User login
Long-Term Outcomes of Allograft Reconstruction of the Anterior Cruciate Ligament
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
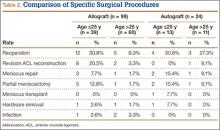
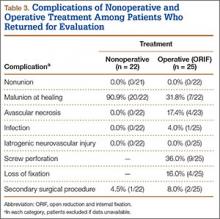
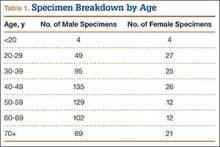
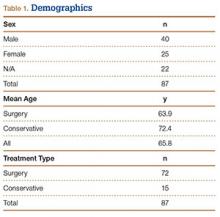
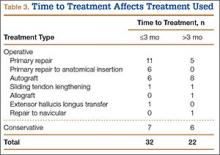
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
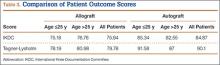
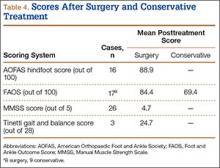
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
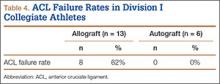
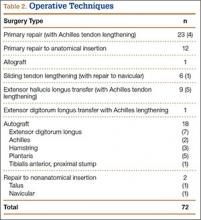
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
Injuries of the anterior cruciate ligament (ACL) are common. Good to excellent long-term results are generally expected in more than 90% of ACL reconstructions.1,2 Although our knowledge of the biomechanics, kinematics, and long-term outcomes of ACL reconstruction is extensive, the ideal graft choice for ACL reconstruction is still up for debate.
Historically, both quadruple-stranded hamstring tendon and bone–patellar tendon–bone (BPTB) autografts have been the most popular graft options for operative reconstruction of the ACL.3 Recently, allograft tissues have become increasingly popular as a graft source. Proponents of allograft ACL reconstruction have cited several advantages over autograft reconstruction, including decreased donor-site morbidity, shorter operative times, and quicker postoperative recovery.4-7 Nevertheless, some authors have recently reported higher rates of both reoperation and graft failure after allograft ACL reconstruction.4,8-11 The 2 senior surgeons in the Sports Medicine Section of the Department of Orthopedic Surgery at the University of Arizona College of Medicine had not recognized such high failure and revision rates in their own clinical practices.
To evaluate the long-term outcomes of allograft ACL reconstruction, we retrospectively reviewed the cases of all patients who underwent allograft or autograft ACL reconstruction by 2 senior surgeons at a single institution over an 8-year period. We hypothesized that the reoperation and revision surgery rates for allograft ACL reconstruction would not be higher than those reported for autograft reconstruction. We also hypothesized that allograft ACL reconstruction failure rates would not be higher for patients younger than 25 years than for patients who are older and less active.
Materials and Methods
This study was approved by the Institutional Review Board at the University of Arizona College of Medicine. We retrospectively reviewed the cases of all patients who underwent primary endoscopic ACL reconstruction at the University of Arizona College of Medicine over an 8-year period (2000–2008). All ACL reconstructions were performed by 2 senior, fellowship-trained sports medicine specialists, including Dr. William A. Grana. Patients were identified from the Current Procedural Terminology (CPT) code for ACL reconstruction. Both autograft and allograft reconstructions were included in the study. Patients undergoing revision ACL reconstruction and patients with multi-ligamentous knee injuries were excluded. All available medical records were reviewed for patient demographics and any concomitant knee pathology. We included patients of all activity levels, patients with acute ACL tears, and patients with chronically ACL-deficient knees. We identified a separate cohort of Division I varsity athletes from the University of Arizona for evaluation. These patients were identified from the injury surveillance system in the athletic training facility of the University of Arizona.
ACL reconstructions at our institution during this 8-year period were performed with both allograft and autograft soft tissue. Allograft tendons were most commonly used. Tibialis anterior allograft was used in the majority of those knees. Tibialis posterior and semitendinosus allografts were used in a small subset of patients. Autograft reconstruction was performed with quadruple-stranded semitendinosus and gracilis tendons. We reviewed operative reports to determine type of graft used for reconstruction.
Patients were assessed clinically by telephone interview and/or mailed survey. They were specifically asked whether there had been any postoperative complications. We reviewed all operative and postoperative follow-up notes for postoperative complications. Objective clinical assessment involved use of the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, the Tegner-Lysholm Knee Scoring Scale, and the Tegner Activity Scale.
Operative Technique
A standard, transtibial arthroscopically assisted ACL reconstruction was performed in all patients. For autograft reconstruction patients, both the semitendinosus and gracilis tendons were harvested through a small anteromedial incision and prepared to form a quadruple-stranded graft. All allograft tendons were obtained from the Musculoskeletal Transplant Foundation (MTF). Tibialis anterior and tibialis posterior allografts were folded in half to form a double-stranded graft. Alternatively, 2 semitendinosus allografts were prepared in the same fashion as that described for autograft hamstring tendons. The tibial tunnel was placed into the center of the ACL tibial footprint. With use of a transtibial approach, an endoscopic offset guide was used to place the femoral tunnel at the 10- and 2-o’clock positions in the right and left knees, respectively. In almost all cases, the graft was secured on the femoral side with a cortical fixation button. Tibial fixation was obtained with a bioabsorbable interference screw.
After ACL reconstruction, each patient participated in the standard accelerated rehabilitation outlined by Shelbourne and Gray.12 Guided rehabilitation was instituted within 1 week after surgery under the guidance of a physical therapist. Range-of-motion exercises and closed-chain strengthening exercises were begun at this time. The protocol emphasized early return of full terminal extension and normalization of gait patterns. Patients were allowed to return to play only after meeting specific criteria, about 6 months after surgery. Many athletes in our Division I university population are allowed to return to play 5 to 6 months after surgery, after meeting return-to-play criteria.
Statistical Analysis
We used Minitab 14 (Minitab, State College, Pennsylvania) to perform all statistical analyses, unpaired Student t tests to compare IKDC and Tegner-Lysholm results between allograft and autograft groups, and χ2 tests to compare revision and reoperation rates between groups. Significance was set at P = .05.
Results
We identified 362 patients who underwent ACL reconstructions at our institution between 2000 and 2008. Of these patients, 302 met the study inclusion criteria. One-hundred twenty-three (40.7%) of the 302 were available for follow-up by telephone interview and/or mailed questionnaire. This follow-up group consisted of 67 males and 56 females. Mean age at surgery was 29 years (range, 17-53 years). Mean follow-up was 50.3 months (range, 11-111 months). Of the 123 patients, 99 underwent allograft ACL reconstruction, and 24 underwent autograft ACL reconstruction. Seventeen (17%) of the 99 allograft cases required additional surgery (Table 1). The reoperation rate for patients under age 25 years (30.8%) was higher than the rate for patients older than 25 years (Table 2). Regarding patients who underwent additional surgeries, mean scores were lower with allograft (Tegner-Lysholm, 59; IKDC, 54) than with autograft (Tegner-Lysholm, 83; IKDC, 79) (Ps = .0025 and .006, respectively).
Revision rates were 10.1% (allograft group) and 4.2% (autograft group) (Table 1). This difference was not statistically significant (P = .18). In the allograft group, the revision rate was higher for patients younger than 25 years (20.5%) than for patients older than 25 years (3.3%) (Table 2). In comparison, in the autograft group, the revision rate was only 4% for patients younger than 25 years. For younger patients, the higher rate of revision with allograft (vs autograft) was statistically significant (P = .038). For older patients, allograft and autograft revision rates did not differ significantly (P = .19). No patient younger than 25 years required revision reconstruction after autograft ACL reconstruction.
IKDC and Tegner-Lysholm outcome scores for allograft and autograft groups are shown in Table 3. In patients 25 years or younger, IKDC scores were 75.18 after allograft reconstruction and 85.34 after autograft reconstruction—a significant difference (P = .045). In addition, Tegner-Lysholm scores were significantly higher after autograft reconstruction (91.58) than allograft reconstruction (78.19) in these younger patients (P = .003) (Table 3). IKDC and Tegner-Lysholm scores were not significantly different for older patients (Ps = .241 and .211, respectively).
The study also included a subset of 19 primary ACL reconstructions (13 allograft, 6 autograft) performed on Division I athletes from the University of Arizona. (Nineteen [91%] of the 21 athletes in our Division I cohort were available for follow-up.) All these patients were younger than 25 years. All autograft reconstructions were performed with quadruple-stranded gracilis and semitendinosus tendons. ACL graft failure occurred in 8 (62%) of the 13 allograft cases; there were no failures in the autograft group (Table 4). One of the 5 allograft cases that did not fail required multiple surgical débridement procedures for infection, but the graft was ultimately retained. There were no infections among the 6 autograft cases.
Discussion
The ideal graft for ACL reconstruction is still a matter of intense debate. There are many graft options for ACL reconstruction. Both BPTB and hamstring autografts are associated with various graft-specific comorbidities. Anterior knee pain, knee extensor weakness, extension loss, patella fracture, patellofemoral crepitance, and infrapatellar nerve injury have been described with BPTB autografts.13-17 In a meta-analysis of 11 studies comparing BPTB autografts with hamstring autograft, Goldblatt and colleagues17 found more extension loss, kneeling pain, and patellofemoral crepitance in the BPTP group.
Knee flexion weakness, knee flexion loss, increased knee laxity, and saphenous nerve injury have all been described with use of hamstring autografts.16-19 Goldblatt and colleagues17 demonstrated a significant flexion loss in the hamstring group in their meta-analysis as well as increased laxity with both the Lachman test and the pivot shift test. They also found that the hamstring autograft group exhibited side-to-side differences of more than 3 mm on KT-1000 testing when compared with the BPTB autograft group.
Proposed advantages of allograft reconstruction include elimination of donor-site morbidity and/or pain from a less invasive procedure, faster initial recovery, more sizing options, and shorter operative times.4-7 In a 5-year follow-up of patients who had ACL reconstruction with either Achilles allograft or BPTB autograft, Poehling and colleagues7 demonstrated overall similar long-term outcomes between the groups. However, the allograft patients reported less pain 1 and 6 weeks after surgery; better function 1 week, 3 months, and 1 year after surgery; and fewer activity limitations throughout the follow-up period. Lamblin and colleagues20 also found no difference between nonirradiated allograft and autograft tissue in ACL reconstruction in a 2013 meta-analysis of ACL studies published over a 32-year period.
Despite the proposed advantages of allograft ACL reconstruction, several recent studies have demonstrated poorer outcomes in both younger patients and more active patients after allograft reconstruction.8-11,21 In a 2007 meta-analysis, Prodromos and colleagues11 compared a series of allograft reconstructions with previously published data sets of both BPTB and hamstring autografts. They found that allograft reconstructions had significantly lower stability rates than autograft reconstructions. In a case–control study by Borchers and colleagues,10 21 patients with ACL graft failure were identified over a 2-year period, and surgical outcomes were compared with those of 42 age- and sex-matched controls. The authors found higher activity level and allograft use to be risk factors for subsequent graft failure after ACL reconstruction. More important, they showed a multiplicative interaction between higher activity level after ACL reconstruction and allograft use—an interaction that greatly increased the odds for ACL graft failure. Last, in a retrospective review, Singhal and colleagues8 evaluated the outcomes of ACL reconstruction using tibialis anterior tendon allograft and reported a 23.1% revision rate. In addition, 37.7% of patients required repeat surgery. The failure/reoperation rate was 55% for patients 25 years or younger and 24% for patients older than 25 years. The authors recommended not using tibialis anterior allografts in patients 25 years or younger and in patients who frequently engage in level I ACL-dependent sports.
The poor outcomes reported by Singhal and colleagues8 may be related to use of irradiated soft-tissue allografts. In a comparison of nonirradiated BPTB allograft and BPTB autograft in patients 25 years or younger, Barber and colleagues22 found equivalent outcomes at 2-year follow-up. They actually found a higher rate of failure for autograft reconstruction (9.4%) than allograft reconstruction (7.1%). A potential critique of their study is the significant difference between the patient groups’ mean ages: 18.6 years (autograft) versus 20.1 years (allograft). Despite this selection bias, Barber and colleagues22 argued that nonirradiated BPTB allograft is equivalent to BPTB autograft for ACL reconstruction.
Our study is one of the largest allograft studies with a comparison group. The principal findings of this study demonstrate that overall reoperation and revision rates after irradiated soft-tissue allograft ACL reconstruction are higher than those historically quoted for autograft ACL reconstruction. Specifically, allograft patients younger than 25 years had a reoperation rate of 30.8% and a revision rate of 20.5%. (Allograft patients older than 25 years had lower rates of reoperation, 8.3%, and revision, 3.3%.) After revision surgery, autograft patients’ subjective outcomes (IKDC and Tegner-Lysholm scores) were significantly improved compared with those of allograft patients (Ps = .0017 and .0031, respectively). Most compelling, however, is the unexpected and quite concerning 62% failure rate in our high-level Division I intercollegiate athletes.
There are multiple hypotheses regarding the higher failure rates of allograft tissues versus autograft tissues in ACL reconstruction. Processing methods, exposure to ionizing radiation, and the incorporation/ligamentization process have all been cited as possible reasons for allograft failure. All the allograft tendons used in the present study were obtained from MTF, which uses a proprietary “aseptic” processing system that includes washing in buffered saline impregnated with antibiotics (imipenem/cilastatin, amphotericin B, gentamicin) followed by final rinsing in phosphate-buffered saline. The majority of grafts are subjected to low-level irradiation (<2 Mrad/20 kGy) based on the outcomes of MTF’s stringent donor-selection process. Although the washing process has not been shown to alter the structural integrity of donor grafts, multiple studies have outlined the detrimental effects of higher levels of gamma radiation on allograft tissues. Although lower levels are effective against potential bacterial contaminants, a radiation level of 4 Mrad is necessary to kill the human immunodeficiency virus (HIV). Thus, a dose of 4 Mrad or higher is needed to truly “sterilize” a graft. This higher dose is an issue, as it has been known for some time that higher levels of ionizing radiation can have adverse effects on the biomechanical strength of soft-tissue allografts. In fact, ionizing radiation has dose-dependent effects.23-26 Schwartz and colleagues27 showed in a caprine model that radiation exposure at 4 Mrad significantly decreased the biomechanical strength of ACL allografts at 6 months. Balsly and colleagues28 found in a biomechanical study that radiation doses of 18 to 22 Mrad did not significantly affect the mechanical integrity of soft-tissue allografts. Conversely, in an in vivo study, Rappe and colleagues29 showed that Achilles allografts irradiated at a dose of 2.0 to 2.5 Mrad had a failure rate (33%) much higher than that of nonirradiated allografts (2.4%). The radiation dose used by MTF is less than 2 Mrad. Although more than needed to kill bacterial contaminants, this dose is considered by MTF to be below the threshold for biomechanical alterations. Only a minority of grafts is treated without irradiation.
It is possible that any level of radiation affects ligamentization of allograft tissues. Multiple studies have outlined the ligamentization process of autograft tendons in vivo. Patellar tendon autografts undergo central degeneration 2 to 6 weeks after reconstruction, but, by 6 to 12 months, these tendons have structural properties similar to those of the native ACL.30-34 Findings are similar for hamstring autografts.35,36 Goradia and colleagues36 found that, by 52 weeks, semitendinosus autografts transform into a histologic structure similar to that of the normal ACL. Remodeling of allograft tendons has been described as occurring at a much slower rate.27,37-40 Bhatia and colleagues37 demonstrated faster remodeling in autograft tissues versus allograft tissues at early time points in an in vivo rabbit model. Ultimately, differences in graft incorporation and ligamentization may be a primary factor in the higher failure rates of allograft ACL reconstruction. Current rehabilitation protocols may not take into account the longer ligamentization process for allograft tissues. These protocols are largely based on our current understanding of the ligamentization process after autograft reconstruction. It is possible that the rehabilitation program and return-to-play schedule for allograft reconstruction need to be altered to help avoid higher failure rates. The return-to-play protocol at the authors’ institution scheduled most varsity athletes to return to play 6 months after surgery. In some cases, the timetable was shortened, and some athletes were returned to play 5 months after surgery, after meeting all return-to-play criteria. Based on the findings of the present study, this return-to-play schedule may be much too aggressive for high-level athletes after allograft reconstruction. It is possible these allografts have not reached “maturity,” as their autograft counterparts have, and thus are not ready for unrestricted return to play.
Our study had multiple strengths. All reconstructions were performed by 2 senior surgeons with extensive clinical experience. The autograft and allograft reconstructions used the same techniques and rehabilitation protocols. This is one of the largest studies of outcomes of allograft ACL reconstruction and one of the largest studies that used a comparison group of autograft reconstructions. Having a comparison group effectively allowed us to contrast the differences between allograft and autograft tissues. Last, this study evaluated a subgroup of high-level NCAA Division I athletes. Follow-up in the overall study was 40.7%, but follow-up in this subgroup was 91%. The very high follow-up rate in the university population helped us validate the overall results of the study. Study results reinforced the fact that irradiated soft-tissue allograft may not be indicated for ACL reconstruction in a younger, more active patient population and led to a change in approach to ACL reconstruction for Division I intercollegiate athletes at the University of Arizona. Allograft ACL reconstruction is no longer recommended for the intercollegiate athletes at the University of Arizona.
Our study had its limitations. First, it had the inherent biases of a retrospective study. Second, many patients were lost to follow-up. We contacted and surveyed 40.7% of the patients who met the inclusion criteria. We tried reaching them in multiple ways—through US mail, all listed phone numbers, family members, and so forth. Tucson, Arizona is a college town and has a larger transient population, which may have added to the difficulty in contacting patients.
Conclusion
Given the high rates of reoperation and revision surgery with allograft reconstruction in younger patients in this study, we recommend against routine use of irradiated soft-tissue allograft tissue for ACL reconstruction in patients 25 years or younger. In our clinical practices, we prefer using autograft tissue for ACL reconstruction in younger, more active individuals. Irradiated soft-tissue allograft ACL reconstruction is a viable option in the older, less active patient population. Although the overall reoperation rate in this cohort study is acceptable, the revision rate for patients younger than 25 years is concerning and should be taken into account when considering use of irradiated soft-tissue allograft for ACL reconstruction in these younger patients.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
1. Schepsis AA, Busconi BD. Sports Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
2. Campbell WC, Canale ST, Beaty JH. Campbell’s Operative Orthopaedics. 11th ed. Philadelphia, PA: Mosby/Elsevier; 2008.
3. Sherman OH, Banffy MB. Anterior cruciate ligament reconstruction: which graft is best? Arthroscopy. 2004;20(9):974-980.
4. Lee JH, Bae DK, Song SJ, Cho SM, Yoon KH. Comparison of clinical results and second-look arthroscopy findings after arthroscopic anterior cruciate ligament reconstruction using 3 different types of grafts. Arthroscopy. 2010;26(1):41-49.
5. Sun K, Tian SQ, Zhang JH, Xia CS, Zhang CL, Yu TB. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25(7):750-759.
6. Kuhn MA, Ross G. Allografts in the treatment of anterior cruciate ligament injuries. Sports Med Arthrosc Rev. 2007;15(3):133-138.
7. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
8. Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469-475.
9. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
10. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case–control study. Am J Sports Med. 2009;37(12):2362-2367.
11. Prodromos C, Joyce B, Shi K. A meta-analysis of stability of autografts compared to allografts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):851-856.
12. Shelbourne KD, Gray T. Anterior cruciate ligament reconstruction with autogenous patellar tendon graft followed by accelerated rehabilitation. A two- to nine-year followup. Am J Sports Med. 1997;25(6):786-795.
13. Rosenberg TD, Franklin JL, Baldwin GN, Nelson KA. Extensor mechanism function after patellar tendon graft harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):519-525.
14. Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone–patellar tendon–bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther. 2009;39(4):278-286.
15. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
16. Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17(9):971-980.
17. Goldblatt JP, Fitzsimmons SE, Balk E, Richmond JC. Reconstruction of the anterior cruciate ligament: meta-analysis of patellar tendon versus hamstring tendon autograft. Arthroscopy. 2005;21(7):791-803.
18. Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2-11.
19. Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248-257.
20. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
21. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246.
22. Barber FA, Cowden CH 3rd, Sanders EJ. Revision rates after anterior cruciate ligament reconstruction using bone–patellar tendon–bone allograft or autograft in a population 25 years old and younger. Arthroscopy. 2014;30(4):483-491.
23. Salehpour A, Butler DL, Proch FS, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone–patellar tendon–bone allografts. J Orthop Res. 1995;13(6):898-906.
24. Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone–patellar tendon–bone allografts. J Orthop Res. 1991;9(2):209-218.
25. Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone–patellar tendon–bone allografts. Am J Sports Med. 1995;23(5):643-646.
26. De Deyne P, Haut RC. Some effects of gamma irradiation on patellar tendon allografts. Connect Tissue Res. 1991;27(1):51-62.
27. Schwartz HE, Matava MJ, Proch FS, et al. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34(11):1747-1755.
28. Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9(4):289-298.
29. Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653-1658.
30. Amiel D, Kleiner JB, Akeson WH. The natural history of the anterior cruciate ligament autograft of patellar tendon origin. Am J Sports Med. 1986;14(6):449-462.
31. Amiel D, Kleiner JB, Roux RD, Harwood FL, Akeson WH. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4(2):162-172.
32. Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217-224.
33. Ballock RT, Woo SL, Lyon RM, Hollis JM, Akeson WH. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7(4):474-485.
34. Clancy WG Jr, Narechania RG, Rosenberg TD, Gmeiner JG, Wisnefske DD, Lange TA. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg Am. 1981;63(8):1270-1284.
35. Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25(4):554-559.
36. Goradia VK, Rochat MC, Kida M, Grana WA. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28(1):40-46.
37. Bhatia S, Bell R, Frank RM, et al. Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789-1798.
38. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
39. Goertzen MJ, Clahsen H, Schulitz KP. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):150-157.
40. Mae T, Shino K, Maeda A, Toritsuka Y, Horibe S, Ochi T. Effect of gamma irradiation on remodeling process of tendon allograft. Clin Orthop. 2003;(414):305-314.
Enhancement of Acute Tendon Repair Using Chitosan Matrix
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
Rotator cuff tears (RCTs) are common tendon injuries that can cause chronic pain and severe functional disability. Massive RCTs do not heal spontaneously and, in many cases, result in poor clinical outcomes. Specifically, muscle atrophy and fatty infiltration correlate with poor outcomes after surgical repair.1 Fatty infiltration of the rotator cuff is a common phenomenon that can lead to permanent structural alterations within the tendon. It has been suggested that changes in muscle fiber orientation (the pennation angle) can cause mesenchymal stem cells to migrate to the interface between muscle fibers and the region of fatty infiltration of the muscle.2 Understanding the factors involved in muscle degeneration and atrophy, and in fatty infiltration, may lead to treatments that improve outcomes for patients with massive RCTs. One proposed treatment involves placing continuous mechanical traction on the ends of the torn tendon.2 Findings from this research have indicated that acute tears that become chronic tears are typified by inelasticity and poor function of the muscle–tendon unit. It is therefore important to develop a method that speeds tendon healing without causing the muscle fiber atrophy and pennation angle changes that lead to fatty atrophy, which appears to be an irreversible structural change.
On the basis of the theory that adding mesenchymal cells may improve tendon healing, investigators have studied use of transcription factors (eg, scleraxis) specific to tendogenesis in the embryonal stage.3,4 Nevertheless, certain transcription factors are associated with formation of fibrocartilage in higher concentrations.4 Moreover, decalcified bone matrix increases cartilage formation when added to the tendon repair site.5 Cartilage formation, however, is associated with poorer functional results.6 Thus, there is a need for a method that facilitates faster tendon healing with higher quality tissue formation and less muscle atrophy.
Chitosan, a linear polysaccharide, is associated with scarless healing of soft tissues and prevention of adhesion formation both intraperitoneally and during tendon healing after surgery.7,8 Chitosan tends to precipitate in physiologic pH, thereby mitigating its potency. Fortunately, a chitosan solution that does not precipitate in physiologic conditions was recently developed.9 The solution’s lack of precipitation, coupled with its in situ gelling, allows it to adhere to the repair site long enough to take effect. These characteristics could allow for intimate contact between gel and tendon, facilitating guided-tissue regeneration and preventing adhesion of the rotator cuff to surrounding tissue. By contrast, other biological agents (eg, platelet-rich plasma) are administered as fluid rather than gel and are therefore more susceptible to diffusing from the repair site, mitigating their effects. Thus, chitosan gel is fairly unique among agents.
In the study reported here, we histologically investigated whether a chitosan gel would help improve healing of rotator cuff tendon (acute supraspinatus) tears in a rat model.
Materials and Methods
Supraspinatus Surgical Model
Forty Wistar rats, each weighing between 300 and 400 g, were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee at Rabin Medical Center in Petah Tikva, Israel. The rats were anesthetized with ketamine 90 mg/kg and xylazine 10 mg/kg, both administered intramuscularly, and anesthesia was prolonged as needed with 2% isoflurane, administered by nose cone. The skin was incised 5 cm along the upper back following the midline of the spine. The resulting skin flaps were retracted and the scapula exposed. Careful blunt dissection allowed visualization of the rotator cuff and the trans-scapular arch. A full-thickness incision of the supraspinatus tendon was then made 2 mm distal to the arch. This procedure was performed on both shoulders. For the right supraspinatus tendon, a bioabsorbable chitosan–hydrochloric acid solution (>70% de-acetylated chitosan, molecular weight of 600 kDa; Heppe Medical Chitosan GmbH, Halle, Germany) was sterilely applied to the ends of the tendon (total volume, 0.5 mL) and automatically gelled in situ by heating to about 37°C (rat’s internal body temperature). The tendon ends were subsequently approximated with a single 4-0 Prolene suture (Ethicon, Somerville, New Jersey). The left shoulder (tendon repaired with suture only) served as a control.
The rats were housed for a maximum of 12 weeks after surgery. They were sacrificed (in groups of 5 each) 2 hours, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, and 12 weeks after surgery. After each rat was sacrificed, both shoulder girdles were harvested, and the sutures were removed from the supraspinatus tendons.
Histologic Analysis
After routine fixation with 4% formalin for 48 hours and decalcification with 10% ethylenediaminetetraacetic acid (EDTA) for 3 weeks, the specimens were sectioned with a microtome blade. Care was taken to ensure the plane of the microtome blade was parallel with the longitudinal plane of the supraspinatus muscle and tendon to allow for evaluation of pennation angle. Hematoxylin-eosin staining and Masson trichrome staining were subsequently performed.
A variety of histologic measurements were obtained with use of ImageJ software (US National Institutes of Health). Percentage of fibrous tissue was determined by examining the slides at low magnification fields (×25) at the tendon healing site. Three such fields were evaluated per specimen. The fibrous tissue was circled manually, and percentage of tissue area was assessed and compared with total region of interest. Cellularity was carefully outlined and measured as percentage of total tendon area occupied by cells. Fatty atrophy was defined as either present or absent. Muscle fiber diameter was defined as average diameter of 10 muscle fibers measured within 2 mm of the tendon laceration site. Inflammatory cell collections were defined as either large (>100 µm in diameter) or small (<100 µm in diameter) and were dichotomized to either present or absent. Pennation angle was defined as average angle between muscle fibers and longitudinal axis of supraspinatus muscle and tendon unit. Ten fibers proximal to and within 2 mm of the laceration site were randomly selected, measured, and averaged.
Statistical Analysis
Statistical analysis was performed with Analyse-it 2.20 for Microsoft Excel 2010 (Analyse-it Software, Leeds, United Kingdom). Data were initially analyzed with the Kolmogorov-Smirnov test to assess for normality of distribution. The t test was used to compare continuous variables when the data were normally distributed and the Mann-Whitney test when the data were not normally distributed.
Results
All tendons (both groups) healed within 12 weeks. Generally, the tissue formed at the repair site exhibited a mixture of tenocyte-like cells (fibrotic tissue) and granulation tissue without clear orientation. As noted in Figure 1, the tendons treated with chitosan had more fibrotic tissue (overall mean, 21.5%) relative to the control group (mean, 12.3%), and the difference was significant (P = .003). The most notable differences were found at time points later than 1 week after surgery. In addition, amount of cellularity (Figure 2) was higher in chitosan-treated tendon and control tendon than in the normal, uninjured adjacent tendon at all time points (P < .001). Chitosan-treated tendons had significantly higher cellularity than untreated control tendons from 1 to 2 weeks (P < .001), and control tendons were significantly hypercellular compared with chitosan-treated tendons from 4 to 8 weeks (P < .001), but both groups exhibited similar cellularity by 12 weeks (P > .05). Fatty atrophy was found at significantly higher rates in control rats than in chitosan-treated rats (P = .001; Table). Furthermore, as noted in Figure 3, muscle fiber diameter decreased in both groups after injury (P < .001).
Figure 4 shows that the amount of inflammatory collections was significantly smaller in the chitosan-treated group than in the control group over the course of the study (P = .01). In addition, pennation angle steadily decreased in the control group throughout the study period, whereas it transiently decreased in the chitosan-treated group (until 2 weeks) before returning to its immediate postoperative level by 12 weeks (Figure 5). Overall, the chitosan-treated group maintained a higher pennation angle than the control group did (P < .001).
Discussion
RCTs affect more than 40% of patients over age 60 years and are a common cause of debilitating pain, reduced shoulder function, and weakness.10 Thirty thousand to 75,000 rotator cuff repairs are performed annually in the United States.11 Although the best treatment for this disorder remains a topic of debate, arthroscopic and (when necessary) open surgical repair is the accepted gold standard for the treatment of tears that do not improve with conservative management. Despite advances in the surgical treatment of these tears, the surgical failure rates are high (range, 20%-90%), with failures attributed to factors beyond patient age, tear size and chronicity, muscle atrophy and degeneration, tendon quality, repair technique, and postoperative rehabilitation.12,13 Repair strategies that biologically enhance the patient’s intrinsic healing potential are needed.
In tendon repair, choice of repair material (eg, graft) is crucial in determining the success of tissue engineering approaches. The ideal scaffold is biocompatible and does not elicit a host inflammatory response. The selected scaffold in its composition and fabricated form must be capable of holding and supporting cells. In addition, the scaffold should be biodegradable, serving as a temporary support for such cells and mechanically augmenting the repaired tendon while allowing for eventual replacement by matrix components. Moreover, the scaffold should have high porosity and a large surface area. Furthermore, the material should mimic the native tendon extracellular matrix (ECM) architecture to allow cells to be distributed throughout the scaffold and to facilitate diffusion of nutrients and factors that promote cellular proliferation and ECM production.
Given the importance of glycosaminoglycans (GAGs) in supporting the reticular structure of the matrix, use of GAGs or GAG-analogues as components of a tendon tissue scaffold for enhancing repair is well documented.14 One such candidate is chitosan, a partially de-acetylated derivative of chitin found in arthropod exoskeletons. Structurally, chitosan shares some characteristics with various GAGs and hyaluronic acid.15 More specifically, chitosan is a linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-glycosidic bonds. Investigators have studied the properties of chitosan, including its biocompatibility, biodegradability, antibacterial activity, mucoadhesivity, and wound healing.16,17
One of the most promising features of chitosan is that it can be processed into porous structures for use in cell transplantation and tissue regeneration.18,19 Porous chitosan structures can be formed by freezing and lyophilizing chitosan-acetic acid solutions; chondrogenic cell adhesion and proliferation onto these structures have been reported.20,21 This chitosan scaffolding method has also been used to test different composites with collagens, gelatins, GAGs, and hyaluronic acid, all of which have also been proposed as useful 3-dimensional materials for tissue repair.22
In the present study, we used chitosan matrix in RCT repair. We hypothesized that chitosan matrix could enhance rotator cuff repair the same way it enhances repair in epidermal tissues.16 Histologic findings demonstrated that the percentage of fibrous tissue was significantly higher in the chitosan-treated group than in the control group. This improved fibroblastic response may be attributed to the ability of chitosan to enhance cell migration and serve as a scaffold for repair. Other studies have indicated that chitin, of which chitosan is the primary derivative, accelerated the healing of skin and subcutaneous tissues by increased cell migration.23 Moreover, Okamoto and colleagues24 reported that chitin implants stimulated abundant angiogenesis through the same mechanism.
Inadequate initial strength of a repair may lead to a recurrent cuff tear or a disability of rotator cuff function in the early healing stages. In our study, the chitosan matrix tended to be absorbed by 6 weeks after surgery. Its adherence to and ultimate absorption at the repair site may be challenged by the flow of irrigation fluid through the subacromial space in the setting of arthroscopic surgery. However, because the chitosan remains in a more robust gel form, it is better able to resist being washed from the repair site. For augmentation, it may be possible to apply a biocompatible patch over the gel to further protect it from being dislodged. In addition, histologic findings showed that the fibrous repair tissue gradually increased until reaching a peak 8 weeks after surgery—an indication that the absorption rate of the chitosan scaffold lags behind full recovery of the repair tissue. Given this relationship, further studies are needed to determine the mechanical strength of the repair between 6 and 8 weeks, which is important for avoiding recurrent tears.
This study had a few limitations. First, as with any animal model, the anatomy and function of the rat shoulder differ from those of the human shoulder. The acromial arch differs in quadruped animals, with less coverage of the supraspinatus and more of the subscapularis.25 These anatomical differences could yield altered stress mechanics that could affect tendon repair. Furthermore, rats and humans differ in their RCT healing rates. Thus, the pathophysiology of muscle atrophy and fat infiltration in rats may slightly differ from that in humans. In addition, no mechanical testing was performed to compare chitosan-treated and untreated rotator cuff repairs, and such testing is needed to clarify the biomechanical importance of augmentation. Furthermore, no immunohistochemical analysis was performed for collagen. In the repair of rotator cuff tendons, surgeons must consider not only the number of cells but also the production of ECM. Although not directly confirmed in this study, chitosan induced fibrous tissue proliferation that mirrored production of a large amount of collagen fibers. Last, we used an open RTC model. As an arthroscopic model was not used, no definitive conclusions can be drawn regarding use of chitosan in arthroscopy.
Conclusion
Use of chitosan as an acellular matrix improved formation of healing fibrous tissue, increased the number of cells, and prevented fatty atrophy and inflammatory aggregates inside repair sites while facilitating recovery of the natural pennation angle of the tissue. These results demonstrate that chitosan can enhance tendon healing in the setting of acute RCT. Further research, including biomechanical testing of repaired tendons, is needed to further delineate the utility of chitosan in regenerating irreparable RCTs.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
1. Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg. 2008;17(1 suppl):1S-7S.
2. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
3. Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow–derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282-1289.
4. Gulotta LV, Rodeo SA. Emerging ideas: evaluation of stem cells genetically modified with scleraxis to improve rotator cuff healing. Clin Orthop. 2011;469(10):2977-2980.
5. Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115-122.
6. Kumagai J, Sarkar K, Uhthoff HK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol. 1994;21(11):2096-2100.
7. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010;33(4):E254-E260.
8. Zhang H, Sheng ZJ, Hou CL. Effect of chitosan membrane on tendon adhesion and healing [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13(6):382-385.
9. Cho MH, Kim KS, Ahn HH, et al. Chitosan gel as an in situ–forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14(6):1099-1108.
10. Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10(3):199-203.
11. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
12. Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3-12.
13. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
14. Hunziker E, Spector M, Libera J, et al. Translation from research to applications. Tissue Eng. 2006;12(12):3341-3364.
15. Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589-2598.
16. Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104(12):6017-6084.
17. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-192.
18. Hsieh WC, Chang CP, Lin SM. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2007;57(2):250-255.
19. Madihally SV, Matthew HW. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20(12):1133-1142.
20. Nettles DL, Elder SH, Gilbert JA. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8(6):1009-1016.
21. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: interconnective pore size and cartilage engineering. Acta Biomater. 2006;2(3):313-320.
22. Manjubala I, Scheler S, Bossert J, Jandt KD. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006;2(1):75-84.
23. Su CH, Sun CS, Juan SW, Ho HO, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes: effects on wound healing and fibroblast. Biomaterials. 1999;20(1):61-68.
24. Okamoto Y, Southwood L, Stashak TS. Effect of chitin on nonwoven fabric implant in tendon healing. Carbohydr Polym. 1997;33:33-38.
25. Gupta R, Lee TQ. Contributions of the different rabbit models to our understanding of rotator cuff pathology. J Shoulder Elbow Surg. 2007;16(5 suppl):S149-S157.
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Emerging Biologics in Orthopedics
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
The discipline of orthopedic medicine and surgery has dramatically advanced over the last several decades. Improved understanding of biomechanics, tissue healing, and the pathogenesis of musculoskeletal diseases has allowed us to make significant progress in the diagnosis, treatment, and rehabilitation of our patients. Despite these advancements, there is still much to be learned, especially in the field of orthobiologics and regenerative medicine. As our understanding of existing technologies, such as bone marrow aspirate, platelet-rich plasma, and adult stem cells, continues to evolve, even newer biologic treatment options are being developed. This issue of The American Journal of Orthopedics focuses on emerging biologics across the spectrum of orthopedic care.
In this issue, on pages 202-205, Mansour and Conway describe a new prone retrograde technique for obtaining bone graft using the Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania). While iliac crest bone graft has been the gold standard for many years, use of the RIA system to obtain bone graft has been studied and has been shown to have decreased morbidity when compared with iliac crest harvest.1 Additionally, intramedullary bone graft from the femur appears to be just as concentrated with biologically active bone marrow as iliac crest harvest.2 This new technique allows increased efficiency, especially for surgeries that are done in the prone position.
Melamed and colleagues examine a new biologic to augment repair of rotator cuff tears (see pages 212-216). Chitosan, a linear polysaccharide, has been shown to help with soft-tissue healing. Although in the past its use has been limited secondary to problems with the compound precipitating at physiologic pH, new formulations mitigate that problem. In the authors’ animal model of acute supraspinatus repair, the use of chitosan gel increased the number of fibroblasts and the amount of repair tissue when compared with untreated controls. Additionally, the experimental group showed a decreased inflammatory response when compared with the control group. This is very exciting research as the biologic enhancement of rotator cuff tendon healing could potentially help decrease the rate of rotator cuff repair failure.
Lenehan and colleagues analyze the long-term outcomes of anterior cruciate ligament reconstruction in a cohort of patients studied over an 8-year period (see pages 217-222). During this period, 99 patients were reconstructed with allograft tissue and 24 with autograft. Their analysis, like other recently published work, shows that the rates of revision were much higher for patients under 25 years of age who were reconstructed using allograft tissue. The rate of revision for NCAA (National Collegiate Athletic Association) Division I athletes reconstructed with allograft tissue was found to be 62%, while the revision rate for all patients under the age of 25 years who received an allograft was found to be 20.5%. Clearly, there is still a great deal to learn about the biology of graft incorporation and healing, especially as it relates to allograft tissue.
These 3 articles exemplify the breadth of orthopedic biologics and their potential role in orthopedic surgery. Through efforts of investigators highlighted in this journal and in others, biologics will become better understood and more widely used when appropriate, leading to improved patient outcomes.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
1. Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116-S120.
2. van der Bel R, Blokhuis TJ. Increased osteogenic capacity of Reamer/Irrigator/Aspirator derived mesenchymal stem cells. Injury. 2014;45(12):2060-2064.
Successful Surgical Treatment of an Intraneural Ganglion of the Common Peroneal Nerve
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Failure of Artelon Interposition Arthroplasty After Partial Trapeziectomy: A Case Report With Histologic and Immunohistochemical Analysis
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Massive Baker Cyst Resulting in Tibial Nerve Compression Neuropathy Secondary to Polyethylene Wear Disease
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Comparison of Locked Plate Fixation and Nonoperative Management for Displaced Proximal Humerus Fractures in Elderly Patients
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
Proximal humerus fractures are increasingly common in the elderly population,1 accounting for 10% of all these patients’ fractures.2 The injuries result in substantial morbidity and are associated with significantly higher mortality rates for up to 4 years.3 With the recent advent of anatomical locking plates,4,5 operative fixation of proximal humerus fractures in elderly patients has become more common.6 Although early clinical studies reported favorable outcomes, high complication rates have also been documented.7-22
Investigators have recently compared outcomes of locked plate fixation and nonoperative treatment of proximal humerus fractures in elderly patients.23-26 Fjalestad and colleagues23 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3- and 4-part fractures in 50 patients age 60 years or older and found no significant differences in Constant score or patient self-assessment at 1 year. Similarly, Olerud and colleagues25 conducted a randomized clinical trial of locked plating versus nonoperative treatment of 3-part fractures in 60 patients age 55 years or older. Although outcomes were better in the operative group, differences did not reach statistical significance, and the operative group’s reoperation rate was 30%.
Given this lack of conclusive outcomes data, optimal treatment of displaced proximal humerus fractures in elderly patients remains unknown. We conducted a study to compare outcomes of operative (locked plate fixation) and nonoperative management of displaced proximal humerus fractures in patients older than 60 years. Our hypothesis was that the clinical outcomes of these 2 treatment methods would be similar.
Materials and Methods
Selection Criteria
Our research protocol was approved by the Partners Human Research Committee. To determine the operative cohort, we queried our trauma database to identify all patients over age 60 years who sustained a displaced proximal humerus fracture between 2006 and 2009 and underwent surgical fixation. Cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if the injury radiographs were absent or inadequate; or if a fixation method other than locked plating was used. Applying these inclusion and exclusion criteria yielded 61 patients over age 60 years who underwent locked plating of a displaced proximal humerus fracture between 2006 and 2009.
The comparison group consisted of all patients who presented to our institutions with a displaced proximal humerus fracture during the same time period but instead had nonoperative treatment. To identify this group, we performed another database search for all patients over age 60 years who sustained a proximal humerus fracture between 2006 and 2009 (n = 452). Twenty-two patients were excluded for inadequate radiographs. To determine which of the other 430 patients had displaced fractures, Dr. Okike and Dr. Lee (orthopedic surgeons) reviewed injury radiographs and any computed tomography scans in duplicate and resolved discrepancies by consensus. Neer’s criteria were used to define displacement: Fractures displaced 1 cm or more and/or with angulation of 45° or more were displaced, and fractures not meeting these criteria were nondisplaced. In the assessment of displacement, interobserver reliability was substantial (overall agreement, 87.0% [374/430]; κ = 0.68). With use of these methods, 311 fractures were classified displaced and 119 nondisplaced. As with the operative group, cases were excluded if they presented more than 4 weeks after injury; if they represented a refracture, nonunion, or pathologic fracture; if the fracture was an isolated greater or lesser tuberosity fracture; if there was an associated neurovascular injury; if injury radiographs were absent or inadequate; or if the treatment method was operative or unknown. Applying these inclusion and exclusion criteria yielded 146 patients over age 60 years who had nonoperative treatment of a displaced proximal humerus fracture between 2006 and 2009.
Patient Characteristics
Dr. Makanji retrospectively reviewed the charts of all 207 patients (61 operative, 146 nonoperative). Information was recorded on patient age and sex, mechanism of injury, number of days between injury and presentation, any associated orthopedic injuries, side of injury, and treatment facility (trauma center A, trauma center B). In addition, a Charlson score was assigned to each patient on the basis of medical comorbidities.27
Radiographs and any computed tomography scans were also assessed by Dr. Okike and Dr. Lee. Each fracture was assigned a Neer classification (2-part, 3-part, 4-part) and an AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification (A, B, C).28 Displacement was categorized as varus angulation (neck–shaft angle, <130°), valgus angulation (neck–shaft angle, >140°), neutral angulation (neck–shaft angle, 135° ± 5°), or translation alone. In addition, all fractures were assessed for dislocation and medial comminution.29
Outcome Measures
All follow-up radiographs were reviewed to assess for nonunion (defined as lack of healing by 12 months), malunion, and humeral head avascular necrosis. Operative patients’ follow-up radiographs were reviewed to determine frequency of screw perforation and/or loss of fixation, and their medical records were reviewed to assess for other complications, including infection, neurovascular injury, and return to operating room for any other reason. Nonoperative patients’ medical records were reviewed to determine if surgical treatment was subsequently required.
To determine clinical outcomes, we asked patients to return for clinical evaluation, which included use of several questionnaires: Constant; DASH (Disabilities of the Arm, Shoulder, and Hand); SMFA (Short Musculoskeletal Functional Assessment); and Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Computer Adaptive Test.
Statistical Analysis
Chi-square test was used to compare the characteristics of patients who returned for clinical evaluation, Fisher exact test was used for tables with multiple cells less than 5, Student t test was used to compare clinical outcomes between operative and nonoperative groups. P < .05 was considered statistically significant, and all tests were 2-sided. Statistical analysis was performed using SAS Version 9 (SAS, Cary, North Carolina).
Results
Of the 207 patients who met the inclusion and exclusion criteria, 61 were treated operatively (locked plate open reduction and internal fixation) and 146 nonoperatively. Mean age was 76.9 years. One hundred fifty-five (74.9%) of the patients were female. Medical comorbidities were common (average Charlson score, 6.6). Most patients (185/207; 89.4%) were injured in a fall. There were 129 two-part fractures, 63 three-part fractures, and 9 four-part fractures (Table 1).
Operative patients’ complications included screw perforation (35.6%; 21 of the 59 cases with radiographs) and loss of fixation (17.5%; 10/57). Four (6.6%) of the 61 operative patients developed an infection. In sum, 8 (13.1%) of operative patients required another surgery (Table 2).
Among nonoperative patients, malunion at time of healing was common (86.9%; 113 of the 130 cases with radiographs). Eighty-six malunions (66.2% of the 130 cases) healed in varus, 25 (19.2%) in valgus, and 2 (1.5%) with translation alone. Uncommon among nonoperative patients were nonunion (1.4%; 2/143) and avascular necrosis (2.2%; 3/136). Two (1.4%) of the 146 nonoperative patients subsequently underwent surgery for malunion (Table 2).
Forty-seven patients accepted our invitation to return for clinical evaluation. Mean follow-up was 3.3 years (range, 1.4-6.4 years). Of these patients, 25 had been treated operatively (Figures 1A, 1B) and 22 nonoperatively (Figures 2A, 2B). Complication rates for patients who returned for clinical evaluation were similar to those for the entire cohort, with the exception of secondary surgical procedures (Table 3). There were no significant differences between operative and nonoperative patients in the group that returned for clinical evaluation (Table 4).
Regarding clinical outcome scores, there were no significant differences between operative and nonoperative patients (Table 5). In particular, there were no differences in SMFA Functional index (18.4 vs 19.7; P = .78), SMFA Bothersome index (20.8 vs 23.6; P = .61), DASH scores (26.5 vs 25.1; P = .79), Constant scores (58.0 vs 59.7; P = .74), or PROMIS Physical Function Computer Adaptive Test scores (43.9 vs 45.0; P = .70).
Discussion
In this observational study of displaced proximal humerus fractures in an elderly population, operative treatment (vs nonoperative treatment) had a lower malunion rate but was associated with more complications, including screw perforation, loss of fixation, and unplanned return to the operating room. Among patients who returned for clinical evaluation at a mean follow-up of 3.3 years, there were no significant operative–nonoperative differences.
Our results are similar to those recently reported by other investigators. In Norway, Fjalestad and colleagues23 conducted a randomized controlled trial of locked plating versus nonoperative treatment in 50 patients over age 60 years with a 3- or 4-part proximal humerus fracture. At 12 months, there was no significant difference between the operative and nonoperative groups’ Constant scores.
Similarly, Olerud and colleagues25 in Sweden conducted a trial in which 60 patients over age 55 years with a 3-part fracture of the proximal humerus were randomized to locked plating or nonoperative treatment. At 2 years, there were no significant operative–nonoperative differences on several outcome measures: Constant scores, DASH scores, EQ-5D (EuroQol) scores. Thirty percent of operative patients required a secondary procedure to treat infection, nonunion, avascular necrosis, screw perforation, stiffness, or impingement.
Our study benefited from having a large sample size (207) of consecutive patients with displaced proximal humerus fractures, but it also had its limitations. In this retrospective study, treatment assignment was not randomized. We were also limited by the large number of patients who did not return for clinical evaluation (160/207; 77.3%), including 52 (25.1%) found to be deceased, 27 (13.0%) who could not be reached, and 81 (39.1%) who declined our request (in many cases because of difficulties traveling to the trauma center). These challenges are inherent to research in the elderly population. As a result, the number of patients who returned for clinical evaluation (47/207; 22.7%) was lower than expected, which may have underpowered the study. In addition, treatment protocols were not standardized; patients were managed by a number of different surgeons. On the other hand, this wide variety of surgeons, including orthopedic trauma and upper extremity specialists, may increase the generalizability of our results.
Conclusion
Although use of locked plate fixation in treating proximal humerus fractures in elderly patients has increased markedly over recent years, definitive evidence supporting such management is lacking. In the present study, the outcomes of locked plate fixation were similar to those of nonoperative treatment. In addition, rates of complications and secondary surgical procedures were higher for operative patients than for nonoperative patients. Research is needed to identify the circumstances under which locked plating improves treatment outcomes for displaced proximal humerus fractures in elderly patients.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
1. Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop. 2006;(442):87-92.
2. Baron JA, Karagas M, Barrett J, et al. Basic epidemiology of fractures of the upper and lower limb among Americans over 65 years of age. Epidemiology. 1996;7(6):612-618.
3. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
4. Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2008;16(5):294-302.
5. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12-26.
6. Bell JE, Leung BC, Spratt KF, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. J Bone Joint Surg Am. 2011;93(2):121-131.
7. Agudelo J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in proximal humerus fractures treated with locking plates. J Orthop Trauma. 2007;21(10):676-681.
8. Bigorre N, Talha A, Cronier P, Hubert L, Toulemonde JL, Massin P. A prospective study of a new locking plate for proximal humeral fracture. Injury. 2009;40(2):192-196.
9. Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75(6):741-745.
10. Brunner F, Sommer C, Bahrs C, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis. J Orthop Trauma. 2009;23(3):163-172.
11. Charalambous CP, Siddique I, Valluripalli K, et al. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127(3):205-210.
12. Egol KA, Ong CC, Walsh M, Jazrawi LM, Tejwani NC, Zuckerman JD. Early complications in proximal humerus fractures (OTA types 11) treated with locked plates. J Orthop Trauma. 2008;22(3):159-164.
13. Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop. 2005;(430):176-181.
14. Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C. Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg. 2009;129(9):1251-1259.
15. Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop. 2006;(442):115-120.
16. Moonot P, Ashwood N, Hamlet M. Early results for treatment of three- and four-part fractures of the proximal humerus using the PHILOS plate system. J Bone Joint Surg Br. 2007;89(9):1206-1209.
17. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures. J Bone Joint Surg Am. 2008;90(2):233-240.
18. Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16(2):202-207.
19. Shahid R, Mushtaq A, Northover J, Maqsood M. Outcome of proximal humerus fractures treated by PHILOS plate internal fixation. Experience of a district general hospital. Acta Orthop Belg. 2008;74(5):602-608.
20. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24.
21. Sudkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study. J Bone Joint Surg Am. 2009;91(6):1320-1328.
22. Thalhammer G, Platzer P, Oberleitner G, Fialka C, Greitbauer M, Vecsei V. Angular stable fixation of proximal humeral fractures. J Trauma. 2009;66(1):204-210.
23. Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. J Orthop Trauma. 2012;26(2):98-106.
24. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(7):1025-1033.
25. Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg. 2011;20(5):747-755.
26. Sanders RJ, Thissen LG, Teepen JC, van Kampen A, Jaarsma RL. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg. 2011;20(7):1118-1124
27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
28. Muller ME, Nazarus C, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
29. Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21(3):185-191.
Lumbar Degenerative Disc Disease and Tibiotalar Joint Arthritis: A 710-Specimen Postmortem Study
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
Osteoarthritis is the most common joint disorder, resulting in significant morbidity and disability. The worldwide prevalence of osteoarthritis was estimated at more than 151 million people, according to data published in 2004.1 In the United States, almost 27 million adults age 25 years and older suffer from clinically apparent disease.2 The spine is one of the most commonly affected joints of arthritis, and idiopathic low back pain is the most frequent complaint in the adult population.3 In adults with low back pain, evidence of lumbar intervertebral disc degeneration is often found on radiography.4 In 1 study, evidence of disc degeneration was found in 90% of adults age 50 to 59 years.5
Degenerative spinal disease most commonly affects the lumbar spine due to its high degree of mobility and weight-loading.6,7 Clinical8,9 and experimental studies10 have suggested that the degenerative changes in the lumbar spine begin in the intervertebral discs. Degenerative disc disease (DDD) results from a continuum of dehydration, degradation, and remodeling of the intervertebral discs and neighboring vertebrae to accommodate the changes in physical loading.11-13 This results in disc-space narrowing, disc bulging and herniation, vertebral rim osteophyte formation, and endplate sclerosis.7,14 Symptomatic neural compression may occur, often manifested by localized lower back and extremity pain, as well as sensory loss and weakness of the lower extremities.15-17 Changes in posture and gait may result because of altered sensation, and the consequent abnormal force transmission may predispose joints to accelerated wear and arthrosis.15,18
Numerous studies have delineated the association between lumbar spinal disorders and lower extremity arthrosis. Of note, research has demonstrated that hip and/or knee pathology and gait alteration may promote low back pain and lumbar disc degeneration.19-21 Although spinal abnormalities, such as scoliosis, may predispose an individual to accelerated hip degeneration,20 no studies have investigated the relationship between lumbar DDD and ankle osteoarthritis.
Ankle arthritis differs from hip and knee arthritis demographically, occurring approximately 9 times less frequently.21 The ankle joint is subjected to more weight-bearing force per square centimeter and is more commonly injured than any other joint in the body.21 Trauma and/or abnormal ankle mechanics are the most common causes of degenerative ankle arthritis.22 Other potential causes include inflammatory arthropathies, neuropathic arthropathy, infection, and tumor. The purpose of this study was to determine if a relationship exists between ankle arthrosis and lumbar disc degeneration, and to delineate if one may promote the onset or progression of the other.
Materials and Methods
We randomly chose 710 cadaveric specimens from the Hamann-Todd Osteological Collection in Cleveland, Ohio. The Hamann-Todd Collection contains skeletal remains from more than 3000 individuals who died in Cleveland, Ohio between 1893 and 1938. The cohort for this study included 583 male and 127 female cadavers, ranging in age from 17 to 105 years at the time of death. Table 1 shows the breakdown of these specimens according to age group; of the 710 specimens, 306 were of African American ancestry, and 404 were Caucasian.
Lumbar DDD was graded at each lumbar spinal level by a single examiner using the Eubanks modification23 of the Kettler and Wilke classification of vertebral endplate osteophytosis24:
Grade 0: normal vertebral endplates;
Grade 1: mild arthrosis, with evidence of osteophytic reaction involving up to 50% of the vertebral endplates;
Grade 2: moderate arthrosis, with evidence of osteophytic reaction involving 50% to 100% of the vertebral endplates;
Grade 3: severe arthrosis, with evidence of osteophytic reaction involving 100% of the vertebral endplates. Osteophytes are hypertrophic and bridging the joint space (Figure 1);
Grade 4: complete ankylosis.
Tibiotalar joint osteoarthritis was evaluated by a single examiner using a modification of the Kellgren-Lawrence classification4 for knee osteoarthritis:
Grade 0: no discernable wear/osteophytes;
Grade 1: 1-mm osteophyte(s) and/or <25% surface wear;
Grade 2: 1- to 2-mm osteophyte(s) and/or 25% to 50% joint surface;
Grade 3: 2- to 3-mm osteophyte(s) and/or >50% joint surface (Figure 2);
Grade 4: multiple large osteophytes and/or definite bony end deformity.
Statistical analysis was performed on the compiled data using Stata software (StataCorp, College Station, Texas). Linear and logistic regression analyses correcting for confounding factors of age, sex, race, and height were performed using a standard P-value cutoff (P < .05) and 95% confidence interval to determine statistical significance.
Results
Patients were considered to have osteoarthritis of the tibiotalar joint if either of the extremities measured grade 1 or higher. Of the 710 specimens selected, 14 specimens did not have adequate bone available for bilateral tibiotalar joint measurement, either from extensive bone degradation or amputation. Of the remaining 696 specimens, 586 had some degree of tibiotalar osteoarthritis present (Table 2). Regression analysis showed a significant positive association between right- and left-ankle osteoarthritis (coefficient: 0.491, P < .01). Tibiotalar joint arthritis was classified as severe if either extremity had arthrosis of grade 3 or higher. Of the 586 specimens that had tibiotalar joint arthritis, only 16% (97 specimens) had severe tibiotalar joint arthritis.
Data regarding lumbar disc degeneration were available for 516 of the 710 specimens selected, 443 of which showed some disc degeneration. Disc degeneration was most prevalent and significant at the L4-L5 and L3-L4 intervertebral levels (Figures 3, 4). Of these 516 specimens, 30 had degeneration at 1 level, 47 specimens had degeneration at 2 levels, 29 specimens had degeneration at 3 levels, 52 had degeneration at 4 levels, and 285 specimens had degeneration at all 5 lumbar levels. The majority of specimens were found to have some degree of degeneration at all 5 lumbar spinal levels (Figure 5). Severe lumbar DDD was defined as grade 3 or higher osteoarthritis present in at least 1 of the 5 lumbar levels. Of the 516 specimens that showed some degree of disc degeneration, 152 were classified as severe. When stratified by number of spinal levels, only 30% of specimens were found to have evidence of severe arthrosis, the majority of which was located at only 1 lumbar segment (Figure 6).
Linear regression analysis of the data showed a statistically significant positive association between lumbar disc degeneration and tibiotalar osteoarthritis (coefficient: 0.844, P < .01), even when correcting for confounding factors, such as age, sex, and race (coefficient: 0.331, P < .01).
Additional analysis of the data demonstrated that tibiotalar joint arthritis remained significantly associated with lumbar DDD across each lumbar level: L1-L2 (coefficient: 0.269, P < .01), L2-L3 (coefficient: 0.283, P < .01), L3-L4 (coefficient: 0.299, P < .01), L4-L5 (coefficient: 0.240, P < .02), L5-S1 (coefficient: 0.167, P < .05).
The presence of 3 or more levels of lumbar DDD significantly increased the possibility of developing severe tibiotalar joint arthritis. Lumbar DDD that encompassed 3 levels showed the highest odds for development of severe tibiotalar joint arthritis with an odds ratio (OR) of 20.542 (Table 3).
When subjects were compared by decade, the mean grade of tibiotalar joint arthritis was significantly higher than lumbar DDD in specimens who died in their 20s and 30s. This difference was insignificant in the fourth decade, and thereafter the mean value of lumbar DDD surpassed that of tibiotalar joint arthritis (Figure 7).
In contrast, severe lumbar DDD was more prevalent than severe tibiotalar joint arthritis in individuals age 20 years or older (Figure 8). There were no specimens under age 20 years with severe lumbar DDD or severe tibiotalar joint arthritis.
Logistic regression showed that individuals with severe lumbar disc degeneration had significantly higher odds of developing severe ankle arthritis (OR: 1.93, P < .05). Similarly, individuals with severe tibiotalar joint arthritis were just as likely to develop severe lumbar DDD with an OR of 1.97 (P < .05).
Discussion
Multiple joint involvement in osteoarthritis is well established with a wide range of evidence linking lower extremity joint pathology and lumbar spinal disease. In 1983, Offierski and MacNab20 were the first to describe hip-spine syndrome. In the next year, a study by Sponseller and colleagues25 of pediatric patients after hip arthrodesis further substantiated the association between spine and extremity disease, and demonstrated a continued cause and effect relationship after surgery.
Lumbar spinal degeneration has also been correlated with knee osteoarthritis. Tsuji and colleagues26 reported that degenerative changes in spinal alignment result in increased thigh muscle tension and knee flexion. Furthermore, in their radiographic analysis of 682 individuals, Horvath and colleagues27 also showed that individuals with spinal degeneration had a higher prevalence of knee and hip osteoarthritis.
One might hypothesize from this evidence that lumbar spinal degeneration and ankle arthritis would also be interrelated, given their interconnected role in lower extremity force transmission. Surprisingly, the literature correlating lumbar degeneration and lower extremity osteoarthritis has overlooked this association and has focused solely on the hip and knee. To our knowledge, this study is the first to identify a statistically significant association between tibiotalar joint osteoarthritis and lumbar disc degeneration.
The literature supported analysis of our data. Miller and colleagues28 evaluated disc degeneration in 600 autopsy specimens using the Nachemson29 grading system. This system categorizes disc degeneration into 4 grades based on macroscopic appearance. Miller and colleagues28 reported evidence of degenerative changes as early as the second decade of life, primarily involving the L3–L4 and L4–L5 levels. Of note, the Nachemson29 classification system includes only evidence of marginal osteophytes in grade 4 disease, which was not identified by Miller and colleagues28 until the fourth decade. These results were similar to those in our study, in which the L3-L4 and L4-L5 intervertebral levels were most commonly affected. However, in our study, significant degenerative changes were found in the third decade of life.
In addition, the percentage of specimens with severe disc degeneration increased with each decade (Figure 8). A substantial amount of histologic evidence demonstrates the progression of disc degeneration with age. With increased age, there is a gradual decrease in the osmotic swelling of intervertebral discs30 and a 2-fold decrease in disc hydration between adolescence and the eighth decade.31 Furthermore, the nucleus pulposus undergoes progressive fibrosis,32,33 with a 5-fold decrease in the fixed-charge density of nucleus glycosaminoglycans,34 and a 2-fold increase in intervertebral disc creep while under compression after age 30 years.35
While analyzing our findings, we had difficulty in determining which pathologic condition debuts and, subsequently, affects the other. According to our results, the mean grade of tibiotalar joint arthritis was higher than that of DDD in specimens through the third and fourth decades of life (Figure 7). After the age of 50 years, the mean grade of DDD surpasses that of tibiotalar arthritis. This may be initially interpreted that development of tibiotalar joint arthritis precedes lumbar disc degeneration. Ankle osteoarthritis is relatively rare, and given that the vast majority of ankle osteoarthritis is secondary to trauma,22 we would expect to see a higher incidence of ankle osteoarthritis in a younger, more active cohort. In addition, given our finding that ankle arthritis is related to lumbar disc degeneration, one could speculate that tibiotalar arthritis at a young age predisposes an individual to developing lumbar degeneration later in life.
However, this conclusion is inherently flawed; closer examination of the data revealed that the mean grade of tibiotalar arthritis and DDD in the third and fourth decades is relatively low, between grade 0 and grade 1 (Figure 7). Therefore, it is difficult to arrive at a conclusion when comparing such small values. Second, we must remember that we are comparing an average value of disc degeneration across all lumbar levels. When a specimen has only 1 disc that is severely degenerated, this value is averaged across all 5 lumbar levels and, thus, the overall mean grade of arthrosis is significantly diminished.
In fact, data from previous studies concur with the second argument. Upper-level lumbar disc degeneration is relatively rare and the vast majority of patients with disc degeneration present with significant disease in only 1 or 2 discs.36,37 Analysis of the specimens in this study revealed bony evidence of disc degeneration present at all 5 lumbar levels in over half of the specimens examined (57%). However, the majority of specimens in this cohort exhibit only low-grade degeneration. When specimens were analyzed for severe arthrosis (grade 3 and higher), nearly half of the specimens were found to have severe disease involving only 1 intervertebral disc (Figure 6). Data from Miller and colleagues28 and the present study show that the upper lumbar levels were relatively spared; the L3-L4 and L4-L5 lumbar levels showed the highest prevalence and severity of degenerative change.
To address this issue, we evaluated the percentage of specimens per decade with severe arthrosis (grade 3 and higher) of at least 1 lumbar intervertebral disc and 1 tibiotalar joint. Severe lumbar disc degeneration was found to be more prevalent than severe ankle arthritis in individuals age 20 years or older (Figure 8). Therefore, we postulate that significant degenerative changes in the lumbar spine precede the development of severe ankle arthritis.
One can further speculate that sequelae from lumbar disc degeneration may lead to the development of tibiotalar arthritis, given our finding that severe lumbar degeneration predisposes an individual to the development of ankle arthritis. Because significant lumbar disc degeneration has long been known to result in both spinal nerve and cord compression, we hypothesize that this resultant neurocompression promotes altered gait and translation of atypical forces to the ankle and foot, thus predisposing to the onset and/or progression of osteoarthritis. In support of this hypothesis, Morag and colleagues15 demonstrated that neurologic compression produced an altered posture and gait because of lost motor function and afferent proprioceptive sensation. This form of neurologic compromise may exert atypical forces upon the foot and ankle, predisposing the joint to accelerated wear and primary arthrosis.
In addition, DDD involving 3 or more lumbar intervertebral levels was found to significantly increase the likelihood of the subject having severe tibiotalar joint arthritis. Provided that lumbar disc degeneration typically involves significant degeneration at 1 level, we assume that significant arthrosis at 3 or more levels correlates to an overall more severe DDD with a higher corresponding likelihood of neural compression. However, compression of peripheral lower extremity nerves has been shown to result in neuropathic arthropathy akin to the diabetic Charcot foot.38 This could be a possible mechanism of accelerated ankle arthritis, but this study did not examine soft-tissue disease nor take into account other medical comorbidities of each specimen, including genetic predispositions towards osteoarthritis.
It should be noted that the aforementioned causative relationship between lumbar disc degeneration and tibiotalar arthritis is speculative and cannot be demonstrated definitively by this investigation. We acknowledge limitations of this study and the need for further research of the possible causative mechanism(s) of accelerated ankle arthrosis secondary to lumbar spinal disease. Ideally, the questions posed by our report would be answered via a large prospective cohort study that utilized both serial imaging and autopsy analysis. Unfortunately, this form of study is logistically and financially difficult to perform.
This was a retrospective cadaveric study in which determination of arthrosis severity was based solely on bony evidence. Therefore, the role of soft-tissue disease in the pathogenesis of arthrosis of the lumbar spine and tibiotalar joint could not be assessed, nor could definitive associations to clinically symptomatic disease. We made the assumption that progression of bone degeneration in both the lumbar spine and tibiotalar joint corresponded equally to the associated soft-tissue changes. Given this assumption, we cannot definitively conclude that degeneration of the lumbar spine precedes that of the ankle, because the absence of magnetic resonance imaging or fresh autopsy specimens in our study misses the early degenerative changes in the discs that precede the bony alteration measured in our study. Furthermore, readers should note that since this study compared only bone morphology, no emphasis was placed on clinical manifestation of lumbar disc degeneration or tibiotalar joint arthritis. As mentioned earlier, radiologic evidence of disc degeneration was found in 90% of adults age 50 to 59 years, according to a study by Hult5; however, it is important to note that not all individuals studied were symptomatic clinically. Unfortunately, medical records were not available for the bony specimens, and clinical correlations could not be assessed during this investigation.
Furthermore, no special attention was given to other pathologic conditions observed during specimen measurement. The presence of diseases, such as osteoporosis, spondylolysis, or previous traumatic injury, may have had implications in the resultant joint degeneration. Finally, the evaluation of arthrosis was performed subjectively without measuring reliability. However, the present analysis includes a large sample, each joint type was reviewed by a single examiner, and used a classification system that was modeled on a validated grading system. Ideally, multiple individuals should have been used for each type of measurement, with subsequent analysis of intraobserver and interobserver reliability.
Conclusion
Based on our study of a large population of adult skeletal specimens, we ascertained that lumbar intervertebral disc degeneration and tibiotalar osteoarthritis are associated. The prevalence of severe lumbar disc degeneration was higher than that of tibiotalar joint arthritis in individuals age 20 years or older. This may suggest that gait changes from disc degeneration or neural compression in the lumbar spine may play a role in the development of ankle osteoarthritis. Additionally, subjects with severe disc degeneration were twice as likely to develop significant tibiotalar osteoarthritis. This must be considered in the differential when treating patients with degenerative changes of the lumbar spine and leg pain.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
1. Mathers C, Fat DM, Boerma JT, for the World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization, 2008.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Kelsey JL, Githens PB, White AA, et al. An epidemiological study of lifting and twisting on the job and risk for acute prolapsed lumbar intervertebral disc. J Orthop Res. 1984;2(1):61-66.
4. Kellgren JH, Lawrence JS. Osteoarthrosis and disc degeneration in an urban population. Ann Rheum Dis. 1958;17(4):388-397.
5. Hult L. Cervical, dorsal and lumbar spinal syndromes; a field investigation of a non-selected material of 1200 workers in different occupations with special reference to disc degeneration and so-called muscular rheumatism. Acta Orthop Scand Suppl. 1954;17:65-73.
6. Hirsch C. The reaction of intervertebral discs to compression forces. J Bone Joint Surg Am. 1955;37(6):1188-1196.
7. Videman T, Nurminen M, Troup JD. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation and physical loading. Spine. 1990;15(8):728-740.
8. Butler D, Trafimow JH, Andersson GB, McNeil TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111-113.
9. Fujiwara A, Tamai K, Yamato M, et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8(5):396-401.
10. Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24(1):12-21.
11. Eisenstein S, Roberts S. The physiology of the disc and its clinical relevance. J Bone Joint Surg Br. 2003;85(5):633-636.
12. Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94(10):1298-1304.
13. Inoue N, Espinoza Orías AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):487-499.
14. Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(suppl 2):3-9.
15. Morag E, Hurwitz DE, Andriacchi TP, Hickey M, Andersson GB. Abnormalities in muscle function during gait in relation to the level of lumbar disc herniation. Spine. 2000;25(7):829-833.
16. Oikawa Y, Ohtori S, Koshi T, et al. Lumbar disc degeneration induces persistent groin pain. Spine. 2012;37(2):114-118.
17. Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21(17):2046-2052.
18. Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: part A. Comparison with healthy subjects. Physiol Meas. 2009;30(11):1171-1186.
19. McGregor AH, Hukins DW. Lower limb involvement in spinal function and low back pain. J Back Musculoskelet Rehabil. 2009;22(4):219-222.
20. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321.
21. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85(5):923-936.
22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop. 2009;467(7):1800-1806.
23. Eubanks JD, Lee MJ, Cassinelli E, Ahn NU. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop. 2007;464:184-189.
24. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
25. Sponseller PD, McBeath AA, Perpich M. Hip arthrodesis in young patients. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(6):853-859.
26. Tsuji T, Matsuyama Y, Goto M, et al. Knee-spine syndrome: correlation between sacral inclination and patellofemoral joint pain. J Orthop Sci. 2002;7(5):519-523.
27. Horvath G, Koroknai G, Acs B, Than P, Illés T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34(8):1245-1249.
28. Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13(2):173-178.
29. Nachemson A. Lumbar intradiscal pressure: experimental studies on post-mortem material. Acta Orthop Scand Suppl. 1960;43:1-104.
30. Kraemer J. Pressure-dependent fluid shifts in the intervertebral disc. Orthop Clin North Am. 1977;8(1):211-216.
31. Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22(2):145-157.
32. Coventry MB, Ghromley RK, Kernohan JW. The intervertebral disc, its macroscopic anatomy and pathology: Part III. Pathologic changes in the intervertebral disc. J Bone Joint Surg Br. 1945;27:460-474.
33. Friberg S, Hirsch C. Anatomical and clinical changes in lumbar disc degeneration. Acta Orthop Scand. 1949;19(2):222-242.
34. Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673(4):443-453.
35. Koeller W, Muehlhaus S, Meier W, Hartmann F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression: influence of age and degeneration. J Biomech. 1986;19(10):807-816.
36. Bosacco SJ, Berman AT, Raisis LW, Zamarin RI. High lumbar herniations. Case reports. Orthopaedics. 1989;12(2):275-278.
37. Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
38. Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
A Systematic Review of Tibialis Anterior Tendon Rupture Treatments and Outcomes
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
Subcutaneous rupture of the tibialis anterior (TA) tendon has been reported predominantly in case reports and small case series because of the relative rarity of the injury. Unlike traumatic lacerations or open injuries to the tendon, subcutaneous injuries often go unnoticed by patients because of compensation by surrounding dorsiflexors of the foot and toes—namely, the extensor hallucis longus (EHL) and the extensor digitorum longus (EDL).1 This can delay presentation to an orthopedic surgeon and lead to difficulties in treatment, such as allograft or autograft being required if primary repair is no longer possible. Case reports and series have described treatment methods as well as anecdotal evidence of outcomes after operative repair or conservative treatment, but there have been no comprehensive systematic reviews of outcomes after various types of treatment. Authors have come to conclusions about expected outcomes based on patient age, time to treatment, treatment used, and other variables, but no reviews have examined these variables across multiple studies. Given the low level of the evidence presented in most of these reports, it is difficult to perform a meta-analysis of the data.
Instead, we systematically reviewed 87 cases from all pertinent studies and examined commonly reported data, such as patient age, time to treatment, treatment used, and outcome. Using the PICO (population, intervention, comparison, outcome) model for systematic reviews, we looked at patients who had closed, spontaneous, complete rupture of the TA tendon and underwent operative repair or conservative treatment of the injury. Outcomes surveyed included successful operative repair or conservative treatment, as measured by objective systems, such as MMSS (Manual Muscle Strength Scale) score, AOFAS (American Orthopaedic Foot and Ankle Society) hindfoot score, and FAOS (Foot and Ankle Outcome Score) testing, or by subjective description of posttreatment outcome.
We intend this review to serve as a guide for surgeons who find themselves treating a ruptured TA tendon, a relatively rare injury. They will be able to select the operative technique or conservative treatment that best matches the patient’s needs, based on comparison with previous case studies.
Materials and Methods
The cases reviewed for this study were found through a comprehensive PubMed search and an independent review of references cited in similar articles. Articles included were published between 1975 and 2012, inclusive. The latest search was performed on March 22, 2013. The search criteria were tibialis anterior [Title/Abstract] OR anterior tibial [Title/Abstract] AND rupture [Title/Abstract]) AND surgery. Only English-language articles, or articles already translated into English, were included. Eligible studies described cases of closed tendon rupture. No traumatic lacerations or open ruptures were included. If a study described both open and subcutaneous ruptures, only the subcutaneous cases were included. Further, partial ruptures were not included. In addition, ruptures caused directly by a known comorbid condition—for example, a rupture caused by a gouty tophaceous deposit at the site of rupture2—were not included. Data were extracted from publications independently and analyzed in a Microsoft Excel workbook (Microsoft, Redmond, Washington). Variables examined included patient age and sex, side involved, time to treatment, mechanism of injury, defect size, predisposing comorbidities, surgery or conservative treatment, type of operative repair (if applicable), graft used (if applicable), pretreatment function (by independent scoring system, if applicable), and posttreatment function. These variables were not necessarily reported in all the studies.
A potential bias exists in our PubMed search. As the query was specific for studies that included operative repair of a ruptured TA tendon, case studies that involved only conservative treatment were excluded. However, the primary goal of this review was to compare operative possibilities and the patient characteristics and outcomes associated with these surgeries.
Results
Figure 1 shows the criteria used to select eligible papers for review. Twenty-three papers matched the criteria.3-25 Data were independently extracted from these papers, as described in the Methods section. Again, not all variables were reported by all authors. Sammarco and colleagues21 reported time to treatment as a mean for 2 groups: 8 cases defined as “early” treatment (mean time to treatment, 0.625 months) and 11 defined as “late” treatment (mean time to treatment, 10.7 months). These mean times were therefore used independently for each case in calculating mean time to treatment for this systematic review.
Table 1 lists the demographics. There were 40 male and 25 female patients, and 22 cases in which sex was not specified. Mean age was 63.9 years (surgery group), 72.4 years (conservative treatment group), and 65.8 years (overall). Of the 87 patients, 72 underwent surgery, and 15 were treated with conservative measures.
Table 2 lists the operative techniques identified. Of the 72 surgeries, 23 were primary repairs, 12 were primary repairs of the anatomical insertion, and 18 involved use of autograft.
Time to treatment was available for 54 of the 87 cases (Table 3). Primary repair was most often performed in cases in which the injury was less than 3 months old, and autograft was most often used in cases in which the injury occurred more than 3 months before presentation.
Posttreatment outcome scores were available for 59 cases. Only 3 authors reported preoperative scores.5,21,24 None of the authors who used conservative treatment measures reported pretreatment scores. Scores used included the MMSS score (26 cases), the AOFAS hindfoot score (16 cases),26 the FAOS (17 cases),27 and the Tinetti gait and balance score (3 cases; the author also used the MMSS score).28Table 4 lists the mean posttreatment scores for patients who underwent surgery and patients treated conservatively. AOFAS, MMSS, and Tinetti scores and FAOS were used by authors presenting operative treatment outcomes. Only posttreatment FAOS was available for both surgery (84.4/100) and conservative treatment (69.4/100).
Discussion
Closed rupture of the TA tendon is a relatively rare entity occurring mostly in older patients without any history of acute, traumatic injury. Some patients, however, recall a particular moment of rupture, often accompanied immediately by pain and swelling, which eventually resolve. Later sequelae include footdrop with associated steppage gait and a palpable mass on the dorsal aspect of the ankle.3,21 Chronic TA tendon rupture can also lead to clawing of the toes as the other foot extensors (EHL, EDL) overcompensate. Cohen and Gordon1 described the case of a patient who ruptured a TA tendon 25 years earlier and then, in the absence of operative repair, developed hypertrophy of the EHL and the EDL. This extensor substitution led to hammer toes and plantar prominence of the metatarsal heads, ultimately leading to moderate pain and a neuroma. Although this particular outcome is likely rare, the more common sequelae of footdrop, flatfoot, Achilles tendon contracture, and compromised gait are reason enough to consider operative repair for any ruptured TA tendon.
Most previous studies of TA tendon rupture were case reports and case studies. In the largest series, Sammarco and colleagues21 described 19 cases of closed rupture. These included 3 traumatic cases, 1 by blunt trauma to the tendon and 2 of open laceration, all treated surgically with various methods. Unfortunately, these 3 traumatic cases were not separated in the authors’ analysis and therefore had to be included in this systematic review. Including them here did not compromise our goals in this review, which included examining typical patient demographics and the most common methods of operative repair.
Conservative measures remain a treatment possibility for some patients. We found that patients treated with conservative measures historically have been older (mean age, 72.4 years) than patients treated surgically (mean age, 63.9 years). However, advanced age itself is not a contraindication for operative repair of a TA tendon rupture, and authors have described positive outcomes for active, elderly (>70 years) patients who wanted to maintain their activity level and therefore opted for operative repair.7,8,10,13,16,24 Ouzounian and Anderson18 described functional limitations (eg, persistent footdrop, slapfoot gait, limitations in walking) after conservative treatment with an ankle-foot orthosis. Operative repair offers the chance for better functional outcome for patients who are surgical candidates and lead even a mildly active lifestyle.
Of operative repair methods, primary repair is used most often. This technique, however, must be allowed by the gap between the 2 ruptured ends after débridement of any necrotic tissue. If the distal stump is not viable, primary repair of the proximal stump to the native anatomical insertion is feasible. Figure 2, reprinted from a case report by Rajagopalan and colleagues,19 shows a ligament–osseous reattachment of the proximal stump using suture anchors to the medial cuneiform. Both primary repair and repair to the anatomical insertion can be augmented with Achilles tendon lengthening if needed to achieve balance between flexor and extensor functions of the ankle.
If the gap between the 2 stumps cannot be covered by the native tendon, then autograft, another surgical technique with positive outcomes, can be used. The most popular autograft sites historically have been the EDL, Achilles, and plantaris tendons. In addition, Goehring and Liakos9 described 3 cases of good results with semitendinosus autograft. Sapkas and colleagues22 used a free-sliding TA graft harvested from the healthy tissue of the proximal tendon stump. Their technique is depicted in Figure 3. Sliding tendon lengthening, well described by Trout and colleagues24 in a case study, is feasible for use of the native tendon when there is a gap to bridge between the 2 stumps of ruptured tendon. EHL or EDL transfer with or without Achilles lengthening is another option, albeit historically less often used.6,7 This technique is depicted in Figure 4, reprinted from a case series by Ellington and colleagues,7 who used EHL transfer with and without Achilles tendon lengthening in 9 cases.
Last, less popular techniques have included repair to sites other than the medial cuneiform, including the neck of the talus and the navicular bone.10,13 An Achilles tendon allograft was used in a case described by Aderinto and Gross3 to repair a ruptured tendon found incidentally on preoperative examination for a scheduled knee arthroplasty. The patient had a postoperative MMSS score of 4/5.
Overall, primary repair is clearly preferred, but successful outcomes can be achieved by other means. As Table 3 shows, primary repair is more often used for ruptures less than 3 months old, and autograft for older ruptures. Although which operative technique to use can be decided after necrotic tissue is débrided, surgeons should try to ascertain age of injury ahead of time so that, going into surgery, they will have a better idea of the feasibility of primary repair.
Posttreatment ankle scores were not widely available. As Table 4 indicates, only FAOS was used for the conservative treatment cases. However, raw mean FAOS and raw mean AOFAS hindfoot, MMSS, and Tinetti scores showed that good outcomes and high scores can be achieved with surgery. Further, the mean FAOS reported by Gwynne-Jones and colleagues10 and Markarian and colleagues13 showed a clinically significant difference between surgery and conservative treatment. DiDomenico and colleagues,5 Sammarco and colleagues,21 and Trout and colleagues24 were the only authors who reported pretreatment and posttreatment scores.
We intend this systematic review of the literature on closed TA rupture to serve as a guide for surgeons who find themselves treating this relatively rare injury, which often presents with only a chief complaint of the foot catching while walking. Overall, the literature shows that operative repair provides very good outcomes for many patients. Patients who are surgical candidates and amenable to surgery can be counseled that operative repair leads to fewer sequelae, such as persistent footdrop and flatfooted gait, with a strong likelihood of return to baseline activity status. Patients who are not surgical candidates or are strongly against surgery can be offered conservative treatment with an ankle-foot orthosis or physical therapy, but they should also be counseled that persistent gait abnormalities and weakness in dorsiflexion are likely outcomes. Surgeons must also consider age of injury (time from probable rupture to presentation), estimating a particular moment of rupture if unknown by the patient. They can then gauge the feasibility of primary repair and, during surgery, decide which technique (primary repair, tendon transfer, autograft, or other technique) will produce the best results. They can also use scores such as the FAOS and the AOFAS hindfoot, MMSS, and Tinetti scores to compare preoperative and postoperative function, though subjective reports of return to previous activity can also serve as markers of successful repair.
This review highlights the need for further study regarding the treatment of TA ruptures. Larger, randomized studies with validated scoring systems for preoperative and postoperative function would offer more insight onto the best treatment options for these complex injuries.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.
1. Cohen DA, Gordon DH. The long-term effects of an untreated tibialis anterior tendon rupture. J Am Podiatr Med Assoc. 1999;89(3):149-152.
2. Jerome JTJ, Varghese M, Sankaran B, Thomas S, Thirumagal SK. Tibialis anterior tendon rupture in gout—case report and literature review. Foot Ankle Surg. 2008;14(3):166-169.
3. Aderinto J, Gross A. Delayed repair of tibialis anterior tendon rupture with Achilles tendon allograft. J Foot Ankle Surg. 2011;50(3):340-342.
4. Constantinou M, Wilson A. Traumatic tear of tibialis anterior during a Gaelic football game: a case report. Br J Sports Med. 2004;38(6):e30.
5. DiDomenico LA, Williams K, Petrolla AF. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Foot Ankle Surg. 2008;47(5):463-467.
6. Dooley BJ, Kudelka P, Menelaus MB. Subcutaneous rupture of the tendon of tibialis anterior. J Bone Joint Surg Br. 1980;62(4):471-472.
7. Ellington JK, McCormick J, Marion C, et al. Surgical outcome following tibialis anterior tendon repair. Foot Ankle Int. 2010;31(5):412-417.
8. ElMaraghy A, Devereaux MW. Bone tunnel fixation for repair of tibialis anterior tendon rupture. Foot Ankle Surg. 2010;16(2):e47-e50.
9. Goehring M, Liakos P. Long-term outcomes following anterior tibialis tendon reconstruction with hamstring autograft in a series of 3 cases. J Foot Ankle Surg. 2009;48(2):196-202.
10. Gwynne-Jones D, Garneti N, Wyatt M. Closed tibialis anterior tendon rupture: a case series. Foot Ankle Int. 2009;30(8):758-762.
11. Kashyap S, Prince R. Spontaneous rupture of the tibialis anterior tendon. A case report. Clin Orthop. 1987;(216):159-161.
12. Kausch T, Rütt J. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch Orthop Trauma Surg. 1998;117(4-5):290-293.
13. Markarian GG, Kelikian AS, Brage M, Trainor T, Dias L. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle Int. 1998;19(12):792-802.
14. Meyn MA Jr. Closed rupture of the anterior tibial tendon. A case report and review of the literature. Clin Orthop. 1975;(113):154-157.
15. Miller RR, Mahan KT. Closed rupture of the anterior tibial tendon. A case report. J Am Podiatr Med Assoc. 1998;88(8):394-399.
16. Neumayer F, Djembi YR, Gerin A, Masquelet AC. Closed rupture of the tibialis anterior tendon: a report of 2 cases. J Foot Ankle Surg. 2009;48(4):457-461.
17. Otte S, Klinger HM, Lorenz F, Haerer T. Operative treatment in case of a closed rupture of the anterior tibial tendon. Arch Orthop Trauma Surg. 2002;122(3):188-190.
18. Ouzounian TJ, Anderson R. Anterior tibial tendon rupture. Foot Ankle Int. 1995;16(7):406-410.
19. Rajagopalan S, Sangar A, Upadhyay V, Lloyd J, Taylor H. Bilateral atraumatic sequential rupture of tibialis anterior tendons. Foot Ankle Spec. 2010;3(6):352-355.
20. Rimoldi RL, Oberlander MA, Waldrop JI, Hunter SC. Acute rupture of the tibialis anterior tendon: a case report. Foot Ankle. 1991;12(3):176-177.
21. Sammarco VJ, Sammarco GJ, Henning C, Chaim S. Surgical repair of acute and chronic tibialis anterior tendon ruptures. J Bone Joint Surg Am. 2009;91(2):325-332.
22. Sapkas GS, Tzoutzopoulos A, Tsoukas FC, Triantafillopoulos IK. Spontaneous tibialis anterior tendon rupture: delayed repair with free-sliding tibialis anterior tendon graft. Am J Orthop. 2008;37(12):E213-E216.
23. Stuart MJ. Traumatic disruption of the anterior tibial tendon while cross-country skiing. A case report. Clin Orthop. 1992;(281):193-194.
24. Trout BM, Hosey G, Wertheimer SJ. Rupture of the tibialis anterior tendon. J Foot Ankle Surg. 2000;39(1):54-58.
25. Van Acker G, Pingen F, Luitse J, Goslings C. Rupture of the tibialis anterior tendon. Acta Orthop Belg. 2006;72(1):105-107.
26. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353.
27. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794.
28. Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429-434.