User login
Applying Military Strategy to Complex Knee Reconstruction: Tips for Planning and Executing Advanced Surgery
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
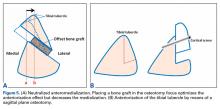
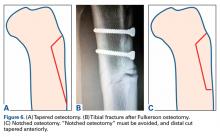
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
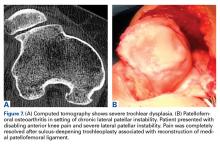
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
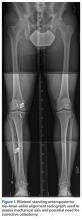
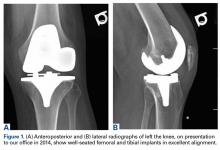
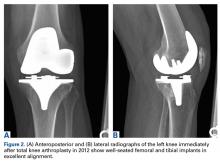
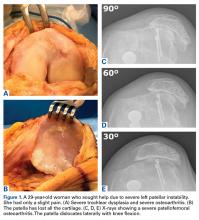
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
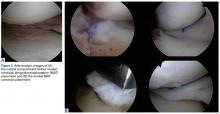
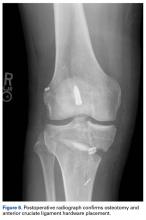
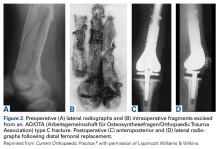
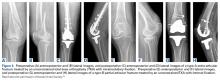
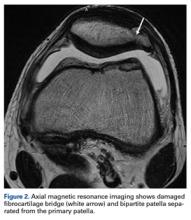
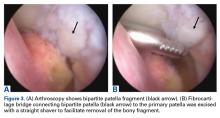
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
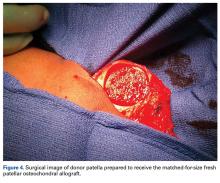
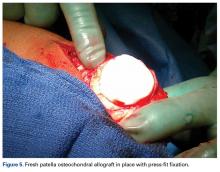
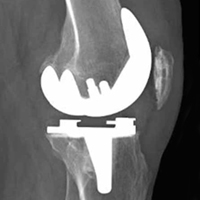
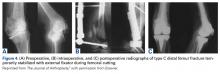
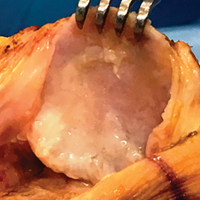
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
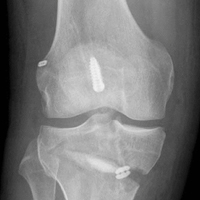
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
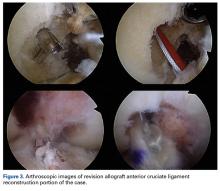
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Thorough preoperative planning is imperative and inclusive of history, physical examination, radiographs, and MRI and potentially CT scan.
- Plan carefully for needed graft sources (autografts and allografts).
- Rehabilitation starts preoperatively and a detailed individualized plan is often warranted.
- Indicated ligamentous repair or augmented repair with reconstruction is more likely to succeed when performed within 2 weeks of injury.
- Complex combined knee restoration surgery can be safely performed in an outpatient setting.
Complex combined knee restoration surgery can be safely performed in an outpatient setting. The term complex knee restoration is used to describe management of knee injuries that are more involved—that is, there is damage to the menisci, cartilage, ligaments, and bones. Management entails not only determining the best treatment options but navigating the more complex logistics of making sure all necessary grafts (fresh and frozen allografts and autografts), implants, and instrumentation are readily available as these cases come to fruition.
The military healthcare paradigm often involves the added logistics of transporting the service member to the correct military treatment facility at the correct time and ensuring the patient’s work-up is complete before he or she arrives for the complex knee restoration. Such cases require significant rehabilitation and time away from family and work, so anything that reduces the morbidity of the surgical undertaking and the overall “morbidity footprint” of time away and that helps the patient return to normal function are value-added and worthy of our attention and diligence in developing an efficient system for managing complex cases.
The globally integrated military healthcare system that is in place has matured over the past decades to allow for the significant majority of the necessary preoperative work-up to be performed at a soldier’s current duty station, wherever in the world that may be, under the guidance of local healthcare providers with specific inputs from the knee restoration surgeon who eventually receives the patient for the planned surgical intervention.
Algorithm for Knee Restoration Planning
Alignment Issues
The first task is to confirm the realignment indication. Realignment may be performed with a proximal opening-wedge medial tibial osteotomy (OWMTO), a distal opening-wedge lateral femoral osteotomy (OWLFO), or a tibial tubercle osteotomy (TTO).1 Given the reproducible clinical improvement achieved and the robust nature of the fixation, these osteotomies are often the first surgical step in complex knee restorations.2 The final determination, made by the surgeon in consultation with the patient, is whether to perform the indicated osteotomy alone or in combination with the rest of the planned restoration surgery. In the vast majority of cases I have managed over the past 2 decades, I have performed the entire knee restoration in a single operation.3 Within the past 5 years, combining the procedures has become even more feasible with the important progress made in multimodal pain management and with the close collaboration of anesthesiologists.4
Meniscus and Cartilage Status
The integration status of meniscus and cartilage within the medial and lateral tibiofemoral compartments is crucial to the comprehensive restoration plan. In fact, the success of the restoration can be said to be dependent on the functional status and health of meniscus and cartilage—which either succeed together or fail apart.
Important covariables are age, prior surgical interventions, activity level expected or allowed after surgery, and size, location, and depth of cartilage injury.5 Whether a cartilage injury is monopolar or bipolar is determined with advanced imaging (magnetic resonance imaging [MRI], computed tomography [CT], weight-bearing radiography) along with analysis of a thorough history (including a review of prior operative reports and arthroscopic images) and a knee examination. Bipolar injuries that involve the condyle and juxtaposed plateau often bode poorly for good clinical outcomes—compared with unipolar lesions, which usually involve the condylar surfaces in isolation. The same thinking regarding the patellofemoral compartment is appropriate. Cartilage lesions that involve the juxtaposed surfaces of the patellar and trochlear groove do poorer than isolated lesions, which are more amenable to cartilage restoration options. The literature on potential cartilage restoration options for the patella and trochlea is expanding. I use the 3-dimensional cartilage restoration option of a fresh patellar osteochondral allograft (OCA) for high-grade cartilage lesions thought to be clinically significant. Other options, such as microfracture, cell-based cartilage restoration, and Osteochondral Autograft Transfer System (Arthrex) procedures (from the thinner condylar cartilage), have varied in their outcomes for patellar lesions. According to more recent literature and a review of my clinical results, fresh patellar OCAs are a good option for patellar lesions.6 Similarly, trochlear lesions can be managed with microfracture, cell-based therapies, or fresh OCAs, depending on surgeon preference.
Functional total or subtotal meniscectomies are often best managed with meniscal allograft transplantation (MAT). An intact or replaced medial or lateral meniscus works synergistically with any planned anterior cruciate ligament (ACL) reconstruction. Again, the adage that meniscus and cartilage succeed together or fail apart is appropriate when planning complex knee restoration. Signs of extrusion or joint-space narrowing and root avulsion or significant loss of meniscal tissue, visualized on MRI or on prior surgical images, often help substantiate a MAT plan. MAT has had the best long-term results when performed in compartments with cartilage damage limited to grade I and grade II changes, in stable knees, and in knees that can be concurrently stabilized.5 Technological advances have increased the value of MAT by limiting the morbidity of the operation and thus allowing for other surgery to be performed concomitantly and safely as part of comprehensive knee restoration. Over the past 20 years, I have arthroscopically performed MAT with bone plugs for medial and lateral procedures, and my results with active-duty soldiers have been promising, paralleling the clinic success reported in the literature.5 Alignment must be considered when performing MAT or cartilage restoration. If the addition of meniscal transplantation or cartilage restoration leaves the knee with residual malalignment of 6° or more, corrective osteotomy is performed.
My view and practice have been to plan for an unloading chondroprotective osteotomy. The goal is a balanced mechanical axis, whether achieved with mere joint-space restoration or with an osteotomy added.
Ligament Status
A comprehensive plan for establishing ligamentous stability is paramount to the overall clinical success of complex knee restorations. Meniscus and cartilage restoration efforts are wasted if clinically significant ligamentous laxity is not concomitantly treated with reconstruction surgery. Revision ACL surgery is by far the most commonly performed surgery in complex knee cases. Diligence in interpreting advanced MRI and physical examination findings is required to make sure there are no concomitant patholaxities in the medial, lateral, posterior, posteromedial, and posterolateral ligamentous complexes. Appropriate ligamentous reconstruction is warranted to maximize clinical results in complex knee restorations. Such cases more commonly require allograft tissue, as the availability of autograft tissue is the limiting issue with 2 or more ligament reconstructions. Military treatment facilities, in which comprehensive knee restorations are performed, have soft-tissue allografts on hand at all times. Having tissue readily available makes it less imperative to determine the most appropriate combined ligamentous reconstruction surgery before the patient arrives—a process that is often difficult. This situation is in contradistinction to the need for specific matched-for-size allograft frozen meniscus and fresh cartilage tissues, both of which require tissue-form procurement in advance of planned restoration surgery.
Rehabilitation Plan
The rehabilitation plan is driven by the part of the complex knee restoration that demands the most caution with respect to weight-bearing and range of motion (ROM) during the first 6 weeks after surgery. The most limiting restorative surgeries involve meniscus and cartilage. Recent clinical trial results support weight-bearing soon after tibial osteotomy performed in the absence of meniscus and cartilage restoration that would otherwise limit weight-bearing for 6 weeks.7 Therefore, most of these complex knee restorations are appropriately managed with a hinged brace locked in extension for toe-touch weight-bearing ambulation, with ROM usually limited to 0° to 90° during the first 6 weeks. Quadriceps rehabilitation with straight-leg raises and isometric contractions is prescribed with a focus on maintaining full extension as the default resting knee position until normalized resting quadriceps tone returns. Full weight-bearing and advancement to full flexion are routinely allowed by 6 weeks.
Case Report
A 41-year-old male service member who was overseas was referred to my clinic for high tibial osteotomy consideration and possible revision ACL reconstruction. His symptoms were medial pain, recurrent instability, and patellofemoral crepitance. Three years earlier, he underwent autograft transtibial ACL reconstruction with significant débridement of the medial meniscus. Before his trip to the United States, I asked that new MRI scans, full-length standing hip–knee–ankle bilateral alignment radiographs, and a 4-view weight-bearing knee series (including a posteroanterior Rosenberg view) be obtained and sent for my review (Figure 1).
Review of the patient’s detailed preoperative imaging work-up and electronic medical record (available through the military’s healthcare system) made it clear that far more surgical intervention was needed than originally assumed. A significant full-thickness chondral lesion of the patella and a subtotal medial meniscectomy would necessitate patellar cartilage restoration and medial MAT in addition to the high tibial osteotomy and revision ACL reconstruction.
Had this patient been sent through the military medical evacuation system, he would have had to make 2 overseas trips—one trip for preoperative evaluation and advanced imaging, whereby he would have been placed on a match list and had to wait for a requested meniscal allograft and an appropriate graft for his patella, and the other trip for his complex surgery. Fortunately, the military’s integrated healthcare network with true 2-way communication and the collaborative use of integrated electronic medical records proved extremely valuable in making management of this complex knee restoration as efficient as possible. From the perspective of the soldier and his military unit, only 1 big overseas trip was needed; from the perspective of the military healthcare system, responsible use of healthcare personnel and monetary resources and well-planned complex knee restoration surgery saved a knee and allowed a soldier-athlete to rejoin the fields of friendly strife.
This patient had undergone functional complete medial meniscectomy and had significant medial compartment pain, varus alignment, and minimal medial joint-space narrowing (assumed grossly intact cartilage about plateau and condyle), plus patellofemoral pain and crepitance with a large high-grade posttraumatic patellar chondral lesion with normal patellofemoral alignment. He also had an isolated failed ACL graft from prior ACL reconstruction. The previous hardware placement was analyzed, and it was determined that the femoral interference screw could be left in place and that the tibial interference screw most likely would be removed. The mechanical axis determined from the bilateral long-leg standing images dictated a need for proximal OWMTO for correction up to 8° to allow the axis to cross the center of the knee. The 8° correction is the measured correction needed to move the axis from its pass through the medial compartment to a more balanced position across the middle of the knee.
The overall plan encompassed major concomitant corrective and restorative surgery: tibial osteotomy, medial MAT, revision ACL reconstruction, and fresh mega-patellar OCA. Once the frozen meniscus and eventually the fresh patella (both matched for size) were obtained, arrangements for the patient’s trip for the complex surgery were finalized.
Surgery was started with brief arthroscopic evaluation to confirm the overall appropriateness of the planned procedure and to determine if any other minor deficiencies would warrant operative intervention. Once confirmed, the restoration proceeded as planned. The OWMTO was performed with a PEEK (polyetheretherketone) wedge implant (iBalance; Arthrex) followed by arthroscopic preparation for medial MAT with removal of any meniscal remnants and placement of passing sutures (Figure 2A).
When the arthroscopic portion of the surgery was finished, a medial parapatellar arthrotomy was made to allow the patella to be inverted and complete fresh mega-patellar OCA placement (Figure 4).
The knee was placed in a ROM brace locked in full extension. The patient was able to do straight-leg raises and calf pumps in the recovery room and was discharged home with a saphenous nerve block and an iPACK (Interspace between the Popliteal Artery and the Capsule of the posterior Knee) nerve block in place. Home-based therapy was started immediately. After the patient’s first postoperative visit, formal therapy (discussed earlier) was initiated (Figure 6).
Discussion
All-inside GraftLink ACL reconstruction with cortical suspensory fixation appears well suited to combined medial and lateral MAT and/or cartilage restoration—whether it be large fresh OCA combined with medial MAT (as in this patient’s case) or another form of cartilage restoration. Arthroscopic MAT with anatomically fashioned and placed bone plugs minimizes the morbidity within the notch footprints and allows for discrete revision socket formation for both femoral and tibial ACL graft placement. In this case, preparation for the medial MAT and ACL sockets was followed by MAT/ACL construct implantation and secure fixation. The arthrotomy was thereby minimized and placed to allow for efficient mega-patellar OCA graft placement.
Over the past decade, I have performed similar concomitant procedures using the same surgical principles that allow for efficient and reproducible complex knee restoration (Figure 7).
Although use of an algorithm for the management of complex knee restorations is not universally feasible, I offer guidelines for complex knee injuries:
- At each decision point, determine whether the knee and the patient can withstand the planned surgical intervention.
- After deciding to proceed with knee restoration, list the meniscus, cartilage, and ligament injuries that must be addressed.
- Determine which repairs (meniscus, cartilage, ligament) are warranted. Repairs generally are best performed within a period of 7 to 14 days.
- Determine which ligament injuries warrant reconstruction. Allograft tissue typically is used for multiligament reconstruction.
- Rank-order the ligament reconstruction requirements. It is fine to proceed with all of the reconstructions if the case is moving smoothly, if there are no developing tourniquet-time issues, and if the soft-tissue envelope is responding as expected.
- Consider autograft and/or allograft tissue needs for concomitant or staged meniscus and cartilage restoration options/requirements.
Am J Orthop. 2017;46(4):170-175, 202. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
1. Uquillas C, Rossy W, Nathasingh CK, Strauss E, Jazrawi L, Gonzalez-Lomas G. Osteotomies about the knee: AAOS exhibit selection. J Bone Joint Surg Am. 2014;96(24):e199.
2. Mehl J, Paul J, Feucht MJ, et al. ACL deficiency and varus osteoarthritis: high tibial osteotomy alone or combined with ACL reconstruction? Arch Orthop Trauma Surg. 2017;137(2):233-240.
3. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
4. Ferrari D, Lopes TJ, França PF, Azevedo FM, Pappas E. Outpatient versus inpatient anterior cruciate ligament reconstruction: a systematic review with meta-analysis. Knee. 2017;24(2):197-206.
5. Weber AE, Gitelis ME, McCarthy MA, Yanke AB, Cole BJ. Malalignment: a requirement for cartilage and organ restoration. Sports Med Arthrosc. 2016;24(2):e14-e22.
6. Prince MR, King AH, Stuart MJ, Dahm DL, Krych AJ. Treatment of patellofemoral cartilage lesions in the young, active patient. J Knee Surg. 2015;28(4):285-295.
7. Scordino LE, DeBerardino TM. Surgical treatment of osteoarthritis in the middle-aged athlete: new horizons in high tibial osteotomies. Sports Med Arthrosc. 2013;21(1):47-51.
VIDEO: Hip, knee replacements fall in Danish RA patients
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – The rates of both total hip and total knee replacement surgeries dropped among Danish patients with rheumatoid arthritis since the mid-1990s, reductions that were coincident with more widespread use of biologic drugs as well as with other improvements in care, according to analyses of Danish national health records.
“The introduction of guidelines [on biologic drug use] in 2002 and increasing use of biologic drugs [as a result] may have contributed to this positive development,” Lene Dreyer, MD, said at the European Congress of Rheumatology. Other factors that may have also contributed include widespread use of conventional disease-modifying antirheumatic drugs (DMARDs) and adoption of a treat-to-target strategy by many clinicians.
In 1996, the first year studied and before any biologic DMARDs were routinely used for rheumatoid arthritis, the rate of total knee replacement was nearly 6/1,000 person-years among RA patients, compared with a 0.42/1,000 person-years rate in the general adult Danish population, a roughly 14-fold excess among the RA patients, Dr. Dreyer reported. But by 2016, ”this gap had almost disappeared,” she said in a video interview. “It seems like rheumatologists in Denmark are doing a good job” treating RA patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That may have been especially true subsequent to 2002, when the Danish Institute for Rational Pharmacotherapy issued recommendations that opened the door to wider use of biologic DMARDs, such as tumor necrosis factor inhibitors, to treat RA patients, noted Dr. Dreyer of Gentofte University Hospital, Copenhagen. During 2003-2011, use of total knee replacement surgery in RA patients fell by an average annualized rate of 0.2 surgeries/1,000 person-years. But among the general Danish population the average annualized rate of knee surgeries rose by 0.08/1,000 person-years.
“This is a very important finding,” commented Robert Landewé, MD, PhD, professor of rheumatology at the Academic Medical Center in Amsterdam. “It is extremely difficult to test the effect of the introduction of the [biologic DMARD] guidelines,” he cautioned. But he highlighted the positive finding that the excess of hip and knee replacement surgeries in patients with RA, compared with the general population, had recently narrowed.
Dr. Dreyer and her associates used records from the Danish National Patient Register to compare 29,427 patients with incident RA during 1996-2011 with more than 290,000 matched control individuals. All people studied had not undergone knee or hip replacement surgery prior to their entry into the study. The researchers used an “interrupted time series analysis” to examine the possible impact of the introduction of widespread access to biologic DMARDs starting in 2003.
The analysis showed that the rate of total hip replacements in 1996 was nearly 9 surgeries/1,000 person-years among RA patients and nearly 3/1,000 person-years in the general population, a threefold excess for RA patients. This rate fell by an average annual rate of 0.38/1,000 person-years among RA patients both before and after 2002, so that by 2011 the rate was roughly half the 1996 rate, about 4.5/1,000 patient-years. The rate in the general population rose during 1996-2011, and by 2011 was nearly 4/1,000 person-years and so nearly the same as RA patients. Wider availability of biologic DMARDs for RA patients starting in 2003 did not have an apparent impact on the rate of total hip replacement.
In contrast, wider use of biologic DMARDs appeared to have an effect on the rate of total knee surgeries among RA patients. During 1996-2001, the rate rose by an annual average of 0.19/1,000 person-years, very similar to the 0.21/1,000 person-years annual rise in the general Danish population. However, during 2003-2011, the average annual rate of total knee surgery fell by 0.20/1,000 person-years in the RA patients but continued to rise at an annual average rate of 0.08/1,000 person-years in the general population, Dr. Dreyer reported.
Additional Danish registry data exist for patients who received biologic DMARDs, and Dr. Dreyer said that she and her associates hope to use this to further examine the impact of these drugs on patient outcomes.
Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: RA patient hip replacements fell from nearly 9/1,000 person-years in 1996 to about 4.5/1,000 person-years in 2011.
Data source: Records from more than 300,000 people in the Danish National Patient Register.
Disclosures: Dr. Dreyer has received lecture fees from Merck Sharp & Dohme and UCB. Dr. Landewé has received consulting fees from several drug companies.
How to prevent secondary posttraumatic knee osteoarthritis
LAS VEGAS – A variety of evidence-based strategies are available for preventing posttraumatic knee osteoarthritis (KOA) in patients who have already sustained an anterior cruciate ligament (ACL) injury. And they’re generally ignored, according to May Arna Risberg, PhD.
“We have a lot of knowledge. We can use secondary prevention strategies. And here I think we, as physical therapists, physicians, and orthopedic surgeons, are doing a lousy job because we are sending these ACL-injured patients back to sports before they have normalized knee function and quadriceps strength,” said Dr. Risberg, professor of sports medicine at the Norwegian School of Sport Sciences in Oslo.
With no proven disease-modifying therapy for KOA available to date, secondary prevention of posttraumatic KOA is worthy of high-priority status, she said at the World Congress on Osteoarthritis. An estimated 250,00 ACL injuries occur annually in the United States, and up to one-half of affected patients, most of whom are young, active people, will experience a second ACL rupture within the first few years after undergoing their initial reconstruction. This second ACL injury greatly increases their risk of developing posttraumatic KOA within 15-20 years, while they are still relatively young, she said.
Moreover, if the second ACL injury involves meniscus surgery, the 5-year risk of posttraumatic KOA roughly triples to up to 48%.
She highlighted a few effective strategies for preventing posttraumatic KOA in patients who already have an ACL injury.
Avoid reinjury
Dr. Risberg was senior author of a recent report from the prospective Delaware-Oslo Cohort Study involving 106 athletes who underwent ACL reconstruction following injury in what she termed level I sports. These are sports that entail lots of pivoting, jumping, and hard cutting, such as basketball, soccer, and handball.
In the first 2 years after ACL repair, 30% of patients who returned to participation in a level 1 sport experienced an ACL reinjury, compared with just 8% who opted for a lower-level sport. Athletes who returned to a level 1 sport had an adjusted 4.3 times greater ACL reinjury rate than those who didn’t, Dr. Risberg noted at the congress sponsored by the Osteoarthritis Research Society International.
The good news is that this sharply increased reinjury risk was mitigated if return to a level 1 sport was delayed for at least 9 months post surgery and if the patient had regained quadriceps strength comparable to the uninjured side. For every month that return to sport was delayed out until 9 months post ACL reconstruction, the knee reinjury rate was reduced by 51% (Br J Sports Med. 2016;50:804-8).
In a meta-analysis by other investigators of 12 studies including 5,707 participants, weakness of the knee extensor muscles was independently associated with a 1.65 times increased risk of developing KOA (Osteoarthritis Cartilage. 2015 Feb;23[2]:171-7).
Attend to BMI
A discussion of the importance of maintaining a healthy body weight is an important aspect of patient education for athletes with knee injuries. In a cohort study of 988 patients who underwent primary ACL reconstruction, being overweight or obese was associated with a significantly increased risk of subsequent meniscal tears and chondral lesions (Am J Sports Med. 2015 Dec;43[12]:2966-73).
Also, it’s well established that obesity is a risk factor for knee OA, and Canadian investigators have shown that young athletes with a sports-related intra-articular knee injury were 3.75 times more likely to be overweight or obese 3-10 years post injury, compared with matched uninjured controls (Osteoarthritis Cartilage. 2015 Jul;23[7]:1122-9).
Consider prehabilitative exercise training
Dr. Risberg and coinvestigators have reported that preoperative quadriceps muscle strength deficits are predictive of impaired knee function, as measured by the Cincinnati Knee Score 2 years post surgery. She said she believes ACL reconstruction shouldn’t be done until quadriceps muscle strength is at least 80% of that in the uninjured limb (Br J Sports Med. 2009 May;43[5]:371-6). She and her coinvestigators have published the details of a 5-week progressive exercise therapy program in which they have shown results in significantly improved early postoperative knee function (J Orthop Sports Phys Ther. 2010 Nov;40[11]:705-21). They now try to have patients complete the twice-weekly, 5-week program before final decisions are reached regarding whether to have ACL reconstruction.
Test all before okaying return to sport
It’s important to know if patients who have undergone ACL reconstruction have gotten full knee function back before determining if they’re ready for full-on sports participation. In the Delaware-Oslo Cohort Study, patients who delayed their return until at least 9 months after surgery and passed the return-to-sports test had a 5.6% reinjury rate within 2 years, while those who failed the return-to-sports criteria had a 38.2% ACL reinjury rate.
The return-to-sports testing utilized in this study entailed isokinetic quadriceps strength testing, the single hop leg test, the 14-item self-rated Knee Outcome Survey–Activities of Daily Living Scale, and a self-rated Global Rating Scale of perceived function on a 0-100 scale. To be cleared for return to sports, a patient had to demonstrate having regained at least 90% of quadriceps muscle strength and hop performance along with scoring in the normative range on both of the self-rating instruments.
Surgical vs. nonsurgical treatment of ACL rupture
The evidence on this score is conflicting, according to Dr. Risberg. While most physical therapists believe ACL reconstruction doesn’t protect against later development of KOA, as reflected in a meta-analysis of published studies (J Bone Joint Surg Am. 2014 Feb 19;96[4]:292-300), a more recent retrospective comparison of 964 patients with an isolated ACL tear and an equal number of matched controls concluded that patients treated nonoperatively were six times more likely to have been diagnosed with KOA and 16.7 times more likely to have undergone total knee replacement at a mean follow-up of 13.7 years than were those treated with ACL reconstruction (Am J Sports Med. 2016 Jul;44[7]:1699-707).
Dr. Risberg’s fellow panelist Jackie Whittaker, PhD, said that, as long as quadriceps muscle strengthening is a priority, it makes sense to strengthen the hamstring as well, particularly if the ACL reconstruction utilized the hamstring tendon.
“Also, I would add that it’s important to develop a relationship with these ACL-injured people, who are often very young. Preventing a disease that they’re going to get 20 years later isn’t a priority for them. You need to develop that relationship and build it up over time. Helping them set realistic expectations is very important. And we need to do what we can to help them find some sort of competitive outlet. A lot of these kids were very competitive, and now they’ve had an injury and can’t compete. They don’t want to go back to playing just any sport. They want to be able to be competitive, and if you don’t help them find another way to express that, they sort of give up on physical activity altogether,” according to Dr. Whittaker of the University of Alberta in Edmonton.
Dr. Risberg and Dr. Whittaker reported having no financial conflicts of interest.
LAS VEGAS – A variety of evidence-based strategies are available for preventing posttraumatic knee osteoarthritis (KOA) in patients who have already sustained an anterior cruciate ligament (ACL) injury. And they’re generally ignored, according to May Arna Risberg, PhD.
“We have a lot of knowledge. We can use secondary prevention strategies. And here I think we, as physical therapists, physicians, and orthopedic surgeons, are doing a lousy job because we are sending these ACL-injured patients back to sports before they have normalized knee function and quadriceps strength,” said Dr. Risberg, professor of sports medicine at the Norwegian School of Sport Sciences in Oslo.
With no proven disease-modifying therapy for KOA available to date, secondary prevention of posttraumatic KOA is worthy of high-priority status, she said at the World Congress on Osteoarthritis. An estimated 250,00 ACL injuries occur annually in the United States, and up to one-half of affected patients, most of whom are young, active people, will experience a second ACL rupture within the first few years after undergoing their initial reconstruction. This second ACL injury greatly increases their risk of developing posttraumatic KOA within 15-20 years, while they are still relatively young, she said.
Moreover, if the second ACL injury involves meniscus surgery, the 5-year risk of posttraumatic KOA roughly triples to up to 48%.
She highlighted a few effective strategies for preventing posttraumatic KOA in patients who already have an ACL injury.
Avoid reinjury
Dr. Risberg was senior author of a recent report from the prospective Delaware-Oslo Cohort Study involving 106 athletes who underwent ACL reconstruction following injury in what she termed level I sports. These are sports that entail lots of pivoting, jumping, and hard cutting, such as basketball, soccer, and handball.
In the first 2 years after ACL repair, 30% of patients who returned to participation in a level 1 sport experienced an ACL reinjury, compared with just 8% who opted for a lower-level sport. Athletes who returned to a level 1 sport had an adjusted 4.3 times greater ACL reinjury rate than those who didn’t, Dr. Risberg noted at the congress sponsored by the Osteoarthritis Research Society International.
The good news is that this sharply increased reinjury risk was mitigated if return to a level 1 sport was delayed for at least 9 months post surgery and if the patient had regained quadriceps strength comparable to the uninjured side. For every month that return to sport was delayed out until 9 months post ACL reconstruction, the knee reinjury rate was reduced by 51% (Br J Sports Med. 2016;50:804-8).
In a meta-analysis by other investigators of 12 studies including 5,707 participants, weakness of the knee extensor muscles was independently associated with a 1.65 times increased risk of developing KOA (Osteoarthritis Cartilage. 2015 Feb;23[2]:171-7).
Attend to BMI
A discussion of the importance of maintaining a healthy body weight is an important aspect of patient education for athletes with knee injuries. In a cohort study of 988 patients who underwent primary ACL reconstruction, being overweight or obese was associated with a significantly increased risk of subsequent meniscal tears and chondral lesions (Am J Sports Med. 2015 Dec;43[12]:2966-73).
Also, it’s well established that obesity is a risk factor for knee OA, and Canadian investigators have shown that young athletes with a sports-related intra-articular knee injury were 3.75 times more likely to be overweight or obese 3-10 years post injury, compared with matched uninjured controls (Osteoarthritis Cartilage. 2015 Jul;23[7]:1122-9).
Consider prehabilitative exercise training
Dr. Risberg and coinvestigators have reported that preoperative quadriceps muscle strength deficits are predictive of impaired knee function, as measured by the Cincinnati Knee Score 2 years post surgery. She said she believes ACL reconstruction shouldn’t be done until quadriceps muscle strength is at least 80% of that in the uninjured limb (Br J Sports Med. 2009 May;43[5]:371-6). She and her coinvestigators have published the details of a 5-week progressive exercise therapy program in which they have shown results in significantly improved early postoperative knee function (J Orthop Sports Phys Ther. 2010 Nov;40[11]:705-21). They now try to have patients complete the twice-weekly, 5-week program before final decisions are reached regarding whether to have ACL reconstruction.
Test all before okaying return to sport
It’s important to know if patients who have undergone ACL reconstruction have gotten full knee function back before determining if they’re ready for full-on sports participation. In the Delaware-Oslo Cohort Study, patients who delayed their return until at least 9 months after surgery and passed the return-to-sports test had a 5.6% reinjury rate within 2 years, while those who failed the return-to-sports criteria had a 38.2% ACL reinjury rate.
The return-to-sports testing utilized in this study entailed isokinetic quadriceps strength testing, the single hop leg test, the 14-item self-rated Knee Outcome Survey–Activities of Daily Living Scale, and a self-rated Global Rating Scale of perceived function on a 0-100 scale. To be cleared for return to sports, a patient had to demonstrate having regained at least 90% of quadriceps muscle strength and hop performance along with scoring in the normative range on both of the self-rating instruments.
Surgical vs. nonsurgical treatment of ACL rupture
The evidence on this score is conflicting, according to Dr. Risberg. While most physical therapists believe ACL reconstruction doesn’t protect against later development of KOA, as reflected in a meta-analysis of published studies (J Bone Joint Surg Am. 2014 Feb 19;96[4]:292-300), a more recent retrospective comparison of 964 patients with an isolated ACL tear and an equal number of matched controls concluded that patients treated nonoperatively were six times more likely to have been diagnosed with KOA and 16.7 times more likely to have undergone total knee replacement at a mean follow-up of 13.7 years than were those treated with ACL reconstruction (Am J Sports Med. 2016 Jul;44[7]:1699-707).
Dr. Risberg’s fellow panelist Jackie Whittaker, PhD, said that, as long as quadriceps muscle strengthening is a priority, it makes sense to strengthen the hamstring as well, particularly if the ACL reconstruction utilized the hamstring tendon.
“Also, I would add that it’s important to develop a relationship with these ACL-injured people, who are often very young. Preventing a disease that they’re going to get 20 years later isn’t a priority for them. You need to develop that relationship and build it up over time. Helping them set realistic expectations is very important. And we need to do what we can to help them find some sort of competitive outlet. A lot of these kids were very competitive, and now they’ve had an injury and can’t compete. They don’t want to go back to playing just any sport. They want to be able to be competitive, and if you don’t help them find another way to express that, they sort of give up on physical activity altogether,” according to Dr. Whittaker of the University of Alberta in Edmonton.
Dr. Risberg and Dr. Whittaker reported having no financial conflicts of interest.
LAS VEGAS – A variety of evidence-based strategies are available for preventing posttraumatic knee osteoarthritis (KOA) in patients who have already sustained an anterior cruciate ligament (ACL) injury. And they’re generally ignored, according to May Arna Risberg, PhD.
“We have a lot of knowledge. We can use secondary prevention strategies. And here I think we, as physical therapists, physicians, and orthopedic surgeons, are doing a lousy job because we are sending these ACL-injured patients back to sports before they have normalized knee function and quadriceps strength,” said Dr. Risberg, professor of sports medicine at the Norwegian School of Sport Sciences in Oslo.
With no proven disease-modifying therapy for KOA available to date, secondary prevention of posttraumatic KOA is worthy of high-priority status, she said at the World Congress on Osteoarthritis. An estimated 250,00 ACL injuries occur annually in the United States, and up to one-half of affected patients, most of whom are young, active people, will experience a second ACL rupture within the first few years after undergoing their initial reconstruction. This second ACL injury greatly increases their risk of developing posttraumatic KOA within 15-20 years, while they are still relatively young, she said.
Moreover, if the second ACL injury involves meniscus surgery, the 5-year risk of posttraumatic KOA roughly triples to up to 48%.
She highlighted a few effective strategies for preventing posttraumatic KOA in patients who already have an ACL injury.
Avoid reinjury
Dr. Risberg was senior author of a recent report from the prospective Delaware-Oslo Cohort Study involving 106 athletes who underwent ACL reconstruction following injury in what she termed level I sports. These are sports that entail lots of pivoting, jumping, and hard cutting, such as basketball, soccer, and handball.
In the first 2 years after ACL repair, 30% of patients who returned to participation in a level 1 sport experienced an ACL reinjury, compared with just 8% who opted for a lower-level sport. Athletes who returned to a level 1 sport had an adjusted 4.3 times greater ACL reinjury rate than those who didn’t, Dr. Risberg noted at the congress sponsored by the Osteoarthritis Research Society International.
The good news is that this sharply increased reinjury risk was mitigated if return to a level 1 sport was delayed for at least 9 months post surgery and if the patient had regained quadriceps strength comparable to the uninjured side. For every month that return to sport was delayed out until 9 months post ACL reconstruction, the knee reinjury rate was reduced by 51% (Br J Sports Med. 2016;50:804-8).
In a meta-analysis by other investigators of 12 studies including 5,707 participants, weakness of the knee extensor muscles was independently associated with a 1.65 times increased risk of developing KOA (Osteoarthritis Cartilage. 2015 Feb;23[2]:171-7).
Attend to BMI
A discussion of the importance of maintaining a healthy body weight is an important aspect of patient education for athletes with knee injuries. In a cohort study of 988 patients who underwent primary ACL reconstruction, being overweight or obese was associated with a significantly increased risk of subsequent meniscal tears and chondral lesions (Am J Sports Med. 2015 Dec;43[12]:2966-73).
Also, it’s well established that obesity is a risk factor for knee OA, and Canadian investigators have shown that young athletes with a sports-related intra-articular knee injury were 3.75 times more likely to be overweight or obese 3-10 years post injury, compared with matched uninjured controls (Osteoarthritis Cartilage. 2015 Jul;23[7]:1122-9).
Consider prehabilitative exercise training
Dr. Risberg and coinvestigators have reported that preoperative quadriceps muscle strength deficits are predictive of impaired knee function, as measured by the Cincinnati Knee Score 2 years post surgery. She said she believes ACL reconstruction shouldn’t be done until quadriceps muscle strength is at least 80% of that in the uninjured limb (Br J Sports Med. 2009 May;43[5]:371-6). She and her coinvestigators have published the details of a 5-week progressive exercise therapy program in which they have shown results in significantly improved early postoperative knee function (J Orthop Sports Phys Ther. 2010 Nov;40[11]:705-21). They now try to have patients complete the twice-weekly, 5-week program before final decisions are reached regarding whether to have ACL reconstruction.
Test all before okaying return to sport
It’s important to know if patients who have undergone ACL reconstruction have gotten full knee function back before determining if they’re ready for full-on sports participation. In the Delaware-Oslo Cohort Study, patients who delayed their return until at least 9 months after surgery and passed the return-to-sports test had a 5.6% reinjury rate within 2 years, while those who failed the return-to-sports criteria had a 38.2% ACL reinjury rate.
The return-to-sports testing utilized in this study entailed isokinetic quadriceps strength testing, the single hop leg test, the 14-item self-rated Knee Outcome Survey–Activities of Daily Living Scale, and a self-rated Global Rating Scale of perceived function on a 0-100 scale. To be cleared for return to sports, a patient had to demonstrate having regained at least 90% of quadriceps muscle strength and hop performance along with scoring in the normative range on both of the self-rating instruments.
Surgical vs. nonsurgical treatment of ACL rupture
The evidence on this score is conflicting, according to Dr. Risberg. While most physical therapists believe ACL reconstruction doesn’t protect against later development of KOA, as reflected in a meta-analysis of published studies (J Bone Joint Surg Am. 2014 Feb 19;96[4]:292-300), a more recent retrospective comparison of 964 patients with an isolated ACL tear and an equal number of matched controls concluded that patients treated nonoperatively were six times more likely to have been diagnosed with KOA and 16.7 times more likely to have undergone total knee replacement at a mean follow-up of 13.7 years than were those treated with ACL reconstruction (Am J Sports Med. 2016 Jul;44[7]:1699-707).
Dr. Risberg’s fellow panelist Jackie Whittaker, PhD, said that, as long as quadriceps muscle strengthening is a priority, it makes sense to strengthen the hamstring as well, particularly if the ACL reconstruction utilized the hamstring tendon.
“Also, I would add that it’s important to develop a relationship with these ACL-injured people, who are often very young. Preventing a disease that they’re going to get 20 years later isn’t a priority for them. You need to develop that relationship and build it up over time. Helping them set realistic expectations is very important. And we need to do what we can to help them find some sort of competitive outlet. A lot of these kids were very competitive, and now they’ve had an injury and can’t compete. They don’t want to go back to playing just any sport. They want to be able to be competitive, and if you don’t help them find another way to express that, they sort of give up on physical activity altogether,” according to Dr. Whittaker of the University of Alberta in Edmonton.
Dr. Risberg and Dr. Whittaker reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM OARSI 2017
Difficult-to-Detect Low-Grade Infections Responsible for Poor Outcomes in Total Knee Arthroplasty
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Despite standardization of diagnostic criteria by the MSIS for the diagnosis of PJI, some low-grade inflections create a diagnostic challenge for clinicians.
- P acnes infection following TJA can be present despite patients having normal serum inflammatory marker levels and synovial fluid aspirations.
- Patients with a PJI with low virulence organisms can present with painful, arthrofibrotic joints that do not appear to be clinically infected.
- Biopsy for pathology and culture can aid in the diagnosis of suspected PJI in patients who fail to meet MSIS criteria.
- If detected and accurately diagnosed, PJI with P acnes can be successfully eradicated with IV antibiotics and 2-stage revision arthroplasty with a good functional outcome.
Total joint arthroplasty (TJA) is a routinely performed, highly efficacious procedure for patients with degenerative osteoarthritis.1,2 In the United States in 2003, more than 450,000 total knee arthroplasties (TKAs) were performed, and this number is projected to increase by more than 673% by 2030, as America’s population continues to age.3 With the increase in primary TJAs has come an increase in revision TJAs. The most common cause of revision TJA is infection (25.2%), which has a rate of 1% to 4% after primary TJA.1,4 Despite advancements in implant technology, preoperative preventive strategies, perioperative techniques, and postoperative management, a recent meta-analysis of patient follow-up data revealed that 15% to 20% of patients remained dissatisfied after TJA, despite having technically well-placed implants.5,6
Recent studies have suggested that prosthetic joint infection (PJI) may be underreported because of the difficulty in diagnosis, which may be one of the reasons why patients remain dissatisfied after TJA.7 As a result, new efforts have been made to develop uniform criteria for PJI diagnosis.8 In 2011, the Musculoskeletal Infection Society (MSIS) developed a new definition for the PJI diagnosis, based on clinical and laboratory criteria, in order to increase diagnostic accuracy. However, MSIS acknowledged that PJI may be present even if these criteria are not met, particularly in the case of low-grade infections, as patients may not present with clinical signs of infection and may have normal inflammatory markers and joint aspirates. The biofilm-forming bacteria Propionibacterium acnes and Staphylococcus epidermidis are 2 such low-virulence organisms—once commonly considered contaminants but now recognized as potential pathogens for postoperative joint infections.9 In a review performed at a major orthopedic hospital, Bjerke-Kroll and colleagues10 found that the rate of PJI with P acnes has been increasing linearly over the past 14 years. According to reports in the literature,11-13P acnes has been isolated in 2% to 4% of all cases of PJI, and Zappe and colleagues13 found a P acnes PJI rate of 6% in a retrospective analysis performed at their institution. Given the high rate of P acnes colonization of the axilla, this organism is now increasingly recognized as a cause of infection after shoulder surgery, as found in a case series of 10 patients with P acnes PJI after total shoulder arthroplasty (TSA).14 However, there is still limited data on the role of P acnes in lower extremity PJI.
Although patients with P acnes PJI can present with overt signs of infection, more often they lack systemic or local signs of infection, making the diagnosis difficult.15 Surgeons may not consider PJI as a cause of TJA failure in patients who do not meet diagnostic criteria.7 In a case series of patients with P acnes PJI after TSA, Millett and colleagues14 concluded that erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are not always reliable indicators of infection with low-virulence organisms. Eighty percent of patients in their study had normal ESR and CRP level before surgery. Zappe and colleagues13 reported on P acnes PJI diagnoses in 4 total hip arthroplasties (THAs), 3 TKAs, and 1 TSA. Of the 8 patients, 6 (75%) had borderline elevated CRP levels, and 4 (50%) had normal synovial fluid analysis and cultures from joint aspirations. In a study using electron microscopy and fluorescence in situ hybridization (FISH) labeling, Stoodley and colleagues16 found, in 8 polyethylene liners removed from culture-negative THA patients for aseptic loosening, extensive biofilm colonization with S epidermidis.
Reports of PJI cases misdiagnosed as aseptic loosening also suggest that screening and diagnostic tools are not sensitive enough to detect all infections and that PJI likely is underdiagnosed. In a prospective cohort study, Portillo and colleagues17 categorized patients who were undergoing revision surgery after TJA by cause of failure: aseptic loosening, mechanical failure, or PJI based on current MSIS guidelines. Intraoperative cultures were taken during the revisions. P acnes was isolated in 2 (3%) of the 63 cases classified as PJI and in 12 (19%) of the 63 classified as aseptic loosening. Tsukayama and colleagues18 reported an 11% rate of positive intraoperative cultures for P acnes during revision surgery in cases that the operating surgeon considered aseptic, based on white blood cell (WBC) count, ESR, and CRP level. Rasouli and colleagues19 used an Ibis biosensor to perform polymerase chain reaction (PCR) on synovial fluid from 44 patients who underwent aseptic revision of TKA failures. The authors detected a pathogen in 17 (38%) of the 44 presumed aseptic patients and concluded some aseptic loosening cases are actually chronic low-grade organism PJIs not diagnosed according to current PJI criteria.
In this article, we present the case of a patient with a stiff, painful knee after TKA and with ESR, CRP level, and synovial fluid analysis within normal limits. Open biopsy for cultures showed P acnes PJI, which was successfully treated with 2-stage revision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 69-year-old man with a past medical history of hypertension underwent left primary TKA in 2012. In 2014, he presented to our office complaining of chronic left knee pain and stiffness that had developed insidiously over the first 3 months after surgery and never improved, despite rigorous physical therapy (Table).
Despite not meeting MSIS diagnostic criteria, the patient elected to undergo open biopsy for synovial culture as a last resort. During surgery, there was no purulence in the joint, and frozen section showed <5 neutrophils per high-power field. All cultures from 5 separate synovial tissue samples grew P acnes, confirming the PJI diagnosis. Cultures turned positive after being incubated an average of 12.2 days (range, 10-14 days). Sensitivities showed the organism was responsive to oxacillin. The risks and benefits of 2-stage revision surgery were discussed with the patient at the next office visit, and he decided on 2-stage revision. On November 4, 2014, he underwent open synovectomy, irrigation and débridement with iodine and Dakin solution, hardware removal, and cement antibiotic spacer placement without complication (Figures 3A, 3B).
Just before stage 2 revision on January 6, 2015, preoperative inflammatory markers were within normal limits. During surgery, additional cultures were taken from synovial tissue. At 15 days, these cultures showed no growth, confirming eradication of the infection. The patient underwent reimplantation without complication and had an uneventful postoperative course with no wound-healing issues (Figures 4A, 4B).
Discussion
Because PJIs with low-virulence organisms can present with normal levels of inflammatory markers and negative fluid analysis and culture from joint aspirations, they pose a diagnostic challenge for arthroplasty surgeons. In this case report, there was a low index of suspicion for PJI based on radiographic, physical examination, and laboratory findings. Our patient did not meet MSIS diagnostic criteria for PJI before undergoing open biopsy. Initial cultures from joint aspiration of synovial fluid were negative, and inflammatory markers were within normal limits. However, all 5 synovial tissue biopsy specimens that were cultured confirmed a low-grade periprosthetic infection with P acnes—likely the reason for the poor outcome. This case supports Zappe and colleagues13 and Millett and colleagues,14 who found that a subset of patients with a low-grade organism PJI had normal to mildly elevated inflammatory markers and negative fluid analysis and cultures from joint aspirations.
Hardware-involved orthopedic infections are often caused by bacteria that form a biofilm, which can be difficult to culture. Biofilm matrix binds cells into aggregates, which grow only a single colony on culture media, decreasing positive yield. Therefore, synovial fluid cultures are often negative, because of the low number of planktonic cells removed by aspirate. Using FISH and PCR, Stoodley and colleagues16 found biofilm on hardware removed for “culture-negative aseptic loosening.” This is especially important for low-grade organism infections that lack a strong inflammatory response in the joint and that may be missed with traditional screening. This may be one reason our patient’s synovial fluid cultures and inflammatory markers were negative.
Another reason these low-grade infections can be missed is that P acnes is notoriously difficult to culture—it may take up to 15 days to grow in a special medium.20 Intraoperative cultures may be read as false-negative if not incubated the right amount of time. In many hospitals, aerobic and anaerobic cultures are discarded if there is no growth after 3 to 5 days. In our patient’s case, the earliest that cultures turned positive was on day 10—which is consistent with other reports, including one by Butler-Wu and colleagues,15 who suggested a minimum incubation of 13 days for optimal recovery of organisms. Our case highlights the importance of lengthening incubation to allow for growth of low-virulent organisms. Given the different types of management used for PJI and aseptic loosening, it is imperative that surgeons take cultures during revision TJA and that cultures are held up to 14 days to allow enough time for low-virulence organisms to grow.
Fortunately, PJI with low-virulence organisms can be treated successfully. Treating P acnes PJI with exchange arthroplasty and IV antibiotics has documented success rates as high as 92%.21 Again, we emphasize the importance of obtaining intraoperative cultures to determine antibiotic sensitivities, which can guide treatment. Our patient’s infection was eradicated with 2-stage revision and IV antibiotics, and his symptoms, ROM, and function improved significantly.
Diagnosing PJI after TJA can be challenging, as there is no definitive test that is sensitive, specific, rapid, and minimally invasive. Researchers have looked for novel serum or synovial fluid biomarkers that may be elevated in PJI. Synovial interleukin 6 (IL-6) and synovial α-defensin show great promise. In 2 separate studies, elevated IL-6 levels strongly correlated with infection.22,23 Jacovides and colleagues23 found that a synovial IL-6 level higher than 4270 pg/mL had a 100% positive predictive value and a 91% negative predictive value for diagnosing PJI. In some trials, synovial α-defensin has shown up to 100% sensitivity and specificity for PJI diagnosis. Most notably, in a trial by Frangiamore and colleagues,24 α-defensin levels were elevated to statistically significant levels in P acnes PJI, indicating this test may help in diagnosing PJI with low-virulence organisms. Finally, PCR has also shown promise in detecting low-grade joint infections. PCR uses 16 primers that allow not only for the identification of pan-genomic bacterial markers, specific bacterial organisms, and Candida, but also for the presence of antibiotic resistance markers. Use of pan-genomic PCR also allows for detection of a wider variety of pathogens, including organisms commonly missed by conventional culture methods.25Early intervention can significantly improve outcomes in PJI. Therefore, we recommend maintaining a high index of suspicion for low-virulence PJI in patients with chronic pain and decreased functionality after TJA with well-placed implants, despite their not meeting current MSIS diagnostic criteria for PJI. As new microbiological tools for detecting PJI with low-grade organisms are developed, use of these technologies can be incorporated into the diagnosis algorithm. Screening tools more sensitive in detecting low-grade organisms can help avoid the morbidity associated with interoperative synovial biopsies for culture and can allow for more efficient surgical planning. These tools, along with increased clinical awareness of potential PJIs, ultimately will lead to earlier detection, accurate diagnosis, and optimal treatment.
Am J Orthop. 2017;46(3):E148-E153. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
1. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
2. Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497.
3. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
4. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589-1593.
5. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
6. Djahani O, Rainer S, Pietsch M, Hofmann S. Systematic analysis of painful total knee prosthesis, a diagnostic algorithm. Arch Bone Jt Surg. 2013;1(2):48-52.
7. Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-1405.
8. Della Valle C, Parvizi J, Bauer TW, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760-770.
9. Dramis A, Aldlyami E, Grimer RJ, Dunlop DJ, O’Connell N, Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33(3):829-833.
10. Bjerke-Kroll BT, Christ AB, Mclawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014;29(5):877-882.
11. Pandey R, Berendt AR, Athanasou NA. Histological and microbiological findings in non-infected and infected revision arthroplasty tissues. The OSIRIS Collaborative Study Group. Oxford Skeletal Infection Research and Intervention Service. Arch Orthop Trauma Surg. 2000;120(10):570-574.
12. Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81(10):1434-1445.
13. Zappe B, Graf S, Ochsner PE, Zimmerli W, Sendi P. Propionibacterium spp. in prosthetic joint infections: a diagnostic challenge. Arch Orthop Trauma Surg. 2008;128(10):1039-1046.
14. Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824-2830.
15. Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490-2495.
16. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563.
17. Portillo ME, Salvadó M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res. 2013;471(11):3672-3678.
18. Tsukayama DT, Strada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512-523.
19. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
20. Schäfer P, Fink B, Sandow D, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Dis. 2008;47(11):1403-1409.
21. Zeller V, Ghorbani A, Strady C, Leonard P, Mamoudy P, Desplaces N. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J Infect. 2007;55(2):119-124.
22. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
23. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
24. Frangiamore SJ, Gajewski ND, Saleh A, Farias-Kovac M, Barsoum WK, Higuera CA. α-Defensin accuracy to diagnose periprosthetic joint infection—best available test? J Arthroplasty. 2016;31(2):456-460.
25. Hartley JC, Harris KA. Molecular techniques for diagnosing prosthetic joint infections. J Antimicrob Chemother. 2014;69(suppl 1):i21-i24.
Primary Total Knee Arthroplasty for Distal Femur Fractures: A Systematic Review of Indications, Implants, Techniques, and Results
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Arthroplasty is a rarely utilized and, therefore, a rarely reported treatment for distal femur fractures.
- Arthroplasty carries certain advantages over fixation, including earlier weight-bearing, a benefit for elderly individuals.
- Arthroplasty is more often described in situations of comminution, often necessitating constrained prostheses.
- It is not unreasonable to utilize arthroplasty in extra-articular fractures in poor-quality bone, which can take the form of unconstrained prosthesis and supplemental fixation.
- The true complication rate is unclear, given that the few papers reporting high complication rates were in sicker populations.
Distal femur fractures (DFFs) in the elderly historically were difficult to treat because of osteoporotic bone, comminution, and intra-articular involvement. DFFs in minimally ambulatory patients were once treated nonoperatively, with traction or immobilization,1,2 but surgery is now considered for displaced and unstable fractures, even in myelopathic and nonambulatory patients, to provide pain relief, ease mobility, and decrease the risks associated with prolonged bed rest.1 Options are constantly evolving, but poor knee function, malunion, nonunion, prolonged immobilization, implant failure, and high morbidity and mortality rates have been reported in several studies regardless of fixation method.
Arthritis after DFF has been reported at rates of 36% to 50% by long-term follow-up.3-5 However, total knee arthroplasty (TKA) for posttraumatic arthritis is more complex because of scarring, arthrofibrosis, malunion, nonunion, and the frequent need for hardware removal. These cases have a higher incidence of infection, aseptic loosening, stiffness,6 and skin necrosis.7 Primary TKA is a rarely used treatment for acute DFF. Several authors have recommended primary TKA for patients with intra-articular DFFs and preexisting osteoarthritis or rheumatoid arthritis, severe comminution, or poor bone stock.7-22 Compared with open reduction and internal fixation (ORIF), primary TKA may allow for earlier mobility and weight-bearing and thereby reduce the rates of complications (eg, respiratory failure, deep vein thrombosis, pulmonary embolism) associated with prolonged immobilization.23As the literature on TKA for acute DFF is scant, and to our knowledge there are no clear indications or guidelines, we performed a systematic review to determine whether TKA has been successful in relieving pain and restoring knee function. In this article, we discuss the indications, implant options, technical considerations, complications, and results (eg, range of motion [ROM], ambulatory status) associated with these procedures.
Methods
On December 1, 2015, we searched the major databases Medline, EMBASE (Excerpta Medica dataBASE), and the Cochrane Library for articles published since 1950. In our searches, we used the conjoint term knee arthroplasty with femur fracture, and knee replacement with femur fracture. Specifically, we queried: ((“knee replacement” OR “knee arthroplasty”) AND (intercondylar OR supracondylar OR femoral OR femur) AND fracture) NOT arthrodesis NOT periprosthetic NOT “posttraumatic arthritis” NOT osteotomy. We also hand-searched the current website of JBJS [Journal of Bone and Joint Surgery] Case Connector, a major case-report repository that was launched in 2011 but is not currently indexed by Medline.
All citations were imported to RefWorks for management and for removal of duplicates. Each article underwent screening and review by Dr. Chen and Dr. Li. Articles were included if titles were relevant to arthroplasty as treatment for acute (within 1 month) DFF. Articles and cases were excluded if they were reviews, published in languages other than English, animal studies, studies regarding nonacute (>3 months or nonunion) DFFs or periprosthetic fractures, or studies that considered only treatments other than TKA (ie, plate osteosynthesis).
Full-text publications were obtained and independently reviewed by Dr. Chen and Dr. Li for relevance and satisfaction of inclusion criteria. Disagreements were resolved by discussion. Given the rarity of publications on the treatment, all study designs from level I to level IV were included.
The same 2 reviewers extracted the data into prearranged summary tables. Data included study size, patient demographics, AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) fracture type either reported or assessed by description and imaging (33A, extra-articular; 33B, partial articular with 1 intact condyle; 33C, complete articular with both condyles involved), baseline comorbidity, implant used and fracture treatment (if separate from arthroplasty), postoperative regimen, respective outcomes, and complication rates.
Results
We identified 728 articles: 389 through Medline, 294 through EMBASE, and 45 through the Cochrane Library (Figure 1).
The current evidence regarding primary TKA for acute DFF is primarily level IV (Table 1). Only 1 level III study16 compared TKA with ORIF. Three case series11,19,24 met our inclusion criteria (Table 1, Table 2). In addition, 5 case series involved patients who met our criteria, but these studies did not separately report results for DFFs and proximal tibia fractures,9,20-22 or separately for acute fractures and nonunions or ORIF failures.8
Modular, hinged, and tumor-type arthroplasty designs accounted for 83% of the treatments included in this review. Trade names are listed in Table 4. Authors who used these implants took a more aggressive approach, often resecting the entire femoral epiphyseal-metaphyseal area, menisci, and collateral ligaments.9,13,15,16,18 The majority of patients who underwent resection had 33C fractures (Tables 1, 3).
The majority of authors who treated fractures with resection and modular implants allowed their patients full weight-bearing soon after surgery (Table 1),11,12,15-18,24 whereas authors who treated their patients partly with fracture fixation often had to delay weight-bearing (Table 1).
Cement use was universally described in the literature. Some authors avoided placing cement in the fracture site (to reduce the risk of nonunion),7,19 whereas others used bone cement to fill metaphyseal defects that remained after fracture resection and implantation.11,24Complication rates were modest, and there were no reports specifically on implant loosening or fracture nonunion.7,10,12-19 The majority of complications were recorded in 2 studies that used megaprostheses in sicker populations: Bell and colleagues11 noted debilitating illnesses in all their patients, and Appleton and colleagues24 included 9 nonambulatory patients and 36 patients who required 2 assistants to ambulate. All deaths were attributed to medical comorbidities and disseminated malignancy. Contrarily, studies by Pearse and colleagues16 and Choi and colleagues19 included previously ambulatory patients and reported no deaths or complications (Table 2). Likewise, in studies that combined results of DFFs and proximal tibia fractures, death and complication rates varied from 7% to 31% (Table 3).
Discussion
DFFs in the elderly historically were difficult to treat. Reported outcomes are largely favorable, but, even with newer plate designs, catastrophic failures still occur in the absence of bony union.26,27 After ORIF, patients’ weight-bearing is often restricted for 12 weeks or longer28—a protocol that is undesirable in elderly patients, especially given that the rate of mortality 1 year after these fractures has been found to be as high as 25%.29
Arthroplasty for DFFs—performed either with ORIF, or independently with a constrained implant—is a documented treatment modality, but the evidence is poor, and results have been mixed. Patients who received hinged TKA with major fracture resection had higher complication rates.8,11,22,24 However, the problems were mostly medical, not associated with surgical technique. Appleton and colleagues24 found a higher than expected 1-year mortality rate, 41%, but used an unhealthy baseline population (44% cognitive impairment, 17% nonambulatory before injury).Although Boureau and colleagues22 found a 1-year mortality rate of 30%, only 1 in 10 deaths was attributable to a perioperative complication. Among the remaining cases involving resection and megaprostheses for previously ambulatory patients, only 1 perioperative death was recorded (Table 2).11,12,16,18 Therefore, the risks associated with patients’ baseline health and ambulatory status must be weighed against the benefits of aggressive arthroplasty.
An overwhelming majority of 33C fractures were treated with megaprostheses—a finding perhaps attributable to the higher likelihood that patients with osteoporosis have intra-articular, comminuted injuries. In addition, surgeons may have been more likely to indicate 33C fractures for joint replacement, whereas 33A and 33B patterns were more amenable to fracture fixation.17,18 Interestingly, few type B fractures (0 in primary analysis and only 9 of 67 cases in Table 3) were treated with megaprostheses. In these situations, 1 condyle and ligamentous constraint remain intact, reducing the need for a constrained implant.
There were no reports of atraumatic or aseptic loosening, though use of rotating platforms with linked prostheses helps minimize this complication. Also surprising is the lack of nonunions in any of the reviewed studies, as nonunion is one of the most devastating complications of ORIF. Only 1 superficial and 2 deep infections were reported in all of the literature—representing 1.8% of all cases, which is comparable to the rate for elective primary TKA.30In elderly patients with significant comorbidities, the main surgical goals are to minimize operative time and reduce time to mobility. It is therefore imperative to keep in mind that arthroplasty is elective. However, functional results of primary TKA for DFF may be more encouraging for healthier patients, as many can achieve satisfactory ROM and early weight-bearing. Therefore, TKA for DFF may benefit healthy and ambulatory patients in the setting of intra-articular comminution. Whether this treatment affects mortality rates remains to be seen.
There were several limitations to this study. First, the literature on the topic is scant. Second, exclusion criteria were kept lax to allow for inclusion of all treatments. This came at a cost to internal validity, given the heterogeneous population and differences in comorbidities between studies. Fracture classification was inconsistent as well: Although AO/OTA classification was dominant, descriptive classifications were used in several cases7,10,12 (these descriptions, however, were sufficient for assigning equivalent AO/OTA classes). Details on preoperative functional status and comorbidity status and on postoperative protocols were also limited, though ROM and ambulatory status were provided in most studies. Last, most of these studies were single case reports or case series, so there may be reporting bias in the body of the literature, as reflected in the discrepancies between encouraging case reports and concerning case series with longer follow-up. Such bias can be avoided with larger, controlled sampling and adequate follow-up.
TKA should be considered for acute DFF in patients who have knee arthritis and are able to tolerate the physiological load of the surgery. In the choice of implant design, several factors should be considered, including bone quality, articular involvement, degree of comminution, and ligamentous injury. Unconstrained knee designs should be considered in cases in which the fracture pattern appears stable and the collateral ligaments are intact (eg, 33A and 33BB fractures). Megaprostheses, which may allow for immediate weight-bearing but require considerable bone resection, would be beneficial in 33C fractures and in fractures with ligamentous compromise. However, their complication rates are unclear, and comparative studies are needed to investigate whether the rates are higher for these patients than for patients treated more traditionally.
Am J Orthop. 2017;46(3):E163-E171. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
1. Cass J, Sems SA. Operative versus nonoperative management of distal femur fracture in myelopathic, nonambulatory patients. Orthopedics. 2008;31(11):1091.
2. Eichenholtz SN. Management of long-bone fracture in paraplegic patients. J Bone Joint Surg Am. 1963;45(2):299-310.
3. Thomson AB, Driver R, Kregor PJ, Obremskey WT. Long-term functional outcomes after intra-articular distal femur fractures: ORIF versus retrograde intramedullary nailing. Orthopedics. 2008;31(8):748-750.
4. Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213-219.
5. Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22(1):20-28.
6. Papadopoulos EC, Parvizi J, Lai CH, Lewallen DG. Total knee arthroplasty following prior distal femoral fracture. Knee. 2002;9(4):267-274.
7. Yoshino N, Takai S, Watanabe Y, Fujiwara H, Ohshima Y, Hirasawa Y. Primary total knee arthroplasty for supracondylar/condylar femoral fracture in osteoarthritic knees. J Arthroplasty. 2001;16(4):471-475.
8. Rosen AL, Strauss E. Primary total knee arthroplasty for complex distal femur fractures in elderly patients. Clin Orthop Relat Res. 2004;(425):101-105.
9. Malviya A, Reed MR, Partington PF. Acute primary total knee arthroplasty for peri-articular knee fractures in patients over 65 years of age. Injury. 2011;42(11):1368-1371.
10. Wolfgang GL. Primary total knee arthroplasty for intercondylar fracture of the femur in a rheumatoid arthritic patient. A case report. Clin Orthop Relat Res. 1982;(171):80-82.
11. Bell KM, Johnstone AJ, Court-Brown CM, Hughes SP. Primary knee arthroplasty for distal femoral fractures in elderly patients. J Bone Joint Surg Br. 1992;74(3):400-402.
12. Shah A, Asirvatham R, Sudlow RA. Primary resection total knee arthroplasty for complicated fracture of the distal femur with an arthritic knee joint. Contemp Orthop. 1993;26(5):463-467.
13. Freedman EL, Hak DJ, Johnson EE, Eckardt JJ. Total knee replacement including a modular distal femoral component in elderly patients with acute fracture or nonunion. J Orthop Trauma. 1995;9(3):231-237.
14. Patterson RH, Earll M. Repair of supracondylar femur fracture and unilateral knee replacement at the same surgery. J Orthop Trauma. 1999;13(5):388-390.
15. Nau T, Pflegerl E, Erhart J, Vecsei V. Primary total knee arthroplasty for periarticular fractures. J Arthroplasty. 2003;18(8):968-971.
16. Pearse EO, Klass B, Bendall SP, Railton GT. Stanmore total knee replacement versus internal fixation for supracondylar fractures of the distal femur in elderly patients. Injury. 2005;36(1):163-168.
17. Mounasamy V, Ma SY, Schoderbek RJ, Mihalko WM, Saleh KJ, Brown TE. Primary total knee arthroplasty with condylar allograft and MCL reconstruction for a comminuted medial condyle fracture in an arthritic knee—a case report. Knee. 2006;13(5):400-403.
18. Mounasamy V, Cui Q, Brown TE, Saleh KJ, Mihalko WM. Primary total knee arthroplasty for a complex distal femur fracture in the elderly: a case report. Eur J Orthop Surg Traumatol. 2007;17(5):491-494.
19. Choi NY, Sohn JM, Cho SG, Kim SC, In Y. Primary total knee arthroplasty for simple distal femoral fractures in elderly patients with knee osteoarthritis. Knee Surg Relat Res. 2013;25(3):141-146.
20. Parratte S, Bonnevialle P, Pietu G, Saragaglia D, Cherrier B, Lafosse JM. Primary total knee arthroplasty in the management of epiphyseal fracture around the knee. Orthop Traumatol Surg Res. 2011;97(6 suppl):S87-S94.
21. Benazzo F, Rossi SM, Ghiara M, Zanardi A, Perticarini L, Combi A. Total knee replacement in acute and chronic traumatic events. Injury. 2014;45(suppl 6):S98-S104.
22. Boureau F, Benad K, Putman S, Dereudre G, Kern G, Chantelot C. Does primary total knee arthroplasty for acute knee joint fracture maintain autonomy in the elderly? A retrospective study of 21 cases. Orthop Traumatol Surg Res. 2015;101(8):947-951.
23. Bishop JA, Suarez P, Diponio L, Ota D, Curtin CM. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: an administrative analysis of risks. Arch Phys Med Rehabil. 2013;94(12):2357-2364.
24. Appleton P, Moran M, Houshian S, Robinson CM. Distal femoral fractures treated by hinged total knee replacement in elderly patients. J Bone Joint Surg Br. 2006;88(8):1065-1070.
25. In Y, Koh HS, Kim SJ. Cruciate-retaining stemmed total knee arthroplasty for supracondylar-intercondylar femoral fractures in elderly patients: a report of three cases. J Arthroplasty. 2006;21(7):1074-1079.
26. Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18(8):509-520.
27. Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur. A report of six cases. J Bone Joint Surg Am. 2006;88(4):846-853.
28. Gwathmey FW Jr, Jones-Quaidoo SM, Kahler D, Hurwitz S, Cui Q. Distal femoral fractures: current concepts. J Am Acad Orthop Surg. 2010;18(10):597-607.
29. Streubel PN, Ricci WM, Wong A, Gardner MJ. Mortality after distal femur fractures in elderly patients. Clin Orthop Relat Res. 2011;469(4):1188-1196.
30. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;(392):15-23.
Think twice before recommending partial meniscectomy
LAS VEGAS – Patients with knee osteoarthritis (OA) and a meniscal tear who underwent arthroscopic partial meniscectomy (APM) subsequently experienced accelerated structural progression of their OA, compared with those randomized to physical therapy alone in the randomized METEOR trial.
“In discussing treatment options for symptomatic meniscal tear, patients and providers must weigh the potential benefits and risks of arthroscopic partial meniscectomy, including this increased risk of structural progression,” Jamie E. Collins, PhD, said at the World Congress on Osteoarthritis.
A thorough physician-patient discussion also needs to mention that several large randomized trials have suggested that patients with meniscal tear and osteoarthritic changes experience similar pain relief with APM plus physical therapy (PT) as compared with PT alone, she added at the congress sponsored by the Osteoarthritis Research Society International.
The METEOR trial was a seven-center U.S. randomized trial of APM plus PT or PT alone in patients with MRI or radiographic evidence of OA changes, a meniscal tear on MRI, and mechanical knee symptoms. Dr. Collins reported on 176 randomized patients with baseline and 18-month follow-up MRIs read by a specialist musculoskeletal radiologist. This analysis excluded the 37 patients in the PT group who crossed over to APM within 6 months after randomization.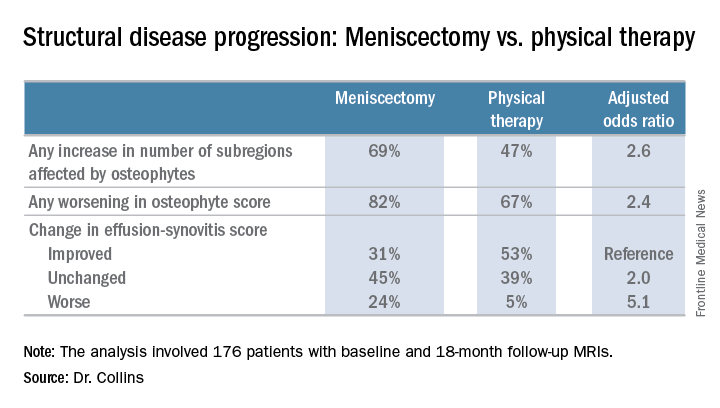
There was no significant difference between the two treatment groups in changes in bone marrow lesions or Hoffa-synovitis.
A secondary analysis that incorporated the 37 crossovers from PT to APM within 6 months showed similarly accelerated OA progression after arthroscopic surgery.
“Future work should focus on determining whether this accelerated structural progression is associated with changes in symptoms and ultimately with a higher risk of total knee replacement. In other words, is 2+ structural worsening something that’s noticeable or important for patients?” Dr. Collins concluded.
She reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
LAS VEGAS – Patients with knee osteoarthritis (OA) and a meniscal tear who underwent arthroscopic partial meniscectomy (APM) subsequently experienced accelerated structural progression of their OA, compared with those randomized to physical therapy alone in the randomized METEOR trial.
“In discussing treatment options for symptomatic meniscal tear, patients and providers must weigh the potential benefits and risks of arthroscopic partial meniscectomy, including this increased risk of structural progression,” Jamie E. Collins, PhD, said at the World Congress on Osteoarthritis.
A thorough physician-patient discussion also needs to mention that several large randomized trials have suggested that patients with meniscal tear and osteoarthritic changes experience similar pain relief with APM plus physical therapy (PT) as compared with PT alone, she added at the congress sponsored by the Osteoarthritis Research Society International.
The METEOR trial was a seven-center U.S. randomized trial of APM plus PT or PT alone in patients with MRI or radiographic evidence of OA changes, a meniscal tear on MRI, and mechanical knee symptoms. Dr. Collins reported on 176 randomized patients with baseline and 18-month follow-up MRIs read by a specialist musculoskeletal radiologist. This analysis excluded the 37 patients in the PT group who crossed over to APM within 6 months after randomization.
There was no significant difference between the two treatment groups in changes in bone marrow lesions or Hoffa-synovitis.
A secondary analysis that incorporated the 37 crossovers from PT to APM within 6 months showed similarly accelerated OA progression after arthroscopic surgery.
“Future work should focus on determining whether this accelerated structural progression is associated with changes in symptoms and ultimately with a higher risk of total knee replacement. In other words, is 2+ structural worsening something that’s noticeable or important for patients?” Dr. Collins concluded.
She reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
LAS VEGAS – Patients with knee osteoarthritis (OA) and a meniscal tear who underwent arthroscopic partial meniscectomy (APM) subsequently experienced accelerated structural progression of their OA, compared with those randomized to physical therapy alone in the randomized METEOR trial.
“In discussing treatment options for symptomatic meniscal tear, patients and providers must weigh the potential benefits and risks of arthroscopic partial meniscectomy, including this increased risk of structural progression,” Jamie E. Collins, PhD, said at the World Congress on Osteoarthritis.
A thorough physician-patient discussion also needs to mention that several large randomized trials have suggested that patients with meniscal tear and osteoarthritic changes experience similar pain relief with APM plus physical therapy (PT) as compared with PT alone, she added at the congress sponsored by the Osteoarthritis Research Society International.
The METEOR trial was a seven-center U.S. randomized trial of APM plus PT or PT alone in patients with MRI or radiographic evidence of OA changes, a meniscal tear on MRI, and mechanical knee symptoms. Dr. Collins reported on 176 randomized patients with baseline and 18-month follow-up MRIs read by a specialist musculoskeletal radiologist. This analysis excluded the 37 patients in the PT group who crossed over to APM within 6 months after randomization.
There was no significant difference between the two treatment groups in changes in bone marrow lesions or Hoffa-synovitis.
A secondary analysis that incorporated the 37 crossovers from PT to APM within 6 months showed similarly accelerated OA progression after arthroscopic surgery.
“Future work should focus on determining whether this accelerated structural progression is associated with changes in symptoms and ultimately with a higher risk of total knee replacement. In other words, is 2+ structural worsening something that’s noticeable or important for patients?” Dr. Collins concluded.
She reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
AT OARSI 2017
Key clinical point:
Major finding: Surgically treated patients were 2.6 times more likely to demonstrate MRI evidence of structural disease progression at 18 months than those who received physical therapy alone.
Data source: This analysis from the multicenter METEOR trial included 176 patients with a symptomatic meniscal tear.
Disclosures: The presenter reported having no financial conflicts regarding her study, which was sponsored by Brigham and Women’s Hospital.
For bone and joint infections, oral antibiotics match IV, cost less
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
VIENNA – Oral antibiotic therapy is just as effective as intravenous treatment in curing bone and joint infections, but costs about $3,500 less.
Treating these infections with oral agents also “improves patient autonomy, as it’s not necessary to have IV lines at home,” and represents a generally wiser use of powerful antibiotics, Matthew Scarborough, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
OVIVA (Oral vs. Intravenous Antibiotics for Bone and Joint Infection) was conducted at 26 sites in the United Kingdom. It randomized 1,054 adults with bone or joint infections to 6 weeks of either oral or intravenous treatment.
An important aspect of the trial was that both oral and IV treatment choices were made before randomization, Dr. Scarborough said. However, the decisions on what drug to use were left up to the treating physician and depended on the infection site and pathogen.
The primary outcome was definite treatment failure (bacteriologic, histologic, and clinical). Patients were followed for 1 year.
Patients were a median of 60 years old. All had surgical treatment before antibiotic therapy, including debridement and, in those with implants, removal of infected devices. The lower limb was involved in 81%, including hip, knee, and foot. The infection was in an upper limb in 10% and in the spine in 7%.
Staphylococcus aureus was present in 38% of cases, coagulase-negative staphylococci in 27%, and streptococci in 15%. Gram-negative bacteria were found in 22%.
For those patients randomized to IV therapy, glycopeptides and cephalosporins were most commonly employed (41% and 33%, respectively). For oral therapy, quinolones and penicillins were most common (37% and 16%). Most patients (74%) continued antibiotic treatment for more than 6 weeks. Forty patients were lost to follow-up.
In the primary intent-to-treat analysis, the failure rate was 13% for oral therapy and 14% for IV therapy, not a significant difference. Results were similar in the other analyses, including a modified intent to treat with only patients who had complete 1-year data, and a per-protocol analysis. All of the point prevalence numbers favored oral therapy, but crossed the null. Curves in the time-to-treatment-failure analysis were virtually superimposable, as were curves in time to discontinuation of therapy.
Another subgroup analysis examined treatment failure by infective organism; again, there were no significant treatment differences in any of the pathogen subgroups examined (S. aureus, coagulase-negative staph, streptococci species, and other gram-negative bacteria).
Nor did the type of antibiotic significantly affect failure rate, Dr. Scarborough noted. The median length of stay was 14 days for patients on IV treatment and 11 days for those taking oral medications. The incidence of serious adverse events was very similar – about 86% in each group.
On a visual analog scale that assessed health-related quality of life, patients taking oral treatment reported better mobility, self-care, and activity level, and less pain, discomfort, anxiety, and depression than those taking IV medications.
Cost represented the other significant difference between the groups. Over 1 year, the mean IV treatment cost was the equivalent of $17,152, and the mean oral treatment cost was $13,611 – a significant difference of $3,541.
“This represents a potential savings to the National Health Service of 16-25 million pounds sterling ($20.6 million-$32.3 million) per year,” Dr. Scarborough said. “All coming at no expense of good clinical outcomes.”
OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: At 1 year, cure rates were identical, but oral treatment cost about $3,500 less than IV treatment.
Data source: The study randomized 1,054 patients.
Disclosures: OVIVA was sponsored by the U.K. National Institute of Health Research. Dr. Scarborough had no financial disclosures.
Arthroscopic Excision of Bipartite Patella With Preservation of Lateral Retinaculum in an Adolescent Ice Hockey Player
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Joint-Preserving Osteotomies for Isolated Patellofemoral Osteoarthritis: Alternatives to Arthroplasty
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patellofemoral osteotomies can provide excellent and reliable symptomatic relief for many patients with symptomatic isolated PFOA.
- PLPF of 1 cm to 1.5 cm of lateral bone can provide excellent pain relief in patients with isolated lateral facet arthritis and overhanging osteophytes without diffuse chondromalacia or hypermobility.
- At 5-year follow-up, >80% of partial lateral facetectomy patients have symptomatic relief.
- Tibial tubercle AMZ (Fulkerson procedure) can provide excellent results in patients with distal and lateral patella chondropathy.
- Avoidance of overmedialization, early range of motion, and limited weight-bearing can help avoid complications associated with tibial tubercle AMZ.
Isolated patellofemoral osteoarthritis (PFOA) is a relatively common disorder. Based on radiological evidence, its prevalence is 24% in women and 11% in men aged over 55 years.1 However, the presence of PFOA on radiographic images does not always correlate with clinical symptoms. PFOA is symptomatic in only 8% of women and 2% of men aged over 55 years,1 and a mismatch often occurs between the symptoms and radiological severity (Figures 1A-1E).
PFOA surgery may be considered when nonsurgical treatment is ineffective and pain becomes disabling. However, which surgical treatment for isolated PFOA is optimal remains controversial. The largest setback in weighing nonarthroplasty surgical options for isolated PFOA is that few studies have been published. Furthermore, published studies offer little scientific evidence; they include case series with few patients and retrospective analyses with limited follow-up and no control group for comparison.
This article focuses on osteotomies, which are described in only 15 articles found through PubMed. The small number is logical given that the prevalence of symptomatic isolated PFOA is low1 and that the majority of patients do not need surgical treatment. A complicating factor is that osteotomy is often associated with other surgical procedures, such as lateral retinaculum release. In descriptions of these cases, it is not clear if the outcome for PFOA is attributable to the osteotomy, is secondary to the associated procedure, or both.
Several alternatives to patellofemoral arthroplasty—partial lateral patellar facetectomy (PLPF), patella-thinning osteotomy (PTO), anteromedialization (AMZ), and sulcus-deepening trochleoplasty (SDT)—are available for the management of isolated PFOA. In this article, we analyze the value of each of these techniques in preserving the patellofemoral joint in the presence of PFOA. These techniques combine the US and European perspectives. The ultimate objective with these surgical techniques is to delay arthroplasty as long as possible.
Partial Lateral Patellar Facetectomy
PLPF is a relatively simple and effective surgical treatment for isolated PFOA in active middle-aged to elderly patients who want to maintain their activity level.3-6 Using an oscillating saw to resect 1 cm to 1.5 cm of the lateral facet of the patella reduces lateral retinaculum tension and thereby decreases lateral patellofemoral contact pressures (Figures 2A, 2B).
PLPF improves pain and function over the long-term and delays the need for major surgery. Wetzels and Bellemans5 evaluated 155 consecutive patients (168 knees) with mean post-PLPF follow-up of 10.9 years. By final follow-up, 62 knees (36.9%) had failed and been revised to total knee arthroplasty (TKA) (60 knees), patellofemoral arthroplasty (1 knee), or total patellectomy (1 knee). Mean time to reoperation was 8 years. Kaplan-Meier survival rates with reoperation as the endpoint were 85% at 5 years, 67.2% at 10 years, and 46.7% at 20 years. At final follow-up, 79 (74.5%) of the 106 knees that had not been revised were rated good or fair, which accounts for 47% of the original group of 168 knees. The key finding is that the effects of PLPF lasted through the 10-year follow-up in half of the patients.5 Paulos and colleagues4 found 5 years of symptomatic relief in more than 80% of carefully selected patients who did not have significant (grade IV) arthritis in the medial or lateral knee compartments.
PLPF is a safe, low-cost, and relatively minor surgery with a low morbidity rate and fast recovery. Also, it does not close the door on other surgery and can easily be converted to TKA. Wetzels and Bellemans5 found that 36.9% of reoperations were TKAs, and López-Franco and colleagues3 found that 30% of knees required secondary TKA.
Patella-Thinning Osteotomy
In patients who are under 65 years old and have disabling anterior knee pain recalcitrant to conservative treatment, PTO may be considered for isolated PFOA with any type of chondral lesion (including severe diffuse chondropathy with exposed bone) (Figures 3A-3C), patellofemoral joint space reduced by more than 50% on skyline view, patellar thickness of 20 mm or more, and normal TT-TG distance.7
Vaquero and colleagues7 analyzed PTO outcomes in 31 patients (35 knees) with mean follow-up of 9 years and noted significant improvements in functional scores and radiologic parameters. All patients except 1 were satisfied with the operation. Radiologic progression of PFOA was slowed, but radiologic femorotibial osteoarthritis progressed in 23 cases (65%), and 4 required TKA. The authors found satisfactory clinical and radiologic outcomes—only 4 patients (12.5%) required TKA—and good functional outcomes.7
PTO, a low-morbidity surgery with good functional outcomes, does not close the door on other surgery, such as TKA.7
Tibial Tubercle Anteromedialization Osteotomy
Whereas PLPF and PTO are indicated in knees with normal TT-TG distance, Fulkerson AMZ osteotomy must be considered in isolated PFOA with articular cartilage lesions at the distal or lateral patellar facets resulting from long-standing malalignment with increased TT-TG distance (Figures 4A, 4B).
AMZ unloads the distal and lateral facets of the patella while improving the extensor mechanism.11,12 A successful AMZ outcome requires preservation of some of the medial and proximal articular cartilage of the patella. In 1983, Fulkerson13 described use of tibial tubercle AMZ osteotomy to address patellofemoral pain associated with patellofemoral chondrosis in conjunction with patellofemoral tilt and/or chronic patellar subluxation. This technique is indicated when the patella needs to be realigned for relief of elevated contact stress and centralization. Currently the technique is used not only in patients with isolated PFOA but in patients with chronic lateral patellar instability. Fulkerson osteotomy combines the benefits of the Maquet technique (unloading) and the Elmslie-Trillat technique (tracking improvement) in a single osteotomy, with no distraction of the osteotomy site with bone graft and without the complication rate of Maquet tibial tubercle elevation. Before surgery, computed tomography (CT) or magnetic resonance imaging (MRI) is routinely used to measure TT-TG distance to determine the tibial tubercle medialization required in the Fulkerson osteotomy. However, TT-TG distance must be used with caution, as it cannot be determined in cases with trochlear dysplasia. Consequently, physical examination and arthroscopic examination for evaluation of patellofemoral tracking and location of chondral defects should be performed before the Fulkerson osteotomy.
Rationale; Indications and Contraindications; Preoperative Planning
As already noted, AMZ unloads the distal and lateral facets of the patella. Beck and colleagues14 suggested AMZ is appropriate for unloading the lateral trochlea. However, it is not useful for central chondral defects and may actually increase the load in patients with medial chondral defects. As AMZ shifts contact force to the medial trochlea, Fulkerson osteotomy is appropriate when distal and lateral chondral lesions must be unloaded. Because this procedure moves the tibial tubercle medially and anteriorly, loads are transferred to the proximal and medial facets of the patella. Therefore, the procedure is contraindicated when diffuse, proximal, or medial chondral lesions are present. Moreover, AMZ is contraindicated in patients with normal TT-TG distance because there is the risk that overmedialization will cause symptomatic medial subluxation. Grade III or IV central trochlear cartilage lesions are also less likely to have successful AMZ outcomes. Therefore, before Fulkerson osteotomy is performed, MRI should be obtained to evaluate the patellofemoral articular surface and TT-TG distance. MRI provides information that is useful for preoperative planning because it allows assessment of articular cartilage lesions, including their location and severity. Moreover, because the osseous and cartilaginous contours of the patella differ, MRI gives a more accurate picture of the patellofemoral congruence than CT does. Last, before the open surgery is performed, the patellofemoral joint should be arthroscopically examined to determine the location of chondral lesions. Cartilage lesion mapping is important because Fulkerson osteotomy outcomes depend on chondral lesion location. Pidoriano and colleagues15 correlated AMZ outcomes with articular lesion location and noted optimal outcomes in patients with distal and lateral patellar articular lesions and intact trochlear cartilage (87% good and excellent outcomes). Patients with medial lesions and proximal or diffuse lesions generally did poorly (55% good and excellent outcomes in medial lesions vs 20% good and excellent outcomes in proximal and diffuse lesions). Central trochlear lesions were associated with medial patellar lesions, and all patients with central trochlear lesions had poor outcomes. Interestingly, Outerbridge grading of patellar lesions was not significantly correlated with overall outcomes.15 Even in cases of severe chondropathy, including bone-on-bone arthritis, AMZ has had reliable outcomes and may be superior to arthroplasty because of joint preservation, duration up to 8 years, and restoration of patellofemoral tracking. It should be noted that a resurfacing technique such as patellofemoral arthroplasty is not a substitute for patella realignment. Any patellofemoral maltracking must be corrected before patellofemoral arthroplasty. Fulkerson osteotomy does not preclude subsequent surgery (eg, TKA). Furthermore, AMZ may prevent the natural progression of PFOA related to chronic lateral tracking.
AMZ osteotomy can be adjusted for the specific indication and for the location of chondral defects. If the primary goal is unloading a lateral lesion, or lateral maltracking, then a flatter osteotomy may be performed to increase the relative medialization of the tubercle; however, if the primary goal is unloading a distal lesion, then a relatively more oblique or vertical osteotomy may be performed to transfer the load more proximally. This is the technique preferred by authors in most cases in which more anteriorization is desired.
When TT-TG distance is used to guide surgical realignment, patellofemoral chondrosis associated with normal TT-TG distance can be addressed with directly anterior displacement of the tibial tubercle. Anteriorization of the tibial tubercle can be obtained by inserting a bone block between the tubercle and the tibial cut (Figure 5A).16 The medialization can be neutralized by making this block as thick as the measured medialization.16
Surgical Outcomes of Anteromedialization in Patellofemoral Osteoarthritis
Fulkerson and colleagues10 followed 30 patients for more than 2 years after they underwent AMZ of the tibial tubercle for persistent patellofemoral pain associated with patellar articular degeneration. Of these 30 patients, 12 were followed for more than 5 years. The authors reported 93% good and excellent subjective outcomes and 89% good and excellent objective outcomes. Quality of improvement was sustained for all 12 patients reevaluated more than 5 years after surgery. When examined separately, 75% of patients with advanced PFOA had a good outcome, but none had an excellent outcome. Carofino and Fulkerson17 retrospectively evaluated tibial tubercle AMZ for isolated PFOA in 22 knees (17 active patients older than 50 years at time of surgery; mean age, 55 years) with minimum follow-up of 2 years (mean, 77 months). Mean postoperative Lysholm score was 83. According to Lysholm scores, outcomes were good to excellent in 12 cases, fair in 6, and poor in 1. The authors concluded that tibial tubercle AMZ is a definitive treatment option for isolated PFOA in active older patients. Morshuis and colleagues18 retrospectively evaluated 22 patients (25 knees) who underwent Fulkerson osteotomy for patellofemoral pain. Outcomes were evaluated a mean of 12 and 30 months after surgery. At the first evaluation, 84% of patients had satisfactory outcomes, and, at the second (≤38 months after surgery), 70%. Only in relatively young patients without signs of PFOA did outcomes remain satisfactory in all cases. At the later evaluation, 60% of patients with PFOA and/or lateralization had satisfactory outcomes.
Tips and Tricks to Avoid Complications
For some patients, AMZ performed technically correctly produced unhappiness—an outcome that may arise from incorrect patient selection or failure to meet patient expectations. It is important to discuss objectives and expectations with the patient before surgery. With correct patient selection and meticulous surgical technique (with customization of osteotomy angle and translation based on underlying lesion), surgeons have obtained excellent outcomes with infrequent complications (Table).
Intraoperative complications may involve neurovascular structures. The anterior tibial artery and the peroneal nerve are at risk during Fulkerson osteotomy. Decreased anterior sensation related to the infrapatellar branch of the saphenous nerve is not uncommon. Reducing the risk of neurovascular injury requires use of retractors and keeping the saw blade visible at all times. Another potential devastating complication is injury of the posterior vascular structures during bicortical tibial drilling for screw placement. According to Kline and colleagues,19 bicortical drilling may occur precariously near the posterior vascular structures of the knee. They advised extreme caution in drilling the posterior cortex during this procedure. To avoid the risk of compartment syndrome, surgeons can leave the anterior compartment fascia open or pie crust it by making multiple small perforations to decrease tension. Tibial fracture is another potential complication with this osteotomy. Reducing the risk of fracture involves tapering the distal cut anteriorly and avoiding a “notched” osteotomy (Figures 6A-6C).
Postoperative complications, which are similar to those associated with any knee surgery, include infection, arthrofibrosis, complex regional pain syndrome, thromboembolism, nonunion, fixation failure, and fracture. Arthrofibrosis has many causes, but the problem decreases with secure osteotomy fixation, early knee motion, and patellar mobilization. Overmedialization can result in medial patella instability, typically subluxation rather than complete dislocation. The instability can be relatively subtle or can cause pain and weakness. Lateralization of the tibial tubercle might be appropiate.23
Sulcus-Deepening Trochleoplasty
High-grade trochlear dysplasia with a prominence, frequently present in lateral patellar instability, is thought to correlate with PFOA because it produces an anti-Maquet effect.24 The dysplasia provokes an increment of the patellofemoral joint pressure that could explain patellofemoral chondropathy and ultimately PFOA. In fact, 33% of patients with isolated PFOA have a history of objective patellar dislocation.24 In these cases, SDT could be considered. Several studies have examined use of this technique in the treatment of instability, but not PFOA.25 After SDT, pain resolves despite the chondral lesions being left alone (Figures 7A, 7B).
Conclusion
Patellofemoral joint replacement is an option for patellofemoral pain only in very select cases. Preserving the joint is always a primary goal. As not all PFOA cases are equal, joint-preserving surgery must be tailored to the patient. The keys to success are good indication, precise surgery, proper rehabilitation, and, above all, doing only what is needed.
Am J Orthop. 2017;46(3):139-145. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
1. McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844-849.
2. Iwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990;(252):190-197.
3. López-Franco M, Murciano-Antón MA, Fernández-Aceñero MJ, De Lucas-Villarrubia JC, López-Martín N, Gómez-Barrena E. Evaluation of a minimally aggressive method of patellofemoral osteoarthritis treatment at 10 years minimum follow-up. Knee. 2013;20(6):476-481.
4. Paulos LE, O’Connor DL, Karistinos A. Partial lateral patellar facetectomy for treatment of arthritis due to lateral patellar compression syndrome. Arthroscopy. 2008;24(5):547-553.
5. Wetzels T, Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: results at long-term follow up. Knee. 2012;19(4):411-415.
6. Yercan HS, Ait Si Selmi T, Neyret P. The treatment of patellofemoral osteoarthritis with partial lateral facetectomy. Clin Orthop Relat Res. 2005;(436):14-19.
7. Vaquero J, Calvo JA, Chana F, Perez-Mañanes R. The patellar thinning osteotomy in patellofemoral arthritis: four to 18 years’ follow-up. J Bone Joint Surg Br. 2010;92(10):1385-1391.
8. Vaquero J, Arriaza R. The patella thinning osteotomy. An experimental study of a new technique for reducing patellofemoral pressure. Int Orthop. 1992;16(4):372-376.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 3rd ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-496.
11. Fulkerson JP. Patellofemoral pain disorders: evaluation and management. J Am Acad Orthop Surg. 1994;2(2):124-132.
12. Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447-456.
13. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;(177):176-181.
14. Beck PR, Thomas AL, Farr J, Lewis PB, Cole BJ. Trochlear contact pressures after anteromedialization of the tibial tubercle. Am J Sports Med. 2005;33(11):1710-1715.
15. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
16. Farr J. Tibial tubercle osteotomy. Tech Knee Surg. 2003;2:28-42.
17. Carofino BC, Fulkerson JP. Anteromedialization of the tibial tubercle for patellofemoral arthritis in patients > 50 years. J Knee Surg. 2008;21(2):101-105.
18. Morshuis WJ, Pavlov PW, de Rooy KP. Anteromedialization of the tibial tuberosity in the treatment of patellofemoral pain and malalignment. Clin Orthop Relat Res. 1990;(255):242-250.
19. Kline AJ, Gonzales J, Beach WR, Miller MD. Vascular risk associated with bicortical tibial drilling during anteromedial tibial tubercle transfer. Am J Orthop. 2006;35(1):30-32.
20. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
21. Fulkerson JP. Fracture of the proximal tibia after Fulkerson anteromedial tibial tubercle transfer. A report of four cases. Am J Sports Med. 1999;27(2):265.
22. Cosgarea AJ, Freedman JA, McFarland EG. Nonunion of the tibial tubercle shingle following Fulkerson osteotomy. Am J Knee Surg. 2001;14(1):51-54.
23. Fulkerson JP. Anterolateralization of the tibial tubercle. Tech Orthop. 1997;12:165-169.
24. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am. 2008;39(3):269-274.
25. Ntagiopoulos PG, Byn P, Dejour D. Midterm results of comprehensive surgical reconstruction including sulcus-deepening trochleoplasty in recurrent patellar dislocations with high-grade trochlear dysplasia. Am J Sports Med. 2013;41(5):998-1004.
26. Oberlander MA, Baker CL, Morgan BE. Patellofemoral arthrosis: the treatment options. Am J Orthop. 1998;27(4):263-270.
27. Scuderi GR. The Patella. New York, NY: Springer-Verlag; 1995.
28. Buuck D, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow up. Oper Tech Sports Med. 2000;8:131-137.
Measuring Malalignment on Imaging in the Treatment of Patellofemoral Instability
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG.
- TT-TG distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.
- TT-TG distance criteria should serve as a guide, rather than a rigid threshold, in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
- Factors such as knee flexion angle, imaging modality, and landmarks used for the measurements should be considered when using TT-TG distance as an indication for surgery.
- There has been significant variability in reported TT-TG measurements. A surgeon using this measurement should understand how it is obtained because many technical factors are involved.
Assessment of malalignment is an important factor in determining surgical treatment options for patellar instability. Although soft-tissue reconstruction of the medial soft-tissue stabilizers is often performed to address patellar instability, bony malalignment may increase stress on the medial soft tissues; therefore, it must be adequately identified and addressed.
Bony malalignment, which is often thought of as lateralization of the tibial tubercle (TT), can be influenced by tibiofemoral alignment, external tibial torsion, and femoral anteversion.
Clinically, coronal alignment can be assessed with a measurement such as quadriceps (Q) angle, but this has been reported to have low interrater reliability and high variability in the reported optimal conditions and positions in which the measurement should be made.1-3An anatomically lateralized TT pulls the extensor mechanism laterally with respect to the trochlear groove (TG), and this can accentuate problems related to patellofemoral instability. A recent biomechanical study found that increased TT lateralization significantly increased lateral patellar translation and tilt in the setting of medial patellofemoral ligament (MPFL) deficiency.4 Although MPFL reconstruction restored patellar kinematics and contact mechanics, this restoration did not occur when the TT was lateralized more than 10 mm relative to its normal position.
Realigning the extensor mechanism by moving the TT medially decreases the lateralizing forces on the patella and the stress on the soft-tissue restraints. This raises the issues of when to correct a lateralized TT and how to identify and measure malalignment.
Radiographic assessment of TT position is most commonly performed by measuring TT-TG distance, which is the distance between the extensor mechanism attachment at the TT and the center of the TG. Originally described on radiographs and subsequently on computed tomography (CT) and magnetic resonance imaging (MRI) scans, distances of more than 15 mm or 20 mm have been reported as indications for TT osteotomy.5,6However, there has been significant variability in reported TT-TG measurements. Studies have found that TT-TG distance is 3.8 mm larger on CT scans than on MRI scans.7 Furthermore, factors such as knee flexion angle at time of imaging have been found to reduce TT-TG distance.1 More recently, patient size and TT-TG ratios relative to patellar and trochlear width were identified as important factors in assessing TT-TG distance.8 Therefore, TT-TG distance measurements should serve as a guide rather than a rigid threshold in the context of imaging and patient factors when deciding whether to perform TT osteotomy for patellar instability.
What You Need to Know About Measuring Patellofemoral Malalignment
TT-TG distance can guide decisions about performing a medializing TT osteotomy for patellar instability because the measurement can aid in assessing bony malalignment caused by an anatomically lateralized tubercle. TT-TG distance can be used to determine when and how far to move the tubercle in TT osteotomy.
Background
A normal TT-TG value is approximately 10 mm. The measurement originally used bony landmarks, including the deepest part of the bony TG and the anterior-most part of the TT, as described by Goutallier and colleagues.9 In their original study, Dejour and colleagues5 found that patients with recurrent symptoms of patellar instability had TT-TG distances >20 mm.
Increased TT-TG distance has been shown to correlate with patellar position, including increased lateral shift and lateral tilt of the patella. In a study using dynamic CT scans of patients with recurrent patellar instability, we found that TT-TG distance increased with knee extension, and that this increase correlated with the lateral shift and lateral tilt of the patella.10An excessively lateralized TT can be corrected with a medializing osteotomy that reduces TT-TG distance to within the normal range. TT surgery can be performed with flat osteotomy, as described by Elmslie and Trillat,11 or with oblique osteotomy, as described by Fulkerson,6 to obtain concomitant anteriorization. In a computational study, Elias and colleagues12 found that medializing TT osteotomy not only reduced TT-TG distance but led to correction of lateral patellar tilt and displacement. Patellofemoral contact forces have also shown to be reduced with anteromedialization.6Although reported outcomes of TT osteotomy have been excellent for patients with patellar instability, the procedure has higher risks and longer rehabilitation relative to a soft-tissue procedure alone. Reported risks associated with TT osteotomy include fracture, nonunion, delayed union, painful screws, and deep vein thrombosis.6,10,13,14Understanding the limitations of and variability in radiographic assessments of TT and TG positions can help when deciding whether to perform TT osteotomy for patellar instability.
Discussion
When considering TT osteotomy for patellar instability, some surgeons use a TT-TG distance of more than 15 mm or 20 mm as a threshold for performing medialization. The variability is based on the multiple patient and imaging factors that can influence TT-TG distance measurement.
Several TG and TT landmarks have been used to measure TT-TG distance. The deepest part of the TG, based on bony anatomy, was used originally, but the cartilaginous landmark at the deepest part of the cartilaginous TG has also been described.15 Similarly, on the TT, the original description of TT-TG distance, by Goutallier and colleagues,9 involved the anterior-most part of the TT on CT scan, though the central part of the TT has also been described.15 We found a 4.2-mm difference in TT-TG distance with use of different landmarks (central tubercle, anterior tubercle) within the same study population.16 Therefore, within a practice, the distance used as an indication for TT osteotomy should be measured consistently.
Knee flexion angle at the time of imaging can also affect measurement of TT-TG distance. Several authors have reported smaller TT-TG distance with increased knee flexion angle.10,16,17 In a study of patients with symptomatic patellar instability, we found that TT-TG distance decreases by an estimated 1 mm for every 4.4° of knee flexion >0°.10 In measurements of TT-TG distance, the sagittal view can be used to assess knee flexion angle because positioning protocols and patient comfort at the time of imaging may produce variable knee flexion angles.
Given the variability that occurs in TT-TG distance with knee flexion angles, some surgeons use TT–posterior cruciate ligament (PCL) distance as another measurement of TT lateralization.18 This measurement is made with both tibial landmarks, from the TT to the medial border of the PCL insertion on the tibia, and theoretically eliminates knee flexion angle as a measurement factor. Seitlinger and colleagues18 found that values >24 mm were associated with symptoms of patellar instability. More study is needed to determine the precise indications for TT osteotomy with use of this measurement.
In addition to patient positioning during knee imaging, patient size should be considered when TT-TG distance is used for malalignment measurement. Camp and colleagues8 discussed the importance of “individualizing” TT-TG distance on the basis of patient size and bony structure. They reported that the ratio of TT-TG distance to trochlear width or patellar width more effectively predicted recurrent patellar instability than TT-TG distance alone.
Measurement of TT-TG distance is valuable in planning surgical treatment for patellar instability because it quantifies a component of malalignment and aids in deciding whether to perform TT osteotomy. However, this distance should be understood in the context of many measurement factors to allow for an individualized procedure that addresses the specific contributors to patellar instability in each patient.
Am J Orthop. 2017;46(3):148-151. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
1. France L, Nester C. Effect of errors in the identification of anatomical landmarks on the accuracy of Q angle values. Clin Biomech (Bristol, Avon). 2001;16(8):710-713.
2. Greene CC, Edwards TB, Wade MR, Carson EW. Reliability of the quadriceps angle measurement. Am J Knee Surg. 2001;14(2):97-103.
3. Smith TO, Hunt NJ, Donell ST. The reliability and validity of the Q-angle: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1068-1079.
4. Stephen JM, Dodds AL, Lumpaopong P, Kader D, Williams A, Amis AA. The ability of medial patellofemoral ligament reconstruction to correct patellar kinematics and contact mechanics in the presence of a lateralized tibial tubercle. Am J Sports Med. 2015;43(9):2198-2207.
5. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
6. Fulkerson JP. Anteromedialization of the tibial tuberosity for patellofemoral malalignment. Clin Orthop Relat Res. 1983;177:176-181.
7. Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med. 2013;41(8):1835-1840.
8. Camp CL, Heidenreich MJ, Dahm DL, Stuart MJ, Levy BA, Krych AJ. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med. 2016;44(2):393-399.
9. Goutallier D, Bernageau J, Lecudonnec B. [The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
10. Tanaka MJ, Elias JJ, Williams AA, Carrino JA, Cosgarea AJ. Correlation between changes in tibial tuberosity-trochlear groove distance and patellar position during active knee extension on dynamic kinematic computed tomography imaging. Arthroscopy. 2015;31(9):1748-1755.
11. Trillat A, Dejour H, Couette A. [Diagnosis and treatment of recurrent dislocations of the patella]. Rev Chir Orthop Reparatrice Appar Motur. 1964;50(6):813-824.
12. Elias JJ, Carrino JA, Saranathan A, Guseila LM, Tanaka MJ, Cosgarea AJ. Variations in kinematics and function following patellar stabilization including tibial tuberosity realignment. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2350-2356.
13. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
14. Wilcox JJ, Snow BJ, Aoki SK, Hung M, Burks RT. Does landmark selection affect the reliability of tibial tubercle-trochlear groove measurements using MRI? Clin Orthop Relat Res. 2012;470(8):2253-2260.
15. Schoettle PB, Zanetti M, Seifert B, Pfirrmann CWA, Fucentese SF, Romero J. The tibial tuberosity-trochlear groove distance; a comparative study between CT and MRI scanning. Knee. 2006;13(1):26-31.
16. Williams AA, Tanaka MJ, Elias JJ, et al. Measuring tibial tuberosity-trochlear groove distance on CT: Where to begin? Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, New Orleans, LA, March 11-15, 2014.
17. Dietrich TJ, Betz M, Pfirrmann CWA, Koch PP, Fucentese SF. End-stage extension of the knee and its influence on tibial tuberosity-trochlear groove distance (TTTG) in asymptomatic volunteers. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):214-218.
18. Seitlinger G, Scheurecker G, Hogler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.