User login
Rheumatoid Arthritis vs Osteoarthritis: Comparison of Demographics and Trends of Joint Replacement Data from the Nationwide Inpatient Sample
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
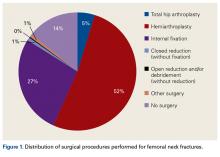
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).
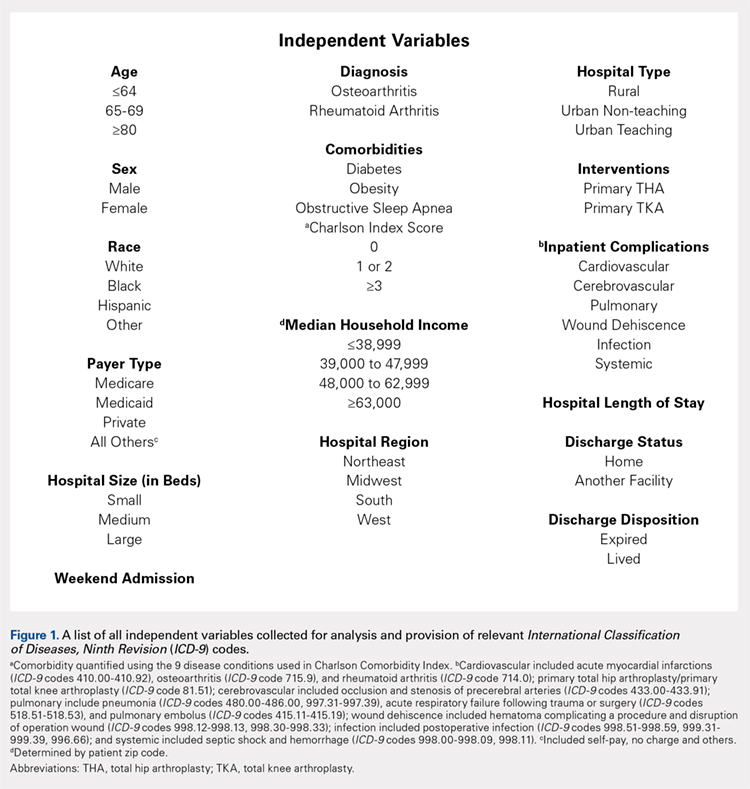
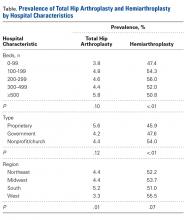
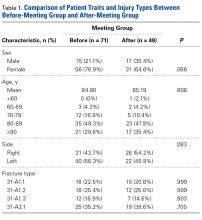
Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
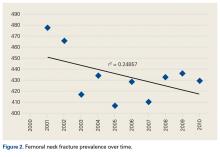
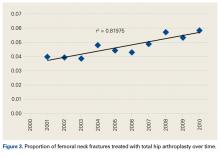
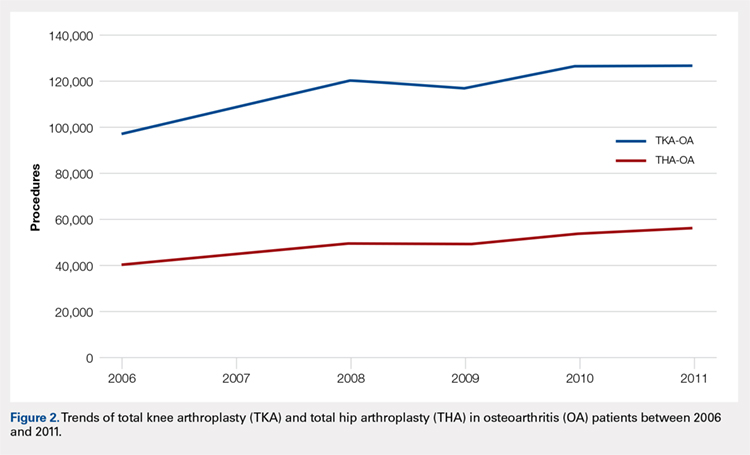
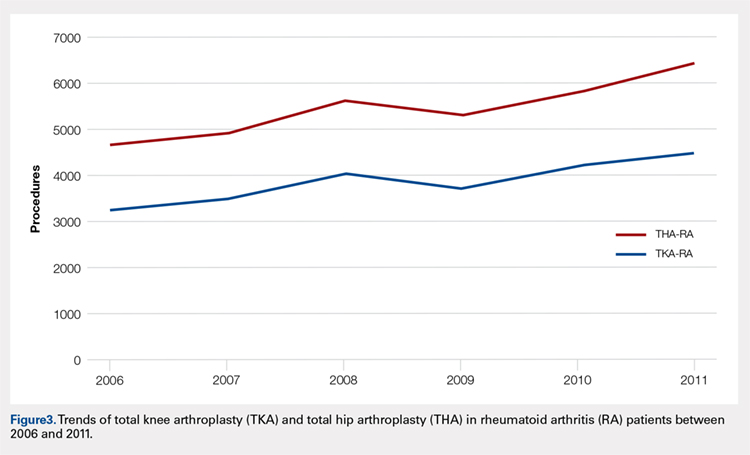
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).
Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
TAKE-HOME POINTS
- Patients undergoing THA for OA, when compared to those with RA undergoing THA, had lower risk for postoperative cardiovascular, pulmonary, wound dehiscence, infections, and systemic complications.
- Patients with OA undergoing THA had statistically significant higher risk of cerebrovascular complication compared to RA patients undergoing the same procedure.
- In TKA, OA patients had significantly higher risk for cardiovascular and cerebrovascular complications, and a significant lower risk for mechanical wounds, infection, and systemic complications.
- RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients.
- These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Outcomes After Peripheral Nerve Block in Hip Arthroscopy
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.
Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.

Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.

Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
TAKE-HOME POINTS
- Postoperative PACU pain was consistently reduced in the PNB group.
- Patients with PNBs had lower postoperative pain medication requirements and lower rates of inpatient admission compared with controls.
- Similar rates of nausea/vomiting and time to discharge were reported for PNB patients and controls.
- PNBs are associated with high rates of satisfaction and few complications.
- Future research should focus on comparing across PNB techniques.
Minimum 5-Year Follow-up of Articular Surface Replacement Acetabular Components Used in Total Hip Arthroplasty
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.
All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).
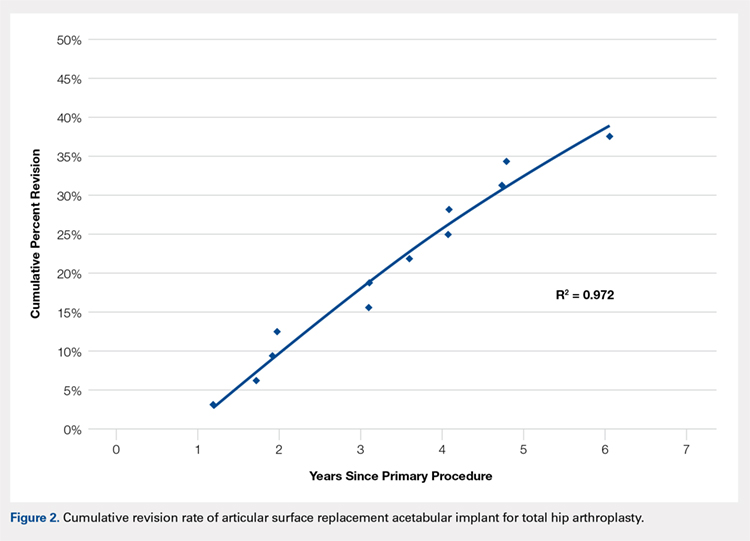
Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.
The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |
Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.

All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).

Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.

The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |

Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.

All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).

Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.

The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |

Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
TAKE-HOME POINTS
- High rate of failure of DePuy Synthes ASR™ XL Acetabular hip system used in THA, approaching 34.4% at 5 years.
- Mean time to revision was 3.1 years with pain being the most common indication for revision surgery.
- Age, gender, acetabular component abduction angle, acetabular size, and serum cobalt or chromium levels were not associated with increased rate of failure.
- Serum cobalt and chromium levels decreased significantly within 6 months of revision surgery.
- Close clinical surveillance and laboratory monitoring of patients is required.
Blood Loss Reduction with Tranexamic Acid and a Bipolar Sealer in Direct Anterior Total Hip Arthroplasty
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
TAKE-HOME POINTS
- TXA reduces blood loss and transfusion requirements in THA.
- The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation.
- The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements.
- The addition of a bipolar sealer did not offer an advantage to transfusion requirements in anterior THA.
- TXA should be used routinely in THA.
Surgery may be best option for hip impingement syndrome
LIVERPOOL, ENGLAND – Hip arthroscopic surgery produced better long-term results than did personalized hip physiotherapy for femoroacetabular impingement syndrome in a randomized trial conduced across multiple U.K. centers.
At 12 months, respective International Hip Outcome Tool-33 (iHOT-33) scores were 58.8 and 49.7, a difference of 9.1 points before and 6.8 points after adjustment for potential confounding factors (P = .0093).
“This trial shows that hip arthroscopic surgery and personalized hip therapy both improved hip-related quality of life for patients with FAI [femoroacetabular impingement] syndrome, but that the surgery did indeed produce a greater improvement at our primary time point of 12 months,” she added. Dr. Foster is professor of musculoskeletal health in primary care at Keele University, Newcastle-under-Lyme, England, one of the 23 centers involved in the FASHIoN study in England, Wales, and Scotland.
“FAI is a very common cause of hip and groin pain in young adults, and it’s associated with the development of hip osteoarthritis,” Dr. Foster noted.
There are three types of FAI – pincer, cam, and combined. The pincer type of FAI is where there is “prominence or overcoverage of the rim of the acetabulum,” and the cam type is where there is a “bony prominence of the femoral head-neck junction,” she explained at the meeting sponsored by the Osteoarthritis Research Society International.
The link to OA comes when the femur and acetabulum prematurely connect, usually during activity, causing damage to the labrum and articular cartilage in the long term. Thus, treating FAI is important, not just for relieving patient’s pain and joint stiffness.
Hip arthroscopic surgery has become an established way of treating FAI syndrome – more than 2,400 operations were performed in 2013 in the United Kingdom alone, Dr. Foster observed. The aim of surgery is to try to reshape the hip joint to prevent impingement, and resect, repair, or reconstruct any intra-articular damage that may be present.
“Physiotherapy aims to improve hip muscle control and strength, and to correct the abnormal movement patterns that we see in these patients,” Dr. Foster said. “Through that, we hope to prevent the premature contact that occurs in FAI syndrome and thereby improve symptoms, allowing patients to return to activities and prevent recurrence.”
Working with physiotherapists, physicians, and surgeons, Dr. Foster and her associates have previously developed a “best conservative care” intervention that they call personalized hip therapy (PHT), which involves the delivery and supervision of an individualized exercise program by experienced physiotherapists over a 3- to 6-month period, and which patients repeat at home (PM R. 2013 May;5[5]:418-26).
The aim of the UK FASHIoN trial was to compare the clinical and cost-effectiveness of hip arthroscopy and PHT for FAI syndrome, as there was no robust clinical trial evidence to demonstrate a benefit of one over the other.
A total of 351 adults with hip and groin pain were randomized to either arthroscopic surgery (n = 173) or PHT (n = 178). The mean age of participants was 35 years, with no significant differences between the two treatment groups in terms of baseline demographics or type or duration of hip impingement.
While surgery was better in terms of patient outcomes, the study didn’t demonstrate its cost-effectiveness within the first 12 months, Dr. Foster observed. Cost-effectiveness, together with various other quality-of-life measurements, was a secondary endpoint of the study.
“Longer-term outcomes are required to establish whether improvement is sustained, and whether surgery is cost-effective at the longer time points for our health service,” she said.
Responding to a question about whether any of the patients in the study had radiographic evidence of osteoarthritis, Dr. Foster said that such patients had been excluded from the study.
“One of the hopes of the trial’s team is that, with the long-term follow-up, we might be able to get data at 5 and 10 years on things like hip osteoarthritis in these patients,” she said.
The study was funded by the National Institute for Health Research. Dr. Foster had no financial relationships or commercial interests to disclose.
SOURCE: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28
LIVERPOOL, ENGLAND – Hip arthroscopic surgery produced better long-term results than did personalized hip physiotherapy for femoroacetabular impingement syndrome in a randomized trial conduced across multiple U.K. centers.
At 12 months, respective International Hip Outcome Tool-33 (iHOT-33) scores were 58.8 and 49.7, a difference of 9.1 points before and 6.8 points after adjustment for potential confounding factors (P = .0093).
“This trial shows that hip arthroscopic surgery and personalized hip therapy both improved hip-related quality of life for patients with FAI [femoroacetabular impingement] syndrome, but that the surgery did indeed produce a greater improvement at our primary time point of 12 months,” she added. Dr. Foster is professor of musculoskeletal health in primary care at Keele University, Newcastle-under-Lyme, England, one of the 23 centers involved in the FASHIoN study in England, Wales, and Scotland.
“FAI is a very common cause of hip and groin pain in young adults, and it’s associated with the development of hip osteoarthritis,” Dr. Foster noted.
There are three types of FAI – pincer, cam, and combined. The pincer type of FAI is where there is “prominence or overcoverage of the rim of the acetabulum,” and the cam type is where there is a “bony prominence of the femoral head-neck junction,” she explained at the meeting sponsored by the Osteoarthritis Research Society International.
The link to OA comes when the femur and acetabulum prematurely connect, usually during activity, causing damage to the labrum and articular cartilage in the long term. Thus, treating FAI is important, not just for relieving patient’s pain and joint stiffness.
Hip arthroscopic surgery has become an established way of treating FAI syndrome – more than 2,400 operations were performed in 2013 in the United Kingdom alone, Dr. Foster observed. The aim of surgery is to try to reshape the hip joint to prevent impingement, and resect, repair, or reconstruct any intra-articular damage that may be present.
“Physiotherapy aims to improve hip muscle control and strength, and to correct the abnormal movement patterns that we see in these patients,” Dr. Foster said. “Through that, we hope to prevent the premature contact that occurs in FAI syndrome and thereby improve symptoms, allowing patients to return to activities and prevent recurrence.”
Working with physiotherapists, physicians, and surgeons, Dr. Foster and her associates have previously developed a “best conservative care” intervention that they call personalized hip therapy (PHT), which involves the delivery and supervision of an individualized exercise program by experienced physiotherapists over a 3- to 6-month period, and which patients repeat at home (PM R. 2013 May;5[5]:418-26).
The aim of the UK FASHIoN trial was to compare the clinical and cost-effectiveness of hip arthroscopy and PHT for FAI syndrome, as there was no robust clinical trial evidence to demonstrate a benefit of one over the other.
A total of 351 adults with hip and groin pain were randomized to either arthroscopic surgery (n = 173) or PHT (n = 178). The mean age of participants was 35 years, with no significant differences between the two treatment groups in terms of baseline demographics or type or duration of hip impingement.
While surgery was better in terms of patient outcomes, the study didn’t demonstrate its cost-effectiveness within the first 12 months, Dr. Foster observed. Cost-effectiveness, together with various other quality-of-life measurements, was a secondary endpoint of the study.
“Longer-term outcomes are required to establish whether improvement is sustained, and whether surgery is cost-effective at the longer time points for our health service,” she said.
Responding to a question about whether any of the patients in the study had radiographic evidence of osteoarthritis, Dr. Foster said that such patients had been excluded from the study.
“One of the hopes of the trial’s team is that, with the long-term follow-up, we might be able to get data at 5 and 10 years on things like hip osteoarthritis in these patients,” she said.
The study was funded by the National Institute for Health Research. Dr. Foster had no financial relationships or commercial interests to disclose.
SOURCE: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28
LIVERPOOL, ENGLAND – Hip arthroscopic surgery produced better long-term results than did personalized hip physiotherapy for femoroacetabular impingement syndrome in a randomized trial conduced across multiple U.K. centers.
At 12 months, respective International Hip Outcome Tool-33 (iHOT-33) scores were 58.8 and 49.7, a difference of 9.1 points before and 6.8 points after adjustment for potential confounding factors (P = .0093).
“This trial shows that hip arthroscopic surgery and personalized hip therapy both improved hip-related quality of life for patients with FAI [femoroacetabular impingement] syndrome, but that the surgery did indeed produce a greater improvement at our primary time point of 12 months,” she added. Dr. Foster is professor of musculoskeletal health in primary care at Keele University, Newcastle-under-Lyme, England, one of the 23 centers involved in the FASHIoN study in England, Wales, and Scotland.
“FAI is a very common cause of hip and groin pain in young adults, and it’s associated with the development of hip osteoarthritis,” Dr. Foster noted.
There are three types of FAI – pincer, cam, and combined. The pincer type of FAI is where there is “prominence or overcoverage of the rim of the acetabulum,” and the cam type is where there is a “bony prominence of the femoral head-neck junction,” she explained at the meeting sponsored by the Osteoarthritis Research Society International.
The link to OA comes when the femur and acetabulum prematurely connect, usually during activity, causing damage to the labrum and articular cartilage in the long term. Thus, treating FAI is important, not just for relieving patient’s pain and joint stiffness.
Hip arthroscopic surgery has become an established way of treating FAI syndrome – more than 2,400 operations were performed in 2013 in the United Kingdom alone, Dr. Foster observed. The aim of surgery is to try to reshape the hip joint to prevent impingement, and resect, repair, or reconstruct any intra-articular damage that may be present.
“Physiotherapy aims to improve hip muscle control and strength, and to correct the abnormal movement patterns that we see in these patients,” Dr. Foster said. “Through that, we hope to prevent the premature contact that occurs in FAI syndrome and thereby improve symptoms, allowing patients to return to activities and prevent recurrence.”
Working with physiotherapists, physicians, and surgeons, Dr. Foster and her associates have previously developed a “best conservative care” intervention that they call personalized hip therapy (PHT), which involves the delivery and supervision of an individualized exercise program by experienced physiotherapists over a 3- to 6-month period, and which patients repeat at home (PM R. 2013 May;5[5]:418-26).
The aim of the UK FASHIoN trial was to compare the clinical and cost-effectiveness of hip arthroscopy and PHT for FAI syndrome, as there was no robust clinical trial evidence to demonstrate a benefit of one over the other.
A total of 351 adults with hip and groin pain were randomized to either arthroscopic surgery (n = 173) or PHT (n = 178). The mean age of participants was 35 years, with no significant differences between the two treatment groups in terms of baseline demographics or type or duration of hip impingement.
While surgery was better in terms of patient outcomes, the study didn’t demonstrate its cost-effectiveness within the first 12 months, Dr. Foster observed. Cost-effectiveness, together with various other quality-of-life measurements, was a secondary endpoint of the study.
“Longer-term outcomes are required to establish whether improvement is sustained, and whether surgery is cost-effective at the longer time points for our health service,” she said.
Responding to a question about whether any of the patients in the study had radiographic evidence of osteoarthritis, Dr. Foster said that such patients had been excluded from the study.
“One of the hopes of the trial’s team is that, with the long-term follow-up, we might be able to get data at 5 and 10 years on things like hip osteoarthritis in these patients,” she said.
The study was funded by the National Institute for Health Research. Dr. Foster had no financial relationships or commercial interests to disclose.
SOURCE: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28
REPORTING FROM OARSI 2018
Key clinical point: Hip arthroscopy produced better results at 12 months than did the best conservative care.
Major finding: iHOT-33 scores at 12 months were 58.3 for surgery and 49.7 for personalized hip therapy (P = .0093)
Study details: Multicenter, randomized controlled UK FASHIoN trial of 351 adults with hip and groin pain.
Disclosures: The study was funded by the National Institute for Health Research. Dr. Foster had nothing to disclose.
Source: Griffin DR et al. Osteoarthritis Cartilage. 2018:26(1):S24-25. Abstract 28.
The Prevention and Treatment of Femoral Trial Head Loss in Total Hip Arthroplasty
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
TAKE-HOME POINTS
- Femoral head trial loss is a complication that can occur during THA.
- This event can be a source of avoidable morbidity.
- Preventative measures can be taken to avoid this complication.
- If preventative measures fail, retrieval of the femoral trial head can be performed.
- A thorough understanding of preventative and retrieval methods is essential for surgeons that perform THA.
Study links RA flares after joint replacement to disease activity, not medications
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Sixty-five percent of RA patients developed flares after joint replacement surgery, and it was more common in those with higher baseline RA activity (odds ratio, 2.11; P = .015).
Study details: Prospective study of 120 patients with RA who underwent hip replacement (44%) or knee replacement (56%).
Disclosures: The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. The lead author disclosed receiving research funding from Novartis and Roche.
Source: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366.
Total Hip Arthroplasty and Hemiarthroplasty: US National Trends in the Treatment of Femoral Neck Fractures
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
Does Knowledge of Implant Cost Affect Fixation Method Choice in the Management of Stable Intertrochanteric Hip Fractures?
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
Take-Home Points
- The incidence of geriatric hip fractures is rising nationally.
- Costs associated with hip fracture care have risen significantly.
- CMN and SHS are effective for stable, intertrochanteric hip fractures.
- Current trends show increased utilization of CMN compared to SHS for stable introchanteric hip fractures.
- Surgeon awareness of implant cost is a critical factor in delivering cost-effective, evidence-based surgical care.
The continuing increase in the population of patients older than 65 years in the United States is well known. For orthopedic surgeons, this trend highlights the importance of effective geriatric fracture care, particularly hip fracture care. Hip fractures in the elderly are expected to increase 50% by 2025 and to number 500,000 by 2040.1 The growing burden of hip fracture cases is accompanied by increasing costs of care. In 2012, 88% of the healthcare dollars spent on these patients were for direct fracture care, a significant increase from 60% in 2009.2 Although fewer than 1 in 5 fractures in the elderly are hip fractures, these injuries account for 72% of the total cost of geriatric fracture care, more than the total cost of all other osteoporosis-related injuries combined.1 Currently, the direct cost of hip fracture care ranges from $8358 to $32,195 and is expected, in total, to reach $25 billion by 2025.2,3
About 50% of geriatric hip fractures are extracapsular intertrochanteric or pertrochanteric.4 Several studies have compared sliding hip screws (SHSs) with cephalomedullary nails (CMNs) in the effective management of stable intertrochanteric fractures.5-11 Although these implants have shown an increased risk for peri-implant fracture and subsequent reoperation, markers such as mortality, medical complications, and restoration of prefracture function have all been equivocal relative to SHSs.12 Ultimately, one implant cannot be definitively recommended over the other for management of stable intertrochanteric hip fractures.13,14 Nevertheless, the current trend increasingly favors CMNs over SHSs.4,15 Most orthopedic surgeons are unaware of or underestimate the cost difference between these implants—a fact even more pronounced for newer implants.4,16 Considering the ever growing cost burden of hip fractures in the United States, orthopedists must consider not only the efficacy of care but the cost of delivery.
We conducted a study to determine the effect that surgeon knowledge of implant cost had on rates of use of SHSs and CMNs in the management of stable intertrochanteric hip fractures.
Patients and Methods
On May 1, 2012, all 9 attending orthopedic surgeons in a private practice group serving a suburban level II trauma center met to discuss implant prices and implant-related costs for the $850 Versafix SHS, the $1950 short Gamma3 nail (SGN), and the $2900 long Gamma3 nail (LGN), all manufactured by Stryker. All surgeons denied previous knowledge of the costs of these implants. During the discussion, no particular implant was recommended for management of any specific type of fracture. Surgeons were not directly instructed to consider implant cost in subsequent hip fracture surgeries and were not informed of our upcoming study of implant utilization.
After obtaining Institutional Review Board approval, we performed a retrospective chart and radiologic review of all hip fractures (Current Procedural Terminology [CPT] code 27244 or 27245) managed with fixation at our institution between May 1, 2011 and April 30, 2013. Two hundred six patients were identified (Figure 1).
One year later, surgeons were again shown their respective hip fracture radiographs, with patient identifying data removed. They were asked to reclassify their respective cases using the OTA system and then indicate the implant they would use for operative fixation in each of their cases.
Patient age, sex, injury side, fracture types, and utilization rates of the SHS, SGN, and LGN implants were compared between the groups. For each eligible patient, implant cost and other financial data were obtained from the hospital’s finance department. Statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) Version 20 for Macintosh. Independent 2-sample t test was used for parametric comparisons, and Fisher exact test was used for nonparametric comparisons.
Results
Examination of implant use per fracture classification revealed an interesting change. In the before group, SHS was the implant most commonly used for 31-A1.1 fractures (7/16, 43.8%), 31-A1.2 fractures (8/18, 44.4%), and 31-A2.1 fractures (10/25, 40.0%), and LGN was used in 66.7% (8/12) of 31-A1.3 fractures. By contrast, in the after group, SHS was most commonly used only for 31-A1.2 fractures (7/12, 58.3%), SGN was used in 90% (9/10) of 31-A1.1 fractures, and LGN was used in 42.1% (8/19) of 31-A2.1 fractures. In addition, 85.7% (6/7) of 31-A1.3 fractures were managed with a version of the Gamma nail.
Reclassification resulted in more A2.1 fractures (42.0% vs 37.0%) and fewer A1.3 fractures (10.1% vs 16.0%). About the same numbers of fractures were classified A1.1 (21.0% vs 21.8%) and A1.2 (26.9% vs 25.2%). SHS was favored for A1.1 fractures (92.0%) and A1.2 fractures (65.6%). SGN was favored for A1.3 fractures (75.0%). Gamma nails of both sizes were favored for A2.1 fractures (88.0%).
Discussion
Comparisons of SHS/plate and CMN constructs in the management of stable intertrochanteric hip fractures have long been discussed in the orthopedic literature. The major concern with CMNs (vs SHSs) is a statistically significantly higher rate of revision surgery, most often for peri-implant fracture. Rates of previous revision surgery for peri- implant fracture have ranged from 2.4% to 6% for CMNs and from 0.6% to 4% for SHSs.5-7,9 In a Cochrane review of 22 studies (3749 patients), Parker and Handoll12 compared CMN and SHS outcomes in 23 categories and found a statistically significant difference only in postoperative fracture rate. However, in a meta-analysis of studies conducted between 2000 and 2005, Bhandari and colleagues8 found no statistically significant difference in risk of femoral shaft fracture between CMNs (0.6%) and SHSs (0.1%). In addition, Utrilla and colleagues10 reported no postoperative fractures with use of Gamma3 CMNs. These recent studies may indicate that newer CMN designs can reduce the incidence of postoperative peri-implant fracture.8,10 Other outcome measures, such as 1-year mortality, functional outcome, and medical complication rate, have shown no statistically significant differences between the 2 implants.10-12 Ultimately, the current recommendation for fixation of stable intertrochanteric hip fractures is either SHS or CMN.13,14
Of our study patients, 78.9% (before group) and 64.6% (after group) were female, and 49.3% (before group) and 47.9% (after group) were between 80 and 89 years of age. Similarly, a review of hip fracture Medicare claims made between 1999 and 2002 revealed that >75% of the patients were females and 48% to 49% were octogenarians.4,18 However, our rates of different fracture types differed from those of Adams and colleagues.5 In a 1-year single-institution study, they found that, for both CMNs and SHSs, the most common stable intertrochanteric fractures were 31-A1.1 fractures; in our study’s before and after groups, more than one-third of injuries were 31-A2.1 fractures. Least common were 31-A1.3 fractures, both in the study by Adams and colleagues5 and in our before (16.9%) and after (14.6%) groups. Although our fracture rates differ from those of previous studies, all 4 classification categories fall under the umbrella of stable intertrochanteric hip fracture, which is the sole focus of this study.14
We hypothesized that cost would be a significant driver of implant choice in the management of these injuries. Given that SHS costs $1186.91 less than SGN and $1625.88 less than LGN at our institution, we expected that the before- discussion group’s overall SHS use rate of 38.0% would increase after discussion. Instead, SGN became the favored implant, with use in almost half of all fractures in the after group. Although the change in overall implant use rate was notable, these findings were not statistically significant. Examination of individual fracture patterns revealed 2 areas of interest. First, SHS was assumed to be the implant of choice in the management of the relatively simple 31-A1.1 fractures. Although this assumption was verified in the before group (SHS use in 43.8% of fractures), SGN was used in almost every case (90%) in the after group. However, when surgeons were asked 1 year later to recommend an implant for A1.1 fractures, 92% suggested SHS. The more cost-effective SHS construct may be the most amenable for use in these injury types given all intertrochanteric hip fracture patterns, though this has not been studied.
On the other hand, for 31-A2.1 fractures, perhaps the most complicated of the stable patterns, LGN became the implant of choice (42.1%). Despite surgeons’ awareness of the cost differences, management of these fractures shifted in the after group to the most expensive implant, indicative of surgeon concern about eventual loss of reduction with SHS and surgeon comfort with a particular procedure. This trend held when surgeons were asked to reclassify fractures 1 year later, as CMNs were recommended for 88% of 31-A2.1 fractures. Although both SHS and CMN were acceptable in 97% of the fractures included in this study, SGNs or LGNs were preferred for almost every fracture pattern involving the lesser trochanter. All 9 attending surgeons described involvement of the lesser trochanter as an indicator of posteromedial calcar injury. Surgeons became particularly concerned when this fracture pattern occurred in patients with significant osteopenia; SHS fixation, in their opinion, would be poor in the setting of a combination of greater posteromedial instability and poor bone quality. In a level I prospective, randomized trial, Barton and colleagues7 found no difference in outcomes between LGN and SHS fixation for 31-A2 proximal femur fractures and recommended SHS implants for the cost savings. In the clinical experience of this group, however, A1.3 and A2.1 fractures were at especially high risk for failure with SHS use, which necessitated greater implant stability through CMN fixation. On the other hand, for simpler fracture patterns, most surgeons suggested SHS implants. In their opinion, SGN and LGN implants offered no additional benefit of stability without evidence of posteromedial injury, even in the setting of osteopenia. For A1.2 fractures, posteromedial involvement was judged on the basis of size of the inferomedial spike or the extent of the inferomedial fracture line. Two surgeons preferred CMN for simple fractures, one because of the increased comfort with the implants and the other because of the minimally invasive surgical technique. Overall, our results indicate that knowledge of implant cost is not a strong enough factor to change surgeon behavior in selecting fixation for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients.
The lack of effect could be a consequence of surgeons’ training and comfort with various implants, especially among younger attending surgeons. Most of the attendings in the practice are under age 50 years, which correlates with a preference for CMN fixation.19 Case loads of >80 intertrochanteric hip fractures per calendar year, as in the after group, also correlates with more CMN use.19 However, the before group had more intertrochanteric hip fractures, and yet SHS was the implant of choice. Resident physician experience and comfort with various implants may play a role too, as teaching hospitals with resident assistance also correlate with CMN use.19 However, no major change in resident physician involvement was undertaken during this period. The institution studied is near a major metropolis in the Northeast, a region that has disfavored SHS in recent years.18 The change from before to after fits an overall trend in changing implant use. Anglen and colleagues15 found a significant decrease in SHS use, from 97% in 1999 to 33% in 2006, for intertrochanteric fracture fixation. Simultaneously, CMN use increased from 3% to 67%.
This study had several limitations. First, its overall sample size was small, and therefore any data fluctuations may be exaggerated. Furthermore, changes in utilization rates were compared over 2 years, which may not be long enough to show a changing trend in implant selection. Post hoc analysis of the sample size determined a power of 0.76 for an α of 0.05 and an effect size of 0.50. Second, radiologic classification was performed in a retrospective review, not officially by the operative surgeon. Fractures that we considered stable may have been considered unstable by the operative surgeon, influencing implant selection. Third, patients were selected from only one hospital, and orthopedic surgeons from other institutions may be more sensitive to cost considerations, changing implant selection more quickly. Fourth, initial selection of patients by CPT code might not have captured all those who satisfied the inclusion criteria. Fifth, only a single intervention was used, and follow-up meetings certainly could have increased the effectiveness of the intervention. Last, this and other retrospective studies are inherently weaker because of possible bias.
Conclusion
Our study results showed that implant cost is not a significant factor in implant selection for uncomplicated stable intertrochanteric hip fractures in previously ambulatory elderly patients. By itself, knowledge of implant cost may not be a strong enough force to change surgeon behavior or preference secondary to consequences of failure or comfort with particular implants. In an economic climate in which healthcare is scrutinized for both its medical effectiveness and cost-effectiveness, further study of forces that could influence orthopedic surgeons to select a more cost-effective implant is warranted.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475.
2. Kilgore ML, Curtis JR, Delzell E, et al. A close examination of healthcare expenditures related to fractures. J Bone Miner Res. 2013;28(4):816-820.
3. Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170.
4. Aros B, Tosteson AN, Gottlieb DJ, Koval KJ. Is a sliding hip screw or IM nail the preferred implant for intertrochanteric fracture fixation? Clin Orthop Relat Res. 2008;466(11):2827-2832.
5. Adams CI, Robinson CM, Court-Brown CM, McQueen MM. Prospective randomized controlled trial of an intramedullary nail versus dynamic screw and plate for intertrochanteric fractures of the femur. J Orthop Trauma. 2001;15(6):394-400.
6. Ahrengart L, Törnkvist H, Fornander P, et al. A randomized study of the compression hip screw and Gamma nail in 426 fractures. Clin Orthop Relat Res. 2002;(401):209-222.
7. Barton TM, Gleeson R, Topliss C, Greenwood R, Harries WJ, Chesser TJ. A comparison of the long Gamma nail with the sliding hip screw for the treatment of AO/OTA 31-A2 fractures of the proximal part of the femur: a prospective randomized trial. J Bone Joint Surg Am. 2010;92(4):792-798.
8. Bhandari M, Schemitsch E, Jönsson A, Zlowodzki M, Haidukewych GJ. Gamma nails revisited: Gamma nails versus compression hip screws in the management of intertrochanteric fractures of the hip: a meta-analysis. J Orthop Trauma. 2009;23(6):460-464.
9. Osnes EK, Lofthus CM, Falch JA, et al. More postoperative femoral fractures with the Gamma nail than the sliding screw plate in the treatment of trochanteric fractures. Acta Orthop Scand. 2001;72(3):252-256.
10. Utrilla AL, Reig JS, Muñoz FM, Tufanisco CB. Trochanteric Gamma nail and compression hip screw for trochanteric fractures. J Orthop Trauma. 2005;19(4):229-233.
11. Verettas DA, Ifantidis P, Chatzipapas CN, et al. Systematic effects of surgical treatment of hip fractures: gliding screw-plating vs intramedullary nailing. Injury. 2010;41(3):279-284.
12. Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst Rev. 2010;(9):CD000093.
13. Kaplan K, Miyamoto R, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. II: intertrochanteric fractures. J Am Acad Orthop Surg. 2008;16(11):665-673.
14. Lindskog DM, Baumgaertner MR. Unstable intertrochanteric hip fractures in the elderly. J Am Acad Orthop Surg. 2004;12(3):179-190.
15. Anglen JO, Weinstein JN; American Board of Orthopaedic Surgery Research Committee. Nail or plate fixation of intertrochanteric hip fractures: changing pattern of practice. A review of the American Board of Orthopaedic Surgery Database. J Bone Joint Surg Am. 2008;90(4):700-707.
16. Streit JJ, Youssef A, Coale RM, Carpenter JE, Marcus RE. Orthopaedic surgeons frequently underestimate the cost of orthopaedic implants. Clin Orthop Relat Res. 2013;471(6):1744-1749.
17. Marsh JL, Slongo TF, Agel J, et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007;21(10 suppl):S1-S133.
18. Forte ML, Virnig BA, Kane RL, et al. Geographic variation in device use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2008;90(4):691-699.
19. Forte ML, Virnig BA, Eberly LE, et al. Provider factors associated with intramedullary nail use for intertrochanteric hip fractures. J Bone Joint Surg Am. 2010;92(5):1105-1114.
For women with RA, small-joint surgery rate nearly twice that of men
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
AT ACR 2017
Key clinical point: Women with RA had a higher rate of small-joint surgery, compared with men.
Major finding: The cumulative incidence of small-joint surgery was significantly higher among women, compared with men (14.4% vs. 7.6%, respectively), but there were no differences between sexes in the rates of large-joint surgery.
Study details: A retrospective, population-based study of 1,077 patients with RA.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.