User login
Sapien 3 performs well (mostly) in bicuspid aortic stenosis
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.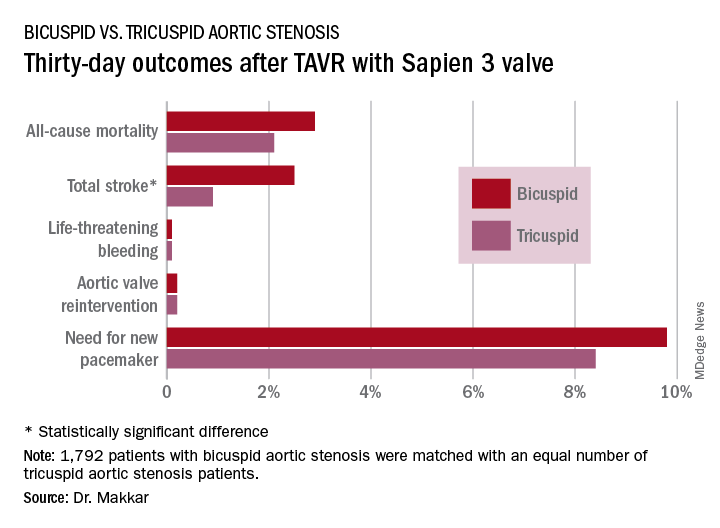
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
REPORTING FROM EUROPCR 2018
Key clinical point: Overall, outcomes were similarly favorable between patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis.
Major finding: The 1-year all-cause mortality and total stroke rates in 1,792 TAVR patients who got the Sapien 3 valve for bicuspid aortic stenosis were 10.4% and 3.4%.
Study details: This was a propensity-matched comparison of TAVR outcomes using the Sapien 3 valve in 1,792 patients with bicuspid aortic stenosis and in an equal number with tricuspid aortic stenosis in the STS/ACC TVT Registry.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
Malpractice reforms reduce invasive cardiac testing
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
Cardiologists in states with payment limits for medical malpractice claims practice less defensive medicine, a study suggests.
Steven A. Farmer, MD, PhD, of George Washington University, Washington, and his colleagues studied the coronary artery disease (CAD) testing practices of 36,647 doctors in nine states that have noneconomic damages caps for medical liability payouts and compared them with the testing practices of 39,154 doctors in 20 no-cap states. (The investigators studied only states that enacted damage limits between 2002 and 2005.) They studied physicians who ordered or performed two or more angiographies on a 5% random sample of Medicare fee-for-service beneficiaries between 1999 and 2013 who were 65 years or older.
Findings showed that in the cap states, doctors ordered 24% fewer angiographies as a first diagnostic test, compared with control physicians (relative change, −24%; 95% confidence interval, −40% to −7%; P = .005), but cap-state doctors also ordered 8% more noninvasive stress tests (7.8%; 95% CI, −3.6% to 19. P = .17), the authors reported in JAMA Cardiology.
Physicians in damages cap states referred 21% fewer patients for angiography following stress testing (−21%; 95% CI, −40% to −2%; P = .03) and fewer of their patients progressed from evaluation to revascularization. Changes in overall ischemic evaluation rates were similar for new-cap and no-cap physicians, the study found.
The authors noted that the decreased tendency for patients of cap-state physicians to progress from ischemic evaluation to revascularization had three possible channels: fewer initial angiographies, less progression from stress testing to angiography, and less progression from angiography to revascularization. The first two channels are statistically significant, while the third is directionally consistent, according to the study.
The overall results show a direct link between damage caps and cardiac care decisions, Dr. Framer wrote, adding that physicians are willing to tolerate greater clinical uncertainty in CAD testing if they face lower malpractice risk. The authors said the analysis builds on previous research showing that 12% of percutaneous coronary interventions for nonacute indications are inappropriate, and that CAD testing and treatments may be overused in the Medicare fee-for-service setting.
“Curtailing marginal or unnecessary angiography and revascularization spares patients invasive procedures and associated risk and saves resources,” Dr. Farmer wrote. “In addition, both the Department of Health and Human Services and commercial payers are moving rapidly toward alternate payment models. A core issue for these models is provider resistance to changing established practice patterns. Our study suggests that physicians who face lower malpractice risk may be less concerned with that risk, and thus more receptive to new care delivery strategies associated with alternate payment models.”
The study is believed to be the first to demonstrate changes in clinical behavior in the CAD testing and treatment setting after damages cap adoption.
SOURCE: Farmer et al. JAMA Cardiol. 2018 Jun 6 doi: 10.1001/jamacardio.2018.1360.
FROM JAMA CARDIOLOGY
Key clinical point: Physicians in states with medical malpractice damages caps perform fewer angiographies.
Major finding: In the damages-cap states, doctors ordered 24% fewer angiographies as a first diagnostic test compared with no-cap physicians.
Study details: A study of 36,647 doctors in nine states that have noneconomic damages caps and 39,154 doctors in 20 no-cap states.
Disclosures: No disclosures were reported.
Source: Farmer et al. JAMA Cardiol. 2018 June 6 doi: 10.1001/jamacardio.2018.1360.
Synergy DES shines in acute MI
PARIS – The Synergy bioabsorbable polymer everolimus-eluting stent performed equally well for treatment of acute MI, compared with other newer-generation drug-eluting stents, through 2 years of follow-up in a massive observational study of all patients undergoing percutaneous coronary intervention in Sweden during a recent multiyear period.
This report from the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR) was undertaken because, even though the Synergy stent has demonstrated outstanding clinical results in randomized trials and observational studies, the stent’s performance specifically in the setting of acute MI had not previously been investigated, Sergio Buccheri, MD, noted at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
SCAAR, which documents every PCI performed in Sweden, provided the capability to fill that important knowledge gap in an unselected real-world population of acute MI patients. Dr. Buccheri, of Uppsala (Sweden) University, reported on 36,292 consecutive patients who underwent PCI with a newer-generation drug-eluting stent (DES) in Sweden from March 2013 to September 2016. Forty percent of them had ST-elevation MI. The Synergy stent was used in 4,889 patients. Among the most commonly used newer DES in the other 31,000-plus patients were the Xience Xpedition, the Resolute Integrity and Resolute Onyx, the Orsiro, BioMatrix, and Promus Element Plus and Promus Premier.
The coprimary endpoints in this analysis were the rates of definite stent thrombosis and clinically relevant restenosis at 2 years of follow-up. Stent thrombosis occurred in 0.69% of the Synergy patients and 0.81% of those who received other newer-generation DES, a nonsignificant difference. Similarly, no significant difference was found in the rate of clinically relevant restenosis: 1.48% and 1.25%, respectively.
“,” Dr. Buccheri noted. “These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice.”
The key secondary outcomes were all-cause mortality and recurrent MI. Again, there were no significant between-group differences. The cumulative all-cause mortality at 2 years was 10.1% in the Synergy group and 9.1% in the others. Recurrent MI occurred in 6.49% of the Synergy group and 6.32% with other DES.
Patients who received the Synergy stent were on average older, had a higher burden of cardiovascular risk factors, and presented more often with left main, triple-vessel disease or vein graft lesions. For that reason, Dr. Buccheri and his coinvestigators developed a propensity score using an array of covariates to adjust for these differences. Plugging those scores into multivariate Cox regression models, there remained no significant differences between the two groups in the adjusted risk of any of the endpoints.
Operators were advised to use dual antiplatelet therapy for 12 months in all patients. However, SCAAR does not include data on adherence to DAPT, which is a study limitation, Dr. Buccheri noted.
The Synergy stent is made up of a thin strut chromium-platinum platform with a bioabsorbable polymer that releases everolimus. The polymer is completely reabsorbed within 4 months, leaving behind a bare metal stent. In animal models, this has been associated with lower levels of inflammation, compared with permanent polymer DES. And inflammation is thought to be one of the main mechanisms underlying stent failure in the late and very late phases after PCI.
The discussion panel was clearly impressed with – and envious of – the sheer size of the SCAAR study population. As one panelist noted, real-life data of this magnitude can really only be obtained in Sweden. Another panelist confessed: “We’re shy of presenting our own studies when we see these numbers.”
Simultaneously with Dr. Buccheri’s presentation, the SCAAR report was published online (EuroIntervention. 2018 May 24. pii: EIJ-D-18-00392. doi: 10.4244/EIJ-D-18-00392).
SCAAR is funded solely by the Swedish government. This study was supported by a grant from Boston Scientific. Dr. Buccheri reported having no financial conflicts of interest.
PARIS – The Synergy bioabsorbable polymer everolimus-eluting stent performed equally well for treatment of acute MI, compared with other newer-generation drug-eluting stents, through 2 years of follow-up in a massive observational study of all patients undergoing percutaneous coronary intervention in Sweden during a recent multiyear period.
This report from the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR) was undertaken because, even though the Synergy stent has demonstrated outstanding clinical results in randomized trials and observational studies, the stent’s performance specifically in the setting of acute MI had not previously been investigated, Sergio Buccheri, MD, noted at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
SCAAR, which documents every PCI performed in Sweden, provided the capability to fill that important knowledge gap in an unselected real-world population of acute MI patients. Dr. Buccheri, of Uppsala (Sweden) University, reported on 36,292 consecutive patients who underwent PCI with a newer-generation drug-eluting stent (DES) in Sweden from March 2013 to September 2016. Forty percent of them had ST-elevation MI. The Synergy stent was used in 4,889 patients. Among the most commonly used newer DES in the other 31,000-plus patients were the Xience Xpedition, the Resolute Integrity and Resolute Onyx, the Orsiro, BioMatrix, and Promus Element Plus and Promus Premier.
The coprimary endpoints in this analysis were the rates of definite stent thrombosis and clinically relevant restenosis at 2 years of follow-up. Stent thrombosis occurred in 0.69% of the Synergy patients and 0.81% of those who received other newer-generation DES, a nonsignificant difference. Similarly, no significant difference was found in the rate of clinically relevant restenosis: 1.48% and 1.25%, respectively.
“,” Dr. Buccheri noted. “These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice.”
The key secondary outcomes were all-cause mortality and recurrent MI. Again, there were no significant between-group differences. The cumulative all-cause mortality at 2 years was 10.1% in the Synergy group and 9.1% in the others. Recurrent MI occurred in 6.49% of the Synergy group and 6.32% with other DES.
Patients who received the Synergy stent were on average older, had a higher burden of cardiovascular risk factors, and presented more often with left main, triple-vessel disease or vein graft lesions. For that reason, Dr. Buccheri and his coinvestigators developed a propensity score using an array of covariates to adjust for these differences. Plugging those scores into multivariate Cox regression models, there remained no significant differences between the two groups in the adjusted risk of any of the endpoints.
Operators were advised to use dual antiplatelet therapy for 12 months in all patients. However, SCAAR does not include data on adherence to DAPT, which is a study limitation, Dr. Buccheri noted.
The Synergy stent is made up of a thin strut chromium-platinum platform with a bioabsorbable polymer that releases everolimus. The polymer is completely reabsorbed within 4 months, leaving behind a bare metal stent. In animal models, this has been associated with lower levels of inflammation, compared with permanent polymer DES. And inflammation is thought to be one of the main mechanisms underlying stent failure in the late and very late phases after PCI.
The discussion panel was clearly impressed with – and envious of – the sheer size of the SCAAR study population. As one panelist noted, real-life data of this magnitude can really only be obtained in Sweden. Another panelist confessed: “We’re shy of presenting our own studies when we see these numbers.”
Simultaneously with Dr. Buccheri’s presentation, the SCAAR report was published online (EuroIntervention. 2018 May 24. pii: EIJ-D-18-00392. doi: 10.4244/EIJ-D-18-00392).
SCAAR is funded solely by the Swedish government. This study was supported by a grant from Boston Scientific. Dr. Buccheri reported having no financial conflicts of interest.
PARIS – The Synergy bioabsorbable polymer everolimus-eluting stent performed equally well for treatment of acute MI, compared with other newer-generation drug-eluting stents, through 2 years of follow-up in a massive observational study of all patients undergoing percutaneous coronary intervention in Sweden during a recent multiyear period.
This report from the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR) was undertaken because, even though the Synergy stent has demonstrated outstanding clinical results in randomized trials and observational studies, the stent’s performance specifically in the setting of acute MI had not previously been investigated, Sergio Buccheri, MD, noted at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
SCAAR, which documents every PCI performed in Sweden, provided the capability to fill that important knowledge gap in an unselected real-world population of acute MI patients. Dr. Buccheri, of Uppsala (Sweden) University, reported on 36,292 consecutive patients who underwent PCI with a newer-generation drug-eluting stent (DES) in Sweden from March 2013 to September 2016. Forty percent of them had ST-elevation MI. The Synergy stent was used in 4,889 patients. Among the most commonly used newer DES in the other 31,000-plus patients were the Xience Xpedition, the Resolute Integrity and Resolute Onyx, the Orsiro, BioMatrix, and Promus Element Plus and Promus Premier.
The coprimary endpoints in this analysis were the rates of definite stent thrombosis and clinically relevant restenosis at 2 years of follow-up. Stent thrombosis occurred in 0.69% of the Synergy patients and 0.81% of those who received other newer-generation DES, a nonsignificant difference. Similarly, no significant difference was found in the rate of clinically relevant restenosis: 1.48% and 1.25%, respectively.
“,” Dr. Buccheri noted. “These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice.”
The key secondary outcomes were all-cause mortality and recurrent MI. Again, there were no significant between-group differences. The cumulative all-cause mortality at 2 years was 10.1% in the Synergy group and 9.1% in the others. Recurrent MI occurred in 6.49% of the Synergy group and 6.32% with other DES.
Patients who received the Synergy stent were on average older, had a higher burden of cardiovascular risk factors, and presented more often with left main, triple-vessel disease or vein graft lesions. For that reason, Dr. Buccheri and his coinvestigators developed a propensity score using an array of covariates to adjust for these differences. Plugging those scores into multivariate Cox regression models, there remained no significant differences between the two groups in the adjusted risk of any of the endpoints.
Operators were advised to use dual antiplatelet therapy for 12 months in all patients. However, SCAAR does not include data on adherence to DAPT, which is a study limitation, Dr. Buccheri noted.
The Synergy stent is made up of a thin strut chromium-platinum platform with a bioabsorbable polymer that releases everolimus. The polymer is completely reabsorbed within 4 months, leaving behind a bare metal stent. In animal models, this has been associated with lower levels of inflammation, compared with permanent polymer DES. And inflammation is thought to be one of the main mechanisms underlying stent failure in the late and very late phases after PCI.
The discussion panel was clearly impressed with – and envious of – the sheer size of the SCAAR study population. As one panelist noted, real-life data of this magnitude can really only be obtained in Sweden. Another panelist confessed: “We’re shy of presenting our own studies when we see these numbers.”
Simultaneously with Dr. Buccheri’s presentation, the SCAAR report was published online (EuroIntervention. 2018 May 24. pii: EIJ-D-18-00392. doi: 10.4244/EIJ-D-18-00392).
SCAAR is funded solely by the Swedish government. This study was supported by a grant from Boston Scientific. Dr. Buccheri reported having no financial conflicts of interest.
REPORTING FROM EUROPCR 2018
Key clinical point: Two years post PCI for acute MI, stent thrombosis and restenosis rates in Synergy stent recipients were as low as with other newer-generation drug eluting stents.
Major finding: The 2-year rate of definite stent thrombosis was 0.69% in the Synergy stent group and 0.81% in recipients of other contemporary drug-eluting stents.
Study details: This was an observational study of 36,292 consecutive Swedish patients with acute MI who received the Synergy stent or other newer-generation drug-eluting stents.
Disclosures: The study was funded by a grant from Boston Scientific. The presenter reported having no financial conflicts of interest.
Guideline: PFO closure best bet for recurrent stroke prevention
In patients younger than 60 years, patent foramen ovale (PFO) closure plus antiplatelet therapy is a better strategy for preventing recurrent ischemic stroke than indefinite anticoagulant therapy without closure, according to a recommendation from an expert panel after a systematic literature review.
When compared with the alternative strategy of indefinite anticoagulant therapy for those patients “open to all options,” the recommendation was labeled only “weak” on the basis of the GRADE classification system used by the panel to rate treatment strategies. However, the recommendation for PFO closure plus antiplatelets becomes “strong” if anticoagulation therapy is contraindicated or declined as a treatment option.
The new recommendations were largely driven by three multicenter studies of that were published simultaneously last year. (N Engl J Med. 2017 Sep 14;377:1011-21; 1022-32; 1033-42). However, these and previous studies did not provide adequate data on risks and benefits for all options, which the authors emphasized in guidelines meant to help clinicians weigh options.
Weak means that clinicians “should recognize that different choices will be appropriate for different patients,” explained Frederick A. Spencer, MD, professor, division of cardiology, McMaster University, Hamilton, Ontario. Quoting from GRADE definitions, Dr. Spencer, who was the guideline panel chair, explained that weak recommendations are the product of uncertainties that may affect choice in specific individuals.
In addition to the recommendation for PFO closure plus antiplatelets as either a weak or strong recommendation in relation to the availability of anticoagulation therapy, the guidelines offered one other recommendation. For patients contraindicated or unwilling to undergo PFO closure, anticoagulation therapy was preferred over antiplatelet therapy. This was also labeled a “weak” recommendation.
These guidelines were the product of collaboration between the BMJ and the nonprofit MAGIC (Making GRADE the Irresistible Choice) project. In addition to providing a methodology and structure to organize guideline deliberations among the participating international experts, MAGIC facilitated communication and collaboration through Web-based technology.
This approach was particularly useful for the detailed analyses and debate about relative risks and benefits of treatment alternatives in which direct comparative data were limited. For example, the authors noted that the only randomized comparison of PFO closure to anticoagulation involved just 353 patients. Indirect evidence was therefore added to improve the precision of the estimated benefits and risks. The panel selected PFO closure plus antiplatelet therapy over anticoagulation therapy, because “the most serious complications of PFO closure are usually short term, whereas anticoagulation imposes a long-term burden and increased risk of major bleeding.”
The estimated rate of adverse events associated with PFO is 3.6%, according to data cited in the expert guidelines. Atrial fibrillation accounts for about half of these events. At 5 years, the absolute reduction in stroke from PFO closure versus no intervention is an estimated 8.7%. The panel assumed that most patients would place greater weight on stroke prevention than the largely reversible adverse events associated with PFO closure.
The newly published guidelines include a detailed review of the available data to allow clinicians to counsel patients appropriately. Dr. Spencer cautioned that no recommendation, strong or weak, should be uniformly applied without considering individual patient factors and preferences.
According to Christopher J. White, MD, system chairman for cardiology, Ochsner Medical Center, New Orleans, the guideline committee had no choice but to label PFO closure plus antiplatelet therapy as a “weak” recommendation within the requirements of GRADE. However, Dr. White believes that it should be considered the best option in most patients despite this terminology.
“What I tell patients is that this is a one-and-done procedure,” Dr. White explained. “Without closure, patients must remain on anticoagulation therapy, which involves refilling prescriptions, remaining compliant with daily therapy for life, and accepting an increased risk of bleeding. Once the PFO is closed and endothelialized, no additional anticoagulation treatment is needed.”
There are adverse events associated with PFO closure, but Dr. White called these uncommon and more acceptable than the risks of indefinite anticoagulation therapy, particularly if patients are not fully compliant.
“When I explain the options to patients, most will opt for PFO closure,” Dr. White said.
He also emphasized that the advantage of PFO closure accrues over time.
“When these patients have a stroke at a relatively young age, they may need to be on anticoagulation for decades. Once the PFO is closed, you are saving the patient the burden of lifelong compliance to anticoagulation therapy as well as the costs,” he added. Although the guidelines compared relative benefits of a given strategy over 5 years, the advantage of PFO closure over anticoagulation will keep increasing beyond this point.
Source: Kuipers et al. BMJ 2018 Jul 25;362:K2515
In patients younger than 60 years, patent foramen ovale (PFO) closure plus antiplatelet therapy is a better strategy for preventing recurrent ischemic stroke than indefinite anticoagulant therapy without closure, according to a recommendation from an expert panel after a systematic literature review.
When compared with the alternative strategy of indefinite anticoagulant therapy for those patients “open to all options,” the recommendation was labeled only “weak” on the basis of the GRADE classification system used by the panel to rate treatment strategies. However, the recommendation for PFO closure plus antiplatelets becomes “strong” if anticoagulation therapy is contraindicated or declined as a treatment option.
The new recommendations were largely driven by three multicenter studies of that were published simultaneously last year. (N Engl J Med. 2017 Sep 14;377:1011-21; 1022-32; 1033-42). However, these and previous studies did not provide adequate data on risks and benefits for all options, which the authors emphasized in guidelines meant to help clinicians weigh options.
Weak means that clinicians “should recognize that different choices will be appropriate for different patients,” explained Frederick A. Spencer, MD, professor, division of cardiology, McMaster University, Hamilton, Ontario. Quoting from GRADE definitions, Dr. Spencer, who was the guideline panel chair, explained that weak recommendations are the product of uncertainties that may affect choice in specific individuals.
In addition to the recommendation for PFO closure plus antiplatelets as either a weak or strong recommendation in relation to the availability of anticoagulation therapy, the guidelines offered one other recommendation. For patients contraindicated or unwilling to undergo PFO closure, anticoagulation therapy was preferred over antiplatelet therapy. This was also labeled a “weak” recommendation.
These guidelines were the product of collaboration between the BMJ and the nonprofit MAGIC (Making GRADE the Irresistible Choice) project. In addition to providing a methodology and structure to organize guideline deliberations among the participating international experts, MAGIC facilitated communication and collaboration through Web-based technology.
This approach was particularly useful for the detailed analyses and debate about relative risks and benefits of treatment alternatives in which direct comparative data were limited. For example, the authors noted that the only randomized comparison of PFO closure to anticoagulation involved just 353 patients. Indirect evidence was therefore added to improve the precision of the estimated benefits and risks. The panel selected PFO closure plus antiplatelet therapy over anticoagulation therapy, because “the most serious complications of PFO closure are usually short term, whereas anticoagulation imposes a long-term burden and increased risk of major bleeding.”
The estimated rate of adverse events associated with PFO is 3.6%, according to data cited in the expert guidelines. Atrial fibrillation accounts for about half of these events. At 5 years, the absolute reduction in stroke from PFO closure versus no intervention is an estimated 8.7%. The panel assumed that most patients would place greater weight on stroke prevention than the largely reversible adverse events associated with PFO closure.
The newly published guidelines include a detailed review of the available data to allow clinicians to counsel patients appropriately. Dr. Spencer cautioned that no recommendation, strong or weak, should be uniformly applied without considering individual patient factors and preferences.
According to Christopher J. White, MD, system chairman for cardiology, Ochsner Medical Center, New Orleans, the guideline committee had no choice but to label PFO closure plus antiplatelet therapy as a “weak” recommendation within the requirements of GRADE. However, Dr. White believes that it should be considered the best option in most patients despite this terminology.
“What I tell patients is that this is a one-and-done procedure,” Dr. White explained. “Without closure, patients must remain on anticoagulation therapy, which involves refilling prescriptions, remaining compliant with daily therapy for life, and accepting an increased risk of bleeding. Once the PFO is closed and endothelialized, no additional anticoagulation treatment is needed.”
There are adverse events associated with PFO closure, but Dr. White called these uncommon and more acceptable than the risks of indefinite anticoagulation therapy, particularly if patients are not fully compliant.
“When I explain the options to patients, most will opt for PFO closure,” Dr. White said.
He also emphasized that the advantage of PFO closure accrues over time.
“When these patients have a stroke at a relatively young age, they may need to be on anticoagulation for decades. Once the PFO is closed, you are saving the patient the burden of lifelong compliance to anticoagulation therapy as well as the costs,” he added. Although the guidelines compared relative benefits of a given strategy over 5 years, the advantage of PFO closure over anticoagulation will keep increasing beyond this point.
Source: Kuipers et al. BMJ 2018 Jul 25;362:K2515
In patients younger than 60 years, patent foramen ovale (PFO) closure plus antiplatelet therapy is a better strategy for preventing recurrent ischemic stroke than indefinite anticoagulant therapy without closure, according to a recommendation from an expert panel after a systematic literature review.
When compared with the alternative strategy of indefinite anticoagulant therapy for those patients “open to all options,” the recommendation was labeled only “weak” on the basis of the GRADE classification system used by the panel to rate treatment strategies. However, the recommendation for PFO closure plus antiplatelets becomes “strong” if anticoagulation therapy is contraindicated or declined as a treatment option.
The new recommendations were largely driven by three multicenter studies of that were published simultaneously last year. (N Engl J Med. 2017 Sep 14;377:1011-21; 1022-32; 1033-42). However, these and previous studies did not provide adequate data on risks and benefits for all options, which the authors emphasized in guidelines meant to help clinicians weigh options.
Weak means that clinicians “should recognize that different choices will be appropriate for different patients,” explained Frederick A. Spencer, MD, professor, division of cardiology, McMaster University, Hamilton, Ontario. Quoting from GRADE definitions, Dr. Spencer, who was the guideline panel chair, explained that weak recommendations are the product of uncertainties that may affect choice in specific individuals.
In addition to the recommendation for PFO closure plus antiplatelets as either a weak or strong recommendation in relation to the availability of anticoagulation therapy, the guidelines offered one other recommendation. For patients contraindicated or unwilling to undergo PFO closure, anticoagulation therapy was preferred over antiplatelet therapy. This was also labeled a “weak” recommendation.
These guidelines were the product of collaboration between the BMJ and the nonprofit MAGIC (Making GRADE the Irresistible Choice) project. In addition to providing a methodology and structure to organize guideline deliberations among the participating international experts, MAGIC facilitated communication and collaboration through Web-based technology.
This approach was particularly useful for the detailed analyses and debate about relative risks and benefits of treatment alternatives in which direct comparative data were limited. For example, the authors noted that the only randomized comparison of PFO closure to anticoagulation involved just 353 patients. Indirect evidence was therefore added to improve the precision of the estimated benefits and risks. The panel selected PFO closure plus antiplatelet therapy over anticoagulation therapy, because “the most serious complications of PFO closure are usually short term, whereas anticoagulation imposes a long-term burden and increased risk of major bleeding.”
The estimated rate of adverse events associated with PFO is 3.6%, according to data cited in the expert guidelines. Atrial fibrillation accounts for about half of these events. At 5 years, the absolute reduction in stroke from PFO closure versus no intervention is an estimated 8.7%. The panel assumed that most patients would place greater weight on stroke prevention than the largely reversible adverse events associated with PFO closure.
The newly published guidelines include a detailed review of the available data to allow clinicians to counsel patients appropriately. Dr. Spencer cautioned that no recommendation, strong or weak, should be uniformly applied without considering individual patient factors and preferences.
According to Christopher J. White, MD, system chairman for cardiology, Ochsner Medical Center, New Orleans, the guideline committee had no choice but to label PFO closure plus antiplatelet therapy as a “weak” recommendation within the requirements of GRADE. However, Dr. White believes that it should be considered the best option in most patients despite this terminology.
“What I tell patients is that this is a one-and-done procedure,” Dr. White explained. “Without closure, patients must remain on anticoagulation therapy, which involves refilling prescriptions, remaining compliant with daily therapy for life, and accepting an increased risk of bleeding. Once the PFO is closed and endothelialized, no additional anticoagulation treatment is needed.”
There are adverse events associated with PFO closure, but Dr. White called these uncommon and more acceptable than the risks of indefinite anticoagulation therapy, particularly if patients are not fully compliant.
“When I explain the options to patients, most will opt for PFO closure,” Dr. White said.
He also emphasized that the advantage of PFO closure accrues over time.
“When these patients have a stroke at a relatively young age, they may need to be on anticoagulation for decades. Once the PFO is closed, you are saving the patient the burden of lifelong compliance to anticoagulation therapy as well as the costs,” he added. Although the guidelines compared relative benefits of a given strategy over 5 years, the advantage of PFO closure over anticoagulation will keep increasing beyond this point.
Source: Kuipers et al. BMJ 2018 Jul 25;362:K2515
FROM BMJ
No benefit of direct stenting in PCI
A patient-level analysis drawn from three randomized, controlled trials finds no evidence that direct stenting improved myocardial reperfusion or clinical outcomes in ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention.
Patients who underwent thrombus aspiration were more likely to receive direct stenting than conventional stenting, but there was no interaction between thrombus aspiration and outcomes, Karim D. Mahmoud, MD, reported in the European Heart Journal.
Direct stenting – stent implantation without balloon predilatation – has been widely adopted in an effort to improve PCI outcomes in STEMI patients, though no formal guidelines call for it. Small trials have suggested a benefit, but no large, definitive trials have been conducted.
Three previous trials have looked at thrombus aspiration in STEMI patients: TAPAS (the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction Study) found that routine manual thrombus aspiration led to better myocardial reperfusion and lower 1-year cardiac mortality (N Engl J Med. 2008 Feb 7;358:557-67). Two larger trials – TASTE (Thrombus Aspiration in ST Elevation Myocardial Infarction in Scandinavia; N Engl J Med. 2013 Oct 24;369[17]:1587-97) and TOTAL (Trial of Routine Aspiration Thrombectomy with PCI vs. PCI Alone in Patients with STEMI; N Engl J Med. 2015 Apr 9;372[15]:1389-98) – both failed to show any benefit of routine thrombus aspiration. As a result, international guidelines recommended use of thrombus aspiration only in selected patients.
The TASTE and TOTAL trials suggested that thrombus aspiration may have led to higher rates of direct stenting, but those rates were lower in TAPAS. Some have suggested that a synergistic effect between thrombus aspiration and DS could explain the positive finding in TAPAS, compared with negative findings in TASTE and TOTAL, said Dr. Mahmoud of Erasmus Medical Center, Rotterdam, the Netherlands.
The researchers tested this idea by pooling patient-level data from the more than 17,000 participants in the three studies, 32% of whom underwent direct stenting. Patients who were randomized to undergo thrombus aspiration were nearly twice as likely to undergo direct stenting (41% vs. 22%, P less than .001). When the researchers 1:1 propensity matched direct stenting versus conventional stenting, 30-day cardiovascular death rates were similar between direct (1.7%) and conventional stenting (1.9%), and there was no interaction between direct stenting and thrombus aspiration. The latter result suggested that there is no synergistic effect. Similar results were found at 1-year follow-up, and with respect to 30-day stroke or transient ischemic attack.
One of the study authors received funding and or honoraria from Bayer, Medtronic, Vascular Solutions, Terumo, Boston Scientific, Abbott Vascular, AstraZeneca, and the Medicines Company.
SOURCE: Mahmoud KD et al. Eur Heart J. 2018;39:2472-9.
A patient-level analysis drawn from three randomized, controlled trials finds no evidence that direct stenting improved myocardial reperfusion or clinical outcomes in ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention.
Patients who underwent thrombus aspiration were more likely to receive direct stenting than conventional stenting, but there was no interaction between thrombus aspiration and outcomes, Karim D. Mahmoud, MD, reported in the European Heart Journal.
Direct stenting – stent implantation without balloon predilatation – has been widely adopted in an effort to improve PCI outcomes in STEMI patients, though no formal guidelines call for it. Small trials have suggested a benefit, but no large, definitive trials have been conducted.
Three previous trials have looked at thrombus aspiration in STEMI patients: TAPAS (the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction Study) found that routine manual thrombus aspiration led to better myocardial reperfusion and lower 1-year cardiac mortality (N Engl J Med. 2008 Feb 7;358:557-67). Two larger trials – TASTE (Thrombus Aspiration in ST Elevation Myocardial Infarction in Scandinavia; N Engl J Med. 2013 Oct 24;369[17]:1587-97) and TOTAL (Trial of Routine Aspiration Thrombectomy with PCI vs. PCI Alone in Patients with STEMI; N Engl J Med. 2015 Apr 9;372[15]:1389-98) – both failed to show any benefit of routine thrombus aspiration. As a result, international guidelines recommended use of thrombus aspiration only in selected patients.
The TASTE and TOTAL trials suggested that thrombus aspiration may have led to higher rates of direct stenting, but those rates were lower in TAPAS. Some have suggested that a synergistic effect between thrombus aspiration and DS could explain the positive finding in TAPAS, compared with negative findings in TASTE and TOTAL, said Dr. Mahmoud of Erasmus Medical Center, Rotterdam, the Netherlands.
The researchers tested this idea by pooling patient-level data from the more than 17,000 participants in the three studies, 32% of whom underwent direct stenting. Patients who were randomized to undergo thrombus aspiration were nearly twice as likely to undergo direct stenting (41% vs. 22%, P less than .001). When the researchers 1:1 propensity matched direct stenting versus conventional stenting, 30-day cardiovascular death rates were similar between direct (1.7%) and conventional stenting (1.9%), and there was no interaction between direct stenting and thrombus aspiration. The latter result suggested that there is no synergistic effect. Similar results were found at 1-year follow-up, and with respect to 30-day stroke or transient ischemic attack.
One of the study authors received funding and or honoraria from Bayer, Medtronic, Vascular Solutions, Terumo, Boston Scientific, Abbott Vascular, AstraZeneca, and the Medicines Company.
SOURCE: Mahmoud KD et al. Eur Heart J. 2018;39:2472-9.
A patient-level analysis drawn from three randomized, controlled trials finds no evidence that direct stenting improved myocardial reperfusion or clinical outcomes in ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention.
Patients who underwent thrombus aspiration were more likely to receive direct stenting than conventional stenting, but there was no interaction between thrombus aspiration and outcomes, Karim D. Mahmoud, MD, reported in the European Heart Journal.
Direct stenting – stent implantation without balloon predilatation – has been widely adopted in an effort to improve PCI outcomes in STEMI patients, though no formal guidelines call for it. Small trials have suggested a benefit, but no large, definitive trials have been conducted.
Three previous trials have looked at thrombus aspiration in STEMI patients: TAPAS (the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction Study) found that routine manual thrombus aspiration led to better myocardial reperfusion and lower 1-year cardiac mortality (N Engl J Med. 2008 Feb 7;358:557-67). Two larger trials – TASTE (Thrombus Aspiration in ST Elevation Myocardial Infarction in Scandinavia; N Engl J Med. 2013 Oct 24;369[17]:1587-97) and TOTAL (Trial of Routine Aspiration Thrombectomy with PCI vs. PCI Alone in Patients with STEMI; N Engl J Med. 2015 Apr 9;372[15]:1389-98) – both failed to show any benefit of routine thrombus aspiration. As a result, international guidelines recommended use of thrombus aspiration only in selected patients.
The TASTE and TOTAL trials suggested that thrombus aspiration may have led to higher rates of direct stenting, but those rates were lower in TAPAS. Some have suggested that a synergistic effect between thrombus aspiration and DS could explain the positive finding in TAPAS, compared with negative findings in TASTE and TOTAL, said Dr. Mahmoud of Erasmus Medical Center, Rotterdam, the Netherlands.
The researchers tested this idea by pooling patient-level data from the more than 17,000 participants in the three studies, 32% of whom underwent direct stenting. Patients who were randomized to undergo thrombus aspiration were nearly twice as likely to undergo direct stenting (41% vs. 22%, P less than .001). When the researchers 1:1 propensity matched direct stenting versus conventional stenting, 30-day cardiovascular death rates were similar between direct (1.7%) and conventional stenting (1.9%), and there was no interaction between direct stenting and thrombus aspiration. The latter result suggested that there is no synergistic effect. Similar results were found at 1-year follow-up, and with respect to 30-day stroke or transient ischemic attack.
One of the study authors received funding and or honoraria from Bayer, Medtronic, Vascular Solutions, Terumo, Boston Scientific, Abbott Vascular, AstraZeneca, and the Medicines Company.
SOURCE: Mahmoud KD et al. Eur Heart J. 2018;39:2472-9.
FROM EUROPEAN HEART JOURNAL
Key clinical point:
Major finding: Thirty-day cardiovascular death rates were similar between direct stenting (1.7%) and conventional stenting (1.9%).
Study details: Propensity-matched analysis of patient data from three previous trials (n = 17,329).
Disclosures: One of the study authors received funding and or honoraria from Bayer, Medtronic, Vascular Solutions, Terumo, Boston Scientific, Abbott Vascular, AstraZeneca, and the Medicines Company.
Source: Mahmoud KD et al. Eur Heart J. 2018;39:2472-9.
Culotte stenting impresses in CELTIC Bifurcation Study
PARIS – Technical success rates were high and major adverse events impressively low with a two-stent culotte strategy using contemporary drug-eluting stents for coronary bifurcation lesions in the randomized CELTIC Bifurcation Study.
“We initiated this study because of a conviction that the story isn’t finished with bifurcation stenting. We’re very much under the impression that the accepted wisdom of a conservative approach is, we think, not correct, and the issue needs to be kept open,” said Dr. Foley, an interventional cardiologist at Beaumont Hospital in Dublin.
The widely accepted provisional single-stent strategy is based on early-days randomized trial evidence using first-generation drug-eluting stents and older techniques that are no longer relevant in contemporary practice. Moreover, this conservative single-stent approach doesn’t address the important issue of ischemia arising from large side branches, he asserted.
“I’ve always been fond of culotte stenting myself because I think it’s a very elegant, simple, repeatable strategy, and with modern stents it becomes easier for modest-volume operators to carry it out well. We’ve kept on trying to convert new colleagues and older colleagues who are set in their ways,” Dr. Foley said.
The CELTIC Bifurcation Study was an investigator-initiated trial in which 177 patients at nine centers in Ireland and the United Kingdom were randomized to culotte stenting using either two-connector, third-generation Synergy everolimus-eluting stents or the three-connector, second-generation Xience everolimus-eluting stents. All participants had Medina 1,1,1 coronary bifurcation lesions, which were left anterior descending/diagonal lesions in more than 80% of cases. A radial approach was used in more than 95% of the procedures. The indication for percutaneous coronary intervention was stable angina in more than 60% of cases. The rate of technical procedural success with final kissing balloon inflation exceeded 96%. The primary outcome – a MACCE (major adverse cardiovascular and cerebrovascular events) composite of death, MI, cerebrovascular accident, and target vessel revascularization over the course of 9 months – occurred in 5.9% of patients: 8.6% of the Synergy group and 3.7% with Xience stents, a nonsignificant difference. This MACCE rate was considerably lower than the 10% figure that the investigators had expected on the basis of published studies of PCI in these complex bifurcation lesions.
“The results were better than expected,” the cardiologist said. “We don’t get excited that easily, to be honest, but nonetheless we’re a little bit excited that the overall MACCE rate in this complex lesion presentation was 5.9%.”
Discussant Volker Schächinger, MD, director of cardiology at Fulda (Germany) Hospital, observed: “It’s always good to reassess what are believed to be answered questions when there are new devices available.” But why not compare culotte stenting to the provisional single-stent strategy? he asked.
“We think provisional versus culotte stenting has been thrashed to death already. And you’d need a bigger trial than we had funding for,” Dr. Foley replied.
“Many of us use the DK [double kissing] crush technique,” another panelist said. “It’s very popular. But if you look at bench testing, perhaps culotte is a better approach by many parameters. So I think it was important for you to highlight the value of culotte and how it can be done properly.”
Discussant James Nolan, MD, a cardiologist at the University Hospital of North Staffordshire (England), said, “The most critical thing with these bifurcation procedures is the operators and how they do it. So you have to do the culotte to the standard done in this trial. If you do a sloppy culotte, it’s not going to be great. It’s probably more important to deliver an excellently performed procedure, whatever it is. You’ll get a better result if you’re good at what you’re doing rather than selecting one procedure or another.”
Dr. Foley agreed, adding: “In some of the DK crush versus culotte randomized trials, I’m not convinced that culotte was done the way I would suggest it should be done.”
Operators in the CELTIC Bifurcation Study were asked to follow a standardized culotte procedure: predilate both limbs of the bifurcation, keep both wires in place, deploy the first stent in the side branch unless the main branch was awkwardly angulated, then cross by going from distally into the optimized first stent, and placing the second stent proximal to the first stent so that the two stents overlap in the proximal main vessel.
“We call that ‘nailing it down,’ ” he explained.
The procedure is completed by sequential high-pressure kissing balloon dilatation of both branches, with intravascular ultrasound or optical coherence tomography recommended but not required.
Simultaneously with this presentation, the study results were published online (EuroIntervention 2018 Jun 8;14[3]:e318-24).
The CELTIC Bifurcation Study was funded by an unrestricted grant from Boston Scientific. Dr. Foley reported having no financial conflicts of interest regarding the study.
PARIS – Technical success rates were high and major adverse events impressively low with a two-stent culotte strategy using contemporary drug-eluting stents for coronary bifurcation lesions in the randomized CELTIC Bifurcation Study.
“We initiated this study because of a conviction that the story isn’t finished with bifurcation stenting. We’re very much under the impression that the accepted wisdom of a conservative approach is, we think, not correct, and the issue needs to be kept open,” said Dr. Foley, an interventional cardiologist at Beaumont Hospital in Dublin.
The widely accepted provisional single-stent strategy is based on early-days randomized trial evidence using first-generation drug-eluting stents and older techniques that are no longer relevant in contemporary practice. Moreover, this conservative single-stent approach doesn’t address the important issue of ischemia arising from large side branches, he asserted.
“I’ve always been fond of culotte stenting myself because I think it’s a very elegant, simple, repeatable strategy, and with modern stents it becomes easier for modest-volume operators to carry it out well. We’ve kept on trying to convert new colleagues and older colleagues who are set in their ways,” Dr. Foley said.
The CELTIC Bifurcation Study was an investigator-initiated trial in which 177 patients at nine centers in Ireland and the United Kingdom were randomized to culotte stenting using either two-connector, third-generation Synergy everolimus-eluting stents or the three-connector, second-generation Xience everolimus-eluting stents. All participants had Medina 1,1,1 coronary bifurcation lesions, which were left anterior descending/diagonal lesions in more than 80% of cases. A radial approach was used in more than 95% of the procedures. The indication for percutaneous coronary intervention was stable angina in more than 60% of cases. The rate of technical procedural success with final kissing balloon inflation exceeded 96%. The primary outcome – a MACCE (major adverse cardiovascular and cerebrovascular events) composite of death, MI, cerebrovascular accident, and target vessel revascularization over the course of 9 months – occurred in 5.9% of patients: 8.6% of the Synergy group and 3.7% with Xience stents, a nonsignificant difference. This MACCE rate was considerably lower than the 10% figure that the investigators had expected on the basis of published studies of PCI in these complex bifurcation lesions.
“The results were better than expected,” the cardiologist said. “We don’t get excited that easily, to be honest, but nonetheless we’re a little bit excited that the overall MACCE rate in this complex lesion presentation was 5.9%.”
Discussant Volker Schächinger, MD, director of cardiology at Fulda (Germany) Hospital, observed: “It’s always good to reassess what are believed to be answered questions when there are new devices available.” But why not compare culotte stenting to the provisional single-stent strategy? he asked.
“We think provisional versus culotte stenting has been thrashed to death already. And you’d need a bigger trial than we had funding for,” Dr. Foley replied.
“Many of us use the DK [double kissing] crush technique,” another panelist said. “It’s very popular. But if you look at bench testing, perhaps culotte is a better approach by many parameters. So I think it was important for you to highlight the value of culotte and how it can be done properly.”
Discussant James Nolan, MD, a cardiologist at the University Hospital of North Staffordshire (England), said, “The most critical thing with these bifurcation procedures is the operators and how they do it. So you have to do the culotte to the standard done in this trial. If you do a sloppy culotte, it’s not going to be great. It’s probably more important to deliver an excellently performed procedure, whatever it is. You’ll get a better result if you’re good at what you’re doing rather than selecting one procedure or another.”
Dr. Foley agreed, adding: “In some of the DK crush versus culotte randomized trials, I’m not convinced that culotte was done the way I would suggest it should be done.”
Operators in the CELTIC Bifurcation Study were asked to follow a standardized culotte procedure: predilate both limbs of the bifurcation, keep both wires in place, deploy the first stent in the side branch unless the main branch was awkwardly angulated, then cross by going from distally into the optimized first stent, and placing the second stent proximal to the first stent so that the two stents overlap in the proximal main vessel.
“We call that ‘nailing it down,’ ” he explained.
The procedure is completed by sequential high-pressure kissing balloon dilatation of both branches, with intravascular ultrasound or optical coherence tomography recommended but not required.
Simultaneously with this presentation, the study results were published online (EuroIntervention 2018 Jun 8;14[3]:e318-24).
The CELTIC Bifurcation Study was funded by an unrestricted grant from Boston Scientific. Dr. Foley reported having no financial conflicts of interest regarding the study.
PARIS – Technical success rates were high and major adverse events impressively low with a two-stent culotte strategy using contemporary drug-eluting stents for coronary bifurcation lesions in the randomized CELTIC Bifurcation Study.
“We initiated this study because of a conviction that the story isn’t finished with bifurcation stenting. We’re very much under the impression that the accepted wisdom of a conservative approach is, we think, not correct, and the issue needs to be kept open,” said Dr. Foley, an interventional cardiologist at Beaumont Hospital in Dublin.
The widely accepted provisional single-stent strategy is based on early-days randomized trial evidence using first-generation drug-eluting stents and older techniques that are no longer relevant in contemporary practice. Moreover, this conservative single-stent approach doesn’t address the important issue of ischemia arising from large side branches, he asserted.
“I’ve always been fond of culotte stenting myself because I think it’s a very elegant, simple, repeatable strategy, and with modern stents it becomes easier for modest-volume operators to carry it out well. We’ve kept on trying to convert new colleagues and older colleagues who are set in their ways,” Dr. Foley said.
The CELTIC Bifurcation Study was an investigator-initiated trial in which 177 patients at nine centers in Ireland and the United Kingdom were randomized to culotte stenting using either two-connector, third-generation Synergy everolimus-eluting stents or the three-connector, second-generation Xience everolimus-eluting stents. All participants had Medina 1,1,1 coronary bifurcation lesions, which were left anterior descending/diagonal lesions in more than 80% of cases. A radial approach was used in more than 95% of the procedures. The indication for percutaneous coronary intervention was stable angina in more than 60% of cases. The rate of technical procedural success with final kissing balloon inflation exceeded 96%. The primary outcome – a MACCE (major adverse cardiovascular and cerebrovascular events) composite of death, MI, cerebrovascular accident, and target vessel revascularization over the course of 9 months – occurred in 5.9% of patients: 8.6% of the Synergy group and 3.7% with Xience stents, a nonsignificant difference. This MACCE rate was considerably lower than the 10% figure that the investigators had expected on the basis of published studies of PCI in these complex bifurcation lesions.
“The results were better than expected,” the cardiologist said. “We don’t get excited that easily, to be honest, but nonetheless we’re a little bit excited that the overall MACCE rate in this complex lesion presentation was 5.9%.”
Discussant Volker Schächinger, MD, director of cardiology at Fulda (Germany) Hospital, observed: “It’s always good to reassess what are believed to be answered questions when there are new devices available.” But why not compare culotte stenting to the provisional single-stent strategy? he asked.
“We think provisional versus culotte stenting has been thrashed to death already. And you’d need a bigger trial than we had funding for,” Dr. Foley replied.
“Many of us use the DK [double kissing] crush technique,” another panelist said. “It’s very popular. But if you look at bench testing, perhaps culotte is a better approach by many parameters. So I think it was important for you to highlight the value of culotte and how it can be done properly.”
Discussant James Nolan, MD, a cardiologist at the University Hospital of North Staffordshire (England), said, “The most critical thing with these bifurcation procedures is the operators and how they do it. So you have to do the culotte to the standard done in this trial. If you do a sloppy culotte, it’s not going to be great. It’s probably more important to deliver an excellently performed procedure, whatever it is. You’ll get a better result if you’re good at what you’re doing rather than selecting one procedure or another.”
Dr. Foley agreed, adding: “In some of the DK crush versus culotte randomized trials, I’m not convinced that culotte was done the way I would suggest it should be done.”
Operators in the CELTIC Bifurcation Study were asked to follow a standardized culotte procedure: predilate both limbs of the bifurcation, keep both wires in place, deploy the first stent in the side branch unless the main branch was awkwardly angulated, then cross by going from distally into the optimized first stent, and placing the second stent proximal to the first stent so that the two stents overlap in the proximal main vessel.
“We call that ‘nailing it down,’ ” he explained.
The procedure is completed by sequential high-pressure kissing balloon dilatation of both branches, with intravascular ultrasound or optical coherence tomography recommended but not required.
Simultaneously with this presentation, the study results were published online (EuroIntervention 2018 Jun 8;14[3]:e318-24).
The CELTIC Bifurcation Study was funded by an unrestricted grant from Boston Scientific. Dr. Foley reported having no financial conflicts of interest regarding the study.
REPORTING FROM EUROPCR 2018
Key clinical point: .
Major finding: The 9-month MACCE rate following culotte stenting for bifurcation lesions was 5.9%, with no significant difference between patients randomized to the Xience or Synergy stents.
Study details: This multicenter randomized trial comprised 177 patients with coronary bifurcation lesions who underwent culotte stenting with either Xience or Synergy everolimus-eluting stents.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by an unrestricted grant from Boston Scientific.
TAVR requirements tweaked in a 6-year update
Transcatheter aortic valve replacement has entered a new stage of development, and so needed a tweaked set of standards for how existing programs operate and what new program need to open, said a panel of experts formed by the four U.S. societies with the closest links to this procedure.
U.S. transcatheter aortic valve replacement (TAVR) programs have “matured as a therapeutic option” since its commercial U.S. introduction in 2012, said a revised statement of operator and institutional recommendations and requirements issued on July 18 by the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. A writing panel formed by these four groups prepared the revision, published online in the Journal of the American College of Cardiology on July 18, to replace the first set of recommendations for running U.S. TAVR programs that came out in 2012 (J Am Coll Cardiol. 2012 May 29;59[22]:2028-42).
“The main thrust is to ensure and allow for the metrics of quality TAVR,” said Joseph E. Bavaria, MD, cochair of the writing panel and professor of surgery and codirector of the transcatheter valve program at the University of Pennsylvania in Philadelphia. “We’re trying to force continuous quality improvement across U.S. TAVR teams,” Dr. Bavaria explained in an interview.
The key to this change will be the data collected on every U.S. TAVR patient in the Transcatheter Valve Therapy registry maintained by the American College of Cardiology and the Society of Thoracic Surgeons, which now has data on more than 120,000 patients who have undergone TAVR at what are now 582 active U.S. TAVR programs, noted Carl L. Tommaso, MD, an interventional cardiologist with NorthShore Medical Group in Bannockburn, Ill., and cochair of the writing panel. “You need to do risk adjustment to measure quality of care,” and the robust database that now exists has begun to make this possible, said Dr. Tommaso, who neither performs TAVR procedures nor participates on a TAVR team. Statistical analyses based on this substantial and always-expanding database of TAVR patients now allows for risk-adjusted assessment of in hospital and 30-day mortality, and risk-adjusted evaluation of 1-year mortality and quality-of-life outcomes are expected within the next couple of years.
“We’re still not yet at the point of having good, risk-adjusted models” for all these measures, but our hope is that in the next 2-7 years we can move completely to quality measures, as has already been done for percutaneous coronary interventions” and away from procedure volume, which currently serves as a surrogate marker for a TAVR program’s competence.
The new document continues to call for TAVR programs to average at least 50 TAVR procedures a year or at least 100 every 2 years, but primarily to insure that each TAVR program can generate enough data about its performance to produce statistically reliable numbers.
“The volume floors are only there because you can’t measure quality without volume,” said Dr. Bavaria. “It’s impossible to measure quality without a certain procedure volume.”
Dr. Bavaria stressed, however, that the goal of the new document is not to limit TAVR programs based on their procedure volume, especially because another goal of the document is to ensure reasonable geographic access to TAVR for U.S. patients. “This document does not advocate for any program to shut down,” he declared. On top of that, “we have no problem with new programs,” although the document noted that “the major threat to low volume sites growing and achieving higher levels of experience is the opening of additional sites in the same geographic region.”
In 2017, 204 of the 525 sites (39%) performing TAVR at that time were performing fewer than 50 procedures annually, the document said, but added that many TAVR centers now operate in what are predominantly rural regions “and it is important that they remain active if they can document acceptable quality even if they should fall below volume thresholds to maintain patient access to care.”
Dr. Tommaso said that perhaps a TAVR center in Alaska, for example, might not meet the 50 cases/year standard, “but I don’t think anyone would worry if the volume was low because we’re serving patients in Alaska.” Currently, 84% of TAVR centers that have been operating for more than 2 years meet the 50 procedures/year threshold, he added. And TAVR centers now operate in 49 of the 50 states, with only Wyoming lacking a TAVR facility within its borders. Despite this, use of TAVR among Wyoming residents is comparable to the rate in Illinois, Dr. Bavaria said.
Both cochairs also highlighted that, with TAVR now approved for patients at moderate risk for aortic-valve surgery, the number of patients who are TAVR candidates has grown, and it’s possible that pending trial results will soon broaden TAVR’s availability to low-risk patients, a step that would greatly further expand the potential patient pool for the procedure.
The revised recommendations and requirements will make it “a little easier to start a new program, except now, for the first time, you need to start with an operator who is already experienced with TAVR,” noted Dr. Bavaria. “The TAVR technology is now mature enough that it’s inappropriate to have learning-curve mortality.” But aside from this the new standards lower the bar a bit for a center’s volume of percutaneous coronary interventions and surgical aortic valve replacements. The revision also maintains that an examination by and consultation with a single cardiac surgeon by a prospective TAVR patient is adequate, similar to the 2012 document, although the Center for Medicare & Medicaid Services mandated in its coverage decision that prospective TAVR patients consult with two cardiac surgeons, the so-called “two-surgeon rule.” If CMS eliminated the two-surgeon rule it would “streamline” the process that patients go through when being assessed for TAVR, Dr. Tommaso observed, and both he and Dr. Bavaria expressed hope that the new document might prompt CMS to reconsider this guidance.
“We felt that two surgeons weren’t needed,” but the document specifies that both the surgeon and the cardiologist whom a prospective patient consults before finalizing plans for the intervention should both be members of the multidisciplinary team that performs the procedure. Until now, these clinicians weren’t specified as necessarily members of the TAVR team, Dr. Bavaria said.
One additional new element in the revised document is specification of shared decision making as the mechanism patients should go through when considering TAVR relative to their other management options, Dr. Tommaso said.Dr. Bavaria and Dr. Tommaso had no disclosures.
SOURCE: Bavaria JE et al. JACC. 2018 Jul18. doi:10.1016/j.jacc.2018.07.002.
Transcatheter aortic valve replacement has entered a new stage of development, and so needed a tweaked set of standards for how existing programs operate and what new program need to open, said a panel of experts formed by the four U.S. societies with the closest links to this procedure.
U.S. transcatheter aortic valve replacement (TAVR) programs have “matured as a therapeutic option” since its commercial U.S. introduction in 2012, said a revised statement of operator and institutional recommendations and requirements issued on July 18 by the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. A writing panel formed by these four groups prepared the revision, published online in the Journal of the American College of Cardiology on July 18, to replace the first set of recommendations for running U.S. TAVR programs that came out in 2012 (J Am Coll Cardiol. 2012 May 29;59[22]:2028-42).
“The main thrust is to ensure and allow for the metrics of quality TAVR,” said Joseph E. Bavaria, MD, cochair of the writing panel and professor of surgery and codirector of the transcatheter valve program at the University of Pennsylvania in Philadelphia. “We’re trying to force continuous quality improvement across U.S. TAVR teams,” Dr. Bavaria explained in an interview.
The key to this change will be the data collected on every U.S. TAVR patient in the Transcatheter Valve Therapy registry maintained by the American College of Cardiology and the Society of Thoracic Surgeons, which now has data on more than 120,000 patients who have undergone TAVR at what are now 582 active U.S. TAVR programs, noted Carl L. Tommaso, MD, an interventional cardiologist with NorthShore Medical Group in Bannockburn, Ill., and cochair of the writing panel. “You need to do risk adjustment to measure quality of care,” and the robust database that now exists has begun to make this possible, said Dr. Tommaso, who neither performs TAVR procedures nor participates on a TAVR team. Statistical analyses based on this substantial and always-expanding database of TAVR patients now allows for risk-adjusted assessment of in hospital and 30-day mortality, and risk-adjusted evaluation of 1-year mortality and quality-of-life outcomes are expected within the next couple of years.
“We’re still not yet at the point of having good, risk-adjusted models” for all these measures, but our hope is that in the next 2-7 years we can move completely to quality measures, as has already been done for percutaneous coronary interventions” and away from procedure volume, which currently serves as a surrogate marker for a TAVR program’s competence.
The new document continues to call for TAVR programs to average at least 50 TAVR procedures a year or at least 100 every 2 years, but primarily to insure that each TAVR program can generate enough data about its performance to produce statistically reliable numbers.
“The volume floors are only there because you can’t measure quality without volume,” said Dr. Bavaria. “It’s impossible to measure quality without a certain procedure volume.”
Dr. Bavaria stressed, however, that the goal of the new document is not to limit TAVR programs based on their procedure volume, especially because another goal of the document is to ensure reasonable geographic access to TAVR for U.S. patients. “This document does not advocate for any program to shut down,” he declared. On top of that, “we have no problem with new programs,” although the document noted that “the major threat to low volume sites growing and achieving higher levels of experience is the opening of additional sites in the same geographic region.”
In 2017, 204 of the 525 sites (39%) performing TAVR at that time were performing fewer than 50 procedures annually, the document said, but added that many TAVR centers now operate in what are predominantly rural regions “and it is important that they remain active if they can document acceptable quality even if they should fall below volume thresholds to maintain patient access to care.”
Dr. Tommaso said that perhaps a TAVR center in Alaska, for example, might not meet the 50 cases/year standard, “but I don’t think anyone would worry if the volume was low because we’re serving patients in Alaska.” Currently, 84% of TAVR centers that have been operating for more than 2 years meet the 50 procedures/year threshold, he added. And TAVR centers now operate in 49 of the 50 states, with only Wyoming lacking a TAVR facility within its borders. Despite this, use of TAVR among Wyoming residents is comparable to the rate in Illinois, Dr. Bavaria said.
Both cochairs also highlighted that, with TAVR now approved for patients at moderate risk for aortic-valve surgery, the number of patients who are TAVR candidates has grown, and it’s possible that pending trial results will soon broaden TAVR’s availability to low-risk patients, a step that would greatly further expand the potential patient pool for the procedure.
The revised recommendations and requirements will make it “a little easier to start a new program, except now, for the first time, you need to start with an operator who is already experienced with TAVR,” noted Dr. Bavaria. “The TAVR technology is now mature enough that it’s inappropriate to have learning-curve mortality.” But aside from this the new standards lower the bar a bit for a center’s volume of percutaneous coronary interventions and surgical aortic valve replacements. The revision also maintains that an examination by and consultation with a single cardiac surgeon by a prospective TAVR patient is adequate, similar to the 2012 document, although the Center for Medicare & Medicaid Services mandated in its coverage decision that prospective TAVR patients consult with two cardiac surgeons, the so-called “two-surgeon rule.” If CMS eliminated the two-surgeon rule it would “streamline” the process that patients go through when being assessed for TAVR, Dr. Tommaso observed, and both he and Dr. Bavaria expressed hope that the new document might prompt CMS to reconsider this guidance.
“We felt that two surgeons weren’t needed,” but the document specifies that both the surgeon and the cardiologist whom a prospective patient consults before finalizing plans for the intervention should both be members of the multidisciplinary team that performs the procedure. Until now, these clinicians weren’t specified as necessarily members of the TAVR team, Dr. Bavaria said.
One additional new element in the revised document is specification of shared decision making as the mechanism patients should go through when considering TAVR relative to their other management options, Dr. Tommaso said.Dr. Bavaria and Dr. Tommaso had no disclosures.
SOURCE: Bavaria JE et al. JACC. 2018 Jul18. doi:10.1016/j.jacc.2018.07.002.
Transcatheter aortic valve replacement has entered a new stage of development, and so needed a tweaked set of standards for how existing programs operate and what new program need to open, said a panel of experts formed by the four U.S. societies with the closest links to this procedure.
U.S. transcatheter aortic valve replacement (TAVR) programs have “matured as a therapeutic option” since its commercial U.S. introduction in 2012, said a revised statement of operator and institutional recommendations and requirements issued on July 18 by the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. A writing panel formed by these four groups prepared the revision, published online in the Journal of the American College of Cardiology on July 18, to replace the first set of recommendations for running U.S. TAVR programs that came out in 2012 (J Am Coll Cardiol. 2012 May 29;59[22]:2028-42).
“The main thrust is to ensure and allow for the metrics of quality TAVR,” said Joseph E. Bavaria, MD, cochair of the writing panel and professor of surgery and codirector of the transcatheter valve program at the University of Pennsylvania in Philadelphia. “We’re trying to force continuous quality improvement across U.S. TAVR teams,” Dr. Bavaria explained in an interview.
The key to this change will be the data collected on every U.S. TAVR patient in the Transcatheter Valve Therapy registry maintained by the American College of Cardiology and the Society of Thoracic Surgeons, which now has data on more than 120,000 patients who have undergone TAVR at what are now 582 active U.S. TAVR programs, noted Carl L. Tommaso, MD, an interventional cardiologist with NorthShore Medical Group in Bannockburn, Ill., and cochair of the writing panel. “You need to do risk adjustment to measure quality of care,” and the robust database that now exists has begun to make this possible, said Dr. Tommaso, who neither performs TAVR procedures nor participates on a TAVR team. Statistical analyses based on this substantial and always-expanding database of TAVR patients now allows for risk-adjusted assessment of in hospital and 30-day mortality, and risk-adjusted evaluation of 1-year mortality and quality-of-life outcomes are expected within the next couple of years.
“We’re still not yet at the point of having good, risk-adjusted models” for all these measures, but our hope is that in the next 2-7 years we can move completely to quality measures, as has already been done for percutaneous coronary interventions” and away from procedure volume, which currently serves as a surrogate marker for a TAVR program’s competence.
The new document continues to call for TAVR programs to average at least 50 TAVR procedures a year or at least 100 every 2 years, but primarily to insure that each TAVR program can generate enough data about its performance to produce statistically reliable numbers.
“The volume floors are only there because you can’t measure quality without volume,” said Dr. Bavaria. “It’s impossible to measure quality without a certain procedure volume.”
Dr. Bavaria stressed, however, that the goal of the new document is not to limit TAVR programs based on their procedure volume, especially because another goal of the document is to ensure reasonable geographic access to TAVR for U.S. patients. “This document does not advocate for any program to shut down,” he declared. On top of that, “we have no problem with new programs,” although the document noted that “the major threat to low volume sites growing and achieving higher levels of experience is the opening of additional sites in the same geographic region.”
In 2017, 204 of the 525 sites (39%) performing TAVR at that time were performing fewer than 50 procedures annually, the document said, but added that many TAVR centers now operate in what are predominantly rural regions “and it is important that they remain active if they can document acceptable quality even if they should fall below volume thresholds to maintain patient access to care.”
Dr. Tommaso said that perhaps a TAVR center in Alaska, for example, might not meet the 50 cases/year standard, “but I don’t think anyone would worry if the volume was low because we’re serving patients in Alaska.” Currently, 84% of TAVR centers that have been operating for more than 2 years meet the 50 procedures/year threshold, he added. And TAVR centers now operate in 49 of the 50 states, with only Wyoming lacking a TAVR facility within its borders. Despite this, use of TAVR among Wyoming residents is comparable to the rate in Illinois, Dr. Bavaria said.
Both cochairs also highlighted that, with TAVR now approved for patients at moderate risk for aortic-valve surgery, the number of patients who are TAVR candidates has grown, and it’s possible that pending trial results will soon broaden TAVR’s availability to low-risk patients, a step that would greatly further expand the potential patient pool for the procedure.
The revised recommendations and requirements will make it “a little easier to start a new program, except now, for the first time, you need to start with an operator who is already experienced with TAVR,” noted Dr. Bavaria. “The TAVR technology is now mature enough that it’s inappropriate to have learning-curve mortality.” But aside from this the new standards lower the bar a bit for a center’s volume of percutaneous coronary interventions and surgical aortic valve replacements. The revision also maintains that an examination by and consultation with a single cardiac surgeon by a prospective TAVR patient is adequate, similar to the 2012 document, although the Center for Medicare & Medicaid Services mandated in its coverage decision that prospective TAVR patients consult with two cardiac surgeons, the so-called “two-surgeon rule.” If CMS eliminated the two-surgeon rule it would “streamline” the process that patients go through when being assessed for TAVR, Dr. Tommaso observed, and both he and Dr. Bavaria expressed hope that the new document might prompt CMS to reconsider this guidance.
“We felt that two surgeons weren’t needed,” but the document specifies that both the surgeon and the cardiologist whom a prospective patient consults before finalizing plans for the intervention should both be members of the multidisciplinary team that performs the procedure. Until now, these clinicians weren’t specified as necessarily members of the TAVR team, Dr. Bavaria said.
One additional new element in the revised document is specification of shared decision making as the mechanism patients should go through when considering TAVR relative to their other management options, Dr. Tommaso said.Dr. Bavaria and Dr. Tommaso had no disclosures.
SOURCE: Bavaria JE et al. JACC. 2018 Jul18. doi:10.1016/j.jacc.2018.07.002.
FROM JACC
Firehawk: A new ‘workhorse’ DES
PARIS – The Xience everolimus-eluting coronary stent is widely considered the current standard treatment, implanted by interventional cardiologists far more often than any other drug-eluting stent (DES). But judging from the results of the TARGET All Comers trial, some serious competition may be headed Xience’s way in the form of the new Firehawk rapamycin-eluting, thin-strut stent featuring a biodegradable polymer drug delivery system.
What’s more, the Firehawk offers a theoretical advantage in that the rapamycin is delivered via a polymer that’s fully absorbed by 9 months, leaving behind a bare metal stent made of cobalt chromium. This structure is believed to be less proinflammatory, atherogenic, and thrombogenic over the long haul, compared with a permanent durable polymer, such as that employed in the Xience stent. This should translate into less late restenosis and in-stent thrombosis.
Also, the Firehawk features thin, 86-mcm struts and the rapamycin, also known as sirolimus, is contained in abluminal grooves directed specifically to the vessel wall. As a result, this DES exposes patients to only one-third as much active drug as other DESs. Ninety percent of the rapamycin is released within 90 days after implantation, according to Dr. Baumbach, director of interventional research at Barts Heart Centre in London and president of the European Association of Percutaneous Cardiovascular Interventions.
The TARGET All Comers trial is a prospective, open-label, noninferiority trial comparing the safety and efficacy of the Firehawk with those of Xience stents in 1,656 DES-eligible patients with symptomatic coronary artery disease randomized at 21 centers.
The primary endpoint was the 12-month composite of target lesion failure, comprising rates of cardiac death, target vessel MI, or ischemia-driven target lesion revascularization. In an intention-to-treat analysis, the rate was 6.1% in the Firehawk patients and 5.9% in the Xience recipients. Results for each of the three components of the composite endpoint were similar in the two groups as well.
A secondary endpoint was in-stent late loss as measured by quantitative coronary angiography at 13 months in a 137-patient subgroup. The rate was 0.17 mm in the Firehawk recipients and similar at 0.11 mm in those receiving the Xience stent, again, which provided solid evidence of noninferiority.
The rate of definite stent thrombosis at 1 year was 1.2% in both study arms.
Discussant Giulio Guagliumi, MD, an interventional cardiologist at Pope Giovanni XXIII Hospital in Bergamo, Italy, pronounced the results “quite reassuring.” But where, he asked, is the evidence of late benefit for the completely biodegradable polymer utilized in the Firehawk?
“We would expect to see such an effect later on, after the stent in question becomes a simple bare metal stent as opposed to a stent with a durable polymer. But we don’t have the ultimate answer yet. In this trial we will have an extended follow-up out to 5 years to see whether there is any translation of these differences into clinical benefit,” Dr. Baumbach replied.
Discussion panelist Julinda Mehilli, MD, inquired how this new stent, which has been approved for the European market, will fit into everyday clinical practice.
“We have many biodegradable polymer DES already. We have the Ultimaster, we have Synergy – and now, the Firehawk. What kind of special features does it have? Is it for use in routine practice or in special populations?” asked Dr. Mehilli, director of interventional cardiology at the German Heart Center at the University of Munich.
“That’s of course the question: What’s the unique point of this stent? I think that the unique point is that there is really no unique point. This is a classic workhorse stent. This is a stent with good radial force and all the other features for everyday use,” according to Dr. Baumbach.
Indeed, he and his Barts colleagues did more than 100 cases in TARGET All Comers and found one of the Firehawk’s strengths was its versatility. It performed well in challenging cases, including left main interventions, as well as in more straightforward cases in this all comers trial.
The Firehawk was developed by MicroPort in China, where its safety and efficacy was established in clinical trials totaling more than 1,000 patients. It then moved to Europe, where it has earned regulatory approval. A pivotal U.S. trial is being planned with the Food and Drug Administration, which has indicated that the European TARGET All Comers data can be incorporated in the study.
Dr. Baumbach reported receiving research grants from Abbott and consultation fees from Keystone Heart, MicroPort, Sinomed, and Stentys.
PARIS – The Xience everolimus-eluting coronary stent is widely considered the current standard treatment, implanted by interventional cardiologists far more often than any other drug-eluting stent (DES). But judging from the results of the TARGET All Comers trial, some serious competition may be headed Xience’s way in the form of the new Firehawk rapamycin-eluting, thin-strut stent featuring a biodegradable polymer drug delivery system.
What’s more, the Firehawk offers a theoretical advantage in that the rapamycin is delivered via a polymer that’s fully absorbed by 9 months, leaving behind a bare metal stent made of cobalt chromium. This structure is believed to be less proinflammatory, atherogenic, and thrombogenic over the long haul, compared with a permanent durable polymer, such as that employed in the Xience stent. This should translate into less late restenosis and in-stent thrombosis.
Also, the Firehawk features thin, 86-mcm struts and the rapamycin, also known as sirolimus, is contained in abluminal grooves directed specifically to the vessel wall. As a result, this DES exposes patients to only one-third as much active drug as other DESs. Ninety percent of the rapamycin is released within 90 days after implantation, according to Dr. Baumbach, director of interventional research at Barts Heart Centre in London and president of the European Association of Percutaneous Cardiovascular Interventions.
The TARGET All Comers trial is a prospective, open-label, noninferiority trial comparing the safety and efficacy of the Firehawk with those of Xience stents in 1,656 DES-eligible patients with symptomatic coronary artery disease randomized at 21 centers.
The primary endpoint was the 12-month composite of target lesion failure, comprising rates of cardiac death, target vessel MI, or ischemia-driven target lesion revascularization. In an intention-to-treat analysis, the rate was 6.1% in the Firehawk patients and 5.9% in the Xience recipients. Results for each of the three components of the composite endpoint were similar in the two groups as well.
A secondary endpoint was in-stent late loss as measured by quantitative coronary angiography at 13 months in a 137-patient subgroup. The rate was 0.17 mm in the Firehawk recipients and similar at 0.11 mm in those receiving the Xience stent, again, which provided solid evidence of noninferiority.
The rate of definite stent thrombosis at 1 year was 1.2% in both study arms.
Discussant Giulio Guagliumi, MD, an interventional cardiologist at Pope Giovanni XXIII Hospital in Bergamo, Italy, pronounced the results “quite reassuring.” But where, he asked, is the evidence of late benefit for the completely biodegradable polymer utilized in the Firehawk?
“We would expect to see such an effect later on, after the stent in question becomes a simple bare metal stent as opposed to a stent with a durable polymer. But we don’t have the ultimate answer yet. In this trial we will have an extended follow-up out to 5 years to see whether there is any translation of these differences into clinical benefit,” Dr. Baumbach replied.
Discussion panelist Julinda Mehilli, MD, inquired how this new stent, which has been approved for the European market, will fit into everyday clinical practice.
“We have many biodegradable polymer DES already. We have the Ultimaster, we have Synergy – and now, the Firehawk. What kind of special features does it have? Is it for use in routine practice or in special populations?” asked Dr. Mehilli, director of interventional cardiology at the German Heart Center at the University of Munich.
“That’s of course the question: What’s the unique point of this stent? I think that the unique point is that there is really no unique point. This is a classic workhorse stent. This is a stent with good radial force and all the other features for everyday use,” according to Dr. Baumbach.
Indeed, he and his Barts colleagues did more than 100 cases in TARGET All Comers and found one of the Firehawk’s strengths was its versatility. It performed well in challenging cases, including left main interventions, as well as in more straightforward cases in this all comers trial.
The Firehawk was developed by MicroPort in China, where its safety and efficacy was established in clinical trials totaling more than 1,000 patients. It then moved to Europe, where it has earned regulatory approval. A pivotal U.S. trial is being planned with the Food and Drug Administration, which has indicated that the European TARGET All Comers data can be incorporated in the study.
Dr. Baumbach reported receiving research grants from Abbott and consultation fees from Keystone Heart, MicroPort, Sinomed, and Stentys.
PARIS – The Xience everolimus-eluting coronary stent is widely considered the current standard treatment, implanted by interventional cardiologists far more often than any other drug-eluting stent (DES). But judging from the results of the TARGET All Comers trial, some serious competition may be headed Xience’s way in the form of the new Firehawk rapamycin-eluting, thin-strut stent featuring a biodegradable polymer drug delivery system.
What’s more, the Firehawk offers a theoretical advantage in that the rapamycin is delivered via a polymer that’s fully absorbed by 9 months, leaving behind a bare metal stent made of cobalt chromium. This structure is believed to be less proinflammatory, atherogenic, and thrombogenic over the long haul, compared with a permanent durable polymer, such as that employed in the Xience stent. This should translate into less late restenosis and in-stent thrombosis.
Also, the Firehawk features thin, 86-mcm struts and the rapamycin, also known as sirolimus, is contained in abluminal grooves directed specifically to the vessel wall. As a result, this DES exposes patients to only one-third as much active drug as other DESs. Ninety percent of the rapamycin is released within 90 days after implantation, according to Dr. Baumbach, director of interventional research at Barts Heart Centre in London and president of the European Association of Percutaneous Cardiovascular Interventions.
The TARGET All Comers trial is a prospective, open-label, noninferiority trial comparing the safety and efficacy of the Firehawk with those of Xience stents in 1,656 DES-eligible patients with symptomatic coronary artery disease randomized at 21 centers.
The primary endpoint was the 12-month composite of target lesion failure, comprising rates of cardiac death, target vessel MI, or ischemia-driven target lesion revascularization. In an intention-to-treat analysis, the rate was 6.1% in the Firehawk patients and 5.9% in the Xience recipients. Results for each of the three components of the composite endpoint were similar in the two groups as well.
A secondary endpoint was in-stent late loss as measured by quantitative coronary angiography at 13 months in a 137-patient subgroup. The rate was 0.17 mm in the Firehawk recipients and similar at 0.11 mm in those receiving the Xience stent, again, which provided solid evidence of noninferiority.
The rate of definite stent thrombosis at 1 year was 1.2% in both study arms.
Discussant Giulio Guagliumi, MD, an interventional cardiologist at Pope Giovanni XXIII Hospital in Bergamo, Italy, pronounced the results “quite reassuring.” But where, he asked, is the evidence of late benefit for the completely biodegradable polymer utilized in the Firehawk?
“We would expect to see such an effect later on, after the stent in question becomes a simple bare metal stent as opposed to a stent with a durable polymer. But we don’t have the ultimate answer yet. In this trial we will have an extended follow-up out to 5 years to see whether there is any translation of these differences into clinical benefit,” Dr. Baumbach replied.
Discussion panelist Julinda Mehilli, MD, inquired how this new stent, which has been approved for the European market, will fit into everyday clinical practice.
“We have many biodegradable polymer DES already. We have the Ultimaster, we have Synergy – and now, the Firehawk. What kind of special features does it have? Is it for use in routine practice or in special populations?” asked Dr. Mehilli, director of interventional cardiology at the German Heart Center at the University of Munich.
“That’s of course the question: What’s the unique point of this stent? I think that the unique point is that there is really no unique point. This is a classic workhorse stent. This is a stent with good radial force and all the other features for everyday use,” according to Dr. Baumbach.
Indeed, he and his Barts colleagues did more than 100 cases in TARGET All Comers and found one of the Firehawk’s strengths was its versatility. It performed well in challenging cases, including left main interventions, as well as in more straightforward cases in this all comers trial.
The Firehawk was developed by MicroPort in China, where its safety and efficacy was established in clinical trials totaling more than 1,000 patients. It then moved to Europe, where it has earned regulatory approval. A pivotal U.S. trial is being planned with the Food and Drug Administration, which has indicated that the European TARGET All Comers data can be incorporated in the study.
Dr. Baumbach reported receiving research grants from Abbott and consultation fees from Keystone Heart, MicroPort, Sinomed, and Stentys.
REPORTING FROM EUROPCR 2018
Key clinical point: The Firehawk has established itself as a versatile workhorse coronary stent.
Major finding:
Study details: This open-label international study randomized 1,656 patients with symptomatic CAD to one of two drug-eluting stents.
Disclosures: The TARGET All Comers trial was sponsored by MicroPort. The presenter reported serving as a consultant to MicroPort and other companies.
TAVR-related stroke risk unrelated to anatomy
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
REPORTING FROM EUROPCR 2018
Key clinical point: .
Major finding: Neither aortic arch type, root angulation, nor calcium burden identifies a subgroup of TAVR patients at higher stroke risk.
Study details: The SENTINEL trial randomized 363 TAVR patients 2:1 to the use of a cerebroembolic protection device or no neuroprotection during the procedure.
Disclosures: The SENTINEL trial was sponsored by Claret Medical. The presenter reported having no financial conflicts of interest.
Source: Jilaihawi H. EuroPCR 2018.
High-bleeding-risk AF patients cut stroke risk with Amplatzer Amulet
Left atrial appendage closure slashes stroke risk in high-risk atrial fibrillation patients with Amplatzer Amulet; how diabetes patients fared with alirocumab in ODYSSEY Outcomes; the new word in stents is “abluminal”; and cheerful news about midlife fitness. Listen to Cardiocast for the week’s top news.
Left atrial appendage closure slashes stroke risk in high-risk atrial fibrillation patients with Amplatzer Amulet; how diabetes patients fared with alirocumab in ODYSSEY Outcomes; the new word in stents is “abluminal”; and cheerful news about midlife fitness. Listen to Cardiocast for the week’s top news.
Left atrial appendage closure slashes stroke risk in high-risk atrial fibrillation patients with Amplatzer Amulet; how diabetes patients fared with alirocumab in ODYSSEY Outcomes; the new word in stents is “abluminal”; and cheerful news about midlife fitness. Listen to Cardiocast for the week’s top news.















