User login
In the management of cesarean scar defects, is there a superior surgical method for treatment?
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
High BMI does not complicate postpartum tubal ligation
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
REPORTING FROM THE PREGNANCY MEETING
What is the role of the ObGyn in preventing and treating obesity?
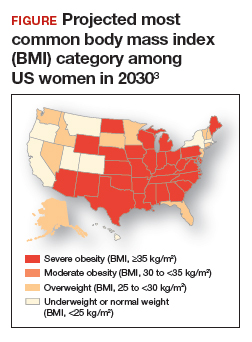
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3
As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
Obesity is a disease causing a public health crisis. In the United States, tobacco use and obesity are the two most important causes of preventable premature death. They result in an estimated 480,0001 and 300,0002 premature deaths per year, respectively. Obesity is a major contributor to diabetes mellitus, hypertension, dyslipidemia, and coronary heart disease. Obesity is also associated with increased rates of colon, breast, and endometrial cancer. Experts predict that in 2030, 50% of adults in the United States will have a body mass index (BMI) ≥ 30 kg/m2, and 25% will have a BMI ≥ 35 kg/m2.3 More women than men are predicted to be severely obese (FIGURE).3

As clinicians we need to increase our efforts to reduce the epidemic of obesity. ObGyns can play an important role in preventing and managing obesity, by recommending primary-care weight management practices, prescribing medications that influence central metabolism, and referring appropriate patients to bariatric surgery centers of excellence.
Primary-care weight management
Measuring BMI and recommending interventions to prevent and treat obesity are important components of a health maintenance encounter. For women who are overweight or obese, dietary changes and exercise are important recommendations. The American Heart Association recommends the following lifestyle interventions4:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Consider using an activity tracker to monitor activity level.
Clinicians should consider referring overweight and obese patients to a nutritionist for a consultation to plan how to consume a high-quality, low-calorie diet. A nutritionist can spend time with patients explaining options for implementing a calorie-restricted diet. In addition, some health insurers will require patients to participate in a supervised calorie-restricted diet plan for at least 6 months before authorizing coverage of expensive weight loss medications or bariatric surgery. In addition to recommending diet and exercise, ObGyns may consider prescribing metformin for their obese patients.
Continue to: Metformin...
Metformin
Metformin is approved for the treatment of type 2 diabetes mellitus. Unlike insulin therapy, which is associated with weight gain, metformin is associated with modest weight loss. The Diabetes Prevention Program (DPP) randomly assigned 3,234 nondiabetic participants with a fasting glucose level between 95 and 125 mg/dL and impaired glucose tolerance (140 to 199 mg/dL) after a 75-g oral glucose load to intensive lifestyle changes (calorie-restricted diet to achieve 7% weight loss plus 150 minutes of exercise weekly), metformin (850 mg twice daily), or placebo.5,6 The mean age of the participants was 51 years, with a mean BMI of 34 kg/m2. Most (68%) of the participants were women.
After 12 months of follow-up, mean weight loss in the intensive lifestyle change, metformin, and placebo groups was 6.5%, 2.7%, and 0.4%, respectively. After 2 years of treatment, weight loss among those who reliably took their metformin pills was approximately 4%, while participants in the placebo group had a 1% weight gain. Among those who continued to reliably take their metformin pills, the weight loss persisted through 9 years of follow up.
The mechanisms by which metformin causes weight loss are not clear. Metformin stimulates phosphorylation of adenosine monophosphate (AMP)-activated protein kinase, which regulates mitochondrial function, hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis.7
Many ObGyns have experience in using metformin for the treatment of polycystic ovary syndrome or gestational diabetes. Hence, the dosing and adverse effects of metformin are familiar to many obstetricians-gynecologists. Metformin is contraindicated in individuals with creatinine clearance less than 30 mL/min. Rarely, metformin can cause lactic acidosis. According to Lexicomp,8 the most common adverse effects of metformin extended release (metformin ER) are diarrhea (17%), nausea and vomiting (7%), and decreased vitamin B12 concentration (7%) due to malabsorption in the terminal ileum. Of note, in the DPP study, hemoglobin concentration was slightly lower over time in the metformin compared with the placebo group (13.6 mg/dL vs 13.8 mg/dL, respectively; P<.001).6 Some experts recommend annual vitamin B12 measurement in individuals taking metformin.
In my practice, I only prescribe metformin ER. I usually start metformin treatment with one 750 mg ER tablet with dinner. If the patient tolerates that dose, I increase the dose to two 750 mg ER tablets with dinner. Metformin-induced adverse effects include diarrhea (17%) and nausea and vomiting (7%). Metformin ER is inexpensive. A one-month supply of metformin (sixty 750 mg tablets) costs between $4 and $21 at major pharmacies.9 Health insurance companies generally do not require preauthorization to cover metformin prescriptions.
Weight loss medications
US Food and Drug Administration (FDA)-approved weight loss medications include: liraglutide (Victoza), orlistat (Xenical, Alli), combination phentermine-extended release topiramate (Qsymia), and combination extended release naltrexone-bupropion (Contrave). All FDA-approved weight loss medications result in mean weight loss in the range of 6% to 10%. Many of these medications are very expensive (more than $200 per month).10 Insurance preauthorization is commonly required for these medications. For ObGyns, it may be best to refer patients who would like to use a weight loss medication to a specialist or specialty center with expertise in using these medications.
Sustainable weight loss is very difficult to achieve through dieting alone. A multitude of dietary interventions have been presented as “revolutionary approaches” to the challenging problem of sustainable weight loss, including the Paleo diet, the Vegan diet, the low-carb diet, the Dukan diet, the ultra-lowfat diet, the Atkins diet, the HCG diet, the Zone diet, the South Beach diet, the plant-based diet, the Mediterranean diet, the Asian diet, and intermittent fasting. Recently, intermittent fasting has been presented as the latest and greatest approach to dieting, with the dual goals of achieving weight loss and improved health.1 In some animal models, intermittent dieting has been shown to increase life-span, a finding that has attracted great interest. A major goal of intermittent fasting is to promote “metabolic switching” with increased reliance on ketones to fuel cellular energy needs.
Two approaches to “prescribing” an intermittent fasting diet are to limit food intake to a period of 6 to 10 hours each day or to markedly reduce caloric intake one or two days per week, for example to 750 calories in a 24-hour period. There are no long-term studies of the health outcomes associated with intermittent fasting. In head-to-head clinical trials of intermittent fasting and daily calorie restriction (classic dieting), both diets result in similar weight loss. For example, in one clinical trial 100 obese participants, with a mean body mass index (BMI) of 34 kg/m2 , including 86 women, were randomly assigned to2:
1. intermittent fasting (25% of energy needs every other day)
2. daily calorie restriction (75% of energy needs every day), or
3. no intervention.
After 12 months of follow up, the participants in the no intervention group had gained 0.5% of their starting weight. The intermittent fasting and the daily calorie restriction groups had similar amounts of weight loss, approximately 5% of their starting weight. More individuals dropped out of the study from the intermittent fasting group than the daily calorie restriction group (38% vs 29%, respectively).
In another clinical trial, 107 overweight or obese premenopausal women, average age 40 years and mean BMI 31 kg/m2 , were randomly assigned to intermittent fasting (25% of energy needs 2 days per week) or daily calorie restriction (75% of energy needs daily) for 6 months. The mean weight of the participants at baseline was 83 kg. Weight loss was similar in the intermittent fasting and daily calorie restriction groups, 6.4 kg (-7.7%) and 5.6 kg (-6.7%), respectively (P=.4).3
The investigators concluded that intermittent fasting and daily calorie restriction could both be offered as effective approaches to weight loss. My conclusion is that intermittent fasting is not a miracle dietary intervention, but it is another important option in the armamentarium of weight loss interventions.
References
1. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging and disease. N Engl J Med. 2019;381:2541-2551.
2. Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177:930-938.
3. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disc disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727.
Sleeve gastrectomy
Two children are playing in a school yard. One child proudly states, “My mother is an endocrinologist. She treats diabetes.” Not to be outdone, the other child replies, “My mother is a bariatric surgeon. She cures diabetes.”
The dialogue reflects the reality that bariatric surgery results in more reliable and significant weight loss than diet, exercise, or weight loss medications. Diet, exercise, and weight loss medications often result in a 5% to 10% decrease in weight, but bariatric surgery typically results in a 25% decrease in weight. Until recently, 3 bariatric surgical procedures were commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (AGB). AGB is now seldom performed because it is less effective than RYGB and SG. Two recently published randomized trials compared the long-term outcomes associated with RYGB and SG. The studies found that SG and RYGB result in a similar degree of weight loss. RYGB resulted in slightly more weight loss than SG, but SG was associated with a lower rate of major complications, such as internal hernias. SG takes much less time to perform than RYGB. SG has become the most commonly performed bariatric surgery in premenopausal women considering pregnancy because of the low risk of internal hernias.
In the Swiss Multicenter Bypass or Sleeve Study (SM-BOSS), 217 participants with a mean BMI of 44 kg/m2 and mean age of 45.5 years were randomly assigned to RYGB or SG and followed for 5 years.11 The majority (72%) of the participants were women. At 5 years of follow-up, in the RYGB and SG groups, mean weight loss was 37 kg and 33 kg, respectively (P=.19). In both groups, weight loss nadir was reached 12 to 24 months after surgery. Expressed as a percentage of original weight, weight loss in the RYGB and SG groups was -29% and -25%, respectively (P=.02). Gastric reflux worsened in both the RYGB and SG groups (6% vs 32%, respectively). The number of reoperations in the RYGB and SG groups was 22% and 16%. Of note, among individuals with prevalent diabetes, RYGB and SG resulted in remission of the diabetes in 68% and 62% of participants, respectively.
In the Sleeve vs Bypass study (SLEEVEPASS), 240 participants, with mean BMI of 46 kg/m2 and mean age of 48 years, were randomly assigned to RYGB or SG and followed for 5 years.12 Most (70%) of the participants were women. Following bariatric surgery, BMI decreased significantly in both groups. In the RYGB group, BMI decreased from 48 kg/m2 preoperatively to 35.4 kg/m2 at 5 years of follow up. In the SG group, BMI decreased from 47 kg/m2 preoperatively to 36.5 kg/m2 at 5 years of follow up. Late major complications (defined as complications occurring from 30 days to 5 years postoperatively) occurred more frequently in the RYGB group (15%) versus the SG group (8%). All the late major complications required reoperation. In the SG group, 7 of 10 reoperations were for severe gastric reflux disease. In the RYGB group 17 of 18 reoperations were for suspected internal hernia, requiring closure of a mesenteric defect at reoperation. There was no treatment-related mortality during the 5-year follow up.
Guidelines for bariatric surgery are BMI ≥ 40 kg/m2 without a comorbid illness or BMI ≥ 35 kg/m2 with at least one serious comorbid disease, such as diabetes.13 ObGyns can build a synergistic relationship with bariatric surgeons by referring eligible patients for surgical consultation and, in return, accepting referrals. A paradox and challenge is that many health insurers require patients to complete a supervised medical weight loss management program prior to being approved for bariatric surgery. However, the medical weight loss program might result in the patient no longer being eligible for insurance coverage of their surgery. For example, a patient who had a BMI of 42 kg/m2 prior to a medical weight loss management program who then lost enough weight to achieve a BMI of 38 kg/m2 might no longer be eligible for insurance coverage of a bariatric operation.14
Continue to: ObGyns need to prioritize treatment for obesity...
ObGyns need to prioritize treatment for obesity
Between 1959 and 2014, US life expectancy increased from 69.9 years to 79.1 years. However, in 2015 and 2016 life expectancy in the United States decreased slightly to 78.9 years, while continuing to improve in other countries.15 What could cause such an unexpected trend? Some experts believe that excess overweight and obesity in the US population, resulting in increased rates of diabetes, hypertension, and heart disease, accounts for a significant proportion of the life expectancy gap between US citizens and those who reside in Australia, Finland, Japan, and Sweden.16,17 All frontline clinicians play an important role in reversing the decades-long trend of increasing rates of overweight and obesity. Interventions that ObGyns could prioritize in their practices for treating overweight and obese patients include: a calorie-restricted diet, exercise, metformin, and SG.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
- Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530-1538.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440-2450.
- American Heart Association. My life check | Life’s simple 7. https://www.heart.org/en/healthyliving/healthy-lifestyle/my-life-check--lifessimple-7. Reviewed May 2, 2018. Accessed February 10, 2020.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
- Diabetes Prevention Program Research Group. Long-term safety, tolerability and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731-737.
- Winder WW, Hardie DG. Inactivation of acetylCoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270(2 pt 1):E299-E304.
- Lexicomp. https://online.lexi.com/lco/action/ home. Accessed February 13, 2020.
- Metformin ER (Glucophage XR). GoodRX website. https://www.goodrx.com/metformin-erglucophage-xr?dosage=750mg&form=tablet&la bel_override=metformin+ER+%28Glucophage+X R%29&quantity=60. Accessed February 13, 2020.
- GoodRX website. www.goodrx.com. Accessed February 10, 2020.
- Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255-265.
- Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241-254.
- Rubino F, Nathan DM, Eckel RH, et al; Delegates of the 2nd Diabetes Surgery Summit. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2-21.
- Gebran SG, Knighton B, Ngaage LM, et al. Insurance coverage criteria for bariatric surgery: a survey of policies. Obes Surg. 2020;30:707-713.
- Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
- Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2019;115:957-961.
- Xu H, Cupples LA, Stokes A, et al. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Network Open. 2018;1:e184587.
Abbreviated MRI bests digital breast tomosynthesis in finding cancer in dense breasts
For women with dense breasts, abbreviated magnetic resonance imaging was more effective than was digital breast tomosynthesis for detecting invasive breast cancer in a cross-sectional study of 1,444 women who underwent both procedures.
Dense breasts are a common reason for failed early diagnosis of breast cancer, wrote Christopher E. Comstock, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues. Digital breast tomosynthesis (DBT) and abbreviated breast magnetic resonance imaging (MRI) are becoming more popular as safe and cost-effective breast cancer screening options, but their effectiveness in women with dense breasts and average breast cancer risk has not been compared.
The researchers reviewed data from 1,444 women aged 40-75 years at 47 institutions in the United States and 1 in Germany. The women underwent both DBT and MRI. The primary endpoint was the detection of invasive cancers, of which 17 were identified at baseline screening. Abbreviated breast MRI detected all 17 cases of invasive cancer, compared with 7 detected by DBT. In addition, MRI detected six of seven women with ductal carcinoma in situ, while DBT identified two of the seven cases, according to the study, which was published in JAMA.
Overall, the invasive cancer detection rate was 11.8 per 1,000 women for MRI compared with 4.8 per 1,000 women for DBT. Sensitivity for MRI and DBT was 96% vs. 39%, and specificity was 87% vs. 97%.
The rate of recommendation for further screening was not significantly different between the procedures (8% for MRI and 10% for DBT). The most common adverse events were three cases of mild allergic reactions and two cases of anxiety.
The study findings were limited by several factors including the inability to show an association between abbreviated breast MRI and breast cancer mortality and the lack of cost-effectiveness comparisons for the two procedures. Because eligibility criteria required a prior breast mammogram to see if the breasts were dense, the study compared an incidence DBT screen to a prevalence abbreviated MRI screen, Dr. Comstock and associates noted.
However, the results show a significantly increased breast cancer detection rate with abbreviated MRI, which merits additional research to examine the relationship between screening strategies and clinical outcomes for women with dense breasts, they said.
The study was supported in part by the National Cancer Institute of the National Institutes of Health, and by Bracco Diagnostics through funding to the ECOG-ACRIN Cancer Research Group. Dr. Comstock disclosed financial relationships with Bracco Diagnostics and Bayer, and three coauthors disclosed financial relationships with other imaging companies. The remaining coauthors had no relevant financial disclosures.
SOURCE: Comstock CK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0572.
For women with dense breasts, abbreviated magnetic resonance imaging was more effective than was digital breast tomosynthesis for detecting invasive breast cancer in a cross-sectional study of 1,444 women who underwent both procedures.
Dense breasts are a common reason for failed early diagnosis of breast cancer, wrote Christopher E. Comstock, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues. Digital breast tomosynthesis (DBT) and abbreviated breast magnetic resonance imaging (MRI) are becoming more popular as safe and cost-effective breast cancer screening options, but their effectiveness in women with dense breasts and average breast cancer risk has not been compared.
The researchers reviewed data from 1,444 women aged 40-75 years at 47 institutions in the United States and 1 in Germany. The women underwent both DBT and MRI. The primary endpoint was the detection of invasive cancers, of which 17 were identified at baseline screening. Abbreviated breast MRI detected all 17 cases of invasive cancer, compared with 7 detected by DBT. In addition, MRI detected six of seven women with ductal carcinoma in situ, while DBT identified two of the seven cases, according to the study, which was published in JAMA.
Overall, the invasive cancer detection rate was 11.8 per 1,000 women for MRI compared with 4.8 per 1,000 women for DBT. Sensitivity for MRI and DBT was 96% vs. 39%, and specificity was 87% vs. 97%.
The rate of recommendation for further screening was not significantly different between the procedures (8% for MRI and 10% for DBT). The most common adverse events were three cases of mild allergic reactions and two cases of anxiety.
The study findings were limited by several factors including the inability to show an association between abbreviated breast MRI and breast cancer mortality and the lack of cost-effectiveness comparisons for the two procedures. Because eligibility criteria required a prior breast mammogram to see if the breasts were dense, the study compared an incidence DBT screen to a prevalence abbreviated MRI screen, Dr. Comstock and associates noted.
However, the results show a significantly increased breast cancer detection rate with abbreviated MRI, which merits additional research to examine the relationship between screening strategies and clinical outcomes for women with dense breasts, they said.
The study was supported in part by the National Cancer Institute of the National Institutes of Health, and by Bracco Diagnostics through funding to the ECOG-ACRIN Cancer Research Group. Dr. Comstock disclosed financial relationships with Bracco Diagnostics and Bayer, and three coauthors disclosed financial relationships with other imaging companies. The remaining coauthors had no relevant financial disclosures.
SOURCE: Comstock CK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0572.
For women with dense breasts, abbreviated magnetic resonance imaging was more effective than was digital breast tomosynthesis for detecting invasive breast cancer in a cross-sectional study of 1,444 women who underwent both procedures.
Dense breasts are a common reason for failed early diagnosis of breast cancer, wrote Christopher E. Comstock, MD, of Memorial Sloan Kettering Cancer Center, New York, and colleagues. Digital breast tomosynthesis (DBT) and abbreviated breast magnetic resonance imaging (MRI) are becoming more popular as safe and cost-effective breast cancer screening options, but their effectiveness in women with dense breasts and average breast cancer risk has not been compared.
The researchers reviewed data from 1,444 women aged 40-75 years at 47 institutions in the United States and 1 in Germany. The women underwent both DBT and MRI. The primary endpoint was the detection of invasive cancers, of which 17 were identified at baseline screening. Abbreviated breast MRI detected all 17 cases of invasive cancer, compared with 7 detected by DBT. In addition, MRI detected six of seven women with ductal carcinoma in situ, while DBT identified two of the seven cases, according to the study, which was published in JAMA.
Overall, the invasive cancer detection rate was 11.8 per 1,000 women for MRI compared with 4.8 per 1,000 women for DBT. Sensitivity for MRI and DBT was 96% vs. 39%, and specificity was 87% vs. 97%.
The rate of recommendation for further screening was not significantly different between the procedures (8% for MRI and 10% for DBT). The most common adverse events were three cases of mild allergic reactions and two cases of anxiety.
The study findings were limited by several factors including the inability to show an association between abbreviated breast MRI and breast cancer mortality and the lack of cost-effectiveness comparisons for the two procedures. Because eligibility criteria required a prior breast mammogram to see if the breasts were dense, the study compared an incidence DBT screen to a prevalence abbreviated MRI screen, Dr. Comstock and associates noted.
However, the results show a significantly increased breast cancer detection rate with abbreviated MRI, which merits additional research to examine the relationship between screening strategies and clinical outcomes for women with dense breasts, they said.
The study was supported in part by the National Cancer Institute of the National Institutes of Health, and by Bracco Diagnostics through funding to the ECOG-ACRIN Cancer Research Group. Dr. Comstock disclosed financial relationships with Bracco Diagnostics and Bayer, and three coauthors disclosed financial relationships with other imaging companies. The remaining coauthors had no relevant financial disclosures.
SOURCE: Comstock CK et al. JAMA. 2020 Feb 25. doi: 10.1001/jama.2020.0572.
FROM JAMA
Community-wide initiative ups teen LARC adoption sixfold
In Rochester, N.Y., a comprehensive community initiative that raised awareness about and delivered training in the use of long-acting reversible contraceptives (LARCs) significantly upped LARC adoption among sexually active female high schoolers.
Over the course of the 3-year project, LARC use rose from about 4% to 24% in this group, a statistically significant increase (P less than .0001). During the same time period, LARC use increased nationally, as well, but at a lower rate, rising from 2% to 5% for the same population, while New York state saw LARC use rise from 2% to 5%.
In New York City, where an unrelated LARC awareness campaign was conducted, LARC use went from 3% to 5% over the study period for sexually active female high school students. Comparing the trend in LARC use in Rochester to the secular trend in these control groups showed significantly higher uptake over time in Rochester (P less than .0001).
Through a series of lunch-and-learn talks given to adults who work with adolescents in community-based settings and in medical settings, the Greater Rochester LARC Initiative reached more than 1,300 individuals during July 2014-June 2017, C. Andrew Aligne, MD, MPH, of the University of Rochester (N.Y.), and coauthors reported in the American Journal of Obstetrics and Gynecology.
Of the 81 total talks delivered, 50 were in medical settings, reaching 703 attendees ranging from front-office personnel to primary care physicians, advanced practice clinicians, and nurses; the talks in community-based settings reached 662 attendees.
“We use the term ‘community detailing’ to describe the design of the intervention because it was an innovative hybrid of academic detailing and community health education,” explained Dr. Aligne and colleagues. This approach is a unique, feasible, and effective approach to unintended adolescent pregnancy programs. “The community detailing approach could be a useful complement to programs for preventing unintended adolescent pregnancy.”
The study’s primary outcome measure was LARC use among sexually active female high school students as identified by responses on the U.S. Centers for Disease Control and Statistics’ Youth Risk Behavior Survey (YRBS).
YRBS data were examined for the years 2013, 2015, and 2017, spanning the period before and after the LARC initiative was begun. A separate question about LARC use wasn’t included in the 2013 YRBS survey, so the investigators used a generous estimate that two-thirds of respondents who reported using the “other” contraceptive category for that year were using LARCs. That category was chosen by a total of 6% of respondents, and encompassed LARC use along with use of the patch, ring, diaphragm, and fertility awareness, explained Dr. Aligne and collaborators.
Addressing the problem of failure to use a condom with LARC use, Dr. Aligne and collaborators found overall low rates of dual-method use, but higher rates in Rochester than in the comparison groups. In Rochester, 78% of respondents reported that they also did not use condoms. This figure was lower than the 91% reported for the United States as a whole, and also was lower than the 93% reported in New York City and the 85% reported in New York state. No increase in sexually transmitted infections was seen in Rochester’s sexually active high school females during the study period.
“Our main finding of increased LARC use is consistent with the literature demonstrating that many sexually active young women, including adolescents, will choose LARC if they are given access not only to birth control itself, but also to accurate information about various contraceptive methods,” concluded Dr. Aligne and his associates.
A practical strength of the Greater Rochester LARC initiative was that it capitalized on existing resources, such as New York state’s preexisting program for free access to contraception and similar provisions in the Affordable Care Act. Also, local Title X clinics that were enrolled in New York’s free contraception initiative already had practitioners who were trained and able to provide same-day LARC insertion.
Pediatricians engaged in the initiative were able to receive free training from LARC manufacturers, as mandated by the Food and Drug Administration. Through collaboration with implant manufacturers, Rochester LARC Initiative staff were able to piggyback on training sessions to add education about contraception counseling and the importance of offering access to all contraception methods.
Taken as a whole, the LARC Initiative could be scaled up, wrote Dr. Aligne and his coauthors, a potential boon in the 21 states where qualifying individuals younger than 19 years of age are eligible for Medicaid reimbursement for family planning services. “Even though easy LARC access is far from universal, there are vast areas of the nation where cost need not be seen as an insurmountable barrier.” Dr. Aligne and coauthors also addressed the fraught history of reproductive justice in the United States, cautioning that universal LARC adoption was not – and should not be – the goal of such initiatives. “There is a history of reproductive coercion in the U.S. including forced sterilization of women of color; therefore, it is critical that LARC methods not be imposed on any particular group. On the other hand, LARC should not be withheld deliberately from adolescents who want it, as this is another form of injustice,” they wrote. “The goal should be to empower individuals to decide what is right for them in a context of social and reproductive justice.”
Using the nationally administered YRBS was a significant strength of the study, commented Dr. Aligne and his collaborators. “This allowed us to employ the study design of pre-post with a nonrandomized control group,” the investigators noted, adding that the “relatively rigorous” methodology reduced the risk of problems with internal validity, and also allowed comparisons between changes in Rochester and those at the state and national level.
However, the researchers acknowledged that the study was not a randomized trial, and there’s always the possibility of unknown confounders contributing to LARC uptake during the study period. Also, the YRBS is a self-report instrument and only includes those enrolled in school.
Dr. Aligne reported that his spouse received compensation for providing contraceptive implant insertion training, as did two coauthors. The LARC initiative was supported by a grant from the Greater Rochester Health Foundation.
SOURCE: Aligne CA et al. Am J Obstet Gynecol. 2020 Jan 22. doi: 10.1016/j.ajog.2020.01.029.
In Rochester, N.Y., a comprehensive community initiative that raised awareness about and delivered training in the use of long-acting reversible contraceptives (LARCs) significantly upped LARC adoption among sexually active female high schoolers.
Over the course of the 3-year project, LARC use rose from about 4% to 24% in this group, a statistically significant increase (P less than .0001). During the same time period, LARC use increased nationally, as well, but at a lower rate, rising from 2% to 5% for the same population, while New York state saw LARC use rise from 2% to 5%.
In New York City, where an unrelated LARC awareness campaign was conducted, LARC use went from 3% to 5% over the study period for sexually active female high school students. Comparing the trend in LARC use in Rochester to the secular trend in these control groups showed significantly higher uptake over time in Rochester (P less than .0001).
Through a series of lunch-and-learn talks given to adults who work with adolescents in community-based settings and in medical settings, the Greater Rochester LARC Initiative reached more than 1,300 individuals during July 2014-June 2017, C. Andrew Aligne, MD, MPH, of the University of Rochester (N.Y.), and coauthors reported in the American Journal of Obstetrics and Gynecology.
Of the 81 total talks delivered, 50 were in medical settings, reaching 703 attendees ranging from front-office personnel to primary care physicians, advanced practice clinicians, and nurses; the talks in community-based settings reached 662 attendees.
“We use the term ‘community detailing’ to describe the design of the intervention because it was an innovative hybrid of academic detailing and community health education,” explained Dr. Aligne and colleagues. This approach is a unique, feasible, and effective approach to unintended adolescent pregnancy programs. “The community detailing approach could be a useful complement to programs for preventing unintended adolescent pregnancy.”
The study’s primary outcome measure was LARC use among sexually active female high school students as identified by responses on the U.S. Centers for Disease Control and Statistics’ Youth Risk Behavior Survey (YRBS).
YRBS data were examined for the years 2013, 2015, and 2017, spanning the period before and after the LARC initiative was begun. A separate question about LARC use wasn’t included in the 2013 YRBS survey, so the investigators used a generous estimate that two-thirds of respondents who reported using the “other” contraceptive category for that year were using LARCs. That category was chosen by a total of 6% of respondents, and encompassed LARC use along with use of the patch, ring, diaphragm, and fertility awareness, explained Dr. Aligne and collaborators.
Addressing the problem of failure to use a condom with LARC use, Dr. Aligne and collaborators found overall low rates of dual-method use, but higher rates in Rochester than in the comparison groups. In Rochester, 78% of respondents reported that they also did not use condoms. This figure was lower than the 91% reported for the United States as a whole, and also was lower than the 93% reported in New York City and the 85% reported in New York state. No increase in sexually transmitted infections was seen in Rochester’s sexually active high school females during the study period.
“Our main finding of increased LARC use is consistent with the literature demonstrating that many sexually active young women, including adolescents, will choose LARC if they are given access not only to birth control itself, but also to accurate information about various contraceptive methods,” concluded Dr. Aligne and his associates.
A practical strength of the Greater Rochester LARC initiative was that it capitalized on existing resources, such as New York state’s preexisting program for free access to contraception and similar provisions in the Affordable Care Act. Also, local Title X clinics that were enrolled in New York’s free contraception initiative already had practitioners who were trained and able to provide same-day LARC insertion.
Pediatricians engaged in the initiative were able to receive free training from LARC manufacturers, as mandated by the Food and Drug Administration. Through collaboration with implant manufacturers, Rochester LARC Initiative staff were able to piggyback on training sessions to add education about contraception counseling and the importance of offering access to all contraception methods.
Taken as a whole, the LARC Initiative could be scaled up, wrote Dr. Aligne and his coauthors, a potential boon in the 21 states where qualifying individuals younger than 19 years of age are eligible for Medicaid reimbursement for family planning services. “Even though easy LARC access is far from universal, there are vast areas of the nation where cost need not be seen as an insurmountable barrier.” Dr. Aligne and coauthors also addressed the fraught history of reproductive justice in the United States, cautioning that universal LARC adoption was not – and should not be – the goal of such initiatives. “There is a history of reproductive coercion in the U.S. including forced sterilization of women of color; therefore, it is critical that LARC methods not be imposed on any particular group. On the other hand, LARC should not be withheld deliberately from adolescents who want it, as this is another form of injustice,” they wrote. “The goal should be to empower individuals to decide what is right for them in a context of social and reproductive justice.”
Using the nationally administered YRBS was a significant strength of the study, commented Dr. Aligne and his collaborators. “This allowed us to employ the study design of pre-post with a nonrandomized control group,” the investigators noted, adding that the “relatively rigorous” methodology reduced the risk of problems with internal validity, and also allowed comparisons between changes in Rochester and those at the state and national level.
However, the researchers acknowledged that the study was not a randomized trial, and there’s always the possibility of unknown confounders contributing to LARC uptake during the study period. Also, the YRBS is a self-report instrument and only includes those enrolled in school.
Dr. Aligne reported that his spouse received compensation for providing contraceptive implant insertion training, as did two coauthors. The LARC initiative was supported by a grant from the Greater Rochester Health Foundation.
SOURCE: Aligne CA et al. Am J Obstet Gynecol. 2020 Jan 22. doi: 10.1016/j.ajog.2020.01.029.
In Rochester, N.Y., a comprehensive community initiative that raised awareness about and delivered training in the use of long-acting reversible contraceptives (LARCs) significantly upped LARC adoption among sexually active female high schoolers.
Over the course of the 3-year project, LARC use rose from about 4% to 24% in this group, a statistically significant increase (P less than .0001). During the same time period, LARC use increased nationally, as well, but at a lower rate, rising from 2% to 5% for the same population, while New York state saw LARC use rise from 2% to 5%.
In New York City, where an unrelated LARC awareness campaign was conducted, LARC use went from 3% to 5% over the study period for sexually active female high school students. Comparing the trend in LARC use in Rochester to the secular trend in these control groups showed significantly higher uptake over time in Rochester (P less than .0001).
Through a series of lunch-and-learn talks given to adults who work with adolescents in community-based settings and in medical settings, the Greater Rochester LARC Initiative reached more than 1,300 individuals during July 2014-June 2017, C. Andrew Aligne, MD, MPH, of the University of Rochester (N.Y.), and coauthors reported in the American Journal of Obstetrics and Gynecology.
Of the 81 total talks delivered, 50 were in medical settings, reaching 703 attendees ranging from front-office personnel to primary care physicians, advanced practice clinicians, and nurses; the talks in community-based settings reached 662 attendees.
“We use the term ‘community detailing’ to describe the design of the intervention because it was an innovative hybrid of academic detailing and community health education,” explained Dr. Aligne and colleagues. This approach is a unique, feasible, and effective approach to unintended adolescent pregnancy programs. “The community detailing approach could be a useful complement to programs for preventing unintended adolescent pregnancy.”
The study’s primary outcome measure was LARC use among sexually active female high school students as identified by responses on the U.S. Centers for Disease Control and Statistics’ Youth Risk Behavior Survey (YRBS).
YRBS data were examined for the years 2013, 2015, and 2017, spanning the period before and after the LARC initiative was begun. A separate question about LARC use wasn’t included in the 2013 YRBS survey, so the investigators used a generous estimate that two-thirds of respondents who reported using the “other” contraceptive category for that year were using LARCs. That category was chosen by a total of 6% of respondents, and encompassed LARC use along with use of the patch, ring, diaphragm, and fertility awareness, explained Dr. Aligne and collaborators.
Addressing the problem of failure to use a condom with LARC use, Dr. Aligne and collaborators found overall low rates of dual-method use, but higher rates in Rochester than in the comparison groups. In Rochester, 78% of respondents reported that they also did not use condoms. This figure was lower than the 91% reported for the United States as a whole, and also was lower than the 93% reported in New York City and the 85% reported in New York state. No increase in sexually transmitted infections was seen in Rochester’s sexually active high school females during the study period.
“Our main finding of increased LARC use is consistent with the literature demonstrating that many sexually active young women, including adolescents, will choose LARC if they are given access not only to birth control itself, but also to accurate information about various contraceptive methods,” concluded Dr. Aligne and his associates.
A practical strength of the Greater Rochester LARC initiative was that it capitalized on existing resources, such as New York state’s preexisting program for free access to contraception and similar provisions in the Affordable Care Act. Also, local Title X clinics that were enrolled in New York’s free contraception initiative already had practitioners who were trained and able to provide same-day LARC insertion.
Pediatricians engaged in the initiative were able to receive free training from LARC manufacturers, as mandated by the Food and Drug Administration. Through collaboration with implant manufacturers, Rochester LARC Initiative staff were able to piggyback on training sessions to add education about contraception counseling and the importance of offering access to all contraception methods.
Taken as a whole, the LARC Initiative could be scaled up, wrote Dr. Aligne and his coauthors, a potential boon in the 21 states where qualifying individuals younger than 19 years of age are eligible for Medicaid reimbursement for family planning services. “Even though easy LARC access is far from universal, there are vast areas of the nation where cost need not be seen as an insurmountable barrier.” Dr. Aligne and coauthors also addressed the fraught history of reproductive justice in the United States, cautioning that universal LARC adoption was not – and should not be – the goal of such initiatives. “There is a history of reproductive coercion in the U.S. including forced sterilization of women of color; therefore, it is critical that LARC methods not be imposed on any particular group. On the other hand, LARC should not be withheld deliberately from adolescents who want it, as this is another form of injustice,” they wrote. “The goal should be to empower individuals to decide what is right for them in a context of social and reproductive justice.”
Using the nationally administered YRBS was a significant strength of the study, commented Dr. Aligne and his collaborators. “This allowed us to employ the study design of pre-post with a nonrandomized control group,” the investigators noted, adding that the “relatively rigorous” methodology reduced the risk of problems with internal validity, and also allowed comparisons between changes in Rochester and those at the state and national level.
However, the researchers acknowledged that the study was not a randomized trial, and there’s always the possibility of unknown confounders contributing to LARC uptake during the study period. Also, the YRBS is a self-report instrument and only includes those enrolled in school.
Dr. Aligne reported that his spouse received compensation for providing contraceptive implant insertion training, as did two coauthors. The LARC initiative was supported by a grant from the Greater Rochester Health Foundation.
SOURCE: Aligne CA et al. Am J Obstet Gynecol. 2020 Jan 22. doi: 10.1016/j.ajog.2020.01.029.
FROM AJOG
June Medical Services v. Russo: Understanding this high-stakes abortion case
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
On March 4, 2020, the Supreme Court of the United States (SCOTUS) will hear opening arguments for June Medical Services v. Russo. (Please note that this case was originally referred to as June Medical Services v. Gee. However, Secretary Rebekah Gee resigned from her position on January 31, 2020, and was replaced by Interim Secretary Stephen Russo.) The case will examine a Louisiana law (Louisiana Act 620, or LA 620), originally passed in 2014, that requires physicians to have hospital admitting privileges within 30 miles of where they provide abortion services.1 When LA 620 was signed into law in 2014, 5 of Louisiana’s 6 abortion clinics would not have met the standards created by this legislation and would have been forced to close, potentially leaving the vast majority of women in Louisiana without access to an abortion provider, and disproportionately impacting poor and rural women. Prior to enactment of this law, physicians at these 5 clinics attempted to obtain admitting privileges, and all were denied. The denials occurred due to two main reasons—because the providers admitted too few patients each year to qualify for hospital privileges or simply because they provided abortion care.2 Shortly after this legislation was signed into law, the Center for Reproductive Rights (CRR) challenged the law, citing the undue burden it created for patients attempting to access abortion care.
Prior case also considered question of hospital privileges for abortion providers
Interestingly, SCOTUS already has ruled on this very question. In 1992, the Court ruled in Planned Parenthood of Southeastern Pennsylvania v. Casey that it is unconstitutional for a state to create an “undue burden” on a woman’s right to abortion prior to fetal viability.3 And in 2016, when considering whether or not requiring abortion providers to obtain hospital privileges creates an undue burden in Whole Women’s Health (WWH) v. Hellerstedt, the Supreme Court’s answer was yes, it does. WWH, with legal aid from CRR, challenged Texas House Bill 2 (H.B. 2), which similar to LA 620, required abortion providers to have local admitting privileges. Based largely on the precedent set in Casey, SCOTUS ruled 5-3 in favor of WWH.
The Louisiana law currently in question was written and challenged in district court simultaneous to the Supreme Court’s review of WWH. The district court declared LA 620 invalid and permanently enjoined its enforcement, finding the law would “drastically burden women’s right to choose abortions.”4 However, the US Court of Appeals for the Fifth Circuit reviewed the case and overturned the district court decision, finding the lower court’s analysis erroneous and stating, “no clinics will likely be forced to close on account of [LA 620].” The Fifth Circuit panel ruled that, despite the precedent of WWH, LA 620 did not create an undue burden because of state-level differences in admitting privileges, demographics, and geography. They also found that only 30% of the 2 million women living in Louisiana would be impacted by the law, predominantly via longer wait times, and argued that this does not represent significant burden. The plaintiffs filed for an emergency stay with SCOTUS, who granted the stay pending a full hearing. On March 4, the Supreme Court will hear arguments to determine if the Fifth Circuit was correct in drawing a distinction between LA 620 and the SCOTUS verdict in WWH.
Targeted restrictions on abortion providers
LA 620 joins a long series of laws meant to enact targeted restrictions on abortion providers, or “TRAP” laws. TRAP laws are written to limit access to abortion under the guise of improving patient safety, despite ample evidence to the contrary, and include such various regulations as admitting privileges, facilities requirements, waiting periods, and parental or partner notification. Many such laws have been enacted in the last decade, and many struck down based on judicial precedent.
How the Supreme Court has ruled in the past
When a case is appealed to the Supreme Court, the court can either decline to hear the case, thereby leaving the lower courts’ ruling in place, or choose to hear the case in full and either affirm or overturn the lower court’s decision. After issuing a ruling in WWH, the 2016-2017 Roberts Court declined to hear challenges from other states with similarly overturned laws, leaving the laws struck down. In electing to hear June Medical Services v. Russo, the court has the opportunity to uphold or overturn the Fifth Circuit Court’s decision. However, today’s Supreme Court differs greatly from the Supreme Court in 2016.
In 2016, the court ruled 5-3 to overturn H.B. 2 in WWH shortly after the death of Justice Antonin Scalia. Scalia was replaced by Justice Neil Gorsuch, a Constitutional originalist who has never directly ruled on an abortion case.5 In 2018, Justice Anthony Kennedy, who authored the court’s majority opinion on Casey and was among the majority on WWH, retired, and was replaced by Justice Brett Kavanaugh. Kavanaugh has ruled once on the right to abortion in Garza v. Hargan in 2017, where he argued that precedent states that the government has “permissible interests in favoring fetal life…and refraining from facilitating abortion,” and that significant delay in care did not constitute undue burden.6 In regard to the 5-4 stay issued by the court in June Medical Services, Kavanaugh joined Gorsuch in voting to deny the application for stay, and was the only justice to issue an opinion alongside the ruling, arguing that because the doctors in question had not applied for and been denied admitting privileges since the WWH ruling, the case hinges on theoretical rather than demonstrable undue burden.7 Appointed by President Donald Trump, both Gorsuch and Kavanaugh are widely considered to be conservative judges, and while neither has a strong judicial record on abortion rights, both are anticipated to side with the conservative majority on the court.
The Supreme Court rarely overturns its own precedent, but concerns are high
The question of precedent will be central in SCOTUS hearing June Medical Services v. Russo so quickly after the WWH decision. Additionally, in hearing this case, the court will have the opportunity to reexamine all relevant precedent, including the Planned Parenthood of Southeastern Pennsylvania v. Casey decision and even Roe v. Wade. With a conservative court and an increasingly charged political environment, reproductive rights advocates fear that the June Medical Services v. Russo ruling may be the first step toward dismantling judicial protection of abortion rights in the United States.
If SCOTUS rules against June Medical Services, stating that admitting privileges do not cause an undue burden for women seeking to access abortion care, other states likely will introduce and enact similar legislation. These TRAP laws have the potential to limit or eliminate access to abortion for 25 million people of reproductive age. Numerous studies have demonstrated that limiting access to abortion care does not decrease the number of abortions but can result in patients using unsafe means to obtain an abortion.8
The medical community recognizes the danger of enacting restrictive legislation. The American College of Obstetricians and Gynecologists (ACOG), along with the American Medical Association, the Society of Maternal-Fetal Medicine, the Association for Sexual and Reproductive Medicine, the American Association of Family Practitioners, and many others, filed an amicus curiae in support of the June Medical Services plaintiffs.9 These brief filings are critical to ensuring the courts hear physician voices in this important legal decision, and ACOG’s briefs have been quoted in several previous Supreme Court opinions, concurrences, and dissents.
Action items
- Although June Medical Services v. Russo’s decision will not be made until early summer 2020, we can continue to use our voices and expertise to speak out against laws designed to limit access to abortion—at the state and federal levels. As women’s health clinicians, we see the impact abortion restrictions have on our patients, especially our low income and rural patients. Sharing these stories with our legislators, testifying for or against legislation, and speaking out in our communities can have a powerful impact. Check with your local ACOG chapter or with ACOG’s state and government affairs office for more information.
- Follow along with this case at SCOTUS Blog.
- Lastly, make sure you are registered to vote. We are in an election year, and using our voices in and out of the ballot box is critical. You can register here.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
- HB338. Louisiana State Legislature. 2014. http://www.legis.la.gov/legis/BillInfo.aspx?s=14RS&b=ACT620&sbi=y. Accessed February 19, 2020.
- Nash E, Donovan MK. Admitting priveleges are back at the U.S. Supreme Court with serious implications for abortion access. Guttmacher Institute. Updated December 3, 2019.
- Planned Parenthood of Southeastern Pennsylvania v. Casey. Cornell Law School Legal Information Institute. https://www.law.cornell.edu/supremecourt/text/505/833. Accessed February 20, 2020.
- June Medical Services LLC v Gee. Oyez. www.oyez.org/cases/2019/18-1323. Accessed February 20, 2020.
- Neil Gorsuch. Oyez. https://www.oyez.org/justices/neil_gorsuch. Accessed February 20, 2020.
- Judge Kavanaugh’s Judicial Record on the Right to Abortion. Center for Reproductive Rights. https://www.reproductiverights.org/sites/crr.civicactions.net/files/documents/factsheets/Judge-Kavanaugh-Judicial-Record-on-the-Right-to-Abortion2.pdf. Accessed February 20, 2020.
- Kavanaugh B. (2019, February 7). June Medical Services, L.L.C, v. Gee, 586 U.S. ____ (2019). Supreme Court of the United States. https://www.supremecourt.gov/opinions/18pdf/18a774_3ebh.pdf. Accessed February 20, 2020.
- Cohen SA. Facts and consequences: Legality, incidence and safety of abortion worldwide. November 20, 2009.
- June Medical Services, LLC v. Russo. SCOTUSblog. February 6, 2020. https://www.scotusblog.com/case-files/cases/june-medical-services-llc-v-russo/. Accessed February 20, 2020.
Resident experience with hysterectomy is on the decline
The total number of hysterectomies performed during residency training has declined significantly since 2008, despite an increase in laparoscopic hysterectomies performed, according to a new analysis of data from graduating ob.gyn. residents that has implications for the structure of resident education.
The investigators abstracted case log data from the Accreditation Council for Graduate Medical Education (ACGME) database to assess trends in residents’ operative experience and found decreases in abdominal and vaginal cases but an increase in experience with laparoscopic hysterectomy.
(from 85 cases to 37), and the median number of vaginal hysterectomies decreased by 36% (from 31 to 20 cases).
Laparoscopic hysterectomy increased by 115% from a median of 20 procedures in 2008-2009 to 43 in 2017-2018. Even so, the median total number of hysterectomies per resident decreased by 6%, from 112 to 105 procedures during those two time periods. (Data on total hysterectomy and laparoscopic hysterectomy were not collected by ACGME until 2008.)
While the absolute decrease in the total number of hysterectomies is “relatively small,” the trend “raises questions about what the appropriate number of hysterectomies per graduating resident should be,” Gregory M. Gressel, MD, MSc, of the Montefiore Medical Center, New York, and coauthors wrote in Obstetrics & Gynecology.
“These data point,” they wrote, “to the necessity of maximizing surgical exposure in the face of a declining availability of procedures and the importance of reflecting on which (and how many) procedures an obstetrics and gynecology resident needs to complete before entering clinical practice.”
The training numbers parallel an increased use of laparoscopic hysterectomy in the United States and other countries, as well as a well-documented decline in the total number of hysterectomies performed in the United States, the latter of which is driven largely by the availability and increasing use of alternatives to the procedure (such as hormone therapy, endometrial ablation, and uterine artery embolization).
Hysterectomy still is a “core procedure of gynecologic surgery,” however, and is “at the heart of surgical training in obstetrics and gynecology,” as surgical techniques developed from learning hysterectomy “are applied broadly in the pelvis,” Saketh R. Guntupalli, MD, wrote in an accompanying editorial.
Dr. Guntupalli, of the University of Colorado at Aurora, Denver, was involved in a survey of fellowship program directors, published in 2015, that found only 20% of first-year fellows were able to independently perform a vaginal hysterectomy and 46% to independently perform an abdominal hysterectomy (Obstet Gynecol. 2015;126:559-68).
This and other research suggest that fellowship training is “used to address deficiencies in residency training rather than to develop new, specialized surgical skills,” he wrote. Given a dearth of fellowship positions in ob.gyn., “it is impossible to adequately use those avenues to train the number of competent surgeons necessary to address the surgical needs of women’s health in the United States.”
To address such concerns, some residency programs have instituted resident tracking to direct more hysterectomy cases toward those residents who plan to pursue surgical subspecialties. The Cleveland Clinic, Dr. Guntupalli noted, has tried the latter approach “with success.”
An increase in the number of accredited training programs and a decrease in the number of residents per program also might help to improve surgical exposure for residents, Dr. Gressel and associates wrote. Over the 16-year study period, the number of graduating residents increased significantly (by 12 per year) and the number of residency programs decreased significantly (0.52 fewer programs per year).
Additionally, Dr. Guntupalli wrote, regulatory bodies may need to reevaluate how competencies are assessed, and whether minimal numbers of cases “continue to carry the same weight as they did in previous generations.”
In the study, one coauthor is a full-time employee of ACGME, and another receives funds as a director for the American Board of Obstetrics and Gynecology. The remaining authors had no relevant financial disclosures. There was no outside funding for the study. Dr. Guntupalli said he had no conflicts of interest.
SOURCES: Gressel GM et al. Obstet Gynecol. 2020 Feb;135(2):268-73; Guntupalli SR. Obstet Gynecol 2020 Feb;135(2):266-7.
This excellent paper by Dr. Gressel and coauthors shows decreasing numbers of hysterectomies – especially open and vaginal approaches – being performed by ob.gyn. residents. Considering also the 2015 publication by Guntupalli et al. showing the low numbers of incoming fellows able to perform hysterectomy, as well as Dr. Guntupalli’s editorial on this new research, we all must question how our patients will be able to undergo safe and effective surgery in the future.
Furthermore, it would truly be disheartening and disconcerting for a young physician to choose a residency with the desire of a specific track, only to lose that choice to a coresident.
In his presidential address to the AAGL some years ago, Javier Magrina, MD, of the Mayo Clinic in Phoenix, discussed separating the “O from the G” (J Minim Invasive Gynecol. 2014;21[4]:501-3). Among his points: From 1979 to 2006, there was a 46% decrease in the number of gynecologic operations (2,852,000 vs. 1,309,000), a 54% increase in the number of American College of Obstetricians and Gynecologists’ fellows (21,364 vs. 51,123), and an 81% decrease in the number of gynecologic operations performed per ACOG fellow (132 vs. 25).
In 1980, he pointed out, the total number of hysterectomy procedures performed in the United States was 647,000. In 2007, this total was 517,000. The total number of ACOG fellows in 1980 was 22,516, compared with 52,385 in 2007. And the total number of hysterectomies performed per ACOG fellow was 28, compared with 9.8 hysterectomies per fellow in 2007.
Dr. Magrina’s data goes hand in hand with Dr. Gressel’s new study. The surgical experience of the gynecologic surgeon certainly is on the wane. The result of this lack of experience is noted by Dr. Guntupalli in his 2015 publication. To us, it is readily apparent that Dr. Magrina is right: The only true solution is to finally realize that we must separate the O from the G.
Charles E. Miller, MD, is director of minimally invasive gynecologic surgery, and director of the AAGL fellowship in minimally invasive gynecologic surgery, at Advocate Lutheran General Hospital, Park Ridge, Ill. Kirsten Sasaki, MD, is associate director of the AAGL fellowship in minimally invasive gynecologic surgery at Advocate Lutheran. They have no other conflicts of interest.
This excellent paper by Dr. Gressel and coauthors shows decreasing numbers of hysterectomies – especially open and vaginal approaches – being performed by ob.gyn. residents. Considering also the 2015 publication by Guntupalli et al. showing the low numbers of incoming fellows able to perform hysterectomy, as well as Dr. Guntupalli’s editorial on this new research, we all must question how our patients will be able to undergo safe and effective surgery in the future.
Furthermore, it would truly be disheartening and disconcerting for a young physician to choose a residency with the desire of a specific track, only to lose that choice to a coresident.
In his presidential address to the AAGL some years ago, Javier Magrina, MD, of the Mayo Clinic in Phoenix, discussed separating the “O from the G” (J Minim Invasive Gynecol. 2014;21[4]:501-3). Among his points: From 1979 to 2006, there was a 46% decrease in the number of gynecologic operations (2,852,000 vs. 1,309,000), a 54% increase in the number of American College of Obstetricians and Gynecologists’ fellows (21,364 vs. 51,123), and an 81% decrease in the number of gynecologic operations performed per ACOG fellow (132 vs. 25).
In 1980, he pointed out, the total number of hysterectomy procedures performed in the United States was 647,000. In 2007, this total was 517,000. The total number of ACOG fellows in 1980 was 22,516, compared with 52,385 in 2007. And the total number of hysterectomies performed per ACOG fellow was 28, compared with 9.8 hysterectomies per fellow in 2007.
Dr. Magrina’s data goes hand in hand with Dr. Gressel’s new study. The surgical experience of the gynecologic surgeon certainly is on the wane. The result of this lack of experience is noted by Dr. Guntupalli in his 2015 publication. To us, it is readily apparent that Dr. Magrina is right: The only true solution is to finally realize that we must separate the O from the G.
Charles E. Miller, MD, is director of minimally invasive gynecologic surgery, and director of the AAGL fellowship in minimally invasive gynecologic surgery, at Advocate Lutheran General Hospital, Park Ridge, Ill. Kirsten Sasaki, MD, is associate director of the AAGL fellowship in minimally invasive gynecologic surgery at Advocate Lutheran. They have no other conflicts of interest.
This excellent paper by Dr. Gressel and coauthors shows decreasing numbers of hysterectomies – especially open and vaginal approaches – being performed by ob.gyn. residents. Considering also the 2015 publication by Guntupalli et al. showing the low numbers of incoming fellows able to perform hysterectomy, as well as Dr. Guntupalli’s editorial on this new research, we all must question how our patients will be able to undergo safe and effective surgery in the future.
Furthermore, it would truly be disheartening and disconcerting for a young physician to choose a residency with the desire of a specific track, only to lose that choice to a coresident.
In his presidential address to the AAGL some years ago, Javier Magrina, MD, of the Mayo Clinic in Phoenix, discussed separating the “O from the G” (J Minim Invasive Gynecol. 2014;21[4]:501-3). Among his points: From 1979 to 2006, there was a 46% decrease in the number of gynecologic operations (2,852,000 vs. 1,309,000), a 54% increase in the number of American College of Obstetricians and Gynecologists’ fellows (21,364 vs. 51,123), and an 81% decrease in the number of gynecologic operations performed per ACOG fellow (132 vs. 25).
In 1980, he pointed out, the total number of hysterectomy procedures performed in the United States was 647,000. In 2007, this total was 517,000. The total number of ACOG fellows in 1980 was 22,516, compared with 52,385 in 2007. And the total number of hysterectomies performed per ACOG fellow was 28, compared with 9.8 hysterectomies per fellow in 2007.
Dr. Magrina’s data goes hand in hand with Dr. Gressel’s new study. The surgical experience of the gynecologic surgeon certainly is on the wane. The result of this lack of experience is noted by Dr. Guntupalli in his 2015 publication. To us, it is readily apparent that Dr. Magrina is right: The only true solution is to finally realize that we must separate the O from the G.
Charles E. Miller, MD, is director of minimally invasive gynecologic surgery, and director of the AAGL fellowship in minimally invasive gynecologic surgery, at Advocate Lutheran General Hospital, Park Ridge, Ill. Kirsten Sasaki, MD, is associate director of the AAGL fellowship in minimally invasive gynecologic surgery at Advocate Lutheran. They have no other conflicts of interest.
The total number of hysterectomies performed during residency training has declined significantly since 2008, despite an increase in laparoscopic hysterectomies performed, according to a new analysis of data from graduating ob.gyn. residents that has implications for the structure of resident education.
The investigators abstracted case log data from the Accreditation Council for Graduate Medical Education (ACGME) database to assess trends in residents’ operative experience and found decreases in abdominal and vaginal cases but an increase in experience with laparoscopic hysterectomy.
(from 85 cases to 37), and the median number of vaginal hysterectomies decreased by 36% (from 31 to 20 cases).
Laparoscopic hysterectomy increased by 115% from a median of 20 procedures in 2008-2009 to 43 in 2017-2018. Even so, the median total number of hysterectomies per resident decreased by 6%, from 112 to 105 procedures during those two time periods. (Data on total hysterectomy and laparoscopic hysterectomy were not collected by ACGME until 2008.)
While the absolute decrease in the total number of hysterectomies is “relatively small,” the trend “raises questions about what the appropriate number of hysterectomies per graduating resident should be,” Gregory M. Gressel, MD, MSc, of the Montefiore Medical Center, New York, and coauthors wrote in Obstetrics & Gynecology.
“These data point,” they wrote, “to the necessity of maximizing surgical exposure in the face of a declining availability of procedures and the importance of reflecting on which (and how many) procedures an obstetrics and gynecology resident needs to complete before entering clinical practice.”
The training numbers parallel an increased use of laparoscopic hysterectomy in the United States and other countries, as well as a well-documented decline in the total number of hysterectomies performed in the United States, the latter of which is driven largely by the availability and increasing use of alternatives to the procedure (such as hormone therapy, endometrial ablation, and uterine artery embolization).
Hysterectomy still is a “core procedure of gynecologic surgery,” however, and is “at the heart of surgical training in obstetrics and gynecology,” as surgical techniques developed from learning hysterectomy “are applied broadly in the pelvis,” Saketh R. Guntupalli, MD, wrote in an accompanying editorial.
Dr. Guntupalli, of the University of Colorado at Aurora, Denver, was involved in a survey of fellowship program directors, published in 2015, that found only 20% of first-year fellows were able to independently perform a vaginal hysterectomy and 46% to independently perform an abdominal hysterectomy (Obstet Gynecol. 2015;126:559-68).
This and other research suggest that fellowship training is “used to address deficiencies in residency training rather than to develop new, specialized surgical skills,” he wrote. Given a dearth of fellowship positions in ob.gyn., “it is impossible to adequately use those avenues to train the number of competent surgeons necessary to address the surgical needs of women’s health in the United States.”
To address such concerns, some residency programs have instituted resident tracking to direct more hysterectomy cases toward those residents who plan to pursue surgical subspecialties. The Cleveland Clinic, Dr. Guntupalli noted, has tried the latter approach “with success.”
An increase in the number of accredited training programs and a decrease in the number of residents per program also might help to improve surgical exposure for residents, Dr. Gressel and associates wrote. Over the 16-year study period, the number of graduating residents increased significantly (by 12 per year) and the number of residency programs decreased significantly (0.52 fewer programs per year).
Additionally, Dr. Guntupalli wrote, regulatory bodies may need to reevaluate how competencies are assessed, and whether minimal numbers of cases “continue to carry the same weight as they did in previous generations.”
In the study, one coauthor is a full-time employee of ACGME, and another receives funds as a director for the American Board of Obstetrics and Gynecology. The remaining authors had no relevant financial disclosures. There was no outside funding for the study. Dr. Guntupalli said he had no conflicts of interest.
SOURCES: Gressel GM et al. Obstet Gynecol. 2020 Feb;135(2):268-73; Guntupalli SR. Obstet Gynecol 2020 Feb;135(2):266-7.
The total number of hysterectomies performed during residency training has declined significantly since 2008, despite an increase in laparoscopic hysterectomies performed, according to a new analysis of data from graduating ob.gyn. residents that has implications for the structure of resident education.
The investigators abstracted case log data from the Accreditation Council for Graduate Medical Education (ACGME) database to assess trends in residents’ operative experience and found decreases in abdominal and vaginal cases but an increase in experience with laparoscopic hysterectomy.
(from 85 cases to 37), and the median number of vaginal hysterectomies decreased by 36% (from 31 to 20 cases).
Laparoscopic hysterectomy increased by 115% from a median of 20 procedures in 2008-2009 to 43 in 2017-2018. Even so, the median total number of hysterectomies per resident decreased by 6%, from 112 to 105 procedures during those two time periods. (Data on total hysterectomy and laparoscopic hysterectomy were not collected by ACGME until 2008.)
While the absolute decrease in the total number of hysterectomies is “relatively small,” the trend “raises questions about what the appropriate number of hysterectomies per graduating resident should be,” Gregory M. Gressel, MD, MSc, of the Montefiore Medical Center, New York, and coauthors wrote in Obstetrics & Gynecology.
“These data point,” they wrote, “to the necessity of maximizing surgical exposure in the face of a declining availability of procedures and the importance of reflecting on which (and how many) procedures an obstetrics and gynecology resident needs to complete before entering clinical practice.”
The training numbers parallel an increased use of laparoscopic hysterectomy in the United States and other countries, as well as a well-documented decline in the total number of hysterectomies performed in the United States, the latter of which is driven largely by the availability and increasing use of alternatives to the procedure (such as hormone therapy, endometrial ablation, and uterine artery embolization).
Hysterectomy still is a “core procedure of gynecologic surgery,” however, and is “at the heart of surgical training in obstetrics and gynecology,” as surgical techniques developed from learning hysterectomy “are applied broadly in the pelvis,” Saketh R. Guntupalli, MD, wrote in an accompanying editorial.
Dr. Guntupalli, of the University of Colorado at Aurora, Denver, was involved in a survey of fellowship program directors, published in 2015, that found only 20% of first-year fellows were able to independently perform a vaginal hysterectomy and 46% to independently perform an abdominal hysterectomy (Obstet Gynecol. 2015;126:559-68).
This and other research suggest that fellowship training is “used to address deficiencies in residency training rather than to develop new, specialized surgical skills,” he wrote. Given a dearth of fellowship positions in ob.gyn., “it is impossible to adequately use those avenues to train the number of competent surgeons necessary to address the surgical needs of women’s health in the United States.”
To address such concerns, some residency programs have instituted resident tracking to direct more hysterectomy cases toward those residents who plan to pursue surgical subspecialties. The Cleveland Clinic, Dr. Guntupalli noted, has tried the latter approach “with success.”
An increase in the number of accredited training programs and a decrease in the number of residents per program also might help to improve surgical exposure for residents, Dr. Gressel and associates wrote. Over the 16-year study period, the number of graduating residents increased significantly (by 12 per year) and the number of residency programs decreased significantly (0.52 fewer programs per year).
Additionally, Dr. Guntupalli wrote, regulatory bodies may need to reevaluate how competencies are assessed, and whether minimal numbers of cases “continue to carry the same weight as they did in previous generations.”
In the study, one coauthor is a full-time employee of ACGME, and another receives funds as a director for the American Board of Obstetrics and Gynecology. The remaining authors had no relevant financial disclosures. There was no outside funding for the study. Dr. Guntupalli said he had no conflicts of interest.
SOURCES: Gressel GM et al. Obstet Gynecol. 2020 Feb;135(2):268-73; Guntupalli SR. Obstet Gynecol 2020 Feb;135(2):266-7.
FROM OBSTETRICS & GYNECOLOGY
Stress incontinence surgery found to improve sexual dysfunction
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
FROM OBSTETRICS & GYNECOLOGY
Can you identify these numerous papules in the groin area?
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2
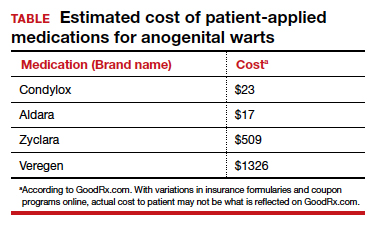
Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2

Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2

Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
CASE Skin tags on the groin
A 47-year-old woman with no personal history of skin cancer presents to a dermatologist for annual skin surveillance examination. She notes multiple “pink skin tags” on the groin, present for 4 months. She says they are asymptomatic and have not been treated previously. She states that she is in a long-term monogamous relationship. Physical examination reveals multiple smooth, flat-topped, pedunculated pink papules on the bilateral upper inner thighs. Shave biopsy of a lesion on the right upper medial thigh is performed to aid in diagnosis (FIGURE 1).
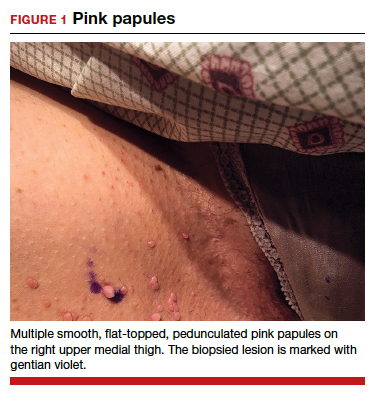
In hysterectomy, consider wider risks of ovary removal
LAS VEGAS – While it’s fading in popularity, ovary removal in hysterectomy is still far from uncommon. A gynecologic surgeon urged colleagues to give deeper consideration to whether the ovaries can stay in place.
“Gynecologists should truly familiarize themselves with the data on cardiovascular, endocrine, bone, and sexual health implications of removing the ovaries when there isn’t a medical indication to do so,” Amanda Nickles Fader, MD, director of the Kelly gynecologic oncology service and the director of the center for rare gynecologic cancers at Johns Hopkins Hospital, Baltimore, said in an interview following her presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Until I started giving this talk, I thought I knew this data. However, once I took a deeper dive into the studies of how hormonally active the postmenopausal ovaries are, as well as the population-based studies demonstrating worse all-cause mortality outcomes in low-risk women who have their ovaries surgically removed prior to their 60s, I was stunned at how compelling this data is,” she said.
The conventional wisdom about ovary removal in hysterectomy has changed dramatically over the decades. As Dr. Nickles Fader explained in the interview, “in the ’80s and early ’90s, the mantra was ‘just take everything out’ at hysterectomy surgery – tubes and ovaries should be removed – without understanding the implications. Then in the late ’90s and early 2000s, it was a more selective strategy of ‘wait until menopause to remove the ovaries.’ ”
Now, “more contemporary data suggests that the ovaries appear to be hormonally active to some degree well into the seventh decade of life, and even women in their early 60s who have their ovaries removed without a medical indication may be harmed.”
Still, ovary removal occurs in about 50%-60% of the 450,000-500,000 hysterectomies performed each year in the United States, Dr. Nickles Fader said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
These findings seem to suggest that messages about the potential benefits of ovary preservation are not getting through to surgeons and patients.
Indeed, a 2017 study of 57,776 benign premenopausal hysterectomies with ovary removal in California from 2005 to 2011 found that 38% had no documented sign of an appropriate diagnosis signaling a need for oophorectomy. These included “ovarian cyst, breast cancer susceptibility gene carrier status, and other diagnoses,” the study authors wrote (Menopause. 2017 Aug;24[8]:947-53).
Dr. Nickles Fader emphasized that ovary removal is appropriate in cases of gynecologic malignancy, while patients at high genetic risk of ovarian cancer may consider salpingo-oophorectomy or salpingectomy.
What about other situations? She offered these pearls in the presentation:
- Don’t remove ovaries before age 60 “without a good reason” because the procedure may lower lifespan and increase cardiovascular risk.
- Ovary removal is linked to cognitive decline, Parkinson’s disease, depression and anxiety, glaucoma, sexual dysfunction, and bone fractures.
- Ovary preservation, in contrast, is linked to improvement of menopausal symptoms, sleep quality, urogenital atrophy, skin conditions, and metabolism.
- Fallopian tubes may be the true trouble area. “The prevailing theory amongst scientists and clinicians is that ‘ovarian cancer’ is in most cases a misnomer, and most of these malignancies start in the fallopian tube,” Dr. Nickles Fader said in the interview.
“It’s a better time than ever to be thoughtful about removing a woman’s ovaries in someone who is at low risk for ovarian cancer. The new, universal guideline is that to best optimize cancer risk reduction and general health outcomes.”
Dr. Nickles Fader disclosed consulting work for Ethicon and Merck.
LAS VEGAS – While it’s fading in popularity, ovary removal in hysterectomy is still far from uncommon. A gynecologic surgeon urged colleagues to give deeper consideration to whether the ovaries can stay in place.
“Gynecologists should truly familiarize themselves with the data on cardiovascular, endocrine, bone, and sexual health implications of removing the ovaries when there isn’t a medical indication to do so,” Amanda Nickles Fader, MD, director of the Kelly gynecologic oncology service and the director of the center for rare gynecologic cancers at Johns Hopkins Hospital, Baltimore, said in an interview following her presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Until I started giving this talk, I thought I knew this data. However, once I took a deeper dive into the studies of how hormonally active the postmenopausal ovaries are, as well as the population-based studies demonstrating worse all-cause mortality outcomes in low-risk women who have their ovaries surgically removed prior to their 60s, I was stunned at how compelling this data is,” she said.
The conventional wisdom about ovary removal in hysterectomy has changed dramatically over the decades. As Dr. Nickles Fader explained in the interview, “in the ’80s and early ’90s, the mantra was ‘just take everything out’ at hysterectomy surgery – tubes and ovaries should be removed – without understanding the implications. Then in the late ’90s and early 2000s, it was a more selective strategy of ‘wait until menopause to remove the ovaries.’ ”
Now, “more contemporary data suggests that the ovaries appear to be hormonally active to some degree well into the seventh decade of life, and even women in their early 60s who have their ovaries removed without a medical indication may be harmed.”
Still, ovary removal occurs in about 50%-60% of the 450,000-500,000 hysterectomies performed each year in the United States, Dr. Nickles Fader said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
These findings seem to suggest that messages about the potential benefits of ovary preservation are not getting through to surgeons and patients.
Indeed, a 2017 study of 57,776 benign premenopausal hysterectomies with ovary removal in California from 2005 to 2011 found that 38% had no documented sign of an appropriate diagnosis signaling a need for oophorectomy. These included “ovarian cyst, breast cancer susceptibility gene carrier status, and other diagnoses,” the study authors wrote (Menopause. 2017 Aug;24[8]:947-53).
Dr. Nickles Fader emphasized that ovary removal is appropriate in cases of gynecologic malignancy, while patients at high genetic risk of ovarian cancer may consider salpingo-oophorectomy or salpingectomy.
What about other situations? She offered these pearls in the presentation:
- Don’t remove ovaries before age 60 “without a good reason” because the procedure may lower lifespan and increase cardiovascular risk.
- Ovary removal is linked to cognitive decline, Parkinson’s disease, depression and anxiety, glaucoma, sexual dysfunction, and bone fractures.
- Ovary preservation, in contrast, is linked to improvement of menopausal symptoms, sleep quality, urogenital atrophy, skin conditions, and metabolism.
- Fallopian tubes may be the true trouble area. “The prevailing theory amongst scientists and clinicians is that ‘ovarian cancer’ is in most cases a misnomer, and most of these malignancies start in the fallopian tube,” Dr. Nickles Fader said in the interview.
“It’s a better time than ever to be thoughtful about removing a woman’s ovaries in someone who is at low risk for ovarian cancer. The new, universal guideline is that to best optimize cancer risk reduction and general health outcomes.”
Dr. Nickles Fader disclosed consulting work for Ethicon and Merck.
LAS VEGAS – While it’s fading in popularity, ovary removal in hysterectomy is still far from uncommon. A gynecologic surgeon urged colleagues to give deeper consideration to whether the ovaries can stay in place.
“Gynecologists should truly familiarize themselves with the data on cardiovascular, endocrine, bone, and sexual health implications of removing the ovaries when there isn’t a medical indication to do so,” Amanda Nickles Fader, MD, director of the Kelly gynecologic oncology service and the director of the center for rare gynecologic cancers at Johns Hopkins Hospital, Baltimore, said in an interview following her presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Until I started giving this talk, I thought I knew this data. However, once I took a deeper dive into the studies of how hormonally active the postmenopausal ovaries are, as well as the population-based studies demonstrating worse all-cause mortality outcomes in low-risk women who have their ovaries surgically removed prior to their 60s, I was stunned at how compelling this data is,” she said.
The conventional wisdom about ovary removal in hysterectomy has changed dramatically over the decades. As Dr. Nickles Fader explained in the interview, “in the ’80s and early ’90s, the mantra was ‘just take everything out’ at hysterectomy surgery – tubes and ovaries should be removed – without understanding the implications. Then in the late ’90s and early 2000s, it was a more selective strategy of ‘wait until menopause to remove the ovaries.’ ”
Now, “more contemporary data suggests that the ovaries appear to be hormonally active to some degree well into the seventh decade of life, and even women in their early 60s who have their ovaries removed without a medical indication may be harmed.”
Still, ovary removal occurs in about 50%-60% of the 450,000-500,000 hysterectomies performed each year in the United States, Dr. Nickles Fader said at the meeting, which was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
These findings seem to suggest that messages about the potential benefits of ovary preservation are not getting through to surgeons and patients.
Indeed, a 2017 study of 57,776 benign premenopausal hysterectomies with ovary removal in California from 2005 to 2011 found that 38% had no documented sign of an appropriate diagnosis signaling a need for oophorectomy. These included “ovarian cyst, breast cancer susceptibility gene carrier status, and other diagnoses,” the study authors wrote (Menopause. 2017 Aug;24[8]:947-53).
Dr. Nickles Fader emphasized that ovary removal is appropriate in cases of gynecologic malignancy, while patients at high genetic risk of ovarian cancer may consider salpingo-oophorectomy or salpingectomy.
What about other situations? She offered these pearls in the presentation:
- Don’t remove ovaries before age 60 “without a good reason” because the procedure may lower lifespan and increase cardiovascular risk.
- Ovary removal is linked to cognitive decline, Parkinson’s disease, depression and anxiety, glaucoma, sexual dysfunction, and bone fractures.
- Ovary preservation, in contrast, is linked to improvement of menopausal symptoms, sleep quality, urogenital atrophy, skin conditions, and metabolism.
- Fallopian tubes may be the true trouble area. “The prevailing theory amongst scientists and clinicians is that ‘ovarian cancer’ is in most cases a misnomer, and most of these malignancies start in the fallopian tube,” Dr. Nickles Fader said in the interview.
“It’s a better time than ever to be thoughtful about removing a woman’s ovaries in someone who is at low risk for ovarian cancer. The new, universal guideline is that to best optimize cancer risk reduction and general health outcomes.”
Dr. Nickles Fader disclosed consulting work for Ethicon and Merck.
EXPERT ANALYSIS FROM PAGS 2019