User login
Bone remodeling associated with CTLA-4 inhibition: an unreported side effect
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
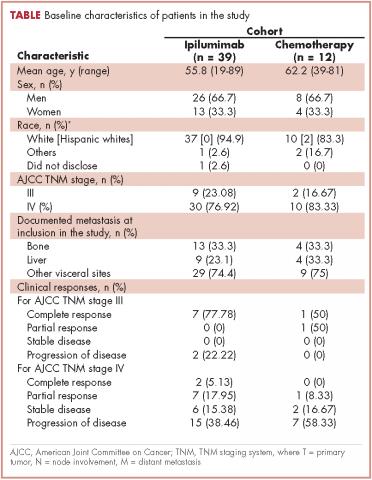
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
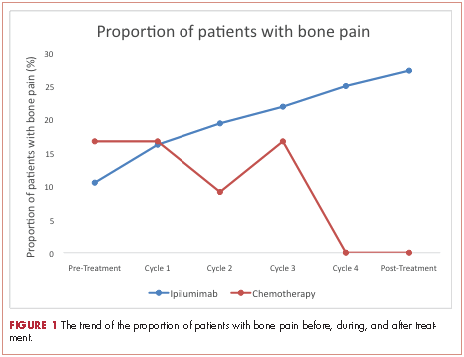
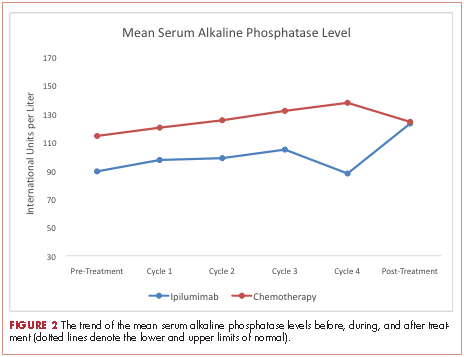
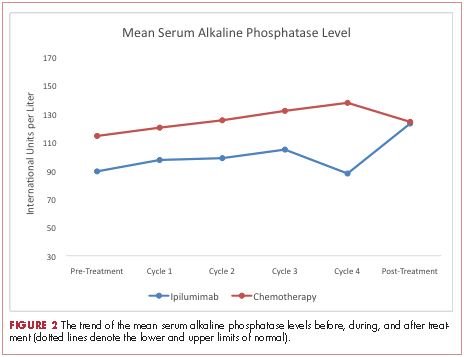
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Intramedullary spinal cord and leptomeningeal metastases presenting as cauda equina syndrome in a patient with melanoma
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
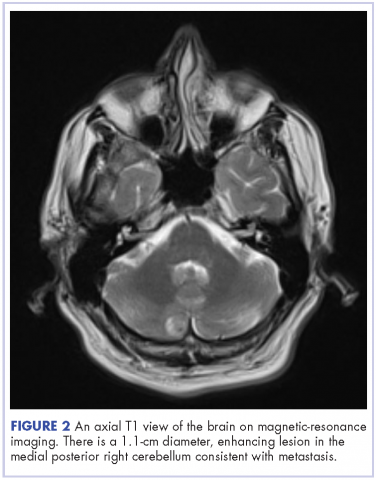
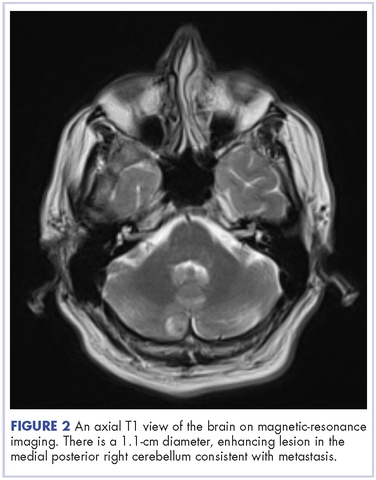
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
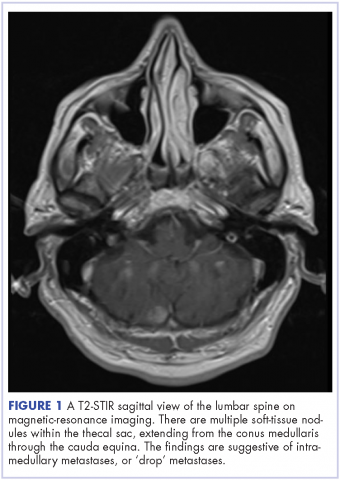
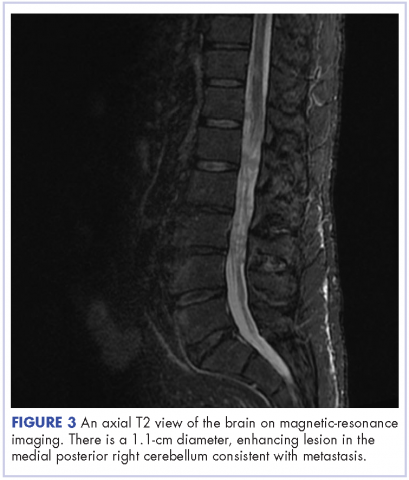
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.
Conclusions
Although metastatic melanoma to the brain is not uncommon, leptomeningeal and intramedullary drop metastases are an infrequent presentation. Even more rare are intramedullary drop metastasis that are significant enough to cause cauda equina syndrome, as with our patient. The incidence of LMD has increased over the years and may continue to increase, likely because of the improved overall survival and a prolonged control of extracranial disease with newly approved systemic therapeutic drugs, such as molecularly targeted therapy and immunotherapy.12 Intramedullary metastases are extremely rare, but reported incidence has seemed to be increasing due to detection with MRI. Currently there are fewer than 100 case reports of intramedullary spinal cord metastasis.6 In one retrospective study, 40 patients with intramedullary metastatic disease secondary to systemic cancer were identified during 1980-1993.6 About half of those cases were from lung cancer, the second most common was breast cancer.
CNS involvement by melanoma can have debilitating complications and confers a poor prognosis. In another retrospective study, several patient characteristics were found to be associated with significantly shorter survival in patients with known brain metastases, including presence of neurologic symptoms and leptomeningeal involvement.3
Malignant cells can reach the CSF by several routes: direct extension, hematogenous, venous access, venous drainage from bone marrow and cranial and peripheral nerves. Once the tumor has reached the CSF, it can seed any portion of the nervous system that has contact with the CSF and become entangled among the cauda equine.13
Given the rarity of leptomeningeal and intramedullary involvement of melanoma, there are no standard treatment guidelines. Treatment for LMD usually consists of intrathecal and systemic chemotherapy. Commonly used intrathecal agents are methotrexate, liposomal cytarabine, and thiopeta.11 The goals of treatment are to improve or stabilize neurologic status of the patient and ideally prolong survival. The choice of agent for intrathecal chemotherapy is guided by the primary tumor, however, there is no strong evidence to choose one agent over the other.12,14 Methotrexate or cytarabine are generally recommended in the National Comprehensive Cancer Network (NCCN) guidelines. Targeted therapy toward the primary tumor is occasionally used for treatment of LMD, for example rituximab can be given intrathecally for lymphoma,15 and trastuzumab has been given intrathecally for breast cancer.16 No intrathecal targeted agents are currently available for melanoma. Administration of intrathecal chemotherapy is given via lumbar puncture or Ommaya reservoir. Induction intrathecal chemotherapy is recommended by NCCN to be given for 4-6 weeks. The schedule of administration varies based on the agent used. Most systemic chemotherapy has poor CSF penetration, which is the basis behind using chemotherapy intrathecally in these patients.14 However, novel therapies for melanoma, such as ipilimumab, have shown activity in the CNS, and it is not known if intrathecal chemotherapy will continue to play role in the management of LMD.17
Systemic therapy for metastatic melanoma has changed with the development of novel agents, which have shown better efficacy than traditional chemotherapy. The recommendation for first-line systemic therapy of metastatic unresectable melanoma is based on several factors: BRAF mutation status, tempo of disease, and presence or absence of cancer-related symptoms. Immunotherapy for metastatic melanoma that is unresectable includes anti-programmed cell death protein-1 (PD-1) monotherapy (nivolumab or pembrolizumab) or combination therapy with nivolumab plus ipilimumab. Targeted therapy is preferred in cases with an identified BRAF mutation. Combination therapy with dabrafenib plus trametinib or with vemurafenib plus cobimetinib is recommended. Single-agent therapy may also be used with dabrafenib or vemurafenib.18
Ipilimumab is a monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 to potentiate an anti-tumor T-cell response that was approved in 2011 by the US Food and Drug Administration for the treatment of melanoma. A randomized, phase 3 clinical trial showed an increase in overall survival in patients with unresectable metastatic disease who had received previous treatment.19 Before that, no therapy had been shown to improve overall survival in patients with metastatic melanoma. Patients with CNS metastases were included in this study.19
The activity of ipilimumab specifically in patients with brain metastasis was further studied in a phase 2 trial that enrolled 72 patients, 1 cohort with symptomatic brain metastases and the other cohort with asymptomatic brain metastases.20 After 12 weeks of therapy, response was assessed by modified World Health Organization criteria for disease control (complete response plus partial response plus stable disease). In all, 18% of patients with asymptomatic brain metastasis achieved disease control, compared with 10% of patients with symptomatic brain metastases. When the brain alone was assessed, 24% of asymptomatic patients and 10% of symptomatic patients achieved disease control. No unexpected toxic effects occurred during the study. Anti-PD1 therapy such as nivolumab, which has shown durable responses in metastatic melanoma, has no published results specifically in patients with active brain metastases.
Of the BRAF-targeted therapy, dabrafenib and vemurafenib have also been studied in patients with brain metastases. For darafenib, 172 patients with BRAF-mutated metastatic melanoma were included in a phase 2 clinical trial that showed an intracranial response of 39% in previously untreated patients and 31% in patients whose brain metastases had progressed after previous local treatment.21 Vemurafenib has also shown intracranial response in a phase 2 clinical trial.22
The role of the aforementioned therapies in patients with metastatic melanoma with CNS disease should not be overlooked because these patients are typically excluded from clinical trials. As already noted, agents such as ipilimumab and the dabrafenib–vemurafenib combination have been studied in patients with brain metastases and have shown disease control, but more studies are needed to truly assess whether there is an improvement in overall survival and whether that will change treatment guidelines. Although patients with parenchymal brain metastases were included in these studies, it is not clear how patients with LMD and intramedullary spinal cord metastases, such as our patient, would be affected. It is also not clear whether intrathecal chemotherapy will continue to play a role in management of metastatic melanoma with LMD, especially if these newer agents have CNS activity in addition to controlling extracranial disease. Although rarely documented, leptomeningeal and intramedullary metastatic disease will likely become increasingly recognized as patients with cancer live longer and diagnostic studies improve. These initial studies showing intracranial disease control show compelling evidence to continue enrolling patients with active CNS disease in clinical trials.
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
1. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S17.e1-S17.e11.
2. Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma: a study of 216 autopsy cases. Am J Surg. 1978;135(6):807-810.
3. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
4. Sun L, Song Y, Gong Q. Easily misdiagnosed delayed metastatic intraspinal extradural melanoma of the lumbar spine: a case report and review of the literature. Oncol Lett. 2013;5(6):1799-1802.
5. Moseley R, Davies A, Bourne S, et al. Neoplastic meningitis in malignant melanoma: diagnosis with monoclonal antibiodies. J Neurol Neurosurg Psychiatry. 1989;52:991-886.
6. Schiff D, O’Neill B. Intramedullary spinal cord metastases clinical features and treatment outcome. Neurology. 1996;47(4):906-912.
7. Fife KM, Colman MH, Stevens G, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293-1300.
8. Raizer J, Hwu W, Panageas K, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol. 2008;10(2):199-207.
9. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
10. Abernethy AP. Central nervous system tumors. In: Loprinzi C, ed. ASCO-SEP: Medical Oncology Self-evaluation Program. 4th ed. Alexandria, VA: American Society of Clinical Oncology, 2015. Page 396. Print.
11. Pape E, Desmedt E, Zairi , et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients. In Vivo. 2012;26(6):1079-1086.
12. Pavlidis N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann Oncol. 2004;15(Suppl 4):iv285-291.
13. DeAngelis L, Posner JB. Neurologic complications of cancer. 2nd ed. New York, NY: Oxford University Press; 2008.
14. Chamberlain, M. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627-635.
15. Chamberlain M, Johnston S, Van Horn A, Glantz MJ. Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol. 2009;91(3):271-277.
16. Zagouri F, Sergentanis T, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22.
17. Silk A, Bassetti M, West BT, Tsien C, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906.
18. [Behind paywall.] National Comprehensive Cancer Network. Melanoma (version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf. November 10, 2016. Accessed February 28, 2016
19. Hodi F, O’Day S, McDermott D, et al. Improved survival with ipilimumab in patients with metastatic melanoma. NEJM. 2010;363(8):711-723.
20. Margolin K, Ernstoff M, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459-465.
21. Long G, Trefzer U, Davies M, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087-1095.
22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicenter study. Ann Oncol. 2017;
Pembrolizumab, nivolumab linked to 3% rate of neurologic events
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
FROM JAMA NEUROLOGY
Key clinical point: Watch for immune-related adverse effects of nivolumab and pembrolizumab.
Major finding: Ten of 347 patients (2.9%) developed subacute neurologic immune-related adverse events, typically neuromuscular syndromes.
Data source: A single-center, retrospective cohort study of 347 patients who received pembrolizumab or nivolumab for metastatic melanoma or solid tumors.
Disclosures: The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Optical Coherence Tomography in Dermatology
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Practice Points
- Optical coherence tomography (OCT) technology has considerable utility in research and clinical settings given its high resolution, wide field of view, moderate penetration depth, straightforward image acquisition, and accessibility to anatomically challenging areas.
- Potential benefits of OCT include its ability to noninvasively diagnose and monitor nonmelanoma skin cancers as well as to delineate presurgical margins and elucidate the course and mechanism of action of skin conditions at the bedside.
- Limitations of OCT include device cost, lack of reimbursement, and training, as well as restricted ability to image advanced deep tumors and differentiate melanocytic lesions.
- Optical coherence tomography recently received 2 category III Current Procedural Terminology (CPT) codes to track its utilization in clinical practice and will hopefully receive category I CPT codes within the next 5 years.
Adverse effects of PD-1/PD-L1 inhibitors varied by tumor type in systematic review
The immune-related adverse effects of inhibitors of programmed cell death protein 1 (PD-1) and its ligand varied by tumor type in a large systematic review and meta-analysis.
Patients with melanoma were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8) compared with patients with non–small cell lung cancer, who were significantly more likely to develop pneumonitis, reported Leila Khoja, MBChB, PhD, of AstraZeneca UK, Melbourn, England, and associates. Patients with melanoma also were significantly more likely to develop arthralgia, hypothyroidism, rash, pruritus, and diarrhea compared with patients with renal cell carcinoma, who were more likely to develop pneumonitis and dyspnea.
“In light of this study, we should be mindful that different tumor types may have different immune-related adverse effect patterns when treated with the same immune checkpoint inhibitor,” the reviewers noted (Ann Oncol. 2017 Aug 8. doi: 10.1093/annonc/mdx286).
The review included 48 trials of nearly 7,000 patients with solid tumors who received CTLA-4 inhibitors (26 studies), PD-1 inhibitors (17 studies), PD-1 ligand (PD-L1) inhibitors (two trials), or both CTLA-4 and PD-1 inhibitors (three trials). The reviewers identified the studies by searching the Medline, EMBASE, and COCHRANE databases for prospective trials published from 2003 through November 2015.
Severe or life-threatening immune-related adverse effects developed in 31% of patients who received CTLA-4 inhibitors and 10% of patients who received PD-1 inhibitors. Inhibitors of CTLA-4 were significantly more likely to cause all grades of colitis (OR, 8.7), hypophysitis (OR, 6.5), and rash (OR, 2.0), while PD-1 inhibitors were more strongly linked with pneumonitis (OR 6.4), hypothyroidism (OR 4.3), arthralgia (OR, 3.5), and vitiligo (OR, 3.5).
The reviewers also looked for significant predictors of immune-related colitis and pneumonitis, because these are potentially fatal. They found that pneumonitis was significantly linked to PD-1/PD-L1 inhibitor therapy (P less than .001) and colitis to CTLA-4 treatment (P = .04), even after accounting for therapeutic dose and tumor type. No other factors reached significance in this multivariable model.
“Clearly, a more thorough understanding of the mechanisms of immune-related adverse effects is needed, which may lead to the identification of biomarkers to predict the occurrence of toxicity in patients or predict those who have immune-related adverse effects that are unlikely to respond to corticosteroids,” the reviewers concluded. Researchers should also study whether clinical factors such as treatment history or comorbidities affect the risk of immune-related adverse effects from immune checkpoint inhibitors, they said.
The reviewers reported having no funding sources and no relevant conflicts of interest.
The immune-related adverse effects of inhibitors of programmed cell death protein 1 (PD-1) and its ligand varied by tumor type in a large systematic review and meta-analysis.
Patients with melanoma were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8) compared with patients with non–small cell lung cancer, who were significantly more likely to develop pneumonitis, reported Leila Khoja, MBChB, PhD, of AstraZeneca UK, Melbourn, England, and associates. Patients with melanoma also were significantly more likely to develop arthralgia, hypothyroidism, rash, pruritus, and diarrhea compared with patients with renal cell carcinoma, who were more likely to develop pneumonitis and dyspnea.
“In light of this study, we should be mindful that different tumor types may have different immune-related adverse effect patterns when treated with the same immune checkpoint inhibitor,” the reviewers noted (Ann Oncol. 2017 Aug 8. doi: 10.1093/annonc/mdx286).
The review included 48 trials of nearly 7,000 patients with solid tumors who received CTLA-4 inhibitors (26 studies), PD-1 inhibitors (17 studies), PD-1 ligand (PD-L1) inhibitors (two trials), or both CTLA-4 and PD-1 inhibitors (three trials). The reviewers identified the studies by searching the Medline, EMBASE, and COCHRANE databases for prospective trials published from 2003 through November 2015.
Severe or life-threatening immune-related adverse effects developed in 31% of patients who received CTLA-4 inhibitors and 10% of patients who received PD-1 inhibitors. Inhibitors of CTLA-4 were significantly more likely to cause all grades of colitis (OR, 8.7), hypophysitis (OR, 6.5), and rash (OR, 2.0), while PD-1 inhibitors were more strongly linked with pneumonitis (OR 6.4), hypothyroidism (OR 4.3), arthralgia (OR, 3.5), and vitiligo (OR, 3.5).
The reviewers also looked for significant predictors of immune-related colitis and pneumonitis, because these are potentially fatal. They found that pneumonitis was significantly linked to PD-1/PD-L1 inhibitor therapy (P less than .001) and colitis to CTLA-4 treatment (P = .04), even after accounting for therapeutic dose and tumor type. No other factors reached significance in this multivariable model.
“Clearly, a more thorough understanding of the mechanisms of immune-related adverse effects is needed, which may lead to the identification of biomarkers to predict the occurrence of toxicity in patients or predict those who have immune-related adverse effects that are unlikely to respond to corticosteroids,” the reviewers concluded. Researchers should also study whether clinical factors such as treatment history or comorbidities affect the risk of immune-related adverse effects from immune checkpoint inhibitors, they said.
The reviewers reported having no funding sources and no relevant conflicts of interest.
The immune-related adverse effects of inhibitors of programmed cell death protein 1 (PD-1) and its ligand varied by tumor type in a large systematic review and meta-analysis.
Patients with melanoma were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8) compared with patients with non–small cell lung cancer, who were significantly more likely to develop pneumonitis, reported Leila Khoja, MBChB, PhD, of AstraZeneca UK, Melbourn, England, and associates. Patients with melanoma also were significantly more likely to develop arthralgia, hypothyroidism, rash, pruritus, and diarrhea compared with patients with renal cell carcinoma, who were more likely to develop pneumonitis and dyspnea.
“In light of this study, we should be mindful that different tumor types may have different immune-related adverse effect patterns when treated with the same immune checkpoint inhibitor,” the reviewers noted (Ann Oncol. 2017 Aug 8. doi: 10.1093/annonc/mdx286).
The review included 48 trials of nearly 7,000 patients with solid tumors who received CTLA-4 inhibitors (26 studies), PD-1 inhibitors (17 studies), PD-1 ligand (PD-L1) inhibitors (two trials), or both CTLA-4 and PD-1 inhibitors (three trials). The reviewers identified the studies by searching the Medline, EMBASE, and COCHRANE databases for prospective trials published from 2003 through November 2015.
Severe or life-threatening immune-related adverse effects developed in 31% of patients who received CTLA-4 inhibitors and 10% of patients who received PD-1 inhibitors. Inhibitors of CTLA-4 were significantly more likely to cause all grades of colitis (OR, 8.7), hypophysitis (OR, 6.5), and rash (OR, 2.0), while PD-1 inhibitors were more strongly linked with pneumonitis (OR 6.4), hypothyroidism (OR 4.3), arthralgia (OR, 3.5), and vitiligo (OR, 3.5).
The reviewers also looked for significant predictors of immune-related colitis and pneumonitis, because these are potentially fatal. They found that pneumonitis was significantly linked to PD-1/PD-L1 inhibitor therapy (P less than .001) and colitis to CTLA-4 treatment (P = .04), even after accounting for therapeutic dose and tumor type. No other factors reached significance in this multivariable model.
“Clearly, a more thorough understanding of the mechanisms of immune-related adverse effects is needed, which may lead to the identification of biomarkers to predict the occurrence of toxicity in patients or predict those who have immune-related adverse effects that are unlikely to respond to corticosteroids,” the reviewers concluded. Researchers should also study whether clinical factors such as treatment history or comorbidities affect the risk of immune-related adverse effects from immune checkpoint inhibitors, they said.
The reviewers reported having no funding sources and no relevant conflicts of interest.
FROM ANNALS OF ONCOLOGY
Key clinical point: Immune-related adverse effects varied by tumor type in patients receiving programmed cell death protein 1 (PD-1) and PD-L1 inhibitors.
Major finding: Patients with melanoma who received PD-1/PD-L1 inhibitors were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8), compared with patients with non-small cell lung cancer, who were significantly more likely to develop pneumonitis.
Data source: A systematic review and meta-analysis of 48 prospective trials of immune checkpoint inhibitors in of 6,938 adults with solid tumors.
Disclosures: The reviewers reported having no funding sources and no relevant conflicts of interest.
Videodermoscopy as a Novel Tool for Dermatologic Education
Dermoscopy, or the noninvasive in vivo examination of the epidermis and superficial dermis using magnification, facilitates the diagnosis of pigmented and nonpigmented skin lesions.1 Despite the benefit of dermoscopy in making early and accurate diagnoses of potentially life-limiting skin cancers, only 48% of dermatologists in the United States use dermoscopy in their practices.2 The most commonly cited reason for not using dermoscopy is lack of training.
Although the use of dermoscopy is associated with younger age and more recent graduation from residency compared to nonusers, dermatology resident physicians continue to receive limited training in dermoscopy.2 In a survey of 139 dermatology chief residents, 48% were not satisfied with the dermoscopy training that they had received during residency. Residents who received bedside instruction in dermoscopy reported greater satisfaction with their dermoscopy training compared to those who did not receive bedside instruction.3 This article provides a brief comparison of standard dermoscopy versus videodermoscopy for the instruction of trainees on common dermatologic diagnoses.
Bedside Dermoscopy
Standard optical dermatoscopes used for patient care and educational purposes typically incorporate 10-fold magnification and permit examination by a single viewer through a lens. With standard dermatoscopes, bedside dermoscopy instruction consists of the independent sequential viewing of skin lesions by instructors and trainees. Trainees must independently search for dermoscopic features noted by the instructor, which may be difficult for novice users. Simultaneous viewing of lesions would allow instructors to clearly indicate in real time pertinent dermoscopic features to their trainees.
Videodermatoscopes facilitate the simultaneous examination of cutaneous lesions by projecting the dermoscopic image onto a digital screen. Furthermore, these devices can incorporate magnifications of up to 200-fold or greater. In recent years, research pertaining to videodermoscopy has focused on the high magnification capabilities of these devices, specifically dermoscopic features that are visualized at magnifications greater than 10-fold, including the light brown nests of basal cell carcinomas that are seen at 50- to 70-fold magnification, twisted red capillary loops seen in active scalp psoriasis at 50-fold magnification, and longitudinal white indentations seen on nail plates affected by onychomycosis at 20-fold magnification.4-6 The potential value of videodermoscopy in medical education lies not only in the high magnification potential, which may make subtle dermoscopic findings more apparent to novice dermoscopists, but also in the ability to facilitate simultaneous dermoscopic examinations by instructors and trainees.
Educational Applications for Videodermoscopy
To illustrate the educational potential of videodermoscopy, images taken with a standard dermatoscope at 10-fold magnification are presented with videodermoscopic images taken at magnifications ranging from 60- to 185-fold (Figures 1–3). These examples demonstrate the potential for videodermoscopy to facilitate the visualization of subtle dermoscopic features by novice dermoscopists, relating to both the enhanced magnification potential and the potential for simultaneous rather than sequential examination.
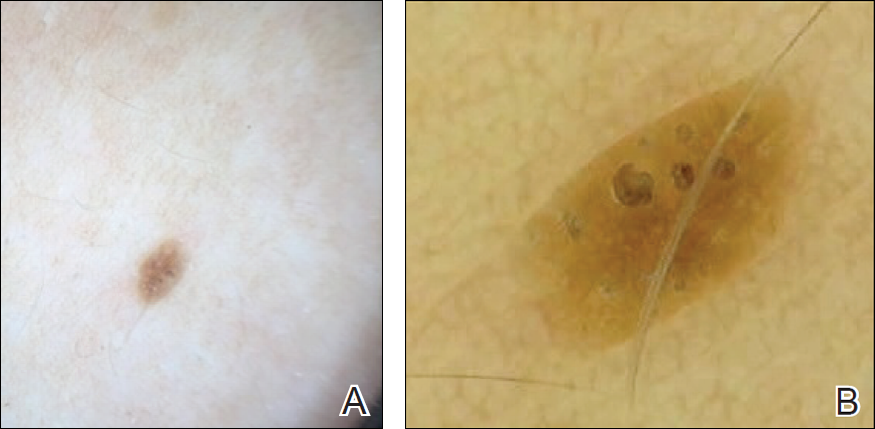
Final Thoughts
High-magnification videodermoscopy may be a useful tool to further dermoscopic education. Videodermatoscopes vary in functionality and cost but are available at price points comparable to those of standard optical dermatoscopes. Owners of standard dermatoscopes can approximate some of the benefits of a digital videodermatoscope by using the standard dermatoscope in conjunction with a camera, including those integrated into mobile phones and tablets. By attaching the standard dermatoscope to a camera with a digital display, the digital zoom of the camera can be used to magnify the standard dermoscopic image, enhancing the ability of novice dermoscopists to visualize subtle findings. By presenting this magnified image on a digital display, dermoscopy instructors and trainees would be able to simultaneously view dermoscopic images of lesions, sometimes with magnifications comparable to videodermatoscopes.
In the setting of a dermatology residency program, videodermoscopy can be incorporated into bedside teaching with experienced dermoscopists and for the live presentation of dermoscopic features at departmental grand rounds. By facilitating the simultaneous, high-magnification and live viewing of skin lesions by dermoscopy instructors and trainees, digital videodermoscopy has the potential to address an area of weakness in dermatologic training.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey [published online July 8, 2010]. J Am Acad Dermatol. 2010;63:412-419, 419.e1-419.e2.
- Wu TP, Newlove T, Smith L, et al. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68:1000-1005.
- Seidenari S, Bellucci C, Bassoli S, et al. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol. 2014;94:677-682.
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
Dermoscopy, or the noninvasive in vivo examination of the epidermis and superficial dermis using magnification, facilitates the diagnosis of pigmented and nonpigmented skin lesions.1 Despite the benefit of dermoscopy in making early and accurate diagnoses of potentially life-limiting skin cancers, only 48% of dermatologists in the United States use dermoscopy in their practices.2 The most commonly cited reason for not using dermoscopy is lack of training.
Although the use of dermoscopy is associated with younger age and more recent graduation from residency compared to nonusers, dermatology resident physicians continue to receive limited training in dermoscopy.2 In a survey of 139 dermatology chief residents, 48% were not satisfied with the dermoscopy training that they had received during residency. Residents who received bedside instruction in dermoscopy reported greater satisfaction with their dermoscopy training compared to those who did not receive bedside instruction.3 This article provides a brief comparison of standard dermoscopy versus videodermoscopy for the instruction of trainees on common dermatologic diagnoses.
Bedside Dermoscopy
Standard optical dermatoscopes used for patient care and educational purposes typically incorporate 10-fold magnification and permit examination by a single viewer through a lens. With standard dermatoscopes, bedside dermoscopy instruction consists of the independent sequential viewing of skin lesions by instructors and trainees. Trainees must independently search for dermoscopic features noted by the instructor, which may be difficult for novice users. Simultaneous viewing of lesions would allow instructors to clearly indicate in real time pertinent dermoscopic features to their trainees.
Videodermatoscopes facilitate the simultaneous examination of cutaneous lesions by projecting the dermoscopic image onto a digital screen. Furthermore, these devices can incorporate magnifications of up to 200-fold or greater. In recent years, research pertaining to videodermoscopy has focused on the high magnification capabilities of these devices, specifically dermoscopic features that are visualized at magnifications greater than 10-fold, including the light brown nests of basal cell carcinomas that are seen at 50- to 70-fold magnification, twisted red capillary loops seen in active scalp psoriasis at 50-fold magnification, and longitudinal white indentations seen on nail plates affected by onychomycosis at 20-fold magnification.4-6 The potential value of videodermoscopy in medical education lies not only in the high magnification potential, which may make subtle dermoscopic findings more apparent to novice dermoscopists, but also in the ability to facilitate simultaneous dermoscopic examinations by instructors and trainees.
Educational Applications for Videodermoscopy
To illustrate the educational potential of videodermoscopy, images taken with a standard dermatoscope at 10-fold magnification are presented with videodermoscopic images taken at magnifications ranging from 60- to 185-fold (Figures 1–3). These examples demonstrate the potential for videodermoscopy to facilitate the visualization of subtle dermoscopic features by novice dermoscopists, relating to both the enhanced magnification potential and the potential for simultaneous rather than sequential examination.



Final Thoughts
High-magnification videodermoscopy may be a useful tool to further dermoscopic education. Videodermatoscopes vary in functionality and cost but are available at price points comparable to those of standard optical dermatoscopes. Owners of standard dermatoscopes can approximate some of the benefits of a digital videodermatoscope by using the standard dermatoscope in conjunction with a camera, including those integrated into mobile phones and tablets. By attaching the standard dermatoscope to a camera with a digital display, the digital zoom of the camera can be used to magnify the standard dermoscopic image, enhancing the ability of novice dermoscopists to visualize subtle findings. By presenting this magnified image on a digital display, dermoscopy instructors and trainees would be able to simultaneously view dermoscopic images of lesions, sometimes with magnifications comparable to videodermatoscopes.
In the setting of a dermatology residency program, videodermoscopy can be incorporated into bedside teaching with experienced dermoscopists and for the live presentation of dermoscopic features at departmental grand rounds. By facilitating the simultaneous, high-magnification and live viewing of skin lesions by dermoscopy instructors and trainees, digital videodermoscopy has the potential to address an area of weakness in dermatologic training.
Dermoscopy, or the noninvasive in vivo examination of the epidermis and superficial dermis using magnification, facilitates the diagnosis of pigmented and nonpigmented skin lesions.1 Despite the benefit of dermoscopy in making early and accurate diagnoses of potentially life-limiting skin cancers, only 48% of dermatologists in the United States use dermoscopy in their practices.2 The most commonly cited reason for not using dermoscopy is lack of training.
Although the use of dermoscopy is associated with younger age and more recent graduation from residency compared to nonusers, dermatology resident physicians continue to receive limited training in dermoscopy.2 In a survey of 139 dermatology chief residents, 48% were not satisfied with the dermoscopy training that they had received during residency. Residents who received bedside instruction in dermoscopy reported greater satisfaction with their dermoscopy training compared to those who did not receive bedside instruction.3 This article provides a brief comparison of standard dermoscopy versus videodermoscopy for the instruction of trainees on common dermatologic diagnoses.
Bedside Dermoscopy
Standard optical dermatoscopes used for patient care and educational purposes typically incorporate 10-fold magnification and permit examination by a single viewer through a lens. With standard dermatoscopes, bedside dermoscopy instruction consists of the independent sequential viewing of skin lesions by instructors and trainees. Trainees must independently search for dermoscopic features noted by the instructor, which may be difficult for novice users. Simultaneous viewing of lesions would allow instructors to clearly indicate in real time pertinent dermoscopic features to their trainees.
Videodermatoscopes facilitate the simultaneous examination of cutaneous lesions by projecting the dermoscopic image onto a digital screen. Furthermore, these devices can incorporate magnifications of up to 200-fold or greater. In recent years, research pertaining to videodermoscopy has focused on the high magnification capabilities of these devices, specifically dermoscopic features that are visualized at magnifications greater than 10-fold, including the light brown nests of basal cell carcinomas that are seen at 50- to 70-fold magnification, twisted red capillary loops seen in active scalp psoriasis at 50-fold magnification, and longitudinal white indentations seen on nail plates affected by onychomycosis at 20-fold magnification.4-6 The potential value of videodermoscopy in medical education lies not only in the high magnification potential, which may make subtle dermoscopic findings more apparent to novice dermoscopists, but also in the ability to facilitate simultaneous dermoscopic examinations by instructors and trainees.
Educational Applications for Videodermoscopy
To illustrate the educational potential of videodermoscopy, images taken with a standard dermatoscope at 10-fold magnification are presented with videodermoscopic images taken at magnifications ranging from 60- to 185-fold (Figures 1–3). These examples demonstrate the potential for videodermoscopy to facilitate the visualization of subtle dermoscopic features by novice dermoscopists, relating to both the enhanced magnification potential and the potential for simultaneous rather than sequential examination.



Final Thoughts
High-magnification videodermoscopy may be a useful tool to further dermoscopic education. Videodermatoscopes vary in functionality and cost but are available at price points comparable to those of standard optical dermatoscopes. Owners of standard dermatoscopes can approximate some of the benefits of a digital videodermatoscope by using the standard dermatoscope in conjunction with a camera, including those integrated into mobile phones and tablets. By attaching the standard dermatoscope to a camera with a digital display, the digital zoom of the camera can be used to magnify the standard dermoscopic image, enhancing the ability of novice dermoscopists to visualize subtle findings. By presenting this magnified image on a digital display, dermoscopy instructors and trainees would be able to simultaneously view dermoscopic images of lesions, sometimes with magnifications comparable to videodermatoscopes.
In the setting of a dermatology residency program, videodermoscopy can be incorporated into bedside teaching with experienced dermoscopists and for the live presentation of dermoscopic features at departmental grand rounds. By facilitating the simultaneous, high-magnification and live viewing of skin lesions by dermoscopy instructors and trainees, digital videodermoscopy has the potential to address an area of weakness in dermatologic training.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey [published online July 8, 2010]. J Am Acad Dermatol. 2010;63:412-419, 419.e1-419.e2.
- Wu TP, Newlove T, Smith L, et al. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68:1000-1005.
- Seidenari S, Bellucci C, Bassoli S, et al. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol. 2014;94:677-682.
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey [published online July 8, 2010]. J Am Acad Dermatol. 2010;63:412-419, 419.e1-419.e2.
- Wu TP, Newlove T, Smith L, et al. The importance of dedicated dermoscopy training during residency: a survey of US dermatology chief residents. J Am Acad Dermatol. 2013;68:1000-1005.
- Seidenari S, Bellucci C, Bassoli S, et al. High magnification digital dermoscopy of basal cell carcinoma: a single-centre study on 400 cases. Acta Derm Venereol. 2014;94:677-682.
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
Resident Pearl
- Bedside dermoscopy training can be enhanced through the use of videodermoscopy, which permits simultaneous, high-magnification viewing.
California study indicates increased melanoma incidence is real
A new analysis in non-Hispanic whites suggests that rising melanoma rates are real, not attributable to increased levels of detection, and that the burden of the disease could rise significantly in the coming years.
The incidence of melanoma in light-skinned individuals has been rising worldwide in recent years, but it remains unclear whether that trend is due to an increase in the disease, or better screening and diagnosis. The new results are drawn from California, and track incidence and stage at diagnosis of melanoma across different socioeconomic status (SES) groups. Across all groups, the researchers found increases not only in incidence, but also in advanced disease.
“Our findings support a true real rise in incidence of melanoma across all thicknesses and stages, and not just thinner, more indolent tumors that may be due to increased screening or diagnosis,” lead researcher Susan Swetter, MD, said in an interview. The study was published online in the Journal of Investigative Dermatology (J Invest Dermatol. 2017 Jul 20. pii: S0022-202X(17)31867-5. doi: 10.1016/j.jid.2017.06.024).
Overall, the incidence rose 25% in men from 1998-2002 to 2008-2012 (an average annual age-adjusted incidence of 34.7 to 43.5 per 100,000 person-years), and by 21% in women between those two time periods (from 21.7 to 26.2 per 100,000). Melanoma incidence rate ratios (IRR) increased across all SES classes: by 27% among men in the highest SES neighborhoods, and by 12% among men in the lowest SES neighborhoods. For women, the rates increased by 28% and 13% respectively.
The highest increases in the incidence of regional and distant disease occurred in the lowest SES neighborhoods, nearly doubling in men (distant disease IRR, 1.87; 95% CI, 1.39-2.53; regional disease IRR, 1.93; 95% CI, 1.51-2.47). Women in these neighborhoods also experienced a significant increase in regional disease (IRR, 1.44; 95% CI, 1.00-2.08), but not distant disease.
Incidence of diagnosis with the thickest tumors (greater than 4 mm) rose significantly in most neighborhood SES quartiles, with the exception of the men in the lowest SES quartiles, who had a lower increase that was of borderline significance.
The results solidify the evidence that melanoma incidence is truly increasing, but they also have public health implications. The rising incidence of more advanced disease suggests a heightening health care burden from melanoma in the coming years, but also points to strategies for prevention, according to Dr. Swetter. “It’s important that we focus not only on primary prevention. We need methods to enhance early detection, especially in areas where there is lower access to dermatologists and even primary care providers, who can assist in this effort,” she said.
The Stanford Cancer Institute funded the study. Dr. Swetter reported having no financial disclosures.
A new analysis in non-Hispanic whites suggests that rising melanoma rates are real, not attributable to increased levels of detection, and that the burden of the disease could rise significantly in the coming years.
The incidence of melanoma in light-skinned individuals has been rising worldwide in recent years, but it remains unclear whether that trend is due to an increase in the disease, or better screening and diagnosis. The new results are drawn from California, and track incidence and stage at diagnosis of melanoma across different socioeconomic status (SES) groups. Across all groups, the researchers found increases not only in incidence, but also in advanced disease.
“Our findings support a true real rise in incidence of melanoma across all thicknesses and stages, and not just thinner, more indolent tumors that may be due to increased screening or diagnosis,” lead researcher Susan Swetter, MD, said in an interview. The study was published online in the Journal of Investigative Dermatology (J Invest Dermatol. 2017 Jul 20. pii: S0022-202X(17)31867-5. doi: 10.1016/j.jid.2017.06.024).
Overall, the incidence rose 25% in men from 1998-2002 to 2008-2012 (an average annual age-adjusted incidence of 34.7 to 43.5 per 100,000 person-years), and by 21% in women between those two time periods (from 21.7 to 26.2 per 100,000). Melanoma incidence rate ratios (IRR) increased across all SES classes: by 27% among men in the highest SES neighborhoods, and by 12% among men in the lowest SES neighborhoods. For women, the rates increased by 28% and 13% respectively.
The highest increases in the incidence of regional and distant disease occurred in the lowest SES neighborhoods, nearly doubling in men (distant disease IRR, 1.87; 95% CI, 1.39-2.53; regional disease IRR, 1.93; 95% CI, 1.51-2.47). Women in these neighborhoods also experienced a significant increase in regional disease (IRR, 1.44; 95% CI, 1.00-2.08), but not distant disease.
Incidence of diagnosis with the thickest tumors (greater than 4 mm) rose significantly in most neighborhood SES quartiles, with the exception of the men in the lowest SES quartiles, who had a lower increase that was of borderline significance.
The results solidify the evidence that melanoma incidence is truly increasing, but they also have public health implications. The rising incidence of more advanced disease suggests a heightening health care burden from melanoma in the coming years, but also points to strategies for prevention, according to Dr. Swetter. “It’s important that we focus not only on primary prevention. We need methods to enhance early detection, especially in areas where there is lower access to dermatologists and even primary care providers, who can assist in this effort,” she said.
The Stanford Cancer Institute funded the study. Dr. Swetter reported having no financial disclosures.
A new analysis in non-Hispanic whites suggests that rising melanoma rates are real, not attributable to increased levels of detection, and that the burden of the disease could rise significantly in the coming years.
The incidence of melanoma in light-skinned individuals has been rising worldwide in recent years, but it remains unclear whether that trend is due to an increase in the disease, or better screening and diagnosis. The new results are drawn from California, and track incidence and stage at diagnosis of melanoma across different socioeconomic status (SES) groups. Across all groups, the researchers found increases not only in incidence, but also in advanced disease.
“Our findings support a true real rise in incidence of melanoma across all thicknesses and stages, and not just thinner, more indolent tumors that may be due to increased screening or diagnosis,” lead researcher Susan Swetter, MD, said in an interview. The study was published online in the Journal of Investigative Dermatology (J Invest Dermatol. 2017 Jul 20. pii: S0022-202X(17)31867-5. doi: 10.1016/j.jid.2017.06.024).
Overall, the incidence rose 25% in men from 1998-2002 to 2008-2012 (an average annual age-adjusted incidence of 34.7 to 43.5 per 100,000 person-years), and by 21% in women between those two time periods (from 21.7 to 26.2 per 100,000). Melanoma incidence rate ratios (IRR) increased across all SES classes: by 27% among men in the highest SES neighborhoods, and by 12% among men in the lowest SES neighborhoods. For women, the rates increased by 28% and 13% respectively.
The highest increases in the incidence of regional and distant disease occurred in the lowest SES neighborhoods, nearly doubling in men (distant disease IRR, 1.87; 95% CI, 1.39-2.53; regional disease IRR, 1.93; 95% CI, 1.51-2.47). Women in these neighborhoods also experienced a significant increase in regional disease (IRR, 1.44; 95% CI, 1.00-2.08), but not distant disease.
Incidence of diagnosis with the thickest tumors (greater than 4 mm) rose significantly in most neighborhood SES quartiles, with the exception of the men in the lowest SES quartiles, who had a lower increase that was of borderline significance.
The results solidify the evidence that melanoma incidence is truly increasing, but they also have public health implications. The rising incidence of more advanced disease suggests a heightening health care burden from melanoma in the coming years, but also points to strategies for prevention, according to Dr. Swetter. “It’s important that we focus not only on primary prevention. We need methods to enhance early detection, especially in areas where there is lower access to dermatologists and even primary care providers, who can assist in this effort,” she said.
The Stanford Cancer Institute funded the study. Dr. Swetter reported having no financial disclosures.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Increased incidences of more advanced disease suggest a rising health care burden.
Major finding: Between 1998-2002 and 2008-2012, incidence rate ratios rose by 25% in men and 21% in women.
Data source: A retrospective study of over 58,000 melanoma cases.
Disclosures: The Stanford Cancer Institute funded the study. Dr. Swetter reported having no financial disclosures.
Nivolumab Linked to Nephritis in Melanoma
Nivolumab and ipilimumab, new immunotherapies for metastatic melanoma, have both been linked to nephritis. Now, researchers from Centre Hospitalier Lyon-Sud and Université Claude Bernard Lyon in France, report on a patient with melanoma who developed acute interstitial immune nephritis after being treated with nivolumab—not once, but twice.
The patient, a 76-year-old woman with pulmonary metastatic melanoma, was given 4 intravenous cycles of ipilimumab as a first-line treatment. After 16 weeks, the disease was progressing; the ipilimumab was discontinued, and 8 weeks later she was started on second-line treatment with nivolumab. After 3 cycles of nivolumab, she developed acute kidney injury. The patient’s creatinine went from 69 µmol/L before nivolumab to 142 µmol/L before the fourth cycle. Immunotherapy was discontinued.
Related: Getting a Better Picture of Skin Cancer
The patient had not received any other drug that could explain the increased creatinine level, the researchers say, and she was otherwise asymptomatic. The renal failure persisted despite an adequate fluid intake over 3 days. Biopsy revealed interstitial edema.
The clinicians treated the patient with oral prednisolone, and her renal function rapidly improved, although her creatinine level remained higher than before the nivolumab.
A follow-up CT scan found a partial response to the nivolumab. Based on that reponse, the multidisciplinary staff elected to continue the treatment at the same dose. The fourth cycle was administered while the patient was still receiving daily corticosteroids.
The infusion did not cause kidney failure relapse. However, after the corticosteroids were stopped, the patient’s creatinine level increased gradually, to 158 µmol/L, and again she was hospitalized with relapse of immune interstitial nephritis. The clinicians reinstituted prednisolone, and the acute interstitial nephritis improved. Nivolumab was discontinued.
Related: Immunotherapy in Melanoma
Drug-induced acute interstitial nephritis often has been more described with nonsteroidal anti-inflammatory drugs and beta-lactams, among others, the researchers say. Immune interstitial nephritis had been reported in a patient treated with nivolumab and ipilimumab concomitantly, and 3 cases of granulomatous interstitial nephritis have been reported with ipilimumab monotherapy. To the authors’ knowledge, this is the first case of immune interstitial nephritis reported with nivolumab monotherapy in metastatic melanoma. It is important to consider, they add, that the patient had also received ipilimumab, and that due to the drug’s elimination half-life (15.4 days), they can’t exclude an “overlap” between the 2 drugs that might have increased the risk of acute interstitial nephritis.
Source:
Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. J Immunother Cancer. 2017;5:56.
doi: 10.1186/s40425-017-0261-2
Nivolumab and ipilimumab, new immunotherapies for metastatic melanoma, have both been linked to nephritis. Now, researchers from Centre Hospitalier Lyon-Sud and Université Claude Bernard Lyon in France, report on a patient with melanoma who developed acute interstitial immune nephritis after being treated with nivolumab—not once, but twice.
The patient, a 76-year-old woman with pulmonary metastatic melanoma, was given 4 intravenous cycles of ipilimumab as a first-line treatment. After 16 weeks, the disease was progressing; the ipilimumab was discontinued, and 8 weeks later she was started on second-line treatment with nivolumab. After 3 cycles of nivolumab, she developed acute kidney injury. The patient’s creatinine went from 69 µmol/L before nivolumab to 142 µmol/L before the fourth cycle. Immunotherapy was discontinued.
Related: Getting a Better Picture of Skin Cancer
The patient had not received any other drug that could explain the increased creatinine level, the researchers say, and she was otherwise asymptomatic. The renal failure persisted despite an adequate fluid intake over 3 days. Biopsy revealed interstitial edema.
The clinicians treated the patient with oral prednisolone, and her renal function rapidly improved, although her creatinine level remained higher than before the nivolumab.
A follow-up CT scan found a partial response to the nivolumab. Based on that reponse, the multidisciplinary staff elected to continue the treatment at the same dose. The fourth cycle was administered while the patient was still receiving daily corticosteroids.
The infusion did not cause kidney failure relapse. However, after the corticosteroids were stopped, the patient’s creatinine level increased gradually, to 158 µmol/L, and again she was hospitalized with relapse of immune interstitial nephritis. The clinicians reinstituted prednisolone, and the acute interstitial nephritis improved. Nivolumab was discontinued.
Related: Immunotherapy in Melanoma
Drug-induced acute interstitial nephritis often has been more described with nonsteroidal anti-inflammatory drugs and beta-lactams, among others, the researchers say. Immune interstitial nephritis had been reported in a patient treated with nivolumab and ipilimumab concomitantly, and 3 cases of granulomatous interstitial nephritis have been reported with ipilimumab monotherapy. To the authors’ knowledge, this is the first case of immune interstitial nephritis reported with nivolumab monotherapy in metastatic melanoma. It is important to consider, they add, that the patient had also received ipilimumab, and that due to the drug’s elimination half-life (15.4 days), they can’t exclude an “overlap” between the 2 drugs that might have increased the risk of acute interstitial nephritis.
Source:
Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. J Immunother Cancer. 2017;5:56.
doi: 10.1186/s40425-017-0261-2
Nivolumab and ipilimumab, new immunotherapies for metastatic melanoma, have both been linked to nephritis. Now, researchers from Centre Hospitalier Lyon-Sud and Université Claude Bernard Lyon in France, report on a patient with melanoma who developed acute interstitial immune nephritis after being treated with nivolumab—not once, but twice.
The patient, a 76-year-old woman with pulmonary metastatic melanoma, was given 4 intravenous cycles of ipilimumab as a first-line treatment. After 16 weeks, the disease was progressing; the ipilimumab was discontinued, and 8 weeks later she was started on second-line treatment with nivolumab. After 3 cycles of nivolumab, she developed acute kidney injury. The patient’s creatinine went from 69 µmol/L before nivolumab to 142 µmol/L before the fourth cycle. Immunotherapy was discontinued.
Related: Getting a Better Picture of Skin Cancer
The patient had not received any other drug that could explain the increased creatinine level, the researchers say, and she was otherwise asymptomatic. The renal failure persisted despite an adequate fluid intake over 3 days. Biopsy revealed interstitial edema.
The clinicians treated the patient with oral prednisolone, and her renal function rapidly improved, although her creatinine level remained higher than before the nivolumab.
A follow-up CT scan found a partial response to the nivolumab. Based on that reponse, the multidisciplinary staff elected to continue the treatment at the same dose. The fourth cycle was administered while the patient was still receiving daily corticosteroids.
The infusion did not cause kidney failure relapse. However, after the corticosteroids were stopped, the patient’s creatinine level increased gradually, to 158 µmol/L, and again she was hospitalized with relapse of immune interstitial nephritis. The clinicians reinstituted prednisolone, and the acute interstitial nephritis improved. Nivolumab was discontinued.
Related: Immunotherapy in Melanoma
Drug-induced acute interstitial nephritis often has been more described with nonsteroidal anti-inflammatory drugs and beta-lactams, among others, the researchers say. Immune interstitial nephritis had been reported in a patient treated with nivolumab and ipilimumab concomitantly, and 3 cases of granulomatous interstitial nephritis have been reported with ipilimumab monotherapy. To the authors’ knowledge, this is the first case of immune interstitial nephritis reported with nivolumab monotherapy in metastatic melanoma. It is important to consider, they add, that the patient had also received ipilimumab, and that due to the drug’s elimination half-life (15.4 days), they can’t exclude an “overlap” between the 2 drugs that might have increased the risk of acute interstitial nephritis.
Source:
Bottlaender L, Breton AL, de Laforcade L, Dijoud F, Thomas L, Dalle S. J Immunother Cancer. 2017;5:56.
doi: 10.1186/s40425-017-0261-2
Ex Vivo Confocal Microscopy: A Diagnostic Tool for Skin Malignancies
Skin cancer is diagnosed in approximately 5.4 million individuals annually in the United States, more than the total number of breast, lung, colon, and prostate cancers diagnosed per year.1 It is estimated that 1 in 5 Americans will develop skin cancer during their lifetime.2 The 2 most common forms of skin cancer are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), accounting for 4 million and 1 million cases diagnosed each year, respectively.3 With the increasing incidence of these skin cancers, the use of noninvasive imaging tools for detection and diagnosis has grown.
Ex vivo confocal microscopy is a diagnostic imaging tool that can be used in real-time at the bedside to assess freshly excised tissue for malignancies. It images tissue samples with cellular resolution and within minutes of biopsy or excision. Ex vivo confocal microscopy is a versatile tool that can assist in the diagnosis and management of skin malignancies such as melanoma, BCC, and SCC.
Reflectance vs Fluorescence Mode
Excised lesions can be examined in reflectance or fluorescence mode in great detail but with slightly varying nuclear-to-dermis contrasts depending on the chromophore that is targeted. In reflectance mode (reflectance confocal microscopy [RCM]), melanin and keratin act as endogenous chromophores because of their high refractive index relative to water,4,5 which allows for the visualization of cellular structures of the skin at low power, as well as microscopic substructures such as melanosomes, cytoplasmic granules, and other cellular organelles at high power. Although an exogenous contrast agent is not required, acetic acid has the capability to highlight nuclei, enhancing the tumor cell-to-dermis contrast in RCM.6 Acetic acid is clinically used as a predictor for certain skin and mucosal membrane neoplasms that blanch when exposed to the solution. In the case of RCM, acetic acid increases the visibility of nuclei by inducing the compaction of chromatin. For the acetowhitening to be effective, the sample must be soaked in the solution for a specific amount of time, depending on the concentration.7 A concentration between 1% and 10% can be used, but the less concentrated the solution, the longer the time of soaking that is required to achieve sufficiently bright nuclei.6
The contrast with acetic acid, however, is quite weak when the tissue is imaged en face, or along the horizontal surface of the sample, due to the collagen in the dermal layer, which has a high reflectance index. This issue is rectified when using the confocal microscope in the fluorescence mode with an exogenous fluorescent dye as a nuclear stain. Fluorescence confocal microscopy (FCM), results in a stronger nuclear-to-dermal contrast because of the role of contrast agents.8 The 1000-fold increase in contrast between nuclei and dermis is the result of dye agents that preferentially bind to nuclear DNA, of which acridine orange is the most commonly used.5,8 Basal cell carcinoma and SCC tumor cells can be visualized with FCM because they appear hyperfluorescent when stained with acridine orange.9 The acridine orange–stained cells display bright nuclei, while the cytoplasm and collagen remains dark. A positive feature of acridine orange is that it does not alter the tissue sample during freezing or formalin fixation and thus has no effect on subsequent histopathology that may need to be performed on the sample.10
High-Resolution Images Aid in Diagnosis
After it is harvested, the tissue sample is soaked in a contrast agent or dye, if needed, depending on the confocal mode to be used. The confocal microscope is then used to take a series of high-resolution individual en face images that are then stitched together to create a final mosaic image that can be up to 12×12 mm.6,11 With a 200-µm depth visibility, confocal microscopy can capture the cellular structures in the epidermis, dermis, and (if compressed enough) subcutaneous fat in just under 3 minutes.12
The images produced through confocal microscopy have an excellent correlation to frozen histological sections and can aid in the diagnosis of many epidermal and dermal malignancies including melanoma, BCC, and SCC. New criteria have been established to aid in the interpretation of the confocal images and identify some of the more common skin cancers.5,12,13 Basal cell carcinoma samples imaged through fluorescence and reflectance in low-power mode display the distinct nodular patterns with well-demarcated edges, as seen on classical histopathology. In the case of FCM, the cells that make up the tumor display hyperfluorescent areas consistent with nucleated cells that are stained with acridine orange. The main features that identify BCC on FCM images include nuclear pleomorphism and crowding, peripheral palisading, clefting of the basaloid islands, increased nucleus-to-cytoplasm ratio, and the presence of a modified dermis surrounding the mass known as the tumoral stroma5,12 (Figure).
In addition to fluorescence and a well-defined tumor silhouette, SCC under FCM displays keratin pearls composed of keratinized squames, nuclear pleomorphism, and fluorescent scales in the stratum corneum that are a result of keratin formation.5,13 The extent of differentiation of the SCC lesion also can be determined by assessing if the silhouette is well defined. A well-defined tumor silhouette is consistent with the diagnosis of a well-differentiated SCC, and vice versa.13 Ex vivo RCM also has been shown to be useful in diagnosing malignant melanomas, with melanin acting as an endogenous chromophore. Some of the features seen on imaging include a disarranged epithelium, hyperreflective roundish and dendritic pagetoid cells, and large hyperreflective polymorphic cells in the superficial chorion.14
Comparison to Conventional Histopathology
Ex vivo confocal microscopy in both the reflectance and fluorescence mode has been shown to perform well compared to conventional histopathology in the diagnosis of biopsy specimens. Ex vivo FCM has been shown to have an overall sensitivity of 88% and specificity of 99% in detecting residual BCC at the margins of excised tissue samples and in the fraction of the time it takes to attain similar results with frozen histopathology.9 Ex vivo RCM has been shown to have a higher prognostic capability, with 100% sensitivity and specificity in identifying BCC when scanning the tissue samples en face.15
Qualitatively, the images produced by RCM and FCM are similar to histopathology in overall architecture. Both techniques enhance the contrast between the epithelium and stroma and create images that can be examined in low as well as high resolution. A substantial difference between confocal microscopy and conventional hematoxylin and eosin–stained histopathology is that the confocal microscope produces images in gray scale. One way to alter the black-and-white images to resemble hematoxylin and eosin–stained slides is through the use of digital staining,16 which could boost clinical acceptance by physicians who are accustomed to the classical pink-purple appearance of pathology slides and could potentially limit the learning curve needed to read the confocal images.
Application in Mohs Micrographic Surgery
An important clinical application of ex vivo FCM imaging that has emerged is the detection of malignant cells at the excision margins during Mohs micrographic surgery. The use of confocal microscopy has the potential to save time by eliminating the need for tissue fixation while still providing good diagnostic accuracy. Implementing FCM as an imaging tool to guide surgical excisions could provide rapid diagnosis of the tissue, expediting excisions and reconstruction or the Mohs procedure while eliminating patient wait time and the need for frozen histopathology. Ex vivo RCM also has been used to establish laser parameters for CO2 laser ablation of superficial and early nodular BCC lesions.17 Other potential uses for ex vivo RCM/FCM could include rapid evaluation of tissue during operating room procedures where rapid frozen sections are currently utilized.
Combining In Vivo and Ex Vivo Confocal Microscopy
Many of the diagnostic guidelines created with the use of ex vivo confocal microscopy have been applied to in vivo use, and therefore the use of both modalities is appealing. In vivo confocal microscopy is a noninvasive technique that has been used to map margins of skin tumors such as BCC and lentigo maligna at the bedside.5 It also has been shown to help plan both surgical and nonsurgical treatment modalities and reconstruction before the tumor is excised.18 This technique also can help the patient understand the extent of the excision and any subsequent reconstruction that may be needed.
Limitations
Ex vivo confocal microscopy used as a diagnostic tool does have some limitations. Its novelty may require surgeons and pathologists to be trained to interpret the images properly and correlate them to conventional diagnostic guidelines. The imaging also is limited to a depth of approximately 200 µm; however, the sample may be flipped so that the underside can be imaged as well, which increases the depth to approximately 400 µm. The tissue being imaged must be fixed flat, which may alter its shape. Complex tissue samples may be difficult to flatten out completely and therefore may be difficult to image. A special mount may be required for the sample to be fixed in a proper position for imaging.6
Final Thoughts
Despite some of these limitations, the need for rapid bedside tissue diagnosis makes ex vivo confocal microscopy an attractive device that can be used as an additional diagnostic tool to histopathology and also has been tested in other disciplines, such as breast cancer pathology. In the future, both in vivo and ex vivo confocal microscopy may be utilized to diagnose cutaneous malignancies, guide surgical excisions, and detect lesion progression, and it may become a basis for rapid diagnosis and detection.19
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [published online January 7, 2016]. CA Cancer J Clin. 2016;66:7-30.
- Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Welzel J, Kästle R, Sattler EC. Fluorescence (multiwave) confocal microscopy. Dermatol Clin. 2016;34:527-533.
- Longo C, Ragazzi M, Rajadhyaksha M, et al. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497-504.
- Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027.
- Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
- Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242-1250.
- Bennàssar A, Vilata A, Puig S, et al. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360-365.
- Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001.
- Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt. 2011;16:076008.
- Bennàssar A, Carrera C, Puig S, et al. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-847.
- Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73:321-322.
- Cinotti E, Haouas M, Grivet D, et al. In vivo and ex vivo confocal microscopy for the management of a melanoma of the eyelid margin. Dermatol Surg. 2015;41:1437-1440.
- , , , ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid [published online December 19, 2016]. Clin Exp Ophthalmol. doi:10.1111/ceo.12904.
- Gareau DS, Jeon H, Nehal KS, et al. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J Surg Res. 2012;178:533-538.
- Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters [published online September 22, 2015]. Lasers Surg Med. 2016;48:133-139.
- Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346-352.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7-19.
Skin cancer is diagnosed in approximately 5.4 million individuals annually in the United States, more than the total number of breast, lung, colon, and prostate cancers diagnosed per year.1 It is estimated that 1 in 5 Americans will develop skin cancer during their lifetime.2 The 2 most common forms of skin cancer are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), accounting for 4 million and 1 million cases diagnosed each year, respectively.3 With the increasing incidence of these skin cancers, the use of noninvasive imaging tools for detection and diagnosis has grown.
Ex vivo confocal microscopy is a diagnostic imaging tool that can be used in real-time at the bedside to assess freshly excised tissue for malignancies. It images tissue samples with cellular resolution and within minutes of biopsy or excision. Ex vivo confocal microscopy is a versatile tool that can assist in the diagnosis and management of skin malignancies such as melanoma, BCC, and SCC.
Reflectance vs Fluorescence Mode
Excised lesions can be examined in reflectance or fluorescence mode in great detail but with slightly varying nuclear-to-dermis contrasts depending on the chromophore that is targeted. In reflectance mode (reflectance confocal microscopy [RCM]), melanin and keratin act as endogenous chromophores because of their high refractive index relative to water,4,5 which allows for the visualization of cellular structures of the skin at low power, as well as microscopic substructures such as melanosomes, cytoplasmic granules, and other cellular organelles at high power. Although an exogenous contrast agent is not required, acetic acid has the capability to highlight nuclei, enhancing the tumor cell-to-dermis contrast in RCM.6 Acetic acid is clinically used as a predictor for certain skin and mucosal membrane neoplasms that blanch when exposed to the solution. In the case of RCM, acetic acid increases the visibility of nuclei by inducing the compaction of chromatin. For the acetowhitening to be effective, the sample must be soaked in the solution for a specific amount of time, depending on the concentration.7 A concentration between 1% and 10% can be used, but the less concentrated the solution, the longer the time of soaking that is required to achieve sufficiently bright nuclei.6
The contrast with acetic acid, however, is quite weak when the tissue is imaged en face, or along the horizontal surface of the sample, due to the collagen in the dermal layer, which has a high reflectance index. This issue is rectified when using the confocal microscope in the fluorescence mode with an exogenous fluorescent dye as a nuclear stain. Fluorescence confocal microscopy (FCM), results in a stronger nuclear-to-dermal contrast because of the role of contrast agents.8 The 1000-fold increase in contrast between nuclei and dermis is the result of dye agents that preferentially bind to nuclear DNA, of which acridine orange is the most commonly used.5,8 Basal cell carcinoma and SCC tumor cells can be visualized with FCM because they appear hyperfluorescent when stained with acridine orange.9 The acridine orange–stained cells display bright nuclei, while the cytoplasm and collagen remains dark. A positive feature of acridine orange is that it does not alter the tissue sample during freezing or formalin fixation and thus has no effect on subsequent histopathology that may need to be performed on the sample.10
High-Resolution Images Aid in Diagnosis
After it is harvested, the tissue sample is soaked in a contrast agent or dye, if needed, depending on the confocal mode to be used. The confocal microscope is then used to take a series of high-resolution individual en face images that are then stitched together to create a final mosaic image that can be up to 12×12 mm.6,11 With a 200-µm depth visibility, confocal microscopy can capture the cellular structures in the epidermis, dermis, and (if compressed enough) subcutaneous fat in just under 3 minutes.12
The images produced through confocal microscopy have an excellent correlation to frozen histological sections and can aid in the diagnosis of many epidermal and dermal malignancies including melanoma, BCC, and SCC. New criteria have been established to aid in the interpretation of the confocal images and identify some of the more common skin cancers.5,12,13 Basal cell carcinoma samples imaged through fluorescence and reflectance in low-power mode display the distinct nodular patterns with well-demarcated edges, as seen on classical histopathology. In the case of FCM, the cells that make up the tumor display hyperfluorescent areas consistent with nucleated cells that are stained with acridine orange. The main features that identify BCC on FCM images include nuclear pleomorphism and crowding, peripheral palisading, clefting of the basaloid islands, increased nucleus-to-cytoplasm ratio, and the presence of a modified dermis surrounding the mass known as the tumoral stroma5,12 (Figure).

In addition to fluorescence and a well-defined tumor silhouette, SCC under FCM displays keratin pearls composed of keratinized squames, nuclear pleomorphism, and fluorescent scales in the stratum corneum that are a result of keratin formation.5,13 The extent of differentiation of the SCC lesion also can be determined by assessing if the silhouette is well defined. A well-defined tumor silhouette is consistent with the diagnosis of a well-differentiated SCC, and vice versa.13 Ex vivo RCM also has been shown to be useful in diagnosing malignant melanomas, with melanin acting as an endogenous chromophore. Some of the features seen on imaging include a disarranged epithelium, hyperreflective roundish and dendritic pagetoid cells, and large hyperreflective polymorphic cells in the superficial chorion.14
Comparison to Conventional Histopathology
Ex vivo confocal microscopy in both the reflectance and fluorescence mode has been shown to perform well compared to conventional histopathology in the diagnosis of biopsy specimens. Ex vivo FCM has been shown to have an overall sensitivity of 88% and specificity of 99% in detecting residual BCC at the margins of excised tissue samples and in the fraction of the time it takes to attain similar results with frozen histopathology.9 Ex vivo RCM has been shown to have a higher prognostic capability, with 100% sensitivity and specificity in identifying BCC when scanning the tissue samples en face.15
Qualitatively, the images produced by RCM and FCM are similar to histopathology in overall architecture. Both techniques enhance the contrast between the epithelium and stroma and create images that can be examined in low as well as high resolution. A substantial difference between confocal microscopy and conventional hematoxylin and eosin–stained histopathology is that the confocal microscope produces images in gray scale. One way to alter the black-and-white images to resemble hematoxylin and eosin–stained slides is through the use of digital staining,16 which could boost clinical acceptance by physicians who are accustomed to the classical pink-purple appearance of pathology slides and could potentially limit the learning curve needed to read the confocal images.
Application in Mohs Micrographic Surgery
An important clinical application of ex vivo FCM imaging that has emerged is the detection of malignant cells at the excision margins during Mohs micrographic surgery. The use of confocal microscopy has the potential to save time by eliminating the need for tissue fixation while still providing good diagnostic accuracy. Implementing FCM as an imaging tool to guide surgical excisions could provide rapid diagnosis of the tissue, expediting excisions and reconstruction or the Mohs procedure while eliminating patient wait time and the need for frozen histopathology. Ex vivo RCM also has been used to establish laser parameters for CO2 laser ablation of superficial and early nodular BCC lesions.17 Other potential uses for ex vivo RCM/FCM could include rapid evaluation of tissue during operating room procedures where rapid frozen sections are currently utilized.
Combining In Vivo and Ex Vivo Confocal Microscopy
Many of the diagnostic guidelines created with the use of ex vivo confocal microscopy have been applied to in vivo use, and therefore the use of both modalities is appealing. In vivo confocal microscopy is a noninvasive technique that has been used to map margins of skin tumors such as BCC and lentigo maligna at the bedside.5 It also has been shown to help plan both surgical and nonsurgical treatment modalities and reconstruction before the tumor is excised.18 This technique also can help the patient understand the extent of the excision and any subsequent reconstruction that may be needed.
Limitations
Ex vivo confocal microscopy used as a diagnostic tool does have some limitations. Its novelty may require surgeons and pathologists to be trained to interpret the images properly and correlate them to conventional diagnostic guidelines. The imaging also is limited to a depth of approximately 200 µm; however, the sample may be flipped so that the underside can be imaged as well, which increases the depth to approximately 400 µm. The tissue being imaged must be fixed flat, which may alter its shape. Complex tissue samples may be difficult to flatten out completely and therefore may be difficult to image. A special mount may be required for the sample to be fixed in a proper position for imaging.6
Final Thoughts
Despite some of these limitations, the need for rapid bedside tissue diagnosis makes ex vivo confocal microscopy an attractive device that can be used as an additional diagnostic tool to histopathology and also has been tested in other disciplines, such as breast cancer pathology. In the future, both in vivo and ex vivo confocal microscopy may be utilized to diagnose cutaneous malignancies, guide surgical excisions, and detect lesion progression, and it may become a basis for rapid diagnosis and detection.19
Skin cancer is diagnosed in approximately 5.4 million individuals annually in the United States, more than the total number of breast, lung, colon, and prostate cancers diagnosed per year.1 It is estimated that 1 in 5 Americans will develop skin cancer during their lifetime.2 The 2 most common forms of skin cancer are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), accounting for 4 million and 1 million cases diagnosed each year, respectively.3 With the increasing incidence of these skin cancers, the use of noninvasive imaging tools for detection and diagnosis has grown.
Ex vivo confocal microscopy is a diagnostic imaging tool that can be used in real-time at the bedside to assess freshly excised tissue for malignancies. It images tissue samples with cellular resolution and within minutes of biopsy or excision. Ex vivo confocal microscopy is a versatile tool that can assist in the diagnosis and management of skin malignancies such as melanoma, BCC, and SCC.
Reflectance vs Fluorescence Mode
Excised lesions can be examined in reflectance or fluorescence mode in great detail but with slightly varying nuclear-to-dermis contrasts depending on the chromophore that is targeted. In reflectance mode (reflectance confocal microscopy [RCM]), melanin and keratin act as endogenous chromophores because of their high refractive index relative to water,4,5 which allows for the visualization of cellular structures of the skin at low power, as well as microscopic substructures such as melanosomes, cytoplasmic granules, and other cellular organelles at high power. Although an exogenous contrast agent is not required, acetic acid has the capability to highlight nuclei, enhancing the tumor cell-to-dermis contrast in RCM.6 Acetic acid is clinically used as a predictor for certain skin and mucosal membrane neoplasms that blanch when exposed to the solution. In the case of RCM, acetic acid increases the visibility of nuclei by inducing the compaction of chromatin. For the acetowhitening to be effective, the sample must be soaked in the solution for a specific amount of time, depending on the concentration.7 A concentration between 1% and 10% can be used, but the less concentrated the solution, the longer the time of soaking that is required to achieve sufficiently bright nuclei.6
The contrast with acetic acid, however, is quite weak when the tissue is imaged en face, or along the horizontal surface of the sample, due to the collagen in the dermal layer, which has a high reflectance index. This issue is rectified when using the confocal microscope in the fluorescence mode with an exogenous fluorescent dye as a nuclear stain. Fluorescence confocal microscopy (FCM), results in a stronger nuclear-to-dermal contrast because of the role of contrast agents.8 The 1000-fold increase in contrast between nuclei and dermis is the result of dye agents that preferentially bind to nuclear DNA, of which acridine orange is the most commonly used.5,8 Basal cell carcinoma and SCC tumor cells can be visualized with FCM because they appear hyperfluorescent when stained with acridine orange.9 The acridine orange–stained cells display bright nuclei, while the cytoplasm and collagen remains dark. A positive feature of acridine orange is that it does not alter the tissue sample during freezing or formalin fixation and thus has no effect on subsequent histopathology that may need to be performed on the sample.10
High-Resolution Images Aid in Diagnosis
After it is harvested, the tissue sample is soaked in a contrast agent or dye, if needed, depending on the confocal mode to be used. The confocal microscope is then used to take a series of high-resolution individual en face images that are then stitched together to create a final mosaic image that can be up to 12×12 mm.6,11 With a 200-µm depth visibility, confocal microscopy can capture the cellular structures in the epidermis, dermis, and (if compressed enough) subcutaneous fat in just under 3 minutes.12
The images produced through confocal microscopy have an excellent correlation to frozen histological sections and can aid in the diagnosis of many epidermal and dermal malignancies including melanoma, BCC, and SCC. New criteria have been established to aid in the interpretation of the confocal images and identify some of the more common skin cancers.5,12,13 Basal cell carcinoma samples imaged through fluorescence and reflectance in low-power mode display the distinct nodular patterns with well-demarcated edges, as seen on classical histopathology. In the case of FCM, the cells that make up the tumor display hyperfluorescent areas consistent with nucleated cells that are stained with acridine orange. The main features that identify BCC on FCM images include nuclear pleomorphism and crowding, peripheral palisading, clefting of the basaloid islands, increased nucleus-to-cytoplasm ratio, and the presence of a modified dermis surrounding the mass known as the tumoral stroma5,12 (Figure).

In addition to fluorescence and a well-defined tumor silhouette, SCC under FCM displays keratin pearls composed of keratinized squames, nuclear pleomorphism, and fluorescent scales in the stratum corneum that are a result of keratin formation.5,13 The extent of differentiation of the SCC lesion also can be determined by assessing if the silhouette is well defined. A well-defined tumor silhouette is consistent with the diagnosis of a well-differentiated SCC, and vice versa.13 Ex vivo RCM also has been shown to be useful in diagnosing malignant melanomas, with melanin acting as an endogenous chromophore. Some of the features seen on imaging include a disarranged epithelium, hyperreflective roundish and dendritic pagetoid cells, and large hyperreflective polymorphic cells in the superficial chorion.14
Comparison to Conventional Histopathology
Ex vivo confocal microscopy in both the reflectance and fluorescence mode has been shown to perform well compared to conventional histopathology in the diagnosis of biopsy specimens. Ex vivo FCM has been shown to have an overall sensitivity of 88% and specificity of 99% in detecting residual BCC at the margins of excised tissue samples and in the fraction of the time it takes to attain similar results with frozen histopathology.9 Ex vivo RCM has been shown to have a higher prognostic capability, with 100% sensitivity and specificity in identifying BCC when scanning the tissue samples en face.15
Qualitatively, the images produced by RCM and FCM are similar to histopathology in overall architecture. Both techniques enhance the contrast between the epithelium and stroma and create images that can be examined in low as well as high resolution. A substantial difference between confocal microscopy and conventional hematoxylin and eosin–stained histopathology is that the confocal microscope produces images in gray scale. One way to alter the black-and-white images to resemble hematoxylin and eosin–stained slides is through the use of digital staining,16 which could boost clinical acceptance by physicians who are accustomed to the classical pink-purple appearance of pathology slides and could potentially limit the learning curve needed to read the confocal images.
Application in Mohs Micrographic Surgery
An important clinical application of ex vivo FCM imaging that has emerged is the detection of malignant cells at the excision margins during Mohs micrographic surgery. The use of confocal microscopy has the potential to save time by eliminating the need for tissue fixation while still providing good diagnostic accuracy. Implementing FCM as an imaging tool to guide surgical excisions could provide rapid diagnosis of the tissue, expediting excisions and reconstruction or the Mohs procedure while eliminating patient wait time and the need for frozen histopathology. Ex vivo RCM also has been used to establish laser parameters for CO2 laser ablation of superficial and early nodular BCC lesions.17 Other potential uses for ex vivo RCM/FCM could include rapid evaluation of tissue during operating room procedures where rapid frozen sections are currently utilized.
Combining In Vivo and Ex Vivo Confocal Microscopy
Many of the diagnostic guidelines created with the use of ex vivo confocal microscopy have been applied to in vivo use, and therefore the use of both modalities is appealing. In vivo confocal microscopy is a noninvasive technique that has been used to map margins of skin tumors such as BCC and lentigo maligna at the bedside.5 It also has been shown to help plan both surgical and nonsurgical treatment modalities and reconstruction before the tumor is excised.18 This technique also can help the patient understand the extent of the excision and any subsequent reconstruction that may be needed.
Limitations
Ex vivo confocal microscopy used as a diagnostic tool does have some limitations. Its novelty may require surgeons and pathologists to be trained to interpret the images properly and correlate them to conventional diagnostic guidelines. The imaging also is limited to a depth of approximately 200 µm; however, the sample may be flipped so that the underside can be imaged as well, which increases the depth to approximately 400 µm. The tissue being imaged must be fixed flat, which may alter its shape. Complex tissue samples may be difficult to flatten out completely and therefore may be difficult to image. A special mount may be required for the sample to be fixed in a proper position for imaging.6
Final Thoughts
Despite some of these limitations, the need for rapid bedside tissue diagnosis makes ex vivo confocal microscopy an attractive device that can be used as an additional diagnostic tool to histopathology and also has been tested in other disciplines, such as breast cancer pathology. In the future, both in vivo and ex vivo confocal microscopy may be utilized to diagnose cutaneous malignancies, guide surgical excisions, and detect lesion progression, and it may become a basis for rapid diagnosis and detection.19
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [published online January 7, 2016]. CA Cancer J Clin. 2016;66:7-30.
- Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Welzel J, Kästle R, Sattler EC. Fluorescence (multiwave) confocal microscopy. Dermatol Clin. 2016;34:527-533.
- Longo C, Ragazzi M, Rajadhyaksha M, et al. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497-504.
- Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027.
- Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
- Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242-1250.
- Bennàssar A, Vilata A, Puig S, et al. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360-365.
- Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001.
- Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt. 2011;16:076008.
- Bennàssar A, Carrera C, Puig S, et al. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-847.
- Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73:321-322.
- Cinotti E, Haouas M, Grivet D, et al. In vivo and ex vivo confocal microscopy for the management of a melanoma of the eyelid margin. Dermatol Surg. 2015;41:1437-1440.
- , , , ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid [published online December 19, 2016]. Clin Exp Ophthalmol. doi:10.1111/ceo.12904.
- Gareau DS, Jeon H, Nehal KS, et al. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J Surg Res. 2012;178:533-538.
- Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters [published online September 22, 2015]. Lasers Surg Med. 2016;48:133-139.
- Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346-352.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7-19.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [published online January 7, 2016]. CA Cancer J Clin. 2016;66:7-30.
- Robinson JK. Sun exposure, sun protection, and vitamin D. JAMA. 2005;294:1541-1543.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Welzel J, Kästle R, Sattler EC. Fluorescence (multiwave) confocal microscopy. Dermatol Clin. 2016;34:527-533.
- Longo C, Ragazzi M, Rajadhyaksha M, et al. In vivo and ex vivo confocal microscopy for dermatologic and Mohs surgeons. Dermatol Clin. 2016;34:497-504.
- Patel YG, Nehal KS, Aranda I, et al. Confocal reflectance mosaicing of basal cell carcinomas in Mohs surgical skin excisions. J Biomed Opt. 2007;12:034027.
- Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323-331.
- Karen JK, Gareau DS, Dusza SW, et al. Detection of basal cell carcinomas in Mohs excisions with fluorescence confocal mosaicing microscopy. Br J Dermatol. 2009;160:1242-1250.
- Bennàssar A, Vilata A, Puig S, et al. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170:360-365.
- Gareau DS, Li Y, Huang B, et al. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001.
- Bini J, Spain J, Nehal K, et al. Confocal mosaicing microscopy of human skin ex vivo: spectral analysis for digital staining to simulate histology-like appearance. J Biomed Opt. 2011;16:076008.
- Bennàssar A, Carrera C, Puig S, et al. Fast evaluation of 69 basal cell carcinomas with ex vivo fluorescence confocal microscopy: criteria description, histopathological correlation, and interobserver agreement. JAMA Dermatol. 2013;149:839-847.
- Longo C, Ragazzi M, Gardini S, et al. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2015;73:321-322.
- Cinotti E, Haouas M, Grivet D, et al. In vivo and ex vivo confocal microscopy for the management of a melanoma of the eyelid margin. Dermatol Surg. 2015;41:1437-1440.
- , , , ‘En face’ ex vivo reflectance confocal microscopy to help the surgery of basal cell carcinoma of the eyelid [published online December 19, 2016]. Clin Exp Ophthalmol. doi:10.1111/ceo.12904.
- Gareau DS, Jeon H, Nehal KS, et al. Rapid screening of cancer margins in tissue with multimodal confocal microscopy. J Surg Res. 2012;178:533-538.
- Sierra H, Damanpour S, Hibler B, et al. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters [published online September 22, 2015]. Lasers Surg Med. 2016;48:133-139.
- Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99:346-352.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Confocal microscopy is an imaging tool that can be used both in vivo and ex vivo to aid in the diagnosis and management of cutaneous neoplasms, including melanoma, basal cell carcinoma, and squamous cell carcinoma, as well as inflammatory dermatoses.
- Ex vivo confocal microscopy can be used in both reflectance and fluorescent modes to render diagnosis in excised tissue or check surgical margins.
- Both in vivo and ex vivo confocal microscopy produces images with cellular resolution with a main limitation being depth of imaging.
What’s on the dermatopathologist’s wish list
NEW YORK – If dermatopathologists had a wish list they could give their dermatologist colleagues, what might it include? High up on the list for many, said Robert Phelps, MD, might be to have them share the clinical picture, treat the specimen gently, and give the best landmarks possible.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Phelps, director of the dermatopathology service at Mount Sinai Medical Center in New York, led off the dermatopathologist-run session – appropriately titled “Help Me Help You” – by asking, “How can the clinician provide the optimal biopsy?”
It’s always helpful to have as much clinical information as possible, said Dr. Phelps, whose discussion focused on tips for neoplastic lesions. This might include prior history of malignancy, autoimmune disease, pathergy, or other relevant medical history, but clinical pictures can also be a big help, although there can be technical and patient privacy issues to overcome, he noted. If, for example, a larger lesion or rash is being biopsied rather than excised, it can be very helpful to see the larger field and full area of distribution of the lesion in question. Submitting multiple specimens for rashes and larger lesions is always a good idea too, he added.
Although curettage can be a great way to biopsy – and perhaps even definitively treat some lesions – problems can arise on the dermatopathologist’s side when melanocytic lesions are curetted for biopsy, according to Dr. Phelps, a practicing dermatologist and a dermatopathologist. “By virtue of the force of the biopsy, the specimen is often fragmented, and histology can be distorted,” he said. One element of that distortion can be that melanocytes can appear to be free floating, which is a problem. “Dyshesion of melanocytes is usually an indication of atypia … It is an important histologic clue as to the possibility of a malignancy supervening.”
These factors can make it tough for a dermatopathologist to make an accurate call. “If there are free-floating melanocytes from a curetted specimen, I can’t rule out invasive melanoma,” explained Dr. Phelps, since he can’t tell if he is seeing true atypia or disruption that’s an artifact of the collection technique.
In this instance, he said, a dermatopathologist would be “obligated to overcall, because one couldn’t really determine the pathology.” The bottom line? “Don’t curette biopsies of melanocytic lesions.”
Another technique that can interfere with the ability to read a tissue specimen accurately is electrodesiccation. Although it’s often performed in conjunction with curettage, electrodesiccation can cause changes in tissue consistent with thermal injury. “Essentially, the tissue has been burned,” Dr. Phelps pointed out. This can result in a characteristic streaming pattern of nuclei, and the dermis can acquire a “peculiar homogenized appearance,” he said.
Although electrodesiccation can be a useful technique to make sure margins are controlled, “when you do this, just be aware that the interpretation is difficult,” he noted. “It’s difficult to tell where the margins are and if they are the appropriate and correct margins,” he said.
When possible, try to avoid squeezing the tumor, Dr. Phelps advised. Excessive pressure on the specimen can distort cell architecture and make pathological diagnosis really challenging, particularly in lymphoid tumors, he said.
“Often, the tumor is not recognizable,” he added. Crush artifact can result in an appearance of small bluish clumps and smearing of collagen fibers. The effect, he said, can be particularly pronounced with small cell carcinoma and lymphoma, and with rapidly proliferating tumors.
Dr. Phelps said that during his training, he was taught not to use forceps to extract a stubborn punch biopsy specimen; rather, he was trained to use a needle to tease out the specimen. Fear of a self-inflicted needle stick with this technique may be a deterrent, he acknowledged. If forceps are used, he suggested being as gentle as possible and using the finest forceps available.
When pathologists receive an intact excised lesion – one not obtained using a Mohs technique, “delineation of the margin is essential,” Dr. Phelps said. Further, accurate mapping is critical to helping the examiner understand the anatomic orientation of the specimen, a key prerequisite that enables accurate communication from the dermatopathologist back to the clinician if there’s a question regarding the need for retreatment, he added.
For an elliptical excision, ideally, both poles of the ellipse would be suture-tagged, and at least one tag is essential, he said. Then superior and inferior borders can be inked with contrasting colors, and the epidermal borders of the lesion should be marked as well. When the specimen is submitted, it should be accompanied by an accurate map that clearly indicates the coding for medial, lateral, inferior, and superior aspects of the specimen. “Always prepare a specimen diagram for oriented specimens,” Dr. Phelps noted.
Don’t forget to make sure that the left-right orientation on the diagram corresponds to the specimen’s orientation on the patient, he added. Some facilities use a clock face system to indicate orientation and positioning, which may be the clearest method of all.
Sometimes, it’s difficult for the dermatopathologist to visualize whether the specimen is aligned in true medial-lateral fashion, or along skin tension lines, which tend to run diagonally, so “the more clinical information, the better,” he said. “With good mapping, precise retreatment can be optimal,” he said.
Dr. Phelps reported that he had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
NEW YORK – If dermatopathologists had a wish list they could give their dermatologist colleagues, what might it include? High up on the list for many, said Robert Phelps, MD, might be to have them share the clinical picture, treat the specimen gently, and give the best landmarks possible.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Phelps, director of the dermatopathology service at Mount Sinai Medical Center in New York, led off the dermatopathologist-run session – appropriately titled “Help Me Help You” – by asking, “How can the clinician provide the optimal biopsy?”
It’s always helpful to have as much clinical information as possible, said Dr. Phelps, whose discussion focused on tips for neoplastic lesions. This might include prior history of malignancy, autoimmune disease, pathergy, or other relevant medical history, but clinical pictures can also be a big help, although there can be technical and patient privacy issues to overcome, he noted. If, for example, a larger lesion or rash is being biopsied rather than excised, it can be very helpful to see the larger field and full area of distribution of the lesion in question. Submitting multiple specimens for rashes and larger lesions is always a good idea too, he added.
Although curettage can be a great way to biopsy – and perhaps even definitively treat some lesions – problems can arise on the dermatopathologist’s side when melanocytic lesions are curetted for biopsy, according to Dr. Phelps, a practicing dermatologist and a dermatopathologist. “By virtue of the force of the biopsy, the specimen is often fragmented, and histology can be distorted,” he said. One element of that distortion can be that melanocytes can appear to be free floating, which is a problem. “Dyshesion of melanocytes is usually an indication of atypia … It is an important histologic clue as to the possibility of a malignancy supervening.”
These factors can make it tough for a dermatopathologist to make an accurate call. “If there are free-floating melanocytes from a curetted specimen, I can’t rule out invasive melanoma,” explained Dr. Phelps, since he can’t tell if he is seeing true atypia or disruption that’s an artifact of the collection technique.
In this instance, he said, a dermatopathologist would be “obligated to overcall, because one couldn’t really determine the pathology.” The bottom line? “Don’t curette biopsies of melanocytic lesions.”
Another technique that can interfere with the ability to read a tissue specimen accurately is electrodesiccation. Although it’s often performed in conjunction with curettage, electrodesiccation can cause changes in tissue consistent with thermal injury. “Essentially, the tissue has been burned,” Dr. Phelps pointed out. This can result in a characteristic streaming pattern of nuclei, and the dermis can acquire a “peculiar homogenized appearance,” he said.
Although electrodesiccation can be a useful technique to make sure margins are controlled, “when you do this, just be aware that the interpretation is difficult,” he noted. “It’s difficult to tell where the margins are and if they are the appropriate and correct margins,” he said.
When possible, try to avoid squeezing the tumor, Dr. Phelps advised. Excessive pressure on the specimen can distort cell architecture and make pathological diagnosis really challenging, particularly in lymphoid tumors, he said.
“Often, the tumor is not recognizable,” he added. Crush artifact can result in an appearance of small bluish clumps and smearing of collagen fibers. The effect, he said, can be particularly pronounced with small cell carcinoma and lymphoma, and with rapidly proliferating tumors.
Dr. Phelps said that during his training, he was taught not to use forceps to extract a stubborn punch biopsy specimen; rather, he was trained to use a needle to tease out the specimen. Fear of a self-inflicted needle stick with this technique may be a deterrent, he acknowledged. If forceps are used, he suggested being as gentle as possible and using the finest forceps available.
When pathologists receive an intact excised lesion – one not obtained using a Mohs technique, “delineation of the margin is essential,” Dr. Phelps said. Further, accurate mapping is critical to helping the examiner understand the anatomic orientation of the specimen, a key prerequisite that enables accurate communication from the dermatopathologist back to the clinician if there’s a question regarding the need for retreatment, he added.
For an elliptical excision, ideally, both poles of the ellipse would be suture-tagged, and at least one tag is essential, he said. Then superior and inferior borders can be inked with contrasting colors, and the epidermal borders of the lesion should be marked as well. When the specimen is submitted, it should be accompanied by an accurate map that clearly indicates the coding for medial, lateral, inferior, and superior aspects of the specimen. “Always prepare a specimen diagram for oriented specimens,” Dr. Phelps noted.
Don’t forget to make sure that the left-right orientation on the diagram corresponds to the specimen’s orientation on the patient, he added. Some facilities use a clock face system to indicate orientation and positioning, which may be the clearest method of all.
Sometimes, it’s difficult for the dermatopathologist to visualize whether the specimen is aligned in true medial-lateral fashion, or along skin tension lines, which tend to run diagonally, so “the more clinical information, the better,” he said. “With good mapping, precise retreatment can be optimal,” he said.
Dr. Phelps reported that he had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
NEW YORK – If dermatopathologists had a wish list they could give their dermatologist colleagues, what might it include? High up on the list for many, said Robert Phelps, MD, might be to have them share the clinical picture, treat the specimen gently, and give the best landmarks possible.
Speaking at the summer meeting of the American Academy of Dermatology, Dr. Phelps, director of the dermatopathology service at Mount Sinai Medical Center in New York, led off the dermatopathologist-run session – appropriately titled “Help Me Help You” – by asking, “How can the clinician provide the optimal biopsy?”
It’s always helpful to have as much clinical information as possible, said Dr. Phelps, whose discussion focused on tips for neoplastic lesions. This might include prior history of malignancy, autoimmune disease, pathergy, or other relevant medical history, but clinical pictures can also be a big help, although there can be technical and patient privacy issues to overcome, he noted. If, for example, a larger lesion or rash is being biopsied rather than excised, it can be very helpful to see the larger field and full area of distribution of the lesion in question. Submitting multiple specimens for rashes and larger lesions is always a good idea too, he added.
Although curettage can be a great way to biopsy – and perhaps even definitively treat some lesions – problems can arise on the dermatopathologist’s side when melanocytic lesions are curetted for biopsy, according to Dr. Phelps, a practicing dermatologist and a dermatopathologist. “By virtue of the force of the biopsy, the specimen is often fragmented, and histology can be distorted,” he said. One element of that distortion can be that melanocytes can appear to be free floating, which is a problem. “Dyshesion of melanocytes is usually an indication of atypia … It is an important histologic clue as to the possibility of a malignancy supervening.”
These factors can make it tough for a dermatopathologist to make an accurate call. “If there are free-floating melanocytes from a curetted specimen, I can’t rule out invasive melanoma,” explained Dr. Phelps, since he can’t tell if he is seeing true atypia or disruption that’s an artifact of the collection technique.
In this instance, he said, a dermatopathologist would be “obligated to overcall, because one couldn’t really determine the pathology.” The bottom line? “Don’t curette biopsies of melanocytic lesions.”
Another technique that can interfere with the ability to read a tissue specimen accurately is electrodesiccation. Although it’s often performed in conjunction with curettage, electrodesiccation can cause changes in tissue consistent with thermal injury. “Essentially, the tissue has been burned,” Dr. Phelps pointed out. This can result in a characteristic streaming pattern of nuclei, and the dermis can acquire a “peculiar homogenized appearance,” he said.
Although electrodesiccation can be a useful technique to make sure margins are controlled, “when you do this, just be aware that the interpretation is difficult,” he noted. “It’s difficult to tell where the margins are and if they are the appropriate and correct margins,” he said.
When possible, try to avoid squeezing the tumor, Dr. Phelps advised. Excessive pressure on the specimen can distort cell architecture and make pathological diagnosis really challenging, particularly in lymphoid tumors, he said.
“Often, the tumor is not recognizable,” he added. Crush artifact can result in an appearance of small bluish clumps and smearing of collagen fibers. The effect, he said, can be particularly pronounced with small cell carcinoma and lymphoma, and with rapidly proliferating tumors.
Dr. Phelps said that during his training, he was taught not to use forceps to extract a stubborn punch biopsy specimen; rather, he was trained to use a needle to tease out the specimen. Fear of a self-inflicted needle stick with this technique may be a deterrent, he acknowledged. If forceps are used, he suggested being as gentle as possible and using the finest forceps available.
When pathologists receive an intact excised lesion – one not obtained using a Mohs technique, “delineation of the margin is essential,” Dr. Phelps said. Further, accurate mapping is critical to helping the examiner understand the anatomic orientation of the specimen, a key prerequisite that enables accurate communication from the dermatopathologist back to the clinician if there’s a question regarding the need for retreatment, he added.
For an elliptical excision, ideally, both poles of the ellipse would be suture-tagged, and at least one tag is essential, he said. Then superior and inferior borders can be inked with contrasting colors, and the epidermal borders of the lesion should be marked as well. When the specimen is submitted, it should be accompanied by an accurate map that clearly indicates the coding for medial, lateral, inferior, and superior aspects of the specimen. “Always prepare a specimen diagram for oriented specimens,” Dr. Phelps noted.
Don’t forget to make sure that the left-right orientation on the diagram corresponds to the specimen’s orientation on the patient, he added. Some facilities use a clock face system to indicate orientation and positioning, which may be the clearest method of all.
Sometimes, it’s difficult for the dermatopathologist to visualize whether the specimen is aligned in true medial-lateral fashion, or along skin tension lines, which tend to run diagonally, so “the more clinical information, the better,” he said. “With good mapping, precise retreatment can be optimal,” he said.
Dr. Phelps reported that he had no relevant conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING