User login
New SVS Task Force Explores Vascular Certification Program
The Society for Vascular Surgery (SVS) executive board has established a task force to explore developing a vascular certification program for inpatient and outpatient care settings.
Noting the shift in professional reimbursement from payment for volume to payment for quality, along with a surge in outpatient endovascular care, “The SVS executive board believes that it is a critical time for vascular surgery to set standards based on quality improvement, efficiency and appropriateness,” said Dr. R. Clement Darling III, SVS president.
Task force chair Dr. Tony Sidawy will oversee two subcommittees, one for inpatient and one for office-based endovascular care (OBEC). Dr. Krishna Jain has been appointed chair of the OBEC subcommittee. A chair for the inpatient subcommittee has yet to be named.
“Vascular surgeons represented by the SVS should take the lead in defining quality and value standards for vascular care before they are defined for us,” said Dr. Sidawy.
“Offering an SVS-led certification process will inspire the most appropriate, high-quality vascular care and optimal outcomes for all patients,” Dr. Jain added.
Many SVS members are pioneers in the design and delivery of care in office-based practice settings, and they have been fierce advocates for this effort, said Dr. Darling. “We have heard our members loud and clear. They want SVS to play a major role in shaping the future of the office-based endovascular center, setting the bar for appropriateness and quality and helping all practitioners achieve it.
“We feel that to provide the best vascular care in a data-driven, quality-based system, the SVS needs to be actively involved in this process," he added. "Vascular surgeons have a long history of making data-driven decisions about which patients need an intervention, and since we treat patients medically as well as by endovascular or open techniques, we have a unique perspective."
A data registry is a critical component and will be provided by the SVS Patient Safety Organization and Vascular Quality Initiative (SVS VQI). VQI registries are already used in more than 430 vascular care settings, ranging from academic to community practice. VQI data can be used to benchmark performance and improve the quality of vascular care.
“Given that the SVS VQI has already been adopted by all types of facilities, including OBECs and vein centers, the SVS VQI is well positioned to help assess and improve quality of care,” said Dr. Jens Eldrup-Jorgensen, SVS PSO medical director.
The process will include discussions and potential collaboration with partners such as the American College of Surgeons, the Outpatient Endovascular and Interventional Society and the Intersociety Accreditation Council, Dr. Darling said, as well as societies such as the American Venous Forum, the Society for Vascular Ultrasound, and the Society for Vascular Nursing.
If established, a pilot program would be launched in 2018 with a full launch planned in 2019.
The Society for Vascular Surgery (SVS) executive board has established a task force to explore developing a vascular certification program for inpatient and outpatient care settings.
Noting the shift in professional reimbursement from payment for volume to payment for quality, along with a surge in outpatient endovascular care, “The SVS executive board believes that it is a critical time for vascular surgery to set standards based on quality improvement, efficiency and appropriateness,” said Dr. R. Clement Darling III, SVS president.
Task force chair Dr. Tony Sidawy will oversee two subcommittees, one for inpatient and one for office-based endovascular care (OBEC). Dr. Krishna Jain has been appointed chair of the OBEC subcommittee. A chair for the inpatient subcommittee has yet to be named.
“Vascular surgeons represented by the SVS should take the lead in defining quality and value standards for vascular care before they are defined for us,” said Dr. Sidawy.
“Offering an SVS-led certification process will inspire the most appropriate, high-quality vascular care and optimal outcomes for all patients,” Dr. Jain added.
Many SVS members are pioneers in the design and delivery of care in office-based practice settings, and they have been fierce advocates for this effort, said Dr. Darling. “We have heard our members loud and clear. They want SVS to play a major role in shaping the future of the office-based endovascular center, setting the bar for appropriateness and quality and helping all practitioners achieve it.
“We feel that to provide the best vascular care in a data-driven, quality-based system, the SVS needs to be actively involved in this process," he added. "Vascular surgeons have a long history of making data-driven decisions about which patients need an intervention, and since we treat patients medically as well as by endovascular or open techniques, we have a unique perspective."
A data registry is a critical component and will be provided by the SVS Patient Safety Organization and Vascular Quality Initiative (SVS VQI). VQI registries are already used in more than 430 vascular care settings, ranging from academic to community practice. VQI data can be used to benchmark performance and improve the quality of vascular care.
“Given that the SVS VQI has already been adopted by all types of facilities, including OBECs and vein centers, the SVS VQI is well positioned to help assess and improve quality of care,” said Dr. Jens Eldrup-Jorgensen, SVS PSO medical director.
The process will include discussions and potential collaboration with partners such as the American College of Surgeons, the Outpatient Endovascular and Interventional Society and the Intersociety Accreditation Council, Dr. Darling said, as well as societies such as the American Venous Forum, the Society for Vascular Ultrasound, and the Society for Vascular Nursing.
If established, a pilot program would be launched in 2018 with a full launch planned in 2019.
The Society for Vascular Surgery (SVS) executive board has established a task force to explore developing a vascular certification program for inpatient and outpatient care settings.
Noting the shift in professional reimbursement from payment for volume to payment for quality, along with a surge in outpatient endovascular care, “The SVS executive board believes that it is a critical time for vascular surgery to set standards based on quality improvement, efficiency and appropriateness,” said Dr. R. Clement Darling III, SVS president.
Task force chair Dr. Tony Sidawy will oversee two subcommittees, one for inpatient and one for office-based endovascular care (OBEC). Dr. Krishna Jain has been appointed chair of the OBEC subcommittee. A chair for the inpatient subcommittee has yet to be named.
“Vascular surgeons represented by the SVS should take the lead in defining quality and value standards for vascular care before they are defined for us,” said Dr. Sidawy.
“Offering an SVS-led certification process will inspire the most appropriate, high-quality vascular care and optimal outcomes for all patients,” Dr. Jain added.
Many SVS members are pioneers in the design and delivery of care in office-based practice settings, and they have been fierce advocates for this effort, said Dr. Darling. “We have heard our members loud and clear. They want SVS to play a major role in shaping the future of the office-based endovascular center, setting the bar for appropriateness and quality and helping all practitioners achieve it.
“We feel that to provide the best vascular care in a data-driven, quality-based system, the SVS needs to be actively involved in this process," he added. "Vascular surgeons have a long history of making data-driven decisions about which patients need an intervention, and since we treat patients medically as well as by endovascular or open techniques, we have a unique perspective."
A data registry is a critical component and will be provided by the SVS Patient Safety Organization and Vascular Quality Initiative (SVS VQI). VQI registries are already used in more than 430 vascular care settings, ranging from academic to community practice. VQI data can be used to benchmark performance and improve the quality of vascular care.
“Given that the SVS VQI has already been adopted by all types of facilities, including OBECs and vein centers, the SVS VQI is well positioned to help assess and improve quality of care,” said Dr. Jens Eldrup-Jorgensen, SVS PSO medical director.
The process will include discussions and potential collaboration with partners such as the American College of Surgeons, the Outpatient Endovascular and Interventional Society and the Intersociety Accreditation Council, Dr. Darling said, as well as societies such as the American Venous Forum, the Society for Vascular Ultrasound, and the Society for Vascular Nursing.
If established, a pilot program would be launched in 2018 with a full launch planned in 2019.
VAM ’17 Will Be a ‘Spectacular Meeting’
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Support GI Research Through a Named Research Award
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research through a gift to the AGA Research Foundation? Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit.
Named award. An AGA pilot award can be renamed after you or a loved one, and targeted for a specific gastrointestinal research area. A new pilot research award can be established with a pledge of $40,000+ or through an estate gift. Gifts of cash or appreciated securities may be used to establish a named award.
Your next step. A named award gift is a wonderful way to acknowledge a loved one’s vision for the future. To learn more about ways to recognize your honoree, contact us at foundation@gastro.org.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research through a gift to the AGA Research Foundation? Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit.
Named award. An AGA pilot award can be renamed after you or a loved one, and targeted for a specific gastrointestinal research area. A new pilot research award can be established with a pledge of $40,000+ or through an estate gift. Gifts of cash or appreciated securities may be used to establish a named award.
Your next step. A named award gift is a wonderful way to acknowledge a loved one’s vision for the future. To learn more about ways to recognize your honoree, contact us at foundation@gastro.org.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research through a gift to the AGA Research Foundation? Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit.
Named award. An AGA pilot award can be renamed after you or a loved one, and targeted for a specific gastrointestinal research area. A new pilot research award can be established with a pledge of $40,000+ or through an estate gift. Gifts of cash or appreciated securities may be used to establish a named award.
Your next step. A named award gift is a wonderful way to acknowledge a loved one’s vision for the future. To learn more about ways to recognize your honoree, contact us at foundation@gastro.org.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
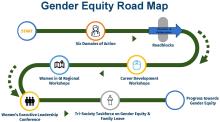
AGA Women’s Committee Outlines Roadmap Towards Gender Equity
Despite the increasing number of women joining the field, GI remains one of the most male-dominated medical subspecialties. and has highlighted future directions to achieve gender equality in GI.
The AGA Gender Equity Framework outlines six domains of action, the current state and desired future state: bias & gender disparities, leadership & career advancement, wellness & balance, retention & recruitment, mentorship & sponsorship, and recognition.
Based on the desired future state, the group created a roadmap towards gender equity with measurable tactics. Career development workshops, including the Women in GI regional workshops and Women’s Executive Leadership Conference, are both crucial tactics.
AGA outlined a few key areas for future gender equity efforts to focus on:
- Clearer GI-specific transparency guidelines regarding recruitment, salary, promotions, funding, and leadership.
- Pathway and research programs that help students from underrepresented backgrounds get involved and stay engaged in GI.
- Support networks (through GI societies, institutions, or other organizations) that help women connect, collaborate, and grow their careers.
The AGA Women’s Committee, along with other AGA committees, will continue to work to achieve the vision laid out in the AGA Gender Equity Framework and Gender Equity Road Map.
Despite the increasing number of women joining the field, GI remains one of the most male-dominated medical subspecialties. and has highlighted future directions to achieve gender equality in GI.
The AGA Gender Equity Framework outlines six domains of action, the current state and desired future state: bias & gender disparities, leadership & career advancement, wellness & balance, retention & recruitment, mentorship & sponsorship, and recognition.
Based on the desired future state, the group created a roadmap towards gender equity with measurable tactics. Career development workshops, including the Women in GI regional workshops and Women’s Executive Leadership Conference, are both crucial tactics.
AGA outlined a few key areas for future gender equity efforts to focus on:
- Clearer GI-specific transparency guidelines regarding recruitment, salary, promotions, funding, and leadership.
- Pathway and research programs that help students from underrepresented backgrounds get involved and stay engaged in GI.
- Support networks (through GI societies, institutions, or other organizations) that help women connect, collaborate, and grow their careers.
The AGA Women’s Committee, along with other AGA committees, will continue to work to achieve the vision laid out in the AGA Gender Equity Framework and Gender Equity Road Map.
Despite the increasing number of women joining the field, GI remains one of the most male-dominated medical subspecialties. and has highlighted future directions to achieve gender equality in GI.
The AGA Gender Equity Framework outlines six domains of action, the current state and desired future state: bias & gender disparities, leadership & career advancement, wellness & balance, retention & recruitment, mentorship & sponsorship, and recognition.
Based on the desired future state, the group created a roadmap towards gender equity with measurable tactics. Career development workshops, including the Women in GI regional workshops and Women’s Executive Leadership Conference, are both crucial tactics.
AGA outlined a few key areas for future gender equity efforts to focus on:
- Clearer GI-specific transparency guidelines regarding recruitment, salary, promotions, funding, and leadership.
- Pathway and research programs that help students from underrepresented backgrounds get involved and stay engaged in GI.
- Support networks (through GI societies, institutions, or other organizations) that help women connect, collaborate, and grow their careers.
The AGA Women’s Committee, along with other AGA committees, will continue to work to achieve the vision laid out in the AGA Gender Equity Framework and Gender Equity Road Map.
Help Sustain GI Research
Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Treatment options for digestive diseases begin with rigorous research, but the limited funding available for physician-scientists to conduct research puts the field at risk of losing talented investigators.
As an AGA member, you have the power to make a difference. By increasing the number of talented women and men doing state-of-the-art research, you can help improve care for all patients suffering from digestive diseases.
Your gift to the AGA Research Foundation will catalyze discovery and career growth for a promising researcher in gastroenterology and hepatology. Please help us fund the next generation of GI researchers by donating today at https://foundation.gastro.org.
Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Treatment options for digestive diseases begin with rigorous research, but the limited funding available for physician-scientists to conduct research puts the field at risk of losing talented investigators.
As an AGA member, you have the power to make a difference. By increasing the number of talented women and men doing state-of-the-art research, you can help improve care for all patients suffering from digestive diseases.
Your gift to the AGA Research Foundation will catalyze discovery and career growth for a promising researcher in gastroenterology and hepatology. Please help us fund the next generation of GI researchers by donating today at https://foundation.gastro.org.
Scientists are working hard to develop new treatments and therapies, and to discover cures to advance the field and better patient care. But they can’t do this without research funding.
A lack of funding can prevent talented individuals from pursuing a research career, thereby denying them the opportunity to conduct work that will ultimately benefit patients with critical needs.
Treatment options for digestive diseases begin with rigorous research, but the limited funding available for physician-scientists to conduct research puts the field at risk of losing talented investigators.
As an AGA member, you have the power to make a difference. By increasing the number of talented women and men doing state-of-the-art research, you can help improve care for all patients suffering from digestive diseases.
Your gift to the AGA Research Foundation will catalyze discovery and career growth for a promising researcher in gastroenterology and hepatology. Please help us fund the next generation of GI researchers by donating today at https://foundation.gastro.org.
Hepatic Encephalopathy: Improve Diagnosis, Management, and Care
.1 HE can be deceptively subtle or profoundly severe, presenting with a wide clinical spectrum – from mild cognitive slowing to life-threatening coma. Without clear disease biomarkers, HE remains a diagnosis of exclusion, making it critical for clinicians to remain vigilant, especially in patients with chronic liver disease (CLD).
The incidence of CLD is climbing, fueled by rising rates of alcohol-associated liver disease, metabolic dysfunction-associated steatotic liver disease (MASLD), and hepatitis C, which is often undiagnosed. For example:
- More than 2 million Americans had alcohol-associated cirrhosis as of 2017.2
- Currently, 38% of all adults and 7–14% of children and adolescents have MASLD. By 2040, the MASLD prevalence rate for adults is projected to increase to more than 55%.3
- The economic burden is staggering – from $1 billion4 in 2003 to over $7 billion5 in hospital costs for cirrhosis-related admissions today.
These figures aren’t just statistics – they represent a growing population of patients who are at risk of developing HE, sometimes without ever receiving a proper diagnosis or follow-up care.
Because HE mimics many other forms of neurological dysfunction – delirium, alcohol intoxication, diabetes-related confusion – it can be easy to miss or misdiagnose. But differentiating HE from other causes of altered mental status is critical, especially for patients who may ultimately require liver transplantation.6, 7
Moreover, patients frequently leave the hospital without adequate education or maintenance medication for episodic overt HE. Without coordinated follow-up between primary care, hepatology, and caregivers, these patients are at risk for recurrence.
To close these practice gaps, education is key. AGA’s course, “Missing the Mark: Hepatic Encephalopathy,” provides clinicians with up-to-date guidance on:
- The changing epidemiology of cirrhosis and undiagnosed cirrhosis for patients with liver disease.
- Assessment guidelines and best practices for HE diagnosis and management.
- How to develop transition-of-care plans with patients, caretakers, and specialty providers after an initial HE diagnosis.
Take the course today: https://tinyurl.com/3muwhmj5
Don’t wait until HE is an emergency. Equip yourself with the tools to recognize it earlier, treat it effectively, and coordinate better care.
References
1. Wolf, DC. Hepatic Encephalopathy. Medscape. 2020 May 1. Retrieved from: https://emedicine.medscape.com/article/186101-overview
2. Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease A review. JAMA. 2021 Jul. doi:10.1001/jama.2021.7683.
3. Younossi ZM, et al. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025 Feb. doi: 10.3350/cmh.2024.0431.
4. Vilstrup H, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug. doi: 10.1002/hep.27210.
5. Desai AP, et al. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019 Jul. doi: 10.14309/ctg.0000000000000062.
6. Serper M, et al. Hepatic encephalopathy predicts early post-transplant cognitive and functional impairment: The Livcog cohort study. Hepatol Commun. 2025 Apr. doi: 10.1097/HC9.0000000000000696.
7. Montagnese S, Bajaj JS. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs. 2019 Jan. doi: 10.1007/s40265-018-1019-y.
.1 HE can be deceptively subtle or profoundly severe, presenting with a wide clinical spectrum – from mild cognitive slowing to life-threatening coma. Without clear disease biomarkers, HE remains a diagnosis of exclusion, making it critical for clinicians to remain vigilant, especially in patients with chronic liver disease (CLD).
The incidence of CLD is climbing, fueled by rising rates of alcohol-associated liver disease, metabolic dysfunction-associated steatotic liver disease (MASLD), and hepatitis C, which is often undiagnosed. For example:
- More than 2 million Americans had alcohol-associated cirrhosis as of 2017.2
- Currently, 38% of all adults and 7–14% of children and adolescents have MASLD. By 2040, the MASLD prevalence rate for adults is projected to increase to more than 55%.3
- The economic burden is staggering – from $1 billion4 in 2003 to over $7 billion5 in hospital costs for cirrhosis-related admissions today.
These figures aren’t just statistics – they represent a growing population of patients who are at risk of developing HE, sometimes without ever receiving a proper diagnosis or follow-up care.
Because HE mimics many other forms of neurological dysfunction – delirium, alcohol intoxication, diabetes-related confusion – it can be easy to miss or misdiagnose. But differentiating HE from other causes of altered mental status is critical, especially for patients who may ultimately require liver transplantation.6, 7
Moreover, patients frequently leave the hospital without adequate education or maintenance medication for episodic overt HE. Without coordinated follow-up between primary care, hepatology, and caregivers, these patients are at risk for recurrence.
To close these practice gaps, education is key. AGA’s course, “Missing the Mark: Hepatic Encephalopathy,” provides clinicians with up-to-date guidance on:
- The changing epidemiology of cirrhosis and undiagnosed cirrhosis for patients with liver disease.
- Assessment guidelines and best practices for HE diagnosis and management.
- How to develop transition-of-care plans with patients, caretakers, and specialty providers after an initial HE diagnosis.
Take the course today: https://tinyurl.com/3muwhmj5
Don’t wait until HE is an emergency. Equip yourself with the tools to recognize it earlier, treat it effectively, and coordinate better care.
References
1. Wolf, DC. Hepatic Encephalopathy. Medscape. 2020 May 1. Retrieved from: https://emedicine.medscape.com/article/186101-overview
2. Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease A review. JAMA. 2021 Jul. doi:10.1001/jama.2021.7683.
3. Younossi ZM, et al. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025 Feb. doi: 10.3350/cmh.2024.0431.
4. Vilstrup H, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug. doi: 10.1002/hep.27210.
5. Desai AP, et al. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019 Jul. doi: 10.14309/ctg.0000000000000062.
6. Serper M, et al. Hepatic encephalopathy predicts early post-transplant cognitive and functional impairment: The Livcog cohort study. Hepatol Commun. 2025 Apr. doi: 10.1097/HC9.0000000000000696.
7. Montagnese S, Bajaj JS. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs. 2019 Jan. doi: 10.1007/s40265-018-1019-y.
.1 HE can be deceptively subtle or profoundly severe, presenting with a wide clinical spectrum – from mild cognitive slowing to life-threatening coma. Without clear disease biomarkers, HE remains a diagnosis of exclusion, making it critical for clinicians to remain vigilant, especially in patients with chronic liver disease (CLD).
The incidence of CLD is climbing, fueled by rising rates of alcohol-associated liver disease, metabolic dysfunction-associated steatotic liver disease (MASLD), and hepatitis C, which is often undiagnosed. For example:
- More than 2 million Americans had alcohol-associated cirrhosis as of 2017.2
- Currently, 38% of all adults and 7–14% of children and adolescents have MASLD. By 2040, the MASLD prevalence rate for adults is projected to increase to more than 55%.3
- The economic burden is staggering – from $1 billion4 in 2003 to over $7 billion5 in hospital costs for cirrhosis-related admissions today.
These figures aren’t just statistics – they represent a growing population of patients who are at risk of developing HE, sometimes without ever receiving a proper diagnosis or follow-up care.
Because HE mimics many other forms of neurological dysfunction – delirium, alcohol intoxication, diabetes-related confusion – it can be easy to miss or misdiagnose. But differentiating HE from other causes of altered mental status is critical, especially for patients who may ultimately require liver transplantation.6, 7
Moreover, patients frequently leave the hospital without adequate education or maintenance medication for episodic overt HE. Without coordinated follow-up between primary care, hepatology, and caregivers, these patients are at risk for recurrence.
To close these practice gaps, education is key. AGA’s course, “Missing the Mark: Hepatic Encephalopathy,” provides clinicians with up-to-date guidance on:
- The changing epidemiology of cirrhosis and undiagnosed cirrhosis for patients with liver disease.
- Assessment guidelines and best practices for HE diagnosis and management.
- How to develop transition-of-care plans with patients, caretakers, and specialty providers after an initial HE diagnosis.
Take the course today: https://tinyurl.com/3muwhmj5
Don’t wait until HE is an emergency. Equip yourself with the tools to recognize it earlier, treat it effectively, and coordinate better care.
References
1. Wolf, DC. Hepatic Encephalopathy. Medscape. 2020 May 1. Retrieved from: https://emedicine.medscape.com/article/186101-overview
2. Singal AK, Mathurin P. Diagnosis and treatment of alcohol-associated liver disease A review. JAMA. 2021 Jul. doi:10.1001/jama.2021.7683.
3. Younossi ZM, et al. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025 Feb. doi: 10.3350/cmh.2024.0431.
4. Vilstrup H, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014 Aug. doi: 10.1002/hep.27210.
5. Desai AP, et al. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clin Transl Gastroenterol. 2019 Jul. doi: 10.14309/ctg.0000000000000062.
6. Serper M, et al. Hepatic encephalopathy predicts early post-transplant cognitive and functional impairment: The Livcog cohort study. Hepatol Commun. 2025 Apr. doi: 10.1097/HC9.0000000000000696.
7. Montagnese S, Bajaj JS. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs. 2019 Jan. doi: 10.1007/s40265-018-1019-y.
Simple Ways to Create Your Legacy
Creating a legacy of giving is easier than you think. As the spring season begins, take some time to start creating your legacy while supporting the AGA Research Foundation.
Here are two ideas to help you get started.
1. Name the AGA Research Foundation as a beneficiary. This arrangement is one of the most tax-smart ways to support the AGA Research Foundation after your lifetime. When you leave retirement plan assets to us, we bypass any taxes and receive the full amount.
2. Include the AGA Research Foundation in your will or living trust. This gift can be made by including as little as one sentence in your will or living trust. Plus, your gift can be modified throughout your lifetime as circumstances change.
Want to learn more about including a gift to the AGA Research Foundation in your future plans? Visit our website at https://foundation.gastro.org/gift-planning/.
Creating a legacy of giving is easier than you think. As the spring season begins, take some time to start creating your legacy while supporting the AGA Research Foundation.
Here are two ideas to help you get started.
1. Name the AGA Research Foundation as a beneficiary. This arrangement is one of the most tax-smart ways to support the AGA Research Foundation after your lifetime. When you leave retirement plan assets to us, we bypass any taxes and receive the full amount.
2. Include the AGA Research Foundation in your will or living trust. This gift can be made by including as little as one sentence in your will or living trust. Plus, your gift can be modified throughout your lifetime as circumstances change.
Want to learn more about including a gift to the AGA Research Foundation in your future plans? Visit our website at https://foundation.gastro.org/gift-planning/.
Creating a legacy of giving is easier than you think. As the spring season begins, take some time to start creating your legacy while supporting the AGA Research Foundation.
Here are two ideas to help you get started.
1. Name the AGA Research Foundation as a beneficiary. This arrangement is one of the most tax-smart ways to support the AGA Research Foundation after your lifetime. When you leave retirement plan assets to us, we bypass any taxes and receive the full amount.
2. Include the AGA Research Foundation in your will or living trust. This gift can be made by including as little as one sentence in your will or living trust. Plus, your gift can be modified throughout your lifetime as circumstances change.
Want to learn more about including a gift to the AGA Research Foundation in your future plans? Visit our website at https://foundation.gastro.org/gift-planning/.
Five Reasons to Update Your Will
You have a will, so you can rest easy, right? Not necessarily. If your will is outdated, it can cause more harm than good.
Even though it can provide for some contingencies, an old will can’t cover every change that may have occurred since it was first drawn. Professionals advise that you review your will every few years and more often if situations such as the following five have occurred since you last updated your will.
1. Family Changes
If you’ve had any changes in your family situation, you will probably need to update your will. Events such as marriage, divorce, death, birth, adoption, or a falling out with a loved one may affect how your estate will be distributed, who should act as guardian for your dependents, and who should be named as executor of your estate.
2. Relocating to a New State
The laws among the states vary. Moving to a new state or purchasing property in another state can affect your estate plan and how property in that state will be taxed and distributed.
3. Tax Law Changes
Federal and state legislatures are continually tinkering with federal estate and state inheritance tax laws. An old will may fail to take advantage of strategies that will minimize estate taxes.
4. You Want to Support a Favorite Cause
If you have developed a connection to a cause, you may want to benefit a particular charity with a gift in your estate. Contact us for sample language you can share with your attorney to include a gift to us in your will.
5. Changes in Your Estate’s Value
When you made your will, your assets may have been relatively modest. Now the value may be larger and your will no longer reflects how you would like your estate divided.
You will help spark future discoveries in GI. Visit our website at https://gastro.planmylegacy.org or contact us at foundation@gastro.org.
You have a will, so you can rest easy, right? Not necessarily. If your will is outdated, it can cause more harm than good.
Even though it can provide for some contingencies, an old will can’t cover every change that may have occurred since it was first drawn. Professionals advise that you review your will every few years and more often if situations such as the following five have occurred since you last updated your will.
1. Family Changes
If you’ve had any changes in your family situation, you will probably need to update your will. Events such as marriage, divorce, death, birth, adoption, or a falling out with a loved one may affect how your estate will be distributed, who should act as guardian for your dependents, and who should be named as executor of your estate.
2. Relocating to a New State
The laws among the states vary. Moving to a new state or purchasing property in another state can affect your estate plan and how property in that state will be taxed and distributed.
3. Tax Law Changes
Federal and state legislatures are continually tinkering with federal estate and state inheritance tax laws. An old will may fail to take advantage of strategies that will minimize estate taxes.
4. You Want to Support a Favorite Cause
If you have developed a connection to a cause, you may want to benefit a particular charity with a gift in your estate. Contact us for sample language you can share with your attorney to include a gift to us in your will.
5. Changes in Your Estate’s Value
When you made your will, your assets may have been relatively modest. Now the value may be larger and your will no longer reflects how you would like your estate divided.
You will help spark future discoveries in GI. Visit our website at https://gastro.planmylegacy.org or contact us at foundation@gastro.org.
You have a will, so you can rest easy, right? Not necessarily. If your will is outdated, it can cause more harm than good.
Even though it can provide for some contingencies, an old will can’t cover every change that may have occurred since it was first drawn. Professionals advise that you review your will every few years and more often if situations such as the following five have occurred since you last updated your will.
1. Family Changes
If you’ve had any changes in your family situation, you will probably need to update your will. Events such as marriage, divorce, death, birth, adoption, or a falling out with a loved one may affect how your estate will be distributed, who should act as guardian for your dependents, and who should be named as executor of your estate.
2. Relocating to a New State
The laws among the states vary. Moving to a new state or purchasing property in another state can affect your estate plan and how property in that state will be taxed and distributed.
3. Tax Law Changes
Federal and state legislatures are continually tinkering with federal estate and state inheritance tax laws. An old will may fail to take advantage of strategies that will minimize estate taxes.
4. You Want to Support a Favorite Cause
If you have developed a connection to a cause, you may want to benefit a particular charity with a gift in your estate. Contact us for sample language you can share with your attorney to include a gift to us in your will.
5. Changes in Your Estate’s Value
When you made your will, your assets may have been relatively modest. Now the value may be larger and your will no longer reflects how you would like your estate divided.
You will help spark future discoveries in GI. Visit our website at https://gastro.planmylegacy.org or contact us at foundation@gastro.org.
Colorectal Cancer Awareness Month is Here!
Happy Colorectal Cancer (CRC) Awareness Month! Today, CRC is the third-most common cancer in men and women in the United States. But there’s good news: We know that screening saves lives. That’s why
We have a variety of resources for both physicians and patients to navigate the CRC screening process.
Clinical Guidance
AGA’s clinical guidelines and clinical practice updates provide evidence-based recommendations to guide your clinical practice decisions. Visit AGA’s new toolkit on CRC for the latest guidance on topics including colonoscopy follow-up, liquid biopsy, appropriate and tailored polypectomy, and more.
Patient Resources
AGA’s GI Patient Center can help your patients understand the need for CRC screening, colorectal cancer symptoms and risks, available screening tests, and the importance of preparing for a colonoscopy. Visit patient.gastro.org to access patient education materials.
Join the Conversation
We’ll be sharing resources and encouraging screenings on social media all month long. Join us as we remind everyone that 45 is the new 50.
Happy Colorectal Cancer (CRC) Awareness Month! Today, CRC is the third-most common cancer in men and women in the United States. But there’s good news: We know that screening saves lives. That’s why
We have a variety of resources for both physicians and patients to navigate the CRC screening process.
Clinical Guidance
AGA’s clinical guidelines and clinical practice updates provide evidence-based recommendations to guide your clinical practice decisions. Visit AGA’s new toolkit on CRC for the latest guidance on topics including colonoscopy follow-up, liquid biopsy, appropriate and tailored polypectomy, and more.
Patient Resources
AGA’s GI Patient Center can help your patients understand the need for CRC screening, colorectal cancer symptoms and risks, available screening tests, and the importance of preparing for a colonoscopy. Visit patient.gastro.org to access patient education materials.
Join the Conversation
We’ll be sharing resources and encouraging screenings on social media all month long. Join us as we remind everyone that 45 is the new 50.
Happy Colorectal Cancer (CRC) Awareness Month! Today, CRC is the third-most common cancer in men and women in the United States. But there’s good news: We know that screening saves lives. That’s why
We have a variety of resources for both physicians and patients to navigate the CRC screening process.
Clinical Guidance
AGA’s clinical guidelines and clinical practice updates provide evidence-based recommendations to guide your clinical practice decisions. Visit AGA’s new toolkit on CRC for the latest guidance on topics including colonoscopy follow-up, liquid biopsy, appropriate and tailored polypectomy, and more.
Patient Resources
AGA’s GI Patient Center can help your patients understand the need for CRC screening, colorectal cancer symptoms and risks, available screening tests, and the importance of preparing for a colonoscopy. Visit patient.gastro.org to access patient education materials.
Join the Conversation
We’ll be sharing resources and encouraging screenings on social media all month long. Join us as we remind everyone that 45 is the new 50.
AGA Research Foundation Memorial and Honorary Gifts: A Special Tribute
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research through a gift to the AGA Research Foundation?
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax. A cash gift of $5,000 or more qualifies for membership in the AGA Supporter Circle.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation in honor of your loved one. A gift in your will of $50,000 or more qualifies for membership in the AGA Legacy Society, which recognizes the foundation’s most generous individual donors.
- Named commentary section funds. You can support a commentary section in a specific AGA journal to honor or memorialize a loved one. This can be established with a gift of $100,000 over the course of 5 years or through an estate gift. The AGA Institute Publications Committee will work with you to provide name recognition for the commentary section in a specific AGA journal for 5 years. All content and editing will be conducted by the editorial board of the journal.
Your Next Step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.foundation.gastro.org.
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research through a gift to the AGA Research Foundation?
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax. A cash gift of $5,000 or more qualifies for membership in the AGA Supporter Circle.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation in honor of your loved one. A gift in your will of $50,000 or more qualifies for membership in the AGA Legacy Society, which recognizes the foundation’s most generous individual donors.
- Named commentary section funds. You can support a commentary section in a specific AGA journal to honor or memorialize a loved one. This can be established with a gift of $100,000 over the course of 5 years or through an estate gift. The AGA Institute Publications Committee will work with you to provide name recognition for the commentary section in a specific AGA journal for 5 years. All content and editing will be conducted by the editorial board of the journal.
Your Next Step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.foundation.gastro.org.
Did you know you can honor a family member, friend, or colleague whose life has been touched by GI research through a gift to the AGA Research Foundation?
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help fund the AGA Research Awards Program. Your gift will assist in furthering basic digestive disease research which can ultimately advance research into all digestive diseases. The financial benefits include an income tax deduction and possible elimination of capital gains tax. A cash gift of $5,000 or more qualifies for membership in the AGA Supporter Circle.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly a percentage of your estate will pass to the AGA Research Foundation in honor of your loved one. A gift in your will of $50,000 or more qualifies for membership in the AGA Legacy Society, which recognizes the foundation’s most generous individual donors.
- Named commentary section funds. You can support a commentary section in a specific AGA journal to honor or memorialize a loved one. This can be established with a gift of $100,000 over the course of 5 years or through an estate gift. The AGA Institute Publications Committee will work with you to provide name recognition for the commentary section in a specific AGA journal for 5 years. All content and editing will be conducted by the editorial board of the journal.
Your Next Step
An honorary gift is a wonderful way to acknowledge someone’s vision for the future. To learn more about ways to recognize your honoree, visit our website at www.foundation.gastro.org.