User login
Widespread prescribing of stimulants with other CNS-active meds
Investigators analyzed prescription drug claims for over 9.1 million U.S. adults over a 1-year period and found that 276,223 (3%) had used a schedule II stimulant, such as methylphenidate and amphetamines, during that time. Of these 276,223 patients, 45% combined these agents with one or more additional CNS-active drugs and almost 25% were simultaneously using two or more additional CNS-active drugs.
Close to half of the stimulant users were taking an antidepressant, while close to one-third filled prescriptions for anxiolytic/sedative/hypnotic meditations, and one-fifth received opioid prescriptions.
The widespread, often off-label use of these stimulants in combination therapy with antidepressants, anxiolytics, opioids, and other psychoactive drugs, “reveals new patterns of utilization beyond the approved use of stimulants as monotherapy for ADHD, but because there are so few studies of these kinds of combination therapy, both the advantages and additional risks [of this type of prescribing] remain unknown,” study investigator Thomas J. Moore, AB, faculty associate in epidemiology, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins Medicine, Baltimore, told this news organization.
The study was published online in BMJ Open.
‘Dangerous’ substances
Amphetamines and methylphenidate are CNS stimulants that have been in use for almost a century. Like opioids and barbiturates, they’re considered “dangerous” and classified as schedule II Controlled Substances because of their high potential for abuse.
Over many years, these stimulants have been used for multiple purposes, including nasal congestion, narcolepsy, appetite suppression, binge eating, depression, senile behavior, lethargy, and ADHD, the researchers note.
Observational studies suggest medical use of these agents has been increasing in the United States. The investigators conducted previous research that revealed a 79% increase from 2013 to 2018 in the number of adults who self-report their use. The current study, said Mr. Moore, explores how these stimulants are being used.
For the study, data was extracted from the MarketScan 2019 and 2020 Commercial Claims and Encounters Databases, focusing on 9.1 million adults aged 19-64 years who were continuously enrolled in an included commercial benefit plan from Oct. 1, 2019 to Dec. 31, 2020.
The primary outcome consisted of an outpatient prescription claim, service date, and days’ supply for the CNS-active drugs.
The researchers defined “combination-2” therapy as 60 or more days of combination treatment with a schedule II stimulant and at least one additional CNS-active drug. “Combination-3” therapy was defined as the addition of at least two additional CNS-active drugs.
The researchers used service date and days’ supply to examine the number of stimulant and other CNS-active drugs for each of the days of 2020.
CNS-active drug classes included antidepressants, anxiolytics/sedatives/hypnotics, antipsychotics, opioids, anticonvulsants, and other CNS-active drugs.
Prescribing cascade
Of the total number of adults enrolled, 3% (n = 276,223) were taking schedule II stimulants during 2020, with a median of 8 (interquartile range, 4-11) prescriptions. These drugs provided 227 (IQR, 110-322) treatment days of exposure.
Among those taking stimulants 45.5% combined the use of at least one additional CNS-active drug for a median of 213 (IQR, 126-301) treatment days; and 24.3% used at least two additional CNS-active drugs for a median of 182 (IQR, 108-276) days.
“Clinicians should beware of the prescribing cascade. Sometimes it begins with an antidepressant that causes too much sedation, so a stimulant gets added, which leads to insomnia, so alprazolam gets added to the mix,” Mr. Moore said.
He cautioned that this “leaves a patient with multiple drugs, all with discontinuation effects of different kinds and clashing effects.”
These new findings, the investigators note, “add new public health concerns to those raised by our previous study. ... this more-detailed profile reveals several new patterns.”
Most patients become “long-term users” once treatment has started, with 75% continuing for a 1-year period.
“This underscores the possible risks of nonmedical use and dependence that have warranted the classification of these drugs as having high potential for psychological or physical dependence and their prominent appearance in toxicology drug rankings of fatal overdose cases,” they write.
They note that the data “do not indicate which intervention may have come first – a stimulant added to compensate for excess sedation from the benzodiazepine, or the alprazolam added to calm excessive CNS stimulation and/or insomnia from the stimulants or other drugs.”
Several limitations cited by the authors include the fact that, although the population encompassed 9.1 million people, it “may not represent all commercially insured adults,” and it doesn’t include people who aren’t covered by commercial insurance.
Moreover, the MarketScan dataset included up to four diagnosis codes for each outpatient and emergency department encounter; therefore, it was not possible to directly link the diagnoses to specific prescription drug claims, and thus the diagnoses were not evaluated.
“Since many providers will not accept a drug claim for a schedule II stimulant without an on-label diagnosis of ADHD,” the authors suspect that “large numbers of this diagnosis were present.”
Complex prescribing regimens
Mark Olfson, MD, MPH, professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said the report “highlights the pharmacological complexity of adults who are treated with stimulants.”
Dr. Olfson, who is a research psychiatrist at the New York State Psychiatric Institute, New York, and was not involved with the study, observed there is “evidence to support stimulants as an adjunctive therapy for treatment-resistant unipolar depression in older adults.”
However, he added, “this indication is unlikely to fully explain the high proportion of nonelderly, stimulant-treated adults who also receive antidepressants.”
These new findings “call for research to increase our understanding of the clinical contexts that motivate these complex prescribing regimens as well as their effectiveness and safety,” said Dr. Olfson.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Mr. Moore declares no relevant financial relationships. Coauthor G. Caleb Alexander, MD, is past chair and a current member of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Olfson declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed prescription drug claims for over 9.1 million U.S. adults over a 1-year period and found that 276,223 (3%) had used a schedule II stimulant, such as methylphenidate and amphetamines, during that time. Of these 276,223 patients, 45% combined these agents with one or more additional CNS-active drugs and almost 25% were simultaneously using two or more additional CNS-active drugs.
Close to half of the stimulant users were taking an antidepressant, while close to one-third filled prescriptions for anxiolytic/sedative/hypnotic meditations, and one-fifth received opioid prescriptions.
The widespread, often off-label use of these stimulants in combination therapy with antidepressants, anxiolytics, opioids, and other psychoactive drugs, “reveals new patterns of utilization beyond the approved use of stimulants as monotherapy for ADHD, but because there are so few studies of these kinds of combination therapy, both the advantages and additional risks [of this type of prescribing] remain unknown,” study investigator Thomas J. Moore, AB, faculty associate in epidemiology, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins Medicine, Baltimore, told this news organization.
The study was published online in BMJ Open.
‘Dangerous’ substances
Amphetamines and methylphenidate are CNS stimulants that have been in use for almost a century. Like opioids and barbiturates, they’re considered “dangerous” and classified as schedule II Controlled Substances because of their high potential for abuse.
Over many years, these stimulants have been used for multiple purposes, including nasal congestion, narcolepsy, appetite suppression, binge eating, depression, senile behavior, lethargy, and ADHD, the researchers note.
Observational studies suggest medical use of these agents has been increasing in the United States. The investigators conducted previous research that revealed a 79% increase from 2013 to 2018 in the number of adults who self-report their use. The current study, said Mr. Moore, explores how these stimulants are being used.
For the study, data was extracted from the MarketScan 2019 and 2020 Commercial Claims and Encounters Databases, focusing on 9.1 million adults aged 19-64 years who were continuously enrolled in an included commercial benefit plan from Oct. 1, 2019 to Dec. 31, 2020.
The primary outcome consisted of an outpatient prescription claim, service date, and days’ supply for the CNS-active drugs.
The researchers defined “combination-2” therapy as 60 or more days of combination treatment with a schedule II stimulant and at least one additional CNS-active drug. “Combination-3” therapy was defined as the addition of at least two additional CNS-active drugs.
The researchers used service date and days’ supply to examine the number of stimulant and other CNS-active drugs for each of the days of 2020.
CNS-active drug classes included antidepressants, anxiolytics/sedatives/hypnotics, antipsychotics, opioids, anticonvulsants, and other CNS-active drugs.
Prescribing cascade
Of the total number of adults enrolled, 3% (n = 276,223) were taking schedule II stimulants during 2020, with a median of 8 (interquartile range, 4-11) prescriptions. These drugs provided 227 (IQR, 110-322) treatment days of exposure.
Among those taking stimulants 45.5% combined the use of at least one additional CNS-active drug for a median of 213 (IQR, 126-301) treatment days; and 24.3% used at least two additional CNS-active drugs for a median of 182 (IQR, 108-276) days.
“Clinicians should beware of the prescribing cascade. Sometimes it begins with an antidepressant that causes too much sedation, so a stimulant gets added, which leads to insomnia, so alprazolam gets added to the mix,” Mr. Moore said.
He cautioned that this “leaves a patient with multiple drugs, all with discontinuation effects of different kinds and clashing effects.”
These new findings, the investigators note, “add new public health concerns to those raised by our previous study. ... this more-detailed profile reveals several new patterns.”
Most patients become “long-term users” once treatment has started, with 75% continuing for a 1-year period.
“This underscores the possible risks of nonmedical use and dependence that have warranted the classification of these drugs as having high potential for psychological or physical dependence and their prominent appearance in toxicology drug rankings of fatal overdose cases,” they write.
They note that the data “do not indicate which intervention may have come first – a stimulant added to compensate for excess sedation from the benzodiazepine, or the alprazolam added to calm excessive CNS stimulation and/or insomnia from the stimulants or other drugs.”
Several limitations cited by the authors include the fact that, although the population encompassed 9.1 million people, it “may not represent all commercially insured adults,” and it doesn’t include people who aren’t covered by commercial insurance.
Moreover, the MarketScan dataset included up to four diagnosis codes for each outpatient and emergency department encounter; therefore, it was not possible to directly link the diagnoses to specific prescription drug claims, and thus the diagnoses were not evaluated.
“Since many providers will not accept a drug claim for a schedule II stimulant without an on-label diagnosis of ADHD,” the authors suspect that “large numbers of this diagnosis were present.”
Complex prescribing regimens
Mark Olfson, MD, MPH, professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said the report “highlights the pharmacological complexity of adults who are treated with stimulants.”
Dr. Olfson, who is a research psychiatrist at the New York State Psychiatric Institute, New York, and was not involved with the study, observed there is “evidence to support stimulants as an adjunctive therapy for treatment-resistant unipolar depression in older adults.”
However, he added, “this indication is unlikely to fully explain the high proportion of nonelderly, stimulant-treated adults who also receive antidepressants.”
These new findings “call for research to increase our understanding of the clinical contexts that motivate these complex prescribing regimens as well as their effectiveness and safety,” said Dr. Olfson.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Mr. Moore declares no relevant financial relationships. Coauthor G. Caleb Alexander, MD, is past chair and a current member of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Olfson declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed prescription drug claims for over 9.1 million U.S. adults over a 1-year period and found that 276,223 (3%) had used a schedule II stimulant, such as methylphenidate and amphetamines, during that time. Of these 276,223 patients, 45% combined these agents with one or more additional CNS-active drugs and almost 25% were simultaneously using two or more additional CNS-active drugs.
Close to half of the stimulant users were taking an antidepressant, while close to one-third filled prescriptions for anxiolytic/sedative/hypnotic meditations, and one-fifth received opioid prescriptions.
The widespread, often off-label use of these stimulants in combination therapy with antidepressants, anxiolytics, opioids, and other psychoactive drugs, “reveals new patterns of utilization beyond the approved use of stimulants as monotherapy for ADHD, but because there are so few studies of these kinds of combination therapy, both the advantages and additional risks [of this type of prescribing] remain unknown,” study investigator Thomas J. Moore, AB, faculty associate in epidemiology, Johns Hopkins Bloomberg School of Public Health and Johns Hopkins Medicine, Baltimore, told this news organization.
The study was published online in BMJ Open.
‘Dangerous’ substances
Amphetamines and methylphenidate are CNS stimulants that have been in use for almost a century. Like opioids and barbiturates, they’re considered “dangerous” and classified as schedule II Controlled Substances because of their high potential for abuse.
Over many years, these stimulants have been used for multiple purposes, including nasal congestion, narcolepsy, appetite suppression, binge eating, depression, senile behavior, lethargy, and ADHD, the researchers note.
Observational studies suggest medical use of these agents has been increasing in the United States. The investigators conducted previous research that revealed a 79% increase from 2013 to 2018 in the number of adults who self-report their use. The current study, said Mr. Moore, explores how these stimulants are being used.
For the study, data was extracted from the MarketScan 2019 and 2020 Commercial Claims and Encounters Databases, focusing on 9.1 million adults aged 19-64 years who were continuously enrolled in an included commercial benefit plan from Oct. 1, 2019 to Dec. 31, 2020.
The primary outcome consisted of an outpatient prescription claim, service date, and days’ supply for the CNS-active drugs.
The researchers defined “combination-2” therapy as 60 or more days of combination treatment with a schedule II stimulant and at least one additional CNS-active drug. “Combination-3” therapy was defined as the addition of at least two additional CNS-active drugs.
The researchers used service date and days’ supply to examine the number of stimulant and other CNS-active drugs for each of the days of 2020.
CNS-active drug classes included antidepressants, anxiolytics/sedatives/hypnotics, antipsychotics, opioids, anticonvulsants, and other CNS-active drugs.
Prescribing cascade
Of the total number of adults enrolled, 3% (n = 276,223) were taking schedule II stimulants during 2020, with a median of 8 (interquartile range, 4-11) prescriptions. These drugs provided 227 (IQR, 110-322) treatment days of exposure.
Among those taking stimulants 45.5% combined the use of at least one additional CNS-active drug for a median of 213 (IQR, 126-301) treatment days; and 24.3% used at least two additional CNS-active drugs for a median of 182 (IQR, 108-276) days.
“Clinicians should beware of the prescribing cascade. Sometimes it begins with an antidepressant that causes too much sedation, so a stimulant gets added, which leads to insomnia, so alprazolam gets added to the mix,” Mr. Moore said.
He cautioned that this “leaves a patient with multiple drugs, all with discontinuation effects of different kinds and clashing effects.”
These new findings, the investigators note, “add new public health concerns to those raised by our previous study. ... this more-detailed profile reveals several new patterns.”
Most patients become “long-term users” once treatment has started, with 75% continuing for a 1-year period.
“This underscores the possible risks of nonmedical use and dependence that have warranted the classification of these drugs as having high potential for psychological or physical dependence and their prominent appearance in toxicology drug rankings of fatal overdose cases,” they write.
They note that the data “do not indicate which intervention may have come first – a stimulant added to compensate for excess sedation from the benzodiazepine, or the alprazolam added to calm excessive CNS stimulation and/or insomnia from the stimulants or other drugs.”
Several limitations cited by the authors include the fact that, although the population encompassed 9.1 million people, it “may not represent all commercially insured adults,” and it doesn’t include people who aren’t covered by commercial insurance.
Moreover, the MarketScan dataset included up to four diagnosis codes for each outpatient and emergency department encounter; therefore, it was not possible to directly link the diagnoses to specific prescription drug claims, and thus the diagnoses were not evaluated.
“Since many providers will not accept a drug claim for a schedule II stimulant without an on-label diagnosis of ADHD,” the authors suspect that “large numbers of this diagnosis were present.”
Complex prescribing regimens
Mark Olfson, MD, MPH, professor of psychiatry, medicine, and law and professor of epidemiology, Columbia University Irving Medical Center, New York, said the report “highlights the pharmacological complexity of adults who are treated with stimulants.”
Dr. Olfson, who is a research psychiatrist at the New York State Psychiatric Institute, New York, and was not involved with the study, observed there is “evidence to support stimulants as an adjunctive therapy for treatment-resistant unipolar depression in older adults.”
However, he added, “this indication is unlikely to fully explain the high proportion of nonelderly, stimulant-treated adults who also receive antidepressants.”
These new findings “call for research to increase our understanding of the clinical contexts that motivate these complex prescribing regimens as well as their effectiveness and safety,” said Dr. Olfson.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors. Mr. Moore declares no relevant financial relationships. Coauthor G. Caleb Alexander, MD, is past chair and a current member of the Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Olfson declares no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
New tool accurately predicts suicide risk in serious mental illness
The 17-question Oxford Mental Illness and Suicide Tool (OxMIS) assessment is designed to predict 12-month suicide risk in people with schizophrenia spectrum disorders and bipolar disorder based on risk factors such as familial traits, antisocial traits, and information about self-harm.
“We have demonstrated the clinical utility of OxMIS in two separate studies and countries. As with any clinical risk prediction tool, it will not improve outcomes unless coupled with effective interventions,” lead investigator Amir Sariaslan, PhD, a senior research fellow in psychiatric epidemiology at the University of Oxford, England, told this news organization.
The findings were published online in Translational Psychiatry.
Twice validated
Dr. Sariaslan and his team originally developed and validated the OxMIS in a cohort of 75,000 people with SMI in Sweden. Recognizing the lack of externally validated prognostic models in the mental health field, the team wanted to validate the instrument in a new, population-based sample in Finland.
The investigators accessed information about patient diagnosis and treatment from the Finnish Care Register for Health Care, which contains de-identified information for all individuals between ages 15 and 65 years diagnosed with an SMI between Jan. 1, 1996, and Dec. 31, 2017.
They included 137,000 patients with somatic symptom disorder or bipolar disorder for a total of more than 5 million episodes of inpatient or outpatient treatment. Investigators linked the cohort to the Causes of Death Register to identify those who had died by suicide within 12 months of an index treatment episode, which investigators randomly selected for each person.
The investigators found that 1,475 individuals in the sample died by suicide within 1 year of their index episode (1.1%).
Each patient was assigned a clinical suicide risk score based on their clinical information, familial traits, prescription information, and comorbid conditions. Using OxMIS, the investigators found that the instrument accurately predicted suicide with an area under the curve of 0.70.
In other words, in 70% of the instances where the investigators randomly selected two people from the sample, one of whom died by suicide and the other of whom did not, the individual who died by suicide had a higher OxMIS risk score.
The investigators note the model overestimated the risk for patients who were at extremely high risk for suicide (those with a predicted suicide risk of > 5%). “In our complementary sensitivity analysis, we observed improved calibration in these patients when we assigned them a suicide risk prediction of no more than 5%,” they write.
Dr. Sariaslan said that the findings highlight the importance of safety planning interventions. “It is also essential to remember that OxMIS is not intended to replace clinical decision-making, but rather to support it,” he said.
As to whether the tool could be used in other populations, such as in the United States, Dr. Sariaslan said, “there is no good evidence that the contribution of risk factors to suicide in this population is different in the U.S. than in northern Europe, so there is no a priori reason to have to do multiple external validations before it can be used for research or clinical purposes.”
One size does not fit all
Commenting on the study, Ronald Kessler, PhD, McNeil Family Professor, department of health care policy at Harvard Medical School, Boston, said that he’d be “surprised” if OxMIS was adopted in the United States because there is already an existing tool that is “slightly more accurate,” which he helped develop.
“In addition, when we start thinking about uses for such scales, it becomes clear that different scales should be used for different segments of the population, depending on intervention options,” Dr. Kessler said.
“So, for example, a different scale would probably be optimal in deciding how to manage psychiatric inpatients in the transition back to the community after hospital discharge than [it would be], say, in deciding how to respond to suicidality among patients presenting at an emergency department. No one scale will fit for all the scenarios in which prediction is desired,” he added.
The study was funded by the Academy of Finland. Dr. Kessler receives funding from the National Institute of Mental Health, Department of Defense, and Veterans Administration to develop suicide prediction models. Dr. Sariaslan has no disclosures to report.
A version of this article first appeared on Medscape.com.
The 17-question Oxford Mental Illness and Suicide Tool (OxMIS) assessment is designed to predict 12-month suicide risk in people with schizophrenia spectrum disorders and bipolar disorder based on risk factors such as familial traits, antisocial traits, and information about self-harm.
“We have demonstrated the clinical utility of OxMIS in two separate studies and countries. As with any clinical risk prediction tool, it will not improve outcomes unless coupled with effective interventions,” lead investigator Amir Sariaslan, PhD, a senior research fellow in psychiatric epidemiology at the University of Oxford, England, told this news organization.
The findings were published online in Translational Psychiatry.
Twice validated
Dr. Sariaslan and his team originally developed and validated the OxMIS in a cohort of 75,000 people with SMI in Sweden. Recognizing the lack of externally validated prognostic models in the mental health field, the team wanted to validate the instrument in a new, population-based sample in Finland.
The investigators accessed information about patient diagnosis and treatment from the Finnish Care Register for Health Care, which contains de-identified information for all individuals between ages 15 and 65 years diagnosed with an SMI between Jan. 1, 1996, and Dec. 31, 2017.
They included 137,000 patients with somatic symptom disorder or bipolar disorder for a total of more than 5 million episodes of inpatient or outpatient treatment. Investigators linked the cohort to the Causes of Death Register to identify those who had died by suicide within 12 months of an index treatment episode, which investigators randomly selected for each person.
The investigators found that 1,475 individuals in the sample died by suicide within 1 year of their index episode (1.1%).
Each patient was assigned a clinical suicide risk score based on their clinical information, familial traits, prescription information, and comorbid conditions. Using OxMIS, the investigators found that the instrument accurately predicted suicide with an area under the curve of 0.70.
In other words, in 70% of the instances where the investigators randomly selected two people from the sample, one of whom died by suicide and the other of whom did not, the individual who died by suicide had a higher OxMIS risk score.
The investigators note the model overestimated the risk for patients who were at extremely high risk for suicide (those with a predicted suicide risk of > 5%). “In our complementary sensitivity analysis, we observed improved calibration in these patients when we assigned them a suicide risk prediction of no more than 5%,” they write.
Dr. Sariaslan said that the findings highlight the importance of safety planning interventions. “It is also essential to remember that OxMIS is not intended to replace clinical decision-making, but rather to support it,” he said.
As to whether the tool could be used in other populations, such as in the United States, Dr. Sariaslan said, “there is no good evidence that the contribution of risk factors to suicide in this population is different in the U.S. than in northern Europe, so there is no a priori reason to have to do multiple external validations before it can be used for research or clinical purposes.”
One size does not fit all
Commenting on the study, Ronald Kessler, PhD, McNeil Family Professor, department of health care policy at Harvard Medical School, Boston, said that he’d be “surprised” if OxMIS was adopted in the United States because there is already an existing tool that is “slightly more accurate,” which he helped develop.
“In addition, when we start thinking about uses for such scales, it becomes clear that different scales should be used for different segments of the population, depending on intervention options,” Dr. Kessler said.
“So, for example, a different scale would probably be optimal in deciding how to manage psychiatric inpatients in the transition back to the community after hospital discharge than [it would be], say, in deciding how to respond to suicidality among patients presenting at an emergency department. No one scale will fit for all the scenarios in which prediction is desired,” he added.
The study was funded by the Academy of Finland. Dr. Kessler receives funding from the National Institute of Mental Health, Department of Defense, and Veterans Administration to develop suicide prediction models. Dr. Sariaslan has no disclosures to report.
A version of this article first appeared on Medscape.com.
The 17-question Oxford Mental Illness and Suicide Tool (OxMIS) assessment is designed to predict 12-month suicide risk in people with schizophrenia spectrum disorders and bipolar disorder based on risk factors such as familial traits, antisocial traits, and information about self-harm.
“We have demonstrated the clinical utility of OxMIS in two separate studies and countries. As with any clinical risk prediction tool, it will not improve outcomes unless coupled with effective interventions,” lead investigator Amir Sariaslan, PhD, a senior research fellow in psychiatric epidemiology at the University of Oxford, England, told this news organization.
The findings were published online in Translational Psychiatry.
Twice validated
Dr. Sariaslan and his team originally developed and validated the OxMIS in a cohort of 75,000 people with SMI in Sweden. Recognizing the lack of externally validated prognostic models in the mental health field, the team wanted to validate the instrument in a new, population-based sample in Finland.
The investigators accessed information about patient diagnosis and treatment from the Finnish Care Register for Health Care, which contains de-identified information for all individuals between ages 15 and 65 years diagnosed with an SMI between Jan. 1, 1996, and Dec. 31, 2017.
They included 137,000 patients with somatic symptom disorder or bipolar disorder for a total of more than 5 million episodes of inpatient or outpatient treatment. Investigators linked the cohort to the Causes of Death Register to identify those who had died by suicide within 12 months of an index treatment episode, which investigators randomly selected for each person.
The investigators found that 1,475 individuals in the sample died by suicide within 1 year of their index episode (1.1%).
Each patient was assigned a clinical suicide risk score based on their clinical information, familial traits, prescription information, and comorbid conditions. Using OxMIS, the investigators found that the instrument accurately predicted suicide with an area under the curve of 0.70.
In other words, in 70% of the instances where the investigators randomly selected two people from the sample, one of whom died by suicide and the other of whom did not, the individual who died by suicide had a higher OxMIS risk score.
The investigators note the model overestimated the risk for patients who were at extremely high risk for suicide (those with a predicted suicide risk of > 5%). “In our complementary sensitivity analysis, we observed improved calibration in these patients when we assigned them a suicide risk prediction of no more than 5%,” they write.
Dr. Sariaslan said that the findings highlight the importance of safety planning interventions. “It is also essential to remember that OxMIS is not intended to replace clinical decision-making, but rather to support it,” he said.
As to whether the tool could be used in other populations, such as in the United States, Dr. Sariaslan said, “there is no good evidence that the contribution of risk factors to suicide in this population is different in the U.S. than in northern Europe, so there is no a priori reason to have to do multiple external validations before it can be used for research or clinical purposes.”
One size does not fit all
Commenting on the study, Ronald Kessler, PhD, McNeil Family Professor, department of health care policy at Harvard Medical School, Boston, said that he’d be “surprised” if OxMIS was adopted in the United States because there is already an existing tool that is “slightly more accurate,” which he helped develop.
“In addition, when we start thinking about uses for such scales, it becomes clear that different scales should be used for different segments of the population, depending on intervention options,” Dr. Kessler said.
“So, for example, a different scale would probably be optimal in deciding how to manage psychiatric inpatients in the transition back to the community after hospital discharge than [it would be], say, in deciding how to respond to suicidality among patients presenting at an emergency department. No one scale will fit for all the scenarios in which prediction is desired,” he added.
The study was funded by the Academy of Finland. Dr. Kessler receives funding from the National Institute of Mental Health, Department of Defense, and Veterans Administration to develop suicide prediction models. Dr. Sariaslan has no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM TRANSLATIONAL PSYCHIATRY
Erratic sleep, lack of activity tied to worsening schizophrenia symptoms
The findings also showed that people with schizophrenia spectrum disorders (SSDs) who lived in residential facilities experienced rigid routines, which correlated with a higher degree of negative symptoms.
The rigid routines were problematic for the patients living in residential settings, lead investigator Fabio Ferrarelli, MD, PhD, told this news organization. Dr. Ferrarelli is an associate professor of psychiatry at the University of Pittsburgh.
“Engaging in different activities at different times in activities associated with motivation and social interaction – this helps to ameliorate difficult-to-treat negative symptoms,” he said.
The findings were published online in Molecular Psychiatry.
Need to increase activity levels
While there is no shortage of research on sleep disturbances among people with schizophrenia, research focusing specifically on rest-activity rhythm disturbances and their relationships to symptoms of schizophrenia has been limited by small sample sizes or the lack of a control group, the investigators note.
To address this research gap, the investigators recruited 230 patients with SSD from participating residential facilities and communities throughout Italy. The participants included 108 healthy control participants, 54 community-dwelling patients with SSD who were receiving outpatient services, and 68 patients with SSD who were living in residential facilities.
All participants wore an actigraph for 7 consecutive days so that investigators could monitor sleep-wake patterns.
Compared with healthy control participants, both SSD groups had more total sleep time and spent more time resting or being passive (P < .001). In contrast, healthy control participants were much more active.
Part of the explanation for this may be that most of the control participants had jobs or attended school. In addition, the investigators note that many medications used to treat SSD can be highly sedating, causing some patients to sleep up to 15 hours per day.
Among residential participants with SSD, there was a higher level of inter-daily stability and higher daily rest-activity-rest fragmentation than occurred among healthy control participants or community-dwelling patients with SSD (P < .001). There was also a higher level of negative symptoms among residential participants with SSD than among the community-dwelling group with SSD.
When the findings were taken together, Dr. Ferrarelli and his team interpreted them to mean that inter-daily stability could reflect premature aging or neurodegenerative processes in patients with more severe forms of schizophrenia.
Another explanation could be that the rigid routine of the residential facility was making negative symptoms worse, Dr. Ferrarelli said. It is important to add variety into the mix – getting people to engage in different activities at different times of day would likely help residential SSD patients overcome negative symptoms of the disorder.
Although participants were recruited in Italy, Dr. Ferrarelli said he believes the findings are generalizable.
Bidirectional relationship?
Commenting on the findings, Matcheri Keshavan, MD, professor of psychiatry at Harvard Medical School in Boston, said the results are consistent with “well-known clinical observations that SSD patients tend to spend more time in bed and have more dysregulated sleep.
“Negative symptoms are also common, especially in residential patients. However, it is difficult to determine causality, as we do not know whether excessive sleepiness and decreased physical activity cause negative symptoms, or vice versa, or whether this is a bidirectional relationship,” Dr. Keshavan said.
He emphasized that physical exercise is known to increase sleep quality for people with mental illness and may also improve negative symptoms. “A useful approach in clinical practice is to increase activity levels, especially physical activities like walking and gardening.”
Dr. Keshavan said he would like to see future research that focuses on whether an intervention such as aerobic exercise would improve sleep quality as well as negative symptoms.
He also said that future research should ideally examine the characteristics of sleep alterations in schizophrenia.
“For example, while sleep duration is increased in schizophrenia, studies suggest that time spent in deep sleep is reduced; sleep spindles, which are important for consolidating memory during sleep, are also reduced. Correcting these deficits may improve negative symptoms and cognitive deficits,” he added.
The study was funded by the Italian Ministry of Health and the National Institute of Mental Health. There were no conflicts of interest.
A version of this article first appeared on Medscape.com.
The findings also showed that people with schizophrenia spectrum disorders (SSDs) who lived in residential facilities experienced rigid routines, which correlated with a higher degree of negative symptoms.
The rigid routines were problematic for the patients living in residential settings, lead investigator Fabio Ferrarelli, MD, PhD, told this news organization. Dr. Ferrarelli is an associate professor of psychiatry at the University of Pittsburgh.
“Engaging in different activities at different times in activities associated with motivation and social interaction – this helps to ameliorate difficult-to-treat negative symptoms,” he said.
The findings were published online in Molecular Psychiatry.
Need to increase activity levels
While there is no shortage of research on sleep disturbances among people with schizophrenia, research focusing specifically on rest-activity rhythm disturbances and their relationships to symptoms of schizophrenia has been limited by small sample sizes or the lack of a control group, the investigators note.
To address this research gap, the investigators recruited 230 patients with SSD from participating residential facilities and communities throughout Italy. The participants included 108 healthy control participants, 54 community-dwelling patients with SSD who were receiving outpatient services, and 68 patients with SSD who were living in residential facilities.
All participants wore an actigraph for 7 consecutive days so that investigators could monitor sleep-wake patterns.
Compared with healthy control participants, both SSD groups had more total sleep time and spent more time resting or being passive (P < .001). In contrast, healthy control participants were much more active.
Part of the explanation for this may be that most of the control participants had jobs or attended school. In addition, the investigators note that many medications used to treat SSD can be highly sedating, causing some patients to sleep up to 15 hours per day.
Among residential participants with SSD, there was a higher level of inter-daily stability and higher daily rest-activity-rest fragmentation than occurred among healthy control participants or community-dwelling patients with SSD (P < .001). There was also a higher level of negative symptoms among residential participants with SSD than among the community-dwelling group with SSD.
When the findings were taken together, Dr. Ferrarelli and his team interpreted them to mean that inter-daily stability could reflect premature aging or neurodegenerative processes in patients with more severe forms of schizophrenia.
Another explanation could be that the rigid routine of the residential facility was making negative symptoms worse, Dr. Ferrarelli said. It is important to add variety into the mix – getting people to engage in different activities at different times of day would likely help residential SSD patients overcome negative symptoms of the disorder.
Although participants were recruited in Italy, Dr. Ferrarelli said he believes the findings are generalizable.
Bidirectional relationship?
Commenting on the findings, Matcheri Keshavan, MD, professor of psychiatry at Harvard Medical School in Boston, said the results are consistent with “well-known clinical observations that SSD patients tend to spend more time in bed and have more dysregulated sleep.
“Negative symptoms are also common, especially in residential patients. However, it is difficult to determine causality, as we do not know whether excessive sleepiness and decreased physical activity cause negative symptoms, or vice versa, or whether this is a bidirectional relationship,” Dr. Keshavan said.
He emphasized that physical exercise is known to increase sleep quality for people with mental illness and may also improve negative symptoms. “A useful approach in clinical practice is to increase activity levels, especially physical activities like walking and gardening.”
Dr. Keshavan said he would like to see future research that focuses on whether an intervention such as aerobic exercise would improve sleep quality as well as negative symptoms.
He also said that future research should ideally examine the characteristics of sleep alterations in schizophrenia.
“For example, while sleep duration is increased in schizophrenia, studies suggest that time spent in deep sleep is reduced; sleep spindles, which are important for consolidating memory during sleep, are also reduced. Correcting these deficits may improve negative symptoms and cognitive deficits,” he added.
The study was funded by the Italian Ministry of Health and the National Institute of Mental Health. There were no conflicts of interest.
A version of this article first appeared on Medscape.com.
The findings also showed that people with schizophrenia spectrum disorders (SSDs) who lived in residential facilities experienced rigid routines, which correlated with a higher degree of negative symptoms.
The rigid routines were problematic for the patients living in residential settings, lead investigator Fabio Ferrarelli, MD, PhD, told this news organization. Dr. Ferrarelli is an associate professor of psychiatry at the University of Pittsburgh.
“Engaging in different activities at different times in activities associated with motivation and social interaction – this helps to ameliorate difficult-to-treat negative symptoms,” he said.
The findings were published online in Molecular Psychiatry.
Need to increase activity levels
While there is no shortage of research on sleep disturbances among people with schizophrenia, research focusing specifically on rest-activity rhythm disturbances and their relationships to symptoms of schizophrenia has been limited by small sample sizes or the lack of a control group, the investigators note.
To address this research gap, the investigators recruited 230 patients with SSD from participating residential facilities and communities throughout Italy. The participants included 108 healthy control participants, 54 community-dwelling patients with SSD who were receiving outpatient services, and 68 patients with SSD who were living in residential facilities.
All participants wore an actigraph for 7 consecutive days so that investigators could monitor sleep-wake patterns.
Compared with healthy control participants, both SSD groups had more total sleep time and spent more time resting or being passive (P < .001). In contrast, healthy control participants were much more active.
Part of the explanation for this may be that most of the control participants had jobs or attended school. In addition, the investigators note that many medications used to treat SSD can be highly sedating, causing some patients to sleep up to 15 hours per day.
Among residential participants with SSD, there was a higher level of inter-daily stability and higher daily rest-activity-rest fragmentation than occurred among healthy control participants or community-dwelling patients with SSD (P < .001). There was also a higher level of negative symptoms among residential participants with SSD than among the community-dwelling group with SSD.
When the findings were taken together, Dr. Ferrarelli and his team interpreted them to mean that inter-daily stability could reflect premature aging or neurodegenerative processes in patients with more severe forms of schizophrenia.
Another explanation could be that the rigid routine of the residential facility was making negative symptoms worse, Dr. Ferrarelli said. It is important to add variety into the mix – getting people to engage in different activities at different times of day would likely help residential SSD patients overcome negative symptoms of the disorder.
Although participants were recruited in Italy, Dr. Ferrarelli said he believes the findings are generalizable.
Bidirectional relationship?
Commenting on the findings, Matcheri Keshavan, MD, professor of psychiatry at Harvard Medical School in Boston, said the results are consistent with “well-known clinical observations that SSD patients tend to spend more time in bed and have more dysregulated sleep.
“Negative symptoms are also common, especially in residential patients. However, it is difficult to determine causality, as we do not know whether excessive sleepiness and decreased physical activity cause negative symptoms, or vice versa, or whether this is a bidirectional relationship,” Dr. Keshavan said.
He emphasized that physical exercise is known to increase sleep quality for people with mental illness and may also improve negative symptoms. “A useful approach in clinical practice is to increase activity levels, especially physical activities like walking and gardening.”
Dr. Keshavan said he would like to see future research that focuses on whether an intervention such as aerobic exercise would improve sleep quality as well as negative symptoms.
He also said that future research should ideally examine the characteristics of sleep alterations in schizophrenia.
“For example, while sleep duration is increased in schizophrenia, studies suggest that time spent in deep sleep is reduced; sleep spindles, which are important for consolidating memory during sleep, are also reduced. Correcting these deficits may improve negative symptoms and cognitive deficits,” he added.
The study was funded by the Italian Ministry of Health and the National Institute of Mental Health. There were no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
Clozapine may curb schizophrenia’s ‘most dreaded outcome’
Investigators reviewed over 53,000 autopsy records, including over 600 from individuals whose autopsies revealed the presence of the antipsychotics clozapine or olanzapine, and found that those who took clozapine were significantly less likely to have died by suicide, compared with their counterparts who were taking olanzapine.
“Clozapine is an important and effective antisuicide medicine and should be strongly considered for treatment-resistant psychotic disorders, especially when the patient may be at risk for suicide,” study investigator Paul Nestadt, MD, associate professor, department of psychiatry and behavioral sciences, Johns Hopkins School of Medicine, Baltimore, told this news organization.
The study was published online in The Journal of Clinical Psychiatry.
Underutilized medication
Clozapine is the only medication indicated for treatment-resistant schizophrenia and is considered “the most efficacious antipsychotic,” the investigators note. Unfortunately, it has “long been underutilized” for several reasons, including prescriber hesitancy and concerns about side effects.
The authors note that its mechanism of action and the basis for superior efficacy are “still poorly understood” but “may extend beyond neurotransmitter receptor binding.”
Importantly, it may have a beneficial impact on domains other than positive symptoms of schizophrenia, including suicidality. Several studies have shown that it’s beneficial in this regard, but it is “unclear whether the unique antisuicidal properties of clozapine are related to better symptom control ... or to the closer monitoring and follow-up mandated for clozapine use,” they note.
A previous trial, the International Suicide Prevention Trial (InterSePT), demonstrated that clozapine is associated with a greater reduction in suicidality, and the findings “led to an FDA indication for clozapine in reducing the risk of recurrent suicidal behavior.”
However, the authors note, “in the severely ill populations in these studies, it is difficult to be certain about patients’ adherence to prescribed clozapine.”
“Other studies, such as InterSePT, have shown some evidence of clozapine working to reduce suicide-related outcomes, such as attempts or suicidal ideation, but few have been sufficiently powered to measure an effect on actual suicide deaths,” said Dr. Nestadt.
“As a suicidologist, I feel it is very important that we understand what treatments and interventions can actually prevent suicide deaths, as most suicides are not associated with past attempts or ideation, with suicide decedents usually looking very different from characteristic nonfatal attempters, from a clinical or epidemiological standpoint,” he added.
“If we could show that clozapine actually decreases the likelihood of suicide deaths in our patients, it gives us more reason to choose it over less effective neuroleptics in our clinics – especially for patients at high risk of suicide,” he said.
For the study, the researchers reviewed 19-year state-wide autopsy records of Maryland’s Office of the Chief Medical Examiner, which “performs uniquely comprehensive death investigations.” Data included in these investigations are full toxicologic panels with postmortem blood levels of antipsychotics.
The researchers compared decedents who tested positive for clozapine and decedents who tested positive for olanzapine. They evaluated demographics, clinical features, and manner-of-death outcomes.
‘Untapped resource’
Of 53,133 decedents, olanzapine or clozapine was detected in the blood of 621 persons (n = 571 and n = 50, respectively).
There were no significant differences in age, sex, race, or urban residence between the decedents who were treated with olanzapine and those who received clozapine.
The odds of a death by suicide in those treated with clozapine were less than half of the odds among decedents who had been treated with olanzapine (odds ratio, 0.47; 95% confidence interval, 0.26-0.84; P = .011).
In sensitivity analyses, the investigators reanalyzed the data to compare clozapine with other antipsychotics, including chlorpromazine, thioridazine, quetiapine, and olanzapine, and the results were similar. The odds of suicide (compared with accident) in those taking clozapine were much lower than in those taking any other tested antipsychotics individually or in combination (OR, 0.42; 95% CI, 0.24-0.73; P = .002).
Dr. Nestadt outlined several hypotheses regarding the mechanism of clozapine’s antisuicidal properties.
“Most theories stem from the differences in its receptor affinity, compared [with] the other neuroleptics,” he said. “In addition to the more typical dopaminergic blockade seen in neuroleptics, clozapine enhances serotonin release and greatly increases peripheral norepinephrine.”
This has been shown to “grant clozapine a greater antidepressant effect than other neuroleptics while also potentially decreasing aggression and impulsivity, which are both strongly associated with suicide risk,” he said.
Clozapine may also “work to reduce the inflammation-triggered activation of the kynurenine pathway, which otherwise contributes to serotonin depletion,” he added.
He noted that some studies have shown that as many as 1 in 10 patients with schizophrenia die by suicide, “so addressing this risk is paramount,” and that clozapine can play an important role in this.
The authors note that the findings “also highlight the utility of state-wide autopsy records, an untapped resource for investigating the potential protective effect of psychiatric medications on suicide at a population level.
“Importantly, we can be certain that this was not an issue of nonadherence to treatment in either group, which is a common issue in the use of these drugs because, instead of prescription records or self-report, we used actual measurements of drug presence in decedents’ blood at death,” said Dr. Nestadt.
‘Strongly suggestive’ data
Commenting on the study, Maria Oquendo, MD, PhD, Ruth Meltzer Professor and chair of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said most work on antisuicidal psychopharmacologic approaches “focuses on suicidal ideation or suicide attempts, due to the rarity of suicide death, even in high-risk populations.”
“Showing that clozapine may decrease risk for the most dreaded outcome of schizophrenia – suicide – is critically important,” said Dr. Oquendo, past president of the American Psychiatric Association.
Nevertheless, some questions remain, said Dr. Oquendo, who was not involved with the study. “Comparison of suicides to only accidental deaths has limitations. Many individuals who die due to accidents, like many suicides, are not similar to the general population,” she added.
However, she acknowledged, the data are strongly suggestive that clozapine protects against suicide.
“While not definitive, ideally these findings will stimulate changes in prescribing practices which may be lifesaving both literally – in terms of preventing suicides – and figuratively, given the drug’s effect on symptoms that impact quality of life and functioning,” said Dr. Oquendo.
The study received no funding or support. Dr. Nestadt is supported by the American Foundation for Suicide prevention and the National Institute on Drug Abuse. The other authors’ disclosures are listed in the original article. Dr. Oquendo receives royalties from the Research Foundation for Mental Hygiene for the commercial use of the Columbia Suicide Severity Rating Scale. She serves as an advisor to Alkermes, Mind Medicine, Sage Therapeutics, St. George’s University, and Fundacion Jimenez Diaz. Her family owns stock in Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Investigators reviewed over 53,000 autopsy records, including over 600 from individuals whose autopsies revealed the presence of the antipsychotics clozapine or olanzapine, and found that those who took clozapine were significantly less likely to have died by suicide, compared with their counterparts who were taking olanzapine.
“Clozapine is an important and effective antisuicide medicine and should be strongly considered for treatment-resistant psychotic disorders, especially when the patient may be at risk for suicide,” study investigator Paul Nestadt, MD, associate professor, department of psychiatry and behavioral sciences, Johns Hopkins School of Medicine, Baltimore, told this news organization.
The study was published online in The Journal of Clinical Psychiatry.
Underutilized medication
Clozapine is the only medication indicated for treatment-resistant schizophrenia and is considered “the most efficacious antipsychotic,” the investigators note. Unfortunately, it has “long been underutilized” for several reasons, including prescriber hesitancy and concerns about side effects.
The authors note that its mechanism of action and the basis for superior efficacy are “still poorly understood” but “may extend beyond neurotransmitter receptor binding.”
Importantly, it may have a beneficial impact on domains other than positive symptoms of schizophrenia, including suicidality. Several studies have shown that it’s beneficial in this regard, but it is “unclear whether the unique antisuicidal properties of clozapine are related to better symptom control ... or to the closer monitoring and follow-up mandated for clozapine use,” they note.
A previous trial, the International Suicide Prevention Trial (InterSePT), demonstrated that clozapine is associated with a greater reduction in suicidality, and the findings “led to an FDA indication for clozapine in reducing the risk of recurrent suicidal behavior.”
However, the authors note, “in the severely ill populations in these studies, it is difficult to be certain about patients’ adherence to prescribed clozapine.”
“Other studies, such as InterSePT, have shown some evidence of clozapine working to reduce suicide-related outcomes, such as attempts or suicidal ideation, but few have been sufficiently powered to measure an effect on actual suicide deaths,” said Dr. Nestadt.
“As a suicidologist, I feel it is very important that we understand what treatments and interventions can actually prevent suicide deaths, as most suicides are not associated with past attempts or ideation, with suicide decedents usually looking very different from characteristic nonfatal attempters, from a clinical or epidemiological standpoint,” he added.
“If we could show that clozapine actually decreases the likelihood of suicide deaths in our patients, it gives us more reason to choose it over less effective neuroleptics in our clinics – especially for patients at high risk of suicide,” he said.
For the study, the researchers reviewed 19-year state-wide autopsy records of Maryland’s Office of the Chief Medical Examiner, which “performs uniquely comprehensive death investigations.” Data included in these investigations are full toxicologic panels with postmortem blood levels of antipsychotics.
The researchers compared decedents who tested positive for clozapine and decedents who tested positive for olanzapine. They evaluated demographics, clinical features, and manner-of-death outcomes.
‘Untapped resource’
Of 53,133 decedents, olanzapine or clozapine was detected in the blood of 621 persons (n = 571 and n = 50, respectively).
There were no significant differences in age, sex, race, or urban residence between the decedents who were treated with olanzapine and those who received clozapine.
The odds of a death by suicide in those treated with clozapine were less than half of the odds among decedents who had been treated with olanzapine (odds ratio, 0.47; 95% confidence interval, 0.26-0.84; P = .011).
In sensitivity analyses, the investigators reanalyzed the data to compare clozapine with other antipsychotics, including chlorpromazine, thioridazine, quetiapine, and olanzapine, and the results were similar. The odds of suicide (compared with accident) in those taking clozapine were much lower than in those taking any other tested antipsychotics individually or in combination (OR, 0.42; 95% CI, 0.24-0.73; P = .002).
Dr. Nestadt outlined several hypotheses regarding the mechanism of clozapine’s antisuicidal properties.
“Most theories stem from the differences in its receptor affinity, compared [with] the other neuroleptics,” he said. “In addition to the more typical dopaminergic blockade seen in neuroleptics, clozapine enhances serotonin release and greatly increases peripheral norepinephrine.”
This has been shown to “grant clozapine a greater antidepressant effect than other neuroleptics while also potentially decreasing aggression and impulsivity, which are both strongly associated with suicide risk,” he said.
Clozapine may also “work to reduce the inflammation-triggered activation of the kynurenine pathway, which otherwise contributes to serotonin depletion,” he added.
He noted that some studies have shown that as many as 1 in 10 patients with schizophrenia die by suicide, “so addressing this risk is paramount,” and that clozapine can play an important role in this.
The authors note that the findings “also highlight the utility of state-wide autopsy records, an untapped resource for investigating the potential protective effect of psychiatric medications on suicide at a population level.
“Importantly, we can be certain that this was not an issue of nonadherence to treatment in either group, which is a common issue in the use of these drugs because, instead of prescription records or self-report, we used actual measurements of drug presence in decedents’ blood at death,” said Dr. Nestadt.
‘Strongly suggestive’ data
Commenting on the study, Maria Oquendo, MD, PhD, Ruth Meltzer Professor and chair of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said most work on antisuicidal psychopharmacologic approaches “focuses on suicidal ideation or suicide attempts, due to the rarity of suicide death, even in high-risk populations.”
“Showing that clozapine may decrease risk for the most dreaded outcome of schizophrenia – suicide – is critically important,” said Dr. Oquendo, past president of the American Psychiatric Association.
Nevertheless, some questions remain, said Dr. Oquendo, who was not involved with the study. “Comparison of suicides to only accidental deaths has limitations. Many individuals who die due to accidents, like many suicides, are not similar to the general population,” she added.
However, she acknowledged, the data are strongly suggestive that clozapine protects against suicide.
“While not definitive, ideally these findings will stimulate changes in prescribing practices which may be lifesaving both literally – in terms of preventing suicides – and figuratively, given the drug’s effect on symptoms that impact quality of life and functioning,” said Dr. Oquendo.
The study received no funding or support. Dr. Nestadt is supported by the American Foundation for Suicide prevention and the National Institute on Drug Abuse. The other authors’ disclosures are listed in the original article. Dr. Oquendo receives royalties from the Research Foundation for Mental Hygiene for the commercial use of the Columbia Suicide Severity Rating Scale. She serves as an advisor to Alkermes, Mind Medicine, Sage Therapeutics, St. George’s University, and Fundacion Jimenez Diaz. Her family owns stock in Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Investigators reviewed over 53,000 autopsy records, including over 600 from individuals whose autopsies revealed the presence of the antipsychotics clozapine or olanzapine, and found that those who took clozapine were significantly less likely to have died by suicide, compared with their counterparts who were taking olanzapine.
“Clozapine is an important and effective antisuicide medicine and should be strongly considered for treatment-resistant psychotic disorders, especially when the patient may be at risk for suicide,” study investigator Paul Nestadt, MD, associate professor, department of psychiatry and behavioral sciences, Johns Hopkins School of Medicine, Baltimore, told this news organization.
The study was published online in The Journal of Clinical Psychiatry.
Underutilized medication
Clozapine is the only medication indicated for treatment-resistant schizophrenia and is considered “the most efficacious antipsychotic,” the investigators note. Unfortunately, it has “long been underutilized” for several reasons, including prescriber hesitancy and concerns about side effects.
The authors note that its mechanism of action and the basis for superior efficacy are “still poorly understood” but “may extend beyond neurotransmitter receptor binding.”
Importantly, it may have a beneficial impact on domains other than positive symptoms of schizophrenia, including suicidality. Several studies have shown that it’s beneficial in this regard, but it is “unclear whether the unique antisuicidal properties of clozapine are related to better symptom control ... or to the closer monitoring and follow-up mandated for clozapine use,” they note.
A previous trial, the International Suicide Prevention Trial (InterSePT), demonstrated that clozapine is associated with a greater reduction in suicidality, and the findings “led to an FDA indication for clozapine in reducing the risk of recurrent suicidal behavior.”
However, the authors note, “in the severely ill populations in these studies, it is difficult to be certain about patients’ adherence to prescribed clozapine.”
“Other studies, such as InterSePT, have shown some evidence of clozapine working to reduce suicide-related outcomes, such as attempts or suicidal ideation, but few have been sufficiently powered to measure an effect on actual suicide deaths,” said Dr. Nestadt.
“As a suicidologist, I feel it is very important that we understand what treatments and interventions can actually prevent suicide deaths, as most suicides are not associated with past attempts or ideation, with suicide decedents usually looking very different from characteristic nonfatal attempters, from a clinical or epidemiological standpoint,” he added.
“If we could show that clozapine actually decreases the likelihood of suicide deaths in our patients, it gives us more reason to choose it over less effective neuroleptics in our clinics – especially for patients at high risk of suicide,” he said.
For the study, the researchers reviewed 19-year state-wide autopsy records of Maryland’s Office of the Chief Medical Examiner, which “performs uniquely comprehensive death investigations.” Data included in these investigations are full toxicologic panels with postmortem blood levels of antipsychotics.
The researchers compared decedents who tested positive for clozapine and decedents who tested positive for olanzapine. They evaluated demographics, clinical features, and manner-of-death outcomes.
‘Untapped resource’
Of 53,133 decedents, olanzapine or clozapine was detected in the blood of 621 persons (n = 571 and n = 50, respectively).
There were no significant differences in age, sex, race, or urban residence between the decedents who were treated with olanzapine and those who received clozapine.
The odds of a death by suicide in those treated with clozapine were less than half of the odds among decedents who had been treated with olanzapine (odds ratio, 0.47; 95% confidence interval, 0.26-0.84; P = .011).
In sensitivity analyses, the investigators reanalyzed the data to compare clozapine with other antipsychotics, including chlorpromazine, thioridazine, quetiapine, and olanzapine, and the results were similar. The odds of suicide (compared with accident) in those taking clozapine were much lower than in those taking any other tested antipsychotics individually or in combination (OR, 0.42; 95% CI, 0.24-0.73; P = .002).
Dr. Nestadt outlined several hypotheses regarding the mechanism of clozapine’s antisuicidal properties.
“Most theories stem from the differences in its receptor affinity, compared [with] the other neuroleptics,” he said. “In addition to the more typical dopaminergic blockade seen in neuroleptics, clozapine enhances serotonin release and greatly increases peripheral norepinephrine.”
This has been shown to “grant clozapine a greater antidepressant effect than other neuroleptics while also potentially decreasing aggression and impulsivity, which are both strongly associated with suicide risk,” he said.
Clozapine may also “work to reduce the inflammation-triggered activation of the kynurenine pathway, which otherwise contributes to serotonin depletion,” he added.
He noted that some studies have shown that as many as 1 in 10 patients with schizophrenia die by suicide, “so addressing this risk is paramount,” and that clozapine can play an important role in this.
The authors note that the findings “also highlight the utility of state-wide autopsy records, an untapped resource for investigating the potential protective effect of psychiatric medications on suicide at a population level.
“Importantly, we can be certain that this was not an issue of nonadherence to treatment in either group, which is a common issue in the use of these drugs because, instead of prescription records or self-report, we used actual measurements of drug presence in decedents’ blood at death,” said Dr. Nestadt.
‘Strongly suggestive’ data
Commenting on the study, Maria Oquendo, MD, PhD, Ruth Meltzer Professor and chair of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said most work on antisuicidal psychopharmacologic approaches “focuses on suicidal ideation or suicide attempts, due to the rarity of suicide death, even in high-risk populations.”
“Showing that clozapine may decrease risk for the most dreaded outcome of schizophrenia – suicide – is critically important,” said Dr. Oquendo, past president of the American Psychiatric Association.
Nevertheless, some questions remain, said Dr. Oquendo, who was not involved with the study. “Comparison of suicides to only accidental deaths has limitations. Many individuals who die due to accidents, like many suicides, are not similar to the general population,” she added.
However, she acknowledged, the data are strongly suggestive that clozapine protects against suicide.
“While not definitive, ideally these findings will stimulate changes in prescribing practices which may be lifesaving both literally – in terms of preventing suicides – and figuratively, given the drug’s effect on symptoms that impact quality of life and functioning,” said Dr. Oquendo.
The study received no funding or support. Dr. Nestadt is supported by the American Foundation for Suicide prevention and the National Institute on Drug Abuse. The other authors’ disclosures are listed in the original article. Dr. Oquendo receives royalties from the Research Foundation for Mental Hygiene for the commercial use of the Columbia Suicide Severity Rating Scale. She serves as an advisor to Alkermes, Mind Medicine, Sage Therapeutics, St. George’s University, and Fundacion Jimenez Diaz. Her family owns stock in Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
New schizophrenia genes identified
The genes were identified through a meta-analysis comparing gene sequences of 35,828 people with schizophrenia to 107,877 people without the condition.
The study builds on a report published last year that identified 10 genes with rare variants that are directly tied to schizophrenia risk. But that study, like most prior genetic analyses on psychiatric illnesses, was done on the DNA from people of European ancestry.
About 40% of the genetic samples included in this new work came from people of non-European ancestry, which researchers say makes it the most ethnically diverse schizophrenia genetics study to date.
Based on the findings, researchers concluded that the schizophrenia risk conferred by the rare genetic variants found on the new genes they discovered and on those previously identified is conserved across ethnicities.
The new genes, SRRM2 and AKAP11, contain rare protein-truncating variants (PTVs) that investigators say could be the cause of schizophrenia in some patients. The results could have significant implications for drug development.
“It’s not curing the illness, but it is taking us a step closer so that we’re able to say that this may be the cause of the illness in a particular patient,” senior investigator Alexander Charney, MD, PhD, associate professor of psychiatry, genetics and genomic sciences, neuroscience, and neurosurgery, at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The findings were published online in Nature Genetics.
Schizophrenia’s genetic architecture
Prior studies suggest the genetic architecture of schizophrenia may be influenced by common single-nucleotide polymorphisms, copy number variants and rare PTVs.
Investigators note that rare PTVs are important because they can link disease risk directly to individual genes. But identifying the PTVs and the genes that harbor them requires large patient cohorts, far bigger than any single institution can provide.
Dr. Charney and other researchers are part of the Psychiatric Genomics Consortium, a collaboration of researchers from hundreds of institutions around the world established in 2007 to create large cohorts for genetic studies of psychiatric disease.
For this study, investigators sequenced a new cohort of 11,580 schizophrenia cases and 10,555 controls of diverse ancestries. The analysis showed that the findings previously established in predominantly European cohorts extended to non-European populations.
They then conducted a meta-analysis of the new cohort combined with datasets from earlier studies, creating a pooled sample of 35,828 cases and 107,877 controls.
This meta-analysis revealed two new genes linked to schizophrenia, SRRM2 and AKAP11. The third gene flagged in the study, PCLO, was previously implicated in schizophrenia but is now identified as having a shared risk for schizophrenia and autism.
The rare PVTs on the 12 genes identified so far through this type of study are probably only involved in a small fraction of schizophrenia cases, Dr. Charney acknowledged. However, the discovery could lead to new treatments that could benefit all patients with the disease, he added.
“There are multiple pathways to psychosis and there’s also multiple pathways to treat psychosis,” Dr. Charney said. “There’s reason to believe if you can find a mechanism by which a human being could develop a psychosis, then reversing that mechanism could help a lot of people who have psychosis for another reason.”
Importance of diverse cohorts
Commenting on the findings, Jennifer Gladys Mulle, MHS, PhD, associate professor of psychiatry at the Robert Wood Johnson Medical School at Rutgers University, Piscataway, N.J., noted that while genetic discoveries have led to new therapies in other medical conditions, that has not been the case with schizophrenia.
“In other disorders, having genetic findings have really opened a window into the molecular mechanisms, which has allowed us to develop pharmaceuticals and understand the disease process better,” said Dr. Mulle, who was not part of this study. “But because we haven’t had that in schizophrenia, it’s really held us back. Having genetic variants associated with schizophrenia may really help us understand the mechanism.”
The inclusion of diverse populations is also a key contribution of this study, Dr. Mulle added.
“So far a lot of the work we’ve done in genetics has been on people of European ancestry,” Dr. Mulle said. “The fact that they have found results that are generalizable across multiple ethnicities really suggests that if we develop pharmaceutical agents based on these findings, it will help many people.”
More attention has been paid recently to a growing problem in the study of genetics of psychiatric disorders: More than 95% of participants in genome-wide association studies that seek to identify gene variants linked to disease are of European ancestry.
Dr. Charney and his colleagues had that in mind when they designed the study.
“We can’t get to a place where genetics is clinically useful if we don’t know the extent to which a particular observation that’s found in one population is also true for other populations,” Dr. Charney said.
The study was funded by the National Institutes of Health. Dr. Charney and Dr. Mulle report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The genes were identified through a meta-analysis comparing gene sequences of 35,828 people with schizophrenia to 107,877 people without the condition.
The study builds on a report published last year that identified 10 genes with rare variants that are directly tied to schizophrenia risk. But that study, like most prior genetic analyses on psychiatric illnesses, was done on the DNA from people of European ancestry.
About 40% of the genetic samples included in this new work came from people of non-European ancestry, which researchers say makes it the most ethnically diverse schizophrenia genetics study to date.
Based on the findings, researchers concluded that the schizophrenia risk conferred by the rare genetic variants found on the new genes they discovered and on those previously identified is conserved across ethnicities.
The new genes, SRRM2 and AKAP11, contain rare protein-truncating variants (PTVs) that investigators say could be the cause of schizophrenia in some patients. The results could have significant implications for drug development.
“It’s not curing the illness, but it is taking us a step closer so that we’re able to say that this may be the cause of the illness in a particular patient,” senior investigator Alexander Charney, MD, PhD, associate professor of psychiatry, genetics and genomic sciences, neuroscience, and neurosurgery, at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The findings were published online in Nature Genetics.
Schizophrenia’s genetic architecture
Prior studies suggest the genetic architecture of schizophrenia may be influenced by common single-nucleotide polymorphisms, copy number variants and rare PTVs.
Investigators note that rare PTVs are important because they can link disease risk directly to individual genes. But identifying the PTVs and the genes that harbor them requires large patient cohorts, far bigger than any single institution can provide.
Dr. Charney and other researchers are part of the Psychiatric Genomics Consortium, a collaboration of researchers from hundreds of institutions around the world established in 2007 to create large cohorts for genetic studies of psychiatric disease.
For this study, investigators sequenced a new cohort of 11,580 schizophrenia cases and 10,555 controls of diverse ancestries. The analysis showed that the findings previously established in predominantly European cohorts extended to non-European populations.
They then conducted a meta-analysis of the new cohort combined with datasets from earlier studies, creating a pooled sample of 35,828 cases and 107,877 controls.
This meta-analysis revealed two new genes linked to schizophrenia, SRRM2 and AKAP11. The third gene flagged in the study, PCLO, was previously implicated in schizophrenia but is now identified as having a shared risk for schizophrenia and autism.
The rare PVTs on the 12 genes identified so far through this type of study are probably only involved in a small fraction of schizophrenia cases, Dr. Charney acknowledged. However, the discovery could lead to new treatments that could benefit all patients with the disease, he added.
“There are multiple pathways to psychosis and there’s also multiple pathways to treat psychosis,” Dr. Charney said. “There’s reason to believe if you can find a mechanism by which a human being could develop a psychosis, then reversing that mechanism could help a lot of people who have psychosis for another reason.”
Importance of diverse cohorts
Commenting on the findings, Jennifer Gladys Mulle, MHS, PhD, associate professor of psychiatry at the Robert Wood Johnson Medical School at Rutgers University, Piscataway, N.J., noted that while genetic discoveries have led to new therapies in other medical conditions, that has not been the case with schizophrenia.
“In other disorders, having genetic findings have really opened a window into the molecular mechanisms, which has allowed us to develop pharmaceuticals and understand the disease process better,” said Dr. Mulle, who was not part of this study. “But because we haven’t had that in schizophrenia, it’s really held us back. Having genetic variants associated with schizophrenia may really help us understand the mechanism.”
The inclusion of diverse populations is also a key contribution of this study, Dr. Mulle added.
“So far a lot of the work we’ve done in genetics has been on people of European ancestry,” Dr. Mulle said. “The fact that they have found results that are generalizable across multiple ethnicities really suggests that if we develop pharmaceutical agents based on these findings, it will help many people.”
More attention has been paid recently to a growing problem in the study of genetics of psychiatric disorders: More than 95% of participants in genome-wide association studies that seek to identify gene variants linked to disease are of European ancestry.
Dr. Charney and his colleagues had that in mind when they designed the study.
“We can’t get to a place where genetics is clinically useful if we don’t know the extent to which a particular observation that’s found in one population is also true for other populations,” Dr. Charney said.
The study was funded by the National Institutes of Health. Dr. Charney and Dr. Mulle report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The genes were identified through a meta-analysis comparing gene sequences of 35,828 people with schizophrenia to 107,877 people without the condition.
The study builds on a report published last year that identified 10 genes with rare variants that are directly tied to schizophrenia risk. But that study, like most prior genetic analyses on psychiatric illnesses, was done on the DNA from people of European ancestry.
About 40% of the genetic samples included in this new work came from people of non-European ancestry, which researchers say makes it the most ethnically diverse schizophrenia genetics study to date.
Based on the findings, researchers concluded that the schizophrenia risk conferred by the rare genetic variants found on the new genes they discovered and on those previously identified is conserved across ethnicities.
The new genes, SRRM2 and AKAP11, contain rare protein-truncating variants (PTVs) that investigators say could be the cause of schizophrenia in some patients. The results could have significant implications for drug development.
“It’s not curing the illness, but it is taking us a step closer so that we’re able to say that this may be the cause of the illness in a particular patient,” senior investigator Alexander Charney, MD, PhD, associate professor of psychiatry, genetics and genomic sciences, neuroscience, and neurosurgery, at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
The findings were published online in Nature Genetics.
Schizophrenia’s genetic architecture
Prior studies suggest the genetic architecture of schizophrenia may be influenced by common single-nucleotide polymorphisms, copy number variants and rare PTVs.
Investigators note that rare PTVs are important because they can link disease risk directly to individual genes. But identifying the PTVs and the genes that harbor them requires large patient cohorts, far bigger than any single institution can provide.
Dr. Charney and other researchers are part of the Psychiatric Genomics Consortium, a collaboration of researchers from hundreds of institutions around the world established in 2007 to create large cohorts for genetic studies of psychiatric disease.
For this study, investigators sequenced a new cohort of 11,580 schizophrenia cases and 10,555 controls of diverse ancestries. The analysis showed that the findings previously established in predominantly European cohorts extended to non-European populations.
They then conducted a meta-analysis of the new cohort combined with datasets from earlier studies, creating a pooled sample of 35,828 cases and 107,877 controls.
This meta-analysis revealed two new genes linked to schizophrenia, SRRM2 and AKAP11. The third gene flagged in the study, PCLO, was previously implicated in schizophrenia but is now identified as having a shared risk for schizophrenia and autism.
The rare PVTs on the 12 genes identified so far through this type of study are probably only involved in a small fraction of schizophrenia cases, Dr. Charney acknowledged. However, the discovery could lead to new treatments that could benefit all patients with the disease, he added.
“There are multiple pathways to psychosis and there’s also multiple pathways to treat psychosis,” Dr. Charney said. “There’s reason to believe if you can find a mechanism by which a human being could develop a psychosis, then reversing that mechanism could help a lot of people who have psychosis for another reason.”
Importance of diverse cohorts
Commenting on the findings, Jennifer Gladys Mulle, MHS, PhD, associate professor of psychiatry at the Robert Wood Johnson Medical School at Rutgers University, Piscataway, N.J., noted that while genetic discoveries have led to new therapies in other medical conditions, that has not been the case with schizophrenia.
“In other disorders, having genetic findings have really opened a window into the molecular mechanisms, which has allowed us to develop pharmaceuticals and understand the disease process better,” said Dr. Mulle, who was not part of this study. “But because we haven’t had that in schizophrenia, it’s really held us back. Having genetic variants associated with schizophrenia may really help us understand the mechanism.”
The inclusion of diverse populations is also a key contribution of this study, Dr. Mulle added.
“So far a lot of the work we’ve done in genetics has been on people of European ancestry,” Dr. Mulle said. “The fact that they have found results that are generalizable across multiple ethnicities really suggests that if we develop pharmaceutical agents based on these findings, it will help many people.”
More attention has been paid recently to a growing problem in the study of genetics of psychiatric disorders: More than 95% of participants in genome-wide association studies that seek to identify gene variants linked to disease are of European ancestry.
Dr. Charney and his colleagues had that in mind when they designed the study.
“We can’t get to a place where genetics is clinically useful if we don’t know the extent to which a particular observation that’s found in one population is also true for other populations,” Dr. Charney said.
The study was funded by the National Institutes of Health. Dr. Charney and Dr. Mulle report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Add-on antipsychotic beats switching meds in older adults with resistant depression
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAGP 2023
Antipsychotic cuts Alzheimer’s-related agitation
results of a phase 3 study suggest.
“In this phase 3 trial of patients with agitation in Alzheimer’s dementia, treatment with brexpiprazole 2 or 3 mg/day resulted in statistically significantly greater improvements in agitation versus placebo on the primary and key secondary endpoints,” said study investigator George Grossberg, MD, professor and director of the division of geriatric psychiatry, department of psychiatry & behavioral neuroscience, Saint Louis University.
Dr. Grossberg presented the findings as part of the annual meeting of the American Association for Geriatric Psychiatry.
Agitation common, distressing
With two previous studies also showing efficacy of brexpiprazole in AD-related agitation, Dr. Grossberg speculated that brexpiprazole will become the first drug to be approved for agitation in AD.
Agitation is one of the most common AD symptoms and is arguably the most distressing for patients and caregivers alike, Dr. Grossberg noted.
The drug was approved by the Food and Drug Administration in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
To investigate the drug at effective doses for AD-related agitation, the researchers conducted a phase 3 multicenter trial that included 345 patients with AD who met criteria for agitation and aggression.
Study participants had a mean Mini-Mental State Examination (MMSE) score between 5 and 22 at screening and baseline and a mean Cohen-Mansfield Agitation Inventory (CMAI) total score of about 79. A score above 45 is considered clinically significant agitation. Use of AD medications were permitted.
Patients had a mean age of 74 years and were randomly assigned in a 2:1 ratio to receive treatment with brexpiprazole 2 mg (n = 75) or 3 mg (n = 153) per day, or placebo (n = 117).
The study’s primary endpoint was improvement as assessed by the CMAI. Over 12 weeks, participants in the brexpiprazole group experienced greater improvement in agitation, with a mean change of –22.6 with brexpiprazole vs. –17.3 with placebo (P = .0026).
Brexpiprazole was also associated with significantly greater improvement in the secondary outcome of change from baseline to week 12 in agitation severity, as assessed using the Clinical Global Impression-Severity of Illness (CGI-S) score (mean change, –1.20 with brexpiprazole vs. –0.93 with placebo; P = .0078).
Specifically, treatment with the drug resulted in improvements in three key subscales of agitation, including aggressive behavior, such as physically striking out (P < .01 vs. placebo); physically nonaggressive; and verbally agitated, such as screaming or cursing (both P < .05).
Treatment-emergent adverse events (TEAEs) associated with brexpiprazole vs. placebo included somnolence (3.5% vs. 0.9%), nasopharyngitis (3.1% vs. 1.7%), dizziness (2.7% vs. 1.7%), diarrhea (2.2% vs. 0.9%), urinary tract infection (2.2% vs. 0.9%), and asthenia (2.2% vs. 0.0%).
“Aside from headache, no other TEAEs had an incidence of more than 5% in the brexpiprazole (2 or 3 mg) group, or in either dose group,” Dr. Grossberg said. “Cognition also remained stable,” he added.
Boxed warnings
Adverse events commonly associated with brexpiprazole include weight change, extrapyramidal events, falls, cardiovascular events, and sedation. In the study, all occurred at an incidence of less than 2% in both study groups, he noted.
Compared with the antipsychotic aripiprazole, brexpiprazole is associated with lower weight gain and akathisia, or motor restlessness.
One death occurred in the brexpiprazole 3 mg group in a patient who had heart failure, pneumonia, and cachexia. At autopsy, it was found the patient had cerebral and coronary atherosclerosis. The death was considered to be unrelated to brexpiprazole, said Dr. Grossberg.
This finding is notable because a caveat is that brexpiprazole, like aripiprazole and other typical and atypical antipsychotics, carries an FDA boxed warning related to an increased risk for death in older patients when used for dementia-related psychosis.
Noting that a black box warning about mortality risk is not a minor issue, Dr. Grossberg added that the risks are relatively low, whereas the risks associated with agitation in dementia can be high.
“If it’s an emergency situation, you have to treat the patient because otherwise they may harm someone else, or harm the staff, or harm their loved ones or themselves, and in those cases, we want to treat the patient first, get them under control, and then we worry about the black box,” he said.
In addition, “the No. 1 reason for getting kicked out of a nursing home is agitation or severe behaviors in the context of a dementia or a major neurocognitive disorder that the facility cannot control,” Dr. Grossberg added.
In such cases, patients may wind up in an emergency department and may not be welcome back at the nursing home.
“There’s always a risk/benefit ratio, and I have that discussion with patients and their families, but I can tell you that I’ve never had a family ask me not to use a medication because of the black box warning, because they see how miserable and how out of control their loved one is and they’re miserable because they see the suffering and will ask that we do anything that we can to get this behavior under control,” Dr. Grossberg said.
Caution still warranted
Commenting on the study, Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry and the Bhatia Family Endowed Chair in Psychiatry at Creighton University, Omaha, Neb., underscored that, owing to the concerns behind the FDA warnings, “nonpharmacologic management is the cornerstone of treating agitation in Alzheimer’s dementia.”
He noted that the lack of an FDA-approved drug for agitation with AD is the result of “the overall benefits of any of the drug classes or drugs trialed to treat agitation in Alzheimer’s dementia vs. their adverse effect profile,” he said.
Therefore, he continued, “any medication or medication class should be used with caution among these individuals who often have polymorbidity.”
Dr. Tampi agreed that “the use of each drug for agitation in AD should be on a case-by-case basis with a clear and documented risk/benefit discussion with the patient and their families.”
“These medications should only be used for refractory symptoms or emergency situations where the agitation is not managed adequately with nonpharmacologic techniques and with a clear and documented risk/benefit discussion with patients and their families,” Dr. Tampi said.
The study was supported by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda. Dr. Tampi had no disclosures to report.
A version of this article first appeared on Medscape.com.
This article was updated 3/14/23.
results of a phase 3 study suggest.
“In this phase 3 trial of patients with agitation in Alzheimer’s dementia, treatment with brexpiprazole 2 or 3 mg/day resulted in statistically significantly greater improvements in agitation versus placebo on the primary and key secondary endpoints,” said study investigator George Grossberg, MD, professor and director of the division of geriatric psychiatry, department of psychiatry & behavioral neuroscience, Saint Louis University.
Dr. Grossberg presented the findings as part of the annual meeting of the American Association for Geriatric Psychiatry.
Agitation common, distressing
With two previous studies also showing efficacy of brexpiprazole in AD-related agitation, Dr. Grossberg speculated that brexpiprazole will become the first drug to be approved for agitation in AD.
Agitation is one of the most common AD symptoms and is arguably the most distressing for patients and caregivers alike, Dr. Grossberg noted.
The drug was approved by the Food and Drug Administration in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
To investigate the drug at effective doses for AD-related agitation, the researchers conducted a phase 3 multicenter trial that included 345 patients with AD who met criteria for agitation and aggression.
Study participants had a mean Mini-Mental State Examination (MMSE) score between 5 and 22 at screening and baseline and a mean Cohen-Mansfield Agitation Inventory (CMAI) total score of about 79. A score above 45 is considered clinically significant agitation. Use of AD medications were permitted.
Patients had a mean age of 74 years and were randomly assigned in a 2:1 ratio to receive treatment with brexpiprazole 2 mg (n = 75) or 3 mg (n = 153) per day, or placebo (n = 117).
The study’s primary endpoint was improvement as assessed by the CMAI. Over 12 weeks, participants in the brexpiprazole group experienced greater improvement in agitation, with a mean change of –22.6 with brexpiprazole vs. –17.3 with placebo (P = .0026).
Brexpiprazole was also associated with significantly greater improvement in the secondary outcome of change from baseline to week 12 in agitation severity, as assessed using the Clinical Global Impression-Severity of Illness (CGI-S) score (mean change, –1.20 with brexpiprazole vs. –0.93 with placebo; P = .0078).
Specifically, treatment with the drug resulted in improvements in three key subscales of agitation, including aggressive behavior, such as physically striking out (P < .01 vs. placebo); physically nonaggressive; and verbally agitated, such as screaming or cursing (both P < .05).
Treatment-emergent adverse events (TEAEs) associated with brexpiprazole vs. placebo included somnolence (3.5% vs. 0.9%), nasopharyngitis (3.1% vs. 1.7%), dizziness (2.7% vs. 1.7%), diarrhea (2.2% vs. 0.9%), urinary tract infection (2.2% vs. 0.9%), and asthenia (2.2% vs. 0.0%).
“Aside from headache, no other TEAEs had an incidence of more than 5% in the brexpiprazole (2 or 3 mg) group, or in either dose group,” Dr. Grossberg said. “Cognition also remained stable,” he added.
Boxed warnings
Adverse events commonly associated with brexpiprazole include weight change, extrapyramidal events, falls, cardiovascular events, and sedation. In the study, all occurred at an incidence of less than 2% in both study groups, he noted.
Compared with the antipsychotic aripiprazole, brexpiprazole is associated with lower weight gain and akathisia, or motor restlessness.
One death occurred in the brexpiprazole 3 mg group in a patient who had heart failure, pneumonia, and cachexia. At autopsy, it was found the patient had cerebral and coronary atherosclerosis. The death was considered to be unrelated to brexpiprazole, said Dr. Grossberg.
This finding is notable because a caveat is that brexpiprazole, like aripiprazole and other typical and atypical antipsychotics, carries an FDA boxed warning related to an increased risk for death in older patients when used for dementia-related psychosis.
Noting that a black box warning about mortality risk is not a minor issue, Dr. Grossberg added that the risks are relatively low, whereas the risks associated with agitation in dementia can be high.
“If it’s an emergency situation, you have to treat the patient because otherwise they may harm someone else, or harm the staff, or harm their loved ones or themselves, and in those cases, we want to treat the patient first, get them under control, and then we worry about the black box,” he said.
In addition, “the No. 1 reason for getting kicked out of a nursing home is agitation or severe behaviors in the context of a dementia or a major neurocognitive disorder that the facility cannot control,” Dr. Grossberg added.
In such cases, patients may wind up in an emergency department and may not be welcome back at the nursing home.
“There’s always a risk/benefit ratio, and I have that discussion with patients and their families, but I can tell you that I’ve never had a family ask me not to use a medication because of the black box warning, because they see how miserable and how out of control their loved one is and they’re miserable because they see the suffering and will ask that we do anything that we can to get this behavior under control,” Dr. Grossberg said.
Caution still warranted
Commenting on the study, Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry and the Bhatia Family Endowed Chair in Psychiatry at Creighton University, Omaha, Neb., underscored that, owing to the concerns behind the FDA warnings, “nonpharmacologic management is the cornerstone of treating agitation in Alzheimer’s dementia.”
He noted that the lack of an FDA-approved drug for agitation with AD is the result of “the overall benefits of any of the drug classes or drugs trialed to treat agitation in Alzheimer’s dementia vs. their adverse effect profile,” he said.
Therefore, he continued, “any medication or medication class should be used with caution among these individuals who often have polymorbidity.”
Dr. Tampi agreed that “the use of each drug for agitation in AD should be on a case-by-case basis with a clear and documented risk/benefit discussion with the patient and their families.”
“These medications should only be used for refractory symptoms or emergency situations where the agitation is not managed adequately with nonpharmacologic techniques and with a clear and documented risk/benefit discussion with patients and their families,” Dr. Tampi said.
The study was supported by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda. Dr. Tampi had no disclosures to report.
A version of this article first appeared on Medscape.com.
This article was updated 3/14/23.
results of a phase 3 study suggest.
“In this phase 3 trial of patients with agitation in Alzheimer’s dementia, treatment with brexpiprazole 2 or 3 mg/day resulted in statistically significantly greater improvements in agitation versus placebo on the primary and key secondary endpoints,” said study investigator George Grossberg, MD, professor and director of the division of geriatric psychiatry, department of psychiatry & behavioral neuroscience, Saint Louis University.
Dr. Grossberg presented the findings as part of the annual meeting of the American Association for Geriatric Psychiatry.
Agitation common, distressing
With two previous studies also showing efficacy of brexpiprazole in AD-related agitation, Dr. Grossberg speculated that brexpiprazole will become the first drug to be approved for agitation in AD.
Agitation is one of the most common AD symptoms and is arguably the most distressing for patients and caregivers alike, Dr. Grossberg noted.
The drug was approved by the Food and Drug Administration in 2015 as an adjunctive therapy to antidepressants for adults with major depressive disorder and for adults with schizophrenia.
To investigate the drug at effective doses for AD-related agitation, the researchers conducted a phase 3 multicenter trial that included 345 patients with AD who met criteria for agitation and aggression.
Study participants had a mean Mini-Mental State Examination (MMSE) score between 5 and 22 at screening and baseline and a mean Cohen-Mansfield Agitation Inventory (CMAI) total score of about 79. A score above 45 is considered clinically significant agitation. Use of AD medications were permitted.
Patients had a mean age of 74 years and were randomly assigned in a 2:1 ratio to receive treatment with brexpiprazole 2 mg (n = 75) or 3 mg (n = 153) per day, or placebo (n = 117).
The study’s primary endpoint was improvement as assessed by the CMAI. Over 12 weeks, participants in the brexpiprazole group experienced greater improvement in agitation, with a mean change of –22.6 with brexpiprazole vs. –17.3 with placebo (P = .0026).
Brexpiprazole was also associated with significantly greater improvement in the secondary outcome of change from baseline to week 12 in agitation severity, as assessed using the Clinical Global Impression-Severity of Illness (CGI-S) score (mean change, –1.20 with brexpiprazole vs. –0.93 with placebo; P = .0078).
Specifically, treatment with the drug resulted in improvements in three key subscales of agitation, including aggressive behavior, such as physically striking out (P < .01 vs. placebo); physically nonaggressive; and verbally agitated, such as screaming or cursing (both P < .05).
Treatment-emergent adverse events (TEAEs) associated with brexpiprazole vs. placebo included somnolence (3.5% vs. 0.9%), nasopharyngitis (3.1% vs. 1.7%), dizziness (2.7% vs. 1.7%), diarrhea (2.2% vs. 0.9%), urinary tract infection (2.2% vs. 0.9%), and asthenia (2.2% vs. 0.0%).
“Aside from headache, no other TEAEs had an incidence of more than 5% in the brexpiprazole (2 or 3 mg) group, or in either dose group,” Dr. Grossberg said. “Cognition also remained stable,” he added.
Boxed warnings
Adverse events commonly associated with brexpiprazole include weight change, extrapyramidal events, falls, cardiovascular events, and sedation. In the study, all occurred at an incidence of less than 2% in both study groups, he noted.
Compared with the antipsychotic aripiprazole, brexpiprazole is associated with lower weight gain and akathisia, or motor restlessness.
One death occurred in the brexpiprazole 3 mg group in a patient who had heart failure, pneumonia, and cachexia. At autopsy, it was found the patient had cerebral and coronary atherosclerosis. The death was considered to be unrelated to brexpiprazole, said Dr. Grossberg.
This finding is notable because a caveat is that brexpiprazole, like aripiprazole and other typical and atypical antipsychotics, carries an FDA boxed warning related to an increased risk for death in older patients when used for dementia-related psychosis.
Noting that a black box warning about mortality risk is not a minor issue, Dr. Grossberg added that the risks are relatively low, whereas the risks associated with agitation in dementia can be high.
“If it’s an emergency situation, you have to treat the patient because otherwise they may harm someone else, or harm the staff, or harm their loved ones or themselves, and in those cases, we want to treat the patient first, get them under control, and then we worry about the black box,” he said.
In addition, “the No. 1 reason for getting kicked out of a nursing home is agitation or severe behaviors in the context of a dementia or a major neurocognitive disorder that the facility cannot control,” Dr. Grossberg added.
In such cases, patients may wind up in an emergency department and may not be welcome back at the nursing home.
“There’s always a risk/benefit ratio, and I have that discussion with patients and their families, but I can tell you that I’ve never had a family ask me not to use a medication because of the black box warning, because they see how miserable and how out of control their loved one is and they’re miserable because they see the suffering and will ask that we do anything that we can to get this behavior under control,” Dr. Grossberg said.
Caution still warranted
Commenting on the study, Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry and the Bhatia Family Endowed Chair in Psychiatry at Creighton University, Omaha, Neb., underscored that, owing to the concerns behind the FDA warnings, “nonpharmacologic management is the cornerstone of treating agitation in Alzheimer’s dementia.”
He noted that the lack of an FDA-approved drug for agitation with AD is the result of “the overall benefits of any of the drug classes or drugs trialed to treat agitation in Alzheimer’s dementia vs. their adverse effect profile,” he said.
Therefore, he continued, “any medication or medication class should be used with caution among these individuals who often have polymorbidity.”
Dr. Tampi agreed that “the use of each drug for agitation in AD should be on a case-by-case basis with a clear and documented risk/benefit discussion with the patient and their families.”
“These medications should only be used for refractory symptoms or emergency situations where the agitation is not managed adequately with nonpharmacologic techniques and with a clear and documented risk/benefit discussion with patients and their families,” Dr. Tampi said.
The study was supported by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda. Dr. Tampi had no disclosures to report.
A version of this article first appeared on Medscape.com.
This article was updated 3/14/23.
AT AAGP 2023
Cognitive remediation training reduces aggression in schizophrenia
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
Aggressive behavior, including verbal or physical threats or violent acts, is at least four times more likely among individuals with schizophrenia, compared with the general population, wrote Anzalee Khan, PhD, of the Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y., and colleagues. Recent studies suggest that psychosocial treatments such as cognitive remediation training (CRT) or social cognition training (SCT) may be helpful, but the potential benefit of combining these strategies has not been explored, they said.
In a study published in Schizophrenia Research , the authors randomized 62 adults with a diagnosis of schizophrenia or schizoaffective disorder to 36 sessions of a combination treatment with cognitive remediation and social cognition; 68 were randomized to cognitive remediation and computer-based control treatment. Participants also had at least one confirmed assault in the past year, or scores of 5 or higher on the Life History of Aggression scale. Complete data were analyzed for 45 patients in the CRT/SRT group and 34 in the CRT control group.
The primary outcome was the measure of aggression using the Modified Overt Aggression Scale (OAS-M) in which higher scores indicate higher levels of aggression. Incidents of aggression were coded based on hospital staff reports and summarized weekly. The mean age of the participants was 34.9 years (ranging from 18 to 60 years), 85% were male, and the mean years of education was 11.5.
At the study’s end (14 weeks), participants in both groups showed significant reductions in measures of aggression from baseline, with the largest effect size for the total global OAS-M score (effect size 1.11 for CRT plus SCT and 0.73 for the CRT plus control group).
The results failed to confirm the hypothesis that the combination of CRT and SCT would significantly increase improvements in aggression compared with CRT alone, the researchers wrote in their discussion. Potential reasons include underdosed SCT intervention (only 12 sessions) and the nature of the SCT used in the study, which had few aggressive social interaction models and more models related to social engagement.
Although adding SCT did not have a significant impact on aggression, patients in the CRT plus SCT group showed greater improvement in cognitive function, emotion recognition, and mentalizing, compared with the controls without SCT, the researchers noted.
“While these findings are not surprising given that participants in the CRT plus SCT group received active social cognition training, they do support the idea that social cognition training may have contributed to further strengthen our effect on cognition,” they wrote.
The findings were limited by several factors including the study population of individuals with chronic schizophrenia and low levels of function in long-term tertiary care, which may limit generalizability, and the inability to control for the effects of pharmacotherapy, the researchers said.
However, the results were strengthened by the multidimensional assessments at both time points and the use of two cognitive and social cognition interventions, and suggest that adding social cognitive training enhanced the effect of CRT on cognitive function, emotion regulation, and mentalizing capacity, they said.
“Future studies are needed to examine the antiaggressive effects of a more intensive and more targeted social cognition intervention combined with CRT,” they concluded.
The study was supported by the Brain and Behavior Research Foundation and the Weill Cornell Clinical and Translational Science Award Program, National Institutes of Health/National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
New insight into preventing antipsychotic-induced weight gain
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.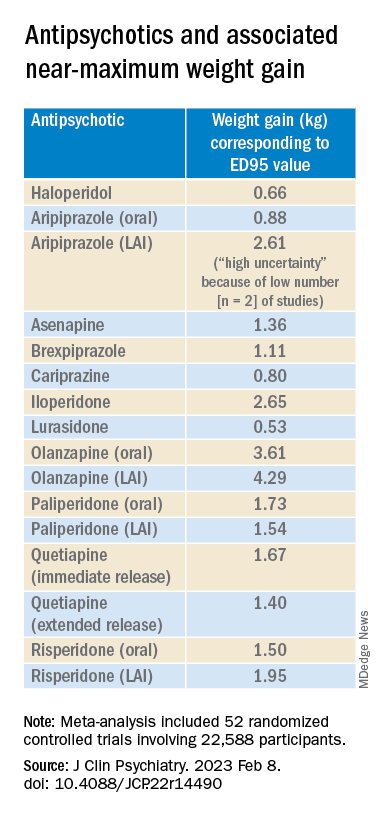
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Encephalitis linked to psychosis, suicidal thoughts
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROPSYCHIATRY AND CLINICAL NEUROSCIENCES













