User login
Inhibitor produces responses in advanced SM
Results of a phase 2 trial suggest the multikinase inhibitor midostaurin can repair organ damage in patients with advanced systemic mastocytosis (SM).
The drug produced a 60% response rate among patients with mastocytosis-related organ damage, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose.
Jason Gotlib, MD, of the Stanford University School of Medicine in California, and his colleagues conducted this study and reported the results in NEJM.
The study was funded by Novartis Inc., which manufactures midostaurin, also known as PKC412.
The researchers noted that roughly 90% of patients with advanced SM have a mutation known as D816V in the gene that encodes the protein KIT, which controls the growth of mast cells.
Unfortunately, the only drug approved to treat advanced SM in the US is the tyrosine kinase inhibitor imatinib, and this drug is not active against the mutated KIT D816V protein. Midostaurin, on the other hand, does inhibit KIT D816V.
With this in mind, Dr Gotlib and his colleagues set out to test midostaurin (given at 100 mg twice daily until disease progression or unacceptable toxicity) in 116 patients.
Eighty-nine of the patients had mastocytosis-related organ damage, 16 had aggressive SM, 57 had SM with an associated hematologic neoplasm, and 16 had mast cell leukemia.
Response
The median follow-up was 26 months (range, 12 to 54), and the study’s primary outcome was the best overall response.
The overall response rate for the primary efficacy population (the 89 patients with mastocytosis-related organ damage) was 60%. Forty-five percent of the patients had a major response, which was defined as complete resolution of at least one type of mastocytosis-related organ damage.
The overall response rate was 75% for patients with aggressive SM, 58% for SM patients with an associated hematologic neoplasm, and 50% for patients with mast-cell leukemia.
Responses occurred in all subgroups, which included patients who were positive for KIT D816V.
The researchers noted that responding patients were less likely to need red blood cell or platelet transfusions, experienced improvements in liver function, and had fewer signs of malabsorption such as weight loss.
The median duration of response for all responders in the primary efficacy population (n=89) was 24.1 months.
Survival
The median overall survival was 28.7 months in the primary efficacy population (n=89) and 33.9 months in the intention-to-treat population (n=116). The median progression-free survival was 14.1 months in the primary efficacy population.
The survival benefit among patients with mast cell leukemia was particularly striking, according to Dr Gotlib. Although most people succumb to this form of the disease within 6 months of diagnosis, the median overall survival of midostaurin-treated patients with mast cell leukemia was 9.4 months.
The median overall survival was not reached among patients with aggressive SM and was 20.7 months among patients who had SM with an associated hematologic neoplasm.
Safety
The most common nonhematologic adverse events were nausea (79%), vomiting (66%), and diarrhea (54%). The most common grade 3/4 nonhematologic adverse events were fatigue (9%) and diarrhea (8%).
Grade 3/4 hematologic adverse events included new or worsening neutropenia (24%), anemia (41%), and thrombocytopenia (29%).
Researchers reduced the dose of midostaurin in 65 patients (56%), mostly due to adverse events (n=48). Twenty-one of these patients (32%) were later able to return to the initial dose.
Eighty-four patients (72%) discontinued treatment, and 32 (28%) were receiving ongoing treatment at the time of data cutoff. The most frequent reasons for treatment discontinuation were disease progression (33%) and adverse events (22%).
Compassionate use
The phase 2 study results are reinforced by a letter published in the same issue of NEJM, which describes a compassionate use program for midostaurin in advanced SM.
The letter includes results in 28 patients. After a median follow-up of 18.5 months, the overall response rate was 71%. The overall survival rate was 42.7%.
The most frequent adverse events were nausea/vomiting in 89% of patients (leading to failure/discontinuation in 18%), lymphocytopenia in 61% (without opportunistic infection), and photosensitivity in 25%. ![]()
Results of a phase 2 trial suggest the multikinase inhibitor midostaurin can repair organ damage in patients with advanced systemic mastocytosis (SM).
The drug produced a 60% response rate among patients with mastocytosis-related organ damage, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose.
Jason Gotlib, MD, of the Stanford University School of Medicine in California, and his colleagues conducted this study and reported the results in NEJM.
The study was funded by Novartis Inc., which manufactures midostaurin, also known as PKC412.
The researchers noted that roughly 90% of patients with advanced SM have a mutation known as D816V in the gene that encodes the protein KIT, which controls the growth of mast cells.
Unfortunately, the only drug approved to treat advanced SM in the US is the tyrosine kinase inhibitor imatinib, and this drug is not active against the mutated KIT D816V protein. Midostaurin, on the other hand, does inhibit KIT D816V.
With this in mind, Dr Gotlib and his colleagues set out to test midostaurin (given at 100 mg twice daily until disease progression or unacceptable toxicity) in 116 patients.
Eighty-nine of the patients had mastocytosis-related organ damage, 16 had aggressive SM, 57 had SM with an associated hematologic neoplasm, and 16 had mast cell leukemia.
Response
The median follow-up was 26 months (range, 12 to 54), and the study’s primary outcome was the best overall response.
The overall response rate for the primary efficacy population (the 89 patients with mastocytosis-related organ damage) was 60%. Forty-five percent of the patients had a major response, which was defined as complete resolution of at least one type of mastocytosis-related organ damage.
The overall response rate was 75% for patients with aggressive SM, 58% for SM patients with an associated hematologic neoplasm, and 50% for patients with mast-cell leukemia.
Responses occurred in all subgroups, which included patients who were positive for KIT D816V.
The researchers noted that responding patients were less likely to need red blood cell or platelet transfusions, experienced improvements in liver function, and had fewer signs of malabsorption such as weight loss.
The median duration of response for all responders in the primary efficacy population (n=89) was 24.1 months.
Survival
The median overall survival was 28.7 months in the primary efficacy population (n=89) and 33.9 months in the intention-to-treat population (n=116). The median progression-free survival was 14.1 months in the primary efficacy population.
The survival benefit among patients with mast cell leukemia was particularly striking, according to Dr Gotlib. Although most people succumb to this form of the disease within 6 months of diagnosis, the median overall survival of midostaurin-treated patients with mast cell leukemia was 9.4 months.
The median overall survival was not reached among patients with aggressive SM and was 20.7 months among patients who had SM with an associated hematologic neoplasm.
Safety
The most common nonhematologic adverse events were nausea (79%), vomiting (66%), and diarrhea (54%). The most common grade 3/4 nonhematologic adverse events were fatigue (9%) and diarrhea (8%).
Grade 3/4 hematologic adverse events included new or worsening neutropenia (24%), anemia (41%), and thrombocytopenia (29%).
Researchers reduced the dose of midostaurin in 65 patients (56%), mostly due to adverse events (n=48). Twenty-one of these patients (32%) were later able to return to the initial dose.
Eighty-four patients (72%) discontinued treatment, and 32 (28%) were receiving ongoing treatment at the time of data cutoff. The most frequent reasons for treatment discontinuation were disease progression (33%) and adverse events (22%).
Compassionate use
The phase 2 study results are reinforced by a letter published in the same issue of NEJM, which describes a compassionate use program for midostaurin in advanced SM.
The letter includes results in 28 patients. After a median follow-up of 18.5 months, the overall response rate was 71%. The overall survival rate was 42.7%.
The most frequent adverse events were nausea/vomiting in 89% of patients (leading to failure/discontinuation in 18%), lymphocytopenia in 61% (without opportunistic infection), and photosensitivity in 25%. ![]()
Results of a phase 2 trial suggest the multikinase inhibitor midostaurin can repair organ damage in patients with advanced systemic mastocytosis (SM).
The drug produced a 60% response rate among patients with mastocytosis-related organ damage, and the median duration of response was 24.1 months.
Fifty-six percent of patients required dose reductions due to toxic effects, but 32% of these patients were able to return to the starting dose.
Jason Gotlib, MD, of the Stanford University School of Medicine in California, and his colleagues conducted this study and reported the results in NEJM.
The study was funded by Novartis Inc., which manufactures midostaurin, also known as PKC412.
The researchers noted that roughly 90% of patients with advanced SM have a mutation known as D816V in the gene that encodes the protein KIT, which controls the growth of mast cells.
Unfortunately, the only drug approved to treat advanced SM in the US is the tyrosine kinase inhibitor imatinib, and this drug is not active against the mutated KIT D816V protein. Midostaurin, on the other hand, does inhibit KIT D816V.
With this in mind, Dr Gotlib and his colleagues set out to test midostaurin (given at 100 mg twice daily until disease progression or unacceptable toxicity) in 116 patients.
Eighty-nine of the patients had mastocytosis-related organ damage, 16 had aggressive SM, 57 had SM with an associated hematologic neoplasm, and 16 had mast cell leukemia.
Response
The median follow-up was 26 months (range, 12 to 54), and the study’s primary outcome was the best overall response.
The overall response rate for the primary efficacy population (the 89 patients with mastocytosis-related organ damage) was 60%. Forty-five percent of the patients had a major response, which was defined as complete resolution of at least one type of mastocytosis-related organ damage.
The overall response rate was 75% for patients with aggressive SM, 58% for SM patients with an associated hematologic neoplasm, and 50% for patients with mast-cell leukemia.
Responses occurred in all subgroups, which included patients who were positive for KIT D816V.
The researchers noted that responding patients were less likely to need red blood cell or platelet transfusions, experienced improvements in liver function, and had fewer signs of malabsorption such as weight loss.
The median duration of response for all responders in the primary efficacy population (n=89) was 24.1 months.
Survival
The median overall survival was 28.7 months in the primary efficacy population (n=89) and 33.9 months in the intention-to-treat population (n=116). The median progression-free survival was 14.1 months in the primary efficacy population.
The survival benefit among patients with mast cell leukemia was particularly striking, according to Dr Gotlib. Although most people succumb to this form of the disease within 6 months of diagnosis, the median overall survival of midostaurin-treated patients with mast cell leukemia was 9.4 months.
The median overall survival was not reached among patients with aggressive SM and was 20.7 months among patients who had SM with an associated hematologic neoplasm.
Safety
The most common nonhematologic adverse events were nausea (79%), vomiting (66%), and diarrhea (54%). The most common grade 3/4 nonhematologic adverse events were fatigue (9%) and diarrhea (8%).
Grade 3/4 hematologic adverse events included new or worsening neutropenia (24%), anemia (41%), and thrombocytopenia (29%).
Researchers reduced the dose of midostaurin in 65 patients (56%), mostly due to adverse events (n=48). Twenty-one of these patients (32%) were later able to return to the initial dose.
Eighty-four patients (72%) discontinued treatment, and 32 (28%) were receiving ongoing treatment at the time of data cutoff. The most frequent reasons for treatment discontinuation were disease progression (33%) and adverse events (22%).
Compassionate use
The phase 2 study results are reinforced by a letter published in the same issue of NEJM, which describes a compassionate use program for midostaurin in advanced SM.
The letter includes results in 28 patients. After a median follow-up of 18.5 months, the overall response rate was 71%. The overall survival rate was 42.7%.
The most frequent adverse events were nausea/vomiting in 89% of patients (leading to failure/discontinuation in 18%), lymphocytopenia in 61% (without opportunistic infection), and photosensitivity in 25%. ![]()
Risk of AML death varies by region

Photo by Rhoda Baer
The risk of death from acute myeloid leukemia (AML) may be influenced by where a patient lives, according to a study published in Cancer.
Three regions in North Carolina were found to be associated with a higher risk of death, when compared to the rest of the state.
Patients had a significantly higher risk of death if they lived in northeastern North Carolina (from Wilson to Roanoke Rapids), in a region around Greenville, and a region around Wake County, including Durham County.
The increased risk remained even when the researchers controlled for other factors.
“The geographic survival disparities we found could not be explained by other sociodemographic variables or proximity to experienced treating facilities,” said study author Ashley Freeman, MD, of the University of North Carolina (UNC) in Chapel Hill.
“This raises the possibility that more complex features of the local healthcare infrastructure, including provider referral and practice patterns, are affecting patient outcomes.”
To study death rates from AML across North Carolina, Dr Freeman and her colleagues analyzed data on 553 adults who were diagnosed with AML between 2003 and 2009 and received inpatient chemotherapy within 30 days of diagnosis.
The team used the UNC Lineberger Integrated Cancer Information and Surveillance System, a database that links insurance claims information to a state information database called the NC Cancer Registry.
The researchers assessed the risk of death in 9 regions defined by the North Carolina Area Health Education Centers (AHEC) Program, a program established in 1972 to address physician shortages and the uneven distribution of healthcare services in North Carolina.
“We looked at geographic disparities because we are trying to improve outcomes for all citizens in North Carolina, consistent with the mission of our cancer center,” said William A. Wood, MD, of UNC.
“We are also trying to find situations in which disparities shouldn’t exist but do for arbitrary reasons—such as where a patient happens to live—so that we can figure out how to improve equity across the state.”
The researchers determined that a region around Greensboro had the lowest risk of death for AML.
Compared to the Greensboro region, the risk of death was 4 times higher in an area of northeastern North Carolina that included Roanoke Rapids, Rocky Mount, and Wilson—the highest in the state.
Compared to the Greensboro region, the risk of death was more than 2 times greater in the eastern region of the state around Greenville, and it was nearly twice as high in the region around Wake County.
“There are areas of the state where there is an elevated mortality, and we need to better understand the factors that are driving that—whether they’re environmental, patient, or provider-related,” said Anne-Marie Meyer, PhD, of UNC.
Nearly half of patients in the study received their care at hospitals not affiliated with one of the state’s 3 National Cancer Institute (NCI) comprehensive cancer centers.
The researchers did not find a significant link between the risk of death and the distance from patients’ homes to their treating facility or the nearest NCI-designated center.
And there was no significant difference in the risk of death at 1 year between patients who received treatment at an NCI-designated cancer center and those who did not. However, patients with a more serious prognosis were more likely to be treated at an NCI-designated cancer center.
The researchers did identify regional differences in healthcare resources. “Area L,” which is the name for the region in northeastern North Carolina that spans from Wilson to Roanoke Rapids, for example, has some of the lowest proportion of general practitioner physicians and radiation oncologists, as well as the highest burden of disease.
However, it is not clear why the disparities continued even after the researchers controlled for regional factors like poverty and education. The team believes other factors could be involved, such as the providers’ experience with treating rare or complex diseases or how supportive care is delivered.
Although there was not a significant association between survival and treatment at an NCI-designated center, there may be other features of treatment facilities, such as patient volume and academic affiliation, that are important for patient outcomes.
The researchers said their next step is to identify those factors and develop programs to try to close the gaps.
“The message here is that acute myeloid leukemia is representative of diseases that are uncommon, involve high-complexity care, and have high risk for morbidity and mortality,” Dr Wood said.
“If we can figure out how to coordinate and improve delivery of effective interventions for this disease throughout the state of North Carolina, then we may be able to develop a model for improving outcomes in many other diseases throughout the state as well.” ![]()

Photo by Rhoda Baer
The risk of death from acute myeloid leukemia (AML) may be influenced by where a patient lives, according to a study published in Cancer.
Three regions in North Carolina were found to be associated with a higher risk of death, when compared to the rest of the state.
Patients had a significantly higher risk of death if they lived in northeastern North Carolina (from Wilson to Roanoke Rapids), in a region around Greenville, and a region around Wake County, including Durham County.
The increased risk remained even when the researchers controlled for other factors.
“The geographic survival disparities we found could not be explained by other sociodemographic variables or proximity to experienced treating facilities,” said study author Ashley Freeman, MD, of the University of North Carolina (UNC) in Chapel Hill.
“This raises the possibility that more complex features of the local healthcare infrastructure, including provider referral and practice patterns, are affecting patient outcomes.”
To study death rates from AML across North Carolina, Dr Freeman and her colleagues analyzed data on 553 adults who were diagnosed with AML between 2003 and 2009 and received inpatient chemotherapy within 30 days of diagnosis.
The team used the UNC Lineberger Integrated Cancer Information and Surveillance System, a database that links insurance claims information to a state information database called the NC Cancer Registry.
The researchers assessed the risk of death in 9 regions defined by the North Carolina Area Health Education Centers (AHEC) Program, a program established in 1972 to address physician shortages and the uneven distribution of healthcare services in North Carolina.
“We looked at geographic disparities because we are trying to improve outcomes for all citizens in North Carolina, consistent with the mission of our cancer center,” said William A. Wood, MD, of UNC.
“We are also trying to find situations in which disparities shouldn’t exist but do for arbitrary reasons—such as where a patient happens to live—so that we can figure out how to improve equity across the state.”
The researchers determined that a region around Greensboro had the lowest risk of death for AML.
Compared to the Greensboro region, the risk of death was 4 times higher in an area of northeastern North Carolina that included Roanoke Rapids, Rocky Mount, and Wilson—the highest in the state.
Compared to the Greensboro region, the risk of death was more than 2 times greater in the eastern region of the state around Greenville, and it was nearly twice as high in the region around Wake County.
“There are areas of the state where there is an elevated mortality, and we need to better understand the factors that are driving that—whether they’re environmental, patient, or provider-related,” said Anne-Marie Meyer, PhD, of UNC.
Nearly half of patients in the study received their care at hospitals not affiliated with one of the state’s 3 National Cancer Institute (NCI) comprehensive cancer centers.
The researchers did not find a significant link between the risk of death and the distance from patients’ homes to their treating facility or the nearest NCI-designated center.
And there was no significant difference in the risk of death at 1 year between patients who received treatment at an NCI-designated cancer center and those who did not. However, patients with a more serious prognosis were more likely to be treated at an NCI-designated cancer center.
The researchers did identify regional differences in healthcare resources. “Area L,” which is the name for the region in northeastern North Carolina that spans from Wilson to Roanoke Rapids, for example, has some of the lowest proportion of general practitioner physicians and radiation oncologists, as well as the highest burden of disease.
However, it is not clear why the disparities continued even after the researchers controlled for regional factors like poverty and education. The team believes other factors could be involved, such as the providers’ experience with treating rare or complex diseases or how supportive care is delivered.
Although there was not a significant association between survival and treatment at an NCI-designated center, there may be other features of treatment facilities, such as patient volume and academic affiliation, that are important for patient outcomes.
The researchers said their next step is to identify those factors and develop programs to try to close the gaps.
“The message here is that acute myeloid leukemia is representative of diseases that are uncommon, involve high-complexity care, and have high risk for morbidity and mortality,” Dr Wood said.
“If we can figure out how to coordinate and improve delivery of effective interventions for this disease throughout the state of North Carolina, then we may be able to develop a model for improving outcomes in many other diseases throughout the state as well.” ![]()

Photo by Rhoda Baer
The risk of death from acute myeloid leukemia (AML) may be influenced by where a patient lives, according to a study published in Cancer.
Three regions in North Carolina were found to be associated with a higher risk of death, when compared to the rest of the state.
Patients had a significantly higher risk of death if they lived in northeastern North Carolina (from Wilson to Roanoke Rapids), in a region around Greenville, and a region around Wake County, including Durham County.
The increased risk remained even when the researchers controlled for other factors.
“The geographic survival disparities we found could not be explained by other sociodemographic variables or proximity to experienced treating facilities,” said study author Ashley Freeman, MD, of the University of North Carolina (UNC) in Chapel Hill.
“This raises the possibility that more complex features of the local healthcare infrastructure, including provider referral and practice patterns, are affecting patient outcomes.”
To study death rates from AML across North Carolina, Dr Freeman and her colleagues analyzed data on 553 adults who were diagnosed with AML between 2003 and 2009 and received inpatient chemotherapy within 30 days of diagnosis.
The team used the UNC Lineberger Integrated Cancer Information and Surveillance System, a database that links insurance claims information to a state information database called the NC Cancer Registry.
The researchers assessed the risk of death in 9 regions defined by the North Carolina Area Health Education Centers (AHEC) Program, a program established in 1972 to address physician shortages and the uneven distribution of healthcare services in North Carolina.
“We looked at geographic disparities because we are trying to improve outcomes for all citizens in North Carolina, consistent with the mission of our cancer center,” said William A. Wood, MD, of UNC.
“We are also trying to find situations in which disparities shouldn’t exist but do for arbitrary reasons—such as where a patient happens to live—so that we can figure out how to improve equity across the state.”
The researchers determined that a region around Greensboro had the lowest risk of death for AML.
Compared to the Greensboro region, the risk of death was 4 times higher in an area of northeastern North Carolina that included Roanoke Rapids, Rocky Mount, and Wilson—the highest in the state.
Compared to the Greensboro region, the risk of death was more than 2 times greater in the eastern region of the state around Greenville, and it was nearly twice as high in the region around Wake County.
“There are areas of the state where there is an elevated mortality, and we need to better understand the factors that are driving that—whether they’re environmental, patient, or provider-related,” said Anne-Marie Meyer, PhD, of UNC.
Nearly half of patients in the study received their care at hospitals not affiliated with one of the state’s 3 National Cancer Institute (NCI) comprehensive cancer centers.
The researchers did not find a significant link between the risk of death and the distance from patients’ homes to their treating facility or the nearest NCI-designated center.
And there was no significant difference in the risk of death at 1 year between patients who received treatment at an NCI-designated cancer center and those who did not. However, patients with a more serious prognosis were more likely to be treated at an NCI-designated cancer center.
The researchers did identify regional differences in healthcare resources. “Area L,” which is the name for the region in northeastern North Carolina that spans from Wilson to Roanoke Rapids, for example, has some of the lowest proportion of general practitioner physicians and radiation oncologists, as well as the highest burden of disease.
However, it is not clear why the disparities continued even after the researchers controlled for regional factors like poverty and education. The team believes other factors could be involved, such as the providers’ experience with treating rare or complex diseases or how supportive care is delivered.
Although there was not a significant association between survival and treatment at an NCI-designated center, there may be other features of treatment facilities, such as patient volume and academic affiliation, that are important for patient outcomes.
The researchers said their next step is to identify those factors and develop programs to try to close the gaps.
“The message here is that acute myeloid leukemia is representative of diseases that are uncommon, involve high-complexity care, and have high risk for morbidity and mortality,” Dr Wood said.
“If we can figure out how to coordinate and improve delivery of effective interventions for this disease throughout the state of North Carolina, then we may be able to develop a model for improving outcomes in many other diseases throughout the state as well.” ![]()
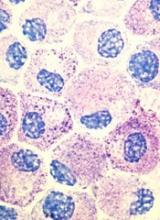
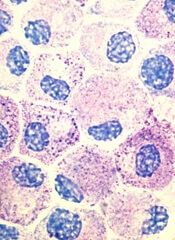
Midostaurin cut organ damage in systemic mastocytosis
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
Midostaurin completely resolved at least one type of organ damage for 45% of patients with advanced systemic mastocytosis, based on a multicenter, open-label, phase II, industry-sponsored trial.
“Response rates were similar regardless of the subtype of advanced systemic mastocytosis, KIT mutation status, or exposure to previous therapy,” reported Jason R. Gotlib, MD, of Stanford (Calif.) University, and his associates. Adverse effects led to dose reductions for 41% of patients, however, and caused 22% of patients to stop treatment, the researchers wrote online June 29 in the New England Journal of Medicine.
Systemic mastocytosis is related to a constitutively activated receptor tyrosine kinase encoded by the KIT D816V mutation. As neoplastic mast cells infiltrate and damage organs, patients develop cytopenias, hypoalbuminemia, osteolytic bone lesions, abnormal liver function, ascites, and weight loss. Mastocytosis lacks effective treatments, and patients with aggressive disease tend to live about 3.5 years, the researchers noted (N Engl J Med. 2016 Jun 29;374:2530-40).
Of 116 patients with advanced systemic mastocytosis, 27 lacked measurable signs of disease or had unrelated signs and symptoms. The remaining 89 patients included 16 with aggressive systemic disease, 57 with systemic disease and an associated hematologic neoplasm, and 16 with mast cell leukemia. Patients received 100 mg oral midostaurin twice daily in continuous 4-week cycles for a median of 11.4 months, with a median follow-up of 26 months.
In all, 53 (60%) patients experienced at least 50% improvement in one type of organ damage or improvement in more than one type of organ damage, said the researchers. These responders included 12 patients with aggressive systemic mastocytosis, 33 patients with systemic mastocytosis and a hematologic neoplasm, and eight patients with mast-cell leukemia. No one achieved complete remission, but after six treatment cycles, 45% of patients had complete resolution of at least one type of organ damage.
Patients typically experienced, at best, a nearly 60% drop in bone marrow mast cell burden and serum tryptase. The median duration of response was 24 months, median overall survival was 28.7 months, and median progression-free survival was 14.1 months. Mast cell leukemia and a history of treatment for mastocytosis were tied to shorter survival, while a 50% decrease in mast cell burden significantly improved survival (hazard ratio, 0.33; P = .01).
Grade 3 or 4 hematologic abnormalities included neutropenia (24% of patients), anemia (41%), and thrombocytopenia (29%). Marked myelosuppression was associated with baseline cytopenia and may have reflected either treatment-related effects or disease progression, the researchers said. The most common grade 3/4 nonhematologic adverse effects were fatigue (9% of patients) and diarrhea (8%).
Novartis Pharmaceuticals sponsored the study, and designed it and collected the data with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. The remaining four coinvestigators had no disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Midostaurin helped to resolve organ damage related to mastocytosis.
Major finding: In all, 45% of patients had complete resolution of at least one type of organ damage within six, 4-week treatment cycles.
Data source: An international, open-label, phase II study of 116 patients given 100 mg oral midostaurin twice daily.
Disclosures: Novartis Pharmaceuticals sponsored the study, designed the study, and collected the data together with the authors. Dr. Gotlib disclosed travel reimbursement from Novartis. Ten coinvestigators disclosed financial ties to Novartis and to several other pharmaceutical companies. Two coinvestigators disclosed direct research support from Novartis. Four coinvestigators had no disclosures.
Study: CMV doesn’t lower risk of relapse, death

Small studies have suggested that early cytomegalovirus (CMV) reactivation may protect against leukemia relapse and even death after hematopoietic stem cell transplant.
However, a new study, based on data from about 9500 patients, suggests otherwise.
Results showed no association between CMV reactivation and relapse but suggested CMV reactivation increases the risk of non-relapse mortality.
Researchers reported these findings in Blood.
“The original purpose of the study was to confirm that CMV infection may prevent leukemia relapse, prevent death, and become a major therapeutic tool for improving patient survival rates,” said study author Pierre Teira, MD, of the University of Montreal in Quebec, Canada.
“However, we found the exact opposite. Our results clearly show that . . . the virus not only does not prevent leukemia relapse [it] also remains a major factor associated with the risk of death. Monitoring of CMV after transplantation remains a priority for patients.”
For this study, Dr Teira and his colleagues analyzed data from 9469 patients who received a transplant between 2003 and 2010.
The patients had acute myeloid leukemia (AML, n=5310), acute lymphoblastic leukemia (ALL, n=1883), chronic myeloid leukemia (CML, n=1079), or myelodysplastic syndromes (MDS, n=1197).
The median time to initial CMV reactivation was 41 days (range, 1-362 days).
The researchers found no significant association between CMV reactivation and disease relapse for AML (P=0.60), ALL (P=0.08), CML (P=0.94), or MDS (P=0.58).
However, CMV reactivation was associated with a significantly higher risk of nonrelapse mortality for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0004), and MDS (P=0.0002).
Therefore, CMV reactivation was associated with significantly lower overall survival for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0005), and MDS (P=0.003).
“Deaths due to uncontrolled CMV reactivation are virtually zero in this study, so uncontrolled CMV reactivation is not what reduces survival rates after transplantation,” Dr Teira noted. “The link between this common virus and increased risk of death remains a biological mystery.”
One possible explanation is that CMV decreases the ability of the patient’s immune system to fight against other types of infection. This is supported by the fact that death rates from infections other than CMV are higher in patients infected with CMV or patients whose donors were.
For researchers, the next step is therefore to verify whether the latest generation of anti-CMV treatments can prevent both reactivation of the virus and weakening of the patient’s immune system against other types of infection in the presence of CMV infection.
“CMV has a complex impact on the outcomes for transplant patients, and, each year, more than 30,000 patients around the world receive bone marrow transplants from donors,” Dr Teira said.
“It is therefore essential for future research to better understand the role played by CMV after bone marrow transplantation and improve the chances of success of the transplant. This will help to better choose the right donor for the right patient.” ![]()

Small studies have suggested that early cytomegalovirus (CMV) reactivation may protect against leukemia relapse and even death after hematopoietic stem cell transplant.
However, a new study, based on data from about 9500 patients, suggests otherwise.
Results showed no association between CMV reactivation and relapse but suggested CMV reactivation increases the risk of non-relapse mortality.
Researchers reported these findings in Blood.
“The original purpose of the study was to confirm that CMV infection may prevent leukemia relapse, prevent death, and become a major therapeutic tool for improving patient survival rates,” said study author Pierre Teira, MD, of the University of Montreal in Quebec, Canada.
“However, we found the exact opposite. Our results clearly show that . . . the virus not only does not prevent leukemia relapse [it] also remains a major factor associated with the risk of death. Monitoring of CMV after transplantation remains a priority for patients.”
For this study, Dr Teira and his colleagues analyzed data from 9469 patients who received a transplant between 2003 and 2010.
The patients had acute myeloid leukemia (AML, n=5310), acute lymphoblastic leukemia (ALL, n=1883), chronic myeloid leukemia (CML, n=1079), or myelodysplastic syndromes (MDS, n=1197).
The median time to initial CMV reactivation was 41 days (range, 1-362 days).
The researchers found no significant association between CMV reactivation and disease relapse for AML (P=0.60), ALL (P=0.08), CML (P=0.94), or MDS (P=0.58).
However, CMV reactivation was associated with a significantly higher risk of nonrelapse mortality for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0004), and MDS (P=0.0002).
Therefore, CMV reactivation was associated with significantly lower overall survival for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0005), and MDS (P=0.003).
“Deaths due to uncontrolled CMV reactivation are virtually zero in this study, so uncontrolled CMV reactivation is not what reduces survival rates after transplantation,” Dr Teira noted. “The link between this common virus and increased risk of death remains a biological mystery.”
One possible explanation is that CMV decreases the ability of the patient’s immune system to fight against other types of infection. This is supported by the fact that death rates from infections other than CMV are higher in patients infected with CMV or patients whose donors were.
For researchers, the next step is therefore to verify whether the latest generation of anti-CMV treatments can prevent both reactivation of the virus and weakening of the patient’s immune system against other types of infection in the presence of CMV infection.
“CMV has a complex impact on the outcomes for transplant patients, and, each year, more than 30,000 patients around the world receive bone marrow transplants from donors,” Dr Teira said.
“It is therefore essential for future research to better understand the role played by CMV after bone marrow transplantation and improve the chances of success of the transplant. This will help to better choose the right donor for the right patient.” ![]()

Small studies have suggested that early cytomegalovirus (CMV) reactivation may protect against leukemia relapse and even death after hematopoietic stem cell transplant.
However, a new study, based on data from about 9500 patients, suggests otherwise.
Results showed no association between CMV reactivation and relapse but suggested CMV reactivation increases the risk of non-relapse mortality.
Researchers reported these findings in Blood.
“The original purpose of the study was to confirm that CMV infection may prevent leukemia relapse, prevent death, and become a major therapeutic tool for improving patient survival rates,” said study author Pierre Teira, MD, of the University of Montreal in Quebec, Canada.
“However, we found the exact opposite. Our results clearly show that . . . the virus not only does not prevent leukemia relapse [it] also remains a major factor associated with the risk of death. Monitoring of CMV after transplantation remains a priority for patients.”
For this study, Dr Teira and his colleagues analyzed data from 9469 patients who received a transplant between 2003 and 2010.
The patients had acute myeloid leukemia (AML, n=5310), acute lymphoblastic leukemia (ALL, n=1883), chronic myeloid leukemia (CML, n=1079), or myelodysplastic syndromes (MDS, n=1197).
The median time to initial CMV reactivation was 41 days (range, 1-362 days).
The researchers found no significant association between CMV reactivation and disease relapse for AML (P=0.60), ALL (P=0.08), CML (P=0.94), or MDS (P=0.58).
However, CMV reactivation was associated with a significantly higher risk of nonrelapse mortality for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0004), and MDS (P=0.0002).
Therefore, CMV reactivation was associated with significantly lower overall survival for AML (P<0.0001), ALL (P<0.0001), CML (P=0.0005), and MDS (P=0.003).
“Deaths due to uncontrolled CMV reactivation are virtually zero in this study, so uncontrolled CMV reactivation is not what reduces survival rates after transplantation,” Dr Teira noted. “The link between this common virus and increased risk of death remains a biological mystery.”
One possible explanation is that CMV decreases the ability of the patient’s immune system to fight against other types of infection. This is supported by the fact that death rates from infections other than CMV are higher in patients infected with CMV or patients whose donors were.
For researchers, the next step is therefore to verify whether the latest generation of anti-CMV treatments can prevent both reactivation of the virus and weakening of the patient’s immune system against other types of infection in the presence of CMV infection.
“CMV has a complex impact on the outcomes for transplant patients, and, each year, more than 30,000 patients around the world receive bone marrow transplants from donors,” Dr Teira said.
“It is therefore essential for future research to better understand the role played by CMV after bone marrow transplantation and improve the chances of success of the transplant. This will help to better choose the right donor for the right patient.” ![]()
Team maps chromatin landscape in CLL

Researchers say they have performed the first large-scale analysis of the chromatin landscape in chronic lymphocytic leukemia (CLL).
And, in doing so, they have identified shared gene regulatory networks as well as heterogeneity between patients and CLL subtypes.
The group says this work should enable deeper investigation into chromatin regulation in CLL and the identification of therapeutically relevant mechanisms of disease.
The work has been published in Nature Communications.
The researchers performed chromatin accessibility mapping—via the assay for transposase-accessible chromatin using sequencing (ATAC-seq)—on 88 CLL samples from 55 patients.
For 10 of the samples, the team also established histone profiles using ChIPmentation for 3 histone marks (H3K4me1, H3K27ac, and H3K27me3) and transcriptome profiles using RNA sequencing.
The researchers then developed a bioinformatic method for linking the chromatin profiles to clinical annotations and molecular diagnostics data, and they analyzed gene regulatory networks that underlie the major disease subtypes of CLL.
The work revealed a “shared core” of regulatory regions in CLL patients as well as variations between the samples.
Furthermore, the chromatin profiles and gene regulatory networks accurately predicted IGHV mutation status and pinpointed differences between IGVH-mutated and IGVH-unmutated CLL.
“Our study has been able to dissect the variability that exists in the epigenome of CLL patients and helped to identify disease-specific changes, which will hopefully be informative for distinguishing disease subtypes or identifying suitable treatments,” said study author Jonathan Strefford, PhD, of the University of Southampton in the UK.
“Epigenetics can offer a useful doorway into ways of improving disease diagnosis and more personalized treatment choices for patients.” ![]()

Researchers say they have performed the first large-scale analysis of the chromatin landscape in chronic lymphocytic leukemia (CLL).
And, in doing so, they have identified shared gene regulatory networks as well as heterogeneity between patients and CLL subtypes.
The group says this work should enable deeper investigation into chromatin regulation in CLL and the identification of therapeutically relevant mechanisms of disease.
The work has been published in Nature Communications.
The researchers performed chromatin accessibility mapping—via the assay for transposase-accessible chromatin using sequencing (ATAC-seq)—on 88 CLL samples from 55 patients.
For 10 of the samples, the team also established histone profiles using ChIPmentation for 3 histone marks (H3K4me1, H3K27ac, and H3K27me3) and transcriptome profiles using RNA sequencing.
The researchers then developed a bioinformatic method for linking the chromatin profiles to clinical annotations and molecular diagnostics data, and they analyzed gene regulatory networks that underlie the major disease subtypes of CLL.
The work revealed a “shared core” of regulatory regions in CLL patients as well as variations between the samples.
Furthermore, the chromatin profiles and gene regulatory networks accurately predicted IGHV mutation status and pinpointed differences between IGVH-mutated and IGVH-unmutated CLL.
“Our study has been able to dissect the variability that exists in the epigenome of CLL patients and helped to identify disease-specific changes, which will hopefully be informative for distinguishing disease subtypes or identifying suitable treatments,” said study author Jonathan Strefford, PhD, of the University of Southampton in the UK.
“Epigenetics can offer a useful doorway into ways of improving disease diagnosis and more personalized treatment choices for patients.” ![]()

Researchers say they have performed the first large-scale analysis of the chromatin landscape in chronic lymphocytic leukemia (CLL).
And, in doing so, they have identified shared gene regulatory networks as well as heterogeneity between patients and CLL subtypes.
The group says this work should enable deeper investigation into chromatin regulation in CLL and the identification of therapeutically relevant mechanisms of disease.
The work has been published in Nature Communications.
The researchers performed chromatin accessibility mapping—via the assay for transposase-accessible chromatin using sequencing (ATAC-seq)—on 88 CLL samples from 55 patients.
For 10 of the samples, the team also established histone profiles using ChIPmentation for 3 histone marks (H3K4me1, H3K27ac, and H3K27me3) and transcriptome profiles using RNA sequencing.
The researchers then developed a bioinformatic method for linking the chromatin profiles to clinical annotations and molecular diagnostics data, and they analyzed gene regulatory networks that underlie the major disease subtypes of CLL.
The work revealed a “shared core” of regulatory regions in CLL patients as well as variations between the samples.
Furthermore, the chromatin profiles and gene regulatory networks accurately predicted IGHV mutation status and pinpointed differences between IGVH-mutated and IGVH-unmutated CLL.
“Our study has been able to dissect the variability that exists in the epigenome of CLL patients and helped to identify disease-specific changes, which will hopefully be informative for distinguishing disease subtypes or identifying suitable treatments,” said study author Jonathan Strefford, PhD, of the University of Southampton in the UK.
“Epigenetics can offer a useful doorway into ways of improving disease diagnosis and more personalized treatment choices for patients.” ![]()
Study explains how a mutation spurs AML development

A set of faulty genetic instructions keeps hematopoietic stem/progenitor cells (HSPCs) from maturing and contributes to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Researchers found that a mutation in the gene DNMT3A removes a “brake” on the activity of stemness genes, which leads to the creation of immature precursor cells that can become AML cells.
Specifically, the DNMT3A mutational hotspot at Arg882 (DNMT3AR882H) cooperates with an NRAS mutation (NRASG12D) to transform HSPCs and induce AML development.
“Due to a large-scale cancer sequencing project, the DNMT3A gene is now appreciated to be one of the top 3 most frequently mutated genes in human acute myeloid leukemia, and yet the role of its mutation in the disease has remained far from clear,” said G. Greg Wang, PhD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“Our findings not only provide a deeper understanding of how this prevalent mutation contributes to the development of AML, but it also offers useful information on how to develop new strategies to treat AML patients.”
In an attempt to understand how the DNMT3A mutation helps drive AML, Dr Wang and his colleagues created one of the first laboratory AML models for studying somatic mutations in DNMT3A.
The DNMT3A gene codes for a protein that binds to specific sections of DNA with a chemical tag that can influence the activity and expression of the underlying genes in cells.
The researchers found that DNMT3AR882H caused AML cells to have a different pattern of chemical tags that affect how the genetic code is interpreted and how the cell develops.
In cancerous cells with DNMT3AR882H, a set of gene enhancers for several stemness genes—including Meis1, Mn1, and the Hoxa gene cluster—were left unchecked. Therefore, HSPCs were left with a constant “on” switch, allowing the cells to “forget” to mature.
“In acute myeloid leukemia, the expression of these stemness genes are aberrantly maintained at a higher level,” Dr Wang said. “As a result, cells ‘forget’ to proceed to normal differentiation and maturation, generating immature precursor blood cells and a prelude to full-blown cancer.”
The researchers also found that, while the DNMT3A mutation is required for AML development, the mutation itself is not sufficient to cause cancer alone. DNMT3AR882H cooperates with another mutation, NRASG12D.
“We found the RAS mutation stimulates these immature blood cells to be hyper-proliferate,” said study author Rui Lu, PhD, of the University of North Carolina Lineberger Comprehensive Cancer Center.
“However, these cells cannot maintain their stem cell properties. While the DNMT3A mutation itself does not have hyper-proliferative effects, [it] does promote stemness properties and generates leukemia stem/initiating cells together with the RAS mutation.”
The researchers also reported testing a potential treatment in cells with the DNMT3A mutation. They found that AML cells with DNMT3AR882H were sensitive to inhibitors of DOT1L, a cellular enzyme involved in modulation of gene expression activities.
As DOT1L inhibitors are currently under clinical investigation, this finding suggests a potential strategy for treating DNMT3A-mutated AML. ![]()

A set of faulty genetic instructions keeps hematopoietic stem/progenitor cells (HSPCs) from maturing and contributes to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Researchers found that a mutation in the gene DNMT3A removes a “brake” on the activity of stemness genes, which leads to the creation of immature precursor cells that can become AML cells.
Specifically, the DNMT3A mutational hotspot at Arg882 (DNMT3AR882H) cooperates with an NRAS mutation (NRASG12D) to transform HSPCs and induce AML development.
“Due to a large-scale cancer sequencing project, the DNMT3A gene is now appreciated to be one of the top 3 most frequently mutated genes in human acute myeloid leukemia, and yet the role of its mutation in the disease has remained far from clear,” said G. Greg Wang, PhD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“Our findings not only provide a deeper understanding of how this prevalent mutation contributes to the development of AML, but it also offers useful information on how to develop new strategies to treat AML patients.”
In an attempt to understand how the DNMT3A mutation helps drive AML, Dr Wang and his colleagues created one of the first laboratory AML models for studying somatic mutations in DNMT3A.
The DNMT3A gene codes for a protein that binds to specific sections of DNA with a chemical tag that can influence the activity and expression of the underlying genes in cells.
The researchers found that DNMT3AR882H caused AML cells to have a different pattern of chemical tags that affect how the genetic code is interpreted and how the cell develops.
In cancerous cells with DNMT3AR882H, a set of gene enhancers for several stemness genes—including Meis1, Mn1, and the Hoxa gene cluster—were left unchecked. Therefore, HSPCs were left with a constant “on” switch, allowing the cells to “forget” to mature.
“In acute myeloid leukemia, the expression of these stemness genes are aberrantly maintained at a higher level,” Dr Wang said. “As a result, cells ‘forget’ to proceed to normal differentiation and maturation, generating immature precursor blood cells and a prelude to full-blown cancer.”
The researchers also found that, while the DNMT3A mutation is required for AML development, the mutation itself is not sufficient to cause cancer alone. DNMT3AR882H cooperates with another mutation, NRASG12D.
“We found the RAS mutation stimulates these immature blood cells to be hyper-proliferate,” said study author Rui Lu, PhD, of the University of North Carolina Lineberger Comprehensive Cancer Center.
“However, these cells cannot maintain their stem cell properties. While the DNMT3A mutation itself does not have hyper-proliferative effects, [it] does promote stemness properties and generates leukemia stem/initiating cells together with the RAS mutation.”
The researchers also reported testing a potential treatment in cells with the DNMT3A mutation. They found that AML cells with DNMT3AR882H were sensitive to inhibitors of DOT1L, a cellular enzyme involved in modulation of gene expression activities.
As DOT1L inhibitors are currently under clinical investigation, this finding suggests a potential strategy for treating DNMT3A-mutated AML. ![]()

A set of faulty genetic instructions keeps hematopoietic stem/progenitor cells (HSPCs) from maturing and contributes to the development of acute myeloid leukemia (AML), according to research published in Cancer Cell.
Researchers found that a mutation in the gene DNMT3A removes a “brake” on the activity of stemness genes, which leads to the creation of immature precursor cells that can become AML cells.
Specifically, the DNMT3A mutational hotspot at Arg882 (DNMT3AR882H) cooperates with an NRAS mutation (NRASG12D) to transform HSPCs and induce AML development.
“Due to a large-scale cancer sequencing project, the DNMT3A gene is now appreciated to be one of the top 3 most frequently mutated genes in human acute myeloid leukemia, and yet the role of its mutation in the disease has remained far from clear,” said G. Greg Wang, PhD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“Our findings not only provide a deeper understanding of how this prevalent mutation contributes to the development of AML, but it also offers useful information on how to develop new strategies to treat AML patients.”
In an attempt to understand how the DNMT3A mutation helps drive AML, Dr Wang and his colleagues created one of the first laboratory AML models for studying somatic mutations in DNMT3A.
The DNMT3A gene codes for a protein that binds to specific sections of DNA with a chemical tag that can influence the activity and expression of the underlying genes in cells.
The researchers found that DNMT3AR882H caused AML cells to have a different pattern of chemical tags that affect how the genetic code is interpreted and how the cell develops.
In cancerous cells with DNMT3AR882H, a set of gene enhancers for several stemness genes—including Meis1, Mn1, and the Hoxa gene cluster—were left unchecked. Therefore, HSPCs were left with a constant “on” switch, allowing the cells to “forget” to mature.
“In acute myeloid leukemia, the expression of these stemness genes are aberrantly maintained at a higher level,” Dr Wang said. “As a result, cells ‘forget’ to proceed to normal differentiation and maturation, generating immature precursor blood cells and a prelude to full-blown cancer.”
The researchers also found that, while the DNMT3A mutation is required for AML development, the mutation itself is not sufficient to cause cancer alone. DNMT3AR882H cooperates with another mutation, NRASG12D.
“We found the RAS mutation stimulates these immature blood cells to be hyper-proliferate,” said study author Rui Lu, PhD, of the University of North Carolina Lineberger Comprehensive Cancer Center.
“However, these cells cannot maintain their stem cell properties. While the DNMT3A mutation itself does not have hyper-proliferative effects, [it] does promote stemness properties and generates leukemia stem/initiating cells together with the RAS mutation.”
The researchers also reported testing a potential treatment in cells with the DNMT3A mutation. They found that AML cells with DNMT3AR882H were sensitive to inhibitors of DOT1L, a cellular enzyme involved in modulation of gene expression activities.
As DOT1L inhibitors are currently under clinical investigation, this finding suggests a potential strategy for treating DNMT3A-mutated AML. ![]()
CHMP rejects ofatumumab as maintenance

Photo courtesy of GSK
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended against expanding the approved indication for ofatumumab (Arzerra).
Novartis, which is developing ofatumumab in cooperation with Genmab, had submitted an application requesting that ofatumumab be authorized as maintenance therapy for patients with relapsed chronic lymphocytic leukemia (CLL).
But the CHMP has advised the European Commission (EC) not to grant this authorization.
The CHMP noted that, in the phase 3 PROLONG trial, ofatumumab maintenance improved progression-free survival (PFS) in CLL patients.
However, the committee said the importance of this improvement is not clear because the PFS results were not supported by other measures, such as overall survival or a significant improvement in patients’ quality of life.
The CHMP also said the use of ofatumumab for maintenance treatment should be seen in the context of its side effects. Common side effects of ofatumumab in the PROLONG trial were infusion reactions, neutropenia, and upper respiratory tract infections.
In the end, the CHMP decided that the PROLONG data were not sufficient to conclude that maintenance treatment with ofatumumab is of more benefit than no treatment. So the committee recommended against expanding the drug’s marketing authorization.
This decision does not have any impact on ongoing clinical trials with ofatumumab.
About ofatumumab
Ofatumumab has been authorized for use in the European Union since April 2010.
The EC first granted ofatumumab conditional approval to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Then, in 2014, the EC granted ofatumumab conditional approval for use in combination with chlorambucil or bendamustine in CLL patients who have not received prior therapy and are not eligible for fludarabine-based therapy.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses in the aforementioned indications. Ofatumumab will not receive full approval until the drug’s developers submit results of additional research to the EC.
About the PROLONG trial
The PROLONG trial was designed to compare ofatumumab maintenance to no further treatment in patients with a complete or partial response after second- or third-line treatment for CLL. Interim results of the study were presented at ASH 2014.
These results—in 474 patients—suggested that ofatumumab can significantly improve PFS. The median PFS was about 29 months in patients who received ofatumumab and about 15 months for patients who did not receive maintenance therapy (P<0.0001).
There was no significant difference in the median overall survival, which was not reached in either treatment arm.
The researchers said there were no unexpected safety findings. The most common adverse events (≥10%) were infusion reactions, neutropenia, and upper respiratory tract infection. ![]()

Photo courtesy of GSK
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended against expanding the approved indication for ofatumumab (Arzerra).
Novartis, which is developing ofatumumab in cooperation with Genmab, had submitted an application requesting that ofatumumab be authorized as maintenance therapy for patients with relapsed chronic lymphocytic leukemia (CLL).
But the CHMP has advised the European Commission (EC) not to grant this authorization.
The CHMP noted that, in the phase 3 PROLONG trial, ofatumumab maintenance improved progression-free survival (PFS) in CLL patients.
However, the committee said the importance of this improvement is not clear because the PFS results were not supported by other measures, such as overall survival or a significant improvement in patients’ quality of life.
The CHMP also said the use of ofatumumab for maintenance treatment should be seen in the context of its side effects. Common side effects of ofatumumab in the PROLONG trial were infusion reactions, neutropenia, and upper respiratory tract infections.
In the end, the CHMP decided that the PROLONG data were not sufficient to conclude that maintenance treatment with ofatumumab is of more benefit than no treatment. So the committee recommended against expanding the drug’s marketing authorization.
This decision does not have any impact on ongoing clinical trials with ofatumumab.
About ofatumumab
Ofatumumab has been authorized for use in the European Union since April 2010.
The EC first granted ofatumumab conditional approval to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Then, in 2014, the EC granted ofatumumab conditional approval for use in combination with chlorambucil or bendamustine in CLL patients who have not received prior therapy and are not eligible for fludarabine-based therapy.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses in the aforementioned indications. Ofatumumab will not receive full approval until the drug’s developers submit results of additional research to the EC.
About the PROLONG trial
The PROLONG trial was designed to compare ofatumumab maintenance to no further treatment in patients with a complete or partial response after second- or third-line treatment for CLL. Interim results of the study were presented at ASH 2014.
These results—in 474 patients—suggested that ofatumumab can significantly improve PFS. The median PFS was about 29 months in patients who received ofatumumab and about 15 months for patients who did not receive maintenance therapy (P<0.0001).
There was no significant difference in the median overall survival, which was not reached in either treatment arm.
The researchers said there were no unexpected safety findings. The most common adverse events (≥10%) were infusion reactions, neutropenia, and upper respiratory tract infection. ![]()

Photo courtesy of GSK
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended against expanding the approved indication for ofatumumab (Arzerra).
Novartis, which is developing ofatumumab in cooperation with Genmab, had submitted an application requesting that ofatumumab be authorized as maintenance therapy for patients with relapsed chronic lymphocytic leukemia (CLL).
But the CHMP has advised the European Commission (EC) not to grant this authorization.
The CHMP noted that, in the phase 3 PROLONG trial, ofatumumab maintenance improved progression-free survival (PFS) in CLL patients.
However, the committee said the importance of this improvement is not clear because the PFS results were not supported by other measures, such as overall survival or a significant improvement in patients’ quality of life.
The CHMP also said the use of ofatumumab for maintenance treatment should be seen in the context of its side effects. Common side effects of ofatumumab in the PROLONG trial were infusion reactions, neutropenia, and upper respiratory tract infections.
In the end, the CHMP decided that the PROLONG data were not sufficient to conclude that maintenance treatment with ofatumumab is of more benefit than no treatment. So the committee recommended against expanding the drug’s marketing authorization.
This decision does not have any impact on ongoing clinical trials with ofatumumab.
About ofatumumab
Ofatumumab has been authorized for use in the European Union since April 2010.
The EC first granted ofatumumab conditional approval to treat CLL patients who are refractory to fludarabine and alemtuzumab.
Then, in 2014, the EC granted ofatumumab conditional approval for use in combination with chlorambucil or bendamustine in CLL patients who have not received prior therapy and are not eligible for fludarabine-based therapy.
Ofatumumab received conditional approval because the drug’s benefits appear to outweigh the risks it poses in the aforementioned indications. Ofatumumab will not receive full approval until the drug’s developers submit results of additional research to the EC.
About the PROLONG trial
The PROLONG trial was designed to compare ofatumumab maintenance to no further treatment in patients with a complete or partial response after second- or third-line treatment for CLL. Interim results of the study were presented at ASH 2014.
These results—in 474 patients—suggested that ofatumumab can significantly improve PFS. The median PFS was about 29 months in patients who received ofatumumab and about 15 months for patients who did not receive maintenance therapy (P<0.0001).
There was no significant difference in the median overall survival, which was not reached in either treatment arm.
The researchers said there were no unexpected safety findings. The most common adverse events (≥10%) were infusion reactions, neutropenia, and upper respiratory tract infection. ![]()
Drug enables transfusion independence in lower-risk MDS

COPENHAGEN—Results from a pair of phase 2 trials suggest luspatercept can produce erythroid responses and enable transfusion independence in patients with lower-risk myelodysplastic syndromes (MDS).
In a 3-month base study, 51% of patients treated with luspatercept had an erythroid response, and 35% achieved transfusion independence.
In an ongoing extension study, 81% of luspatercept-treated patients have had an erythroid response, and 50% have achieved transfusion independence.
The majority of adverse events in both trials were grade 1 and 2.
Uwe Platzbecker, MD, of the University Hospital in Dresden, Germany, presented these results at the 21st Congress of the European Hematology Association (abstract S131*). The studies were sponsored by Acceleron Pharma, Inc.
Luspatercept (formerly ACE-536) is a modified activin receptor type IIB fusion protein that increases red blood cell (RBC) levels by targeting molecules in the TGF-β superfamily. Acceleron and Celgene are developing luspatercept to treat anemia in patients with rare blood disorders.
The phase 2 base study was a dose-escalation trial in which MDS patients received luspatercept for 3 months. In the ongoing extension study, patients from the base study are receiving luspatercept for an additional 24 months.
In both studies, patients with high transfusion burden (≥4 RBC units/8 weeks) and those with low transfusion burden (<4 RBC units/8 weeks) received luspatercept once every 3 weeks.
Base study
This study included 58 patients with a median age of 71.5 (range, 27-90). Their median time since diagnosis was 2.4 years (range, 0-14). Seventeen percent of patients had prior lenalidomide treatment, and 66% had previously received erythropoiesis-stimulating agents (ESAs).
In patients with low transfusion burden (n=19), the median hemoglobin at baseline was 8.7 g/dL (range, 6.4-10.1). In patients with high transfusion burden (n=39), the median number of RBC units transfused per 8 weeks was 6 (range, 4-18).
Patients received luspatercept subcutaneously every 3 weeks for up to 5 doses. The study included 7 dose-escalation cohorts (n=27, 0.125 to 1.75 mg/kg) and an expansion cohort (n=31, 1.0 to 1.75 mg/kg).
The primary outcome measure was the proportion of patients who had an erythroid response. In non-transfusion-dependent patients, an erythroid response was defined as a hemoglobin increase of at least 1.5 g/dL from baseline for at least 14 days.
In transfusion-dependent patients, an erythroid response was defined as a reduction of at least 4 RBC units transfused or a reduction of at least 50% of RBC units transfused compared to pretreatment.
Fifty-one percent (25/49) of patients treated at the higher dose levels had an erythroid response. And 35% (14/40) of transfused patients treated at the higher dose levels were transfusion independent for at least 8 weeks.
Extension study
This study includes 32 patients with a median age of 71.5 (range, 29-90). Their median time since diagnosis was 2.9 years (range, 0-14). Nineteen percent of patients had prior lenalidomide treatment, and 59% had previously received ESAs.
In patients with low transfusion burden (n=13), the median hemoglobin at baseline was 8.5 g/dL (range, 6.4-10.1). In patients with high transfusion burden (n=19), the median number of RBC units transfused per 8 weeks was 6 (range, 4-14).
In this ongoing study, patients are receiving luspatercept (1.0 to 1.75 mg/kg) subcutaneously every 3 weeks for an additional 24 months.
At last follow-up (March 4, 2016), 81% (26/32) of patients had an erythroid response. And 50% (11/22) of patients who were transfused prior to study initiation achieved transfusion independence for at least 8 weeks (range, 9-80+ weeks).
Dr Platzbecker noted that, in both studies, erythroid responses were observed whether or not patients previously received ESAs and regardless of patients’ baseline erythropoietin levels.
Safety
There were three grade 3 adverse events that were possibly or probably related to luspatercept—an increase in blast cell count, myalgia, and worsening of general condition.
Adverse events that were possibly or probably related to luspatercept and occurred in at least 2 patients were fatigue (7%, n=4), bone pain (5%, n=3), diarrhea (5%, n=3), myalgia (5%, n=3), headache (3%, n=2), hypertension (3%, n=2), and injection site erythema (3%, n=2).
Dr Platzbecker said luspatercept was generally safe and well-tolerated in these studies. And the results of these trials supported the initiation of a phase 3 study (MEDALIST, NCT02631070) in patients with lower-risk MDS. ![]()
*Data in the abstract differ from data presented at the meeting.

COPENHAGEN—Results from a pair of phase 2 trials suggest luspatercept can produce erythroid responses and enable transfusion independence in patients with lower-risk myelodysplastic syndromes (MDS).
In a 3-month base study, 51% of patients treated with luspatercept had an erythroid response, and 35% achieved transfusion independence.
In an ongoing extension study, 81% of luspatercept-treated patients have had an erythroid response, and 50% have achieved transfusion independence.
The majority of adverse events in both trials were grade 1 and 2.
Uwe Platzbecker, MD, of the University Hospital in Dresden, Germany, presented these results at the 21st Congress of the European Hematology Association (abstract S131*). The studies were sponsored by Acceleron Pharma, Inc.
Luspatercept (formerly ACE-536) is a modified activin receptor type IIB fusion protein that increases red blood cell (RBC) levels by targeting molecules in the TGF-β superfamily. Acceleron and Celgene are developing luspatercept to treat anemia in patients with rare blood disorders.
The phase 2 base study was a dose-escalation trial in which MDS patients received luspatercept for 3 months. In the ongoing extension study, patients from the base study are receiving luspatercept for an additional 24 months.
In both studies, patients with high transfusion burden (≥4 RBC units/8 weeks) and those with low transfusion burden (<4 RBC units/8 weeks) received luspatercept once every 3 weeks.
Base study
This study included 58 patients with a median age of 71.5 (range, 27-90). Their median time since diagnosis was 2.4 years (range, 0-14). Seventeen percent of patients had prior lenalidomide treatment, and 66% had previously received erythropoiesis-stimulating agents (ESAs).
In patients with low transfusion burden (n=19), the median hemoglobin at baseline was 8.7 g/dL (range, 6.4-10.1). In patients with high transfusion burden (n=39), the median number of RBC units transfused per 8 weeks was 6 (range, 4-18).
Patients received luspatercept subcutaneously every 3 weeks for up to 5 doses. The study included 7 dose-escalation cohorts (n=27, 0.125 to 1.75 mg/kg) and an expansion cohort (n=31, 1.0 to 1.75 mg/kg).
The primary outcome measure was the proportion of patients who had an erythroid response. In non-transfusion-dependent patients, an erythroid response was defined as a hemoglobin increase of at least 1.5 g/dL from baseline for at least 14 days.
In transfusion-dependent patients, an erythroid response was defined as a reduction of at least 4 RBC units transfused or a reduction of at least 50% of RBC units transfused compared to pretreatment.
Fifty-one percent (25/49) of patients treated at the higher dose levels had an erythroid response. And 35% (14/40) of transfused patients treated at the higher dose levels were transfusion independent for at least 8 weeks.
Extension study
This study includes 32 patients with a median age of 71.5 (range, 29-90). Their median time since diagnosis was 2.9 years (range, 0-14). Nineteen percent of patients had prior lenalidomide treatment, and 59% had previously received ESAs.
In patients with low transfusion burden (n=13), the median hemoglobin at baseline was 8.5 g/dL (range, 6.4-10.1). In patients with high transfusion burden (n=19), the median number of RBC units transfused per 8 weeks was 6 (range, 4-14).
In this ongoing study, patients are receiving luspatercept (1.0 to 1.75 mg/kg) subcutaneously every 3 weeks for an additional 24 months.
At last follow-up (March 4, 2016), 81% (26/32) of patients had an erythroid response. And 50% (11/22) of patients who were transfused prior to study initiation achieved transfusion independence for at least 8 weeks (range, 9-80+ weeks).
Dr Platzbecker noted that, in both studies, erythroid responses were observed whether or not patients previously received ESAs and regardless of patients’ baseline erythropoietin levels.
Safety
There were three grade 3 adverse events that were possibly or probably related to luspatercept—an increase in blast cell count, myalgia, and worsening of general condition.
Adverse events that were possibly or probably related to luspatercept and occurred in at least 2 patients were fatigue (7%, n=4), bone pain (5%, n=3), diarrhea (5%, n=3), myalgia (5%, n=3), headache (3%, n=2), hypertension (3%, n=2), and injection site erythema (3%, n=2).
Dr Platzbecker said luspatercept was generally safe and well-tolerated in these studies. And the results of these trials supported the initiation of a phase 3 study (MEDALIST, NCT02631070) in patients with lower-risk MDS. ![]()
*Data in the abstract differ from data presented at the meeting.

COPENHAGEN—Results from a pair of phase 2 trials suggest luspatercept can produce erythroid responses and enable transfusion independence in patients with lower-risk myelodysplastic syndromes (MDS).
In a 3-month base study, 51% of patients treated with luspatercept had an erythroid response, and 35% achieved transfusion independence.
In an ongoing extension study, 81% of luspatercept-treated patients have had an erythroid response, and 50% have achieved transfusion independence.
The majority of adverse events in both trials were grade 1 and 2.
Uwe Platzbecker, MD, of the University Hospital in Dresden, Germany, presented these results at the 21st Congress of the European Hematology Association (abstract S131*). The studies were sponsored by Acceleron Pharma, Inc.
Luspatercept (formerly ACE-536) is a modified activin receptor type IIB fusion protein that increases red blood cell (RBC) levels by targeting molecules in the TGF-β superfamily. Acceleron and Celgene are developing luspatercept to treat anemia in patients with rare blood disorders.
The phase 2 base study was a dose-escalation trial in which MDS patients received luspatercept for 3 months. In the ongoing extension study, patients from the base study are receiving luspatercept for an additional 24 months.
In both studies, patients with high transfusion burden (≥4 RBC units/8 weeks) and those with low transfusion burden (<4 RBC units/8 weeks) received luspatercept once every 3 weeks.
Base study
This study included 58 patients with a median age of 71.5 (range, 27-90). Their median time since diagnosis was 2.4 years (range, 0-14). Seventeen percent of patients had prior lenalidomide treatment, and 66% had previously received erythropoiesis-stimulating agents (ESAs).
In patients with low transfusion burden (n=19), the median hemoglobin at baseline was 8.7 g/dL (range, 6.4-10.1). In patients with high transfusion burden (n=39), the median number of RBC units transfused per 8 weeks was 6 (range, 4-18).
Patients received luspatercept subcutaneously every 3 weeks for up to 5 doses. The study included 7 dose-escalation cohorts (n=27, 0.125 to 1.75 mg/kg) and an expansion cohort (n=31, 1.0 to 1.75 mg/kg).
The primary outcome measure was the proportion of patients who had an erythroid response. In non-transfusion-dependent patients, an erythroid response was defined as a hemoglobin increase of at least 1.5 g/dL from baseline for at least 14 days.
In transfusion-dependent patients, an erythroid response was defined as a reduction of at least 4 RBC units transfused or a reduction of at least 50% of RBC units transfused compared to pretreatment.
Fifty-one percent (25/49) of patients treated at the higher dose levels had an erythroid response. And 35% (14/40) of transfused patients treated at the higher dose levels were transfusion independent for at least 8 weeks.
Extension study
This study includes 32 patients with a median age of 71.5 (range, 29-90). Their median time since diagnosis was 2.9 years (range, 0-14). Nineteen percent of patients had prior lenalidomide treatment, and 59% had previously received ESAs.
In patients with low transfusion burden (n=13), the median hemoglobin at baseline was 8.5 g/dL (range, 6.4-10.1). In patients with high transfusion burden (n=19), the median number of RBC units transfused per 8 weeks was 6 (range, 4-14).
In this ongoing study, patients are receiving luspatercept (1.0 to 1.75 mg/kg) subcutaneously every 3 weeks for an additional 24 months.
At last follow-up (March 4, 2016), 81% (26/32) of patients had an erythroid response. And 50% (11/22) of patients who were transfused prior to study initiation achieved transfusion independence for at least 8 weeks (range, 9-80+ weeks).
Dr Platzbecker noted that, in both studies, erythroid responses were observed whether or not patients previously received ESAs and regardless of patients’ baseline erythropoietin levels.
Safety
There were three grade 3 adverse events that were possibly or probably related to luspatercept—an increase in blast cell count, myalgia, and worsening of general condition.
Adverse events that were possibly or probably related to luspatercept and occurred in at least 2 patients were fatigue (7%, n=4), bone pain (5%, n=3), diarrhea (5%, n=3), myalgia (5%, n=3), headache (3%, n=2), hypertension (3%, n=2), and injection site erythema (3%, n=2).
Dr Platzbecker said luspatercept was generally safe and well-tolerated in these studies. And the results of these trials supported the initiation of a phase 3 study (MEDALIST, NCT02631070) in patients with lower-risk MDS.
*Data in the abstract differ from data presented at the meeting.
Team describes method of targeting LSCs in BC-CML

Photo courtesy of
UC San Diego Health
New research has revealed a method of targeting leukemia stem cells (LSCs) in blast crisis chronic myeloid leukemia (BC-CML).
For this study, investigators used human cells and mouse models to define the role of ADAR1, an RNA editing enzyme, in BC-CML.
The team discovered how ADAR1 promotes LSC generation and identified a small molecule that can disrupt this process to fight BC-CML.
Catriona Jamieson, MD, PhD, of the University of California, San Diego, and her colleagues described this work in Cell Stem Cell.
“In this study, we showed that cancer stem cells co-opt an RNA editing system to clone themselves,” Dr Jamieson said. “What’s more, we found a method to dial it down.”
The investigators knew that ADAR1 can edit the sequence of microRNAs. By swapping out just one microRNA building block for another, ADAR1 alters the carefully orchestrated system cells use to control which genes are turned on or off at which times.
ADAR1 is also known to promote cancer progression and resistance to therapy. But Dr Jamieson’s team wanted to determine ADAR1’s role in governing LSCs.
The investigators conducted experiments with human BC-CML cells and mouse models of BC-CML. And they found that increased JAK2 signaling and BCR-ABL1 amplification activate ADAR1 in BC-CML cells. Then, hyper-ADAR1 editing slows down microRNAs known as let-7.
Ultimately, this activity increases cellular regeneration, turning white blood cell precursors into LSCs. And LSCs promote BC-CML.
After learning how the ADAR1 system works, Dr Jamieson and her colleagues looked for a way to stop it.
By inhibiting ADAR1 with a small-molecule compound known as 8-Aza, the investigators were able to counter ADAR1’s effect on LSC self-renewal and restore let-7.
Treatment with 8-Aza reduced self-renewal of BC-CML cells by approximately 40%, when compared to untreated cells.
“Based on this research, we believe that detecting ADAR1 activity will be important for predicting cancer progression,” Dr Jamieson said.
“In addition, inhibiting this enzyme represents a unique therapeutic vulnerability in cancer stem cells with active inflammatory signaling that may respond to pharmacologic inhibitors of inflammation sensitivity or selective ADAR1 inhibitors that are currently being developed.”

Photo courtesy of
UC San Diego Health
New research has revealed a method of targeting leukemia stem cells (LSCs) in blast crisis chronic myeloid leukemia (BC-CML).
For this study, investigators used human cells and mouse models to define the role of ADAR1, an RNA editing enzyme, in BC-CML.
The team discovered how ADAR1 promotes LSC generation and identified a small molecule that can disrupt this process to fight BC-CML.
Catriona Jamieson, MD, PhD, of the University of California, San Diego, and her colleagues described this work in Cell Stem Cell.
“In this study, we showed that cancer stem cells co-opt an RNA editing system to clone themselves,” Dr Jamieson said. “What’s more, we found a method to dial it down.”
The investigators knew that ADAR1 can edit the sequence of microRNAs. By swapping out just one microRNA building block for another, ADAR1 alters the carefully orchestrated system cells use to control which genes are turned on or off at which times.
ADAR1 is also known to promote cancer progression and resistance to therapy. But Dr Jamieson’s team wanted to determine ADAR1’s role in governing LSCs.
The investigators conducted experiments with human BC-CML cells and mouse models of BC-CML. And they found that increased JAK2 signaling and BCR-ABL1 amplification activate ADAR1 in BC-CML cells. Then, hyper-ADAR1 editing slows down microRNAs known as let-7.
Ultimately, this activity increases cellular regeneration, turning white blood cell precursors into LSCs. And LSCs promote BC-CML.
After learning how the ADAR1 system works, Dr Jamieson and her colleagues looked for a way to stop it.
By inhibiting ADAR1 with a small-molecule compound known as 8-Aza, the investigators were able to counter ADAR1’s effect on LSC self-renewal and restore let-7.
Treatment with 8-Aza reduced self-renewal of BC-CML cells by approximately 40%, when compared to untreated cells.
“Based on this research, we believe that detecting ADAR1 activity will be important for predicting cancer progression,” Dr Jamieson said.
“In addition, inhibiting this enzyme represents a unique therapeutic vulnerability in cancer stem cells with active inflammatory signaling that may respond to pharmacologic inhibitors of inflammation sensitivity or selective ADAR1 inhibitors that are currently being developed.”

Photo courtesy of
UC San Diego Health
New research has revealed a method of targeting leukemia stem cells (LSCs) in blast crisis chronic myeloid leukemia (BC-CML).
For this study, investigators used human cells and mouse models to define the role of ADAR1, an RNA editing enzyme, in BC-CML.
The team discovered how ADAR1 promotes LSC generation and identified a small molecule that can disrupt this process to fight BC-CML.
Catriona Jamieson, MD, PhD, of the University of California, San Diego, and her colleagues described this work in Cell Stem Cell.
“In this study, we showed that cancer stem cells co-opt an RNA editing system to clone themselves,” Dr Jamieson said. “What’s more, we found a method to dial it down.”
The investigators knew that ADAR1 can edit the sequence of microRNAs. By swapping out just one microRNA building block for another, ADAR1 alters the carefully orchestrated system cells use to control which genes are turned on or off at which times.
ADAR1 is also known to promote cancer progression and resistance to therapy. But Dr Jamieson’s team wanted to determine ADAR1’s role in governing LSCs.
The investigators conducted experiments with human BC-CML cells and mouse models of BC-CML. And they found that increased JAK2 signaling and BCR-ABL1 amplification activate ADAR1 in BC-CML cells. Then, hyper-ADAR1 editing slows down microRNAs known as let-7.
Ultimately, this activity increases cellular regeneration, turning white blood cell precursors into LSCs. And LSCs promote BC-CML.
After learning how the ADAR1 system works, Dr Jamieson and her colleagues looked for a way to stop it.
By inhibiting ADAR1 with a small-molecule compound known as 8-Aza, the investigators were able to counter ADAR1’s effect on LSC self-renewal and restore let-7.
Treatment with 8-Aza reduced self-renewal of BC-CML cells by approximately 40%, when compared to untreated cells.
“Based on this research, we believe that detecting ADAR1 activity will be important for predicting cancer progression,” Dr Jamieson said.
“In addition, inhibiting this enzyme represents a unique therapeutic vulnerability in cancer stem cells with active inflammatory signaling that may respond to pharmacologic inhibitors of inflammation sensitivity or selective ADAR1 inhibitors that are currently being developed.”
Acalabrutinib shows efficacy as monotherapy in untreated CLL
CHICAGO – Acalabrutinib, which has shown efficacy in relapsed chronic lymphocytic leukemia, has now shown efficacy as a monotherapy for patients with previously untreated CLL, based on results from an ongoing phase I-II study presented as a poster at the annual meeting of the American Society of Clinical Oncology.
In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months. CLL has not progressed in any of the patients, and none have experienced Richter’s transformation, Dr. John C. Byrd, the D. Warren Brown Chair of Leukemia Research at The Ohio State University, Columbus, and his colleagues reported. Based on its favorable safety profile and durable response rates, a phase III trialof acalabrutinib therapy has been initiated (NCT02475681).
Oral acalabrutinib was given at doses of 100 mg twice daily to 37 patients or 200 mg daily to 37 other patients. About half of the patients had bulky lymph nodes of at least 5 cm and 38 of 67 patients had an unmutated IGHV gene. Median time on the study was 11 months.
All patients had rapid declines in lymphadenopathy. Both dose schedules were associated with clinical activity, with Bruton’s tyrosine kinase occupancy highest at 98% with twice-daily dosing and 93% with once-daily dosing. Treatment-related lymphocytosis occurred in 39 of 74 patients and resolved in 38 of the 39. In general, lymphocytosis peaked at a median of 1 week and resolved by a median of 7 weeks.
Acalabrutinib was well tolerated with 72 of 74 patients continuing on the drug. Most adverse events were grade 2 or less, and included headache (42%), diarrhea (35%), arthralgia (22%), contusion (18%), nausea (18%) and increased weight (18%). Grade 3-4 adverse events that occurred in at least two patients included syncope (two patients) and hypertension (two patients). There was one grade 3 upper GI bleed from a gastric ulcer and aspirin use, and one grade 5 case of pneumonia. No atrial fibrillation was reported.
Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
On Twitter @maryjodales
CHICAGO – Acalabrutinib, which has shown efficacy in relapsed chronic lymphocytic leukemia, has now shown efficacy as a monotherapy for patients with previously untreated CLL, based on results from an ongoing phase I-II study presented as a poster at the annual meeting of the American Society of Clinical Oncology.
In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months. CLL has not progressed in any of the patients, and none have experienced Richter’s transformation, Dr. John C. Byrd, the D. Warren Brown Chair of Leukemia Research at The Ohio State University, Columbus, and his colleagues reported. Based on its favorable safety profile and durable response rates, a phase III trialof acalabrutinib therapy has been initiated (NCT02475681).
Oral acalabrutinib was given at doses of 100 mg twice daily to 37 patients or 200 mg daily to 37 other patients. About half of the patients had bulky lymph nodes of at least 5 cm and 38 of 67 patients had an unmutated IGHV gene. Median time on the study was 11 months.
All patients had rapid declines in lymphadenopathy. Both dose schedules were associated with clinical activity, with Bruton’s tyrosine kinase occupancy highest at 98% with twice-daily dosing and 93% with once-daily dosing. Treatment-related lymphocytosis occurred in 39 of 74 patients and resolved in 38 of the 39. In general, lymphocytosis peaked at a median of 1 week and resolved by a median of 7 weeks.
Acalabrutinib was well tolerated with 72 of 74 patients continuing on the drug. Most adverse events were grade 2 or less, and included headache (42%), diarrhea (35%), arthralgia (22%), contusion (18%), nausea (18%) and increased weight (18%). Grade 3-4 adverse events that occurred in at least two patients included syncope (two patients) and hypertension (two patients). There was one grade 3 upper GI bleed from a gastric ulcer and aspirin use, and one grade 5 case of pneumonia. No atrial fibrillation was reported.
Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
On Twitter @maryjodales
CHICAGO – Acalabrutinib, which has shown efficacy in relapsed chronic lymphocytic leukemia, has now shown efficacy as a monotherapy for patients with previously untreated CLL, based on results from an ongoing phase I-II study presented as a poster at the annual meeting of the American Society of Clinical Oncology.
In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months. CLL has not progressed in any of the patients, and none have experienced Richter’s transformation, Dr. John C. Byrd, the D. Warren Brown Chair of Leukemia Research at The Ohio State University, Columbus, and his colleagues reported. Based on its favorable safety profile and durable response rates, a phase III trialof acalabrutinib therapy has been initiated (NCT02475681).
Oral acalabrutinib was given at doses of 100 mg twice daily to 37 patients or 200 mg daily to 37 other patients. About half of the patients had bulky lymph nodes of at least 5 cm and 38 of 67 patients had an unmutated IGHV gene. Median time on the study was 11 months.
All patients had rapid declines in lymphadenopathy. Both dose schedules were associated with clinical activity, with Bruton’s tyrosine kinase occupancy highest at 98% with twice-daily dosing and 93% with once-daily dosing. Treatment-related lymphocytosis occurred in 39 of 74 patients and resolved in 38 of the 39. In general, lymphocytosis peaked at a median of 1 week and resolved by a median of 7 weeks.
Acalabrutinib was well tolerated with 72 of 74 patients continuing on the drug. Most adverse events were grade 2 or less, and included headache (42%), diarrhea (35%), arthralgia (22%), contusion (18%), nausea (18%) and increased weight (18%). Grade 3-4 adverse events that occurred in at least two patients included syncope (two patients) and hypertension (two patients). There was one grade 3 upper GI bleed from a gastric ulcer and aspirin use, and one grade 5 case of pneumonia. No atrial fibrillation was reported.
Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.
On Twitter @maryjodales
FROM 2016 ASCO ANNUAL MEETING
Key clinical point: Acalabrutinib, which has shown efficacy in relapsed CLL, has now shown efficacy as a monotherapy for patients with previously untreated CLL.
Major finding: In a 74-patient study, best overall response rate was 96%, and median time to response was 2 months.
Data source: An ongoing phase I-II study.
Disclosures: Dr. Byrd receives research funding from Acerta Pharma, the maker of acalabrutinib, as well as from Genentech and Pharmacyclics.