User login
Bazedoxifene effectively prevents glucocorticoid-induced bone loss in RA
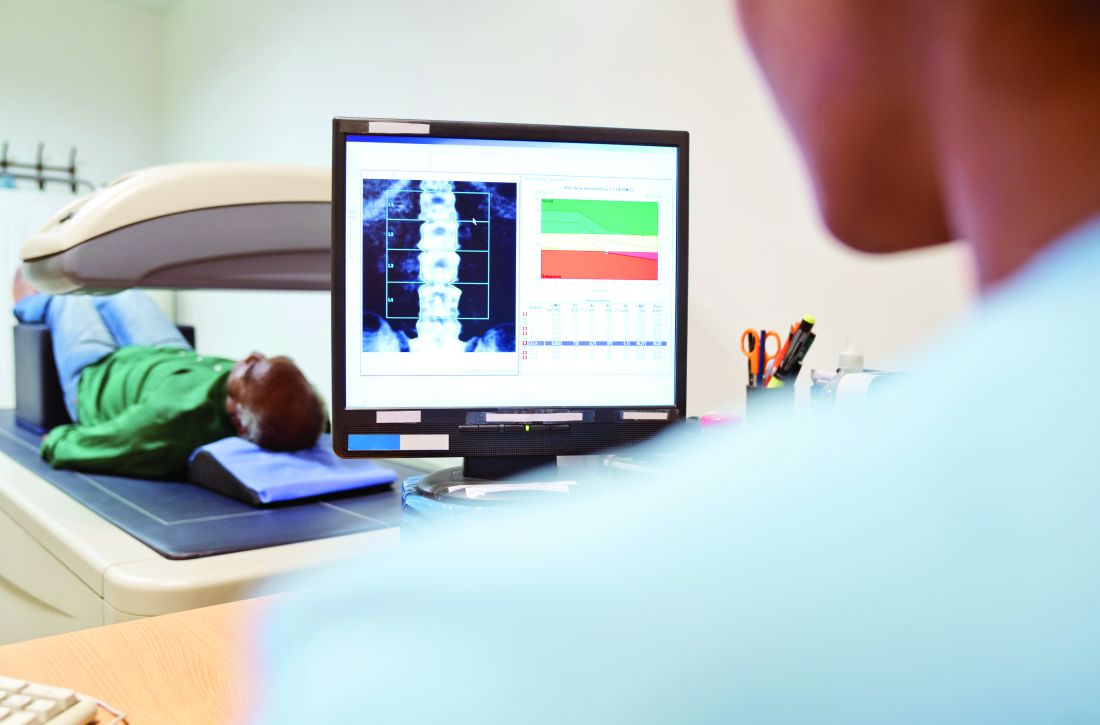
Key clinical point: Treatment with bazedoxifene for 48 weeks significantly increased bone mineral density (BMD) of L-spine and decreased bone turnover markers in postmenopausal women with rheumatoid arthritis (RA) receiving low-dose glucocorticoids.
Major finding: Treatment with bazedoxifene significantly increased L-spine BMD (0.015 g/cm2; P = .007), whereas the change in the control group was not significant (0.002 g/cm2; P = .694). At 24 weeks, all bone turnover biomarkers decreased significantly with bazedoxifene, which persisted to 48 weeks (all P less than .01), whereas recovery observed in the control group was not significant.
Study details: The data come from an open-label study involving 114 postmenopausal women with osteopenia who had been receiving low-dose glucocorticoids for RA and were randomly assigned to either daily bazedoxifene (20 mg/day) with calcium and vitamin D or only calcium and vitamin D (control group).
Disclosures: The study was supported by a research grant from Pfizer Pharmaceuticals Korea Ltd. YK Sung reported receiving research grants from Bristol-Myers Squibb, Eisai, Pfizer, and JW Pharmaceutical.
Source: Cho SK et al. Arthritis Res Ther. 2021 Jul 2. doi: 10.1186/s13075-021-02564-1.
Key clinical point: Treatment with bazedoxifene for 48 weeks significantly increased bone mineral density (BMD) of L-spine and decreased bone turnover markers in postmenopausal women with rheumatoid arthritis (RA) receiving low-dose glucocorticoids.
Major finding: Treatment with bazedoxifene significantly increased L-spine BMD (0.015 g/cm2; P = .007), whereas the change in the control group was not significant (0.002 g/cm2; P = .694). At 24 weeks, all bone turnover biomarkers decreased significantly with bazedoxifene, which persisted to 48 weeks (all P less than .01), whereas recovery observed in the control group was not significant.
Study details: The data come from an open-label study involving 114 postmenopausal women with osteopenia who had been receiving low-dose glucocorticoids for RA and were randomly assigned to either daily bazedoxifene (20 mg/day) with calcium and vitamin D or only calcium and vitamin D (control group).
Disclosures: The study was supported by a research grant from Pfizer Pharmaceuticals Korea Ltd. YK Sung reported receiving research grants from Bristol-Myers Squibb, Eisai, Pfizer, and JW Pharmaceutical.
Source: Cho SK et al. Arthritis Res Ther. 2021 Jul 2. doi: 10.1186/s13075-021-02564-1.
Key clinical point: Treatment with bazedoxifene for 48 weeks significantly increased bone mineral density (BMD) of L-spine and decreased bone turnover markers in postmenopausal women with rheumatoid arthritis (RA) receiving low-dose glucocorticoids.
Major finding: Treatment with bazedoxifene significantly increased L-spine BMD (0.015 g/cm2; P = .007), whereas the change in the control group was not significant (0.002 g/cm2; P = .694). At 24 weeks, all bone turnover biomarkers decreased significantly with bazedoxifene, which persisted to 48 weeks (all P less than .01), whereas recovery observed in the control group was not significant.
Study details: The data come from an open-label study involving 114 postmenopausal women with osteopenia who had been receiving low-dose glucocorticoids for RA and were randomly assigned to either daily bazedoxifene (20 mg/day) with calcium and vitamin D or only calcium and vitamin D (control group).
Disclosures: The study was supported by a research grant from Pfizer Pharmaceuticals Korea Ltd. YK Sung reported receiving research grants from Bristol-Myers Squibb, Eisai, Pfizer, and JW Pharmaceutical.
Source: Cho SK et al. Arthritis Res Ther. 2021 Jul 2. doi: 10.1186/s13075-021-02564-1.
RA: No significant change in efficacy and safety after adalimumab to CT-P17 switch
Key clinical point: In patients with active rheumatoid arthritis (RA), adalimumab biosimilar CT-P17 and reference adalimumab showed comparable efficacy and safety during 1 year of treatment even after switching to CT-P17 from reference adalimumab.
Major finding: At 52 weeks, the proportion of patients achieving a 20%/50%/70% improvement by American College of Rheumatology criteria was comparable among those who continued CT-P17 (80.5%/66.3%/44.6%), continued reference adalimumab (77.8%/62.1%/49.0%), or switched to CT-P17 (82.2%/66.4%/47.4%), respectively. Similar proportions of patients in each treatment group experienced 1 or more treatment-emergent adverse events.
Study details: This was a 52-week, randomized, phase 3 study of 607 patients with active RA who received either 40 mg (100 mg/mL) CT-P17 or reference adalimumab subcutaneously every 2 weeks until week 24. Later, patients receiving CT-P17 continued to receive the same, whereas those receiving reference adalimumab either continued the same or switched to CT-P17.
Disclosures: This work was supported by Celltrion, Inc. Some of the authors reported receiving research grants, consulting, speaker fees, and/or honoraria from various sources. SJ Lee, SH Kim, YJ Bae, GE Yang, and JK Yoo declared being employees of Celltrion, Inc.
Source: Furst DE et al. Rheumatology (Oxford). 2021 Jun 17. doi: 10.1093/rheumatology/keab460.
Key clinical point: In patients with active rheumatoid arthritis (RA), adalimumab biosimilar CT-P17 and reference adalimumab showed comparable efficacy and safety during 1 year of treatment even after switching to CT-P17 from reference adalimumab.
Major finding: At 52 weeks, the proportion of patients achieving a 20%/50%/70% improvement by American College of Rheumatology criteria was comparable among those who continued CT-P17 (80.5%/66.3%/44.6%), continued reference adalimumab (77.8%/62.1%/49.0%), or switched to CT-P17 (82.2%/66.4%/47.4%), respectively. Similar proportions of patients in each treatment group experienced 1 or more treatment-emergent adverse events.
Study details: This was a 52-week, randomized, phase 3 study of 607 patients with active RA who received either 40 mg (100 mg/mL) CT-P17 or reference adalimumab subcutaneously every 2 weeks until week 24. Later, patients receiving CT-P17 continued to receive the same, whereas those receiving reference adalimumab either continued the same or switched to CT-P17.
Disclosures: This work was supported by Celltrion, Inc. Some of the authors reported receiving research grants, consulting, speaker fees, and/or honoraria from various sources. SJ Lee, SH Kim, YJ Bae, GE Yang, and JK Yoo declared being employees of Celltrion, Inc.
Source: Furst DE et al. Rheumatology (Oxford). 2021 Jun 17. doi: 10.1093/rheumatology/keab460.
Key clinical point: In patients with active rheumatoid arthritis (RA), adalimumab biosimilar CT-P17 and reference adalimumab showed comparable efficacy and safety during 1 year of treatment even after switching to CT-P17 from reference adalimumab.
Major finding: At 52 weeks, the proportion of patients achieving a 20%/50%/70% improvement by American College of Rheumatology criteria was comparable among those who continued CT-P17 (80.5%/66.3%/44.6%), continued reference adalimumab (77.8%/62.1%/49.0%), or switched to CT-P17 (82.2%/66.4%/47.4%), respectively. Similar proportions of patients in each treatment group experienced 1 or more treatment-emergent adverse events.
Study details: This was a 52-week, randomized, phase 3 study of 607 patients with active RA who received either 40 mg (100 mg/mL) CT-P17 or reference adalimumab subcutaneously every 2 weeks until week 24. Later, patients receiving CT-P17 continued to receive the same, whereas those receiving reference adalimumab either continued the same or switched to CT-P17.
Disclosures: This work was supported by Celltrion, Inc. Some of the authors reported receiving research grants, consulting, speaker fees, and/or honoraria from various sources. SJ Lee, SH Kim, YJ Bae, GE Yang, and JK Yoo declared being employees of Celltrion, Inc.
Source: Furst DE et al. Rheumatology (Oxford). 2021 Jun 17. doi: 10.1093/rheumatology/keab460.
Commentary: RA August 2021
Recently, ACR guidelines were published on the prevention and treatment of glucocorticoid-induced osteoporosis, emphasizing use of supplemental calcium and vitamin D and antiresorptive therapy. This randomized, controlled, open-label trial by Cho et al looks at the efficacy of selective estrogen receptor modulators (SERMs) in preventing bone loss and fractures in postmenopausal RA patients with osteopenia on long-term glucocorticoids. Patients were randomized to bazedoxefine, a 3rd-generation SERM vs no therapy in addition to background calcium and vitamin D supplementation and bone mineral density (BMD) and trabecular bone score (TBS). Bone turnover markers were assessed over the course of 48 weeks, in addition to adverse events. Prednisone dose was relatively low, with a baseline of ~3 mg/day. Bone density increased by almost 2% in the L spine (P < 0.05), but the increases in BMD at the femoral neck and TBS were not significant. Bone turnover markers did decrease in the bazedoxefine group, though not in the control group. These findings are not striking but are promising given the short duration of the study (<1 year), and potentially deserves longer-term follow-up, both for evaluation of efficacy as well as long-term safety. In the US, the medication is only available in combination with estrogen, and thus it is not currently a practical option for RA patients with GIOP.
Despite wider availability of both synthetic and biologic DMARDs for treatment of RA in recent years, persistent pain is an issue for many patients, perhaps due to peripheral sensitization. Though it seems almost unnecessary to investigate the utility of glucocorticoids for treatment of RA-related pain, the magnitude and duration of their benefit is unknown. McWilliams et al take the approach of quantitatively analyzing the benefit of glucocorticoids in RA over time in their systematic review and meta-analysis. The study looked at both spontaneous and evoked pain, and evaluated 65 studies that mostly used pain visual analogue scale, tender joint count, and Ritchie Articular Index. Reduction in spontaneous pain was greatest in the 0-3-month time period, with smaller reductions in 3-6 months and >6 month. A similar pattern was seen in evaluation of the time course of evoked pain. As such, the benefits of long-term systemic glucocorticoid therapy may not outweigh its well-known risks, especially in patients with persistent pain. Whether this is due to non-inflammatory sources of pain or other mechanisms remains unknown and is likely heavily impacted by the specific clinical scenario.
Due to the potential long-terms side effects of chronic immunosuppression, including long-term glucocorticoid therapy in patients with persistent pain in RA, the course and predictors of unacceptable pain are of interest. Eberhard et al examined an inception cohort of RA patients in a single center in Sweden over the course of 5 years. They were found to have reduction of VAS pain from inclusion to 6 months, but pain levels largely stayed the same during the rest of the follow-up period. The proportion of patients with unacceptable pain also decreased but remained stable at about 30% after 1 year, with 20% having unacceptable pain and low inflammation and about 10% having high inflammation and unacceptable pain. The study found seropositivity, high inflammatory parameters, high DAS28 and severe patient-reported outcomes to be predictive of high inflammation and unacceptable pain and lower age, higher VAS pain, and low ESR to be predictive of low inflammation and unacceptable pain. These findings highlight the importance of examining non-inflammatory mechanisms of pain as well as identifying treatments; however, in evaluating the risk of immunosuppression and glucocorticoid therapy, it does not guide increasing or reducing treatment in RA patients with persistent pain.
Recently, ACR guidelines were published on the prevention and treatment of glucocorticoid-induced osteoporosis, emphasizing use of supplemental calcium and vitamin D and antiresorptive therapy. This randomized, controlled, open-label trial by Cho et al looks at the efficacy of selective estrogen receptor modulators (SERMs) in preventing bone loss and fractures in postmenopausal RA patients with osteopenia on long-term glucocorticoids. Patients were randomized to bazedoxefine, a 3rd-generation SERM vs no therapy in addition to background calcium and vitamin D supplementation and bone mineral density (BMD) and trabecular bone score (TBS). Bone turnover markers were assessed over the course of 48 weeks, in addition to adverse events. Prednisone dose was relatively low, with a baseline of ~3 mg/day. Bone density increased by almost 2% in the L spine (P < 0.05), but the increases in BMD at the femoral neck and TBS were not significant. Bone turnover markers did decrease in the bazedoxefine group, though not in the control group. These findings are not striking but are promising given the short duration of the study (<1 year), and potentially deserves longer-term follow-up, both for evaluation of efficacy as well as long-term safety. In the US, the medication is only available in combination with estrogen, and thus it is not currently a practical option for RA patients with GIOP.
Despite wider availability of both synthetic and biologic DMARDs for treatment of RA in recent years, persistent pain is an issue for many patients, perhaps due to peripheral sensitization. Though it seems almost unnecessary to investigate the utility of glucocorticoids for treatment of RA-related pain, the magnitude and duration of their benefit is unknown. McWilliams et al take the approach of quantitatively analyzing the benefit of glucocorticoids in RA over time in their systematic review and meta-analysis. The study looked at both spontaneous and evoked pain, and evaluated 65 studies that mostly used pain visual analogue scale, tender joint count, and Ritchie Articular Index. Reduction in spontaneous pain was greatest in the 0-3-month time period, with smaller reductions in 3-6 months and >6 month. A similar pattern was seen in evaluation of the time course of evoked pain. As such, the benefits of long-term systemic glucocorticoid therapy may not outweigh its well-known risks, especially in patients with persistent pain. Whether this is due to non-inflammatory sources of pain or other mechanisms remains unknown and is likely heavily impacted by the specific clinical scenario.
Due to the potential long-terms side effects of chronic immunosuppression, including long-term glucocorticoid therapy in patients with persistent pain in RA, the course and predictors of unacceptable pain are of interest. Eberhard et al examined an inception cohort of RA patients in a single center in Sweden over the course of 5 years. They were found to have reduction of VAS pain from inclusion to 6 months, but pain levels largely stayed the same during the rest of the follow-up period. The proportion of patients with unacceptable pain also decreased but remained stable at about 30% after 1 year, with 20% having unacceptable pain and low inflammation and about 10% having high inflammation and unacceptable pain. The study found seropositivity, high inflammatory parameters, high DAS28 and severe patient-reported outcomes to be predictive of high inflammation and unacceptable pain and lower age, higher VAS pain, and low ESR to be predictive of low inflammation and unacceptable pain. These findings highlight the importance of examining non-inflammatory mechanisms of pain as well as identifying treatments; however, in evaluating the risk of immunosuppression and glucocorticoid therapy, it does not guide increasing or reducing treatment in RA patients with persistent pain.
Recently, ACR guidelines were published on the prevention and treatment of glucocorticoid-induced osteoporosis, emphasizing use of supplemental calcium and vitamin D and antiresorptive therapy. This randomized, controlled, open-label trial by Cho et al looks at the efficacy of selective estrogen receptor modulators (SERMs) in preventing bone loss and fractures in postmenopausal RA patients with osteopenia on long-term glucocorticoids. Patients were randomized to bazedoxefine, a 3rd-generation SERM vs no therapy in addition to background calcium and vitamin D supplementation and bone mineral density (BMD) and trabecular bone score (TBS). Bone turnover markers were assessed over the course of 48 weeks, in addition to adverse events. Prednisone dose was relatively low, with a baseline of ~3 mg/day. Bone density increased by almost 2% in the L spine (P < 0.05), but the increases in BMD at the femoral neck and TBS were not significant. Bone turnover markers did decrease in the bazedoxefine group, though not in the control group. These findings are not striking but are promising given the short duration of the study (<1 year), and potentially deserves longer-term follow-up, both for evaluation of efficacy as well as long-term safety. In the US, the medication is only available in combination with estrogen, and thus it is not currently a practical option for RA patients with GIOP.
Despite wider availability of both synthetic and biologic DMARDs for treatment of RA in recent years, persistent pain is an issue for many patients, perhaps due to peripheral sensitization. Though it seems almost unnecessary to investigate the utility of glucocorticoids for treatment of RA-related pain, the magnitude and duration of their benefit is unknown. McWilliams et al take the approach of quantitatively analyzing the benefit of glucocorticoids in RA over time in their systematic review and meta-analysis. The study looked at both spontaneous and evoked pain, and evaluated 65 studies that mostly used pain visual analogue scale, tender joint count, and Ritchie Articular Index. Reduction in spontaneous pain was greatest in the 0-3-month time period, with smaller reductions in 3-6 months and >6 month. A similar pattern was seen in evaluation of the time course of evoked pain. As such, the benefits of long-term systemic glucocorticoid therapy may not outweigh its well-known risks, especially in patients with persistent pain. Whether this is due to non-inflammatory sources of pain or other mechanisms remains unknown and is likely heavily impacted by the specific clinical scenario.
Due to the potential long-terms side effects of chronic immunosuppression, including long-term glucocorticoid therapy in patients with persistent pain in RA, the course and predictors of unacceptable pain are of interest. Eberhard et al examined an inception cohort of RA patients in a single center in Sweden over the course of 5 years. They were found to have reduction of VAS pain from inclusion to 6 months, but pain levels largely stayed the same during the rest of the follow-up period. The proportion of patients with unacceptable pain also decreased but remained stable at about 30% after 1 year, with 20% having unacceptable pain and low inflammation and about 10% having high inflammation and unacceptable pain. The study found seropositivity, high inflammatory parameters, high DAS28 and severe patient-reported outcomes to be predictive of high inflammation and unacceptable pain and lower age, higher VAS pain, and low ESR to be predictive of low inflammation and unacceptable pain. These findings highlight the importance of examining non-inflammatory mechanisms of pain as well as identifying treatments; however, in evaluating the risk of immunosuppression and glucocorticoid therapy, it does not guide increasing or reducing treatment in RA patients with persistent pain.
COVID-19 vaccine hesitancy still weighs heavy for some rheumatic disease patients
With 49% of the U.S. population fully vaccinated against SARS-CoV-2, a new study highlights the degree of vaccine hesitancy among patients with rheumatic disease to get the vaccine.
The international study, published in May 2021 in Rheumatology, suggests that, of 1,258 patients surveyed worldwide, approximately 40% of patients said they would decline the vaccine.
“Sometimes it’s helpful to talk through their concerns,” said Jeffrey Curtis, MD, MPH, a University of Alabama at Birmingham rheumatologist who leads the American College of Rheumatology COVID-19 vaccine task force. Dr. Curtis recently reviewed the current literature on COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases (RMDs) at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
COVID-19 vaccinations for patients with autoimmune inflammatory rheumatic disease (AIIRD) is not straightforward. The immune response can be blunted by existing treatments and disease flares can occur.
The latest version of COVID-19 vaccination guidance for patients with RMDs from the ACR addresses vaccine use and implementation strategies. The guidance was issued as conditional or provisional because of the lack of evidence. Its principals are largely based on accepted practice for other vaccines. The guidance is routinely updated as new evidence becomes available. In his presentation at GRAPPA, Dr. Curtis reviewed the latest version of the guidance, which he emphasized is a guidance only and not meant to replace clinical judgment or shared decision-making with patients.
“This is a platform for you to start from as you are thinking about and discussing with your patient what might be best for him or her,” he said.
Concerns about impact of disease activity, treatments on effectiveness
Dr. Curtis highlighted some controversial aspects of COVID-19 vaccines, including heterogeneity of rheumatic diseases and treatment. Patients with AIIRD, including psoriatic arthritis, spondyloarthritis, RA, and lupus, are at higher risk for hospitalized COVID-19 and worse outcomes, and as such, they are prioritized for vaccination by the Centers for Disease Control and Prevention.
However, for AIIRD patients, the immune response to COVID-19 vaccination can be “blunted,” according to one study. This may be because of glucocorticoid use or high disease activity. Immunomodulatory therapies, such as methotrexate, rituximab, and abatacept, are known to diminish vaccine response in general. The evidence is less clear for tumor necrosis factor and Janus kinase inhibitors, but they are thought to have the same impact on vaccine effectiveness, Dr. Curtis said. But in these cases, if the effect of a COVID-19 vaccine drops from 90% to 70%, the benefits of vaccination still far outweighs the risk of contracting COVID-19.
“Although we don’t have strong data with clinical outcomes for autoimmune disease or inflammatory disease patients, I’ll run a hypothetical and say: ‘Look, if this vaccine starts 90%-95% effective, even if it’s only 70% effective in somebody with lupus or vasculitis or someone who is taking a higher dose of steroids, I’ll take 70% over nothing if you chose to be vaccinated,’ ” he said.
The benefit of vaccination also outweighs the potential risk of disease flare, he said. The risk is real, but to date, no studies have pointed to a significant risk of disease flare or worsening. However, there have been reported cases of myocardial infarction.
Autoimmune manifestations after vaccination vs. after infection
Researchers writing in the June 29, 2021, issue of JAMA Cardiology described case reports of acute myocarditis in 23 people who received the BNT162b2-mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) messenger RNA (mRNA) COVID-19 vaccines. Plus, there been subsequent reports of myocarditis in other patients, wrote David K. Shay, MD, MPH, in an accompanying editorial. Dr. Shay is a member of the CDC COVID-19 Response Team.
“What do we know about this possible association between myocarditis and immunization with mRNA-based COVID-19 vaccines, and what remains unclear? Acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism,” he said.
The cases of myocarditis are concerning, Dr. Curtis said, but the risk is very low with relatively few cases reported among 161 million fully vaccinated people in the United States.
“Certainly, we’re not seeking to minimize that, but the risk of getting COVID and some of the downstream sequelae (autoimmune manifestations) almost certainly outweigh the risks for some of the autoimmune manifestations or worsening [condition],” he said.
A nationwide cohort study from Denmark of 58,052 patients with inflammatory rheumatic disease published in December 2020 in Rheumatology, found that patients with COVID-19 who had an inflammatory rheumatic disease were more likely to be admitted to the hospital, compared with COVID-19 patients without rheumatic disease. Patients with rheumatic disease had a higher risk of a severe COVID-19 outcome, but it was not a statistically significant difference, said Dr. Curtis, adding that the individual factors such as age and treatment currently received largely determines the risk. The strongest associations between hospitalization for COVID-19 and rheumatic disease were found among patients with RA, vasculitis, and connective tissue disease. Dr. Curtis noted that his own new study results show that risk of death from a COVID-19 infection is higher for patients who have RA or psoriatic arthritis.
There have been published case reports of patients who have developed new-onset lupus, vasculitis, Kawasaki disease, multiple sclerosis, autoimmune cytopenias, and other manifestations after a COVID-19 infection. “These authors suggest that perhaps there is a transient influence on the immune system that leads to a loss of self-tolerance to antigens,” Dr. Curtis said. “Some patients may have an underlying predisposition to autoimmunity in which infections just unmask as we sometimes see with other infections – chronic hepatitis for example.”
Antibody tests not recommended
In its COVID-19 guidance, the ACR, like the Food and Drug Administration, recommends health care providers not to routinely order antibody tests for IgM or IgG to assess immunity after a person has been vaccinated or to assess the need for vaccination in an unvaccinated person. More research is needed to determine if antibodies provide protection, and if so, for how long and how much. Plus, the antibody testing process is not clear cut, so ordering the wrong test is possible, Dr. Curtis said. The tests should clearly differentiate between spike proteins or nucleocapsid proteins.
“The bottom line is that you might be ordering the wrong lab test. Even if you’re ordering the right lab test, I would assert that you probably don’t know what to do with the result. I would then ask you, ‘Does it mean they are protected? Does it mean they are not protected? What are you going to do with the results?’ ” he asked.
Kevin Winthrop, MD, MPH, a specialist in infectious diseases at Oregon Health & Science University, Portland, said that, at this point, it’s too early to know what antibody tests mean. “I think it is tempting to test some people, especially patients on B-cell depletion therapy and those on mycophenolate mofetil (MMF). Outside of those two types of [disease-modifying antirheumatic drug] users, I wouldn’t be tempted to test. We don’t know how well protected they are, but we assume they are protected to some extent,” he said. “They’re probably partially protected and as such, they should take the same precautions they were taking a year ago: masking and avoidance. I think that’s just how it’s going to be for those folks for another year until we get this thing sorted out.”
Modifications to existing rheumatic disease therapies
In its COVID-19 vaccine guidance, the ACR issued recommendations for some common rheumatic disease therapeutics before and/or after the COVID-19 vaccine is administered. The modifications are limited to MMF, methotrexate, JAK inhibitors, subcutaneous abatacept, acetaminophen, and NSAIDs. The recommendations include: hold mycophenolate for 1 week after vaccination if disease is stable; for patients with well-controlled disease, hold methotrexate for 1 week after each of the two mRNA vaccine doses; for patients with well-controlled disease receiving the Johnson & Johnson vaccine, hold methotrexate for 2 weeks after receiving the vaccine; hold JAK inhibitors for 1 week after each dose; for abatacept subcutaneous, hold treatment for 1 week before and after the first dose; and in patients with stable disease, hold acetaminophen and NSAIDs for 24 hours before vaccination, because taking either before vaccination could blunt the vaccine response, Dr. Curtis said.
Holding medication, such as methotrexate, could risk having a flare-up of disease. One study showed the rate of disease flare-up because of withholding standard treatment may be up to 11%, compared with 5.1% in patients who did not hold treatment, he said.
“The point is, if you hold some of these therapies, whether methotrexate or tofacitinib, arthritis will get a little bit worse,” Dr. Curtis said.
A study published on the preprint server medRxiv found that immunosuppressive therapies blunted the response of SARS-CoV-2 vaccines in patients with chronic inflammatory diseases, most significantly with glucocorticoids and B-cell therapies.
“That’s what’s led to a lot of the guidance statements about holding treatments for a week or 2 for rituximab. If you’re giving it at 6-month intervals, you want to schedule the vaccine dose or series at about month 5, or a month before the next cycle,” he said.
Talking with patients about COVID-19 vaccination
In talking with patients about vaccine safety, Dr. Curtis recommends addressing a few common misperceptions. First, COVID-19 viruses were not created with a live-attenuated virus (which would be contraindicated for immunosuppressed patients). “You can put patients’ mind at ease that none of the vaccine candidates or platforms – even those that say viral vector – put patients at risk for contracting the infection. These are nonreplicating. So, it’s like you extracted the engine that would allow this virus to replicate,” he said.
Of three COVID-19 vaccinations available in the United States, is one better than the other? The ACR COVID-19 vaccine task force did not reach a consensus on safety profiles of the vaccines because, without head-to-head comparisons, it’s impossible to know, he said.
In talking with patients, review the protocol for continuing with prescribed treatment modalities before the patient receives a COVID-19 vaccine. Safety concerns and concerns about the possibility of having a disease flare-up should be addressed, he said.
With 49% of the U.S. population fully vaccinated against SARS-CoV-2, a new study highlights the degree of vaccine hesitancy among patients with rheumatic disease to get the vaccine.
The international study, published in May 2021 in Rheumatology, suggests that, of 1,258 patients surveyed worldwide, approximately 40% of patients said they would decline the vaccine.
“Sometimes it’s helpful to talk through their concerns,” said Jeffrey Curtis, MD, MPH, a University of Alabama at Birmingham rheumatologist who leads the American College of Rheumatology COVID-19 vaccine task force. Dr. Curtis recently reviewed the current literature on COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases (RMDs) at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
COVID-19 vaccinations for patients with autoimmune inflammatory rheumatic disease (AIIRD) is not straightforward. The immune response can be blunted by existing treatments and disease flares can occur.
The latest version of COVID-19 vaccination guidance for patients with RMDs from the ACR addresses vaccine use and implementation strategies. The guidance was issued as conditional or provisional because of the lack of evidence. Its principals are largely based on accepted practice for other vaccines. The guidance is routinely updated as new evidence becomes available. In his presentation at GRAPPA, Dr. Curtis reviewed the latest version of the guidance, which he emphasized is a guidance only and not meant to replace clinical judgment or shared decision-making with patients.
“This is a platform for you to start from as you are thinking about and discussing with your patient what might be best for him or her,” he said.
Concerns about impact of disease activity, treatments on effectiveness
Dr. Curtis highlighted some controversial aspects of COVID-19 vaccines, including heterogeneity of rheumatic diseases and treatment. Patients with AIIRD, including psoriatic arthritis, spondyloarthritis, RA, and lupus, are at higher risk for hospitalized COVID-19 and worse outcomes, and as such, they are prioritized for vaccination by the Centers for Disease Control and Prevention.
However, for AIIRD patients, the immune response to COVID-19 vaccination can be “blunted,” according to one study. This may be because of glucocorticoid use or high disease activity. Immunomodulatory therapies, such as methotrexate, rituximab, and abatacept, are known to diminish vaccine response in general. The evidence is less clear for tumor necrosis factor and Janus kinase inhibitors, but they are thought to have the same impact on vaccine effectiveness, Dr. Curtis said. But in these cases, if the effect of a COVID-19 vaccine drops from 90% to 70%, the benefits of vaccination still far outweighs the risk of contracting COVID-19.
“Although we don’t have strong data with clinical outcomes for autoimmune disease or inflammatory disease patients, I’ll run a hypothetical and say: ‘Look, if this vaccine starts 90%-95% effective, even if it’s only 70% effective in somebody with lupus or vasculitis or someone who is taking a higher dose of steroids, I’ll take 70% over nothing if you chose to be vaccinated,’ ” he said.
The benefit of vaccination also outweighs the potential risk of disease flare, he said. The risk is real, but to date, no studies have pointed to a significant risk of disease flare or worsening. However, there have been reported cases of myocardial infarction.
Autoimmune manifestations after vaccination vs. after infection
Researchers writing in the June 29, 2021, issue of JAMA Cardiology described case reports of acute myocarditis in 23 people who received the BNT162b2-mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) messenger RNA (mRNA) COVID-19 vaccines. Plus, there been subsequent reports of myocarditis in other patients, wrote David K. Shay, MD, MPH, in an accompanying editorial. Dr. Shay is a member of the CDC COVID-19 Response Team.
“What do we know about this possible association between myocarditis and immunization with mRNA-based COVID-19 vaccines, and what remains unclear? Acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism,” he said.
The cases of myocarditis are concerning, Dr. Curtis said, but the risk is very low with relatively few cases reported among 161 million fully vaccinated people in the United States.
“Certainly, we’re not seeking to minimize that, but the risk of getting COVID and some of the downstream sequelae (autoimmune manifestations) almost certainly outweigh the risks for some of the autoimmune manifestations or worsening [condition],” he said.
A nationwide cohort study from Denmark of 58,052 patients with inflammatory rheumatic disease published in December 2020 in Rheumatology, found that patients with COVID-19 who had an inflammatory rheumatic disease were more likely to be admitted to the hospital, compared with COVID-19 patients without rheumatic disease. Patients with rheumatic disease had a higher risk of a severe COVID-19 outcome, but it was not a statistically significant difference, said Dr. Curtis, adding that the individual factors such as age and treatment currently received largely determines the risk. The strongest associations between hospitalization for COVID-19 and rheumatic disease were found among patients with RA, vasculitis, and connective tissue disease. Dr. Curtis noted that his own new study results show that risk of death from a COVID-19 infection is higher for patients who have RA or psoriatic arthritis.
There have been published case reports of patients who have developed new-onset lupus, vasculitis, Kawasaki disease, multiple sclerosis, autoimmune cytopenias, and other manifestations after a COVID-19 infection. “These authors suggest that perhaps there is a transient influence on the immune system that leads to a loss of self-tolerance to antigens,” Dr. Curtis said. “Some patients may have an underlying predisposition to autoimmunity in which infections just unmask as we sometimes see with other infections – chronic hepatitis for example.”
Antibody tests not recommended
In its COVID-19 guidance, the ACR, like the Food and Drug Administration, recommends health care providers not to routinely order antibody tests for IgM or IgG to assess immunity after a person has been vaccinated or to assess the need for vaccination in an unvaccinated person. More research is needed to determine if antibodies provide protection, and if so, for how long and how much. Plus, the antibody testing process is not clear cut, so ordering the wrong test is possible, Dr. Curtis said. The tests should clearly differentiate between spike proteins or nucleocapsid proteins.
“The bottom line is that you might be ordering the wrong lab test. Even if you’re ordering the right lab test, I would assert that you probably don’t know what to do with the result. I would then ask you, ‘Does it mean they are protected? Does it mean they are not protected? What are you going to do with the results?’ ” he asked.
Kevin Winthrop, MD, MPH, a specialist in infectious diseases at Oregon Health & Science University, Portland, said that, at this point, it’s too early to know what antibody tests mean. “I think it is tempting to test some people, especially patients on B-cell depletion therapy and those on mycophenolate mofetil (MMF). Outside of those two types of [disease-modifying antirheumatic drug] users, I wouldn’t be tempted to test. We don’t know how well protected they are, but we assume they are protected to some extent,” he said. “They’re probably partially protected and as such, they should take the same precautions they were taking a year ago: masking and avoidance. I think that’s just how it’s going to be for those folks for another year until we get this thing sorted out.”
Modifications to existing rheumatic disease therapies
In its COVID-19 vaccine guidance, the ACR issued recommendations for some common rheumatic disease therapeutics before and/or after the COVID-19 vaccine is administered. The modifications are limited to MMF, methotrexate, JAK inhibitors, subcutaneous abatacept, acetaminophen, and NSAIDs. The recommendations include: hold mycophenolate for 1 week after vaccination if disease is stable; for patients with well-controlled disease, hold methotrexate for 1 week after each of the two mRNA vaccine doses; for patients with well-controlled disease receiving the Johnson & Johnson vaccine, hold methotrexate for 2 weeks after receiving the vaccine; hold JAK inhibitors for 1 week after each dose; for abatacept subcutaneous, hold treatment for 1 week before and after the first dose; and in patients with stable disease, hold acetaminophen and NSAIDs for 24 hours before vaccination, because taking either before vaccination could blunt the vaccine response, Dr. Curtis said.
Holding medication, such as methotrexate, could risk having a flare-up of disease. One study showed the rate of disease flare-up because of withholding standard treatment may be up to 11%, compared with 5.1% in patients who did not hold treatment, he said.
“The point is, if you hold some of these therapies, whether methotrexate or tofacitinib, arthritis will get a little bit worse,” Dr. Curtis said.
A study published on the preprint server medRxiv found that immunosuppressive therapies blunted the response of SARS-CoV-2 vaccines in patients with chronic inflammatory diseases, most significantly with glucocorticoids and B-cell therapies.
“That’s what’s led to a lot of the guidance statements about holding treatments for a week or 2 for rituximab. If you’re giving it at 6-month intervals, you want to schedule the vaccine dose or series at about month 5, or a month before the next cycle,” he said.
Talking with patients about COVID-19 vaccination
In talking with patients about vaccine safety, Dr. Curtis recommends addressing a few common misperceptions. First, COVID-19 viruses were not created with a live-attenuated virus (which would be contraindicated for immunosuppressed patients). “You can put patients’ mind at ease that none of the vaccine candidates or platforms – even those that say viral vector – put patients at risk for contracting the infection. These are nonreplicating. So, it’s like you extracted the engine that would allow this virus to replicate,” he said.
Of three COVID-19 vaccinations available in the United States, is one better than the other? The ACR COVID-19 vaccine task force did not reach a consensus on safety profiles of the vaccines because, without head-to-head comparisons, it’s impossible to know, he said.
In talking with patients, review the protocol for continuing with prescribed treatment modalities before the patient receives a COVID-19 vaccine. Safety concerns and concerns about the possibility of having a disease flare-up should be addressed, he said.
With 49% of the U.S. population fully vaccinated against SARS-CoV-2, a new study highlights the degree of vaccine hesitancy among patients with rheumatic disease to get the vaccine.
The international study, published in May 2021 in Rheumatology, suggests that, of 1,258 patients surveyed worldwide, approximately 40% of patients said they would decline the vaccine.
“Sometimes it’s helpful to talk through their concerns,” said Jeffrey Curtis, MD, MPH, a University of Alabama at Birmingham rheumatologist who leads the American College of Rheumatology COVID-19 vaccine task force. Dr. Curtis recently reviewed the current literature on COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases (RMDs) at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
COVID-19 vaccinations for patients with autoimmune inflammatory rheumatic disease (AIIRD) is not straightforward. The immune response can be blunted by existing treatments and disease flares can occur.
The latest version of COVID-19 vaccination guidance for patients with RMDs from the ACR addresses vaccine use and implementation strategies. The guidance was issued as conditional or provisional because of the lack of evidence. Its principals are largely based on accepted practice for other vaccines. The guidance is routinely updated as new evidence becomes available. In his presentation at GRAPPA, Dr. Curtis reviewed the latest version of the guidance, which he emphasized is a guidance only and not meant to replace clinical judgment or shared decision-making with patients.
“This is a platform for you to start from as you are thinking about and discussing with your patient what might be best for him or her,” he said.
Concerns about impact of disease activity, treatments on effectiveness
Dr. Curtis highlighted some controversial aspects of COVID-19 vaccines, including heterogeneity of rheumatic diseases and treatment. Patients with AIIRD, including psoriatic arthritis, spondyloarthritis, RA, and lupus, are at higher risk for hospitalized COVID-19 and worse outcomes, and as such, they are prioritized for vaccination by the Centers for Disease Control and Prevention.
However, for AIIRD patients, the immune response to COVID-19 vaccination can be “blunted,” according to one study. This may be because of glucocorticoid use or high disease activity. Immunomodulatory therapies, such as methotrexate, rituximab, and abatacept, are known to diminish vaccine response in general. The evidence is less clear for tumor necrosis factor and Janus kinase inhibitors, but they are thought to have the same impact on vaccine effectiveness, Dr. Curtis said. But in these cases, if the effect of a COVID-19 vaccine drops from 90% to 70%, the benefits of vaccination still far outweighs the risk of contracting COVID-19.
“Although we don’t have strong data with clinical outcomes for autoimmune disease or inflammatory disease patients, I’ll run a hypothetical and say: ‘Look, if this vaccine starts 90%-95% effective, even if it’s only 70% effective in somebody with lupus or vasculitis or someone who is taking a higher dose of steroids, I’ll take 70% over nothing if you chose to be vaccinated,’ ” he said.
The benefit of vaccination also outweighs the potential risk of disease flare, he said. The risk is real, but to date, no studies have pointed to a significant risk of disease flare or worsening. However, there have been reported cases of myocardial infarction.
Autoimmune manifestations after vaccination vs. after infection
Researchers writing in the June 29, 2021, issue of JAMA Cardiology described case reports of acute myocarditis in 23 people who received the BNT162b2-mRNA (Pfizer-BioNTech) or mRNA-1273 (Moderna) messenger RNA (mRNA) COVID-19 vaccines. Plus, there been subsequent reports of myocarditis in other patients, wrote David K. Shay, MD, MPH, in an accompanying editorial. Dr. Shay is a member of the CDC COVID-19 Response Team.
“What do we know about this possible association between myocarditis and immunization with mRNA-based COVID-19 vaccines, and what remains unclear? Acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism,” he said.
The cases of myocarditis are concerning, Dr. Curtis said, but the risk is very low with relatively few cases reported among 161 million fully vaccinated people in the United States.
“Certainly, we’re not seeking to minimize that, but the risk of getting COVID and some of the downstream sequelae (autoimmune manifestations) almost certainly outweigh the risks for some of the autoimmune manifestations or worsening [condition],” he said.
A nationwide cohort study from Denmark of 58,052 patients with inflammatory rheumatic disease published in December 2020 in Rheumatology, found that patients with COVID-19 who had an inflammatory rheumatic disease were more likely to be admitted to the hospital, compared with COVID-19 patients without rheumatic disease. Patients with rheumatic disease had a higher risk of a severe COVID-19 outcome, but it was not a statistically significant difference, said Dr. Curtis, adding that the individual factors such as age and treatment currently received largely determines the risk. The strongest associations between hospitalization for COVID-19 and rheumatic disease were found among patients with RA, vasculitis, and connective tissue disease. Dr. Curtis noted that his own new study results show that risk of death from a COVID-19 infection is higher for patients who have RA or psoriatic arthritis.
There have been published case reports of patients who have developed new-onset lupus, vasculitis, Kawasaki disease, multiple sclerosis, autoimmune cytopenias, and other manifestations after a COVID-19 infection. “These authors suggest that perhaps there is a transient influence on the immune system that leads to a loss of self-tolerance to antigens,” Dr. Curtis said. “Some patients may have an underlying predisposition to autoimmunity in which infections just unmask as we sometimes see with other infections – chronic hepatitis for example.”
Antibody tests not recommended
In its COVID-19 guidance, the ACR, like the Food and Drug Administration, recommends health care providers not to routinely order antibody tests for IgM or IgG to assess immunity after a person has been vaccinated or to assess the need for vaccination in an unvaccinated person. More research is needed to determine if antibodies provide protection, and if so, for how long and how much. Plus, the antibody testing process is not clear cut, so ordering the wrong test is possible, Dr. Curtis said. The tests should clearly differentiate between spike proteins or nucleocapsid proteins.
“The bottom line is that you might be ordering the wrong lab test. Even if you’re ordering the right lab test, I would assert that you probably don’t know what to do with the result. I would then ask you, ‘Does it mean they are protected? Does it mean they are not protected? What are you going to do with the results?’ ” he asked.
Kevin Winthrop, MD, MPH, a specialist in infectious diseases at Oregon Health & Science University, Portland, said that, at this point, it’s too early to know what antibody tests mean. “I think it is tempting to test some people, especially patients on B-cell depletion therapy and those on mycophenolate mofetil (MMF). Outside of those two types of [disease-modifying antirheumatic drug] users, I wouldn’t be tempted to test. We don’t know how well protected they are, but we assume they are protected to some extent,” he said. “They’re probably partially protected and as such, they should take the same precautions they were taking a year ago: masking and avoidance. I think that’s just how it’s going to be for those folks for another year until we get this thing sorted out.”
Modifications to existing rheumatic disease therapies
In its COVID-19 vaccine guidance, the ACR issued recommendations for some common rheumatic disease therapeutics before and/or after the COVID-19 vaccine is administered. The modifications are limited to MMF, methotrexate, JAK inhibitors, subcutaneous abatacept, acetaminophen, and NSAIDs. The recommendations include: hold mycophenolate for 1 week after vaccination if disease is stable; for patients with well-controlled disease, hold methotrexate for 1 week after each of the two mRNA vaccine doses; for patients with well-controlled disease receiving the Johnson & Johnson vaccine, hold methotrexate for 2 weeks after receiving the vaccine; hold JAK inhibitors for 1 week after each dose; for abatacept subcutaneous, hold treatment for 1 week before and after the first dose; and in patients with stable disease, hold acetaminophen and NSAIDs for 24 hours before vaccination, because taking either before vaccination could blunt the vaccine response, Dr. Curtis said.
Holding medication, such as methotrexate, could risk having a flare-up of disease. One study showed the rate of disease flare-up because of withholding standard treatment may be up to 11%, compared with 5.1% in patients who did not hold treatment, he said.
“The point is, if you hold some of these therapies, whether methotrexate or tofacitinib, arthritis will get a little bit worse,” Dr. Curtis said.
A study published on the preprint server medRxiv found that immunosuppressive therapies blunted the response of SARS-CoV-2 vaccines in patients with chronic inflammatory diseases, most significantly with glucocorticoids and B-cell therapies.
“That’s what’s led to a lot of the guidance statements about holding treatments for a week or 2 for rituximab. If you’re giving it at 6-month intervals, you want to schedule the vaccine dose or series at about month 5, or a month before the next cycle,” he said.
Talking with patients about COVID-19 vaccination
In talking with patients about vaccine safety, Dr. Curtis recommends addressing a few common misperceptions. First, COVID-19 viruses were not created with a live-attenuated virus (which would be contraindicated for immunosuppressed patients). “You can put patients’ mind at ease that none of the vaccine candidates or platforms – even those that say viral vector – put patients at risk for contracting the infection. These are nonreplicating. So, it’s like you extracted the engine that would allow this virus to replicate,” he said.
Of three COVID-19 vaccinations available in the United States, is one better than the other? The ACR COVID-19 vaccine task force did not reach a consensus on safety profiles of the vaccines because, without head-to-head comparisons, it’s impossible to know, he said.
In talking with patients, review the protocol for continuing with prescribed treatment modalities before the patient receives a COVID-19 vaccine. Safety concerns and concerns about the possibility of having a disease flare-up should be addressed, he said.
FROM THE GRAPPA 2021 ANNUAL MEETING
Vertebral fractures still a risk with low-dose oral glucocorticoids for RA
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
FROM RHEUMATOLOGY
Clinical Edge Commentary: RA July 2021
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Several recent studies have evaluated risks of therapy in rheumatoid arthritis (RA). One question regarding treatment of early RA is whether different initial treatment strategies confer different risks. In a systematic review with network meta-analysis, Adas et al reviewed differences between methotrexate, biologic disease-modifying antirheumatic drug (bDMARD), and steroid use in early RA. Overall, risk of serious adverse events was higher with bDMARD monotherapy than methotrexate monotherapy. Of note, while generally long-term steroid use is disfavored due to adverse effects, serious adverse events were not increased in patients treated with methotrexate and steroids together. The size of differences in risk was small and study heterogeneity, including the class of bDMARDs, limits generalizability of this information; thus, variations in the studies themselves may account for these differences.
Pazmino et al also looked at treatment strategies in early RA in a post hoc analysis of participants at “low-risk” for poor prognosis in the CareRA trial, in which patients were randomized to step-up methotrexate without glucocorticoids or step-down with glucocorticoids. While pain scores and disease activity scores were similar among the two groups, analgesic use (including non-steroidal anti-inflammatory drugs [NSAIDs] and opioids) was significantly lower among those randomized to the glucocorticoid-bridging arm. Though this information is reassuring as to the utility of glucocorticoids, it is not clear that this correlation is broadly applicable, for example, among the “higher-risk” patients who might otherwise be more likely to receive glucocorticoids.
A recent analysis of the COVID-19 global rheumatology alliance physician registry by Sparks et al of cases of COVID-19 in patients with rheumatic disease looked more specifically at COVID-19 outcomes in patients with RA on biologic therapy. These are of interest due both to the risk of immunosuppression overall as well as the use of immunosuppressive medications in COVID-19-associated hyperinflammation. The study evaluated outcomes of hospitalization (including respiratory support and mortality). While hospitalization is difficult to evaluate as an outcome without knowing the background rate of COVID in the different areas, of the hospitalized patients, patients who used Janus kinase inhibitors (JAKi) and rituximab received oxygen or ventilator support and had higher mortality than those who were on abatacept, IL-6 inhibitors, or TNF inhibitors. Wider interpretation is difficult due to lack of knowledge of when medications were given (including rituximab dosing), but the results suggest that concern is warranted in improving outcomes for patients with RA on these therapies.
Finally, regarding the well-known cardiovascular risk associated with RA, several observational studies have suggested that methotrexate is associated with reduction in risk of cardiovascular events. This cohort study of the Veterans Affairs RA registry followed over 2000 patients for a mean of about 5 years; a reduction in incidence of cardiovascular events was associated with methotrexate use, independent of age, body mass index (BMI), cardiovascular risk factors, RA disease activity, and other RA therapies. It may be that methotrexate use is associated with an unknown mediator of cardiovascular disease not evaluated in this study, such as reduced glucocorticoid or NSAID use, but this area deserves further investigation.
Risk for ischemic stroke higher in seropositive RA patients
Key clinical point: The patients with seropositive rheumatoid arthritis (RA) are at an increased risk for ischemic stroke, particularly females and those without diabetes and dyslipidemia.
Major finding: The risk for ischemic stroke was significantly higher in the seropositive RA vs. control group (adjusted hazard ratio [aHR], 1.40; 95% confidence interval, 1.07-1.82). The risk for ischemic stroke was higher in females with seropositive RA vs. males (aHR, 1.44 vs. 1.12; P less than .001) and those without vs. with diabetes (aHR, 1.47 vs. 0.98; P less than .001) and dyslipidemia (aHR, 1.43 vs. 0.92; P = .008).
Study details: This was a nationwide, longitudinal 12-year follow-up study involving patients with seropositive RA (n=2,765) who were prescribed any disease-modifying antirheumatic drug and age- and sex-matched with control participants (n=13,825).
Disclosures: This study was funded by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning. The authors declared no conflicts of interest.
Source: Lee DH et al. PLoS One. 2021 May 17. doi: 10.1371/journal.pone.0251851.
Key clinical point: The patients with seropositive rheumatoid arthritis (RA) are at an increased risk for ischemic stroke, particularly females and those without diabetes and dyslipidemia.
Major finding: The risk for ischemic stroke was significantly higher in the seropositive RA vs. control group (adjusted hazard ratio [aHR], 1.40; 95% confidence interval, 1.07-1.82). The risk for ischemic stroke was higher in females with seropositive RA vs. males (aHR, 1.44 vs. 1.12; P less than .001) and those without vs. with diabetes (aHR, 1.47 vs. 0.98; P less than .001) and dyslipidemia (aHR, 1.43 vs. 0.92; P = .008).
Study details: This was a nationwide, longitudinal 12-year follow-up study involving patients with seropositive RA (n=2,765) who were prescribed any disease-modifying antirheumatic drug and age- and sex-matched with control participants (n=13,825).
Disclosures: This study was funded by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning. The authors declared no conflicts of interest.
Source: Lee DH et al. PLoS One. 2021 May 17. doi: 10.1371/journal.pone.0251851.
Key clinical point: The patients with seropositive rheumatoid arthritis (RA) are at an increased risk for ischemic stroke, particularly females and those without diabetes and dyslipidemia.
Major finding: The risk for ischemic stroke was significantly higher in the seropositive RA vs. control group (adjusted hazard ratio [aHR], 1.40; 95% confidence interval, 1.07-1.82). The risk for ischemic stroke was higher in females with seropositive RA vs. males (aHR, 1.44 vs. 1.12; P less than .001) and those without vs. with diabetes (aHR, 1.47 vs. 0.98; P less than .001) and dyslipidemia (aHR, 1.43 vs. 0.92; P = .008).
Study details: This was a nationwide, longitudinal 12-year follow-up study involving patients with seropositive RA (n=2,765) who were prescribed any disease-modifying antirheumatic drug and age- and sex-matched with control participants (n=13,825).
Disclosures: This study was funded by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning. The authors declared no conflicts of interest.
Source: Lee DH et al. PLoS One. 2021 May 17. doi: 10.1371/journal.pone.0251851.
Baricitinib shows long-term promise in treatment-refractory RA
Key clinical point: Long-term treatment with baricitinib was effective for up to 120 weeks and was well tolerated in patients with moderately to severely active rheumatoid arthritis (RA) with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs or tumor necrosis factor inhibitor.
Major finding: In RA-BUILD-BEYOND and RA-BEACON-BEYOND, 85.1% and 86.5% of patients treated with baricitinib achieved Simple Disease Activity Index (SDAI) low-disease activity (LDA); 40.5% and 24.3% were in SDAI remission; 62.2% and 50.0% had Health Assessment Questionnaire Disability Index of 0.5 or less; and 81.1% and 73.7% achieved 0.22 or greater change from baseline at week 120, respectively. Rates of adverse events of special interest were similar as reported previously.
Study details: Findings are from the post hoc analysis of 2 24-week phase 3 studies, RA-BUILD and RA-BEACON. Patients who completed either trial entered the ongoing 120-week RA-BEYOND long-term extension study.
Disclosures: This study was supported by Eli Lilly and Company and Incyte Corporation. The authors reported receiving research grant, consulting/speakers fees, and/or honoraria from various sources. Five of the authors reported being employees and stockholders of Eli Lilly and Company.
Source: Wells AF et al. Rheumatol Ther. 2021 May 24. doi: 10.1007/s40744-021-00317-9.
Key clinical point: Long-term treatment with baricitinib was effective for up to 120 weeks and was well tolerated in patients with moderately to severely active rheumatoid arthritis (RA) with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs or tumor necrosis factor inhibitor.
Major finding: In RA-BUILD-BEYOND and RA-BEACON-BEYOND, 85.1% and 86.5% of patients treated with baricitinib achieved Simple Disease Activity Index (SDAI) low-disease activity (LDA); 40.5% and 24.3% were in SDAI remission; 62.2% and 50.0% had Health Assessment Questionnaire Disability Index of 0.5 or less; and 81.1% and 73.7% achieved 0.22 or greater change from baseline at week 120, respectively. Rates of adverse events of special interest were similar as reported previously.
Study details: Findings are from the post hoc analysis of 2 24-week phase 3 studies, RA-BUILD and RA-BEACON. Patients who completed either trial entered the ongoing 120-week RA-BEYOND long-term extension study.
Disclosures: This study was supported by Eli Lilly and Company and Incyte Corporation. The authors reported receiving research grant, consulting/speakers fees, and/or honoraria from various sources. Five of the authors reported being employees and stockholders of Eli Lilly and Company.
Source: Wells AF et al. Rheumatol Ther. 2021 May 24. doi: 10.1007/s40744-021-00317-9.
Key clinical point: Long-term treatment with baricitinib was effective for up to 120 weeks and was well tolerated in patients with moderately to severely active rheumatoid arthritis (RA) with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs or tumor necrosis factor inhibitor.
Major finding: In RA-BUILD-BEYOND and RA-BEACON-BEYOND, 85.1% and 86.5% of patients treated with baricitinib achieved Simple Disease Activity Index (SDAI) low-disease activity (LDA); 40.5% and 24.3% were in SDAI remission; 62.2% and 50.0% had Health Assessment Questionnaire Disability Index of 0.5 or less; and 81.1% and 73.7% achieved 0.22 or greater change from baseline at week 120, respectively. Rates of adverse events of special interest were similar as reported previously.
Study details: Findings are from the post hoc analysis of 2 24-week phase 3 studies, RA-BUILD and RA-BEACON. Patients who completed either trial entered the ongoing 120-week RA-BEYOND long-term extension study.
Disclosures: This study was supported by Eli Lilly and Company and Incyte Corporation. The authors reported receiving research grant, consulting/speakers fees, and/or honoraria from various sources. Five of the authors reported being employees and stockholders of Eli Lilly and Company.
Source: Wells AF et al. Rheumatol Ther. 2021 May 24. doi: 10.1007/s40744-021-00317-9.
Clusterin could serve as a potential predictive biomarker for disease activity and treatment response in early RA
Key clinical point: Patients with treatment-naive early rheumatoid arthritis (RA) had higher serum clusterin levels, which decreased significantly after the initiation of conventional therapy. Moreover, baseline clusterin levels predicted the achievement of low disease activity and remission during the first year.
Major finding: Patients with treatment-naive early RA vs. healthy controls (HCs) had significantly higher clusterin levels (P less than .0001), which declined significantly after 3 months of therapy (P less than .0001) and was comparable with that of HCs (P = .865). Lower clusterin levels at baseline predicted achieving remission (P = .018) and low disease activity (P = .025) at 12 months.
Study details: The data come from an analysis of patients with treatment-naive early RA (n=52) who were age-/sex-matched with HCs (n=52).
Disclosures: This study received support from the Ministry of Health of the Czech Republic. No competing interest was reported.
Source: Kropáčková T et al. Sci Rep. 2021 Jun 1. doi: 10.1038/s41598-021-90973-2.
Key clinical point: Patients with treatment-naive early rheumatoid arthritis (RA) had higher serum clusterin levels, which decreased significantly after the initiation of conventional therapy. Moreover, baseline clusterin levels predicted the achievement of low disease activity and remission during the first year.
Major finding: Patients with treatment-naive early RA vs. healthy controls (HCs) had significantly higher clusterin levels (P less than .0001), which declined significantly after 3 months of therapy (P less than .0001) and was comparable with that of HCs (P = .865). Lower clusterin levels at baseline predicted achieving remission (P = .018) and low disease activity (P = .025) at 12 months.
Study details: The data come from an analysis of patients with treatment-naive early RA (n=52) who were age-/sex-matched with HCs (n=52).
Disclosures: This study received support from the Ministry of Health of the Czech Republic. No competing interest was reported.
Source: Kropáčková T et al. Sci Rep. 2021 Jun 1. doi: 10.1038/s41598-021-90973-2.
Key clinical point: Patients with treatment-naive early rheumatoid arthritis (RA) had higher serum clusterin levels, which decreased significantly after the initiation of conventional therapy. Moreover, baseline clusterin levels predicted the achievement of low disease activity and remission during the first year.
Major finding: Patients with treatment-naive early RA vs. healthy controls (HCs) had significantly higher clusterin levels (P less than .0001), which declined significantly after 3 months of therapy (P less than .0001) and was comparable with that of HCs (P = .865). Lower clusterin levels at baseline predicted achieving remission (P = .018) and low disease activity (P = .025) at 12 months.
Study details: The data come from an analysis of patients with treatment-naive early RA (n=52) who were age-/sex-matched with HCs (n=52).
Disclosures: This study received support from the Ministry of Health of the Czech Republic. No competing interest was reported.
Source: Kropáčková T et al. Sci Rep. 2021 Jun 1. doi: 10.1038/s41598-021-90973-2.
HR-pQCT scanning has comparable diagnostic precision to conventional radiography for classifying erosive RA
Key clinical point: High-resolution peripheral quantitative computed tomography (HR-pQCT) of 2 metacarpophalangeal joints and conventional radiography (CR) of 44 joints had comparable diagnostic accuracy for classifying patients with established rheumatoid arthritis (RA) as having erosive disease.
Major finding: Using CR as reference, the sensitivity and specificity of HR-pQCT for classifying patients having erosive RA was 89% (95% confidence interval [CI], 84%-92%) and 30% (95% Cl, 20%-43%), respectively. In contrast, the sensitivity and specificity of CR of 44 joints was 85% (95% Cl, 80%-89%) and 38% (95% Cl, 25%-52%), respectively, when HR-pQCT was used as reference. There was no significant difference between the sensitivities of patients classified as having erosive RA by HR-pQCT or CR (2.14; P = .177).
Study details: Data come from a single-center cross-sectional study of 353 patients with established RA.
Disclosures: The study received support from the Aarhus University Research Foundation, Danish Rheumatism Association, Novo Nordic Foundation, and A.P. Møller Fonden. E M Hauge and B Langdahl reported receiving grants and personal fees from various sources. All the other authors reported no conflicts of interest.
Source: Klose-Jensen R et al. Rheumatology (Oxford). 2021 May 20. doi: 10.1093/rheumatology/keab446.
Key clinical point: High-resolution peripheral quantitative computed tomography (HR-pQCT) of 2 metacarpophalangeal joints and conventional radiography (CR) of 44 joints had comparable diagnostic accuracy for classifying patients with established rheumatoid arthritis (RA) as having erosive disease.
Major finding: Using CR as reference, the sensitivity and specificity of HR-pQCT for classifying patients having erosive RA was 89% (95% confidence interval [CI], 84%-92%) and 30% (95% Cl, 20%-43%), respectively. In contrast, the sensitivity and specificity of CR of 44 joints was 85% (95% Cl, 80%-89%) and 38% (95% Cl, 25%-52%), respectively, when HR-pQCT was used as reference. There was no significant difference between the sensitivities of patients classified as having erosive RA by HR-pQCT or CR (2.14; P = .177).
Study details: Data come from a single-center cross-sectional study of 353 patients with established RA.
Disclosures: The study received support from the Aarhus University Research Foundation, Danish Rheumatism Association, Novo Nordic Foundation, and A.P. Møller Fonden. E M Hauge and B Langdahl reported receiving grants and personal fees from various sources. All the other authors reported no conflicts of interest.
Source: Klose-Jensen R et al. Rheumatology (Oxford). 2021 May 20. doi: 10.1093/rheumatology/keab446.
Key clinical point: High-resolution peripheral quantitative computed tomography (HR-pQCT) of 2 metacarpophalangeal joints and conventional radiography (CR) of 44 joints had comparable diagnostic accuracy for classifying patients with established rheumatoid arthritis (RA) as having erosive disease.
Major finding: Using CR as reference, the sensitivity and specificity of HR-pQCT for classifying patients having erosive RA was 89% (95% confidence interval [CI], 84%-92%) and 30% (95% Cl, 20%-43%), respectively. In contrast, the sensitivity and specificity of CR of 44 joints was 85% (95% Cl, 80%-89%) and 38% (95% Cl, 25%-52%), respectively, when HR-pQCT was used as reference. There was no significant difference between the sensitivities of patients classified as having erosive RA by HR-pQCT or CR (2.14; P = .177).
Study details: Data come from a single-center cross-sectional study of 353 patients with established RA.
Disclosures: The study received support from the Aarhus University Research Foundation, Danish Rheumatism Association, Novo Nordic Foundation, and A.P. Møller Fonden. E M Hauge and B Langdahl reported receiving grants and personal fees from various sources. All the other authors reported no conflicts of interest.
Source: Klose-Jensen R et al. Rheumatology (Oxford). 2021 May 20. doi: 10.1093/rheumatology/keab446.