User login
Knotless Tape Suture Fixation of Quadriceps Tendon Rupture: A Novel Technique
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
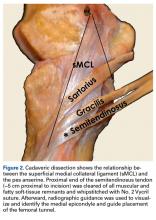
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
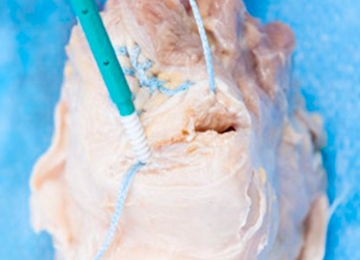
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
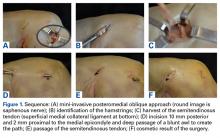
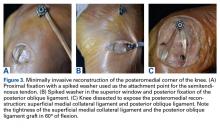
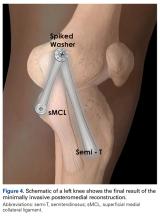
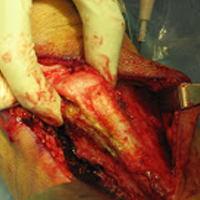
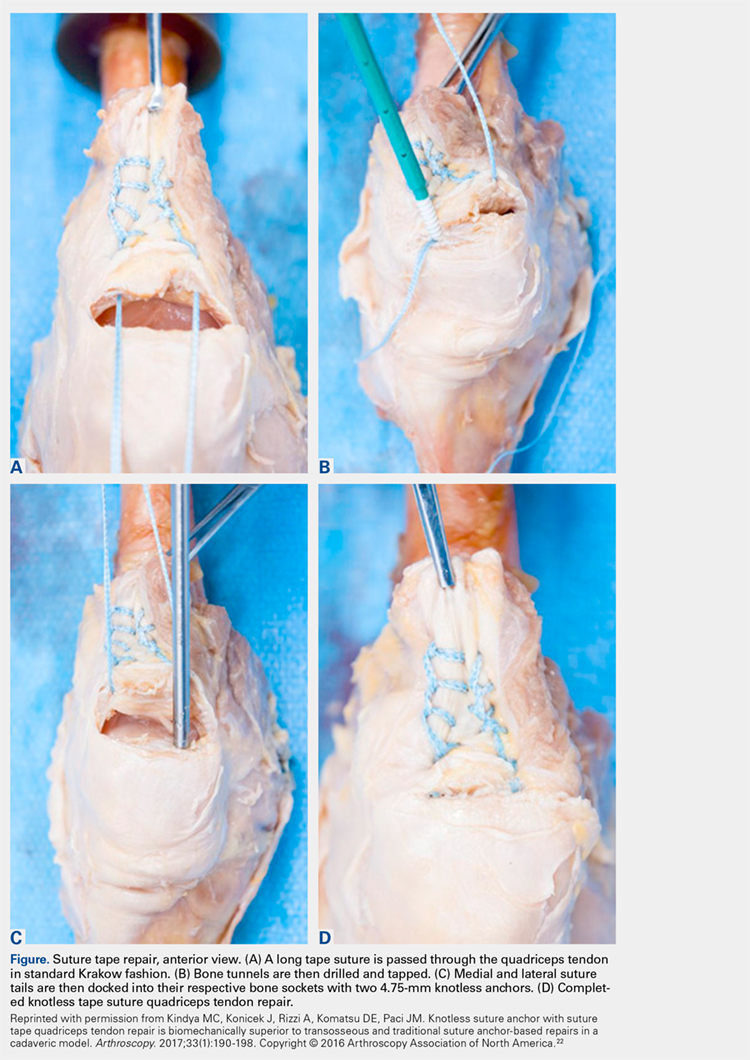
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).
After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
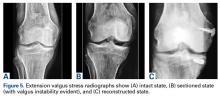
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).

After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).

After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
TAKE-HOME POINTS
- Knotless tape suture fixation of the quadriceps tendon is biomechanically superior to traditional fixation techniques.
- When passing locking Krackow stitches, be sure to take all slack out with each pass.
- Consider double tapping the patella pilot holes prior to placing anchors, as the bone is very hard.
- Palpate the articular surface of the patella when drilling pilot holes for safe placement.
- Perform an adequate retinacular repair to complete the repair.
Biomechanical Evaluation of a Novel Suture Augment in Patella Fixation
Take-Home Points
- Suture augmentation improves construct strength for patella fixation.
- Krackow sutures may be placed in the quadriceps and patella tendons, then secured over the anterior patella (much like an anterior tension band).
- The Krackow technique described was superior to the suture cerclage technique based on mean load values, but did not reach statistical significance.
- The Krackow suture technique is a viable and easily applied technique for suture augmentation of patella fixation constructs.
Patella fractures are relatively uncommon, accounting for only 1% of skeletal injuries.1 Restoration of the function of the patella and the extensor mechanism is vital for knee extension and gait. However, patella fractures have an inherently high rate of complications, making these injuries challenging to treat.2-4 In patients with intact extensor function, displacement of <4 mm, and articular step-off of <3 mm, nonoperative management is extremely effective, with 99% of patients reporting favorable results.5 However, for fractures in which the extensor mechanism is disrupted, surgical intervention typically is indicated.6
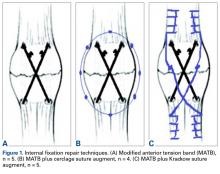
Authors have reported various surgical interventions, one of the most commonly used being the anterior tension band (ATB) technique, first described by the AO (Arbeitsgemeinschaft für Osteosynthesefragen) group in the 1950s.7 By converting distractive anterior force during knee flexion to compressive force at the fracture site, the ATB technique provides a repair stronger than the previously used cerclage repair.8 Although initially considered standard of care, the ATB technique was soon found to be associated with implant failure and subcutaneous irritation prompting implant removal.9
To address these issues, Berg10 and Carpenter and colleagues11 evaluated an ATB technique that used cannulated screws instead of Kirschner wires (K-wires). This variation on the ATB technique reduced the implant-related issues while maintaining the mechanical advantage of the tension band. The more rigid design also permitted earlier postoperative rehabilitation, which significantly reduced development of arthrofibrosis.6,7,10 This modified ATB (MATB) technique has since been investigated for additional augments, mainly focusing on use of different tension band materials, including polyester suture and braided composite suture.12-14
However, there is little research on augments that incorporate the surrounding soft tissue, specifically the quadriceps and patellar tendons. In a recent retrospective clinical study, Oh and colleagues15 found positive clinical results with use of Krackow sutures, though 2 or 3 vertically oriented stainless steel wires were used instead of cannulated screws.
We conducted a study to determine the biomechanical efficacy of using a cerclage suture augment and a Krackow suture augment coupled with and compared with conventional MATB repair. If effective, this technique may represent another strategy for increasing repair strength and thereby improve postoperative outcomes.
Materials and Methods
Specimen Preparation
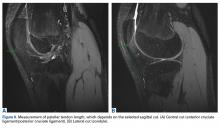
Fresh-frozen cadaver extensor mechanisms (quadriceps tendon, patella, surrounding retinaculum, patellar tendon) were kept frozen at –4°C until preparation. Fifteen specimens were selected. Mean (SD) age at death was 68 (10) years (range, 51-85 years). One specimen was excluded for a short patella tendon, which precluded adequate attachment for testing. All specimens were free of overt osseous pathology.
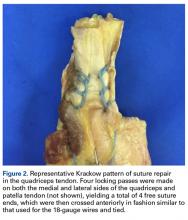
After specimens were thawed overnight, the patellae were transversely osteotomized with an osteotome at the junction of the middle and distal thirds of the patella. Sharp dissection was performed to carry the division through the medial and lateral retinaculum at the same level. All 14 specimens were then repaired using the MATB technique. First, the transverse fracture was reduced with a reduction clamp. Then, two 4-mm cannulated screws (DePuy Synthes) were inserted parallel to each other and perpendicular to the fracture. An 18-gauge stainless steel wire was then passed through each screw, crossed anteriorly, and tightened to create a figure-of-8 ATB. The specimens were then randomly divided into 3 groups—MATB; MATB with cerclage suture augment; MATB with Krackow suture augment—while ensuring specimens from a single cadaver were placed in different groups to avoid confounding based on bone density differences.
Experimental Setup
Repaired specimens were secured with tissue clamps at the quadriceps and patellar tendons on an MTS Bionix 858 (MTS Systems) hydraulic arm.
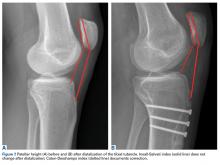
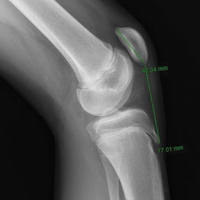
Each patella was secured for cyclic testing. Initially it was placed under 10 N of tension. Then it underwent tensile loading from 10 N to 300 N at 50 N/s for 10 cycles. These parameters were based on previous biomechanical patella studies.10,11 Load was measured with the MTS load cell and displacement with the displacement transducer. Fracture displacement associated with 300-N cyclic tension was recorded. Displacement was calculated as the difference between 10th cycle and 2nd cycle values, which accounted for any degree of initial tissue slippage. After cyclic testing, the patella was placed back in 10 N of tensile loading and subjected to maximum force loading to determine ultimate repair strength. For maximum loading, the patella was stretched progressively at 50 N/s until failure. Again, load and displacement were measured with MTS.
Statistical Analysis
After testing, fracture displacement and maximum load force data were compiled for analysis. One-way analysis of variance with Bonferroni correction was used to determine if there were significant differences between groups. Significance level was set at P < .05.
Results
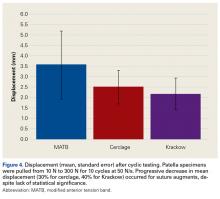
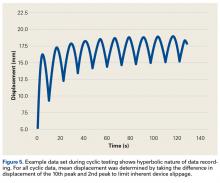
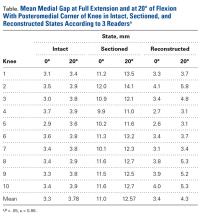
For cyclic testing, mean total displacement was measured over 10 cycles for each group. Again, displacement was determined by taking the difference between 10th cycle and 2nd cycle values, allowing for system stabilization.
Discussion
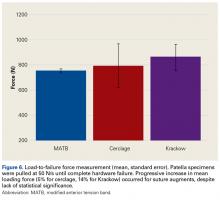
Our main objective was to compare the efficacy of a novel suture augment technique with that of other patella fracture repair techniques. Our hypothesis—that adding a Krackow suture augment would increase strength in both cyclic and maximum loading—was supported. Although testing results were not statistically significant because of the small sample size, we think this novel technique has clinically relevant descriptive significance and warrants further investigation.
Proper anatomical reduction and postoperative stabilization are of utmost importance in clinical approaches to patella fractures. In addition, regardless of which technical procedure is used, open reduction should also allow for early range of motion to prevent joint arthrofibrosis. Ever since the ATB technique was first described by the AO group, postoperative outcomes have improved significantly. In a retrospective study by Levack and colleagues,17 30 of 64 patients with patella fractures underwent internal fixation. Mean follow-up was 6.2 years. By both objective and subjective measures, the best functional outcomes were associated with internal tension band fixation (vs cerclage repair). Lotke and Ecker18 also documented the efficacy of the tension band technique. Sixteen patients with patella fractures underwent anterior tension banding; those with a comminuted fracture also underwent cerclage repair for patella stabilization during tension banding. At 6-week follow-up, all patients had good range of motion (≥90° flexion), relatively few symptoms, and no implant failures. Results were similar to those of Levack and colleagues.17
Although it improves stability and functional outcomes over conventional patellectomy and cerclage wiring, the ATB technique has been associated with subcutaneous irritation caused by the K-wires used to secure the band. Hung and colleagues9 followed up 68 patients with patellar fractures. Five of these patients underwent tension banding. Although there was a high level of adequate functional outcomes, implant irritation was found to be “quite frequent.”
To address this issue, Carpenter and colleagues11 evaluated an ATB technique that uses K-wires instead of cannulated screws. Biomechanical testing in a cadaver model revealed less fracture displacement and overall more repair strength through cyclic and maximum load testing. Clinically, these results were supported by Berg,10 who followed up 10 patients with transverse patella fractures repaired with the MATB technique. At a mean follow-up of 24 months, 7 of the 10 patients had good to excellent outcomes, and there were no implant failures.
Further investigation into patella repairs has mainly focused on improving the MATB technique and experimenting with different tension band materials. Rabalais and colleagues13 biomechanically tested high-strength polyethylene suture as a replacement for standard 18-gauge wire, and Bryant and colleagues14 tested a braided composite suture (FiberWire; Arthrex) as a replacement for standard 18-gauge stainless steel wire. Both found no significant difference with use of augmented tension band material, but Rabalais and colleagues13 did find more advantages with a parallel tension band construct than with a standard figure-of-8 arrangement.
In developing our novel technique, we considered that Krackow sutures are routinely used in both quadriceps tendon repair and patellar tendon repair, including partial patellectomy for distal patella fracture. With a suture placed in both tendons, the augment could be expected to resist longitudinal gapping and augment the tension band across the anterior patella. First described by Krackow and colleagues,19 the Krackow suture is widely used for tendon reconstruction. In an interlocking system of sutures, the Krackow suture provides a repair that is more stable than repair with conventional suture techniques, specifically in the context of tendon repair.20 Given the sesamoidal nature of the patella, its repair shares the goal of gap prevention with other tendon repairs. In theory, anchoring the supporting structures that are above and below the patella provides support for the intervening patella and ultimately improves fracture fixation strength.
Oh and colleagues15 reported on the clinical efficacy of a Krackow augment in distal pole patella repairs. Similarly, we found a Krackow augment to be efficacious, supporting its potential in clinical approaches to patella repairs. Our results indicate this augment can be a useful clinical adjunct in biomechanical evaluation.
Limitations of this study include its use of dissected extensor mechanisms, which may have less biofidelity than whole-knee specimens. In our model, specimens were secured at the patellar tendon and the quadriceps tendons, as opposed to the quadriceps tendon and the tibia distally. Use of this model could have led to an increase in early displacement during cyclic testing as a result of tissue slippage. Furthermore, our small sample size could have affected our ability to demonstrate a difference between these techniques.
Given its increased strength as demonstrated by mean displacement during cyclic loading and mean load to failure, as well as the early clinical data recently published, the Krackow suture augment represents a feasible technique for patella fixation. It likely will be most useful in cases in which conventional techniques are prone to failure or cannot be applied, such as severe distal comminution or poor bone density. Further biomechanical testing with a larger number of specimens may be required for statistical significance.
Conclusion
In patella fracture repair strategies, the Krackow suture augment increased strength when used with a MATB technique. Failure to reach statistical significance likely resulted from our small sample size. Further biomechanical testing and clinical studies are needed for more complete evaluation of this technique. We think it will be most useful in the setting of poor bone quality or severe comminution, which can limit fixation options. As increased repair strength allows earlier postoperative rehabilitation and maintains fracture reduction, patient outcomes should improve. This novel technique represents another strategy for managing challenging patella fractures.
1. Boström Å. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
2. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
3. LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426.
4. Kaufer H. Mechanical function of the patella. J Bone Joint Surg Am. 1971;53(8):1551-1560.
5. Braun W, Wiedemann M, Rüter A, Kundel K, Kolbinger S. Indications and results of nonoperative treatment of patellar fractures. Clin Orthop Relat Res. 1993;(289):197-201.
6. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19(4):198-207.
7. Müller M, Allgöwer M, Schneider R, Willeneger H. Manual of Internal Fixation: Techniques Recommended by the AO Group. Berlin, Germany: Springer; 1979.
8. Weber MJ, Janecki CJ, McLeod P, Nelson CL, Thompson JA. Efficacy of various forms of fixation of transverse fractures of the patella. J Bone Joint Surg Am. 1980;62(2):215-220.
9. Hung LK, Chan KM, Chow YN, Leung PC. Fractured patella: operative treatment using the tension band principle. Injury. 1985;16(5):343-347.
10. Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
11. Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
12. Hughes SC, Stott PM, Hearnden AJ, Ripley LG. A new and effective tension-band braided polyester suture technique for transverse patellar fracture fixation. Injury. 2007;38(2):212-222.
13. Rabalais RD, Burger E, Lu Y, Mansour A, Baratta RV. Comparison of two tension-band fixation materials and techniques in transverse patella fractures: a biomechanical study. Orthopedics. 2008;31(2):128.
14. Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: a pilot study. J Orthop. 2014;12(2):92-96.
15. Oh HK, Choo SK, Kim JW, Lee M. Internal fixation of displaced inferior pole of the patella fractures using vertical wiring augmented with Krachow suturing. Injury. 2015;46(12):2512-2515.
16. Goodfellow J, Hungerford DS, Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58(3):287-290.
17. Levack B, Flannagan JP, Hobbs S. Results of surgical treatment of patellar fractures. J Bone Joint Surg Br. 1985;67(3):416-419.
18. Lotke PA, Ecker ML. Transverse fractures of the patella. Clin Orthop Relat Res. 1981;(158):180-184.
19. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
20. Hahn JM, Inceoğlu S, Wongworawat MD. Biomechanical comparison of Krackow locking stitch versus nonlocking loop stitch with varying number of throws. Am J Sports Med. 2014;42(12):3003-3008.
Take-Home Points
- Suture augmentation improves construct strength for patella fixation.
- Krackow sutures may be placed in the quadriceps and patella tendons, then secured over the anterior patella (much like an anterior tension band).
- The Krackow technique described was superior to the suture cerclage technique based on mean load values, but did not reach statistical significance.
- The Krackow suture technique is a viable and easily applied technique for suture augmentation of patella fixation constructs.
Patella fractures are relatively uncommon, accounting for only 1% of skeletal injuries.1 Restoration of the function of the patella and the extensor mechanism is vital for knee extension and gait. However, patella fractures have an inherently high rate of complications, making these injuries challenging to treat.2-4 In patients with intact extensor function, displacement of <4 mm, and articular step-off of <3 mm, nonoperative management is extremely effective, with 99% of patients reporting favorable results.5 However, for fractures in which the extensor mechanism is disrupted, surgical intervention typically is indicated.6
Authors have reported various surgical interventions, one of the most commonly used being the anterior tension band (ATB) technique, first described by the AO (Arbeitsgemeinschaft für Osteosynthesefragen) group in the 1950s.7 By converting distractive anterior force during knee flexion to compressive force at the fracture site, the ATB technique provides a repair stronger than the previously used cerclage repair.8 Although initially considered standard of care, the ATB technique was soon found to be associated with implant failure and subcutaneous irritation prompting implant removal.9
To address these issues, Berg10 and Carpenter and colleagues11 evaluated an ATB technique that used cannulated screws instead of Kirschner wires (K-wires). This variation on the ATB technique reduced the implant-related issues while maintaining the mechanical advantage of the tension band. The more rigid design also permitted earlier postoperative rehabilitation, which significantly reduced development of arthrofibrosis.6,7,10 This modified ATB (MATB) technique has since been investigated for additional augments, mainly focusing on use of different tension band materials, including polyester suture and braided composite suture.12-14
However, there is little research on augments that incorporate the surrounding soft tissue, specifically the quadriceps and patellar tendons. In a recent retrospective clinical study, Oh and colleagues15 found positive clinical results with use of Krackow sutures, though 2 or 3 vertically oriented stainless steel wires were used instead of cannulated screws.
We conducted a study to determine the biomechanical efficacy of using a cerclage suture augment and a Krackow suture augment coupled with and compared with conventional MATB repair. If effective, this technique may represent another strategy for increasing repair strength and thereby improve postoperative outcomes.
Materials and Methods
Specimen Preparation
Fresh-frozen cadaver extensor mechanisms (quadriceps tendon, patella, surrounding retinaculum, patellar tendon) were kept frozen at –4°C until preparation. Fifteen specimens were selected. Mean (SD) age at death was 68 (10) years (range, 51-85 years). One specimen was excluded for a short patella tendon, which precluded adequate attachment for testing. All specimens were free of overt osseous pathology.
After specimens were thawed overnight, the patellae were transversely osteotomized with an osteotome at the junction of the middle and distal thirds of the patella. Sharp dissection was performed to carry the division through the medial and lateral retinaculum at the same level. All 14 specimens were then repaired using the MATB technique. First, the transverse fracture was reduced with a reduction clamp. Then, two 4-mm cannulated screws (DePuy Synthes) were inserted parallel to each other and perpendicular to the fracture. An 18-gauge stainless steel wire was then passed through each screw, crossed anteriorly, and tightened to create a figure-of-8 ATB. The specimens were then randomly divided into 3 groups—MATB; MATB with cerclage suture augment; MATB with Krackow suture augment—while ensuring specimens from a single cadaver were placed in different groups to avoid confounding based on bone density differences.
Experimental Setup
Repaired specimens were secured with tissue clamps at the quadriceps and patellar tendons on an MTS Bionix 858 (MTS Systems) hydraulic arm.
Each patella was secured for cyclic testing. Initially it was placed under 10 N of tension. Then it underwent tensile loading from 10 N to 300 N at 50 N/s for 10 cycles. These parameters were based on previous biomechanical patella studies.10,11 Load was measured with the MTS load cell and displacement with the displacement transducer. Fracture displacement associated with 300-N cyclic tension was recorded. Displacement was calculated as the difference between 10th cycle and 2nd cycle values, which accounted for any degree of initial tissue slippage. After cyclic testing, the patella was placed back in 10 N of tensile loading and subjected to maximum force loading to determine ultimate repair strength. For maximum loading, the patella was stretched progressively at 50 N/s until failure. Again, load and displacement were measured with MTS.
Statistical Analysis
After testing, fracture displacement and maximum load force data were compiled for analysis. One-way analysis of variance with Bonferroni correction was used to determine if there were significant differences between groups. Significance level was set at P < .05.
Results
For cyclic testing, mean total displacement was measured over 10 cycles for each group. Again, displacement was determined by taking the difference between 10th cycle and 2nd cycle values, allowing for system stabilization.
Discussion
Our main objective was to compare the efficacy of a novel suture augment technique with that of other patella fracture repair techniques. Our hypothesis—that adding a Krackow suture augment would increase strength in both cyclic and maximum loading—was supported. Although testing results were not statistically significant because of the small sample size, we think this novel technique has clinically relevant descriptive significance and warrants further investigation.
Proper anatomical reduction and postoperative stabilization are of utmost importance in clinical approaches to patella fractures. In addition, regardless of which technical procedure is used, open reduction should also allow for early range of motion to prevent joint arthrofibrosis. Ever since the ATB technique was first described by the AO group, postoperative outcomes have improved significantly. In a retrospective study by Levack and colleagues,17 30 of 64 patients with patella fractures underwent internal fixation. Mean follow-up was 6.2 years. By both objective and subjective measures, the best functional outcomes were associated with internal tension band fixation (vs cerclage repair). Lotke and Ecker18 also documented the efficacy of the tension band technique. Sixteen patients with patella fractures underwent anterior tension banding; those with a comminuted fracture also underwent cerclage repair for patella stabilization during tension banding. At 6-week follow-up, all patients had good range of motion (≥90° flexion), relatively few symptoms, and no implant failures. Results were similar to those of Levack and colleagues.17
Although it improves stability and functional outcomes over conventional patellectomy and cerclage wiring, the ATB technique has been associated with subcutaneous irritation caused by the K-wires used to secure the band. Hung and colleagues9 followed up 68 patients with patellar fractures. Five of these patients underwent tension banding. Although there was a high level of adequate functional outcomes, implant irritation was found to be “quite frequent.”
To address this issue, Carpenter and colleagues11 evaluated an ATB technique that uses K-wires instead of cannulated screws. Biomechanical testing in a cadaver model revealed less fracture displacement and overall more repair strength through cyclic and maximum load testing. Clinically, these results were supported by Berg,10 who followed up 10 patients with transverse patella fractures repaired with the MATB technique. At a mean follow-up of 24 months, 7 of the 10 patients had good to excellent outcomes, and there were no implant failures.
Further investigation into patella repairs has mainly focused on improving the MATB technique and experimenting with different tension band materials. Rabalais and colleagues13 biomechanically tested high-strength polyethylene suture as a replacement for standard 18-gauge wire, and Bryant and colleagues14 tested a braided composite suture (FiberWire; Arthrex) as a replacement for standard 18-gauge stainless steel wire. Both found no significant difference with use of augmented tension band material, but Rabalais and colleagues13 did find more advantages with a parallel tension band construct than with a standard figure-of-8 arrangement.
In developing our novel technique, we considered that Krackow sutures are routinely used in both quadriceps tendon repair and patellar tendon repair, including partial patellectomy for distal patella fracture. With a suture placed in both tendons, the augment could be expected to resist longitudinal gapping and augment the tension band across the anterior patella. First described by Krackow and colleagues,19 the Krackow suture is widely used for tendon reconstruction. In an interlocking system of sutures, the Krackow suture provides a repair that is more stable than repair with conventional suture techniques, specifically in the context of tendon repair.20 Given the sesamoidal nature of the patella, its repair shares the goal of gap prevention with other tendon repairs. In theory, anchoring the supporting structures that are above and below the patella provides support for the intervening patella and ultimately improves fracture fixation strength.
Oh and colleagues15 reported on the clinical efficacy of a Krackow augment in distal pole patella repairs. Similarly, we found a Krackow augment to be efficacious, supporting its potential in clinical approaches to patella repairs. Our results indicate this augment can be a useful clinical adjunct in biomechanical evaluation.
Limitations of this study include its use of dissected extensor mechanisms, which may have less biofidelity than whole-knee specimens. In our model, specimens were secured at the patellar tendon and the quadriceps tendons, as opposed to the quadriceps tendon and the tibia distally. Use of this model could have led to an increase in early displacement during cyclic testing as a result of tissue slippage. Furthermore, our small sample size could have affected our ability to demonstrate a difference between these techniques.
Given its increased strength as demonstrated by mean displacement during cyclic loading and mean load to failure, as well as the early clinical data recently published, the Krackow suture augment represents a feasible technique for patella fixation. It likely will be most useful in cases in which conventional techniques are prone to failure or cannot be applied, such as severe distal comminution or poor bone density. Further biomechanical testing with a larger number of specimens may be required for statistical significance.
Conclusion
In patella fracture repair strategies, the Krackow suture augment increased strength when used with a MATB technique. Failure to reach statistical significance likely resulted from our small sample size. Further biomechanical testing and clinical studies are needed for more complete evaluation of this technique. We think it will be most useful in the setting of poor bone quality or severe comminution, which can limit fixation options. As increased repair strength allows earlier postoperative rehabilitation and maintains fracture reduction, patient outcomes should improve. This novel technique represents another strategy for managing challenging patella fractures.
Take-Home Points
- Suture augmentation improves construct strength for patella fixation.
- Krackow sutures may be placed in the quadriceps and patella tendons, then secured over the anterior patella (much like an anterior tension band).
- The Krackow technique described was superior to the suture cerclage technique based on mean load values, but did not reach statistical significance.
- The Krackow suture technique is a viable and easily applied technique for suture augmentation of patella fixation constructs.
Patella fractures are relatively uncommon, accounting for only 1% of skeletal injuries.1 Restoration of the function of the patella and the extensor mechanism is vital for knee extension and gait. However, patella fractures have an inherently high rate of complications, making these injuries challenging to treat.2-4 In patients with intact extensor function, displacement of <4 mm, and articular step-off of <3 mm, nonoperative management is extremely effective, with 99% of patients reporting favorable results.5 However, for fractures in which the extensor mechanism is disrupted, surgical intervention typically is indicated.6
Authors have reported various surgical interventions, one of the most commonly used being the anterior tension band (ATB) technique, first described by the AO (Arbeitsgemeinschaft für Osteosynthesefragen) group in the 1950s.7 By converting distractive anterior force during knee flexion to compressive force at the fracture site, the ATB technique provides a repair stronger than the previously used cerclage repair.8 Although initially considered standard of care, the ATB technique was soon found to be associated with implant failure and subcutaneous irritation prompting implant removal.9
To address these issues, Berg10 and Carpenter and colleagues11 evaluated an ATB technique that used cannulated screws instead of Kirschner wires (K-wires). This variation on the ATB technique reduced the implant-related issues while maintaining the mechanical advantage of the tension band. The more rigid design also permitted earlier postoperative rehabilitation, which significantly reduced development of arthrofibrosis.6,7,10 This modified ATB (MATB) technique has since been investigated for additional augments, mainly focusing on use of different tension band materials, including polyester suture and braided composite suture.12-14
However, there is little research on augments that incorporate the surrounding soft tissue, specifically the quadriceps and patellar tendons. In a recent retrospective clinical study, Oh and colleagues15 found positive clinical results with use of Krackow sutures, though 2 or 3 vertically oriented stainless steel wires were used instead of cannulated screws.
We conducted a study to determine the biomechanical efficacy of using a cerclage suture augment and a Krackow suture augment coupled with and compared with conventional MATB repair. If effective, this technique may represent another strategy for increasing repair strength and thereby improve postoperative outcomes.
Materials and Methods
Specimen Preparation
Fresh-frozen cadaver extensor mechanisms (quadriceps tendon, patella, surrounding retinaculum, patellar tendon) were kept frozen at –4°C until preparation. Fifteen specimens were selected. Mean (SD) age at death was 68 (10) years (range, 51-85 years). One specimen was excluded for a short patella tendon, which precluded adequate attachment for testing. All specimens were free of overt osseous pathology.
After specimens were thawed overnight, the patellae were transversely osteotomized with an osteotome at the junction of the middle and distal thirds of the patella. Sharp dissection was performed to carry the division through the medial and lateral retinaculum at the same level. All 14 specimens were then repaired using the MATB technique. First, the transverse fracture was reduced with a reduction clamp. Then, two 4-mm cannulated screws (DePuy Synthes) were inserted parallel to each other and perpendicular to the fracture. An 18-gauge stainless steel wire was then passed through each screw, crossed anteriorly, and tightened to create a figure-of-8 ATB. The specimens were then randomly divided into 3 groups—MATB; MATB with cerclage suture augment; MATB with Krackow suture augment—while ensuring specimens from a single cadaver were placed in different groups to avoid confounding based on bone density differences.
Experimental Setup
Repaired specimens were secured with tissue clamps at the quadriceps and patellar tendons on an MTS Bionix 858 (MTS Systems) hydraulic arm.
Each patella was secured for cyclic testing. Initially it was placed under 10 N of tension. Then it underwent tensile loading from 10 N to 300 N at 50 N/s for 10 cycles. These parameters were based on previous biomechanical patella studies.10,11 Load was measured with the MTS load cell and displacement with the displacement transducer. Fracture displacement associated with 300-N cyclic tension was recorded. Displacement was calculated as the difference between 10th cycle and 2nd cycle values, which accounted for any degree of initial tissue slippage. After cyclic testing, the patella was placed back in 10 N of tensile loading and subjected to maximum force loading to determine ultimate repair strength. For maximum loading, the patella was stretched progressively at 50 N/s until failure. Again, load and displacement were measured with MTS.
Statistical Analysis
After testing, fracture displacement and maximum load force data were compiled for analysis. One-way analysis of variance with Bonferroni correction was used to determine if there were significant differences between groups. Significance level was set at P < .05.
Results
For cyclic testing, mean total displacement was measured over 10 cycles for each group. Again, displacement was determined by taking the difference between 10th cycle and 2nd cycle values, allowing for system stabilization.
Discussion
Our main objective was to compare the efficacy of a novel suture augment technique with that of other patella fracture repair techniques. Our hypothesis—that adding a Krackow suture augment would increase strength in both cyclic and maximum loading—was supported. Although testing results were not statistically significant because of the small sample size, we think this novel technique has clinically relevant descriptive significance and warrants further investigation.
Proper anatomical reduction and postoperative stabilization are of utmost importance in clinical approaches to patella fractures. In addition, regardless of which technical procedure is used, open reduction should also allow for early range of motion to prevent joint arthrofibrosis. Ever since the ATB technique was first described by the AO group, postoperative outcomes have improved significantly. In a retrospective study by Levack and colleagues,17 30 of 64 patients with patella fractures underwent internal fixation. Mean follow-up was 6.2 years. By both objective and subjective measures, the best functional outcomes were associated with internal tension band fixation (vs cerclage repair). Lotke and Ecker18 also documented the efficacy of the tension band technique. Sixteen patients with patella fractures underwent anterior tension banding; those with a comminuted fracture also underwent cerclage repair for patella stabilization during tension banding. At 6-week follow-up, all patients had good range of motion (≥90° flexion), relatively few symptoms, and no implant failures. Results were similar to those of Levack and colleagues.17
Although it improves stability and functional outcomes over conventional patellectomy and cerclage wiring, the ATB technique has been associated with subcutaneous irritation caused by the K-wires used to secure the band. Hung and colleagues9 followed up 68 patients with patellar fractures. Five of these patients underwent tension banding. Although there was a high level of adequate functional outcomes, implant irritation was found to be “quite frequent.”
To address this issue, Carpenter and colleagues11 evaluated an ATB technique that uses K-wires instead of cannulated screws. Biomechanical testing in a cadaver model revealed less fracture displacement and overall more repair strength through cyclic and maximum load testing. Clinically, these results were supported by Berg,10 who followed up 10 patients with transverse patella fractures repaired with the MATB technique. At a mean follow-up of 24 months, 7 of the 10 patients had good to excellent outcomes, and there were no implant failures.
Further investigation into patella repairs has mainly focused on improving the MATB technique and experimenting with different tension band materials. Rabalais and colleagues13 biomechanically tested high-strength polyethylene suture as a replacement for standard 18-gauge wire, and Bryant and colleagues14 tested a braided composite suture (FiberWire; Arthrex) as a replacement for standard 18-gauge stainless steel wire. Both found no significant difference with use of augmented tension band material, but Rabalais and colleagues13 did find more advantages with a parallel tension band construct than with a standard figure-of-8 arrangement.
In developing our novel technique, we considered that Krackow sutures are routinely used in both quadriceps tendon repair and patellar tendon repair, including partial patellectomy for distal patella fracture. With a suture placed in both tendons, the augment could be expected to resist longitudinal gapping and augment the tension band across the anterior patella. First described by Krackow and colleagues,19 the Krackow suture is widely used for tendon reconstruction. In an interlocking system of sutures, the Krackow suture provides a repair that is more stable than repair with conventional suture techniques, specifically in the context of tendon repair.20 Given the sesamoidal nature of the patella, its repair shares the goal of gap prevention with other tendon repairs. In theory, anchoring the supporting structures that are above and below the patella provides support for the intervening patella and ultimately improves fracture fixation strength.
Oh and colleagues15 reported on the clinical efficacy of a Krackow augment in distal pole patella repairs. Similarly, we found a Krackow augment to be efficacious, supporting its potential in clinical approaches to patella repairs. Our results indicate this augment can be a useful clinical adjunct in biomechanical evaluation.
Limitations of this study include its use of dissected extensor mechanisms, which may have less biofidelity than whole-knee specimens. In our model, specimens were secured at the patellar tendon and the quadriceps tendons, as opposed to the quadriceps tendon and the tibia distally. Use of this model could have led to an increase in early displacement during cyclic testing as a result of tissue slippage. Furthermore, our small sample size could have affected our ability to demonstrate a difference between these techniques.
Given its increased strength as demonstrated by mean displacement during cyclic loading and mean load to failure, as well as the early clinical data recently published, the Krackow suture augment represents a feasible technique for patella fixation. It likely will be most useful in cases in which conventional techniques are prone to failure or cannot be applied, such as severe distal comminution or poor bone density. Further biomechanical testing with a larger number of specimens may be required for statistical significance.
Conclusion
In patella fracture repair strategies, the Krackow suture augment increased strength when used with a MATB technique. Failure to reach statistical significance likely resulted from our small sample size. Further biomechanical testing and clinical studies are needed for more complete evaluation of this technique. We think it will be most useful in the setting of poor bone quality or severe comminution, which can limit fixation options. As increased repair strength allows earlier postoperative rehabilitation and maintains fracture reduction, patient outcomes should improve. This novel technique represents another strategy for managing challenging patella fractures.
1. Boström Å. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
2. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
3. LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426.
4. Kaufer H. Mechanical function of the patella. J Bone Joint Surg Am. 1971;53(8):1551-1560.
5. Braun W, Wiedemann M, Rüter A, Kundel K, Kolbinger S. Indications and results of nonoperative treatment of patellar fractures. Clin Orthop Relat Res. 1993;(289):197-201.
6. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19(4):198-207.
7. Müller M, Allgöwer M, Schneider R, Willeneger H. Manual of Internal Fixation: Techniques Recommended by the AO Group. Berlin, Germany: Springer; 1979.
8. Weber MJ, Janecki CJ, McLeod P, Nelson CL, Thompson JA. Efficacy of various forms of fixation of transverse fractures of the patella. J Bone Joint Surg Am. 1980;62(2):215-220.
9. Hung LK, Chan KM, Chow YN, Leung PC. Fractured patella: operative treatment using the tension band principle. Injury. 1985;16(5):343-347.
10. Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
11. Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
12. Hughes SC, Stott PM, Hearnden AJ, Ripley LG. A new and effective tension-band braided polyester suture technique for transverse patellar fracture fixation. Injury. 2007;38(2):212-222.
13. Rabalais RD, Burger E, Lu Y, Mansour A, Baratta RV. Comparison of two tension-band fixation materials and techniques in transverse patella fractures: a biomechanical study. Orthopedics. 2008;31(2):128.
14. Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: a pilot study. J Orthop. 2014;12(2):92-96.
15. Oh HK, Choo SK, Kim JW, Lee M. Internal fixation of displaced inferior pole of the patella fractures using vertical wiring augmented with Krachow suturing. Injury. 2015;46(12):2512-2515.
16. Goodfellow J, Hungerford DS, Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58(3):287-290.
17. Levack B, Flannagan JP, Hobbs S. Results of surgical treatment of patellar fractures. J Bone Joint Surg Br. 1985;67(3):416-419.
18. Lotke PA, Ecker ML. Transverse fractures of the patella. Clin Orthop Relat Res. 1981;(158):180-184.
19. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
20. Hahn JM, Inceoğlu S, Wongworawat MD. Biomechanical comparison of Krackow locking stitch versus nonlocking loop stitch with varying number of throws. Am J Sports Med. 2014;42(12):3003-3008.
1. Boström Å. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
2. Hungerford DS, Barry M. Biomechanics of the patellofemoral joint. Clin Orthop Relat Res. 1979;(144):9-15.
3. LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426.
4. Kaufer H. Mechanical function of the patella. J Bone Joint Surg Am. 1971;53(8):1551-1560.
5. Braun W, Wiedemann M, Rüter A, Kundel K, Kolbinger S. Indications and results of nonoperative treatment of patellar fractures. Clin Orthop Relat Res. 1993;(289):197-201.
6. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19(4):198-207.
7. Müller M, Allgöwer M, Schneider R, Willeneger H. Manual of Internal Fixation: Techniques Recommended by the AO Group. Berlin, Germany: Springer; 1979.
8. Weber MJ, Janecki CJ, McLeod P, Nelson CL, Thompson JA. Efficacy of various forms of fixation of transverse fractures of the patella. J Bone Joint Surg Am. 1980;62(2):215-220.
9. Hung LK, Chan KM, Chow YN, Leung PC. Fractured patella: operative treatment using the tension band principle. Injury. 1985;16(5):343-347.
10. Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
11. Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
12. Hughes SC, Stott PM, Hearnden AJ, Ripley LG. A new and effective tension-band braided polyester suture technique for transverse patellar fracture fixation. Injury. 2007;38(2):212-222.
13. Rabalais RD, Burger E, Lu Y, Mansour A, Baratta RV. Comparison of two tension-band fixation materials and techniques in transverse patella fractures: a biomechanical study. Orthopedics. 2008;31(2):128.
14. Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: a pilot study. J Orthop. 2014;12(2):92-96.
15. Oh HK, Choo SK, Kim JW, Lee M. Internal fixation of displaced inferior pole of the patella fractures using vertical wiring augmented with Krachow suturing. Injury. 2015;46(12):2512-2515.
16. Goodfellow J, Hungerford DS, Zindel M. Patello-femoral joint mechanics and pathology. 1. Functional anatomy of the patello-femoral joint. J Bone Joint Surg Br. 1976;58(3):287-290.
17. Levack B, Flannagan JP, Hobbs S. Results of surgical treatment of patellar fractures. J Bone Joint Surg Br. 1985;67(3):416-419.
18. Lotke PA, Ecker ML. Transverse fractures of the patella. Clin Orthop Relat Res. 1981;(158):180-184.
19. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
20. Hahn JM, Inceoğlu S, Wongworawat MD. Biomechanical comparison of Krackow locking stitch versus nonlocking loop stitch with varying number of throws. Am J Sports Med. 2014;42(12):3003-3008.
Minimally Invasive Anatomical Reconstruction of Posteromedial Corner of Knee: A Cadaveric Study
Take-Home Points
- Injuries to the medial knee are the most common knee ligament injuries, and often occur in the athletic population.
- Complete posteromedial corner injuries require surgical treatment to restore joint stability and biomechanics.
- Biomechanical evidence has demonstrated an important load-sharing distribution between the sMCL and the POL.
- Valgus instability caused by a medial side injury, can lead to both ACL/posterior cruciate ligament reconstruction graft failure if the medial sided injury is not concurrently repaired or reconstructed.
- Anatomic posteromedial corner reconstruction yields excellent biomechanical and patient-reported outcomes.
Most injuries of the medial structures of the knee are treated conservatively.1-3 In severe acute injuries and chronic symptomatic instabilities, however, surgical treatment is needed to restore knee stability and to prevent degenerative changes secondary to instability.4 Three structures involved in medial stability are the superficial medial collateral ligament (sMCL), which is the primary valgus restraint; the posterior oblique ligament (POL), which is the primary restraint to internal rotation and the secondary valgus restraint; and the semimembranosus.5,6
Surgical techniques for posteromedial knee reconstruction include direct repair,7 repair with augmentation,8,9 advancement of the tibial insertion of the sMCL,10 and transfer of the pes anserine tendons.11 In anatomical reconstruction of the posteromedial corner, which has been described before, the sMCL and the POL are reconstructed to reproduce the native motion and stability of the knee.12 Clinically, repair and reconstruction have similar patient-reported outcomes and medial opening evaluations over the short term.
These approaches require large incisions and extensive dissection of soft tissue on the medial aspect of the knee.5 Given these drawbacks, it is reasonable to consider less invasive options. Minimally invasive surgery has the advantages of reduced scarring and blood loss, less disruption of surrounding tissue, faster recovery, and improved aesthetics.4
We conducted a study of a minimally invasive technique for reconstructing the posteromedial structures of the knee. We compared medial compartment stability measured on valgus stress radiographs in intact, sectioned, and reconstructed states in cadaveric knees. We hypothesized that a minimally invasive technique using autogenous hamstring graft in the appropriate anatomical location would return valgus stability to its nearly native state.
Materials and Methods
This study was conducted at the Buenos Aires British Hospital in Buenos Aires, Argentina, and at the University of Colorado Hospital in Aurora. Ten fresh-frozen cadaveric knees with no evidence of ligamentous injuries, osteoarthritis, or previous surgery were used. Mean donor age was 69.4 years (range, 45-87 years). Each specimen was maintained at room temperature for 24 hours before use. The femur was sectioned 20 cm proximal to the knee joint. The tibia was sectioned 12.5 cm distal to the knee joint.
Identification and Sectioning of Posteromedial Structures
After intact-state evaluation, each knee’s sMCL, dMCL, and POL were sectioned at their tibial insertion. Valgus stress radiograph was repeated and medial compartment gap was remeasured for comparison of the sectioned state with the intact and reconstructed states.
Anatomical Reconstruction With Mini-Invasive Technique
After sectioning of medial stabilizing structures, minimally invasive reconstruction was performed through 2 small incisions on the medial aspect of each of the 10 knees, as follows. First, the semitendinosus tendon was identified through the oblique incision that had been used for sectioning. Then, an open-ended tendon stripper was placed around the circumference of the semitendinosus and was passed proximomedially, transecting the tendon at its musculotendinous junction. While the tendon stripper was being passed, care was taken to maintain the nearby tibial insertion of the sartorius fascia (Figures 1D-1F).
With the semitendinosus tendon looped around the wire, isometricity was tested by pulling the suture within the tendon and moving the knee through a full range of motion. The isometric point was confirmed by tendon migration of <2 mm.13 Migration was measured by marking the graft 2 mm from its insertion; the graft was then pulled to ensure correct isometric point position. An 18-mm cannulated spiked screw and washer (Arthrex) were then passed over the wire and partially secured to the femur—the attachment point for the proximal sMCL portion of the semitendinosus graft. The semitendinosus tendon was then secured beneath the spiked washer with the knee in 20° of flexion with neutral rotation, recreating the sMCL.
Posteriorly, the distal insertion site of the POL was identified at the posteromedial aspect of the tibia through the oblique incision previously described. A 7-mm tunnel was drilled starting posteromedial (10 mm under tibial articular surface) and exiting just distal and medial to the Gerdy tubercle.
After final fixation, the medial knee was openly dissected to assess the inverted-V ligament reconstruction for anatomical placement and avoidance of crucial structures.
Stability Testing
Per International Knee Documentation Committee guidelines for stressing the medial compartment,14 valgus stress radiographs were obtained for all specimens at 0° and 20° of flexion in intact, sectioned, and reconstructed states.
The medial gap formed by the femoral condyle and its corresponding tibial plateau (at site of maximal separation) was tested in all 3 state conditions (intact, sectioned, reconstructed). Distances were digitally measured with a picture archiving and communication system viewer (Imagecast; IDX Systems Corporation). Medial gap was measured by taking the shortest distance between the subchondral bone surface of the most distal aspect of the medial femoral condyle and the corresponding medial tibial plateau. Three independent examiners took all the measurements; each examiner was blinded to the others’ measurements.
Statistics
Paired Student t tests were used to compare the 3 conditions, and the Shapiro-Wilk test was used to check for a normally distributed population. Statistical significance was set at P < .05. Statistical analyses were performed with GraphPad software.
Results
In all 10 specimens, the sMCL, the dMCL, and the POL were successfully identified and sectioned through a medial oblique incision over the distal insertion of the structures.
During all valgus testing states, there was no loss of graft fixation, and there was no gross graft slippage. In addition, all grafts remained in continuity with no evidence of failure, and there were no failures or breakages of the proximal or distal screw.
After posteromedial sectioning, mean medial gap was statistically significantly larger (P = .0002) at full extension (11 mm vs 3.3 mm) and at 20° of flexion (12.6 mm vs 3.8 mm). There was no statistically significant difference between the value of the intact state and the value after minimally invasive reconstruction at 0° (P = .56) or 20° (P = .102) of flexion.
Discussion
In this article, we describe a minimally invasive technique for anatomical posteromedial reconstruction of the knee in a cadaveric model. This technique restores the knee’s native valgus stability without causing extensive damage to the surrounding soft tissues and thereby potentially prevents scar formation and reduces blood loss.
Superficial MCL injury, one of the most common knee ligament injuries, is often associated with POL injury.7 Although most sMCL injuries are treated nonoperatively, with good results,3 surgical treatment is needed for severe (grade III) instabilities, symptomatic chronic instabilities, and knee dislocations.12,17 Most posteromedial reconstruction techniques require an extensive approach that causes damage to surrounding soft tissue,6,7,9,10 which in turn may compromise healing and positive patient outcomes. Surgical techniques include direct repair with sutures or anchors,18 capsular procedures,19 augmentations,9 internal bracing,6 and complete reconstruction of the posteromedial corner.20
LaPrade and Wijdicks12 have previously described anatomical reconstruction of the posteromedial corner. In their technique, a split semitendinosus autograft is used to reconstruct the sMCL and the POL separately, using 4 implants and reproducing each ligament’s anatomical attachment site. In this proposed technique, the distal attachment of the semitendinosus insertion is left intact, and uses 1 attachment point on the distal femur and 1 on the proximal tibia, allowing use of only 2 implants. In addition, it is performed with a minimally invasive approach, reduces cost, limits surgical exposure, and with experience may shorten operative time. To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL tibial attachment as posterior as possible, which can be performed with this minimally invasive approach as well.
To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL as posterior as possible. Despite the potential for increased graft stress with an anterior position, as in our modified technique, our group of 10 knees had no graft fixation failures in isolated valgus stress testing in either extension or flexion. Our minimally invasive posteromedial knee reconstruction significantly improved knee stability over the sectioned state as well as medial compartment gapping with valgus stress. There was no significant difference in medial compartment gapping between the intact and reconstructed states.
Our technique was built on open procedures (described by Kim and colleagues13) that carefully identify the isometric point of the graft. In addition, it adopted the modification (proposed by Lind and colleagues21) in which a fixation point is added at the distal insertion of the POL instead of being sutured to the direct arm of the semitendinosus tendon.
Furthermore, our technique, despite being similar to those described by Dong and colleagues22 and Borden and colleagues,23 has the advantages of minimally invasive surgery and reduced disruption of soft tissues. Dong and colleagues22 reported on 64 patients with a mean follow-up of 34 months; patients’ medial opening measurements were significantly decreased at follow-up and fell within the normal range.
The present study had several limitations. First, the age of our specimens was higher than the mean age of patients with knee ligament injury, potentially leading to firmer or more fibrotic tendons less susceptible to elongation. Second, we did not evaluate the knees’ rotational stability, and anterior cruciate ligaments (ACLs) were intact. As most posteromedial injuries co-occur with ACL injuries, a more realistic situation would have been reproduced by assessing rotational stability while performing both ACL reconstruction and the proposed posteromedial reconstruction. Third, static specimen measurements do not reflect the dynamic function of the posteromedial corner. Prospective clinical studies are needed to assess the true effectiveness of the posteromedial corner in the clinical scenario.
Knowledge of the anatomy of the medial aspect of the knee is vital to reconstruction of the medial side of the knee. Our results suggest that a minimally invasive technique can restore valgus stability without the need for extensive dissection and disruption of surrounding soft tissues. More research is needed to determine the results of this technique in vivo.
1. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
2. Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65(3):323-329.
3. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Rel Res. 1990;(256):174-177.
4. Jeng CL, Bluman EM, Myerson MS. Minimally invasive deltoid ligament reconstruction for stage IV flatfoot deformity. Foot Ankle Int. 2011;32(1):21-30.
5. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347.
6. Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505-e508.
7. Hughston JC, Eilers AF. The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am. 1973;55(5):923-940.
8. Gorin S, Paul DD, Wilkinson EJ. An anterior cruciate ligament and medial collateral ligament tear in a skeletally immature patient: a new technique to augment primary repair of the medial collateral ligament and an allograft reconstruction of the anterior cruciate ligament. Arthroscopy. 2003;19(10):E21-E26.
Take-Home Points
- Injuries to the medial knee are the most common knee ligament injuries, and often occur in the athletic population.
- Complete posteromedial corner injuries require surgical treatment to restore joint stability and biomechanics.
- Biomechanical evidence has demonstrated an important load-sharing distribution between the sMCL and the POL.
- Valgus instability caused by a medial side injury, can lead to both ACL/posterior cruciate ligament reconstruction graft failure if the medial sided injury is not concurrently repaired or reconstructed.
- Anatomic posteromedial corner reconstruction yields excellent biomechanical and patient-reported outcomes.
Most injuries of the medial structures of the knee are treated conservatively.1-3 In severe acute injuries and chronic symptomatic instabilities, however, surgical treatment is needed to restore knee stability and to prevent degenerative changes secondary to instability.4 Three structures involved in medial stability are the superficial medial collateral ligament (sMCL), which is the primary valgus restraint; the posterior oblique ligament (POL), which is the primary restraint to internal rotation and the secondary valgus restraint; and the semimembranosus.5,6
Surgical techniques for posteromedial knee reconstruction include direct repair,7 repair with augmentation,8,9 advancement of the tibial insertion of the sMCL,10 and transfer of the pes anserine tendons.11 In anatomical reconstruction of the posteromedial corner, which has been described before, the sMCL and the POL are reconstructed to reproduce the native motion and stability of the knee.12 Clinically, repair and reconstruction have similar patient-reported outcomes and medial opening evaluations over the short term.
These approaches require large incisions and extensive dissection of soft tissue on the medial aspect of the knee.5 Given these drawbacks, it is reasonable to consider less invasive options. Minimally invasive surgery has the advantages of reduced scarring and blood loss, less disruption of surrounding tissue, faster recovery, and improved aesthetics.4
We conducted a study of a minimally invasive technique for reconstructing the posteromedial structures of the knee. We compared medial compartment stability measured on valgus stress radiographs in intact, sectioned, and reconstructed states in cadaveric knees. We hypothesized that a minimally invasive technique using autogenous hamstring graft in the appropriate anatomical location would return valgus stability to its nearly native state.
Materials and Methods
This study was conducted at the Buenos Aires British Hospital in Buenos Aires, Argentina, and at the University of Colorado Hospital in Aurora. Ten fresh-frozen cadaveric knees with no evidence of ligamentous injuries, osteoarthritis, or previous surgery were used. Mean donor age was 69.4 years (range, 45-87 years). Each specimen was maintained at room temperature for 24 hours before use. The femur was sectioned 20 cm proximal to the knee joint. The tibia was sectioned 12.5 cm distal to the knee joint.
Identification and Sectioning of Posteromedial Structures
After intact-state evaluation, each knee’s sMCL, dMCL, and POL were sectioned at their tibial insertion. Valgus stress radiograph was repeated and medial compartment gap was remeasured for comparison of the sectioned state with the intact and reconstructed states.
Anatomical Reconstruction With Mini-Invasive Technique
After sectioning of medial stabilizing structures, minimally invasive reconstruction was performed through 2 small incisions on the medial aspect of each of the 10 knees, as follows. First, the semitendinosus tendon was identified through the oblique incision that had been used for sectioning. Then, an open-ended tendon stripper was placed around the circumference of the semitendinosus and was passed proximomedially, transecting the tendon at its musculotendinous junction. While the tendon stripper was being passed, care was taken to maintain the nearby tibial insertion of the sartorius fascia (Figures 1D-1F).
With the semitendinosus tendon looped around the wire, isometricity was tested by pulling the suture within the tendon and moving the knee through a full range of motion. The isometric point was confirmed by tendon migration of <2 mm.13 Migration was measured by marking the graft 2 mm from its insertion; the graft was then pulled to ensure correct isometric point position. An 18-mm cannulated spiked screw and washer (Arthrex) were then passed over the wire and partially secured to the femur—the attachment point for the proximal sMCL portion of the semitendinosus graft. The semitendinosus tendon was then secured beneath the spiked washer with the knee in 20° of flexion with neutral rotation, recreating the sMCL.
Posteriorly, the distal insertion site of the POL was identified at the posteromedial aspect of the tibia through the oblique incision previously described. A 7-mm tunnel was drilled starting posteromedial (10 mm under tibial articular surface) and exiting just distal and medial to the Gerdy tubercle.
After final fixation, the medial knee was openly dissected to assess the inverted-V ligament reconstruction for anatomical placement and avoidance of crucial structures.
Stability Testing
Per International Knee Documentation Committee guidelines for stressing the medial compartment,14 valgus stress radiographs were obtained for all specimens at 0° and 20° of flexion in intact, sectioned, and reconstructed states.
The medial gap formed by the femoral condyle and its corresponding tibial plateau (at site of maximal separation) was tested in all 3 state conditions (intact, sectioned, reconstructed). Distances were digitally measured with a picture archiving and communication system viewer (Imagecast; IDX Systems Corporation). Medial gap was measured by taking the shortest distance between the subchondral bone surface of the most distal aspect of the medial femoral condyle and the corresponding medial tibial plateau. Three independent examiners took all the measurements; each examiner was blinded to the others’ measurements.
Statistics
Paired Student t tests were used to compare the 3 conditions, and the Shapiro-Wilk test was used to check for a normally distributed population. Statistical significance was set at P < .05. Statistical analyses were performed with GraphPad software.
Results
In all 10 specimens, the sMCL, the dMCL, and the POL were successfully identified and sectioned through a medial oblique incision over the distal insertion of the structures.
During all valgus testing states, there was no loss of graft fixation, and there was no gross graft slippage. In addition, all grafts remained in continuity with no evidence of failure, and there were no failures or breakages of the proximal or distal screw.
After posteromedial sectioning, mean medial gap was statistically significantly larger (P = .0002) at full extension (11 mm vs 3.3 mm) and at 20° of flexion (12.6 mm vs 3.8 mm). There was no statistically significant difference between the value of the intact state and the value after minimally invasive reconstruction at 0° (P = .56) or 20° (P = .102) of flexion.
Discussion
In this article, we describe a minimally invasive technique for anatomical posteromedial reconstruction of the knee in a cadaveric model. This technique restores the knee’s native valgus stability without causing extensive damage to the surrounding soft tissues and thereby potentially prevents scar formation and reduces blood loss.
Superficial MCL injury, one of the most common knee ligament injuries, is often associated with POL injury.7 Although most sMCL injuries are treated nonoperatively, with good results,3 surgical treatment is needed for severe (grade III) instabilities, symptomatic chronic instabilities, and knee dislocations.12,17 Most posteromedial reconstruction techniques require an extensive approach that causes damage to surrounding soft tissue,6,7,9,10 which in turn may compromise healing and positive patient outcomes. Surgical techniques include direct repair with sutures or anchors,18 capsular procedures,19 augmentations,9 internal bracing,6 and complete reconstruction of the posteromedial corner.20
LaPrade and Wijdicks12 have previously described anatomical reconstruction of the posteromedial corner. In their technique, a split semitendinosus autograft is used to reconstruct the sMCL and the POL separately, using 4 implants and reproducing each ligament’s anatomical attachment site. In this proposed technique, the distal attachment of the semitendinosus insertion is left intact, and uses 1 attachment point on the distal femur and 1 on the proximal tibia, allowing use of only 2 implants. In addition, it is performed with a minimally invasive approach, reduces cost, limits surgical exposure, and with experience may shorten operative time. To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL tibial attachment as posterior as possible, which can be performed with this minimally invasive approach as well.
To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL as posterior as possible. Despite the potential for increased graft stress with an anterior position, as in our modified technique, our group of 10 knees had no graft fixation failures in isolated valgus stress testing in either extension or flexion. Our minimally invasive posteromedial knee reconstruction significantly improved knee stability over the sectioned state as well as medial compartment gapping with valgus stress. There was no significant difference in medial compartment gapping between the intact and reconstructed states.
Our technique was built on open procedures (described by Kim and colleagues13) that carefully identify the isometric point of the graft. In addition, it adopted the modification (proposed by Lind and colleagues21) in which a fixation point is added at the distal insertion of the POL instead of being sutured to the direct arm of the semitendinosus tendon.
Furthermore, our technique, despite being similar to those described by Dong and colleagues22 and Borden and colleagues,23 has the advantages of minimally invasive surgery and reduced disruption of soft tissues. Dong and colleagues22 reported on 64 patients with a mean follow-up of 34 months; patients’ medial opening measurements were significantly decreased at follow-up and fell within the normal range.
The present study had several limitations. First, the age of our specimens was higher than the mean age of patients with knee ligament injury, potentially leading to firmer or more fibrotic tendons less susceptible to elongation. Second, we did not evaluate the knees’ rotational stability, and anterior cruciate ligaments (ACLs) were intact. As most posteromedial injuries co-occur with ACL injuries, a more realistic situation would have been reproduced by assessing rotational stability while performing both ACL reconstruction and the proposed posteromedial reconstruction. Third, static specimen measurements do not reflect the dynamic function of the posteromedial corner. Prospective clinical studies are needed to assess the true effectiveness of the posteromedial corner in the clinical scenario.
Knowledge of the anatomy of the medial aspect of the knee is vital to reconstruction of the medial side of the knee. Our results suggest that a minimally invasive technique can restore valgus stability without the need for extensive dissection and disruption of surrounding soft tissues. More research is needed to determine the results of this technique in vivo.
Take-Home Points
- Injuries to the medial knee are the most common knee ligament injuries, and often occur in the athletic population.
- Complete posteromedial corner injuries require surgical treatment to restore joint stability and biomechanics.
- Biomechanical evidence has demonstrated an important load-sharing distribution between the sMCL and the POL.
- Valgus instability caused by a medial side injury, can lead to both ACL/posterior cruciate ligament reconstruction graft failure if the medial sided injury is not concurrently repaired or reconstructed.
- Anatomic posteromedial corner reconstruction yields excellent biomechanical and patient-reported outcomes.
Most injuries of the medial structures of the knee are treated conservatively.1-3 In severe acute injuries and chronic symptomatic instabilities, however, surgical treatment is needed to restore knee stability and to prevent degenerative changes secondary to instability.4 Three structures involved in medial stability are the superficial medial collateral ligament (sMCL), which is the primary valgus restraint; the posterior oblique ligament (POL), which is the primary restraint to internal rotation and the secondary valgus restraint; and the semimembranosus.5,6
Surgical techniques for posteromedial knee reconstruction include direct repair,7 repair with augmentation,8,9 advancement of the tibial insertion of the sMCL,10 and transfer of the pes anserine tendons.11 In anatomical reconstruction of the posteromedial corner, which has been described before, the sMCL and the POL are reconstructed to reproduce the native motion and stability of the knee.12 Clinically, repair and reconstruction have similar patient-reported outcomes and medial opening evaluations over the short term.
These approaches require large incisions and extensive dissection of soft tissue on the medial aspect of the knee.5 Given these drawbacks, it is reasonable to consider less invasive options. Minimally invasive surgery has the advantages of reduced scarring and blood loss, less disruption of surrounding tissue, faster recovery, and improved aesthetics.4
We conducted a study of a minimally invasive technique for reconstructing the posteromedial structures of the knee. We compared medial compartment stability measured on valgus stress radiographs in intact, sectioned, and reconstructed states in cadaveric knees. We hypothesized that a minimally invasive technique using autogenous hamstring graft in the appropriate anatomical location would return valgus stability to its nearly native state.
Materials and Methods
This study was conducted at the Buenos Aires British Hospital in Buenos Aires, Argentina, and at the University of Colorado Hospital in Aurora. Ten fresh-frozen cadaveric knees with no evidence of ligamentous injuries, osteoarthritis, or previous surgery were used. Mean donor age was 69.4 years (range, 45-87 years). Each specimen was maintained at room temperature for 24 hours before use. The femur was sectioned 20 cm proximal to the knee joint. The tibia was sectioned 12.5 cm distal to the knee joint.
Identification and Sectioning of Posteromedial Structures
After intact-state evaluation, each knee’s sMCL, dMCL, and POL were sectioned at their tibial insertion. Valgus stress radiograph was repeated and medial compartment gap was remeasured for comparison of the sectioned state with the intact and reconstructed states.
Anatomical Reconstruction With Mini-Invasive Technique
After sectioning of medial stabilizing structures, minimally invasive reconstruction was performed through 2 small incisions on the medial aspect of each of the 10 knees, as follows. First, the semitendinosus tendon was identified through the oblique incision that had been used for sectioning. Then, an open-ended tendon stripper was placed around the circumference of the semitendinosus and was passed proximomedially, transecting the tendon at its musculotendinous junction. While the tendon stripper was being passed, care was taken to maintain the nearby tibial insertion of the sartorius fascia (Figures 1D-1F).
With the semitendinosus tendon looped around the wire, isometricity was tested by pulling the suture within the tendon and moving the knee through a full range of motion. The isometric point was confirmed by tendon migration of <2 mm.13 Migration was measured by marking the graft 2 mm from its insertion; the graft was then pulled to ensure correct isometric point position. An 18-mm cannulated spiked screw and washer (Arthrex) were then passed over the wire and partially secured to the femur—the attachment point for the proximal sMCL portion of the semitendinosus graft. The semitendinosus tendon was then secured beneath the spiked washer with the knee in 20° of flexion with neutral rotation, recreating the sMCL.
Posteriorly, the distal insertion site of the POL was identified at the posteromedial aspect of the tibia through the oblique incision previously described. A 7-mm tunnel was drilled starting posteromedial (10 mm under tibial articular surface) and exiting just distal and medial to the Gerdy tubercle.
After final fixation, the medial knee was openly dissected to assess the inverted-V ligament reconstruction for anatomical placement and avoidance of crucial structures.
Stability Testing
Per International Knee Documentation Committee guidelines for stressing the medial compartment,14 valgus stress radiographs were obtained for all specimens at 0° and 20° of flexion in intact, sectioned, and reconstructed states.
The medial gap formed by the femoral condyle and its corresponding tibial plateau (at site of maximal separation) was tested in all 3 state conditions (intact, sectioned, reconstructed). Distances were digitally measured with a picture archiving and communication system viewer (Imagecast; IDX Systems Corporation). Medial gap was measured by taking the shortest distance between the subchondral bone surface of the most distal aspect of the medial femoral condyle and the corresponding medial tibial plateau. Three independent examiners took all the measurements; each examiner was blinded to the others’ measurements.
Statistics
Paired Student t tests were used to compare the 3 conditions, and the Shapiro-Wilk test was used to check for a normally distributed population. Statistical significance was set at P < .05. Statistical analyses were performed with GraphPad software.
Results
In all 10 specimens, the sMCL, the dMCL, and the POL were successfully identified and sectioned through a medial oblique incision over the distal insertion of the structures.
During all valgus testing states, there was no loss of graft fixation, and there was no gross graft slippage. In addition, all grafts remained in continuity with no evidence of failure, and there were no failures or breakages of the proximal or distal screw.
After posteromedial sectioning, mean medial gap was statistically significantly larger (P = .0002) at full extension (11 mm vs 3.3 mm) and at 20° of flexion (12.6 mm vs 3.8 mm). There was no statistically significant difference between the value of the intact state and the value after minimally invasive reconstruction at 0° (P = .56) or 20° (P = .102) of flexion.
Discussion
In this article, we describe a minimally invasive technique for anatomical posteromedial reconstruction of the knee in a cadaveric model. This technique restores the knee’s native valgus stability without causing extensive damage to the surrounding soft tissues and thereby potentially prevents scar formation and reduces blood loss.
Superficial MCL injury, one of the most common knee ligament injuries, is often associated with POL injury.7 Although most sMCL injuries are treated nonoperatively, with good results,3 surgical treatment is needed for severe (grade III) instabilities, symptomatic chronic instabilities, and knee dislocations.12,17 Most posteromedial reconstruction techniques require an extensive approach that causes damage to surrounding soft tissue,6,7,9,10 which in turn may compromise healing and positive patient outcomes. Surgical techniques include direct repair with sutures or anchors,18 capsular procedures,19 augmentations,9 internal bracing,6 and complete reconstruction of the posteromedial corner.20
LaPrade and Wijdicks12 have previously described anatomical reconstruction of the posteromedial corner. In their technique, a split semitendinosus autograft is used to reconstruct the sMCL and the POL separately, using 4 implants and reproducing each ligament’s anatomical attachment site. In this proposed technique, the distal attachment of the semitendinosus insertion is left intact, and uses 1 attachment point on the distal femur and 1 on the proximal tibia, allowing use of only 2 implants. In addition, it is performed with a minimally invasive approach, reduces cost, limits surgical exposure, and with experience may shorten operative time. To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL tibial attachment as posterior as possible, which can be performed with this minimally invasive approach as well.
To reduce the graft failure rate, the technique of LaPrade and Wijdicks12 positions the sMCL as posterior as possible. Despite the potential for increased graft stress with an anterior position, as in our modified technique, our group of 10 knees had no graft fixation failures in isolated valgus stress testing in either extension or flexion. Our minimally invasive posteromedial knee reconstruction significantly improved knee stability over the sectioned state as well as medial compartment gapping with valgus stress. There was no significant difference in medial compartment gapping between the intact and reconstructed states.
Our technique was built on open procedures (described by Kim and colleagues13) that carefully identify the isometric point of the graft. In addition, it adopted the modification (proposed by Lind and colleagues21) in which a fixation point is added at the distal insertion of the POL instead of being sutured to the direct arm of the semitendinosus tendon.
Furthermore, our technique, despite being similar to those described by Dong and colleagues22 and Borden and colleagues,23 has the advantages of minimally invasive surgery and reduced disruption of soft tissues. Dong and colleagues22 reported on 64 patients with a mean follow-up of 34 months; patients’ medial opening measurements were significantly decreased at follow-up and fell within the normal range.
The present study had several limitations. First, the age of our specimens was higher than the mean age of patients with knee ligament injury, potentially leading to firmer or more fibrotic tendons less susceptible to elongation. Second, we did not evaluate the knees’ rotational stability, and anterior cruciate ligaments (ACLs) were intact. As most posteromedial injuries co-occur with ACL injuries, a more realistic situation would have been reproduced by assessing rotational stability while performing both ACL reconstruction and the proposed posteromedial reconstruction. Third, static specimen measurements do not reflect the dynamic function of the posteromedial corner. Prospective clinical studies are needed to assess the true effectiveness of the posteromedial corner in the clinical scenario.
Knowledge of the anatomy of the medial aspect of the knee is vital to reconstruction of the medial side of the knee. Our results suggest that a minimally invasive technique can restore valgus stability without the need for extensive dissection and disruption of surrounding soft tissues. More research is needed to determine the results of this technique in vivo.
1. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
2. Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65(3):323-329.
3. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Rel Res. 1990;(256):174-177.
4. Jeng CL, Bluman EM, Myerson MS. Minimally invasive deltoid ligament reconstruction for stage IV flatfoot deformity. Foot Ankle Int. 2011;32(1):21-30.
5. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347.
6. Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505-e508.
7. Hughston JC, Eilers AF. The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am. 1973;55(5):923-940.
8. Gorin S, Paul DD, Wilkinson EJ. An anterior cruciate ligament and medial collateral ligament tear in a skeletally immature patient: a new technique to augment primary repair of the medial collateral ligament and an allograft reconstruction of the anterior cruciate ligament. Arthroscopy. 2003;19(10):E21-E26.
1. Ellsasser JC, Reynolds FC, Omohundro JR. The non-operative treatment of collateral ligament injuries of the knee in professional football players. An analysis of seventy-four injuries treated non-operatively and twenty-four injuries treated surgically. J Bone Joint Surg Am. 1974;56(6):1185-1190.
2. Indelicato PA. Non-operative treatment of complete tears of the medial collateral ligament of the knee. J Bone Joint Surg Am. 1983;65(3):323-329.
3. Indelicato PA, Hermansdorfer J, Huegel M. Nonoperative management of complete tears of the medial collateral ligament of the knee in intercollegiate football players. Clin Orthop Rel Res. 1990;(256):174-177.
4. Jeng CL, Bluman EM, Myerson MS. Minimally invasive deltoid ligament reconstruction for stage IV flatfoot deformity. Foot Ankle Int. 2011;32(1):21-30.
5. Coobs BR, Wijdicks CA, Armitage BM, et al. An in vitro analysis of an anatomical medial knee reconstruction. Am J Sports Med. 2010;38(2):339-347.
6. Lubowitz JH, MacKay G, Gilmer B. Knee medial collateral ligament and posteromedial corner anatomic repair with internal bracing. Arthrosc Tech. 2014;3(4):e505-e508.
7. Hughston JC, Eilers AF. The role of the posterior oblique ligament in repairs of acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am. 1973;55(5):923-940.
8. Gorin S, Paul DD, Wilkinson EJ. An anterior cruciate ligament and medial collateral ligament tear in a skeletally immature patient: a new technique to augment primary repair of the medial collateral ligament and an allograft reconstruction of the anterior cruciate ligament. Arthroscopy. 2003;19(10):E21-E26.
A Systematic Review of 21 Tibial Tubercle Osteotomy Studies and More Than 1000 Knees: Indications, Clinical Outcomes, Complications, and Reoperations
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
Take-Home Points
- TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects.
- Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
- TTO was most commonly performed for isolated patellar instability in the presence of knee pain.
- Young women with prior surgery on the affected knee made up the primary patient population for this procedure.
- While TTO significantly improves knee pain and clinical outcome scores, >1 in 5 patients required reoperation for hardware removal.
Patellofemoral pain and patellofemoral instability are common orthopedic problems. Studies have found that 30% of patients 13 to 19 years old have patellofemoral pain and that 29 in 100,000 patients 10 to 17 years old have patellofemoral instability.1-3 The reported rate of recurrence after nonoperative management of patellofemoral instability is 33%.4 Tibial tubercle osteotomy (TTO), first described by Hauser5 in 1938, is an effective treatment option for many patellofemoral disorders.
TTO indications include patellofemoral maltracking or malalignment, patellar instability, patellofemoral arthritis, and focal patellofemoral chondral defects.6 With TTO, the goal is to move the tibial tubercle in a direction that will either improve patellar tracking or offload the medial or lateral patellar facet to improve pain and function.7,8 This action typically involves anterior, medial, lateral, or distal translation of the tibial tubercle, as posteriorization can lead to increased contact forces across the patellofemoral joint, resulting in accelerated patellofemoral wear and increased pain.9
We systematically reviewed the TTO literature to identify indications, clinical outcomes, complications, and reoperations. We hypothesized that the overall complication rate and the overall reoperation rate would both be <10%.
Clinical Evaluation of Patellofemoral Pathology
Patients with patellofemoral pain often report anterior knee pain, which typically begins gradually and is often activity related. Several symptoms may be present: pain with prolonged sitting with knees bent; pain on rising from a seated position; pain or crepitus with climbing stairs; and pain during repetitive activity such as running, squatting, or jumping. Location, duration, and onset of symptoms should be elicited. Patellofemoral instability can be described as dislocation events or subluxation events; number of events, mechanisms of injury, and resulting need for reduction should be documented. As age, sex, body mass index, and physical fitness are relevant to risk of recurrence, the physician should ask about general ligamentous laxity, other joint dislocations, and prior surgical intervention. Swelling or mechanical symptoms may indicate patellofemoral joint pathology.6,10
Physical examination of patients with patellofemoral pathology begins with assessment for overall limb alignment (including resting position of patella and corresponding quadriceps angle [Q-angle]), generalized ligamentous laxity (including hypermobile joints, evaluated with Brighton criteria), overall peri-knee muscle tone and strength, effusion, and gait pattern.
Common TTO Procedures
TTO specifics depend on anatomy, radiographic alignment characteristics, and presence of chondral defects. Essentially, the patella is translated to offload the affected areas. Osteotomy and movement of the tibial tubercle can include anteriorization, anteromedialization, proximalization, medialization, or distalization.
Methods
Search Strategy and Data Collection
We searched the PubMed (Medline) database for all English-language TTO studies published between database inception and April 9, 2015. After PROSPERO registration, and following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we used the algorithm (“tibial” AND “tubercle” AND “osteotomy”) NOT (“total” AND “knee” AND “arthroplasty”) to search the literature. Inclusion criteria included level I-IV studies on TTO indications, operative findings, and outcomes. Exclusion criteria were non-English studies, unpublished studies, level V evidence, letters to the editor, editorials, review articles, basic science articles, technique articles, revision procedures, articles without clinical outcomes, and conference proceeding abstracts. Studies that reported on duplicate populations were included only with the most recent available clinical outcomes. All abstracts were reviewed in duplicate by Dr. Levy and Dr. Rao and assessed with respect to the criteria outlined. Then the same authors performed full-text reviews of eligible studies before including these studies in the systematic review.
Assessment of Study Quality
The quality of each TTO study in the review was assessed with a modified Coleman methodology score (MCMS), which ranges from 0 to 100. A study with an MCMS of <55 points is considered a poor-quality study.11
Data Synthesis and Statistical Analysis
Given that most of the included studies were level IV, a formal meta-analysis was not indicated. In this article, we report categorical data as frequencies with percentages and preoperative and postoperative continuous data as means (SDs), with weighted means based on number of patients in each study, where applicable. We used 2-tailed t tests for comparisons made with the free Meta-Analysis Calculator and Grapher (http://www.healthstrategy.com/meta/meta.pl ). Statistical significance was set at P < .05.
Results
Search Results and Included Studies
Only 1 study provided preoperative body mass index (27 kg/m2). There were 55.35% of patients who had prior surgery on the affected knee (6 studies reporting).
Preoperative Data
Preoperative pathologic, radiographic, and clinical scoring data were scarcely reported and nonuniform (Table 2). The most common pathology treated with TTO was isolated patellofemoral instability (746/1055 patients, 70.7%). The other pathologies addressed were isolated patellofemoral osteoarthritis/chondromalacia patellae (143, 13.6%), patellofemoral instability with patella alta (61, 5.8%), patellofemoral instability with patellofemoral osteoarthritis (45, 4.3%), isolated patella baja (41, 3.9%), isolated patella alta (19, 1.8%), and patellofemoral osteoarthritis with patella baja (2, 0.2%). Five hundred fifty-five patients (53%) had a preoperative complaint that included knee pain, and 809 (77%) reported preoperative patellar laxity or instability events. The imaging data reported were Q-angle, Insall-Salvati ratio, Caton-Deschamps index, Blackburne-Peel ratio, Outerbridge osteoarthritis grade, and TT-TG distance. Preoperative clinical scoring data most prominently included a visual analog scale (VAS) score of 70.50 (4 studies reporting), a Lysholm score of 59.19 (5 studies), and a Kujala score of 41.16 (4 studies). Shelbourne-Trumper and Cox-Insall scores were reported in 1 and 2 studies, respectively.
Operative Characteristics
Of the 21 studies, 12 reported only on patients who had TTO performed in isolation; in the other 9 studies, cohorts included patients who underwent concurrent procedures. In the 17 studies (856 patients) that listed numbers of patients who underwent specific concomitant procedures, 715 patients (83.5%) underwent an isolated TTO procedure, and the other 141 (16.5%) underwent either concomitant lateral femoral trochleoplasty, arthroscopic drilling of chondral lesions, patellar shaving chondroplasty, partial meniscectomy or concomitant meniscal repair, intra-articular loose body removal, and/or lateral release with or without medial plication.
Postoperative Data
There was a cumulative total of 79 complications (8% of cohort): 17 recurrent patellar dislocations (1.9%), 4 recurrent patellar subluxations (0.4%), 10 wound complications (1.0%), 2 intraoperative complications (0.2%), 14 tibial tubercle fractures (1.3%), 19 proximal tibia fractures (1.8%), 4 cases of anterior knee pain (0.4%), 4 cases of neuropraxia (0.4%), and 5 infections (0.5%). Of note, 219 knees (21%) required reoperation, but 170 (16.3%) of these were for painful hardware removal. Sixteen knees (1.5%) required revision TTO, 1 (0.1%) required subsequent high tibial osteotomy, 2 (0.2%) underwent patellofemoral arthroplasty for advanced arthritic changes, and 5 (0.5%) underwent total knee arthroplasty for advanced arthritic changes.
Studies With TTO Performed in Isolation
Twelve studies reported outcomes of isolated TTO procedures. In the 638 patients who underwent isolated TTO, the pathologies addressed were instability/laxity (429 patients, 67%), patellofemoral osteoarthritis (74, 12%), patella alta with instability (61, 10%), patellofemoral osteoarthritis with instability (31, 5%), patella baja (24, 4%), and patella alta (19, 3%). Pain was a preoperative issue in 289 (45%) of these patients and instability in 472 (74%).
Only 2.8% of patients experienced postoperative patellar dislocation events. Of the 12 studies, 2 reported VAS scores (34-point weighted mean improvement, 65 points before surgery to 31 after surgery), 3 reported Lysholm scores (30-point improvement, from 60 to 90), and 2 reported Kujala scores (21-point improvement, from 46 to 67).
Complication rates for this isolated-TTO pooled cohort of patients were 1.2% for revision TTOs, 0.5% for wound complications, 0.8% for tibial tubercle fractures, and 1.9% for proximal tibia fractures. In total, 16% of patients required hardware removal after surgery.
Discussion
This study found that TTO improved patient pain and clinical outcome scores despite having a high (16%) rate of reoperation for painful hardware in patients with preoperative pain or instability, or with patellofemoral osteoarthritis or aberrant patellar anatomy. This reoperation rate and the overall complication rate both exceeded our hypothesized 10% cumulative rate. However, <1% of patients required conversion to a definitive end-stage surgery (patellofemoral arthroplasty or total knee arthroplasty) by final follow-up, and the rates of comorbidities (anterior knee pain, wound infection, recurrent patellar subluxation/dislocation, tibial fracture) were relatively low.
Patellofemoral disorders are common in the general population and a frequent primary complaint on presentation to orthopedic offices. Having a thorough understanding of knee joint biomechanics is imperative when trying to determine whether surgery is appropriate for these complaints and how to proceed. Extensor mechanism abnormalities, including high lateral force vectors (or larger TT-TG distances) and excessive patellar tilt, can affect alignment and increase the risk for patellofemoral dislocations, patellofemoral anterior- based knee pains, and chondral lesions. Patella alta, an elevated patella, risks increased contact stresses between the patella and the trochlear groove33 and decreases the osseous constraints that inhibit dislocation of the patella with physiologic flexion of the joint.34 With TTO, the change in tuberosity position can alter angles in the extensor mechanism and thereby decrease joint reaction forces and patellofemoral contact area forces.35,36
Although its use began as an option for combating patellar instability events in patients with predisposed patellofemoral kinematics,5 TTO has evolved in its therapeutic uses to include offloading patellar and trochlear focal chondral lesions and slowing progression of patellofemoral arthritis. Multiple iterations and modifications of the procedure have involved distal and medial transfer of the tibial tuberosity, medialization alone, concurrent anterior and medial elevation of the tuberosity, and proximal or distal transfers, depending on the pathology being corrected. Although TTO is highly versatile in treating multiple patellofemoral joint pathologies, this study found that its primary indication continues to be patellar instability, with anteromedialization as the most common direction of tubercle transfer in support of the medial structures providing the medial force vector that keeps the patella in place. These medial structures include the medial patellofemoral ligament, the vastus medialis obliquus, the medial patellotibial ligament, and the medial retinaculum.
Also notable was the relatively high rate of reoperation after TTO. However, >75% of reoperations were performed to remove painful hardware, and the need for reoperation seemed to have no effect on the statistically significant overall preoperative-to-postoperative improvement in VAS, Lysholm, and Kujala scores. Rates of definitive surgery for end-stage patellofemoral changes, including patellofemoral arthroplasty and total knee arthroplasty, were quite low at the weighted mean follow-up of several years after surgery, suggesting a role for TTO in avoiding arthroplasty. Although the infection rate was <1%, the rate of tibial tubercle or proximal tibia fractures was a cumulative 3.1%. Patients should be counseled on this complication risk, as treatment can require cast immobilization and weight-bearing limitations.24
The 69% proportion of women in the overall cohort and the mean (SD) age of 27.68 (10.45) years highlight the primary patient population that undergoes TTO. Compared with men, young women are more likely to have aberrant patellofemoral biomechanics, owing to their native anatomy, including their relatively larger Q-angle and TT-TG distance and thus increased lateral translational force vectors on the patella.37 In addition, more than half of patients who are having TTO underwent previous surgery on the affected knee—an indication that TTO is still not universally considered first-line in addressing patellofemoral pathology.
Limitations of the Analysis
The limitations of this analysis derive from the limitations of the included studies, which were mostly retrospective case series with relatively short follow-up. The low MCMS (<55) of all 21 studies highlights their low quality as well. These studies showed considerable heterogeneity in their reporting of specific preoperative, intraoperative, and postoperative radiographic, physical examination, and clinical outcome scores, which may be indicative of the relatively low rate of use of TTO, a procedure originally described decades ago. These studies also showed ample heterogeneity in the specific radiographic parameters or outcome scales they used to present their data. We were therefore limited in our ability to cohesively summarize and provide cumulative data points from the patients as a unified cohort. There was substantial variety in the procedures performed, surgical techniques used, concomitant pathologies addressed at time of surgery, and diagnoses treated—indicating a performance bias. This additionally precluded any significant meta-analysis within the patient cohort. A higher quality study, a randomized controlled trial, is needed to answer more definitively and completely the questions we left unanswered, including the effect on radiographic parameters, additional clinical outcomes, and patient satisfaction.
Conclusion
TTO is most commonly performed for isolated patellar instability in the presence of knee pain. Other pathologies addressed are patellofemoral osteoarthritis, and patella alta and patella baja with and without associated knee pain. TTO significantly improves knee pain and clinical outcome scores, though 21% of patients (>1 in 5) require reoperation for hardware removal. Young women with prior surgery on the affected knee are the primary patient population.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
1. Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7- year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64(4):393-400.
2. Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685-693.
3. Mehta VM, Inoue M, Nomura E, Fithian DC. An algorithm guiding the evaluation and treatment of acute primary patellar dislocations. Sports Med Arthrosc. 2007;15(2):78-81.
4. Erickson BJ, Mascarenhas R, Sayegh ET, et al. Does operative treatment of first-time patellar dislocations lead to increased patellofemoral stability? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1207-1215.
5. Hauser E. Total tendon transplant for slipping patella. Surg Gynecol Obstet. 1938;66:199-214.
6. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
7. Hall MJ, Mandalia VI. Tibial tubercle osteotomy for patello-femoral joint disorders. Knee Surg Sports Traumatol Arthrosc. 2016;24(3):855-861.
8. Grawe B, Stein BS. Tibial tubercle osteotomy: indication and techniques. J Knee Surg. 2015;28(4):279-284.
9. Fulkerson JP. Disorders of the Patellofemoral Joint. 4th ed. Baltimore, MD: Williams & Wilkins; 1997.
10. Koh JL, Stewart C. Patellar instability. Clin Sports Med. 2014;33(3):461-476.
11. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11.
12. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Rel Res. 2002;(396):152-162.
13. Atkinson HD, Bailey CA, Anand S, Johal P, Oakeshott RD. Tibial tubercle advancement osteotomy with bone allograft for patellofemoral arthritis: a retrospective cohort study of 50 knees. Arch Orthop Trauma Surg. 2012;132(4):437-445.
14. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
15. Dantas P, Nunes C, Moreira J, Amaral LB. Antero-medialisation of the tibial tubercle for patellar instability. Int Orthop. 2005;29(6):390-391.
16. Drexler M, Dwyer T, Marmor M, Sternheim A, Cameron HU, Cameron JC. The treatment of acquired patella baja with proximalize the tibial tuberosity. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2578-2583.
17. Eager MR, Bader DA, Kelly JD 4th, Moyer RA. Delayed fracture of the tibia following anteromedialization osteotomy of the tibial tubercle: a report of 5 cases. Am J Sports Med. 2004;32(4):1041-1048.
18. Ebinger TP, Boezaart A, Albright JP. Modifications of the Fulkerson osteotomy: a pilot study assessment of a novel technique of dynamic intraoperative determination of the adequacy of tubercle transfer. Iowa Orthop J. 2007;27:61-64.
19. Fulkerson JP, Becker GJ, Meaney JA, Miranda M, Folcik MA. Anteromedial tibial tubercle transfer without bone graft. Am J Sports Med. 1990;18(5):490-498.
20. Heatley FW, Allen PR, Patrick JH. Tibial tubercle advancement for anterior knee pain: a temporary or permanent solution. Clin Orthop Relat Res. 1986;(208):216-225.
21. Hirsh DM, Reddy DK. Experience with Maquet anterior tibial tubercle advancement for patellofemoral arthralgia. Clin Orthop Relat Res. 1980;(148):136-139.
22. Jack CM, Rajaratnam SS, Khan HO, Keast-Butler O, Butler-Manuel PA, Heatley FW. The modified tibial tubercle osteotomy for anterior knee pain due to chondromalacia patellae in adults: a five-year prospective study. Bone Joint Res. 2012;1(8):167-173.
23. Koëter S, Diks MJ, Anderson PG, Wymenga AB. A modified tibial tubercle osteotomy for patellar maltracking: results at two years. J Bone Joint Surg Br. 2007;89(2):180-185.
24. Luhmann SJ, Fuhrhop S, O’Donnell JC, Gordon JE. Tibial fractures after tibial tubercle osteotomies for patellar instability: a comparison of three osteotomy configurations. J Child Orthop. 2011;5(1):19-26.
25. Naranja RJ Jr, Reilly PJ, Kuhlman JR, Haut E, Torg JS. Long-term evaluation of the Elmslie-Trillat-Maquet procedure for patellofemoral dysfunction. Am J Sports Med. 1996;24(6):779-784.
26. Naveed MA, Ackroyd CE, Porteous AJ. Long-term (ten- to 15-year) outcome of arthroscopically assisted Elmslie-Trillat tibial tubercle osteotomy. Bone Joint J. 2013;95(4):478-485.
27. Paulos L, Swanson SC, Stoddard GJ, Barber-Westin S. Surgical correction of limb malalignment for instability of the patella: a comparison of 2 techniques. Am J Sports Med. 2009;37(7):1288-1300.
28. Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP. Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med. 1997;25(4):533-537.
29. Shen HC, Chao KH, Huang GS, Pan RY, Lee CH. Combined proximal and distal realignment procedures to treat the habitual dislocation of the patella in adults. Am J Sports Med. 2007;35(12):2101-2108.
30. Stetson WB, Friedman MJ, Fulkerson JP, Cheng M, Buuck D. Fracture of the proximal tibia with immediate weightbearing after a Fulkerson osteotomy. Am J Sports Med. 1997;25(4):570-574.
31. Valenzuela L, Nemtala F, Orrego M, et al. Treatment of patellofemoral chondropathy with the Bandi tibial tubercle osteotomy: more than 10 years follow-up. Knee. 2011;18(2):94-97.
32. Wang CJ, Wong T, Ko JY, Siu KK. Triple positioning of tibial tubercle osteotomy for patellofemoral disorders. Knee. 2014;21(1):133-137.
33. Luyckx T, Didden K, Vandenneucker H, Labey L, Innocenti B, Bellemans J. Is there a biomechanical explanation for anterior knee pain in patients with patella alta? Influence of patellar height on patellofemoral contact force, contact area and contact pressure. J Bone Joint Surg Br. 2009;91(3):344-350.
34. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
35. Maquet P. Advancement of the tibial tuberosity. Clin Orthop Relat Res. 1976;(115):225-230.
36. Lewallen DG, Riegger CL, Myers ER, Hayes WC. Effects of retinacular release and tibial tubercle elevation in patellofemoral degenerative joint disease. J Orthop Res. 1990;8(6):856-862.
37. Aglietti P, Insall JN, Cerulli G. Patellar pain and incongruence, I: measurements of incongruence. Clin Orthop Relat Res. 1983;(176):217-224.
For women with RA, small-joint surgery rate nearly twice that of men
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
AT ACR 2017
Key clinical point: Women with RA had a higher rate of small-joint surgery, compared with men.
Major finding: The cumulative incidence of small-joint surgery was significantly higher among women, compared with men (14.4% vs. 7.6%, respectively), but there were no differences between sexes in the rates of large-joint surgery.
Study details: A retrospective, population-based study of 1,077 patients with RA.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
Return to Activities After Patellofemoral Arthroplasty
Take-Home Points
- PFA improved knee function and pain scores in patients with isolated patellofemoral arthritis.
- The majority (84.2%) of patients undergoing PFA were female.
- Regardless of age or gender, 72.2% of patients returned to their desired preoperative activity after PFA, and 52.8% returned at the same or higher level.
- The rate of conversion from PFA to TKA was 6.3%.
- PFA is an alternative to TKA in active patients with isolated patellofemoral arthritis.
Compared with total knee arthroplasty (TKA), single-compartment knee arthroplasty may provide better physiologic function, faster recovery, and higher rates of return to activities in patients with unicompartmental knee disease.1-3 In 1955, McKeever4 introduced patellar arthroplasty for surgical management of isolated patellofemoral arthritis. In 1979, Lubinus5 improved on the technique and design by adding a femoral component. Since then, implants and techniques have been developed to effect better clinical outcomes. Patellofemoral arthroplasty (PFA) has many advantages over TKA in the treatment of patellofemoral arthritis. PFA is less invasive, requires shorter tourniquet times, has faster recovery, and spares the tibiofemoral compartment, leaving more native bone for potential conversion to TKA. Regarding activity and function, the resurfacing arthroplasty (vs TKA) allows maintenance of nearly normal knee kinematics.
Despite these advantages, the broader orthopedic surgery community has only cautiously accepted PFA. The procedure has high complication rates. Persistent instability, malalignment, wear, impingement, and tibiofemoral arthritis progression can occur after PFA.6 Although first-generation PFA prostheses often failed because of mechanical problems, loosening, maltracking, or instability,7 the most common indication for PFA revision has been, according to a recent large retrospective study,8 unexplained pain. More than 10 to 15 years after PFA, tibiofemoral arthritis may be the primary mechanism of failure.9 Nevertheless, compared with standard TKA for isolated patellofemoral arthritis, modern PFA does not have significantly different clinical outcomes, including complication and revision rates.6Numerous patient factors influence functional prognosis before and after knee arthroplasty, regardless of surgical technique and implant used. Age, comorbidities, athletic status, mental health, pain, functional limitations, excessive caution, “artificial joint”–related worries, and rehabilitation protocol all influence function.10 Return to activity and other quality-of-life indices are important aspects of postoperative patient satisfaction.
Methods
We conducted a retrospective cohort study to describe functional status after PFA for patellofemoral arthritis. We identified 48 consecutive PFAs (39 patients) performed by a team of 2 orthopedic surgeons (specialists in treating patellofemoral pathology) between 2009 and 2014.
Three validated patient-reported outcome measures (PROMs) were used to determine preoperative (baseline) and postoperative functional status: Kujala score, Lysholm score, and International Knee Documentation Committee (IKDC) score. The Kujala score is a measure of knee function specific to the patellofemoral joint; the Lysholm score focuses on activities related to the knee; and the IKDC score is a general measure of knee function. Charts were reviewed to extract patients’ clinical data, including preoperative outcome scores, medical history, physical examination data, intraoperative characteristics, and postoperative course. By telephone, patients answered questions about their postoperative clinical course and completed final follow-up questionnaires. They were also asked which sporting or fitness activity they had preferred before surgery and whether they were able to return to that activity after surgery.
Statistical analysis included the study population’s descriptive statistics. Means and SDs were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Paired t tests were used to analyze changes in PROM scores. For comparison of differences between characteristics of patients who did and did not return to their previous activity level, independent-samples t tests were used for continuous variables. Chi-square tests or Fisher exact tests were used to compare discrete variables. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 22.0 (IBM).
Results
Postoperative knee-specific PROM scores and general pain score (reported by the patient on a scale of 0-10) were statistically significantly improved (P < .001 for all measures) over preoperative scores (Table 4).
After surgery, 1 patient (2.6%) developed a pulmonary embolus, which was successfully identified and treated without incident. Five patients (10.4%) had another surgery on the same knee. Three patients (6.3%) underwent conversion to TKA: 1 for continued symptoms in the setting of newly diagnosed inflammatory arthritis, 1 for arthritic pain, and 1 for patellofemoral instability. Two patients (4.2%) underwent irrigation and débridement: 1 for hematoma and 1 for suspected (culture-negative) infection.
Discussion
Historically, the literature evaluating knee arthroplasty outcomes has focused on implant survivorship, pain relief, and patient satisfaction. Since the advent of partial knee arthroplasty options, more attention has been given to functional outcomes and return to activities after single-compartment knee resurfacing. TKA remains the gold standard by which newer, less invasive surgical options are measured. In a large prospective study, 97% of patients (age, >55 years) who had TKA for patellofemoral arthritis reported good or excellent clinical results, the majority being excellent.11 Post-TKA functional status and activity levels may not be rated as highly. After TKA, many patients switch to lower impact sports or reduce or stop their participation in sports.12 A small study of competitive adult tennis players found high levels of post-TKA satisfaction, ability to resume playing tennis, pain relief, and increased or continued enjoyment in playing.13 In a study of 355 patients (417 knees) who had underwent TKA, improvement in Knee Society function score showed a moderate correlation to an increase in weighted activity score (R = 0.362).14
Unicondylar knee arthroplasty (UKA) is becoming a popular treatment option for single-compartment tibiofemoral arthritis. A systematic review of 18 original studies of patients with knee osteoarthritis found that overall return to sports varied from 36% to 89% after TKA and from 75% to 100% after UKA.15 In another study, return-to-sports rates were similar for UKA (87%) and TKA (83%); the only significant difference was UKA patients returned quicker.16 The authors of a large meta-analysis conceded that significant heterogeneity of data prevented them from drawing definitive conclusions, but UKA patients seemed to return to low- and high-impact sports 2 weeks faster than their TKA counterparts.10 Overall, UKA and TKA patients (age, 51-71 years) had comparable return-to-sports rates at an average of 4 years after surgery.10 A smaller study corroborated faster return to sports for UKA over TKA patients and also found that, compared with TKA patients, UKA patients participated in sports more regularly and over a longer period.17 On the other hand, Walton and colleagues18 found similar return-to-sports rates but higher frequency of and satisfaction with sports participation in UKA over TKA patients.
A large retrospective study found no differences in rates of return to sports after TKA, UKA, patellar resurfacing, hip resurfacing, and total hip arthroplasty.19 Pain was the most common barrier to return. UKA patients who returned to sports tended to be younger than those who did not.20 Naal and colleagues3 found that 95% of UKA patients returned to their activities—hiking, walking, cycling, and swimming being most common. Although 90.3% of patients said surgery maintained or improved their ability to participate in sports, participation in high-impact sports (eg, running) decreased after surgery.
Outcomes of PFA vary because of evolving patient selection, implant design, surgical technique, and return-to-activity expectations.21,22 Most PFA outcome studies focus on implant survivorship, complication rates, and postoperative knee scores.23-28 PFA studies focused on return to activities are limited. Kooijman and colleagues7 and Mertl and colleagues29 reported good or excellent clinical results of PFA in 86% and 82% of patients, respectively. Neither study included a comprehensive analysis of postoperative functional status. Similarly, De Cloedt and colleagues30 reported good PFA outcomes in 43% of patients with degenerative joint disease and in 83% of patients with instability. Specific activity status was not described. Dahm and colleagues31 and Farr and colleagues32 suggested postoperative pain resolution motivates some PFA patients not only to resume preoperative activities but to start participating in new, higher level activities after pain has subsided. However, the studies did not examine the characteristics of patients who returned to baseline activities and did not examine return-to-sports rates.
Study Strengths and Limitations
Our study focused on the PFA patient population of a surgical team of 2 fellowship-trained orthopedic surgeons (specialists in treating patellofemoral pathology). Although generalization of our findings to other surgeons and different implants may be limited, the study design standardized treatment in a way that makes these findings more reliable. The 100% follow-up strengthens these findings as well. Last, though the patient population was relatively small, it was consistent with or larger than the PFA patient groups studied previously.
Conclusion
In this study, PROM and pain scores were significantly improved after PFA. That almost 75% of patients returned to their preferred activities and >50% of patients returned at the same or a higher activity level provides useful information for preoperative discussions with patients who want to remain active after PFA. Prospective studies are needed to evaluate the longevity and durability of PFA, particularly in active patients.
1. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
2. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
3. Naal FD, Fischer M, Preuss A, et al. Return to sports and recreational activity after unicompartmental knee arthroplasty. Am J Sports Med. 2007;35(10):1688-1695.
4. McKeever DC. Patellar prosthesis. J Bone Joint Surg Am. 1955;37(5):1074-1084.
5. Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119-127.
6. Dy CJ, Franco N, Ma Y, Mazumdar M, McCarthy MM, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174-2190.
7. Kooijman HJ, Driessen AP, van Horn JR. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(6):836-840.
8. Baker PN, Refaie R, Gregg P, Deehan D. Revision following patello-femoral arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2047-2053.
9. Lonner JH, Bloomfield MR. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44(3):271-280.
10. Papalia R, Del Buono A, Zampogna B, Maffulli N, Denaro V. Sport activity following joint arthroplasty: a systematic review. Br Med Bull. 2012;101:81-103.
11. Mont MA, Haas S, Mullick T, Hungerford DS. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84(11):1977-1981.
12. Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75(6):405-408.
13. Mont MA, Rajadhyaksha AD, Marxen JL, Silberstein CE, Hungerford DS. Tennis after total knee arthroplasty. Am J Sports Med. 2002;30(2):163-166.
14. Marker DR, Mont MA, Seyler TM, McGrath MS, Kolisek FR, Bonutti PM. Does functional improvement following TKA correlate to increased sports activity? Iowa Orthop J. 2009;29:11-16.
15. Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med. 2016;46(2):269-292.
16. Ho JC, Stitzlein RN, Green CJ, Stoner T, Froimson MI. Return to sports activity following UKA and TKA. J Knee Surg. 2016;29(3):254-259.
17. Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979.
18. Walton NP, Jahromi I, Lewis PL, Dobson PJ, Angel KR, Campbell DG. Patient-perceived outcomes and return to sport and work: TKA versus mini-incision unicompartmental knee arthroplasty. J Knee Surg. 2006;19(2):112-116.
19. Wylde V, Blom A, Dieppe P, Hewlett S, Learmonth I. Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920-923.
20. Pietschmann MF, Wohlleb L, Weber P, et al. Sports activities after medial unicompartmental knee arthroplasty Oxford III—what can we expect? Int Orthop. 2013;37(1):31-37.
21. Lonner JH. Patellofemoral arthroplasty. Orthopedics. 2010;33(9):653.
22. Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(1 suppl):S35-S43.
23. Krajca-Radcliffe JB, Coker TP. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143-151.
24. Mihalko WM, Boachie-Adjei Y, Spang JT, Fulkerson JP, Arendt EA, Saleh KJ. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
25. Lonner JH. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158-165.
26. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3):347-354.
27. Farr J 2nd, Barrett D. Optimizing patellofemoral arthroplasty. Knee. 2008;15(5):339-347.
28. Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(suppl 4):122-137.
29. Mertl P, Van FT, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83(8):712-718.
30. De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [in French]. Acta Orthop Belg. 1999;65(2):170-175.
31. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487-491.
32. Farr J, Arendt E, Dahm D, Daynes J. Patellofemoral arthroplasty in the athlete. Clin Sports Med. 2014;33(3):547-552.
Take-Home Points
- PFA improved knee function and pain scores in patients with isolated patellofemoral arthritis.
- The majority (84.2%) of patients undergoing PFA were female.
- Regardless of age or gender, 72.2% of patients returned to their desired preoperative activity after PFA, and 52.8% returned at the same or higher level.
- The rate of conversion from PFA to TKA was 6.3%.
- PFA is an alternative to TKA in active patients with isolated patellofemoral arthritis.
Compared with total knee arthroplasty (TKA), single-compartment knee arthroplasty may provide better physiologic function, faster recovery, and higher rates of return to activities in patients with unicompartmental knee disease.1-3 In 1955, McKeever4 introduced patellar arthroplasty for surgical management of isolated patellofemoral arthritis. In 1979, Lubinus5 improved on the technique and design by adding a femoral component. Since then, implants and techniques have been developed to effect better clinical outcomes. Patellofemoral arthroplasty (PFA) has many advantages over TKA in the treatment of patellofemoral arthritis. PFA is less invasive, requires shorter tourniquet times, has faster recovery, and spares the tibiofemoral compartment, leaving more native bone for potential conversion to TKA. Regarding activity and function, the resurfacing arthroplasty (vs TKA) allows maintenance of nearly normal knee kinematics.
Despite these advantages, the broader orthopedic surgery community has only cautiously accepted PFA. The procedure has high complication rates. Persistent instability, malalignment, wear, impingement, and tibiofemoral arthritis progression can occur after PFA.6 Although first-generation PFA prostheses often failed because of mechanical problems, loosening, maltracking, or instability,7 the most common indication for PFA revision has been, according to a recent large retrospective study,8 unexplained pain. More than 10 to 15 years after PFA, tibiofemoral arthritis may be the primary mechanism of failure.9 Nevertheless, compared with standard TKA for isolated patellofemoral arthritis, modern PFA does not have significantly different clinical outcomes, including complication and revision rates.6Numerous patient factors influence functional prognosis before and after knee arthroplasty, regardless of surgical technique and implant used. Age, comorbidities, athletic status, mental health, pain, functional limitations, excessive caution, “artificial joint”–related worries, and rehabilitation protocol all influence function.10 Return to activity and other quality-of-life indices are important aspects of postoperative patient satisfaction.
Methods
We conducted a retrospective cohort study to describe functional status after PFA for patellofemoral arthritis. We identified 48 consecutive PFAs (39 patients) performed by a team of 2 orthopedic surgeons (specialists in treating patellofemoral pathology) between 2009 and 2014.
Three validated patient-reported outcome measures (PROMs) were used to determine preoperative (baseline) and postoperative functional status: Kujala score, Lysholm score, and International Knee Documentation Committee (IKDC) score. The Kujala score is a measure of knee function specific to the patellofemoral joint; the Lysholm score focuses on activities related to the knee; and the IKDC score is a general measure of knee function. Charts were reviewed to extract patients’ clinical data, including preoperative outcome scores, medical history, physical examination data, intraoperative characteristics, and postoperative course. By telephone, patients answered questions about their postoperative clinical course and completed final follow-up questionnaires. They were also asked which sporting or fitness activity they had preferred before surgery and whether they were able to return to that activity after surgery.
Statistical analysis included the study population’s descriptive statistics. Means and SDs were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Paired t tests were used to analyze changes in PROM scores. For comparison of differences between characteristics of patients who did and did not return to their previous activity level, independent-samples t tests were used for continuous variables. Chi-square tests or Fisher exact tests were used to compare discrete variables. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 22.0 (IBM).
Results
Postoperative knee-specific PROM scores and general pain score (reported by the patient on a scale of 0-10) were statistically significantly improved (P < .001 for all measures) over preoperative scores (Table 4).
After surgery, 1 patient (2.6%) developed a pulmonary embolus, which was successfully identified and treated without incident. Five patients (10.4%) had another surgery on the same knee. Three patients (6.3%) underwent conversion to TKA: 1 for continued symptoms in the setting of newly diagnosed inflammatory arthritis, 1 for arthritic pain, and 1 for patellofemoral instability. Two patients (4.2%) underwent irrigation and débridement: 1 for hematoma and 1 for suspected (culture-negative) infection.
Discussion
Historically, the literature evaluating knee arthroplasty outcomes has focused on implant survivorship, pain relief, and patient satisfaction. Since the advent of partial knee arthroplasty options, more attention has been given to functional outcomes and return to activities after single-compartment knee resurfacing. TKA remains the gold standard by which newer, less invasive surgical options are measured. In a large prospective study, 97% of patients (age, >55 years) who had TKA for patellofemoral arthritis reported good or excellent clinical results, the majority being excellent.11 Post-TKA functional status and activity levels may not be rated as highly. After TKA, many patients switch to lower impact sports or reduce or stop their participation in sports.12 A small study of competitive adult tennis players found high levels of post-TKA satisfaction, ability to resume playing tennis, pain relief, and increased or continued enjoyment in playing.13 In a study of 355 patients (417 knees) who had underwent TKA, improvement in Knee Society function score showed a moderate correlation to an increase in weighted activity score (R = 0.362).14
Unicondylar knee arthroplasty (UKA) is becoming a popular treatment option for single-compartment tibiofemoral arthritis. A systematic review of 18 original studies of patients with knee osteoarthritis found that overall return to sports varied from 36% to 89% after TKA and from 75% to 100% after UKA.15 In another study, return-to-sports rates were similar for UKA (87%) and TKA (83%); the only significant difference was UKA patients returned quicker.16 The authors of a large meta-analysis conceded that significant heterogeneity of data prevented them from drawing definitive conclusions, but UKA patients seemed to return to low- and high-impact sports 2 weeks faster than their TKA counterparts.10 Overall, UKA and TKA patients (age, 51-71 years) had comparable return-to-sports rates at an average of 4 years after surgery.10 A smaller study corroborated faster return to sports for UKA over TKA patients and also found that, compared with TKA patients, UKA patients participated in sports more regularly and over a longer period.17 On the other hand, Walton and colleagues18 found similar return-to-sports rates but higher frequency of and satisfaction with sports participation in UKA over TKA patients.
A large retrospective study found no differences in rates of return to sports after TKA, UKA, patellar resurfacing, hip resurfacing, and total hip arthroplasty.19 Pain was the most common barrier to return. UKA patients who returned to sports tended to be younger than those who did not.20 Naal and colleagues3 found that 95% of UKA patients returned to their activities—hiking, walking, cycling, and swimming being most common. Although 90.3% of patients said surgery maintained or improved their ability to participate in sports, participation in high-impact sports (eg, running) decreased after surgery.
Outcomes of PFA vary because of evolving patient selection, implant design, surgical technique, and return-to-activity expectations.21,22 Most PFA outcome studies focus on implant survivorship, complication rates, and postoperative knee scores.23-28 PFA studies focused on return to activities are limited. Kooijman and colleagues7 and Mertl and colleagues29 reported good or excellent clinical results of PFA in 86% and 82% of patients, respectively. Neither study included a comprehensive analysis of postoperative functional status. Similarly, De Cloedt and colleagues30 reported good PFA outcomes in 43% of patients with degenerative joint disease and in 83% of patients with instability. Specific activity status was not described. Dahm and colleagues31 and Farr and colleagues32 suggested postoperative pain resolution motivates some PFA patients not only to resume preoperative activities but to start participating in new, higher level activities after pain has subsided. However, the studies did not examine the characteristics of patients who returned to baseline activities and did not examine return-to-sports rates.
Study Strengths and Limitations
Our study focused on the PFA patient population of a surgical team of 2 fellowship-trained orthopedic surgeons (specialists in treating patellofemoral pathology). Although generalization of our findings to other surgeons and different implants may be limited, the study design standardized treatment in a way that makes these findings more reliable. The 100% follow-up strengthens these findings as well. Last, though the patient population was relatively small, it was consistent with or larger than the PFA patient groups studied previously.
Conclusion
In this study, PROM and pain scores were significantly improved after PFA. That almost 75% of patients returned to their preferred activities and >50% of patients returned at the same or a higher activity level provides useful information for preoperative discussions with patients who want to remain active after PFA. Prospective studies are needed to evaluate the longevity and durability of PFA, particularly in active patients.
Take-Home Points
- PFA improved knee function and pain scores in patients with isolated patellofemoral arthritis.
- The majority (84.2%) of patients undergoing PFA were female.
- Regardless of age or gender, 72.2% of patients returned to their desired preoperative activity after PFA, and 52.8% returned at the same or higher level.
- The rate of conversion from PFA to TKA was 6.3%.
- PFA is an alternative to TKA in active patients with isolated patellofemoral arthritis.
Compared with total knee arthroplasty (TKA), single-compartment knee arthroplasty may provide better physiologic function, faster recovery, and higher rates of return to activities in patients with unicompartmental knee disease.1-3 In 1955, McKeever4 introduced patellar arthroplasty for surgical management of isolated patellofemoral arthritis. In 1979, Lubinus5 improved on the technique and design by adding a femoral component. Since then, implants and techniques have been developed to effect better clinical outcomes. Patellofemoral arthroplasty (PFA) has many advantages over TKA in the treatment of patellofemoral arthritis. PFA is less invasive, requires shorter tourniquet times, has faster recovery, and spares the tibiofemoral compartment, leaving more native bone for potential conversion to TKA. Regarding activity and function, the resurfacing arthroplasty (vs TKA) allows maintenance of nearly normal knee kinematics.
Despite these advantages, the broader orthopedic surgery community has only cautiously accepted PFA. The procedure has high complication rates. Persistent instability, malalignment, wear, impingement, and tibiofemoral arthritis progression can occur after PFA.6 Although first-generation PFA prostheses often failed because of mechanical problems, loosening, maltracking, or instability,7 the most common indication for PFA revision has been, according to a recent large retrospective study,8 unexplained pain. More than 10 to 15 years after PFA, tibiofemoral arthritis may be the primary mechanism of failure.9 Nevertheless, compared with standard TKA for isolated patellofemoral arthritis, modern PFA does not have significantly different clinical outcomes, including complication and revision rates.6Numerous patient factors influence functional prognosis before and after knee arthroplasty, regardless of surgical technique and implant used. Age, comorbidities, athletic status, mental health, pain, functional limitations, excessive caution, “artificial joint”–related worries, and rehabilitation protocol all influence function.10 Return to activity and other quality-of-life indices are important aspects of postoperative patient satisfaction.
Methods
We conducted a retrospective cohort study to describe functional status after PFA for patellofemoral arthritis. We identified 48 consecutive PFAs (39 patients) performed by a team of 2 orthopedic surgeons (specialists in treating patellofemoral pathology) between 2009 and 2014.
Three validated patient-reported outcome measures (PROMs) were used to determine preoperative (baseline) and postoperative functional status: Kujala score, Lysholm score, and International Knee Documentation Committee (IKDC) score. The Kujala score is a measure of knee function specific to the patellofemoral joint; the Lysholm score focuses on activities related to the knee; and the IKDC score is a general measure of knee function. Charts were reviewed to extract patients’ clinical data, including preoperative outcome scores, medical history, physical examination data, intraoperative characteristics, and postoperative course. By telephone, patients answered questions about their postoperative clinical course and completed final follow-up questionnaires. They were also asked which sporting or fitness activity they had preferred before surgery and whether they were able to return to that activity after surgery.
Statistical analysis included the study population’s descriptive statistics. Means and SDs were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Paired t tests were used to analyze changes in PROM scores. For comparison of differences between characteristics of patients who did and did not return to their previous activity level, independent-samples t tests were used for continuous variables. Chi-square tests or Fisher exact tests were used to compare discrete variables. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 22.0 (IBM).
Results
Postoperative knee-specific PROM scores and general pain score (reported by the patient on a scale of 0-10) were statistically significantly improved (P < .001 for all measures) over preoperative scores (Table 4).
After surgery, 1 patient (2.6%) developed a pulmonary embolus, which was successfully identified and treated without incident. Five patients (10.4%) had another surgery on the same knee. Three patients (6.3%) underwent conversion to TKA: 1 for continued symptoms in the setting of newly diagnosed inflammatory arthritis, 1 for arthritic pain, and 1 for patellofemoral instability. Two patients (4.2%) underwent irrigation and débridement: 1 for hematoma and 1 for suspected (culture-negative) infection.
Discussion
Historically, the literature evaluating knee arthroplasty outcomes has focused on implant survivorship, pain relief, and patient satisfaction. Since the advent of partial knee arthroplasty options, more attention has been given to functional outcomes and return to activities after single-compartment knee resurfacing. TKA remains the gold standard by which newer, less invasive surgical options are measured. In a large prospective study, 97% of patients (age, >55 years) who had TKA for patellofemoral arthritis reported good or excellent clinical results, the majority being excellent.11 Post-TKA functional status and activity levels may not be rated as highly. After TKA, many patients switch to lower impact sports or reduce or stop their participation in sports.12 A small study of competitive adult tennis players found high levels of post-TKA satisfaction, ability to resume playing tennis, pain relief, and increased or continued enjoyment in playing.13 In a study of 355 patients (417 knees) who had underwent TKA, improvement in Knee Society function score showed a moderate correlation to an increase in weighted activity score (R = 0.362).14
Unicondylar knee arthroplasty (UKA) is becoming a popular treatment option for single-compartment tibiofemoral arthritis. A systematic review of 18 original studies of patients with knee osteoarthritis found that overall return to sports varied from 36% to 89% after TKA and from 75% to 100% after UKA.15 In another study, return-to-sports rates were similar for UKA (87%) and TKA (83%); the only significant difference was UKA patients returned quicker.16 The authors of a large meta-analysis conceded that significant heterogeneity of data prevented them from drawing definitive conclusions, but UKA patients seemed to return to low- and high-impact sports 2 weeks faster than their TKA counterparts.10 Overall, UKA and TKA patients (age, 51-71 years) had comparable return-to-sports rates at an average of 4 years after surgery.10 A smaller study corroborated faster return to sports for UKA over TKA patients and also found that, compared with TKA patients, UKA patients participated in sports more regularly and over a longer period.17 On the other hand, Walton and colleagues18 found similar return-to-sports rates but higher frequency of and satisfaction with sports participation in UKA over TKA patients.
A large retrospective study found no differences in rates of return to sports after TKA, UKA, patellar resurfacing, hip resurfacing, and total hip arthroplasty.19 Pain was the most common barrier to return. UKA patients who returned to sports tended to be younger than those who did not.20 Naal and colleagues3 found that 95% of UKA patients returned to their activities—hiking, walking, cycling, and swimming being most common. Although 90.3% of patients said surgery maintained or improved their ability to participate in sports, participation in high-impact sports (eg, running) decreased after surgery.
Outcomes of PFA vary because of evolving patient selection, implant design, surgical technique, and return-to-activity expectations.21,22 Most PFA outcome studies focus on implant survivorship, complication rates, and postoperative knee scores.23-28 PFA studies focused on return to activities are limited. Kooijman and colleagues7 and Mertl and colleagues29 reported good or excellent clinical results of PFA in 86% and 82% of patients, respectively. Neither study included a comprehensive analysis of postoperative functional status. Similarly, De Cloedt and colleagues30 reported good PFA outcomes in 43% of patients with degenerative joint disease and in 83% of patients with instability. Specific activity status was not described. Dahm and colleagues31 and Farr and colleagues32 suggested postoperative pain resolution motivates some PFA patients not only to resume preoperative activities but to start participating in new, higher level activities after pain has subsided. However, the studies did not examine the characteristics of patients who returned to baseline activities and did not examine return-to-sports rates.
Study Strengths and Limitations
Our study focused on the PFA patient population of a surgical team of 2 fellowship-trained orthopedic surgeons (specialists in treating patellofemoral pathology). Although generalization of our findings to other surgeons and different implants may be limited, the study design standardized treatment in a way that makes these findings more reliable. The 100% follow-up strengthens these findings as well. Last, though the patient population was relatively small, it was consistent with or larger than the PFA patient groups studied previously.
Conclusion
In this study, PROM and pain scores were significantly improved after PFA. That almost 75% of patients returned to their preferred activities and >50% of patients returned at the same or a higher activity level provides useful information for preoperative discussions with patients who want to remain active after PFA. Prospective studies are needed to evaluate the longevity and durability of PFA, particularly in active patients.
1. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
2. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
3. Naal FD, Fischer M, Preuss A, et al. Return to sports and recreational activity after unicompartmental knee arthroplasty. Am J Sports Med. 2007;35(10):1688-1695.
4. McKeever DC. Patellar prosthesis. J Bone Joint Surg Am. 1955;37(5):1074-1084.
5. Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119-127.
6. Dy CJ, Franco N, Ma Y, Mazumdar M, McCarthy MM, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174-2190.
7. Kooijman HJ, Driessen AP, van Horn JR. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(6):836-840.
8. Baker PN, Refaie R, Gregg P, Deehan D. Revision following patello-femoral arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2047-2053.
9. Lonner JH, Bloomfield MR. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44(3):271-280.
10. Papalia R, Del Buono A, Zampogna B, Maffulli N, Denaro V. Sport activity following joint arthroplasty: a systematic review. Br Med Bull. 2012;101:81-103.
11. Mont MA, Haas S, Mullick T, Hungerford DS. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84(11):1977-1981.
12. Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75(6):405-408.
13. Mont MA, Rajadhyaksha AD, Marxen JL, Silberstein CE, Hungerford DS. Tennis after total knee arthroplasty. Am J Sports Med. 2002;30(2):163-166.
14. Marker DR, Mont MA, Seyler TM, McGrath MS, Kolisek FR, Bonutti PM. Does functional improvement following TKA correlate to increased sports activity? Iowa Orthop J. 2009;29:11-16.
15. Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med. 2016;46(2):269-292.
16. Ho JC, Stitzlein RN, Green CJ, Stoner T, Froimson MI. Return to sports activity following UKA and TKA. J Knee Surg. 2016;29(3):254-259.
17. Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979.
18. Walton NP, Jahromi I, Lewis PL, Dobson PJ, Angel KR, Campbell DG. Patient-perceived outcomes and return to sport and work: TKA versus mini-incision unicompartmental knee arthroplasty. J Knee Surg. 2006;19(2):112-116.
19. Wylde V, Blom A, Dieppe P, Hewlett S, Learmonth I. Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920-923.
20. Pietschmann MF, Wohlleb L, Weber P, et al. Sports activities after medial unicompartmental knee arthroplasty Oxford III—what can we expect? Int Orthop. 2013;37(1):31-37.
21. Lonner JH. Patellofemoral arthroplasty. Orthopedics. 2010;33(9):653.
22. Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(1 suppl):S35-S43.
23. Krajca-Radcliffe JB, Coker TP. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143-151.
24. Mihalko WM, Boachie-Adjei Y, Spang JT, Fulkerson JP, Arendt EA, Saleh KJ. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
25. Lonner JH. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158-165.
26. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3):347-354.
27. Farr J 2nd, Barrett D. Optimizing patellofemoral arthroplasty. Knee. 2008;15(5):339-347.
28. Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(suppl 4):122-137.
29. Mertl P, Van FT, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83(8):712-718.
30. De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [in French]. Acta Orthop Belg. 1999;65(2):170-175.
31. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487-491.
32. Farr J, Arendt E, Dahm D, Daynes J. Patellofemoral arthroplasty in the athlete. Clin Sports Med. 2014;33(3):547-552.
1. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
2. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
3. Naal FD, Fischer M, Preuss A, et al. Return to sports and recreational activity after unicompartmental knee arthroplasty. Am J Sports Med. 2007;35(10):1688-1695.
4. McKeever DC. Patellar prosthesis. J Bone Joint Surg Am. 1955;37(5):1074-1084.
5. Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119-127.
6. Dy CJ, Franco N, Ma Y, Mazumdar M, McCarthy MM, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174-2190.
7. Kooijman HJ, Driessen AP, van Horn JR. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(6):836-840.
8. Baker PN, Refaie R, Gregg P, Deehan D. Revision following patello-femoral arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2047-2053.
9. Lonner JH, Bloomfield MR. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44(3):271-280.
10. Papalia R, Del Buono A, Zampogna B, Maffulli N, Denaro V. Sport activity following joint arthroplasty: a systematic review. Br Med Bull. 2012;101:81-103.
11. Mont MA, Haas S, Mullick T, Hungerford DS. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84(11):1977-1981.
12. Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75(6):405-408.
13. Mont MA, Rajadhyaksha AD, Marxen JL, Silberstein CE, Hungerford DS. Tennis after total knee arthroplasty. Am J Sports Med. 2002;30(2):163-166.
14. Marker DR, Mont MA, Seyler TM, McGrath MS, Kolisek FR, Bonutti PM. Does functional improvement following TKA correlate to increased sports activity? Iowa Orthop J. 2009;29:11-16.
15. Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med. 2016;46(2):269-292.
16. Ho JC, Stitzlein RN, Green CJ, Stoner T, Froimson MI. Return to sports activity following UKA and TKA. J Knee Surg. 2016;29(3):254-259.
17. Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979.
18. Walton NP, Jahromi I, Lewis PL, Dobson PJ, Angel KR, Campbell DG. Patient-perceived outcomes and return to sport and work: TKA versus mini-incision unicompartmental knee arthroplasty. J Knee Surg. 2006;19(2):112-116.
19. Wylde V, Blom A, Dieppe P, Hewlett S, Learmonth I. Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920-923.
20. Pietschmann MF, Wohlleb L, Weber P, et al. Sports activities after medial unicompartmental knee arthroplasty Oxford III—what can we expect? Int Orthop. 2013;37(1):31-37.
21. Lonner JH. Patellofemoral arthroplasty. Orthopedics. 2010;33(9):653.
22. Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(1 suppl):S35-S43.
23. Krajca-Radcliffe JB, Coker TP. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143-151.
24. Mihalko WM, Boachie-Adjei Y, Spang JT, Fulkerson JP, Arendt EA, Saleh KJ. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
25. Lonner JH. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158-165.
26. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3):347-354.
27. Farr J 2nd, Barrett D. Optimizing patellofemoral arthroplasty. Knee. 2008;15(5):339-347.
28. Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(suppl 4):122-137.
29. Mertl P, Van FT, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83(8):712-718.
30. De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [in French]. Acta Orthop Belg. 1999;65(2):170-175.
31. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487-491.
32. Farr J, Arendt E, Dahm D, Daynes J. Patellofemoral arthroplasty in the athlete. Clin Sports Med. 2014;33(3):547-552.
Genotype-guided warfarin dosing reduced adverse events in arthroplasty patients
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
FROM JAMA
Key clinical point:
Major finding: Genotype-guided dosing reduced adverse events – primarily elevated INRs – by almost 4% compared to clinically based warfarin dosing.
Data source: A randomized trial comprising 1,650 elderly patients undergoing elective knee or hip arthroplasty.
Disclosures: Dr. Gage had no financial disclosures, but several of his coauthors noted relationships with pharmaceutical and imaging companies.
Patella Alta: A Comprehensive Review of Current Knowledge
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
Use of Intravenous Tranexamic Acid Improves Early Ambulation After Total Knee Arthroplasty and Anterior and Posterior Total Hip Arthroplasty
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
Paraskiing Crash and Knee Dislocation With Multiligament Reconstruction and Iliotibial Band Repair
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.