User login
Trends in Utilization of Total Hip Arthroplasty for Femoral Neck Fractures in the United States
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.
Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
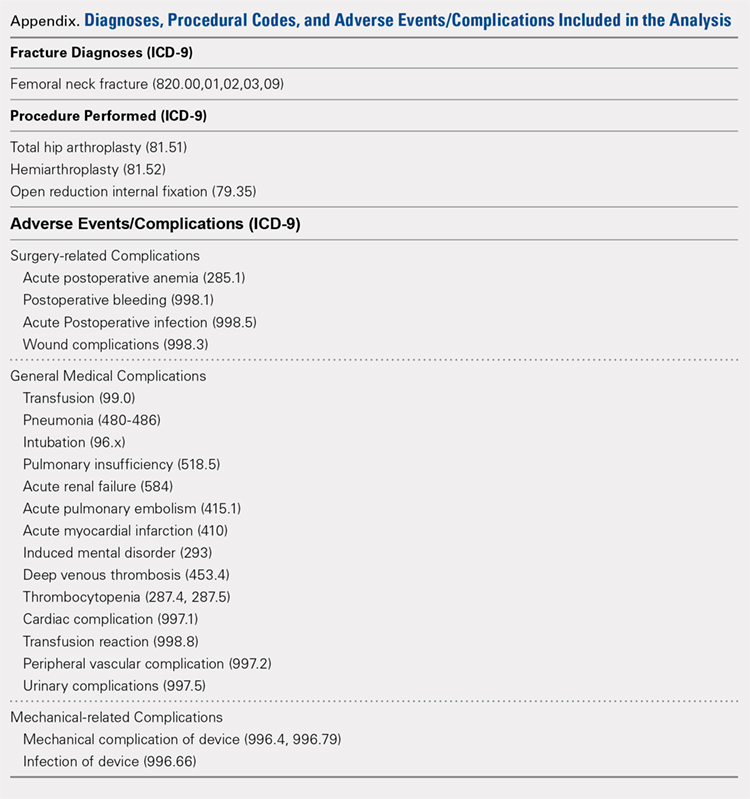
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.
Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
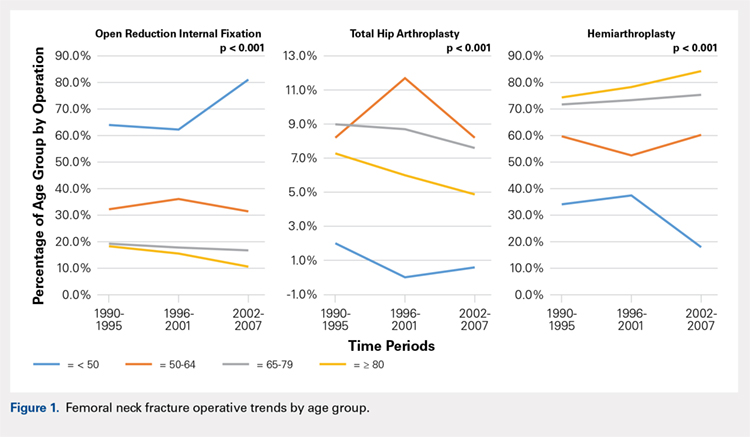
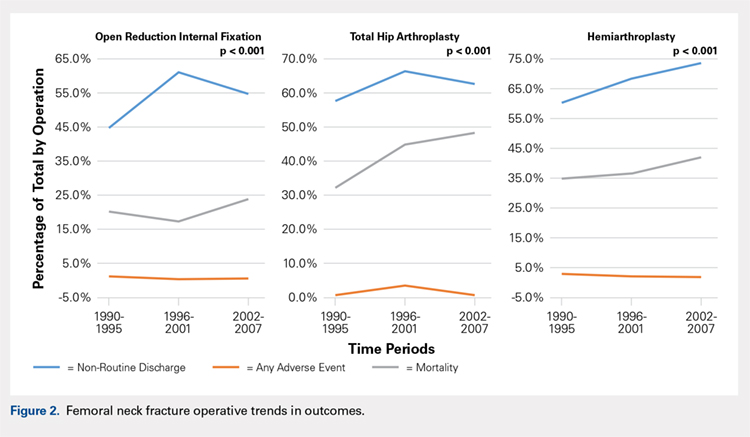
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.
Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).
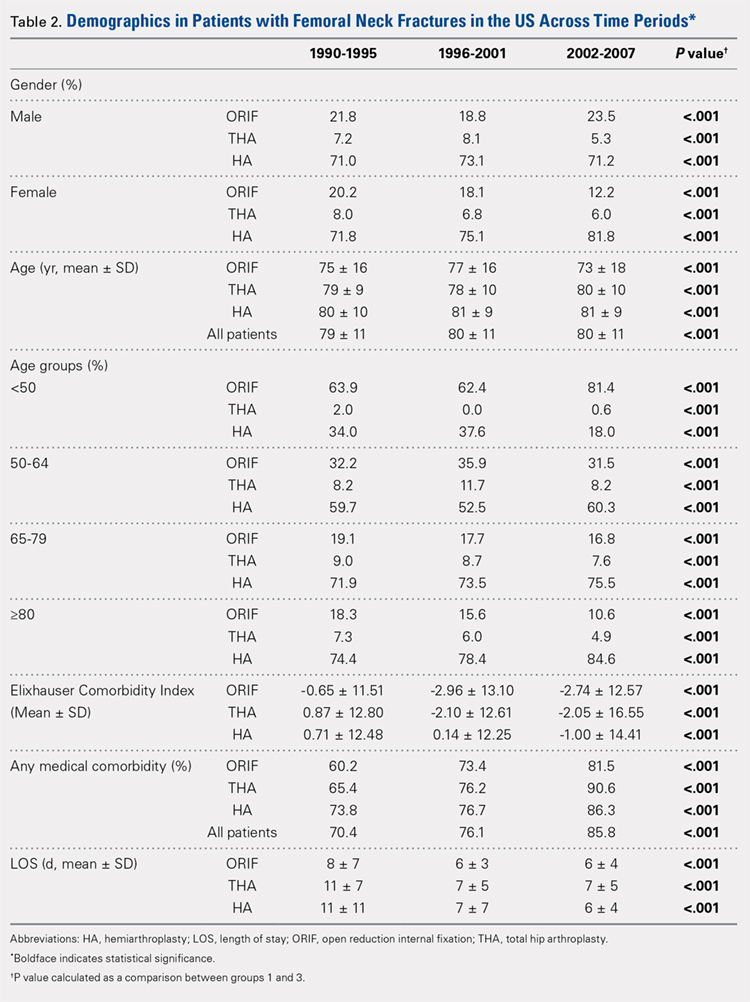
Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
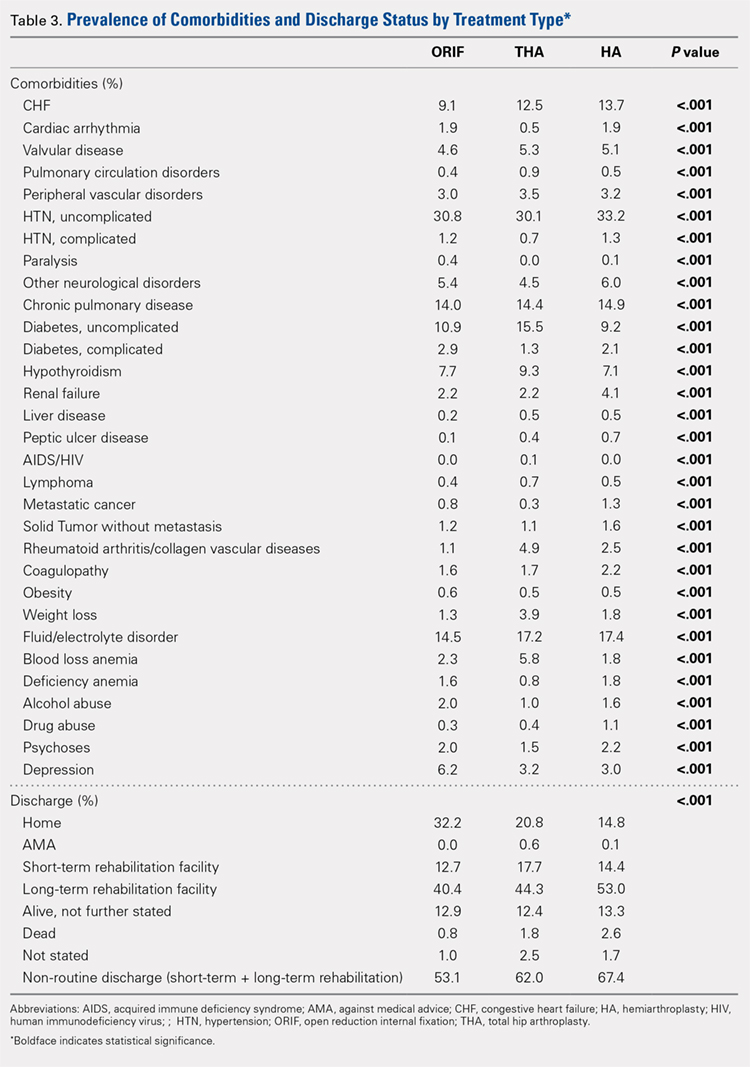
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.
DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.
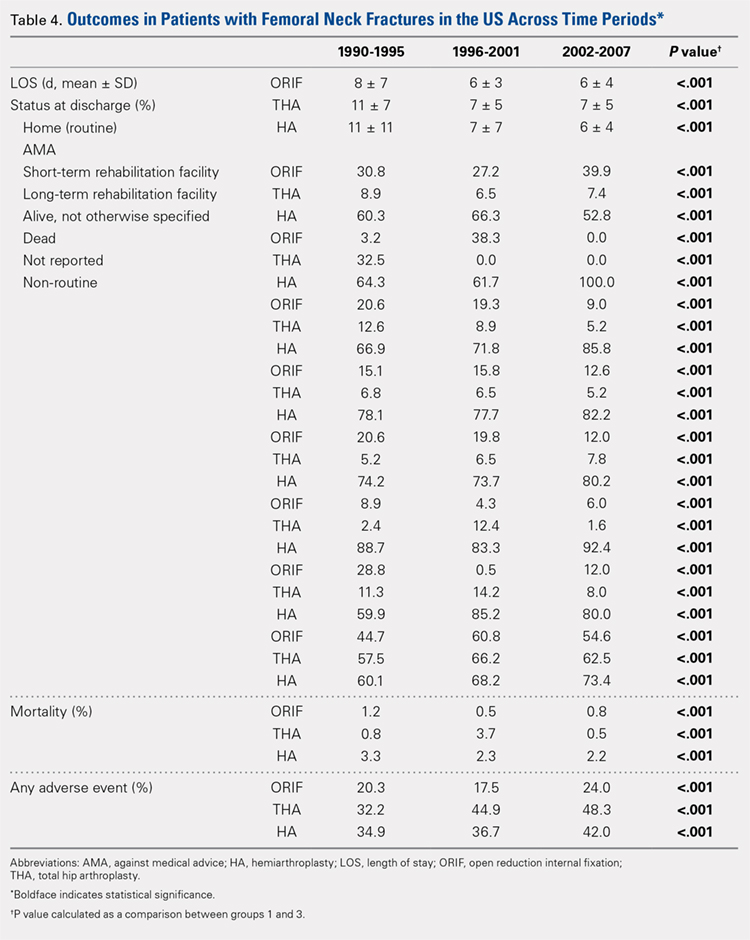
Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).
GENERAL ADVERSE EVENTS
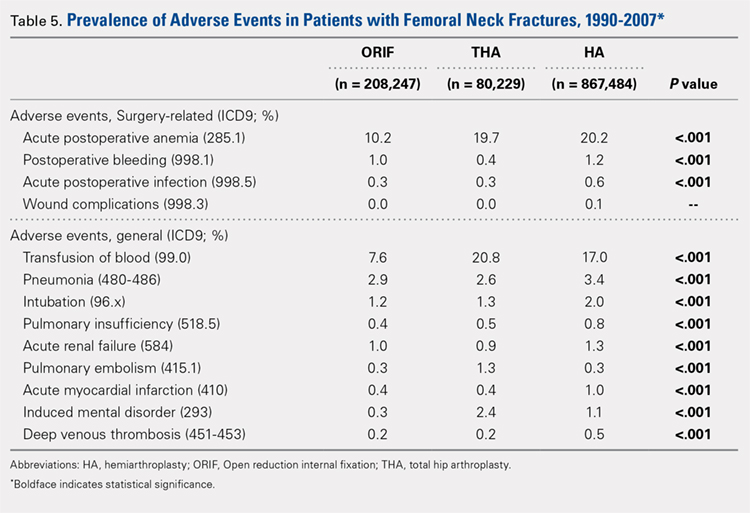
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.
Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).
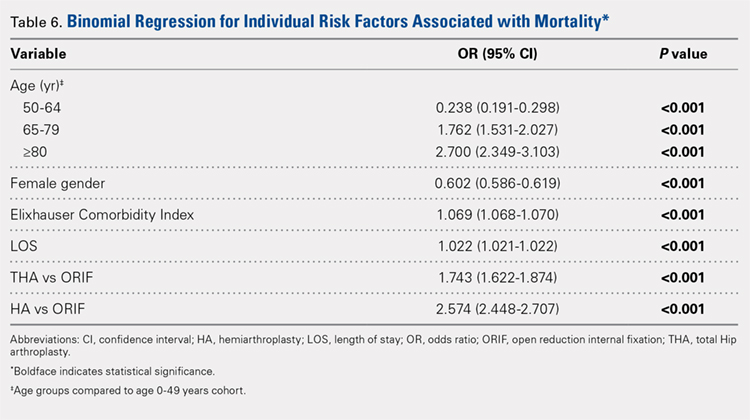
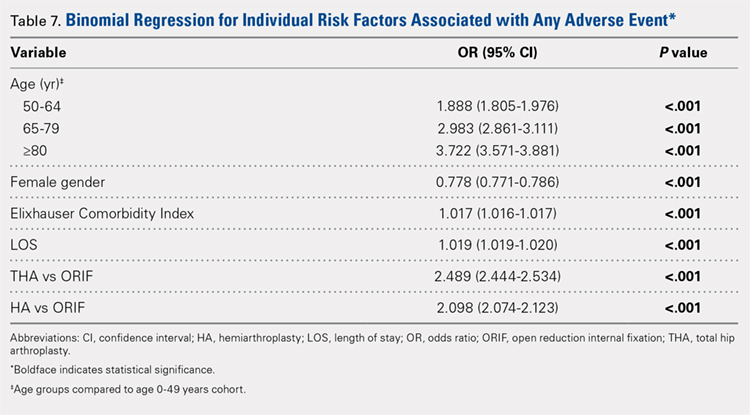
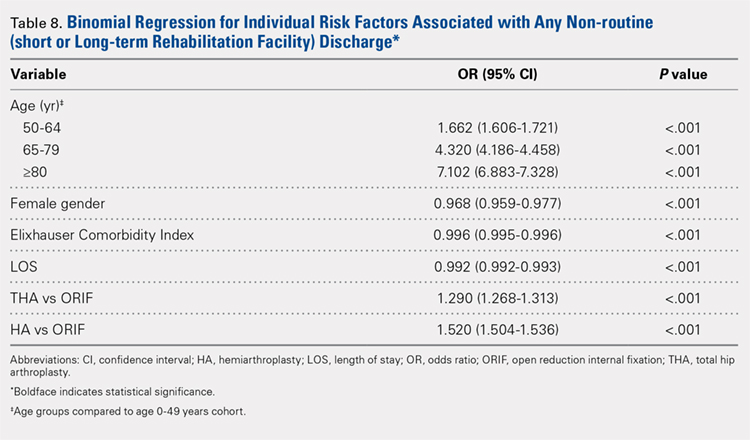
Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).
DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.
Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
TAKE-HOME POINTS
- The femoral neck patient population is older and has more medical comorbidities.
- Hemiarthroplasty (HA) is being performed more commonly in patients > 50 years old for femoral neck fractures.
- Open reduction and internal fixation is being performed more commonly in patients > 80 years old for femoral neck fractures.
- The rate of adverse events following femoral neck fracture is higher in the total hip arthroplasty (THA) group than in the HA group.
- THA is an independent risk factor for adverse events following femoral neck fracture.
Hip and Core Muscle Injuries in Soccer
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
TAKE-HOME POINTS
- Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need surgical intervention.
- Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain.
- Acute groin pain in soccer players is most commonly caused by muscle strain involving the adductor longus, the iliopsoas or the rectus femoris.
- Inguinal-related groin pain is a common cause of chronic groin pain and typically is the most challenging to treat with a complex pathophysiology and a high association with femoroacetabular impingement.
- Hip-related groin pain (femoroacetabular impingement, labral tears, and chondral injuries) usually respond well to surgical intervention and has high rates of return to sport.
Timing of Adverse Events Following Geriatric Hip Fracture Surgery: A Study of 19,873 Patients in the American College of Surgeons National Surgical Quality Improvement Program
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
TAKE-HOME POINTS
- The median postoperative day of diagnosis for myocardial infarction was 3, 3 for cardiac arrest requiring cardiopulmonary resuscitation, 3 for stroke, 4 for pneumonia, 4 for pulmonary embolism, 7 for urinary tract infection, 9 for deep vein thrombosis, 9 for sepsis, 11 for mortality, and 16 for surgical site infection.
- For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30; however, for the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
- The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
- These results facilitate targeted clinical surveillance, guide patient counseling, and inform the duration of follow-up required in research studies.
- Clinicians should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Analysis of Incidence and Outcome Predictors for Patients Admitted to US Hospitals with Acetabular Fractures from 1990 to 2010
ABSTRACT
The incidence of acetabular fractures and associated in-hospital complication rates in the United States are poorly defined. Studies evaluating predictors of outcome for isolated acetabular fractures are weakly generalizable due to small sample sizes or the inclusion of all types of pelvic fractures. This study sought to analyze trends in acetabular fractures and associated complications in the US using the largest and most recent national dataset available.
The National Hospital Discharge Survey was queried to identify all patients admitted to US hospitals with acetabular fractures between 1990 and 2010. A representative cohort of 497,389 patients was identified, and multivariable logistic regression was used to identify independent predictors of mortality, adverse events, requirement of blood transfusion, and operative treatment with open reduction and internal fixation (ORIF).
Between 1990 and 2010, the population-adjusted incidence of acetabular fractures increased from 7.8 to 9.5/100,000 capita (P < .001). Mortality declined from 5.9% to 0.4% (P < .001), paralleling an increase in the proportion of patients treated with ORIF (12.6%-20.4%, P < .001), which was the variable associated with the lowest odds of mortality. Surgical intervention was associated with higher odds of adverse events and a requirement for blood transfusion. The average in-hospital length of stay decreased from 17.0 days to 10.3 days (P < .001).
This study provides the largest and most comprehensive epidemiologic analysis of acetabular fractures in the US. Knowledge of the increasing incidence of acetabular fractures and prognostic factors associated with poor outcomes may improve outcomes.
Continue to: Acetabular fractures are major injuries...
Acetabular fractures are major injuries frequently associated with life-altering sequelae1 and a significant resulting cost to society.2 Acetabular fractures are most often the result of a high-energy trauma3-5 or fall from a height.5,6 Functional outcomes and the prevention of post-traumatic arthritis have been shown to depend upon the accuracy of operative reduction.7-9 However, literature on the epidemiology of acetabular fractures is largely limited to European countries,1,10 and their incidence in the United States is more poorly defined.11 Published mortality rates in the existing literature vary widely from 2% to 45%,12-14 and few studies have identified the risk factors associated with in-hospital complications.15 While age, gender, and high-velocity mechanisms have been linked to increased mortality and complications,14-16 the evidence for these associations is poorly generalizable due to the inclusion of all pelvic fractures in these studies. Some reports suggest that advances in surgical management have improved survival and functional outcome,15,17 but these are based upon small cohorts. Knowledge of the incidence and patterns of disease burden are crucial for the allocation of limited healthcare resources.
This study sought to describe the trends in incidence as well as the factors influencing mortality and the risk of complications for patients admitted to US hospitals with an acetabular fracture using the National Hospital Discharge Survey (NHDS), the most recently available Centers for Disease Control and Prevention data, which is also one of the largest inpatient databases in the US. Knowledge of the factors influencing outcomes for patients admitted with acetabular fractures may improve management and decrease complications.
METHODS
NATIONAL HOSPITAL DISCHARGE SURVEY
The NHDS, developed by the National Center for Healthcare Statistics division of the Centers for Disease Control and Prevention,18 was used to estimate the incidence of acetabular fractures and to evaluate the risk factors for ensuing mortality and inpatient complications. The NHDS is a publically available survey providing demographic and medical data for inpatients discharged from non-federal, short-stay hospitals in the US.19 The NHDS is the principal database used by the US government for monitoring hospital use and is considered the most comprehensive of all inpatient surgical databases in use today.19 The survey uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes20 to classify medical diagnoses and procedures. The NHDS uses a stratified, multistage probability design to collect demographic information (age, gender, race), expected source of payment (insurance status), medical information of up to 7 discharge diagnoses and up to 4 procedures, length of care, hospital size, US region, and inpatient outcomes including discharge destination.21 To ensure unbiased national sampling of inpatient records, the NHDS uses a complex, 3-stage probability design including inflation by reciprocals of the probabilities of sample selection, adjustment for no response, and population weighting ratio adjustments.19 This study did not require approval by the Institutional Review Board because the NHDS is a publically available database with no patient-identifying information.
Continue to: PATIENT SELECTION...
PATIENT SELECTION
All patients admitted to hospitals in the US with a fracture of the acetabulum between 1990 and 2010 were identified using ICD-9-CM codes. Discharges with a diagnosis code (ICD-9-CM) of closed fracture of the acetabulum (808.0) or open fracture of the acetabulum (808.1) were identified using previously described techniques.22 The database was subsequently queried to identify patients treated using open reduction and internal fixation (ORIF) (ICD-9-CM, 79.30/79.39), closed reduction and internal fixation (CRIF) (ICD-9-CM, 79.10/79.19), or external (ICD-9-CM, 78.10/78.19) or internal (ICD-9-CM, 78.50/78.59) fixation without reduction. Demographic variables were then collected, including age, sex, primary diagnosis, associated diagnoses, type of fracture (open vs closed), prevalence of comorbidities, length of stay, and discharge destination. The complication screening package23 was used to determine the incidence of complications. The variable adverse event was created on the basis of the variables postoperative bleeding (998.1), acute postoperative infection (998.5), acute postoperative anemia (285.1), acute renal failure (584), acute myocardial infarction (410), pulmonary embolism (415.1), induced mental disorder (293), pneumonia (480-486), pulmonary insufficiency (518.5), deep venous thrombosis (453.4), intubation (96.xx), and blood transfusion (99.x).
STATISTICAL ANALYSIS
Because of the large sample size, a normal distribution of the data was assumed. Differences between categorical variables were compared using the Pearson chi square test, while the independent-samples t test was used to compare differences between continuous variables. To determine independent predictors of in-hospital outcomes (death, adverse events, requirement for blood transfusion, or treatment with ORIF), all variables present in at least 2% of the population24 were included in a multivariable binary logistic regression model. For in-hospital adverse events, a 1% cutoff was used due to their lower rates of occurrence, as previously described.25The dichotomous variables were death, presence of adverse events, receipt of blood transfusion, and treatment with ORIF. A multivariable regression model allows for the control of potential confounders, isolating the effect of individual variables on inpatient outcomes. Covariates accounted for in the regression model included gender, age, region of the country, and preexisting comorbidities (diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation). To assess the association between individual variables and inpatient outcomes, odds ratios and confidence intervals were calculated. A P value of <.001 was used to define statistical significance, correcting for multiple comparisons, as previously described.25 US census data were used to obtain national population estimates for each year of the study from 1990 to 2010.26 Rates were presented as the number of acetabular fractures per 100,000 standard population. All data were analyzed using the software Statistical Package for the Social Sciences [SPSS] version 20.
RESULTS
INCIDENCE AND DEMOGRAPHICS
A cohort representative of 497,389 patients with a diagnosis of acetabular fracture was identified between 1990 and 2010 (Table 1). In 1990, 19,560 cases (7.84 per 100,000 capita) of acetabular fractures were recoded, while in 2010, the number of cases increased to 29,373 or 9.5 per 100,000 capita (P < .001) (Table 2). The mean age of patients with an acetabular fracture was 52.6 years (standard deviation [SD], 23.7) and 60.6% were male (Table 1). The most frequently associated diagnosis was closed fracture of the pelvis (29.8%) followed by fracture of the femur (13.1%) and closed fracture of the ilium (3.8%) (Table 1). Of the total cohort, 23.2% underwent ORIF (Table 1). In 1990, 12.6% of patients with a diagnosis of acetabular fracture underwent ORIF, whereas 20.4% of patients underwent ORIF in 2010 (P < .001) (Table 2). Average length of hospital stay was 8.3 days (SD, 17.9) overall (Table 1). In 1990 the average length of stay was 17.0 days (SD, 14.9), decreasing to 10.3 days (SD, 9.3) in 2010 (P < .001) (Table 2).
Table 1. Patient Characteristics for Patients with Acetabular Fractures in the United States from 1990 to 2007
Parameter | Total 1990-2010 |
Total Number | 497,389 |
Gender (%) |
|
Male | 60.6 |
Female | 39.4 |
Age, years (%) |
|
<20 | 6.7 |
20-40 | 31.5 |
41-60 | 22.3 |
61-85 | 30.4 |
>85 | 23.5 |
Race (%) |
|
White | 66.4 |
Black | 9.3 |
Asian | 1.7 |
Other | 2.4 |
Not stated | 20.2 |
Primary Diagnosis (%) |
|
Closed fracture of acetabulum (808.0) | 98.9 |
Open fracture of acetabulum (808.1) | 1.1 |
Associated diagnoses (%) |
|
Closed fracture of pubis (808.2) | 26.1 |
Open fracture of pubis (808.3) | 0.1 |
Closed fracture of ischium (808.42) | 1.7 |
Open fracture of ischium (808.52) | 0.0 |
Closed fracture of ilium (808.41) | 3.8 |
Open fracture of ilium (808.51) | 0.0 |
Closed fracture other part pelvis (808.49) | 0.7 |
Open fracture other part pelvis (808.59) | 0.0 |
Multiple closed pelvic fractures (808.43) | 0.5 |
Multiple open pelvic fractures (808.53) | 0.0 |
Any pelvic fracture from above | 29.8 |
Fracture of neck of femur (820) | 7.2 |
Fracture of any part of femur (820/821) | 13.1 |
Head trauma (959.01) | 0.7 |
Head/face trauma (959.0/959.01) | 0.7 |
Chest trauma (959.11) | 0.1 |
Chest/trunk trauma (959.1/959.11) | 0.1 |
Procedures (%) |
|
Open reduction internal fixation (79.30/79.39) | 23.2 |
Closed reduction internal fixation (79.10/79.19) | 1.3 |
External fixation (78.10/78.19) | 0.7 |
Internal fixation without reduction (78.50/78.59) | 0.4 |
Comorbidities (%) |
|
No | 72.9 |
Yes | 27.1 |
Adverse Events (%) |
|
No | 74.1 |
Yes | 25.9 |
Discharge Disposition (%) |
|
Routine/home (1) | 45.4 |
Left against medical advice (2) | 0.2 |
Short term fac (3) | 13.1 |
Long term fac (4) | 22.2 |
Alive, not stated (5) | 12 |
Dead (6) | 3.5 |
Not reported (9) | 3.6 |
Mortality (%) | 3.5 |
Age (y), mean (SD) | 52.6 (23.7) |
Days of Care, mean (SD) | 8.3 (17.9) |
Principal Source of Payment (%) |
|
Private insurance | 39 |
Medicare | 30.5 |
Medicaid | 7.7 |
Other government | 1.9 |
Self-pay | 7.9 |
Workmen’s comp | 4 |
Other | 4.7 |
Not stated | 4.4 |
Abbreviation: SD, standard deviation.
Table 2. Patient Characteristics in 1990, 1995, 1999, 2003, and 2007 Among Patients with Acetabular Fractures
Variable | 1990 | 1995 | 1999 | 2003 | 2007 | 2010 |
Total number | 19,560 | 17,506 | 22,767 | 27,133 | 34,027 | 29,373 |
Incidence per 100,000 capita | 7.84 | 6.57 | 8.16 | 9.35 | 11.30 | 9.5 |
Gender (%) |
| |||||
Male | 51.0 | 70.7 | 61.2 | 62.6 | 62.5 | 64.9 |
Female | 49.0 | 29.3 | 38.8 | 37.4 | 37.5 | 35.1 |
Fracture (%) |
| |||||
Open | 2.1 | 1.7 | 3.3 | 1.4 | 0.1 | 1.8 |
Closed | 97.9 | 98.3 | 96.7 | 98.6 | 99.9 | 98.2 |
Underwent ORIF (%) | 12.6 | 20.9 | 20.2 | 22.9 | 27.8 | 20.4 |
Adverse events (%) | 10.9 | 16.2 | 23.7 | 31 | 35.1 | 37.6 |
Transfusion (%) | 0.3 | 2.2 | 7.4 | 6.5 | 10.5 | 9.5 |
Discharge (%) |
| |||||
Routine | 58 | 65.6 | 35.6 | 45.9 | 40.2 | 41.6 |
Non-routine to inpatient facility | 26.8 | 23.1 | 46.4 | 33.8 | 40.8 | 34.6 |
Mortality (%) | 5.9 | 3.6 | 2 | 2.9 | 1.5 | 0.4 |
Mean Age (y) | 52.9 | 48.4 | 52.3 | 56.3 | 57 | 53.2 |
Mean DOC (days) | 17.0 | 13.4 | 8.7 | 10.8 | 8.5 | 10.3 |
Abbreviations: DOC, days of care; ORIF, open reduction internal fixation.
Continue to: MORTALITY...
MORTALITY
In-hospital mortality decreased from 5.9% in 1990 to 0.4% in 2010 (P < .001) (3.5% for the total cohort) (Tables 1 and 2). Multivariable logistic regression analysis demonstrated pulmonary insufficiency (odds ratio [OR], 9.07; 95% confidence interval [CI], 8.52-9.66; P < .01), pneumonia (OR, 3.22; 95% CI, 3.05-3.39; P < .01), and age >85 years (OR, 2.28; 95% CI, 2.16-2.40; P < .01) to be associated with the highest odds of inpatient mortality. CRIF (OR, 1.99; 95% CI, 1.78-2.23; P < .01), external fixator (OR, 1.82; 95% CI, 1.45-2.29; P < .01), and having received a blood transfusion (OR, 1.81; 95% CI, 1.71-1.91; P < .01) were also associated with increased odds of mortality. Treatment with ORIF (OR, 0.19; 95% CI, 0.17-0.20; P < .01) was independently associated with decreased odds of inpatient mortality, as was age <20 years (OR, 0.26; 95% CI, 0.23-0.30; P < .01) (model fit: for omnibus test of model coefficients, X = 25,966 P < .01; Nagelkerke, R2 = 0.20) (Table 3).
Table 3. Logistic Regression for Predictors of Mortality Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Pulmonary insufficiency | 9.07 (8.52–9.66) | < 0.01 |
Pneumonia | 3.22 (3.05–3.39) | < 0.01 |
Age >85 years | 2.28 (2.16–2.40) | < 0.01 |
Closed reduction internal fixation | 1.99 (1.78–2.23) | < 0.01 |
External Fixator | 1.82 (1.45–2.29) | < 0.01 |
Blood transfusion | 1.81 (1.71–1.91) | < 0.01 |
Gender (male) | 1.76 (1.70–1.83) | < 0.01 |
Associated femoral neck fracture | 1.23 (1.15–1.30) | < 0.01 |
Age 41-60 years | 1.19 (1.11–1.29) | < 0.01 |
Age 61-85 years | 1.17 (1.11–1.23) | < 0.01 |
Congestive heart failure | 1.14 (1.07–1.22) | < 0.01 |
Associated pelvic fracture | 1.13 (1.10–1.17) | < 0.01 |
Geographic region | 1.11 (1.09–1.12) | < 0.01 |
Source of payment | 1.02 (1.01–1.02) | < 0.01 |
Race | 0.99 (0.98–0.99) | < 0.01 |
DOC | 0.98 (0.98–0.98) | < 0.01 |
Hypertension | 0.67 (0.64–0.71) | < 0.01 |
Atrial fibrillation | 0.52 (0.48–0.57) | < 0.01 |
Diabetes mellitus | 0.35 (0.32–0.38) | < 0.01 |
Age 20-40 years | 0.32 (0.30–0.35) | < 0.01 |
Age <20 years | 0.26 (0.23–0.30) | < 0.01 |
Coronary artery disease | 0.21 (0.18–0.24) | < 0.01 |
Open reduction internal fixation | 0.19 (0.17–0.20) | < 0.01 |
Omnibus X 25,966, P < .01 | ||
Nagelkerke R2= 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
COMORBIDITIES AND ADVERSE EVENTS
The prevalence of comorbidities and adverse events is listed in Tables 4 and 5, respectively. Hypertensive disease was the most common comorbidity at 15.3%, followed by diabetes mellitus at 6.9%. Overall, 25.9% of patients experienced an in-hospital adverse event, with the most common being postoperative anemia (7.3%) and blood transfusion (8.1%) (Tables 1 and 5). The percentage of patients experiencing an adverse event increased from 10.9% in 1990 to 37.6% in 2010 (P < .01) (Table 2). Multivariable logistic regression analysis revealed CRIF (OR, 3.08; 95% CI, 2.91-3.26; P < .01), coronary artery disease (OR, 2.02; 95% CI, 1.91-2.15; P < .01), associated femoral neck fracture (OR, 1.53; 95% CI, 1.47-1.60; P < .01), and ORIF (OR, 1.22; 95% CI, 1.20-1.24; P < .01) to be associated with higher odds of inpatient adverse events (model fit: for omnibus test of model coefficients, X = 160,275, P < .01; Nagelkerke, R2 = 0.41) (Table 6).
Table 4. Prevalence of Comorbidities in Patients with Acetabular Fractures Between 1990 and 2007 (n = 403.927)
Parameter (ICD-9) | Percentage of Total |
Hypertensive disease (401–405) | 15.3% |
Diabetes mellitus (250) | 6.9% |
Atrial fibrillation (427.31) | 4.0% |
Congestive heart failure (428) | 3.9% |
Osteoporosis (733.0) | 2.1% |
Coronary artery disease (414.01) | 2.0% |
Obesity (278.00, 278.01) | 2.0% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 5. Prevalence of In-Hospital Adverse Events Among Patients with Acetabular Fractures Between 1990 and 2007 (n = 403,927)
Parameter (ICD-9) | Percentage of Total |
Transfusion of blood (99.0) | 8.1% |
Acute postoperative anemia (285.1) | 7.3% |
Intubation (96.x) | 4.9% |
Acute renal failure (584) | 3.4% |
Pneumonia (480-486) | 3.2% |
Pulmonary insufficiency (518.5) | 2.3% |
Pulmonary embolism (415.1) | 1.6% |
Deep venous thrombosis (453.4) | 1.0% |
Acute myocardial infarction (410) | 0.9% |
Postoperative bleeding (998.1) | 0.7% |
Acute postoperative infection (998.5) | 0.5% |
Induced mental disorder (293) | 0.4% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 6. Logistic Regression for Predictors of Adverse Events Among Patients Hospitalized for Acetabular Fracture (n = 403,927)
Variable | OR (95% CI) | P |
Closed reduction internal fixation | 3.08 (2.91-3.26) | < 0.01 |
Coronary artery disease | 2.02 (1.91-2.15) | < 0.01 |
Associated femoral neck fracture | 1.53 (1.47-1.60) | < 0.01 |
Open reduction internal fixation | 1.22 (1.20-1.24) | < 0.01 |
Gender (male) | 1.16 (1.14-1.18) | < 0.01 |
Associated fracture of any part of femur | 1.13 (1.10-1.17) | < 0.01 |
Age >85 years | 1.08 (1.05-1.12) | < 0.01 |
Geographic region | 1.07 (1.06-1.07) | < 0.01 |
DOC | 1.04 (1.04-1.04) | < 0.01 |
Race | 1.02 (1.02-1.03) | < 0.01 |
Source of payment | 1.01 (1.01-1.01) | < 0.01 |
Congestive heart failure | 1.01 (0.96-1.06) | 0.78 |
Atrial fibrillation | 0.88 (0.84-0.92) | < 0.01 |
Age 61-85 years | 0.68 (0.66-0.71) | < 0.01 |
Age <20 years | 0.67 (0.64-0.70) | < 0.01 |
Associated pelvis fracture | 0.64 (0.63-0.66) | < 0.01 |
Age 41-60 years | 0.58 (0.56-0.61) | < 0.01 |
Diabetes mellitus | 0.48 (0.46-0.50) | < 0.01 |
Age 20-40 years | 0.45 (0.43-0.47) | < 0.01 |
Hypertension | 0.44 (0.43-0.45) | < 0.01 |
External Fixator | 0.39 (0.35-0.44) | < 0.01 |
Omnibus X 160,275, P < .01 | ||
Nagelkerke R2 = 0.41 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
BLOOD TRANSFUSION
Overall, 7.3% of patients experienced acute postoperative anemia (Table 5). Between 1990 and 2010, the percentage of patients receiving blood transfusions increased from 0.3% to 9.5%, respectively (P < .01) (Table 2). In multivariable logistic regression analysis, patients treated with ORIF (OR, 8.13; 95% CI, 7.91-8.36; P < .01), those with congestive heart failure (OR, 4.23; 95% CI, 4.06-4.41; P < .01), those with an associated femur fracture (OR, 3.13; 95% CI, 2.99-3.27; P < .01), those with atrial fibrillation (OR, 1.96; 95% CI, 1.88-2.05; P < .01), and those treated with CRIF (OR, 1.42; 95% CI, 1.29-1.56; P < .01) were associated with significantly higher odds of blood transfusion (model fit: omnibus test of model coefficients, X = 42,653, P < .01; Nagelkerke, R2 = 0.19) (Table 7).
Table 7. Logistic Regression for Predictors of the Requirement for Blood Transfusion Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Open reduction internal fixation | 8.13 (7.91-8.36) | < 0.01 |
Congestive heart failure | 4.23 (4.06-4.41) | < 0.01 |
Associated fracture of any part of femur | 3.13 (2.99-3.27) | < 0.01 |
Atrial fibrillation | 1.96 (1.88-2.05) | < 0.01 |
Closed reduction internal fixation | 1.42 (1.29-1.56) | < 0.01 |
Geographic region | 1.38 (1.36-1.39) | < 0.01 |
Hypertension | 1.38 (1.34-1.42) | < 0.01 |
Associated pelvic fracture | 1.28 (1.25-1.31) | < 0.01 |
Age 61-85 years | 1.06 (1.02-1.11) | 0.01 |
Source of payment | 0.99 (0.98-0.99) | < 0.01 |
Race | 0.98 (0.97-0.98) | < 0.01 |
DOC | 0.96 (0.96-0.96) | < 0.01 |
Age >85 years | 0.74 (0.72-0.77) | < 0.01 |
External fixator | 0.69 (0.59-0.80) | < 0.01 |
Coronary artery disease | 0.62 (0.57-0.68) | < 0.01 |
Age 41-60 years | 0.57 (0.54-0.60) | < 0.01 |
Gender (male) | 0.54 (0.52-0.55) | < 0.01 |
Diabetes mellitus | 0.38 (0.36-0.41) | < 0.01 |
Age 20-40 years | 0.32 (0.30-0.34) | < 0.01 |
Associated femoral neck fracture | 0.29 (0.27-0.31) | < 0.01 |
Age <20 years | 0.24 (0.22-0.26) | < 0.01 |
Omnibus X = 42,653, P < .01 | ||
Nagelkerke R2 = 0.19 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
TREATMENT WITH ORIF
Over the 20-year study period, 23.2% of patients with acetabular fractures were treated with ORIF (Table 1). In 1990, 12.6% of patients underwent ORIF, while in 2010 this percentage increased to 20.4% (P < .001) (Table 2). Multivariable logistic regression analysis demonstrated that age between 41 and 60 years (OR, 1.88; 95% CI, 1.78-1.98; P < .01) was associated with the highest odds of undergoing ORIF. Age 20 to 40 years (OR, 1.86; 95% CI, 1.76-1.97; P < .01), age <20 years (OR, 1.82; 95% CI, 1.72-1.93; P < .01), and male gender (OR, 1.65; 95% CI, 1.63-1.68; P < .01) were also associated with being treated by ORIF. In contrast, coronary artery disease (OR, 0.27; 95% CI, 0.25-0.30; P < .01), age >85 years (OR, 0.46; 95% CI, 0.44-0.47; P < .01), and congestive heart failure (OR, 0.48; 95% CI, 0.46-0.51; P < .01) were associated with the lowest odds of undergoing ORIF (model fit: omnibus test of model coefficients, X = 71,118, P < .01; Nagelkerke, R2 = 0.20) (Table 8).
Table 8. Logistic Regression for Predictors of the Requirement for Discharge to Another Inpatient Facility Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Age 41-60 years | 1.88 (1.78-1.98) | < 0.01 |
Age 20-40 years | 1.86 (1.76-1.97) | < 0.01 |
Age <20 years | 1.82 (1.72-1.93) | < 0.01 |
Gender (male) | 1.65 (1.63-1.68) | < 0.01 |
Larger hospital bed size | 1.46 (1.45-1.47) | < 0.01 |
Hypertension | 1.35 (1.32-1.38) | < 0.01 |
Diabetes mellitus | 1.09 (1.05-1.13) | < 0.01 |
DOC | 1.02 (1.02-1.02) | < 0.01 |
Source of payment | 1.01 (1.01-1.02) | < 0.01 |
Race | 1.00 (0.99-1.00) | 0.17 |
Age 61-85 years | 0.94 (0.90-0.99) | 0.02 |
Region | 0.92 (0.91-0.93) | < 0.01 |
Atrial fibrillation | 0.83 (0.79-0.87) | < 0.01 |
Congestive heart failure | 0.48 (0.46-0.51) | < 0.01 |
Age >85 years | 0.46 (0.44-0.47) | < 0.01 |
Coronary artery disease | 0.27 (0.25-0.30) | < 0.01 |
Omnibus X 71,118, P < .01 | ||
Nagelkerke R2 = 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
Continue to: DISCUSSION...
DISCUSSION
This study evaluates the incidence of acetabular fractures in the US between 1990 and 2010, and identifies prognostic factors associated with complications and death. The study demonstrates an increase in the population-adjusted incidence of acetabular fractures between 1990 and 2010 (7.84 cases per 100,000 capita to 9.5 cases per 100,000 capita), in contrast to the decreasing trend reported by Mauffrey and colleagues.11 Some studies suggest that up to 80% of acetabular fractures are associated with motor vehicle collisions and motorcycle accidents.9,27 While the rate of motor vehicle accidents has remained stable over the study period, motorcycle ownership and deaths more than doubled between 2001 and 2008,28 primarily among individuals over 40 years of age. In this study, the mean age of patients with acetabular fractures ranged from 48 to 57 years. The dramatic increase in motorcycle ownership and deaths in these age groups may partially explain the rising incidence of acetabular fractures. The other possibility is that changes in automobile design and safety equipment may have altered the injury patterns observed in patients surviving motor vehicle crashes. Compared to the United Kingdom, in which studies report a fixed incidence of 3 per 100,000 capita1 between 1988 and 2003, the incidence of acetabular fractures in the US is greater. In contrast, the incidence of acetabular fractures reported in this study is less than the 20 per 100,000 reported in Sweden between 1976 and 1985,29 or the 37 per 100,000 reported in Rochester, Minnesota between 1968 and 1977,30 which may be due to increased seatbelt usage.31
In addition to the national incidence, this study demonstrated that the proportion of patients with acetabular fractures treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010. This is substantially lower than the 77% reported by Ochs and colleagues32 in a German population. Concurrent with the increase in ORIF, there was a decrease in in-hospital mortality from 5.9% in 1990 to 0.4% in 2010. The initial mortality rates in this study are comparable to much earlier reports and some small studies,9,32-37 but the rates reported in the later years of this study show a substantial decrease that is likely a more accurate estimation of the current incidence. The improved survival rates may be due to advances in the operative treatment of acetabular fractures, in which mechanical stabilization allows for early patient mobilization and facilitation of optimal nursing care.38 With ORIF becoming the standard of care for displaced acetabular fractures,9 numerous reports have demonstrated an association between early definitive fixation and improved survival.17,39,40 This is similar to our study, which found ORIF to be associated with the lowest odds of mortality in multivariate logistic regression analysis. It is possible that advances in patient care by intensivists over this period have also contributed to the decrease in mortality, but the correlation with operative treatment in this study is very strong and agrees well with prior studies.16 Moreover, multiple studies have demonstrated decreased in-hospital mortality among patients undergoing various orthopedic surgical procedures during this period.41-43 The correlation with operative treatment in this study agrees well with prior studies.16
In contrast, higher odds of mortality were seen in patients over the age of 85 years with pulmonary insufficiency, congestive heart failure, pneumonia, or an associated femur or pelvic fracture. This is similar to prior reports in which patients with combined acetabulum and pelvic ring injuries fared worse than those with isolated injures,44,45 as did patients with associated non-musculoskeletal injuries.46 The finding that age over 85 years was associated with higher odds of mortality likely reflects the increased number of comorbidities and decreased physiologic reserve seen in this patient population. Finally, male gender was associated with higher odds of in-hospital mortality. There are 2 possible explanations for this: Either there is gender dimorphism in sex hormones and cytokine activity in response to hemorrhage and sepsis,38,47 or there is a greater tendency for males to be involved in higher energy accidents with more severe concomitant injuries.
The results of multivariable regression analysis demonstrated that patients were more likely to require blood transfusion if they were managed surgically or had atrial fibrillation, congestive heart failure, or associated femur fracture. Not surprisingly, concurrent pelvic fracture was also associated with higher odds of blood transfusion, as pelvic hemorrhage is reported to be the cause of death in up to half of patients who die following a pelvic fracture.46
Between 1990 and 2010, in-hospital days of care decreased from 17.0 days to 10.3 days. While a decreased length of stay has been demonstrated in other orthopedic conditions over the study period,41 it is possible the decrease in length of stay demonstrated in this study is due to improved surgical technique and the implementation of early surgical intervention.39,48-50 Plaisier and colleagues17 demonstrated superior functional outcomes, quicker return to baseline function, and decreased length of stay in patients treated with early ORIF of their acetabular fractures. Other studies have shown that the benefits of early surgery include improved reduction quality and ease of reduction,51 as well as control of bleeding, pain relief, and mobilization of the patient.39 Another possible explanation for the decreased length of stay is the increased rate of discharge to other inpatient facilities, such as rehabilitation facilities, which was demonstrated in this study.
Continue to: Interestingly, male gender and younger age...
Interestingly, male gender and younger age were associated with operative management of the acetabular fracture. In contrast, there was a decreased likelihood of operative treatment among elderly patients and those patients with cardiac comorbidities. It is possible that the relationship we found between the likelihood of ORIF and age relates to the bimodal distribution of fractures, with higher energy and potentially more displaced fractures occurring in younger patients3-5 and lower energy fractures in the elderly.
In contrast to decreasing in-hospital days of care, there was a rise in the number of adverse events between 1990 (10.9%) and 2010 (37.6%). This can be partially attributed to the increased rates of blood transfusion, which was received by 9.5% of patients with acetabular fractures in the final study year. Additionally, surgical intervention was associated with increased adverse events in this study, and surgical intervention increased over the study period. Other factors that may have contributed to an increase in adverse events include an aging population,52 as advanced age was independently associated with higher odds of adverse events in this study.
Despite the strengths of using large, national databases for epidemiological research,53 this study has several limitations. Like all large databases, the NHDS is subject to error in coding and data entry.54 Additionally, the database only allows for 7 diagnostic codes and 4 procedure codes per entry. As a result, the prevalence of comorbid conditions and adverse events may be underreported.25 Moreover, the severity of a comorbid disease cannot be appreciated when dichotomously classified.55 Another limitation is that the database only provides inpatient data, so complications that arise after discharge, as well as follow-up data, are unknown. Furthermore, the results of this study are limited to practice patterns in the US from 1990 to 2010. This database does not provide injury mechanisms, so we cannot distinguish between high-energy and low-energy injuries. Lastly, analysis of the different types of acetabular fractures was not performed since classification of acetabular fractures cannot be assessed with ICD-9 codes.
CONCLUSION
This study is the largest epidemiologic analysis of acetabular fractures in the US and also provides predictors of in-hospital mortality. The incidence of acetabular fractures in the US is increasing, while mortality is decreasing. Identifying risk factors associated with poor outcomes has the potential to change treatment strategies, resource allocation, in-hospital monitoring, and discharge planning for this patient population.
This paper will be judged for the Resident Writer’s Award.
1. Laird A, Keating JF. Acetabular fractures: a 16-year prospective epidemiological study. J Bone Joint Surg Br. 2005;87(7):969-973. doi:10.1302/0301-620X.87B7.16017.
2. Geoghegan JM, Longdon EJ, Hassan K, Calthorpe D. Acetabular fractures in the UK. What are the numbers? Injury. 2007;38(3):329-333. doi:10.1016/j.injury.2006.09.015.
3. Tavakoli Darestani R, Kazemian G, Emami Moghaddam M, Manafi Rasi A, Alipour Y, Bagherian Lemraski MM. An unusual combination of acetabular and pelvic fracture: is this a new subtype of acetabular fracture? Trauma Mon. 2013;18(1):37-40. doi:10.5812/traumamon.9613.
4. McDonnell M, Schachter AK, Phillips DP, Liporace FA. Acetabular fracture through the triradiate cartilage after low-energy trauma. J Orthop Trauma. 2007;21(7):495-498. doi:10.1097/BOT.0b013e31812f67ff.
5. Giannoudis PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875-883. doi:10.1097/01.ta.0000242259.67486.15.
6. Gänsslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27 Suppl 1:S-A13-A20. doi:10.1016/S0020-1383(96)90106-0.
7. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645. doi:10.2106/00004623-199611000-00002.
8. Wright R, Barrett K, Christie MJ, Johnson KD. Acetabular fractures: long-term follow-up of open reduction and internal fixation. J Orthop Trauma. 1994;8(5):397-403. doi:10.1097/00005131-199410000-00005.
9. Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2-9.
10. Davarinos N, Ellanti P, Morris S, Mc Elwain JP. Epidemiology of pelvic and acetabular trauma in a Dublin tertiary hospital: a 10-year experience. Ir J Med Sci. 2012;181(2):243-246. doi:10.1007/s11845-011-0791-4.
11. Mauffrey C, Hao J, Cuellar DO 3rd, et al. The epidemiology and injury patterns of acetabular fractures: are the USA and China comparable? Clin Orthop Relat Res. 2014;472(11):3332-3337. doi:10.1007/s11999-014-3462-8.
12. Dente CJ, Feliciano DV, Rozycki GS, et al. The outcome of open pelvic fractures in the modern era. Am J Surg. 2005;190(6):830-835. doi:10.1016/j.amjsurg.2005.05.050.
13. Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1-13. doi:10.1016/j.injury.2004.05.029.
14. Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985-991. doi:10.1016/j.injury.2011.06.003.
15. Arroyo W, Nelson KJ, Belmont PJ Jr, Bader JO, Schoenfeld AJ. Pelvic trauma: what are the predictors of mortality and cardiac, venous thrombo-embolic and infectious complications following injury? Injury. 2013;44(12):1745-1749. doi:10.1016/j.injury.2013.08.007.
16. Flint L, Cryer HG. Pelvic fracture: the last 50 years. J Trauma. 2010;69(3):483-488. doi:10.1097/TA.0b013e3181ef9ce1.
17. Plaisier BR, Meldon SW, Super DM, Malangoni MA. Improved outcome after early fixation of acetabular fractures. Injury. 2000;31(2):81-84. doi:10.1016/S0020-1383(99)00233-8.
18. Centers for Disease Control and Prevention: National Hospital. Discharge survey. http://www.cdc.gov/nchs/nhds.htm. Accessed August 22, 2013.
19. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;(39):1-42.
20. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed June 18, 2013.
21. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627. doi:10.1007/s11999-008-0402-5.
22. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157. doi:10.1016/j.psym.2012.08.009.
23. Iezzoni LI, Daley J, Heeren T, et al. Using administrative data to screen hospitals for high complication rates. Inquiry. 1994;31(1):40-55.
24. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478-2486.
25. Bot AG, Menendez ME, Neuhaus V, Ring D. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(4):519-527. doi:10.1016/j.jse.2013.12.006.
26. United States Census Bureau. Population. https://www.census.gov/topics/population.html. Accessed December 4, 2012.
27. Porter SE, Schroeder AC, Dzugan SS, Graves ML, Zhang L, Russell GV. Acetabular fracture patterns and their associated injuries. J Orthop Trauma. 2008;22(3):165-170. doi:10.1097/BOT.0b013e318165918b.
28. Centers for Disease Control and Prevention. Motorcycle Crash-Related Data. https://www.cdc.gov/motorvehiclesafety/mc/index.html Accessed September 23, 2018
29. Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300. doi:10.3109/17453679209154786.
30. Melton LJ 3rd, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;155(155):43-47. doi:10.1097/00003086-198103000-00008.
31. al-Qahtani S, O'Connor G. Acetabular fractures before and after the introduction of seatbelt legislation. Can J Surg. 1996;39(4):317-320.
32. Ochs BG, Marintschev I, Hoyer H, et al. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2010;41(8):839-851. doi:10.1016/j.injury.2010.04.010.
33. Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151(151):81-106. doi:10.1055/s-2007-980136.
34. de Ridder VA, de Lange S, Kingma L, Hogervorst M. Results of 75 consecutive patients with an acetabular fracture. Clin Orthop Relat Res. 1994;305(305):53-57. doi:10.1097/00003086-199408000-00008.
35. Aho AJ, Isberg UK, Katevuo VK. Acetabular posterior wall fracture. 38 Cases followed for 5 years. Acta Orthop Scand. 1986;57(2):101-105. doi:10.3109/17453678609000878.
36. Stöckle U, Hoffmann R, Südkamp NP, Reindl R, Haas NP. Treatment of complex acetabular fractures through a modified extended iliofemoral approach. J Orthop Trauma. 2002;16(4):220-230. doi:10.1097/00005131-200204000-00002.
37. Tibbs BM, Kopar P, Dente CJ. Acetabular and isolated pelvic ring fractures: a comparison of initial assessment and outcome. Am Surg. 2008;74(6):538-541; discussion 541.
38. Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012;470(8):2090-2097. doi:10.1007/s11999-012-2276-9.
39. Vallier HA, Cureton BA, Ekstein C, Oldenburg FP, Wilber JH. Early definitive stabilization of unstable pelvis and acetabulum fractures reduces morbidity. J Trauma. 2010;69(3):677-684. doi:10.1097/TA.0b013e3181e50914.
40. Enninghorst N, Toth L, King KL, McDougall D, Mackenzie S, Balogh ZJ. Acute definitive internal fixation of pelvic ring fractures in polytrauma patients: a feasible option. J Trauma. 2010;68(4):935-941. doi:10.1097/TA.0b013e3181d27b48.
41. Buller LT, Best MJ, Quinnan SM. A nationwide analysis of pelvic ring fractures: incidence and trends in treatment, length of stay, and mortality. Geriatr Orthop Surg Rehabil. 2016;7(1):9-17. doi:10.1177/2151458515616250.
42. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191. doi:10.2106/JBJS.M.01126.
43. Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214.
44. Halvorson JJ, Lamothe J, Martin CR, et al. Combined acetabulum and pelvic ring injuries. J Am Acad Orthop Surg. 2014;22(5):304-314. doi:10.5435/JAAOS-22-05-304.
45. Osgood GM, Manson TT, O'Toole RV, Turen CH. Combined pelvic ring disruption and acetabular fracture: associated injury patterns in 40 patients. J Orthop Trauma. 2013;27(5):243-247. doi:10.1097/BOT.0b013e31826c2751.
46. Poole GV, Ward EF, Muakkassa FF. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg. 1991;213(6):532-538; discussion 538.
47. Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi:10.1097/00000658-200201000-00014.
48. Goldstein A, Phillips T, Sclafani SJ, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26(4):325-333. doi:10.1097/00005373-198604000-00004.
49. Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28-31. doi:10.1097/00005373-199101000-00006.
50. Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35(5):671-675; discussion 676.
51. Madhu R, Kotnis R, Al-Mousawi A, et al. Outcome of surgery for reconstruction of fractures of the acetabulum. The time dependent effect of delay. J Bone Joint Surg Br. 2006;88(9):1197-1203. doi:10.1302/0301-620X.88B9.17588.
52. Centers for Disease Control and Prevention. The State of Aging & Health in America 2013. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Accessed December 5, 2013.
53. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
54. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449. [author reply:450-451]. doi:10.1097/ALN.0b013e3181adf739.
55. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706. doi:10.1007/s11999-013-2876-z.
ABSTRACT
The incidence of acetabular fractures and associated in-hospital complication rates in the United States are poorly defined. Studies evaluating predictors of outcome for isolated acetabular fractures are weakly generalizable due to small sample sizes or the inclusion of all types of pelvic fractures. This study sought to analyze trends in acetabular fractures and associated complications in the US using the largest and most recent national dataset available.
The National Hospital Discharge Survey was queried to identify all patients admitted to US hospitals with acetabular fractures between 1990 and 2010. A representative cohort of 497,389 patients was identified, and multivariable logistic regression was used to identify independent predictors of mortality, adverse events, requirement of blood transfusion, and operative treatment with open reduction and internal fixation (ORIF).
Between 1990 and 2010, the population-adjusted incidence of acetabular fractures increased from 7.8 to 9.5/100,000 capita (P < .001). Mortality declined from 5.9% to 0.4% (P < .001), paralleling an increase in the proportion of patients treated with ORIF (12.6%-20.4%, P < .001), which was the variable associated with the lowest odds of mortality. Surgical intervention was associated with higher odds of adverse events and a requirement for blood transfusion. The average in-hospital length of stay decreased from 17.0 days to 10.3 days (P < .001).
This study provides the largest and most comprehensive epidemiologic analysis of acetabular fractures in the US. Knowledge of the increasing incidence of acetabular fractures and prognostic factors associated with poor outcomes may improve outcomes.
Continue to: Acetabular fractures are major injuries...
Acetabular fractures are major injuries frequently associated with life-altering sequelae1 and a significant resulting cost to society.2 Acetabular fractures are most often the result of a high-energy trauma3-5 or fall from a height.5,6 Functional outcomes and the prevention of post-traumatic arthritis have been shown to depend upon the accuracy of operative reduction.7-9 However, literature on the epidemiology of acetabular fractures is largely limited to European countries,1,10 and their incidence in the United States is more poorly defined.11 Published mortality rates in the existing literature vary widely from 2% to 45%,12-14 and few studies have identified the risk factors associated with in-hospital complications.15 While age, gender, and high-velocity mechanisms have been linked to increased mortality and complications,14-16 the evidence for these associations is poorly generalizable due to the inclusion of all pelvic fractures in these studies. Some reports suggest that advances in surgical management have improved survival and functional outcome,15,17 but these are based upon small cohorts. Knowledge of the incidence and patterns of disease burden are crucial for the allocation of limited healthcare resources.
This study sought to describe the trends in incidence as well as the factors influencing mortality and the risk of complications for patients admitted to US hospitals with an acetabular fracture using the National Hospital Discharge Survey (NHDS), the most recently available Centers for Disease Control and Prevention data, which is also one of the largest inpatient databases in the US. Knowledge of the factors influencing outcomes for patients admitted with acetabular fractures may improve management and decrease complications.
METHODS
NATIONAL HOSPITAL DISCHARGE SURVEY
The NHDS, developed by the National Center for Healthcare Statistics division of the Centers for Disease Control and Prevention,18 was used to estimate the incidence of acetabular fractures and to evaluate the risk factors for ensuing mortality and inpatient complications. The NHDS is a publically available survey providing demographic and medical data for inpatients discharged from non-federal, short-stay hospitals in the US.19 The NHDS is the principal database used by the US government for monitoring hospital use and is considered the most comprehensive of all inpatient surgical databases in use today.19 The survey uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes20 to classify medical diagnoses and procedures. The NHDS uses a stratified, multistage probability design to collect demographic information (age, gender, race), expected source of payment (insurance status), medical information of up to 7 discharge diagnoses and up to 4 procedures, length of care, hospital size, US region, and inpatient outcomes including discharge destination.21 To ensure unbiased national sampling of inpatient records, the NHDS uses a complex, 3-stage probability design including inflation by reciprocals of the probabilities of sample selection, adjustment for no response, and population weighting ratio adjustments.19 This study did not require approval by the Institutional Review Board because the NHDS is a publically available database with no patient-identifying information.
Continue to: PATIENT SELECTION...
PATIENT SELECTION
All patients admitted to hospitals in the US with a fracture of the acetabulum between 1990 and 2010 were identified using ICD-9-CM codes. Discharges with a diagnosis code (ICD-9-CM) of closed fracture of the acetabulum (808.0) or open fracture of the acetabulum (808.1) were identified using previously described techniques.22 The database was subsequently queried to identify patients treated using open reduction and internal fixation (ORIF) (ICD-9-CM, 79.30/79.39), closed reduction and internal fixation (CRIF) (ICD-9-CM, 79.10/79.19), or external (ICD-9-CM, 78.10/78.19) or internal (ICD-9-CM, 78.50/78.59) fixation without reduction. Demographic variables were then collected, including age, sex, primary diagnosis, associated diagnoses, type of fracture (open vs closed), prevalence of comorbidities, length of stay, and discharge destination. The complication screening package23 was used to determine the incidence of complications. The variable adverse event was created on the basis of the variables postoperative bleeding (998.1), acute postoperative infection (998.5), acute postoperative anemia (285.1), acute renal failure (584), acute myocardial infarction (410), pulmonary embolism (415.1), induced mental disorder (293), pneumonia (480-486), pulmonary insufficiency (518.5), deep venous thrombosis (453.4), intubation (96.xx), and blood transfusion (99.x).
STATISTICAL ANALYSIS
Because of the large sample size, a normal distribution of the data was assumed. Differences between categorical variables were compared using the Pearson chi square test, while the independent-samples t test was used to compare differences between continuous variables. To determine independent predictors of in-hospital outcomes (death, adverse events, requirement for blood transfusion, or treatment with ORIF), all variables present in at least 2% of the population24 were included in a multivariable binary logistic regression model. For in-hospital adverse events, a 1% cutoff was used due to their lower rates of occurrence, as previously described.25The dichotomous variables were death, presence of adverse events, receipt of blood transfusion, and treatment with ORIF. A multivariable regression model allows for the control of potential confounders, isolating the effect of individual variables on inpatient outcomes. Covariates accounted for in the regression model included gender, age, region of the country, and preexisting comorbidities (diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation). To assess the association between individual variables and inpatient outcomes, odds ratios and confidence intervals were calculated. A P value of <.001 was used to define statistical significance, correcting for multiple comparisons, as previously described.25 US census data were used to obtain national population estimates for each year of the study from 1990 to 2010.26 Rates were presented as the number of acetabular fractures per 100,000 standard population. All data were analyzed using the software Statistical Package for the Social Sciences [SPSS] version 20.
RESULTS
INCIDENCE AND DEMOGRAPHICS
A cohort representative of 497,389 patients with a diagnosis of acetabular fracture was identified between 1990 and 2010 (Table 1). In 1990, 19,560 cases (7.84 per 100,000 capita) of acetabular fractures were recoded, while in 2010, the number of cases increased to 29,373 or 9.5 per 100,000 capita (P < .001) (Table 2). The mean age of patients with an acetabular fracture was 52.6 years (standard deviation [SD], 23.7) and 60.6% were male (Table 1). The most frequently associated diagnosis was closed fracture of the pelvis (29.8%) followed by fracture of the femur (13.1%) and closed fracture of the ilium (3.8%) (Table 1). Of the total cohort, 23.2% underwent ORIF (Table 1). In 1990, 12.6% of patients with a diagnosis of acetabular fracture underwent ORIF, whereas 20.4% of patients underwent ORIF in 2010 (P < .001) (Table 2). Average length of hospital stay was 8.3 days (SD, 17.9) overall (Table 1). In 1990 the average length of stay was 17.0 days (SD, 14.9), decreasing to 10.3 days (SD, 9.3) in 2010 (P < .001) (Table 2).
Table 1. Patient Characteristics for Patients with Acetabular Fractures in the United States from 1990 to 2007
Parameter | Total 1990-2010 |
Total Number | 497,389 |
Gender (%) |
|
Male | 60.6 |
Female | 39.4 |
Age, years (%) |
|
<20 | 6.7 |
20-40 | 31.5 |
41-60 | 22.3 |
61-85 | 30.4 |
>85 | 23.5 |
Race (%) |
|
White | 66.4 |
Black | 9.3 |
Asian | 1.7 |
Other | 2.4 |
Not stated | 20.2 |
Primary Diagnosis (%) |
|
Closed fracture of acetabulum (808.0) | 98.9 |
Open fracture of acetabulum (808.1) | 1.1 |
Associated diagnoses (%) |
|
Closed fracture of pubis (808.2) | 26.1 |
Open fracture of pubis (808.3) | 0.1 |
Closed fracture of ischium (808.42) | 1.7 |
Open fracture of ischium (808.52) | 0.0 |
Closed fracture of ilium (808.41) | 3.8 |
Open fracture of ilium (808.51) | 0.0 |
Closed fracture other part pelvis (808.49) | 0.7 |
Open fracture other part pelvis (808.59) | 0.0 |
Multiple closed pelvic fractures (808.43) | 0.5 |
Multiple open pelvic fractures (808.53) | 0.0 |
Any pelvic fracture from above | 29.8 |
Fracture of neck of femur (820) | 7.2 |
Fracture of any part of femur (820/821) | 13.1 |
Head trauma (959.01) | 0.7 |
Head/face trauma (959.0/959.01) | 0.7 |
Chest trauma (959.11) | 0.1 |
Chest/trunk trauma (959.1/959.11) | 0.1 |
Procedures (%) |
|
Open reduction internal fixation (79.30/79.39) | 23.2 |
Closed reduction internal fixation (79.10/79.19) | 1.3 |
External fixation (78.10/78.19) | 0.7 |
Internal fixation without reduction (78.50/78.59) | 0.4 |
Comorbidities (%) |
|
No | 72.9 |
Yes | 27.1 |
Adverse Events (%) |
|
No | 74.1 |
Yes | 25.9 |
Discharge Disposition (%) |
|
Routine/home (1) | 45.4 |
Left against medical advice (2) | 0.2 |
Short term fac (3) | 13.1 |
Long term fac (4) | 22.2 |
Alive, not stated (5) | 12 |
Dead (6) | 3.5 |
Not reported (9) | 3.6 |
Mortality (%) | 3.5 |
Age (y), mean (SD) | 52.6 (23.7) |
Days of Care, mean (SD) | 8.3 (17.9) |
Principal Source of Payment (%) |
|
Private insurance | 39 |
Medicare | 30.5 |
Medicaid | 7.7 |
Other government | 1.9 |
Self-pay | 7.9 |
Workmen’s comp | 4 |
Other | 4.7 |
Not stated | 4.4 |
Abbreviation: SD, standard deviation.
Table 2. Patient Characteristics in 1990, 1995, 1999, 2003, and 2007 Among Patients with Acetabular Fractures
Variable | 1990 | 1995 | 1999 | 2003 | 2007 | 2010 |
Total number | 19,560 | 17,506 | 22,767 | 27,133 | 34,027 | 29,373 |
Incidence per 100,000 capita | 7.84 | 6.57 | 8.16 | 9.35 | 11.30 | 9.5 |
Gender (%) |
| |||||
Male | 51.0 | 70.7 | 61.2 | 62.6 | 62.5 | 64.9 |
Female | 49.0 | 29.3 | 38.8 | 37.4 | 37.5 | 35.1 |
Fracture (%) |
| |||||
Open | 2.1 | 1.7 | 3.3 | 1.4 | 0.1 | 1.8 |
Closed | 97.9 | 98.3 | 96.7 | 98.6 | 99.9 | 98.2 |
Underwent ORIF (%) | 12.6 | 20.9 | 20.2 | 22.9 | 27.8 | 20.4 |
Adverse events (%) | 10.9 | 16.2 | 23.7 | 31 | 35.1 | 37.6 |
Transfusion (%) | 0.3 | 2.2 | 7.4 | 6.5 | 10.5 | 9.5 |
Discharge (%) |
| |||||
Routine | 58 | 65.6 | 35.6 | 45.9 | 40.2 | 41.6 |
Non-routine to inpatient facility | 26.8 | 23.1 | 46.4 | 33.8 | 40.8 | 34.6 |
Mortality (%) | 5.9 | 3.6 | 2 | 2.9 | 1.5 | 0.4 |
Mean Age (y) | 52.9 | 48.4 | 52.3 | 56.3 | 57 | 53.2 |
Mean DOC (days) | 17.0 | 13.4 | 8.7 | 10.8 | 8.5 | 10.3 |
Abbreviations: DOC, days of care; ORIF, open reduction internal fixation.
Continue to: MORTALITY...
MORTALITY
In-hospital mortality decreased from 5.9% in 1990 to 0.4% in 2010 (P < .001) (3.5% for the total cohort) (Tables 1 and 2). Multivariable logistic regression analysis demonstrated pulmonary insufficiency (odds ratio [OR], 9.07; 95% confidence interval [CI], 8.52-9.66; P < .01), pneumonia (OR, 3.22; 95% CI, 3.05-3.39; P < .01), and age >85 years (OR, 2.28; 95% CI, 2.16-2.40; P < .01) to be associated with the highest odds of inpatient mortality. CRIF (OR, 1.99; 95% CI, 1.78-2.23; P < .01), external fixator (OR, 1.82; 95% CI, 1.45-2.29; P < .01), and having received a blood transfusion (OR, 1.81; 95% CI, 1.71-1.91; P < .01) were also associated with increased odds of mortality. Treatment with ORIF (OR, 0.19; 95% CI, 0.17-0.20; P < .01) was independently associated with decreased odds of inpatient mortality, as was age <20 years (OR, 0.26; 95% CI, 0.23-0.30; P < .01) (model fit: for omnibus test of model coefficients, X = 25,966 P < .01; Nagelkerke, R2 = 0.20) (Table 3).
Table 3. Logistic Regression for Predictors of Mortality Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Pulmonary insufficiency | 9.07 (8.52–9.66) | < 0.01 |
Pneumonia | 3.22 (3.05–3.39) | < 0.01 |
Age >85 years | 2.28 (2.16–2.40) | < 0.01 |
Closed reduction internal fixation | 1.99 (1.78–2.23) | < 0.01 |
External Fixator | 1.82 (1.45–2.29) | < 0.01 |
Blood transfusion | 1.81 (1.71–1.91) | < 0.01 |
Gender (male) | 1.76 (1.70–1.83) | < 0.01 |
Associated femoral neck fracture | 1.23 (1.15–1.30) | < 0.01 |
Age 41-60 years | 1.19 (1.11–1.29) | < 0.01 |
Age 61-85 years | 1.17 (1.11–1.23) | < 0.01 |
Congestive heart failure | 1.14 (1.07–1.22) | < 0.01 |
Associated pelvic fracture | 1.13 (1.10–1.17) | < 0.01 |
Geographic region | 1.11 (1.09–1.12) | < 0.01 |
Source of payment | 1.02 (1.01–1.02) | < 0.01 |
Race | 0.99 (0.98–0.99) | < 0.01 |
DOC | 0.98 (0.98–0.98) | < 0.01 |
Hypertension | 0.67 (0.64–0.71) | < 0.01 |
Atrial fibrillation | 0.52 (0.48–0.57) | < 0.01 |
Diabetes mellitus | 0.35 (0.32–0.38) | < 0.01 |
Age 20-40 years | 0.32 (0.30–0.35) | < 0.01 |
Age <20 years | 0.26 (0.23–0.30) | < 0.01 |
Coronary artery disease | 0.21 (0.18–0.24) | < 0.01 |
Open reduction internal fixation | 0.19 (0.17–0.20) | < 0.01 |
Omnibus X 25,966, P < .01 | ||
Nagelkerke R2= 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
COMORBIDITIES AND ADVERSE EVENTS
The prevalence of comorbidities and adverse events is listed in Tables 4 and 5, respectively. Hypertensive disease was the most common comorbidity at 15.3%, followed by diabetes mellitus at 6.9%. Overall, 25.9% of patients experienced an in-hospital adverse event, with the most common being postoperative anemia (7.3%) and blood transfusion (8.1%) (Tables 1 and 5). The percentage of patients experiencing an adverse event increased from 10.9% in 1990 to 37.6% in 2010 (P < .01) (Table 2). Multivariable logistic regression analysis revealed CRIF (OR, 3.08; 95% CI, 2.91-3.26; P < .01), coronary artery disease (OR, 2.02; 95% CI, 1.91-2.15; P < .01), associated femoral neck fracture (OR, 1.53; 95% CI, 1.47-1.60; P < .01), and ORIF (OR, 1.22; 95% CI, 1.20-1.24; P < .01) to be associated with higher odds of inpatient adverse events (model fit: for omnibus test of model coefficients, X = 160,275, P < .01; Nagelkerke, R2 = 0.41) (Table 6).
Table 4. Prevalence of Comorbidities in Patients with Acetabular Fractures Between 1990 and 2007 (n = 403.927)
Parameter (ICD-9) | Percentage of Total |
Hypertensive disease (401–405) | 15.3% |
Diabetes mellitus (250) | 6.9% |
Atrial fibrillation (427.31) | 4.0% |
Congestive heart failure (428) | 3.9% |
Osteoporosis (733.0) | 2.1% |
Coronary artery disease (414.01) | 2.0% |
Obesity (278.00, 278.01) | 2.0% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 5. Prevalence of In-Hospital Adverse Events Among Patients with Acetabular Fractures Between 1990 and 2007 (n = 403,927)
Parameter (ICD-9) | Percentage of Total |
Transfusion of blood (99.0) | 8.1% |
Acute postoperative anemia (285.1) | 7.3% |
Intubation (96.x) | 4.9% |
Acute renal failure (584) | 3.4% |
Pneumonia (480-486) | 3.2% |
Pulmonary insufficiency (518.5) | 2.3% |
Pulmonary embolism (415.1) | 1.6% |
Deep venous thrombosis (453.4) | 1.0% |
Acute myocardial infarction (410) | 0.9% |
Postoperative bleeding (998.1) | 0.7% |
Acute postoperative infection (998.5) | 0.5% |
Induced mental disorder (293) | 0.4% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 6. Logistic Regression for Predictors of Adverse Events Among Patients Hospitalized for Acetabular Fracture (n = 403,927)
Variable | OR (95% CI) | P |
Closed reduction internal fixation | 3.08 (2.91-3.26) | < 0.01 |
Coronary artery disease | 2.02 (1.91-2.15) | < 0.01 |
Associated femoral neck fracture | 1.53 (1.47-1.60) | < 0.01 |
Open reduction internal fixation | 1.22 (1.20-1.24) | < 0.01 |
Gender (male) | 1.16 (1.14-1.18) | < 0.01 |
Associated fracture of any part of femur | 1.13 (1.10-1.17) | < 0.01 |
Age >85 years | 1.08 (1.05-1.12) | < 0.01 |
Geographic region | 1.07 (1.06-1.07) | < 0.01 |
DOC | 1.04 (1.04-1.04) | < 0.01 |
Race | 1.02 (1.02-1.03) | < 0.01 |
Source of payment | 1.01 (1.01-1.01) | < 0.01 |
Congestive heart failure | 1.01 (0.96-1.06) | 0.78 |
Atrial fibrillation | 0.88 (0.84-0.92) | < 0.01 |
Age 61-85 years | 0.68 (0.66-0.71) | < 0.01 |
Age <20 years | 0.67 (0.64-0.70) | < 0.01 |
Associated pelvis fracture | 0.64 (0.63-0.66) | < 0.01 |
Age 41-60 years | 0.58 (0.56-0.61) | < 0.01 |
Diabetes mellitus | 0.48 (0.46-0.50) | < 0.01 |
Age 20-40 years | 0.45 (0.43-0.47) | < 0.01 |
Hypertension | 0.44 (0.43-0.45) | < 0.01 |
External Fixator | 0.39 (0.35-0.44) | < 0.01 |
Omnibus X 160,275, P < .01 | ||
Nagelkerke R2 = 0.41 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
BLOOD TRANSFUSION
Overall, 7.3% of patients experienced acute postoperative anemia (Table 5). Between 1990 and 2010, the percentage of patients receiving blood transfusions increased from 0.3% to 9.5%, respectively (P < .01) (Table 2). In multivariable logistic regression analysis, patients treated with ORIF (OR, 8.13; 95% CI, 7.91-8.36; P < .01), those with congestive heart failure (OR, 4.23; 95% CI, 4.06-4.41; P < .01), those with an associated femur fracture (OR, 3.13; 95% CI, 2.99-3.27; P < .01), those with atrial fibrillation (OR, 1.96; 95% CI, 1.88-2.05; P < .01), and those treated with CRIF (OR, 1.42; 95% CI, 1.29-1.56; P < .01) were associated with significantly higher odds of blood transfusion (model fit: omnibus test of model coefficients, X = 42,653, P < .01; Nagelkerke, R2 = 0.19) (Table 7).
Table 7. Logistic Regression for Predictors of the Requirement for Blood Transfusion Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Open reduction internal fixation | 8.13 (7.91-8.36) | < 0.01 |
Congestive heart failure | 4.23 (4.06-4.41) | < 0.01 |
Associated fracture of any part of femur | 3.13 (2.99-3.27) | < 0.01 |
Atrial fibrillation | 1.96 (1.88-2.05) | < 0.01 |
Closed reduction internal fixation | 1.42 (1.29-1.56) | < 0.01 |
Geographic region | 1.38 (1.36-1.39) | < 0.01 |
Hypertension | 1.38 (1.34-1.42) | < 0.01 |
Associated pelvic fracture | 1.28 (1.25-1.31) | < 0.01 |
Age 61-85 years | 1.06 (1.02-1.11) | 0.01 |
Source of payment | 0.99 (0.98-0.99) | < 0.01 |
Race | 0.98 (0.97-0.98) | < 0.01 |
DOC | 0.96 (0.96-0.96) | < 0.01 |
Age >85 years | 0.74 (0.72-0.77) | < 0.01 |
External fixator | 0.69 (0.59-0.80) | < 0.01 |
Coronary artery disease | 0.62 (0.57-0.68) | < 0.01 |
Age 41-60 years | 0.57 (0.54-0.60) | < 0.01 |
Gender (male) | 0.54 (0.52-0.55) | < 0.01 |
Diabetes mellitus | 0.38 (0.36-0.41) | < 0.01 |
Age 20-40 years | 0.32 (0.30-0.34) | < 0.01 |
Associated femoral neck fracture | 0.29 (0.27-0.31) | < 0.01 |
Age <20 years | 0.24 (0.22-0.26) | < 0.01 |
Omnibus X = 42,653, P < .01 | ||
Nagelkerke R2 = 0.19 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
TREATMENT WITH ORIF
Over the 20-year study period, 23.2% of patients with acetabular fractures were treated with ORIF (Table 1). In 1990, 12.6% of patients underwent ORIF, while in 2010 this percentage increased to 20.4% (P < .001) (Table 2). Multivariable logistic regression analysis demonstrated that age between 41 and 60 years (OR, 1.88; 95% CI, 1.78-1.98; P < .01) was associated with the highest odds of undergoing ORIF. Age 20 to 40 years (OR, 1.86; 95% CI, 1.76-1.97; P < .01), age <20 years (OR, 1.82; 95% CI, 1.72-1.93; P < .01), and male gender (OR, 1.65; 95% CI, 1.63-1.68; P < .01) were also associated with being treated by ORIF. In contrast, coronary artery disease (OR, 0.27; 95% CI, 0.25-0.30; P < .01), age >85 years (OR, 0.46; 95% CI, 0.44-0.47; P < .01), and congestive heart failure (OR, 0.48; 95% CI, 0.46-0.51; P < .01) were associated with the lowest odds of undergoing ORIF (model fit: omnibus test of model coefficients, X = 71,118, P < .01; Nagelkerke, R2 = 0.20) (Table 8).
Table 8. Logistic Regression for Predictors of the Requirement for Discharge to Another Inpatient Facility Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Age 41-60 years | 1.88 (1.78-1.98) | < 0.01 |
Age 20-40 years | 1.86 (1.76-1.97) | < 0.01 |
Age <20 years | 1.82 (1.72-1.93) | < 0.01 |
Gender (male) | 1.65 (1.63-1.68) | < 0.01 |
Larger hospital bed size | 1.46 (1.45-1.47) | < 0.01 |
Hypertension | 1.35 (1.32-1.38) | < 0.01 |
Diabetes mellitus | 1.09 (1.05-1.13) | < 0.01 |
DOC | 1.02 (1.02-1.02) | < 0.01 |
Source of payment | 1.01 (1.01-1.02) | < 0.01 |
Race | 1.00 (0.99-1.00) | 0.17 |
Age 61-85 years | 0.94 (0.90-0.99) | 0.02 |
Region | 0.92 (0.91-0.93) | < 0.01 |
Atrial fibrillation | 0.83 (0.79-0.87) | < 0.01 |
Congestive heart failure | 0.48 (0.46-0.51) | < 0.01 |
Age >85 years | 0.46 (0.44-0.47) | < 0.01 |
Coronary artery disease | 0.27 (0.25-0.30) | < 0.01 |
Omnibus X 71,118, P < .01 | ||
Nagelkerke R2 = 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
Continue to: DISCUSSION...
DISCUSSION
This study evaluates the incidence of acetabular fractures in the US between 1990 and 2010, and identifies prognostic factors associated with complications and death. The study demonstrates an increase in the population-adjusted incidence of acetabular fractures between 1990 and 2010 (7.84 cases per 100,000 capita to 9.5 cases per 100,000 capita), in contrast to the decreasing trend reported by Mauffrey and colleagues.11 Some studies suggest that up to 80% of acetabular fractures are associated with motor vehicle collisions and motorcycle accidents.9,27 While the rate of motor vehicle accidents has remained stable over the study period, motorcycle ownership and deaths more than doubled between 2001 and 2008,28 primarily among individuals over 40 years of age. In this study, the mean age of patients with acetabular fractures ranged from 48 to 57 years. The dramatic increase in motorcycle ownership and deaths in these age groups may partially explain the rising incidence of acetabular fractures. The other possibility is that changes in automobile design and safety equipment may have altered the injury patterns observed in patients surviving motor vehicle crashes. Compared to the United Kingdom, in which studies report a fixed incidence of 3 per 100,000 capita1 between 1988 and 2003, the incidence of acetabular fractures in the US is greater. In contrast, the incidence of acetabular fractures reported in this study is less than the 20 per 100,000 reported in Sweden between 1976 and 1985,29 or the 37 per 100,000 reported in Rochester, Minnesota between 1968 and 1977,30 which may be due to increased seatbelt usage.31
In addition to the national incidence, this study demonstrated that the proportion of patients with acetabular fractures treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010. This is substantially lower than the 77% reported by Ochs and colleagues32 in a German population. Concurrent with the increase in ORIF, there was a decrease in in-hospital mortality from 5.9% in 1990 to 0.4% in 2010. The initial mortality rates in this study are comparable to much earlier reports and some small studies,9,32-37 but the rates reported in the later years of this study show a substantial decrease that is likely a more accurate estimation of the current incidence. The improved survival rates may be due to advances in the operative treatment of acetabular fractures, in which mechanical stabilization allows for early patient mobilization and facilitation of optimal nursing care.38 With ORIF becoming the standard of care for displaced acetabular fractures,9 numerous reports have demonstrated an association between early definitive fixation and improved survival.17,39,40 This is similar to our study, which found ORIF to be associated with the lowest odds of mortality in multivariate logistic regression analysis. It is possible that advances in patient care by intensivists over this period have also contributed to the decrease in mortality, but the correlation with operative treatment in this study is very strong and agrees well with prior studies.16 Moreover, multiple studies have demonstrated decreased in-hospital mortality among patients undergoing various orthopedic surgical procedures during this period.41-43 The correlation with operative treatment in this study agrees well with prior studies.16
In contrast, higher odds of mortality were seen in patients over the age of 85 years with pulmonary insufficiency, congestive heart failure, pneumonia, or an associated femur or pelvic fracture. This is similar to prior reports in which patients with combined acetabulum and pelvic ring injuries fared worse than those with isolated injures,44,45 as did patients with associated non-musculoskeletal injuries.46 The finding that age over 85 years was associated with higher odds of mortality likely reflects the increased number of comorbidities and decreased physiologic reserve seen in this patient population. Finally, male gender was associated with higher odds of in-hospital mortality. There are 2 possible explanations for this: Either there is gender dimorphism in sex hormones and cytokine activity in response to hemorrhage and sepsis,38,47 or there is a greater tendency for males to be involved in higher energy accidents with more severe concomitant injuries.
The results of multivariable regression analysis demonstrated that patients were more likely to require blood transfusion if they were managed surgically or had atrial fibrillation, congestive heart failure, or associated femur fracture. Not surprisingly, concurrent pelvic fracture was also associated with higher odds of blood transfusion, as pelvic hemorrhage is reported to be the cause of death in up to half of patients who die following a pelvic fracture.46
Between 1990 and 2010, in-hospital days of care decreased from 17.0 days to 10.3 days. While a decreased length of stay has been demonstrated in other orthopedic conditions over the study period,41 it is possible the decrease in length of stay demonstrated in this study is due to improved surgical technique and the implementation of early surgical intervention.39,48-50 Plaisier and colleagues17 demonstrated superior functional outcomes, quicker return to baseline function, and decreased length of stay in patients treated with early ORIF of their acetabular fractures. Other studies have shown that the benefits of early surgery include improved reduction quality and ease of reduction,51 as well as control of bleeding, pain relief, and mobilization of the patient.39 Another possible explanation for the decreased length of stay is the increased rate of discharge to other inpatient facilities, such as rehabilitation facilities, which was demonstrated in this study.
Continue to: Interestingly, male gender and younger age...
Interestingly, male gender and younger age were associated with operative management of the acetabular fracture. In contrast, there was a decreased likelihood of operative treatment among elderly patients and those patients with cardiac comorbidities. It is possible that the relationship we found between the likelihood of ORIF and age relates to the bimodal distribution of fractures, with higher energy and potentially more displaced fractures occurring in younger patients3-5 and lower energy fractures in the elderly.
In contrast to decreasing in-hospital days of care, there was a rise in the number of adverse events between 1990 (10.9%) and 2010 (37.6%). This can be partially attributed to the increased rates of blood transfusion, which was received by 9.5% of patients with acetabular fractures in the final study year. Additionally, surgical intervention was associated with increased adverse events in this study, and surgical intervention increased over the study period. Other factors that may have contributed to an increase in adverse events include an aging population,52 as advanced age was independently associated with higher odds of adverse events in this study.
Despite the strengths of using large, national databases for epidemiological research,53 this study has several limitations. Like all large databases, the NHDS is subject to error in coding and data entry.54 Additionally, the database only allows for 7 diagnostic codes and 4 procedure codes per entry. As a result, the prevalence of comorbid conditions and adverse events may be underreported.25 Moreover, the severity of a comorbid disease cannot be appreciated when dichotomously classified.55 Another limitation is that the database only provides inpatient data, so complications that arise after discharge, as well as follow-up data, are unknown. Furthermore, the results of this study are limited to practice patterns in the US from 1990 to 2010. This database does not provide injury mechanisms, so we cannot distinguish between high-energy and low-energy injuries. Lastly, analysis of the different types of acetabular fractures was not performed since classification of acetabular fractures cannot be assessed with ICD-9 codes.
CONCLUSION
This study is the largest epidemiologic analysis of acetabular fractures in the US and also provides predictors of in-hospital mortality. The incidence of acetabular fractures in the US is increasing, while mortality is decreasing. Identifying risk factors associated with poor outcomes has the potential to change treatment strategies, resource allocation, in-hospital monitoring, and discharge planning for this patient population.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The incidence of acetabular fractures and associated in-hospital complication rates in the United States are poorly defined. Studies evaluating predictors of outcome for isolated acetabular fractures are weakly generalizable due to small sample sizes or the inclusion of all types of pelvic fractures. This study sought to analyze trends in acetabular fractures and associated complications in the US using the largest and most recent national dataset available.
The National Hospital Discharge Survey was queried to identify all patients admitted to US hospitals with acetabular fractures between 1990 and 2010. A representative cohort of 497,389 patients was identified, and multivariable logistic regression was used to identify independent predictors of mortality, adverse events, requirement of blood transfusion, and operative treatment with open reduction and internal fixation (ORIF).
Between 1990 and 2010, the population-adjusted incidence of acetabular fractures increased from 7.8 to 9.5/100,000 capita (P < .001). Mortality declined from 5.9% to 0.4% (P < .001), paralleling an increase in the proportion of patients treated with ORIF (12.6%-20.4%, P < .001), which was the variable associated with the lowest odds of mortality. Surgical intervention was associated with higher odds of adverse events and a requirement for blood transfusion. The average in-hospital length of stay decreased from 17.0 days to 10.3 days (P < .001).
This study provides the largest and most comprehensive epidemiologic analysis of acetabular fractures in the US. Knowledge of the increasing incidence of acetabular fractures and prognostic factors associated with poor outcomes may improve outcomes.
Continue to: Acetabular fractures are major injuries...
Acetabular fractures are major injuries frequently associated with life-altering sequelae1 and a significant resulting cost to society.2 Acetabular fractures are most often the result of a high-energy trauma3-5 or fall from a height.5,6 Functional outcomes and the prevention of post-traumatic arthritis have been shown to depend upon the accuracy of operative reduction.7-9 However, literature on the epidemiology of acetabular fractures is largely limited to European countries,1,10 and their incidence in the United States is more poorly defined.11 Published mortality rates in the existing literature vary widely from 2% to 45%,12-14 and few studies have identified the risk factors associated with in-hospital complications.15 While age, gender, and high-velocity mechanisms have been linked to increased mortality and complications,14-16 the evidence for these associations is poorly generalizable due to the inclusion of all pelvic fractures in these studies. Some reports suggest that advances in surgical management have improved survival and functional outcome,15,17 but these are based upon small cohorts. Knowledge of the incidence and patterns of disease burden are crucial for the allocation of limited healthcare resources.
This study sought to describe the trends in incidence as well as the factors influencing mortality and the risk of complications for patients admitted to US hospitals with an acetabular fracture using the National Hospital Discharge Survey (NHDS), the most recently available Centers for Disease Control and Prevention data, which is also one of the largest inpatient databases in the US. Knowledge of the factors influencing outcomes for patients admitted with acetabular fractures may improve management and decrease complications.
METHODS
NATIONAL HOSPITAL DISCHARGE SURVEY
The NHDS, developed by the National Center for Healthcare Statistics division of the Centers for Disease Control and Prevention,18 was used to estimate the incidence of acetabular fractures and to evaluate the risk factors for ensuing mortality and inpatient complications. The NHDS is a publically available survey providing demographic and medical data for inpatients discharged from non-federal, short-stay hospitals in the US.19 The NHDS is the principal database used by the US government for monitoring hospital use and is considered the most comprehensive of all inpatient surgical databases in use today.19 The survey uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes20 to classify medical diagnoses and procedures. The NHDS uses a stratified, multistage probability design to collect demographic information (age, gender, race), expected source of payment (insurance status), medical information of up to 7 discharge diagnoses and up to 4 procedures, length of care, hospital size, US region, and inpatient outcomes including discharge destination.21 To ensure unbiased national sampling of inpatient records, the NHDS uses a complex, 3-stage probability design including inflation by reciprocals of the probabilities of sample selection, adjustment for no response, and population weighting ratio adjustments.19 This study did not require approval by the Institutional Review Board because the NHDS is a publically available database with no patient-identifying information.
Continue to: PATIENT SELECTION...
PATIENT SELECTION
All patients admitted to hospitals in the US with a fracture of the acetabulum between 1990 and 2010 were identified using ICD-9-CM codes. Discharges with a diagnosis code (ICD-9-CM) of closed fracture of the acetabulum (808.0) or open fracture of the acetabulum (808.1) were identified using previously described techniques.22 The database was subsequently queried to identify patients treated using open reduction and internal fixation (ORIF) (ICD-9-CM, 79.30/79.39), closed reduction and internal fixation (CRIF) (ICD-9-CM, 79.10/79.19), or external (ICD-9-CM, 78.10/78.19) or internal (ICD-9-CM, 78.50/78.59) fixation without reduction. Demographic variables were then collected, including age, sex, primary diagnosis, associated diagnoses, type of fracture (open vs closed), prevalence of comorbidities, length of stay, and discharge destination. The complication screening package23 was used to determine the incidence of complications. The variable adverse event was created on the basis of the variables postoperative bleeding (998.1), acute postoperative infection (998.5), acute postoperative anemia (285.1), acute renal failure (584), acute myocardial infarction (410), pulmonary embolism (415.1), induced mental disorder (293), pneumonia (480-486), pulmonary insufficiency (518.5), deep venous thrombosis (453.4), intubation (96.xx), and blood transfusion (99.x).
STATISTICAL ANALYSIS
Because of the large sample size, a normal distribution of the data was assumed. Differences between categorical variables were compared using the Pearson chi square test, while the independent-samples t test was used to compare differences between continuous variables. To determine independent predictors of in-hospital outcomes (death, adverse events, requirement for blood transfusion, or treatment with ORIF), all variables present in at least 2% of the population24 were included in a multivariable binary logistic regression model. For in-hospital adverse events, a 1% cutoff was used due to their lower rates of occurrence, as previously described.25The dichotomous variables were death, presence of adverse events, receipt of blood transfusion, and treatment with ORIF. A multivariable regression model allows for the control of potential confounders, isolating the effect of individual variables on inpatient outcomes. Covariates accounted for in the regression model included gender, age, region of the country, and preexisting comorbidities (diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, atrial fibrillation). To assess the association between individual variables and inpatient outcomes, odds ratios and confidence intervals were calculated. A P value of <.001 was used to define statistical significance, correcting for multiple comparisons, as previously described.25 US census data were used to obtain national population estimates for each year of the study from 1990 to 2010.26 Rates were presented as the number of acetabular fractures per 100,000 standard population. All data were analyzed using the software Statistical Package for the Social Sciences [SPSS] version 20.
RESULTS
INCIDENCE AND DEMOGRAPHICS
A cohort representative of 497,389 patients with a diagnosis of acetabular fracture was identified between 1990 and 2010 (Table 1). In 1990, 19,560 cases (7.84 per 100,000 capita) of acetabular fractures were recoded, while in 2010, the number of cases increased to 29,373 or 9.5 per 100,000 capita (P < .001) (Table 2). The mean age of patients with an acetabular fracture was 52.6 years (standard deviation [SD], 23.7) and 60.6% were male (Table 1). The most frequently associated diagnosis was closed fracture of the pelvis (29.8%) followed by fracture of the femur (13.1%) and closed fracture of the ilium (3.8%) (Table 1). Of the total cohort, 23.2% underwent ORIF (Table 1). In 1990, 12.6% of patients with a diagnosis of acetabular fracture underwent ORIF, whereas 20.4% of patients underwent ORIF in 2010 (P < .001) (Table 2). Average length of hospital stay was 8.3 days (SD, 17.9) overall (Table 1). In 1990 the average length of stay was 17.0 days (SD, 14.9), decreasing to 10.3 days (SD, 9.3) in 2010 (P < .001) (Table 2).
Table 1. Patient Characteristics for Patients with Acetabular Fractures in the United States from 1990 to 2007
Parameter | Total 1990-2010 |
Total Number | 497,389 |
Gender (%) |
|
Male | 60.6 |
Female | 39.4 |
Age, years (%) |
|
<20 | 6.7 |
20-40 | 31.5 |
41-60 | 22.3 |
61-85 | 30.4 |
>85 | 23.5 |
Race (%) |
|
White | 66.4 |
Black | 9.3 |
Asian | 1.7 |
Other | 2.4 |
Not stated | 20.2 |
Primary Diagnosis (%) |
|
Closed fracture of acetabulum (808.0) | 98.9 |
Open fracture of acetabulum (808.1) | 1.1 |
Associated diagnoses (%) |
|
Closed fracture of pubis (808.2) | 26.1 |
Open fracture of pubis (808.3) | 0.1 |
Closed fracture of ischium (808.42) | 1.7 |
Open fracture of ischium (808.52) | 0.0 |
Closed fracture of ilium (808.41) | 3.8 |
Open fracture of ilium (808.51) | 0.0 |
Closed fracture other part pelvis (808.49) | 0.7 |
Open fracture other part pelvis (808.59) | 0.0 |
Multiple closed pelvic fractures (808.43) | 0.5 |
Multiple open pelvic fractures (808.53) | 0.0 |
Any pelvic fracture from above | 29.8 |
Fracture of neck of femur (820) | 7.2 |
Fracture of any part of femur (820/821) | 13.1 |
Head trauma (959.01) | 0.7 |
Head/face trauma (959.0/959.01) | 0.7 |
Chest trauma (959.11) | 0.1 |
Chest/trunk trauma (959.1/959.11) | 0.1 |
Procedures (%) |
|
Open reduction internal fixation (79.30/79.39) | 23.2 |
Closed reduction internal fixation (79.10/79.19) | 1.3 |
External fixation (78.10/78.19) | 0.7 |
Internal fixation without reduction (78.50/78.59) | 0.4 |
Comorbidities (%) |
|
No | 72.9 |
Yes | 27.1 |
Adverse Events (%) |
|
No | 74.1 |
Yes | 25.9 |
Discharge Disposition (%) |
|
Routine/home (1) | 45.4 |
Left against medical advice (2) | 0.2 |
Short term fac (3) | 13.1 |
Long term fac (4) | 22.2 |
Alive, not stated (5) | 12 |
Dead (6) | 3.5 |
Not reported (9) | 3.6 |
Mortality (%) | 3.5 |
Age (y), mean (SD) | 52.6 (23.7) |
Days of Care, mean (SD) | 8.3 (17.9) |
Principal Source of Payment (%) |
|
Private insurance | 39 |
Medicare | 30.5 |
Medicaid | 7.7 |
Other government | 1.9 |
Self-pay | 7.9 |
Workmen’s comp | 4 |
Other | 4.7 |
Not stated | 4.4 |
Abbreviation: SD, standard deviation.
Table 2. Patient Characteristics in 1990, 1995, 1999, 2003, and 2007 Among Patients with Acetabular Fractures
Variable | 1990 | 1995 | 1999 | 2003 | 2007 | 2010 |
Total number | 19,560 | 17,506 | 22,767 | 27,133 | 34,027 | 29,373 |
Incidence per 100,000 capita | 7.84 | 6.57 | 8.16 | 9.35 | 11.30 | 9.5 |
Gender (%) |
| |||||
Male | 51.0 | 70.7 | 61.2 | 62.6 | 62.5 | 64.9 |
Female | 49.0 | 29.3 | 38.8 | 37.4 | 37.5 | 35.1 |
Fracture (%) |
| |||||
Open | 2.1 | 1.7 | 3.3 | 1.4 | 0.1 | 1.8 |
Closed | 97.9 | 98.3 | 96.7 | 98.6 | 99.9 | 98.2 |
Underwent ORIF (%) | 12.6 | 20.9 | 20.2 | 22.9 | 27.8 | 20.4 |
Adverse events (%) | 10.9 | 16.2 | 23.7 | 31 | 35.1 | 37.6 |
Transfusion (%) | 0.3 | 2.2 | 7.4 | 6.5 | 10.5 | 9.5 |
Discharge (%) |
| |||||
Routine | 58 | 65.6 | 35.6 | 45.9 | 40.2 | 41.6 |
Non-routine to inpatient facility | 26.8 | 23.1 | 46.4 | 33.8 | 40.8 | 34.6 |
Mortality (%) | 5.9 | 3.6 | 2 | 2.9 | 1.5 | 0.4 |
Mean Age (y) | 52.9 | 48.4 | 52.3 | 56.3 | 57 | 53.2 |
Mean DOC (days) | 17.0 | 13.4 | 8.7 | 10.8 | 8.5 | 10.3 |
Abbreviations: DOC, days of care; ORIF, open reduction internal fixation.
Continue to: MORTALITY...
MORTALITY
In-hospital mortality decreased from 5.9% in 1990 to 0.4% in 2010 (P < .001) (3.5% for the total cohort) (Tables 1 and 2). Multivariable logistic regression analysis demonstrated pulmonary insufficiency (odds ratio [OR], 9.07; 95% confidence interval [CI], 8.52-9.66; P < .01), pneumonia (OR, 3.22; 95% CI, 3.05-3.39; P < .01), and age >85 years (OR, 2.28; 95% CI, 2.16-2.40; P < .01) to be associated with the highest odds of inpatient mortality. CRIF (OR, 1.99; 95% CI, 1.78-2.23; P < .01), external fixator (OR, 1.82; 95% CI, 1.45-2.29; P < .01), and having received a blood transfusion (OR, 1.81; 95% CI, 1.71-1.91; P < .01) were also associated with increased odds of mortality. Treatment with ORIF (OR, 0.19; 95% CI, 0.17-0.20; P < .01) was independently associated with decreased odds of inpatient mortality, as was age <20 years (OR, 0.26; 95% CI, 0.23-0.30; P < .01) (model fit: for omnibus test of model coefficients, X = 25,966 P < .01; Nagelkerke, R2 = 0.20) (Table 3).
Table 3. Logistic Regression for Predictors of Mortality Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Pulmonary insufficiency | 9.07 (8.52–9.66) | < 0.01 |
Pneumonia | 3.22 (3.05–3.39) | < 0.01 |
Age >85 years | 2.28 (2.16–2.40) | < 0.01 |
Closed reduction internal fixation | 1.99 (1.78–2.23) | < 0.01 |
External Fixator | 1.82 (1.45–2.29) | < 0.01 |
Blood transfusion | 1.81 (1.71–1.91) | < 0.01 |
Gender (male) | 1.76 (1.70–1.83) | < 0.01 |
Associated femoral neck fracture | 1.23 (1.15–1.30) | < 0.01 |
Age 41-60 years | 1.19 (1.11–1.29) | < 0.01 |
Age 61-85 years | 1.17 (1.11–1.23) | < 0.01 |
Congestive heart failure | 1.14 (1.07–1.22) | < 0.01 |
Associated pelvic fracture | 1.13 (1.10–1.17) | < 0.01 |
Geographic region | 1.11 (1.09–1.12) | < 0.01 |
Source of payment | 1.02 (1.01–1.02) | < 0.01 |
Race | 0.99 (0.98–0.99) | < 0.01 |
DOC | 0.98 (0.98–0.98) | < 0.01 |
Hypertension | 0.67 (0.64–0.71) | < 0.01 |
Atrial fibrillation | 0.52 (0.48–0.57) | < 0.01 |
Diabetes mellitus | 0.35 (0.32–0.38) | < 0.01 |
Age 20-40 years | 0.32 (0.30–0.35) | < 0.01 |
Age <20 years | 0.26 (0.23–0.30) | < 0.01 |
Coronary artery disease | 0.21 (0.18–0.24) | < 0.01 |
Open reduction internal fixation | 0.19 (0.17–0.20) | < 0.01 |
Omnibus X 25,966, P < .01 | ||
Nagelkerke R2= 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
COMORBIDITIES AND ADVERSE EVENTS
The prevalence of comorbidities and adverse events is listed in Tables 4 and 5, respectively. Hypertensive disease was the most common comorbidity at 15.3%, followed by diabetes mellitus at 6.9%. Overall, 25.9% of patients experienced an in-hospital adverse event, with the most common being postoperative anemia (7.3%) and blood transfusion (8.1%) (Tables 1 and 5). The percentage of patients experiencing an adverse event increased from 10.9% in 1990 to 37.6% in 2010 (P < .01) (Table 2). Multivariable logistic regression analysis revealed CRIF (OR, 3.08; 95% CI, 2.91-3.26; P < .01), coronary artery disease (OR, 2.02; 95% CI, 1.91-2.15; P < .01), associated femoral neck fracture (OR, 1.53; 95% CI, 1.47-1.60; P < .01), and ORIF (OR, 1.22; 95% CI, 1.20-1.24; P < .01) to be associated with higher odds of inpatient adverse events (model fit: for omnibus test of model coefficients, X = 160,275, P < .01; Nagelkerke, R2 = 0.41) (Table 6).
Table 4. Prevalence of Comorbidities in Patients with Acetabular Fractures Between 1990 and 2007 (n = 403.927)
Parameter (ICD-9) | Percentage of Total |
Hypertensive disease (401–405) | 15.3% |
Diabetes mellitus (250) | 6.9% |
Atrial fibrillation (427.31) | 4.0% |
Congestive heart failure (428) | 3.9% |
Osteoporosis (733.0) | 2.1% |
Coronary artery disease (414.01) | 2.0% |
Obesity (278.00, 278.01) | 2.0% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 5. Prevalence of In-Hospital Adverse Events Among Patients with Acetabular Fractures Between 1990 and 2007 (n = 403,927)
Parameter (ICD-9) | Percentage of Total |
Transfusion of blood (99.0) | 8.1% |
Acute postoperative anemia (285.1) | 7.3% |
Intubation (96.x) | 4.9% |
Acute renal failure (584) | 3.4% |
Pneumonia (480-486) | 3.2% |
Pulmonary insufficiency (518.5) | 2.3% |
Pulmonary embolism (415.1) | 1.6% |
Deep venous thrombosis (453.4) | 1.0% |
Acute myocardial infarction (410) | 0.9% |
Postoperative bleeding (998.1) | 0.7% |
Acute postoperative infection (998.5) | 0.5% |
Induced mental disorder (293) | 0.4% |
Abbreviation: ICD-9, International Classifications of Diseases, 9th Revision.
Table 6. Logistic Regression for Predictors of Adverse Events Among Patients Hospitalized for Acetabular Fracture (n = 403,927)
Variable | OR (95% CI) | P |
Closed reduction internal fixation | 3.08 (2.91-3.26) | < 0.01 |
Coronary artery disease | 2.02 (1.91-2.15) | < 0.01 |
Associated femoral neck fracture | 1.53 (1.47-1.60) | < 0.01 |
Open reduction internal fixation | 1.22 (1.20-1.24) | < 0.01 |
Gender (male) | 1.16 (1.14-1.18) | < 0.01 |
Associated fracture of any part of femur | 1.13 (1.10-1.17) | < 0.01 |
Age >85 years | 1.08 (1.05-1.12) | < 0.01 |
Geographic region | 1.07 (1.06-1.07) | < 0.01 |
DOC | 1.04 (1.04-1.04) | < 0.01 |
Race | 1.02 (1.02-1.03) | < 0.01 |
Source of payment | 1.01 (1.01-1.01) | < 0.01 |
Congestive heart failure | 1.01 (0.96-1.06) | 0.78 |
Atrial fibrillation | 0.88 (0.84-0.92) | < 0.01 |
Age 61-85 years | 0.68 (0.66-0.71) | < 0.01 |
Age <20 years | 0.67 (0.64-0.70) | < 0.01 |
Associated pelvis fracture | 0.64 (0.63-0.66) | < 0.01 |
Age 41-60 years | 0.58 (0.56-0.61) | < 0.01 |
Diabetes mellitus | 0.48 (0.46-0.50) | < 0.01 |
Age 20-40 years | 0.45 (0.43-0.47) | < 0.01 |
Hypertension | 0.44 (0.43-0.45) | < 0.01 |
External Fixator | 0.39 (0.35-0.44) | < 0.01 |
Omnibus X 160,275, P < .01 | ||
Nagelkerke R2 = 0.41 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
BLOOD TRANSFUSION
Overall, 7.3% of patients experienced acute postoperative anemia (Table 5). Between 1990 and 2010, the percentage of patients receiving blood transfusions increased from 0.3% to 9.5%, respectively (P < .01) (Table 2). In multivariable logistic regression analysis, patients treated with ORIF (OR, 8.13; 95% CI, 7.91-8.36; P < .01), those with congestive heart failure (OR, 4.23; 95% CI, 4.06-4.41; P < .01), those with an associated femur fracture (OR, 3.13; 95% CI, 2.99-3.27; P < .01), those with atrial fibrillation (OR, 1.96; 95% CI, 1.88-2.05; P < .01), and those treated with CRIF (OR, 1.42; 95% CI, 1.29-1.56; P < .01) were associated with significantly higher odds of blood transfusion (model fit: omnibus test of model coefficients, X = 42,653, P < .01; Nagelkerke, R2 = 0.19) (Table 7).
Table 7. Logistic Regression for Predictors of the Requirement for Blood Transfusion Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Open reduction internal fixation | 8.13 (7.91-8.36) | < 0.01 |
Congestive heart failure | 4.23 (4.06-4.41) | < 0.01 |
Associated fracture of any part of femur | 3.13 (2.99-3.27) | < 0.01 |
Atrial fibrillation | 1.96 (1.88-2.05) | < 0.01 |
Closed reduction internal fixation | 1.42 (1.29-1.56) | < 0.01 |
Geographic region | 1.38 (1.36-1.39) | < 0.01 |
Hypertension | 1.38 (1.34-1.42) | < 0.01 |
Associated pelvic fracture | 1.28 (1.25-1.31) | < 0.01 |
Age 61-85 years | 1.06 (1.02-1.11) | 0.01 |
Source of payment | 0.99 (0.98-0.99) | < 0.01 |
Race | 0.98 (0.97-0.98) | < 0.01 |
DOC | 0.96 (0.96-0.96) | < 0.01 |
Age >85 years | 0.74 (0.72-0.77) | < 0.01 |
External fixator | 0.69 (0.59-0.80) | < 0.01 |
Coronary artery disease | 0.62 (0.57-0.68) | < 0.01 |
Age 41-60 years | 0.57 (0.54-0.60) | < 0.01 |
Gender (male) | 0.54 (0.52-0.55) | < 0.01 |
Diabetes mellitus | 0.38 (0.36-0.41) | < 0.01 |
Age 20-40 years | 0.32 (0.30-0.34) | < 0.01 |
Associated femoral neck fracture | 0.29 (0.27-0.31) | < 0.01 |
Age <20 years | 0.24 (0.22-0.26) | < 0.01 |
Omnibus X = 42,653, P < .01 | ||
Nagelkerke R2 = 0.19 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
TREATMENT WITH ORIF
Over the 20-year study period, 23.2% of patients with acetabular fractures were treated with ORIF (Table 1). In 1990, 12.6% of patients underwent ORIF, while in 2010 this percentage increased to 20.4% (P < .001) (Table 2). Multivariable logistic regression analysis demonstrated that age between 41 and 60 years (OR, 1.88; 95% CI, 1.78-1.98; P < .01) was associated with the highest odds of undergoing ORIF. Age 20 to 40 years (OR, 1.86; 95% CI, 1.76-1.97; P < .01), age <20 years (OR, 1.82; 95% CI, 1.72-1.93; P < .01), and male gender (OR, 1.65; 95% CI, 1.63-1.68; P < .01) were also associated with being treated by ORIF. In contrast, coronary artery disease (OR, 0.27; 95% CI, 0.25-0.30; P < .01), age >85 years (OR, 0.46; 95% CI, 0.44-0.47; P < .01), and congestive heart failure (OR, 0.48; 95% CI, 0.46-0.51; P < .01) were associated with the lowest odds of undergoing ORIF (model fit: omnibus test of model coefficients, X = 71,118, P < .01; Nagelkerke, R2 = 0.20) (Table 8).
Table 8. Logistic Regression for Predictors of the Requirement for Discharge to Another Inpatient Facility Among Patients with Acetabular Fractures (n = 403,927)
Variable | OR (95% CI) | P |
Age 41-60 years | 1.88 (1.78-1.98) | < 0.01 |
Age 20-40 years | 1.86 (1.76-1.97) | < 0.01 |
Age <20 years | 1.82 (1.72-1.93) | < 0.01 |
Gender (male) | 1.65 (1.63-1.68) | < 0.01 |
Larger hospital bed size | 1.46 (1.45-1.47) | < 0.01 |
Hypertension | 1.35 (1.32-1.38) | < 0.01 |
Diabetes mellitus | 1.09 (1.05-1.13) | < 0.01 |
DOC | 1.02 (1.02-1.02) | < 0.01 |
Source of payment | 1.01 (1.01-1.02) | < 0.01 |
Race | 1.00 (0.99-1.00) | 0.17 |
Age 61-85 years | 0.94 (0.90-0.99) | 0.02 |
Region | 0.92 (0.91-0.93) | < 0.01 |
Atrial fibrillation | 0.83 (0.79-0.87) | < 0.01 |
Congestive heart failure | 0.48 (0.46-0.51) | < 0.01 |
Age >85 years | 0.46 (0.44-0.47) | < 0.01 |
Coronary artery disease | 0.27 (0.25-0.30) | < 0.01 |
Omnibus X 71,118, P < .01 | ||
Nagelkerke R2 = 0.20 |
Abbreviations: CI, confidence interval; DOC, days of care; OR, odds ratio.
Continue to: DISCUSSION...
DISCUSSION
This study evaluates the incidence of acetabular fractures in the US between 1990 and 2010, and identifies prognostic factors associated with complications and death. The study demonstrates an increase in the population-adjusted incidence of acetabular fractures between 1990 and 2010 (7.84 cases per 100,000 capita to 9.5 cases per 100,000 capita), in contrast to the decreasing trend reported by Mauffrey and colleagues.11 Some studies suggest that up to 80% of acetabular fractures are associated with motor vehicle collisions and motorcycle accidents.9,27 While the rate of motor vehicle accidents has remained stable over the study period, motorcycle ownership and deaths more than doubled between 2001 and 2008,28 primarily among individuals over 40 years of age. In this study, the mean age of patients with acetabular fractures ranged from 48 to 57 years. The dramatic increase in motorcycle ownership and deaths in these age groups may partially explain the rising incidence of acetabular fractures. The other possibility is that changes in automobile design and safety equipment may have altered the injury patterns observed in patients surviving motor vehicle crashes. Compared to the United Kingdom, in which studies report a fixed incidence of 3 per 100,000 capita1 between 1988 and 2003, the incidence of acetabular fractures in the US is greater. In contrast, the incidence of acetabular fractures reported in this study is less than the 20 per 100,000 reported in Sweden between 1976 and 1985,29 or the 37 per 100,000 reported in Rochester, Minnesota between 1968 and 1977,30 which may be due to increased seatbelt usage.31
In addition to the national incidence, this study demonstrated that the proportion of patients with acetabular fractures treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010. This is substantially lower than the 77% reported by Ochs and colleagues32 in a German population. Concurrent with the increase in ORIF, there was a decrease in in-hospital mortality from 5.9% in 1990 to 0.4% in 2010. The initial mortality rates in this study are comparable to much earlier reports and some small studies,9,32-37 but the rates reported in the later years of this study show a substantial decrease that is likely a more accurate estimation of the current incidence. The improved survival rates may be due to advances in the operative treatment of acetabular fractures, in which mechanical stabilization allows for early patient mobilization and facilitation of optimal nursing care.38 With ORIF becoming the standard of care for displaced acetabular fractures,9 numerous reports have demonstrated an association between early definitive fixation and improved survival.17,39,40 This is similar to our study, which found ORIF to be associated with the lowest odds of mortality in multivariate logistic regression analysis. It is possible that advances in patient care by intensivists over this period have also contributed to the decrease in mortality, but the correlation with operative treatment in this study is very strong and agrees well with prior studies.16 Moreover, multiple studies have demonstrated decreased in-hospital mortality among patients undergoing various orthopedic surgical procedures during this period.41-43 The correlation with operative treatment in this study agrees well with prior studies.16
In contrast, higher odds of mortality were seen in patients over the age of 85 years with pulmonary insufficiency, congestive heart failure, pneumonia, or an associated femur or pelvic fracture. This is similar to prior reports in which patients with combined acetabulum and pelvic ring injuries fared worse than those with isolated injures,44,45 as did patients with associated non-musculoskeletal injuries.46 The finding that age over 85 years was associated with higher odds of mortality likely reflects the increased number of comorbidities and decreased physiologic reserve seen in this patient population. Finally, male gender was associated with higher odds of in-hospital mortality. There are 2 possible explanations for this: Either there is gender dimorphism in sex hormones and cytokine activity in response to hemorrhage and sepsis,38,47 or there is a greater tendency for males to be involved in higher energy accidents with more severe concomitant injuries.
The results of multivariable regression analysis demonstrated that patients were more likely to require blood transfusion if they were managed surgically or had atrial fibrillation, congestive heart failure, or associated femur fracture. Not surprisingly, concurrent pelvic fracture was also associated with higher odds of blood transfusion, as pelvic hemorrhage is reported to be the cause of death in up to half of patients who die following a pelvic fracture.46
Between 1990 and 2010, in-hospital days of care decreased from 17.0 days to 10.3 days. While a decreased length of stay has been demonstrated in other orthopedic conditions over the study period,41 it is possible the decrease in length of stay demonstrated in this study is due to improved surgical technique and the implementation of early surgical intervention.39,48-50 Plaisier and colleagues17 demonstrated superior functional outcomes, quicker return to baseline function, and decreased length of stay in patients treated with early ORIF of their acetabular fractures. Other studies have shown that the benefits of early surgery include improved reduction quality and ease of reduction,51 as well as control of bleeding, pain relief, and mobilization of the patient.39 Another possible explanation for the decreased length of stay is the increased rate of discharge to other inpatient facilities, such as rehabilitation facilities, which was demonstrated in this study.
Continue to: Interestingly, male gender and younger age...
Interestingly, male gender and younger age were associated with operative management of the acetabular fracture. In contrast, there was a decreased likelihood of operative treatment among elderly patients and those patients with cardiac comorbidities. It is possible that the relationship we found between the likelihood of ORIF and age relates to the bimodal distribution of fractures, with higher energy and potentially more displaced fractures occurring in younger patients3-5 and lower energy fractures in the elderly.
In contrast to decreasing in-hospital days of care, there was a rise in the number of adverse events between 1990 (10.9%) and 2010 (37.6%). This can be partially attributed to the increased rates of blood transfusion, which was received by 9.5% of patients with acetabular fractures in the final study year. Additionally, surgical intervention was associated with increased adverse events in this study, and surgical intervention increased over the study period. Other factors that may have contributed to an increase in adverse events include an aging population,52 as advanced age was independently associated with higher odds of adverse events in this study.
Despite the strengths of using large, national databases for epidemiological research,53 this study has several limitations. Like all large databases, the NHDS is subject to error in coding and data entry.54 Additionally, the database only allows for 7 diagnostic codes and 4 procedure codes per entry. As a result, the prevalence of comorbid conditions and adverse events may be underreported.25 Moreover, the severity of a comorbid disease cannot be appreciated when dichotomously classified.55 Another limitation is that the database only provides inpatient data, so complications that arise after discharge, as well as follow-up data, are unknown. Furthermore, the results of this study are limited to practice patterns in the US from 1990 to 2010. This database does not provide injury mechanisms, so we cannot distinguish between high-energy and low-energy injuries. Lastly, analysis of the different types of acetabular fractures was not performed since classification of acetabular fractures cannot be assessed with ICD-9 codes.
CONCLUSION
This study is the largest epidemiologic analysis of acetabular fractures in the US and also provides predictors of in-hospital mortality. The incidence of acetabular fractures in the US is increasing, while mortality is decreasing. Identifying risk factors associated with poor outcomes has the potential to change treatment strategies, resource allocation, in-hospital monitoring, and discharge planning for this patient population.
This paper will be judged for the Resident Writer’s Award.
1. Laird A, Keating JF. Acetabular fractures: a 16-year prospective epidemiological study. J Bone Joint Surg Br. 2005;87(7):969-973. doi:10.1302/0301-620X.87B7.16017.
2. Geoghegan JM, Longdon EJ, Hassan K, Calthorpe D. Acetabular fractures in the UK. What are the numbers? Injury. 2007;38(3):329-333. doi:10.1016/j.injury.2006.09.015.
3. Tavakoli Darestani R, Kazemian G, Emami Moghaddam M, Manafi Rasi A, Alipour Y, Bagherian Lemraski MM. An unusual combination of acetabular and pelvic fracture: is this a new subtype of acetabular fracture? Trauma Mon. 2013;18(1):37-40. doi:10.5812/traumamon.9613.
4. McDonnell M, Schachter AK, Phillips DP, Liporace FA. Acetabular fracture through the triradiate cartilage after low-energy trauma. J Orthop Trauma. 2007;21(7):495-498. doi:10.1097/BOT.0b013e31812f67ff.
5. Giannoudis PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875-883. doi:10.1097/01.ta.0000242259.67486.15.
6. Gänsslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27 Suppl 1:S-A13-A20. doi:10.1016/S0020-1383(96)90106-0.
7. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645. doi:10.2106/00004623-199611000-00002.
8. Wright R, Barrett K, Christie MJ, Johnson KD. Acetabular fractures: long-term follow-up of open reduction and internal fixation. J Orthop Trauma. 1994;8(5):397-403. doi:10.1097/00005131-199410000-00005.
9. Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2-9.
10. Davarinos N, Ellanti P, Morris S, Mc Elwain JP. Epidemiology of pelvic and acetabular trauma in a Dublin tertiary hospital: a 10-year experience. Ir J Med Sci. 2012;181(2):243-246. doi:10.1007/s11845-011-0791-4.
11. Mauffrey C, Hao J, Cuellar DO 3rd, et al. The epidemiology and injury patterns of acetabular fractures: are the USA and China comparable? Clin Orthop Relat Res. 2014;472(11):3332-3337. doi:10.1007/s11999-014-3462-8.
12. Dente CJ, Feliciano DV, Rozycki GS, et al. The outcome of open pelvic fractures in the modern era. Am J Surg. 2005;190(6):830-835. doi:10.1016/j.amjsurg.2005.05.050.
13. Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1-13. doi:10.1016/j.injury.2004.05.029.
14. Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985-991. doi:10.1016/j.injury.2011.06.003.
15. Arroyo W, Nelson KJ, Belmont PJ Jr, Bader JO, Schoenfeld AJ. Pelvic trauma: what are the predictors of mortality and cardiac, venous thrombo-embolic and infectious complications following injury? Injury. 2013;44(12):1745-1749. doi:10.1016/j.injury.2013.08.007.
16. Flint L, Cryer HG. Pelvic fracture: the last 50 years. J Trauma. 2010;69(3):483-488. doi:10.1097/TA.0b013e3181ef9ce1.
17. Plaisier BR, Meldon SW, Super DM, Malangoni MA. Improved outcome after early fixation of acetabular fractures. Injury. 2000;31(2):81-84. doi:10.1016/S0020-1383(99)00233-8.
18. Centers for Disease Control and Prevention: National Hospital. Discharge survey. http://www.cdc.gov/nchs/nhds.htm. Accessed August 22, 2013.
19. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;(39):1-42.
20. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed June 18, 2013.
21. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627. doi:10.1007/s11999-008-0402-5.
22. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157. doi:10.1016/j.psym.2012.08.009.
23. Iezzoni LI, Daley J, Heeren T, et al. Using administrative data to screen hospitals for high complication rates. Inquiry. 1994;31(1):40-55.
24. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478-2486.
25. Bot AG, Menendez ME, Neuhaus V, Ring D. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(4):519-527. doi:10.1016/j.jse.2013.12.006.
26. United States Census Bureau. Population. https://www.census.gov/topics/population.html. Accessed December 4, 2012.
27. Porter SE, Schroeder AC, Dzugan SS, Graves ML, Zhang L, Russell GV. Acetabular fracture patterns and their associated injuries. J Orthop Trauma. 2008;22(3):165-170. doi:10.1097/BOT.0b013e318165918b.
28. Centers for Disease Control and Prevention. Motorcycle Crash-Related Data. https://www.cdc.gov/motorvehiclesafety/mc/index.html Accessed September 23, 2018
29. Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300. doi:10.3109/17453679209154786.
30. Melton LJ 3rd, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;155(155):43-47. doi:10.1097/00003086-198103000-00008.
31. al-Qahtani S, O'Connor G. Acetabular fractures before and after the introduction of seatbelt legislation. Can J Surg. 1996;39(4):317-320.
32. Ochs BG, Marintschev I, Hoyer H, et al. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2010;41(8):839-851. doi:10.1016/j.injury.2010.04.010.
33. Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151(151):81-106. doi:10.1055/s-2007-980136.
34. de Ridder VA, de Lange S, Kingma L, Hogervorst M. Results of 75 consecutive patients with an acetabular fracture. Clin Orthop Relat Res. 1994;305(305):53-57. doi:10.1097/00003086-199408000-00008.
35. Aho AJ, Isberg UK, Katevuo VK. Acetabular posterior wall fracture. 38 Cases followed for 5 years. Acta Orthop Scand. 1986;57(2):101-105. doi:10.3109/17453678609000878.
36. Stöckle U, Hoffmann R, Südkamp NP, Reindl R, Haas NP. Treatment of complex acetabular fractures through a modified extended iliofemoral approach. J Orthop Trauma. 2002;16(4):220-230. doi:10.1097/00005131-200204000-00002.
37. Tibbs BM, Kopar P, Dente CJ. Acetabular and isolated pelvic ring fractures: a comparison of initial assessment and outcome. Am Surg. 2008;74(6):538-541; discussion 541.
38. Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012;470(8):2090-2097. doi:10.1007/s11999-012-2276-9.
39. Vallier HA, Cureton BA, Ekstein C, Oldenburg FP, Wilber JH. Early definitive stabilization of unstable pelvis and acetabulum fractures reduces morbidity. J Trauma. 2010;69(3):677-684. doi:10.1097/TA.0b013e3181e50914.
40. Enninghorst N, Toth L, King KL, McDougall D, Mackenzie S, Balogh ZJ. Acute definitive internal fixation of pelvic ring fractures in polytrauma patients: a feasible option. J Trauma. 2010;68(4):935-941. doi:10.1097/TA.0b013e3181d27b48.
41. Buller LT, Best MJ, Quinnan SM. A nationwide analysis of pelvic ring fractures: incidence and trends in treatment, length of stay, and mortality. Geriatr Orthop Surg Rehabil. 2016;7(1):9-17. doi:10.1177/2151458515616250.
42. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191. doi:10.2106/JBJS.M.01126.
43. Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214.
44. Halvorson JJ, Lamothe J, Martin CR, et al. Combined acetabulum and pelvic ring injuries. J Am Acad Orthop Surg. 2014;22(5):304-314. doi:10.5435/JAAOS-22-05-304.
45. Osgood GM, Manson TT, O'Toole RV, Turen CH. Combined pelvic ring disruption and acetabular fracture: associated injury patterns in 40 patients. J Orthop Trauma. 2013;27(5):243-247. doi:10.1097/BOT.0b013e31826c2751.
46. Poole GV, Ward EF, Muakkassa FF. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg. 1991;213(6):532-538; discussion 538.
47. Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi:10.1097/00000658-200201000-00014.
48. Goldstein A, Phillips T, Sclafani SJ, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26(4):325-333. doi:10.1097/00005373-198604000-00004.
49. Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28-31. doi:10.1097/00005373-199101000-00006.
50. Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35(5):671-675; discussion 676.
51. Madhu R, Kotnis R, Al-Mousawi A, et al. Outcome of surgery for reconstruction of fractures of the acetabulum. The time dependent effect of delay. J Bone Joint Surg Br. 2006;88(9):1197-1203. doi:10.1302/0301-620X.88B9.17588.
52. Centers for Disease Control and Prevention. The State of Aging & Health in America 2013. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Accessed December 5, 2013.
53. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
54. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449. [author reply:450-451]. doi:10.1097/ALN.0b013e3181adf739.
55. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706. doi:10.1007/s11999-013-2876-z.
1. Laird A, Keating JF. Acetabular fractures: a 16-year prospective epidemiological study. J Bone Joint Surg Br. 2005;87(7):969-973. doi:10.1302/0301-620X.87B7.16017.
2. Geoghegan JM, Longdon EJ, Hassan K, Calthorpe D. Acetabular fractures in the UK. What are the numbers? Injury. 2007;38(3):329-333. doi:10.1016/j.injury.2006.09.015.
3. Tavakoli Darestani R, Kazemian G, Emami Moghaddam M, Manafi Rasi A, Alipour Y, Bagherian Lemraski MM. An unusual combination of acetabular and pelvic fracture: is this a new subtype of acetabular fracture? Trauma Mon. 2013;18(1):37-40. doi:10.5812/traumamon.9613.
4. McDonnell M, Schachter AK, Phillips DP, Liporace FA. Acetabular fracture through the triradiate cartilage after low-energy trauma. J Orthop Trauma. 2007;21(7):495-498. doi:10.1097/BOT.0b013e31812f67ff.
5. Giannoudis PV, Grotz MR, Tzioupis C, et al. Prevalence of pelvic fractures, associated injuries, and mortality: the United Kingdom perspective. J Trauma. 2007;63(4):875-883. doi:10.1097/01.ta.0000242259.67486.15.
6. Gänsslen A, Pohlemann T, Paul C, Lobenhoffer P, Tscherne H. Epidemiology of pelvic ring injuries. Injury. 1996;27 Suppl 1:S-A13-A20. doi:10.1016/S0020-1383(96)90106-0.
7. Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78(11):1632-1645. doi:10.2106/00004623-199611000-00002.
8. Wright R, Barrett K, Christie MJ, Johnson KD. Acetabular fractures: long-term follow-up of open reduction and internal fixation. J Orthop Trauma. 1994;8(5):397-403. doi:10.1097/00005131-199410000-00005.
9. Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2-9.
10. Davarinos N, Ellanti P, Morris S, Mc Elwain JP. Epidemiology of pelvic and acetabular trauma in a Dublin tertiary hospital: a 10-year experience. Ir J Med Sci. 2012;181(2):243-246. doi:10.1007/s11845-011-0791-4.
11. Mauffrey C, Hao J, Cuellar DO 3rd, et al. The epidemiology and injury patterns of acetabular fractures: are the USA and China comparable? Clin Orthop Relat Res. 2014;472(11):3332-3337. doi:10.1007/s11999-014-3462-8.
12. Dente CJ, Feliciano DV, Rozycki GS, et al. The outcome of open pelvic fractures in the modern era. Am J Surg. 2005;190(6):830-835. doi:10.1016/j.amjsurg.2005.05.050.
13. Grotz MR, Allami MK, Harwood P, Pape HC, Krettek C, Giannoudis PV. Open pelvic fractures: epidemiology, current concepts of management and outcome. Injury. 2005;36(1):1-13. doi:10.1016/j.injury.2004.05.029.
14. Gabbe BJ, de Steiger R, Esser M, Bucknill A, Russ MK, Cameron PA. Predictors of mortality following severe pelvic ring fracture: results of a population-based study. Injury. 2011;42(10):985-991. doi:10.1016/j.injury.2011.06.003.
15. Arroyo W, Nelson KJ, Belmont PJ Jr, Bader JO, Schoenfeld AJ. Pelvic trauma: what are the predictors of mortality and cardiac, venous thrombo-embolic and infectious complications following injury? Injury. 2013;44(12):1745-1749. doi:10.1016/j.injury.2013.08.007.
16. Flint L, Cryer HG. Pelvic fracture: the last 50 years. J Trauma. 2010;69(3):483-488. doi:10.1097/TA.0b013e3181ef9ce1.
17. Plaisier BR, Meldon SW, Super DM, Malangoni MA. Improved outcome after early fixation of acetabular fractures. Injury. 2000;31(2):81-84. doi:10.1016/S0020-1383(99)00233-8.
18. Centers for Disease Control and Prevention: National Hospital. Discharge survey. http://www.cdc.gov/nchs/nhds.htm. Accessed August 22, 2013.
19. Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;(39):1-42.
20. Centers for Disease Control and Prevention, National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed June 18, 2013.
21. Memtsoudis SG, González Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617-2627. doi:10.1007/s11999-008-0402-5.
22. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population-based study. Psychosomatics. 2013;54(2):149-157. doi:10.1016/j.psym.2012.08.009.
23. Iezzoni LI, Daley J, Heeren T, et al. Using administrative data to screen hospitals for high complication rates. Inquiry. 1994;31(1):40-55.
24. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993;270(20):2478-2486.
25. Bot AG, Menendez ME, Neuhaus V, Ring D. The influence of psychiatric comorbidity on perioperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(4):519-527. doi:10.1016/j.jse.2013.12.006.
26. United States Census Bureau. Population. https://www.census.gov/topics/population.html. Accessed December 4, 2012.
27. Porter SE, Schroeder AC, Dzugan SS, Graves ML, Zhang L, Russell GV. Acetabular fracture patterns and their associated injuries. J Orthop Trauma. 2008;22(3):165-170. doi:10.1097/BOT.0b013e318165918b.
28. Centers for Disease Control and Prevention. Motorcycle Crash-Related Data. https://www.cdc.gov/motorvehiclesafety/mc/index.html Accessed September 23, 2018
29. Ragnarsson B, Jacobsson B. Epidemiology of pelvic fractures in a Swedish county. Acta Orthop Scand. 1992;63(3):297-300. doi:10.3109/17453679209154786.
30. Melton LJ 3rd, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;155(155):43-47. doi:10.1097/00003086-198103000-00008.
31. al-Qahtani S, O'Connor G. Acetabular fractures before and after the introduction of seatbelt legislation. Can J Surg. 1996;39(4):317-320.
32. Ochs BG, Marintschev I, Hoyer H, et al. Changes in the treatment of acetabular fractures over 15 years: analysis of 1266 cases treated by the German Pelvic Multicentre Study Group (DAO/DGU). Injury. 2010;41(8):839-851. doi:10.1016/j.injury.2010.04.010.
33. Letournel E. Acetabulum fractures: classification and management. Clin Orthop Relat Res. 1980;151(151):81-106. doi:10.1055/s-2007-980136.
34. de Ridder VA, de Lange S, Kingma L, Hogervorst M. Results of 75 consecutive patients with an acetabular fracture. Clin Orthop Relat Res. 1994;305(305):53-57. doi:10.1097/00003086-199408000-00008.
35. Aho AJ, Isberg UK, Katevuo VK. Acetabular posterior wall fracture. 38 Cases followed for 5 years. Acta Orthop Scand. 1986;57(2):101-105. doi:10.3109/17453678609000878.
36. Stöckle U, Hoffmann R, Südkamp NP, Reindl R, Haas NP. Treatment of complex acetabular fractures through a modified extended iliofemoral approach. J Orthop Trauma. 2002;16(4):220-230. doi:10.1097/00005131-200204000-00002.
37. Tibbs BM, Kopar P, Dente CJ. Acetabular and isolated pelvic ring fractures: a comparison of initial assessment and outcome. Am Surg. 2008;74(6):538-541; discussion 541.
38. Holstein JH, Culemann U, Pohlemann T, Working Group Mortality in Pelvic Fracture Patients. What are predictors of mortality in patients with pelvic fractures? Clin Orthop Relat Res. 2012;470(8):2090-2097. doi:10.1007/s11999-012-2276-9.
39. Vallier HA, Cureton BA, Ekstein C, Oldenburg FP, Wilber JH. Early definitive stabilization of unstable pelvis and acetabulum fractures reduces morbidity. J Trauma. 2010;69(3):677-684. doi:10.1097/TA.0b013e3181e50914.
40. Enninghorst N, Toth L, King KL, McDougall D, Mackenzie S, Balogh ZJ. Acute definitive internal fixation of pelvic ring fractures in polytrauma patients: a feasible option. J Trauma. 2010;68(4):935-941. doi:10.1097/TA.0b013e3181d27b48.
41. Buller LT, Best MJ, Quinnan SM. A nationwide analysis of pelvic ring fractures: incidence and trends in treatment, length of stay, and mortality. Geriatr Orthop Surg Rehabil. 2016;7(1):9-17. doi:10.1177/2151458515616250.
42. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191. doi:10.2106/JBJS.M.01126.
43. Lo JC, Srinivasan S, Chandra M, et al. Trends in mortality following hip fracture in older women. Am J Manag Care. 2015;21(3):e206-e214.
44. Halvorson JJ, Lamothe J, Martin CR, et al. Combined acetabulum and pelvic ring injuries. J Am Acad Orthop Surg. 2014;22(5):304-314. doi:10.5435/JAAOS-22-05-304.
45. Osgood GM, Manson TT, O'Toole RV, Turen CH. Combined pelvic ring disruption and acetabular fracture: associated injury patterns in 40 patients. J Orthop Trauma. 2013;27(5):243-247. doi:10.1097/BOT.0b013e31826c2751.
46. Poole GV, Ward EF, Muakkassa FF. Pelvic fracture from major blunt trauma. Outcome is determined by associated injuries. Ann Surg. 1991;213(6):532-538; discussion 538.
47. Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi:10.1097/00000658-200201000-00014.
48. Goldstein A, Phillips T, Sclafani SJ, et al. Early open reduction and internal fixation of the disrupted pelvic ring. J Trauma. 1986;26(4):325-333. doi:10.1097/00005373-198604000-00004.
49. Latenser BA, Gentilello LM, Tarver AA, Thalgott JS, Batdorf JW. Improved outcome with early fixation of skeletally unstable pelvic fractures. J Trauma. 1991;31(1):28-31. doi:10.1097/00005373-199101000-00006.
50. Riemer BL, Butterfield SL, Diamond DL, et al. Acute mortality associated with injuries to the pelvic ring: the role of early patient mobilization and external fixation. J Trauma. 1993;35(5):671-675; discussion 676.
51. Madhu R, Kotnis R, Al-Mousawi A, et al. Outcome of surgery for reconstruction of fractures of the acetabulum. The time dependent effect of delay. J Bone Joint Surg Br. 2006;88(9):1197-1203. doi:10.1302/0301-620X.88B9.17588.
52. Centers for Disease Control and Prevention. The State of Aging & Health in America 2013. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Accessed December 5, 2013.
53. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
54. Memtsoudis SG. Limitations associated with the analysis of data from administrative databases. Anesthesiology. 2009;111(2):449. [author reply:450-451]. doi:10.1097/ALN.0b013e3181adf739.
55. Neuhaus V, Swellengrebel CH, Bossen JK, Ring D. What are the factors influencing outcome among patients admitted to a hospital with a proximal humeral fracture? Clin Orthop Relat Res. 2013;471(5):1698-1706. doi:10.1007/s11999-013-2876-z.
TAKE-HOME POINTS
- The population-adjusted incidence of acetabular fractures increased between 1990 and 2010. Mortality associated with acetabular fractures decreased from 5.9% to 0.4% between 1990 and 2010.
- The proportion of patients treated with ORIF increased from 12.6% to 20.4% between 1990 and 2010.
- The average in-patient hospital length of stay following acetabular fracture decreased from 17.0 to 10.4 days between 1990 and 2010.
- ORIF is associated with the lowest odds of mortality following acetabular fracture.
A Three-View Radiographic Approach to Femoroacetabular Impingement
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40°may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140°may indicate coxa valga
<130°may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.
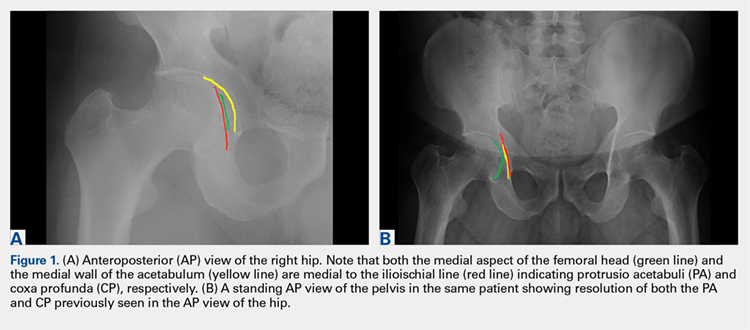
A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40°may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140°may indicate coxa valga
<130°may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40°may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140°may indicate coxa valga
<130°may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
TAKE-HOME POINTS
- FAI is a frequently unrecognized cause of hip pain in adolescents and young adults.
- Understanding the potential sites of impingement and the specific radiographs to visualize these sites can help avoid unnecessary imaging and delayed diagnosis.
- A simple radiographic approach consisting of a standing AP view of the pelvis, a cross-table lateral view, and a false profile view is often a sufficient screening tool.
- While we tend to classify FAI into cam and pincer osseous bumps, alterations in hip dynamics can result in functional impingement even in the absence of the osseous bumps.
- Advanced imaging is reserved for patients who have failed conservative management or are considering surgical intervention.
Time-to-Surgery for Definitive Fixation of Hip Fractures: A Look at Outcomes Based Upon Delay
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.
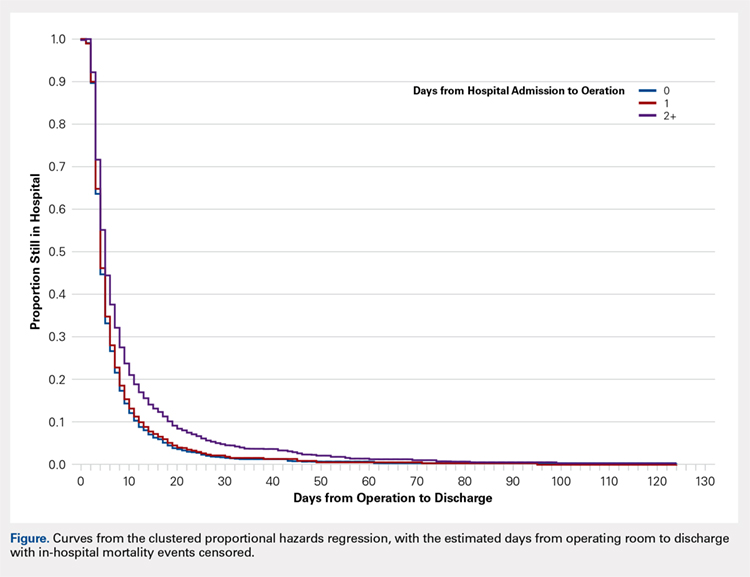
All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).
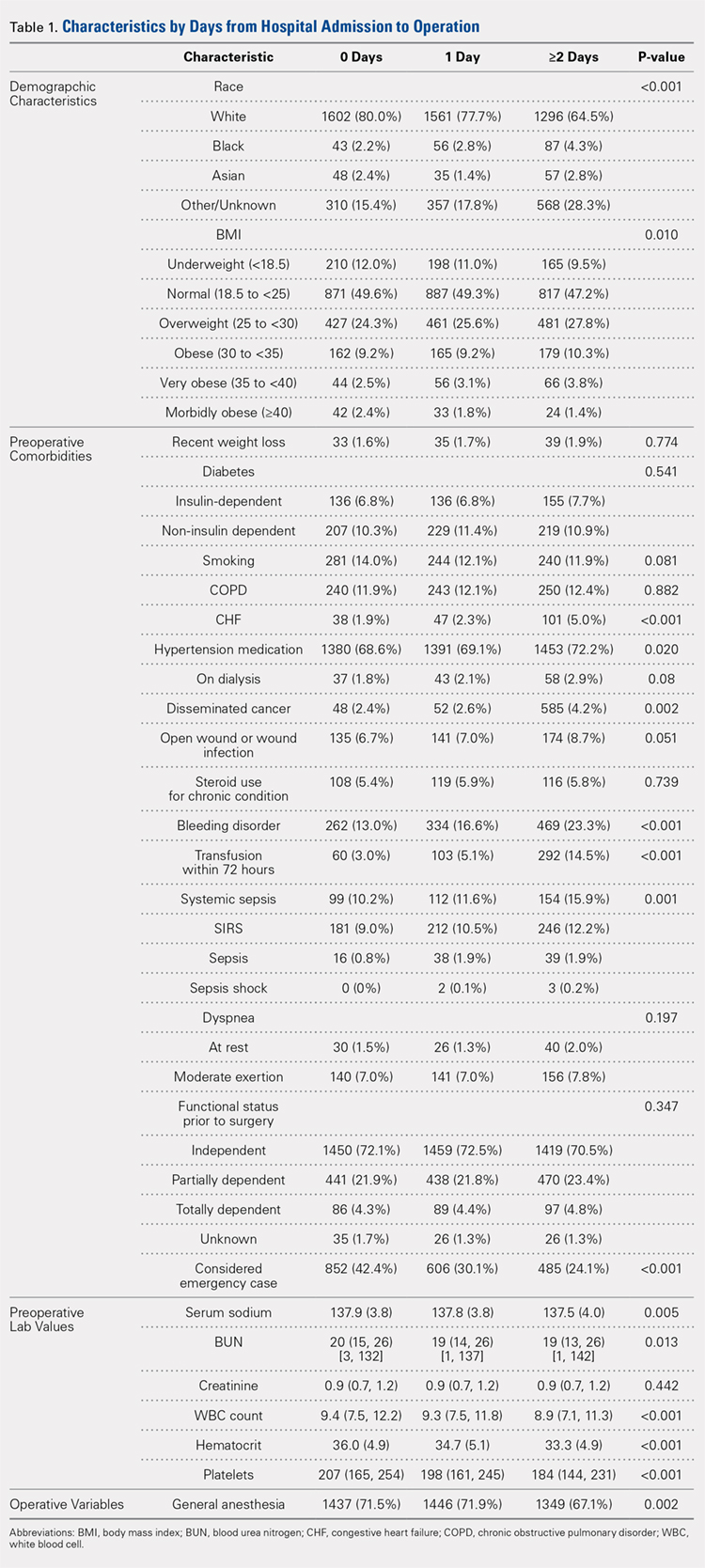
The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.

All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).

The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
ABSTRACT
The morbidity and mortality after hip fracture in the elderly are influenced by non-modifiable comorbidities. Time-to-surgery is a modifiable factor that may play a role in postoperative morbidity. This study investigates the outcomes and complications in the elderly hip fracture surgery as a function of time-to-surgery.
Using the American College of Surgeons-National Surgical Quality Improvement Program data from 2011 to 2012, a study population was generated using the Current Procedural Terminology codes for percutaneous or open treatment of femoral neck fractures (27235, 27236) and fixation with a screw and side plate or intramedullary fixation (27244, 27245) for peritrochanteric fractures. Three time-to-surgery groups (<24 hours to surgical intervention, 24-48 hours, and >48 hours) were created and matched for surgery type, sex, age, and American Society of Anesthesiologists class. Time-to-surgery was then studied for its effect on the post-surgical outcomes using the adjusted regression modeling.
A study population of 6036 hip fractures was created, and 2012 patients were assigned to each matched time-to-surgery group. The unadjusted models showed that the earlier surgical intervention groups (<24 hours and 24-48 hours) exhibited a lower overall complication rate (P = .034) compared with the group waiting for surgery >48 hours. The unadjusted mortality rates increased with delay to surgical intervention (P = .039). Time-to-surgery caused no effect on the return to the operating room rate (P = .554) nor readmission rate (P = .285). Compared with other time-to-surgeries, the time-to-surgery of >48 hours was associated with prolonged total hospital length of stay (10.9 days) (P < .001) and a longer surgery-to-discharge time (hazard ratio, 95% confidence interval: 0.74, 0.69-0.79) (P < .001). Adjusted analyses showed no time-to-surgery related difference in complications (P = .143) but presented an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001).
Timeliness of surgical intervention in a comorbidity-adjusted population of elderly hip fracture patients causes no effect on the overall complications, readmissions, nor 30-day mortality. However, time-to-surgery of >48 hours is associated with costly increase in the total length of stay, including an increased post-surgery-to-discharge time.
Continue to: Despite the best efforts to optimize surgical care...
Despite the best efforts to optimize surgical care and postoperative rehabilitation following hip fracture, elderly patients feature alarmingly high in-hospital and 1-year mortality rates of 4.35% to 9.2%1-4 and 36%,5 respectively. Those who survive are unlikely to return to independent living, with only 17% of the patients following hip fracture being able to walk independently 6 months postoperatively, and 12% being able to climb stairs6. Possibly, these poor outcomes reflect a preoperative medical comorbidity burden rather than a measure of medical or surgical quality. Given the absence of consensus regarding optimal time-to-surgery, treating physicians often opt to delay surgical intervention for the purposes of medically optimizing highly comorbid patients without significant data to suggest clinical benefit of such practice.
Numerous investigators have attempted to identify the modifiable risk factors for complication after surgical care of elderly hip fracture patients. However, consensus guidelines of care are missing. This condition is largely due to the difficulties in effectively modifying preoperative demographic and medical comorbidities on a semi-urgent basis. However, timeliness to surgery is one area for study that the care team can affect. Although time-to-surgery is dependent on multiple factors, including time of presentation, day of week of admission, difficulties with scheduling, and administrative delays, the care team plays a role in hastening or retarding time-to-surgery. Several studies have considered various time cut-offs (24, 48, 72, and 120 hours) to define early intervention, but none have defined a specific role for early or delayed surgery. Several investigators have discovered a positive association between delayed time-to-surgery and mortality;4,8-14 however, the most rigorously conducted studies that stringently control for preoperative comorbidities and demographics conclude that variance in time-to-surgery causes no effect on the in-hospital or 1-year mortality risk.1-3,15-18
Other investigators have shown that with early surgical intervention for hip fracture, patients experience shorter hospital stays,1,3,16,17,19-22 less days in pain,19 decreased risk of decubitus ulcers,15,17,19,22 and an increased likelihood of independence following fracture,22-25 regardless of preoperative medical status. Despite this evidence of improved outcomes with early surgery, 40% to 54% of hip fracture patients in the United States experience surgical delays of more than 24 to 48 hours. Additionally, with the recent (2013) national estimates of cost per day spent in the hospital falling between $1791 to $2289,26 minimizing the days spent in the hospital would likely lead to significant cost-savings, presuming no adverse effect on health-related outcomes. To this end, we hypothesize that the value (outcomes per associated cost)7 of hip fracture surgical care can be positively influenced by minimizing surgical wait-times. We assessed the effect of early surgical intervention, within 24 or 48 hours of presentation, on 30-day mortality, postoperative morbidity, hospital length of stay, and readmission rates in a comorbidity-adjusted population from a nationally representative cohort.
Continue to: METHODS AND MATERIALS...
METHODS AND MATERIALS
This study used the data from the American College of Surgeon-National Surgical Quality Improvement Program (ACS-NSQIP) database. With over 258 participating hospitals, this database has been widely used to identify national trends in various surgical specialties.27-34 The database includes information from participants in 43 states with hospitals ranging from rural community hospitals to large academic centers. Each site employs surgical clinical reviewers who are rigorously trained to collect data through chart review and discussion with the treating surgeon and/or patient,35 allowing for the use of robust and quality data with proven inter-rater reliability.36,37
Using the 2011 to 2012 NSQIP database, we used primary Current Procedural Terminology codes to identify all patients who underwent percutaneous (27235) or open (27236) fixation of femoral neck fractures; and fixation with a screw and side plate (27244) or intramedullary fixation (27245) for peritrochanteric fractures. The sample was divided into 3 time-to-surgery groups (<24 hours from presentation to surgery, 24-48 hours, and >48 hours) which were matched for fracture type (femoral neck or peritrochanteric), sex, age (under 75 years or ≥75 years), and American Society of Anesthesiologists (ASA) class used as a surrogate for severity of medical infirmary. The subjects were randomly matched 1:1:1 to create 3 statistically equivalent time-to-surgery groups using Proc SurveySelect (SAS version 9.2, SAS Institute).
Generalized linear models using logit link function for binary variables and identity link function for normally distributed characteristics were used to compare the 3 time-to-surgery groups. Descriptive statistics are presented as counts and percentages or least-square means with standard deviations. Preoperative lab values that were not normally distributed were log transformed and presented in their original scales with median values and 25th to 75th percentiles. Outcomes were similarly modeled.
Total hospital stay was modeled with a negative binomial distribution. Proportional hazards models were used to model the time from operating room (OR) to discharge, censoring patients who died before discharge, with results presented as hazard ratios (HR) and 95% confidence intervals (CI) (Figure). The assumption of the proportional hazards was tested using a Wald test. Using this model, a HR of <1 denotes a longer postoperative hospital stay, as a longer hospital stay decreases the “risk” for discharge.

All models were adjusted for confounders, including race, body mass index (BMI), hypertension, chronic obstructive pulmonary disease, cancer, bleeding disorders, transfusion within 72 hours before surgery, preoperative levels of creatinine, platelet count, white blood cells (WBCs), hematocrit anesthesia type, and wound infection. These covariates were selected based upon their observed relationship to the studied outcomes and time-to-surgery groups, and were evaluated across the models for all outcomes for consistency and clarity. All statistical analyses were run at a type I error rate of 5% and performed in SAS version 9.2 software.
Continue to: RESULTS...
RESULTS
A study population of 6036 hip fractures was identified and divided into 3 groups of 2012 subjects each based upon time-to-surgery. The groups were successfully matched for surgery type, age (≥75 years old), gender, and ASA class. In each group, 594 of the 2012 (29.5%) patients were male, 1525 (75.8%) were ≥75 years of age, 9 (.5%) were ASA Class I, 269 (13.4%) were ASA Class II, 1424 (70.8%) were ASA class III, and 309 (15.4%) were ASA class IV.
Significant differences in preoperative comorbidity burden and preoperative lab values were identified between the 3 cohorts. Increased time-to-surgery was associated with differences in race (P < .001), elevated BMI (P = .010), higher rates of congestive heart failure (P < .001), hypertension medication (P = .020), bleeding disorders (P < .001), blood transfusion within 72 hours of surgery (P < .001), and systemic sepsis (P = .001). Delay to surgery was also associated with lower preoperative sodium (P = .005), blood urea nitrogen (P = .013), serum WBC (P < .001), hematocrit (P < .001), and platelets (P < .001) (Table 1).

The unadjusted analyses revealed no association between time-to-surgery and return to OR (P = .554) nor readmission (P = .285). However, increasing time-to-surgery was associated with an increase in overall complications (P = .034), total length of hospital stay (P < .001), and 30-day mortality (P = .039) (Table 2).
Table 2. Estimated Event Rates from Matched Cohorts (Unadjusted)
| Time From Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 15.30% | 15.30% | 17.90% | 0.034 |
Total length of stay | 5.4 | 6.7 | 10.9 | <0.001 |
(mean days, 95% confidence interval) | (5.2, 5.7) | (6.5, 7.0) | (10.3, 11.5) | |
Time from OR to discharge | -ref- | 0.96 | 0.74 | <0.001 |
(Hazard ratio) | (0.90,1.02) | (0.69, 0.79) | ||
Return to OR | 2.40% | 2.40% | 2.00% | 0.554 |
Readmission | 9.60% | 8.40% | 8.30% | 0.285 |
30-day mortality rate | 5.80% | 5.30% | 7.20% | 0.039 |
Abbreviation: OR, operating room.
The adjusted analysis controlling for preoperative demographic and comorbidity variables revealed trends toward the increased overall complications and 30-day mortality with increased time-to-surgery; these trends showed no statistical significance (P = .143 and P = .08). No statistical relationship was observed between return to OR nor readmission and time-to-surgery. Increasing time-to-surgery remained significantly associated with the increased total length of hospital stay (P < .001). The adjusted analysis also revealed that the delay of >48 hours in time-to-surgery resulted in a longer surgery-to-discharge time (P < .001) (Table 3). No evidence of violation of the proportional hazards assumption was observed in the unadjusted nor adjusted clustered proportional hazards models (Wald test, P = .27 and P = .25, respectively).
Table 3. Estimated Event Rates from Matched Cohorts (Adjusteda)
| Time from Presentation to Definitive Fixation | |||
Outcomes | <24 hours | 24-48 hours | >48 hours | P-value |
Overall complication rate | 11.70% | 10.70% | 12.60% | 0.143 |
Total length of stay | 4.2 | 5.1 | 7.6 | <0.001 |
(mean days, 95% confidence interval) | (4.0, 4.5) | (4.8, 5.5) | (7.1, 8.3) | |
Time from OR to discharge | -ref- | 1.03 | 0.87 | <0.001 |
(Hazard ratio) | (0.97, 1.09) | (0.81, 0.92) | ||
Return to OR | 2.10% | 2.10% | 1.60% | 0.541 |
Readmission | 7.20% | 6.40% | 6.00% | 0.304 |
30-day mortality rate | 4.20% | 3.70% | 5.20% | 0.08 |
aModel adjusted for race, hypertension medication, cancer, bleeding disorders, transfusion within 72 hours before surgery, emergency status, wound infection, anesthesia type (general), body mass index (18.5-25), history of chronic obstructive pulmonary disease, and preoperative levels of creatinine, platelet count, white blood cell count, and hematocrit.
Continue to: DISCUSSION...
DISCUSSION
Previous research has demonstrated an association between age,3,4,25 comorbidity burden,1,3,25 gender,3,4 and ASA class4,18,21 with outcomes following hip fractures and serves as the basis of our matched analysis statistical methodology in assessing the effect of time-to-surgery on the outcome following hip fracture surgery. Prior investigators have also established the positive correlation between increased preoperative comorbidity burden and delay in time-to-surgery.10,15 This finding was confirmed in our unadjusted comparison of 3 time-to-surgery groups. However, prior investigations have not established a clear association between time-to-surgical intervention and postoperative morbidity and mortality.1,15,16,18,20,38 This study utilized a nationally representative dataset known for its data integrity and from which 6036 patients with surgically treated hip fractures, matched for surgery type, age, gender, and ASA class (a surrogate for severity of medical infirmary), were studied using adjusted regression modeling to afford an isolated statistical assessment of the effect of time-to-surgery on outcomes following hip fracture surgery.
Despite a large sample size and rigorous statistical methodology, for many outcome measures, our results show no support for the early or late operative intervention following hip fracture. We found no difference in 30-day mortality, readmission rate, nor total complication rate between the 3 time-to-surgery cohorts. This result indicates that the care of elderly patients following hip fracture is inherently complicated and that perioperative complication risk is probably only modestly modifiable by best medical practices, including optimizing time from clinical presentation to surgery.
As expected, patients who experienced longer delays from presentation to surgery were on average, more comorbid and more likely to yield abnormal preoperative lab values. However, in the adjusted analysis, delay in time-to-surgery, presumably for medical management, was not found to be associated with improved outcomes. In the same adjusted analysis, we uniquely identified that in the patients whose surgeries were delayed for more than 48 hours, the time from surgery-to-discharge was significantly increased. As a result, these patients spent extra days in the hospital both preoperatively and postoperatively, but without any corollary improvement in the outcomes.
Continue to: Recent estimates of the cost of hospital admission...
Recent estimates of the cost of hospital admission is approximated nationally at $2000/day.26 Although our data fail to support the formal cost-analysis of the effect of time-to-surgery in hip fracture care, a simple value-based analysis indicates that quality is preserved (no difference in outcome), whereas costly hospital days are eliminated with earlier surgery. The value in elderly hip fracture care. defined as the outcomes relative to the costs,7 is ultimately optimized by earlier time-to-surgery.
Although using a large, multi-institutional database is advantageous for finding population-based trends that are representative of a large cohort, using the ACS-NSQIP database features its limitations. Our analysis was limited to the defined scope of NSQIP and nature of the injury, whereas root cause for delay was not available for study. We were unable to identify which patients were delayed for administrative reasons or surgical convenience and which were delayed for medical optimization. Participation in the ACS-NSQIP database is voluntary, and no randomized hospital sampling was conducted. Participating hospitals were de-identified in the database. As expected, we were unable to identify the specific institution-based hip fracture protocols that may affect the outcomes following treatment for these fractures. Further, socioeconomic information and payer-status are unavailable for the study. Additionally, observations are limited to 30 days postoperative, and we cannot comment on longer-term outcomes. Finally, discharge disposition and functional outcome data are not represented, and we were unable to correlate time-to-surgery and functional recovery. However, previous studies have established that delay in time-to-surgery following hip fractures is negatively correlated with functional outcomes.22-25
Nevertheless, the ACS-NSQIP database remains one of the largest American surgical databases available, and includes care centers from nearly every state with variable demographics including rural, urban, and academic centers. The ACS performs broad-based inter-rater reliability audits on every participating site and has found an overall disagreement rate of only 1.8%. As such, although discrepancies exist between the complete patient chart and the data entered, the data found in the ACS-NSQIP database are reliable and considered a valid source of study.34,35 The large sample size, quality of data collection, wide geographic representation, and varied hospital types within the dataset possibly make our findings relevant in the majority of American healthcare settings.
CONCLUSION
This study demonstrates an associated increased length of hospital stay, including the increased time from surgery-to-discharge, in patients with hip fractures whose surgical intervention is delayed for >48 hours after presentation. Given the prior evidence that early surgical intervention improves the functional outcomes and the current evidence that surgical delay for any cause increases costly hospital length of stay without corollary improvement in the outcomes, a value-based assessment of hip fracture care argues for early surgical intervention whenever possible. Our findings should inform physician, institution, and policy maker value-based decision making regarding the best practices in geriatric hip fracture care.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
1. Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233. doi:10.7326/0003-4819-155-4-201108160-00006.
2. Verbeek DO, Ponsen KJ, Goslings JC, Heetveld MJ. Effect of surgical delay on outcome in hip fracture patients: a retrospective multivariate analysis of 192 patients. Int Orthop. 2008;32(1):13-18. doi:10.1007/s00264-006-0290-9.
3. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922-927. doi:10.1302/0301-620X.91B7.22446.
4. Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra-early surgery in hip fracture patients improves survival. Injury. 2013;44(6):726-729. doi:10.1016/j.injury.2012.08.025.
5. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525. doi:10.1056/NEJM199606063342307.
6. Marottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. J Am Geriatr Soc. 1992;40(9):861-866. doi:10.1111/j.1532-5415.1992.tb01980.x.
7. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477-2481. doi:10.1056/NEJMp1011024.
8. Radcliff TA, Henderson WG, Stoner TJ, Khuri SF, Dohm M, Hutt E. Patient risk factors, operative care, and outcomes among older community-dwelling male veterans with hip fracture. J Bone Joint Surg Am. 2008;90(1):34-42. doi:10.2106/JBJS.G.00065.
9. Novack V, Jotkowitz A, Etzion O, Porath A. Does delay in surgery after hip fracture lead to worse outcomes? A multicenter survey. Int J Qual Health Care. 2007;19(3):170-176. doi:10.1093/intqhc/mzm003.
10. Weller I, Wai EK, Jaglal S, Kreder HJ. The effect of hospital type and surgical delay on mortality after surgery for hip fracture. J Bone Joint Surg Br. 2005;87(3):361-366. doi:10.1302/0301-620X.87B3.15300.
11. Moran CG, Wenn RT, Sikand M, Taylor AM. Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am. 2005;87(3):483-489. doi:10.2106/JBJS.D.01796.
12. Holt G, Smith R, Duncan K, McKeown DW. Does delay to theatre for medical reasons affect the peri-operative mortality in patients with a fracture of the hip? J Bone Joint Surg Br. 2010;92(6):835-841. doi:10.1302/0301-620X.92B6.24463.
13. Pioli G, Lauretani F, Davoli ML, et al. Older people with hip fracture and IADL disability require earlier surgery. J Gerontol A Biol Sci Med Sci. 2012;67(11):1272-1277. doi:10.1093/gerona/gls097.
14. Mackenzie DG, Wild S, Muir R. Mortality associated with delay in operation after hip fracture: Scottish data provide additional information. BMJ. 2006;332(7549):1093. doi:10.1136/bmj.332.7549.1093.
15. Grimes JP, Gregory PM, Noveck H, Butler MS, Carson JL. The effects of time-to-surgery on mortality and morbidity in patients following hip fracture. Am J Med. 2002;112(9):702-709. doi:10.1016/S0002-9343(02)01119-1.
16. Majumdar SR, Beaupre LA, Johnston DW, Dick DA, Cinats JG, Jiang HX. Lack of association between mortality and timing of surgical fixation in elderly patients with hip fracture: results of a retrospective population-based cohort study. Med Care. 2006;44(6):552-559. doi:10.1097/01.mlr.0000215812.13720.2e.
17. Hommel A, Ulander K, Bjorkelund KB, Norrman PO, Wingstrand H, Thorngren KG. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury. 2008;39(10):1164-1174. doi:10.1016/j.injury.2008.01.048.
18. Rae HC, Harris IA, McEvoy L, Todorova T. Delay to surgery and mortality after hip fracture. ANZ J Surg. 2007;77(10):889-891. doi:10.1111/j.1445-2197.2007.04267.x.
19. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743. doi:10.1001/jama.291.14.1738.
20. Bergeron E, Lavoie A, Moore L, et al. Is the delay to surgery for isolated hip fracture predictive of outcome in efficient systems? J Trauma. 2006;60(4):753-757. doi:10.1097/01.ta.0000214649.53190.2a.
21. Siegmeth AW, Gurusamy K, Parker MJ. Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur. J Bone Joint Surg Br. 2005;87(8):1123-1126. doi:10.1302/0301-620X.87B8.16357.
22. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436-1442. doi:10.2106/JBJS.G.00890.
23. Hoenig H, Rubenstein LV, Sloane R, Horner R, Kahn K. What is the role of timing in the surgical and rehabilitative care of community-dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157(5):513-520.
24. Doruk H, Mas MR, Yildiz C, Sonmez A, Kýrdemir V. The effect of the timing of hip fracture surgery on the activity of daily living and mortality in elderly. Arch Gerontol Geriatr. 2004;39(2):179-185. doi:10.1016/j.archger.2004.03.004.
25. Yonezawa T, Yamazaki K, Atsumi T, Obara S. Influence of the timing of surgery on mortality and activity of hip fracture in elderly patients. J Orthop Sci Off J Jpn Orthop Assoc. 2009;14(5):566-573. doi:10.1007/s00776-009-1380-5.
26. Henry J Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed March 14, 2013.
27. Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2,911 patients from the national surgical quality improvement program. J Am Coll Surg. 2011;213(3):370-378. doi:10.1016/j.jamcollsurg.2011.05.019.
28. Davis SS Jr, Husain FA, Lin E, Nandipati KC, Perez S, Sweeney JF. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216(1):96-104. doi:10.1016/j.jamcollsurg.2012.08.014.
29. Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216(2):229-238. doi:10.1016/j.jamcollsurg.2012.11.003.
30. Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-854. doi:10.1016/j.jamcollsurg.2011.01.005.
31. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199. doi:10.2106/JBJS.K.01682.
32. Rao RD. Risk factors for complications and mortality after spine surgery assessed with the NSQIP database: where do we go from here? Commentary on an article by Andrew J Schoenfeld, MD, et al.: "Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program". J Bone Joint Surg Am. 2011;93(17):e101:(101-102). doi:10.2106/JBJS.K.00786.
33. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/JBJS.I.00735.
34. Tsilimparis N, Perez S, Dayama A, Ricotta JJ 2nd. Age-stratified results from 20,095 aortoiliac aneurysm repairs: should we approach octogenarians and nonagenarians differently? J Am Coll Surg. 2012;215(5):690-701. doi:10.1016/j.jamcollsurg.2012.06.411.
35. ACS National Surgical Quality Improvement Program. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip?. Accessed March 14, 2013.
36. Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg. 2009;198(5 Suppl):S19-S27. doi:10.1016/j.amjsurg.2009.07.025.
37. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
38. Khan SK, Kalra S, Khanna A, Thiruvengada MM, Parker MJ. Timing of surgery for hip fractures: a systematic review of 52 published studies involving 291,413 patients. Injury. 2009;40(7):692-697. doi:10.1016/j.injury.2009.01.010.
TAKE-HOME POINTS
- Time-to-surgery for definitive fixation of hip fractures is a modifiable risk factor.
- This study fails to demonstrate a benefit in delaying surgery for medical optimization as there were no time-to-surgery related differences in complications (P = 1.43).
- Delay in definitive surgery results in an increase in the total length of stay (P < .001) and surgery-to-discharge time (P < .001) without an improvement in overall complications, readmission or 30-day mortality rates.
- Despite numerous investigations, there are no consensus guidelines to decrease complications and mortality rates following hip fracture surgery.
- ACS-NSQIP database is a reliable and validated database.
High Body Mass Index is Related to Increased Perioperative Complications After Periacetabular Osteotomy
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1
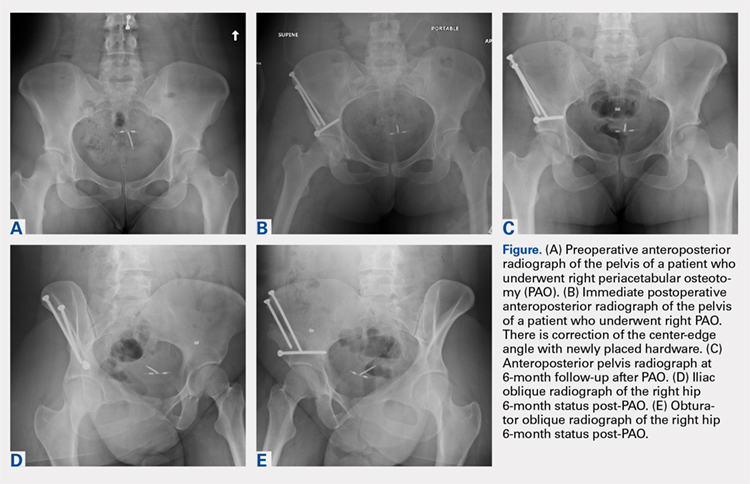
There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1

There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
ABSTRACT
The purpose of this study is to determine the relationship of body mass index (BMI), age, smoking status, and other comorbid conditions to the rate and type of complications occurring in the perioperative period following periacetabular osteotomy. A retrospective review was performed on 80 hips to determine demographic information as well as pre- and postoperative pain scores, center-edge angle, Tönnis angle, intraoperative blood loss, and perioperative complications within 90 days of surgery. Patients were placed into high- (>30) and low- (<30) BMI groups to determine any correlation between complications and BMI. The high-BMI group had a significantly greater rate of perioperative complications than the low-BMI group (30% vs 8%) and, correspondingly, patients with complications had significantly higher BMI than those without (30.9 ± 9.5, 26.2 ± 5.6) (P = .03). Center-edge angle and Tönnis angle were corrected in both groups. Improvement in postoperative pain scores and radiographically measured acetabular correction can be achieved in high- and low-BMI patients. High-BMI patients have a higher rate of perioperative wound complications.
Continue to: The Bernese periacetabular osteotomy...
The Bernese periacetabular osteotomy (PAO) has become a widely used procedure for hip preservation in adolescent and young adult patients with symptomatic anatomic aberrancies of the acetabulum due to developmental hip dysplasia, trauma, infection, femoroacetabular impingement, and other causes.1-6 Acetabular dysplasia is one of the most common causes of secondary osteoarthritis, and the goal of PAO is to slow or halt the progression of arthrosis to prolong or potentially eliminate the need for total hip arthroplasty while relieving pain and increasing function and activity.1,7,8
The PAO involves realigning the acetabulum to improve anterior and lateral coverage of the femoral head, acetabular anteversion, and medicalization of the joint.5,6 It is preferred over other described acetabular osteotomies due to its inherent stability given that the posterior column is not violated.3,5,6,9 Since its initial description in 1988,5 short-, medium- and long-term outcomes have been reported with excellent patient satisfaction and function.2,7,10-15 The radiographic, functional, and patient satisfaction outcomes are excellent; therefore, this has become an accepted form of treatment for acetabular dysplasia.16 Additional procedures, such as hip arthroscopy, have also been combined with PAO to treat intra-articular pathologies without open arthrotomy.17 Several studies have evaluated preoperative radiographic factors, such as Tönnis grade, previous surgeries, and morphology of the hip; as well as demographic factors, such as age, body mass index (BMI), comorbid diseases, and activity level, which seem to play a role in the final outcome.11,18,19 This work has advanced our understanding and allowed surgeons to apply selection criteria to improve patient outcomes.
There are multiple reported complications of the PAO procedure, including infection,2 wound dehiscence,20 periacetabular fracture,21 intra-articular extension of the osteotomy,22 excessive acetabular retroversion,23,24 hardware failure, femoral or sciatic nerve palsy,25 heterotopic ossification, prominent hardware, deep vein thrombosis or pulmonary embolism,26 osteonecrosis of the femoral head or acetabulum,24 non-union,24 intrapelvic bleeding,24 incisional hernia,27 lateral femoral cutaneous nerve palsy,20,28 and reflex sympathetic dystrophy.1,2,29 There are also several studies reporting a learning curve phenomenon, in which the proportion of complications is higher in the initial series of surgeries performed by each specific surgeon.22,20,29
Despite the widely reported short-, medium-, and long-term results of this treatment, no study thus far has attempted to correlate preoperative patient factors with early perioperative outcomes and complications. This information would be useful in patient counseling and decision making in the early postoperative period. Therefore, the purpose of this study is to analyze data from the perioperative period in patients who have undergone the PAO performed by a single surgeon at our institution to determine any correlation between patient characteristics such as age, comorbid disease, hip pathologic diagnosis, BMI, or previous procedures and perioperative complications occurring within the first 90 days.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a search was performed on the basis of operative report Current Procedural Terminology (CPT) codes for all patients who underwent PAO performed by a single surgeon between 2005 and 2013. Patients were included if they had PAO surgery with at least 90 days of follow-up. There was no exclusion for age, previous surgery, or underlying hip or medical diagnosis. A retrospective review of electronic medical records and radiographic imaging was undertaken to determine pre- and postoperative demographic information, pain scores, center-edge angle of Weiberg and Tönnis angles, intraoperative estimated blood loss, and all perioperative complications. Weight and height were recorded from the immediate preoperative visit and measured in kilograms (kg) and meters (m), respectively. BMI was derived from these measurements. Pain was assessed via visual analog scale at the preoperative visit as well as at 12 weeks postoperatively. Preoperative and 12-week postoperative Tönnis and center-edge angles were measured by a single orthopedic surgeon. All radiographs were deemed adequate in position and penetration for measurement of these parameters. Evidence of osteonecrosis of the femoral head was evaluated on all postoperative radiographs within this perioperative period. Estimated blood loss was established by review of operative records and anesthesia notes.
Perioperative complications were classified using the Clavien-Dindo system, which has previously been validated for use in hip preservation surgery.30 This includes 5 grades of complications based on the treatment needed and severity of resulting long-term disability. Grade I complications do not require any change in the postoperative course and were therefore left out of our statistical analysis. Examples include symptomatic hardware, mild heterotopic ossification, and iliopsoas tendonitis. Grade II complications are those that require a change in outpatient management, such as delayed wound healing, superficial infection, transient nerve palsy, violation of the posterior column, and intra-articular osteotomy. Grade III complications require invasive or surgical treatment but leave the patient with no long-term disability. Examples include wound dehiscence, hematoma or infection necessitating surgical débridement and irrigation, and revision of the osteotomy due to hardware malposition or hip instability. Grade IV complications involve both surgery and long-term disability. Grade IV complications applicable to hip preservation surgery are osteonecrosis, permanent nerve injury, major vascular injury, or pulmonary embolism. A grade V complication is death.
For analysis and correlation between demographics and perioperative outcomes and complications, patients were grouped into several groups for comparison. Low (<30) vs high (>30) BMI, smokers vs non-smokers, diabetic vs non-diabetic patients, and those who had previous surgery vs those who did not were compared. A two-tailed t test was used for normally distributed continuous variables and a Mann-Whitney U test, for non-parametric data to compare postoperative radiographic correction, pain scores, and complication rates between each of these groups.
The operative technique for PAO as described by Ganz and colleagues5 in 1988 was utilized in all patients. When preoperative imaging showed evidence of labral pathology, a Cam lesion of the femoral head and neck junction, abnormal proximal femoral anatomy, osteonecrosis of the femoral head, or an os acetabulum, a concomitant procedure was performed. Seventeen patients underwent débridement of a Cam lesion noted to be impinging following PAO. Seventeen patients underwent labral débridement and 4 underwent labral repair. Four patients underwent intertrochanteric osteotomy and 1 underwent greater trochanteric slide. Two patients underwent free-vascularized fibular grafting to the ipsilateral femoral head and 5 underwent fixation of an os acetabulum.
Continue to: RESULTS...
RESULTS
A total of 80 hips in 73 patients underwent PAO with adequate perioperative follow-up and records in the inclusion period. Figures A-E represent a patient pre-procedure, immediately post procedure, and 6 months after successful PAO. The average age was 27.5 years (12.8-43.6 years), and the average BMI was 26.8 (18.7-52.2). Four patients had diabetes, 8 were smokers, and 10 had undergone previous surgeries including arthroscopic labral débridement, 3 open reduction with Salter osteotomy, 3 open reduction with internal fixation of a femoral neck fracture, 1 core decompression for femoral head osteonecrosis, 3 subtrochanteric osteotomy and subsequent non-union treated with cephalomedullary nailing, and 1 previous PAO requiring revision.1

There were 11 perioperative complications in 10 patients (12.5%). The majority of these were infection (n = 10). Overall complications categorized by BMI are summarized in Table 1. Age was similar in patients with complications (27.4 ± 8.8 years) and those without (27.5 ± 8.2 years) (P = .99). Patients with complications had significantly higher BMI than those without (30.9.3 ± 9.5, 26.2 ± 5.6) (P = .03). There was no effect of concomitant procedures on the complication rate. Of the patients who had complications, 60% (6/10) had concomitant procedures, vs 63% (44/70) of those who had no complications (P = .86) Two of 4 patients with diabetes mellitus developed complications, both of which were wound infections. One of these required incision and débridement. There were no perioperative complications in any of the 7 smokers.
Table 1. Complications in Low- and High-BMI Patients | ||||
Complications | Total | BMI <30 | BMI >30 | |
Infection | 10 | 4 | 6 | |
| Superficial | 8 | 4 | 4 |
| Deep | 2 | 0 | 2 |
Long screw | 1 | 1 | 0 | |
Total | 13 | 5 | 6 | |
Abbreviation: BMI, body mass index.
Twenty hips were in the high-BMI (>30) and 60 were in the low-BMI (<30) patient groups. There were 6 total perioperative complications in the high-BMI group (30%) and 5 in the low-BMI group (8%). The most common complications in the low-BMI group were superficial infections.4 There were 6 total complications in the high-BMI group: 2 deep and 4 superficial infections. There were 3 reoperations (5%) in the low-BMI group during the perioperative period. Two patients underwent successful débridement and irrigation of a superficial wound, and 1 patient required removal of a prominent screw. There were 3 reoperations in the high-BMI group, all of which were débridement and irrigations for wound infections. The rate of wound dehiscence and wound infection was significantly higher in high-BMI patients (30% [6/20]) than in low-BMI patients (8.3% [4/60]) (P = .006). The mean estimated blood loss in the high-BMI group was greater at 923.75 mL vs 779.25 mL in the low-BMI patients; however, this did not reach statistical significance (P = .350). Seventy percent (14/20) of patients who were obese had concomitant procedures vs 60% (36/60) of those who had normal BMI (P = .42 by chi-square analysis). There was no difference in estimated blood loss in patients who underwent concomitant procedures (Table 2).
Table 2. Average Estimated Blood Loss (mL) | |||
| Average EBL | BMI <30 | BMI >30 |
Concomitant procedure | 765 | 759 | 779 |
No concomitant procedure | 900 | 810 | 1263 |
Total | 815 | 779 | 924 |
Abbreviations: BMI, body mass index; EBL, estimated blood loss.
Preoperative pain scores improved from 4.9 (range, 0-10) to 1.9 (range, 0-6) in the high-BMI group and 4.2 (range, 0-10) to 1.2 (range, 0-6) in the low-BMI group (P = .260). The preoperative center-edge angle in the high-BMI group improved from 6.63° ± 6.5° to 28.53° ± 6.7°, and the Tönnis angle from 24.96° ± 6.3° to 10.06° ± 7.7°. In the low-BMI group the center-edge angle improved from 10.53° ± 11.77° to 27.07° ± 13.9°, and the Tönnis angle from 19.00° ± 10.3° to 2.79° ± 8.3°. There was no difference in postoperative center-edge angle between the high-BMI and low-BMI groups (P = .66). There was a trend toward significance in the postoperative Tönnis angle between the high-BMI and low-BMI groups (P = .051).
Continue to: DISCUSSION...
DISCUSSION
There have been 4 previously published articles specifically on complications following PAO. Each of these encompassed follow-up visits including both the perioperative period and at least 2 years of follow-up.20,22,24,29 Davey and Santore29 reported an overall rate of complications of 10% in a series of 70 patients. These authors classified complications into minor, moderate, and major for purposes of research and discussion, and this classification system has been utilized or modified within the literature to discuss complications in most other articles. Complications within the perioperative period included 2 cases of excessive intraoperative bleeding, 2 cases of reflex sympathetic dystrophy, and 1 case each of unresolved sciatic nerve palsy and deep vein thrombosis.29 Hussell and colleagues22 reported on a large series of 508 PAOs and analyzed the technical complications that occurred during the procedure and caused either immediate or longer-term problems for the patients. Notably, they concluded that 85% of the technical complications occurred with the initial 50 PAOs performed, signifying a steep learning curve for this technically demanding procedure. Perioperative complications reported were intra-articular osteotomy in 2.2%, femoral nerve palsy in 0.6%, sciatic nerve palsy in 1.0%, posterior column insufficiency in 1.2%, and symptomatic hardware in 3.0%.22 Biedermann and colleagues20 found that 47 out of 60 PAOs in their series had at least 1 minor complication. The most common perioperative complications were lateral femoral cutaneous nerve dysesthesia in 33%, delayed wound healing infection in 15%, major blood loss in 8.3%, sciatic or peroneal nerve palsy in 10%, posterior column discontinuity in 6.7%, and intra-articular osteotomy in 1.6%.20 Most recently, complications of PAO in an adolescent population were evaluated.24 The overall rate of complications was 37%. Major perioperative complications included 1 patient with excessive bleeding due to an aberrant artery at the medial wall of the pelvis thought to be due to revascularization following a previous Dega osteotomy. Two patients required immediate revision of the osteotomy due to excessive anterior coverage noted on postoperative radiographs. There were 5% with superficial stitch abscess causing minor infection, 5% with transient lateral femoral cutaneous nerve palsy, and 15 patients with symptomatic hardware.24
At 12.5%, our overall complication rate is slightly lower than that previously reported in the literature. This may be due to the difference in the scope of this study, which reported only perioperative complications. We also chose to utilize the modified Clavien-Dindo classification system for reporting our complications rather than classifying them as minor or major as in the above studies. This classification system has been validated for use in reporting complications of hip preservation surgery. We considered only Grade II complications and higher for statistical analysis as these required a change in postoperative management, which may have artificially lowered our complication rate.
The data in this study indicate that, compared with patients with a BMI of <30, obese patients have a higher rate of perioperative complications and reoperations. Additionally, the proportion of Grade II and higher complications, importantly deep infection, was higher in obese patients. We did not have any reported incidence of deep vein thrombosis or pulmonary embolism, urinary tract infection, intra-articular osteotomy, acetabular or pelvic fracture, femoral or sciatic nerve palsy, or long-term lateral femoral cutaneous nerve palsy in this series of patients. The most common complication in the low-BMI group was symptomatic hardware. Sixteen patients had this complaint; however, this was not considered a Grade II complication as there would be no change in management during the study period, including the perioperative time frame. Two out of 4 patients with diabetes mellitus developed wound infections, both of which required reoperation. However, the number of patients with diabetes mellitus was not large enough to draw any conclusions from this information. There were no perioperative complications in smokers. We hypothesized that there may be a higher rate of wound complications in this population, and although the data in our patients did not support this hypothesis, a larger cohort of smokers is needed to make this determination. Another potential complication in smokers is non-union, which was not reported in this study on perioperative complications. Although it did not reach statistical significance, the intraoperative blood loss was almost 150 mL greater in high-BMI patients (924 mL vs 779 mL). Additionally, there appears to be no effect of concomitant procedure on estimated blood loss in either low- or high-BMI groups. Age was not a risk factor for the development of perioperative complications in this cohort. Pain was reliably improved in both the high- and low-BMI groups at the 12-week follow-up visit. The center-edge angle could be normalized in both groups to 28.53° in the high-BMI group and 27.07° in the low-BMI group, with a similar final correction between groups. The Tönnis angle was also improved in both groups, but the final Tönnis angle strongly trended toward statistical significance (2.79° in the low-BMI group vs 10.06° in the high-BMI group).
This study has limitations in that it is a retrospective review of patient information based on medical records and therefore relied on documentation performed at the time of service. There also may have been a difference in the intraoperative or postoperative protocol for wound monitoring or rehabilitation among patients based on body habitus, which we are not able to detect from the medical records. Although the overall number of patients in this cohort is comparable to other studies on the outcomes of patients after PAO, the number of patients in each BMI group was not evenly matched. Without randomization, selection bias occurred at the time of the procedure as some obese patients were not offered this procedure based on the senior surgeon’s discretion. Additionally, when subgroups such as patients with diabetes mellitus or smokers were analyzed, the number of subjects was too small for statistical analysis; therefore, no conclusions could be made as to the risk of perioperative complications in these populations.
CONCLUSION
Despite the limitations in this study, based on the data from this cohort, we concluded that the goal of PAO of restoring more normal hip joint anatomy can be achieved in both low- and high-BMI patients. However, patients with a BMI >30 should be counseled on their increased risk of major perioperative complications, specifically wound dehiscence and infection, and the higher likelihood of reoperation for treatment of these complications. Diabetic patients can be counseled that they may have a higher risk of infection as well, but future studies with larger numbers will be needed to confirm this. Patients with low BMI should be counseled about the potential for prominent or symptomatic hardware, which may necessitate removal following osteotomy union.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
1. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. J Bone Joint Surg Am. 2005;87(2):254-259. doi:10.2106/JBJS.E.00887.
2. Clohisy JC, Schutz AL, St John L, Schoenecker PL, Wright RW. Periacetabular osteotomy: a systematic literature review. Clin Orthop Relat Res. 2009;467(8):2041-2052. doi:10.1007/s11999-009-0842-6.
3. Gillingham BL, Sanchez AA, Wenger DR. Pelvic osteotomies for the treatment of hip dysplasia in children and young adults. J Am Acad Orthop Surg. 1999;7(5):325-337. doi:10.5435/00124635-199909000-00005.
4. Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85-A(2):278-286. doi:10.2106/00004623-200302000-00015.
5. Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res. 1988;(232):26-36. doi:10.1097/00003086-198807000-00006.
6. Tibor LM, Sink EL. Periacetabular osteotomy for hip preservation. Orthop Clin North Am. 2012;43(3):343-357. doi:10.1016/j.ocl.2012.05.011.
7. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724. doi:10.1302/0301-620X.89B6.18805.
8. Novais EN, Heyworth B, Murray K, Johnson VM, Kim YJ, Millis MB. Physical activity level improves after periacetabular osteotomy for the treatment of symptomatic hip dysplasia. Clin Orthop Relat Res. 2013;471(3):981-988. doi:10.1007/s11999-012-2578-y.
9. Clohisy JC, Barrett SE, Gordon JE, Delgado ED, Schoenecker PL. Periacetabular osteotomy in the treatment of severe acetabular dysplasia. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:65-83. doi:10.2106/JBJS.E.00887.
10. Badra MI, Anand A, Straight JJ, Sala DA, Ruchelsman DE, Feldman DS. Functional outcome in adult patients following Bernese periacetabular osteotomy. Orthopedics 2008;31(1):69. doi:10.3928/01477447-20080101-03.
11. Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470(11):2978-2987. doi:10.1007/s11999-012-2386-4.
12. Ito H, Tanino H, Yamanaka Y, Minami A, Matsuno T. Intermediate to long-term results of periacetabular osteotomy in patients younger and older than forty years of age. J Bone Joint Surg Am. 2011;93(14):1347-1354. doi:10.2106/JBJS.J.01059.
13. Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91(9):2113-2123. doi:10.2106/JBJS.G.00143.
14. Pogliacomi F, Stark A, Wallensten R. Periacetabular osteotomy. Good pain relief in symptomatic hip dysplasia, 32 patients followed for 4 years. Acta Orthop. 2005;76(1):67-74. doi:10.1080/00016470510030346.
15. Zhu J, Chen X, Cui Y, Shen C, Cai G. Mid-term results of Bernese periacetabular osteotomy for developmental dysplasia of hip in middle aged patients. Int Orthop. 2013;37(4):589-594. doi:10.1007/s00264-013-1790-z.
16. Lehmann CL, Nepple JJ, Baca G, Schoenecker PL, Clohisy JC. Do fluoroscopy and postoperative radiographs correlate for periacetabular osteotomy corrections? Clin Orthop Relat Res. 2012;470(12):3508-3514. doi:10.1007/s11999-012-2483-4.
17. Nakayama H, Fukunishi S, Fukui T, Yoshiya S. Arthroscopic labral repair concomitantly performed with curved periacetabular osteotomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):938-941. doi:10.1007/s00167-013-2362-x.
18. Sambandam SN, Hull J, Jiranek WA. Factors predicting the failure of Bernese periacetabular osteotomy: a meta-regression analysis. Int Orthop. 2009;33(6):1483-1488. doi:10.1007/s00264-008-0643-7.
19. Yasunaga Y, Yamasaki T, Ochi M. Patient selection criteria for periacetabular osteotomy or rotational acetabular osteotomy. Clin Orthop Relat Res. 2012;470(12):3342-3354. doi:10.1007/s11999-012-2516-z.
20. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617. doi:10.1007/s00264-007-0372-3.
21. Espinosa N, Strassberg J, Belzile EL, Millis MB, Kim YJ. Extraarticular fractures after periacetabular osteotomy. Clin Orthop Relat Res. 2008;466(7):1645-1651. doi:10.1007/s11999-008-0280-x.
22. Hussell JG, Rodriguez JA, Ganz R. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):81-92.
23. Tannast M, Pfander G, Steppacher SD, Mast JW, Ganz R. Total acetabular retroversion following pelvic osteotomy: presentation, management, and outcome. Hip Int. 2013;23 Suppl 9:S14-S26. doi:10.5301/hipint.5000089.
24. Thawrani D, Sucato DJ, Podeszwa DA, DeLaRocha A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. J Bone Joint Surg Am. 2010;92(8):1707-1714. doi:10.2106/JBJS.I.00829.
25. Sierra RJ, Beaule P, Zaltz I, Millis MB, Clohisy JC, Trousdale RT; ANCHOR Group. Prevention of nerve injury after periacetabular osteotomy. Clin Orthop Relat Res. 2012;470(8):2209-2219. doi:10.1007/s11999-012-2409-1.
26. Zaltz I, Beaulé P, Clohisy J, et al. Incidence of deep vein thrombosis and pulmonary embolus following periacetabular osteotomy. J Bone Joint Surg Am. 2011;93 Suppl 2:62-65. doi:10.2106/JBJS.J.01769.
27. Burmeister H, Kaiser B, Siebenrock KA, Ganz R. Incisional hernia after periacetabular osteotomy. Clin Orthop Relat Res. 2004;(425):177-179. doi:10.1097/01.blo.0000130203.28818.da.
28. Kiyama T, Naito M, Shiramizu K, Shinoda T, Maeyama A. Ischemia of the lateral femoral cutaneous nerve during periacetabular osteotomy using Smith-Petersen approach. J Orthop Traumatol. 2009;10(3):123-126. doi:10.1007/s10195-009-0055-5.
29. Davey JP, Santore RF. Complications of periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):33-37. doi:10.1097/00003086-199906000-00005.
30. Sink EL, Leunig M, Zaltz I, Gilbert JC, Clohisy J; Academic Network for Conservational Hip Outcomes Research Group. Reliability of a complication classification system for orthopaedic surgery. Clin Orthop Relat Res. 2012;470(8):2220-2226. doi:10.1007/s11999-012-2343-2.
TAKE-HOME POINTS
- PAO is an effective procedure to treat symptomatic hip dysplasia in patients without degenerative changes.
- The postoperative correction of dysplasia as measured by center-edge angles were similar in low and high BMI groups.
- Patients with obesity (BMI >30) have a higher incidence of postoperative complications following PAO.
- There were too few patients with diabetes or smoking to determine a significantly increased rate of complications. However, we believe based on the literature these patient populations are at higher risk for complications in the early postoperative period.
- Patients with BMI >30 can have a successful outcome with a PAO procedure. However, this patient population should have counseling about their increased risk of complications, and be given opportunity to lose weight when possible preoperatively.
Hip fracture outcomes are the next ERAS improvement goal
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
ORLANDO – compared with patients treated before the intervention, an investigator reported at the American College of Surgeons Quality and Safety Conference.
These patients had a lower pneumonia rate and were more often discharged to home from acute care after the program was implemented, according to Lila Gottenbos, RN, BSN, of Langley (B.C.) Memorial Hospital.
The intervention incorporated some traditional enhanced recovery after surgery (ERAS) process measures, along with others that were not so traditional, Ms. Gottenbos said. “Implementing ERAS in a fractured hip patient population is possible, and by doing so, more patients go home faster to their previous places of residence with fewer complications.”
A multidisciplinary team at Langley Memorial Hospital, a 200-bed community hospital with approximately 6,000 surgical procedures performed each year, has used ERAS measures in their colorectal patient population since 2013. Those measures have been successful in creating a sustained reduction in morbidity and length of stay, according to Ms. Gottenbos.
The team began searching for other patient populations who might also benefit. They chose to focus on the fractured hip population, which in 2015 had a 9.7% mortality rate, 17% morbidity rate, 5% pneumonia rate, and 19% rate of discharge to home from acute care. “We looked at this data and we realized we had a significant opportunity to do better for our patients,” Ms. Gottenbos told meeting attendees.
The team developed ERAS-based process measures tailored specifically to pre- and postoperative challenges in the fractured hip patient population, Ms. Gottenbos said. Measures included preoperative patient and family education, elimination of prolonged preoperative NPO status, early mobilization, assessment of mentation, and use of standardized order sets. The protocol has been applied to every hip fracture patient who has had surgery from January 2016 to the present. The hospital averages 110 of these procedures per year.
Fractured hip mortality dropped after the modified ERAS process measures were adopted, Ms. Gottenbos reported. Measured to 30 days postoperatively, mortality decreased from 9.7% in 2015 to 4.2% by 2017. Similarly, fractured hip morbidity within 30 days, excluding transfusion, dropped from 17.7% in 2015 to 11.7% in 2017, and fractured hip pneumonia dropped from 5.4% to 2.5%.
Perhaps the most telling evidence of success, according to the presenter, was the increase in the number of patients going home from acute care: “Before ERAS, fractured hip patients were going home to their place of residence less than 20% of the time from the acute care setting, meaning they were languishing in the hospital, in a convalescent unit, in a rehab unit, or worse, residential care,” she said. “We’ve been able to increase that to over 43%.”
The program is ongoing. A multidisciplinary team meets monthly to review outcomes data and devise strategies to improve compliance with the process measures. “It’s an iterative process, and it’s one that’s worked very well for us so far,” Ms. Gottenbos remarked.
The investigator had no disclosures.
REPORTING FROM ACSQSC 2018
Key clinical point: Fractured hip patients managed with the ERAS protocol had improved outcomes.
Major finding: After implementation of the ERAS protocol, 43% of fractured hip patients were discharged to home, which is up from 20% before the project.
Study details: More than 200 patients treated for hip fracture during 2016-2017 at the Langley (B.C.) Memorial Hospital.
Disclosures: The investigator had no disclosures. .
Nearly one-quarter of presurgery patients already using opioids
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
at a large academic medical center, a cross-sectional observational study has determined.
Prescription or illegal opioid use can have profound implications for surgical outcomes and continued postoperative medication abuse. “Preoperative opioid use was associated with a greater burden of comorbid disease and multiple risk factors for poor recovery. ... Opioid-tolerant patients are at risk for opioid-associated adverse events and are less likely to discontinue opioid-based therapy after their surgery,” wrote Paul E. Hilliard, MD, and a team of researchers at the University of Michigan Health System. Although the question of preoperative opioid use has been examined and the Michigan findings are consistent with earlier estimates of prevalence (Ann Surg. 2017;265[4]:695-701), this study sought a more detailed profile of both the characteristics of these patients and the types of procedures correlated with opioid use.
Patient data were derived primarily from two ongoing institutional registries, the Michigan Genomics Initiative and the Analgesic Outcomes Study. Each of these projects involved recruiting nonemergency surgery patients to participate and self-report on pain and affect issues. Opioid use data were extracted from the preop anesthesia history and from physical examination. A total of 34,186 patients were recruited for this study; 54.2% were women, 89.1% were white, and the mean age was 53.1 years. Overall, 23.1% of these patients were taking opioids of various kinds, mostly by prescription along with nonprescription opioids and illegal drugs of other kinds.
The most common opioids found in this patient sample were hydrocodone bitartrate (59.4%), tramadol hydrochloride (21.2%) and oxycodone hydrochloride (18.5%), although the duration or frequency of use was not determined.
“In our experience, in surveys like this patients are pretty honest. [The data do not] track to their medical record, but was done privately for research. That having been said, I am sure there is significant underreporting,” study coauthor Michael J. Englesbe, MD, FACS, said in an interview. In addition to some nondisclosure by study participants, the exclusion of patients admitted to surgery from the ED could mean that 23.1% is a conservative estimate, he noted.
Patient characteristics included in the study (tobacco use, alcohol use, sleep apnea, pain, life satisfaction, depression, anxiety) were self-reported and validated using tools such as the Brief Pain Inventory, the Fibromyalgia Survey, and the Hospital Anxiety and Depression Scale. Procedural data were derived from patient records and ICD-10 data and rated via the ASA score and Charlson Comorbidity Index.
A multivariate analysis of patient characteristics found that age between 31 and 40, tobacco use, heavy alcohol use, pain score, depression, comorbidities reflected in a higher ASA score, and Charlson Comorbidity Score were all significant risk factors for presurgical opiate use.
Patients who were scheduled for surgical procedures involving lower extremities (adjusted odds ratio 3.61, 95% confidence interval, 2.81-4.64) were at the highest risk for opioid use, followed by pelvis surgery, excluding hip (aOR, 3.09, 95% CI, 1.88-5.08), upper arm or elbow (aOR, 3.07, 95% CI, 2.12-4.45), and spine surgery (aOR, 2.68, 95% CI, 2.15-3.32).
The study also broke out the data by presurgery opioid usage and surgery service. Of patients having spine neurosurgery, 55.1% were already taking opioids, and among those having orthopedic spine surgery, 65.1% were taking opioids. General surgery patients were not among those mostly likely to be using opioids (gastrointestinal surgery, 19.3% and endocrine surgery 14.3%). “Certain surgical services may be more likely to encounter patients with high comorbidities for opioid use, and more targeted opioid education strategies aimed at those services may help to mitigate risk in the postoperative period,” the authors wrote.
“All surgeons should take a preop pain history. They should ask about current pain and previous pain experiences. They should also ask about a history of substance use disorder. This should lead into a discussion of the pain expectations from the procedure. Patients should expect to be in pain, that is normal. Pain-free surgery is rare. If a patient has a complex pain history or takes chronic opioids, the surgeon should consider referring them to anesthesia for formal preop pain management planning and potentially weaning of opioid dose prior to elective surgery,” noted Dr. Englesbe, the Cyrenus G. Darling Sr., MD and Cyrenus G Darling Jr., MD Professor of Surgery, and faculty at the Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor.
Surgeons are likely to see patients with a past history of opioid dependence or who are recovering from substance abuse. “Every effort should be made to avoid opioids in these patients. We have developed a Pain Optimization Pathway which facilitates no postoperative opioids for these and other patients. These patients are at high risk to relapse and surgeons must know who these patients are so they can provide optimal care,” Dr. Englesbe added.The limitations of this study as reported by the authors include the single-center design, the nondiverse racial makeup of the sample, and the difficulty of ascertaining the dosing and duration of opioid use, both prescription and illegal.
The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
SOURCE: Hilliard PE et al. JAMA Surg. 2018 Jul 11. doi: 10.1001/jamasurg.2018.2102.
FROM JAMA SURGERY
Key clinical point: Preoperative opioid use is prevalent in patients who are having spinal surgery and have depression.
Major finding:
Study details: An observational study of 34,186 surgical patients in the University of Michigan Health system.
Disclosures: The investigators reported no disclosures relevant to this study. This study was supported by the National Institute on Drug Abuse, National Institutes of Health, the American College of Surgeons, and other noncommercial sources.
Source: Hilliard P E et al. JAMA Surg. 2018 Jul 11;. doi:10.1001/jamasurg.2018.2102.
Glucocorticoids linked with surgical infections in RA patients
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
AMSTERDAM – Patients with rheumatoid arthritis who underwent elective knee or hip arthroplasty had a doubled rate of hospitalization for infection when they averaged more than 10 mg/day oral prednisone during the 3 months before surgery, based on a review of about 11,000 U.S. insurance claims.
“Limiting glucocorticoid exposure before surgery should be a focus of perioperative management,” Michael D. George, MD, said at the European Congress of Rheumatology. “Glucocorticoid use, especially greater than 10 mg/day, is associated with a greater risk of infection and hospital readmission,” said Dr. George, a rheumatologist at the University of Pennsylvania in Philadelphia.
The analysis also showed that treatment with any biologic drug – including abatacept (Orencia), rituximab (Rituxan), tocilizumab (Actemra), and any of several tumor necrosis factor (TNF) inhibitors – had a similar impact on both postsurgical infections requiring hospitalization and 30-day hospital readmissions.
The findings suggest “it’s more important to reduce glucocorticoids than biological drugs,” commented John D. Isaacs, MD, professor of clinical rheumatology at Newcastle University in Newcastle upon Tyne, England. “This is a really important question that has been very difficult to answer.”
Dr. George and his associates used data from patients with rheumatoid arthritis during 2006-2015 who underwent knee or hip arthroplasty and were in databases from Medicare, or MarketScan, which includes commercial insurers. This identified 11,021 RA patients on any of several biologic drugs before their surgery: 16% on abatacept, 4% on rituximab, 4% on tocilizumab, and the remaining 76% on a TNF inhibitor, either adalimumab (Humira), etanercept (Enbrel), or infliximab (Remicade). About 43% of all patients were on a glucocorticoid during the 3 months before surgery. Biologic use was defined as a minimum of one dose within 8 weeks of surgery, and at least three total dosages during the prior year, except for rituximab, which was at least one dose given 16 weeks before surgery and at least two doses during the prior year.
The rate of hospitalized infections ranged from 6.6% to 8.5% depending on the biologic drug used, and 30-day readmissions ranged from 4.8% to 6.8%. A third outcome the analysis assessed was prosthetic joint infection during 1-year follow-up, which was again similar across most of the biologics, except for patients on tocilizumab, who had prosthetic joint infections roughly threefold more often than the other patients. Although this was a statistically significant difference, Dr. George discounted the finding given the very small number of tocilizumab-treated patients who had these infections and said that any conclusion about tocilizumab’s effect on this outcome had to await data from more patients.
The glucocorticoid analysis divided patients into four subgroups: those not on a glucocorticoid, those on an average daily dosage of 5 mg/day prednisone or equivalent or less, patients on 6-10 mg/day prednisone, and those on more than 10 mg/day. In a propensity-weighted analysis, these three escalating levels of glucocorticoid use showed a dose-response relationship to the rates of both hospitalized infections and 30-day readmissions. At the highest level of glucocorticoid use, hospitalized infections occurred twice as often as in patients not on a glucocorticoid, and 30-day readmissions were more than 50% higher than in those not on an oral steroid, both statistically significant differences. For the outcome of 1-year prosthetic joint infections, the analysis again showed a dose-related link among glucocorticoid users, topping out with a greater than 50% increased rate among those on the highest glucocorticoid dosages when compared with nonusers, but this difference was not statistically significant.
The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept. Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
SOURCE: George MD et al. EULAR 2018. Abstract OP0228.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point:
Major finding: RA patients on more than 10 mg/day prednisone had a more than twofold higher rate of postsurgical hospitalized infections.
Study details: Review of Medicare and MarketScan administrative claims data for 11,021 patients with rheumatoid arthritis who underwent joint surgery.
Disclosures: The study was partially funded by Bristol-Myers Squibb, the company that markets abatacept (Orencia). Dr. George has received research funding from Bristol-Myers Squibb, and some of his coauthors on the study are employees of the company.
Source: George MD et al. EULAR 2018. Abstract OP0228.