User login
Medical Management of Ectopic Pregnancy: Early Diagnosis is Key
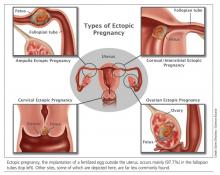
Ectopic pregnancy is a significant health risk to women during their childbearing years; approximately 6% of all pregnancy-related deaths are due to ectopic pregnancy.1-3 Some 1% to 2% of all pregnancies in the United States each year—approximately 100,000 cases—are ectopic, with an estimated annual cost of care approaching $1.1 billion.4 The incidence of ectopic pregnancy has increased in the past 20 years; in one analysis, ectopic pregnancy was diagnosed in 18% of women who presented to an emergency department (ED) with first trimester vaginal bleeding, abdominal pain, or both.5 This growing prevalence is attributed to a number of factors, including the sensitivity of current diagnostic methods in detecting early ectopic pregnancy, the greater incidence of salpingitis, and the growing use of assisted reproductive technologies.2,6
While the number of ectopic pregnancies is on the rise, the proportion of patients requiring hospitalization for surgical treatment of ectopic pregnancy has decreased significantly. Today, for appropriate patients, many clinicians manage ectopic pregnancy on an outpatient basis using the drug methotrexate.6
In this article, we will present an overview of the current status of medical management of ectopic pregnancy, along with a case study. The case study describes a patient diagnosed with an unruptured ectopic pregnancy who was managed medically with methotrexate. It illustrates how, with early diagnosis, clinicians can intervene to make medical management an effective treatment option in selected situations.
The patient reported a history of oral contraceptive use until approximately three months prior to this pregnancy. She was taking no medications and had no known drug allergies. Her previous pregnancies included two uncomplicated vaginal births at term and one miscarriage at six to seven weeks’ gestation two years ago. She also reported a dilation and curettage after the miscarriage. Her medical, surgical, and gynecologic histories were otherwise noncontributory. A review of systems was otherwise negative.
Sexual history revealed that the patient was married and monogamous with her husband of five years. She disclosed four previous sexual partners and inconsistent use of condoms with those partners; no current condom use was reported. Seven years ago, she tested positive for gonorrhea and chlamydia and was treated concurrently with her partner. Subsequent diagnostics were negative. She reported vaginal intercourse but no oral sex and denied any other sexual contact. All partners had been male.
On the next page: Diagnosis and case continuation >>
DIAGNOSIS
There is some variation in the presentation of women experiencing ectopic pregnancy; this may be due to differences in the pathologic mechanisms of ectopic pregnancy. Patients may be asymptomatic, hemodynamically compromised, or somewhere in between.3 Typical clinical signs include abdominal pain, amenorrhea, and vaginal bleeding. Approximately 40% to 50% of patients present with vaginal bleeding, 50% may have a palpable adnexal mass, and 75% may have abdominal tenderness.3 Only about 50% of women with ectopic pregnancies present with these typical symptoms.3
The patient may also experience common symptoms of early pregnancy, such as nausea, fatigue, and breast fullness. Worrisome signs and symptoms, including abdominal guarding, hypotension, tachycardia, shock, shoulder pain from peritoneal irritation, dizziness, fever, and vomiting, may also be present.3,7 Approximately 20% of patients with ectopic pregnancies are hemodynamically compromised at presentation, which is highly suggestive of rupture.3
Risk factors
Risk factors for ectopic pregnancy include previous ectopic pregnancy; previous tubal procedures; history of sexually transmitted disease or genital infections; infertility; use of assisted reproductive technology; previous abdominal or pelvic surgery; smoking; pelvic inflammatory disease; exposure in utero to diethylstilbestrol; and previous intrauterine device use.2,5,7,8 Knowledge of these risk factors can help identify a patient with an ectopic pregnancy.
The diagnosis of ectopic pregnancy is most certainly a clinical challenge. The differential diagnosis is based upon history and physical findings; the list can be lengthy if both vaginal bleeding and abdominal pain (nonspecific symptoms common in women who miscarry) are present.7 Prompt completion of diagnostic testing is critical in making a definitive diagnosis. Possible diagnoses are listed in Table 1.
CASE Upon examination, the patient appeared comfortable and relaxed, and there were no signs of distress. Blood pressure was 100/65 mm Hg, pulse rate was 72 beats/min, and temperature was 99.0°F. There was no tenderness upon abdominal examination. Pelvic examination revealed a small amount of brown vaginal discharge but no active bleeding or pooled blood, clots, or tissue. The cervical os was closed, and positive Chadwick sign was present. Bimanual examination revealed no cervical motion tenderness. The uterus was soft, mobile, and nontender, and consistent in size with a gestation at eight weeks. There were no palpable adnexa, ovaries, or masses. There was no pain with bimanual examination and no evidence of tenderness at the posterior fornix. The remainder of the physical examination was unremarkable.
It is important to note that examination results in the case patient are not unusual in a woman with a small, unruptured ectopic pregnancy. All findings were normal except for the scant brown vaginal discharge. Abdominal and adnexal tenderness are common, as is a palpable adnexal mass; but absence of a detectable mass does not exclude ectopic pregnancy.1 Pathologic findings may include severe abdominal tenderness and pain, significant vaginal bleeding, passage of clots, tachycardia, and orthostatic hypotension.
Diagnostic workup
Laboratory tests are critical to making an accurate diagnosis for women whose history and physical examination results are consistent with ectopic pregnancy. Assessment for ectopic pregnancy should include a urine pregnancy test, transvaginal ultrasound, measurement of serum ß-human chorionic gonadotropin (ß-hCG) level, and occasionally, diagnostic curettage.1 Once the diagnosis is confirmed, a complete blood count (CBC) is necessary to assess anemia and platelet functioning. Coagulation tests may be required for worrisome bleeding. Blood type, Rh status, and antibody screen are also necessary to determine whether a patient who is Rh D-negative will require Rh immune globulin. See Table 2 for the patient’s laboratory test results.
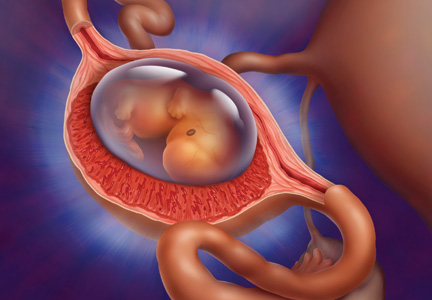
In a patient with a ß-hCG level greater than the discriminatory cutoff value of 1,500 to 1,800 mIU/mL, the level above which an intrauterine gestational sac is visible on transvaginal ultrasound in a normal pregnancy, an empty uterus is considered an ectopic pregnancy until proven otherwise.3 In a definite intrauterine pregnancy of about six weeks’ gestation, transvaginal ultrasound reveals a gestational sac that contains a yolk sac and a fetal pole.3
CASE The patient’s presenting symptoms, combined with a positive pregnancy test, ß-hCG level of 1,850 mIU/mL, and a complex adnexal mass in the right fallopian tube, were highly suggestive of an unruptured ectopic pregnancy (see Table 3 for the patient’s transvaginal ultrasound findings). There was also a secondary finding of a corpus luteum cyst. Other diagnoses were ruled out, and the patient was diagnosed with an unruptured ectopic pregnancy.
On the next page: Treatment >>
TREATMENT
A patient with an ectopic pregnancy who presents with pain and hemodynamic instability should be referred immediately for appropriate surgical care.7 Otherwise, once the diagnosis of ectopic pregnancy is confirmed, the patient should be referred to an obstetric specialist. Treatments for ectopic pregnancy include expectant management and surgery—which will be discussed briefly—and medical management, which is the focus of this review.5
Expectant management
Most ectopic pregnancies are diagnosed early as a result of accurate, minimally invasive and noninvasive diagnostic tools and greater awareness of risk factors. Since the natural course of early ectopic pregnancy is often self-limited, eventually resulting in tubal abortion or reabsorption, expectant management is a viable option.9
This treatment option may be considered if the patient is asymptomatic; ß-hCG is < 200 mIU/mL; the ectopic mass is < 3 cm; and no fetal heartbeat is present.1,2 With this approach, patients must be willing to accept the risk for tubal rupture and agree to close monitoring of ß-hCG levels. The ß-hCG level must be measured every 24 to 48 hours in order to determine if it is declining adequately, plateauing, or increasing.2,5
Surgery
For the hemodynamically unstable patient, the treatment decision is relatively straightforward. Optimal treatment for a ruptured ectopic pregnancy is immediate surgery, which may include salpingostomy or salpingectomy.10 Surgery may also be considered for hemodynamically stable patients with nonruptured ectopic pregnancies; in addition to her clinical presentation, overall management may be driven by a patient’s preferences.5 Salpingostomy and salpingectomy can be performed either laparoscopically or via laparotomy, depending on the specific situation.
Medical management
The use of methotrexate for the management of unruptured ectopic pregnancy was introduced in the early 1980s.11 Initially, protocols called for multiple doses administered during the course of an inpatient stay. Further research led to revised treatment recommendations and today, medical management most often consists of a single dose of methotrexate with outpatient follow-up.3
Methotrexate is a folic acid antagonist often used as an antimetabolite chemotherapeutic agent. In ectopic pregnancy, it inhibits growth of the rapidly dividing trophoblastic cells and ultimately ends the pregnancy.2 Outcomes of medical management are comparable to those of surgical treatment, including the potential for future normal pregnancies.2,5
An analysis of US trends in ectopic pregnancy management from 2002-2007 revealed that the use of methotrexate increased from 11.1% to 35.1% during that time, while the use of surgical approaches declined from 90% to 65%.10 Medical management of ectopic pregnancy eliminates the costs of surgery, anesthesia, and hospitalization and avoids potential complications of surgery and anesthesia.
Appropriate candidates
A hemodynamically stable patient with a confirmed or high clinical suspicion of ectopic pregnancy, an unruptured mass, no active bleeding, and low ß-hCG levels (< 5,000 mIU/mL) can be considered for methotrexate therapy.2,3,9 It is critical that medically managed patients be willing and able to adhere to all follow-up appointments.9 Before initiating treatment, normal serum creatinine and transaminase levels should be confirmed, and there should be no evidence of significant anemia, leukopenia, or thrombocytopenia.2 To detect any adverse effects of methotrexate on renal, hepatic, and hematologic functioning, these tests are repeated one week after administration.2
Contraindications
Contraindications to methotrexate treatment include breastfeeding, immunodeficiency, alcoholism, alcoholic liver disease or other chronic liver disease, preexisting blood dyscrasias (eg, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia), known sensitivity to methotrexate, active pulmonary disease, peptic ulcer, and hepatic, renal, or hematologic dysfunction. Relative contraindications are a gestational sac larger than 3.5 cm and embryonic cardiac motion.2
On the next page: Patient education >>
PATIENT EDUCATION AND INFORMED CONSENT
A diagnosis of unruptured ectopic pregnancy requires patient education about the condition and its treatment options. The clinician should explain what an ectopic pregnancy is and distinguish between unruptured and ruptured. A discussion of the benefits and risks of each treatment option for which the patient is an appropriate candidate, as well as what to anticipate during treatment, is needed. Emotional support for impending pregnancy loss should also be provided.
For patients who choose medical management, education includes methotrexate-specific information and written instructions to follow after methotrexate administration. Patients must be instructed about the use of safety precautions after treatment (eg, the toilet should be double-flushed with the lid closed during the first 72 hours after treatment to prevent exposing others to methotrexate in urine and stool), the need for adherence to follow-up visits, and warning signs of a possible rupture.5 These warning signs are listed in Table 4.
The most common adverse effects of methotrexate are gastrointestinal (nausea, vomiting, stomatitis). Patients should be advised to avoid alcohol, NSAIDs, folic acid supplements, excessive sun exposure (due to photosensitivity), strenuous exercise, and sexual intercourse until ß-hCG has returned to nonpregnant levels. Other adverse effects may include a temporary elevation in liver enzymes and rarely, alopecia. Abdominal pain may occur a few days after methotrexate administration, likely from the cytotoxic effects of the drug on the trophoblastic tissue.
Informed consent is required prior to methotrexate administration. The patient must be advised of the potential risks of medical management with methotrexate, including rupture of the ectopic pregnancy during treatment, inadvertent administration of methotrexate in the presence of an early intrauterine embryo, allergic reaction to methotrexate, and methotrexate-induced pneumonitis.5
CASE After lengthy discussion of the treatment options, the patient chose medical management with methotrexate. She verbalized her understanding of the teaching provided and signed an informed consent document.
METHOTREXATE REGIMENS
Protocols for single-dose, two-dose, and fixed multidose methotrexate regimens are described in the medical literature, according to a 2008 American Congress of Obstetricians and Gynecologists practice bulletin.2 A 2013 practice committee opinion of the American Society for Reproductive Medicine (ASRM) indicates that single-dose and multiple-dose regimens are used most often.12
With methotrexate treatment, complete resolution of ectopic pregnancy usually occurs in two to three weeks but may require up to six to eight weeks, depending on how high the ß-hCG level is when treatment begins.12
Single-dose
In the single-dose regimen, an intramuscular (IM) injection of methotrexate 50 mg/m2 is administered on day 1. The ß-hCG levels are measured on days 4 and 7 after administration; a decrease of at least 15% in the ß-hCG level should be observed. The ß-hCG level is then measured weekly until it reaches < 2 mIU/mL or is undetectable.2 If the level does not decline, a repeat dose of methotrexate can be given, with measurement of ß-hCG on days 4 and 7 after the repeat dose. If the ß-hCG level fails to decrease, additional methotrexate or surgical intervention should be considered.
The single-dose regimen is more frequently used and is most successful when ß-hCG levels are low (< 5,000 mIU/mL), the ectopic mass is small
(< 3.5 cm), and embryonic cardiac activity is not observed on ultrasound.2,3 Patients with ß-hCG levels > 5,000 mIU/mL may be appropriate candidates for additional doses of methotrexate.2 In fact, the single-dose protocol provides for repeat doses of methotrexate if the ß-hCG level is not decreasing adequately.12
Multiple-dose
With the multiple-dose regimen, methotrexate 1 mg/kg IM is administered on days 1, 3, 5, and 7; on days 2, 4, 6, and 8, the patient receives leucovorin (folinic acid) 0.1 mg/kg IM. The ß-hCG level is measured on days methotrexate is administered; once the minimum 15% decline is observed, ß-hCG is measured weekly until a nonpregnant level is reached.12
The patient received a single dose of methotrexate 50 mg/m2 IM on day 1 and returned to the clinic for follow-up on days 4 and 7 posttreatment. On day 4, her ß-hCG level was 1,060 mIU/mL; on day 7, it was 470 mIU/mL. Also on day 7, blood was drawn for a CBC and comprehensive metabolic panel; results were within normal limits. The patient continued weekly follow-up until her ß-hCG level decreased to < 2 mIU/mL.
On the next page: Follow-up and conclusion >>
FOLLOW-UP AND REFERRALS
Close monitoring of ß-hCG levels, as described previously, is essential after methotrexate treatment in order to confirm that the pregnancy has been terminated and reduce the risk for tubal rupture. Clinicians should also be sensitive to the sequelae of loss of a pregnancy and refer patients as needed to appropriate health care professionals for grief support.
CASE The patient was referred to an obstetrics clinic and reported for all scheduled follow-up appointments. She was discharged from care after a full reduction in her ß-hCG to nonpregnant levels. While at the clinic, the patient was referred to social services for psychosocial counseling.
CONCLUSION
Ectopic implantation is a serious complication that may occur during the first trimester of pregnancy. Worldwide, it is the leading cause of maternal death in the first trimester. For women who meet specific criteria, outpatient treatment of early ectopic pregnancy with methotrexate avoids surgery and decreases the overall cost of care. Medical management and conservative surgical management offer the patient comparable outcomes for tubal patency preservation and risk for ectopic pregnancy recurrence.11
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72(9):1707-1714.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479-1485.
3. Sepilian VP, Wood E. Ectopic pregnancy. http://emedicine.medscape.com/article/2041923-overview. Medscape. Accessed June 19, 2014.
4. Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med. 2010;56(6):674-683.
5. Murtaza UI, Ortmann MJ, Mando-Vandrick J, Lee ASD. Management of first-trimester complications in the emergency department. Am J Health Syst Pharm. 2013;70(2):99-111.
6. Sewell CA, Cundiff GW. Trends for inpatient treatment of tubal pregnancy in Maryland. Am J Obstet Gynecol. 2002;186(3):404-408.
7. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet. 2009;279(4):443-453.
8. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
9. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.
10. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3): 495-502.
11. Autry A. Medical treatment of ectopic pregnancy: is there something new? Obstet Gynecol. 2013;122(4):733.
12. The Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Ectopic pregnancy is a significant health risk to women during their childbearing years; approximately 6% of all pregnancy-related deaths are due to ectopic pregnancy.1-3 Some 1% to 2% of all pregnancies in the United States each year—approximately 100,000 cases—are ectopic, with an estimated annual cost of care approaching $1.1 billion.4 The incidence of ectopic pregnancy has increased in the past 20 years; in one analysis, ectopic pregnancy was diagnosed in 18% of women who presented to an emergency department (ED) with first trimester vaginal bleeding, abdominal pain, or both.5 This growing prevalence is attributed to a number of factors, including the sensitivity of current diagnostic methods in detecting early ectopic pregnancy, the greater incidence of salpingitis, and the growing use of assisted reproductive technologies.2,6
While the number of ectopic pregnancies is on the rise, the proportion of patients requiring hospitalization for surgical treatment of ectopic pregnancy has decreased significantly. Today, for appropriate patients, many clinicians manage ectopic pregnancy on an outpatient basis using the drug methotrexate.6
In this article, we will present an overview of the current status of medical management of ectopic pregnancy, along with a case study. The case study describes a patient diagnosed with an unruptured ectopic pregnancy who was managed medically with methotrexate. It illustrates how, with early diagnosis, clinicians can intervene to make medical management an effective treatment option in selected situations.
The patient reported a history of oral contraceptive use until approximately three months prior to this pregnancy. She was taking no medications and had no known drug allergies. Her previous pregnancies included two uncomplicated vaginal births at term and one miscarriage at six to seven weeks’ gestation two years ago. She also reported a dilation and curettage after the miscarriage. Her medical, surgical, and gynecologic histories were otherwise noncontributory. A review of systems was otherwise negative.
Sexual history revealed that the patient was married and monogamous with her husband of five years. She disclosed four previous sexual partners and inconsistent use of condoms with those partners; no current condom use was reported. Seven years ago, she tested positive for gonorrhea and chlamydia and was treated concurrently with her partner. Subsequent diagnostics were negative. She reported vaginal intercourse but no oral sex and denied any other sexual contact. All partners had been male.
On the next page: Diagnosis and case continuation >>
DIAGNOSIS
There is some variation in the presentation of women experiencing ectopic pregnancy; this may be due to differences in the pathologic mechanisms of ectopic pregnancy. Patients may be asymptomatic, hemodynamically compromised, or somewhere in between.3 Typical clinical signs include abdominal pain, amenorrhea, and vaginal bleeding. Approximately 40% to 50% of patients present with vaginal bleeding, 50% may have a palpable adnexal mass, and 75% may have abdominal tenderness.3 Only about 50% of women with ectopic pregnancies present with these typical symptoms.3
The patient may also experience common symptoms of early pregnancy, such as nausea, fatigue, and breast fullness. Worrisome signs and symptoms, including abdominal guarding, hypotension, tachycardia, shock, shoulder pain from peritoneal irritation, dizziness, fever, and vomiting, may also be present.3,7 Approximately 20% of patients with ectopic pregnancies are hemodynamically compromised at presentation, which is highly suggestive of rupture.3
Risk factors
Risk factors for ectopic pregnancy include previous ectopic pregnancy; previous tubal procedures; history of sexually transmitted disease or genital infections; infertility; use of assisted reproductive technology; previous abdominal or pelvic surgery; smoking; pelvic inflammatory disease; exposure in utero to diethylstilbestrol; and previous intrauterine device use.2,5,7,8 Knowledge of these risk factors can help identify a patient with an ectopic pregnancy.
The diagnosis of ectopic pregnancy is most certainly a clinical challenge. The differential diagnosis is based upon history and physical findings; the list can be lengthy if both vaginal bleeding and abdominal pain (nonspecific symptoms common in women who miscarry) are present.7 Prompt completion of diagnostic testing is critical in making a definitive diagnosis. Possible diagnoses are listed in Table 1.
CASE Upon examination, the patient appeared comfortable and relaxed, and there were no signs of distress. Blood pressure was 100/65 mm Hg, pulse rate was 72 beats/min, and temperature was 99.0°F. There was no tenderness upon abdominal examination. Pelvic examination revealed a small amount of brown vaginal discharge but no active bleeding or pooled blood, clots, or tissue. The cervical os was closed, and positive Chadwick sign was present. Bimanual examination revealed no cervical motion tenderness. The uterus was soft, mobile, and nontender, and consistent in size with a gestation at eight weeks. There were no palpable adnexa, ovaries, or masses. There was no pain with bimanual examination and no evidence of tenderness at the posterior fornix. The remainder of the physical examination was unremarkable.
It is important to note that examination results in the case patient are not unusual in a woman with a small, unruptured ectopic pregnancy. All findings were normal except for the scant brown vaginal discharge. Abdominal and adnexal tenderness are common, as is a palpable adnexal mass; but absence of a detectable mass does not exclude ectopic pregnancy.1 Pathologic findings may include severe abdominal tenderness and pain, significant vaginal bleeding, passage of clots, tachycardia, and orthostatic hypotension.
Diagnostic workup
Laboratory tests are critical to making an accurate diagnosis for women whose history and physical examination results are consistent with ectopic pregnancy. Assessment for ectopic pregnancy should include a urine pregnancy test, transvaginal ultrasound, measurement of serum ß-human chorionic gonadotropin (ß-hCG) level, and occasionally, diagnostic curettage.1 Once the diagnosis is confirmed, a complete blood count (CBC) is necessary to assess anemia and platelet functioning. Coagulation tests may be required for worrisome bleeding. Blood type, Rh status, and antibody screen are also necessary to determine whether a patient who is Rh D-negative will require Rh immune globulin. See Table 2 for the patient’s laboratory test results.
In a patient with a ß-hCG level greater than the discriminatory cutoff value of 1,500 to 1,800 mIU/mL, the level above which an intrauterine gestational sac is visible on transvaginal ultrasound in a normal pregnancy, an empty uterus is considered an ectopic pregnancy until proven otherwise.3 In a definite intrauterine pregnancy of about six weeks’ gestation, transvaginal ultrasound reveals a gestational sac that contains a yolk sac and a fetal pole.3
CASE The patient’s presenting symptoms, combined with a positive pregnancy test, ß-hCG level of 1,850 mIU/mL, and a complex adnexal mass in the right fallopian tube, were highly suggestive of an unruptured ectopic pregnancy (see Table 3 for the patient’s transvaginal ultrasound findings). There was also a secondary finding of a corpus luteum cyst. Other diagnoses were ruled out, and the patient was diagnosed with an unruptured ectopic pregnancy.
On the next page: Treatment >>
TREATMENT
A patient with an ectopic pregnancy who presents with pain and hemodynamic instability should be referred immediately for appropriate surgical care.7 Otherwise, once the diagnosis of ectopic pregnancy is confirmed, the patient should be referred to an obstetric specialist. Treatments for ectopic pregnancy include expectant management and surgery—which will be discussed briefly—and medical management, which is the focus of this review.5
Expectant management
Most ectopic pregnancies are diagnosed early as a result of accurate, minimally invasive and noninvasive diagnostic tools and greater awareness of risk factors. Since the natural course of early ectopic pregnancy is often self-limited, eventually resulting in tubal abortion or reabsorption, expectant management is a viable option.9
This treatment option may be considered if the patient is asymptomatic; ß-hCG is < 200 mIU/mL; the ectopic mass is < 3 cm; and no fetal heartbeat is present.1,2 With this approach, patients must be willing to accept the risk for tubal rupture and agree to close monitoring of ß-hCG levels. The ß-hCG level must be measured every 24 to 48 hours in order to determine if it is declining adequately, plateauing, or increasing.2,5
Surgery
For the hemodynamically unstable patient, the treatment decision is relatively straightforward. Optimal treatment for a ruptured ectopic pregnancy is immediate surgery, which may include salpingostomy or salpingectomy.10 Surgery may also be considered for hemodynamically stable patients with nonruptured ectopic pregnancies; in addition to her clinical presentation, overall management may be driven by a patient’s preferences.5 Salpingostomy and salpingectomy can be performed either laparoscopically or via laparotomy, depending on the specific situation.
Medical management
The use of methotrexate for the management of unruptured ectopic pregnancy was introduced in the early 1980s.11 Initially, protocols called for multiple doses administered during the course of an inpatient stay. Further research led to revised treatment recommendations and today, medical management most often consists of a single dose of methotrexate with outpatient follow-up.3
Methotrexate is a folic acid antagonist often used as an antimetabolite chemotherapeutic agent. In ectopic pregnancy, it inhibits growth of the rapidly dividing trophoblastic cells and ultimately ends the pregnancy.2 Outcomes of medical management are comparable to those of surgical treatment, including the potential for future normal pregnancies.2,5
An analysis of US trends in ectopic pregnancy management from 2002-2007 revealed that the use of methotrexate increased from 11.1% to 35.1% during that time, while the use of surgical approaches declined from 90% to 65%.10 Medical management of ectopic pregnancy eliminates the costs of surgery, anesthesia, and hospitalization and avoids potential complications of surgery and anesthesia.
Appropriate candidates
A hemodynamically stable patient with a confirmed or high clinical suspicion of ectopic pregnancy, an unruptured mass, no active bleeding, and low ß-hCG levels (< 5,000 mIU/mL) can be considered for methotrexate therapy.2,3,9 It is critical that medically managed patients be willing and able to adhere to all follow-up appointments.9 Before initiating treatment, normal serum creatinine and transaminase levels should be confirmed, and there should be no evidence of significant anemia, leukopenia, or thrombocytopenia.2 To detect any adverse effects of methotrexate on renal, hepatic, and hematologic functioning, these tests are repeated one week after administration.2
Contraindications
Contraindications to methotrexate treatment include breastfeeding, immunodeficiency, alcoholism, alcoholic liver disease or other chronic liver disease, preexisting blood dyscrasias (eg, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia), known sensitivity to methotrexate, active pulmonary disease, peptic ulcer, and hepatic, renal, or hematologic dysfunction. Relative contraindications are a gestational sac larger than 3.5 cm and embryonic cardiac motion.2
On the next page: Patient education >>
PATIENT EDUCATION AND INFORMED CONSENT
A diagnosis of unruptured ectopic pregnancy requires patient education about the condition and its treatment options. The clinician should explain what an ectopic pregnancy is and distinguish between unruptured and ruptured. A discussion of the benefits and risks of each treatment option for which the patient is an appropriate candidate, as well as what to anticipate during treatment, is needed. Emotional support for impending pregnancy loss should also be provided.
For patients who choose medical management, education includes methotrexate-specific information and written instructions to follow after methotrexate administration. Patients must be instructed about the use of safety precautions after treatment (eg, the toilet should be double-flushed with the lid closed during the first 72 hours after treatment to prevent exposing others to methotrexate in urine and stool), the need for adherence to follow-up visits, and warning signs of a possible rupture.5 These warning signs are listed in Table 4.
The most common adverse effects of methotrexate are gastrointestinal (nausea, vomiting, stomatitis). Patients should be advised to avoid alcohol, NSAIDs, folic acid supplements, excessive sun exposure (due to photosensitivity), strenuous exercise, and sexual intercourse until ß-hCG has returned to nonpregnant levels. Other adverse effects may include a temporary elevation in liver enzymes and rarely, alopecia. Abdominal pain may occur a few days after methotrexate administration, likely from the cytotoxic effects of the drug on the trophoblastic tissue.
Informed consent is required prior to methotrexate administration. The patient must be advised of the potential risks of medical management with methotrexate, including rupture of the ectopic pregnancy during treatment, inadvertent administration of methotrexate in the presence of an early intrauterine embryo, allergic reaction to methotrexate, and methotrexate-induced pneumonitis.5
CASE After lengthy discussion of the treatment options, the patient chose medical management with methotrexate. She verbalized her understanding of the teaching provided and signed an informed consent document.
METHOTREXATE REGIMENS
Protocols for single-dose, two-dose, and fixed multidose methotrexate regimens are described in the medical literature, according to a 2008 American Congress of Obstetricians and Gynecologists practice bulletin.2 A 2013 practice committee opinion of the American Society for Reproductive Medicine (ASRM) indicates that single-dose and multiple-dose regimens are used most often.12
With methotrexate treatment, complete resolution of ectopic pregnancy usually occurs in two to three weeks but may require up to six to eight weeks, depending on how high the ß-hCG level is when treatment begins.12
Single-dose
In the single-dose regimen, an intramuscular (IM) injection of methotrexate 50 mg/m2 is administered on day 1. The ß-hCG levels are measured on days 4 and 7 after administration; a decrease of at least 15% in the ß-hCG level should be observed. The ß-hCG level is then measured weekly until it reaches < 2 mIU/mL or is undetectable.2 If the level does not decline, a repeat dose of methotrexate can be given, with measurement of ß-hCG on days 4 and 7 after the repeat dose. If the ß-hCG level fails to decrease, additional methotrexate or surgical intervention should be considered.
The single-dose regimen is more frequently used and is most successful when ß-hCG levels are low (< 5,000 mIU/mL), the ectopic mass is small
(< 3.5 cm), and embryonic cardiac activity is not observed on ultrasound.2,3 Patients with ß-hCG levels > 5,000 mIU/mL may be appropriate candidates for additional doses of methotrexate.2 In fact, the single-dose protocol provides for repeat doses of methotrexate if the ß-hCG level is not decreasing adequately.12
Multiple-dose
With the multiple-dose regimen, methotrexate 1 mg/kg IM is administered on days 1, 3, 5, and 7; on days 2, 4, 6, and 8, the patient receives leucovorin (folinic acid) 0.1 mg/kg IM. The ß-hCG level is measured on days methotrexate is administered; once the minimum 15% decline is observed, ß-hCG is measured weekly until a nonpregnant level is reached.12
The patient received a single dose of methotrexate 50 mg/m2 IM on day 1 and returned to the clinic for follow-up on days 4 and 7 posttreatment. On day 4, her ß-hCG level was 1,060 mIU/mL; on day 7, it was 470 mIU/mL. Also on day 7, blood was drawn for a CBC and comprehensive metabolic panel; results were within normal limits. The patient continued weekly follow-up until her ß-hCG level decreased to < 2 mIU/mL.
On the next page: Follow-up and conclusion >>
FOLLOW-UP AND REFERRALS
Close monitoring of ß-hCG levels, as described previously, is essential after methotrexate treatment in order to confirm that the pregnancy has been terminated and reduce the risk for tubal rupture. Clinicians should also be sensitive to the sequelae of loss of a pregnancy and refer patients as needed to appropriate health care professionals for grief support.
CASE The patient was referred to an obstetrics clinic and reported for all scheduled follow-up appointments. She was discharged from care after a full reduction in her ß-hCG to nonpregnant levels. While at the clinic, the patient was referred to social services for psychosocial counseling.
CONCLUSION
Ectopic implantation is a serious complication that may occur during the first trimester of pregnancy. Worldwide, it is the leading cause of maternal death in the first trimester. For women who meet specific criteria, outpatient treatment of early ectopic pregnancy with methotrexate avoids surgery and decreases the overall cost of care. Medical management and conservative surgical management offer the patient comparable outcomes for tubal patency preservation and risk for ectopic pregnancy recurrence.11
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72(9):1707-1714.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479-1485.
3. Sepilian VP, Wood E. Ectopic pregnancy. http://emedicine.medscape.com/article/2041923-overview. Medscape. Accessed June 19, 2014.
4. Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med. 2010;56(6):674-683.
5. Murtaza UI, Ortmann MJ, Mando-Vandrick J, Lee ASD. Management of first-trimester complications in the emergency department. Am J Health Syst Pharm. 2013;70(2):99-111.
6. Sewell CA, Cundiff GW. Trends for inpatient treatment of tubal pregnancy in Maryland. Am J Obstet Gynecol. 2002;186(3):404-408.
7. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet. 2009;279(4):443-453.
8. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
9. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.
10. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3): 495-502.
11. Autry A. Medical treatment of ectopic pregnancy: is there something new? Obstet Gynecol. 2013;122(4):733.
12. The Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Ectopic pregnancy is a significant health risk to women during their childbearing years; approximately 6% of all pregnancy-related deaths are due to ectopic pregnancy.1-3 Some 1% to 2% of all pregnancies in the United States each year—approximately 100,000 cases—are ectopic, with an estimated annual cost of care approaching $1.1 billion.4 The incidence of ectopic pregnancy has increased in the past 20 years; in one analysis, ectopic pregnancy was diagnosed in 18% of women who presented to an emergency department (ED) with first trimester vaginal bleeding, abdominal pain, or both.5 This growing prevalence is attributed to a number of factors, including the sensitivity of current diagnostic methods in detecting early ectopic pregnancy, the greater incidence of salpingitis, and the growing use of assisted reproductive technologies.2,6
While the number of ectopic pregnancies is on the rise, the proportion of patients requiring hospitalization for surgical treatment of ectopic pregnancy has decreased significantly. Today, for appropriate patients, many clinicians manage ectopic pregnancy on an outpatient basis using the drug methotrexate.6
In this article, we will present an overview of the current status of medical management of ectopic pregnancy, along with a case study. The case study describes a patient diagnosed with an unruptured ectopic pregnancy who was managed medically with methotrexate. It illustrates how, with early diagnosis, clinicians can intervene to make medical management an effective treatment option in selected situations.
The patient reported a history of oral contraceptive use until approximately three months prior to this pregnancy. She was taking no medications and had no known drug allergies. Her previous pregnancies included two uncomplicated vaginal births at term and one miscarriage at six to seven weeks’ gestation two years ago. She also reported a dilation and curettage after the miscarriage. Her medical, surgical, and gynecologic histories were otherwise noncontributory. A review of systems was otherwise negative.
Sexual history revealed that the patient was married and monogamous with her husband of five years. She disclosed four previous sexual partners and inconsistent use of condoms with those partners; no current condom use was reported. Seven years ago, she tested positive for gonorrhea and chlamydia and was treated concurrently with her partner. Subsequent diagnostics were negative. She reported vaginal intercourse but no oral sex and denied any other sexual contact. All partners had been male.
On the next page: Diagnosis and case continuation >>
DIAGNOSIS
There is some variation in the presentation of women experiencing ectopic pregnancy; this may be due to differences in the pathologic mechanisms of ectopic pregnancy. Patients may be asymptomatic, hemodynamically compromised, or somewhere in between.3 Typical clinical signs include abdominal pain, amenorrhea, and vaginal bleeding. Approximately 40% to 50% of patients present with vaginal bleeding, 50% may have a palpable adnexal mass, and 75% may have abdominal tenderness.3 Only about 50% of women with ectopic pregnancies present with these typical symptoms.3
The patient may also experience common symptoms of early pregnancy, such as nausea, fatigue, and breast fullness. Worrisome signs and symptoms, including abdominal guarding, hypotension, tachycardia, shock, shoulder pain from peritoneal irritation, dizziness, fever, and vomiting, may also be present.3,7 Approximately 20% of patients with ectopic pregnancies are hemodynamically compromised at presentation, which is highly suggestive of rupture.3
Risk factors
Risk factors for ectopic pregnancy include previous ectopic pregnancy; previous tubal procedures; history of sexually transmitted disease or genital infections; infertility; use of assisted reproductive technology; previous abdominal or pelvic surgery; smoking; pelvic inflammatory disease; exposure in utero to diethylstilbestrol; and previous intrauterine device use.2,5,7,8 Knowledge of these risk factors can help identify a patient with an ectopic pregnancy.
The diagnosis of ectopic pregnancy is most certainly a clinical challenge. The differential diagnosis is based upon history and physical findings; the list can be lengthy if both vaginal bleeding and abdominal pain (nonspecific symptoms common in women who miscarry) are present.7 Prompt completion of diagnostic testing is critical in making a definitive diagnosis. Possible diagnoses are listed in Table 1.
CASE Upon examination, the patient appeared comfortable and relaxed, and there were no signs of distress. Blood pressure was 100/65 mm Hg, pulse rate was 72 beats/min, and temperature was 99.0°F. There was no tenderness upon abdominal examination. Pelvic examination revealed a small amount of brown vaginal discharge but no active bleeding or pooled blood, clots, or tissue. The cervical os was closed, and positive Chadwick sign was present. Bimanual examination revealed no cervical motion tenderness. The uterus was soft, mobile, and nontender, and consistent in size with a gestation at eight weeks. There were no palpable adnexa, ovaries, or masses. There was no pain with bimanual examination and no evidence of tenderness at the posterior fornix. The remainder of the physical examination was unremarkable.
It is important to note that examination results in the case patient are not unusual in a woman with a small, unruptured ectopic pregnancy. All findings were normal except for the scant brown vaginal discharge. Abdominal and adnexal tenderness are common, as is a palpable adnexal mass; but absence of a detectable mass does not exclude ectopic pregnancy.1 Pathologic findings may include severe abdominal tenderness and pain, significant vaginal bleeding, passage of clots, tachycardia, and orthostatic hypotension.
Diagnostic workup
Laboratory tests are critical to making an accurate diagnosis for women whose history and physical examination results are consistent with ectopic pregnancy. Assessment for ectopic pregnancy should include a urine pregnancy test, transvaginal ultrasound, measurement of serum ß-human chorionic gonadotropin (ß-hCG) level, and occasionally, diagnostic curettage.1 Once the diagnosis is confirmed, a complete blood count (CBC) is necessary to assess anemia and platelet functioning. Coagulation tests may be required for worrisome bleeding. Blood type, Rh status, and antibody screen are also necessary to determine whether a patient who is Rh D-negative will require Rh immune globulin. See Table 2 for the patient’s laboratory test results.
In a patient with a ß-hCG level greater than the discriminatory cutoff value of 1,500 to 1,800 mIU/mL, the level above which an intrauterine gestational sac is visible on transvaginal ultrasound in a normal pregnancy, an empty uterus is considered an ectopic pregnancy until proven otherwise.3 In a definite intrauterine pregnancy of about six weeks’ gestation, transvaginal ultrasound reveals a gestational sac that contains a yolk sac and a fetal pole.3
CASE The patient’s presenting symptoms, combined with a positive pregnancy test, ß-hCG level of 1,850 mIU/mL, and a complex adnexal mass in the right fallopian tube, were highly suggestive of an unruptured ectopic pregnancy (see Table 3 for the patient’s transvaginal ultrasound findings). There was also a secondary finding of a corpus luteum cyst. Other diagnoses were ruled out, and the patient was diagnosed with an unruptured ectopic pregnancy.
On the next page: Treatment >>
TREATMENT
A patient with an ectopic pregnancy who presents with pain and hemodynamic instability should be referred immediately for appropriate surgical care.7 Otherwise, once the diagnosis of ectopic pregnancy is confirmed, the patient should be referred to an obstetric specialist. Treatments for ectopic pregnancy include expectant management and surgery—which will be discussed briefly—and medical management, which is the focus of this review.5
Expectant management
Most ectopic pregnancies are diagnosed early as a result of accurate, minimally invasive and noninvasive diagnostic tools and greater awareness of risk factors. Since the natural course of early ectopic pregnancy is often self-limited, eventually resulting in tubal abortion or reabsorption, expectant management is a viable option.9
This treatment option may be considered if the patient is asymptomatic; ß-hCG is < 200 mIU/mL; the ectopic mass is < 3 cm; and no fetal heartbeat is present.1,2 With this approach, patients must be willing to accept the risk for tubal rupture and agree to close monitoring of ß-hCG levels. The ß-hCG level must be measured every 24 to 48 hours in order to determine if it is declining adequately, plateauing, or increasing.2,5
Surgery
For the hemodynamically unstable patient, the treatment decision is relatively straightforward. Optimal treatment for a ruptured ectopic pregnancy is immediate surgery, which may include salpingostomy or salpingectomy.10 Surgery may also be considered for hemodynamically stable patients with nonruptured ectopic pregnancies; in addition to her clinical presentation, overall management may be driven by a patient’s preferences.5 Salpingostomy and salpingectomy can be performed either laparoscopically or via laparotomy, depending on the specific situation.
Medical management
The use of methotrexate for the management of unruptured ectopic pregnancy was introduced in the early 1980s.11 Initially, protocols called for multiple doses administered during the course of an inpatient stay. Further research led to revised treatment recommendations and today, medical management most often consists of a single dose of methotrexate with outpatient follow-up.3
Methotrexate is a folic acid antagonist often used as an antimetabolite chemotherapeutic agent. In ectopic pregnancy, it inhibits growth of the rapidly dividing trophoblastic cells and ultimately ends the pregnancy.2 Outcomes of medical management are comparable to those of surgical treatment, including the potential for future normal pregnancies.2,5
An analysis of US trends in ectopic pregnancy management from 2002-2007 revealed that the use of methotrexate increased from 11.1% to 35.1% during that time, while the use of surgical approaches declined from 90% to 65%.10 Medical management of ectopic pregnancy eliminates the costs of surgery, anesthesia, and hospitalization and avoids potential complications of surgery and anesthesia.
Appropriate candidates
A hemodynamically stable patient with a confirmed or high clinical suspicion of ectopic pregnancy, an unruptured mass, no active bleeding, and low ß-hCG levels (< 5,000 mIU/mL) can be considered for methotrexate therapy.2,3,9 It is critical that medically managed patients be willing and able to adhere to all follow-up appointments.9 Before initiating treatment, normal serum creatinine and transaminase levels should be confirmed, and there should be no evidence of significant anemia, leukopenia, or thrombocytopenia.2 To detect any adverse effects of methotrexate on renal, hepatic, and hematologic functioning, these tests are repeated one week after administration.2
Contraindications
Contraindications to methotrexate treatment include breastfeeding, immunodeficiency, alcoholism, alcoholic liver disease or other chronic liver disease, preexisting blood dyscrasias (eg, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia), known sensitivity to methotrexate, active pulmonary disease, peptic ulcer, and hepatic, renal, or hematologic dysfunction. Relative contraindications are a gestational sac larger than 3.5 cm and embryonic cardiac motion.2
On the next page: Patient education >>
PATIENT EDUCATION AND INFORMED CONSENT
A diagnosis of unruptured ectopic pregnancy requires patient education about the condition and its treatment options. The clinician should explain what an ectopic pregnancy is and distinguish between unruptured and ruptured. A discussion of the benefits and risks of each treatment option for which the patient is an appropriate candidate, as well as what to anticipate during treatment, is needed. Emotional support for impending pregnancy loss should also be provided.
For patients who choose medical management, education includes methotrexate-specific information and written instructions to follow after methotrexate administration. Patients must be instructed about the use of safety precautions after treatment (eg, the toilet should be double-flushed with the lid closed during the first 72 hours after treatment to prevent exposing others to methotrexate in urine and stool), the need for adherence to follow-up visits, and warning signs of a possible rupture.5 These warning signs are listed in Table 4.
The most common adverse effects of methotrexate are gastrointestinal (nausea, vomiting, stomatitis). Patients should be advised to avoid alcohol, NSAIDs, folic acid supplements, excessive sun exposure (due to photosensitivity), strenuous exercise, and sexual intercourse until ß-hCG has returned to nonpregnant levels. Other adverse effects may include a temporary elevation in liver enzymes and rarely, alopecia. Abdominal pain may occur a few days after methotrexate administration, likely from the cytotoxic effects of the drug on the trophoblastic tissue.
Informed consent is required prior to methotrexate administration. The patient must be advised of the potential risks of medical management with methotrexate, including rupture of the ectopic pregnancy during treatment, inadvertent administration of methotrexate in the presence of an early intrauterine embryo, allergic reaction to methotrexate, and methotrexate-induced pneumonitis.5
CASE After lengthy discussion of the treatment options, the patient chose medical management with methotrexate. She verbalized her understanding of the teaching provided and signed an informed consent document.
METHOTREXATE REGIMENS
Protocols for single-dose, two-dose, and fixed multidose methotrexate regimens are described in the medical literature, according to a 2008 American Congress of Obstetricians and Gynecologists practice bulletin.2 A 2013 practice committee opinion of the American Society for Reproductive Medicine (ASRM) indicates that single-dose and multiple-dose regimens are used most often.12
With methotrexate treatment, complete resolution of ectopic pregnancy usually occurs in two to three weeks but may require up to six to eight weeks, depending on how high the ß-hCG level is when treatment begins.12
Single-dose
In the single-dose regimen, an intramuscular (IM) injection of methotrexate 50 mg/m2 is administered on day 1. The ß-hCG levels are measured on days 4 and 7 after administration; a decrease of at least 15% in the ß-hCG level should be observed. The ß-hCG level is then measured weekly until it reaches < 2 mIU/mL or is undetectable.2 If the level does not decline, a repeat dose of methotrexate can be given, with measurement of ß-hCG on days 4 and 7 after the repeat dose. If the ß-hCG level fails to decrease, additional methotrexate or surgical intervention should be considered.
The single-dose regimen is more frequently used and is most successful when ß-hCG levels are low (< 5,000 mIU/mL), the ectopic mass is small
(< 3.5 cm), and embryonic cardiac activity is not observed on ultrasound.2,3 Patients with ß-hCG levels > 5,000 mIU/mL may be appropriate candidates for additional doses of methotrexate.2 In fact, the single-dose protocol provides for repeat doses of methotrexate if the ß-hCG level is not decreasing adequately.12
Multiple-dose
With the multiple-dose regimen, methotrexate 1 mg/kg IM is administered on days 1, 3, 5, and 7; on days 2, 4, 6, and 8, the patient receives leucovorin (folinic acid) 0.1 mg/kg IM. The ß-hCG level is measured on days methotrexate is administered; once the minimum 15% decline is observed, ß-hCG is measured weekly until a nonpregnant level is reached.12
The patient received a single dose of methotrexate 50 mg/m2 IM on day 1 and returned to the clinic for follow-up on days 4 and 7 posttreatment. On day 4, her ß-hCG level was 1,060 mIU/mL; on day 7, it was 470 mIU/mL. Also on day 7, blood was drawn for a CBC and comprehensive metabolic panel; results were within normal limits. The patient continued weekly follow-up until her ß-hCG level decreased to < 2 mIU/mL.
On the next page: Follow-up and conclusion >>
FOLLOW-UP AND REFERRALS
Close monitoring of ß-hCG levels, as described previously, is essential after methotrexate treatment in order to confirm that the pregnancy has been terminated and reduce the risk for tubal rupture. Clinicians should also be sensitive to the sequelae of loss of a pregnancy and refer patients as needed to appropriate health care professionals for grief support.
CASE The patient was referred to an obstetrics clinic and reported for all scheduled follow-up appointments. She was discharged from care after a full reduction in her ß-hCG to nonpregnant levels. While at the clinic, the patient was referred to social services for psychosocial counseling.
CONCLUSION
Ectopic implantation is a serious complication that may occur during the first trimester of pregnancy. Worldwide, it is the leading cause of maternal death in the first trimester. For women who meet specific criteria, outpatient treatment of early ectopic pregnancy with methotrexate avoids surgery and decreases the overall cost of care. Medical management and conservative surgical management offer the patient comparable outcomes for tubal patency preservation and risk for ectopic pregnancy recurrence.11
REFERENCES
1. Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician. 2005;72(9):1707-1714.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479-1485.
3. Sepilian VP, Wood E. Ectopic pregnancy. http://emedicine.medscape.com/article/2041923-overview. Medscape. Accessed June 19, 2014.
4. Stein JC, Wang R, Adler N, et al. Emergency physician ultrasonography for evaluating patients at risk for ectopic pregnancy: a meta-analysis. Ann Emerg Med. 2010;56(6):674-683.
5. Murtaza UI, Ortmann MJ, Mando-Vandrick J, Lee ASD. Management of first-trimester complications in the emergency department. Am J Health Syst Pharm. 2013;70(2):99-111.
6. Sewell CA, Cundiff GW. Trends for inpatient treatment of tubal pregnancy in Maryland. Am J Obstet Gynecol. 2002;186(3):404-408.
7. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet. 2009;279(4):443-453.
8. Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
9. Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007;(1):CD000324.
10. Hoover KW, Tao G, Kent CK. Trends in the diagnosis and treatment of ectopic pregnancy in the United States. Obstet Gynecol. 2010;115(3): 495-502.
11. Autry A. Medical treatment of ectopic pregnancy: is there something new? Obstet Gynecol. 2013;122(4):733.
12. The Practice Committee of the American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Adding rapid trichomonas test to the sexually transmitted infection panel boosts rapid treatment
ATLANTA – Adding a rapid Trichomonas vaginalis test to the adolescent sexually transmitted infection laboratory regimen facilitated on-site diagnosis and treatment at an urban medical center emergency department.
The OSOM rapid trichomonas test provided point of care testing results in 10 minutes, with a sensitivity of 83% and specificity of 99%, Dr. Heather Territo reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention. The prevalence of Trichomonas vaginalis approaches 25% in inner-city adolescents. Prior studies have demonstrated an association between Trichomonas vaginalis and cervical neoplasia, enhanced HIV transmission, and pregnancy complications.
After routine Trichomonas vaginalis testing was implemented at the Women’s and Children’s Hospital of Buffalo, N.Y., in November 2011, 212 females aged 13-20 years were tested in the following year; 13.6% tested positive by rapid test and 15.5% by nucleic acid amplification test (NAAT). In the year before routine testing was implemented, 31 of 234 patients were tested, based on a retrospective chart review. Treatment was administered to 24% and to 7% of patients during the respective study periods, said Dr. Territo of the hospital’s division of emergency medicine.
Additionally, two positive results were found in 20 males tested.
In the emergency department, Trichomonas vaginalis is often diagnosed on the basis of clinical findings; the positive predictive value of this approach is less than 50%. Direct microscopy of vaginal secretions has a sensitivity of about 60%-70%.
The rapid test and the NAAT test results were concordant in 178 of 188 cases (94.6%). Ten of the 33 positive NAAT tests (30%) were negative on the rapid test.
Dr. Territo reported having no relevant financial disclosures.
ATLANTA – Adding a rapid Trichomonas vaginalis test to the adolescent sexually transmitted infection laboratory regimen facilitated on-site diagnosis and treatment at an urban medical center emergency department.
The OSOM rapid trichomonas test provided point of care testing results in 10 minutes, with a sensitivity of 83% and specificity of 99%, Dr. Heather Territo reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention. The prevalence of Trichomonas vaginalis approaches 25% in inner-city adolescents. Prior studies have demonstrated an association between Trichomonas vaginalis and cervical neoplasia, enhanced HIV transmission, and pregnancy complications.
After routine Trichomonas vaginalis testing was implemented at the Women’s and Children’s Hospital of Buffalo, N.Y., in November 2011, 212 females aged 13-20 years were tested in the following year; 13.6% tested positive by rapid test and 15.5% by nucleic acid amplification test (NAAT). In the year before routine testing was implemented, 31 of 234 patients were tested, based on a retrospective chart review. Treatment was administered to 24% and to 7% of patients during the respective study periods, said Dr. Territo of the hospital’s division of emergency medicine.
Additionally, two positive results were found in 20 males tested.
In the emergency department, Trichomonas vaginalis is often diagnosed on the basis of clinical findings; the positive predictive value of this approach is less than 50%. Direct microscopy of vaginal secretions has a sensitivity of about 60%-70%.
The rapid test and the NAAT test results were concordant in 178 of 188 cases (94.6%). Ten of the 33 positive NAAT tests (30%) were negative on the rapid test.
Dr. Territo reported having no relevant financial disclosures.
ATLANTA – Adding a rapid Trichomonas vaginalis test to the adolescent sexually transmitted infection laboratory regimen facilitated on-site diagnosis and treatment at an urban medical center emergency department.
The OSOM rapid trichomonas test provided point of care testing results in 10 minutes, with a sensitivity of 83% and specificity of 99%, Dr. Heather Territo reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention. The prevalence of Trichomonas vaginalis approaches 25% in inner-city adolescents. Prior studies have demonstrated an association between Trichomonas vaginalis and cervical neoplasia, enhanced HIV transmission, and pregnancy complications.
After routine Trichomonas vaginalis testing was implemented at the Women’s and Children’s Hospital of Buffalo, N.Y., in November 2011, 212 females aged 13-20 years were tested in the following year; 13.6% tested positive by rapid test and 15.5% by nucleic acid amplification test (NAAT). In the year before routine testing was implemented, 31 of 234 patients were tested, based on a retrospective chart review. Treatment was administered to 24% and to 7% of patients during the respective study periods, said Dr. Territo of the hospital’s division of emergency medicine.
Additionally, two positive results were found in 20 males tested.
In the emergency department, Trichomonas vaginalis is often diagnosed on the basis of clinical findings; the positive predictive value of this approach is less than 50%. Direct microscopy of vaginal secretions has a sensitivity of about 60%-70%.
The rapid test and the NAAT test results were concordant in 178 of 188 cases (94.6%). Ten of the 33 positive NAAT tests (30%) were negative on the rapid test.
Dr. Territo reported having no relevant financial disclosures.
AT THE 2014 STD PREVENTION CONFERENCE
Key clinical point: A positive rapid trichomonas vaginalis test allows immediate treatment in the ED.
Major finding: Treatment was administered to 24% vs. 7% of patients before and after implementing routine testing.
Data source: A retrospective review of 234 patients, and a prospective evaluation of 212 patients.
Disclosures: Dr. Territo reported having no disclosures.
U.S. gestational diabetes prevalence may be as high as 9.4%
The rate of gestational diabetes in the United States is somewhere between 4.6% and 9.4%, according to investigators from the Centers for Disease Control and Prevention.
Researchers compared data from birth certificates collected from 16 states to data from the Pregnancy Risk Assessment Monitoring System (PRAMS) in 21 states. PRAMS is a CDC-led surveillance project that works with state health departments to collect population-based data on maternal experiences before, during, and after pregnancy.
In 2010, the prevalence of gestational diabetes mellitus was 4.6% as listed on birth certificates, 8.7% on the PRAMS scale, and 9.2% as reported on either the PRAMS scale or birth certificates. The percent agreement between the sources was 94.1%, wrote Carla L. DeSisto, M.P.H., and her associates at the CDC (Prev. Chronic Dis. 2014;11:130415 [doi:10.5888/pcd11.130415]).
There was no significant difference in gestational diabetes prevalence between the two study periods examined – 8.1% in 2007-08 and 8.5% in 2009-10, according to an analysis of PRAMS data from 123,373 women. In both time periods, Utah had the lowest prevalence (5.7% and 5.6%, respectively) and Rhode Island had the highest (10.4% and 11.7%).
The rate of gestational diabetes in the United States is somewhere between 4.6% and 9.4%, according to investigators from the Centers for Disease Control and Prevention.
Researchers compared data from birth certificates collected from 16 states to data from the Pregnancy Risk Assessment Monitoring System (PRAMS) in 21 states. PRAMS is a CDC-led surveillance project that works with state health departments to collect population-based data on maternal experiences before, during, and after pregnancy.
In 2010, the prevalence of gestational diabetes mellitus was 4.6% as listed on birth certificates, 8.7% on the PRAMS scale, and 9.2% as reported on either the PRAMS scale or birth certificates. The percent agreement between the sources was 94.1%, wrote Carla L. DeSisto, M.P.H., and her associates at the CDC (Prev. Chronic Dis. 2014;11:130415 [doi:10.5888/pcd11.130415]).
There was no significant difference in gestational diabetes prevalence between the two study periods examined – 8.1% in 2007-08 and 8.5% in 2009-10, according to an analysis of PRAMS data from 123,373 women. In both time periods, Utah had the lowest prevalence (5.7% and 5.6%, respectively) and Rhode Island had the highest (10.4% and 11.7%).
The rate of gestational diabetes in the United States is somewhere between 4.6% and 9.4%, according to investigators from the Centers for Disease Control and Prevention.
Researchers compared data from birth certificates collected from 16 states to data from the Pregnancy Risk Assessment Monitoring System (PRAMS) in 21 states. PRAMS is a CDC-led surveillance project that works with state health departments to collect population-based data on maternal experiences before, during, and after pregnancy.
In 2010, the prevalence of gestational diabetes mellitus was 4.6% as listed on birth certificates, 8.7% on the PRAMS scale, and 9.2% as reported on either the PRAMS scale or birth certificates. The percent agreement between the sources was 94.1%, wrote Carla L. DeSisto, M.P.H., and her associates at the CDC (Prev. Chronic Dis. 2014;11:130415 [doi:10.5888/pcd11.130415]).
There was no significant difference in gestational diabetes prevalence between the two study periods examined – 8.1% in 2007-08 and 8.5% in 2009-10, according to an analysis of PRAMS data from 123,373 women. In both time periods, Utah had the lowest prevalence (5.7% and 5.6%, respectively) and Rhode Island had the highest (10.4% and 11.7%).
FROM PREVENTING CHRONIC DISEASE
Major finding: The prevalence of gestational diabetes mellitus in the United States is between 4.6% and 9.4%.
Data source: Statistical analysis of birth certificate data from 15 states and PRAMS data from 21 states.
Disclosures: No disclosures were reported.
Maternal antidepressants don’t increase infant cardiac malformations
Infants born to women who took antidepressants during the first trimester of their pregnancies showed no increase in cardiac malformations overall or in specific cardiac defects that earlier research had linked to fetal exposure to the drugs, investigators have reported.
Previously, paroxetine and sertraline had been associated with congenital cardiac malformations, chiefly septal defects, but "considerable controversy" remains on the subject, the researchers noted in a report published online June 19 in the New England Journal of Medicine.
In the current large, population-based cohort study, the investigators drew from Medicaid records for 46 states and the District of Columbia and identified 949,504 completed pregnancies during a 7-year period. A total of 64,389 of these women (6.8%) used an antidepressant during their first trimester, including SSRIs, tricyclics, serotonin–norepinephrine reuptake inhibitors (SNRIs), and bupropion.
The SSRIs taken by women most frequently were sertraline, paroxetine, and fluoxetine, wrote Krista F. Huybrechts, Ph.D., of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital, Boston, and her associates.
In the study’s initial analysis, cardiac malformations were diagnosed in 6,403 infants not exposed to antidepressants, for a rate of 72.3 malformations per 10,000 infants, and in 580 infants exposed to antidepressants, for a rate of 90.1 malformations per 10,000 infants. Once these data were adjusted to account for the effect of the underlying depression (indication bias), however, there was no association between the use of antidepressants in general and infant cardiac malformations, nor between specific antidepressants and specific cardiac malformations.
In particular, "we found no significant associations between paroxetine and right ventricular outflow tract obstruction or between sertraline and ventricular septal defect," Dr. Huybrechts and her associates said (N. Engl. J. Med. 2014 June 19 [doi:10.1056/NEJMoa1312828]).
In addition, numerous subgroup and sensitivity analyses found no such associations regardless of the women’s age, race, or use of monotherapy vs. polytherapy, and no dose-response relationships. In contrast, the data confirmed well-known associations between infant cardiac malformations and maternal diabetes, maternal use of anticonvulsants, and multiple gestations, which supports the premise that "the outcomes of interest were well captured in our study," the investigators said.
This study was supported by the Agency for Healthcare Research and Quality, the National Institute of Mental Health, and the National Institute of Child Health and Human Development. Dr. Huybrechts reported no financial conflicts of interest; her associates reported numerous ties to industry sources.
Infants born to women who took antidepressants during the first trimester of their pregnancies showed no increase in cardiac malformations overall or in specific cardiac defects that earlier research had linked to fetal exposure to the drugs, investigators have reported.
Previously, paroxetine and sertraline had been associated with congenital cardiac malformations, chiefly septal defects, but "considerable controversy" remains on the subject, the researchers noted in a report published online June 19 in the New England Journal of Medicine.
In the current large, population-based cohort study, the investigators drew from Medicaid records for 46 states and the District of Columbia and identified 949,504 completed pregnancies during a 7-year period. A total of 64,389 of these women (6.8%) used an antidepressant during their first trimester, including SSRIs, tricyclics, serotonin–norepinephrine reuptake inhibitors (SNRIs), and bupropion.
The SSRIs taken by women most frequently were sertraline, paroxetine, and fluoxetine, wrote Krista F. Huybrechts, Ph.D., of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital, Boston, and her associates.
In the study’s initial analysis, cardiac malformations were diagnosed in 6,403 infants not exposed to antidepressants, for a rate of 72.3 malformations per 10,000 infants, and in 580 infants exposed to antidepressants, for a rate of 90.1 malformations per 10,000 infants. Once these data were adjusted to account for the effect of the underlying depression (indication bias), however, there was no association between the use of antidepressants in general and infant cardiac malformations, nor between specific antidepressants and specific cardiac malformations.
In particular, "we found no significant associations between paroxetine and right ventricular outflow tract obstruction or between sertraline and ventricular septal defect," Dr. Huybrechts and her associates said (N. Engl. J. Med. 2014 June 19 [doi:10.1056/NEJMoa1312828]).
In addition, numerous subgroup and sensitivity analyses found no such associations regardless of the women’s age, race, or use of monotherapy vs. polytherapy, and no dose-response relationships. In contrast, the data confirmed well-known associations between infant cardiac malformations and maternal diabetes, maternal use of anticonvulsants, and multiple gestations, which supports the premise that "the outcomes of interest were well captured in our study," the investigators said.
This study was supported by the Agency for Healthcare Research and Quality, the National Institute of Mental Health, and the National Institute of Child Health and Human Development. Dr. Huybrechts reported no financial conflicts of interest; her associates reported numerous ties to industry sources.
Infants born to women who took antidepressants during the first trimester of their pregnancies showed no increase in cardiac malformations overall or in specific cardiac defects that earlier research had linked to fetal exposure to the drugs, investigators have reported.
Previously, paroxetine and sertraline had been associated with congenital cardiac malformations, chiefly septal defects, but "considerable controversy" remains on the subject, the researchers noted in a report published online June 19 in the New England Journal of Medicine.
In the current large, population-based cohort study, the investigators drew from Medicaid records for 46 states and the District of Columbia and identified 949,504 completed pregnancies during a 7-year period. A total of 64,389 of these women (6.8%) used an antidepressant during their first trimester, including SSRIs, tricyclics, serotonin–norepinephrine reuptake inhibitors (SNRIs), and bupropion.
The SSRIs taken by women most frequently were sertraline, paroxetine, and fluoxetine, wrote Krista F. Huybrechts, Ph.D., of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital, Boston, and her associates.
In the study’s initial analysis, cardiac malformations were diagnosed in 6,403 infants not exposed to antidepressants, for a rate of 72.3 malformations per 10,000 infants, and in 580 infants exposed to antidepressants, for a rate of 90.1 malformations per 10,000 infants. Once these data were adjusted to account for the effect of the underlying depression (indication bias), however, there was no association between the use of antidepressants in general and infant cardiac malformations, nor between specific antidepressants and specific cardiac malformations.
In particular, "we found no significant associations between paroxetine and right ventricular outflow tract obstruction or between sertraline and ventricular septal defect," Dr. Huybrechts and her associates said (N. Engl. J. Med. 2014 June 19 [doi:10.1056/NEJMoa1312828]).
In addition, numerous subgroup and sensitivity analyses found no such associations regardless of the women’s age, race, or use of monotherapy vs. polytherapy, and no dose-response relationships. In contrast, the data confirmed well-known associations between infant cardiac malformations and maternal diabetes, maternal use of anticonvulsants, and multiple gestations, which supports the premise that "the outcomes of interest were well captured in our study," the investigators said.
This study was supported by the Agency for Healthcare Research and Quality, the National Institute of Mental Health, and the National Institute of Child Health and Human Development. Dr. Huybrechts reported no financial conflicts of interest; her associates reported numerous ties to industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: First trimester use of antidepressants does not significantly increase fetal cardiac malformation risk.
Major finding: After adjustment, there was no association between the use of antidepressants in general and infant cardiac malformations, nor between specific antidepressants and specific cardiac malformations.
Data source: A nationwide cohort study involving 949,504 pregnancies in which 6.8% of the women took antidepressants during the first trimester.
Disclosures: This study was supported by the Agency for Healthcare Research and Quality, the National Institute of Mental Health, and the National Institute of Child Health and Human Development. Dr. Huybrechts reported no financial conflicts of interest; her associates reported numerous ties to industry sources.
Maternal infections associated with increased risk of cerebral palsy
VANCOUVER, B.C. – Intra- or extra-amniotic fluid infections during pregnancy are associated with an increased risk of having a child with cerebral palsy, according to analysis of six million California birth records.
Researchers found that pregnant women who were hospitalized with diagnosis of chorioamnionitis had a fourfold increase in risk of having a child with cerebral palsy (CP), while genitourinary and respiratory infections increased that risk by twofold, each.
"I think this is a very important study, and it took us a step further in trying to understand what could cause cerebral palsy," said Dr. Lee M. Sanders, associate professor of pediatrics and codirector of the Center for Policy, Outcomes, and Prevention at Stanford (Calif.) University. But because the study shows an association only, "it’s important not to cause alarm," said Dr. Sanders, who was not involved in the study.
Dr. Joshua Bear of the University of California, San Francisco, and his colleagues analyzed the California birth records from 1991 to 2001, in addition to records of all children receiving services for CP from the California Department of Developmental Services through 2006.
There were close to 8,500 CP cases, or 1.4 per 1,000 live births, which is at the lower end of some of the reported statistics, Dr. Bear said.
Analysis of infection diagnoses showed that 6% of the women had unaffected births, while 15.3% had children with CP, with a relative risk of 2.7. Among the latter group, 7.6% had chorioamnionitis, 5.2% had other genitourinary infections, and 3.5% had respiratory infections.
Among women hospitalized with chorioamnionitis, there was a fourfold increase in risk of having CP in preterm infants and twofold increase in term births. Most women hospitalized with chorioamnionitis gave birth at that hospitalization, said Dr. Bear.
For other genitourinary infections, there was a 1.4-fold increase in the risk of CP for prenatal hospitalization, and 1.9-fold increase for birth hospitalization.
Respiratory infections showed a similar trend, with a twofold increased risk for prenatal hospitalization and 2.6-fold increase risk for birth hospitalization.
Increased risk of CP with other genitourinary and respiratory infections, even during prenatal hospitalization, suggest that "these infections may be directly or indirectly contributing to harm on the developing fetal brain and not be simply another complication during delivery," Dr. Bear said during his presentation at the annual meeting of Pediatric Academic Societies.
Analysis of sociodemographic risk factors showed that women younger than 18 and older than 35 were more likely to have a child with CP. Black women were at a higher risk of CP, compared with white and Hispanic women, and Asian women were at lower risk. Years of education was inversely associated with the risk of CP. Also, male infants were at a slightly higher risk of CP than were their female counterparts.
Yet when researchers adjusted for maternal age, race, education, socioeconomic status, and infant sex, the association between infections and increased risk of CP remained the same.
Dr. Bear and his colleagues also stratified the data by presence of birth asphyxia, to find clues if it could be a cause for CP. The results showed a large drop in rate ratios of chorioamnionitis, from 4 to 1.5 when asphyxia was diagnosed at birth, but the rates for other genitourinary and respiratory infections remained the same. "This suggests that there’s some interaction between chorioamnionitis and presence of birth asphyxia, although the nature of that interaction is unclear." said Dr. Bear.
The study had several limitations, including the use of ICD-9 codes, researchers’ inability to account for comorbid conditions related to hospitalization, and being limited to hospital-based diagnoses.
The findings, said Dr. Bear, highlighted the need for further research in several areas, including the association between CP and respiratory infections, and whether risk of CP can be decreased by preventing or treating the infections.
The findings can also "help us with public health interventions, such as better rates of influenza vaccinations among pregnant women," said Dr. Sanders.
Dr. Bear and Dr. Sanders had no relevant disclosures.
On Twitter @naseemmiller
VANCOUVER, B.C. – Intra- or extra-amniotic fluid infections during pregnancy are associated with an increased risk of having a child with cerebral palsy, according to analysis of six million California birth records.
Researchers found that pregnant women who were hospitalized with diagnosis of chorioamnionitis had a fourfold increase in risk of having a child with cerebral palsy (CP), while genitourinary and respiratory infections increased that risk by twofold, each.
"I think this is a very important study, and it took us a step further in trying to understand what could cause cerebral palsy," said Dr. Lee M. Sanders, associate professor of pediatrics and codirector of the Center for Policy, Outcomes, and Prevention at Stanford (Calif.) University. But because the study shows an association only, "it’s important not to cause alarm," said Dr. Sanders, who was not involved in the study.
Dr. Joshua Bear of the University of California, San Francisco, and his colleagues analyzed the California birth records from 1991 to 2001, in addition to records of all children receiving services for CP from the California Department of Developmental Services through 2006.
There were close to 8,500 CP cases, or 1.4 per 1,000 live births, which is at the lower end of some of the reported statistics, Dr. Bear said.
Analysis of infection diagnoses showed that 6% of the women had unaffected births, while 15.3% had children with CP, with a relative risk of 2.7. Among the latter group, 7.6% had chorioamnionitis, 5.2% had other genitourinary infections, and 3.5% had respiratory infections.
Among women hospitalized with chorioamnionitis, there was a fourfold increase in risk of having CP in preterm infants and twofold increase in term births. Most women hospitalized with chorioamnionitis gave birth at that hospitalization, said Dr. Bear.
For other genitourinary infections, there was a 1.4-fold increase in the risk of CP for prenatal hospitalization, and 1.9-fold increase for birth hospitalization.
Respiratory infections showed a similar trend, with a twofold increased risk for prenatal hospitalization and 2.6-fold increase risk for birth hospitalization.
Increased risk of CP with other genitourinary and respiratory infections, even during prenatal hospitalization, suggest that "these infections may be directly or indirectly contributing to harm on the developing fetal brain and not be simply another complication during delivery," Dr. Bear said during his presentation at the annual meeting of Pediatric Academic Societies.
Analysis of sociodemographic risk factors showed that women younger than 18 and older than 35 were more likely to have a child with CP. Black women were at a higher risk of CP, compared with white and Hispanic women, and Asian women were at lower risk. Years of education was inversely associated with the risk of CP. Also, male infants were at a slightly higher risk of CP than were their female counterparts.
Yet when researchers adjusted for maternal age, race, education, socioeconomic status, and infant sex, the association between infections and increased risk of CP remained the same.
Dr. Bear and his colleagues also stratified the data by presence of birth asphyxia, to find clues if it could be a cause for CP. The results showed a large drop in rate ratios of chorioamnionitis, from 4 to 1.5 when asphyxia was diagnosed at birth, but the rates for other genitourinary and respiratory infections remained the same. "This suggests that there’s some interaction between chorioamnionitis and presence of birth asphyxia, although the nature of that interaction is unclear." said Dr. Bear.
The study had several limitations, including the use of ICD-9 codes, researchers’ inability to account for comorbid conditions related to hospitalization, and being limited to hospital-based diagnoses.
The findings, said Dr. Bear, highlighted the need for further research in several areas, including the association between CP and respiratory infections, and whether risk of CP can be decreased by preventing or treating the infections.
The findings can also "help us with public health interventions, such as better rates of influenza vaccinations among pregnant women," said Dr. Sanders.
Dr. Bear and Dr. Sanders had no relevant disclosures.
On Twitter @naseemmiller
VANCOUVER, B.C. – Intra- or extra-amniotic fluid infections during pregnancy are associated with an increased risk of having a child with cerebral palsy, according to analysis of six million California birth records.
Researchers found that pregnant women who were hospitalized with diagnosis of chorioamnionitis had a fourfold increase in risk of having a child with cerebral palsy (CP), while genitourinary and respiratory infections increased that risk by twofold, each.
"I think this is a very important study, and it took us a step further in trying to understand what could cause cerebral palsy," said Dr. Lee M. Sanders, associate professor of pediatrics and codirector of the Center for Policy, Outcomes, and Prevention at Stanford (Calif.) University. But because the study shows an association only, "it’s important not to cause alarm," said Dr. Sanders, who was not involved in the study.
Dr. Joshua Bear of the University of California, San Francisco, and his colleagues analyzed the California birth records from 1991 to 2001, in addition to records of all children receiving services for CP from the California Department of Developmental Services through 2006.
There were close to 8,500 CP cases, or 1.4 per 1,000 live births, which is at the lower end of some of the reported statistics, Dr. Bear said.
Analysis of infection diagnoses showed that 6% of the women had unaffected births, while 15.3% had children with CP, with a relative risk of 2.7. Among the latter group, 7.6% had chorioamnionitis, 5.2% had other genitourinary infections, and 3.5% had respiratory infections.
Among women hospitalized with chorioamnionitis, there was a fourfold increase in risk of having CP in preterm infants and twofold increase in term births. Most women hospitalized with chorioamnionitis gave birth at that hospitalization, said Dr. Bear.
For other genitourinary infections, there was a 1.4-fold increase in the risk of CP for prenatal hospitalization, and 1.9-fold increase for birth hospitalization.
Respiratory infections showed a similar trend, with a twofold increased risk for prenatal hospitalization and 2.6-fold increase risk for birth hospitalization.
Increased risk of CP with other genitourinary and respiratory infections, even during prenatal hospitalization, suggest that "these infections may be directly or indirectly contributing to harm on the developing fetal brain and not be simply another complication during delivery," Dr. Bear said during his presentation at the annual meeting of Pediatric Academic Societies.
Analysis of sociodemographic risk factors showed that women younger than 18 and older than 35 were more likely to have a child with CP. Black women were at a higher risk of CP, compared with white and Hispanic women, and Asian women were at lower risk. Years of education was inversely associated with the risk of CP. Also, male infants were at a slightly higher risk of CP than were their female counterparts.
Yet when researchers adjusted for maternal age, race, education, socioeconomic status, and infant sex, the association between infections and increased risk of CP remained the same.
Dr. Bear and his colleagues also stratified the data by presence of birth asphyxia, to find clues if it could be a cause for CP. The results showed a large drop in rate ratios of chorioamnionitis, from 4 to 1.5 when asphyxia was diagnosed at birth, but the rates for other genitourinary and respiratory infections remained the same. "This suggests that there’s some interaction between chorioamnionitis and presence of birth asphyxia, although the nature of that interaction is unclear." said Dr. Bear.
The study had several limitations, including the use of ICD-9 codes, researchers’ inability to account for comorbid conditions related to hospitalization, and being limited to hospital-based diagnoses.
The findings, said Dr. Bear, highlighted the need for further research in several areas, including the association between CP and respiratory infections, and whether risk of CP can be decreased by preventing or treating the infections.
The findings can also "help us with public health interventions, such as better rates of influenza vaccinations among pregnant women," said Dr. Sanders.
Dr. Bear and Dr. Sanders had no relevant disclosures.
On Twitter @naseemmiller
AT THE PAS ANNUAL MEETING
Key clinical finding: The association between maternal infections and increased risk of CP could be a call for public health interventions, such as higher rate of flu vaccination for pregnant women.
Major finding: Pregnant women who were hospitalized with diagnosis of chorioamnionitis had a fourfold increase in risk of having a child with cerebral palsy (CP), while genitourinary and respiratory infections increased that risk by twofold, each.
Data source: Analysis of California birth records from 1991 to 2001, and records of all children receiving services for CP from the California Department of Developmental Services through 2006.
Disclosures: Dr. Bear and Dr. Sanders had no relevant disclosures.
Higher risk of adverse outcomes among pregnant women with PCOS
The low-grade chronic inflammation that accompanies polycystic ovary syndrome increases during pregnancy, thereby increasing the risk of adverse pregnancy outcomes, according to a recent study of 300 women.
The results "confirmed higher markers of chronic low-grade inflammation in PCOS patients," and suggested "that pregnancy enhances the chronic low-grade inflammation typical of the syndrome," Dr. Stefano Palomba of the Istituto di Ricovero e Cura a Carattere Scientifico in Reggio Emilia, Italy, and his associates reported online May 29 in the Journal of Clinical Endocrinology & Metabolism (doi: 10.1210/jc.2014-1214).
The researchers recruited 150 women with PCOS and 150 healthy controls, matched by age and body mass index, who were pregnant for the first time and at less than 7 weeks’ gestation when they enrolled in the study between February 2003 and April 2012. Ovulation was stimulated by using gonadotropins or clomiphene citrate in 83 of the 150 women with PCOS to induce pregnancy; 118 of the PCOS patients had clinical or biochemical hyperandrogenism, or both.
At the start of the study, and then at 12, 20, and 32 weeks’ gestation, the women underwent a standard clinical assessment, received ultrasounds, and provided serum for white blood cell (WBC) counts and measurements of C-reactive protein (CRP) and ferritin. Each woman’s pregnancy and perinatal outcomes were classified as normal or pathological. Among the adverse (pathological) outcomes were miscarriages, gestational diabetes, preterm birth, pregnancy-induced hypertension, and being large or small for gestational age.
Nearly a third (32%) of the women with PCOS had adverse outcomes, compared with 11.3% of women without PCOS. The researchers identified in the PCOS patients an increased risk for adverse obstetric/neonatal outcomes for WBC (for 1 standard-deviation increase in WBC distribution, the hazard ratio was 1.52), as well as CRP and ferritin (for 1 SD increase in the distributions of CRP and ferritin, HR was 1.19 and 1.12, respectively). Yet in the women without PCOS, only an increased CRP (HR, 1.21) was associated with adverse outcomes.
No funding source was noted. The authors reported no disclosures.
The low-grade chronic inflammation that accompanies polycystic ovary syndrome increases during pregnancy, thereby increasing the risk of adverse pregnancy outcomes, according to a recent study of 300 women.
The results "confirmed higher markers of chronic low-grade inflammation in PCOS patients," and suggested "that pregnancy enhances the chronic low-grade inflammation typical of the syndrome," Dr. Stefano Palomba of the Istituto di Ricovero e Cura a Carattere Scientifico in Reggio Emilia, Italy, and his associates reported online May 29 in the Journal of Clinical Endocrinology & Metabolism (doi: 10.1210/jc.2014-1214).
The researchers recruited 150 women with PCOS and 150 healthy controls, matched by age and body mass index, who were pregnant for the first time and at less than 7 weeks’ gestation when they enrolled in the study between February 2003 and April 2012. Ovulation was stimulated by using gonadotropins or clomiphene citrate in 83 of the 150 women with PCOS to induce pregnancy; 118 of the PCOS patients had clinical or biochemical hyperandrogenism, or both.
At the start of the study, and then at 12, 20, and 32 weeks’ gestation, the women underwent a standard clinical assessment, received ultrasounds, and provided serum for white blood cell (WBC) counts and measurements of C-reactive protein (CRP) and ferritin. Each woman’s pregnancy and perinatal outcomes were classified as normal or pathological. Among the adverse (pathological) outcomes were miscarriages, gestational diabetes, preterm birth, pregnancy-induced hypertension, and being large or small for gestational age.
Nearly a third (32%) of the women with PCOS had adverse outcomes, compared with 11.3% of women without PCOS. The researchers identified in the PCOS patients an increased risk for adverse obstetric/neonatal outcomes for WBC (for 1 standard-deviation increase in WBC distribution, the hazard ratio was 1.52), as well as CRP and ferritin (for 1 SD increase in the distributions of CRP and ferritin, HR was 1.19 and 1.12, respectively). Yet in the women without PCOS, only an increased CRP (HR, 1.21) was associated with adverse outcomes.
No funding source was noted. The authors reported no disclosures.
The low-grade chronic inflammation that accompanies polycystic ovary syndrome increases during pregnancy, thereby increasing the risk of adverse pregnancy outcomes, according to a recent study of 300 women.
The results "confirmed higher markers of chronic low-grade inflammation in PCOS patients," and suggested "that pregnancy enhances the chronic low-grade inflammation typical of the syndrome," Dr. Stefano Palomba of the Istituto di Ricovero e Cura a Carattere Scientifico in Reggio Emilia, Italy, and his associates reported online May 29 in the Journal of Clinical Endocrinology & Metabolism (doi: 10.1210/jc.2014-1214).
The researchers recruited 150 women with PCOS and 150 healthy controls, matched by age and body mass index, who were pregnant for the first time and at less than 7 weeks’ gestation when they enrolled in the study between February 2003 and April 2012. Ovulation was stimulated by using gonadotropins or clomiphene citrate in 83 of the 150 women with PCOS to induce pregnancy; 118 of the PCOS patients had clinical or biochemical hyperandrogenism, or both.
At the start of the study, and then at 12, 20, and 32 weeks’ gestation, the women underwent a standard clinical assessment, received ultrasounds, and provided serum for white blood cell (WBC) counts and measurements of C-reactive protein (CRP) and ferritin. Each woman’s pregnancy and perinatal outcomes were classified as normal or pathological. Among the adverse (pathological) outcomes were miscarriages, gestational diabetes, preterm birth, pregnancy-induced hypertension, and being large or small for gestational age.
Nearly a third (32%) of the women with PCOS had adverse outcomes, compared with 11.3% of women without PCOS. The researchers identified in the PCOS patients an increased risk for adverse obstetric/neonatal outcomes for WBC (for 1 standard-deviation increase in WBC distribution, the hazard ratio was 1.52), as well as CRP and ferritin (for 1 SD increase in the distributions of CRP and ferritin, HR was 1.19 and 1.12, respectively). Yet in the women without PCOS, only an increased CRP (HR, 1.21) was associated with adverse outcomes.
No funding source was noted. The authors reported no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Feds urge pregnant women, young children to eat more fish
The Food and Drug Administration and the Environmental Protection Agency are encouraging pregnant women, as well as women who may become pregnant or are breastfeeding, and young children to eat more fish, setting for the first time a recommended lower limit of fish consumption to promote health.
In draft guidance released June 10, the agencies are calling for women to consume 8-12 ounces of a variety of fish that are lower in mercury; that amount works out to an average of two to three servings per week. Among the types of fish highlighted that are lower in mercury are salmon, shrimp, pollock, tuna (light canned), tilapia, catfish, and cod.
The U.S. Department of Agriculture suggests an amount of 3-5 ounces per week for children under the age of 6 and 4-6 ounces per week for children aged 6-8, the agencies said in an accompanying question-and-answer set. Appropriate amounts of fish for older children would increase up to the adult recommendation. Children should not be given fish until they are at least 6 months of age.
The agencies recommend four types of fish to avoid because of mercury content: tilefish from the Gulf of Mexico, shark, swordfish, and king mackerel. They also recommend limiting white (albacore) tuna to 6 ounces per week for adults and even less for children.
In addition, the agency is advising that those eating fish caught in local waters should listen for local advisories regarding those fish and, if no advice is available, fish consumption from those sources should be limited to 6 ounces per week in pregnant women and 1-3 ounces for young children. The recommendation reiterates that pregnant women and young children should avoid eating raw fish.
The agencies noted that they are issuing this draft guidance because recent reports "show many pregnant women in the United States are not consuming fish recommended" by 2010 United States Department of Agriculture guidelines. The agencies added that there is "longstanding evidence of the nutritional value of fish in the diet. Fish contain high-quality protein, many vitamins and minerals, omega-3 fatty acid, and are mostly low in saturated fat, and some fish even contain vitamin D. The nutritional value of fish is especially important during growth and development before birth, in early infancy for breastfed infants, and in childhood."
Citing its own analysis of seafood consumption data from more than 1,000 pregnant women in the United States, the FDA noted that 21% ate no fish in the previous month and those who ate fish consumed far less than recommended in USDA guidelines, with 50% eating fewer than 2 ounces per week and 75% eating fewer than 4 ounces per week.
This draft guidance would replace similar recommendations issued by the FDA/EPA in March 2004, with a recommendation of simply up to 12 ounces of a variety of fish per week (two average meals), avoiding the same four identified types of fish because of their high mercury content. Previous guidance did not set a recommended lower limit of consumption.
The agencies will begin accepting comments on new guidance June 11.
The Food and Drug Administration and the Environmental Protection Agency are encouraging pregnant women, as well as women who may become pregnant or are breastfeeding, and young children to eat more fish, setting for the first time a recommended lower limit of fish consumption to promote health.
In draft guidance released June 10, the agencies are calling for women to consume 8-12 ounces of a variety of fish that are lower in mercury; that amount works out to an average of two to three servings per week. Among the types of fish highlighted that are lower in mercury are salmon, shrimp, pollock, tuna (light canned), tilapia, catfish, and cod.
The U.S. Department of Agriculture suggests an amount of 3-5 ounces per week for children under the age of 6 and 4-6 ounces per week for children aged 6-8, the agencies said in an accompanying question-and-answer set. Appropriate amounts of fish for older children would increase up to the adult recommendation. Children should not be given fish until they are at least 6 months of age.
The agencies recommend four types of fish to avoid because of mercury content: tilefish from the Gulf of Mexico, shark, swordfish, and king mackerel. They also recommend limiting white (albacore) tuna to 6 ounces per week for adults and even less for children.
In addition, the agency is advising that those eating fish caught in local waters should listen for local advisories regarding those fish and, if no advice is available, fish consumption from those sources should be limited to 6 ounces per week in pregnant women and 1-3 ounces for young children. The recommendation reiterates that pregnant women and young children should avoid eating raw fish.
The agencies noted that they are issuing this draft guidance because recent reports "show many pregnant women in the United States are not consuming fish recommended" by 2010 United States Department of Agriculture guidelines. The agencies added that there is "longstanding evidence of the nutritional value of fish in the diet. Fish contain high-quality protein, many vitamins and minerals, omega-3 fatty acid, and are mostly low in saturated fat, and some fish even contain vitamin D. The nutritional value of fish is especially important during growth and development before birth, in early infancy for breastfed infants, and in childhood."
Citing its own analysis of seafood consumption data from more than 1,000 pregnant women in the United States, the FDA noted that 21% ate no fish in the previous month and those who ate fish consumed far less than recommended in USDA guidelines, with 50% eating fewer than 2 ounces per week and 75% eating fewer than 4 ounces per week.
This draft guidance would replace similar recommendations issued by the FDA/EPA in March 2004, with a recommendation of simply up to 12 ounces of a variety of fish per week (two average meals), avoiding the same four identified types of fish because of their high mercury content. Previous guidance did not set a recommended lower limit of consumption.
The agencies will begin accepting comments on new guidance June 11.
The Food and Drug Administration and the Environmental Protection Agency are encouraging pregnant women, as well as women who may become pregnant or are breastfeeding, and young children to eat more fish, setting for the first time a recommended lower limit of fish consumption to promote health.
In draft guidance released June 10, the agencies are calling for women to consume 8-12 ounces of a variety of fish that are lower in mercury; that amount works out to an average of two to three servings per week. Among the types of fish highlighted that are lower in mercury are salmon, shrimp, pollock, tuna (light canned), tilapia, catfish, and cod.
The U.S. Department of Agriculture suggests an amount of 3-5 ounces per week for children under the age of 6 and 4-6 ounces per week for children aged 6-8, the agencies said in an accompanying question-and-answer set. Appropriate amounts of fish for older children would increase up to the adult recommendation. Children should not be given fish until they are at least 6 months of age.
The agencies recommend four types of fish to avoid because of mercury content: tilefish from the Gulf of Mexico, shark, swordfish, and king mackerel. They also recommend limiting white (albacore) tuna to 6 ounces per week for adults and even less for children.
In addition, the agency is advising that those eating fish caught in local waters should listen for local advisories regarding those fish and, if no advice is available, fish consumption from those sources should be limited to 6 ounces per week in pregnant women and 1-3 ounces for young children. The recommendation reiterates that pregnant women and young children should avoid eating raw fish.
The agencies noted that they are issuing this draft guidance because recent reports "show many pregnant women in the United States are not consuming fish recommended" by 2010 United States Department of Agriculture guidelines. The agencies added that there is "longstanding evidence of the nutritional value of fish in the diet. Fish contain high-quality protein, many vitamins and minerals, omega-3 fatty acid, and are mostly low in saturated fat, and some fish even contain vitamin D. The nutritional value of fish is especially important during growth and development before birth, in early infancy for breastfed infants, and in childhood."
Citing its own analysis of seafood consumption data from more than 1,000 pregnant women in the United States, the FDA noted that 21% ate no fish in the previous month and those who ate fish consumed far less than recommended in USDA guidelines, with 50% eating fewer than 2 ounces per week and 75% eating fewer than 4 ounces per week.
This draft guidance would replace similar recommendations issued by the FDA/EPA in March 2004, with a recommendation of simply up to 12 ounces of a variety of fish per week (two average meals), avoiding the same four identified types of fish because of their high mercury content. Previous guidance did not set a recommended lower limit of consumption.
The agencies will begin accepting comments on new guidance June 11.
Azathioprine, Anti-TNF Monotherapy Safe in IBD Pregnancy
CHICAGO – Women with inflammatory bowel disease can safely continue monotherapy with azathioprine/6-mercaptopurine or antitumor necrosis–alpha agents during pregnancy to maintain remission, according to an analysis of 1,289 women and 1,039 live births.
Infants exposed to azathioprine/6 MP and anti-TNF-alpha agents achieve developmental milestone scores at rates similar to unexposed infants and do not have higher rates of congenital anomalies, lead author Dr. Uma Mahadevan said at the annual Digestive Disease Week.
Overall, there were 55 congenital anomalies, with 21 diagnosed at birth. Events were similar among infants with in utero exposure to azathioprine, anti-TNF agents, combination therapy, and those with no drug exposure (12 vs. 17 vs. 7 vs. 19 events).
Exposure was defined as any use of azathioprine/6 MP or the anti-TNF-alpha agents infliximab (Remicade), adalimumab (Humira), or certolizumab pegol (Cimzia) at any time from 3 months prior to conception to the end of the pregnancy. Nine women had exposure to the recombinant monoclonal antibody natalizumab (Tysabri) and were included in the anti-TNF agents group.
The analysis was based on the PIANO registry, a prospective cohort of pregnant women with IBD who were followed by telephone or in-person questionnaire at every trimester, at delivery, at 4, 9, and 12 months after delivery, and annually for the first 4 years of their child’s life. Seven women received their diagnosis of IBD during pregnancy, 59.4% had Crohn’s disease, 38.3% ulcerative colitis, and 2.3% were indeterminate.
Two-thirds of women reported breastfeeding, with unexposed women significantly more likely to breastfeed than women with exposure to azathioprine, anti-TNF agents, or combination therapy (85% vs. 65% vs. 71% vs. 61%; P less than .0001).
After controlling for drug exposure, breastfeeding was not associated with an increased risk of infant infection or reduced height or weight, reported Dr. Mahadevan, with the University of California San Francisco.
Infants exposed to combination therapy, however, had higher rates of preterm birth, after adjustment for none/mild vs. moderate/severe IBD activity (odds ratio, 2.6; P less than .05).
Infants exposed to combination therapy who were born to mothers with ulcerative colitis also had increased rates of preterm birth (odds ratio, 4.9), low birth weight (OR, 6.1), and NICU stay (OR, 3.9).
One possible reason for this signal is that women with ulcerative colitis in the PIANO registry, as well as two other studies, have more disease activity during pregnancy, Dr. Mahadevan suggested. Another possibility is that mothers with ulcerative colitis may have low levels of untreated inflammation.
With just 161 women available for analysis, the PIANO investigators previously reported a significant increase in infant infections at 12 months of age in those exposed to combination infliximab or adalimumab plus azathioprine. Most anti-TNF agents cross the placenta in the second and third trimester, which has raised concerns about immune system development and risk of subsequent infections.
In the current analysis, there was no increase in infant infections when the investigators looked at all anti-TNF drugs. "But when we removed certolizumab from the analysis, it was significant just as it was 2 years ago," Dr. Mahadevan said in an interview. "Certolizumab is not actively transported across the placenta. Fortunately, the majority of the infections were minor, including otitis media and upper respiratory infections."
Developmental milestones
Overall, infants exposed to azathioprine or anti-TNF agents were found to achieve developmental milestones at rates similar to unexposed infants of mothers with IBD based on Denver Childhood Developmental Test and Ages and Stages Questionnaire scores, she said.
After controlling for preterm birth, the mean developmental milestone score at 4 months was 0.92 for infants with no drug exposure, with a nonsignificant mean delta difference of 0.01, 0.01, 0.00 points for infants exposed to azathioprine, anti-TNF agents, and combination therapy, respectively.
In a surprising twist, infants exposed to anti-TNF agents alone actually had significantly better scores at 12 months than unexposed infants, bettering the average score of 0.81 in the unexposed group by a mean delta of 0.02 points (P = .01), Dr. Mahadevan said.
"I wouldn’t interpret this data [as indicating] that biologics make your baby smarter. I think the key thing is that it doesn’t make it worse," she remarked. "When you see the deltas, they are pretty small."
On the Ages and Stages Questionnaire, the biologic group had significantly better mean scores than the unexposed group for gross motor skills at 36 months (55.94 vs. 47.53; P = .01), gross motor skills at 48 months (60 vs. 51.20; P = .02), fine motor skills at 48 months (53.50 vs. 42.11; P = .01), and personal/social interaction at 48 months (58 vs. 51.93; P = .04).
The azathioprine-exposed group always had better scores than the unexposed group, Dr. Mahadevan said. Where it was statistically significant was for personal/social interaction at 24 months (50.75 vs. 47.34; P = .04), problem solving at 36 months (mean 52.04 vs. 48.66; P = .05), and problem solving at 48 months (mean 59.92 vs. 57.66; P = .02).
Dr. Mahadevan observed that the data may change because not all infants have reached each time point and may not reflect outcomes in lower socioeconomic groups since more than 90% of women were of similar socioeconomic and educational status.
Still, the findings are reassuring in the absence of any randomized controlled trials. A recent report found children born to mothers with IBD had higher rates of attention-deficit hyperactivity disorder and gross motor abnormalities (J. Crohns Colitis 2013;7:542-50), while a case series reported normal neuropsychological development in 25 consecutive children exposed in utero to TNF-alpha inhibitors (Inflamm. Bowel Dis. 2014;20:495-501), she noted.
A recent systematic review of 58 articles or abstracts also found no association between TNF-alpha inhibitor use during pregnancy with IBD and adverse pregnancy outcomes, congenital abnormalities, or infections in the first year of life (BMC Medicine 2013;11:174).
The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
CHICAGO – Women with inflammatory bowel disease can safely continue monotherapy with azathioprine/6-mercaptopurine or antitumor necrosis–alpha agents during pregnancy to maintain remission, according to an analysis of 1,289 women and 1,039 live births.
Infants exposed to azathioprine/6 MP and anti-TNF-alpha agents achieve developmental milestone scores at rates similar to unexposed infants and do not have higher rates of congenital anomalies, lead author Dr. Uma Mahadevan said at the annual Digestive Disease Week.
Overall, there were 55 congenital anomalies, with 21 diagnosed at birth. Events were similar among infants with in utero exposure to azathioprine, anti-TNF agents, combination therapy, and those with no drug exposure (12 vs. 17 vs. 7 vs. 19 events).
Exposure was defined as any use of azathioprine/6 MP or the anti-TNF-alpha agents infliximab (Remicade), adalimumab (Humira), or certolizumab pegol (Cimzia) at any time from 3 months prior to conception to the end of the pregnancy. Nine women had exposure to the recombinant monoclonal antibody natalizumab (Tysabri) and were included in the anti-TNF agents group.
The analysis was based on the PIANO registry, a prospective cohort of pregnant women with IBD who were followed by telephone or in-person questionnaire at every trimester, at delivery, at 4, 9, and 12 months after delivery, and annually for the first 4 years of their child’s life. Seven women received their diagnosis of IBD during pregnancy, 59.4% had Crohn’s disease, 38.3% ulcerative colitis, and 2.3% were indeterminate.
Two-thirds of women reported breastfeeding, with unexposed women significantly more likely to breastfeed than women with exposure to azathioprine, anti-TNF agents, or combination therapy (85% vs. 65% vs. 71% vs. 61%; P less than .0001).
After controlling for drug exposure, breastfeeding was not associated with an increased risk of infant infection or reduced height or weight, reported Dr. Mahadevan, with the University of California San Francisco.
Infants exposed to combination therapy, however, had higher rates of preterm birth, after adjustment for none/mild vs. moderate/severe IBD activity (odds ratio, 2.6; P less than .05).
Infants exposed to combination therapy who were born to mothers with ulcerative colitis also had increased rates of preterm birth (odds ratio, 4.9), low birth weight (OR, 6.1), and NICU stay (OR, 3.9).
One possible reason for this signal is that women with ulcerative colitis in the PIANO registry, as well as two other studies, have more disease activity during pregnancy, Dr. Mahadevan suggested. Another possibility is that mothers with ulcerative colitis may have low levels of untreated inflammation.
With just 161 women available for analysis, the PIANO investigators previously reported a significant increase in infant infections at 12 months of age in those exposed to combination infliximab or adalimumab plus azathioprine. Most anti-TNF agents cross the placenta in the second and third trimester, which has raised concerns about immune system development and risk of subsequent infections.
In the current analysis, there was no increase in infant infections when the investigators looked at all anti-TNF drugs. "But when we removed certolizumab from the analysis, it was significant just as it was 2 years ago," Dr. Mahadevan said in an interview. "Certolizumab is not actively transported across the placenta. Fortunately, the majority of the infections were minor, including otitis media and upper respiratory infections."
Developmental milestones
Overall, infants exposed to azathioprine or anti-TNF agents were found to achieve developmental milestones at rates similar to unexposed infants of mothers with IBD based on Denver Childhood Developmental Test and Ages and Stages Questionnaire scores, she said.
After controlling for preterm birth, the mean developmental milestone score at 4 months was 0.92 for infants with no drug exposure, with a nonsignificant mean delta difference of 0.01, 0.01, 0.00 points for infants exposed to azathioprine, anti-TNF agents, and combination therapy, respectively.
In a surprising twist, infants exposed to anti-TNF agents alone actually had significantly better scores at 12 months than unexposed infants, bettering the average score of 0.81 in the unexposed group by a mean delta of 0.02 points (P = .01), Dr. Mahadevan said.
"I wouldn’t interpret this data [as indicating] that biologics make your baby smarter. I think the key thing is that it doesn’t make it worse," she remarked. "When you see the deltas, they are pretty small."
On the Ages and Stages Questionnaire, the biologic group had significantly better mean scores than the unexposed group for gross motor skills at 36 months (55.94 vs. 47.53; P = .01), gross motor skills at 48 months (60 vs. 51.20; P = .02), fine motor skills at 48 months (53.50 vs. 42.11; P = .01), and personal/social interaction at 48 months (58 vs. 51.93; P = .04).
The azathioprine-exposed group always had better scores than the unexposed group, Dr. Mahadevan said. Where it was statistically significant was for personal/social interaction at 24 months (50.75 vs. 47.34; P = .04), problem solving at 36 months (mean 52.04 vs. 48.66; P = .05), and problem solving at 48 months (mean 59.92 vs. 57.66; P = .02).
Dr. Mahadevan observed that the data may change because not all infants have reached each time point and may not reflect outcomes in lower socioeconomic groups since more than 90% of women were of similar socioeconomic and educational status.
Still, the findings are reassuring in the absence of any randomized controlled trials. A recent report found children born to mothers with IBD had higher rates of attention-deficit hyperactivity disorder and gross motor abnormalities (J. Crohns Colitis 2013;7:542-50), while a case series reported normal neuropsychological development in 25 consecutive children exposed in utero to TNF-alpha inhibitors (Inflamm. Bowel Dis. 2014;20:495-501), she noted.
A recent systematic review of 58 articles or abstracts also found no association between TNF-alpha inhibitor use during pregnancy with IBD and adverse pregnancy outcomes, congenital abnormalities, or infections in the first year of life (BMC Medicine 2013;11:174).
The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
CHICAGO – Women with inflammatory bowel disease can safely continue monotherapy with azathioprine/6-mercaptopurine or antitumor necrosis–alpha agents during pregnancy to maintain remission, according to an analysis of 1,289 women and 1,039 live births.
Infants exposed to azathioprine/6 MP and anti-TNF-alpha agents achieve developmental milestone scores at rates similar to unexposed infants and do not have higher rates of congenital anomalies, lead author Dr. Uma Mahadevan said at the annual Digestive Disease Week.
Overall, there were 55 congenital anomalies, with 21 diagnosed at birth. Events were similar among infants with in utero exposure to azathioprine, anti-TNF agents, combination therapy, and those with no drug exposure (12 vs. 17 vs. 7 vs. 19 events).
Exposure was defined as any use of azathioprine/6 MP or the anti-TNF-alpha agents infliximab (Remicade), adalimumab (Humira), or certolizumab pegol (Cimzia) at any time from 3 months prior to conception to the end of the pregnancy. Nine women had exposure to the recombinant monoclonal antibody natalizumab (Tysabri) and were included in the anti-TNF agents group.
The analysis was based on the PIANO registry, a prospective cohort of pregnant women with IBD who were followed by telephone or in-person questionnaire at every trimester, at delivery, at 4, 9, and 12 months after delivery, and annually for the first 4 years of their child’s life. Seven women received their diagnosis of IBD during pregnancy, 59.4% had Crohn’s disease, 38.3% ulcerative colitis, and 2.3% were indeterminate.
Two-thirds of women reported breastfeeding, with unexposed women significantly more likely to breastfeed than women with exposure to azathioprine, anti-TNF agents, or combination therapy (85% vs. 65% vs. 71% vs. 61%; P less than .0001).
After controlling for drug exposure, breastfeeding was not associated with an increased risk of infant infection or reduced height or weight, reported Dr. Mahadevan, with the University of California San Francisco.
Infants exposed to combination therapy, however, had higher rates of preterm birth, after adjustment for none/mild vs. moderate/severe IBD activity (odds ratio, 2.6; P less than .05).
Infants exposed to combination therapy who were born to mothers with ulcerative colitis also had increased rates of preterm birth (odds ratio, 4.9), low birth weight (OR, 6.1), and NICU stay (OR, 3.9).
One possible reason for this signal is that women with ulcerative colitis in the PIANO registry, as well as two other studies, have more disease activity during pregnancy, Dr. Mahadevan suggested. Another possibility is that mothers with ulcerative colitis may have low levels of untreated inflammation.
With just 161 women available for analysis, the PIANO investigators previously reported a significant increase in infant infections at 12 months of age in those exposed to combination infliximab or adalimumab plus azathioprine. Most anti-TNF agents cross the placenta in the second and third trimester, which has raised concerns about immune system development and risk of subsequent infections.
In the current analysis, there was no increase in infant infections when the investigators looked at all anti-TNF drugs. "But when we removed certolizumab from the analysis, it was significant just as it was 2 years ago," Dr. Mahadevan said in an interview. "Certolizumab is not actively transported across the placenta. Fortunately, the majority of the infections were minor, including otitis media and upper respiratory infections."
Developmental milestones
Overall, infants exposed to azathioprine or anti-TNF agents were found to achieve developmental milestones at rates similar to unexposed infants of mothers with IBD based on Denver Childhood Developmental Test and Ages and Stages Questionnaire scores, she said.
After controlling for preterm birth, the mean developmental milestone score at 4 months was 0.92 for infants with no drug exposure, with a nonsignificant mean delta difference of 0.01, 0.01, 0.00 points for infants exposed to azathioprine, anti-TNF agents, and combination therapy, respectively.
In a surprising twist, infants exposed to anti-TNF agents alone actually had significantly better scores at 12 months than unexposed infants, bettering the average score of 0.81 in the unexposed group by a mean delta of 0.02 points (P = .01), Dr. Mahadevan said.
"I wouldn’t interpret this data [as indicating] that biologics make your baby smarter. I think the key thing is that it doesn’t make it worse," she remarked. "When you see the deltas, they are pretty small."
On the Ages and Stages Questionnaire, the biologic group had significantly better mean scores than the unexposed group for gross motor skills at 36 months (55.94 vs. 47.53; P = .01), gross motor skills at 48 months (60 vs. 51.20; P = .02), fine motor skills at 48 months (53.50 vs. 42.11; P = .01), and personal/social interaction at 48 months (58 vs. 51.93; P = .04).
The azathioprine-exposed group always had better scores than the unexposed group, Dr. Mahadevan said. Where it was statistically significant was for personal/social interaction at 24 months (50.75 vs. 47.34; P = .04), problem solving at 36 months (mean 52.04 vs. 48.66; P = .05), and problem solving at 48 months (mean 59.92 vs. 57.66; P = .02).
Dr. Mahadevan observed that the data may change because not all infants have reached each time point and may not reflect outcomes in lower socioeconomic groups since more than 90% of women were of similar socioeconomic and educational status.
Still, the findings are reassuring in the absence of any randomized controlled trials. A recent report found children born to mothers with IBD had higher rates of attention-deficit hyperactivity disorder and gross motor abnormalities (J. Crohns Colitis 2013;7:542-50), while a case series reported normal neuropsychological development in 25 consecutive children exposed in utero to TNF-alpha inhibitors (Inflamm. Bowel Dis. 2014;20:495-501), she noted.
A recent systematic review of 58 articles or abstracts also found no association between TNF-alpha inhibitor use during pregnancy with IBD and adverse pregnancy outcomes, congenital abnormalities, or infections in the first year of life (BMC Medicine 2013;11:174).
The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
AT DDW 2014
Azathioprine, anti-TNF monotherapy safe in IBD pregnancy
CHICAGO – Women with inflammatory bowel disease can safely continue monotherapy with azathioprine/6-mercaptopurine or antitumor necrosis–alpha agents during pregnancy to maintain remission, according to an analysis of 1,289 women and 1,039 live births.
Infants exposed to azathioprine/6 MP and anti-TNF-alpha agents achieve developmental milestone scores at rates similar to unexposed infants and do not have higher rates of congenital anomalies, lead author Dr. Uma Mahadevan said at the annual Digestive Disease Week.
Overall, there were 55 congenital anomalies, with 21 diagnosed at birth. Events were similar among infants with in utero exposure to azathioprine, anti-TNF agents, combination therapy, and those with no drug exposure (12 vs. 17 vs. 7 vs. 19 events).
Exposure was defined as any use of azathioprine/6 MP or the anti-TNF-alpha agents infliximab (Remicade), adalimumab (Humira), or certolizumab pegol (Cimzia) at any time from 3 months prior to conception to the end of the pregnancy. Nine women had exposure to the recombinant monoclonal antibody natalizumab (Tysabri) and were included in the anti-TNF agents group.
The analysis was based on the PIANO registry, a prospective cohort of pregnant women with IBD who were followed by telephone or in-person questionnaire at every trimester, at delivery, at 4, 9, and 12 months after delivery, and annually for the first 4 years of their child’s life. Seven women received their diagnosis of IBD during pregnancy, 59.4% had Crohn’s disease, 38.3% ulcerative colitis, and 2.3% were indeterminate.
Two-thirds of women reported breastfeeding, with unexposed women significantly more likely to breastfeed than women with exposure to azathioprine, anti-TNF agents, or combination therapy (85% vs. 65% vs. 71% vs. 61%; P less than .0001).
After controlling for drug exposure, breastfeeding was not associated with an increased risk of infant infection or reduced height or weight, reported Dr. Mahadevan, with the University of California San Francisco.
Infants exposed to combination therapy, however, had higher rates of preterm birth, after adjustment for none/mild vs. moderate/severe IBD activity (odds ratio, 2.6; P less than .05).
Infants exposed to combination therapy who were born to mothers with ulcerative colitis also had increased rates of preterm birth (odds ratio, 4.9), low birth weight (OR, 6.1), and NICU stay (OR, 3.9).
One possible reason for this signal is that women with ulcerative colitis in the PIANO registry, as well as two other studies, have more disease activity during pregnancy, Dr. Mahadevan suggested. Another possibility is that mothers with ulcerative colitis may have low levels of untreated inflammation.
With just 161 women available for analysis, the PIANO investigators previously reported a significant increase in infant infections at 12 months of age in those exposed to combination infliximab or adalimumab plus azathioprine. Most anti-TNF agents cross the placenta in the second and third trimester, which has raised concerns about immune system development and risk of subsequent infections.
In the current analysis, there was no increase in infant infections when the investigators looked at all anti-TNF drugs. "But when we removed certolizumab from the analysis, it was significant just as it was 2 years ago," Dr. Mahadevan said in an interview. "Certolizumab is not actively transported across the placenta. Fortunately, the majority of the infections were minor, including otitis media and upper respiratory infections."
Developmental milestones
Overall, infants exposed to azathioprine or anti-TNF agents were found to achieve developmental milestones at rates similar to unexposed infants of mothers with IBD based on Denver Childhood Developmental Test and Ages and Stages Questionnaire scores, she said.
After controlling for preterm birth, the mean developmental milestone score at 4 months was 0.92 for infants with no drug exposure, with a nonsignificant mean delta difference of 0.01, 0.01, 0.00 points for infants exposed to azathioprine, anti-TNF agents, and combination therapy, respectively.
In a surprising twist, infants exposed to anti-TNF agents alone actually had significantly better scores at 12 months than unexposed infants, bettering the average score of 0.81 in the unexposed group by a mean delta of 0.02 points (P = .01), Dr. Mahadevan said.
"I wouldn’t interpret this data [as indicating] that biologics make your baby smarter. I think the key thing is that it doesn’t make it worse," she remarked. "When you see the deltas, they are pretty small."
On the Ages and Stages Questionnaire, the biologic group had significantly better mean scores than the unexposed group for gross motor skills at 36 months (55.94 vs. 47.53; P = .01), gross motor skills at 48 months (60 vs. 51.20; P = .02), fine motor skills at 48 months (53.50 vs. 42.11; P = .01), and personal/social interaction at 48 months (58 vs. 51.93; P = .04).
The azathioprine-exposed group always had better scores than the unexposed group, Dr. Mahadevan said. Where it was statistically significant was for personal/social interaction at 24 months (50.75 vs. 47.34; P = .04), problem solving at 36 months (mean 52.04 vs. 48.66; P = .05), and problem solving at 48 months (mean 59.92 vs. 57.66; P = .02).
Dr. Mahadevan observed that the data may change because not all infants have reached each time point and may not reflect outcomes in lower socioeconomic groups since more than 90% of women were of similar socioeconomic and educational status.
Still, the findings are reassuring in the absence of any randomized controlled trials. A recent report found children born to mothers with IBD had higher rates of attention-deficit hyperactivity disorder and gross motor abnormalities (J. Crohns Colitis 2013;7:542-50), while a case series reported normal neuropsychological development in 25 consecutive children exposed in utero to TNF-alpha inhibitors (Inflamm. Bowel Dis. 2014;20:495-501), she noted.
A recent systematic review of 58 articles or abstracts also found no association between TNF-alpha inhibitor use during pregnancy with IBD and adverse pregnancy outcomes, congenital abnormalities, or infections in the first year of life (BMC Medicine 2013;11:174).
The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
CHICAGO – Women with inflammatory bowel disease can safely continue monotherapy with azathioprine/6-mercaptopurine or antitumor necrosis–alpha agents during pregnancy to maintain remission, according to an analysis of 1,289 women and 1,039 live births.
Infants exposed to azathioprine/6 MP and anti-TNF-alpha agents achieve developmental milestone scores at rates similar to unexposed infants and do not have higher rates of congenital anomalies, lead author Dr. Uma Mahadevan said at the annual Digestive Disease Week.
Overall, there were 55 congenital anomalies, with 21 diagnosed at birth. Events were similar among infants with in utero exposure to azathioprine, anti-TNF agents, combination therapy, and those with no drug exposure (12 vs. 17 vs. 7 vs. 19 events).
Exposure was defined as any use of azathioprine/6 MP or the anti-TNF-alpha agents infliximab (Remicade), adalimumab (Humira), or certolizumab pegol (Cimzia) at any time from 3 months prior to conception to the end of the pregnancy. Nine women had exposure to the recombinant monoclonal antibody natalizumab (Tysabri) and were included in the anti-TNF agents group.
The analysis was based on the PIANO registry, a prospective cohort of pregnant women with IBD who were followed by telephone or in-person questionnaire at every trimester, at delivery, at 4, 9, and 12 months after delivery, and annually for the first 4 years of their child’s life. Seven women received their diagnosis of IBD during pregnancy, 59.4% had Crohn’s disease, 38.3% ulcerative colitis, and 2.3% were indeterminate.
Two-thirds of women reported breastfeeding, with unexposed women significantly more likely to breastfeed than women with exposure to azathioprine, anti-TNF agents, or combination therapy (85% vs. 65% vs. 71% vs. 61%; P less than .0001).
After controlling for drug exposure, breastfeeding was not associated with an increased risk of infant infection or reduced height or weight, reported Dr. Mahadevan, with the University of California San Francisco.
Infants exposed to combination therapy, however, had higher rates of preterm birth, after adjustment for none/mild vs. moderate/severe IBD activity (odds ratio, 2.6; P less than .05).
Infants exposed to combination therapy who were born to mothers with ulcerative colitis also had increased rates of preterm birth (odds ratio, 4.9), low birth weight (OR, 6.1), and NICU stay (OR, 3.9).
One possible reason for this signal is that women with ulcerative colitis in the PIANO registry, as well as two other studies, have more disease activity during pregnancy, Dr. Mahadevan suggested. Another possibility is that mothers with ulcerative colitis may have low levels of untreated inflammation.
With just 161 women available for analysis, the PIANO investigators previously reported a significant increase in infant infections at 12 months of age in those exposed to combination infliximab or adalimumab plus azathioprine. Most anti-TNF agents cross the placenta in the second and third trimester, which has raised concerns about immune system development and risk of subsequent infections.
In the current analysis, there was no increase in infant infections when the investigators looked at all anti-TNF drugs. "But when we removed certolizumab from the analysis, it was significant just as it was 2 years ago," Dr. Mahadevan said in an interview. "Certolizumab is not actively transported across the placenta. Fortunately, the majority of the infections were minor, including otitis media and upper respiratory infections."
Developmental milestones
Overall, infants exposed to azathioprine or anti-TNF agents were found to achieve developmental milestones at rates similar to unexposed infants of mothers with IBD based on Denver Childhood Developmental Test and Ages and Stages Questionnaire scores, she said.
After controlling for preterm birth, the mean developmental milestone score at 4 months was 0.92 for infants with no drug exposure, with a nonsignificant mean delta difference of 0.01, 0.01, 0.00 points for infants exposed to azathioprine, anti-TNF agents, and combination therapy, respectively.
In a surprising twist, infants exposed to anti-TNF agents alone actually had significantly better scores at 12 months than unexposed infants, bettering the average score of 0.81 in the unexposed group by a mean delta of 0.02 points (P = .01), Dr. Mahadevan said.
"I wouldn’t interpret this data [as indicating] that biologics make your baby smarter. I think the key thing is that it doesn’t make it worse," she remarked. "When you see the deltas, they are pretty small."
On the Ages and Stages Questionnaire, the biologic group had significantly better mean scores than the unexposed group for gross motor skills at 36 months (55.94 vs. 47.53; P = .01), gross motor skills at 48 months (60 vs. 51.20; P = .02), fine motor skills at 48 months (53.50 vs. 42.11; P = .01), and personal/social interaction at 48 months (58 vs. 51.93; P = .04).
The azathioprine-exposed group always had better scores than the unexposed group, Dr. Mahadevan said. Where it was statistically significant was for personal/social interaction at 24 months (50.75 vs. 47.34; P = .04), problem solving at 36 months (mean 52.04 vs. 48.66; P = .05), and problem solving at 48 months (mean 59.92 vs. 57.66; P = .02).
Dr. Mahadevan observed that the data may change because not all infants have reached each time point and may not reflect outcomes in lower socioeconomic groups since more than 90% of women were of similar socioeconomic and educational status.
Still, the findings are reassuring in the absence of any randomized controlled trials. A recent report found children born to mothers with IBD had higher rates of attention-deficit hyperactivity disorder and gross motor abnormalities (J. Crohns Colitis 2013;7:542-50), while a case series reported normal neuropsychological development in 25 consecutive children exposed in utero to TNF-alpha inhibitors (Inflamm. Bowel Dis. 2014;20:495-501), she noted.
A recent systematic review of 58 articles or abstracts also found no association between TNF-alpha inhibitor use during pregnancy with IBD and adverse pregnancy outcomes, congenital abnormalities, or infections in the first year of life (BMC Medicine 2013;11:174).
The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
CHICAGO – Women with inflammatory bowel disease can safely continue monotherapy with azathioprine/6-mercaptopurine or antitumor necrosis–alpha agents during pregnancy to maintain remission, according to an analysis of 1,289 women and 1,039 live births.
Infants exposed to azathioprine/6 MP and anti-TNF-alpha agents achieve developmental milestone scores at rates similar to unexposed infants and do not have higher rates of congenital anomalies, lead author Dr. Uma Mahadevan said at the annual Digestive Disease Week.
Overall, there were 55 congenital anomalies, with 21 diagnosed at birth. Events were similar among infants with in utero exposure to azathioprine, anti-TNF agents, combination therapy, and those with no drug exposure (12 vs. 17 vs. 7 vs. 19 events).
Exposure was defined as any use of azathioprine/6 MP or the anti-TNF-alpha agents infliximab (Remicade), adalimumab (Humira), or certolizumab pegol (Cimzia) at any time from 3 months prior to conception to the end of the pregnancy. Nine women had exposure to the recombinant monoclonal antibody natalizumab (Tysabri) and were included in the anti-TNF agents group.
The analysis was based on the PIANO registry, a prospective cohort of pregnant women with IBD who were followed by telephone or in-person questionnaire at every trimester, at delivery, at 4, 9, and 12 months after delivery, and annually for the first 4 years of their child’s life. Seven women received their diagnosis of IBD during pregnancy, 59.4% had Crohn’s disease, 38.3% ulcerative colitis, and 2.3% were indeterminate.
Two-thirds of women reported breastfeeding, with unexposed women significantly more likely to breastfeed than women with exposure to azathioprine, anti-TNF agents, or combination therapy (85% vs. 65% vs. 71% vs. 61%; P less than .0001).
After controlling for drug exposure, breastfeeding was not associated with an increased risk of infant infection or reduced height or weight, reported Dr. Mahadevan, with the University of California San Francisco.
Infants exposed to combination therapy, however, had higher rates of preterm birth, after adjustment for none/mild vs. moderate/severe IBD activity (odds ratio, 2.6; P less than .05).
Infants exposed to combination therapy who were born to mothers with ulcerative colitis also had increased rates of preterm birth (odds ratio, 4.9), low birth weight (OR, 6.1), and NICU stay (OR, 3.9).
One possible reason for this signal is that women with ulcerative colitis in the PIANO registry, as well as two other studies, have more disease activity during pregnancy, Dr. Mahadevan suggested. Another possibility is that mothers with ulcerative colitis may have low levels of untreated inflammation.
With just 161 women available for analysis, the PIANO investigators previously reported a significant increase in infant infections at 12 months of age in those exposed to combination infliximab or adalimumab plus azathioprine. Most anti-TNF agents cross the placenta in the second and third trimester, which has raised concerns about immune system development and risk of subsequent infections.
In the current analysis, there was no increase in infant infections when the investigators looked at all anti-TNF drugs. "But when we removed certolizumab from the analysis, it was significant just as it was 2 years ago," Dr. Mahadevan said in an interview. "Certolizumab is not actively transported across the placenta. Fortunately, the majority of the infections were minor, including otitis media and upper respiratory infections."
Developmental milestones
Overall, infants exposed to azathioprine or anti-TNF agents were found to achieve developmental milestones at rates similar to unexposed infants of mothers with IBD based on Denver Childhood Developmental Test and Ages and Stages Questionnaire scores, she said.
After controlling for preterm birth, the mean developmental milestone score at 4 months was 0.92 for infants with no drug exposure, with a nonsignificant mean delta difference of 0.01, 0.01, 0.00 points for infants exposed to azathioprine, anti-TNF agents, and combination therapy, respectively.
In a surprising twist, infants exposed to anti-TNF agents alone actually had significantly better scores at 12 months than unexposed infants, bettering the average score of 0.81 in the unexposed group by a mean delta of 0.02 points (P = .01), Dr. Mahadevan said.
"I wouldn’t interpret this data [as indicating] that biologics make your baby smarter. I think the key thing is that it doesn’t make it worse," she remarked. "When you see the deltas, they are pretty small."
On the Ages and Stages Questionnaire, the biologic group had significantly better mean scores than the unexposed group for gross motor skills at 36 months (55.94 vs. 47.53; P = .01), gross motor skills at 48 months (60 vs. 51.20; P = .02), fine motor skills at 48 months (53.50 vs. 42.11; P = .01), and personal/social interaction at 48 months (58 vs. 51.93; P = .04).
The azathioprine-exposed group always had better scores than the unexposed group, Dr. Mahadevan said. Where it was statistically significant was for personal/social interaction at 24 months (50.75 vs. 47.34; P = .04), problem solving at 36 months (mean 52.04 vs. 48.66; P = .05), and problem solving at 48 months (mean 59.92 vs. 57.66; P = .02).
Dr. Mahadevan observed that the data may change because not all infants have reached each time point and may not reflect outcomes in lower socioeconomic groups since more than 90% of women were of similar socioeconomic and educational status.
Still, the findings are reassuring in the absence of any randomized controlled trials. A recent report found children born to mothers with IBD had higher rates of attention-deficit hyperactivity disorder and gross motor abnormalities (J. Crohns Colitis 2013;7:542-50), while a case series reported normal neuropsychological development in 25 consecutive children exposed in utero to TNF-alpha inhibitors (Inflamm. Bowel Dis. 2014;20:495-501), she noted.
A recent systematic review of 58 articles or abstracts also found no association between TNF-alpha inhibitor use during pregnancy with IBD and adverse pregnancy outcomes, congenital abnormalities, or infections in the first year of life (BMC Medicine 2013;11:174).
The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
AT DDW 2014
Key clinical point: Monotherapy with azathioprine and anti-TNF agents can be continued in pregnancy with IBD to maintain remission.
Major finding: Congenital anomalies were not higher in children with exposure to azathioprine, anti-TNF agents, or combination therapy, compared with no drug exposure (12 vs. 17 vs. 7 vs. 19 anomalies).
Data source: Analysis of 1,289 women with IBD and 1,039 live births.
Disclosures: The study was supported by the Crohn’s and Colitis Foundation of America. Dr. Mahadevan reported serving as an advisor for Janssen-Cilag, AbbVie, Takeda, and UCB Pharma, and receiving research grants from Prometheus Laboratories and GlaxoSmithKline.
Hypertension and pregnancy and preventing the first cesarean delivery
This peer to peer discussion focuses on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines1 and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.2
In this 20-minute audiocast, listen to these experts discuss:
Changing diagnostic tools for preeclampsia
- The 24-hour urinary protein estimation: When is it necessary?
- Use of magnesium sulfate for seizure prophylaxis
Preventing the first cesarean delivery
- Redefining the stages of labor: When is the second-stage too long?
- The lost skill of forceps delivery
- Is cesarean delivery rate the optimal metric for measuring neonatal outcome?
John T. Repke, MD, is University Professor and Chairman of the Department of Obstetrics and Gynecology at Penn State University College of Medicine, and Obstetrician-Gynecologist-in-Chief at the Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Repke is a member of the Board of Editors of OBG Management and is author of the June 2014 Guest Editorial on hypertension and pregnancy.
Errol R. Norwitz, MD, PhD, is the Louis E. Phaneuf Professor and Chairman of the Department of Obstetrics and Gynecology at Tufts Medical Center and Tufts University School of Medicine in Boston, Massachusetts. Dr. Norwitz is a member of the Board of Editors of OBG Management and is author of the June 2014 Update on operative vaginal delivery.
The speakers report no financial relationships relevant to this audiocast.
Click here for a downloadable transcript
TRANSCRIPT
ACOG guidelines on hypertension and pregnancy raise some questions
John T. Repke, MD: So, Errol, I was impressed over the first couple of days of being at the meeting. As you know, we had a postgraduate course, and one of the items that we talked about was the new hypertension and pregnancy document that was released by the Task Force on Hypertension and Pregnancy1 charged by the American College of Obstetricians and Gynecologists. I’ve got to say that while the goal of the document was to provide some standardization and clarification, there still seems to be a lot of confusion in my audience about how to interpret some of the guidelines. Have you found that?
Errol R. Norwitz, MD, PhD: Yes, I have. I found it interesting that it was put out as an executive summary, and not as a practice bulletin, which will probably follow in months. That document, which came out in November 2013, helped to address many of the issues we’ve had over the years of preeclampsia, in terms of its definition and some of the management issues. But, it also raised a number of questions that still need to be resolved.
Dr. Repke: Yes. I think one of the things to keep in mind, and I’ve tabulated all of the recommendations, is that about 60 recommendations came out of that document and only six of the 60 were accompanied by a strong quality of evidence, or rather, a high quality of evidence, and a strong recommendation. And a lot of those things were addressing issues that I think most practitioners already did, in so far as using antenatal steroids for maturation; using magnesium sulfate for patients with preeclampsia with severe features; and using magnesium sulfate as a treatment of eclampsia. But a lot of the other recommendations really were based on either moderate- or low-quality evidence, and had qualified recommendations. And, I think that’s what has led to some of the confusion.
Changing diagnostic tools for preeclampsia
Dr. Repke: What sort of specific things are your practitioners asking you about as far as, “Is this gestational hypertension or is this preeclampsia?” The guidelines say proteinuria is not required anymore. How are you dealing with that?
Should we still do the 24-hour urinary protein estimation?
Dr. Norwitz: The biggest change, in my mind, is the statement that you no longer require significant proteinuria to make the diagnosis of preeclampsia, and, indeed, of severe preeclampsia. So, if you do have significant proteinuria, then that would confirm the diagnosis. But, you can also have preeclampsia in the presence of other endorgan injuries, such as kidney injury and liver injury in the absence of significant proteinuria.
So, one of the questions that comes up is, “Should we actually do the 24-hour urinary protein estimation?”
And, my answer is, “yes.” If you have significant proteinuria, then that would confirm the diagnosis. If you don’t, you can still make the diagnosis in the setting of low platelets, elevated liver enzymes, or abnormal renal function. So, the issue is, and I’d be curious to hear your answer, if you have someone with platelets of, let’s say, 78, a new onset of sustained elevation of blood pressure, would you do the 24-hour urine estimation or just defer it?
Dr. Repke: We wouldn’t perform the 24-hour urine test under those circumstances. And, we would consider that nuance of hypertension with a severe feature that is now preeclampsia with severe feature, and the management would be based on gestational age. With a platelet count that low, the management would be stabilization and delivery. Although, if stabilized, I think that’s the type of patient that potentially could have delivery delayed until you could get an effective antenatal steroid if she was less that 34 weeks’ gestation.
Dr. Norwitz: So, that’s one issue I think needs to be clarified. If there’s other evidence of endorgan damage, then you can defer the 24-hour urinary protein. That’s another question that comes up. I’m pleased they could resolve the issue of repeated 24-hour urinary estimations. Once you have your 300 mg suggestive of the diagnosis of preeclampsia, there’s no reason to then repeat it looking for elevation and increased leakage of protein into the urine, because it doesn’t correlate with adverse outcome for the mother or fetus. So, that issue was clarified.
Dr. Repke: I think that two questions that came up in our course, and I think they were very legitimate, are, “Do we even need to do urine protein at all?” Because if you look at the guidelines for management, the only difference between preeclampsia management without severe features and gestational hypertension is frequency of antenatal testing until you decide to begin delivery. Now, in the old days, one would say, “Well, another difference would be that the preeclamptic would get magnesium sulfate.” But the current Hypertension in Pregnancy Guidelines1 suggest that preeclampsia without severe features doesn’t necessarily have to be managed with magnesium sulfate. So, I’m still wrestling with whether, other than the fact that it might be for study purposes or for categorization or research, whether proteinuria adds anything to the equation.
And, then the second question is, “How do you resolve the issue of disagreement?” So, the example is protein:creatinine ratio allows for a more rapid diagnosis of significant proteinuria. If that patient doesn’t have to deliver immediately and a 24-hour urine sample is obtained, which do you believe if you have a protein:creatinine ratio greater than 0.3, but now your 24-hour urine is 212 mg/dL? And, I don’t have the answer to that, but that’s another area of confusion.
Dr. Norwitz: And, I think that confusion will persist. I don’t think this document is going to resolve it.
New terminology: Preeclampsia with or without severe features
Dr. Norwitz: I do like the difference in terminology between preeclampsia with severe features and preeclampsia without severe features. I think the old terminology of severe and mild preeclampsia was somewhat confusing. I certainly appreciate that alteration in terminology, although it may take a while for it to catch on. I’m still seeing the term “mild preeclampsia” used quite widely.
Use of magnesium sulfate for seizure prophylaxis
Dr. Norwitz: You did raise the issue of magnesium sulfate for seizure prophylaxis in the setting of severe preeclampsia without severe features. And I was struck by the statement. Not only is it not necessary to give it, but in the Executive Summary, as you suggest, it is not indicated and you recommended against starting it. Is that how you interpret it as the well?
Dr. Repke: Well, I might have interpreted the statement the way I wanted to interpret it. And, as you know, in our institution, because we feel we are a teaching program, people can progress very quickly intrapartum from not having severe features to having severe features, and we don’t want to miss that window of opportunity. Our practice in that regard does not follow the guidelines. We use intrapartum magnesium prophylaxis for all patients with the diagnosis of preeclampsia, and continue it for 24 hours postpartum.
Dr. Norwitz: And I would have to say we decided do the same. So, once a diagnosis of preeclampsia is made, we would give intrapartum, and then postpartum magnesium seizure prophylaxis for 24 hours, regardless of whether there’s evidence of severe features or no severe features.
Dr. Repke: And there again, I think it’s why, for you and I, it will still be important to assess the proteinuria because that diagnostic difference between preeclampsia and gestational hypertension is going to alter management. But if you follow the document word for word, if you’re not going to use magnesium without severe features, I’m not really sure what proteinuria adds. I guess, at the end of the day, you’ve got to be a good doctor. And, you’ve got to be physically assessing your patient on a very regular basis.
Preventing the first cesarean delivery. Will cesarean rates decline?
Dr. Repke: So, speaking of guidelines, the Society for Maternal Fetal Medicine (SMFM) just came out with a document trying to address this issue of the cesarean-section rate in the United States and are there things that we can be doing to lower the primary C-section rate.2 My feeling is probably disseminated from the recognition that vaginal birth after C-section never got to the levels of acceptance that anybody hoped back when Healthy People 2010 was first written. And, we could eliminate that issue or, at least significantly reduce that issue, if the first C-section never took place.
And, I guess I’d like some of your thoughts about some of the things in that document, some of the things we need to be reconsidering in terms of how we define labor and so on.
Dr. Norwitz: It is true, I think, that there’s been an epidemic of cesarean deliveries in the last decade in the US, but also throughout the world, I think, even in countries that have traditionally had very low cesarean delivery rates, the classic one being Ireland and the UK countries. Their rates are now increasing significantly.
And there are a number of different reasons as to why this may be. I think, certainly the obesity epidemic has contributed to this. You want to deliver patients who have an elevated BMI prior to the postterm period. But, it’s often difficult to monitor these patients, and the cesarean-delivery rate overall is much higher in that population. So, that might be one reason why cesarean-delivery rates overall are going up. But, certainly there are many others.
Dr. Repke: Yes, I think you’re absolutely right: the demographics of change. Childbearing is being delayed. We know that uterine contractility dynamics alter with advanced maternal age. We’ve got a higher incidence of multiple gestations with advanced maternal age. We have more patients that require induction because of supervening medical complications of pregnancy, whether that be Class A2 gestational diabetes, or whether that be pregnancy-induced hypertension.
Redefining the stages of labor
Dr. Repke: I think some of the intriguing data, to me, is a willingness to re-look at how we define the stages of labor. And what are acceptable norms? And, while I have some concerns about how that may be interpreted in the rank and file, I think it’s at least heightened the awareness of my faculty that we just can’t tolerate the C-section at 4 cm, or whether the latent phase of labor should be allowed to go to 6 cm. I don’t think we really have the data for that. But I’d be happy if I could just start to see a reduction in “failure to progress at 4 cm.”
And then the issue of second stage, I think, is also important. What do you think about the guidelines’ recommendation that there may not really be an upper limit of allowability for second-stage labors?
Dr. Norwitz: Well, certainly I think it’s important to think back to the historical context of where the labor curves developed. The original labor curves were actually developed in Zimbabwe (at that time it was Rhodesia) by an obstetrician working in the community called Philpott. And he was trying to determine when it was appropriate to send patients into the tertiary care center. So he designed the labor curve and said if patients failed to progress over a certain number of hours, those are patients that are likely to need an operative delivery, and he would then send them into the tertiary care center.
And, then Dr. Freidman picked up on that idea and developed the Freidman Curve in Boston. But that was an era, again, many years ago when the population demographics were very different, when not many patients received regional anesthesia. I think if you look at the current guidelines, there is a huge discrepancy between women who get epidural anesthesia and those that don’t in terms of the progress of labor, both the first stage and the second stage.
Is it anesthesia?
Dr. Repke: One of the things that, you know, I haven’t looked at this paper in a long time, but you remember at Brigham and Women’s Hospital, probably 20 years ago, we were winding down the Active Management of Labor Study3 that was designed to try to replicate what had gone on at the Dublin Maternity Hospital,4 and if I’m remembering correctly, one of the remarkable things about that is that in Dublin, there were virtually no C-sections in the second stage. And so people assumed that while they were more aggressive with forceps, the operative vaginal delivery rate was no different between Brigham and Women’s Hospital and the Dublin Maternity Hospital. And so there needed to be another explanation.
And, I know I’m going to incur some of the ire of our anesthesia colleagues, but I really wonder whether there is a contribution of regional anesthesia to some of the labor dystocias that we see, and whether that’s a new demographic that we haven’t really adequately assessed. Even though I recognize some of the anesthesia literature5 seems to suggest very strongly that it has no effect. You know, if you were to plot a graph of regional anesthesia rates and cesarean section rates, they would probably parallel each other.
Dr. Norwitz: I think they do. I think we’ve long known that epidural anesthesia slows down the second stage of labor. These analyses suggest that it also has a significant effect on the first stage. And, I think that needs to be taken into account.
The lost skill of forceps delivery
Dr. Norwitz: I personally think that the skill set, in terms of operative vaginal delivery with forceps and vacuum, has really been lost. And I do feel that’s one of the factors contributing to the increase in cesarean delivery rates. I certainly see that in my practice: that I’m comfortable doing rotational forceps and mid-cavity forceps deliveries, where many of my colleagues have lost that skill, and rely now on the vacuum, which in certain circumstances is a less-than-ideal instrument. So, I believe that’s part of the reason why the cesarean delivery rates have gone up.
Lengthy second stage
Dr. Norwitz: But, certainly, I think, epidural anesthesia has made a difference, and I think we need to be cognizant of the fact that there is no “hard stop” now, in terms of the length of the second stage. If you get to 3 hours, even 4 hours, I would say, and there’s continued descent with pushing and fetal heart-rate tracing is reassuring, it’s reasonable to continue beyond those cutoffs.
Dr. Repke: I agree. I also have a concern about that, and I’m going to use a little bit of a parallel example of, you know, 7 or 8 years ago, there was a big push, and I think it was an appropriate push, to try to avoid elective deliveries prior to 39 weeks.6,7 What ended up happening was that people forgot about the term “elective,” and all they heard was 39 weeks. And what we would see on Board Examinations was, “Why do you have this placenta previa delivering at 39 weeks?”
“Well, that’s our hospital policy. We can’t deliver before 39 weeks.”
And, I think, the complications started to arise, and that’s what led to SMFM and ACOG coming out with guidelines for when it is acceptable to deliver prior to 39 weeks.2,6–8
So, the analogy is: I’m afraid that people are only going to see there is no upper limit for latent phase, there is no upper limit for second stage; that clinical judgment may not get its due in making these decisions. And we’ve all been in situations where, when you are trying to extract the head out of the pelvis, a cesarean section after a 5- or 6-hour second stage has its own set of complications. So my concern is that I hope we will recognize that we have to still use some clinical judgment, what I term the so-called “art of obstetrics,” into managing these patients.
Are you optimistic that we’re going to the lower C-section rate?
Dr. Norwitz: No, I think, it’s going to continue to go up. I think, with the increasing number of multiple pregnancies, obesity, maternal age getting further and further along, I think this is only going to continue to rise. And to be honest, I don’t know the correct cesarean delivery rate, or even if that is the metric that we should be measuring.
What is the right metric to measure neonatal outcome?
Dr. Norwitz: Maybe we should be looking at perinatal outcome. If perinatal outcome is improved, then maybe the cesarean rate is less important. Obviously, the first cesarean does have implications for subsequent pregnancy outcomes, and if we do continue to see this rise in cesarean deliveries, we are going to end up with many more placental accretas and hemorrhages in women in years to come.
So, careful counseling is important. If patients plan to have one or two kids only, maybe a cesarean delivery is very reasonable. If they are planning on having six or seven kids, then maybe you have to have a more careful discussion.
Dr. Repke: Yes, I think, that’s a very good point: the number of cesareans and the potential risks for abnormal placentation. I think societal expectations have changed in terms of what they want. Most mothers are willing to sacrifice maternal risk for presumed benefits to the fetus.
I think, where we’ve gotten into trouble as a specialty, though, is that we’ve had a hard time proving that neonatal outcome, in fact, has improved—despite an almost tripling of the cesarean section rate since, probably, the early 1970s. Although, anecdotally, what my pediatric and neonatal colleagues will tell me is they don’t get the kind of damaged babies they used to get. So the neonatologists that are closer to my age that have been doing this for a long time, they’re not seeing the really severe meconium aspiration syndromes; they’re not seeing really severe forceps-related injuries, or vacuum-related injuries that they used to see. So, those may be data that we’re going to need to accumulate with a little bit more rigor, and see if that’s true.
But I tend to agree with you. I don’t know what the right cesarean section rate is. I often tell people, I have yet to meet a patient who doesn’t think her cesarean section was indicated. And that’s where I think we hit the crossroads of individual patient-care management. So, we know across all other disciplines in medicine we’re entering the era of personalized medicine, yet we want to make broad public health policy that may not apply to individuals, and run with that. So, that’s also a concern. But, as they say, a story we will follow with interest.
Dr. Norwitz: I think so. I think the other part of that equation is the stillbirth rate, and the fact that there’s a push now to avoid elective inductions before 39 weeks, which I think is very reasonable, with a focus there again on elective inductions.
There’s also a push to induce patients before 42 weeks. And that bar has been pushed back, and in most practices around the country now, deliveries are being affected and recommended at 41 weeks. And clearly, if you take a nulliparous patient with an unfavorable cervix and induce at 41 weeks, you are going to increase the cesarean rate. I would argue that you are also decreasing the chance that there will be a stillbirth. But that data has not been forthcoming.
So this issue is by no means resolved. I think there are going to be many more years of data and studies and consensus opinions before we have a much better sense of what the right cesarean
rate is.
Dr. Repke: Yes, I think that’s a great point. And, one thing that I think people aren’t maybe that familiar with is when this push came, and again, it is an appropriate push to minimize elective deliveries before 39 weeks. When they looked at neonatal outcomes, all they looked at were the group that delivered at 37 weeks, and the group that delivered at 39 weeks. And they didn’t look at what happened with the other ones.9
So, they did look at the stillbirths of fetal distress or the other complications that happened between 37 and 1, and 38 and 6. They just looked at neonates that were born at 37 weeks and compared them to neonates that were born at 39 weeks, and found reduced instances of things like transient tachypnea of the newborn, hyperbilirubinemia, and thermoregulation issues, and those sorts of things. But, never looked at the neonates in that window, so no question 39 is better than 37, but, 37 is better than not making it to 39. So that, as you said, we’ve got a lot more information we’ve got to gather.
Errol, good talking with you.
Dr. Norwitz: Thank you.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus: Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193.
- Frigoletto FD, Lieberman E, Lang J, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–750.
- O’Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd ed. London: Mosby- Yearbook; 1993.
- Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia effect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80(6):1201–1208.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 561: Nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911–915.
- Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–333.
- Tita ATN, Landon MB, Spong CY, et al; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat Cesarean Delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
This peer to peer discussion focuses on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines1 and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.2
In this 20-minute audiocast, listen to these experts discuss:
Changing diagnostic tools for preeclampsia
- The 24-hour urinary protein estimation: When is it necessary?
- Use of magnesium sulfate for seizure prophylaxis
Preventing the first cesarean delivery
- Redefining the stages of labor: When is the second-stage too long?
- The lost skill of forceps delivery
- Is cesarean delivery rate the optimal metric for measuring neonatal outcome?
John T. Repke, MD, is University Professor and Chairman of the Department of Obstetrics and Gynecology at Penn State University College of Medicine, and Obstetrician-Gynecologist-in-Chief at the Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Repke is a member of the Board of Editors of OBG Management and is author of the June 2014 Guest Editorial on hypertension and pregnancy.
Errol R. Norwitz, MD, PhD, is the Louis E. Phaneuf Professor and Chairman of the Department of Obstetrics and Gynecology at Tufts Medical Center and Tufts University School of Medicine in Boston, Massachusetts. Dr. Norwitz is a member of the Board of Editors of OBG Management and is author of the June 2014 Update on operative vaginal delivery.
The speakers report no financial relationships relevant to this audiocast.
Click here for a downloadable transcript
TRANSCRIPT
ACOG guidelines on hypertension and pregnancy raise some questions
John T. Repke, MD: So, Errol, I was impressed over the first couple of days of being at the meeting. As you know, we had a postgraduate course, and one of the items that we talked about was the new hypertension and pregnancy document that was released by the Task Force on Hypertension and Pregnancy1 charged by the American College of Obstetricians and Gynecologists. I’ve got to say that while the goal of the document was to provide some standardization and clarification, there still seems to be a lot of confusion in my audience about how to interpret some of the guidelines. Have you found that?
Errol R. Norwitz, MD, PhD: Yes, I have. I found it interesting that it was put out as an executive summary, and not as a practice bulletin, which will probably follow in months. That document, which came out in November 2013, helped to address many of the issues we’ve had over the years of preeclampsia, in terms of its definition and some of the management issues. But, it also raised a number of questions that still need to be resolved.
Dr. Repke: Yes. I think one of the things to keep in mind, and I’ve tabulated all of the recommendations, is that about 60 recommendations came out of that document and only six of the 60 were accompanied by a strong quality of evidence, or rather, a high quality of evidence, and a strong recommendation. And a lot of those things were addressing issues that I think most practitioners already did, in so far as using antenatal steroids for maturation; using magnesium sulfate for patients with preeclampsia with severe features; and using magnesium sulfate as a treatment of eclampsia. But a lot of the other recommendations really were based on either moderate- or low-quality evidence, and had qualified recommendations. And, I think that’s what has led to some of the confusion.
Changing diagnostic tools for preeclampsia
Dr. Repke: What sort of specific things are your practitioners asking you about as far as, “Is this gestational hypertension or is this preeclampsia?” The guidelines say proteinuria is not required anymore. How are you dealing with that?
Should we still do the 24-hour urinary protein estimation?
Dr. Norwitz: The biggest change, in my mind, is the statement that you no longer require significant proteinuria to make the diagnosis of preeclampsia, and, indeed, of severe preeclampsia. So, if you do have significant proteinuria, then that would confirm the diagnosis. But, you can also have preeclampsia in the presence of other endorgan injuries, such as kidney injury and liver injury in the absence of significant proteinuria.
So, one of the questions that comes up is, “Should we actually do the 24-hour urinary protein estimation?”
And, my answer is, “yes.” If you have significant proteinuria, then that would confirm the diagnosis. If you don’t, you can still make the diagnosis in the setting of low platelets, elevated liver enzymes, or abnormal renal function. So, the issue is, and I’d be curious to hear your answer, if you have someone with platelets of, let’s say, 78, a new onset of sustained elevation of blood pressure, would you do the 24-hour urine estimation or just defer it?
Dr. Repke: We wouldn’t perform the 24-hour urine test under those circumstances. And, we would consider that nuance of hypertension with a severe feature that is now preeclampsia with severe feature, and the management would be based on gestational age. With a platelet count that low, the management would be stabilization and delivery. Although, if stabilized, I think that’s the type of patient that potentially could have delivery delayed until you could get an effective antenatal steroid if she was less that 34 weeks’ gestation.
Dr. Norwitz: So, that’s one issue I think needs to be clarified. If there’s other evidence of endorgan damage, then you can defer the 24-hour urinary protein. That’s another question that comes up. I’m pleased they could resolve the issue of repeated 24-hour urinary estimations. Once you have your 300 mg suggestive of the diagnosis of preeclampsia, there’s no reason to then repeat it looking for elevation and increased leakage of protein into the urine, because it doesn’t correlate with adverse outcome for the mother or fetus. So, that issue was clarified.
Dr. Repke: I think that two questions that came up in our course, and I think they were very legitimate, are, “Do we even need to do urine protein at all?” Because if you look at the guidelines for management, the only difference between preeclampsia management without severe features and gestational hypertension is frequency of antenatal testing until you decide to begin delivery. Now, in the old days, one would say, “Well, another difference would be that the preeclamptic would get magnesium sulfate.” But the current Hypertension in Pregnancy Guidelines1 suggest that preeclampsia without severe features doesn’t necessarily have to be managed with magnesium sulfate. So, I’m still wrestling with whether, other than the fact that it might be for study purposes or for categorization or research, whether proteinuria adds anything to the equation.
And, then the second question is, “How do you resolve the issue of disagreement?” So, the example is protein:creatinine ratio allows for a more rapid diagnosis of significant proteinuria. If that patient doesn’t have to deliver immediately and a 24-hour urine sample is obtained, which do you believe if you have a protein:creatinine ratio greater than 0.3, but now your 24-hour urine is 212 mg/dL? And, I don’t have the answer to that, but that’s another area of confusion.
Dr. Norwitz: And, I think that confusion will persist. I don’t think this document is going to resolve it.
New terminology: Preeclampsia with or without severe features
Dr. Norwitz: I do like the difference in terminology between preeclampsia with severe features and preeclampsia without severe features. I think the old terminology of severe and mild preeclampsia was somewhat confusing. I certainly appreciate that alteration in terminology, although it may take a while for it to catch on. I’m still seeing the term “mild preeclampsia” used quite widely.
Use of magnesium sulfate for seizure prophylaxis
Dr. Norwitz: You did raise the issue of magnesium sulfate for seizure prophylaxis in the setting of severe preeclampsia without severe features. And I was struck by the statement. Not only is it not necessary to give it, but in the Executive Summary, as you suggest, it is not indicated and you recommended against starting it. Is that how you interpret it as the well?
Dr. Repke: Well, I might have interpreted the statement the way I wanted to interpret it. And, as you know, in our institution, because we feel we are a teaching program, people can progress very quickly intrapartum from not having severe features to having severe features, and we don’t want to miss that window of opportunity. Our practice in that regard does not follow the guidelines. We use intrapartum magnesium prophylaxis for all patients with the diagnosis of preeclampsia, and continue it for 24 hours postpartum.
Dr. Norwitz: And I would have to say we decided do the same. So, once a diagnosis of preeclampsia is made, we would give intrapartum, and then postpartum magnesium seizure prophylaxis for 24 hours, regardless of whether there’s evidence of severe features or no severe features.
Dr. Repke: And there again, I think it’s why, for you and I, it will still be important to assess the proteinuria because that diagnostic difference between preeclampsia and gestational hypertension is going to alter management. But if you follow the document word for word, if you’re not going to use magnesium without severe features, I’m not really sure what proteinuria adds. I guess, at the end of the day, you’ve got to be a good doctor. And, you’ve got to be physically assessing your patient on a very regular basis.
Preventing the first cesarean delivery. Will cesarean rates decline?
Dr. Repke: So, speaking of guidelines, the Society for Maternal Fetal Medicine (SMFM) just came out with a document trying to address this issue of the cesarean-section rate in the United States and are there things that we can be doing to lower the primary C-section rate.2 My feeling is probably disseminated from the recognition that vaginal birth after C-section never got to the levels of acceptance that anybody hoped back when Healthy People 2010 was first written. And, we could eliminate that issue or, at least significantly reduce that issue, if the first C-section never took place.
And, I guess I’d like some of your thoughts about some of the things in that document, some of the things we need to be reconsidering in terms of how we define labor and so on.
Dr. Norwitz: It is true, I think, that there’s been an epidemic of cesarean deliveries in the last decade in the US, but also throughout the world, I think, even in countries that have traditionally had very low cesarean delivery rates, the classic one being Ireland and the UK countries. Their rates are now increasing significantly.
And there are a number of different reasons as to why this may be. I think, certainly the obesity epidemic has contributed to this. You want to deliver patients who have an elevated BMI prior to the postterm period. But, it’s often difficult to monitor these patients, and the cesarean-delivery rate overall is much higher in that population. So, that might be one reason why cesarean-delivery rates overall are going up. But, certainly there are many others.
Dr. Repke: Yes, I think you’re absolutely right: the demographics of change. Childbearing is being delayed. We know that uterine contractility dynamics alter with advanced maternal age. We’ve got a higher incidence of multiple gestations with advanced maternal age. We have more patients that require induction because of supervening medical complications of pregnancy, whether that be Class A2 gestational diabetes, or whether that be pregnancy-induced hypertension.
Redefining the stages of labor
Dr. Repke: I think some of the intriguing data, to me, is a willingness to re-look at how we define the stages of labor. And what are acceptable norms? And, while I have some concerns about how that may be interpreted in the rank and file, I think it’s at least heightened the awareness of my faculty that we just can’t tolerate the C-section at 4 cm, or whether the latent phase of labor should be allowed to go to 6 cm. I don’t think we really have the data for that. But I’d be happy if I could just start to see a reduction in “failure to progress at 4 cm.”
And then the issue of second stage, I think, is also important. What do you think about the guidelines’ recommendation that there may not really be an upper limit of allowability for second-stage labors?
Dr. Norwitz: Well, certainly I think it’s important to think back to the historical context of where the labor curves developed. The original labor curves were actually developed in Zimbabwe (at that time it was Rhodesia) by an obstetrician working in the community called Philpott. And he was trying to determine when it was appropriate to send patients into the tertiary care center. So he designed the labor curve and said if patients failed to progress over a certain number of hours, those are patients that are likely to need an operative delivery, and he would then send them into the tertiary care center.
And, then Dr. Freidman picked up on that idea and developed the Freidman Curve in Boston. But that was an era, again, many years ago when the population demographics were very different, when not many patients received regional anesthesia. I think if you look at the current guidelines, there is a huge discrepancy between women who get epidural anesthesia and those that don’t in terms of the progress of labor, both the first stage and the second stage.
Is it anesthesia?
Dr. Repke: One of the things that, you know, I haven’t looked at this paper in a long time, but you remember at Brigham and Women’s Hospital, probably 20 years ago, we were winding down the Active Management of Labor Study3 that was designed to try to replicate what had gone on at the Dublin Maternity Hospital,4 and if I’m remembering correctly, one of the remarkable things about that is that in Dublin, there were virtually no C-sections in the second stage. And so people assumed that while they were more aggressive with forceps, the operative vaginal delivery rate was no different between Brigham and Women’s Hospital and the Dublin Maternity Hospital. And so there needed to be another explanation.
And, I know I’m going to incur some of the ire of our anesthesia colleagues, but I really wonder whether there is a contribution of regional anesthesia to some of the labor dystocias that we see, and whether that’s a new demographic that we haven’t really adequately assessed. Even though I recognize some of the anesthesia literature5 seems to suggest very strongly that it has no effect. You know, if you were to plot a graph of regional anesthesia rates and cesarean section rates, they would probably parallel each other.
Dr. Norwitz: I think they do. I think we’ve long known that epidural anesthesia slows down the second stage of labor. These analyses suggest that it also has a significant effect on the first stage. And, I think that needs to be taken into account.
The lost skill of forceps delivery
Dr. Norwitz: I personally think that the skill set, in terms of operative vaginal delivery with forceps and vacuum, has really been lost. And I do feel that’s one of the factors contributing to the increase in cesarean delivery rates. I certainly see that in my practice: that I’m comfortable doing rotational forceps and mid-cavity forceps deliveries, where many of my colleagues have lost that skill, and rely now on the vacuum, which in certain circumstances is a less-than-ideal instrument. So, I believe that’s part of the reason why the cesarean delivery rates have gone up.
Lengthy second stage
Dr. Norwitz: But, certainly, I think, epidural anesthesia has made a difference, and I think we need to be cognizant of the fact that there is no “hard stop” now, in terms of the length of the second stage. If you get to 3 hours, even 4 hours, I would say, and there’s continued descent with pushing and fetal heart-rate tracing is reassuring, it’s reasonable to continue beyond those cutoffs.
Dr. Repke: I agree. I also have a concern about that, and I’m going to use a little bit of a parallel example of, you know, 7 or 8 years ago, there was a big push, and I think it was an appropriate push, to try to avoid elective deliveries prior to 39 weeks.6,7 What ended up happening was that people forgot about the term “elective,” and all they heard was 39 weeks. And what we would see on Board Examinations was, “Why do you have this placenta previa delivering at 39 weeks?”
“Well, that’s our hospital policy. We can’t deliver before 39 weeks.”
And, I think, the complications started to arise, and that’s what led to SMFM and ACOG coming out with guidelines for when it is acceptable to deliver prior to 39 weeks.2,6–8
So, the analogy is: I’m afraid that people are only going to see there is no upper limit for latent phase, there is no upper limit for second stage; that clinical judgment may not get its due in making these decisions. And we’ve all been in situations where, when you are trying to extract the head out of the pelvis, a cesarean section after a 5- or 6-hour second stage has its own set of complications. So my concern is that I hope we will recognize that we have to still use some clinical judgment, what I term the so-called “art of obstetrics,” into managing these patients.
Are you optimistic that we’re going to the lower C-section rate?
Dr. Norwitz: No, I think, it’s going to continue to go up. I think, with the increasing number of multiple pregnancies, obesity, maternal age getting further and further along, I think this is only going to continue to rise. And to be honest, I don’t know the correct cesarean delivery rate, or even if that is the metric that we should be measuring.
What is the right metric to measure neonatal outcome?
Dr. Norwitz: Maybe we should be looking at perinatal outcome. If perinatal outcome is improved, then maybe the cesarean rate is less important. Obviously, the first cesarean does have implications for subsequent pregnancy outcomes, and if we do continue to see this rise in cesarean deliveries, we are going to end up with many more placental accretas and hemorrhages in women in years to come.
So, careful counseling is important. If patients plan to have one or two kids only, maybe a cesarean delivery is very reasonable. If they are planning on having six or seven kids, then maybe you have to have a more careful discussion.
Dr. Repke: Yes, I think, that’s a very good point: the number of cesareans and the potential risks for abnormal placentation. I think societal expectations have changed in terms of what they want. Most mothers are willing to sacrifice maternal risk for presumed benefits to the fetus.
I think, where we’ve gotten into trouble as a specialty, though, is that we’ve had a hard time proving that neonatal outcome, in fact, has improved—despite an almost tripling of the cesarean section rate since, probably, the early 1970s. Although, anecdotally, what my pediatric and neonatal colleagues will tell me is they don’t get the kind of damaged babies they used to get. So the neonatologists that are closer to my age that have been doing this for a long time, they’re not seeing the really severe meconium aspiration syndromes; they’re not seeing really severe forceps-related injuries, or vacuum-related injuries that they used to see. So, those may be data that we’re going to need to accumulate with a little bit more rigor, and see if that’s true.
But I tend to agree with you. I don’t know what the right cesarean section rate is. I often tell people, I have yet to meet a patient who doesn’t think her cesarean section was indicated. And that’s where I think we hit the crossroads of individual patient-care management. So, we know across all other disciplines in medicine we’re entering the era of personalized medicine, yet we want to make broad public health policy that may not apply to individuals, and run with that. So, that’s also a concern. But, as they say, a story we will follow with interest.
Dr. Norwitz: I think so. I think the other part of that equation is the stillbirth rate, and the fact that there’s a push now to avoid elective inductions before 39 weeks, which I think is very reasonable, with a focus there again on elective inductions.
There’s also a push to induce patients before 42 weeks. And that bar has been pushed back, and in most practices around the country now, deliveries are being affected and recommended at 41 weeks. And clearly, if you take a nulliparous patient with an unfavorable cervix and induce at 41 weeks, you are going to increase the cesarean rate. I would argue that you are also decreasing the chance that there will be a stillbirth. But that data has not been forthcoming.
So this issue is by no means resolved. I think there are going to be many more years of data and studies and consensus opinions before we have a much better sense of what the right cesarean
rate is.
Dr. Repke: Yes, I think that’s a great point. And, one thing that I think people aren’t maybe that familiar with is when this push came, and again, it is an appropriate push to minimize elective deliveries before 39 weeks. When they looked at neonatal outcomes, all they looked at were the group that delivered at 37 weeks, and the group that delivered at 39 weeks. And they didn’t look at what happened with the other ones.9
So, they did look at the stillbirths of fetal distress or the other complications that happened between 37 and 1, and 38 and 6. They just looked at neonates that were born at 37 weeks and compared them to neonates that were born at 39 weeks, and found reduced instances of things like transient tachypnea of the newborn, hyperbilirubinemia, and thermoregulation issues, and those sorts of things. But, never looked at the neonates in that window, so no question 39 is better than 37, but, 37 is better than not making it to 39. So that, as you said, we’ve got a lot more information we’ve got to gather.
Errol, good talking with you.
Dr. Norwitz: Thank you.
This peer to peer discussion focuses on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines1 and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.2
In this 20-minute audiocast, listen to these experts discuss:
Changing diagnostic tools for preeclampsia
- The 24-hour urinary protein estimation: When is it necessary?
- Use of magnesium sulfate for seizure prophylaxis
Preventing the first cesarean delivery
- Redefining the stages of labor: When is the second-stage too long?
- The lost skill of forceps delivery
- Is cesarean delivery rate the optimal metric for measuring neonatal outcome?
John T. Repke, MD, is University Professor and Chairman of the Department of Obstetrics and Gynecology at Penn State University College of Medicine, and Obstetrician-Gynecologist-in-Chief at the Milton S. Hershey Medical Center in Hershey, Pennsylvania. Dr. Repke is a member of the Board of Editors of OBG Management and is author of the June 2014 Guest Editorial on hypertension and pregnancy.
Errol R. Norwitz, MD, PhD, is the Louis E. Phaneuf Professor and Chairman of the Department of Obstetrics and Gynecology at Tufts Medical Center and Tufts University School of Medicine in Boston, Massachusetts. Dr. Norwitz is a member of the Board of Editors of OBG Management and is author of the June 2014 Update on operative vaginal delivery.
The speakers report no financial relationships relevant to this audiocast.
Click here for a downloadable transcript
TRANSCRIPT
ACOG guidelines on hypertension and pregnancy raise some questions
John T. Repke, MD: So, Errol, I was impressed over the first couple of days of being at the meeting. As you know, we had a postgraduate course, and one of the items that we talked about was the new hypertension and pregnancy document that was released by the Task Force on Hypertension and Pregnancy1 charged by the American College of Obstetricians and Gynecologists. I’ve got to say that while the goal of the document was to provide some standardization and clarification, there still seems to be a lot of confusion in my audience about how to interpret some of the guidelines. Have you found that?
Errol R. Norwitz, MD, PhD: Yes, I have. I found it interesting that it was put out as an executive summary, and not as a practice bulletin, which will probably follow in months. That document, which came out in November 2013, helped to address many of the issues we’ve had over the years of preeclampsia, in terms of its definition and some of the management issues. But, it also raised a number of questions that still need to be resolved.
Dr. Repke: Yes. I think one of the things to keep in mind, and I’ve tabulated all of the recommendations, is that about 60 recommendations came out of that document and only six of the 60 were accompanied by a strong quality of evidence, or rather, a high quality of evidence, and a strong recommendation. And a lot of those things were addressing issues that I think most practitioners already did, in so far as using antenatal steroids for maturation; using magnesium sulfate for patients with preeclampsia with severe features; and using magnesium sulfate as a treatment of eclampsia. But a lot of the other recommendations really were based on either moderate- or low-quality evidence, and had qualified recommendations. And, I think that’s what has led to some of the confusion.
Changing diagnostic tools for preeclampsia
Dr. Repke: What sort of specific things are your practitioners asking you about as far as, “Is this gestational hypertension or is this preeclampsia?” The guidelines say proteinuria is not required anymore. How are you dealing with that?
Should we still do the 24-hour urinary protein estimation?
Dr. Norwitz: The biggest change, in my mind, is the statement that you no longer require significant proteinuria to make the diagnosis of preeclampsia, and, indeed, of severe preeclampsia. So, if you do have significant proteinuria, then that would confirm the diagnosis. But, you can also have preeclampsia in the presence of other endorgan injuries, such as kidney injury and liver injury in the absence of significant proteinuria.
So, one of the questions that comes up is, “Should we actually do the 24-hour urinary protein estimation?”
And, my answer is, “yes.” If you have significant proteinuria, then that would confirm the diagnosis. If you don’t, you can still make the diagnosis in the setting of low platelets, elevated liver enzymes, or abnormal renal function. So, the issue is, and I’d be curious to hear your answer, if you have someone with platelets of, let’s say, 78, a new onset of sustained elevation of blood pressure, would you do the 24-hour urine estimation or just defer it?
Dr. Repke: We wouldn’t perform the 24-hour urine test under those circumstances. And, we would consider that nuance of hypertension with a severe feature that is now preeclampsia with severe feature, and the management would be based on gestational age. With a platelet count that low, the management would be stabilization and delivery. Although, if stabilized, I think that’s the type of patient that potentially could have delivery delayed until you could get an effective antenatal steroid if she was less that 34 weeks’ gestation.
Dr. Norwitz: So, that’s one issue I think needs to be clarified. If there’s other evidence of endorgan damage, then you can defer the 24-hour urinary protein. That’s another question that comes up. I’m pleased they could resolve the issue of repeated 24-hour urinary estimations. Once you have your 300 mg suggestive of the diagnosis of preeclampsia, there’s no reason to then repeat it looking for elevation and increased leakage of protein into the urine, because it doesn’t correlate with adverse outcome for the mother or fetus. So, that issue was clarified.
Dr. Repke: I think that two questions that came up in our course, and I think they were very legitimate, are, “Do we even need to do urine protein at all?” Because if you look at the guidelines for management, the only difference between preeclampsia management without severe features and gestational hypertension is frequency of antenatal testing until you decide to begin delivery. Now, in the old days, one would say, “Well, another difference would be that the preeclamptic would get magnesium sulfate.” But the current Hypertension in Pregnancy Guidelines1 suggest that preeclampsia without severe features doesn’t necessarily have to be managed with magnesium sulfate. So, I’m still wrestling with whether, other than the fact that it might be for study purposes or for categorization or research, whether proteinuria adds anything to the equation.
And, then the second question is, “How do you resolve the issue of disagreement?” So, the example is protein:creatinine ratio allows for a more rapid diagnosis of significant proteinuria. If that patient doesn’t have to deliver immediately and a 24-hour urine sample is obtained, which do you believe if you have a protein:creatinine ratio greater than 0.3, but now your 24-hour urine is 212 mg/dL? And, I don’t have the answer to that, but that’s another area of confusion.
Dr. Norwitz: And, I think that confusion will persist. I don’t think this document is going to resolve it.
New terminology: Preeclampsia with or without severe features
Dr. Norwitz: I do like the difference in terminology between preeclampsia with severe features and preeclampsia without severe features. I think the old terminology of severe and mild preeclampsia was somewhat confusing. I certainly appreciate that alteration in terminology, although it may take a while for it to catch on. I’m still seeing the term “mild preeclampsia” used quite widely.
Use of magnesium sulfate for seizure prophylaxis
Dr. Norwitz: You did raise the issue of magnesium sulfate for seizure prophylaxis in the setting of severe preeclampsia without severe features. And I was struck by the statement. Not only is it not necessary to give it, but in the Executive Summary, as you suggest, it is not indicated and you recommended against starting it. Is that how you interpret it as the well?
Dr. Repke: Well, I might have interpreted the statement the way I wanted to interpret it. And, as you know, in our institution, because we feel we are a teaching program, people can progress very quickly intrapartum from not having severe features to having severe features, and we don’t want to miss that window of opportunity. Our practice in that regard does not follow the guidelines. We use intrapartum magnesium prophylaxis for all patients with the diagnosis of preeclampsia, and continue it for 24 hours postpartum.
Dr. Norwitz: And I would have to say we decided do the same. So, once a diagnosis of preeclampsia is made, we would give intrapartum, and then postpartum magnesium seizure prophylaxis for 24 hours, regardless of whether there’s evidence of severe features or no severe features.
Dr. Repke: And there again, I think it’s why, for you and I, it will still be important to assess the proteinuria because that diagnostic difference between preeclampsia and gestational hypertension is going to alter management. But if you follow the document word for word, if you’re not going to use magnesium without severe features, I’m not really sure what proteinuria adds. I guess, at the end of the day, you’ve got to be a good doctor. And, you’ve got to be physically assessing your patient on a very regular basis.
Preventing the first cesarean delivery. Will cesarean rates decline?
Dr. Repke: So, speaking of guidelines, the Society for Maternal Fetal Medicine (SMFM) just came out with a document trying to address this issue of the cesarean-section rate in the United States and are there things that we can be doing to lower the primary C-section rate.2 My feeling is probably disseminated from the recognition that vaginal birth after C-section never got to the levels of acceptance that anybody hoped back when Healthy People 2010 was first written. And, we could eliminate that issue or, at least significantly reduce that issue, if the first C-section never took place.
And, I guess I’d like some of your thoughts about some of the things in that document, some of the things we need to be reconsidering in terms of how we define labor and so on.
Dr. Norwitz: It is true, I think, that there’s been an epidemic of cesarean deliveries in the last decade in the US, but also throughout the world, I think, even in countries that have traditionally had very low cesarean delivery rates, the classic one being Ireland and the UK countries. Their rates are now increasing significantly.
And there are a number of different reasons as to why this may be. I think, certainly the obesity epidemic has contributed to this. You want to deliver patients who have an elevated BMI prior to the postterm period. But, it’s often difficult to monitor these patients, and the cesarean-delivery rate overall is much higher in that population. So, that might be one reason why cesarean-delivery rates overall are going up. But, certainly there are many others.
Dr. Repke: Yes, I think you’re absolutely right: the demographics of change. Childbearing is being delayed. We know that uterine contractility dynamics alter with advanced maternal age. We’ve got a higher incidence of multiple gestations with advanced maternal age. We have more patients that require induction because of supervening medical complications of pregnancy, whether that be Class A2 gestational diabetes, or whether that be pregnancy-induced hypertension.
Redefining the stages of labor
Dr. Repke: I think some of the intriguing data, to me, is a willingness to re-look at how we define the stages of labor. And what are acceptable norms? And, while I have some concerns about how that may be interpreted in the rank and file, I think it’s at least heightened the awareness of my faculty that we just can’t tolerate the C-section at 4 cm, or whether the latent phase of labor should be allowed to go to 6 cm. I don’t think we really have the data for that. But I’d be happy if I could just start to see a reduction in “failure to progress at 4 cm.”
And then the issue of second stage, I think, is also important. What do you think about the guidelines’ recommendation that there may not really be an upper limit of allowability for second-stage labors?
Dr. Norwitz: Well, certainly I think it’s important to think back to the historical context of where the labor curves developed. The original labor curves were actually developed in Zimbabwe (at that time it was Rhodesia) by an obstetrician working in the community called Philpott. And he was trying to determine when it was appropriate to send patients into the tertiary care center. So he designed the labor curve and said if patients failed to progress over a certain number of hours, those are patients that are likely to need an operative delivery, and he would then send them into the tertiary care center.
And, then Dr. Freidman picked up on that idea and developed the Freidman Curve in Boston. But that was an era, again, many years ago when the population demographics were very different, when not many patients received regional anesthesia. I think if you look at the current guidelines, there is a huge discrepancy between women who get epidural anesthesia and those that don’t in terms of the progress of labor, both the first stage and the second stage.
Is it anesthesia?
Dr. Repke: One of the things that, you know, I haven’t looked at this paper in a long time, but you remember at Brigham and Women’s Hospital, probably 20 years ago, we were winding down the Active Management of Labor Study3 that was designed to try to replicate what had gone on at the Dublin Maternity Hospital,4 and if I’m remembering correctly, one of the remarkable things about that is that in Dublin, there were virtually no C-sections in the second stage. And so people assumed that while they were more aggressive with forceps, the operative vaginal delivery rate was no different between Brigham and Women’s Hospital and the Dublin Maternity Hospital. And so there needed to be another explanation.
And, I know I’m going to incur some of the ire of our anesthesia colleagues, but I really wonder whether there is a contribution of regional anesthesia to some of the labor dystocias that we see, and whether that’s a new demographic that we haven’t really adequately assessed. Even though I recognize some of the anesthesia literature5 seems to suggest very strongly that it has no effect. You know, if you were to plot a graph of regional anesthesia rates and cesarean section rates, they would probably parallel each other.
Dr. Norwitz: I think they do. I think we’ve long known that epidural anesthesia slows down the second stage of labor. These analyses suggest that it also has a significant effect on the first stage. And, I think that needs to be taken into account.
The lost skill of forceps delivery
Dr. Norwitz: I personally think that the skill set, in terms of operative vaginal delivery with forceps and vacuum, has really been lost. And I do feel that’s one of the factors contributing to the increase in cesarean delivery rates. I certainly see that in my practice: that I’m comfortable doing rotational forceps and mid-cavity forceps deliveries, where many of my colleagues have lost that skill, and rely now on the vacuum, which in certain circumstances is a less-than-ideal instrument. So, I believe that’s part of the reason why the cesarean delivery rates have gone up.
Lengthy second stage
Dr. Norwitz: But, certainly, I think, epidural anesthesia has made a difference, and I think we need to be cognizant of the fact that there is no “hard stop” now, in terms of the length of the second stage. If you get to 3 hours, even 4 hours, I would say, and there’s continued descent with pushing and fetal heart-rate tracing is reassuring, it’s reasonable to continue beyond those cutoffs.
Dr. Repke: I agree. I also have a concern about that, and I’m going to use a little bit of a parallel example of, you know, 7 or 8 years ago, there was a big push, and I think it was an appropriate push, to try to avoid elective deliveries prior to 39 weeks.6,7 What ended up happening was that people forgot about the term “elective,” and all they heard was 39 weeks. And what we would see on Board Examinations was, “Why do you have this placenta previa delivering at 39 weeks?”
“Well, that’s our hospital policy. We can’t deliver before 39 weeks.”
And, I think, the complications started to arise, and that’s what led to SMFM and ACOG coming out with guidelines for when it is acceptable to deliver prior to 39 weeks.2,6–8
So, the analogy is: I’m afraid that people are only going to see there is no upper limit for latent phase, there is no upper limit for second stage; that clinical judgment may not get its due in making these decisions. And we’ve all been in situations where, when you are trying to extract the head out of the pelvis, a cesarean section after a 5- or 6-hour second stage has its own set of complications. So my concern is that I hope we will recognize that we have to still use some clinical judgment, what I term the so-called “art of obstetrics,” into managing these patients.
Are you optimistic that we’re going to the lower C-section rate?
Dr. Norwitz: No, I think, it’s going to continue to go up. I think, with the increasing number of multiple pregnancies, obesity, maternal age getting further and further along, I think this is only going to continue to rise. And to be honest, I don’t know the correct cesarean delivery rate, or even if that is the metric that we should be measuring.
What is the right metric to measure neonatal outcome?
Dr. Norwitz: Maybe we should be looking at perinatal outcome. If perinatal outcome is improved, then maybe the cesarean rate is less important. Obviously, the first cesarean does have implications for subsequent pregnancy outcomes, and if we do continue to see this rise in cesarean deliveries, we are going to end up with many more placental accretas and hemorrhages in women in years to come.
So, careful counseling is important. If patients plan to have one or two kids only, maybe a cesarean delivery is very reasonable. If they are planning on having six or seven kids, then maybe you have to have a more careful discussion.
Dr. Repke: Yes, I think, that’s a very good point: the number of cesareans and the potential risks for abnormal placentation. I think societal expectations have changed in terms of what they want. Most mothers are willing to sacrifice maternal risk for presumed benefits to the fetus.
I think, where we’ve gotten into trouble as a specialty, though, is that we’ve had a hard time proving that neonatal outcome, in fact, has improved—despite an almost tripling of the cesarean section rate since, probably, the early 1970s. Although, anecdotally, what my pediatric and neonatal colleagues will tell me is they don’t get the kind of damaged babies they used to get. So the neonatologists that are closer to my age that have been doing this for a long time, they’re not seeing the really severe meconium aspiration syndromes; they’re not seeing really severe forceps-related injuries, or vacuum-related injuries that they used to see. So, those may be data that we’re going to need to accumulate with a little bit more rigor, and see if that’s true.
But I tend to agree with you. I don’t know what the right cesarean section rate is. I often tell people, I have yet to meet a patient who doesn’t think her cesarean section was indicated. And that’s where I think we hit the crossroads of individual patient-care management. So, we know across all other disciplines in medicine we’re entering the era of personalized medicine, yet we want to make broad public health policy that may not apply to individuals, and run with that. So, that’s also a concern. But, as they say, a story we will follow with interest.
Dr. Norwitz: I think so. I think the other part of that equation is the stillbirth rate, and the fact that there’s a push now to avoid elective inductions before 39 weeks, which I think is very reasonable, with a focus there again on elective inductions.
There’s also a push to induce patients before 42 weeks. And that bar has been pushed back, and in most practices around the country now, deliveries are being affected and recommended at 41 weeks. And clearly, if you take a nulliparous patient with an unfavorable cervix and induce at 41 weeks, you are going to increase the cesarean rate. I would argue that you are also decreasing the chance that there will be a stillbirth. But that data has not been forthcoming.
So this issue is by no means resolved. I think there are going to be many more years of data and studies and consensus opinions before we have a much better sense of what the right cesarean
rate is.
Dr. Repke: Yes, I think that’s a great point. And, one thing that I think people aren’t maybe that familiar with is when this push came, and again, it is an appropriate push to minimize elective deliveries before 39 weeks. When they looked at neonatal outcomes, all they looked at were the group that delivered at 37 weeks, and the group that delivered at 39 weeks. And they didn’t look at what happened with the other ones.9
So, they did look at the stillbirths of fetal distress or the other complications that happened between 37 and 1, and 38 and 6. They just looked at neonates that were born at 37 weeks and compared them to neonates that were born at 39 weeks, and found reduced instances of things like transient tachypnea of the newborn, hyperbilirubinemia, and thermoregulation issues, and those sorts of things. But, never looked at the neonates in that window, so no question 39 is better than 37, but, 37 is better than not making it to 39. So that, as you said, we’ve got a lot more information we’ve got to gather.
Errol, good talking with you.
Dr. Norwitz: Thank you.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus: Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193.
- Frigoletto FD, Lieberman E, Lang J, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–750.
- O’Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd ed. London: Mosby- Yearbook; 1993.
- Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia effect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80(6):1201–1208.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 561: Nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911–915.
- Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–333.
- Tita ATN, Landon MB, Spong CY, et al; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat Cesarean Delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Caughey AB, Cahill AG, Guise JM, Rouse DJ; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric Care Consensus: Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–193.
- Frigoletto FD, Lieberman E, Lang J, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–750.
- O’Driscoll K, Meagher D, Boylan P. Active Management of Labor. 3rd ed. London: Mosby- Yearbook; 1993.
- Chestnut DH, McGrath JM, Vincent RD, et al. Does early administration of epidural analgesia effect obstetric outcome in nulliparous women who are in spontaneous labor? Anesthesiology. 1994;80(6):1201–1208.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 560: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121(4):908–910.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 561: Nonmedically indicated early-term deliveries. Obstet Gynecol. 2013;121(4):911–915.
- Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118(2 Pt 1):323–333.
- Tita ATN, Landon MB, Spong CY, et al; Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat Cesarean Delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111–120.