User login
Treatment-free remissions achieved in patients with chronic myeloid leukemia
Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia (CML) in chronic phase, Timothy P. Hughes, MD, and his colleagues in the international ENESTop trial reported at the annual meeting of the American Society of Clinical Oncology.
The conclusion is based on follow-up data on 126 patients who achieved a sustained deep molecular response (MR4.5) after switching from imatinib (Gleevec) to nilotinib (Tasigna) and discontinued nilotinib. So far, these are the largest prospective treatment-free remission data set in a population of patients who achieved a sustained deep molecular response after switching from imatinib to nilotinib, Dr. Hughes, head of hematology at the University of Adelaide and his colleagues wrote in a poster presentation.
The ENESTop study is a single-arm, phase II study. Patients eligible for the study started treatment with imatinib when they were first diagnosed with CML, then switched to nilotinib for at least 2 years with the combined time on the drugs of at least 3 years and small amounts of leukemia cells remaining after the nilotinib treatment.
For the consolidation phase of the study, patients continued their nilotinib therapy for 1 year. Patients without confirmed loss of MR4.5 after 1 year were eligible to stop nilotinib. RQ-PCR (reverse transcriptase–polymerase chain reaction) was monitored every 12 weeks in the consolidation phase of the study and every 4 weeks during first 48 weeks of treatment-free remission. Nilotinib was restarted if patients had confirmed loss of deep molecular response (MR4 [consecutive BCR-ABL1IS greater than 0.01%]) or loss of major molecular response ([MMR] BCR-ABL1IS greater than 0.1%).
Of the 163 patients in the consolidation phase of the study, 126 entered treatment-free remission. Their median duration of tyrosine kinase inhibitor use prior to treatment-free remission was nearly 88 months, with a 53-month median duration of nilotinib therapy. At data cut-off, with median follow up of 50 weeks, 58% of the 126 patients were still in treatment-free remission at 48 weeks.
During treatment-free remission, 18 patients had confirmed loss of MR4 and 34 lost MMR. One patient had atypical transcript and came off the study. All but one of the 52 patients reinitiated nilotinib; 50 (98%) regained at least MMR by data cut-off, 48 (94%) regained MR4, and 47 (92%) regained MR4.5. One patient switched to another tyrosine kinase inhibitor at 22 weeks after restarting therapy.
Of those who restarted therapy, the median time was 12 weeks to regain MR4 and was 13 weeks to regain MR4.5. No new safety findings were observed on treatment.
The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
On Twitter @maryjodales
Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia (CML) in chronic phase, Timothy P. Hughes, MD, and his colleagues in the international ENESTop trial reported at the annual meeting of the American Society of Clinical Oncology.
The conclusion is based on follow-up data on 126 patients who achieved a sustained deep molecular response (MR4.5) after switching from imatinib (Gleevec) to nilotinib (Tasigna) and discontinued nilotinib. So far, these are the largest prospective treatment-free remission data set in a population of patients who achieved a sustained deep molecular response after switching from imatinib to nilotinib, Dr. Hughes, head of hematology at the University of Adelaide and his colleagues wrote in a poster presentation.
The ENESTop study is a single-arm, phase II study. Patients eligible for the study started treatment with imatinib when they were first diagnosed with CML, then switched to nilotinib for at least 2 years with the combined time on the drugs of at least 3 years and small amounts of leukemia cells remaining after the nilotinib treatment.
For the consolidation phase of the study, patients continued their nilotinib therapy for 1 year. Patients without confirmed loss of MR4.5 after 1 year were eligible to stop nilotinib. RQ-PCR (reverse transcriptase–polymerase chain reaction) was monitored every 12 weeks in the consolidation phase of the study and every 4 weeks during first 48 weeks of treatment-free remission. Nilotinib was restarted if patients had confirmed loss of deep molecular response (MR4 [consecutive BCR-ABL1IS greater than 0.01%]) or loss of major molecular response ([MMR] BCR-ABL1IS greater than 0.1%).
Of the 163 patients in the consolidation phase of the study, 126 entered treatment-free remission. Their median duration of tyrosine kinase inhibitor use prior to treatment-free remission was nearly 88 months, with a 53-month median duration of nilotinib therapy. At data cut-off, with median follow up of 50 weeks, 58% of the 126 patients were still in treatment-free remission at 48 weeks.
During treatment-free remission, 18 patients had confirmed loss of MR4 and 34 lost MMR. One patient had atypical transcript and came off the study. All but one of the 52 patients reinitiated nilotinib; 50 (98%) regained at least MMR by data cut-off, 48 (94%) regained MR4, and 47 (92%) regained MR4.5. One patient switched to another tyrosine kinase inhibitor at 22 weeks after restarting therapy.
Of those who restarted therapy, the median time was 12 weeks to regain MR4 and was 13 weeks to regain MR4.5. No new safety findings were observed on treatment.
The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
On Twitter @maryjodales
Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia (CML) in chronic phase, Timothy P. Hughes, MD, and his colleagues in the international ENESTop trial reported at the annual meeting of the American Society of Clinical Oncology.
The conclusion is based on follow-up data on 126 patients who achieved a sustained deep molecular response (MR4.5) after switching from imatinib (Gleevec) to nilotinib (Tasigna) and discontinued nilotinib. So far, these are the largest prospective treatment-free remission data set in a population of patients who achieved a sustained deep molecular response after switching from imatinib to nilotinib, Dr. Hughes, head of hematology at the University of Adelaide and his colleagues wrote in a poster presentation.
The ENESTop study is a single-arm, phase II study. Patients eligible for the study started treatment with imatinib when they were first diagnosed with CML, then switched to nilotinib for at least 2 years with the combined time on the drugs of at least 3 years and small amounts of leukemia cells remaining after the nilotinib treatment.
For the consolidation phase of the study, patients continued their nilotinib therapy for 1 year. Patients without confirmed loss of MR4.5 after 1 year were eligible to stop nilotinib. RQ-PCR (reverse transcriptase–polymerase chain reaction) was monitored every 12 weeks in the consolidation phase of the study and every 4 weeks during first 48 weeks of treatment-free remission. Nilotinib was restarted if patients had confirmed loss of deep molecular response (MR4 [consecutive BCR-ABL1IS greater than 0.01%]) or loss of major molecular response ([MMR] BCR-ABL1IS greater than 0.1%).
Of the 163 patients in the consolidation phase of the study, 126 entered treatment-free remission. Their median duration of tyrosine kinase inhibitor use prior to treatment-free remission was nearly 88 months, with a 53-month median duration of nilotinib therapy. At data cut-off, with median follow up of 50 weeks, 58% of the 126 patients were still in treatment-free remission at 48 weeks.
During treatment-free remission, 18 patients had confirmed loss of MR4 and 34 lost MMR. One patient had atypical transcript and came off the study. All but one of the 52 patients reinitiated nilotinib; 50 (98%) regained at least MMR by data cut-off, 48 (94%) regained MR4, and 47 (92%) regained MR4.5. One patient switched to another tyrosine kinase inhibitor at 22 weeks after restarting therapy.
Of those who restarted therapy, the median time was 12 weeks to regain MR4 and was 13 weeks to regain MR4.5. No new safety findings were observed on treatment.
The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
On Twitter @maryjodales
FROM 2016 ASCO ANNUAL MEETING
Key clinical point: Treatment-free remission attempts are safe and are achievable in most patients with chronic myeloid leukemia in chronic phase.
Major finding: At data cut-off, with median follow-up of 50 weeks, 58% of the 126 patients who entered the treatment-free stage of the study were still in treatment-free remission at 48 weeks.
Data source: The ENESTop study is a single-arm, phase II study that included 163 patients.
Disclosures: The study is sponsored by Novartis, the maker of nilotinib (Tasigna). Dr. Hughes receives research support and honoraria from, and is a consultant or advisor to Novartis as well as Ariad and Bristol-Myers Squibb.
FDA places CAR-T cell trial on hold following patient deaths
The Food and Drug Administration placed Juno Therapeutics’ phase II ROCKET trial, involving CAR-T cell therapy, on clinical hold following two treatment-related patient deaths caused by excess fluid accumulation in the brain.
The ROCKET trial is a single-arm, multicenter phase II study treating adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia with an infusion of the patient’s own T cells that have been genetically modified to express a chimeric antigen receptor (CAR) that will bind to CD19-expressing leukemia cells. This treatment is referred to as JCAR015, and the ROCKET trial is only one of three current clinical trials testing its safety and efficacy.
Just before the ROCKET trial commenced, researchers added the chemotherapy drug fludarabine, which was successful in improving the performance of other immunotherapies, to the JCAR015 infusion. Researchers involved in the trial reported that the addition of this drug was likely the cause of the patient deaths.
Juno Therapeuticswill submit a revised trial protocol and patient consent form to the FDA before the hold is lifted, Juno reported in a written statement. The other trials led by Juno Therapeutics involving CAR-T cell product candidates are not affected.
On Twitter @jessnicolecraig
The Food and Drug Administration placed Juno Therapeutics’ phase II ROCKET trial, involving CAR-T cell therapy, on clinical hold following two treatment-related patient deaths caused by excess fluid accumulation in the brain.
The ROCKET trial is a single-arm, multicenter phase II study treating adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia with an infusion of the patient’s own T cells that have been genetically modified to express a chimeric antigen receptor (CAR) that will bind to CD19-expressing leukemia cells. This treatment is referred to as JCAR015, and the ROCKET trial is only one of three current clinical trials testing its safety and efficacy.
Just before the ROCKET trial commenced, researchers added the chemotherapy drug fludarabine, which was successful in improving the performance of other immunotherapies, to the JCAR015 infusion. Researchers involved in the trial reported that the addition of this drug was likely the cause of the patient deaths.
Juno Therapeuticswill submit a revised trial protocol and patient consent form to the FDA before the hold is lifted, Juno reported in a written statement. The other trials led by Juno Therapeutics involving CAR-T cell product candidates are not affected.
On Twitter @jessnicolecraig
The Food and Drug Administration placed Juno Therapeutics’ phase II ROCKET trial, involving CAR-T cell therapy, on clinical hold following two treatment-related patient deaths caused by excess fluid accumulation in the brain.
The ROCKET trial is a single-arm, multicenter phase II study treating adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia with an infusion of the patient’s own T cells that have been genetically modified to express a chimeric antigen receptor (CAR) that will bind to CD19-expressing leukemia cells. This treatment is referred to as JCAR015, and the ROCKET trial is only one of three current clinical trials testing its safety and efficacy.
Just before the ROCKET trial commenced, researchers added the chemotherapy drug fludarabine, which was successful in improving the performance of other immunotherapies, to the JCAR015 infusion. Researchers involved in the trial reported that the addition of this drug was likely the cause of the patient deaths.
Juno Therapeuticswill submit a revised trial protocol and patient consent form to the FDA before the hold is lifted, Juno reported in a written statement. The other trials led by Juno Therapeutics involving CAR-T cell product candidates are not affected.
On Twitter @jessnicolecraig
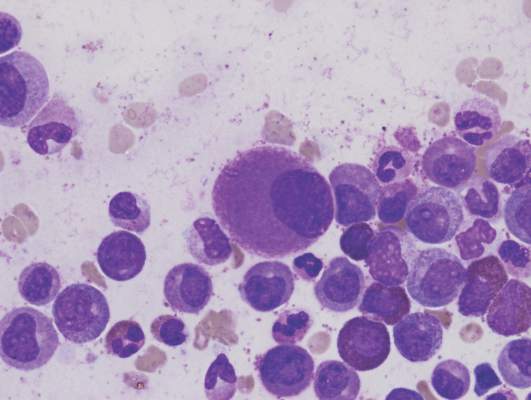
Short telomeres predicted delayed bone marrow recovery in pediatric AML
Among children with de novo acute myeloid leukemia, shorter telomere length at the end of induction chemotherapy predicted delayed bone marrow recovery in later courses, according to a study of 115 patients published online in the Journal of Clinical Oncology.
“This association was not related to differences in host factors, telomere maintenance gene variants, AML disease characteristics, or therapeutic exposures,” wrote Robert Gerbing of Children’s Oncology Group (Monrovia, Calif.), and his associates. “If validated in a larger cohort, prospective ascertainment of telomere length at end of AML induction may permit individualized risk assessment for severe myelosuppression and toxicities with subsequent therapy, as well as clarify the influence of age and cytogenetic or molecular disease characteristics.”
Acute myeloid leukemia comprises about one in five childhood leukemias and requires intensive treatment that has led to mortality in up to 19% of patients, the researchers noted (Blood 2008 Feb 1;111[3]:1044-53).
“Prolonged, profound neutropenia is a well recognized risk factor for sepsis and invasive fungal infections, both major contributors to treatment-related mortality,” they added. In nonleukemic hematopoietic cells, telomere length is a “quantifiable host factor that may indicate potential risk for impaired bone marrow recovery after chemotherapy,” they wrote (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.67.3467).
To test this hypothesis, the researchers analyzed paired diagnostic and remission bone marrow samples for 115 children with de novo AML enrolled in a Children’s Oncology Group protocol (AAML0531) that involved five chemotherapy courses. After each course, 62 patients reached absolute neutrophil count (ANC) recovery (500 cells/mcL) within the expected time frame, meaning that the recovery time was always less than one standard deviation of the group average. The remaining 53 patients had significantly delayed ANC recovery, meaning that they exceeded the group average by at least one standard deviation after at least two courses of chemotherapy.
The study size was adequate to detect a 0.2-unit difference in average telomere length between the two groups, the investigators noted. To measure telomere length, they estimated telomere content based on quantitative polymerase chain reaction (PCR) of bone marrow samples taken after induction chemotherapy. Then they compared patients who fell within the lowest quartile of telomere content to those in quartiles 2 through 4.
Telomere content was not associated with days to ANC recovery after the first three chemotherapy courses. After the fourth and fifth courses (intensifications two and three), patients had longer ANC recovery times than during the first three courses (mean, 45.2 days for intensification two and 43.7 days for intensification three). But patients with the shortest telomeres (that is, the patients in telomere content quartile 1) had significantly longer average ANC recovery times compared with patients in telomere content quartiles 2 through 4, both for intensifications two (P less than .001) and three (P = .002).
“Analysis of individual quartiles confirmed the association between less telomere content in quartile 1 and delays in ANC recovery,” the investigators noted. After they accounted for age at diagnosis, short telomere length remained a significant predictor of delayed ANC recovery after the fourth (P = .002) and fifth (P = .009) courses. Finally, DNA sequencing revealed evidence of telomere biology disorders, the investigators said.
The work was supported by an Alex’s Lemonade Stand Young Investigators Award, by a St. Baldrick’s Foundation Scholar Award, and by the National Institutes of Health. Mr. Gerbing and senior author Maria Gramatges, MD, had no disclosures. Two coinvestigators disclosed ties to Pfizer, Novartis, Dexcom, and several other pharmaceutical companies.
Among children with de novo acute myeloid leukemia, shorter telomere length at the end of induction chemotherapy predicted delayed bone marrow recovery in later courses, according to a study of 115 patients published online in the Journal of Clinical Oncology.
“This association was not related to differences in host factors, telomere maintenance gene variants, AML disease characteristics, or therapeutic exposures,” wrote Robert Gerbing of Children’s Oncology Group (Monrovia, Calif.), and his associates. “If validated in a larger cohort, prospective ascertainment of telomere length at end of AML induction may permit individualized risk assessment for severe myelosuppression and toxicities with subsequent therapy, as well as clarify the influence of age and cytogenetic or molecular disease characteristics.”
Acute myeloid leukemia comprises about one in five childhood leukemias and requires intensive treatment that has led to mortality in up to 19% of patients, the researchers noted (Blood 2008 Feb 1;111[3]:1044-53).
“Prolonged, profound neutropenia is a well recognized risk factor for sepsis and invasive fungal infections, both major contributors to treatment-related mortality,” they added. In nonleukemic hematopoietic cells, telomere length is a “quantifiable host factor that may indicate potential risk for impaired bone marrow recovery after chemotherapy,” they wrote (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.67.3467).
To test this hypothesis, the researchers analyzed paired diagnostic and remission bone marrow samples for 115 children with de novo AML enrolled in a Children’s Oncology Group protocol (AAML0531) that involved five chemotherapy courses. After each course, 62 patients reached absolute neutrophil count (ANC) recovery (500 cells/mcL) within the expected time frame, meaning that the recovery time was always less than one standard deviation of the group average. The remaining 53 patients had significantly delayed ANC recovery, meaning that they exceeded the group average by at least one standard deviation after at least two courses of chemotherapy.
The study size was adequate to detect a 0.2-unit difference in average telomere length between the two groups, the investigators noted. To measure telomere length, they estimated telomere content based on quantitative polymerase chain reaction (PCR) of bone marrow samples taken after induction chemotherapy. Then they compared patients who fell within the lowest quartile of telomere content to those in quartiles 2 through 4.
Telomere content was not associated with days to ANC recovery after the first three chemotherapy courses. After the fourth and fifth courses (intensifications two and three), patients had longer ANC recovery times than during the first three courses (mean, 45.2 days for intensification two and 43.7 days for intensification three). But patients with the shortest telomeres (that is, the patients in telomere content quartile 1) had significantly longer average ANC recovery times compared with patients in telomere content quartiles 2 through 4, both for intensifications two (P less than .001) and three (P = .002).
“Analysis of individual quartiles confirmed the association between less telomere content in quartile 1 and delays in ANC recovery,” the investigators noted. After they accounted for age at diagnosis, short telomere length remained a significant predictor of delayed ANC recovery after the fourth (P = .002) and fifth (P = .009) courses. Finally, DNA sequencing revealed evidence of telomere biology disorders, the investigators said.
The work was supported by an Alex’s Lemonade Stand Young Investigators Award, by a St. Baldrick’s Foundation Scholar Award, and by the National Institutes of Health. Mr. Gerbing and senior author Maria Gramatges, MD, had no disclosures. Two coinvestigators disclosed ties to Pfizer, Novartis, Dexcom, and several other pharmaceutical companies.
Among children with de novo acute myeloid leukemia, shorter telomere length at the end of induction chemotherapy predicted delayed bone marrow recovery in later courses, according to a study of 115 patients published online in the Journal of Clinical Oncology.
“This association was not related to differences in host factors, telomere maintenance gene variants, AML disease characteristics, or therapeutic exposures,” wrote Robert Gerbing of Children’s Oncology Group (Monrovia, Calif.), and his associates. “If validated in a larger cohort, prospective ascertainment of telomere length at end of AML induction may permit individualized risk assessment for severe myelosuppression and toxicities with subsequent therapy, as well as clarify the influence of age and cytogenetic or molecular disease characteristics.”
Acute myeloid leukemia comprises about one in five childhood leukemias and requires intensive treatment that has led to mortality in up to 19% of patients, the researchers noted (Blood 2008 Feb 1;111[3]:1044-53).
“Prolonged, profound neutropenia is a well recognized risk factor for sepsis and invasive fungal infections, both major contributors to treatment-related mortality,” they added. In nonleukemic hematopoietic cells, telomere length is a “quantifiable host factor that may indicate potential risk for impaired bone marrow recovery after chemotherapy,” they wrote (J Clin Oncol. 2016 Jun 27. doi: 10.1200/JCO.2016.67.3467).
To test this hypothesis, the researchers analyzed paired diagnostic and remission bone marrow samples for 115 children with de novo AML enrolled in a Children’s Oncology Group protocol (AAML0531) that involved five chemotherapy courses. After each course, 62 patients reached absolute neutrophil count (ANC) recovery (500 cells/mcL) within the expected time frame, meaning that the recovery time was always less than one standard deviation of the group average. The remaining 53 patients had significantly delayed ANC recovery, meaning that they exceeded the group average by at least one standard deviation after at least two courses of chemotherapy.
The study size was adequate to detect a 0.2-unit difference in average telomere length between the two groups, the investigators noted. To measure telomere length, they estimated telomere content based on quantitative polymerase chain reaction (PCR) of bone marrow samples taken after induction chemotherapy. Then they compared patients who fell within the lowest quartile of telomere content to those in quartiles 2 through 4.
Telomere content was not associated with days to ANC recovery after the first three chemotherapy courses. After the fourth and fifth courses (intensifications two and three), patients had longer ANC recovery times than during the first three courses (mean, 45.2 days for intensification two and 43.7 days for intensification three). But patients with the shortest telomeres (that is, the patients in telomere content quartile 1) had significantly longer average ANC recovery times compared with patients in telomere content quartiles 2 through 4, both for intensifications two (P less than .001) and three (P = .002).
“Analysis of individual quartiles confirmed the association between less telomere content in quartile 1 and delays in ANC recovery,” the investigators noted. After they accounted for age at diagnosis, short telomere length remained a significant predictor of delayed ANC recovery after the fourth (P = .002) and fifth (P = .009) courses. Finally, DNA sequencing revealed evidence of telomere biology disorders, the investigators said.
The work was supported by an Alex’s Lemonade Stand Young Investigators Award, by a St. Baldrick’s Foundation Scholar Award, and by the National Institutes of Health. Mr. Gerbing and senior author Maria Gramatges, MD, had no disclosures. Two coinvestigators disclosed ties to Pfizer, Novartis, Dexcom, and several other pharmaceutical companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Children with acute myeloid leukemia who had shorter bone marrow remission telomeres were significantly more likely to experience delayed absolute neutrophil recovery after later chemotherapy courses than were children with longer telomeres.
Major finding: Telomere length predicted time to ANC recovery after the fourth (P = .002) and fifth (P = .009) chemotherapy courses, even after adjustment for age.
Data source: A study of 115 children with de novo AML: 53 with delayed ANC recovery times and 62 with normal ANC recovery times.
Disclosures: The work was supported by an Alex’s Lemonade Stand Young Investigators Award, by a St. Baldrick’s Foundation Scholar Award, and by the National Institutes of Health. Dr. Gerbing and senior author Maria Gramatges, MD, had no disclosures. Two coinvestigators disclosed ties to Pfizer, Novartis, Dexcom, and several other pharmaceutical companies.
Cancer cell lines predict drug response, study shows

Image from PNAS
A study published in Cell has shown that patient-derived cancer cell lines harbor most of the same genetic changes found in patients’ tumors and could therefore be used to learn how cancers are likely to respond to new drugs.
Researchers believe this discovery could help advance personalized cancer medicine by leading to results that help doctors predict the best available drugs or the most suitable clinical trials for each individual patient.
“We need better ways to figure out which groups of patients are more likely to respond to a new drug before we run complex and expensive clinical trials,” said study author Ultan McDermott, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“Our research shows that cancer cell lines do capture the molecular alterations found in tumors and so can be predictive of how a tumor will respond to a drug. This means the cell lines could tell us much more about how a tumor is likely to respond to a new drug before we try to test it in patients. We hope this information will ultimately help in the design of clinical trials that target those patients with the greatest likelihood of benefiting from treatment.”
The researchers said this is the first systematic, large-scale study to combine molecular data from patients, cancer cell lines, and drug sensitivity.
For the study, the team looked at genetic mutations known to cause cancer in more than 11,000 patient samples of 29 different cancer types, including acute lymphoblastic leukemia, acute myeloid leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, diffuse large B-cell lymphoma, and multiple myeloma.
The researchers built a catalogue of the genetic changes that cause cancer in patients and mapped these alterations onto 1000 cancer cell lines. Next, they tested the cell lines for sensitivity to 265 different cancer drugs to understand which of these changes affect sensitivity.
This revealed that the majority of molecular abnormalities found in patients’ cancers are also found in cancer cells in the laboratory.
The work also showed that many of the molecular abnormalities detected in the thousands of patient samples can, both individually and in combination, have a strong effect on whether a particular drug affects a cancer cell’s survival.
The results suggest cancer cell lines could be better exploited to learn which drugs offer the most effective treatment to which patients.
“If a cell line has the same genetic features as a patient’s tumor, and that cell line responded to a specific drug, we can focus new research on this finding,” said study author Francesco Iorio, PhD, of the European Bioinformatics Institute in Cambridge, UK.
“This could ultimately help assign cancer patients into more precise groups based on how likely they are to respond to therapy. This resource can really help cancer research. Most importantly, it can be used to create tools for doctors to select a clinical trial which is most promising for their cancer patient. That is still a way off, but we are heading in the right direction.” ![]()

Image from PNAS
A study published in Cell has shown that patient-derived cancer cell lines harbor most of the same genetic changes found in patients’ tumors and could therefore be used to learn how cancers are likely to respond to new drugs.
Researchers believe this discovery could help advance personalized cancer medicine by leading to results that help doctors predict the best available drugs or the most suitable clinical trials for each individual patient.
“We need better ways to figure out which groups of patients are more likely to respond to a new drug before we run complex and expensive clinical trials,” said study author Ultan McDermott, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“Our research shows that cancer cell lines do capture the molecular alterations found in tumors and so can be predictive of how a tumor will respond to a drug. This means the cell lines could tell us much more about how a tumor is likely to respond to a new drug before we try to test it in patients. We hope this information will ultimately help in the design of clinical trials that target those patients with the greatest likelihood of benefiting from treatment.”
The researchers said this is the first systematic, large-scale study to combine molecular data from patients, cancer cell lines, and drug sensitivity.
For the study, the team looked at genetic mutations known to cause cancer in more than 11,000 patient samples of 29 different cancer types, including acute lymphoblastic leukemia, acute myeloid leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, diffuse large B-cell lymphoma, and multiple myeloma.
The researchers built a catalogue of the genetic changes that cause cancer in patients and mapped these alterations onto 1000 cancer cell lines. Next, they tested the cell lines for sensitivity to 265 different cancer drugs to understand which of these changes affect sensitivity.
This revealed that the majority of molecular abnormalities found in patients’ cancers are also found in cancer cells in the laboratory.
The work also showed that many of the molecular abnormalities detected in the thousands of patient samples can, both individually and in combination, have a strong effect on whether a particular drug affects a cancer cell’s survival.
The results suggest cancer cell lines could be better exploited to learn which drugs offer the most effective treatment to which patients.
“If a cell line has the same genetic features as a patient’s tumor, and that cell line responded to a specific drug, we can focus new research on this finding,” said study author Francesco Iorio, PhD, of the European Bioinformatics Institute in Cambridge, UK.
“This could ultimately help assign cancer patients into more precise groups based on how likely they are to respond to therapy. This resource can really help cancer research. Most importantly, it can be used to create tools for doctors to select a clinical trial which is most promising for their cancer patient. That is still a way off, but we are heading in the right direction.” ![]()

Image from PNAS
A study published in Cell has shown that patient-derived cancer cell lines harbor most of the same genetic changes found in patients’ tumors and could therefore be used to learn how cancers are likely to respond to new drugs.
Researchers believe this discovery could help advance personalized cancer medicine by leading to results that help doctors predict the best available drugs or the most suitable clinical trials for each individual patient.
“We need better ways to figure out which groups of patients are more likely to respond to a new drug before we run complex and expensive clinical trials,” said study author Ultan McDermott, MD, PhD, of the Wellcome Trust Sanger Institute in Cambridge, UK.
“Our research shows that cancer cell lines do capture the molecular alterations found in tumors and so can be predictive of how a tumor will respond to a drug. This means the cell lines could tell us much more about how a tumor is likely to respond to a new drug before we try to test it in patients. We hope this information will ultimately help in the design of clinical trials that target those patients with the greatest likelihood of benefiting from treatment.”
The researchers said this is the first systematic, large-scale study to combine molecular data from patients, cancer cell lines, and drug sensitivity.
For the study, the team looked at genetic mutations known to cause cancer in more than 11,000 patient samples of 29 different cancer types, including acute lymphoblastic leukemia, acute myeloid leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, diffuse large B-cell lymphoma, and multiple myeloma.
The researchers built a catalogue of the genetic changes that cause cancer in patients and mapped these alterations onto 1000 cancer cell lines. Next, they tested the cell lines for sensitivity to 265 different cancer drugs to understand which of these changes affect sensitivity.
This revealed that the majority of molecular abnormalities found in patients’ cancers are also found in cancer cells in the laboratory.
The work also showed that many of the molecular abnormalities detected in the thousands of patient samples can, both individually and in combination, have a strong effect on whether a particular drug affects a cancer cell’s survival.
The results suggest cancer cell lines could be better exploited to learn which drugs offer the most effective treatment to which patients.
“If a cell line has the same genetic features as a patient’s tumor, and that cell line responded to a specific drug, we can focus new research on this finding,” said study author Francesco Iorio, PhD, of the European Bioinformatics Institute in Cambridge, UK.
“This could ultimately help assign cancer patients into more precise groups based on how likely they are to respond to therapy. This resource can really help cancer research. Most importantly, it can be used to create tools for doctors to select a clinical trial which is most promising for their cancer patient. That is still a way off, but we are heading in the right direction.” ![]()
Mutations may be a ‘missing link’ in AML

Research published in Nature Communications suggests that mutations in the ZBTB7A gene are associated with t(8;21)-rearranged acute myeloid leukemia (AML).
Investigators believe these mutations may be one of the “missing links” in RUNX1/RUNX1T1-driven leukemogenesis.
The team analyzed samples from 56 patients with t(8;21)-rearranged AML and identified recurring ZBTB7A mutations in 23% of those samples.
This included missense and truncating mutations that resulted in alteration or loss of the C-terminal zinc-finger domain of ZBTB7A.
The investigators noted that the transcription factor ZBTB7A is important for hematopoietic lineage fate decisions and for the regulation of glycolysis.
So the team was not surprised to find that ZBTB7A mutations boosted the energy metabolism in leukemia cells.
“In healthy cells, the active ZBTB7A gene acts like a parking brake on metabolism,” said study author Philipp Greif, MD, of Ludwig-Maximilians-Universität München in Munich, Germany.
“If the gene is defective, cancer cells get more energy to use for proliferation.”
Dr Greif and his colleagues also found they could reduce the growth rate of AML cells by increasing levels of active ZBTB7A.
And the team observed an indication of ZBTB7A’s growth-inhibiting effects in the clinic. Leukemia patients with higher levels of ZBTB7A expression had significantly better chances of survival than patients in whom the gene was hardly active or not active at all.
Now, the investigators plan to explore whether ZBTB7A expression can be used to customize therapies for individual patients. They also believe their discovery is a promising starting point for developing new approaches to treat AML.
“It might be possible to use specially modified glucose molecules to block the energy production process in AML cells,” said study author Luise Hartmann, of Ludwig-Maximilians-Universität München.
“Initial clinical trials in other cancers have already shown that these agents are well-tolerated by patients.” ![]()

Research published in Nature Communications suggests that mutations in the ZBTB7A gene are associated with t(8;21)-rearranged acute myeloid leukemia (AML).
Investigators believe these mutations may be one of the “missing links” in RUNX1/RUNX1T1-driven leukemogenesis.
The team analyzed samples from 56 patients with t(8;21)-rearranged AML and identified recurring ZBTB7A mutations in 23% of those samples.
This included missense and truncating mutations that resulted in alteration or loss of the C-terminal zinc-finger domain of ZBTB7A.
The investigators noted that the transcription factor ZBTB7A is important for hematopoietic lineage fate decisions and for the regulation of glycolysis.
So the team was not surprised to find that ZBTB7A mutations boosted the energy metabolism in leukemia cells.
“In healthy cells, the active ZBTB7A gene acts like a parking brake on metabolism,” said study author Philipp Greif, MD, of Ludwig-Maximilians-Universität München in Munich, Germany.
“If the gene is defective, cancer cells get more energy to use for proliferation.”
Dr Greif and his colleagues also found they could reduce the growth rate of AML cells by increasing levels of active ZBTB7A.
And the team observed an indication of ZBTB7A’s growth-inhibiting effects in the clinic. Leukemia patients with higher levels of ZBTB7A expression had significantly better chances of survival than patients in whom the gene was hardly active or not active at all.
Now, the investigators plan to explore whether ZBTB7A expression can be used to customize therapies for individual patients. They also believe their discovery is a promising starting point for developing new approaches to treat AML.
“It might be possible to use specially modified glucose molecules to block the energy production process in AML cells,” said study author Luise Hartmann, of Ludwig-Maximilians-Universität München.
“Initial clinical trials in other cancers have already shown that these agents are well-tolerated by patients.” ![]()

Research published in Nature Communications suggests that mutations in the ZBTB7A gene are associated with t(8;21)-rearranged acute myeloid leukemia (AML).
Investigators believe these mutations may be one of the “missing links” in RUNX1/RUNX1T1-driven leukemogenesis.
The team analyzed samples from 56 patients with t(8;21)-rearranged AML and identified recurring ZBTB7A mutations in 23% of those samples.
This included missense and truncating mutations that resulted in alteration or loss of the C-terminal zinc-finger domain of ZBTB7A.
The investigators noted that the transcription factor ZBTB7A is important for hematopoietic lineage fate decisions and for the regulation of glycolysis.
So the team was not surprised to find that ZBTB7A mutations boosted the energy metabolism in leukemia cells.
“In healthy cells, the active ZBTB7A gene acts like a parking brake on metabolism,” said study author Philipp Greif, MD, of Ludwig-Maximilians-Universität München in Munich, Germany.
“If the gene is defective, cancer cells get more energy to use for proliferation.”
Dr Greif and his colleagues also found they could reduce the growth rate of AML cells by increasing levels of active ZBTB7A.
And the team observed an indication of ZBTB7A’s growth-inhibiting effects in the clinic. Leukemia patients with higher levels of ZBTB7A expression had significantly better chances of survival than patients in whom the gene was hardly active or not active at all.
Now, the investigators plan to explore whether ZBTB7A expression can be used to customize therapies for individual patients. They also believe their discovery is a promising starting point for developing new approaches to treat AML.
“It might be possible to use specially modified glucose molecules to block the energy production process in AML cells,” said study author Luise Hartmann, of Ludwig-Maximilians-Universität München.
“Initial clinical trials in other cancers have already shown that these agents are well-tolerated by patients.” ![]()
NICE recommends approval for bosutinib

Photo courtesy of CDC
The National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending approval for bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML).
NICE is recommending that bosutinib be made available through normal National Health Service (NHS) funding channels so patients don’t have to apply to the Cancer Drugs Fund (CDF) to obtain it.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib.
“People with this type of chronic myeloid leukemia, who haven’t responded to first- and second-line treatment or who experience severe side effects, have few or no treatment options left,” said Carole Longson, director of the Centre for Health Technology Evaluation at NICE.
“New patients who need this drug can be reassured that bosutinib should be made available for routine use within the NHS.”
The current list price of bosutinib is £45,000 per patient per year. However, the NHS has been offered a discount by Pfizer, the drug’s manufacturer.
NICE previously looked at bosutinib in 2013 but did not recommend the drug for use on the NHS at that time, saying the drug was not cost-effective. Bosutinib was then made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
“The company positively engaged with our CDF reconsideration process and demonstrated that their drug can be cost-effective, which resulted in a positive recommendation,” Longson said. “This decision, when implemented, frees up funding in the CDF, which can be spent on other new and innovative cancer treatments.”
NICE’s final draft guidance is now with consultees who have the opportunity to appeal against the decision or notify NICE of any factual errors. The appeal period will close at 5 pm on July 21, 2016.
Until the final decision is published, bosutinib will still be available to new and existing patients through the old CDF. ![]()

Photo courtesy of CDC
The National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending approval for bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML).
NICE is recommending that bosutinib be made available through normal National Health Service (NHS) funding channels so patients don’t have to apply to the Cancer Drugs Fund (CDF) to obtain it.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib.
“People with this type of chronic myeloid leukemia, who haven’t responded to first- and second-line treatment or who experience severe side effects, have few or no treatment options left,” said Carole Longson, director of the Centre for Health Technology Evaluation at NICE.
“New patients who need this drug can be reassured that bosutinib should be made available for routine use within the NHS.”
The current list price of bosutinib is £45,000 per patient per year. However, the NHS has been offered a discount by Pfizer, the drug’s manufacturer.
NICE previously looked at bosutinib in 2013 but did not recommend the drug for use on the NHS at that time, saying the drug was not cost-effective. Bosutinib was then made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
“The company positively engaged with our CDF reconsideration process and demonstrated that their drug can be cost-effective, which resulted in a positive recommendation,” Longson said. “This decision, when implemented, frees up funding in the CDF, which can be spent on other new and innovative cancer treatments.”
NICE’s final draft guidance is now with consultees who have the opportunity to appeal against the decision or notify NICE of any factual errors. The appeal period will close at 5 pm on July 21, 2016.
Until the final decision is published, bosutinib will still be available to new and existing patients through the old CDF. ![]()

Photo courtesy of CDC
The National Institute for Health and Care Excellence (NICE) has issued a final draft guidance recommending approval for bosutinib (Bosulif), a tyrosine kinase inhibitor used to treat certain patients with chronic myeloid leukemia (CML).
NICE is recommending that bosutinib be made available through normal National Health Service (NHS) funding channels so patients don’t have to apply to the Cancer Drugs Fund (CDF) to obtain it.
The CDF is money the government sets aside to pay for cancer drugs that haven’t been approved by NICE and aren’t available within the NHS in England.
Following the decision to reform the CDF earlier this year, NICE began to reappraise all drugs currently in the CDF in April. Bosutinib is the first drug to be looked at through this reconsideration process.
Bosutinib has conditional approval from the European Commission to treat adults with Philadelphia-chromosome-positive CML in chronic phase, accelerated phase, or blast phase, but only if those patients have previously received one or more tyrosine kinase inhibitors and are not considered eligible for treatment with imatinib, nilotinib, or dasatinib.
“People with this type of chronic myeloid leukemia, who haven’t responded to first- and second-line treatment or who experience severe side effects, have few or no treatment options left,” said Carole Longson, director of the Centre for Health Technology Evaluation at NICE.
“New patients who need this drug can be reassured that bosutinib should be made available for routine use within the NHS.”
The current list price of bosutinib is £45,000 per patient per year. However, the NHS has been offered a discount by Pfizer, the drug’s manufacturer.
NICE previously looked at bosutinib in 2013 but did not recommend the drug for use on the NHS at that time, saying the drug was not cost-effective. Bosutinib was then made available to patients via the CDF.
As part of the reappraisal process, Pfizer offered a discount for bosutinib. Taking this discount into consideration, as well as the limited treatment options for CML patients, NICE decided bosutinib is cost-effective.
“The company positively engaged with our CDF reconsideration process and demonstrated that their drug can be cost-effective, which resulted in a positive recommendation,” Longson said. “This decision, when implemented, frees up funding in the CDF, which can be spent on other new and innovative cancer treatments.”
NICE’s final draft guidance is now with consultees who have the opportunity to appeal against the decision or notify NICE of any factual errors. The appeal period will close at 5 pm on July 21, 2016.
Until the final decision is published, bosutinib will still be available to new and existing patients through the old CDF. ![]()
Drugs produce comparable results in CP-CML

Long-term results from the DASISION trial suggest that dasatinib and imatinib produce similar outcomes in patients with newly diagnosed chronic phase chronic myeloid leukemia (CP-CML).
Although patients who received dasatinib experienced faster and deeper molecular responses than patients who received imatinib, the overall survival and progression-free survival rates were similar between the treatment arms.
Overall, adverse events (AEs) were similar between the arms as well.
Researchers said these results suggest that dasatinib should continue to be considered an option for patients with newly diagnosed CP-CML.
The team reported the results of this study in the Journal of Clinical Oncology. The research was sponsored by Bristol-Myers Squibb.
The trial enrolled 519 patients with newly diagnosed CP-CML. They were randomized to receive dasatinib at 100 mg once daily (n=259) or imatinib at 400 mg once daily (n=260). Baseline characteristics were well-balanced between the arms.
At 5 years of follow-up, 61% of patients in the dasatinib arm and 63% of patients in the imatinib arm remained on treatment.
Response and survival
The cumulative 5-year rate of major molecular response was 76% in the dasatinib arm and 64% in the imatinib arm (P=0.0022). The rates of MR4.5 were 42% and 33%, respectively (P=0.0251).
The estimated 5-year overall survival was 91% in the dasatinib arm and 90% in the imatinib arm (hazard ratio=1.01; 95% CI, 0.58 to 1.73).
The estimated 5-year progression-free survival was 85% and 86%, respectively (hazard ratio=1.06; 95% CI, 0.68 to 1.66).
Safety
In both treatment arms, most AEs were grade 1 or 2. Grade 3/4 AEs occurred in 15% of patients in the dasatinib arm and 11% of patients in the imatinib arm.
Rates of grade 3/4 hematologic AEs tended to be higher in the dasatinib arm than the imatinib arm.
But the rates of most drug-related, nonhematologic AEs were lower in the dasatinib arm than the imatinib arm or were comparable between the arms.
The exception was drug-related pleural effusion, which was more common with dasatinib (28%) than with imatinib (0.8%).
Drug-related AEs were largely manageable, although they led to treatment discontinuation in 16% of dasatinib-treated patients and 7% of imatinib-treated patients.
By 5 years, 26 patients (10%) in each treatment arm had died. Nine patients in the dasatinib arm died of disease progression, as did 17 patients in the imatinib arm. ![]()

Long-term results from the DASISION trial suggest that dasatinib and imatinib produce similar outcomes in patients with newly diagnosed chronic phase chronic myeloid leukemia (CP-CML).
Although patients who received dasatinib experienced faster and deeper molecular responses than patients who received imatinib, the overall survival and progression-free survival rates were similar between the treatment arms.
Overall, adverse events (AEs) were similar between the arms as well.
Researchers said these results suggest that dasatinib should continue to be considered an option for patients with newly diagnosed CP-CML.
The team reported the results of this study in the Journal of Clinical Oncology. The research was sponsored by Bristol-Myers Squibb.
The trial enrolled 519 patients with newly diagnosed CP-CML. They were randomized to receive dasatinib at 100 mg once daily (n=259) or imatinib at 400 mg once daily (n=260). Baseline characteristics were well-balanced between the arms.
At 5 years of follow-up, 61% of patients in the dasatinib arm and 63% of patients in the imatinib arm remained on treatment.
Response and survival
The cumulative 5-year rate of major molecular response was 76% in the dasatinib arm and 64% in the imatinib arm (P=0.0022). The rates of MR4.5 were 42% and 33%, respectively (P=0.0251).
The estimated 5-year overall survival was 91% in the dasatinib arm and 90% in the imatinib arm (hazard ratio=1.01; 95% CI, 0.58 to 1.73).
The estimated 5-year progression-free survival was 85% and 86%, respectively (hazard ratio=1.06; 95% CI, 0.68 to 1.66).
Safety
In both treatment arms, most AEs were grade 1 or 2. Grade 3/4 AEs occurred in 15% of patients in the dasatinib arm and 11% of patients in the imatinib arm.
Rates of grade 3/4 hematologic AEs tended to be higher in the dasatinib arm than the imatinib arm.
But the rates of most drug-related, nonhematologic AEs were lower in the dasatinib arm than the imatinib arm or were comparable between the arms.
The exception was drug-related pleural effusion, which was more common with dasatinib (28%) than with imatinib (0.8%).
Drug-related AEs were largely manageable, although they led to treatment discontinuation in 16% of dasatinib-treated patients and 7% of imatinib-treated patients.
By 5 years, 26 patients (10%) in each treatment arm had died. Nine patients in the dasatinib arm died of disease progression, as did 17 patients in the imatinib arm. ![]()

Long-term results from the DASISION trial suggest that dasatinib and imatinib produce similar outcomes in patients with newly diagnosed chronic phase chronic myeloid leukemia (CP-CML).
Although patients who received dasatinib experienced faster and deeper molecular responses than patients who received imatinib, the overall survival and progression-free survival rates were similar between the treatment arms.
Overall, adverse events (AEs) were similar between the arms as well.
Researchers said these results suggest that dasatinib should continue to be considered an option for patients with newly diagnosed CP-CML.
The team reported the results of this study in the Journal of Clinical Oncology. The research was sponsored by Bristol-Myers Squibb.
The trial enrolled 519 patients with newly diagnosed CP-CML. They were randomized to receive dasatinib at 100 mg once daily (n=259) or imatinib at 400 mg once daily (n=260). Baseline characteristics were well-balanced between the arms.
At 5 years of follow-up, 61% of patients in the dasatinib arm and 63% of patients in the imatinib arm remained on treatment.
Response and survival
The cumulative 5-year rate of major molecular response was 76% in the dasatinib arm and 64% in the imatinib arm (P=0.0022). The rates of MR4.5 were 42% and 33%, respectively (P=0.0251).
The estimated 5-year overall survival was 91% in the dasatinib arm and 90% in the imatinib arm (hazard ratio=1.01; 95% CI, 0.58 to 1.73).
The estimated 5-year progression-free survival was 85% and 86%, respectively (hazard ratio=1.06; 95% CI, 0.68 to 1.66).
Safety
In both treatment arms, most AEs were grade 1 or 2. Grade 3/4 AEs occurred in 15% of patients in the dasatinib arm and 11% of patients in the imatinib arm.
Rates of grade 3/4 hematologic AEs tended to be higher in the dasatinib arm than the imatinib arm.
But the rates of most drug-related, nonhematologic AEs were lower in the dasatinib arm than the imatinib arm or were comparable between the arms.
The exception was drug-related pleural effusion, which was more common with dasatinib (28%) than with imatinib (0.8%).
Drug-related AEs were largely manageable, although they led to treatment discontinuation in 16% of dasatinib-treated patients and 7% of imatinib-treated patients.
By 5 years, 26 patients (10%) in each treatment arm had died. Nine patients in the dasatinib arm died of disease progression, as did 17 patients in the imatinib arm. ![]()
Team identifies potential therapeutic target for AML

New research suggests that E proteins and their antagonists, Id proteins, can play key roles in acute myeloid leukemia (AML).
The study showed that overexpression of the Id2 protein or knockdown of the E2-2 protein can suppress both mixed-lineage leukemia (MLL)-rearranged AML and t(8;21) AML.
These findings, published in Cancer Cell, suggest the Id2/E-protein axis may be a promising therapeutic target for AML.
“There is a particularly urgent need for new, targeted, drug-based therapies for AML, and with every discovery of what’s driving the cancer, we take a step closer to achieving that,” said study author Ricky Johnstone, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
“What we found in this case was the suppression of Id2 protein plays an important, and previously unrecognized, role in allowing MLL re-arranged AML cancer cells to take hold and spread. Drugs that influence levels of this protein, or stop it being suppressed by the cancer, could provide a much-needed new avenue to combatting this disease.”
The researchers first found that Id2 regulates leukemia stem cell (LSC) potential. Specifically, low Id2 expression is associated with LSC enrichment, and Id2 overexpression hinders leukemia development.
Further investigation revealed that the fusion protein MLL-AF9 suppresses Id2 and activates E2-2 expression, while E2-2 depletion phenocopies Id2 overexpression in MLL-AF9-AML cells.
The team also found that Id2’s tumor-suppressive function is conserved in t(8;21) AML. And low expression of Id2 and its associated gene signature are associated with poor prognosis in patients with MLL-rearranged AML or t(8;21) AML. ![]()

New research suggests that E proteins and their antagonists, Id proteins, can play key roles in acute myeloid leukemia (AML).
The study showed that overexpression of the Id2 protein or knockdown of the E2-2 protein can suppress both mixed-lineage leukemia (MLL)-rearranged AML and t(8;21) AML.
These findings, published in Cancer Cell, suggest the Id2/E-protein axis may be a promising therapeutic target for AML.
“There is a particularly urgent need for new, targeted, drug-based therapies for AML, and with every discovery of what’s driving the cancer, we take a step closer to achieving that,” said study author Ricky Johnstone, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
“What we found in this case was the suppression of Id2 protein plays an important, and previously unrecognized, role in allowing MLL re-arranged AML cancer cells to take hold and spread. Drugs that influence levels of this protein, or stop it being suppressed by the cancer, could provide a much-needed new avenue to combatting this disease.”
The researchers first found that Id2 regulates leukemia stem cell (LSC) potential. Specifically, low Id2 expression is associated with LSC enrichment, and Id2 overexpression hinders leukemia development.
Further investigation revealed that the fusion protein MLL-AF9 suppresses Id2 and activates E2-2 expression, while E2-2 depletion phenocopies Id2 overexpression in MLL-AF9-AML cells.
The team also found that Id2’s tumor-suppressive function is conserved in t(8;21) AML. And low expression of Id2 and its associated gene signature are associated with poor prognosis in patients with MLL-rearranged AML or t(8;21) AML. ![]()

New research suggests that E proteins and their antagonists, Id proteins, can play key roles in acute myeloid leukemia (AML).
The study showed that overexpression of the Id2 protein or knockdown of the E2-2 protein can suppress both mixed-lineage leukemia (MLL)-rearranged AML and t(8;21) AML.
These findings, published in Cancer Cell, suggest the Id2/E-protein axis may be a promising therapeutic target for AML.
“There is a particularly urgent need for new, targeted, drug-based therapies for AML, and with every discovery of what’s driving the cancer, we take a step closer to achieving that,” said study author Ricky Johnstone, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
“What we found in this case was the suppression of Id2 protein plays an important, and previously unrecognized, role in allowing MLL re-arranged AML cancer cells to take hold and spread. Drugs that influence levels of this protein, or stop it being suppressed by the cancer, could provide a much-needed new avenue to combatting this disease.”
The researchers first found that Id2 regulates leukemia stem cell (LSC) potential. Specifically, low Id2 expression is associated with LSC enrichment, and Id2 overexpression hinders leukemia development.
Further investigation revealed that the fusion protein MLL-AF9 suppresses Id2 and activates E2-2 expression, while E2-2 depletion phenocopies Id2 overexpression in MLL-AF9-AML cells.
The team also found that Id2’s tumor-suppressive function is conserved in t(8;21) AML. And low expression of Id2 and its associated gene signature are associated with poor prognosis in patients with MLL-rearranged AML or t(8;21) AML. ![]()
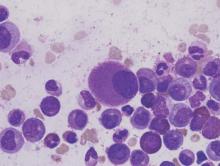
Telomere length linked to neutrophil recovery in AML

Image by Volker Brinkmann
Researchers say they have discovered a way to predict which children with acute myeloid leukemia (AML) are at the highest risk of delayed neutrophil recovery.
The team examined the role of telomeres in neutrophil recovery and found that the length of a patient’s telomeres can indicate the rate of recovery following chemotherapy.
The group reported their findings in the Journal of Clinical Oncology.
“We were interested in telomere length as a marker of blood count recovery because defects in telomere maintenance are known risks for bone marrow failure and aplastic anemia,” said study author Maria Monica Gramatges, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“We know that up to 15% to 20% of children can take 2 months or longer to recover their blood counts after a course of AML chemotherapy. Our goal was to understand if these children had an underlying genetic predisposition associated with an impaired capacity for recovery.”
Dr Gramatges and her colleagues hypothesized that short telomere length could be associated with a delay in neutrophil recovery.
So they obtained bone marrow samples from AML patients who recovered as expected (within 30 days) after each chemotherapy course (n=62), and from AML patients who experienced significant delays in recovery after chemotherapy (n=53).
The team then measured telomere length on each subject and categorized the group by quartile, from shortest to longest.
Subjects in the quartile with the shortest telomere lengths took the longest to recover, especially during the last 2 courses of chemotherapy. In an adjusted analysis, lower telomere content was significantly associated with prolonged neutropenia after the fourth (P=0.002) and fifth courses of chemotherapy (P=0.009).
The researchers said these results support the hypothesis that telomeres are an indicator of capacity for neutrophil recovery following chemotherapy.
Dr Gramatges hopes the results of this study will be helpful in further understanding which children are at a higher risk for prolonged myelosuppression and how to target those children with modified treatments, improved supportive care, and closer monitoring in order to prevent potential complications such as severe infections.
“A significant proportion of children with AML suffer from treatment-related toxicities, with some succumbing to complications of the therapies we give, rather than from the actual cancer itself,” Dr Gramatges said.
“We hope this research will help us identify those who are at a higher risk for delayed recovery and use this knowledge to reduce the morbidity and mortality associated with AML treatment.” ![]()

Image by Volker Brinkmann
Researchers say they have discovered a way to predict which children with acute myeloid leukemia (AML) are at the highest risk of delayed neutrophil recovery.
The team examined the role of telomeres in neutrophil recovery and found that the length of a patient’s telomeres can indicate the rate of recovery following chemotherapy.
The group reported their findings in the Journal of Clinical Oncology.
“We were interested in telomere length as a marker of blood count recovery because defects in telomere maintenance are known risks for bone marrow failure and aplastic anemia,” said study author Maria Monica Gramatges, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“We know that up to 15% to 20% of children can take 2 months or longer to recover their blood counts after a course of AML chemotherapy. Our goal was to understand if these children had an underlying genetic predisposition associated with an impaired capacity for recovery.”
Dr Gramatges and her colleagues hypothesized that short telomere length could be associated with a delay in neutrophil recovery.
So they obtained bone marrow samples from AML patients who recovered as expected (within 30 days) after each chemotherapy course (n=62), and from AML patients who experienced significant delays in recovery after chemotherapy (n=53).
The team then measured telomere length on each subject and categorized the group by quartile, from shortest to longest.
Subjects in the quartile with the shortest telomere lengths took the longest to recover, especially during the last 2 courses of chemotherapy. In an adjusted analysis, lower telomere content was significantly associated with prolonged neutropenia after the fourth (P=0.002) and fifth courses of chemotherapy (P=0.009).
The researchers said these results support the hypothesis that telomeres are an indicator of capacity for neutrophil recovery following chemotherapy.
Dr Gramatges hopes the results of this study will be helpful in further understanding which children are at a higher risk for prolonged myelosuppression and how to target those children with modified treatments, improved supportive care, and closer monitoring in order to prevent potential complications such as severe infections.
“A significant proportion of children with AML suffer from treatment-related toxicities, with some succumbing to complications of the therapies we give, rather than from the actual cancer itself,” Dr Gramatges said.
“We hope this research will help us identify those who are at a higher risk for delayed recovery and use this knowledge to reduce the morbidity and mortality associated with AML treatment.” ![]()

Image by Volker Brinkmann
Researchers say they have discovered a way to predict which children with acute myeloid leukemia (AML) are at the highest risk of delayed neutrophil recovery.
The team examined the role of telomeres in neutrophil recovery and found that the length of a patient’s telomeres can indicate the rate of recovery following chemotherapy.
The group reported their findings in the Journal of Clinical Oncology.
“We were interested in telomere length as a marker of blood count recovery because defects in telomere maintenance are known risks for bone marrow failure and aplastic anemia,” said study author Maria Monica Gramatges, MD, PhD, of Baylor College of Medicine in Houston, Texas.
“We know that up to 15% to 20% of children can take 2 months or longer to recover their blood counts after a course of AML chemotherapy. Our goal was to understand if these children had an underlying genetic predisposition associated with an impaired capacity for recovery.”
Dr Gramatges and her colleagues hypothesized that short telomere length could be associated with a delay in neutrophil recovery.
So they obtained bone marrow samples from AML patients who recovered as expected (within 30 days) after each chemotherapy course (n=62), and from AML patients who experienced significant delays in recovery after chemotherapy (n=53).
The team then measured telomere length on each subject and categorized the group by quartile, from shortest to longest.
Subjects in the quartile with the shortest telomere lengths took the longest to recover, especially during the last 2 courses of chemotherapy. In an adjusted analysis, lower telomere content was significantly associated with prolonged neutropenia after the fourth (P=0.002) and fifth courses of chemotherapy (P=0.009).
The researchers said these results support the hypothesis that telomeres are an indicator of capacity for neutrophil recovery following chemotherapy.
Dr Gramatges hopes the results of this study will be helpful in further understanding which children are at a higher risk for prolonged myelosuppression and how to target those children with modified treatments, improved supportive care, and closer monitoring in order to prevent potential complications such as severe infections.
“A significant proportion of children with AML suffer from treatment-related toxicities, with some succumbing to complications of the therapies we give, rather than from the actual cancer itself,” Dr Gramatges said.
“We hope this research will help us identify those who are at a higher risk for delayed recovery and use this knowledge to reduce the morbidity and mortality associated with AML treatment.” ![]()
New type of CAR T cells can produce responses in NHL

Image courtesy of NIAID
Results of a phase 1 study suggest that chimeric antigen receptor T cells specific for the κ light chain (κ.CAR T cells) can produce responses in patients with relapsed or refractory B-cell malignancies, largely without side effects.
The therapy induced complete and partial responses in some patients with non-Hodgkin lymphoma (NHL), and it allowed other patients with chronic lymphocytic leukemia (CLL) or multiple myeloma (MM) to maintain stable disease.
There was 1 adverse event considered possibly related to the treatment.
The researchers reported these results in The Journal of Clinical Investigation.
The κ.CAR T cells were designed to recognize κ-restricted cells and spare normal B cells expressing the nontargeted λ light chain.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said study author Carlos Ramos, MD, of Baylor College of Medicine in Houston, Texas.
He and his colleagues tested the κ.CAR T cells in 16 patients with relapsed or refractory κ+ NHL (n=7), CLL (n=2), or MM (n=7).
The team isolated T cells from these patients and modified the cells so they could target the κ light chain on the surface of malignant B cells. The modified T cells were infused back into the patients, and each patient was monitored for disease progression and side effects.
Eleven patients stopped receiving other treatments at least 4 weeks prior to T-cell infusion. Six patients without lymphopenia received cyclophosphamide at 12.5 mg/kg 4 days before κ.CAR T-cell infusion (0.2×108 to 2×108 κ.CAR T cells/m2).
“We found the treatment to be feasible and safe at all the dose levels studied,” Dr Ramos said.
One MM patient had grade 3 lymphopenia that was deemed possibly related to treatment, but none of the other adverse events were thought to result from the κ.CAR T cells.
Two patients with NHL achieved a complete response to treatment, 1 lasting more than 32 months and the other lasting 6 weeks. A third NHL patient had a partial response lasting 3 months, and a CLL patient had stable disease lasting 6 weeks.
Five of the 7 MM patients had stable disease, 3 lasting 6 weeks, 1 lasting 17 months, and 1 lasting 24 months.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” Dr Ramos concluded. ![]()

Image courtesy of NIAID
Results of a phase 1 study suggest that chimeric antigen receptor T cells specific for the κ light chain (κ.CAR T cells) can produce responses in patients with relapsed or refractory B-cell malignancies, largely without side effects.
The therapy induced complete and partial responses in some patients with non-Hodgkin lymphoma (NHL), and it allowed other patients with chronic lymphocytic leukemia (CLL) or multiple myeloma (MM) to maintain stable disease.
There was 1 adverse event considered possibly related to the treatment.
The researchers reported these results in The Journal of Clinical Investigation.
The κ.CAR T cells were designed to recognize κ-restricted cells and spare normal B cells expressing the nontargeted λ light chain.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said study author Carlos Ramos, MD, of Baylor College of Medicine in Houston, Texas.
He and his colleagues tested the κ.CAR T cells in 16 patients with relapsed or refractory κ+ NHL (n=7), CLL (n=2), or MM (n=7).
The team isolated T cells from these patients and modified the cells so they could target the κ light chain on the surface of malignant B cells. The modified T cells were infused back into the patients, and each patient was monitored for disease progression and side effects.
Eleven patients stopped receiving other treatments at least 4 weeks prior to T-cell infusion. Six patients without lymphopenia received cyclophosphamide at 12.5 mg/kg 4 days before κ.CAR T-cell infusion (0.2×108 to 2×108 κ.CAR T cells/m2).
“We found the treatment to be feasible and safe at all the dose levels studied,” Dr Ramos said.
One MM patient had grade 3 lymphopenia that was deemed possibly related to treatment, but none of the other adverse events were thought to result from the κ.CAR T cells.
Two patients with NHL achieved a complete response to treatment, 1 lasting more than 32 months and the other lasting 6 weeks. A third NHL patient had a partial response lasting 3 months, and a CLL patient had stable disease lasting 6 weeks.
Five of the 7 MM patients had stable disease, 3 lasting 6 weeks, 1 lasting 17 months, and 1 lasting 24 months.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” Dr Ramos concluded. ![]()

Image courtesy of NIAID
Results of a phase 1 study suggest that chimeric antigen receptor T cells specific for the κ light chain (κ.CAR T cells) can produce responses in patients with relapsed or refractory B-cell malignancies, largely without side effects.
The therapy induced complete and partial responses in some patients with non-Hodgkin lymphoma (NHL), and it allowed other patients with chronic lymphocytic leukemia (CLL) or multiple myeloma (MM) to maintain stable disease.
There was 1 adverse event considered possibly related to the treatment.
The researchers reported these results in The Journal of Clinical Investigation.
The κ.CAR T cells were designed to recognize κ-restricted cells and spare normal B cells expressing the nontargeted λ light chain.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said study author Carlos Ramos, MD, of Baylor College of Medicine in Houston, Texas.
He and his colleagues tested the κ.CAR T cells in 16 patients with relapsed or refractory κ+ NHL (n=7), CLL (n=2), or MM (n=7).
The team isolated T cells from these patients and modified the cells so they could target the κ light chain on the surface of malignant B cells. The modified T cells were infused back into the patients, and each patient was monitored for disease progression and side effects.
Eleven patients stopped receiving other treatments at least 4 weeks prior to T-cell infusion. Six patients without lymphopenia received cyclophosphamide at 12.5 mg/kg 4 days before κ.CAR T-cell infusion (0.2×108 to 2×108 κ.CAR T cells/m2).
“We found the treatment to be feasible and safe at all the dose levels studied,” Dr Ramos said.
One MM patient had grade 3 lymphopenia that was deemed possibly related to treatment, but none of the other adverse events were thought to result from the κ.CAR T cells.
Two patients with NHL achieved a complete response to treatment, 1 lasting more than 32 months and the other lasting 6 weeks. A third NHL patient had a partial response lasting 3 months, and a CLL patient had stable disease lasting 6 weeks.
Five of the 7 MM patients had stable disease, 3 lasting 6 weeks, 1 lasting 17 months, and 1 lasting 24 months.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” Dr Ramos concluded.