User login
Study links severe childhood eczema to sedentary behaviors
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
SCOTTSDALE, ARIZ. – Children with severe atopic dermatitis were significantly more likely to log at least 5 hours of screen time a day, and were significantly less likely to exercise than were nonatopic controls, said the lead investigator of a large national study.
“Atopic dermatitis overall was not associated with sedentary behavior. It was severe disease only,” said Mark Strom of the department of dermatology, Northwestern University, Chicago, during an oral presentation at the annual meeting of the Society for Investigative Dermatology. Patients tended to be even more sedentary if they suffered from disturbed sleep in addition to severe eczema, he added.
Heat and sweat worsen the intense itch of atopic dermatitis. Hypothesizing that this would deter affected children from physical activity, Mr. Strom and his associates analyzed data for 131,783 respondents aged 18 and under from the National Survey of Children’s Health. The survey assesses physical activity by asking how many days a week the respondent sweated and breathed hard for at least 20 minutes. Screen time is measured by asking about daily hours spent watching television and playing video games, and sleep quality is assessed by asking how many nights a week the child slept the normal amount for his or her age.
Simply having atopic dermatitis was linked with only a slight increase in the chance of having a sedentary lifestyle after controlling for demographic factors, insurance status, geographic location, and educational level, according to Mr. Strom. Specifically, eczema was significantly associated with a 12% lower odds of having exercised on at least 3 days of the previous week (odds ratio, 0.88). However, severe atopic dermatitis significantly reduced the odds that a child exercised at least one day a week by 61% (OR, 0.39). Furthermore, severe atopic dermatitis was associated with more than double the odds of having at least 5 hours of daily screen time (OR, 2.62). And having either moderate or severe eczema was tied to a significant decrease in the odds of having participated in sports in the past year, Mr. Strom said.
“Atopic dermatitis and sleep disturbance each contribute to sedentary behavior,” he reported. Nonatopic children who did not sleep enough on most nights had nearly double the odds of heavy television and video game use, compared with children who slept more, a significant difference. When poor sleepers also had atopic dermatitis, their odds of heavy screen use more than tripled. Poor sleepers were also significantly less likely to join sports teams, even when they did not have eczema, Mr. Strom said.
“Children with more severe atopic dermatitis may have more profound exacerbations of activity-related symptoms, which would lead to these findings,” he concluded. Future studies should explore whether better symptom control can help improve sedentary behaviors, he added.
The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A large national study linked severe atopic dermatitis to sedentary behaviors and screen time.
Major finding: Compared with children without eczema, those with severe disease were about 60% less likely to exercise at least once a week (OR, 0.39).
Data source: An analysis of data for 131,783 children from the National Survey of Children’s Health.
Disclosures: The study was sponsored by the Maternal and Child Health Bureau of the U.S. Department of Health and Human Services. Mr. Strom had no disclosures.
Nonwhite race, lower socioeconomic status predicts persistently active AD
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Persistently active atopic dermatitis is associated with nonwhite race, annual household income under $50,000, female sex, and history of atopy.
Major finding: Nonwhite race and history of atopy each lowered the odds of complete disease control by about 43% (odds ratios, 0.53; P less than .05).
Data source: A longitudinal cohort study of 6,237 patients aged 2-26 years from the Pediatric Eczema Elective Registry (PEER).
Disclosures: Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
Nonwhite race, lower socioeconomic status predicts persistently active AD
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Persistently active atopic dermatitis is associated with nonwhite race, annual household income under $50,000, female sex, and history of atopy.
Major finding: Nonwhite race and history of atopy each lowered the odds of complete disease control by about 43% (odds ratios, 0.53; P less than .05).
Data source: A longitudinal cohort study of 6,237 patients aged 2-26 years from the Pediatric Eczema Elective Registry (PEER).
Disclosures: Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
Febrile, Immunocompromised Man With Rash
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
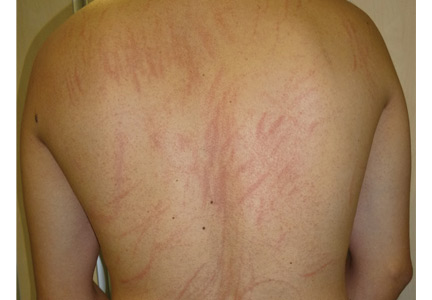
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
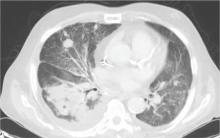
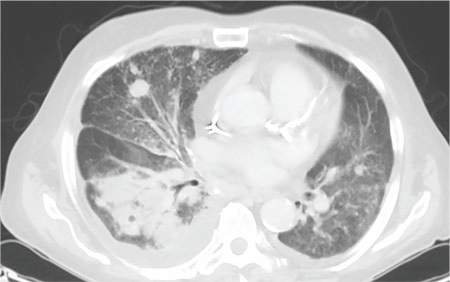
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
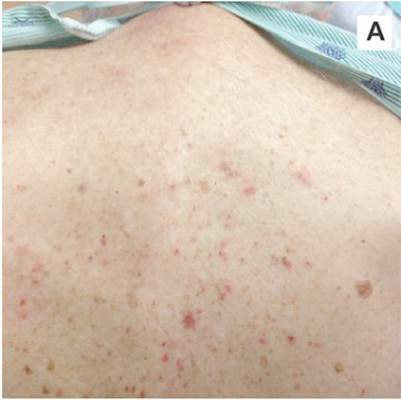 | 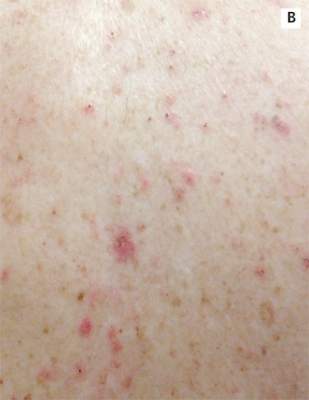 |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
 |  |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
IN THIS ARTICLE
- Conditions associated with increased risk for case disease
- Outcome for the case patient
- Differential diagnosis
A 78-year-old white man with chronic lymphocytic leukemia is admitted to the hospital with worsening cough, shortness of breath, and fever. His medical history is significant for pneumonia caused by Pneumocystis jirovecii in the past year. In the weeks preceding hospital admission, the patient developed an erythematous rash over his trunk (see photographs).
During the man’s hospital stay, this eruption becomes increasingly pruritic and spreads to his proximal extremities. His pulmonary symptoms improve slightly following the initiation of broad-spectrum antibiotic therapy (piperacillin/tazobactam and vancomycin), but CT performed one week after admission reveals worsening pulmonary disease (see image). The radiologist’s differential diagnosis includes neoplasm, fungal infection, Kaposi sarcoma, and autoimmune disease.
 |  |
| A. The patient's back shows a distribution of lesions, with areas of excoriation caused by scratching. | B. A close-up reveals erythematous papules and keratotic papules. |
Suspecting that the progressive rash is related to the systemic process, the provider orders a punch biopsy in an effort to reach a diagnosis with minimally invasive studies. When the patient’s clinical status further declines, he undergoes video-assisted thoracoscopic surgery to obtain an excisional biopsy of one of the pulmonary nodules. Subsequent analysis reveals fungal organisms consistent with histoplasmosis. Interestingly, in the histologic review of the skin biopsy, focal acantholytic dyskeratosis—suggestive of Grover disease—is identified.
Continue for discussion >>
DISCUSSION
Grover disease (GD), also known as transient acantholytic dermatosis, is a skin condition of uncertain pathophysiology. Its clinical presentation can be difficult to distinguish from other dermopathies.1,2
Incidence
GD most commonly appears in fair-skinned persons of late middle age, with men affected at two to three times the rate seen in women.1,2 Although GD has been documented in patients ranging in age from 4 to 100, this dermopathy is rare in younger patients.1-3 Persons with a prior history of atopic dermatitis, contact dermatitis, or xerosis cutis are at increased risk for GD—likely due to an increased dermatologic sensitivity to irritants resulting from the aforementioned disorders.1,4 Risk for GD is also elevated in patients with chronic medical conditions, immunodeficiency, febrile illnesses, or malignancies (see Table 1).2-5
The true incidence of GD is not known; biopsy-proven GD is uncommon, and specific data on the incidence and prevalence of the condition are lacking. Swiss researchers who reviewed more than 30,000 skin biopsies in the late 1990s noted only 24 diagnosed cases of GD, and similar findings have been reported in the United States.1,6 However, the variable presentation and often mild nature of GD may result in cases of misdiagnosis, lack of diagnosis, or empiric treatment in the absence of a formal diagnosis.7
Causative factors
Although the pathophysiology of GD is uncertain, the most likely cause is an occlusion of the eccrine glands.3 This is followed by acantholysis, or separation of keratinocytes within the epidermis, which in turn leads to the development of vesicular lesions.
Though diagnosed most often in the winter, GD has also been associated with exposure to sunlight, heat, xerosis, and diaphoresis.1,3 Hospitalized or bedridden patients are at risk for occlusion of the eccrine glands and thus for GD. Use of certain therapies, including sulfadoxine/pyrimethamine (an antimalarial treatment), ionizing radiation, and interleukin-4, may also be precursors for the condition.2
Other exacerbating factors have been suggested, but reports are largely limited to case studies and other anecdotal publications.2 Concrete data regarding the etiology and pathophysiology of GD are still relatively scarce.
Clinical presentation
Patients with GD present with pruritic dermatitis on the trunk and proximal extremities, most classically on the anterior chest and mid back.2,3 The severity of the rash does not necessarily correlate to the degree of pruritus. Some patients report only mild pruritus, while others experience debilitating discomfort and pain. In most cases, erythematous and violaceous papules and vesicles appear first, followed by keratotic erosions.3
GD is a self-limited disorder that often resolves within a few weeks, although some cases will persist for several months.3,5 Severity and duration of symptoms appear to be correlated with increasing age; elderly patients experience worse pruritus for longer periods than do younger patients.2
Although the condition is sometimes referred to as transient acantholytic dermatosis, there are three typical presentations of GD: transient eruptive, persistent pruritic, and chronic asymptomatic.4 Transient eruptive GD presents suddenly, with intense pruritus, and tends to subside over several weeks. Persistent pruritic disease generally causes a milder pruritus, with symptoms that last for several months and are not well controlled by medication. Chronic asymptomatic GD can be difficult to treat medically, yet this form of the disease typically causes little to no irritation and requires minimal therapeutic intervention.4
Systemic symptoms of GD have not been observed. Pruritus and rash are the main features in most affected patients. However, pruritic papulovesicular eruptions are commonly seen in other conditions with similar characteristics (see Table 2,3,4), and GD is comparatively rare. While clinical appearance alone may suggest a diagnosis of GD, further testing may be needed to eliminate other conditions from the differential.
Treatment and prognosis
In the absence of randomized therapeutic trials for GD, there are no strict guidelines for treatment. When irritation, inflammation, and pruritus become bothersome, several interventions may be considered. The first step may consist of efforts to modify aggravating factors, such as dry skin, occlusion, excess heat, and rapid temperature changes. Indeed, for mild cases of GD, this may be all that is required.
The firstline pharmacotherapy for GD is medium- to high-potency topical corticosteroids, which reduce inflammation and pruritus in approximately half of affected patients.3,6,8 Topical emollients and oral antihistamines can also provide symptom relief. Vitamin D analogues are considered secondline therapy, and retinoids (both topical and systemic) have also been shown to reduce GD severity.3,4,8
Severe, refractory cases may require more aggressive systemic therapy with corticosteroids or retinoids. For pruritic relief, several weeks of oral corticosteroids may be necessary—and GD may rebound after treatment ceases.3,4 Therefore, oral corticosteroids should only be considered for severe or persistent cases, since the systemic adverse effects (eg, immunosuppression, weight gain, dysglycemia) of these drugs may outweigh the benefits in patients with GD. Other interventions, including phototherapy and immunosuppressive drugs (eg, etanercept) have also demonstrated benefit in select patients.4,9,10
The self-limited nature of GD, along with its lack of systemic symptoms, is associated with a generally benign course of disease and no long-term sequelae.3,5
Continue to outcome for the case patient >>
OUTCOME FOR THE CASE PATIENT
This case involved an immunocompromised patient with systemic symptoms, vasculitic cutaneous lesions, and significant pulmonary disease. The differential diagnosis was extensive, and diagnosis based on clinical grounds alone was extremely challenging. In these circumstances, diagnostic testing was essential to reach a final diagnosis.
In this case, the skin biopsy yielded a diagnosis of GD, and the rash was found to be unrelated to the patient’s systemic and pulmonary symptoms. The providers were then able to focus on the diagnosis of histoplasmosis, with only minimal intervention for the patient’s GD (ie, oral diphenhydramine prn for pruritus).
CONCLUSION
In many cases of GD, skin biopsy can guide providers when the history and physical examination do not yield a clear diagnosis. The histopathology of affected tissue can provide invaluable information about an underlying disease process, particularly in complex cases such as this patient’s. Skin biopsy provides a minimally invasive opportunity to obtain a diagnosis in patients with a condition that affects multiple organ systems, and its use should be considered in disease processes with cutaneous manifestations.
REFERENCES
1. Scheinfeld N, Mones J. Seasonal variation of transient acantholytic dyskeratosis (Grover’s disease). J Am Acad Dermatol. 2006;55(2): 263-268.
2. Parsons JM. Transient acantholytic dermatosis (Grover’s disease): a global perspective. J Am Acad Dermatol. 1996;35(5 part 1):653-666.
3. Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133(9):1490-1494.
4. Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86.
5. Ippoliti G, Paulli M, Lucioni M, et al. Grover’s disease after heart transplantation: a case report. Case Rep Transplant. 2012;2012:126592.
6. Streit M, Paredes BE, Braathen LR, Brand CU. Transitory acantholytic dermatosis (Grover’s disease): an analysis of the clinical spectrum based on 21 histologically assessed cases [in German]. Hautarzt. 2000;51:244-249.
7. Joshi R, Taneja A. Grover’s disease with acrosyringeal acantholysis: a rare histological presentation of an uncommon disease. Indian J Dermatol. 2014;59(6):621-623.
8. Riemann H, High WA. Grover’s disease (transient and persistent acantholytic dermatosis). UpToDate. 2015. www.uptodate.com/contents/grovers-disease-transient-and-persistent-acantholytic-dermatosis. Accessed June 4, 2016.
9. Breuckmann F, Appelhans C, Altmeyer P, Kreuter A. Medium-dose ultraviolet A1 phototherapy in transient acantholytic dermatosis (Grover’s disease). J Am Acad Dermatol. 2005;52(1):169-170.
10. Norman R, Chau V. Use of etanercept in treating pruritus and preventing new lesions in Grover disease. J Am Acad Dermatol. 2011;64(4):796-798.
The Promise of Peanut Allergy Prevention Lies in Draft Guidelines
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
The promise of peanut allergy prevention lies in draft guidelines
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Updated guidelines from the National Institute of Allergy and Infectious Diseases for the early introduction of peanut-containing foods to children at increased risk for peanut allergies are on the horizon, pending final approval.
“Two studies recently showed that infants at high risk of developing peanut allergy [infants with egg allergy and or severe eczema] were much less likely to have peanut allergy at age 5 years if they were able to incorporate peanut regularly into the diet between 4 and 11 months of age,” said Dr. Scott H. Sicherer, the Elliot and Roslyn Jaffe Professor of Pediatrics, Allergy and Immunology, and chief of the division of allergy and immunology in the department of pediatrics at the Icahn School of Medicine at Mount Sinai, New York.
“However, adding peanut to the diet at this age requires caution because these infants may already be allergic to peanut, and so allergy testing and care in adding peanut to the diet with medical supervision is needed in this high-risk group,” noted Dr. Sicherer, a member of the expert panel that worked on the guidelines.
The draft guidelines include 43 clinical recommendations for the diagnosis and management of food allergies in children, according to the NIAID website. In particular, the draft guidelines recommend introducing peanut-containing foods to infants aged 4-6 months who are at increased risk for peanut allergy because of severe eczema and/or egg allergies, after an evaluation with skin prick testing or peanut-specific IgE testing.
“Peanut allergy is relatively common and often persistent, and so a strategy that could prevent the allergy is very important,” Dr. Sicherer said in an interview. “However, peanut can be a choking hazard as peanuts or peanut butter, and so families should talk to their pediatrician about how and when to incorporate peanut into the diet, and whether allergy testing and referral to an allergist is needed.”
Support for the guidelines comes from several large studies with promising results, notably the LEAP (Learning Early about Peanut Allergy) trial. A recent extension of that study, known as LEAP-On (Persistence of Oral Tolerance to Peanut), showed that regular consumption of peanut-containing foods from infancy to 5 years provided ongoing protection against allergies, even 6 years after peanut consumption was discontinued for a 1-year period in 550 children (N Eng J Med. 2016 Apr 14;374:1435-43).
In the original LEAP study, 640 infants aged 4-11 months with severe eczema, egg allergy, or both were randomized to dietary peanut consumption or avoidance (N Engl J Med. 2015 Feb 26;372[9]:803-13). The prevalence of peanut allergy at 5 years of age was approximately 2% in the peanut-consumption group, compared with 14% in the peanut-avoidance group.
Another significant randomized trial, the EAT study (Enquiring About Tolerance) tested not only peanut, but also the early introduction of cooked egg, cow’s milk, sesame, wheat, and fish to 1,303 infants aged 3 months and older in the general population. The study’s strict protocol made adherence difficult, but researchers found a significant 67% reduction in the prevalence of food allergies at age 3 years among the children who followed the protocol, compared with controls, with relative risk reductions of 100% and 75%, respectively, for peanut and egg allergies (N Engl J Med. 2016 May 5;374:1733-43).
The next steps for research should make early introduction of peanut-containing foods even more effective at allergy prevention, Dr. Sicherer noted.
“We need to learn more about how much peanut should be incorporated into the diet, how long the protein has to be kept in the diet to have the best preventative effect, and whether this strategy applies to other foods,” he said.
Skin patch testing pinpoints dietary triggers of IBS
SAN DIEGO – About 90% of patients reported improvement in symptoms of irritable bowel syndrome after avoiding type 4 food allergens identified by skin patch testing, according to an uncontrolled study.
Furthermore, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted, said Dr. Michael Stierstorfer, a dermatologist at East Penn Dermatology in North Wales, Pa., who partnered with gastroenterologists at Temple University to conduct the study. “This raises questions about a possible overlap between IBS and allergic contact enteritis,” the researchers stated in a poster presented at the annual Digestive Disease Week.
Irritable bowel syndrome is often treatment refractory and tends to elude conventional diagnostics. That was the case for Dr. Stierstorfer, who several years ago developed symptoms of IBS with constipation (IBS-C) that eventually affected him about half the time, he said in an interview. A hydrogen breath test, upper endoscopy, colonoscopy, abdominal/pelvic CT, and tests for gluten-sensitive enteropathy and parasites revealed no abnormalities except decreased small intestinal motility, he said.
But after “flaring badly” twice when he ate Indian food, he began to suspect a cause. “I stopped eating garlic and within a day, I was absolutely fine,” Dr. Stierstorfer said. “The symptoms recurred only if I accidentally ate garlic again.”
Studies had refuted links between IBS and type 1 hypersensitivity but had not explored the role of type 4 (delayed) hypersensitivity in the disorder, Dr. Stierstorfer discovered. “Dermatologists do patch testing all the time for patients with refractory eczema to search for type 4 allergic contact factors that might be causing their rash,” he said. “I performed a patch test of garlic on myself to look for a type 4 allergy, and it was strongly positive. I thought I probably wasn’t the only person walking around with symptoms that mimicked IBS but were really from a type 4 food allergy.”
He tested that idea by skin patch testing 50 patients with IBS symptoms whom he recruited through his dermatology practice. In all, 30 (60%) patients reacted to at least one food allergen, of whom 14 (46%) reported symptomatic improvement after eliminating the suspected triggers from their diets. The findings appeared in the March 2013 Journal of the American Academy of Dermatology (68:377-84).
Next, Dr. Stierstorfer partnered with Dr. Grace Shin, a 3rd-year gastroenterology fellow at Temple University, Philadelphia, and her colleagues. Together, they tested 57 patients with physician-diagnosed IBS with diarrhea (about 43% of patients), IBS with constipation (16%), mixed IBS (30%), or unsubtyped IBS (11%). Patients averaged 41 years of age (standard deviation, 15 years) and 77% were female. Each patient had between 118 and 122 individual allergen patches placed on his or her back. Two days later, the patches were removed and the skin evaluated for macular erythema consistent with a type 4 hypersensitivity reaction. The patients were checked again a day or 2 later to catch any highly delayed reactions.
In all, 56 patients (98%) showed evidence of at least one hypersensitivity, and most reacted to between two and three allergens, Dr. Stierstorfer said. The most commonly identified triggers were cinnamon bark (35 patients; 61%) and sodium bisulfite (26 patients; 46%). At baseline, patients rated their abdominal pain or discomfort at an average of 6.7 on a 10-point severity scale (SD, 2.3 points). After 2-4 weeks of avoiding allergens to which they developed macular edema, they reported a mean 4.4-point improvement in their abdominal symptoms (SD, 2.7 points; P less than .001).
The patients also reported an average 5.8-point improvement on a 10-point scale of global IBS symptom severity (SD, 3.2 points; P less than .001). Overall, 91% of patients reported at least partial relief of abdominal symptoms, while 89% of patients reported at least partial relief of global symptoms, the investigators reported.
Based on these results, “food-related type 4 hypersensitivity reactions may contribute to the pathogenesis of IBS and IBS-like symptoms,” Dr. Shin said in an interview. “The idea of allergic contact enteritis intrigued me, because it made me think that some patients diagnosed with IBS, especially IBS with diarrhea, might benefit from allergy testing when the standard approaches don’t work.”
Another dietary intervention for IBS, the low-FODMAP diet, can help relieve symptoms, “but it’s a hard diet to follow,” Dr. Shin added. “Being able to focus on eliminating one or two things would be easier than eliminating multiple classes of foods that are so common to an American diet.”
Next, the team is planning a controlled trial of the skin patch test. “There is still more validation work to do,” said Dr. Stierstorfer. “But I think this shows that looking at something from a unique perspective – in this case, a dermatologic perspective for a GI problem – can result in a new approach, and potentially an advance in medicine.”
Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
SAN DIEGO – About 90% of patients reported improvement in symptoms of irritable bowel syndrome after avoiding type 4 food allergens identified by skin patch testing, according to an uncontrolled study.
Furthermore, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted, said Dr. Michael Stierstorfer, a dermatologist at East Penn Dermatology in North Wales, Pa., who partnered with gastroenterologists at Temple University to conduct the study. “This raises questions about a possible overlap between IBS and allergic contact enteritis,” the researchers stated in a poster presented at the annual Digestive Disease Week.
Irritable bowel syndrome is often treatment refractory and tends to elude conventional diagnostics. That was the case for Dr. Stierstorfer, who several years ago developed symptoms of IBS with constipation (IBS-C) that eventually affected him about half the time, he said in an interview. A hydrogen breath test, upper endoscopy, colonoscopy, abdominal/pelvic CT, and tests for gluten-sensitive enteropathy and parasites revealed no abnormalities except decreased small intestinal motility, he said.
But after “flaring badly” twice when he ate Indian food, he began to suspect a cause. “I stopped eating garlic and within a day, I was absolutely fine,” Dr. Stierstorfer said. “The symptoms recurred only if I accidentally ate garlic again.”
Studies had refuted links between IBS and type 1 hypersensitivity but had not explored the role of type 4 (delayed) hypersensitivity in the disorder, Dr. Stierstorfer discovered. “Dermatologists do patch testing all the time for patients with refractory eczema to search for type 4 allergic contact factors that might be causing their rash,” he said. “I performed a patch test of garlic on myself to look for a type 4 allergy, and it was strongly positive. I thought I probably wasn’t the only person walking around with symptoms that mimicked IBS but were really from a type 4 food allergy.”
He tested that idea by skin patch testing 50 patients with IBS symptoms whom he recruited through his dermatology practice. In all, 30 (60%) patients reacted to at least one food allergen, of whom 14 (46%) reported symptomatic improvement after eliminating the suspected triggers from their diets. The findings appeared in the March 2013 Journal of the American Academy of Dermatology (68:377-84).
Next, Dr. Stierstorfer partnered with Dr. Grace Shin, a 3rd-year gastroenterology fellow at Temple University, Philadelphia, and her colleagues. Together, they tested 57 patients with physician-diagnosed IBS with diarrhea (about 43% of patients), IBS with constipation (16%), mixed IBS (30%), or unsubtyped IBS (11%). Patients averaged 41 years of age (standard deviation, 15 years) and 77% were female. Each patient had between 118 and 122 individual allergen patches placed on his or her back. Two days later, the patches were removed and the skin evaluated for macular erythema consistent with a type 4 hypersensitivity reaction. The patients were checked again a day or 2 later to catch any highly delayed reactions.
In all, 56 patients (98%) showed evidence of at least one hypersensitivity, and most reacted to between two and three allergens, Dr. Stierstorfer said. The most commonly identified triggers were cinnamon bark (35 patients; 61%) and sodium bisulfite (26 patients; 46%). At baseline, patients rated their abdominal pain or discomfort at an average of 6.7 on a 10-point severity scale (SD, 2.3 points). After 2-4 weeks of avoiding allergens to which they developed macular edema, they reported a mean 4.4-point improvement in their abdominal symptoms (SD, 2.7 points; P less than .001).
The patients also reported an average 5.8-point improvement on a 10-point scale of global IBS symptom severity (SD, 3.2 points; P less than .001). Overall, 91% of patients reported at least partial relief of abdominal symptoms, while 89% of patients reported at least partial relief of global symptoms, the investigators reported.
Based on these results, “food-related type 4 hypersensitivity reactions may contribute to the pathogenesis of IBS and IBS-like symptoms,” Dr. Shin said in an interview. “The idea of allergic contact enteritis intrigued me, because it made me think that some patients diagnosed with IBS, especially IBS with diarrhea, might benefit from allergy testing when the standard approaches don’t work.”
Another dietary intervention for IBS, the low-FODMAP diet, can help relieve symptoms, “but it’s a hard diet to follow,” Dr. Shin added. “Being able to focus on eliminating one or two things would be easier than eliminating multiple classes of foods that are so common to an American diet.”
Next, the team is planning a controlled trial of the skin patch test. “There is still more validation work to do,” said Dr. Stierstorfer. “But I think this shows that looking at something from a unique perspective – in this case, a dermatologic perspective for a GI problem – can result in a new approach, and potentially an advance in medicine.”
Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
SAN DIEGO – About 90% of patients reported improvement in symptoms of irritable bowel syndrome after avoiding type 4 food allergens identified by skin patch testing, according to an uncontrolled study.
Furthermore, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted, said Dr. Michael Stierstorfer, a dermatologist at East Penn Dermatology in North Wales, Pa., who partnered with gastroenterologists at Temple University to conduct the study. “This raises questions about a possible overlap between IBS and allergic contact enteritis,” the researchers stated in a poster presented at the annual Digestive Disease Week.
Irritable bowel syndrome is often treatment refractory and tends to elude conventional diagnostics. That was the case for Dr. Stierstorfer, who several years ago developed symptoms of IBS with constipation (IBS-C) that eventually affected him about half the time, he said in an interview. A hydrogen breath test, upper endoscopy, colonoscopy, abdominal/pelvic CT, and tests for gluten-sensitive enteropathy and parasites revealed no abnormalities except decreased small intestinal motility, he said.
But after “flaring badly” twice when he ate Indian food, he began to suspect a cause. “I stopped eating garlic and within a day, I was absolutely fine,” Dr. Stierstorfer said. “The symptoms recurred only if I accidentally ate garlic again.”
Studies had refuted links between IBS and type 1 hypersensitivity but had not explored the role of type 4 (delayed) hypersensitivity in the disorder, Dr. Stierstorfer discovered. “Dermatologists do patch testing all the time for patients with refractory eczema to search for type 4 allergic contact factors that might be causing their rash,” he said. “I performed a patch test of garlic on myself to look for a type 4 allergy, and it was strongly positive. I thought I probably wasn’t the only person walking around with symptoms that mimicked IBS but were really from a type 4 food allergy.”
He tested that idea by skin patch testing 50 patients with IBS symptoms whom he recruited through his dermatology practice. In all, 30 (60%) patients reacted to at least one food allergen, of whom 14 (46%) reported symptomatic improvement after eliminating the suspected triggers from their diets. The findings appeared in the March 2013 Journal of the American Academy of Dermatology (68:377-84).
Next, Dr. Stierstorfer partnered with Dr. Grace Shin, a 3rd-year gastroenterology fellow at Temple University, Philadelphia, and her colleagues. Together, they tested 57 patients with physician-diagnosed IBS with diarrhea (about 43% of patients), IBS with constipation (16%), mixed IBS (30%), or unsubtyped IBS (11%). Patients averaged 41 years of age (standard deviation, 15 years) and 77% were female. Each patient had between 118 and 122 individual allergen patches placed on his or her back. Two days later, the patches were removed and the skin evaluated for macular erythema consistent with a type 4 hypersensitivity reaction. The patients were checked again a day or 2 later to catch any highly delayed reactions.
In all, 56 patients (98%) showed evidence of at least one hypersensitivity, and most reacted to between two and three allergens, Dr. Stierstorfer said. The most commonly identified triggers were cinnamon bark (35 patients; 61%) and sodium bisulfite (26 patients; 46%). At baseline, patients rated their abdominal pain or discomfort at an average of 6.7 on a 10-point severity scale (SD, 2.3 points). After 2-4 weeks of avoiding allergens to which they developed macular edema, they reported a mean 4.4-point improvement in their abdominal symptoms (SD, 2.7 points; P less than .001).
The patients also reported an average 5.8-point improvement on a 10-point scale of global IBS symptom severity (SD, 3.2 points; P less than .001). Overall, 91% of patients reported at least partial relief of abdominal symptoms, while 89% of patients reported at least partial relief of global symptoms, the investigators reported.
Based on these results, “food-related type 4 hypersensitivity reactions may contribute to the pathogenesis of IBS and IBS-like symptoms,” Dr. Shin said in an interview. “The idea of allergic contact enteritis intrigued me, because it made me think that some patients diagnosed with IBS, especially IBS with diarrhea, might benefit from allergy testing when the standard approaches don’t work.”
Another dietary intervention for IBS, the low-FODMAP diet, can help relieve symptoms, “but it’s a hard diet to follow,” Dr. Shin added. “Being able to focus on eliminating one or two things would be easier than eliminating multiple classes of foods that are so common to an American diet.”
Next, the team is planning a controlled trial of the skin patch test. “There is still more validation work to do,” said Dr. Stierstorfer. “But I think this shows that looking at something from a unique perspective – in this case, a dermatologic perspective for a GI problem – can result in a new approach, and potentially an advance in medicine.”
Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
AT DDW® 2016
Key clinical point: Avoiding food allergens identified by skin patch testing significantly improved self-reported symptoms of irritable bowel syndrome.
Major finding: In all, 69% of patients reported at least moderate improvement after eliminating foods to which they reacted.
Data source: A single-arm proof-of-concept study of 57 patients with physician-diagnosed IBS.
Disclosures: Dr. Shin had no disclosures. Dr. Stierstorfer disclosed financial ties to IBS Centers for Advanced Food Allergy Testing.
Whiplash-shaped acute rash
A previously healthy 32-year-old man presented to the emergency room with a persistent, nonpruritic rash on his trunk, which had suddenly appeared 2 days after he ate Chinese food.
Physical examination revealed multiple crosslinked linear plaques that appeared like scratches over his chest, back, and shoulders (Figures 1 and 2). He had no dermatographism, and his scalp, nails, palms, and soles were not affected. He had no signs of lymphadenopathy or systemic involvement.
Basic blood and urinary laboratory testing, blood cultures, and serologic studies showed normal or negative results.
Given the presentation and results of initial testing, his rash was diagnosed as flagellate erythema, likely due to shiitake mushroom intake. The diagnosis does not require histopathologic confirmation.
The rash resolved spontaneously over the next 2 weeks with use of a topical emollient and without scarring or residual hyperpigmentation.
FLAGELLATE ERYTHEMA
Flagellate erythema is a peculiar cutaneous eruption characterized by the progressive or sudden onset of parallel linear or curvilinear plaques, most commonly on the trunk. The plaques are typically arranged in a scratch pattern resembling marks left by the lashes of a whip.1 In contrast to other itchy dermatoses and neurotic excoriations that may present with self-induced linear marks, flagellate erythema appears spontaneously.
Drug-related causes, disease associations
Originally described in association with bleomycin treatment, flagellate erythema is currently considered a distinct feature of several dermatologic and systemic disorders, and therefore the ability to recognize it is valuable in daily practice.2 In addition to bleomycin analogues and anticancer agents such as peplomycin,1 bendamustine,3 and docetaxel,4 physicians should consider shiitake dermatitis5 and other less commonly reported associations such as dermatomyositis,6 lupus,7 Still disease,8 and parvovirus infection.9
Diagnostic features
The diagnosis of flagellate erythema is mainly based on the morphologic features of the clinical lesions.1 Shiitake dermatitis and flagellate erythema related to rheumatologic disease usually present with more inflammatory and erythematous plaques. Chemotherapy-induced flagellate rash typically has a violaceous or purpuric coloration, which tends to leave noticeable hyperpigmentation for several months.2
Skin biopsy may be necessary to distinguish it from similar-looking dermatoses with different histologic findings, such as dermatographism, phytophotodermatitis, erythema gyratum repens, and factitious dermatoses, which may require specific treatments or be related to important underlying pathology.1,2
Treatment
Treatment includes both specific treatment of the underlying cause and symptomatic care of the skin with topical emollients and, in cases of associated pruritus, oral antihistamines. The patient should also be reassured about the self-healing nature of shiitake dermatitis rash.5
- Yamamoto T, Nishioka K. Flagellate erythema. Int J Dermatol 2006; 45:627–631.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014; 80:149–152.
- Mahmoud BH, Eide MJ. Bendamustine-induced “flagellate dermatitis.” Dermatol Online J 2012; 18:12.
- Tallon B, Lamb S. Flagellate erythema induced by docetaxel. Clin Exp Dermatol 2008; 33:276–277.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol 2012; 67:140–141.
- Jara M, Amérigo J, Duce S, Borbujo J. Dermatomyositis and flagellate erythema. Clin Exp Dermatol 1996; 21:440–441.
- Niiyama S, Katsuoka K. Systemic lupus erythematosus with flagellate erythema. Eur J Dermatol 2012; 22:808–809.
- Ciliberto H, Kumar MG, Musiek A. Flagellate erythema in a patient with fever. JAMA Dermatol 2013; 149:1425–1426.
- Miguélez A, Dueñas J, Hervás D, Hervás JA, Salva F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol 2014; 53:e583–e585.
A previously healthy 32-year-old man presented to the emergency room with a persistent, nonpruritic rash on his trunk, which had suddenly appeared 2 days after he ate Chinese food.
Physical examination revealed multiple crosslinked linear plaques that appeared like scratches over his chest, back, and shoulders (Figures 1 and 2). He had no dermatographism, and his scalp, nails, palms, and soles were not affected. He had no signs of lymphadenopathy or systemic involvement.
Basic blood and urinary laboratory testing, blood cultures, and serologic studies showed normal or negative results.
Given the presentation and results of initial testing, his rash was diagnosed as flagellate erythema, likely due to shiitake mushroom intake. The diagnosis does not require histopathologic confirmation.
The rash resolved spontaneously over the next 2 weeks with use of a topical emollient and without scarring or residual hyperpigmentation.
FLAGELLATE ERYTHEMA
Flagellate erythema is a peculiar cutaneous eruption characterized by the progressive or sudden onset of parallel linear or curvilinear plaques, most commonly on the trunk. The plaques are typically arranged in a scratch pattern resembling marks left by the lashes of a whip.1 In contrast to other itchy dermatoses and neurotic excoriations that may present with self-induced linear marks, flagellate erythema appears spontaneously.
Drug-related causes, disease associations
Originally described in association with bleomycin treatment, flagellate erythema is currently considered a distinct feature of several dermatologic and systemic disorders, and therefore the ability to recognize it is valuable in daily practice.2 In addition to bleomycin analogues and anticancer agents such as peplomycin,1 bendamustine,3 and docetaxel,4 physicians should consider shiitake dermatitis5 and other less commonly reported associations such as dermatomyositis,6 lupus,7 Still disease,8 and parvovirus infection.9
Diagnostic features
The diagnosis of flagellate erythema is mainly based on the morphologic features of the clinical lesions.1 Shiitake dermatitis and flagellate erythema related to rheumatologic disease usually present with more inflammatory and erythematous plaques. Chemotherapy-induced flagellate rash typically has a violaceous or purpuric coloration, which tends to leave noticeable hyperpigmentation for several months.2
Skin biopsy may be necessary to distinguish it from similar-looking dermatoses with different histologic findings, such as dermatographism, phytophotodermatitis, erythema gyratum repens, and factitious dermatoses, which may require specific treatments or be related to important underlying pathology.1,2
Treatment
Treatment includes both specific treatment of the underlying cause and symptomatic care of the skin with topical emollients and, in cases of associated pruritus, oral antihistamines. The patient should also be reassured about the self-healing nature of shiitake dermatitis rash.5
A previously healthy 32-year-old man presented to the emergency room with a persistent, nonpruritic rash on his trunk, which had suddenly appeared 2 days after he ate Chinese food.
Physical examination revealed multiple crosslinked linear plaques that appeared like scratches over his chest, back, and shoulders (Figures 1 and 2). He had no dermatographism, and his scalp, nails, palms, and soles were not affected. He had no signs of lymphadenopathy or systemic involvement.
Basic blood and urinary laboratory testing, blood cultures, and serologic studies showed normal or negative results.
Given the presentation and results of initial testing, his rash was diagnosed as flagellate erythema, likely due to shiitake mushroom intake. The diagnosis does not require histopathologic confirmation.
The rash resolved spontaneously over the next 2 weeks with use of a topical emollient and without scarring or residual hyperpigmentation.
FLAGELLATE ERYTHEMA
Flagellate erythema is a peculiar cutaneous eruption characterized by the progressive or sudden onset of parallel linear or curvilinear plaques, most commonly on the trunk. The plaques are typically arranged in a scratch pattern resembling marks left by the lashes of a whip.1 In contrast to other itchy dermatoses and neurotic excoriations that may present with self-induced linear marks, flagellate erythema appears spontaneously.
Drug-related causes, disease associations
Originally described in association with bleomycin treatment, flagellate erythema is currently considered a distinct feature of several dermatologic and systemic disorders, and therefore the ability to recognize it is valuable in daily practice.2 In addition to bleomycin analogues and anticancer agents such as peplomycin,1 bendamustine,3 and docetaxel,4 physicians should consider shiitake dermatitis5 and other less commonly reported associations such as dermatomyositis,6 lupus,7 Still disease,8 and parvovirus infection.9
Diagnostic features
The diagnosis of flagellate erythema is mainly based on the morphologic features of the clinical lesions.1 Shiitake dermatitis and flagellate erythema related to rheumatologic disease usually present with more inflammatory and erythematous plaques. Chemotherapy-induced flagellate rash typically has a violaceous or purpuric coloration, which tends to leave noticeable hyperpigmentation for several months.2
Skin biopsy may be necessary to distinguish it from similar-looking dermatoses with different histologic findings, such as dermatographism, phytophotodermatitis, erythema gyratum repens, and factitious dermatoses, which may require specific treatments or be related to important underlying pathology.1,2
Treatment
Treatment includes both specific treatment of the underlying cause and symptomatic care of the skin with topical emollients and, in cases of associated pruritus, oral antihistamines. The patient should also be reassured about the self-healing nature of shiitake dermatitis rash.5
- Yamamoto T, Nishioka K. Flagellate erythema. Int J Dermatol 2006; 45:627–631.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014; 80:149–152.
- Mahmoud BH, Eide MJ. Bendamustine-induced “flagellate dermatitis.” Dermatol Online J 2012; 18:12.
- Tallon B, Lamb S. Flagellate erythema induced by docetaxel. Clin Exp Dermatol 2008; 33:276–277.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol 2012; 67:140–141.
- Jara M, Amérigo J, Duce S, Borbujo J. Dermatomyositis and flagellate erythema. Clin Exp Dermatol 1996; 21:440–441.
- Niiyama S, Katsuoka K. Systemic lupus erythematosus with flagellate erythema. Eur J Dermatol 2012; 22:808–809.
- Ciliberto H, Kumar MG, Musiek A. Flagellate erythema in a patient with fever. JAMA Dermatol 2013; 149:1425–1426.
- Miguélez A, Dueñas J, Hervás D, Hervás JA, Salva F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol 2014; 53:e583–e585.
- Yamamoto T, Nishioka K. Flagellate erythema. Int J Dermatol 2006; 45:627–631.
- Bhushan P, Manjul P, Baliyan V. Flagellate dermatoses. Indian J Dermatol Venereol Leprol 2014; 80:149–152.
- Mahmoud BH, Eide MJ. Bendamustine-induced “flagellate dermatitis.” Dermatol Online J 2012; 18:12.
- Tallon B, Lamb S. Flagellate erythema induced by docetaxel. Clin Exp Dermatol 2008; 33:276–277.
- Adler MJ, Larsen WG. Clinical variability of shiitake dermatitis. J Am Acad Dermatol 2012; 67:140–141.
- Jara M, Amérigo J, Duce S, Borbujo J. Dermatomyositis and flagellate erythema. Clin Exp Dermatol 1996; 21:440–441.
- Niiyama S, Katsuoka K. Systemic lupus erythematosus with flagellate erythema. Eur J Dermatol 2012; 22:808–809.
- Ciliberto H, Kumar MG, Musiek A. Flagellate erythema in a patient with fever. JAMA Dermatol 2013; 149:1425–1426.
- Miguélez A, Dueñas J, Hervás D, Hervás JA, Salva F, Martín-Santiago A. Flagellate erythema in parvovirus B19 infection. Int J Dermatol 2014; 53:e583–e585.
Multiple linear subcutaneous nodules
A 34-year-old woman sought consultation at our clinic for an asymptomatic swelling on her right foot that had been growing very slowly over the last 15 years. She said she had presented to other healthcare facilities, but no diagnosis had been made and no treatment had been offered.
Examination revealed a linear swelling extending from the lower third to the mid-dorsal surface of the right foot (Figure 1). Palpation revealed multiple, closely set nodules arranged in a linear fashion. This finding along with the history raised the suspicion of neurofibroma and other conditions in the differential diagnosis, eg, pure neuritic Hansen disease, phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The rest of the mucocutaneous examination results were normal. No café-au-lait spots, axillary freckling, or other swelling suggestive of neurofibroma was seen. She had no family history of mucocutaneous disease or other systemic disorder.
Because of the suspicion of neurofibromatosis, slit-lamp examination of the eyes was done to rule out Lisch nodules, a common feature of neurofibromatosis; the results were normal. Plain radiography of the right foot showed only soft-tissue swelling. Magnetic resonance imaging with contrast, done to determine the extent of the lesions, revealed multiple dumbbell-shaped lesions with homogeneous enhancement (Figure 2). Histopathologic study of a biopsy specimen of the lesions showed tumor cells in the dermis. The cells were long, with elongated nuclei with pointed ends, arranged in long and short fascicles—an appearance characteristic of neurofibroma. Areas of hypocellularity and hypercellularity were seen, and on S100 protein immunostaining, the tumor cells showed strong nuclear and cytoplasmic positivity (Figure 3).
The histologic evaluation confirmed neurofibroma. The specific diagnosis of sporadic solitary neurofibroma was made based on the onset of the lesions, the number of lesions (one in this patient), and the absence of features suggestive of neurofibromatosis.
SPORADIC SOLITARY NEUROFIBROMA
Neurofibroma is a common tumor of the peripheral nerve sheath and, when present with features such as café-au-lait spots, axillary freckling, and characteristic bone changes, it is pathognomic of neurofibromatosis type 1.1 But solitary neurofibromas can occur sporadically in the absence of other features of neurofibromatosis.
Sporadic solitary neurofibroma arises from small nerves, is benign in nature, and carries a lower rate of malignant transformation than its counterpart that occurs in the setting of neurofibromatosis.2 Though sporadic solitary neurofibroma can occur in any part of the body, it is commonly seen on the head and neck, and occasionally on the presacral and parasacral space, thigh, intrascrotal area,3 the ankle and foot,4,5 and the subungual region.6 A series of 397 peripheral neural sheath tumors examined over 30 years showed 55 sporadic solitary neurofibromas occurring in the brachial plexus region, 45 in the upper extremities, 10 in the pelvic plexus, and 31 in the lower extremities.7
Management of sporadic solitary neurofibroma depends on the patient’s discomfort. For asymptomatic lesions, serial observation is all that is required. Complete surgical excision including the parent nerve is the treatment for large lesions. More research is needed to define the potential role of drugs such as pirfenidone and tipifarnib.
THE DIFFERENTIAL DIAGNOSIS
Sporadic solitary neurofibroma can masquerade as pure neuritic Hansen disease (leprosy), phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The absence of neural symptoms and no evidence of trophic changes exclude pure neuritic Hansen disease. Phaeohyphomycosis clinically presents as a single cyst that may evolve into pigmented plaques,8 and the diagnosis relies on the presence of fungus in tissue. The absence of cystic changes clinically and fungi histopathologically in this patient did not favor phaeohyphomycosis. Palisaded neutrophilic granulomatous dermatitis is characterized clinically by cordlike skin lesions (the “rope sign”) and is accompanied by extracutaneous, mostly articular features. Histopathologically, it shows intense neutrophilic infiltrate and interstitial histiocytic infiltrate along with collagen degeneration. The absence of extracutaneous and classical histologic features negated this possibility in this patient.
Though sporotrichosis and cutaneous atypical mycobacterial infections may present in linear fashion following the course of the lymphatic vessels, the absence of epidermal changes after a disease course of 15 years and the absence of granulomatous infiltrate in histopathology excluded these possibilities in this patient.
The patient was referred to a plastic surgeon, and the lesions were successfully resected. She did not return for additional review after that.
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014; 13:834–843.
- Pulathan Z, Imamoglu M, Cay A, Guven YK. Intermittent claudication due to right common femoral artery compression by a solitary neurofibroma. Eur J Pediatr 2005; 164:463–465.
- Hosseini MM, Geramizadeh B, Shakeri S, Karimi MH. Intrascrotal solitary neurofibroma: a case report and review of the literature. Urol Ann 2012; 4:119–121.
- Carvajal JA, Cuartas E, Qadir R, Levi AD, Temple HT. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int 2011; 32:163–167.
- Tahririan MA, Hekmatnia A, Ahrar H, Heidarpour M, Hekmatnia F. Solitary giant neurofibroma of thigh. Adv Biomed Res 2014; 3:158.
- Huajun J, Wei Q, Ming L, Chongyang F, Weiguo Z, Decheng L. Solitary subungual neurofibroma in the right first finger. Int J Dermatol 2012; 51:335–338.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102:246–255.
- Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis 2009; 22:559–563.
A 34-year-old woman sought consultation at our clinic for an asymptomatic swelling on her right foot that had been growing very slowly over the last 15 years. She said she had presented to other healthcare facilities, but no diagnosis had been made and no treatment had been offered.
Examination revealed a linear swelling extending from the lower third to the mid-dorsal surface of the right foot (Figure 1). Palpation revealed multiple, closely set nodules arranged in a linear fashion. This finding along with the history raised the suspicion of neurofibroma and other conditions in the differential diagnosis, eg, pure neuritic Hansen disease, phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The rest of the mucocutaneous examination results were normal. No café-au-lait spots, axillary freckling, or other swelling suggestive of neurofibroma was seen. She had no family history of mucocutaneous disease or other systemic disorder.
Because of the suspicion of neurofibromatosis, slit-lamp examination of the eyes was done to rule out Lisch nodules, a common feature of neurofibromatosis; the results were normal. Plain radiography of the right foot showed only soft-tissue swelling. Magnetic resonance imaging with contrast, done to determine the extent of the lesions, revealed multiple dumbbell-shaped lesions with homogeneous enhancement (Figure 2). Histopathologic study of a biopsy specimen of the lesions showed tumor cells in the dermis. The cells were long, with elongated nuclei with pointed ends, arranged in long and short fascicles—an appearance characteristic of neurofibroma. Areas of hypocellularity and hypercellularity were seen, and on S100 protein immunostaining, the tumor cells showed strong nuclear and cytoplasmic positivity (Figure 3).
The histologic evaluation confirmed neurofibroma. The specific diagnosis of sporadic solitary neurofibroma was made based on the onset of the lesions, the number of lesions (one in this patient), and the absence of features suggestive of neurofibromatosis.
SPORADIC SOLITARY NEUROFIBROMA
Neurofibroma is a common tumor of the peripheral nerve sheath and, when present with features such as café-au-lait spots, axillary freckling, and characteristic bone changes, it is pathognomic of neurofibromatosis type 1.1 But solitary neurofibromas can occur sporadically in the absence of other features of neurofibromatosis.
Sporadic solitary neurofibroma arises from small nerves, is benign in nature, and carries a lower rate of malignant transformation than its counterpart that occurs in the setting of neurofibromatosis.2 Though sporadic solitary neurofibroma can occur in any part of the body, it is commonly seen on the head and neck, and occasionally on the presacral and parasacral space, thigh, intrascrotal area,3 the ankle and foot,4,5 and the subungual region.6 A series of 397 peripheral neural sheath tumors examined over 30 years showed 55 sporadic solitary neurofibromas occurring in the brachial plexus region, 45 in the upper extremities, 10 in the pelvic plexus, and 31 in the lower extremities.7
Management of sporadic solitary neurofibroma depends on the patient’s discomfort. For asymptomatic lesions, serial observation is all that is required. Complete surgical excision including the parent nerve is the treatment for large lesions. More research is needed to define the potential role of drugs such as pirfenidone and tipifarnib.
THE DIFFERENTIAL DIAGNOSIS
Sporadic solitary neurofibroma can masquerade as pure neuritic Hansen disease (leprosy), phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The absence of neural symptoms and no evidence of trophic changes exclude pure neuritic Hansen disease. Phaeohyphomycosis clinically presents as a single cyst that may evolve into pigmented plaques,8 and the diagnosis relies on the presence of fungus in tissue. The absence of cystic changes clinically and fungi histopathologically in this patient did not favor phaeohyphomycosis. Palisaded neutrophilic granulomatous dermatitis is characterized clinically by cordlike skin lesions (the “rope sign”) and is accompanied by extracutaneous, mostly articular features. Histopathologically, it shows intense neutrophilic infiltrate and interstitial histiocytic infiltrate along with collagen degeneration. The absence of extracutaneous and classical histologic features negated this possibility in this patient.
Though sporotrichosis and cutaneous atypical mycobacterial infections may present in linear fashion following the course of the lymphatic vessels, the absence of epidermal changes after a disease course of 15 years and the absence of granulomatous infiltrate in histopathology excluded these possibilities in this patient.
The patient was referred to a plastic surgeon, and the lesions were successfully resected. She did not return for additional review after that.
A 34-year-old woman sought consultation at our clinic for an asymptomatic swelling on her right foot that had been growing very slowly over the last 15 years. She said she had presented to other healthcare facilities, but no diagnosis had been made and no treatment had been offered.
Examination revealed a linear swelling extending from the lower third to the mid-dorsal surface of the right foot (Figure 1). Palpation revealed multiple, closely set nodules arranged in a linear fashion. This finding along with the history raised the suspicion of neurofibroma and other conditions in the differential diagnosis, eg, pure neuritic Hansen disease, phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The rest of the mucocutaneous examination results were normal. No café-au-lait spots, axillary freckling, or other swelling suggestive of neurofibroma was seen. She had no family history of mucocutaneous disease or other systemic disorder.
Because of the suspicion of neurofibromatosis, slit-lamp examination of the eyes was done to rule out Lisch nodules, a common feature of neurofibromatosis; the results were normal. Plain radiography of the right foot showed only soft-tissue swelling. Magnetic resonance imaging with contrast, done to determine the extent of the lesions, revealed multiple dumbbell-shaped lesions with homogeneous enhancement (Figure 2). Histopathologic study of a biopsy specimen of the lesions showed tumor cells in the dermis. The cells were long, with elongated nuclei with pointed ends, arranged in long and short fascicles—an appearance characteristic of neurofibroma. Areas of hypocellularity and hypercellularity were seen, and on S100 protein immunostaining, the tumor cells showed strong nuclear and cytoplasmic positivity (Figure 3).
The histologic evaluation confirmed neurofibroma. The specific diagnosis of sporadic solitary neurofibroma was made based on the onset of the lesions, the number of lesions (one in this patient), and the absence of features suggestive of neurofibromatosis.
SPORADIC SOLITARY NEUROFIBROMA
Neurofibroma is a common tumor of the peripheral nerve sheath and, when present with features such as café-au-lait spots, axillary freckling, and characteristic bone changes, it is pathognomic of neurofibromatosis type 1.1 But solitary neurofibromas can occur sporadically in the absence of other features of neurofibromatosis.
Sporadic solitary neurofibroma arises from small nerves, is benign in nature, and carries a lower rate of malignant transformation than its counterpart that occurs in the setting of neurofibromatosis.2 Though sporadic solitary neurofibroma can occur in any part of the body, it is commonly seen on the head and neck, and occasionally on the presacral and parasacral space, thigh, intrascrotal area,3 the ankle and foot,4,5 and the subungual region.6 A series of 397 peripheral neural sheath tumors examined over 30 years showed 55 sporadic solitary neurofibromas occurring in the brachial plexus region, 45 in the upper extremities, 10 in the pelvic plexus, and 31 in the lower extremities.7
Management of sporadic solitary neurofibroma depends on the patient’s discomfort. For asymptomatic lesions, serial observation is all that is required. Complete surgical excision including the parent nerve is the treatment for large lesions. More research is needed to define the potential role of drugs such as pirfenidone and tipifarnib.
THE DIFFERENTIAL DIAGNOSIS
Sporadic solitary neurofibroma can masquerade as pure neuritic Hansen disease (leprosy), phaeohyphomycosis, and palisaded neutrophilic granulomatous dermatitis. The absence of neural symptoms and no evidence of trophic changes exclude pure neuritic Hansen disease. Phaeohyphomycosis clinically presents as a single cyst that may evolve into pigmented plaques,8 and the diagnosis relies on the presence of fungus in tissue. The absence of cystic changes clinically and fungi histopathologically in this patient did not favor phaeohyphomycosis. Palisaded neutrophilic granulomatous dermatitis is characterized clinically by cordlike skin lesions (the “rope sign”) and is accompanied by extracutaneous, mostly articular features. Histopathologically, it shows intense neutrophilic infiltrate and interstitial histiocytic infiltrate along with collagen degeneration. The absence of extracutaneous and classical histologic features negated this possibility in this patient.
Though sporotrichosis and cutaneous atypical mycobacterial infections may present in linear fashion following the course of the lymphatic vessels, the absence of epidermal changes after a disease course of 15 years and the absence of granulomatous infiltrate in histopathology excluded these possibilities in this patient.
The patient was referred to a plastic surgeon, and the lesions were successfully resected. She did not return for additional review after that.
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014; 13:834–843.
- Pulathan Z, Imamoglu M, Cay A, Guven YK. Intermittent claudication due to right common femoral artery compression by a solitary neurofibroma. Eur J Pediatr 2005; 164:463–465.
- Hosseini MM, Geramizadeh B, Shakeri S, Karimi MH. Intrascrotal solitary neurofibroma: a case report and review of the literature. Urol Ann 2012; 4:119–121.
- Carvajal JA, Cuartas E, Qadir R, Levi AD, Temple HT. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int 2011; 32:163–167.
- Tahririan MA, Hekmatnia A, Ahrar H, Heidarpour M, Hekmatnia F. Solitary giant neurofibroma of thigh. Adv Biomed Res 2014; 3:158.
- Huajun J, Wei Q, Ming L, Chongyang F, Weiguo Z, Decheng L. Solitary subungual neurofibroma in the right first finger. Int J Dermatol 2012; 51:335–338.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102:246–255.
- Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis 2009; 22:559–563.
- Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014; 13:834–843.
- Pulathan Z, Imamoglu M, Cay A, Guven YK. Intermittent claudication due to right common femoral artery compression by a solitary neurofibroma. Eur J Pediatr 2005; 164:463–465.
- Hosseini MM, Geramizadeh B, Shakeri S, Karimi MH. Intrascrotal solitary neurofibroma: a case report and review of the literature. Urol Ann 2012; 4:119–121.
- Carvajal JA, Cuartas E, Qadir R, Levi AD, Temple HT. Peripheral nerve sheath tumors of the foot and ankle. Foot Ankle Int 2011; 32:163–167.
- Tahririan MA, Hekmatnia A, Ahrar H, Heidarpour M, Hekmatnia F. Solitary giant neurofibroma of thigh. Adv Biomed Res 2014; 3:158.
- Huajun J, Wei Q, Ming L, Chongyang F, Weiguo Z, Decheng L. Solitary subungual neurofibroma in the right first finger. Int J Dermatol 2012; 51:335–338.
- Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005; 102:246–255.
- Garnica M, Nucci M, Queiroz-Telles F. Difficult mycoses of the skin: advances in the epidemiology and management of eumycetoma, phaeohyphomycosis and chromoblastomycosis. Curr Opin Infect Dis 2009; 22:559–563.
Lebrikizumab boosts lung function in asthma
LOS ANGELES – The investigational interleukin-13 inhibitor lebrikizumab provides a clinically meaningful improvement in measures of lung function within 1 week after the first dose in patients with moderate-to-severe uncontrolled asthma on standard-of-care therapy and a high baseline serum periostin level, Dr. Jonathan Corren reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a post hoc analysis of three phase II randomized trials of lebrikizumab as add-on therapy in a total of 558 patients with uncontrolled asthma while on a moderate- or high-dose inhaled corticosteroid plus at least one other controller medication, most often a long-acting beta agonist. The post hoc analysis included 333 asthma patients who received lebrikizumab subcutaneously at 125 or 250 mg every 4 weeks for 12 weeks and 225 who got placebo. Baseline serum periostin levels were 50 ng/mL or higher in 252 participants.
One week after the first dose of lebrikizumab, the high serum periostin group demonstrated a placebo-subtracted mean 147-mL improvement from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1). The week 1 improvement in FEV1 with lebrikizumab in the low serum periostin group was more modest: a placebo-subtracted 57 mL.
The response to lebrikizumab was maintained through 12 weeks of once-monthly therapy, with a mean placebo-subtracted week 12 improvement in FEV1 of 198 mL in the high-periostin group, compared with 74 mL in low-periostin patients. The lebrikizumab-treated group with high baseline periostin had a 16% improvement from baseline in FEV1 as compared with a 5% improvement in placebo-treated patients with high periostin.
The three trials were known by the acronyms MILLY, LUTE, and VERSE. Dr. Corren was first author of the MILLY study (N Engl J Med. 2011 Sep 22;365(12):1088-98), which was the initial report that lebrikizumab performed markedly better in patients with uncontrolled asthma and a high baseline serum periostin – a biomarker for IL-13 activity – and that periostin was a better predictor of response to lebrikizumab than either blood eosinophil count or serum IgE.
The new pooled post hoc analysis was performed to boost sample size and confirm the key MILLY findings, as well as to more closely examine the speed of improvement in airflow in response to therapy, said Dr. Corren of the University of California, Los Angeles.
Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 with high affinity. Its efficacy in the phase II trials confirms the importance of IL-13 as a mediator of disease activity in a subset of asthma patients with activation of Type 2 lymphocytes.
“We know specifically that IL-13 has some very important effects in asthma, including upregulation of adhesion molecules that allow eosinophils to stick in the lung, as well as promoting hyperplasia of smooth muscle and mucus cell hyperplasia with increased mucus secretion. Immunologically, it allows switching from IgM to IgE on the surface of B cells. So IL-13 is a cytokine that literally makes people atopic,” Dr. Corren explained in an interview.
Several ongoing phase III randomized trials of lebrikizumab in adults with uncontrolled asthma despite standard-of-care therapy are due to be completed in the first half of 2017. A phase III trial in adolescents is also underway.
Dr. Corren reported receiving research funding from Roche/Genentech, which sponsored the studies.
LOS ANGELES – The investigational interleukin-13 inhibitor lebrikizumab provides a clinically meaningful improvement in measures of lung function within 1 week after the first dose in patients with moderate-to-severe uncontrolled asthma on standard-of-care therapy and a high baseline serum periostin level, Dr. Jonathan Corren reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a post hoc analysis of three phase II randomized trials of lebrikizumab as add-on therapy in a total of 558 patients with uncontrolled asthma while on a moderate- or high-dose inhaled corticosteroid plus at least one other controller medication, most often a long-acting beta agonist. The post hoc analysis included 333 asthma patients who received lebrikizumab subcutaneously at 125 or 250 mg every 4 weeks for 12 weeks and 225 who got placebo. Baseline serum periostin levels were 50 ng/mL or higher in 252 participants.
One week after the first dose of lebrikizumab, the high serum periostin group demonstrated a placebo-subtracted mean 147-mL improvement from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1). The week 1 improvement in FEV1 with lebrikizumab in the low serum periostin group was more modest: a placebo-subtracted 57 mL.
The response to lebrikizumab was maintained through 12 weeks of once-monthly therapy, with a mean placebo-subtracted week 12 improvement in FEV1 of 198 mL in the high-periostin group, compared with 74 mL in low-periostin patients. The lebrikizumab-treated group with high baseline periostin had a 16% improvement from baseline in FEV1 as compared with a 5% improvement in placebo-treated patients with high periostin.
The three trials were known by the acronyms MILLY, LUTE, and VERSE. Dr. Corren was first author of the MILLY study (N Engl J Med. 2011 Sep 22;365(12):1088-98), which was the initial report that lebrikizumab performed markedly better in patients with uncontrolled asthma and a high baseline serum periostin – a biomarker for IL-13 activity – and that periostin was a better predictor of response to lebrikizumab than either blood eosinophil count or serum IgE.
The new pooled post hoc analysis was performed to boost sample size and confirm the key MILLY findings, as well as to more closely examine the speed of improvement in airflow in response to therapy, said Dr. Corren of the University of California, Los Angeles.
Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 with high affinity. Its efficacy in the phase II trials confirms the importance of IL-13 as a mediator of disease activity in a subset of asthma patients with activation of Type 2 lymphocytes.
“We know specifically that IL-13 has some very important effects in asthma, including upregulation of adhesion molecules that allow eosinophils to stick in the lung, as well as promoting hyperplasia of smooth muscle and mucus cell hyperplasia with increased mucus secretion. Immunologically, it allows switching from IgM to IgE on the surface of B cells. So IL-13 is a cytokine that literally makes people atopic,” Dr. Corren explained in an interview.
Several ongoing phase III randomized trials of lebrikizumab in adults with uncontrolled asthma despite standard-of-care therapy are due to be completed in the first half of 2017. A phase III trial in adolescents is also underway.
Dr. Corren reported receiving research funding from Roche/Genentech, which sponsored the studies.
LOS ANGELES – The investigational interleukin-13 inhibitor lebrikizumab provides a clinically meaningful improvement in measures of lung function within 1 week after the first dose in patients with moderate-to-severe uncontrolled asthma on standard-of-care therapy and a high baseline serum periostin level, Dr. Jonathan Corren reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
He presented a post hoc analysis of three phase II randomized trials of lebrikizumab as add-on therapy in a total of 558 patients with uncontrolled asthma while on a moderate- or high-dose inhaled corticosteroid plus at least one other controller medication, most often a long-acting beta agonist. The post hoc analysis included 333 asthma patients who received lebrikizumab subcutaneously at 125 or 250 mg every 4 weeks for 12 weeks and 225 who got placebo. Baseline serum periostin levels were 50 ng/mL or higher in 252 participants.
One week after the first dose of lebrikizumab, the high serum periostin group demonstrated a placebo-subtracted mean 147-mL improvement from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1). The week 1 improvement in FEV1 with lebrikizumab in the low serum periostin group was more modest: a placebo-subtracted 57 mL.
The response to lebrikizumab was maintained through 12 weeks of once-monthly therapy, with a mean placebo-subtracted week 12 improvement in FEV1 of 198 mL in the high-periostin group, compared with 74 mL in low-periostin patients. The lebrikizumab-treated group with high baseline periostin had a 16% improvement from baseline in FEV1 as compared with a 5% improvement in placebo-treated patients with high periostin.
The three trials were known by the acronyms MILLY, LUTE, and VERSE. Dr. Corren was first author of the MILLY study (N Engl J Med. 2011 Sep 22;365(12):1088-98), which was the initial report that lebrikizumab performed markedly better in patients with uncontrolled asthma and a high baseline serum periostin – a biomarker for IL-13 activity – and that periostin was a better predictor of response to lebrikizumab than either blood eosinophil count or serum IgE.
The new pooled post hoc analysis was performed to boost sample size and confirm the key MILLY findings, as well as to more closely examine the speed of improvement in airflow in response to therapy, said Dr. Corren of the University of California, Los Angeles.
Lebrikizumab is an IgG4 humanized monoclonal antibody that binds to IL-13 with high affinity. Its efficacy in the phase II trials confirms the importance of IL-13 as a mediator of disease activity in a subset of asthma patients with activation of Type 2 lymphocytes.
“We know specifically that IL-13 has some very important effects in asthma, including upregulation of adhesion molecules that allow eosinophils to stick in the lung, as well as promoting hyperplasia of smooth muscle and mucus cell hyperplasia with increased mucus secretion. Immunologically, it allows switching from IgM to IgE on the surface of B cells. So IL-13 is a cytokine that literally makes people atopic,” Dr. Corren explained in an interview.
Several ongoing phase III randomized trials of lebrikizumab in adults with uncontrolled asthma despite standard-of-care therapy are due to be completed in the first half of 2017. A phase III trial in adolescents is also underway.
Dr. Corren reported receiving research funding from Roche/Genentech, which sponsored the studies.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: Lebrikizumab shows promise for treatment of patients with moderate-to-severe type 2 lymphocyte-mediated asthma.
Major finding: One week after patients with uncontrolled asthma and a high baseline serum periostin level received their first dose of lebrikizumab, they demonstrated a mean 16% improvement over baseline in forced expiratory volume in 1 second.
Data source: This was a post hoc analysis of three phase II, randomized, placebo-controlled clinical trials totaling 558 patients with moderate-to-severe uncontrolled asthma while on standard of care therapy.
Disclosures: The study presenter reported receiving research funding from Roche/Genentech, which is developing lebrikizumab.