User login
2020 Update on fertility
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
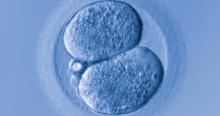
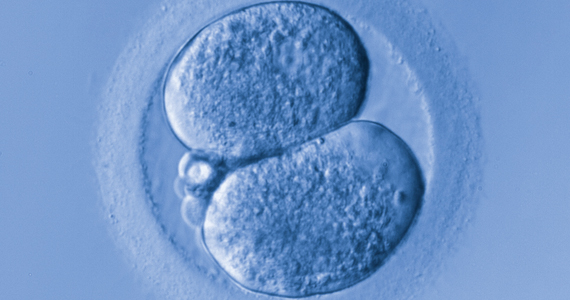
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
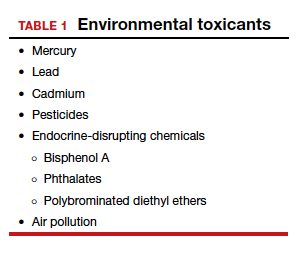
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.

Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
ObGyn malpractice liability risk: 2020 developments and probabilities
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
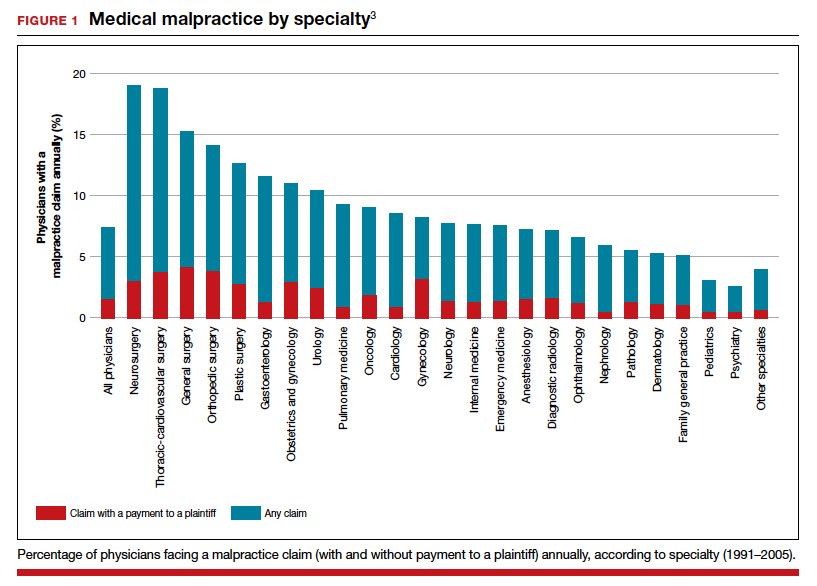
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)
Continue to: The number of ObGyn paid malpractice claims has decreased over time...
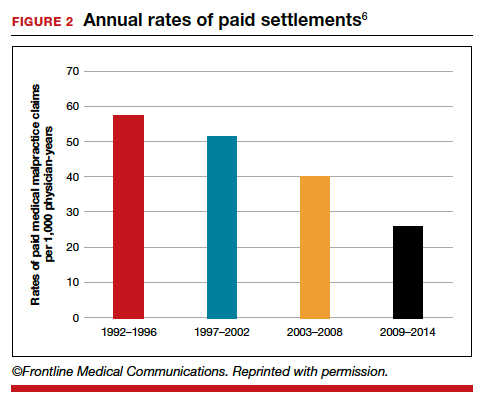
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4
It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M
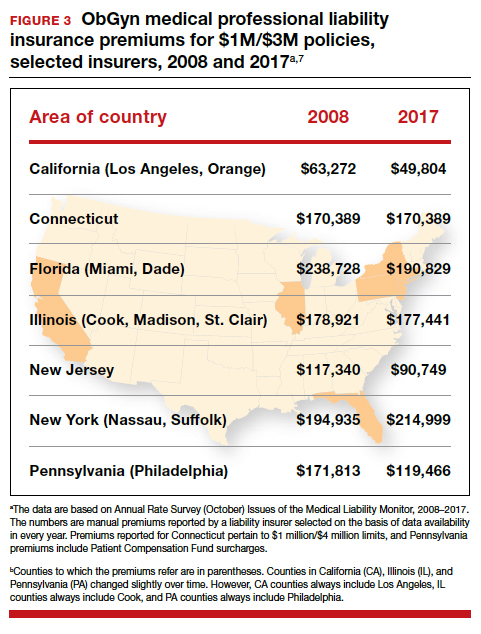
Why have malpractice payouts declined overall?
Have medical errors declined?
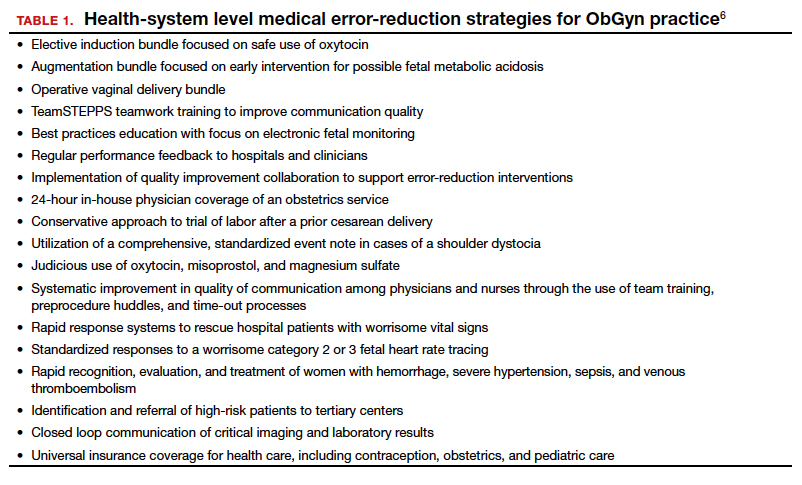
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.
The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.
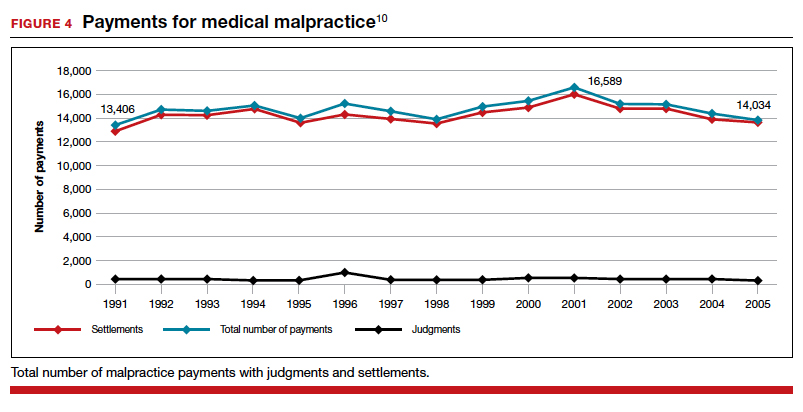
Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.
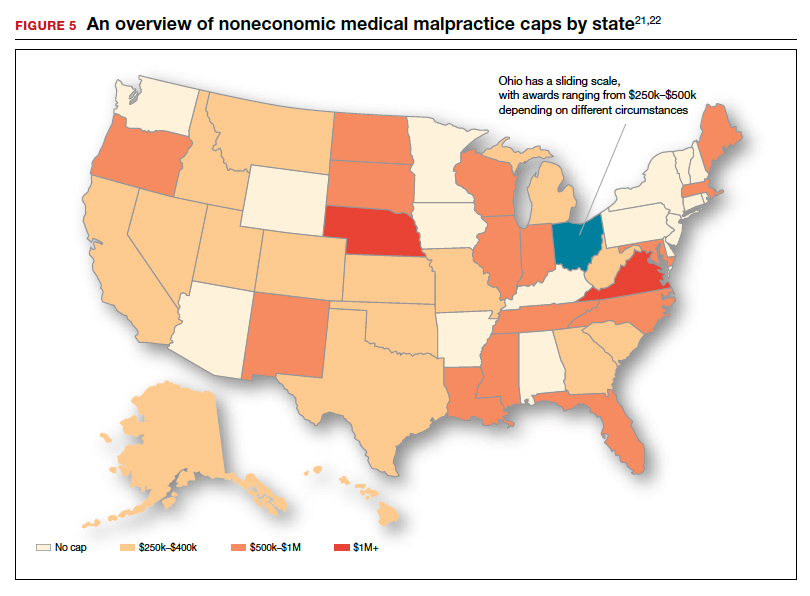
Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
Lidocaine-prilocaine cream tops lidocaine injections for vulvar biopsy pain
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
aotto@mdedge.com
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
aotto@mdedge.com
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
The median highest pain score in a randomized trial of 38 women undergoing vulvar biopsies was 25.7 mm lower, on a 100 mm visual analogue scale, when they received 5% lidocaine-prilocaine cream instead of a 1% lidocaine injection, according to a report from Duke University, in Durham, N.C.
“In the current study, we found that application of lidocaine-prilocaine cream, alone, for a minimum of 10 minutes before vulvar biopsy on a non–hair-bearing surface results in a significantly lower maximum pain score and a significantly better patient rating of the biopsy experience,” said investigators led by Logan K. Williams, MD, of the department of obstetrics and gynecology at Duke University, Durham, N.C.
Given the “clear advantage” of the cream, it “should be considered as an anesthetic method for vulvar biopsy in a non-hair-bearing area,” Dr. Williams and colleagues concluded (Obstet Gynecol. 2020 Feb;135{2]:311-8).
Studies have pitted the cream against the injection before, but they did not compare patients’ maximal pain scores. The team wanted to do that because “comparing the highest score allows us to consider the possibility that the pain of anesthesia application” – injection versus cream – “may be greater than the pain of any other portion of the biopsy procedure.”
They randomized 19 women to the cream, approximately 5 g at the site of biopsy at least 10 minutes beforehand, and 18 others to the injection, 2 mL using a 27-gauge needle, at least 1 minute prior.
The median highest pain score in the lidocaine-prilocaine group was 20 mm, but 56.5 mm in the injection group. Patients randomized to lidocaine-prilocaine also had a significantly better (P = 0.02) experience than those receiving injected lidocaine, also assessed by visual analog scale (VAS). The median baseline pain level was 0 mm.
Anxiety was assessed after patients knew whether they were going to get the cream or the injection, but before the biopsy. The median score in the cream group was of 19 mm on another VAS, compared with 31.5 mm.
Participants were 60 years old on average, and almost all had prior vulvar biopsies. Two in the cream group and three in the injection group had punch biopsies; cervical biopsy forceps were used for the rest. More than half the women had benign findings, and most of the others had vulvar intraepithelial neoplasia, but there was one invasive cancer. At Duke, the cost of the injection was $0.99, compared with $7.36 for the cream.
Dr. Williams and colleagues cited a few limitations. One is that the patients and clinicians in the study were not blinded. Another is that most of the patients had undergone vulvar biopsy before, possibly predisposing them to bias.
“In the future, consideration could be taken to studying lidocaine-prilocaine cream applications to hair-bearing surfaces, which were excluded in this study.” Also, “there is a question of the histologic effect of lidocaine-prilocaine on tissues and whether this could affect pathologic diagnoses.
“We are conducting a separate ancillary study in conjunction with our dermatopathology colleagues to investigate this question,” the investigators said.
The work was funded by Duke and the National Institutes of Health. Dr. Williams had no disclosures.
aotto@mdedge.com
SOURCE: Williams LK et al. Obstet Gynecol. 2020 Feb;135(2):311-8.
FROM OBSTETRICS AND GYNECOLOGY
Progestin-only systemic hormone therapy for menopausal hot flashes
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
The field of menopause medicine is dominated by studies documenting the effectiveness of systemic estrogen or estrogen-progestin hormone therapy for the treatment of hot flashes caused by hypoestrogenism. The effectiveness of progestin-only systemic hormone therapy for the treatment of hot flashes is much less studied and seldom is utilized in clinical practice. A small number of studies have reported that progestins, including micronized progesterone, medroxyprogesterone acetate, and norethindrone acetate, are effective treatment for hot flashes. Progestin-only systemic hormone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment.
Micronized progesterone
Micronized progesterone (Prometrium) 300 mg daily taken at bedtime has been reported to effectively treat hot flashes in postmenopausal women. In one study, 133 postmenopausal women with an average age of 55 years and approximately 3 years from their last menstrual period were randomly assigned to 12 weeks of treatment with placebo or micronized progesterone 300 mg daily taken at bedtime.1 Mean serum progesterone levels were 0.28 ng/mL (0.89 nM) and 27 ng/mL (86 nM) in the women taking placebo and micronized progesterone, respectively. Compared with placebo, micronized progesterone reduced daytime and nighttime hot flash frequency and severity. In addition, compared with placebo, micronized progesterone improved the quality of sleep.1
Most reviews conclude that micronized progesterone has minimal cardiovascular risk.2 Micronized progesterone therapy might be especially helpful for postmenopausal women with moderate to severe hot flashes who have a contraindication to estrogen treatment such as those at increased risk for cardiovascular disease or women with a thrombophilia. Many experts believe that systemic estrogen therapy is contraindicated in postmenopausal women with an American Heart Association risk score greater than 10% over 10 years.3 Additional contraindications to systemic estrogen include women with cardiac disease who have a thrombophilia, such as the Factor V Leiden mutation.4
For women who are at high risk for estrogen-induced cardiovascular events, micronized progesterone may be a better option than systemic estrogen for treating hot flashes. Alternatively, in these women at risk of cardiovascular disease a selective serotonin reuptake inhibitor, such as escitalopram, 10 mg to 20 mg daily, may be a good option for treating postmenopausal hot flashes.5
Medroxyprogesterone acetate
Medroxyprogesterone acetate, at a dosage of 20 mg daily, is an effective treatment for hot flashes. In a randomized clinical trial 27 postmenopausal women with hot flashes were randomly assigned to treatment with placebo or medroxyprogesterone acetate 20 mg daily for 4 weeks. Vasomotor flushes were decreased by 26% and 74% in the placebo and medroxyprogesterone groups, respectively.6
Depot medroxyprogesterone acetate injections at dosages from 150 mg to 400 mg also have been reported to effectively treat hot flashes.7,8 In a trial comparing the effectiveness of estrogen monotherapy (conjugated equine estrogen 0.6 mg daily) with progestin monotherapy (medroxyprogesterone acetate 10 mg daily), both treatments were equally effective in reducing hot flashes.9
Continue to: Micronized progesterone vs medroxyprogesterone acetate...
Micronized progesterone vs medroxyprogesterone acetate
Experts in menopause medicine have suggested that in postmenopausal women micronized progesterone has a better pattern of benefits and fewer risks than medroxyprogesterone acetate.10,11 For example, in the E3N observational study of hormones and breast cancer risk, among 80,377 French postmenopausal women followed for a mean of 8 years, the combination of transdermal estradiol plus oral micronized progesterone was associated with no significantly increased risk of breast cancer (relative risk [RR], 1.08, 95% confidence interval [CI], 0.89–1.31) compared with never users of postmenopausal hormone therapy.12 By contrast, the combination of oral estrogen plus medroxyprogesterone acetate was associated with an increased risk of breast cancer (RR, 1.48; 95% CI, 1.02–2.16) compared with never users of postmenopausal hormone therapy. The E3N study indicates that micronized progesterone may have a more favorable breast health profile than medroxyprogesterone acetate.12

Norethindrone acetate
Norethindrone acetate monotherapy is not commonly prescribed for the treatment of menopausal hot flashes. However, a large clinical trial has demonstrated that norethindrone acetate effectively suppresses hot flashes in women with endometriosis treated with depot leuprolide acetate (LA). In one trial 201 women with endometriosis were randomly assigned to 12 months of treatment with13:
- LA plus placebo pills
- LA plus norethindrone acetate (NEA) 5 mg daily
- LA plus NEA 5 mg daily plus conjugated equine estrogen (CEE) 0.625 mg daily, or
- LA plus NEA 5 mg daily plus CEE 1.25 mg daily.
The median number of hot flashes in 24 hours was 6 in the LA plus placebo group and 0 in both the LA plus NEA 5 mg daily group and the LA plus NEA 5 mg plus CEE 1.25 mg daily group. This study demonstrates that NEA 5 mg daily is an effective treatment for hot flashes.
In the same study, LA plus placebo was associated with a significant decrease in lumbar spine bone mineral density. No significant decrease in bone mineral density was observed in the women who received LA plus NEA 5 mg daily. This finding indicates that NEA 5 mg reduces bone absorption caused by hypoestrogenism. In humans, norethindrone is a substrate for the aromatase enzyme system.14 Small quantities of ethinyl estradiol may be formed by aromatization of norethindrone in vivo,15,16 contributing to the effectiveness of NEA in suppressing hot flashes and preserving bone density.
Progestin: The estrogen alternative to hot flashes
For postmenopausal women with moderate to severe hot flashes, estrogen treatment reliably suppresses hot flashes and often improves sleep quality and mood. For postmenopausal women with a contraindication to estrogen treatment, progestin-only treatment with micronized progesterone or norethindrone acetate may be an effective option.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
- Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886-893.
- Spark MJ, Willis J. Systematic review of progesterone use by midlife menopausal women. Maturitas 2012; 72: 192-202.
- Manson JE, Ames JM, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/nonhormonal therapy decision making: a clinical decision-suport tool from The North American Menopause Society. Menopause. 2015;22:247-253.
- Herrington DM, Vittinghoff E, Howard TD, et al. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002;22:1012-1017.
- Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848-855.
- Schiff I, Tulchinsky D, Cramer D, et al. Oral medroxyprogesterone in the treatment of postmenopausal symptoms. JAMA. 1980;244:1443-1445.
- Bullock JL, Massey FM, Gambrell RD Jr. Use of medroxyprogesterone acetate to prevent menopausal symptoms. Obstet Gynecol. 1975;46:165-168.
- Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depot medroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409-1414.
- Prior JC, Nielsen JD, Hitchcock CL, et al. Medroxyprogesterone and conjugated oestrogen are equivalent for hot flushes: 1-year randomized double-blind trial following premenopausal ovariectomy. Clin Sci (Lond). 2007;112:517-525.
- L’hermite M, Simoncini T, Fuller S, et al. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas. 2008;60:185-201.
- Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? Menopause. 2014;21:769-783.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Barbieri RL, Canick JA, Ryan KJ. High-affinity steroid binding to rat testis 17 alpha-hydroxylase and human placental aromatase. J Steroid Biochem. 1981;14:387-393.
- Chu MC, Zhang X, Gentzschein E, et al. Formation of ethinyl estradiol in women during treatment with norethindrone acetate. J Clin Endocrinol Metab. 2007;92:2205-2207.
- Chwalisz K, Surrey E, Stanczyk FZ. The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci. 2012;19:563-571.
The removal of the multiple-kilogram uterus using MIGSs
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
Safely pushing the limits of MIGS surgery
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
Performing gender-reaffirming surgery: Guidelines for the general ob.gyn.
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
Are providers asking about menstrual bleeding before/during anticoagulant therapy?
ORLANDO – A small study suggests health care providers may fail to ask patients about heavy menstrual bleeding before or during treatment with oral anticoagulants.
Researchers performed a chart review at a single center, which indicated that 60% of women were not asked about heavy menstrual bleeding before they were prescribed an oral anticoagulant.
Six months after the women started anticoagulant therapy, 29% required treatment for heavy menstrual bleeding. Charts for the remaining 71% of women contained no information about heavy menstrual bleeding.
“We were unable to distinguish between true absence of heavy menstrual bleeding and absence of reporting,” said Bethany T. Samuelson Bannow, MD, of Oregon Health & Science University, Portland.
Dr. Samuelson Bannow presented these findings at the annual meeting of the American Society of Hematology.
She explained that heavy menstrual bleeding is defined as more than 80 mL of blood loss per cycle. It affects 10%-15% of women in their lifetime, and anticoagulants increase the risk of heavy menstrual bleeding.
Studies have shown that heavy menstrual bleeding occurs in 22%-65% of women treated with vitamin K agonists and 20%-27% of women treated with rivaroxaban (Blood. 2017;130[24]:2603-9). However, many anticoagulant studies don’t include heavy menstrual bleeding as an outcome.
To gain more insight, Dr. Samuelson Bannow and colleagues conducted a chart review. Their study included 236 women of reproductive age treated at Oregon Health & Science University between Jan. 1, 2012, and Dec. 31, 2018.
The patients’ median age was 37 years (range, 18-50 years). Most patients (67%) were receiving an oral anticoagulant for venous thromboembolism. The rest were on anticoagulant therapy for arterial thrombosis (6%), atrial fibrillation (6%), a mechanical valve (1%), or “other” reasons (20%).
Dr. Samuelson Bannow said the other group was “almost exclusively women who were receiving prophylaxis” postoperatively or for travel. Most women in this group were receiving rivaroxaban.
Rivaroxaban was the most commonly prescribed anticoagulant in the entire cohort (41%), followed by warfarin (34%) and apixaban (25%).
At the time of anticoagulant prescription, 12% of women reported a history of heavy menstrual bleeding, and 28% did not. For most patients – 60% – there was no discussion of menstrual history documented.
Six months after starting oral anticoagulant therapy, 29% of patients required treatment for heavy menstrual bleeding. For 71% of patients, there was no documentation on the treatment of heavy menstrual bleeding.
Treatment for heavy menstrual bleeding was required in 33% of patients on rivaroxaban, 24% of those on apixaban, and 29% of those on warfarin, a significant difference (P less than .001).
“Rates of heavy menstrual bleeding … are higher in rivaroxaban users,” Dr. Samuelson Bannow said. “This is not the first study to demonstrate this. However, [the rate of heavy menstrual bleeding in this study] is still a lot lower than we would expect based on past levels with warfarin. This tells us we’re probably missing a lot of heavy menstrual bleeding. That’s not too surprising considering how few providers are actually asking about the menses.”
Dr. Samuelson Bannow and colleagues disclosed no conflicts of interest.
SOURCE: Samuelson Bannow BT et al. ASH 2019, Abstract 60.
ORLANDO – A small study suggests health care providers may fail to ask patients about heavy menstrual bleeding before or during treatment with oral anticoagulants.
Researchers performed a chart review at a single center, which indicated that 60% of women were not asked about heavy menstrual bleeding before they were prescribed an oral anticoagulant.
Six months after the women started anticoagulant therapy, 29% required treatment for heavy menstrual bleeding. Charts for the remaining 71% of women contained no information about heavy menstrual bleeding.
“We were unable to distinguish between true absence of heavy menstrual bleeding and absence of reporting,” said Bethany T. Samuelson Bannow, MD, of Oregon Health & Science University, Portland.
Dr. Samuelson Bannow presented these findings at the annual meeting of the American Society of Hematology.
She explained that heavy menstrual bleeding is defined as more than 80 mL of blood loss per cycle. It affects 10%-15% of women in their lifetime, and anticoagulants increase the risk of heavy menstrual bleeding.
Studies have shown that heavy menstrual bleeding occurs in 22%-65% of women treated with vitamin K agonists and 20%-27% of women treated with rivaroxaban (Blood. 2017;130[24]:2603-9). However, many anticoagulant studies don’t include heavy menstrual bleeding as an outcome.
To gain more insight, Dr. Samuelson Bannow and colleagues conducted a chart review. Their study included 236 women of reproductive age treated at Oregon Health & Science University between Jan. 1, 2012, and Dec. 31, 2018.
The patients’ median age was 37 years (range, 18-50 years). Most patients (67%) were receiving an oral anticoagulant for venous thromboembolism. The rest were on anticoagulant therapy for arterial thrombosis (6%), atrial fibrillation (6%), a mechanical valve (1%), or “other” reasons (20%).
Dr. Samuelson Bannow said the other group was “almost exclusively women who were receiving prophylaxis” postoperatively or for travel. Most women in this group were receiving rivaroxaban.
Rivaroxaban was the most commonly prescribed anticoagulant in the entire cohort (41%), followed by warfarin (34%) and apixaban (25%).
At the time of anticoagulant prescription, 12% of women reported a history of heavy menstrual bleeding, and 28% did not. For most patients – 60% – there was no discussion of menstrual history documented.
Six months after starting oral anticoagulant therapy, 29% of patients required treatment for heavy menstrual bleeding. For 71% of patients, there was no documentation on the treatment of heavy menstrual bleeding.
Treatment for heavy menstrual bleeding was required in 33% of patients on rivaroxaban, 24% of those on apixaban, and 29% of those on warfarin, a significant difference (P less than .001).
“Rates of heavy menstrual bleeding … are higher in rivaroxaban users,” Dr. Samuelson Bannow said. “This is not the first study to demonstrate this. However, [the rate of heavy menstrual bleeding in this study] is still a lot lower than we would expect based on past levels with warfarin. This tells us we’re probably missing a lot of heavy menstrual bleeding. That’s not too surprising considering how few providers are actually asking about the menses.”
Dr. Samuelson Bannow and colleagues disclosed no conflicts of interest.
SOURCE: Samuelson Bannow BT et al. ASH 2019, Abstract 60.
ORLANDO – A small study suggests health care providers may fail to ask patients about heavy menstrual bleeding before or during treatment with oral anticoagulants.
Researchers performed a chart review at a single center, which indicated that 60% of women were not asked about heavy menstrual bleeding before they were prescribed an oral anticoagulant.
Six months after the women started anticoagulant therapy, 29% required treatment for heavy menstrual bleeding. Charts for the remaining 71% of women contained no information about heavy menstrual bleeding.
“We were unable to distinguish between true absence of heavy menstrual bleeding and absence of reporting,” said Bethany T. Samuelson Bannow, MD, of Oregon Health & Science University, Portland.
Dr. Samuelson Bannow presented these findings at the annual meeting of the American Society of Hematology.
She explained that heavy menstrual bleeding is defined as more than 80 mL of blood loss per cycle. It affects 10%-15% of women in their lifetime, and anticoagulants increase the risk of heavy menstrual bleeding.
Studies have shown that heavy menstrual bleeding occurs in 22%-65% of women treated with vitamin K agonists and 20%-27% of women treated with rivaroxaban (Blood. 2017;130[24]:2603-9). However, many anticoagulant studies don’t include heavy menstrual bleeding as an outcome.
To gain more insight, Dr. Samuelson Bannow and colleagues conducted a chart review. Their study included 236 women of reproductive age treated at Oregon Health & Science University between Jan. 1, 2012, and Dec. 31, 2018.
The patients’ median age was 37 years (range, 18-50 years). Most patients (67%) were receiving an oral anticoagulant for venous thromboembolism. The rest were on anticoagulant therapy for arterial thrombosis (6%), atrial fibrillation (6%), a mechanical valve (1%), or “other” reasons (20%).
Dr. Samuelson Bannow said the other group was “almost exclusively women who were receiving prophylaxis” postoperatively or for travel. Most women in this group were receiving rivaroxaban.
Rivaroxaban was the most commonly prescribed anticoagulant in the entire cohort (41%), followed by warfarin (34%) and apixaban (25%).
At the time of anticoagulant prescription, 12% of women reported a history of heavy menstrual bleeding, and 28% did not. For most patients – 60% – there was no discussion of menstrual history documented.
Six months after starting oral anticoagulant therapy, 29% of patients required treatment for heavy menstrual bleeding. For 71% of patients, there was no documentation on the treatment of heavy menstrual bleeding.
Treatment for heavy menstrual bleeding was required in 33% of patients on rivaroxaban, 24% of those on apixaban, and 29% of those on warfarin, a significant difference (P less than .001).
“Rates of heavy menstrual bleeding … are higher in rivaroxaban users,” Dr. Samuelson Bannow said. “This is not the first study to demonstrate this. However, [the rate of heavy menstrual bleeding in this study] is still a lot lower than we would expect based on past levels with warfarin. This tells us we’re probably missing a lot of heavy menstrual bleeding. That’s not too surprising considering how few providers are actually asking about the menses.”
Dr. Samuelson Bannow and colleagues disclosed no conflicts of interest.
SOURCE: Samuelson Bannow BT et al. ASH 2019, Abstract 60.
REPORTING FROM ASH 2019
Consider sparing the uterus in prolapse procedures
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Unnecessary pelvic exams, Pap tests common in young women
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.