User login
Mass shooters and mental illness: Reexamining the connection
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Delirious mania: Presentation, pathogenesis, and management
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
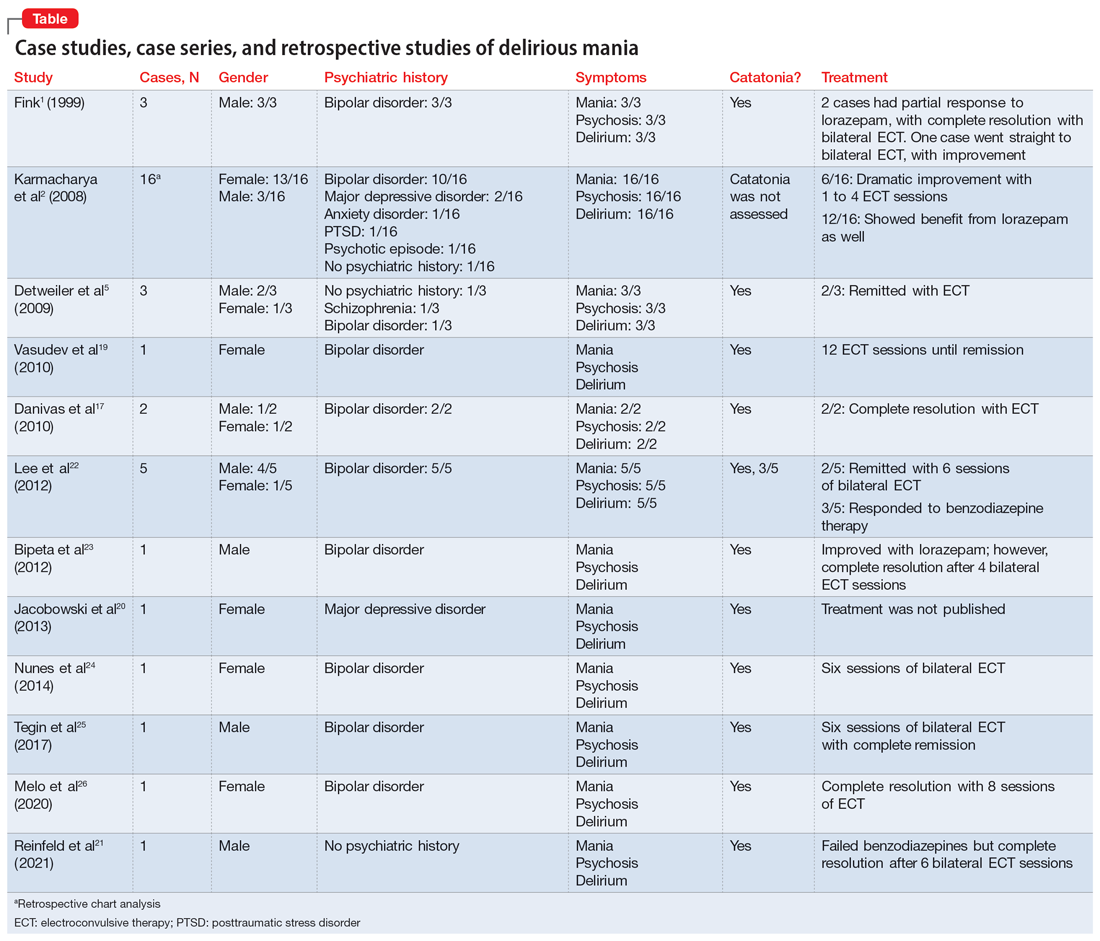
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).
Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
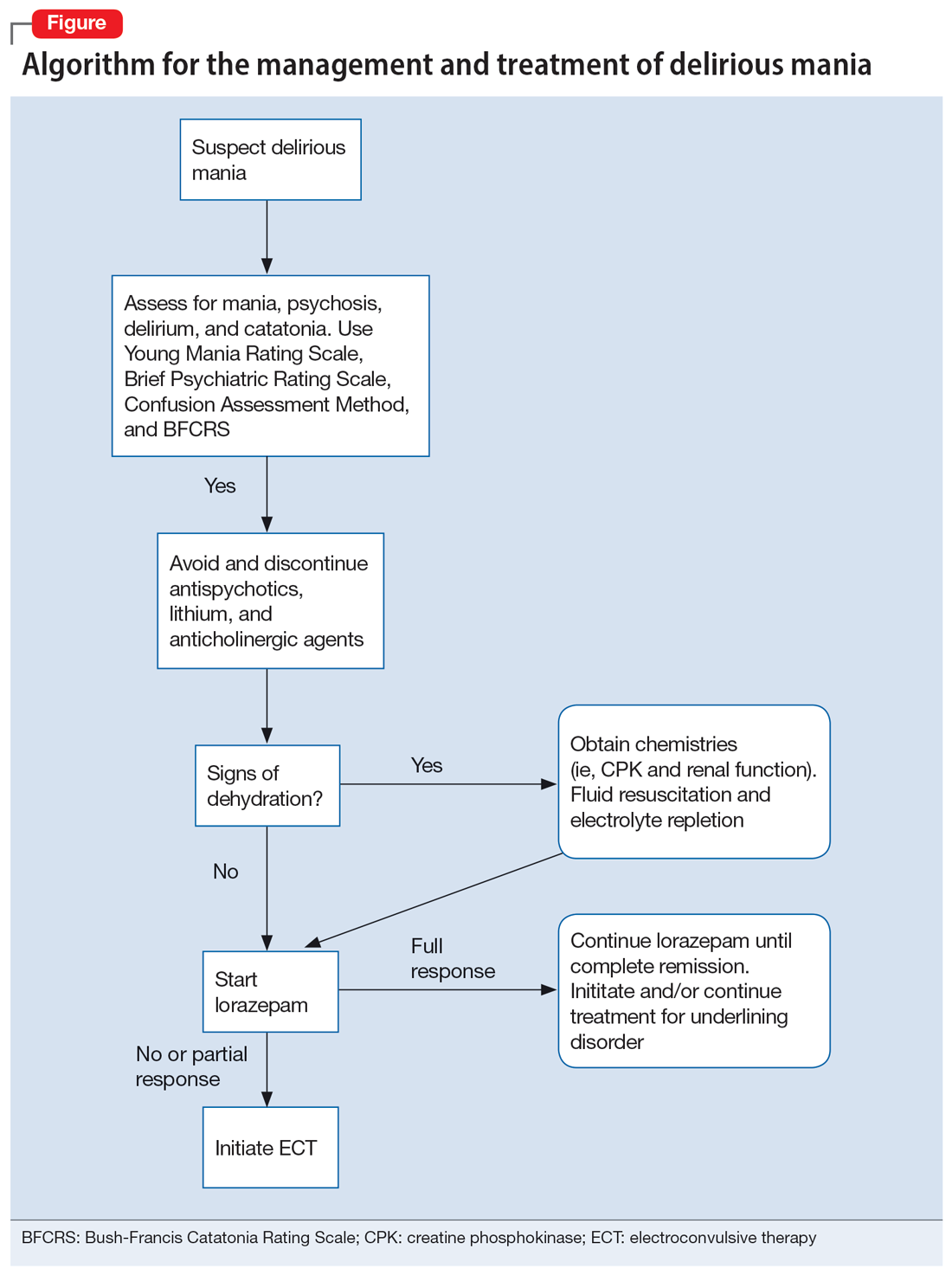
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.
Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).

Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.

Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Delirious mania is a syndrome characterized by the acute onset of severe hyperactivity, psychosis, catatonia, and intermittent confusion. While there have been growing reports of this phenomenon over the last 2 decades, it remains poorly recognized and understood.1,2 There is no widely accepted nosology for delirious mania and the condition is absent from DSM-5, which magnifies the difficulties in making a timely diagnosis and initiating appropriate treatment. Delayed diagnosis and treatment may result in a detrimental outcome.2,3 Delirious mania has also been labeled as lethal catatonia, specific febrile delirium, hyperactive or exhaustive mania, and Bell’s mania.2,4,5 The characterization and diagnosis of this condition have a long and inconsistent history (Box1,6-11).
Box
Delirious mania was originally recognized in 1849 by Luther Bell in McLean Hospital after he observed 40 cases that were uniquely distinct from 1,700 other cases from 1836 to 1849.6 He described these patients as being suddenly confused, demonstrating unprovoked combativeness, remarkable decreased need for sleep, excessive motor restlessness, extreme fearfulness, and certain physiological signs, including rapid pulse and sweating. Bell was limited to the psychiatric treatment of his time, which largely was confined to physical restraints. Approximately three-fourths of these patients died.6
Following Bell’s report, this syndrome remained unexplored and rarely described. Some researchers postulated that the development of confusion was a natural progression of late-phase mania in close to 20% of patients.7 However, this did not account for the rapid onset of symptoms as well as certain unexplained movement abnormalities. In 1980, Bond8 presented 3 cases that were similar in nature to Bell’s depiction: acute onset with extraordinary irritability, withdrawal, delirium, and mania.
For the next 2 decades, delirious mania was seldom reported in the literature. The term was often reserved to illustrate when a patient had nothing more than mania with features of delirium.9
By 1996, catatonia became better recognized in its wide array of symptomology and diagnostic scales.10,11 In 1999, in addition to the sudden onset of excitement, paranoia, grandiosity, and disorientation, Fink1 reported catatonic signs including negativism, stereotypy, posturing, grimacing, and echo phenomena in patients with delirious mania. He identified its sensitive response to electroconvulsive therapy.
Delirious mania continues to be met with incertitude in clinical practice, and numerous inconsistencies have been reported in the literature. For example, some cases that have been reported as delirious mania had more evidence of primary delirium due to another medical condition or primary mania.12,13 Other cases have demonstrated swift improvement of symptoms after monotherapy with antipsychotics without a trial of benzodiazepines or electroconvulsive therapy (ECT); the exclusion of a sudden onset questions the validity of the diagnosis and promotes the use of less efficacious treatments.14,15 Other reports have confirmed that the diagnosis is missed when certain symptoms are more predominant, such as a thought disorder (acute schizophrenia), grandiosity and delusional ideation (bipolar disorder [BD]), and less commonly assessed catatonic signs (ambitendency, automatic obedience). These symptoms are mistakenly attributed to the respective disease.1,16 This especially holds true when delirious mania is initially diagnosed as a primary psychosis, which leads to the administration of antipsychotics.17 Other cases have reported that delirious mania was resistant to treatment, but ECT was never pursued.18
In this review, we provide a more comprehensive perspective of the clinical presentation, pathogenesis, and management of delirious mania. We searched PubMed and Google Scholar using the keywords “delirious mania,” “delirious mania AND catatonia,” or “manic delirium.” Most articles we found were case reports, case series, or retrospective chart reviews. There were no systematic reviews, meta analyses, or randomized control trials (RCTs). The 12 articles included in this review consist of 7 individual case reports, 4 case series, and 1 retrospective chart review that describe a total of 36 cases (Table1,2,5,17,19-26).

Clinical presentation: What to look for
Patients with delirious mania typically develop symptoms extremely rapidly. In virtually all published literature, symptoms were reported to emerge within hours to days and consisted of severe forms of mania, psychosis, and delirium; 100% of the cases in our review had these symptoms. Commonly reported symptoms were:
- intense excitement
- emotional lability
- grandiose delusions
- profound insomnia
- pressured and rapid speech
- auditory and visual hallucinations
- hypersexuality
- thought disorganization.
Exquisite paranoia can also result in violent aggression (and may require the use of physical restraints). Patients may confine themselves to very small spaces (such as a closet) in response to the intense paranoia. Impairments in various neurocognitive domains—including inability to focus; disorientation; language and visuospatial disturbances; difficulty with shifting and sustaining attention; and short-term memory impairments—have been reported. Patients often cannot recall the events during the episode.1,2,5,27,28
Catatonia has been closely associated with delirious mania.29 Features of excited catatonia—such as excessive motor activity, negativism, grimacing, posturing, echolalia, echopraxia, stereotypy, automatic obedience, verbigeration, combativeness, impulsivity, and rigidity—typically accompany delirious mania.1,5,10,19,27
In addition to these symptoms, patients may engage in specific behaviors. They may exhibit inappropriate toileting such as smearing feces on walls or in bags, fecal or urinary incontinence, disrobing or running naked in public places, or pouring liquid on the floor or on one’s head.1,2
Continue to: Of the 36 cases...
Of the 36 cases reported in the literature we reviewed, 20 (55%) were female. Most patients had an underlining psychiatric condition, including BD (72%), major depressive disorder (8%), and schizophrenia (2%). Three patients had no psychiatric history.
Physical examination
On initial presentation, a patient with delirious mania may be dehydrated, with dry mucous membranes, pale conjunctiva, tongue dryness, and poor skin turgor.28,30 Due to excessive motor activity, diaphoresis with tachycardia, fluctuating blood pressure, and fever may be present.31
Certain basic cognitive tasks should be assessed to determine the patient’s orientation to place, date, and time. Assess if the patient can recall recent events, names of objects, or perform serial 7s; clock drawing capabilities also should be ascertained.1,2,5 A Mini-Mental State Examination is useful.32
The Bush-Francis Catatonia Rating Scale should be used to elicit features of catatonia, such as waxy flexibility, negativism, gegenhalten, mitgehen, catalepsy, ambitendency, automatic obedience, and grasp reflex.10
Laboratory findings are nonspecific
Although no specific laboratory findings are associated with delirious mania, bloodwork and imaging are routinely investigated, especially if delirium characteristics are most striking. A complete blood count, chemistries, hepatic panel, thyroid functioning, blood and/or urine cultures, creatinine phosphokinase (CPK), and urinalysis can be ordered. Head imaging such as MRI and CT to rule out intracranial pathology are typically performed.19 However, the diagnosis of delirious mania is based on the presence of the phenotypic features, by verification of catatonia, and by the responsiveness to the treatment delivered.29
Continue to: Pathogenisis: Several hypotheses
Pathogenesis: Several hypotheses
The pathogenesis of delirious mania is not well understood. There are several postulations but no salient theory. Most patients with delirious mania have an underlying systemic medical or psychiatric condition.
Mood disorders. Patients with BD or schizoaffective disorder are especially susceptible to delirious mania. The percentage of manic patients who present with delirious mania varies by study. One study suggested approximately 19% have features of the phenomenon,33 while others estimated 15% to 25%.34 Elias et al35 calculated that 15% of patients with mania succumb to manic exhaustion; from this it can be reasonably concluded that these were cases of misdiagnosed delirious mania.
Delirium hypothesis. Patients with delirious mania typically have features of delirium, including fluctuation of consciousness, disorientation, and/or poor sleep-wake cycle.36 During rapid eye movement (REM) and non-REM sleep, memory circuits are fortified. When there is a substantial loss of REM and non-REM sleep, these circuits become faulty, even after 1 night. Pathological brain waves on EEG reflect the inability to reinforce the memory circuits. Patients with these waves may develop hallucinations, bizarre delusions, and altered sensorium. ECT reduces the pathological slow wave morphologies, thus restoring the synaptic maintenance and correcting the incompetent circuitry. This can explain the robust and rapid response of ECT in a patient with delirious mania.37,38
Neurotransmitter hypothesis. It has been shown that in patients with delirious mania there is dysregulation of dopamine transport, which leads to dopamine overflow in the synapse. In contrast to a drug effect (ie, cocaine or methamphetamine) that acts by inhibiting dopamine reuptake, dopamine overflow in delirious mania is caused by the loss of dopamine transporter regulation. This results in a dysfunctional dopaminergic state that precipitates an acute state of delirium and agitation.39,40
Serotonin plays a role in mood disorders, including mania and depression.41,42 More specifically, serotonin has been implicated in impulsivity and aggression as shown by reduced levels of CSF 5-hydroxyindoleacetic acid (5-HIAA) and depletion of 5-hydroxytryptophan (5-HTP).43
Continue to: Alterations in gamma-aminobutyric acid (GABA) transmission...
Alterations in gamma-aminobutyric acid (GABA) transmission are known to occur in delirium and catatonia. In delirium, GABA signaling is increased, which disrupts the circadian rhythm and melatonin release, thus impairing the sleep-wake cycle.44 Deficiencies in acetylcholine and melatonin are seen as well as excess of other neurotransmitters, including norepinephrine and glutamate.45 Conversely, in catatonia, functional imaging studies found decreased GABA-A binding in orbitofrontal, prefrontal, parietal, and motor cortical regions.46 A study analyzing 10 catatonic patients found decreased density of GABA-A receptors in the left sensorimotor cortex compared to psychiatric and healthy controls.47
Other neurotransmitters, such as glutamate, at the N-methyl-D-aspartate receptors (NMDAR) have been hypothesized to be hyperactive, causing downstream dysregulation of GABA functioning.48 However, the exact connection between delirious mania and all these receptors and neurotransmitters remains unknown.
Encephalitis hypothesis. The relationship between delirious mania and autoimmune encephalitis suggests delirious mania has etiologies other than a primary psychiatric illness. In a 2020 retrospective study49 that analyzed 79 patients with anti-NMDAR encephalitis, 25.3% met criteria for delirious mania, and 95% of these patients had catatonic features. Dalmau et al50 found that in many cases, patients tend to respond to ECT; in a cases series of 3 patients, 2 responded to benzodiazepines.
COVID-19 hypothesis. The SARS-CoV-2 virion has been associated with many neuropsychiatric complications, including mood, psychotic, and neurocognitive disorders.51,52 There also have been cases of COVID-19–induced catatonia.53-55 One case of delirious mania in a patient with COVID-19 has been reported.21 The general mechanism has been proposed to be related to the stimulation of the proinflammatory cytokines, such as tumor necrosis factor-alpha and interleukin-6, which the virus produces in large quantities.56 These cytokines have been linked to psychosis and other psychiatric disorders.57 The patient with COVID-19–induced delirious mania had elevated inflammatory markers, including erythrocyte sedimentation rate, C-reactive protein, ferritin, and D-dimer, which supports a proinflammatory state. This patient had a complete resolution of symptoms with ECT.21
Management: Benzodiazepines and ECT
A step-by-step algorithm for managing delirious mania is outlined in the Figure. Regardless of the underlining etiology, management of delirious mania consists of benzodiazepines (lorazepam and diazepam); prompt use of ECT, particularly for patients who do not improve with large doses of lorazepam; or (if applicable) continued treatment of the underlining medical condition, which does not preclude the use of benzodiazepines or ECT. Recent reports27,58 have described details for using ECT for delirious mania, highlighting the use of high-energy dosing, bilateral electrode placement, and frequent sessions.

Continue to: Knowing which medications...
Knowing which medications to avoid is as important as knowing which agents to administer. Be vigilant in avoiding high-potency antipsychotics, as these medications can worsen extrapyramidal symptoms and may precipitate seizures or neuroleptic malignant syndrome (NMS).28 Anticholinergic agents should also be avoided because they worsen confusion. Although lithium is effective in BD, in delirious mania, high doses of lithium and haloperidol may cause severe encephalopathic syndromes, with symptoms that can include lethargy, tremors, cerebellar dysfunction, and worsened confusion; it may also cause widespread and irreversible brain damage.59
Due to long periods of hyperactivity, withdrawal, and diaphoresis, patients with delirious mania may be severely dehydrated with metabolic derangements, including elevated CPK due to rhabdomyolysis from prolonged exertion, IM antipsychotics, or rigidity. To prevent acute renal failure, this must be immediately addressed with rapid fluid resuscitation and electrolyte repletion.61
Benzodiazepines. The rapid use of lorazepam should be initiated when delirious mania is suspected. Doses of 6 to 20 mg have been reported to be effective if tolerated.5,20 Typically, high-dose lorazepam will not have the sedative effect that would normally occur in a patient who does not have delirious mania.2 Lorazepam should be titrated until full resolution of symptoms. Doses up to 30 mg have been reported as effective and tolerable.62 In our literature review, 50% of patients (18/36) responded or partially responded to lorazepam. However, only 3 case reports documented a complete remission with lorazepam, and many patients needed ECT for remission of symptoms.
ECT is generally reserved for patients who are not helped by benzodiazepine therapy, which is estimated to be up to 20%.5 ECT is highly effective in delirious mania, with remission rates ranging from 80% to 100%.1 ECT is also effective in acute nondelirious mania (comparable to depression); however, it is only used in a small minority of cases (0.2% to 12%).35 In our review, 58% of cases (21/36) reported using ECT, and in all cases it resulted in complete remission.
A dramatic improvement can be seen even after a single ECT session, though most patients show improvement after 4 sessions or 3 to 7 days.1,2,5 In our review, most patients received 4 to 12 sessions until achieving complete remission.
Continue to: No RCTs have evaluated...
No RCTs have evaluated ECT electrode placement in patients with delirious mania. However, several RCTs have investigated electrode placement in patients with acute nondelirious mania. Hiremani et al63 found that bitemporal placement had a more rapid response rate than bifrontal placement, but there was no overall difference in response rate. Barekatain et al64 found no difference between these 2 bilateral approaches. Many of the delirious mania cases report using a bilateral placement (including 42% of the ECT cases in our review) due to the emergent need for rapid relief of symptoms, which is especially necessary if the patient is experiencing hemodynamic instability, excessive violence, risk for self-harm, worsening delirium, or resistance to lorazepam.
Prognosis: Often fatal if left untreated
Patients with delirious mania are at high risk to progress to a more severe form of NMS or malignant catatonia. Therefore, high-potency antipsychotics should be avoided; mortality can be elevated from 60% without antipsychotics to 78% with antipsychotics.4 Some researchers estimate 75% to 78% of cases of delirious mania can be fatal if left untreated.3,6
Bottom Line
Delirious mania is routinely mistaken for more conventional manic or psychotic disorders. Clinicians need to be able to rapidly recognize the symptoms of this syndrome, which include mania, psychosis, delirium, and possible catatonia, so they can avoid administering toxic agents and instead initiate effective treatments such as benzodiazepines and electroconvulsive therapy.
Related Resources
- Arsan C, Baker C, Wong J, et al. Delirious mania: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2021;23(1):20f02744. doi:10.4088/PCC.20f02744
- Lamba G, Kennedy EA, Vu CP. Case report: ECT for delirious mania. Clinical Psychiatry News. December 14, 2021. https://www.mdedge.com/psychiatry/article/249909/bipolar-disorder/case-report-ect-delirious-mania
Drug Brand Names
Diazepam • Valium
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
1. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
2. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
3. Friedman RS, Mufson MJ, Eisenberg TD, et al. Medically and psychiatrically ill: the challenge of delirious mania. Harv Rev Psychiatry. 2003;11(2):91-98.
4. Mann SC, Caroff SN, Bleier HR, et al. Lethal catatonia. Am J Psychiatry. 1986;143(11):1374-1381.
5. Detweiler MB, Mehra A, Rowell T, et al. Delirious mania and malignant catatonia: a report of 3 cases and review. Psychiatr Q. 2009;80(1):23-40.
6. Bell L. On a form of disease resembling some advanced stages of mania and fever. American Journal of Insanity. 1849;6(2):97-127.
7. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28(2):221-228.
8. Bond TC. Recognition of acute delirious mania. Arch Gen Psychiatry. 1980;37(5):553-554.
9. Hutchinson G, David A. Manic pseudo-delirium - two case reports. Behav Neurol. 1997;10(1):21-23.
10. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
11. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
12. Cordeiro CR, Saraiva R, Côrte-Real B, et al. When the bell rings: clinical features of Bell’s mania. Prim Care Companion CNS Disord. 2020;22(2):19l02511. doi:10.4088/PCC.19l02511
13. Yeo LX, Kuo TC, Hu KC, et al. Lurasidone-induced delirious mania. Am J Ther. 2019;26(6):e786-e787.
14. Jung WY, Lee BD. Quetiapine treatment for delirious mania in a military soldier. Prim Care Companion J Clin Psychiatry. 2010;12(2):PCC.09l00830. doi:10.4088/PCC.09l00830yel
15. Wahid N, Chin G, Turner AH, et al. Clinical response of clozapine as a treatment for delirious mania. Ment Illn. 2017;9(2):7182. doi:10.4081/mi.2017.7182
16. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
17. Danivas V, Behere RV, Varambally S, et al. Electroconvulsive therapy in the treatment of delirious mania: a report of 2 patients. J ECT. 2010;26(4):278-279.
18. O’Callaghan N, McDonald C, Hallahan B. Delirious mania intractable to treatment. Ir J Psychol Med. 2016;33(2):129-132.
19. Vasudev K, Grunze H. What works for delirious catatonic mania? BMJ Case Rep. 2010;2010:bcr0220102713. doi:10.1136/bcr.02.2010.2713
20. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
21. Reinfeld S, Yacoub A. A case of delirious mania induced by COVID-19 treated with electroconvulsive therapy. J ECT. 2021;37(4):e38-e39.
22. Lee BS, Huang SS, Hsu WY, et al. Clinical features of delirious mania: a series of five cases and a brief literature review. BMC Psychiatry. 2012;12:65. doi:10.1186/1471-244X-12-65
23. Bipeta R, Khan MA. Delirious mania: can we get away with this concept? A case report and review of the literature. Case Rep Psychiatry. 2012;2012:720354. doi:10.1155/2012/720354
24. Nunes AL, Cheniaux E. Delirium and mania with catatonic features in a Brazilian patient: response to ECT. J Neuropsychiatry Clin Neurosci. 2014;26(1):E1-E3.
25. Tegin C, Kalayil G, Lippmann S. Electroconvulsive therapy and delirious catatonic mania. J ECT. 2017;33(4):e33-e34.
26. Melo AL, Serra M. Delirious mania and catatonia. Bipolar Disord. 2020;22(6):647-649.
27. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
28. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
29. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251 Suppl 1:I8-I13.
30. Vivanti A, Harvey K, Ash S, et al. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47(3):340-355.
31. Ware MR, Feller DB, Hall KL. Neuroleptic malignant syndrome: diagnosis and management. Prim Care Companion CNS Disord. 2018;20(1):17r02185. doi:10.4088/PCC.17r0218
32. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
33. Taylor MA, Abrams R. The phenomenology of mania. A new look at some old patients. Arch Gen Psychiatry. 1973;29(4):520-522.
34. Klerman GL. The spectrum of mania. Compr Psychiatry. 1981;22(1):11-20.
35. Elias A, Thomas N, Sackeim HA. Electroconvulsive therapy in mania: a review of 80 years of clinical experience. Am J Psychiatry. 2021;178(3):229-239.
36. Thom RP, Levy-Carrick NC, Bui M, et al. Delirium. Am J Psychiatry. 2019;176(10):785-793.
37. Charlton BG, Kavanau JL. Delirium and psychotic symptoms--an integrative model. Med Hypotheses. 2002;58(1):24-27.
38. Kramp P, Bolwig TG. Electroconvulsive therapy in acute delirious states. Compr Psychiatry. 1981;22(4):368-371.
39. Mash DC. Excited delirium and sudden death: a syndromal disorder at the extreme end of the neuropsychiatric continuum. Front Physiol. 2016;7:435.
40. Strawn JR, Keck PE Jr, Caroff SN. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870-876.
41. Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59 Suppl 14:11-14.
42. Shiah IS, Yatham LN. Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disord. 2000;2(2):77-92.
43. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42-58.
44. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789-856, ix.
45. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222.
46. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
47. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67(4):445-450.
48. Daniels J. Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci. 2009;21(4):371-380.
49. Restrepo-Martínez M, Chacón-González J, Bayliss L, et al. Delirious mania as a neuropsychiatric presentation in patients with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):64-69.
50. Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18(11):1045-1057.
51. Steardo L Jr, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261.
52. Iqbal Y, Al Abdulla MA, Albrahim S, et al. Psychiatric presentation of patients with acute SARS-CoV-2 infection: a retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6(5):e109.
53. Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529-530.
54. Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556-560.
55. Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia - a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891.
56. Chowdhury MA, Hossain N, Kashem MA, et al. Immune response in COVID-19: a review. J Infect Public Health. 2020;13(11):1619-1629.
57. Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693-697.
58. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
59. Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA. 1974;230(9):1283-1287.
60. Davis MJ, de Nesnera A, Folks DG. Confused and nearly naked after going on spending sprees. Current Psychiatry. 2014;13(7):56-62.
61. Stanley M, Chippa V, Aeddula NR, et al. Rhabdomyolysis. StatPearls Publishing; 2021.
62. Fink M, Taylor MA. The catatonia syndrome: forgotten but not gone. Arch Gen Psychiatry. 2009;66(11):1173-1177.
63. Hiremani RM, Thirthalli J, Tharayil BS, et al. Double-blind randomized controlled study comparing short-term efficacy of bifrontal and bitemporal electroconvulsive therapy in acute mania. Bipolar Disord. 2008;10(6):701-707.
64. Barekatain M, Jahangard L, Haghighi M, et al. Bifrontal versus bitemporal electroconvulsive therapy in severe manic patients. J ECT. 2008;24(3):199-202.
Cannabis and schizophrenia: A complex relationship
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
Approximately 1 in 200 individuals will be diagnosed with schizophrenia in their lifetime.1 DSM-5 criteria for the diagnosis of schizophrenia require the presence of ≥2 of 5 symptoms: delusions, hallucinations, disordered speech, grossly disorganized (or catatonic) behavior, and negative symptoms such as flat affect or avolition.2 Multiple studies have found increased rates of cannabis use among patients with schizophrenia. Because cognitive deficits are the chief predictor of clinical outcomes and quality of life in individuals with schizophrenia, the cognitive effects of cannabis use among these patients are of clinical significance.3 As legislation increasingly allows for the sale, possession, and consumption of cannabis, it is crucial to provide clinicians with evidence-based recommendations for treating patients who regularly use cannabis (approximately 8% of the adult population3). In this article, we analyze several peer-reviewed studies to investigate the impact of cannabis use on the onset and development of schizophrenia.
A look at substance-induced psychosis
Schizophrenia is associated with several structural brain changes, and some of these changes may be influenced by cannabis use (Box4). The biochemical etiology of schizophrenia is poorly understood but thought to involve dopamine, glutamate, serotonin, and gamma-aminobutyric acid. Certain positive symptoms, such as hallucinations, are uniquely human and difficult to study in animal models.5 Psychoactive substance use, especially cannabis, is frequently comorbid with schizophrenia. Additionally, certain individuals may be more predisposed to substance-induced psychosis than others based on genetic variation and underlying brain structure changes.4 Substance-induced psychosis is a psychotic state following the ingestion of a psychoactive substance or drug withdrawal lasting ≥48 hours.6 The psychoactive effects of cannabis have been associated with an exacerbation of existing schizophrenia symptoms.7 In 1998, Hall7 proposed 2 hypotheses to explain the relationship between cannabis and psychosis. The first was that heavy consumption of cannabis triggers a specific type of cannabis psychosis.7 The second was that cannabis use exacerbates existing schizophrenia, making the symptoms worse.7 Hall7 concluded that there was a complicated interaction among an individual’s vulnerability to their stressors, environment, and genetics.
Box
Schizophrenia is associated with several structural changes in the brain, including lateral ventriculomegaly, reduced prefrontal cortex volume, and generalized atrophy. These changes may precede illness and act as a risk marker.4 A multivariate regression analysis that compared patients with schizophrenia who were cannabis users vs patients with schizophrenia who were nonusers found that those with high-level cannabis use had relatively higher left and right lateral ventricle volume (r = 0.208, P = .13, and r = 0.226, P = .007, respectively) as well as increased third ventricle volume (r = 0.271, P = .001).4 These changes were dose-dependent and may lead to worse disease outcomes.4
Cannabis, COMT, and homocysteine
Great advances have been made in our ability to examine the association between genetics and metabolism. One example of this is the interaction between the catechol-O-methyltransferase (COMT) gene and the active component of cannabis, delta-9-tetrahydrocannabinol (THC). COMT codes for an enzyme that degrades cortical dopamine. The Val158Met polymorphism of this gene increases COMT activity, leading to increased dopamine catabolism, and thus decreased levels of extracellular dopamine, which induces an increase in mesolimbic dopaminergic activity, thereby increasing susceptibility to psychosis.3
In a study that genotyped 135 patients with schizophrenia, the Val158Met polymorphism was present in 29.63% of participants.3 Because THC can induce episodes of psychosis, individuals with this polymorphism may be at a higher risk of developing schizophrenia. Compared to Met carrier control participants with similar histories of cannabis consumption, those with the Val158Met polymorphism demonstrated markedly worse performance on tests of verbal fluency and processing speed.3 This is clinically significant because cognitive impairments are a major prognostic factor in schizophrenia, and identifying patients with this polymorphism could help personalize interventions for those who consume cannabis and are at risk of developing schizophrenia.
A study that evaluated 56 patients with first-episode schizophrenia found that having a history of cannabis abuse was associated with significantly higher levels of homocysteine as well as lower levels of high-density lipoprotein and vitamin B12.8 Homocysteine is an agonist at the glutamate binding site and a partial antagonist at the glycine co-agonist site in the N-methyl-
The C677T polymorphism in MTHFR may predict the risk of developing metabolic syndrome in patients taking second-generation antipsychotics.8 Elevations in homocysteine by as little as 5 μmol/L may increase schizophrenia risk by 70% compared to controls, possibly due to homocysteine initiating neuronal apoptosis, catalyzing dysfunction of the mitochondria, or increasing oxidative stress.8 There is a positive correlation between homocysteine levels and severity of negative symptoms (P = .006) and general psychopathology (P = .008) of schizophrenia when analyzed using the Positive and Negative Syndrome Scale.8 Negative symptoms such as blunted affect, apathy, anhedonia, and loss of motivation significantly impact the social and economic outcomes of patients diagnosed with schizophrenia.
Research paints a mixed picture
A Danish study analyzed the rates of conversion to schizophrenia or bipolar disorder (BD) among 6,788 individuals who received a diagnosis of substance-induced psychosis from 1994 to 2014.6 Ten comparison participants were selected for each case participant, matched on sex and year/month of birth. Participants were followed until the first occurrence of schizophrenia or BD, death, or emigration from Denmark. Substances implicated in the initial psychotic episode included cannabis, alcohol, opioids, sedatives, cocaine, amphetamines, hallucinogens, and combinations of substances.
Continue to: The overall conversion rate...
The overall conversion rate over 20 years was 32.2% (95% CI, 29.7 to 34.9), with 26.0% developing schizophrenia vs 8.4% developing BD.6 Of the substances involved, cannabis was the most common, implicated in 41.2% (95% CI, 36.6 to 46.2) of cases.6 One-half of male patients converted within 2.0 years and one-half of female patients converted within 4.4 years after a cannabis-induced psychosis.6
This study had several limitations. It could not account for any short-term psychotic symptoms experienced by the general population, especially after cannabis use. Such patients might not seek treatment. Thus, the results might not be generalizable to the general population. The study did not evaluate if conversion rates differed based on continued substance use following the psychosis episode, or the amount of each substance taken prior to the episode. Dose-dependence was not well elucidated, and this study only looked at patients from Denmark and did not account for socioeconomic status.6
Another Danish study looked at the influences of gender and cannabis use in the early course of the disease in 133 patients with schizophrenia.9 These researchers found that male gender was a significant predictor of earlier onset of dysfunction socially and in the workplace, as well as a higher risk of developing negative symptoms. However, compared to gender, cannabis use was a stronger predictor of age at first psychotic episode. For cannabis users, the median age of onset of negative symptoms was 23.7, compared to 38.4 for nonusers (P < .001).9
Cannabis use is significantly elevated among individuals with psychosis, with a 12-month prevalence of 29.2% compared to 4.0% among the general population of the United States.10 In a study that assessed 229 patients with a schizophrenia spectrum disorder during their first hospitalization and 6 months, 2 years, 4 years, and 10 years later, Foti et al10 found that the lifetime rate of cannabis use was 66.2%. Survival analysis found cannabis use doubled the risk of the onset of psychosis compared to nonusers of the same age (hazard ratio [HR] = 1.97; 95% CI, 1.48 to 2.62, P < .001), even after adjusting for socioeconomic status, age, and gender (HR = 1.34; 95% CI, 1.01 to 1.77, P < .05).10 Additionally, Foti et al10 found significant positive correlations between psychotic symptoms and cannabis use in patients with schizophrenia over the course of 10 years. An increase in symptoms was associated with a higher likelihood of cannabis use, and a decrease in symptoms was correlated with a lower likelihood of use (adjusted odds ratio = 1.64; 95% CI, 1.12 to 2.43, P < .0125).10
Ortiz-Medina et al11 conducted a meta-analysis of 22 studies of 15 cohorts from healthy populations and 12 other cohort follow-up studies that evaluated the onset of psychotic symptoms in individuals who used cannabis. Most studies found associations between cannabis use and the onset of symptoms of schizophrenia, and most determined cannabis was also a major risk factor for other psychotic disorders. Analyses of dose-dependence indicated that repeated cannabis use increased the risk of developing psychotic symptoms. This risk is increased when an individual starts using cannabis before age 15.11 Age seemed to be a stronger predictor of onset and outcome than sex, with no significant differences between men and women. One study in this review found that approximately 8% to 13% cases of schizophrenia may have been solely due to cannabis.11 The most significant limitation to the studies analyzed in this review is that retrospective studies utilize self-reported questionnaires.
Continue to: Other researchers have found...
Other researchers have found it would take a relatively high number of individuals to stop using cannabis to prevent 1 case of schizophrenia. In a study of data from England and Wales, Hickman et al12 evaluated the best available estimates of the incidence of schizophrenia, rates of heavy and light cannabis use, and risk that cannabis causes schizophrenia to determine the number needed to prevent (NNP) 1 case of schizophrenia. They estimated that it would require approximately 2,800 men age 20 to 24 (90% CI, 2,018 to 4,530) and 4,700 men age 35 to 39 (90% CI, 3,114 to 8,416) who heavily used cannabis to stop their consumption to prevent 1 case of schizophrenia.12 For women with heavy cannabis use, the mean NNP was 5,470 for women age 25 to 29 (90% CI, 3,640 to 9,839) and 10,870 for women age 35 to 39 (90% CI, 6,786 to 22,732).12 For light cannabis users, the NNP was 4 to 5 times higher than the NNP for heavy cannabis users. This suggests that clinical interventions aimed at preventing dependence on cannabis would be more effective than interventions aimed at eliminating cannabis use.
Medical cannabis and increased potency
In recent years, the use of medical cannabis, which is used to address adverse effects of chemotherapy as well as neuropathic pain, Parkinson’s disease, and epilepsy, has been increasing.13 However, there is a lack of well-conducted randomized clinical trials evaluating medical cannabis’ efficacy and safety. As medical cannabis continues to gain public acceptance and more states permit its legal use, patients and physicians should be fully informed of the known adverse effects, including impaired attention, learning, and motivation.13
Several studies have drawn attention to the dose-dependence of many of cannabis’ effects. Since at least the 1960s, the concentration of THC in cannabis has increased substantially, thus increasing its potency. Based on 66,747 samples across 8 studies, 1 meta-analysis estimated that THC concentrations in herbal cannabis increased by 0.29% (P < .001) each year between 1970 and 2017.14 Similarly, THC concentrations in cannabis resins were found to have increased by 0.57% (P = .017) each year between 1975 and 2017.14 Cannabis products with high concentrations of THC carry an increased risk of addiction and mental health disorders.14
Identifying those at highest risk
Despite ongoing research, scientific consensus on the relationship of cannabis to schizophrenia and psychosis has yet to be reached. The disparity between the relatively high prevalence of regular adult use of cannabis (8%7)and the low incidence of cannabis-induced psychosis suggests that cannabis use alone is unlikely to lead to episodes of psychosis in individuals who are not predisposed to such episodes. Sarrazin et al15 evaluated 170 patients with schizophrenia, 31 of whom had cannabis use disorder. They found no significant difference in lifetime symptom dimensions between groups, and proposed that cannabis-associated schizophrenia should not be categorized as a distinct clinical entity of schizophrenia with specific features.15
Policies that encourage follow-up of patients after episodes of drug-induced psychosis may mitigate the adverse social and economic effects of schizophrenia. Currently, these policies are not widely implemented in health care institutions, possibly because psychotic symptoms may fade after the drug’s effects have dissipated. Despite this, these patients are at high risk of developing schizophrenia and self-harm. New-onset schizophrenia should be promptly identified because delayed diagnosis is associated with worse prognosis.6 Additionally, identifying genetic susceptibilities to schizophrenia—such as the Val158Met polymorphisms—in individuals who use cannabis could help clinicians manage or slow the onset or progression of schizophrenia.3 Motivational interviewing strategies should be used to minimize or eliminate cannabis use in individuals with active schizophrenia or psychosis, thus preventing worse outcomes.
Bottom Line
Identifying susceptibilities to schizophrenia may guide interventions in patients who use cannabis. Several large studies have suggested that cannabis use may exacerbate symptoms and worsen the prognosis of schizophrenia. Motivational interviewing strategies aimed at minimizing cannabis use may improve outcomes in patients with schizophrenia.
Related Resources
- Khokhar JY, Dwiel LL, Henricks AM, et al. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78-85. doi:10.1016/j. schres.2017.04.016
- Otite ES, Solanky A, Doumas S. Adolescents, THC, and the risk of psychosis. Current Psychiatry. 2021;20(12):e1-e2. doi:10.12788/cp.0197
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
1. Simeone JC, Ward AJ, Rotella P, et al. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15(1):193. doi:10.1186/s12888-015-0578-7
2. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10. doi:10.1016/j.schres.2013.05.028
3. Bosia M, Buonocore M, Bechi M, et al. Schizophrenia, cannabis use and catechol-O-methyltransferase (COMT): modeling the interplay on cognition. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:363-368. doi:10.1016/j.pnpbp.2019.02.009
4. Welch KA, McIntosh AM, Job DE, et al. The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull. 2011;37(5):1066-1076. doi:10.1093/schbul/sbq013
5. Winship IR, Dursun SM, Baker GB, et al. An overview of animal models related to schizophrenia. Can J Psychiatry. 2019;64(1):5-17. doi:10.1177/0706743718773728
6. Starzer MSK, Nordentoft M, Hjorthøj C. Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry. 2018;175(4):343-350. doi:10.1176/appi.ajp.2017.17020223
7. Hall W. Cannabis use and psychosis. Drug Alcohol Rev. 1998;17(4):433-444. doi:10.1080/09595239800187271
8. Misiak B, Frydecka D, Slezak R, et al. Elevated homocysteine level in first-episode schizophrenia patients—the relevance of family history of schizophrenia and lifetime diagnosis of cannabis abuse. Metab Brain Dis. 2014;29(3):661-670. doi:10.1007/s11011-014-9534-3
9. Veen ND, Selten J, van der Tweel I, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161(3):501-506. doi:10.1176/appi.ajp.161.3.501
10. Foti DJ, Kotov R, Guey LT, et al. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167(8):987-993. doi:10.1176/appi.ajp.2010.09020189
11. Ortiz-Medina MB, Perea M, Torales J, et al. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018;64(7):690-704. doi:10.1177/0020764018801690
12. Hickman M, Vickerman P, Macleod J, et al. If cannabis caused schizophrenia—how many cannabis users may need to be prevented in order to prevent one case of schizophrenia? England and Wales calculations. Addiction. 2009;104(11):1856-1861. doi:10.1111/j.1360-0443.2009.02736.x
13. Gupta S, Phalen T, Gupta S. Medical marijuana: do the benefits outweigh the risks? Current Psychiatry. 2018;17(1):34-41.
14. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. 2021;116(5):1000-1010. doi:10.1111/add.15253
15. Sarrazin S, Louppe F, Doukhan R, et al. A clinical comparison of schizophrenia with and without pre-onset cannabis use disorder: a retrospective cohort study using categorical and dimensional approaches. Ann Gen Psychiatry. 2015;14:44. doi:10.1186/s12991-015-0083-x
Symptoms of psychosis and OCD in a patient with postpartum depression
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.
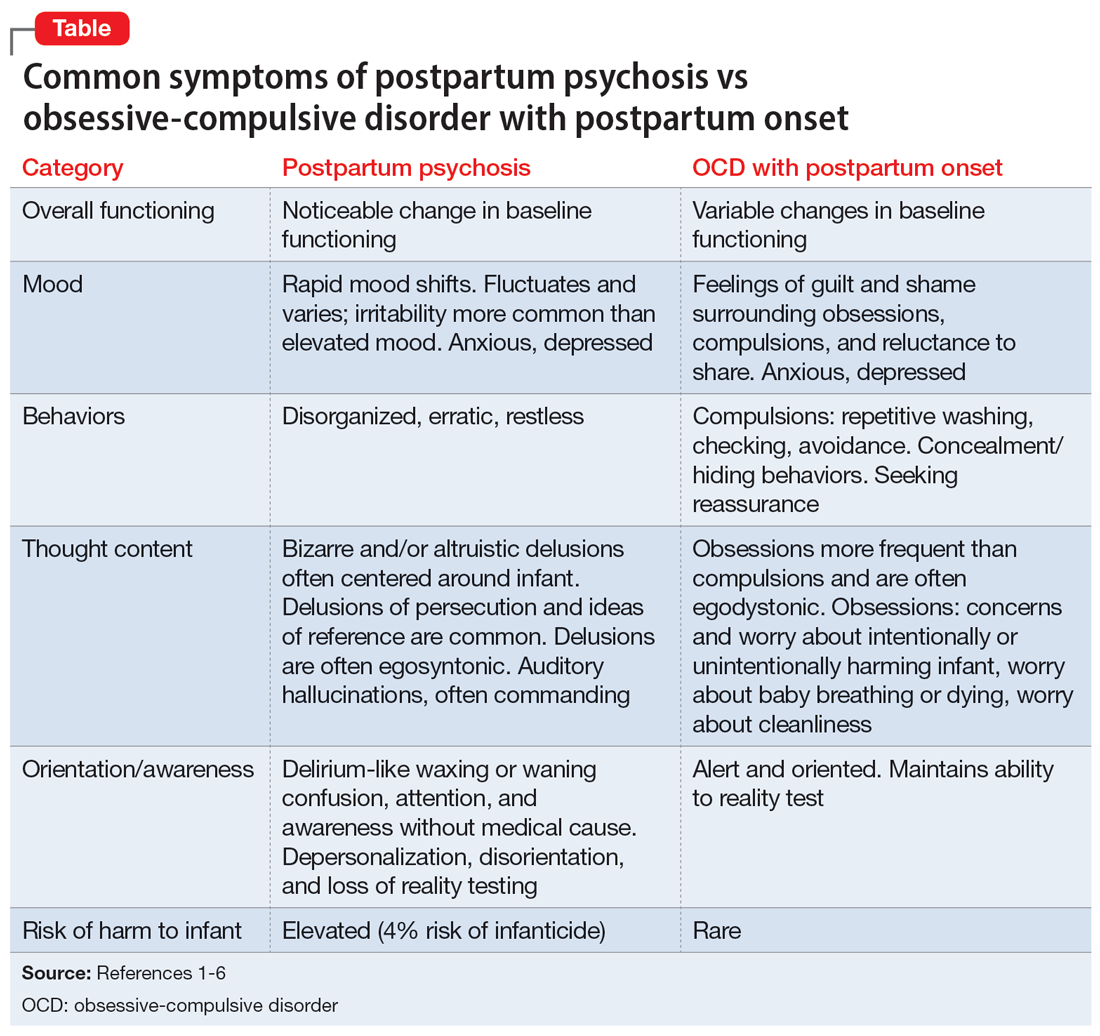
Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.
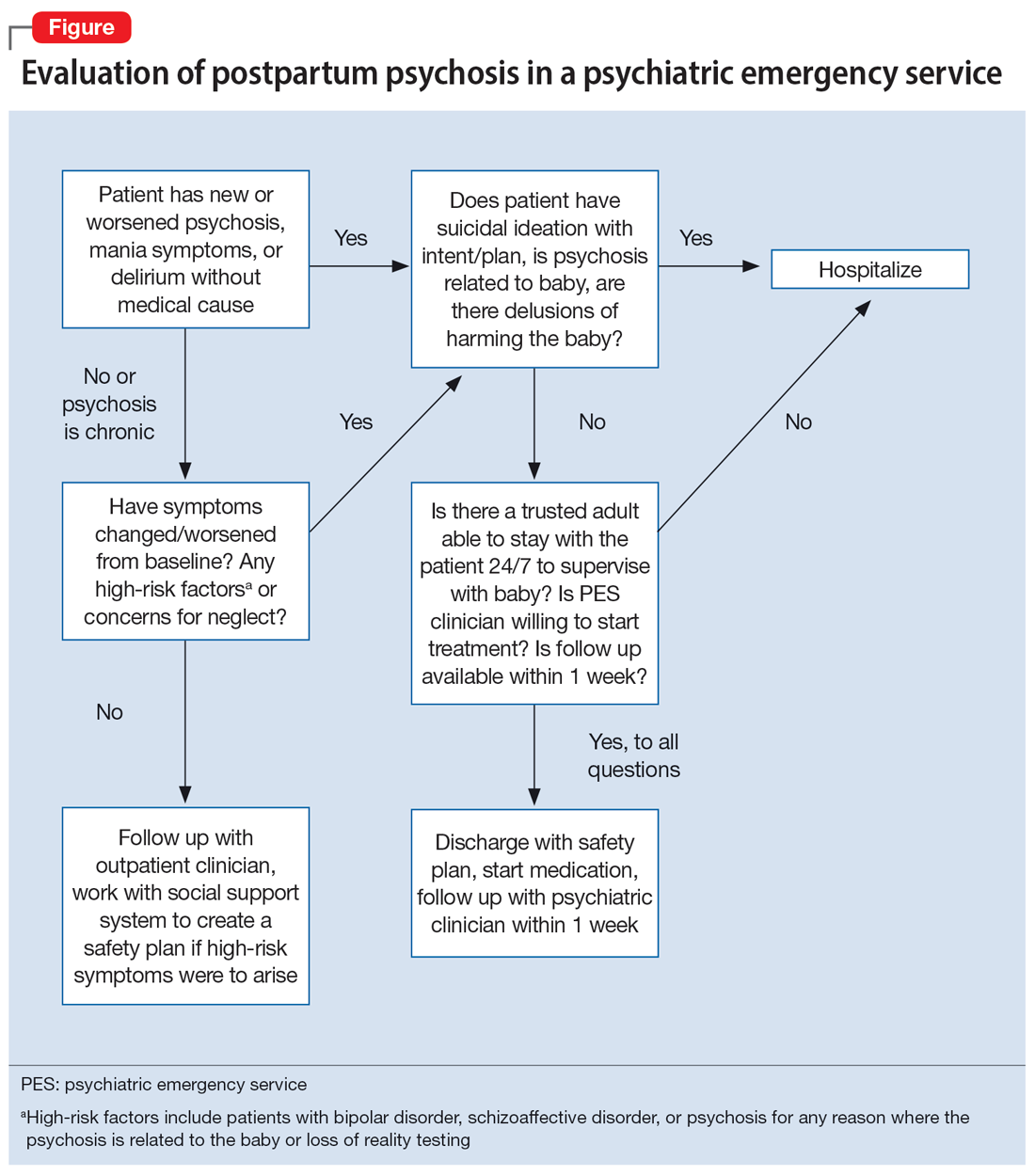
If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
CASE Thoughts of harming baby
Ms. A, age 37, is G4P2, 4 months postpartum, and breastfeeding. She has major depressive disorder (MDD) with peripartum onset, posttraumatic stress disorder, and mild intellectual disability. For years she has been stable on fluoxetine 40 mg/d and prazosin 2 mg/d. Despite recent titration of her medications, at her most recent outpatient appointment Ms. A reports having a depressed mood with frequent crying, insomnia, a lack of desire to bond with her baby, and feelings of shame. She also says she has had auditory hallucinations and thoughts of harming her baby. Ms. A’s outpatient physician makes an urgent request for her to be evaluated at the psychiatric emergency department (ED).
HISTORY Depression and possible auditory hallucinations
Ms. A developed MDD following the birth of her first child, for which her care team initiated fluoxetine at 20 mg/d and titrated it to 40 mg/d,which was effective. At that time, her outpatient physician documented potential psychotic features, including vague descriptions of derogatory auditory hallucinations. However, it was unclear if these auditory hallucinations were more representative of a distressing inner monologue without the quality of an external voice. The team determined that Ms. A was not at acute risk for harm to herself or her baby and was appropriate for outpatient care. Because the nature of these possible auditory hallucinations was mild, nondistressing, and nonthreatening, the treatment team did not initiate an antipsychotic and Ms. A was not hospitalized. She has no history of hypomanic/manic episodes and has never met criteria for a psychotic disorder.
EVALUATION Distressing thoughts and discontinued medications
During the evaluation by psychiatric emergency services, Ms. A reports that 2 weeks after giving birth she experienced a worsening of her depressive symptoms. She says she began hearing voices telling her to harm herself and her baby and describes frequent distressing thoughts, such as stabbing her baby with a knife and running over her baby with a car. Ms. A says she repeatedly wakes up at night to check on her baby’s breathing, overfeeds her baby due to a fear of inadequate nutrition, and notes intermittent feelings of confusion. Afraid of being alone with her infant, Ms. A asks her partner and mother to move in with her. Additionally, she says 2 weeks ago she discontinued all her medications at the suggestion of her partner, who recommended herbal supplements. Ms. A’s initial routine laboratory results are unremarkable and her urine drug screen is negative for all substances.
[polldaddy:13041928]
The authors’ observations
Approximately 85% of birthing parents experience some form of postpartum mood disturbance; 10% to 15% develop more significant symptoms of anxiety or depression.3 The etiology of postpartum illness is multifactorial, and includes psychiatric personal/family history, insomnia, acute and chronic psychosocial stressors, and rapid hormone fluctuations.1 As a result, the postpartum period represents a vulnerable time for birthing parents, particularly those with previously established psychiatric illness.
Ms. A’s initial presentation was concerning for a possible diagnosis of postpartum psychosis vs obsessive-compulsive disorder (OCD) with postpartum onset; other differential diagnoses included MDD with peripartum onset and psychotic features (Table1-6). Ms. A’s subjective clinical history was significant for critical pertinent findings of both OCD with postpartum onset (ie, egodystonic intrusive thoughts, checking behaviors, feelings of shame, and seeking reassurance) and postpartum psychosis (ie, command auditory hallucinations and waxing/waning confusion), which added to diagnostic complexity.

Although postpartum psychosis is rare (1 to 2 cases per 1,000 women),5 it is considered a psychiatric emergency because it has significant potential for infanticide, morbidity, and mortality. Most symptoms develop within the first 2 weeks of the postpartum period.2 There are many risk factors for the development of postpartum psychosis; however, in first-time pregnancies, a previous diagnosis of BD I is the single most important risk factor.1 Approximately 20% to 30% of women with BD experience postpartum psychosis.4
For many patients (approximately 56.7%, according to 1 meta-analysis7), postpartum psychosis denotes an episode of BD, representing a more severe form of illness with increased risk of recurrence. Most manic or mixed mood episodes reoccur within the first year removed from the perinatal period. In contrast, for some patients (approximately 43.5% according to the same meta-analysis), the episode denotes “isolated postpartum psychosis.”7 Isolated postpartum psychosis is a psychotic episode that occurs only in the postpartum period with no recurrence of psychosis or recurrence of psychosis exclusive to postpartum periods. If treated, this type of postpartum psychosis has a more favorable prognosis than postpartum psychosis in a patient with BD.7 As such, a BD diagnosis should not be established at the onset of a patient’s first postpartum psychosis presentation. Regardless of type, all presentations of postpartum psychosis are considered a psychiatry emergency.
Continue to: The prevalence of OCD...
The prevalence of OCD with postpartum onset varies. One study estimated it occurs in 2.43% of cases.4 However, the true prevalence is likely underreported due to feelings of guilt or shame associated with intrusive thoughts, and fear of stigmatization and separation from the baby. Approximately 70.6% of women experiencing OCD with postpartum onset have a comorbid depressive disorder.4
Ms. A’s presentation to the psychiatric ED carried with it diagnostic complexity and uncertainty. Her initial presentation was concerning for elements of both postpartum psychosis and OCD with postpartum onset. After her evaluation in the psychiatric ED, there remained a lack of clear and convincing evidence for a diagnosis of OCD with postpartum onset, which eliminated the possibility of discharging Ms. A with robust safety planning and reinitiation of a selective serotonin reuptake inhibitor.
Additionally, because auditory hallucinations are atypical in OCD, the treatment team remained concerned for a diagnosis of postpartum psychosis, which would warrant hospitalization. With assistance from the institution’s reproductive psychiatrists, the treatment team discussed the importance of inpatient hospitalization for risk mitigation, close observation, and thorough evaluation for greater diagnostic clarity and certainty.
TREATMENT Involuntary hospitalization
The treatment team counsels Ms. A and her partner on her differential diagnoses, including the elevated acute risk of harm to herself and her baby if she has postpartum psychosis, as well as the need for continued observation and evaluation. When alone with a clinician, Ms. A says she understands and agrees to voluntary hospitalization. However, following a subsequent risk-benefit discussion with her partner, they both grew increasingly concerned about her separation from the baby and reinitiating her medications. Amid these concerns, the treatment team notices that Ms. A attempts to minimize her symptoms. Ms. A changes her mind and no longer consents to hospitalization. She is placed on a psychiatric hold for involuntary hospitalization on the psychiatric inpatient unit.
On the inpatient unit, the inpatient clinicians and a reproductive psychiatrist continue to evaluate Ms. A. Though her diagnosis remains unclear, Ms. A agrees to start a trial of quetiapine 100 mg/d titrated to 150 mg/d to manage her potential postpartum psychosis, depressed mood, insomnia (off-label), anxiety (off-label), and OCD (off-label). Lithium is deferred because Ms. A is breastfeeding.
[polldaddy:13041932]
Continue to: The authors' observations
The authors’ observations
Due to an elevated acute risk of suicide and infanticide, postpartum psychosis represents a psychiatric emergency and often requires hospitalization. The Figure outlines steps in evaluating a patient with concerns for postpartum psychosis in a psychiatric emergency service setting. Due to the waxing and waning nature of symptoms, patients may appear psychiatrically stable at any time but remain at an overall elevated acute risk of harm to self and/or their baby.

If a patient is being considered for discharge based on yes answers to all questions in Step 2 of the Figure, the emergency psychiatric clinician must initiate appropriate psychotropic medications and complete robust safety planning with the patient and a trusted adult who will provide direct supervision. Safety planning may include (but is not limited to) strict return precautions, education on concerning symptoms and behaviors, psychotropic education and agreement of compliance, and detailed instructions on outpatient follow-up within 1 week. Ideally—and as was the case for Ms. A—a reproductive psychiatrist should be consulted in the emergency setting for shared decision-making on admission vs discharge, medication management, and outpatient follow-up considerations.
Because postpartum psychosis carries significant risks and hospitalization generally results in separating the patient from their baby, initiating psychotropics should not be delayed. Clinicians must consider the patient’s psychiatric history, allergies, and breastfeeding status.
Based on current evidence, first-line treatment for postpartum psychosis includes a mood stabilizer, an antipsychotic, and possibly a benzodiazepine.6 Thus, an appropriate initial treatment regimen would be a benzodiazepine (particularly lorazepam due to its relatively shorter half-life) and an antipsychotic (eg, haloperidol, olanzapine, or quetiapine) for acute psychosis, plus lithium for mood stabilization.1,5
If the postpartum psychosis represents an episode of BD, use of a long-term mood stabilizer may be required. In contrast, for isolated postpartum psychosis, clinicians may consider initiating psychotropics only in the immediate postpartum period, with an eventual slow taper. In future pregnancies, psychotropics may be reintroduced postpartum, which will avoid peripartum fetal exposure.8 If the patient is breastfeeding, lithium may be deferred in an acute care setting. For patients with evidence of catatonia, severe suicidality, refusal of oral intake with compromised nutrition, severe agitation, or treatment resistance, electroconvulsive therapy remains a safe and effective treatment option.6 Additionally, the safety of continued breastfeeding in acute psychosis must be considered, with the potential for recommending discontinuation, which would decrease sleep disruptions at night and increase the ability of others to feed the baby. Comprehensive care requires nonpharmacologic interventions, including psychoeducation for the patient and their family, individual psychotherapy, and expansion of psychosocial supports.
Continue to: Patients who have experienced...
Patients who have experienced an episode of postpartum psychosis are predisposed to another episode in future pregnancies.1 Current research recommends prophylaxis of recurrence with lithium monotherapy.1,2,5,6 Similar to other psychotropics in reproductive psychiatry, maintenance therapy on lithium requires a thorough “risk vs risk” discussion with the patient. The risk of lithium use while pregnant and/or breastfeeding must be weighed against the risks associated with postpartum psychosis (ie, infanticide, suicide, poor peripartum care, or poor infant bonding).
OUTCOME Improved mood
After 7 days of inpatient treatment with quetiapine, Ms. A demonstrates improvement in the targeted depressive symptoms (including improved motivation/energy and insomnia, decreased feelings of guilt, and denial of ongoing suicidal ideation). Additionally, the thoughts of harming her baby are less frequent, and command auditory hallucinations resolve. Upon discharge, Ms. A and her partner meet with inpatient clinicians for continued counseling, safety planning, and plans for outpatient follow-up with the institution’s reproductive psychiatrist.
The authors’ observations
Many aspects of Ms. A’s initial presentation in the psychiatric ED were challenging. Given the presence of symptoms of both psychosis and OCD, a diagnosis was difficult to ascertain in the emergency setting. Since command auditory hallucinations are atypical in patients with postpartum OCD, the treatment team maintained high suspicion for postpartum psychosis, which represented an emergency requiring inpatient care.
Hospitalization separated Ms. A from her baby, for whom she was the primary caregiver. Additional considerations for inpatient admission and psychotropic initiation were necessary, because Ms. A was breastfeeding. Although Ms. A’s partner was able to provide full-time childcare, the patient ultimately did not agree to hospitalization and required an emergency hold for involuntary admission, which was an additional barrier to care. Furthermore, her partner held unfavorable beliefs regarding psychotropic medications and Ms. A’s need for hospital admission, which required ongoing patient and partner education in the emergency, inpatient, and outpatient settings. Moreover, if Ms. A’s symptoms were ultimately attributable to postpartum OCD, the patient’s involuntary hospitalization might have increased the risk of stigmatization of mental illness and treatment with psychotropics.
Bottom Line
The peripartum period is a vulnerable time for patients, particularly those with previously diagnosed psychiatric illnesses. Postpartum psychosis is the most severe form of postpartum psychiatric illness and often represents an episode of bipolar disorder. Due to an elevated acute risk of suicide and infanticide, postpartum psychosis is a psychiatric emergency and warrants inpatient hospitalization for immediate intervention.
Related Resources
- Sharma V. Does your patient have postpartum OCD? Current Psychiatry. 2019;18(5):9-10.
- Hatters Friedman S, Prakash C, Nagel-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
Drug Brand Names
Fluoxetine • Prozac
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Valproic acid • Depakene
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
1. Raza SK, Raza S. Postpartum Psychosis. StatPearls Publishing; 2023. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK544304/
2. MGH Center for Women’s Mental Health. What Is Postpartum Psychosis: This Is What You Need to Know. MGH Center for Women’s Mental Health. Published November 15, 2019. Accessed June 22, 2023. https://womensmentalhealth.org/posts/postpartum-psychosis-ten-things-need-know-2/
3. MGH Center for Women’s Mental Health. Postpartum Psychiatric Disorders. MGH Center for Women’s Mental Health. Accessed October 7, 2023. https://womensmentalhealth.org/specialty-clinics-2/postpartum-psychiatric-disorders-2/
4. Sharma V, Sommerdyk C. Obsessive-compulsive disorder in the postpartum period: diagnosis, differential diagnosis and management. Womens Health (Lond). 2015;11(4):543-552. doi:10.2217/whe.15.20
5. Osborne LM. Recognizing and managing postpartum psychosis: a clinical guide for obstetric providers. Obstet Gynecol Clin North Am. 2018;45(3):455-468. doi:10.1016/j.ogc.2018.04.005
6. Hutner LA, Catapano LA, Nagle-Yang SM, et al, eds. Textbook of Women’s Reproductive Mental Health. American Psychiatric Association; 2022.
7. Gilden J, Kamperman AM, Munk-Olsen T, et al. Long-term outcomes of postpartum psychosis: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(2):19r12906. doi:10.4088/JCP.19r12906
8. Bergink V, Boyce P, Munk-Olsen T. Postpartum psychosis: a valuable misnomer. Aust N Z J Psychiatry. 2015;49(2):102-103. doi:10.1177/0004867414564698
Black psychiatric inpatients more likely to be restrained and for longer
TOPLINE:
, new research suggests.
METHODOLOGY:
- The study, part of a larger retrospective chart review of inpatient psychiatric electronic medical records (EMRs), included 29,739 adolescents (aged 12-17 years) and adults admitted because of severe and disruptive psychiatric illness or concerns about self-harm.
- A restraint event was defined as a physician-ordered physical or mechanical hold in which patients are unable to move their limbs, body, or head or are given medication to restrict their movement.
- Researchers used scores on the Dynamic Appraisal of Situational Aggression (DASA) at admission to assess risk for aggression among high-risk psychiatric inpatients (scores ranged from a low of 0 to a high of 7).
- From restraint event data extracted from the EMR, researchers investigated whether restraint frequency or duration was affected by “adultification,” a form of racial bias in which adolescents are perceived as being older than their actual age and are treated accordingly.
TAKEAWAY:
- Of the entire cohort, 1867 (6.3%) experienced a restraint event, and 27,872 (93.7%) did not.
- Compared with White patients, restraint was 85% more likely among Black patients (adjusted odds ratio, 1.85; P < .001) and 36% more likely among multiracial patients (aOR, 1.36; P = .006), which researchers suggest may reflect systemic racism within psychiatry and medicine, as well as an implicit or learned perception that people of color are more aggressive and dangerous.
- Lower DASA score at admission (P = .001), shorter length of stay (P < .001), adult age (P = .001), female sex (P = .042), and Black race, compared with White race (P = .001), were significantly associated with longer restraint duration, which may serve as a proxy for psychiatric symptom severity.
- Neither age group alone (adolescent or adult) nor the interaction of race and age group was significantly associated with experiencing a restraint event, suggesting no evidence of “adultification.”
IN PRACTICE:
It’s important to raise awareness about racial differences in restraint events in inpatient psychiatric settings, the authors write, adding that addressing overcrowding and investing in bias assessment and restraint education may reduce bias in the care of agitated patients and the use of restraints.
SOURCE:
The study was carried out by Sonali Singal, BS, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., and colleagues. It was published online in Psychiatric Services.
LIMITATIONS:
The variables analyzed in the study were limited by the retrospective chart review and by the available EMR data, which may have contained entry errors. Although the investigators used DASA scores to control for differences in aggression, they could not control for symptom severity. The study could not examine the impact of race on seclusion (involuntary confinement), a variable often examined in tandem with restraint, because there were too few such events. The analysis also did not control for substance use disorder, which can influence a patient’s behavior and be related to restraint use.
DISCLOSURES:
Ms. Singal reported no relevant financial relationships. The original article has disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- The study, part of a larger retrospective chart review of inpatient psychiatric electronic medical records (EMRs), included 29,739 adolescents (aged 12-17 years) and adults admitted because of severe and disruptive psychiatric illness or concerns about self-harm.
- A restraint event was defined as a physician-ordered physical or mechanical hold in which patients are unable to move their limbs, body, or head or are given medication to restrict their movement.
- Researchers used scores on the Dynamic Appraisal of Situational Aggression (DASA) at admission to assess risk for aggression among high-risk psychiatric inpatients (scores ranged from a low of 0 to a high of 7).
- From restraint event data extracted from the EMR, researchers investigated whether restraint frequency or duration was affected by “adultification,” a form of racial bias in which adolescents are perceived as being older than their actual age and are treated accordingly.
TAKEAWAY:
- Of the entire cohort, 1867 (6.3%) experienced a restraint event, and 27,872 (93.7%) did not.
- Compared with White patients, restraint was 85% more likely among Black patients (adjusted odds ratio, 1.85; P < .001) and 36% more likely among multiracial patients (aOR, 1.36; P = .006), which researchers suggest may reflect systemic racism within psychiatry and medicine, as well as an implicit or learned perception that people of color are more aggressive and dangerous.
- Lower DASA score at admission (P = .001), shorter length of stay (P < .001), adult age (P = .001), female sex (P = .042), and Black race, compared with White race (P = .001), were significantly associated with longer restraint duration, which may serve as a proxy for psychiatric symptom severity.
- Neither age group alone (adolescent or adult) nor the interaction of race and age group was significantly associated with experiencing a restraint event, suggesting no evidence of “adultification.”
IN PRACTICE:
It’s important to raise awareness about racial differences in restraint events in inpatient psychiatric settings, the authors write, adding that addressing overcrowding and investing in bias assessment and restraint education may reduce bias in the care of agitated patients and the use of restraints.
SOURCE:
The study was carried out by Sonali Singal, BS, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., and colleagues. It was published online in Psychiatric Services.
LIMITATIONS:
The variables analyzed in the study were limited by the retrospective chart review and by the available EMR data, which may have contained entry errors. Although the investigators used DASA scores to control for differences in aggression, they could not control for symptom severity. The study could not examine the impact of race on seclusion (involuntary confinement), a variable often examined in tandem with restraint, because there were too few such events. The analysis also did not control for substance use disorder, which can influence a patient’s behavior and be related to restraint use.
DISCLOSURES:
Ms. Singal reported no relevant financial relationships. The original article has disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- The study, part of a larger retrospective chart review of inpatient psychiatric electronic medical records (EMRs), included 29,739 adolescents (aged 12-17 years) and adults admitted because of severe and disruptive psychiatric illness or concerns about self-harm.
- A restraint event was defined as a physician-ordered physical or mechanical hold in which patients are unable to move their limbs, body, or head or are given medication to restrict their movement.
- Researchers used scores on the Dynamic Appraisal of Situational Aggression (DASA) at admission to assess risk for aggression among high-risk psychiatric inpatients (scores ranged from a low of 0 to a high of 7).
- From restraint event data extracted from the EMR, researchers investigated whether restraint frequency or duration was affected by “adultification,” a form of racial bias in which adolescents are perceived as being older than their actual age and are treated accordingly.
TAKEAWAY:
- Of the entire cohort, 1867 (6.3%) experienced a restraint event, and 27,872 (93.7%) did not.
- Compared with White patients, restraint was 85% more likely among Black patients (adjusted odds ratio, 1.85; P < .001) and 36% more likely among multiracial patients (aOR, 1.36; P = .006), which researchers suggest may reflect systemic racism within psychiatry and medicine, as well as an implicit or learned perception that people of color are more aggressive and dangerous.
- Lower DASA score at admission (P = .001), shorter length of stay (P < .001), adult age (P = .001), female sex (P = .042), and Black race, compared with White race (P = .001), were significantly associated with longer restraint duration, which may serve as a proxy for psychiatric symptom severity.
- Neither age group alone (adolescent or adult) nor the interaction of race and age group was significantly associated with experiencing a restraint event, suggesting no evidence of “adultification.”
IN PRACTICE:
It’s important to raise awareness about racial differences in restraint events in inpatient psychiatric settings, the authors write, adding that addressing overcrowding and investing in bias assessment and restraint education may reduce bias in the care of agitated patients and the use of restraints.
SOURCE:
The study was carried out by Sonali Singal, BS, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., and colleagues. It was published online in Psychiatric Services.
LIMITATIONS:
The variables analyzed in the study were limited by the retrospective chart review and by the available EMR data, which may have contained entry errors. Although the investigators used DASA scores to control for differences in aggression, they could not control for symptom severity. The study could not examine the impact of race on seclusion (involuntary confinement), a variable often examined in tandem with restraint, because there were too few such events. The analysis also did not control for substance use disorder, which can influence a patient’s behavior and be related to restraint use.
DISCLOSURES:
Ms. Singal reported no relevant financial relationships. The original article has disclosures of other authors.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
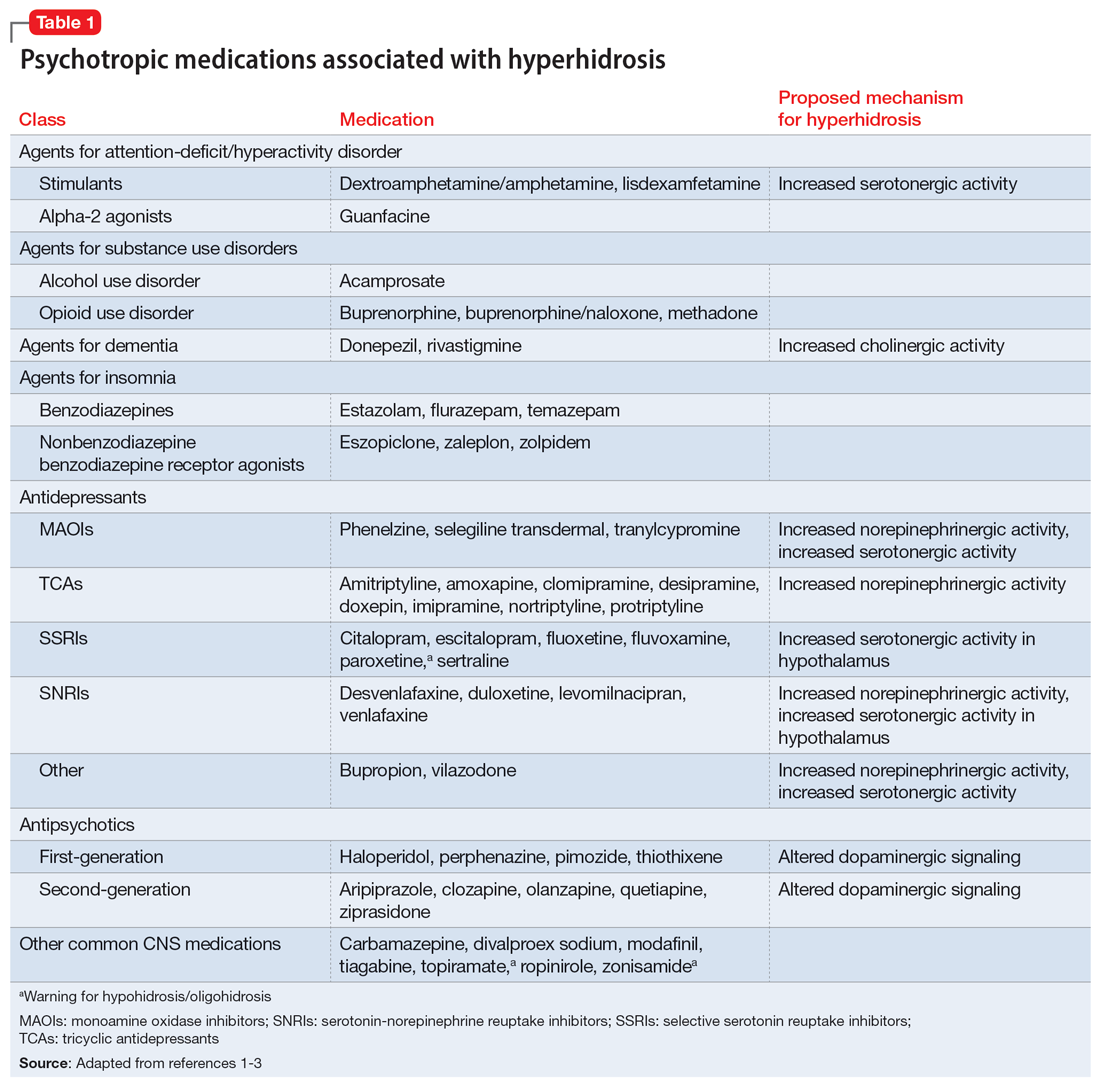
The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
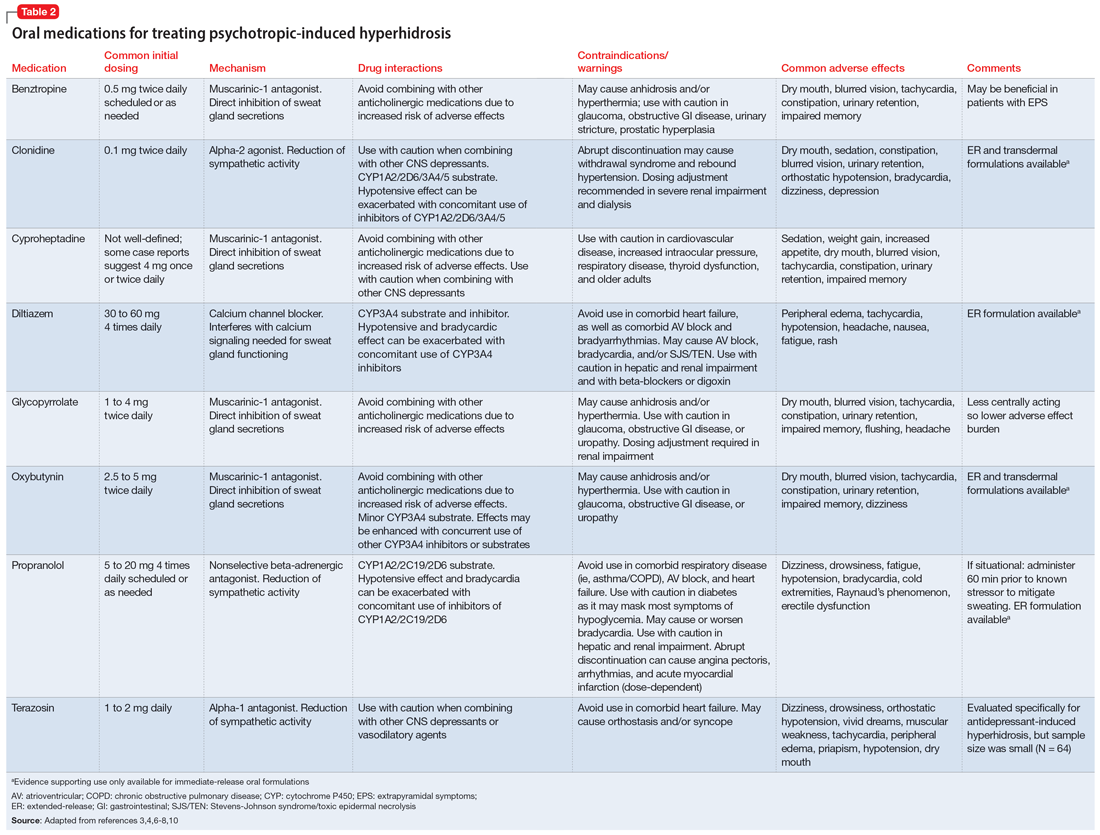
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown,but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Interviewing a patient experiencing psychosis
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
Clinicians of all experience levels, particularly trainees, may struggle when interviewing an individual experiencing psychosis. Many clinicians feel unsure what to say when a patient expresses fixed beliefs that are not amenable to change despite conflicting evidence, or worry about inadvertently affirming these beliefs. Supporting and empathizing with a person experiencing psychosis while avoiding reinforcing delusional beliefs is an important skillset for clinicians to have. While there is no single “correct” approach to interviewing individuals with psychosis, key principles include:
1. Do not begin by challenging delusions
People experiencing delusions often feel strongly about the validity of their beliefs and find evidence to support them. Directly challenging these beliefs from the beginning may alienate them. Instead, explore with neutral questioning: “Can you tell me more about X?” “What did you notice that made you believe Y?” Later, when rapport is established, it may be appropriate to explore discrepancies that provide insight into their delusions, a technique used in cognitive-behavioral therapy for psychosis.
2. Validate the emotion, not the psychosis
Many interviewers worry that talking about a patient’s delusions or voices will inadvertently reinforce them. Instead of agreeing with the content, listen for and empathize with the emotion (which is often fear): “That sounds frightening.” If the emotion is unclear, ask: “How did you feel when that happened?” When unsure what to say, sometimes a neutral “mmm” conveys listening without reinforcing the psychosis.
3. Explicitly state emotions and intentions
People with psychosis may have difficulty processing others’ emotions and facial expressions.1 We recommend using verbal cues to assist them in recognizing emotions and intentions: “It makes me sad to hear how alone you felt,” or “I’m here to help you.” The interviewer may mildly “amplify” their facial expressions so that the person experiencing psychosis can more clearly identify the expressed emotion, though not all individuals with psychosis respond well to this.
4. Reflect the patient’s own words
We recommend using the patient’s exact (typically nonclinical) words in referring to their experiences to build rapport and a shared understanding of their subjective experience.2 Avoid introducing clinical jargon, such as “delusion” or “hallucination.” For example, the interviewer might follow a patient’s explanation of their experiences by asking: “You heard voices in the walls—what did they say?” If the patient uses clinical jargon, the interviewer should clarify their meaning: “When you say ‘paranoid,’ what does that mean to you?”
5. Be intentional with gestures and positioning
People with schizophrenia-spectrum disorders may have difficulty interpreting gestures and are more likely to perceive gestures as self-referential.1 We recommend minimizing gestures or using simple, neutral-to-positive movements appropriate to cultural context. For example, in the United States, hands with palms up in front of the body generally convey openness, whereas arms crossed over the chest may convey anger. We recommend that to avoid appearing confrontational, interviewers do not position themselves directly in front of the patient, instead positioning themselves at an angle. Consider mirroring patients’ gestures or postures to convey empathy and build rapport.3
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
1. Chapellier V, Pavlidou A, Maderthaner L, et al. The impact of poor nonverbal social perception on functional capacity in schizophrenia. Front Psychol. 2022;13:804093. doi:10.3389/fpsyg.2022.804093
2. Olson M, Seikkula J, Ziedonis D. The key elements of dialogic practice in Open Dialogue: fidelity criteria. University of Massachusetts Medical School. Published September 2, 2014. Accessed August 16, 2023. https://www.umassmed.edu/globalassets/psychiatry/open-dialogue/keyelementsv1.109022014.pdf
3. Raffard S, Salesse RN, Bortolon C, et al. Using mimicry of body movements by a virtual agent to increase synchronization behavior and rapport in individuals with schizophrenia. Sci Rep. 2018;8(1):17356. doi:10.1038/s41598-018-35813-6
Substance-induced psychosis tied to schizophrenia risk
TOPLINE:
Three years after an initial ER visit, 18.5% of those with substance-induced psychosis were diagnosed with an SSD. Cannabis-induced psychosis was associated with the greatest risk.
METHODOLOGY:
- In this retrospective, population-based cohort study, investigators evaluated the risk of transition to a diagnosis of SSD for individuals with an ER visit for substance use versus the general population.
- Investigators at The Ottawa Hospital and the Institute for Clinical Evaluative Sciences, both in Ontario, analyzed data from six linked databases containing health information on nearly 10 million Ontario residents aged 14-65 years eligible for medical coverage.
- Investigators collected the health data between January 2008 and March 2022 on residents with substance use–related ER visits with, and without, psychosis.
TAKEAWAY:
- There were nearly 408,000 individuals with an ER visit for substance use, of which 13,800 (3.4%) of the visits were for substance-induced psychosis.
- Individuals with substance-induced psychosis were at a 163-fold (age- and sex-adjusted hazard ratio, 163.2; 95% confidence interval, 156.1-170.5) increased risk of transitioning to an SSD, relative to the general population (3-year risk, 18.5% vs. 0.1%).
- Individuals with an ER visit for substance use without psychosis had a lower relative risk of transitioning (aHR, 9.8; 95% CI, 9.5-10.2; 3-year risk, 1.4%) but incurred more than three times the absolute number of transitions (9,969 vs. 3,029).
- ER visits related to cannabis use had the highest transition risk among visits with psychosis (aHR, 241.6; 95% CI, 225.5-258.9) and the third-highest risk among visits without psychosis (aHR, 14.3; 95% CI, 13.5-15.2).
- Younger age and male sex were associated with a higher risk of transition, and the risk of male sex was greater in younger, compared with older, individuals particularly for cannabis use.
IN PRACTICE:
“Primary prevention efforts aimed at reducing substance use and substance use disorders could substantially reduce the population-level burden of chronic psychoses,” the investigators write. “Our findings also highlight the need for targeted secondary prevention providing early intervention and reducing substance use in the highest-risk groups, which may delay or prevent transition to schizophrenia spectrum disorders.”
SOURCE:
Daniel T. Myran, MD, MPH, of the Ottawa Hospital Research Institute, led the study, which was funded by the Canadian Institutes of Health Research and the University of Ottawa department of family medicine. The study was published online in JAMA Psychiatry.
LIMITATIONS:
Investigators did not have access to detailed data on substance-related outpatient visits or patterns of substance use, which could provide additional prognostic information.
DISCLOSURES:
Dr. Myran reported receiving grants from the Canadian Institutes of Health Research during the conduct of the study. Dr. Solmi reported receiving honoraria for participation on advisory boards or presentations from AbbVie, Angelini, Lundbeck, and Otsuka outside the submitted work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Three years after an initial ER visit, 18.5% of those with substance-induced psychosis were diagnosed with an SSD. Cannabis-induced psychosis was associated with the greatest risk.
METHODOLOGY:
- In this retrospective, population-based cohort study, investigators evaluated the risk of transition to a diagnosis of SSD for individuals with an ER visit for substance use versus the general population.
- Investigators at The Ottawa Hospital and the Institute for Clinical Evaluative Sciences, both in Ontario, analyzed data from six linked databases containing health information on nearly 10 million Ontario residents aged 14-65 years eligible for medical coverage.
- Investigators collected the health data between January 2008 and March 2022 on residents with substance use–related ER visits with, and without, psychosis.
TAKEAWAY:
- There were nearly 408,000 individuals with an ER visit for substance use, of which 13,800 (3.4%) of the visits were for substance-induced psychosis.
- Individuals with substance-induced psychosis were at a 163-fold (age- and sex-adjusted hazard ratio, 163.2; 95% confidence interval, 156.1-170.5) increased risk of transitioning to an SSD, relative to the general population (3-year risk, 18.5% vs. 0.1%).
- Individuals with an ER visit for substance use without psychosis had a lower relative risk of transitioning (aHR, 9.8; 95% CI, 9.5-10.2; 3-year risk, 1.4%) but incurred more than three times the absolute number of transitions (9,969 vs. 3,029).
- ER visits related to cannabis use had the highest transition risk among visits with psychosis (aHR, 241.6; 95% CI, 225.5-258.9) and the third-highest risk among visits without psychosis (aHR, 14.3; 95% CI, 13.5-15.2).
- Younger age and male sex were associated with a higher risk of transition, and the risk of male sex was greater in younger, compared with older, individuals particularly for cannabis use.
IN PRACTICE:
“Primary prevention efforts aimed at reducing substance use and substance use disorders could substantially reduce the population-level burden of chronic psychoses,” the investigators write. “Our findings also highlight the need for targeted secondary prevention providing early intervention and reducing substance use in the highest-risk groups, which may delay or prevent transition to schizophrenia spectrum disorders.”
SOURCE:
Daniel T. Myran, MD, MPH, of the Ottawa Hospital Research Institute, led the study, which was funded by the Canadian Institutes of Health Research and the University of Ottawa department of family medicine. The study was published online in JAMA Psychiatry.
LIMITATIONS:
Investigators did not have access to detailed data on substance-related outpatient visits or patterns of substance use, which could provide additional prognostic information.
DISCLOSURES:
Dr. Myran reported receiving grants from the Canadian Institutes of Health Research during the conduct of the study. Dr. Solmi reported receiving honoraria for participation on advisory boards or presentations from AbbVie, Angelini, Lundbeck, and Otsuka outside the submitted work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Three years after an initial ER visit, 18.5% of those with substance-induced psychosis were diagnosed with an SSD. Cannabis-induced psychosis was associated with the greatest risk.
METHODOLOGY:
- In this retrospective, population-based cohort study, investigators evaluated the risk of transition to a diagnosis of SSD for individuals with an ER visit for substance use versus the general population.
- Investigators at The Ottawa Hospital and the Institute for Clinical Evaluative Sciences, both in Ontario, analyzed data from six linked databases containing health information on nearly 10 million Ontario residents aged 14-65 years eligible for medical coverage.
- Investigators collected the health data between January 2008 and March 2022 on residents with substance use–related ER visits with, and without, psychosis.
TAKEAWAY:
- There were nearly 408,000 individuals with an ER visit for substance use, of which 13,800 (3.4%) of the visits were for substance-induced psychosis.
- Individuals with substance-induced psychosis were at a 163-fold (age- and sex-adjusted hazard ratio, 163.2; 95% confidence interval, 156.1-170.5) increased risk of transitioning to an SSD, relative to the general population (3-year risk, 18.5% vs. 0.1%).
- Individuals with an ER visit for substance use without psychosis had a lower relative risk of transitioning (aHR, 9.8; 95% CI, 9.5-10.2; 3-year risk, 1.4%) but incurred more than three times the absolute number of transitions (9,969 vs. 3,029).
- ER visits related to cannabis use had the highest transition risk among visits with psychosis (aHR, 241.6; 95% CI, 225.5-258.9) and the third-highest risk among visits without psychosis (aHR, 14.3; 95% CI, 13.5-15.2).
- Younger age and male sex were associated with a higher risk of transition, and the risk of male sex was greater in younger, compared with older, individuals particularly for cannabis use.
IN PRACTICE:
“Primary prevention efforts aimed at reducing substance use and substance use disorders could substantially reduce the population-level burden of chronic psychoses,” the investigators write. “Our findings also highlight the need for targeted secondary prevention providing early intervention and reducing substance use in the highest-risk groups, which may delay or prevent transition to schizophrenia spectrum disorders.”
SOURCE:
Daniel T. Myran, MD, MPH, of the Ottawa Hospital Research Institute, led the study, which was funded by the Canadian Institutes of Health Research and the University of Ottawa department of family medicine. The study was published online in JAMA Psychiatry.
LIMITATIONS:
Investigators did not have access to detailed data on substance-related outpatient visits or patterns of substance use, which could provide additional prognostic information.
DISCLOSURES:
Dr. Myran reported receiving grants from the Canadian Institutes of Health Research during the conduct of the study. Dr. Solmi reported receiving honoraria for participation on advisory boards or presentations from AbbVie, Angelini, Lundbeck, and Otsuka outside the submitted work. The remaining authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
New insight into genetic link between schizophrenia and CVD
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Auditory hallucinations in a patient who is hearing impaired
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.
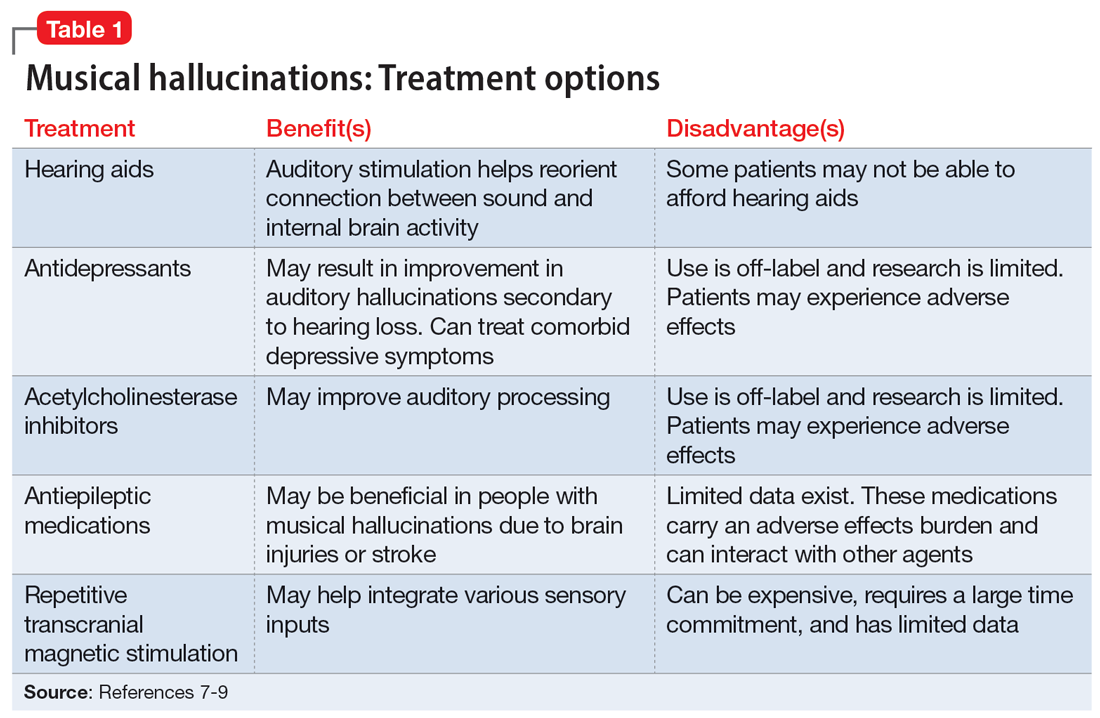
Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11
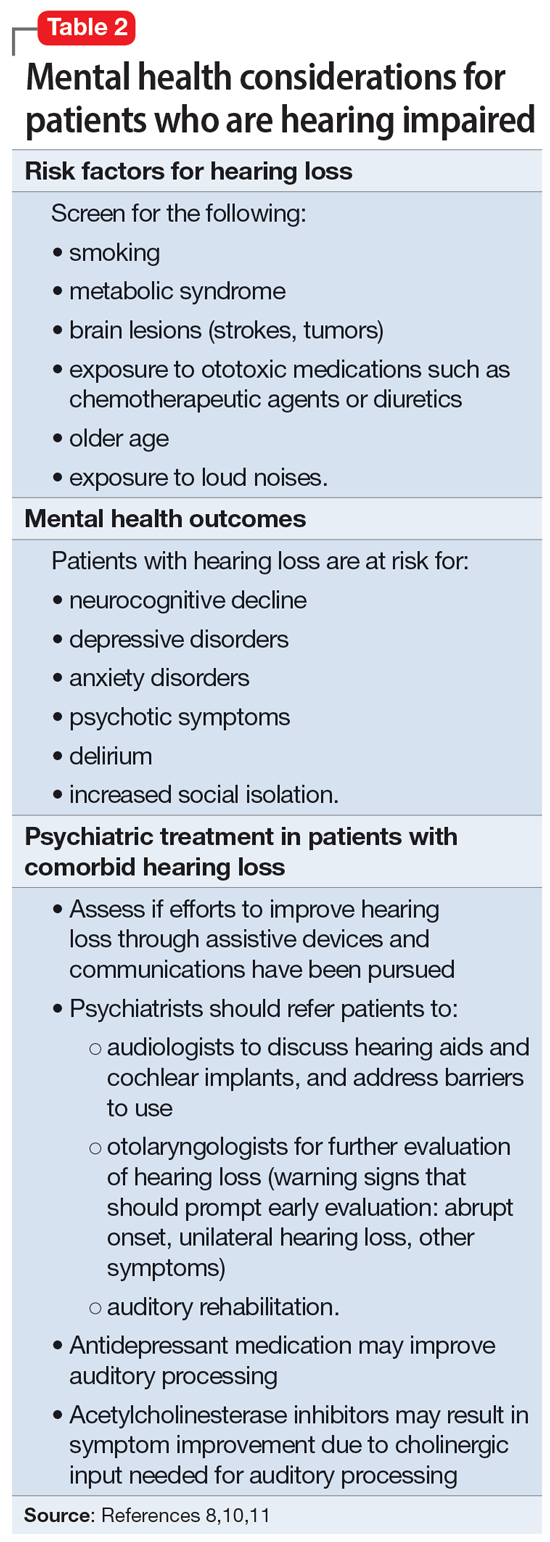
Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11
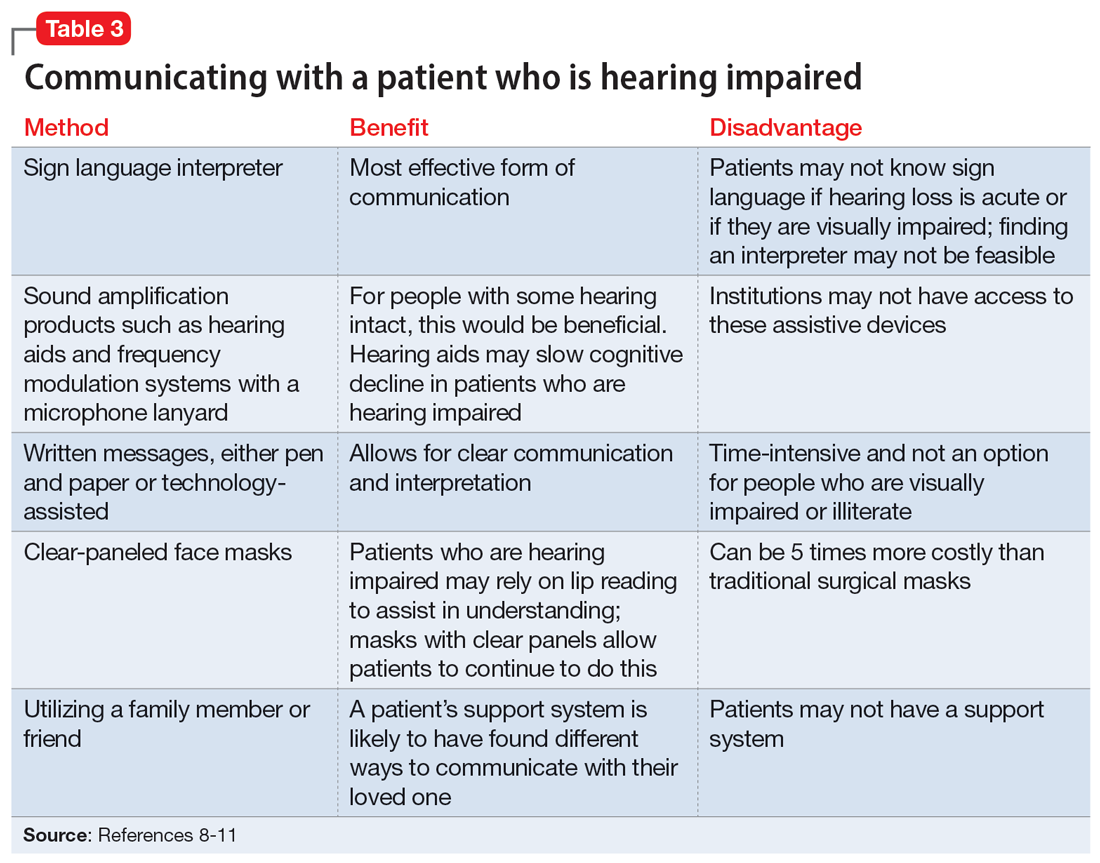
OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
CASE New-onset auditory hallucinations
Ms. L, age 78, presents to our hospital with worsening anxiety due to auditory hallucinations. She has been hearing music, which she reports is worse at night and consists of songs, usually the song Jingle Bells, sometimes just melodies and other times with lyrics. Ms. L denies paranoia, visual hallucinations, or worsening mood.
Two weeks ago, Ms. L had visited another hospital, describing 5 days of right-side hearing loss accompanied by pain and burning in her ear and face, along with vesicular lesions in a dermatomal pattern extending into her auditory canal. During this visit, Ms. L’s complete blood count, urine culture, urine drug screen, electrolytes, liver panel, thyroid studies, and vitamin levels were unremarkable. A CT scan of her head showed no abnormalities.
Ms. L was diagnosed with Ramsay Hunt syndrome (herpes zoster oticus), which affects cranial nerves, because of physical examination findings with a dermatomal pattern of lesion distribution and associated pain. Ramsay Hunt syndrome can cause facial paralysis and hearing loss in the affected ear. She was discharged with prescriptions for prednisone 60 mg/d for 7 days and valacyclovir 1 g/d for 7 days and told to follow up with her primary care physician. During the present visit to our hospital, Ms. L’s home health nurse reports that she still has her entire bottles of valacyclovir and prednisone left. Ms. L also has left-side hearing loss that began 5 years ago and a history of recurrent major depressive disorder (MDD) and generalized anxiety disorder. Due to the recent onset of right-side hearing loss, her hearing impairment requires her to communicate via writing or via a voice-to-text app.
HISTORY Depressed and living alone
Ms. L was diagnosed with MDD more than 4 decades ago and has been receiving medication since then. She reports no prior psychiatric hospitalizations, suicide attempts, manic symptoms, or psychotic symptoms. For more than 20 years, she has seen a nurse practitioner, who had prescribed mirtazapine 30 mg/d for MDD, poor appetite, and sleep. Within the last 5 years, her nurse practitioner added risperidone 0.5 mg/d at night to augment the mirtazapine for tearfulness, irritability, and mood swings.
Ms. L’s medical history also includes hypertension and chronic obstructive pulmonary disease. She is a retired teacher and lives alone. She has a chore worker who visits her home for 1 hour 5 days a week to help with cleaning and lifting, and support from her son. Ms. L no longer drives and relies on others for transportation, but is able to manage her finances, activities of daily living, cooking, and walking without any assistance.
[polldaddy:12807642]
EVALUATION Identifying the cause of the music
Ms. L is alert and oriented to time and situation, her concentration is appropriate, and her recent and remote memories are preserved. A full cognitive screen is not performed, but she is able to spell WORLD forwards and backwards and adequately perform a serial 7s test. An examination of her ear does not reveal any open vesicular lesions or swelling, but she continues to report pain and tingling in the C7 dermatomal pattern. Her urine drug screen and infectious and autoimmune laboratory testing are unremarkable. She does not have electrolyte, renal function, or blood count abnormalities. An MRI of her brain that is performed to rule out intracranial pathology due to acute hearing loss shows no acute intracranial abnormalities, with some artifact effect due to motion. Because temporal lobe epilepsy can present with hallucinations,1 an EEG is performed to rule out seizure activity; it shows a normal wake pattern.
Psychiatry is consulted for management of the auditory hallucinations because Ms. L is distressed by hearing music. Ms. L is evaluated by Neurology and Otolaryngology. Neurology recommends a repeat brain MRI in the outpatient setting after seeing an artifact in the inpatient imaging, as well as follow-up with her primary care physician. Otolaryngology believes her symptoms are secondary to Ramsay Hunt syndrome with incomplete treatment, which is consistent with the initial diagnosis from her previous hospital visit, and recommends another course of oral corticosteroids, along with Audiology and Otolaryngology follow-up.
Continue to: The authors' observations
The authors’ observations
This is the first case we have seen detailing musical hallucinations (MH) secondary to Ramsay Hunt syndrome, although musical hallucinations have been associated with other etiologies of hearing loss. MH is a “release phenomenon” believed to be caused by deprivation of stimulation of the auditory cortex.2 They are categorized as complex auditory hallucinations made up of melodies and rhythms and may be present in up to 2.5% of patients with hearing impairment.1 The condition is mostly seen in older adults because this population is more likely to experience hearing loss. MH is more common among women (70% to 80% of cases) and is highly comorbid with psychiatric disorders such as schizophrenia, obsessive-compulsive disorder, or (as was the case for Ms. L) MDD.3 Hallucinations secondary to hearing loss may be more common in left-side hearing loss.4 In a 2005 study, Warner et al5 found religious music such as hymns or Christmas carols was most commonly heard, possibly due to repetitive past exposure.
There is no consensus on treatment for MH. Current treatment guidance comes from case reports and case series. Treatment is generally most successful when the etiology of the hallucination is both apparent and treatable, such as an infectious eitiology.3 In the case of MH due to hearing loss, hallucinations may improve following treatment with hearing aids or cochlear implants,1,3,6,7 which is what was advised for Ms. L. Table 17-9 outlines other possible measures for addressing musical hallucinations.

Anticholinesterases, antidepressants, and antiepileptics may provide some benefit.8 However, pharmacotherapy is generally less efficacious and can cause adverse effects, so environmental support and hearing aids may be a safer approach. No medications have been shown to completely cure MH.
TREATMENT Hearing loss management and follow-up
When speaking with the consulting psychiatry team, Ms. L reports her outpatient psychotropic regimen has been helpful. The team decides to continue mirtazapine 30 mg/d and risperidone 0.5 mg/d at night. We recommend that Ms. L discuss tapering off risperidone with her outpatient clinician if they feel it may be indicated to reduce the risk of adverse effects. The treatment team decides not to start corticosteroids due to the risk of steroid-induced psychotic symptoms. The team discusses hallucinations related to hearing loss with Ms. L and advise her to follow up with Audiology and Otolaryngology in the outpatient setting.
The authors’ observations
Approximately 40% of people age >60 struggle with hearing impairment4,9; this impacts their general quality of life and how clinicians communicate with such patients.10 People with hearing loss are more likely to develop feelings of social isolation, depression, and delirium (Table 28,10,11).11

Risk factors for hearing loss include tobacco use, metabolic syndrome, exposure to loud noises, and exposure to certain ototoxic medications such as chemotherapeutic agents.11 As psychiatrists, it is important to identify patients who may be at risk for hearing loss and refer them to the appropriate medical professional. If hearing loss is new onset, refer the patient to an otolaryngologist for a full evaluation. Unilateral hearing loss should warrant further workup because this could be due to an acoustic neuroma.11
When providing care for a patient who uses a hearing aid, discuss adherence, barriers to adherence, and difficulties with adjusting the hearing aid. A referral to an audiologist may help patients address these barriers. Patients with hearing impairment or loss may benefit from auditory rehabilitation programs that provide communication strategies, ways to adapt to hearing loss, and information about different assistive options.11 Such programs are often run by audiologists or speech language pathologists and contain both counseling and group components.
Continue to: Is is critical for psychiatrists...
It is critical for psychiatrists to ensure appropriate communication with patients who are hearing impaired (Table 38-11). The use of assistive devices such as sound amplifiers, written messages, or family members to assist in communication is needed to prevent miscommunication.9-11

OUTCOME Lack of follow-up
A home health worker visits Ms. L, communicating with her using voice-to-text. Ms. L has not yet gone to her primary care physician, audiologist, or outpatient psychiatrist for follow-up because she needs to arrange transportation. Ms. L remains distressed by the music she is hearing, which is worse at night, along with her acute hearing loss.
Bottom Line
Hearing loss can predispose a person to psychiatric disorders and symptoms, including depression, delirium, and auditory hallucinations. Psychiatrists should strive to ensure clear communication with patients who are hearing impaired and should refer such patients to appropriate resources to improve outcomes.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- Sosland MD, Pinninti N. 5 ways to quiet auditory hallucinations. Current Psychiatry. 2005;4(4):110.
- Convery E, Keidser G, McLelland M, et al. A smartphone app to facilitate remote patient-provider communication in hearing health care: usability and effect on hearing aid outcomes. Telemed E-Health. 2020;26(6):798-804. doi:10.1089/ tmj.2019.0109
Drug Brand Names
Mirtazapine • Remeron
Prednisone • Rayos
Risperidone • Risperdal
Valacyclovir • Valtrex
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409
1. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. doi:10.1002/gps.618
2. Alvarez Perez P, Garcia-Antelo MJ, Rubio-Nazabal E. “Doctor, I hear music”: a brief review about musical hallucinations. Open Neurol J. 2017;11:11-14. doi:10.2174/1874205X01711010011
3. Sanchez TG, Rocha SCM, Knobel KAB, et al. Musical hallucination associated with hearing loss. Arq Neuropsiquiatr. 2011;69(2B):395-400. doi:10.1590/S0004-282X2011000300024
4. Teunisse RJ, Olde Rikkert MGM. Prevalence of musical hallucinations in patients referred for audiometric testing. Am J Geriatr Psychiatry. 2012;20(12):1075-1077. doi:10.1097/JGP.0b013e31823e31c4
5. Warner N, Aziz V. Hymns and arias: musical hallucinations in older people in Wales. Int J Geriatr Psychiatry. 2005;20(7):658-660. doi:10.1002/gps.1338
6. Low WK, Tham CA, D’Souza VD, et al. Musical ear syndrome in adult cochlear implant patients. J Laryngol Otol. 2013;127(9):854-858. doi:10.1017/S0022215113001758
7. Brunner JP, Amedee RG. Musical hallucinations in a patient with presbycusis: a case report. Ochsner J. 2015;15(1):89-91.
8. Coebergh JAF, Lauw RF, Bots R, et al. Musical hallucinations: review of treatment effects. Front Psychol. 2015;6:814. doi:10.3389/fpsyg.2015.00814
9. Ten Hulzen RD, Fabry DA. Impact of hearing loss and universal face masking in the COVID-19 era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
10. Shukla A, Nieman CL, Price C, et al. Impact of hearing loss on patient-provider communication among hospitalized patients: a systematic review. Am J Med Qual. 2019;34(3):284-292. doi:10.1177/1062860618798926
11. Blazer DG, Tucci DL. Hearing loss and psychiatric disorders: a review. Psychol Med. 2019;49(6):891-897. doi:10.1017/S0033291718003409









