User login
FDA: Etanercept first biologic approved for pediatric psoriasis
Etanercept has been received Food and Drug Administration approval for treating chronic moderate to severe plaque psoriasis in children and adolescents, aged 4-17 years, making this the first biologic and first systemic treatment approved in the United States for pediatric psoriasis.
Etanercept, a tumor necrosis factor blocker marketed as Enbrel, was approved in 1998 for treating moderately to severely active rheumatoid arthritis and has been approved for several other indications since then, including psoriatic arthritis and moderate to severe psoriasis in adults, and polyarticular juvenile idiopathic arthritis in patients aged 2 years and older.
Etanercept has been received Food and Drug Administration approval for treating chronic moderate to severe plaque psoriasis in children and adolescents, aged 4-17 years, making this the first biologic and first systemic treatment approved in the United States for pediatric psoriasis.
Etanercept, a tumor necrosis factor blocker marketed as Enbrel, was approved in 1998 for treating moderately to severely active rheumatoid arthritis and has been approved for several other indications since then, including psoriatic arthritis and moderate to severe psoriasis in adults, and polyarticular juvenile idiopathic arthritis in patients aged 2 years and older.
Etanercept has been received Food and Drug Administration approval for treating chronic moderate to severe plaque psoriasis in children and adolescents, aged 4-17 years, making this the first biologic and first systemic treatment approved in the United States for pediatric psoriasis.
Etanercept, a tumor necrosis factor blocker marketed as Enbrel, was approved in 1998 for treating moderately to severely active rheumatoid arthritis and has been approved for several other indications since then, including psoriatic arthritis and moderate to severe psoriasis in adults, and polyarticular juvenile idiopathic arthritis in patients aged 2 years and older.
Close monitoring of psoriasis patients can delay PsA onset
NEWPORT BEACH, CALIF. – A patient with psoriasis can develop crippling psoriatic arthritis (PsA) within 5 to 10 years of diagnosis, but monitoring patients for signs of trouble can help prevent the onset of PsA, according to Alan Menter, MD.
Even a simple foot examination can make a huge difference, noted Dr. Menter, chief of the division of dermatology and director of the Psoriasis Research Institute at Baylor University Medical Center, Dallas. “At every visit, you and I should be looking for early signs of joint disease,” he said at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar. “We should not let these patients develop any joint disease because we have drugs that can prevent joint destruction.”
Dr. Menter pointed out that PsA is a disease that is distinct from psoriasis. “It’s linked to psoriasis, but genetically, there are differences,” he said, “and immunologically, what goes on in skin is not identical.”
He provided the following pearls regarding diagnosing PsA:
• Be on the lookout for “sausage fingers” and “sausage toes,” both signs of PsA. “You and I are very visual people, and we can see a swollen toe or finger very easily,” Dr. Menter said. “I take the shoes off every psoriasis patient at every visit and run my thumb and index finger down the Achilles. I look for a swollen Achilles – classic enthesitis.” In some cases, swollen big toes in psoriasis patients may be misdiagnosed as gout instead of PsA, he noted.
• Ask patients about how their joints feel when they wake up in the morning: Do they have swelling and tenderness? “That’s an early marker of psoriatic arthritis disease,” Dr. Menter said. In contrast, in a patient with osteoarthritis, “the more they use their joints, the worse it gets.”
• The severity of psoriasis has nothing to do with the severity of PsA. “You can have 50% of the body covered with psoriasis but no arthritis,” he said. “Or you can have someone with one patch of psoriasis on the scalp with devastating joint disease.”
• Be aware that there are five PsA subtypes that can occur in combination with each other:
1. Dactylitis. This is the form that causes the “sausage digit.”
2. Asymmetric oligoarthritis. This is the type most commonly seen on presentation, when there are few joints affected.
3. Symmetric arthritis. This form is more common in females and difficult to differentiate from rheumatoid arthritis.
4. Distal interphalangeal joint arthritis. This type is often linked to dactylitis and nail dystrophy.
5. Arthritis mutilans. This is more common in females, linked to long disease duration, and present in an estimated 5% of cases.
Dr. Menter suggested that dermatologists refer suspected cases of PsA to a rheumatologist. Since patients may have to wait 6-10 weeks for an appointment, he recommended that dermatologists consider NSAIDs, such as the over-the-counter naproxen and prescription meloxicam and celecoxib in the meantime. Dermatologists may also consider bringing up the use of methotrexate and biologics, he said.
Dr. Menter disclosed relationships with multiple pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by Frontline Medical Communications.
NEWPORT BEACH, CALIF. – A patient with psoriasis can develop crippling psoriatic arthritis (PsA) within 5 to 10 years of diagnosis, but monitoring patients for signs of trouble can help prevent the onset of PsA, according to Alan Menter, MD.
Even a simple foot examination can make a huge difference, noted Dr. Menter, chief of the division of dermatology and director of the Psoriasis Research Institute at Baylor University Medical Center, Dallas. “At every visit, you and I should be looking for early signs of joint disease,” he said at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar. “We should not let these patients develop any joint disease because we have drugs that can prevent joint destruction.”
Dr. Menter pointed out that PsA is a disease that is distinct from psoriasis. “It’s linked to psoriasis, but genetically, there are differences,” he said, “and immunologically, what goes on in skin is not identical.”
He provided the following pearls regarding diagnosing PsA:
• Be on the lookout for “sausage fingers” and “sausage toes,” both signs of PsA. “You and I are very visual people, and we can see a swollen toe or finger very easily,” Dr. Menter said. “I take the shoes off every psoriasis patient at every visit and run my thumb and index finger down the Achilles. I look for a swollen Achilles – classic enthesitis.” In some cases, swollen big toes in psoriasis patients may be misdiagnosed as gout instead of PsA, he noted.
• Ask patients about how their joints feel when they wake up in the morning: Do they have swelling and tenderness? “That’s an early marker of psoriatic arthritis disease,” Dr. Menter said. In contrast, in a patient with osteoarthritis, “the more they use their joints, the worse it gets.”
• The severity of psoriasis has nothing to do with the severity of PsA. “You can have 50% of the body covered with psoriasis but no arthritis,” he said. “Or you can have someone with one patch of psoriasis on the scalp with devastating joint disease.”
• Be aware that there are five PsA subtypes that can occur in combination with each other:
1. Dactylitis. This is the form that causes the “sausage digit.”
2. Asymmetric oligoarthritis. This is the type most commonly seen on presentation, when there are few joints affected.
3. Symmetric arthritis. This form is more common in females and difficult to differentiate from rheumatoid arthritis.
4. Distal interphalangeal joint arthritis. This type is often linked to dactylitis and nail dystrophy.
5. Arthritis mutilans. This is more common in females, linked to long disease duration, and present in an estimated 5% of cases.
Dr. Menter suggested that dermatologists refer suspected cases of PsA to a rheumatologist. Since patients may have to wait 6-10 weeks for an appointment, he recommended that dermatologists consider NSAIDs, such as the over-the-counter naproxen and prescription meloxicam and celecoxib in the meantime. Dermatologists may also consider bringing up the use of methotrexate and biologics, he said.
Dr. Menter disclosed relationships with multiple pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by Frontline Medical Communications.
NEWPORT BEACH, CALIF. – A patient with psoriasis can develop crippling psoriatic arthritis (PsA) within 5 to 10 years of diagnosis, but monitoring patients for signs of trouble can help prevent the onset of PsA, according to Alan Menter, MD.
Even a simple foot examination can make a huge difference, noted Dr. Menter, chief of the division of dermatology and director of the Psoriasis Research Institute at Baylor University Medical Center, Dallas. “At every visit, you and I should be looking for early signs of joint disease,” he said at the Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar. “We should not let these patients develop any joint disease because we have drugs that can prevent joint destruction.”
Dr. Menter pointed out that PsA is a disease that is distinct from psoriasis. “It’s linked to psoriasis, but genetically, there are differences,” he said, “and immunologically, what goes on in skin is not identical.”
He provided the following pearls regarding diagnosing PsA:
• Be on the lookout for “sausage fingers” and “sausage toes,” both signs of PsA. “You and I are very visual people, and we can see a swollen toe or finger very easily,” Dr. Menter said. “I take the shoes off every psoriasis patient at every visit and run my thumb and index finger down the Achilles. I look for a swollen Achilles – classic enthesitis.” In some cases, swollen big toes in psoriasis patients may be misdiagnosed as gout instead of PsA, he noted.
• Ask patients about how their joints feel when they wake up in the morning: Do they have swelling and tenderness? “That’s an early marker of psoriatic arthritis disease,” Dr. Menter said. In contrast, in a patient with osteoarthritis, “the more they use their joints, the worse it gets.”
• The severity of psoriasis has nothing to do with the severity of PsA. “You can have 50% of the body covered with psoriasis but no arthritis,” he said. “Or you can have someone with one patch of psoriasis on the scalp with devastating joint disease.”
• Be aware that there are five PsA subtypes that can occur in combination with each other:
1. Dactylitis. This is the form that causes the “sausage digit.”
2. Asymmetric oligoarthritis. This is the type most commonly seen on presentation, when there are few joints affected.
3. Symmetric arthritis. This form is more common in females and difficult to differentiate from rheumatoid arthritis.
4. Distal interphalangeal joint arthritis. This type is often linked to dactylitis and nail dystrophy.
5. Arthritis mutilans. This is more common in females, linked to long disease duration, and present in an estimated 5% of cases.
Dr. Menter suggested that dermatologists refer suspected cases of PsA to a rheumatologist. Since patients may have to wait 6-10 weeks for an appointment, he recommended that dermatologists consider NSAIDs, such as the over-the-counter naproxen and prescription meloxicam and celecoxib in the meantime. Dermatologists may also consider bringing up the use of methotrexate and biologics, he said.
Dr. Menter disclosed relationships with multiple pharmaceutical companies, including AbbVie, Allergan, Amgen, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, and Pfizer.
SDEF and this news organization are owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM SDEF WOMEN'S & PEDIATRIC DERMATOLOGY SEMINAR
PsA bone loss measurement: A surrogate for radiographic progression?
An advanced computer assisted digital x-ray radiogrammetry technique that measures bone thickness has the potential to be a surrogate marker of radiographic progression in psoriatic arthritis, according to a report in Arthritis Research & Therapy.
The method uses software called BoneXpert to sensitively differentiate between the different stages of disease manifestation affecting bone integrity. Digital x-ray radiogrammetry (DXR) with BoneXpert has a clinical advantage over standard techniques such as radiographs through its ability to be integrated into a picture archiving and communication system that allows direct image analysis and quantification of bone loss, according to the study authors, led by Alexander Pfeil, MD, of Jena (Germany) University Hospital – Friedrich Schiller University.
The researchers used the computer-assisted diagnosis software to measure the metacarpal index (MCI) and its cortical thickness score (MCI T-score) in the metacarpal bones of 104 psoriatic arthritis (PsA) patients who fulfilled the CASPAR criteria. All patients were treated either with nonsteroidal anti-inflammatory drugs or disease-modifying antirheumatic drugs (Arthritis Res Ther. 2016;18:248. doi: 10.1186/s13075-016-1145-4).
In the total PsA cohort, the MCI T-score showed a significantly reduced negative value of –1.289. “The reduced MCI T-score was clearly associated with a reduced bone mineral density of the metacarpal bones in PsA,” the investigators wrote.
For all scores, the researchers found a severity-dependent reduction for the BoneXpert parameters of MCI, MCI T-score, T, and Bone Health Index.
The strongest reductions were seen for MCI and T using the Proliferation Score (MCI: –28.3%; T: –31.9%) and the Destruction Score (MCI: –30.8%; T: –30.9%) of the Psoriatic Arthritis Ratingen Score.
A reduced MCI and T-score was directly associated with cortical thinning and the periarticular demineralization of the metacarpal bones, and highlighted a direct association with bone destruction and bone proliferation in PsA, the investigators said.
“The measurement of periarticular bone loss can be considered a complementary approach to verify PsA-related bony changes and a surrogate marker for PsA progression,” the researchers suggested.
The technique’s high reproducibility can also be used to optimize an appropriate individual therapeutic strategy, they added.
The study had no specific funding source, and the authors declared no conflicts of interest.
An advanced computer assisted digital x-ray radiogrammetry technique that measures bone thickness has the potential to be a surrogate marker of radiographic progression in psoriatic arthritis, according to a report in Arthritis Research & Therapy.
The method uses software called BoneXpert to sensitively differentiate between the different stages of disease manifestation affecting bone integrity. Digital x-ray radiogrammetry (DXR) with BoneXpert has a clinical advantage over standard techniques such as radiographs through its ability to be integrated into a picture archiving and communication system that allows direct image analysis and quantification of bone loss, according to the study authors, led by Alexander Pfeil, MD, of Jena (Germany) University Hospital – Friedrich Schiller University.
The researchers used the computer-assisted diagnosis software to measure the metacarpal index (MCI) and its cortical thickness score (MCI T-score) in the metacarpal bones of 104 psoriatic arthritis (PsA) patients who fulfilled the CASPAR criteria. All patients were treated either with nonsteroidal anti-inflammatory drugs or disease-modifying antirheumatic drugs (Arthritis Res Ther. 2016;18:248. doi: 10.1186/s13075-016-1145-4).
In the total PsA cohort, the MCI T-score showed a significantly reduced negative value of –1.289. “The reduced MCI T-score was clearly associated with a reduced bone mineral density of the metacarpal bones in PsA,” the investigators wrote.
For all scores, the researchers found a severity-dependent reduction for the BoneXpert parameters of MCI, MCI T-score, T, and Bone Health Index.
The strongest reductions were seen for MCI and T using the Proliferation Score (MCI: –28.3%; T: –31.9%) and the Destruction Score (MCI: –30.8%; T: –30.9%) of the Psoriatic Arthritis Ratingen Score.
A reduced MCI and T-score was directly associated with cortical thinning and the periarticular demineralization of the metacarpal bones, and highlighted a direct association with bone destruction and bone proliferation in PsA, the investigators said.
“The measurement of periarticular bone loss can be considered a complementary approach to verify PsA-related bony changes and a surrogate marker for PsA progression,” the researchers suggested.
The technique’s high reproducibility can also be used to optimize an appropriate individual therapeutic strategy, they added.
The study had no specific funding source, and the authors declared no conflicts of interest.
An advanced computer assisted digital x-ray radiogrammetry technique that measures bone thickness has the potential to be a surrogate marker of radiographic progression in psoriatic arthritis, according to a report in Arthritis Research & Therapy.
The method uses software called BoneXpert to sensitively differentiate between the different stages of disease manifestation affecting bone integrity. Digital x-ray radiogrammetry (DXR) with BoneXpert has a clinical advantage over standard techniques such as radiographs through its ability to be integrated into a picture archiving and communication system that allows direct image analysis and quantification of bone loss, according to the study authors, led by Alexander Pfeil, MD, of Jena (Germany) University Hospital – Friedrich Schiller University.
The researchers used the computer-assisted diagnosis software to measure the metacarpal index (MCI) and its cortical thickness score (MCI T-score) in the metacarpal bones of 104 psoriatic arthritis (PsA) patients who fulfilled the CASPAR criteria. All patients were treated either with nonsteroidal anti-inflammatory drugs or disease-modifying antirheumatic drugs (Arthritis Res Ther. 2016;18:248. doi: 10.1186/s13075-016-1145-4).
In the total PsA cohort, the MCI T-score showed a significantly reduced negative value of –1.289. “The reduced MCI T-score was clearly associated with a reduced bone mineral density of the metacarpal bones in PsA,” the investigators wrote.
For all scores, the researchers found a severity-dependent reduction for the BoneXpert parameters of MCI, MCI T-score, T, and Bone Health Index.
The strongest reductions were seen for MCI and T using the Proliferation Score (MCI: –28.3%; T: –31.9%) and the Destruction Score (MCI: –30.8%; T: –30.9%) of the Psoriatic Arthritis Ratingen Score.
A reduced MCI and T-score was directly associated with cortical thinning and the periarticular demineralization of the metacarpal bones, and highlighted a direct association with bone destruction and bone proliferation in PsA, the investigators said.
“The measurement of periarticular bone loss can be considered a complementary approach to verify PsA-related bony changes and a surrogate marker for PsA progression,” the researchers suggested.
The technique’s high reproducibility can also be used to optimize an appropriate individual therapeutic strategy, they added.
The study had no specific funding source, and the authors declared no conflicts of interest.
FROM ARTHRITIS RESEARCH & THERAPY
Key clinical point:
Main finding: In the total PsA cohort, the MCI T-score showed a significantly reduced negative value of –1.289.
Data source: A cohort of 104 PsA patients fulfilling the CASPAR criteria who were taking nonsteroidal inflammatory drugs or disease-modifying antirheumatic drugs.
Disclosures: The study had no specific funding source, and the authors declared no conflicts of interest.
Erratum
Due to a submission error, the article “A Boxed Warning for Inadequate Psoriasis Treatment” (Cutis. 2016;98:206-207) did not contain the complete author disclosure information. The corrected disclosure statement appears below:
Ms. Kagha and Ms. Anderson report no conflict of interest. Dr. Blauvelt has served as a clinical study investigator and scientific adviser for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Dermira Inc; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Janssen Biotech, Inc; Merck & Co; Novartis; Pfizer Inc; Regeneron Pharmaceuticals, Inc; Sandoz, a Novartis Division; Sanofi; Sun Pharmaceutical Industries, Ltd; UCB; and Valeant Pharmaceuticals International, Inc, as well as a paid speaker for Eli Lilly and Company. Dr. Leonardi has served as an advisory board member and consultant for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Dermira Inc; Eli Lilly and Company; Janssen Biotech, Inc; LEO Pharma; Pfizer Inc; Sandoz, a Novartis Division; UCB; and Vitae Pharmaceuticals. He also has been an investigator for AbbVie Inc; Actavis Pharma, Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Coherus BioSciences; Corrona, LLC; Dermira Inc; Eli Lilly and Company; Galderma Laboratories, LP; Glenmark Pharmaceuticals Inc; Janssen Biotech, Inc; LEO Pharma; Merck & Co; Novartis; Pfizer Inc; Sandoz, a Novartis Division; Stiefel, a GSK company; and Wyeth Pharmaceuticals, Inc. Dr. Leonardi also has been on the speaker’s bureau for AbbVie Inc; Celgene Corporation; Eli Lilly and Company; and Novartis. Dr. Feldman is a consultant, researcher, and/or speaker for AbbVie Inc; Amgen, Inc; Baxter; Boehringer Ingelheim; Celgene Corporation; Janssen Biotech, Inc; Merck & Co; Mylan; Novartis; Pfizer Inc; and Valeant Pharmaceuticals International, Inc.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
Due to a submission error, the article “A Boxed Warning for Inadequate Psoriasis Treatment” (Cutis. 2016;98:206-207) did not contain the complete author disclosure information. The corrected disclosure statement appears below:
Ms. Kagha and Ms. Anderson report no conflict of interest. Dr. Blauvelt has served as a clinical study investigator and scientific adviser for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Dermira Inc; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Janssen Biotech, Inc; Merck & Co; Novartis; Pfizer Inc; Regeneron Pharmaceuticals, Inc; Sandoz, a Novartis Division; Sanofi; Sun Pharmaceutical Industries, Ltd; UCB; and Valeant Pharmaceuticals International, Inc, as well as a paid speaker for Eli Lilly and Company. Dr. Leonardi has served as an advisory board member and consultant for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Dermira Inc; Eli Lilly and Company; Janssen Biotech, Inc; LEO Pharma; Pfizer Inc; Sandoz, a Novartis Division; UCB; and Vitae Pharmaceuticals. He also has been an investigator for AbbVie Inc; Actavis Pharma, Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Coherus BioSciences; Corrona, LLC; Dermira Inc; Eli Lilly and Company; Galderma Laboratories, LP; Glenmark Pharmaceuticals Inc; Janssen Biotech, Inc; LEO Pharma; Merck & Co; Novartis; Pfizer Inc; Sandoz, a Novartis Division; Stiefel, a GSK company; and Wyeth Pharmaceuticals, Inc. Dr. Leonardi also has been on the speaker’s bureau for AbbVie Inc; Celgene Corporation; Eli Lilly and Company; and Novartis. Dr. Feldman is a consultant, researcher, and/or speaker for AbbVie Inc; Amgen, Inc; Baxter; Boehringer Ingelheim; Celgene Corporation; Janssen Biotech, Inc; Merck & Co; Mylan; Novartis; Pfizer Inc; and Valeant Pharmaceuticals International, Inc.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
Due to a submission error, the article “A Boxed Warning for Inadequate Psoriasis Treatment” (Cutis. 2016;98:206-207) did not contain the complete author disclosure information. The corrected disclosure statement appears below:
Ms. Kagha and Ms. Anderson report no conflict of interest. Dr. Blauvelt has served as a clinical study investigator and scientific adviser for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Dermira Inc; Eli Lilly and Company; Genentech, Inc; GlaxoSmithKline; Janssen Biotech, Inc; Merck & Co; Novartis; Pfizer Inc; Regeneron Pharmaceuticals, Inc; Sandoz, a Novartis Division; Sanofi; Sun Pharmaceutical Industries, Ltd; UCB; and Valeant Pharmaceuticals International, Inc, as well as a paid speaker for Eli Lilly and Company. Dr. Leonardi has served as an advisory board member and consultant for AbbVie Inc; Amgen, Inc; Boehringer Ingelheim; Dermira Inc; Eli Lilly and Company; Janssen Biotech, Inc; LEO Pharma; Pfizer Inc; Sandoz, a Novartis Division; UCB; and Vitae Pharmaceuticals. He also has been an investigator for AbbVie Inc; Actavis Pharma, Inc; Amgen, Inc; Boehringer Ingelheim; Celgene Corporation; Coherus BioSciences; Corrona, LLC; Dermira Inc; Eli Lilly and Company; Galderma Laboratories, LP; Glenmark Pharmaceuticals Inc; Janssen Biotech, Inc; LEO Pharma; Merck & Co; Novartis; Pfizer Inc; Sandoz, a Novartis Division; Stiefel, a GSK company; and Wyeth Pharmaceuticals, Inc. Dr. Leonardi also has been on the speaker’s bureau for AbbVie Inc; Celgene Corporation; Eli Lilly and Company; and Novartis. Dr. Feldman is a consultant, researcher, and/or speaker for AbbVie Inc; Amgen, Inc; Baxter; Boehringer Ingelheim; Celgene Corporation; Janssen Biotech, Inc; Merck & Co; Mylan; Novartis; Pfizer Inc; and Valeant Pharmaceuticals International, Inc.
The staff of Cutis® makes every possible effort to ensure accuracy in its articles and apologizes for the mistake.
Tildrakizumab for psoriasis scores high marks in phase III
VIENNA – The investigational interleukin-23 p-19 subunit inhibitor tildrakizumab achieved PASI 90 improvement rates approaching 60% in patients with moderate to severe plaque psoriasis at week 28 of the pivotal phase III reSURFACE 1 and reSURFACE 2 trials, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Moreover, the PASI 100 rate at week 28 in the two clinical trials was 24% with tildrakizumab, a humanized monoclonal antibody, at the 100-mg dose and 30% at 200 mg.
It was at these highest efficacy thresholds that the p-19 inhibitor really separated itself from etanercept (Enbrel) in reSURFACE 2, where the two biologics went head-to-head in randomized fashion. Patients on etanercept had a PASI 90 rate at week 28 of 31%, roughly only half that of tildrakizumab at the higher dose.
Guselkumab was the other IL-23 p-19 inhibitor that was a featured attraction at the EADV congress, with 48-week outcomes presented from the 837-patient, pivotal phase III VOYAGE 1 trial. Although caution is always warranted in comparing results across clinical trials because of differences in study populations, guselkumab achieved better top-end efficacy numbers than did tildrakizumab: a PASI 90 of 73.3% at 16 weeks and 80.2% at 24 weeks, along with PASI 100 responses of 34.4% at 16 weeks and 44.4% at 24 weeks.
“I believe there will be characteristics of the new drugs beyond efficacy that will come into play when making treatment decisions: Is dosing every 8 weeks or every 12 weeks? What is the price? What is the outcome after 1 year? I think it’s too early to close the book in trying to understand what these different drugs do, but these phase III results do give us the insight that IL-23 p-19 is actually the sweet spot in psoriasis. By targeting it we are able to keep the disease under control with drugs that are very convenient to use,” Dr. Reich said.
He added that his psoriasis patients really appreciate the convenience of quarterly as opposed to more frequent dosing of biologics, and he does, too.
reSURFACE 1 is a 64-week, randomized, phase III trial conducted in the United States, Canada, and Europe in which 772 patients were randomized 2:2:1 to tildrakizumab at 100 mg or 200 mg or to placebo. reSURFACE 2 is a 64-week trial in which 1,090 patients were randomized 2:2:1:2 to tildrakizumab at 100 mg or 200 mg, placebo, or etanercept at 50 mg twice weekly for the first 12 weeks and once weekly thereafter. At week 12 in both trials, patients on placebo were rerandomized to tildrakizumab at 100 or 200 mg for the duration. Participants averaged a baseline Psoriasis Area and Severity index score of 20, a body weight of 88 kg, and disease involvement over 31% of their body surface area.
Tildrakizumab was dosed in a regimen that’s the same as for ustekinumab (Stelara), which inhibits IL-12 as well as IL-23: one subcutaneous injection at baseline, another 1 month later, and every 12 weeks thereafter.
Dr. Reich presented results of the two pivotal trials through week 28. The coprimary efficacy endpoints in both studies were the PASI 75 response and proportion of subjects with a Physician’s Global Assessment (PGA) score of 0 or 1, meaning clear or minimal disease, compared with placebo at week 12. In hindsight, he said, those were not the best endpoints to have employed.
“We have here a drug that takes a little while to get to full throttle. The primary endpoint selected here at week 12 does not show efficacy data that really separates tildrakizumab from a drug like Stelara. But at week 28 you move toward levels of PASI 90 and PASI 100 response that we really want to see,” the dermatologist said.
Combining the results of reSURFACE 1 and 2, the PASI 75 response rate at week 12 – after only two doses – was 63% in the 100-mg arm and 64% at 200 mg. But the rates kept climbing thereafter such that by week 28 the PASI 75 rates were 77% and 78%.
Fifty-seven percent of patients on tildrakizumab at 100 mg had a PGA score of 0 or 1 at week 12, as did 6% of placebo-treated controls. By week 28, 66% of patients on the lower dose of the p-19 inhibitor had a PGA of 0 or 1. Rates in patients on tildrakizumab at 200 mg were 57% and 66% at 12 and 28 weeks, respectively.
Rates of adverse events of special interest in new biologic agents, including severe infections, malignancies, and major cardiovascular events, were similarly low across all study arms.
“My feeling is that looking at week 12 and week 28 safety data is of limited value. All I can say here is that through week 28 in these two studies I don’t see a safety signal. But for me, the real insight will have to come from larger studies with longer follow-up,” Dr. Reich said.
Asked why he thinks tildrakizumab is a slow starter, with only middling efficacy at the 12-week mark before subsequently picking up steam, he said it’s probably not a matter of the wrong doses being selected for reSURFACE 1 and 2, since the outcomes with 100 and 200 mg are fairly similar. More likely, the monoclonal antibody takes a bit longer to bind to its target and neutralize it than do some of the other biologics.
“It could be that if you dosed tildrakizumab at weeks 0, 2, and 8 as induction therapy you’d hit the mark at 12 weeks,” he added.
The reSURFACE trials are funded by Sun Pharma and Merck. Dr. Reich reported having received research grants from and serving as a consultant to Merck and numerous other pharmaceutical companies interested in new treatments for inflammatory skin diseases.
VIENNA – The investigational interleukin-23 p-19 subunit inhibitor tildrakizumab achieved PASI 90 improvement rates approaching 60% in patients with moderate to severe plaque psoriasis at week 28 of the pivotal phase III reSURFACE 1 and reSURFACE 2 trials, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Moreover, the PASI 100 rate at week 28 in the two clinical trials was 24% with tildrakizumab, a humanized monoclonal antibody, at the 100-mg dose and 30% at 200 mg.
It was at these highest efficacy thresholds that the p-19 inhibitor really separated itself from etanercept (Enbrel) in reSURFACE 2, where the two biologics went head-to-head in randomized fashion. Patients on etanercept had a PASI 90 rate at week 28 of 31%, roughly only half that of tildrakizumab at the higher dose.
Guselkumab was the other IL-23 p-19 inhibitor that was a featured attraction at the EADV congress, with 48-week outcomes presented from the 837-patient, pivotal phase III VOYAGE 1 trial. Although caution is always warranted in comparing results across clinical trials because of differences in study populations, guselkumab achieved better top-end efficacy numbers than did tildrakizumab: a PASI 90 of 73.3% at 16 weeks and 80.2% at 24 weeks, along with PASI 100 responses of 34.4% at 16 weeks and 44.4% at 24 weeks.
“I believe there will be characteristics of the new drugs beyond efficacy that will come into play when making treatment decisions: Is dosing every 8 weeks or every 12 weeks? What is the price? What is the outcome after 1 year? I think it’s too early to close the book in trying to understand what these different drugs do, but these phase III results do give us the insight that IL-23 p-19 is actually the sweet spot in psoriasis. By targeting it we are able to keep the disease under control with drugs that are very convenient to use,” Dr. Reich said.
He added that his psoriasis patients really appreciate the convenience of quarterly as opposed to more frequent dosing of biologics, and he does, too.
reSURFACE 1 is a 64-week, randomized, phase III trial conducted in the United States, Canada, and Europe in which 772 patients were randomized 2:2:1 to tildrakizumab at 100 mg or 200 mg or to placebo. reSURFACE 2 is a 64-week trial in which 1,090 patients were randomized 2:2:1:2 to tildrakizumab at 100 mg or 200 mg, placebo, or etanercept at 50 mg twice weekly for the first 12 weeks and once weekly thereafter. At week 12 in both trials, patients on placebo were rerandomized to tildrakizumab at 100 or 200 mg for the duration. Participants averaged a baseline Psoriasis Area and Severity index score of 20, a body weight of 88 kg, and disease involvement over 31% of their body surface area.
Tildrakizumab was dosed in a regimen that’s the same as for ustekinumab (Stelara), which inhibits IL-12 as well as IL-23: one subcutaneous injection at baseline, another 1 month later, and every 12 weeks thereafter.
Dr. Reich presented results of the two pivotal trials through week 28. The coprimary efficacy endpoints in both studies were the PASI 75 response and proportion of subjects with a Physician’s Global Assessment (PGA) score of 0 or 1, meaning clear or minimal disease, compared with placebo at week 12. In hindsight, he said, those were not the best endpoints to have employed.
“We have here a drug that takes a little while to get to full throttle. The primary endpoint selected here at week 12 does not show efficacy data that really separates tildrakizumab from a drug like Stelara. But at week 28 you move toward levels of PASI 90 and PASI 100 response that we really want to see,” the dermatologist said.
Combining the results of reSURFACE 1 and 2, the PASI 75 response rate at week 12 – after only two doses – was 63% in the 100-mg arm and 64% at 200 mg. But the rates kept climbing thereafter such that by week 28 the PASI 75 rates were 77% and 78%.
Fifty-seven percent of patients on tildrakizumab at 100 mg had a PGA score of 0 or 1 at week 12, as did 6% of placebo-treated controls. By week 28, 66% of patients on the lower dose of the p-19 inhibitor had a PGA of 0 or 1. Rates in patients on tildrakizumab at 200 mg were 57% and 66% at 12 and 28 weeks, respectively.
Rates of adverse events of special interest in new biologic agents, including severe infections, malignancies, and major cardiovascular events, were similarly low across all study arms.
“My feeling is that looking at week 12 and week 28 safety data is of limited value. All I can say here is that through week 28 in these two studies I don’t see a safety signal. But for me, the real insight will have to come from larger studies with longer follow-up,” Dr. Reich said.
Asked why he thinks tildrakizumab is a slow starter, with only middling efficacy at the 12-week mark before subsequently picking up steam, he said it’s probably not a matter of the wrong doses being selected for reSURFACE 1 and 2, since the outcomes with 100 and 200 mg are fairly similar. More likely, the monoclonal antibody takes a bit longer to bind to its target and neutralize it than do some of the other biologics.
“It could be that if you dosed tildrakizumab at weeks 0, 2, and 8 as induction therapy you’d hit the mark at 12 weeks,” he added.
The reSURFACE trials are funded by Sun Pharma and Merck. Dr. Reich reported having received research grants from and serving as a consultant to Merck and numerous other pharmaceutical companies interested in new treatments for inflammatory skin diseases.
VIENNA – The investigational interleukin-23 p-19 subunit inhibitor tildrakizumab achieved PASI 90 improvement rates approaching 60% in patients with moderate to severe plaque psoriasis at week 28 of the pivotal phase III reSURFACE 1 and reSURFACE 2 trials, Kristian Reich, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Moreover, the PASI 100 rate at week 28 in the two clinical trials was 24% with tildrakizumab, a humanized monoclonal antibody, at the 100-mg dose and 30% at 200 mg.
It was at these highest efficacy thresholds that the p-19 inhibitor really separated itself from etanercept (Enbrel) in reSURFACE 2, where the two biologics went head-to-head in randomized fashion. Patients on etanercept had a PASI 90 rate at week 28 of 31%, roughly only half that of tildrakizumab at the higher dose.
Guselkumab was the other IL-23 p-19 inhibitor that was a featured attraction at the EADV congress, with 48-week outcomes presented from the 837-patient, pivotal phase III VOYAGE 1 trial. Although caution is always warranted in comparing results across clinical trials because of differences in study populations, guselkumab achieved better top-end efficacy numbers than did tildrakizumab: a PASI 90 of 73.3% at 16 weeks and 80.2% at 24 weeks, along with PASI 100 responses of 34.4% at 16 weeks and 44.4% at 24 weeks.
“I believe there will be characteristics of the new drugs beyond efficacy that will come into play when making treatment decisions: Is dosing every 8 weeks or every 12 weeks? What is the price? What is the outcome after 1 year? I think it’s too early to close the book in trying to understand what these different drugs do, but these phase III results do give us the insight that IL-23 p-19 is actually the sweet spot in psoriasis. By targeting it we are able to keep the disease under control with drugs that are very convenient to use,” Dr. Reich said.
He added that his psoriasis patients really appreciate the convenience of quarterly as opposed to more frequent dosing of biologics, and he does, too.
reSURFACE 1 is a 64-week, randomized, phase III trial conducted in the United States, Canada, and Europe in which 772 patients were randomized 2:2:1 to tildrakizumab at 100 mg or 200 mg or to placebo. reSURFACE 2 is a 64-week trial in which 1,090 patients were randomized 2:2:1:2 to tildrakizumab at 100 mg or 200 mg, placebo, or etanercept at 50 mg twice weekly for the first 12 weeks and once weekly thereafter. At week 12 in both trials, patients on placebo were rerandomized to tildrakizumab at 100 or 200 mg for the duration. Participants averaged a baseline Psoriasis Area and Severity index score of 20, a body weight of 88 kg, and disease involvement over 31% of their body surface area.
Tildrakizumab was dosed in a regimen that’s the same as for ustekinumab (Stelara), which inhibits IL-12 as well as IL-23: one subcutaneous injection at baseline, another 1 month later, and every 12 weeks thereafter.
Dr. Reich presented results of the two pivotal trials through week 28. The coprimary efficacy endpoints in both studies were the PASI 75 response and proportion of subjects with a Physician’s Global Assessment (PGA) score of 0 or 1, meaning clear or minimal disease, compared with placebo at week 12. In hindsight, he said, those were not the best endpoints to have employed.
“We have here a drug that takes a little while to get to full throttle. The primary endpoint selected here at week 12 does not show efficacy data that really separates tildrakizumab from a drug like Stelara. But at week 28 you move toward levels of PASI 90 and PASI 100 response that we really want to see,” the dermatologist said.
Combining the results of reSURFACE 1 and 2, the PASI 75 response rate at week 12 – after only two doses – was 63% in the 100-mg arm and 64% at 200 mg. But the rates kept climbing thereafter such that by week 28 the PASI 75 rates were 77% and 78%.
Fifty-seven percent of patients on tildrakizumab at 100 mg had a PGA score of 0 or 1 at week 12, as did 6% of placebo-treated controls. By week 28, 66% of patients on the lower dose of the p-19 inhibitor had a PGA of 0 or 1. Rates in patients on tildrakizumab at 200 mg were 57% and 66% at 12 and 28 weeks, respectively.
Rates of adverse events of special interest in new biologic agents, including severe infections, malignancies, and major cardiovascular events, were similarly low across all study arms.
“My feeling is that looking at week 12 and week 28 safety data is of limited value. All I can say here is that through week 28 in these two studies I don’t see a safety signal. But for me, the real insight will have to come from larger studies with longer follow-up,” Dr. Reich said.
Asked why he thinks tildrakizumab is a slow starter, with only middling efficacy at the 12-week mark before subsequently picking up steam, he said it’s probably not a matter of the wrong doses being selected for reSURFACE 1 and 2, since the outcomes with 100 and 200 mg are fairly similar. More likely, the monoclonal antibody takes a bit longer to bind to its target and neutralize it than do some of the other biologics.
“It could be that if you dosed tildrakizumab at weeks 0, 2, and 8 as induction therapy you’d hit the mark at 12 weeks,” he added.
The reSURFACE trials are funded by Sun Pharma and Merck. Dr. Reich reported having received research grants from and serving as a consultant to Merck and numerous other pharmaceutical companies interested in new treatments for inflammatory skin diseases.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Tildrakizumab dosed quarterly at 100 or 200 mg achieved PASI 75 response rates of 63%-64% at week 12 and 77%-78% at week 28.
Data source: reSURFACE 1 and 2: two pivotal, phase III, randomized clinical trials comprising 1,862 patients with moderate to severe plaque psoriasis.
Disclosures: The reSURFACE trials are funded by Sun Pharma and Merck. The presenter reported having received research grants from and serving as a consultant to Merck and numerous other pharmaceutical companies developing treatments for inflammatory skin diseases.
Secukinumab for psoriasis at 4 years: undiminished efficacy and safety
VIENNA – Four-year follow-up of patients on secukinumab for psoriasis shows sustained very high efficacy, with almost 100% of patients who had a Psoriasis Area and Severity Index (PASI) 90 or 100 response at 1 year maintaining it through 4 years, Robert Bissonnette, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I must warn you that my presentation will be very boring as compared to what I’ve seen earlier at this meeting, the very cutting edge phase II and phase III data being presented. My presentation doesn’t contain any surprises. However, as a clinician who is using interleukin-17A inhibition in my practice to treat psoriasis patients, that’s probably what I want,” said Dr. Bissonnette, president of Innovaderm Research in Montreal.
“This is the longest-term safety and efficacy data available to date for patients treated with an IL-17 antagonist using an approved dose,” he noted.
Dr. Bissonnette presented 4-year results in the 165 participants who took the approved regimen from the start of the study. These were patients at the serious end of the disease severity spectrum. Their mean baseline PASI score was 23.5, with 33% of their body surface area being affected. Their mean Dermatology Life Quality Index (DLQI) score was 13.1. The mean body mass index was 28.7 kg/m2. A total of 71% of subjects had previously been on systemic therapy. One-third of participants had been on other biologics.
At 1 year, 88.9% of subjects had a PASI 75 response; at 4 years, the PASI 75 rate was 88.5%. Similarly, the PASI 90 rate was 68.5% at 1 year and 66.4% after 4 years. The PASI 100 rate was 43.8% at 1 year and 43.5% at year 4.
After 1 year on secukinumab, patients showed a mean 91.1% improvement, compared with their baseline PASI score. At 4 years, the figure was 90.8%.
Bearing in mind that the average baseline DLQI score at baseline was 13.1, it’s noteworthy that after 1 year on secukinumab, 72.7% of patients had a DLQI of 0 or 1, indicating psoriasis had no impact on their life. At year 4, the rate was 70.8%, Dr. Bissonnette continued.
As an audience member observed, however, the study population decreased from 165 patients to 131 over the course of 4 years. And since this was an “as observed” analysis, outcomes were counted only in those patients still in the study. It’s accepted as a legitimate statistical method, but it casts outcomes in a particularly favorable light.
“The main reason for dropouts was for personal reasons,” Dr. Bissonnette explained in response. “Number two was lack of or loss of efficacy. Loss of efficacy over time occurred at an absolute rate of 4%-8% per year.”
Overall, adverse event rates declined over the course of 4 years of follow-up.
“This is reassuring, but I don’t think it’s evidence that adverse events actually decrease over time because of longer use of secukinumab. I think it’s probably due to something we usually see in long-term clinical trials: a phenomenon of underreporting. When patients are treated with a new agent they tend to be very, very conscientious about what’s going on with their well-being. They will report a slight sore throat, a slight congestion. But once they’ve been on treatment for a longer time they’re less likely to report those very minor adverse events,” according to the dermatologist.
The Food and Drug Administration requires clinical trialists to keep careful track of selected adverse events in studies of biologic agents. In 4 years on secukinumab, there were no cases of tuberculosis, neutropenia, major adverse cardiovascular events, or Crohn’s disease. There were two cases of ulcerative colitis in year 2; however, one involved an exacerbation of preexisting disease. Also, two patients developed cancer other than nonmelanoma skin cancer in year 2. The incidence of vulvovaginal candidiasis was 1.8% during years 1 and 2, 0.6% in year 3, and zero in year 4.
Thus, the safety profile was favorable, with no pattern of increasing adverse events with longer medication use, Dr. Bissonnette concluded.
The study was sponsored by Novartis. Dr. Bissonnette reported serving as an investigator for and consultant to Novartis and 16 other pharmaceutical companies.
VIENNA – Four-year follow-up of patients on secukinumab for psoriasis shows sustained very high efficacy, with almost 100% of patients who had a Psoriasis Area and Severity Index (PASI) 90 or 100 response at 1 year maintaining it through 4 years, Robert Bissonnette, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I must warn you that my presentation will be very boring as compared to what I’ve seen earlier at this meeting, the very cutting edge phase II and phase III data being presented. My presentation doesn’t contain any surprises. However, as a clinician who is using interleukin-17A inhibition in my practice to treat psoriasis patients, that’s probably what I want,” said Dr. Bissonnette, president of Innovaderm Research in Montreal.
“This is the longest-term safety and efficacy data available to date for patients treated with an IL-17 antagonist using an approved dose,” he noted.
Dr. Bissonnette presented 4-year results in the 165 participants who took the approved regimen from the start of the study. These were patients at the serious end of the disease severity spectrum. Their mean baseline PASI score was 23.5, with 33% of their body surface area being affected. Their mean Dermatology Life Quality Index (DLQI) score was 13.1. The mean body mass index was 28.7 kg/m2. A total of 71% of subjects had previously been on systemic therapy. One-third of participants had been on other biologics.
At 1 year, 88.9% of subjects had a PASI 75 response; at 4 years, the PASI 75 rate was 88.5%. Similarly, the PASI 90 rate was 68.5% at 1 year and 66.4% after 4 years. The PASI 100 rate was 43.8% at 1 year and 43.5% at year 4.
After 1 year on secukinumab, patients showed a mean 91.1% improvement, compared with their baseline PASI score. At 4 years, the figure was 90.8%.
Bearing in mind that the average baseline DLQI score at baseline was 13.1, it’s noteworthy that after 1 year on secukinumab, 72.7% of patients had a DLQI of 0 or 1, indicating psoriasis had no impact on their life. At year 4, the rate was 70.8%, Dr. Bissonnette continued.
As an audience member observed, however, the study population decreased from 165 patients to 131 over the course of 4 years. And since this was an “as observed” analysis, outcomes were counted only in those patients still in the study. It’s accepted as a legitimate statistical method, but it casts outcomes in a particularly favorable light.
“The main reason for dropouts was for personal reasons,” Dr. Bissonnette explained in response. “Number two was lack of or loss of efficacy. Loss of efficacy over time occurred at an absolute rate of 4%-8% per year.”
Overall, adverse event rates declined over the course of 4 years of follow-up.
“This is reassuring, but I don’t think it’s evidence that adverse events actually decrease over time because of longer use of secukinumab. I think it’s probably due to something we usually see in long-term clinical trials: a phenomenon of underreporting. When patients are treated with a new agent they tend to be very, very conscientious about what’s going on with their well-being. They will report a slight sore throat, a slight congestion. But once they’ve been on treatment for a longer time they’re less likely to report those very minor adverse events,” according to the dermatologist.
The Food and Drug Administration requires clinical trialists to keep careful track of selected adverse events in studies of biologic agents. In 4 years on secukinumab, there were no cases of tuberculosis, neutropenia, major adverse cardiovascular events, or Crohn’s disease. There were two cases of ulcerative colitis in year 2; however, one involved an exacerbation of preexisting disease. Also, two patients developed cancer other than nonmelanoma skin cancer in year 2. The incidence of vulvovaginal candidiasis was 1.8% during years 1 and 2, 0.6% in year 3, and zero in year 4.
Thus, the safety profile was favorable, with no pattern of increasing adverse events with longer medication use, Dr. Bissonnette concluded.
The study was sponsored by Novartis. Dr. Bissonnette reported serving as an investigator for and consultant to Novartis and 16 other pharmaceutical companies.
VIENNA – Four-year follow-up of patients on secukinumab for psoriasis shows sustained very high efficacy, with almost 100% of patients who had a Psoriasis Area and Severity Index (PASI) 90 or 100 response at 1 year maintaining it through 4 years, Robert Bissonnette, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“I must warn you that my presentation will be very boring as compared to what I’ve seen earlier at this meeting, the very cutting edge phase II and phase III data being presented. My presentation doesn’t contain any surprises. However, as a clinician who is using interleukin-17A inhibition in my practice to treat psoriasis patients, that’s probably what I want,” said Dr. Bissonnette, president of Innovaderm Research in Montreal.
“This is the longest-term safety and efficacy data available to date for patients treated with an IL-17 antagonist using an approved dose,” he noted.
Dr. Bissonnette presented 4-year results in the 165 participants who took the approved regimen from the start of the study. These were patients at the serious end of the disease severity spectrum. Their mean baseline PASI score was 23.5, with 33% of their body surface area being affected. Their mean Dermatology Life Quality Index (DLQI) score was 13.1. The mean body mass index was 28.7 kg/m2. A total of 71% of subjects had previously been on systemic therapy. One-third of participants had been on other biologics.
At 1 year, 88.9% of subjects had a PASI 75 response; at 4 years, the PASI 75 rate was 88.5%. Similarly, the PASI 90 rate was 68.5% at 1 year and 66.4% after 4 years. The PASI 100 rate was 43.8% at 1 year and 43.5% at year 4.
After 1 year on secukinumab, patients showed a mean 91.1% improvement, compared with their baseline PASI score. At 4 years, the figure was 90.8%.
Bearing in mind that the average baseline DLQI score at baseline was 13.1, it’s noteworthy that after 1 year on secukinumab, 72.7% of patients had a DLQI of 0 or 1, indicating psoriasis had no impact on their life. At year 4, the rate was 70.8%, Dr. Bissonnette continued.
As an audience member observed, however, the study population decreased from 165 patients to 131 over the course of 4 years. And since this was an “as observed” analysis, outcomes were counted only in those patients still in the study. It’s accepted as a legitimate statistical method, but it casts outcomes in a particularly favorable light.
“The main reason for dropouts was for personal reasons,” Dr. Bissonnette explained in response. “Number two was lack of or loss of efficacy. Loss of efficacy over time occurred at an absolute rate of 4%-8% per year.”
Overall, adverse event rates declined over the course of 4 years of follow-up.
“This is reassuring, but I don’t think it’s evidence that adverse events actually decrease over time because of longer use of secukinumab. I think it’s probably due to something we usually see in long-term clinical trials: a phenomenon of underreporting. When patients are treated with a new agent they tend to be very, very conscientious about what’s going on with their well-being. They will report a slight sore throat, a slight congestion. But once they’ve been on treatment for a longer time they’re less likely to report those very minor adverse events,” according to the dermatologist.
The Food and Drug Administration requires clinical trialists to keep careful track of selected adverse events in studies of biologic agents. In 4 years on secukinumab, there were no cases of tuberculosis, neutropenia, major adverse cardiovascular events, or Crohn’s disease. There were two cases of ulcerative colitis in year 2; however, one involved an exacerbation of preexisting disease. Also, two patients developed cancer other than nonmelanoma skin cancer in year 2. The incidence of vulvovaginal candidiasis was 1.8% during years 1 and 2, 0.6% in year 3, and zero in year 4.
Thus, the safety profile was favorable, with no pattern of increasing adverse events with longer medication use, Dr. Bissonnette concluded.
The study was sponsored by Novartis. Dr. Bissonnette reported serving as an investigator for and consultant to Novartis and 16 other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: After 1 year on secukinumab, 43.8% of psoriasis patients had a PASI 100 response. After 3 additional years on the interleukin-17A inhibitor, the rate was virtually unchanged at 43.5%.
Data source: This was analysis of 165 psoriasis patients on secukinumab at the approved dose prospectively followed for 4 years in an extension of a phase III clinical trial.
Disclosures: Novartis sponsored the study. The presenter reported serving as an investigator for and consultant to Novartis and 16 other pharmaceutical companies.
Surgical Risks From Systemic Psoriasis Therapies

I am a coauthor on a recent literature review (J Am Acad Dermatol. 2016;75:798.e7-805.e7) that addressed a common question regarding the use of systemic agents: What should a clinician do if a patient on one of these therapies has an upcoming elective surgery?
Treatment with systemic immunomodulatory agents commonly is employed in patients with moderate to severe plaque psoriasis and psoriatic arthritis. In these individuals, the concern is that surgery may carry an increased risk for infectious or surgical complications. Based on the available literature, my coauthors and I sought to create recommendations for the perioperative management of systemic immunosuppressive therapies in patients with psoriasis and psoriatic arthritis. We conducted a literature review to examine studies that addressed the use of methotrexate, cyclosporine, and biologic agents in patients undergoing surgery. A total of 46 studies were examined, nearly all retrospective studies in patients with inflammatory bowel disease and rheumatoid arthritis.
Based on level III evidence, we concluded that infliximab, adalimumab, etanercept, methotrexate, and cyclosporine can be safely continued through low-risk operations in patients with psoriasis and psoriatic arthritis. For moderate- and high-risk surgeries, a case-by-case approach should be taken based on the patient’s individual risk factors and comorbidities.
What’s the issue?
This study does not provide specific guidelines because of limited and conflicting literature. However, it does provide general guidelines that hopefully will be augmented in the future. How will you handle this situation when it arises in your practice?

I am a coauthor on a recent literature review (J Am Acad Dermatol. 2016;75:798.e7-805.e7) that addressed a common question regarding the use of systemic agents: What should a clinician do if a patient on one of these therapies has an upcoming elective surgery?
Treatment with systemic immunomodulatory agents commonly is employed in patients with moderate to severe plaque psoriasis and psoriatic arthritis. In these individuals, the concern is that surgery may carry an increased risk for infectious or surgical complications. Based on the available literature, my coauthors and I sought to create recommendations for the perioperative management of systemic immunosuppressive therapies in patients with psoriasis and psoriatic arthritis. We conducted a literature review to examine studies that addressed the use of methotrexate, cyclosporine, and biologic agents in patients undergoing surgery. A total of 46 studies were examined, nearly all retrospective studies in patients with inflammatory bowel disease and rheumatoid arthritis.
Based on level III evidence, we concluded that infliximab, adalimumab, etanercept, methotrexate, and cyclosporine can be safely continued through low-risk operations in patients with psoriasis and psoriatic arthritis. For moderate- and high-risk surgeries, a case-by-case approach should be taken based on the patient’s individual risk factors and comorbidities.
What’s the issue?
This study does not provide specific guidelines because of limited and conflicting literature. However, it does provide general guidelines that hopefully will be augmented in the future. How will you handle this situation when it arises in your practice?

I am a coauthor on a recent literature review (J Am Acad Dermatol. 2016;75:798.e7-805.e7) that addressed a common question regarding the use of systemic agents: What should a clinician do if a patient on one of these therapies has an upcoming elective surgery?
Treatment with systemic immunomodulatory agents commonly is employed in patients with moderate to severe plaque psoriasis and psoriatic arthritis. In these individuals, the concern is that surgery may carry an increased risk for infectious or surgical complications. Based on the available literature, my coauthors and I sought to create recommendations for the perioperative management of systemic immunosuppressive therapies in patients with psoriasis and psoriatic arthritis. We conducted a literature review to examine studies that addressed the use of methotrexate, cyclosporine, and biologic agents in patients undergoing surgery. A total of 46 studies were examined, nearly all retrospective studies in patients with inflammatory bowel disease and rheumatoid arthritis.
Based on level III evidence, we concluded that infliximab, adalimumab, etanercept, methotrexate, and cyclosporine can be safely continued through low-risk operations in patients with psoriasis and psoriatic arthritis. For moderate- and high-risk surgeries, a case-by-case approach should be taken based on the patient’s individual risk factors and comorbidities.
What’s the issue?
This study does not provide specific guidelines because of limited and conflicting literature. However, it does provide general guidelines that hopefully will be augmented in the future. How will you handle this situation when it arises in your practice?
Scalp Psoriasis: Weighing Treatment Options

Scalp psoriasis often is the initial presentation of psoriasis, and it can be one of the most challenging aspects of the disease. It can be difficult to treat for several reasons. First, hair can interfere with topical therapy reaching its site of action on the scalp. Second, facial skin also can be exposed to these treatments with the associated risk for adverse events. Finally, compliance often is difficult.
An evidence-based review published online on September 21 in the American Journal of Clinical Dermatology examined treatments for scalp psoriasis, including newer systemic therapies. Of 475 studies initially identified from PubMed and 845 from Embase (up to May 2016), the review included 27 clinical trials, 4 papers reporting pooled analyses of other clinical trials, 10 open-label trials, 1 case series, and 2 case reports after excluding non-English literature.
Wang and Tsai noted that few randomized controlled trials have been performed specifically in scalp psoriasis. The authors found that topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is more highly effective than either of its individual components.
The analysis also suggested that localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but they located no published randomized controlled trials specifically evaluating these agents in scalp psoriasis. Wang and Tsai also commented that biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis. They suggested that more controlled studies are needed for an evidence-based approach to scalp psoriasis.
What’s the issue?
Scalp psoriasis can be an isolated condition or may occur in association with more extensive disease. There has been increased attention to its treatment over the last several years, with several new options. What is your preferred approach to scalp psoriasis?

Scalp psoriasis often is the initial presentation of psoriasis, and it can be one of the most challenging aspects of the disease. It can be difficult to treat for several reasons. First, hair can interfere with topical therapy reaching its site of action on the scalp. Second, facial skin also can be exposed to these treatments with the associated risk for adverse events. Finally, compliance often is difficult.
An evidence-based review published online on September 21 in the American Journal of Clinical Dermatology examined treatments for scalp psoriasis, including newer systemic therapies. Of 475 studies initially identified from PubMed and 845 from Embase (up to May 2016), the review included 27 clinical trials, 4 papers reporting pooled analyses of other clinical trials, 10 open-label trials, 1 case series, and 2 case reports after excluding non-English literature.
Wang and Tsai noted that few randomized controlled trials have been performed specifically in scalp psoriasis. The authors found that topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is more highly effective than either of its individual components.
The analysis also suggested that localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but they located no published randomized controlled trials specifically evaluating these agents in scalp psoriasis. Wang and Tsai also commented that biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis. They suggested that more controlled studies are needed for an evidence-based approach to scalp psoriasis.
What’s the issue?
Scalp psoriasis can be an isolated condition or may occur in association with more extensive disease. There has been increased attention to its treatment over the last several years, with several new options. What is your preferred approach to scalp psoriasis?

Scalp psoriasis often is the initial presentation of psoriasis, and it can be one of the most challenging aspects of the disease. It can be difficult to treat for several reasons. First, hair can interfere with topical therapy reaching its site of action on the scalp. Second, facial skin also can be exposed to these treatments with the associated risk for adverse events. Finally, compliance often is difficult.
An evidence-based review published online on September 21 in the American Journal of Clinical Dermatology examined treatments for scalp psoriasis, including newer systemic therapies. Of 475 studies initially identified from PubMed and 845 from Embase (up to May 2016), the review included 27 clinical trials, 4 papers reporting pooled analyses of other clinical trials, 10 open-label trials, 1 case series, and 2 case reports after excluding non-English literature.
Wang and Tsai noted that few randomized controlled trials have been performed specifically in scalp psoriasis. The authors found that topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is more highly effective than either of its individual components.
The analysis also suggested that localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but they located no published randomized controlled trials specifically evaluating these agents in scalp psoriasis. Wang and Tsai also commented that biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis. They suggested that more controlled studies are needed for an evidence-based approach to scalp psoriasis.
What’s the issue?
Scalp psoriasis can be an isolated condition or may occur in association with more extensive disease. There has been increased attention to its treatment over the last several years, with several new options. What is your preferred approach to scalp psoriasis?
Guselkumab achieves highest-ever response rates in psoriasis
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
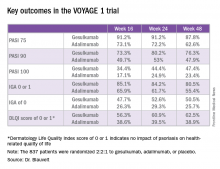
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Key clinical point:
Major finding: The PASI 90 response rate at 24 weeks was 80% in psoriasis patients on guselkumab compared with 53% in those on adalimumab.
Data source: A randomized, multinational, 48-week, pivotal phase III clinical trial involving 837 psoriasis patients assigned to guselkumab, adalimumab, or placebo.
Disclosures: The VOYAGE 1 trial was funded by Janssen, which is developing guselkumab. The study presenter reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Birth outcomes unaffected by paternal immunosuppressive therapy
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
VIENNA – The use of classic systemic immunosuppressive agents by men in the months shortly before conception was not associated with increased risk of low birthweight, preterm birth, or congenital anomalies in their offspring in a large Danish national registry.
“We didn’t see any real safety signals,” Dr. Alexander Egeberg reported at the annual congress of the European Academy of Dermatology and Venereology.
He and his coinvestigators at the University of Copenhagen decided to examine this issue for a simple reason: “We know quite a lot from registry studies about the safety of these drugs when used by women during pregnancy, but very little about the safety of paternal use,” Dr. Egeberg explained.
Methotrexate, azathioprine, and cyclosporine are often prescribed for patients with moderate to severe psoriasis and psoriatic arthritis as well as other chronic inflammatory disorders. Female patients are typically told to stop using these medications if they’re trying to become pregnant, or as soon as they think they might be pregnant, but nearly half of all pregnancies are unintended.
Using linked comprehensive national Danish databases, the investigators scrutinized the medical records of all children born in Denmark during 2004-2010, as well as those of their parents. They identified 2,235 children whose fathers had been on immunosuppressive therapy for a medical condition at any time prior to conception. There were 1,246 fathers who had been on azathioprine, 848 on methotrexate, and 141 on cyclosporine.
Rates of preterm birth, congenital anomalies, and low birthweight were compared in children born to fathers using immunosuppression and in 415,589 children born to fathers with no history of exposure to the medications. These comparisons entailed multivariate regression analyses adjusted for maternal age, parity, smoking status, and the child’s gender. Dr. Egeberg and his colleagues also compared rates of these reproductive complications in the subgroup of children whose fathers had been on the medications within 3 months prior to the estimated time of conception and in children whose fathers had stopped taking the drugs by that point.
None of the adverse neonatal outcomes were significantly increased in ever or recent paternal users of the medications under study, with one exception. Paternal use of cyclosporine within the last 3 months prior to conception was associated with an adjusted 3.7-fold increased likelihood of having a baby with a congenital anomaly. Dr. Egeberg, however, was quick to state that this finding was based on small numbers of exposures: 18 paternal exposures and four affected offspring.
“The cyclosporine finding should be interpreted quite cautiously,” he emphasized.
The reproductive outcomes study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from and serving as a consultant to Pfizer and Eli Lilly.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Adjusted rates of congenital anomalies, preterm birth, and low birthweight are not increased in children with paternal use of azathioprine, methotrexate, or cyclosporine prior to conception.
Data source: This retrospective study utilized linked Danish national registries to compare rates of low birthweight, congenital anomalies, and preterm birth in all Danish children born in 2004-2010 depending upon whether or not the father had been on methotrexate, azathioprine, or cyclosporine prior to the pregnancy.
Disclosures: The study was supported by Danish governmental research funds. Dr. Egeberg reported having received research funding from, and serving as a consultant to, Pfizer and Eli Lilly.