User login
Debunking Psoriasis Myths: Does UVB Phototherapy Cause Skin Cancer?
Myth: UVB phototherapy causes skin cancer
Phototherapy is a common treatment modality for psoriasis patients that can be used in the physician’s office or psoriasis clinic or at home. Options include UVB phototherapy (broadband and narrowband), which slows the growth of affected skin cells; psoralen plus UVA (PUVA), which slows excessive skin cell growth; and excimer laser therapy, which targets select areas of the skin affected by mild to moderate psoriasis and is particularly useful for scalp psoriasis. Each of these therapies may be combined with other topical and/or systemic psoriasis treatments. The effects of UV light on the skin and the connection to skin cancer is widely known. Therefore, patient education on the risk for skin cancer with phototherapy is essential.
Evidence suggests that UVB phototherapy remains a safe treatment modality. In a 2005 analysis of prospective and retrospective studies on skin cancer risk from UVB phototherapy, 11 studies (10 concerning psoriasis patients) were reviewed and the researchers concluded that all studies eventually showed no increased skin cancer risk with UVB phototherapy. One of the PUVA cohort studies examined genital skin cancers and found an increased rate of genital tumors associated with UVB phototherapy.
Another analysis to define the long-term carcinogenic risk for narrowband UVB treatment found that there was no association between narrowband UVB exposure alone (without PUVA) and any skin cancer. For patients treated with narrowband UVB and PUVA, there was a small increase in basal cell carcinomas.
Dermatologists should monitor psoriasis patients for self-administered treatment with tanning beds. Based on a questionnaire sent to approximately 14,000 subscribers of National Psoriasis Foundation emails, 62% of 617 tanners started tanning to treat psoriasis; they were more likely to have received medical phototherapy and had more severe psoriasis. Approximately 30% of these patients indicated that they used tanning as a self-treatment for psoriasis because of the inconvenience and cost of UV light treatment in a physician’s office as well as treatment failure of other therapies prescribed by the physician. “Our results imply that tanning bed usage among psoriasis sufferers is widespread and linked with tanning addiction,” reported Felton et al. “Practitioners should be particularly vigilant to the possibility of tanning bed usage in at-risk patients.” These patients may be at increased risk for skin cancer. Problematic tanning behaviors may be seen in younger female patients diagnosed with psoriasis at an early age as well as patients with severe psoriasis who were previously prescribed phototherapy treatment.
Expert Commentary on next page
Expert Commentary
UVB phototherapy is an effective therapy that does not increase the risk of nonmelanoma skin cancers (NMSCs), according to the 2 analyses mentioned above. When I discuss the risks and benefits of UVB phototherapy with psoriasis patients, I do say that there is a theoretical increased risk for NMSC but that the 2005 study mentioned above does not indicate an increased risk. However, UVB phototherapy and cyclosporine should not be combined, as this combination does increase the risk for NMSC.
Psoralen plus UVA definitely will increase the risk for NMSC, particularly squamous cell carcinoma. However, in this age of the biologics, PUVA use has fallen out of favor, partly due to the increased risk for NMSC, and many patients will not encounter dermatology practices that still use PUVA.
—Jashin J. Wu, MD (Los Angeles, California)
Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159:931-935.
Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355-360.
Phototherapy. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/treatments/phototherapy . Accessed October 4, 2016.
Myth: UVB phototherapy causes skin cancer
Phototherapy is a common treatment modality for psoriasis patients that can be used in the physician’s office or psoriasis clinic or at home. Options include UVB phototherapy (broadband and narrowband), which slows the growth of affected skin cells; psoralen plus UVA (PUVA), which slows excessive skin cell growth; and excimer laser therapy, which targets select areas of the skin affected by mild to moderate psoriasis and is particularly useful for scalp psoriasis. Each of these therapies may be combined with other topical and/or systemic psoriasis treatments. The effects of UV light on the skin and the connection to skin cancer is widely known. Therefore, patient education on the risk for skin cancer with phototherapy is essential.
Evidence suggests that UVB phototherapy remains a safe treatment modality. In a 2005 analysis of prospective and retrospective studies on skin cancer risk from UVB phototherapy, 11 studies (10 concerning psoriasis patients) were reviewed and the researchers concluded that all studies eventually showed no increased skin cancer risk with UVB phototherapy. One of the PUVA cohort studies examined genital skin cancers and found an increased rate of genital tumors associated with UVB phototherapy.
Another analysis to define the long-term carcinogenic risk for narrowband UVB treatment found that there was no association between narrowband UVB exposure alone (without PUVA) and any skin cancer. For patients treated with narrowband UVB and PUVA, there was a small increase in basal cell carcinomas.
Dermatologists should monitor psoriasis patients for self-administered treatment with tanning beds. Based on a questionnaire sent to approximately 14,000 subscribers of National Psoriasis Foundation emails, 62% of 617 tanners started tanning to treat psoriasis; they were more likely to have received medical phototherapy and had more severe psoriasis. Approximately 30% of these patients indicated that they used tanning as a self-treatment for psoriasis because of the inconvenience and cost of UV light treatment in a physician’s office as well as treatment failure of other therapies prescribed by the physician. “Our results imply that tanning bed usage among psoriasis sufferers is widespread and linked with tanning addiction,” reported Felton et al. “Practitioners should be particularly vigilant to the possibility of tanning bed usage in at-risk patients.” These patients may be at increased risk for skin cancer. Problematic tanning behaviors may be seen in younger female patients diagnosed with psoriasis at an early age as well as patients with severe psoriasis who were previously prescribed phototherapy treatment.
Expert Commentary on next page
Expert Commentary
UVB phototherapy is an effective therapy that does not increase the risk of nonmelanoma skin cancers (NMSCs), according to the 2 analyses mentioned above. When I discuss the risks and benefits of UVB phototherapy with psoriasis patients, I do say that there is a theoretical increased risk for NMSC but that the 2005 study mentioned above does not indicate an increased risk. However, UVB phototherapy and cyclosporine should not be combined, as this combination does increase the risk for NMSC.
Psoralen plus UVA definitely will increase the risk for NMSC, particularly squamous cell carcinoma. However, in this age of the biologics, PUVA use has fallen out of favor, partly due to the increased risk for NMSC, and many patients will not encounter dermatology practices that still use PUVA.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: UVB phototherapy causes skin cancer
Phototherapy is a common treatment modality for psoriasis patients that can be used in the physician’s office or psoriasis clinic or at home. Options include UVB phototherapy (broadband and narrowband), which slows the growth of affected skin cells; psoralen plus UVA (PUVA), which slows excessive skin cell growth; and excimer laser therapy, which targets select areas of the skin affected by mild to moderate psoriasis and is particularly useful for scalp psoriasis. Each of these therapies may be combined with other topical and/or systemic psoriasis treatments. The effects of UV light on the skin and the connection to skin cancer is widely known. Therefore, patient education on the risk for skin cancer with phototherapy is essential.
Evidence suggests that UVB phototherapy remains a safe treatment modality. In a 2005 analysis of prospective and retrospective studies on skin cancer risk from UVB phototherapy, 11 studies (10 concerning psoriasis patients) were reviewed and the researchers concluded that all studies eventually showed no increased skin cancer risk with UVB phototherapy. One of the PUVA cohort studies examined genital skin cancers and found an increased rate of genital tumors associated with UVB phototherapy.
Another analysis to define the long-term carcinogenic risk for narrowband UVB treatment found that there was no association between narrowband UVB exposure alone (without PUVA) and any skin cancer. For patients treated with narrowband UVB and PUVA, there was a small increase in basal cell carcinomas.
Dermatologists should monitor psoriasis patients for self-administered treatment with tanning beds. Based on a questionnaire sent to approximately 14,000 subscribers of National Psoriasis Foundation emails, 62% of 617 tanners started tanning to treat psoriasis; they were more likely to have received medical phototherapy and had more severe psoriasis. Approximately 30% of these patients indicated that they used tanning as a self-treatment for psoriasis because of the inconvenience and cost of UV light treatment in a physician’s office as well as treatment failure of other therapies prescribed by the physician. “Our results imply that tanning bed usage among psoriasis sufferers is widespread and linked with tanning addiction,” reported Felton et al. “Practitioners should be particularly vigilant to the possibility of tanning bed usage in at-risk patients.” These patients may be at increased risk for skin cancer. Problematic tanning behaviors may be seen in younger female patients diagnosed with psoriasis at an early age as well as patients with severe psoriasis who were previously prescribed phototherapy treatment.
Expert Commentary on next page
Expert Commentary
UVB phototherapy is an effective therapy that does not increase the risk of nonmelanoma skin cancers (NMSCs), according to the 2 analyses mentioned above. When I discuss the risks and benefits of UVB phototherapy with psoriasis patients, I do say that there is a theoretical increased risk for NMSC but that the 2005 study mentioned above does not indicate an increased risk. However, UVB phototherapy and cyclosporine should not be combined, as this combination does increase the risk for NMSC.
Psoralen plus UVA definitely will increase the risk for NMSC, particularly squamous cell carcinoma. However, in this age of the biologics, PUVA use has fallen out of favor, partly due to the increased risk for NMSC, and many patients will not encounter dermatology practices that still use PUVA.
—Jashin J. Wu, MD (Los Angeles, California)
Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159:931-935.
Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355-360.
Phototherapy. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/treatments/phototherapy . Accessed October 4, 2016.
Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
Hearn RM, Kerr AC, Rahim KF, et al. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol. 2008;159:931-935.
Lee E, Koo J, Berger T. UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol. 2005;44:355-360.
Phototherapy. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/treatments/phototherapy . Accessed October 4, 2016.
Presenting Treatment Safety Data: Subjective Interpretations of Objective Information
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
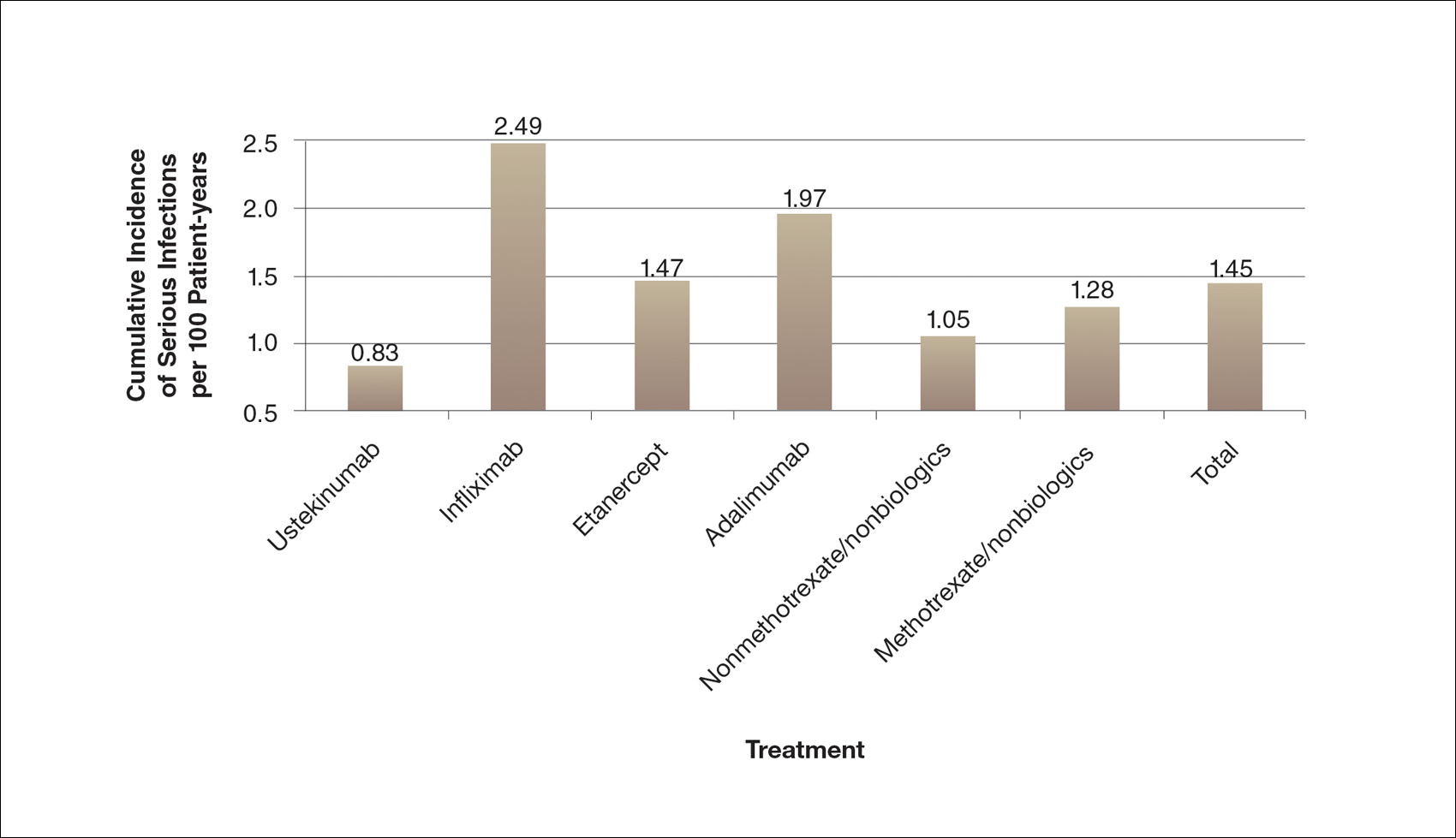
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.
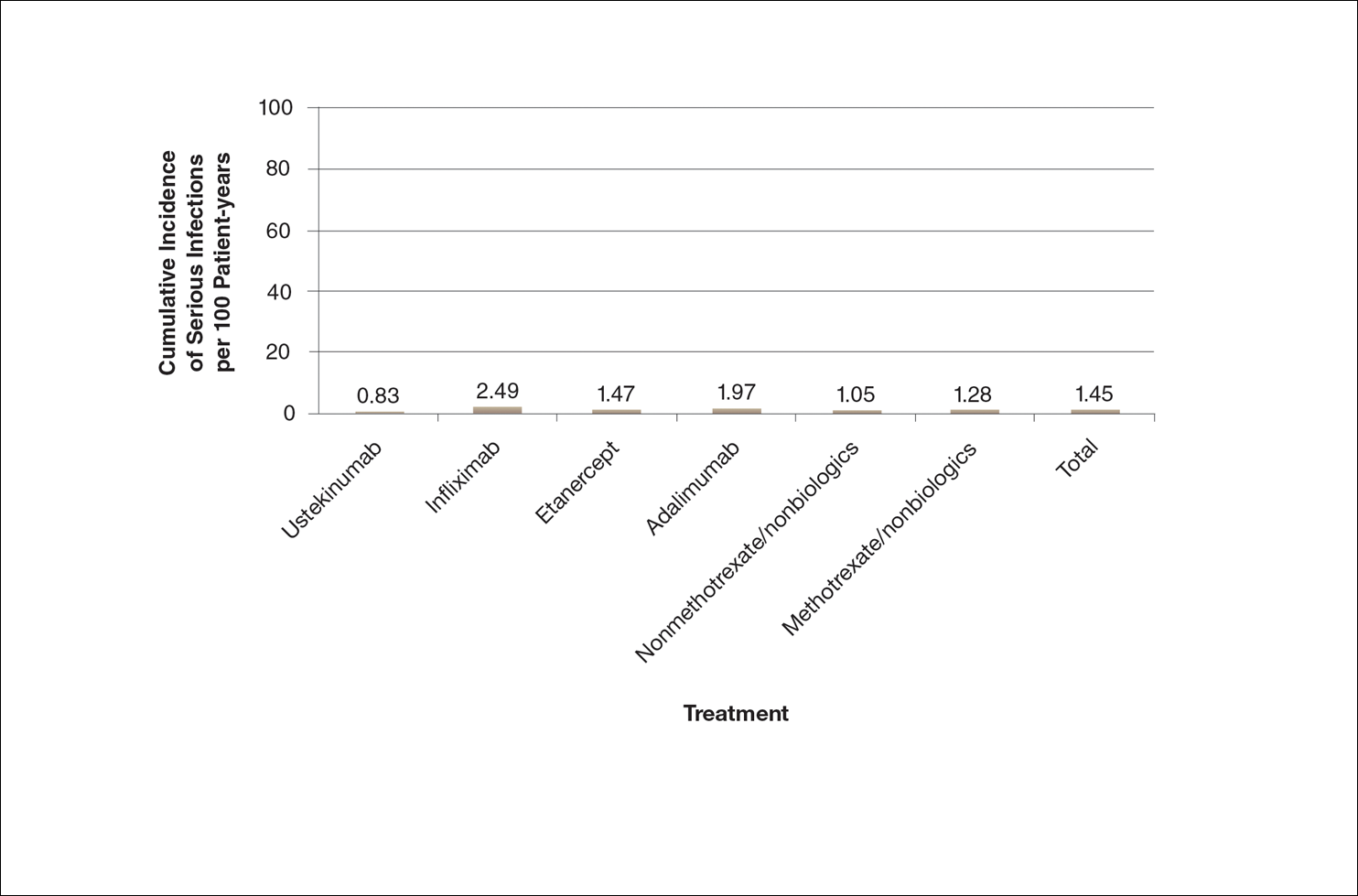
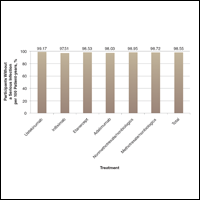
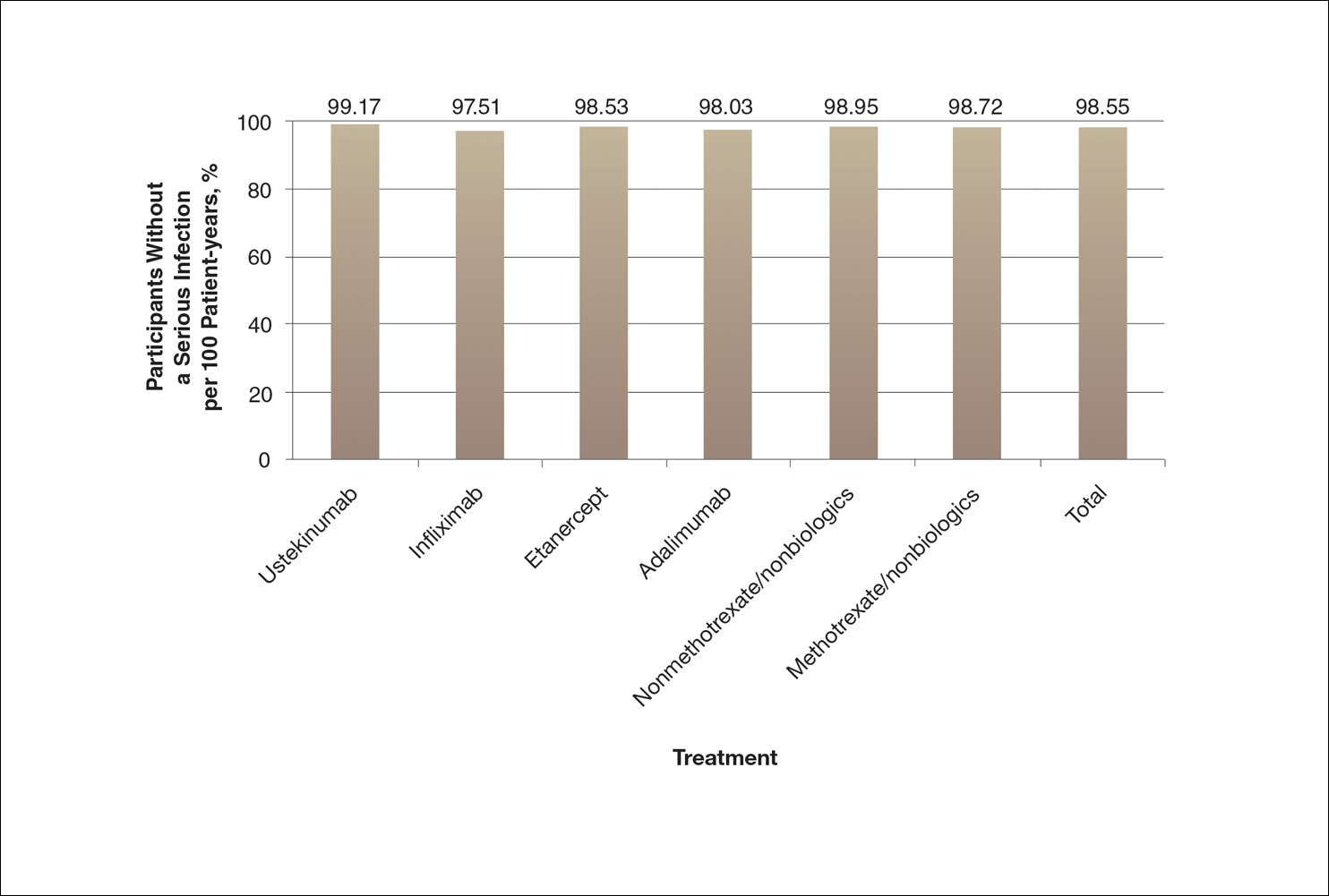
A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.
Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
The Nuremberg Code in 1947,1 the Declaration of Helsinki in 1964,2 and the Belmont Report in 19793 were cornerstones in the establishment of ethical principles in the medical field. These documents specifically highlight the concept of informed consent, which maintains that to practice ethical medicine, physicians must fully inform patients of all therapeutic benefits and especially risks as well as treatment alternatives before they consent to therapeutic intervention. Educating patients about risks of treatment is obligatory. Risk communication involves a mutual exchange of information between physicians and patients; the physician presents risk information in an understandable manner that adequately conveys pertinent data that is critical for the patient to make an informed therapeutic decision.4
An inherent problem with risk education is that patients may be terrified about risks associated with treatment. Some patients will refuse needed treatment because of fear.5 When patients have concerns about the safety profile of a treatment regimen and potential adverse effects, they may be less compliant with treatment.6 The intelligent noncompliance phenomenon occurs when a patient knowingly makes the choice to not adhere to treatment, and concern regarding treatment risks relative to benefits is a common reason underlying this phenomenon.7,8
Behavioral economists have studied how individuals weigh risks. Kahneman and Tversky’s9 prospect theory asserts that individuals tend to overweigh unlikely risks and underweigh more certain risks, which they call the certainty effect; it is the basis of the human tendency to avoid risks in situations of likely gain and to pursue risks in situations of likely loss. The tendency to overweigh rare risks is even more pronounced for affect-rich events such as serious side effects.10 The way data are presented can affect how patients interpret the information. Context and framing of data affect patients’ perceptions.11 We describe several ways to present safety data using graphical presentation of psoriasis treatment safety data as an example and explain how each one can affect patients’ perception of treatment risks.
Approaches to Presenting Safety Data
There are numerous ways to present safety data to patients, including verbal, numeric, and visual strategies.12 Many methods of presentation are a combination of these strategies. Graphs are visual strategies to further categorize and present numeric data, and physicians may choose to incorporate these aids when presenting safety information to patients. Graphical presentations give the patient a mental picture of the data. Numerous types of graphs can be constructed. Kalb et al13 determined the effect of psoriasis treatment on the risk of serious infection from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). We used the results from this study to demonstrate multiple ways of presenting safety data (Figures 1–3).
A graphical presentation with a truncated y-axis is a common approach (Figure 1). Graphs with truncated axes are sometimes used to conserve space or to accentuate certain differences in the graph that would otherwise be less obvious without the zoomed in y-axis.14 These graphs present quantitatively accurate information that can be visually misleading at the same time. Truncated axes accentuate differences, creating mental impressions that are not reflective of the magnitude of the numeric differences. Alternatively, a graph with a full y-axis includes both the maximum and minimum data values on the y-axis (Figure 2). The y-axis also extends maximally to the total number of patients or patient-years studied. This type of graph presents all of the numeric data without distortion.


A graph also can present the percentage of patients or patient-years that do not have an adverse effect (Figure 3). This inverse presentation of the data does not emphasize rare cases of patients who have had adverse effects; instead, it emphasizes the large percentage of patients who did not have adverse effects and presents a far more reassuring perspective, even though mathematically the information is identical.

Focus on the Patients Who Do Not Have Adverse Effects of Treatments
Fear of adverse effects is one of the most commonly reported causes of poor treatment adherence.15 New therapies for psoriasis are highly effective and safe, but as with all treatments, they also are associated with some risks. Patients may latch onto those risks too tightly or perhaps, in other circumstances, not tightly enough. The method used by a physician to present safety data to a patient may determine the patient’s perception about treatments.
When trying to give patients an accurate impression of treatment risks, it may be helpful to avoid approaches that focus on presenting the (few) cases of severe adverse drug effects since patients (and physicians) are likely to overweigh the unlikely risk of having an adverse effect if presented with this information. It may be more reassuring to focus on presenting information about the chance of not having an adverse drug effect, assuming the physician’s goal is to be reassuring.
Poor communication with patients when presenting safety data can foster exaggerated fears of an unlikely consequence to the point that patients can be left undertreated and sustaining disease symptoms.16 Physicians may strive to do no harm to their patients, but without careful presentation of safety data in the process of helping the patient make an informed decision, it is possible to do mental harm to patients in the form of fear or even, in the case of nonadherence or treatment refusal, physical harm in the form of continued disease symptoms.
One limitation of this review is that we only used graphical presentation of data as an example. Similar concerns apply to numerical data presentation. Telling a patient the risk of a severe adverse reaction is doubled by a certain treatment may be terrifying, though if the baseline risk is rare, doubling the baseline risk may represent only a minimal increase in the absolute risk. Telling a patient the risk is only 1 in 1000 may still be alarming because many patients tend to focus on the 1, but telling a patient that 999 of 1000 patients do not have a problem can be much more reassuring.
The physician’s goal—to help patients make informed decisions about their treatment—calls for him/her to assimilate safety data into useful information that the patient can use to make an informed decision.17 Overly comforting or alarming, confusing, and inaccurate information can misguide the patient, violating the ethical principle of nonmaleficence. Although there is an obligation to educate patients about risks, there may not be a purely objective way to do it. When physicians present objective data to patients, whether in numerical or graphical form, there will be an unavoidable subjective interpretation of the data. The form of presentation will have a critical effect on patients’ subjective perceptions. Physicians can present objective data in such a way as to be reassuring or frightening.
Conclusion
Despite physicians’ best-intentioned efforts, it may be impossible to avoid presenting safety data in a way that will be subjectively interpreted by patients. Physicians have a choice in how they present data to patients; their best judgment should be used in how they present data to inform patients, guide them, and offer them the best treatment outcomes.
Acknowledgment
We thank Scott Jaros, BA (Winston-Salem, North Carolina), for his assistance in the revision of the manuscript.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
- Freyhofer HH. The Nuremberg Medical Trial: The Holocaust and the Origin of the Nuremberg Medical Code. New York, NY: Peter Lang Publishing; 2004.
- Carlson R, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57:695-713.
- Office for Human Research Protections. The Belmont Report. Rockville, MD: US Department of Health and Human Services; 1979.
- Edwards A, Elwyn G, Mulley A. Explaining risks: turning numerical data into meaningful pictures. BMJ. 2002;324:827-830.
- Hayden C, Neame R, Tarrant C. Patients’ adherence-related beliefs about methotrexate: a qualitative study of the role of written patient information. BMJ Open. 2015;5:e006918.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Weintraub M. Intelligent noncompliance with special emphasis on the elderly. Contemp Pharm Pract. 1981;4:8-11.
- Horne R. Representations of medication and treatment: advances in theory and measurement. In: Petrie KJ, Weinman JA, eds. Perceptions of Health and Illness: Current Research and Applications. London, England: Routledge, Taylor & Francis Group; 1997:155-188.
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263-291.
- Rottenstreich Y, Hsee CK. Money, kisses, and electric shocks: on the affective psychology of risk. Psychol Sci. 2001;12:185-190.
- Kessler JB, Zhang CY. Behavioural economics and health. In: Detels R, Gulliford M, Abdool Karim Q, et al, eds. Oxford Textbook of Global Public Health. 6th ed. Oxford, UK: Oxford University Press; 2015:775-789.
- Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations [published online September 14, 2007]. Med Decis Making. 2007;27:696-713.
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151:961-969.
- Rensberger B. Slanting the slopes of graphs. The Washington Post. May 10, 1995. http://www.washingtonpost.com/archive/1995/05/10/slanting-the-slope-of-graphs/08a34412-60a2-4719-86e5-d7433938c166/. Accessed September 21, 2016.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555-567.
- Hahn RA. The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med. 1997;26(5, pt 1):607-611.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327:745-748.
Practice Points
- Physicians can guide patients’ perceptions of drug safety by the way safety data are presented.
- For patients who are concerned about rare treatment risks, presenting data on the patients who have not experienced adverse effects can be reassuring.
Psoriasis not consistently linked to adverse pregnancy outcomes
Psoriasis was not consistently associated with adverse pregnancy outcomes across nine studies in a systematic review of the literature, but four of the studies reported significant increases in at least one adverse outcome among women with psoriasis, according to a report in the British Journal of Dermatology.
Many women with psoriasis develop the disorder during their reproductive years, and more than 100,000 births to such patients are estimated to occur in the United States each year. Other autoimmune diseases are known to adversely affect pregnancy outcomes, but the issue has not been well studied among women with psoriasis, said Robert Bobotsis, a medical student at Western University, London (Ont.), and his associates.
They performed a systematic review of the literature to examine a possible link, but were only able to find nine fair- or good-quality studies involving a total of 4,756 pregnancies from which to extract data concerning a possible association. This small sample size may have been underpowered to detect the uncommon adverse pregnancy outcomes being assessed. Moreover, the investigators were unable to conduct a meta-analysis pooling the data because the effect measures were inconsistent across the nine studies, Mr. Bobotsis and his associates noted.
The review included a retrospective case series, a retrospective case control study, three retrospective cohort studies, two prospective cohort studies, one cross-sectional study, and one study combining prospective and retrospective cohorts. It “did not demonstrate an increased risk of poor outcomes in pregnant women with psoriasis” (Br J Dermatol. 2016 Jul 24;175:464-72).
However, four studies showed that compared with women who didn’t have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6. “Our results should be viewed as an opportunity to further research pregnancy outcomes in psoriasis,” the investigators said.
Psoriasis was not consistently associated with adverse pregnancy outcomes across nine studies in a systematic review of the literature, but four of the studies reported significant increases in at least one adverse outcome among women with psoriasis, according to a report in the British Journal of Dermatology.
Many women with psoriasis develop the disorder during their reproductive years, and more than 100,000 births to such patients are estimated to occur in the United States each year. Other autoimmune diseases are known to adversely affect pregnancy outcomes, but the issue has not been well studied among women with psoriasis, said Robert Bobotsis, a medical student at Western University, London (Ont.), and his associates.
They performed a systematic review of the literature to examine a possible link, but were only able to find nine fair- or good-quality studies involving a total of 4,756 pregnancies from which to extract data concerning a possible association. This small sample size may have been underpowered to detect the uncommon adverse pregnancy outcomes being assessed. Moreover, the investigators were unable to conduct a meta-analysis pooling the data because the effect measures were inconsistent across the nine studies, Mr. Bobotsis and his associates noted.
The review included a retrospective case series, a retrospective case control study, three retrospective cohort studies, two prospective cohort studies, one cross-sectional study, and one study combining prospective and retrospective cohorts. It “did not demonstrate an increased risk of poor outcomes in pregnant women with psoriasis” (Br J Dermatol. 2016 Jul 24;175:464-72).
However, four studies showed that compared with women who didn’t have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6. “Our results should be viewed as an opportunity to further research pregnancy outcomes in psoriasis,” the investigators said.
Psoriasis was not consistently associated with adverse pregnancy outcomes across nine studies in a systematic review of the literature, but four of the studies reported significant increases in at least one adverse outcome among women with psoriasis, according to a report in the British Journal of Dermatology.
Many women with psoriasis develop the disorder during their reproductive years, and more than 100,000 births to such patients are estimated to occur in the United States each year. Other autoimmune diseases are known to adversely affect pregnancy outcomes, but the issue has not been well studied among women with psoriasis, said Robert Bobotsis, a medical student at Western University, London (Ont.), and his associates.
They performed a systematic review of the literature to examine a possible link, but were only able to find nine fair- or good-quality studies involving a total of 4,756 pregnancies from which to extract data concerning a possible association. This small sample size may have been underpowered to detect the uncommon adverse pregnancy outcomes being assessed. Moreover, the investigators were unable to conduct a meta-analysis pooling the data because the effect measures were inconsistent across the nine studies, Mr. Bobotsis and his associates noted.
The review included a retrospective case series, a retrospective case control study, three retrospective cohort studies, two prospective cohort studies, one cross-sectional study, and one study combining prospective and retrospective cohorts. It “did not demonstrate an increased risk of poor outcomes in pregnant women with psoriasis” (Br J Dermatol. 2016 Jul 24;175:464-72).
However, four studies showed that compared with women who didn’t have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6. “Our results should be viewed as an opportunity to further research pregnancy outcomes in psoriasis,” the investigators said.
Key clinical point: Psoriasis is not consistently associated with adverse pregnancy outcomes across studies, but individual studies reported such links.
Major finding: Four of nine studies showed that compared with women who did not have psoriasis, those who did were at significantly increased risk for spontaneous abortion, cesarean delivery, low birth weight, macrosomia, large for gestational age, and prematurity, with odds ratios as high as 5.6.
Data source: A systematic review of nine reports in the literature concerning adverse outcomes in 4,756 pregnancies among women with psoriasis.
Disclosures: The authors reported that this work had no funding sources. Mr. Bobotsis reported having no relevant financial disclosures; two of his associates reported ties to AbbVie, Actelion, Amgen, Bio-K, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Roche, and Valeant.
Development of Bullous Pemphigoid in a Patient With Psoriasis and Metabolic Syndrome
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
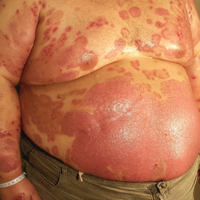
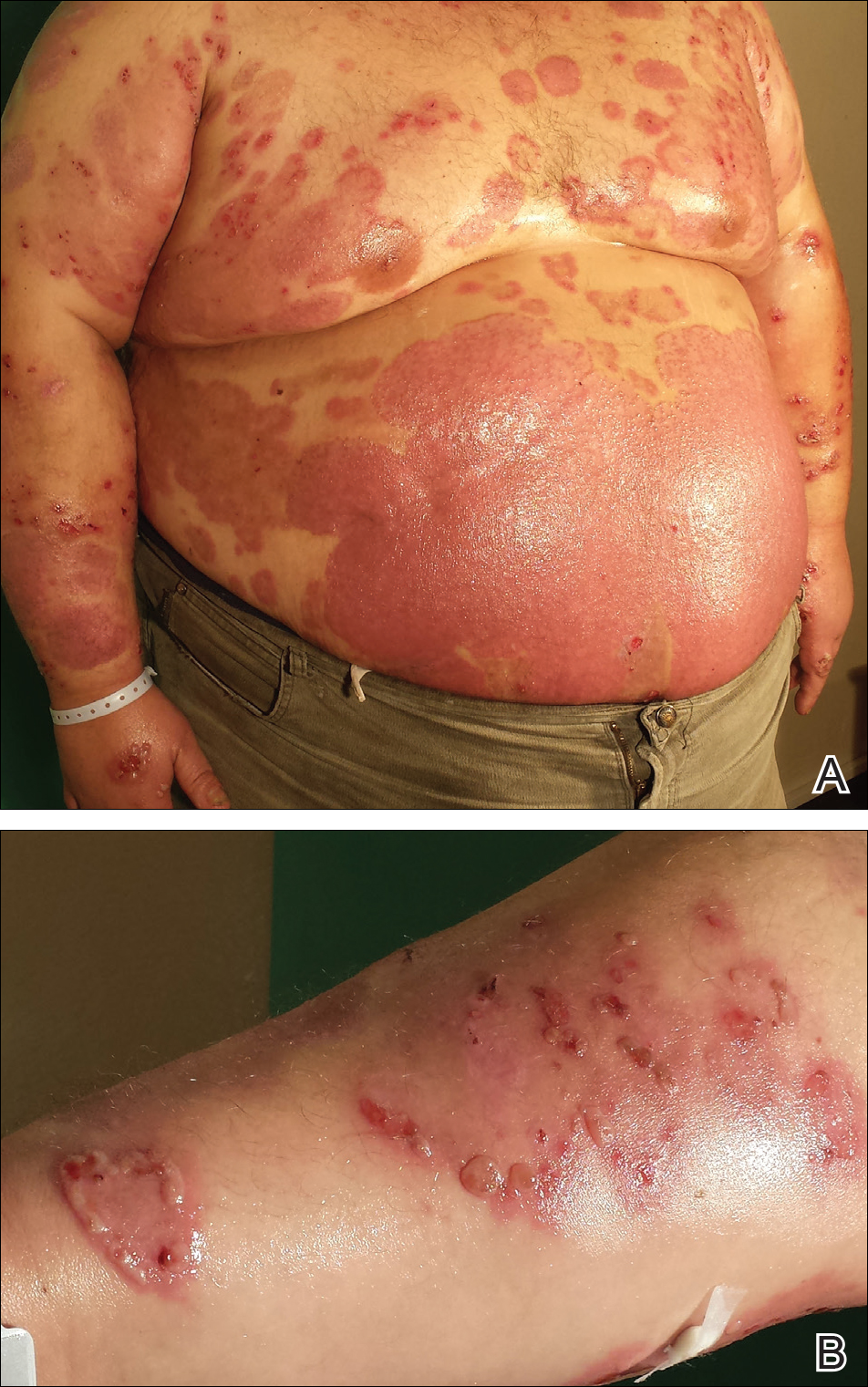
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.
The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
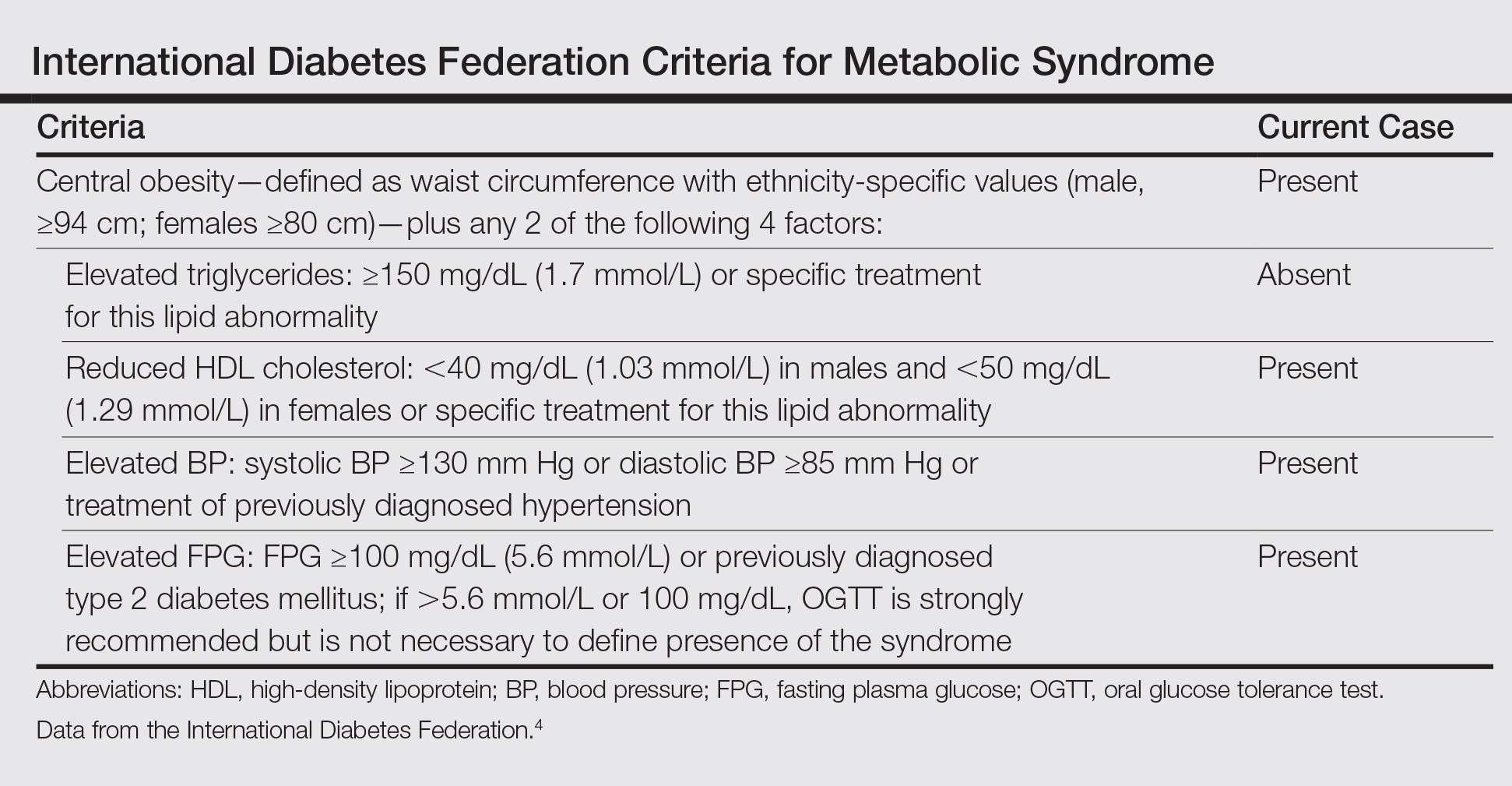
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.

The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering disease.1 The majority of BP cases are idiopathic and occur in patients older than 60 years. The disease is characterized by the development of circulating IgG autoantibodies reacting with the BP180 antigen of the basement membrane zone.1 Psoriasis vulgaris (PV) is a common, chronic, immune-mediated disease affecting approximately 2% of the world’s population including children and adults.2 Both entities may coexist with internal disorders such as hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, hyperlipidemia, and cerebrovascular accident. It has been postulated that BP more often coexists with neurological disorders, such as stroke and Parkinson disease,3 whereas PV usually is associated with cardiovascular disorders and diabetes mellitus.2 We report the case of a 35-year-old man with chronic PV and metabolic syndrome who developed BP that was successfully treated with methotrexate (MTX).
Case Report
A 35-year-old man with a 15-year history of PV, class 3 obesity (body mass index, 69.2), and thrombosis of the left leg was referred to the dermatology department due to a sudden extensive erythematous and bullous eruption located on the trunk, arms, and legs with involvement of the oral mucosa that had started 4 weeks prior. The skin lesions were accompanied by severe pruritus. On admission to the hospital, the patient presented with stable psoriatic plaques located on the trunk, arms, and proximal part of the lower legs with a psoriasis area severity index score of 11.8 (Figure 1A). He also had disseminated tense blisters and erosions partially arranged in an annular pattern located on the border of the psoriatic plaques as well as on an erythematous base or within unaffected skin (Figure 1B). Additionally, a few small erosions were present on the oral mucosa.

The patient’s father had a history of PV, but there was no family history of obesity or autoimmune blistering disorders. On physical examination, central obesity was noted with a waist circumference of 180 cm and a body mass index of 69.2; his blood pressure was 220/150 mm Hg. Laboratory tests revealed leukocytosis (20.06×109/L [reference range, 4.5–11.0×109/L]) with neutrophilia (16.2×109/L [reference range, 1.6–7.6×109/L]; 80.9% [reference range, 40.0%–70.0%]), eosinophilia (1.01×109/L [reference range, 0–0.5×109/L]), elevated C-reactive protein levels (49.4 mg/L [reference range, 0.0–9.0 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–12 mm/h]), elevated γ-glutamyltransferase (66 U/L [reference range, 0–55 U/L]), decreased high-density lipoprotein levels (38 mg/dL [reference range, ≥40 mg/dL]), elevated fasting plasma glucose (116 mg/dL or 6.4 mmol/L [reference range, 70–99 mg/dL or 3.9–5.5 mmol/L]), elevated total IgE (1540 µg/L [reference range, 0–1000 µg/L]), elevated D-dimer (3.21 µg/mL [reference range, <0.5 µg/mL]), and low free triiodothyronine levels (130 pg/dL [reference range, 171–371 pg/dL]). The total protein level was 6.5 g/dL (reference range, 6.0–8.0 g/dL) and albumin level was 3.2 g/dL (reference range, 4.02–4.76 g/dL). A chest radiograph showed no abnormalities.
Based on the physical examination and laboratory testing, it was determined that the patient fulfilled 4 of 5 criteria for metabolic syndrome described by the International Diabetes Federation in 2006 (Table).4 Direct immunofluorescence performed on normal-appearing perilesional skin demonstrated linear IgG and C3 deposits along the basement membrane zone. Indirect immunofluorescence detected circulating IgG autoantibodies at a titer of 1:80. Serum studies using biochip mosaics5 revealed the reactivity of circulating IgG antibodies to the epidermal side of salt-split skin and with antigen dots of tetrameric BP180-NC16a, which prompted the diagnosis of BP (Figure 2).

Oral treatment with MTX 12.5 mg once weekly with clobetasol propionate cream applied to affected skin was initiated for 4 weeks. The PV resolved completely and blister formation stopped. A few weeks later BP reappeared, even though the patient was still taking MTX. The treatment failure may have been related to the patient’s class 3 obesity; therefore, the dose was increased to 20 mg once weekly for 8 weeks, which led to rapid healing of BP erosions. The patient was monitored for 2 months with no symptoms of recurrence.
Comment
Psoriasis Comorbidities
The correlation between PV and cardiovascular disorders such as myocardial infarction, cerebrovascular accident, and pulmonary embolism has been well established and is widely accepted.2 It also has been documented that the risk for metabolic syndrome with components such as diabetes mellitus, hypertension, lipid abnormalities, obesity, and arteriosclerosis is notably increased in PV patients.6 Moreover, associated internal disorders are responsible for a 3- to 4-year reduction in life expectancy in patients with moderate to severe PV.7
Correlation of PV and BP
Psoriasis also may coexist with autoimmune disorders such as rheumatoid arthritis, lupus erythematosus, and blistering disorders.8 There are more than 60 known cases reporting PV in association with various types of subepidermal blistering diseases, including pemphigus vulgaris, epidermolysis bullosa acquisita, anti-p200 pemphigoid, and BP.8,9 The pathogenetic relationship between BP and PV remains obscure. In most published cases, PV preceded BP by 5 to 30 years, possibly ascribable to patients being diagnosed with PV at a younger age.9 In general, patients with BP and PV are younger than patients with BP only, with a mean age of 62 years.9 Because our patient was in his mid-30s when he developed BP, in such cases physicians should take under consideration any triggering factors (eg, drugs). Physical examination and detailed laboratory findings allowed us to make the patient aware of the potential for development of metabolic syndrome. This condition in combination with PV could be a predisposing factor for BP development. According to more recent research, PV is considered a generalized inflammatory process rather than a disorder limited to the skin and joints.10 The chronic inflammatory process in psoriatic skin results in exposure of autoantigens, leading to an immune response and the production of BP antibodies. The neutrophil elastase enzyme present in psoriatic lesions also may take part in dermoepidermal junction degradation and blister formation of BP.11 According to other observations, some antipsoriatic therapies (eg, psoralen plus UVA, UVB, dithranol, coal tar) could be associated with development of BP.12 Moreover, it was shown that psoralen plus UVA therapy, which is widely used in PV treatment, alters the cytokine profile from helper T cells TH1 to TH2.12 TH2-dependent cytokines predominate the sera and erosions in BP patients and seem to be notably relevant to the pathophysiology of the disease.13 The history of our patient’s psoriatic treatment included only topical corticosteroids, keratolytic agents, and occasionally dithranol and coal tar; however, UV phototherapy or any other systemic therapies had never been utilized. Three previously reported cases of patients with PV and BP also revealed no history of UV phototherapy,8,9 which suggests that mechanisms responsible for coexistence of PV and BP are more complex. It has been proven that proinflammatory cytokines secreted by TH1 and TH17 cells, in particular tumor necrosis factor α, IL-17, IL-22, and IL-23, play an important role in the development of psoriatic lesions.10 On the other hand, these cytokines are known to contribute to vascular inflammation, leading to development of arteriosclerosis, as well as to regulate adipogenesis and obesity.14,15 Arakawa et al16 reported increased expression of IL-17 in lesional skin in BP. They concluded that IL-17 may contribute to the recruitment of eosinophils and neutrophils and tissue damage in BP. Therefore, it is highly likely that IL-17 might be a common factor underlying the coexistence of BP with PV and metabolic syndrome. More such reports are required for better understanding this association.
BP Treatment
Selecting a therapy for BP with coexistent PV is challenging, especially in patients with extreme obesity and metabolic syndrome. It is well established that obesity correlates with a higher incidence of PV and more severe disease. On the other hand, obesity also influences response to therapy. Systemic corticosteroids are contraindicated in psoriasis patients because of severe side effects, such as rebound phenomenon of psoriatic lesions and risk for development of generalized pustular PV. Although systemic corticosteroids are effective in BP, high-dose therapy may potentially be life-threatening, particularly in these obese patients with conditions such as hypertension and diabetes mellitus, among others,1 as was observed in our case. Taking into consideration the above mentioned conditions and our experience on such cases, the current patient had received MTX (12.5 mg once weekly) and clobetasol propionate cream, which led to the rapid healing of the psoriatic plaques, whereas BP was more resistant to this therapy. This response may be explained by our patient’s class 3 obesity (body mass index, 69.2). Therefore, the dose of MTX was increased to 20 mg once weekly and was successful. The decision to use MTX was supported by evidence that this medicine may reduce the risk for arteriosclerosis and cardiovascular disorders.17
There are some alternative therapeutic options for patients with coexisting BP and PV, such as cyclosporine,18 combination low-dose cyclosporine and low-dose systemic corticosteroids,19 dapsone,20 azathioprine,21 mycophenolate mofetil,22 and acitretin.23 It also has been shown that biologics (eg, ustekinumab) may be a successful solution in patients with PV and antilaminin-γ1 pemphigoid.24 However, these alternative therapeutic regimens could not be considered in our patient because of serious coexisting internal disorders.
Conclusion
We present a case of concomitant BP and PV in a patient with metabolic syndrome. Although the pathogenic role of this unique coexistence is not fully understood, MTX proved suitable and effective in this single case. Further studies should be performed to elucidate the pathogenic relationship and therapeutic solutions for cases with coexisting PV, BP, and metabolic syndrome.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
- Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of gluco-corticosteroids, and old age. Arch Dermatol. 2002;138:903-908.
- Pietrzak A, Bartosinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis vulgaris. Int J Dermatol. 2013;52:153-162.
- Stinco G, Codutti R, Scarbolo M, et al. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85:136-139.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: International Diabetes Foundation; 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed September 14, 2016.
- Van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- Sommer DM, Jenisch S, Suchan M, et al. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321-328.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Lazarczyk M, Wozniak K, Ishii N, et al. Coexistence of psoriasis and pemphigoid—only a coincidence? Int J Mol Med. 2006;18:619-623.
- Yasuda H, Tomita Y, Shibaki A, et al. Two cases of subepidermal blistering disease with anti-p200 or 180-kD bullous pemphigoid antigen associated with psoriasis. Dermatology. 2004;209:149-155.
- Malakouti M, Brown GE, Wang E, et al. The role of IL-17 in psoriasis [published online February 20, 2014]. J Dermatolog Treat. 2015;26:41-44.
- Glinski W, Jarzabek-Chorzelska M, Pierozynska-Dubowska M, et al. Basement membrane zone as a target for human neutrophil elastase in psoriasis. Arch Dermatol Res. 1990;282:506-511.
- Klosner G, Trautinger F, Knobler R, et al. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459-462.
- Gounni AS, Wellemans V, Agouli M, et al. Increased expression of Th2-associated chemokines in bullous pemphigoid disease. role of eosinophils in the production and release of these chemokines. Clin Immunol. 2006;120:220-231.
- Gao Q, Jiang Y, Ma T, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820-5827.
- Zúñiga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959.
- Arakawa M, Dainichi T, Ishii N, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20:1022-1024.
- Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199-207.
- Boixeda JP, Soria C, Medina S, et al. Bullous pemphigoid and psoriasis: treatment with cyclosporine. J Am Acad Dermatol. 1991;24:152.
- Bianchi L, Gatti S, Nini G. Bullous pemphigoid and severe erythrodermic psoriasis: combined low-dose treatment with cyclosporine and systemic steroids. J Am Acad Dermatol. 1992;27(2, pt 1):278.
- Hisler BM, Blumenthal NC, Aronson PJ, et al. Bullous pemphigoid in psoriatic lesions. J Am Acad Dermatol. 1989;20:683-684.
- Primka EJ III, Camisa C. Psoriasis and bullous pemphigoid treated with azathioprine. J Am Acad Dermatol. 1998;39:121-123.
- Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265-268.
- Kobayashi TT, Elston DM, Libow LF, et al. A case of bullous pemphigoid limited to psoriatic plaques. Cutis. 2002;70:283-287.
- Maijima Y, Yagi H, Tateishi C, et al. A successful treatment with ustekinumab in case of antilaminin-γ1 pemphigoid associated with psoriasis. Br J Dermatol. 2013;168:1367-1369.
Practice Points
- Metabolic syndrome and psoriasis vulgaris (PV) may promote development of bullous pemphigoid (BP) in patients younger than 60 years.
- Methotrexate may be a therapeutic solution for BP coexisting with PV and metabolic syndrome.
In Orbit

When using biologic therapies for psoriasis, it is important to evaluate long-term efficacy (>4 years of follow-up). Biologic drug survival in psoriasis reflects long-term performance in real-life settings. Prior studies have yielded inconsistent results.
Vilarrasa et al (J Am Acad Dermatol. 2016;74:1066-1072) conducted an observational retrospective study called ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis) to determine drug survival (the mean length of time patients remain on a drug) in a cohort of 427 patients (63.5% male; mean age, 50.2 years) with moderate to severe psoriasis vulgaris (mean baseline psoriasis area and severity index [PASI], 16.4). In addition to determining mean drug survival times for etanercept, infliximab, adalimumab, and ustekinumab, investigators searched for variables that positively or negatively affected drug survival times. Data were extracted from clinical records of patients treated with biologic agents over a 4-year period. Drug survival was analyzed using the Kaplan-Meier method and the influence of several covariates was assessed using Cox regression.
The investigators evaluated 703 treatment courses. The overall median drug survival was 31.0 months. Cumulative probability of drug survival was lower in obese patients (23.0 months; 95% CI, 17.4-28.6) than in patients with body mass index less than 30 (37.3 months; 95% CI, 29.4-45.1; P=.001). Drug survival was significantly higher for ustekinumab than for any other biologic agent (log-rank test, P<.001). Multivariate analysis showed that obesity, etanercept treatment, and strict adherence to approved doses were associated with an increased probability of drug withdrawal, whereas ustekinumab treatment and PASI 75 and PASI 90 responses at week 16 prolonged drug survival. Data were collected retrospectively.
What’s the issue?
These results should help to educate patients and to manage expectations about drug efficacy.

When using biologic therapies for psoriasis, it is important to evaluate long-term efficacy (>4 years of follow-up). Biologic drug survival in psoriasis reflects long-term performance in real-life settings. Prior studies have yielded inconsistent results.
Vilarrasa et al (J Am Acad Dermatol. 2016;74:1066-1072) conducted an observational retrospective study called ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis) to determine drug survival (the mean length of time patients remain on a drug) in a cohort of 427 patients (63.5% male; mean age, 50.2 years) with moderate to severe psoriasis vulgaris (mean baseline psoriasis area and severity index [PASI], 16.4). In addition to determining mean drug survival times for etanercept, infliximab, adalimumab, and ustekinumab, investigators searched for variables that positively or negatively affected drug survival times. Data were extracted from clinical records of patients treated with biologic agents over a 4-year period. Drug survival was analyzed using the Kaplan-Meier method and the influence of several covariates was assessed using Cox regression.
The investigators evaluated 703 treatment courses. The overall median drug survival was 31.0 months. Cumulative probability of drug survival was lower in obese patients (23.0 months; 95% CI, 17.4-28.6) than in patients with body mass index less than 30 (37.3 months; 95% CI, 29.4-45.1; P=.001). Drug survival was significantly higher for ustekinumab than for any other biologic agent (log-rank test, P<.001). Multivariate analysis showed that obesity, etanercept treatment, and strict adherence to approved doses were associated with an increased probability of drug withdrawal, whereas ustekinumab treatment and PASI 75 and PASI 90 responses at week 16 prolonged drug survival. Data were collected retrospectively.
What’s the issue?
These results should help to educate patients and to manage expectations about drug efficacy.

When using biologic therapies for psoriasis, it is important to evaluate long-term efficacy (>4 years of follow-up). Biologic drug survival in psoriasis reflects long-term performance in real-life settings. Prior studies have yielded inconsistent results.
Vilarrasa et al (J Am Acad Dermatol. 2016;74:1066-1072) conducted an observational retrospective study called ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis) to determine drug survival (the mean length of time patients remain on a drug) in a cohort of 427 patients (63.5% male; mean age, 50.2 years) with moderate to severe psoriasis vulgaris (mean baseline psoriasis area and severity index [PASI], 16.4). In addition to determining mean drug survival times for etanercept, infliximab, adalimumab, and ustekinumab, investigators searched for variables that positively or negatively affected drug survival times. Data were extracted from clinical records of patients treated with biologic agents over a 4-year period. Drug survival was analyzed using the Kaplan-Meier method and the influence of several covariates was assessed using Cox regression.
The investigators evaluated 703 treatment courses. The overall median drug survival was 31.0 months. Cumulative probability of drug survival was lower in obese patients (23.0 months; 95% CI, 17.4-28.6) than in patients with body mass index less than 30 (37.3 months; 95% CI, 29.4-45.1; P=.001). Drug survival was significantly higher for ustekinumab than for any other biologic agent (log-rank test, P<.001). Multivariate analysis showed that obesity, etanercept treatment, and strict adherence to approved doses were associated with an increased probability of drug withdrawal, whereas ustekinumab treatment and PASI 75 and PASI 90 responses at week 16 prolonged drug survival. Data were collected retrospectively.
What’s the issue?
These results should help to educate patients and to manage expectations about drug efficacy.
Debunking Psoriasis Myths: Is Psoriasis Infectious?
Myth: Psoriasis Is Infectious The precise cause of psoriasis is unknown, but researchers believe the immune system and genetics play major roles in its development, according to the National Psoriasis Foundation. The skin cells in patients with psoriasis grow at an abnormally fast rate, which causes the buildup of psoriasis lesions. Usually, something triggers psoriasis to flare.
A common misconception among patients is that psoriasis is caused by an infection. Psoriasis is not contagious and psoriasis lesions are not infectious.
However, psoriasis patients are more prone to infections than those without psoriasis. Risk factors for serious infections in psoriasis patients include immune dysregulation, systemic immunosuppressive medications, and comorbid health conditions such as diabetes mellitus or obesity. A 2016 study revealed an increased incidence of serious infections (eg, cellulitis, herpes simplex virus infection, any fungal infection, infectious arthritis, methicillin-resistant Staphylococcus aureus) in hospitalized patients with psoriasis. Higher rates were seen among nonwhite and non-privately insured patients.
In a 2011 study, the likelihood of infectious diseases in patients with psoriasis was twice as high as the reference population. The risk was highest in patients with more severe psoriasis but was not associated with recent systemic antipsoriatic drug dispensing. Respiratory tract, abdominal, and skin infections occurred most frequently in patients with psoriasis.
Poor access to adequate dermatologic care may contribute to higher rates of infections. Dermatologists must closely monitor patients with psoriasis for infection. More research is needed to develop interventions for prevention.
Expert Commentary Psoriasis patients have long faced discrimination because of an irrational fear that their disease was somehow contagious. In fact this is completely false. This highlights the need for education of the public, so that they understand the true causes and nature of the disease.
—Jeffrey M. Weinberg, MD (New York, New York)
About psoriasis. National Psoriasis Foundation website. http://www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States [published online June 17, 2016]. J Am Acad Dermatol. 2016;75:287-296.
Wakkee M, de Vries E, van den Haak P, et al. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135-1144.
Myth: Psoriasis Is Infectious The precise cause of psoriasis is unknown, but researchers believe the immune system and genetics play major roles in its development, according to the National Psoriasis Foundation. The skin cells in patients with psoriasis grow at an abnormally fast rate, which causes the buildup of psoriasis lesions. Usually, something triggers psoriasis to flare.
A common misconception among patients is that psoriasis is caused by an infection. Psoriasis is not contagious and psoriasis lesions are not infectious.
However, psoriasis patients are more prone to infections than those without psoriasis. Risk factors for serious infections in psoriasis patients include immune dysregulation, systemic immunosuppressive medications, and comorbid health conditions such as diabetes mellitus or obesity. A 2016 study revealed an increased incidence of serious infections (eg, cellulitis, herpes simplex virus infection, any fungal infection, infectious arthritis, methicillin-resistant Staphylococcus aureus) in hospitalized patients with psoriasis. Higher rates were seen among nonwhite and non-privately insured patients.
In a 2011 study, the likelihood of infectious diseases in patients with psoriasis was twice as high as the reference population. The risk was highest in patients with more severe psoriasis but was not associated with recent systemic antipsoriatic drug dispensing. Respiratory tract, abdominal, and skin infections occurred most frequently in patients with psoriasis.
Poor access to adequate dermatologic care may contribute to higher rates of infections. Dermatologists must closely monitor patients with psoriasis for infection. More research is needed to develop interventions for prevention.
Expert Commentary Psoriasis patients have long faced discrimination because of an irrational fear that their disease was somehow contagious. In fact this is completely false. This highlights the need for education of the public, so that they understand the true causes and nature of the disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Myth: Psoriasis Is Infectious The precise cause of psoriasis is unknown, but researchers believe the immune system and genetics play major roles in its development, according to the National Psoriasis Foundation. The skin cells in patients with psoriasis grow at an abnormally fast rate, which causes the buildup of psoriasis lesions. Usually, something triggers psoriasis to flare.
A common misconception among patients is that psoriasis is caused by an infection. Psoriasis is not contagious and psoriasis lesions are not infectious.
However, psoriasis patients are more prone to infections than those without psoriasis. Risk factors for serious infections in psoriasis patients include immune dysregulation, systemic immunosuppressive medications, and comorbid health conditions such as diabetes mellitus or obesity. A 2016 study revealed an increased incidence of serious infections (eg, cellulitis, herpes simplex virus infection, any fungal infection, infectious arthritis, methicillin-resistant Staphylococcus aureus) in hospitalized patients with psoriasis. Higher rates were seen among nonwhite and non-privately insured patients.
In a 2011 study, the likelihood of infectious diseases in patients with psoriasis was twice as high as the reference population. The risk was highest in patients with more severe psoriasis but was not associated with recent systemic antipsoriatic drug dispensing. Respiratory tract, abdominal, and skin infections occurred most frequently in patients with psoriasis.
Poor access to adequate dermatologic care may contribute to higher rates of infections. Dermatologists must closely monitor patients with psoriasis for infection. More research is needed to develop interventions for prevention.
Expert Commentary Psoriasis patients have long faced discrimination because of an irrational fear that their disease was somehow contagious. In fact this is completely false. This highlights the need for education of the public, so that they understand the true causes and nature of the disease.
—Jeffrey M. Weinberg, MD (New York, New York)
About psoriasis. National Psoriasis Foundation website. http://www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States [published online June 17, 2016]. J Am Acad Dermatol. 2016;75:287-296.
Wakkee M, de Vries E, van den Haak P, et al. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135-1144.
About psoriasis. National Psoriasis Foundation website. http://www.psoriasis.org/about-psoriasis. Accessed September 9, 2016.
Hsu DY, Gordon K, Silverberg JI. Serious infections in hospitalized patients with psoriasis in the United States [published online June 17, 2016]. J Am Acad Dermatol. 2016;75:287-296.
Wakkee M, de Vries E, van den Haak P, et al. Increased risk of infectious disease requiring hospitalization among patients with psoriasis: a population-based cohort. J Am Acad Dermatol. 2011;65:1135-1144.
A Boxed Warning for Inadequate Psoriasis Treatment
The US Food and Drug Administration uses the term boxed warning to highlight potentially dangerous situations associated with prescription drugs. A boxed warning is used when “[T]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”1 However, drugs are not the only potential cause of severe adverse outcomes in patients with psoriasis. Untreated psoriasis also is a well-established cause of serious morbidity and mortality. What are the risks of inadequate psoriasis treatment?
Psoriasis is associated with an increased risk for cardiovascular disease.2-4 Patients with psoriasis also have a higher prevalence of classic cardiovascular risk factors including smoking, diabetes mellitus, hypertension, obesity, and hyperlipidemia.5,6 Psoriasis is a T-cell mediated disease process driven by IL-23 and TH17 helper cell–derived proinflammatory cytokines, sharing certain genetic aspects with metabolic syndrome.6 Cytokine actions on insulin signaling, lipid metabolism, and adipogenesis may underlie the increased prevalence of metabolic syndrome and cardiovascular risk factors in patients with psoriasis. In addition to treating the cutaneous manifestations of psoriasis, reducing inflammation in these patients reduces C-reactive protein and lipid peroxidation and increases high-density lipoprotein levels.6 Tumor necrosis factor α blockers decrease the risk for cardiovascular disease in patients with psoriasis.7,8 Lower than expected rates of cardiovascular disease also have been reported in a large cohort of psoriasis patients (ie, PSOLAR [Psoriasis Longitudinal Assessment and Registry] registry) being treated with either ustekinumab or tumor necrosis factor α blockers.9
Psoriatic arthritis is a chronic inflammatory disease in which active inflammation results in progressive joint destruction.10 Tumor necrosis factor α inhibitors suppress disease progression, preserve function, and delay destruction of the joints. Ustekinumab also helps control psoriatic arthritis and inhibits radiographic progression of joint disease.11
Importantly, untreated moderate to severe psoriasis is associated with several comorbidities that may lead to early death such as heart attacks and strokes.12 Furthermore, patients not taking biologic medications may have higher death rates than patients taking biologic medications.9 Psoriasis also is associated with tremendous suffering and negative psychosocial effects. The mental and physical impact of the disease is comparable to other major medical conditions (eg, cancer, arthritis, hypertension, heart disease, diabetes, depression).13 Patients also may experience physical discomfort from pain and itching.14 Children with psoriasis may experience bullying, which is associated with an increased number of depressive episodes, thereby increasing their risk for developing psychiatric conditions such as depression and anxiety as adults.15 The stigma associated with psoriasis may affect patients’ ability to build relationships. Patients with psoriasis experience higher divorce rates than patients with other chronic medical conditions, and direct involvement of genital regions may negatively impact patients’ sex lives. Patients have noted that the stigma of psoriasis also is associated with the inability to obtain employment.15 Almost one-third of patients with psoriasis who are either not working or are retired base their work status on their skin condition.16 Furthermore, psoriasis may contribute to economic burden for patients due to indirect costs associated with work absenteeism.17
Adequate treatment of psoriasis improves patients’ physical and psychological health as well as their ability to function in the workplace. However, despite the benefits of treatment, 30% of patients with severe psoriasis and 53% of patients with moderate psoriasis receive no treatment or only topical medications instead of systemic therapies.16 The potential adverse events of inadequate psoriasis treatment far outweigh any potential benefits of withholding treatment. Perhaps a boxed warning should be issued for inadequate treatment of psoriasis patients.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: warning and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. US Food and Drug Administration website. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. Published October 6, 2011. Accessed August 10, 2016.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Rose S, Sheth NH, Baker JF, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273-278.
- Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148-1153.
- Lee MK, Kim HS, Cho EB, et al. A study of awareness and screening behavior of cardiovascular risk factors in patients with psoriasis and dermatologists. Ann Dermatol. 2015;27:59-65.
- Voiculescu VM, Lupu M, Papagheorghe L, et al. Psoriasis and metabolic syndrome—scientific evidence and therapeutic implications. J Med Life. 2014;7:468-471.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular comorbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Chimenti MS, Graceffa D, Perricone R. Anti-TNFα discontinuation in rheumatoid and psoriatic arthritis: is it possible after disease remission [published online Apr 21, 2011]? Autoimmun Rev. 2011;10:636-640.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000-1006.
- Pietrzak A, Bartosinska J, Blaszczyk R, et al. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401-407.
- Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
- Garshick MK, Kimball AB. Psoriasis and the life cycle of persistent life effects. Dermatol Clin. 2015;33:25-39.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. August 17, 2014;20. pii:13030/qt48r4w8h2.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
The US Food and Drug Administration uses the term boxed warning to highlight potentially dangerous situations associated with prescription drugs. A boxed warning is used when “[T]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”1 However, drugs are not the only potential cause of severe adverse outcomes in patients with psoriasis. Untreated psoriasis also is a well-established cause of serious morbidity and mortality. What are the risks of inadequate psoriasis treatment?
Psoriasis is associated with an increased risk for cardiovascular disease.2-4 Patients with psoriasis also have a higher prevalence of classic cardiovascular risk factors including smoking, diabetes mellitus, hypertension, obesity, and hyperlipidemia.5,6 Psoriasis is a T-cell mediated disease process driven by IL-23 and TH17 helper cell–derived proinflammatory cytokines, sharing certain genetic aspects with metabolic syndrome.6 Cytokine actions on insulin signaling, lipid metabolism, and adipogenesis may underlie the increased prevalence of metabolic syndrome and cardiovascular risk factors in patients with psoriasis. In addition to treating the cutaneous manifestations of psoriasis, reducing inflammation in these patients reduces C-reactive protein and lipid peroxidation and increases high-density lipoprotein levels.6 Tumor necrosis factor α blockers decrease the risk for cardiovascular disease in patients with psoriasis.7,8 Lower than expected rates of cardiovascular disease also have been reported in a large cohort of psoriasis patients (ie, PSOLAR [Psoriasis Longitudinal Assessment and Registry] registry) being treated with either ustekinumab or tumor necrosis factor α blockers.9
Psoriatic arthritis is a chronic inflammatory disease in which active inflammation results in progressive joint destruction.10 Tumor necrosis factor α inhibitors suppress disease progression, preserve function, and delay destruction of the joints. Ustekinumab also helps control psoriatic arthritis and inhibits radiographic progression of joint disease.11
Importantly, untreated moderate to severe psoriasis is associated with several comorbidities that may lead to early death such as heart attacks and strokes.12 Furthermore, patients not taking biologic medications may have higher death rates than patients taking biologic medications.9 Psoriasis also is associated with tremendous suffering and negative psychosocial effects. The mental and physical impact of the disease is comparable to other major medical conditions (eg, cancer, arthritis, hypertension, heart disease, diabetes, depression).13 Patients also may experience physical discomfort from pain and itching.14 Children with psoriasis may experience bullying, which is associated with an increased number of depressive episodes, thereby increasing their risk for developing psychiatric conditions such as depression and anxiety as adults.15 The stigma associated with psoriasis may affect patients’ ability to build relationships. Patients with psoriasis experience higher divorce rates than patients with other chronic medical conditions, and direct involvement of genital regions may negatively impact patients’ sex lives. Patients have noted that the stigma of psoriasis also is associated with the inability to obtain employment.15 Almost one-third of patients with psoriasis who are either not working or are retired base their work status on their skin condition.16 Furthermore, psoriasis may contribute to economic burden for patients due to indirect costs associated with work absenteeism.17
Adequate treatment of psoriasis improves patients’ physical and psychological health as well as their ability to function in the workplace. However, despite the benefits of treatment, 30% of patients with severe psoriasis and 53% of patients with moderate psoriasis receive no treatment or only topical medications instead of systemic therapies.16 The potential adverse events of inadequate psoriasis treatment far outweigh any potential benefits of withholding treatment. Perhaps a boxed warning should be issued for inadequate treatment of psoriasis patients.
The US Food and Drug Administration uses the term boxed warning to highlight potentially dangerous situations associated with prescription drugs. A boxed warning is used when “[T]here is an adverse reaction so serious in proportion to the potential benefit from the drug (e.g., a fatal, life-threatening or permanently disabling adverse reaction) that it is essential that it be considered in assessing the risks and benefits of using the drug.”1 However, drugs are not the only potential cause of severe adverse outcomes in patients with psoriasis. Untreated psoriasis also is a well-established cause of serious morbidity and mortality. What are the risks of inadequate psoriasis treatment?
Psoriasis is associated with an increased risk for cardiovascular disease.2-4 Patients with psoriasis also have a higher prevalence of classic cardiovascular risk factors including smoking, diabetes mellitus, hypertension, obesity, and hyperlipidemia.5,6 Psoriasis is a T-cell mediated disease process driven by IL-23 and TH17 helper cell–derived proinflammatory cytokines, sharing certain genetic aspects with metabolic syndrome.6 Cytokine actions on insulin signaling, lipid metabolism, and adipogenesis may underlie the increased prevalence of metabolic syndrome and cardiovascular risk factors in patients with psoriasis. In addition to treating the cutaneous manifestations of psoriasis, reducing inflammation in these patients reduces C-reactive protein and lipid peroxidation and increases high-density lipoprotein levels.6 Tumor necrosis factor α blockers decrease the risk for cardiovascular disease in patients with psoriasis.7,8 Lower than expected rates of cardiovascular disease also have been reported in a large cohort of psoriasis patients (ie, PSOLAR [Psoriasis Longitudinal Assessment and Registry] registry) being treated with either ustekinumab or tumor necrosis factor α blockers.9
Psoriatic arthritis is a chronic inflammatory disease in which active inflammation results in progressive joint destruction.10 Tumor necrosis factor α inhibitors suppress disease progression, preserve function, and delay destruction of the joints. Ustekinumab also helps control psoriatic arthritis and inhibits radiographic progression of joint disease.11
Importantly, untreated moderate to severe psoriasis is associated with several comorbidities that may lead to early death such as heart attacks and strokes.12 Furthermore, patients not taking biologic medications may have higher death rates than patients taking biologic medications.9 Psoriasis also is associated with tremendous suffering and negative psychosocial effects. The mental and physical impact of the disease is comparable to other major medical conditions (eg, cancer, arthritis, hypertension, heart disease, diabetes, depression).13 Patients also may experience physical discomfort from pain and itching.14 Children with psoriasis may experience bullying, which is associated with an increased number of depressive episodes, thereby increasing their risk for developing psychiatric conditions such as depression and anxiety as adults.15 The stigma associated with psoriasis may affect patients’ ability to build relationships. Patients with psoriasis experience higher divorce rates than patients with other chronic medical conditions, and direct involvement of genital regions may negatively impact patients’ sex lives. Patients have noted that the stigma of psoriasis also is associated with the inability to obtain employment.15 Almost one-third of patients with psoriasis who are either not working or are retired base their work status on their skin condition.16 Furthermore, psoriasis may contribute to economic burden for patients due to indirect costs associated with work absenteeism.17
Adequate treatment of psoriasis improves patients’ physical and psychological health as well as their ability to function in the workplace. However, despite the benefits of treatment, 30% of patients with severe psoriasis and 53% of patients with moderate psoriasis receive no treatment or only topical medications instead of systemic therapies.16 The potential adverse events of inadequate psoriasis treatment far outweigh any potential benefits of withholding treatment. Perhaps a boxed warning should be issued for inadequate treatment of psoriasis patients.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: warning and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. US Food and Drug Administration website. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. Published October 6, 2011. Accessed August 10, 2016.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Rose S, Sheth NH, Baker JF, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273-278.
- Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148-1153.
- Lee MK, Kim HS, Cho EB, et al. A study of awareness and screening behavior of cardiovascular risk factors in patients with psoriasis and dermatologists. Ann Dermatol. 2015;27:59-65.
- Voiculescu VM, Lupu M, Papagheorghe L, et al. Psoriasis and metabolic syndrome—scientific evidence and therapeutic implications. J Med Life. 2014;7:468-471.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular comorbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Chimenti MS, Graceffa D, Perricone R. Anti-TNFα discontinuation in rheumatoid and psoriatic arthritis: is it possible after disease remission [published online Apr 21, 2011]? Autoimmun Rev. 2011;10:636-640.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000-1006.
- Pietrzak A, Bartosinska J, Blaszczyk R, et al. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401-407.
- Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
- Garshick MK, Kimball AB. Psoriasis and the life cycle of persistent life effects. Dermatol Clin. 2015;33:25-39.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. August 17, 2014;20. pii:13030/qt48r4w8h2.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry: warning and precautions, contraindications, and boxed warning sections of labeling for human prescription drug and biological products—content and format. US Food and Drug Administration website. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm075096.pdf. Published October 6, 2011. Accessed August 10, 2016.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Rose S, Sheth NH, Baker JF, et al. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273-278.
- Shlyankevich J, Mehta NN, Krueger JG, et al. Accumulating evidence for the association and shared pathogenic mechanisms between psoriasis and cardiovascular-related comorbidities. Am J Med. 2014;127:1148-1153.
- Lee MK, Kim HS, Cho EB, et al. A study of awareness and screening behavior of cardiovascular risk factors in patients with psoriasis and dermatologists. Ann Dermatol. 2015;27:59-65.
- Voiculescu VM, Lupu M, Papagheorghe L, et al. Psoriasis and metabolic syndrome—scientific evidence and therapeutic implications. J Med Life. 2014;7:468-471.
- Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
- Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular comorbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Chimenti MS, Graceffa D, Perricone R. Anti-TNFα discontinuation in rheumatoid and psoriatic arthritis: is it possible after disease remission [published online Apr 21, 2011]? Autoimmun Rev. 2011;10:636-640.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73:1000-1006.
- Pietrzak A, Bartosinska J, Blaszczyk R, et al. Increased serum level of N-terminal Pro-B-type natriuretic peptide as a possible biomarker of cardiovascular risk in psoriatic patients. J Eur Acad Dermatol Venereol. 2015;29:1010-1014.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3, pt 1):401-407.
- Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
- Garshick MK, Kimball AB. Psoriasis and the life cycle of persistent life effects. Dermatol Clin. 2015;33:25-39.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. August 17, 2014;20. pii:13030/qt48r4w8h2.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
Some psoriasis patients benefit from switching anti-TNF agents
Psoriasis patients may have more success with a second tumor necrosis factor (TNF) antagonist after failure with a first, report Paul S. Yamauchi, MD, PhD, and coauthors.
Investigators analyzed 15 studies evaluating the efficacy of switching TNF antagonists after primary or secondary failure. Response rates at 24 weeks for a second antagonist were 30%-74% for a 75% improvement in Psoriasis Area and Severity Index score, and 20%-70% for achieving a Physician Global Assessment score of 0/1. Mean improvements in Dermatology Life Quality Index ranged from –3.5 to –13, Dr. Yamauchi and colleagues reported.
Patients who experienced secondary failure with initial treatment generally achieved better responses than those with primary failure, the authors said.
Though response rates to a second anti-TNF agent were lower than for a first, “a substantial proportion of patients in every study achieved treatment success,” they added.
Read the full article in the Journal of the American Academy of Dermatology.
Psoriasis patients may have more success with a second tumor necrosis factor (TNF) antagonist after failure with a first, report Paul S. Yamauchi, MD, PhD, and coauthors.
Investigators analyzed 15 studies evaluating the efficacy of switching TNF antagonists after primary or secondary failure. Response rates at 24 weeks for a second antagonist were 30%-74% for a 75% improvement in Psoriasis Area and Severity Index score, and 20%-70% for achieving a Physician Global Assessment score of 0/1. Mean improvements in Dermatology Life Quality Index ranged from –3.5 to –13, Dr. Yamauchi and colleagues reported.
Patients who experienced secondary failure with initial treatment generally achieved better responses than those with primary failure, the authors said.
Though response rates to a second anti-TNF agent were lower than for a first, “a substantial proportion of patients in every study achieved treatment success,” they added.
Read the full article in the Journal of the American Academy of Dermatology.
Psoriasis patients may have more success with a second tumor necrosis factor (TNF) antagonist after failure with a first, report Paul S. Yamauchi, MD, PhD, and coauthors.
Investigators analyzed 15 studies evaluating the efficacy of switching TNF antagonists after primary or secondary failure. Response rates at 24 weeks for a second antagonist were 30%-74% for a 75% improvement in Psoriasis Area and Severity Index score, and 20%-70% for achieving a Physician Global Assessment score of 0/1. Mean improvements in Dermatology Life Quality Index ranged from –3.5 to –13, Dr. Yamauchi and colleagues reported.
Patients who experienced secondary failure with initial treatment generally achieved better responses than those with primary failure, the authors said.
Though response rates to a second anti-TNF agent were lower than for a first, “a substantial proportion of patients in every study achieved treatment success,” they added.
Read the full article in the Journal of the American Academy of Dermatology.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Biosimilar version of etanercept gains FDA approval
A biosimilar of etanercept received clearance for marketing from the Food and Drug Administration on Aug. 30 for all of the inflammatory disease indications held by the reference originator etanercept product, Enbrel, according to an announcement from the agency.
Approval for all of Enbrel’s indications – rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis – was initially met with skepticism by members of the agency’s Arthritis Advisory Committee at a meeting in July because the biosimilar was compared against Enbrel in patients with plaque psoriasis only, but eventually all panel members voted to recommend approval.
The approval allows the biosimilar etanercept, called etanercept-szzs, to be marketed as a biosimilar only, not as an interchangeable product. The FDA has not yet developed guidance for manufacturers to follow to get approval for interchangeability, which means that a biosimilar “may be substituted for the reference product by a pharmacist without the intervention of the health care provider who prescribed the reference product,” according to the agency.
“We carefully evaluate the structural and functional characteristics of these complex molecules. Patients and providers can have confidence that there are no clinically meaningful differences in safety and efficacy from the reference product,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the agency’s announcement.
Etanercept-szzs will be marketed by Sandoz as Erelzi. Erelzi’s prescribing information can be found here. The biosimilar is currently undergoing review with the European Medicines Agency.
A biosimilar of etanercept received clearance for marketing from the Food and Drug Administration on Aug. 30 for all of the inflammatory disease indications held by the reference originator etanercept product, Enbrel, according to an announcement from the agency.
Approval for all of Enbrel’s indications – rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis – was initially met with skepticism by members of the agency’s Arthritis Advisory Committee at a meeting in July because the biosimilar was compared against Enbrel in patients with plaque psoriasis only, but eventually all panel members voted to recommend approval.
The approval allows the biosimilar etanercept, called etanercept-szzs, to be marketed as a biosimilar only, not as an interchangeable product. The FDA has not yet developed guidance for manufacturers to follow to get approval for interchangeability, which means that a biosimilar “may be substituted for the reference product by a pharmacist without the intervention of the health care provider who prescribed the reference product,” according to the agency.
“We carefully evaluate the structural and functional characteristics of these complex molecules. Patients and providers can have confidence that there are no clinically meaningful differences in safety and efficacy from the reference product,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the agency’s announcement.
Etanercept-szzs will be marketed by Sandoz as Erelzi. Erelzi’s prescribing information can be found here. The biosimilar is currently undergoing review with the European Medicines Agency.
A biosimilar of etanercept received clearance for marketing from the Food and Drug Administration on Aug. 30 for all of the inflammatory disease indications held by the reference originator etanercept product, Enbrel, according to an announcement from the agency.
Approval for all of Enbrel’s indications – rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and polyarticular juvenile idiopathic arthritis – was initially met with skepticism by members of the agency’s Arthritis Advisory Committee at a meeting in July because the biosimilar was compared against Enbrel in patients with plaque psoriasis only, but eventually all panel members voted to recommend approval.
The approval allows the biosimilar etanercept, called etanercept-szzs, to be marketed as a biosimilar only, not as an interchangeable product. The FDA has not yet developed guidance for manufacturers to follow to get approval for interchangeability, which means that a biosimilar “may be substituted for the reference product by a pharmacist without the intervention of the health care provider who prescribed the reference product,” according to the agency.
“We carefully evaluate the structural and functional characteristics of these complex molecules. Patients and providers can have confidence that there are no clinically meaningful differences in safety and efficacy from the reference product,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the agency’s announcement.
Etanercept-szzs will be marketed by Sandoz as Erelzi. Erelzi’s prescribing information can be found here. The biosimilar is currently undergoing review with the European Medicines Agency.
Ixekizumab improved psoriatic arthritis in patients who had not taken biologics
Two different doses of the humanized monoclonal antibody ixekizumab improved signs and symptoms of active psoriatic arthritis in a phase III manufacturer-sponsored trial of patients who had not taken a biologic drug before.
The agent selectively binds and neutralizes interleukin (IL)-17A, which promotes joint inflammation and damage via several mechanisms. So the study findings support the view that IL-17A is a key cytokine in the pathogenesis of psoriatic arthritis and an appropriate therapeutic target, said Philip J. Mease, MD, of the department of rheumatology at Swedish Medical Center and the University of Washington, Seattle, and his associates.
They are performing the ongoing, 3-year, randomized, double-blind trial (SPIRIT-P1) comparing responses with an 80-mg dose of ixekizumab every 2 weeks (103 patients), an 80-mg dose every 4 weeks (107 patients), a 40-mg dose of adalimumab (Humira) every 2 weeks (101 patients, active control group), and matching placebo (106 patients, placebo-control group). Each of the two ixekizumab arms received a starting dose of 160 mg given as two injections at week 0. This report presented the findings after the initial 24-week, double-blind treatment period of the trial.
The study participants are adults with active psoriatic arthritis who had never been treated with biologic agents and who continued taking their usual doses of conventional disease-modifying antirheumatic drugs, oral corticosteroids, opiates, and/or nonsteroidal anti-inflammatory drugs/Cox-2 inhibitors during the study. The mean patient age was 49.5 years. Of the 382 who completed this portion of the study, 57 showed an inadequate response and required rescue medication, including 10 on the lower dose of ixekizumab, 11 on the higher dose of ixekizumab, 9 taking adalimumab, and 27 taking placebo.
The primary efficacy endpoint, ACR20 response at week 24, was met by 62.1% of the higher-dose ixekizumab group, 57.9% of the lower-dose ixekizumab group, and 57.4% of the adalimumab group, all of which were significantly greater than the 30.2% rate in the placebo group. Both doses of the study drug as well as the active control drug also improved secondary endpoints: reducing mean levels of disease activity as measured by the 28-joint Disease Activity Score using on C-reactive protein, improving patient-reported physical function on the Health Assessment Questionnaire–Disability Index, and improving disease-related physical health as measured by the SF-36, the investigators said (Ann Rheum Dis. 2016 Aug 23. doi: 10.1136/annrheumdis-2016-209709).
In addition, the progression of structural joint damage, as assessed on radiographs of bone erosions and joint-space narrowing in the hands and feet, was significantly less with the three active treatments than with placebo. Among patients with the most extensive disease, a significantly greater percentage achieved Psoriasis Area and Severity Index 75 level of improvement with the three active treatments than with placebo. And among patients with nail involvement, mean improvements in Nail Psoriasis Severity Index scores were significantly higher with the three active treatments than with placebo.
Adverse effects included grade 1 and 2 neutropenia, herpes zoster involving the eyelid, gastroenteritis, esophageal candidiasis, and depression-related symptoms. All infections resolved with treatment, and none required discontinuation of the study drug.
This study was funded and sponsored by Eli Lilly, maker of ixekizumab. Dr. Mease reported receiving grants, personal fees, and other support from Eli Lilly, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Pfizer, UCB Pharma, Merck, Novartis, and Corrona. His associates reported ties to numerous industry sources.
Two different doses of the humanized monoclonal antibody ixekizumab improved signs and symptoms of active psoriatic arthritis in a phase III manufacturer-sponsored trial of patients who had not taken a biologic drug before.
The agent selectively binds and neutralizes interleukin (IL)-17A, which promotes joint inflammation and damage via several mechanisms. So the study findings support the view that IL-17A is a key cytokine in the pathogenesis of psoriatic arthritis and an appropriate therapeutic target, said Philip J. Mease, MD, of the department of rheumatology at Swedish Medical Center and the University of Washington, Seattle, and his associates.
They are performing the ongoing, 3-year, randomized, double-blind trial (SPIRIT-P1) comparing responses with an 80-mg dose of ixekizumab every 2 weeks (103 patients), an 80-mg dose every 4 weeks (107 patients), a 40-mg dose of adalimumab (Humira) every 2 weeks (101 patients, active control group), and matching placebo (106 patients, placebo-control group). Each of the two ixekizumab arms received a starting dose of 160 mg given as two injections at week 0. This report presented the findings after the initial 24-week, double-blind treatment period of the trial.
The study participants are adults with active psoriatic arthritis who had never been treated with biologic agents and who continued taking their usual doses of conventional disease-modifying antirheumatic drugs, oral corticosteroids, opiates, and/or nonsteroidal anti-inflammatory drugs/Cox-2 inhibitors during the study. The mean patient age was 49.5 years. Of the 382 who completed this portion of the study, 57 showed an inadequate response and required rescue medication, including 10 on the lower dose of ixekizumab, 11 on the higher dose of ixekizumab, 9 taking adalimumab, and 27 taking placebo.
The primary efficacy endpoint, ACR20 response at week 24, was met by 62.1% of the higher-dose ixekizumab group, 57.9% of the lower-dose ixekizumab group, and 57.4% of the adalimumab group, all of which were significantly greater than the 30.2% rate in the placebo group. Both doses of the study drug as well as the active control drug also improved secondary endpoints: reducing mean levels of disease activity as measured by the 28-joint Disease Activity Score using on C-reactive protein, improving patient-reported physical function on the Health Assessment Questionnaire–Disability Index, and improving disease-related physical health as measured by the SF-36, the investigators said (Ann Rheum Dis. 2016 Aug 23. doi: 10.1136/annrheumdis-2016-209709).
In addition, the progression of structural joint damage, as assessed on radiographs of bone erosions and joint-space narrowing in the hands and feet, was significantly less with the three active treatments than with placebo. Among patients with the most extensive disease, a significantly greater percentage achieved Psoriasis Area and Severity Index 75 level of improvement with the three active treatments than with placebo. And among patients with nail involvement, mean improvements in Nail Psoriasis Severity Index scores were significantly higher with the three active treatments than with placebo.
Adverse effects included grade 1 and 2 neutropenia, herpes zoster involving the eyelid, gastroenteritis, esophageal candidiasis, and depression-related symptoms. All infections resolved with treatment, and none required discontinuation of the study drug.
This study was funded and sponsored by Eli Lilly, maker of ixekizumab. Dr. Mease reported receiving grants, personal fees, and other support from Eli Lilly, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Pfizer, UCB Pharma, Merck, Novartis, and Corrona. His associates reported ties to numerous industry sources.
Two different doses of the humanized monoclonal antibody ixekizumab improved signs and symptoms of active psoriatic arthritis in a phase III manufacturer-sponsored trial of patients who had not taken a biologic drug before.
The agent selectively binds and neutralizes interleukin (IL)-17A, which promotes joint inflammation and damage via several mechanisms. So the study findings support the view that IL-17A is a key cytokine in the pathogenesis of psoriatic arthritis and an appropriate therapeutic target, said Philip J. Mease, MD, of the department of rheumatology at Swedish Medical Center and the University of Washington, Seattle, and his associates.
They are performing the ongoing, 3-year, randomized, double-blind trial (SPIRIT-P1) comparing responses with an 80-mg dose of ixekizumab every 2 weeks (103 patients), an 80-mg dose every 4 weeks (107 patients), a 40-mg dose of adalimumab (Humira) every 2 weeks (101 patients, active control group), and matching placebo (106 patients, placebo-control group). Each of the two ixekizumab arms received a starting dose of 160 mg given as two injections at week 0. This report presented the findings after the initial 24-week, double-blind treatment period of the trial.
The study participants are adults with active psoriatic arthritis who had never been treated with biologic agents and who continued taking their usual doses of conventional disease-modifying antirheumatic drugs, oral corticosteroids, opiates, and/or nonsteroidal anti-inflammatory drugs/Cox-2 inhibitors during the study. The mean patient age was 49.5 years. Of the 382 who completed this portion of the study, 57 showed an inadequate response and required rescue medication, including 10 on the lower dose of ixekizumab, 11 on the higher dose of ixekizumab, 9 taking adalimumab, and 27 taking placebo.
The primary efficacy endpoint, ACR20 response at week 24, was met by 62.1% of the higher-dose ixekizumab group, 57.9% of the lower-dose ixekizumab group, and 57.4% of the adalimumab group, all of which were significantly greater than the 30.2% rate in the placebo group. Both doses of the study drug as well as the active control drug also improved secondary endpoints: reducing mean levels of disease activity as measured by the 28-joint Disease Activity Score using on C-reactive protein, improving patient-reported physical function on the Health Assessment Questionnaire–Disability Index, and improving disease-related physical health as measured by the SF-36, the investigators said (Ann Rheum Dis. 2016 Aug 23. doi: 10.1136/annrheumdis-2016-209709).
In addition, the progression of structural joint damage, as assessed on radiographs of bone erosions and joint-space narrowing in the hands and feet, was significantly less with the three active treatments than with placebo. Among patients with the most extensive disease, a significantly greater percentage achieved Psoriasis Area and Severity Index 75 level of improvement with the three active treatments than with placebo. And among patients with nail involvement, mean improvements in Nail Psoriasis Severity Index scores were significantly higher with the three active treatments than with placebo.
Adverse effects included grade 1 and 2 neutropenia, herpes zoster involving the eyelid, gastroenteritis, esophageal candidiasis, and depression-related symptoms. All infections resolved with treatment, and none required discontinuation of the study drug.
This study was funded and sponsored by Eli Lilly, maker of ixekizumab. Dr. Mease reported receiving grants, personal fees, and other support from Eli Lilly, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Pfizer, UCB Pharma, Merck, Novartis, and Corrona. His associates reported ties to numerous industry sources.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Ixekizumab improved signs and symptoms of active psoriatic arthritis in a phase III manufacturer-sponsored trial of patients who had not taken biologics before.
Major finding: The primary endpoint, ACR20 response at week 24, was met by 62.1% of the lower-dose ixekizumab group, 57.9% of the higher-dose ixekizumab group, and 57.4% of the adalimumab group, which was significantly greater than the 30.2% rate in the placebo group.
Data source: A randomized, double-blind, placebo- and active treatment-controlled clinical trial involving 417 adults naive to biologic therapy.
Disclosures: This study was funded and sponsored by Eli Lilly, maker of ixekizumab. Dr. Mease reported receiving grants, personal fees, and other support from Eli Lilly, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Pfizer, UCB Pharma, Merck, Novartis, and Corrona. His associates reported ties to numerous industry sources.