User login
Study links radon and hematologic cancers in women

New research suggests there is a significant positive association between high levels of residential radon and the risk of hematologic malignancies in women.
The study is the first prospective, population-based study of residential radon exposure and hematologic malignancy risk.
Therefore, the researchers caution that it requires replication to better understand the association and whether it truly differs by sex.
Lauren Teras, PhD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in Environmental Research.
Radon is a naturally occurring byproduct of the decay of radium and is a known human lung carcinogen. It is the second-leading cause of lung cancer in the US.
Modeling studies have shown that radon delivers a non-negligible dose of alpha radiation to the bone marrow and therefore could increase the risk of hematologic malignancies. However, studies investigating the link between radon and hematologic malignancies have produced inconsistent results.
For the current study, Dr Teras and her colleagues used data from the American Cancer Society Cancer Prevention Study-II Nutrition Cohort to examine the association between county-level residential radon exposure and the risk of hematologic cancer.
The analysis included 140,652 participants, including 3019 who had hematologic malignancies during 19 years of follow-up (1992 to 2011).
The researchers found that women living in counties with the highest mean radon concentration (> 148 Bq/m3) had a significantly higher risk of developing a hematologic malignancy than women living in counties with the lowest radon levels (< 74 Bq/m3).
The adjusted hazard ratio (adjusted for age, race, family history of hematologic malignancy, etc.) was 1.63 (P=0.0010).
The researchers also found evidence of a dose-response relationship, with an adjusted hazard ratio of 1.38 (P=0.001).
The team said there was evidence of a positive exposure-response relationship between radon concentration and the risk of all lymphoid malignancy subtypes in women. But the highest risk was observed for follicular lymphoma, with an adjusted hazard ratio of 2.74 (P=0.02).
On the other hand, there was a non-significant inverse association between radon and myeloid leukemias in women.
There was no association between hematologic malignancy and radon exposure among the men.
The researchers said a possible explanation for this finding is that men may have a higher baseline risk of hematologic malignancy, possibly because of more exposure to occupational or other risk factors, which would reduce the impact of any additional risk from residential radon.
In women, who have a smaller baseline risk, residential radon exposure might be a larger contributor to overall risk.
Another reason for the sex difference observed in this study may be that the women of this generation spent more time in their homes, so they had more residential exposure than men.
“The overall lifetime risk of hematological cancers in the United States is about 2%, so even a 60% relative increase would still mean a relatively small absolute risk,” Dr Teras noted.
“Nonetheless, radon is already associated with lung cancer, and if other studies confirm the link to blood cancers, we think it would warrant strengthened public health efforts to mitigate residential radon risks.” ![]()

New research suggests there is a significant positive association between high levels of residential radon and the risk of hematologic malignancies in women.
The study is the first prospective, population-based study of residential radon exposure and hematologic malignancy risk.
Therefore, the researchers caution that it requires replication to better understand the association and whether it truly differs by sex.
Lauren Teras, PhD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in Environmental Research.
Radon is a naturally occurring byproduct of the decay of radium and is a known human lung carcinogen. It is the second-leading cause of lung cancer in the US.
Modeling studies have shown that radon delivers a non-negligible dose of alpha radiation to the bone marrow and therefore could increase the risk of hematologic malignancies. However, studies investigating the link between radon and hematologic malignancies have produced inconsistent results.
For the current study, Dr Teras and her colleagues used data from the American Cancer Society Cancer Prevention Study-II Nutrition Cohort to examine the association between county-level residential radon exposure and the risk of hematologic cancer.
The analysis included 140,652 participants, including 3019 who had hematologic malignancies during 19 years of follow-up (1992 to 2011).
The researchers found that women living in counties with the highest mean radon concentration (> 148 Bq/m3) had a significantly higher risk of developing a hematologic malignancy than women living in counties with the lowest radon levels (< 74 Bq/m3).
The adjusted hazard ratio (adjusted for age, race, family history of hematologic malignancy, etc.) was 1.63 (P=0.0010).
The researchers also found evidence of a dose-response relationship, with an adjusted hazard ratio of 1.38 (P=0.001).
The team said there was evidence of a positive exposure-response relationship between radon concentration and the risk of all lymphoid malignancy subtypes in women. But the highest risk was observed for follicular lymphoma, with an adjusted hazard ratio of 2.74 (P=0.02).
On the other hand, there was a non-significant inverse association between radon and myeloid leukemias in women.
There was no association between hematologic malignancy and radon exposure among the men.
The researchers said a possible explanation for this finding is that men may have a higher baseline risk of hematologic malignancy, possibly because of more exposure to occupational or other risk factors, which would reduce the impact of any additional risk from residential radon.
In women, who have a smaller baseline risk, residential radon exposure might be a larger contributor to overall risk.
Another reason for the sex difference observed in this study may be that the women of this generation spent more time in their homes, so they had more residential exposure than men.
“The overall lifetime risk of hematological cancers in the United States is about 2%, so even a 60% relative increase would still mean a relatively small absolute risk,” Dr Teras noted.
“Nonetheless, radon is already associated with lung cancer, and if other studies confirm the link to blood cancers, we think it would warrant strengthened public health efforts to mitigate residential radon risks.” ![]()

New research suggests there is a significant positive association between high levels of residential radon and the risk of hematologic malignancies in women.
The study is the first prospective, population-based study of residential radon exposure and hematologic malignancy risk.
Therefore, the researchers caution that it requires replication to better understand the association and whether it truly differs by sex.
Lauren Teras, PhD, of the American Cancer Society in Atlanta, Georgia, and her colleagues conducted this study and reported the results in Environmental Research.
Radon is a naturally occurring byproduct of the decay of radium and is a known human lung carcinogen. It is the second-leading cause of lung cancer in the US.
Modeling studies have shown that radon delivers a non-negligible dose of alpha radiation to the bone marrow and therefore could increase the risk of hematologic malignancies. However, studies investigating the link between radon and hematologic malignancies have produced inconsistent results.
For the current study, Dr Teras and her colleagues used data from the American Cancer Society Cancer Prevention Study-II Nutrition Cohort to examine the association between county-level residential radon exposure and the risk of hematologic cancer.
The analysis included 140,652 participants, including 3019 who had hematologic malignancies during 19 years of follow-up (1992 to 2011).
The researchers found that women living in counties with the highest mean radon concentration (> 148 Bq/m3) had a significantly higher risk of developing a hematologic malignancy than women living in counties with the lowest radon levels (< 74 Bq/m3).
The adjusted hazard ratio (adjusted for age, race, family history of hematologic malignancy, etc.) was 1.63 (P=0.0010).
The researchers also found evidence of a dose-response relationship, with an adjusted hazard ratio of 1.38 (P=0.001).
The team said there was evidence of a positive exposure-response relationship between radon concentration and the risk of all lymphoid malignancy subtypes in women. But the highest risk was observed for follicular lymphoma, with an adjusted hazard ratio of 2.74 (P=0.02).
On the other hand, there was a non-significant inverse association between radon and myeloid leukemias in women.
There was no association between hematologic malignancy and radon exposure among the men.
The researchers said a possible explanation for this finding is that men may have a higher baseline risk of hematologic malignancy, possibly because of more exposure to occupational or other risk factors, which would reduce the impact of any additional risk from residential radon.
In women, who have a smaller baseline risk, residential radon exposure might be a larger contributor to overall risk.
Another reason for the sex difference observed in this study may be that the women of this generation spent more time in their homes, so they had more residential exposure than men.
“The overall lifetime risk of hematological cancers in the United States is about 2%, so even a 60% relative increase would still mean a relatively small absolute risk,” Dr Teras noted.
“Nonetheless, radon is already associated with lung cancer, and if other studies confirm the link to blood cancers, we think it would warrant strengthened public health efforts to mitigate residential radon risks.” ![]()
CHMP recommends approving drug to treat FL

The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that obinutuzumab (Gazyvaro), an anti-CD20 monoclonal antibody, be approved for use in patients with follicular lymphoma (FL).
The recommended indication is for obinutuzumab to be given first in combination with bendamustine and then as maintenance therapy in FL patients who did not respond to, progressed during, or progressed up to 6 months after treatment with rituximab or a rituximab-containing regimen.
Based on the CHMP’s recommendation, a final decision regarding the approval of obinutuzumab in FL is expected from the European Commission in the coming months.
Obinutuzumab is already approved in the European Union for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia and comorbidities that make them unsuitable for full-dose fludarabine-based therapy.
Obinutuzumab is being developed by Roche.
GADOLIN trial
The CHMP’s recommendation to approve obinutuzumab in FL is based on results from the phase 3 GADOLIN trial. The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()

The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that obinutuzumab (Gazyvaro), an anti-CD20 monoclonal antibody, be approved for use in patients with follicular lymphoma (FL).
The recommended indication is for obinutuzumab to be given first in combination with bendamustine and then as maintenance therapy in FL patients who did not respond to, progressed during, or progressed up to 6 months after treatment with rituximab or a rituximab-containing regimen.
Based on the CHMP’s recommendation, a final decision regarding the approval of obinutuzumab in FL is expected from the European Commission in the coming months.
Obinutuzumab is already approved in the European Union for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia and comorbidities that make them unsuitable for full-dose fludarabine-based therapy.
Obinutuzumab is being developed by Roche.
GADOLIN trial
The CHMP’s recommendation to approve obinutuzumab in FL is based on results from the phase 3 GADOLIN trial. The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()

The European Medicine Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended that obinutuzumab (Gazyvaro), an anti-CD20 monoclonal antibody, be approved for use in patients with follicular lymphoma (FL).
The recommended indication is for obinutuzumab to be given first in combination with bendamustine and then as maintenance therapy in FL patients who did not respond to, progressed during, or progressed up to 6 months after treatment with rituximab or a rituximab-containing regimen.
Based on the CHMP’s recommendation, a final decision regarding the approval of obinutuzumab in FL is expected from the European Commission in the coming months.
Obinutuzumab is already approved in the European Union for use in combination with chlorambucil to treat patients with previously untreated chronic lymphocytic leukemia and comorbidities that make them unsuitable for full-dose fludarabine-based therapy.
Obinutuzumab is being developed by Roche.
GADOLIN trial
The CHMP’s recommendation to approve obinutuzumab in FL is based on results from the phase 3 GADOLIN trial. The study included 413 patients with rituximab-refractory non-Hodgkin lymphoma, including 321 patients with FL, 46 with marginal zone lymphoma, and 28 with small lymphocytic lymphoma.
The patients were randomized to receive bendamustine alone (control arm) or a combination of bendamustine and obinutuzumab followed by obinutuzumab maintenance (every 2 months for 2 years or until progression).
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC). The secondary endpoints were PFS assessed by investigator review, best overall response, complete response (CR), partial response (PR), duration of response, overall survival, and safety profile.
Among patients with FL, the obinutuzumab regimen improved PFS compared to bendamustine alone, as assessed by IRC (hazard ratio [HR]=0.48, P<0.0001). The median PFS was not reached in patients receiving the obinutuzumab regimen but was 13.8 months in those receiving bendamustine alone.
Investigator-assessed PFS was consistent with IRC-assessed PFS. Investigators said the median PFS with the obinutuzumab regimen was more than double that with bendamustine alone—29.2 months vs 13.7 months (HR=0.48, P<0.0001).
The best overall response for patients receiving the obinutuzumab regimen was 78.7% (15.5% CR, 63.2% PR), compared to 74.7% for those receiving bendamustine alone (18.7% CR, 56% PR), as assessed by the IRC.
The median duration of response was not reached for patients receiving the obinutuzumab regimen and was 11.6 months for those receiving bendamustine alone.
The median overall survival has not yet been reached in either study arm.
The most common grade 3/4 adverse events observed in patients receiving the obinutuzumab regimen were neutropenia (33%), infusion reactions (11%), and thrombocytopenia (10%).
The most common adverse events of any grade were infusion reactions (69%), neutropenia (35%), nausea (54%), fatigue (39%), cough (26%), diarrhea (27%), constipation (19%), fever (18%), thrombocytopenia (15%), vomiting (22%), upper respiratory tract infection (13%), decreased appetite (18%), joint or muscle pain (12%), sinusitis (12%), anemia (12%), general weakness (11%), and urinary tract infection (10%). ![]()
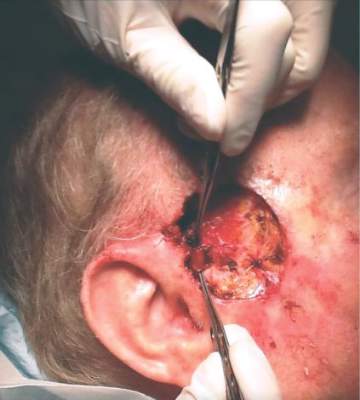
Search is on for cases of aggressive, ruxolitinib-associated skin cancers
ORLANDO – The hematologic cancer drug ruxolitinib seems to be associated with cases of aggressive nonmelanoma skin cancer.
After treating a very aggressive squamous cell carcinoma in a 55-year-old man treated with ruxolitinib for polycythemia vera, and hearing firsthand of three other similar cases, Dr. Fiona Zwald is collecting additional data on the association. She intends to publish these cases in a monograph as a warning to dermatologists, hematologists, oncologists, and other physicians who manage patients with hematologic malignancies, she said at the annual meeting of the American College of Mohs Surgery.
The prescribing information for ruxolitinib (Jakafi, Incyte Pharmaceuticals; Jakavi, Novartis) was updated in 2014 to warn that patients taking the drug face an increased risk of nonmelanoma skin cancers. The label also recommends that physicians inspect the skin regularly and urge patients to be alert for and report any new or changing lesions.
Despite the warnings and recommendations, cases are occurring – and some are quite serious, said Dr. Zwald, a Mohs surgeon in Atlanta.
“People should know this is actually happening. If you have experience with this medication, please let us know so we can compile this report. We are trying to assess the number of skin cancers before and after initiating this medication,” she said.
Ruxolitinib is an inhibitor of Janus kinase with a special affinity for the JAK1 and JAK2 subtypes. Like other cytokine-signaling molecules, their function depends on cell context; it may inhibit cell growth in one setting, and, in another, stimulate it. Ruxolitinib was initially approved in 2011 for the treatment of intermediate- and high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis.
In 2014, indications for ruxolitinib were expanded to include treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea.
Dr. Zwald’s patient had a 10-year history of polycythemia vera. He was initially well controlled on the standard hydroxyurea treatment. In the meantime, he began working as a caddy at a major U.S. golf club. He developed many facial squamous cell carcinomas that were treated with excision and radiation. A year before he presented to Dr. Zwald, he stopped responding to hydroxyurea and was placed on ruxolitinib.
The patient presented with a 4-cm ulcerated lesion over part of his right temple and to the right helical crus; the lesion had developed over 3 months. Dr. Zwald consulted with the patient’s medical oncologist; treatment with ruxolitinib continued, albeit at a reduced dosage in light of recent events.
She performed Mohs surgery on the patient. It was a challenging case, she said, not the least because adequate anesthesia could not be achieved with local anesthetic. Preoperative staging showed no nodal spread.
“He did, unfortunately demonstrate a large, indurated mass located over one branch of the superficial temporal artery. At the helical crus there was an area of bound-down, fixed tumor. Knowing that I would not be able to fully resect this, I passed him on to the operating room,” Dr. Zwald said. “This tumor was found to extend down to the parotid capsule, but margins were clear.” The surgical defect was successfully repaired with a split-thickness skin graft.
The tumor recurred about 3 months later, and the patient underwent another surgery.
“This time we could not get clear surgical margins, and the tumor was approaching the external auditory meatus. Surgery was abandoned due to fears of complications to that area,” she said.
She presented the case at tumor board, during which she and her colleagues discussed adjuvant radiation. They initially abandoned this idea because he had already had so much radiation to his face. After the second surgery, they decide to proceed with radiation. “The next conversation we have will be whether to add another adjuvant therapy to treatment.”
She sent out the case and requests for feedback to the International Transplant Skin Cancer Collaborative, an 800-member consortium of dermatologists and Mohs surgeons who take care of transplant patients. She received information on three additional cases of aggressive squamous cell carcinoma (SCC) associated with ruxolitinib treatment:
• A patient with myelodysplastic syndrome with aggressive scalp SCC with cutaneous metastases.
• A patient with undifferentiated pleomorphic sarcoma of the scalp, several cutaneous SCCs.
• A patient with a myelodysplastic syndrome with in-transit metastases and explosive cutaneous SCCs. The patient has had the ruxolitinib dose reduced and may be switched to capecitabine.
Dr. Zwald noted that her patient was at risk for aggressive skin cancers for reasons in addition to ruxolitinib treatment.
“He was already immunosuppressed from his malignancy. He was on hydroxyurea, a drug that’s a cumulative phototoxin, and he’s out in the sun playing golf every day, and then was put on ruxolitinib. But the question we face now is how to try and stop this medication so we can get better treatment for him which will, of course, be very difficult.”
To contribute to Dr. Zwald’s case series, please email her at Fiona.Zwald@gmail.com.
She had no relevant financial disclosures.
On Twitter @Alz_Gal
ORLANDO – The hematologic cancer drug ruxolitinib seems to be associated with cases of aggressive nonmelanoma skin cancer.
After treating a very aggressive squamous cell carcinoma in a 55-year-old man treated with ruxolitinib for polycythemia vera, and hearing firsthand of three other similar cases, Dr. Fiona Zwald is collecting additional data on the association. She intends to publish these cases in a monograph as a warning to dermatologists, hematologists, oncologists, and other physicians who manage patients with hematologic malignancies, she said at the annual meeting of the American College of Mohs Surgery.
The prescribing information for ruxolitinib (Jakafi, Incyte Pharmaceuticals; Jakavi, Novartis) was updated in 2014 to warn that patients taking the drug face an increased risk of nonmelanoma skin cancers. The label also recommends that physicians inspect the skin regularly and urge patients to be alert for and report any new or changing lesions.
Despite the warnings and recommendations, cases are occurring – and some are quite serious, said Dr. Zwald, a Mohs surgeon in Atlanta.
“People should know this is actually happening. If you have experience with this medication, please let us know so we can compile this report. We are trying to assess the number of skin cancers before and after initiating this medication,” she said.
Ruxolitinib is an inhibitor of Janus kinase with a special affinity for the JAK1 and JAK2 subtypes. Like other cytokine-signaling molecules, their function depends on cell context; it may inhibit cell growth in one setting, and, in another, stimulate it. Ruxolitinib was initially approved in 2011 for the treatment of intermediate- and high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis.
In 2014, indications for ruxolitinib were expanded to include treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea.
Dr. Zwald’s patient had a 10-year history of polycythemia vera. He was initially well controlled on the standard hydroxyurea treatment. In the meantime, he began working as a caddy at a major U.S. golf club. He developed many facial squamous cell carcinomas that were treated with excision and radiation. A year before he presented to Dr. Zwald, he stopped responding to hydroxyurea and was placed on ruxolitinib.
The patient presented with a 4-cm ulcerated lesion over part of his right temple and to the right helical crus; the lesion had developed over 3 months. Dr. Zwald consulted with the patient’s medical oncologist; treatment with ruxolitinib continued, albeit at a reduced dosage in light of recent events.
She performed Mohs surgery on the patient. It was a challenging case, she said, not the least because adequate anesthesia could not be achieved with local anesthetic. Preoperative staging showed no nodal spread.
“He did, unfortunately demonstrate a large, indurated mass located over one branch of the superficial temporal artery. At the helical crus there was an area of bound-down, fixed tumor. Knowing that I would not be able to fully resect this, I passed him on to the operating room,” Dr. Zwald said. “This tumor was found to extend down to the parotid capsule, but margins were clear.” The surgical defect was successfully repaired with a split-thickness skin graft.
The tumor recurred about 3 months later, and the patient underwent another surgery.
“This time we could not get clear surgical margins, and the tumor was approaching the external auditory meatus. Surgery was abandoned due to fears of complications to that area,” she said.
She presented the case at tumor board, during which she and her colleagues discussed adjuvant radiation. They initially abandoned this idea because he had already had so much radiation to his face. After the second surgery, they decide to proceed with radiation. “The next conversation we have will be whether to add another adjuvant therapy to treatment.”
She sent out the case and requests for feedback to the International Transplant Skin Cancer Collaborative, an 800-member consortium of dermatologists and Mohs surgeons who take care of transplant patients. She received information on three additional cases of aggressive squamous cell carcinoma (SCC) associated with ruxolitinib treatment:
• A patient with myelodysplastic syndrome with aggressive scalp SCC with cutaneous metastases.
• A patient with undifferentiated pleomorphic sarcoma of the scalp, several cutaneous SCCs.
• A patient with a myelodysplastic syndrome with in-transit metastases and explosive cutaneous SCCs. The patient has had the ruxolitinib dose reduced and may be switched to capecitabine.
Dr. Zwald noted that her patient was at risk for aggressive skin cancers for reasons in addition to ruxolitinib treatment.
“He was already immunosuppressed from his malignancy. He was on hydroxyurea, a drug that’s a cumulative phototoxin, and he’s out in the sun playing golf every day, and then was put on ruxolitinib. But the question we face now is how to try and stop this medication so we can get better treatment for him which will, of course, be very difficult.”
To contribute to Dr. Zwald’s case series, please email her at Fiona.Zwald@gmail.com.
She had no relevant financial disclosures.
On Twitter @Alz_Gal
ORLANDO – The hematologic cancer drug ruxolitinib seems to be associated with cases of aggressive nonmelanoma skin cancer.
After treating a very aggressive squamous cell carcinoma in a 55-year-old man treated with ruxolitinib for polycythemia vera, and hearing firsthand of three other similar cases, Dr. Fiona Zwald is collecting additional data on the association. She intends to publish these cases in a monograph as a warning to dermatologists, hematologists, oncologists, and other physicians who manage patients with hematologic malignancies, she said at the annual meeting of the American College of Mohs Surgery.
The prescribing information for ruxolitinib (Jakafi, Incyte Pharmaceuticals; Jakavi, Novartis) was updated in 2014 to warn that patients taking the drug face an increased risk of nonmelanoma skin cancers. The label also recommends that physicians inspect the skin regularly and urge patients to be alert for and report any new or changing lesions.
Despite the warnings and recommendations, cases are occurring – and some are quite serious, said Dr. Zwald, a Mohs surgeon in Atlanta.
“People should know this is actually happening. If you have experience with this medication, please let us know so we can compile this report. We are trying to assess the number of skin cancers before and after initiating this medication,” she said.
Ruxolitinib is an inhibitor of Janus kinase with a special affinity for the JAK1 and JAK2 subtypes. Like other cytokine-signaling molecules, their function depends on cell context; it may inhibit cell growth in one setting, and, in another, stimulate it. Ruxolitinib was initially approved in 2011 for the treatment of intermediate- and high-risk myelofibrosis, including primary myelofibrosis, post–polycythemia vera myelofibrosis, and post–essential thrombocythemia myelofibrosis.
In 2014, indications for ruxolitinib were expanded to include treatment of patients with polycythemia vera who have had an inadequate response to or are intolerant of hydroxyurea.
Dr. Zwald’s patient had a 10-year history of polycythemia vera. He was initially well controlled on the standard hydroxyurea treatment. In the meantime, he began working as a caddy at a major U.S. golf club. He developed many facial squamous cell carcinomas that were treated with excision and radiation. A year before he presented to Dr. Zwald, he stopped responding to hydroxyurea and was placed on ruxolitinib.
The patient presented with a 4-cm ulcerated lesion over part of his right temple and to the right helical crus; the lesion had developed over 3 months. Dr. Zwald consulted with the patient’s medical oncologist; treatment with ruxolitinib continued, albeit at a reduced dosage in light of recent events.
She performed Mohs surgery on the patient. It was a challenging case, she said, not the least because adequate anesthesia could not be achieved with local anesthetic. Preoperative staging showed no nodal spread.
“He did, unfortunately demonstrate a large, indurated mass located over one branch of the superficial temporal artery. At the helical crus there was an area of bound-down, fixed tumor. Knowing that I would not be able to fully resect this, I passed him on to the operating room,” Dr. Zwald said. “This tumor was found to extend down to the parotid capsule, but margins were clear.” The surgical defect was successfully repaired with a split-thickness skin graft.
The tumor recurred about 3 months later, and the patient underwent another surgery.
“This time we could not get clear surgical margins, and the tumor was approaching the external auditory meatus. Surgery was abandoned due to fears of complications to that area,” she said.
She presented the case at tumor board, during which she and her colleagues discussed adjuvant radiation. They initially abandoned this idea because he had already had so much radiation to his face. After the second surgery, they decide to proceed with radiation. “The next conversation we have will be whether to add another adjuvant therapy to treatment.”
She sent out the case and requests for feedback to the International Transplant Skin Cancer Collaborative, an 800-member consortium of dermatologists and Mohs surgeons who take care of transplant patients. She received information on three additional cases of aggressive squamous cell carcinoma (SCC) associated with ruxolitinib treatment:
• A patient with myelodysplastic syndrome with aggressive scalp SCC with cutaneous metastases.
• A patient with undifferentiated pleomorphic sarcoma of the scalp, several cutaneous SCCs.
• A patient with a myelodysplastic syndrome with in-transit metastases and explosive cutaneous SCCs. The patient has had the ruxolitinib dose reduced and may be switched to capecitabine.
Dr. Zwald noted that her patient was at risk for aggressive skin cancers for reasons in addition to ruxolitinib treatment.
“He was already immunosuppressed from his malignancy. He was on hydroxyurea, a drug that’s a cumulative phototoxin, and he’s out in the sun playing golf every day, and then was put on ruxolitinib. But the question we face now is how to try and stop this medication so we can get better treatment for him which will, of course, be very difficult.”
To contribute to Dr. Zwald’s case series, please email her at Fiona.Zwald@gmail.com.
She had no relevant financial disclosures.
On Twitter @Alz_Gal
AT THE ACMS ANNUAL MEETING
Cancer diagnosis linked to mental health disorders

A recent cancer diagnosis is associated with an increased risk for mental health disorders and increased use of psychiatric medications, according to a large, nationwide study conducted in Sweden.
Overall, there was an increased risk of mental health disorders from 10 months before a cancer diagnosis that peaked during the first week after diagnosis and decreased after that, although the risk remained elevated at 10 years after diagnosis.
In addition, there was an increased use of psychiatric medications from 1 month before cancer diagnosis that peaked at about 3 months after diagnosis and remained elevated 2 years after diagnosis.
Donghao Lu, MD, of the Karolinska Institutet in Stockholm, Sweden and colleagues conducted this study and reported the results in JAMA Oncology.
The study included 304,118 patients with cancer and 3,041,174 cancer-free individuals randomly selected from the Swedish population for comparison.
The researchers investigated changes in risk for several common and potentially stress-related mental disorders—including depression, anxiety, substance abuse, somatoform/conversion disorder, and stress reaction/adjustment disorder—from the cancer diagnostic workup through to post-diagnosis.
They found the relative rate for all of the mental disorders studied started to increase from 10 months before cancer diagnosis, with a hazard ratio [HR] of 1.1 (95%CI, 1.1-1.2).
The rate peaked during the first week after diagnosis, with an HR of 6.7 (95%CI, 6.1-7.4). It decreased rapidly thereafter but was still elevated 10 years after diagnosis, with an HR of 1.1 (95%CI, 1.1-1.2).
The rate elevation was clear for all of the main cancers, including hematologic malignancies, except for nonmelanoma skin cancer.
Among the cancer patients, the mental disorder with the highest cumulative incidence was depression. This was followed by anxiety and stress reaction/adjustment disorder.
When compared to controls, the cancer patients had a higher cumulative incidence of most of the mental disorders. The exception was somatoform/conversion disorder.
The researchers also examined the use of psychiatric medications for patients with cancer to assess milder mental health conditions and symptoms.
The team found an increased use of psychiatric medications in cancer patients compared to controls, from 1 month before diagnosis—12.2% vs 11.7% (P=0.04)—that peaked at about 3 months after diagnosis—18.1% vs 11.9% (P<0.001)—and was still elevated 2 years after diagnosis—15.4% vs 12.7% (P<0.001).
The researchers said the results of this study support the existing guidelines of integrating psychological management into cancer care and call for extended vigilance for multiple mental disorders starting from the time of the cancer diagnostic workup. ![]()

A recent cancer diagnosis is associated with an increased risk for mental health disorders and increased use of psychiatric medications, according to a large, nationwide study conducted in Sweden.
Overall, there was an increased risk of mental health disorders from 10 months before a cancer diagnosis that peaked during the first week after diagnosis and decreased after that, although the risk remained elevated at 10 years after diagnosis.
In addition, there was an increased use of psychiatric medications from 1 month before cancer diagnosis that peaked at about 3 months after diagnosis and remained elevated 2 years after diagnosis.
Donghao Lu, MD, of the Karolinska Institutet in Stockholm, Sweden and colleagues conducted this study and reported the results in JAMA Oncology.
The study included 304,118 patients with cancer and 3,041,174 cancer-free individuals randomly selected from the Swedish population for comparison.
The researchers investigated changes in risk for several common and potentially stress-related mental disorders—including depression, anxiety, substance abuse, somatoform/conversion disorder, and stress reaction/adjustment disorder—from the cancer diagnostic workup through to post-diagnosis.
They found the relative rate for all of the mental disorders studied started to increase from 10 months before cancer diagnosis, with a hazard ratio [HR] of 1.1 (95%CI, 1.1-1.2).
The rate peaked during the first week after diagnosis, with an HR of 6.7 (95%CI, 6.1-7.4). It decreased rapidly thereafter but was still elevated 10 years after diagnosis, with an HR of 1.1 (95%CI, 1.1-1.2).
The rate elevation was clear for all of the main cancers, including hematologic malignancies, except for nonmelanoma skin cancer.
Among the cancer patients, the mental disorder with the highest cumulative incidence was depression. This was followed by anxiety and stress reaction/adjustment disorder.
When compared to controls, the cancer patients had a higher cumulative incidence of most of the mental disorders. The exception was somatoform/conversion disorder.
The researchers also examined the use of psychiatric medications for patients with cancer to assess milder mental health conditions and symptoms.
The team found an increased use of psychiatric medications in cancer patients compared to controls, from 1 month before diagnosis—12.2% vs 11.7% (P=0.04)—that peaked at about 3 months after diagnosis—18.1% vs 11.9% (P<0.001)—and was still elevated 2 years after diagnosis—15.4% vs 12.7% (P<0.001).
The researchers said the results of this study support the existing guidelines of integrating psychological management into cancer care and call for extended vigilance for multiple mental disorders starting from the time of the cancer diagnostic workup. ![]()

A recent cancer diagnosis is associated with an increased risk for mental health disorders and increased use of psychiatric medications, according to a large, nationwide study conducted in Sweden.
Overall, there was an increased risk of mental health disorders from 10 months before a cancer diagnosis that peaked during the first week after diagnosis and decreased after that, although the risk remained elevated at 10 years after diagnosis.
In addition, there was an increased use of psychiatric medications from 1 month before cancer diagnosis that peaked at about 3 months after diagnosis and remained elevated 2 years after diagnosis.
Donghao Lu, MD, of the Karolinska Institutet in Stockholm, Sweden and colleagues conducted this study and reported the results in JAMA Oncology.
The study included 304,118 patients with cancer and 3,041,174 cancer-free individuals randomly selected from the Swedish population for comparison.
The researchers investigated changes in risk for several common and potentially stress-related mental disorders—including depression, anxiety, substance abuse, somatoform/conversion disorder, and stress reaction/adjustment disorder—from the cancer diagnostic workup through to post-diagnosis.
They found the relative rate for all of the mental disorders studied started to increase from 10 months before cancer diagnosis, with a hazard ratio [HR] of 1.1 (95%CI, 1.1-1.2).
The rate peaked during the first week after diagnosis, with an HR of 6.7 (95%CI, 6.1-7.4). It decreased rapidly thereafter but was still elevated 10 years after diagnosis, with an HR of 1.1 (95%CI, 1.1-1.2).
The rate elevation was clear for all of the main cancers, including hematologic malignancies, except for nonmelanoma skin cancer.
Among the cancer patients, the mental disorder with the highest cumulative incidence was depression. This was followed by anxiety and stress reaction/adjustment disorder.
When compared to controls, the cancer patients had a higher cumulative incidence of most of the mental disorders. The exception was somatoform/conversion disorder.
The researchers also examined the use of psychiatric medications for patients with cancer to assess milder mental health conditions and symptoms.
The team found an increased use of psychiatric medications in cancer patients compared to controls, from 1 month before diagnosis—12.2% vs 11.7% (P=0.04)—that peaked at about 3 months after diagnosis—18.1% vs 11.9% (P<0.001)—and was still elevated 2 years after diagnosis—15.4% vs 12.7% (P<0.001).
The researchers said the results of this study support the existing guidelines of integrating psychological management into cancer care and call for extended vigilance for multiple mental disorders starting from the time of the cancer diagnostic workup. ![]()
Costs for orally administered cancer drugs on the rise

Photo courtesy of the CDC
New orally administered cancer drugs are much more expensive in their first year on the market than such drugs launched about 15 years ago, according to a study published in JAMA Oncology.
The research showed that a month of treatment with orally administered cancer drugs introduced in 2014 was, on average, 6 times more expensive at launch than monthly treatment costs for such drugs introduced in 2000, after adjusting for inflation.
In addition, most existing therapies had substantial price increases from the time they were launched to 2014.
“The major trend here is that these products are just getting more expensive over time,” said study author Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
For this study, Dr Dusetzina evaluated what commercial health insurance companies and patients paid for prescription fills—before rebates and discounts—for 32 orally administered cancer drugs from 2000 to 2014. The information came from the TruvenHealth MarketScan Commercial Claims and Encounters database.
The data showed that orally administered drugs approved in 2000 cost an average of $1869 (95% CI, $1648-$2121) per month, compared to $11,325 (95% CI, $10 989-$11 671) for those approved in 2014.
When Dr Dusetzina compared changes in spending by year from a product’s launch to 2014, she observed increases in most of the drugs studied.
The drugs with the largest increases in monthly spending were thalidomide, which increased from $1869 to $7564 ($5695) and imatinib, which increased from $3346 to $8479 ($5133).
However, 2 drugs showed decreases in mean monthly spending between their launch and 2014. Monthly spending for lenalidomide decreased from $10,109 to $9640 ($469), and monthly spending for vorinostat decreased from $9755 to $7592 ($2163).
Dr Dusetzina pointed out that the amount patients pay for these drugs depends on their healthcare benefits. However, the high prices are being passed along to patients more and more, potentially affecting the patients’ access to these drugs.
“Patients are increasingly taking on the burden of paying for these high-cost specialty drugs as plans move toward use of higher deductibles and co-insurance—where a patient will pay a percentage of the drug cost rather than a flat copay,” Dr Dusetzina said.
She noted that while this study did account for payments by commercial health plans, it did not account for spending by Medicaid and Medicare, which may differ. In addition, only the products that were dispensed and reimbursed by commercial health plans were included, which may have excluded rarely used or recently approved products. ![]()

Photo courtesy of the CDC
New orally administered cancer drugs are much more expensive in their first year on the market than such drugs launched about 15 years ago, according to a study published in JAMA Oncology.
The research showed that a month of treatment with orally administered cancer drugs introduced in 2014 was, on average, 6 times more expensive at launch than monthly treatment costs for such drugs introduced in 2000, after adjusting for inflation.
In addition, most existing therapies had substantial price increases from the time they were launched to 2014.
“The major trend here is that these products are just getting more expensive over time,” said study author Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
For this study, Dr Dusetzina evaluated what commercial health insurance companies and patients paid for prescription fills—before rebates and discounts—for 32 orally administered cancer drugs from 2000 to 2014. The information came from the TruvenHealth MarketScan Commercial Claims and Encounters database.
The data showed that orally administered drugs approved in 2000 cost an average of $1869 (95% CI, $1648-$2121) per month, compared to $11,325 (95% CI, $10 989-$11 671) for those approved in 2014.
When Dr Dusetzina compared changes in spending by year from a product’s launch to 2014, she observed increases in most of the drugs studied.
The drugs with the largest increases in monthly spending were thalidomide, which increased from $1869 to $7564 ($5695) and imatinib, which increased from $3346 to $8479 ($5133).
However, 2 drugs showed decreases in mean monthly spending between their launch and 2014. Monthly spending for lenalidomide decreased from $10,109 to $9640 ($469), and monthly spending for vorinostat decreased from $9755 to $7592 ($2163).
Dr Dusetzina pointed out that the amount patients pay for these drugs depends on their healthcare benefits. However, the high prices are being passed along to patients more and more, potentially affecting the patients’ access to these drugs.
“Patients are increasingly taking on the burden of paying for these high-cost specialty drugs as plans move toward use of higher deductibles and co-insurance—where a patient will pay a percentage of the drug cost rather than a flat copay,” Dr Dusetzina said.
She noted that while this study did account for payments by commercial health plans, it did not account for spending by Medicaid and Medicare, which may differ. In addition, only the products that were dispensed and reimbursed by commercial health plans were included, which may have excluded rarely used or recently approved products. ![]()

Photo courtesy of the CDC
New orally administered cancer drugs are much more expensive in their first year on the market than such drugs launched about 15 years ago, according to a study published in JAMA Oncology.
The research showed that a month of treatment with orally administered cancer drugs introduced in 2014 was, on average, 6 times more expensive at launch than monthly treatment costs for such drugs introduced in 2000, after adjusting for inflation.
In addition, most existing therapies had substantial price increases from the time they were launched to 2014.
“The major trend here is that these products are just getting more expensive over time,” said study author Stacie Dusetzina, PhD, of the University of North Carolina at Chapel Hill.
For this study, Dr Dusetzina evaluated what commercial health insurance companies and patients paid for prescription fills—before rebates and discounts—for 32 orally administered cancer drugs from 2000 to 2014. The information came from the TruvenHealth MarketScan Commercial Claims and Encounters database.
The data showed that orally administered drugs approved in 2000 cost an average of $1869 (95% CI, $1648-$2121) per month, compared to $11,325 (95% CI, $10 989-$11 671) for those approved in 2014.
When Dr Dusetzina compared changes in spending by year from a product’s launch to 2014, she observed increases in most of the drugs studied.
The drugs with the largest increases in monthly spending were thalidomide, which increased from $1869 to $7564 ($5695) and imatinib, which increased from $3346 to $8479 ($5133).
However, 2 drugs showed decreases in mean monthly spending between their launch and 2014. Monthly spending for lenalidomide decreased from $10,109 to $9640 ($469), and monthly spending for vorinostat decreased from $9755 to $7592 ($2163).
Dr Dusetzina pointed out that the amount patients pay for these drugs depends on their healthcare benefits. However, the high prices are being passed along to patients more and more, potentially affecting the patients’ access to these drugs.
“Patients are increasingly taking on the burden of paying for these high-cost specialty drugs as plans move toward use of higher deductibles and co-insurance—where a patient will pay a percentage of the drug cost rather than a flat copay,” Dr Dusetzina said.
She noted that while this study did account for payments by commercial health plans, it did not account for spending by Medicaid and Medicare, which may differ. In addition, only the products that were dispensed and reimbursed by commercial health plans were included, which may have excluded rarely used or recently approved products. ![]()
‘Universal’ CAR T cell may overcome limitations
FROM THE AACR ANNUAL MEETING
A differently engineered chimeric antigen receptor (CAR) T cell promises to overcome major limitations of current CAR T cell therapies. Rather than engineer the CAR T cells to have a receptor that recognizes specific tumor antigens one at a time and requiring different CAR T cells for every antigen, this technique engineers a T cell receptor that can bind one invariant end of a bifunctional molecule. The molecule is constructed such that the other end can bind to whatever tumor cell surface marker is of interest. In this way, the CAR T cells can be constructed once and be directed to various tumor markers.
Standard CAR T cells are engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, potentially providing immune surveillance in case cancer cells arise again. But they uniquely recognize just CD19 – a problem, in that they kill even normal B cells, so-called off-target toxicity.
Beyond the unique specificity of standard CAR T cells, Philip Low, Ph.D., director of the Center for Drug Discovery at Purdue University in West Lafayette, Indiana, said these cells have three major limitations. First, they may lyse tumor cells so rapidly that a systemic tumor lysis syndrome or “cytokine storm” occurs. Second, the persisting CAR T cells can kill normal cells – for example, ones directed against CD19 killing normal B cells. Third, tumor cells have unstable genomes, leading to tumor heterogeneity, with some cells potentially losing the targeted antigens and therefore becoming “invisible” to the CAR T cells.
“So what we have done is basically designed a solution to all three, and we call it a universal CAR T cell because of its ability, with the help of an adapter molecule, to recognize all of these mutated tumor cells within a heterogeneous tumor,” he said at the annual meeting of the American Association for Cancer Research. The key was to make a CAR T cell with a surface receptor that binds to the dye fluorescein. Then fluorescein is coupled through a short linker to a molecule that binds specifically to a molecule expressed on tumor cells. In this way the CAR T cell can be made to interact with any tumor cell, depending on what is coupled to the fluorescein. The technique is analogous to a socket wrench. Every socket has the same size hole that the ratchet handle fits into regardless of the size of the “business end” of the sockets, which recognize different size nuts.
Dr. Low gave an example of folic acid, for which he says a receptor is overexpressed on about 40% of human tumors but almost never on normal cells. “We link fluorescein to the vitamin folic acid,” he said. CAR T cells are injected into an animal, and nothing happens unless a folate-fluorescein conjugate is also injected. “As soon as we inject folate-fluorescein, the folate binds to the tumor cell surface, the fluorescein part of the folate-fluorescein binds to the CAR T cell, this forces a very tight interaction between the engineered T cell and the cancer cell, and we found it leads to melting away of the tumor,” he said.
This technique addresses the three major problems with standard CAR T cell therapy. By titrating the binding affinity, concentration, and rate of administration of the fluorescein conjugate, the rate of tumor killing can be controlled, mitigating tumor lysis syndrome. Plus, normal cells may be spared if the parameters are adjusted so that the conjugate binds only to cells with high levels of the target molecule, such as tumor cells.
Because its low molecular weight, the bi-specific conjugate rapidly disappears from the circulation, and the cell killing can be terminated, allowing normal cells to regenerate – for example, in the case of normal B cells that carry CD19. Since CAR T cells generate progeny that stay in the body, the progeny remain “dormant” but are ready to be activated again by addition of the conjugate to attack tumor cells if they arise.
A major issue is dealing with tumor heterogeneity; Dr. Low’s method seems to address that, as well. “We have tumor-specific ligands for over 90% of all human cancers,” he said. “Within another couple of months we’ll have them for 100%.”
Tumors typically contain lots of hypoxic cells, and hypoxic cells overexpress carbonic anhydrase-9. “Virtually every tumor has large fractions of the tumor mass that overexpress carbonic anhydrase-9, and we have a ligand that binds very specifically to that,” Dr. Low said.
To address the problem of tumor heterogeneity, with different mutations within different areas of the tumor or over time because of genetic instability in the cells, Dr. Low said, “We have a cocktail of about five of these small molecules… they are inexpensive to produce… and they clear very rapidly… and with the cocktail we can hit nearly all cancer cells, even in heterogeneous cancers.”
One limitation, as with standard CAR T cell therapy, is that the technique will still depend on using an individual patient’s T cells to modify through use of a lentiviral vector, so there would not be a universal, off-the-shelf T cell to use for everyone.
The technique and materials have been tested only in animals so far, using tumor-specific ligands for the folate receptor, a prostate-specific membrane antigen, and an antigen overexpressed on neuroendocrine tumors. Dr. Low has intentions to move the technology into human trials. He said the bridging molecules exist in highly purified form, and CAR T cell technology has already been developed by others. “Today we see great success in animal models and have no reason to believe that it won’t translate at least to a good extent to the clinic,” he said. Still, he expects some obstacles along the way and is willing to partner with others working on similar problems as well as large pharmaceutical companies.
The research has been supported by Endocyte, a company that Dr. Low founded and for which he is Chief Scientific Officer and a member of the board of directors. He has filed two patents on the technology, which are held by Purdue University and licensed to Endocyte.
FROM THE AACR ANNUAL MEETING
A differently engineered chimeric antigen receptor (CAR) T cell promises to overcome major limitations of current CAR T cell therapies. Rather than engineer the CAR T cells to have a receptor that recognizes specific tumor antigens one at a time and requiring different CAR T cells for every antigen, this technique engineers a T cell receptor that can bind one invariant end of a bifunctional molecule. The molecule is constructed such that the other end can bind to whatever tumor cell surface marker is of interest. In this way, the CAR T cells can be constructed once and be directed to various tumor markers.
Standard CAR T cells are engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, potentially providing immune surveillance in case cancer cells arise again. But they uniquely recognize just CD19 – a problem, in that they kill even normal B cells, so-called off-target toxicity.
Beyond the unique specificity of standard CAR T cells, Philip Low, Ph.D., director of the Center for Drug Discovery at Purdue University in West Lafayette, Indiana, said these cells have three major limitations. First, they may lyse tumor cells so rapidly that a systemic tumor lysis syndrome or “cytokine storm” occurs. Second, the persisting CAR T cells can kill normal cells – for example, ones directed against CD19 killing normal B cells. Third, tumor cells have unstable genomes, leading to tumor heterogeneity, with some cells potentially losing the targeted antigens and therefore becoming “invisible” to the CAR T cells.
“So what we have done is basically designed a solution to all three, and we call it a universal CAR T cell because of its ability, with the help of an adapter molecule, to recognize all of these mutated tumor cells within a heterogeneous tumor,” he said at the annual meeting of the American Association for Cancer Research. The key was to make a CAR T cell with a surface receptor that binds to the dye fluorescein. Then fluorescein is coupled through a short linker to a molecule that binds specifically to a molecule expressed on tumor cells. In this way the CAR T cell can be made to interact with any tumor cell, depending on what is coupled to the fluorescein. The technique is analogous to a socket wrench. Every socket has the same size hole that the ratchet handle fits into regardless of the size of the “business end” of the sockets, which recognize different size nuts.
Dr. Low gave an example of folic acid, for which he says a receptor is overexpressed on about 40% of human tumors but almost never on normal cells. “We link fluorescein to the vitamin folic acid,” he said. CAR T cells are injected into an animal, and nothing happens unless a folate-fluorescein conjugate is also injected. “As soon as we inject folate-fluorescein, the folate binds to the tumor cell surface, the fluorescein part of the folate-fluorescein binds to the CAR T cell, this forces a very tight interaction between the engineered T cell and the cancer cell, and we found it leads to melting away of the tumor,” he said.
This technique addresses the three major problems with standard CAR T cell therapy. By titrating the binding affinity, concentration, and rate of administration of the fluorescein conjugate, the rate of tumor killing can be controlled, mitigating tumor lysis syndrome. Plus, normal cells may be spared if the parameters are adjusted so that the conjugate binds only to cells with high levels of the target molecule, such as tumor cells.
Because its low molecular weight, the bi-specific conjugate rapidly disappears from the circulation, and the cell killing can be terminated, allowing normal cells to regenerate – for example, in the case of normal B cells that carry CD19. Since CAR T cells generate progeny that stay in the body, the progeny remain “dormant” but are ready to be activated again by addition of the conjugate to attack tumor cells if they arise.
A major issue is dealing with tumor heterogeneity; Dr. Low’s method seems to address that, as well. “We have tumor-specific ligands for over 90% of all human cancers,” he said. “Within another couple of months we’ll have them for 100%.”
Tumors typically contain lots of hypoxic cells, and hypoxic cells overexpress carbonic anhydrase-9. “Virtually every tumor has large fractions of the tumor mass that overexpress carbonic anhydrase-9, and we have a ligand that binds very specifically to that,” Dr. Low said.
To address the problem of tumor heterogeneity, with different mutations within different areas of the tumor or over time because of genetic instability in the cells, Dr. Low said, “We have a cocktail of about five of these small molecules… they are inexpensive to produce… and they clear very rapidly… and with the cocktail we can hit nearly all cancer cells, even in heterogeneous cancers.”
One limitation, as with standard CAR T cell therapy, is that the technique will still depend on using an individual patient’s T cells to modify through use of a lentiviral vector, so there would not be a universal, off-the-shelf T cell to use for everyone.
The technique and materials have been tested only in animals so far, using tumor-specific ligands for the folate receptor, a prostate-specific membrane antigen, and an antigen overexpressed on neuroendocrine tumors. Dr. Low has intentions to move the technology into human trials. He said the bridging molecules exist in highly purified form, and CAR T cell technology has already been developed by others. “Today we see great success in animal models and have no reason to believe that it won’t translate at least to a good extent to the clinic,” he said. Still, he expects some obstacles along the way and is willing to partner with others working on similar problems as well as large pharmaceutical companies.
The research has been supported by Endocyte, a company that Dr. Low founded and for which he is Chief Scientific Officer and a member of the board of directors. He has filed two patents on the technology, which are held by Purdue University and licensed to Endocyte.
FROM THE AACR ANNUAL MEETING
A differently engineered chimeric antigen receptor (CAR) T cell promises to overcome major limitations of current CAR T cell therapies. Rather than engineer the CAR T cells to have a receptor that recognizes specific tumor antigens one at a time and requiring different CAR T cells for every antigen, this technique engineers a T cell receptor that can bind one invariant end of a bifunctional molecule. The molecule is constructed such that the other end can bind to whatever tumor cell surface marker is of interest. In this way, the CAR T cells can be constructed once and be directed to various tumor markers.
Standard CAR T cells are engineered to express on their surfaces receptors that recognize a specific antigen. These cells have been used up to now to recognize and kill tumor cells – for example, B cell leukemias carrying the pan-B cell marker CD19. The CAR T cells and their progeny, including memory T cells, remain in the body and continue to carry out their functions, potentially providing immune surveillance in case cancer cells arise again. But they uniquely recognize just CD19 – a problem, in that they kill even normal B cells, so-called off-target toxicity.
Beyond the unique specificity of standard CAR T cells, Philip Low, Ph.D., director of the Center for Drug Discovery at Purdue University in West Lafayette, Indiana, said these cells have three major limitations. First, they may lyse tumor cells so rapidly that a systemic tumor lysis syndrome or “cytokine storm” occurs. Second, the persisting CAR T cells can kill normal cells – for example, ones directed against CD19 killing normal B cells. Third, tumor cells have unstable genomes, leading to tumor heterogeneity, with some cells potentially losing the targeted antigens and therefore becoming “invisible” to the CAR T cells.
“So what we have done is basically designed a solution to all three, and we call it a universal CAR T cell because of its ability, with the help of an adapter molecule, to recognize all of these mutated tumor cells within a heterogeneous tumor,” he said at the annual meeting of the American Association for Cancer Research. The key was to make a CAR T cell with a surface receptor that binds to the dye fluorescein. Then fluorescein is coupled through a short linker to a molecule that binds specifically to a molecule expressed on tumor cells. In this way the CAR T cell can be made to interact with any tumor cell, depending on what is coupled to the fluorescein. The technique is analogous to a socket wrench. Every socket has the same size hole that the ratchet handle fits into regardless of the size of the “business end” of the sockets, which recognize different size nuts.
Dr. Low gave an example of folic acid, for which he says a receptor is overexpressed on about 40% of human tumors but almost never on normal cells. “We link fluorescein to the vitamin folic acid,” he said. CAR T cells are injected into an animal, and nothing happens unless a folate-fluorescein conjugate is also injected. “As soon as we inject folate-fluorescein, the folate binds to the tumor cell surface, the fluorescein part of the folate-fluorescein binds to the CAR T cell, this forces a very tight interaction between the engineered T cell and the cancer cell, and we found it leads to melting away of the tumor,” he said.
This technique addresses the three major problems with standard CAR T cell therapy. By titrating the binding affinity, concentration, and rate of administration of the fluorescein conjugate, the rate of tumor killing can be controlled, mitigating tumor lysis syndrome. Plus, normal cells may be spared if the parameters are adjusted so that the conjugate binds only to cells with high levels of the target molecule, such as tumor cells.
Because its low molecular weight, the bi-specific conjugate rapidly disappears from the circulation, and the cell killing can be terminated, allowing normal cells to regenerate – for example, in the case of normal B cells that carry CD19. Since CAR T cells generate progeny that stay in the body, the progeny remain “dormant” but are ready to be activated again by addition of the conjugate to attack tumor cells if they arise.
A major issue is dealing with tumor heterogeneity; Dr. Low’s method seems to address that, as well. “We have tumor-specific ligands for over 90% of all human cancers,” he said. “Within another couple of months we’ll have them for 100%.”
Tumors typically contain lots of hypoxic cells, and hypoxic cells overexpress carbonic anhydrase-9. “Virtually every tumor has large fractions of the tumor mass that overexpress carbonic anhydrase-9, and we have a ligand that binds very specifically to that,” Dr. Low said.
To address the problem of tumor heterogeneity, with different mutations within different areas of the tumor or over time because of genetic instability in the cells, Dr. Low said, “We have a cocktail of about five of these small molecules… they are inexpensive to produce… and they clear very rapidly… and with the cocktail we can hit nearly all cancer cells, even in heterogeneous cancers.”
One limitation, as with standard CAR T cell therapy, is that the technique will still depend on using an individual patient’s T cells to modify through use of a lentiviral vector, so there would not be a universal, off-the-shelf T cell to use for everyone.
The technique and materials have been tested only in animals so far, using tumor-specific ligands for the folate receptor, a prostate-specific membrane antigen, and an antigen overexpressed on neuroendocrine tumors. Dr. Low has intentions to move the technology into human trials. He said the bridging molecules exist in highly purified form, and CAR T cell technology has already been developed by others. “Today we see great success in animal models and have no reason to believe that it won’t translate at least to a good extent to the clinic,” he said. Still, he expects some obstacles along the way and is willing to partner with others working on similar problems as well as large pharmaceutical companies.
The research has been supported by Endocyte, a company that Dr. Low founded and for which he is Chief Scientific Officer and a member of the board of directors. He has filed two patents on the technology, which are held by Purdue University and licensed to Endocyte.
CAR T-cell trial explores new territory

Photo courtesy of
Fred Hutch News Service
Researchers say they have conducted the first trial of CD19-directed chimeric antigen receptor (CAR) T-cell therapy in which CD4+ and CD8+ cells were administered in equal proportions.
And the assurance that each patient received the same mixture of cells allowed the team to draw clear conclusions about the effects of administering CAR T-cell therapy at different doses.
The researchers detailed these conclusions in The Journal of Clinical Investigation.
This phase 1/2 trial (NCT01865617) was funded, in part, by Juno Therapeutics, the company developing the CAR T-cell therapy as JCAR014.
The work was also funded by the National Cancer Institute, private philanthropists, and the Life Sciences Discovery Fund.
Patients and treatment
The researchers reported data on 32 patients who had relapsed or refractory, CD19+ B-cell acute lymphoblastic leukemia and a median age of 40 (range, 20–73). Two of these patients were excluded due to complications prior to receiving treatment.
The 30 patients who were treated in this study had received a median of 3 prior intensive chemotherapy regimens (range, 1–11). Eleven patients had failed a hematopoietic stem cell transplant (HSCT). And all patients had detectable disease in the bone marrow, extramedullary sites, or cerebrospinal fluid at baseline.
To create the CAR T-cell therapy, the researchers modified CD8+ and CD4+ T-cell subsets separately to express a CD19-targeted CAR incorporating 4-1BB and CD3ζ signaling domains. The cells were then formulated in a defined ratio of CD4+:CD8+ CAR T cells.
Patients underwent lymphodepletion with a cyclophosphamide-based regimen (alone or with fludarabine or etoposide) and then received CAR T cells 48 to 96 hours later. The CAR T cells were given at 3 dose levels—2 × 105/kg (DL1), 2 × 106/kg (DL2), and 2 × 107/kg (DL3).
Toxicity
The first 2 patients who received CAR T-cell therapy at DL3 developed severe toxicities, including 1 patient who died. So DL3 was not given to any subsequent patients.
Two patients died after CAR T-cell infusion. One patient had severe cytokine release syndrome (CRS) and multiorgan failure and died on day 3. The other patient had transient severe CRS with irreversible neurologic toxicity and died on day 122.
The most common adverse event observed in the first 14 days after CAR T-cell infusion was CRS, which occurred in 25 patients. Seven patients had severe CRS that put them in the intensive care unit.
However, for the patients treated at DL1 and DL2, dexamethasone alone or with tocilizumab resolved CRS.
Fifteen patients developed severe neurotoxicity (grade 3 or higher), either with CRS or after it resolved. For all but 1 patient (the aforementioned patient who died), neurologic symptoms and signs resolved.
Response
One patient died before response assessment, so 29 patients were evaluable. Twenty-seven of these patients (93%) achieved bone marrow remission by flow cytometry.
Of the 2 patients who did not achieve a complete response (CR), 1 underwent an allogeneic HSCT after receiving CAR T cells. After HSCT, she relapsed, was re-enrolled on the trial, and achieved a CR after receiving a higher dose of CAR T cells (DL2).
Twenty-five patients (86%) achieved a CR without evidence of minimal residual disease by flow cytometry and conventional karyotyping, FISH, or QPCR.
“Patients who come onto the trial have really limited options for treatment,” said study author Cameron Turtle, MBBS, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“They have refractory acute leukemia. So the fact that we’re getting so many into remission is giving these people a way forward.”
Unfortunately, not all patients stayed in CR. Some relapsed and were treated again with CAR T cells, and 2 patients relapsed with leukemias that were immune to the CAR T cells. The researchers said it is still too early to know the long-term outcomes of the therapy.
“This is just the beginning,” Dr Turtle said. “It sounds fantastic to say that we get over 90% remissions, but there’s so much more work to do make sure they’re durable remissions, to work out who’s going to benefit the most, and extend this work to other diseases.”
Lessons learned
The researchers said that, because these CAR T cells had a defined CD4+:CD8+ composition, this study provides the first clear evidence of the relationships between the CAR T-cell dose patients receive and their outcomes after infusion.
The team found that high doses of CAR T cells and high tumor burden increase the risks of severe CRS and neurotoxicity. However, certain biomarkers can identify patients at the highest risk of toxicity.
Levels of IL-6, IFN-γ, and TNF-α on the first day after CAR T-cell infusion were significantly higher in patients who later developed severe neurotoxicity. Levels of these biomarkers were also higher in patients who were later sent to the intensive care unit.
Furthermore, risk-stratified CAR T-cell dosing based on bone marrow disease burden decreased toxicity.
The researchers also said they observed CD8+ T-cell-mediated anti-CAR transgene product immune responses in some patients, which limited CAR T-cell persistence and increased the risk of relapse.
And including fludarabine in the lymphodepletion regimen resulted in better CAR T-cell persistence and disease-free survival than when cyclophosphamide was given alone or with etoposide. ![]()

Photo courtesy of
Fred Hutch News Service
Researchers say they have conducted the first trial of CD19-directed chimeric antigen receptor (CAR) T-cell therapy in which CD4+ and CD8+ cells were administered in equal proportions.
And the assurance that each patient received the same mixture of cells allowed the team to draw clear conclusions about the effects of administering CAR T-cell therapy at different doses.
The researchers detailed these conclusions in The Journal of Clinical Investigation.
This phase 1/2 trial (NCT01865617) was funded, in part, by Juno Therapeutics, the company developing the CAR T-cell therapy as JCAR014.
The work was also funded by the National Cancer Institute, private philanthropists, and the Life Sciences Discovery Fund.
Patients and treatment
The researchers reported data on 32 patients who had relapsed or refractory, CD19+ B-cell acute lymphoblastic leukemia and a median age of 40 (range, 20–73). Two of these patients were excluded due to complications prior to receiving treatment.
The 30 patients who were treated in this study had received a median of 3 prior intensive chemotherapy regimens (range, 1–11). Eleven patients had failed a hematopoietic stem cell transplant (HSCT). And all patients had detectable disease in the bone marrow, extramedullary sites, or cerebrospinal fluid at baseline.
To create the CAR T-cell therapy, the researchers modified CD8+ and CD4+ T-cell subsets separately to express a CD19-targeted CAR incorporating 4-1BB and CD3ζ signaling domains. The cells were then formulated in a defined ratio of CD4+:CD8+ CAR T cells.
Patients underwent lymphodepletion with a cyclophosphamide-based regimen (alone or with fludarabine or etoposide) and then received CAR T cells 48 to 96 hours later. The CAR T cells were given at 3 dose levels—2 × 105/kg (DL1), 2 × 106/kg (DL2), and 2 × 107/kg (DL3).
Toxicity
The first 2 patients who received CAR T-cell therapy at DL3 developed severe toxicities, including 1 patient who died. So DL3 was not given to any subsequent patients.
Two patients died after CAR T-cell infusion. One patient had severe cytokine release syndrome (CRS) and multiorgan failure and died on day 3. The other patient had transient severe CRS with irreversible neurologic toxicity and died on day 122.
The most common adverse event observed in the first 14 days after CAR T-cell infusion was CRS, which occurred in 25 patients. Seven patients had severe CRS that put them in the intensive care unit.
However, for the patients treated at DL1 and DL2, dexamethasone alone or with tocilizumab resolved CRS.
Fifteen patients developed severe neurotoxicity (grade 3 or higher), either with CRS or after it resolved. For all but 1 patient (the aforementioned patient who died), neurologic symptoms and signs resolved.
Response
One patient died before response assessment, so 29 patients were evaluable. Twenty-seven of these patients (93%) achieved bone marrow remission by flow cytometry.
Of the 2 patients who did not achieve a complete response (CR), 1 underwent an allogeneic HSCT after receiving CAR T cells. After HSCT, she relapsed, was re-enrolled on the trial, and achieved a CR after receiving a higher dose of CAR T cells (DL2).
Twenty-five patients (86%) achieved a CR without evidence of minimal residual disease by flow cytometry and conventional karyotyping, FISH, or QPCR.
“Patients who come onto the trial have really limited options for treatment,” said study author Cameron Turtle, MBBS, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“They have refractory acute leukemia. So the fact that we’re getting so many into remission is giving these people a way forward.”
Unfortunately, not all patients stayed in CR. Some relapsed and were treated again with CAR T cells, and 2 patients relapsed with leukemias that were immune to the CAR T cells. The researchers said it is still too early to know the long-term outcomes of the therapy.
“This is just the beginning,” Dr Turtle said. “It sounds fantastic to say that we get over 90% remissions, but there’s so much more work to do make sure they’re durable remissions, to work out who’s going to benefit the most, and extend this work to other diseases.”
Lessons learned
The researchers said that, because these CAR T cells had a defined CD4+:CD8+ composition, this study provides the first clear evidence of the relationships between the CAR T-cell dose patients receive and their outcomes after infusion.
The team found that high doses of CAR T cells and high tumor burden increase the risks of severe CRS and neurotoxicity. However, certain biomarkers can identify patients at the highest risk of toxicity.
Levels of IL-6, IFN-γ, and TNF-α on the first day after CAR T-cell infusion were significantly higher in patients who later developed severe neurotoxicity. Levels of these biomarkers were also higher in patients who were later sent to the intensive care unit.
Furthermore, risk-stratified CAR T-cell dosing based on bone marrow disease burden decreased toxicity.
The researchers also said they observed CD8+ T-cell-mediated anti-CAR transgene product immune responses in some patients, which limited CAR T-cell persistence and increased the risk of relapse.
And including fludarabine in the lymphodepletion regimen resulted in better CAR T-cell persistence and disease-free survival than when cyclophosphamide was given alone or with etoposide. ![]()

Photo courtesy of
Fred Hutch News Service
Researchers say they have conducted the first trial of CD19-directed chimeric antigen receptor (CAR) T-cell therapy in which CD4+ and CD8+ cells were administered in equal proportions.
And the assurance that each patient received the same mixture of cells allowed the team to draw clear conclusions about the effects of administering CAR T-cell therapy at different doses.
The researchers detailed these conclusions in The Journal of Clinical Investigation.
This phase 1/2 trial (NCT01865617) was funded, in part, by Juno Therapeutics, the company developing the CAR T-cell therapy as JCAR014.
The work was also funded by the National Cancer Institute, private philanthropists, and the Life Sciences Discovery Fund.
Patients and treatment
The researchers reported data on 32 patients who had relapsed or refractory, CD19+ B-cell acute lymphoblastic leukemia and a median age of 40 (range, 20–73). Two of these patients were excluded due to complications prior to receiving treatment.
The 30 patients who were treated in this study had received a median of 3 prior intensive chemotherapy regimens (range, 1–11). Eleven patients had failed a hematopoietic stem cell transplant (HSCT). And all patients had detectable disease in the bone marrow, extramedullary sites, or cerebrospinal fluid at baseline.
To create the CAR T-cell therapy, the researchers modified CD8+ and CD4+ T-cell subsets separately to express a CD19-targeted CAR incorporating 4-1BB and CD3ζ signaling domains. The cells were then formulated in a defined ratio of CD4+:CD8+ CAR T cells.
Patients underwent lymphodepletion with a cyclophosphamide-based regimen (alone or with fludarabine or etoposide) and then received CAR T cells 48 to 96 hours later. The CAR T cells were given at 3 dose levels—2 × 105/kg (DL1), 2 × 106/kg (DL2), and 2 × 107/kg (DL3).
Toxicity
The first 2 patients who received CAR T-cell therapy at DL3 developed severe toxicities, including 1 patient who died. So DL3 was not given to any subsequent patients.
Two patients died after CAR T-cell infusion. One patient had severe cytokine release syndrome (CRS) and multiorgan failure and died on day 3. The other patient had transient severe CRS with irreversible neurologic toxicity and died on day 122.
The most common adverse event observed in the first 14 days after CAR T-cell infusion was CRS, which occurred in 25 patients. Seven patients had severe CRS that put them in the intensive care unit.
However, for the patients treated at DL1 and DL2, dexamethasone alone or with tocilizumab resolved CRS.
Fifteen patients developed severe neurotoxicity (grade 3 or higher), either with CRS or after it resolved. For all but 1 patient (the aforementioned patient who died), neurologic symptoms and signs resolved.
Response
One patient died before response assessment, so 29 patients were evaluable. Twenty-seven of these patients (93%) achieved bone marrow remission by flow cytometry.
Of the 2 patients who did not achieve a complete response (CR), 1 underwent an allogeneic HSCT after receiving CAR T cells. After HSCT, she relapsed, was re-enrolled on the trial, and achieved a CR after receiving a higher dose of CAR T cells (DL2).
Twenty-five patients (86%) achieved a CR without evidence of minimal residual disease by flow cytometry and conventional karyotyping, FISH, or QPCR.
“Patients who come onto the trial have really limited options for treatment,” said study author Cameron Turtle, MBBS, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“They have refractory acute leukemia. So the fact that we’re getting so many into remission is giving these people a way forward.”
Unfortunately, not all patients stayed in CR. Some relapsed and were treated again with CAR T cells, and 2 patients relapsed with leukemias that were immune to the CAR T cells. The researchers said it is still too early to know the long-term outcomes of the therapy.
“This is just the beginning,” Dr Turtle said. “It sounds fantastic to say that we get over 90% remissions, but there’s so much more work to do make sure they’re durable remissions, to work out who’s going to benefit the most, and extend this work to other diseases.”
Lessons learned
The researchers said that, because these CAR T cells had a defined CD4+:CD8+ composition, this study provides the first clear evidence of the relationships between the CAR T-cell dose patients receive and their outcomes after infusion.
The team found that high doses of CAR T cells and high tumor burden increase the risks of severe CRS and neurotoxicity. However, certain biomarkers can identify patients at the highest risk of toxicity.
Levels of IL-6, IFN-γ, and TNF-α on the first day after CAR T-cell infusion were significantly higher in patients who later developed severe neurotoxicity. Levels of these biomarkers were also higher in patients who were later sent to the intensive care unit.
Furthermore, risk-stratified CAR T-cell dosing based on bone marrow disease burden decreased toxicity.
The researchers also said they observed CD8+ T-cell-mediated anti-CAR transgene product immune responses in some patients, which limited CAR T-cell persistence and increased the risk of relapse.
And including fludarabine in the lymphodepletion regimen resulted in better CAR T-cell persistence and disease-free survival than when cyclophosphamide was given alone or with etoposide. ![]()
Manipulating a microRNA to treat AML

Image by Su Jung Song
The microRNA miR-22 is “an essential antitumor gatekeeper” in acute myeloid leukemia (AML), researchers have reported in Nature Communications.
The team found that miR-22 was significantly downregulated in AML, and forced expression of miR-22 produced antileukemic effects in AML cells
and mouse models of the disease.
Futhermore, nanoparticles carrying miR-22 oligonucleotides appeared to cure AML in some mice.
“Previous research has shown that microRNA miR-22 is linked to breast cancer and other blood disorders [myelodysplastic syndromes], which sometimes turn into AML,” said study author Jianjun Chen, PhD, of the University of Cincinnati in Ohio.
“But we found in this study that it could be an essential antitumor gatekeeper in AML when it is downregulated. When we forced miR-22 expression, we saw difficulty in leukemia cells developing, growing, and thriving.”
Dr Chen and his colleagues first found that miR-22 was significantly downregulated (P<0.05) in samples from AML patients, when compared with normal CD34+ hematopoietic stem/progenitor cells, CD33+ myeloid progenitor cells, and mononuclear cells. The set of AML samples included MLL, t(15;17), t(8;21), and inv(16) AML.
When the researchers forced expression of miR-22 in human AML cells, they found the microRNA significantly inhibited cell viability, growth, and proliferation, while promoting apoptosis.
The team also investigated the role of miR-22 in colony formation induced by MLL-AF10/t(10;11), PML-RARA/t(15;17), and AML1-ETO9a/t(8;21). They found that forced expression of miR-22 significantly inhibited colony formation induced by all of these oncogenic fusion genes.
In mice, forced expression of miR-22 blocked MLL-AF9-mediated leukemogenesis and MLL-AF10-mediated leukemogenesis.
Forced expression of miR-22 also inhibited progression of AML induced by MLL-AF9, AE9a, or FLT3-ITD/NPM1c+ in secondary recipient mice. The researchers said this resulted in “largely normal” morphologies in the peripheral blood, bone marrow, spleen, and liver tissues of these mice.
In addition, the team found that nanoparticles carrying miR-22 oligonucleotides significantly delayed AML progression in secondary recipient mice with MLL-AF9 and AE9a-induced AML. At least 40% of the mice appeared to be completely cured.
In a xenotransplantation model, miR-22 nanoparticles significantly delayed AML progression induced by human MV4;11/t(4;11) cells.
Further investigation into the role miR-22 plays in AML revealed that 3 oncogenes—CRTC1, FLT3, and MYCBP—are “functionally important” targets of miR-22 in AML. And miR-22 represses the CREB and MYC signaling pathways.
The researchers also found DNA copy-number loss in the miR-22 gene locus in AML cases, and they discovered the expression of miR-22 is epigenetically repressed in AML.
“The downregulation, or decreased output, of miR-22 in AML is caused by the loss of the number of DNA being copied and/or stopping their expression through a pathway called TET1/GFI1/EZH2/SIN3A,” Dr Chen explained.
“Our study uncovers a previously unappreciated signaling pathway—TET1/GFI1/EZH2/SIN3A/miR-22/CREB-MYC—and provides new insights into genetic mechanisms causing and progressing AML and also highlights the clinical potential of miR-22-based AML therapy. More research on this pathway and ways to target it are necessary.” ![]()

Image by Su Jung Song
The microRNA miR-22 is “an essential antitumor gatekeeper” in acute myeloid leukemia (AML), researchers have reported in Nature Communications.
The team found that miR-22 was significantly downregulated in AML, and forced expression of miR-22 produced antileukemic effects in AML cells
and mouse models of the disease.
Futhermore, nanoparticles carrying miR-22 oligonucleotides appeared to cure AML in some mice.
“Previous research has shown that microRNA miR-22 is linked to breast cancer and other blood disorders [myelodysplastic syndromes], which sometimes turn into AML,” said study author Jianjun Chen, PhD, of the University of Cincinnati in Ohio.
“But we found in this study that it could be an essential antitumor gatekeeper in AML when it is downregulated. When we forced miR-22 expression, we saw difficulty in leukemia cells developing, growing, and thriving.”
Dr Chen and his colleagues first found that miR-22 was significantly downregulated (P<0.05) in samples from AML patients, when compared with normal CD34+ hematopoietic stem/progenitor cells, CD33+ myeloid progenitor cells, and mononuclear cells. The set of AML samples included MLL, t(15;17), t(8;21), and inv(16) AML.
When the researchers forced expression of miR-22 in human AML cells, they found the microRNA significantly inhibited cell viability, growth, and proliferation, while promoting apoptosis.
The team also investigated the role of miR-22 in colony formation induced by MLL-AF10/t(10;11), PML-RARA/t(15;17), and AML1-ETO9a/t(8;21). They found that forced expression of miR-22 significantly inhibited colony formation induced by all of these oncogenic fusion genes.
In mice, forced expression of miR-22 blocked MLL-AF9-mediated leukemogenesis and MLL-AF10-mediated leukemogenesis.
Forced expression of miR-22 also inhibited progression of AML induced by MLL-AF9, AE9a, or FLT3-ITD/NPM1c+ in secondary recipient mice. The researchers said this resulted in “largely normal” morphologies in the peripheral blood, bone marrow, spleen, and liver tissues of these mice.
In addition, the team found that nanoparticles carrying miR-22 oligonucleotides significantly delayed AML progression in secondary recipient mice with MLL-AF9 and AE9a-induced AML. At least 40% of the mice appeared to be completely cured.
In a xenotransplantation model, miR-22 nanoparticles significantly delayed AML progression induced by human MV4;11/t(4;11) cells.
Further investigation into the role miR-22 plays in AML revealed that 3 oncogenes—CRTC1, FLT3, and MYCBP—are “functionally important” targets of miR-22 in AML. And miR-22 represses the CREB and MYC signaling pathways.
The researchers also found DNA copy-number loss in the miR-22 gene locus in AML cases, and they discovered the expression of miR-22 is epigenetically repressed in AML.
“The downregulation, or decreased output, of miR-22 in AML is caused by the loss of the number of DNA being copied and/or stopping their expression through a pathway called TET1/GFI1/EZH2/SIN3A,” Dr Chen explained.
“Our study uncovers a previously unappreciated signaling pathway—TET1/GFI1/EZH2/SIN3A/miR-22/CREB-MYC—and provides new insights into genetic mechanisms causing and progressing AML and also highlights the clinical potential of miR-22-based AML therapy. More research on this pathway and ways to target it are necessary.” ![]()

Image by Su Jung Song
The microRNA miR-22 is “an essential antitumor gatekeeper” in acute myeloid leukemia (AML), researchers have reported in Nature Communications.
The team found that miR-22 was significantly downregulated in AML, and forced expression of miR-22 produced antileukemic effects in AML cells
and mouse models of the disease.
Futhermore, nanoparticles carrying miR-22 oligonucleotides appeared to cure AML in some mice.
“Previous research has shown that microRNA miR-22 is linked to breast cancer and other blood disorders [myelodysplastic syndromes], which sometimes turn into AML,” said study author Jianjun Chen, PhD, of the University of Cincinnati in Ohio.
“But we found in this study that it could be an essential antitumor gatekeeper in AML when it is downregulated. When we forced miR-22 expression, we saw difficulty in leukemia cells developing, growing, and thriving.”
Dr Chen and his colleagues first found that miR-22 was significantly downregulated (P<0.05) in samples from AML patients, when compared with normal CD34+ hematopoietic stem/progenitor cells, CD33+ myeloid progenitor cells, and mononuclear cells. The set of AML samples included MLL, t(15;17), t(8;21), and inv(16) AML.
When the researchers forced expression of miR-22 in human AML cells, they found the microRNA significantly inhibited cell viability, growth, and proliferation, while promoting apoptosis.
The team also investigated the role of miR-22 in colony formation induced by MLL-AF10/t(10;11), PML-RARA/t(15;17), and AML1-ETO9a/t(8;21). They found that forced expression of miR-22 significantly inhibited colony formation induced by all of these oncogenic fusion genes.
In mice, forced expression of miR-22 blocked MLL-AF9-mediated leukemogenesis and MLL-AF10-mediated leukemogenesis.
Forced expression of miR-22 also inhibited progression of AML induced by MLL-AF9, AE9a, or FLT3-ITD/NPM1c+ in secondary recipient mice. The researchers said this resulted in “largely normal” morphologies in the peripheral blood, bone marrow, spleen, and liver tissues of these mice.
In addition, the team found that nanoparticles carrying miR-22 oligonucleotides significantly delayed AML progression in secondary recipient mice with MLL-AF9 and AE9a-induced AML. At least 40% of the mice appeared to be completely cured.
In a xenotransplantation model, miR-22 nanoparticles significantly delayed AML progression induced by human MV4;11/t(4;11) cells.
Further investigation into the role miR-22 plays in AML revealed that 3 oncogenes—CRTC1, FLT3, and MYCBP—are “functionally important” targets of miR-22 in AML. And miR-22 represses the CREB and MYC signaling pathways.
The researchers also found DNA copy-number loss in the miR-22 gene locus in AML cases, and they discovered the expression of miR-22 is epigenetically repressed in AML.
“The downregulation, or decreased output, of miR-22 in AML is caused by the loss of the number of DNA being copied and/or stopping their expression through a pathway called TET1/GFI1/EZH2/SIN3A,” Dr Chen explained.
“Our study uncovers a previously unappreciated signaling pathway—TET1/GFI1/EZH2/SIN3A/miR-22/CREB-MYC—and provides new insights into genetic mechanisms causing and progressing AML and also highlights the clinical potential of miR-22-based AML therapy. More research on this pathway and ways to target it are necessary.” ![]()
VIDEO: Adding ixazomib to len-dex boosts progression-free survival in multiple myeloma
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
FROM NEJM
Key clinical point: Adding ixazomib to lenalidomide and dexamethasone was associated with a longer progression-free survival and limited additional toxic effects in patients with multiple myeloma.
Major finding: At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group.
Data source: Phase III results on 722 patients in the TOURMALINE trial.
Disclosures: The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
New single-tube assay detects one CLL cell in 1 million leukocytes
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
FROM LEUKEMIA
Key clinical point: Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay.
Major finding: In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, and also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
Data source: The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis.
Disclosures: Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.