User login
Deucravacitinib Improves Patient-Reported Outcomes in PsA
Key clinical point: Deucravacitinib improved patient-reported outcomes (PRO) for physical and social functioning, mental health, fatigue, and pain in patients with active psoriatic arthritis (PsA).
Major finding: At week 16, 6 mg deucravacitinib vs placebo led to significant changes in functional ability as assessed by the Health Assessment Questionnaire-Disability Index (−0.26; 95% CI −0.42 to −0.10) and the 36-Item Short-Form Health Survey physical component summary (3.3; 95% CI 0.9 to 5.7), with similar outcomes for 12 mg deucravacitinib. Improvements were also noted in mental health and quality of life at week 16 with deucravacitinib vs placebo.
Study details: Findings are from a phase 2, double-blind trial that included 203 patients with active PsA who were randomly assigned (1:1:1) to receive 6 mg deucravacitinib daily (n = 70), 12 mg deucravacitinib daily (n = 67), or placebo (n = 66) for 16 weeks.
Disclosures: This study was sponsored by Bristol Myers Squibb. The authors declared no conflicts of interest.
Source: Strand V, Gossec L, Coates LC, et al. Improvements in patient-reported outcomes after treatment with deucravacitinib in patients with psoriatic arthritis: Results from a randomized phase 2 trial. Arthritis Care Res (Hoboken). 2024 (Mar 26). doi: 10.1002/acr.25333 Source
Key clinical point: Deucravacitinib improved patient-reported outcomes (PRO) for physical and social functioning, mental health, fatigue, and pain in patients with active psoriatic arthritis (PsA).
Major finding: At week 16, 6 mg deucravacitinib vs placebo led to significant changes in functional ability as assessed by the Health Assessment Questionnaire-Disability Index (−0.26; 95% CI −0.42 to −0.10) and the 36-Item Short-Form Health Survey physical component summary (3.3; 95% CI 0.9 to 5.7), with similar outcomes for 12 mg deucravacitinib. Improvements were also noted in mental health and quality of life at week 16 with deucravacitinib vs placebo.
Study details: Findings are from a phase 2, double-blind trial that included 203 patients with active PsA who were randomly assigned (1:1:1) to receive 6 mg deucravacitinib daily (n = 70), 12 mg deucravacitinib daily (n = 67), or placebo (n = 66) for 16 weeks.
Disclosures: This study was sponsored by Bristol Myers Squibb. The authors declared no conflicts of interest.
Source: Strand V, Gossec L, Coates LC, et al. Improvements in patient-reported outcomes after treatment with deucravacitinib in patients with psoriatic arthritis: Results from a randomized phase 2 trial. Arthritis Care Res (Hoboken). 2024 (Mar 26). doi: 10.1002/acr.25333 Source
Key clinical point: Deucravacitinib improved patient-reported outcomes (PRO) for physical and social functioning, mental health, fatigue, and pain in patients with active psoriatic arthritis (PsA).
Major finding: At week 16, 6 mg deucravacitinib vs placebo led to significant changes in functional ability as assessed by the Health Assessment Questionnaire-Disability Index (−0.26; 95% CI −0.42 to −0.10) and the 36-Item Short-Form Health Survey physical component summary (3.3; 95% CI 0.9 to 5.7), with similar outcomes for 12 mg deucravacitinib. Improvements were also noted in mental health and quality of life at week 16 with deucravacitinib vs placebo.
Study details: Findings are from a phase 2, double-blind trial that included 203 patients with active PsA who were randomly assigned (1:1:1) to receive 6 mg deucravacitinib daily (n = 70), 12 mg deucravacitinib daily (n = 67), or placebo (n = 66) for 16 weeks.
Disclosures: This study was sponsored by Bristol Myers Squibb. The authors declared no conflicts of interest.
Source: Strand V, Gossec L, Coates LC, et al. Improvements in patient-reported outcomes after treatment with deucravacitinib in patients with psoriatic arthritis: Results from a randomized phase 2 trial. Arthritis Care Res (Hoboken). 2024 (Mar 26). doi: 10.1002/acr.25333 Source
Enthesitis or Dactylitis Remission Associated with Improved Patient-Reported Outcomes in PsA
Key clinical point: Among biologic-naive, guselkumab-treated patients with psoriatic arthritis (PsA), enthesitis resolution (ER) was associated with dactylitis resolution (DR), and those achieving ER or DR showed improvements in patient-reported outcomes.
Major finding: At weeks 24, 52, and 100, guselkumab-treated patients who achieved DR were more likely to achieve ER, and vice versa (all P < .05). At week 24, a higher proportion of patients who did vs did not achieve ER reported minimal pain (30%-45% vs 11%-21%; all P < .001), with similar pain outcomes in patients who did vs did not achieve DR.
Study details: This post hoc analysis included 739 biologic-naive patients with PsA who were randomly assigned to receive guselkumab (100 mg every 4 or 8 weeks) or placebo with crossover to guselkumab (100 mg every 4 weeks) at week 24, of whom 68.6% and 44.9% of patients had enthesitis and dactylitis, respectively.
Disclosures: This study was supported by Janssen Research & Development, LLC. Six authors declared being employees of Janssen and owning Johnson and Johnson stock or stock options. The other authors declared receiving consulting fees from or having other ties with various sources, including Janssen.
Source: Rahman P, McInnes IB, Deodhar A, et al. Association between enthesitis/dactylitis resolution and patient-reported outcomes in guselkumab-treated patients with psoriatic arthritis. Clin Rheumatol. 2024;43:1591-1604 (Mar 12). doi: 10.1007/s10067-024-06921-8 Source
Key clinical point: Among biologic-naive, guselkumab-treated patients with psoriatic arthritis (PsA), enthesitis resolution (ER) was associated with dactylitis resolution (DR), and those achieving ER or DR showed improvements in patient-reported outcomes.
Major finding: At weeks 24, 52, and 100, guselkumab-treated patients who achieved DR were more likely to achieve ER, and vice versa (all P < .05). At week 24, a higher proportion of patients who did vs did not achieve ER reported minimal pain (30%-45% vs 11%-21%; all P < .001), with similar pain outcomes in patients who did vs did not achieve DR.
Study details: This post hoc analysis included 739 biologic-naive patients with PsA who were randomly assigned to receive guselkumab (100 mg every 4 or 8 weeks) or placebo with crossover to guselkumab (100 mg every 4 weeks) at week 24, of whom 68.6% and 44.9% of patients had enthesitis and dactylitis, respectively.
Disclosures: This study was supported by Janssen Research & Development, LLC. Six authors declared being employees of Janssen and owning Johnson and Johnson stock or stock options. The other authors declared receiving consulting fees from or having other ties with various sources, including Janssen.
Source: Rahman P, McInnes IB, Deodhar A, et al. Association between enthesitis/dactylitis resolution and patient-reported outcomes in guselkumab-treated patients with psoriatic arthritis. Clin Rheumatol. 2024;43:1591-1604 (Mar 12). doi: 10.1007/s10067-024-06921-8 Source
Key clinical point: Among biologic-naive, guselkumab-treated patients with psoriatic arthritis (PsA), enthesitis resolution (ER) was associated with dactylitis resolution (DR), and those achieving ER or DR showed improvements in patient-reported outcomes.
Major finding: At weeks 24, 52, and 100, guselkumab-treated patients who achieved DR were more likely to achieve ER, and vice versa (all P < .05). At week 24, a higher proportion of patients who did vs did not achieve ER reported minimal pain (30%-45% vs 11%-21%; all P < .001), with similar pain outcomes in patients who did vs did not achieve DR.
Study details: This post hoc analysis included 739 biologic-naive patients with PsA who were randomly assigned to receive guselkumab (100 mg every 4 or 8 weeks) or placebo with crossover to guselkumab (100 mg every 4 weeks) at week 24, of whom 68.6% and 44.9% of patients had enthesitis and dactylitis, respectively.
Disclosures: This study was supported by Janssen Research & Development, LLC. Six authors declared being employees of Janssen and owning Johnson and Johnson stock or stock options. The other authors declared receiving consulting fees from or having other ties with various sources, including Janssen.
Source: Rahman P, McInnes IB, Deodhar A, et al. Association between enthesitis/dactylitis resolution and patient-reported outcomes in guselkumab-treated patients with psoriatic arthritis. Clin Rheumatol. 2024;43:1591-1604 (Mar 12). doi: 10.1007/s10067-024-06921-8 Source
Durable Improvements Across PsA Disease Domains with Guselkumab
Key clinical point: Guselkumab treatment led to durable improvements in key Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (PsA)-recognized domains through 2 years and showed a consistent safety profile in biologic or Janus kinase inhibitor-naive patients with active PsA.
Major finding: At week 100, more than 50% of patients receiving guselkumab (100 mg every 4 or 8 weeks) achieved achieved a low PsA Disease Activity Index, had enthesitis resolution, dactylitis resolution, and 100% improvement in Psoriasis Area and Severity Index. No new safety signals were observed.
Study details: This post hoc analysis included 442 biologic or Janus kinase inhibitor-naive patients with active PsA and previous inadequate response or intolerance to standard nonbiologics who received 100 mg guselkumab every 4 or 8 weeks through week 100.
Disclosures: This study was supported by Janssen Research & Development (R&D), LLC. Three authors declared being employees of Janssen R&D and owning Johnson and Johnson stocks or stock options. Several authors declared receiving honoraria from or having other ties with various sources, including Janssen.
Source: Coates LC, Gossec L, Zimmermann M, et al. Guselkumab provides durable improvement across psoriatic arthritis disease domains: Post hoc analysis of a phase 3, randomised, double-blind, placebo-controlled study. RMD Open. 2024;10:e003977 (Mar 26). doi: 10.1136/rmdopen-2023-003977 Source
Key clinical point: Guselkumab treatment led to durable improvements in key Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (PsA)-recognized domains through 2 years and showed a consistent safety profile in biologic or Janus kinase inhibitor-naive patients with active PsA.
Major finding: At week 100, more than 50% of patients receiving guselkumab (100 mg every 4 or 8 weeks) achieved achieved a low PsA Disease Activity Index, had enthesitis resolution, dactylitis resolution, and 100% improvement in Psoriasis Area and Severity Index. No new safety signals were observed.
Study details: This post hoc analysis included 442 biologic or Janus kinase inhibitor-naive patients with active PsA and previous inadequate response or intolerance to standard nonbiologics who received 100 mg guselkumab every 4 or 8 weeks through week 100.
Disclosures: This study was supported by Janssen Research & Development (R&D), LLC. Three authors declared being employees of Janssen R&D and owning Johnson and Johnson stocks or stock options. Several authors declared receiving honoraria from or having other ties with various sources, including Janssen.
Source: Coates LC, Gossec L, Zimmermann M, et al. Guselkumab provides durable improvement across psoriatic arthritis disease domains: Post hoc analysis of a phase 3, randomised, double-blind, placebo-controlled study. RMD Open. 2024;10:e003977 (Mar 26). doi: 10.1136/rmdopen-2023-003977 Source
Key clinical point: Guselkumab treatment led to durable improvements in key Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (PsA)-recognized domains through 2 years and showed a consistent safety profile in biologic or Janus kinase inhibitor-naive patients with active PsA.
Major finding: At week 100, more than 50% of patients receiving guselkumab (100 mg every 4 or 8 weeks) achieved achieved a low PsA Disease Activity Index, had enthesitis resolution, dactylitis resolution, and 100% improvement in Psoriasis Area and Severity Index. No new safety signals were observed.
Study details: This post hoc analysis included 442 biologic or Janus kinase inhibitor-naive patients with active PsA and previous inadequate response or intolerance to standard nonbiologics who received 100 mg guselkumab every 4 or 8 weeks through week 100.
Disclosures: This study was supported by Janssen Research & Development (R&D), LLC. Three authors declared being employees of Janssen R&D and owning Johnson and Johnson stocks or stock options. Several authors declared receiving honoraria from or having other ties with various sources, including Janssen.
Source: Coates LC, Gossec L, Zimmermann M, et al. Guselkumab provides durable improvement across psoriatic arthritis disease domains: Post hoc analysis of a phase 3, randomised, double-blind, placebo-controlled study. RMD Open. 2024;10:e003977 (Mar 26). doi: 10.1136/rmdopen-2023-003977 Source
Risankizumab Offers Long-term Protection Against PsA
Key clinical point: Risankizumab showed long-term efficacy and tolerability in patients having active psoriatic arthritis (PsA) with previous inadequate response or intolerance to one or more conventional synthetic disease-modifying antirheumatic drugs (csDMARD-IR).
Major finding: At week 100, more than half the patients who received risankizumab continuously (64.3%) or switched from placebo to risankizumab (62.1%) achieved ≥20% improvement in the American College of Rheumatology criteria (ACR20), with Minimal Disease Activity being reported by nearly 35% of patients in both cohorts. Risankizumab showed a consistent safety profile with no new concerns.
Study details: This long-term efficacy and safety analysis of the KEEPsAKE 1 trial included 828 csDMARD-IR patients with active PsA who received risankizumab or placebo followed by risankizumab till week 100.
Disclosures: This study was funded by AbbVie. Seven authors declared being employees of or holding stocks or stock options in AbbVie. Several authors declared serving as consultants or speakers for or having other ties with various sources, including AbbVie.
Source: Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 100-week results from the phase 3 KEEPsAKE 1 randomized clinical trial. Rheumatol Ther. 2024 (Mar 18). doi: 10.1007/s40744-024-00654-5 Source
Key clinical point: Risankizumab showed long-term efficacy and tolerability in patients having active psoriatic arthritis (PsA) with previous inadequate response or intolerance to one or more conventional synthetic disease-modifying antirheumatic drugs (csDMARD-IR).
Major finding: At week 100, more than half the patients who received risankizumab continuously (64.3%) or switched from placebo to risankizumab (62.1%) achieved ≥20% improvement in the American College of Rheumatology criteria (ACR20), with Minimal Disease Activity being reported by nearly 35% of patients in both cohorts. Risankizumab showed a consistent safety profile with no new concerns.
Study details: This long-term efficacy and safety analysis of the KEEPsAKE 1 trial included 828 csDMARD-IR patients with active PsA who received risankizumab or placebo followed by risankizumab till week 100.
Disclosures: This study was funded by AbbVie. Seven authors declared being employees of or holding stocks or stock options in AbbVie. Several authors declared serving as consultants or speakers for or having other ties with various sources, including AbbVie.
Source: Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 100-week results from the phase 3 KEEPsAKE 1 randomized clinical trial. Rheumatol Ther. 2024 (Mar 18). doi: 10.1007/s40744-024-00654-5 Source
Key clinical point: Risankizumab showed long-term efficacy and tolerability in patients having active psoriatic arthritis (PsA) with previous inadequate response or intolerance to one or more conventional synthetic disease-modifying antirheumatic drugs (csDMARD-IR).
Major finding: At week 100, more than half the patients who received risankizumab continuously (64.3%) or switched from placebo to risankizumab (62.1%) achieved ≥20% improvement in the American College of Rheumatology criteria (ACR20), with Minimal Disease Activity being reported by nearly 35% of patients in both cohorts. Risankizumab showed a consistent safety profile with no new concerns.
Study details: This long-term efficacy and safety analysis of the KEEPsAKE 1 trial included 828 csDMARD-IR patients with active PsA who received risankizumab or placebo followed by risankizumab till week 100.
Disclosures: This study was funded by AbbVie. Seven authors declared being employees of or holding stocks or stock options in AbbVie. Several authors declared serving as consultants or speakers for or having other ties with various sources, including AbbVie.
Source: Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 100-week results from the phase 3 KEEPsAKE 1 randomized clinical trial. Rheumatol Ther. 2024 (Mar 18). doi: 10.1007/s40744-024-00654-5 Source
Low Risk for Serious Infections Among New Users of Targeted Therapies in PsA
Key clinical point: The overall risk for serious infections was low in patients with psoriatic arthritis (PsA) who were new users of targeted therapies, with etanercept and ustekinumab being safer treatment options than adalimumab.
Major finding: The incidence of serious infections in new users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93).
Study details: This cohort study included 12,071 patients with PsA (age ≥ 18 years) from the French National Health Insurance Database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib).
Disclosures: This study did not receive any specific funding. Two authors declared receiving meeting support, consulting fees, etc., from or having other ties with various sources. The other authors declared no conflicts of interest.
Source: Bastard L, Claudepierre P, Penso L, et al. Risk of serious infection associated with different classes of targeted therapies used in psoriatic arthritis: A nationwide cohort study from the French Health Insurance Database (SNDS). RMD Open. 2024;10:e003865 (Mar 14). doi: 10.1136/rmdopen-2023-003865 Source
Key clinical point: The overall risk for serious infections was low in patients with psoriatic arthritis (PsA) who were new users of targeted therapies, with etanercept and ustekinumab being safer treatment options than adalimumab.
Major finding: The incidence of serious infections in new users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93).
Study details: This cohort study included 12,071 patients with PsA (age ≥ 18 years) from the French National Health Insurance Database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib).
Disclosures: This study did not receive any specific funding. Two authors declared receiving meeting support, consulting fees, etc., from or having other ties with various sources. The other authors declared no conflicts of interest.
Source: Bastard L, Claudepierre P, Penso L, et al. Risk of serious infection associated with different classes of targeted therapies used in psoriatic arthritis: A nationwide cohort study from the French Health Insurance Database (SNDS). RMD Open. 2024;10:e003865 (Mar 14). doi: 10.1136/rmdopen-2023-003865 Source
Key clinical point: The overall risk for serious infections was low in patients with psoriatic arthritis (PsA) who were new users of targeted therapies, with etanercept and ustekinumab being safer treatment options than adalimumab.
Major finding: The incidence of serious infections in new users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93).
Study details: This cohort study included 12,071 patients with PsA (age ≥ 18 years) from the French National Health Insurance Database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib).
Disclosures: This study did not receive any specific funding. Two authors declared receiving meeting support, consulting fees, etc., from or having other ties with various sources. The other authors declared no conflicts of interest.
Source: Bastard L, Claudepierre P, Penso L, et al. Risk of serious infection associated with different classes of targeted therapies used in psoriatic arthritis: A nationwide cohort study from the French Health Insurance Database (SNDS). RMD Open. 2024;10:e003865 (Mar 14). doi: 10.1136/rmdopen-2023-003865 Source
Favorable Efficacy Outcomes with Bimekizumab vs Guselkumab in PsA
Key clinical point: Bimekizumab showed better long-term efficacy than guselkumab in patients with psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD) or had previous inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: In bDMARD-naive patients, bimekizumab (160 mg every 4 weeks [Q4W]) was associated with a greater likelihood of achievement of ≥70% improvement in the American College of Rheumatology response (odds ratio [OR] > 2.0; P ≤ .001) and minimal disease activity outcome (OR > 1.5; P ≤ .005) at week 52 compared with guselkumab (100 mg Q4W or every 8 weeks). Similar outcomes were observed in the TNFi-IR subgroup.
Study details: This matching-adjusted indirect comparison study included bDMARD-naive and TNFi-IR patients with PsA who received bimekizumab (431 and 267 patients, respectively) and guselkumab (495 and 189 patients, respectively).
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees and stockholders of UCB Pharma. The other authors declared receiving consulting fees or honoraria from or having other ties with various sources, including UCB Pharma.
Source: Warren RB, McInnes IB, Nash P, et al. Comparative effectiveness of bimekizumab and guselkumab in patients with psoriatic arthritis at 52 weeks assessed using a matching-adjusted indirect comparison. Rheumatol Ther. 2024 (Mar 15). doi: 10.1007/s40744-024-00659-0 Source
Key clinical point: Bimekizumab showed better long-term efficacy than guselkumab in patients with psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD) or had previous inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: In bDMARD-naive patients, bimekizumab (160 mg every 4 weeks [Q4W]) was associated with a greater likelihood of achievement of ≥70% improvement in the American College of Rheumatology response (odds ratio [OR] > 2.0; P ≤ .001) and minimal disease activity outcome (OR > 1.5; P ≤ .005) at week 52 compared with guselkumab (100 mg Q4W or every 8 weeks). Similar outcomes were observed in the TNFi-IR subgroup.
Study details: This matching-adjusted indirect comparison study included bDMARD-naive and TNFi-IR patients with PsA who received bimekizumab (431 and 267 patients, respectively) and guselkumab (495 and 189 patients, respectively).
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees and stockholders of UCB Pharma. The other authors declared receiving consulting fees or honoraria from or having other ties with various sources, including UCB Pharma.
Source: Warren RB, McInnes IB, Nash P, et al. Comparative effectiveness of bimekizumab and guselkumab in patients with psoriatic arthritis at 52 weeks assessed using a matching-adjusted indirect comparison. Rheumatol Ther. 2024 (Mar 15). doi: 10.1007/s40744-024-00659-0 Source
Key clinical point: Bimekizumab showed better long-term efficacy than guselkumab in patients with psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD) or had previous inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: In bDMARD-naive patients, bimekizumab (160 mg every 4 weeks [Q4W]) was associated with a greater likelihood of achievement of ≥70% improvement in the American College of Rheumatology response (odds ratio [OR] > 2.0; P ≤ .001) and minimal disease activity outcome (OR > 1.5; P ≤ .005) at week 52 compared with guselkumab (100 mg Q4W or every 8 weeks). Similar outcomes were observed in the TNFi-IR subgroup.
Study details: This matching-adjusted indirect comparison study included bDMARD-naive and TNFi-IR patients with PsA who received bimekizumab (431 and 267 patients, respectively) and guselkumab (495 and 189 patients, respectively).
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees and stockholders of UCB Pharma. The other authors declared receiving consulting fees or honoraria from or having other ties with various sources, including UCB Pharma.
Source: Warren RB, McInnes IB, Nash P, et al. Comparative effectiveness of bimekizumab and guselkumab in patients with psoriatic arthritis at 52 weeks assessed using a matching-adjusted indirect comparison. Rheumatol Ther. 2024 (Mar 15). doi: 10.1007/s40744-024-00659-0 Source
Worldwide Prevalence of Psoriatic Arthritis More Precisely Determined
TOPLINE:
According to this meta-analysis, psoriatic arthritis (PsA) affects 112 out of every 100,000 adults globally, with higher rates observed in Europe and North America than in Asia and South America, according to an analysis of 30 studies.
METHODOLOGY:
- Many previous epidemiological studies have estimated the global prevalence of PsA but have reported marked variations, which could be explained by differences in methodology and inclusion criteria.
- This meta-analysis used data from 30 studies conducted between 1982 and 2020 to estimate the worldwide prevalence of PsA in the general adult population, giving particular attention to methodological differences among the included studies.
- The included studies were either population-based (n = 13) or based on health administrative records (n = 17) and covered over 180 million adults across 24 countries.
- Overall, 15 studies were from Europe, seven from Asia, six from North America, and two from South America.
TAKEAWAY:
- The global prevalence of PsA was estimated at 113 (95% CI, 64-198) and 109 (75-158) cases per 100,000 based on population-based studies and health administrative data studies, respectively.
- The pooled global prevalence of PsA (combining the population-based and health administrative studies) was 112 cases per 100,000 (95% CI, 83-151).
- Combining both study designs, the global prevalence rates of PsA were 188 (95% CI, 128-289) cases per 100,000 for Europe, 48 (95% CI, 20-115) for Asia, 133 (95% CI, 93-191) for North America, and 17 (95% CI, 4-70) for South America.
IN PRACTICE:
“Robust estimates of prevalence are crucial for healthcare planning and resource allocation,” wrote the authors.
SOURCE:
The study was conducted by Stephanie Lembke, MSc, and colleagues from the Aberdeen Centre for Arthritis and Musculoskeletal Health, University of Aberdeen, Scotland. It was published online in Rheumatology (Oxford).
LIMITATIONS:
The meta-analysis had high levels of uncertainty and high heterogeneity between studies. In countries with unequal healthcare access, using data from statutory or private insurance databases to calculate PsA prevalence may systematically exclude uninsured individuals or those covered by private insurers. Moreover, the data were insufficient for a statistically meaningful subgroup analysis.
DISCLOSURES:
The study did not receive any specific funding from any public, commercial, or nonprofit sectors to carry out this work. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
According to this meta-analysis, psoriatic arthritis (PsA) affects 112 out of every 100,000 adults globally, with higher rates observed in Europe and North America than in Asia and South America, according to an analysis of 30 studies.
METHODOLOGY:
- Many previous epidemiological studies have estimated the global prevalence of PsA but have reported marked variations, which could be explained by differences in methodology and inclusion criteria.
- This meta-analysis used data from 30 studies conducted between 1982 and 2020 to estimate the worldwide prevalence of PsA in the general adult population, giving particular attention to methodological differences among the included studies.
- The included studies were either population-based (n = 13) or based on health administrative records (n = 17) and covered over 180 million adults across 24 countries.
- Overall, 15 studies were from Europe, seven from Asia, six from North America, and two from South America.
TAKEAWAY:
- The global prevalence of PsA was estimated at 113 (95% CI, 64-198) and 109 (75-158) cases per 100,000 based on population-based studies and health administrative data studies, respectively.
- The pooled global prevalence of PsA (combining the population-based and health administrative studies) was 112 cases per 100,000 (95% CI, 83-151).
- Combining both study designs, the global prevalence rates of PsA were 188 (95% CI, 128-289) cases per 100,000 for Europe, 48 (95% CI, 20-115) for Asia, 133 (95% CI, 93-191) for North America, and 17 (95% CI, 4-70) for South America.
IN PRACTICE:
“Robust estimates of prevalence are crucial for healthcare planning and resource allocation,” wrote the authors.
SOURCE:
The study was conducted by Stephanie Lembke, MSc, and colleagues from the Aberdeen Centre for Arthritis and Musculoskeletal Health, University of Aberdeen, Scotland. It was published online in Rheumatology (Oxford).
LIMITATIONS:
The meta-analysis had high levels of uncertainty and high heterogeneity between studies. In countries with unequal healthcare access, using data from statutory or private insurance databases to calculate PsA prevalence may systematically exclude uninsured individuals or those covered by private insurers. Moreover, the data were insufficient for a statistically meaningful subgroup analysis.
DISCLOSURES:
The study did not receive any specific funding from any public, commercial, or nonprofit sectors to carry out this work. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
According to this meta-analysis, psoriatic arthritis (PsA) affects 112 out of every 100,000 adults globally, with higher rates observed in Europe and North America than in Asia and South America, according to an analysis of 30 studies.
METHODOLOGY:
- Many previous epidemiological studies have estimated the global prevalence of PsA but have reported marked variations, which could be explained by differences in methodology and inclusion criteria.
- This meta-analysis used data from 30 studies conducted between 1982 and 2020 to estimate the worldwide prevalence of PsA in the general adult population, giving particular attention to methodological differences among the included studies.
- The included studies were either population-based (n = 13) or based on health administrative records (n = 17) and covered over 180 million adults across 24 countries.
- Overall, 15 studies were from Europe, seven from Asia, six from North America, and two from South America.
TAKEAWAY:
- The global prevalence of PsA was estimated at 113 (95% CI, 64-198) and 109 (75-158) cases per 100,000 based on population-based studies and health administrative data studies, respectively.
- The pooled global prevalence of PsA (combining the population-based and health administrative studies) was 112 cases per 100,000 (95% CI, 83-151).
- Combining both study designs, the global prevalence rates of PsA were 188 (95% CI, 128-289) cases per 100,000 for Europe, 48 (95% CI, 20-115) for Asia, 133 (95% CI, 93-191) for North America, and 17 (95% CI, 4-70) for South America.
IN PRACTICE:
“Robust estimates of prevalence are crucial for healthcare planning and resource allocation,” wrote the authors.
SOURCE:
The study was conducted by Stephanie Lembke, MSc, and colleagues from the Aberdeen Centre for Arthritis and Musculoskeletal Health, University of Aberdeen, Scotland. It was published online in Rheumatology (Oxford).
LIMITATIONS:
The meta-analysis had high levels of uncertainty and high heterogeneity between studies. In countries with unequal healthcare access, using data from statutory or private insurance databases to calculate PsA prevalence may systematically exclude uninsured individuals or those covered by private insurers. Moreover, the data were insufficient for a statistically meaningful subgroup analysis.
DISCLOSURES:
The study did not receive any specific funding from any public, commercial, or nonprofit sectors to carry out this work. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
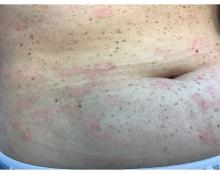
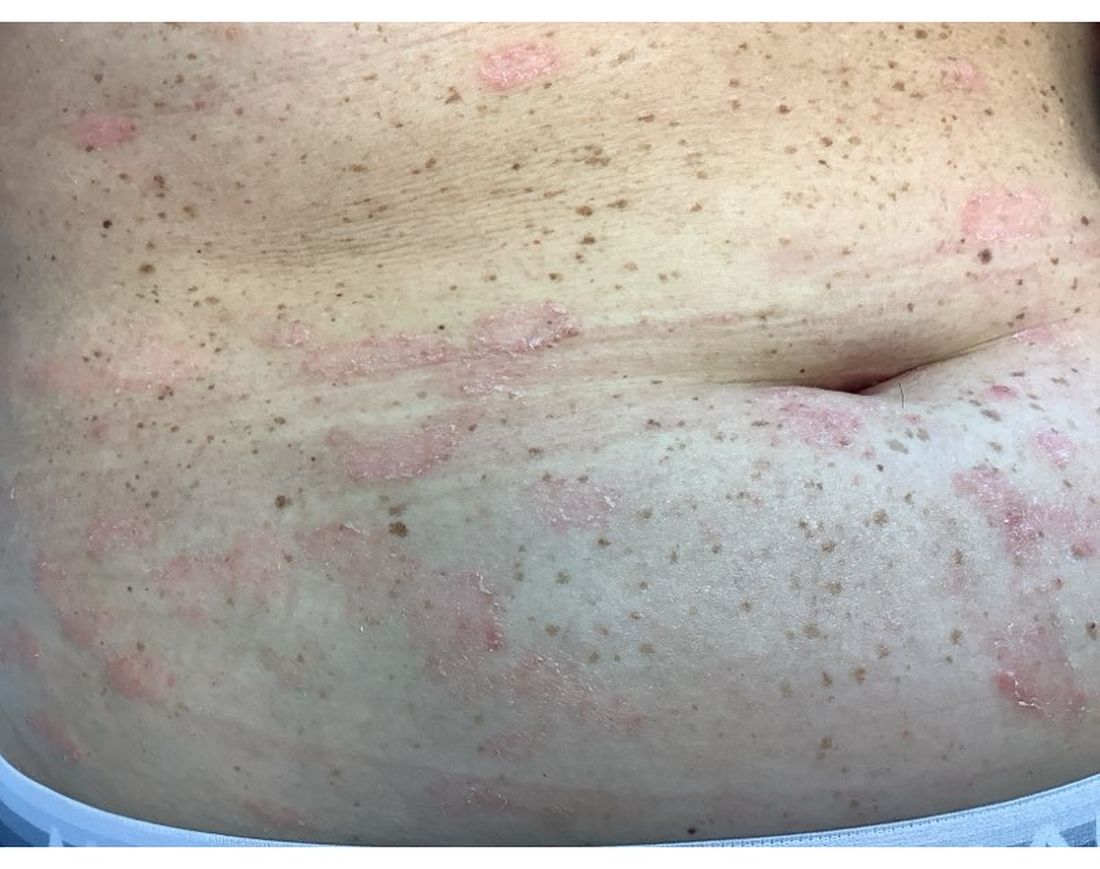
A 30-Year-Old White Female Presented With a 4-Month History of Scaly, Erythematous Patches and Plaques on Her Trunk and Extremities
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Tumor necrosis factor (TNF)-alpha inhibitors are used to treat a variety of autoimmune conditions including psoriasis, psoriatic arthritis, rheumatoid arthritis (RA), spondyloarthritis, and inflammatory bowel disease (IBD). Interestingly, they have also been observed to cause paradoxical psoriasis with an incidence between 0.6%-5.3%, most commonly occurring in patients with underlying Crohn’s disease and rheumatoid arthritis (RA). Infliximab is the most common TNF inhibitor associated with this condition (52.6%-62.6% of cases) followed by etanercept (12%-29%). .
Psoriasis is traditionally divided into two types. Patients with type I psoriasis have a family history, develop symptoms before the age of 40 and are often positive for HLA-Cw6. Type II psoriasis is not related to HLA-Cw6, lacks a family history, and typically manifests after age 40. Psoriatic lesions are well-defined, erythematous plaques with silvery scales most commonly appearing on extensor surfaces and the scalp. Variants include nail psoriasis, pustular psoriasis, inverse psoriasis, and guttate psoriasis.
Although psoriasis is typically a clinical diagnosis, histologic examination may be used to differentiate from other dermatoses if necessary. The lesions of TNF inhibitor-induced psoriasis characteristically display patterns similar to primary psoriasis, including parakeratosis, microabscesses, and rete ridges. Eosinophilic hypersensitivity reactions and features overlapping with eczematous hypersensitivity (psoriasiform dermatitis) may also be present.
The pathogenesis of this condition is not well understood, but theories include a variety of immune processes including interferon overproduction, interleukin and T-cell activation, and the presence of an infectious nidus. Classical psoriasis is related to type 1 interferon release, so theoretically, immunosuppression caused by TNF inhibitor treatment may permit uncontrolled production of interferons, resulting in psoriatic lesions. Another theory is that interleukin (IL)-23, a pro-inflammatory cytokine, promotes activation of T-helper 17 (Th17) cells. Th17 cells are part of the pathogenesis of primary psoriasis and other inflammatory conditions, such as RA and inflammatory bowel disease. Of note, individuals with gastrointestinal inflammatory diseases are already known to be at a greater risk for developing psoriasis. Immunosuppression caused by a TNF inhibitor may leave patients more susceptible to other infections, which may induce psoriatic plaques.
There are multiple approaches to treatment depending on the severity of the disease. If the psoriatic eruption is mild, the medication may be continued. This “treat-through” method is often considered when stopping the current immunotherapy would cause the patient significant issues. Moderate to severe cases of TNF inhibitor-induced psoriasis may warrant switching TNF inhibitor therapy or completely changing the drug class used in the treatment of the underlying autoimmune condition. Additional treatments include topical and oral steroids, UV therapy, methotrexate, cyclosporine, and acitretin.
This case and the photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Leon S. Maratchi, MD, Gastro Health, Hollywood, Florida. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Li SJ et al. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80. doi: 10.1177/2475530318810851.
2. Lu J and Lu Y. J Transl Autoimmun. 2023 Sep 6:7:100211. doi: 10.1016/j.jtauto.2023.100211.
3. Nair PA and Badri T. Psoriasis. [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK448194/
Congress Directly Provides $10 Million for Arthritis Research for First Time
Congress provided $10 million to fund arthritis research in the recently passed federal fiscal year 2024 budget.
The new arthritis program is part of the Department of Defense’s (DOD’s) Congressionally Directed Medical Research Programs (CDMRP), which provides dedicated funding to study certain diseases and health conditions.
This is the first stand-alone research program for arthritis of the CDMRP, though the organization had previously funded arthritis-related research through their other programs, including chronic pain management, joint warfighter medical, peer-reviewed orthopedic, peer-reviewed medical, and tick-borne disease programs.
It is not yet known what specific aspects of arthritis this funding will go toward. The standard process for new programs involves speaking with researchers, clinicians, and individuals with these targeted health conditions to better understand research gaps and narrow focus, Akua Roach, PhD, the program manager for this new CDMRP arthritis research program, told this news organization.
“We’re not going to be able to solve every question,” she said, though the allocated $10 million is “a great number to do a lot of great work.”
While the CDMRP is under the DOD, research funding can go to studying patient populations outside of military personnel or veterans, she added.
“I think that is perhaps a common misconception that if you are getting funding from the DOD, that you have to have a DOD population, and that is not true,” she said.
Another misconception is that CDMRP funding only goes to military treatment facilities. In fact, on average, 92% of CDMRP funding goes to academia, industry, and other nonmilitary recipients, noted CDMRP Director Colonel Sarah Goldman.
“Anyone around the world can apply for funding,” she told this news organization. “We want to fund the best research.”
Because the funding is provided under the defense bill, there will be discussions around the military relevance of research, she added, which not only includes service members but also their families.
CDMRP anticipates that funding opportunities through this new arthritis research program will be available by July or August 2024.
A version of this article appeared on Medscape.com.
Congress provided $10 million to fund arthritis research in the recently passed federal fiscal year 2024 budget.
The new arthritis program is part of the Department of Defense’s (DOD’s) Congressionally Directed Medical Research Programs (CDMRP), which provides dedicated funding to study certain diseases and health conditions.
This is the first stand-alone research program for arthritis of the CDMRP, though the organization had previously funded arthritis-related research through their other programs, including chronic pain management, joint warfighter medical, peer-reviewed orthopedic, peer-reviewed medical, and tick-borne disease programs.
It is not yet known what specific aspects of arthritis this funding will go toward. The standard process for new programs involves speaking with researchers, clinicians, and individuals with these targeted health conditions to better understand research gaps and narrow focus, Akua Roach, PhD, the program manager for this new CDMRP arthritis research program, told this news organization.
“We’re not going to be able to solve every question,” she said, though the allocated $10 million is “a great number to do a lot of great work.”
While the CDMRP is under the DOD, research funding can go to studying patient populations outside of military personnel or veterans, she added.
“I think that is perhaps a common misconception that if you are getting funding from the DOD, that you have to have a DOD population, and that is not true,” she said.
Another misconception is that CDMRP funding only goes to military treatment facilities. In fact, on average, 92% of CDMRP funding goes to academia, industry, and other nonmilitary recipients, noted CDMRP Director Colonel Sarah Goldman.
“Anyone around the world can apply for funding,” she told this news organization. “We want to fund the best research.”
Because the funding is provided under the defense bill, there will be discussions around the military relevance of research, she added, which not only includes service members but also their families.
CDMRP anticipates that funding opportunities through this new arthritis research program will be available by July or August 2024.
A version of this article appeared on Medscape.com.
Congress provided $10 million to fund arthritis research in the recently passed federal fiscal year 2024 budget.
The new arthritis program is part of the Department of Defense’s (DOD’s) Congressionally Directed Medical Research Programs (CDMRP), which provides dedicated funding to study certain diseases and health conditions.
This is the first stand-alone research program for arthritis of the CDMRP, though the organization had previously funded arthritis-related research through their other programs, including chronic pain management, joint warfighter medical, peer-reviewed orthopedic, peer-reviewed medical, and tick-borne disease programs.
It is not yet known what specific aspects of arthritis this funding will go toward. The standard process for new programs involves speaking with researchers, clinicians, and individuals with these targeted health conditions to better understand research gaps and narrow focus, Akua Roach, PhD, the program manager for this new CDMRP arthritis research program, told this news organization.
“We’re not going to be able to solve every question,” she said, though the allocated $10 million is “a great number to do a lot of great work.”
While the CDMRP is under the DOD, research funding can go to studying patient populations outside of military personnel or veterans, she added.
“I think that is perhaps a common misconception that if you are getting funding from the DOD, that you have to have a DOD population, and that is not true,” she said.
Another misconception is that CDMRP funding only goes to military treatment facilities. In fact, on average, 92% of CDMRP funding goes to academia, industry, and other nonmilitary recipients, noted CDMRP Director Colonel Sarah Goldman.
“Anyone around the world can apply for funding,” she told this news organization. “We want to fund the best research.”
Because the funding is provided under the defense bill, there will be discussions around the military relevance of research, she added, which not only includes service members but also their families.
CDMRP anticipates that funding opportunities through this new arthritis research program will be available by July or August 2024.
A version of this article appeared on Medscape.com.
Tuberculosis Screening Gaps Persist in New DMARD Users
TOPLINE:
The rates of screening for latent tuberculosis remain suboptimal among new users of biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), with notable variations by medication type and demographic characteristics.
METHODOLOGY:
- Professional society guidelines recommend screening for tuberculosis before starting treatment with most b/tsDMARDs.
- In an attempt to estimate the extent of latent tuberculosis screening, researchers combined claims and electronic health record datasets to evaluate 2853 new b/tsDMARD users (mean age, 73 years; 72% women; and 73% non-Hispanic White).
- The primary analysis focused on assessing the proportion of patients screened for latent tuberculosis in the year before starting a new b/tsDMARD.
- A sensitivity analysis evaluated the extent of screening within the 3 years preceding the initiation of a new b/tsDMARD.
- A total of 65.6% of patients received screening for latent tuberculosis in the year before initiating a new b/tsDMARD.
- Screening rates improved only slightly on expanding the window to 3 years, with 72.9% of patients receiving any tuberculosis screening.
- When stratified by drug type, over half of new users of Janus kinase inhibitors and nearly 90% of new users of interleukin-17 inhibitors had not received screening.
- Hispanic patients had lower odds of tuberculosis screening within 1 year than White patients (odds ratio [OR], 0.64; 95% CI, 0.46-0.90), as did those in the highest socioeconomic quartile, compared with the lowest (OR, 0.61; 95% CI, 0.40-0.94).
IN PRACTICE:
“Educational initiatives, team-based care delivery, task shifting, and technological interventions to address observed gaps in patient safety procedures are needed,” the authors wrote.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco, and published online in Arthritis Care & Research
LIMITATIONS:
The study lacked access to scanned documents or clinical notes, which may have resulted in the omission of a small number of tests that had no Medicare billing. Moreover, the study was restricted to a 3-year lookback period, potentially missing some remote screenings. The findings may have limited generalizability to younger patients or those not dually eligible for Medicare and Medicaid.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute for Arthritis and Musculoskeletal and Skin Diseases. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
The rates of screening for latent tuberculosis remain suboptimal among new users of biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), with notable variations by medication type and demographic characteristics.
METHODOLOGY:
- Professional society guidelines recommend screening for tuberculosis before starting treatment with most b/tsDMARDs.
- In an attempt to estimate the extent of latent tuberculosis screening, researchers combined claims and electronic health record datasets to evaluate 2853 new b/tsDMARD users (mean age, 73 years; 72% women; and 73% non-Hispanic White).
- The primary analysis focused on assessing the proportion of patients screened for latent tuberculosis in the year before starting a new b/tsDMARD.
- A sensitivity analysis evaluated the extent of screening within the 3 years preceding the initiation of a new b/tsDMARD.
- A total of 65.6% of patients received screening for latent tuberculosis in the year before initiating a new b/tsDMARD.
- Screening rates improved only slightly on expanding the window to 3 years, with 72.9% of patients receiving any tuberculosis screening.
- When stratified by drug type, over half of new users of Janus kinase inhibitors and nearly 90% of new users of interleukin-17 inhibitors had not received screening.
- Hispanic patients had lower odds of tuberculosis screening within 1 year than White patients (odds ratio [OR], 0.64; 95% CI, 0.46-0.90), as did those in the highest socioeconomic quartile, compared with the lowest (OR, 0.61; 95% CI, 0.40-0.94).
IN PRACTICE:
“Educational initiatives, team-based care delivery, task shifting, and technological interventions to address observed gaps in patient safety procedures are needed,” the authors wrote.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco, and published online in Arthritis Care & Research
LIMITATIONS:
The study lacked access to scanned documents or clinical notes, which may have resulted in the omission of a small number of tests that had no Medicare billing. Moreover, the study was restricted to a 3-year lookback period, potentially missing some remote screenings. The findings may have limited generalizability to younger patients or those not dually eligible for Medicare and Medicaid.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute for Arthritis and Musculoskeletal and Skin Diseases. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
The rates of screening for latent tuberculosis remain suboptimal among new users of biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), with notable variations by medication type and demographic characteristics.
METHODOLOGY:
- Professional society guidelines recommend screening for tuberculosis before starting treatment with most b/tsDMARDs.
- In an attempt to estimate the extent of latent tuberculosis screening, researchers combined claims and electronic health record datasets to evaluate 2853 new b/tsDMARD users (mean age, 73 years; 72% women; and 73% non-Hispanic White).
- The primary analysis focused on assessing the proportion of patients screened for latent tuberculosis in the year before starting a new b/tsDMARD.
- A sensitivity analysis evaluated the extent of screening within the 3 years preceding the initiation of a new b/tsDMARD.
- A total of 65.6% of patients received screening for latent tuberculosis in the year before initiating a new b/tsDMARD.
- Screening rates improved only slightly on expanding the window to 3 years, with 72.9% of patients receiving any tuberculosis screening.
- When stratified by drug type, over half of new users of Janus kinase inhibitors and nearly 90% of new users of interleukin-17 inhibitors had not received screening.
- Hispanic patients had lower odds of tuberculosis screening within 1 year than White patients (odds ratio [OR], 0.64; 95% CI, 0.46-0.90), as did those in the highest socioeconomic quartile, compared with the lowest (OR, 0.61; 95% CI, 0.40-0.94).
IN PRACTICE:
“Educational initiatives, team-based care delivery, task shifting, and technological interventions to address observed gaps in patient safety procedures are needed,” the authors wrote.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco, and published online in Arthritis Care & Research
LIMITATIONS:
The study lacked access to scanned documents or clinical notes, which may have resulted in the omission of a small number of tests that had no Medicare billing. Moreover, the study was restricted to a 3-year lookback period, potentially missing some remote screenings. The findings may have limited generalizability to younger patients or those not dually eligible for Medicare and Medicaid.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute for Arthritis and Musculoskeletal and Skin Diseases. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.