User login
Sex toys for science
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study: Uterine polyp removal in office possible via ultrasound
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
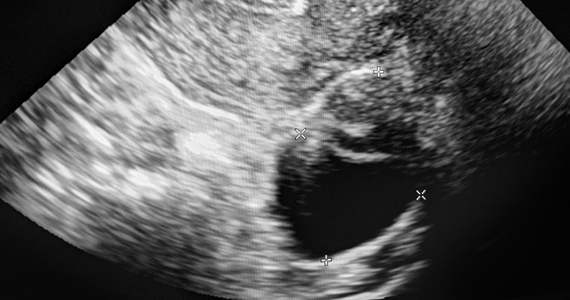
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IUD cuts heavy menses in nulliparous patients with obesity
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ACOG 2022
Society of Gynecologic Surgeons meeting champions training of future gynecologic surgeons
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
Commonly used antibiotics in ObGyn practice
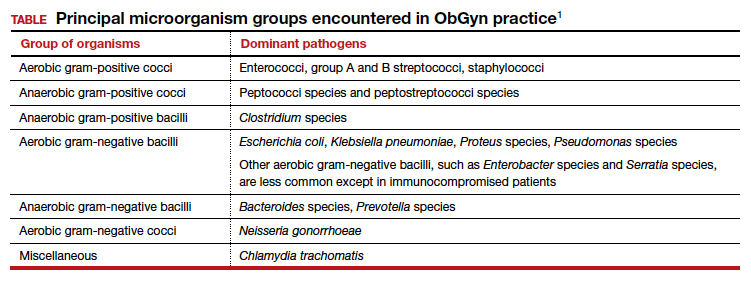
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.
Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.

Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.

Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
Can US “pattern recognition” of classic adnexal lesions reduce surgery, and even referrals for other imaging, in average-risk women?
Gupta A, Jha P, Baran TM, et al. Ovarian cancer detection in average-risk women: classic- versus nonclassic-appearing adnexal lesions at US. Radiology. 2022;212338. doi: 10.1148/radiol.212338.
Expert commentary
Gupta and colleagues conducted a multicenter, retrospective review of 970 adnexal lesions among 878 women—75% were premenopausal and 25% were postmenopausal.
Imaging details
The lesions were characterized by pattern recognition as “classic” (simple cysts, endometriomas, hemorrhagic cysts, or dermoids) or “nonclassic.” Out of 673 classic lesions, there were 4 malignancies (0.6%), of which 1 was an endometrioma and 3 were classified as simple cysts. However, out of 297 nonclassic lesions (multilocular, unilocular with solid areas or wall irregularity, or mostly solid), 32% (33/103) were malignant when vascularity was present, while 8% (16/184) were malignant when no intralesional vascularity was appreciated.
The authors pointed out that, especially because their study was retrospective, there was no standardization of scan technique or equipment employed. However, this point adds credibility to the “real world” nature of such imaging.
Other data corroborate findings
Other studies have looked at pattern recognition in efforts to optimize a conservative approach to benign masses and referral to oncology for suspected malignant masses, as described above. This was the main cornerstone of the International Consensus Conference,2 which also identified next steps for indeterminate masses, including evidence-based risk assessment algorithms and referral (to an expert imager or gynecologic oncologist). A multicenter trial in Europe3 found that ultrasound experience substantially impacts on diagnostic performance when adnexal masses are classified using pattern recognition. This occurred in a stepwise fashion with increasing accuracy directly related to the level of expertise. Shetty and colleagues4 found that pattern recognition performed better than the risk of malignancy index (sensitivities of 95% and 79%, respectively). ●
While the concept of pattern recognition for some “classic” benign ovarian masses has been around for some time, this is the first time a large United States–based study (albeit retrospective) has corroborated that when ultrasonography reveals a classic, or “almost certainly benign” finding, patients can be reassured that the lesion is benign, thereby avoiding extensive further workup. When a lesion is “nonclassic” in appearance and without any blood flow, further imaging with follow-up magnetic resonance imaging or repeat ultrasound could be considered. In women with a nonclassic lesion with blood flow, particularly in older women, referral to a gynecologic oncologic surgeon will help ensure expeditious treatment of possible ovarian cancer.
- Boll D, Geomini PM, Brölmann HA. The pre-operative assessment of the adnexal mass: the accuracy of clinical estimates versus clinical prediction rules. BJOG. 2003;110:519-523.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36:849-863. doi: 10.1002/jum.14197.
- Van Holsbeke C, Daemen A, Yazbek J, et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest. 2010;69:160-168. doi: 10.1159/000265012.
- Shetty J, Reddy G, Pandey D. Role of sonographic grayscale pattern recognition in the diagnosis of adnexal masses. J Clin Diagn Res. 2017;11:QC12-QC15. doi: 10.7860 /JCDR/2017/28533.10614.
Gupta A, Jha P, Baran TM, et al. Ovarian cancer detection in average-risk women: classic- versus nonclassic-appearing adnexal lesions at US. Radiology. 2022;212338. doi: 10.1148/radiol.212338.
Expert commentary
Gupta and colleagues conducted a multicenter, retrospective review of 970 adnexal lesions among 878 women—75% were premenopausal and 25% were postmenopausal.
Imaging details
The lesions were characterized by pattern recognition as “classic” (simple cysts, endometriomas, hemorrhagic cysts, or dermoids) or “nonclassic.” Out of 673 classic lesions, there were 4 malignancies (0.6%), of which 1 was an endometrioma and 3 were classified as simple cysts. However, out of 297 nonclassic lesions (multilocular, unilocular with solid areas or wall irregularity, or mostly solid), 32% (33/103) were malignant when vascularity was present, while 8% (16/184) were malignant when no intralesional vascularity was appreciated.
The authors pointed out that, especially because their study was retrospective, there was no standardization of scan technique or equipment employed. However, this point adds credibility to the “real world” nature of such imaging.
Other data corroborate findings
Other studies have looked at pattern recognition in efforts to optimize a conservative approach to benign masses and referral to oncology for suspected malignant masses, as described above. This was the main cornerstone of the International Consensus Conference,2 which also identified next steps for indeterminate masses, including evidence-based risk assessment algorithms and referral (to an expert imager or gynecologic oncologist). A multicenter trial in Europe3 found that ultrasound experience substantially impacts on diagnostic performance when adnexal masses are classified using pattern recognition. This occurred in a stepwise fashion with increasing accuracy directly related to the level of expertise. Shetty and colleagues4 found that pattern recognition performed better than the risk of malignancy index (sensitivities of 95% and 79%, respectively). ●
While the concept of pattern recognition for some “classic” benign ovarian masses has been around for some time, this is the first time a large United States–based study (albeit retrospective) has corroborated that when ultrasonography reveals a classic, or “almost certainly benign” finding, patients can be reassured that the lesion is benign, thereby avoiding extensive further workup. When a lesion is “nonclassic” in appearance and without any blood flow, further imaging with follow-up magnetic resonance imaging or repeat ultrasound could be considered. In women with a nonclassic lesion with blood flow, particularly in older women, referral to a gynecologic oncologic surgeon will help ensure expeditious treatment of possible ovarian cancer.
Gupta A, Jha P, Baran TM, et al. Ovarian cancer detection in average-risk women: classic- versus nonclassic-appearing adnexal lesions at US. Radiology. 2022;212338. doi: 10.1148/radiol.212338.
Expert commentary
Gupta and colleagues conducted a multicenter, retrospective review of 970 adnexal lesions among 878 women—75% were premenopausal and 25% were postmenopausal.
Imaging details
The lesions were characterized by pattern recognition as “classic” (simple cysts, endometriomas, hemorrhagic cysts, or dermoids) or “nonclassic.” Out of 673 classic lesions, there were 4 malignancies (0.6%), of which 1 was an endometrioma and 3 were classified as simple cysts. However, out of 297 nonclassic lesions (multilocular, unilocular with solid areas or wall irregularity, or mostly solid), 32% (33/103) were malignant when vascularity was present, while 8% (16/184) were malignant when no intralesional vascularity was appreciated.
The authors pointed out that, especially because their study was retrospective, there was no standardization of scan technique or equipment employed. However, this point adds credibility to the “real world” nature of such imaging.
Other data corroborate findings
Other studies have looked at pattern recognition in efforts to optimize a conservative approach to benign masses and referral to oncology for suspected malignant masses, as described above. This was the main cornerstone of the International Consensus Conference,2 which also identified next steps for indeterminate masses, including evidence-based risk assessment algorithms and referral (to an expert imager or gynecologic oncologist). A multicenter trial in Europe3 found that ultrasound experience substantially impacts on diagnostic performance when adnexal masses are classified using pattern recognition. This occurred in a stepwise fashion with increasing accuracy directly related to the level of expertise. Shetty and colleagues4 found that pattern recognition performed better than the risk of malignancy index (sensitivities of 95% and 79%, respectively). ●
While the concept of pattern recognition for some “classic” benign ovarian masses has been around for some time, this is the first time a large United States–based study (albeit retrospective) has corroborated that when ultrasonography reveals a classic, or “almost certainly benign” finding, patients can be reassured that the lesion is benign, thereby avoiding extensive further workup. When a lesion is “nonclassic” in appearance and without any blood flow, further imaging with follow-up magnetic resonance imaging or repeat ultrasound could be considered. In women with a nonclassic lesion with blood flow, particularly in older women, referral to a gynecologic oncologic surgeon will help ensure expeditious treatment of possible ovarian cancer.
- Boll D, Geomini PM, Brölmann HA. The pre-operative assessment of the adnexal mass: the accuracy of clinical estimates versus clinical prediction rules. BJOG. 2003;110:519-523.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36:849-863. doi: 10.1002/jum.14197.
- Van Holsbeke C, Daemen A, Yazbek J, et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest. 2010;69:160-168. doi: 10.1159/000265012.
- Shetty J, Reddy G, Pandey D. Role of sonographic grayscale pattern recognition in the diagnosis of adnexal masses. J Clin Diagn Res. 2017;11:QC12-QC15. doi: 10.7860 /JCDR/2017/28533.10614.
- Boll D, Geomini PM, Brölmann HA. The pre-operative assessment of the adnexal mass: the accuracy of clinical estimates versus clinical prediction rules. BJOG. 2003;110:519-523.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36:849-863. doi: 10.1002/jum.14197.
- Van Holsbeke C, Daemen A, Yazbek J, et al. Ultrasound experience substantially impacts on diagnostic performance and confidence when adnexal masses are classified using pattern recognition. Gynecol Obstet Invest. 2010;69:160-168. doi: 10.1159/000265012.
- Shetty J, Reddy G, Pandey D. Role of sonographic grayscale pattern recognition in the diagnosis of adnexal masses. J Clin Diagn Res. 2017;11:QC12-QC15. doi: 10.7860 /JCDR/2017/28533.10614.
Relugolix combo eases a long-neglected fibroid symptom: Pain
Combination therapy with relugolix (Orgovyx, Relumina), a gonadotropin-releasing hormone antagonist, significantly reduced the pain of uterine fibroids, an undertreated aspect of this disease.
In pooled results from the multicenter randomized placebo-controlled LIBERTY 1 and 2 trials, relugolix combination therapy (CT) with the progestin norethindrone (Aygestin, Camila) markedly decreased both menstrual and nonmenstrual fibroid pain, as well as heavy bleeding and other symptoms of leiomyomas. This hormone-dependent condition occurs in 70%-80% of premenopausal women.
“Historically, studies of uterine fibroids have not asked about pain, so one of strengths of these studies is that they asked women to rate their pain and found a substantial proportion listed pain as a symptom,” lead author Elizabeth A. Stewart, MD, director of reproductive endocrinology at the Mayo Clinic in Rochester, Minn., said in an interview.
The combination was effective against all categories of leiomyoma symptoms, she said, and adverse events were few.
Bleeding has been the main focus of studies of leiomyoma therapies, while chronic pain has been largely neglected, said James H. Segars Jr., MD, director of the division of reproductive science and women’s health research at Johns Hopkins Medicine in Baltimore, who was not involved in the studies. Across both of the LIBERTY trials, involving 509 women randomized during the period April 2017 to December 2018, more than half overall (54.4%) met their pain reduction goals in a subpopulation analysis. Pain reduction was a secondary outcome of the trials, with bleeding reduction the primary endpoint. Other fibroid symptoms are abdominal bloating and pressure.
“The consistent and significant reduction in measures of pain with relugolix-CT observed in the LIBERTY program is clinically meaningful, patient-relevant, and together with an improvement of heavy menstrual bleeding and other uterine leiomyoma–associated symptoms, is likely to have a substantial effect on the life of women with symptomatic uterine leiomyomas,” Dr. Stewart and colleagues wrote. Their report was published online in Obstetrics & Gynecology.
Dr. Segars concurred. “This study is important because sometimes the only fibroid symptom women have is pain. If we ignore that, we miss a lot of women who have pain but no bleeding.”
The study
The premenopausal participants had a mean age of just over 42 years (range, 18-50) and were enrolled from North and South America, Europe, and Africa. All reported leiomyoma-associated heavy menstrual bleeding of 80 mL or greater per cycle for two cycles, or 160 mL or greater during one cycle.
In both arms, the mean body mass index was 32 kg/m2, while menstrual blood loss volume was 245.4 (± 186.4) mL in the relugolix-CT and 207.4 (± 114.3) mL in the placebo group.
Pain was a frequent symptom, with approximately 70% in the intervention group and 74% in the placebo group reporting fibroid pain at baseline.
Women were randomized 1:1:1 to receive:
- Relugolix-CT (relugolix 40 mg, estradiol 1 mg, norethindrone acetate 0.5 mg)
- Delayed relugolix-CT (relugolix 40 mg monotherapy followed by relugolix-CT, each for 12 weeks)
- Placebo, taken orally once daily for 24 weeks
The therapy was well tolerated and adverse events were low.
The subpopulation analysis found that over the study period, the proportion of women achieving minimal to no pain (level 0 to 1) during the last 35 days of treatment was notably higher in the relugolix-CT arm than in the placebo arm: 45.2% (95% confidence interval [CI], 36.4%-54.3%) versus 13.9% (95% CI, 8.8%-20.5%) in the placebo group (nominal P = .001).
Moreover, the proportions of women with minimal to no pain during both menstrual days and nonmenstrual days were significantly higher with relugolix-CT: 65.0% (95% CI, 55.6%-73.5%) and 44.6% (95% CI, 32.3%-7.5%), respectively, compared with placebo: 19.3% (95% CI 13.2%–26.7%, nominal P = 001), and 21.6% (95% CI, 12.9%-32.7%, nominal P = 004), respectively.
Studies of relugolix monotherapy in Japanese women with uterine leiomyomas have demonstrated reductions in pain.
“Significantly, this combination therapy allows women to be treated over 2 years and to take the oral tablet themselves, unlike Lupron [leuprolide], which is injected and can only be taken for a couple of months because of bone loss,” Dr. Segars said. And the add-back component of combination therapy prevents the adverse symptoms of a hypoestrogenic state.
“The pain of fibroids is chronic, and the longer treatment allows time for the pain fibers to revert to a normal state,” he explained. “The pain pathways get etched into the nerves and it takes time to revert.”
He noted that the LIBERTY trials showed a slight downward trend in pain continuing after 24 weeks of treatment. Other studies of similar hormonal treatments have shown a reduction in the size of fibroids, which can be as large as a tennis ball.
As in endometriosis, leiomyomas are associated with elevated circulating cytokines and a systemic proinflammatory state. In endometriosis, this milieu is linked to the risk of inflammatory arthritis, fibromyalgia, lupus, and cardiovascular disease, Dr. Segars said. “If we did a deeper dive, we might find the same associations for fibroids.” Apart from chronic depression and fatigue, fibroids are linked to downstream pregnancy complications and poor outcomes such as miscarriage and preterm birth, he said.
“There remains a high unmet need for effective treatments, especially nonsurgical interventions, for women with uterine leiomyomas,” the authors wrote. Dr. Stewart added that “it would be helpful to learn more about how relugolix and other drugs in its class work in fibroids. No category of symptoms has been unresponsive to these medications – they are powerful drugs to help women with uterine fibroids.” She noted that relugolix-CT has already been approved outside the United States for symptoms beyond heavy menstrual bleeding.
Future research should focus on developing a therapy that does not interfere with fertility, Dr. Segars said. “We need a treatment that will allow women to get pregnant on it.”
Myovant Sciences GmbH sponsored LIBERTY 1 and 2 and oversaw all aspects of the studies. Dr. Stewart has provided consulting services to Myovant, Bayer, AbbVie, and ObsEva. She has received royalties from UpToDate and fees from Med Learning Group, Med-IQ, Medscape, Peer View, and PER, as well as honoraria from the American College of Obstetricians and Gynecologists and Massachusetts Medical Society. She holds a patent for methods and compounds for treatment of abnormal uterine bleeding. Dr. Segars has consulted for Bayer and Organon. Several coauthors reported similar financial relationships with private-sector entities and two coauthors are employees of Myovant.
Combination therapy with relugolix (Orgovyx, Relumina), a gonadotropin-releasing hormone antagonist, significantly reduced the pain of uterine fibroids, an undertreated aspect of this disease.
In pooled results from the multicenter randomized placebo-controlled LIBERTY 1 and 2 trials, relugolix combination therapy (CT) with the progestin norethindrone (Aygestin, Camila) markedly decreased both menstrual and nonmenstrual fibroid pain, as well as heavy bleeding and other symptoms of leiomyomas. This hormone-dependent condition occurs in 70%-80% of premenopausal women.
“Historically, studies of uterine fibroids have not asked about pain, so one of strengths of these studies is that they asked women to rate their pain and found a substantial proportion listed pain as a symptom,” lead author Elizabeth A. Stewart, MD, director of reproductive endocrinology at the Mayo Clinic in Rochester, Minn., said in an interview.
The combination was effective against all categories of leiomyoma symptoms, she said, and adverse events were few.
Bleeding has been the main focus of studies of leiomyoma therapies, while chronic pain has been largely neglected, said James H. Segars Jr., MD, director of the division of reproductive science and women’s health research at Johns Hopkins Medicine in Baltimore, who was not involved in the studies. Across both of the LIBERTY trials, involving 509 women randomized during the period April 2017 to December 2018, more than half overall (54.4%) met their pain reduction goals in a subpopulation analysis. Pain reduction was a secondary outcome of the trials, with bleeding reduction the primary endpoint. Other fibroid symptoms are abdominal bloating and pressure.
“The consistent and significant reduction in measures of pain with relugolix-CT observed in the LIBERTY program is clinically meaningful, patient-relevant, and together with an improvement of heavy menstrual bleeding and other uterine leiomyoma–associated symptoms, is likely to have a substantial effect on the life of women with symptomatic uterine leiomyomas,” Dr. Stewart and colleagues wrote. Their report was published online in Obstetrics & Gynecology.
Dr. Segars concurred. “This study is important because sometimes the only fibroid symptom women have is pain. If we ignore that, we miss a lot of women who have pain but no bleeding.”
The study
The premenopausal participants had a mean age of just over 42 years (range, 18-50) and were enrolled from North and South America, Europe, and Africa. All reported leiomyoma-associated heavy menstrual bleeding of 80 mL or greater per cycle for two cycles, or 160 mL or greater during one cycle.
In both arms, the mean body mass index was 32 kg/m2, while menstrual blood loss volume was 245.4 (± 186.4) mL in the relugolix-CT and 207.4 (± 114.3) mL in the placebo group.
Pain was a frequent symptom, with approximately 70% in the intervention group and 74% in the placebo group reporting fibroid pain at baseline.
Women were randomized 1:1:1 to receive:
- Relugolix-CT (relugolix 40 mg, estradiol 1 mg, norethindrone acetate 0.5 mg)
- Delayed relugolix-CT (relugolix 40 mg monotherapy followed by relugolix-CT, each for 12 weeks)
- Placebo, taken orally once daily for 24 weeks
The therapy was well tolerated and adverse events were low.
The subpopulation analysis found that over the study period, the proportion of women achieving minimal to no pain (level 0 to 1) during the last 35 days of treatment was notably higher in the relugolix-CT arm than in the placebo arm: 45.2% (95% confidence interval [CI], 36.4%-54.3%) versus 13.9% (95% CI, 8.8%-20.5%) in the placebo group (nominal P = .001).
Moreover, the proportions of women with minimal to no pain during both menstrual days and nonmenstrual days were significantly higher with relugolix-CT: 65.0% (95% CI, 55.6%-73.5%) and 44.6% (95% CI, 32.3%-7.5%), respectively, compared with placebo: 19.3% (95% CI 13.2%–26.7%, nominal P = 001), and 21.6% (95% CI, 12.9%-32.7%, nominal P = 004), respectively.
Studies of relugolix monotherapy in Japanese women with uterine leiomyomas have demonstrated reductions in pain.
“Significantly, this combination therapy allows women to be treated over 2 years and to take the oral tablet themselves, unlike Lupron [leuprolide], which is injected and can only be taken for a couple of months because of bone loss,” Dr. Segars said. And the add-back component of combination therapy prevents the adverse symptoms of a hypoestrogenic state.
“The pain of fibroids is chronic, and the longer treatment allows time for the pain fibers to revert to a normal state,” he explained. “The pain pathways get etched into the nerves and it takes time to revert.”
He noted that the LIBERTY trials showed a slight downward trend in pain continuing after 24 weeks of treatment. Other studies of similar hormonal treatments have shown a reduction in the size of fibroids, which can be as large as a tennis ball.
As in endometriosis, leiomyomas are associated with elevated circulating cytokines and a systemic proinflammatory state. In endometriosis, this milieu is linked to the risk of inflammatory arthritis, fibromyalgia, lupus, and cardiovascular disease, Dr. Segars said. “If we did a deeper dive, we might find the same associations for fibroids.” Apart from chronic depression and fatigue, fibroids are linked to downstream pregnancy complications and poor outcomes such as miscarriage and preterm birth, he said.
“There remains a high unmet need for effective treatments, especially nonsurgical interventions, for women with uterine leiomyomas,” the authors wrote. Dr. Stewart added that “it would be helpful to learn more about how relugolix and other drugs in its class work in fibroids. No category of symptoms has been unresponsive to these medications – they are powerful drugs to help women with uterine fibroids.” She noted that relugolix-CT has already been approved outside the United States for symptoms beyond heavy menstrual bleeding.
Future research should focus on developing a therapy that does not interfere with fertility, Dr. Segars said. “We need a treatment that will allow women to get pregnant on it.”
Myovant Sciences GmbH sponsored LIBERTY 1 and 2 and oversaw all aspects of the studies. Dr. Stewart has provided consulting services to Myovant, Bayer, AbbVie, and ObsEva. She has received royalties from UpToDate and fees from Med Learning Group, Med-IQ, Medscape, Peer View, and PER, as well as honoraria from the American College of Obstetricians and Gynecologists and Massachusetts Medical Society. She holds a patent for methods and compounds for treatment of abnormal uterine bleeding. Dr. Segars has consulted for Bayer and Organon. Several coauthors reported similar financial relationships with private-sector entities and two coauthors are employees of Myovant.
Combination therapy with relugolix (Orgovyx, Relumina), a gonadotropin-releasing hormone antagonist, significantly reduced the pain of uterine fibroids, an undertreated aspect of this disease.
In pooled results from the multicenter randomized placebo-controlled LIBERTY 1 and 2 trials, relugolix combination therapy (CT) with the progestin norethindrone (Aygestin, Camila) markedly decreased both menstrual and nonmenstrual fibroid pain, as well as heavy bleeding and other symptoms of leiomyomas. This hormone-dependent condition occurs in 70%-80% of premenopausal women.
“Historically, studies of uterine fibroids have not asked about pain, so one of strengths of these studies is that they asked women to rate their pain and found a substantial proportion listed pain as a symptom,” lead author Elizabeth A. Stewart, MD, director of reproductive endocrinology at the Mayo Clinic in Rochester, Minn., said in an interview.
The combination was effective against all categories of leiomyoma symptoms, she said, and adverse events were few.
Bleeding has been the main focus of studies of leiomyoma therapies, while chronic pain has been largely neglected, said James H. Segars Jr., MD, director of the division of reproductive science and women’s health research at Johns Hopkins Medicine in Baltimore, who was not involved in the studies. Across both of the LIBERTY trials, involving 509 women randomized during the period April 2017 to December 2018, more than half overall (54.4%) met their pain reduction goals in a subpopulation analysis. Pain reduction was a secondary outcome of the trials, with bleeding reduction the primary endpoint. Other fibroid symptoms are abdominal bloating and pressure.
“The consistent and significant reduction in measures of pain with relugolix-CT observed in the LIBERTY program is clinically meaningful, patient-relevant, and together with an improvement of heavy menstrual bleeding and other uterine leiomyoma–associated symptoms, is likely to have a substantial effect on the life of women with symptomatic uterine leiomyomas,” Dr. Stewart and colleagues wrote. Their report was published online in Obstetrics & Gynecology.
Dr. Segars concurred. “This study is important because sometimes the only fibroid symptom women have is pain. If we ignore that, we miss a lot of women who have pain but no bleeding.”
The study
The premenopausal participants had a mean age of just over 42 years (range, 18-50) and were enrolled from North and South America, Europe, and Africa. All reported leiomyoma-associated heavy menstrual bleeding of 80 mL or greater per cycle for two cycles, or 160 mL or greater during one cycle.
In both arms, the mean body mass index was 32 kg/m2, while menstrual blood loss volume was 245.4 (± 186.4) mL in the relugolix-CT and 207.4 (± 114.3) mL in the placebo group.
Pain was a frequent symptom, with approximately 70% in the intervention group and 74% in the placebo group reporting fibroid pain at baseline.
Women were randomized 1:1:1 to receive:
- Relugolix-CT (relugolix 40 mg, estradiol 1 mg, norethindrone acetate 0.5 mg)
- Delayed relugolix-CT (relugolix 40 mg monotherapy followed by relugolix-CT, each for 12 weeks)
- Placebo, taken orally once daily for 24 weeks
The therapy was well tolerated and adverse events were low.
The subpopulation analysis found that over the study period, the proportion of women achieving minimal to no pain (level 0 to 1) during the last 35 days of treatment was notably higher in the relugolix-CT arm than in the placebo arm: 45.2% (95% confidence interval [CI], 36.4%-54.3%) versus 13.9% (95% CI, 8.8%-20.5%) in the placebo group (nominal P = .001).
Moreover, the proportions of women with minimal to no pain during both menstrual days and nonmenstrual days were significantly higher with relugolix-CT: 65.0% (95% CI, 55.6%-73.5%) and 44.6% (95% CI, 32.3%-7.5%), respectively, compared with placebo: 19.3% (95% CI 13.2%–26.7%, nominal P = 001), and 21.6% (95% CI, 12.9%-32.7%, nominal P = 004), respectively.
Studies of relugolix monotherapy in Japanese women with uterine leiomyomas have demonstrated reductions in pain.
“Significantly, this combination therapy allows women to be treated over 2 years and to take the oral tablet themselves, unlike Lupron [leuprolide], which is injected and can only be taken for a couple of months because of bone loss,” Dr. Segars said. And the add-back component of combination therapy prevents the adverse symptoms of a hypoestrogenic state.
“The pain of fibroids is chronic, and the longer treatment allows time for the pain fibers to revert to a normal state,” he explained. “The pain pathways get etched into the nerves and it takes time to revert.”
He noted that the LIBERTY trials showed a slight downward trend in pain continuing after 24 weeks of treatment. Other studies of similar hormonal treatments have shown a reduction in the size of fibroids, which can be as large as a tennis ball.
As in endometriosis, leiomyomas are associated with elevated circulating cytokines and a systemic proinflammatory state. In endometriosis, this milieu is linked to the risk of inflammatory arthritis, fibromyalgia, lupus, and cardiovascular disease, Dr. Segars said. “If we did a deeper dive, we might find the same associations for fibroids.” Apart from chronic depression and fatigue, fibroids are linked to downstream pregnancy complications and poor outcomes such as miscarriage and preterm birth, he said.
“There remains a high unmet need for effective treatments, especially nonsurgical interventions, for women with uterine leiomyomas,” the authors wrote. Dr. Stewart added that “it would be helpful to learn more about how relugolix and other drugs in its class work in fibroids. No category of symptoms has been unresponsive to these medications – they are powerful drugs to help women with uterine fibroids.” She noted that relugolix-CT has already been approved outside the United States for symptoms beyond heavy menstrual bleeding.
Future research should focus on developing a therapy that does not interfere with fertility, Dr. Segars said. “We need a treatment that will allow women to get pregnant on it.”
Myovant Sciences GmbH sponsored LIBERTY 1 and 2 and oversaw all aspects of the studies. Dr. Stewart has provided consulting services to Myovant, Bayer, AbbVie, and ObsEva. She has received royalties from UpToDate and fees from Med Learning Group, Med-IQ, Medscape, Peer View, and PER, as well as honoraria from the American College of Obstetricians and Gynecologists and Massachusetts Medical Society. She holds a patent for methods and compounds for treatment of abnormal uterine bleeding. Dr. Segars has consulted for Bayer and Organon. Several coauthors reported similar financial relationships with private-sector entities and two coauthors are employees of Myovant.
FROM OBSTETRICS & GYNECOLOGY
FDA approves oteseconazole for chronic yeast infections
The Food and Drug Administration has approved oteseconazole capsules (Vivjoa), an azole antifungal agent, for the prevention of recurrent yeast infections in women who are not of reproductive potential.
Oteseconazole inhibits CYP51, an enzyme fungi require to preserve the integrity of their cell walls and to grow properly, according to Mycovia, the drug’s manufacturer. It is the first FDA-approved product for the treatment of recurrent vulvovaginal candidiasis (RVVC).
Recurrent vulvovaginal candidiasis, or chronic yeast infection, affects an estimated 138 million women worldwide annually. The condition is defined as three or more symptomatic acute episodes of yeast infection within a 12-month period. The primary symptoms of RVVC include vaginal itching, burning, irritation, and inflammation. Some patients may also experience abnormal vaginal discharge and pain during sex or urination.
“A medicine with Vivjoa’s sustained efficacy combined with the clinical safety profile has been long needed, as until now, physicians and their patients have had no FDA-approved medications for RVVC,” Stephen Brand, PhD, chief development officer of Mycovia, said in a statement. “We are excited to be the first to offer a medication designed specifically for RVVC, a challenging and chronic condition that is expected to increase in prevalence over the next decade.”
Approval for oteseconazole was based on results of three phase 3 trials involving 875 patients at 232 sites across 11 countries. In the U.S.-only ultraVIOLET trial, 89.7% of women with RVVC who received oteseconazole cleared their initial yeast infection and did not experience a recurrence during the 50-week maintenance period, compared with 57.1% of those who received fluconazole (Diflucan) followed by placebo (P < .001), according to Mycovia.
The most common side effects reported in phase 3 clinical studies were headache (7.4%) and nausea (3.6%), the company said. Patients with a hypersensitivity to oteseconazole should not take the drug, nor should those who are of reproductive potential, pregnant, or lactating.
Mycovia said it plans to launch the drug in the second quarter of 2022.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved oteseconazole capsules (Vivjoa), an azole antifungal agent, for the prevention of recurrent yeast infections in women who are not of reproductive potential.
Oteseconazole inhibits CYP51, an enzyme fungi require to preserve the integrity of their cell walls and to grow properly, according to Mycovia, the drug’s manufacturer. It is the first FDA-approved product for the treatment of recurrent vulvovaginal candidiasis (RVVC).
Recurrent vulvovaginal candidiasis, or chronic yeast infection, affects an estimated 138 million women worldwide annually. The condition is defined as three or more symptomatic acute episodes of yeast infection within a 12-month period. The primary symptoms of RVVC include vaginal itching, burning, irritation, and inflammation. Some patients may also experience abnormal vaginal discharge and pain during sex or urination.
“A medicine with Vivjoa’s sustained efficacy combined with the clinical safety profile has been long needed, as until now, physicians and their patients have had no FDA-approved medications for RVVC,” Stephen Brand, PhD, chief development officer of Mycovia, said in a statement. “We are excited to be the first to offer a medication designed specifically for RVVC, a challenging and chronic condition that is expected to increase in prevalence over the next decade.”
Approval for oteseconazole was based on results of three phase 3 trials involving 875 patients at 232 sites across 11 countries. In the U.S.-only ultraVIOLET trial, 89.7% of women with RVVC who received oteseconazole cleared their initial yeast infection and did not experience a recurrence during the 50-week maintenance period, compared with 57.1% of those who received fluconazole (Diflucan) followed by placebo (P < .001), according to Mycovia.
The most common side effects reported in phase 3 clinical studies were headache (7.4%) and nausea (3.6%), the company said. Patients with a hypersensitivity to oteseconazole should not take the drug, nor should those who are of reproductive potential, pregnant, or lactating.
Mycovia said it plans to launch the drug in the second quarter of 2022.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved oteseconazole capsules (Vivjoa), an azole antifungal agent, for the prevention of recurrent yeast infections in women who are not of reproductive potential.
Oteseconazole inhibits CYP51, an enzyme fungi require to preserve the integrity of their cell walls and to grow properly, according to Mycovia, the drug’s manufacturer. It is the first FDA-approved product for the treatment of recurrent vulvovaginal candidiasis (RVVC).
Recurrent vulvovaginal candidiasis, or chronic yeast infection, affects an estimated 138 million women worldwide annually. The condition is defined as three or more symptomatic acute episodes of yeast infection within a 12-month period. The primary symptoms of RVVC include vaginal itching, burning, irritation, and inflammation. Some patients may also experience abnormal vaginal discharge and pain during sex or urination.
“A medicine with Vivjoa’s sustained efficacy combined with the clinical safety profile has been long needed, as until now, physicians and their patients have had no FDA-approved medications for RVVC,” Stephen Brand, PhD, chief development officer of Mycovia, said in a statement. “We are excited to be the first to offer a medication designed specifically for RVVC, a challenging and chronic condition that is expected to increase in prevalence over the next decade.”
Approval for oteseconazole was based on results of three phase 3 trials involving 875 patients at 232 sites across 11 countries. In the U.S.-only ultraVIOLET trial, 89.7% of women with RVVC who received oteseconazole cleared their initial yeast infection and did not experience a recurrence during the 50-week maintenance period, compared with 57.1% of those who received fluconazole (Diflucan) followed by placebo (P < .001), according to Mycovia.
The most common side effects reported in phase 3 clinical studies were headache (7.4%) and nausea (3.6%), the company said. Patients with a hypersensitivity to oteseconazole should not take the drug, nor should those who are of reproductive potential, pregnant, or lactating.
Mycovia said it plans to launch the drug in the second quarter of 2022.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
Childhood abuse may increase risk of MS in women
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
, according to the first prospective cohort study of its kind.
More research is needed to uncover underlying mechanisms of action, according to lead author Karine Eid, MD, a PhD candidate at Haukeland University Hospital, Bergen, Norway, and colleagues.
“Trauma and stressful life events have been associated with an increased risk of autoimmune disorders,” the investigators wrote in the Journal Of Neurology, Neurosurgery, & Psychiatry. “Whether adverse events in childhood can have an impact on MS susceptibility is not known.”
The present study recruited participants from the Norwegian Mother, Father and Child cohort, a population consisting of Norwegian women who were pregnant from 1999 to 2008. Of the 77,997 participating women, 14,477 reported emotional, sexual, and/or physical abuse in childhood, while the remaining 63,520 women reported no abuse. After a mean follow-up of 13 years, 300 women were diagnosed with MS, among whom 24% reported a history of childhood abuse, compared with 19% among women who did not develop MS.
To look for associations between childhood abuse and risk of MS, the investigators used a Cox model adjusted for confounders and mediators, including smoking, obesity, adult socioeconomic factors, and childhood social status. The model revealed that emotional abuse increased the risk of MS by 40% (hazard ratio [HR] 1.40; 95% confidence interval [CI], 1.03-1.90), and sexual abuse increased the risk of MS by 65% (HR 1.65; 95% CI, 1.13-2.39).
Although physical abuse alone did not significantly increase risk of MS (HR 1.31; 95% CI, 0.83-2.06), it did contribute to a dose-response relationship when women were exposed to more than one type of childhood abuse. Women exposed to two out of three abuse categories had a 66% increased risk of MS (HR 1.66; 95% CI, 1.04-2.67), whereas women exposed to all three types of abuse had the highest risk of MS, at 93% (HR 1.93; 95% CI, 1.02-3.67).
Dr. Eid and colleagues noted that their findings are supported by previous retrospective research, and discussed possible mechanisms of action.
“The increased risk of MS after exposure to childhood sexual and emotional abuse may have a biological explanation,” they wrote. “Childhood abuse can cause dysregulation of the hypothalamic-pituitary-adrenal axis, lead to oxidative stress, and induce a proinflammatory state decades into adulthood. Psychological stress has been shown to disrupt the blood-brain barrier and cause epigenetic changes that may increase the risk of neurodegenerative disorders, including MS.
“The underlying mechanisms behind this association should be investigated further,” they concluded.
Study findings should guide interventions
Commenting on the research, Ruth Ann Marrie, MD, PhD, professor of medicine and community health sciences and director of the multiple sclerosis clinic at Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, said that the present study “has several strengths compared to prior studies – including that it is prospective and the sample size.”
Dr. Marrie, who was not involved in the study, advised clinicians in the field to take note of the findings, as patients with a history of abuse may need unique interventions.
“Providers need to recognize the higher prevalence of childhood maltreatment in people with MS,” Dr. Marrie said in an interview. “These findings dovetail with others that suggest that adverse childhood experiences are associated with increased mental health concerns and pain catastrophizing in people with MS. Affected individuals may benefit from additional psychological supports and trauma-informed care.”
Tiffany Joy Braley, MD, associate professor of neurology, and Carri Polick, RN and PhD candidate at the school of nursing, University of Michigan, Ann Arbor, who published a case report last year highlighting the importance of evaluating stress exposure in MS, suggested that the findings should guide interventions at both a system and patient level.
“Although a cause-and-effect relationship cannot be established by the current study, these and related findings should be considered in the context of system level and policy interventions that address links between environment and health care disparities,” they said in a joint, written comment. “Given recent impetus to provide trauma-informed health care, these data could be particularly informative in neurological conditions which are associated with high mental health comorbidity. Traumatic stress screening practices could lead to referrals for appropriate support services and more personalized health care.”
While several mechanisms have been proposed to explain the link between traumatic stress and MS, more work is needed in this area, they added.
This knowledge gap was acknowledged by Dr. Marrie.
“Our understanding of the etiology of MS remains incomplete,” Dr. Marrie said. “We still need a better understanding of mechanisms by which adverse childhood experiences lead to MS, how they interact with other risk factors for MS (beyond smoking and obesity), and whether there are any interventions that can mitigate the risk of developing MS that is associated with adverse childhood experiences.”
The investigators disclosed relationships with Novartis, Biogen, Merck, and others. Dr. Marrie receives research support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, MS Society of Canada, the Consortium of Multiple Sclerosis Centers, Crohn’s and Colitis Canada, Research Manitoba, and the Arthritis Society; she has no pharmaceutical support. Dr. Braley and Ms. Polick reported no conflicts of interest.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY, & PSYCHIATRY
Tebipenem pivoxil hydrobromide offers oral option for complex UTIs
“No new oral antibiotic alternative has emerged to treat these conditions in more than 25 years,” corresponding author Angela K. Talley, MD, said in an interview. The new research was published in the New England Journal of Medicine.
Patients with complicated urinary tract infection (cUTI), including acute pyelonephritis (AP), are often hospitalized and treated with intravenous therapy because of the lack of oral options, especially in cases of antibiotic-resistant pathogens, explained Dr. Talley, of Spero Therapeutics.
In their new phase 3, double-blind randomized trial, the researchers evaluated the safety and effectiveness of oral TBP-PI-HBr, compared with intravenous ertapenem in hospitalized patients with cUTIs or AP. Oral tebipenem is an investigational carbapenem with demonstrated activity against uropathogenic Enterobacterales, and it has shown effectiveness in animal models, the researchers noted in their paper.
Methods and results
The researchers randomized 1,372 adult patients. The microbiologic intent-to-treat population included 449 patients who received TBP-PI-HBr (600 mg every 8 hours) and 419 who received ertapenem (1 g every 24 hours) for 7-10 days or up to 14 days for patients with bacteremia.
The primary endpoint was a composite of clinical cure and favorable microbiologic response, assessed at a test-of-cure visit on day 19. Clinical cure was defined as “complete resolution or clinically significant alleviation of baseline signs and symptoms of complicated urinary tract infection or acute pyelonephritis and no new symptoms, such that no further antimicrobial therapy was warranted,” the researchers wrote. Microbiologic response was defined as a reduction to less than 103 CFU per milliliter in uropathogen levels from baseline at day 19.
Overall, the clinical response occurred in 58.8% of patients who received TBP-PI-HBr and 61.6% of those who received ertapenem at the test-of-cure visit.
Clinical cure rates were similar in the TBP-PI-HBr and ertapenem groups (93.1% vs. 93.6%) at the test-of-cure visit.
Both treatment groups showed similar responses to Enterobacterales pathogens at the test-of-cure visit (62.7% for TBP-PI-HBr and 65.2% for ertapenem).
Among patients with bacteremia at baseline, overall response rates were 72.3% and 66.0% for TBP-PI-HBr and ertapenem, respectively, at the test-of-cure visit, and 93.6% and 96.2%, respectively, at the end-of-treatment visit on or around day 25.
The overall incidence of adverse events was approximately 26% in both treatment groups. Most adverse events were mild or moderate in severity and did not limit treatment, the researchers wrote.
The mean age of the patients was 58.1 years; 46.1% were aged 65 and older, and 11.5% had bacteremia at baseline.
The study findings were limited by several factors, including the mandated 7- to 10-day course of antibiotics, which may not reflect the standard of care in other settings in the United States. The study’s trial sites were located in the United States, South Africa, and Europe. The study population was primarily White and from Central and Eastern Europe. Other limitations included the randomization of patients before confirming the baseline pathogen, although this was done to limit potential confounding from previous antibiotics, the researchers noted.
Safety and efficacy support application for approval
“To our knowledge, this is the first head-to-head evaluation of an IV vs. an oral drug for the treatment of cUTI and acute pyelonephritis,” Dr. Talley said in an interview.
“The findings demonstrate that almost all patients in the study achieved complete resolution of the signs and symptoms of their infection,” she said.
TBP-PI-HBr has not been approved by the Food and Drug Administration, but a new drug application that included data from the current study was submitted to the FDA and is currently under review, Dr. Talley noted.
As for additional research, the current study was conducted in hospitalized patients, and the use of TBP-PI-HBr in the outpatient setting has not yet been evaluated, she said.
Approval and use of oral carbapenem will change practice
The current study is very important because it provides a viable and effective alternative form of antibiotic delivery for the patients with complicated UTI, Noel N. Deep, MD, emphasized in an interview.
“Currently these patients have to be treated with IV carbapenem antibiotics either in a hospital or through a home health nurse,” Dr. Deep, a general internist in group practice in Antigo, Wisc., explained.
Current IV strategies also carry the inherent risk associated with the insertion of an IV catheter that is left in place for several days or replaced periodically. “The oral antibiotic eliminates these risks and higher health care costs and provides a safer and equally efficacious option,” Dr. Deep said.
In the current study, “I was definitely surprised at the effectiveness of the oral carbapenem,” Dr. Deep said. “I am absolutely delighted with this new treatment option that physicians can now add to their armamentarium [assuming FDA approval] as we provide care to our patients,” he said.
If approved, TBP-PI-HBr will definitely change the treatment spectrum for the multidrug-resistant bacterial UTIs, said Dr. Deep. “Carbapenems have continued to be effective and low antibiotic resistance to carbapenems has been recorded.”
As for additional research, “I would like to see studies done in other ethnicities and different countries to ascertain the effectiveness of this antibiotic in those populations and against other bacterial strains with potentially different resistance mechanisms,” Dr. Deep said.
The study was supported by Spero Therapeutics and the Department of Health and Human Services. Lead author Paul B. Eckburg, MD, of Stanford (Calif.) University, and Dr. Talley are employees of Spero Therapeutics. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
“No new oral antibiotic alternative has emerged to treat these conditions in more than 25 years,” corresponding author Angela K. Talley, MD, said in an interview. The new research was published in the New England Journal of Medicine.
Patients with complicated urinary tract infection (cUTI), including acute pyelonephritis (AP), are often hospitalized and treated with intravenous therapy because of the lack of oral options, especially in cases of antibiotic-resistant pathogens, explained Dr. Talley, of Spero Therapeutics.
In their new phase 3, double-blind randomized trial, the researchers evaluated the safety and effectiveness of oral TBP-PI-HBr, compared with intravenous ertapenem in hospitalized patients with cUTIs or AP. Oral tebipenem is an investigational carbapenem with demonstrated activity against uropathogenic Enterobacterales, and it has shown effectiveness in animal models, the researchers noted in their paper.
Methods and results
The researchers randomized 1,372 adult patients. The microbiologic intent-to-treat population included 449 patients who received TBP-PI-HBr (600 mg every 8 hours) and 419 who received ertapenem (1 g every 24 hours) for 7-10 days or up to 14 days for patients with bacteremia.
The primary endpoint was a composite of clinical cure and favorable microbiologic response, assessed at a test-of-cure visit on day 19. Clinical cure was defined as “complete resolution or clinically significant alleviation of baseline signs and symptoms of complicated urinary tract infection or acute pyelonephritis and no new symptoms, such that no further antimicrobial therapy was warranted,” the researchers wrote. Microbiologic response was defined as a reduction to less than 103 CFU per milliliter in uropathogen levels from baseline at day 19.
Overall, the clinical response occurred in 58.8% of patients who received TBP-PI-HBr and 61.6% of those who received ertapenem at the test-of-cure visit.
Clinical cure rates were similar in the TBP-PI-HBr and ertapenem groups (93.1% vs. 93.6%) at the test-of-cure visit.
Both treatment groups showed similar responses to Enterobacterales pathogens at the test-of-cure visit (62.7% for TBP-PI-HBr and 65.2% for ertapenem).
Among patients with bacteremia at baseline, overall response rates were 72.3% and 66.0% for TBP-PI-HBr and ertapenem, respectively, at the test-of-cure visit, and 93.6% and 96.2%, respectively, at the end-of-treatment visit on or around day 25.
The overall incidence of adverse events was approximately 26% in both treatment groups. Most adverse events were mild or moderate in severity and did not limit treatment, the researchers wrote.
The mean age of the patients was 58.1 years; 46.1% were aged 65 and older, and 11.5% had bacteremia at baseline.
The study findings were limited by several factors, including the mandated 7- to 10-day course of antibiotics, which may not reflect the standard of care in other settings in the United States. The study’s trial sites were located in the United States, South Africa, and Europe. The study population was primarily White and from Central and Eastern Europe. Other limitations included the randomization of patients before confirming the baseline pathogen, although this was done to limit potential confounding from previous antibiotics, the researchers noted.
Safety and efficacy support application for approval
“To our knowledge, this is the first head-to-head evaluation of an IV vs. an oral drug for the treatment of cUTI and acute pyelonephritis,” Dr. Talley said in an interview.
“The findings demonstrate that almost all patients in the study achieved complete resolution of the signs and symptoms of their infection,” she said.
TBP-PI-HBr has not been approved by the Food and Drug Administration, but a new drug application that included data from the current study was submitted to the FDA and is currently under review, Dr. Talley noted.
As for additional research, the current study was conducted in hospitalized patients, and the use of TBP-PI-HBr in the outpatient setting has not yet been evaluated, she said.
Approval and use of oral carbapenem will change practice
The current study is very important because it provides a viable and effective alternative form of antibiotic delivery for the patients with complicated UTI, Noel N. Deep, MD, emphasized in an interview.
“Currently these patients have to be treated with IV carbapenem antibiotics either in a hospital or through a home health nurse,” Dr. Deep, a general internist in group practice in Antigo, Wisc., explained.
Current IV strategies also carry the inherent risk associated with the insertion of an IV catheter that is left in place for several days or replaced periodically. “The oral antibiotic eliminates these risks and higher health care costs and provides a safer and equally efficacious option,” Dr. Deep said.
In the current study, “I was definitely surprised at the effectiveness of the oral carbapenem,” Dr. Deep said. “I am absolutely delighted with this new treatment option that physicians can now add to their armamentarium [assuming FDA approval] as we provide care to our patients,” he said.
If approved, TBP-PI-HBr will definitely change the treatment spectrum for the multidrug-resistant bacterial UTIs, said Dr. Deep. “Carbapenems have continued to be effective and low antibiotic resistance to carbapenems has been recorded.”
As for additional research, “I would like to see studies done in other ethnicities and different countries to ascertain the effectiveness of this antibiotic in those populations and against other bacterial strains with potentially different resistance mechanisms,” Dr. Deep said.
The study was supported by Spero Therapeutics and the Department of Health and Human Services. Lead author Paul B. Eckburg, MD, of Stanford (Calif.) University, and Dr. Talley are employees of Spero Therapeutics. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
“No new oral antibiotic alternative has emerged to treat these conditions in more than 25 years,” corresponding author Angela K. Talley, MD, said in an interview. The new research was published in the New England Journal of Medicine.
Patients with complicated urinary tract infection (cUTI), including acute pyelonephritis (AP), are often hospitalized and treated with intravenous therapy because of the lack of oral options, especially in cases of antibiotic-resistant pathogens, explained Dr. Talley, of Spero Therapeutics.
In their new phase 3, double-blind randomized trial, the researchers evaluated the safety and effectiveness of oral TBP-PI-HBr, compared with intravenous ertapenem in hospitalized patients with cUTIs or AP. Oral tebipenem is an investigational carbapenem with demonstrated activity against uropathogenic Enterobacterales, and it has shown effectiveness in animal models, the researchers noted in their paper.
Methods and results
The researchers randomized 1,372 adult patients. The microbiologic intent-to-treat population included 449 patients who received TBP-PI-HBr (600 mg every 8 hours) and 419 who received ertapenem (1 g every 24 hours) for 7-10 days or up to 14 days for patients with bacteremia.
The primary endpoint was a composite of clinical cure and favorable microbiologic response, assessed at a test-of-cure visit on day 19. Clinical cure was defined as “complete resolution or clinically significant alleviation of baseline signs and symptoms of complicated urinary tract infection or acute pyelonephritis and no new symptoms, such that no further antimicrobial therapy was warranted,” the researchers wrote. Microbiologic response was defined as a reduction to less than 103 CFU per milliliter in uropathogen levels from baseline at day 19.
Overall, the clinical response occurred in 58.8% of patients who received TBP-PI-HBr and 61.6% of those who received ertapenem at the test-of-cure visit.
Clinical cure rates were similar in the TBP-PI-HBr and ertapenem groups (93.1% vs. 93.6%) at the test-of-cure visit.
Both treatment groups showed similar responses to Enterobacterales pathogens at the test-of-cure visit (62.7% for TBP-PI-HBr and 65.2% for ertapenem).
Among patients with bacteremia at baseline, overall response rates were 72.3% and 66.0% for TBP-PI-HBr and ertapenem, respectively, at the test-of-cure visit, and 93.6% and 96.2%, respectively, at the end-of-treatment visit on or around day 25.
The overall incidence of adverse events was approximately 26% in both treatment groups. Most adverse events were mild or moderate in severity and did not limit treatment, the researchers wrote.
The mean age of the patients was 58.1 years; 46.1% were aged 65 and older, and 11.5% had bacteremia at baseline.
The study findings were limited by several factors, including the mandated 7- to 10-day course of antibiotics, which may not reflect the standard of care in other settings in the United States. The study’s trial sites were located in the United States, South Africa, and Europe. The study population was primarily White and from Central and Eastern Europe. Other limitations included the randomization of patients before confirming the baseline pathogen, although this was done to limit potential confounding from previous antibiotics, the researchers noted.
Safety and efficacy support application for approval
“To our knowledge, this is the first head-to-head evaluation of an IV vs. an oral drug for the treatment of cUTI and acute pyelonephritis,” Dr. Talley said in an interview.
“The findings demonstrate that almost all patients in the study achieved complete resolution of the signs and symptoms of their infection,” she said.
TBP-PI-HBr has not been approved by the Food and Drug Administration, but a new drug application that included data from the current study was submitted to the FDA and is currently under review, Dr. Talley noted.
As for additional research, the current study was conducted in hospitalized patients, and the use of TBP-PI-HBr in the outpatient setting has not yet been evaluated, she said.
Approval and use of oral carbapenem will change practice
The current study is very important because it provides a viable and effective alternative form of antibiotic delivery for the patients with complicated UTI, Noel N. Deep, MD, emphasized in an interview.
“Currently these patients have to be treated with IV carbapenem antibiotics either in a hospital or through a home health nurse,” Dr. Deep, a general internist in group practice in Antigo, Wisc., explained.
Current IV strategies also carry the inherent risk associated with the insertion of an IV catheter that is left in place for several days or replaced periodically. “The oral antibiotic eliminates these risks and higher health care costs and provides a safer and equally efficacious option,” Dr. Deep said.
In the current study, “I was definitely surprised at the effectiveness of the oral carbapenem,” Dr. Deep said. “I am absolutely delighted with this new treatment option that physicians can now add to their armamentarium [assuming FDA approval] as we provide care to our patients,” he said.
If approved, TBP-PI-HBr will definitely change the treatment spectrum for the multidrug-resistant bacterial UTIs, said Dr. Deep. “Carbapenems have continued to be effective and low antibiotic resistance to carbapenems has been recorded.”
As for additional research, “I would like to see studies done in other ethnicities and different countries to ascertain the effectiveness of this antibiotic in those populations and against other bacterial strains with potentially different resistance mechanisms,” Dr. Deep said.
The study was supported by Spero Therapeutics and the Department of Health and Human Services. Lead author Paul B. Eckburg, MD, of Stanford (Calif.) University, and Dr. Talley are employees of Spero Therapeutics. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE