallbladder could not be safely dissected away from the common bile duct, a remnant of the gallbladder might be left behind, the mucosa cauterized, and the right upper quadrant drained.
Fast forward 40 years, and we find a distinctly different landscape. As the Boomer generation reaches geriatric age in expanding numbers, surgeons encounter an increasingly older patient population, often with numerous comorbidities and high surgical risk. Our increased critical care capability to rescue patients from sepsis and organ failure also introduces new challenges in decision-making about whether immediate cholecystectomy or a temporizing option is better for the elderly septic ICU patient before us.
At the same time, our overwhelmingly most common elective biliary procedure has become a laparoscopic cholecystectomy (LC), with which our younger surgeons have become comfortable and facile. Multiple randomized studies also confirm the superiority of early LC for acute cholecystitis, although LC is associated with a higher rate of conversion to open cholecystectomy in acute cholecystitis than in the elective setting. Since it is acknowledged that the mortality and morbidity of an open cholecystectomy is greater than that of its laparoscopic counterpart, especially in the setting of severe inflammation, and the younger surgeons are less confident in performing open cholecystectomy, it is not surprising that they embrace a strategy that allows them to avoid surgical management of acute cholecystitis in the high-risk patient with severe disease.
The ready availability of interventional radiologists in the past 30 years also offers a less invasive option than surgery – the percutaneous tube cholecystostomy (PC). It is no wonder that PC has increasingly become the “go-to” early option when the patient is old and sick or the surgeon lacks confidence in his/her open surgical skills in a potentially hostile, inflamed right upper quadrant. If the increasing number of articles on PC appearing in the literature is any indication, its use has proliferated in the recent past. As yet, no randomized clinical trials or other high-quality evidence have emerged to support its increased use, but a consensus panel of experts has issued the Tokyo Guidelines, recommending PC as primary therapy for stage III acute cholecystitis, the form of disease associated with organ failure, but not citing evidence to support this recommendation (J Hepatobiliary Pancreat Surg. 2007;14[1]:91-7). Although the rate of PC use in Medicare patients with stage III acute cholecystitis has more than doubled in the past 20 years, Tokyo Guidelines have clearly not been uniformly adopted in the U.S., since PC use in patients with stage III acute cholecystitis is only 10% (J Am Coll Surg. 2017;224[4]: 502-14).
Whether the increase in PC use is appropriate or not remains undetermined. Other uncertainties about PC need clarification. When patients have a PC placed for acute cholecystitis, do they always need their gallbladders removed later? The rate of recurrent acute cholecystitis after PC is variable in the literature, although it appears to be more likely in patients with acute calculous than acalculous cholecystitis. The likelihood that the patient will later undergo a cholecystectomy varies from a low of 3% to a high of 57% in various studies.(Surgery. 2014;155[4]:615-22; J Am Coll Surg. 2012; 214[2]:196-201).
The exact rate may not even accurately be known, since some patients may be lost to follow-up or get subsequent care in another facility. The decision to perform cholecystectomy after PC involves assessment of patient risk for surgery and, ultimately, surgeon judgment. Other questions also remain unanswered: What is the role of surgeon experience in the decision to defer surgical therapy for acute cholecystitis? Is the surgeon even the one who is in charge of the decision in all cases, or is that decision being made by an internist, intensivist, or hospitalist, who may judge the patient’s risk differently than a surgeon would? Are we witnessing an evolution in management of severe cholecystitis in the high-risk, septic and elderly patient towards antibiotics and PC unless the patient fails that treatment? This strategy appears to be gaining in popularity, since several studies have shown that the minority of patients who have PC end up having their gallbladders removed. If symptoms recur and nonoperative treatment has clearly failed, should the decision be made to refer to a highly experienced surgeon (by virtue of laparoscopic skills or reputation as a hepatic-pancreatic biliary specialist)? Recent studies show that 46%-86% of elective interval cholecystectomies after successful PC can be performed laparoscopically with low complication rates, although those studies came from institutions with notable laparoscopic expertise (J Am Coll Surg. 2012;214[2]:196-201; J Gastrointest Surg. 2017; 21[5]:761-69).
One of my most revered senior surgical mentors recently opined that the safest strategy for the high-risk patient with severe acute cholecystitis was indeed PC and antibiotics followed by watchful waiting, and reserving cholecystectomy only for those who fail nonoperative therapy. I initially bristled at that concept as being antithetic to the surgical bias in favor of cholecystectomy as the answer to all gallbladder evils. But after reflecting further on the changing landscape of our therapeutic options and our changing surgical training, I’m thinking that his strategy may be reasonable.
After all, it’s about choosing the safest path for the patient. All cholecystectomies are not routine.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
Outpatient appendectomy success depends on patient selection, communication
Outpatient laparoscopic surgery for uncomplicated appendicitis can be safely implemented in a large county hospital that serves a poor, underserved population, findings from a prospective, observational trial have shown.
Outpatient appendectomy has gradually gained acceptance in the United States, and numerous studies support the practice. David R. Rosen, MD, of the University of Southern California, Los Angeles, and his colleagues considered the possible advantages of outpatient laparoscopic appendectomy for their institution, such as decreased length of stay, decreased costs, and fewer admissions.
The research team hypothesized that with a “well-defined protocol consisting of strict inclusion and exclusion criteria, clear patient instructions, and close observation to identify patients who would not succeed with the outpatient appendectomy treatment strategy, outpatient appendectomy would be feasible without worsening patient outcomes or satisfaction.”
The findings were published in the Journal of the American College of Surgeons (2017 May;224[5]:862-7).
The investigators conducted a study of patients presenting at a safety-net county hospital and diagnosed with acute appendicitis. A year-long observation period produced a control group of 178 admitted patients.
The outpatient protocol was then introduced. Patients were counseled on the possibility of their being discharged from the postanesthesia care unit (PACU), depending on intraoperative findings and their capacity to arrange a ride home and willingness to participate in postoperative follow-up. Patient education was a key element of the protocol. In all, 173 patients were identified for the outpatient program.
The intraoperative criteria for discharge from the PACU included no evidence of perforation or gangrene, and no surgical complications or adverse events. Patients were cleared for discharge if they met the following criteria: heart rate less than 100 beats/min; systolic blood pressure greater than 110 mm Hg; pain well controlled (less than 4 on a 1-10 scale); ambulatory; urinated since surgery; oral intake; and dressings dry without evidence of bleeding.
The patients had been thoroughly briefed on what to expect and problems that would necessitate a return to the emergency department. The physician assessed each patient’s readiness to be discharged, wrote a discharge order, and confirmed the pain medication prescription and follow-up appointment.
Of the 173 patients selected for the outpatient program, 113 (65%) ended up being discharged from the PACU. The reasons for these admissions included interoperative findings, failure to pass the discharge criteria, homelessness, and no transportation to get home.
The control and outpatient groups were similar demographically, except that the latter were on average significantly older (mean age 32.4 years vs. 36.6 years, respectively). The outpatient group had a significantly shorter operative time (69 minutes vs. 83 minutes), a significantly longer stay in the PACU (242 minutes vs.141 minutes), and a significantly shorter total postoperative length of stay (9 hours vs.19 hours).
There were no differences between the groups in terms of complications, postdischarge ED visits, or readmissions. Those who were discharged from the PACU had no postoperative complications and no readmissions.
The length of stay in the PACU gradually decreased for the outpatient group. “This can be attributed to the adoption of a new protocol,” the researchers noted. However, “we purposely did not want to rush the discharge process to ensure our patients and families had all questions answered and were comfortable leaving the hospital.”
A key component of the protocol was the follow-up appointment for all appendectomy patients; about one-third of both groups did not return for their follow-up appointments. Those missed follow-ups could mean some patients returned to another hospital, but the investigators suggested that this was unlikely.
“Because our hospital serves a patient population of low socioeconomic status and often without health insurance, our public hospital is often the only hospital to which they would present,” the investigators wrote.
Most of those who did return completed a questionnaire on their level of satisfaction. Survey results showed no differences in satisfaction between the groups and a generally positive view of the protocol among the outpatient group.
The study did not account for actual cost savings, but reduced hospital admissions and readmissions were achieved. Investigators assert that other studies have shown that each day of hospitalization avoided saves about $1,900.
“It is challenging to deliver high-quality, efficient care to an underserved population in a public hospital,” Dr. Rosen said in an interview. “In this setting, communication and patient education are vital components for success. By setting clear expectations and empowering patients to participate in their care, we can maximize our patients’ outcomes.”
The investigators had no disclosures.
Outpatient laparoscopic surgery for uncomplicated appendicitis can be safely implemented in a large county hospital that serves a poor, underserved population, findings from a prospective, observational trial have shown.
Outpatient appendectomy has gradually gained acceptance in the United States, and numerous studies support the practice. David R. Rosen, MD, of the University of Southern California, Los Angeles, and his colleagues considered the possible advantages of outpatient laparoscopic appendectomy for their institution, such as decreased length of stay, decreased costs, and fewer admissions.
The research team hypothesized that with a “well-defined protocol consisting of strict inclusion and exclusion criteria, clear patient instructions, and close observation to identify patients who would not succeed with the outpatient appendectomy treatment strategy, outpatient appendectomy would be feasible without worsening patient outcomes or satisfaction.”
The findings were published in the Journal of the American College of Surgeons (2017 May;224[5]:862-7).
The investigators conducted a study of patients presenting at a safety-net county hospital and diagnosed with acute appendicitis. A year-long observation period produced a control group of 178 admitted patients.
The outpatient protocol was then introduced. Patients were counseled on the possibility of their being discharged from the postanesthesia care unit (PACU), depending on intraoperative findings and their capacity to arrange a ride home and willingness to participate in postoperative follow-up. Patient education was a key element of the protocol. In all, 173 patients were identified for the outpatient program.
The intraoperative criteria for discharge from the PACU included no evidence of perforation or gangrene, and no surgical complications or adverse events. Patients were cleared for discharge if they met the following criteria: heart rate less than 100 beats/min; systolic blood pressure greater than 110 mm Hg; pain well controlled (less than 4 on a 1-10 scale); ambulatory; urinated since surgery; oral intake; and dressings dry without evidence of bleeding.
The patients had been thoroughly briefed on what to expect and problems that would necessitate a return to the emergency department. The physician assessed each patient’s readiness to be discharged, wrote a discharge order, and confirmed the pain medication prescription and follow-up appointment.
Of the 173 patients selected for the outpatient program, 113 (65%) ended up being discharged from the PACU. The reasons for these admissions included interoperative findings, failure to pass the discharge criteria, homelessness, and no transportation to get home.
The control and outpatient groups were similar demographically, except that the latter were on average significantly older (mean age 32.4 years vs. 36.6 years, respectively). The outpatient group had a significantly shorter operative time (69 minutes vs. 83 minutes), a significantly longer stay in the PACU (242 minutes vs.141 minutes), and a significantly shorter total postoperative length of stay (9 hours vs.19 hours).
There were no differences between the groups in terms of complications, postdischarge ED visits, or readmissions. Those who were discharged from the PACU had no postoperative complications and no readmissions.
The length of stay in the PACU gradually decreased for the outpatient group. “This can be attributed to the adoption of a new protocol,” the researchers noted. However, “we purposely did not want to rush the discharge process to ensure our patients and families had all questions answered and were comfortable leaving the hospital.”
A key component of the protocol was the follow-up appointment for all appendectomy patients; about one-third of both groups did not return for their follow-up appointments. Those missed follow-ups could mean some patients returned to another hospital, but the investigators suggested that this was unlikely.
“Because our hospital serves a patient population of low socioeconomic status and often without health insurance, our public hospital is often the only hospital to which they would present,” the investigators wrote.
Most of those who did return completed a questionnaire on their level of satisfaction. Survey results showed no differences in satisfaction between the groups and a generally positive view of the protocol among the outpatient group.
The study did not account for actual cost savings, but reduced hospital admissions and readmissions were achieved. Investigators assert that other studies have shown that each day of hospitalization avoided saves about $1,900.
“It is challenging to deliver high-quality, efficient care to an underserved population in a public hospital,” Dr. Rosen said in an interview. “In this setting, communication and patient education are vital components for success. By setting clear expectations and empowering patients to participate in their care, we can maximize our patients’ outcomes.”
The investigators had no disclosures.
Outpatient laparoscopic surgery for uncomplicated appendicitis can be safely implemented in a large county hospital that serves a poor, underserved population, findings from a prospective, observational trial have shown.
Outpatient appendectomy has gradually gained acceptance in the United States, and numerous studies support the practice. David R. Rosen, MD, of the University of Southern California, Los Angeles, and his colleagues considered the possible advantages of outpatient laparoscopic appendectomy for their institution, such as decreased length of stay, decreased costs, and fewer admissions.
The research team hypothesized that with a “well-defined protocol consisting of strict inclusion and exclusion criteria, clear patient instructions, and close observation to identify patients who would not succeed with the outpatient appendectomy treatment strategy, outpatient appendectomy would be feasible without worsening patient outcomes or satisfaction.”
The findings were published in the Journal of the American College of Surgeons (2017 May;224[5]:862-7).
The investigators conducted a study of patients presenting at a safety-net county hospital and diagnosed with acute appendicitis. A year-long observation period produced a control group of 178 admitted patients.
The outpatient protocol was then introduced. Patients were counseled on the possibility of their being discharged from the postanesthesia care unit (PACU), depending on intraoperative findings and their capacity to arrange a ride home and willingness to participate in postoperative follow-up. Patient education was a key element of the protocol. In all, 173 patients were identified for the outpatient program.
The intraoperative criteria for discharge from the PACU included no evidence of perforation or gangrene, and no surgical complications or adverse events. Patients were cleared for discharge if they met the following criteria: heart rate less than 100 beats/min; systolic blood pressure greater than 110 mm Hg; pain well controlled (less than 4 on a 1-10 scale); ambulatory; urinated since surgery; oral intake; and dressings dry without evidence of bleeding.
The patients had been thoroughly briefed on what to expect and problems that would necessitate a return to the emergency department. The physician assessed each patient’s readiness to be discharged, wrote a discharge order, and confirmed the pain medication prescription and follow-up appointment.
Of the 173 patients selected for the outpatient program, 113 (65%) ended up being discharged from the PACU. The reasons for these admissions included interoperative findings, failure to pass the discharge criteria, homelessness, and no transportation to get home.
The control and outpatient groups were similar demographically, except that the latter were on average significantly older (mean age 32.4 years vs. 36.6 years, respectively). The outpatient group had a significantly shorter operative time (69 minutes vs. 83 minutes), a significantly longer stay in the PACU (242 minutes vs.141 minutes), and a significantly shorter total postoperative length of stay (9 hours vs.19 hours).
There were no differences between the groups in terms of complications, postdischarge ED visits, or readmissions. Those who were discharged from the PACU had no postoperative complications and no readmissions.
The length of stay in the PACU gradually decreased for the outpatient group. “This can be attributed to the adoption of a new protocol,” the researchers noted. However, “we purposely did not want to rush the discharge process to ensure our patients and families had all questions answered and were comfortable leaving the hospital.”
A key component of the protocol was the follow-up appointment for all appendectomy patients; about one-third of both groups did not return for their follow-up appointments. Those missed follow-ups could mean some patients returned to another hospital, but the investigators suggested that this was unlikely.
“Because our hospital serves a patient population of low socioeconomic status and often without health insurance, our public hospital is often the only hospital to which they would present,” the investigators wrote.
Most of those who did return completed a questionnaire on their level of satisfaction. Survey results showed no differences in satisfaction between the groups and a generally positive view of the protocol among the outpatient group.
The study did not account for actual cost savings, but reduced hospital admissions and readmissions were achieved. Investigators assert that other studies have shown that each day of hospitalization avoided saves about $1,900.
“It is challenging to deliver high-quality, efficient care to an underserved population in a public hospital,” Dr. Rosen said in an interview. “In this setting, communication and patient education are vital components for success. By setting clear expectations and empowering patients to participate in their care, we can maximize our patients’ outcomes.”
The investigators had no disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: .
Major finding: The outpatient group had a shorter postoperative hospital length of stay (9 hours vs. 19 hours).
Data source: A prospective, observational study of 351 patients with a diagnosis of acute appendicitis at a public safety-net hospital that serves poor and mostly uninsured patients.
Disclosures: The authors had no disclosures.
Mesh use for lap paraesophageal hernia repair held steady
Utilization of mesh in laparoscopic paraesophageal hernia repair (PEHR) remained steady from 2011 to 2014, despite a lack of evidence supporting its use, according to Francisco Schlottmann, MD, and his associates.
In an analysis of 9,590 laparoscopic PEHR performed from 2011 to 2014, 60.6% procedures were done without mesh and 39.4% were done with mesh. Over the 3-year study period, mesh utilization fell only 1.2% overall, with laparoscopic PEHR with mesh accounting for 39.4% of procedures in 2011 and 38.2% in 2014.
Patients who received mesh were slightly older and significantly more likely to be an inpatient admission. Postoperative urinary tract infection was less common in patients with mesh, occurring in 1% of patients, compared with 1.5% of patients without mesh. No significant difference in demographics was seen, and 30-day risk of comorbidity and mortality was the same. Mean length of stay was 2.7 days for PEHR with mesh and 2.5 days for PEHR without mesh.
“The use of mesh is associated with high expenses, and biomedical technology continues to offer newer and more expensive mesh products on the market. Given the progressive aging of the U.S. population, PEHR are expected to increase in the future. The indiscriminate and not supported by evidence use of mesh may determine unnecessary costs for the health care system,” the investigators noted.
Find the full study in the Journal of Gastrointestinal Surgery (2017 May 26. doi: 10.1007/s11605-017-3452-8).
Utilization of mesh in laparoscopic paraesophageal hernia repair (PEHR) remained steady from 2011 to 2014, despite a lack of evidence supporting its use, according to Francisco Schlottmann, MD, and his associates.
In an analysis of 9,590 laparoscopic PEHR performed from 2011 to 2014, 60.6% procedures were done without mesh and 39.4% were done with mesh. Over the 3-year study period, mesh utilization fell only 1.2% overall, with laparoscopic PEHR with mesh accounting for 39.4% of procedures in 2011 and 38.2% in 2014.
Patients who received mesh were slightly older and significantly more likely to be an inpatient admission. Postoperative urinary tract infection was less common in patients with mesh, occurring in 1% of patients, compared with 1.5% of patients without mesh. No significant difference in demographics was seen, and 30-day risk of comorbidity and mortality was the same. Mean length of stay was 2.7 days for PEHR with mesh and 2.5 days for PEHR without mesh.
“The use of mesh is associated with high expenses, and biomedical technology continues to offer newer and more expensive mesh products on the market. Given the progressive aging of the U.S. population, PEHR are expected to increase in the future. The indiscriminate and not supported by evidence use of mesh may determine unnecessary costs for the health care system,” the investigators noted.
Find the full study in the Journal of Gastrointestinal Surgery (2017 May 26. doi: 10.1007/s11605-017-3452-8).
Utilization of mesh in laparoscopic paraesophageal hernia repair (PEHR) remained steady from 2011 to 2014, despite a lack of evidence supporting its use, according to Francisco Schlottmann, MD, and his associates.
In an analysis of 9,590 laparoscopic PEHR performed from 2011 to 2014, 60.6% procedures were done without mesh and 39.4% were done with mesh. Over the 3-year study period, mesh utilization fell only 1.2% overall, with laparoscopic PEHR with mesh accounting for 39.4% of procedures in 2011 and 38.2% in 2014.
Patients who received mesh were slightly older and significantly more likely to be an inpatient admission. Postoperative urinary tract infection was less common in patients with mesh, occurring in 1% of patients, compared with 1.5% of patients without mesh. No significant difference in demographics was seen, and 30-day risk of comorbidity and mortality was the same. Mean length of stay was 2.7 days for PEHR with mesh and 2.5 days for PEHR without mesh.
“The use of mesh is associated with high expenses, and biomedical technology continues to offer newer and more expensive mesh products on the market. Given the progressive aging of the U.S. population, PEHR are expected to increase in the future. The indiscriminate and not supported by evidence use of mesh may determine unnecessary costs for the health care system,” the investigators noted.
Find the full study in the Journal of Gastrointestinal Surgery (2017 May 26. doi: 10.1007/s11605-017-3452-8).
FROM THE JOURNAL OF GASTROINTESTINAL SURGERY
Big data study looks at safety of concurrent surgical procedures
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Concurrent operations were not associated with an increased risk for worse outcomes, compared with nonconcurrent operations.
Major finding: After propensity-score matching and risk adjustment, the adjusted odds ratio for death or serious mortality was 1.08, and for reoperation, 1.16, for concurrent operations vs. nonconcurrent operations.
Data source: Propensity-score-matched concurrent and nonconcurrent operations (n = 11,044 for each) done in 2014 and 2015 in the American College of Surgeons National Surgical Quality Improvement Program registry.
Disclosures: Dr. Liu and coauthors had no financial relationships to disclose.
Choice of lap vs. open SBO surgery still hinges on patient selection
PHILADELPHIA – In surgery for small-bowel obstruction, laparoscopy is more likely to result in bowel injury than is open surgery but with ultimately better outcomes overall. This means that surgeons should proceed with “considerable caution” when performing minimally invasive surgery in the small bowel, researchers at University of Toronto reported during the annual meeting of the American Surgical Association.
In explaining the rationale for the study, Dr. Behman said, “Several studies have shown that over the last several years [that] the utilization of laparoscopic techniques for adhesive small-bowel obstruction has become increasingly common. However, these procedures have some inherent challenges.” Those technical challenges include introducing the trocar into an abdomen filled with distended bowel, trying to manipulate the distended bowel with laparoscopic bowel graspers, and the potentially ischemic bowel wall, he said.
The University of Toronto researchers performed a population-based retrospective cohort study of 8,584 patients in the Ministry of Health Ontario database from 2005 to 2014. The primary outcome was a composite, consisting of bowel repair, including operative billing codes for either via intraoperative enterotomy or suture repair of the intestine, or bowel resection. Secondary outcomes were serious complications and 30-day mortality.
During the study period, the share of SBO procedures performed laparoscopically increased from 4% in 2005 to 14.3% in 2014, Dr. Behman said.
“Patients in the laparoscopic group were slightly younger, had a lower overall comorbidity burden, and tended to be treated at larger hospitals and hospitals without teaching designations,” Dr. Behman said. Specifically, the average age of the 673 patients who had laparoscopy was 63 years vs. 67 years for the 7,911 open-procedure patients. As for comorbidities, 9% and 50% in the laparoscopic group were healthy and low users and high and very high users, respectively, vs. 7% and 57% of the open-procedure group.
The incidence of any bowel intervention was 53.5% in the laparoscopic group vs. 42% in the open group, Dr. Behman said. After a multivariable regression analysis, the researchers determined the following factors raised a patient’s odds of having a bowel intervention: older age; female sex; having had an after-hours procedure; and having had the procedure earlier in the study period. “However, even after [the researchers adjusted] for all of these covariates, the variable in our model that was most significantly associated with a bowel intervention was having had a laparoscopic procedure, with an odds ratio of 1.6,” Dr. Behman said.
When analyzing secondary outcomes, the researchers found that laparoscopy was associated with a lower risk of 30-day mortality (OR, 0.6) and serious complication (OR, 0.8). “So, there does appear to be a direct trade-off with laparoscopy probably having better clinical outcomes, but also being a risk factor for bowel intervention,” Dr. Behman said. So the researchers preformed a secondary analysis that divided the cohort into four subgroups: laparoscopy with and without bowel intervention; and open surgery with and without bowel intervention.
“The next question we wanted to ask was, in patients that are at high risk of a laparoscopic bowel injury, is an open approach or early conversion to open beneficial?” Dr. Behman said. “To put this another way, is it more important to avoid a bowel intervention or to avoid the morbidity associated with an open procedure?”
The secondary analysis confirmed that laparoscopic patients had lower rates of serious complications: in those without bowel intervention, 5% for laparoscopy vs. 10% for open; and in those who had a bowel intervention, 15% for laparoscopy vs. 22% for open. However, those who had an open procedure without a bowel intervention had lower complication rates than did those who had a laparoscopic operation with a bowel intervention, Dr. Behman said, “suggesting that perhaps the bowel intervention is a greater driver of serious complications than having an open procedure.”
These findings can inform patient selection, Dr. Behman added. “In light of the fact that laparoscopic approaches are likely associated with a greater risk of bowel intervention, appropriate caution should accompany these procedures,” he said. “In patients who are at high risk for a bowel intervention, an open approach or early conversion to a laparotomy should be considered.”
In his discussion of the study, Lawrence Diebel, MD, FACS, of Wayne State University, Detroit, asked if the study accounted for timing of surgery, the etiology of laparoscopic-related bowel interventions, and if serosal tears were included as bowel injuries. Dr. Behman said that the study did include timing of surgery in the initial analysis and found it to be a nonfactor. However, the database did not specify the causes for bowel resections or reasons for conversions.
Dr. Behman and Dr. Diebel reported having no financial disclosures
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – In surgery for small-bowel obstruction, laparoscopy is more likely to result in bowel injury than is open surgery but with ultimately better outcomes overall. This means that surgeons should proceed with “considerable caution” when performing minimally invasive surgery in the small bowel, researchers at University of Toronto reported during the annual meeting of the American Surgical Association.
In explaining the rationale for the study, Dr. Behman said, “Several studies have shown that over the last several years [that] the utilization of laparoscopic techniques for adhesive small-bowel obstruction has become increasingly common. However, these procedures have some inherent challenges.” Those technical challenges include introducing the trocar into an abdomen filled with distended bowel, trying to manipulate the distended bowel with laparoscopic bowel graspers, and the potentially ischemic bowel wall, he said.
The University of Toronto researchers performed a population-based retrospective cohort study of 8,584 patients in the Ministry of Health Ontario database from 2005 to 2014. The primary outcome was a composite, consisting of bowel repair, including operative billing codes for either via intraoperative enterotomy or suture repair of the intestine, or bowel resection. Secondary outcomes were serious complications and 30-day mortality.
During the study period, the share of SBO procedures performed laparoscopically increased from 4% in 2005 to 14.3% in 2014, Dr. Behman said.
“Patients in the laparoscopic group were slightly younger, had a lower overall comorbidity burden, and tended to be treated at larger hospitals and hospitals without teaching designations,” Dr. Behman said. Specifically, the average age of the 673 patients who had laparoscopy was 63 years vs. 67 years for the 7,911 open-procedure patients. As for comorbidities, 9% and 50% in the laparoscopic group were healthy and low users and high and very high users, respectively, vs. 7% and 57% of the open-procedure group.
The incidence of any bowel intervention was 53.5% in the laparoscopic group vs. 42% in the open group, Dr. Behman said. After a multivariable regression analysis, the researchers determined the following factors raised a patient’s odds of having a bowel intervention: older age; female sex; having had an after-hours procedure; and having had the procedure earlier in the study period. “However, even after [the researchers adjusted] for all of these covariates, the variable in our model that was most significantly associated with a bowel intervention was having had a laparoscopic procedure, with an odds ratio of 1.6,” Dr. Behman said.
When analyzing secondary outcomes, the researchers found that laparoscopy was associated with a lower risk of 30-day mortality (OR, 0.6) and serious complication (OR, 0.8). “So, there does appear to be a direct trade-off with laparoscopy probably having better clinical outcomes, but also being a risk factor for bowel intervention,” Dr. Behman said. So the researchers preformed a secondary analysis that divided the cohort into four subgroups: laparoscopy with and without bowel intervention; and open surgery with and without bowel intervention.
“The next question we wanted to ask was, in patients that are at high risk of a laparoscopic bowel injury, is an open approach or early conversion to open beneficial?” Dr. Behman said. “To put this another way, is it more important to avoid a bowel intervention or to avoid the morbidity associated with an open procedure?”
The secondary analysis confirmed that laparoscopic patients had lower rates of serious complications: in those without bowel intervention, 5% for laparoscopy vs. 10% for open; and in those who had a bowel intervention, 15% for laparoscopy vs. 22% for open. However, those who had an open procedure without a bowel intervention had lower complication rates than did those who had a laparoscopic operation with a bowel intervention, Dr. Behman said, “suggesting that perhaps the bowel intervention is a greater driver of serious complications than having an open procedure.”
These findings can inform patient selection, Dr. Behman added. “In light of the fact that laparoscopic approaches are likely associated with a greater risk of bowel intervention, appropriate caution should accompany these procedures,” he said. “In patients who are at high risk for a bowel intervention, an open approach or early conversion to a laparotomy should be considered.”
In his discussion of the study, Lawrence Diebel, MD, FACS, of Wayne State University, Detroit, asked if the study accounted for timing of surgery, the etiology of laparoscopic-related bowel interventions, and if serosal tears were included as bowel injuries. Dr. Behman said that the study did include timing of surgery in the initial analysis and found it to be a nonfactor. However, the database did not specify the causes for bowel resections or reasons for conversions.
Dr. Behman and Dr. Diebel reported having no financial disclosures
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – In surgery for small-bowel obstruction, laparoscopy is more likely to result in bowel injury than is open surgery but with ultimately better outcomes overall. This means that surgeons should proceed with “considerable caution” when performing minimally invasive surgery in the small bowel, researchers at University of Toronto reported during the annual meeting of the American Surgical Association.
In explaining the rationale for the study, Dr. Behman said, “Several studies have shown that over the last several years [that] the utilization of laparoscopic techniques for adhesive small-bowel obstruction has become increasingly common. However, these procedures have some inherent challenges.” Those technical challenges include introducing the trocar into an abdomen filled with distended bowel, trying to manipulate the distended bowel with laparoscopic bowel graspers, and the potentially ischemic bowel wall, he said.
The University of Toronto researchers performed a population-based retrospective cohort study of 8,584 patients in the Ministry of Health Ontario database from 2005 to 2014. The primary outcome was a composite, consisting of bowel repair, including operative billing codes for either via intraoperative enterotomy or suture repair of the intestine, or bowel resection. Secondary outcomes were serious complications and 30-day mortality.
During the study period, the share of SBO procedures performed laparoscopically increased from 4% in 2005 to 14.3% in 2014, Dr. Behman said.
“Patients in the laparoscopic group were slightly younger, had a lower overall comorbidity burden, and tended to be treated at larger hospitals and hospitals without teaching designations,” Dr. Behman said. Specifically, the average age of the 673 patients who had laparoscopy was 63 years vs. 67 years for the 7,911 open-procedure patients. As for comorbidities, 9% and 50% in the laparoscopic group were healthy and low users and high and very high users, respectively, vs. 7% and 57% of the open-procedure group.
The incidence of any bowel intervention was 53.5% in the laparoscopic group vs. 42% in the open group, Dr. Behman said. After a multivariable regression analysis, the researchers determined the following factors raised a patient’s odds of having a bowel intervention: older age; female sex; having had an after-hours procedure; and having had the procedure earlier in the study period. “However, even after [the researchers adjusted] for all of these covariates, the variable in our model that was most significantly associated with a bowel intervention was having had a laparoscopic procedure, with an odds ratio of 1.6,” Dr. Behman said.
When analyzing secondary outcomes, the researchers found that laparoscopy was associated with a lower risk of 30-day mortality (OR, 0.6) and serious complication (OR, 0.8). “So, there does appear to be a direct trade-off with laparoscopy probably having better clinical outcomes, but also being a risk factor for bowel intervention,” Dr. Behman said. So the researchers preformed a secondary analysis that divided the cohort into four subgroups: laparoscopy with and without bowel intervention; and open surgery with and without bowel intervention.
“The next question we wanted to ask was, in patients that are at high risk of a laparoscopic bowel injury, is an open approach or early conversion to open beneficial?” Dr. Behman said. “To put this another way, is it more important to avoid a bowel intervention or to avoid the morbidity associated with an open procedure?”
The secondary analysis confirmed that laparoscopic patients had lower rates of serious complications: in those without bowel intervention, 5% for laparoscopy vs. 10% for open; and in those who had a bowel intervention, 15% for laparoscopy vs. 22% for open. However, those who had an open procedure without a bowel intervention had lower complication rates than did those who had a laparoscopic operation with a bowel intervention, Dr. Behman said, “suggesting that perhaps the bowel intervention is a greater driver of serious complications than having an open procedure.”
These findings can inform patient selection, Dr. Behman added. “In light of the fact that laparoscopic approaches are likely associated with a greater risk of bowel intervention, appropriate caution should accompany these procedures,” he said. “In patients who are at high risk for a bowel intervention, an open approach or early conversion to a laparotomy should be considered.”
In his discussion of the study, Lawrence Diebel, MD, FACS, of Wayne State University, Detroit, asked if the study accounted for timing of surgery, the etiology of laparoscopic-related bowel interventions, and if serosal tears were included as bowel injuries. Dr. Behman said that the study did include timing of surgery in the initial analysis and found it to be a nonfactor. However, the database did not specify the causes for bowel resections or reasons for conversions.
Dr. Behman and Dr. Diebel reported having no financial disclosures
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Laparoscopic surgery for SBO is associated with a greater risk of bowel intervention.
Major finding: The incidence of bowel intervention was 53.5% vs. 43.4% in laparoscopic and open procedures, respectively.
Data source: Review of 8,584 procedures for SBO in the Ministry of Health Ontario database performed from 2005 to 2014.
Disclosures: Dr. Behman and Dr. Diebel reported having no financial disclosures.
Low-income uninsured trauma patients at risk for ruinous medical costs
Almost three-quarters of uninsured adults admitted for traumatic injury are at risk of catastrophic health expenditures (CHEs), according to a large retrospective study from a national patient database.
Since enactment of the Affordable Care Act in 2010, the number of uninsured individuals has dropped substantially, but there remains a large population of younger adults, many from low-income areas, who still are not covered. The Centers for Disease Control and Prevention reported in 2015 that 12.8% of individuals aged 18-64 years were uninsured. The financial impact of a traumatic injury is likely to be significant for those paying out of pocket, but the question of who is at risk and to what degree is understudied, according to John W. Scott, MD, of Brigham and Women’s Hospital, Boston, and his colleagues.
“Defining populations at risk of financial catastrophe after medical expense is a necessary step towards elucidating the effect of health care reforms intended to increase access to healthcare through insurance expansion,” they wrote in the Annals of Surgery (2017 Apr 7. doi: 10.1097/SLA.0000000000002254).
Dr. Scott and his colleagues analyzed trauma diagnoses in adults aged 18-64 years from Nationwide Inpatient Sample 2007-2011. They used the U.S. Census data to estimate post-subsistence income (income remaining after paying for food) and hospital charges for trauma diagnoses. The sample of 117,502 patient encounters was weighted to represent 579,683 trauma patients in the national database.
CHEs were defined conservatively as expenses that exceed 40% of a patient’s post-subsistence income; they also applied a lower threshold of CHEs – 10% of a patient’s entire income – without accounting for subsistence needs.
The investigators wanted to look at who was at greatest risk for CHEs and used the Census data to sort trauma patients by residential zip code to identify the distribution of income in the sample.
Costs to trauma patients varied by injury severity score (ISS) and by income quartile. Patients in the 25th income quartile paid less for their treatment, even accounting for ISS, but despite this difference, poorer patients were at a much greater risk for CHEs.
The median charge for all of these uninsured trauma patients was $27,420. Those trauma patients in the 25th percentile of income were charged an average of $15,196, while those in 75th quartile were charged $49,696. The difference in costs also was evident when comparing patients in different ISS categories: for ISS 9-15, median trauma costs for the lower-quartile patients was $31,095, but costs for the upper quartile patients was $52,639.
With cost, income, and injury severity data, the investigators found that overall the proportion of adults aged 18-64 years at risk for CHEs was 70.8% (95% confidence interval, 70.7%-71.1%). Subpopulations (ethnicity/race, age, sex) were within a few percentage point of each other.
Risk of CHEs varied widely by income quartile and ISS. Uninsured trauma patients in the highest income quartile had a 52.9% risk of CHEs, compared with 77.5% of those in the lowest-income quartile. Overall, 81.8% of patients with ISS greater than 24 were at risk for CHEs. In addition, the study found that patients treated at large or urban teaching hospitals – those most likely to handle the most-severely injured patients – were at a heightened risk for CHEs.
When the investigators conducted the same analysis with a lower threshold of CHEs (10% of income, not including subsistence expenses), the overall risk for uninsured trauma patients for catastrophic health expenses was more than 90%.
“Trauma patients are at particularly high risk for CHE because trauma disproportionately affects uninsured patients with little disposable income and because trauma injuries commonly affect multiple organ systems and incur expensive, multispecialty care,” Dr. Scott and his colleagues wrote. “Large unpaid medical debts may have longstanding impacts on patients’ financial well-being, affecting credit ratings and overall financial solvency. These financial stresses may be further compounded by an inability to return to work caused by injury.”
Dr. Scott and his coauthors added that having health insurance does not entirely eliminate the risk for CHEs and that individuals in the lower income brackets covered by employer-based plans or Medicaid had difficulty paying medical bills.
Only hospital costs were examined, which may not reflect forgiven debts, the investigators noted. In addition, the costs may be underreported because they do not include physician charges, postacute care, home health care, or rehabilitation. Incomes were estimated by zip code analysis, which may misclassify some individuals.
“Efforts are needed to ensure that the lifesaving care provided by our highly coordinated trauma systems do not have the unintended consequence of curing patients into destitution,” Dr. Scott and his colleagues wrote
The authors declared no conflicts of interest.
Almost three-quarters of uninsured adults admitted for traumatic injury are at risk of catastrophic health expenditures (CHEs), according to a large retrospective study from a national patient database.
Since enactment of the Affordable Care Act in 2010, the number of uninsured individuals has dropped substantially, but there remains a large population of younger adults, many from low-income areas, who still are not covered. The Centers for Disease Control and Prevention reported in 2015 that 12.8% of individuals aged 18-64 years were uninsured. The financial impact of a traumatic injury is likely to be significant for those paying out of pocket, but the question of who is at risk and to what degree is understudied, according to John W. Scott, MD, of Brigham and Women’s Hospital, Boston, and his colleagues.
“Defining populations at risk of financial catastrophe after medical expense is a necessary step towards elucidating the effect of health care reforms intended to increase access to healthcare through insurance expansion,” they wrote in the Annals of Surgery (2017 Apr 7. doi: 10.1097/SLA.0000000000002254).
Dr. Scott and his colleagues analyzed trauma diagnoses in adults aged 18-64 years from Nationwide Inpatient Sample 2007-2011. They used the U.S. Census data to estimate post-subsistence income (income remaining after paying for food) and hospital charges for trauma diagnoses. The sample of 117,502 patient encounters was weighted to represent 579,683 trauma patients in the national database.
CHEs were defined conservatively as expenses that exceed 40% of a patient’s post-subsistence income; they also applied a lower threshold of CHEs – 10% of a patient’s entire income – without accounting for subsistence needs.
The investigators wanted to look at who was at greatest risk for CHEs and used the Census data to sort trauma patients by residential zip code to identify the distribution of income in the sample.
Costs to trauma patients varied by injury severity score (ISS) and by income quartile. Patients in the 25th income quartile paid less for their treatment, even accounting for ISS, but despite this difference, poorer patients were at a much greater risk for CHEs.
The median charge for all of these uninsured trauma patients was $27,420. Those trauma patients in the 25th percentile of income were charged an average of $15,196, while those in 75th quartile were charged $49,696. The difference in costs also was evident when comparing patients in different ISS categories: for ISS 9-15, median trauma costs for the lower-quartile patients was $31,095, but costs for the upper quartile patients was $52,639.
With cost, income, and injury severity data, the investigators found that overall the proportion of adults aged 18-64 years at risk for CHEs was 70.8% (95% confidence interval, 70.7%-71.1%). Subpopulations (ethnicity/race, age, sex) were within a few percentage point of each other.
Risk of CHEs varied widely by income quartile and ISS. Uninsured trauma patients in the highest income quartile had a 52.9% risk of CHEs, compared with 77.5% of those in the lowest-income quartile. Overall, 81.8% of patients with ISS greater than 24 were at risk for CHEs. In addition, the study found that patients treated at large or urban teaching hospitals – those most likely to handle the most-severely injured patients – were at a heightened risk for CHEs.
When the investigators conducted the same analysis with a lower threshold of CHEs (10% of income, not including subsistence expenses), the overall risk for uninsured trauma patients for catastrophic health expenses was more than 90%.
“Trauma patients are at particularly high risk for CHE because trauma disproportionately affects uninsured patients with little disposable income and because trauma injuries commonly affect multiple organ systems and incur expensive, multispecialty care,” Dr. Scott and his colleagues wrote. “Large unpaid medical debts may have longstanding impacts on patients’ financial well-being, affecting credit ratings and overall financial solvency. These financial stresses may be further compounded by an inability to return to work caused by injury.”
Dr. Scott and his coauthors added that having health insurance does not entirely eliminate the risk for CHEs and that individuals in the lower income brackets covered by employer-based plans or Medicaid had difficulty paying medical bills.
Only hospital costs were examined, which may not reflect forgiven debts, the investigators noted. In addition, the costs may be underreported because they do not include physician charges, postacute care, home health care, or rehabilitation. Incomes were estimated by zip code analysis, which may misclassify some individuals.
“Efforts are needed to ensure that the lifesaving care provided by our highly coordinated trauma systems do not have the unintended consequence of curing patients into destitution,” Dr. Scott and his colleagues wrote
The authors declared no conflicts of interest.
Almost three-quarters of uninsured adults admitted for traumatic injury are at risk of catastrophic health expenditures (CHEs), according to a large retrospective study from a national patient database.
Since enactment of the Affordable Care Act in 2010, the number of uninsured individuals has dropped substantially, but there remains a large population of younger adults, many from low-income areas, who still are not covered. The Centers for Disease Control and Prevention reported in 2015 that 12.8% of individuals aged 18-64 years were uninsured. The financial impact of a traumatic injury is likely to be significant for those paying out of pocket, but the question of who is at risk and to what degree is understudied, according to John W. Scott, MD, of Brigham and Women’s Hospital, Boston, and his colleagues.
“Defining populations at risk of financial catastrophe after medical expense is a necessary step towards elucidating the effect of health care reforms intended to increase access to healthcare through insurance expansion,” they wrote in the Annals of Surgery (2017 Apr 7. doi: 10.1097/SLA.0000000000002254).
Dr. Scott and his colleagues analyzed trauma diagnoses in adults aged 18-64 years from Nationwide Inpatient Sample 2007-2011. They used the U.S. Census data to estimate post-subsistence income (income remaining after paying for food) and hospital charges for trauma diagnoses. The sample of 117,502 patient encounters was weighted to represent 579,683 trauma patients in the national database.
CHEs were defined conservatively as expenses that exceed 40% of a patient’s post-subsistence income; they also applied a lower threshold of CHEs – 10% of a patient’s entire income – without accounting for subsistence needs.
The investigators wanted to look at who was at greatest risk for CHEs and used the Census data to sort trauma patients by residential zip code to identify the distribution of income in the sample.
Costs to trauma patients varied by injury severity score (ISS) and by income quartile. Patients in the 25th income quartile paid less for their treatment, even accounting for ISS, but despite this difference, poorer patients were at a much greater risk for CHEs.
The median charge for all of these uninsured trauma patients was $27,420. Those trauma patients in the 25th percentile of income were charged an average of $15,196, while those in 75th quartile were charged $49,696. The difference in costs also was evident when comparing patients in different ISS categories: for ISS 9-15, median trauma costs for the lower-quartile patients was $31,095, but costs for the upper quartile patients was $52,639.
With cost, income, and injury severity data, the investigators found that overall the proportion of adults aged 18-64 years at risk for CHEs was 70.8% (95% confidence interval, 70.7%-71.1%). Subpopulations (ethnicity/race, age, sex) were within a few percentage point of each other.
Risk of CHEs varied widely by income quartile and ISS. Uninsured trauma patients in the highest income quartile had a 52.9% risk of CHEs, compared with 77.5% of those in the lowest-income quartile. Overall, 81.8% of patients with ISS greater than 24 were at risk for CHEs. In addition, the study found that patients treated at large or urban teaching hospitals – those most likely to handle the most-severely injured patients – were at a heightened risk for CHEs.
When the investigators conducted the same analysis with a lower threshold of CHEs (10% of income, not including subsistence expenses), the overall risk for uninsured trauma patients for catastrophic health expenses was more than 90%.
“Trauma patients are at particularly high risk for CHE because trauma disproportionately affects uninsured patients with little disposable income and because trauma injuries commonly affect multiple organ systems and incur expensive, multispecialty care,” Dr. Scott and his colleagues wrote. “Large unpaid medical debts may have longstanding impacts on patients’ financial well-being, affecting credit ratings and overall financial solvency. These financial stresses may be further compounded by an inability to return to work caused by injury.”
Dr. Scott and his coauthors added that having health insurance does not entirely eliminate the risk for CHEs and that individuals in the lower income brackets covered by employer-based plans or Medicaid had difficulty paying medical bills.
Only hospital costs were examined, which may not reflect forgiven debts, the investigators noted. In addition, the costs may be underreported because they do not include physician charges, postacute care, home health care, or rehabilitation. Incomes were estimated by zip code analysis, which may misclassify some individuals.
“Efforts are needed to ensure that the lifesaving care provided by our highly coordinated trauma systems do not have the unintended consequence of curing patients into destitution,” Dr. Scott and his colleagues wrote
The authors declared no conflicts of interest.
FROM ANNALS OF SURGERY
Drainage may be nonfactor after distal pancreatectomy outcomes
PHILADELPHIA – A randomized multicenter trial that compared patients who had distal pancreatectomy with and without routine peritoneal drainage found no appreciable difference in the complication rates between the two groups, the lead investigator reported at the annual meeting of the American Surgical Association here.
The trial randomized 202 patients to the intraperitoneal drainage group and 197 to the nondrainage group. The groups were well matched in terms of patients who dropped out because of metastatic disease or other reason, as well as demographics and operative data. “They were equally matched for laparoscopic vs. open, equally matched for vascular resection, equally matched for pancreatic texture and duct size, equally matched for method of transection, equally matched for operative time and blood loss and equally matched for surgical pathology,” Dr. Van Buren said.
The primary outcome was frequency of grade 2 complications or greater, which occurred in 44% (76) of the drainage group and 42% (72) of the nondrainage group (P = .804). “Similarly, the groups were equal for grade 3 severe complications as well as median complication severity and median number of complications,” Dr. Van Buren said. The rates of complications of grade 3 or greater were 29% (51) for the drainage group and 26% (44) for the nondrainage group (P = .477). Ninety-day mortality also was similar between the two groups: None died in the drainage group and two (1%) died in the nondrainage group (P = .24).
Drilling down into types of complications, Dr. Van Buren added that rates were similar across the board. “There was no difference in clinically relevant postoperative pancreatic fistula between the drain and no-drain group,” he said. “There was no difference in intra-abdominal abscess, no difference in the rates of postoperative imaging, no difference in the rates of postoperative percutaneous drain placements, and there was no difference in readmission or reoperation.”
One outcome that was noticeably different between the two groups was intra-abdominal fluid collections, reported in 9% (15) of the drainage group and 22% (38) of the nondrainage group (P = .0004). “However,” Dr. Van Buren said, “these were asymptomatic.”
In his discussion of the presentation, Charles Yeo, MD, FACS, of Thomas Jefferson University, Philadelphia, said that “the scope and the rigor of this study are impressive and quite laudable” but raised a number of questions, including concerns about the two deaths in the nondrainage group in the context of two other trials: a smaller multicenter trial, coauthored by Dr. Van Buren, that compared postoperative use of drains and nondrainage in pancreaticoduodenectomy but was halted because of eight deaths in the nondrainage group (Ann Surg. 2014;259:605-12); and the German PANDRA trial reported at last year’s ASA meeting that found nondrainage after pancreaticoduodenectomy to be superior to drainage in terms of reintervention and fistula rates.
Dr. Van Buren replied that the first study from Baylor – referenced by Dr. Yeo – involved “well-balanced groups, and they were equally matched and had minimal dropout throughout.” Because of that, the finding that drain placement for the pancreaticoduodenectomy “was definitive,” whereas the PANDRA trial was subject to some criticisms. The screening and randomization processes in PANDRA have been criticized because 3,200 patients were eligible for enrollment, suggesting a screening bias, Dr. Van Buren said. In addition, drains were placed in 21% of patients who were allocated to the no-drain group, suggesting surgeons deviated from the protocol in higher-risk situations, resulting in additional selection bias. This implies PANDRA was more of a selective draining trial, he said.
Dr. Van Buren and Dr. Yeo reported having no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – A randomized multicenter trial that compared patients who had distal pancreatectomy with and without routine peritoneal drainage found no appreciable difference in the complication rates between the two groups, the lead investigator reported at the annual meeting of the American Surgical Association here.
The trial randomized 202 patients to the intraperitoneal drainage group and 197 to the nondrainage group. The groups were well matched in terms of patients who dropped out because of metastatic disease or other reason, as well as demographics and operative data. “They were equally matched for laparoscopic vs. open, equally matched for vascular resection, equally matched for pancreatic texture and duct size, equally matched for method of transection, equally matched for operative time and blood loss and equally matched for surgical pathology,” Dr. Van Buren said.
The primary outcome was frequency of grade 2 complications or greater, which occurred in 44% (76) of the drainage group and 42% (72) of the nondrainage group (P = .804). “Similarly, the groups were equal for grade 3 severe complications as well as median complication severity and median number of complications,” Dr. Van Buren said. The rates of complications of grade 3 or greater were 29% (51) for the drainage group and 26% (44) for the nondrainage group (P = .477). Ninety-day mortality also was similar between the two groups: None died in the drainage group and two (1%) died in the nondrainage group (P = .24).
Drilling down into types of complications, Dr. Van Buren added that rates were similar across the board. “There was no difference in clinically relevant postoperative pancreatic fistula between the drain and no-drain group,” he said. “There was no difference in intra-abdominal abscess, no difference in the rates of postoperative imaging, no difference in the rates of postoperative percutaneous drain placements, and there was no difference in readmission or reoperation.”
One outcome that was noticeably different between the two groups was intra-abdominal fluid collections, reported in 9% (15) of the drainage group and 22% (38) of the nondrainage group (P = .0004). “However,” Dr. Van Buren said, “these were asymptomatic.”
In his discussion of the presentation, Charles Yeo, MD, FACS, of Thomas Jefferson University, Philadelphia, said that “the scope and the rigor of this study are impressive and quite laudable” but raised a number of questions, including concerns about the two deaths in the nondrainage group in the context of two other trials: a smaller multicenter trial, coauthored by Dr. Van Buren, that compared postoperative use of drains and nondrainage in pancreaticoduodenectomy but was halted because of eight deaths in the nondrainage group (Ann Surg. 2014;259:605-12); and the German PANDRA trial reported at last year’s ASA meeting that found nondrainage after pancreaticoduodenectomy to be superior to drainage in terms of reintervention and fistula rates.
Dr. Van Buren replied that the first study from Baylor – referenced by Dr. Yeo – involved “well-balanced groups, and they were equally matched and had minimal dropout throughout.” Because of that, the finding that drain placement for the pancreaticoduodenectomy “was definitive,” whereas the PANDRA trial was subject to some criticisms. The screening and randomization processes in PANDRA have been criticized because 3,200 patients were eligible for enrollment, suggesting a screening bias, Dr. Van Buren said. In addition, drains were placed in 21% of patients who were allocated to the no-drain group, suggesting surgeons deviated from the protocol in higher-risk situations, resulting in additional selection bias. This implies PANDRA was more of a selective draining trial, he said.
Dr. Van Buren and Dr. Yeo reported having no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – A randomized multicenter trial that compared patients who had distal pancreatectomy with and without routine peritoneal drainage found no appreciable difference in the complication rates between the two groups, the lead investigator reported at the annual meeting of the American Surgical Association here.
The trial randomized 202 patients to the intraperitoneal drainage group and 197 to the nondrainage group. The groups were well matched in terms of patients who dropped out because of metastatic disease or other reason, as well as demographics and operative data. “They were equally matched for laparoscopic vs. open, equally matched for vascular resection, equally matched for pancreatic texture and duct size, equally matched for method of transection, equally matched for operative time and blood loss and equally matched for surgical pathology,” Dr. Van Buren said.
The primary outcome was frequency of grade 2 complications or greater, which occurred in 44% (76) of the drainage group and 42% (72) of the nondrainage group (P = .804). “Similarly, the groups were equal for grade 3 severe complications as well as median complication severity and median number of complications,” Dr. Van Buren said. The rates of complications of grade 3 or greater were 29% (51) for the drainage group and 26% (44) for the nondrainage group (P = .477). Ninety-day mortality also was similar between the two groups: None died in the drainage group and two (1%) died in the nondrainage group (P = .24).
Drilling down into types of complications, Dr. Van Buren added that rates were similar across the board. “There was no difference in clinically relevant postoperative pancreatic fistula between the drain and no-drain group,” he said. “There was no difference in intra-abdominal abscess, no difference in the rates of postoperative imaging, no difference in the rates of postoperative percutaneous drain placements, and there was no difference in readmission or reoperation.”
One outcome that was noticeably different between the two groups was intra-abdominal fluid collections, reported in 9% (15) of the drainage group and 22% (38) of the nondrainage group (P = .0004). “However,” Dr. Van Buren said, “these were asymptomatic.”
In his discussion of the presentation, Charles Yeo, MD, FACS, of Thomas Jefferson University, Philadelphia, said that “the scope and the rigor of this study are impressive and quite laudable” but raised a number of questions, including concerns about the two deaths in the nondrainage group in the context of two other trials: a smaller multicenter trial, coauthored by Dr. Van Buren, that compared postoperative use of drains and nondrainage in pancreaticoduodenectomy but was halted because of eight deaths in the nondrainage group (Ann Surg. 2014;259:605-12); and the German PANDRA trial reported at last year’s ASA meeting that found nondrainage after pancreaticoduodenectomy to be superior to drainage in terms of reintervention and fistula rates.
Dr. Van Buren replied that the first study from Baylor – referenced by Dr. Yeo – involved “well-balanced groups, and they were equally matched and had minimal dropout throughout.” Because of that, the finding that drain placement for the pancreaticoduodenectomy “was definitive,” whereas the PANDRA trial was subject to some criticisms. The screening and randomization processes in PANDRA have been criticized because 3,200 patients were eligible for enrollment, suggesting a screening bias, Dr. Van Buren said. In addition, drains were placed in 21% of patients who were allocated to the no-drain group, suggesting surgeons deviated from the protocol in higher-risk situations, resulting in additional selection bias. This implies PANDRA was more of a selective draining trial, he said.
Dr. Van Buren and Dr. Yeo reported having no financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, is to be published in Annals of Surgery pending editorial review.
AT THE ASA ANNUAL
Key clinical point: A randomized clinical trial has provided Level 1 evidence that placement of intraperitoneal drains does not alter clinical outcomes after distal pancreatectomy.
Major finding: No significant difference was found in the rate of complications between those who had drains and those who did not (44% vs. 42% of complications greater than grade 2).
Data source: Randomized trial of 399 patients who underwent distal pancreatectomy at 14 high-volume pancreas centers.
Disclosures: Dr. Van Buren reported having no financial disclosures.
Point/Counterpoint: Should all suspected mucinous cystic neoplasms be resected?
Dr. Pawlik is a pretty clever guy and a strong adversary. Mucinous cystic neoplasm is literally the only topic in hepato-pancreato-biliary surgery he has not written about – yet.1
But I will argue that all suspected mucinous cystic neoplasms (MCNs) should be surgically removed. Reasons include: the natural history of a benign MCN in a typical 45-year-old remains unknown, clinical features are not always reliable, and it’s difficult to distinguish benign from malignant neoplasms without surgery.
Although many MCNs are benign when resected, all have malignant potential.
The prevalence of cancer at time of diagnosis with an MCN is fairly low, about 15%. This means most of these cysts are benign when resected. However, discrimination between benign and malignant is difficult without surgery, and the degree of epithelial dysplasia at time of resection ranges from mild to severe with invasive carcinoma.
When cancer is present, patients tend to do poorly. In one study where 44 out of 344 patients developed invasive cancer, the group with cancer had a 3-year overall survival rate of 59%.2
In addition, aspiration of cyst fluid is often of limited utility. It is poor at distinguishing whether a cyst is benign or malignant. For example, in a series of 55 patients with MCNs that underwent fine-needle aspiration of cyst epithelium, 71% of assays were nondiagnostic.3 So this diagnostic test is very insensitive and may miss at least half of cancers – another point in favor of surgical resection for all MCNs.
It is true that evidence in the literature associates certain clinical factors with a higher risk for malignancy in suspected MCNs. These include male gender, larger cysts, and location in the pancreatic head or neck or larger cyst diameter and presence of nodules.2,4 However, use of clinical features is not perfect.
In another study, of 163 resected MCNs, those with invasive cancer were often – but not always – larger than 4 cm with nodules.5 These same series revealed that those with invasive cancer were often, but again not always, larger than 4 cm.
We see this in our practice as well – that a large cyst or presence of nodules is not a foolproof indicator of malignancy. We had a 38-year-old woman with a large cyst and nodules who did not have cancer. In contrast, a 45-year-old woman at our institution presented with small cysts and no cancer on fine-needle aspiration. However, final pathology in her case revealed high-grade dysplasia.
Also, we only know what happens to cysts that have been resected. We know nothing about duration or other best practices for following patients who do not undergo surgery. For this reason, we really don’t know what these tumors will do if left alone.
Surgery is curative and carries a pretty low risk. Following resection, these patients do well. We’re talking about curative operations.
Dr. Katz is an Associate Professor, Department of Surgical Oncology, Division of Surgery at the University of Texas MD Anderson Cancer Center in Houston. He is also Chief of the Pancreas Surgery Service at MD Anderson. Dr. Katz noted he was asked to provide the pro side of the argument, and he may not necessarily uphold these positions in his own practice. Dr. Katz had no relevant financial disclosures.
References:
1. Dr. Pawlik’s list of selected publications: http://pathology.jhu.edu/liver/pawlik.cfm.
2. JAMA Surg. 2017;152:19-25.
3. Cancer. 2017;125:169-77.
4. Pancreas. 2011;40:67-71.
5. Ann Surg. 2008;247:571-9.
Some mucinous cystic neoplasms can be safely followed.
Data in the literature suggest some of these suspected mucinous cystic neoplasms can be followed; surgery may not be indicated solely because “as surgeons we tend to take all masses out” and because operative complications occur. Therefore, resection is not a benign procedure.
Dr. Katz, you were done before you got started. The evidence shows that some of the MCNs can be followed rather than resected.
Most of the published studies focus on IPMN, but I think the topic of MCNs is becoming increasingly important; they follow IPMNs as the second most common type of cystic neoplasms. So we’re going to be finding more small MCNs. In this debate, we are not talking about an 8-cm MCN, but rather what do we do when we see a 2- to 3-cm MCN. Do these patients all need to undergo resection?
What Dr. Katz is concerned about is that we are going to miss a cancer. We should operate on all patients because – at least as Dr. Katz’s argues – the surgery can always be done without complications.
However, even at the very, very experienced centers, morbidity was 30%-50%, and a pancreatic fistula developed for 1 in 10 patients. So it’s not a benign procedure.1
As surgeons, we tend to take every mass out. Although operative mortality is low, it is still in a measurable range, 1%-2%, and that is even at expert centers. Many of these small cysts are being found at smaller and community hospitals, and undue morbidity and mortality may weigh more heavily in these settings.2
Rather than “being a hammer and seeing everything as a nail,” we need a more rational approach. For example, we should identify a subgroup that will the most benefit from resection.
Investigators from Memorial Sloan Kettering Cancer Center reported that certain clinical factors identify patients at higher risk for mortality, such as nodules.3 Patients with invasive cancer almost all had nodules. And all patients with invasive cancer without nodules had a tumor larger than 4 cm.
Maybe using both presence of nodules and size is the right approach to identifying underling malignancy in suspected MCNs. Again, size is important, as is male gender, presence of solid nodules, and duct dilatation.4
We are arguing whether all MCNs should be resected. I’m positing that many 2-cm to 3-cm MCNs can be followed. There is only a small chance they will grow over time, and it’s unlikely they are harboring a malignancy.
Dr. Pawlik is Chair of Surgery at the Ohio State Wexner Medical Center in Columbus. He is also the Urban Meyer III and Shelley Meyer Chair for Cancer Research at Ohio State. He has no disclosures.
References
1. HPB (Oxford). 2007;9:8-15.
2. Diagnosis and Management of Cystic Lesions of the Pancreas. Diagnostic and Therapeutic Endoscopy Volume 2011 (2011), Article ID 478913.
3. JAMA Surg. 2017;152:19-25.
4. Ann Surg. 2006;244:572-82.
This Point/Counterpoint feature is based on comments Dr. Katz and Dr. Pawlik made during a debate at AHPBA 2017, the annual meeting of the Americas Hepato-Pancreato-Biliary Association.
Dr. Pawlik is a pretty clever guy and a strong adversary. Mucinous cystic neoplasm is literally the only topic in hepato-pancreato-biliary surgery he has not written about – yet.1
But I will argue that all suspected mucinous cystic neoplasms (MCNs) should be surgically removed. Reasons include: the natural history of a benign MCN in a typical 45-year-old remains unknown, clinical features are not always reliable, and it’s difficult to distinguish benign from malignant neoplasms without surgery.
Although many MCNs are benign when resected, all have malignant potential.
The prevalence of cancer at time of diagnosis with an MCN is fairly low, about 15%. This means most of these cysts are benign when resected. However, discrimination between benign and malignant is difficult without surgery, and the degree of epithelial dysplasia at time of resection ranges from mild to severe with invasive carcinoma.
When cancer is present, patients tend to do poorly. In one study where 44 out of 344 patients developed invasive cancer, the group with cancer had a 3-year overall survival rate of 59%.2
In addition, aspiration of cyst fluid is often of limited utility. It is poor at distinguishing whether a cyst is benign or malignant. For example, in a series of 55 patients with MCNs that underwent fine-needle aspiration of cyst epithelium, 71% of assays were nondiagnostic.3 So this diagnostic test is very insensitive and may miss at least half of cancers – another point in favor of surgical resection for all MCNs.
It is true that evidence in the literature associates certain clinical factors with a higher risk for malignancy in suspected MCNs. These include male gender, larger cysts, and location in the pancreatic head or neck or larger cyst diameter and presence of nodules.2,4 However, use of clinical features is not perfect.
In another study, of 163 resected MCNs, those with invasive cancer were often – but not always – larger than 4 cm with nodules.5 These same series revealed that those with invasive cancer were often, but again not always, larger than 4 cm.
We see this in our practice as well – that a large cyst or presence of nodules is not a foolproof indicator of malignancy. We had a 38-year-old woman with a large cyst and nodules who did not have cancer. In contrast, a 45-year-old woman at our institution presented with small cysts and no cancer on fine-needle aspiration. However, final pathology in her case revealed high-grade dysplasia.
Also, we only know what happens to cysts that have been resected. We know nothing about duration or other best practices for following patients who do not undergo surgery. For this reason, we really don’t know what these tumors will do if left alone.
Surgery is curative and carries a pretty low risk. Following resection, these patients do well. We’re talking about curative operations.
Dr. Katz is an Associate Professor, Department of Surgical Oncology, Division of Surgery at the University of Texas MD Anderson Cancer Center in Houston. He is also Chief of the Pancreas Surgery Service at MD Anderson. Dr. Katz noted he was asked to provide the pro side of the argument, and he may not necessarily uphold these positions in his own practice. Dr. Katz had no relevant financial disclosures.
References:
1. Dr. Pawlik’s list of selected publications: http://pathology.jhu.edu/liver/pawlik.cfm.
2. JAMA Surg. 2017;152:19-25.
3. Cancer. 2017;125:169-77.
4. Pancreas. 2011;40:67-71.
5. Ann Surg. 2008;247:571-9.
Some mucinous cystic neoplasms can be safely followed.
Data in the literature suggest some of these suspected mucinous cystic neoplasms can be followed; surgery may not be indicated solely because “as surgeons we tend to take all masses out” and because operative complications occur. Therefore, resection is not a benign procedure.
Dr. Katz, you were done before you got started. The evidence shows that some of the MCNs can be followed rather than resected.
Most of the published studies focus on IPMN, but I think the topic of MCNs is becoming increasingly important; they follow IPMNs as the second most common type of cystic neoplasms. So we’re going to be finding more small MCNs. In this debate, we are not talking about an 8-cm MCN, but rather what do we do when we see a 2- to 3-cm MCN. Do these patients all need to undergo resection?
What Dr. Katz is concerned about is that we are going to miss a cancer. We should operate on all patients because – at least as Dr. Katz’s argues – the surgery can always be done without complications.
However, even at the very, very experienced centers, morbidity was 30%-50%, and a pancreatic fistula developed for 1 in 10 patients. So it’s not a benign procedure.1
As surgeons, we tend to take every mass out. Although operative mortality is low, it is still in a measurable range, 1%-2%, and that is even at expert centers. Many of these small cysts are being found at smaller and community hospitals, and undue morbidity and mortality may weigh more heavily in these settings.2
Rather than “being a hammer and seeing everything as a nail,” we need a more rational approach. For example, we should identify a subgroup that will the most benefit from resection.
Investigators from Memorial Sloan Kettering Cancer Center reported that certain clinical factors identify patients at higher risk for mortality, such as nodules.3 Patients with invasive cancer almost all had nodules. And all patients with invasive cancer without nodules had a tumor larger than 4 cm.
Maybe using both presence of nodules and size is the right approach to identifying underling malignancy in suspected MCNs. Again, size is important, as is male gender, presence of solid nodules, and duct dilatation.4
We are arguing whether all MCNs should be resected. I’m positing that many 2-cm to 3-cm MCNs can be followed. There is only a small chance they will grow over time, and it’s unlikely they are harboring a malignancy.
Dr. Pawlik is Chair of Surgery at the Ohio State Wexner Medical Center in Columbus. He is also the Urban Meyer III and Shelley Meyer Chair for Cancer Research at Ohio State. He has no disclosures.
References
1. HPB (Oxford). 2007;9:8-15.
2. Diagnosis and Management of Cystic Lesions of the Pancreas. Diagnostic and Therapeutic Endoscopy Volume 2011 (2011), Article ID 478913.
3. JAMA Surg. 2017;152:19-25.
4. Ann Surg. 2006;244:572-82.
This Point/Counterpoint feature is based on comments Dr. Katz and Dr. Pawlik made during a debate at AHPBA 2017, the annual meeting of the Americas Hepato-Pancreato-Biliary Association.
Dr. Pawlik is a pretty clever guy and a strong adversary. Mucinous cystic neoplasm is literally the only topic in hepato-pancreato-biliary surgery he has not written about – yet.1
But I will argue that all suspected mucinous cystic neoplasms (MCNs) should be surgically removed. Reasons include: the natural history of a benign MCN in a typical 45-year-old remains unknown, clinical features are not always reliable, and it’s difficult to distinguish benign from malignant neoplasms without surgery.
Although many MCNs are benign when resected, all have malignant potential.
The prevalence of cancer at time of diagnosis with an MCN is fairly low, about 15%. This means most of these cysts are benign when resected. However, discrimination between benign and malignant is difficult without surgery, and the degree of epithelial dysplasia at time of resection ranges from mild to severe with invasive carcinoma.
When cancer is present, patients tend to do poorly. In one study where 44 out of 344 patients developed invasive cancer, the group with cancer had a 3-year overall survival rate of 59%.2
In addition, aspiration of cyst fluid is often of limited utility. It is poor at distinguishing whether a cyst is benign or malignant. For example, in a series of 55 patients with MCNs that underwent fine-needle aspiration of cyst epithelium, 71% of assays were nondiagnostic.3 So this diagnostic test is very insensitive and may miss at least half of cancers – another point in favor of surgical resection for all MCNs.
It is true that evidence in the literature associates certain clinical factors with a higher risk for malignancy in suspected MCNs. These include male gender, larger cysts, and location in the pancreatic head or neck or larger cyst diameter and presence of nodules.2,4 However, use of clinical features is not perfect.
In another study, of 163 resected MCNs, those with invasive cancer were often – but not always – larger than 4 cm with nodules.5 These same series revealed that those with invasive cancer were often, but again not always, larger than 4 cm.
We see this in our practice as well – that a large cyst or presence of nodules is not a foolproof indicator of malignancy. We had a 38-year-old woman with a large cyst and nodules who did not have cancer. In contrast, a 45-year-old woman at our institution presented with small cysts and no cancer on fine-needle aspiration. However, final pathology in her case revealed high-grade dysplasia.
Also, we only know what happens to cysts that have been resected. We know nothing about duration or other best practices for following patients who do not undergo surgery. For this reason, we really don’t know what these tumors will do if left alone.
Surgery is curative and carries a pretty low risk. Following resection, these patients do well. We’re talking about curative operations.
Dr. Katz is an Associate Professor, Department of Surgical Oncology, Division of Surgery at the University of Texas MD Anderson Cancer Center in Houston. He is also Chief of the Pancreas Surgery Service at MD Anderson. Dr. Katz noted he was asked to provide the pro side of the argument, and he may not necessarily uphold these positions in his own practice. Dr. Katz had no relevant financial disclosures.
References:
1. Dr. Pawlik’s list of selected publications: http://pathology.jhu.edu/liver/pawlik.cfm.
2. JAMA Surg. 2017;152:19-25.
3. Cancer. 2017;125:169-77.
4. Pancreas. 2011;40:67-71.
5. Ann Surg. 2008;247:571-9.
Some mucinous cystic neoplasms can be safely followed.
Data in the literature suggest some of these suspected mucinous cystic neoplasms can be followed; surgery may not be indicated solely because “as surgeons we tend to take all masses out” and because operative complications occur. Therefore, resection is not a benign procedure.
Dr. Katz, you were done before you got started. The evidence shows that some of the MCNs can be followed rather than resected.
Most of the published studies focus on IPMN, but I think the topic of MCNs is becoming increasingly important; they follow IPMNs as the second most common type of cystic neoplasms. So we’re going to be finding more small MCNs. In this debate, we are not talking about an 8-cm MCN, but rather what do we do when we see a 2- to 3-cm MCN. Do these patients all need to undergo resection?
What Dr. Katz is concerned about is that we are going to miss a cancer. We should operate on all patients because – at least as Dr. Katz’s argues – the surgery can always be done without complications.
However, even at the very, very experienced centers, morbidity was 30%-50%, and a pancreatic fistula developed for 1 in 10 patients. So it’s not a benign procedure.1
As surgeons, we tend to take every mass out. Although operative mortality is low, it is still in a measurable range, 1%-2%, and that is even at expert centers. Many of these small cysts are being found at smaller and community hospitals, and undue morbidity and mortality may weigh more heavily in these settings.2
Rather than “being a hammer and seeing everything as a nail,” we need a more rational approach. For example, we should identify a subgroup that will the most benefit from resection.
Investigators from Memorial Sloan Kettering Cancer Center reported that certain clinical factors identify patients at higher risk for mortality, such as nodules.3 Patients with invasive cancer almost all had nodules. And all patients with invasive cancer without nodules had a tumor larger than 4 cm.
Maybe using both presence of nodules and size is the right approach to identifying underling malignancy in suspected MCNs. Again, size is important, as is male gender, presence of solid nodules, and duct dilatation.4
We are arguing whether all MCNs should be resected. I’m positing that many 2-cm to 3-cm MCNs can be followed. There is only a small chance they will grow over time, and it’s unlikely they are harboring a malignancy.
Dr. Pawlik is Chair of Surgery at the Ohio State Wexner Medical Center in Columbus. He is also the Urban Meyer III and Shelley Meyer Chair for Cancer Research at Ohio State. He has no disclosures.
References
1. HPB (Oxford). 2007;9:8-15.
2. Diagnosis and Management of Cystic Lesions of the Pancreas. Diagnostic and Therapeutic Endoscopy Volume 2011 (2011), Article ID 478913.
3. JAMA Surg. 2017;152:19-25.
4. Ann Surg. 2006;244:572-82.
This Point/Counterpoint feature is based on comments Dr. Katz and Dr. Pawlik made during a debate at AHPBA 2017, the annual meeting of the Americas Hepato-Pancreato-Biliary Association.
Study identifies gaps in surgical trainees’ readiness
PHILADELPHIA – The question of how prepared general surgery residents are to operate independently after their training is longstanding, but clear definitions of competency and readiness have been elusive. A consortium of general surgery residencies has developed a metric for assessing surgeon readiness, but what the metric revealed may be a cause for concern for the surgical profession.
Brian C. George, MD, of the University of Michigan, Ann Arbor, reported at the annual meeting of the American Surgical Association on results of a study designed to measure the autonomy and readiness for independent practice of residents at 14 general surgery programs.
The study found that in the final 6 months of training, 96% of residents were rated competent by their observers to perform a straightforward appendectomy on their own, but only 71% were rated the same for partial colectomy, Dr. George said.
The participating general surgery attendings rated residents according to three scales (J Surg Educ. 2016;73:e118-130):
•“Performance” scale to measure readiness for independent practice, with competence defined as practice-ready and exceptional performance.
• “Zwisch” scale, named after Jay Zwischenberger, MD, FACS, of the University of Kentucky, to assess the amount of autonomy granted to a resident by the supervising surgical attending.
• “Complexity” scale to measure the patient-related complexity of the case at hand.
The study used a smartphone-based app, SIMPL, to collect data from September 2015 through December 2016. In evaluating performance, 437 observers provided 8,526 different ratings of 522 residents.
In a subset analysis, the study authors focused on the 132 operations the Surgical Council on Resident Education considers the core procedures of general surgery and found that 77% of fifth-year residents were rated as competent. Further restricting the analysis to residents in their final 6 months of training performing the five most commonly rated core procedures – appendectomy, cholecystectomy, ventral hernia repair, inguinal/femoral hernia repair, and partial colectomy – the researchers found that competency ranged from a high of 96% for appendectomy to a low of 71% for partial colectomy.
“If you combine all five procedures into one category, on average the residents are rated competent 84% of the time during the last 6 months of training,” Dr. George said. “But what’s really interesting – and I think this is probably the most important result for this study – is that looking at just the less-frequently rated core procedures and excluding the top five, residents are deemed competent in the last 6 months of training for 74% of those observed procedures.”
According to the Zwisch” scale to measure autonomy, residents in their last 6 months of training displayed what Dr. George termed “meaningful autonomy” in 77% of their observed operations for the core procedures. “Interestingly,” he added, “they were observed to have maximum autonomy, which is the supervision-only level, for 33% of all observed procedures.”
The researchers did an adjusted analysis to account for confounding factors such as the stringency of individual raters and patient complexity. “Using this type of analysis, the likelihood that a typical trainee rated by a typical attendee would be deemed competent by the end of training for a relatively straightforward laparoscopy appendectomy is 97%,” Dr. George said. “For a difficult laparoscopic appendectomy, by the end of training, they’re likely to be deemed competent 92% of time.” In the adjusted analysis, for a straightforward partial colectomy the raters predicted trainees to be competent 91.8% of time, but only 81.8% of the time for a complicated partial colectomy. “For less-frequently performed core procedures, there are many for which residents are likely to be deemed competent at a much lower level,” Dr. George added.
In discussing the study, Ara Darzi, MD, of St. Mary’s Hospital, London, called the methodology “unique and commendable,” and asked “Would you make the case to say we should increase the number of years of trainees from PG5 to PG6 or at least make your fellowships compulsory?”
Dr. George answered that 80% of general surgery residents already go into fellowships. “Whether it’s required or not is above my pay grade,” he said. “I hope not. Five years is already a big ask for a lot of medical students who are considering this profession. If we increase the training requirements, we’re going to have a supply problem that we will then need to address. We will be trading one problem for another.” He later added “The 20,000 hours of surgical residency should be enough to train a general surgeon to competence – its up to us to figure out how.”
The following organizations supported the research: American Board of Surgery, Association for Surgical Education, Association for Program Directors in Surgery, Massachusetts General Hospital, Northwestern University, Indiana University, and the members of the Procedural Learning and Safety Collaborative.
Neither Dr. George nor Dr. Darzi had any relevant financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – The question of how prepared general surgery residents are to operate independently after their training is longstanding, but clear definitions of competency and readiness have been elusive. A consortium of general surgery residencies has developed a metric for assessing surgeon readiness, but what the metric revealed may be a cause for concern for the surgical profession.
Brian C. George, MD, of the University of Michigan, Ann Arbor, reported at the annual meeting of the American Surgical Association on results of a study designed to measure the autonomy and readiness for independent practice of residents at 14 general surgery programs.
The study found that in the final 6 months of training, 96% of residents were rated competent by their observers to perform a straightforward appendectomy on their own, but only 71% were rated the same for partial colectomy, Dr. George said.
The participating general surgery attendings rated residents according to three scales (J Surg Educ. 2016;73:e118-130):
•“Performance” scale to measure readiness for independent practice, with competence defined as practice-ready and exceptional performance.
• “Zwisch” scale, named after Jay Zwischenberger, MD, FACS, of the University of Kentucky, to assess the amount of autonomy granted to a resident by the supervising surgical attending.
• “Complexity” scale to measure the patient-related complexity of the case at hand.
The study used a smartphone-based app, SIMPL, to collect data from September 2015 through December 2016. In evaluating performance, 437 observers provided 8,526 different ratings of 522 residents.
In a subset analysis, the study authors focused on the 132 operations the Surgical Council on Resident Education considers the core procedures of general surgery and found that 77% of fifth-year residents were rated as competent. Further restricting the analysis to residents in their final 6 months of training performing the five most commonly rated core procedures – appendectomy, cholecystectomy, ventral hernia repair, inguinal/femoral hernia repair, and partial colectomy – the researchers found that competency ranged from a high of 96% for appendectomy to a low of 71% for partial colectomy.
“If you combine all five procedures into one category, on average the residents are rated competent 84% of the time during the last 6 months of training,” Dr. George said. “But what’s really interesting – and I think this is probably the most important result for this study – is that looking at just the less-frequently rated core procedures and excluding the top five, residents are deemed competent in the last 6 months of training for 74% of those observed procedures.”
According to the Zwisch” scale to measure autonomy, residents in their last 6 months of training displayed what Dr. George termed “meaningful autonomy” in 77% of their observed operations for the core procedures. “Interestingly,” he added, “they were observed to have maximum autonomy, which is the supervision-only level, for 33% of all observed procedures.”
The researchers did an adjusted analysis to account for confounding factors such as the stringency of individual raters and patient complexity. “Using this type of analysis, the likelihood that a typical trainee rated by a typical attendee would be deemed competent by the end of training for a relatively straightforward laparoscopy appendectomy is 97%,” Dr. George said. “For a difficult laparoscopic appendectomy, by the end of training, they’re likely to be deemed competent 92% of time.” In the adjusted analysis, for a straightforward partial colectomy the raters predicted trainees to be competent 91.8% of time, but only 81.8% of the time for a complicated partial colectomy. “For less-frequently performed core procedures, there are many for which residents are likely to be deemed competent at a much lower level,” Dr. George added.
In discussing the study, Ara Darzi, MD, of St. Mary’s Hospital, London, called the methodology “unique and commendable,” and asked “Would you make the case to say we should increase the number of years of trainees from PG5 to PG6 or at least make your fellowships compulsory?”
Dr. George answered that 80% of general surgery residents already go into fellowships. “Whether it’s required or not is above my pay grade,” he said. “I hope not. Five years is already a big ask for a lot of medical students who are considering this profession. If we increase the training requirements, we’re going to have a supply problem that we will then need to address. We will be trading one problem for another.” He later added “The 20,000 hours of surgical residency should be enough to train a general surgeon to competence – its up to us to figure out how.”
The following organizations supported the research: American Board of Surgery, Association for Surgical Education, Association for Program Directors in Surgery, Massachusetts General Hospital, Northwestern University, Indiana University, and the members of the Procedural Learning and Safety Collaborative.
Neither Dr. George nor Dr. Darzi had any relevant financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – The question of how prepared general surgery residents are to operate independently after their training is longstanding, but clear definitions of competency and readiness have been elusive. A consortium of general surgery residencies has developed a metric for assessing surgeon readiness, but what the metric revealed may be a cause for concern for the surgical profession.
Brian C. George, MD, of the University of Michigan, Ann Arbor, reported at the annual meeting of the American Surgical Association on results of a study designed to measure the autonomy and readiness for independent practice of residents at 14 general surgery programs.
The study found that in the final 6 months of training, 96% of residents were rated competent by their observers to perform a straightforward appendectomy on their own, but only 71% were rated the same for partial colectomy, Dr. George said.
The participating general surgery attendings rated residents according to three scales (J Surg Educ. 2016;73:e118-130):
•“Performance” scale to measure readiness for independent practice, with competence defined as practice-ready and exceptional performance.
• “Zwisch” scale, named after Jay Zwischenberger, MD, FACS, of the University of Kentucky, to assess the amount of autonomy granted to a resident by the supervising surgical attending.
• “Complexity” scale to measure the patient-related complexity of the case at hand.
The study used a smartphone-based app, SIMPL, to collect data from September 2015 through December 2016. In evaluating performance, 437 observers provided 8,526 different ratings of 522 residents.
In a subset analysis, the study authors focused on the 132 operations the Surgical Council on Resident Education considers the core procedures of general surgery and found that 77% of fifth-year residents were rated as competent. Further restricting the analysis to residents in their final 6 months of training performing the five most commonly rated core procedures – appendectomy, cholecystectomy, ventral hernia repair, inguinal/femoral hernia repair, and partial colectomy – the researchers found that competency ranged from a high of 96% for appendectomy to a low of 71% for partial colectomy.
“If you combine all five procedures into one category, on average the residents are rated competent 84% of the time during the last 6 months of training,” Dr. George said. “But what’s really interesting – and I think this is probably the most important result for this study – is that looking at just the less-frequently rated core procedures and excluding the top five, residents are deemed competent in the last 6 months of training for 74% of those observed procedures.”
According to the Zwisch” scale to measure autonomy, residents in their last 6 months of training displayed what Dr. George termed “meaningful autonomy” in 77% of their observed operations for the core procedures. “Interestingly,” he added, “they were observed to have maximum autonomy, which is the supervision-only level, for 33% of all observed procedures.”
The researchers did an adjusted analysis to account for confounding factors such as the stringency of individual raters and patient complexity. “Using this type of analysis, the likelihood that a typical trainee rated by a typical attendee would be deemed competent by the end of training for a relatively straightforward laparoscopy appendectomy is 97%,” Dr. George said. “For a difficult laparoscopic appendectomy, by the end of training, they’re likely to be deemed competent 92% of time.” In the adjusted analysis, for a straightforward partial colectomy the raters predicted trainees to be competent 91.8% of time, but only 81.8% of the time for a complicated partial colectomy. “For less-frequently performed core procedures, there are many for which residents are likely to be deemed competent at a much lower level,” Dr. George added.
In discussing the study, Ara Darzi, MD, of St. Mary’s Hospital, London, called the methodology “unique and commendable,” and asked “Would you make the case to say we should increase the number of years of trainees from PG5 to PG6 or at least make your fellowships compulsory?”
Dr. George answered that 80% of general surgery residents already go into fellowships. “Whether it’s required or not is above my pay grade,” he said. “I hope not. Five years is already a big ask for a lot of medical students who are considering this profession. If we increase the training requirements, we’re going to have a supply problem that we will then need to address. We will be trading one problem for another.” He later added “The 20,000 hours of surgical residency should be enough to train a general surgeon to competence – its up to us to figure out how.”
The following organizations supported the research: American Board of Surgery, Association for Surgical Education, Association for Program Directors in Surgery, Massachusetts General Hospital, Northwestern University, Indiana University, and the members of the Procedural Learning and Safety Collaborative.
Neither Dr. George nor Dr. Darzi had any relevant financial disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: General surgery residents are often but not universally ready to independently perform core surgical procedures upon completion of surgical training.
Major finding: In the last 6 months of training, residents were rated competent 84% of the time in performing the five leading core procedures and 64% of the time for less-frequently performed procedures.
Data source: Ratings of 437 of 8,526 different observations of 522 residents at 14 institutions of the Procedural Learning and Safety Collaborative.
Disclosures: Dr. George and his coauthors reported having no financial disclosures.