User login
Line complications plague dose-adjusted EPOCH-R in non-Hodgkin lymphoma
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), which is used to treat several types of aggressive non-Hodgkin lymphomas, is associated with high rates of line-associated complications, a new study suggests.
These findings, published in Clinical Lymphoma, Myeloma & Leukemia, confirm other recent findings that DA-EPOCH-R has a significantly greater rate of complications, compared with that of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy.
The authors note that the use of DA-EPOCH-R is based on data from early phase trials, as well as retrospective data, that support its use as induction chemotherapy in high-grade B-cell lymphoma with MYC, BCL2, and/or BCL6 translocations. But currently, there are no data published from randomized trials that support the use of upfront DA-EPOCH-R therapy.
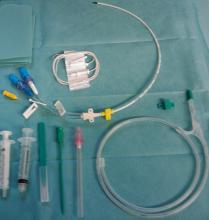
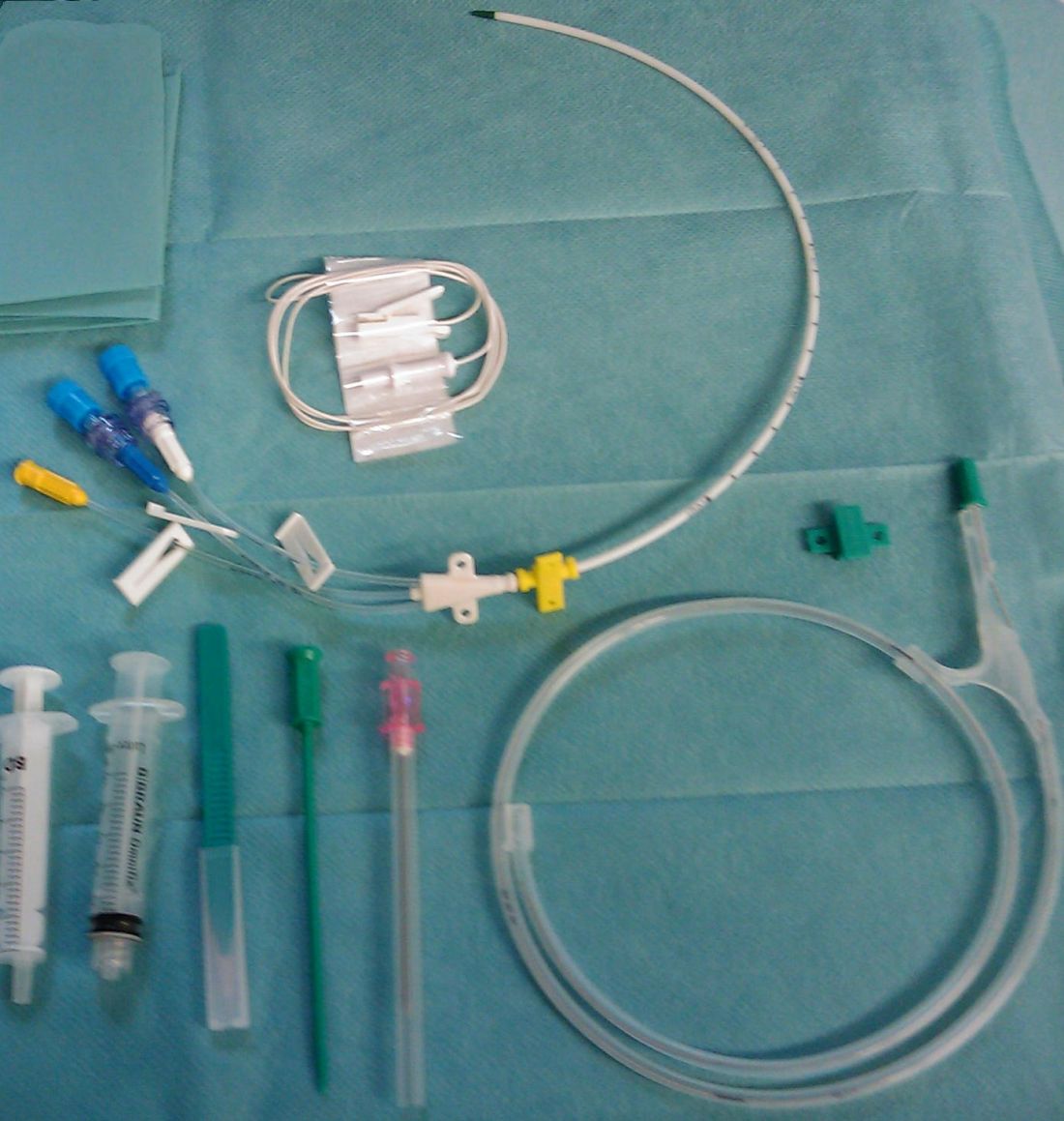
DA-EPOCH-R is an infusion-based therapy that requires a central venous catheter.
In their study, Rachel J. David, MD, of Wilmot Cancer Institute, Rochester, N.Y., and her colleagues conducted a retrospective study that included all patients treated with DA-EPOCH-R at their institution between March 2011 and July 2016, and also included a concurrent cohort of patients with diffuse large B-cell lymphoma (DLBCL) who were treated with R-CHOP. The goal was to identify the rates and predictors of line-associated complications linked with the use of DA-EPOCH-R therapy in this population.
The patient cohort comprised 43 patients who received DA-EPOCH-R and 44 patients who received RCHOP.
Patients in the DA-EPOCH-R cohort experienced a significantly higher rate of complications (P =.03), compared with the R-CHOP group.
In the DA-EPOCH-R cohort, 17 patients (39.5%) reported at least one LAC, which included venous thromboembolism, chemotherapy extravasation, and line-associated infection, during the study period. Grade 3 toxicity was observed in 41% of these patients.
In contrast, eight patients (18.2%) in the R-CHOP arm experienced at least one complication, with five of the eight patients experiencing grade 3-4 toxicity.
In a univariate analysis, body mass index of 35 kg/m2 and the use of a peripherally inserted central catheter line were both significantly associated with a higher risk of venous thromboembolism (P = .04 and P = .02, respectively).
“For patients undergoing treatment with DA-EPOCH-R in whom the use of [central venous catheters] cannot be avoided, the morbidity of [line-associated complications] should be factored in by the clinician when determining upfront treatment,” the researchers wrote.
They reported having no conflicts of interest.
SOURCE: David RJ et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 29. doi: 10.1016/j.clml.2018.08.014.
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), which is used to treat several types of aggressive non-Hodgkin lymphomas, is associated with high rates of line-associated complications, a new study suggests.
These findings, published in Clinical Lymphoma, Myeloma & Leukemia, confirm other recent findings that DA-EPOCH-R has a significantly greater rate of complications, compared with that of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy.
The authors note that the use of DA-EPOCH-R is based on data from early phase trials, as well as retrospective data, that support its use as induction chemotherapy in high-grade B-cell lymphoma with MYC, BCL2, and/or BCL6 translocations. But currently, there are no data published from randomized trials that support the use of upfront DA-EPOCH-R therapy.
DA-EPOCH-R is an infusion-based therapy that requires a central venous catheter.
In their study, Rachel J. David, MD, of Wilmot Cancer Institute, Rochester, N.Y., and her colleagues conducted a retrospective study that included all patients treated with DA-EPOCH-R at their institution between March 2011 and July 2016, and also included a concurrent cohort of patients with diffuse large B-cell lymphoma (DLBCL) who were treated with R-CHOP. The goal was to identify the rates and predictors of line-associated complications linked with the use of DA-EPOCH-R therapy in this population.
The patient cohort comprised 43 patients who received DA-EPOCH-R and 44 patients who received RCHOP.
Patients in the DA-EPOCH-R cohort experienced a significantly higher rate of complications (P =.03), compared with the R-CHOP group.
In the DA-EPOCH-R cohort, 17 patients (39.5%) reported at least one LAC, which included venous thromboembolism, chemotherapy extravasation, and line-associated infection, during the study period. Grade 3 toxicity was observed in 41% of these patients.
In contrast, eight patients (18.2%) in the R-CHOP arm experienced at least one complication, with five of the eight patients experiencing grade 3-4 toxicity.
In a univariate analysis, body mass index of 35 kg/m2 and the use of a peripherally inserted central catheter line were both significantly associated with a higher risk of venous thromboembolism (P = .04 and P = .02, respectively).
“For patients undergoing treatment with DA-EPOCH-R in whom the use of [central venous catheters] cannot be avoided, the morbidity of [line-associated complications] should be factored in by the clinician when determining upfront treatment,” the researchers wrote.
They reported having no conflicts of interest.
SOURCE: David RJ et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 29. doi: 10.1016/j.clml.2018.08.014.
Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R), which is used to treat several types of aggressive non-Hodgkin lymphomas, is associated with high rates of line-associated complications, a new study suggests.
These findings, published in Clinical Lymphoma, Myeloma & Leukemia, confirm other recent findings that DA-EPOCH-R has a significantly greater rate of complications, compared with that of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy.
The authors note that the use of DA-EPOCH-R is based on data from early phase trials, as well as retrospective data, that support its use as induction chemotherapy in high-grade B-cell lymphoma with MYC, BCL2, and/or BCL6 translocations. But currently, there are no data published from randomized trials that support the use of upfront DA-EPOCH-R therapy.
DA-EPOCH-R is an infusion-based therapy that requires a central venous catheter.
In their study, Rachel J. David, MD, of Wilmot Cancer Institute, Rochester, N.Y., and her colleagues conducted a retrospective study that included all patients treated with DA-EPOCH-R at their institution between March 2011 and July 2016, and also included a concurrent cohort of patients with diffuse large B-cell lymphoma (DLBCL) who were treated with R-CHOP. The goal was to identify the rates and predictors of line-associated complications linked with the use of DA-EPOCH-R therapy in this population.
The patient cohort comprised 43 patients who received DA-EPOCH-R and 44 patients who received RCHOP.
Patients in the DA-EPOCH-R cohort experienced a significantly higher rate of complications (P =.03), compared with the R-CHOP group.
In the DA-EPOCH-R cohort, 17 patients (39.5%) reported at least one LAC, which included venous thromboembolism, chemotherapy extravasation, and line-associated infection, during the study period. Grade 3 toxicity was observed in 41% of these patients.
In contrast, eight patients (18.2%) in the R-CHOP arm experienced at least one complication, with five of the eight patients experiencing grade 3-4 toxicity.
In a univariate analysis, body mass index of 35 kg/m2 and the use of a peripherally inserted central catheter line were both significantly associated with a higher risk of venous thromboembolism (P = .04 and P = .02, respectively).
“For patients undergoing treatment with DA-EPOCH-R in whom the use of [central venous catheters] cannot be avoided, the morbidity of [line-associated complications] should be factored in by the clinician when determining upfront treatment,” the researchers wrote.
They reported having no conflicts of interest.
SOURCE: David RJ et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 29. doi: 10.1016/j.clml.2018.08.014.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: In all, 17 dose-adjusted R-EPOCH patients (39.5%) experienced at least one line-associated complication, versus 8 patients (18.2%) in the R-CHOP group.
Study details: A retrospective single-institution study with 87 patients.
Disclosures: The researchers reported having no conflicts of interest.
Source: David RJ et al. Clin Lymphoma Myeloma Leuk. 2018 Aug 29. doi: 10.1016/j.clml.2018.08.014.
First-line bortezomib prolongs survival in MCL
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
The proteasome inhibitor, bortezomib, represents a “substantial advance” for the treatment of newly diagnosed mantle cell lymphoma, according to Simon Rule, MD.
In an accompanying commentary, he stated that bortezomib-based VR-CAP (rituximab plus cyclophosphamide, doxorubicin, and prednisone) showed a clear survival benefit in the LYM-3002 trial, compared with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). However, in order to use this combination in elderly patients, the administration method must be considered. Additionally, it makes sense to routinely use rituximab maintenance.
While the final analysis of the LYM-3002 trial is positive, there are caveats to consider before changing practice, particularly for elderly patients. First, the study had a somewhat younger population and fewer high-risk patients, compared with the only similar study of R-CHOP regimen in an elderly population. The bortezomib plus VR-CAP combination also had significant toxicity that could limit its widespread use in elderly patients.
Dr. Rule also noted that, internationally, bendamustine-based therapy is increasingly being chosen over R-CHOP for older patients with mantle cell lymphoma.
“Whether VR-CAP or the combination of bortezomib and bendamustine-based regimens will be the optimal approach has yet to be established. However, if R-CHOP is being considered, then the long-term survival results reported by Robak and colleagues strongly support the use of VR-CAP as an alternative,” Dr. Rule wrote.
Dr. Rule is with the University of Plymouth (England). These comments are adapted from his commentary (Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045[18]30743-5). Dr. Rule reported receiving grants and personal fees from Janssen Pharmaceuticals.
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
Bortezomib in combination with rituximab plus chemotherapy significantly improved overall survival in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL), compared with standard treatment, according to final results from the international, phase 3 LYM-3002 trial.
After a median follow-up period of 82.0 months, median overall survival was 90.7 months among participants who were given first-line bortezomib in addition to rituximab plus cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus 55.7 months in the control arm, where patients were given rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), for a hazard ratio of 0.66 (95% confidence interval, 0.51-0.85; P = .001).
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues also reported that patients in the bortezomib arm experienced two novel adverse effects, which were different from findings reported in the primary analysis. Each case was classified as grade 4; there was one case of gastric cancer and one case of lung adenocarcinoma.
The findings were reported in the Lancet Oncology.
Among 268 patients in the follow-up analysis set, the median age was 66 years and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI). For those considered high risk, no significant difference was noted when comparing the two groups on the basis of overall survival.
“When analyzed according to MIPI risk category, VR-CAP was associated with significantly improved overall survival, compared with R-CHOP in the low-risk and intermediate-risk categories, but not in the high-risk category,” the investigators wrote.
The authors acknowledged a key limitation of the study was that rituximab was not given as a maintenance therapy since it was not considered standard of care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommended that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties to Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
SOURCE: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
FROM THE LANCET ONCOLOGY
Key clinical point:
Major finding: Median overall survival was 90.7 months in the intervention arm (bortezomib in addition to rituximab plus chemotherapy) versus 55.7 months in the control arm (hazard ratio, 0.66; 95% confidence interval, 0.51-0.85; P = .001).
Study details: LYM-3002 was a phase 3, randomized, open-label study of 487 transplant-ineligible patients with untreated mantle cell lymphoma.
Disclosures: The study was sponsored by Janssen Pharmaceuticals. The authors reported financial ties with Janssen, Celgene, Ipsen Biopharmaceuticals, Johnson & Johnson, Novartis, and others.
Source: Robak T et al. Lancet Oncol. 2018 Oct 19. doi: 10.1016/S1470-2045(18)30685-5.
‘Mechanoprimed’ MSCs aid hematopoietic recovery
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Combo can prolong overall survival in MCL
Final results of a phase 3 trial suggest bortezomib plus rituximab and chemotherapy can significantly improve overall survival (OS) in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL).
In the LYM-3002 trial, researchers compared bortezomib plus rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
The median OS was significantly longer in patients who received VR-CAP than in those who received R-CHOP—90.7 months and 55.7 months, respectively.
This survival benefit was observed in patients with low- and intermediate-risk disease but not high-risk disease.
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues reported these results in The Lancet Oncology alongside a related commentary.
The LYM-3002 trial began more than a decade ago, and initial results were published in 2015. At that time, the VR-CAP group showed a significant increase in progression-free survival compared with the R-CHOP group.
The final analysis of LYM-3002 included 268 of the original 487 MCL patients. Twenty-three percent of patients in the VR-CAP group (n=32) discontinued due to death, as did 40% of patients in the R-CHOP group (n=51). The main cause of death was progression—29% and 14%, respectively.
Among the 268 patients in the final analysis, 140 belonged to the VR-CAP group and 128 to the R-CHOP group. The patients’ median age was 66 (range, 26-83), 71% (n=190) were male, 74% (n=199) had stage IV disease, and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI).
About half of patients received therapies after the trial interventions (n=255, 52%)—43% (n=104) in the VR-CAP group and 62% (n=151) in the R-CHOP group. Most patients received subsequent antineoplastic therapy—77% (n=80) and 81% (n=123), respectively—and more than half received rituximab as second-line therapy—53% (n=55) and 59% (n=89), respectively.
Results
At a median follow-up of 82.0 months, the median OS was significantly longer in the VR-CAP group than in the R-CHOP group—90.7 months (95% CI, 71.4 to not estimable) and 55.7 months (95% CI, 47.2 to 68.9), respectively (hazard ratio [HR]=0.66 [95% CI, 0.51–0.85]; P=0.001).
The 4-year OS was 67.3% in the VR-CAP group and 54.3% in the R-CHOP group. The 6-year OS was 56.6% and 42.0%, respectively.
The researchers noted that VR-CAP was associated with significantly improved OS among patients in the low-risk and intermediate-risk MIPI categories but not in the high-risk category.
In the low-risk cohort, the median OS was 81.7 months in the R-CHOP group and not estimable in the VR-CAP group (HR=0.54 [95% CI, 0.30–0.95]; P≤0.05).
In the intermediate-risk cohort, the median OS was 62.2 months in the R-CHOP group and not estimable in the VR-CAP group (HR=0.55 [95% CI, 0.36–0.85]; P≤0.01).
In the high-risk cohort, the median OS was 37.1 months in the R-CHOP group and 30.4 months in the VR-CAP group (HR=1.02 [95% CI, 0.69–1.50]).
The researchers reported three new adverse events in the final analysis—grade 4 lung adenocarcinoma and grade 4 gastric cancer in the VR-CAP group as well as grade 2 pneumonia in the R-CHOP group.
The team acknowledged that a key limitation of this study was that rituximab was not given as maintenance since it was not considered standard care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommend that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The LYM-3002 study was sponsored by Janssen Research & Development. The study authors reported financial ties to Janssen, Celgene, Ipsen, Johnson & Johnson, Novartis, and other companies.
Final results of a phase 3 trial suggest bortezomib plus rituximab and chemotherapy can significantly improve overall survival (OS) in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL).
In the LYM-3002 trial, researchers compared bortezomib plus rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
The median OS was significantly longer in patients who received VR-CAP than in those who received R-CHOP—90.7 months and 55.7 months, respectively.
This survival benefit was observed in patients with low- and intermediate-risk disease but not high-risk disease.
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues reported these results in The Lancet Oncology alongside a related commentary.
The LYM-3002 trial began more than a decade ago, and initial results were published in 2015. At that time, the VR-CAP group showed a significant increase in progression-free survival compared with the R-CHOP group.
The final analysis of LYM-3002 included 268 of the original 487 MCL patients. Twenty-three percent of patients in the VR-CAP group (n=32) discontinued due to death, as did 40% of patients in the R-CHOP group (n=51). The main cause of death was progression—29% and 14%, respectively.
Among the 268 patients in the final analysis, 140 belonged to the VR-CAP group and 128 to the R-CHOP group. The patients’ median age was 66 (range, 26-83), 71% (n=190) were male, 74% (n=199) had stage IV disease, and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI).
About half of patients received therapies after the trial interventions (n=255, 52%)—43% (n=104) in the VR-CAP group and 62% (n=151) in the R-CHOP group. Most patients received subsequent antineoplastic therapy—77% (n=80) and 81% (n=123), respectively—and more than half received rituximab as second-line therapy—53% (n=55) and 59% (n=89), respectively.
Results
At a median follow-up of 82.0 months, the median OS was significantly longer in the VR-CAP group than in the R-CHOP group—90.7 months (95% CI, 71.4 to not estimable) and 55.7 months (95% CI, 47.2 to 68.9), respectively (hazard ratio [HR]=0.66 [95% CI, 0.51–0.85]; P=0.001).
The 4-year OS was 67.3% in the VR-CAP group and 54.3% in the R-CHOP group. The 6-year OS was 56.6% and 42.0%, respectively.
The researchers noted that VR-CAP was associated with significantly improved OS among patients in the low-risk and intermediate-risk MIPI categories but not in the high-risk category.
In the low-risk cohort, the median OS was 81.7 months in the R-CHOP group and not estimable in the VR-CAP group (HR=0.54 [95% CI, 0.30–0.95]; P≤0.05).
In the intermediate-risk cohort, the median OS was 62.2 months in the R-CHOP group and not estimable in the VR-CAP group (HR=0.55 [95% CI, 0.36–0.85]; P≤0.01).
In the high-risk cohort, the median OS was 37.1 months in the R-CHOP group and 30.4 months in the VR-CAP group (HR=1.02 [95% CI, 0.69–1.50]).
The researchers reported three new adverse events in the final analysis—grade 4 lung adenocarcinoma and grade 4 gastric cancer in the VR-CAP group as well as grade 2 pneumonia in the R-CHOP group.
The team acknowledged that a key limitation of this study was that rituximab was not given as maintenance since it was not considered standard care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommend that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The LYM-3002 study was sponsored by Janssen Research & Development. The study authors reported financial ties to Janssen, Celgene, Ipsen, Johnson & Johnson, Novartis, and other companies.
Final results of a phase 3 trial suggest bortezomib plus rituximab and chemotherapy can significantly improve overall survival (OS) in transplant-ineligible patients with newly diagnosed mantle cell lymphoma (MCL).
In the LYM-3002 trial, researchers compared bortezomib plus rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).
The median OS was significantly longer in patients who received VR-CAP than in those who received R-CHOP—90.7 months and 55.7 months, respectively.
This survival benefit was observed in patients with low- and intermediate-risk disease but not high-risk disease.
Tadeusz Robak, MD, of the Medical University of Lodz in Poland, and his colleagues reported these results in The Lancet Oncology alongside a related commentary.
The LYM-3002 trial began more than a decade ago, and initial results were published in 2015. At that time, the VR-CAP group showed a significant increase in progression-free survival compared with the R-CHOP group.
The final analysis of LYM-3002 included 268 of the original 487 MCL patients. Twenty-three percent of patients in the VR-CAP group (n=32) discontinued due to death, as did 40% of patients in the R-CHOP group (n=51). The main cause of death was progression—29% and 14%, respectively.
Among the 268 patients in the final analysis, 140 belonged to the VR-CAP group and 128 to the R-CHOP group. The patients’ median age was 66 (range, 26-83), 71% (n=190) were male, 74% (n=199) had stage IV disease, and 31% were classified as high risk based on the MCL-specific International Prognostic Index (MIPI).
About half of patients received therapies after the trial interventions (n=255, 52%)—43% (n=104) in the VR-CAP group and 62% (n=151) in the R-CHOP group. Most patients received subsequent antineoplastic therapy—77% (n=80) and 81% (n=123), respectively—and more than half received rituximab as second-line therapy—53% (n=55) and 59% (n=89), respectively.
Results
At a median follow-up of 82.0 months, the median OS was significantly longer in the VR-CAP group than in the R-CHOP group—90.7 months (95% CI, 71.4 to not estimable) and 55.7 months (95% CI, 47.2 to 68.9), respectively (hazard ratio [HR]=0.66 [95% CI, 0.51–0.85]; P=0.001).
The 4-year OS was 67.3% in the VR-CAP group and 54.3% in the R-CHOP group. The 6-year OS was 56.6% and 42.0%, respectively.
The researchers noted that VR-CAP was associated with significantly improved OS among patients in the low-risk and intermediate-risk MIPI categories but not in the high-risk category.
In the low-risk cohort, the median OS was 81.7 months in the R-CHOP group and not estimable in the VR-CAP group (HR=0.54 [95% CI, 0.30–0.95]; P≤0.05).
In the intermediate-risk cohort, the median OS was 62.2 months in the R-CHOP group and not estimable in the VR-CAP group (HR=0.55 [95% CI, 0.36–0.85]; P≤0.01).
In the high-risk cohort, the median OS was 37.1 months in the R-CHOP group and 30.4 months in the VR-CAP group (HR=1.02 [95% CI, 0.69–1.50]).
The researchers reported three new adverse events in the final analysis—grade 4 lung adenocarcinoma and grade 4 gastric cancer in the VR-CAP group as well as grade 2 pneumonia in the R-CHOP group.
The team acknowledged that a key limitation of this study was that rituximab was not given as maintenance since it was not considered standard care at the time of study initiation.
Moving forward, Dr. Robak and his colleagues recommend that bortezomib be investigated in combination with newer targeted therapies in order to establish best practice for treating MCL.
The LYM-3002 study was sponsored by Janssen Research & Development. The study authors reported financial ties to Janssen, Celgene, Ipsen, Johnson & Johnson, Novartis, and other companies.
Older age predicts mortality after alloHCT in NHL, but not relapse
Elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within 1 year of allogeneic hematopoietic cell transplantation (alloHCT), compared with younger or middle-age patients, according to investigators.
Comorbidities also increased risks of nonrelapse mortality (NRM) at 1 year, but to a lesser extent than that of elderly status, reported lead author Charalampia Kyriakou, MD, PhD, of the department of haematology at University College London Hospital and London North West University Healthcare NHS Trust, and her colleagues.
“Although alloHCT is feasible and effective in very old patients, the increased NRM risk must be taken into account when assessing the indication for alloHCT for NHL in this age group,” the investigators wrote in Biology of Blood and Marrow Transplantation.
This decision is becoming more common, they noted. “With the advent of reduced-intensity conditioning (RIC) strategies and other improvements in transplantation technology, alloHCT is being increasingly considered in elderly patients with [relapsed and refractory] NHL.”
The retrospective study analyzed 3,919 patients with NHL who underwent alloHCT between 2003 and 2013. Patients were sorted into three age groups: young (18-50 years), middle age (51-65 years), or elderly (66-77 years).
Disease types also were reported: 1,461 patients had follicular lymphoma (FL; 37%), 1,192 had diffuse large B cell lymphoma (DLBCL; 30%), 823 had mantle cell lymphoma (MCL; 21%), and 443 had peripheral T cell lymphoma (PTCL; 11%).
At the time of alloHCT, about 85% of patients were chemosensitive, with the remainder being chemorefractory. The age groups had similar patient characteristics, with exceptions noted for unrelated donors, MCL, and RIC, which became increasingly overrepresented with age.
The results showed that NRM at 1 year was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001). Overall survival at 3 years followed an inverse trend, decreasing with age from 60% in young patients to 54% in middle-age patients, before dropping more dramatically to 38% in the elderly (P less than .001).
In contrast to these significant associations between age and survival, relapse risk at 3 years remained relatively consistent, with young patients at 30%, middle-age patients at 31%, and elderly patients at 28% (P = .355).
The investigators noted that the risk of NRM increased most dramatically between middle age and old age, with less significant differences between the middle-age and young groups. They suggested that “age per se should have a limited impact on the indication for alloHCT for NHL in patients up to age 65 years.”
The increased risk with elderly status could not be fully explained by comorbidities, although these were more common in elderly patients. After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.” Therefore, age remains an independent risk factor.
“The information provided in this cohort of patients with NHL, the largest reported to date, is useful and relevant, especially in the era of evolving therapies,” the investigators wrote. They added that the information is “even more relevant now with the availability of treatment with ... chimeric antigen receptor (CAR) T cells ... after relapse post-alloHCT.”
The investigators reported having no financial disclosures.
SOURCE: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
Elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within 1 year of allogeneic hematopoietic cell transplantation (alloHCT), compared with younger or middle-age patients, according to investigators.
Comorbidities also increased risks of nonrelapse mortality (NRM) at 1 year, but to a lesser extent than that of elderly status, reported lead author Charalampia Kyriakou, MD, PhD, of the department of haematology at University College London Hospital and London North West University Healthcare NHS Trust, and her colleagues.
“Although alloHCT is feasible and effective in very old patients, the increased NRM risk must be taken into account when assessing the indication for alloHCT for NHL in this age group,” the investigators wrote in Biology of Blood and Marrow Transplantation.
This decision is becoming more common, they noted. “With the advent of reduced-intensity conditioning (RIC) strategies and other improvements in transplantation technology, alloHCT is being increasingly considered in elderly patients with [relapsed and refractory] NHL.”
The retrospective study analyzed 3,919 patients with NHL who underwent alloHCT between 2003 and 2013. Patients were sorted into three age groups: young (18-50 years), middle age (51-65 years), or elderly (66-77 years).
Disease types also were reported: 1,461 patients had follicular lymphoma (FL; 37%), 1,192 had diffuse large B cell lymphoma (DLBCL; 30%), 823 had mantle cell lymphoma (MCL; 21%), and 443 had peripheral T cell lymphoma (PTCL; 11%).
At the time of alloHCT, about 85% of patients were chemosensitive, with the remainder being chemorefractory. The age groups had similar patient characteristics, with exceptions noted for unrelated donors, MCL, and RIC, which became increasingly overrepresented with age.
The results showed that NRM at 1 year was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001). Overall survival at 3 years followed an inverse trend, decreasing with age from 60% in young patients to 54% in middle-age patients, before dropping more dramatically to 38% in the elderly (P less than .001).
In contrast to these significant associations between age and survival, relapse risk at 3 years remained relatively consistent, with young patients at 30%, middle-age patients at 31%, and elderly patients at 28% (P = .355).
The investigators noted that the risk of NRM increased most dramatically between middle age and old age, with less significant differences between the middle-age and young groups. They suggested that “age per se should have a limited impact on the indication for alloHCT for NHL in patients up to age 65 years.”
The increased risk with elderly status could not be fully explained by comorbidities, although these were more common in elderly patients. After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.” Therefore, age remains an independent risk factor.
“The information provided in this cohort of patients with NHL, the largest reported to date, is useful and relevant, especially in the era of evolving therapies,” the investigators wrote. They added that the information is “even more relevant now with the availability of treatment with ... chimeric antigen receptor (CAR) T cells ... after relapse post-alloHCT.”
The investigators reported having no financial disclosures.
SOURCE: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
Elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within 1 year of allogeneic hematopoietic cell transplantation (alloHCT), compared with younger or middle-age patients, according to investigators.
Comorbidities also increased risks of nonrelapse mortality (NRM) at 1 year, but to a lesser extent than that of elderly status, reported lead author Charalampia Kyriakou, MD, PhD, of the department of haematology at University College London Hospital and London North West University Healthcare NHS Trust, and her colleagues.
“Although alloHCT is feasible and effective in very old patients, the increased NRM risk must be taken into account when assessing the indication for alloHCT for NHL in this age group,” the investigators wrote in Biology of Blood and Marrow Transplantation.
This decision is becoming more common, they noted. “With the advent of reduced-intensity conditioning (RIC) strategies and other improvements in transplantation technology, alloHCT is being increasingly considered in elderly patients with [relapsed and refractory] NHL.”
The retrospective study analyzed 3,919 patients with NHL who underwent alloHCT between 2003 and 2013. Patients were sorted into three age groups: young (18-50 years), middle age (51-65 years), or elderly (66-77 years).
Disease types also were reported: 1,461 patients had follicular lymphoma (FL; 37%), 1,192 had diffuse large B cell lymphoma (DLBCL; 30%), 823 had mantle cell lymphoma (MCL; 21%), and 443 had peripheral T cell lymphoma (PTCL; 11%).
At the time of alloHCT, about 85% of patients were chemosensitive, with the remainder being chemorefractory. The age groups had similar patient characteristics, with exceptions noted for unrelated donors, MCL, and RIC, which became increasingly overrepresented with age.
The results showed that NRM at 1 year was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001). Overall survival at 3 years followed an inverse trend, decreasing with age from 60% in young patients to 54% in middle-age patients, before dropping more dramatically to 38% in the elderly (P less than .001).
In contrast to these significant associations between age and survival, relapse risk at 3 years remained relatively consistent, with young patients at 30%, middle-age patients at 31%, and elderly patients at 28% (P = .355).
The investigators noted that the risk of NRM increased most dramatically between middle age and old age, with less significant differences between the middle-age and young groups. They suggested that “age per se should have a limited impact on the indication for alloHCT for NHL in patients up to age 65 years.”
The increased risk with elderly status could not be fully explained by comorbidities, although these were more common in elderly patients. After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.” Therefore, age remains an independent risk factor.
“The information provided in this cohort of patients with NHL, the largest reported to date, is useful and relevant, especially in the era of evolving therapies,” the investigators wrote. They added that the information is “even more relevant now with the availability of treatment with ... chimeric antigen receptor (CAR) T cells ... after relapse post-alloHCT.”
The investigators reported having no financial disclosures.
SOURCE: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point:
Major finding: One-year nonrelapse mortality (NRM) was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001).
Study details: A retrospective analysis of 3,919 patients with NHL who underwent alloHCT between 2003 and 2013.
Disclosures: The researchers reported having no financial disclosures.
Source: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
When to choose stem cell transplant in PTCL
DUBROVNIK, CROATIA – , according to one expert.
The success of HSCT varies according to the subtype of PTCL and the type of transplant, Ali Bazarbachi, MD, PhD, of the American University of Beirut, Lebanon, said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with nonlocalized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired, Dr. Bazarbachi said.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable – localized ENKTL and anaplastic lymphoma kinase–positive (ALK-positive) ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 2012, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival – compared with previous results with CHOP – in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma (J Clin Oncol. 2012 Sep 1;30[25]:3093-9; ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924).
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients – those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study showed improved survival in patients who received consolidation with auto- or allo-HSCT, compared with patients who did not receive a transplant (Blood. 2017;130:342).
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, Dr. Bazarbachi said.
However, chemosensitive patients who have relapsed should receive auto-HSCT only if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
ATLL
ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative, Dr. Bazarbachi said. It is most effective in patients who have achieved a complete or partial response to induction (Blood. 2012 Aug 23;120[8]:1734-41).
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Also, allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time, as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 2014, all four ATLL patients who underwent auto-HSCT “rapidly” died (Bone Marrow Transplant. 2014 Oct;49[10]:1266-8).
ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients, and it is most effective when patients have achieved a complete response to induction (Biol Blood Marrow Transplant. 2008 Dec;14[12]:1356-64).
Allo-HSCT also is an option for frontline consolidation in patients with nonlocalized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should receive auto-HSCT only if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, nonlocalized ENKTL, nasal type, should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
Dr. Bazarbachi did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – , according to one expert.
The success of HSCT varies according to the subtype of PTCL and the type of transplant, Ali Bazarbachi, MD, PhD, of the American University of Beirut, Lebanon, said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with nonlocalized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired, Dr. Bazarbachi said.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable – localized ENKTL and anaplastic lymphoma kinase–positive (ALK-positive) ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 2012, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival – compared with previous results with CHOP – in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma (J Clin Oncol. 2012 Sep 1;30[25]:3093-9; ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924).
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients – those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study showed improved survival in patients who received consolidation with auto- or allo-HSCT, compared with patients who did not receive a transplant (Blood. 2017;130:342).
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, Dr. Bazarbachi said.
However, chemosensitive patients who have relapsed should receive auto-HSCT only if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
ATLL
ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative, Dr. Bazarbachi said. It is most effective in patients who have achieved a complete or partial response to induction (Blood. 2012 Aug 23;120[8]:1734-41).
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Also, allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time, as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 2014, all four ATLL patients who underwent auto-HSCT “rapidly” died (Bone Marrow Transplant. 2014 Oct;49[10]:1266-8).
ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients, and it is most effective when patients have achieved a complete response to induction (Biol Blood Marrow Transplant. 2008 Dec;14[12]:1356-64).
Allo-HSCT also is an option for frontline consolidation in patients with nonlocalized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should receive auto-HSCT only if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, nonlocalized ENKTL, nasal type, should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
Dr. Bazarbachi did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
DUBROVNIK, CROATIA – , according to one expert.
The success of HSCT varies according to the subtype of PTCL and the type of transplant, Ali Bazarbachi, MD, PhD, of the American University of Beirut, Lebanon, said at Leukemia and Lymphoma, a meeting jointly sponsored by the University of Texas MD Anderson Cancer Center and the School of Medicine at the University of Zagreb, Croatia.
For example, autologous (auto) HSCT given as frontline consolidation can be considered the standard of care for PTCL–not otherwise specified (NOS), angioimmunoblastic T-cell lymphoma (AITL), and certain patients with anaplastic large-cell lymphoma (ALCL), according to Dr. Bazarbachi.
On the other hand, auto-HSCT should never be used in patients with adult T-cell leukemia/lymphoma (ATLL).
Both auto-HSCT and allogeneic (allo) HSCT are options for patients with nonlocalized, extranodal natural killer T-cell lymphoma (ENKTL), nasal type, but only at certain times.
State of PTCL treatment
Patients with newly diagnosed PTCL are no longer treated like patients with B-cell lymphoma, but treatment outcomes in PTCL still leave a lot to be desired, Dr. Bazarbachi said.
He noted that, with any of the chemotherapy regimens used, typically, about a third of patients are primary refractory, a third relapse, and a quarter are cured. Only two forms of PTCL are frequently curable – localized ENKTL and anaplastic lymphoma kinase–positive (ALK-positive) ALCL.
Current treatment strategies for PTCL do include HSCT, but recommendations vary. Dr. Bazarbachi made the following recommendations, supported by evidence from clinical trials.
PTCL-NOS, AITL, and ALCL
For patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, auto-HSCT as frontline consolidation can be considered the standard of care in patients who responded to induction, Dr. Bazarbachi said.
In a study published in 2012, high-dose chemotherapy and auto-HSCT as consolidation improved 5-year overall survival – compared with previous results with CHOP – in patients with ALK-negative ALCL, AITL, PTCL-NOS, and enteropathy-associated T-cell lymphoma (J Clin Oncol. 2012 Sep 1;30[25]:3093-9; ISRN Hematol. 2011 Jun 16. doi: 10.5402/2011/623924).
Allo-HSCT may also be an option for frontline consolidation in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, according to Dr. Bazarbachi.
“Allo-transplant is not dead in this indication,” he said. “But it should be either part of a clinical trial or [given] to some selected patients – those with persistent bone marrow involvement, very young patients, or patients with primary refractory disease.”
Results from the COMPLETE study showed improved survival in patients who received consolidation with auto- or allo-HSCT, compared with patients who did not receive a transplant (Blood. 2017;130:342).
COMPLETE patients with AITL or PTCL-NOS had improvements in progression-free and overall survival with HSCT. The survival advantage was “less evident” in patients with ALCL, the researchers said, but this trial included both ALK-negative and ALK-positive patients.
Allo- and auto-HSCT can be options after relapse in patients with PTCL-NOS, AITL, or ALK-negative, non-DUSP22 ALCL, Dr. Bazarbachi said.
However, chemosensitive patients who have relapsed should receive auto-HSCT only if they did not receive it frontline. Patients who have already undergone auto-HSCT can receive allo-HSCT, Dr. Bazarbachi said.
He added that refractory patients should not undergo auto-HSCT and should receive allo-HSCT only within the context of a clinical trial.
ATLL
ATLL has a dismal prognosis, but allo-HSCT as frontline consolidation is potentially curative, Dr. Bazarbachi said. It is most effective in patients who have achieved a complete or partial response to induction (Blood. 2012 Aug 23;120[8]:1734-41).
However, allo-HSCT should not be given as consolidation to ATLL patients who have received prior mogamulizumab. These patients have an increased risk of morbidity and mortality if they undergo allo-HSCT.
Also, allo-HSCT should not be given to refractory ATLL patients, although it may be an option for relapsed patients.
Dr. Bazarbachi stressed that ATLL patients should not receive auto-HSCT at any time, as frontline consolidation, after relapse, or if they have refractory disease.
Auto-HSCT “does not work in this disease,” he said. In a study published in 2014, all four ATLL patients who underwent auto-HSCT “rapidly” died (Bone Marrow Transplant. 2014 Oct;49[10]:1266-8).
ENKTL
Dr. Bazarbachi said frontline consolidation with auto-HSCT should be considered the standard of care for patients with non-localized ENKTL, nasal type.
Auto-HSCT has been shown to improve survival in these patients, and it is most effective when patients have achieved a complete response to induction (Biol Blood Marrow Transplant. 2008 Dec;14[12]:1356-64).
Allo-HSCT also is an option for frontline consolidation in patients with nonlocalized ENKTL, nasal type, Dr. Bazarbachi said.
He added that chemosensitive patients who have relapsed can receive allo-HSCT, but they should receive auto-HSCT only if they did not receive it in the frontline setting. Both types of transplant should take place when patients are in complete remission.
Patients with refractory, nonlocalized ENKTL, nasal type, should not receive auto-HSCT, but allo-HSCT is an option, Dr. Bazarbachi said.
Dr. Bazarbachi did not declare any conflicts of interest.
The Leukemia and Lymphoma meeting is organized by Jonathan Wood & Associates, which is owned by the parent company of this news organization.
EXPERT ANALYSIS FROM LEUKEMIA AND LYMPHOMA 2018
Age limits restrict AYA participation in relevant trials
MUNICH—Age limits imposed in European countries can prevent adolescents and young adults (AYAs) from enrolling in appropriate clinical trials, a new study suggests.
Investigators reviewed phase 1 and 2 trials conducted over a 6-year period at a single center in France.
The results showed that adolescents were prevented from enrolling in potentially beneficial adult trials, and young adults were unable to enroll in potentially beneficial pediatric trials.
These results were presented at the ESMO 2018 Congress (abstract 424P_PR).
In Europe, the legal minimum age to participate in adult clinical trials is typically 18.
“We know, however, that certain girls will develop genetically driven breast cancers very early in life,” said Dr. Aurore Vozy, of Gustave Roussy Institut de Cancérologie in Villejuif, France.
“There are no pediatric trials for this disease, yet these patients are systematically barred from participating in the relevant adult trials. The situation is similar for some adolescents with lymphomas or sarcomas, whose tumors often resemble those of adults much more closely than those found in children.”
On the other hand, adults in their early twenties may be diagnosed with cancers most commonly seen in children. And pediatric clinical trials typically set an upper age limit of 18 or 21.
To assess the availability and accessibility of new treatments to AYA cancer patients, Dr. Vozy and her colleagues conducted a review of all phase 1 and 2 trials opened at Gustave Roussy from 2012 through 2017 for patients with solid tumors or lymphomas.
Over the 6-year period, 465 trials were open—403 adult trials and 62 pediatric trials.
Only 65 of the trials (14%) included patients between the ages of 12 and 17.
“In other words, patients in this age group had access to less than 15% of all the early phase trials at our institute,” Dr. Vozy said.
In all, there were 389 trials that were not open to adolescents, and the investigators found that 55% of these trials could have been relevant for underage patients. Twenty-eight of the trials targeted tumor types that are particularly common among teenagers.
“This means that patients have been denied access to innovative medicines which were available at the very center where they were being treated, and to which they may have had a better response than to conventional therapy,” Dr. Vozy said.
She and her colleagues also found that young adults were often unable to enroll in pediatric trials.
There were 62 pediatric trials open over the period studied, and more than half of them (n=36, 58%) did not recruit patients aged 19 to 25, even though 10 of these trials targeted tumor types that also occur in this age group.
“Raising the age bar in pediatric trials to 25 years would clearly make sense in certain cases,” Dr. Vozy said.
She argued, however, that the more pressing issue is the current age limit in adult trials.
“We know that the diseases, toxicities, and pharmacology seen in 12- to 17-year-olds are similar to what we find in adults, so it would be feasible to include these patients in adult trials at no additional risk to them,” Dr. Vozy said.
This has already been done successfully in the United States, where the minimum age for trial participation has been lowered to 12 years.
An additional measure to consider, Dr. Vozy said, is creating dedicated trial cohorts for adolescents within adult trials.
“In a context where, today, most phase 1 trials in oncology are launched with multiple study populations for different tumor types, it would be easy to cater to the specific needs of adolescents by including them in cohorts of their own,” she said.
“The main constraint is that trials which include underage patients should only be conducted in centers that also have pediatric services onsite. Adolescents may be affected by disease similarly to adults, but they still need to be treated and followed up on by pediatric specialists.”
One investigator involved in this study reported relationships with Amgen, Astellas, Astra Zeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. All other investigators declared no conflicts of interest.
MUNICH—Age limits imposed in European countries can prevent adolescents and young adults (AYAs) from enrolling in appropriate clinical trials, a new study suggests.
Investigators reviewed phase 1 and 2 trials conducted over a 6-year period at a single center in France.
The results showed that adolescents were prevented from enrolling in potentially beneficial adult trials, and young adults were unable to enroll in potentially beneficial pediatric trials.
These results were presented at the ESMO 2018 Congress (abstract 424P_PR).
In Europe, the legal minimum age to participate in adult clinical trials is typically 18.
“We know, however, that certain girls will develop genetically driven breast cancers very early in life,” said Dr. Aurore Vozy, of Gustave Roussy Institut de Cancérologie in Villejuif, France.
“There are no pediatric trials for this disease, yet these patients are systematically barred from participating in the relevant adult trials. The situation is similar for some adolescents with lymphomas or sarcomas, whose tumors often resemble those of adults much more closely than those found in children.”
On the other hand, adults in their early twenties may be diagnosed with cancers most commonly seen in children. And pediatric clinical trials typically set an upper age limit of 18 or 21.
To assess the availability and accessibility of new treatments to AYA cancer patients, Dr. Vozy and her colleagues conducted a review of all phase 1 and 2 trials opened at Gustave Roussy from 2012 through 2017 for patients with solid tumors or lymphomas.
Over the 6-year period, 465 trials were open—403 adult trials and 62 pediatric trials.
Only 65 of the trials (14%) included patients between the ages of 12 and 17.
“In other words, patients in this age group had access to less than 15% of all the early phase trials at our institute,” Dr. Vozy said.
In all, there were 389 trials that were not open to adolescents, and the investigators found that 55% of these trials could have been relevant for underage patients. Twenty-eight of the trials targeted tumor types that are particularly common among teenagers.
“This means that patients have been denied access to innovative medicines which were available at the very center where they were being treated, and to which they may have had a better response than to conventional therapy,” Dr. Vozy said.
She and her colleagues also found that young adults were often unable to enroll in pediatric trials.
There were 62 pediatric trials open over the period studied, and more than half of them (n=36, 58%) did not recruit patients aged 19 to 25, even though 10 of these trials targeted tumor types that also occur in this age group.
“Raising the age bar in pediatric trials to 25 years would clearly make sense in certain cases,” Dr. Vozy said.
She argued, however, that the more pressing issue is the current age limit in adult trials.
“We know that the diseases, toxicities, and pharmacology seen in 12- to 17-year-olds are similar to what we find in adults, so it would be feasible to include these patients in adult trials at no additional risk to them,” Dr. Vozy said.
This has already been done successfully in the United States, where the minimum age for trial participation has been lowered to 12 years.
An additional measure to consider, Dr. Vozy said, is creating dedicated trial cohorts for adolescents within adult trials.
“In a context where, today, most phase 1 trials in oncology are launched with multiple study populations for different tumor types, it would be easy to cater to the specific needs of adolescents by including them in cohorts of their own,” she said.
“The main constraint is that trials which include underage patients should only be conducted in centers that also have pediatric services onsite. Adolescents may be affected by disease similarly to adults, but they still need to be treated and followed up on by pediatric specialists.”
One investigator involved in this study reported relationships with Amgen, Astellas, Astra Zeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. All other investigators declared no conflicts of interest.
MUNICH—Age limits imposed in European countries can prevent adolescents and young adults (AYAs) from enrolling in appropriate clinical trials, a new study suggests.
Investigators reviewed phase 1 and 2 trials conducted over a 6-year period at a single center in France.
The results showed that adolescents were prevented from enrolling in potentially beneficial adult trials, and young adults were unable to enroll in potentially beneficial pediatric trials.
These results were presented at the ESMO 2018 Congress (abstract 424P_PR).
In Europe, the legal minimum age to participate in adult clinical trials is typically 18.
“We know, however, that certain girls will develop genetically driven breast cancers very early in life,” said Dr. Aurore Vozy, of Gustave Roussy Institut de Cancérologie in Villejuif, France.
“There are no pediatric trials for this disease, yet these patients are systematically barred from participating in the relevant adult trials. The situation is similar for some adolescents with lymphomas or sarcomas, whose tumors often resemble those of adults much more closely than those found in children.”
On the other hand, adults in their early twenties may be diagnosed with cancers most commonly seen in children. And pediatric clinical trials typically set an upper age limit of 18 or 21.
To assess the availability and accessibility of new treatments to AYA cancer patients, Dr. Vozy and her colleagues conducted a review of all phase 1 and 2 trials opened at Gustave Roussy from 2012 through 2017 for patients with solid tumors or lymphomas.
Over the 6-year period, 465 trials were open—403 adult trials and 62 pediatric trials.
Only 65 of the trials (14%) included patients between the ages of 12 and 17.
“In other words, patients in this age group had access to less than 15% of all the early phase trials at our institute,” Dr. Vozy said.
In all, there were 389 trials that were not open to adolescents, and the investigators found that 55% of these trials could have been relevant for underage patients. Twenty-eight of the trials targeted tumor types that are particularly common among teenagers.
“This means that patients have been denied access to innovative medicines which were available at the very center where they were being treated, and to which they may have had a better response than to conventional therapy,” Dr. Vozy said.
She and her colleagues also found that young adults were often unable to enroll in pediatric trials.
There were 62 pediatric trials open over the period studied, and more than half of them (n=36, 58%) did not recruit patients aged 19 to 25, even though 10 of these trials targeted tumor types that also occur in this age group.
“Raising the age bar in pediatric trials to 25 years would clearly make sense in certain cases,” Dr. Vozy said.
She argued, however, that the more pressing issue is the current age limit in adult trials.
“We know that the diseases, toxicities, and pharmacology seen in 12- to 17-year-olds are similar to what we find in adults, so it would be feasible to include these patients in adult trials at no additional risk to them,” Dr. Vozy said.
This has already been done successfully in the United States, where the minimum age for trial participation has been lowered to 12 years.
An additional measure to consider, Dr. Vozy said, is creating dedicated trial cohorts for adolescents within adult trials.
“In a context where, today, most phase 1 trials in oncology are launched with multiple study populations for different tumor types, it would be easy to cater to the specific needs of adolescents by including them in cohorts of their own,” she said.
“The main constraint is that trials which include underage patients should only be conducted in centers that also have pediatric services onsite. Adolescents may be affected by disease similarly to adults, but they still need to be treated and followed up on by pediatric specialists.”
One investigator involved in this study reported relationships with Amgen, Astellas, Astra Zeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. All other investigators declared no conflicts of interest.
Study challenges LVEF assessment before DLBCL treatment
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
FROM MAYO CLINIC PROCEEDINGS: INNOVATIONS, QUALITY & OUTCOMES
Key clinical point:
Major finding: Among diffuse large B-cell lymphoma (DLBCL) patients who had LVEF measured, the incidence of heart failure post treatment did not differ between patients who received doxorubicin and those who did not (P = 1.0).
Study details: A retrospective analysis of 291 patients diagnosed with DLBCL between 2001 and 2013.
Disclosures: The researchers reported having no competing interests.
Source: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Study supports sequencing in kids with cancer
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
SAN DIEGO—Comprehensive next-generation sequencing is both feasible and clinically useful in pediatric cancer patients, a new study suggests.
Researchers sequenced samples from 253 pediatric cancer patients and found that, in 79% of cases, there was at least one finding that could help guide care.
Scott Newman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, presented these findings at the American Society of Human Genetics (ASHG) 2018 Annual Meeting (abstract 52).
The researchers conducted whole-genome, exome, and transcriptome sequencing of the patients’ tumors, as well as sequencing non-cancerous tissues from the same patients.
Of the 253 patients studied, 123 had hematologic malignancies.
The researchers found a mean of four pathogenic or likely pathogenic variants per patient (range, 0-18). This included prognostic (21.8%) and diagnostic (15.1%) variants as well as variants that could be targeted therapeutically (6.8%).
In all, 79% of the patients had at least one variant that was targetable, diagnostic, or prognostic. And test results were available within about 40 days, quickly enough that they could be used to guide care.
“With results available in a clinically relevant time frame, and pricing becoming increasingly comparable to the radiology and pathology tests, WGS [whole-genome sequencing] is becoming more accessible to pediatric oncology patients,” Dr. Newman said.
This work was part of the Genomes for Kids study (G4K), an effort to understand how best to use genetic data for pediatric cancer diagnosis and treatment. St. Jude has compiled the information from G4K into a publicly accessible online database.
The researchers have continued to perform sequencing on current patients, and, since the original study ended, have successfully used this method on roughly 300 additional patients. The team plans to continue studying sequencing methods in hopes of producing clinically applicable data more quickly.
G4K was sponsored by St. Jude.
Ibrutinib discontinuation harms survival in CLL
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
Key clinical point:
Major finding: Median overall survival after discontinuation of ibrutinib due to disease progression was 1.7 months.
Study details: A single-institution study of 170 patients with CLL or non-Hodgkin lymphoma who were taking ibrutinib.
Disclosures: The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
Source: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.