User login
Risk of Osteoporotic Fracture After Steroid Injections in Patients With Medicare
Take-Home Points
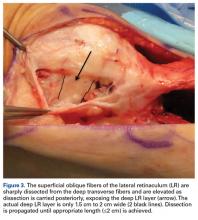
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
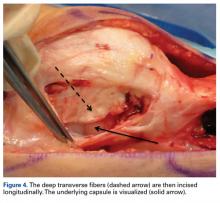
Although statistically significant, this may not be clinically relevant.
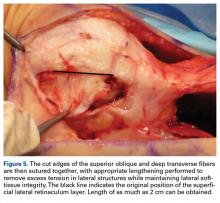
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
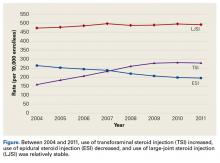
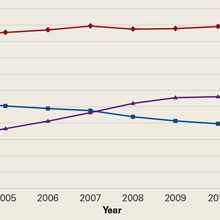
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
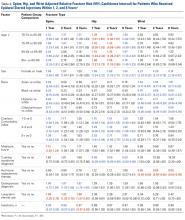
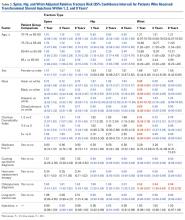
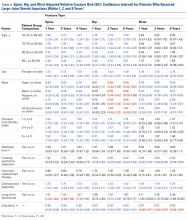
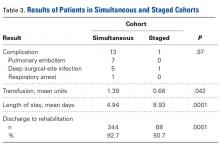
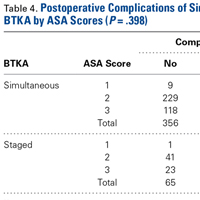
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Medial Oblique Meniscomeniscal Ligament of Knee
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
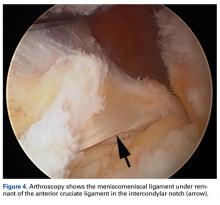
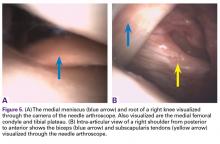
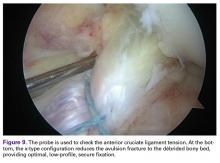
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
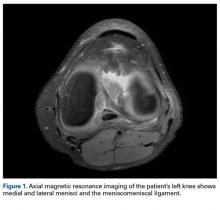
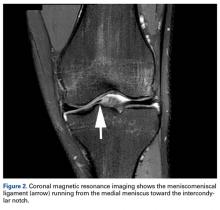
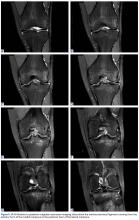
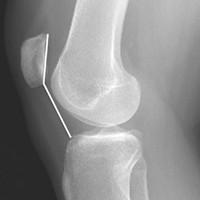
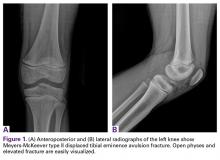
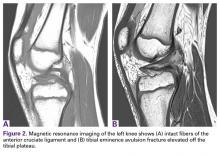
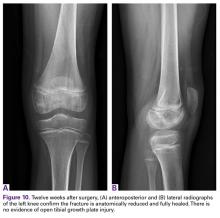
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
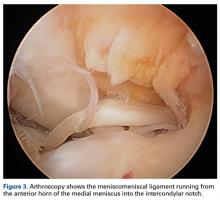
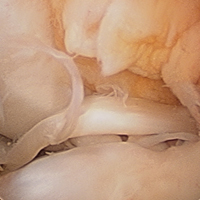
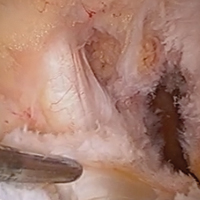
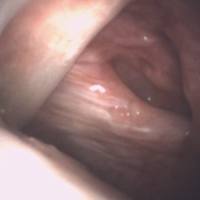
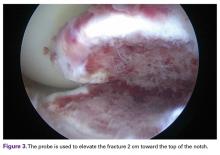
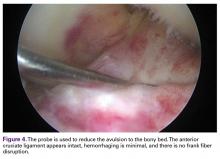
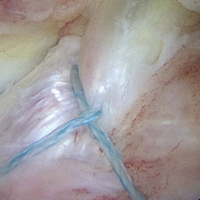
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Medial Oblique Meniscomeniscal Ligament
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
In-Office Diagnostic Needle Arthroscopy: Understanding the Potential Value for the US Healthcare System
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
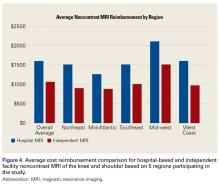
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
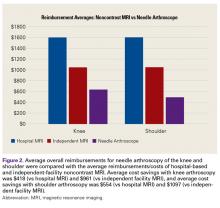
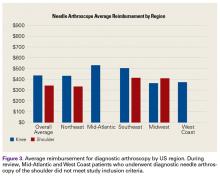
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
Comparison of Lateral Retinaculum Release and Lengthening in the Treatment of Patellofemoral Disorders
Take-Home Points
- Understanding the indications for treatment is essential.
- Identifying the superficial (oblique fibers) and deep layers (transverse fibers) of the LR is very important and can lengthen the LR by as much as 20 mm.
- Open procedures reduce the risk of hematomas and related pain.
- The goal is to obtain 1 or 2 patellar quadrants of medial and lateral patellar glide in extensino and a neutral patella.
- If the Z-plasty is combined with the MPFL reconstruction or tibial tubercle transfer, the LR is set to length after the tubercle transfer and before the MPFL reconstruction (to avoid overconstraint).
Anterior knee pain is a common clinical problem that can be challenging to correct, in large part because of multiple causative factors, including structural/anatomical, functional, alignment, and neuroperception/pain pathway factors. One difficult aspect of anatomical assessment is judging the soft-tissue balance between the medial restraints (medial patellofemoral ligament [MPFL]; medial quadriceps tendon to femoral ligament; medial patellotibial and patellomeniscal ligaments) and the lateral restraints (lateral retinaculum [LR] specifically). Both LR tightness and patellar instability can be interpreted as anterior knee pain. Differentiating these entities is one of the most difficult clinical challenges in orthopedics.
LR release (LRR) has been found to improve patellar mobility and tracking.1 In the absence of clearly defined guidelines, the procedure quickly gained in popularity because of its technical simplicity and the enticing "one tool fits all" treatment approach suggested in early reviews. Injudicious use of LRR, alone or in combination with other procedures, led to iatrogenic instability and chronic pain. LR lengthening (LRL) was introduced to address LR tightness while maintaining lateral soft-tissue integrity and avoiding some of the severe complications of LRR.2
Today, isolated use of LRR/LRL is recommended only for treatment of LR tightness and pain secondary to lateral patellar hypercompression.3 It can also be used as an adjunct treatment in the setting of patellofemoral instability. LRR/LRL should never be used as primary treatment for patellofemoral instability.
In this review of treatments for LR tightness and patellofemoral disorders, we compare the use of LRR and LRL.
Discussion
LR procedures are indicated for LR tightness, which is assessed by taking a history, performing a physical examination, and obtaining diagnostic imaging. Decisions should be based on all findings considered together and never on imaging findings alone.
Physical Examination
The physical examination should include assessment of limb alignment, patellar mobility, muscle balance, and dynamic patellar tracking.
Limb Alignment. Abnormal valgus, rotational deformities, and increased Q-angle are associated with LR tightness. Valgus alignment can be assessed on standing inspection; rotational deformities with increased hip anteversion by hip motion with the patient in the prone position (increased hip internal rotation, decreased hip external rotation); and Q-angle on weight-bearing standing examination and with the patient flexing and extending the knee while seated.
Patellar Mobility. The patellar glide and tilt tests provide the most direct evaluations of LR tightness. Medial displacement of <1 quadrant is consistent with tightness, and displacement of >3 quadrants is consistent with laxity. In full extension, the patellar glide test evaluates only the soft-tissue restraints; at 30° flexion, it also evaluates patellofemoral engagement. The patellar tilt test measures the lifting of the lateral edge of the patella. With normal elevation being 0° to 20°, lack of patellar tilt means the LR is tight, and tilt of >20° means it is loose. MPFL patency can be examined with the Lachman test; the examiner rapidly moves the patella laterally while feeling for the characteristic hard endpoint of lateral translation.
Muscle Balance. The tone, strength, and tightness of the core (abdomen, dorsal, and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius) should be evaluated.
Dynamic Patellar Tracking. The J-sign is the course (shaped like an inverted J) that the patella takes when it is medialized into the trochlea from its laterally displaced resting position as the knee goes from full extension to flexion. The J-sign can be associated with LR tightness, trochlear dysplasia, and patella alta.
Imaging
Although we cannot provide a comprehensive review of the imaging literature, the following radiologic examinations should be used to assess the patellofemoral joint.
30° Lateral Radiograph. Increased tilt is seen when the lateral facet is not anterior to the patellar ridge. Also evaluated are trochlear anatomy, patellar height, and other factors involved in patellofemoral disorders.
30° Flexed Axial (Merchant) Radiograph. Patellar tilt, subluxation, and trochlear dysplasia are evaluated. Images obtained with progressive flexion can be very useful in verifying patellar tilt reduction. Lack of reduction during early flexion suggests LR tightness.4
Alignment Axial Radiographs (Scanogram). Valgus alignment is assessed with this full-length, standing, long-leg examination.
Computed Tomography/Magnetic Resonance Imaging. Many parameters of patellar alignment have been described. Basic assessment should include evaluation of patellar tilt, angle by the line across posterior condyles and a line through the greatest patellar width (>20° indicates abnormality and LR tightness) and tibial tubercle-trochlear groove distance (computed tomography or magnetic resonance imaging scan of the knee is used to measure this distance, and to confirm a significant amount in light of complex patellofemoral malalignment5).
Indications
Lateral compression syndrome with LR tightness is often successfully treated with isolated LRR, and results are reproducible and predictable.6 Surgical intervention for patellofemoral pain should be undertaken only after failed extensive nonoperative treatment with physical therapy and bracing/taping. Patients with LR tightness on preoperative examination, lateral patellar tilt on imaging, and normal Q-angle can obtain satisfactory results with this procedure. Patellar subluxation or dislocation history, high Q-angle (>20°), grade 3 or 4 chondral injury, and patellofemoral arthritis are associated with poorer outcomes when the procedure is performed in isolation.6International Patellofemoral Study Group members agreed that LRR/LRL is a valid treatment option when indicated, but it is rarely performed in isolation and constitutes only 1% to 2% of surgeries performed by this group of experts.7 When lateral compression syndrome progresses to arthritis, LRR/LRL can be performed with lateral patella facetectomy for maximal improvement.4 In the setting of patellofemoral instability, LRR/LRL can be combined with proximal and/or distal realignment surgery if the LR is tight. The LR is the last line of defense limiting lateral translation in the setting of an incompetent MPFL. Isolated LRR/LRL in the setting of instability further destabilizes the patella and worsens the instability. Therefore, LRR/LRL
is a poor surgical option as an isolated procedure for this condition and should be used only as an adjunct in cases of patellofemoral instability with LR tightness that does not allow the patella to be centralized into the trochlea.8 LRR/LRL can also be performed to improve patellar tracking in patellofemoral arthroplasty and total knee arthroplasty.
Lateral Retinaculum Release Versus Lengthening
LRR was first described for the treatment of patellar instability in 1891.9 It was also used for the treatment of lateral patellar hypercompression syndrome associated with LR tightness that led to lateral patellar tracking, joint overload, degeneration, and anterior knee pain.10 Metcalf10 further popularized the procedure by describing a minimally invasive arthroscopic version. However, the arthroscopic technique is as aggressive as the open technique and may be performed with less control, potentially making its results more variable. As proximal and distal releases are performed from the "inside out," more capsule and muscle disruption is needed to release the more superficial layers.
Z-plasty lengthening of the LR was described as an alternative for maintaining lateral patellar soft-tissue integrity while reducing the tension of the lateral tissue restraints.3 This is our preferred method.
Performing LRL instead of LRR avoids iatrogenic medial patellar instability, avoids overrelease and muscle injury, and improves soft-tissue balance.3 Open release or lengthening reduces inadvertent injury to the lateral superior/inferior geniculate arteries and allows direct hemostasis. Two prospective randomized studies found functional knee outcomes and return to athletic activities were improved more after LRL than LRR.11,12 These procedures had similar rates of postoperative knee stiffness, decreased muscle mass, and decreased strength. Each prospective study used an extensive LRR technique for LRR cases (various authors have recommended performing the release until the patella is perpendicular to the trochlea), which may have affected outcomes. In any case, with lengthening, the surgeon is less likely to excessively disrupt the lateral tissues.
Lateral Retinaculum Release. LRR can be openly performed by lateral parapatellar incision,1 a mini-open percutaneous technique, or arthroscopy. For these open techniques, incisions of various sizes have been used to access the LR and incise it about 1 cm lateral to the patella starting at the distal end of the vastus lateralis and extending distally until patellar tilt reduction is sufficient. If tightness in deep flexion persists, the LRR can be extended distally to the tibial tubercle. Open techniques have the advantage of sparing the joint capsule. All-arthroscopic techniques involve using electrocautery to cut through the capsule and access the LR.
Lateral Retinaculum Lengthening.
The LR is sharply divided into a superficial layer of superficial oblique fibers from the anterior iliotibial band and a deep layer of transverse fibers from the femur. For LRR, these 2 layers must be identified separate from the articular capsule.13
Complications
Complications of performing LRR/LRL to change the lateral restraint include medial patellar instability, increased lateral pain, repair failure, recurrent lateral instability, quadriceps weakness and atrophy, postoperative hemarthrosis, knee stiffness, wound complications, and thermal skin injury.7 These complications often result from poor surgical technique and too aggressive release. Although recommended patellar tilt historically has varied from 45° to 90°, the current goal is to normalize the tight soft-tissue restraints without creating secondary instability.
The most significant complication of LRR is medial patellar instability caused by muscle atrophy and loss of soft-tissue restraint.14 Medial instability can be difficult to diagnose and should be considered in any patient with patellofemoral pain, popping, or patellar instability after LRR.15 A positive medial subluxation test or medial patellar apprehension test suggests medial instability.
Medial patellar instability usually requires surgical treatment. Direct LR repair, lateral soft-tissue reconstruction, and other procedures can be used to restore lateral restraint.15 However, these are salvage techniques, and patients often remain significantly limited by pain or instability. Therefore, the LR must be carefully addressed and preferably should undergo lengthening rather than release.
1. Merchant AC, Mercer RL. Lateral release of the patella. A preliminary report. Clin Orthop Relat Res. 1974;(103):40-45.
2. Ceder LC, Larson RL. Z-plasty lateral retinacular release for the treatment of patellar compression syndrome. Clin Orthop Relat Res. 1979;(144):110-113.
3. Biedert R. Lateral patellar hypercompression, tilt and mild lateral subluxation. In: Biedert R, ed. Patellofemoral Disorders. Chichester, England: Wiley; 2004:161-166.
4. Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100-106.
5. Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle–posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
6. Lattermann C, Toth J, Bach BR Jr. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc. 2007;15(2):57-60.
7. Fithian DC, Paxton EW, Post WR, Panni AS; International Patellofemoral Study Group. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20(5):463-468.
8. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
9. Pollard B. Old dislocation of patella by intra-articular operation. Lancet. 1891;(988):17-22.
10. Metcalf RW. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
11. Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788-797.
12. O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759-1769.
13. Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br. 2008;90(4):527-534.
14. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
15. McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26-33.
Take-Home Points
- Understanding the indications for treatment is essential.
- Identifying the superficial (oblique fibers) and deep layers (transverse fibers) of the LR is very important and can lengthen the LR by as much as 20 mm.
- Open procedures reduce the risk of hematomas and related pain.
- The goal is to obtain 1 or 2 patellar quadrants of medial and lateral patellar glide in extensino and a neutral patella.
- If the Z-plasty is combined with the MPFL reconstruction or tibial tubercle transfer, the LR is set to length after the tubercle transfer and before the MPFL reconstruction (to avoid overconstraint).
Anterior knee pain is a common clinical problem that can be challenging to correct, in large part because of multiple causative factors, including structural/anatomical, functional, alignment, and neuroperception/pain pathway factors. One difficult aspect of anatomical assessment is judging the soft-tissue balance between the medial restraints (medial patellofemoral ligament [MPFL]; medial quadriceps tendon to femoral ligament; medial patellotibial and patellomeniscal ligaments) and the lateral restraints (lateral retinaculum [LR] specifically). Both LR tightness and patellar instability can be interpreted as anterior knee pain. Differentiating these entities is one of the most difficult clinical challenges in orthopedics.
LR release (LRR) has been found to improve patellar mobility and tracking.1 In the absence of clearly defined guidelines, the procedure quickly gained in popularity because of its technical simplicity and the enticing "one tool fits all" treatment approach suggested in early reviews. Injudicious use of LRR, alone or in combination with other procedures, led to iatrogenic instability and chronic pain. LR lengthening (LRL) was introduced to address LR tightness while maintaining lateral soft-tissue integrity and avoiding some of the severe complications of LRR.2
Today, isolated use of LRR/LRL is recommended only for treatment of LR tightness and pain secondary to lateral patellar hypercompression.3 It can also be used as an adjunct treatment in the setting of patellofemoral instability. LRR/LRL should never be used as primary treatment for patellofemoral instability.
In this review of treatments for LR tightness and patellofemoral disorders, we compare the use of LRR and LRL.
Discussion
LR procedures are indicated for LR tightness, which is assessed by taking a history, performing a physical examination, and obtaining diagnostic imaging. Decisions should be based on all findings considered together and never on imaging findings alone.
Physical Examination
The physical examination should include assessment of limb alignment, patellar mobility, muscle balance, and dynamic patellar tracking.
Limb Alignment. Abnormal valgus, rotational deformities, and increased Q-angle are associated with LR tightness. Valgus alignment can be assessed on standing inspection; rotational deformities with increased hip anteversion by hip motion with the patient in the prone position (increased hip internal rotation, decreased hip external rotation); and Q-angle on weight-bearing standing examination and with the patient flexing and extending the knee while seated.
Patellar Mobility. The patellar glide and tilt tests provide the most direct evaluations of LR tightness. Medial displacement of <1 quadrant is consistent with tightness, and displacement of >3 quadrants is consistent with laxity. In full extension, the patellar glide test evaluates only the soft-tissue restraints; at 30° flexion, it also evaluates patellofemoral engagement. The patellar tilt test measures the lifting of the lateral edge of the patella. With normal elevation being 0° to 20°, lack of patellar tilt means the LR is tight, and tilt of >20° means it is loose. MPFL patency can be examined with the Lachman test; the examiner rapidly moves the patella laterally while feeling for the characteristic hard endpoint of lateral translation.
Muscle Balance. The tone, strength, and tightness of the core (abdomen, dorsal, and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius) should be evaluated.
Dynamic Patellar Tracking. The J-sign is the course (shaped like an inverted J) that the patella takes when it is medialized into the trochlea from its laterally displaced resting position as the knee goes from full extension to flexion. The J-sign can be associated with LR tightness, trochlear dysplasia, and patella alta.
Imaging
Although we cannot provide a comprehensive review of the imaging literature, the following radiologic examinations should be used to assess the patellofemoral joint.
30° Lateral Radiograph. Increased tilt is seen when the lateral facet is not anterior to the patellar ridge. Also evaluated are trochlear anatomy, patellar height, and other factors involved in patellofemoral disorders.
30° Flexed Axial (Merchant) Radiograph. Patellar tilt, subluxation, and trochlear dysplasia are evaluated. Images obtained with progressive flexion can be very useful in verifying patellar tilt reduction. Lack of reduction during early flexion suggests LR tightness.4
Alignment Axial Radiographs (Scanogram). Valgus alignment is assessed with this full-length, standing, long-leg examination.
Computed Tomography/Magnetic Resonance Imaging. Many parameters of patellar alignment have been described. Basic assessment should include evaluation of patellar tilt, angle by the line across posterior condyles and a line through the greatest patellar width (>20° indicates abnormality and LR tightness) and tibial tubercle-trochlear groove distance (computed tomography or magnetic resonance imaging scan of the knee is used to measure this distance, and to confirm a significant amount in light of complex patellofemoral malalignment5).
Indications
Lateral compression syndrome with LR tightness is often successfully treated with isolated LRR, and results are reproducible and predictable.6 Surgical intervention for patellofemoral pain should be undertaken only after failed extensive nonoperative treatment with physical therapy and bracing/taping. Patients with LR tightness on preoperative examination, lateral patellar tilt on imaging, and normal Q-angle can obtain satisfactory results with this procedure. Patellar subluxation or dislocation history, high Q-angle (>20°), grade 3 or 4 chondral injury, and patellofemoral arthritis are associated with poorer outcomes when the procedure is performed in isolation.6International Patellofemoral Study Group members agreed that LRR/LRL is a valid treatment option when indicated, but it is rarely performed in isolation and constitutes only 1% to 2% of surgeries performed by this group of experts.7 When lateral compression syndrome progresses to arthritis, LRR/LRL can be performed with lateral patella facetectomy for maximal improvement.4 In the setting of patellofemoral instability, LRR/LRL can be combined with proximal and/or distal realignment surgery if the LR is tight. The LR is the last line of defense limiting lateral translation in the setting of an incompetent MPFL. Isolated LRR/LRL in the setting of instability further destabilizes the patella and worsens the instability. Therefore, LRR/LRL
is a poor surgical option as an isolated procedure for this condition and should be used only as an adjunct in cases of patellofemoral instability with LR tightness that does not allow the patella to be centralized into the trochlea.8 LRR/LRL can also be performed to improve patellar tracking in patellofemoral arthroplasty and total knee arthroplasty.
Lateral Retinaculum Release Versus Lengthening
LRR was first described for the treatment of patellar instability in 1891.9 It was also used for the treatment of lateral patellar hypercompression syndrome associated with LR tightness that led to lateral patellar tracking, joint overload, degeneration, and anterior knee pain.10 Metcalf10 further popularized the procedure by describing a minimally invasive arthroscopic version. However, the arthroscopic technique is as aggressive as the open technique and may be performed with less control, potentially making its results more variable. As proximal and distal releases are performed from the "inside out," more capsule and muscle disruption is needed to release the more superficial layers.
Z-plasty lengthening of the LR was described as an alternative for maintaining lateral patellar soft-tissue integrity while reducing the tension of the lateral tissue restraints.3 This is our preferred method.
Performing LRL instead of LRR avoids iatrogenic medial patellar instability, avoids overrelease and muscle injury, and improves soft-tissue balance.3 Open release or lengthening reduces inadvertent injury to the lateral superior/inferior geniculate arteries and allows direct hemostasis. Two prospective randomized studies found functional knee outcomes and return to athletic activities were improved more after LRL than LRR.11,12 These procedures had similar rates of postoperative knee stiffness, decreased muscle mass, and decreased strength. Each prospective study used an extensive LRR technique for LRR cases (various authors have recommended performing the release until the patella is perpendicular to the trochlea), which may have affected outcomes. In any case, with lengthening, the surgeon is less likely to excessively disrupt the lateral tissues.
Lateral Retinaculum Release. LRR can be openly performed by lateral parapatellar incision,1 a mini-open percutaneous technique, or arthroscopy. For these open techniques, incisions of various sizes have been used to access the LR and incise it about 1 cm lateral to the patella starting at the distal end of the vastus lateralis and extending distally until patellar tilt reduction is sufficient. If tightness in deep flexion persists, the LRR can be extended distally to the tibial tubercle. Open techniques have the advantage of sparing the joint capsule. All-arthroscopic techniques involve using electrocautery to cut through the capsule and access the LR.
Lateral Retinaculum Lengthening.
The LR is sharply divided into a superficial layer of superficial oblique fibers from the anterior iliotibial band and a deep layer of transverse fibers from the femur. For LRR, these 2 layers must be identified separate from the articular capsule.13
Complications
Complications of performing LRR/LRL to change the lateral restraint include medial patellar instability, increased lateral pain, repair failure, recurrent lateral instability, quadriceps weakness and atrophy, postoperative hemarthrosis, knee stiffness, wound complications, and thermal skin injury.7 These complications often result from poor surgical technique and too aggressive release. Although recommended patellar tilt historically has varied from 45° to 90°, the current goal is to normalize the tight soft-tissue restraints without creating secondary instability.
The most significant complication of LRR is medial patellar instability caused by muscle atrophy and loss of soft-tissue restraint.14 Medial instability can be difficult to diagnose and should be considered in any patient with patellofemoral pain, popping, or patellar instability after LRR.15 A positive medial subluxation test or medial patellar apprehension test suggests medial instability.
Medial patellar instability usually requires surgical treatment. Direct LR repair, lateral soft-tissue reconstruction, and other procedures can be used to restore lateral restraint.15 However, these are salvage techniques, and patients often remain significantly limited by pain or instability. Therefore, the LR must be carefully addressed and preferably should undergo lengthening rather than release.
Take-Home Points
- Understanding the indications for treatment is essential.
- Identifying the superficial (oblique fibers) and deep layers (transverse fibers) of the LR is very important and can lengthen the LR by as much as 20 mm.
- Open procedures reduce the risk of hematomas and related pain.
- The goal is to obtain 1 or 2 patellar quadrants of medial and lateral patellar glide in extensino and a neutral patella.
- If the Z-plasty is combined with the MPFL reconstruction or tibial tubercle transfer, the LR is set to length after the tubercle transfer and before the MPFL reconstruction (to avoid overconstraint).
Anterior knee pain is a common clinical problem that can be challenging to correct, in large part because of multiple causative factors, including structural/anatomical, functional, alignment, and neuroperception/pain pathway factors. One difficult aspect of anatomical assessment is judging the soft-tissue balance between the medial restraints (medial patellofemoral ligament [MPFL]; medial quadriceps tendon to femoral ligament; medial patellotibial and patellomeniscal ligaments) and the lateral restraints (lateral retinaculum [LR] specifically). Both LR tightness and patellar instability can be interpreted as anterior knee pain. Differentiating these entities is one of the most difficult clinical challenges in orthopedics.
LR release (LRR) has been found to improve patellar mobility and tracking.1 In the absence of clearly defined guidelines, the procedure quickly gained in popularity because of its technical simplicity and the enticing "one tool fits all" treatment approach suggested in early reviews. Injudicious use of LRR, alone or in combination with other procedures, led to iatrogenic instability and chronic pain. LR lengthening (LRL) was introduced to address LR tightness while maintaining lateral soft-tissue integrity and avoiding some of the severe complications of LRR.2
Today, isolated use of LRR/LRL is recommended only for treatment of LR tightness and pain secondary to lateral patellar hypercompression.3 It can also be used as an adjunct treatment in the setting of patellofemoral instability. LRR/LRL should never be used as primary treatment for patellofemoral instability.
In this review of treatments for LR tightness and patellofemoral disorders, we compare the use of LRR and LRL.
Discussion
LR procedures are indicated for LR tightness, which is assessed by taking a history, performing a physical examination, and obtaining diagnostic imaging. Decisions should be based on all findings considered together and never on imaging findings alone.
Physical Examination
The physical examination should include assessment of limb alignment, patellar mobility, muscle balance, and dynamic patellar tracking.
Limb Alignment. Abnormal valgus, rotational deformities, and increased Q-angle are associated with LR tightness. Valgus alignment can be assessed on standing inspection; rotational deformities with increased hip anteversion by hip motion with the patient in the prone position (increased hip internal rotation, decreased hip external rotation); and Q-angle on weight-bearing standing examination and with the patient flexing and extending the knee while seated.
Patellar Mobility. The patellar glide and tilt tests provide the most direct evaluations of LR tightness. Medial displacement of <1 quadrant is consistent with tightness, and displacement of >3 quadrants is consistent with laxity. In full extension, the patellar glide test evaluates only the soft-tissue restraints; at 30° flexion, it also evaluates patellofemoral engagement. The patellar tilt test measures the lifting of the lateral edge of the patella. With normal elevation being 0° to 20°, lack of patellar tilt means the LR is tight, and tilt of >20° means it is loose. MPFL patency can be examined with the Lachman test; the examiner rapidly moves the patella laterally while feeling for the characteristic hard endpoint of lateral translation.
Muscle Balance. The tone, strength, and tightness of the core (abdomen, dorsal, and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius) should be evaluated.
Dynamic Patellar Tracking. The J-sign is the course (shaped like an inverted J) that the patella takes when it is medialized into the trochlea from its laterally displaced resting position as the knee goes from full extension to flexion. The J-sign can be associated with LR tightness, trochlear dysplasia, and patella alta.
Imaging
Although we cannot provide a comprehensive review of the imaging literature, the following radiologic examinations should be used to assess the patellofemoral joint.
30° Lateral Radiograph. Increased tilt is seen when the lateral facet is not anterior to the patellar ridge. Also evaluated are trochlear anatomy, patellar height, and other factors involved in patellofemoral disorders.
30° Flexed Axial (Merchant) Radiograph. Patellar tilt, subluxation, and trochlear dysplasia are evaluated. Images obtained with progressive flexion can be very useful in verifying patellar tilt reduction. Lack of reduction during early flexion suggests LR tightness.4
Alignment Axial Radiographs (Scanogram). Valgus alignment is assessed with this full-length, standing, long-leg examination.
Computed Tomography/Magnetic Resonance Imaging. Many parameters of patellar alignment have been described. Basic assessment should include evaluation of patellar tilt, angle by the line across posterior condyles and a line through the greatest patellar width (>20° indicates abnormality and LR tightness) and tibial tubercle-trochlear groove distance (computed tomography or magnetic resonance imaging scan of the knee is used to measure this distance, and to confirm a significant amount in light of complex patellofemoral malalignment5).
Indications
Lateral compression syndrome with LR tightness is often successfully treated with isolated LRR, and results are reproducible and predictable.6 Surgical intervention for patellofemoral pain should be undertaken only after failed extensive nonoperative treatment with physical therapy and bracing/taping. Patients with LR tightness on preoperative examination, lateral patellar tilt on imaging, and normal Q-angle can obtain satisfactory results with this procedure. Patellar subluxation or dislocation history, high Q-angle (>20°), grade 3 or 4 chondral injury, and patellofemoral arthritis are associated with poorer outcomes when the procedure is performed in isolation.6International Patellofemoral Study Group members agreed that LRR/LRL is a valid treatment option when indicated, but it is rarely performed in isolation and constitutes only 1% to 2% of surgeries performed by this group of experts.7 When lateral compression syndrome progresses to arthritis, LRR/LRL can be performed with lateral patella facetectomy for maximal improvement.4 In the setting of patellofemoral instability, LRR/LRL can be combined with proximal and/or distal realignment surgery if the LR is tight. The LR is the last line of defense limiting lateral translation in the setting of an incompetent MPFL. Isolated LRR/LRL in the setting of instability further destabilizes the patella and worsens the instability. Therefore, LRR/LRL
is a poor surgical option as an isolated procedure for this condition and should be used only as an adjunct in cases of patellofemoral instability with LR tightness that does not allow the patella to be centralized into the trochlea.8 LRR/LRL can also be performed to improve patellar tracking in patellofemoral arthroplasty and total knee arthroplasty.
Lateral Retinaculum Release Versus Lengthening
LRR was first described for the treatment of patellar instability in 1891.9 It was also used for the treatment of lateral patellar hypercompression syndrome associated with LR tightness that led to lateral patellar tracking, joint overload, degeneration, and anterior knee pain.10 Metcalf10 further popularized the procedure by describing a minimally invasive arthroscopic version. However, the arthroscopic technique is as aggressive as the open technique and may be performed with less control, potentially making its results more variable. As proximal and distal releases are performed from the "inside out," more capsule and muscle disruption is needed to release the more superficial layers.
Z-plasty lengthening of the LR was described as an alternative for maintaining lateral patellar soft-tissue integrity while reducing the tension of the lateral tissue restraints.3 This is our preferred method.
Performing LRL instead of LRR avoids iatrogenic medial patellar instability, avoids overrelease and muscle injury, and improves soft-tissue balance.3 Open release or lengthening reduces inadvertent injury to the lateral superior/inferior geniculate arteries and allows direct hemostasis. Two prospective randomized studies found functional knee outcomes and return to athletic activities were improved more after LRL than LRR.11,12 These procedures had similar rates of postoperative knee stiffness, decreased muscle mass, and decreased strength. Each prospective study used an extensive LRR technique for LRR cases (various authors have recommended performing the release until the patella is perpendicular to the trochlea), which may have affected outcomes. In any case, with lengthening, the surgeon is less likely to excessively disrupt the lateral tissues.
Lateral Retinaculum Release. LRR can be openly performed by lateral parapatellar incision,1 a mini-open percutaneous technique, or arthroscopy. For these open techniques, incisions of various sizes have been used to access the LR and incise it about 1 cm lateral to the patella starting at the distal end of the vastus lateralis and extending distally until patellar tilt reduction is sufficient. If tightness in deep flexion persists, the LRR can be extended distally to the tibial tubercle. Open techniques have the advantage of sparing the joint capsule. All-arthroscopic techniques involve using electrocautery to cut through the capsule and access the LR.
Lateral Retinaculum Lengthening.
The LR is sharply divided into a superficial layer of superficial oblique fibers from the anterior iliotibial band and a deep layer of transverse fibers from the femur. For LRR, these 2 layers must be identified separate from the articular capsule.13
Complications
Complications of performing LRR/LRL to change the lateral restraint include medial patellar instability, increased lateral pain, repair failure, recurrent lateral instability, quadriceps weakness and atrophy, postoperative hemarthrosis, knee stiffness, wound complications, and thermal skin injury.7 These complications often result from poor surgical technique and too aggressive release. Although recommended patellar tilt historically has varied from 45° to 90°, the current goal is to normalize the tight soft-tissue restraints without creating secondary instability.
The most significant complication of LRR is medial patellar instability caused by muscle atrophy and loss of soft-tissue restraint.14 Medial instability can be difficult to diagnose and should be considered in any patient with patellofemoral pain, popping, or patellar instability after LRR.15 A positive medial subluxation test or medial patellar apprehension test suggests medial instability.
Medial patellar instability usually requires surgical treatment. Direct LR repair, lateral soft-tissue reconstruction, and other procedures can be used to restore lateral restraint.15 However, these are salvage techniques, and patients often remain significantly limited by pain or instability. Therefore, the LR must be carefully addressed and preferably should undergo lengthening rather than release.
1. Merchant AC, Mercer RL. Lateral release of the patella. A preliminary report. Clin Orthop Relat Res. 1974;(103):40-45.
2. Ceder LC, Larson RL. Z-plasty lateral retinacular release for the treatment of patellar compression syndrome. Clin Orthop Relat Res. 1979;(144):110-113.
3. Biedert R. Lateral patellar hypercompression, tilt and mild lateral subluxation. In: Biedert R, ed. Patellofemoral Disorders. Chichester, England: Wiley; 2004:161-166.
4. Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100-106.
5. Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle–posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
6. Lattermann C, Toth J, Bach BR Jr. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc. 2007;15(2):57-60.
7. Fithian DC, Paxton EW, Post WR, Panni AS; International Patellofemoral Study Group. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20(5):463-468.
8. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
9. Pollard B. Old dislocation of patella by intra-articular operation. Lancet. 1891;(988):17-22.
10. Metcalf RW. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
11. Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788-797.
12. O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759-1769.
13. Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br. 2008;90(4):527-534.
14. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
15. McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26-33.
1. Merchant AC, Mercer RL. Lateral release of the patella. A preliminary report. Clin Orthop Relat Res. 1974;(103):40-45.
2. Ceder LC, Larson RL. Z-plasty lateral retinacular release for the treatment of patellar compression syndrome. Clin Orthop Relat Res. 1979;(144):110-113.
3. Biedert R. Lateral patellar hypercompression, tilt and mild lateral subluxation. In: Biedert R, ed. Patellofemoral Disorders. Chichester, England: Wiley; 2004:161-166.
4. Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100-106.
5. Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle–posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
6. Lattermann C, Toth J, Bach BR Jr. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc. 2007;15(2):57-60.
7. Fithian DC, Paxton EW, Post WR, Panni AS; International Patellofemoral Study Group. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20(5):463-468.
8. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
9. Pollard B. Old dislocation of patella by intra-articular operation. Lancet. 1891;(988):17-22.
10. Metcalf RW. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
11. Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788-797.
12. O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759-1769.
13. Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br. 2008;90(4):527-534.
14. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
15. McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26-33.
Patella Alta Sees You, Do You See It?
Take-Home Points
The decision to adda TTDO to an MPFL reconstruction is dependent on patellar height as assessed with the CDI, as well as multiple other patient and anatomical factors.
TTDOs that include a complete detachment of the tibial tubercle (as required for distalization) have increased risk of nonunion and hardware failure.
Poor surgical technique (failure to make a flat osteotomy cut, cortical only bone block, poor bony apposition of the detached bone block- particularly at the location of any transverse plane cut, and failure to minimize thermal damage through copious irrigation) can increase nonunion risk.
Postoperative rehabilitation should include a 6-week period of limited weight-bearing.
Reconstruction of the MPFL should be performed after any TTO is performed.
Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased tibial tubercle-trochlear groove (TT-TG) or tibial tubercle-posterior cruciate ligament distance.2,5 Of all the factors, TT-TG distance and the medial patellofemoral ligament (MPFL) have received the ost attention. Patellar height remains a crucial yet underappreciated contributor that is amenable to surgical correction with tibial tubercle distalization osteotomy (TTDO). The obvious question is how severe patella alta must be to require surgical correction. In other words, when is patella alta so severe that isolated MPFL reconstruction is insufficient to restore patellar stability?
The indications for TTDO are not completely clear and depend on multiple factors. Patient factors, physical examination findings, and radiographic measures must be considered. In general, adding TTDO to MPFL reconstruction should be considered when the degree of patella alta exceeds 1.4 on the Caton-Deschamps Index (CDI). Presence of trochlear dysplasia, patellar maltracking (J-sign), lateral patellar apprehension that persists at higher flexion angles, and decreased patellotrochlear articular cartilage contact on sagittal magnetic resonance imaging may drive the decision to proceed with TTDO when the CDI is lower.
Why You Need To Know About Patella Alta
Recurrent lateral patellar dislocation is a debilitating knee condition that often involves young, active patients and significantly affects their quality of life. The MPFL is a primary restraint to lateral patellar dislocation, and an MPFL injury is a key contributor to loss of patellar stability. MPFL reconstruction is increasingly being performed to treat recurrent lateral patellar instability.6 Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased TT-TG distance.2,5 This review focuses on patella alta.
The classic teaching of the Lyon School of Knee Surgery in France, the menu à la carte, is that patella alta exceeding 1.2 on the CDI is an indication for TTDO.2 Although this teaching is an excellent guide for normal anatomy, we must keep in mind that the classic surgical menu does not consider the influence of MPFL reconstruction, as development of the menu predated this surgical option. At that time, the proximal soft-tissue procedures included vastus medialis obliquus plasty and advancement, which are performed to balance soft tissues and treat patellar tilt. These procedures and MPFL reconstruction have different functions, and the difference may be important. Furthermore, performing TTDO alongside MPFL reconstruction significantly increases the risk of complications and alters the rehabilitation protocol. However, significant untreated patella alta has been implicated as a cause of failure of isolated MPFL reconstruction.7 Establishing when MPFL reconstruction alone is sufficient is therefore crucial in avoiding the increased morbidity associated with the addition of TTDO.
Discussion
Above a certain degree of patella alta, isolated MPFL reconstruction fails to restore patellar stability. What remains unknown is the appropriate CDI cutoff (1.4) and whether the same cutoff can be used for all patients. In 2013, Wagner and colleagues8 assessed the influence of patella alta on isolated MPFL reconstruction outcomes and found no significant difference, though their study did not include many patients with significant alta and was underpowered. In 2014, Feller and colleagues9 reported on a series of patients who were successfully treated with isolated MPFL reconstruction despite patella alta significantly exceeding the traditional CDI cutoff of 1.2. Their indication for performing the isolated procedure was normal patellar tracking—in particular, absence of the J-sign. Further analysis of these patients revealed a preponderance of relatively normal TT-TG distances and low-grade, if any, trochlear dysplasia in comparison with other patients treated with a combination of MPFL reconstruction and tibial tubercle osteotomy.
Together, the work of Wagner and colleagues8 and Feller and colleagues9 suggests the historical use of the CDI of 1.2 as a hard and fast indication for adding TTDO is aggressive. In fact, it is probably the case that there really is no single CDI cutoff that is an appropriate indication for adding TTDO in all patients with instability. This decision is, and should be, influenced by a multitude of other factors, including other anatomical factors, physical examination findings, patient factors, and, of course, patient preference.
An interesting idea to consider in treating patellar instability is the interplay of patella alta and trochlear dysplasia. Patella alta is theorized as contributing to patellar instability in part by delaying entry of the patella into the TG as the knee flexes, therefore requiring less force to laterally displace
the patella.10 Similarly, in the setting of trochlear dysplasia, a shallow TG leads to less bony constraint of the patella, particularly in the groove’s superior portions, which are more involved in lower grade dysplasia. Because trochlear dysplasia and patella alta decrease patellar stability by similar mechanisms, they clearly interact, and a patient with both is at higher risk for instability than a patient who exhibits either in isolation.11 Therefore, trochlear dysplasia, particularly higher grade, may be an indication for adding TTDO at lower CDI.
Other imaging and physical examination factors can provide additional insight into the process of patellar engagement into the trochlea in each patient. The patellotrochlear index (PTI) directly measures the relationship between the patella and the trochlea, rather than relative to the tibia, as with other measures of patellar height.12[[{"fid":"201853","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]The PTI is correlated with tibia-based measures of height, but the correlation is not perfect. Lower degrees of overlap between the patella and the trochlea (PTI <0.15) and significant functional patella alta may warrant adding TTDO in cases of borderline CDI (1.2-1.4). Figures 1A, 1B and 2A, 2B show the imaging of 2 patients with relatively similar patellar height (assessed with CDI) but quite different degrees of overlap between the patella and trochlea. [[{"fid":"201854","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The patient with less overlap is more likely to have delayed patellar engagement and symptomatic patella alta and thus may be a poorer candidate for isolated MPFL reconstruction. For additional information, please refer to the work by Roland Biedert, MD, who has proposed trochlear lengthening in these situations.13
Physical examination (even in the era of advance imaging) continues to provide useful insight into whether to add TTDO. One physical examination test that can help in understanding patellar-
trochlear dynamics is the patellar apprehension and relief test. Patellar apprehension has been widely discussed, but equally important is the degree of knee flexion above which apprehension dissipates. As patella alta and trochlear dysplasia become more severe, more knee flexion is required to relieve apprehension. Apprehension that is relieved at 30° to 40° of flexion suggests that patellar stability stands a good chance of being restored with isolated MPFL reconstruction, whereas persistent instability >45° or especially 60° of knee flexion suggests that there is significant patella alta, trochlear dysplasia, or both and that TTDO should be added. A large J-sign during knee flexion and extension provides further evidence that entry of the patella into the TG is delayed, typically because of patella alta, trochlear dysplasia, or both, and possibly tight lateral structures or a lateralized tibial tubercle. This sign is another clue that isolated MPFL reconstruction may be insufficient to completely restore patellar stability.
1. Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45-54.
2. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
3. Geenen E, Molenaers G, Martens M. Patella alta in patellofemoral instability. Acta Orthop Belg. 1989;55(3):387-393.
4. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
5. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl) [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
6. Feller JA, Lind M, Nelson J, Diduch DR, Arendt E. Repair and reconstruction of the medial patellofemoral ligament for treatment of lateral patellar dislocations. In: Scott WN, ed. Insall & Scott—Surgery of the Knee. 5th ed. Philadelphia, PA: Churchill Livingstone; 2011:677-687.
7. Thaunat M, Erasmus PJ. Recurrent patellar dislocation after medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):40-43.
8. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
9. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
10. Ward SR, Terk MR, Powers CM. Patella alta: association
with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):
1749-1755.
11. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309
12. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
13. Biedert RM. Trochlear lengthening osteotomy with or without elevation of the lateral trochlear facet. In: Zaffagnini S, Dejour D, Arendt EA, eds. Patellofemoral Pain, Instability, and Arthritis. Germany: Springer-Verlag Berlin Heidelberg; 2010:
209-215.
Take-Home Points
The decision to adda TTDO to an MPFL reconstruction is dependent on patellar height as assessed with the CDI, as well as multiple other patient and anatomical factors.
TTDOs that include a complete detachment of the tibial tubercle (as required for distalization) have increased risk of nonunion and hardware failure.
Poor surgical technique (failure to make a flat osteotomy cut, cortical only bone block, poor bony apposition of the detached bone block- particularly at the location of any transverse plane cut, and failure to minimize thermal damage through copious irrigation) can increase nonunion risk.
Postoperative rehabilitation should include a 6-week period of limited weight-bearing.
Reconstruction of the MPFL should be performed after any TTO is performed.
Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased tibial tubercle-trochlear groove (TT-TG) or tibial tubercle-posterior cruciate ligament distance.2,5 Of all the factors, TT-TG distance and the medial patellofemoral ligament (MPFL) have received the ost attention. Patellar height remains a crucial yet underappreciated contributor that is amenable to surgical correction with tibial tubercle distalization osteotomy (TTDO). The obvious question is how severe patella alta must be to require surgical correction. In other words, when is patella alta so severe that isolated MPFL reconstruction is insufficient to restore patellar stability?
The indications for TTDO are not completely clear and depend on multiple factors. Patient factors, physical examination findings, and radiographic measures must be considered. In general, adding TTDO to MPFL reconstruction should be considered when the degree of patella alta exceeds 1.4 on the Caton-Deschamps Index (CDI). Presence of trochlear dysplasia, patellar maltracking (J-sign), lateral patellar apprehension that persists at higher flexion angles, and decreased patellotrochlear articular cartilage contact on sagittal magnetic resonance imaging may drive the decision to proceed with TTDO when the CDI is lower.
Why You Need To Know About Patella Alta
Recurrent lateral patellar dislocation is a debilitating knee condition that often involves young, active patients and significantly affects their quality of life. The MPFL is a primary restraint to lateral patellar dislocation, and an MPFL injury is a key contributor to loss of patellar stability. MPFL reconstruction is increasingly being performed to treat recurrent lateral patellar instability.6 Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased TT-TG distance.2,5 This review focuses on patella alta.
The classic teaching of the Lyon School of Knee Surgery in France, the menu à la carte, is that patella alta exceeding 1.2 on the CDI is an indication for TTDO.2 Although this teaching is an excellent guide for normal anatomy, we must keep in mind that the classic surgical menu does not consider the influence of MPFL reconstruction, as development of the menu predated this surgical option. At that time, the proximal soft-tissue procedures included vastus medialis obliquus plasty and advancement, which are performed to balance soft tissues and treat patellar tilt. These procedures and MPFL reconstruction have different functions, and the difference may be important. Furthermore, performing TTDO alongside MPFL reconstruction significantly increases the risk of complications and alters the rehabilitation protocol. However, significant untreated patella alta has been implicated as a cause of failure of isolated MPFL reconstruction.7 Establishing when MPFL reconstruction alone is sufficient is therefore crucial in avoiding the increased morbidity associated with the addition of TTDO.
Discussion
Above a certain degree of patella alta, isolated MPFL reconstruction fails to restore patellar stability. What remains unknown is the appropriate CDI cutoff (1.4) and whether the same cutoff can be used for all patients. In 2013, Wagner and colleagues8 assessed the influence of patella alta on isolated MPFL reconstruction outcomes and found no significant difference, though their study did not include many patients with significant alta and was underpowered. In 2014, Feller and colleagues9 reported on a series of patients who were successfully treated with isolated MPFL reconstruction despite patella alta significantly exceeding the traditional CDI cutoff of 1.2. Their indication for performing the isolated procedure was normal patellar tracking—in particular, absence of the J-sign. Further analysis of these patients revealed a preponderance of relatively normal TT-TG distances and low-grade, if any, trochlear dysplasia in comparison with other patients treated with a combination of MPFL reconstruction and tibial tubercle osteotomy.
Together, the work of Wagner and colleagues8 and Feller and colleagues9 suggests the historical use of the CDI of 1.2 as a hard and fast indication for adding TTDO is aggressive. In fact, it is probably the case that there really is no single CDI cutoff that is an appropriate indication for adding TTDO in all patients with instability. This decision is, and should be, influenced by a multitude of other factors, including other anatomical factors, physical examination findings, patient factors, and, of course, patient preference.
An interesting idea to consider in treating patellar instability is the interplay of patella alta and trochlear dysplasia. Patella alta is theorized as contributing to patellar instability in part by delaying entry of the patella into the TG as the knee flexes, therefore requiring less force to laterally displace
the patella.10 Similarly, in the setting of trochlear dysplasia, a shallow TG leads to less bony constraint of the patella, particularly in the groove’s superior portions, which are more involved in lower grade dysplasia. Because trochlear dysplasia and patella alta decrease patellar stability by similar mechanisms, they clearly interact, and a patient with both is at higher risk for instability than a patient who exhibits either in isolation.11 Therefore, trochlear dysplasia, particularly higher grade, may be an indication for adding TTDO at lower CDI.
Other imaging and physical examination factors can provide additional insight into the process of patellar engagement into the trochlea in each patient. The patellotrochlear index (PTI) directly measures the relationship between the patella and the trochlea, rather than relative to the tibia, as with other measures of patellar height.12[[{"fid":"201853","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]The PTI is correlated with tibia-based measures of height, but the correlation is not perfect. Lower degrees of overlap between the patella and the trochlea (PTI <0.15) and significant functional patella alta may warrant adding TTDO in cases of borderline CDI (1.2-1.4). Figures 1A, 1B and 2A, 2B show the imaging of 2 patients with relatively similar patellar height (assessed with CDI) but quite different degrees of overlap between the patella and trochlea. [[{"fid":"201854","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The patient with less overlap is more likely to have delayed patellar engagement and symptomatic patella alta and thus may be a poorer candidate for isolated MPFL reconstruction. For additional information, please refer to the work by Roland Biedert, MD, who has proposed trochlear lengthening in these situations.13
Physical examination (even in the era of advance imaging) continues to provide useful insight into whether to add TTDO. One physical examination test that can help in understanding patellar-
trochlear dynamics is the patellar apprehension and relief test. Patellar apprehension has been widely discussed, but equally important is the degree of knee flexion above which apprehension dissipates. As patella alta and trochlear dysplasia become more severe, more knee flexion is required to relieve apprehension. Apprehension that is relieved at 30° to 40° of flexion suggests that patellar stability stands a good chance of being restored with isolated MPFL reconstruction, whereas persistent instability >45° or especially 60° of knee flexion suggests that there is significant patella alta, trochlear dysplasia, or both and that TTDO should be added. A large J-sign during knee flexion and extension provides further evidence that entry of the patella into the TG is delayed, typically because of patella alta, trochlear dysplasia, or both, and possibly tight lateral structures or a lateralized tibial tubercle. This sign is another clue that isolated MPFL reconstruction may be insufficient to completely restore patellar stability.
Take-Home Points
The decision to adda TTDO to an MPFL reconstruction is dependent on patellar height as assessed with the CDI, as well as multiple other patient and anatomical factors.
TTDOs that include a complete detachment of the tibial tubercle (as required for distalization) have increased risk of nonunion and hardware failure.
Poor surgical technique (failure to make a flat osteotomy cut, cortical only bone block, poor bony apposition of the detached bone block- particularly at the location of any transverse plane cut, and failure to minimize thermal damage through copious irrigation) can increase nonunion risk.
Postoperative rehabilitation should include a 6-week period of limited weight-bearing.
Reconstruction of the MPFL should be performed after any TTO is performed.
Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased tibial tubercle-trochlear groove (TT-TG) or tibial tubercle-posterior cruciate ligament distance.2,5 Of all the factors, TT-TG distance and the medial patellofemoral ligament (MPFL) have received the ost attention. Patellar height remains a crucial yet underappreciated contributor that is amenable to surgical correction with tibial tubercle distalization osteotomy (TTDO). The obvious question is how severe patella alta must be to require surgical correction. In other words, when is patella alta so severe that isolated MPFL reconstruction is insufficient to restore patellar stability?
The indications for TTDO are not completely clear and depend on multiple factors. Patient factors, physical examination findings, and radiographic measures must be considered. In general, adding TTDO to MPFL reconstruction should be considered when the degree of patella alta exceeds 1.4 on the Caton-Deschamps Index (CDI). Presence of trochlear dysplasia, patellar maltracking (J-sign), lateral patellar apprehension that persists at higher flexion angles, and decreased patellotrochlear articular cartilage contact on sagittal magnetic resonance imaging may drive the decision to proceed with TTDO when the CDI is lower.
Why You Need To Know About Patella Alta
Recurrent lateral patellar dislocation is a debilitating knee condition that often involves young, active patients and significantly affects their quality of life. The MPFL is a primary restraint to lateral patellar dislocation, and an MPFL injury is a key contributor to loss of patellar stability. MPFL reconstruction is increasingly being performed to treat recurrent lateral patellar instability.6 Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased TT-TG distance.2,5 This review focuses on patella alta.
The classic teaching of the Lyon School of Knee Surgery in France, the menu à la carte, is that patella alta exceeding 1.2 on the CDI is an indication for TTDO.2 Although this teaching is an excellent guide for normal anatomy, we must keep in mind that the classic surgical menu does not consider the influence of MPFL reconstruction, as development of the menu predated this surgical option. At that time, the proximal soft-tissue procedures included vastus medialis obliquus plasty and advancement, which are performed to balance soft tissues and treat patellar tilt. These procedures and MPFL reconstruction have different functions, and the difference may be important. Furthermore, performing TTDO alongside MPFL reconstruction significantly increases the risk of complications and alters the rehabilitation protocol. However, significant untreated patella alta has been implicated as a cause of failure of isolated MPFL reconstruction.7 Establishing when MPFL reconstruction alone is sufficient is therefore crucial in avoiding the increased morbidity associated with the addition of TTDO.
Discussion
Above a certain degree of patella alta, isolated MPFL reconstruction fails to restore patellar stability. What remains unknown is the appropriate CDI cutoff (1.4) and whether the same cutoff can be used for all patients. In 2013, Wagner and colleagues8 assessed the influence of patella alta on isolated MPFL reconstruction outcomes and found no significant difference, though their study did not include many patients with significant alta and was underpowered. In 2014, Feller and colleagues9 reported on a series of patients who were successfully treated with isolated MPFL reconstruction despite patella alta significantly exceeding the traditional CDI cutoff of 1.2. Their indication for performing the isolated procedure was normal patellar tracking—in particular, absence of the J-sign. Further analysis of these patients revealed a preponderance of relatively normal TT-TG distances and low-grade, if any, trochlear dysplasia in comparison with other patients treated with a combination of MPFL reconstruction and tibial tubercle osteotomy.
Together, the work of Wagner and colleagues8 and Feller and colleagues9 suggests the historical use of the CDI of 1.2 as a hard and fast indication for adding TTDO is aggressive. In fact, it is probably the case that there really is no single CDI cutoff that is an appropriate indication for adding TTDO in all patients with instability. This decision is, and should be, influenced by a multitude of other factors, including other anatomical factors, physical examination findings, patient factors, and, of course, patient preference.
An interesting idea to consider in treating patellar instability is the interplay of patella alta and trochlear dysplasia. Patella alta is theorized as contributing to patellar instability in part by delaying entry of the patella into the TG as the knee flexes, therefore requiring less force to laterally displace
the patella.10 Similarly, in the setting of trochlear dysplasia, a shallow TG leads to less bony constraint of the patella, particularly in the groove’s superior portions, which are more involved in lower grade dysplasia. Because trochlear dysplasia and patella alta decrease patellar stability by similar mechanisms, they clearly interact, and a patient with both is at higher risk for instability than a patient who exhibits either in isolation.11 Therefore, trochlear dysplasia, particularly higher grade, may be an indication for adding TTDO at lower CDI.
Other imaging and physical examination factors can provide additional insight into the process of patellar engagement into the trochlea in each patient. The patellotrochlear index (PTI) directly measures the relationship between the patella and the trochlea, rather than relative to the tibia, as with other measures of patellar height.12[[{"fid":"201853","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]The PTI is correlated with tibia-based measures of height, but the correlation is not perfect. Lower degrees of overlap between the patella and the trochlea (PTI <0.15) and significant functional patella alta may warrant adding TTDO in cases of borderline CDI (1.2-1.4). Figures 1A, 1B and 2A, 2B show the imaging of 2 patients with relatively similar patellar height (assessed with CDI) but quite different degrees of overlap between the patella and trochlea. [[{"fid":"201854","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The patient with less overlap is more likely to have delayed patellar engagement and symptomatic patella alta and thus may be a poorer candidate for isolated MPFL reconstruction. For additional information, please refer to the work by Roland Biedert, MD, who has proposed trochlear lengthening in these situations.13
Physical examination (even in the era of advance imaging) continues to provide useful insight into whether to add TTDO. One physical examination test that can help in understanding patellar-
trochlear dynamics is the patellar apprehension and relief test. Patellar apprehension has been widely discussed, but equally important is the degree of knee flexion above which apprehension dissipates. As patella alta and trochlear dysplasia become more severe, more knee flexion is required to relieve apprehension. Apprehension that is relieved at 30° to 40° of flexion suggests that patellar stability stands a good chance of being restored with isolated MPFL reconstruction, whereas persistent instability >45° or especially 60° of knee flexion suggests that there is significant patella alta, trochlear dysplasia, or both and that TTDO should be added. A large J-sign during knee flexion and extension provides further evidence that entry of the patella into the TG is delayed, typically because of patella alta, trochlear dysplasia, or both, and possibly tight lateral structures or a lateralized tibial tubercle. This sign is another clue that isolated MPFL reconstruction may be insufficient to completely restore patellar stability.
1. Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45-54.
2. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
3. Geenen E, Molenaers G, Martens M. Patella alta in patellofemoral instability. Acta Orthop Belg. 1989;55(3):387-393.
4. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
5. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl) [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
6. Feller JA, Lind M, Nelson J, Diduch DR, Arendt E. Repair and reconstruction of the medial patellofemoral ligament for treatment of lateral patellar dislocations. In: Scott WN, ed. Insall & Scott—Surgery of the Knee. 5th ed. Philadelphia, PA: Churchill Livingstone; 2011:677-687.
7. Thaunat M, Erasmus PJ. Recurrent patellar dislocation after medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):40-43.
8. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
9. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
10. Ward SR, Terk MR, Powers CM. Patella alta: association
with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):
1749-1755.
11. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309
12. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
13. Biedert RM. Trochlear lengthening osteotomy with or without elevation of the lateral trochlear facet. In: Zaffagnini S, Dejour D, Arendt EA, eds. Patellofemoral Pain, Instability, and Arthritis. Germany: Springer-Verlag Berlin Heidelberg; 2010:
209-215.
1. Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45-54.
2. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
3. Geenen E, Molenaers G, Martens M. Patella alta in patellofemoral instability. Acta Orthop Belg. 1989;55(3):387-393.
4. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
5. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl) [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
6. Feller JA, Lind M, Nelson J, Diduch DR, Arendt E. Repair and reconstruction of the medial patellofemoral ligament for treatment of lateral patellar dislocations. In: Scott WN, ed. Insall & Scott—Surgery of the Knee. 5th ed. Philadelphia, PA: Churchill Livingstone; 2011:677-687.
7. Thaunat M, Erasmus PJ. Recurrent patellar dislocation after medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):40-43.
8. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
9. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
10. Ward SR, Terk MR, Powers CM. Patella alta: association
with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):
1749-1755.
11. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309
12. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
13. Biedert RM. Trochlear lengthening osteotomy with or without elevation of the lateral trochlear facet. In: Zaffagnini S, Dejour D, Arendt EA, eds. Patellofemoral Pain, Instability, and Arthritis. Germany: Springer-Verlag Berlin Heidelberg; 2010:
209-215.
Cartilage Restoration in the Patellofemoral Joint
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
Practice Makes Perfect?
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
Is Simultaneous Bilateral Total Knee Arthroplasty (BTKA) as Safe as Staged BTKA?
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Knotless Arthroscopic Reduction and Internal Fixation of a Displaced Anterior Cruciate Ligament Tibial Eminence Avulsion Fracture
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Technique provides optimal fixation while simultaneously protecting open growth plates.
- Self tensioning feature insures both optimal ACL tension and fracture reduction.
- No need for future hardware removal.
- 10Cross suture configuration optimizes strength of fixation for highly consistent results.
- Use fluoroscopy to avoid violation of tibial physis.
Generally occurring in the 8- to 14-year-old population, tibial eminence avulsion (TEA) fractures are a common variant of anterior cruciate ligament (ACL) ruptures and represent 2% to 5% of all knee injuries in skeletally immature individuals.1,2 Compared with adults, children likely experience this anomaly more often because of the weakness of their incompletely ossified tibial plateau relative to the strength of their native ACL.3
The open repair techniques that have been described have multiple disadvantages, including open incisions, difficult visualization of the fracture owing to the location of the fat pad, and increased risk for arthrofibrosis. Arthroscopic fixation is considered the treatment of choice for TEA fractures because it allows for direct visualization of injury, accurate reduction of fracture fragments, removal of loose fragments, and easy treatment of associated soft-tissue injuries.4-6Several fixation techniques for ACL-TEA fractures were recently described: arthroscopic reduction and internal fixation (ARIF) with Kirschner wires,7 cannulated screws,4 the Meniscus Arrow device (Bionx Implants),8 pull-out sutures,9,10 bioabsorbable nails,11 Herbert screws,12 TightRope fixation (Arthrex),13 and various other rotator cuff and meniscal repair systems.14,15 These approaches tend to have good outcomes for TEA fractures, but there are risks associated with ACL tensioning and potential tibial growth plate violation or hardware problems. Likewise, there are no studies with large numbers of patients treated with these new techniques, so the optimal method of reduction and fixation is still unknown.
In this article, we describe a new ARIF technique that involves 2 absorbable anchors with adjustable suture-tensioning technology. This technique optimizes reduction and helps surgeons avoid proximal tibial physeal damage, procedure-related morbidity, and additional surgery.
Case Report
History
The patient, an 8-year-old boy, sustained a noncontact twisting injury of the left knee during a cutting maneuver in a flag football game. He experienced immediate pain and subsequent swelling. Clinical examination revealed a moderate effusion with motion limitations secondary to swelling and irritability. The patient’s Lachman test result was 2+. Pivot shift testing was not possible because of guarding. The knee was stable to varus and valgus stress at 0° and 30° of flexion. Limited knee flexion prohibited placement of the patient in the position needed for anterior and posterior drawer testing. His patella was stable on lateral stress testing at 20° of flexion with no apprehension. Neurovascular status was intact throughout the lower extremity.
Anteroposterior and lateral radiographs showed a minimally displaced Meyers-McKeever type II TEA fracture (Figures 1A, 1B).
After discussing potential treatment options with the parents, Dr. Smith proceeded with arthroscopic surgery for definitive reduction and internal fixation of the patient’s left knee displaced ACL-TEA fracture. The new adjustable suture-tensioning fixation technique was used. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Examination Under Anesthesia
Examination with the patient under general anesthesia revealed 3+ Lachman, 2+ pivot shift with foot in internal and external rotation, and 1+ anterior drawer with foot in neutral and internal rotation. The knee was stable to varus and valgus stress testing.
Surgical Technique
Proper patient positioning and padding of bony prominences were ensured, and the limb was sterilely prepared and draped.
Given the young age of the patient, it was imperative to avoid the open proximal tibial growth plate. The surgical plan for stabilization involved use of two 3.0-mm BioComposite Knotless SutureTak anchors (Arthrex). This anchor configuration is based on a No. 2 FiberWire suture shuttled through itself to create a locking splice mechanism that allows for adjustable tensioning. The anchors were placed on each side of the tibial bony avulsion site with two No. 2 FiberWire sutures and were then crossed about the avulsion fracture fragment in an “x-type” configuration to secure the ACL back down to the bony bed.
First, a curette was used to débride fibrous tissue on the underside of the fracture fragment and on the fracture bed. Minimal amounts of cancellous bone were débrided from the tibial fracture bed to optimize fracture reduction by slightly recessing the fracture fragment to ensure optimal ACL tensioning (Figure 5).
Next, from the accessory superior medial portal, the end of the wire that had been passed through the medial aspect of the bony avulsion was retrieved through the lateral portal. This wire was used to shuttle the repair suture from the laterally positioned SutureTak anchor over and through the medial aspect of the bony fragment out of the accessory superior medial (Figure 7).
Follow-Up
Two weeks after surgery, the patient returned to clinic for suture removal. Four weeks after surgery, radiographs confirmed anatomical reduction of the TEA fracture, and outpatient physical therapy (range-of-motion exercises as tolerated) and isometric quadriceps strengthening were instituted. Twelve weeks after surgery, examination revealed full knee motion, negative Lachman and pivot shift test results, and residual quadriceps muscle atrophy, and radiographs confirmed complete fracture healing with maintenance of a normal proximal tibial growth plate (Figures 10A, 10B).
Discussion
The highlight of this case is the simplicity of an excellent reduction of a displaced ACL-TEA fracture. Minimally invasive absorbable implants did not violate the proximal tibial physis, and the unique adjustable suture-tensioning technology allowed the degree of reduction and ACL tension to be “dialed in.” SutureTak implants have strong No. 2 FiberWire suture for excellent stability with an overall small suture load, and their small size avoids the risk of violating the proximal tibial physis and avoids potential growth disturbances.
Despite the obvious risks it poses to the open proximal tibial physis, surgical reduction of Meyers-McKeever type II and type III fractures is the norm for restoring ACL stability. Screws and suture fixation are the most common and reliable methods of TEA fracture reduction.16,17 In recent systematic reviews, however, Osti and colleagues17 and Gans and colleagues18 noted there is not enough evidence to warrant a “gold standard” in pediatric tibial avulsion cases.
Other fixation methods for TEA fractures must be investigated. Anderson and colleagues19 described the biomechanics of 4 different physeal-sparing avulsion fracture reduction techniques: an ultra-high-molecular-weight polyethylene (UHMWPE) suture-suture button, a suture anchor, a polydioxanone suture-suture button, and screw fixation. Using techniques described by Kocher and colleagues,4 Berg,20 Mah and colleagues,21 Vega and colleagues,22 and Lu and colleagues,23 Anderson and colleagues19 reduced TEA fractures in skeletally immature porcine knees. Compared with suture anchors, UHMWPE suture-suture buttons provided biomechanically superior cyclic and load-to-failure results as well as more consistent fixation.
Screw fixation has shown good results but has disadvantages. Incorrect positioning of a screw can lead to impingement and articular cartilage damage, and screw removal may be needed if discomfort at the fixation site persists.24,25 Likewise, screws generally are an option only for large fracture fragments, as there is an inherent risk of fracturing small TEA fractures, which can be common in skeletally immature patients.
Brunner and colleagues26 recently found that TEA fracture repair with absorbable sutures and distal bone bridge fixation yielded 3-month radiographic and clinical healing rates similar to those obtained with nonabsorbable sutures tied around a screw. However, other authors have reported growth disturbances with use of a similar technique, owing to a disturbance of the open proximal tibial growth plate.9 In that regard, a major advantage of this new knotless suturing technique is that distal fixation is not necessary.
The minimally invasive TEA fraction reduction technique described in this article has 6 advantages: It provides excellent fixation while avoiding proximal tibial growth plate injury; the degree of tensioning is easily controlled during reduction; it uses strong suture instead of metal screws or pins; the reduction construct is low-profile; distal fixation is unnecessary; and implant removal is unnecessary, thus limiting subsequent surgical intervention. With respect to long-term outcomes, however, it is not known how this procedure will compare with other commonly used ARIF methods in physeal-sparing techniques for TEA fracture fixation.
This case report highlights a novel pediatric displaced ACL-TEA fracture reduction technique that allows for adjustable reduction and resultant ACL tensioning with excellent strong suture fixation without violating the proximal tibial physis, which could make it invaluable in the surgical treatment of this injury in skeletally immature patients.
Am J Orthop. 2017;46(4):203-208. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.
1. Eiskjaer S, Larsen ST, Schmidt MB. The significance of hemarthrosis of the knee in children. Arch Orthop Trauma Surg. 1988;107(2):96-98.
2. Luhmann SJ. Acute traumatic knee effusions in children and adolescents. J Pediatr Orthop. 2003;23(2):199-202.
3. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217-225.
4. Kocher MS, Foreman ES, Micheli LJ. Laxity and functional outcome after arthroscopic reduction and internal fixation of displaced tibial spine fractures in children. Arthroscopy. 2003;19(10):1085-1090.
5. Lubowitz JH, Elson WS, Guttmann D. Part II: arthroscopic treatment of tibial plateau fractures: intercondylar eminence avulsion fractures. Arthroscopy. 2005;21(1):86-92.
6. Vargas B, Lutz N, Dutoit M, Zambelli PY. Nonunion after fracture of the anterior tibial spine: case report and review of the literature. J Pediatr Orthop B. 2009;18(2):90-92.
7. Sommerfeldt DW. Arthroscopically assisted internal fixation of avulsion fractures of the anterior cruciate ligament during childhood and adolescence [in German]. Oper Orthop Traumatol. 2008;20(4-5):310-320.
8. Wouters DB, de Graaf JS, Hemmer PH, Burgerhof JG, Kramer WL. The arthroscopic treatment of displaced tibial spine fractures in children and adolescents using Meniscus Arrows®. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):736-739.
9. Ahn JH, Yoo JC. Clinical outcome of arthroscopic reduction and suture for displaced acute and chronic tibial spine fractures. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):116-121.
10. Huang TW, Hsu KY, Cheng CY, et al. Arthroscopic suture fixation of tibial eminence avulsion fractures. Arthroscopy. 2008;24(11):1232-1238.
11. Liljeros K, Werner S, Janarv PM. Arthroscopic fixation of anterior tibial spine fractures with bioabsorbable nails in skeletally immature patients. Am J Sports Med. 2009;37(5):923-928.
12. Wiegand N, Naumov I, Vamhidy L, Not LG. Arthroscopic treatment of tibial spine fracture in children with a cannulated Herbert screw. Knee. 2014;21(2):481-485.
13. Faivre B, Benea H, Klouche S, Lespagnol F, Bauer T, Hardy P. An original arthroscopic fixation of adult’s tibial eminence fractures using the Tightrope® device: a report of 8 cases and review of literature. Knee. 2014;21(4):833-839.
14. Kluemper CT, Snyder GM, Coats AC, Johnson DL, Mair SD. Arthroscopic suture fixation of tibial eminence fractures. Orthopedics. 2013;36(11):e1401-e1406.
15. Ochiai S, Hagino T, Watanabe Y, Senga S, Haro H. One strategy for arthroscopic suture fixation of tibial intercondylar eminence fractures using the Meniscal Viper Repair System. Sports Med Arthrosc Rehabil Ther Technol. 2011;3:17.
16. Bogunovic L, Tarabichi M, Harris D, Wright R. Treatment of tibial eminence fractures: a systematic review. J Knee Surg. 2015;28(3):255-262.
17. Osti L, Buda M, Soldati F, Del Buono A, Osti R, Maffulli N. Arthroscopic treatment of tibial eminence fracture: a systematic review of different fixation methods. Br Med Bull. 2016;118(1):73-90.
18. Gans I, Baldwin KD, Ganley TJ. Treatment and management outcomes of tibial eminence fractures in pediatric patients: a systematic review. Am J Sports Med. 2014;42(7):1743-1750.
19. Anderson CN, Nyman JS, McCullough KA, et al. Biomechanical evaluation of physeal-sparing fixation methods in tibial eminence fractures. Am J Sports Med. 2013;41(7):1586-1594.
20. Berg EE. Pediatric tibial eminence fractures: arthroscopic cannulated screw fixation. Arthroscopy. 1995;11(3):328-331.
21. Mah JY, Otsuka NY, McLean J. An arthroscopic technique for the reduction and fixation of tibial-eminence fractures. J Pediatr Orthop. 1996;16(1):119-121.
22. Vega JR, Irribarra LA, Baar AK, Iniguez M, Salgado M, Gana N. Arthroscopic fixation of displaced tibial eminence fractures: a new growth plate-sparing method. Arthroscopy. 2008;24(11):1239-1243.
23. Lu XW, Hu XP, Jin C, Zhu T, Ding Y, Dai LY. Reduction and fixation of the avulsion fracture of the tibial eminence using mini-open technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1476-1480.
24. Bonin N, Jeunet L, Obert L, Dejour D. Adult tibial eminence fracture fixation: arthroscopic procedure using K-wire folded fixation. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):857-862.
25. Senekovic V, Veselko M. Anterograde arthroscopic fixation of avulsion fractures of the tibial eminence with a cannulated screw: five-year results. Arthroscopy. 2003;19(1):54-61.
26. Brunner S, Vavken P, Kilger R, et al. Absorbable and non-absorbable suture fixation results in similar outcomes for tibial eminence fractures in children and adolescents. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):723-729.