User login
Levetiracetam found effective in controlling seizures in pregnancy
Levetiracetam monotherapy appears to be an effective strategy for controlling seizures in pregnancy, results from a large Australian study demonstrated.
In fact, levetiracetam provided seizure control comparable with that of older antiepileptic drugs (AEDs) carbamazepine and valproate but superior when compared with other newer AEDs, including lamotrigine and topiramate. The findings suggest that levetiracetam can be considered "among the agents of first choice in women contemplating pregnancy, and to be suitable for use in most circumstances in women who have the potential for pregnancy, unless the type of seizure disorder present, or other consideration, makes a different agent clinically preferable," wrote Dr. Frank J. E. Vajda of the department of medicine at the University of Melbourne and his associates (Epilepsia 2014 July 3 [doi:10.1111/epi.12711]).
Using a national registry, the researchers analyzed data regarding seizure control in 1,111 pregnancies that began between the middle of 1998 and November of 2013, during which the patients took only one AED for at least the first trimester. The seizure occurrence rates for patients on levetiracetam monotherapy were 31.7% for any seizure and 13.4% for convulsive seizures, which were similar to the rates of all older AEDs combined (34.8% and 18.9%, respectively). Compared with levetiracetam, however, the rate of seizure-affected pregnancy was about 50% and 30% higher among patients on lamotrigine and topiramate, respectively.
The authors acknowledged certain limitations of the study, including the fact that the allocation of particular AEDs "was not randomized, and there can be no guarantee that the drugs were always taken as prescribed or necessarily employed optimally, guided by plasma AED concentration monitoring when that was available."
The researchers stated that they had no relevant financial conflicts.
Levetiracetam monotherapy appears to be an effective strategy for controlling seizures in pregnancy, results from a large Australian study demonstrated.
In fact, levetiracetam provided seizure control comparable with that of older antiepileptic drugs (AEDs) carbamazepine and valproate but superior when compared with other newer AEDs, including lamotrigine and topiramate. The findings suggest that levetiracetam can be considered "among the agents of first choice in women contemplating pregnancy, and to be suitable for use in most circumstances in women who have the potential for pregnancy, unless the type of seizure disorder present, or other consideration, makes a different agent clinically preferable," wrote Dr. Frank J. E. Vajda of the department of medicine at the University of Melbourne and his associates (Epilepsia 2014 July 3 [doi:10.1111/epi.12711]).
Using a national registry, the researchers analyzed data regarding seizure control in 1,111 pregnancies that began between the middle of 1998 and November of 2013, during which the patients took only one AED for at least the first trimester. The seizure occurrence rates for patients on levetiracetam monotherapy were 31.7% for any seizure and 13.4% for convulsive seizures, which were similar to the rates of all older AEDs combined (34.8% and 18.9%, respectively). Compared with levetiracetam, however, the rate of seizure-affected pregnancy was about 50% and 30% higher among patients on lamotrigine and topiramate, respectively.
The authors acknowledged certain limitations of the study, including the fact that the allocation of particular AEDs "was not randomized, and there can be no guarantee that the drugs were always taken as prescribed or necessarily employed optimally, guided by plasma AED concentration monitoring when that was available."
The researchers stated that they had no relevant financial conflicts.
Levetiracetam monotherapy appears to be an effective strategy for controlling seizures in pregnancy, results from a large Australian study demonstrated.
In fact, levetiracetam provided seizure control comparable with that of older antiepileptic drugs (AEDs) carbamazepine and valproate but superior when compared with other newer AEDs, including lamotrigine and topiramate. The findings suggest that levetiracetam can be considered "among the agents of first choice in women contemplating pregnancy, and to be suitable for use in most circumstances in women who have the potential for pregnancy, unless the type of seizure disorder present, or other consideration, makes a different agent clinically preferable," wrote Dr. Frank J. E. Vajda of the department of medicine at the University of Melbourne and his associates (Epilepsia 2014 July 3 [doi:10.1111/epi.12711]).
Using a national registry, the researchers analyzed data regarding seizure control in 1,111 pregnancies that began between the middle of 1998 and November of 2013, during which the patients took only one AED for at least the first trimester. The seizure occurrence rates for patients on levetiracetam monotherapy were 31.7% for any seizure and 13.4% for convulsive seizures, which were similar to the rates of all older AEDs combined (34.8% and 18.9%, respectively). Compared with levetiracetam, however, the rate of seizure-affected pregnancy was about 50% and 30% higher among patients on lamotrigine and topiramate, respectively.
The authors acknowledged certain limitations of the study, including the fact that the allocation of particular AEDs "was not randomized, and there can be no guarantee that the drugs were always taken as prescribed or necessarily employed optimally, guided by plasma AED concentration monitoring when that was available."
The researchers stated that they had no relevant financial conflicts.
FROM EPILEPSIA
Key clinical point: Compared with older antiepileptic drugs, levetiracetam was equally effective in keeping pregnant women with the major types of epilepsy seizure free.
Major finding: Seizure occurrence rates for patients on levetiracetam monotherapy were 31.7% for any seizure and 13.4% for convulsive seizures, similar to the rates of all older AEDs combined (34.8% and 18.9%, respectively).
Data source: A study of 1,111 pregnancies in Australian women that began between mid-1998 and November of 2013.
Disclosures: The authors stated that they had no relevant financial conflicts.
Donated IVF eggs linked to gestational hypertension
MUNICH – Women pregnant with an embryo produced by in vitro fertilization of a donated egg developed gestational hypertension at nearly four times the rate of matched women who were pregnant following IVF with a self egg, in a multicenter, case-control study with 580 participants.
In addition, preeclampsia was more than four times higher in the women who had received a donor egg as part of an IVF procedure, compared with women who used a self egg for their IVF, Dr. Hélène Letur-Könirsch reported at the annual meeting of the European Society of Human Reproduction and Embryology.
Although the mechanism underlying this association is not known, one possible explanation is that when a woman carries an embryo that is completely allogenic, it triggers modified immune tolerance that leads to impaired trophoblast implantation. Other candidate hypotheses are that defective genes in the mother trigger abnormal metabolic pathways that cause hypertension and preeclampsia, and that ovarian insufficiency features reduced ovarian steroid production, causing vascular and immunologic changes that lead to pregnancy-induced hypertension.
Results from several prior studies also had shown a link between use of a donor egg for IVF and an increased rate of gestational hypertension and preeclampsia, but most prior studies had been smaller and failed to adequately control for possible confounders, said Dr. Letur-Könirsch, an endocrinologist at the Fertility Center of the Institut Mutualiste Montsouris in Paris.
She and her associates from seven IVF centers in France pooled data from their entire series of IVF cases that involved donated eggs during the period from February 2005 to September 2012, a total of 217 pregnant women. The analysis matched these cases with 363 control women who had IVF pregnancies during the study period using self eggs by parameters such as age, parity, time of pregnancy, and whether the IVF procedure involved transfer of a fresh or frozen embryo.
Women in the study averaged 35 years of age, with 82% aged 39 years or younger. Average body mass index was virtually identical in both groups, about 24 kg/m2, and the live-birth rate was 79% in both groups. All women in both groups were normotensive prior to their IVF procedure.
The incidence of pregnancy-induced hypertension, defined as blood pressure that reached at least 140/90 mm Hg when measured in office on both arms with two separate measurements, was about 29% in the women who received a donated egg and 14% in those who used a self egg. Development of preeclampsia, defined as persistent gestational hypertension plus proteinuria of at least 0.3 g/day, occurred in about 18% of women who received a donated egg and 7% in those who used a self egg.
In a multivariate analysis that controlled for age, pregnancy history, and use of a fresh or frozen embryo, women who used a donated egg had a 3.9-fold increased rate of pregnancy-induced hypertension and a 4.6-fold increased rate of preeclampsia, compared with women who used a self egg – both statistically significant differences.
The findings suggested that before a woman embarks on pregnancy using a donated egg, she should undergo thorough evaluation for preexisting risk factors for gestational hypertension, including hypertension, obesity, diabetes, renal disease, chronic infection, autoimmune diseases such as systemic lupus erythematosus, a family history of preeclampsia, and living at a high altitude. If these risk factors are present, they should be controlled if possible. Women who have one or more of these risk factors may also be candidates for prophylactic treatment with aspirin to prevent development of preeclampsia, especially if they develop pregnancy-induced hypertension.
During pregnancy, women who received a donor egg should undergo regular and frequent blood pressure measurement, as well as assessment for other possible abnormalities such as Doppler ultrasound examination of uterine arteries and measuring serum and urine markers.
"Physicians and patients must be aware of the risk [from donor eggs] to assure that these women get adequate monitoring and management during pregnancy," Dr. Letur-Könirsch said.
Dr. Letur-Könirsch had no disclosures.
On Twitter @mitchelzoler
MUNICH – Women pregnant with an embryo produced by in vitro fertilization of a donated egg developed gestational hypertension at nearly four times the rate of matched women who were pregnant following IVF with a self egg, in a multicenter, case-control study with 580 participants.
In addition, preeclampsia was more than four times higher in the women who had received a donor egg as part of an IVF procedure, compared with women who used a self egg for their IVF, Dr. Hélène Letur-Könirsch reported at the annual meeting of the European Society of Human Reproduction and Embryology.
Although the mechanism underlying this association is not known, one possible explanation is that when a woman carries an embryo that is completely allogenic, it triggers modified immune tolerance that leads to impaired trophoblast implantation. Other candidate hypotheses are that defective genes in the mother trigger abnormal metabolic pathways that cause hypertension and preeclampsia, and that ovarian insufficiency features reduced ovarian steroid production, causing vascular and immunologic changes that lead to pregnancy-induced hypertension.
Results from several prior studies also had shown a link between use of a donor egg for IVF and an increased rate of gestational hypertension and preeclampsia, but most prior studies had been smaller and failed to adequately control for possible confounders, said Dr. Letur-Könirsch, an endocrinologist at the Fertility Center of the Institut Mutualiste Montsouris in Paris.
She and her associates from seven IVF centers in France pooled data from their entire series of IVF cases that involved donated eggs during the period from February 2005 to September 2012, a total of 217 pregnant women. The analysis matched these cases with 363 control women who had IVF pregnancies during the study period using self eggs by parameters such as age, parity, time of pregnancy, and whether the IVF procedure involved transfer of a fresh or frozen embryo.
Women in the study averaged 35 years of age, with 82% aged 39 years or younger. Average body mass index was virtually identical in both groups, about 24 kg/m2, and the live-birth rate was 79% in both groups. All women in both groups were normotensive prior to their IVF procedure.
The incidence of pregnancy-induced hypertension, defined as blood pressure that reached at least 140/90 mm Hg when measured in office on both arms with two separate measurements, was about 29% in the women who received a donated egg and 14% in those who used a self egg. Development of preeclampsia, defined as persistent gestational hypertension plus proteinuria of at least 0.3 g/day, occurred in about 18% of women who received a donated egg and 7% in those who used a self egg.
In a multivariate analysis that controlled for age, pregnancy history, and use of a fresh or frozen embryo, women who used a donated egg had a 3.9-fold increased rate of pregnancy-induced hypertension and a 4.6-fold increased rate of preeclampsia, compared with women who used a self egg – both statistically significant differences.
The findings suggested that before a woman embarks on pregnancy using a donated egg, she should undergo thorough evaluation for preexisting risk factors for gestational hypertension, including hypertension, obesity, diabetes, renal disease, chronic infection, autoimmune diseases such as systemic lupus erythematosus, a family history of preeclampsia, and living at a high altitude. If these risk factors are present, they should be controlled if possible. Women who have one or more of these risk factors may also be candidates for prophylactic treatment with aspirin to prevent development of preeclampsia, especially if they develop pregnancy-induced hypertension.
During pregnancy, women who received a donor egg should undergo regular and frequent blood pressure measurement, as well as assessment for other possible abnormalities such as Doppler ultrasound examination of uterine arteries and measuring serum and urine markers.
"Physicians and patients must be aware of the risk [from donor eggs] to assure that these women get adequate monitoring and management during pregnancy," Dr. Letur-Könirsch said.
Dr. Letur-Könirsch had no disclosures.
On Twitter @mitchelzoler
MUNICH – Women pregnant with an embryo produced by in vitro fertilization of a donated egg developed gestational hypertension at nearly four times the rate of matched women who were pregnant following IVF with a self egg, in a multicenter, case-control study with 580 participants.
In addition, preeclampsia was more than four times higher in the women who had received a donor egg as part of an IVF procedure, compared with women who used a self egg for their IVF, Dr. Hélène Letur-Könirsch reported at the annual meeting of the European Society of Human Reproduction and Embryology.
Although the mechanism underlying this association is not known, one possible explanation is that when a woman carries an embryo that is completely allogenic, it triggers modified immune tolerance that leads to impaired trophoblast implantation. Other candidate hypotheses are that defective genes in the mother trigger abnormal metabolic pathways that cause hypertension and preeclampsia, and that ovarian insufficiency features reduced ovarian steroid production, causing vascular and immunologic changes that lead to pregnancy-induced hypertension.
Results from several prior studies also had shown a link between use of a donor egg for IVF and an increased rate of gestational hypertension and preeclampsia, but most prior studies had been smaller and failed to adequately control for possible confounders, said Dr. Letur-Könirsch, an endocrinologist at the Fertility Center of the Institut Mutualiste Montsouris in Paris.
She and her associates from seven IVF centers in France pooled data from their entire series of IVF cases that involved donated eggs during the period from February 2005 to September 2012, a total of 217 pregnant women. The analysis matched these cases with 363 control women who had IVF pregnancies during the study period using self eggs by parameters such as age, parity, time of pregnancy, and whether the IVF procedure involved transfer of a fresh or frozen embryo.
Women in the study averaged 35 years of age, with 82% aged 39 years or younger. Average body mass index was virtually identical in both groups, about 24 kg/m2, and the live-birth rate was 79% in both groups. All women in both groups were normotensive prior to their IVF procedure.
The incidence of pregnancy-induced hypertension, defined as blood pressure that reached at least 140/90 mm Hg when measured in office on both arms with two separate measurements, was about 29% in the women who received a donated egg and 14% in those who used a self egg. Development of preeclampsia, defined as persistent gestational hypertension plus proteinuria of at least 0.3 g/day, occurred in about 18% of women who received a donated egg and 7% in those who used a self egg.
In a multivariate analysis that controlled for age, pregnancy history, and use of a fresh or frozen embryo, women who used a donated egg had a 3.9-fold increased rate of pregnancy-induced hypertension and a 4.6-fold increased rate of preeclampsia, compared with women who used a self egg – both statistically significant differences.
The findings suggested that before a woman embarks on pregnancy using a donated egg, she should undergo thorough evaluation for preexisting risk factors for gestational hypertension, including hypertension, obesity, diabetes, renal disease, chronic infection, autoimmune diseases such as systemic lupus erythematosus, a family history of preeclampsia, and living at a high altitude. If these risk factors are present, they should be controlled if possible. Women who have one or more of these risk factors may also be candidates for prophylactic treatment with aspirin to prevent development of preeclampsia, especially if they develop pregnancy-induced hypertension.
During pregnancy, women who received a donor egg should undergo regular and frequent blood pressure measurement, as well as assessment for other possible abnormalities such as Doppler ultrasound examination of uterine arteries and measuring serum and urine markers.
"Physicians and patients must be aware of the risk [from donor eggs] to assure that these women get adequate monitoring and management during pregnancy," Dr. Letur-Könirsch said.
Dr. Letur-Könirsch had no disclosures.
On Twitter @mitchelzoler
AT ESHRE 2014
Key clinical point: Women who became pregnant by in vitro fertilization with a donated egg had a substantially increased risk of pregnancy-induced hypertension and preeclampsia.
Major finding: Women with IVF pregnancy using donated eggs had a 3.9-fold increased rate of gestational hypertension, compared with women using self eggs.
Data source: A case-control study with 580 women who underwent IVF at any of seven French fertility clinics.
Disclosures: Dr. Letur-Könirsch had no disclosures.
VIDEO: Kisspeptin outperforms HCG for early miscarriage prediction
CHICAGO – A one-time measurement of plasma kisspeptin – a family of placental peptides also being studied for fertility treatment – better predicts miscarriage than does serial measurement of human chorionic gonadotropin (HCG), a widely used measure.
British researchers measured both in 993 asymptomatic women at approximately 11 weeks’ gestation, 50 of whom later miscarried. Plasma kisspeptin proved a more accurate predictor of miscarriage than HCG: the area under the receiver operating characteristic (ROC) curve for plasma kisspeptin was 0.899, compared with 0.775 for serum HCG. Plasma kisspeptin above 1,306 pmol/L was associated with a highly significant 87% reduced risk of miscarriage, even after adjusting for age, body mass index, gestational age, smoking, and blood pressure.
Lead investigator Dr. Ali Abbara of Imperial College London explained why that matters at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A one-time measurement of plasma kisspeptin – a family of placental peptides also being studied for fertility treatment – better predicts miscarriage than does serial measurement of human chorionic gonadotropin (HCG), a widely used measure.
British researchers measured both in 993 asymptomatic women at approximately 11 weeks’ gestation, 50 of whom later miscarried. Plasma kisspeptin proved a more accurate predictor of miscarriage than HCG: the area under the receiver operating characteristic (ROC) curve for plasma kisspeptin was 0.899, compared with 0.775 for serum HCG. Plasma kisspeptin above 1,306 pmol/L was associated with a highly significant 87% reduced risk of miscarriage, even after adjusting for age, body mass index, gestational age, smoking, and blood pressure.
Lead investigator Dr. Ali Abbara of Imperial College London explained why that matters at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A one-time measurement of plasma kisspeptin – a family of placental peptides also being studied for fertility treatment – better predicts miscarriage than does serial measurement of human chorionic gonadotropin (HCG), a widely used measure.
British researchers measured both in 993 asymptomatic women at approximately 11 weeks’ gestation, 50 of whom later miscarried. Plasma kisspeptin proved a more accurate predictor of miscarriage than HCG: the area under the receiver operating characteristic (ROC) curve for plasma kisspeptin was 0.899, compared with 0.775 for serum HCG. Plasma kisspeptin above 1,306 pmol/L was associated with a highly significant 87% reduced risk of miscarriage, even after adjusting for age, body mass index, gestational age, smoking, and blood pressure.
Lead investigator Dr. Ali Abbara of Imperial College London explained why that matters at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ICE/ENDO 2014
The short cervix and preterm birth: 8 key questions and evidence-based answers
CASE: NULLIPAROUS WOMAN WITH A SHORT CERVIX
Your ultrasound technician telephones to report that your 32-year-old nulliparous patient, who is currently at 20 weeks’ gestation, was incidentally found to have a short cervix (18 mm) at the time of her routine fetal anatomy survey.
How do you proceed? And how do you counsel the patient? What interventions might reduce the risk of preterm birth (PTB)? Would your recommendations change if she had a history of PTB or was carrying twins?
Preterm birth, defined as delivery prior to 37 weeks’ gestation, is the leading cause of neonatal morbidity and mortality in the United States. The rate of PTB peaked at 12.8% in 2006 and has slowly declined since but remains unacceptably high at 11.5%.1 Most PTBs are spontaneous, arising from the onset of labor or from preterm premature rupture of membranes. Regrettably, tocolytics remain largely ineffective once the process of preterm parturition has begun.
Ideally, women at highest risk for PTB could be identified so that additional screening and interventions could be initiated. Few prognostic tests are available to predict which women will deliver preterm. Generally, the greatest risk factor for spontaneous PTB is a history of spontaneous PTB.2,3 However, women with such a history account for only 10% of all births before 34 weeks’ gestation.
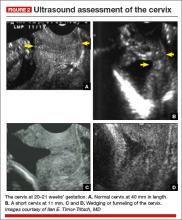
The appearance and length of the cervix during the second trimester appears to be an even better predictor of spontaneous PTB than history alone (FIGURE 1).4,5 For example, in one study of unselected pregnant women at 22 to 24 weeks’ gestation, only 1.7% had a cervical length less than 15 mm, but they accounted for 58% of births before 32 weeks.6 The shorter the cervix, the greater the risk of spontaneous PTB.7 The presence of a short cervix is even more ominous in a woman with a history of spontaneous PTB.8
Optimal pregnancy management after detection of a short cervix remains somewhat unclear and varies, based on the rest of the patient’s clinical picture and obstetric history.
In this article, I address 8 critical questions about diagnosis and management of the short cervix in the second trimester and offer evidence-based answers for clinical practice.
1. How is a short cervix defined?
A cervical length below the 10th centile for gestational age is considered “short.” At 18 to 24 weeks’ gestation, the 10th centile corresponds to a cervical length of less than 25 mm.9
The cervix undergoes physiologic shortening that begins at 28 to 30 weeks of gestation. At 32 weeks, the 50th centile for cervical length is 25 mm. Therefore, cervical-length measurements that appear moderately short between 28 and 32 weeks and beyond are of limited clinical utility, and the clinician should incorporate gestational age into prematurity risk assessment.7
2. Who should be screened?
The question of whether universal cervical-length assessment should be performed is controversial. Several decision analyses in recent years suggest that universal sonographic screening for a short cervix is cost-effective.10,11 Overall, however, the effectiveness of universal cervical-length screening remains clinically understudied, and it is difficult to draw conclusions from decision analyses. Moreover, there is considerable concern about resources and feasibility of implementing universal vaginal cervical-length assessment, as well as significant disagreement about the accuracy of transabdominal cervical-length assessment in the detection of a short cervix.
Transabdominal ultrasound may overestimate cervical length by as much as 10 to 15 mm. One recent study demonstrated that, using a transabdominal cutoff of 30 mm, the sensitivity of detecting a transvaginal cervical length of less than 20 mm was 90%; if the cutoff was increased to 35 mm, sensitivity increased to 100%.12
A collaborative practice guideline on obstetric ultrasound from the American College of Radiology, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, and the Society of Radiologists in Ultrasound recommends that the maternal cervix be examined “as clinically appropriate when technically feasible” during a standard second- or third-trimester ultrasound examination (FIGURE 2).13 The guideline also states that transvaginal or transperineal ultrasound may be considered if the cervix appears shortened or cannot be adequately visualized during the transabdominal ultrasound. However, no specific protocols are suggested.
Given the uncertainty, it is recommended that each practice or ultrasound unit adopt a standard protocol for cervical-length assessment during pregnancy. This protocol can entail either routine abdominal or vaginal assessment of the cervix, or a combination of abdominal and vaginal assessment. Clinical risk factors can be used to help stratify low-risk women when abdominal cervical-length assessment is the initial approach to evaluation.
In my practice, all women undergo cervical-length assessment at the time of the routine anatomy survey (18–22 weeks). Those who are at low risk for PTB are screened initially with transabdominal ultrasound, and a transvaginal examination is performed if the cervix cannot be seen or appears to be less than 30 mm in length.
Women with a history of spontaneous PTB undergo screening by transvaginal cervical-length assessment. Typically, the first measurement is obtained at the time of the fetal anatomic survey (18–22 weeks), when the lower uterine segment is sufficiently developed to accurately measure the cervix. We perform serial cervical-length assessment every 1 or 2 weeks until 28 weeks’ gestation in women with a prior early spontaneous PTB (<34 weeks), those with a history of recurrent PTB, and those who have an initial short cervix. Serial monitoring has been shown to increase the prediction of spontaneous PTB in high-risk women.14
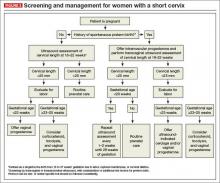
See the algorithm presented in (FIGURE 3) for the screening and treatment of women with singleton gestations.
3. How do I counsel patients about the risk of prematurity?
The risk of spontaneous PTB varies with the gestational age that the short cervix is detected and with the degree of cervical shortening. The earlier in the pregnancy the cervix is found to be short, the higher the risk for spontaneous PTB. For example, results of one large multicenter study of almost 3,000 unselected women pregnant with a singleton gestation across the United States showed that a cervical length of 25 mm was associated with a 15% to 20% incidence of PTB when detected at 28 weeks’ gestation; the incidence rose to 30% to 35% if the short cervix was detected at 20 weeks.9 In this cohort, 84% of women had no history of PTB.
A short cervix and a prior PTB (particularly a very early prior PTB) are two major risk factors for PTB. Together, they significantly increase the risk of an early delivery over individual or single factors alone. Among women who have had a prior PTB and who now have a cervical length of less than 25 mm, the risk of recurrent PTB is 35% to 40%. In contrast, women with a prior PTB and a normal cervical length have a significantly lower risk of recurrence—around 10%.15
If the physical examination is concerning for cervical dilation or prolapsing membranes, women should be counseled about the poor prognosis for the pregnancy, particularly when these findings are detected at a previable or periviable gestational age, regardless of their history of PTB. In these circumstances, in the absence of labor or intra-amniotic infection, a “rescue” cervical cerclage may be considered as a last resort (see page 34 for more on cerclage).
4. What evaluation or monitoring is needed once a short cervix is identified?
Women found to have a short cervix should be evaluated for the presence of preterm labor and intra-amniotic infection. This evaluation may include a sterile speculum examination or digital cervical examination, or both, as well as screening for genitourinary tract infection. Other testing may include a complete blood count with a white blood cell differential and external tocometry with or without fetal heart rate monitoring (based on the gestational age, as appropriate).
For some women with a short cervix, intra-amniotic infection may be a contributing factor (or it may develop if there are exposed membranes in the vagina). The presence of intra-amniotic infection precludes further expectant management of the pregnancy because of the risk of maternal infectious morbidity, including sepsis.
Women who have intra-amniotic infection are not candidates for any intervention such as cerclage or progesterone supplementation.
If the patient is at or beyond the point of fetal viability at the time her short cervix is detected, consider external fetal heart rate monitoring.
Antenatal corticosteroids can be administered, as appropriate, depending on the perceived risk of delivery.
5. Should I place a cerclage?
Numerous studies have examined the efficacy of ultrasound-indicated cerclage, a surgical procedure to stitch the cervix closed once a short cervix has been detected.
In general, placement of a cervical cerclage is not offered past the point of fetal viability, which is generally in the range of 23 to 25 weeks’ gestation, depending on local institutional and neonatal intensive care unit policies. Confirmed or suspected chorioamnionitis is also a contraindication to cerclage placement.
Among women without a history of PTB who are found to have a short cervix, existing data do not suggest a benefit for cerclage, although vaginal progesterone appears to be a reasonable option (see page 36).16,17
As for women with a history of PTB, Owen and colleagues studied 302 patients with a cervical length less than 25 mm and a history of spontaneous PTB before 34 weeks.18 The women were randomly assigned to ultrasound-indicated cerclage or “usual care,” which consisted of recommendations of pelvic rest, physical activity restriction, and education about the symptoms of preterm labor. Otherwise, management was directed by clinical practice at each center. All women treated with cerclage had a reduced risk of previable PTB (<24 weeks’ gestation), and those who had the shortest cervical length (<15 mm) also had a lower risk of delivery before 35 weeks.
The degree of cervical shortening that has “qualified” women for study enrollment has varied between studies, with upper limits ranging from 15 to 25 mm. Berghella and colleagues performed a patient-level meta-analysis to determine whether efficacy of the cerclage varied by cervical length at the time of placement.8 They examined 552 women with singleton gestations from four randomized controlled trials that included 208 women with a short cervix and a history of spontaneous PTB. They found a significant reduction in the rate of preterm delivery before 35 weeks’ gestation among women with singleton pregnancies, a short cervix, and a history of spontaneous PTB; the reduction did not vary by the degree of cervical shortening. However, there was no significant reduction in the rate of PTB among the subset of women without a history of spontaneous PTB.
Berghella and colleagues estimated that, if a cervical cerclage were offered to the 8% of women with a prior spontaneous PTB and a cervical length of less than 25 mm, more than 6,500 newborns would be saved each year from perinatal death associated with prematurity.8
The placement of a “rescue” cerclage in the setting of cervical dilation with or without prolapsing membranes is associated with high rates of maternal and neonatal morbidity, regardless of the patient’s obstetric history. However, cerclage placement in this setting may be associated with better outcomes than expectant management with bed rest alone.19 Patients should be carefully counseled about this procedure, including the risk of infection and the possibility that pregnancy may be prolonged only from a previable to a periviable gestational age. Decisions as to whether a patient is a candidate for rescue cerclage should be made in consultation with a maternal-fetal medicine specialist.20
6. Is a cervical pessary beneficial?
The pessary is another “mechanical” treatment similar to cerclage, and it may be helpful in reducing the incidence of PTB among women with a short cervix. In the largest study of this approach, 16,000 primarily low-risk women with singleton gestations were screened for a short cervix. Of these, 385 women with a cervical length of less than 25 mm were randomly assigned to undergo Arabin pessary placement or expectant management.21
Among those who received the pessary, the odds ratio (OR) for PTB before 34 weeks was significantly reduced (OR, 0.18; 95% confidence interval [CI], 0.08–0.37), and the OR for adverse composite neonatal outcome also was significantly reduced (OR, 0.14; 95% CI, 0.04–0.39).21
The Arabin pessary is not currently approved by the US Food and Drug Administration for this indication in the United States, nor is it available for insertion outside of research studies. Other ring-shaped pessaries are available in the United States, but their use in the setting of a short cervix is considered experimental and off-label. Additional studies of this promising intervention are currently under way.
7. Who is a candidate for supplemental progesterone?
Progesterone is a naturally occurring hormone essential to the maintenance of pregnancy. It has an overall quiescent effect on the myometrium, is known to have anti-inflammatory properties, and inhibits cervical ripening.22 It has been studied in a number of different formulations and doses.
Vaginal progesterone supplementation has been shown to reduce the risk of PTB among women with a shortened cervix regardless of their pregnancy history.16,17,23,24 In the largest trials of unselected general obstetrics populations (including women with and without a history of PTB), use of vaginal progesterone among women with a short cervix reduced the rates of very early spontaneous PTB (<28 and <32 weeks’ gestation) by 40% to 50%.16,17 As expected, progesterone also was associated with a significant reduction in the rates of respiratory distress syndrome and composite neonatal morbidity.
Intramuscular (IM) progesterone supplementation has been shown to reduce the rate of recurrent PTB among women with a history of spontaneous PTB. When caring for a woman with a history of PTB who is found to have a short cervix, IM progesterone should be offered if the woman is not already taking it. IM progesterone has not been proven effective among nulliparous women who are incidentally found to have a short cervix; it should not be offered in this situation.25
8. Should the care of women carrying twins or triplets be managed differently?
Yes. Although women with multiple gestations are at higher risk for PTB than women carrying a singleton fetus, no interventions have proven to be effective in this population. Many studies have been limited to twin gestations as a group, with an inability to perform subgroup analyses or enroll women who also have a short cervix, due to sample size and power issues.
Progesterone in multiple gestations
Although several formulations of progesterone—including IM 17-alpha hydroxyprogesterone caproate, micronized progesterone, and progesterone suppositories—have been studied, no randomized trial data have demonstrated a reduction in PTB or neonatal morbidity.26–29 Individual patient-level data from a meta-analysis of vaginal progesterone in the setting of multiple gestations with a short cervical length suggest trends toward a reduced rate of PTB before 33 weeks’ gestation (relative risk [RR], 0.70; 95% CI, 0.34–1.44) and lower composite neonatal morbidity and mortality (RR, 0.56; 95% CI, 0.30–0.97).30
Mechanical strategies in multiple gestations
No randomized data suggest that a pessary is effective in multiple gestations. In one study of 813 multiple gestations in the Netherlands, women were randomly assigned—regardless of cervical length—to receive a pessary at 16 to 20 weeks versus standard care; no difference in adverse perinatal outcomes was detected between groups.31
As for cerclage, although data are limited, some studies suggest that placement of a cerclage in twin gestations with cervical shortening may increase the rate of PTB.32
Bottom line for multiples
Although women carrying multiple gestations are at higher risk for PTB, data are extremely limited. At present, data do not support routine use of cerclage for a short cervix—and some suggest possible harm. Vaginal progesterone or placement of a pessary may be of benefit but should be used with caution and with the understanding that data are sparse.
CASE RESOLVED
You counsel your nulliparous patient that she has an elevated risk of PTB, based on her cervical length of 18 mm at 20 weeks’ gestation, and evaluate her clinically for evidence of preterm labor. Apart from the short cervix, her examination is unremarkable. You offer her nightly vaginal progesterone suppositories and schedule a visit to reevaluate her cervix in 1 week. If cervical dilation or prolapsing membranes are noted before the age of fetal viability, you will consider placing a “rescue” cervical cerclage.
Had this patient experienced a prior PTB, you would first ensure that she is taking IM 17-alpha hydroxyprogesterone caproate. It also would be reasonable to place an ultrasound-indicated cerclage or begin vaginal progesterone suppositories. Although data are limited on concomitant use of IM and vaginal progesterone, some experts may consider it, on an experimental basis, for patients with a short cervix and a prior PTB.
If this patient were carrying a twin gestation, vaginal progesterone would still be a consideration, provided she is counseled about the limited evidence of its efficacy in this setting. Cerclage would not be appropriate, given the possible risk of harm.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, city and state. Stay in touch! Your feedback is important to us!
1. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
2. Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–1596.
3. Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 2006;195(3):643–650.
4. To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: A population-based prospective study. Ultrasound Obstet Gynecol. 2006;27(4):362–367.
5. Guzman ER, Ananth CV. Cervical length and spontaneous prematurity: Laying the foundation for future interventional randomized trials for the short cervix. Ultrasound Obstet Gynecol. 2001;18(3):195–199.
6. Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: Prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12(5):312–317.
7. Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110(2 Pt 1):311–317.
8. Berghella V, Keeler SM, To MS, Althuisius SM, Rust OA. Effectiveness of cerclage according to severity of cervical length shortening: A meta-analysis. Ultrasound Obstet Gynecol. 2010;35(4):468–473.
9. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567–572.
10. Werner EF, Han CS, Pettker CM, et al. Universal cervical-length screening to prevent preterm birth: A cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38 (1):32–37.
11. Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: A decision and economic analysis. Am J Obstet Gynecol. 2010;202(6):548.e1–e8.
12. Friedman AM, Srinivas SK, Parry S, Elovitz MA, Wang E, Schwartz N. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1–e7.
13. American College of Radiology, American College of Obstetricians and Gynecologists, American Institute of Ultrasound in Medicine, Society of Radiologists in Ultrasound. ACR–ACOG–AIUM–SRU Practice Guideline for the Performance of Obstetrical Ultrasound. http://www.acr.org/Quality-Safety/Standards-Guidelines/Practice-Guidelines-by-Modality/~/media/ACR/Documents/PGTS/guidelines/US_Obstetrical.pdf. Accessed June 16, 2014.
14. Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340–1348.
15. Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: Recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178(5):1035–1040.
16. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469.
17. Hassan SS, Romero R, Vidyadhari D, et al; PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: A multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31.
18. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1–e8.
19. Namouz S, Porat S, Okun N, Windrim R, Farine D. Emergency cerclage: literature review. Obstet Gynecol Surv. 2013;68(5):379–388.
20. Cockwell HA, Smith GN. Cervical incompetence and the role of emergency cerclage. J Obstet Gynaecol Can. 2005;27(2):123–129.
21. Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): An open-label randomised controlled trial. Lancet. 2012;379(9828):1800–1806.
22. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23(7):947–954.
23. Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. Erratum: N England J Med. 2003;349(13):1299.
24. O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: Primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30(5):687–696.
25. Grobman WA, Thom EA, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207(5):390.e1–e8.
26. Rouse DJ, Caritis SN, Peaceman AM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454–461.
27. Caritis SN, Rouse DJ, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Maternal-Fetal Medicine Units (MFMU) Network. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: A randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):285–292.
28. Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A; PREDICT Group. Prevention of preterm delivery in twin gestations (PREDICT): A multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38(3):272–280.
29. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): A randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034–2040.
30. Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1–e19.
31. Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): A multicentre, open-label randomised controlled trial. Lancet. 2013;382(9901):1341–1349.
32. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: Meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106(1):181–189.
CASE: NULLIPAROUS WOMAN WITH A SHORT CERVIX
Your ultrasound technician telephones to report that your 32-year-old nulliparous patient, who is currently at 20 weeks’ gestation, was incidentally found to have a short cervix (18 mm) at the time of her routine fetal anatomy survey.
How do you proceed? And how do you counsel the patient? What interventions might reduce the risk of preterm birth (PTB)? Would your recommendations change if she had a history of PTB or was carrying twins?
Preterm birth, defined as delivery prior to 37 weeks’ gestation, is the leading cause of neonatal morbidity and mortality in the United States. The rate of PTB peaked at 12.8% in 2006 and has slowly declined since but remains unacceptably high at 11.5%.1 Most PTBs are spontaneous, arising from the onset of labor or from preterm premature rupture of membranes. Regrettably, tocolytics remain largely ineffective once the process of preterm parturition has begun.
Ideally, women at highest risk for PTB could be identified so that additional screening and interventions could be initiated. Few prognostic tests are available to predict which women will deliver preterm. Generally, the greatest risk factor for spontaneous PTB is a history of spontaneous PTB.2,3 However, women with such a history account for only 10% of all births before 34 weeks’ gestation.
The appearance and length of the cervix during the second trimester appears to be an even better predictor of spontaneous PTB than history alone (FIGURE 1).4,5 For example, in one study of unselected pregnant women at 22 to 24 weeks’ gestation, only 1.7% had a cervical length less than 15 mm, but they accounted for 58% of births before 32 weeks.6 The shorter the cervix, the greater the risk of spontaneous PTB.7 The presence of a short cervix is even more ominous in a woman with a history of spontaneous PTB.8
Optimal pregnancy management after detection of a short cervix remains somewhat unclear and varies, based on the rest of the patient’s clinical picture and obstetric history.
In this article, I address 8 critical questions about diagnosis and management of the short cervix in the second trimester and offer evidence-based answers for clinical practice.
1. How is a short cervix defined?
A cervical length below the 10th centile for gestational age is considered “short.” At 18 to 24 weeks’ gestation, the 10th centile corresponds to a cervical length of less than 25 mm.9
The cervix undergoes physiologic shortening that begins at 28 to 30 weeks of gestation. At 32 weeks, the 50th centile for cervical length is 25 mm. Therefore, cervical-length measurements that appear moderately short between 28 and 32 weeks and beyond are of limited clinical utility, and the clinician should incorporate gestational age into prematurity risk assessment.7
2. Who should be screened?
The question of whether universal cervical-length assessment should be performed is controversial. Several decision analyses in recent years suggest that universal sonographic screening for a short cervix is cost-effective.10,11 Overall, however, the effectiveness of universal cervical-length screening remains clinically understudied, and it is difficult to draw conclusions from decision analyses. Moreover, there is considerable concern about resources and feasibility of implementing universal vaginal cervical-length assessment, as well as significant disagreement about the accuracy of transabdominal cervical-length assessment in the detection of a short cervix.
Transabdominal ultrasound may overestimate cervical length by as much as 10 to 15 mm. One recent study demonstrated that, using a transabdominal cutoff of 30 mm, the sensitivity of detecting a transvaginal cervical length of less than 20 mm was 90%; if the cutoff was increased to 35 mm, sensitivity increased to 100%.12
A collaborative practice guideline on obstetric ultrasound from the American College of Radiology, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, and the Society of Radiologists in Ultrasound recommends that the maternal cervix be examined “as clinically appropriate when technically feasible” during a standard second- or third-trimester ultrasound examination (FIGURE 2).13 The guideline also states that transvaginal or transperineal ultrasound may be considered if the cervix appears shortened or cannot be adequately visualized during the transabdominal ultrasound. However, no specific protocols are suggested.
Given the uncertainty, it is recommended that each practice or ultrasound unit adopt a standard protocol for cervical-length assessment during pregnancy. This protocol can entail either routine abdominal or vaginal assessment of the cervix, or a combination of abdominal and vaginal assessment. Clinical risk factors can be used to help stratify low-risk women when abdominal cervical-length assessment is the initial approach to evaluation.
In my practice, all women undergo cervical-length assessment at the time of the routine anatomy survey (18–22 weeks). Those who are at low risk for PTB are screened initially with transabdominal ultrasound, and a transvaginal examination is performed if the cervix cannot be seen or appears to be less than 30 mm in length.
Women with a history of spontaneous PTB undergo screening by transvaginal cervical-length assessment. Typically, the first measurement is obtained at the time of the fetal anatomic survey (18–22 weeks), when the lower uterine segment is sufficiently developed to accurately measure the cervix. We perform serial cervical-length assessment every 1 or 2 weeks until 28 weeks’ gestation in women with a prior early spontaneous PTB (<34 weeks), those with a history of recurrent PTB, and those who have an initial short cervix. Serial monitoring has been shown to increase the prediction of spontaneous PTB in high-risk women.14
See the algorithm presented in (FIGURE 3) for the screening and treatment of women with singleton gestations.
3. How do I counsel patients about the risk of prematurity?
The risk of spontaneous PTB varies with the gestational age that the short cervix is detected and with the degree of cervical shortening. The earlier in the pregnancy the cervix is found to be short, the higher the risk for spontaneous PTB. For example, results of one large multicenter study of almost 3,000 unselected women pregnant with a singleton gestation across the United States showed that a cervical length of 25 mm was associated with a 15% to 20% incidence of PTB when detected at 28 weeks’ gestation; the incidence rose to 30% to 35% if the short cervix was detected at 20 weeks.9 In this cohort, 84% of women had no history of PTB.
A short cervix and a prior PTB (particularly a very early prior PTB) are two major risk factors for PTB. Together, they significantly increase the risk of an early delivery over individual or single factors alone. Among women who have had a prior PTB and who now have a cervical length of less than 25 mm, the risk of recurrent PTB is 35% to 40%. In contrast, women with a prior PTB and a normal cervical length have a significantly lower risk of recurrence—around 10%.15
If the physical examination is concerning for cervical dilation or prolapsing membranes, women should be counseled about the poor prognosis for the pregnancy, particularly when these findings are detected at a previable or periviable gestational age, regardless of their history of PTB. In these circumstances, in the absence of labor or intra-amniotic infection, a “rescue” cervical cerclage may be considered as a last resort (see page 34 for more on cerclage).
4. What evaluation or monitoring is needed once a short cervix is identified?
Women found to have a short cervix should be evaluated for the presence of preterm labor and intra-amniotic infection. This evaluation may include a sterile speculum examination or digital cervical examination, or both, as well as screening for genitourinary tract infection. Other testing may include a complete blood count with a white blood cell differential and external tocometry with or without fetal heart rate monitoring (based on the gestational age, as appropriate).
For some women with a short cervix, intra-amniotic infection may be a contributing factor (or it may develop if there are exposed membranes in the vagina). The presence of intra-amniotic infection precludes further expectant management of the pregnancy because of the risk of maternal infectious morbidity, including sepsis.
Women who have intra-amniotic infection are not candidates for any intervention such as cerclage or progesterone supplementation.
If the patient is at or beyond the point of fetal viability at the time her short cervix is detected, consider external fetal heart rate monitoring.
Antenatal corticosteroids can be administered, as appropriate, depending on the perceived risk of delivery.
5. Should I place a cerclage?
Numerous studies have examined the efficacy of ultrasound-indicated cerclage, a surgical procedure to stitch the cervix closed once a short cervix has been detected.
In general, placement of a cervical cerclage is not offered past the point of fetal viability, which is generally in the range of 23 to 25 weeks’ gestation, depending on local institutional and neonatal intensive care unit policies. Confirmed or suspected chorioamnionitis is also a contraindication to cerclage placement.
Among women without a history of PTB who are found to have a short cervix, existing data do not suggest a benefit for cerclage, although vaginal progesterone appears to be a reasonable option (see page 36).16,17
As for women with a history of PTB, Owen and colleagues studied 302 patients with a cervical length less than 25 mm and a history of spontaneous PTB before 34 weeks.18 The women were randomly assigned to ultrasound-indicated cerclage or “usual care,” which consisted of recommendations of pelvic rest, physical activity restriction, and education about the symptoms of preterm labor. Otherwise, management was directed by clinical practice at each center. All women treated with cerclage had a reduced risk of previable PTB (<24 weeks’ gestation), and those who had the shortest cervical length (<15 mm) also had a lower risk of delivery before 35 weeks.
The degree of cervical shortening that has “qualified” women for study enrollment has varied between studies, with upper limits ranging from 15 to 25 mm. Berghella and colleagues performed a patient-level meta-analysis to determine whether efficacy of the cerclage varied by cervical length at the time of placement.8 They examined 552 women with singleton gestations from four randomized controlled trials that included 208 women with a short cervix and a history of spontaneous PTB. They found a significant reduction in the rate of preterm delivery before 35 weeks’ gestation among women with singleton pregnancies, a short cervix, and a history of spontaneous PTB; the reduction did not vary by the degree of cervical shortening. However, there was no significant reduction in the rate of PTB among the subset of women without a history of spontaneous PTB.
Berghella and colleagues estimated that, if a cervical cerclage were offered to the 8% of women with a prior spontaneous PTB and a cervical length of less than 25 mm, more than 6,500 newborns would be saved each year from perinatal death associated with prematurity.8
The placement of a “rescue” cerclage in the setting of cervical dilation with or without prolapsing membranes is associated with high rates of maternal and neonatal morbidity, regardless of the patient’s obstetric history. However, cerclage placement in this setting may be associated with better outcomes than expectant management with bed rest alone.19 Patients should be carefully counseled about this procedure, including the risk of infection and the possibility that pregnancy may be prolonged only from a previable to a periviable gestational age. Decisions as to whether a patient is a candidate for rescue cerclage should be made in consultation with a maternal-fetal medicine specialist.20
6. Is a cervical pessary beneficial?
The pessary is another “mechanical” treatment similar to cerclage, and it may be helpful in reducing the incidence of PTB among women with a short cervix. In the largest study of this approach, 16,000 primarily low-risk women with singleton gestations were screened for a short cervix. Of these, 385 women with a cervical length of less than 25 mm were randomly assigned to undergo Arabin pessary placement or expectant management.21
Among those who received the pessary, the odds ratio (OR) for PTB before 34 weeks was significantly reduced (OR, 0.18; 95% confidence interval [CI], 0.08–0.37), and the OR for adverse composite neonatal outcome also was significantly reduced (OR, 0.14; 95% CI, 0.04–0.39).21
The Arabin pessary is not currently approved by the US Food and Drug Administration for this indication in the United States, nor is it available for insertion outside of research studies. Other ring-shaped pessaries are available in the United States, but their use in the setting of a short cervix is considered experimental and off-label. Additional studies of this promising intervention are currently under way.
7. Who is a candidate for supplemental progesterone?
Progesterone is a naturally occurring hormone essential to the maintenance of pregnancy. It has an overall quiescent effect on the myometrium, is known to have anti-inflammatory properties, and inhibits cervical ripening.22 It has been studied in a number of different formulations and doses.
Vaginal progesterone supplementation has been shown to reduce the risk of PTB among women with a shortened cervix regardless of their pregnancy history.16,17,23,24 In the largest trials of unselected general obstetrics populations (including women with and without a history of PTB), use of vaginal progesterone among women with a short cervix reduced the rates of very early spontaneous PTB (<28 and <32 weeks’ gestation) by 40% to 50%.16,17 As expected, progesterone also was associated with a significant reduction in the rates of respiratory distress syndrome and composite neonatal morbidity.
Intramuscular (IM) progesterone supplementation has been shown to reduce the rate of recurrent PTB among women with a history of spontaneous PTB. When caring for a woman with a history of PTB who is found to have a short cervix, IM progesterone should be offered if the woman is not already taking it. IM progesterone has not been proven effective among nulliparous women who are incidentally found to have a short cervix; it should not be offered in this situation.25
8. Should the care of women carrying twins or triplets be managed differently?
Yes. Although women with multiple gestations are at higher risk for PTB than women carrying a singleton fetus, no interventions have proven to be effective in this population. Many studies have been limited to twin gestations as a group, with an inability to perform subgroup analyses or enroll women who also have a short cervix, due to sample size and power issues.
Progesterone in multiple gestations
Although several formulations of progesterone—including IM 17-alpha hydroxyprogesterone caproate, micronized progesterone, and progesterone suppositories—have been studied, no randomized trial data have demonstrated a reduction in PTB or neonatal morbidity.26–29 Individual patient-level data from a meta-analysis of vaginal progesterone in the setting of multiple gestations with a short cervical length suggest trends toward a reduced rate of PTB before 33 weeks’ gestation (relative risk [RR], 0.70; 95% CI, 0.34–1.44) and lower composite neonatal morbidity and mortality (RR, 0.56; 95% CI, 0.30–0.97).30
Mechanical strategies in multiple gestations
No randomized data suggest that a pessary is effective in multiple gestations. In one study of 813 multiple gestations in the Netherlands, women were randomly assigned—regardless of cervical length—to receive a pessary at 16 to 20 weeks versus standard care; no difference in adverse perinatal outcomes was detected between groups.31
As for cerclage, although data are limited, some studies suggest that placement of a cerclage in twin gestations with cervical shortening may increase the rate of PTB.32
Bottom line for multiples
Although women carrying multiple gestations are at higher risk for PTB, data are extremely limited. At present, data do not support routine use of cerclage for a short cervix—and some suggest possible harm. Vaginal progesterone or placement of a pessary may be of benefit but should be used with caution and with the understanding that data are sparse.
CASE RESOLVED
You counsel your nulliparous patient that she has an elevated risk of PTB, based on her cervical length of 18 mm at 20 weeks’ gestation, and evaluate her clinically for evidence of preterm labor. Apart from the short cervix, her examination is unremarkable. You offer her nightly vaginal progesterone suppositories and schedule a visit to reevaluate her cervix in 1 week. If cervical dilation or prolapsing membranes are noted before the age of fetal viability, you will consider placing a “rescue” cervical cerclage.
Had this patient experienced a prior PTB, you would first ensure that she is taking IM 17-alpha hydroxyprogesterone caproate. It also would be reasonable to place an ultrasound-indicated cerclage or begin vaginal progesterone suppositories. Although data are limited on concomitant use of IM and vaginal progesterone, some experts may consider it, on an experimental basis, for patients with a short cervix and a prior PTB.
If this patient were carrying a twin gestation, vaginal progesterone would still be a consideration, provided she is counseled about the limited evidence of its efficacy in this setting. Cerclage would not be appropriate, given the possible risk of harm.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, city and state. Stay in touch! Your feedback is important to us!
CASE: NULLIPAROUS WOMAN WITH A SHORT CERVIX
Your ultrasound technician telephones to report that your 32-year-old nulliparous patient, who is currently at 20 weeks’ gestation, was incidentally found to have a short cervix (18 mm) at the time of her routine fetal anatomy survey.
How do you proceed? And how do you counsel the patient? What interventions might reduce the risk of preterm birth (PTB)? Would your recommendations change if she had a history of PTB or was carrying twins?
Preterm birth, defined as delivery prior to 37 weeks’ gestation, is the leading cause of neonatal morbidity and mortality in the United States. The rate of PTB peaked at 12.8% in 2006 and has slowly declined since but remains unacceptably high at 11.5%.1 Most PTBs are spontaneous, arising from the onset of labor or from preterm premature rupture of membranes. Regrettably, tocolytics remain largely ineffective once the process of preterm parturition has begun.
Ideally, women at highest risk for PTB could be identified so that additional screening and interventions could be initiated. Few prognostic tests are available to predict which women will deliver preterm. Generally, the greatest risk factor for spontaneous PTB is a history of spontaneous PTB.2,3 However, women with such a history account for only 10% of all births before 34 weeks’ gestation.
The appearance and length of the cervix during the second trimester appears to be an even better predictor of spontaneous PTB than history alone (FIGURE 1).4,5 For example, in one study of unselected pregnant women at 22 to 24 weeks’ gestation, only 1.7% had a cervical length less than 15 mm, but they accounted for 58% of births before 32 weeks.6 The shorter the cervix, the greater the risk of spontaneous PTB.7 The presence of a short cervix is even more ominous in a woman with a history of spontaneous PTB.8
Optimal pregnancy management after detection of a short cervix remains somewhat unclear and varies, based on the rest of the patient’s clinical picture and obstetric history.
In this article, I address 8 critical questions about diagnosis and management of the short cervix in the second trimester and offer evidence-based answers for clinical practice.
1. How is a short cervix defined?
A cervical length below the 10th centile for gestational age is considered “short.” At 18 to 24 weeks’ gestation, the 10th centile corresponds to a cervical length of less than 25 mm.9
The cervix undergoes physiologic shortening that begins at 28 to 30 weeks of gestation. At 32 weeks, the 50th centile for cervical length is 25 mm. Therefore, cervical-length measurements that appear moderately short between 28 and 32 weeks and beyond are of limited clinical utility, and the clinician should incorporate gestational age into prematurity risk assessment.7
2. Who should be screened?
The question of whether universal cervical-length assessment should be performed is controversial. Several decision analyses in recent years suggest that universal sonographic screening for a short cervix is cost-effective.10,11 Overall, however, the effectiveness of universal cervical-length screening remains clinically understudied, and it is difficult to draw conclusions from decision analyses. Moreover, there is considerable concern about resources and feasibility of implementing universal vaginal cervical-length assessment, as well as significant disagreement about the accuracy of transabdominal cervical-length assessment in the detection of a short cervix.
Transabdominal ultrasound may overestimate cervical length by as much as 10 to 15 mm. One recent study demonstrated that, using a transabdominal cutoff of 30 mm, the sensitivity of detecting a transvaginal cervical length of less than 20 mm was 90%; if the cutoff was increased to 35 mm, sensitivity increased to 100%.12
A collaborative practice guideline on obstetric ultrasound from the American College of Radiology, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, and the Society of Radiologists in Ultrasound recommends that the maternal cervix be examined “as clinically appropriate when technically feasible” during a standard second- or third-trimester ultrasound examination (FIGURE 2).13 The guideline also states that transvaginal or transperineal ultrasound may be considered if the cervix appears shortened or cannot be adequately visualized during the transabdominal ultrasound. However, no specific protocols are suggested.
Given the uncertainty, it is recommended that each practice or ultrasound unit adopt a standard protocol for cervical-length assessment during pregnancy. This protocol can entail either routine abdominal or vaginal assessment of the cervix, or a combination of abdominal and vaginal assessment. Clinical risk factors can be used to help stratify low-risk women when abdominal cervical-length assessment is the initial approach to evaluation.
In my practice, all women undergo cervical-length assessment at the time of the routine anatomy survey (18–22 weeks). Those who are at low risk for PTB are screened initially with transabdominal ultrasound, and a transvaginal examination is performed if the cervix cannot be seen or appears to be less than 30 mm in length.
Women with a history of spontaneous PTB undergo screening by transvaginal cervical-length assessment. Typically, the first measurement is obtained at the time of the fetal anatomic survey (18–22 weeks), when the lower uterine segment is sufficiently developed to accurately measure the cervix. We perform serial cervical-length assessment every 1 or 2 weeks until 28 weeks’ gestation in women with a prior early spontaneous PTB (<34 weeks), those with a history of recurrent PTB, and those who have an initial short cervix. Serial monitoring has been shown to increase the prediction of spontaneous PTB in high-risk women.14
See the algorithm presented in (FIGURE 3) for the screening and treatment of women with singleton gestations.
3. How do I counsel patients about the risk of prematurity?
The risk of spontaneous PTB varies with the gestational age that the short cervix is detected and with the degree of cervical shortening. The earlier in the pregnancy the cervix is found to be short, the higher the risk for spontaneous PTB. For example, results of one large multicenter study of almost 3,000 unselected women pregnant with a singleton gestation across the United States showed that a cervical length of 25 mm was associated with a 15% to 20% incidence of PTB when detected at 28 weeks’ gestation; the incidence rose to 30% to 35% if the short cervix was detected at 20 weeks.9 In this cohort, 84% of women had no history of PTB.
A short cervix and a prior PTB (particularly a very early prior PTB) are two major risk factors for PTB. Together, they significantly increase the risk of an early delivery over individual or single factors alone. Among women who have had a prior PTB and who now have a cervical length of less than 25 mm, the risk of recurrent PTB is 35% to 40%. In contrast, women with a prior PTB and a normal cervical length have a significantly lower risk of recurrence—around 10%.15
If the physical examination is concerning for cervical dilation or prolapsing membranes, women should be counseled about the poor prognosis for the pregnancy, particularly when these findings are detected at a previable or periviable gestational age, regardless of their history of PTB. In these circumstances, in the absence of labor or intra-amniotic infection, a “rescue” cervical cerclage may be considered as a last resort (see page 34 for more on cerclage).
4. What evaluation or monitoring is needed once a short cervix is identified?
Women found to have a short cervix should be evaluated for the presence of preterm labor and intra-amniotic infection. This evaluation may include a sterile speculum examination or digital cervical examination, or both, as well as screening for genitourinary tract infection. Other testing may include a complete blood count with a white blood cell differential and external tocometry with or without fetal heart rate monitoring (based on the gestational age, as appropriate).
For some women with a short cervix, intra-amniotic infection may be a contributing factor (or it may develop if there are exposed membranes in the vagina). The presence of intra-amniotic infection precludes further expectant management of the pregnancy because of the risk of maternal infectious morbidity, including sepsis.
Women who have intra-amniotic infection are not candidates for any intervention such as cerclage or progesterone supplementation.
If the patient is at or beyond the point of fetal viability at the time her short cervix is detected, consider external fetal heart rate monitoring.
Antenatal corticosteroids can be administered, as appropriate, depending on the perceived risk of delivery.
5. Should I place a cerclage?
Numerous studies have examined the efficacy of ultrasound-indicated cerclage, a surgical procedure to stitch the cervix closed once a short cervix has been detected.
In general, placement of a cervical cerclage is not offered past the point of fetal viability, which is generally in the range of 23 to 25 weeks’ gestation, depending on local institutional and neonatal intensive care unit policies. Confirmed or suspected chorioamnionitis is also a contraindication to cerclage placement.
Among women without a history of PTB who are found to have a short cervix, existing data do not suggest a benefit for cerclage, although vaginal progesterone appears to be a reasonable option (see page 36).16,17
As for women with a history of PTB, Owen and colleagues studied 302 patients with a cervical length less than 25 mm and a history of spontaneous PTB before 34 weeks.18 The women were randomly assigned to ultrasound-indicated cerclage or “usual care,” which consisted of recommendations of pelvic rest, physical activity restriction, and education about the symptoms of preterm labor. Otherwise, management was directed by clinical practice at each center. All women treated with cerclage had a reduced risk of previable PTB (<24 weeks’ gestation), and those who had the shortest cervical length (<15 mm) also had a lower risk of delivery before 35 weeks.
The degree of cervical shortening that has “qualified” women for study enrollment has varied between studies, with upper limits ranging from 15 to 25 mm. Berghella and colleagues performed a patient-level meta-analysis to determine whether efficacy of the cerclage varied by cervical length at the time of placement.8 They examined 552 women with singleton gestations from four randomized controlled trials that included 208 women with a short cervix and a history of spontaneous PTB. They found a significant reduction in the rate of preterm delivery before 35 weeks’ gestation among women with singleton pregnancies, a short cervix, and a history of spontaneous PTB; the reduction did not vary by the degree of cervical shortening. However, there was no significant reduction in the rate of PTB among the subset of women without a history of spontaneous PTB.
Berghella and colleagues estimated that, if a cervical cerclage were offered to the 8% of women with a prior spontaneous PTB and a cervical length of less than 25 mm, more than 6,500 newborns would be saved each year from perinatal death associated with prematurity.8
The placement of a “rescue” cerclage in the setting of cervical dilation with or without prolapsing membranes is associated with high rates of maternal and neonatal morbidity, regardless of the patient’s obstetric history. However, cerclage placement in this setting may be associated with better outcomes than expectant management with bed rest alone.19 Patients should be carefully counseled about this procedure, including the risk of infection and the possibility that pregnancy may be prolonged only from a previable to a periviable gestational age. Decisions as to whether a patient is a candidate for rescue cerclage should be made in consultation with a maternal-fetal medicine specialist.20
6. Is a cervical pessary beneficial?
The pessary is another “mechanical” treatment similar to cerclage, and it may be helpful in reducing the incidence of PTB among women with a short cervix. In the largest study of this approach, 16,000 primarily low-risk women with singleton gestations were screened for a short cervix. Of these, 385 women with a cervical length of less than 25 mm were randomly assigned to undergo Arabin pessary placement or expectant management.21
Among those who received the pessary, the odds ratio (OR) for PTB before 34 weeks was significantly reduced (OR, 0.18; 95% confidence interval [CI], 0.08–0.37), and the OR for adverse composite neonatal outcome also was significantly reduced (OR, 0.14; 95% CI, 0.04–0.39).21
The Arabin pessary is not currently approved by the US Food and Drug Administration for this indication in the United States, nor is it available for insertion outside of research studies. Other ring-shaped pessaries are available in the United States, but their use in the setting of a short cervix is considered experimental and off-label. Additional studies of this promising intervention are currently under way.
7. Who is a candidate for supplemental progesterone?
Progesterone is a naturally occurring hormone essential to the maintenance of pregnancy. It has an overall quiescent effect on the myometrium, is known to have anti-inflammatory properties, and inhibits cervical ripening.22 It has been studied in a number of different formulations and doses.
Vaginal progesterone supplementation has been shown to reduce the risk of PTB among women with a shortened cervix regardless of their pregnancy history.16,17,23,24 In the largest trials of unselected general obstetrics populations (including women with and without a history of PTB), use of vaginal progesterone among women with a short cervix reduced the rates of very early spontaneous PTB (<28 and <32 weeks’ gestation) by 40% to 50%.16,17 As expected, progesterone also was associated with a significant reduction in the rates of respiratory distress syndrome and composite neonatal morbidity.
Intramuscular (IM) progesterone supplementation has been shown to reduce the rate of recurrent PTB among women with a history of spontaneous PTB. When caring for a woman with a history of PTB who is found to have a short cervix, IM progesterone should be offered if the woman is not already taking it. IM progesterone has not been proven effective among nulliparous women who are incidentally found to have a short cervix; it should not be offered in this situation.25
8. Should the care of women carrying twins or triplets be managed differently?
Yes. Although women with multiple gestations are at higher risk for PTB than women carrying a singleton fetus, no interventions have proven to be effective in this population. Many studies have been limited to twin gestations as a group, with an inability to perform subgroup analyses or enroll women who also have a short cervix, due to sample size and power issues.
Progesterone in multiple gestations
Although several formulations of progesterone—including IM 17-alpha hydroxyprogesterone caproate, micronized progesterone, and progesterone suppositories—have been studied, no randomized trial data have demonstrated a reduction in PTB or neonatal morbidity.26–29 Individual patient-level data from a meta-analysis of vaginal progesterone in the setting of multiple gestations with a short cervical length suggest trends toward a reduced rate of PTB before 33 weeks’ gestation (relative risk [RR], 0.70; 95% CI, 0.34–1.44) and lower composite neonatal morbidity and mortality (RR, 0.56; 95% CI, 0.30–0.97).30
Mechanical strategies in multiple gestations
No randomized data suggest that a pessary is effective in multiple gestations. In one study of 813 multiple gestations in the Netherlands, women were randomly assigned—regardless of cervical length—to receive a pessary at 16 to 20 weeks versus standard care; no difference in adverse perinatal outcomes was detected between groups.31
As for cerclage, although data are limited, some studies suggest that placement of a cerclage in twin gestations with cervical shortening may increase the rate of PTB.32
Bottom line for multiples
Although women carrying multiple gestations are at higher risk for PTB, data are extremely limited. At present, data do not support routine use of cerclage for a short cervix—and some suggest possible harm. Vaginal progesterone or placement of a pessary may be of benefit but should be used with caution and with the understanding that data are sparse.
CASE RESOLVED
You counsel your nulliparous patient that she has an elevated risk of PTB, based on her cervical length of 18 mm at 20 weeks’ gestation, and evaluate her clinically for evidence of preterm labor. Apart from the short cervix, her examination is unremarkable. You offer her nightly vaginal progesterone suppositories and schedule a visit to reevaluate her cervix in 1 week. If cervical dilation or prolapsing membranes are noted before the age of fetal viability, you will consider placing a “rescue” cervical cerclage.
Had this patient experienced a prior PTB, you would first ensure that she is taking IM 17-alpha hydroxyprogesterone caproate. It also would be reasonable to place an ultrasound-indicated cerclage or begin vaginal progesterone suppositories. Although data are limited on concomitant use of IM and vaginal progesterone, some experts may consider it, on an experimental basis, for patients with a short cervix and a prior PTB.
If this patient were carrying a twin gestation, vaginal progesterone would still be a consideration, provided she is counseled about the limited evidence of its efficacy in this setting. Cerclage would not be appropriate, given the possible risk of harm.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, city and state. Stay in touch! Your feedback is important to us!
1. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
2. Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–1596.
3. Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 2006;195(3):643–650.
4. To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: A population-based prospective study. Ultrasound Obstet Gynecol. 2006;27(4):362–367.
5. Guzman ER, Ananth CV. Cervical length and spontaneous prematurity: Laying the foundation for future interventional randomized trials for the short cervix. Ultrasound Obstet Gynecol. 2001;18(3):195–199.
6. Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: Prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12(5):312–317.
7. Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110(2 Pt 1):311–317.
8. Berghella V, Keeler SM, To MS, Althuisius SM, Rust OA. Effectiveness of cerclage according to severity of cervical length shortening: A meta-analysis. Ultrasound Obstet Gynecol. 2010;35(4):468–473.
9. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567–572.
10. Werner EF, Han CS, Pettker CM, et al. Universal cervical-length screening to prevent preterm birth: A cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38 (1):32–37.
11. Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: A decision and economic analysis. Am J Obstet Gynecol. 2010;202(6):548.e1–e8.
12. Friedman AM, Srinivas SK, Parry S, Elovitz MA, Wang E, Schwartz N. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1–e7.
13. American College of Radiology, American College of Obstetricians and Gynecologists, American Institute of Ultrasound in Medicine, Society of Radiologists in Ultrasound. ACR–ACOG–AIUM–SRU Practice Guideline for the Performance of Obstetrical Ultrasound. http://www.acr.org/Quality-Safety/Standards-Guidelines/Practice-Guidelines-by-Modality/~/media/ACR/Documents/PGTS/guidelines/US_Obstetrical.pdf. Accessed June 16, 2014.
14. Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340–1348.
15. Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: Recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178(5):1035–1040.
16. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469.
17. Hassan SS, Romero R, Vidyadhari D, et al; PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: A multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31.
18. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1–e8.
19. Namouz S, Porat S, Okun N, Windrim R, Farine D. Emergency cerclage: literature review. Obstet Gynecol Surv. 2013;68(5):379–388.
20. Cockwell HA, Smith GN. Cervical incompetence and the role of emergency cerclage. J Obstet Gynaecol Can. 2005;27(2):123–129.
21. Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): An open-label randomised controlled trial. Lancet. 2012;379(9828):1800–1806.
22. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23(7):947–954.
23. Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. Erratum: N England J Med. 2003;349(13):1299.
24. O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: Primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30(5):687–696.
25. Grobman WA, Thom EA, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207(5):390.e1–e8.
26. Rouse DJ, Caritis SN, Peaceman AM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454–461.
27. Caritis SN, Rouse DJ, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Maternal-Fetal Medicine Units (MFMU) Network. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: A randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):285–292.
28. Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A; PREDICT Group. Prevention of preterm delivery in twin gestations (PREDICT): A multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38(3):272–280.
29. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): A randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034–2040.
30. Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1–e19.
31. Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): A multicentre, open-label randomised controlled trial. Lancet. 2013;382(9901):1341–1349.
32. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: Meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106(1):181–189.
1. Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
2. Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–1596.
3. Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol 2006;195(3):643–650.
4. To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: A population-based prospective study. Ultrasound Obstet Gynecol. 2006;27(4):362–367.
5. Guzman ER, Ananth CV. Cervical length and spontaneous prematurity: Laying the foundation for future interventional randomized trials for the short cervix. Ultrasound Obstet Gynecol. 2001;18(3):195–199.
6. Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: Prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12(5):312–317.
7. Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110(2 Pt 1):311–317.
8. Berghella V, Keeler SM, To MS, Althuisius SM, Rust OA. Effectiveness of cerclage according to severity of cervical length shortening: A meta-analysis. Ultrasound Obstet Gynecol. 2010;35(4):468–473.
9. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567–572.
10. Werner EF, Han CS, Pettker CM, et al. Universal cervical-length screening to prevent preterm birth: A cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38 (1):32–37.
11. Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: A decision and economic analysis. Am J Obstet Gynecol. 2010;202(6):548.e1–e8.
12. Friedman AM, Srinivas SK, Parry S, Elovitz MA, Wang E, Schwartz N. Can transabdominal ultrasound be used as a screening test for short cervical length? Am J Obstet Gynecol. 2013;208(3):190.e1–e7.
13. American College of Radiology, American College of Obstetricians and Gynecologists, American Institute of Ultrasound in Medicine, Society of Radiologists in Ultrasound. ACR–ACOG–AIUM–SRU Practice Guideline for the Performance of Obstetrical Ultrasound. http://www.acr.org/Quality-Safety/Standards-Guidelines/Practice-Guidelines-by-Modality/~/media/ACR/Documents/PGTS/guidelines/US_Obstetrical.pdf. Accessed June 16, 2014.
14. Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340–1348.
15. Iams JD, Goldenberg RL, Mercer BM, et al. The Preterm Prediction Study: Recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178(5):1035–1040.
16. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469.
17. Hassan SS, Romero R, Vidyadhari D, et al; PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: A multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31.
18. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1–e8.
19. Namouz S, Porat S, Okun N, Windrim R, Farine D. Emergency cerclage: literature review. Obstet Gynecol Surv. 2013;68(5):379–388.
20. Cockwell HA, Smith GN. Cervical incompetence and the role of emergency cerclage. J Obstet Gynaecol Can. 2005;27(2):123–129.
21. Goya M, Pratcorona L, Merced C, et al. Cervical pessary in pregnant women with a short cervix (PECEP): An open-label randomised controlled trial. Lancet. 2012;379(9828):1800–1806.
22. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23(7):947–954.
23. Meis PJ, Klebanoff M, Thom E, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. Erratum: N England J Med. 2003;349(13):1299.
24. O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: Primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2007;30(5):687–696.
25. Grobman WA, Thom EA, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207(5):390.e1–e8.
26. Rouse DJ, Caritis SN, Peaceman AM, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454–461.
27. Caritis SN, Rouse DJ, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Maternal-Fetal Medicine Units (MFMU) Network. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: A randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):285–292.
28. Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A; PREDICT Group. Prevention of preterm delivery in twin gestations (PREDICT): A multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38(3):272–280.
29. Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): A randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034–2040.
30. Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124.e1–e19.
31. Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): A multicentre, open-label randomised controlled trial. Lancet. 2013;382(9901):1341–1349.
32. Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: Meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106(1):181–189.
2014 Update on infectious disease
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
Does prenatal acetaminophen exposure increase the risk of behavioral problems in the child?
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
Increased risk of stillbirth, ectopic pregnancy linked with primary C-sections
Both elective and emergency cesarean sections in first births appear to slightly increase the risk of stillbirth and ectopic pregnancy in subsequent pregnancies, according to a new study.
Compared with women whose first birth was a spontaneous vaginal delivery, primiparous women with a primary C-section were 14% more likely to have a subsequent stillbirth and 9% more likely to have a later ectopic pregnancy, but were no more likely to have a subsequent miscarriage, reported Sinéad O’Neill of Cork University Maternity Hospital, Ireland, and her associates (PLoS Med. 2014 July 1 [doi:10.1371/journal.pmed.1001670]).
The analysis was limited by incomplete data, and the increased rate of stillbirths and/or ectopic pregnancies could be driven by underlying factors that contributed to the need for a C-section, the researchers noted.
They analyzed Danish national registry data on 832,996 primiparous women with a live birth between Jan. 1, 1982, and Dec. 31, 2010, followed until the next stillbirth, miscarriage, ectopic pregnancy, live birth, death or emigration. Miscarriage was defined as loss before 28 weeks’ gestation until April 2004, and before 22 weeks’ gestation from 2004 onward.
The fully-adjusted analysis controlled for the following:
• Maternal age, origin, and marital status.
• Previous stillbirth, miscarriage, or ectopic pregnancy.
• Birth year.
• Socioeconomic status (mother’s education and both parents’ gross income).
• Medical complications in the first live birth, including multiples, diabetes, gestational diabetes, placental abruption, placenta previa, and hypertensive disorders.
• Gestational age at birth and birth weight.
The researchers lacked data on maternal body mass index, smoking status, and fertility treatment, as well as causes of stillbirth, maternally requested C-sections, and the gestational ages of the stillbirths and miscarriages.
The increased rate of stillbirth (hazard ratio 1.14) among women with a primary C-section, compared with women with an initial spontaneous vaginal birth, translated to an absolute risk increase of 0.03% and a number needed to harm of 3,333. Emergency C-sections showed a barely higher risk (HR 1.15), but the risk with elective C-sections (HR 1.11) did not reach statistical significance (95% CI 0.91, 1.35).
"Almost 50% of the explained stillbirths in this cohort were due to antenatal complications (including placental abruption/infarction, intrauterine growth restriction, preeclampsia, prematurity, and poor placental growth)," the researchers wrote. "The clinical importance is that although many of these complications are largely not preventable, an increased awareness that the fetus is at risk may facilitate increased surveillance and optimally timed delivery and may lead to improved perinatal outcomes."
The increased risk of ectopic pregnancy (HR 1.09) with overall primary C-sections translated to an absolute risk increase of 0.1% and number needed to harm of 1,000 women. The increased risk was similar for emergency (HR 1.09) and elective (HR 1.12) C-sections, both statistically significant. Overall, primary C-sections were not associated with miscarriage, and miscarriage risk was decreased (HR 0.72) for maternally requested C-sections.
The authors reported that C-section rates among first-time Danish mothers increased during the study period from 12.8% in 1982 to 23% in 2010. "This increase, coupled with better surveillance and detection of adverse or underlying complications earlier in pregnancy, could explain the increased hazard of subsequent stillbirth found in this study among women with a prior cesarean section," they wrote.
Although the findings may be particularly relevant for expectant mothers requesting a non–medically indicated C-section, "it must be acknowledged that a cesarean section can be a vital intervention, and the likelihood of adverse outcome may be decreased, for example, by choosing an elective cesarean section to avoid fetal death due to a failed vaginal birth after cesarean or to prevent sudden stillbirth post-term," the researchers wrote.
The research was supported by the National Perinatal Epidemiology Centre in Cork, Ireland, and as part of the Health Research Board PhD Scholars program in Health Services Research. A coauthor, Dr. Louise C. Kenny, is a Science Foundation Ireland Principal Investigator and director of Centre, INFANT, funded by Science Foundation Ireland.
Despite the increase in the
number of cesarean deliveries around the world over the past decades, there is
not a clinically significant increase in attendant maternal-fetal
complications. Thus, although the Danish study findings are neither novel nor
surprising, ultimately this study supports the observation that cesarean
delivery is another safe route for both the mother and child. It also is crucial
to recognize that this study is not a strict comparison of whether vaginal
delivery is safer than cesarean delivery, but an examination of how C-sections
may be linked to subsequent pregnancy complications.
The data for ectopic pregnancies presented in this
study have only marginal statistical relevance, but not a great clinical
significance. Additionally, a woman’s prior gynecologic history, such as
infections or surgical scarring due to the C-section procedure, greatly
influences the risk for ectopic pregnancy. Because many ectopic pregnancies can
abort prior to the detection of pregnancy, drawing any major conclusions from
these results is quite difficult.
It also is unclear to what extent the incomplete data
could influence how we interpret the findings of this study. As the fields of
developmental biology, teratology, biochemistry, and reproductive immunology
have advanced, so too has our understanding of the complex mechanisms involved
in successful pregnancy outcomes. Basing clinical applications on the findings from
this study, where gaps exist in the investigators’ knowledge of the women’s
overall health, becomes much more nebulous, and any conclusions should be
cautiously made and not extrapolated too much.
Dr. E. Albert Reece, Ph.D., MBA, is vice president for medical affairs at the University of Maryland, Baltimore, the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He made these comments in an interview.
Despite the increase in the
number of cesarean deliveries around the world over the past decades, there is
not a clinically significant increase in attendant maternal-fetal
complications. Thus, although the Danish study findings are neither novel nor
surprising, ultimately this study supports the observation that cesarean
delivery is another safe route for both the mother and child. It also is crucial
to recognize that this study is not a strict comparison of whether vaginal
delivery is safer than cesarean delivery, but an examination of how C-sections
may be linked to subsequent pregnancy complications.
The data for ectopic pregnancies presented in this
study have only marginal statistical relevance, but not a great clinical
significance. Additionally, a woman’s prior gynecologic history, such as
infections or surgical scarring due to the C-section procedure, greatly
influences the risk for ectopic pregnancy. Because many ectopic pregnancies can
abort prior to the detection of pregnancy, drawing any major conclusions from
these results is quite difficult.
It also is unclear to what extent the incomplete data
could influence how we interpret the findings of this study. As the fields of
developmental biology, teratology, biochemistry, and reproductive immunology
have advanced, so too has our understanding of the complex mechanisms involved
in successful pregnancy outcomes. Basing clinical applications on the findings from
this study, where gaps exist in the investigators’ knowledge of the women’s
overall health, becomes much more nebulous, and any conclusions should be
cautiously made and not extrapolated too much.
Dr. E. Albert Reece, Ph.D., MBA, is vice president for medical affairs at the University of Maryland, Baltimore, the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He made these comments in an interview.
Despite the increase in the
number of cesarean deliveries around the world over the past decades, there is
not a clinically significant increase in attendant maternal-fetal
complications. Thus, although the Danish study findings are neither novel nor
surprising, ultimately this study supports the observation that cesarean
delivery is another safe route for both the mother and child. It also is crucial
to recognize that this study is not a strict comparison of whether vaginal
delivery is safer than cesarean delivery, but an examination of how C-sections
may be linked to subsequent pregnancy complications.
The data for ectopic pregnancies presented in this
study have only marginal statistical relevance, but not a great clinical
significance. Additionally, a woman’s prior gynecologic history, such as
infections or surgical scarring due to the C-section procedure, greatly
influences the risk for ectopic pregnancy. Because many ectopic pregnancies can
abort prior to the detection of pregnancy, drawing any major conclusions from
these results is quite difficult.
It also is unclear to what extent the incomplete data
could influence how we interpret the findings of this study. As the fields of
developmental biology, teratology, biochemistry, and reproductive immunology
have advanced, so too has our understanding of the complex mechanisms involved
in successful pregnancy outcomes. Basing clinical applications on the findings from
this study, where gaps exist in the investigators’ knowledge of the women’s
overall health, becomes much more nebulous, and any conclusions should be
cautiously made and not extrapolated too much.
Dr. E. Albert Reece, Ph.D., MBA, is vice president for medical affairs at the University of Maryland, Baltimore, the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He made these comments in an interview.
Both elective and emergency cesarean sections in first births appear to slightly increase the risk of stillbirth and ectopic pregnancy in subsequent pregnancies, according to a new study.
Compared with women whose first birth was a spontaneous vaginal delivery, primiparous women with a primary C-section were 14% more likely to have a subsequent stillbirth and 9% more likely to have a later ectopic pregnancy, but were no more likely to have a subsequent miscarriage, reported Sinéad O’Neill of Cork University Maternity Hospital, Ireland, and her associates (PLoS Med. 2014 July 1 [doi:10.1371/journal.pmed.1001670]).
The analysis was limited by incomplete data, and the increased rate of stillbirths and/or ectopic pregnancies could be driven by underlying factors that contributed to the need for a C-section, the researchers noted.
They analyzed Danish national registry data on 832,996 primiparous women with a live birth between Jan. 1, 1982, and Dec. 31, 2010, followed until the next stillbirth, miscarriage, ectopic pregnancy, live birth, death or emigration. Miscarriage was defined as loss before 28 weeks’ gestation until April 2004, and before 22 weeks’ gestation from 2004 onward.
The fully-adjusted analysis controlled for the following:
• Maternal age, origin, and marital status.
• Previous stillbirth, miscarriage, or ectopic pregnancy.
• Birth year.
• Socioeconomic status (mother’s education and both parents’ gross income).
• Medical complications in the first live birth, including multiples, diabetes, gestational diabetes, placental abruption, placenta previa, and hypertensive disorders.
• Gestational age at birth and birth weight.
The researchers lacked data on maternal body mass index, smoking status, and fertility treatment, as well as causes of stillbirth, maternally requested C-sections, and the gestational ages of the stillbirths and miscarriages.
The increased rate of stillbirth (hazard ratio 1.14) among women with a primary C-section, compared with women with an initial spontaneous vaginal birth, translated to an absolute risk increase of 0.03% and a number needed to harm of 3,333. Emergency C-sections showed a barely higher risk (HR 1.15), but the risk with elective C-sections (HR 1.11) did not reach statistical significance (95% CI 0.91, 1.35).
"Almost 50% of the explained stillbirths in this cohort were due to antenatal complications (including placental abruption/infarction, intrauterine growth restriction, preeclampsia, prematurity, and poor placental growth)," the researchers wrote. "The clinical importance is that although many of these complications are largely not preventable, an increased awareness that the fetus is at risk may facilitate increased surveillance and optimally timed delivery and may lead to improved perinatal outcomes."
The increased risk of ectopic pregnancy (HR 1.09) with overall primary C-sections translated to an absolute risk increase of 0.1% and number needed to harm of 1,000 women. The increased risk was similar for emergency (HR 1.09) and elective (HR 1.12) C-sections, both statistically significant. Overall, primary C-sections were not associated with miscarriage, and miscarriage risk was decreased (HR 0.72) for maternally requested C-sections.
The authors reported that C-section rates among first-time Danish mothers increased during the study period from 12.8% in 1982 to 23% in 2010. "This increase, coupled with better surveillance and detection of adverse or underlying complications earlier in pregnancy, could explain the increased hazard of subsequent stillbirth found in this study among women with a prior cesarean section," they wrote.
Although the findings may be particularly relevant for expectant mothers requesting a non–medically indicated C-section, "it must be acknowledged that a cesarean section can be a vital intervention, and the likelihood of adverse outcome may be decreased, for example, by choosing an elective cesarean section to avoid fetal death due to a failed vaginal birth after cesarean or to prevent sudden stillbirth post-term," the researchers wrote.
The research was supported by the National Perinatal Epidemiology Centre in Cork, Ireland, and as part of the Health Research Board PhD Scholars program in Health Services Research. A coauthor, Dr. Louise C. Kenny, is a Science Foundation Ireland Principal Investigator and director of Centre, INFANT, funded by Science Foundation Ireland.
Both elective and emergency cesarean sections in first births appear to slightly increase the risk of stillbirth and ectopic pregnancy in subsequent pregnancies, according to a new study.
Compared with women whose first birth was a spontaneous vaginal delivery, primiparous women with a primary C-section were 14% more likely to have a subsequent stillbirth and 9% more likely to have a later ectopic pregnancy, but were no more likely to have a subsequent miscarriage, reported Sinéad O’Neill of Cork University Maternity Hospital, Ireland, and her associates (PLoS Med. 2014 July 1 [doi:10.1371/journal.pmed.1001670]).
The analysis was limited by incomplete data, and the increased rate of stillbirths and/or ectopic pregnancies could be driven by underlying factors that contributed to the need for a C-section, the researchers noted.
They analyzed Danish national registry data on 832,996 primiparous women with a live birth between Jan. 1, 1982, and Dec. 31, 2010, followed until the next stillbirth, miscarriage, ectopic pregnancy, live birth, death or emigration. Miscarriage was defined as loss before 28 weeks’ gestation until April 2004, and before 22 weeks’ gestation from 2004 onward.
The fully-adjusted analysis controlled for the following:
• Maternal age, origin, and marital status.
• Previous stillbirth, miscarriage, or ectopic pregnancy.
• Birth year.
• Socioeconomic status (mother’s education and both parents’ gross income).
• Medical complications in the first live birth, including multiples, diabetes, gestational diabetes, placental abruption, placenta previa, and hypertensive disorders.
• Gestational age at birth and birth weight.
The researchers lacked data on maternal body mass index, smoking status, and fertility treatment, as well as causes of stillbirth, maternally requested C-sections, and the gestational ages of the stillbirths and miscarriages.
The increased rate of stillbirth (hazard ratio 1.14) among women with a primary C-section, compared with women with an initial spontaneous vaginal birth, translated to an absolute risk increase of 0.03% and a number needed to harm of 3,333. Emergency C-sections showed a barely higher risk (HR 1.15), but the risk with elective C-sections (HR 1.11) did not reach statistical significance (95% CI 0.91, 1.35).
"Almost 50% of the explained stillbirths in this cohort were due to antenatal complications (including placental abruption/infarction, intrauterine growth restriction, preeclampsia, prematurity, and poor placental growth)," the researchers wrote. "The clinical importance is that although many of these complications are largely not preventable, an increased awareness that the fetus is at risk may facilitate increased surveillance and optimally timed delivery and may lead to improved perinatal outcomes."
The increased risk of ectopic pregnancy (HR 1.09) with overall primary C-sections translated to an absolute risk increase of 0.1% and number needed to harm of 1,000 women. The increased risk was similar for emergency (HR 1.09) and elective (HR 1.12) C-sections, both statistically significant. Overall, primary C-sections were not associated with miscarriage, and miscarriage risk was decreased (HR 0.72) for maternally requested C-sections.
The authors reported that C-section rates among first-time Danish mothers increased during the study period from 12.8% in 1982 to 23% in 2010. "This increase, coupled with better surveillance and detection of adverse or underlying complications earlier in pregnancy, could explain the increased hazard of subsequent stillbirth found in this study among women with a prior cesarean section," they wrote.
Although the findings may be particularly relevant for expectant mothers requesting a non–medically indicated C-section, "it must be acknowledged that a cesarean section can be a vital intervention, and the likelihood of adverse outcome may be decreased, for example, by choosing an elective cesarean section to avoid fetal death due to a failed vaginal birth after cesarean or to prevent sudden stillbirth post-term," the researchers wrote.
The research was supported by the National Perinatal Epidemiology Centre in Cork, Ireland, and as part of the Health Research Board PhD Scholars program in Health Services Research. A coauthor, Dr. Louise C. Kenny, is a Science Foundation Ireland Principal Investigator and director of Centre, INFANT, funded by Science Foundation Ireland.
FROM PLOS MEDICINE
Birth defect risk low in celiac disease
Pregnant mothers with celiac disease have little to fear when it comes to birth defects, despite previous underpowered reports to the contrary, wrote Daniela Zugna, Ph.D., and her colleagues in the July issue of Clinical Gastroenterology and Hepatology (doi.org/10.1016/j.cgh.2013.10.012).
Dr. Zugna of University of Turin, in Italy, looked at a total of 5,774 mothers with celiac disease (CD) and 3,039 fathers with CD, cross-referenced with the Swedish Medical Birth Register, the Patient Register, the Register of Congenital Malformations and the Multigeneration Register.
From 1973 through 2009, the mothers gave birth to 11,382 children, and 6,002 children were born to fathers with CD.
These were compared with nearly 41,000 and 20,000 children of healthy mothers and fathers born over the same time period, respectively.
The authors found that 672 babies of mothers with CD (5.9%) and 2,098 control offspring (5.1%) were born with congenital malformations; these included heart defects, neural tube defects, limb deficiencies, and orofacial clefts, among others.
That amounted to an adjusted prevalence odds ratio of having a child with birth defects of 1.15 for mothers with CD (95% confidence interval, 1.05-1.26).
For fathers, the figures were 352 children with birth defects born to CD patients (5.9%) and 1,009 to healthy controls (5.1%), for a similar odds ratio of 1.14 (95% CI, 1.00-1.29).
However, when the data were restricted to births between 2000 and 2009, the significance of these differences vanished: in this most modern era, mothers with CD carried a nonsignificant OR of 1.11 (95% CI, 0.79-1.56).
By the same token, fathers of babies born between 2000 and 2009 had an OR of 1.01 for having a child with birth defects (95% CI, 0.81-1.26).
In postulating reasons for the slightly increased number of birth defects seen over the span of the study, the authors wrote: "While folic acid deficiency is common in newly diagnosed CD, it is sometimes seen after diagnosis, perhaps because of a lack of folic acid in the gluten-free diet."
And regarding the possible connection between fathers with CD and their children, "If spouses to men with CD are also primarily on a gluten-free diet, this may actually explain the excess risk for congenital malformation in paternal offspring."
Alternatively, "Low sperm quality in men with CD could potentially influence the risk of malformations."
The authors conceded several important weaknesses in this study. For one, they did not have data on diet adherence in CD, nor on the periconceptional or perinatal use of folic acid supplementation.
Moreover, a substantial proportion of pregnancies with neural tube defects discovered during prenatal screening were likely to have been terminated, potentially resulting in artificially low rates of this malformation: indeed, the authors pointed to data showing that between 1999 and 2009, between 51% and 93% of all NTD pregnancies were terminated, depending on the specific defect.
Finally, "Over time, symptoms have changed in CD," they wrote.
"It is possible that, with increased use of celiac serology, milder cases of CD are now diagnosed in which there is no association with congenital malformation."
The authors disclosed no conflicts of interest; individual authors disclosed several grants from nonprofit and research agencies in Europe, including the Italian Association for Cancer Research, the Stockholm County Council, the Danish Medical Research Council, and the Karolinska Institutet, among others.
Pregnant mothers with celiac disease have little to fear when it comes to birth defects, despite previous underpowered reports to the contrary, wrote Daniela Zugna, Ph.D., and her colleagues in the July issue of Clinical Gastroenterology and Hepatology (doi.org/10.1016/j.cgh.2013.10.012).
Dr. Zugna of University of Turin, in Italy, looked at a total of 5,774 mothers with celiac disease (CD) and 3,039 fathers with CD, cross-referenced with the Swedish Medical Birth Register, the Patient Register, the Register of Congenital Malformations and the Multigeneration Register.
From 1973 through 2009, the mothers gave birth to 11,382 children, and 6,002 children were born to fathers with CD.
These were compared with nearly 41,000 and 20,000 children of healthy mothers and fathers born over the same time period, respectively.
The authors found that 672 babies of mothers with CD (5.9%) and 2,098 control offspring (5.1%) were born with congenital malformations; these included heart defects, neural tube defects, limb deficiencies, and orofacial clefts, among others.
That amounted to an adjusted prevalence odds ratio of having a child with birth defects of 1.15 for mothers with CD (95% confidence interval, 1.05-1.26).
For fathers, the figures were 352 children with birth defects born to CD patients (5.9%) and 1,009 to healthy controls (5.1%), for a similar odds ratio of 1.14 (95% CI, 1.00-1.29).
However, when the data were restricted to births between 2000 and 2009, the significance of these differences vanished: in this most modern era, mothers with CD carried a nonsignificant OR of 1.11 (95% CI, 0.79-1.56).
By the same token, fathers of babies born between 2000 and 2009 had an OR of 1.01 for having a child with birth defects (95% CI, 0.81-1.26).
In postulating reasons for the slightly increased number of birth defects seen over the span of the study, the authors wrote: "While folic acid deficiency is common in newly diagnosed CD, it is sometimes seen after diagnosis, perhaps because of a lack of folic acid in the gluten-free diet."
And regarding the possible connection between fathers with CD and their children, "If spouses to men with CD are also primarily on a gluten-free diet, this may actually explain the excess risk for congenital malformation in paternal offspring."
Alternatively, "Low sperm quality in men with CD could potentially influence the risk of malformations."
The authors conceded several important weaknesses in this study. For one, they did not have data on diet adherence in CD, nor on the periconceptional or perinatal use of folic acid supplementation.
Moreover, a substantial proportion of pregnancies with neural tube defects discovered during prenatal screening were likely to have been terminated, potentially resulting in artificially low rates of this malformation: indeed, the authors pointed to data showing that between 1999 and 2009, between 51% and 93% of all NTD pregnancies were terminated, depending on the specific defect.
Finally, "Over time, symptoms have changed in CD," they wrote.
"It is possible that, with increased use of celiac serology, milder cases of CD are now diagnosed in which there is no association with congenital malformation."
The authors disclosed no conflicts of interest; individual authors disclosed several grants from nonprofit and research agencies in Europe, including the Italian Association for Cancer Research, the Stockholm County Council, the Danish Medical Research Council, and the Karolinska Institutet, among others.
Pregnant mothers with celiac disease have little to fear when it comes to birth defects, despite previous underpowered reports to the contrary, wrote Daniela Zugna, Ph.D., and her colleagues in the July issue of Clinical Gastroenterology and Hepatology (doi.org/10.1016/j.cgh.2013.10.012).
Dr. Zugna of University of Turin, in Italy, looked at a total of 5,774 mothers with celiac disease (CD) and 3,039 fathers with CD, cross-referenced with the Swedish Medical Birth Register, the Patient Register, the Register of Congenital Malformations and the Multigeneration Register.
From 1973 through 2009, the mothers gave birth to 11,382 children, and 6,002 children were born to fathers with CD.
These were compared with nearly 41,000 and 20,000 children of healthy mothers and fathers born over the same time period, respectively.
The authors found that 672 babies of mothers with CD (5.9%) and 2,098 control offspring (5.1%) were born with congenital malformations; these included heart defects, neural tube defects, limb deficiencies, and orofacial clefts, among others.
That amounted to an adjusted prevalence odds ratio of having a child with birth defects of 1.15 for mothers with CD (95% confidence interval, 1.05-1.26).
For fathers, the figures were 352 children with birth defects born to CD patients (5.9%) and 1,009 to healthy controls (5.1%), for a similar odds ratio of 1.14 (95% CI, 1.00-1.29).
However, when the data were restricted to births between 2000 and 2009, the significance of these differences vanished: in this most modern era, mothers with CD carried a nonsignificant OR of 1.11 (95% CI, 0.79-1.56).
By the same token, fathers of babies born between 2000 and 2009 had an OR of 1.01 for having a child with birth defects (95% CI, 0.81-1.26).
In postulating reasons for the slightly increased number of birth defects seen over the span of the study, the authors wrote: "While folic acid deficiency is common in newly diagnosed CD, it is sometimes seen after diagnosis, perhaps because of a lack of folic acid in the gluten-free diet."
And regarding the possible connection between fathers with CD and their children, "If spouses to men with CD are also primarily on a gluten-free diet, this may actually explain the excess risk for congenital malformation in paternal offspring."
Alternatively, "Low sperm quality in men with CD could potentially influence the risk of malformations."
The authors conceded several important weaknesses in this study. For one, they did not have data on diet adherence in CD, nor on the periconceptional or perinatal use of folic acid supplementation.
Moreover, a substantial proportion of pregnancies with neural tube defects discovered during prenatal screening were likely to have been terminated, potentially resulting in artificially low rates of this malformation: indeed, the authors pointed to data showing that between 1999 and 2009, between 51% and 93% of all NTD pregnancies were terminated, depending on the specific defect.
Finally, "Over time, symptoms have changed in CD," they wrote.
"It is possible that, with increased use of celiac serology, milder cases of CD are now diagnosed in which there is no association with congenital malformation."
The authors disclosed no conflicts of interest; individual authors disclosed several grants from nonprofit and research agencies in Europe, including the Italian Association for Cancer Research, the Stockholm County Council, the Danish Medical Research Council, and the Karolinska Institutet, among others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Parents with celiac disease do not now have an increased risk of having a child with birth defects.
Major finding: Mothers with celiac disease have a very slightly increased risk of having a child with birth defects (adjusted prevalence OR, 1.15), but the risk disappears when restricted to births occurring after the year 2000.
Data source: A nationwide study of health registries in Sweden from 1973 through 2009.
Disclosures: The authors disclosed no conflicts of interest; they disclosed several grants from nonprofit and research agencies in Europe.
Supreme Court: Companies can deny contraceptive coverage
WASHINGTON – For-profit companies cannot be compelled to provide insurance coverage for contraception if doing so violates the religious beliefs of the company’s owners, the Supreme Court ruled June 30.
Dissenting justices said that it had the potential to allow employers to use their religious beliefs as a way to object to providing coverage for vaccinations, blood transfusions, and other procedures, and that it could open the door to deny employment to certain individuals or groups.
The Court ruled 5-4 in the cases of Burwell v. Hobby Lobby Stores Inc. and Conestoga Wood Specialties Corp. v. Burwell.
Justice Samuel Alito, reading the opinion for the majority, said that the Religious Freedom Restoration Act (RFRA) of 1993 protected the rights of not just individuals, but also of individuals who run closely held for-profit corporations.
The terms of that law "make it perfectly clear that Congress did not discriminate in this way against men and women who wish to run their businesses as for-profit corporations in the manner required by their religious beliefs," according to the opinion signed by Justice Alito, Justice Anthony P. Kennedy, Justice Antonin Scalia, and Justice Clarence Thomas, and Chief Justice John P. Roberts.
The plaintiffs in the cases said that being required by the Affordable Care Act to provide insurance coverage for contraception violated their religious beliefs. In particular, Hobby Lobby and Conestoga Wood both objected to four specific types of contraception that they said interfere with conception and thus constitute abortion: the Ella morning-after pill, the Plan B morning-after pill, and hormonal and copper intrauterine devices (IUDs).
The majority opinion said that by requiring companies that did not comply to pay fines, the government was placing an undue burden on these employers. Justice Alito noted that Hobby Lobby would have to either give up its religious tenets or pay up to $1.3 million a day in fines*.
"If these consequences don’t amount to a heavy burden, I’m not sure what would," he said.
The majority also said that since the government already makes exceptions to the contraception requirement for religious organizations and nonprofit religiously affiliated groups, that it could have made the same allowances for for-profit companies. The government also could have offered to pay for the contraceptives that companies refuse to cover, they said.
Justice Ruth Bader Ginsburg, who read the dissenting opinion, called the majority’s opinion "a decision of startling breadth." If allowed to stand, it could raise what she called "a host of ‘me, too’ questions," such as whether an employer could opt out of coverage for antidepressants, vaccinations, or medications derived from pigs, "based on the employer’s sincerely held religious beliefs opposing those medical practices."
Joined by Justice Stephen G. Breyer, Justice Elena Kagan, and Justice Sonia Sotomayor, she also said that while the ruling might seem to be limited to access to the four contraceptives in the cases, it was likely that companies would seek to deny coverage for all available contraceptive technologies.
The dissenters also said that they doubted that Congress intended for the RFRA to be used to allow a company to stand in between a woman and her physician. Any decision to use a contraceptive "will be the woman’s autonomous choice, informed by the physician she consults," they wrote.
Many physicians’ and women’s health organizations called it a dangerous precedent.
The American College of Obstetricians and Gynecologists President John C. Jennings said in a statement that the group was “profoundly disappointed” in the decision, as it “inappropriately allows employers to interfere in women’s health care decisions.” Dr. Jennings also said that contraceptives should not be treated differently than other health care services for women. “The value of family planning – including contraception – has been clearly demonstrated,” he said. “The ability of a woman to time and space her children reduces infant, child, and maternal morbidity and mortality, and can lead to more optimal health outcomes for mother and for baby.”
Contraception also helps prevent unintended pregnancy, he said, adding, “This is absolutely essential in America, where nearly one half of all pregnancies are unintended.”
In a statement, Dr.Reid Blackwelder, president of the American Academy of Family Physicians, said, "With this decision, the court has moved health care decisions out of the exam room, where patients can consult with their physicians — and where such decisions should be made — and put them into the hands of business owners who base decisions on personal beliefs rather than [on] medical science." Dr. Blackwelder added, "Personal or institutional beliefs should not put Americans’ health and lives at risk, simply because employers control the insurance available to their workers. Unfortunately, the Supreme Court decision opens the gate for just such consequences."
Dr. James M. Perrin, president of the American Academy of Pediatrics, said, "For pediatricians, the science is clear: contraception is a safe, effective tool to prevent unintended pregnancy in young women of any reproductive age. Today, we are disappointed that justice did not side with science."
Some physician groups expressed their support for the decision.
The American Association of Pro-Life Obstetricians and Gynecologists called the ruling a "tremendous victory for all Americans and their freedom of conscience." The group added that it was "hopeful that this recognition of conscientious objection will help to strengthen the rights of physicians to continue to refuse to participate in any medical procedure or to prescribe any drug that is designed and purposed to kill a living human being."
The Christian Medical Association also applauded the decision. In a statement, Dr. David Stevens, CEO, said, "We are very thankful that the Supreme Court acted to protect family businesses from government coercion and fines for simply honoring the tenets of their faith," and called it a "much-needed victory for faith freedoms."
In a joint press briefing, representatives from Planned Parenthood, NARAL, the American Civil Liberties Union, and the National Women’s Law Center said that they would work with Congress to try to reverse the Supreme Court ruling through legislation. The AAP will do the same, said Dr. Perrin.
Some Senate Democrats have said they will prepare legislation to address the case immediately. Sen. Patty Murray (D-Wash.), who filed a friend of the court brief on behalf of 18 Senators that sided with the government’s case, said in a statement that, "Since the Supreme Court decided it will not protect women’s access to health care, I will. In the coming days I will work with my colleagues and the Administration to protect this access, regardless of who signs your paycheck."
Senate Republican leader Mitch McConnell (R-Ky.), however, said that the decision was a validation of religious freedom. "Obamacare is the single worst piece of legislation to pass in the last 50 years, and I was glad to see the Supreme Court agree that this particular Obamacare mandate violates the Religious Freedom Restoration Act," Sen. McConnell said, in a statement.
On Twitter @aliciaault
*Clarification, 7/2/2014: An earlier version of this article did not include the intact quote from the majority opinion.
*This story was updated 7/2/2014.
WASHINGTON – For-profit companies cannot be compelled to provide insurance coverage for contraception if doing so violates the religious beliefs of the company’s owners, the Supreme Court ruled June 30.
Dissenting justices said that it had the potential to allow employers to use their religious beliefs as a way to object to providing coverage for vaccinations, blood transfusions, and other procedures, and that it could open the door to deny employment to certain individuals or groups.
The Court ruled 5-4 in the cases of Burwell v. Hobby Lobby Stores Inc. and Conestoga Wood Specialties Corp. v. Burwell.
Justice Samuel Alito, reading the opinion for the majority, said that the Religious Freedom Restoration Act (RFRA) of 1993 protected the rights of not just individuals, but also of individuals who run closely held for-profit corporations.
The terms of that law "make it perfectly clear that Congress did not discriminate in this way against men and women who wish to run their businesses as for-profit corporations in the manner required by their religious beliefs," according to the opinion signed by Justice Alito, Justice Anthony P. Kennedy, Justice Antonin Scalia, and Justice Clarence Thomas, and Chief Justice John P. Roberts.
The plaintiffs in the cases said that being required by the Affordable Care Act to provide insurance coverage for contraception violated their religious beliefs. In particular, Hobby Lobby and Conestoga Wood both objected to four specific types of contraception that they said interfere with conception and thus constitute abortion: the Ella morning-after pill, the Plan B morning-after pill, and hormonal and copper intrauterine devices (IUDs).
The majority opinion said that by requiring companies that did not comply to pay fines, the government was placing an undue burden on these employers. Justice Alito noted that Hobby Lobby would have to either give up its religious tenets or pay up to $1.3 million a day in fines*.
"If these consequences don’t amount to a heavy burden, I’m not sure what would," he said.
The majority also said that since the government already makes exceptions to the contraception requirement for religious organizations and nonprofit religiously affiliated groups, that it could have made the same allowances for for-profit companies. The government also could have offered to pay for the contraceptives that companies refuse to cover, they said.
Justice Ruth Bader Ginsburg, who read the dissenting opinion, called the majority’s opinion "a decision of startling breadth." If allowed to stand, it could raise what she called "a host of ‘me, too’ questions," such as whether an employer could opt out of coverage for antidepressants, vaccinations, or medications derived from pigs, "based on the employer’s sincerely held religious beliefs opposing those medical practices."
Joined by Justice Stephen G. Breyer, Justice Elena Kagan, and Justice Sonia Sotomayor, she also said that while the ruling might seem to be limited to access to the four contraceptives in the cases, it was likely that companies would seek to deny coverage for all available contraceptive technologies.
The dissenters also said that they doubted that Congress intended for the RFRA to be used to allow a company to stand in between a woman and her physician. Any decision to use a contraceptive "will be the woman’s autonomous choice, informed by the physician she consults," they wrote.
Many physicians’ and women’s health organizations called it a dangerous precedent.
The American College of Obstetricians and Gynecologists President John C. Jennings said in a statement that the group was “profoundly disappointed” in the decision, as it “inappropriately allows employers to interfere in women’s health care decisions.” Dr. Jennings also said that contraceptives should not be treated differently than other health care services for women. “The value of family planning – including contraception – has been clearly demonstrated,” he said. “The ability of a woman to time and space her children reduces infant, child, and maternal morbidity and mortality, and can lead to more optimal health outcomes for mother and for baby.”
Contraception also helps prevent unintended pregnancy, he said, adding, “This is absolutely essential in America, where nearly one half of all pregnancies are unintended.”
In a statement, Dr.Reid Blackwelder, president of the American Academy of Family Physicians, said, "With this decision, the court has moved health care decisions out of the exam room, where patients can consult with their physicians — and where such decisions should be made — and put them into the hands of business owners who base decisions on personal beliefs rather than [on] medical science." Dr. Blackwelder added, "Personal or institutional beliefs should not put Americans’ health and lives at risk, simply because employers control the insurance available to their workers. Unfortunately, the Supreme Court decision opens the gate for just such consequences."
Dr. James M. Perrin, president of the American Academy of Pediatrics, said, "For pediatricians, the science is clear: contraception is a safe, effective tool to prevent unintended pregnancy in young women of any reproductive age. Today, we are disappointed that justice did not side with science."
Some physician groups expressed their support for the decision.
The American Association of Pro-Life Obstetricians and Gynecologists called the ruling a "tremendous victory for all Americans and their freedom of conscience." The group added that it was "hopeful that this recognition of conscientious objection will help to strengthen the rights of physicians to continue to refuse to participate in any medical procedure or to prescribe any drug that is designed and purposed to kill a living human being."
The Christian Medical Association also applauded the decision. In a statement, Dr. David Stevens, CEO, said, "We are very thankful that the Supreme Court acted to protect family businesses from government coercion and fines for simply honoring the tenets of their faith," and called it a "much-needed victory for faith freedoms."
In a joint press briefing, representatives from Planned Parenthood, NARAL, the American Civil Liberties Union, and the National Women’s Law Center said that they would work with Congress to try to reverse the Supreme Court ruling through legislation. The AAP will do the same, said Dr. Perrin.
Some Senate Democrats have said they will prepare legislation to address the case immediately. Sen. Patty Murray (D-Wash.), who filed a friend of the court brief on behalf of 18 Senators that sided with the government’s case, said in a statement that, "Since the Supreme Court decided it will not protect women’s access to health care, I will. In the coming days I will work with my colleagues and the Administration to protect this access, regardless of who signs your paycheck."
Senate Republican leader Mitch McConnell (R-Ky.), however, said that the decision was a validation of religious freedom. "Obamacare is the single worst piece of legislation to pass in the last 50 years, and I was glad to see the Supreme Court agree that this particular Obamacare mandate violates the Religious Freedom Restoration Act," Sen. McConnell said, in a statement.
On Twitter @aliciaault
*Clarification, 7/2/2014: An earlier version of this article did not include the intact quote from the majority opinion.
*This story was updated 7/2/2014.
WASHINGTON – For-profit companies cannot be compelled to provide insurance coverage for contraception if doing so violates the religious beliefs of the company’s owners, the Supreme Court ruled June 30.
Dissenting justices said that it had the potential to allow employers to use their religious beliefs as a way to object to providing coverage for vaccinations, blood transfusions, and other procedures, and that it could open the door to deny employment to certain individuals or groups.
The Court ruled 5-4 in the cases of Burwell v. Hobby Lobby Stores Inc. and Conestoga Wood Specialties Corp. v. Burwell.
Justice Samuel Alito, reading the opinion for the majority, said that the Religious Freedom Restoration Act (RFRA) of 1993 protected the rights of not just individuals, but also of individuals who run closely held for-profit corporations.
The terms of that law "make it perfectly clear that Congress did not discriminate in this way against men and women who wish to run their businesses as for-profit corporations in the manner required by their religious beliefs," according to the opinion signed by Justice Alito, Justice Anthony P. Kennedy, Justice Antonin Scalia, and Justice Clarence Thomas, and Chief Justice John P. Roberts.
The plaintiffs in the cases said that being required by the Affordable Care Act to provide insurance coverage for contraception violated their religious beliefs. In particular, Hobby Lobby and Conestoga Wood both objected to four specific types of contraception that they said interfere with conception and thus constitute abortion: the Ella morning-after pill, the Plan B morning-after pill, and hormonal and copper intrauterine devices (IUDs).
The majority opinion said that by requiring companies that did not comply to pay fines, the government was placing an undue burden on these employers. Justice Alito noted that Hobby Lobby would have to either give up its religious tenets or pay up to $1.3 million a day in fines*.
"If these consequences don’t amount to a heavy burden, I’m not sure what would," he said.
The majority also said that since the government already makes exceptions to the contraception requirement for religious organizations and nonprofit religiously affiliated groups, that it could have made the same allowances for for-profit companies. The government also could have offered to pay for the contraceptives that companies refuse to cover, they said.
Justice Ruth Bader Ginsburg, who read the dissenting opinion, called the majority’s opinion "a decision of startling breadth." If allowed to stand, it could raise what she called "a host of ‘me, too’ questions," such as whether an employer could opt out of coverage for antidepressants, vaccinations, or medications derived from pigs, "based on the employer’s sincerely held religious beliefs opposing those medical practices."
Joined by Justice Stephen G. Breyer, Justice Elena Kagan, and Justice Sonia Sotomayor, she also said that while the ruling might seem to be limited to access to the four contraceptives in the cases, it was likely that companies would seek to deny coverage for all available contraceptive technologies.
The dissenters also said that they doubted that Congress intended for the RFRA to be used to allow a company to stand in between a woman and her physician. Any decision to use a contraceptive "will be the woman’s autonomous choice, informed by the physician she consults," they wrote.
Many physicians’ and women’s health organizations called it a dangerous precedent.
The American College of Obstetricians and Gynecologists President John C. Jennings said in a statement that the group was “profoundly disappointed” in the decision, as it “inappropriately allows employers to interfere in women’s health care decisions.” Dr. Jennings also said that contraceptives should not be treated differently than other health care services for women. “The value of family planning – including contraception – has been clearly demonstrated,” he said. “The ability of a woman to time and space her children reduces infant, child, and maternal morbidity and mortality, and can lead to more optimal health outcomes for mother and for baby.”
Contraception also helps prevent unintended pregnancy, he said, adding, “This is absolutely essential in America, where nearly one half of all pregnancies are unintended.”
In a statement, Dr.Reid Blackwelder, president of the American Academy of Family Physicians, said, "With this decision, the court has moved health care decisions out of the exam room, where patients can consult with their physicians — and where such decisions should be made — and put them into the hands of business owners who base decisions on personal beliefs rather than [on] medical science." Dr. Blackwelder added, "Personal or institutional beliefs should not put Americans’ health and lives at risk, simply because employers control the insurance available to their workers. Unfortunately, the Supreme Court decision opens the gate for just such consequences."
Dr. James M. Perrin, president of the American Academy of Pediatrics, said, "For pediatricians, the science is clear: contraception is a safe, effective tool to prevent unintended pregnancy in young women of any reproductive age. Today, we are disappointed that justice did not side with science."
Some physician groups expressed their support for the decision.
The American Association of Pro-Life Obstetricians and Gynecologists called the ruling a "tremendous victory for all Americans and their freedom of conscience." The group added that it was "hopeful that this recognition of conscientious objection will help to strengthen the rights of physicians to continue to refuse to participate in any medical procedure or to prescribe any drug that is designed and purposed to kill a living human being."
The Christian Medical Association also applauded the decision. In a statement, Dr. David Stevens, CEO, said, "We are very thankful that the Supreme Court acted to protect family businesses from government coercion and fines for simply honoring the tenets of their faith," and called it a "much-needed victory for faith freedoms."
In a joint press briefing, representatives from Planned Parenthood, NARAL, the American Civil Liberties Union, and the National Women’s Law Center said that they would work with Congress to try to reverse the Supreme Court ruling through legislation. The AAP will do the same, said Dr. Perrin.
Some Senate Democrats have said they will prepare legislation to address the case immediately. Sen. Patty Murray (D-Wash.), who filed a friend of the court brief on behalf of 18 Senators that sided with the government’s case, said in a statement that, "Since the Supreme Court decided it will not protect women’s access to health care, I will. In the coming days I will work with my colleagues and the Administration to protect this access, regardless of who signs your paycheck."
Senate Republican leader Mitch McConnell (R-Ky.), however, said that the decision was a validation of religious freedom. "Obamacare is the single worst piece of legislation to pass in the last 50 years, and I was glad to see the Supreme Court agree that this particular Obamacare mandate violates the Religious Freedom Restoration Act," Sen. McConnell said, in a statement.
On Twitter @aliciaault
*Clarification, 7/2/2014: An earlier version of this article did not include the intact quote from the majority opinion.
*This story was updated 7/2/2014.
AT THE U.S. SUPREME COURT
Live births seen in half of pregnancies exposed to belimumab
PARIS – The live birth rate among women taking belimumab for systemic lupus erythematosus is similar to the background rate for women with the disorder, a study showed.
An analysis of clinical trials found that women who were taking the drug when they became pregnant had a live birth rate of 48% – in line with studies that place the rate at 55%-88%, Dr. Marcy Powell said at the annual European Congress of Rheumatology.
Although the number of pregnancies examined in the studies was small – with 80 exposed to belimumab (Benlysta) and 6 to placebo – the results were echoed by the initial findings in a recently established belimumab pregnancy registry, said Dr. Powell, director of safety evaluation and risk management at GlaxoSmithKline, which manufactures the drug.
More than 3,000 adult patients have been treated with belimumab – many for more than 10 years, but there are scant data on pregnancy outcomes. It’s a category C drug; women are advised to avoid pregnancy while taking it and for at least 4 months after stopping it. Thus, the only available pregnancy data are from inadvertent exposures in which the drug was stopped as soon as the pregnancy was discovered.
The data were drawn from the BLISS 52 and 78 studies, which enrolled more than 1,600 patients, 94% of whom were women. At baseline, the patients’ mean age was 38 years. Other medications were common in the cohort: 86% of patients were using corticosteroids, with 58% of those taking a prednisone equivalent of more than 5.7 mg/day. More than half (65%) were using antimalarials, and 49% were taking another immunosuppressant, including azathioprine, methotrexate, or mycophenolate mofetil.
Of the six pregnancies among patients assigned to placebo, there were three elective terminations, two spontaneous miscarriages, and one stillbirth, for a total fetal loss rate of 50%. The three pregnancies with fetal loss included two women who tested positive for anticardiolipin antibodies.
Among the 80 drug-exposed pregnancies, there were 21 elective terminations as well as 20 miscarriages and 1 stillbirth, yielding a total fetal loss rate of 26%. The background miscarriage rate was 12%-22%, and the background stillbirth rate was 2%-5%, both of which are similar to rates observed in the exposed pregnancies. Overall, women tested positive for anticardiolipin antibodies in 20 pregnancies exposed to belimumab, including 21% of the live births and 38% of the fetal losses.
There were 38 live births (48%). Four neonates had a congenital anomaly:
• One with Dandy-Walker syndrome.
• One with an unbalanced translocation (11 and 13) of maternal origin, a strictly genetic anomaly.
• One born at 27 weeks’ gestation with patent ductus arteriosus.
• One with bilateral enlarged kidneys with abnormal function whose mother was receiving ambrisentan, a known teratogen, for portal hypertension. (The infant was placed on dialysis and had the right kidney removed.)
The stillbirths in the placebo and belimumab patients occurred in conjunction with severe, very early third-trimester preeclampsia, Dr. Powell said. She noted that the sample size is so small that it’s unclear whether the results can be extrapolated to larger populations.
However, the Belimumab Pregnancy Registry, established by GlaxoSmithKline, could provide more reliable data, she said. So far, the registry has enrolled nine pregnancies, seven of which have been completed. Among those, there have been six live births and one miscarriage. Three of the infants were preterm, born at 32, 35, and 36 weeks’ gestation. There was one birth defect (mild Epstein’s anomaly of the tricuspid valve).
On Twitter @alz_gal
PARIS – The live birth rate among women taking belimumab for systemic lupus erythematosus is similar to the background rate for women with the disorder, a study showed.
An analysis of clinical trials found that women who were taking the drug when they became pregnant had a live birth rate of 48% – in line with studies that place the rate at 55%-88%, Dr. Marcy Powell said at the annual European Congress of Rheumatology.
Although the number of pregnancies examined in the studies was small – with 80 exposed to belimumab (Benlysta) and 6 to placebo – the results were echoed by the initial findings in a recently established belimumab pregnancy registry, said Dr. Powell, director of safety evaluation and risk management at GlaxoSmithKline, which manufactures the drug.
More than 3,000 adult patients have been treated with belimumab – many for more than 10 years, but there are scant data on pregnancy outcomes. It’s a category C drug; women are advised to avoid pregnancy while taking it and for at least 4 months after stopping it. Thus, the only available pregnancy data are from inadvertent exposures in which the drug was stopped as soon as the pregnancy was discovered.
The data were drawn from the BLISS 52 and 78 studies, which enrolled more than 1,600 patients, 94% of whom were women. At baseline, the patients’ mean age was 38 years. Other medications were common in the cohort: 86% of patients were using corticosteroids, with 58% of those taking a prednisone equivalent of more than 5.7 mg/day. More than half (65%) were using antimalarials, and 49% were taking another immunosuppressant, including azathioprine, methotrexate, or mycophenolate mofetil.
Of the six pregnancies among patients assigned to placebo, there were three elective terminations, two spontaneous miscarriages, and one stillbirth, for a total fetal loss rate of 50%. The three pregnancies with fetal loss included two women who tested positive for anticardiolipin antibodies.
Among the 80 drug-exposed pregnancies, there were 21 elective terminations as well as 20 miscarriages and 1 stillbirth, yielding a total fetal loss rate of 26%. The background miscarriage rate was 12%-22%, and the background stillbirth rate was 2%-5%, both of which are similar to rates observed in the exposed pregnancies. Overall, women tested positive for anticardiolipin antibodies in 20 pregnancies exposed to belimumab, including 21% of the live births and 38% of the fetal losses.
There were 38 live births (48%). Four neonates had a congenital anomaly:
• One with Dandy-Walker syndrome.
• One with an unbalanced translocation (11 and 13) of maternal origin, a strictly genetic anomaly.
• One born at 27 weeks’ gestation with patent ductus arteriosus.
• One with bilateral enlarged kidneys with abnormal function whose mother was receiving ambrisentan, a known teratogen, for portal hypertension. (The infant was placed on dialysis and had the right kidney removed.)
The stillbirths in the placebo and belimumab patients occurred in conjunction with severe, very early third-trimester preeclampsia, Dr. Powell said. She noted that the sample size is so small that it’s unclear whether the results can be extrapolated to larger populations.
However, the Belimumab Pregnancy Registry, established by GlaxoSmithKline, could provide more reliable data, she said. So far, the registry has enrolled nine pregnancies, seven of which have been completed. Among those, there have been six live births and one miscarriage. Three of the infants were preterm, born at 32, 35, and 36 weeks’ gestation. There was one birth defect (mild Epstein’s anomaly of the tricuspid valve).
On Twitter @alz_gal
PARIS – The live birth rate among women taking belimumab for systemic lupus erythematosus is similar to the background rate for women with the disorder, a study showed.
An analysis of clinical trials found that women who were taking the drug when they became pregnant had a live birth rate of 48% – in line with studies that place the rate at 55%-88%, Dr. Marcy Powell said at the annual European Congress of Rheumatology.
Although the number of pregnancies examined in the studies was small – with 80 exposed to belimumab (Benlysta) and 6 to placebo – the results were echoed by the initial findings in a recently established belimumab pregnancy registry, said Dr. Powell, director of safety evaluation and risk management at GlaxoSmithKline, which manufactures the drug.
More than 3,000 adult patients have been treated with belimumab – many for more than 10 years, but there are scant data on pregnancy outcomes. It’s a category C drug; women are advised to avoid pregnancy while taking it and for at least 4 months after stopping it. Thus, the only available pregnancy data are from inadvertent exposures in which the drug was stopped as soon as the pregnancy was discovered.
The data were drawn from the BLISS 52 and 78 studies, which enrolled more than 1,600 patients, 94% of whom were women. At baseline, the patients’ mean age was 38 years. Other medications were common in the cohort: 86% of patients were using corticosteroids, with 58% of those taking a prednisone equivalent of more than 5.7 mg/day. More than half (65%) were using antimalarials, and 49% were taking another immunosuppressant, including azathioprine, methotrexate, or mycophenolate mofetil.
Of the six pregnancies among patients assigned to placebo, there were three elective terminations, two spontaneous miscarriages, and one stillbirth, for a total fetal loss rate of 50%. The three pregnancies with fetal loss included two women who tested positive for anticardiolipin antibodies.
Among the 80 drug-exposed pregnancies, there were 21 elective terminations as well as 20 miscarriages and 1 stillbirth, yielding a total fetal loss rate of 26%. The background miscarriage rate was 12%-22%, and the background stillbirth rate was 2%-5%, both of which are similar to rates observed in the exposed pregnancies. Overall, women tested positive for anticardiolipin antibodies in 20 pregnancies exposed to belimumab, including 21% of the live births and 38% of the fetal losses.
There were 38 live births (48%). Four neonates had a congenital anomaly:
• One with Dandy-Walker syndrome.
• One with an unbalanced translocation (11 and 13) of maternal origin, a strictly genetic anomaly.
• One born at 27 weeks’ gestation with patent ductus arteriosus.
• One with bilateral enlarged kidneys with abnormal function whose mother was receiving ambrisentan, a known teratogen, for portal hypertension. (The infant was placed on dialysis and had the right kidney removed.)
The stillbirths in the placebo and belimumab patients occurred in conjunction with severe, very early third-trimester preeclampsia, Dr. Powell said. She noted that the sample size is so small that it’s unclear whether the results can be extrapolated to larger populations.
However, the Belimumab Pregnancy Registry, established by GlaxoSmithKline, could provide more reliable data, she said. So far, the registry has enrolled nine pregnancies, seven of which have been completed. Among those, there have been six live births and one miscarriage. Three of the infants were preterm, born at 32, 35, and 36 weeks’ gestation. There was one birth defect (mild Epstein’s anomaly of the tricuspid valve).
On Twitter @alz_gal
AT THE EULAR CONGRESS 2014
Key clinical point: Successful pregnancies are possible in SLE patients exposed to belimumab.
Major finding: The live birth rate was 48%, and the total fetal loss rate was 26%.
Data source: A cohort of 86 pregnancies and 9 additional pregnancies in a registry database.
Disclosures: Dr. Powell is director of safety evaluation and risk management at GlaxoSmithKline.