User login
Laparoscopic hysterectomy best in morbidly obese uterine cancer patients
BOSTON – Total laparoscopic hysterectomy had fewer complications and a shorter length of stay among morbidly obese women with uterine cancer, compared with an abdominal approach, according to findings presented at the annual Minimally Invasive Surgery Week.
“We wanted to look at current trends and see if we’re doing more the abdominal route or adopting more minimally invasive surgery, but also find out what obesity has to do with it,” Emad Mikhail, MD, of the University of South Florida in Tampa, said in an interview. “Morbidly obese patients are a really vulnerable group of patients; they have a lot of medical comorbidities, and when they need surgery they have an increased risk of having poor perioperative outcomes because of their BMI [body mass index].”
More than half of the cases were total laparoscopic hysterectomy (1,025), a third were total abdominal hysterectomy (672), about 12% were laparoscopic assisted vaginal hysterectomy (248), and less than 3% were total vaginal hysterectomy (57).
Comparing total abdominal and total laparoscopic hysterectomy – the two most commonly performed type of hysterectomy in the study – median operative times were notably higher in total laparoscopic hysterectomy: 171 minutes versus 150 minutes (P less than .05). But the laparoscopic approach had a shorter length of stay, a lower rate of hospital readmissions, and fewer surgical complications.
Within the total laparoscopic hysterectomy cohort, length of stay averaged 1 day. Dr. Mikhail reported that this cohort also had 14 blood transfusions, 13 superficial surgical site infections, 5 deep incisional surgical site infections, and 41 readmissions within 30 days, all of which were significantly lower than in the total abdominal hysterectomy cohort (P less than .05).
Mean BMI was similar in the two groups, with 34.0 in the total abdominal hysterectomy cohort and 33.7 in the total laparoscopic hysterectomy cohort.
“As the BMI goes up, more [minimally invasive surgery] is adopted, which is a great finding,” Dr. Mikhail said. “It actually serves the purpose of vulnerable patients. Instead of having an increase in perioperative morbidity, they should benefit more from a [minimally invasive surgery] approach, which this study is showing.”
Next, Dr. Mikhail said he wants to tease out what types of procedures subspecialists are performing among morbidly obese patients with uterine cancer. This type of data collection would be easier, he added, if minimally invasive gynecologic surgeons had their own taxonomy code.
“Without a code, we cannot pull data and see if being a fellowship-trained, minimally invasive surgeon has a benefit for patients,” he said. “Are those surgeons offering more minimally invasive techniques for morbidly obese patients?”
Dr. Mikhail reported having no relevant financial disclosures. The meeting was held by the Society of Laparoendoscopic Surgeons.
BOSTON – Total laparoscopic hysterectomy had fewer complications and a shorter length of stay among morbidly obese women with uterine cancer, compared with an abdominal approach, according to findings presented at the annual Minimally Invasive Surgery Week.
“We wanted to look at current trends and see if we’re doing more the abdominal route or adopting more minimally invasive surgery, but also find out what obesity has to do with it,” Emad Mikhail, MD, of the University of South Florida in Tampa, said in an interview. “Morbidly obese patients are a really vulnerable group of patients; they have a lot of medical comorbidities, and when they need surgery they have an increased risk of having poor perioperative outcomes because of their BMI [body mass index].”
More than half of the cases were total laparoscopic hysterectomy (1,025), a third were total abdominal hysterectomy (672), about 12% were laparoscopic assisted vaginal hysterectomy (248), and less than 3% were total vaginal hysterectomy (57).
Comparing total abdominal and total laparoscopic hysterectomy – the two most commonly performed type of hysterectomy in the study – median operative times were notably higher in total laparoscopic hysterectomy: 171 minutes versus 150 minutes (P less than .05). But the laparoscopic approach had a shorter length of stay, a lower rate of hospital readmissions, and fewer surgical complications.
Within the total laparoscopic hysterectomy cohort, length of stay averaged 1 day. Dr. Mikhail reported that this cohort also had 14 blood transfusions, 13 superficial surgical site infections, 5 deep incisional surgical site infections, and 41 readmissions within 30 days, all of which were significantly lower than in the total abdominal hysterectomy cohort (P less than .05).
Mean BMI was similar in the two groups, with 34.0 in the total abdominal hysterectomy cohort and 33.7 in the total laparoscopic hysterectomy cohort.
“As the BMI goes up, more [minimally invasive surgery] is adopted, which is a great finding,” Dr. Mikhail said. “It actually serves the purpose of vulnerable patients. Instead of having an increase in perioperative morbidity, they should benefit more from a [minimally invasive surgery] approach, which this study is showing.”
Next, Dr. Mikhail said he wants to tease out what types of procedures subspecialists are performing among morbidly obese patients with uterine cancer. This type of data collection would be easier, he added, if minimally invasive gynecologic surgeons had their own taxonomy code.
“Without a code, we cannot pull data and see if being a fellowship-trained, minimally invasive surgeon has a benefit for patients,” he said. “Are those surgeons offering more minimally invasive techniques for morbidly obese patients?”
Dr. Mikhail reported having no relevant financial disclosures. The meeting was held by the Society of Laparoendoscopic Surgeons.
BOSTON – Total laparoscopic hysterectomy had fewer complications and a shorter length of stay among morbidly obese women with uterine cancer, compared with an abdominal approach, according to findings presented at the annual Minimally Invasive Surgery Week.
“We wanted to look at current trends and see if we’re doing more the abdominal route or adopting more minimally invasive surgery, but also find out what obesity has to do with it,” Emad Mikhail, MD, of the University of South Florida in Tampa, said in an interview. “Morbidly obese patients are a really vulnerable group of patients; they have a lot of medical comorbidities, and when they need surgery they have an increased risk of having poor perioperative outcomes because of their BMI [body mass index].”
More than half of the cases were total laparoscopic hysterectomy (1,025), a third were total abdominal hysterectomy (672), about 12% were laparoscopic assisted vaginal hysterectomy (248), and less than 3% were total vaginal hysterectomy (57).
Comparing total abdominal and total laparoscopic hysterectomy – the two most commonly performed type of hysterectomy in the study – median operative times were notably higher in total laparoscopic hysterectomy: 171 minutes versus 150 minutes (P less than .05). But the laparoscopic approach had a shorter length of stay, a lower rate of hospital readmissions, and fewer surgical complications.
Within the total laparoscopic hysterectomy cohort, length of stay averaged 1 day. Dr. Mikhail reported that this cohort also had 14 blood transfusions, 13 superficial surgical site infections, 5 deep incisional surgical site infections, and 41 readmissions within 30 days, all of which were significantly lower than in the total abdominal hysterectomy cohort (P less than .05).
Mean BMI was similar in the two groups, with 34.0 in the total abdominal hysterectomy cohort and 33.7 in the total laparoscopic hysterectomy cohort.
“As the BMI goes up, more [minimally invasive surgery] is adopted, which is a great finding,” Dr. Mikhail said. “It actually serves the purpose of vulnerable patients. Instead of having an increase in perioperative morbidity, they should benefit more from a [minimally invasive surgery] approach, which this study is showing.”
Next, Dr. Mikhail said he wants to tease out what types of procedures subspecialists are performing among morbidly obese patients with uterine cancer. This type of data collection would be easier, he added, if minimally invasive gynecologic surgeons had their own taxonomy code.
“Without a code, we cannot pull data and see if being a fellowship-trained, minimally invasive surgeon has a benefit for patients,” he said. “Are those surgeons offering more minimally invasive techniques for morbidly obese patients?”
Dr. Mikhail reported having no relevant financial disclosures. The meeting was held by the Society of Laparoendoscopic Surgeons.
Key clinical point:
Major finding: Total laparoscopic hysterectomy had shorter length of stay, fewer transfusions, fewer surgical site infections, and fewer readmissions, compared with total abdominal hysterectomy (P less than .05).
Data source: Retrospective review of data on 2,002 morbidly obese patients with uterine cancer in the ACS-NSQIP database.
Disclosures: Dr. Mikhail reported having no relevant financial disclosures.
Absorbable suture performs well in sacrocolpopexy with mesh
DENVER – Using absorbable polydioxanone suture during laparoscopic sacrocolpopexy was associated with a mesh erosion rate of just 1.6%, according to a single-center, 1-year prospective study of 64 patients.
That is substantially less than typical erosion rates of about 5% when permanent suture is used, Danielle Taylor, DO, of Akron (Ohio ) General Medical Center said at Pelvic Floor Disorders Week sponsored by the American Urogynecologic Society.
The researchers observed no anatomic failures or suture extrusions, and patients reported significant postoperative improvements on several validated measures of quality of life.
“Larger samples and longer follow-up may be needed,” said Dr. Taylor. “But our study suggests that permanent, nondissolving suture material may not be necessary for sacrocolpopexy.”
Sacrocolpopexy with mesh usually involves using nonabsorbable suture to attach its anterior and posterior arms to the vaginal mucosa. Instead, Dr. Taylor and colleagues used 90-day delayed absorbable 2.0 V-Loc (Covidien) suture during laparoscopic sacrocolpopexy for patients with baseline Pelvic Organ Prolapse Quantification (POP-Q) scores of at least 2 and symptomatic uterovaginal prolapse.
Two permanent Gore-Tex sutures were also placed at the apex of the cervix in each of the 64 patients, said Dr. Taylor, a urogynecology fellow at the University of Massachusetts, Worcester, who worked on the study as a resident at the Cleveland Clinic Akron General, in Ohio. She and her colleagues rechecked patients at postoperative weeks 2 and 6, and at months 6 and 12. They lost two patients to follow-up, both after week 2.
At baseline, 37 patients (58%) were in stage II pelvic organ prolapse, 27% were in stage III, and 14% were in stage IV. At 6 months after surgery, 85% had no detectable prolapse, 8% had stage I, and 6% had stage II. At 1 year, 82% remained in pelvic organ prolapse stage 0 and the rest were in stage I or II. All stage II patients remained asymptomatic, Dr. Taylor said.
At baseline, the median value for POP-Q point C was -3 (range, –8 to +6). At 6 months and 1 year later, the median value had improved to –8, and patients ranged between –10 and –8.
Quality of life surveys of 54 patients reflected these outcomes, Dr. Taylor said. A year after surgery, average scores on the Pelvic Floor Distress Index (PFDI) dropped by 67 points, from 103 to 35 (P less than .0001). Likewise, average scores on the Pelvic Floor Impact Questionnaire (PFIQ) dropped by 29 points (P less than .0001), and scores on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) indicated a significant decrease in the effects of pelvic organ prolapse on sexual functioning (P = .008).
In addition to a single case of mesh erosion, one patient developed postoperative ileus and one experienced small bowel obstruction, both of which resolved, Dr. Taylor reported. The researchers aim to continue the study with longer follow-up intervals and detailed analyses of postoperative pain.
Dr. Taylor reported no funding sources and had no disclosures. One coauthor disclosed ties to Coloplast Corp.
DENVER – Using absorbable polydioxanone suture during laparoscopic sacrocolpopexy was associated with a mesh erosion rate of just 1.6%, according to a single-center, 1-year prospective study of 64 patients.
That is substantially less than typical erosion rates of about 5% when permanent suture is used, Danielle Taylor, DO, of Akron (Ohio ) General Medical Center said at Pelvic Floor Disorders Week sponsored by the American Urogynecologic Society.
The researchers observed no anatomic failures or suture extrusions, and patients reported significant postoperative improvements on several validated measures of quality of life.
“Larger samples and longer follow-up may be needed,” said Dr. Taylor. “But our study suggests that permanent, nondissolving suture material may not be necessary for sacrocolpopexy.”
Sacrocolpopexy with mesh usually involves using nonabsorbable suture to attach its anterior and posterior arms to the vaginal mucosa. Instead, Dr. Taylor and colleagues used 90-day delayed absorbable 2.0 V-Loc (Covidien) suture during laparoscopic sacrocolpopexy for patients with baseline Pelvic Organ Prolapse Quantification (POP-Q) scores of at least 2 and symptomatic uterovaginal prolapse.
Two permanent Gore-Tex sutures were also placed at the apex of the cervix in each of the 64 patients, said Dr. Taylor, a urogynecology fellow at the University of Massachusetts, Worcester, who worked on the study as a resident at the Cleveland Clinic Akron General, in Ohio. She and her colleagues rechecked patients at postoperative weeks 2 and 6, and at months 6 and 12. They lost two patients to follow-up, both after week 2.
At baseline, 37 patients (58%) were in stage II pelvic organ prolapse, 27% were in stage III, and 14% were in stage IV. At 6 months after surgery, 85% had no detectable prolapse, 8% had stage I, and 6% had stage II. At 1 year, 82% remained in pelvic organ prolapse stage 0 and the rest were in stage I or II. All stage II patients remained asymptomatic, Dr. Taylor said.
At baseline, the median value for POP-Q point C was -3 (range, –8 to +6). At 6 months and 1 year later, the median value had improved to –8, and patients ranged between –10 and –8.
Quality of life surveys of 54 patients reflected these outcomes, Dr. Taylor said. A year after surgery, average scores on the Pelvic Floor Distress Index (PFDI) dropped by 67 points, from 103 to 35 (P less than .0001). Likewise, average scores on the Pelvic Floor Impact Questionnaire (PFIQ) dropped by 29 points (P less than .0001), and scores on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) indicated a significant decrease in the effects of pelvic organ prolapse on sexual functioning (P = .008).
In addition to a single case of mesh erosion, one patient developed postoperative ileus and one experienced small bowel obstruction, both of which resolved, Dr. Taylor reported. The researchers aim to continue the study with longer follow-up intervals and detailed analyses of postoperative pain.
Dr. Taylor reported no funding sources and had no disclosures. One coauthor disclosed ties to Coloplast Corp.
DENVER – Using absorbable polydioxanone suture during laparoscopic sacrocolpopexy was associated with a mesh erosion rate of just 1.6%, according to a single-center, 1-year prospective study of 64 patients.
That is substantially less than typical erosion rates of about 5% when permanent suture is used, Danielle Taylor, DO, of Akron (Ohio ) General Medical Center said at Pelvic Floor Disorders Week sponsored by the American Urogynecologic Society.
The researchers observed no anatomic failures or suture extrusions, and patients reported significant postoperative improvements on several validated measures of quality of life.
“Larger samples and longer follow-up may be needed,” said Dr. Taylor. “But our study suggests that permanent, nondissolving suture material may not be necessary for sacrocolpopexy.”
Sacrocolpopexy with mesh usually involves using nonabsorbable suture to attach its anterior and posterior arms to the vaginal mucosa. Instead, Dr. Taylor and colleagues used 90-day delayed absorbable 2.0 V-Loc (Covidien) suture during laparoscopic sacrocolpopexy for patients with baseline Pelvic Organ Prolapse Quantification (POP-Q) scores of at least 2 and symptomatic uterovaginal prolapse.
Two permanent Gore-Tex sutures were also placed at the apex of the cervix in each of the 64 patients, said Dr. Taylor, a urogynecology fellow at the University of Massachusetts, Worcester, who worked on the study as a resident at the Cleveland Clinic Akron General, in Ohio. She and her colleagues rechecked patients at postoperative weeks 2 and 6, and at months 6 and 12. They lost two patients to follow-up, both after week 2.
At baseline, 37 patients (58%) were in stage II pelvic organ prolapse, 27% were in stage III, and 14% were in stage IV. At 6 months after surgery, 85% had no detectable prolapse, 8% had stage I, and 6% had stage II. At 1 year, 82% remained in pelvic organ prolapse stage 0 and the rest were in stage I or II. All stage II patients remained asymptomatic, Dr. Taylor said.
At baseline, the median value for POP-Q point C was -3 (range, –8 to +6). At 6 months and 1 year later, the median value had improved to –8, and patients ranged between –10 and –8.
Quality of life surveys of 54 patients reflected these outcomes, Dr. Taylor said. A year after surgery, average scores on the Pelvic Floor Distress Index (PFDI) dropped by 67 points, from 103 to 35 (P less than .0001). Likewise, average scores on the Pelvic Floor Impact Questionnaire (PFIQ) dropped by 29 points (P less than .0001), and scores on the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) indicated a significant decrease in the effects of pelvic organ prolapse on sexual functioning (P = .008).
In addition to a single case of mesh erosion, one patient developed postoperative ileus and one experienced small bowel obstruction, both of which resolved, Dr. Taylor reported. The researchers aim to continue the study with longer follow-up intervals and detailed analyses of postoperative pain.
Dr. Taylor reported no funding sources and had no disclosures. One coauthor disclosed ties to Coloplast Corp.
Key clinical point:
Major finding: When 90-day delayed absorbable polydioxanone suture was used, the mesh erosion rate was 1.6%. There were no anatomic failures or cases of suture extrusion.
Data source: A single-center prospective case series of 64 patients.
Disclosures: Dr. Taylor reported having no financial disclosures. One coauthor reported ties to Coloplast Corp.
Robotic surgery boasts fewer postoperative complications in radical hysterectomy
BOSTON – Robot-assisted radical hysterectomy is just as safe, or perhaps safer, than open surgery, according to a new study that examined perioperative and postoperative outcomes with long-term follow-ups for both types of procedures.
“Robotic surgery has been expanding for the last 20 years, but still the recurrence rate with cancer patients is missing data because very few studies are published; they don’t have long-term oncologic outcomes, and if [the technology] works properly we have to put it into the literature,” M. Bilal Sert, MD, of Oslo University, said at the annual Minimally Invasive Surgery Week.
Dr. Sert and his coinvestigators identified 215 women who underwent either open or robot-assisted radical hysterectomy between November 2005 and December 2012. All of the procedures were elective and the robot-assisted operations were performed using the da Vinci robotic surgical platform. After excluding neoadjuvant cases, which totaled 19, the researchers looked at data on 196 patients (122 open radical hysterectomy cases and 74 robot-assisted radical hysterectomy cases).
On average, operating time for open radical hysterectomy was 171 minutes, versus 263 minutes for robot-assisted radical hysterectomy. However, the robotic surgery arm had lower mean estimated blood loss than the open surgery cohort: 80 milliliters versus 468 milliliters, respectively (P = .003). Follow-up time frames were shorter in the robotic surgery cohort by 6 months: 46 months reported for robotic surgery, compared with a 52-month average experienced by those in the open surgery cohort.
Both groups experienced recurrences, including 12 patients in the open surgery cohort (9.8%) and 9 patients in the robotic surgery cohort (12.1%) (P = .3), indicating a statistically insignificant difference. Similarly, rates of perioperative complications were 8% for open surgery and 11% for robotic surgery (P = .3), which was not significantly different.
However, rates of postoperative complications were 36% for open surgery and 12% for robotic surgery (P = .001), which was statistically significant.
“Based on our data, I can say that [robot-assisted radical hysterectomy] is safe, and in fact I prefer to use the robot,” Dr. Sert said at the meeting, which was held by the Society of Laparoendoscopic Surgeons. “Of course, robot-assisted surgery will not automatically make you a better surgeon, but on more complicated radical hysterectomy patients, it will help make the surgeon more precise.”
No funding source was disclosed for this study. Dr. Sert reported having no relevant financial disclosures.
BOSTON – Robot-assisted radical hysterectomy is just as safe, or perhaps safer, than open surgery, according to a new study that examined perioperative and postoperative outcomes with long-term follow-ups for both types of procedures.
“Robotic surgery has been expanding for the last 20 years, but still the recurrence rate with cancer patients is missing data because very few studies are published; they don’t have long-term oncologic outcomes, and if [the technology] works properly we have to put it into the literature,” M. Bilal Sert, MD, of Oslo University, said at the annual Minimally Invasive Surgery Week.
Dr. Sert and his coinvestigators identified 215 women who underwent either open or robot-assisted radical hysterectomy between November 2005 and December 2012. All of the procedures were elective and the robot-assisted operations were performed using the da Vinci robotic surgical platform. After excluding neoadjuvant cases, which totaled 19, the researchers looked at data on 196 patients (122 open radical hysterectomy cases and 74 robot-assisted radical hysterectomy cases).
On average, operating time for open radical hysterectomy was 171 minutes, versus 263 minutes for robot-assisted radical hysterectomy. However, the robotic surgery arm had lower mean estimated blood loss than the open surgery cohort: 80 milliliters versus 468 milliliters, respectively (P = .003). Follow-up time frames were shorter in the robotic surgery cohort by 6 months: 46 months reported for robotic surgery, compared with a 52-month average experienced by those in the open surgery cohort.
Both groups experienced recurrences, including 12 patients in the open surgery cohort (9.8%) and 9 patients in the robotic surgery cohort (12.1%) (P = .3), indicating a statistically insignificant difference. Similarly, rates of perioperative complications were 8% for open surgery and 11% for robotic surgery (P = .3), which was not significantly different.
However, rates of postoperative complications were 36% for open surgery and 12% for robotic surgery (P = .001), which was statistically significant.
“Based on our data, I can say that [robot-assisted radical hysterectomy] is safe, and in fact I prefer to use the robot,” Dr. Sert said at the meeting, which was held by the Society of Laparoendoscopic Surgeons. “Of course, robot-assisted surgery will not automatically make you a better surgeon, but on more complicated radical hysterectomy patients, it will help make the surgeon more precise.”
No funding source was disclosed for this study. Dr. Sert reported having no relevant financial disclosures.
BOSTON – Robot-assisted radical hysterectomy is just as safe, or perhaps safer, than open surgery, according to a new study that examined perioperative and postoperative outcomes with long-term follow-ups for both types of procedures.
“Robotic surgery has been expanding for the last 20 years, but still the recurrence rate with cancer patients is missing data because very few studies are published; they don’t have long-term oncologic outcomes, and if [the technology] works properly we have to put it into the literature,” M. Bilal Sert, MD, of Oslo University, said at the annual Minimally Invasive Surgery Week.
Dr. Sert and his coinvestigators identified 215 women who underwent either open or robot-assisted radical hysterectomy between November 2005 and December 2012. All of the procedures were elective and the robot-assisted operations were performed using the da Vinci robotic surgical platform. After excluding neoadjuvant cases, which totaled 19, the researchers looked at data on 196 patients (122 open radical hysterectomy cases and 74 robot-assisted radical hysterectomy cases).
On average, operating time for open radical hysterectomy was 171 minutes, versus 263 minutes for robot-assisted radical hysterectomy. However, the robotic surgery arm had lower mean estimated blood loss than the open surgery cohort: 80 milliliters versus 468 milliliters, respectively (P = .003). Follow-up time frames were shorter in the robotic surgery cohort by 6 months: 46 months reported for robotic surgery, compared with a 52-month average experienced by those in the open surgery cohort.
Both groups experienced recurrences, including 12 patients in the open surgery cohort (9.8%) and 9 patients in the robotic surgery cohort (12.1%) (P = .3), indicating a statistically insignificant difference. Similarly, rates of perioperative complications were 8% for open surgery and 11% for robotic surgery (P = .3), which was not significantly different.
However, rates of postoperative complications were 36% for open surgery and 12% for robotic surgery (P = .001), which was statistically significant.
“Based on our data, I can say that [robot-assisted radical hysterectomy] is safe, and in fact I prefer to use the robot,” Dr. Sert said at the meeting, which was held by the Society of Laparoendoscopic Surgeons. “Of course, robot-assisted surgery will not automatically make you a better surgeon, but on more complicated radical hysterectomy patients, it will help make the surgeon more precise.”
No funding source was disclosed for this study. Dr. Sert reported having no relevant financial disclosures.
Key clinical point:
Major finding: Postoperative complications were 36% for patients who underwent open radical hysterectomy, compared with 12% for those undergoing robot-assisted radical hysterectomy (P = .001).
Data source: Retrospective review of data on 215 patients who underwent open or robot-assisted radical hysterectomy between November 2005 and December 2012.
Disclosures: Dr. Sert reported having no relevant financial disclosures.
Modern breast surgery: What you should know
In a striking trend, the rate of contralateral prophylactic mastectomy (CPM) has risen by 30% over the last 10 years in the United States.1 Many women undergo CPM because of the fear and anxiety of cancer recurrence and their perceived risk of contralateral breast cancer; however, few women have a medical condition that necessitates removal of the contralateral breast. The medical indications for CPM include having a pathogenic genetic mutation (eg, BRCA1 and BRCA2), a strong family history of breast cancer, or prior mediastina chest radiation.
The actual risk of contralateral breast cancer is much lower than perceived. In women without a genetic mutation, the 10-year risk of contralateral breast cancer is only 3% to 5%.1 Also, CPM does not prevent the development of metastatic disease and offers no survival benefit over breast conservation or unilateral mastectomy.2 Furthermore, compared with unilateral therapeutic mastectomy, the “upgrade” to a CPM carries a 2.7-fold risk of a major surgical complication.3 It is therefore important that patients receive appropriate counseling regarding CPM, and that this counseling include cancer stage at diagnosis, family history and genetic risk, and cancer versus surgical risk (see “Counseling patients on contralateral prophylactic mastectomy” for key points to cover in patient discussions).
Counseling patients on contralateral prophylactic mastectomy
Commonly, patients diagnosed with breast cancer consider having their contralateral healthy breast removed as part of a bilateral mastectomy. They often experience severe anxiety about the cancer coming back and believe that removing both breasts will enable them to live longer. Keep the following key facts in mind when discussing treatment options with breast cancer patients.
Cancer stage at diagnosis. How long a patient lives from the time of her breast cancer diagnosis depends on the stage of the cancer at diagnosis, not the type of surgery performed. A woman with early stage I or stage II breast cancer has an 80% to 90% chance of being cancer free in 5 years.1 The chance of cancer recurring in the bones, liver, or lungs (metastatic breast cancer) will not be changed by removing the healthy breast. The risk of metastatic recurrence can be reduced, however, with chemotherapy and/or with hormone-blocker therapy.
Family history and genetic risk. Few women have a strong family history of breast and/or ovarian and other cancers, and this issue should be addressed with genetic counseling and testing prior to surgery. Those who carry a cancer-causing gene, such as BRCA1 or BRCA2, are at increased risk (40% to 60%) for a second or third breast cancer, especially if they are diagnosed at a young age (<50 years).2,3 In women who have a genetic mutation, removing both breasts and sometimes the ovaries can prevent development of another breast cancer. But this will not prevent spread of the cancer that is already present. Only chemotherapy and hormone blockers can prevent the spread of cancer.
Cancer risk versus surgical risk. For women with no family history of breast cancer, no genetic mutation, and no prior chest wall radiation, the risk of developing a new breast cancer in their other breast is only 3% to 5% every 10 years.3,4 This means that they have a 95% chance of not developing a new breast cancer in their healthy breast. Notably, removing the healthy breast can double the risk of postsurgical complications, including bleeding, infection, and loss of tissue and implant. The mastectomy site will be numb and the skin and nipple areola will not have any function other than cosmetic. Finally, wound complications from surgery could delay the start of important cancer treatment, such as chemotherapy or radiation.
The bottom line. Unless a woman has a strong family history of breast cancer, is diagnosed at a very young age, or has a genetic cancer-causing mutation, removing the contralateral healthy breast is not medically necessary and is not routinely recommended.
References
- Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5992.
- Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute. NIH Publ. No. 05-5302. 2006:181–205. http://seer.cancer.gov/archive/publications/mpmono. Accessed September 18, 2016.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
Women should be made aware that there are alternatives to mastectomy that have similar, or even better, outcomes with improved quality of life. Furthermore, a multi‑disciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care. Knowledge of this team approach and of modern breast cancer treatments is essential for general ObGyns as this understanding improves the overall care and guidance—specifically regarding referral to expert, high-volume breast surgeons—provided to those women most in need.
Expanded treatment options for breast cancer
Advancements in breast surgery, better imaging, and targeted therapies are changing the paradigm of breast cancer treatment.
Image-guided biopsy is key in decision making
When an abnormality is found in the breast, surgical excision of an undiagnosed breast lesion is no longer considered an appropriate first step. Use of image-guided biopsy or minimally invasive core needle biopsy allows for accurate diagnosis of a breast lesion while avoiding a potentially breast deforming and expensive surgical operation. It is always better to go into the operating room (OR) with a diagnosis and do the right operation the first time.
A core needle biopsy, results of which demonstrate a benign lesion, helps avoid breast surgery in women who do not need it. If cancer is diagnosed on biopsy, the extent of disease can be better evaluated and decision making can be more informed, with a multidisciplinary approach used to consider the various options, including genetic counseling, plastic surgery consultation, or neoadjuvant therapy. Some lesions, such as those too close to the skin, chest wall, or an implant, may not be amenable to core needle biopsy and therefore require surgical excision for diagnosis.
Benefits of a multidisciplinary tumor conference
It is important for a multidisciplinary group of cancer specialists to review a patient’s case and discuss the ideal treatment plan prior to surgery. Some breast cancer subtypes (such as human epidermal growth factor receptor 2 [HER2]–overamplified breast cancer and many triple-negative breast cancers) are very sensitive to chemotherapy, and patients with these tumor types may benefit from receiving neoadjuvant chemotherapy prior to surgery. New types of chemotherapy may allow up to 60% of some breast cancers to diminish almost completely, with subsequent improved cosmetic results of breast surgery.4 It may also allow time for genetic counseling and testing prior to surgery. (See “How to code for a multidisciplinary tumor conference” for appropriate coding procedure.)
How to code for a multidisciplinary tumor conference
Melanie Witt, RN, MA
There are two coding choices for team conferences involving physician participation. If the patient and/or family is present, the CPT instruction is to bill a problem E/M service code (99201-99215) based on the time spent during this coordination of care/counseling. Documentation would include details about the conference decisions and implications for care, rather than history or examination.
If the patient is not present, report 99367 (Medical team conference with interdisciplinary team of health care professionals, patient and/or family not present, 30 minutes or more; participation by physician), but note that this code was developed under the assumption that the conference would be performed in a facility setting. Diagnostic coding would be breast cancer.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
This is an excerpt from a companion coding resource for breast cancer–related procedures by Ms. Witt. To read the companion article, “Coding for breast cancer–related procedures: A how-to guide,” in its entirety, click here.
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.
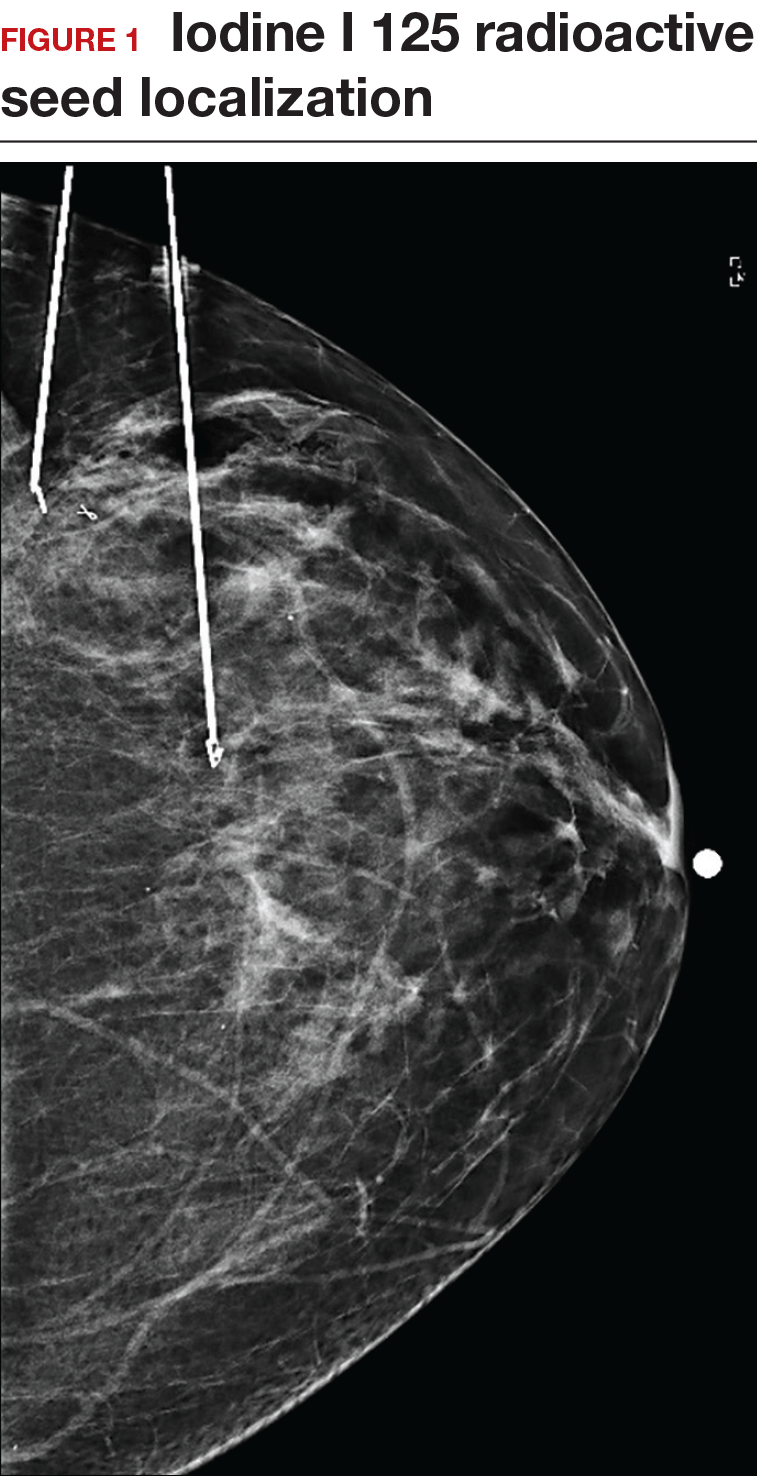
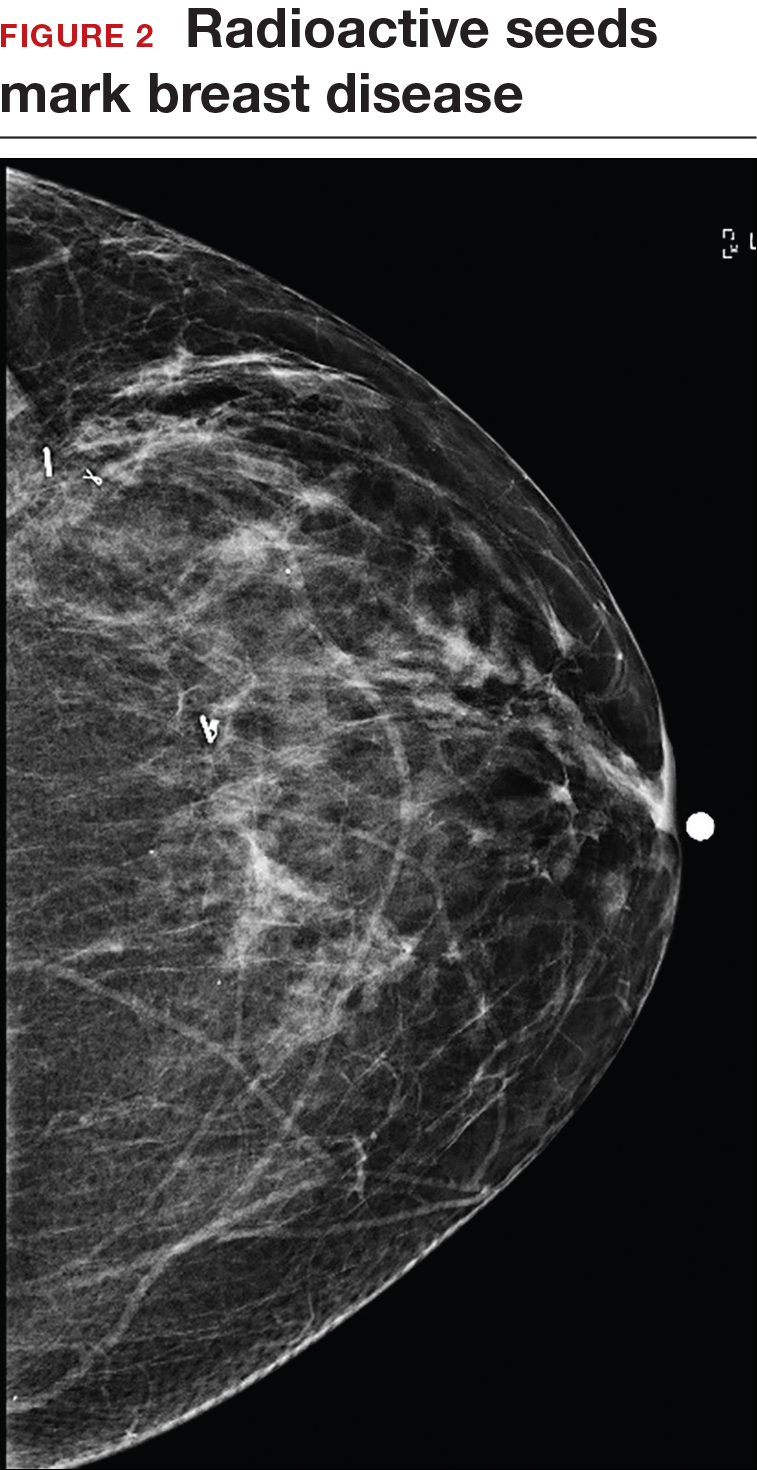
Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.
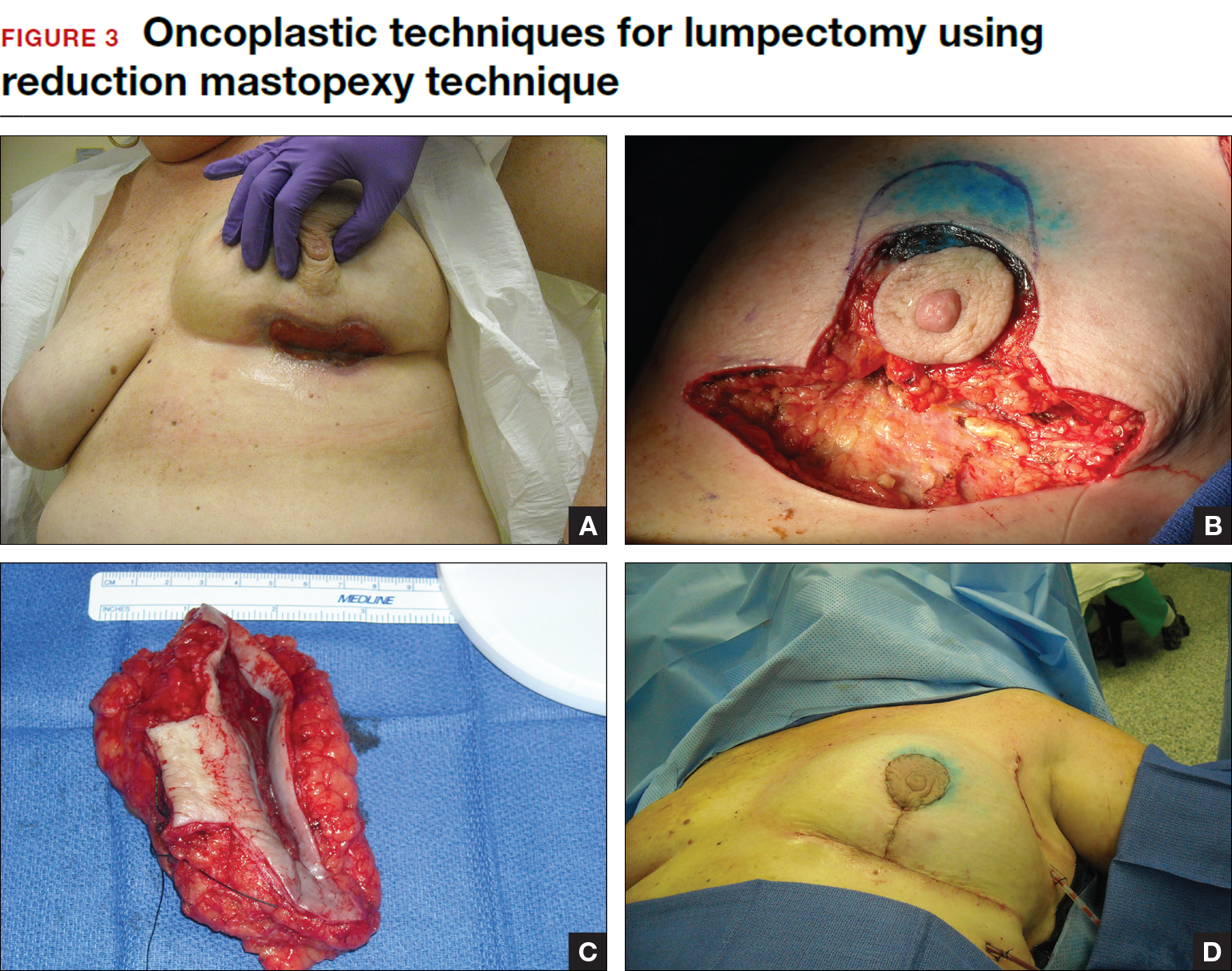
Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
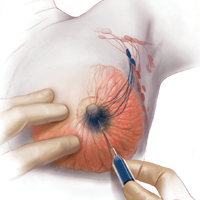
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.
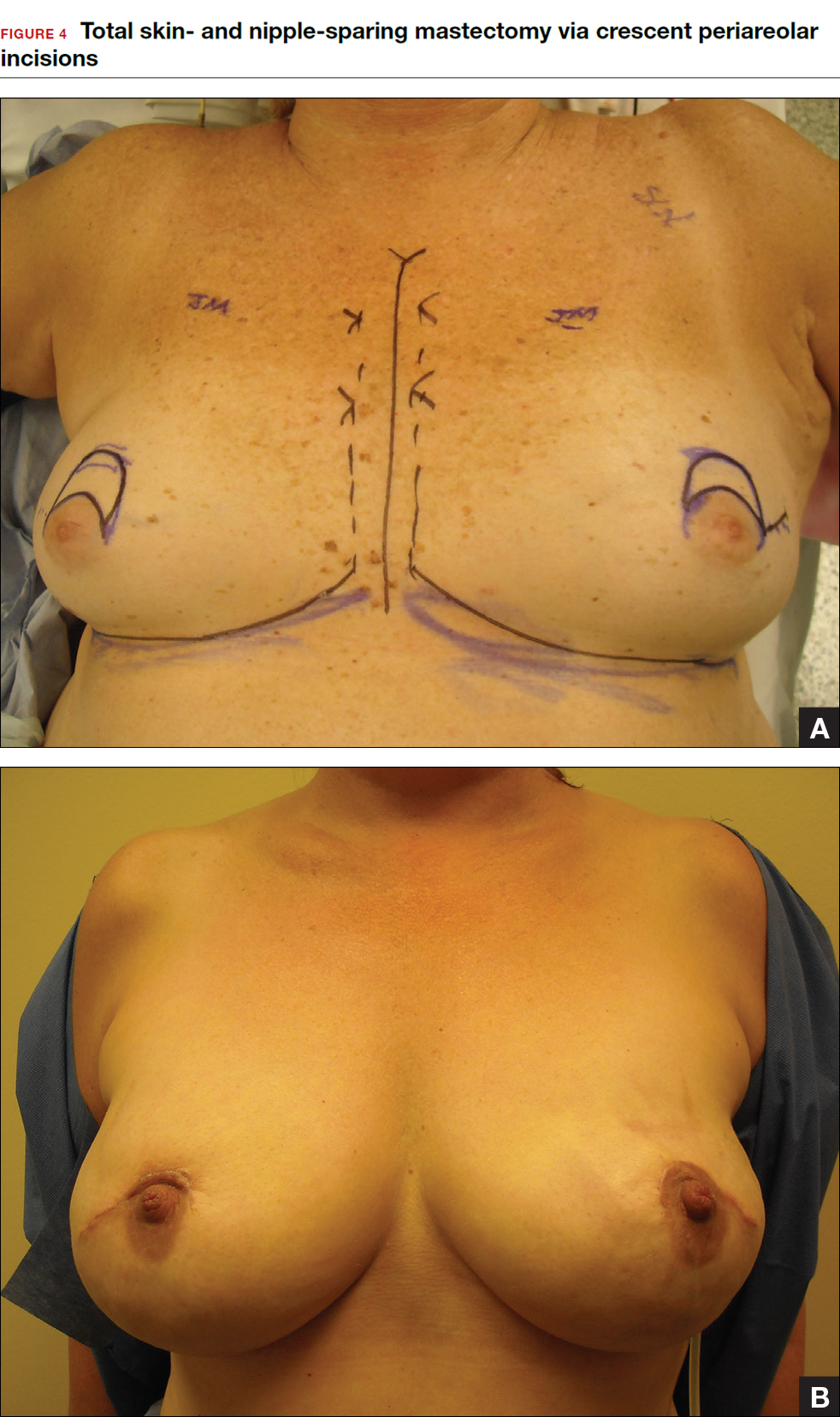
Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12
How do we manage the lymph nodes: Axillary dissection vs sentinel node biopsy?
Evaluation of the axillary nodes is currently part of breast cancer staging and can help the clinician determine the need for adjuvant chemotherapy. It also may assist in assessing the need for extending the radiation field beyond the breast to include the regional lymph nodes. Patients with early stage (stage I and II) breast cancer who do not have abnormal palpable lymph nodes or biopsy-proven metastasis to axillary nodes qualify for sentinel lymph node (SLN) biopsy.
Sentinel node biopsy = less morbidity with no loss of accuracy. Compared with axillary lymph node dissection (ALND; removing all the level I and II nodes in the axilla), SLN biopsy has a 98% accuracy and is associated with less morbidity from lymphedema. The procedure involves injecting the breast with 2 tracers: a radioactive isotope, injected into the breast within 24 hours of the operation, and isosulfan blue dye, injected into the breast in the OR at the time of surgery (see illustration). Both tracers travel through the breast lymphatics and concentrate in the first few lymph nodes that drain the breast. The surgery is performed through a separate axillary incision, and the blue and radioactive lymph nodes are individually dissected and removed for pathologic evaluation. On average, 2 to 4 sentinel nodes are removed, including any suspicious palpable nodes. In experienced hands, this procedure has a false-negative rate of less than 5% to 10%.13
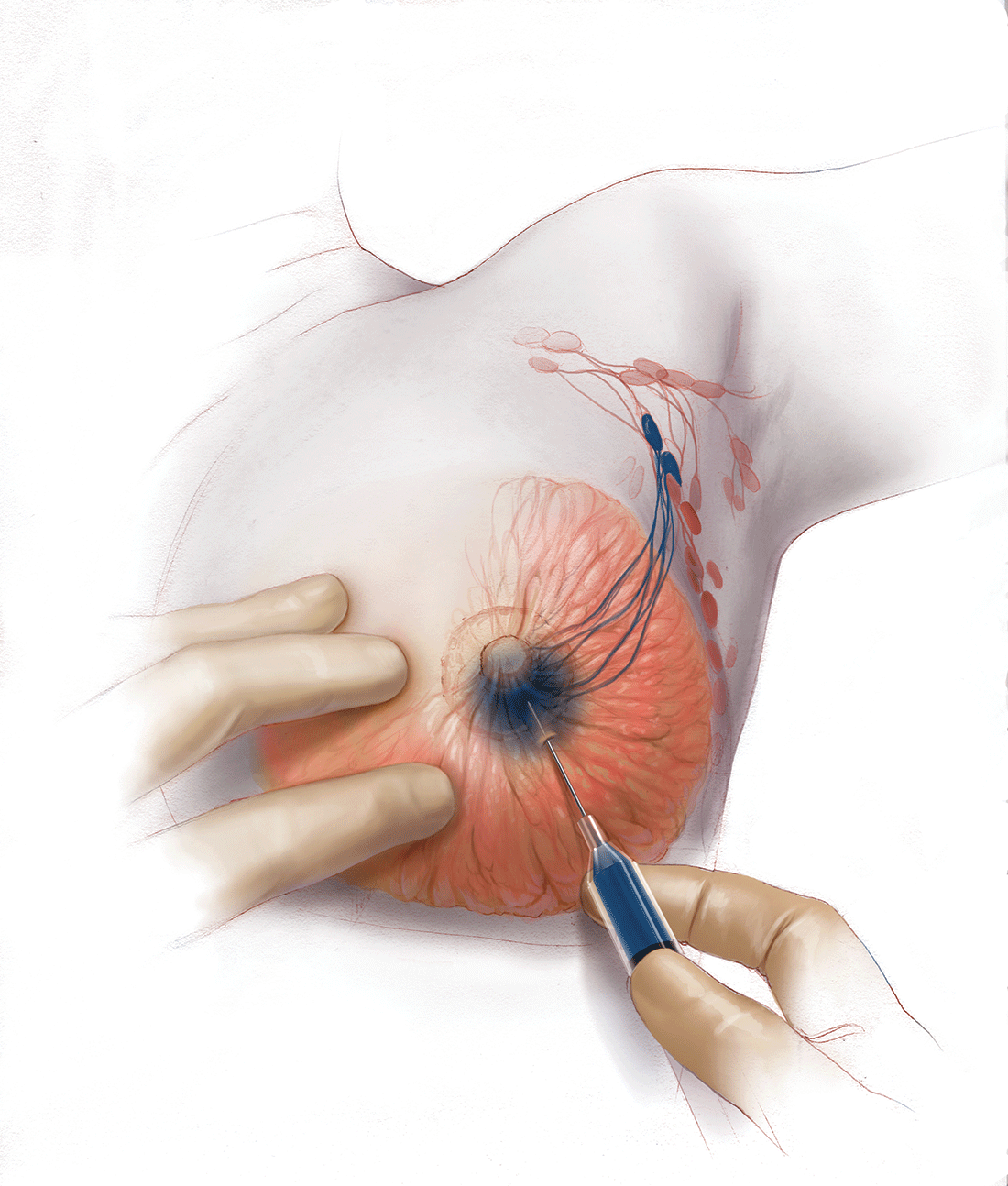
Axillary node dissection no longer standard of care. The indication for a completion ALND has changed based on the results of the randomized trial, ACOSOG Z0011.14 In this trial, patients with early stage breast cancer and 1 to 2 positive SLNs who were undergoing breast conservation therapy with radiation and adjuvant systemic therapy were randomly assigned to ALND or no ALND. (The trial did not include patients who were undergoing mastectomy, neoadjuvant chemotherapy, or who had more than 2 metastatic lymph nodes.) The investigators found no difference in overall or disease-free survival or local-regional recurrence between the 2 treatment groups over 9.2 years of follow up.14
Based on this practice-changing trial result, guidelines of the National Comprehensive Cancer Network no longer recommend completion ALND for patients who meet the ACOSOG Z0011 criteria. For patients who do not meet ACOSOG Z0011 criteria, we do intraoperative pathologic lymph node assessment with either frozen section or imprint cytology, and we perform immediate ALND when results are positive.
Indications for SLN biopsy include:
- invasive breast cancer with clinically negative axillary nodes
- ductal carcinoma in situ (DCIS) with microinvasion or extensive enough to require mastectomy
- clinically negative axillary nodes after neoadjuvant chemotherapy.
Contraindications for SLN biopsy include:
- bulky palpable lymphadenopathy
- pregnancy, as the safety of radioactive isotope and blue dye is not well studied; in isotope mapping the radiation dose is small and within safety limits for pregnant patients
- inflammatory breast cancer.
Complications of any axillary surgery may include risk of lymphedema (5% with SLN biopsy and 30% to 40% with ALND).15 Other complications include neuropathy of the affected arm with chronic pain and numbness of the skin.
Positive trends: Improved patient outcomes, specialized clinician training
Management of breast cancer has changed dramatically over the past several decades. More women are surviving breast cancer thanks to improvements in early detection, an individualized treatment approach with less aggressive surgery, and more effective targeted systemic therapies. A multidisciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care.
Complexity in breast disease management has led to the development of formal fellowship training in breast surgical oncology. Studies have demonstrated that patients treated by high-volume breast surgeons are more satisfied with their care and have improved cancer outcomes.16,17 Women should be aware that they have different options for their breast cancer care, and surgeons with advanced specialization in this field may provide optimal results and better quality of care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
- Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer [published online ahead of print March 8, 2016]. Ann Surg. doi:10.1097/SLA.0000000000001698.
- Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20(13):4113–4120.
- Zhang X, Zhang XJ, Zhang TY, et al. Effect and safety of dual anti-human epidermal growth factor receptor 2 therapy compared to monotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a systematic review. BMC Cancer. 2014;14:625.
- Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645–663.
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systemic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–446.
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77.
- De La Cruz L, Moody AM, Tappy EE, Blankenship AA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–3249.
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol. 2015;22(2):370–376.
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–212.
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600.
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). In: 2016 ASCO Annual Meeting; June 3-7, 2016. J Clin Oncol. 2016;34(15; May 20 suppl): Abstract 1007.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–615.
- Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698.
In a striking trend, the rate of contralateral prophylactic mastectomy (CPM) has risen by 30% over the last 10 years in the United States.1 Many women undergo CPM because of the fear and anxiety of cancer recurrence and their perceived risk of contralateral breast cancer; however, few women have a medical condition that necessitates removal of the contralateral breast. The medical indications for CPM include having a pathogenic genetic mutation (eg, BRCA1 and BRCA2), a strong family history of breast cancer, or prior mediastina chest radiation.
The actual risk of contralateral breast cancer is much lower than perceived. In women without a genetic mutation, the 10-year risk of contralateral breast cancer is only 3% to 5%.1 Also, CPM does not prevent the development of metastatic disease and offers no survival benefit over breast conservation or unilateral mastectomy.2 Furthermore, compared with unilateral therapeutic mastectomy, the “upgrade” to a CPM carries a 2.7-fold risk of a major surgical complication.3 It is therefore important that patients receive appropriate counseling regarding CPM, and that this counseling include cancer stage at diagnosis, family history and genetic risk, and cancer versus surgical risk (see “Counseling patients on contralateral prophylactic mastectomy” for key points to cover in patient discussions).
Counseling patients on contralateral prophylactic mastectomy
Commonly, patients diagnosed with breast cancer consider having their contralateral healthy breast removed as part of a bilateral mastectomy. They often experience severe anxiety about the cancer coming back and believe that removing both breasts will enable them to live longer. Keep the following key facts in mind when discussing treatment options with breast cancer patients.
Cancer stage at diagnosis. How long a patient lives from the time of her breast cancer diagnosis depends on the stage of the cancer at diagnosis, not the type of surgery performed. A woman with early stage I or stage II breast cancer has an 80% to 90% chance of being cancer free in 5 years.1 The chance of cancer recurring in the bones, liver, or lungs (metastatic breast cancer) will not be changed by removing the healthy breast. The risk of metastatic recurrence can be reduced, however, with chemotherapy and/or with hormone-blocker therapy.
Family history and genetic risk. Few women have a strong family history of breast and/or ovarian and other cancers, and this issue should be addressed with genetic counseling and testing prior to surgery. Those who carry a cancer-causing gene, such as BRCA1 or BRCA2, are at increased risk (40% to 60%) for a second or third breast cancer, especially if they are diagnosed at a young age (<50 years).2,3 In women who have a genetic mutation, removing both breasts and sometimes the ovaries can prevent development of another breast cancer. But this will not prevent spread of the cancer that is already present. Only chemotherapy and hormone blockers can prevent the spread of cancer.
Cancer risk versus surgical risk. For women with no family history of breast cancer, no genetic mutation, and no prior chest wall radiation, the risk of developing a new breast cancer in their other breast is only 3% to 5% every 10 years.3,4 This means that they have a 95% chance of not developing a new breast cancer in their healthy breast. Notably, removing the healthy breast can double the risk of postsurgical complications, including bleeding, infection, and loss of tissue and implant. The mastectomy site will be numb and the skin and nipple areola will not have any function other than cosmetic. Finally, wound complications from surgery could delay the start of important cancer treatment, such as chemotherapy or radiation.
The bottom line. Unless a woman has a strong family history of breast cancer, is diagnosed at a very young age, or has a genetic cancer-causing mutation, removing the contralateral healthy breast is not medically necessary and is not routinely recommended.
References
- Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5992.
- Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute. NIH Publ. No. 05-5302. 2006:181–205. http://seer.cancer.gov/archive/publications/mpmono. Accessed September 18, 2016.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
Women should be made aware that there are alternatives to mastectomy that have similar, or even better, outcomes with improved quality of life. Furthermore, a multi‑disciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care. Knowledge of this team approach and of modern breast cancer treatments is essential for general ObGyns as this understanding improves the overall care and guidance—specifically regarding referral to expert, high-volume breast surgeons—provided to those women most in need.
Expanded treatment options for breast cancer
Advancements in breast surgery, better imaging, and targeted therapies are changing the paradigm of breast cancer treatment.
Image-guided biopsy is key in decision making
When an abnormality is found in the breast, surgical excision of an undiagnosed breast lesion is no longer considered an appropriate first step. Use of image-guided biopsy or minimally invasive core needle biopsy allows for accurate diagnosis of a breast lesion while avoiding a potentially breast deforming and expensive surgical operation. It is always better to go into the operating room (OR) with a diagnosis and do the right operation the first time.
A core needle biopsy, results of which demonstrate a benign lesion, helps avoid breast surgery in women who do not need it. If cancer is diagnosed on biopsy, the extent of disease can be better evaluated and decision making can be more informed, with a multidisciplinary approach used to consider the various options, including genetic counseling, plastic surgery consultation, or neoadjuvant therapy. Some lesions, such as those too close to the skin, chest wall, or an implant, may not be amenable to core needle biopsy and therefore require surgical excision for diagnosis.
Benefits of a multidisciplinary tumor conference
It is important for a multidisciplinary group of cancer specialists to review a patient’s case and discuss the ideal treatment plan prior to surgery. Some breast cancer subtypes (such as human epidermal growth factor receptor 2 [HER2]–overamplified breast cancer and many triple-negative breast cancers) are very sensitive to chemotherapy, and patients with these tumor types may benefit from receiving neoadjuvant chemotherapy prior to surgery. New types of chemotherapy may allow up to 60% of some breast cancers to diminish almost completely, with subsequent improved cosmetic results of breast surgery.4 It may also allow time for genetic counseling and testing prior to surgery. (See “How to code for a multidisciplinary tumor conference” for appropriate coding procedure.)
How to code for a multidisciplinary tumor conference
Melanie Witt, RN, MA
There are two coding choices for team conferences involving physician participation. If the patient and/or family is present, the CPT instruction is to bill a problem E/M service code (99201-99215) based on the time spent during this coordination of care/counseling. Documentation would include details about the conference decisions and implications for care, rather than history or examination.
If the patient is not present, report 99367 (Medical team conference with interdisciplinary team of health care professionals, patient and/or family not present, 30 minutes or more; participation by physician), but note that this code was developed under the assumption that the conference would be performed in a facility setting. Diagnostic coding would be breast cancer.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
This is an excerpt from a companion coding resource for breast cancer–related procedures by Ms. Witt. To read the companion article, “Coding for breast cancer–related procedures: A how-to guide,” in its entirety, click here.
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.


Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.

Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.

Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12
How do we manage the lymph nodes: Axillary dissection vs sentinel node biopsy?
Evaluation of the axillary nodes is currently part of breast cancer staging and can help the clinician determine the need for adjuvant chemotherapy. It also may assist in assessing the need for extending the radiation field beyond the breast to include the regional lymph nodes. Patients with early stage (stage I and II) breast cancer who do not have abnormal palpable lymph nodes or biopsy-proven metastasis to axillary nodes qualify for sentinel lymph node (SLN) biopsy.
Sentinel node biopsy = less morbidity with no loss of accuracy. Compared with axillary lymph node dissection (ALND; removing all the level I and II nodes in the axilla), SLN biopsy has a 98% accuracy and is associated with less morbidity from lymphedema. The procedure involves injecting the breast with 2 tracers: a radioactive isotope, injected into the breast within 24 hours of the operation, and isosulfan blue dye, injected into the breast in the OR at the time of surgery (see illustration). Both tracers travel through the breast lymphatics and concentrate in the first few lymph nodes that drain the breast. The surgery is performed through a separate axillary incision, and the blue and radioactive lymph nodes are individually dissected and removed for pathologic evaluation. On average, 2 to 4 sentinel nodes are removed, including any suspicious palpable nodes. In experienced hands, this procedure has a false-negative rate of less than 5% to 10%.13

Axillary node dissection no longer standard of care. The indication for a completion ALND has changed based on the results of the randomized trial, ACOSOG Z0011.14 In this trial, patients with early stage breast cancer and 1 to 2 positive SLNs who were undergoing breast conservation therapy with radiation and adjuvant systemic therapy were randomly assigned to ALND or no ALND. (The trial did not include patients who were undergoing mastectomy, neoadjuvant chemotherapy, or who had more than 2 metastatic lymph nodes.) The investigators found no difference in overall or disease-free survival or local-regional recurrence between the 2 treatment groups over 9.2 years of follow up.14
Based on this practice-changing trial result, guidelines of the National Comprehensive Cancer Network no longer recommend completion ALND for patients who meet the ACOSOG Z0011 criteria. For patients who do not meet ACOSOG Z0011 criteria, we do intraoperative pathologic lymph node assessment with either frozen section or imprint cytology, and we perform immediate ALND when results are positive.
Indications for SLN biopsy include:
- invasive breast cancer with clinically negative axillary nodes
- ductal carcinoma in situ (DCIS) with microinvasion or extensive enough to require mastectomy
- clinically negative axillary nodes after neoadjuvant chemotherapy.
Contraindications for SLN biopsy include:
- bulky palpable lymphadenopathy
- pregnancy, as the safety of radioactive isotope and blue dye is not well studied; in isotope mapping the radiation dose is small and within safety limits for pregnant patients
- inflammatory breast cancer.
Complications of any axillary surgery may include risk of lymphedema (5% with SLN biopsy and 30% to 40% with ALND).15 Other complications include neuropathy of the affected arm with chronic pain and numbness of the skin.
Positive trends: Improved patient outcomes, specialized clinician training
Management of breast cancer has changed dramatically over the past several decades. More women are surviving breast cancer thanks to improvements in early detection, an individualized treatment approach with less aggressive surgery, and more effective targeted systemic therapies. A multidisciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care.
Complexity in breast disease management has led to the development of formal fellowship training in breast surgical oncology. Studies have demonstrated that patients treated by high-volume breast surgeons are more satisfied with their care and have improved cancer outcomes.16,17 Women should be aware that they have different options for their breast cancer care, and surgeons with advanced specialization in this field may provide optimal results and better quality of care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In a striking trend, the rate of contralateral prophylactic mastectomy (CPM) has risen by 30% over the last 10 years in the United States.1 Many women undergo CPM because of the fear and anxiety of cancer recurrence and their perceived risk of contralateral breast cancer; however, few women have a medical condition that necessitates removal of the contralateral breast. The medical indications for CPM include having a pathogenic genetic mutation (eg, BRCA1 and BRCA2), a strong family history of breast cancer, or prior mediastina chest radiation.
The actual risk of contralateral breast cancer is much lower than perceived. In women without a genetic mutation, the 10-year risk of contralateral breast cancer is only 3% to 5%.1 Also, CPM does not prevent the development of metastatic disease and offers no survival benefit over breast conservation or unilateral mastectomy.2 Furthermore, compared with unilateral therapeutic mastectomy, the “upgrade” to a CPM carries a 2.7-fold risk of a major surgical complication.3 It is therefore important that patients receive appropriate counseling regarding CPM, and that this counseling include cancer stage at diagnosis, family history and genetic risk, and cancer versus surgical risk (see “Counseling patients on contralateral prophylactic mastectomy” for key points to cover in patient discussions).
Counseling patients on contralateral prophylactic mastectomy
Commonly, patients diagnosed with breast cancer consider having their contralateral healthy breast removed as part of a bilateral mastectomy. They often experience severe anxiety about the cancer coming back and believe that removing both breasts will enable them to live longer. Keep the following key facts in mind when discussing treatment options with breast cancer patients.
Cancer stage at diagnosis. How long a patient lives from the time of her breast cancer diagnosis depends on the stage of the cancer at diagnosis, not the type of surgery performed. A woman with early stage I or stage II breast cancer has an 80% to 90% chance of being cancer free in 5 years.1 The chance of cancer recurring in the bones, liver, or lungs (metastatic breast cancer) will not be changed by removing the healthy breast. The risk of metastatic recurrence can be reduced, however, with chemotherapy and/or with hormone-blocker therapy.
Family history and genetic risk. Few women have a strong family history of breast and/or ovarian and other cancers, and this issue should be addressed with genetic counseling and testing prior to surgery. Those who carry a cancer-causing gene, such as BRCA1 or BRCA2, are at increased risk (40% to 60%) for a second or third breast cancer, especially if they are diagnosed at a young age (<50 years).2,3 In women who have a genetic mutation, removing both breasts and sometimes the ovaries can prevent development of another breast cancer. But this will not prevent spread of the cancer that is already present. Only chemotherapy and hormone blockers can prevent the spread of cancer.
Cancer risk versus surgical risk. For women with no family history of breast cancer, no genetic mutation, and no prior chest wall radiation, the risk of developing a new breast cancer in their other breast is only 3% to 5% every 10 years.3,4 This means that they have a 95% chance of not developing a new breast cancer in their healthy breast. Notably, removing the healthy breast can double the risk of postsurgical complications, including bleeding, infection, and loss of tissue and implant. The mastectomy site will be numb and the skin and nipple areola will not have any function other than cosmetic. Finally, wound complications from surgery could delay the start of important cancer treatment, such as chemotherapy or radiation.
The bottom line. Unless a woman has a strong family history of breast cancer, is diagnosed at a very young age, or has a genetic cancer-causing mutation, removing the contralateral healthy breast is not medically necessary and is not routinely recommended.
References
- Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5992.
- Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute. NIH Publ. No. 05-5302. 2006:181–205. http://seer.cancer.gov/archive/publications/mpmono. Accessed September 18, 2016.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
Women should be made aware that there are alternatives to mastectomy that have similar, or even better, outcomes with improved quality of life. Furthermore, a multi‑disciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care. Knowledge of this team approach and of modern breast cancer treatments is essential for general ObGyns as this understanding improves the overall care and guidance—specifically regarding referral to expert, high-volume breast surgeons—provided to those women most in need.
Expanded treatment options for breast cancer
Advancements in breast surgery, better imaging, and targeted therapies are changing the paradigm of breast cancer treatment.
Image-guided biopsy is key in decision making
When an abnormality is found in the breast, surgical excision of an undiagnosed breast lesion is no longer considered an appropriate first step. Use of image-guided biopsy or minimally invasive core needle biopsy allows for accurate diagnosis of a breast lesion while avoiding a potentially breast deforming and expensive surgical operation. It is always better to go into the operating room (OR) with a diagnosis and do the right operation the first time.
A core needle biopsy, results of which demonstrate a benign lesion, helps avoid breast surgery in women who do not need it. If cancer is diagnosed on biopsy, the extent of disease can be better evaluated and decision making can be more informed, with a multidisciplinary approach used to consider the various options, including genetic counseling, plastic surgery consultation, or neoadjuvant therapy. Some lesions, such as those too close to the skin, chest wall, or an implant, may not be amenable to core needle biopsy and therefore require surgical excision for diagnosis.
Benefits of a multidisciplinary tumor conference
It is important for a multidisciplinary group of cancer specialists to review a patient’s case and discuss the ideal treatment plan prior to surgery. Some breast cancer subtypes (such as human epidermal growth factor receptor 2 [HER2]–overamplified breast cancer and many triple-negative breast cancers) are very sensitive to chemotherapy, and patients with these tumor types may benefit from receiving neoadjuvant chemotherapy prior to surgery. New types of chemotherapy may allow up to 60% of some breast cancers to diminish almost completely, with subsequent improved cosmetic results of breast surgery.4 It may also allow time for genetic counseling and testing prior to surgery. (See “How to code for a multidisciplinary tumor conference” for appropriate coding procedure.)
How to code for a multidisciplinary tumor conference
Melanie Witt, RN, MA
There are two coding choices for team conferences involving physician participation. If the patient and/or family is present, the CPT instruction is to bill a problem E/M service code (99201-99215) based on the time spent during this coordination of care/counseling. Documentation would include details about the conference decisions and implications for care, rather than history or examination.
If the patient is not present, report 99367 (Medical team conference with interdisciplinary team of health care professionals, patient and/or family not present, 30 minutes or more; participation by physician), but note that this code was developed under the assumption that the conference would be performed in a facility setting. Diagnostic coding would be breast cancer.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
This is an excerpt from a companion coding resource for breast cancer–related procedures by Ms. Witt. To read the companion article, “Coding for breast cancer–related procedures: A how-to guide,” in its entirety, click here.
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.


Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.

Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.

Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12
How do we manage the lymph nodes: Axillary dissection vs sentinel node biopsy?
Evaluation of the axillary nodes is currently part of breast cancer staging and can help the clinician determine the need for adjuvant chemotherapy. It also may assist in assessing the need for extending the radiation field beyond the breast to include the regional lymph nodes. Patients with early stage (stage I and II) breast cancer who do not have abnormal palpable lymph nodes or biopsy-proven metastasis to axillary nodes qualify for sentinel lymph node (SLN) biopsy.
Sentinel node biopsy = less morbidity with no loss of accuracy. Compared with axillary lymph node dissection (ALND; removing all the level I and II nodes in the axilla), SLN biopsy has a 98% accuracy and is associated with less morbidity from lymphedema. The procedure involves injecting the breast with 2 tracers: a radioactive isotope, injected into the breast within 24 hours of the operation, and isosulfan blue dye, injected into the breast in the OR at the time of surgery (see illustration). Both tracers travel through the breast lymphatics and concentrate in the first few lymph nodes that drain the breast. The surgery is performed through a separate axillary incision, and the blue and radioactive lymph nodes are individually dissected and removed for pathologic evaluation. On average, 2 to 4 sentinel nodes are removed, including any suspicious palpable nodes. In experienced hands, this procedure has a false-negative rate of less than 5% to 10%.13

Axillary node dissection no longer standard of care. The indication for a completion ALND has changed based on the results of the randomized trial, ACOSOG Z0011.14 In this trial, patients with early stage breast cancer and 1 to 2 positive SLNs who were undergoing breast conservation therapy with radiation and adjuvant systemic therapy were randomly assigned to ALND or no ALND. (The trial did not include patients who were undergoing mastectomy, neoadjuvant chemotherapy, or who had more than 2 metastatic lymph nodes.) The investigators found no difference in overall or disease-free survival or local-regional recurrence between the 2 treatment groups over 9.2 years of follow up.14
Based on this practice-changing trial result, guidelines of the National Comprehensive Cancer Network no longer recommend completion ALND for patients who meet the ACOSOG Z0011 criteria. For patients who do not meet ACOSOG Z0011 criteria, we do intraoperative pathologic lymph node assessment with either frozen section or imprint cytology, and we perform immediate ALND when results are positive.
Indications for SLN biopsy include:
- invasive breast cancer with clinically negative axillary nodes
- ductal carcinoma in situ (DCIS) with microinvasion or extensive enough to require mastectomy
- clinically negative axillary nodes after neoadjuvant chemotherapy.
Contraindications for SLN biopsy include:
- bulky palpable lymphadenopathy
- pregnancy, as the safety of radioactive isotope and blue dye is not well studied; in isotope mapping the radiation dose is small and within safety limits for pregnant patients
- inflammatory breast cancer.
Complications of any axillary surgery may include risk of lymphedema (5% with SLN biopsy and 30% to 40% with ALND).15 Other complications include neuropathy of the affected arm with chronic pain and numbness of the skin.
Positive trends: Improved patient outcomes, specialized clinician training
Management of breast cancer has changed dramatically over the past several decades. More women are surviving breast cancer thanks to improvements in early detection, an individualized treatment approach with less aggressive surgery, and more effective targeted systemic therapies. A multidisciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care.
Complexity in breast disease management has led to the development of formal fellowship training in breast surgical oncology. Studies have demonstrated that patients treated by high-volume breast surgeons are more satisfied with their care and have improved cancer outcomes.16,17 Women should be aware that they have different options for their breast cancer care, and surgeons with advanced specialization in this field may provide optimal results and better quality of care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
- Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer [published online ahead of print March 8, 2016]. Ann Surg. doi:10.1097/SLA.0000000000001698.
- Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20(13):4113–4120.
- Zhang X, Zhang XJ, Zhang TY, et al. Effect and safety of dual anti-human epidermal growth factor receptor 2 therapy compared to monotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a systematic review. BMC Cancer. 2014;14:625.
- Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645–663.
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systemic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–446.
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77.
- De La Cruz L, Moody AM, Tappy EE, Blankenship AA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–3249.
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol. 2015;22(2):370–376.
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–212.
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600.
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). In: 2016 ASCO Annual Meeting; June 3-7, 2016. J Clin Oncol. 2016;34(15; May 20 suppl): Abstract 1007.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–615.
- Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
- Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer [published online ahead of print March 8, 2016]. Ann Surg. doi:10.1097/SLA.0000000000001698.
- Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20(13):4113–4120.
- Zhang X, Zhang XJ, Zhang TY, et al. Effect and safety of dual anti-human epidermal growth factor receptor 2 therapy compared to monotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a systematic review. BMC Cancer. 2014;14:625.
- Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645–663.
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systemic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–446.
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77.
- De La Cruz L, Moody AM, Tappy EE, Blankenship AA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–3249.
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol. 2015;22(2):370–376.
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–212.
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600.
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). In: 2016 ASCO Annual Meeting; June 3-7, 2016. J Clin Oncol. 2016;34(15; May 20 suppl): Abstract 1007.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–615.
- Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698.
In this Article
- Counseling patients on CPM
- Oncoplastic lumpectomy approach
- How to manage the lymph nodes
Letter to the Editor: Tubal occlusion device removal
VIDEO: “LAPAROSCOPIC SALPINGECTOMY AND CORNUAL RESECTION REPURPOSED: A NOVEL APPROACH TO TUBAL OCCLUSION DEVICE REMOVAL”
MICHELLE PACIS, MD, MPH (JULY 2016)
Easier technique for removing tubal occlusion devices?
My patient’s rheumatologist recently asked me to remove the tubal occlusion device (Essure) inserts that I had placed approximately 5 years ago. I think the technique I used was a little easier than the one shown in the video by Dr. Pacis and featured by Dr. Advincula in his video series. I started with a standard salpingectomy from the fimbriated end, as did the technique in the video. Then I made a circumferential incision of the tubal serosa at the junction of the tube as it enters the cornua, taking care to not cut the device insert, which could be visualized and felt with cold shears. The proximal end of the device insert, including the post and coil, then easily pulled out with some elongation of the coil. Since I did not need to resect the cornua, I was able to easily seal off the small defect without need to suture.
Alexander Lin, MD
Chicago, Illinois
Dr. Pacis responds
Thank you for sharing your method for tubal occlusion device removal. Your technique would certainly work for devices that reside predominantly in the tube. We have found that many of the devices become quite anchored and adherent to the tubal mucosa. While there are many surgical approaches to device removal, our preference is to perform salpingectomy with cornual resection, so as to avoid traction on the microinsert, and remove the device intact. We are then able to give the specimen, which contains the insert, to pathology so they can comment on the status of the device.
VIDEO: “LAPAROSCOPIC SALPINGECTOMY AND CORNUAL RESECTION REPURPOSED: A NOVEL APPROACH TO TUBAL OCCLUSION DEVICE REMOVAL”
MICHELLE PACIS, MD, MPH (JULY 2016)
Easier technique for removing tubal occlusion devices?
My patient’s rheumatologist recently asked me to remove the tubal occlusion device (Essure) inserts that I had placed approximately 5 years ago. I think the technique I used was a little easier than the one shown in the video by Dr. Pacis and featured by Dr. Advincula in his video series. I started with a standard salpingectomy from the fimbriated end, as did the technique in the video. Then I made a circumferential incision of the tubal serosa at the junction of the tube as it enters the cornua, taking care to not cut the device insert, which could be visualized and felt with cold shears. The proximal end of the device insert, including the post and coil, then easily pulled out with some elongation of the coil. Since I did not need to resect the cornua, I was able to easily seal off the small defect without need to suture.
Alexander Lin, MD
Chicago, Illinois
Dr. Pacis responds
Thank you for sharing your method for tubal occlusion device removal. Your technique would certainly work for devices that reside predominantly in the tube. We have found that many of the devices become quite anchored and adherent to the tubal mucosa. While there are many surgical approaches to device removal, our preference is to perform salpingectomy with cornual resection, so as to avoid traction on the microinsert, and remove the device intact. We are then able to give the specimen, which contains the insert, to pathology so they can comment on the status of the device.
VIDEO: “LAPAROSCOPIC SALPINGECTOMY AND CORNUAL RESECTION REPURPOSED: A NOVEL APPROACH TO TUBAL OCCLUSION DEVICE REMOVAL”
MICHELLE PACIS, MD, MPH (JULY 2016)
Easier technique for removing tubal occlusion devices?
My patient’s rheumatologist recently asked me to remove the tubal occlusion device (Essure) inserts that I had placed approximately 5 years ago. I think the technique I used was a little easier than the one shown in the video by Dr. Pacis and featured by Dr. Advincula in his video series. I started with a standard salpingectomy from the fimbriated end, as did the technique in the video. Then I made a circumferential incision of the tubal serosa at the junction of the tube as it enters the cornua, taking care to not cut the device insert, which could be visualized and felt with cold shears. The proximal end of the device insert, including the post and coil, then easily pulled out with some elongation of the coil. Since I did not need to resect the cornua, I was able to easily seal off the small defect without need to suture.
Alexander Lin, MD
Chicago, Illinois
Dr. Pacis responds
Thank you for sharing your method for tubal occlusion device removal. Your technique would certainly work for devices that reside predominantly in the tube. We have found that many of the devices become quite anchored and adherent to the tubal mucosa. While there are many surgical approaches to device removal, our preference is to perform salpingectomy with cornual resection, so as to avoid traction on the microinsert, and remove the device intact. We are then able to give the specimen, which contains the insert, to pathology so they can comment on the status of the device.
Study eyes anastomotic failure in stapled vs. hand-sewn techniques
WAIKOLOA, HAWAII – The risk of anastomotic failure among emergency general surgery patients requiring bowel resection and anastomosis stands at 12.5% and is similar between stapled and hand-sewn techniques, results from a multicenter analysis demonstrated.
Surgeons participating in the study, known as Stapled vs. Handsewn: A Prospective Emergency Surgery Study (SHAPES), “appear to be performing hand-sewn techniques in patients who have a higher burden of disease,” Brandon R. Bruns, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “Without adjustment and despite being performed in a more ill population, there was no difference in failure rate between hand-sewn and stapled techniques. After modeling, only being managed with an open abdomen and contamination at initial operation were associated with anastomotic failure.”
For SHAPES, which is the largest study of its kind and was sponsored by the AAST Multi-Institutional Studies Committee, Dr. Bruns and his associates set out to prospectively evaluate anastomotic failure rates for hand-sewn and stapled anastomoses in patients undergoing urgent/emergent operations. A 1999 study by Seattle researchers found that stapled techniques seemed to be associated with anastomotic failure (J Trauma. 1999;47[3]:500-08). Two years later, the same researchers pooled 4-year retrospective data from four trauma centers and concluded that again, hand-sewn techniques appeared to be superior to stapled techniques after traumatic bowel resection and anastomosis (J Trauma. 2001;51[6]:1054-61). Also in 2001, AAST sponsored a multi-institutional study that examined the same question, this time in penetrative colonic injury. Investigators found no difference in complications between the two groups (J Trauma. 2002;52[1]:117-21). They did, however, find a 22.7% overall incidence of colon-related complications.
“With this background we hypothesized that anastomotic failure rate would be high for emergency general surgery patients undergoing bowel resection and anastomosis,” said Dr. Bruns, an acute care surgeon at the R. Adams Cowley Shock Trauma Center at the University of Maryland Medical Center, Baltimore. “We also hypothesized that failure rates would be higher for stapled techniques, compared with hand-sewn.”
The SHAPES researchers prospectively enrolled 595 patients at 15 medical centers in the United States who underwent urgent/emergent bowel resection for emergency general surgery pathology between July 22, 2013, and Dec. 31, 2015. The patients were grouped by hand-sewn vs. stapled anastomoses and demographic and clinical variables were collected. The primary outcome was anastomotic failure. As in other studies, anastomotic failure was evaluated at the anastomosis level. Multivariable logistic regression was done, controlling for age and risk factors for anastomotic failure.
Dr. Bruns reported that the 595 patients had 649 anastomoses. Of these, 61% were stapled and 39% were hand-sewn. The mean age of patients was 62 years, 51% were male, and the overall mortality rate was 7.7%. More than two-thirds of the patients (35%) had more than one indication for operative intervention. The most common single indication for operation was small bowel obstruction, at 23%. The majority of the anastomoses were small bowel to small bowel (72%), while 21% were small bowel to large bowel, and 7% were large bowel to large bowel. There were a total of 81 anastomotic failures, for a rate of 12.5%.
When the researchers compared the hand-sewn and stapled groups, they were similar with respect to sex and age, with higher Charlson comorbidity indices in stapled and a higher body mass index in the hand-sewn group. They also observed a lower hemoglobin, higher INR, higher lactate, lower albumin, and worse renal function in the hand-sewn group, compared with the stapled group. Moreover, hand-sewn anastomotic techniques were performed more frequently in patients that were on vasopressor support. “Despite patients receiving hand-sewn techniques, having worse laboratory values, and more intraoperative vasopressors, the two techniques had equivalent failure rates, at 15.4% for hand-sewn and 10.6% for stapled,” Dr. Bruns said. “Patients with hand-sewn techniques had longer hospital length of stay, longer ICU length of stay, and a significantly higher mortality, compared with those who underwent stapled techniques. Interestingly and different from most other studies on the topic, operating time between the two groups was equivalent.”
On multivariate regression, the presence of contamination at initial bowel resection (OR 1.96) and the patient being managed with an open abdomen (OR 2.53) were independently associated with anastomotic failure, while the type of anastomosis (hand-sewn vs. stapled) was not.
The researchers also conducted a subanalysis of 165 patients managed with an open abdomen who had bowel resection and anastomosis. These patients had higher BMIs, higher lactates, higher INRs, and more negative base deficits, compared with those who were not managed with an open abdomen. “Perhaps not unexpectedly, open abdomen patients were more likely to be on vasopressor agents,” Dr. Bruns said. “They had longer hospital lengths of stay, more ICU days, and an 18.2% overall mortality. Overall there was an almost 22% anastomotic failure rate in patients managed with an open abdomen, compared with an 8.5% rate in patients managed with non-open techniques.” Comparing hand-sewn and stapled techniques, there was no difference in failure rate in patients managed with an open abdomen (25.2% vs. 17.5%, respectively; P = .20).
“An overall mortality rate of 8% and an anastomotic complication rate of 12.5% should emphasize the dire needs for these operations and the need for meticulous operative as well as surgically directed perioperative care in these patients,” the invited discussant, Gregory Jurkovich, MD, professor of surgery at the Davis Medical Center, University of California, said at the meeting. “We as surgeons must pay attention to all aspects of care in these patients.”
Dr. Bruns acknowledged certain limitations of the study, including the fact that it was not a randomized, controlled trial. Also, “surgeon preference did dictate the type of anastomosis that was created, and the patient and surgeon populations were heterogeneous,” he said. “The multivariable model was limited by missing laboratory data, likely given the urgent nature of some of the operative procedures.”
He reported having no financial disclosures.
WAIKOLOA, HAWAII – The risk of anastomotic failure among emergency general surgery patients requiring bowel resection and anastomosis stands at 12.5% and is similar between stapled and hand-sewn techniques, results from a multicenter analysis demonstrated.
Surgeons participating in the study, known as Stapled vs. Handsewn: A Prospective Emergency Surgery Study (SHAPES), “appear to be performing hand-sewn techniques in patients who have a higher burden of disease,” Brandon R. Bruns, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “Without adjustment and despite being performed in a more ill population, there was no difference in failure rate between hand-sewn and stapled techniques. After modeling, only being managed with an open abdomen and contamination at initial operation were associated with anastomotic failure.”
For SHAPES, which is the largest study of its kind and was sponsored by the AAST Multi-Institutional Studies Committee, Dr. Bruns and his associates set out to prospectively evaluate anastomotic failure rates for hand-sewn and stapled anastomoses in patients undergoing urgent/emergent operations. A 1999 study by Seattle researchers found that stapled techniques seemed to be associated with anastomotic failure (J Trauma. 1999;47[3]:500-08). Two years later, the same researchers pooled 4-year retrospective data from four trauma centers and concluded that again, hand-sewn techniques appeared to be superior to stapled techniques after traumatic bowel resection and anastomosis (J Trauma. 2001;51[6]:1054-61). Also in 2001, AAST sponsored a multi-institutional study that examined the same question, this time in penetrative colonic injury. Investigators found no difference in complications between the two groups (J Trauma. 2002;52[1]:117-21). They did, however, find a 22.7% overall incidence of colon-related complications.
“With this background we hypothesized that anastomotic failure rate would be high for emergency general surgery patients undergoing bowel resection and anastomosis,” said Dr. Bruns, an acute care surgeon at the R. Adams Cowley Shock Trauma Center at the University of Maryland Medical Center, Baltimore. “We also hypothesized that failure rates would be higher for stapled techniques, compared with hand-sewn.”
The SHAPES researchers prospectively enrolled 595 patients at 15 medical centers in the United States who underwent urgent/emergent bowel resection for emergency general surgery pathology between July 22, 2013, and Dec. 31, 2015. The patients were grouped by hand-sewn vs. stapled anastomoses and demographic and clinical variables were collected. The primary outcome was anastomotic failure. As in other studies, anastomotic failure was evaluated at the anastomosis level. Multivariable logistic regression was done, controlling for age and risk factors for anastomotic failure.
Dr. Bruns reported that the 595 patients had 649 anastomoses. Of these, 61% were stapled and 39% were hand-sewn. The mean age of patients was 62 years, 51% were male, and the overall mortality rate was 7.7%. More than two-thirds of the patients (35%) had more than one indication for operative intervention. The most common single indication for operation was small bowel obstruction, at 23%. The majority of the anastomoses were small bowel to small bowel (72%), while 21% were small bowel to large bowel, and 7% were large bowel to large bowel. There were a total of 81 anastomotic failures, for a rate of 12.5%.
When the researchers compared the hand-sewn and stapled groups, they were similar with respect to sex and age, with higher Charlson comorbidity indices in stapled and a higher body mass index in the hand-sewn group. They also observed a lower hemoglobin, higher INR, higher lactate, lower albumin, and worse renal function in the hand-sewn group, compared with the stapled group. Moreover, hand-sewn anastomotic techniques were performed more frequently in patients that were on vasopressor support. “Despite patients receiving hand-sewn techniques, having worse laboratory values, and more intraoperative vasopressors, the two techniques had equivalent failure rates, at 15.4% for hand-sewn and 10.6% for stapled,” Dr. Bruns said. “Patients with hand-sewn techniques had longer hospital length of stay, longer ICU length of stay, and a significantly higher mortality, compared with those who underwent stapled techniques. Interestingly and different from most other studies on the topic, operating time between the two groups was equivalent.”
On multivariate regression, the presence of contamination at initial bowel resection (OR 1.96) and the patient being managed with an open abdomen (OR 2.53) were independently associated with anastomotic failure, while the type of anastomosis (hand-sewn vs. stapled) was not.
The researchers also conducted a subanalysis of 165 patients managed with an open abdomen who had bowel resection and anastomosis. These patients had higher BMIs, higher lactates, higher INRs, and more negative base deficits, compared with those who were not managed with an open abdomen. “Perhaps not unexpectedly, open abdomen patients were more likely to be on vasopressor agents,” Dr. Bruns said. “They had longer hospital lengths of stay, more ICU days, and an 18.2% overall mortality. Overall there was an almost 22% anastomotic failure rate in patients managed with an open abdomen, compared with an 8.5% rate in patients managed with non-open techniques.” Comparing hand-sewn and stapled techniques, there was no difference in failure rate in patients managed with an open abdomen (25.2% vs. 17.5%, respectively; P = .20).
“An overall mortality rate of 8% and an anastomotic complication rate of 12.5% should emphasize the dire needs for these operations and the need for meticulous operative as well as surgically directed perioperative care in these patients,” the invited discussant, Gregory Jurkovich, MD, professor of surgery at the Davis Medical Center, University of California, said at the meeting. “We as surgeons must pay attention to all aspects of care in these patients.”
Dr. Bruns acknowledged certain limitations of the study, including the fact that it was not a randomized, controlled trial. Also, “surgeon preference did dictate the type of anastomosis that was created, and the patient and surgeon populations were heterogeneous,” he said. “The multivariable model was limited by missing laboratory data, likely given the urgent nature of some of the operative procedures.”
He reported having no financial disclosures.
WAIKOLOA, HAWAII – The risk of anastomotic failure among emergency general surgery patients requiring bowel resection and anastomosis stands at 12.5% and is similar between stapled and hand-sewn techniques, results from a multicenter analysis demonstrated.
Surgeons participating in the study, known as Stapled vs. Handsewn: A Prospective Emergency Surgery Study (SHAPES), “appear to be performing hand-sewn techniques in patients who have a higher burden of disease,” Brandon R. Bruns, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “Without adjustment and despite being performed in a more ill population, there was no difference in failure rate between hand-sewn and stapled techniques. After modeling, only being managed with an open abdomen and contamination at initial operation were associated with anastomotic failure.”
For SHAPES, which is the largest study of its kind and was sponsored by the AAST Multi-Institutional Studies Committee, Dr. Bruns and his associates set out to prospectively evaluate anastomotic failure rates for hand-sewn and stapled anastomoses in patients undergoing urgent/emergent operations. A 1999 study by Seattle researchers found that stapled techniques seemed to be associated with anastomotic failure (J Trauma. 1999;47[3]:500-08). Two years later, the same researchers pooled 4-year retrospective data from four trauma centers and concluded that again, hand-sewn techniques appeared to be superior to stapled techniques after traumatic bowel resection and anastomosis (J Trauma. 2001;51[6]:1054-61). Also in 2001, AAST sponsored a multi-institutional study that examined the same question, this time in penetrative colonic injury. Investigators found no difference in complications between the two groups (J Trauma. 2002;52[1]:117-21). They did, however, find a 22.7% overall incidence of colon-related complications.
“With this background we hypothesized that anastomotic failure rate would be high for emergency general surgery patients undergoing bowel resection and anastomosis,” said Dr. Bruns, an acute care surgeon at the R. Adams Cowley Shock Trauma Center at the University of Maryland Medical Center, Baltimore. “We also hypothesized that failure rates would be higher for stapled techniques, compared with hand-sewn.”
The SHAPES researchers prospectively enrolled 595 patients at 15 medical centers in the United States who underwent urgent/emergent bowel resection for emergency general surgery pathology between July 22, 2013, and Dec. 31, 2015. The patients were grouped by hand-sewn vs. stapled anastomoses and demographic and clinical variables were collected. The primary outcome was anastomotic failure. As in other studies, anastomotic failure was evaluated at the anastomosis level. Multivariable logistic regression was done, controlling for age and risk factors for anastomotic failure.
Dr. Bruns reported that the 595 patients had 649 anastomoses. Of these, 61% were stapled and 39% were hand-sewn. The mean age of patients was 62 years, 51% were male, and the overall mortality rate was 7.7%. More than two-thirds of the patients (35%) had more than one indication for operative intervention. The most common single indication for operation was small bowel obstruction, at 23%. The majority of the anastomoses were small bowel to small bowel (72%), while 21% were small bowel to large bowel, and 7% were large bowel to large bowel. There were a total of 81 anastomotic failures, for a rate of 12.5%.
When the researchers compared the hand-sewn and stapled groups, they were similar with respect to sex and age, with higher Charlson comorbidity indices in stapled and a higher body mass index in the hand-sewn group. They also observed a lower hemoglobin, higher INR, higher lactate, lower albumin, and worse renal function in the hand-sewn group, compared with the stapled group. Moreover, hand-sewn anastomotic techniques were performed more frequently in patients that were on vasopressor support. “Despite patients receiving hand-sewn techniques, having worse laboratory values, and more intraoperative vasopressors, the two techniques had equivalent failure rates, at 15.4% for hand-sewn and 10.6% for stapled,” Dr. Bruns said. “Patients with hand-sewn techniques had longer hospital length of stay, longer ICU length of stay, and a significantly higher mortality, compared with those who underwent stapled techniques. Interestingly and different from most other studies on the topic, operating time between the two groups was equivalent.”
On multivariate regression, the presence of contamination at initial bowel resection (OR 1.96) and the patient being managed with an open abdomen (OR 2.53) were independently associated with anastomotic failure, while the type of anastomosis (hand-sewn vs. stapled) was not.
The researchers also conducted a subanalysis of 165 patients managed with an open abdomen who had bowel resection and anastomosis. These patients had higher BMIs, higher lactates, higher INRs, and more negative base deficits, compared with those who were not managed with an open abdomen. “Perhaps not unexpectedly, open abdomen patients were more likely to be on vasopressor agents,” Dr. Bruns said. “They had longer hospital lengths of stay, more ICU days, and an 18.2% overall mortality. Overall there was an almost 22% anastomotic failure rate in patients managed with an open abdomen, compared with an 8.5% rate in patients managed with non-open techniques.” Comparing hand-sewn and stapled techniques, there was no difference in failure rate in patients managed with an open abdomen (25.2% vs. 17.5%, respectively; P = .20).
“An overall mortality rate of 8% and an anastomotic complication rate of 12.5% should emphasize the dire needs for these operations and the need for meticulous operative as well as surgically directed perioperative care in these patients,” the invited discussant, Gregory Jurkovich, MD, professor of surgery at the Davis Medical Center, University of California, said at the meeting. “We as surgeons must pay attention to all aspects of care in these patients.”
Dr. Bruns acknowledged certain limitations of the study, including the fact that it was not a randomized, controlled trial. Also, “surgeon preference did dictate the type of anastomosis that was created, and the patient and surgeon populations were heterogeneous,” he said. “The multivariable model was limited by missing laboratory data, likely given the urgent nature of some of the operative procedures.”
He reported having no financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: In patients requiring emergency bowel resection and anastomosis, surgeons appear to be performing hand-sewn techniques in patients who have a higher burden of disease.
Major finding: There were 81 anastomotic failures in the study group, for a rate of 12.5%.
Data source: A prospective evaluation of 595 patients at 15 medical centers in the United States who underwent urgent/emergent bowel resection for emergency general surgery pathology between July 22, 2013, and Dec. 31, 2015.
Disclosures: Dr. Bruns reported having no financial disclosures.
Alternative options for visualizing ureteral patency during intraoperative cystoscopy

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related Articles:
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related Articles:
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related Articles:
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
This video brought to you by 
Post-Discharge Methicillin-Resistant Staphylococcus aureus Infections: Epidemiology and Potential Approaches to Control
From the Division of Adult Infectious Diseases, University of Colorado Denver, Aurora, CO, and the Department of Veterans Affairs, Eastern Colorado Healthcare System, Denver, CO.
Abstract
- Objective: To review the published literature on methicillin-resistant Staphylococcus aureus (MRSA) infections among patients recently discharged from hospital, with a focus on possible prevention measures.
- Methods: Literature review.
- Results: MRSA is a major cause of post-discharge infections. Risk factors for post-discharge MRSA include colonization, dependent ambulatory status, duration of hospitalization > 5 days, discharge to a long-term care facility, presence of a central venous catheter (CVC), presence of a non-CVC invasive device, a chronic wound in the post-discharge period, hemodialysis, systemic corticosteroids, and receiving anti-MRSA antimicrobial agents. Potential approaches to control include prevention of incident colonization during hospital stay, removal of nonessential CVCs and other devices, good wound debridement and care, and antimicrobial stewardship. Hand hygiene and environmental cleaning are horizontal measures that are also recommended. Decolonization may be useful in selected cases.
- Conclusion: Post-discharge MRSA infections are an important and underestimated source of morbidity and mortality. The future research agenda should include identification of post-discharge patients who are most likely to benefit from decolonization strategies, and testing those strategies.
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of morbidity and mortality due to infections of the bloodstream, lung, surgical sites, bone, and skin and soft tissues. The mortality associated with S. aureus bloodstream infections is 14% to 45% [1–4]. A bloodstream infection caused by MRSA is associated with a twofold increased mortality as compared to one caused by methicillin-sensitive S. aureus [5]. MRSA pneumonia carries a mortality of 8%, which increases to 39% when bacteremia is also present [6]. S. aureus bloodstream infection also carries a high risk of functional disability, with 65% of patients in a recent series requiring nursing home care in the recovery period [7]. In 2011 there were more than 11,000 deaths due to invasive MRSA infection in the United States [8]. Clearly S. aureus, and particularly MRSA, is a pathogen of major clinical significance.
Methicillin resistance was described in 1961, soon after methicillin became available in the 1950s. Prevalence of MRSA remained low until the 1980s, when it rapidly increased in health care settings. The predominant health care–associated strain in the United States is USA100, a member of clonal complex 5. Community-acquired MRSA infection has garnered much attention since it was recognized in 1996 [9]. The predominant community-associated strain has been USA300, a member of clonal complex 8 [10]. Following its emergence in the community, USA300 became a significant health care–associated pathogen as well [11]. The larger share of MRSA disease remains health care–associated [8]. The most recent data from the Center for Disease Control and Prevention Active Bacterial Core Surveillance system indicate that 77.6% of invasive MRSA infection is health care–associated, resulting in 9127 deaths in 2011 [8].
This article reviews the published literature on MRSA infections among patients recently discharged from hospital, with a focus on possible prevention measures.
MRSA Epidemiologic Categories
Epidemiologic investigations of MRSA categorize infections according to the presumed acquisition site, ie, in the community or in a health care setting. Older literature refers to nosocomial MRSA infection, which is now commonly referred to as hospital-onset health care–associated (HO-HCA) MRSA. A common definition of HO-HCA MRSA infection is an infection with the first positive culture on hospital day 4 or later [12]. Community-onset health care–associated MRSA (CO-HCA MRSA) is defined as infection that is diagnosed in the outpatient setting, or prior to day 4 of hospitalization, in a patient with recent health care exposure, eg, hospitalization within the past year, hemodialysis, surgery, or presence of a central venous catheter at time of presentation to the hospital [12]. Community-associated MRSA (CA-MSRSA) is infection in patients who do not meet criteria for either type of health care associated MRSA. Post-discharge MRSA infections would be included in the CO-HCA MRSA group.
Infection Control Programs
Classic infection control programs, developed in the 1960s, focused on infections that presented more than 48 to 72 hours after admission and prior to discharge from hospital. In that era, the average length of hospital stay was 1 week or more, and there was sufficient time for health care–associated infections to become clinically apparent. In recent years, length of stay has progressively shortened [13]. As hospital stays shortened, the risk that an infection caused by a health care–acquired pathogen would be identified after discharge grew. More recent studies have documented that the majority of HO-HCA infections become apparent after the index hospitalization [8,14].
Data from the Active Bacterial Core Surveillance System quantify the burden of CO-HCA MRSA disease at a national level [8,14]. However, it is not readily detected by many hospital infection surveillance programs. Avery et al studied a database constructed with California state mandated reports of MRSA infection and identified cases with MRSA present on admission. They then searched for a previous admission, within 30 days. If a prior admission was identified, the MRSA case was assigned to the hospital that had recently discharged the patient. Using this approach, they found that the incidence of health care–associated MRSA infection increased from 12.2 cases/10,000 admissions when traditional surveillance methods were used to 35.7/10,000 admissions using the revised method of assignment of health care exposure [15]. These data suggest that post-discharge MRSA disease is underappreciated by hospital infection control programs.
Lessons from Hospital-Onset MRSA
The morbidity and mortality associated with MRSA have led to the development of vigorous infection control programs to reduce the risk of health care–associated MRSA infection [16–18]. Vertical infection control strategies, ie, those focused on MRSA specifically, have included active screening for colonization, and nursing colonized patients in contact precautions. Since colonization is the antecedent to infection in most cases, prevention of transmission of MRSA from patient to patient should prevent most infections. There is ample evidence that colonized patients contaminate their immediate environment with MRSA, creating a reservoir of resistant pathogens that can be transmitted to other patients on the hands and clothing of health care workers [19,20]. Quasi-experimental studies of active screening and isolation strategies have shown decreases in MRSA transmission and infection following implementation [18]. The only randomized comparative trial of active screening and isolation versus usual care did not demonstrate benefit, possibly due to delays in lab confirmation of colonization status [21]. Horizontal infection control strategies are applied to all patients, regardless of colonization with resistant pathogens, in an attempt to decrease health care–associated infections with all pathogens. Examples of horizontal strategies are hand hygiene, environmental cleaning, and the prevention bundles for central line–associated bloodstream infection.
The Burden of Community-Onset MRSA
CO-HCA MRSA represents 60% of the burden of invasive MRSA infection [8]. While this category includes cases that have not been hospitalized, eg, patients on hemodialysis, post-discharge MRSA infection accounts for the majority of cases [15]. Recent data indicate that the incidence of HO-HCA MRSA decreased 54.2% between 2005 and 2011 [8]. This decrease in HO-HCA MRSA infection occurred concurrently with widespread implementation of vigorous horizontal infection control measures, such as bundled prevention strategies for central line–associated bloodstream infection and ventilator-associated pneumonia. The decline in CO-HCA MRSA infection has been much less steep, at 27.7%. The majority of the CO-HCA infections are in post-discharge patients. Furthermore, the incidence of CO-HCA MRSA infection may be underestimated [15].
Post-Discharge MRSA Colonization and Infection
Hospital-associated MRSA infection is reportable in many jurisdictions, but post-discharge MRSA infection is not a specific reportable condition, limiting the available surveillance data. Avery et al [15] studied ICD-9 code data for all hospitals in Orange County, California, and found that 23.5/10,000 hospital admissions were associated with a post-discharge MRSA infection. This nearly tripled the incidence of health care–associated MRSA infection, compared to surveillance that included only hospital-onset cases. Future research should refine these observations, as ICD-9 code data correlate imperfectly with chart reviews and have not yet been well validated for MRSA research.
The CDC estimated that in 2011 there were 48,353 CO-HCA MRSA infections resulting in 10,934 deaths. This estimate is derived from study of the Active Bacterial Core surveillance sample [8]. In that sample, 79% of CO-HCA MRSA infections occurred in patients hospitalized within the last year. Thus, we can estimate that there were 34,249 post-discharge MRSA infections resulting in 8638 deaths in the United States in 2011.
MRSA colonization is the antecedent to infection in the majority of cases [22]. Thus we can assess the health care burden of post-discharge MRSA by analyzing colonization as well as infection. Furthermore, the risk of MRSA colonization of household members can be addressed. Lucet et al evaluated hospital inpatients preparing for discharge to a home health care setting, and found that 12.7% of them were colonized with MRSA at the time of discharge, and 45% of them remained colonized for more than a year [23]. Patients who regained independence in activities of daily living were more likely to become free of MRSA colonization. The study provided no data on the risk of MRSA infection in the colonized patients. 19.1% of household contacts became colonized with MRSA, demonstrating that the burden of MRSA extends beyond the index patient. None of the colonized household contacts developed MRSA infection during the study period.
Risk Factors for Post-Discharge MRSA
Case control studies of patients with post-discharge invasive MRSA have shed light on risk factors for infection. While many risk factors are not modifiable, these studies may provide a road map to development of prevention strategies for the post-discharge setting. A study of hospitals in New York that participated in the Active Bacterial Core surveillance system identified a statistically significant increased risk of MRSA invasive infection among patients with several factors associated with physical disability, including a physical therapy evaluation, dependent ambulatory status, duration of hospitalization > 5 days, and discharge to a long-term care facility. Additional risk factors identified in the bivariate analysis were presence of a central venous catheter, hemodialysis, systemic corticosteroids, and receiving anti-MRSA antimicrobial agents. When subjected to multivariate analysis, however, the most significant and potent risk factor was a previous positive MRSA clinical culture (matched odds ratio 23, P < 0.001). Other significant risk factors in the multivariate analysis were hemodialysis, presence of a central venous catheter in the outpatient setting, and a visit to the emergency department [24]. A second, larger, multistate study also based on data from the Active Bacterial Core surveillance system showed that 5 risk factors were significantly associated with post-discharge invasive MRSA infection: (1) MRSA colonization, (2) a central venous catheter (CVC) present at discharge, (3) presence of a non-CVC invasive device, (4) a chronic wound in the post-discharge period, and (5) discharge to a nursing home. MRSA colonization was associated with a 7.7-fold increased odds of invasive MRSA infection, a much greater increase than any of the other risk factors [25]. Based on these results, strategies to consider include enhanced infection measures for prevention of incident MRSA colonization in the inpatient setting, decolonization therapy for those who become colonized, removal of non-essential medical devices, including central venous catheters, excellent nursing care for essential devices and wounds, hand hygiene, environmental cleaning, and antimicrobial stewardship.
Development of Strategies to Decrease Post-Discharge MRSA
While the epidemiology of post-discharge health care–associated MRSA infections has become a topic of interest to researchers, approaches to control are in their infancy. Few of the approaches have been subjected to rigorous study in the post-discharge environment. Nevertheless, some low risk, common sense strategies may be considered. Furthermore, an outline of research objectives may be constructed.
Prevention of Colonization in the Inpatient Setting
Robust infection control measures must be implemented in inpatient settings to prevent incident MRSA colonization [16,17]. Key recommendations include surveillance and monitoring of MRSA infections, adherence to standard hand hygiene guidance, environmental cleanliness, and use of dedicated equipment for patients who are colonized or infected with MRSA. Active screening for asymptomatic MRSA carriage and isolation of carriers may be implemented if routine measures are not successful.
Decolonization
Despite the best infection control programs, some patients will be colonized with MRSA at the time of hospital discharge. As detailed above, MRSA colonization is a potent risk factor for infection in the post-discharge setting, as well as in hospital inpatients [22]. A logical approach to this would be to attempt to eradicate colonization. There are several strategies for decolonization therapy, which may be used alone or in combination, including nasal mupirocin, nasal povidone-iodine, systemic antistaphylococcal drugs alone or in combination with oral rifampin, chlorhexidine bathing, or bleach baths [26–29].
A preliminary step in approaching the idea of post-discharge decolonization therapy is to show that patients can be successfully decolonized. With those data in hand, randomized trials seeking to demonstrate a decrease in invasive MRSA infections can be planned. Decolonization using nasal mupirocin has an initial success rate of 60% to 100% in a variety of patient populations [30–35]. Poor adherence to the decolonization protocol may limit success in the outpatient setting. Patients are more likely to resolve their MRSA colonization spontaneously when they regain their general health and independence in activities of daily living [23]. Colonization of other household members may provide a reservoir of MRSA leading to recolonization of the index case. Treatment of the household members may be offered, to provide more durable maintenance of the decolonized state [35]. When chronically ill patients who have been decolonized are followed longitudinally, up to 39% become colonized again, most often with the same strain [30,31]. Attempts to maintain a MRSA-free state in nursing home residents using prolonged mupirocin therapy resulted in emergence of mupirocin resistance [31]. Thus decolonization can be achieved, but is difficult to maintain, especially in debilitated, chronically ill patients. Mupirocin resistance can occur, limiting success of decolonization therapies.
Successful decolonization has been proven to reduce the risk of MRSA infection in the perioperative, dialysis, and intensive care unit settings [33,36–38]. In dialysis patients the risk of S. aureus bloodstream infection, including MRSA, can be reduced 59% with the use of mupirocin decolonization of the nares, with or without treatment of dialysis access exit sites [37]. A placebo-controlled trial demonstrated that decolonization of the nares with mupirocin reduced surgical site infections with S. aureus. All S. aureus isolates in the study were methicillin-susceptible. A second randomized controlled trial of nasal mupirocin did not achieve a statistically significant decrease in S. aureus surgical site infections, but it showed that mupirocin decolonization therapy decreased nosocomial S. aureus infections among nasal carriers [33]. 99.2% of isolates in that study et al were methicillin-susceptible. Quasi-experimental studies have shown similar benefits for surgical patients who are colonized with MRSA [39–41]. A more recent randomized trial, in ICU patients, demonstrated decreased incidence of invasive infection in patients treated with nasal mupirocin and chlorhexidine baths [38]. The common themeof these studies is that they enrolled patients who had a short-term condition, eg, surgery or critical illness, placing them at high risk for invasive MRSA infection. This maximizes the potential benefit of decolonization and minimizes the risk of emergence of resistance. Furthermore, adherence to decolonization protocols is likely to be high in the perioperative and ICU settings. To extrapolate the ICU and perioperative data to the post-discharge setting would be imprudent.
In summary, decolonization may be a useful strategy to reduce invasive MRSA infection in post-discharge patients, but more data are needed for most patient populations. The evidence for decolonization therapy is strongest for dialysis patients, in whom implementation of routine decolonization of MRSA colonized nares is a useful intervention [37]. There are not yet clinical trials of decolonization therapy in patients at time of hospital discharge showing a reduction in invasive MRSA infection. Decolonization strategies have important drawbacks, including emergence of resistance to mupirocin, chlorhexidine, and systemic agents. Furthermore, there is a risk of hypersensitivity reactions, Clostridium difficile infection, and potential for negative impacts onthe normal microbiome. The potential for lesser efficacy in a chronically ill outpatient population must also be considered in the post-discharge setting. Randomized controlled trials with invasive infection outcomes should be performed prior to implementing routine decolonization therapy of hospital discharge patients.
Care of Invasive Devices
Discharge with a central venous catheter was associated with a 2.16-fold increased risk of invasive MRSA infection; other invasive devices were associated with a 3.03-fold increased risk [25]. Clinicians must carefully assess patients nearing discharge for any opportunity to remove invasive devices. Idle devices have been reported in inpatient settings [42] and could occur in other settings. Antimicrobial therapy is a common indication for an outpatient central venous catheter and can also be associated with increased risk of invasive MRSA infection [25,43]. Duration and route of administration of antimicrobial agents should be carefully considered, with an eye to switching to oral therapy whenever possible. When a central venous catheter must be utilized, it should be maintained as carefully as in the inpatient setting. Tools for reducing risk of catheter-associated bloodstream infection include keeping the site dry, scrubbing the hub whenever accessing the catheter, aseptic techniques for dressing changes, and chlorhexidine sponges at the insertion site [44,45]. Reporting of central line–associated bloodstream infection rates by home care agencies is an important quality measure.
Wound Care
The presence of a chronic wound in the post-discharge period is associated with a 4.41-fold increased risk of invasive MRSA infection [25]. Although randomized controlled trials are lacking, it is prudent to ensure that wounds are fully debrided to remove devitalized tissue that can be fertile ground for a MRSA infection. The burden of organisms on a chronic wound is often very large, creating high risk of resistance when exposed to antimicrobial agents. Decolonization therapy is not likely to meet with durable success in such cases and should probably be avoided, except in special circumstances, eg, in preparation for cardiothoracic surgery.
Infection Control in Nursing Home Settings
In the Active Bacterial Core cohort, discharge to a nursing home was associated with a 2.1- to 2.65-fold increased risk of invasive MRSA infection [24,25]. It is notable that the authors controlled for the Charlson comorbidity index, suggesting that nursing home care is more than a marker for comorbidity [25]. The tension between the demands of careful infection control and the home-like setting that is desirable for long-term care creates challenges in the prevention of invasive MRSA infection. Nevertheless, careful management of invasive devices and wounds and antimicrobial stewardship are strategies that may reduce the risk of invasive MRSA infection in long-term care settings. Contact precautions for colonized nursing home residents are recommended only during an outbreak [46]. Staff should be trained in proper application of standard precautions, including use of gowns and gloves when handling body fluids. A study of an aggressive program of screening, decolonization with nasal mupirocin and chlorhexidine bathing, enhanced hand hygiene and environmental cleaning demonstrated a significant reduction in MRSA colonization [47]. An increase in mupirocin resistance during the study led to a switch to retapamulin for nasal application. The Association of Practitioners of Infection Control has issued guidance for MRSA prevention in long-term care facilities [48]. The guidance focuses on surveillance for MRSA infection, performing a MRSA risk assessment, hand hygiene, and environmental cleaning.
Antimicrobial Stewardship
Antimicrobial therapy, especially with fluoroquinolones and third- or fourth-generation cephalosporins, is associated with increased risk of MRSA colonization and infection [43,49,50]. Implementation of an antimicrobial stewardship program, coupled with infection control measures, in a region of Scotland resulted in decreased incidence of MRSA infections among hospital inpatients and in the surrounding community [51]. Thus a robust antimicrobial stewardship program is likely to reduce post-discharge MRSA infections.
Role of Hand Hygiene
The importance of hand hygiene in the prevention of infection has been observed for nearly 2 centuries [52]. Multiple quasi-experimental studies have demonstrated a decreased infection rate when hand hygiene practices for health care workers were introduced or strengthened. A randomized trial in a newborn nursery documented a decrease in transmission of S. aureus when nurses washed their hands after handling a colonized infant [53]. In addition to health care providers, patient hand hygiene can reduce health care–associated infections [54]. Traditional handwashing with soap and water will be familiar to most patients and families. Waterless hand hygiene, typically using alcohol-based hand rubs, is more efficacious and convenient for cleaning hands that are not visibly soiled [52]. If products containing emollients are used, it can also reduce skin drying and cracking. Patients and families should be taught to wash their hands before and after manipulating any medical devices and caring for wounds. Education of patients and family members on the techniques and importance of hand hygiene during hospitalization and at the time of discharge is a simple, low-cost strategy to reduce post-discharge MRSA infections. Teaching can be incorporated into the daily care of patients by nursing and medical staff, both verbally and by example. As a horizontal infection control measure, hand hygiene education has the additional benefit of reducing infections due to all pathogens.
Role of Environmental Cleaning in the Home Setting
Multiple studies have found that the immediate environment of patients who are colonized or infected with MRSA is contaminated with the organism, with greater organism burdens associated with infected patients compared to those who are only colonized [55–59]. Greater environmental contamination is observed when MRSA is present in the urine or wounds of patients [59]. This can lead to transmission of MRSA to family members [23,60,61]. Risk factors for transmission include participation in the care of the patient, older age, and being the partner of the case patient. For the patient, there can be transmission to uninfected body sites and a cycle of recolonization and re-infection. Successful decolonization strategies have included frequent laundering of bedclothes and towels, as well as screening and decolonization of family members. While these strategies may succeed in decolonization, there is no consensus on efficacy in preventing infection in patients or family members. More research in this area is needed, particularly for decolonization strategies, which carry risk of resistance. Attention to cleanliness in the home is a basic hygiene measure that can be recommended.
Conclusion
Post-discharge MRSA infections are an important and underestimated source of morbidity and mortality. Strategies for prevention include infection control measures to prevent incident colonization during hospitalization, removal of any nonessential invasive devices, nursing care for essential devices, wound care, avoiding nonessential antimicrobial therapy, hand hygiene for patients and caregivers, and cleaning of the home environment. Decolonization therapies currently play a limited role, particularly in outbreak situations. The future research agenda should include identification of post-discharge patients who are most likely to benefit from decolonization strategies, and testing those strategies.
Corresponding author: Mary Bessesen, MD, InfectiousDiseases (111L), 1055 Clermont St., Denver, CO 80220, Mary.Bessesen@ucdenver.edu.
1. Chen SY, Wang JT, Chen TH et al. Impact of traditional hospital strain of methicillin-resistant Staphylococcus aureus (MRSA) and community strain of MRSA on mortality in patients with community-onset S aureus bacteremia. Medicine 2010;89:285–94.
2. Lahey T, Shah R, Gittzus J,et al. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine 2009;88:263–7.
3. Chang FY, MacDonald BB, Peacock JE Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine 2003;82:322–32.
4. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002;162:2229–35.
5. Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53–9.
6. Schreiber MP, Chan CM, Shorr AF. Bacteremia in Staphylococcus aureus pneumonia: outcomes and epidemiology. J Crit Care 2011;26:395–401.
7. Malani PN, Rana MM, Banerjee M, Bradley SF. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc 2008;56:1485–9.
8. Dantes RM, Mu YP, Belflower RR, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013;173:1970–8.
9. Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus - Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep 1999;48:707–10.
10. Diep BA, Carleton HA, Chang RF, et al. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis 2006;193:1495–503.
11. Jenkins TC, McCollister BD, Sharma R, et al. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect Control Hosp Epidemiol 2009;30:233–41.
12. Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005-2008. JAMA 2010;304:641–8.
13. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA 2010;303:2141–7.
14. Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis 2006;42:389–91.
15. Avery TR, Kleinman KP, Klompas M, et al. Inclusion of 30-day postdischarge detection triples the incidence of hospital-onset methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2012;33:114–21.
16. Calfee DP, Salgado CD, Classen D, et al. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect Control Hosp Epidemiol 2008;29:Suppl 80.
17. Yokoe DS, Anderson DJ, Berenholtz SM, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 2014;35:967–77.
18. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
19. Stiefel U, Cadnum JL, Eckstein BC, et al. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect Control Hosp Epidemiol 2011;32:185–7.
20. Chang S, Sethi AK, Eckstein BC, et al. Skin and environmental contamination with methicillin-resistant Staphylococcus aureus among carriers identified clinically versus through active surveillance. Clin Infect Dis 2009;48:1423–8.
21. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011;364:1407–18.
22. Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
23. Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med 2009;169:1372–8.
24. Duffy J, Dumyati G, Bulens S, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control 2013;41:782–6.
25. Epstein L, Mu Y, Belflower R, et al. Risk factors for invasive methicillin-resistant Staphylococcus aureus infection after recent discharge from an acute-care hospitalization, 2011-2013. Clin Infect Dis 2016;62:45–52.
26. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 2007;44:178–85.
27. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol 2008;29:510–6.
28. Anderson MJ, David ML, Scholz M, et al. Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares. Antimicrob Agents Chemother 2015;59:2765–73.
29. Strausbaugh LJ, Jacobson C, Sewell DL, et al. Antimicrobial therapy for methicillin-resistant Staphylococcus aureus colonization in residents and staff of a Veterans Affairs nursing home care unit. Infect Control Hosp Epidemiol 1992;13:151–9.
30. Mody L, Kauffman CA, McNeil SA, et al. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:1467–74.
31. Kauffman CA, Terpenning MS, He X, et al. Attempts to eradicate methicillin-resistant Staphylococcus aureus from a long-term-care facility with the use of mupirocin ointment. Am J Med 1993;94:371–8.
32. Cederna JE, Terpenning MS, Ensberg M, et al. Staphylococcus aureus nasal colonization in a nursing home: eradication with mupirocin. Infect Control Hosp Epidemiol 1990;11:13–6.
33. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002;346:1871–7.
34. Kohler P, Bregenzer-Witteck A, Rettenmund G, et al. MRSA decolonization: success rate, risk factors for failure and optimal duration of follow-up. Infection 2013;41:33–40.
35. Ammerlaan HS, Kluytmans JA, Berkhout H, et al. Eradication of carriage with methicillin-resistant Staphylococcus aureus: effectiveness of a national guideline. J Antimicrob Chemother 2011;66:2409–17.
36. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17.
37. Nair R, Perencevich EN, Blevins AE, et al. Clinical effectiveness of mupirocin for preventing Staphylococcus aureus infections in nonsurgical settings: a meta-analysis. Clin Infect Dis 2016;62:618–30.
38. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent icu infection. N Engl J Med 2013;368:2255–65.
39. Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–71.
40. Walsh EE, Greene L, Kirshner R. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med 2011;171:68–73.
41. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am 2010;92:1820–6.
42. Lederle FA, Parenti CM, Berskow LC, Ellingson KJ. The idle intravenous catheter. Ann Intern Med 1992;116:737–8.
43. Avery CM, Ameerally P, Castling B, Swann RA. Infection of surgical wounds in the maxillofacial region and free flap donor sites with methicillin-resistant Staphylococcus aureus. Br J Oral Maxillofac Surg 2006;44:217–21.
44. Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 2009;301:1231–41.
45. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:753–71.
46. Manzur A, Gudiol F. Methicillin-resistant Staphylococcus aureus in long-term-care facilities. Clin Microbiol Infect 2009;15 Suppl 7:26–30.
47. Schora DM, Boehm S, Das S, et al. Impact of Detection, Education, Research and Decolonization without Isolation in Long-term care (DERAIL) on methicillin-resistant Staphylococcus aureus colonization and transmission at 3 long-term care facilities. Am J Infect Control 2014;42(10 Suppl):S269–73.
48. Rebmann T, Aureden K, Association for Professionals in Infection Control and Epidemiology. Preventing methicillin-resistant Staphylococcus aureus transmission in long-term care facilities: an executive summary of the APIC Elimination Guide. Am J Infect Control 2011;39:235–8.
49. Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med 2014;174:1660–7.
50. Couderc C, Jolivet S, Thiebaut AC, et al. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis 2014;59:206–15.
51. Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis 2015;15:1438–49.
52. Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep 2002;51(RR-16):1–48.
53. Mortimer EA Jr, Lipsitz PJ, Wolinsky E, et al. Transmission of staphylococci between newborns. Importance of the hands to personnel. Am J Dis Child 1962;104:289–95.
54. Gagne D, Bedard G, Maziade PJ. Systematic patients’ hand disinfection: impact on meticillin-resistant Staphylococcus aureus infection rates in a community hospital. J Hosp Infect 2010;75:269–72.
55. Knelson LP, Williams DA, Gergen MF, et al. A comparison of environmental contamination by patients infected or colonized with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a multicenter study. Infect Control Hosp Epidemiol 2014;35:872–5.
56. Murphy CR, Eells SJ, Quan V, et al. Methicillin-resistant Staphylococcus aureus burden in nursing homes associated with environmental contamination of common areas. J Am Geriatr Soc 2012;60:1012–8.
57. Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med 2011;171:491–4.
58. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
59. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
60. Mollema FP, Richardus JH, Behrendt M, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 2010;48:202–7.
61. Calfee DP, Durbin LJ, Germanson TP, et al. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol 2003;24:422–6.
From the Division of Adult Infectious Diseases, University of Colorado Denver, Aurora, CO, and the Department of Veterans Affairs, Eastern Colorado Healthcare System, Denver, CO.
Abstract
- Objective: To review the published literature on methicillin-resistant Staphylococcus aureus (MRSA) infections among patients recently discharged from hospital, with a focus on possible prevention measures.
- Methods: Literature review.
- Results: MRSA is a major cause of post-discharge infections. Risk factors for post-discharge MRSA include colonization, dependent ambulatory status, duration of hospitalization > 5 days, discharge to a long-term care facility, presence of a central venous catheter (CVC), presence of a non-CVC invasive device, a chronic wound in the post-discharge period, hemodialysis, systemic corticosteroids, and receiving anti-MRSA antimicrobial agents. Potential approaches to control include prevention of incident colonization during hospital stay, removal of nonessential CVCs and other devices, good wound debridement and care, and antimicrobial stewardship. Hand hygiene and environmental cleaning are horizontal measures that are also recommended. Decolonization may be useful in selected cases.
- Conclusion: Post-discharge MRSA infections are an important and underestimated source of morbidity and mortality. The future research agenda should include identification of post-discharge patients who are most likely to benefit from decolonization strategies, and testing those strategies.
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of morbidity and mortality due to infections of the bloodstream, lung, surgical sites, bone, and skin and soft tissues. The mortality associated with S. aureus bloodstream infections is 14% to 45% [1–4]. A bloodstream infection caused by MRSA is associated with a twofold increased mortality as compared to one caused by methicillin-sensitive S. aureus [5]. MRSA pneumonia carries a mortality of 8%, which increases to 39% when bacteremia is also present [6]. S. aureus bloodstream infection also carries a high risk of functional disability, with 65% of patients in a recent series requiring nursing home care in the recovery period [7]. In 2011 there were more than 11,000 deaths due to invasive MRSA infection in the United States [8]. Clearly S. aureus, and particularly MRSA, is a pathogen of major clinical significance.
Methicillin resistance was described in 1961, soon after methicillin became available in the 1950s. Prevalence of MRSA remained low until the 1980s, when it rapidly increased in health care settings. The predominant health care–associated strain in the United States is USA100, a member of clonal complex 5. Community-acquired MRSA infection has garnered much attention since it was recognized in 1996 [9]. The predominant community-associated strain has been USA300, a member of clonal complex 8 [10]. Following its emergence in the community, USA300 became a significant health care–associated pathogen as well [11]. The larger share of MRSA disease remains health care–associated [8]. The most recent data from the Center for Disease Control and Prevention Active Bacterial Core Surveillance system indicate that 77.6% of invasive MRSA infection is health care–associated, resulting in 9127 deaths in 2011 [8].
This article reviews the published literature on MRSA infections among patients recently discharged from hospital, with a focus on possible prevention measures.
MRSA Epidemiologic Categories
Epidemiologic investigations of MRSA categorize infections according to the presumed acquisition site, ie, in the community or in a health care setting. Older literature refers to nosocomial MRSA infection, which is now commonly referred to as hospital-onset health care–associated (HO-HCA) MRSA. A common definition of HO-HCA MRSA infection is an infection with the first positive culture on hospital day 4 or later [12]. Community-onset health care–associated MRSA (CO-HCA MRSA) is defined as infection that is diagnosed in the outpatient setting, or prior to day 4 of hospitalization, in a patient with recent health care exposure, eg, hospitalization within the past year, hemodialysis, surgery, or presence of a central venous catheter at time of presentation to the hospital [12]. Community-associated MRSA (CA-MSRSA) is infection in patients who do not meet criteria for either type of health care associated MRSA. Post-discharge MRSA infections would be included in the CO-HCA MRSA group.
Infection Control Programs
Classic infection control programs, developed in the 1960s, focused on infections that presented more than 48 to 72 hours after admission and prior to discharge from hospital. In that era, the average length of hospital stay was 1 week or more, and there was sufficient time for health care–associated infections to become clinically apparent. In recent years, length of stay has progressively shortened [13]. As hospital stays shortened, the risk that an infection caused by a health care–acquired pathogen would be identified after discharge grew. More recent studies have documented that the majority of HO-HCA infections become apparent after the index hospitalization [8,14].
Data from the Active Bacterial Core Surveillance System quantify the burden of CO-HCA MRSA disease at a national level [8,14]. However, it is not readily detected by many hospital infection surveillance programs. Avery et al studied a database constructed with California state mandated reports of MRSA infection and identified cases with MRSA present on admission. They then searched for a previous admission, within 30 days. If a prior admission was identified, the MRSA case was assigned to the hospital that had recently discharged the patient. Using this approach, they found that the incidence of health care–associated MRSA infection increased from 12.2 cases/10,000 admissions when traditional surveillance methods were used to 35.7/10,000 admissions using the revised method of assignment of health care exposure [15]. These data suggest that post-discharge MRSA disease is underappreciated by hospital infection control programs.
Lessons from Hospital-Onset MRSA
The morbidity and mortality associated with MRSA have led to the development of vigorous infection control programs to reduce the risk of health care–associated MRSA infection [16–18]. Vertical infection control strategies, ie, those focused on MRSA specifically, have included active screening for colonization, and nursing colonized patients in contact precautions. Since colonization is the antecedent to infection in most cases, prevention of transmission of MRSA from patient to patient should prevent most infections. There is ample evidence that colonized patients contaminate their immediate environment with MRSA, creating a reservoir of resistant pathogens that can be transmitted to other patients on the hands and clothing of health care workers [19,20]. Quasi-experimental studies of active screening and isolation strategies have shown decreases in MRSA transmission and infection following implementation [18]. The only randomized comparative trial of active screening and isolation versus usual care did not demonstrate benefit, possibly due to delays in lab confirmation of colonization status [21]. Horizontal infection control strategies are applied to all patients, regardless of colonization with resistant pathogens, in an attempt to decrease health care–associated infections with all pathogens. Examples of horizontal strategies are hand hygiene, environmental cleaning, and the prevention bundles for central line–associated bloodstream infection.
The Burden of Community-Onset MRSA
CO-HCA MRSA represents 60% of the burden of invasive MRSA infection [8]. While this category includes cases that have not been hospitalized, eg, patients on hemodialysis, post-discharge MRSA infection accounts for the majority of cases [15]. Recent data indicate that the incidence of HO-HCA MRSA decreased 54.2% between 2005 and 2011 [8]. This decrease in HO-HCA MRSA infection occurred concurrently with widespread implementation of vigorous horizontal infection control measures, such as bundled prevention strategies for central line–associated bloodstream infection and ventilator-associated pneumonia. The decline in CO-HCA MRSA infection has been much less steep, at 27.7%. The majority of the CO-HCA infections are in post-discharge patients. Furthermore, the incidence of CO-HCA MRSA infection may be underestimated [15].
Post-Discharge MRSA Colonization and Infection
Hospital-associated MRSA infection is reportable in many jurisdictions, but post-discharge MRSA infection is not a specific reportable condition, limiting the available surveillance data. Avery et al [15] studied ICD-9 code data for all hospitals in Orange County, California, and found that 23.5/10,000 hospital admissions were associated with a post-discharge MRSA infection. This nearly tripled the incidence of health care–associated MRSA infection, compared to surveillance that included only hospital-onset cases. Future research should refine these observations, as ICD-9 code data correlate imperfectly with chart reviews and have not yet been well validated for MRSA research.
The CDC estimated that in 2011 there were 48,353 CO-HCA MRSA infections resulting in 10,934 deaths. This estimate is derived from study of the Active Bacterial Core surveillance sample [8]. In that sample, 79% of CO-HCA MRSA infections occurred in patients hospitalized within the last year. Thus, we can estimate that there were 34,249 post-discharge MRSA infections resulting in 8638 deaths in the United States in 2011.
MRSA colonization is the antecedent to infection in the majority of cases [22]. Thus we can assess the health care burden of post-discharge MRSA by analyzing colonization as well as infection. Furthermore, the risk of MRSA colonization of household members can be addressed. Lucet et al evaluated hospital inpatients preparing for discharge to a home health care setting, and found that 12.7% of them were colonized with MRSA at the time of discharge, and 45% of them remained colonized for more than a year [23]. Patients who regained independence in activities of daily living were more likely to become free of MRSA colonization. The study provided no data on the risk of MRSA infection in the colonized patients. 19.1% of household contacts became colonized with MRSA, demonstrating that the burden of MRSA extends beyond the index patient. None of the colonized household contacts developed MRSA infection during the study period.
Risk Factors for Post-Discharge MRSA
Case control studies of patients with post-discharge invasive MRSA have shed light on risk factors for infection. While many risk factors are not modifiable, these studies may provide a road map to development of prevention strategies for the post-discharge setting. A study of hospitals in New York that participated in the Active Bacterial Core surveillance system identified a statistically significant increased risk of MRSA invasive infection among patients with several factors associated with physical disability, including a physical therapy evaluation, dependent ambulatory status, duration of hospitalization > 5 days, and discharge to a long-term care facility. Additional risk factors identified in the bivariate analysis were presence of a central venous catheter, hemodialysis, systemic corticosteroids, and receiving anti-MRSA antimicrobial agents. When subjected to multivariate analysis, however, the most significant and potent risk factor was a previous positive MRSA clinical culture (matched odds ratio 23, P < 0.001). Other significant risk factors in the multivariate analysis were hemodialysis, presence of a central venous catheter in the outpatient setting, and a visit to the emergency department [24]. A second, larger, multistate study also based on data from the Active Bacterial Core surveillance system showed that 5 risk factors were significantly associated with post-discharge invasive MRSA infection: (1) MRSA colonization, (2) a central venous catheter (CVC) present at discharge, (3) presence of a non-CVC invasive device, (4) a chronic wound in the post-discharge period, and (5) discharge to a nursing home. MRSA colonization was associated with a 7.7-fold increased odds of invasive MRSA infection, a much greater increase than any of the other risk factors [25]. Based on these results, strategies to consider include enhanced infection measures for prevention of incident MRSA colonization in the inpatient setting, decolonization therapy for those who become colonized, removal of non-essential medical devices, including central venous catheters, excellent nursing care for essential devices and wounds, hand hygiene, environmental cleaning, and antimicrobial stewardship.
Development of Strategies to Decrease Post-Discharge MRSA
While the epidemiology of post-discharge health care–associated MRSA infections has become a topic of interest to researchers, approaches to control are in their infancy. Few of the approaches have been subjected to rigorous study in the post-discharge environment. Nevertheless, some low risk, common sense strategies may be considered. Furthermore, an outline of research objectives may be constructed.
Prevention of Colonization in the Inpatient Setting
Robust infection control measures must be implemented in inpatient settings to prevent incident MRSA colonization [16,17]. Key recommendations include surveillance and monitoring of MRSA infections, adherence to standard hand hygiene guidance, environmental cleanliness, and use of dedicated equipment for patients who are colonized or infected with MRSA. Active screening for asymptomatic MRSA carriage and isolation of carriers may be implemented if routine measures are not successful.
Decolonization
Despite the best infection control programs, some patients will be colonized with MRSA at the time of hospital discharge. As detailed above, MRSA colonization is a potent risk factor for infection in the post-discharge setting, as well as in hospital inpatients [22]. A logical approach to this would be to attempt to eradicate colonization. There are several strategies for decolonization therapy, which may be used alone or in combination, including nasal mupirocin, nasal povidone-iodine, systemic antistaphylococcal drugs alone or in combination with oral rifampin, chlorhexidine bathing, or bleach baths [26–29].
A preliminary step in approaching the idea of post-discharge decolonization therapy is to show that patients can be successfully decolonized. With those data in hand, randomized trials seeking to demonstrate a decrease in invasive MRSA infections can be planned. Decolonization using nasal mupirocin has an initial success rate of 60% to 100% in a variety of patient populations [30–35]. Poor adherence to the decolonization protocol may limit success in the outpatient setting. Patients are more likely to resolve their MRSA colonization spontaneously when they regain their general health and independence in activities of daily living [23]. Colonization of other household members may provide a reservoir of MRSA leading to recolonization of the index case. Treatment of the household members may be offered, to provide more durable maintenance of the decolonized state [35]. When chronically ill patients who have been decolonized are followed longitudinally, up to 39% become colonized again, most often with the same strain [30,31]. Attempts to maintain a MRSA-free state in nursing home residents using prolonged mupirocin therapy resulted in emergence of mupirocin resistance [31]. Thus decolonization can be achieved, but is difficult to maintain, especially in debilitated, chronically ill patients. Mupirocin resistance can occur, limiting success of decolonization therapies.
Successful decolonization has been proven to reduce the risk of MRSA infection in the perioperative, dialysis, and intensive care unit settings [33,36–38]. In dialysis patients the risk of S. aureus bloodstream infection, including MRSA, can be reduced 59% with the use of mupirocin decolonization of the nares, with or without treatment of dialysis access exit sites [37]. A placebo-controlled trial demonstrated that decolonization of the nares with mupirocin reduced surgical site infections with S. aureus. All S. aureus isolates in the study were methicillin-susceptible. A second randomized controlled trial of nasal mupirocin did not achieve a statistically significant decrease in S. aureus surgical site infections, but it showed that mupirocin decolonization therapy decreased nosocomial S. aureus infections among nasal carriers [33]. 99.2% of isolates in that study et al were methicillin-susceptible. Quasi-experimental studies have shown similar benefits for surgical patients who are colonized with MRSA [39–41]. A more recent randomized trial, in ICU patients, demonstrated decreased incidence of invasive infection in patients treated with nasal mupirocin and chlorhexidine baths [38]. The common themeof these studies is that they enrolled patients who had a short-term condition, eg, surgery or critical illness, placing them at high risk for invasive MRSA infection. This maximizes the potential benefit of decolonization and minimizes the risk of emergence of resistance. Furthermore, adherence to decolonization protocols is likely to be high in the perioperative and ICU settings. To extrapolate the ICU and perioperative data to the post-discharge setting would be imprudent.
In summary, decolonization may be a useful strategy to reduce invasive MRSA infection in post-discharge patients, but more data are needed for most patient populations. The evidence for decolonization therapy is strongest for dialysis patients, in whom implementation of routine decolonization of MRSA colonized nares is a useful intervention [37]. There are not yet clinical trials of decolonization therapy in patients at time of hospital discharge showing a reduction in invasive MRSA infection. Decolonization strategies have important drawbacks, including emergence of resistance to mupirocin, chlorhexidine, and systemic agents. Furthermore, there is a risk of hypersensitivity reactions, Clostridium difficile infection, and potential for negative impacts onthe normal microbiome. The potential for lesser efficacy in a chronically ill outpatient population must also be considered in the post-discharge setting. Randomized controlled trials with invasive infection outcomes should be performed prior to implementing routine decolonization therapy of hospital discharge patients.
Care of Invasive Devices
Discharge with a central venous catheter was associated with a 2.16-fold increased risk of invasive MRSA infection; other invasive devices were associated with a 3.03-fold increased risk [25]. Clinicians must carefully assess patients nearing discharge for any opportunity to remove invasive devices. Idle devices have been reported in inpatient settings [42] and could occur in other settings. Antimicrobial therapy is a common indication for an outpatient central venous catheter and can also be associated with increased risk of invasive MRSA infection [25,43]. Duration and route of administration of antimicrobial agents should be carefully considered, with an eye to switching to oral therapy whenever possible. When a central venous catheter must be utilized, it should be maintained as carefully as in the inpatient setting. Tools for reducing risk of catheter-associated bloodstream infection include keeping the site dry, scrubbing the hub whenever accessing the catheter, aseptic techniques for dressing changes, and chlorhexidine sponges at the insertion site [44,45]. Reporting of central line–associated bloodstream infection rates by home care agencies is an important quality measure.
Wound Care
The presence of a chronic wound in the post-discharge period is associated with a 4.41-fold increased risk of invasive MRSA infection [25]. Although randomized controlled trials are lacking, it is prudent to ensure that wounds are fully debrided to remove devitalized tissue that can be fertile ground for a MRSA infection. The burden of organisms on a chronic wound is often very large, creating high risk of resistance when exposed to antimicrobial agents. Decolonization therapy is not likely to meet with durable success in such cases and should probably be avoided, except in special circumstances, eg, in preparation for cardiothoracic surgery.
Infection Control in Nursing Home Settings
In the Active Bacterial Core cohort, discharge to a nursing home was associated with a 2.1- to 2.65-fold increased risk of invasive MRSA infection [24,25]. It is notable that the authors controlled for the Charlson comorbidity index, suggesting that nursing home care is more than a marker for comorbidity [25]. The tension between the demands of careful infection control and the home-like setting that is desirable for long-term care creates challenges in the prevention of invasive MRSA infection. Nevertheless, careful management of invasive devices and wounds and antimicrobial stewardship are strategies that may reduce the risk of invasive MRSA infection in long-term care settings. Contact precautions for colonized nursing home residents are recommended only during an outbreak [46]. Staff should be trained in proper application of standard precautions, including use of gowns and gloves when handling body fluids. A study of an aggressive program of screening, decolonization with nasal mupirocin and chlorhexidine bathing, enhanced hand hygiene and environmental cleaning demonstrated a significant reduction in MRSA colonization [47]. An increase in mupirocin resistance during the study led to a switch to retapamulin for nasal application. The Association of Practitioners of Infection Control has issued guidance for MRSA prevention in long-term care facilities [48]. The guidance focuses on surveillance for MRSA infection, performing a MRSA risk assessment, hand hygiene, and environmental cleaning.
Antimicrobial Stewardship
Antimicrobial therapy, especially with fluoroquinolones and third- or fourth-generation cephalosporins, is associated with increased risk of MRSA colonization and infection [43,49,50]. Implementation of an antimicrobial stewardship program, coupled with infection control measures, in a region of Scotland resulted in decreased incidence of MRSA infections among hospital inpatients and in the surrounding community [51]. Thus a robust antimicrobial stewardship program is likely to reduce post-discharge MRSA infections.
Role of Hand Hygiene
The importance of hand hygiene in the prevention of infection has been observed for nearly 2 centuries [52]. Multiple quasi-experimental studies have demonstrated a decreased infection rate when hand hygiene practices for health care workers were introduced or strengthened. A randomized trial in a newborn nursery documented a decrease in transmission of S. aureus when nurses washed their hands after handling a colonized infant [53]. In addition to health care providers, patient hand hygiene can reduce health care–associated infections [54]. Traditional handwashing with soap and water will be familiar to most patients and families. Waterless hand hygiene, typically using alcohol-based hand rubs, is more efficacious and convenient for cleaning hands that are not visibly soiled [52]. If products containing emollients are used, it can also reduce skin drying and cracking. Patients and families should be taught to wash their hands before and after manipulating any medical devices and caring for wounds. Education of patients and family members on the techniques and importance of hand hygiene during hospitalization and at the time of discharge is a simple, low-cost strategy to reduce post-discharge MRSA infections. Teaching can be incorporated into the daily care of patients by nursing and medical staff, both verbally and by example. As a horizontal infection control measure, hand hygiene education has the additional benefit of reducing infections due to all pathogens.
Role of Environmental Cleaning in the Home Setting
Multiple studies have found that the immediate environment of patients who are colonized or infected with MRSA is contaminated with the organism, with greater organism burdens associated with infected patients compared to those who are only colonized [55–59]. Greater environmental contamination is observed when MRSA is present in the urine or wounds of patients [59]. This can lead to transmission of MRSA to family members [23,60,61]. Risk factors for transmission include participation in the care of the patient, older age, and being the partner of the case patient. For the patient, there can be transmission to uninfected body sites and a cycle of recolonization and re-infection. Successful decolonization strategies have included frequent laundering of bedclothes and towels, as well as screening and decolonization of family members. While these strategies may succeed in decolonization, there is no consensus on efficacy in preventing infection in patients or family members. More research in this area is needed, particularly for decolonization strategies, which carry risk of resistance. Attention to cleanliness in the home is a basic hygiene measure that can be recommended.
Conclusion
Post-discharge MRSA infections are an important and underestimated source of morbidity and mortality. Strategies for prevention include infection control measures to prevent incident colonization during hospitalization, removal of any nonessential invasive devices, nursing care for essential devices, wound care, avoiding nonessential antimicrobial therapy, hand hygiene for patients and caregivers, and cleaning of the home environment. Decolonization therapies currently play a limited role, particularly in outbreak situations. The future research agenda should include identification of post-discharge patients who are most likely to benefit from decolonization strategies, and testing those strategies.
Corresponding author: Mary Bessesen, MD, InfectiousDiseases (111L), 1055 Clermont St., Denver, CO 80220, Mary.Bessesen@ucdenver.edu.
From the Division of Adult Infectious Diseases, University of Colorado Denver, Aurora, CO, and the Department of Veterans Affairs, Eastern Colorado Healthcare System, Denver, CO.
Abstract
- Objective: To review the published literature on methicillin-resistant Staphylococcus aureus (MRSA) infections among patients recently discharged from hospital, with a focus on possible prevention measures.
- Methods: Literature review.
- Results: MRSA is a major cause of post-discharge infections. Risk factors for post-discharge MRSA include colonization, dependent ambulatory status, duration of hospitalization > 5 days, discharge to a long-term care facility, presence of a central venous catheter (CVC), presence of a non-CVC invasive device, a chronic wound in the post-discharge period, hemodialysis, systemic corticosteroids, and receiving anti-MRSA antimicrobial agents. Potential approaches to control include prevention of incident colonization during hospital stay, removal of nonessential CVCs and other devices, good wound debridement and care, and antimicrobial stewardship. Hand hygiene and environmental cleaning are horizontal measures that are also recommended. Decolonization may be useful in selected cases.
- Conclusion: Post-discharge MRSA infections are an important and underestimated source of morbidity and mortality. The future research agenda should include identification of post-discharge patients who are most likely to benefit from decolonization strategies, and testing those strategies.
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of morbidity and mortality due to infections of the bloodstream, lung, surgical sites, bone, and skin and soft tissues. The mortality associated with S. aureus bloodstream infections is 14% to 45% [1–4]. A bloodstream infection caused by MRSA is associated with a twofold increased mortality as compared to one caused by methicillin-sensitive S. aureus [5]. MRSA pneumonia carries a mortality of 8%, which increases to 39% when bacteremia is also present [6]. S. aureus bloodstream infection also carries a high risk of functional disability, with 65% of patients in a recent series requiring nursing home care in the recovery period [7]. In 2011 there were more than 11,000 deaths due to invasive MRSA infection in the United States [8]. Clearly S. aureus, and particularly MRSA, is a pathogen of major clinical significance.
Methicillin resistance was described in 1961, soon after methicillin became available in the 1950s. Prevalence of MRSA remained low until the 1980s, when it rapidly increased in health care settings. The predominant health care–associated strain in the United States is USA100, a member of clonal complex 5. Community-acquired MRSA infection has garnered much attention since it was recognized in 1996 [9]. The predominant community-associated strain has been USA300, a member of clonal complex 8 [10]. Following its emergence in the community, USA300 became a significant health care–associated pathogen as well [11]. The larger share of MRSA disease remains health care–associated [8]. The most recent data from the Center for Disease Control and Prevention Active Bacterial Core Surveillance system indicate that 77.6% of invasive MRSA infection is health care–associated, resulting in 9127 deaths in 2011 [8].
This article reviews the published literature on MRSA infections among patients recently discharged from hospital, with a focus on possible prevention measures.
MRSA Epidemiologic Categories
Epidemiologic investigations of MRSA categorize infections according to the presumed acquisition site, ie, in the community or in a health care setting. Older literature refers to nosocomial MRSA infection, which is now commonly referred to as hospital-onset health care–associated (HO-HCA) MRSA. A common definition of HO-HCA MRSA infection is an infection with the first positive culture on hospital day 4 or later [12]. Community-onset health care–associated MRSA (CO-HCA MRSA) is defined as infection that is diagnosed in the outpatient setting, or prior to day 4 of hospitalization, in a patient with recent health care exposure, eg, hospitalization within the past year, hemodialysis, surgery, or presence of a central venous catheter at time of presentation to the hospital [12]. Community-associated MRSA (CA-MSRSA) is infection in patients who do not meet criteria for either type of health care associated MRSA. Post-discharge MRSA infections would be included in the CO-HCA MRSA group.
Infection Control Programs
Classic infection control programs, developed in the 1960s, focused on infections that presented more than 48 to 72 hours after admission and prior to discharge from hospital. In that era, the average length of hospital stay was 1 week or more, and there was sufficient time for health care–associated infections to become clinically apparent. In recent years, length of stay has progressively shortened [13]. As hospital stays shortened, the risk that an infection caused by a health care–acquired pathogen would be identified after discharge grew. More recent studies have documented that the majority of HO-HCA infections become apparent after the index hospitalization [8,14].
Data from the Active Bacterial Core Surveillance System quantify the burden of CO-HCA MRSA disease at a national level [8,14]. However, it is not readily detected by many hospital infection surveillance programs. Avery et al studied a database constructed with California state mandated reports of MRSA infection and identified cases with MRSA present on admission. They then searched for a previous admission, within 30 days. If a prior admission was identified, the MRSA case was assigned to the hospital that had recently discharged the patient. Using this approach, they found that the incidence of health care–associated MRSA infection increased from 12.2 cases/10,000 admissions when traditional surveillance methods were used to 35.7/10,000 admissions using the revised method of assignment of health care exposure [15]. These data suggest that post-discharge MRSA disease is underappreciated by hospital infection control programs.
Lessons from Hospital-Onset MRSA
The morbidity and mortality associated with MRSA have led to the development of vigorous infection control programs to reduce the risk of health care–associated MRSA infection [16–18]. Vertical infection control strategies, ie, those focused on MRSA specifically, have included active screening for colonization, and nursing colonized patients in contact precautions. Since colonization is the antecedent to infection in most cases, prevention of transmission of MRSA from patient to patient should prevent most infections. There is ample evidence that colonized patients contaminate their immediate environment with MRSA, creating a reservoir of resistant pathogens that can be transmitted to other patients on the hands and clothing of health care workers [19,20]. Quasi-experimental studies of active screening and isolation strategies have shown decreases in MRSA transmission and infection following implementation [18]. The only randomized comparative trial of active screening and isolation versus usual care did not demonstrate benefit, possibly due to delays in lab confirmation of colonization status [21]. Horizontal infection control strategies are applied to all patients, regardless of colonization with resistant pathogens, in an attempt to decrease health care–associated infections with all pathogens. Examples of horizontal strategies are hand hygiene, environmental cleaning, and the prevention bundles for central line–associated bloodstream infection.
The Burden of Community-Onset MRSA
CO-HCA MRSA represents 60% of the burden of invasive MRSA infection [8]. While this category includes cases that have not been hospitalized, eg, patients on hemodialysis, post-discharge MRSA infection accounts for the majority of cases [15]. Recent data indicate that the incidence of HO-HCA MRSA decreased 54.2% between 2005 and 2011 [8]. This decrease in HO-HCA MRSA infection occurred concurrently with widespread implementation of vigorous horizontal infection control measures, such as bundled prevention strategies for central line–associated bloodstream infection and ventilator-associated pneumonia. The decline in CO-HCA MRSA infection has been much less steep, at 27.7%. The majority of the CO-HCA infections are in post-discharge patients. Furthermore, the incidence of CO-HCA MRSA infection may be underestimated [15].
Post-Discharge MRSA Colonization and Infection
Hospital-associated MRSA infection is reportable in many jurisdictions, but post-discharge MRSA infection is not a specific reportable condition, limiting the available surveillance data. Avery et al [15] studied ICD-9 code data for all hospitals in Orange County, California, and found that 23.5/10,000 hospital admissions were associated with a post-discharge MRSA infection. This nearly tripled the incidence of health care–associated MRSA infection, compared to surveillance that included only hospital-onset cases. Future research should refine these observations, as ICD-9 code data correlate imperfectly with chart reviews and have not yet been well validated for MRSA research.
The CDC estimated that in 2011 there were 48,353 CO-HCA MRSA infections resulting in 10,934 deaths. This estimate is derived from study of the Active Bacterial Core surveillance sample [8]. In that sample, 79% of CO-HCA MRSA infections occurred in patients hospitalized within the last year. Thus, we can estimate that there were 34,249 post-discharge MRSA infections resulting in 8638 deaths in the United States in 2011.
MRSA colonization is the antecedent to infection in the majority of cases [22]. Thus we can assess the health care burden of post-discharge MRSA by analyzing colonization as well as infection. Furthermore, the risk of MRSA colonization of household members can be addressed. Lucet et al evaluated hospital inpatients preparing for discharge to a home health care setting, and found that 12.7% of them were colonized with MRSA at the time of discharge, and 45% of them remained colonized for more than a year [23]. Patients who regained independence in activities of daily living were more likely to become free of MRSA colonization. The study provided no data on the risk of MRSA infection in the colonized patients. 19.1% of household contacts became colonized with MRSA, demonstrating that the burden of MRSA extends beyond the index patient. None of the colonized household contacts developed MRSA infection during the study period.
Risk Factors for Post-Discharge MRSA
Case control studies of patients with post-discharge invasive MRSA have shed light on risk factors for infection. While many risk factors are not modifiable, these studies may provide a road map to development of prevention strategies for the post-discharge setting. A study of hospitals in New York that participated in the Active Bacterial Core surveillance system identified a statistically significant increased risk of MRSA invasive infection among patients with several factors associated with physical disability, including a physical therapy evaluation, dependent ambulatory status, duration of hospitalization > 5 days, and discharge to a long-term care facility. Additional risk factors identified in the bivariate analysis were presence of a central venous catheter, hemodialysis, systemic corticosteroids, and receiving anti-MRSA antimicrobial agents. When subjected to multivariate analysis, however, the most significant and potent risk factor was a previous positive MRSA clinical culture (matched odds ratio 23, P < 0.001). Other significant risk factors in the multivariate analysis were hemodialysis, presence of a central venous catheter in the outpatient setting, and a visit to the emergency department [24]. A second, larger, multistate study also based on data from the Active Bacterial Core surveillance system showed that 5 risk factors were significantly associated with post-discharge invasive MRSA infection: (1) MRSA colonization, (2) a central venous catheter (CVC) present at discharge, (3) presence of a non-CVC invasive device, (4) a chronic wound in the post-discharge period, and (5) discharge to a nursing home. MRSA colonization was associated with a 7.7-fold increased odds of invasive MRSA infection, a much greater increase than any of the other risk factors [25]. Based on these results, strategies to consider include enhanced infection measures for prevention of incident MRSA colonization in the inpatient setting, decolonization therapy for those who become colonized, removal of non-essential medical devices, including central venous catheters, excellent nursing care for essential devices and wounds, hand hygiene, environmental cleaning, and antimicrobial stewardship.
Development of Strategies to Decrease Post-Discharge MRSA
While the epidemiology of post-discharge health care–associated MRSA infections has become a topic of interest to researchers, approaches to control are in their infancy. Few of the approaches have been subjected to rigorous study in the post-discharge environment. Nevertheless, some low risk, common sense strategies may be considered. Furthermore, an outline of research objectives may be constructed.
Prevention of Colonization in the Inpatient Setting
Robust infection control measures must be implemented in inpatient settings to prevent incident MRSA colonization [16,17]. Key recommendations include surveillance and monitoring of MRSA infections, adherence to standard hand hygiene guidance, environmental cleanliness, and use of dedicated equipment for patients who are colonized or infected with MRSA. Active screening for asymptomatic MRSA carriage and isolation of carriers may be implemented if routine measures are not successful.
Decolonization
Despite the best infection control programs, some patients will be colonized with MRSA at the time of hospital discharge. As detailed above, MRSA colonization is a potent risk factor for infection in the post-discharge setting, as well as in hospital inpatients [22]. A logical approach to this would be to attempt to eradicate colonization. There are several strategies for decolonization therapy, which may be used alone or in combination, including nasal mupirocin, nasal povidone-iodine, systemic antistaphylococcal drugs alone or in combination with oral rifampin, chlorhexidine bathing, or bleach baths [26–29].
A preliminary step in approaching the idea of post-discharge decolonization therapy is to show that patients can be successfully decolonized. With those data in hand, randomized trials seeking to demonstrate a decrease in invasive MRSA infections can be planned. Decolonization using nasal mupirocin has an initial success rate of 60% to 100% in a variety of patient populations [30–35]. Poor adherence to the decolonization protocol may limit success in the outpatient setting. Patients are more likely to resolve their MRSA colonization spontaneously when they regain their general health and independence in activities of daily living [23]. Colonization of other household members may provide a reservoir of MRSA leading to recolonization of the index case. Treatment of the household members may be offered, to provide more durable maintenance of the decolonized state [35]. When chronically ill patients who have been decolonized are followed longitudinally, up to 39% become colonized again, most often with the same strain [30,31]. Attempts to maintain a MRSA-free state in nursing home residents using prolonged mupirocin therapy resulted in emergence of mupirocin resistance [31]. Thus decolonization can be achieved, but is difficult to maintain, especially in debilitated, chronically ill patients. Mupirocin resistance can occur, limiting success of decolonization therapies.
Successful decolonization has been proven to reduce the risk of MRSA infection in the perioperative, dialysis, and intensive care unit settings [33,36–38]. In dialysis patients the risk of S. aureus bloodstream infection, including MRSA, can be reduced 59% with the use of mupirocin decolonization of the nares, with or without treatment of dialysis access exit sites [37]. A placebo-controlled trial demonstrated that decolonization of the nares with mupirocin reduced surgical site infections with S. aureus. All S. aureus isolates in the study were methicillin-susceptible. A second randomized controlled trial of nasal mupirocin did not achieve a statistically significant decrease in S. aureus surgical site infections, but it showed that mupirocin decolonization therapy decreased nosocomial S. aureus infections among nasal carriers [33]. 99.2% of isolates in that study et al were methicillin-susceptible. Quasi-experimental studies have shown similar benefits for surgical patients who are colonized with MRSA [39–41]. A more recent randomized trial, in ICU patients, demonstrated decreased incidence of invasive infection in patients treated with nasal mupirocin and chlorhexidine baths [38]. The common themeof these studies is that they enrolled patients who had a short-term condition, eg, surgery or critical illness, placing them at high risk for invasive MRSA infection. This maximizes the potential benefit of decolonization and minimizes the risk of emergence of resistance. Furthermore, adherence to decolonization protocols is likely to be high in the perioperative and ICU settings. To extrapolate the ICU and perioperative data to the post-discharge setting would be imprudent.
In summary, decolonization may be a useful strategy to reduce invasive MRSA infection in post-discharge patients, but more data are needed for most patient populations. The evidence for decolonization therapy is strongest for dialysis patients, in whom implementation of routine decolonization of MRSA colonized nares is a useful intervention [37]. There are not yet clinical trials of decolonization therapy in patients at time of hospital discharge showing a reduction in invasive MRSA infection. Decolonization strategies have important drawbacks, including emergence of resistance to mupirocin, chlorhexidine, and systemic agents. Furthermore, there is a risk of hypersensitivity reactions, Clostridium difficile infection, and potential for negative impacts onthe normal microbiome. The potential for lesser efficacy in a chronically ill outpatient population must also be considered in the post-discharge setting. Randomized controlled trials with invasive infection outcomes should be performed prior to implementing routine decolonization therapy of hospital discharge patients.
Care of Invasive Devices
Discharge with a central venous catheter was associated with a 2.16-fold increased risk of invasive MRSA infection; other invasive devices were associated with a 3.03-fold increased risk [25]. Clinicians must carefully assess patients nearing discharge for any opportunity to remove invasive devices. Idle devices have been reported in inpatient settings [42] and could occur in other settings. Antimicrobial therapy is a common indication for an outpatient central venous catheter and can also be associated with increased risk of invasive MRSA infection [25,43]. Duration and route of administration of antimicrobial agents should be carefully considered, with an eye to switching to oral therapy whenever possible. When a central venous catheter must be utilized, it should be maintained as carefully as in the inpatient setting. Tools for reducing risk of catheter-associated bloodstream infection include keeping the site dry, scrubbing the hub whenever accessing the catheter, aseptic techniques for dressing changes, and chlorhexidine sponges at the insertion site [44,45]. Reporting of central line–associated bloodstream infection rates by home care agencies is an important quality measure.
Wound Care
The presence of a chronic wound in the post-discharge period is associated with a 4.41-fold increased risk of invasive MRSA infection [25]. Although randomized controlled trials are lacking, it is prudent to ensure that wounds are fully debrided to remove devitalized tissue that can be fertile ground for a MRSA infection. The burden of organisms on a chronic wound is often very large, creating high risk of resistance when exposed to antimicrobial agents. Decolonization therapy is not likely to meet with durable success in such cases and should probably be avoided, except in special circumstances, eg, in preparation for cardiothoracic surgery.
Infection Control in Nursing Home Settings
In the Active Bacterial Core cohort, discharge to a nursing home was associated with a 2.1- to 2.65-fold increased risk of invasive MRSA infection [24,25]. It is notable that the authors controlled for the Charlson comorbidity index, suggesting that nursing home care is more than a marker for comorbidity [25]. The tension between the demands of careful infection control and the home-like setting that is desirable for long-term care creates challenges in the prevention of invasive MRSA infection. Nevertheless, careful management of invasive devices and wounds and antimicrobial stewardship are strategies that may reduce the risk of invasive MRSA infection in long-term care settings. Contact precautions for colonized nursing home residents are recommended only during an outbreak [46]. Staff should be trained in proper application of standard precautions, including use of gowns and gloves when handling body fluids. A study of an aggressive program of screening, decolonization with nasal mupirocin and chlorhexidine bathing, enhanced hand hygiene and environmental cleaning demonstrated a significant reduction in MRSA colonization [47]. An increase in mupirocin resistance during the study led to a switch to retapamulin for nasal application. The Association of Practitioners of Infection Control has issued guidance for MRSA prevention in long-term care facilities [48]. The guidance focuses on surveillance for MRSA infection, performing a MRSA risk assessment, hand hygiene, and environmental cleaning.
Antimicrobial Stewardship
Antimicrobial therapy, especially with fluoroquinolones and third- or fourth-generation cephalosporins, is associated with increased risk of MRSA colonization and infection [43,49,50]. Implementation of an antimicrobial stewardship program, coupled with infection control measures, in a region of Scotland resulted in decreased incidence of MRSA infections among hospital inpatients and in the surrounding community [51]. Thus a robust antimicrobial stewardship program is likely to reduce post-discharge MRSA infections.
Role of Hand Hygiene
The importance of hand hygiene in the prevention of infection has been observed for nearly 2 centuries [52]. Multiple quasi-experimental studies have demonstrated a decreased infection rate when hand hygiene practices for health care workers were introduced or strengthened. A randomized trial in a newborn nursery documented a decrease in transmission of S. aureus when nurses washed their hands after handling a colonized infant [53]. In addition to health care providers, patient hand hygiene can reduce health care–associated infections [54]. Traditional handwashing with soap and water will be familiar to most patients and families. Waterless hand hygiene, typically using alcohol-based hand rubs, is more efficacious and convenient for cleaning hands that are not visibly soiled [52]. If products containing emollients are used, it can also reduce skin drying and cracking. Patients and families should be taught to wash their hands before and after manipulating any medical devices and caring for wounds. Education of patients and family members on the techniques and importance of hand hygiene during hospitalization and at the time of discharge is a simple, low-cost strategy to reduce post-discharge MRSA infections. Teaching can be incorporated into the daily care of patients by nursing and medical staff, both verbally and by example. As a horizontal infection control measure, hand hygiene education has the additional benefit of reducing infections due to all pathogens.
Role of Environmental Cleaning in the Home Setting
Multiple studies have found that the immediate environment of patients who are colonized or infected with MRSA is contaminated with the organism, with greater organism burdens associated with infected patients compared to those who are only colonized [55–59]. Greater environmental contamination is observed when MRSA is present in the urine or wounds of patients [59]. This can lead to transmission of MRSA to family members [23,60,61]. Risk factors for transmission include participation in the care of the patient, older age, and being the partner of the case patient. For the patient, there can be transmission to uninfected body sites and a cycle of recolonization and re-infection. Successful decolonization strategies have included frequent laundering of bedclothes and towels, as well as screening and decolonization of family members. While these strategies may succeed in decolonization, there is no consensus on efficacy in preventing infection in patients or family members. More research in this area is needed, particularly for decolonization strategies, which carry risk of resistance. Attention to cleanliness in the home is a basic hygiene measure that can be recommended.
Conclusion
Post-discharge MRSA infections are an important and underestimated source of morbidity and mortality. Strategies for prevention include infection control measures to prevent incident colonization during hospitalization, removal of any nonessential invasive devices, nursing care for essential devices, wound care, avoiding nonessential antimicrobial therapy, hand hygiene for patients and caregivers, and cleaning of the home environment. Decolonization therapies currently play a limited role, particularly in outbreak situations. The future research agenda should include identification of post-discharge patients who are most likely to benefit from decolonization strategies, and testing those strategies.
Corresponding author: Mary Bessesen, MD, InfectiousDiseases (111L), 1055 Clermont St., Denver, CO 80220, Mary.Bessesen@ucdenver.edu.
1. Chen SY, Wang JT, Chen TH et al. Impact of traditional hospital strain of methicillin-resistant Staphylococcus aureus (MRSA) and community strain of MRSA on mortality in patients with community-onset S aureus bacteremia. Medicine 2010;89:285–94.
2. Lahey T, Shah R, Gittzus J,et al. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine 2009;88:263–7.
3. Chang FY, MacDonald BB, Peacock JE Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine 2003;82:322–32.
4. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002;162:2229–35.
5. Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53–9.
6. Schreiber MP, Chan CM, Shorr AF. Bacteremia in Staphylococcus aureus pneumonia: outcomes and epidemiology. J Crit Care 2011;26:395–401.
7. Malani PN, Rana MM, Banerjee M, Bradley SF. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc 2008;56:1485–9.
8. Dantes RM, Mu YP, Belflower RR, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013;173:1970–8.
9. Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus - Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep 1999;48:707–10.
10. Diep BA, Carleton HA, Chang RF, et al. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis 2006;193:1495–503.
11. Jenkins TC, McCollister BD, Sharma R, et al. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect Control Hosp Epidemiol 2009;30:233–41.
12. Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005-2008. JAMA 2010;304:641–8.
13. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA 2010;303:2141–7.
14. Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis 2006;42:389–91.
15. Avery TR, Kleinman KP, Klompas M, et al. Inclusion of 30-day postdischarge detection triples the incidence of hospital-onset methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2012;33:114–21.
16. Calfee DP, Salgado CD, Classen D, et al. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect Control Hosp Epidemiol 2008;29:Suppl 80.
17. Yokoe DS, Anderson DJ, Berenholtz SM, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 2014;35:967–77.
18. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
19. Stiefel U, Cadnum JL, Eckstein BC, et al. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect Control Hosp Epidemiol 2011;32:185–7.
20. Chang S, Sethi AK, Eckstein BC, et al. Skin and environmental contamination with methicillin-resistant Staphylococcus aureus among carriers identified clinically versus through active surveillance. Clin Infect Dis 2009;48:1423–8.
21. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011;364:1407–18.
22. Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
23. Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med 2009;169:1372–8.
24. Duffy J, Dumyati G, Bulens S, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control 2013;41:782–6.
25. Epstein L, Mu Y, Belflower R, et al. Risk factors for invasive methicillin-resistant Staphylococcus aureus infection after recent discharge from an acute-care hospitalization, 2011-2013. Clin Infect Dis 2016;62:45–52.
26. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 2007;44:178–85.
27. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol 2008;29:510–6.
28. Anderson MJ, David ML, Scholz M, et al. Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares. Antimicrob Agents Chemother 2015;59:2765–73.
29. Strausbaugh LJ, Jacobson C, Sewell DL, et al. Antimicrobial therapy for methicillin-resistant Staphylococcus aureus colonization in residents and staff of a Veterans Affairs nursing home care unit. Infect Control Hosp Epidemiol 1992;13:151–9.
30. Mody L, Kauffman CA, McNeil SA, et al. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:1467–74.
31. Kauffman CA, Terpenning MS, He X, et al. Attempts to eradicate methicillin-resistant Staphylococcus aureus from a long-term-care facility with the use of mupirocin ointment. Am J Med 1993;94:371–8.
32. Cederna JE, Terpenning MS, Ensberg M, et al. Staphylococcus aureus nasal colonization in a nursing home: eradication with mupirocin. Infect Control Hosp Epidemiol 1990;11:13–6.
33. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002;346:1871–7.
34. Kohler P, Bregenzer-Witteck A, Rettenmund G, et al. MRSA decolonization: success rate, risk factors for failure and optimal duration of follow-up. Infection 2013;41:33–40.
35. Ammerlaan HS, Kluytmans JA, Berkhout H, et al. Eradication of carriage with methicillin-resistant Staphylococcus aureus: effectiveness of a national guideline. J Antimicrob Chemother 2011;66:2409–17.
36. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17.
37. Nair R, Perencevich EN, Blevins AE, et al. Clinical effectiveness of mupirocin for preventing Staphylococcus aureus infections in nonsurgical settings: a meta-analysis. Clin Infect Dis 2016;62:618–30.
38. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent icu infection. N Engl J Med 2013;368:2255–65.
39. Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–71.
40. Walsh EE, Greene L, Kirshner R. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med 2011;171:68–73.
41. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am 2010;92:1820–6.
42. Lederle FA, Parenti CM, Berskow LC, Ellingson KJ. The idle intravenous catheter. Ann Intern Med 1992;116:737–8.
43. Avery CM, Ameerally P, Castling B, Swann RA. Infection of surgical wounds in the maxillofacial region and free flap donor sites with methicillin-resistant Staphylococcus aureus. Br J Oral Maxillofac Surg 2006;44:217–21.
44. Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 2009;301:1231–41.
45. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:753–71.
46. Manzur A, Gudiol F. Methicillin-resistant Staphylococcus aureus in long-term-care facilities. Clin Microbiol Infect 2009;15 Suppl 7:26–30.
47. Schora DM, Boehm S, Das S, et al. Impact of Detection, Education, Research and Decolonization without Isolation in Long-term care (DERAIL) on methicillin-resistant Staphylococcus aureus colonization and transmission at 3 long-term care facilities. Am J Infect Control 2014;42(10 Suppl):S269–73.
48. Rebmann T, Aureden K, Association for Professionals in Infection Control and Epidemiology. Preventing methicillin-resistant Staphylococcus aureus transmission in long-term care facilities: an executive summary of the APIC Elimination Guide. Am J Infect Control 2011;39:235–8.
49. Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med 2014;174:1660–7.
50. Couderc C, Jolivet S, Thiebaut AC, et al. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis 2014;59:206–15.
51. Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis 2015;15:1438–49.
52. Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep 2002;51(RR-16):1–48.
53. Mortimer EA Jr, Lipsitz PJ, Wolinsky E, et al. Transmission of staphylococci between newborns. Importance of the hands to personnel. Am J Dis Child 1962;104:289–95.
54. Gagne D, Bedard G, Maziade PJ. Systematic patients’ hand disinfection: impact on meticillin-resistant Staphylococcus aureus infection rates in a community hospital. J Hosp Infect 2010;75:269–72.
55. Knelson LP, Williams DA, Gergen MF, et al. A comparison of environmental contamination by patients infected or colonized with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a multicenter study. Infect Control Hosp Epidemiol 2014;35:872–5.
56. Murphy CR, Eells SJ, Quan V, et al. Methicillin-resistant Staphylococcus aureus burden in nursing homes associated with environmental contamination of common areas. J Am Geriatr Soc 2012;60:1012–8.
57. Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med 2011;171:491–4.
58. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
59. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
60. Mollema FP, Richardus JH, Behrendt M, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 2010;48:202–7.
61. Calfee DP, Durbin LJ, Germanson TP, et al. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol 2003;24:422–6.
1. Chen SY, Wang JT, Chen TH et al. Impact of traditional hospital strain of methicillin-resistant Staphylococcus aureus (MRSA) and community strain of MRSA on mortality in patients with community-onset S aureus bacteremia. Medicine 2010;89:285–94.
2. Lahey T, Shah R, Gittzus J,et al. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine 2009;88:263–7.
3. Chang FY, MacDonald BB, Peacock JE Jr, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine 2003;82:322–32.
4. Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002;162:2229–35.
5. Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53–9.
6. Schreiber MP, Chan CM, Shorr AF. Bacteremia in Staphylococcus aureus pneumonia: outcomes and epidemiology. J Crit Care 2011;26:395–401.
7. Malani PN, Rana MM, Banerjee M, Bradley SF. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J Am Geriatr Soc 2008;56:1485–9.
8. Dantes RM, Mu YP, Belflower RR, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013;173:1970–8.
9. Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus - Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep 1999;48:707–10.
10. Diep BA, Carleton HA, Chang RF, et al. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J Infect Dis 2006;193:1495–503.
11. Jenkins TC, McCollister BD, Sharma R, et al. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect Control Hosp Epidemiol 2009;30:233–41.
12. Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005-2008. JAMA 2010;304:641–8.
13. Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA 2010;303:2141–7.
14. Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis 2006;42:389–91.
15. Avery TR, Kleinman KP, Klompas M, et al. Inclusion of 30-day postdischarge detection triples the incidence of hospital-onset methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2012;33:114–21.
16. Calfee DP, Salgado CD, Classen D, et al. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect Control Hosp Epidemiol 2008;29:Suppl 80.
17. Yokoe DS, Anderson DJ, Berenholtz SM, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 2014;35:967–77.
18. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30.
19. Stiefel U, Cadnum JL, Eckstein BC, et al. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect Control Hosp Epidemiol 2011;32:185–7.
20. Chang S, Sethi AK, Eckstein BC, et al. Skin and environmental contamination with methicillin-resistant Staphylococcus aureus among carriers identified clinically versus through active surveillance. Clin Infect Dis 2009;48:1423–8.
21. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011;364:1407–18.
22. Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004;364:703–5.
23. Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med 2009;169:1372–8.
24. Duffy J, Dumyati G, Bulens S, et al. Community-onset invasive methicillin-resistant Staphylococcus aureus infections following hospital discharge. Am J Infect Control 2013;41:782–6.
25. Epstein L, Mu Y, Belflower R, et al. Risk factors for invasive methicillin-resistant Staphylococcus aureus infection after recent discharge from an acute-care hospitalization, 2011-2013. Clin Infect Dis 2016;62:45–52.
26. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 2007;44:178–85.
27. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol 2008;29:510–6.
28. Anderson MJ, David ML, Scholz M, et al. Efficacy of skin and nasal povidone-iodine preparation against mupirocin-resistant methicillin-resistant Staphylococcus aureus and S. aureus within the anterior nares. Antimicrob Agents Chemother 2015;59:2765–73.
29. Strausbaugh LJ, Jacobson C, Sewell DL, et al. Antimicrobial therapy for methicillin-resistant Staphylococcus aureus colonization in residents and staff of a Veterans Affairs nursing home care unit. Infect Control Hosp Epidemiol 1992;13:151–9.
30. Mody L, Kauffman CA, McNeil SA, et al. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:1467–74.
31. Kauffman CA, Terpenning MS, He X, et al. Attempts to eradicate methicillin-resistant Staphylococcus aureus from a long-term-care facility with the use of mupirocin ointment. Am J Med 1993;94:371–8.
32. Cederna JE, Terpenning MS, Ensberg M, et al. Staphylococcus aureus nasal colonization in a nursing home: eradication with mupirocin. Infect Control Hosp Epidemiol 1990;11:13–6.
33. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002;346:1871–7.
34. Kohler P, Bregenzer-Witteck A, Rettenmund G, et al. MRSA decolonization: success rate, risk factors for failure and optimal duration of follow-up. Infection 2013;41:33–40.
35. Ammerlaan HS, Kluytmans JA, Berkhout H, et al. Eradication of carriage with methicillin-resistant Staphylococcus aureus: effectiveness of a national guideline. J Antimicrob Chemother 2011;66:2409–17.
36. Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17.
37. Nair R, Perencevich EN, Blevins AE, et al. Clinical effectiveness of mupirocin for preventing Staphylococcus aureus infections in nonsurgical settings: a meta-analysis. Clin Infect Dis 2016;62:618–30.
38. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent icu infection. N Engl J Med 2013;368:2255–65.
39. Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–71.
40. Walsh EE, Greene L, Kirshner R. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med 2011;171:68–73.
41. Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg Am 2010;92:1820–6.
42. Lederle FA, Parenti CM, Berskow LC, Ellingson KJ. The idle intravenous catheter. Ann Intern Med 1992;116:737–8.
43. Avery CM, Ameerally P, Castling B, Swann RA. Infection of surgical wounds in the maxillofacial region and free flap donor sites with methicillin-resistant Staphylococcus aureus. Br J Oral Maxillofac Surg 2006;44:217–21.
44. Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 2009;301:1231–41.
45. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:753–71.
46. Manzur A, Gudiol F. Methicillin-resistant Staphylococcus aureus in long-term-care facilities. Clin Microbiol Infect 2009;15 Suppl 7:26–30.
47. Schora DM, Boehm S, Das S, et al. Impact of Detection, Education, Research and Decolonization without Isolation in Long-term care (DERAIL) on methicillin-resistant Staphylococcus aureus colonization and transmission at 3 long-term care facilities. Am J Infect Control 2014;42(10 Suppl):S269–73.
48. Rebmann T, Aureden K, Association for Professionals in Infection Control and Epidemiology. Preventing methicillin-resistant Staphylococcus aureus transmission in long-term care facilities: an executive summary of the APIC Elimination Guide. Am J Infect Control 2011;39:235–8.
49. Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med 2014;174:1660–7.
50. Couderc C, Jolivet S, Thiebaut AC, et al. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis 2014;59:206–15.
51. Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis 2015;15:1438–49.
52. Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep 2002;51(RR-16):1–48.
53. Mortimer EA Jr, Lipsitz PJ, Wolinsky E, et al. Transmission of staphylococci between newborns. Importance of the hands to personnel. Am J Dis Child 1962;104:289–95.
54. Gagne D, Bedard G, Maziade PJ. Systematic patients’ hand disinfection: impact on meticillin-resistant Staphylococcus aureus infection rates in a community hospital. J Hosp Infect 2010;75:269–72.
55. Knelson LP, Williams DA, Gergen MF, et al. A comparison of environmental contamination by patients infected or colonized with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a multicenter study. Infect Control Hosp Epidemiol 2014;35:872–5.
56. Murphy CR, Eells SJ, Quan V, et al. Methicillin-resistant Staphylococcus aureus burden in nursing homes associated with environmental contamination of common areas. J Am Geriatr Soc 2012;60:1012–8.
57. Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med 2011;171:491–4.
58. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 2009;73:378–85.
59. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997;18:622–7.
60. Mollema FP, Richardus JH, Behrendt M, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 2010;48:202–7.
61. Calfee DP, Durbin LJ, Germanson TP, et al. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol 2003;24:422–6.
Taking hysteroscopy to the office
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
The benefits of integrating in-office hysteroscopy
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.
Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.