User login
Adenomyosis: While a last resort, surgery remains an option
Adenomyosis causing severe dysmenorrhea, dyspareunia, and heavy menstrual bleeding has been thought to affect primarily multiparous women in their mid- to late 40s. Often women who experience pain and heavy bleeding will tolerate their symptoms until they are done with childbearing, at which point they often go on to have a hysterectomy to relieve them of these symptoms. Tissue histology obtained at the time of hysterectomy confirms the diagnosis of adenomyosis.
Because the diagnosis is made at the time of hysterectomy, the published incidence and prevalence of adenomyosis is more a reflection of a risk for hysterectomy and not for the disease itself. MRI has been used to evaluate the junctional zone in patients with symptoms of endometriosis. This screen tool is an expensive one, however, and has not been used extensively to evaluate women with symptoms of adenomyosis who are not candidates for a hysterectomy.
Ultrasound studies
Over the past 5-7 years, numerous studies have been performed that demonstrate ultrasound changes consistent with adenomyosis within the uterus. These changes include asymmetry and heterogeneity of the anterior and posterior myometrium, cystic lesions in the myometrium, ultrasound striations, and streaking and irregular junctional zone thickening seen on 3-D scans.
Our newfound ability to demonstrate changes consistent with adenomyosis by ultrasound – a tool that is much less expensive than MRI and more available to patients – means that we can and should consider adenomyosis in patients suffering from dysmenorrhea, heavy menstrual bleeding, back pain, dyspareunia, and infertility – regardless of the patient’s age.
In the last 5 years, adenomyosis has been increasingly recognized as a disorder affecting women of all reproductive ages, including teenagers whose dysmenorrhea disrupts their education and young women undergoing infertility evaluations. In one study, 12% of adolescent girls and young women aged 14–20 years lost days of school or work each month because of dysmenorrhea.1 This disruption is not “normal.”
Several meta-analyses have also demonstrated that ultrasound and MRI changes consistent with adenomyosis can affect embryo implantation rates in women undergoing in vitro fertilization. The implantation rates can be as low as one half the expected rate without adenomyosis. Additionally, adenomyosis has been shown to increase the risk of miscarriage and preterm delivery.2,3
The clinicians who order and carefully look at the ultrasound themselves, rather than rely on the radiologist to make the diagnosis, will be able to see the changes consistent with adenomyosis. Over time – I anticipate the next several years – a standardized radiologic definition for adenomyosis will evolve, and radiologists will become more familiar with these changes. In the meantime, our patients should not have missed diagnoses.
Considerations for surgery
For the majority of younger patients who are not trying to conceive but want to maintain their fertility, medical treatment with oral contraceptives, progestins, or the levonorgestrel-releasing intrauterine device (Mirena) will relieve symptoms. The Mirena IUD has been found in studies of 6-36 months’ treatment duration to decrease the size of the uterus by 25%4 and improve dysmenorrhea and menorrhagia with a low profile of adverse effects in most women.
The Mirena IUD should be considered as a first-line therapy for all women with heavy menstrual bleeding and dyspareunia who want to preserve their fertility.
Patients who do not respond to or cannot tolerate medical therapy, and do not want to preserve their fertility, may consider hysterectomy, long regarded as the preferred method of treatment. Endometrial ablation can also be considered in those who no longer desire to preserve fertility and are experiencing heavy menstrual bleeding. Those with extensive adenomyosis, however, often experience poor results with endometrial ablation and may ultimately require hysterectomy. Endometrial ablation has a history of a high failure rate in women younger than 45 years old.
Patients with adenomyosis who wish to preserve their fertility and cannot tolerate or are unresponsive to hormonal therapy, or those with infertility thought to be caused by adenomyosis, should consider these three management options:
- Do nothing. The embryo implantation rate is not zero with adenomyosis, and we have no data on the number of patients who conceive with adenomyotic changes detected by MRI or ultrasound.
- Pretreat with a GnRH agonist for 2-3 months prior to a frozen embryo transfer (FET). Suppressing the disease prior to an FET seems to increase the implantation rate to what is expected for that patient given her age and other fertility factors.3 While this approach is often successful, an estimated 15%-20% of patients are unable to tolerate GnRH agonist treatment because of its side effects.
- Seek surgical resection of adenomyosis. Unlike uterine fibroids, adenomyosis has no pseudocapsule. When resecting the disease via laparotomy, laparoscopy, or hysteroscopy, the process is more of a debulking procedure. Surgical resection should be reserved for those who cannot tolerate hormonal suppression or have failed the other two options.
Surgical approaches
Surgical excision can be challenging because adenomyosis burrows its way through the muscle, is often diffuse, and cannot necessarily be resected with clean margins as can a fibroid. Yet, as demonstrated in a systematic review of 27 observational studies of conservative surgery for adenomyosis – 10 prospective and 17 retrospective studies with a total of almost 1,400 patients and all with adenomyosis confirmed histopathologically – surgery can improve pain, menorrhagia, and adenomyosis-related infertility in a significant number of cases.5
Disease may be resected through laparotomy, laparoscopy, or as we are currently doing with focal disease that is close to the endometrium, hysteroscopy. The type of surgery will depend on the location and characteristics of the disease, and on the surgeon’s skills. The principles are the same with all three approaches: to remove as much diseased tissue – and preserve as much healthy myometrial tissue – as possible and to reconstruct the uterine wall so that it maintains its integrity and can sustain a pregnancy.
The open approach known as the Osada procedure, after Hisao Osada, MD, PhD, in Tokyo, is well described in the literature, with a relatively large number of cases reported in prospective studies. Dr. Osada performs a radical adenomyosis excision with a triple flap method of uterine wall reconstruction. The uterus is bisected in the mid-sagittal plane all the way down through the adenomyosis until the uterine cavity is reached. Excision of the adenomyotic tissue is guided by palpation with the index finger, and a myometrial thickness of 1 cm from the serosa and the endometrium is preserved.
The endometrium is closed, and the myometrial defect is closed with a triple flap method that avoids overlapping suture lines. On one side of the uterus, the myometrium and serosa are sutured in the antero-posterior plane. The seromuscular layer of the opposite side of the uterine wall is then brought over the first seromuscular suture line.6
Others, such as Grigoris H. Grimbizis, MD, PhD, in Greece, have used a laparoscopic approach and closed the myometrium in layers similar to those of a myomectomy.7 There are no comparative trials that demonstrate one technique is superior to the other.
While there are no textbook techniques published for resecting adenomyotic tissue laparoscopically or hysteroscopically from the normal myometrium, there are some general principals the surgeon should keep in mind. Adenomyosis is defined as the presence of endometrial glands and stroma within myometrium, but biopsy studies have demonstrated that there are relatively few glands and stroma within the diseased tissue. Mostly, the adenomyotic tissue we encounter comprises smooth muscle hyperplasia and fibrosis.
Since there is no pseudocapsule surrounding adenomyotic tissue, the visual cue for the cytoreductive procedure is the presence of normal-appearing myometrium. The normal myometrium can be delineated by palpation with laparoscopic instruments or hysteroscopic loops as it clearly feels less fibrotic and firm than the adenomyotic tissue. For this reason, the adenomyotic tissue is removed in a piecemeal fashion until normal tissue is encountered. (This same philosophy can be applied to removing fibrotic, glandular, or cystic tissue hysteroscopically.)
If the disease involves the inner myometrium, it should resected as this may be very important to restoring normal uterine contractions needed for embryo implantation and development, even if it means entering the cavity laparoscopically.
Hysteroscopically, there is no ability to suture a myometrial defect. This limitation is concerning because the adenomyosis is thought to invade the myometrium and not displace it as seen with monoclonal uterine fibroids. There are no case reports of uterine rupture after hysteroscopic resection of adenomyosis, but the number of cases reported with this type of resection in general is very small.
Laparoscopically, the myometrial defect should be repaired similarly to a myomectomy defect. Chromic or polydioxanone (PDS) suture is appropriate. We have used 2-0 PDS V-loc and a 2-3 layer closure in our laparoscopic cases.
Diffuse adenomyosis can involve the entire anterior or posterior wall of the uterus or both. The surgeon should not attempt to remove all of the disease in this situation and must leave enough tissue, even diseased, to allow for structural integrity during pregnancy. Uterine rupture has not been reported in all published case series and studies, but overall, it is a concern with surgical excision of adenomyosis. An analysis of over 2,000 cases of adenomyomectomies reported worldwide since 1990 shows a uterine rupture rate in the 6% rate, with a pregnancy rate ranging from 7%-72%.8
When the disease is focal and close to the endometrium, as opposed to diffuse and affecting the entire back wall of the uterus, hysteroscopic excision may be an appropriate, less invasive approach.
One of the patients for whom we’ve taken this approach was a 37-year-old patient who presented with a history of six miscarriages, a negative work-up for recurrent pregnancy loss, an enlarged uterus, 8 years of heavy menstrual bleeding, and only mild dysmenorrhea. She had undergone in vitro fertilization with failed embryo transfers but normal genetic screens of the embryos. She was referred with a suspicion of fibroids. An MRI and ultrasound showed heterogeneous myometrium adjacent to the endometrium. This tissue was resected using a bipolar loop electrode until normal myometrium was encountered.
Hysteroscopic resections are currently described in the literature through case reports rather than larger prospective or retrospective studies, and much more research is needed to demonstrate the efficacy and safety of this approach.
At this point in time, while surgery to excise adenomyosis is a last resort and best methods are deliberated, it is still important to appreciate that surgery is an option. Continued infertility is not the only choice, nor is hysterectomy.
References
1. J Pediatr Adolesc Gynecol 2014;27:258-65.
2. Minerva Ginecol. 2018 Jun;70(3):295-302.
3. Fertil Steril. 2017;108(3):483-490.e3.
4. Am J Obstet Gynecol. 2008;198(4):373.e1-7.
5. J. Minim Invasive Gynecol. 2018 Feb;25:265-76.
6. Reproductive BioMed Online. 2011 Jan;22(1):94-9.
7. Fertil Steril. 2014 Feb;101(2):472-87.
8. Fertil Steril. 2018 Mar;109(3):406-17.
Adenomyosis causing severe dysmenorrhea, dyspareunia, and heavy menstrual bleeding has been thought to affect primarily multiparous women in their mid- to late 40s. Often women who experience pain and heavy bleeding will tolerate their symptoms until they are done with childbearing, at which point they often go on to have a hysterectomy to relieve them of these symptoms. Tissue histology obtained at the time of hysterectomy confirms the diagnosis of adenomyosis.
Because the diagnosis is made at the time of hysterectomy, the published incidence and prevalence of adenomyosis is more a reflection of a risk for hysterectomy and not for the disease itself. MRI has been used to evaluate the junctional zone in patients with symptoms of endometriosis. This screen tool is an expensive one, however, and has not been used extensively to evaluate women with symptoms of adenomyosis who are not candidates for a hysterectomy.
Ultrasound studies
Over the past 5-7 years, numerous studies have been performed that demonstrate ultrasound changes consistent with adenomyosis within the uterus. These changes include asymmetry and heterogeneity of the anterior and posterior myometrium, cystic lesions in the myometrium, ultrasound striations, and streaking and irregular junctional zone thickening seen on 3-D scans.
Our newfound ability to demonstrate changes consistent with adenomyosis by ultrasound – a tool that is much less expensive than MRI and more available to patients – means that we can and should consider adenomyosis in patients suffering from dysmenorrhea, heavy menstrual bleeding, back pain, dyspareunia, and infertility – regardless of the patient’s age.
In the last 5 years, adenomyosis has been increasingly recognized as a disorder affecting women of all reproductive ages, including teenagers whose dysmenorrhea disrupts their education and young women undergoing infertility evaluations. In one study, 12% of adolescent girls and young women aged 14–20 years lost days of school or work each month because of dysmenorrhea.1 This disruption is not “normal.”
Several meta-analyses have also demonstrated that ultrasound and MRI changes consistent with adenomyosis can affect embryo implantation rates in women undergoing in vitro fertilization. The implantation rates can be as low as one half the expected rate without adenomyosis. Additionally, adenomyosis has been shown to increase the risk of miscarriage and preterm delivery.2,3
The clinicians who order and carefully look at the ultrasound themselves, rather than rely on the radiologist to make the diagnosis, will be able to see the changes consistent with adenomyosis. Over time – I anticipate the next several years – a standardized radiologic definition for adenomyosis will evolve, and radiologists will become more familiar with these changes. In the meantime, our patients should not have missed diagnoses.
Considerations for surgery
For the majority of younger patients who are not trying to conceive but want to maintain their fertility, medical treatment with oral contraceptives, progestins, or the levonorgestrel-releasing intrauterine device (Mirena) will relieve symptoms. The Mirena IUD has been found in studies of 6-36 months’ treatment duration to decrease the size of the uterus by 25%4 and improve dysmenorrhea and menorrhagia with a low profile of adverse effects in most women.
The Mirena IUD should be considered as a first-line therapy for all women with heavy menstrual bleeding and dyspareunia who want to preserve their fertility.
Patients who do not respond to or cannot tolerate medical therapy, and do not want to preserve their fertility, may consider hysterectomy, long regarded as the preferred method of treatment. Endometrial ablation can also be considered in those who no longer desire to preserve fertility and are experiencing heavy menstrual bleeding. Those with extensive adenomyosis, however, often experience poor results with endometrial ablation and may ultimately require hysterectomy. Endometrial ablation has a history of a high failure rate in women younger than 45 years old.
Patients with adenomyosis who wish to preserve their fertility and cannot tolerate or are unresponsive to hormonal therapy, or those with infertility thought to be caused by adenomyosis, should consider these three management options:
- Do nothing. The embryo implantation rate is not zero with adenomyosis, and we have no data on the number of patients who conceive with adenomyotic changes detected by MRI or ultrasound.
- Pretreat with a GnRH agonist for 2-3 months prior to a frozen embryo transfer (FET). Suppressing the disease prior to an FET seems to increase the implantation rate to what is expected for that patient given her age and other fertility factors.3 While this approach is often successful, an estimated 15%-20% of patients are unable to tolerate GnRH agonist treatment because of its side effects.
- Seek surgical resection of adenomyosis. Unlike uterine fibroids, adenomyosis has no pseudocapsule. When resecting the disease via laparotomy, laparoscopy, or hysteroscopy, the process is more of a debulking procedure. Surgical resection should be reserved for those who cannot tolerate hormonal suppression or have failed the other two options.
Surgical approaches
Surgical excision can be challenging because adenomyosis burrows its way through the muscle, is often diffuse, and cannot necessarily be resected with clean margins as can a fibroid. Yet, as demonstrated in a systematic review of 27 observational studies of conservative surgery for adenomyosis – 10 prospective and 17 retrospective studies with a total of almost 1,400 patients and all with adenomyosis confirmed histopathologically – surgery can improve pain, menorrhagia, and adenomyosis-related infertility in a significant number of cases.5
Disease may be resected through laparotomy, laparoscopy, or as we are currently doing with focal disease that is close to the endometrium, hysteroscopy. The type of surgery will depend on the location and characteristics of the disease, and on the surgeon’s skills. The principles are the same with all three approaches: to remove as much diseased tissue – and preserve as much healthy myometrial tissue – as possible and to reconstruct the uterine wall so that it maintains its integrity and can sustain a pregnancy.
The open approach known as the Osada procedure, after Hisao Osada, MD, PhD, in Tokyo, is well described in the literature, with a relatively large number of cases reported in prospective studies. Dr. Osada performs a radical adenomyosis excision with a triple flap method of uterine wall reconstruction. The uterus is bisected in the mid-sagittal plane all the way down through the adenomyosis until the uterine cavity is reached. Excision of the adenomyotic tissue is guided by palpation with the index finger, and a myometrial thickness of 1 cm from the serosa and the endometrium is preserved.
The endometrium is closed, and the myometrial defect is closed with a triple flap method that avoids overlapping suture lines. On one side of the uterus, the myometrium and serosa are sutured in the antero-posterior plane. The seromuscular layer of the opposite side of the uterine wall is then brought over the first seromuscular suture line.6
Others, such as Grigoris H. Grimbizis, MD, PhD, in Greece, have used a laparoscopic approach and closed the myometrium in layers similar to those of a myomectomy.7 There are no comparative trials that demonstrate one technique is superior to the other.
While there are no textbook techniques published for resecting adenomyotic tissue laparoscopically or hysteroscopically from the normal myometrium, there are some general principals the surgeon should keep in mind. Adenomyosis is defined as the presence of endometrial glands and stroma within myometrium, but biopsy studies have demonstrated that there are relatively few glands and stroma within the diseased tissue. Mostly, the adenomyotic tissue we encounter comprises smooth muscle hyperplasia and fibrosis.
Since there is no pseudocapsule surrounding adenomyotic tissue, the visual cue for the cytoreductive procedure is the presence of normal-appearing myometrium. The normal myometrium can be delineated by palpation with laparoscopic instruments or hysteroscopic loops as it clearly feels less fibrotic and firm than the adenomyotic tissue. For this reason, the adenomyotic tissue is removed in a piecemeal fashion until normal tissue is encountered. (This same philosophy can be applied to removing fibrotic, glandular, or cystic tissue hysteroscopically.)
If the disease involves the inner myometrium, it should resected as this may be very important to restoring normal uterine contractions needed for embryo implantation and development, even if it means entering the cavity laparoscopically.
Hysteroscopically, there is no ability to suture a myometrial defect. This limitation is concerning because the adenomyosis is thought to invade the myometrium and not displace it as seen with monoclonal uterine fibroids. There are no case reports of uterine rupture after hysteroscopic resection of adenomyosis, but the number of cases reported with this type of resection in general is very small.
Laparoscopically, the myometrial defect should be repaired similarly to a myomectomy defect. Chromic or polydioxanone (PDS) suture is appropriate. We have used 2-0 PDS V-loc and a 2-3 layer closure in our laparoscopic cases.
Diffuse adenomyosis can involve the entire anterior or posterior wall of the uterus or both. The surgeon should not attempt to remove all of the disease in this situation and must leave enough tissue, even diseased, to allow for structural integrity during pregnancy. Uterine rupture has not been reported in all published case series and studies, but overall, it is a concern with surgical excision of adenomyosis. An analysis of over 2,000 cases of adenomyomectomies reported worldwide since 1990 shows a uterine rupture rate in the 6% rate, with a pregnancy rate ranging from 7%-72%.8
When the disease is focal and close to the endometrium, as opposed to diffuse and affecting the entire back wall of the uterus, hysteroscopic excision may be an appropriate, less invasive approach.
One of the patients for whom we’ve taken this approach was a 37-year-old patient who presented with a history of six miscarriages, a negative work-up for recurrent pregnancy loss, an enlarged uterus, 8 years of heavy menstrual bleeding, and only mild dysmenorrhea. She had undergone in vitro fertilization with failed embryo transfers but normal genetic screens of the embryos. She was referred with a suspicion of fibroids. An MRI and ultrasound showed heterogeneous myometrium adjacent to the endometrium. This tissue was resected using a bipolar loop electrode until normal myometrium was encountered.
Hysteroscopic resections are currently described in the literature through case reports rather than larger prospective or retrospective studies, and much more research is needed to demonstrate the efficacy and safety of this approach.
At this point in time, while surgery to excise adenomyosis is a last resort and best methods are deliberated, it is still important to appreciate that surgery is an option. Continued infertility is not the only choice, nor is hysterectomy.
References
1. J Pediatr Adolesc Gynecol 2014;27:258-65.
2. Minerva Ginecol. 2018 Jun;70(3):295-302.
3. Fertil Steril. 2017;108(3):483-490.e3.
4. Am J Obstet Gynecol. 2008;198(4):373.e1-7.
5. J. Minim Invasive Gynecol. 2018 Feb;25:265-76.
6. Reproductive BioMed Online. 2011 Jan;22(1):94-9.
7. Fertil Steril. 2014 Feb;101(2):472-87.
8. Fertil Steril. 2018 Mar;109(3):406-17.
Adenomyosis causing severe dysmenorrhea, dyspareunia, and heavy menstrual bleeding has been thought to affect primarily multiparous women in their mid- to late 40s. Often women who experience pain and heavy bleeding will tolerate their symptoms until they are done with childbearing, at which point they often go on to have a hysterectomy to relieve them of these symptoms. Tissue histology obtained at the time of hysterectomy confirms the diagnosis of adenomyosis.
Because the diagnosis is made at the time of hysterectomy, the published incidence and prevalence of adenomyosis is more a reflection of a risk for hysterectomy and not for the disease itself. MRI has been used to evaluate the junctional zone in patients with symptoms of endometriosis. This screen tool is an expensive one, however, and has not been used extensively to evaluate women with symptoms of adenomyosis who are not candidates for a hysterectomy.
Ultrasound studies
Over the past 5-7 years, numerous studies have been performed that demonstrate ultrasound changes consistent with adenomyosis within the uterus. These changes include asymmetry and heterogeneity of the anterior and posterior myometrium, cystic lesions in the myometrium, ultrasound striations, and streaking and irregular junctional zone thickening seen on 3-D scans.
Our newfound ability to demonstrate changes consistent with adenomyosis by ultrasound – a tool that is much less expensive than MRI and more available to patients – means that we can and should consider adenomyosis in patients suffering from dysmenorrhea, heavy menstrual bleeding, back pain, dyspareunia, and infertility – regardless of the patient’s age.
In the last 5 years, adenomyosis has been increasingly recognized as a disorder affecting women of all reproductive ages, including teenagers whose dysmenorrhea disrupts their education and young women undergoing infertility evaluations. In one study, 12% of adolescent girls and young women aged 14–20 years lost days of school or work each month because of dysmenorrhea.1 This disruption is not “normal.”
Several meta-analyses have also demonstrated that ultrasound and MRI changes consistent with adenomyosis can affect embryo implantation rates in women undergoing in vitro fertilization. The implantation rates can be as low as one half the expected rate without adenomyosis. Additionally, adenomyosis has been shown to increase the risk of miscarriage and preterm delivery.2,3
The clinicians who order and carefully look at the ultrasound themselves, rather than rely on the radiologist to make the diagnosis, will be able to see the changes consistent with adenomyosis. Over time – I anticipate the next several years – a standardized radiologic definition for adenomyosis will evolve, and radiologists will become more familiar with these changes. In the meantime, our patients should not have missed diagnoses.
Considerations for surgery
For the majority of younger patients who are not trying to conceive but want to maintain their fertility, medical treatment with oral contraceptives, progestins, or the levonorgestrel-releasing intrauterine device (Mirena) will relieve symptoms. The Mirena IUD has been found in studies of 6-36 months’ treatment duration to decrease the size of the uterus by 25%4 and improve dysmenorrhea and menorrhagia with a low profile of adverse effects in most women.
The Mirena IUD should be considered as a first-line therapy for all women with heavy menstrual bleeding and dyspareunia who want to preserve their fertility.
Patients who do not respond to or cannot tolerate medical therapy, and do not want to preserve their fertility, may consider hysterectomy, long regarded as the preferred method of treatment. Endometrial ablation can also be considered in those who no longer desire to preserve fertility and are experiencing heavy menstrual bleeding. Those with extensive adenomyosis, however, often experience poor results with endometrial ablation and may ultimately require hysterectomy. Endometrial ablation has a history of a high failure rate in women younger than 45 years old.
Patients with adenomyosis who wish to preserve their fertility and cannot tolerate or are unresponsive to hormonal therapy, or those with infertility thought to be caused by adenomyosis, should consider these three management options:
- Do nothing. The embryo implantation rate is not zero with adenomyosis, and we have no data on the number of patients who conceive with adenomyotic changes detected by MRI or ultrasound.
- Pretreat with a GnRH agonist for 2-3 months prior to a frozen embryo transfer (FET). Suppressing the disease prior to an FET seems to increase the implantation rate to what is expected for that patient given her age and other fertility factors.3 While this approach is often successful, an estimated 15%-20% of patients are unable to tolerate GnRH agonist treatment because of its side effects.
- Seek surgical resection of adenomyosis. Unlike uterine fibroids, adenomyosis has no pseudocapsule. When resecting the disease via laparotomy, laparoscopy, or hysteroscopy, the process is more of a debulking procedure. Surgical resection should be reserved for those who cannot tolerate hormonal suppression or have failed the other two options.
Surgical approaches
Surgical excision can be challenging because adenomyosis burrows its way through the muscle, is often diffuse, and cannot necessarily be resected with clean margins as can a fibroid. Yet, as demonstrated in a systematic review of 27 observational studies of conservative surgery for adenomyosis – 10 prospective and 17 retrospective studies with a total of almost 1,400 patients and all with adenomyosis confirmed histopathologically – surgery can improve pain, menorrhagia, and adenomyosis-related infertility in a significant number of cases.5
Disease may be resected through laparotomy, laparoscopy, or as we are currently doing with focal disease that is close to the endometrium, hysteroscopy. The type of surgery will depend on the location and characteristics of the disease, and on the surgeon’s skills. The principles are the same with all three approaches: to remove as much diseased tissue – and preserve as much healthy myometrial tissue – as possible and to reconstruct the uterine wall so that it maintains its integrity and can sustain a pregnancy.
The open approach known as the Osada procedure, after Hisao Osada, MD, PhD, in Tokyo, is well described in the literature, with a relatively large number of cases reported in prospective studies. Dr. Osada performs a radical adenomyosis excision with a triple flap method of uterine wall reconstruction. The uterus is bisected in the mid-sagittal plane all the way down through the adenomyosis until the uterine cavity is reached. Excision of the adenomyotic tissue is guided by palpation with the index finger, and a myometrial thickness of 1 cm from the serosa and the endometrium is preserved.
The endometrium is closed, and the myometrial defect is closed with a triple flap method that avoids overlapping suture lines. On one side of the uterus, the myometrium and serosa are sutured in the antero-posterior plane. The seromuscular layer of the opposite side of the uterine wall is then brought over the first seromuscular suture line.6
Others, such as Grigoris H. Grimbizis, MD, PhD, in Greece, have used a laparoscopic approach and closed the myometrium in layers similar to those of a myomectomy.7 There are no comparative trials that demonstrate one technique is superior to the other.
While there are no textbook techniques published for resecting adenomyotic tissue laparoscopically or hysteroscopically from the normal myometrium, there are some general principals the surgeon should keep in mind. Adenomyosis is defined as the presence of endometrial glands and stroma within myometrium, but biopsy studies have demonstrated that there are relatively few glands and stroma within the diseased tissue. Mostly, the adenomyotic tissue we encounter comprises smooth muscle hyperplasia and fibrosis.
Since there is no pseudocapsule surrounding adenomyotic tissue, the visual cue for the cytoreductive procedure is the presence of normal-appearing myometrium. The normal myometrium can be delineated by palpation with laparoscopic instruments or hysteroscopic loops as it clearly feels less fibrotic and firm than the adenomyotic tissue. For this reason, the adenomyotic tissue is removed in a piecemeal fashion until normal tissue is encountered. (This same philosophy can be applied to removing fibrotic, glandular, or cystic tissue hysteroscopically.)
If the disease involves the inner myometrium, it should resected as this may be very important to restoring normal uterine contractions needed for embryo implantation and development, even if it means entering the cavity laparoscopically.
Hysteroscopically, there is no ability to suture a myometrial defect. This limitation is concerning because the adenomyosis is thought to invade the myometrium and not displace it as seen with monoclonal uterine fibroids. There are no case reports of uterine rupture after hysteroscopic resection of adenomyosis, but the number of cases reported with this type of resection in general is very small.
Laparoscopically, the myometrial defect should be repaired similarly to a myomectomy defect. Chromic or polydioxanone (PDS) suture is appropriate. We have used 2-0 PDS V-loc and a 2-3 layer closure in our laparoscopic cases.
Diffuse adenomyosis can involve the entire anterior or posterior wall of the uterus or both. The surgeon should not attempt to remove all of the disease in this situation and must leave enough tissue, even diseased, to allow for structural integrity during pregnancy. Uterine rupture has not been reported in all published case series and studies, but overall, it is a concern with surgical excision of adenomyosis. An analysis of over 2,000 cases of adenomyomectomies reported worldwide since 1990 shows a uterine rupture rate in the 6% rate, with a pregnancy rate ranging from 7%-72%.8
When the disease is focal and close to the endometrium, as opposed to diffuse and affecting the entire back wall of the uterus, hysteroscopic excision may be an appropriate, less invasive approach.
One of the patients for whom we’ve taken this approach was a 37-year-old patient who presented with a history of six miscarriages, a negative work-up for recurrent pregnancy loss, an enlarged uterus, 8 years of heavy menstrual bleeding, and only mild dysmenorrhea. She had undergone in vitro fertilization with failed embryo transfers but normal genetic screens of the embryos. She was referred with a suspicion of fibroids. An MRI and ultrasound showed heterogeneous myometrium adjacent to the endometrium. This tissue was resected using a bipolar loop electrode until normal myometrium was encountered.
Hysteroscopic resections are currently described in the literature through case reports rather than larger prospective or retrospective studies, and much more research is needed to demonstrate the efficacy and safety of this approach.
At this point in time, while surgery to excise adenomyosis is a last resort and best methods are deliberated, it is still important to appreciate that surgery is an option. Continued infertility is not the only choice, nor is hysterectomy.
References
1. J Pediatr Adolesc Gynecol 2014;27:258-65.
2. Minerva Ginecol. 2018 Jun;70(3):295-302.
3. Fertil Steril. 2017;108(3):483-490.e3.
4. Am J Obstet Gynecol. 2008;198(4):373.e1-7.
5. J. Minim Invasive Gynecol. 2018 Feb;25:265-76.
6. Reproductive BioMed Online. 2011 Jan;22(1):94-9.
7. Fertil Steril. 2014 Feb;101(2):472-87.
8. Fertil Steril. 2018 Mar;109(3):406-17.
Menstrual irregularity appears to be predictor of early death
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
FROM THE BMJ
POP surgeries not tied to decreased sexual functioning
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.
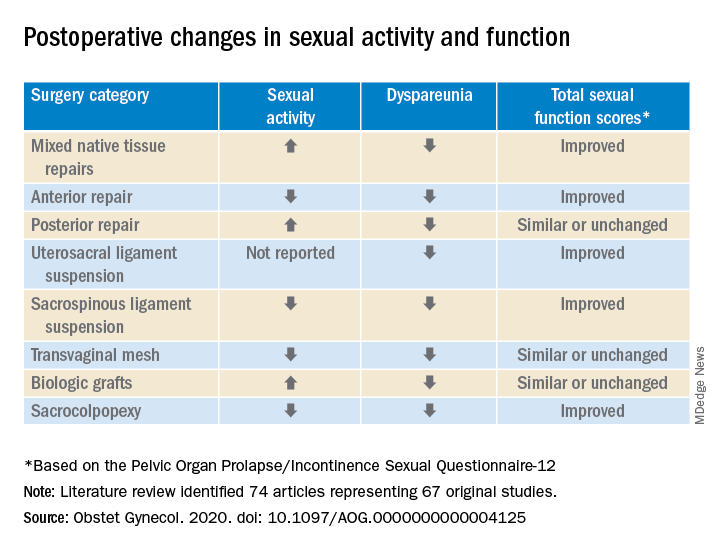
In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.

In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
Danielle D. Antosh, MD, of the Houston Methodist Hospital and colleagues reported in a systematic review of prospective comparative studies on pelvic organ prolapse surgery, which was published in Obstetrics & Gynecology.

In a preliminary search of 3,124 citations, Dr. Antosh and her colleagues, who are members of the Society of Gynecologic Surgeons Systematic Review Group responsible for the study, identified and accepted 74 articles representing 67 original studies. Ten of these were ancillary studies with different reported outcomes or follow-up times, and 44 were randomized control trials. They compared the pre- and postoperative effects of pelvic organ prolapse (POP) surgery on sexual function for changes in sexual activity and function across eight different prolapse surgery categories: mixed native tissue repairs, anterior repair, posterior repair, uterosacral ligament suspension, sacrospinous ligament suspension, transvaginal mesh, biologic grafts, and sacrocolpopexy. In only three categories – posterior repair, transvaginal mesh, and biological grafts – postoperative changes in sexual function scores were similar or remained unchanged. In all other categories, total sexual function scores improved. Dyspareunia was lower after surgery for all prolapse surgery types.
“Although sexual function improves in the majority of women, it is important to note that a small proportion of women can develop de novo (new onset) dyspareunia after surgery. The rate of this ranges from 0%-9% for prolapse surgeries. However, there is limited data on posterior repairs,” Dr. Antosh said in a later interview.*
POP surgeries positively impact sexual function, dyspareunia outcomes
The researchers determined that there is “moderate to high quality data” supporting the influence of POP on sexual activity and function. They also observed a lower prevalence of dyspareunia postoperatively across all surgery types, compared with baseline. Additionally, no decrease in sexual function was reported across surgical categories; in fact, sexual activity and function improved or stayed the same after POP surgery in this review.
Across most POP surgery groups, Dr. Antosh and colleagues found that with the exception of the sacrospinous ligament suspension, transvaginal mesh, and sacrocolpopexy groups, the rate of postoperative sexual activity was modestly higher. Several studies attributed this finding to a lack of partner or partner-related issues. Another 16 studies (7.7%) cited pain as the primary factor for postoperative sexual inactivity.
Few studies included in the review “reported both preoperative and postoperative rates of sexual activity and dyspareunia, and no study reported patient-level changes in sexual activity or dyspareunia (except occasionally, for de novo activity or dyspareunia),” Dr. Antosh and associates clarified. As a result, they concluded that their findings are based primarily on qualitative comparisons of events reported pre- and postoperatively from different but overlapping sets of studies.
The finding that the prevalence of dyspareunia decreased following all types of POP surgery is consistent with previous reviews. Because the studies did not account for minimally important differences in sexual function scores, it is important to consider this when interpreting results of the review. Dr. Antosh and colleagues also noted that some studies did not define dyspareunia, and those that did frequently used measures that were not validated. They also were unable to identify the persistence of dyspareunia following surgery as this was not recorded in the literature.
Menopausal status and other considerations
Also worth noting, the mean age of women in the studies were postmenopausal, yet the “studies did not stratify sexual function outcomes based on premenopause compared with postmenopause status.”
The researchers advised that future studies using validated definitions of sexual activity, function, and dyspareunia, as well as reporting both their preoperative and postoperative measures would do much to improve the quality of research reported.
It is widely recognized that women with pelvic floor disorders experience a high rate of sexual dysfunction, so the need to achieve optimum outcomes that at least maintain if not improve sexual function postoperatively should be of key concern when planning POP surgery for patients, they cautioned. Previous studies have observed that women experiencing POP rated the need for improved sexual function second only to resolved bulge symptoms and improvement in overall function. The women also classified sexual dysfunction in the same category of adversity as having chronic pain or having to be admitted to an intensive care unit.
Study provides preoperative counseling help
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology and Natasha Abdullah, MD, obstetrics and gynecology resident, both of Einstein Medical Center, Philadelphia, collaboratively commented on the study findings: “This review provides physicians with data to add to the preoperative counseling for patients undergoing pelvic organ prolapse surgery.”
In particular, they noted that, while the article “is a review of previous literature, Table 1 provides an opportunity for physicians to share with their patients the important answers to their concerns surrounding postoperative sexual activity, dyspareunia, and total changes in sexual function scores based on the Pelvic Organ Prolapse/Incontinence Sexual Questionnaire 12 (PISQ-12).”
Noting the clinical usefulness of the questionnaire, they added: “The PISQ-12 is a validated and reliable short form that evaluates sexual function in women with urinary incontinence and/or pelvic organ prolapse and predicts PISQ-31 scores. It was developed from the data of 99 of 182 women surveyed to create the long form (PISQ-31); 46 patients were recruited for further validation. Test-retest reliability was checked with a subset of 20 patients. All subsets regression analysis with R greater than 0.92 identified 12 items that predicted PISQ-31 scores. The PISQ-12 covers three domains of function: Behavioral/Emotive, Physical, and Partner-Related.”
Because ob.gyns. are trained to recognize the “multifactorial reasons (age, partner relationship, other health conditions etc.) that surround sexual activity,” Dr. Jaspan and Dr. Abdullah cautioned against prematurely concluding that the lower sexual activity score is directly related to POP surgery.
“Because sexual function is such an important postsurgical outcome for patients, this article provides significant preoperative counseling data for patients on all POP repair options,” they observed. “No surgical option worsens sexual function. The article concludes that individually validated definitions of sexual activity, function, and dyspareunia in a measuring instrument would improve the quality of data for future studies.”
The study authors reported no relevant financial disclosures. Although no direct funding was provided by the Society of Gynecologic Surgeons, they did provide meeting space, oversight, stipends for expert and statistical support, and aid in disseminating findings. Dr. Abdullah and Dr. Jaspan had no relevant financial disclosures.
SOURCE: Antosh DD et al. Obstet Gynecol. 2020. doi: 10.1097/AOG.0000000000004125.
*This article was updated on 10/28.
FROM OBSTETRICS & GYNECOLOGY
PCOS tied to risk for cardiovascular disease after menopause
Women with polycystic ovarian syndrome (PCOS) before menopause appear to have a greater risk of stroke, heart attack, and other cardiovascular events after menopause, according to findings presented at the virtual American Society for Reproductive Medicine (ASRM) 2020 Scientific Congress.
“We found a PCOS diagnosis prior to menopause was associated with a 64% increased risk of cardiovascular disease after menopause independent of age at enrollment, race, body mass index, and smoking status,” presenter Jacob Christ, MD, a resident at the University of Washington in Seattle, told attendees. “Taken together, our results suggest that women with PCOS have more risk factors for future cardiovascular disease at baseline, and a present PCOS diagnosis prior to menopause is associated with an increased risk of cardiovascular disease after menopause.”
The results are important to consider in women seeking care related to fertility, according to Amanda N. Kallen, MD, assistant professor of reproductive endocrinology and infertility at Yale Medicine in New Haven, Conn.
“As fertility specialists, we often see women with PCOS visit us when they are having trouble conceiving, but often [they] do not return to our care once they’ve built their family,” said Dr. Kallen, who was not involved in the research.
“This excellent talk emphasized how critical it is for us as reproductive endocrinologists to have ongoing discussions with PCOS patients about long-term cardiovascular risks at every opportunity, and to emphasize that these risks persist long after the reproductive years have ended,” Dr. Kallen said in an interview.
Identifying women at higher risk
Characteristics of PCOS in adolescence are already understood, including hyperandrogenism, acne, irregular bleeding, and variable ages of menarche, Dr. Christ explained. Similarly, in women’s reproductive years, PCOS is linked to abnormal uterine bleeding, hirsutism, dyslipidemia, infertility, impaired glucose tolerance, gestational diabetes, and preeclampsia.
“What is less clear is if baseline cardiometabolic dysfunction during reproductive years translates into cardiovascular disease after menopause,” Dr. Christ said. “Menopausal changes may reduce risk of cardiovascular disease among PCOS women, as it is known that overall, androgen levels decline during menopause. Furthermore, ovarian aging may be delayed in PCOS women, which may be protective against cardiovascular disease.”
To learn more, the researchers completed a secondary analysis of data from the Study of Women’s Health Across the Nation (SWAN), a prospective cohort study. Women enrolled in the study were aged 42-52 years at baseline, had a uterus and at least one ovary, and menstruated within the previous 3 months. Women were considered to have PCOS if they had both biochemical hyperandrogenism and a history of irregular menses.
The researchers included participants in the secondary analysis if they had complete data on the women’s baseline menstrual status and total testosterone and if the women had at least one follow-up visit after menopause. Menopause was approximated as 51 years old if not otherwise reported (or 1 year after study entry if age 51 at entry). At the follow-up visit, women self-reported any cardiovascular disease events since menopause.
The study’s primary outcome was the first postmenopausal cardiovascular event. These included any of the following: myocardial infarction, cerebrovascular accident or stroke, angina, percutaneous coronary intervention or angioplasty, coronary artery bypass graft, heart failure, carotid artery procedure, peripheral artery disease or lower extremity procedure, renal artery procedure, deep vein thrombosis, pulmonary embolism, and abdominal aortic aneurysm.
Among 1,340 women included in the analysis, 174 (13%) women had PCOS and 1,166 did not. The average age at screening and at menopause were not significantly different between the groups, but they did differ based on other baseline characteristics.
More women with PCOS frequently smoked cigarettes (22%) vs. those without PCOS (12.7%), and women with PCOS had an average body mass index of 31.3, vs. 26.7 among those without PCOS. Women with PCOS also had higher systolic blood pressure (120.7 vs. 115.8 mm Hg), higher total cholesterol (202 vs. 192 mg/dL), and higher fasting blood glucose (103.7 vs. 89.2 mg/dL; P < .01 for all).
After the researchers controlled for age at enrollment, race, BMI, and smoking status, women with PCOS had 1.6 times greater odds of a cardiovascular event after menopause compared with women without PCOS (odds ratio [OR], 1.6; P = .029). Those with PCOS also had a significantly higher Atherosclerotic Cardiovascular Disease risk scores (P = .024), but their Framingham 10-year risk score was not significantly different from those without PCOS.
Although the findings are not necessarily surprising, the study’s value particularly lay in its size, prospective data collection, and rigorous methods, said Ginny Ryan, MD, MA, professor and division chief of reproductive endocrinology and infertility at the University of Washington in Seattle.
“While this study’s criteria used to identify subjects with PCOS selected a population with a particularly severe phenotype of PCOS and thus a higher risk population for cardiovascular disease, it is vital for women’s health providers to truly understand the medium- and long-term life-threatening associations found more commonly in many with PCOS,” Dr. Ryan, who attended the talk and was not involved in the research, said in an interview.
“This study emphasizes the importance of identifying PCOS before menopause, not just for the patient’s immediate well-being, but also so that appropriate counseling and referral can take place to optimize primary, secondary, and tertiary prevention efforts against CVD and related morbidity and mortality,” Dr. Ryan said. “Providers who focus on reproductive health and reproductive-aged women have the opportunity to play a vital role in optimizing the long-term health of their patients.”
Aside from being a prospective cohort with more than 2 decades of follow-up, the study’s other strengths included the definition of PCOS before menopause and use of multiple markers of postmenopausal cardiovascular disease, Dr. Christ said. The study’s main weaknesses were the exclusion of mild PCOS, the self-reporting of cardiovascular events, and the multiple ways of defining menopause.
Dr. Kallen is a consultant for Gynaesight and a reviewer for Healthline. Dr. Christ and Dr. Ryan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with polycystic ovarian syndrome (PCOS) before menopause appear to have a greater risk of stroke, heart attack, and other cardiovascular events after menopause, according to findings presented at the virtual American Society for Reproductive Medicine (ASRM) 2020 Scientific Congress.
“We found a PCOS diagnosis prior to menopause was associated with a 64% increased risk of cardiovascular disease after menopause independent of age at enrollment, race, body mass index, and smoking status,” presenter Jacob Christ, MD, a resident at the University of Washington in Seattle, told attendees. “Taken together, our results suggest that women with PCOS have more risk factors for future cardiovascular disease at baseline, and a present PCOS diagnosis prior to menopause is associated with an increased risk of cardiovascular disease after menopause.”
The results are important to consider in women seeking care related to fertility, according to Amanda N. Kallen, MD, assistant professor of reproductive endocrinology and infertility at Yale Medicine in New Haven, Conn.
“As fertility specialists, we often see women with PCOS visit us when they are having trouble conceiving, but often [they] do not return to our care once they’ve built their family,” said Dr. Kallen, who was not involved in the research.
“This excellent talk emphasized how critical it is for us as reproductive endocrinologists to have ongoing discussions with PCOS patients about long-term cardiovascular risks at every opportunity, and to emphasize that these risks persist long after the reproductive years have ended,” Dr. Kallen said in an interview.
Identifying women at higher risk
Characteristics of PCOS in adolescence are already understood, including hyperandrogenism, acne, irregular bleeding, and variable ages of menarche, Dr. Christ explained. Similarly, in women’s reproductive years, PCOS is linked to abnormal uterine bleeding, hirsutism, dyslipidemia, infertility, impaired glucose tolerance, gestational diabetes, and preeclampsia.
“What is less clear is if baseline cardiometabolic dysfunction during reproductive years translates into cardiovascular disease after menopause,” Dr. Christ said. “Menopausal changes may reduce risk of cardiovascular disease among PCOS women, as it is known that overall, androgen levels decline during menopause. Furthermore, ovarian aging may be delayed in PCOS women, which may be protective against cardiovascular disease.”
To learn more, the researchers completed a secondary analysis of data from the Study of Women’s Health Across the Nation (SWAN), a prospective cohort study. Women enrolled in the study were aged 42-52 years at baseline, had a uterus and at least one ovary, and menstruated within the previous 3 months. Women were considered to have PCOS if they had both biochemical hyperandrogenism and a history of irregular menses.
The researchers included participants in the secondary analysis if they had complete data on the women’s baseline menstrual status and total testosterone and if the women had at least one follow-up visit after menopause. Menopause was approximated as 51 years old if not otherwise reported (or 1 year after study entry if age 51 at entry). At the follow-up visit, women self-reported any cardiovascular disease events since menopause.
The study’s primary outcome was the first postmenopausal cardiovascular event. These included any of the following: myocardial infarction, cerebrovascular accident or stroke, angina, percutaneous coronary intervention or angioplasty, coronary artery bypass graft, heart failure, carotid artery procedure, peripheral artery disease or lower extremity procedure, renal artery procedure, deep vein thrombosis, pulmonary embolism, and abdominal aortic aneurysm.
Among 1,340 women included in the analysis, 174 (13%) women had PCOS and 1,166 did not. The average age at screening and at menopause were not significantly different between the groups, but they did differ based on other baseline characteristics.
More women with PCOS frequently smoked cigarettes (22%) vs. those without PCOS (12.7%), and women with PCOS had an average body mass index of 31.3, vs. 26.7 among those without PCOS. Women with PCOS also had higher systolic blood pressure (120.7 vs. 115.8 mm Hg), higher total cholesterol (202 vs. 192 mg/dL), and higher fasting blood glucose (103.7 vs. 89.2 mg/dL; P < .01 for all).
After the researchers controlled for age at enrollment, race, BMI, and smoking status, women with PCOS had 1.6 times greater odds of a cardiovascular event after menopause compared with women without PCOS (odds ratio [OR], 1.6; P = .029). Those with PCOS also had a significantly higher Atherosclerotic Cardiovascular Disease risk scores (P = .024), but their Framingham 10-year risk score was not significantly different from those without PCOS.
Although the findings are not necessarily surprising, the study’s value particularly lay in its size, prospective data collection, and rigorous methods, said Ginny Ryan, MD, MA, professor and division chief of reproductive endocrinology and infertility at the University of Washington in Seattle.
“While this study’s criteria used to identify subjects with PCOS selected a population with a particularly severe phenotype of PCOS and thus a higher risk population for cardiovascular disease, it is vital for women’s health providers to truly understand the medium- and long-term life-threatening associations found more commonly in many with PCOS,” Dr. Ryan, who attended the talk and was not involved in the research, said in an interview.
“This study emphasizes the importance of identifying PCOS before menopause, not just for the patient’s immediate well-being, but also so that appropriate counseling and referral can take place to optimize primary, secondary, and tertiary prevention efforts against CVD and related morbidity and mortality,” Dr. Ryan said. “Providers who focus on reproductive health and reproductive-aged women have the opportunity to play a vital role in optimizing the long-term health of their patients.”
Aside from being a prospective cohort with more than 2 decades of follow-up, the study’s other strengths included the definition of PCOS before menopause and use of multiple markers of postmenopausal cardiovascular disease, Dr. Christ said. The study’s main weaknesses were the exclusion of mild PCOS, the self-reporting of cardiovascular events, and the multiple ways of defining menopause.
Dr. Kallen is a consultant for Gynaesight and a reviewer for Healthline. Dr. Christ and Dr. Ryan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with polycystic ovarian syndrome (PCOS) before menopause appear to have a greater risk of stroke, heart attack, and other cardiovascular events after menopause, according to findings presented at the virtual American Society for Reproductive Medicine (ASRM) 2020 Scientific Congress.
“We found a PCOS diagnosis prior to menopause was associated with a 64% increased risk of cardiovascular disease after menopause independent of age at enrollment, race, body mass index, and smoking status,” presenter Jacob Christ, MD, a resident at the University of Washington in Seattle, told attendees. “Taken together, our results suggest that women with PCOS have more risk factors for future cardiovascular disease at baseline, and a present PCOS diagnosis prior to menopause is associated with an increased risk of cardiovascular disease after menopause.”
The results are important to consider in women seeking care related to fertility, according to Amanda N. Kallen, MD, assistant professor of reproductive endocrinology and infertility at Yale Medicine in New Haven, Conn.
“As fertility specialists, we often see women with PCOS visit us when they are having trouble conceiving, but often [they] do not return to our care once they’ve built their family,” said Dr. Kallen, who was not involved in the research.
“This excellent talk emphasized how critical it is for us as reproductive endocrinologists to have ongoing discussions with PCOS patients about long-term cardiovascular risks at every opportunity, and to emphasize that these risks persist long after the reproductive years have ended,” Dr. Kallen said in an interview.
Identifying women at higher risk
Characteristics of PCOS in adolescence are already understood, including hyperandrogenism, acne, irregular bleeding, and variable ages of menarche, Dr. Christ explained. Similarly, in women’s reproductive years, PCOS is linked to abnormal uterine bleeding, hirsutism, dyslipidemia, infertility, impaired glucose tolerance, gestational diabetes, and preeclampsia.
“What is less clear is if baseline cardiometabolic dysfunction during reproductive years translates into cardiovascular disease after menopause,” Dr. Christ said. “Menopausal changes may reduce risk of cardiovascular disease among PCOS women, as it is known that overall, androgen levels decline during menopause. Furthermore, ovarian aging may be delayed in PCOS women, which may be protective against cardiovascular disease.”
To learn more, the researchers completed a secondary analysis of data from the Study of Women’s Health Across the Nation (SWAN), a prospective cohort study. Women enrolled in the study were aged 42-52 years at baseline, had a uterus and at least one ovary, and menstruated within the previous 3 months. Women were considered to have PCOS if they had both biochemical hyperandrogenism and a history of irregular menses.
The researchers included participants in the secondary analysis if they had complete data on the women’s baseline menstrual status and total testosterone and if the women had at least one follow-up visit after menopause. Menopause was approximated as 51 years old if not otherwise reported (or 1 year after study entry if age 51 at entry). At the follow-up visit, women self-reported any cardiovascular disease events since menopause.
The study’s primary outcome was the first postmenopausal cardiovascular event. These included any of the following: myocardial infarction, cerebrovascular accident or stroke, angina, percutaneous coronary intervention or angioplasty, coronary artery bypass graft, heart failure, carotid artery procedure, peripheral artery disease or lower extremity procedure, renal artery procedure, deep vein thrombosis, pulmonary embolism, and abdominal aortic aneurysm.
Among 1,340 women included in the analysis, 174 (13%) women had PCOS and 1,166 did not. The average age at screening and at menopause were not significantly different between the groups, but they did differ based on other baseline characteristics.
More women with PCOS frequently smoked cigarettes (22%) vs. those without PCOS (12.7%), and women with PCOS had an average body mass index of 31.3, vs. 26.7 among those without PCOS. Women with PCOS also had higher systolic blood pressure (120.7 vs. 115.8 mm Hg), higher total cholesterol (202 vs. 192 mg/dL), and higher fasting blood glucose (103.7 vs. 89.2 mg/dL; P < .01 for all).
After the researchers controlled for age at enrollment, race, BMI, and smoking status, women with PCOS had 1.6 times greater odds of a cardiovascular event after menopause compared with women without PCOS (odds ratio [OR], 1.6; P = .029). Those with PCOS also had a significantly higher Atherosclerotic Cardiovascular Disease risk scores (P = .024), but their Framingham 10-year risk score was not significantly different from those without PCOS.
Although the findings are not necessarily surprising, the study’s value particularly lay in its size, prospective data collection, and rigorous methods, said Ginny Ryan, MD, MA, professor and division chief of reproductive endocrinology and infertility at the University of Washington in Seattle.
“While this study’s criteria used to identify subjects with PCOS selected a population with a particularly severe phenotype of PCOS and thus a higher risk population for cardiovascular disease, it is vital for women’s health providers to truly understand the medium- and long-term life-threatening associations found more commonly in many with PCOS,” Dr. Ryan, who attended the talk and was not involved in the research, said in an interview.
“This study emphasizes the importance of identifying PCOS before menopause, not just for the patient’s immediate well-being, but also so that appropriate counseling and referral can take place to optimize primary, secondary, and tertiary prevention efforts against CVD and related morbidity and mortality,” Dr. Ryan said. “Providers who focus on reproductive health and reproductive-aged women have the opportunity to play a vital role in optimizing the long-term health of their patients.”
Aside from being a prospective cohort with more than 2 decades of follow-up, the study’s other strengths included the definition of PCOS before menopause and use of multiple markers of postmenopausal cardiovascular disease, Dr. Christ said. The study’s main weaknesses were the exclusion of mild PCOS, the self-reporting of cardiovascular events, and the multiple ways of defining menopause.
Dr. Kallen is a consultant for Gynaesight and a reviewer for Healthline. Dr. Christ and Dr. Ryan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Relugolix combo effective for uterine fibroids through 1 year
A combination therapy using the experimental drug relugolix was effective in treating pain and heavy bleeding from uterine fibroids for a full year, according to findings from a long-term extension study of the phase 3, open-label LIBERTY trials.
The drug was also well tolerated, with retention of bone mineral density and no new adverse events, said Ayman Al-Hendy, MD, PhD, who presented the results Oct. 17 at the virtual American Society for Reproductive Medicine 2020 Scientific Congress.
“Relugolix combination therapy represents a potential long-term treatment for women with heavy menstrual bleeding associated with uterine fibroids,” said Al-Hendy, a gynecologist and endoscopic surgeon at the University of Chicago.
Dr. Al-Hendy, who consults for the company that makes the drug, on Oct. 20 presented results showing improvement in quality of life with relugolix therapy.
“The fact that this longer-term study shows continued, persistent results at a year really gives us confidence that we’ll be able to use these drugs as a long-term therapy to treat fibroids,” Hugh S. Taylor, MD, president-elect of ASRM, said in an interview. Dr. Taylor, a professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn., was not involved in the study.
“A drug like this is so necessary,” Dr. Taylor continued. “We don’t have any other drugs on the market approved for long-term use.”
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist under investigation for long-term management of uterine fibroids. The once-daily combination therapy includes 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate.
Extension study shows prolonged benefits
The extension trial enrolled women aged 18-50 years who were experiencing heavy menstrual bleeding from uterine fibroids and who completed the 24-week phase 3, double-blind, placebo-controlled LIBERTY 1 or 2 trials. Heavy menstrual bleeding was defined as bleeding in which at least 80 mL of blood was lost per cycle for two cycles or 160 mL was lost during one cycle. Ultrasound imaging was used to confirm the presence of fibroids.
In LIBERTY 1 and 2, women were randomly assigned to receive relugolix combination therapy, placebo, or relugolix alone for 12 weeks followed by combination therapy for the remaining 12 weeks (delayed group). Those trials found that relugolix combination therapy was effective through 6 months in reducing menstrual blood loss and pain in women with uterine fibroids without loss of bone mineral density.
LIBERTY 3 extended the trial to 52 weeks, with all participants receiving relugolix combination therapy.
As in the earlier trials, the primary endpoint was reduced menstrual blood loss. By the end of the study, women needed to have at least a 50% reduction in blood loss from the initial study’s baseline while maintaining a blood loss of <80 mL. The investigators also evaluated the mean percentage of menstrual blood loss reduction, amenorrhea rate, and improvements in anemia as secondary endpoints and assessed changes in bone mineral density.
The extension study enrolled 78% (n = 477) of the 610 women who completed the initial study; of those, 363 women completed the extension study.
Among the 163 women who began with relugolix combination therapy in the first two trials, 87.7% met the primary endpoint in a per-protocol analysis through week 52. The proportion of responders in the extension study was 75.6% among the group that formerly received placebo (n = 164) and 79.9% in the delayed group (n = 149).
The overall average reduction in menstrual blood volume was 89.9%. Most of the women experienced amenorrhea at the end of the year: 70.6% in the relugolix group, 57.9% in the group that formerly received placebo, and 68.5% in the delayed group.
Reductions in uterine volume and uterine fibroid volume were also sustained from week 24 to week 52. For the relugolix combination therapy group, the mean loss of uterine fibroid volume from baseline was 13.5% at week 24 and 18.3% at week 52. Similarly, the delayed group’s average loss in fibroid volume was 28.1% at week 24 and 33.9% at week 52. The placebo group, which only had a 7% loss in fibroid volume at week 24, had an 18.4% loss in volume from baseline at week 52.
Among patients with anemia, defined as hemoglobin concentrations of <10.5 g/dL at baseline, 59% of those in the original relugolix group saw an improvement of at least 2 g/dL hemoglobin. The women’s improvement in pain symptoms also continued through week 52, with a 51.3-point reduction in scores on the bleeding pain and discomfort scale from baseline to the end of the study.
Adverse events were the same in the extension study and in the initial study. Those most commonly reported were headache and hot flashes. No serious safety signals occurred. The average reduction in bone mineral density was 0.80% at week 52, indicating no concerning loss.
A new drug class to treat uterine fibroids
Relugolix is one of three GnRH antagonists being studied for the long-term treatment of fibroids. The Food and Drug Administration approved the combination of elagolix, estradiol, and norethindrone acetate (Oriahnn) in May. Linzagolix, another GnRH antagonist, is currently in clinical trials.
“We’ll have a whole class of new drugs that are likely to fulfill this long sought-after goal of reducing the need for surgery for fibroids and doing it without a lot of side effects,” Dr. Taylor said. “The quality-of-life improvements seen here, the lack of significant adverse effects – none that were surprising in long term – the relatively low reduction in bone mineral density in a year are all very exciting [and suggest] that this will be a safe and effective long-term treatment.”
Significant improvement in quality of life
In the presentation on quality of life with relugolix therapy, Dr. Al-Hendy shared results regarding the severity of women’s symptoms as well as health-related quality of life, as determined on the basis of the Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire at baseline, week 12, and week 24 in LIBERTY 1 and 2. Higher UFS-QoL scores correlate with more severe symptoms. With the subscale of health-related quality of life, higher scores indicate a better quality of life.
The substudy enrolled 253 patients who received relugolix combination therapy and 256 patients who received placebo. The average menstrual blood loss was 243 mL in the relugolix group and 215 mL in the placebo group at baseline. Mean fibroid volume was the same in both groups at baseline, 73 cm3.
The proportion of Black patients was similar in both groups: 48% of the relugolix group and 54% of the placebo group.
The severity of women’s symptoms dropped from a baseline UFS-QoL score of 57 to 22.4 at 6 months among those who received relugolix combination therapy. In the placebo group, the initial score of 59.6 only dropped to 46.9 (P < .0001, for –21.4 difference in change).
Health-related quality of life increased from 38.3 to 76.6 among those who received relugolix. In the placebo group, it increased from 35.7 to 48.2 (P < .0001, for 24.5 difference). Subscales of health-related quality of life – including concern, control, activities, energy/mood, self-consciousness, and sexual function – also all improved significantly in the relugolix group, compared with the placebo group (P < .0001).
“This is a condition we see all the time that’s easily diagnosed, and we have first-line drugs we’ve been using to treat them, but none are good long-term fixes,” Dr. Taylor said. The current first-line treatments, oral contraceptives, can stabilize bleeding, “but they don’t make the fibroids shrink, they don’t stop the bleeding, women continue to have breakthrough bleeding, and the fibroids can continue to grow.”
He said most of the estimated 600,000 hysterectomies performed in the United States each year are for uterine fibroids.
“It’s a major surgery that no one wants to go through if they don’t have to,” Dr. Taylor said. “Here we have a drug that really has potential to stop the growth of the fibroids, that can stop the bleeding or dramatically improve it, and, really, for the first time, directly impact the fibroids and give us a long-term alternative.”
The studies were funded by Myovant Sciences. Dr. Al-Hendy reported consulting for AbbVie, Bayer, and Myovant Sciences, and he owns a patent for novel diagnostics and therapeutics for uterine sarcoma. Dr. Taylor has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A combination therapy using the experimental drug relugolix was effective in treating pain and heavy bleeding from uterine fibroids for a full year, according to findings from a long-term extension study of the phase 3, open-label LIBERTY trials.
The drug was also well tolerated, with retention of bone mineral density and no new adverse events, said Ayman Al-Hendy, MD, PhD, who presented the results Oct. 17 at the virtual American Society for Reproductive Medicine 2020 Scientific Congress.
“Relugolix combination therapy represents a potential long-term treatment for women with heavy menstrual bleeding associated with uterine fibroids,” said Al-Hendy, a gynecologist and endoscopic surgeon at the University of Chicago.
Dr. Al-Hendy, who consults for the company that makes the drug, on Oct. 20 presented results showing improvement in quality of life with relugolix therapy.
“The fact that this longer-term study shows continued, persistent results at a year really gives us confidence that we’ll be able to use these drugs as a long-term therapy to treat fibroids,” Hugh S. Taylor, MD, president-elect of ASRM, said in an interview. Dr. Taylor, a professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn., was not involved in the study.
“A drug like this is so necessary,” Dr. Taylor continued. “We don’t have any other drugs on the market approved for long-term use.”
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist under investigation for long-term management of uterine fibroids. The once-daily combination therapy includes 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate.
Extension study shows prolonged benefits
The extension trial enrolled women aged 18-50 years who were experiencing heavy menstrual bleeding from uterine fibroids and who completed the 24-week phase 3, double-blind, placebo-controlled LIBERTY 1 or 2 trials. Heavy menstrual bleeding was defined as bleeding in which at least 80 mL of blood was lost per cycle for two cycles or 160 mL was lost during one cycle. Ultrasound imaging was used to confirm the presence of fibroids.
In LIBERTY 1 and 2, women were randomly assigned to receive relugolix combination therapy, placebo, or relugolix alone for 12 weeks followed by combination therapy for the remaining 12 weeks (delayed group). Those trials found that relugolix combination therapy was effective through 6 months in reducing menstrual blood loss and pain in women with uterine fibroids without loss of bone mineral density.
LIBERTY 3 extended the trial to 52 weeks, with all participants receiving relugolix combination therapy.
As in the earlier trials, the primary endpoint was reduced menstrual blood loss. By the end of the study, women needed to have at least a 50% reduction in blood loss from the initial study’s baseline while maintaining a blood loss of <80 mL. The investigators also evaluated the mean percentage of menstrual blood loss reduction, amenorrhea rate, and improvements in anemia as secondary endpoints and assessed changes in bone mineral density.
The extension study enrolled 78% (n = 477) of the 610 women who completed the initial study; of those, 363 women completed the extension study.
Among the 163 women who began with relugolix combination therapy in the first two trials, 87.7% met the primary endpoint in a per-protocol analysis through week 52. The proportion of responders in the extension study was 75.6% among the group that formerly received placebo (n = 164) and 79.9% in the delayed group (n = 149).
The overall average reduction in menstrual blood volume was 89.9%. Most of the women experienced amenorrhea at the end of the year: 70.6% in the relugolix group, 57.9% in the group that formerly received placebo, and 68.5% in the delayed group.
Reductions in uterine volume and uterine fibroid volume were also sustained from week 24 to week 52. For the relugolix combination therapy group, the mean loss of uterine fibroid volume from baseline was 13.5% at week 24 and 18.3% at week 52. Similarly, the delayed group’s average loss in fibroid volume was 28.1% at week 24 and 33.9% at week 52. The placebo group, which only had a 7% loss in fibroid volume at week 24, had an 18.4% loss in volume from baseline at week 52.
Among patients with anemia, defined as hemoglobin concentrations of <10.5 g/dL at baseline, 59% of those in the original relugolix group saw an improvement of at least 2 g/dL hemoglobin. The women’s improvement in pain symptoms also continued through week 52, with a 51.3-point reduction in scores on the bleeding pain and discomfort scale from baseline to the end of the study.
Adverse events were the same in the extension study and in the initial study. Those most commonly reported were headache and hot flashes. No serious safety signals occurred. The average reduction in bone mineral density was 0.80% at week 52, indicating no concerning loss.
A new drug class to treat uterine fibroids
Relugolix is one of three GnRH antagonists being studied for the long-term treatment of fibroids. The Food and Drug Administration approved the combination of elagolix, estradiol, and norethindrone acetate (Oriahnn) in May. Linzagolix, another GnRH antagonist, is currently in clinical trials.
“We’ll have a whole class of new drugs that are likely to fulfill this long sought-after goal of reducing the need for surgery for fibroids and doing it without a lot of side effects,” Dr. Taylor said. “The quality-of-life improvements seen here, the lack of significant adverse effects – none that were surprising in long term – the relatively low reduction in bone mineral density in a year are all very exciting [and suggest] that this will be a safe and effective long-term treatment.”
Significant improvement in quality of life
In the presentation on quality of life with relugolix therapy, Dr. Al-Hendy shared results regarding the severity of women’s symptoms as well as health-related quality of life, as determined on the basis of the Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire at baseline, week 12, and week 24 in LIBERTY 1 and 2. Higher UFS-QoL scores correlate with more severe symptoms. With the subscale of health-related quality of life, higher scores indicate a better quality of life.
The substudy enrolled 253 patients who received relugolix combination therapy and 256 patients who received placebo. The average menstrual blood loss was 243 mL in the relugolix group and 215 mL in the placebo group at baseline. Mean fibroid volume was the same in both groups at baseline, 73 cm3.
The proportion of Black patients was similar in both groups: 48% of the relugolix group and 54% of the placebo group.
The severity of women’s symptoms dropped from a baseline UFS-QoL score of 57 to 22.4 at 6 months among those who received relugolix combination therapy. In the placebo group, the initial score of 59.6 only dropped to 46.9 (P < .0001, for –21.4 difference in change).
Health-related quality of life increased from 38.3 to 76.6 among those who received relugolix. In the placebo group, it increased from 35.7 to 48.2 (P < .0001, for 24.5 difference). Subscales of health-related quality of life – including concern, control, activities, energy/mood, self-consciousness, and sexual function – also all improved significantly in the relugolix group, compared with the placebo group (P < .0001).
“This is a condition we see all the time that’s easily diagnosed, and we have first-line drugs we’ve been using to treat them, but none are good long-term fixes,” Dr. Taylor said. The current first-line treatments, oral contraceptives, can stabilize bleeding, “but they don’t make the fibroids shrink, they don’t stop the bleeding, women continue to have breakthrough bleeding, and the fibroids can continue to grow.”
He said most of the estimated 600,000 hysterectomies performed in the United States each year are for uterine fibroids.
“It’s a major surgery that no one wants to go through if they don’t have to,” Dr. Taylor said. “Here we have a drug that really has potential to stop the growth of the fibroids, that can stop the bleeding or dramatically improve it, and, really, for the first time, directly impact the fibroids and give us a long-term alternative.”
The studies were funded by Myovant Sciences. Dr. Al-Hendy reported consulting for AbbVie, Bayer, and Myovant Sciences, and he owns a patent for novel diagnostics and therapeutics for uterine sarcoma. Dr. Taylor has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A combination therapy using the experimental drug relugolix was effective in treating pain and heavy bleeding from uterine fibroids for a full year, according to findings from a long-term extension study of the phase 3, open-label LIBERTY trials.
The drug was also well tolerated, with retention of bone mineral density and no new adverse events, said Ayman Al-Hendy, MD, PhD, who presented the results Oct. 17 at the virtual American Society for Reproductive Medicine 2020 Scientific Congress.
“Relugolix combination therapy represents a potential long-term treatment for women with heavy menstrual bleeding associated with uterine fibroids,” said Al-Hendy, a gynecologist and endoscopic surgeon at the University of Chicago.
Dr. Al-Hendy, who consults for the company that makes the drug, on Oct. 20 presented results showing improvement in quality of life with relugolix therapy.
“The fact that this longer-term study shows continued, persistent results at a year really gives us confidence that we’ll be able to use these drugs as a long-term therapy to treat fibroids,” Hugh S. Taylor, MD, president-elect of ASRM, said in an interview. Dr. Taylor, a professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn., was not involved in the study.
“A drug like this is so necessary,” Dr. Taylor continued. “We don’t have any other drugs on the market approved for long-term use.”
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist under investigation for long-term management of uterine fibroids. The once-daily combination therapy includes 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate.
Extension study shows prolonged benefits
The extension trial enrolled women aged 18-50 years who were experiencing heavy menstrual bleeding from uterine fibroids and who completed the 24-week phase 3, double-blind, placebo-controlled LIBERTY 1 or 2 trials. Heavy menstrual bleeding was defined as bleeding in which at least 80 mL of blood was lost per cycle for two cycles or 160 mL was lost during one cycle. Ultrasound imaging was used to confirm the presence of fibroids.
In LIBERTY 1 and 2, women were randomly assigned to receive relugolix combination therapy, placebo, or relugolix alone for 12 weeks followed by combination therapy for the remaining 12 weeks (delayed group). Those trials found that relugolix combination therapy was effective through 6 months in reducing menstrual blood loss and pain in women with uterine fibroids without loss of bone mineral density.
LIBERTY 3 extended the trial to 52 weeks, with all participants receiving relugolix combination therapy.
As in the earlier trials, the primary endpoint was reduced menstrual blood loss. By the end of the study, women needed to have at least a 50% reduction in blood loss from the initial study’s baseline while maintaining a blood loss of <80 mL. The investigators also evaluated the mean percentage of menstrual blood loss reduction, amenorrhea rate, and improvements in anemia as secondary endpoints and assessed changes in bone mineral density.
The extension study enrolled 78% (n = 477) of the 610 women who completed the initial study; of those, 363 women completed the extension study.
Among the 163 women who began with relugolix combination therapy in the first two trials, 87.7% met the primary endpoint in a per-protocol analysis through week 52. The proportion of responders in the extension study was 75.6% among the group that formerly received placebo (n = 164) and 79.9% in the delayed group (n = 149).
The overall average reduction in menstrual blood volume was 89.9%. Most of the women experienced amenorrhea at the end of the year: 70.6% in the relugolix group, 57.9% in the group that formerly received placebo, and 68.5% in the delayed group.
Reductions in uterine volume and uterine fibroid volume were also sustained from week 24 to week 52. For the relugolix combination therapy group, the mean loss of uterine fibroid volume from baseline was 13.5% at week 24 and 18.3% at week 52. Similarly, the delayed group’s average loss in fibroid volume was 28.1% at week 24 and 33.9% at week 52. The placebo group, which only had a 7% loss in fibroid volume at week 24, had an 18.4% loss in volume from baseline at week 52.
Among patients with anemia, defined as hemoglobin concentrations of <10.5 g/dL at baseline, 59% of those in the original relugolix group saw an improvement of at least 2 g/dL hemoglobin. The women’s improvement in pain symptoms also continued through week 52, with a 51.3-point reduction in scores on the bleeding pain and discomfort scale from baseline to the end of the study.
Adverse events were the same in the extension study and in the initial study. Those most commonly reported were headache and hot flashes. No serious safety signals occurred. The average reduction in bone mineral density was 0.80% at week 52, indicating no concerning loss.
A new drug class to treat uterine fibroids
Relugolix is one of three GnRH antagonists being studied for the long-term treatment of fibroids. The Food and Drug Administration approved the combination of elagolix, estradiol, and norethindrone acetate (Oriahnn) in May. Linzagolix, another GnRH antagonist, is currently in clinical trials.
“We’ll have a whole class of new drugs that are likely to fulfill this long sought-after goal of reducing the need for surgery for fibroids and doing it without a lot of side effects,” Dr. Taylor said. “The quality-of-life improvements seen here, the lack of significant adverse effects – none that were surprising in long term – the relatively low reduction in bone mineral density in a year are all very exciting [and suggest] that this will be a safe and effective long-term treatment.”
Significant improvement in quality of life
In the presentation on quality of life with relugolix therapy, Dr. Al-Hendy shared results regarding the severity of women’s symptoms as well as health-related quality of life, as determined on the basis of the Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire at baseline, week 12, and week 24 in LIBERTY 1 and 2. Higher UFS-QoL scores correlate with more severe symptoms. With the subscale of health-related quality of life, higher scores indicate a better quality of life.
The substudy enrolled 253 patients who received relugolix combination therapy and 256 patients who received placebo. The average menstrual blood loss was 243 mL in the relugolix group and 215 mL in the placebo group at baseline. Mean fibroid volume was the same in both groups at baseline, 73 cm3.
The proportion of Black patients was similar in both groups: 48% of the relugolix group and 54% of the placebo group.
The severity of women’s symptoms dropped from a baseline UFS-QoL score of 57 to 22.4 at 6 months among those who received relugolix combination therapy. In the placebo group, the initial score of 59.6 only dropped to 46.9 (P < .0001, for –21.4 difference in change).
Health-related quality of life increased from 38.3 to 76.6 among those who received relugolix. In the placebo group, it increased from 35.7 to 48.2 (P < .0001, for 24.5 difference). Subscales of health-related quality of life – including concern, control, activities, energy/mood, self-consciousness, and sexual function – also all improved significantly in the relugolix group, compared with the placebo group (P < .0001).
“This is a condition we see all the time that’s easily diagnosed, and we have first-line drugs we’ve been using to treat them, but none are good long-term fixes,” Dr. Taylor said. The current first-line treatments, oral contraceptives, can stabilize bleeding, “but they don’t make the fibroids shrink, they don’t stop the bleeding, women continue to have breakthrough bleeding, and the fibroids can continue to grow.”
He said most of the estimated 600,000 hysterectomies performed in the United States each year are for uterine fibroids.
“It’s a major surgery that no one wants to go through if they don’t have to,” Dr. Taylor said. “Here we have a drug that really has potential to stop the growth of the fibroids, that can stop the bleeding or dramatically improve it, and, really, for the first time, directly impact the fibroids and give us a long-term alternative.”
The studies were funded by Myovant Sciences. Dr. Al-Hendy reported consulting for AbbVie, Bayer, and Myovant Sciences, and he owns a patent for novel diagnostics and therapeutics for uterine sarcoma. Dr. Taylor has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fetal estrogens show promise for safer therapy for menopause
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Don’t discount ultrapotent topical corticosteroids for vulvar lichen sclerosus
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
according to an expert speaking at the virtual conference on diseases of the vulva and vagina.
If needed, intralesional steroid injections or calcineurin inhibitors can be added to a topical corticosteroid regimen, Libby Edwards, MD, suggested at the meeting, hosted by the International Society for the Study of Vulvovaginal Disease. In addition, early reports indicate that newer interventions such as fractional CO2 laser treatments may help patients with refractory disease.
Still, “there is no question, there is no argument: First-, second- and third-line treatment for lichen sclerosus is an ultrapotent or superpotent topical corticosteroid,” she said. Steroids include halobetasol, clobetasol, or betamethasone dipropionate in augmented vehicle ointment once or twice per day. Patients should continue this regimen until the skin texture is normal or the disease is controlled as well as possible, which usually takes several months, said Dr. Edwards, of Southeast Vulvar Clinic in Charlotte, N.C.
Patients then should continue treatment, but less frequently or with a lower potency steroid.
Although corticosteroids are not Food and Drug Administration–approved for the treatment of lichen sclerosus, double-blind, placebo-controlled trials support their use, Dr. Edwards said.
Getting patients to use topical corticosteroids as directed can be a challenge, however, and patient education is crucial.
After about 10 days, many patients start to feel better and stop the medication prematurely, which may lead to recurrence.
“That is such an important counseling point,” Aruna Venkatesan, MD, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center in San Jose, Calif., said during a panel discussion. “Tell them, listen, I may not see you back for a couple months, and you may start feeling better sooner. But I want you to keep using this at this frequency so that when you come back we can make a good decision about whether you’re ready” for a lower potency regimen.
To encourage daily use, Hope K. Haefner, MD, asks patients whether they brush their teeth every night. “When they say yes, I tell them to put the steroid ointment by their toothpaste and use it after brushing,” Dr. Haefner, the Harold A. Furlong Professor of Women’s Health at Michigan Medicine in Ann Arbor, said during the discussion. “But don’t mix up the tubes.”
Once lichen sclerosus is controlled, options include decreasing the superpotent steroid to once, three times per week or changing to a midpotency steroid such as triamcinolone ointment every day, Dr. Edwards said.
Evidence suggests that controlling lichen sclerosus may prevent squamous cell carcinoma and scarring. In a study of more than 500 patients, about 70% complied with treatment instructions, whereas about 30% were considered partially compliant (JAMA Dermatol. 2015 Oct;151[10]:1061-7.). Patients who adhered to their therapy were less likely to have cancer or ongoing scarring during an average of 4.7 years of follow-up.
Beyond topical steroids
“Almost always, topical steroids are all you need,” said Dr. Edwards. “Before I go beyond that, I think of other issues that may be causing symptoms,” such as atrophic vagina, steroid dermatitis, or vulvodynia.
For patients with refractory lichen sclerosus, other treatments “can add more oomph to your topical steroid, but they are not better,” she said.
Intralesional corticosteroid injections are one option.
Another option is adding a calcineurin inhibitor such as tacrolimus or pimecrolimus, although these medications can burn with application and irritate. In addition, they carry warnings about rare cases of cancer associated with their use.
Dr. Edwards also uses methotrexate, which is supported by case reports and an open-label study. In a recently published study that included 21 patients with vulvar lichen sclerosus and 24 patients with extragenital lichen sclerosus, about half improved after receiving methotrexate (Dermatol Ther. 2020 Apr 29;e13473.).
What about lasers?
Fractional CO2 laser treatments, which are pulsed to minimize damage from heat, have “a lot of providers very excited,” Dr. Edwards said. In one open-label study of 40 patients, most reported a decrease in symptoms. (J Low Genit Tract Dis. 2020 Apr;24[2]:225-8.)
“We’re awaiting blinded, controlled studies,” Dr. Edwards said. “We don’t have those available yet although they are in progress.”
Ten of Dr. Edwards’ patients who did not improve enough with medication have received laser treatments. One patient stopped laser therapy after one treatment. One did not improve. Two were completely cleared, and six had significant improvement.
If patients who improved stopped steroids against recommendations, lichen sclerosus recurred, Dr. Edwards said.
The ISSVD does not recommend laser for the routine treatment of lichen sclerosus because of a lack of adequate studies and long-term safety data and biologic implausibility, Dr. Edwards noted (J Low Genit Tract Dis. 2019 Apr;23[2]:151-60.) Laser treatments for lichen sclerosus should not be used outside of clinical trials or without special arrangements for clinical governance, consent, and audit, according to a consensus document from the society.
“I mostly agree with that,” Dr. Edwards said. “But I now think that this is a reasonable thing to use when other treatments have been exhausted.”
Dr. Edwards and Dr. Venkatesan had no conflicts of interest. Dr. Haefner is an author for UpToDate.
EXPERT ANALYSIS FROM THE ISSVD BIENNIAL CONFERENCE
Endometriosis, surgical approach impact risk of bowel injury in hysterectomy
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
FROM OBSTETRICS & GYNECOLOGY
Work-life balance: How 5 surgeons manage life in and out of the operating room
Patrick J. Culligan, MD:
My impression of our younger colleagues, however, is that many of them are not attracted to the traditional ivory tower research model of academic advancement to which many in previous generations aspired. They seem more concerned with work-life balance as their measure of success rather than the classic metrics of money and prestige. Everyone still needs role models and mentors, though, and that’s where all of you come in. I asked each of you to be on this panel because I admire you for your varying approaches to work-life balance while achieving success as gynecologic surgeons. I thought others in the field might be inspired by hearing your stories.
Cultivating your passions
Kristie Greene, MD: What I have come to learn and appreciate is a really simple point: you do not have to do everything. Determining who you want to be both personally and professionally is step 1.
Granted, answering the question, “Who do I want to be?” is not as simple as it sounds. Many factors figure into the decisions we make in our personal and professional lives. Also, it is not a question we often stop and ask ourselves. From early on, we are placed on an escalator moving up through medical school, residency, fellowship, good job, better job, etc. We are so accustomed to being competitive, to winning, and to wanting to be the best that we sometimes forget to ask ourselves, “What is it exactly that I want, and why? What is my endpoint? And does it make me happy?”
Multitasking is regarded as a talent. As much as we would like to believe that we can do everything at the same time and do it all well, we actually can’t. A friend of mine made me read a book a couple of years ago, called Feeling Good, by David Burns. The book encourages you to consider the different tasks you do in a day and rate how good you are at each of them on a scale of 1 to 10. It then asks you to think about how much enjoyment you derive from each of the tasks and about why you are doing the ones that bring you little to no enjoyment.
I ultimately decided that, for me professionally, the most important thing was my interest in global health. So I decided to do whatever it took to make this happen. But you don’t get something for nothing, and everything comes with sacrifices.
Continue to: Charles Rardin, MD...
Charles Rardin, MD: How exactly did you decide that you were going to focus your career toward pursuing international health? How did you know it was more important? And how did you overcome some of those obstacles?
Dr. Greene: You have to ask the hard question again about what brings you the most joy professionally and personally. That was the easy part of it for me because global health has always been that source of happiness and fulfillment for me. The more challenging parts are the sacrifices and hard choices that come with it. With global health, it can be difficult to balance the demands of a clinical practice.
All of our jobs are a business. I am still struggling with the money part of it. For my husband and I, that meant we had to start small—do what we could afford. But then it blossomed into something that was involving residents, fellows, and med students, which requires far more funding than we had. So I reached out to family. Most of our families donate to different organizations or charities every year, so why not donate to a loved one for something they are passionate about?
At the University of South Florida (USF), we set up a fund, a foundation for global health, which helps support our work abroad as well as the costs associated with involvement of our trainees. Right now, what we have is still small potatoes to a country, but we are making it happen by starting at a small level and growing it.
Beyond the money aspect, traveling abroad means less involvement in meetings, missed opportunities to teach courses that might interest me, and time away from my family. I guess my advice on this whole thing is that you can make things happen if they are important enough to you, and if you are willing to make sacrifices in other areas because you can’t have it all.
Making time for you
Dr. Culligan: So you have found what is important to you, and you have found a way to make it happen. But you are faced with more work; you have given yourself additional work on top of your regular work. How do you make time for a personal life?
Catherine Matthews, MD: In preparing for this discussion, I decided to break down my advice into 3 buckets: The first bucket is discovering and knowing your authentic self. The second is building a community, which I’ll elaborate on. And the third, which we have discussed, is to let go of the money.
Dr. Culligan: I love the concept of the authentic self, but how does that jive with a tendency to strive for perfection? We all think we can do it all. How do we narrow down to what really matters?
Dr. Matthews: We often focus on the things that bring us happiness and what we are good at, but it’s the things that make us unhappy that tend to bring us down. It’s the presence of unhappiness, not the absence of happiness, that seems to be the undoing of many, including myself.
None of us are born with dramatic insight. It is experience that leads to insight. People who are actually present are able to gain insight through observation. A person becomes a better surgeon by observing the outcome of doing a stitch this way versus that; you learn how to do it by seeing what it looks like afterward.
Finding our authentic selves happens in much the same way. Having the presence of mind to ask the right questions, such as, “How am I feeling while I’m doing this?” leads to insights into the true self.
Continue to: It takes a village...
It takes a village
Dr. Greene: Catherine mentioned community earlier, and that is extremely important. The people who surround us can have a huge impact on the way we perceive things, including ourselves. Having a mix of people in our lives—some who practice medicine and others who don’t—helps us stay balanced and answer some of the tough questions. Catherine, for example, has helped me in various stages of my career to ask myself meaningful questions and get real answers.
Dr. Rardin: Part of finding balance is luck, and part of it is making a choice between money and everything else. In considering my first job out of training, I knew that money had the potential to distract me from what was important to me. So I chose a position that was almost entirely salaried so that the decisions I made clinically, surgically, and regarding work-life balance would be less likely to directly impact what was important to me.
Sally Huber, MD: I am still in the “getting there” phase of my life, but one thing I have found is that getting my family involved and excited about what I do has made them much more accepting of when I have longer work days or work to do on the weekend. My spouse has become quite involved with what I have been doing with transgender health in Atlanta. It has been a great bonding experience; she shares my passions, and together we are creating something about which we both can be proud.
When work invades home life
Dr. Culligan: That is great. Sally, I think when we talked, you were just learning about the necessity of mental separation and of not taking your work home with you, which is so hard for all of us with all of our devices.
Dr. Huber: Yes, this year has been about seeing what works best as far as being efficient at work and having quality time at home. At the end of every day I ask myself, “What worked well today? What didn’t work well? What else can I do to maximize time with my family?” I am slowly becoming more efficient, but it has been a challenge. During fellowship, your day is pretty set, but once you are practicing on your own, your hours and responsibilities are completely different, and you have to figure out what works best for you, your values, and your expectations of private life. It takes some time, and I am still figuring it out.
Dr. Culligan: How often would you say that you bring work home? I try hard once I am home to quit working, but sometimes on the weekends I break that rule.
Dr. Matthews: I must say that I do feel like there are certain times when I am better at that than others. Work comes in waves with pressing deadlines. If I averaged it out, probably a third of the time I have some email or some conference call or something that I have got to do at home. I do really try to limit the obligations that I have after 5:30 or 6:00 pm. I resent intrusions after that time. As far as weekends, I delegate about one weekend every 2 months to work, instead of doing a little bit every weekend.
Dr. Greene: I agree. I try hard to make 5:30 to 7:30 pm unequivocal time for a family dinner and time for my kids. During that time, I do not have my phone near me so I can’t look at email or texts. I try not to schedule conference calls. I try to be there to read books to my kids at night. Then if I need to do work, I do it later at night, which interferes with time with my spouse, and is not ideal, but that’s what happens.
Dr. Matthews: One of the things that I think is a huge part of work-life balance is work-related travel. When you are present at work on a consistent basis, the work does not pile up to the extent that it does when you are absent on a trip. When you come back, you invariably pay the price by seeing more patients and doing more surgery. Then it becomes a stressful event.
My advice to young people is to be very thoughtful about planning trips, especially distant ones. You do not want to sit on a plane all day when you could be doing something more productive. If I could have done something differently in my mid-career, I would have traveled less.
Continue to: Prioritizing “out of office” time...
Prioritizing “out of office” time
Dr. Greene: How do you all mentally separate yourself from work, so that when you are on vacation with your family you are not thinking about the office, the patients, and all of the things on your to-do list?
Dr. Rardin: I don’t have a great answer for that except that it is about being present. You have to decide that now is the time when I am home, now is the time when I am a parent, now is the time when I am a boy scout leader, etc. I guess maybe it’s a skill, or maybe it’s about making something a priority. Work will always be waiting for you when you turn your attention back to it.
Dr. Matthews: Kristie, the answer to your question goes back to community. Partners in a practice cover for each other. You have to trust them to take care of things so that you can relax during your time away.
Some people recommend not scheduling challenging cases right before going away because invariably something goes wrong, and then you are asking, “Why did I schedule 3 colpopexies before getting on a plane?”
Dr. Rardin: Yes, I completely agree with all of that. Personally, I feel fortunate that I can compartmentalize pretty well. When I am home with my kids, I allow myself to shed some of the doctor/surgeon/leadership persona; I am able to be goofy and completely non–doctor-like. It works to help me leave work behind.
Dr. Matthews: Other things you can do include setting up an out-of-office notice on your email that says when you will be back and what to do in case of urgent matters. This basically says to the world, “Don’t expect to hear from me until X date.” It removes the expectation that you will respond sooner. Otherwise, we would all be on our smartphones all the time and not enjoying our time away.
What I wish I knew then
Dr. Culligan: How would you complete the sentence, “I wish they had told me X when I was embarking on my career?”
Dr. Rardin: I keep coming back to the phrase, “Don’t do anything that you can reasonably pay someone else to do.” By that I mean, if you don’t get energy from housework, consider spending some of your money to get help with the housework. Resolve to make a relatively small expenditure to maximize the quality of the time that you give to yourself and your family. Those are the sorts of things that I think can go a long way.
Dr. Culligan: Charley, your wife is an ObGyn. How do you navigate a dual medical career household? What advice do you have for others?
Dr. Rardin: When I was going into fellowship, we had a conversation about how hard it is for both people in a relationship to have an academic fire in the belly and to be truly engaged in climbing the academic ladder. We made a decision that Jane would go into private practice. There has got to be some give and take in a dual medical relationship; a lot of sacrifices and compromises need to happen. We are fortunate in that there are complementary aspects to our jobs. We both spend about the same number of nights away from the house, but my travel is more in chunks and hers is overnight calls for labor and delivery. We have different ways of (briefly) single-parenting, and you have to come up with ways to handle the domestic chores.
Dr. Matthews: I wish someone had explained to me that the people you work with are much more important than the place. The human connection is what defines your experience, much more than any ego-driven outcome.
Dr. Greene: I wish someone had explained to me the competing aspects of academic medicine. The cards are stacked in a way that make it difficult for you to win. For example, you may love to teach and may be really good at it, but if you let your students handle too many cases, your relative value units plummet and then the hospital is on your back. There are the interests of people, and there are the interests of the business. Everything is a balance, and it’s really tricky.
Dr. Huber: Luckily, Pat counselled me as I was finishing my fellowship about the importance of negotiating a good contract, of being pushy and knowing what you want out of it and knowing what your limitations are. I joined a private practice that had 3 different physical locations. If I had to drive to all of them, as they wanted, it would have meant up to a one-and-a-half-hour commute. But I pushed to stay in one location and to put that extra hour to better use. I am glad I did, but it was terrifying at the time because I didn’t want to lose the offer. I know people that did not do that and took the first thing they got. Now, they are driving all over the place or they have these crazy hours or terrible call responsibilities that if they had just been a little firmer, they probably could have gotten out of. As they start trying to find work-life balance, they are already handicapped.
Continue to: Passions outside the office...
Passions outside the office
Dr. Culligan: One thing I would like to touch on is what is going on in each of your personal lives because all of you have interesting stories to tell outside of what you do professionally. What drives you other than medicine?
Dr. Rardin: I am the father of 3 boys. The oldest one just got his Eagle Scout rank yesterday in Boy Scouts. I would be a woodworker if I wasn’t in medicine. I am a Deacon at church. And I love to spend my downtime reading with my family in front of the fireplace.
Dr. Matthews: For me, it’s music. When my husband and I first met, he asked me if I played a musical instrument. I said I played the cello in primary school. He said, “Great, go rent a cello.” I was never at all interested in playing the cello by myself, but because he plays guitar and piano we became able to play a lot of music together. Our son, Alexander, plays drums. We now have a family band.
In addition, I do yoga. I would never have labeled myself an anxious person, but I learned through this process that I am and need to manage it. It took a lot of years to figure that out. If I don’t leave myself an hour each day to go to a yoga class, I am not a happy person and neither is anyone around me. Also, I get tremendous pleasure from reading books and magazines as opposed to watching a screen.
Dr. Greene: I have found that my passions outside of work often change depending on my stage of life. Right now, I have two young babies and so my life outside of work revolves around them. Before the babies, my dad, who lives in Buffalo, was ill. So for awhile, we were flying to Buffalo almost every weekend that I was not on call. I would say, in general what fuels me is connecting with the people I love as often as I can. A typical night involves me and my husband going for a walk with our kids and dog after dinner and talking to each other. We connect with neighbors and chat on the front porch. It doesn’t really matter what we are doing; it is about being surrounded by people who matter.
Dr. Huber: It’s similar for me. Having a child completely shifts your world view. My goal every day is to give my daughter her first feeding in the morning and to get home as soon as possible at the end of the day to do her last feeding and put her to sleep. She crawled for the first time yesterday, and I was so excited that I could be there for that.
Also, I love being outdoors. I love hiking and camping. Going on a hike and being outside with nature is my way of decompressing.
Continue to: Thinking about upcoming generations...
Thinking about upcoming generations
Dr. Matthews: One other thing I would like to propose is looking at what can we do to make the profession better for the next generation. As a group, our profession is somewhat inflexible. We tend to fall into the trap of, “since this is the way we have always done it this is how we should continue doing it.” The OR still starts at 7:00 or 7:30 am, ignoring the need for school drop-offs, etc. We are not innovative about flexibility in the work week. Honestly, it does not work well for many people, patients and physicians alike. Flexible scheduling should be something that is on the table for both men and women who are trying to balance being full-time parents and full-time surgeons. We need to create an environment in which it is okay for you to spend 10 years instead of 6 as an assistant professor because you are also a young parent, and it will not count against you when you come up for promotion.
Dr. Culligan: I agree with you, Catherine. Full “Professor” is a nice title, but it means time away from family and a lot of other things. Each of us has to decide whether it is worth it, especially since it often does not come with any extra money.
Dr. Huber: A question on a recent survey of residents asked, “Do you see yourself going into private practice or academic medicine when you’ve completed your residency?” When I was a resident, everyone wanted to go into academic medicine, but now it seems like more and more residents have their sights set on private practice because that is where they see the opportunities to create work-life balance.
In the academic world, you have to try to get a promotion in X number of years, and get X number of publications, and be a great teacher, doctor, and administrator all at the same time. I am wondering if we are going to start seeing more and more residents and fellows going into private or hospital-owned practice where there aren’t those added expectations.
Dr. Rardin: I agree, and we are back to what we said in the beginning about doing an honest assessment of what is meaningful and important. We are all trained to try to reach for that shiny brass ring, but do we really want that brass ring? Will it be an asset or a hindrance once we get it? It is okay to be honest and say, “I really don’t want that promotion. I would rather spend more time with my family.” ●
Patrick J. Culligan, MD:
My impression of our younger colleagues, however, is that many of them are not attracted to the traditional ivory tower research model of academic advancement to which many in previous generations aspired. They seem more concerned with work-life balance as their measure of success rather than the classic metrics of money and prestige. Everyone still needs role models and mentors, though, and that’s where all of you come in. I asked each of you to be on this panel because I admire you for your varying approaches to work-life balance while achieving success as gynecologic surgeons. I thought others in the field might be inspired by hearing your stories.
Cultivating your passions
Kristie Greene, MD: What I have come to learn and appreciate is a really simple point: you do not have to do everything. Determining who you want to be both personally and professionally is step 1.
Granted, answering the question, “Who do I want to be?” is not as simple as it sounds. Many factors figure into the decisions we make in our personal and professional lives. Also, it is not a question we often stop and ask ourselves. From early on, we are placed on an escalator moving up through medical school, residency, fellowship, good job, better job, etc. We are so accustomed to being competitive, to winning, and to wanting to be the best that we sometimes forget to ask ourselves, “What is it exactly that I want, and why? What is my endpoint? And does it make me happy?”
Multitasking is regarded as a talent. As much as we would like to believe that we can do everything at the same time and do it all well, we actually can’t. A friend of mine made me read a book a couple of years ago, called Feeling Good, by David Burns. The book encourages you to consider the different tasks you do in a day and rate how good you are at each of them on a scale of 1 to 10. It then asks you to think about how much enjoyment you derive from each of the tasks and about why you are doing the ones that bring you little to no enjoyment.
I ultimately decided that, for me professionally, the most important thing was my interest in global health. So I decided to do whatever it took to make this happen. But you don’t get something for nothing, and everything comes with sacrifices.
Continue to: Charles Rardin, MD...
Charles Rardin, MD: How exactly did you decide that you were going to focus your career toward pursuing international health? How did you know it was more important? And how did you overcome some of those obstacles?
Dr. Greene: You have to ask the hard question again about what brings you the most joy professionally and personally. That was the easy part of it for me because global health has always been that source of happiness and fulfillment for me. The more challenging parts are the sacrifices and hard choices that come with it. With global health, it can be difficult to balance the demands of a clinical practice.
All of our jobs are a business. I am still struggling with the money part of it. For my husband and I, that meant we had to start small—do what we could afford. But then it blossomed into something that was involving residents, fellows, and med students, which requires far more funding than we had. So I reached out to family. Most of our families donate to different organizations or charities every year, so why not donate to a loved one for something they are passionate about?
At the University of South Florida (USF), we set up a fund, a foundation for global health, which helps support our work abroad as well as the costs associated with involvement of our trainees. Right now, what we have is still small potatoes to a country, but we are making it happen by starting at a small level and growing it.
Beyond the money aspect, traveling abroad means less involvement in meetings, missed opportunities to teach courses that might interest me, and time away from my family. I guess my advice on this whole thing is that you can make things happen if they are important enough to you, and if you are willing to make sacrifices in other areas because you can’t have it all.
Making time for you
Dr. Culligan: So you have found what is important to you, and you have found a way to make it happen. But you are faced with more work; you have given yourself additional work on top of your regular work. How do you make time for a personal life?
Catherine Matthews, MD: In preparing for this discussion, I decided to break down my advice into 3 buckets: The first bucket is discovering and knowing your authentic self. The second is building a community, which I’ll elaborate on. And the third, which we have discussed, is to let go of the money.
Dr. Culligan: I love the concept of the authentic self, but how does that jive with a tendency to strive for perfection? We all think we can do it all. How do we narrow down to what really matters?
Dr. Matthews: We often focus on the things that bring us happiness and what we are good at, but it’s the things that make us unhappy that tend to bring us down. It’s the presence of unhappiness, not the absence of happiness, that seems to be the undoing of many, including myself.
None of us are born with dramatic insight. It is experience that leads to insight. People who are actually present are able to gain insight through observation. A person becomes a better surgeon by observing the outcome of doing a stitch this way versus that; you learn how to do it by seeing what it looks like afterward.
Finding our authentic selves happens in much the same way. Having the presence of mind to ask the right questions, such as, “How am I feeling while I’m doing this?” leads to insights into the true self.
Continue to: It takes a village...
It takes a village
Dr. Greene: Catherine mentioned community earlier, and that is extremely important. The people who surround us can have a huge impact on the way we perceive things, including ourselves. Having a mix of people in our lives—some who practice medicine and others who don’t—helps us stay balanced and answer some of the tough questions. Catherine, for example, has helped me in various stages of my career to ask myself meaningful questions and get real answers.
Dr. Rardin: Part of finding balance is luck, and part of it is making a choice between money and everything else. In considering my first job out of training, I knew that money had the potential to distract me from what was important to me. So I chose a position that was almost entirely salaried so that the decisions I made clinically, surgically, and regarding work-life balance would be less likely to directly impact what was important to me.
Sally Huber, MD: I am still in the “getting there” phase of my life, but one thing I have found is that getting my family involved and excited about what I do has made them much more accepting of when I have longer work days or work to do on the weekend. My spouse has become quite involved with what I have been doing with transgender health in Atlanta. It has been a great bonding experience; she shares my passions, and together we are creating something about which we both can be proud.
When work invades home life
Dr. Culligan: That is great. Sally, I think when we talked, you were just learning about the necessity of mental separation and of not taking your work home with you, which is so hard for all of us with all of our devices.
Dr. Huber: Yes, this year has been about seeing what works best as far as being efficient at work and having quality time at home. At the end of every day I ask myself, “What worked well today? What didn’t work well? What else can I do to maximize time with my family?” I am slowly becoming more efficient, but it has been a challenge. During fellowship, your day is pretty set, but once you are practicing on your own, your hours and responsibilities are completely different, and you have to figure out what works best for you, your values, and your expectations of private life. It takes some time, and I am still figuring it out.
Dr. Culligan: How often would you say that you bring work home? I try hard once I am home to quit working, but sometimes on the weekends I break that rule.
Dr. Matthews: I must say that I do feel like there are certain times when I am better at that than others. Work comes in waves with pressing deadlines. If I averaged it out, probably a third of the time I have some email or some conference call or something that I have got to do at home. I do really try to limit the obligations that I have after 5:30 or 6:00 pm. I resent intrusions after that time. As far as weekends, I delegate about one weekend every 2 months to work, instead of doing a little bit every weekend.
Dr. Greene: I agree. I try hard to make 5:30 to 7:30 pm unequivocal time for a family dinner and time for my kids. During that time, I do not have my phone near me so I can’t look at email or texts. I try not to schedule conference calls. I try to be there to read books to my kids at night. Then if I need to do work, I do it later at night, which interferes with time with my spouse, and is not ideal, but that’s what happens.
Dr. Matthews: One of the things that I think is a huge part of work-life balance is work-related travel. When you are present at work on a consistent basis, the work does not pile up to the extent that it does when you are absent on a trip. When you come back, you invariably pay the price by seeing more patients and doing more surgery. Then it becomes a stressful event.
My advice to young people is to be very thoughtful about planning trips, especially distant ones. You do not want to sit on a plane all day when you could be doing something more productive. If I could have done something differently in my mid-career, I would have traveled less.
Continue to: Prioritizing “out of office” time...
Prioritizing “out of office” time
Dr. Greene: How do you all mentally separate yourself from work, so that when you are on vacation with your family you are not thinking about the office, the patients, and all of the things on your to-do list?
Dr. Rardin: I don’t have a great answer for that except that it is about being present. You have to decide that now is the time when I am home, now is the time when I am a parent, now is the time when I am a boy scout leader, etc. I guess maybe it’s a skill, or maybe it’s about making something a priority. Work will always be waiting for you when you turn your attention back to it.
Dr. Matthews: Kristie, the answer to your question goes back to community. Partners in a practice cover for each other. You have to trust them to take care of things so that you can relax during your time away.
Some people recommend not scheduling challenging cases right before going away because invariably something goes wrong, and then you are asking, “Why did I schedule 3 colpopexies before getting on a plane?”
Dr. Rardin: Yes, I completely agree with all of that. Personally, I feel fortunate that I can compartmentalize pretty well. When I am home with my kids, I allow myself to shed some of the doctor/surgeon/leadership persona; I am able to be goofy and completely non–doctor-like. It works to help me leave work behind.
Dr. Matthews: Other things you can do include setting up an out-of-office notice on your email that says when you will be back and what to do in case of urgent matters. This basically says to the world, “Don’t expect to hear from me until X date.” It removes the expectation that you will respond sooner. Otherwise, we would all be on our smartphones all the time and not enjoying our time away.
What I wish I knew then
Dr. Culligan: How would you complete the sentence, “I wish they had told me X when I was embarking on my career?”
Dr. Rardin: I keep coming back to the phrase, “Don’t do anything that you can reasonably pay someone else to do.” By that I mean, if you don’t get energy from housework, consider spending some of your money to get help with the housework. Resolve to make a relatively small expenditure to maximize the quality of the time that you give to yourself and your family. Those are the sorts of things that I think can go a long way.
Dr. Culligan: Charley, your wife is an ObGyn. How do you navigate a dual medical career household? What advice do you have for others?
Dr. Rardin: When I was going into fellowship, we had a conversation about how hard it is for both people in a relationship to have an academic fire in the belly and to be truly engaged in climbing the academic ladder. We made a decision that Jane would go into private practice. There has got to be some give and take in a dual medical relationship; a lot of sacrifices and compromises need to happen. We are fortunate in that there are complementary aspects to our jobs. We both spend about the same number of nights away from the house, but my travel is more in chunks and hers is overnight calls for labor and delivery. We have different ways of (briefly) single-parenting, and you have to come up with ways to handle the domestic chores.
Dr. Matthews: I wish someone had explained to me that the people you work with are much more important than the place. The human connection is what defines your experience, much more than any ego-driven outcome.
Dr. Greene: I wish someone had explained to me the competing aspects of academic medicine. The cards are stacked in a way that make it difficult for you to win. For example, you may love to teach and may be really good at it, but if you let your students handle too many cases, your relative value units plummet and then the hospital is on your back. There are the interests of people, and there are the interests of the business. Everything is a balance, and it’s really tricky.
Dr. Huber: Luckily, Pat counselled me as I was finishing my fellowship about the importance of negotiating a good contract, of being pushy and knowing what you want out of it and knowing what your limitations are. I joined a private practice that had 3 different physical locations. If I had to drive to all of them, as they wanted, it would have meant up to a one-and-a-half-hour commute. But I pushed to stay in one location and to put that extra hour to better use. I am glad I did, but it was terrifying at the time because I didn’t want to lose the offer. I know people that did not do that and took the first thing they got. Now, they are driving all over the place or they have these crazy hours or terrible call responsibilities that if they had just been a little firmer, they probably could have gotten out of. As they start trying to find work-life balance, they are already handicapped.
Continue to: Passions outside the office...
Passions outside the office
Dr. Culligan: One thing I would like to touch on is what is going on in each of your personal lives because all of you have interesting stories to tell outside of what you do professionally. What drives you other than medicine?
Dr. Rardin: I am the father of 3 boys. The oldest one just got his Eagle Scout rank yesterday in Boy Scouts. I would be a woodworker if I wasn’t in medicine. I am a Deacon at church. And I love to spend my downtime reading with my family in front of the fireplace.
Dr. Matthews: For me, it’s music. When my husband and I first met, he asked me if I played a musical instrument. I said I played the cello in primary school. He said, “Great, go rent a cello.” I was never at all interested in playing the cello by myself, but because he plays guitar and piano we became able to play a lot of music together. Our son, Alexander, plays drums. We now have a family band.
In addition, I do yoga. I would never have labeled myself an anxious person, but I learned through this process that I am and need to manage it. It took a lot of years to figure that out. If I don’t leave myself an hour each day to go to a yoga class, I am not a happy person and neither is anyone around me. Also, I get tremendous pleasure from reading books and magazines as opposed to watching a screen.
Dr. Greene: I have found that my passions outside of work often change depending on my stage of life. Right now, I have two young babies and so my life outside of work revolves around them. Before the babies, my dad, who lives in Buffalo, was ill. So for awhile, we were flying to Buffalo almost every weekend that I was not on call. I would say, in general what fuels me is connecting with the people I love as often as I can. A typical night involves me and my husband going for a walk with our kids and dog after dinner and talking to each other. We connect with neighbors and chat on the front porch. It doesn’t really matter what we are doing; it is about being surrounded by people who matter.
Dr. Huber: It’s similar for me. Having a child completely shifts your world view. My goal every day is to give my daughter her first feeding in the morning and to get home as soon as possible at the end of the day to do her last feeding and put her to sleep. She crawled for the first time yesterday, and I was so excited that I could be there for that.
Also, I love being outdoors. I love hiking and camping. Going on a hike and being outside with nature is my way of decompressing.
Continue to: Thinking about upcoming generations...
Thinking about upcoming generations
Dr. Matthews: One other thing I would like to propose is looking at what can we do to make the profession better for the next generation. As a group, our profession is somewhat inflexible. We tend to fall into the trap of, “since this is the way we have always done it this is how we should continue doing it.” The OR still starts at 7:00 or 7:30 am, ignoring the need for school drop-offs, etc. We are not innovative about flexibility in the work week. Honestly, it does not work well for many people, patients and physicians alike. Flexible scheduling should be something that is on the table for both men and women who are trying to balance being full-time parents and full-time surgeons. We need to create an environment in which it is okay for you to spend 10 years instead of 6 as an assistant professor because you are also a young parent, and it will not count against you when you come up for promotion.
Dr. Culligan: I agree with you, Catherine. Full “Professor” is a nice title, but it means time away from family and a lot of other things. Each of us has to decide whether it is worth it, especially since it often does not come with any extra money.
Dr. Huber: A question on a recent survey of residents asked, “Do you see yourself going into private practice or academic medicine when you’ve completed your residency?” When I was a resident, everyone wanted to go into academic medicine, but now it seems like more and more residents have their sights set on private practice because that is where they see the opportunities to create work-life balance.
In the academic world, you have to try to get a promotion in X number of years, and get X number of publications, and be a great teacher, doctor, and administrator all at the same time. I am wondering if we are going to start seeing more and more residents and fellows going into private or hospital-owned practice where there aren’t those added expectations.
Dr. Rardin: I agree, and we are back to what we said in the beginning about doing an honest assessment of what is meaningful and important. We are all trained to try to reach for that shiny brass ring, but do we really want that brass ring? Will it be an asset or a hindrance once we get it? It is okay to be honest and say, “I really don’t want that promotion. I would rather spend more time with my family.” ●
Patrick J. Culligan, MD:
My impression of our younger colleagues, however, is that many of them are not attracted to the traditional ivory tower research model of academic advancement to which many in previous generations aspired. They seem more concerned with work-life balance as their measure of success rather than the classic metrics of money and prestige. Everyone still needs role models and mentors, though, and that’s where all of you come in. I asked each of you to be on this panel because I admire you for your varying approaches to work-life balance while achieving success as gynecologic surgeons. I thought others in the field might be inspired by hearing your stories.
Cultivating your passions
Kristie Greene, MD: What I have come to learn and appreciate is a really simple point: you do not have to do everything. Determining who you want to be both personally and professionally is step 1.
Granted, answering the question, “Who do I want to be?” is not as simple as it sounds. Many factors figure into the decisions we make in our personal and professional lives. Also, it is not a question we often stop and ask ourselves. From early on, we are placed on an escalator moving up through medical school, residency, fellowship, good job, better job, etc. We are so accustomed to being competitive, to winning, and to wanting to be the best that we sometimes forget to ask ourselves, “What is it exactly that I want, and why? What is my endpoint? And does it make me happy?”
Multitasking is regarded as a talent. As much as we would like to believe that we can do everything at the same time and do it all well, we actually can’t. A friend of mine made me read a book a couple of years ago, called Feeling Good, by David Burns. The book encourages you to consider the different tasks you do in a day and rate how good you are at each of them on a scale of 1 to 10. It then asks you to think about how much enjoyment you derive from each of the tasks and about why you are doing the ones that bring you little to no enjoyment.
I ultimately decided that, for me professionally, the most important thing was my interest in global health. So I decided to do whatever it took to make this happen. But you don’t get something for nothing, and everything comes with sacrifices.
Continue to: Charles Rardin, MD...
Charles Rardin, MD: How exactly did you decide that you were going to focus your career toward pursuing international health? How did you know it was more important? And how did you overcome some of those obstacles?
Dr. Greene: You have to ask the hard question again about what brings you the most joy professionally and personally. That was the easy part of it for me because global health has always been that source of happiness and fulfillment for me. The more challenging parts are the sacrifices and hard choices that come with it. With global health, it can be difficult to balance the demands of a clinical practice.
All of our jobs are a business. I am still struggling with the money part of it. For my husband and I, that meant we had to start small—do what we could afford. But then it blossomed into something that was involving residents, fellows, and med students, which requires far more funding than we had. So I reached out to family. Most of our families donate to different organizations or charities every year, so why not donate to a loved one for something they are passionate about?
At the University of South Florida (USF), we set up a fund, a foundation for global health, which helps support our work abroad as well as the costs associated with involvement of our trainees. Right now, what we have is still small potatoes to a country, but we are making it happen by starting at a small level and growing it.
Beyond the money aspect, traveling abroad means less involvement in meetings, missed opportunities to teach courses that might interest me, and time away from my family. I guess my advice on this whole thing is that you can make things happen if they are important enough to you, and if you are willing to make sacrifices in other areas because you can’t have it all.
Making time for you
Dr. Culligan: So you have found what is important to you, and you have found a way to make it happen. But you are faced with more work; you have given yourself additional work on top of your regular work. How do you make time for a personal life?
Catherine Matthews, MD: In preparing for this discussion, I decided to break down my advice into 3 buckets: The first bucket is discovering and knowing your authentic self. The second is building a community, which I’ll elaborate on. And the third, which we have discussed, is to let go of the money.
Dr. Culligan: I love the concept of the authentic self, but how does that jive with a tendency to strive for perfection? We all think we can do it all. How do we narrow down to what really matters?
Dr. Matthews: We often focus on the things that bring us happiness and what we are good at, but it’s the things that make us unhappy that tend to bring us down. It’s the presence of unhappiness, not the absence of happiness, that seems to be the undoing of many, including myself.
None of us are born with dramatic insight. It is experience that leads to insight. People who are actually present are able to gain insight through observation. A person becomes a better surgeon by observing the outcome of doing a stitch this way versus that; you learn how to do it by seeing what it looks like afterward.
Finding our authentic selves happens in much the same way. Having the presence of mind to ask the right questions, such as, “How am I feeling while I’m doing this?” leads to insights into the true self.
Continue to: It takes a village...
It takes a village
Dr. Greene: Catherine mentioned community earlier, and that is extremely important. The people who surround us can have a huge impact on the way we perceive things, including ourselves. Having a mix of people in our lives—some who practice medicine and others who don’t—helps us stay balanced and answer some of the tough questions. Catherine, for example, has helped me in various stages of my career to ask myself meaningful questions and get real answers.
Dr. Rardin: Part of finding balance is luck, and part of it is making a choice between money and everything else. In considering my first job out of training, I knew that money had the potential to distract me from what was important to me. So I chose a position that was almost entirely salaried so that the decisions I made clinically, surgically, and regarding work-life balance would be less likely to directly impact what was important to me.
Sally Huber, MD: I am still in the “getting there” phase of my life, but one thing I have found is that getting my family involved and excited about what I do has made them much more accepting of when I have longer work days or work to do on the weekend. My spouse has become quite involved with what I have been doing with transgender health in Atlanta. It has been a great bonding experience; she shares my passions, and together we are creating something about which we both can be proud.
When work invades home life
Dr. Culligan: That is great. Sally, I think when we talked, you were just learning about the necessity of mental separation and of not taking your work home with you, which is so hard for all of us with all of our devices.
Dr. Huber: Yes, this year has been about seeing what works best as far as being efficient at work and having quality time at home. At the end of every day I ask myself, “What worked well today? What didn’t work well? What else can I do to maximize time with my family?” I am slowly becoming more efficient, but it has been a challenge. During fellowship, your day is pretty set, but once you are practicing on your own, your hours and responsibilities are completely different, and you have to figure out what works best for you, your values, and your expectations of private life. It takes some time, and I am still figuring it out.
Dr. Culligan: How often would you say that you bring work home? I try hard once I am home to quit working, but sometimes on the weekends I break that rule.
Dr. Matthews: I must say that I do feel like there are certain times when I am better at that than others. Work comes in waves with pressing deadlines. If I averaged it out, probably a third of the time I have some email or some conference call or something that I have got to do at home. I do really try to limit the obligations that I have after 5:30 or 6:00 pm. I resent intrusions after that time. As far as weekends, I delegate about one weekend every 2 months to work, instead of doing a little bit every weekend.
Dr. Greene: I agree. I try hard to make 5:30 to 7:30 pm unequivocal time for a family dinner and time for my kids. During that time, I do not have my phone near me so I can’t look at email or texts. I try not to schedule conference calls. I try to be there to read books to my kids at night. Then if I need to do work, I do it later at night, which interferes with time with my spouse, and is not ideal, but that’s what happens.
Dr. Matthews: One of the things that I think is a huge part of work-life balance is work-related travel. When you are present at work on a consistent basis, the work does not pile up to the extent that it does when you are absent on a trip. When you come back, you invariably pay the price by seeing more patients and doing more surgery. Then it becomes a stressful event.
My advice to young people is to be very thoughtful about planning trips, especially distant ones. You do not want to sit on a plane all day when you could be doing something more productive. If I could have done something differently in my mid-career, I would have traveled less.
Continue to: Prioritizing “out of office” time...
Prioritizing “out of office” time
Dr. Greene: How do you all mentally separate yourself from work, so that when you are on vacation with your family you are not thinking about the office, the patients, and all of the things on your to-do list?
Dr. Rardin: I don’t have a great answer for that except that it is about being present. You have to decide that now is the time when I am home, now is the time when I am a parent, now is the time when I am a boy scout leader, etc. I guess maybe it’s a skill, or maybe it’s about making something a priority. Work will always be waiting for you when you turn your attention back to it.
Dr. Matthews: Kristie, the answer to your question goes back to community. Partners in a practice cover for each other. You have to trust them to take care of things so that you can relax during your time away.
Some people recommend not scheduling challenging cases right before going away because invariably something goes wrong, and then you are asking, “Why did I schedule 3 colpopexies before getting on a plane?”
Dr. Rardin: Yes, I completely agree with all of that. Personally, I feel fortunate that I can compartmentalize pretty well. When I am home with my kids, I allow myself to shed some of the doctor/surgeon/leadership persona; I am able to be goofy and completely non–doctor-like. It works to help me leave work behind.
Dr. Matthews: Other things you can do include setting up an out-of-office notice on your email that says when you will be back and what to do in case of urgent matters. This basically says to the world, “Don’t expect to hear from me until X date.” It removes the expectation that you will respond sooner. Otherwise, we would all be on our smartphones all the time and not enjoying our time away.
What I wish I knew then
Dr. Culligan: How would you complete the sentence, “I wish they had told me X when I was embarking on my career?”
Dr. Rardin: I keep coming back to the phrase, “Don’t do anything that you can reasonably pay someone else to do.” By that I mean, if you don’t get energy from housework, consider spending some of your money to get help with the housework. Resolve to make a relatively small expenditure to maximize the quality of the time that you give to yourself and your family. Those are the sorts of things that I think can go a long way.
Dr. Culligan: Charley, your wife is an ObGyn. How do you navigate a dual medical career household? What advice do you have for others?
Dr. Rardin: When I was going into fellowship, we had a conversation about how hard it is for both people in a relationship to have an academic fire in the belly and to be truly engaged in climbing the academic ladder. We made a decision that Jane would go into private practice. There has got to be some give and take in a dual medical relationship; a lot of sacrifices and compromises need to happen. We are fortunate in that there are complementary aspects to our jobs. We both spend about the same number of nights away from the house, but my travel is more in chunks and hers is overnight calls for labor and delivery. We have different ways of (briefly) single-parenting, and you have to come up with ways to handle the domestic chores.
Dr. Matthews: I wish someone had explained to me that the people you work with are much more important than the place. The human connection is what defines your experience, much more than any ego-driven outcome.
Dr. Greene: I wish someone had explained to me the competing aspects of academic medicine. The cards are stacked in a way that make it difficult for you to win. For example, you may love to teach and may be really good at it, but if you let your students handle too many cases, your relative value units plummet and then the hospital is on your back. There are the interests of people, and there are the interests of the business. Everything is a balance, and it’s really tricky.
Dr. Huber: Luckily, Pat counselled me as I was finishing my fellowship about the importance of negotiating a good contract, of being pushy and knowing what you want out of it and knowing what your limitations are. I joined a private practice that had 3 different physical locations. If I had to drive to all of them, as they wanted, it would have meant up to a one-and-a-half-hour commute. But I pushed to stay in one location and to put that extra hour to better use. I am glad I did, but it was terrifying at the time because I didn’t want to lose the offer. I know people that did not do that and took the first thing they got. Now, they are driving all over the place or they have these crazy hours or terrible call responsibilities that if they had just been a little firmer, they probably could have gotten out of. As they start trying to find work-life balance, they are already handicapped.
Continue to: Passions outside the office...
Passions outside the office
Dr. Culligan: One thing I would like to touch on is what is going on in each of your personal lives because all of you have interesting stories to tell outside of what you do professionally. What drives you other than medicine?
Dr. Rardin: I am the father of 3 boys. The oldest one just got his Eagle Scout rank yesterday in Boy Scouts. I would be a woodworker if I wasn’t in medicine. I am a Deacon at church. And I love to spend my downtime reading with my family in front of the fireplace.
Dr. Matthews: For me, it’s music. When my husband and I first met, he asked me if I played a musical instrument. I said I played the cello in primary school. He said, “Great, go rent a cello.” I was never at all interested in playing the cello by myself, but because he plays guitar and piano we became able to play a lot of music together. Our son, Alexander, plays drums. We now have a family band.
In addition, I do yoga. I would never have labeled myself an anxious person, but I learned through this process that I am and need to manage it. It took a lot of years to figure that out. If I don’t leave myself an hour each day to go to a yoga class, I am not a happy person and neither is anyone around me. Also, I get tremendous pleasure from reading books and magazines as opposed to watching a screen.
Dr. Greene: I have found that my passions outside of work often change depending on my stage of life. Right now, I have two young babies and so my life outside of work revolves around them. Before the babies, my dad, who lives in Buffalo, was ill. So for awhile, we were flying to Buffalo almost every weekend that I was not on call. I would say, in general what fuels me is connecting with the people I love as often as I can. A typical night involves me and my husband going for a walk with our kids and dog after dinner and talking to each other. We connect with neighbors and chat on the front porch. It doesn’t really matter what we are doing; it is about being surrounded by people who matter.
Dr. Huber: It’s similar for me. Having a child completely shifts your world view. My goal every day is to give my daughter her first feeding in the morning and to get home as soon as possible at the end of the day to do her last feeding and put her to sleep. She crawled for the first time yesterday, and I was so excited that I could be there for that.
Also, I love being outdoors. I love hiking and camping. Going on a hike and being outside with nature is my way of decompressing.
Continue to: Thinking about upcoming generations...
Thinking about upcoming generations
Dr. Matthews: One other thing I would like to propose is looking at what can we do to make the profession better for the next generation. As a group, our profession is somewhat inflexible. We tend to fall into the trap of, “since this is the way we have always done it this is how we should continue doing it.” The OR still starts at 7:00 or 7:30 am, ignoring the need for school drop-offs, etc. We are not innovative about flexibility in the work week. Honestly, it does not work well for many people, patients and physicians alike. Flexible scheduling should be something that is on the table for both men and women who are trying to balance being full-time parents and full-time surgeons. We need to create an environment in which it is okay for you to spend 10 years instead of 6 as an assistant professor because you are also a young parent, and it will not count against you when you come up for promotion.
Dr. Culligan: I agree with you, Catherine. Full “Professor” is a nice title, but it means time away from family and a lot of other things. Each of us has to decide whether it is worth it, especially since it often does not come with any extra money.
Dr. Huber: A question on a recent survey of residents asked, “Do you see yourself going into private practice or academic medicine when you’ve completed your residency?” When I was a resident, everyone wanted to go into academic medicine, but now it seems like more and more residents have their sights set on private practice because that is where they see the opportunities to create work-life balance.
In the academic world, you have to try to get a promotion in X number of years, and get X number of publications, and be a great teacher, doctor, and administrator all at the same time. I am wondering if we are going to start seeing more and more residents and fellows going into private or hospital-owned practice where there aren’t those added expectations.
Dr. Rardin: I agree, and we are back to what we said in the beginning about doing an honest assessment of what is meaningful and important. We are all trained to try to reach for that shiny brass ring, but do we really want that brass ring? Will it be an asset or a hindrance once we get it? It is okay to be honest and say, “I really don’t want that promotion. I would rather spend more time with my family.” ●
The professional advancement of drug and device innovation

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●

I often say that there are both “guardrail” days and very good days when it comes to the ins and outs of health care builds and product launches. The process is much like starting down the path of a country road in the middle of a blizzard—unless you have dependable wipers and a good defrost system, that path can get murky very quickly. With this article I hope to offer my counsel to inventors, featuring a few of my prior launches as well as case studies of health care launches I was not involved with, and sharing the lessons learned and hurdles that were overcome. I encourage all entrepreneurs to act on their ideas because, in the world of health care startups, the only failure is not acting on an invention.
Case study 1: Cerezyme
Today, Cerezyme is indicated for patients with Gaucher, which is a lysosomal storage disorder. Cerezyme’s first-generation product, called Ceredase, was a human tissue-derived protein that we extracted from human placentas. At the time, the concept of moving this program forward was denied by the Board of Directors because they said that even if you could collect enough placentas to make the enzyme, it would be too expensive to manufacture. In fact, early scale-up modeling for manufacturing the protein demonstrated that Genzyme would need 4 tons of placentas per Gaucher patient per year.
Gaucher is a severe, early-onset disease that has a significant negative outcome for patients. Patients with Gaucher are in dire need of treatment. Genzyme went forward with the Ceredase program by financing it through the families of patients with the disease, by starting an LLC separate from the business and funding the initial clinical trial and the development of the protein through the families of Gaucher patients. That approach was a successful endeavor. A great example of a creative capital structure to advance a program.
This was in the late 1980s/early 1990s, and at the height of the AIDS challenge. Genzyme based the manufacturing in Lille, France, and we cryopreserved placentas in the United States and Europe and shipped them to Lille to be processed into therapy. Genzyme eventually received approval for Ceredase from the US Food and Drug Administration (FDA) and the European Medicines Agency. At the height of the placenta collection, we were gathering about 10% to 15% of the placentas in the United States and 30% to 40% of the placentas in Europe. Resources supply became an issue until we developed a recombinant form of the protein, accomplished by using a manufacturing system called a CHO cell line.
This is a very good success story: If this invention was not pursued, Gaucher patients would not benefit from the treatment today. In addition, there are a plethora of patients with different lysosomal storage disorders treated with additional proteins that have been aided by us going through the entire development, manufacturing, and global commercialization process. We figured out how to manufacture and deliver the treatment, working through multiple countries’ political systems, and today the therapy is paid for by insurance and government systems on a worldwide basis.
Continue to: Case study 2...
Case study 2: ThinPrep
I like to use the approval of ThinPrep as an example of avoiding a false negative—a stoppage in the development of the product or drug for the wrong reasons. False negatives, in my mind, occur when you are developing a technology and you run into issues during the clinical phase and/or with FDA approval, or with a technical failure or you run out of capital prior to knowing whether or not the innovation actually works. In the case of ThinPrep, a poorly run clinical trial almost resulted in a false negative.
The company at the time was Cytyc, and an initial clinical study presented to the FDA yielded a neutral-negative outcome. The FDA said that there were not enough data to show the differentiation from the current Pap smear standard of care.
The founders of the company at that time had inherited the study protocol from a prior leadership team, so they had to finish the trial with the initial protocol. Given the FDA’s advisement, they developed a new trial. It took the persistence of these two founders, who mortgaged their homes and spent their personal dollars to take this through the next wave of clinical development. In the end it was successful. The revised clinical trial yielded an approval for ThinPrep, which is now considered a standard of care.
The use of ThinPrep reduced cervical cancer deaths by 40% from preapproval. The challenging path from clinical development to eventual commercial launch and physician leadership in advancing patient care makes the story of ThinPrep a great example of not allowing an early false negative of a poorly designed and run clinical trial stop important innovation.
Case study 3: Cologuard
The development of Cologuard is a case study demonstrating that, sometimes, when your first attempt does not work, you need to have the persistence to raise additional capital and/or use a slightly different technical approach. The approval story of Cologuard is important to share because it is an important cancer screening diagnostic, using DNA from stool samples to test for colon cancer, giving access to important colon cancer screening to many patients. Currently, caregivers are only scraping the surface with Cologuard’s ability to screen the population. There are many more patients that need access to the test, and I believe they will get it in the years ahead.
Cologuard went through a first- and second-generational technical failure. They could not get the test’s specificity and sensitivity to be at the level of a screening tool; there were too many false-positive results. With the third iteration came the technical breakthrough, and a very large, expensive study was conducted—one the leadership team was criticized for. However, that study yielded the data that achieved a New England Journal of Medicine article, and reimbursement support across the country. The combination of the right technical team and the right leadership team, who planned a proper commercial launch, with a CEO that supported the extensive clinical study, has resulted in the fourth generation of Cologuard—an important breakthrough offering a very useful new standard of care in colon cancer detection and screening.
Continue to: Pearls for moving your innovations forward...
Pearls for moving your innovations forward
Because of my experience in undergoing health care start-ups, and contributing to several of those advancements of innovation, many inventors approach me for advice on their paths from idea to full-concept company. Here are a few of my lessons learned.
Consider purpose, not financial gain, first and foremost. Financial gain is typically the by-product or outcome of a standard-of-care breakthrough for inventors, but it’s a very hard road. Pursue your invention for advancing patient care and moving a new standard of care forward in health care versus financial gain at the end.
Determine whether your invention is a product or a company, or potentially, not capitalizable at all. Figure this out early. Analyze your idea to make sure it is sound and truly novel. Analyze the competition and to make sure it is sound and truly novel. Analyze the competition and the market dynamics to support a new product. Can the development path be defined very clearly to raise capital? Is your innovation a big enough breakthrough in the market with several current products to actually make a difference in patient outcomes (and eventually achieve product reimbursement)? The creation of a company may be the right strategy if the innovation can support a differentiated enough breakthrough where you can actually support all the infrastructure to build the business. If you find that the market is not there to support and develop your idea to eventual success, backing off early is important to preserve invested capital.
Protect early. Is your invention patentable, or has someone else already thought of the idea? What kind of patent(s) are appropriate? Where, geographically, do you want to protect your invention? Find a good patent attorney in your local area, early in the process, to help you answer all of these critical questions. Patents are expensive to file and maintain, but it is not expensive to do a literature search to find out if your idea is novel. A provisional patent, which would be your first step, is an important cost-effective step.
Capital is out there. If your invention or idea deserves capital, it is available. I will address raising capital in more detail in the next section.
Consider regulatory and manufacturing as achievable hurdles. Inventors often get tripped up here, considering the regulatory hurdles and manufacturing too challenging and abandoning their ideas because the risk is too great. Regulatory and manufacturing are very important aspects of health care standard-of-care builds. Cutting corners is not an option. That said, regulatory and manufacturing should not stop you. Challenges often can be worked through as long as the clinical need is there, and the clinical data support bringing that technology forward.
Consider corporate partnerships. I am a fan of corporate partners. But which ones should you target, and when and why? Corporate partnerships can bring significant capital, which is great, but there is enough investor capital out there that you should not pursue a corporate partner just for capital. The main benefit of a corporate partner is enterprise intellect. They typically know more about the field that you are entering than the investors or a small company leadership team.
Establish and listen to advisors. When thinking about who to trust, research their track record. Advisors who have gone through this process before, and specifically in your product area, are important to have access to.
Persistence is key. I have observed a tremendous “compression of innovation” in the health care areas that I have been involved with—human tissue-derived proteins, robotic surgery, stem cell therapy, and digital health (which is still in its infancy). For each of these breakthrough categories, early on, it appeared that it couldn’t be done. However, after the first 2 or 3 major breakthroughs in each one of these areas, a compression of innovation occurred. For instance, after approximately 15 years of protein development, we came out with the recombinant manufacturing systems for proteins. Very quickly, within 10 years, there were more than 70 proteins on the market. The persistence of the inventors to overcome early obstacles in each of these health care areas was critical to future success in each area.
Continue to: Raising capital...
Raising capital
There are different investors who specialize in different types of investment opportunities. The first phase of raising capital is the seed round—where there is typically early data, or even no data and just a concept. From this seed round forward, there is less risk as you develop your technology; thus, there are different investors that support different stages of development and that specialize in different types of investing. It is important to target the right investors and raise enough capital to be able to go achieve multiple operational milestones. Otherwise, when you go through your first round of capital, or the Series A or B financing rounds, there may not be a set of investors out there to fund the company moving forward. Health care investors will make it known that they invest in certain rounds of capital. You can determine who those investors are by doing a search online.
A mistake health care inventors can make is not taking enough capital from investors, because they are concerned about dilution. I advise investors not to focus on dilution but rather on, how big can you make “the pie” (value of the company) worth? The entire process is about bringing a true product through to a new standard-of-care curve.
Trust is the most important thing to earn with investors, and there is zero tolerance for a lack of trust. Share your vision as the inventor with investors, who want to know where this category could be in the next 5 or 10 years. Clinical data will always win, and health care investors and industry leaders should be focused on executing the most robust clinical data to demonstrate the clearest potential clinical outcome. Investors will follow a good plan that has been developed to achieve FDA approval, successful commercialization or “go to market” launch, and eventual reimbursement to support a true standard-of-care change.
Failure is defined by inaction
The 3 case studies that I have shared were success stories because the ideas and inventions were acted upon. When I was at Genzyme, we built the company up to more than $1 billion in revenue. We commercialized proteins in over 50 countries. Most importantly, many patients benefited from the innovation. If you have an invention and an idea, act on it—and surround yourself with great people in every discipline. Having the right people and team is extremely important. ●