User login
Most FGR patients deliver vaginally after induction of labor
CHICAGO – Induction of labor is reasonable in cases involving fetal growth restriction, as most patients who are induced deliver vaginally rather than by cesarean section, according to findings from a retrospective cohort study.
Of 134 patients who underwent induction of labor for fetal growth restriction (FGR), 81% delivered vaginally, Dr. Kari Horowitz reported in a poster at the annual meeting of the American Congress of Obstetricians and Gynecologists.
In those who delivered by C-section, the indication was nonreassuring fetal heart rate in 88% of cases, according to Dr. Horowitz, of the University of Connecticut, Farmington.
The cesarean delivery rates were highest in cases involving nulliparity, prematurity, hypertension, oligohydramnios, and use of prostaglandins. Logistic regression analysis showed that only prematurity was significantly associated with cesarean delivery (odds ratio, 3.81).
The findings are based on a chart review of all patients with singleton pregnancy, a non-anomalous fetus, and suspected FGR (estimated fetal weight and/or fetal abdominal circumference less than the 10th percentile, or no interval growth) who delivered between January 2008 and December 2012. Patients were excluded from the study if there was multiple gestation; fetal anomalies or aneuploidy; malpresentation; a history of prior cesarean delivery; or other contraindications to vaginal delivery.
Although FGR is associated with neonatal risks, including intrauterine demise, neonatal morbidity and mortality, and postnatal morbidity, it is not considered an indication for cesarean delivery. Affected fetuses, however, may be at risk for nonreassuring fetal heart rate tracing and fetal distress due to uteroplacental insufficiency. Delivery at 38 to 39 6/7 weeks is recommended in cases of isolated FGR, and delivery before 38 weeks is recommended in cases with additional risk factors for adverse outcomes.
"Prior studies have found increased risk of cesarean delivery in FGR neonates undergoing induction of labor as compared to spontaneous labor, and no difference in rates of cesarean delivery or adverse outcomes in patients undergoing induction of labor versus expectant management. Term FGR fetuses have been found to have significantly higher rates of cesarean delivery for nonreassuring fetal heart rate tracing," Dr. Horowitz noted.
"It is recommended that these patients undergo a trial of labor," Dr. Horowitz concluded, noting that preterm patients should be counseled about the increased risk of cesarean delivery for nonreassuring fetal heart tracing.
Dr. Horowitz reported having no disclosures.
CHICAGO – Induction of labor is reasonable in cases involving fetal growth restriction, as most patients who are induced deliver vaginally rather than by cesarean section, according to findings from a retrospective cohort study.
Of 134 patients who underwent induction of labor for fetal growth restriction (FGR), 81% delivered vaginally, Dr. Kari Horowitz reported in a poster at the annual meeting of the American Congress of Obstetricians and Gynecologists.
In those who delivered by C-section, the indication was nonreassuring fetal heart rate in 88% of cases, according to Dr. Horowitz, of the University of Connecticut, Farmington.
The cesarean delivery rates were highest in cases involving nulliparity, prematurity, hypertension, oligohydramnios, and use of prostaglandins. Logistic regression analysis showed that only prematurity was significantly associated with cesarean delivery (odds ratio, 3.81).
The findings are based on a chart review of all patients with singleton pregnancy, a non-anomalous fetus, and suspected FGR (estimated fetal weight and/or fetal abdominal circumference less than the 10th percentile, or no interval growth) who delivered between January 2008 and December 2012. Patients were excluded from the study if there was multiple gestation; fetal anomalies or aneuploidy; malpresentation; a history of prior cesarean delivery; or other contraindications to vaginal delivery.
Although FGR is associated with neonatal risks, including intrauterine demise, neonatal morbidity and mortality, and postnatal morbidity, it is not considered an indication for cesarean delivery. Affected fetuses, however, may be at risk for nonreassuring fetal heart rate tracing and fetal distress due to uteroplacental insufficiency. Delivery at 38 to 39 6/7 weeks is recommended in cases of isolated FGR, and delivery before 38 weeks is recommended in cases with additional risk factors for adverse outcomes.
"Prior studies have found increased risk of cesarean delivery in FGR neonates undergoing induction of labor as compared to spontaneous labor, and no difference in rates of cesarean delivery or adverse outcomes in patients undergoing induction of labor versus expectant management. Term FGR fetuses have been found to have significantly higher rates of cesarean delivery for nonreassuring fetal heart rate tracing," Dr. Horowitz noted.
"It is recommended that these patients undergo a trial of labor," Dr. Horowitz concluded, noting that preterm patients should be counseled about the increased risk of cesarean delivery for nonreassuring fetal heart tracing.
Dr. Horowitz reported having no disclosures.
CHICAGO – Induction of labor is reasonable in cases involving fetal growth restriction, as most patients who are induced deliver vaginally rather than by cesarean section, according to findings from a retrospective cohort study.
Of 134 patients who underwent induction of labor for fetal growth restriction (FGR), 81% delivered vaginally, Dr. Kari Horowitz reported in a poster at the annual meeting of the American Congress of Obstetricians and Gynecologists.
In those who delivered by C-section, the indication was nonreassuring fetal heart rate in 88% of cases, according to Dr. Horowitz, of the University of Connecticut, Farmington.
The cesarean delivery rates were highest in cases involving nulliparity, prematurity, hypertension, oligohydramnios, and use of prostaglandins. Logistic regression analysis showed that only prematurity was significantly associated with cesarean delivery (odds ratio, 3.81).
The findings are based on a chart review of all patients with singleton pregnancy, a non-anomalous fetus, and suspected FGR (estimated fetal weight and/or fetal abdominal circumference less than the 10th percentile, or no interval growth) who delivered between January 2008 and December 2012. Patients were excluded from the study if there was multiple gestation; fetal anomalies or aneuploidy; malpresentation; a history of prior cesarean delivery; or other contraindications to vaginal delivery.
Although FGR is associated with neonatal risks, including intrauterine demise, neonatal morbidity and mortality, and postnatal morbidity, it is not considered an indication for cesarean delivery. Affected fetuses, however, may be at risk for nonreassuring fetal heart rate tracing and fetal distress due to uteroplacental insufficiency. Delivery at 38 to 39 6/7 weeks is recommended in cases of isolated FGR, and delivery before 38 weeks is recommended in cases with additional risk factors for adverse outcomes.
"Prior studies have found increased risk of cesarean delivery in FGR neonates undergoing induction of labor as compared to spontaneous labor, and no difference in rates of cesarean delivery or adverse outcomes in patients undergoing induction of labor versus expectant management. Term FGR fetuses have been found to have significantly higher rates of cesarean delivery for nonreassuring fetal heart rate tracing," Dr. Horowitz noted.
"It is recommended that these patients undergo a trial of labor," Dr. Horowitz concluded, noting that preterm patients should be counseled about the increased risk of cesarean delivery for nonreassuring fetal heart tracing.
Dr. Horowitz reported having no disclosures.
AT THE ACOG ANNUAL CLINICAL MEETING
A brief scale IDs sleep-disordered breathing in pregnancy
CHICAGO – A four-item pregnancy-specific sleep disturbance scale proved valid as a screening tool for sleep-disordered breathing in pregnancy, and was associated with preeclampsia in a study of more than 1,100 women.
After adjustment for potential confounders – including sociodemographics, body mass index, and high blood pressure – a higher score on the short-form pregnancy-specific questionnaire (SF-SPQ) was significantly associated with an increase in the risk of preeclampsia (adjusted risk ratio, 1.54) Alpna Agrawal, Ph.D., of the University of Texas Health Science Center, Houston, reported in a poster at the annual meeting of the American Congress of Obstetricians and Gynecologists.
"In a clinical setting, the short format and validity of SF-SPQ shown in this study suggests it may be a quick and effective method to screen women at risk for sleep-disordered breathing," Dr. Agrawal wrote.
The SF-SPQ was developed based on data collected from 1,153 pregnant women seen in three outpatient clinics between 2010 and 2013. Sleep patterns were assessed by the Berlin Questionnaire, Epworth Sleepiness Scale, and by questions regarding napping behavior. A confirmatory factor analysis (CFA) was performed to develop a sensitive and specific sleep scale derived from the findings. The scale ultimately included "snoring frequently," "bothersome snoring," "stopped breathing while sleeping," and "falling asleep while driving."
"These items were conceptually related to sleep-disordered breathing during pregnancy, statistically intercorrelated, and/or associated with adverse outcomes. CFA factor loadings were significant and model fit was good," Dr. Agrawal wrote.
In addition to higher score on the SF-SPQ, adjusted relative risks of adverse outcomes were associated with BMI greater than 30 (adjusted relative risk, 1.55), hypertension (adjusted RR, 5.07), and screening positive on the Berlin Questionnaire (adjusted RR, 2.45).
"These data suggest that comorbid conditions such as obesity and hypertension (which are themselves a part of the Berlin Questionnaire) drive association with pregnancy outcomes. However, preeclampsia was independently associated with the SF-SPQ," Dr. Agrawal noted.
Prior studies have demonstrated that sleep-disordered breathing during pregnancy is associated with adverse pregnancy outcomes, but an efficient and efficacious screening tool for sleep disorders has been lacking.
The findings are important, because in the United States, preeclampsia affects up to 6% of pregnancies and is linked with other morbidities such as intrauterine growth restriction, and because studies suggest that the use of continuous positive airway pressure (CPAP) can reduce the risk of preeclampsia in pregnant patients with sleep-disordered breathing.
Additional research is needed to evaluate the efficacy of the SF-SPQ for detecting women at risk, Dr. Agrawal concluded.
Dr. Agrawal reported having no disclosures.
CHICAGO – A four-item pregnancy-specific sleep disturbance scale proved valid as a screening tool for sleep-disordered breathing in pregnancy, and was associated with preeclampsia in a study of more than 1,100 women.
After adjustment for potential confounders – including sociodemographics, body mass index, and high blood pressure – a higher score on the short-form pregnancy-specific questionnaire (SF-SPQ) was significantly associated with an increase in the risk of preeclampsia (adjusted risk ratio, 1.54) Alpna Agrawal, Ph.D., of the University of Texas Health Science Center, Houston, reported in a poster at the annual meeting of the American Congress of Obstetricians and Gynecologists.
"In a clinical setting, the short format and validity of SF-SPQ shown in this study suggests it may be a quick and effective method to screen women at risk for sleep-disordered breathing," Dr. Agrawal wrote.
The SF-SPQ was developed based on data collected from 1,153 pregnant women seen in three outpatient clinics between 2010 and 2013. Sleep patterns were assessed by the Berlin Questionnaire, Epworth Sleepiness Scale, and by questions regarding napping behavior. A confirmatory factor analysis (CFA) was performed to develop a sensitive and specific sleep scale derived from the findings. The scale ultimately included "snoring frequently," "bothersome snoring," "stopped breathing while sleeping," and "falling asleep while driving."
"These items were conceptually related to sleep-disordered breathing during pregnancy, statistically intercorrelated, and/or associated with adverse outcomes. CFA factor loadings were significant and model fit was good," Dr. Agrawal wrote.
In addition to higher score on the SF-SPQ, adjusted relative risks of adverse outcomes were associated with BMI greater than 30 (adjusted relative risk, 1.55), hypertension (adjusted RR, 5.07), and screening positive on the Berlin Questionnaire (adjusted RR, 2.45).
"These data suggest that comorbid conditions such as obesity and hypertension (which are themselves a part of the Berlin Questionnaire) drive association with pregnancy outcomes. However, preeclampsia was independently associated with the SF-SPQ," Dr. Agrawal noted.
Prior studies have demonstrated that sleep-disordered breathing during pregnancy is associated with adverse pregnancy outcomes, but an efficient and efficacious screening tool for sleep disorders has been lacking.
The findings are important, because in the United States, preeclampsia affects up to 6% of pregnancies and is linked with other morbidities such as intrauterine growth restriction, and because studies suggest that the use of continuous positive airway pressure (CPAP) can reduce the risk of preeclampsia in pregnant patients with sleep-disordered breathing.
Additional research is needed to evaluate the efficacy of the SF-SPQ for detecting women at risk, Dr. Agrawal concluded.
Dr. Agrawal reported having no disclosures.
CHICAGO – A four-item pregnancy-specific sleep disturbance scale proved valid as a screening tool for sleep-disordered breathing in pregnancy, and was associated with preeclampsia in a study of more than 1,100 women.
After adjustment for potential confounders – including sociodemographics, body mass index, and high blood pressure – a higher score on the short-form pregnancy-specific questionnaire (SF-SPQ) was significantly associated with an increase in the risk of preeclampsia (adjusted risk ratio, 1.54) Alpna Agrawal, Ph.D., of the University of Texas Health Science Center, Houston, reported in a poster at the annual meeting of the American Congress of Obstetricians and Gynecologists.
"In a clinical setting, the short format and validity of SF-SPQ shown in this study suggests it may be a quick and effective method to screen women at risk for sleep-disordered breathing," Dr. Agrawal wrote.
The SF-SPQ was developed based on data collected from 1,153 pregnant women seen in three outpatient clinics between 2010 and 2013. Sleep patterns were assessed by the Berlin Questionnaire, Epworth Sleepiness Scale, and by questions regarding napping behavior. A confirmatory factor analysis (CFA) was performed to develop a sensitive and specific sleep scale derived from the findings. The scale ultimately included "snoring frequently," "bothersome snoring," "stopped breathing while sleeping," and "falling asleep while driving."
"These items were conceptually related to sleep-disordered breathing during pregnancy, statistically intercorrelated, and/or associated with adverse outcomes. CFA factor loadings were significant and model fit was good," Dr. Agrawal wrote.
In addition to higher score on the SF-SPQ, adjusted relative risks of adverse outcomes were associated with BMI greater than 30 (adjusted relative risk, 1.55), hypertension (adjusted RR, 5.07), and screening positive on the Berlin Questionnaire (adjusted RR, 2.45).
"These data suggest that comorbid conditions such as obesity and hypertension (which are themselves a part of the Berlin Questionnaire) drive association with pregnancy outcomes. However, preeclampsia was independently associated with the SF-SPQ," Dr. Agrawal noted.
Prior studies have demonstrated that sleep-disordered breathing during pregnancy is associated with adverse pregnancy outcomes, but an efficient and efficacious screening tool for sleep disorders has been lacking.
The findings are important, because in the United States, preeclampsia affects up to 6% of pregnancies and is linked with other morbidities such as intrauterine growth restriction, and because studies suggest that the use of continuous positive airway pressure (CPAP) can reduce the risk of preeclampsia in pregnant patients with sleep-disordered breathing.
Additional research is needed to evaluate the efficacy of the SF-SPQ for detecting women at risk, Dr. Agrawal concluded.
Dr. Agrawal reported having no disclosures.
AT THE ACOG ANNUAL CLINICAL MEETING
Key clinical point: A short pregnancy-specific sleep disturbance scale may be useful as a screening tool for sleep-disordered breathing in pregnancy, which is associated with preeclampsia.
Major finding: Higher SF-SPQ score was significantly associated with an increase in the risk of preeclampsia (adjusted risk ratio, 1.54).
Data source: Confirmatory factor analysis of data from 1,153 pregnant women.
Disclosures: Dr. Agrawal reported having no disclosures
Jury Finds Fault With Midwife’s Care
During her pregnancy, a Wisconsin woman received care from a nurse-midwife. In late July 2001, misoprostol was administered to induce labor. The woman was admitted to the hospital in active labor at approximately 2 pm. She was 6 cm dilated. Dilation arrested three times, and then there was a two-hour failure to dilate.
The nurse-midwife then tried putting the mother in a water-birthing tub to stimulate contractions, without success. Oxytocin was then administered. Immediately thereafter, the patient developed uterine hyperstimulation, and the fetal heart rate strip showed late decelerations, indicative of fetal distress.
Despite these abnormalities, the nurse-midwife continued increasing oxytocin throughout labor, which continued for about 12 hours. The oxytocin dose exceeded the hospital’s recommended protocols. The patient reached a point at which she was having strong contractions every 1.5 min. Full dilation was not reached until 10:38 pm.
Around midnight, the electronic fetal monitor allegedly showed an abnormal heart pattern, with decelerations with almost every contraction. The mother was allowed to continue with labor, and the fetal monitoring equipment was removed at 1:26 am so the mother could be placed in the water-birthing tub again. Nurses took the fetal heart rate during this time and recorded it as normal.
When the child was delivered at approximately 2 am, she had a heart rate of 80 beats/min; she was apneic, cyanotic, and virtually lifeless. Apgar scores were recorded as 1 at one minute and 3 at five minutes. Arterial blood gas/pH was 7.165.
An attending physician was called and arrived within 20 min. The infant was resuscitated, intubated, and transferred to another hospital. A CT scan taken at 56 hours of life was read as normal. An MRl at nine months was also read as normal.
The child was subsequently diagnosed as having cerebral palsy. She requires a walker for ambulation and has arm and leg impairments and significant cognitive deficits, necessitating 24-hour assistance.
The plaintiff claimed that if oxytocin had been discontinued, she would have reached full dilation hours earlier than she did. She also claimed that a cesarean delivery or operative vaginal delivery should have been performed when the fetal monitor indicated an abnormal heart pattern. (She contended that the “normal” heart rate recorded around this time was actually the maternal heart rate.)
The parties did not dispute that the infant had experienced a hypoxic/ischemic event but disagreed on when it occurred. The plaintiff claimed that the ischemic event occurred during delivery. The defendants claimed that it occurred in utero prior to delivery. The plaintiff also disputed the MRI findings, which the plaintiff argued showed significant brain injury.
On the next page: Outcome >>
OUTCOME
A jury found the nurse-midwife 80% at fault and the hospital 20% at fault. The jury awarded $13.5 million to the child and $100,000 to the plaintiff. An additional $110,000 in past medical expenses was added to the verdict.
COMMENT
Obstetrics/midwifery accounts for a significant percentage of malpractice cases filed and monetary damages awarded. In this case, a substantial $13.5 million verdict was awarded, with 80% of the verdict against the midwife for inappropriate use of oxytocin and failure to refer for cesarean delivery.
A detailed discussion of obstetric management is beyond the scope of this article (in part because we don’t have access to much data, including fetal heart rate tracings). However, there are a few points to consider.
First, consider surgical options when appropriate. Without doubt, operative delivery by cesarean section is overused for all the wrong reasons. Some mothers, families, and clinicians strongly desire a more natural childbirth and strive to create such an experience—forgoing traditional medications and anesthesia. Often, this is perfectly safe, reasonable, and preferable.
However, if the perinatal course is rocky, it is wise to monitor closely and adopt a collaborative approach. When prenatal screening suggests a difficult delivery, exercise caution and have a fallback plan. Known high-risk deliveries should have a team approach from the outset, with all assets available to bedside on short notice.
While operative delivery is overused, it shouldn’t be demonized either. When genuinely needed, it can be lifesaving. Some patients do not want a cesarean delivery, and both the patient and the clinician may equate operative delivery with personal failure. However, that view may present a barrier to calling for consultation when it is genuinely needed.
For natural childbirth enthusiasts, think of the surgical delivery option as sealed in a glass case. Break that glass for all the right reasons: to preserve life or avoid significant fetal morbidity. Discuss the indications for surgical management ahead of time, so the mother is not surprised by a sudden rush to the operating room, feeling frightened and out of control.
It is recognized that patients and clinicians have firmly held beliefs, and opinions are strong on this subject. Patients have a right to self-determination and to select the birthing experience that will suit them best. Yet there is tension because jurors will expect a clinician to fully communicate known risks to patients and use all available resources to safeguard the mother and fetus at all times. In this case, the jury concluded that the midwife failed to refer for cesarean delivery after about 10 hours of labor, when the fetal heart rate pattern was nonreassuring. One of the plaintiff’s expert witnesses who criticized the defendant midwife’s care was herself a highly regarded midwife.
Continued on the next page >>
Second, use oxytocin carefully, slowing or stopping it when required. While we do not have access to the fetal heart rate monitor strips in this case, we do know that the plaintiff met her burden of proof and persuaded the jurors that the midwife inappropriately increased the drug in the setting of uterine hyperstimulation, with evidence of fetal distress. It seems surprising that the allegedly “normal” pattern recorded at 1:26 am could have been the maternal heart rate—but apparently, the jurors were convinced of this.
Third, when a facility has a medication protocol, follow it unless there is good cause not to. Medication protocols can be useful to establish operating guidelines and reduce medication errors. But they can also shackle clinicians by substituting tables and algorithms for clinical judgment. Problems arise when a protocol is sidestepped, and the clinician is raked over the coals for failing to adhere. If your facility has protocols that are important to your practice, read the documentation. Learn it, know it, live it.
If you operate outside a protocol, and your case goes to trial, the expert witness defending your care will be forced to take on both the plaintiff’s allegations and your own facility’s recommendations. The plaintiff’s closing argument will include a variation of “Mr. A did not even bother to follow his hospital’s own rules.” This argument is easy for jurors to understand, and many will reach a finding of negligence based on this fact alone. If you disagree with the protocol, or it is not reflective of your actual practice, either clinician practice or the protocol must be changed. Do not routinely circumvent protocols without good reason.
Ideally, protocols should be constructed to give clinicians flexibility based on clinical judgment and patient response. If you have a role in forming a protocol, consider advocating for less rigidity and allowing for professional judgment. If the protocol is rigid, be sure that everyone understands it and that it can be strictly followed in a real world practice environment. Put plainly, don’t install a set of rules you can’t live with—it is professionally constraining and legally risky.
IN SUM
From a legal standpoint, it is not safe to completely discard surgical delivery; when needed, it is required. Patients given oxytocin must be monitored closely, and the drug should be discontinued in the setting of uterine hyperactivity with fetal distress. Follow medication protocols or change them—but whatever you do, don’t ignore them.
During her pregnancy, a Wisconsin woman received care from a nurse-midwife. In late July 2001, misoprostol was administered to induce labor. The woman was admitted to the hospital in active labor at approximately 2 pm. She was 6 cm dilated. Dilation arrested three times, and then there was a two-hour failure to dilate.
The nurse-midwife then tried putting the mother in a water-birthing tub to stimulate contractions, without success. Oxytocin was then administered. Immediately thereafter, the patient developed uterine hyperstimulation, and the fetal heart rate strip showed late decelerations, indicative of fetal distress.
Despite these abnormalities, the nurse-midwife continued increasing oxytocin throughout labor, which continued for about 12 hours. The oxytocin dose exceeded the hospital’s recommended protocols. The patient reached a point at which she was having strong contractions every 1.5 min. Full dilation was not reached until 10:38 pm.
Around midnight, the electronic fetal monitor allegedly showed an abnormal heart pattern, with decelerations with almost every contraction. The mother was allowed to continue with labor, and the fetal monitoring equipment was removed at 1:26 am so the mother could be placed in the water-birthing tub again. Nurses took the fetal heart rate during this time and recorded it as normal.
When the child was delivered at approximately 2 am, she had a heart rate of 80 beats/min; she was apneic, cyanotic, and virtually lifeless. Apgar scores were recorded as 1 at one minute and 3 at five minutes. Arterial blood gas/pH was 7.165.
An attending physician was called and arrived within 20 min. The infant was resuscitated, intubated, and transferred to another hospital. A CT scan taken at 56 hours of life was read as normal. An MRl at nine months was also read as normal.
The child was subsequently diagnosed as having cerebral palsy. She requires a walker for ambulation and has arm and leg impairments and significant cognitive deficits, necessitating 24-hour assistance.
The plaintiff claimed that if oxytocin had been discontinued, she would have reached full dilation hours earlier than she did. She also claimed that a cesarean delivery or operative vaginal delivery should have been performed when the fetal monitor indicated an abnormal heart pattern. (She contended that the “normal” heart rate recorded around this time was actually the maternal heart rate.)
The parties did not dispute that the infant had experienced a hypoxic/ischemic event but disagreed on when it occurred. The plaintiff claimed that the ischemic event occurred during delivery. The defendants claimed that it occurred in utero prior to delivery. The plaintiff also disputed the MRI findings, which the plaintiff argued showed significant brain injury.
On the next page: Outcome >>
OUTCOME
A jury found the nurse-midwife 80% at fault and the hospital 20% at fault. The jury awarded $13.5 million to the child and $100,000 to the plaintiff. An additional $110,000 in past medical expenses was added to the verdict.
COMMENT
Obstetrics/midwifery accounts for a significant percentage of malpractice cases filed and monetary damages awarded. In this case, a substantial $13.5 million verdict was awarded, with 80% of the verdict against the midwife for inappropriate use of oxytocin and failure to refer for cesarean delivery.
A detailed discussion of obstetric management is beyond the scope of this article (in part because we don’t have access to much data, including fetal heart rate tracings). However, there are a few points to consider.
First, consider surgical options when appropriate. Without doubt, operative delivery by cesarean section is overused for all the wrong reasons. Some mothers, families, and clinicians strongly desire a more natural childbirth and strive to create such an experience—forgoing traditional medications and anesthesia. Often, this is perfectly safe, reasonable, and preferable.
However, if the perinatal course is rocky, it is wise to monitor closely and adopt a collaborative approach. When prenatal screening suggests a difficult delivery, exercise caution and have a fallback plan. Known high-risk deliveries should have a team approach from the outset, with all assets available to bedside on short notice.
While operative delivery is overused, it shouldn’t be demonized either. When genuinely needed, it can be lifesaving. Some patients do not want a cesarean delivery, and both the patient and the clinician may equate operative delivery with personal failure. However, that view may present a barrier to calling for consultation when it is genuinely needed.
For natural childbirth enthusiasts, think of the surgical delivery option as sealed in a glass case. Break that glass for all the right reasons: to preserve life or avoid significant fetal morbidity. Discuss the indications for surgical management ahead of time, so the mother is not surprised by a sudden rush to the operating room, feeling frightened and out of control.
It is recognized that patients and clinicians have firmly held beliefs, and opinions are strong on this subject. Patients have a right to self-determination and to select the birthing experience that will suit them best. Yet there is tension because jurors will expect a clinician to fully communicate known risks to patients and use all available resources to safeguard the mother and fetus at all times. In this case, the jury concluded that the midwife failed to refer for cesarean delivery after about 10 hours of labor, when the fetal heart rate pattern was nonreassuring. One of the plaintiff’s expert witnesses who criticized the defendant midwife’s care was herself a highly regarded midwife.
Continued on the next page >>
Second, use oxytocin carefully, slowing or stopping it when required. While we do not have access to the fetal heart rate monitor strips in this case, we do know that the plaintiff met her burden of proof and persuaded the jurors that the midwife inappropriately increased the drug in the setting of uterine hyperstimulation, with evidence of fetal distress. It seems surprising that the allegedly “normal” pattern recorded at 1:26 am could have been the maternal heart rate—but apparently, the jurors were convinced of this.
Third, when a facility has a medication protocol, follow it unless there is good cause not to. Medication protocols can be useful to establish operating guidelines and reduce medication errors. But they can also shackle clinicians by substituting tables and algorithms for clinical judgment. Problems arise when a protocol is sidestepped, and the clinician is raked over the coals for failing to adhere. If your facility has protocols that are important to your practice, read the documentation. Learn it, know it, live it.
If you operate outside a protocol, and your case goes to trial, the expert witness defending your care will be forced to take on both the plaintiff’s allegations and your own facility’s recommendations. The plaintiff’s closing argument will include a variation of “Mr. A did not even bother to follow his hospital’s own rules.” This argument is easy for jurors to understand, and many will reach a finding of negligence based on this fact alone. If you disagree with the protocol, or it is not reflective of your actual practice, either clinician practice or the protocol must be changed. Do not routinely circumvent protocols without good reason.
Ideally, protocols should be constructed to give clinicians flexibility based on clinical judgment and patient response. If you have a role in forming a protocol, consider advocating for less rigidity and allowing for professional judgment. If the protocol is rigid, be sure that everyone understands it and that it can be strictly followed in a real world practice environment. Put plainly, don’t install a set of rules you can’t live with—it is professionally constraining and legally risky.
IN SUM
From a legal standpoint, it is not safe to completely discard surgical delivery; when needed, it is required. Patients given oxytocin must be monitored closely, and the drug should be discontinued in the setting of uterine hyperactivity with fetal distress. Follow medication protocols or change them—but whatever you do, don’t ignore them.
During her pregnancy, a Wisconsin woman received care from a nurse-midwife. In late July 2001, misoprostol was administered to induce labor. The woman was admitted to the hospital in active labor at approximately 2 pm. She was 6 cm dilated. Dilation arrested three times, and then there was a two-hour failure to dilate.
The nurse-midwife then tried putting the mother in a water-birthing tub to stimulate contractions, without success. Oxytocin was then administered. Immediately thereafter, the patient developed uterine hyperstimulation, and the fetal heart rate strip showed late decelerations, indicative of fetal distress.
Despite these abnormalities, the nurse-midwife continued increasing oxytocin throughout labor, which continued for about 12 hours. The oxytocin dose exceeded the hospital’s recommended protocols. The patient reached a point at which she was having strong contractions every 1.5 min. Full dilation was not reached until 10:38 pm.
Around midnight, the electronic fetal monitor allegedly showed an abnormal heart pattern, with decelerations with almost every contraction. The mother was allowed to continue with labor, and the fetal monitoring equipment was removed at 1:26 am so the mother could be placed in the water-birthing tub again. Nurses took the fetal heart rate during this time and recorded it as normal.
When the child was delivered at approximately 2 am, she had a heart rate of 80 beats/min; she was apneic, cyanotic, and virtually lifeless. Apgar scores were recorded as 1 at one minute and 3 at five minutes. Arterial blood gas/pH was 7.165.
An attending physician was called and arrived within 20 min. The infant was resuscitated, intubated, and transferred to another hospital. A CT scan taken at 56 hours of life was read as normal. An MRl at nine months was also read as normal.
The child was subsequently diagnosed as having cerebral palsy. She requires a walker for ambulation and has arm and leg impairments and significant cognitive deficits, necessitating 24-hour assistance.
The plaintiff claimed that if oxytocin had been discontinued, she would have reached full dilation hours earlier than she did. She also claimed that a cesarean delivery or operative vaginal delivery should have been performed when the fetal monitor indicated an abnormal heart pattern. (She contended that the “normal” heart rate recorded around this time was actually the maternal heart rate.)
The parties did not dispute that the infant had experienced a hypoxic/ischemic event but disagreed on when it occurred. The plaintiff claimed that the ischemic event occurred during delivery. The defendants claimed that it occurred in utero prior to delivery. The plaintiff also disputed the MRI findings, which the plaintiff argued showed significant brain injury.
On the next page: Outcome >>
OUTCOME
A jury found the nurse-midwife 80% at fault and the hospital 20% at fault. The jury awarded $13.5 million to the child and $100,000 to the plaintiff. An additional $110,000 in past medical expenses was added to the verdict.
COMMENT
Obstetrics/midwifery accounts for a significant percentage of malpractice cases filed and monetary damages awarded. In this case, a substantial $13.5 million verdict was awarded, with 80% of the verdict against the midwife for inappropriate use of oxytocin and failure to refer for cesarean delivery.
A detailed discussion of obstetric management is beyond the scope of this article (in part because we don’t have access to much data, including fetal heart rate tracings). However, there are a few points to consider.
First, consider surgical options when appropriate. Without doubt, operative delivery by cesarean section is overused for all the wrong reasons. Some mothers, families, and clinicians strongly desire a more natural childbirth and strive to create such an experience—forgoing traditional medications and anesthesia. Often, this is perfectly safe, reasonable, and preferable.
However, if the perinatal course is rocky, it is wise to monitor closely and adopt a collaborative approach. When prenatal screening suggests a difficult delivery, exercise caution and have a fallback plan. Known high-risk deliveries should have a team approach from the outset, with all assets available to bedside on short notice.
While operative delivery is overused, it shouldn’t be demonized either. When genuinely needed, it can be lifesaving. Some patients do not want a cesarean delivery, and both the patient and the clinician may equate operative delivery with personal failure. However, that view may present a barrier to calling for consultation when it is genuinely needed.
For natural childbirth enthusiasts, think of the surgical delivery option as sealed in a glass case. Break that glass for all the right reasons: to preserve life or avoid significant fetal morbidity. Discuss the indications for surgical management ahead of time, so the mother is not surprised by a sudden rush to the operating room, feeling frightened and out of control.
It is recognized that patients and clinicians have firmly held beliefs, and opinions are strong on this subject. Patients have a right to self-determination and to select the birthing experience that will suit them best. Yet there is tension because jurors will expect a clinician to fully communicate known risks to patients and use all available resources to safeguard the mother and fetus at all times. In this case, the jury concluded that the midwife failed to refer for cesarean delivery after about 10 hours of labor, when the fetal heart rate pattern was nonreassuring. One of the plaintiff’s expert witnesses who criticized the defendant midwife’s care was herself a highly regarded midwife.
Continued on the next page >>
Second, use oxytocin carefully, slowing or stopping it when required. While we do not have access to the fetal heart rate monitor strips in this case, we do know that the plaintiff met her burden of proof and persuaded the jurors that the midwife inappropriately increased the drug in the setting of uterine hyperstimulation, with evidence of fetal distress. It seems surprising that the allegedly “normal” pattern recorded at 1:26 am could have been the maternal heart rate—but apparently, the jurors were convinced of this.
Third, when a facility has a medication protocol, follow it unless there is good cause not to. Medication protocols can be useful to establish operating guidelines and reduce medication errors. But they can also shackle clinicians by substituting tables and algorithms for clinical judgment. Problems arise when a protocol is sidestepped, and the clinician is raked over the coals for failing to adhere. If your facility has protocols that are important to your practice, read the documentation. Learn it, know it, live it.
If you operate outside a protocol, and your case goes to trial, the expert witness defending your care will be forced to take on both the plaintiff’s allegations and your own facility’s recommendations. The plaintiff’s closing argument will include a variation of “Mr. A did not even bother to follow his hospital’s own rules.” This argument is easy for jurors to understand, and many will reach a finding of negligence based on this fact alone. If you disagree with the protocol, or it is not reflective of your actual practice, either clinician practice or the protocol must be changed. Do not routinely circumvent protocols without good reason.
Ideally, protocols should be constructed to give clinicians flexibility based on clinical judgment and patient response. If you have a role in forming a protocol, consider advocating for less rigidity and allowing for professional judgment. If the protocol is rigid, be sure that everyone understands it and that it can be strictly followed in a real world practice environment. Put plainly, don’t install a set of rules you can’t live with—it is professionally constraining and legally risky.
IN SUM
From a legal standpoint, it is not safe to completely discard surgical delivery; when needed, it is required. Patients given oxytocin must be monitored closely, and the drug should be discontinued in the setting of uterine hyperactivity with fetal distress. Follow medication protocols or change them—but whatever you do, don’t ignore them.
VIDEO: Abnormal endocrinology labs? Look beyond ‘usual suspects’
PHILADELPHIA – Psychiatric medications can affect prolactin levels, while antibodies can affect thyroid-stimulating hormone levels. Hirsutism may be the result of polycystic ovary syndrome – but it may also be caused by congenital adrenal hyperplasia.
And if that’s not confounding enough, physicians should add to the medical factors that can influence lab reports what Dr. Ellen L. Connor says is the importance of "knowing the typical ranges of the assays you are using, and what the ranges considered normal are at the [laboratory] you’re working with."
In a video interview at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology, Dr. Connor of the department of pediatric endocrinology at the University of Wisconsin, Madison, reviews what can change prolactin levels, how to get the most clinical utility out of thyroid tests, what is the gold standard for testosterone testing in women, how best to test and interpret vitamin D levels, and what adrenal malfunctions are possible in young women. She also stresses the value of working with knowledgeable lab personnel.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – Psychiatric medications can affect prolactin levels, while antibodies can affect thyroid-stimulating hormone levels. Hirsutism may be the result of polycystic ovary syndrome – but it may also be caused by congenital adrenal hyperplasia.
And if that’s not confounding enough, physicians should add to the medical factors that can influence lab reports what Dr. Ellen L. Connor says is the importance of "knowing the typical ranges of the assays you are using, and what the ranges considered normal are at the [laboratory] you’re working with."
In a video interview at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology, Dr. Connor of the department of pediatric endocrinology at the University of Wisconsin, Madison, reviews what can change prolactin levels, how to get the most clinical utility out of thyroid tests, what is the gold standard for testosterone testing in women, how best to test and interpret vitamin D levels, and what adrenal malfunctions are possible in young women. She also stresses the value of working with knowledgeable lab personnel.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – Psychiatric medications can affect prolactin levels, while antibodies can affect thyroid-stimulating hormone levels. Hirsutism may be the result of polycystic ovary syndrome – but it may also be caused by congenital adrenal hyperplasia.
And if that’s not confounding enough, physicians should add to the medical factors that can influence lab reports what Dr. Ellen L. Connor says is the importance of "knowing the typical ranges of the assays you are using, and what the ranges considered normal are at the [laboratory] you’re working with."
In a video interview at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology, Dr. Connor of the department of pediatric endocrinology at the University of Wisconsin, Madison, reviews what can change prolactin levels, how to get the most clinical utility out of thyroid tests, what is the gold standard for testosterone testing in women, how best to test and interpret vitamin D levels, and what adrenal malfunctions are possible in young women. She also stresses the value of working with knowledgeable lab personnel.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM NASPAG 2014
How to identify and manage cesarean-scar pregnancy
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
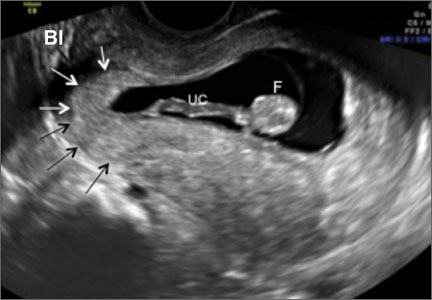
Accurate identification of CSP depends on the following sonographic criteria:
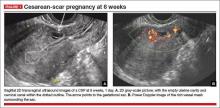
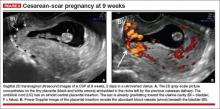
- empty uterine cavity and cervical canal (FIGURE 1A)
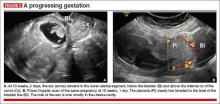
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
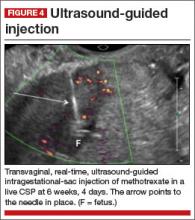
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
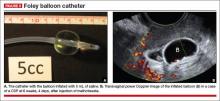
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
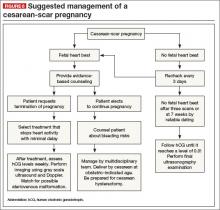
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: rbarbieri@frontlinemedcom.com Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: rbarbieri@frontlinemedcom.com Please include the city and state in which you practice.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: rbarbieri@frontlinemedcom.com Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
2014 Update on operative vaginal delivery
The past year has seen the publication of four studies with relevance for clinicians:
- a retrospective cohort study that examined the maternal risks of operative vaginal delivery using forceps, vacuum extraction (FIGURE 1), or a combination of forceps and vacuum
- a prospective cohort study that investigated the efficacy and safety of three different techniques for midcavity rotational delivery in the setting of transverse arrest, namely manual rotation, vacuum rotation, and rotational forceps
- another retrospective cohort study that compared maternal morbidity among operative vaginal deliveries performed by midwives and physician providers in the United Kingdom
- a description of a new technique for instrumental vaginal delivery that is low-cost, simple, and easy to perform.
FIGURE 1. In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery when nonreassuring fetal
testing is noted during the second stage of labor.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of the June Guest Editorial titled "Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a 're-run' or as a 'new series'?" recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
DO NOT SWITCH INSTRUMENTS
Fong A, Wu E, Pan D, Chung HJ, Ogunyemi DA. Temporal trends and morbidities of vacuum, forceps, and combined use of both [published online ahead of print April 9, 2014]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.904282.
In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery in the setting of nonreassuring fetal testing (“fetal distress”) in the second stage of labor. It takes just a few minutes to perform and can avert a frantic dash to the operating room for an emergent cesarean delivery. What to do then in a situation where the vacuum extractor keeps popping off, the vertex is at +3/+5 station, and the fetal heart rate has been at 80 bpm for 8 minutes? It is extremely tempting to discard the ventouse and grab the forceps. But would that be the right decision?
Related article: Is the rate of progress the same for induced and spontaneous labors? William F. Rayburn, MD, MBA (Examining the Evidence; November 2012)
Details of the study
Earlier studies suggested that the combination of vacuum and forceps is associated with an increased risk of fetal injury. Whether this is also true of injury to the mother was not known. To address this issue, Fong and colleagues performed a retrospective cohort study of all successful operative vaginal deliveries identified using ICD-9 procedure codes in the California Health Discharge Dataset from 2001 through 2007. Maternal outcomes were compared between the 202,439 fetuses delivered by vacuum extraction (reference group), 13,555 fetuses delivered by forceps, and 710 fetuses delivered using a combination of the two methods.
Using multivariate analysis modeling, Fong and colleagues demonstrated that, when compared with the vacuum alone, the combined use of vacuum and forceps was associated with significantly higher odds of:
- third- and fourth-degree perineal lacerations (adjusted odds ratio [aOR], 2.86; 95% confidence interval [CI], 2.43–3.36)
- postpartum hemorrhage (aOR, 1.81; 95% CI, 1.33–2.46)
- operative delivery failure (aOR, 2.81; 95% CI, 2.27–3.48).
Related articles:
• Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
• Postpartum hemorrhage: 11 critical questions, answered by an expert. Q&A with Haywood L. Brown, MD (January 2011)
Fortunately, combined vacuum/forceps deliveries are uncommon, comprising only 0.33% of operative deliveries in this cohort.
Despite the large dataset used, the study was underpowered to examine the effect of combined vacuum/forceps on the incidence of rare events, such as pelvic hematoma, cervical laceration, thromboembolism, and maternal death.
What this EVIDENCE means for practice
The message is clear: Avoid combined vacuum/forceps deliveries. Choose your initial instrument with care because a failed operative vaginal delivery means a cesarean. You don’t get to choose again. The American College of Obstetricians and Gynecologists also recommends against using multiple instruments “unless there is a compelling and justifiable reason.”1
LEARN TO PERFORM MIDCAVITY ROTATIONAL DELIVERIES
Bahl R, Van de Venne M, Macleod M, Strachan B, Murphy DJ. Maternal and neonatal morbidity in relation to the instrument used for midcavity rotational operative vaginal delivery: A prospective cohort study. BJOG. 2013;120(12):1526–1532.
Cesarean delivery during the second stage of labor used to be an uncommon event. It was said that if labor progressed adequately to achieve full cervical dilatation, a vaginal delivery should be achieved. Over the past few decades, however, the rate of cesarean delivery at full cervical dilatation has increased substantially, thereby contributing to the well-documented cesarean epidemic.
The most common indication for cesarean delivery during the second stage of labor is arrest of descent due to malposition of the fetal head, typically a transverse arrest. A number of alternatives to cesarean are available, all of which involve assisted rotation of the fetal head. Historical case series reporting increased neonatal morbidity have led to a reduction in the use of rotational forceps to facilitate this rotation. Attempted manual rotation and “rotational vacuum extraction” are now preferred, particularly by less experienced providers. Which of these three approaches is most effective is unknown.
Related article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; July 2011)
Details of the study
A prospective cohort study was carried out at two university hospitals in Scotland and England to compare maternal and neonatal morbidity associated with alternative techniques for midcavity rotational delivery. The choice of instrument was left to the provider.
Of the 381 nulliparous women who had an attempted midcavity rotational operative vaginal delivery, 163 (42.8%) underwent manual rotation followed by nonrotational forceps delivery, 73 (19.1%) had a rotational vacuum delivery, and 145 (38.1%) delivered with the assistance of rotational (Kielland) forceps.
Regardless of the instrument used, successful rotation and vaginal delivery were achieved in more than 90% of cases, with a cesarean rate of 4.2%, 6.8%, and 9.6% for manual rotation, vacuum, and rotational forceps, respectively (aOR, 0.39; 95% CI, 0.14–1.06). There were no significant differences in maternal complications (postpartum hemorrhage, third- and fourth-degree perineal lacerations) and neonatal morbidity (low cord pH, neonatal trauma, and neonatal intensive care unit admission) between the three instruments.
What this EVIDENCE means for practice
Midcavity rotational delivery can be achieved with a high degree of success and few adverse events in women who develop transverse arrest in the second stage of labor. Maternal and perinatal outcomes are comparable with rotational forceps, vacuum extraction, and manual rotation. With appropriate training, midcavity rotational delivery can be practiced safely, including the use of Kielland forceps.
SHOULD MIDWIVES PERFORM OPERATIVE VAGINAL DELIVERIES?
Black M, Mitchell E, Danielian P. Instrumental vaginal deliveries; are midwives safer practitioners? A retrospective cohort study. Acta Obstet Gynecol Scand. 2013;92(12):1383–1387.
In the United States, instrumental vaginal deliveries are performed only by physicians. In the United Kingdom, the opportunity to perform such deliveries has recently become available to midwives as well. Because midwives have less experience in performing surgical procedures, the question has arisen as to whether their complication rate is higher than that of physicians. Alternatively, because midwives typically are more patient than physicians and more reluctant to resort to obstetric interventions, it is possible that their complication rate may be lower.
Details of the study
To address this issue, Black and colleagues performed a retrospective cohort study of consecutive women who had a successful nonrotational instrumental vaginal delivery of a liveborn singleton infant outside of the operating room between June 2005 and June 2010 at Aberdeen Maternity Hospital in the United Kingdom.
Of the 2,540 women included in the final analysis, 330 (13%) were delivered by midwives and the remaining 2,210 (87%) by physicians—1,049 (41%) by junior doctors and 1,161 (46%) by more senior doctors. All midwives had undergone formal training at the University of Bradford. There were no differences between groups in demographic characteristics (maternal age, gestational age, parity, body mass index, or birth weight) or in the indications for instrumental delivery.
Major findings were that midwives were significantly less likely than junior and senior physicians to use forceps as the instrument of choice for delivery (OR, 0.6; 95% CI, 0.4–0.7). Mean blood loss was significantly lower in the midwife group (57 mL), although it is unlikely that this finding was clinically significant. There were no differences in severe perineal injury (third- or fourth-degree perineal lacerations), arterial cord pH, or postpartum hemorrhage.
A secondary analysis comparing the outcome of operative vaginal deliveries by trained midwives with the outcome by junior physicians alone produced almost identical results.
Strengths of the study include the fact that it was conducted at a single center and had a large sample number. Weaknesses include its retrospective design and the fact that one major outcome (namely, failed operative vaginal delivery leading to cesarean) was not examined. This study was not designed or powered to examine neonatal outcomes.
What this EVIDENCE means for practice
These data demonstrate that midwives can perform operative vaginal deliveries using either forceps or vacuum with a rate of maternal morbidity equivalent to those performed by physicians.
Are these findings truly revolutionary? Although midwives do not perform cesarean deliveries, they do perform and repair episiotomies when indicated. Restricting instrumental vaginal deliveries to physicians alone may be motivated more by tradition and logistics than concerns over patient safety. Indeed, the ability of a midwife working in a remote area to perform an instrumental vaginal delivery in an emergency situation may be highly beneficial to perinatal outcome, although it should be stressed that such an approach ought to be limited to practitioners who have undergone rigorous formal training.
Other benefits of midwives performing operative vaginal deliveries may include increased autonomy for the midwifery providers, improvements in physician-midwife interactions, and enhanced continuity of care for women.
IN THE PIPELINE: THE ODÓN DEVICE FOR OPERATIVE VAGINAL DELIVERY
World Health Organization Odón Device Research Group. Feasibility and safety study of a new device (Odón device) for assisted vaginal deliveries: Study protocol. Reprod Health. 2013;10:33.
Childbirth remains a risky venture. According to the World Health Organization (WHO), approximately 2.6 million babies are stillborn and 260,000 women die in childbirth each year, with developing countries disproportionately affected. Many of these adverse events result from complications at the time of delivery. Instrumental vaginal delivery is used to shorten the second stage of labor and improve perinatal and maternal outcomes.
Operative vaginal delivery likely does reduce the rate of stillbirth and early neonatal death and lower the cesarean delivery rate, but the instruments themselves do occasionally cause maternal and fetal injury, including cephalohematoma, retinal hemorrhage, facial nerve palsy, and skull fractures. Although numerous modifications to the design of forceps and the vacuum extractor have been made over the years, no new technology has been introduced for centuries.
In 2005, Mr. Jorge Odón, a car mechanic from Argentina with no formal training in medicine or obstetrics (aside from being the father of five), came up with an idea for a novel technique to assist in delivery. He was inspired by a simple trick he used to entertain his friends. It involved removing a loose cork from the inside of an empty bottle using a plastic bag. It occurred to him one day that this same scientific principle could be used to expedite delivery of the fetal head from the birth canal, and so he built the first prototype. The device has since been named in his honor.
Description of the Odón device
The Odón device consists of a tube containing a polyethylene bag. The tube is inserted into the birth canal and the bag is deployed and inflated to create a plastic sleeve that hugs the baby’s head. The applicator tube is then discarded and traction is applied to the plastic bag to move the head (and the entire fetus) down the birth canal (FIGURE 2).
![]()
The advantages of the Odón device are that it is:
- low-cost
- simple to use
- compact, easy to transport and store
- designed to minimize trauma to the mother and fetus.
Current stage of development
The Odón device already has been piloted in the United States and South America. The WHO plans to introduce it into the obstetric armamentarium in a three-phase clinical trial outlined in the Odón Device Research Project report. The first phase is under way and involves testing the device under normal delivery conditions in tertiary hospitals in Argentina and South Africa. The next two phases will 1) assess its efficacy in women with a prolonged second stage of labor but no “fetal distress” and 2) compare its performance head-to-head against the vacuum extractor and forceps.
What this EVIDENCE means for practice
Enthusiasm for the Odón device is fueled by its simplicity and the likelihood that midlevel providers working in remote obstetric units can be trained in its use, thereby increasing access to an important modality of emergency obstetric care. This is particularly important in centers that lack immediate access to cesarean delivery capabilities. Whether the device can be used in developing countries to more effectively manage the second stage of labor and thereby reduce infectious morbidity and pelvic floor injuries has yet to be confirmed but is a testable hypothesis.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Reference
- American College of Obstetricians and Gynecologists. ACOG practice bulletin #17: Operative vaginal delivery. Washington, DC: ACOG; 2000.
The past year has seen the publication of four studies with relevance for clinicians:
- a retrospective cohort study that examined the maternal risks of operative vaginal delivery using forceps, vacuum extraction (FIGURE 1), or a combination of forceps and vacuum
- a prospective cohort study that investigated the efficacy and safety of three different techniques for midcavity rotational delivery in the setting of transverse arrest, namely manual rotation, vacuum rotation, and rotational forceps
- another retrospective cohort study that compared maternal morbidity among operative vaginal deliveries performed by midwives and physician providers in the United Kingdom
- a description of a new technique for instrumental vaginal delivery that is low-cost, simple, and easy to perform.
FIGURE 1. In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery when nonreassuring fetal
testing is noted during the second stage of labor.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of the June Guest Editorial titled "Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a 're-run' or as a 'new series'?" recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
DO NOT SWITCH INSTRUMENTS
Fong A, Wu E, Pan D, Chung HJ, Ogunyemi DA. Temporal trends and morbidities of vacuum, forceps, and combined use of both [published online ahead of print April 9, 2014]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.904282.
In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery in the setting of nonreassuring fetal testing (“fetal distress”) in the second stage of labor. It takes just a few minutes to perform and can avert a frantic dash to the operating room for an emergent cesarean delivery. What to do then in a situation where the vacuum extractor keeps popping off, the vertex is at +3/+5 station, and the fetal heart rate has been at 80 bpm for 8 minutes? It is extremely tempting to discard the ventouse and grab the forceps. But would that be the right decision?
Related article: Is the rate of progress the same for induced and spontaneous labors? William F. Rayburn, MD, MBA (Examining the Evidence; November 2012)
Details of the study
Earlier studies suggested that the combination of vacuum and forceps is associated with an increased risk of fetal injury. Whether this is also true of injury to the mother was not known. To address this issue, Fong and colleagues performed a retrospective cohort study of all successful operative vaginal deliveries identified using ICD-9 procedure codes in the California Health Discharge Dataset from 2001 through 2007. Maternal outcomes were compared between the 202,439 fetuses delivered by vacuum extraction (reference group), 13,555 fetuses delivered by forceps, and 710 fetuses delivered using a combination of the two methods.
Using multivariate analysis modeling, Fong and colleagues demonstrated that, when compared with the vacuum alone, the combined use of vacuum and forceps was associated with significantly higher odds of:
- third- and fourth-degree perineal lacerations (adjusted odds ratio [aOR], 2.86; 95% confidence interval [CI], 2.43–3.36)
- postpartum hemorrhage (aOR, 1.81; 95% CI, 1.33–2.46)
- operative delivery failure (aOR, 2.81; 95% CI, 2.27–3.48).
Related articles:
• Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
• Postpartum hemorrhage: 11 critical questions, answered by an expert. Q&A with Haywood L. Brown, MD (January 2011)
Fortunately, combined vacuum/forceps deliveries are uncommon, comprising only 0.33% of operative deliveries in this cohort.
Despite the large dataset used, the study was underpowered to examine the effect of combined vacuum/forceps on the incidence of rare events, such as pelvic hematoma, cervical laceration, thromboembolism, and maternal death.
What this EVIDENCE means for practice
The message is clear: Avoid combined vacuum/forceps deliveries. Choose your initial instrument with care because a failed operative vaginal delivery means a cesarean. You don’t get to choose again. The American College of Obstetricians and Gynecologists also recommends against using multiple instruments “unless there is a compelling and justifiable reason.”1
LEARN TO PERFORM MIDCAVITY ROTATIONAL DELIVERIES
Bahl R, Van de Venne M, Macleod M, Strachan B, Murphy DJ. Maternal and neonatal morbidity in relation to the instrument used for midcavity rotational operative vaginal delivery: A prospective cohort study. BJOG. 2013;120(12):1526–1532.
Cesarean delivery during the second stage of labor used to be an uncommon event. It was said that if labor progressed adequately to achieve full cervical dilatation, a vaginal delivery should be achieved. Over the past few decades, however, the rate of cesarean delivery at full cervical dilatation has increased substantially, thereby contributing to the well-documented cesarean epidemic.
The most common indication for cesarean delivery during the second stage of labor is arrest of descent due to malposition of the fetal head, typically a transverse arrest. A number of alternatives to cesarean are available, all of which involve assisted rotation of the fetal head. Historical case series reporting increased neonatal morbidity have led to a reduction in the use of rotational forceps to facilitate this rotation. Attempted manual rotation and “rotational vacuum extraction” are now preferred, particularly by less experienced providers. Which of these three approaches is most effective is unknown.
Related article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; July 2011)
Details of the study
A prospective cohort study was carried out at two university hospitals in Scotland and England to compare maternal and neonatal morbidity associated with alternative techniques for midcavity rotational delivery. The choice of instrument was left to the provider.
Of the 381 nulliparous women who had an attempted midcavity rotational operative vaginal delivery, 163 (42.8%) underwent manual rotation followed by nonrotational forceps delivery, 73 (19.1%) had a rotational vacuum delivery, and 145 (38.1%) delivered with the assistance of rotational (Kielland) forceps.
Regardless of the instrument used, successful rotation and vaginal delivery were achieved in more than 90% of cases, with a cesarean rate of 4.2%, 6.8%, and 9.6% for manual rotation, vacuum, and rotational forceps, respectively (aOR, 0.39; 95% CI, 0.14–1.06). There were no significant differences in maternal complications (postpartum hemorrhage, third- and fourth-degree perineal lacerations) and neonatal morbidity (low cord pH, neonatal trauma, and neonatal intensive care unit admission) between the three instruments.
What this EVIDENCE means for practice
Midcavity rotational delivery can be achieved with a high degree of success and few adverse events in women who develop transverse arrest in the second stage of labor. Maternal and perinatal outcomes are comparable with rotational forceps, vacuum extraction, and manual rotation. With appropriate training, midcavity rotational delivery can be practiced safely, including the use of Kielland forceps.
SHOULD MIDWIVES PERFORM OPERATIVE VAGINAL DELIVERIES?
Black M, Mitchell E, Danielian P. Instrumental vaginal deliveries; are midwives safer practitioners? A retrospective cohort study. Acta Obstet Gynecol Scand. 2013;92(12):1383–1387.
In the United States, instrumental vaginal deliveries are performed only by physicians. In the United Kingdom, the opportunity to perform such deliveries has recently become available to midwives as well. Because midwives have less experience in performing surgical procedures, the question has arisen as to whether their complication rate is higher than that of physicians. Alternatively, because midwives typically are more patient than physicians and more reluctant to resort to obstetric interventions, it is possible that their complication rate may be lower.
Details of the study
To address this issue, Black and colleagues performed a retrospective cohort study of consecutive women who had a successful nonrotational instrumental vaginal delivery of a liveborn singleton infant outside of the operating room between June 2005 and June 2010 at Aberdeen Maternity Hospital in the United Kingdom.
Of the 2,540 women included in the final analysis, 330 (13%) were delivered by midwives and the remaining 2,210 (87%) by physicians—1,049 (41%) by junior doctors and 1,161 (46%) by more senior doctors. All midwives had undergone formal training at the University of Bradford. There were no differences between groups in demographic characteristics (maternal age, gestational age, parity, body mass index, or birth weight) or in the indications for instrumental delivery.
Major findings were that midwives were significantly less likely than junior and senior physicians to use forceps as the instrument of choice for delivery (OR, 0.6; 95% CI, 0.4–0.7). Mean blood loss was significantly lower in the midwife group (57 mL), although it is unlikely that this finding was clinically significant. There were no differences in severe perineal injury (third- or fourth-degree perineal lacerations), arterial cord pH, or postpartum hemorrhage.
A secondary analysis comparing the outcome of operative vaginal deliveries by trained midwives with the outcome by junior physicians alone produced almost identical results.
Strengths of the study include the fact that it was conducted at a single center and had a large sample number. Weaknesses include its retrospective design and the fact that one major outcome (namely, failed operative vaginal delivery leading to cesarean) was not examined. This study was not designed or powered to examine neonatal outcomes.
What this EVIDENCE means for practice
These data demonstrate that midwives can perform operative vaginal deliveries using either forceps or vacuum with a rate of maternal morbidity equivalent to those performed by physicians.
Are these findings truly revolutionary? Although midwives do not perform cesarean deliveries, they do perform and repair episiotomies when indicated. Restricting instrumental vaginal deliveries to physicians alone may be motivated more by tradition and logistics than concerns over patient safety. Indeed, the ability of a midwife working in a remote area to perform an instrumental vaginal delivery in an emergency situation may be highly beneficial to perinatal outcome, although it should be stressed that such an approach ought to be limited to practitioners who have undergone rigorous formal training.
Other benefits of midwives performing operative vaginal deliveries may include increased autonomy for the midwifery providers, improvements in physician-midwife interactions, and enhanced continuity of care for women.
IN THE PIPELINE: THE ODÓN DEVICE FOR OPERATIVE VAGINAL DELIVERY
World Health Organization Odón Device Research Group. Feasibility and safety study of a new device (Odón device) for assisted vaginal deliveries: Study protocol. Reprod Health. 2013;10:33.
Childbirth remains a risky venture. According to the World Health Organization (WHO), approximately 2.6 million babies are stillborn and 260,000 women die in childbirth each year, with developing countries disproportionately affected. Many of these adverse events result from complications at the time of delivery. Instrumental vaginal delivery is used to shorten the second stage of labor and improve perinatal and maternal outcomes.
Operative vaginal delivery likely does reduce the rate of stillbirth and early neonatal death and lower the cesarean delivery rate, but the instruments themselves do occasionally cause maternal and fetal injury, including cephalohematoma, retinal hemorrhage, facial nerve palsy, and skull fractures. Although numerous modifications to the design of forceps and the vacuum extractor have been made over the years, no new technology has been introduced for centuries.
In 2005, Mr. Jorge Odón, a car mechanic from Argentina with no formal training in medicine or obstetrics (aside from being the father of five), came up with an idea for a novel technique to assist in delivery. He was inspired by a simple trick he used to entertain his friends. It involved removing a loose cork from the inside of an empty bottle using a plastic bag. It occurred to him one day that this same scientific principle could be used to expedite delivery of the fetal head from the birth canal, and so he built the first prototype. The device has since been named in his honor.
Description of the Odón device
The Odón device consists of a tube containing a polyethylene bag. The tube is inserted into the birth canal and the bag is deployed and inflated to create a plastic sleeve that hugs the baby’s head. The applicator tube is then discarded and traction is applied to the plastic bag to move the head (and the entire fetus) down the birth canal (FIGURE 2).
![]()
The advantages of the Odón device are that it is:
- low-cost
- simple to use
- compact, easy to transport and store
- designed to minimize trauma to the mother and fetus.
Current stage of development
The Odón device already has been piloted in the United States and South America. The WHO plans to introduce it into the obstetric armamentarium in a three-phase clinical trial outlined in the Odón Device Research Project report. The first phase is under way and involves testing the device under normal delivery conditions in tertiary hospitals in Argentina and South Africa. The next two phases will 1) assess its efficacy in women with a prolonged second stage of labor but no “fetal distress” and 2) compare its performance head-to-head against the vacuum extractor and forceps.
What this EVIDENCE means for practice
Enthusiasm for the Odón device is fueled by its simplicity and the likelihood that midlevel providers working in remote obstetric units can be trained in its use, thereby increasing access to an important modality of emergency obstetric care. This is particularly important in centers that lack immediate access to cesarean delivery capabilities. Whether the device can be used in developing countries to more effectively manage the second stage of labor and thereby reduce infectious morbidity and pelvic floor injuries has yet to be confirmed but is a testable hypothesis.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
The past year has seen the publication of four studies with relevance for clinicians:
- a retrospective cohort study that examined the maternal risks of operative vaginal delivery using forceps, vacuum extraction (FIGURE 1), or a combination of forceps and vacuum
- a prospective cohort study that investigated the efficacy and safety of three different techniques for midcavity rotational delivery in the setting of transverse arrest, namely manual rotation, vacuum rotation, and rotational forceps
- another retrospective cohort study that compared maternal morbidity among operative vaginal deliveries performed by midwives and physician providers in the United Kingdom
- a description of a new technique for instrumental vaginal delivery that is low-cost, simple, and easy to perform.
FIGURE 1. In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery when nonreassuring fetal
testing is noted during the second stage of labor.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of the June Guest Editorial titled "Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a 're-run' or as a 'new series'?" recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
DO NOT SWITCH INSTRUMENTS
Fong A, Wu E, Pan D, Chung HJ, Ogunyemi DA. Temporal trends and morbidities of vacuum, forceps, and combined use of both [published online ahead of print April 9, 2014]. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.904282.
In trained hands, operative vaginal delivery can be an extremely effective intervention to expedite delivery in the setting of nonreassuring fetal testing (“fetal distress”) in the second stage of labor. It takes just a few minutes to perform and can avert a frantic dash to the operating room for an emergent cesarean delivery. What to do then in a situation where the vacuum extractor keeps popping off, the vertex is at +3/+5 station, and the fetal heart rate has been at 80 bpm for 8 minutes? It is extremely tempting to discard the ventouse and grab the forceps. But would that be the right decision?
Related article: Is the rate of progress the same for induced and spontaneous labors? William F. Rayburn, MD, MBA (Examining the Evidence; November 2012)
Details of the study
Earlier studies suggested that the combination of vacuum and forceps is associated with an increased risk of fetal injury. Whether this is also true of injury to the mother was not known. To address this issue, Fong and colleagues performed a retrospective cohort study of all successful operative vaginal deliveries identified using ICD-9 procedure codes in the California Health Discharge Dataset from 2001 through 2007. Maternal outcomes were compared between the 202,439 fetuses delivered by vacuum extraction (reference group), 13,555 fetuses delivered by forceps, and 710 fetuses delivered using a combination of the two methods.
Using multivariate analysis modeling, Fong and colleagues demonstrated that, when compared with the vacuum alone, the combined use of vacuum and forceps was associated with significantly higher odds of:
- third- and fourth-degree perineal lacerations (adjusted odds ratio [aOR], 2.86; 95% confidence interval [CI], 2.43–3.36)
- postpartum hemorrhage (aOR, 1.81; 95% CI, 1.33–2.46)
- operative delivery failure (aOR, 2.81; 95% CI, 2.27–3.48).
Related articles:
• Develop and use a checklist for 3rd- and 4th-degree perineal lacerations. Robert L. Barbieri, MD (Editorial; August 2013)
• Postpartum hemorrhage: 11 critical questions, answered by an expert. Q&A with Haywood L. Brown, MD (January 2011)
Fortunately, combined vacuum/forceps deliveries are uncommon, comprising only 0.33% of operative deliveries in this cohort.
Despite the large dataset used, the study was underpowered to examine the effect of combined vacuum/forceps on the incidence of rare events, such as pelvic hematoma, cervical laceration, thromboembolism, and maternal death.
What this EVIDENCE means for practice
The message is clear: Avoid combined vacuum/forceps deliveries. Choose your initial instrument with care because a failed operative vaginal delivery means a cesarean. You don’t get to choose again. The American College of Obstetricians and Gynecologists also recommends against using multiple instruments “unless there is a compelling and justifiable reason.”1
LEARN TO PERFORM MIDCAVITY ROTATIONAL DELIVERIES
Bahl R, Van de Venne M, Macleod M, Strachan B, Murphy DJ. Maternal and neonatal morbidity in relation to the instrument used for midcavity rotational operative vaginal delivery: A prospective cohort study. BJOG. 2013;120(12):1526–1532.
Cesarean delivery during the second stage of labor used to be an uncommon event. It was said that if labor progressed adequately to achieve full cervical dilatation, a vaginal delivery should be achieved. Over the past few decades, however, the rate of cesarean delivery at full cervical dilatation has increased substantially, thereby contributing to the well-documented cesarean epidemic.
The most common indication for cesarean delivery during the second stage of labor is arrest of descent due to malposition of the fetal head, typically a transverse arrest. A number of alternatives to cesarean are available, all of which involve assisted rotation of the fetal head. Historical case series reporting increased neonatal morbidity have led to a reduction in the use of rotational forceps to facilitate this rotation. Attempted manual rotation and “rotational vacuum extraction” are now preferred, particularly by less experienced providers. Which of these three approaches is most effective is unknown.
Related article: You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; July 2011)
Details of the study
A prospective cohort study was carried out at two university hospitals in Scotland and England to compare maternal and neonatal morbidity associated with alternative techniques for midcavity rotational delivery. The choice of instrument was left to the provider.
Of the 381 nulliparous women who had an attempted midcavity rotational operative vaginal delivery, 163 (42.8%) underwent manual rotation followed by nonrotational forceps delivery, 73 (19.1%) had a rotational vacuum delivery, and 145 (38.1%) delivered with the assistance of rotational (Kielland) forceps.
Regardless of the instrument used, successful rotation and vaginal delivery were achieved in more than 90% of cases, with a cesarean rate of 4.2%, 6.8%, and 9.6% for manual rotation, vacuum, and rotational forceps, respectively (aOR, 0.39; 95% CI, 0.14–1.06). There were no significant differences in maternal complications (postpartum hemorrhage, third- and fourth-degree perineal lacerations) and neonatal morbidity (low cord pH, neonatal trauma, and neonatal intensive care unit admission) between the three instruments.
What this EVIDENCE means for practice
Midcavity rotational delivery can be achieved with a high degree of success and few adverse events in women who develop transverse arrest in the second stage of labor. Maternal and perinatal outcomes are comparable with rotational forceps, vacuum extraction, and manual rotation. With appropriate training, midcavity rotational delivery can be practiced safely, including the use of Kielland forceps.
SHOULD MIDWIVES PERFORM OPERATIVE VAGINAL DELIVERIES?
Black M, Mitchell E, Danielian P. Instrumental vaginal deliveries; are midwives safer practitioners? A retrospective cohort study. Acta Obstet Gynecol Scand. 2013;92(12):1383–1387.
In the United States, instrumental vaginal deliveries are performed only by physicians. In the United Kingdom, the opportunity to perform such deliveries has recently become available to midwives as well. Because midwives have less experience in performing surgical procedures, the question has arisen as to whether their complication rate is higher than that of physicians. Alternatively, because midwives typically are more patient than physicians and more reluctant to resort to obstetric interventions, it is possible that their complication rate may be lower.
Details of the study
To address this issue, Black and colleagues performed a retrospective cohort study of consecutive women who had a successful nonrotational instrumental vaginal delivery of a liveborn singleton infant outside of the operating room between June 2005 and June 2010 at Aberdeen Maternity Hospital in the United Kingdom.
Of the 2,540 women included in the final analysis, 330 (13%) were delivered by midwives and the remaining 2,210 (87%) by physicians—1,049 (41%) by junior doctors and 1,161 (46%) by more senior doctors. All midwives had undergone formal training at the University of Bradford. There were no differences between groups in demographic characteristics (maternal age, gestational age, parity, body mass index, or birth weight) or in the indications for instrumental delivery.
Major findings were that midwives were significantly less likely than junior and senior physicians to use forceps as the instrument of choice for delivery (OR, 0.6; 95% CI, 0.4–0.7). Mean blood loss was significantly lower in the midwife group (57 mL), although it is unlikely that this finding was clinically significant. There were no differences in severe perineal injury (third- or fourth-degree perineal lacerations), arterial cord pH, or postpartum hemorrhage.
A secondary analysis comparing the outcome of operative vaginal deliveries by trained midwives with the outcome by junior physicians alone produced almost identical results.
Strengths of the study include the fact that it was conducted at a single center and had a large sample number. Weaknesses include its retrospective design and the fact that one major outcome (namely, failed operative vaginal delivery leading to cesarean) was not examined. This study was not designed or powered to examine neonatal outcomes.
What this EVIDENCE means for practice
These data demonstrate that midwives can perform operative vaginal deliveries using either forceps or vacuum with a rate of maternal morbidity equivalent to those performed by physicians.
Are these findings truly revolutionary? Although midwives do not perform cesarean deliveries, they do perform and repair episiotomies when indicated. Restricting instrumental vaginal deliveries to physicians alone may be motivated more by tradition and logistics than concerns over patient safety. Indeed, the ability of a midwife working in a remote area to perform an instrumental vaginal delivery in an emergency situation may be highly beneficial to perinatal outcome, although it should be stressed that such an approach ought to be limited to practitioners who have undergone rigorous formal training.
Other benefits of midwives performing operative vaginal deliveries may include increased autonomy for the midwifery providers, improvements in physician-midwife interactions, and enhanced continuity of care for women.
IN THE PIPELINE: THE ODÓN DEVICE FOR OPERATIVE VAGINAL DELIVERY
World Health Organization Odón Device Research Group. Feasibility and safety study of a new device (Odón device) for assisted vaginal deliveries: Study protocol. Reprod Health. 2013;10:33.
Childbirth remains a risky venture. According to the World Health Organization (WHO), approximately 2.6 million babies are stillborn and 260,000 women die in childbirth each year, with developing countries disproportionately affected. Many of these adverse events result from complications at the time of delivery. Instrumental vaginal delivery is used to shorten the second stage of labor and improve perinatal and maternal outcomes.
Operative vaginal delivery likely does reduce the rate of stillbirth and early neonatal death and lower the cesarean delivery rate, but the instruments themselves do occasionally cause maternal and fetal injury, including cephalohematoma, retinal hemorrhage, facial nerve palsy, and skull fractures. Although numerous modifications to the design of forceps and the vacuum extractor have been made over the years, no new technology has been introduced for centuries.
In 2005, Mr. Jorge Odón, a car mechanic from Argentina with no formal training in medicine or obstetrics (aside from being the father of five), came up with an idea for a novel technique to assist in delivery. He was inspired by a simple trick he used to entertain his friends. It involved removing a loose cork from the inside of an empty bottle using a plastic bag. It occurred to him one day that this same scientific principle could be used to expedite delivery of the fetal head from the birth canal, and so he built the first prototype. The device has since been named in his honor.
Description of the Odón device
The Odón device consists of a tube containing a polyethylene bag. The tube is inserted into the birth canal and the bag is deployed and inflated to create a plastic sleeve that hugs the baby’s head. The applicator tube is then discarded and traction is applied to the plastic bag to move the head (and the entire fetus) down the birth canal (FIGURE 2).
![]()
The advantages of the Odón device are that it is:
- low-cost
- simple to use
- compact, easy to transport and store
- designed to minimize trauma to the mother and fetus.
Current stage of development
The Odón device already has been piloted in the United States and South America. The WHO plans to introduce it into the obstetric armamentarium in a three-phase clinical trial outlined in the Odón Device Research Project report. The first phase is under way and involves testing the device under normal delivery conditions in tertiary hospitals in Argentina and South Africa. The next two phases will 1) assess its efficacy in women with a prolonged second stage of labor but no “fetal distress” and 2) compare its performance head-to-head against the vacuum extractor and forceps.
What this EVIDENCE means for practice
Enthusiasm for the Odón device is fueled by its simplicity and the likelihood that midlevel providers working in remote obstetric units can be trained in its use, thereby increasing access to an important modality of emergency obstetric care. This is particularly important in centers that lack immediate access to cesarean delivery capabilities. Whether the device can be used in developing countries to more effectively manage the second stage of labor and thereby reduce infectious morbidity and pelvic floor injuries has yet to be confirmed but is a testable hypothesis.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Reference
- American College of Obstetricians and Gynecologists. ACOG practice bulletin #17: Operative vaginal delivery. Washington, DC: ACOG; 2000.
Reference
- American College of Obstetricians and Gynecologists. ACOG practice bulletin #17: Operative vaginal delivery. Washington, DC: ACOG; 2000.
Have you read the June Guest Editorial? Click here to access Dr. John T. Repke's Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”?
Is neonatal injury more likely outside of a 30-minute decision-to-incision time interval for cesarean delivery?
Cesarean section is one of the most common surgical procedures worldwide. In a review of more than 13 million deliveries, cesarean delivery for nonreassuring fetal heart rate tracing occurred in about 3% of cases.1 Most of these urgent deliveries occur without known predisposing factors.1 A source of consternation for clinicians related to labor and delivery is the decision-to-incision time (DIT) interval for cesarean delivery for nonreassuring fetal heart rate tracing.
Previously, The American College of Obstetricians and Gynecologists (ACOG) suggested the DIT interval should be 30 minutes or less, for prolonged DIT increased the likelihood of neonatal injury.2 A DIT interval of more than 30 minutes became the sine qua non for poor neonatal outcomes and the linchpin for obstetric litigation.3 Starting in the 1990s, publications indicated that neonatal morbidity is not related to DIT and adverse neonatal outcomes may occur with a DIT interval of only a few minutes.4 Most studies, however, were hampered by small sample size.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai, MD (March 2012)
In an attempt to clarify whether neonatal outcomes differed among cesarean deliveries performed before or after 30 minutes lapsed, Tolcher and colleagues recently published a systematic review and meta-analysis evaluating all published reports that assessed adherence to a DIT policy for cesarean deliveries to be performed within 30 minutes of a nonreassuring fetal heart rate tracing. They reported on the number of emergent (Category 1) and urgent (Category 2) cesarean deliveries accomplished within 30 minutes and compared neonatal outcomes for cesarean deliveries before and after the 30-minute DIT.
Some important observations:
- First, all the studies were observational; only one paper focused exclusively on preterm infants, and only five of the identified 34 publications, involving 22,936 women, were determined to be “high quality.”
- Second, one of five neonates (21%) requiring emergent cesarean delivery were not delivered within 30 minutes. And 64% of urgent deliveries were not performed within 30 minutes.
- Third, and most surprisingly, in the 13 studies that included neonatal outcomes, 5-minute Apgar scores less than 7 and cord pH values less than 7.10 were significantly more common among neonates delivered within 30 minutes than among neonates delivered outside of 30 minutes. When the authors limited analysis to infants requiring emergent versus urgent delivery, however, the difference in Apgar scores and pH values was nonsignificant.
Several strengths of this analysis should be mentioned. The careful study design—meticulous and systematic evaluation of all publications and adherence to established publication evaluation and meta-analysis reporting protocols—strengthen the validity of these results. This report is clinically useful because the authors not only evaluated time frames from decision-to-incision but also reported and correlated neonatal outcomes.
Despite the multiple strengths, some weaknesses are worth mentioning. No maternal outcomes were reported. Mothers who require emergent cesarean delivery are at increased risk for adverse outcomes due to the requirement for general anesthesia and urgency with which the surgery is performed. The report only focused on 5-minute Apgar scores less than 7, neonatal intensive care admissions, and cord pH values less than 7.10 as adverse neonatal outcomes. The absence of additional adverse outcomes, as well as long-term neonatal and infant outcomes, hampers our ability to present the patient with all the facts. Lastly, the authors promulgated the classification of degree of urgency for cesarean delivery proposed by Lucas and colleagues5 without providing evidence that this classification is linked with clinically meaningful outcomes.
While a randomized trial would be unethical, a fact acknowledged by the authors, prospective cohort studies with long-term neonatal and infant follow-up could provide us with much needed information that would help us counsel our patients. The frequency with which cesarean deliveries are performed requires us to offer our patients the best and most comprehensive information available.
Related article:
• Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence; December 2013)
• Mother-, baby-, and family- centered cesarean delivery: It is possible. William Camann, MD, and Robert L. Barbieri, MD (Editorial; March 2013)
What this evidence means for practice
The ideal decision-to-incision time is probably best determined individually and may not encompass a “one-size-fits-all” approach. More studies are needed to elucidate this critical clinical question. In the meantime, we suggest: 1) consulting colleagues if interpretation of the tracing is uncertain, especially with preterm parturients, 2) intrauterine resuscitation, including tocolytics and amnioinfusion when appropriate, 3) scalp or vibroacoustic stimulation to elicit acceleration, 4) administering ephedrine if hypotensive, 5) expeditious delivery considering the clinical situation and logistics, 6) documenting decision-to-incision time in operative notes, and 7) sending umbilical arterial and venous blood for acid-base analysis and the placenta to pathology for evaluation.
Suneet P. Chauhan, MD, and Hector Mendez-Figueroa, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: Rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337−350.
- American Academy of Pediatrics; American College of Obstetrics and Gynecology. Guidelines for perinatal care. 2nd ed. Washington, DC: American College of Obstetrics and Gynecology; 1988:71.
- Chauhan SP, Chauhan VB, Cowan BD, Hendrix NW, Magann EF, Morrison JC. Professional liability claims and Central Association of Obstetricians and Gynecologists members: Myth versus reality. Am J Obstet Gynecol. 2005;192(6):1820−1826.
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force. Neonatal encephalopathy and neurologic outcomes. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014.
- Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: A new classification. J R Soc Med. 2000;93(7):346–350.
Cesarean section is one of the most common surgical procedures worldwide. In a review of more than 13 million deliveries, cesarean delivery for nonreassuring fetal heart rate tracing occurred in about 3% of cases.1 Most of these urgent deliveries occur without known predisposing factors.1 A source of consternation for clinicians related to labor and delivery is the decision-to-incision time (DIT) interval for cesarean delivery for nonreassuring fetal heart rate tracing.
Previously, The American College of Obstetricians and Gynecologists (ACOG) suggested the DIT interval should be 30 minutes or less, for prolonged DIT increased the likelihood of neonatal injury.2 A DIT interval of more than 30 minutes became the sine qua non for poor neonatal outcomes and the linchpin for obstetric litigation.3 Starting in the 1990s, publications indicated that neonatal morbidity is not related to DIT and adverse neonatal outcomes may occur with a DIT interval of only a few minutes.4 Most studies, however, were hampered by small sample size.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai, MD (March 2012)
In an attempt to clarify whether neonatal outcomes differed among cesarean deliveries performed before or after 30 minutes lapsed, Tolcher and colleagues recently published a systematic review and meta-analysis evaluating all published reports that assessed adherence to a DIT policy for cesarean deliveries to be performed within 30 minutes of a nonreassuring fetal heart rate tracing. They reported on the number of emergent (Category 1) and urgent (Category 2) cesarean deliveries accomplished within 30 minutes and compared neonatal outcomes for cesarean deliveries before and after the 30-minute DIT.
Some important observations:
- First, all the studies were observational; only one paper focused exclusively on preterm infants, and only five of the identified 34 publications, involving 22,936 women, were determined to be “high quality.”
- Second, one of five neonates (21%) requiring emergent cesarean delivery were not delivered within 30 minutes. And 64% of urgent deliveries were not performed within 30 minutes.
- Third, and most surprisingly, in the 13 studies that included neonatal outcomes, 5-minute Apgar scores less than 7 and cord pH values less than 7.10 were significantly more common among neonates delivered within 30 minutes than among neonates delivered outside of 30 minutes. When the authors limited analysis to infants requiring emergent versus urgent delivery, however, the difference in Apgar scores and pH values was nonsignificant.
Several strengths of this analysis should be mentioned. The careful study design—meticulous and systematic evaluation of all publications and adherence to established publication evaluation and meta-analysis reporting protocols—strengthen the validity of these results. This report is clinically useful because the authors not only evaluated time frames from decision-to-incision but also reported and correlated neonatal outcomes.
Despite the multiple strengths, some weaknesses are worth mentioning. No maternal outcomes were reported. Mothers who require emergent cesarean delivery are at increased risk for adverse outcomes due to the requirement for general anesthesia and urgency with which the surgery is performed. The report only focused on 5-minute Apgar scores less than 7, neonatal intensive care admissions, and cord pH values less than 7.10 as adverse neonatal outcomes. The absence of additional adverse outcomes, as well as long-term neonatal and infant outcomes, hampers our ability to present the patient with all the facts. Lastly, the authors promulgated the classification of degree of urgency for cesarean delivery proposed by Lucas and colleagues5 without providing evidence that this classification is linked with clinically meaningful outcomes.
While a randomized trial would be unethical, a fact acknowledged by the authors, prospective cohort studies with long-term neonatal and infant follow-up could provide us with much needed information that would help us counsel our patients. The frequency with which cesarean deliveries are performed requires us to offer our patients the best and most comprehensive information available.
Related article:
• Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence; December 2013)
• Mother-, baby-, and family- centered cesarean delivery: It is possible. William Camann, MD, and Robert L. Barbieri, MD (Editorial; March 2013)
What this evidence means for practice
The ideal decision-to-incision time is probably best determined individually and may not encompass a “one-size-fits-all” approach. More studies are needed to elucidate this critical clinical question. In the meantime, we suggest: 1) consulting colleagues if interpretation of the tracing is uncertain, especially with preterm parturients, 2) intrauterine resuscitation, including tocolytics and amnioinfusion when appropriate, 3) scalp or vibroacoustic stimulation to elicit acceleration, 4) administering ephedrine if hypotensive, 5) expeditious delivery considering the clinical situation and logistics, 6) documenting decision-to-incision time in operative notes, and 7) sending umbilical arterial and venous blood for acid-base analysis and the placenta to pathology for evaluation.
Suneet P. Chauhan, MD, and Hector Mendez-Figueroa, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Cesarean section is one of the most common surgical procedures worldwide. In a review of more than 13 million deliveries, cesarean delivery for nonreassuring fetal heart rate tracing occurred in about 3% of cases.1 Most of these urgent deliveries occur without known predisposing factors.1 A source of consternation for clinicians related to labor and delivery is the decision-to-incision time (DIT) interval for cesarean delivery for nonreassuring fetal heart rate tracing.
Previously, The American College of Obstetricians and Gynecologists (ACOG) suggested the DIT interval should be 30 minutes or less, for prolonged DIT increased the likelihood of neonatal injury.2 A DIT interval of more than 30 minutes became the sine qua non for poor neonatal outcomes and the linchpin for obstetric litigation.3 Starting in the 1990s, publications indicated that neonatal morbidity is not related to DIT and adverse neonatal outcomes may occur with a DIT interval of only a few minutes.4 Most studies, however, were hampered by small sample size.
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery. Baha M. Sibai, MD (March 2012)
In an attempt to clarify whether neonatal outcomes differed among cesarean deliveries performed before or after 30 minutes lapsed, Tolcher and colleagues recently published a systematic review and meta-analysis evaluating all published reports that assessed adherence to a DIT policy for cesarean deliveries to be performed within 30 minutes of a nonreassuring fetal heart rate tracing. They reported on the number of emergent (Category 1) and urgent (Category 2) cesarean deliveries accomplished within 30 minutes and compared neonatal outcomes for cesarean deliveries before and after the 30-minute DIT.
Some important observations:
- First, all the studies were observational; only one paper focused exclusively on preterm infants, and only five of the identified 34 publications, involving 22,936 women, were determined to be “high quality.”
- Second, one of five neonates (21%) requiring emergent cesarean delivery were not delivered within 30 minutes. And 64% of urgent deliveries were not performed within 30 minutes.
- Third, and most surprisingly, in the 13 studies that included neonatal outcomes, 5-minute Apgar scores less than 7 and cord pH values less than 7.10 were significantly more common among neonates delivered within 30 minutes than among neonates delivered outside of 30 minutes. When the authors limited analysis to infants requiring emergent versus urgent delivery, however, the difference in Apgar scores and pH values was nonsignificant.
Several strengths of this analysis should be mentioned. The careful study design—meticulous and systematic evaluation of all publications and adherence to established publication evaluation and meta-analysis reporting protocols—strengthen the validity of these results. This report is clinically useful because the authors not only evaluated time frames from decision-to-incision but also reported and correlated neonatal outcomes.
Despite the multiple strengths, some weaknesses are worth mentioning. No maternal outcomes were reported. Mothers who require emergent cesarean delivery are at increased risk for adverse outcomes due to the requirement for general anesthesia and urgency with which the surgery is performed. The report only focused on 5-minute Apgar scores less than 7, neonatal intensive care admissions, and cord pH values less than 7.10 as adverse neonatal outcomes. The absence of additional adverse outcomes, as well as long-term neonatal and infant outcomes, hampers our ability to present the patient with all the facts. Lastly, the authors promulgated the classification of degree of urgency for cesarean delivery proposed by Lucas and colleagues5 without providing evidence that this classification is linked with clinically meaningful outcomes.
While a randomized trial would be unethical, a fact acknowledged by the authors, prospective cohort studies with long-term neonatal and infant follow-up could provide us with much needed information that would help us counsel our patients. The frequency with which cesarean deliveries are performed requires us to offer our patients the best and most comprehensive information available.
Related article:
• Is the risk of placenta accreta in a subsequent pregnancy higher after emergent primary cesarean or after elective primary cesarean? Yinka Oyelese, MD (Examining the Evidence; December 2013)
• Mother-, baby-, and family- centered cesarean delivery: It is possible. William Camann, MD, and Robert L. Barbieri, MD (Editorial; March 2013)
What this evidence means for practice
The ideal decision-to-incision time is probably best determined individually and may not encompass a “one-size-fits-all” approach. More studies are needed to elucidate this critical clinical question. In the meantime, we suggest: 1) consulting colleagues if interpretation of the tracing is uncertain, especially with preterm parturients, 2) intrauterine resuscitation, including tocolytics and amnioinfusion when appropriate, 3) scalp or vibroacoustic stimulation to elicit acceleration, 4) administering ephedrine if hypotensive, 5) expeditious delivery considering the clinical situation and logistics, 6) documenting decision-to-incision time in operative notes, and 7) sending umbilical arterial and venous blood for acid-base analysis and the placenta to pathology for evaluation.
Suneet P. Chauhan, MD, and Hector Mendez-Figueroa, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: Rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337−350.
- American Academy of Pediatrics; American College of Obstetrics and Gynecology. Guidelines for perinatal care. 2nd ed. Washington, DC: American College of Obstetrics and Gynecology; 1988:71.
- Chauhan SP, Chauhan VB, Cowan BD, Hendrix NW, Magann EF, Morrison JC. Professional liability claims and Central Association of Obstetricians and Gynecologists members: Myth versus reality. Am J Obstet Gynecol. 2005;192(6):1820−1826.
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force. Neonatal encephalopathy and neurologic outcomes. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014.
- Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: A new classification. J R Soc Med. 2000;93(7):346–350.
- Chauhan SP, Magann EF, Scott JR, Scardo JA, Hendrix NW, Martin JN Jr. Cesarean delivery for fetal distress: Rate and risk factors. Obstet Gynecol Surv. 2003;58(5):337−350.
- American Academy of Pediatrics; American College of Obstetrics and Gynecology. Guidelines for perinatal care. 2nd ed. Washington, DC: American College of Obstetrics and Gynecology; 1988:71.
- Chauhan SP, Chauhan VB, Cowan BD, Hendrix NW, Magann EF, Morrison JC. Professional liability claims and Central Association of Obstetricians and Gynecologists members: Myth versus reality. Am J Obstet Gynecol. 2005;192(6):1820−1826.
- American College of Obstetricians and Gynecologists and American Academy of Pediatrics Task Force. Neonatal encephalopathy and neurologic outcomes. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014.
- Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: A new classification. J R Soc Med. 2000;93(7):346–350.
Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”?
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery
John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
Have you read Dr. Errol R. Norwitz's Update on Operative Vaginal Delivery? Click here to access.
Study identified cutoff for vertical HBV transmission
Mothers did not transmit hepatitis B virus to their offspring when their viral load was less than 5 x 107 IU/mL, even when the mothers tested positive for e antigen, researchers reported online May 26 in Annals of Internal Medicine.
"Prenatal treatments, such as oral antiviral agents, are unlikely to be of additional benefit in this population and may cause harm, even if used only for short periods, by inducing viral resistance or post-treatment hepatitis flares," said Ai Kubo, Ph.D., of the Kaiser Permanente division of research in Oakland, Calif., and her associates.
Vertical transmission occurred in 3.61 of every 100 live births to mothers whose viral loads exceeded the 5 x 107 IU/mL cutoff, the researchers reported (95% confidence interval, 0.75-10.56). "Future studies to evaluate the effectiveness of additional prophylaxis measures, such as late-pregnancy antiviral treatment, are warranted among these high-risk women," they said.
The observational study included 4,446 infants born to 3,253 hepatitis B virus (HBV)-positive mothers between 1997 and 2010. The mothers were members of the Kaiser Permanente Northern California integrated health services delivery organization, which has a centralized program of prenatal screening and neonatal immunoprophylaxis for HBV. The investigators searched electronic health data records to identify women who tested positive for HBV surface antigen and were due to deliver within 4 months, then tracked their infants to verify that they received HBV immunoglobulin and HBV vaccine (Ann. Int. Med. 2014 May 27 [doi: 10.7326/M13-2529]).
An estimated 0.75 infants were infected with HBV per 100 live births, the researchers reported (Poisson 95% confidence interval, 0.48-1.10). Infants born to e antigen–positive mothers had an infection rate of 3.37 per 100 live births (95% CI, 2.08-5.14), compared with 0.04 (95% CI, 0.001-0.24) for mothers who tested negative for e antigen. The lowest viral level associated with transmission was 6.32 x 107 IU/mL, the investigators said.
The centralized program expanded over time, so by 2010 most mothers who tested positive for HBV surface antigen underwent prenatal testing for e antigen status and HBV viral load, the researchers also reported. In addition, almost all offspring of infected mothers received HBV immunoglobulin, a complete series of HBV vaccine within recommended time frames, and follow-up testing for HBV immune response and infection.
"Our results suggest that an organized program with high rates of prenatal screening, detection, and immunoprophylaxis can effectively prevent vertical transmission of hepatitis B," said Dr. Kubo and her associates. But they cautioned that "a centralized approach may be difficult to implement in many prenatal settings."
Kaiser Permanente Community Benefit and the National Institutes of Health funded the study. Dr. Kubo reported no conflicts of interest.
hepatitis vertical transmission,
Mothers did not transmit hepatitis B virus to their offspring when their viral load was less than 5 x 107 IU/mL, even when the mothers tested positive for e antigen, researchers reported online May 26 in Annals of Internal Medicine.
"Prenatal treatments, such as oral antiviral agents, are unlikely to be of additional benefit in this population and may cause harm, even if used only for short periods, by inducing viral resistance or post-treatment hepatitis flares," said Ai Kubo, Ph.D., of the Kaiser Permanente division of research in Oakland, Calif., and her associates.
Vertical transmission occurred in 3.61 of every 100 live births to mothers whose viral loads exceeded the 5 x 107 IU/mL cutoff, the researchers reported (95% confidence interval, 0.75-10.56). "Future studies to evaluate the effectiveness of additional prophylaxis measures, such as late-pregnancy antiviral treatment, are warranted among these high-risk women," they said.
The observational study included 4,446 infants born to 3,253 hepatitis B virus (HBV)-positive mothers between 1997 and 2010. The mothers were members of the Kaiser Permanente Northern California integrated health services delivery organization, which has a centralized program of prenatal screening and neonatal immunoprophylaxis for HBV. The investigators searched electronic health data records to identify women who tested positive for HBV surface antigen and were due to deliver within 4 months, then tracked their infants to verify that they received HBV immunoglobulin and HBV vaccine (Ann. Int. Med. 2014 May 27 [doi: 10.7326/M13-2529]).
An estimated 0.75 infants were infected with HBV per 100 live births, the researchers reported (Poisson 95% confidence interval, 0.48-1.10). Infants born to e antigen–positive mothers had an infection rate of 3.37 per 100 live births (95% CI, 2.08-5.14), compared with 0.04 (95% CI, 0.001-0.24) for mothers who tested negative for e antigen. The lowest viral level associated with transmission was 6.32 x 107 IU/mL, the investigators said.
The centralized program expanded over time, so by 2010 most mothers who tested positive for HBV surface antigen underwent prenatal testing for e antigen status and HBV viral load, the researchers also reported. In addition, almost all offspring of infected mothers received HBV immunoglobulin, a complete series of HBV vaccine within recommended time frames, and follow-up testing for HBV immune response and infection.
"Our results suggest that an organized program with high rates of prenatal screening, detection, and immunoprophylaxis can effectively prevent vertical transmission of hepatitis B," said Dr. Kubo and her associates. But they cautioned that "a centralized approach may be difficult to implement in many prenatal settings."
Kaiser Permanente Community Benefit and the National Institutes of Health funded the study. Dr. Kubo reported no conflicts of interest.
Mothers did not transmit hepatitis B virus to their offspring when their viral load was less than 5 x 107 IU/mL, even when the mothers tested positive for e antigen, researchers reported online May 26 in Annals of Internal Medicine.
"Prenatal treatments, such as oral antiviral agents, are unlikely to be of additional benefit in this population and may cause harm, even if used only for short periods, by inducing viral resistance or post-treatment hepatitis flares," said Ai Kubo, Ph.D., of the Kaiser Permanente division of research in Oakland, Calif., and her associates.
Vertical transmission occurred in 3.61 of every 100 live births to mothers whose viral loads exceeded the 5 x 107 IU/mL cutoff, the researchers reported (95% confidence interval, 0.75-10.56). "Future studies to evaluate the effectiveness of additional prophylaxis measures, such as late-pregnancy antiviral treatment, are warranted among these high-risk women," they said.
The observational study included 4,446 infants born to 3,253 hepatitis B virus (HBV)-positive mothers between 1997 and 2010. The mothers were members of the Kaiser Permanente Northern California integrated health services delivery organization, which has a centralized program of prenatal screening and neonatal immunoprophylaxis for HBV. The investigators searched electronic health data records to identify women who tested positive for HBV surface antigen and were due to deliver within 4 months, then tracked their infants to verify that they received HBV immunoglobulin and HBV vaccine (Ann. Int. Med. 2014 May 27 [doi: 10.7326/M13-2529]).
An estimated 0.75 infants were infected with HBV per 100 live births, the researchers reported (Poisson 95% confidence interval, 0.48-1.10). Infants born to e antigen–positive mothers had an infection rate of 3.37 per 100 live births (95% CI, 2.08-5.14), compared with 0.04 (95% CI, 0.001-0.24) for mothers who tested negative for e antigen. The lowest viral level associated with transmission was 6.32 x 107 IU/mL, the investigators said.
The centralized program expanded over time, so by 2010 most mothers who tested positive for HBV surface antigen underwent prenatal testing for e antigen status and HBV viral load, the researchers also reported. In addition, almost all offspring of infected mothers received HBV immunoglobulin, a complete series of HBV vaccine within recommended time frames, and follow-up testing for HBV immune response and infection.
"Our results suggest that an organized program with high rates of prenatal screening, detection, and immunoprophylaxis can effectively prevent vertical transmission of hepatitis B," said Dr. Kubo and her associates. But they cautioned that "a centralized approach may be difficult to implement in many prenatal settings."
Kaiser Permanente Community Benefit and the National Institutes of Health funded the study. Dr. Kubo reported no conflicts of interest.
hepatitis vertical transmission,
hepatitis vertical transmission,
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Prenatal HBV screening followed by postnatal prophylaxis is effective at preventing vertical transmission of HBV, but prenatal treatment is unlikely to be of additional benefit in women with a viral load less than 5 x 107 IU/mL or a negative e antigen status.
Major finding: Mothers did not transmit hepatitis B virus to their offspring when their viral load was less than 5 x 107 IU/mL, even when they were e antigen–positive.
Data source: Observational study of 4,446 infants born to 3,253 mothers with hepatitis B virus infection between 1997 and 2010.
Disclosures: Kaiser Permanente Community Benefit and the National Institutes of Health funded the study. Dr. Kubo reported no conflicts of interest.
Several factors help predict labor onset, emergent outcome in PPROM
CHICAGO – A novel composite prognostic index score helped predict the onset of labor in a retrospective cohort of patients with preterm premature rupture of membranes.
In a separate study, researchers identified three independent predictors of emergent outcomes in patients with preterm premature rupture of membranes (PPROM).
The findings of both studies were presented in posters at the annual meeting of the American Congress of Obstetricians and Gynecologists.
The composite prognostic index score in the first study predicted the likelihood of labor within 12 hours while maintaining a significantly high negative predictive value in 78 patients between 24 and 34 weeks of gestation who were admitted with PPROM over a 2-year period, according to Dr. Yelena Feldman of Trihealth, Cincinnati.
In fact, differences in all variables included in the score were significant at 12 hours prior to labor, she noted.
Variables included as part of the score were deepest vertical pocket of amniotic fluid by ultrasound, fetal heart rate, changes in fetal heart rate variability, presence of decelerations, number of contractions, vaginal bleeding, and record of nursing concern. Each variable was scored at four time points prior to spontaneous labor onset (48, 36, 24, and 12 hours), and a model using the presence of dichotomous variables at 12 hours prior to labor onset was used to make the composite score.
A binary model with the outcome of 12 hours until labor onset had the best results (91.9% specificity; 51.25% sensitivity, 85% negative predictive value, and 67.8% positive predictive value).
Each variable was assigned a number of points based on its beta coefficient in the multivariate model, and the patient could be assigned a score based on the presence of these characteristics.
"The value would correspond to the risk of labor starting within 12 hours," she noted.
The cutoff score, determined by the receiver operating characteristic curves that signified the likelihood of starting labor in 12 hours, was 18, Dr. Feldman explained in the poster.
A score of 18 yielded a negative predictive value of 90.5%, a positive predictive value of 52%, and a sensitivity of 80.9%.
"This composite score may serve as a useful tool in clinical settings where patients admitted with PPROM need decisions regarding patient transfer, administering magnesium sulfate for neuroprotection, or administering a rescue dose of steroid," she concluded.
In the second study, Dr. Tripp Nelson of the Medical University of South Carolina, Charleston, found that malpresentation, bleeding, and sexually transmitted infection each predicted emergent outcomes in PPROM patients.
An admission test utilizing these three factors had 96.4% negative predictive value for emergent outcomes, 57.4% positive predictive value, 91% specificity, and 75.9% sensitivity. For the retrospective case-control study, Dr. Nelson and his colleagues identified 624 subjects, including 83 with at least one emergent outcome.
The emergent group had significantly higher rates of perinatal death and acidosis, and while bivariable comparison showed increased incidence of leukocytosis, urinary tract infection, sexually transmitted infection (STI), malpresentation, latency, vaginal bleeding, and fundal tenderness; only vaginal bleeding, STI, and malpresentation remained significant on logistic regression analysis.
Further randomized testing is needed for model validation, Dr. Nelson concluded.
The authors of both studies reported having no disclosures.
CHICAGO – A novel composite prognostic index score helped predict the onset of labor in a retrospective cohort of patients with preterm premature rupture of membranes.
In a separate study, researchers identified three independent predictors of emergent outcomes in patients with preterm premature rupture of membranes (PPROM).
The findings of both studies were presented in posters at the annual meeting of the American Congress of Obstetricians and Gynecologists.
The composite prognostic index score in the first study predicted the likelihood of labor within 12 hours while maintaining a significantly high negative predictive value in 78 patients between 24 and 34 weeks of gestation who were admitted with PPROM over a 2-year period, according to Dr. Yelena Feldman of Trihealth, Cincinnati.
In fact, differences in all variables included in the score were significant at 12 hours prior to labor, she noted.
Variables included as part of the score were deepest vertical pocket of amniotic fluid by ultrasound, fetal heart rate, changes in fetal heart rate variability, presence of decelerations, number of contractions, vaginal bleeding, and record of nursing concern. Each variable was scored at four time points prior to spontaneous labor onset (48, 36, 24, and 12 hours), and a model using the presence of dichotomous variables at 12 hours prior to labor onset was used to make the composite score.
A binary model with the outcome of 12 hours until labor onset had the best results (91.9% specificity; 51.25% sensitivity, 85% negative predictive value, and 67.8% positive predictive value).
Each variable was assigned a number of points based on its beta coefficient in the multivariate model, and the patient could be assigned a score based on the presence of these characteristics.
"The value would correspond to the risk of labor starting within 12 hours," she noted.
The cutoff score, determined by the receiver operating characteristic curves that signified the likelihood of starting labor in 12 hours, was 18, Dr. Feldman explained in the poster.
A score of 18 yielded a negative predictive value of 90.5%, a positive predictive value of 52%, and a sensitivity of 80.9%.
"This composite score may serve as a useful tool in clinical settings where patients admitted with PPROM need decisions regarding patient transfer, administering magnesium sulfate for neuroprotection, or administering a rescue dose of steroid," she concluded.
In the second study, Dr. Tripp Nelson of the Medical University of South Carolina, Charleston, found that malpresentation, bleeding, and sexually transmitted infection each predicted emergent outcomes in PPROM patients.
An admission test utilizing these three factors had 96.4% negative predictive value for emergent outcomes, 57.4% positive predictive value, 91% specificity, and 75.9% sensitivity. For the retrospective case-control study, Dr. Nelson and his colleagues identified 624 subjects, including 83 with at least one emergent outcome.
The emergent group had significantly higher rates of perinatal death and acidosis, and while bivariable comparison showed increased incidence of leukocytosis, urinary tract infection, sexually transmitted infection (STI), malpresentation, latency, vaginal bleeding, and fundal tenderness; only vaginal bleeding, STI, and malpresentation remained significant on logistic regression analysis.
Further randomized testing is needed for model validation, Dr. Nelson concluded.
The authors of both studies reported having no disclosures.
CHICAGO – A novel composite prognostic index score helped predict the onset of labor in a retrospective cohort of patients with preterm premature rupture of membranes.
In a separate study, researchers identified three independent predictors of emergent outcomes in patients with preterm premature rupture of membranes (PPROM).
The findings of both studies were presented in posters at the annual meeting of the American Congress of Obstetricians and Gynecologists.
The composite prognostic index score in the first study predicted the likelihood of labor within 12 hours while maintaining a significantly high negative predictive value in 78 patients between 24 and 34 weeks of gestation who were admitted with PPROM over a 2-year period, according to Dr. Yelena Feldman of Trihealth, Cincinnati.
In fact, differences in all variables included in the score were significant at 12 hours prior to labor, she noted.
Variables included as part of the score were deepest vertical pocket of amniotic fluid by ultrasound, fetal heart rate, changes in fetal heart rate variability, presence of decelerations, number of contractions, vaginal bleeding, and record of nursing concern. Each variable was scored at four time points prior to spontaneous labor onset (48, 36, 24, and 12 hours), and a model using the presence of dichotomous variables at 12 hours prior to labor onset was used to make the composite score.
A binary model with the outcome of 12 hours until labor onset had the best results (91.9% specificity; 51.25% sensitivity, 85% negative predictive value, and 67.8% positive predictive value).
Each variable was assigned a number of points based on its beta coefficient in the multivariate model, and the patient could be assigned a score based on the presence of these characteristics.
"The value would correspond to the risk of labor starting within 12 hours," she noted.
The cutoff score, determined by the receiver operating characteristic curves that signified the likelihood of starting labor in 12 hours, was 18, Dr. Feldman explained in the poster.
A score of 18 yielded a negative predictive value of 90.5%, a positive predictive value of 52%, and a sensitivity of 80.9%.
"This composite score may serve as a useful tool in clinical settings where patients admitted with PPROM need decisions regarding patient transfer, administering magnesium sulfate for neuroprotection, or administering a rescue dose of steroid," she concluded.
In the second study, Dr. Tripp Nelson of the Medical University of South Carolina, Charleston, found that malpresentation, bleeding, and sexually transmitted infection each predicted emergent outcomes in PPROM patients.
An admission test utilizing these three factors had 96.4% negative predictive value for emergent outcomes, 57.4% positive predictive value, 91% specificity, and 75.9% sensitivity. For the retrospective case-control study, Dr. Nelson and his colleagues identified 624 subjects, including 83 with at least one emergent outcome.
The emergent group had significantly higher rates of perinatal death and acidosis, and while bivariable comparison showed increased incidence of leukocytosis, urinary tract infection, sexually transmitted infection (STI), malpresentation, latency, vaginal bleeding, and fundal tenderness; only vaginal bleeding, STI, and malpresentation remained significant on logistic regression analysis.
Further randomized testing is needed for model validation, Dr. Nelson concluded.
The authors of both studies reported having no disclosures.
AT THE ACOG ANNUAL CLINICAL MEETING
Key clinical point: Prognostic tests for onset of labor and emergent outcome in PPROM look promising.
Major finding: A composite prognostic index score of 18 yielded a negative predictive value of 90.5%, positive predictive value of 52%, and sensitivity of 80.9% for labor onset within 12 hours. An admission test utilizing malpresentation, bleeding, and sexually transmitted infection had 96.4% negative predictive value for emergent outcomes.
Data source: Two retrospective studies in 78 and 624 patients, respectively.
Disclosures: The authors of both studies reported having no disclosures.