User login
Baseline neuromotor abnormalities persist in schizophrenia
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
Subtle visual dysfunctions often precede early-stage psychosis
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Neuropsychiatric symptoms after stroke
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.
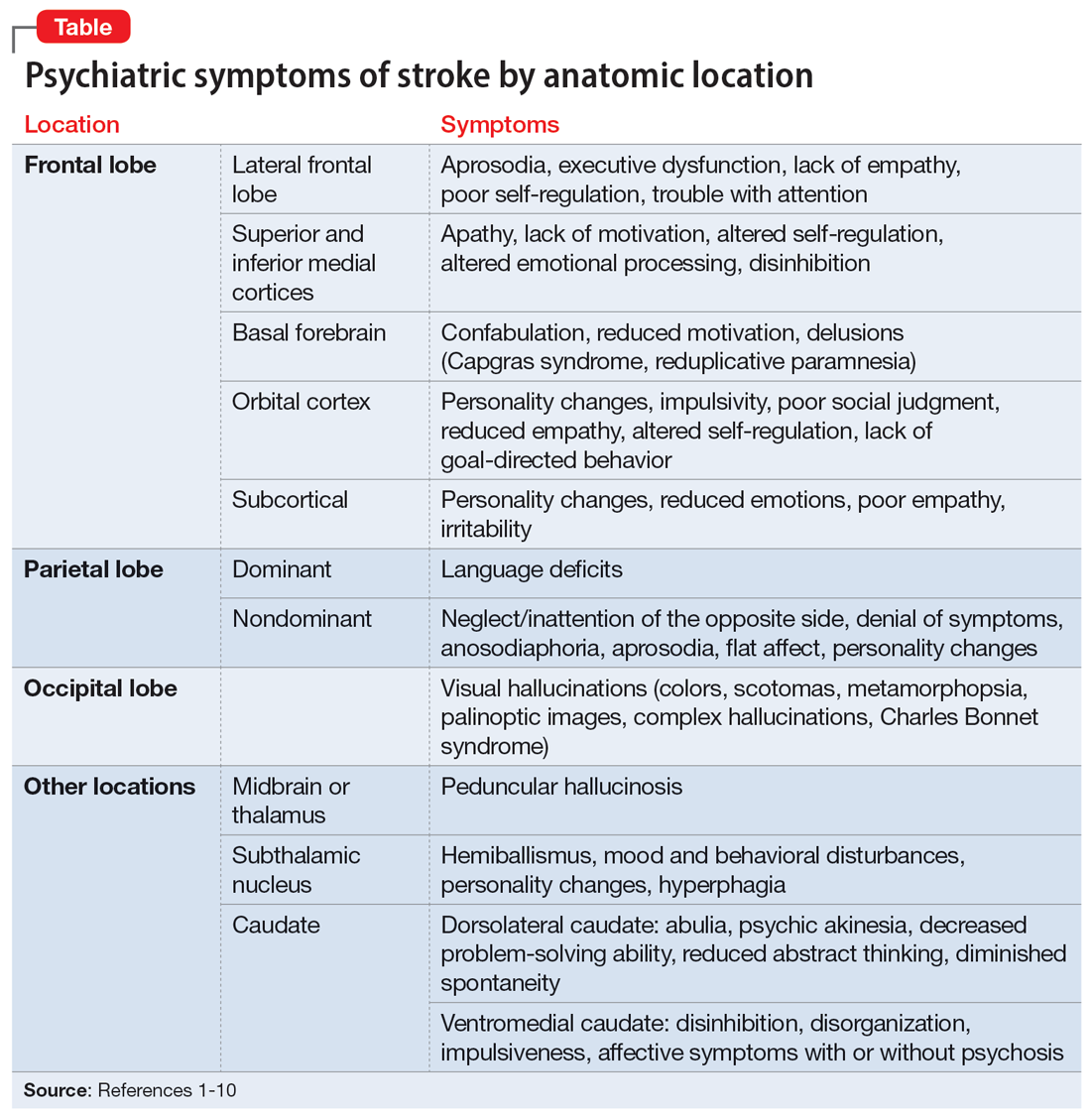
Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Preparing patients with serious mental illness for extreme HEAT
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Brief Psychiatric Rating Scale succeeds as transdiagnostic measure
“Current DSM and ICD diagnoses do not depict psychopathology accurately, therefore their validity in research and utility in clinical practice is questioned,” wrote Andreas B. Hofmann, PhD, of the University of Zürich and colleagues.
The BPRS was developed to assess changes in psychopathology across a range of severe psychiatric disorders, but its potential to assess symptoms in nonpsychotic disorders has not been explored, the researchers said.
In a study published in Psychiatry Research, the investigators analyzed data from 600 adult psychiatric inpatients divided equally into six diagnostic categories: alcohol use disorder, major depressive disorder, anxiety disorders, bipolar disorder, schizophrenia, and personality disorders. The mean age of the patients was 41.5 years and 45.5% were women. The demographic characteristics were similar across most groups, although patients with a personality disorder were significantly more likely than other patients to be younger and female.
Patients were assessed using the BPRS based on their main diagnosis. The mini-ICF-APP, another validated measure for assessing psychiatric disorders, served as a comparator, and both were compared to the Clinical Global Impression Scale (CGI).
Overall, the BPRS and mini-ICF-APP showed moderate correlation and good agreement, the researchers said. The Pearson correlation coefficient for the BPRS and mini-ICF-APP scales was 0.53 and the concordance correlation coefficient was 0.52. The mean sum scores for the BPRS, the mini-ICF-APP, and the CGI were 45.4 (standard deviation, 14.4), 19.93 (SD, 8.21), and 5.55 (SD, 0.84), respectively, which indicated “markedly ill” to “severely ill” patients, the researchers said.
The researchers were able to detect three clusters of symptoms corresponding to externalizing, internalizing, and thought disturbance domains using the BPRS, and four clusters using the mini-ICF-APP.
The symptoms using BPRS and the functionality domains using the mini-ICF-APP “showed a close interplay,” the researchers noted.
“The symptoms and functional domains we found to be central within the network structure are among the first targets of any psychiatric or psychotherapeutic intervention, namely the building of a common language and understanding as well as the establishment of confidence in relationships and a trustworthy therapeutic alliance,” they wrote in their discussion.
The study findings were limited by several factors including the collection of data from routine practice rather than clinical trials, the focus on only the main diagnosis without comorbidities, and the inclusion only of patients requiring hospitalization, the researchers noted.
However, the results were strengthened by the large sample size, and demonstrate the validity of the BPRS as a measurement tool across a range of psychiatric diagnoses, they said.
“Since the BPRS is a widely known and readily available psychometric scale, our results support its use as a transdiagnostic measurement instrument of psychopathology,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“Current DSM and ICD diagnoses do not depict psychopathology accurately, therefore their validity in research and utility in clinical practice is questioned,” wrote Andreas B. Hofmann, PhD, of the University of Zürich and colleagues.
The BPRS was developed to assess changes in psychopathology across a range of severe psychiatric disorders, but its potential to assess symptoms in nonpsychotic disorders has not been explored, the researchers said.
In a study published in Psychiatry Research, the investigators analyzed data from 600 adult psychiatric inpatients divided equally into six diagnostic categories: alcohol use disorder, major depressive disorder, anxiety disorders, bipolar disorder, schizophrenia, and personality disorders. The mean age of the patients was 41.5 years and 45.5% were women. The demographic characteristics were similar across most groups, although patients with a personality disorder were significantly more likely than other patients to be younger and female.
Patients were assessed using the BPRS based on their main diagnosis. The mini-ICF-APP, another validated measure for assessing psychiatric disorders, served as a comparator, and both were compared to the Clinical Global Impression Scale (CGI).
Overall, the BPRS and mini-ICF-APP showed moderate correlation and good agreement, the researchers said. The Pearson correlation coefficient for the BPRS and mini-ICF-APP scales was 0.53 and the concordance correlation coefficient was 0.52. The mean sum scores for the BPRS, the mini-ICF-APP, and the CGI were 45.4 (standard deviation, 14.4), 19.93 (SD, 8.21), and 5.55 (SD, 0.84), respectively, which indicated “markedly ill” to “severely ill” patients, the researchers said.
The researchers were able to detect three clusters of symptoms corresponding to externalizing, internalizing, and thought disturbance domains using the BPRS, and four clusters using the mini-ICF-APP.
The symptoms using BPRS and the functionality domains using the mini-ICF-APP “showed a close interplay,” the researchers noted.
“The symptoms and functional domains we found to be central within the network structure are among the first targets of any psychiatric or psychotherapeutic intervention, namely the building of a common language and understanding as well as the establishment of confidence in relationships and a trustworthy therapeutic alliance,” they wrote in their discussion.
The study findings were limited by several factors including the collection of data from routine practice rather than clinical trials, the focus on only the main diagnosis without comorbidities, and the inclusion only of patients requiring hospitalization, the researchers noted.
However, the results were strengthened by the large sample size, and demonstrate the validity of the BPRS as a measurement tool across a range of psychiatric diagnoses, they said.
“Since the BPRS is a widely known and readily available psychometric scale, our results support its use as a transdiagnostic measurement instrument of psychopathology,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
“Current DSM and ICD diagnoses do not depict psychopathology accurately, therefore their validity in research and utility in clinical practice is questioned,” wrote Andreas B. Hofmann, PhD, of the University of Zürich and colleagues.
The BPRS was developed to assess changes in psychopathology across a range of severe psychiatric disorders, but its potential to assess symptoms in nonpsychotic disorders has not been explored, the researchers said.
In a study published in Psychiatry Research, the investigators analyzed data from 600 adult psychiatric inpatients divided equally into six diagnostic categories: alcohol use disorder, major depressive disorder, anxiety disorders, bipolar disorder, schizophrenia, and personality disorders. The mean age of the patients was 41.5 years and 45.5% were women. The demographic characteristics were similar across most groups, although patients with a personality disorder were significantly more likely than other patients to be younger and female.
Patients were assessed using the BPRS based on their main diagnosis. The mini-ICF-APP, another validated measure for assessing psychiatric disorders, served as a comparator, and both were compared to the Clinical Global Impression Scale (CGI).
Overall, the BPRS and mini-ICF-APP showed moderate correlation and good agreement, the researchers said. The Pearson correlation coefficient for the BPRS and mini-ICF-APP scales was 0.53 and the concordance correlation coefficient was 0.52. The mean sum scores for the BPRS, the mini-ICF-APP, and the CGI were 45.4 (standard deviation, 14.4), 19.93 (SD, 8.21), and 5.55 (SD, 0.84), respectively, which indicated “markedly ill” to “severely ill” patients, the researchers said.
The researchers were able to detect three clusters of symptoms corresponding to externalizing, internalizing, and thought disturbance domains using the BPRS, and four clusters using the mini-ICF-APP.
The symptoms using BPRS and the functionality domains using the mini-ICF-APP “showed a close interplay,” the researchers noted.
“The symptoms and functional domains we found to be central within the network structure are among the first targets of any psychiatric or psychotherapeutic intervention, namely the building of a common language and understanding as well as the establishment of confidence in relationships and a trustworthy therapeutic alliance,” they wrote in their discussion.
The study findings were limited by several factors including the collection of data from routine practice rather than clinical trials, the focus on only the main diagnosis without comorbidities, and the inclusion only of patients requiring hospitalization, the researchers noted.
However, the results were strengthened by the large sample size, and demonstrate the validity of the BPRS as a measurement tool across a range of psychiatric diagnoses, they said.
“Since the BPRS is a widely known and readily available psychometric scale, our results support its use as a transdiagnostic measurement instrument of psychopathology,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
APA task force highlights U.S. psychiatric bed crisis
The model, introduced in a recent report from the organization, can predict how changes in any component of mental health care in a community, including mobile trauma teams and assertive community treatment, will affect other components and the overall capacity to care for patients with mental illness.
Leaders of the APA task force that drafted the report noted that communities can use the model to confront the ongoing mental health crisis brought about by a lack of inpatient beds, a shortage of mental health professionals, shorter inpatient stays, and a rising number of individuals with mental illness.
The report was first released at the APA’s annual meeting in May 2022 and was discussed in further detail at a press briefing in mid-August.
“Part of the wisdom of the APA leadership of releasing this report in this format now is to keep attention and awareness on the issue and acknowledge that there is a terrible shortage of beds,” Anita Everett, MD, past president of the APA and chair of the report’s task force, told briefing attendees.
“We need to have ongoing conversations about how we can solve this problem,” said Dr. Everett, who is also director of the Center for Mental Health Services at the Substance Abuse and Mental Health Services Administration.
A virtual world
The report describes both historic and current psychiatric bed use and discusses how the availability of community resources affects the need for inpatient care. It includes analyses of inpatient medical care spending and describes barriers to accessing inpatient psychiatric care.
Historically, the number of state-operated psychiatric hospital beds in the United States was 337 per 100,000 people in the mid-1950s. Today, that figure is about 11.7 state psychiatric hospital beds per 100,000 people, the report says.
The average length of an inpatient stay has also decreased significantly both for adults and children. Pediatric length of stay declined from 12.2 days to 4.4 days between 1990 and 2000.
Launched in 2020, the APA Presidential Task Force on the Assessment of Psychiatric Bed Needs in the United States includes more than 30 mental health professionals and members of the APA administration.
The group was charged with drafting a report that explains and defines the current mental health crisis. They were also charged with developing a method for calculating the number of psychiatric beds needed in any given community.
Task force leaders said the model considers how individuals enter the mental health care system and are routed to appropriate services, how long they remain in the system, and the capacity of the system to respond to demand.
The model is based on a “virtual world” that has a number of care components. These include mobile crisis teams, intensive team-based outpatient care, community-based crisis beds, psychiatric hospital beds, and residential and step-down programs.
The model factors in the magnitude of the need for beds in many service areas. Factors include population size, estimates of the rate of acute mental health crises per 100,000 population, adequacy of the community mental health system, the intersection between the mental health and criminal justice systems, and outpatient and inpatient capacities.
The model computes the estimated number of patients waiting in the emergency department, crisis receiving centers, and jail, as well as average wait times. It also calculates the percentage of use of the various services.
The model will be continually updated and can be modified to better reflect the current situation in any given community.
Real-world testing
A team led by the University of Michigan, Ann Arbor, and two area hospitals is testing the APA model by using it to calculate the number of beds needed in their community.
“Because the model is focused on the continuum of care services, it allows communities to try to focus on what is the right mix of services needed to try to reduce the need for in-patient hospitalization and measure the impact of development of resources across the continuum, including inpatient beds, to try to achieve the right mix,” Gregory Dalack, MD, chair of the department of psychiatry at the University of Michigan Health System, told this news organization.
Ultimately, Dr. Dalack expects that the model will tell the team something they already know: that additional psychiatric beds are needed in their community.
However, meeting the needs of patients and families is not just about beds, he noted. The model will help provide a fuller picture of psychiatric care and will take into account existing services from many aspects of the care field.
“If we put all the focus just on hospital beds, we are only addressing one part of the challenge,” Dr. Dalack said.
The challenge is also about “identifying what resources/services are already in the continuum of care, where expansion of those or development of new programs might be needed, and what the impact is on the system, particularly with folks who arrive in the emergency room who might need inpatient admission,” he added.
Dr. Everett said the APA leadership team is now actively recruiting others to test the model in their communities, which will help to calibrate the system.
A version of this article first appeared on Medscape.com.
The model, introduced in a recent report from the organization, can predict how changes in any component of mental health care in a community, including mobile trauma teams and assertive community treatment, will affect other components and the overall capacity to care for patients with mental illness.
Leaders of the APA task force that drafted the report noted that communities can use the model to confront the ongoing mental health crisis brought about by a lack of inpatient beds, a shortage of mental health professionals, shorter inpatient stays, and a rising number of individuals with mental illness.
The report was first released at the APA’s annual meeting in May 2022 and was discussed in further detail at a press briefing in mid-August.
“Part of the wisdom of the APA leadership of releasing this report in this format now is to keep attention and awareness on the issue and acknowledge that there is a terrible shortage of beds,” Anita Everett, MD, past president of the APA and chair of the report’s task force, told briefing attendees.
“We need to have ongoing conversations about how we can solve this problem,” said Dr. Everett, who is also director of the Center for Mental Health Services at the Substance Abuse and Mental Health Services Administration.
A virtual world
The report describes both historic and current psychiatric bed use and discusses how the availability of community resources affects the need for inpatient care. It includes analyses of inpatient medical care spending and describes barriers to accessing inpatient psychiatric care.
Historically, the number of state-operated psychiatric hospital beds in the United States was 337 per 100,000 people in the mid-1950s. Today, that figure is about 11.7 state psychiatric hospital beds per 100,000 people, the report says.
The average length of an inpatient stay has also decreased significantly both for adults and children. Pediatric length of stay declined from 12.2 days to 4.4 days between 1990 and 2000.
Launched in 2020, the APA Presidential Task Force on the Assessment of Psychiatric Bed Needs in the United States includes more than 30 mental health professionals and members of the APA administration.
The group was charged with drafting a report that explains and defines the current mental health crisis. They were also charged with developing a method for calculating the number of psychiatric beds needed in any given community.
Task force leaders said the model considers how individuals enter the mental health care system and are routed to appropriate services, how long they remain in the system, and the capacity of the system to respond to demand.
The model is based on a “virtual world” that has a number of care components. These include mobile crisis teams, intensive team-based outpatient care, community-based crisis beds, psychiatric hospital beds, and residential and step-down programs.
The model factors in the magnitude of the need for beds in many service areas. Factors include population size, estimates of the rate of acute mental health crises per 100,000 population, adequacy of the community mental health system, the intersection between the mental health and criminal justice systems, and outpatient and inpatient capacities.
The model computes the estimated number of patients waiting in the emergency department, crisis receiving centers, and jail, as well as average wait times. It also calculates the percentage of use of the various services.
The model will be continually updated and can be modified to better reflect the current situation in any given community.
Real-world testing
A team led by the University of Michigan, Ann Arbor, and two area hospitals is testing the APA model by using it to calculate the number of beds needed in their community.
“Because the model is focused on the continuum of care services, it allows communities to try to focus on what is the right mix of services needed to try to reduce the need for in-patient hospitalization and measure the impact of development of resources across the continuum, including inpatient beds, to try to achieve the right mix,” Gregory Dalack, MD, chair of the department of psychiatry at the University of Michigan Health System, told this news organization.
Ultimately, Dr. Dalack expects that the model will tell the team something they already know: that additional psychiatric beds are needed in their community.
However, meeting the needs of patients and families is not just about beds, he noted. The model will help provide a fuller picture of psychiatric care and will take into account existing services from many aspects of the care field.
“If we put all the focus just on hospital beds, we are only addressing one part of the challenge,” Dr. Dalack said.
The challenge is also about “identifying what resources/services are already in the continuum of care, where expansion of those or development of new programs might be needed, and what the impact is on the system, particularly with folks who arrive in the emergency room who might need inpatient admission,” he added.
Dr. Everett said the APA leadership team is now actively recruiting others to test the model in their communities, which will help to calibrate the system.
A version of this article first appeared on Medscape.com.
The model, introduced in a recent report from the organization, can predict how changes in any component of mental health care in a community, including mobile trauma teams and assertive community treatment, will affect other components and the overall capacity to care for patients with mental illness.
Leaders of the APA task force that drafted the report noted that communities can use the model to confront the ongoing mental health crisis brought about by a lack of inpatient beds, a shortage of mental health professionals, shorter inpatient stays, and a rising number of individuals with mental illness.
The report was first released at the APA’s annual meeting in May 2022 and was discussed in further detail at a press briefing in mid-August.
“Part of the wisdom of the APA leadership of releasing this report in this format now is to keep attention and awareness on the issue and acknowledge that there is a terrible shortage of beds,” Anita Everett, MD, past president of the APA and chair of the report’s task force, told briefing attendees.
“We need to have ongoing conversations about how we can solve this problem,” said Dr. Everett, who is also director of the Center for Mental Health Services at the Substance Abuse and Mental Health Services Administration.
A virtual world
The report describes both historic and current psychiatric bed use and discusses how the availability of community resources affects the need for inpatient care. It includes analyses of inpatient medical care spending and describes barriers to accessing inpatient psychiatric care.
Historically, the number of state-operated psychiatric hospital beds in the United States was 337 per 100,000 people in the mid-1950s. Today, that figure is about 11.7 state psychiatric hospital beds per 100,000 people, the report says.
The average length of an inpatient stay has also decreased significantly both for adults and children. Pediatric length of stay declined from 12.2 days to 4.4 days between 1990 and 2000.
Launched in 2020, the APA Presidential Task Force on the Assessment of Psychiatric Bed Needs in the United States includes more than 30 mental health professionals and members of the APA administration.
The group was charged with drafting a report that explains and defines the current mental health crisis. They were also charged with developing a method for calculating the number of psychiatric beds needed in any given community.
Task force leaders said the model considers how individuals enter the mental health care system and are routed to appropriate services, how long they remain in the system, and the capacity of the system to respond to demand.
The model is based on a “virtual world” that has a number of care components. These include mobile crisis teams, intensive team-based outpatient care, community-based crisis beds, psychiatric hospital beds, and residential and step-down programs.
The model factors in the magnitude of the need for beds in many service areas. Factors include population size, estimates of the rate of acute mental health crises per 100,000 population, adequacy of the community mental health system, the intersection between the mental health and criminal justice systems, and outpatient and inpatient capacities.
The model computes the estimated number of patients waiting in the emergency department, crisis receiving centers, and jail, as well as average wait times. It also calculates the percentage of use of the various services.
The model will be continually updated and can be modified to better reflect the current situation in any given community.
Real-world testing
A team led by the University of Michigan, Ann Arbor, and two area hospitals is testing the APA model by using it to calculate the number of beds needed in their community.
“Because the model is focused on the continuum of care services, it allows communities to try to focus on what is the right mix of services needed to try to reduce the need for in-patient hospitalization and measure the impact of development of resources across the continuum, including inpatient beds, to try to achieve the right mix,” Gregory Dalack, MD, chair of the department of psychiatry at the University of Michigan Health System, told this news organization.
Ultimately, Dr. Dalack expects that the model will tell the team something they already know: that additional psychiatric beds are needed in their community.
However, meeting the needs of patients and families is not just about beds, he noted. The model will help provide a fuller picture of psychiatric care and will take into account existing services from many aspects of the care field.
“If we put all the focus just on hospital beds, we are only addressing one part of the challenge,” Dr. Dalack said.
The challenge is also about “identifying what resources/services are already in the continuum of care, where expansion of those or development of new programs might be needed, and what the impact is on the system, particularly with folks who arrive in the emergency room who might need inpatient admission,” he added.
Dr. Everett said the APA leadership team is now actively recruiting others to test the model in their communities, which will help to calibrate the system.
A version of this article first appeared on Medscape.com.
Postpartum psychosis: Does longitudinal course inform treatment?
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at obnews@mdedge.com.
LAIAs safer, more effective than oral antipsychotics for schizophrenia
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Positive phase 3 results for novel schizophrenia drug
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
The investigational agent xanomeline-trospium (KarXT, Karuna Therapeutics), which combines a muscarinic receptor agonist with an anticholinergic agent, helps improve psychosis symptoms and is not associated with weight gain or sedation in adults with schizophrenia, new research shows.
Top-line results from the phase 3 EMERGENT-2 trial showed a significantly greater reduction from baseline on Positive and Negative Syndrome Scale (PANSS) total scores for those receiving the active drug than for those receiving placebo, meeting its primary endpoint.
and potentially usher in the first new class of medicine for these patients in more than 50 years,” Steve Paul, MD, chief executive officer, president, and chairman of Karuna Therapeutics, said in a press release.
Primary outcome met
About 20%-33% of patients with schizophrenia do not respond to conventional treatments, the company noted. Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
Unlike current therapies, KarXT doesn’t rely on the dopaminergic or serotonergic pathways. It comprises the muscarinic agonist xanomeline and the muscarinic antagonist trospium and is designed to preferentially stimulate muscarinic receptors in the central nervous system.
Results from a phase 2 trial of almost 200 patients with schizophrenia were published last year in the New England Journal of Medicine. The findings showed that those who received xanomeline-trospium had a significantly greater reduction in psychosis symptoms than those who received placebo.
In the current phase 3 EMERGENT-2 trial, investigators included 252 adults aged 18-65 years who were diagnosed with schizophrenia and were experiencing symptoms of psychosis. Patients were randomly assigned to receive either a flexible dose of xanomeline-trospium or placebo twice daily.
The primary endpoint was change from baseline in the PANSS total score at week 5. Results showed a statistically significant and clinically meaningful 9.6-point reduction in the PANSS total score in participants taking the active drug, compared with those taking placebo (–21.2 vs. –11.6, respectively; P < .0001; Cohen’s d effect size, 0.61).
In addition, there was an early and sustained significant reduction of schizophrenia symptoms, as assessed by the PANSS total score, starting at week 2. This reduction was maintained through all trial timepoints.
Safety profile
The novel drug also met key secondary endpoints. In the active treatment group, there was a significant reduction on the PANSS subscales in both positive symptoms of schizophrenia, such as hallucinations or delusions, and negative symptoms, such as difficulty enjoying life or withdrawal from others.
Overall, the agent was generally well tolerated. The treatment-emergent adverse events (TEAEs) rate for xanomeline-trospium and placebo was 75% versus 58%, respectively.
The most common TEAEs for the active treatment were all mild-to-moderate in severity and included constipation, dyspepsia, nausea, vomiting, headache, increases in blood pressure, dizziness, gastroesophageal reflux disease, abdominal discomfort, and diarrhea.
As in prior trials, an increase in heart rate was also associated with the active treatment and decreased in magnitude by the end of the current study.
Discontinuation rates related to TEAEs were similar between xanomeline-trospium (7%) and placebo (6%), as were rates of serious TEAEs (2% in each group) – which included suicidal ideation, worsening of schizophrenia symptoms, and appendicitis.
Notably, the drug was not associated with common problematic adverse events of current therapies, such as weight gain, sedation, and movement disorders.
Karuna plans to submit a New Drug Application with the U.S. Food and Drug Administration for KarXT in mid-2023. In addition to schizophrenia, the drug is in development for the treatment of other psychiatric and neurological conditions, including Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
Mixed results for intensive home care for psychiatric crises
Intensive home treatment may offer an alternative to inpatient care for patients in acute psychiatric crisis – but the intervention is no outright substitute, new research suggests.
However, there was no difference between treatment groups in improvement in quality of life or patient satisfaction; and a reduction in symptom severity noted after 6 weeks of home treatment faded within 6 months.
“We found no differences in admission rates either, which suggests that intensive home treatment is not a substitute for inpatient care but a different treatment opportunity for psychiatric patients in crisis,” Jurgen Cornelis, MD, Arkin Institute for Mental Health, Amsterdam, and colleagues write.
The findings were published online in The Lancet Psychiatry.
Increasingly popular
“Intensive home treatment is increasingly popular as an alternative to hospitalization. It was developed to prevent or reduce levels of inpatient care and facilitate the transition between inpatient care and low-intensity outpatient care,” the investigators write.
However, there have previously been only two randomized controlled trials published that assessed this type of care, resulting in “somewhat conflicting findings,” they add.
For the current study, participants presented to psychiatric emergency wards at two medical centers in the Netherlands. They were included only if they were able to offer informed consent within 14 days.
The intensive home treatment group (n = 183) worked with a multidisciplinary team that designed a care plan tailored to their specific crisis. Treatment components included pharmacotherapy, up to three home visits each day, psychoeducation, brief supportive and cognitive behavioral therapy, social care, and support and empowerment of the patient’s informal care system.
The usual care group (n = 63) commonly received a combination of highly intensive inpatient treatment in the first phase and outpatient treatment up to two times a week in the second phase. Treatment included similar components as those in intensive home treatment.
The most common primary clinical diagnosis in both groups was mood disorder, followed by psychotic disorders, personality disorders, or anxiety disorders.
The home treatment group had a significantly higher total mean item score on the Brief Psychiatric Rating Scale (BPRS) at baseline (2.23 vs. 2.04, P = .04).
Mixed results
Results at 6 weeks showed the number of hospital days was 25.3% lower in the home treatment group, compared with those who received usual care.
That trend continued at 1 year, with the intensive home treatment group recording 36.6% fewer hospital days than the usual care group (mean, 42.5 days vs. 67 days, respectively; P = .03).
However, the number of patients who were admitted in the first 6 weeks and at 1 year stayed the same, as did the mean number of admissions per patient over 12 months.
The home treatment group reported significantly fewer symptoms on the BPRS depression and anxiety scale at 6 weeks, compared with the usual treatment group (P = .025), but that difference was not maintained after 6 months.
The number of adverse events, including suicide attempts, was similar between the groups. Three patients in the home treatment group and two in the usual care group died by suicide.
“Future research should focus on which components of intensive home treatment or hospitalization can be used when, for whom, and meet which goals, so that both hospital care and intensive home treatment can be used proportionally and efficiently for patients in psychiatric crisis,” the investigators write.
Not generalizable?
In an accompanying editorial, Claire Henderson, PhD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, noted that generalizing the study’s results to other countries could be problematic, especially to regions such as North America, which have shorter lengths of stay for psychiatric hospitalization.
“Future trials looking at intensive home treatment would be most informative if done in countries with relatively short lengths of stay, and without separate crisis services for people receiving assertive community treatment,” Dr. Henderson writes.
The study was funded by De Stichting tot Steun Vereniging voor Christelijke Verzorging van Geestes-en Zenuwzieken. The investigators and Dr. Henderson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intensive home treatment may offer an alternative to inpatient care for patients in acute psychiatric crisis – but the intervention is no outright substitute, new research suggests.
However, there was no difference between treatment groups in improvement in quality of life or patient satisfaction; and a reduction in symptom severity noted after 6 weeks of home treatment faded within 6 months.
“We found no differences in admission rates either, which suggests that intensive home treatment is not a substitute for inpatient care but a different treatment opportunity for psychiatric patients in crisis,” Jurgen Cornelis, MD, Arkin Institute for Mental Health, Amsterdam, and colleagues write.
The findings were published online in The Lancet Psychiatry.
Increasingly popular
“Intensive home treatment is increasingly popular as an alternative to hospitalization. It was developed to prevent or reduce levels of inpatient care and facilitate the transition between inpatient care and low-intensity outpatient care,” the investigators write.
However, there have previously been only two randomized controlled trials published that assessed this type of care, resulting in “somewhat conflicting findings,” they add.
For the current study, participants presented to psychiatric emergency wards at two medical centers in the Netherlands. They were included only if they were able to offer informed consent within 14 days.
The intensive home treatment group (n = 183) worked with a multidisciplinary team that designed a care plan tailored to their specific crisis. Treatment components included pharmacotherapy, up to three home visits each day, psychoeducation, brief supportive and cognitive behavioral therapy, social care, and support and empowerment of the patient’s informal care system.
The usual care group (n = 63) commonly received a combination of highly intensive inpatient treatment in the first phase and outpatient treatment up to two times a week in the second phase. Treatment included similar components as those in intensive home treatment.
The most common primary clinical diagnosis in both groups was mood disorder, followed by psychotic disorders, personality disorders, or anxiety disorders.
The home treatment group had a significantly higher total mean item score on the Brief Psychiatric Rating Scale (BPRS) at baseline (2.23 vs. 2.04, P = .04).
Mixed results
Results at 6 weeks showed the number of hospital days was 25.3% lower in the home treatment group, compared with those who received usual care.
That trend continued at 1 year, with the intensive home treatment group recording 36.6% fewer hospital days than the usual care group (mean, 42.5 days vs. 67 days, respectively; P = .03).
However, the number of patients who were admitted in the first 6 weeks and at 1 year stayed the same, as did the mean number of admissions per patient over 12 months.
The home treatment group reported significantly fewer symptoms on the BPRS depression and anxiety scale at 6 weeks, compared with the usual treatment group (P = .025), but that difference was not maintained after 6 months.
The number of adverse events, including suicide attempts, was similar between the groups. Three patients in the home treatment group and two in the usual care group died by suicide.
“Future research should focus on which components of intensive home treatment or hospitalization can be used when, for whom, and meet which goals, so that both hospital care and intensive home treatment can be used proportionally and efficiently for patients in psychiatric crisis,” the investigators write.
Not generalizable?
In an accompanying editorial, Claire Henderson, PhD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, noted that generalizing the study’s results to other countries could be problematic, especially to regions such as North America, which have shorter lengths of stay for psychiatric hospitalization.
“Future trials looking at intensive home treatment would be most informative if done in countries with relatively short lengths of stay, and without separate crisis services for people receiving assertive community treatment,” Dr. Henderson writes.
The study was funded by De Stichting tot Steun Vereniging voor Christelijke Verzorging van Geestes-en Zenuwzieken. The investigators and Dr. Henderson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intensive home treatment may offer an alternative to inpatient care for patients in acute psychiatric crisis – but the intervention is no outright substitute, new research suggests.
However, there was no difference between treatment groups in improvement in quality of life or patient satisfaction; and a reduction in symptom severity noted after 6 weeks of home treatment faded within 6 months.
“We found no differences in admission rates either, which suggests that intensive home treatment is not a substitute for inpatient care but a different treatment opportunity for psychiatric patients in crisis,” Jurgen Cornelis, MD, Arkin Institute for Mental Health, Amsterdam, and colleagues write.
The findings were published online in The Lancet Psychiatry.
Increasingly popular
“Intensive home treatment is increasingly popular as an alternative to hospitalization. It was developed to prevent or reduce levels of inpatient care and facilitate the transition between inpatient care and low-intensity outpatient care,” the investigators write.
However, there have previously been only two randomized controlled trials published that assessed this type of care, resulting in “somewhat conflicting findings,” they add.
For the current study, participants presented to psychiatric emergency wards at two medical centers in the Netherlands. They were included only if they were able to offer informed consent within 14 days.
The intensive home treatment group (n = 183) worked with a multidisciplinary team that designed a care plan tailored to their specific crisis. Treatment components included pharmacotherapy, up to three home visits each day, psychoeducation, brief supportive and cognitive behavioral therapy, social care, and support and empowerment of the patient’s informal care system.
The usual care group (n = 63) commonly received a combination of highly intensive inpatient treatment in the first phase and outpatient treatment up to two times a week in the second phase. Treatment included similar components as those in intensive home treatment.
The most common primary clinical diagnosis in both groups was mood disorder, followed by psychotic disorders, personality disorders, or anxiety disorders.
The home treatment group had a significantly higher total mean item score on the Brief Psychiatric Rating Scale (BPRS) at baseline (2.23 vs. 2.04, P = .04).
Mixed results
Results at 6 weeks showed the number of hospital days was 25.3% lower in the home treatment group, compared with those who received usual care.
That trend continued at 1 year, with the intensive home treatment group recording 36.6% fewer hospital days than the usual care group (mean, 42.5 days vs. 67 days, respectively; P = .03).
However, the number of patients who were admitted in the first 6 weeks and at 1 year stayed the same, as did the mean number of admissions per patient over 12 months.
The home treatment group reported significantly fewer symptoms on the BPRS depression and anxiety scale at 6 weeks, compared with the usual treatment group (P = .025), but that difference was not maintained after 6 months.
The number of adverse events, including suicide attempts, was similar between the groups. Three patients in the home treatment group and two in the usual care group died by suicide.
“Future research should focus on which components of intensive home treatment or hospitalization can be used when, for whom, and meet which goals, so that both hospital care and intensive home treatment can be used proportionally and efficiently for patients in psychiatric crisis,” the investigators write.
Not generalizable?
In an accompanying editorial, Claire Henderson, PhD, Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, noted that generalizing the study’s results to other countries could be problematic, especially to regions such as North America, which have shorter lengths of stay for psychiatric hospitalization.
“Future trials looking at intensive home treatment would be most informative if done in countries with relatively short lengths of stay, and without separate crisis services for people receiving assertive community treatment,” Dr. Henderson writes.
The study was funded by De Stichting tot Steun Vereniging voor Christelijke Verzorging van Geestes-en Zenuwzieken. The investigators and Dr. Henderson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY












