User login
How to Manage Psoriasis Safely in Pregnant Women
Pregnant women should avoid biologics and other systemic medications for psoriasis management, according to Jenny Eileen Murase, MD, Assistant Clinical Professor of Dermatology, University of California, San Francisco, at the 74th Annual Meeting of the American Academy of Dermatology (March 4-8, 2016) in Washington, DC. Patients should opt to use topical treatments such as moisturizers, emollients, and low- to moderate-dose corticosteroids.
Some women may experience improvement of their psoriasis during pregnancy because of an autoimmune system shift, often to the point of not needing treatment to manage the condition. “About half of pregnant women experience a dramatic improvement that may allow them to temporarily discontinue treatment,” Dr. Murase explained.
If additional therapy is needed, phototherapy may be utilized. Narrowband UVB is the best option for pregnant women but broadband UVB may be considered. Psoralen plus UVA should be avoided, as psoralen may enter breast milk and lead to light sensitivity in babies.
If the treatment regimen for the condition is discontinued or altered during pregnancy, Dr. Murase recommends that patients restart their prepregnancy regimen as soon as possible after giving birth, as the condition may flare postpregnancy.
In a recent installment of “Practical Pearls From the Cutis Board,” Jeffrey M. Weinberg, MD, discussed first-line treatments for psoriasis in pregnant women. “Pregnant patients need to know that it is important to carefully monitor them throughout their pregnancy,” he stated. “Although many drugs are not contraindicated, it is still important for the dermatologist to consult with the patient’s obstetrician to discuss risks and benefits of different therapies.”
He also indicated that if a pregnant woman does wish to continue biologic therapy, close monitoring and enrollment in a pregnancy registry (http://www.pregnancystudies.org) would be good options. “This registry is analyzing whether medications that are used to treat autoimmune diseases are safe to take during pregnancy.”
Dermatologists need to see these patients regularly to keep the dialogue ongoing and to monitor their condition.
Pregnant women should avoid biologics and other systemic medications for psoriasis management, according to Jenny Eileen Murase, MD, Assistant Clinical Professor of Dermatology, University of California, San Francisco, at the 74th Annual Meeting of the American Academy of Dermatology (March 4-8, 2016) in Washington, DC. Patients should opt to use topical treatments such as moisturizers, emollients, and low- to moderate-dose corticosteroids.
Some women may experience improvement of their psoriasis during pregnancy because of an autoimmune system shift, often to the point of not needing treatment to manage the condition. “About half of pregnant women experience a dramatic improvement that may allow them to temporarily discontinue treatment,” Dr. Murase explained.
If additional therapy is needed, phototherapy may be utilized. Narrowband UVB is the best option for pregnant women but broadband UVB may be considered. Psoralen plus UVA should be avoided, as psoralen may enter breast milk and lead to light sensitivity in babies.
If the treatment regimen for the condition is discontinued or altered during pregnancy, Dr. Murase recommends that patients restart their prepregnancy regimen as soon as possible after giving birth, as the condition may flare postpregnancy.
In a recent installment of “Practical Pearls From the Cutis Board,” Jeffrey M. Weinberg, MD, discussed first-line treatments for psoriasis in pregnant women. “Pregnant patients need to know that it is important to carefully monitor them throughout their pregnancy,” he stated. “Although many drugs are not contraindicated, it is still important for the dermatologist to consult with the patient’s obstetrician to discuss risks and benefits of different therapies.”
He also indicated that if a pregnant woman does wish to continue biologic therapy, close monitoring and enrollment in a pregnancy registry (http://www.pregnancystudies.org) would be good options. “This registry is analyzing whether medications that are used to treat autoimmune diseases are safe to take during pregnancy.”
Dermatologists need to see these patients regularly to keep the dialogue ongoing and to monitor their condition.
Pregnant women should avoid biologics and other systemic medications for psoriasis management, according to Jenny Eileen Murase, MD, Assistant Clinical Professor of Dermatology, University of California, San Francisco, at the 74th Annual Meeting of the American Academy of Dermatology (March 4-8, 2016) in Washington, DC. Patients should opt to use topical treatments such as moisturizers, emollients, and low- to moderate-dose corticosteroids.
Some women may experience improvement of their psoriasis during pregnancy because of an autoimmune system shift, often to the point of not needing treatment to manage the condition. “About half of pregnant women experience a dramatic improvement that may allow them to temporarily discontinue treatment,” Dr. Murase explained.
If additional therapy is needed, phototherapy may be utilized. Narrowband UVB is the best option for pregnant women but broadband UVB may be considered. Psoralen plus UVA should be avoided, as psoralen may enter breast milk and lead to light sensitivity in babies.
If the treatment regimen for the condition is discontinued or altered during pregnancy, Dr. Murase recommends that patients restart their prepregnancy regimen as soon as possible after giving birth, as the condition may flare postpregnancy.
In a recent installment of “Practical Pearls From the Cutis Board,” Jeffrey M. Weinberg, MD, discussed first-line treatments for psoriasis in pregnant women. “Pregnant patients need to know that it is important to carefully monitor them throughout their pregnancy,” he stated. “Although many drugs are not contraindicated, it is still important for the dermatologist to consult with the patient’s obstetrician to discuss risks and benefits of different therapies.”
He also indicated that if a pregnant woman does wish to continue biologic therapy, close monitoring and enrollment in a pregnancy registry (http://www.pregnancystudies.org) would be good options. “This registry is analyzing whether medications that are used to treat autoimmune diseases are safe to take during pregnancy.”
Dermatologists need to see these patients regularly to keep the dialogue ongoing and to monitor their condition.
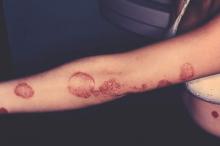
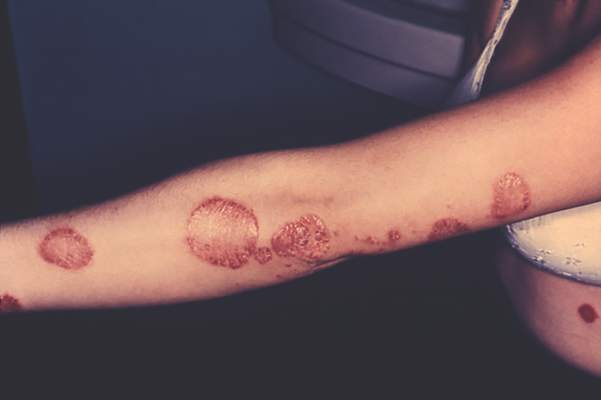
Don’t overlook topical tazarotene for psoriasis
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Tazarotene remains an important and effective albeit greatly underutilized topical therapy in psoriasis – but it’s on its way to becoming an even better drug, Dr. Linda Stein Gold said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The topical retinoid has been formulated together with the superpotent steroid halobetasol propionate 0.01% in an investigational fixed combination lotion known for now as IDP-118. This medication, under development by Valeant, is in an ongoing, phase III multicenter, double-blind randomized trial with the vehicle lotion serving as control in adults with moderate to severe plaque psoriasis. A year-long, open-label, phase III safety study is also in progress, according to Dr. Stein Gold of Henry Ford Hospital in Detroit.
Tazarotene (Tazorac) is approved by the Food and Drug Administration as a 0.05% and 0.1% cream or gel for psoriasis and in the 0.1% cream or gel for acne. But when Dr. Stein Gold asked her large Hawaii audience for a show of hands as to who is prescribing tazarotene for their psoriasis patients, not a hand went up.
“Tazarotene carries some baggage,” she observed. “It’s pregnancy category X, and it also is quite irritating. If you use tazarotene on psoriatic skin, you’ll get a lot of irritation. But if you do so in combination with a potent or superpotent topical steroid, you’re not only able to increase the efficacy, but you also minimize the tolerability issues.
These dual benefits are the result of the two treatments’ differing mechanisms of action. This has been known for a long time. Indeed, it was demonstrated in a randomized trial nearly 2 decades ago (J Am Acad Dermatol. 1998 Oct;39[4 Pt 2]:S139-43). But only with the recent appreciation that 80% of psoriasis patients treat their disease exclusively with topical therapies has a pharmaceutical company moved to take advantage of these synergistic effects.
Dr. Stein Gold, director of dermatology research at Detroit’s Henry Ford Health System, said that the pharmaceutical industry has finally noted the considerable unmet need for additional topical psoriasis therapies that will be more effective and/or cosmetically elegant or have a novel mechanism of action. A slew of novel topical agents are now in the developmental pipeline in phase II studies for psoriasis. Among these new molecules are a topical formulation of methotrexate in a proprietary vehicle; the Janus kinase (JAK) 1 and 2 inhibitor ruxolitinib (Jakafi) in a cream for treatment of both psoriasis and atopic dermatitis; a tyrosine kinase inhibitor cream and ointment; an integrin inhibitor cream, and a phosphodiesterase-4 inhibitor ointment.
In addition, in October 2015, the FDA approved an aerosol foam fixed combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% (Enstilar) for psoriasis. It’s more effective and cosmetically elegant than the fixed-combination ointment, she noted.
“Topical therapy is still going strong. I think there is always going to be a need for topical psoriasis therapies,” the dermatologist declared.
She reported serving as a consultant to and/or scientific advisory board member for numerous pharmaceutical companies.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
60 weeks of ixekizumab effective, well tolerated in moderate-to-severe plaque psoriasis
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
WASHINGTON – After 60 weeks of continuous therapy with the investigational drug ixekizumab, 722 patients who initially had moderate to severe plaque psoriasis had Psoriasis Area Severity Index (PASI) 75, 90, and 100 response rates of 87%, 78%, and 57%, respectively, based on study results presented by Dr. Andrew Blauvelt at the annual meeting of the American Academy of Dermatology.
Additionally, the rate of complete clearance or mild severity of plaque psoriasis based on the Physician Global Assessment measure was 79% in the study patients. Dr. Blauvelt said “these were tremendous results over time.”
The findings come from the open-label continuation study of ixekizumab in UNCOVER-3. At week 12 of that study, 722 ixekizumab-treated patients were continued on or converted to a regimen of 80 mg ixekizumab every 4 weeks.
In UNCOVER-3, all study participants initially received induction therapy with ixekizumab and then were randomized to placebo (193 patients), etanercept 50 mg twice weekly (382 patients), 80 mg ixekizumab every 4 weeks (386 patients) after a 160 mg starting dose, or 80 mg ixekizumab every 2 weeks (385 patients) after an initial 160 mg starting dose. After 12 weeks, patients entered open-label treatment with the study drug once every 4 weeks.
The long-term safety and tolerability profile was similar to the one seen during the induction period and cited in previously published data. Dr. Blauvelt, President of the Oregon Medical Research Center, said the finding was “good news, in that there were no unexpected safety signals [not already] seen with initial therapy.”
Ixekizumab is an IgG4 monoclonal antibody that selectively binds with interleukin 17A (IL-17A) cytokines, inhibiting interaction with the IL-17 receptor.
According to a spokesperson from Eli Lilly, sponsor of the UNCOVER trial and maker of ixekizumab, a decision from the U.S. Food and Drug Administration on whether to approve ixekizumab for use in psoriasis is expected during the first quarter of 2016.
Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
On Twitter @whitneymcknight
AT AAD 2016
Key clinical point: Continuous therapy with the investigational drug ixekizumab appears to be safe and effective for long-term use.
Major finding: At Week 60, the PASI 75, 90, and 100 response rates were 87%, 78%, and 57%, respectively.
Data source: Phase 3 UNCOVER-3 trial of 1,346 patients randomized to placebo, active control, or ixekizumab at every 4 weeks or every 2 weeks after induction.
Disclosures: This trial was sponsored by Eli Lilly, maker of ixekizumab. Dr. Blauvelt is an advisor or consultant for and has received research grants from a wide variety of drug companies including Eli Lilly.
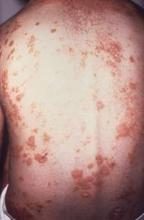
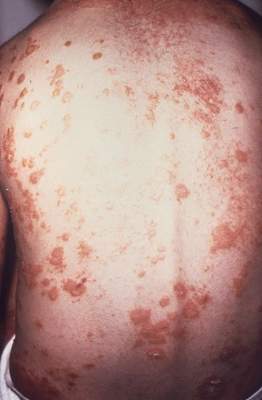
Minimal disease activity criteria in PsA fall short
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
The minimal disease activity criteria used to identify low disease activity in psoriatic arthritis (PsA) may not be useful as a treatment target in patients with extended skin involvement, according to the results of a small study.
Minimal disease activity (MDA) for PsA is a composite measure encompassing clinically important aspects of the disease: arthritis, psoriasis, enthesitis, pain, patient-assessed global disease activity, and physical function and provides an objective target for therapy in clinical trials, Dr. Josefina Marin of the Hospital Italiano de Buenos Aires and her associates wrote in the Journal of Rheumatology.
To evaluate components of the MDA criteria, the research team enrolled 83 consecutive patients with PsA aged over 18 years who were attending their clinic. Patients needed to fulfill five out of the seven criteria to be classified as having MDA.
An assessment by a single experienced rheumatologist revealed that 41 patients met MDA criteria, with only 7.4% of these patients not satisfying the tender/swollen joint–count criteria.
However, only 51% (n = 21) of patients fulfilling MDA fulfilled the skin criteria (Psoriasis Area and Severity Index [PASI] and body surface area [BSA]), a percentage not statistically different from the 36% of patients not in MDA (n = 15; P = .154). “This is important because MDA may therefore not be useful as a treatment target in patients with extended skin involvement,” the investigators wrote (J Rheum. 2016 Mar 1. doi: 10.3899/jrheum.151101).
The investigators noted that the selection of the target was of crucial importance when implementing a treat-to-target and tight control strategy. “In this sense our study provides reassurance that almost all patients at MDA will have no swollen/tender joints but also raises some concerns that the skin might not be well controlled if the MDA is used as the only target to treat patients with both joint and skin involvement,” they wrote. One explanation could be that the cutoff values selected in the MDA criteria for skin involvement are too stringent for therapies currently available, they suggested.
MDA seems to be a valuable tool to define low disease activity in patients with PsA, but the skin component requires further evaluation, they concluded.
The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point: Minimal disease activity criteria in PsA may not be stringent enough for people with extended skin involvement.
Major finding: Only half of the PsA patients classified as having minimal disease activity met PASI and body surface area criteria.
Data source: A single-center study of 83 consecutive PsA patients aged over 18 years.
Disclosures: The research was partially supported by the PANLAR/Abbvie Rheumatology prize 2012.
Effects of Tumor Necrosis Factor α Inhibitors Extend Beyond Psoriasis: Insulin Sensitivity in Psoriasis Patients With Type 2 Diabetes Mellitus
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
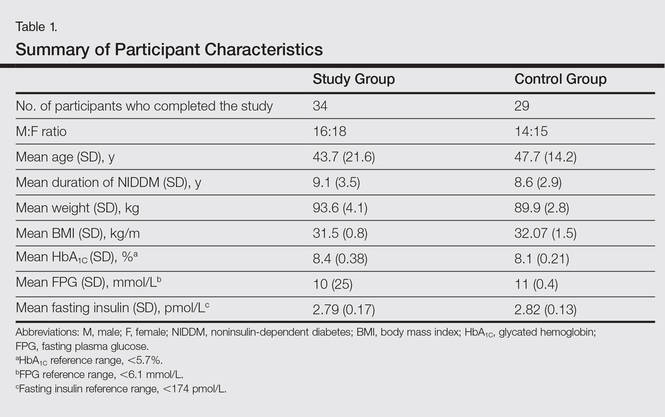
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.
A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
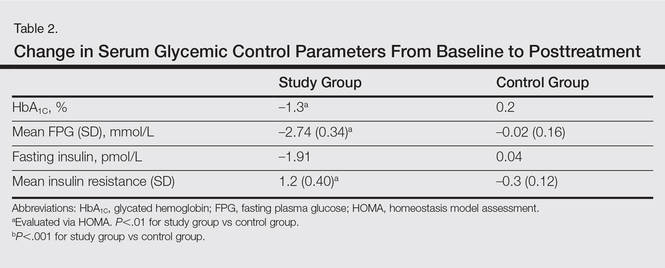
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).
Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.

A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).

Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
Psoriasis is a chronic inflammatory disorder associated with increased expression of proinflammatory mediators such as tumor necrosis factor (TNF) α.1 Anti-TNF drugs (eg, etanercept, adalimumab, infliximab) were proven to be highly effective for the treatment of psoriasis over the last 2 decades.2 Interestingly, TNF inhibitors have been thought to be effective in improving insulin resistance in patients with type 2 diabetes mellitus (DM) by blocking TNF, which is involved in the inflammatory condition in DM.
Type 2 DM is a common chronic condition characterized by hyperglycemia resulting from a combination of peripheral and hepatic insulin resistance and impaired insulin secretion.3 It is characterized by defects in both insulin secretion and insulin sensitivity.4,5 Type 2 DM has been linked with a marked increase in cardiovascular disease, morbidity, and mortality.6 Evidence-based literature regarding the role of chronic inflammation as an important pathogenetic factor in type 2 DM has been growing.7-9 It also has been suggested that pharmacological strategies to reduce this underlying associated silent inflammation are useful in treating DM, which also is true for other conditions such as obesity, metabolic syndrome, and cardiovascular diseases.10
Psoriasis predisposes patients to insulin resistance and may put them at risk for developing DM.11,12 The association between psoriasis and DM suggests that systemic immunosuppression also may diminish the risk for developing DM. Several longitudinal studies have found that TNF inhibitors improve insulin resistance.13,14 Dandona et al15 reported a considerable decrease of TNF-α levels with the concurrent restoration of insulin sensitivity during weight loss.
Pereira et al16 found a notable connection between psoriasis, DM, and insulin resistance with an odds ratio of 2.63 of abnormal glucose homeostasis in patients with psoriasis compared to controls. Yazdani-Biuki et al17 proved that extended administration of anti–TNF-α antibody was able to improve insulin sensitivity in insulin-resistant patients. The same finding was established by Kiortsis et al.14
In this prospective controlled study, we evaluated the effects of anti-TNF agents on insulin resistance and sensitivity in psoriasis patients with type 2 DM treated with anti-TNF agents.
Methods
A total of 70 patients attending the dermatological outpatient clinics at Farwaniya Hospital (Kuwait City, Kuwait) between January 2012 and September 2014 were enrolled in the study and were randomly distributed into 2 equal groups (n=35 each). The study was approved by the hospital ethics committee. Patients were included in the study if they had moderate to severe psoriasis (ie, psoriasis area severity index score ≥10) with documented type 2 DM and high fasting plasma glucose (FPG) levels (ie, >10 mmol/L). Patients who were currently being treated with oral hypoglycemic agents but not insulin therapy were included in the study. Patients were excluded if they had phototherapy within the last 4 weeks, prior biologic therapy, current and prior insulin therapy, a change in oral hypoglycemic drug dosage in the last 2 months, other serious systemic illness (eg, malignancy, hepatitis B or C virus, metabolic or endocrine disease), and/or abnormal laboratory investigations (eg, liver/kidney profile, chest radiograph abnormality, positive Mantoux test). All of the patients enrolled in the study provided informed consent and underwent routine baseline investigations including complete blood cell counts, general health profile, chest radiograph, antinuclear antibody test, Mantoux test, FPG and insulin levels, glycated hemoglobin (HbA1C), homeostasis model assessment (HOMA), routine urine examination, and enzyme-linked immunosorbent assay for tuberculosis. The study group was treated with anti-TNF agents, and the control group received conventional antipsoriatic medications.
All the patients included in the study had high FPG levels (ie, >10 mmol/L) at the time of enrollment and were currently being treated with oral hypoglycemic agents. The dose of oral hypoglycemic agents was unchanged for at least 2 months before entry into the run-in period and throughout the 24-week study period. Demographic details including sex, age, medical history (eg, type of psoriasis, prior and concomitant treatments) were collected from the participants’ clinical histories. Participants from both groups were appropriately matched in terms of age, sex, body weight, body mass index, and duration of type 2 DM (Table 1). The primary end point of the study was to analyze and compare clinical and serum data collected at baseline and after 24 weeks of therapy.

A complete biochemical profile was repeated in both groups after 24 weeks of treatment. Each participant underwent a baseline short insulin sensitivity test immediately before treatment and at 4 and 24 weeks of treatment. We assessed insulin resistance via HOMA, calculated as follows: FPG [mmol/L] × fasting serum insulin [pmol/L] / 22.5.18 Oral glucose tolerance tests were performed to calculate the HOMA of insulin resistance. Serum insulin concentration was determined via enzyme-linked immunosorbent assay.
Statistical analysis was performed using SPSS software (version 12.0). Continuous patient characteristics were analyzed using mean and SD as well as discrete data as counts and proportions. Association was examined using χ2 tests for categorical variables and 2-sided t test/Wilcoxon rank sum test for continuous variables. Analysis of variance was used to compare the results in 3 different anti-TNF agents used in the study group.
Results
Of the 35 participants enrolled in the study group, 34 (97.1%) completed the study and were evaluated. The study group included 16 men and 18 women aged 19 to 63 years (mean age [SD], 43.7 [21.6] years) who were treated with TNF-α inhibitors—8 participants with etanercept, 14 with adalimumab, and 12 with infliximab—according to the standard dosage schedule for 24 weeks.
Of the 35 participants enrolled in the control group, 29 (82.9%) completed the study and were evaluated. Six patients did not follow up for the complete duration of the 24-week study period and were not evaluated. The control group included 14 men and 15 women aged 18 to 65 years (mean age [SD], 47.7 [14.2] years) who were treated with other systemic therapies—8 participants with topical corticosteroids or calcipotriol only, 7 with cyclosporine A, and 14 with methotrexate. The dose of the drug was kept stable throughout the 24-week study period.
Demographic and baseline characteristics for all participants are shown in Table 1. There were no significant differences in demographic or baseline characteristics among the study group versus the control group, and all participants were similar in age; body mass index; as well as FPG, fasting insulin, and HbA1C levels.
At baseline, both study and control participants had elevated mean (SD) FPG levels (10 [25] mmol/L and 11 [0.4] mmol/L, respectively), fasting insulin levels (2.79 [0.17] pmol/L and 2.82 [0.13] pmol/L, respectively), and HbA1C levels (8.4% [0.38%] and 8.1% [0.21%], respectively)(Table 1).
The study group showed significant improvements in glycemic control at the end of the study (Table 2). At week 24, study group participants had a mean (SD) decrease in FPG levels of 2.74 (0.34) mmol/L versus 0.02 (0.16) mmol/L in the control group. This difference between the 2 groups after 24 weeks was found to be statistically significant (P<.01). On further analysis of the study group, no statistically significant difference (P>.01) was noted in the 3 anti-TNF agents used. Compared to the control group, the study group showed a significant decrease from baseline values of FPG and HbA1C (P<.01). Fasting insulin levels decreased significantly for study group participants as compared with control (–1.91 pmol/L vs 0.04 pmol/L)(P<.001)(Table 2). However, on analysis of the 3 anti-TNF agents, no statistically significant difference was found (P>.05). Participants in the control group showed no significant change in fasting insulin and FPG levels.
To confirm that there was a change in insulin sensitivity in response to TNF-α inhibitors, we analyzed FPG and fasting insulin values using the HOMA method. There was no change in mean relative insulin resistance in the control group in response to therapy (mean [SD], 5.4 [0.31] vs 5.6 [0.15], before vs after therapy), while there was mild improvement in relative insulin resistance in the study group (5.9 [0.52] vs 4.8 [0.34], before vs after therapy). There also was a significant difference in the change in relative insulin resistance in response to treatment between the study and control groups (1.2 [0.40] vs –0.3 [0.12]; P<.01)(Table 2).

Comment
There has been an unprecedented rise in the rate of obesity and associated metabolic diseases such as type 2 DM. Following the current trend, it is estimated that the world will have approximately 592 million cases of type 2 DM by the year 2035.19 Almost two-thirds of these patients are estimated to die of cardiovascular diseases.
Although the pathophysiology of type 2 DM is not known, insulin resistance in the muscles and liver as well as failure of pancreatic β cells represent the core of the complex pathophysiology. The associated underlying silent inflammation was thought to have a key role in both insulin resistance and insulin secretory defects seen in type 2 DM. Furthermore, recent data suggest the central role of TNF-α, IL-1, and IL-6 pathways in this inflammation.10 Tumor necrosis factor α has been shown to have a dual effect on insulin resistance as well as pancreatic β cell function. It blocks the function of insulin at the receptor level and has been implicated as a causative factor in obesity-associated insulin resistance and also in the pathogenesis of type 2 DM.20,21 Furthermore, cytokines that activate nuclear factor κβ (a nuclear transcription factor closely involved in the regulation of cellular inflammatory response), such as TNF-α, are thought to be a common denominator for β-cell apoptosis in types 1 and 2 DM.22 Additionally, it has been suggested that TNF-α is a powerful regulator of adipose tissue.23 Neutralizing TNF-α in obese Zucker rats has shown increased insulin sensitivity.3 Tumor necrosis factor α and IL-6 as well as C-reactive protein and plasminogen activator inhibitor 1 are negatively associated with insulin sensitivity.24-27 These findings have led researchers to investigate the role of anti-TNF agents for the management of type 2 DM.28
Psoriasis has now come to be known as a systemic inflammatory disorder and is associated with increased expression of TNF-α. It predisposes patients to insulin resistance and places them at higher risk for developing DM.11,12 Systematic reports recommend that there is a link between psoriasis and DM featured by helper T cell (TH1) cytokines.29 This link can stimulate insulin resistance and metabolic syndrome as well as inflammatory cytokines identified to motivate psoriasis.29,30 The association between psoriasis and type 2 DM proposes a possible pathophysiologic connection between the 2 diseases. Patients with psoriasis have altered T-cell subtype 1 pathways and dysregulated oxidative and angiogenic mechanisms.31,32 Many of these immune pathways may similarly predispose psoriasis patients to impaired glucose tolerance and DM. Inflammation may cause insulin resistance and DM through numerous mechanisms. Systemic inflammation linked with psoriasis may lead to high levels of circulating IL-1, IL-6, and TNF-α that predispose patients to impaired glucose tolerance and type 2 DM.33
Several longitudinal investigations have found that TNF inhibitors improve insulin resistance.13,14,34-38 Gonzalez-Gay et al13 confirmed a rapid beneficial effect of infliximab on insulin resistance and insulin sensitivity in rheumatoid arthritis (RA) patients, which might support the long-term use of drugs that act by blocking TNF-α to diminish the mechanisms implicated in the development of atherosclerosis in patients with RA. Kiortsis et al14 performed a complete biochemical profile before and after 6 months of treatment with infliximab in 17 patients with ankylosing spondylitis and 28 patients with RA. The researchers found a significant decrease of the HOMA index in the percentile of their patients with the highest insulin resistance (P<.01).14
Stagakis et al34 found that 12 weeks of treatment with anti-TNF agents may improve insulin resistance in patients with active RA and high insulin resistance. Treatment with anti-TNF agents was shown to restore the phosphorylation status of serine phosphorylation of insulin receptor substrate 1 (Ser312-IRS-1) and AKT (protein kinase B), which are important mediators in the insulin signaling cascade. The investigators concluded that treatment with anti-TNF agents may improve insulin resistance and sensitivity in RA patients with active disease and high insulin resistance.34
Solomon et al35 studied the link between disease-modifying antirheumatic drugs and DM risk in patients with RA and psoriasis. The authors proposed that initiation of treatment with TNF inhibitors in psoriasis patients was associated with a reduced incidence of DM. The results showed a lower risk for developing DM in patients with psoriasis who were treated with a TNF inhibitor compared with numerous other drugs.35
Marra et al36 studied the effects of etanercept on insulin sensitivity in 9 patients with psoriasis. They reported a decrease in insulin resistance evaluated by HOMA after 24 weeks of etanercept treatment.36 Wambier et al37 reported severe hypoglycemia after initiation of anti-TNF therapy with etanercept in a patient with generalized pustular psoriasis and type 2 DM.
Yazdani-Biuki et al38 reported the case of a patient who demonstrated a relapse of type 2 DM after an interruption of prolonged treatment with infliximab, an anti–TNF-α antibody for psoriatic arthritis. The improvement in insulin sensitivity of this patient has been reported along with post hoc evidence that chronic administration of infliximab improves insulin resistance in a small sample of patients with inflammatory joint diseases.17
Other studies on the effects of TNF inhibitors on insulin resistance and sensitivity have yielded conflicting results. Martínez-Abundis et al39 studied the effects of etanercept on insulin resistance and sensitivity in a randomized trial of psoriatic patients at risk for developing type 2 DM. Results indicated that anti-TNF therapy had no significant influence on insulin sensitivity measured using a hyperinsulinemic clamp during 2 weeks of etanercept treatment in psoriatic patients with risk factors for type 2 DM. The explanation of this discrepancy may be due to the short duration of the study period.
It is still unknown if psoriasis treatment affects a patient’s risk for developing DM. However, Solomon et al35 evaluated the association of incidental DM among patients with prescribed TNF inhibitors or methotrexate and proposed that initiation of treatment with TNF inhibitors was associated with a diminished incidence of DM.
Our study supports and confirms that psoriasis patients treated with TNF-α inhibitors showed improved glycemic indices and insulin resistance compared with control patients treated with other common systemic drugs for psoriasis. We did not take into consideration other conventional risk factors such as hypertension and coronary artery disease. The number of participants included in the current study was not large enough to evaluate each of the anti-TNF agents in a separate group, and participants were not followed up long enough to see the impact of the biochemical changes noted in the results on long-term morbidity or mortality.
Conclusion
Our study confirms a beneficial effect of TNF-α inhibitors on insulin resistance and insulin sensitivity in psoriasis patients with type 2 DM. Treatment with TNF-α inhibitors may have beneficial effects on insulin sensitivity in even the most insulin-resistant patients with psoriasis. The study results may support the hypothesis that long-term use of TNF inhibitors may reduce the mechanisms involved in the development of DM in patients with psoriasis. The improvement in insulin sensitivity may in turn decrease the coronary artery disease risk in these patients. Additional large, prospective, multicenter studies are required to further analyze the effects of anti–TNF-α antibodies on insulin sensitivity and β cell function in insulin-resistant or diabetic psoriasis patients.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866-873.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. a systematic review. J Rheumatol. 2006;33:1447-1451.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. a balanced overview. Diabetes Care. 1992;15:318-368.
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;4:177-269.
- Reaven GM. Banting Lecture 1988. role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
- Erkelens DW. Insulin resistance syndrome and type 2 diabetes mellitus. Am J Cardiol. 2001;88(7B):38J-42J.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91.
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278.
- Van der Poll T, Romijn JA, Endert E, et al. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol. 1991;261:457-465.
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-172.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis. 2010;69:2114-2117.
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83-86.
- Kiortsis DN, Mavridis AK, Vasakos S, et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765-766.
- Dandona P, Weinstock R, Thusu K, et al. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metabol. 1998;83:2907-2910.
- Pereira RR, Amladi ST, Varthakavi PK. A study of the prevalence of diabetes, insulin resistance, lipid abnormalities, and cardiovascular risk factors in patients with chronic plaque psoriasis. Ind J Dermatol. 2011;56:520-526.
- Yazdani-Biuki B, Stelzl H, Brezinschek HP, et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-α antibody infliximab. Eur J Clin Invest. 2004;34:641-642.
- Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders. The Bruneck Study. Diabetes. 1998;47:1643-1649.
- Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, prediabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087.
- Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169-175.
- Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212-217.
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433-1440.
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349-356.
- Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334.
- Festa A, D’Agostino R Jr, Tracy RP, et al. Insulin Resistance Atherosclerosis Study. elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137.
- Meigs JB, O’Donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537.
- Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676-1685.
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Exp Opin Invest Drugs. 2014;24:1-25.
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119.
- Karadag AS, Yavuz B, Ertugrul DT, et al. Is psoriasis a pre-atherosclerotic disease? increased insulin resistance and impaired endothelial function in patients with psoriasis. Int J Dermatol. 2010;49:642-646.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. A tale of two plaques: convergent mechanisms of T-cell–mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549.
- Armstrong AW, Voyles SV, Armstrong EJ, et al. Angiogenesis and oxidative stress: common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci. 2011;63:1-9.
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157:1249-1251.
- Stagakis I, Bertsias G, Karvounaris S, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14:R141.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Marra M, Campanati A, Testa R, et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731-736.
- Wambier CG, Foss-Freitas MC, Paschoal RS, et al. Severe hypoglycemia after initiation of anti-tumor necrosis factor therapy with etanercept in a patient with generalized pustular psoriasis and type 2 diabetes mellitus. J Am Acad Dermatol. 2009;60:883-885.
- Yazdani-Biuki B, Mueller T, Brezinschek HP, et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712.
- Martínez-Abundis E, Reynoso-von Drateln C, Hernández-Salazar E, et al. Effect of etanercept on insulin secretion and insulin sensitivity in a randomized trial with psoriatic patients at risk for developing type 2 diabetes mellitus. Arch Dermatol Res. 2007;299:461-465.
Practice Points
- Psoriasis is associated with an increased incidence of insulin resistance and type 2 diabetes mellitus (DM).
- Anti–tumor necrosis factor drugs, which are effective for the treatment of psoriasis, were found to improve insulin resistance in psoriasis patients with type 2 DM.
Brodalumab met primary endpoints, deaths called ‘unrelated’
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
The investigational interleukin-17 inhibitor brodalumab met both primary endpoints in a phase III, double-blind, placebo-controlled trial of 661 patients with moderate to severe plaque psoriasis, investigators reported in the British Journal of Dermatology.
By week 12, 83% of patients on 210 mg brodalumab achieved Psoriasis Area and Severity Index (PASI) 75, as did 60% of patients on 140 mg and 3% of placebo patients, said Dr. Kim Papp of Probity Medical Research in Waterloo, Ont., and associates. The coprimary endpoint, a static Physician’s Global Assessment (sPGA) score of 0 or 1 (clear or almost clear skin), was achieved by 76%, 54%, and 1% of patients, respectively. “Skin clearance continues to improve beyond 12 weeks, and is sustained through 1 year of therapy,” and the safety profile “was considered to be acceptable,” the researchers said.
The AMAGINE-1 trial included a 12-week induction period followed by a withdrawal-retreatment period lasting up to 52 weeks. At the end of induction, brodalumab patients who achieved sPGA 0/1 were rerandomized to placebo or to induction dose. Four weeks later, those with sPGA scores of at least 3 received another induction dose. After 12 weeks of retreatment, patients scoring sPGA 2/3 were rescued with brodalumab 210 mg every 2 weeks (Br J Dermatol. 2016 Feb 23. doi: 10.1111/bjd.14493).
The psoriasis community has closely watched brodalumab, which handily beat ustekinumab in the AMAGINE-2 psoriasis trial before Amgen abruptly pulled out of development in the wake of two suicides by trial participants. AstraZeneca and Valeant Pharmaceuticals then partnered on the agent, and the Food and Drug Administration accepted a Biologics License Application in January 2016.
Dr. Papp and associates published details of the suicides, noting that one involved a 59-year-old man who died 2 months after his last 210-mg dose. He had no history of psychiatric disorders and normal baseline Hospital Anxiety and Depression Scale scores, which worsened on placebo but normalized by week 52. “The investigator reported that there was no reasonable possibility that the event of suicide was related to investigational product,” the researchers said. The second suicide involved a 56-year-old man who also received 210 mg brodalumab. He had a history of severe depression, but his Hospital Anxiety and Depression Scale (HADS) scores were normal when last assessed, his psoriasis was well controlled, and his death was considered unrelated to treatment. Indeed, moderate or severe baseline HADS scores were more likely to improve when patients received brodalumab instead of placebo, the researchers said. The other two deaths on study involved patients with substantial cardiovascular and hepatic comorbidities and also were considered unrelated to treatment.
Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
Key clinical point: The interleukin-17 inhibitor brodalumab met its coprimary endpoints in a phase III trial of patients with moderate to severe plaque psoriasis.
Major finding: At week 12, 83% of patients on 210 mg brodalumab achieved PASI 75, as did 60% of 140-mg patients and 3% of the placebo group. The coprimary endpoint, a static Physician Global Assessment score of 0/1, was met for 76% of 210-mg patients, 54% of 140-mg patients, and 1% of placebo patients. All four deaths were considered unrelated to treatment.
Data source: AMAGINE-1, a phase III, multicenter, randomized, double-blind study of brodalumab (210 or 140 mg) or placebo in 661 patients.
Disclosures: Amgen and AstraZeneca/MedImmune funded the study, and Amgen analyzed the data. Dr. Papp reported having served as a consultant, investigator, and speaker for Amgen and numerous other pharmaceutical companies. Ten coinvestigators also reported relationships with Amgen and other pharmaceutical companies, and six coinvestigators reported current or former employment with Amgen.
Ixekizumab for plaque psoriasis improves work productivity
Ixekizumab improved work productivity among patients with moderate to severe plaque psoriasis in three manufacturer-sponsored phase III clinical trials, according to a report published online March 7 in JAMA Dermatology.
In previous studies, many adults with plaque psoriasis have reported that their skin condition forces them to miss work, reduces their productivity at work, requires them to take early retirement, and restricts them to part-time work when they would prefer to work full time. This is associated with substantial economic consequences for the patient and his or her family, for employers, and for society at large.
“A recent systematic review estimated the annual indirect economic burden of psoriasis in the United States to be $23.9 billion to $35.4 billion from productivity losses due to absenteeism and presenteeism,” said Dr. April W. Armstrong of the University of Southern California, Los Angeles, and her associates.
They assessed whether ixekizumab improved work productivity by analyzing data regarding that secondary endpoint in the three randomized double-blind UNCOVER trials, which compared the agent against either a matching placebo or etanercept in 3,866 patients with chronic plaque psoriasis involving at least 10% of their body surface area, sPGA (static Physician Global Assessment) scores of 3 or higher, and PASI (Psoriasis Area Severity Index) scores of 12 or higher. The study participants were treated for 12 weeks with either the active drug or a comparator; in two of the studies, those who responded to ixekizumab could then continue on maintenance therapy for up to a further 60 weeks.
Work productivity was measured using the WPAI-PSO (Work Productivity and Activity Impairment-Psoriasis) questionnaire, which assesses psoriasis-associated absenteeism (time missed from work), presenteeism (impairment and reduced effectiveness at work), and overall loss of work productivity.
Across all three trials, 12 weeks of ixekizumab was significantly better than placebo or etanercept was at increasing work productivity and reducing impairment at work. These benefits were sustained in patients who continued long-term treatment, while patients who discontinued treatment lost these benefits and also showed significant worsening in absenteeism, Dr. Armstrong and her associates said (JAMA Dermatol. 2016 Mar 7. doi: 10.1001/jamadermatol.2016.0269).
The improvements in work productivity paralleled improvements in patients’ PASI scores while taking ixekizumab.
Ixekizumab improved work productivity among patients with moderate to severe plaque psoriasis in three manufacturer-sponsored phase III clinical trials, according to a report published online March 7 in JAMA Dermatology.
In previous studies, many adults with plaque psoriasis have reported that their skin condition forces them to miss work, reduces their productivity at work, requires them to take early retirement, and restricts them to part-time work when they would prefer to work full time. This is associated with substantial economic consequences for the patient and his or her family, for employers, and for society at large.
“A recent systematic review estimated the annual indirect economic burden of psoriasis in the United States to be $23.9 billion to $35.4 billion from productivity losses due to absenteeism and presenteeism,” said Dr. April W. Armstrong of the University of Southern California, Los Angeles, and her associates.
They assessed whether ixekizumab improved work productivity by analyzing data regarding that secondary endpoint in the three randomized double-blind UNCOVER trials, which compared the agent against either a matching placebo or etanercept in 3,866 patients with chronic plaque psoriasis involving at least 10% of their body surface area, sPGA (static Physician Global Assessment) scores of 3 or higher, and PASI (Psoriasis Area Severity Index) scores of 12 or higher. The study participants were treated for 12 weeks with either the active drug or a comparator; in two of the studies, those who responded to ixekizumab could then continue on maintenance therapy for up to a further 60 weeks.
Work productivity was measured using the WPAI-PSO (Work Productivity and Activity Impairment-Psoriasis) questionnaire, which assesses psoriasis-associated absenteeism (time missed from work), presenteeism (impairment and reduced effectiveness at work), and overall loss of work productivity.
Across all three trials, 12 weeks of ixekizumab was significantly better than placebo or etanercept was at increasing work productivity and reducing impairment at work. These benefits were sustained in patients who continued long-term treatment, while patients who discontinued treatment lost these benefits and also showed significant worsening in absenteeism, Dr. Armstrong and her associates said (JAMA Dermatol. 2016 Mar 7. doi: 10.1001/jamadermatol.2016.0269).
The improvements in work productivity paralleled improvements in patients’ PASI scores while taking ixekizumab.
Ixekizumab improved work productivity among patients with moderate to severe plaque psoriasis in three manufacturer-sponsored phase III clinical trials, according to a report published online March 7 in JAMA Dermatology.
In previous studies, many adults with plaque psoriasis have reported that their skin condition forces them to miss work, reduces their productivity at work, requires them to take early retirement, and restricts them to part-time work when they would prefer to work full time. This is associated with substantial economic consequences for the patient and his or her family, for employers, and for society at large.
“A recent systematic review estimated the annual indirect economic burden of psoriasis in the United States to be $23.9 billion to $35.4 billion from productivity losses due to absenteeism and presenteeism,” said Dr. April W. Armstrong of the University of Southern California, Los Angeles, and her associates.
They assessed whether ixekizumab improved work productivity by analyzing data regarding that secondary endpoint in the three randomized double-blind UNCOVER trials, which compared the agent against either a matching placebo or etanercept in 3,866 patients with chronic plaque psoriasis involving at least 10% of their body surface area, sPGA (static Physician Global Assessment) scores of 3 or higher, and PASI (Psoriasis Area Severity Index) scores of 12 or higher. The study participants were treated for 12 weeks with either the active drug or a comparator; in two of the studies, those who responded to ixekizumab could then continue on maintenance therapy for up to a further 60 weeks.
Work productivity was measured using the WPAI-PSO (Work Productivity and Activity Impairment-Psoriasis) questionnaire, which assesses psoriasis-associated absenteeism (time missed from work), presenteeism (impairment and reduced effectiveness at work), and overall loss of work productivity.
Across all three trials, 12 weeks of ixekizumab was significantly better than placebo or etanercept was at increasing work productivity and reducing impairment at work. These benefits were sustained in patients who continued long-term treatment, while patients who discontinued treatment lost these benefits and also showed significant worsening in absenteeism, Dr. Armstrong and her associates said (JAMA Dermatol. 2016 Mar 7. doi: 10.1001/jamadermatol.2016.0269).
The improvements in work productivity paralleled improvements in patients’ PASI scores while taking ixekizumab.
FROM JAMA DERMATOLOGY
Key clinical point: Ixekizumab improved work productivity among patients with moderate to severe plaque psoriasis in three manufacturer-sponsored phase III clinical trials.
Major finding: Across all three trials, 12 weeks of ixekizumab was significantly better than placebo or etanercept was at increasing work productivity and reducing impairment at work.
Data source: An analysis of the secondary endpoint of work productivity in three international randomized, controlled phase III trials involving 3,866 patients.
Disclosures: The three UNCOVER trials were supported by Eli Lilly, maker of ixekizumab. Dr. Armstrong reported being a consultant for, receiving honoraria from, and receiving grant support from Eli Lilly and additional pharmaceutical companies. Her associates reported ties to numerous industry sources.
Subclinical inflammation predicts progression from psoriasis to PsA
People with cutaneous psoriasis who have arthralgia and signs of subclinical inflammation on MRI are at an increased risk of progressing to psoriatic arthritis, according to findings from a cross-sectional, longitudinal study.
First author Dr. Francesca Faustini of the University of Erlangen-Nuremberg, Erlangen, Germany, and her colleagues said the fact that psoriatic skin disease has a higher prevalence than arthritis raises the question whether patients with psoriasis without psoriatic arthritis (PsA) are spared from joint inflammation or whether mild changes, which escape physical examination, can be found in some patients (Ann Rheum Dis. 2016 Feb. 25. doi: 10.1136/annrheumdis-2015-208821).
Research published last year by the researchers shed some light on this by showing that psoriasis patients without PsA exhibit enthesiophytes as the result of pathological bone formation in the joint (Ann Rheum Dis. 2015 Feb 4. doi: 10.1136/annrheumdis-2014-206347).
“The presence of similar changes in patients with psoriasis strongly supports the hypothesis of subclinical joint pathology that antedates the clinical onset of PsA,” they said. The current study help to build on that finding by examining the extent to which inflammatory changes in the joints precede the onset of PsA, how such changes are related to structural pathology, and whether they influence the progression of psoriasis to PsA.
Dr. Faustini and her colleagues studied 55 patients with psoriasis who were attending a dermatology clinic and 30 healthy controls who underwent MRI of the hand and were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Patients with psoriasis also received a clinical investigation, high-resolution CT for detecting erosions and enthesiophytes, and were followed up for at least 1 year for the development of PsA.
Results showed that almost half of the patients with psoriasis (47%) had a least one inflammatory lesion shown on MRI.
Synovitis was the most prevalent inflammatory lesion (38%), while osteitis (11%), tenosynovitis (4%) and periarticular inflammation (4%) were less frequent.
Based on the PsA MRI scoring (PsAMRIS) system in patients with MRI lesions, the extent of inflammation was scored 3.0 units for synovitis, 1.8 for osteitis, 10.5 for tenosynovitis, and 3.0 for periarticular inflammation. Synovitis was deemed moderate (PsAMRIS of 2) in five joints and mild (score of 1) in all others where it was present; none of the joints scored 3 (severe).
Enthesiophytes and bone erosions were not different between patients with psoriasis with or without inflammatory MRI changes.
Among 41 patients who completed a follow-up examination a mean of about 14 months after the first, the risk for developing PsA at 1 year was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia (at least one tender joint), but was as low as 15% if patients had normal MRIs and did not report arthralgia. A total of 12 (30%) developed PsA according to CASPAR criteria.
“Subclinical inflammation appears to substantially influence the risk of patients with psoriasis to progress to PsA ... these findings indicate the possibility to define patients with psoriasis, in which preventive treatment for the development of PsA may be feasible,” they concluded.
However, they noted that it was important to consider that the presence of inflammatory lesions does not necessarily indicate that cutaneous psoriasis is causally linked to such lesions, particularly because neither disease activity and duration nor scalp and nail involvement was associated with MRI lesions.
“These findings suggest that skin and joint inflammation occur uncoupled and that skin disease may not represent the key pacemaker for joint inflammation,” they said.
The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
People with cutaneous psoriasis who have arthralgia and signs of subclinical inflammation on MRI are at an increased risk of progressing to psoriatic arthritis, according to findings from a cross-sectional, longitudinal study.
First author Dr. Francesca Faustini of the University of Erlangen-Nuremberg, Erlangen, Germany, and her colleagues said the fact that psoriatic skin disease has a higher prevalence than arthritis raises the question whether patients with psoriasis without psoriatic arthritis (PsA) are spared from joint inflammation or whether mild changes, which escape physical examination, can be found in some patients (Ann Rheum Dis. 2016 Feb. 25. doi: 10.1136/annrheumdis-2015-208821).
Research published last year by the researchers shed some light on this by showing that psoriasis patients without PsA exhibit enthesiophytes as the result of pathological bone formation in the joint (Ann Rheum Dis. 2015 Feb 4. doi: 10.1136/annrheumdis-2014-206347).
“The presence of similar changes in patients with psoriasis strongly supports the hypothesis of subclinical joint pathology that antedates the clinical onset of PsA,” they said. The current study help to build on that finding by examining the extent to which inflammatory changes in the joints precede the onset of PsA, how such changes are related to structural pathology, and whether they influence the progression of psoriasis to PsA.
Dr. Faustini and her colleagues studied 55 patients with psoriasis who were attending a dermatology clinic and 30 healthy controls who underwent MRI of the hand and were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Patients with psoriasis also received a clinical investigation, high-resolution CT for detecting erosions and enthesiophytes, and were followed up for at least 1 year for the development of PsA.
Results showed that almost half of the patients with psoriasis (47%) had a least one inflammatory lesion shown on MRI.
Synovitis was the most prevalent inflammatory lesion (38%), while osteitis (11%), tenosynovitis (4%) and periarticular inflammation (4%) were less frequent.
Based on the PsA MRI scoring (PsAMRIS) system in patients with MRI lesions, the extent of inflammation was scored 3.0 units for synovitis, 1.8 for osteitis, 10.5 for tenosynovitis, and 3.0 for periarticular inflammation. Synovitis was deemed moderate (PsAMRIS of 2) in five joints and mild (score of 1) in all others where it was present; none of the joints scored 3 (severe).
Enthesiophytes and bone erosions were not different between patients with psoriasis with or without inflammatory MRI changes.
Among 41 patients who completed a follow-up examination a mean of about 14 months after the first, the risk for developing PsA at 1 year was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia (at least one tender joint), but was as low as 15% if patients had normal MRIs and did not report arthralgia. A total of 12 (30%) developed PsA according to CASPAR criteria.
“Subclinical inflammation appears to substantially influence the risk of patients with psoriasis to progress to PsA ... these findings indicate the possibility to define patients with psoriasis, in which preventive treatment for the development of PsA may be feasible,” they concluded.
However, they noted that it was important to consider that the presence of inflammatory lesions does not necessarily indicate that cutaneous psoriasis is causally linked to such lesions, particularly because neither disease activity and duration nor scalp and nail involvement was associated with MRI lesions.
“These findings suggest that skin and joint inflammation occur uncoupled and that skin disease may not represent the key pacemaker for joint inflammation,” they said.
The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
People with cutaneous psoriasis who have arthralgia and signs of subclinical inflammation on MRI are at an increased risk of progressing to psoriatic arthritis, according to findings from a cross-sectional, longitudinal study.
First author Dr. Francesca Faustini of the University of Erlangen-Nuremberg, Erlangen, Germany, and her colleagues said the fact that psoriatic skin disease has a higher prevalence than arthritis raises the question whether patients with psoriasis without psoriatic arthritis (PsA) are spared from joint inflammation or whether mild changes, which escape physical examination, can be found in some patients (Ann Rheum Dis. 2016 Feb. 25. doi: 10.1136/annrheumdis-2015-208821).
Research published last year by the researchers shed some light on this by showing that psoriasis patients without PsA exhibit enthesiophytes as the result of pathological bone formation in the joint (Ann Rheum Dis. 2015 Feb 4. doi: 10.1136/annrheumdis-2014-206347).
“The presence of similar changes in patients with psoriasis strongly supports the hypothesis of subclinical joint pathology that antedates the clinical onset of PsA,” they said. The current study help to build on that finding by examining the extent to which inflammatory changes in the joints precede the onset of PsA, how such changes are related to structural pathology, and whether they influence the progression of psoriasis to PsA.
Dr. Faustini and her colleagues studied 55 patients with psoriasis who were attending a dermatology clinic and 30 healthy controls who underwent MRI of the hand and were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Patients with psoriasis also received a clinical investigation, high-resolution CT for detecting erosions and enthesiophytes, and were followed up for at least 1 year for the development of PsA.
Results showed that almost half of the patients with psoriasis (47%) had a least one inflammatory lesion shown on MRI.
Synovitis was the most prevalent inflammatory lesion (38%), while osteitis (11%), tenosynovitis (4%) and periarticular inflammation (4%) were less frequent.
Based on the PsA MRI scoring (PsAMRIS) system in patients with MRI lesions, the extent of inflammation was scored 3.0 units for synovitis, 1.8 for osteitis, 10.5 for tenosynovitis, and 3.0 for periarticular inflammation. Synovitis was deemed moderate (PsAMRIS of 2) in five joints and mild (score of 1) in all others where it was present; none of the joints scored 3 (severe).
Enthesiophytes and bone erosions were not different between patients with psoriasis with or without inflammatory MRI changes.
Among 41 patients who completed a follow-up examination a mean of about 14 months after the first, the risk for developing PsA at 1 year was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia (at least one tender joint), but was as low as 15% if patients had normal MRIs and did not report arthralgia. A total of 12 (30%) developed PsA according to CASPAR criteria.
“Subclinical inflammation appears to substantially influence the risk of patients with psoriasis to progress to PsA ... these findings indicate the possibility to define patients with psoriasis, in which preventive treatment for the development of PsA may be feasible,” they concluded.
However, they noted that it was important to consider that the presence of inflammatory lesions does not necessarily indicate that cutaneous psoriasis is causally linked to such lesions, particularly because neither disease activity and duration nor scalp and nail involvement was associated with MRI lesions.
“These findings suggest that skin and joint inflammation occur uncoupled and that skin disease may not represent the key pacemaker for joint inflammation,” they said.
The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Subclinical inflammation appears to substantially influence the risk of patients with psoriasis progressing to PsA.
Major finding: The risk for developing PsA was as high as 56% if patients had subclinical synovitis and symptoms related to arthralgia.
Data source: A cross-sectional longitudinal analysis of 55 patients with cutaneous psoriasis and 30 healthy controls.
Disclosures: The study received funding from the German Research Foundation, the Marie Curie project OSTEOIMMUNE, the German Ministry of Science and Education, the Innovative Medicines Initiative, and the Pfizer Competitive Grant Award Germany. The authors declared no conflicts of interest.
NNTs show once-unimaginable psoriasis outcomes now readily attainable
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.
Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.

Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Scrutiny of the number needed to treat with various systemic drugs to achieve a Psoriasis Area and Severity Index–90 (PASI-90) response in psoriasis highlights the folly of current stepwise treatment strategies imposed upon dermatologists by many payers, Dr. Craig L. Leonardi asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“It’s time to rethink our goals,” declared Dr. Leonardi, a dermatologist at Saint Louis University and a noted clinical trialist.
Most health plans insist that patients with moderate to severe psoriasis must have tried methotrexate and failed to achieve an adequate response before moving on to costlier biologic agents. But a PASI-90 response rate, indicative of 90% improvement in psoriasis area and severity, was achieved in only 13.6% of patients on methotrexate in the CHAMPION trial, compared with 59.2% and 70.9% of patients in phase III randomized trials of the interleukin-17 antagonists secukinumab (Cosentyx) and ixekizumab, respectively.
In other words, it is now routinely possible using highly effective medications to achieve a PASI-90 response in the majority of psoriasis patients, he noted.
Although statisticians say it isn’t appropriate to compare outcomes across clinical trials because study populations may differ, Dr. Leonardi decided it nevertheless would be illuminating to compare NNTs (the number of patients who needed to be treated with a medication instead of placebo to achieve one additional responder in a defined time period). In this analysis, he used data from phase III randomized, placebo-controlled clinical trials and Food and Drug Administration–regulated package inserts to calculate NNTs for systemic medications for psoriasis for the 10- to 16-week duration of phase III trials. The NNTs for a PASI-90 response ranged from a whopping 43.5 for methotrexate to 1.7 with secukinumab and 1.4 for ixekizumab.
“You can see immediately that methotrexate is outed as a weak and ineffective drug. And yet which drug are we usually asked to use first? Methotrexate. This is a structural problem that we have to solve in our specialty. We have to get with the insurance industry, we have to get with our guidelines of care and rewrite them. There is no reason that this drug should be placed in front of any of the other drugs, which are much more effective,” Dr. Leonardi said.

Viewing the data another way, the proportion of patients who achieved a PASI-75 response at the time of a major placebo-controlled clinical trial’s primary endpoint ranged from a low of 35.5% with methotrexate to 81.6% with secukinumab and 89.1% with ixekizumab. The NNTs to get a PASI-75 improvement with secukinumab and ixekizumab were, respectively, 1.3 and 1.2, compared to 6.0 for methotrexate.
“With an NNT of 1.2, if you treat 12 patients with ixekizumab, 10 of them are going to achieve PASI-75. This is a very different world than we had at the beginning of this adventure back in the early 2000s,” he observed.
In the modern era of highly effective biologic therapies for psoriasis, it makes sense to push as hard as possible in an effort to try to clear patients, according to Dr. Leonardi. That’s in part because the closer patients come to that once-nearly-unattainable goal, the better they actually feel, as underscored in the phase III results for ixekizumab.
In that study, the proportion of patients with a Dermatology Life Quality Index (DLQI) score of 0 or 1 at week 12 rose stepwise with the size of their PASI response. Among patients with a week-12 PASI response of 50 to less than 75, 18.8% had a DLQI of 0 or 1. For patients with a PASI-75 to less than PASI-90, it jumped to 52.3%. Among subjects with a PASI-90 to less than 100, 66.9% had a DLQI of 0 or 1. And among the 35.3% of ixekizumab-treated patients who had a PASI-100 response, the likelihood of a DLQI of 0 or 1 rose to 71.1%.
Noting that etanercept (Enbrel) is the only biologic on the market that achieves a PASI-75 response in less than half of treated patients, Dr. Leonardi said, “I’m over using etanercept as a first-line biologic. It’s the weakest biologic we can pick right now. I really like the highly efficacious drugs. I like adalimumab. I like the efficacy I see with ustekinumab. I like secukinumab. They are all preferred first-line drugs if I can get them, but it’s an insurance company world. I can’t tell you how many times I’ve heard, ‘Well, we’re not saying you can’t prescribe it, Dr. Leonardi, we’re just not going to pay for it.’ ”
Ixekizumab is under FDA review for psoriasis and a decision is expected within the first quarter of 2016, according to a spokesperson for Eli Lilly.
Dr. Leonardi is a recipient of research grants from well over a dozen pharmaceutical companies and is a consultant to and/or member of the speakers bureaus for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Janssen, Eli Lilly, Leo, Novartis, Pfizer, Sandoz, UCB, and Vitae.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Registry shows no increased cancer risk with biologics for psoriasis
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The latest update from the ongoing PSOLAR registry provides “very reassuring” evidence that the use of biologic agents to treat moderate to severe psoriasis doesn’t significantly increase malignancy risk other than for skin cancer, according to Dr. Kristina Callis Duffin.
Dr. Duffin of the department of dermatology at the University of Utah, Salt Lake City, cited a report presented by Dr. David Fiorentino, professor of dermatology at Stanford (Calif.) University, at the annual meeting of the European Academy of Dermatology and Venereology last October in Copenhagen. This update from the prospective international Psoriasis Longitudinal Assessment and Registry (PSOLAR) included 12,093 psoriasis patients deemed candidates for biologics, including 2,084 who did not go on a biologic agent while the rest did.
During 40,388 patient-years of prospective follow-up, or an average of 3.3 years, 455 patients were diagnosed with a malignancy other than skin cancer. The cumulative malignancy rate was 0.75 cases per 100 patient-years in patients on nonbiologic therapies, which was not significantly different from the rates of 0.51 per 100 patient-years in participants who started on ustekinumab (Stelara) at enrollment, 0.81 in patients on infliximab (Remicade), or 0.73 per 100 patient-years in those on other biologics, Dr. Duffin said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“These are all very low rates,” commented Dr. Duffin.
The PSOLAR data are particularly valuable because the registry was set up specifically to prospectively examine the long-term safety and efficacy of biologic agents in psoriasis patients. In contrast, the landmark epidemiologic study led by Dr. Joel M. Gelfand of the University of Pennsylvania, Philadelphia, which concluded that mild psoriasis was associated with a 34% increased risk of lymphoma and that severe psoriasis carried a 59% greater risk than in nonpsoriatic controls (J Invest Dermatol. 2006 Oct;126[10]:2194-201), involved a retrospective analysis of the U.K. General Practice Research Database. And while that study had strength in numbers – it included more than 153,000 British psoriasis patients and nearly 800,000 controls – it wasn’t designed to look specifically at psoriasis patients.
It’s reassuring that the lymphoma rate of 0.47 cases per 100 patient-years in patients with severe psoriasis in the U.K. registry during the prebiologics era is virtually identical to the rates associated with biologic agents in PSOLAR to date, which ranged from 0.3 to 0.5 cases per 100 patient-years, Dr. Duffin said.
The PSOLAR findings are worth sharing with patients. As a result of direct-to-consumer advertising by pharmaceutical companies, psoriasis patients are typically quite concerned about the risk of cancer associated with biologic agents, she added.
“They hear the comment in the ad that serious infections and malignancies have been reported in patients on these drugs as ‘these drugs increase the risk of malignancy.’ So where I start this conversation is, ‘Actually, patients with psoriasis do have some increased risk of malignancy, but those malignancies are mostly nonmelanoma skin cancers and lymphoproliferative diseases,’” the dermatologist explained.
Much of this risk is probably related to the fact that patients with moderate to severe psoriasis often have an extensive history of exposure to immunosuppressive agents such as cyclosporine as well as UV light therapies, which increase the risk of skin cancer.
“You also have to consider the fact that psoriasis patients tend to have a lot of smoking behaviors and alcohol behaviors that increase cancer risk,” Dr. Duffin continued.
In shared decision making regarding the option of biologic therapy in psoriasis patients having a history of cancer or who develop cancer while on a biologic, she likes to pose a question: What scares you more: the risk of your cancer coming back or not being able to have a good quality of life?
“That gets them thinking,” she said.
She stressed that as part of discussions regarding the risk/benefit profile of biologic therapy in an individual with a history of cancer, or of continuing a biologic in someone diagnosed with a malignancy while on treatment, it’s important for the dermatologist to talk with the patient’s oncologist, who is best positioned to provide insight into the risk of cancer recurrence.
Dr. Duffin is a recipient of research grants from and a consultant to Janssen, which sponsors the PSOLAR registry, as well as to more than half a dozen other pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR