User login
Novel antibody evokes responses in relapsed/refractory myeloma
CHICAGO – An investigational targeted therapy for multiple myeloma delayed disease progression in patients for whom as many as five prior lines of therapy had failed.
Daratumumab, an experimental antibody targeted to the CD38 receptor expressed at high levels on the surface of myeloma cells, was associated with a 29.2% overall response rate (ORR) at a median follow-up of 9.4 months, including three patients with a complete remission, said Dr. Saad Zafar Usmani of Levine Cancer Institute–Carolinas Healthcare System, Charlotte, N.C.
“Daratumumab monotherapy produced unprecedented overall responses that deepened over time in this heavily pretreated multiple myeloma patient population. These results highlight the potential of daratumumab as a novel, well-tolerated, single-agent therapy in various stages of myeloma treatment,” Dr. Usmani said at a briefing prior to the presentation of the data in a session at the annual meeting of the American Society of Clinical Oncology.
The results support further exploration of daratumumab in combination with currently available therapies for multiple myeloma, he added.
In the dose-selection portion of a phase II open-label, international, multicenter study, patients with multiple myeloma who had received at least three prior lines of therapy including a proteasome inhibitor and an immunomodulatory agent, or who had no treatment response to the most recent proteasome inhibitor and immunomodulator, were randomly assigned to receive either 8 mg/kg or 16 mg/kg daratumumab. After a response evaluation, the 16 mg/kg dose was chosen, and an additional 90 patients were enrolled at this dose.
As noted, the overall response rate, the primary endpoint, was 29.2%, consisting of three stringent complete responses, 10 very good partial responses, and 18 partial responses. The median duration of response was 7.4 months. The responses were consistent across all clinically relevant subgroups, Dr. Usmani said.
The median time to progression was 3.7 months. Median overall survival had not been reached at the time of the last data analysis, and the estimated 1-year overall survival rate was 65%.
At a median follow-up of 9.4 months, 14 of 31 patients with responses remained on therapy.
Patients tolerated the 16 mg/kg dose well, and none discontinued because of drug-related events.
Minor infusion site reactions (grade 1 or 2) were common during the first infusion and were managed with standard therapies, Dr. Usmani said.
“This is the first monoclonal antibody in myeloma that has shown single-agent activity,” Dr. Usmani said.
ASCO expert Dr. Merry-Jennifer Markham from the University of Florida in Gainesville, who was not involved in the study, commented that “there have been substantial treatment advances in multiple myeloma over the last decade, and daratumumab will be an important addition to the list of options. This therapy may offer a glimmer of hope for those myeloma patients who have run out of treatment options.
The study was funded by Janssen Research and Development. Dr. Usmani is on the speaker’s bureau for Janssen Oncology.
CHICAGO – An investigational targeted therapy for multiple myeloma delayed disease progression in patients for whom as many as five prior lines of therapy had failed.
Daratumumab, an experimental antibody targeted to the CD38 receptor expressed at high levels on the surface of myeloma cells, was associated with a 29.2% overall response rate (ORR) at a median follow-up of 9.4 months, including three patients with a complete remission, said Dr. Saad Zafar Usmani of Levine Cancer Institute–Carolinas Healthcare System, Charlotte, N.C.
“Daratumumab monotherapy produced unprecedented overall responses that deepened over time in this heavily pretreated multiple myeloma patient population. These results highlight the potential of daratumumab as a novel, well-tolerated, single-agent therapy in various stages of myeloma treatment,” Dr. Usmani said at a briefing prior to the presentation of the data in a session at the annual meeting of the American Society of Clinical Oncology.
The results support further exploration of daratumumab in combination with currently available therapies for multiple myeloma, he added.
In the dose-selection portion of a phase II open-label, international, multicenter study, patients with multiple myeloma who had received at least three prior lines of therapy including a proteasome inhibitor and an immunomodulatory agent, or who had no treatment response to the most recent proteasome inhibitor and immunomodulator, were randomly assigned to receive either 8 mg/kg or 16 mg/kg daratumumab. After a response evaluation, the 16 mg/kg dose was chosen, and an additional 90 patients were enrolled at this dose.
As noted, the overall response rate, the primary endpoint, was 29.2%, consisting of three stringent complete responses, 10 very good partial responses, and 18 partial responses. The median duration of response was 7.4 months. The responses were consistent across all clinically relevant subgroups, Dr. Usmani said.
The median time to progression was 3.7 months. Median overall survival had not been reached at the time of the last data analysis, and the estimated 1-year overall survival rate was 65%.
At a median follow-up of 9.4 months, 14 of 31 patients with responses remained on therapy.
Patients tolerated the 16 mg/kg dose well, and none discontinued because of drug-related events.
Minor infusion site reactions (grade 1 or 2) were common during the first infusion and were managed with standard therapies, Dr. Usmani said.
“This is the first monoclonal antibody in myeloma that has shown single-agent activity,” Dr. Usmani said.
ASCO expert Dr. Merry-Jennifer Markham from the University of Florida in Gainesville, who was not involved in the study, commented that “there have been substantial treatment advances in multiple myeloma over the last decade, and daratumumab will be an important addition to the list of options. This therapy may offer a glimmer of hope for those myeloma patients who have run out of treatment options.
The study was funded by Janssen Research and Development. Dr. Usmani is on the speaker’s bureau for Janssen Oncology.
CHICAGO – An investigational targeted therapy for multiple myeloma delayed disease progression in patients for whom as many as five prior lines of therapy had failed.
Daratumumab, an experimental antibody targeted to the CD38 receptor expressed at high levels on the surface of myeloma cells, was associated with a 29.2% overall response rate (ORR) at a median follow-up of 9.4 months, including three patients with a complete remission, said Dr. Saad Zafar Usmani of Levine Cancer Institute–Carolinas Healthcare System, Charlotte, N.C.
“Daratumumab monotherapy produced unprecedented overall responses that deepened over time in this heavily pretreated multiple myeloma patient population. These results highlight the potential of daratumumab as a novel, well-tolerated, single-agent therapy in various stages of myeloma treatment,” Dr. Usmani said at a briefing prior to the presentation of the data in a session at the annual meeting of the American Society of Clinical Oncology.
The results support further exploration of daratumumab in combination with currently available therapies for multiple myeloma, he added.
In the dose-selection portion of a phase II open-label, international, multicenter study, patients with multiple myeloma who had received at least three prior lines of therapy including a proteasome inhibitor and an immunomodulatory agent, or who had no treatment response to the most recent proteasome inhibitor and immunomodulator, were randomly assigned to receive either 8 mg/kg or 16 mg/kg daratumumab. After a response evaluation, the 16 mg/kg dose was chosen, and an additional 90 patients were enrolled at this dose.
As noted, the overall response rate, the primary endpoint, was 29.2%, consisting of three stringent complete responses, 10 very good partial responses, and 18 partial responses. The median duration of response was 7.4 months. The responses were consistent across all clinically relevant subgroups, Dr. Usmani said.
The median time to progression was 3.7 months. Median overall survival had not been reached at the time of the last data analysis, and the estimated 1-year overall survival rate was 65%.
At a median follow-up of 9.4 months, 14 of 31 patients with responses remained on therapy.
Patients tolerated the 16 mg/kg dose well, and none discontinued because of drug-related events.
Minor infusion site reactions (grade 1 or 2) were common during the first infusion and were managed with standard therapies, Dr. Usmani said.
“This is the first monoclonal antibody in myeloma that has shown single-agent activity,” Dr. Usmani said.
ASCO expert Dr. Merry-Jennifer Markham from the University of Florida in Gainesville, who was not involved in the study, commented that “there have been substantial treatment advances in multiple myeloma over the last decade, and daratumumab will be an important addition to the list of options. This therapy may offer a glimmer of hope for those myeloma patients who have run out of treatment options.
The study was funded by Janssen Research and Development. Dr. Usmani is on the speaker’s bureau for Janssen Oncology.
AT ASCO 2015
Key clinical point: The investigational monoclonal antibody daratumumab was effective in patients with heavily pretreated multiple myeloma.
Major finding: The overall response rate to daratumumab monotherapy was 29.2%.
Data source: A phase II open-label trial in 108 patients with previously treated or refractory multiple myeloma.
Disclosures: The study was funded by Janssen Research and Development. Dr. Usmani is on the speaker’s bureau for Janssen Oncology.
Drug improves upon standard therapy for relapsed CLL/SLL, speaker says

Chanan-Khan, MD
© ASCO/Zach Boyden-Holmes
CHICAGO—Interim results of the phase 3 HELIOS trial suggest that adding ibrutinib to treatment with bendamustine and rituximab (BR) improves outcomes for patients with relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Patients who received ibrutinib and BR had significantly higher response rates and a significantly longer progression-free survival than patients who received BR with placebo.
There was no significant difference between the arms with regard to overall survival, but the researchers said these results were confounded by the fact that 31% of patients in the placebo arm crossed over to the ibrutinib arm after they progressed.
“We found that ibrutinib can be safely paired with existing therapy to powerfully prolong remissions and improve patients’ well-being,” said study investigator Asher Alban Akmal Chanan-Khan, MD, of the Mayo Clinic in Jacksonville, Florida.
Dr Chanan-Khan presented these findings at the 2015 ASCO Annual Meeting (abstract LBA7005). The research was funded by Janssen Research & Development, LLC, the company co-developing ibrutinib with Pharmacyclics.
The study included 578 patients with previously treated CLL/SLL, excluding those with del(17p). The patients were randomized to receive 6 cycles of BR plus once-daily ibrutinib (n=289) or 6 cycles of BR plus placebo (n=289). Ibrutinib and placebo were given until disease progression or unacceptable toxicity.
Dr Chanan-Khan said baseline characteristics were comparable between the treatment arms. For each arm, the median number of prior treatments was 2, more than 50% of patients had bulky disease, and about 80% of patients had unmutated IGVH.
“[However,] advanced Rai-stage disease was observed in a slightly [greater] proportion of patients in the control arm versus the ibrutinib arm,” Dr Chanan-Khan noted. “Conversely, a higher proportion of patients with del(11q) was noted in the ibrutinib-containing arm.”
Ultimately, 81.9% (n=235) of patients in the ibrutinib arm and 77.4% (n=222) of those in the placebo arm received their assigned 6 cycles of BR. At the time of analysis, the rate of treatment discontinuation was 29.1% (n=84) in the ibrutinib arm and 64.7% in the placebo arm (n=187).
Those patients who progressed on placebo were allowed to cross over to the ibrutinib arm, and 90 patients had crossed over at the time of the interim analysis.
Response and survival
The study’s primary endpoint was progression-free survival, as assessed by an independent review committee (IRC), in the intent-to-treat population (n=289 in each arm).
At a median follow-up of 17 months, progression-free survival was 13.3 months in the placebo arm and was not reached in the ibrutinib arm (P<0.0001).
“The hazard ratio on this particular survival curve is 0.20, which translates into a reduced risk of progression or death by 80%,” Dr Chanan-Khan said. “This is remarkable. You cannot get a better hazard ratio than this.”
Dr Chanan-Khan also noted that the overall response rate was significantly higher in the ibrutinib arm than the placebo arm. The rates were 82.7% and 67.8%, respectively (P<0.0001), according to the IRC, and 86.2% and 68.9%, respectively (P<0.0001), according to investigator assessment.
The rate of complete response plus complete response with incomplete blood count recovery was 10.4% in the ibrutinib arm and 2.8% in the placebo arm, according to the IRC. According to investigator assessment, the rates were 21.4% and 5.9%, respectively.
The median overall survival was not reached in either arm, and the hazard ratio was 0.628 (P=0.0598).
Adverse events
Dr Chanan-Kahn said the safety profile of the ibrutinib-BR combination was consistent with the safety profiles of each individual drug.
The incidence of adverse events was 70.7% in the ibrutinib-BR arm and 70% in the placebo-BR arm. The most common events were neutropenia (58.2% and 54.7%, respectively), nausea (36.9% vs 35.2%), diarrhea (35.5% vs 23.7%), thrombocytopenia (30.7% vs 24.4%), pyrexia (24.7% vs 22%), anemia (22.6% vs 28.9%), and fatigue (21.6% vs 22.6%).
The incidence of grade 3/4 adverse events was 28.9% in the ibrutinib arm and 25% in the placebo arm. The most common of these were neutropenia (53.7% vs 50.5%) and thrombocytopenia (15% in both arms).
Atrial fibrillation was seen in 7.3% of patients in the ibrutinib arm and 2.8% in the placebo arm. Grade 3/4 atrial fibrillation occurred in 2.8% and 0.7% of patients, respectively. The incidence of tumor lysis syndrome was 3.5% in both arms.
The rate of bleeding was 31% in the ibrutinib arm and 14.6% in the placebo arm. And the rates of major hemorrhage were 3.8% and 1.7%, respectively.
Adverse events were the primary reason for discontinuation in patients who received ibrutinib—14.2%, compared to 11.8% of patients who received placebo. The primary reason for discontinuation in the placebo arm was progressive disease or relapse—45%, compared to 4.8% in the ibrutinib arm.
Taken together, the results of this trial suggest treatment with ibrutinib and BR is superior to standard BR therapy in patients with relapsed CLL/SLL, Dr Chanan-Kahn said.
“This was one of the most rigorous clinical trials ever conducted in CLL,” he said, “and it truly validates ibrutinib as an important drug for this cancer.” ![]()

Chanan-Khan, MD
© ASCO/Zach Boyden-Holmes
CHICAGO—Interim results of the phase 3 HELIOS trial suggest that adding ibrutinib to treatment with bendamustine and rituximab (BR) improves outcomes for patients with relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Patients who received ibrutinib and BR had significantly higher response rates and a significantly longer progression-free survival than patients who received BR with placebo.
There was no significant difference between the arms with regard to overall survival, but the researchers said these results were confounded by the fact that 31% of patients in the placebo arm crossed over to the ibrutinib arm after they progressed.
“We found that ibrutinib can be safely paired with existing therapy to powerfully prolong remissions and improve patients’ well-being,” said study investigator Asher Alban Akmal Chanan-Khan, MD, of the Mayo Clinic in Jacksonville, Florida.
Dr Chanan-Khan presented these findings at the 2015 ASCO Annual Meeting (abstract LBA7005). The research was funded by Janssen Research & Development, LLC, the company co-developing ibrutinib with Pharmacyclics.
The study included 578 patients with previously treated CLL/SLL, excluding those with del(17p). The patients were randomized to receive 6 cycles of BR plus once-daily ibrutinib (n=289) or 6 cycles of BR plus placebo (n=289). Ibrutinib and placebo were given until disease progression or unacceptable toxicity.
Dr Chanan-Khan said baseline characteristics were comparable between the treatment arms. For each arm, the median number of prior treatments was 2, more than 50% of patients had bulky disease, and about 80% of patients had unmutated IGVH.
“[However,] advanced Rai-stage disease was observed in a slightly [greater] proportion of patients in the control arm versus the ibrutinib arm,” Dr Chanan-Khan noted. “Conversely, a higher proportion of patients with del(11q) was noted in the ibrutinib-containing arm.”
Ultimately, 81.9% (n=235) of patients in the ibrutinib arm and 77.4% (n=222) of those in the placebo arm received their assigned 6 cycles of BR. At the time of analysis, the rate of treatment discontinuation was 29.1% (n=84) in the ibrutinib arm and 64.7% in the placebo arm (n=187).
Those patients who progressed on placebo were allowed to cross over to the ibrutinib arm, and 90 patients had crossed over at the time of the interim analysis.
Response and survival
The study’s primary endpoint was progression-free survival, as assessed by an independent review committee (IRC), in the intent-to-treat population (n=289 in each arm).
At a median follow-up of 17 months, progression-free survival was 13.3 months in the placebo arm and was not reached in the ibrutinib arm (P<0.0001).
“The hazard ratio on this particular survival curve is 0.20, which translates into a reduced risk of progression or death by 80%,” Dr Chanan-Khan said. “This is remarkable. You cannot get a better hazard ratio than this.”
Dr Chanan-Khan also noted that the overall response rate was significantly higher in the ibrutinib arm than the placebo arm. The rates were 82.7% and 67.8%, respectively (P<0.0001), according to the IRC, and 86.2% and 68.9%, respectively (P<0.0001), according to investigator assessment.
The rate of complete response plus complete response with incomplete blood count recovery was 10.4% in the ibrutinib arm and 2.8% in the placebo arm, according to the IRC. According to investigator assessment, the rates were 21.4% and 5.9%, respectively.
The median overall survival was not reached in either arm, and the hazard ratio was 0.628 (P=0.0598).
Adverse events
Dr Chanan-Kahn said the safety profile of the ibrutinib-BR combination was consistent with the safety profiles of each individual drug.
The incidence of adverse events was 70.7% in the ibrutinib-BR arm and 70% in the placebo-BR arm. The most common events were neutropenia (58.2% and 54.7%, respectively), nausea (36.9% vs 35.2%), diarrhea (35.5% vs 23.7%), thrombocytopenia (30.7% vs 24.4%), pyrexia (24.7% vs 22%), anemia (22.6% vs 28.9%), and fatigue (21.6% vs 22.6%).
The incidence of grade 3/4 adverse events was 28.9% in the ibrutinib arm and 25% in the placebo arm. The most common of these were neutropenia (53.7% vs 50.5%) and thrombocytopenia (15% in both arms).
Atrial fibrillation was seen in 7.3% of patients in the ibrutinib arm and 2.8% in the placebo arm. Grade 3/4 atrial fibrillation occurred in 2.8% and 0.7% of patients, respectively. The incidence of tumor lysis syndrome was 3.5% in both arms.
The rate of bleeding was 31% in the ibrutinib arm and 14.6% in the placebo arm. And the rates of major hemorrhage were 3.8% and 1.7%, respectively.
Adverse events were the primary reason for discontinuation in patients who received ibrutinib—14.2%, compared to 11.8% of patients who received placebo. The primary reason for discontinuation in the placebo arm was progressive disease or relapse—45%, compared to 4.8% in the ibrutinib arm.
Taken together, the results of this trial suggest treatment with ibrutinib and BR is superior to standard BR therapy in patients with relapsed CLL/SLL, Dr Chanan-Kahn said.
“This was one of the most rigorous clinical trials ever conducted in CLL,” he said, “and it truly validates ibrutinib as an important drug for this cancer.” ![]()

Chanan-Khan, MD
© ASCO/Zach Boyden-Holmes
CHICAGO—Interim results of the phase 3 HELIOS trial suggest that adding ibrutinib to treatment with bendamustine and rituximab (BR) improves outcomes for patients with relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Patients who received ibrutinib and BR had significantly higher response rates and a significantly longer progression-free survival than patients who received BR with placebo.
There was no significant difference between the arms with regard to overall survival, but the researchers said these results were confounded by the fact that 31% of patients in the placebo arm crossed over to the ibrutinib arm after they progressed.
“We found that ibrutinib can be safely paired with existing therapy to powerfully prolong remissions and improve patients’ well-being,” said study investigator Asher Alban Akmal Chanan-Khan, MD, of the Mayo Clinic in Jacksonville, Florida.
Dr Chanan-Khan presented these findings at the 2015 ASCO Annual Meeting (abstract LBA7005). The research was funded by Janssen Research & Development, LLC, the company co-developing ibrutinib with Pharmacyclics.
The study included 578 patients with previously treated CLL/SLL, excluding those with del(17p). The patients were randomized to receive 6 cycles of BR plus once-daily ibrutinib (n=289) or 6 cycles of BR plus placebo (n=289). Ibrutinib and placebo were given until disease progression or unacceptable toxicity.
Dr Chanan-Khan said baseline characteristics were comparable between the treatment arms. For each arm, the median number of prior treatments was 2, more than 50% of patients had bulky disease, and about 80% of patients had unmutated IGVH.
“[However,] advanced Rai-stage disease was observed in a slightly [greater] proportion of patients in the control arm versus the ibrutinib arm,” Dr Chanan-Khan noted. “Conversely, a higher proportion of patients with del(11q) was noted in the ibrutinib-containing arm.”
Ultimately, 81.9% (n=235) of patients in the ibrutinib arm and 77.4% (n=222) of those in the placebo arm received their assigned 6 cycles of BR. At the time of analysis, the rate of treatment discontinuation was 29.1% (n=84) in the ibrutinib arm and 64.7% in the placebo arm (n=187).
Those patients who progressed on placebo were allowed to cross over to the ibrutinib arm, and 90 patients had crossed over at the time of the interim analysis.
Response and survival
The study’s primary endpoint was progression-free survival, as assessed by an independent review committee (IRC), in the intent-to-treat population (n=289 in each arm).
At a median follow-up of 17 months, progression-free survival was 13.3 months in the placebo arm and was not reached in the ibrutinib arm (P<0.0001).
“The hazard ratio on this particular survival curve is 0.20, which translates into a reduced risk of progression or death by 80%,” Dr Chanan-Khan said. “This is remarkable. You cannot get a better hazard ratio than this.”
Dr Chanan-Khan also noted that the overall response rate was significantly higher in the ibrutinib arm than the placebo arm. The rates were 82.7% and 67.8%, respectively (P<0.0001), according to the IRC, and 86.2% and 68.9%, respectively (P<0.0001), according to investigator assessment.
The rate of complete response plus complete response with incomplete blood count recovery was 10.4% in the ibrutinib arm and 2.8% in the placebo arm, according to the IRC. According to investigator assessment, the rates were 21.4% and 5.9%, respectively.
The median overall survival was not reached in either arm, and the hazard ratio was 0.628 (P=0.0598).
Adverse events
Dr Chanan-Kahn said the safety profile of the ibrutinib-BR combination was consistent with the safety profiles of each individual drug.
The incidence of adverse events was 70.7% in the ibrutinib-BR arm and 70% in the placebo-BR arm. The most common events were neutropenia (58.2% and 54.7%, respectively), nausea (36.9% vs 35.2%), diarrhea (35.5% vs 23.7%), thrombocytopenia (30.7% vs 24.4%), pyrexia (24.7% vs 22%), anemia (22.6% vs 28.9%), and fatigue (21.6% vs 22.6%).
The incidence of grade 3/4 adverse events was 28.9% in the ibrutinib arm and 25% in the placebo arm. The most common of these were neutropenia (53.7% vs 50.5%) and thrombocytopenia (15% in both arms).
Atrial fibrillation was seen in 7.3% of patients in the ibrutinib arm and 2.8% in the placebo arm. Grade 3/4 atrial fibrillation occurred in 2.8% and 0.7% of patients, respectively. The incidence of tumor lysis syndrome was 3.5% in both arms.
The rate of bleeding was 31% in the ibrutinib arm and 14.6% in the placebo arm. And the rates of major hemorrhage were 3.8% and 1.7%, respectively.
Adverse events were the primary reason for discontinuation in patients who received ibrutinib—14.2%, compared to 11.8% of patients who received placebo. The primary reason for discontinuation in the placebo arm was progressive disease or relapse—45%, compared to 4.8% in the ibrutinib arm.
Taken together, the results of this trial suggest treatment with ibrutinib and BR is superior to standard BR therapy in patients with relapsed CLL/SLL, Dr Chanan-Kahn said.
“This was one of the most rigorous clinical trials ever conducted in CLL,” he said, “and it truly validates ibrutinib as an important drug for this cancer.” ![]()
GADOLIN: Combination improves PFS in rituximab-refractory indolent NHL
CHICAGO – Progression-free survival for patients with rituximab-refractory indolent non-Hodgkin’s lymphoma was effectively doubled with a combination of obinutuzumab and bendamustine, compared with bendamustine alone.
In a randomized trial halted early because of the evident superiority of the combination over bendamustine monotherapy, at a median follow-up of 20 months the median progression-free survival as assessed by an independent radiology facility had not been reached for patients treated with obinutuzumab (Gazyva) and bendamustine, vs. 14.9 months for bendamustine alone. As assessed by the investigators, the median PFS rates were 29 months and 14 months, respectively, reported Dr. Laurie Helen Sehn, a medical oncologist at the British Columbia (Canada) Cancer Agency in Vancouver.
“The combination of obinutuzumab [and bendamustine], followed by obinutuzumab maintenance, resulted in a statistically significant, but more importantly, a clinically meaningful increase in progression-free survival compared with bendamustine alone,” Dr. Sehn said at a briefing at the annual meeting of the American Society of Clinical Oncology.
“This study is remarkable, because it does demonstrate the first randomized evidence of a clinical benefit of a novel anti-CD20 monoclonal antibody for patients who are rituximab refractory,” she added.
The advent of the anti-CD20 antibody rituximab in the later 1990s transformed treatment of hematologic malignancies, including indolent non-Hodgkin’s lymphomas. However, some patients have disease that is resistant to rituximab or recurs after rituximab therapy, and for these patients treatment options are limited.
Bendamustine (Treanda) has been shown to be effective in patients with rituximab-refractory indolent NHL, but with remission durations of only 7-9 months.
Obinutuzumab is a glycoengineered anti-CD20 antibody that ASCO expert Dr. Merry-Jennifer Markham from the University of Florida in Gainesville called “a super-rituximab.” It has been shown in preclinical studies to have activity against malignancies when combined with bendamustine.
In the GADOLIN trial, 413 patients with rituximab-refractory indolent NHL histologies were randomly assigned to receive either obinutuzumab 1,000 mg on days 1, 8 and 15 of the first 28-day cycle and then on day 1 of subsequent cycles plus bendamustine 90 mg/m2 per day on days 1 and 2 of the first six cycles, or only bendamustine 120 mg/m2 per day on the same schedule.
The NHL subtypes treated included follicular lymphomas, marginal zone lymphomas and small lymphocytic lymphomas.
For patients assigned to obinutuzumab, treatment continued until a determination of complete or partial response or stable disease, and were then continued on obinutuzumab maintenance, at a dose of 1,000 mg every 2 months for up to 2 years, or until disease progression.
Median follow-up was 23 months for the combination arm, and 20 months in the bendamustine only arm.
At the first planned interim analysis, the median PFS by independent radiology review, the primary endpoint, was not reached among patients in the combination, compared with 14.9 months for bendamustine. This translated into a hazard ratio of 0.55 (P = .0001), and prompted the data safety monitoring committee to recommend ending the trial.
The safety analysis showed that the overall rates of adverse events, serious adverse events, grade 3 or great events, deaths and withdrawals were similar between the two trial arms, Dr. Sehn said.
“The safety profile revealed no new safety finding and was in keeping with what was expected with the combination of drugs,” Dr. Sehn said.
“The fact that these responses and the progression-free survival responses were so robust in a population that has already received an anti-CD20 agent is remarkable, and I think this really does open up the therapies that will lead to substantial time for these patients, who are all incurable,” Dr. Markham said in an interview. She was not involved in the GADOLIN trial.
CHICAGO – Progression-free survival for patients with rituximab-refractory indolent non-Hodgkin’s lymphoma was effectively doubled with a combination of obinutuzumab and bendamustine, compared with bendamustine alone.
In a randomized trial halted early because of the evident superiority of the combination over bendamustine monotherapy, at a median follow-up of 20 months the median progression-free survival as assessed by an independent radiology facility had not been reached for patients treated with obinutuzumab (Gazyva) and bendamustine, vs. 14.9 months for bendamustine alone. As assessed by the investigators, the median PFS rates were 29 months and 14 months, respectively, reported Dr. Laurie Helen Sehn, a medical oncologist at the British Columbia (Canada) Cancer Agency in Vancouver.
“The combination of obinutuzumab [and bendamustine], followed by obinutuzumab maintenance, resulted in a statistically significant, but more importantly, a clinically meaningful increase in progression-free survival compared with bendamustine alone,” Dr. Sehn said at a briefing at the annual meeting of the American Society of Clinical Oncology.
“This study is remarkable, because it does demonstrate the first randomized evidence of a clinical benefit of a novel anti-CD20 monoclonal antibody for patients who are rituximab refractory,” she added.
The advent of the anti-CD20 antibody rituximab in the later 1990s transformed treatment of hematologic malignancies, including indolent non-Hodgkin’s lymphomas. However, some patients have disease that is resistant to rituximab or recurs after rituximab therapy, and for these patients treatment options are limited.
Bendamustine (Treanda) has been shown to be effective in patients with rituximab-refractory indolent NHL, but with remission durations of only 7-9 months.
Obinutuzumab is a glycoengineered anti-CD20 antibody that ASCO expert Dr. Merry-Jennifer Markham from the University of Florida in Gainesville called “a super-rituximab.” It has been shown in preclinical studies to have activity against malignancies when combined with bendamustine.
In the GADOLIN trial, 413 patients with rituximab-refractory indolent NHL histologies were randomly assigned to receive either obinutuzumab 1,000 mg on days 1, 8 and 15 of the first 28-day cycle and then on day 1 of subsequent cycles plus bendamustine 90 mg/m2 per day on days 1 and 2 of the first six cycles, or only bendamustine 120 mg/m2 per day on the same schedule.
The NHL subtypes treated included follicular lymphomas, marginal zone lymphomas and small lymphocytic lymphomas.
For patients assigned to obinutuzumab, treatment continued until a determination of complete or partial response or stable disease, and were then continued on obinutuzumab maintenance, at a dose of 1,000 mg every 2 months for up to 2 years, or until disease progression.
Median follow-up was 23 months for the combination arm, and 20 months in the bendamustine only arm.
At the first planned interim analysis, the median PFS by independent radiology review, the primary endpoint, was not reached among patients in the combination, compared with 14.9 months for bendamustine. This translated into a hazard ratio of 0.55 (P = .0001), and prompted the data safety monitoring committee to recommend ending the trial.
The safety analysis showed that the overall rates of adverse events, serious adverse events, grade 3 or great events, deaths and withdrawals were similar between the two trial arms, Dr. Sehn said.
“The safety profile revealed no new safety finding and was in keeping with what was expected with the combination of drugs,” Dr. Sehn said.
“The fact that these responses and the progression-free survival responses were so robust in a population that has already received an anti-CD20 agent is remarkable, and I think this really does open up the therapies that will lead to substantial time for these patients, who are all incurable,” Dr. Markham said in an interview. She was not involved in the GADOLIN trial.
CHICAGO – Progression-free survival for patients with rituximab-refractory indolent non-Hodgkin’s lymphoma was effectively doubled with a combination of obinutuzumab and bendamustine, compared with bendamustine alone.
In a randomized trial halted early because of the evident superiority of the combination over bendamustine monotherapy, at a median follow-up of 20 months the median progression-free survival as assessed by an independent radiology facility had not been reached for patients treated with obinutuzumab (Gazyva) and bendamustine, vs. 14.9 months for bendamustine alone. As assessed by the investigators, the median PFS rates were 29 months and 14 months, respectively, reported Dr. Laurie Helen Sehn, a medical oncologist at the British Columbia (Canada) Cancer Agency in Vancouver.
“The combination of obinutuzumab [and bendamustine], followed by obinutuzumab maintenance, resulted in a statistically significant, but more importantly, a clinically meaningful increase in progression-free survival compared with bendamustine alone,” Dr. Sehn said at a briefing at the annual meeting of the American Society of Clinical Oncology.
“This study is remarkable, because it does demonstrate the first randomized evidence of a clinical benefit of a novel anti-CD20 monoclonal antibody for patients who are rituximab refractory,” she added.
The advent of the anti-CD20 antibody rituximab in the later 1990s transformed treatment of hematologic malignancies, including indolent non-Hodgkin’s lymphomas. However, some patients have disease that is resistant to rituximab or recurs after rituximab therapy, and for these patients treatment options are limited.
Bendamustine (Treanda) has been shown to be effective in patients with rituximab-refractory indolent NHL, but with remission durations of only 7-9 months.
Obinutuzumab is a glycoengineered anti-CD20 antibody that ASCO expert Dr. Merry-Jennifer Markham from the University of Florida in Gainesville called “a super-rituximab.” It has been shown in preclinical studies to have activity against malignancies when combined with bendamustine.
In the GADOLIN trial, 413 patients with rituximab-refractory indolent NHL histologies were randomly assigned to receive either obinutuzumab 1,000 mg on days 1, 8 and 15 of the first 28-day cycle and then on day 1 of subsequent cycles plus bendamustine 90 mg/m2 per day on days 1 and 2 of the first six cycles, or only bendamustine 120 mg/m2 per day on the same schedule.
The NHL subtypes treated included follicular lymphomas, marginal zone lymphomas and small lymphocytic lymphomas.
For patients assigned to obinutuzumab, treatment continued until a determination of complete or partial response or stable disease, and were then continued on obinutuzumab maintenance, at a dose of 1,000 mg every 2 months for up to 2 years, or until disease progression.
Median follow-up was 23 months for the combination arm, and 20 months in the bendamustine only arm.
At the first planned interim analysis, the median PFS by independent radiology review, the primary endpoint, was not reached among patients in the combination, compared with 14.9 months for bendamustine. This translated into a hazard ratio of 0.55 (P = .0001), and prompted the data safety monitoring committee to recommend ending the trial.
The safety analysis showed that the overall rates of adverse events, serious adverse events, grade 3 or great events, deaths and withdrawals were similar between the two trial arms, Dr. Sehn said.
“The safety profile revealed no new safety finding and was in keeping with what was expected with the combination of drugs,” Dr. Sehn said.
“The fact that these responses and the progression-free survival responses were so robust in a population that has already received an anti-CD20 agent is remarkable, and I think this really does open up the therapies that will lead to substantial time for these patients, who are all incurable,” Dr. Markham said in an interview. She was not involved in the GADOLIN trial.
AT ASCO 2015
Key clinical point: Adding obinutuzumab to bendamustine extended progression-free survival in patients with rituximab-refractory indolent non-Hodgkin’s lymphoma.
Major finding: The hazard ratio for progression-free survival was 0.55 with the combination compared with bendamustine monotherapy.
Data source: Randomized controlled trial in 413 patients with rituximab-refractory indolent NHL.
Disclosures: The study was supported by Genetech and F. Hoffman-La Roche, Ltd. Dr. Sehn has received honoraria and research funding and has served as an advisor to the companies.
Hodgkin lymphoma incidence on the decline worldwide

Photo courtesy of NIH
In trying to estimate the global cancer burden, researchers found that cases of Hodgkin lymphoma (HL) have decreased worldwide over the last 2 decades.
The team studied 28 cancer types in 188 countries, and HL was the only malignancy whose incidence decreased from 1990 to 2013.
And the number of HL deaths in 2013 was comparatively low. When the researchers ranked cancers according to the number of global deaths, HL was 26th on the list of 28.
The researchers disclosed these results in JAMA Oncology.
The team collected data from cancer registries, vital records, verbal autopsy reports, and other sources to estimate the global cancer burden.
The data suggested that, in 2013, there were 14.9 million new cancer cases and 8.2 million cancer deaths worldwide. The proportion of cancer deaths as part of all deaths increased from 12% in 1990 to 15% in 2013.
The most common malignancy in men was prostate cancer, with 1.4 million cases in 2013. For women, it was breast cancer, with 1.8 million cases in 2013.
Tracheal, bronchus, and lung cancers were the leading cause of cancer death in men and women, with 1.6 million deaths in 2013.
Hematologic malignancies
Globally, the age-standardized incidence of HL per 100,000 people decreased by 34% during the time period studied. Cases of HL fell from about 103,000 in 1990 to 93,000 in 2013.
When the researchers ranked cancer types according to the number of global deaths in 2013, HL came in 26th. There were about 24,000 HL deaths in 2013—14,000 among men and 10,000 among women.
Non-Hodgkin lymphoma (NHL) came in 11th for global cancer deaths in 2013. There were about 226,000 NHL deaths—133,000 among men and 92,000 among women.
In addition, the incidence of NHL more than doubled from 1990 to 2013, rising from about 227,000 to 465,000. According to 2013 data, 1 in 103 men and 1 in 151 women developed NHL between birth and 79 years of age.
The researchers observed an increase in cases of multiple myeloma (MM) as well, from about 63,000 in 1990 to 117,000 in 2013.
In 2013, there were about 79,000 MM deaths—42,000 among men and 37,000 among women. MM ranked 19th on the list of global cancer deaths in 2013.
Leukemia ranked 9th on the list. There were about 265,000 leukemia deaths in 2013—149,000 among men and 116,000 among women.
Cases of leukemia increased from 297,000 in 1990 to 414,000 in 2013. According to 2013 data, 1 in 127 men and 1 in 203 women developed leukemia between birth and 79 years of age.
This research shows that cancer remains a major threat to people’s health around the world, said study author Christina Fitzmaurice, MD, of the University of Washington in Seattle.
“Cancer prevention, screening, and treatment programs are costly,” she noted, “and it is very important for countries to know which cancers cause the highest disease burden in order to allocate scarce resources appropriately.” ![]()

Photo courtesy of NIH
In trying to estimate the global cancer burden, researchers found that cases of Hodgkin lymphoma (HL) have decreased worldwide over the last 2 decades.
The team studied 28 cancer types in 188 countries, and HL was the only malignancy whose incidence decreased from 1990 to 2013.
And the number of HL deaths in 2013 was comparatively low. When the researchers ranked cancers according to the number of global deaths, HL was 26th on the list of 28.
The researchers disclosed these results in JAMA Oncology.
The team collected data from cancer registries, vital records, verbal autopsy reports, and other sources to estimate the global cancer burden.
The data suggested that, in 2013, there were 14.9 million new cancer cases and 8.2 million cancer deaths worldwide. The proportion of cancer deaths as part of all deaths increased from 12% in 1990 to 15% in 2013.
The most common malignancy in men was prostate cancer, with 1.4 million cases in 2013. For women, it was breast cancer, with 1.8 million cases in 2013.
Tracheal, bronchus, and lung cancers were the leading cause of cancer death in men and women, with 1.6 million deaths in 2013.
Hematologic malignancies
Globally, the age-standardized incidence of HL per 100,000 people decreased by 34% during the time period studied. Cases of HL fell from about 103,000 in 1990 to 93,000 in 2013.
When the researchers ranked cancer types according to the number of global deaths in 2013, HL came in 26th. There were about 24,000 HL deaths in 2013—14,000 among men and 10,000 among women.
Non-Hodgkin lymphoma (NHL) came in 11th for global cancer deaths in 2013. There were about 226,000 NHL deaths—133,000 among men and 92,000 among women.
In addition, the incidence of NHL more than doubled from 1990 to 2013, rising from about 227,000 to 465,000. According to 2013 data, 1 in 103 men and 1 in 151 women developed NHL between birth and 79 years of age.
The researchers observed an increase in cases of multiple myeloma (MM) as well, from about 63,000 in 1990 to 117,000 in 2013.
In 2013, there were about 79,000 MM deaths—42,000 among men and 37,000 among women. MM ranked 19th on the list of global cancer deaths in 2013.
Leukemia ranked 9th on the list. There were about 265,000 leukemia deaths in 2013—149,000 among men and 116,000 among women.
Cases of leukemia increased from 297,000 in 1990 to 414,000 in 2013. According to 2013 data, 1 in 127 men and 1 in 203 women developed leukemia between birth and 79 years of age.
This research shows that cancer remains a major threat to people’s health around the world, said study author Christina Fitzmaurice, MD, of the University of Washington in Seattle.
“Cancer prevention, screening, and treatment programs are costly,” she noted, “and it is very important for countries to know which cancers cause the highest disease burden in order to allocate scarce resources appropriately.” ![]()

Photo courtesy of NIH
In trying to estimate the global cancer burden, researchers found that cases of Hodgkin lymphoma (HL) have decreased worldwide over the last 2 decades.
The team studied 28 cancer types in 188 countries, and HL was the only malignancy whose incidence decreased from 1990 to 2013.
And the number of HL deaths in 2013 was comparatively low. When the researchers ranked cancers according to the number of global deaths, HL was 26th on the list of 28.
The researchers disclosed these results in JAMA Oncology.
The team collected data from cancer registries, vital records, verbal autopsy reports, and other sources to estimate the global cancer burden.
The data suggested that, in 2013, there were 14.9 million new cancer cases and 8.2 million cancer deaths worldwide. The proportion of cancer deaths as part of all deaths increased from 12% in 1990 to 15% in 2013.
The most common malignancy in men was prostate cancer, with 1.4 million cases in 2013. For women, it was breast cancer, with 1.8 million cases in 2013.
Tracheal, bronchus, and lung cancers were the leading cause of cancer death in men and women, with 1.6 million deaths in 2013.
Hematologic malignancies
Globally, the age-standardized incidence of HL per 100,000 people decreased by 34% during the time period studied. Cases of HL fell from about 103,000 in 1990 to 93,000 in 2013.
When the researchers ranked cancer types according to the number of global deaths in 2013, HL came in 26th. There were about 24,000 HL deaths in 2013—14,000 among men and 10,000 among women.
Non-Hodgkin lymphoma (NHL) came in 11th for global cancer deaths in 2013. There were about 226,000 NHL deaths—133,000 among men and 92,000 among women.
In addition, the incidence of NHL more than doubled from 1990 to 2013, rising from about 227,000 to 465,000. According to 2013 data, 1 in 103 men and 1 in 151 women developed NHL between birth and 79 years of age.
The researchers observed an increase in cases of multiple myeloma (MM) as well, from about 63,000 in 1990 to 117,000 in 2013.
In 2013, there were about 79,000 MM deaths—42,000 among men and 37,000 among women. MM ranked 19th on the list of global cancer deaths in 2013.
Leukemia ranked 9th on the list. There were about 265,000 leukemia deaths in 2013—149,000 among men and 116,000 among women.
Cases of leukemia increased from 297,000 in 1990 to 414,000 in 2013. According to 2013 data, 1 in 127 men and 1 in 203 women developed leukemia between birth and 79 years of age.
This research shows that cancer remains a major threat to people’s health around the world, said study author Christina Fitzmaurice, MD, of the University of Washington in Seattle.
“Cancer prevention, screening, and treatment programs are costly,” she noted, “and it is very important for countries to know which cancers cause the highest disease burden in order to allocate scarce resources appropriately.” ![]()
CAR T-cell therapy appears feasible in HL

LONDON—Results of a small, phase 1 trial suggest CD30-directed chimeric antigen receptor (CAR) T-cell therapy is feasible in patients with aggressive Hodgkin lymphoma (HL).
The trial included 7 patients with relapsed or refractory HL.
Five of the patients achieved stable disease or better after infusions of CAR T cells, and the researchers said treatment-related adverse events were manageable.
William (Wei) Cao, PhD, of Cellular Biomedicine Group, presented these results at the 10th Annual World Stem Cells & Regenerative Medicine Congress.
The research was funded by Cellular Biomedicine Group, the company developing the CAR T-cell therapy (known as CBM-C30.1), as well as by grants from the National Natural Science Foundation of China and the National Basic Science and Development Program of China.
The trial included 7 patients with progressive HL. Two patients had stage III disease, and 5 had stage IV. The patients had a median of 16 prior treatments (range, 8-24) and limited prognosis (several months to less than 2-year survival) with currently available therapies.
The patients received escalating doses of autologous T cells transduced with a CD30-directed CAR moiety for 3 to 5 days, following a conditioning regimen. The researchers measured the level of CAR transgenes in peripheral blood and biopsied tumor tissues by quantitative PCR.
Two patients achieved a partial response to CAR T-cell therapy, and 3 attained stable disease. So the therapy resulted in an overall disease control rate of 71.4% (5/7) and an objective response rate of 28.6% (2/7).
Stable disease lasted 2 months in 2 of the patients and more than 3.5 months in the third patient. Partial response lasted more than 2 months in 1 patient and more than 3.5 months in the other.
Dr Cao said adverse events consisted largely of fever and were manageable with medical intervention. One patient experienced 5-day self-limiting arthralgia, myalgia, and dual knee swelling 2 weeks after cell infusion. There were no delayed or severe adverse events.
“We are very encouraged by the efficacy and toxicity profile of our CAR-T CD30 technology,” Dr Cao said, “given that the [patients] were diagnosed with stage III and IV Hodgkin’s lymphoma.” ![]()

LONDON—Results of a small, phase 1 trial suggest CD30-directed chimeric antigen receptor (CAR) T-cell therapy is feasible in patients with aggressive Hodgkin lymphoma (HL).
The trial included 7 patients with relapsed or refractory HL.
Five of the patients achieved stable disease or better after infusions of CAR T cells, and the researchers said treatment-related adverse events were manageable.
William (Wei) Cao, PhD, of Cellular Biomedicine Group, presented these results at the 10th Annual World Stem Cells & Regenerative Medicine Congress.
The research was funded by Cellular Biomedicine Group, the company developing the CAR T-cell therapy (known as CBM-C30.1), as well as by grants from the National Natural Science Foundation of China and the National Basic Science and Development Program of China.
The trial included 7 patients with progressive HL. Two patients had stage III disease, and 5 had stage IV. The patients had a median of 16 prior treatments (range, 8-24) and limited prognosis (several months to less than 2-year survival) with currently available therapies.
The patients received escalating doses of autologous T cells transduced with a CD30-directed CAR moiety for 3 to 5 days, following a conditioning regimen. The researchers measured the level of CAR transgenes in peripheral blood and biopsied tumor tissues by quantitative PCR.
Two patients achieved a partial response to CAR T-cell therapy, and 3 attained stable disease. So the therapy resulted in an overall disease control rate of 71.4% (5/7) and an objective response rate of 28.6% (2/7).
Stable disease lasted 2 months in 2 of the patients and more than 3.5 months in the third patient. Partial response lasted more than 2 months in 1 patient and more than 3.5 months in the other.
Dr Cao said adverse events consisted largely of fever and were manageable with medical intervention. One patient experienced 5-day self-limiting arthralgia, myalgia, and dual knee swelling 2 weeks after cell infusion. There were no delayed or severe adverse events.
“We are very encouraged by the efficacy and toxicity profile of our CAR-T CD30 technology,” Dr Cao said, “given that the [patients] were diagnosed with stage III and IV Hodgkin’s lymphoma.” ![]()

LONDON—Results of a small, phase 1 trial suggest CD30-directed chimeric antigen receptor (CAR) T-cell therapy is feasible in patients with aggressive Hodgkin lymphoma (HL).
The trial included 7 patients with relapsed or refractory HL.
Five of the patients achieved stable disease or better after infusions of CAR T cells, and the researchers said treatment-related adverse events were manageable.
William (Wei) Cao, PhD, of Cellular Biomedicine Group, presented these results at the 10th Annual World Stem Cells & Regenerative Medicine Congress.
The research was funded by Cellular Biomedicine Group, the company developing the CAR T-cell therapy (known as CBM-C30.1), as well as by grants from the National Natural Science Foundation of China and the National Basic Science and Development Program of China.
The trial included 7 patients with progressive HL. Two patients had stage III disease, and 5 had stage IV. The patients had a median of 16 prior treatments (range, 8-24) and limited prognosis (several months to less than 2-year survival) with currently available therapies.
The patients received escalating doses of autologous T cells transduced with a CD30-directed CAR moiety for 3 to 5 days, following a conditioning regimen. The researchers measured the level of CAR transgenes in peripheral blood and biopsied tumor tissues by quantitative PCR.
Two patients achieved a partial response to CAR T-cell therapy, and 3 attained stable disease. So the therapy resulted in an overall disease control rate of 71.4% (5/7) and an objective response rate of 28.6% (2/7).
Stable disease lasted 2 months in 2 of the patients and more than 3.5 months in the third patient. Partial response lasted more than 2 months in 1 patient and more than 3.5 months in the other.
Dr Cao said adverse events consisted largely of fever and were manageable with medical intervention. One patient experienced 5-day self-limiting arthralgia, myalgia, and dual knee swelling 2 weeks after cell infusion. There were no delayed or severe adverse events.
“We are very encouraged by the efficacy and toxicity profile of our CAR-T CD30 technology,” Dr Cao said, “given that the [patients] were diagnosed with stage III and IV Hodgkin’s lymphoma.” ![]()
Mantle Cell Lymphoma: An Evolving Therapeutic Landscape
Mantle cell lymphoma (MCL) is an uncommon B-cell non-Hodgkin lymphoma (NHL) characterized by the translocation, t(11;14), that results in aberrant expression of cyclin D1.1 The clinical presentation varies significantly from asymptomatic to rapidly enlarging lymph nodes, necessitating immediate treatment. Treatment approaches to newly diagnosed MCL correspondingly vary to match the clinical presentation, but they also reflect the bias of individual providers. No treatment is curative, so different treatment philosophies heavily influence management strategies. Mantle cell lymphoma was first described in the 1990s as a unique pathobiologic entity, and it is only now developing its own set of treatment principles distinct from other lymphomas.2 As novel targeted therapies become available, fundamental questions regarding the best treatment approach are certain to evolve.
Traditional Intensive Treatment
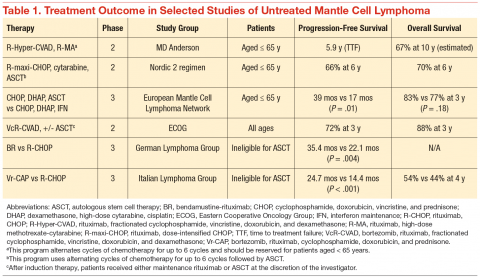
The clinical challenge of treating patients with MCL centers on its propensity to relapse quickly after initial therapy. Although most patients will respond to the initial therapy, the duration of their remissions are disappointingly short. The R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) regimens can induce a complete response in the majority of patients, but they invariably relapse within 18 months of finishing therapy. Recognition of this problem led investigators to test highly intensive regimens designed to maximize the depth of remission often followed by autologous stem cell transplant. The highly intensive regimens are successful at extending remission durations to > 5 years in most cases, but at the cost of significant myelotoxicity and a nontrivial risk of death during induction therapy (Table 1).
In a subset analysis of a hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) study in MCL, it was observed that patients aged < 65 years had a better survival rate than did older patients.3 Accordingly, these regimens are largely restricted to patients aged < 65 years, which is only a minority of patients with MCL. Importantly, despite initial enthusiasm for the impressive rates of remission, most data suggest that these regimens are not curative.
Nonintensive Treatment
Until recently, patients aged > 65 years or those with significant comorbidities did not have effective treatment options that resulted in durable remissions. Most patients were treated with R-CHOP therapy, and the median survival was only 2 to 3 years.4 Interferon maintenance was frequently used but was largely ineffective at prolongation of remission. Treatment patterns changed quickly after the preliminary results of the randomized StiL (Study Group Indolent Lymphomas) study demonstrated that bendamustine-rituximab (BR) could induce durable remissions with less toxicity than that of R-CHOP therapy (Table 1).5 The superiority of BR over R-CHOP at inducing remissions in MCL was subsequently confirmed in the U.S.-led BRIGHT study; this regimen has become a popular regimen for patients aged > 65 years.6 Despite improved remission rate with less toxicity, however, the BR induction regimen still does not adequately address the problem of short durations of remission.
In a phase 3 study, the European MCL Network demonstrated that the use of maintenance rituximab after induction therapy prolonged both progression-free survival (PFS) as well as overall survival (when given after R-CHOP) compared with interferon maintenance.7 Many providers have extrapolated the benefits seen with rituximab maintenance after R-CHOP to all induction regimens, and rituximab maintenance is increasingly offered to older patients after their induction therapy. An important detail of this study is that rituximab was given indefinitely during maintenance and was not capped at 2 years, as often is done for maintenance treatment in other indolent lymphomas.
A landmark randomized study was recently published that demonstrated an advantage of substituting bortezomib for vincristine in the initial regimen.8 In a phase 3 study of 487 patients, the Vr-CAP (bortezomib in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone) regimen demonstrated an improvement in median PFS over R-CHOP after 40 months of followup (24.7 months vs 14.4 months; hazard radio [HR] 0.63; P < .001) (Table 1). Of particular note, however, is that for patients who achieved a complete remission, the median duration of remission was significantly longer than that of R-CHOP (42.1 months vs 18.0 months; HR 0.63; P < .001). These data suggest that not only is Vr-CAP superior to R-CHOP as a whole, but also that some patients are particularly sensitive to bortezomib, resulting in durable remissions.
Novel Targeted Agents
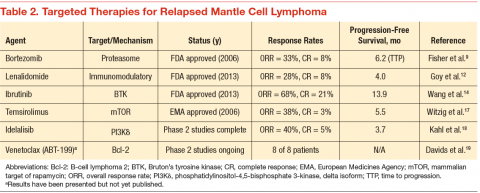
Since 2006, 3 novel targeted agents have been approved for use in relapsed MCL, and many others are currently in clinical trials (Table 2). Bortezomib is approved for use in both the relapsed and upfront setting and demonstrates clear activity in MCL; however, no biomarker currently exists to help understand which patients will respond. Furthermore, most responses to single agents are partial and short-lived.9
Lenalidomide is an oral immunomodulatory agent that works in NHL through a variety of direct and indirect mechanisms.10 As a single agent, lenalidomide has shown activity in a number of studies and can be combined with rituximab without an apparent increase in the toxicity profile.11-13 The EMERGE trial tested lenalidomide as a single agent at 25 mg (every 21 days out of a 28-day cycle) in patients who had previously been treated with bortezomib. In 128 patients, the overall response rate (RR) was 28% with a complete RR of 7.5%. Although these numbers are modest, long-term data from the NHL-003 trial show a subset of patients with durations of remissions nearing 4 years.11 Thus, a biomarker for lenalidomide that predicts response would be of great
clinical utility.
Perhaps of even greater interest have been the clinical results seen with the use of the oral Bruton’s tyrosine kinase inhibitor, ibrutinib. In 111 patients with relapsed MCL treated with ibrutinib 560 mg, the overall RR was 68% with a complete RR of 21%.14 Most patients tolerated therapy very well, and the duration of remission was estimated at 17.5 months. This was a very impressive result with an agent that is tolerable by most patients with MCL.
Conclusions and Future Directions
Mantle cell lymphoma remains a clinical challenge to many providers due to the heterogeneity in the clinical presentation and the lack of consensus regarding the optimal management strategy. The most commonly recommended approach remains to offer highly intensive chemotherapy programs to patients aged < 65 years, but the introduction of novel active agents into the treatment paradigm is beginning to challenge the assumption that all patients need aggressive therapy.
Future research directions should include predictive biomarkers to enhance treatment decisions. Researchers also should begin to understand the toxicity profile and efficacy of the novel targeted agents when used in combination. Early, informative reports are available regarding ibrutinib when added to both rituximab and BR.15,16 Important research questions will include the long-term effects of extended duration therapy (maintenance) paradigms as well as the introduction of novel molecular monitoring methods, such as circulating tumor DNA and circulating tumor cells, to help guide clinical decisions. In a disease with a reportedly dismal outcome, the near future holds much promise as the MCL treatment landscape evolves at a rapid pace.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26-38.
2. Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991;78(2):259-263.
3. Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013-7023.
4. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992.
5. Rummel MJ, Niederle N, Maschmeyer G, et al; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210.
6. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustinerituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952.
7. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
8. Ro bak T, Huang H, Jin J, et al; LYM-3002 Investigators. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944-953.
9. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867-4874.
10 . Kritharis A, Coyle M, Sharma J, Evens AM. Lenalidomide in non-Hodgkin lymphoma: biologic perspectives and therapeutic opportunities [published online ahead of print March 3, 2015]. Blood. pii: blood-2014-11-567792.
11 . Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892-2897.
12 . Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) Study [published online ahead of print September 3, 2013]. J Clin Oncol. doi: 10.1200/JCO.2013.49.2835.
13 . Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab-resistance in patients with indolent B-cell and mantle cell lymphomas [published online ahead of print January 28, 2015]. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-14-2221.
14 . Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516.
15 . Wang ML, Hagemeister F, Westin JR, et al. Ibrutinib and rituximab are an efficacious and safe combination in relapsed mantle cell lymphoma: preliminary results from a phase II clinical trial. Blood. 2014;124(21):627.
16 . Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125(2):242-248.
17 . Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347-5356.
18 . Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3K inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398-3405.
19. Davids MS, Roberts AW, Anderson MA, et al. The BCL-2-specific BH3-mimetic ABT-199 (GDC-0199) is active and well-tolerated in patients with relapsed nonhodgkin lymphoma: interim results of a phase I study. Blood. 2012;120(21): Abstract 304.
Mantle cell lymphoma (MCL) is an uncommon B-cell non-Hodgkin lymphoma (NHL) characterized by the translocation, t(11;14), that results in aberrant expression of cyclin D1.1 The clinical presentation varies significantly from asymptomatic to rapidly enlarging lymph nodes, necessitating immediate treatment. Treatment approaches to newly diagnosed MCL correspondingly vary to match the clinical presentation, but they also reflect the bias of individual providers. No treatment is curative, so different treatment philosophies heavily influence management strategies. Mantle cell lymphoma was first described in the 1990s as a unique pathobiologic entity, and it is only now developing its own set of treatment principles distinct from other lymphomas.2 As novel targeted therapies become available, fundamental questions regarding the best treatment approach are certain to evolve.
Traditional Intensive Treatment
The clinical challenge of treating patients with MCL centers on its propensity to relapse quickly after initial therapy. Although most patients will respond to the initial therapy, the duration of their remissions are disappointingly short. The R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) regimens can induce a complete response in the majority of patients, but they invariably relapse within 18 months of finishing therapy. Recognition of this problem led investigators to test highly intensive regimens designed to maximize the depth of remission often followed by autologous stem cell transplant. The highly intensive regimens are successful at extending remission durations to > 5 years in most cases, but at the cost of significant myelotoxicity and a nontrivial risk of death during induction therapy (Table 1).
In a subset analysis of a hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) study in MCL, it was observed that patients aged < 65 years had a better survival rate than did older patients.3 Accordingly, these regimens are largely restricted to patients aged < 65 years, which is only a minority of patients with MCL. Importantly, despite initial enthusiasm for the impressive rates of remission, most data suggest that these regimens are not curative.
Nonintensive Treatment
Until recently, patients aged > 65 years or those with significant comorbidities did not have effective treatment options that resulted in durable remissions. Most patients were treated with R-CHOP therapy, and the median survival was only 2 to 3 years.4 Interferon maintenance was frequently used but was largely ineffective at prolongation of remission. Treatment patterns changed quickly after the preliminary results of the randomized StiL (Study Group Indolent Lymphomas) study demonstrated that bendamustine-rituximab (BR) could induce durable remissions with less toxicity than that of R-CHOP therapy (Table 1).5 The superiority of BR over R-CHOP at inducing remissions in MCL was subsequently confirmed in the U.S.-led BRIGHT study; this regimen has become a popular regimen for patients aged > 65 years.6 Despite improved remission rate with less toxicity, however, the BR induction regimen still does not adequately address the problem of short durations of remission.
In a phase 3 study, the European MCL Network demonstrated that the use of maintenance rituximab after induction therapy prolonged both progression-free survival (PFS) as well as overall survival (when given after R-CHOP) compared with interferon maintenance.7 Many providers have extrapolated the benefits seen with rituximab maintenance after R-CHOP to all induction regimens, and rituximab maintenance is increasingly offered to older patients after their induction therapy. An important detail of this study is that rituximab was given indefinitely during maintenance and was not capped at 2 years, as often is done for maintenance treatment in other indolent lymphomas.
A landmark randomized study was recently published that demonstrated an advantage of substituting bortezomib for vincristine in the initial regimen.8 In a phase 3 study of 487 patients, the Vr-CAP (bortezomib in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone) regimen demonstrated an improvement in median PFS over R-CHOP after 40 months of followup (24.7 months vs 14.4 months; hazard radio [HR] 0.63; P < .001) (Table 1). Of particular note, however, is that for patients who achieved a complete remission, the median duration of remission was significantly longer than that of R-CHOP (42.1 months vs 18.0 months; HR 0.63; P < .001). These data suggest that not only is Vr-CAP superior to R-CHOP as a whole, but also that some patients are particularly sensitive to bortezomib, resulting in durable remissions.
Novel Targeted Agents
Since 2006, 3 novel targeted agents have been approved for use in relapsed MCL, and many others are currently in clinical trials (Table 2). Bortezomib is approved for use in both the relapsed and upfront setting and demonstrates clear activity in MCL; however, no biomarker currently exists to help understand which patients will respond. Furthermore, most responses to single agents are partial and short-lived.9
Lenalidomide is an oral immunomodulatory agent that works in NHL through a variety of direct and indirect mechanisms.10 As a single agent, lenalidomide has shown activity in a number of studies and can be combined with rituximab without an apparent increase in the toxicity profile.11-13 The EMERGE trial tested lenalidomide as a single agent at 25 mg (every 21 days out of a 28-day cycle) in patients who had previously been treated with bortezomib. In 128 patients, the overall response rate (RR) was 28% with a complete RR of 7.5%. Although these numbers are modest, long-term data from the NHL-003 trial show a subset of patients with durations of remissions nearing 4 years.11 Thus, a biomarker for lenalidomide that predicts response would be of great
clinical utility.
Perhaps of even greater interest have been the clinical results seen with the use of the oral Bruton’s tyrosine kinase inhibitor, ibrutinib. In 111 patients with relapsed MCL treated with ibrutinib 560 mg, the overall RR was 68% with a complete RR of 21%.14 Most patients tolerated therapy very well, and the duration of remission was estimated at 17.5 months. This was a very impressive result with an agent that is tolerable by most patients with MCL.
Conclusions and Future Directions
Mantle cell lymphoma remains a clinical challenge to many providers due to the heterogeneity in the clinical presentation and the lack of consensus regarding the optimal management strategy. The most commonly recommended approach remains to offer highly intensive chemotherapy programs to patients aged < 65 years, but the introduction of novel active agents into the treatment paradigm is beginning to challenge the assumption that all patients need aggressive therapy.
Future research directions should include predictive biomarkers to enhance treatment decisions. Researchers also should begin to understand the toxicity profile and efficacy of the novel targeted agents when used in combination. Early, informative reports are available regarding ibrutinib when added to both rituximab and BR.15,16 Important research questions will include the long-term effects of extended duration therapy (maintenance) paradigms as well as the introduction of novel molecular monitoring methods, such as circulating tumor DNA and circulating tumor cells, to help guide clinical decisions. In a disease with a reportedly dismal outcome, the near future holds much promise as the MCL treatment landscape evolves at a rapid pace.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
Mantle cell lymphoma (MCL) is an uncommon B-cell non-Hodgkin lymphoma (NHL) characterized by the translocation, t(11;14), that results in aberrant expression of cyclin D1.1 The clinical presentation varies significantly from asymptomatic to rapidly enlarging lymph nodes, necessitating immediate treatment. Treatment approaches to newly diagnosed MCL correspondingly vary to match the clinical presentation, but they also reflect the bias of individual providers. No treatment is curative, so different treatment philosophies heavily influence management strategies. Mantle cell lymphoma was first described in the 1990s as a unique pathobiologic entity, and it is only now developing its own set of treatment principles distinct from other lymphomas.2 As novel targeted therapies become available, fundamental questions regarding the best treatment approach are certain to evolve.
Traditional Intensive Treatment
The clinical challenge of treating patients with MCL centers on its propensity to relapse quickly after initial therapy. Although most patients will respond to the initial therapy, the duration of their remissions are disappointingly short. The R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) regimens can induce a complete response in the majority of patients, but they invariably relapse within 18 months of finishing therapy. Recognition of this problem led investigators to test highly intensive regimens designed to maximize the depth of remission often followed by autologous stem cell transplant. The highly intensive regimens are successful at extending remission durations to > 5 years in most cases, but at the cost of significant myelotoxicity and a nontrivial risk of death during induction therapy (Table 1).
In a subset analysis of a hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) study in MCL, it was observed that patients aged < 65 years had a better survival rate than did older patients.3 Accordingly, these regimens are largely restricted to patients aged < 65 years, which is only a minority of patients with MCL. Importantly, despite initial enthusiasm for the impressive rates of remission, most data suggest that these regimens are not curative.
Nonintensive Treatment
Until recently, patients aged > 65 years or those with significant comorbidities did not have effective treatment options that resulted in durable remissions. Most patients were treated with R-CHOP therapy, and the median survival was only 2 to 3 years.4 Interferon maintenance was frequently used but was largely ineffective at prolongation of remission. Treatment patterns changed quickly after the preliminary results of the randomized StiL (Study Group Indolent Lymphomas) study demonstrated that bendamustine-rituximab (BR) could induce durable remissions with less toxicity than that of R-CHOP therapy (Table 1).5 The superiority of BR over R-CHOP at inducing remissions in MCL was subsequently confirmed in the U.S.-led BRIGHT study; this regimen has become a popular regimen for patients aged > 65 years.6 Despite improved remission rate with less toxicity, however, the BR induction regimen still does not adequately address the problem of short durations of remission.
In a phase 3 study, the European MCL Network demonstrated that the use of maintenance rituximab after induction therapy prolonged both progression-free survival (PFS) as well as overall survival (when given after R-CHOP) compared with interferon maintenance.7 Many providers have extrapolated the benefits seen with rituximab maintenance after R-CHOP to all induction regimens, and rituximab maintenance is increasingly offered to older patients after their induction therapy. An important detail of this study is that rituximab was given indefinitely during maintenance and was not capped at 2 years, as often is done for maintenance treatment in other indolent lymphomas.
A landmark randomized study was recently published that demonstrated an advantage of substituting bortezomib for vincristine in the initial regimen.8 In a phase 3 study of 487 patients, the Vr-CAP (bortezomib in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone) regimen demonstrated an improvement in median PFS over R-CHOP after 40 months of followup (24.7 months vs 14.4 months; hazard radio [HR] 0.63; P < .001) (Table 1). Of particular note, however, is that for patients who achieved a complete remission, the median duration of remission was significantly longer than that of R-CHOP (42.1 months vs 18.0 months; HR 0.63; P < .001). These data suggest that not only is Vr-CAP superior to R-CHOP as a whole, but also that some patients are particularly sensitive to bortezomib, resulting in durable remissions.
Novel Targeted Agents
Since 2006, 3 novel targeted agents have been approved for use in relapsed MCL, and many others are currently in clinical trials (Table 2). Bortezomib is approved for use in both the relapsed and upfront setting and demonstrates clear activity in MCL; however, no biomarker currently exists to help understand which patients will respond. Furthermore, most responses to single agents are partial and short-lived.9
Lenalidomide is an oral immunomodulatory agent that works in NHL through a variety of direct and indirect mechanisms.10 As a single agent, lenalidomide has shown activity in a number of studies and can be combined with rituximab without an apparent increase in the toxicity profile.11-13 The EMERGE trial tested lenalidomide as a single agent at 25 mg (every 21 days out of a 28-day cycle) in patients who had previously been treated with bortezomib. In 128 patients, the overall response rate (RR) was 28% with a complete RR of 7.5%. Although these numbers are modest, long-term data from the NHL-003 trial show a subset of patients with durations of remissions nearing 4 years.11 Thus, a biomarker for lenalidomide that predicts response would be of great
clinical utility.
Perhaps of even greater interest have been the clinical results seen with the use of the oral Bruton’s tyrosine kinase inhibitor, ibrutinib. In 111 patients with relapsed MCL treated with ibrutinib 560 mg, the overall RR was 68% with a complete RR of 21%.14 Most patients tolerated therapy very well, and the duration of remission was estimated at 17.5 months. This was a very impressive result with an agent that is tolerable by most patients with MCL.
Conclusions and Future Directions
Mantle cell lymphoma remains a clinical challenge to many providers due to the heterogeneity in the clinical presentation and the lack of consensus regarding the optimal management strategy. The most commonly recommended approach remains to offer highly intensive chemotherapy programs to patients aged < 65 years, but the introduction of novel active agents into the treatment paradigm is beginning to challenge the assumption that all patients need aggressive therapy.
Future research directions should include predictive biomarkers to enhance treatment decisions. Researchers also should begin to understand the toxicity profile and efficacy of the novel targeted agents when used in combination. Early, informative reports are available regarding ibrutinib when added to both rituximab and BR.15,16 Important research questions will include the long-term effects of extended duration therapy (maintenance) paradigms as well as the introduction of novel molecular monitoring methods, such as circulating tumor DNA and circulating tumor cells, to help guide clinical decisions. In a disease with a reportedly dismal outcome, the near future holds much promise as the MCL treatment landscape evolves at a rapid pace.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26-38.
2. Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991;78(2):259-263.
3. Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013-7023.
4. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992.
5. Rummel MJ, Niederle N, Maschmeyer G, et al; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210.
6. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustinerituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952.
7. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
8. Ro bak T, Huang H, Jin J, et al; LYM-3002 Investigators. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944-953.
9. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867-4874.
10 . Kritharis A, Coyle M, Sharma J, Evens AM. Lenalidomide in non-Hodgkin lymphoma: biologic perspectives and therapeutic opportunities [published online ahead of print March 3, 2015]. Blood. pii: blood-2014-11-567792.
11 . Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892-2897.
12 . Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) Study [published online ahead of print September 3, 2013]. J Clin Oncol. doi: 10.1200/JCO.2013.49.2835.
13 . Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab-resistance in patients with indolent B-cell and mantle cell lymphomas [published online ahead of print January 28, 2015]. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-14-2221.
14 . Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516.
15 . Wang ML, Hagemeister F, Westin JR, et al. Ibrutinib and rituximab are an efficacious and safe combination in relapsed mantle cell lymphoma: preliminary results from a phase II clinical trial. Blood. 2014;124(21):627.
16 . Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125(2):242-248.
17 . Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347-5356.
18 . Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3K inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398-3405.
19. Davids MS, Roberts AW, Anderson MA, et al. The BCL-2-specific BH3-mimetic ABT-199 (GDC-0199) is active and well-tolerated in patients with relapsed nonhodgkin lymphoma: interim results of a phase I study. Blood. 2012;120(21): Abstract 304.
1. Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26-38.
2. Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991;78(2):259-263.
3. Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013-7023.
4. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992.
5. Rummel MJ, Niederle N, Maschmeyer G, et al; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210.
6. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustinerituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944-2952.
7. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531.
8. Ro bak T, Huang H, Jin J, et al; LYM-3002 Investigators. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med. 2015;372(10):944-953.
9. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867-4874.
10 . Kritharis A, Coyle M, Sharma J, Evens AM. Lenalidomide in non-Hodgkin lymphoma: biologic perspectives and therapeutic opportunities [published online ahead of print March 3, 2015]. Blood. pii: blood-2014-11-567792.
11 . Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892-2897.
12 . Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) Study [published online ahead of print September 3, 2013]. J Clin Oncol. doi: 10.1200/JCO.2013.49.2835.
13 . Chong EA, Ahmadi T, Aqui NA, et al. Combination of lenalidomide and rituximab overcomes rituximab-resistance in patients with indolent B-cell and mantle cell lymphomas [published online ahead of print January 28, 2015]. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-14-2221.
14 . Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516.
15 . Wang ML, Hagemeister F, Westin JR, et al. Ibrutinib and rituximab are an efficacious and safe combination in relapsed mantle cell lymphoma: preliminary results from a phase II clinical trial. Blood. 2014;124(21):627.
16 . Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125(2):242-248.
17 . Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347-5356.
18 . Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3K inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. 2014;123(22):3398-3405.
19. Davids MS, Roberts AW, Anderson MA, et al. The BCL-2-specific BH3-mimetic ABT-199 (GDC-0199) is active and well-tolerated in patients with relapsed nonhodgkin lymphoma: interim results of a phase I study. Blood. 2012;120(21): Abstract 304.
Histone variant may contribute to lymphoma

Image by Eric Smith
Researchers say they have identified histone chaperones that play an important role in the structure of chromatin.
The team believes this finding, published in Molecular Cell, could lead to a better understanding of lymphomas and other cancers.
“Maintaining an appropriate chromatin structure is essential for normal development, and, not surprisingly, defects in chromatin components can lead to several diseases,” said study author François Robert, PhD, of Institut de Recherches Cliniques de Montréal in Québec, Canada.
In studying chromatin, Dr Robert and his colleagues have been interested in a histone variant called H2A.Z.
The researchers knew that H2A.Z is incorporated into promoter regions of the gene by SWR-C-related chromatin remodeling complexes, but they wanted to determine if H2A.Z is actively excluded from non-promoter regions.
“With this study, we discovered that 2 other proteins, FACT and Spt6, play an important role in the location of H2A.Z,” said Célia Jeronimo, PhD, a research associate in Dr Robert’s lab.
The team found that FACT and SPt6 both help keep H2A.Z from accumulating in intragenic regions. When either histone chaperone is absent, H2A.Z is mislocalized, which alters chromatin composition and contributes to cryptic transcription.
“Inappropriate H2A.Z localization has previously been observed in cancer cells, but little was understood about the consequences of this phenomenon,” Dr Robert said.
“Although our study was performed in yeast cells, it suggests that mislocalization of H2A.Z may lead to cryptic transcription in some types of cancer such as lymphoma, and this may contribute to the disease. Our next step is therefore to investigate the possible role of H2A.Z and its associated gene expression defects in cancer cells.” ![]()

Image by Eric Smith
Researchers say they have identified histone chaperones that play an important role in the structure of chromatin.
The team believes this finding, published in Molecular Cell, could lead to a better understanding of lymphomas and other cancers.
“Maintaining an appropriate chromatin structure is essential for normal development, and, not surprisingly, defects in chromatin components can lead to several diseases,” said study author François Robert, PhD, of Institut de Recherches Cliniques de Montréal in Québec, Canada.
In studying chromatin, Dr Robert and his colleagues have been interested in a histone variant called H2A.Z.
The researchers knew that H2A.Z is incorporated into promoter regions of the gene by SWR-C-related chromatin remodeling complexes, but they wanted to determine if H2A.Z is actively excluded from non-promoter regions.
“With this study, we discovered that 2 other proteins, FACT and Spt6, play an important role in the location of H2A.Z,” said Célia Jeronimo, PhD, a research associate in Dr Robert’s lab.
The team found that FACT and SPt6 both help keep H2A.Z from accumulating in intragenic regions. When either histone chaperone is absent, H2A.Z is mislocalized, which alters chromatin composition and contributes to cryptic transcription.
“Inappropriate H2A.Z localization has previously been observed in cancer cells, but little was understood about the consequences of this phenomenon,” Dr Robert said.
“Although our study was performed in yeast cells, it suggests that mislocalization of H2A.Z may lead to cryptic transcription in some types of cancer such as lymphoma, and this may contribute to the disease. Our next step is therefore to investigate the possible role of H2A.Z and its associated gene expression defects in cancer cells.” ![]()

Image by Eric Smith
Researchers say they have identified histone chaperones that play an important role in the structure of chromatin.
The team believes this finding, published in Molecular Cell, could lead to a better understanding of lymphomas and other cancers.
“Maintaining an appropriate chromatin structure is essential for normal development, and, not surprisingly, defects in chromatin components can lead to several diseases,” said study author François Robert, PhD, of Institut de Recherches Cliniques de Montréal in Québec, Canada.
In studying chromatin, Dr Robert and his colleagues have been interested in a histone variant called H2A.Z.
The researchers knew that H2A.Z is incorporated into promoter regions of the gene by SWR-C-related chromatin remodeling complexes, but they wanted to determine if H2A.Z is actively excluded from non-promoter regions.
“With this study, we discovered that 2 other proteins, FACT and Spt6, play an important role in the location of H2A.Z,” said Célia Jeronimo, PhD, a research associate in Dr Robert’s lab.
The team found that FACT and SPt6 both help keep H2A.Z from accumulating in intragenic regions. When either histone chaperone is absent, H2A.Z is mislocalized, which alters chromatin composition and contributes to cryptic transcription.
“Inappropriate H2A.Z localization has previously been observed in cancer cells, but little was understood about the consequences of this phenomenon,” Dr Robert said.
“Although our study was performed in yeast cells, it suggests that mislocalization of H2A.Z may lead to cryptic transcription in some types of cancer such as lymphoma, and this may contribute to the disease. Our next step is therefore to investigate the possible role of H2A.Z and its associated gene expression defects in cancer cells.” ![]()
Dexrazoxane Tx did not affect overall survival in pediatric leukemia and lymphoma
Exposure to dexrazoxane among pediatric patients with leukemia or lymphoma did not affect overall mortality during a median follow-up period of 12.6 years, according to a report published online in the Journal of Clinical Oncology.
Aggregated data from three Children’s Oncology Group trials showed that among 1,008 pediatric patients who received treatment with doxorubicin with or without dexrazoxane (DRZ) from 1996 to 2001, exposure to DRZ was not associated with an increased risk of relapse (HR, 0.81; 95% CI, 0.60-1.08) or death (HR, 1.03; 0.73-1.45). Comparing DRZ with non-DRZ treatment groups at 10 years, the cumulative incidence of relapse was 16.1% vs. 19.1% (difference, – 3.0%; 95% CI, – 7.9% to 0.2%) and overall mortality was 12.8% vs. 12.2% (difference, – 0.6%; 95% CI, – 3.5% to 4.7%). The three trials (P9404, P9425, and P9426) evaluated individually likewise did not show significant differences in relapse or mortality rates.
Although studies in adults show a positive effect of DRZ on heart failure rates after anthracycline therapy, concern over DRZ interference with cancer therapies and a possible link to second cancers have limited its use in children and prompted Dr. Eric Chow of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues to assess the effect of DRZ on mortality.
The investigators wrote that DRZ “does not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS [acute myeloid leukemia/myelodysplastic syndrome]) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant,” the investigators said. (J. Clin. Oncol. 2015 May 26 [doi:10.1200/JCO.2014.59.4473])
Aggregated data from the three trials shows that the 10-year mortality rate of AML/MDS was 1.4% for those treated with DRZ (seven patients), compared with 0.8% for those treated without DRZ (five patients).
The beneficial effects of DRZ in decreasing the risk of heart failure have been observed in trials of adult patients, but the results for survivors of childhood cancers have been inconclusive because heart failure may develop over a longer time period in children. With the median age of survivors in this study of 24 years, significant differences in cardiac mortality due to DRZ use are not detectable. To evaluate DRZ as a cardioprotectant, a new Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]) will determine the cardiovascular health of individuals in the three trials P9404, P9425, and P9426.
“Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group … with cumulative incidences of each approaching 20% by age 50 years, any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive,” Dr. Chow and his associates wrote.
The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
Exposure to dexrazoxane among pediatric patients with leukemia or lymphoma did not affect overall mortality during a median follow-up period of 12.6 years, according to a report published online in the Journal of Clinical Oncology.
Aggregated data from three Children’s Oncology Group trials showed that among 1,008 pediatric patients who received treatment with doxorubicin with or without dexrazoxane (DRZ) from 1996 to 2001, exposure to DRZ was not associated with an increased risk of relapse (HR, 0.81; 95% CI, 0.60-1.08) or death (HR, 1.03; 0.73-1.45). Comparing DRZ with non-DRZ treatment groups at 10 years, the cumulative incidence of relapse was 16.1% vs. 19.1% (difference, – 3.0%; 95% CI, – 7.9% to 0.2%) and overall mortality was 12.8% vs. 12.2% (difference, – 0.6%; 95% CI, – 3.5% to 4.7%). The three trials (P9404, P9425, and P9426) evaluated individually likewise did not show significant differences in relapse or mortality rates.
Although studies in adults show a positive effect of DRZ on heart failure rates after anthracycline therapy, concern over DRZ interference with cancer therapies and a possible link to second cancers have limited its use in children and prompted Dr. Eric Chow of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues to assess the effect of DRZ on mortality.
The investigators wrote that DRZ “does not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS [acute myeloid leukemia/myelodysplastic syndrome]) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant,” the investigators said. (J. Clin. Oncol. 2015 May 26 [doi:10.1200/JCO.2014.59.4473])
Aggregated data from the three trials shows that the 10-year mortality rate of AML/MDS was 1.4% for those treated with DRZ (seven patients), compared with 0.8% for those treated without DRZ (five patients).
The beneficial effects of DRZ in decreasing the risk of heart failure have been observed in trials of adult patients, but the results for survivors of childhood cancers have been inconclusive because heart failure may develop over a longer time period in children. With the median age of survivors in this study of 24 years, significant differences in cardiac mortality due to DRZ use are not detectable. To evaluate DRZ as a cardioprotectant, a new Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]) will determine the cardiovascular health of individuals in the three trials P9404, P9425, and P9426.
“Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group … with cumulative incidences of each approaching 20% by age 50 years, any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive,” Dr. Chow and his associates wrote.
The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
Exposure to dexrazoxane among pediatric patients with leukemia or lymphoma did not affect overall mortality during a median follow-up period of 12.6 years, according to a report published online in the Journal of Clinical Oncology.
Aggregated data from three Children’s Oncology Group trials showed that among 1,008 pediatric patients who received treatment with doxorubicin with or without dexrazoxane (DRZ) from 1996 to 2001, exposure to DRZ was not associated with an increased risk of relapse (HR, 0.81; 95% CI, 0.60-1.08) or death (HR, 1.03; 0.73-1.45). Comparing DRZ with non-DRZ treatment groups at 10 years, the cumulative incidence of relapse was 16.1% vs. 19.1% (difference, – 3.0%; 95% CI, – 7.9% to 0.2%) and overall mortality was 12.8% vs. 12.2% (difference, – 0.6%; 95% CI, – 3.5% to 4.7%). The three trials (P9404, P9425, and P9426) evaluated individually likewise did not show significant differences in relapse or mortality rates.
Although studies in adults show a positive effect of DRZ on heart failure rates after anthracycline therapy, concern over DRZ interference with cancer therapies and a possible link to second cancers have limited its use in children and prompted Dr. Eric Chow of the Fred Hutchinson Cancer Research Center, Seattle, and his colleagues to assess the effect of DRZ on mortality.
The investigators wrote that DRZ “does not appear to interfere with cancer treatment efficacy, in terms of original cancer mortality or overall risk of relapse. Although the risk for secondary cancer mortality (mainly as a result of AML/MDS [acute myeloid leukemia/myelodysplastic syndrome]) was greater among those exposed to DRZ, the overall number of events was small, and the differences were not statistically significant,” the investigators said. (J. Clin. Oncol. 2015 May 26 [doi:10.1200/JCO.2014.59.4473])
Aggregated data from the three trials shows that the 10-year mortality rate of AML/MDS was 1.4% for those treated with DRZ (seven patients), compared with 0.8% for those treated without DRZ (five patients).
The beneficial effects of DRZ in decreasing the risk of heart failure have been observed in trials of adult patients, but the results for survivors of childhood cancers have been inconclusive because heart failure may develop over a longer time period in children. With the median age of survivors in this study of 24 years, significant differences in cardiac mortality due to DRZ use are not detectable. To evaluate DRZ as a cardioprotectant, a new Children’s Oncology Group study (Effects of Dexrazoxane Hydrochloride on Biomarkers Associated With Cardiomyopathy and Heart Failure After Cancer Treatment [HEART]) will determine the cardiovascular health of individuals in the three trials P9404, P9425, and P9426.
“Given that second cancers and symptomatic cardiac disease appear to be by far the two most common categories of serious late effects (in terms of both absolute and relative risks) among long-term childhood cancer survivors as a group … with cumulative incidences of each approaching 20% by age 50 years, any strategy that offers the promise of reduced cardiotoxicity without being offset by second cancers is highly attractive,” Dr. Chow and his associates wrote.
The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Treatment with dexrazoxane was not associated with an increased risk for cancer relapse or death.
Major finding: For pediatric patients with leukemia and lymphoma, the cumulative incidence of relapse at 10 years was 16.1% with DRZ, compared with 19.1% without DRZ (difference, – 3.0%; 95% CI, – 7.9% to 0.2%); overall mortality was 12.8% with DRZ vs. 12.2% without DRZ (difference, – 0.6%; 95% CI, – 3.5% to 4.7%).
Data source: Aggregated Children’s Oncology Group trials enrolling 1,008 pediatric patients with leukemia or lymphoma who were randomized to receive doxorubicin with or without DRZ from 1996 to 2001.
Disclosures: The study was supported by the National Institutes of Health, St. Baldrick’s Foundation, and the Leukemia and Lymphoma Society. Dr. Chow reported having no relevant financial conflicts. Three of his coauthors reported having financial relationships with industry.
Herbs reduce fatigue in cancer patients

Photo by Alexander Baxevanis
An herbal mixture used in traditional Chinese medicine can reduce fatigue in cancer patients, results of a phase 1/2 study suggest.
The mixture, Ren Shen Yangrong Tang (RSYRT), is a soup containing 12 herbs.
In the study, cancer patients suffering from moderate to severe fatigue reported significantly less fatigue after taking RSYRT for 2 to 3 weeks.
Researchers reported these results in the Journal of Alternative and Complementary Medicine.
Yichen Xu, MD, of Beijing Cancer Hospital & Institute in China, and colleagues evaluated RSYRT in 33 patients who had completed cancer treatment. The patients had stable disease and no anemia.
Eleven patients had moderate fatigue (a score of 4-6 on a 0-10 scale), and 22 had severe fatigue (a score of 7-10). All patients had experienced fatigue for at least 4 months.
Patients took RSYRT twice a day for 6 weeks and experienced a significant decrease in fatigue severity. The mean fatigue score decreased from 7.06 at baseline to 3.30 at the 6-week mark (P<0.001).
The fatigue category also changed significantly (P=0.024). Among the 22 patients who had severe fatigue before RSYRT, half had mild fatigue after therapy, and half had moderate fatigue.
Among the 11 patients who had moderate fatigue at baseline, only 1 still had moderate fatigue after receiving RSYRT. The rest had mild fatigue.
All of the patients said they felt better after taking RSYRT for 4 weeks.
There were no “uncomfortable events” related to RSYRT, such as gastrointestinal upset, insomnia, headache, or rash. None of the patients required a dose reduction or dose interruption.
None of the patients had blood chemistry abnormalities or abnormal liver/kidney function. Two patients who had a change in ST segment before RSYRT had normal electrocardiogram results after treatment. ![]()

Photo by Alexander Baxevanis
An herbal mixture used in traditional Chinese medicine can reduce fatigue in cancer patients, results of a phase 1/2 study suggest.
The mixture, Ren Shen Yangrong Tang (RSYRT), is a soup containing 12 herbs.
In the study, cancer patients suffering from moderate to severe fatigue reported significantly less fatigue after taking RSYRT for 2 to 3 weeks.
Researchers reported these results in the Journal of Alternative and Complementary Medicine.
Yichen Xu, MD, of Beijing Cancer Hospital & Institute in China, and colleagues evaluated RSYRT in 33 patients who had completed cancer treatment. The patients had stable disease and no anemia.
Eleven patients had moderate fatigue (a score of 4-6 on a 0-10 scale), and 22 had severe fatigue (a score of 7-10). All patients had experienced fatigue for at least 4 months.
Patients took RSYRT twice a day for 6 weeks and experienced a significant decrease in fatigue severity. The mean fatigue score decreased from 7.06 at baseline to 3.30 at the 6-week mark (P<0.001).
The fatigue category also changed significantly (P=0.024). Among the 22 patients who had severe fatigue before RSYRT, half had mild fatigue after therapy, and half had moderate fatigue.
Among the 11 patients who had moderate fatigue at baseline, only 1 still had moderate fatigue after receiving RSYRT. The rest had mild fatigue.
All of the patients said they felt better after taking RSYRT for 4 weeks.
There were no “uncomfortable events” related to RSYRT, such as gastrointestinal upset, insomnia, headache, or rash. None of the patients required a dose reduction or dose interruption.
None of the patients had blood chemistry abnormalities or abnormal liver/kidney function. Two patients who had a change in ST segment before RSYRT had normal electrocardiogram results after treatment. ![]()

Photo by Alexander Baxevanis
An herbal mixture used in traditional Chinese medicine can reduce fatigue in cancer patients, results of a phase 1/2 study suggest.
The mixture, Ren Shen Yangrong Tang (RSYRT), is a soup containing 12 herbs.
In the study, cancer patients suffering from moderate to severe fatigue reported significantly less fatigue after taking RSYRT for 2 to 3 weeks.
Researchers reported these results in the Journal of Alternative and Complementary Medicine.
Yichen Xu, MD, of Beijing Cancer Hospital & Institute in China, and colleagues evaluated RSYRT in 33 patients who had completed cancer treatment. The patients had stable disease and no anemia.
Eleven patients had moderate fatigue (a score of 4-6 on a 0-10 scale), and 22 had severe fatigue (a score of 7-10). All patients had experienced fatigue for at least 4 months.
Patients took RSYRT twice a day for 6 weeks and experienced a significant decrease in fatigue severity. The mean fatigue score decreased from 7.06 at baseline to 3.30 at the 6-week mark (P<0.001).
The fatigue category also changed significantly (P=0.024). Among the 22 patients who had severe fatigue before RSYRT, half had mild fatigue after therapy, and half had moderate fatigue.
Among the 11 patients who had moderate fatigue at baseline, only 1 still had moderate fatigue after receiving RSYRT. The rest had mild fatigue.
All of the patients said they felt better after taking RSYRT for 4 weeks.
There were no “uncomfortable events” related to RSYRT, such as gastrointestinal upset, insomnia, headache, or rash. None of the patients required a dose reduction or dose interruption.
None of the patients had blood chemistry abnormalities or abnormal liver/kidney function. Two patients who had a change in ST segment before RSYRT had normal electrocardiogram results after treatment. ![]()
Inhibitor promotes chemosensitization in CLL

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.

PHILADELPHIA—A DNA-dependent protein kinase (DNA-PK) inhibitor can sensitize chronic lymphocytic leukemia (CLL) cells to chemotherapy, according to
preclinical research.
The inhibitor, NDD0004, sensitized CLL cells—even those from patients with high-risk cytogenetics—to treatment with mitoxantrone.
However, not all CLL samples were sensitive to treatment, so researchers are now trying to determine which patients might derive benefit from DNA-PK inhibitors.
Gesa Junge, a PhD student at Newcastle University in the UK, and her colleagues conducted this research and presented the results at the AACR Annual Meeting 2015 (abstract 3624*). The work was supported by AstraZeneca.
The researchers’ goal was to validate that DNA-PK inhibition is a valid approach to chemosensitization in CLL. So the team tested NU7441—a compound that inhibits DNA-PK and PI3 kinase—and NDD0004—a more selective DNA-PK inhibitor.
The team isolated CLL cells from patients’ peripheral blood, cultured the cells, and treated them with mitoxantrone and/or 1μM of NDD0004 or 1μM of NU7441.
Junge and her colleagues found that NDD0004 sensitized cells to mitoxantrone more effectively than NU7441. Sensitization was 202-fold higher with NDD004 plus mitoxantrone than with mitoxantrone alone and 69-fold higher with NU7441 plus mitoxantrone than with mitoxantrone alone (P=0.02).
However, sensitization varied between CLL samples, and the researchers have yet to determine why. Their experiments showed that variability was not a result of DNA-PK levels.
Still, the team found that CLL cells from patients with poor prognostic markers were sensitive to DNA-PK inhibition.
Sensitization with NU7441 plus mitoxantrone was 69-fold higher than mitoxantrone alone in CLL samples with del(13q), 25-fold higher in samples with del(11q), 12-fold higher in samples with TP53 mutation, and 16-fold higher in samples with ATM dysfunction.
Sensitization with NDD0004 plus mitoxantrone was 201-fold higher than mitoxantrone alone in CLL samples with del(13q), 314-fold higher in samples with del(11q), 27-fold higher in samples with TP53 mutation, and 18-fold higher in samples with ATM dysfunction.
To confirm that sensitization was a result of DNA-PK inhibition, Junge and her colleagues tested NDD0004 in an isogenic pair of DNA-PK-deficient and DNA-PK-proficient HCT116 cells. They found that HCT116 cells lacking DNA-PK were not sensitive to NDD0004, but cells with DNA-PK were sensitive.
The researchers also investigated the mechanism of NDD0004. Their results suggest the drug works by inhibiting the repair of DNA double-strand breaks.
“What we think is happening is that we are inducing DNA damage with mitoxantrone, and that gets repaired by 24 hours,” Junge said. “But if the DNA-PK inhibitor is there, the damage persists, and that seems to translate quite nicely into an apoptosis response.”
To further this research, Junge and her colleagues are hoping to identify biomarkers that can help them determine which CLL patients are likely to respond to DNA-PK inhibitors. ![]()
*Information in the abstract differs from that presented at the meeting.