User login
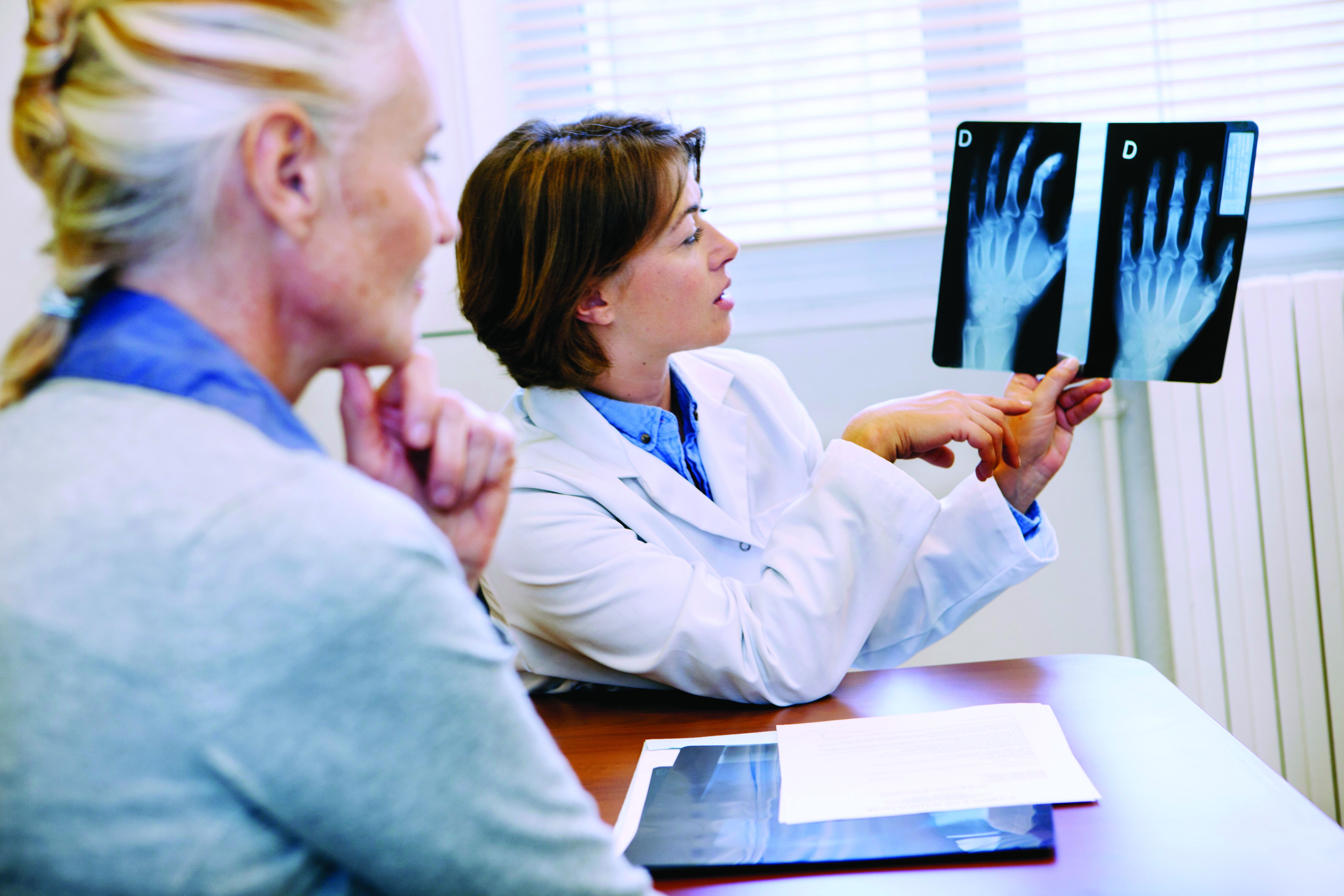
Are repeat radiographs necessary in rheumatoid and psoriatic arthritis?
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
FROM RWCS 2023
PsA prediction tool approaches clinical utility
Easily collected variables establish risk
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
Easily collected variables establish risk
Easily collected variables establish risk
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
A new tool for predicting which patients with psoriasis will develop psoriatic arthritis (PsA) is showing promise for such clinical applications as early treatment in those at risk or trials to prevent PsA, according to a summary of progress at the annual meeting of the Canadian Rheumatology Association.
Based on current levels of sensitivity and specificity, psoriasis “can be predicted with reasonable accuracy,” reported Lihi Eder, MD, PhD, director of research in the rheumatology division at the University of Toronto.
The predictive method, called PRESTO (Prediction of Psoriatic Arthritis Tool), is based on variables readily available in clinical practice, according to Dr. Eder. Once values are assigned to the risk factors, the risk of PsA over a 1-year or 5-year time frame can be estimated with a calculator.
She called PRESTO the “first clinical tool for predicting PsA among psoriasis patients.”
The work on this tool began in 2006 when the International Psoriasis and Arthritis Research Team (IPART) initiated a prospectively collected cohort of psoriasis patients. To be enrolled, patients had to be free of signs and symptoms of arthritis upon examination by a rheumatologist. They were then invited to return annually for follow-up that again included screening for joint involvement by a rheumatologist.
At baseline and at follow-up evaluations, 13 predictors were evaluated. These involved psoriasis characteristics, such as nail pitting; symptoms, such as stiffness; comorbidities, such as additional inflammatory diseases; and laboratory values, such as upregulated markers of inflammation.
Symptoms and signs used to predict PsA
Dr. Eder and her colleagues applied regression models to select an optimal combination of variables weighted for predictive value. Variables offering predictive value included higher PASI (Psoriasis Area and Severity Index), greater fatigue score as measured by FACIT (Functional Assessment of Chronic Illness Therapy) score, greater morning stiffness, and greater pain.
When applied to 635 patients in the IPART cohort, in which there were 51 incident PsA cases over 1 year and 75 incident cases over 5 years, the area under the curve (AUC) for PRESTO at the cutoffs studied was 72% for the 1-year time window and 75% for the 5-year time window.
These levels are associated with adequate accuracy, according to Dr. Eder, who explained that “an AUC greater than 70% is considered reasonable” for clinical applicability.
Moreover, the cutoffs can be adjusted for the specific purpose of the predictive tool. For example, to screen patients for risk, lower cutoffs could be employed to increase sensitivity. In order to select patients for a clinical trial to prevent PsA, higher cutoffs could be employed to increase specificity.
But sensitivities and specificities move in opposite directions when cutoffs are adjusted. Showing data from the 5-year prediction model, Dr. Eder reported that specificities climbed from about 58% to 97% as cutoffs were increased. The sensitivities with these adjustments fell from 79% to 14%.
In general, Dr. Eder said there was “excellent calibration” for the cutoffs employed when they compared the predicted and observed rates of PsA according to quintile of predictive probability. The differences were particularly minor over a 1-year time period. Over the 5-year period, observed rates were somewhat higher than predicted in the fourth and fifth quintile, but, again, this discrepancy could be modified for specific applications with cutoff adjustments.
Validation studies are planned
Even though psoriasis patients in IPART represents one of the largest cohorts of prospectively collected psoriasis patients, Dr. Eder acknowledged that the sample size would be considered “moderate” for developing a predictive model. However, the fact that the data were collected prospectively using standardized methodology strengthens the findings and provides the basis for the next step.
“Validation studies are planned with external cohorts,” said Dr. Eder, who indicated that a viable tool for identifying psoriasis patients at risk for PsA is likely. Even if it is not employed routinely in its current form at the level of individual patient care, she predicted that it will have value at a research level for understanding the relationship of psoriasis to PsA.
Christopher T. Ritchlin, MD, a professor and researcher at the University of Rochester (N.Y.), agreed that PRESTO has important potential as a clinical tool. Dr. Ritchlin has been involved in the development of PRESTO but was not involved in the presentation made at the CRA annual meeting.
“The PRESTO tool has the ability to predict the 2- and 5-year risk of developing psoriatic arthritis, which is an important advance if confirmed,” he said in an interview. He pointed out that approximately 25%-30% who develop psoriasis will go on to develop PsA but until now there has been no way to identify them.
“This tool may provide a pathway to early intervention,” he said.
Dr. Eder has financial relationships with AbbVie, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer, Sandoz, and UCB. Dr. Ritchlin has financial relationships with many of the same companies.
FROM CRA 2023
Health plans get very poor scores for access to autoimmune drugs
Both public and private health plans score poorly when it comes to providing access to autoimmune medication, according to a report commissioned by the Autoimmune Association and Let My Doctors Decide, a national partnership of health care professionals. The analysis, published Jan. 26, found that 75% of insurers in the United States have policies that can limit coverage for Food and Drug Administration–approved medications for Crohn’s disease, lupus nephritis, multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
“Choice among health plans is a hallmark of the American health insurance system, yet this analysis shows that people living with autoimmune conditions have few, if any, coverage choices that do not involve significant to severe access restrictions,” the authors wrote.
The study looked at three common utilization management policies by health plans that can limit coverage of certain medications: step therapy, formulary/tier placement, and prior authorization. To compare health plans, researchers weighted these policies using a point system. Each medication indicated for each condition was given a score of 0-4 based on access restrictions in a health plan. If a plan used step therapy, it received one point, and requiring prior authorization added an additional point. They also added points based on where a drug appeared on a plan’s formulary. A lower total score meant fewer access barriers. The numbers were then added, and each health plan received a grade of A, B, C, or F based on their average score. The datasets and analysis were provided and performed by the data analytics firm MMIT.
Nearly 9 in 10 Medicare plans received a C or worse for coverage of medication received via mail order or the pharmacy. In commercial plans, the majority of plans scored Cs or Fs for six of the seven conditions, excluding lupus nephritis, where 67% of all commercial health plans scored a B for access to these medications.
Physician-administered medications tended to receive poorer coverage than drugs received via pharmacy. Across all conditions, 65% of Medicare Advantage plans scored an F for physician-administered medication access. For both psoriasis and multiple sclerosis, at least 80% of Medicare plans earned failing scores because of these restrictions. Coverage was poorer on both commercial and health exchange plans, where across all conditions, 83% achieved failing scores. Two exceptions were the Southern and Northern California PPO plans by the Kaiser Foundation Health Plan. Out of the largest 25 health plans in the United States, these two plans earned As in coverage for physician-administered medications across all seven autoimmune conditions.
The report shows “a growing disconnect between science and health insurance benefit designs that were developed in the 1960s and 1970s,” Kenneth Thorpe, PhD, of Emory University, Atlanta, said in an interview. Insurers originally designed these benefits to prevent excessive utilization in a population of mostly acutely ill patients, he said, whereas now, 90% of healthcare spending is linked to chronic conditions. For these patients, research shows that incentivizing patients to adhere to medications results in fewer hospitalizations and, therefore, more cost savings, Thorpe noted. These plans also do not consider that there is no average patient, he said, and healthcare providers should be able to match each patient to the best treatment option for them rather than trying out other less expensive medications first. “To the extent that physicians can have the flexibility to provide medications and treatments to patients that are going to have the best clinical response, that’s better outcomes at lower cost,” Dr. Thorpe said. While research shows heterogeneity in patient outcomes with different medication, “benefit designs from the past just don’t recognize that.”
Neither America’s Health Insurance Plans nor Pharmaceutical Care Management Association responded to a request for comment.
Quardricos Driskell, executive director of Let My Doctors Decide and vice president of government relations and public policy at the Autoimmune Association, hopes the study will spur action by policy makers and health plans to improve access to medications for the people who need them. Another larger point of the report is to “uphold the sanctity of protecting the doctor and patient relationship,” he said in an interview, adding “that decisions fundamentally need to be made not by insurance plans or middleman pharmacy benefit managers, but by the provider and patient.”
Mr. Driskell and Dr. Thorpe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both public and private health plans score poorly when it comes to providing access to autoimmune medication, according to a report commissioned by the Autoimmune Association and Let My Doctors Decide, a national partnership of health care professionals. The analysis, published Jan. 26, found that 75% of insurers in the United States have policies that can limit coverage for Food and Drug Administration–approved medications for Crohn’s disease, lupus nephritis, multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
“Choice among health plans is a hallmark of the American health insurance system, yet this analysis shows that people living with autoimmune conditions have few, if any, coverage choices that do not involve significant to severe access restrictions,” the authors wrote.
The study looked at three common utilization management policies by health plans that can limit coverage of certain medications: step therapy, formulary/tier placement, and prior authorization. To compare health plans, researchers weighted these policies using a point system. Each medication indicated for each condition was given a score of 0-4 based on access restrictions in a health plan. If a plan used step therapy, it received one point, and requiring prior authorization added an additional point. They also added points based on where a drug appeared on a plan’s formulary. A lower total score meant fewer access barriers. The numbers were then added, and each health plan received a grade of A, B, C, or F based on their average score. The datasets and analysis were provided and performed by the data analytics firm MMIT.
Nearly 9 in 10 Medicare plans received a C or worse for coverage of medication received via mail order or the pharmacy. In commercial plans, the majority of plans scored Cs or Fs for six of the seven conditions, excluding lupus nephritis, where 67% of all commercial health plans scored a B for access to these medications.
Physician-administered medications tended to receive poorer coverage than drugs received via pharmacy. Across all conditions, 65% of Medicare Advantage plans scored an F for physician-administered medication access. For both psoriasis and multiple sclerosis, at least 80% of Medicare plans earned failing scores because of these restrictions. Coverage was poorer on both commercial and health exchange plans, where across all conditions, 83% achieved failing scores. Two exceptions were the Southern and Northern California PPO plans by the Kaiser Foundation Health Plan. Out of the largest 25 health plans in the United States, these two plans earned As in coverage for physician-administered medications across all seven autoimmune conditions.
The report shows “a growing disconnect between science and health insurance benefit designs that were developed in the 1960s and 1970s,” Kenneth Thorpe, PhD, of Emory University, Atlanta, said in an interview. Insurers originally designed these benefits to prevent excessive utilization in a population of mostly acutely ill patients, he said, whereas now, 90% of healthcare spending is linked to chronic conditions. For these patients, research shows that incentivizing patients to adhere to medications results in fewer hospitalizations and, therefore, more cost savings, Thorpe noted. These plans also do not consider that there is no average patient, he said, and healthcare providers should be able to match each patient to the best treatment option for them rather than trying out other less expensive medications first. “To the extent that physicians can have the flexibility to provide medications and treatments to patients that are going to have the best clinical response, that’s better outcomes at lower cost,” Dr. Thorpe said. While research shows heterogeneity in patient outcomes with different medication, “benefit designs from the past just don’t recognize that.”
Neither America’s Health Insurance Plans nor Pharmaceutical Care Management Association responded to a request for comment.
Quardricos Driskell, executive director of Let My Doctors Decide and vice president of government relations and public policy at the Autoimmune Association, hopes the study will spur action by policy makers and health plans to improve access to medications for the people who need them. Another larger point of the report is to “uphold the sanctity of protecting the doctor and patient relationship,” he said in an interview, adding “that decisions fundamentally need to be made not by insurance plans or middleman pharmacy benefit managers, but by the provider and patient.”
Mr. Driskell and Dr. Thorpe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both public and private health plans score poorly when it comes to providing access to autoimmune medication, according to a report commissioned by the Autoimmune Association and Let My Doctors Decide, a national partnership of health care professionals. The analysis, published Jan. 26, found that 75% of insurers in the United States have policies that can limit coverage for Food and Drug Administration–approved medications for Crohn’s disease, lupus nephritis, multiple sclerosis, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
“Choice among health plans is a hallmark of the American health insurance system, yet this analysis shows that people living with autoimmune conditions have few, if any, coverage choices that do not involve significant to severe access restrictions,” the authors wrote.
The study looked at three common utilization management policies by health plans that can limit coverage of certain medications: step therapy, formulary/tier placement, and prior authorization. To compare health plans, researchers weighted these policies using a point system. Each medication indicated for each condition was given a score of 0-4 based on access restrictions in a health plan. If a plan used step therapy, it received one point, and requiring prior authorization added an additional point. They also added points based on where a drug appeared on a plan’s formulary. A lower total score meant fewer access barriers. The numbers were then added, and each health plan received a grade of A, B, C, or F based on their average score. The datasets and analysis were provided and performed by the data analytics firm MMIT.
Nearly 9 in 10 Medicare plans received a C or worse for coverage of medication received via mail order or the pharmacy. In commercial plans, the majority of plans scored Cs or Fs for six of the seven conditions, excluding lupus nephritis, where 67% of all commercial health plans scored a B for access to these medications.
Physician-administered medications tended to receive poorer coverage than drugs received via pharmacy. Across all conditions, 65% of Medicare Advantage plans scored an F for physician-administered medication access. For both psoriasis and multiple sclerosis, at least 80% of Medicare plans earned failing scores because of these restrictions. Coverage was poorer on both commercial and health exchange plans, where across all conditions, 83% achieved failing scores. Two exceptions were the Southern and Northern California PPO plans by the Kaiser Foundation Health Plan. Out of the largest 25 health plans in the United States, these two plans earned As in coverage for physician-administered medications across all seven autoimmune conditions.
The report shows “a growing disconnect between science and health insurance benefit designs that were developed in the 1960s and 1970s,” Kenneth Thorpe, PhD, of Emory University, Atlanta, said in an interview. Insurers originally designed these benefits to prevent excessive utilization in a population of mostly acutely ill patients, he said, whereas now, 90% of healthcare spending is linked to chronic conditions. For these patients, research shows that incentivizing patients to adhere to medications results in fewer hospitalizations and, therefore, more cost savings, Thorpe noted. These plans also do not consider that there is no average patient, he said, and healthcare providers should be able to match each patient to the best treatment option for them rather than trying out other less expensive medications first. “To the extent that physicians can have the flexibility to provide medications and treatments to patients that are going to have the best clinical response, that’s better outcomes at lower cost,” Dr. Thorpe said. While research shows heterogeneity in patient outcomes with different medication, “benefit designs from the past just don’t recognize that.”
Neither America’s Health Insurance Plans nor Pharmaceutical Care Management Association responded to a request for comment.
Quardricos Driskell, executive director of Let My Doctors Decide and vice president of government relations and public policy at the Autoimmune Association, hopes the study will spur action by policy makers and health plans to improve access to medications for the people who need them. Another larger point of the report is to “uphold the sanctity of protecting the doctor and patient relationship,” he said in an interview, adding “that decisions fundamentally need to be made not by insurance plans or middleman pharmacy benefit managers, but by the provider and patient.”
Mr. Driskell and Dr. Thorpe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PsA: Baseline disease activity predicts DAPSA response in patients treated with apremilast
Key clinical point: Nearly half of the patients with psoriatic arthritis (PsA) treated with apremilast achieved disease activity index for psoriatic arthritis (DAPSA) low disease activity/remission at 6 or 12 months, with lower baseline disease activity being the only factor associated with the achievement of low disease activity or remission.
Major finding: Overall, 42.7% and 54.9% of patients achieved DAPSA low disease activity or remission at 6 and 12 months, respectively. Baseline DAPSA was inversely associated with the odds of achieving low disease activity or remission at 6 months (odds ratio [OR] 0.84) and 12 months (OR 0.91; both P < .01).
Study details: Findings are from a retrospective study including 293 patients with PsA who were treated with apremilast.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Becciolini A et al. Predictors of DAPSA response in psoriatic arthritis patients treated with apremilast in a retrospective observational multi-centric study. Biomedicines. 2023;11(2):433 (Feb 2). Doi: 10.3390/biomedicines11020433
Key clinical point: Nearly half of the patients with psoriatic arthritis (PsA) treated with apremilast achieved disease activity index for psoriatic arthritis (DAPSA) low disease activity/remission at 6 or 12 months, with lower baseline disease activity being the only factor associated with the achievement of low disease activity or remission.
Major finding: Overall, 42.7% and 54.9% of patients achieved DAPSA low disease activity or remission at 6 and 12 months, respectively. Baseline DAPSA was inversely associated with the odds of achieving low disease activity or remission at 6 months (odds ratio [OR] 0.84) and 12 months (OR 0.91; both P < .01).
Study details: Findings are from a retrospective study including 293 patients with PsA who were treated with apremilast.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Becciolini A et al. Predictors of DAPSA response in psoriatic arthritis patients treated with apremilast in a retrospective observational multi-centric study. Biomedicines. 2023;11(2):433 (Feb 2). Doi: 10.3390/biomedicines11020433
Key clinical point: Nearly half of the patients with psoriatic arthritis (PsA) treated with apremilast achieved disease activity index for psoriatic arthritis (DAPSA) low disease activity/remission at 6 or 12 months, with lower baseline disease activity being the only factor associated with the achievement of low disease activity or remission.
Major finding: Overall, 42.7% and 54.9% of patients achieved DAPSA low disease activity or remission at 6 and 12 months, respectively. Baseline DAPSA was inversely associated with the odds of achieving low disease activity or remission at 6 months (odds ratio [OR] 0.84) and 12 months (OR 0.91; both P < .01).
Study details: Findings are from a retrospective study including 293 patients with PsA who were treated with apremilast.
Disclosures: This study did not receive any external funding. The authors declared no conflicts of interest.
Source: Becciolini A et al. Predictors of DAPSA response in psoriatic arthritis patients treated with apremilast in a retrospective observational multi-centric study. Biomedicines. 2023;11(2):433 (Feb 2). Doi: 10.3390/biomedicines11020433
Long-term safety and tolerability of upadacitinib in PsA
Key clinical point: Upadacitinib demonstrated an acceptable long-term safety profile and was generally well tolerated with no new safety signals in patients with psoriatic arthritis (PsA).
Major finding: Overall, patients with PsA receiving 15 mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and deaths (0.8/100 PY).
Study details: This integrated safety analysis of 12 phase 3 trials included 6991 patients with PsA (n = 907), rheumatoid arthritis (n = 3,209), ankylosing spondylitis (n = 182), and atopic dermatitis (n = 2693) who received upadacitinib (15 or 30 mg once daily); 1008 patients with RA (n = 579) and PsA (n = 429) who received 40 mg adalimumab every other week; and 314 patients with RA who received methotrexate.
Disclosures: This study was funded by AbbVie. Five authors declared being full-time employees of AbbVie or Mount Sinai or holding stock or stock options in AbbVie. Several authors reported ties with various sources, including AbbVie.
Source: Burmester GR et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735;15(Feb 8). Doi: 10.1136/rmdopen-2022-002735
Key clinical point: Upadacitinib demonstrated an acceptable long-term safety profile and was generally well tolerated with no new safety signals in patients with psoriatic arthritis (PsA).
Major finding: Overall, patients with PsA receiving 15 mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and deaths (0.8/100 PY).
Study details: This integrated safety analysis of 12 phase 3 trials included 6991 patients with PsA (n = 907), rheumatoid arthritis (n = 3,209), ankylosing spondylitis (n = 182), and atopic dermatitis (n = 2693) who received upadacitinib (15 or 30 mg once daily); 1008 patients with RA (n = 579) and PsA (n = 429) who received 40 mg adalimumab every other week; and 314 patients with RA who received methotrexate.
Disclosures: This study was funded by AbbVie. Five authors declared being full-time employees of AbbVie or Mount Sinai or holding stock or stock options in AbbVie. Several authors reported ties with various sources, including AbbVie.
Source: Burmester GR et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735;15(Feb 8). Doi: 10.1136/rmdopen-2022-002735
Key clinical point: Upadacitinib demonstrated an acceptable long-term safety profile and was generally well tolerated with no new safety signals in patients with psoriatic arthritis (PsA).
Major finding: Overall, patients with PsA receiving 15 mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and deaths (0.8/100 PY).
Study details: This integrated safety analysis of 12 phase 3 trials included 6991 patients with PsA (n = 907), rheumatoid arthritis (n = 3,209), ankylosing spondylitis (n = 182), and atopic dermatitis (n = 2693) who received upadacitinib (15 or 30 mg once daily); 1008 patients with RA (n = 579) and PsA (n = 429) who received 40 mg adalimumab every other week; and 314 patients with RA who received methotrexate.
Disclosures: This study was funded by AbbVie. Five authors declared being full-time employees of AbbVie or Mount Sinai or holding stock or stock options in AbbVie. Several authors reported ties with various sources, including AbbVie.
Source: Burmester GR et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735;15(Feb 8). Doi: 10.1136/rmdopen-2022-002735
Baseline cardiovascular risk may influence MACE and malignancy incidences in tofacitinib-treated PsA patients
Key clinical point: Preventive monitoring for cardiovascular risk is suggested in tofacitinib-treated patients with psoriatic arthritis (PsA) as higher atherosclerotic cardiovascular disease (ASCVD) risk appears to be associated with a higher incidence of major cardiovascular events (MACE) and malignancies.
Major finding: The risk for MACE appeared to be higher among patients with PsA and high vs low 10-year ASCVD risk (incidence rate [IR] 1.37 [95% CI 0.03-7.63] vs 0.08 [95% CI 0.0-0.42]), with the incidence of malignancies being the highest in patients with PsA and an intermediate 10-year ASCVD risk (IR 2.56, 95% CI 1.11-5.05).
Study details: Findings are from a post hoc analysis of 10 clinical trials including patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib.
Disclosures: This study was sponsored by Pfizer. Some authors declared receiving speaker fees or grant or research support or serving as consultants for various sources, including Pfizer. Some authors declared being employees and shareholders of Pfizer or Syneos Health, a paid contractor to Pfizer.
Source: Kristensen LE et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023 (Feb 7). Doi: 10.1177/1759720X221149965
Key clinical point: Preventive monitoring for cardiovascular risk is suggested in tofacitinib-treated patients with psoriatic arthritis (PsA) as higher atherosclerotic cardiovascular disease (ASCVD) risk appears to be associated with a higher incidence of major cardiovascular events (MACE) and malignancies.
Major finding: The risk for MACE appeared to be higher among patients with PsA and high vs low 10-year ASCVD risk (incidence rate [IR] 1.37 [95% CI 0.03-7.63] vs 0.08 [95% CI 0.0-0.42]), with the incidence of malignancies being the highest in patients with PsA and an intermediate 10-year ASCVD risk (IR 2.56, 95% CI 1.11-5.05).
Study details: Findings are from a post hoc analysis of 10 clinical trials including patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib.
Disclosures: This study was sponsored by Pfizer. Some authors declared receiving speaker fees or grant or research support or serving as consultants for various sources, including Pfizer. Some authors declared being employees and shareholders of Pfizer or Syneos Health, a paid contractor to Pfizer.
Source: Kristensen LE et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023 (Feb 7). Doi: 10.1177/1759720X221149965
Key clinical point: Preventive monitoring for cardiovascular risk is suggested in tofacitinib-treated patients with psoriatic arthritis (PsA) as higher atherosclerotic cardiovascular disease (ASCVD) risk appears to be associated with a higher incidence of major cardiovascular events (MACE) and malignancies.
Major finding: The risk for MACE appeared to be higher among patients with PsA and high vs low 10-year ASCVD risk (incidence rate [IR] 1.37 [95% CI 0.03-7.63] vs 0.08 [95% CI 0.0-0.42]), with the incidence of malignancies being the highest in patients with PsA and an intermediate 10-year ASCVD risk (IR 2.56, 95% CI 1.11-5.05).
Study details: Findings are from a post hoc analysis of 10 clinical trials including patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib.
Disclosures: This study was sponsored by Pfizer. Some authors declared receiving speaker fees or grant or research support or serving as consultants for various sources, including Pfizer. Some authors declared being employees and shareholders of Pfizer or Syneos Health, a paid contractor to Pfizer.
Source: Kristensen LE et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023 (Feb 7). Doi: 10.1177/1759720X221149965
Circulating microRNA can differentiate between psoriasis and psoriatic arthritis
Key clinical point: Signatures of circulating microRNA in patients with psoriatic arthritis (PsA) and patients with psoriasis were significantly different from those in control individuals, with some even being differentially regulated between PsA and psoriasis.
Major finding: Overall, 9 microRNA best differentiated patients with PsA and psoriasis from control individuals (area under the curve [AUC] ≥0.70; all P < .05) and 4 microRNA best differentiated patients with PsA from patients with psoriasis (all P < .05). A combination of 4 microRNA (miR-19b-3p, miR-21-5p, miR-92a-3p, and let-7b-5p) vs miR-92a-3p alone could better differentiate between patients with PsA and psoriasis (AUC 0.92 vs 0.82).
Study details: This cross-sectional study included 51 patients with PsA, 40 patients with psoriasis, and 50 control individuals.
Disclosures: This study did not receive any specific funding. Two authors declared being employees or shareholders of TAmiRNA GmbH or holding intellectual property for the diagnostic use of microRNA in bone diseases.
Source: Haschka J et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology (Oxford). 2023 (Feb 3). Doi: 10.1093/rheumatology/kead059
Key clinical point: Signatures of circulating microRNA in patients with psoriatic arthritis (PsA) and patients with psoriasis were significantly different from those in control individuals, with some even being differentially regulated between PsA and psoriasis.
Major finding: Overall, 9 microRNA best differentiated patients with PsA and psoriasis from control individuals (area under the curve [AUC] ≥0.70; all P < .05) and 4 microRNA best differentiated patients with PsA from patients with psoriasis (all P < .05). A combination of 4 microRNA (miR-19b-3p, miR-21-5p, miR-92a-3p, and let-7b-5p) vs miR-92a-3p alone could better differentiate between patients with PsA and psoriasis (AUC 0.92 vs 0.82).
Study details: This cross-sectional study included 51 patients with PsA, 40 patients with psoriasis, and 50 control individuals.
Disclosures: This study did not receive any specific funding. Two authors declared being employees or shareholders of TAmiRNA GmbH or holding intellectual property for the diagnostic use of microRNA in bone diseases.
Source: Haschka J et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology (Oxford). 2023 (Feb 3). Doi: 10.1093/rheumatology/kead059
Key clinical point: Signatures of circulating microRNA in patients with psoriatic arthritis (PsA) and patients with psoriasis were significantly different from those in control individuals, with some even being differentially regulated between PsA and psoriasis.
Major finding: Overall, 9 microRNA best differentiated patients with PsA and psoriasis from control individuals (area under the curve [AUC] ≥0.70; all P < .05) and 4 microRNA best differentiated patients with PsA from patients with psoriasis (all P < .05). A combination of 4 microRNA (miR-19b-3p, miR-21-5p, miR-92a-3p, and let-7b-5p) vs miR-92a-3p alone could better differentiate between patients with PsA and psoriasis (AUC 0.92 vs 0.82).
Study details: This cross-sectional study included 51 patients with PsA, 40 patients with psoriasis, and 50 control individuals.
Disclosures: This study did not receive any specific funding. Two authors declared being employees or shareholders of TAmiRNA GmbH or holding intellectual property for the diagnostic use of microRNA in bone diseases.
Source: Haschka J et al. Identification of circulating microRNA patterns in patients in psoriasis and psoriatic arthritis. Rheumatology (Oxford). 2023 (Feb 3). Doi: 10.1093/rheumatology/kead059
Crude mortality rate doubled in PsA patients during COVID-19 pandemic
Key clinical point: The crude mortality rate increased by two-fold in patients with psoriatic arthritis (PsA) during the COVID-19 pandemic compared with the pre-pandemic era, with pulmonary reasons being the major risk factors for mortality.
Major finding: Although standard mortality rates were similar between patients with PsA and the general population during the pre-pandemic period (0.95; 95% CI 0.61-1.49), the crude mortality rate, which was 5.07 before the pandemic, increased by 2.12-fold during the pandemic in the PsA population. Interestingly, deaths due to pulmonary reasons increased from 6% during the pre-pandemic era to 66% during the pandemic.
Study details: Findings are from an international multicenter registry study including 1216 patients with PsA who were followed up for 7500 patient-years.
Disclosures: This study did not report the source of funding. Some authors declared receiving honoraria or research grants from several sources.
Source: Erden A et al. Mortality in psoriatic arthritis patients, changes over time, and the impact of COVID-19: Results from a multicenter Psoriatic Arthritis Registry (PsART-ID). Clin Rheumatol. 2023;42(2):385-390(Jan 13). Doi: 10.1007/s10067-022-06492-6
Key clinical point: The crude mortality rate increased by two-fold in patients with psoriatic arthritis (PsA) during the COVID-19 pandemic compared with the pre-pandemic era, with pulmonary reasons being the major risk factors for mortality.
Major finding: Although standard mortality rates were similar between patients with PsA and the general population during the pre-pandemic period (0.95; 95% CI 0.61-1.49), the crude mortality rate, which was 5.07 before the pandemic, increased by 2.12-fold during the pandemic in the PsA population. Interestingly, deaths due to pulmonary reasons increased from 6% during the pre-pandemic era to 66% during the pandemic.
Study details: Findings are from an international multicenter registry study including 1216 patients with PsA who were followed up for 7500 patient-years.
Disclosures: This study did not report the source of funding. Some authors declared receiving honoraria or research grants from several sources.
Source: Erden A et al. Mortality in psoriatic arthritis patients, changes over time, and the impact of COVID-19: Results from a multicenter Psoriatic Arthritis Registry (PsART-ID). Clin Rheumatol. 2023;42(2):385-390(Jan 13). Doi: 10.1007/s10067-022-06492-6
Key clinical point: The crude mortality rate increased by two-fold in patients with psoriatic arthritis (PsA) during the COVID-19 pandemic compared with the pre-pandemic era, with pulmonary reasons being the major risk factors for mortality.
Major finding: Although standard mortality rates were similar between patients with PsA and the general population during the pre-pandemic period (0.95; 95% CI 0.61-1.49), the crude mortality rate, which was 5.07 before the pandemic, increased by 2.12-fold during the pandemic in the PsA population. Interestingly, deaths due to pulmonary reasons increased from 6% during the pre-pandemic era to 66% during the pandemic.
Study details: Findings are from an international multicenter registry study including 1216 patients with PsA who were followed up for 7500 patient-years.
Disclosures: This study did not report the source of funding. Some authors declared receiving honoraria or research grants from several sources.
Source: Erden A et al. Mortality in psoriatic arthritis patients, changes over time, and the impact of COVID-19: Results from a multicenter Psoriatic Arthritis Registry (PsART-ID). Clin Rheumatol. 2023;42(2):385-390(Jan 13). Doi: 10.1007/s10067-022-06492-6
Real-world evidence on impact of PsA manifestation on patient outcomes
Key clinical point: All multiple manifestations of psoriatic arthritis (PsA) negatively affect quality of life (QoL), with dactylitis, peripheral joint disease, and psoriasis impairing functional status, whereas joint, skin, and periarticular symptoms independently impair work productivity.
Major finding: Presence vs absence of enthesitis, dactylitis, inflammatory back pain, and peripheral joint involvement was associated with worse QoL and self-rated health (all P < .05), whereas increasing number of affected joints and greater body surface area involvement significantly correlated with poorer functional state and greater work productivity impairment (all P < .05).
Study details: Findings are from a cross-sectional observational study including 2222 patients with physician-confirmed diagnosis of PsA.
Disclosures: This study did not receive any specific funding. Some authors declared receiving grants from, serving as a consultant for, being an employee of, or owning shares in different sources.
Source: Walsh JA et al. Impact of key manifestations of psoriatic arthritis on patient quality of life, functional status, and work productivity: Findings from a real-world study in the United States and Europe. Joint Bone Spine. 2023;105534 (Jan 25). Doi: 10.1016/j.jbspin.2023.105534
Key clinical point: All multiple manifestations of psoriatic arthritis (PsA) negatively affect quality of life (QoL), with dactylitis, peripheral joint disease, and psoriasis impairing functional status, whereas joint, skin, and periarticular symptoms independently impair work productivity.
Major finding: Presence vs absence of enthesitis, dactylitis, inflammatory back pain, and peripheral joint involvement was associated with worse QoL and self-rated health (all P < .05), whereas increasing number of affected joints and greater body surface area involvement significantly correlated with poorer functional state and greater work productivity impairment (all P < .05).
Study details: Findings are from a cross-sectional observational study including 2222 patients with physician-confirmed diagnosis of PsA.
Disclosures: This study did not receive any specific funding. Some authors declared receiving grants from, serving as a consultant for, being an employee of, or owning shares in different sources.
Source: Walsh JA et al. Impact of key manifestations of psoriatic arthritis on patient quality of life, functional status, and work productivity: Findings from a real-world study in the United States and Europe. Joint Bone Spine. 2023;105534 (Jan 25). Doi: 10.1016/j.jbspin.2023.105534
Key clinical point: All multiple manifestations of psoriatic arthritis (PsA) negatively affect quality of life (QoL), with dactylitis, peripheral joint disease, and psoriasis impairing functional status, whereas joint, skin, and periarticular symptoms independently impair work productivity.
Major finding: Presence vs absence of enthesitis, dactylitis, inflammatory back pain, and peripheral joint involvement was associated with worse QoL and self-rated health (all P < .05), whereas increasing number of affected joints and greater body surface area involvement significantly correlated with poorer functional state and greater work productivity impairment (all P < .05).
Study details: Findings are from a cross-sectional observational study including 2222 patients with physician-confirmed diagnosis of PsA.
Disclosures: This study did not receive any specific funding. Some authors declared receiving grants from, serving as a consultant for, being an employee of, or owning shares in different sources.
Source: Walsh JA et al. Impact of key manifestations of psoriatic arthritis on patient quality of life, functional status, and work productivity: Findings from a real-world study in the United States and Europe. Joint Bone Spine. 2023;105534 (Jan 25). Doi: 10.1016/j.jbspin.2023.105534
Concomitant PsA tied with higher comorbidities and low treatment persistence in psoriasis
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533
Key clinical point: Patients with psoriasis and concomitant psoriatic arthritis (PsA) had a greater comorbidity burden compared with those with psoriasis alone, which negatively impacted treatment persistence.
Major finding: Among patients receiving ustekinumab, those with concomitant PsA vs psoriasis alone had higher comorbidity burden, including diabetes (odds ratio [OR] 1.52; 95% CI 1.16-1.97), hypertension (OR 1.55; 95% CI 1.27-1.89), and obesity (OR 1.33; 95% CI 1.1-1.61), and a shorter time to ustekinumab discontinuation (hazard ratio 1.98; P < .0001).
Study details: This was a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs.
Disclosures: This study was funded by Janssen-Cilag Ltd. W Tillett and A Ogdie declared receiving fees and grants or research support from various sources, including Janssen. A Passey and P Gorecki declared being employees of Janssen-Cilag Ltd.
Source: Tillett W et al. Impact of psoriatic arthritis and comorbidities on ustekinumab outcomes in psoriasis: A retrospective, observational BADBIR cohort study. RMD Open. 2023;9(1):e002533 (Jan 17). Doi: 10.1136/rmdopen-2022-002533