User login
Uterine balloon tamponade found safe in postpartum hemorrhage
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Product update: Neuromodulation device, cystoscopy simplified, hysteroscopy seal, next immunization frontier
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
NEW SACRAL NEUROMODULATION DEVICE
FOR MORE INFORMATION, VISIT: https://www.axonics.com/
CERVICAL SEAL FOR HYSTEROSCOPIC DEVICES
For more information, visit: https://gynsurgicalsolutions.com/product/omni-lok/
UNIVERSAL CYSTOSCOPY SIMPLIFIED
FOR MORE INFORMATION, VISIT: https://cystosure.com/
NEXT FRONTIER IN VACCINE IMMUNIZATION
Globally, there are 410,000 cases of GBS every year. GBS is most common in newborns; women who are carriers of the GBS bacteria may pass it on to their newborns during labor and birth. An estimated 10% to 30% of pregnant women carry the GBS bacteria. The disease can manifest as sepsis, pneumonia, and meningitis, with potentially fatal outcomes for some. A maternal vaccine may prevent 231,000 infant and maternal GBS cases, says Pfizer.
According to Pfizer, RSV causes more hospitalizations each year than influenza among young children, with an estimated 33 million cases globally each year in children less than age 5 years.
FOR MORE INFORMATION, VISIT: https://www.pfizer.com/
Should secondary cytoreduction be performed for platinum-sensitive recurrent ovarian cancer?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
EXPERT COMMENTARY
Ovarian cancer represents the most lethal gynecologic cancer, with an estimated 14,000 deaths in 2019.1 While the incidence of this disease is low in comparison to uterine cancer, the advanced stage at diagnosis portends poor prognosis. While stage is an independent risk factor for death, it is also a risk for recurrence, with more than 80% of women developing recurrent disease.2-4 Secondary cytoreduction remains an option for patients in which disease recurs; up until now this management option was driven by retrospective data.5
Details of the study
Coleman and colleagues conducted the Gynecologic Oncology Group (GOG) 0213 trial—a phase 3, multicenter, randomized clinical trial that included 485 women with recurrent ovarian cancer. The surgical objective of the trial was to determine whether secondary cytoreduction in operable, platinum-sensitive (PS) patients improved overall survival (OS).
Patients were eligible to participate in the surgical portion of the trial if they had PS measurable disease and had the intention to achieve complete gross resection. Women with ascites, evidence of extraabdominal disease, and “diffuse carcinomatosis” were excluded. The primary and secondary end points were OS and progression-free survival (PFS), respectively.
Results. There were no statistical differences between the surgery and no surgery groups with regard to median OS (50.6 months vs 64.7 months, respectively; hazard ratio [HR], 1.29; 95% confidence interval [CI], 0.97–1.72) or median PFS (18.9 months vs 16.2 months; HR, 0.82; 95% CI, 0.66 to 1.01). When comparing patients in which complete gross resection was achieved (150 patients vs 245 who did not receive surgery), there was only a statistical difference in PFS in favor of the surgical group (22.4 months vs 16.2 months; HR, 0.62; 95% CI, 0.48–0.80).
Of note, 67% of the patients who received surgery (63% intention-to-treat) were debulked to complete gross resection. There were 33% more patients with extraabdominal disease (10% vs 7% of total patients in each group) and 15% more patients with upper abdominal disease (40% vs 33% of total patients in each group) included in the surgical group. Finally, the median time to chemotherapy was 40 days in the surgery group versus 7 days in the no surgery group.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
The authors deserve to be commended for this well-designed and laborious trial, which is the first of its kind. The strength of the study is its randomized design producing level I data.
Study weaknesses include lack of reporting of BRCA status and the impact of receiving targeted therapies after the trial was over. It is well established that BRCA-mutated patients have an independent survival advantage, even when taking into account platinum sensitivity.6-8BRCA status of the study population is not specifically addressed in this paper. The authors noted in the first GOG 0213 trial publication, which assessed bevacizumab in the recurrent setting, that BRCA status has an impact on patient outcomes. Subsequently, they state that they do not report BRCA status because “…its independent effect on response to an anti-angiogenesis agent was unknown,” but it clearly would affect survival analysis if unbalanced between groups.9
Similarly, in the introduction to their study, Coleman and colleagues list availability of maintenance therapy, for instance poly ADP (adenosine diphosphate–ribose) polymerase (PARP) inhibitors, as rationale for conducting their trial. They subsequently cite this as a possible reason that the median overall survival was 3 times longer than expected. However, they provide no data on which patients received maintenance therapy, which again could have drastically affected survival outcomes.10 They do report in the supplementary information that, when stratifying those receiving bevacizumab adjuvantly during the trial, the median OS was comparable between the surgical and nonsurgical groups (58.5 months vs 61.7 months).
The authors discuss the presence of patient selection bias as a weakness in the study. Selection bias is evident in this trial (as in many surgical trials) because patients with a limited volume of disease were selected to participate over those with large-volume disease. It is reasonable to conclude that this study is likely selecting patients with less aggressive tumor biology, not only evident by low-volume disease at recurrence but also by the 20.4-month median platinum-free interval in the surgical group, which certainly affects the trial’s validity. Despite being considered PS, the disease biology in a patient with a platinum-free interval of 20.4 months is surely different from the disease biology in a patient with a 6.4-month platinum-free interval; therefore, it is difficult to generalize these data to all PS recurrent ovarian cancer patients. Similarly, other research has suggested strict selection criteria, which was not apparent in this study’s methodology.11 While the number of metastatic sites were relatively equal between the surgery and no surgery groups, there were more patients in the surgical group with extraabdominal disease, which the authors used as an exclusion criterion.
Lastly, the time to treatment commencement in each arm, which was 40 days for the surgical arm and 7 days in the nonsurgical arm, could represent a flaw in this trial. While we expect a difference in duration to account for recovery time, many centers start chemotherapy as soon as 21 days after surgery, which is almost half of the median interval in the surgical group in this trial. While the authors address this by stating that they completed a landmark analysis, no data or information about what time points they used for the analysis are provided. They simply report an interquartile range of 28 to 51 days. It is hard to know what effect this may have had on the outcome.
This is the first randomized clinical trial conducted to assess whether secondary surgical cytoreduction is beneficial in PS recurrent ovarian cancer patients. It provides compelling evidence to critically evaluate whether surgical cytoreduction is appropriate in a similar patient population. However, we would recommend using caution applying these data to patients who have different platinum-free intervals or low-volume disease limited to the pelvis.
The trial is not without flaws, as the authors point out in their discussion, but currently, it is the best evidence afforded to gynecologic oncologists. There are multiple trials currently ongoing, including DESTOP-III, which had similar PFS results as GOG 0213. If consensus is reached with these 2 trials, we believe that secondary cytoreduction will be utilized far less often in patients with recurrent ovarian cancer and a long platinum-free interval, thereby changing the current treatment paradigm for these patients.
MICHAEL D. TOBONI, MD, MPH, AND DAVID G. MUTCH, MD
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34.
- Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099-2106.
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505-515.
- Mullen MM, Kuroki LM, Thaker PH. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecol Oncol. 2019;152:416-425.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer. November 26, 2019. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 18, 2019.
- Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187-2195.
- Gallagher DJ, Konner JA, Bell-McGuinn KM, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22:1127-1132.
- Sun C, Li N, Ding D, et al. The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One. 2014;9:e95285.
- Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
What is optimal hormonal treatment for women with polycystic ovary syndrome?
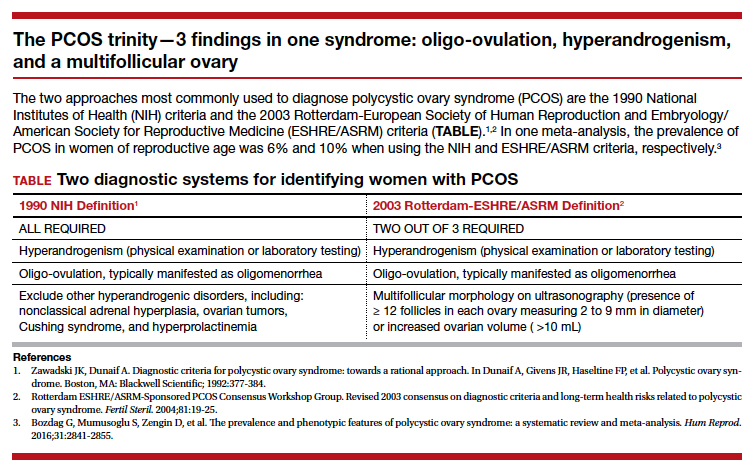
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.
The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.
Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Eating for 2: Managing eating disorders in pregnancy
Eating disorders affect nearly 1% of US adults,1 and disordered eating, or unspecified eating disorder, affects at least 1% of all pregnancies.2 Among 739 pregnant women assessed with the Eating Disorder Diagnostic scale, 7.5% of patients met criteria for an eating disorder, with 8.8% of women reporting binge eating and 2.3% of pregnant women engaging in regular compensatory behaviors. In fact, 23.4% of the study population expressed concerns about pregnancy-related weight gain and body shape.3 Eating disorders during pregnancy are more common than previously thought, and they create unique clinical challenges for obstetric providers.
Types of eating disorders
There are 3 major types of eating disorders: anorexia nervosa, bulimia nervosa, and binge eating disorder, with significant fluidity existing between all 3 conditions.
Anorexia nervosa is a condition in which an individual believes he or she is significantly overweight despite being underweight. Patients with anorexia nervosa often restrict food intake and have compulsive rituals around eating and exercise, leading to weight loss and starvation.4
Bulimia nervosa is marked by intensive dieting, uncontrolled episodes of overeating, and compensatory behaviors.4 Compensatory behaviors include self-induced vomiting; excessive exercise; and misuse of laxatives, diuretics, or other medications.
Binge eating disorder is classified as recurrent episodes of uncontrolled overeating without compensatory purging behaviors, leading to excessive weight gain.4
Eating disorders and pregnancy
Pregnancy can impact the course of preexisting eating disorders, and women also can develop symptoms of eating disorders for the first time during pregnancy. This is clinically significant as there are both maternal and fetal consequences to a mother’s disordered eating.
The risks of anorexia nervosa include vitamin deficiencies (vitamin B12/folate), dehydration leading to renal injury and electrolyte imbalances, hypoglycemia, abnormal lipid profiles, cardiac arrhythmia, and even death. The mortality rate of patients with anorexia nervosa may approach 10%; however, death during pregnancy is quite rare.2 Bulimia nervosa also carries the risks of protein and vitamin deficiencies, hypoglycemia and hyperglycemia, and death, with mortality estimated at 7% for those with a 5-year history of the illness. However, death in pregnancy due to the condition is again quite rare.5
Eating disorders can cause significant maternal and fetal complications during pregnancy and postpartum.
Maternal complications. When women with eating disorders become pregnant, they have increased risks of some pregnancy complications. Approximately 10% to 25% of pregnant women with eating disorders develop hyperemesis gravidarum.6 The nausea can serve as a trigger for a woman with an eating disorder, particularly among women with a history of purging behaviors.
Cesarean delivery is more common among women with eating disorders, which may be due to preexisting fetal compromise, leading to poor tolerance of labor, or to clinicians perceiving these pregnancies as higher risk.7
It is well known that eating disorders are highly comorbid with depression and other psychiatric conditions. In fact, 30% to 40% of women with an eating disorder develop symptoms of postpartum depression.8
Continue to: Fetal risks and complications...
Fetal risks and complications. Excessive caloric restriction and dieting can lead to folate deficiency, which in turn increases the risk of neural tube defects. Such defects are more common among women with eating disorders.9 Intrauterine growth restriction also can be a concern, most likely because of maternal malnutrition and poor maternal weight gain.10 In addition, women with eating disorders are more likely to have a preterm delivery or experience perinatal mortality or stillbirth.10
Bulimia nervosa is associated with low birthweight, while anorexia nervosa is associated with the very premature birth, low birthweight, and perinatal death.11 Eating disorders during pregnancy can have long-term psychological impacts on children, including increased likelihood of childhood hyperactivity, conduct, and adjustment disorder.12
When a patient presents showing concerning signs or symptoms of an eating disorder, it is best to start by giving her a validated assessment tool. Normalize this questioning as routine amongst populations of obstetric patients. If concerning behaviors are identified, it is best to have an open and honest conversation with the patient about her history and current disordered eating behaviors, including restrictive, binging, or purging. It is also important to address concerns and fears about pregnancy and its associated triggers. If patients are willing to accept care, it is best to connect them with a multidisciplinary treatment team, including psychiatry, nutrition, obstetrics, and social work.
Assessing patients for an eating disorder
Diagnosis of eating disorders is an interview-guided process using clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.4 The Eating Disorder Examination is a semi-structured interview composed of 4 subsections (restraint, eating concern, shape concern, and weight concern). The interview’s aim is to assess the psychopathology associated with eating disorders, and it is used in research settings rather than clinically.

Clinical diagnosis. The SCOFF questionnaire is a quick, validated tool that can be used to clinically assess for an eating disorder.13 It is composed of 5 questions, with a positive test resulting from 2 yes answers:
- Do you make yourself sick because you feel uncomfortably full?
- Do you worry that you have lost control over how much you eat?
- Have you recently lost more than one stone (14 lb) in a 3-month period?
- Do you believe yourself to be fat when others say you are too thin?
- Would you say that food dominates your life?
Referral. Patients for whom you have a concern for any eating disorder should be referred to a psychiatrist for formal diagnosis. Integrated multidisciplinary care of pregnant patients with eating disorders is necessary to improve maternal and fetal outcomes. Care teams should include obstetricians or maternal-fetal medicine clinicians experienced in caring for patients with eating disorders, psychiatrists, psychologists, nutritionists, and social workers. General treatment principles require an assessment for appropriate setting of intervention, which depends on presentation severity, assessment of nutritional status, treatment of psychiatric comorbidity, and psychotherapeutic intervention.
Overall management strategy
The initial treatment strategy for pregnant women with eating disorders should involve evaluating for severe illness and life-threatening complications of the specific disorder. All patients should be screened for suicidal ideation, severe malnutrition, electrolyte abnormalities, dehydration, hemodynamic instability, and cardiac arrhythmia. Patients with any of these severe features should be admitted for medical hospitalization and psychiatric evaluation.14 Patients that are hospitalized should be watched closely for refeeding syndrome—potentially life threatening metabolic disturbances that occur when nutrition is reinstituted to patients who are severely malnourished.
Patients without severe features or acute life-threatening complications can be managed safely on an outpatient basis with close medical monitoring. Psychiatric providers should be involved to assess for treatment needs including psychotherapy and psychotropic medications. There are numerous pharmacologic options available for patients, with the use of selective serotonin reuptake inhibitors (SSRIs) most common. While SSRI use has been controversial in pregnancy in the past, the risks of untreated illness carry risk to the mother and unborn child that outweigh the small risks associated with SSRI exposure in pregnancy.15
Women should have established care with a nutritionist or dietician who can ensure adequate counseling regarding meal planning and multivitamin supplementation. The numerous food restrictions in pregnancy, such as avoidance of unpasteurized cheese or deli meats, may be triggering for many patients with a history of restrictive eating.
One of the greatest difficulties for women with disordered eating in pregnancy revolves around weight gain. Many patients find the various measurements of pregnancy (maternal weight gain, fetal weight, fetal heart rate, and fundal height) triggering, which can make appropriate maternal and fetal weight gain in pregnancy very challenging. One strategy for managing this includes using fetal weight and growth as a surrogate for appropriate maternal gestational weight gain. One other strategy involves blind weights, where the woman is turned away from the scale so her weight is not disclosed to her. Patients often will not be able to achieve the expected 28 to 40 lb of pregnancy weight gain. It is best to have an open, honest conversation in early pregnancy to discuss how she would like to address weight in her pregnancy.
A 38-year-old woman (G1) at 32 weeks' gestation presents for a routine visit. Her bulimia had been in relatively good control until the nausea of pregnancy triggered a return to purging behaviors. She reports searching her online medical record for any recording of weights, and has now started restrictive eating because a routine recent growth scan revealed the baby to be in the 80th percentile for growth. She is concerned about her mood, and thinks she may be depressed. Because her bulimia was present before pregnancy, during her pregnancy she is followed by a multidisciplinary team, including maternal-fetal medicine, perinatal psychiatry, and nutrition. At pregnancy, she elected for outpatient day program management during her pregnancy.
Continue to: Postpregnancy concerns...
Postpregnancy concerns
Patients with eating disorders are at high risk of relapse in the postpartum period, even if they are able to achieve full remission in pregnancy. Rapid postpartum weight loss may be a sign of disordered eating. Postpartum depression also is a concern, and women should be followed closely for surveillance of symptoms. Finally, postpartum contraception is extremely important. The menstrual irregularities that are common among women with eating disorders along with common misconceptions regarding fertility in the postpartum period increase the risk of unplanned pregnancy.
Remain cognizant of eating disorders
A clear surveillance plan early in the pregnancy that is developed in conjunction with the patient and her care team is crucial in improving maternal and fetal outcomes among women with an eating disorder. Clinician knowledge of complications and risks specific to disordered eating and pregnancy can affect outcomes for both mother and baby.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21:340-344.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348-358.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatry Association: Arlington, VA; 2013.
- Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. Br J Psychiatry. 1999;174:135-140.
- Franko DL, Spirrell EB. Detection and management of eating disorders during pregnancy. Obstet Gynecol. 2000;95:942-946.
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child Cohort Study. Int J Eat Disord. 2009;42:9-18.
- Mitchell-Gieleghem A, Mittelstaedt ME, Bulik CM. Eating disorders and childbearing: concealment and consequences. Birth. 2002;29:182.
- Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127-1131.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190-255.
- Linna MS, Raevuori A, Haukka J. Pregnancy, obstetrics, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211:392.e1-e8.
- Barona M, Nybo Andersen AM, Micali N. Childhood psychopathology in children of women with eating disorders. Acta Psychiatr Scand. 2016;134:295-304.
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol. 2009;114:1353-1367.
- Weisskopf E, Fischer CJ, Bickle Graz M, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf. 2015;14:413-427.
Eating disorders affect nearly 1% of US adults,1 and disordered eating, or unspecified eating disorder, affects at least 1% of all pregnancies.2 Among 739 pregnant women assessed with the Eating Disorder Diagnostic scale, 7.5% of patients met criteria for an eating disorder, with 8.8% of women reporting binge eating and 2.3% of pregnant women engaging in regular compensatory behaviors. In fact, 23.4% of the study population expressed concerns about pregnancy-related weight gain and body shape.3 Eating disorders during pregnancy are more common than previously thought, and they create unique clinical challenges for obstetric providers.
Types of eating disorders
There are 3 major types of eating disorders: anorexia nervosa, bulimia nervosa, and binge eating disorder, with significant fluidity existing between all 3 conditions.
Anorexia nervosa is a condition in which an individual believes he or she is significantly overweight despite being underweight. Patients with anorexia nervosa often restrict food intake and have compulsive rituals around eating and exercise, leading to weight loss and starvation.4
Bulimia nervosa is marked by intensive dieting, uncontrolled episodes of overeating, and compensatory behaviors.4 Compensatory behaviors include self-induced vomiting; excessive exercise; and misuse of laxatives, diuretics, or other medications.
Binge eating disorder is classified as recurrent episodes of uncontrolled overeating without compensatory purging behaviors, leading to excessive weight gain.4
Eating disorders and pregnancy
Pregnancy can impact the course of preexisting eating disorders, and women also can develop symptoms of eating disorders for the first time during pregnancy. This is clinically significant as there are both maternal and fetal consequences to a mother’s disordered eating.
The risks of anorexia nervosa include vitamin deficiencies (vitamin B12/folate), dehydration leading to renal injury and electrolyte imbalances, hypoglycemia, abnormal lipid profiles, cardiac arrhythmia, and even death. The mortality rate of patients with anorexia nervosa may approach 10%; however, death during pregnancy is quite rare.2 Bulimia nervosa also carries the risks of protein and vitamin deficiencies, hypoglycemia and hyperglycemia, and death, with mortality estimated at 7% for those with a 5-year history of the illness. However, death in pregnancy due to the condition is again quite rare.5
Eating disorders can cause significant maternal and fetal complications during pregnancy and postpartum.
Maternal complications. When women with eating disorders become pregnant, they have increased risks of some pregnancy complications. Approximately 10% to 25% of pregnant women with eating disorders develop hyperemesis gravidarum.6 The nausea can serve as a trigger for a woman with an eating disorder, particularly among women with a history of purging behaviors.
Cesarean delivery is more common among women with eating disorders, which may be due to preexisting fetal compromise, leading to poor tolerance of labor, or to clinicians perceiving these pregnancies as higher risk.7
It is well known that eating disorders are highly comorbid with depression and other psychiatric conditions. In fact, 30% to 40% of women with an eating disorder develop symptoms of postpartum depression.8
Continue to: Fetal risks and complications...
Fetal risks and complications. Excessive caloric restriction and dieting can lead to folate deficiency, which in turn increases the risk of neural tube defects. Such defects are more common among women with eating disorders.9 Intrauterine growth restriction also can be a concern, most likely because of maternal malnutrition and poor maternal weight gain.10 In addition, women with eating disorders are more likely to have a preterm delivery or experience perinatal mortality or stillbirth.10
Bulimia nervosa is associated with low birthweight, while anorexia nervosa is associated with the very premature birth, low birthweight, and perinatal death.11 Eating disorders during pregnancy can have long-term psychological impacts on children, including increased likelihood of childhood hyperactivity, conduct, and adjustment disorder.12
When a patient presents showing concerning signs or symptoms of an eating disorder, it is best to start by giving her a validated assessment tool. Normalize this questioning as routine amongst populations of obstetric patients. If concerning behaviors are identified, it is best to have an open and honest conversation with the patient about her history and current disordered eating behaviors, including restrictive, binging, or purging. It is also important to address concerns and fears about pregnancy and its associated triggers. If patients are willing to accept care, it is best to connect them with a multidisciplinary treatment team, including psychiatry, nutrition, obstetrics, and social work.
Assessing patients for an eating disorder
Diagnosis of eating disorders is an interview-guided process using clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.4 The Eating Disorder Examination is a semi-structured interview composed of 4 subsections (restraint, eating concern, shape concern, and weight concern). The interview’s aim is to assess the psychopathology associated with eating disorders, and it is used in research settings rather than clinically.

Clinical diagnosis. The SCOFF questionnaire is a quick, validated tool that can be used to clinically assess for an eating disorder.13 It is composed of 5 questions, with a positive test resulting from 2 yes answers:
- Do you make yourself sick because you feel uncomfortably full?
- Do you worry that you have lost control over how much you eat?
- Have you recently lost more than one stone (14 lb) in a 3-month period?
- Do you believe yourself to be fat when others say you are too thin?
- Would you say that food dominates your life?
Referral. Patients for whom you have a concern for any eating disorder should be referred to a psychiatrist for formal diagnosis. Integrated multidisciplinary care of pregnant patients with eating disorders is necessary to improve maternal and fetal outcomes. Care teams should include obstetricians or maternal-fetal medicine clinicians experienced in caring for patients with eating disorders, psychiatrists, psychologists, nutritionists, and social workers. General treatment principles require an assessment for appropriate setting of intervention, which depends on presentation severity, assessment of nutritional status, treatment of psychiatric comorbidity, and psychotherapeutic intervention.
Overall management strategy
The initial treatment strategy for pregnant women with eating disorders should involve evaluating for severe illness and life-threatening complications of the specific disorder. All patients should be screened for suicidal ideation, severe malnutrition, electrolyte abnormalities, dehydration, hemodynamic instability, and cardiac arrhythmia. Patients with any of these severe features should be admitted for medical hospitalization and psychiatric evaluation.14 Patients that are hospitalized should be watched closely for refeeding syndrome—potentially life threatening metabolic disturbances that occur when nutrition is reinstituted to patients who are severely malnourished.
Patients without severe features or acute life-threatening complications can be managed safely on an outpatient basis with close medical monitoring. Psychiatric providers should be involved to assess for treatment needs including psychotherapy and psychotropic medications. There are numerous pharmacologic options available for patients, with the use of selective serotonin reuptake inhibitors (SSRIs) most common. While SSRI use has been controversial in pregnancy in the past, the risks of untreated illness carry risk to the mother and unborn child that outweigh the small risks associated with SSRI exposure in pregnancy.15
Women should have established care with a nutritionist or dietician who can ensure adequate counseling regarding meal planning and multivitamin supplementation. The numerous food restrictions in pregnancy, such as avoidance of unpasteurized cheese or deli meats, may be triggering for many patients with a history of restrictive eating.
One of the greatest difficulties for women with disordered eating in pregnancy revolves around weight gain. Many patients find the various measurements of pregnancy (maternal weight gain, fetal weight, fetal heart rate, and fundal height) triggering, which can make appropriate maternal and fetal weight gain in pregnancy very challenging. One strategy for managing this includes using fetal weight and growth as a surrogate for appropriate maternal gestational weight gain. One other strategy involves blind weights, where the woman is turned away from the scale so her weight is not disclosed to her. Patients often will not be able to achieve the expected 28 to 40 lb of pregnancy weight gain. It is best to have an open, honest conversation in early pregnancy to discuss how she would like to address weight in her pregnancy.
A 38-year-old woman (G1) at 32 weeks' gestation presents for a routine visit. Her bulimia had been in relatively good control until the nausea of pregnancy triggered a return to purging behaviors. She reports searching her online medical record for any recording of weights, and has now started restrictive eating because a routine recent growth scan revealed the baby to be in the 80th percentile for growth. She is concerned about her mood, and thinks she may be depressed. Because her bulimia was present before pregnancy, during her pregnancy she is followed by a multidisciplinary team, including maternal-fetal medicine, perinatal psychiatry, and nutrition. At pregnancy, she elected for outpatient day program management during her pregnancy.
Continue to: Postpregnancy concerns...
Postpregnancy concerns
Patients with eating disorders are at high risk of relapse in the postpartum period, even if they are able to achieve full remission in pregnancy. Rapid postpartum weight loss may be a sign of disordered eating. Postpartum depression also is a concern, and women should be followed closely for surveillance of symptoms. Finally, postpartum contraception is extremely important. The menstrual irregularities that are common among women with eating disorders along with common misconceptions regarding fertility in the postpartum period increase the risk of unplanned pregnancy.
Remain cognizant of eating disorders
A clear surveillance plan early in the pregnancy that is developed in conjunction with the patient and her care team is crucial in improving maternal and fetal outcomes among women with an eating disorder. Clinician knowledge of complications and risks specific to disordered eating and pregnancy can affect outcomes for both mother and baby.
Eating disorders affect nearly 1% of US adults,1 and disordered eating, or unspecified eating disorder, affects at least 1% of all pregnancies.2 Among 739 pregnant women assessed with the Eating Disorder Diagnostic scale, 7.5% of patients met criteria for an eating disorder, with 8.8% of women reporting binge eating and 2.3% of pregnant women engaging in regular compensatory behaviors. In fact, 23.4% of the study population expressed concerns about pregnancy-related weight gain and body shape.3 Eating disorders during pregnancy are more common than previously thought, and they create unique clinical challenges for obstetric providers.
Types of eating disorders
There are 3 major types of eating disorders: anorexia nervosa, bulimia nervosa, and binge eating disorder, with significant fluidity existing between all 3 conditions.
Anorexia nervosa is a condition in which an individual believes he or she is significantly overweight despite being underweight. Patients with anorexia nervosa often restrict food intake and have compulsive rituals around eating and exercise, leading to weight loss and starvation.4
Bulimia nervosa is marked by intensive dieting, uncontrolled episodes of overeating, and compensatory behaviors.4 Compensatory behaviors include self-induced vomiting; excessive exercise; and misuse of laxatives, diuretics, or other medications.
Binge eating disorder is classified as recurrent episodes of uncontrolled overeating without compensatory purging behaviors, leading to excessive weight gain.4
Eating disorders and pregnancy
Pregnancy can impact the course of preexisting eating disorders, and women also can develop symptoms of eating disorders for the first time during pregnancy. This is clinically significant as there are both maternal and fetal consequences to a mother’s disordered eating.
The risks of anorexia nervosa include vitamin deficiencies (vitamin B12/folate), dehydration leading to renal injury and electrolyte imbalances, hypoglycemia, abnormal lipid profiles, cardiac arrhythmia, and even death. The mortality rate of patients with anorexia nervosa may approach 10%; however, death during pregnancy is quite rare.2 Bulimia nervosa also carries the risks of protein and vitamin deficiencies, hypoglycemia and hyperglycemia, and death, with mortality estimated at 7% for those with a 5-year history of the illness. However, death in pregnancy due to the condition is again quite rare.5
Eating disorders can cause significant maternal and fetal complications during pregnancy and postpartum.
Maternal complications. When women with eating disorders become pregnant, they have increased risks of some pregnancy complications. Approximately 10% to 25% of pregnant women with eating disorders develop hyperemesis gravidarum.6 The nausea can serve as a trigger for a woman with an eating disorder, particularly among women with a history of purging behaviors.
Cesarean delivery is more common among women with eating disorders, which may be due to preexisting fetal compromise, leading to poor tolerance of labor, or to clinicians perceiving these pregnancies as higher risk.7
It is well known that eating disorders are highly comorbid with depression and other psychiatric conditions. In fact, 30% to 40% of women with an eating disorder develop symptoms of postpartum depression.8
Continue to: Fetal risks and complications...
Fetal risks and complications. Excessive caloric restriction and dieting can lead to folate deficiency, which in turn increases the risk of neural tube defects. Such defects are more common among women with eating disorders.9 Intrauterine growth restriction also can be a concern, most likely because of maternal malnutrition and poor maternal weight gain.10 In addition, women with eating disorders are more likely to have a preterm delivery or experience perinatal mortality or stillbirth.10
Bulimia nervosa is associated with low birthweight, while anorexia nervosa is associated with the very premature birth, low birthweight, and perinatal death.11 Eating disorders during pregnancy can have long-term psychological impacts on children, including increased likelihood of childhood hyperactivity, conduct, and adjustment disorder.12
When a patient presents showing concerning signs or symptoms of an eating disorder, it is best to start by giving her a validated assessment tool. Normalize this questioning as routine amongst populations of obstetric patients. If concerning behaviors are identified, it is best to have an open and honest conversation with the patient about her history and current disordered eating behaviors, including restrictive, binging, or purging. It is also important to address concerns and fears about pregnancy and its associated triggers. If patients are willing to accept care, it is best to connect them with a multidisciplinary treatment team, including psychiatry, nutrition, obstetrics, and social work.
Assessing patients for an eating disorder
Diagnosis of eating disorders is an interview-guided process using clinical criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition.4 The Eating Disorder Examination is a semi-structured interview composed of 4 subsections (restraint, eating concern, shape concern, and weight concern). The interview’s aim is to assess the psychopathology associated with eating disorders, and it is used in research settings rather than clinically.

Clinical diagnosis. The SCOFF questionnaire is a quick, validated tool that can be used to clinically assess for an eating disorder.13 It is composed of 5 questions, with a positive test resulting from 2 yes answers:
- Do you make yourself sick because you feel uncomfortably full?
- Do you worry that you have lost control over how much you eat?
- Have you recently lost more than one stone (14 lb) in a 3-month period?
- Do you believe yourself to be fat when others say you are too thin?
- Would you say that food dominates your life?
Referral. Patients for whom you have a concern for any eating disorder should be referred to a psychiatrist for formal diagnosis. Integrated multidisciplinary care of pregnant patients with eating disorders is necessary to improve maternal and fetal outcomes. Care teams should include obstetricians or maternal-fetal medicine clinicians experienced in caring for patients with eating disorders, psychiatrists, psychologists, nutritionists, and social workers. General treatment principles require an assessment for appropriate setting of intervention, which depends on presentation severity, assessment of nutritional status, treatment of psychiatric comorbidity, and psychotherapeutic intervention.
Overall management strategy
The initial treatment strategy for pregnant women with eating disorders should involve evaluating for severe illness and life-threatening complications of the specific disorder. All patients should be screened for suicidal ideation, severe malnutrition, electrolyte abnormalities, dehydration, hemodynamic instability, and cardiac arrhythmia. Patients with any of these severe features should be admitted for medical hospitalization and psychiatric evaluation.14 Patients that are hospitalized should be watched closely for refeeding syndrome—potentially life threatening metabolic disturbances that occur when nutrition is reinstituted to patients who are severely malnourished.
Patients without severe features or acute life-threatening complications can be managed safely on an outpatient basis with close medical monitoring. Psychiatric providers should be involved to assess for treatment needs including psychotherapy and psychotropic medications. There are numerous pharmacologic options available for patients, with the use of selective serotonin reuptake inhibitors (SSRIs) most common. While SSRI use has been controversial in pregnancy in the past, the risks of untreated illness carry risk to the mother and unborn child that outweigh the small risks associated with SSRI exposure in pregnancy.15
Women should have established care with a nutritionist or dietician who can ensure adequate counseling regarding meal planning and multivitamin supplementation. The numerous food restrictions in pregnancy, such as avoidance of unpasteurized cheese or deli meats, may be triggering for many patients with a history of restrictive eating.
One of the greatest difficulties for women with disordered eating in pregnancy revolves around weight gain. Many patients find the various measurements of pregnancy (maternal weight gain, fetal weight, fetal heart rate, and fundal height) triggering, which can make appropriate maternal and fetal weight gain in pregnancy very challenging. One strategy for managing this includes using fetal weight and growth as a surrogate for appropriate maternal gestational weight gain. One other strategy involves blind weights, where the woman is turned away from the scale so her weight is not disclosed to her. Patients often will not be able to achieve the expected 28 to 40 lb of pregnancy weight gain. It is best to have an open, honest conversation in early pregnancy to discuss how she would like to address weight in her pregnancy.
A 38-year-old woman (G1) at 32 weeks' gestation presents for a routine visit. Her bulimia had been in relatively good control until the nausea of pregnancy triggered a return to purging behaviors. She reports searching her online medical record for any recording of weights, and has now started restrictive eating because a routine recent growth scan revealed the baby to be in the 80th percentile for growth. She is concerned about her mood, and thinks she may be depressed. Because her bulimia was present before pregnancy, during her pregnancy she is followed by a multidisciplinary team, including maternal-fetal medicine, perinatal psychiatry, and nutrition. At pregnancy, she elected for outpatient day program management during her pregnancy.
Continue to: Postpregnancy concerns...
Postpregnancy concerns
Patients with eating disorders are at high risk of relapse in the postpartum period, even if they are able to achieve full remission in pregnancy. Rapid postpartum weight loss may be a sign of disordered eating. Postpartum depression also is a concern, and women should be followed closely for surveillance of symptoms. Finally, postpartum contraception is extremely important. The menstrual irregularities that are common among women with eating disorders along with common misconceptions regarding fertility in the postpartum period increase the risk of unplanned pregnancy.
Remain cognizant of eating disorders
A clear surveillance plan early in the pregnancy that is developed in conjunction with the patient and her care team is crucial in improving maternal and fetal outcomes among women with an eating disorder. Clinician knowledge of complications and risks specific to disordered eating and pregnancy can affect outcomes for both mother and baby.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21:340-344.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348-358.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatry Association: Arlington, VA; 2013.
- Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. Br J Psychiatry. 1999;174:135-140.
- Franko DL, Spirrell EB. Detection and management of eating disorders during pregnancy. Obstet Gynecol. 2000;95:942-946.
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child Cohort Study. Int J Eat Disord. 2009;42:9-18.
- Mitchell-Gieleghem A, Mittelstaedt ME, Bulik CM. Eating disorders and childbearing: concealment and consequences. Birth. 2002;29:182.
- Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127-1131.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190-255.
- Linna MS, Raevuori A, Haukka J. Pregnancy, obstetrics, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211:392.e1-e8.
- Barona M, Nybo Andersen AM, Micali N. Childhood psychopathology in children of women with eating disorders. Acta Psychiatr Scand. 2016;134:295-304.
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol. 2009;114:1353-1367.
- Weisskopf E, Fischer CJ, Bickle Graz M, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf. 2015;14:413-427.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Easter A, Bye A, Taborelli E, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. 2013;21:340-344.
- Hudson JI, Hiripi E, Pope HG Jr, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348-358.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). American Psychiatry Association: Arlington, VA; 2013.
- Morgan JF, Lacey JH, Sedgwick PM. Impact of pregnancy on bulimia nervosa. Br J Psychiatry. 1999;174:135-140.
- Franko DL, Spirrell EB. Detection and management of eating disorders during pregnancy. Obstet Gynecol. 2000;95:942-946.
- Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child Cohort Study. Int J Eat Disord. 2009;42:9-18.
- Mitchell-Gieleghem A, Mittelstaedt ME, Bulik CM. Eating disorders and childbearing: concealment and consequences. Birth. 2002;29:182.
- Carmichael SL, Shaw GM, Schaffer DM, et al. Dieting behaviors and risk of neural tube defects. Am J Epidemiol. 2003;158:1127-1131.
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190-255.
- Linna MS, Raevuori A, Haukka J. Pregnancy, obstetrics, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211:392.e1-e8.
- Barona M, Nybo Andersen AM, Micali N. Childhood psychopathology in children of women with eating disorders. Acta Psychiatr Scand. 2016;134:295-304.
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319:1467.
- Andersen AE, Ryan GL. Eating disorders in the obstetric and gynecologic patient population. Obstet Gynecol. 2009;114:1353-1367.
- Weisskopf E, Fischer CJ, Bickle Graz M, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence. Expert Opin Drug Saf. 2015;14:413-427.
Is elagolix effective at reducing HMB for women with varying fibroid sizes and types?
Whether or not women experience symptoms from uterine fibroid(s) can be dependent on a fibroid’s size and location. Heavy menstrual bleeding (HMB) is the most common symptom resulting from fibroids, and it occurs in up to one-third of women with fibroids. For fibroids that are large (>10 cm), “bulk” symptoms may occur, including pelvic pressure, urinary urgency or frequency, incontinence, constipation, abdominal protrusion, etc.1
Elagolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, was US Food and Drug Administration–approved in 2018 to treat moderate to severe pain caused by endometriosis. 2 Elagolix is being evaluated in 2 phase 3 randomized, double-blind trials for the additional treatment of HMB associated with uterine fibroids. The results of these studies were presented at the 2019 AAGL meeting on November 12, in Vancouver, Canada.
Phase 3 study details
Premenopausal women aged 18 to 51 years were included in the Elaris UF-1 and UF-2 studies if they had HMB (defined using the alkaline hematin methodology as menstrual blood loss [MBL] >80 mL/cycle) and uterine fibroids as confirmed through ultrasound. Because elagolix suppresses estrogen and progesterone, treatment results in dose- and duration-dependent decreases in bone mineral density (BMD),2 and add-back therapy can lessen these adverse effects. Subsequently, participants were randomly assigned 1:1:2 to placebo, elagolix 300 mg twice daily, or elagolix 300 mg twice daily with add-back therapy (1 mg estradiol/0.5 mg norethidrone acetate [E2/NETA]) once daily. Uterine volume and size and location of uterine fibroid(s) were assessed by ultrasound. Subgroups were defined by baseline FIGO categories, grouped FIGO 0-3, FIGO 4, or FIGO 5-8.3
Over the 6-month studies, 72.2% (95% confidence interval [CI], 67.65–76.73) of the 395 women who received elagolix plus E2/NETA achieved < 80 mL MBL during the final month and ≥ 50% MBL reduction from baseline to the final month. When stratified by FIGO classification, the results were similar for all subgroups: FIGO 0-3, 77.7% (95% CI, 67.21–80.85). Similar results were seen in women with a primary fibroid volume of either greater or less than 36.2 cm3 (median).3
The most frequently reported adverse events among women taking elagolix plus E2/NETA were hot flushes, night sweats, nausea, and headache. Changes in BMD among these women were not significant compared with women taking placebo.3
The Elaris UF-1 and UF-2 studies are funded by AbbVie Inc.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480.
- Orilissa [package insert]. North Chicago, IL: AbbVie; August 2019.
- Al-Hendy A, Simon J, Hurtado S, et al. Effect of fibroid location and size on efficacy in elagolix: results from phase 3 clinical trials. Paper presented at: 48th Annual Meeting of the AAGL; November 2019; Vancouver, Canada.
Whether or not women experience symptoms from uterine fibroid(s) can be dependent on a fibroid’s size and location. Heavy menstrual bleeding (HMB) is the most common symptom resulting from fibroids, and it occurs in up to one-third of women with fibroids. For fibroids that are large (>10 cm), “bulk” symptoms may occur, including pelvic pressure, urinary urgency or frequency, incontinence, constipation, abdominal protrusion, etc.1
Elagolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, was US Food and Drug Administration–approved in 2018 to treat moderate to severe pain caused by endometriosis. 2 Elagolix is being evaluated in 2 phase 3 randomized, double-blind trials for the additional treatment of HMB associated with uterine fibroids. The results of these studies were presented at the 2019 AAGL meeting on November 12, in Vancouver, Canada.
Phase 3 study details
Premenopausal women aged 18 to 51 years were included in the Elaris UF-1 and UF-2 studies if they had HMB (defined using the alkaline hematin methodology as menstrual blood loss [MBL] >80 mL/cycle) and uterine fibroids as confirmed through ultrasound. Because elagolix suppresses estrogen and progesterone, treatment results in dose- and duration-dependent decreases in bone mineral density (BMD),2 and add-back therapy can lessen these adverse effects. Subsequently, participants were randomly assigned 1:1:2 to placebo, elagolix 300 mg twice daily, or elagolix 300 mg twice daily with add-back therapy (1 mg estradiol/0.5 mg norethidrone acetate [E2/NETA]) once daily. Uterine volume and size and location of uterine fibroid(s) were assessed by ultrasound. Subgroups were defined by baseline FIGO categories, grouped FIGO 0-3, FIGO 4, or FIGO 5-8.3
Over the 6-month studies, 72.2% (95% confidence interval [CI], 67.65–76.73) of the 395 women who received elagolix plus E2/NETA achieved < 80 mL MBL during the final month and ≥ 50% MBL reduction from baseline to the final month. When stratified by FIGO classification, the results were similar for all subgroups: FIGO 0-3, 77.7% (95% CI, 67.21–80.85). Similar results were seen in women with a primary fibroid volume of either greater or less than 36.2 cm3 (median).3
The most frequently reported adverse events among women taking elagolix plus E2/NETA were hot flushes, night sweats, nausea, and headache. Changes in BMD among these women were not significant compared with women taking placebo.3
The Elaris UF-1 and UF-2 studies are funded by AbbVie Inc.
Whether or not women experience symptoms from uterine fibroid(s) can be dependent on a fibroid’s size and location. Heavy menstrual bleeding (HMB) is the most common symptom resulting from fibroids, and it occurs in up to one-third of women with fibroids. For fibroids that are large (>10 cm), “bulk” symptoms may occur, including pelvic pressure, urinary urgency or frequency, incontinence, constipation, abdominal protrusion, etc.1
Elagolix, an oral gonadotropin-releasing hormone (GnRH) receptor antagonist, was US Food and Drug Administration–approved in 2018 to treat moderate to severe pain caused by endometriosis. 2 Elagolix is being evaluated in 2 phase 3 randomized, double-blind trials for the additional treatment of HMB associated with uterine fibroids. The results of these studies were presented at the 2019 AAGL meeting on November 12, in Vancouver, Canada.
Phase 3 study details
Premenopausal women aged 18 to 51 years were included in the Elaris UF-1 and UF-2 studies if they had HMB (defined using the alkaline hematin methodology as menstrual blood loss [MBL] >80 mL/cycle) and uterine fibroids as confirmed through ultrasound. Because elagolix suppresses estrogen and progesterone, treatment results in dose- and duration-dependent decreases in bone mineral density (BMD),2 and add-back therapy can lessen these adverse effects. Subsequently, participants were randomly assigned 1:1:2 to placebo, elagolix 300 mg twice daily, or elagolix 300 mg twice daily with add-back therapy (1 mg estradiol/0.5 mg norethidrone acetate [E2/NETA]) once daily. Uterine volume and size and location of uterine fibroid(s) were assessed by ultrasound. Subgroups were defined by baseline FIGO categories, grouped FIGO 0-3, FIGO 4, or FIGO 5-8.3
Over the 6-month studies, 72.2% (95% confidence interval [CI], 67.65–76.73) of the 395 women who received elagolix plus E2/NETA achieved < 80 mL MBL during the final month and ≥ 50% MBL reduction from baseline to the final month. When stratified by FIGO classification, the results were similar for all subgroups: FIGO 0-3, 77.7% (95% CI, 67.21–80.85). Similar results were seen in women with a primary fibroid volume of either greater or less than 36.2 cm3 (median).3
The most frequently reported adverse events among women taking elagolix plus E2/NETA were hot flushes, night sweats, nausea, and headache. Changes in BMD among these women were not significant compared with women taking placebo.3
The Elaris UF-1 and UF-2 studies are funded by AbbVie Inc.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480.
- Orilissa [package insert]. North Chicago, IL: AbbVie; August 2019.
- Al-Hendy A, Simon J, Hurtado S, et al. Effect of fibroid location and size on efficacy in elagolix: results from phase 3 clinical trials. Paper presented at: 48th Annual Meeting of the AAGL; November 2019; Vancouver, Canada.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480.
- Orilissa [package insert]. North Chicago, IL: AbbVie; August 2019.
- Al-Hendy A, Simon J, Hurtado S, et al. Effect of fibroid location and size on efficacy in elagolix: results from phase 3 clinical trials. Paper presented at: 48th Annual Meeting of the AAGL; November 2019; Vancouver, Canada.
Can the office visit interval for routine pessary care be extended safely?
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Propst K, Mellen C, O’Sullivan DM, et al. Timing of office-based pessary care: a randomized controlled trial. Obstet Gynecol. 2019 Dec 5. Doi: 10.1097/AOG.0000000000003580.
EXPERT COMMENTARY
Vaginal pessaries are a common and effective approach for managing pelvic organ prolapse (POP) as well as stress urinary incontinence (SUI). Vaginal mucosal erosions, however, may complicate pessary use. The risk for erosions may be associated with the frequency of pessary change, which involves removing the pessary, washing it, and replacing it in the vagina. Existing data do not address the frequency of pessary change. Recently, however, investigators conducted a randomized noninferiority trial to evaluate the effect of pessary visit intervals on the development of vaginal epithelial abnormalities.
Details of the study
At a single US hospital, Propst and colleagues randomly assigned women who used pessaries for POP, SUI, or both to routine pessary care (offices visits every 12 weeks) or to extended interval pessary care (office visits every 24 weeks). The women used ring, incontinence dish, or Gelhorn pessaries, did not change their pessaries on their own, and had no vaginal mucosal abnormalities.
A total of 130 women were randomly assigned, 64 to the routine care group and 66 to the extended interval care group. The mean age was 79 years and 90% were white, 4.6% were black, and 4% were Hispanic. Approximately 74% of the women used vaginal estrogen.
The primary outcome was the rate of vaginal epithelial abnormalities, including epithelial breaks or erosions. The predetermined noninferiority margin was set at 7.5%.
Results. At the 48-week follow-up, the rate of epithelial erosion was 7.4% in the routine care group and 1.7% in the extended interval care group, thus meeting the prespecified criteria for noninferiority of extended interval pessary care.
Women in each care group reported a similar amount of bothersome vaginal discharge. This was reported on a 5-point scale, with higher numbers indicating greater degree of bother. The mean scores were 1.39 in the routine care group and 1.34 in the extended interval care group. No other pessary-related adverse events occurred in either care group.
Study strengths and limitations
This trial provides good evidence that the timing of office pessary care can be extended to 24 weeks without compromising outcomes. However, since nearly three-quarters of the study participants used vaginal estrogen, the results may not be applicable to pessary users who do not use vaginal estrogen.
Many women change their pessary at home as often as weekly or daily. For women who rely on office visits for pessary care, however, the trial by Propst and colleagues provides good quality evidence that pessaries can be changed as infrequently as every 24 weeks without compromising outcomes. An important limitation of these data is that since most study participants used vaginal estrogen, the findings may not apply to pessary use among women who do not use vaginal estrogen.
ANDREW M. KAUNITZ, MD, NCMP
Don’t neglect urinary tract in gynecologic procedures
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Beware the dangers of nerve injury in vaginal surgery
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Pelvic organ prolapse surgery isn’t as ‘simple’ as you think
LAS VEGAS – Surgical repair of pelvic organ prolapse often may seem like an uncomplicated procedure. But many factors play roles into decisions, and surgeons around the world vary widely in how they handle the operations, Mark D. Walters, MD, told colleagues at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“These prolapse repairs seem relatively simple at first, but they’re not simple at all,” he said.
Questions to ask prior to surgery
It’s important to first answer a number of questions, said Dr. Walters, professor and vice-chair of gynecology at the Cleveland Clinic. “When you see a patient like this, you may not realize how many decisions you’re making.”
These questions include:
- Is the patient sexually active or planning to be?
- Has she had a hysterectomy, and or is one necessary? If so, how should it be done? What does the patient think about a hysterectomy?
- Should the prolapse procedure be performed vaginally, open, laparoscopically, or robotically?
- Is adding a graft advisable? What kind?
- Should there be a sling to prevent stress urinary incontinence?”
Worldwide differences in surgical technique choice
Dr. Walters talked to colleagues from several nations and learned about these variations in surgical techniques.
Chinese surgeons use a variety of techniques with transvaginal mesh (TVM). Their use is more common in more populated cities because of the effect of medical education; native tissue procedures are more common in less-populated regions that are considered “backward.”
TVM with hysteropexy (“apical sling”) also is common in Latin America, while Middle Eastern surgeons have little training in female pelvic medicine and reconstructive surgery.
In Europe, France embraces mesh surgery and laparoscopy, while the United Kingdom has “completely abandoned” mesh surgery, and the Netherlands rarely uses it in favor of vaginal procedures.
In the United States, he said, TVM is “discouraged” while a variety of other procedures are used.
What procedures should surgeons embrace? There are many topics of debate, Dr. Walters said, including type of transvaginal repair (native tissue or mesh-augmented or sacrocolpopexy?), repair of “defects” in the vagina (even if they’re nonsymptomatic?) and the removal of the uterus (yes or no?).
Dr. Walters pointed to several explanations for this variation, including lack of high-quality research, confirmation bias, economic conflicts – surgeons are in the business of surgery, after all – and lack of insight into what women prefer.
Consider patient choice
In a survey, Dr. Walters polled women in their 50s with this question: “How much do you value your uterus?” Three women, he said, had widely varied opinions on a scale of 1-10, with one at 10 and another at 0.
“A doctor doesn’t know this and doesn’t have a way to ask, and the doctor has [his/her] own opinion about the value of the uterus,” he said. “Shouldn’t we know what patients think?”
How to measure success
He offered these tips about measuring success:
- Focus on symptomatic cure more than clinical cure.
- Remember that perfect anatomic support isn’t linked to health-related quality of life, and some loss of anatomic support is normal.
- Understand that commonly used definitions of anatomic success often aren’t clinically relevant.
Dr. Walters’ disclosures: royalties (Elsevier, UpToDate), website/lecturer (International Academy of Pelvic Surgery), and website editor (Foundation for Female Health Awareness).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – Surgical repair of pelvic organ prolapse often may seem like an uncomplicated procedure. But many factors play roles into decisions, and surgeons around the world vary widely in how they handle the operations, Mark D. Walters, MD, told colleagues at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“These prolapse repairs seem relatively simple at first, but they’re not simple at all,” he said.
Questions to ask prior to surgery
It’s important to first answer a number of questions, said Dr. Walters, professor and vice-chair of gynecology at the Cleveland Clinic. “When you see a patient like this, you may not realize how many decisions you’re making.”
These questions include:
- Is the patient sexually active or planning to be?
- Has she had a hysterectomy, and or is one necessary? If so, how should it be done? What does the patient think about a hysterectomy?
- Should the prolapse procedure be performed vaginally, open, laparoscopically, or robotically?
- Is adding a graft advisable? What kind?
- Should there be a sling to prevent stress urinary incontinence?”
Worldwide differences in surgical technique choice
Dr. Walters talked to colleagues from several nations and learned about these variations in surgical techniques.
Chinese surgeons use a variety of techniques with transvaginal mesh (TVM). Their use is more common in more populated cities because of the effect of medical education; native tissue procedures are more common in less-populated regions that are considered “backward.”
TVM with hysteropexy (“apical sling”) also is common in Latin America, while Middle Eastern surgeons have little training in female pelvic medicine and reconstructive surgery.
In Europe, France embraces mesh surgery and laparoscopy, while the United Kingdom has “completely abandoned” mesh surgery, and the Netherlands rarely uses it in favor of vaginal procedures.
In the United States, he said, TVM is “discouraged” while a variety of other procedures are used.
What procedures should surgeons embrace? There are many topics of debate, Dr. Walters said, including type of transvaginal repair (native tissue or mesh-augmented or sacrocolpopexy?), repair of “defects” in the vagina (even if they’re nonsymptomatic?) and the removal of the uterus (yes or no?).
Dr. Walters pointed to several explanations for this variation, including lack of high-quality research, confirmation bias, economic conflicts – surgeons are in the business of surgery, after all – and lack of insight into what women prefer.
Consider patient choice
In a survey, Dr. Walters polled women in their 50s with this question: “How much do you value your uterus?” Three women, he said, had widely varied opinions on a scale of 1-10, with one at 10 and another at 0.
“A doctor doesn’t know this and doesn’t have a way to ask, and the doctor has [his/her] own opinion about the value of the uterus,” he said. “Shouldn’t we know what patients think?”
How to measure success
He offered these tips about measuring success:
- Focus on symptomatic cure more than clinical cure.
- Remember that perfect anatomic support isn’t linked to health-related quality of life, and some loss of anatomic support is normal.
- Understand that commonly used definitions of anatomic success often aren’t clinically relevant.
Dr. Walters’ disclosures: royalties (Elsevier, UpToDate), website/lecturer (International Academy of Pelvic Surgery), and website editor (Foundation for Female Health Awareness).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – Surgical repair of pelvic organ prolapse often may seem like an uncomplicated procedure. But many factors play roles into decisions, and surgeons around the world vary widely in how they handle the operations, Mark D. Walters, MD, told colleagues at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“These prolapse repairs seem relatively simple at first, but they’re not simple at all,” he said.
Questions to ask prior to surgery
It’s important to first answer a number of questions, said Dr. Walters, professor and vice-chair of gynecology at the Cleveland Clinic. “When you see a patient like this, you may not realize how many decisions you’re making.”
These questions include:
- Is the patient sexually active or planning to be?
- Has she had a hysterectomy, and or is one necessary? If so, how should it be done? What does the patient think about a hysterectomy?
- Should the prolapse procedure be performed vaginally, open, laparoscopically, or robotically?
- Is adding a graft advisable? What kind?
- Should there be a sling to prevent stress urinary incontinence?”
Worldwide differences in surgical technique choice
Dr. Walters talked to colleagues from several nations and learned about these variations in surgical techniques.
Chinese surgeons use a variety of techniques with transvaginal mesh (TVM). Their use is more common in more populated cities because of the effect of medical education; native tissue procedures are more common in less-populated regions that are considered “backward.”
TVM with hysteropexy (“apical sling”) also is common in Latin America, while Middle Eastern surgeons have little training in female pelvic medicine and reconstructive surgery.
In Europe, France embraces mesh surgery and laparoscopy, while the United Kingdom has “completely abandoned” mesh surgery, and the Netherlands rarely uses it in favor of vaginal procedures.
In the United States, he said, TVM is “discouraged” while a variety of other procedures are used.
What procedures should surgeons embrace? There are many topics of debate, Dr. Walters said, including type of transvaginal repair (native tissue or mesh-augmented or sacrocolpopexy?), repair of “defects” in the vagina (even if they’re nonsymptomatic?) and the removal of the uterus (yes or no?).
Dr. Walters pointed to several explanations for this variation, including lack of high-quality research, confirmation bias, economic conflicts – surgeons are in the business of surgery, after all – and lack of insight into what women prefer.
Consider patient choice
In a survey, Dr. Walters polled women in their 50s with this question: “How much do you value your uterus?” Three women, he said, had widely varied opinions on a scale of 1-10, with one at 10 and another at 0.
“A doctor doesn’t know this and doesn’t have a way to ask, and the doctor has [his/her] own opinion about the value of the uterus,” he said. “Shouldn’t we know what patients think?”
How to measure success
He offered these tips about measuring success:
- Focus on symptomatic cure more than clinical cure.
- Remember that perfect anatomic support isn’t linked to health-related quality of life, and some loss of anatomic support is normal.
- Understand that commonly used definitions of anatomic success often aren’t clinically relevant.
Dr. Walters’ disclosures: royalties (Elsevier, UpToDate), website/lecturer (International Academy of Pelvic Surgery), and website editor (Foundation for Female Health Awareness).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019