User login
Pembrolizumab plus SBRT shows promise for advanced solid tumors
SAN FRANCISCO – Pembrolizumab immunotherapy with multi-site stereotactic body radiotherapy (SBRT) appears to be a safe and effective treatment in patients with advanced solid tumors, according to findings from a phase 1 study.
Of 79 patients with metastatic solid tumors who progressed on standard treatment and who were enrolled in the study, 68 underwent multi-site SBRT, received at least one cycle of pembrolizumab (Keytruda), and had imaging follow-up. The overall objective response rate in those 68 patients was 13.2%, Jeffrey Lemons, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
When responses in the non-irradiated lesions (out-of-field responses) were measured based on a 30% reduction in any single lesion, the rate was 26.9%. But when defined by a 30% reduction in aggregate diameter of the non-irradiated measurable lesions, the rate was 13.5%, he said. While both approaches for measuring response are acceptable, Dr. Lemons noted, it’s important to be sure which one is being used in a given study.
Overall, 73 patients received both SBRT and pembrolizumab (5 had no imaging follow-up). They had a mean age of 62 years and a median of five prior therapies. Cancer types included ovarian/fallopian tube cancer (12.3%), non–small cell lung cancer (9.6%), breast cancer (8.2%), cholangiocarcinoma (8.2%), endometrial cancer (8.2%), colorectal cancer (6.8%), head and neck cancer (5.5%), and other tumors, each with less than 5% accrual (41.2%).
The number of sites treated with SBRT was two in 94.5% of patients, three in 4.1%, and four in 1.3%; 151 lesions in total were treated.
The premise for combining pembrolizumab and SBRT is that response to anti-programmed cell death-1 (PD1) therapy seems to correspond with interferon-gamma signaling, and that SBRT can stimulate innate and adaptive immunity to potentially augment immunotherapy, Dr. Lemons explained. In addition, anti-PD1 treatment outcomes are improved with lower disease burden.
Multi-site radiation is an emerging paradigm for eradicating metastatic disease, he said.
Patients included in the study had metastatic solid tumors and had progressed on standard treatment. They had measurable disease by RECIST, and metastases amenable to SBRT with 0.25 cc to 65 cc of viable tumor.
Tumors larger than 65 cc were partially targeted with radiotherapy. Radiation doses were adapted from recently completed and ongoing National Cancer Institute trials and ranged from 30-50 Gy (3-5 fractions) based on anatomic location.
Pembrolizumab was initiated within 7 days of the final SBRT treatment.
Dose-limiting toxicities, all grade 3, occurred in six patients during a median follow-up of 5.5 months, and included pneumonitis in three patients, hepatic failure in one patient, and colitis in two patients, but there were no radiation dose reductions, Dr. Lemons said.
“This is the first and largest prospective trial to determine the safety of this combination,” he explained. “There was some intriguing clinical activity ... and we feel that this justifies further randomized studies
The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures.
sworcester@frontlinemedcom.com
SOURCE: Lemons J et al., ASCO-SITC abstract #20.
SAN FRANCISCO – Pembrolizumab immunotherapy with multi-site stereotactic body radiotherapy (SBRT) appears to be a safe and effective treatment in patients with advanced solid tumors, according to findings from a phase 1 study.
Of 79 patients with metastatic solid tumors who progressed on standard treatment and who were enrolled in the study, 68 underwent multi-site SBRT, received at least one cycle of pembrolizumab (Keytruda), and had imaging follow-up. The overall objective response rate in those 68 patients was 13.2%, Jeffrey Lemons, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
When responses in the non-irradiated lesions (out-of-field responses) were measured based on a 30% reduction in any single lesion, the rate was 26.9%. But when defined by a 30% reduction in aggregate diameter of the non-irradiated measurable lesions, the rate was 13.5%, he said. While both approaches for measuring response are acceptable, Dr. Lemons noted, it’s important to be sure which one is being used in a given study.
Overall, 73 patients received both SBRT and pembrolizumab (5 had no imaging follow-up). They had a mean age of 62 years and a median of five prior therapies. Cancer types included ovarian/fallopian tube cancer (12.3%), non–small cell lung cancer (9.6%), breast cancer (8.2%), cholangiocarcinoma (8.2%), endometrial cancer (8.2%), colorectal cancer (6.8%), head and neck cancer (5.5%), and other tumors, each with less than 5% accrual (41.2%).
The number of sites treated with SBRT was two in 94.5% of patients, three in 4.1%, and four in 1.3%; 151 lesions in total were treated.
The premise for combining pembrolizumab and SBRT is that response to anti-programmed cell death-1 (PD1) therapy seems to correspond with interferon-gamma signaling, and that SBRT can stimulate innate and adaptive immunity to potentially augment immunotherapy, Dr. Lemons explained. In addition, anti-PD1 treatment outcomes are improved with lower disease burden.
Multi-site radiation is an emerging paradigm for eradicating metastatic disease, he said.
Patients included in the study had metastatic solid tumors and had progressed on standard treatment. They had measurable disease by RECIST, and metastases amenable to SBRT with 0.25 cc to 65 cc of viable tumor.
Tumors larger than 65 cc were partially targeted with radiotherapy. Radiation doses were adapted from recently completed and ongoing National Cancer Institute trials and ranged from 30-50 Gy (3-5 fractions) based on anatomic location.
Pembrolizumab was initiated within 7 days of the final SBRT treatment.
Dose-limiting toxicities, all grade 3, occurred in six patients during a median follow-up of 5.5 months, and included pneumonitis in three patients, hepatic failure in one patient, and colitis in two patients, but there were no radiation dose reductions, Dr. Lemons said.
“This is the first and largest prospective trial to determine the safety of this combination,” he explained. “There was some intriguing clinical activity ... and we feel that this justifies further randomized studies
The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures.
sworcester@frontlinemedcom.com
SOURCE: Lemons J et al., ASCO-SITC abstract #20.
SAN FRANCISCO – Pembrolizumab immunotherapy with multi-site stereotactic body radiotherapy (SBRT) appears to be a safe and effective treatment in patients with advanced solid tumors, according to findings from a phase 1 study.
Of 79 patients with metastatic solid tumors who progressed on standard treatment and who were enrolled in the study, 68 underwent multi-site SBRT, received at least one cycle of pembrolizumab (Keytruda), and had imaging follow-up. The overall objective response rate in those 68 patients was 13.2%, Jeffrey Lemons, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
When responses in the non-irradiated lesions (out-of-field responses) were measured based on a 30% reduction in any single lesion, the rate was 26.9%. But when defined by a 30% reduction in aggregate diameter of the non-irradiated measurable lesions, the rate was 13.5%, he said. While both approaches for measuring response are acceptable, Dr. Lemons noted, it’s important to be sure which one is being used in a given study.
Overall, 73 patients received both SBRT and pembrolizumab (5 had no imaging follow-up). They had a mean age of 62 years and a median of five prior therapies. Cancer types included ovarian/fallopian tube cancer (12.3%), non–small cell lung cancer (9.6%), breast cancer (8.2%), cholangiocarcinoma (8.2%), endometrial cancer (8.2%), colorectal cancer (6.8%), head and neck cancer (5.5%), and other tumors, each with less than 5% accrual (41.2%).
The number of sites treated with SBRT was two in 94.5% of patients, three in 4.1%, and four in 1.3%; 151 lesions in total were treated.
The premise for combining pembrolizumab and SBRT is that response to anti-programmed cell death-1 (PD1) therapy seems to correspond with interferon-gamma signaling, and that SBRT can stimulate innate and adaptive immunity to potentially augment immunotherapy, Dr. Lemons explained. In addition, anti-PD1 treatment outcomes are improved with lower disease burden.
Multi-site radiation is an emerging paradigm for eradicating metastatic disease, he said.
Patients included in the study had metastatic solid tumors and had progressed on standard treatment. They had measurable disease by RECIST, and metastases amenable to SBRT with 0.25 cc to 65 cc of viable tumor.
Tumors larger than 65 cc were partially targeted with radiotherapy. Radiation doses were adapted from recently completed and ongoing National Cancer Institute trials and ranged from 30-50 Gy (3-5 fractions) based on anatomic location.
Pembrolizumab was initiated within 7 days of the final SBRT treatment.
Dose-limiting toxicities, all grade 3, occurred in six patients during a median follow-up of 5.5 months, and included pneumonitis in three patients, hepatic failure in one patient, and colitis in two patients, but there were no radiation dose reductions, Dr. Lemons said.
“This is the first and largest prospective trial to determine the safety of this combination,” he explained. “There was some intriguing clinical activity ... and we feel that this justifies further randomized studies
The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures.
sworcester@frontlinemedcom.com
SOURCE: Lemons J et al., ASCO-SITC abstract #20.
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point: Pembrolizumab plus multi-site SBRT appears safe and effective for advanced solid tumors.
Major finding: The overall objective response rate was 13.2%.
Study details: A phase 1 study of 79 patients.
Disclosures: The University of Chicago sponsored the study. Dr. Lemons reported having no disclosures
Source: Lemons J et al. ASCO-SITC abstract #20.
OS similar among mRCC patients enrolled in clinical trials across different geographic regions
Overall survival was similar among patients enrolled in clinical trials for metastatic renal cell carcinoma (mRCC) across different geographic regions, according to a pooled retrospective analysis.
Demographic characteristics, clinicopathologic variables, survival, and toxicity data were collected across five geographic regions, including, United States/Canada (USC), Western Europe (WE), Eastern Europe (EE), Latin America (LA), and Asia/Africa/Oceania (AAO) for 4,736 patients who had mRCC treated between 2003 and 2013 and were enrolled in phase 2 and phase 3 clinical trials.
Patients in USC and WE were slightly older (mean ages, 60.6 and 60.5 years, respectively) and with higher numbers undergoing prior nephrectomy. Higher BMI was also observed in patients in the USC and LA regions. While ECOG performance status of 0 was more frequent in LA patients, treatment-related adverse events and use of statin and angiotensin inhibitor system was higher in USC.
“We highlight that, despite different baseline characteristics, OS was similar among patients enrolled in clinical trials across different geographic regions,” reported Andre P. Fay, MD, PhD, and colleagues from Dana Farber Cancer Institute, Boston, in Journal of Global Oncology. “Access to clinical trials may be an important alternative to eliminate health disparities and promote health equity in patients with mRCC.”
This study was supported by Pfizer and in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE, DF/HCC Kidney Cancer Program, and the Trust Family, Loker Pinard, and Michael Brigham Funds for Kidney Cancer Research at Dana-Farber Cancer Institute. All of the study authors reported disclosures with the sponsor, Pfizer, or other pharmaceutical companies.
SOURCE: Fay AP et al. J Global Oncol. 2018 Jan 17. doi: 10.1200/JGO.17.00119.
Overall survival was similar among patients enrolled in clinical trials for metastatic renal cell carcinoma (mRCC) across different geographic regions, according to a pooled retrospective analysis.
Demographic characteristics, clinicopathologic variables, survival, and toxicity data were collected across five geographic regions, including, United States/Canada (USC), Western Europe (WE), Eastern Europe (EE), Latin America (LA), and Asia/Africa/Oceania (AAO) for 4,736 patients who had mRCC treated between 2003 and 2013 and were enrolled in phase 2 and phase 3 clinical trials.
Patients in USC and WE were slightly older (mean ages, 60.6 and 60.5 years, respectively) and with higher numbers undergoing prior nephrectomy. Higher BMI was also observed in patients in the USC and LA regions. While ECOG performance status of 0 was more frequent in LA patients, treatment-related adverse events and use of statin and angiotensin inhibitor system was higher in USC.
“We highlight that, despite different baseline characteristics, OS was similar among patients enrolled in clinical trials across different geographic regions,” reported Andre P. Fay, MD, PhD, and colleagues from Dana Farber Cancer Institute, Boston, in Journal of Global Oncology. “Access to clinical trials may be an important alternative to eliminate health disparities and promote health equity in patients with mRCC.”
This study was supported by Pfizer and in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE, DF/HCC Kidney Cancer Program, and the Trust Family, Loker Pinard, and Michael Brigham Funds for Kidney Cancer Research at Dana-Farber Cancer Institute. All of the study authors reported disclosures with the sponsor, Pfizer, or other pharmaceutical companies.
SOURCE: Fay AP et al. J Global Oncol. 2018 Jan 17. doi: 10.1200/JGO.17.00119.
Overall survival was similar among patients enrolled in clinical trials for metastatic renal cell carcinoma (mRCC) across different geographic regions, according to a pooled retrospective analysis.
Demographic characteristics, clinicopathologic variables, survival, and toxicity data were collected across five geographic regions, including, United States/Canada (USC), Western Europe (WE), Eastern Europe (EE), Latin America (LA), and Asia/Africa/Oceania (AAO) for 4,736 patients who had mRCC treated between 2003 and 2013 and were enrolled in phase 2 and phase 3 clinical trials.
Patients in USC and WE were slightly older (mean ages, 60.6 and 60.5 years, respectively) and with higher numbers undergoing prior nephrectomy. Higher BMI was also observed in patients in the USC and LA regions. While ECOG performance status of 0 was more frequent in LA patients, treatment-related adverse events and use of statin and angiotensin inhibitor system was higher in USC.
“We highlight that, despite different baseline characteristics, OS was similar among patients enrolled in clinical trials across different geographic regions,” reported Andre P. Fay, MD, PhD, and colleagues from Dana Farber Cancer Institute, Boston, in Journal of Global Oncology. “Access to clinical trials may be an important alternative to eliminate health disparities and promote health equity in patients with mRCC.”
This study was supported by Pfizer and in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE, DF/HCC Kidney Cancer Program, and the Trust Family, Loker Pinard, and Michael Brigham Funds for Kidney Cancer Research at Dana-Farber Cancer Institute. All of the study authors reported disclosures with the sponsor, Pfizer, or other pharmaceutical companies.
SOURCE: Fay AP et al. J Global Oncol. 2018 Jan 17. doi: 10.1200/JGO.17.00119.
FROM journal of global oncology
Key clinical point: The potential differences in clinical outcomes may be contributed by differences in access to clinical trials, disease biology, reporting of adverse events, and quality of care.
Major finding: Patient characteristics differed according to geographic region. No statistically significant differences in OS were observed when the United States/Canada (USC) was compared with other regions: Latin America, Asia/Oceania/Africa, and Eastern Europe.
Study details: Pooled retrospective analysis of 4,736 patients who had mRCC treated between 2003 and 2013 and were enrolled in phase 2 and phase 3 clinical trials.
Disclosures: The study was funded by Pfizer and in part by the Dana Farber/Harvard Cancer Center. All of the study authors reported conflicts of interest involving the sponsor, Pfizer, or other pharmaceutical companies.
Source: Fay AP et al. J Global Oncol. 2018 Jan 17. doi: 10.1200/JGO.17.00119.
In Brazil, few patients get second- and third-line treatment for metastatic RCC
, a retrospective study showed.
Of 3,990 patients with metastatic renal cell carcinoma (mRCC), 79% received an appropriate first-line treatment – mainly a vascular endothelial growth factor agent. But only 20% went on to get a second-line agent, and just 5% received a third-line agent, Paulo G. Bergerot, MD, and his colleagues reported in the Journal of Global Oncology.
Patients in private institutions were significantly more likely to receive appropriate first- and second-line treatment than those in public institutions, although the numbers receiving third-line agents were similarly low, reported Dr. Bergerot of the Federal University of São Paulo and his coauthors.
The study highlights sharp discrepancies between treatment in Brazil and more developed countries, the team noted.
“Previous reports from the International Metastatic Renal Cell Carcinoma Database Consortium suggest that approximately 48% of patients who receive first-line therapy proceed to second-line therapy. In addition, among patients who received first-line therapy in this experience, approximately 21% received third-line therapy,” the investigators wrote.
The reasons behind the differences aren’t entirely clear, but cost and clinicians’ knowledge of emerging study data could be major factors, they suggested.
“In particular, we suspect limited availability and cost of second-line treatments to be a barrier, although our data set did not have the capability of confirming this. Another barrier to receipt of second-line therapy might be educational gaps among practitioners. Emerging data from phase 3 studies supporting the use of agents in the refractory setting may not be widely broadcast. The discordance in receipt of therapies in private and public settings is perhaps the greatest indication that financial and social barriers likely affect treatment paradigms in Brazil,” the authors wrote.
Slow dissemination of clinical knowledge may also be reflected in another of the team’s findings: 240 patients received “nontraditional” first-line cytotoxic treatments, which lacked regulatory approval and had little supporting evidence for treating mRCC, the investigators reported.
Dr. Bergerot had no relevant financial disclosures, although several of his coauthors reported financial relationships with various pharmaceutical companies.
SOURCE: Bergerot et al. J Glob Oncol. 2017 Dec 27. doi: 10.1200/JGO.17.00113.
, a retrospective study showed.
Of 3,990 patients with metastatic renal cell carcinoma (mRCC), 79% received an appropriate first-line treatment – mainly a vascular endothelial growth factor agent. But only 20% went on to get a second-line agent, and just 5% received a third-line agent, Paulo G. Bergerot, MD, and his colleagues reported in the Journal of Global Oncology.
Patients in private institutions were significantly more likely to receive appropriate first- and second-line treatment than those in public institutions, although the numbers receiving third-line agents were similarly low, reported Dr. Bergerot of the Federal University of São Paulo and his coauthors.
The study highlights sharp discrepancies between treatment in Brazil and more developed countries, the team noted.
“Previous reports from the International Metastatic Renal Cell Carcinoma Database Consortium suggest that approximately 48% of patients who receive first-line therapy proceed to second-line therapy. In addition, among patients who received first-line therapy in this experience, approximately 21% received third-line therapy,” the investigators wrote.
The reasons behind the differences aren’t entirely clear, but cost and clinicians’ knowledge of emerging study data could be major factors, they suggested.
“In particular, we suspect limited availability and cost of second-line treatments to be a barrier, although our data set did not have the capability of confirming this. Another barrier to receipt of second-line therapy might be educational gaps among practitioners. Emerging data from phase 3 studies supporting the use of agents in the refractory setting may not be widely broadcast. The discordance in receipt of therapies in private and public settings is perhaps the greatest indication that financial and social barriers likely affect treatment paradigms in Brazil,” the authors wrote.
Slow dissemination of clinical knowledge may also be reflected in another of the team’s findings: 240 patients received “nontraditional” first-line cytotoxic treatments, which lacked regulatory approval and had little supporting evidence for treating mRCC, the investigators reported.
Dr. Bergerot had no relevant financial disclosures, although several of his coauthors reported financial relationships with various pharmaceutical companies.
SOURCE: Bergerot et al. J Glob Oncol. 2017 Dec 27. doi: 10.1200/JGO.17.00113.
, a retrospective study showed.
Of 3,990 patients with metastatic renal cell carcinoma (mRCC), 79% received an appropriate first-line treatment – mainly a vascular endothelial growth factor agent. But only 20% went on to get a second-line agent, and just 5% received a third-line agent, Paulo G. Bergerot, MD, and his colleagues reported in the Journal of Global Oncology.
Patients in private institutions were significantly more likely to receive appropriate first- and second-line treatment than those in public institutions, although the numbers receiving third-line agents were similarly low, reported Dr. Bergerot of the Federal University of São Paulo and his coauthors.
The study highlights sharp discrepancies between treatment in Brazil and more developed countries, the team noted.
“Previous reports from the International Metastatic Renal Cell Carcinoma Database Consortium suggest that approximately 48% of patients who receive first-line therapy proceed to second-line therapy. In addition, among patients who received first-line therapy in this experience, approximately 21% received third-line therapy,” the investigators wrote.
The reasons behind the differences aren’t entirely clear, but cost and clinicians’ knowledge of emerging study data could be major factors, they suggested.
“In particular, we suspect limited availability and cost of second-line treatments to be a barrier, although our data set did not have the capability of confirming this. Another barrier to receipt of second-line therapy might be educational gaps among practitioners. Emerging data from phase 3 studies supporting the use of agents in the refractory setting may not be widely broadcast. The discordance in receipt of therapies in private and public settings is perhaps the greatest indication that financial and social barriers likely affect treatment paradigms in Brazil,” the authors wrote.
Slow dissemination of clinical knowledge may also be reflected in another of the team’s findings: 240 patients received “nontraditional” first-line cytotoxic treatments, which lacked regulatory approval and had little supporting evidence for treating mRCC, the investigators reported.
Dr. Bergerot had no relevant financial disclosures, although several of his coauthors reported financial relationships with various pharmaceutical companies.
SOURCE: Bergerot et al. J Glob Oncol. 2017 Dec 27. doi: 10.1200/JGO.17.00113.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
Key clinical point: Few Brazilians with mRCC receive anything after their first-line treatment.
Major finding: First-line agents were used in 79% of the cohort, but only 20% got second-line treatments and just 5%, third-line treatment.
Study details: A retrospective database study involving 3,990 patients with mRCC.
Disclosures: Dr. Bergerot had no relevant financial disclosures, although several of his coauthors disclosed financial relationships with pharmaceutical companies.
Source: Bergerot et al. J Glob Oncol. 2017 Dec 27. doi: 10.1200/JGO.17.00113.
Mutations linked to checkpoint inhibitor response in RCC
in a derivation and validation study involving a total of 98 patients.
This finding “has important implications as a molecular tool for considering immunotherapy responsiveness” in patients with ccRCC and possibly patients with other cancer types, wrote Eliezer M. Van Allen, MD, of Dana Farber Cancer Institute in Boston and coauthors.
The derivation cohort included 35 patients with metastatic ccRCC treated with nivolumab in a prospective clinical trial. Genome sequencing of pretreatment tumor specimens showed that improved survival after treatment was significantly linked with truncating mutations in a gene, PBRM1, that codes for a protein in the SWI/SNF chromatin-remodeling complex. Patients in the derivation cohort who had these mutations were nearly 13-fold more likely to have clinical benefit from treatment, compared with those without these mutations.
The validation study included specimens and treatment-outcome results from 63 patients with metastatic ccRCC treated with either nivolumab or a different checkpoint inhibitor, such as atezolizumab (Tecentriq). In the validation study, PBRM1 mutations linked with a sixfold higher rate of clinical benefit from treatment.
The researchers noted that the types of mutations they identified as likely involved occur in more than 20% of all cancer types. Results from mouse studies have suggested that tumor cells with these types of mutations are more sensitive to T cell–mediated cytotoxicity, an observation that “lends a mechanistic basis” to the observed findings.
The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
SOURCE: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951
in a derivation and validation study involving a total of 98 patients.
This finding “has important implications as a molecular tool for considering immunotherapy responsiveness” in patients with ccRCC and possibly patients with other cancer types, wrote Eliezer M. Van Allen, MD, of Dana Farber Cancer Institute in Boston and coauthors.
The derivation cohort included 35 patients with metastatic ccRCC treated with nivolumab in a prospective clinical trial. Genome sequencing of pretreatment tumor specimens showed that improved survival after treatment was significantly linked with truncating mutations in a gene, PBRM1, that codes for a protein in the SWI/SNF chromatin-remodeling complex. Patients in the derivation cohort who had these mutations were nearly 13-fold more likely to have clinical benefit from treatment, compared with those without these mutations.
The validation study included specimens and treatment-outcome results from 63 patients with metastatic ccRCC treated with either nivolumab or a different checkpoint inhibitor, such as atezolizumab (Tecentriq). In the validation study, PBRM1 mutations linked with a sixfold higher rate of clinical benefit from treatment.
The researchers noted that the types of mutations they identified as likely involved occur in more than 20% of all cancer types. Results from mouse studies have suggested that tumor cells with these types of mutations are more sensitive to T cell–mediated cytotoxicity, an observation that “lends a mechanistic basis” to the observed findings.
The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
SOURCE: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951
in a derivation and validation study involving a total of 98 patients.
This finding “has important implications as a molecular tool for considering immunotherapy responsiveness” in patients with ccRCC and possibly patients with other cancer types, wrote Eliezer M. Van Allen, MD, of Dana Farber Cancer Institute in Boston and coauthors.
The derivation cohort included 35 patients with metastatic ccRCC treated with nivolumab in a prospective clinical trial. Genome sequencing of pretreatment tumor specimens showed that improved survival after treatment was significantly linked with truncating mutations in a gene, PBRM1, that codes for a protein in the SWI/SNF chromatin-remodeling complex. Patients in the derivation cohort who had these mutations were nearly 13-fold more likely to have clinical benefit from treatment, compared with those without these mutations.
The validation study included specimens and treatment-outcome results from 63 patients with metastatic ccRCC treated with either nivolumab or a different checkpoint inhibitor, such as atezolizumab (Tecentriq). In the validation study, PBRM1 mutations linked with a sixfold higher rate of clinical benefit from treatment.
The researchers noted that the types of mutations they identified as likely involved occur in more than 20% of all cancer types. Results from mouse studies have suggested that tumor cells with these types of mutations are more sensitive to T cell–mediated cytotoxicity, an observation that “lends a mechanistic basis” to the observed findings.
The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
SOURCE: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951
FROM SCIENCE
Key clinical point: Mutations in PBRM1 linked with better survival after immune checkpoint inhibitor therapy.
Major finding: Patients with a PBRM1 mutation were 6- to 13-fold more likely to have clinical benefit from checkpoint inhibitor treatment.
Study details: Derivation and validation studies that included 98 total patients with metastatic clear cell renal cell carcinoma.
Disclosures: The study received funding in part from Bristol-Myers Squibb, the company that markets nivolumab (Obdivo). Several researchers involved in this study have received honoraria and research support from Bristol-Myers Squibb and from several other drug companies.
Source: Miao D et al. Science. 2018 Jan 4. doi: 10.1126/science.aan5951.
Supportive medications and interventions received by prostate cancer survivors: results from the PiCTure study
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
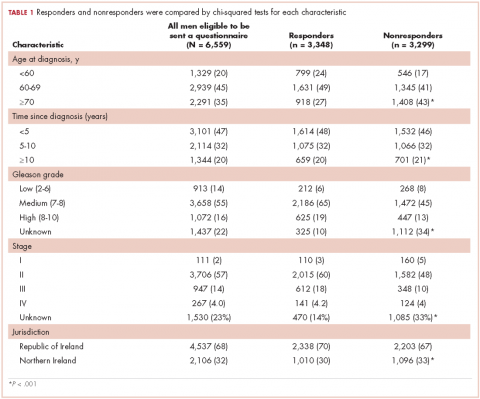
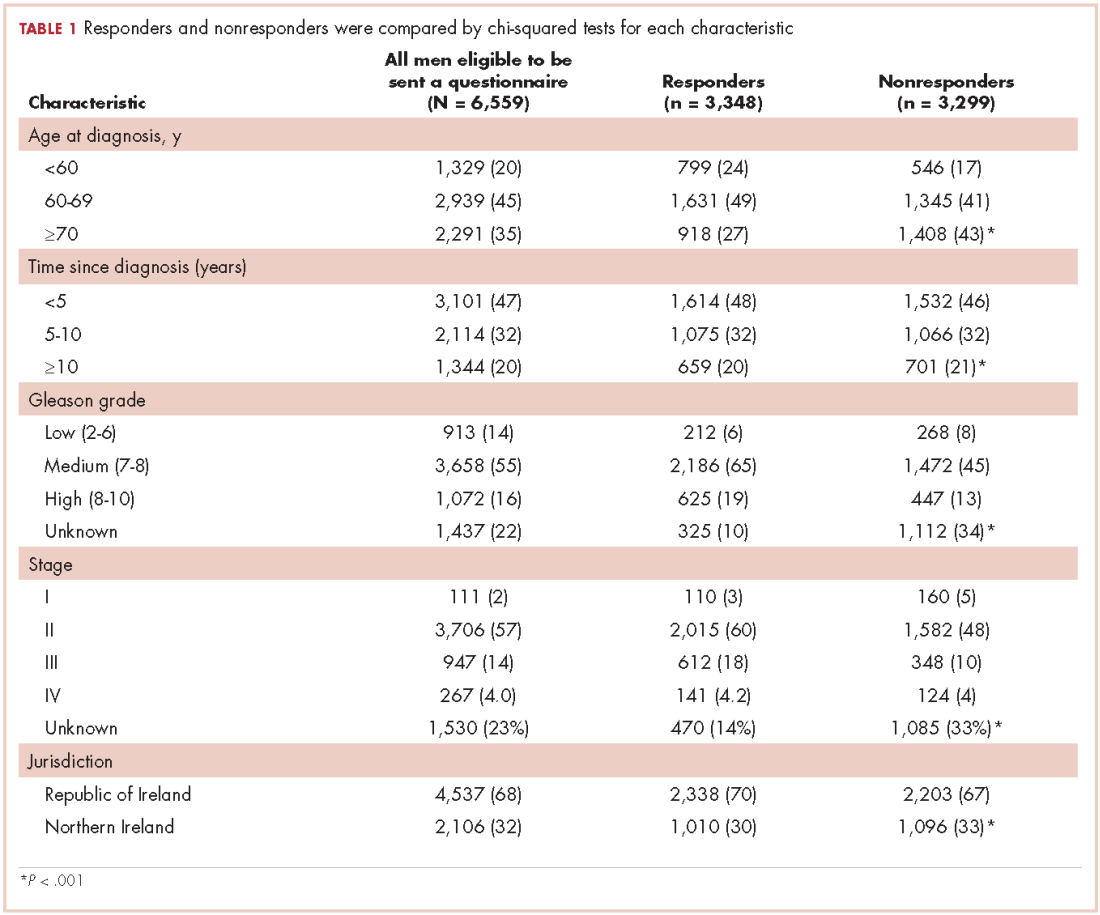
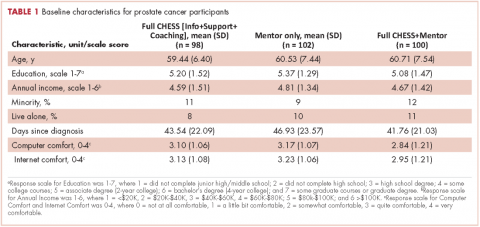
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
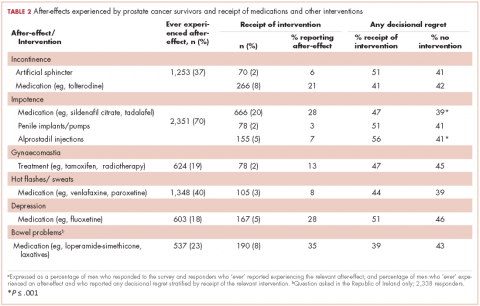
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
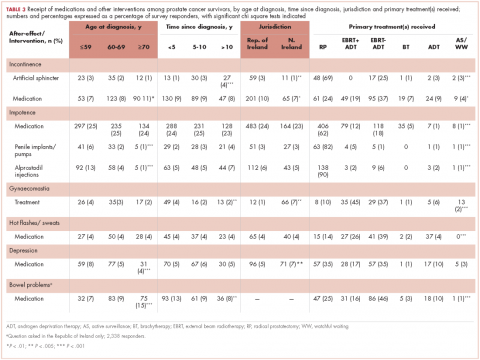
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
The impact of combining human and online supportive resources for prostate cancer patients
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
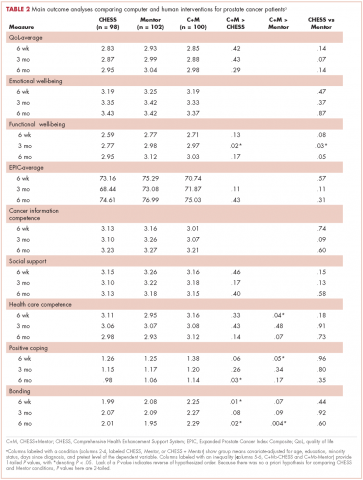
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
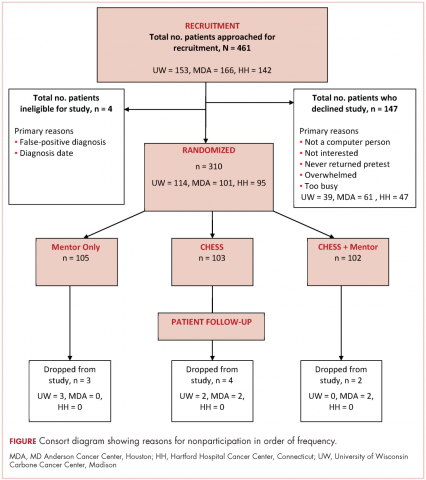
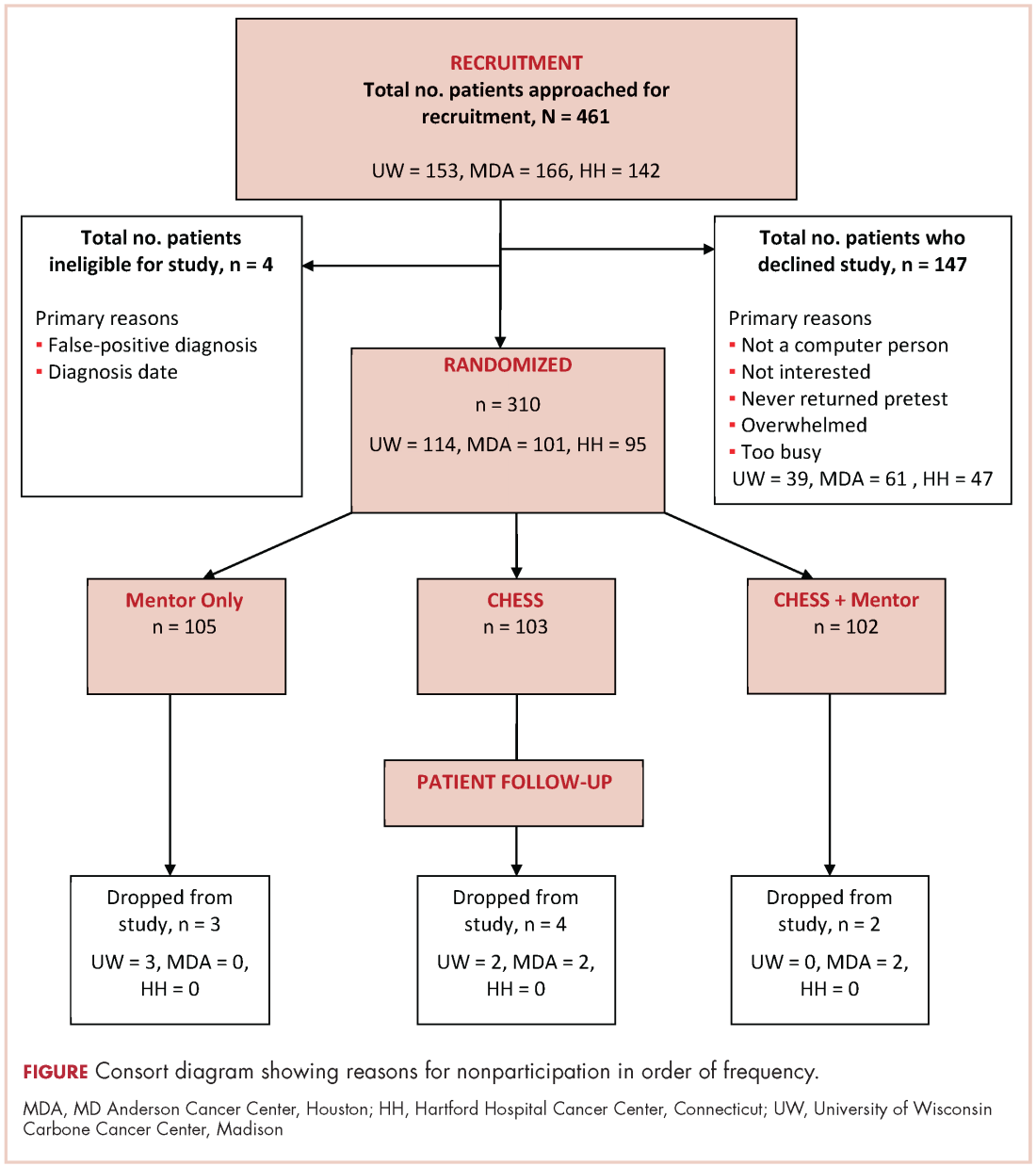
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
Hallmark tumor metabolism becomes a validated therapeutic target
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
Altered cell metabolism has long been recognized as a distinctive feature of malignant cells but, until recently, research efforts had focused on a single aspect. It has become increasingly evident that many metabolic pathways are altered in cancer cells. Improved understanding has yielded the first regulatory approval in this new class of drugs. Here, we discuss the latest developments in the therapeutic targeting of the cancer metabolism hallmark.
A cancer cell’s sweet tooth
The metabolism of cancer cells differs from that of normal cells, an observation that has spawned a dedicated field of research and new targeted drug development. The German physiologist Otto Warburg is credited as the father of the field with his observations about the way in which cancer cells derive energy from glucose.1
In normal cells, glucose is converted into pyruvate in the cytoplasm, which is then, most often, fed to the mitochondria that use oxidative phosphorylation to produce energy in the form of adenosine triphosphate (ATP). Cancer cells seem instead to favor using the pyruvate to produce lactate through glycolysis (Figure 1).
Glycolysis is usually reserved for conditions of poor oxygen availability, but although the tumor microenvironment is often hypoxic, cancer cells have been shown to use glycolysis even when oxygen is plentiful. As a result, the phenomenon is known as aerobic glycolysis, although it is most often referred to as the Warburg effect.2
Glycolysis is much less efficient than oxidative phosphorylation at producing energy, yielding only 2 ATP. In order to meet their energy demands in this way, cancer cells ramp up their glucose intake, an effect that has been exploited for the detection of cancer with positron-emission tomography.
Warburg postulated that this metabolic shift was a result of mitochondrial damage and defective oxidative phosphorylation, even going so far as to suggest that cancer was a mitochondrial disease. It has subsequently been shown that the mitochondria are mostly intact in cancer cells and that oxidative phosphorylation can still occur.3
The Warburg effect has been the subject of significant investigative efforts as researchers have attempted to better understand how this phenomenon comes about. Studies have shown that it is driven in large part by the transcription factors hypoxia inducible factor 1 alpha (HIF-1α) and c-Myc. In addition, numerous other signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway, and the activation of oncogenes and inactivation of tumor suppressors, are thought to play a central role.
HIF-1α is an oxygen-sensing transcription factor that coordinates cellular responses to reduced oxygen levels by binding to specific regions, known as hypoxia response elements, on target genes in the nucleus and regulating their subsequent expression. Oxygen levels and metabolism are tightly linked, and HIF-1α sits at the intersection of the 2 since many of its target genes are involved in metabolic pathways, including many glycolytic enzymes, but it also directly inhibits oxidative phosphorylation by suppressing key enzymes in this metabolic pathway.
The expression of HIF-1α and numerous glycolytic enzymes, including lactate dehydrogenase (LDH), phosphofructokinase (PFK), hexokinase II (HKII), and pyruvate dehydrogenase kinase (PDK) is increased in many tumor types. Other molecules that are associated with glucose uptake and metabolism are also dysregulated, such as the GLUT-1 glucose transporter.2,4-6
Targeting glycolysis and glucose uptake
According to one study, glucose transporters and glycolytic enzymes are overexpressed in 24 different types of cancer, representing more than 70% of all cancer cases.7 This enables cancer cells to respond metabolically as though they are experiencing hypoxia, even when oxygen is plentiful and, indeed, when hypoxia is a concern, to mount a faster response. It also provides a tempting avenue for anticancer drug design by exploiting the dependency of cancer cells on glycolysis to survive and thrive.
Inhibitors of HKII, LDH, PFK, PDK, and GLUT-1 have been and continue to be developed. For example, 2-deoxy-D-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced by hydrogen, preventing further glycolysis; it acts as a competitive inhibitor of HKII. Dichloroacetate (DCA) activates the pyruvate dehydrogenase complex and inhibits the actions of the PDKs. Although development of DCA itself was unsuccessful, DCA derivatives continue to be pursued. WZB117 and STF-31 are novel small-molecule inhibitors of GLUT-1-mediated glucose transport. To date, where inhibitors of glycolysis have progressed into clinical trials, they have not proved successful, often limited by off-target effects and low potency.8-11
A variety of cell signaling pathways are implicated in metabolism by tightly regulating the ability of cells to gain access to and use nutrients. Through aberrations in these pathways, cancer cells can essentially go rogue, ignoring regulatory signals and taking up nutrients in an autonomous manner. One of the most frequently altered signaling pathways in human cancer, the PI3K-Akt-mTOR pathway, is also an important regulator of metabolism, coordinating the uptake of multiple nutrients, including glucose.
Akt in particular is thought to have a critical role in glucose metabolism and increased Akt pathway signaling has been shown to correlate with increased rates of glycolysis in cancer cells. Thus, Akt inhibitors could double as glycolytic or glucose transport inhibitors.12,13
A number of Akt inhibitors are being evaluated in clinical trials (Table) and results from the phase 2 LOTUS trial of ipatasertib (GDC-0068) were recently published.
Among 124 patients randomly assigned to paclitaxel in combination with either ipatasertib or placebo, there was a modest improvement in progression-free survival (PFS) in the ipatasertib arm in patients with triple-negative breast cancer (TNBC; 6 months vs 4.2 months, respectively; hazard ratio [HR], 0.60; P = .037). The effect was more pronounced, though not statistically significant, in patients with phosphatase and tensin homolog (PTEN)-low tumors (6.2 months vs 3.7 months; HR, 0.59; P = .18). The most common grade 3 and higher adverse events (AEs) were diarrhea, reduced neutrophil count, and neutropenia.14
The Warburg paradox
Although the molecular mechanisms underlying the Warburg effect have been revealed to some extent, why cancer cells would choose to use such an energy-inefficient process when they have such high energy demands, remains something of a paradox. It’s still not entirely clear, but several explanations that are not necessarily mutually exclusive have been proposed and relate to the inherent benefits of glycolysis and might explain why cancer cells favor this pathway despite its poor energy yield. First, ATP is produced much more rapidly through glycolysis than oxidative phosphorylation, up to 100 times faster. Thus, using glycolysis is a trade-off, between making less energy and making it more quickly.
Second, cancer cells require more than just ATP to meet their metabolic demands. They need amino acids for protein synthesis; nucleotides for DNA replication; lipids for cell membrane synthesis; nicotinamide adenine dinucleotide phosphate (NADPH), which helps the cancer cell deal with oxidative stress; and various other metabolites. Glycolysis branches off into other metabolic pathways that generate many of these metabolites. Among these branched pathways is the pentose phosphate pathway (PPP), which is required for the generation of ribonucleotides and is a major source for NADPH. Cancer cells have been shown to upregulate the flux of glucose into the PPP to meet their anabolic demands and counter oxidative stress.
Third, the lactic acid produced through glycolysis is actively exported from tumor cells by monocarboxylate transporters (MCTs). This creates a highly acidic tumor microenvironment, which can promote several cancer-related processes and also plays a role in tumor-induced immunosuppression, by inhibiting the activity of tumor-infiltrating T cells, reducing dendritic cell maturation, and promoting the transformation of macrophages to a protumorigenic form.2,4,6
Beyond the Warburg effect
Although the focus has been on glucose metabolism and glycolysis, it has been increasingly recognized that many different metabolic pathways are altered. Fundamental changes to the metabolism of all 4 major classes of macromolecules – carbohydrates, lipids, proteins, and nucleic acids – have been observed, encompassing all aspects of cellular metabolism and enabling cancer cells to meet their complete metabolic requirements. There is also evidence that cancer cells are able to switch between different metabolic pathways depending on the availability of oxygen, their energetic needs, environmental stresses, and many other factors. Certainly, there is significant heterogeneity in the metabolic changes that occur in tumors, which vary from tumor to tumor and even within the same tumor and across the lifespan of a tumor as it progresses from an early stage to more advanced or metastatic disease.
The notion of the Warburg effect as a universal phenomenon in cancer cells is now being widely disregarded. Many tumors continue to use oxidative phosphorylation, particularly slower growing tumors, to meet their energy needs. More recently a “reverse” Warburg effect was described, whereby cancer cells are thought to influence the metabolism of the surrounding stromal fibroblasts and essentially outsource aerobic glycolysis to these cells, while performing energy-efficient oxidative phosphorylation themselves (Figure 2).5,15,16
There is thought to be a “lactate shuttle” between the stromal and cancer cells. The stromal cells express high levels of efflux MCTs so that they can remove the subsequently high levels of lactate from the cytoplasm and avoid pickling themselves. The lactate is then shuttled to the cancer cells that have MCTs on their surface that are involved in lactate uptake. The cancer cells oxidize the lactate back into pyruvate, which can then be used in the tricarboxylic acid (TCA) cycle to feed oxidative phosphorylation for efficient ATP production. This hypothesis reflects a broader appreciation of the role of the microenvironment in contributing to cancer metabolism.17,18
An improved holistic understanding of cancer cell metabolism has led to the recognition of altered cancer metabolism as one of the hallmark abilities required for transformation of a normal cell into a cancerous one. It is categorized as “deregulation of bioenergetics” in the most up to date review of the cancer hallmarks.19 It has also begun to shape the therapeutic landscape as new drug targets have emerged.
IDH inhibitors first to market
A number of new metabolically-targeted treatment strategies are being developed. Most promising are small molecule inhibitors of the isocitrate dehydrogenase (IDH) enzymes. These enzymes play an essential role in the TCA cycle, catalyzing the conversion of isocitrate to alpha-ketoglutarate, generating carbon dioxide and NADPH. Recurrent mutations in the IDH1 and IDH2 genes have been observed in several different types of cancer, including glioma, acute myeloid leukemia (AML), and cholangiocarcinoma.
IDH mutations are known as neomorphic mutations because they confer a new function on the altered gene product. In this case, the mutant IDH enzyme converts alpha-ketoglutarate further into D-2-hydroxyglutarate (D-2HG). This molecule has a number of different effects that promote tumorigenesis, including fostering defective DNA repair (Figure 3).20,21
Intriguing research presented at the American Association of Cancer Research Annual Meeting revealed that IDH mutations may make cancer cells more vulnerable to poly (ADP-ribose) polymerase (PARP) inhibition, likely as a result of defects in homologous recombination pathways of DNA repair.22The pursuit of IDH as a potential therapeutic target has yielded the first regulatory approval for a metabolically targeted anticancer therapy. In August 2017, the United States Food and Drug Administration (FDA) approved enasidenib, an IDH2 inhibitor, for the treatment of relapsed or refractory AML with an IDH2 mutation. It was approved in combination with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect IDH2 mutations.
The approval was based on a single-arm trial in which responses occurred in almost a quarter of the 199 patients treated with 100 mg oral enasidenib daily. After a median follow-up of 6.6 months, 23% of the patients experienced a complete response or a complete response with partial hematologic recovery lasting a median of 8.2 months. The most common AEs were nausea, vomiting, diarrhea, elevated bilirubin levels, and reduced appetite.23
Several other IDH inhibitors are also showing encouraging efficacy. Ivosidenib is an IDH1 inhibitor and the results of a phase 1 study in patients with cholangiocarcinoma were recently presented at a leading conference. Escalating doses of ivosidenib (100 mg twice daily to 1,200 mg once daily) were administered to 73 patients (as of December 2016). The confirmed partial response (PR) rate was 6%, the rate of stable disease was 56%, and PFS at 6 months was 40%. There were no dose-limiting toxicities (DLTs) and treatment-emergent AEs included fatigue, nausea, vomiting, diarrhea, decreased appetite, dysgeusia, and QT prolongation.24
Another study of ivosidenib was presented at the 2017 annual meeting of the Society for Neuro-Oncology. In that study, patients with glioma received daily doses of ivosidenib ranging from 300 mg to 900 mg. Two patients had a minor response, 83% had stable disease, and the median PFS was 13 months. There were no DLTs and most AEs were mild to moderate and included, most commonly, headache, nausea, diarrhea, and vomiting.25
Pursuing alternative targets and repurposing drugs
Other metabolic targets that are being pursued include glutaminase, given the observation of significantly enhanced glutamine uptake in cancer cells. CB-839 is a glutaminase inhibitor that is currently being evaluated in phase 1 and 2 clinical trials. Updated clinical trial data from a phase 1 trial of CB-839 in combination with paclitaxel in patients with advanced/metastatic TNBC were presented at the San Antonio Breast Cancer Symposium last year.26
As of October 2017, 49 patients had been treated with 400 mg, 600 mg, or 800 mg CB-839 twice daily in combination with 80 mg/m2 intravenous paclitaxel weekly. Among the 44 patients evaluable for response, the rate of PR was 22% and of disease control, 59%. The one DLT was grade 3 neutropenia at the 400 mg dose. Overall AEs were mostly low grade and reversible.
In recent years, lactate has emerged as more than just a by-product of altered cancer cell metabolism. It is responsible, at least in part, for the highly acidic tumor microenvironment that fosters many of the other hallmarks of cancer. In addition, lactate promotes angiogenesis by upregulating HIF-1α in endothelial cells. Depriving tumor cells of the ability to export lactate is a potentially promising therapeutic strategy. An MCT-1 inhibitor, AZD3965, is being evaluated in early stage clinical trials.
Finally, several drugs that are renowned for their use in other disease settings are being repurposed for cancer therapy because of their potential effects on cancer cell metabolism. Ritonavir, an antiretroviral drug used in the treatment of HIV, is an inhibitor of GLUT-1 and is being evaluated in phase 1 and 2 clinical trials. Meanwhile, long-term studies of metformin, a drug that has revolutionized the treatment of diabetes, have revealed a reduction in the emergence of new cancers in diabetic patients treated who are treated with it, and the drug has been shown to improve breast cancer survival rates. Its precise anticancer effects are somewhat unclear, but it is thought to act in part by inhibiting oxidative phosphorylation. Numerous clinical trials of metformin in different types of cancer are ongoing.27,2
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
1. Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269-270.
2. Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908-38926.
3. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309-314.
4. Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013-1019.
5. Gupta S, Roy A, Dwarakanath BS. Metabolic cooperation and competition in the tumor microenvironment: implications for therapy. Front Oncol. 2017;7:68.
6. Marchiq I, Pouyssegur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J Mol Med (Berl). 2016;94(2):155-171.
7. Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014-1020.
8. Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8(17):3430-3440.
9. Zhang W, Zhang SL, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390-1400.
10. Talekar M, Boreddy SR, Singh A, Amiji M. Tumor aerobic glycolysis: new insights into therapeutic strategies with targeted delivery. Expert Opin Biol Ther. 2014;14(8):1145-1159.
11. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
12. Lien EC, Lyssiotis CA, Cantley LC. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In: Cramer T, Schmitt CA, eds. Metabolism in Cancer. Cham, Switzerland: Springer International Publishing; 2016:39-72.
13. Simons AL, Orcutt KP, Madsen JM, Scarbrough PM, Spitz DR. The role of Akt pathway signaling in glucose metabolism and metabolic oxidative stress. In: Spitz DR, Dornfeld KJ, Krishnan K, Gius D (eds). Oxidative stress in cancer biology and therapy. Humana Press (copyright holder, Springer Science+Business Media, LLC); 2012:21-46.
14. Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372.
15. Fu Y, Liu S, Yin S, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813-57825.
16. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44(3):198-203.
17. Brooks GA. Cell–cell and intracellular lactate shuttles. Journal Physiol. 2009;587(23):5591-5600.
18. Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272-280.
19. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
20. Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016;21(117):373-380.
21. Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370.
22. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017;77(7):1709-1718.
23. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731.
24. Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015-4015.
25. Mellinghoff IK, Touat M, Maher E, et al. ACTR-46. AG-120, a first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(suppl_6):vi10-vi11.
26. Kalinsky K, Harding J, DeMichele A, et al. Phase 1 study of CB-839, a first-in-class oral inhibitor of glutaminase, in combination with paclitaxel in patients with advanced triple negative breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas.
27. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? Biomed Res Int. 2015;2015:548436.
28. Leone A, Di Gennaro E, Bruzzese F, Avallone A, Budillon A. New perspective for an old antidiabetic drug: metformin as anticancer agent. Cancer Treat Res. 2014;159:355-376.
DNA vaccine + PD-1 blockade shows promise in mCRPC
NATIONAL HARBOR, MD. – Combining programmed death (PD)-1 blockade with tumor-targeted T-cell activation by a novel DNA vaccine safely enhanced antitumor immune responses in metastatic castration-resistant prostate cancer (mCRPC) patients in a randomized clinical study.
Of 26 patients with mCRPC who were evaluable for response, 13 received treatment with an investigational DNA vaccine (pTVG-HP) that encodes prostatic acid phosphatase (PAP) and concurrent PD-1 blockade, and 13 received sequential vaccination and PD-1 blockade. No difference was seen between the groups with respect to progression-free survival at 6 months, but of eight patients in the concurrent therapy arm who had measurable disease, one experienced a partial response and two experienced a reduction in tumor volume, Douglas G. McNeel, MD, PhD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“We did not see objective responses in [six patients with measurable disease in the sequential treatment arm], said Dr. McNeel, a professor at the University of Wisconsin, Madison.
Prostate specific antigen (PSA) responses, which may be a more sensitive marker, were also more common in the concurrent treatment group; 8 of 13 patients in that group had a PSA decline from baseline, and 4 of those had a decline of greater than 25% from baseline, whereas only 1 of the 13 patients in the sequential treatment arm experienced any decline in PSA vs. baseline, Dr. McNeel said.
Responses to the vaccine’s target antigen, prostatic acid phosphatase, were seen in both arms, but only those who received the combined treatment and who had evidence of immune response experienced PSA decline, he added.
Pre- and postvaccination biopsies of metastatic sites showed that concurrent treatment, compared with sequential treatment, elicited tumor-infiltrating CD8+ T cells, PD-L1 expression in tumors, and changes associated with CD8+ T-cell activation, he said, adding that immunization with concurrent PD-1 blockade also elicits changes in proliferation detected by (18F) fluorothymidine PET/CT.
“We’ve been interested in vaccines for cancer, because we know that having the right kind of T cells in the tumor microenvironment is associated with better long-term outcomes,” Dr. McNeel said, noting that the ability of vaccines to activate T cells and augment cytolytic T cells, in particular, should have anticancer activity.
However, the clinical activity of single-agent tumor vaccines has been underwhelming, he noted.
PAP has been a focus in vaccine development, because it is essentially restricted to prostate tissue in humans. A nearly identical prostate-specific rat homologue was used in early studies, and PAP permits evaluation of serum PSA as an independent assessment of response in human trials, he explained.
“It’s the same target as the sipuleucel-T vaccine,” he said, referring to a Food and Drug Administration–approved vaccine for prostate cancer(Provenge).
Two prior phase 1/2 trials looking at DNA vaccine encoding PAP in patients with early biochemically recurrent prostate cancer showed that PAP-specific T-cell immune responses were elicited and that no significant adverse events occurred.
In both trials, the development of persistent PAP-specific, interferon-gamma–secreting T cells was associated with favorable change in PSA doubling time (suggesting a possible impact on the disease), and with PD-L1 expression in circulating tumor cells (suggesting a potential mechanism of resistance), he said.
Laboratory studies helped identify mechanisms of immune resistance following DNA immunization, he said, explaining that immunization elicits T cells secreting interferon-gamma, which leads to an increase in PD-L1 expression on tumor cells.
Encoding epitopes with increased major histocompatibility complex class 1 affinity elicited CD+ t cells with increased and persistent PD-1 expression, and blockade of PD-1 or PD-L1 with vaccination led to improved antitumor responses, he said.
The findings led to the new model focused on timing of PD-1 blockade with vaccine T-cell activation studied in the current trial.
It was hypothesized that PD-1 blockade at the time of T-cell activation with vaccination would be more effective than was blockade of PD-1-regulated T cells previously elicited with vaccination.
Study subjects had mCRPC and evidence of disease progression. Previous treatment with abiraterone(Zytiga), enzalutamide(Xtandi), or chemotherapy, was allowed, but patients with prior sipuleucel-T vaccine exposure were excluded.
Patients in the concurrent treatment arm received both the vaccine and PD-1 blockade with pembrolizumab (Keytruda)over 12 weeks, and those in the sequential therapy arm received vaccination first followed by PD-1 blockade, each for 12 weeks.
Both approaches were well tolerated.
“Essentially, we saw nothing that was unexpected,” Dr. McNeel said.
Adverse events greater than grade 2 included fatigue in one patient, diarrhea in one patient, and autoimmune hepatitis in one patient. No patients discontinued treatment from toxicity, he noted.
One death occurred during follow-up in a patient who had evidence of progression and refused further follow-up, therefore it could not be determined if the death was related to treatment.
The current findings, which are notable in part because PD-1 pathway inhibitors have demonstrated little clinical activity when used as single agents for prostate cancer and which expand upon data presented in a scientific poster at SITC 2016, demonstrate that combining this blockade with tumor-targeted T-cell activation by a DNA vaccine is safe and can augment tumor-specific T cells – as detectable within the peripheral blood and by imaging – and can result in objective antitumor changes.
“To summarize, plasmid DNA vaccines can elicit antigen-specific CD8+ T cells; immunization can increase PD-L1 expression on tumor cells – and we’ve demonstrated, in mice anyway, that this is mediated by the T cells elicited with immunization; PD-1 expression increases on CD8+ T cells following vaccination; and we think this is an opportunity to use checkpoint blockade at the point of vaccination to improve antitumor responses,” Dr. McNeel said.
“So we can look at this as PD-1 blockade to improve the effect of vaccination, but we can also look at it the other way around, and that is that anti-tumor vaccines can elicit the tumor-specific CD8+ T cells needed to enable PD-1 blockade to work,” he said. “ I think this has implications for the choice of vaccine approach, the antigen, and the timing of PD-1 blockade.”
Based on these results, an expansion arm has been opened to evaluate the safety and clinical efficacy of combination treatment beyond 12 weeks, and future studies will look at the combination of two different vaccines to improve antitumor response, he said.
Dr. McNeel disclosed financial relationships (intellectual property rights/patent holder, consultant, ownership interest) with Madison Vaccines Inc. The study is funded by a 2014 Movember PCF Challenge Award and Madison Vaccines.
sworcester@frontlinemedcom.com
SOURCE: McNeel D et al., J Immunother Cancer. 2017 5(Suppl 2):86 Abstract O11.
NATIONAL HARBOR, MD. – Combining programmed death (PD)-1 blockade with tumor-targeted T-cell activation by a novel DNA vaccine safely enhanced antitumor immune responses in metastatic castration-resistant prostate cancer (mCRPC) patients in a randomized clinical study.
Of 26 patients with mCRPC who were evaluable for response, 13 received treatment with an investigational DNA vaccine (pTVG-HP) that encodes prostatic acid phosphatase (PAP) and concurrent PD-1 blockade, and 13 received sequential vaccination and PD-1 blockade. No difference was seen between the groups with respect to progression-free survival at 6 months, but of eight patients in the concurrent therapy arm who had measurable disease, one experienced a partial response and two experienced a reduction in tumor volume, Douglas G. McNeel, MD, PhD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“We did not see objective responses in [six patients with measurable disease in the sequential treatment arm], said Dr. McNeel, a professor at the University of Wisconsin, Madison.
Prostate specific antigen (PSA) responses, which may be a more sensitive marker, were also more common in the concurrent treatment group; 8 of 13 patients in that group had a PSA decline from baseline, and 4 of those had a decline of greater than 25% from baseline, whereas only 1 of the 13 patients in the sequential treatment arm experienced any decline in PSA vs. baseline, Dr. McNeel said.
Responses to the vaccine’s target antigen, prostatic acid phosphatase, were seen in both arms, but only those who received the combined treatment and who had evidence of immune response experienced PSA decline, he added.
Pre- and postvaccination biopsies of metastatic sites showed that concurrent treatment, compared with sequential treatment, elicited tumor-infiltrating CD8+ T cells, PD-L1 expression in tumors, and changes associated with CD8+ T-cell activation, he said, adding that immunization with concurrent PD-1 blockade also elicits changes in proliferation detected by (18F) fluorothymidine PET/CT.
“We’ve been interested in vaccines for cancer, because we know that having the right kind of T cells in the tumor microenvironment is associated with better long-term outcomes,” Dr. McNeel said, noting that the ability of vaccines to activate T cells and augment cytolytic T cells, in particular, should have anticancer activity.
However, the clinical activity of single-agent tumor vaccines has been underwhelming, he noted.
PAP has been a focus in vaccine development, because it is essentially restricted to prostate tissue in humans. A nearly identical prostate-specific rat homologue was used in early studies, and PAP permits evaluation of serum PSA as an independent assessment of response in human trials, he explained.
“It’s the same target as the sipuleucel-T vaccine,” he said, referring to a Food and Drug Administration–approved vaccine for prostate cancer(Provenge).
Two prior phase 1/2 trials looking at DNA vaccine encoding PAP in patients with early biochemically recurrent prostate cancer showed that PAP-specific T-cell immune responses were elicited and that no significant adverse events occurred.
In both trials, the development of persistent PAP-specific, interferon-gamma–secreting T cells was associated with favorable change in PSA doubling time (suggesting a possible impact on the disease), and with PD-L1 expression in circulating tumor cells (suggesting a potential mechanism of resistance), he said.
Laboratory studies helped identify mechanisms of immune resistance following DNA immunization, he said, explaining that immunization elicits T cells secreting interferon-gamma, which leads to an increase in PD-L1 expression on tumor cells.
Encoding epitopes with increased major histocompatibility complex class 1 affinity elicited CD+ t cells with increased and persistent PD-1 expression, and blockade of PD-1 or PD-L1 with vaccination led to improved antitumor responses, he said.
The findings led to the new model focused on timing of PD-1 blockade with vaccine T-cell activation studied in the current trial.
It was hypothesized that PD-1 blockade at the time of T-cell activation with vaccination would be more effective than was blockade of PD-1-regulated T cells previously elicited with vaccination.
Study subjects had mCRPC and evidence of disease progression. Previous treatment with abiraterone(Zytiga), enzalutamide(Xtandi), or chemotherapy, was allowed, but patients with prior sipuleucel-T vaccine exposure were excluded.
Patients in the concurrent treatment arm received both the vaccine and PD-1 blockade with pembrolizumab (Keytruda)over 12 weeks, and those in the sequential therapy arm received vaccination first followed by PD-1 blockade, each for 12 weeks.
Both approaches were well tolerated.
“Essentially, we saw nothing that was unexpected,” Dr. McNeel said.
Adverse events greater than grade 2 included fatigue in one patient, diarrhea in one patient, and autoimmune hepatitis in one patient. No patients discontinued treatment from toxicity, he noted.
One death occurred during follow-up in a patient who had evidence of progression and refused further follow-up, therefore it could not be determined if the death was related to treatment.
The current findings, which are notable in part because PD-1 pathway inhibitors have demonstrated little clinical activity when used as single agents for prostate cancer and which expand upon data presented in a scientific poster at SITC 2016, demonstrate that combining this blockade with tumor-targeted T-cell activation by a DNA vaccine is safe and can augment tumor-specific T cells – as detectable within the peripheral blood and by imaging – and can result in objective antitumor changes.
“To summarize, plasmid DNA vaccines can elicit antigen-specific CD8+ T cells; immunization can increase PD-L1 expression on tumor cells – and we’ve demonstrated, in mice anyway, that this is mediated by the T cells elicited with immunization; PD-1 expression increases on CD8+ T cells following vaccination; and we think this is an opportunity to use checkpoint blockade at the point of vaccination to improve antitumor responses,” Dr. McNeel said.
“So we can look at this as PD-1 blockade to improve the effect of vaccination, but we can also look at it the other way around, and that is that anti-tumor vaccines can elicit the tumor-specific CD8+ T cells needed to enable PD-1 blockade to work,” he said. “ I think this has implications for the choice of vaccine approach, the antigen, and the timing of PD-1 blockade.”
Based on these results, an expansion arm has been opened to evaluate the safety and clinical efficacy of combination treatment beyond 12 weeks, and future studies will look at the combination of two different vaccines to improve antitumor response, he said.
Dr. McNeel disclosed financial relationships (intellectual property rights/patent holder, consultant, ownership interest) with Madison Vaccines Inc. The study is funded by a 2014 Movember PCF Challenge Award and Madison Vaccines.
sworcester@frontlinemedcom.com
SOURCE: McNeel D et al., J Immunother Cancer. 2017 5(Suppl 2):86 Abstract O11.
NATIONAL HARBOR, MD. – Combining programmed death (PD)-1 blockade with tumor-targeted T-cell activation by a novel DNA vaccine safely enhanced antitumor immune responses in metastatic castration-resistant prostate cancer (mCRPC) patients in a randomized clinical study.
Of 26 patients with mCRPC who were evaluable for response, 13 received treatment with an investigational DNA vaccine (pTVG-HP) that encodes prostatic acid phosphatase (PAP) and concurrent PD-1 blockade, and 13 received sequential vaccination and PD-1 blockade. No difference was seen between the groups with respect to progression-free survival at 6 months, but of eight patients in the concurrent therapy arm who had measurable disease, one experienced a partial response and two experienced a reduction in tumor volume, Douglas G. McNeel, MD, PhD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“We did not see objective responses in [six patients with measurable disease in the sequential treatment arm], said Dr. McNeel, a professor at the University of Wisconsin, Madison.
Prostate specific antigen (PSA) responses, which may be a more sensitive marker, were also more common in the concurrent treatment group; 8 of 13 patients in that group had a PSA decline from baseline, and 4 of those had a decline of greater than 25% from baseline, whereas only 1 of the 13 patients in the sequential treatment arm experienced any decline in PSA vs. baseline, Dr. McNeel said.
Responses to the vaccine’s target antigen, prostatic acid phosphatase, were seen in both arms, but only those who received the combined treatment and who had evidence of immune response experienced PSA decline, he added.
Pre- and postvaccination biopsies of metastatic sites showed that concurrent treatment, compared with sequential treatment, elicited tumor-infiltrating CD8+ T cells, PD-L1 expression in tumors, and changes associated with CD8+ T-cell activation, he said, adding that immunization with concurrent PD-1 blockade also elicits changes in proliferation detected by (18F) fluorothymidine PET/CT.
“We’ve been interested in vaccines for cancer, because we know that having the right kind of T cells in the tumor microenvironment is associated with better long-term outcomes,” Dr. McNeel said, noting that the ability of vaccines to activate T cells and augment cytolytic T cells, in particular, should have anticancer activity.
However, the clinical activity of single-agent tumor vaccines has been underwhelming, he noted.
PAP has been a focus in vaccine development, because it is essentially restricted to prostate tissue in humans. A nearly identical prostate-specific rat homologue was used in early studies, and PAP permits evaluation of serum PSA as an independent assessment of response in human trials, he explained.
“It’s the same target as the sipuleucel-T vaccine,” he said, referring to a Food and Drug Administration–approved vaccine for prostate cancer(Provenge).
Two prior phase 1/2 trials looking at DNA vaccine encoding PAP in patients with early biochemically recurrent prostate cancer showed that PAP-specific T-cell immune responses were elicited and that no significant adverse events occurred.
In both trials, the development of persistent PAP-specific, interferon-gamma–secreting T cells was associated with favorable change in PSA doubling time (suggesting a possible impact on the disease), and with PD-L1 expression in circulating tumor cells (suggesting a potential mechanism of resistance), he said.
Laboratory studies helped identify mechanisms of immune resistance following DNA immunization, he said, explaining that immunization elicits T cells secreting interferon-gamma, which leads to an increase in PD-L1 expression on tumor cells.
Encoding epitopes with increased major histocompatibility complex class 1 affinity elicited CD+ t cells with increased and persistent PD-1 expression, and blockade of PD-1 or PD-L1 with vaccination led to improved antitumor responses, he said.
The findings led to the new model focused on timing of PD-1 blockade with vaccine T-cell activation studied in the current trial.
It was hypothesized that PD-1 blockade at the time of T-cell activation with vaccination would be more effective than was blockade of PD-1-regulated T cells previously elicited with vaccination.
Study subjects had mCRPC and evidence of disease progression. Previous treatment with abiraterone(Zytiga), enzalutamide(Xtandi), or chemotherapy, was allowed, but patients with prior sipuleucel-T vaccine exposure were excluded.
Patients in the concurrent treatment arm received both the vaccine and PD-1 blockade with pembrolizumab (Keytruda)over 12 weeks, and those in the sequential therapy arm received vaccination first followed by PD-1 blockade, each for 12 weeks.
Both approaches were well tolerated.
“Essentially, we saw nothing that was unexpected,” Dr. McNeel said.
Adverse events greater than grade 2 included fatigue in one patient, diarrhea in one patient, and autoimmune hepatitis in one patient. No patients discontinued treatment from toxicity, he noted.
One death occurred during follow-up in a patient who had evidence of progression and refused further follow-up, therefore it could not be determined if the death was related to treatment.
The current findings, which are notable in part because PD-1 pathway inhibitors have demonstrated little clinical activity when used as single agents for prostate cancer and which expand upon data presented in a scientific poster at SITC 2016, demonstrate that combining this blockade with tumor-targeted T-cell activation by a DNA vaccine is safe and can augment tumor-specific T cells – as detectable within the peripheral blood and by imaging – and can result in objective antitumor changes.
“To summarize, plasmid DNA vaccines can elicit antigen-specific CD8+ T cells; immunization can increase PD-L1 expression on tumor cells – and we’ve demonstrated, in mice anyway, that this is mediated by the T cells elicited with immunization; PD-1 expression increases on CD8+ T cells following vaccination; and we think this is an opportunity to use checkpoint blockade at the point of vaccination to improve antitumor responses,” Dr. McNeel said.
“So we can look at this as PD-1 blockade to improve the effect of vaccination, but we can also look at it the other way around, and that is that anti-tumor vaccines can elicit the tumor-specific CD8+ T cells needed to enable PD-1 blockade to work,” he said. “ I think this has implications for the choice of vaccine approach, the antigen, and the timing of PD-1 blockade.”
Based on these results, an expansion arm has been opened to evaluate the safety and clinical efficacy of combination treatment beyond 12 weeks, and future studies will look at the combination of two different vaccines to improve antitumor response, he said.
Dr. McNeel disclosed financial relationships (intellectual property rights/patent holder, consultant, ownership interest) with Madison Vaccines Inc. The study is funded by a 2014 Movember PCF Challenge Award and Madison Vaccines.
sworcester@frontlinemedcom.com
SOURCE: McNeel D et al., J Immunother Cancer. 2017 5(Suppl 2):86 Abstract O11.
REPORTING FROM SITC 2017
Key clinical point: Concurrent PD-1 blockade enhanced DNA vaccine activity in mCRPC.
Major finding: A partial response and tumor volume reduction occurred in one and two patients, respectively.
Study details: A randomized clinical study of 26 patients.
Disclosures: Dr. McNeel disclosed financial relationships (intellectual property rights/patent holder, consultant, ownership interest) with Madison Vaccines. The study is funded by a 2014 Movember PCF Challenge Award and Madison Vaccines.
Source: Douglas McNeel D et al. J Immunother Cancer. 2017 Nov; 5(Suppl 2):86 Abstract O11.
FDA approves sunitinib malate as adjuvant treatment for RCC
The Food and Drug Administration has approved sunitinib malate for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma (RCC) following nephrectomy.
“This is the first adjuvant treatment approved for patients with renal cell carcinoma, which is significant because patients with this disease who have a nephrectomy are often at high risk of the cancer returning,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a written statement.
Approval for adjuvant treatment of RCC was based on median disease-free survival of 6.8 years for patients receiving sunitinib malate, compared with 5.6 years for patients receiving placebo in S-TRAC, a phase III trial of 615 patients with high risk of recurrent RCC following nephrectomy. In the trial, presented at the European Society for Medical Oncology Congress in 2016 and published in the New England Journal of Medicine, patients were randomized 1:1 to receive either 50 mg sunitinib malate once daily, 4 weeks on treatment followed by 2 weeks off, or placebo. Overall survival data were not mature at the time of data analysis.
The most common adverse reactions to sunitinib in the trial were fatigue/asthenia, diarrhea, mucositis/stomatitis, nausea, decreased appetite/anorexia, vomiting, abdominal pain, hand-foot syndrome, hypertension, bleeding events, dysgeusia, dyspepsia, and thrombocytopenia.
Severe side effects included hepatotoxicity, low left ventricular ejection fraction, myocardial ischemia/infarction, prolonged QT intervals/torsade de pointes, hypertension, hemorrhagic events, tumor lysis syndrome, thrombotic microangiopathy (including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome), proteinuria, thyroid dysfunction, hypoglycemia, osteonecrosis, and wound-healing complications. A boxed warning alerts health care professionals and patients about the risk of hepatoxicity, which may result in liver failure or death.
Sunitinib malate is marketed as Sutent by Pfizer. The recommended dose for the adjuvant treatment of RCC is 50 mg orally once daily, with or without food, 4 weeks on treatment followed by 2 weeks off for nine 6-week cycles.
Full prescribing information is available here.
The Food and Drug Administration has approved sunitinib malate for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma (RCC) following nephrectomy.
“This is the first adjuvant treatment approved for patients with renal cell carcinoma, which is significant because patients with this disease who have a nephrectomy are often at high risk of the cancer returning,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a written statement.
Approval for adjuvant treatment of RCC was based on median disease-free survival of 6.8 years for patients receiving sunitinib malate, compared with 5.6 years for patients receiving placebo in S-TRAC, a phase III trial of 615 patients with high risk of recurrent RCC following nephrectomy. In the trial, presented at the European Society for Medical Oncology Congress in 2016 and published in the New England Journal of Medicine, patients were randomized 1:1 to receive either 50 mg sunitinib malate once daily, 4 weeks on treatment followed by 2 weeks off, or placebo. Overall survival data were not mature at the time of data analysis.
The most common adverse reactions to sunitinib in the trial were fatigue/asthenia, diarrhea, mucositis/stomatitis, nausea, decreased appetite/anorexia, vomiting, abdominal pain, hand-foot syndrome, hypertension, bleeding events, dysgeusia, dyspepsia, and thrombocytopenia.
Severe side effects included hepatotoxicity, low left ventricular ejection fraction, myocardial ischemia/infarction, prolonged QT intervals/torsade de pointes, hypertension, hemorrhagic events, tumor lysis syndrome, thrombotic microangiopathy (including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome), proteinuria, thyroid dysfunction, hypoglycemia, osteonecrosis, and wound-healing complications. A boxed warning alerts health care professionals and patients about the risk of hepatoxicity, which may result in liver failure or death.
Sunitinib malate is marketed as Sutent by Pfizer. The recommended dose for the adjuvant treatment of RCC is 50 mg orally once daily, with or without food, 4 weeks on treatment followed by 2 weeks off for nine 6-week cycles.
Full prescribing information is available here.
The Food and Drug Administration has approved sunitinib malate for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma (RCC) following nephrectomy.
“This is the first adjuvant treatment approved for patients with renal cell carcinoma, which is significant because patients with this disease who have a nephrectomy are often at high risk of the cancer returning,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a written statement.
Approval for adjuvant treatment of RCC was based on median disease-free survival of 6.8 years for patients receiving sunitinib malate, compared with 5.6 years for patients receiving placebo in S-TRAC, a phase III trial of 615 patients with high risk of recurrent RCC following nephrectomy. In the trial, presented at the European Society for Medical Oncology Congress in 2016 and published in the New England Journal of Medicine, patients were randomized 1:1 to receive either 50 mg sunitinib malate once daily, 4 weeks on treatment followed by 2 weeks off, or placebo. Overall survival data were not mature at the time of data analysis.
The most common adverse reactions to sunitinib in the trial were fatigue/asthenia, diarrhea, mucositis/stomatitis, nausea, decreased appetite/anorexia, vomiting, abdominal pain, hand-foot syndrome, hypertension, bleeding events, dysgeusia, dyspepsia, and thrombocytopenia.
Severe side effects included hepatotoxicity, low left ventricular ejection fraction, myocardial ischemia/infarction, prolonged QT intervals/torsade de pointes, hypertension, hemorrhagic events, tumor lysis syndrome, thrombotic microangiopathy (including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome), proteinuria, thyroid dysfunction, hypoglycemia, osteonecrosis, and wound-healing complications. A boxed warning alerts health care professionals and patients about the risk of hepatoxicity, which may result in liver failure or death.
Sunitinib malate is marketed as Sutent by Pfizer. The recommended dose for the adjuvant treatment of RCC is 50 mg orally once daily, with or without food, 4 weeks on treatment followed by 2 weeks off for nine 6-week cycles.
Full prescribing information is available here.
NCI-MATCH: Nivolumab shows promising activity in noncolorectal cancers
NATIONAL HARBOR, MD. – The immune checkpoint inhibitor nivolumab has promising activity in mismatch repair–deficient noncolorectal cancers, according to preliminary findings from the first sub-arm of the National Cancer Institute’s landmark Molecular Analysis for Therapy Choice (NCI-MATCH) trial.
NCI-MATCH is a 1,173-site precision medicine trial launched in 2015 to study targeted therapies for patients with relapsed/refractory solid tumors, lymphomas, and myelomas. In the first substudy (arm Z1D), the investigators identified 4,900 subjects with samples that could be tested for “actionable molecular abnormalities,” and from among those, they identified 77 with loss of mismatch repair proteins MLH1 or MSH2. Ultimately 47 patients were treated with nivolumab in the substudy.
The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease, said Dr. Azad of Johns Hopkins University, Baltimore.
The patients had a median age of 60 years and were heavily pretreated with a median of three prior therapies. The most common histologies among them were endometrioid endometrial cancer (10 patients), prostate cancer (6 patients), and breast cancer (3 patients).
The safety and tolerability of treatment was as expected for single-agent nivolumab treatment. Toxicity was predominantly low-grade fatigue. Anemia was the most common grade 3 toxicity.
“DNA repair defects due to mismatch repair–deficiency are most commonly caused by silencing of mismatch repair proteins MLH1 or MSH2 and, a little less commonly, MSH6 or PMS2. This can happen through DNA mutation, as well as promoter methylation,” Dr. Azad explained. “In fact, nivolumab has already been tested in patients with mismatch repair–deficient colorectal cancer, both alone and in combination with anti-CTLA-4 ipilimumab ... in addition, pembrolizumab was approved earlier this year for pretreated mismatch repair–deficient cancer.”
“So this formed the nidus for our interest and hypothesis that nivolumab would also have activity in mismatch repair–deficient noncolorectal cancer,” she said.
Study subjects had relapsed/refractory cancers, good end-organ function, and good performance status. They were screened for molecular alterations by centralized testing on fresh biopsy tissue, and mismatch repair deficiency was defined through immunohistochemistry as loss of nuclear expression of MLH1 or MSH2. Patients with mismatch repair–deficient colorectal cancer were excluded.
Those in the nivolumab arm received 3 mg/kg every 2 weeks, and after cycle 4, they could be switched to receive treatment every 4 weeks. Imaging was performed every 2 weeks, and patients were allowed to remain in the study as long as their disease had not progressed. A caveat was that patients with progression within the first 24 weeks, but with no more than four new lesions or 40% increase in tumor index lesions, could remain in the study as long as they were clinically stable.
The overall response rate was compared against a null value of 5%.
“We enrolled 35 patients so that we could have 31 evaluable patients, looking for a signal of 5 or greater responses in that patient group to conclude that the arm was promising and worth further testing,” Dr. Azad said. “This gave us 91.8% power to conclude that an agent was promising if the overall response was truly 25%.”
The study met its primary endpoint, with 8 responses out of 34 evaluable patients, she reported.
“Of note, we had five more patients that had unconfirmed responses. Two of those remained on study at the time of data cutoff, so these response numbers may change as the study matures,” she said.
The disease control rate was 56%, and benefit was seen across tumor histologies, she noted.
“The duration of benefit was compelling for these patients,” she said. “The median time to response was 2.1 cycles, and the 6-month progression-free survival was 49%.”
The median duration of response has not been reached.
Follow-up is ongoing, and 12 patients are enrolled in an expansion cohort; results should be reported within the next year.
“Future work includes interrogating tumor tissue and blood to identify possible predictive markers of response and resistance,” Dr. Azad concluded.
Dr. Azad reported having no disclosures.
NATIONAL HARBOR, MD. – The immune checkpoint inhibitor nivolumab has promising activity in mismatch repair–deficient noncolorectal cancers, according to preliminary findings from the first sub-arm of the National Cancer Institute’s landmark Molecular Analysis for Therapy Choice (NCI-MATCH) trial.
NCI-MATCH is a 1,173-site precision medicine trial launched in 2015 to study targeted therapies for patients with relapsed/refractory solid tumors, lymphomas, and myelomas. In the first substudy (arm Z1D), the investigators identified 4,900 subjects with samples that could be tested for “actionable molecular abnormalities,” and from among those, they identified 77 with loss of mismatch repair proteins MLH1 or MSH2. Ultimately 47 patients were treated with nivolumab in the substudy.
The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease, said Dr. Azad of Johns Hopkins University, Baltimore.
The patients had a median age of 60 years and were heavily pretreated with a median of three prior therapies. The most common histologies among them were endometrioid endometrial cancer (10 patients), prostate cancer (6 patients), and breast cancer (3 patients).
The safety and tolerability of treatment was as expected for single-agent nivolumab treatment. Toxicity was predominantly low-grade fatigue. Anemia was the most common grade 3 toxicity.
“DNA repair defects due to mismatch repair–deficiency are most commonly caused by silencing of mismatch repair proteins MLH1 or MSH2 and, a little less commonly, MSH6 or PMS2. This can happen through DNA mutation, as well as promoter methylation,” Dr. Azad explained. “In fact, nivolumab has already been tested in patients with mismatch repair–deficient colorectal cancer, both alone and in combination with anti-CTLA-4 ipilimumab ... in addition, pembrolizumab was approved earlier this year for pretreated mismatch repair–deficient cancer.”
“So this formed the nidus for our interest and hypothesis that nivolumab would also have activity in mismatch repair–deficient noncolorectal cancer,” she said.
Study subjects had relapsed/refractory cancers, good end-organ function, and good performance status. They were screened for molecular alterations by centralized testing on fresh biopsy tissue, and mismatch repair deficiency was defined through immunohistochemistry as loss of nuclear expression of MLH1 or MSH2. Patients with mismatch repair–deficient colorectal cancer were excluded.
Those in the nivolumab arm received 3 mg/kg every 2 weeks, and after cycle 4, they could be switched to receive treatment every 4 weeks. Imaging was performed every 2 weeks, and patients were allowed to remain in the study as long as their disease had not progressed. A caveat was that patients with progression within the first 24 weeks, but with no more than four new lesions or 40% increase in tumor index lesions, could remain in the study as long as they were clinically stable.
The overall response rate was compared against a null value of 5%.
“We enrolled 35 patients so that we could have 31 evaluable patients, looking for a signal of 5 or greater responses in that patient group to conclude that the arm was promising and worth further testing,” Dr. Azad said. “This gave us 91.8% power to conclude that an agent was promising if the overall response was truly 25%.”
The study met its primary endpoint, with 8 responses out of 34 evaluable patients, she reported.
“Of note, we had five more patients that had unconfirmed responses. Two of those remained on study at the time of data cutoff, so these response numbers may change as the study matures,” she said.
The disease control rate was 56%, and benefit was seen across tumor histologies, she noted.
“The duration of benefit was compelling for these patients,” she said. “The median time to response was 2.1 cycles, and the 6-month progression-free survival was 49%.”
The median duration of response has not been reached.
Follow-up is ongoing, and 12 patients are enrolled in an expansion cohort; results should be reported within the next year.
“Future work includes interrogating tumor tissue and blood to identify possible predictive markers of response and resistance,” Dr. Azad concluded.
Dr. Azad reported having no disclosures.
NATIONAL HARBOR, MD. – The immune checkpoint inhibitor nivolumab has promising activity in mismatch repair–deficient noncolorectal cancers, according to preliminary findings from the first sub-arm of the National Cancer Institute’s landmark Molecular Analysis for Therapy Choice (NCI-MATCH) trial.
NCI-MATCH is a 1,173-site precision medicine trial launched in 2015 to study targeted therapies for patients with relapsed/refractory solid tumors, lymphomas, and myelomas. In the first substudy (arm Z1D), the investigators identified 4,900 subjects with samples that could be tested for “actionable molecular abnormalities,” and from among those, they identified 77 with loss of mismatch repair proteins MLH1 or MSH2. Ultimately 47 patients were treated with nivolumab in the substudy.
The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease, said Dr. Azad of Johns Hopkins University, Baltimore.
The patients had a median age of 60 years and were heavily pretreated with a median of three prior therapies. The most common histologies among them were endometrioid endometrial cancer (10 patients), prostate cancer (6 patients), and breast cancer (3 patients).
The safety and tolerability of treatment was as expected for single-agent nivolumab treatment. Toxicity was predominantly low-grade fatigue. Anemia was the most common grade 3 toxicity.
“DNA repair defects due to mismatch repair–deficiency are most commonly caused by silencing of mismatch repair proteins MLH1 or MSH2 and, a little less commonly, MSH6 or PMS2. This can happen through DNA mutation, as well as promoter methylation,” Dr. Azad explained. “In fact, nivolumab has already been tested in patients with mismatch repair–deficient colorectal cancer, both alone and in combination with anti-CTLA-4 ipilimumab ... in addition, pembrolizumab was approved earlier this year for pretreated mismatch repair–deficient cancer.”
“So this formed the nidus for our interest and hypothesis that nivolumab would also have activity in mismatch repair–deficient noncolorectal cancer,” she said.
Study subjects had relapsed/refractory cancers, good end-organ function, and good performance status. They were screened for molecular alterations by centralized testing on fresh biopsy tissue, and mismatch repair deficiency was defined through immunohistochemistry as loss of nuclear expression of MLH1 or MSH2. Patients with mismatch repair–deficient colorectal cancer were excluded.
Those in the nivolumab arm received 3 mg/kg every 2 weeks, and after cycle 4, they could be switched to receive treatment every 4 weeks. Imaging was performed every 2 weeks, and patients were allowed to remain in the study as long as their disease had not progressed. A caveat was that patients with progression within the first 24 weeks, but with no more than four new lesions or 40% increase in tumor index lesions, could remain in the study as long as they were clinically stable.
The overall response rate was compared against a null value of 5%.
“We enrolled 35 patients so that we could have 31 evaluable patients, looking for a signal of 5 or greater responses in that patient group to conclude that the arm was promising and worth further testing,” Dr. Azad said. “This gave us 91.8% power to conclude that an agent was promising if the overall response was truly 25%.”
The study met its primary endpoint, with 8 responses out of 34 evaluable patients, she reported.
“Of note, we had five more patients that had unconfirmed responses. Two of those remained on study at the time of data cutoff, so these response numbers may change as the study matures,” she said.
The disease control rate was 56%, and benefit was seen across tumor histologies, she noted.
“The duration of benefit was compelling for these patients,” she said. “The median time to response was 2.1 cycles, and the 6-month progression-free survival was 49%.”
The median duration of response has not been reached.
Follow-up is ongoing, and 12 patients are enrolled in an expansion cohort; results should be reported within the next year.
“Future work includes interrogating tumor tissue and blood to identify possible predictive markers of response and resistance,” Dr. Azad concluded.
Dr. Azad reported having no disclosures.
AT SITC 2017
Key clinical point:
Major finding: The confirmed overall response rate was 24%, and an additional 27% of patients had stable disease.
Data source: Arm Z1D (35 patients) of the NCI-MATCH trial.
Disclosures: Dr. Azad reported having no disclosures.