User login
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
TOPLINE:
A novel, blood-based test developed using fragmentomic features of cell-free DNA (cfDNA) detects colorectal cancer (CRC) with a 90.4% sensitivity and shows consistent performance across stages and tumor locations.
METHODOLOGY:
- Researchers conducted a prospective case-control study to develop and validate a noninvasive cfDNA-based screening test for CRC.
- Adults aged 40-89 years with CRC or advanced adenomas were enrolled at a tertiary center in South Korea between 2021 and 2024.
- Blood samples were drawn after colonoscopy, but prior to treatment, in patients with CRC, advanced adenomas, and asymptomatic controls with normal colonoscopy results.
- A model was trained on fragmentonic features derived from whole genome sequencing of cfDNA from 1250 participants and validated for its diagnostic performance in the remaining 427 participants, including all with advanced adenomas.
- The primary endpoint was the sensitivity of the cfDNA test for detecting CRC. The area under the receiver operating characteristic curve (AUROC) was also calculated.
TAKEAWAY:
- The cfDNA test detected CRC with 90.4% sensitivity and an AUROC of 0.978.
- Sensitivity by CRC stage was 84.2% for stage I, 85.0% for stage II, 94.4% for stage III, 100% for stage IV.
- Advanced adenomas were detected with 58.3% sensitivity and an AUROC of 0.862.
- Among individuals with normal colonoscopy findings, the test was correctly negative 94.7% of the time.
- Diagnostic sensitivities were consistent between left- and right-sided CRC tumors, among participants aged < 60 years and ≥ 60 years, and across left- and right-sided advanced adenomas.
IN PRACTICE:
"This highlights the potential clinical utility of the test in identifying candidates for minimally invasive therapeutic approaches tool for CRC," the authors wrote. "Notably, the high sensitivity observed for early-stage CRC and the favorable sensitivity for [advanced adenoma] suggest that this cfDNA test may offer benefits not only in diagnosis but also in prognosis and ultimately in CRC prevention."
SOURCE:
This study was led by Seung Wook Hong, MD, Asan Medical Center in Seoul, South Korea. It was published online on November 19, 2025, in the American Journal of Gastroenterology.
LIMITATIONS:
The case-control design introduced spectrum bias by comparing clearly defined CRC and advanced adenomas cases with individuals who had normal colonoscopy results. The CRC prevalence of 17% to 18% was higher than that observed in true screening populations, limiting generalizability. The exclusively Korean cohort limited extrapolation to non-Asian populations.
DISCLOSURES:
The study received support from GC Genome, Yongin, South Korea. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A novel, blood-based test developed using fragmentomic features of cell-free DNA (cfDNA) detects colorectal cancer (CRC) with a 90.4% sensitivity and shows consistent performance across stages and tumor locations.
METHODOLOGY:
- Researchers conducted a prospective case-control study to develop and validate a noninvasive cfDNA-based screening test for CRC.
- Adults aged 40-89 years with CRC or advanced adenomas were enrolled at a tertiary center in South Korea between 2021 and 2024.
- Blood samples were drawn after colonoscopy, but prior to treatment, in patients with CRC, advanced adenomas, and asymptomatic controls with normal colonoscopy results.
- A model was trained on fragmentonic features derived from whole genome sequencing of cfDNA from 1250 participants and validated for its diagnostic performance in the remaining 427 participants, including all with advanced adenomas.
- The primary endpoint was the sensitivity of the cfDNA test for detecting CRC. The area under the receiver operating characteristic curve (AUROC) was also calculated.
TAKEAWAY:
- The cfDNA test detected CRC with 90.4% sensitivity and an AUROC of 0.978.
- Sensitivity by CRC stage was 84.2% for stage I, 85.0% for stage II, 94.4% for stage III, 100% for stage IV.
- Advanced adenomas were detected with 58.3% sensitivity and an AUROC of 0.862.
- Among individuals with normal colonoscopy findings, the test was correctly negative 94.7% of the time.
- Diagnostic sensitivities were consistent between left- and right-sided CRC tumors, among participants aged < 60 years and ≥ 60 years, and across left- and right-sided advanced adenomas.
IN PRACTICE:
"This highlights the potential clinical utility of the test in identifying candidates for minimally invasive therapeutic approaches tool for CRC," the authors wrote. "Notably, the high sensitivity observed for early-stage CRC and the favorable sensitivity for [advanced adenoma] suggest that this cfDNA test may offer benefits not only in diagnosis but also in prognosis and ultimately in CRC prevention."
SOURCE:
This study was led by Seung Wook Hong, MD, Asan Medical Center in Seoul, South Korea. It was published online on November 19, 2025, in the American Journal of Gastroenterology.
LIMITATIONS:
The case-control design introduced spectrum bias by comparing clearly defined CRC and advanced adenomas cases with individuals who had normal colonoscopy results. The CRC prevalence of 17% to 18% was higher than that observed in true screening populations, limiting generalizability. The exclusively Korean cohort limited extrapolation to non-Asian populations.
DISCLOSURES:
The study received support from GC Genome, Yongin, South Korea. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A novel, blood-based test developed using fragmentomic features of cell-free DNA (cfDNA) detects colorectal cancer (CRC) with a 90.4% sensitivity and shows consistent performance across stages and tumor locations.
METHODOLOGY:
- Researchers conducted a prospective case-control study to develop and validate a noninvasive cfDNA-based screening test for CRC.
- Adults aged 40-89 years with CRC or advanced adenomas were enrolled at a tertiary center in South Korea between 2021 and 2024.
- Blood samples were drawn after colonoscopy, but prior to treatment, in patients with CRC, advanced adenomas, and asymptomatic controls with normal colonoscopy results.
- A model was trained on fragmentonic features derived from whole genome sequencing of cfDNA from 1250 participants and validated for its diagnostic performance in the remaining 427 participants, including all with advanced adenomas.
- The primary endpoint was the sensitivity of the cfDNA test for detecting CRC. The area under the receiver operating characteristic curve (AUROC) was also calculated.
TAKEAWAY:
- The cfDNA test detected CRC with 90.4% sensitivity and an AUROC of 0.978.
- Sensitivity by CRC stage was 84.2% for stage I, 85.0% for stage II, 94.4% for stage III, 100% for stage IV.
- Advanced adenomas were detected with 58.3% sensitivity and an AUROC of 0.862.
- Among individuals with normal colonoscopy findings, the test was correctly negative 94.7% of the time.
- Diagnostic sensitivities were consistent between left- and right-sided CRC tumors, among participants aged < 60 years and ≥ 60 years, and across left- and right-sided advanced adenomas.
IN PRACTICE:
"This highlights the potential clinical utility of the test in identifying candidates for minimally invasive therapeutic approaches tool for CRC," the authors wrote. "Notably, the high sensitivity observed for early-stage CRC and the favorable sensitivity for [advanced adenoma] suggest that this cfDNA test may offer benefits not only in diagnosis but also in prognosis and ultimately in CRC prevention."
SOURCE:
This study was led by Seung Wook Hong, MD, Asan Medical Center in Seoul, South Korea. It was published online on November 19, 2025, in the American Journal of Gastroenterology.
LIMITATIONS:
The case-control design introduced spectrum bias by comparing clearly defined CRC and advanced adenomas cases with individuals who had normal colonoscopy results. The CRC prevalence of 17% to 18% was higher than that observed in true screening populations, limiting generalizability. The exclusively Korean cohort limited extrapolation to non-Asian populations.
DISCLOSURES:
The study received support from GC Genome, Yongin, South Korea. The authors reported no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
Cell-Free DNA Blood Test Shows Strong Performance in Detecting Early-Stage CRC
Geographic Clusters Show Uneven Cancer Screening in the US
Geographic Clusters Show Uneven Cancer Screening in the US
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An analysis of 3142 US counties revealed that county-level screening for breast, cervical, and colorectal cancer increased overall between 1997 and 2019; however, despite the reduced geographic variation, persistently high-screening clusters remained in the Northeast, whereas persistently low-screening clusters remained in the Southwest.
METHODOLOGY:
- Cancer screening reduces mortality. Despite guideline recommendation, the uptake of breast, cervical, and colorectal cancer screening in the US falls short of national goals and varies across sociodemographic groups. To date, only a few studies have examined geographic and temporal patterns of screening.
- To address this gap, researchers conducted a cross-sectional study using an ecological panel design to analyze county-level screening prevalence across 3142 US mainland counties from 1997 to 2019, deriving prevalence estimates from Behavioral Risk Factor Surveillance System (BRFSS) and National Health Interview Survey (NHIS) data over 3- to 5-year periods.
- Spatial autocorrelation analyses, including Global Moran I and the bivariate local indicator of spatial autocorrelation, were performed to assess geographic clusters of cancer screening within each period. Four types of local geographic clusters of county-level cancer screening were identified: counties with persistently high screening rates, counties with persistently low screening rates, counties in which screening rates decreased from high to low, and counties in which screening rates increased from low to high.
- Screening prevalence was compared across multiple time windows for different modalities (mammography, a Papanicolaou test, colonoscopy, colorectal cancer test, endoscopy, and a fecal occult blood test [FOBT]). Overall, 3101 counties were analyzed for mammography and the Papanicolaou test, 3107 counties for colonoscopy, 3100 counties for colorectal cancer test, 3089 counties for endoscopy, and 3090 counties for the FOBT.
TAKEAWAY:
- Overall screening prevalence increased from 1997 to 2019, and global spatial autocorrelation declined over time. For instance, the distribution of mammography screening became 83% more uniform in more recent years (Moran I, 0.57 in 1997-1999 vs 0.10 in 2017-2019). Similarly, Papanicolaou test screening became more uniform in more recent years (Moran I, 0.44 vs. 0.07). These changes indicate reduced geographic heterogeneity.
- Colonoscopy and endoscopy use increased, surpassing a 50% prevalence in many counties for 2010; however, FOBT use declined. Spatial clustering also attenuated, with a 23.4% declined in Moran I for colonoscopy from 2011-2016 to 2017-2019, a 12.3% decline in the colorectal cancer test from 2004-2007 to 2008-2010, and a 14.0% decline for endoscopy from 2004-2007 to 2008-2010.
- Persistently high-/high-screening clusters were concentrated in the Northeast for mammography and colorectal cancer screening and in the East for Papanicolaou test screening, whereas persistently low-/low-screening clusters were concentrated in the Southwest for the same modalities.
- Clusters of low- and high-screening counties were more disadvantaged -- with lower socioeconomic status and a higher proportion of non-White residents -- than other cluster types, suggesting some improvement in screening uptake in more disadvantaged areas. Counties with persistently low screening exhibited greater socioeconomic disadvantages -- lower media household income, higher poverty, lower home values, and lower educational attainment -- than those with persistently high screening.
IN PRACTICE:
"This cross-sectional study found that despite secular increases that reduced geographic variation in screening, local clusters of high and low screening persisted in the Northeast and Southwest US, respectively. Future studies could incorporate health care access characteristics to explain why areas of low screening did not catch up to optimize cancer screening practice," the authors wrote.
SOURCE:
The study, led by Pranoti Pradhan, PhD, Harvard T.H. Chan School of Public Health, Boston, was published online in JAMA Network Open.
LIMITATIONS:
The county-level estimates were modeled using BRFSS, NHIS, and US Census data, which might be susceptible to sampling biases despite corrections for nonresponse and noncoverage. Researchers lacked data on specific health systems characteristics that may have directly driven changes in prevalence and were restricted to using screening time intervals available from the Small Area Estimates for Cancer-Relates Measures from the National Cancer Institute, rather than those according to US Preventive Services Task Force guidelines. Additionally, the spatial cluster method was sensitive to county size and arrangement, which may have influenced local cluster detection.
DISCLOSURES:
This research was supported by the T32 Cancer Prevention and Control Funding Fellowship and T32 Cancer Epidemiology Fellowship at the Harvard T.H. Chan School of Public Health. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Geographic Clusters Show Uneven Cancer Screening in the US
Geographic Clusters Show Uneven Cancer Screening in the US
Improving Colorectal Cancer Screening via Mailed Fecal Immunochemical Testing in a Veterans Affairs Health System
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
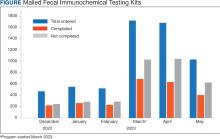
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
Colorectal cancer (CRC) is among the most common cancers and causes of cancer-related deaths in the United States.1 Reflective of a nationwide trend, CRC screening rates at the Veterans Affairs Connecticut Healthcare System (VACHS) decreased during the COVID-19 pandemic.2-5 Contributing factors to this decrease included cancellations of elective colonoscopies during the initial phase of the pandemic and concurrent turnover of endoscopists. In 2021, the US Preventive Services Task Force lowered the recommended initial CRC screening age from 50 years to 45 years, further increasing the backlog of unscreened patients.6
Fecal immunochemical testing (FIT) is a noninvasive screening method in which antibodies are used to detect hemoglobin in the stool. The sensitivity and specificity of 1-time FIT are 79% to 80% and 94%, respectively, for the detection of CRC, with sensitivity improving with successive testing.7,8 Annual FIT is recognized as a tier 1 preferred screening method by the US Multi-Society Task Force on Colorectal Cancer.7,9 Programs that mail FIT kits to eligible patients outside of physician visits have been successfully implemented in health care systems.10,11
The VACHS designed and implemented a mailed FIT program using existing infrastructure and staffing.
Program Description
A team of local stakeholders comprised of VACHS leadership, primary care, nursing, and gastroenterology staff, as well as representatives from laboratory, informatics, mail services, and group practice management, was established to execute the project. The team met monthly to plan the project.
The team developed a dataset consisting of patients aged 45 to 75 years who were at average risk for CRC and due for CRC screening. Patients were defined as due for CRC screening if they had not had a colonoscopy in the previous 9 years or a FIT or fecal occult blood test in the previous 11 months. Average risk for CRC was defined by excluding patients with associated diagnosis codes for CRC, colectomy, inflammatory bowel disease, and anemia. The program also excluded patients with diagnosis codes associated with dementia, deferring discussions about cancer screening to their primary care practitioners (PCPs). Patients with invalid mailing addresses were also excluded, as well as those whose PCPs had indicated in the electronic health record that the patient received CRC screening outside the US Department of Veterans Affairs (VA) system.
Letter Templates
Two patient letter electronic health record templates were developed. The first was a primer letter, which was mailed to patients 2 to 3 weeks before the mailed FIT kit as an introduction to the program.12 The purpose of the primer letter was to give advance notice to patients that they could expect a FIT kit to arrive in the mail. The goal was to prepare patients to complete FIT when the kit arrived and prompt them to call the VA to opt out of the mailed FIT program if they were up to date with CRC screening or if they had a condition which made them at high risk for CRC.
The second FIT letter arrived with the FIT kit, introduced FIT and described the importance of CRC screening. The letter detailed instructions for completing FIT and automatically created a FIT order. It also included a list of common conditions that may exclude patients, with a recommendation for patients to contact their medical team if they felt they were not candidates for FIT.
Staff Education
A previous VACHS pilot project demonstrated the success of a mailed FIT program to increase FIT use. Implemented as part of the pilot program, staff education consisted of a session for clinicians about the role of FIT in CRC screening and an all-staff education session. An additional education session about CRC and FIT for all staff was repeated with the program launch.
Program Launch
The mailed FIT program was introduced during a VACHS primary care all-staff meeting. After the meeting, each patient aligned care team (PACT) received an encrypted email that included a list of the patients on their team who were candidates for the program, a patient-facing FIT instruction sheet, detailed instructions on how to send the FIT primer letter, and a FIT package consisting of the labeled FIT kit, FIT letter, and patient instruction sheet. A reminder letter was sent to each patient 3 weeks after the FIT package was mailed. The patient lists were populated into a shared, encrypted Microsoft Teams folder that was edited in real time by PACT teams and viewed by VACHS leadership to track progress.
Program Metrics
At program launch, the VACHS had 4642 patients due for CRC screening who were eligible for the mailed FIT program. On March 7, 2023, the data consisting of FIT tests ordered between December 2022 and May 2023—3 months before and after the launch of the program—were reviewed and categorized. In the 3 months before program launch, 1528 FIT were ordered and 714 were returned (46.7%). In the 3 months after the launch of the program, 4383 FIT were ordered and 1712 were returned (39.1%) (Figure). Test orders increased 287% from the preintervention to the postintervention period. The mean (SD) number of monthly FIT tests prelaunch was 509 (32.7), which increased to 1461 (331.6) postlaunch.
At the VACHS, 61.4% of patients aged 45 to 75 years were up to date with CRC screening before the program launch. In the 3 months after program launch, the rate increased to 63.8% among patients aged 45 to 75 years, the highest rate in our Veterans Integrated Services Network and exceeding the VA national average CRC screening rate, according to unpublished VA Monthly Management Report data.
In the 3 months following the program launch, 139 FIT kits tested positive for potential CRC. Of these, 79 (56.8%) patients had completed a diagnostic colonoscopy. PACT PCPs and nurses received reports on patients with positive FIT tests and those with no colonoscopy scheduled or completed and were asked to follow up.
Discussion
Through a proactive, population-based CRC screening program centered on mailed FIT kits outside of the traditional patient visit, the VACHS increased the use of FIT and rates of CRC screening. The numbers of FIT kits ordered and completed substantially increased in the 3 months after program launch.
Compared to mailed FIT programs described in the literature that rely on centralized processes in that a separate team operates the mailed FIT program for the entire organization, this program used existing PACT infrastructure and staff.10,11 This strategy allowed VACHS to design and implement the program in several months. Not needing to hire new staff or create a central team for the sole purpose of implementing the program allowed us to save on any organizational funding and efforts that would have accompanied the additional staff. The program described in this article may be more attainable for primary care practices or smaller health systems that do not have the capacity for the creation of a centralized process.
Limitations
Although the total number of FIT completions substantially increased during the program, the rate of FIT completion during the mailed FIT program was lower than the rate of completion prior to program launch. This decreased rate of FIT kit completion may be related to separation from a patient visit and potential loss of real-time education with a clinician. The program’s decentralized design increased the existing workload for primary care staff, and as a result, consideration must be given to local staffing levels. Additionally, the report of eligible patients depended on diagnosis codes and may have captured patients with higher-than-average risk of CRC, such as patients with prior history of adenomatous polyps, family history of CRC, or other medical or genetic conditions. We attempted to mitigate this by including a list of conditions that would exclude patients from FIT eligibility in the FIT letter and giving them the option to opt out.
Conclusions
CRC screening rates improved following implementation of a primary care team-centered quality improvement process to proactively identify patients appropriate for FIT and mail them FIT kits. This project highlights that population-health interventions around CRC screening via use of FIT can be successful within a primary care patient-centered medical home model, considering the increases in both CRC screening rates and increase in FIT tests ordered.
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
1. American Cancer Society. Key statistics for colorectal cancer. Revised January 29, 2024. Accessed June 11, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
2. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878-884. doi:10.1001/jamaoncol.2021.0884
3. Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. 2022;53(3):730-744. doi:10.1007/s12029-021-00679-x
4. Adams MA, Kurlander JE, Gao Y, Yankey N, Saini SD. Impact of coronavirus disease 2019 on screening colonoscopy utilization in a large integrated health system. Gastroenterology. 2022;162(7):2098-2100.e2. doi:10.1053/j.gastro.2022.02.034
5. Sundaram S, Olson S, Sharma P, Rajendra S. A review of the impact of the COVID-19 pandemic on colorectal cancer screening: implications and solutions. Pathogens. 2021;10(11):558. doi:10.3390/pathogens10111508
6. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi:10.1001/jama.2021.6238
7. Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2017;85(1):2-21.e3. doi:10.1016/j.gie.2016.09.025
8. Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171. doi:10.7326/M13-1484
9. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307-323. doi:10.1053/j.gastro.2017.05.013
10. Deeds SA, Moore CB, Gunnink EJ, et al. Implementation of a mailed faecal immunochemical test programme for colorectal cancer screening among veterans. BMJ Open Qual. 2022;11(4):e001927. doi:10.1136/bmjoq-2022-001927
11. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2022;20(1):145-152. doi:10.1016/j.cgh.2020.09.042
12. Deeds S, Liu T, Schuttner L, et al. A postcard primer prior to mailed fecal immunochemical test among veterans: a randomized controlled trial. J Gen Intern Med. 2023:38(14):3235-3241. doi:10.1007/s11606-023-08248-7
AI Tool Helps Patients Assess Bowel Preparation for Colonoscopy
AI Tool Helps Patients Assess Bowel Preparation for Colonoscopy
TOPLINE:
An artificial intelligence (AI) model, developed using stool images, accurately assessed whether a patient’s bowel preparation was sufficient for colonoscopy. The best version of the model achieved an area under the receiver operating characteristic curve (AUC) of 0.95, accuracy of 0.90, sensitivity of 0.93, and specificity of 0.86.
METHODOLOGY:
- Patients often need help during bowel preparation for colonoscopy, which increases staff workload; up to 20%-25% of colonoscopies are reported to be inadequately prepared. Researchers developed and tested an AI tool (AI-PREPOO) using stool images to assess whether patients were ready for colonoscopy.
- They conducted a multicenter observational study in Japan between 2022 and 2023 that included 37 patients scheduled for colonoscopy (median age, 57 years; 45.9% women).
- After starting consumption of a 2-liter polyethylene glycol solution, patients used smartphones to take photos of their stool in the toilet after each bowel movement and uploaded the images to a secure web server.
- The images were divided into training and test sets. Images were classified as “ready” for colonoscopy when the stool was clear or light yellow and watery with no solid content.
- Four image-recognition models based on different deep learning architectures were developed using transfer learning to classify readiness for colonoscopy.
TAKEAWAY:
- Researchers collected 282 stool images, with 141 classified as ready and 141 as not ready. Of these, 224 images were used for training (the number augmented to 2240 images) and 58 for testing.
- All four AI-PREPOO models showed high performance, with AUCs ranging from 0.92 to 0.95; pairwise differences in AUCs were not significant.
- The AI-PREPOO 1 model, based on the MobileNetV3-Small architecture, showed the most balanced performance, with an AUC of 0.95, accuracy of 0.90, sensitivity of 0.93, and specificity of 0.86 on the test set.
- During colonoscopy, all patients had a Boston Bowel Preparation Scale score of 6 or higher, indicating that “ready” images corresponded to an adequately prepared bowel.
IN PRACTICE:
“If implemented as a mobile application, our model would allow patients to quickly and independently assess bowel preparation adequacy, reducing reliance on nurses and alleviating embarrassment associated with sharing stool images. This approach could also lessen nurses’ workload by minimizing unnecessary inquiries and preventing excessive or insufficient bowel preparation due to uncertainty,” the authors wrote.
SOURCE:
This study was led by Kosuke Kojima, Graduate School of Medical and Dental Sciences, Niigata University in Niigata, Japan. It was published online in the Journal of Gastroenterology and Hepatology.
LIMITATIONS:
The small dataset limited generalizability and increased the risk for overfitting. In real-world practice, stool images might vary in lighting, angle, focus, zoom, and background; thus, a larger and more diverse dataset was needed. The model lacked external validation in an independent or prospective cohort.
DISCLOSURES:
This study received support from the Japanese Foundation for Research and Promotion of Endoscopy. The authors reported having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI) model, developed using stool images, accurately assessed whether a patient’s bowel preparation was sufficient for colonoscopy. The best version of the model achieved an area under the receiver operating characteristic curve (AUC) of 0.95, accuracy of 0.90, sensitivity of 0.93, and specificity of 0.86.
METHODOLOGY:
- Patients often need help during bowel preparation for colonoscopy, which increases staff workload; up to 20%-25% of colonoscopies are reported to be inadequately prepared. Researchers developed and tested an AI tool (AI-PREPOO) using stool images to assess whether patients were ready for colonoscopy.
- They conducted a multicenter observational study in Japan between 2022 and 2023 that included 37 patients scheduled for colonoscopy (median age, 57 years; 45.9% women).
- After starting consumption of a 2-liter polyethylene glycol solution, patients used smartphones to take photos of their stool in the toilet after each bowel movement and uploaded the images to a secure web server.
- The images were divided into training and test sets. Images were classified as “ready” for colonoscopy when the stool was clear or light yellow and watery with no solid content.
- Four image-recognition models based on different deep learning architectures were developed using transfer learning to classify readiness for colonoscopy.
TAKEAWAY:
- Researchers collected 282 stool images, with 141 classified as ready and 141 as not ready. Of these, 224 images were used for training (the number augmented to 2240 images) and 58 for testing.
- All four AI-PREPOO models showed high performance, with AUCs ranging from 0.92 to 0.95; pairwise differences in AUCs were not significant.
- The AI-PREPOO 1 model, based on the MobileNetV3-Small architecture, showed the most balanced performance, with an AUC of 0.95, accuracy of 0.90, sensitivity of 0.93, and specificity of 0.86 on the test set.
- During colonoscopy, all patients had a Boston Bowel Preparation Scale score of 6 or higher, indicating that “ready” images corresponded to an adequately prepared bowel.
IN PRACTICE:
“If implemented as a mobile application, our model would allow patients to quickly and independently assess bowel preparation adequacy, reducing reliance on nurses and alleviating embarrassment associated with sharing stool images. This approach could also lessen nurses’ workload by minimizing unnecessary inquiries and preventing excessive or insufficient bowel preparation due to uncertainty,” the authors wrote.
SOURCE:
This study was led by Kosuke Kojima, Graduate School of Medical and Dental Sciences, Niigata University in Niigata, Japan. It was published online in the Journal of Gastroenterology and Hepatology.
LIMITATIONS:
The small dataset limited generalizability and increased the risk for overfitting. In real-world practice, stool images might vary in lighting, angle, focus, zoom, and background; thus, a larger and more diverse dataset was needed. The model lacked external validation in an independent or prospective cohort.
DISCLOSURES:
This study received support from the Japanese Foundation for Research and Promotion of Endoscopy. The authors reported having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI) model, developed using stool images, accurately assessed whether a patient’s bowel preparation was sufficient for colonoscopy. The best version of the model achieved an area under the receiver operating characteristic curve (AUC) of 0.95, accuracy of 0.90, sensitivity of 0.93, and specificity of 0.86.
METHODOLOGY:
- Patients often need help during bowel preparation for colonoscopy, which increases staff workload; up to 20%-25% of colonoscopies are reported to be inadequately prepared. Researchers developed and tested an AI tool (AI-PREPOO) using stool images to assess whether patients were ready for colonoscopy.
- They conducted a multicenter observational study in Japan between 2022 and 2023 that included 37 patients scheduled for colonoscopy (median age, 57 years; 45.9% women).
- After starting consumption of a 2-liter polyethylene glycol solution, patients used smartphones to take photos of their stool in the toilet after each bowel movement and uploaded the images to a secure web server.
- The images were divided into training and test sets. Images were classified as “ready” for colonoscopy when the stool was clear or light yellow and watery with no solid content.
- Four image-recognition models based on different deep learning architectures were developed using transfer learning to classify readiness for colonoscopy.
TAKEAWAY:
- Researchers collected 282 stool images, with 141 classified as ready and 141 as not ready. Of these, 224 images were used for training (the number augmented to 2240 images) and 58 for testing.
- All four AI-PREPOO models showed high performance, with AUCs ranging from 0.92 to 0.95; pairwise differences in AUCs were not significant.
- The AI-PREPOO 1 model, based on the MobileNetV3-Small architecture, showed the most balanced performance, with an AUC of 0.95, accuracy of 0.90, sensitivity of 0.93, and specificity of 0.86 on the test set.
- During colonoscopy, all patients had a Boston Bowel Preparation Scale score of 6 or higher, indicating that “ready” images corresponded to an adequately prepared bowel.
IN PRACTICE:
“If implemented as a mobile application, our model would allow patients to quickly and independently assess bowel preparation adequacy, reducing reliance on nurses and alleviating embarrassment associated with sharing stool images. This approach could also lessen nurses’ workload by minimizing unnecessary inquiries and preventing excessive or insufficient bowel preparation due to uncertainty,” the authors wrote.
SOURCE:
This study was led by Kosuke Kojima, Graduate School of Medical and Dental Sciences, Niigata University in Niigata, Japan. It was published online in the Journal of Gastroenterology and Hepatology.
LIMITATIONS:
The small dataset limited generalizability and increased the risk for overfitting. In real-world practice, stool images might vary in lighting, angle, focus, zoom, and background; thus, a larger and more diverse dataset was needed. The model lacked external validation in an independent or prospective cohort.
DISCLOSURES:
This study received support from the Japanese Foundation for Research and Promotion of Endoscopy. The authors reported having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
AI Tool Helps Patients Assess Bowel Preparation for Colonoscopy
AI Tool Helps Patients Assess Bowel Preparation for Colonoscopy
Alcohol and CRC: These Drinking Patterns May Influence Risk
Alcohol and CRC: These Drinking Patterns May Influence Risk
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
New research sheds light on how chronic heavy alcohol use may contribute to colorectal cancer (CRC) development and how quitting may lower the risk for precancerous colorectal adenomas.
In a large US cancer screening trial, current heavy drinkers — with an average lifetime alcohol intake of 14 or more drinks per week — had a 25% higher risk for CRC and an almost twofold higher risk for rectal cancer than light drinkers averaging less than one drink per week.
When the research team further considered drinking consistency, steady heavy drinking throughout adulthood was associated with a 91% higher risk for CRC than consistent light drinking.
Additionally, no increased risk for CRC was found among former drinkers, and former drinkers were less likely than light drinkers to develop nonadvanced colorectal adenomas.
This analysis “adds to the growing amount of concerning literature showing that chronic heavy alcohol use can potentially contribute to colorectal cancer development,” Benjamin H. Levy III, MD, gastroenterologist and clinical associate of medicine at UChicago Medicine in Chicago, who wasn’t involved in the study, told Medscape Medical News.
The study’s co-senior author, Erikka Loftfield, PhD, MPH, also noted that the study “provides new evidence indicating that drinking cessation, compared to consistent light drinking, may lower adenoma risk.”
Current cancer prevention guidelines recommend limiting alcohol intake or ideally not drinking at all, and “our findings do not change this advice,” said Loftfield, with the National Cancer Institute (NCI) in Bethesda, Maryland.
The study was published online on January 26 in the journal Cancer.
Addressing a Data Gap
Alcoholic beverages are classified as carcinogenic to humans and are causally associated with CRC, Loftfield told Medscape Medical News. However, much of the evidence for this comes from cohort studies that only measure recent drinking patterns, generally among older adults, at study baseline. Fewer studies have looked at how drinking over a person’s lifetime and alcohol consumption patterns relate to colorectal adenoma and CRC risk, she explained.
To address these gaps, Loftfield and colleagues leveraged data on alcohol intake gathered as part of the NCI’s Prostate, Long, Colorectal, and Ovarian Cancer Screening Trial.
Average lifetime alcohol intake was calculated as drinks per week from age 18 through study baseline, and drinking patterns were further classified based on consistency and intensity over time.
During 20 years of follow-up, 1679 incident CRC cases occurred among 88,092 study participants. In multivariable-adjusted analyses, current heavy drinkers had a higher risk for CRC than those averaging less than one drink per week (hazard ratio [HR], 1.25), with the strongest association observed for rectal cancer (HR, 1.95).
“The increase in rectal cancer risk for heavy drinkers seen in this 20-year observational study was especially concerning,” Levy told Medscape Medical News.
What About Moderate Drinking?
Perhaps counterintuitively, moderate current drinkers (those consuming an average of 7 to less than 14 drinks per week) had a lower risk for CRC (HR, 0.79), especially distal colon cancer (HR, 0.64), than light drinkers.
Loftfield said that research in rodents suggests moderate alcohol intake may reduce inflammation and lower DNA damage, but it’s possible that the observed inverse association is due to residual confounding by unmeasured or poorly measured confounders, such as socioeconomic status.
She said it’s also important to note that the inverse association of moderate alcohol intake was strongest for distal colon cancer and in the screening arm of the trial. Those in the screening arm who screened positive with flexible sigmoidoscopy had polyps removed and were referred for colonoscopy during the trial period, making screening a potential intervention as well.
“Screening with flexible sigmoidoscopy has previously been found to decrease CRC incidence, specifically distal colon cancer, in this population. Thus, it is possible that better adherence to screening among moderate drinkers over the course of follow-up contributed to this finding,” Loftfield explained.
When looking at consistency of drinking, her team found that current drinkers who were consistent heavy drinkers throughout adulthood had a higher risk for CRC than consistent light drinkers (HR, 1.91).
Separate analyses of incident colorectal adenomas were directionally consistent with the CRC findings. These analyses included 12,327 participants with a negative baseline sigmoidoscopy, among whom 812 adenomas were detected on repeat screening.
Compared with current light drinkers, former drinkers had significantly lower odds of nonadvanced adenomas (odds ratio [OR], 0.58), but no significant association was observed for advanced adenomas (OR, 1.08; 95% CI, 0.62-1.90). The authors cautioned, however, that overall adenoma case numbers were limited, and estimates for advanced lesions were imprecise.
Educating Patients
Reached for comment, William Dahut, chief scientific officer for the American Cancer Society, told Medscape Medical News that this “very well done, large perspective study clearly demonstrates the significant increased risk of colorectal cancer for those that are heavy drinkers.”
He noted that the nearly twofold increased risk for rectal cancer among heavy drinkers “makes biological sense because the rectum is the area of the body where the toxins produced by alcohol potentially spend the most period of time.”
Heavy drinkers are at the highest risk, Dahut said, and “for them, screenings are particularly important.”
Even with this growing body of evidence, Levy noted that many patients in America and worldwide “have not been educated yet about the potential carcinogenic dangers of chronic alcohol use.”
Levy recommended that physicians get “accurate social histories about alcohol use” and “spend several minutes educating patients about their increased risk of cancer and liver problems from heavy alcohol use.”
Dahut encouraged health providers to tell patients that the risk for CRC from alcohol is also based on one’s lifetime alcohol consumption, “not simply what they had last weekend.”
Overall, this important research study, along with the Surgeon General’s recent publication about Alcohol and Cancer Risk, will hopefully “encourage physicians to have important conversations about alcohol reduction with their patients,” Levy said.
The study had no commercial funding. Loftfield, Dahult, and Levy reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Alcohol and CRC: These Drinking Patterns May Influence Risk
Alcohol and CRC: These Drinking Patterns May Influence Risk
A New First-Line Option in BRAF-Mutant Metastatic CRC?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
The targeted therapy combination of encorafenib and cetuximab with FOLFIRI (leucovorin/5-fluorouracil [FU]/irinotecan) chemotherapy may be a new first-line option for patients with BRAF V600E-mutant metastatic colorectal cancer (CRC), according to new results from the BREAKWATER trial.
After a median follow-up of about 10 months, response rates were significantly better with encorafenib and cetuximab plus FOLFIRI than with FOLFIRI alone — without increasing side effects.
The findings, presented at the ASCO Gastrointestinal Cancers Symposium 2026, point to a potential new option for the 20%-25% of patients with BRAF V600E-mutant metastatic CRC who receive FOLFIRI as their chemotherapy.
Based on previous results from BREAKWATER, the FDA granted accelerated approval to first-line encorafenib (Braftovi) and cetuximab (Erbitux) plus mFOLFOX6 for this patient population. That regimen doubled median overall survival compared with standard chemotherapy with or without bevacizumab.
Cohort 3 of BREAKWATER was designed to address a specific question: Are the benefits with the targeted therapy duo a “FOLFOX-specific phenomenon?” lead investigator Scott Kopetz, MD, PhD, of MD Anderson Cancer Center, Houston, said during a press briefing.
Based on these early results, the answer is no. Instead, Kopetz said, there appears to be a “broader synergy” between the targeted therapies and cytotoxic chemotherapy.
Joel Saltzman, MD, ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic in Cleveland, agreed.
“The additional data from the BREAKWATER trial reveals that it is the targeted therapy backbone that provides the better disease control and response rate in BRAF V600E-mutant colorectal cancers,” he said.
BRAF V600E mutations occur in up to 12% of patients with metastatic CRC and are associated with poor outcomes. While many newly diagnosed patients receive FOLFOX (leucovorin/5-FU/oxaliplatin) in the first line, FOLFIRI is a common alternative — often due to concerns about oxaliplatin-associated peripheral neuropathy, Kopetz noted.
The safety lead-in portion of BREAKWATER showed that encorafenib and cetuximab plus FOLFIRI were tolerable and had promising antitumor activity.
Cohort 3 of the trial included 147 patients (mean age, 62 years; 46% male) with BRAF V600E-mutant metastatic CRC, no prior systemic treatment, and good performance status (Eastern Cooperative Oncology Group PS 0 or 1); 73 patients were randomly allocated to encorafenib and cetuximab plus FOLFIRI and 74 to FOLFIRI with or without bevacizumab. The primary endpoint was objective response rate assessed by blinded independent central review.
After a median follow-up of 10 months, patients in the targeted therapy group had an objective response rate of 64.4% vs 39.2% among patients who received FOLFIRI alone or with bevacizumab (odds ratio, 2.76; P = .001).
Responses to the targeted therapies were “rapid and durable,” Kopetz said. More than half (57.4%) of patients treated with encorafenib and cetuximab and FOLFIRI had a duration of response of 6 months or longer than 34.5% in the control group.
Data on overall survival, a secondary endpoint, were not yet mature, but there was a trend toward improved survival with targeted therapy.
Importantly, Kopetz reported, there were no new safety signals, and serious treatment-emergent adverse events occurred at a similar rate in both treatment groups: 39.4% in the targeted therapy group and 36.8% in the control group.
The most common adverse events in both groups included nausea, diarrhea, vomiting, fatigue, appetite loss, and alopecia. About 10% of patients in the targeted therapy group and 9% of those in the control group discontinued their treatment early, suggesting the severity of side effects was similar between the groups.
Kopetz cautioned that the data are still early and follow-up is ongoing. However, he said, the findings support the targeted drugs plus FOLFIRI as a “potential new standard of care” for this patient population.
“The addition of FOLFIRI chemotherapy in the frontline setting will give oncologists and patients more options when selecting a first-line regimen,” Saltzman said. “To have as many options as possible is certainly something we all hope for.”
The trial was funded by Pfizer. Kopetz reported consulting for Pfizer and several other pharmaceutical companies. Saltzman reported having no disclosures.
A version of this article first appeared on Medscape.com.
A New First-Line Option in BRAF-Mutant Metastatic CRC?
A New First-Line Option in BRAF-Mutant Metastatic CRC?
GLP-1 Drugs Tied to Lower CRC Risk and Better Outcomes
GLP-1 Drugs Tied to Lower CRC Risk and Better Outcomes
The GLP-1 drugs widely prescribed for diabetes and weight loss might also help reduce the risk for colorectal cancer and possibly improve outcomes in people who have the disease, according to a series of studies presented at ASCO Gastrointestinal Cancers Symposium 2026.
In one study, researchers observed a 36% lower risk for colorectal cancer among people who used GLP-1 receptor agonists vs those who used aspirin — a drug long investigated for colorectal cancer primary prevention.
While aspirin has shown “modest efficacy” in that regard, it also carries a bleeding risk that limits its use, Colton Jones, MD, a hematology and oncology fellow with The University of Texas San Antonio, told conference attendees.
Emerging evidence suggests that GLP-1s possess anti-inflammatory and anti-neoplastic properties, while some recent observational studies have linked the medications to reduced risks for certain cancers, particularly obesity-related types.
However, Jones said, research into a possible role for GLP-1s in cancer risk reduction is still in the early stages.
Prevention Potential
To conduct a “real-world” analysis, Jones and his colleagues turned to the TriNetX database, which contains electronic health records from about 150 million patients at more than 100 US healthcare organizations.
The researchers created two propensity score-matched cohorts of GLP-1 users and aspirin users, with 140,828 patients (average age, 58 years) in each. None had a history of colorectal cancer, and none were using anti-inflammatory medications other than aspirin or glucose-lowering drugs other than a GLP-1.
During a median follow-up of 5-6 years, GLP-1 use was significantly associated with reduced colorectal cancer incidence compared with aspirin use (hazard ratio [HR], 0.64). The findings were similar among people considered to be at an increased colorectal cancer risk due to health or family history: In that group, GLP-1 users had a roughly 42% lower risk of the disease (HR, 0.58).
Overall, the risk reduction with GLP-1 use was seen regardless of obesity or diabetes status, but the association was strongest among people who began treatment before age 45.
When the researchers examined individual GLP-1 medications, only semaglutide (Ozempic), liraglutide (Saxenda/Victoza), and dulaglutide (Trulicity) were associated with significant risk reductions.
As for safety outcomes, aspirin users had slightly higher rates of gastrointestinal bleeding and gastric ulcers and were more likely to suffer acute kidney injury (2.8% vs 1.15% among GLP-1 users; HR, 0.37). GLP-1 users experienced more diarrhea (6.8% vs 5.4%) and abdominal pain (19% vs 16.3%) than aspirin users did.
Jones said that both the risk reduction and safety profile associated with GLP-1s “underscore a potential public health impact” and warrant prospective validation.
Study discussant Joel Saltzman, MD, an ASCO gastrointestinal cancer expert, called the findings “thought-provoking.”
Broadly, he said, the study raises important questions about how metabolic disease, obesity, and cancer risk are interconnected — and how prevention strategies might evolve as more data emerge.
“It will certainly be interesting over the upcoming years to see how [GLP-1s] fit into colorectal cancer prevention,” said Saltzman, of Taussig Cancer Center, Cleveland Clinic, Cleveland.
Improved CRC Outcomes?
Looking beyond prevention, Jones and his colleagues conducted a separate analysis of patients diagnosed with colorectal cancer, to see whether GLP-1 therapy was associated with outcomes.
In that analysis, also using the TriNetX database, they matched 5170 patients with colorectal cancer who were on GLP-1 therapy with the same number of patients who were not on a GLP-1 medication.
Over 10 years, GLP-1 use was associated with a 53% reduction in all-cause mortality compared with nonuse (HR, 0.47), corresponding to an absolute risk reduction of 5.6% and a number needed to treat of 18.
The survival benefit was consistent across age, diabetes status, BMI, cancer stage, and treatment subgroups. GLP-1 use was not associated with a statistically significant change in the risk for metastases (HR, 0.895).
Meanwhile, another study presented at the meeting, by researchers at Mayo Clinic, Jacksonville, Florida, yielded similar findings.
Researchers led by Yajur Arya, MD, focused specifically on patients with colon cancer and comorbid obesity comparing outcomes in nearly 2000 patients taking a GLP-1 with more than 16,000 matched patients who were not on a GLP-1 agent.
Over 5 years of follow-up, GLP-1 users had a lower risk for overall mortality (HR, 0.46). They also showed decreased risks for myocardial infarction (HR, 0.83), sepsis (risk difference, -3.48%), and need for mechanical ventilation (HR, 0.49).
Both Jones and Arya stressed, however, that the findings only serve to highlight possible benefits of GLP-1 use beyond diabetes and weight management. Prospective studies, they said, are needed to better understand why these associations exist, and to potentially guide practice in the future.
None of the studies had commercial funding. Jones, Arya, and Saltzman had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The GLP-1 drugs widely prescribed for diabetes and weight loss might also help reduce the risk for colorectal cancer and possibly improve outcomes in people who have the disease, according to a series of studies presented at ASCO Gastrointestinal Cancers Symposium 2026.
In one study, researchers observed a 36% lower risk for colorectal cancer among people who used GLP-1 receptor agonists vs those who used aspirin — a drug long investigated for colorectal cancer primary prevention.
While aspirin has shown “modest efficacy” in that regard, it also carries a bleeding risk that limits its use, Colton Jones, MD, a hematology and oncology fellow with The University of Texas San Antonio, told conference attendees.
Emerging evidence suggests that GLP-1s possess anti-inflammatory and anti-neoplastic properties, while some recent observational studies have linked the medications to reduced risks for certain cancers, particularly obesity-related types.
However, Jones said, research into a possible role for GLP-1s in cancer risk reduction is still in the early stages.
Prevention Potential
To conduct a “real-world” analysis, Jones and his colleagues turned to the TriNetX database, which contains electronic health records from about 150 million patients at more than 100 US healthcare organizations.
The researchers created two propensity score-matched cohorts of GLP-1 users and aspirin users, with 140,828 patients (average age, 58 years) in each. None had a history of colorectal cancer, and none were using anti-inflammatory medications other than aspirin or glucose-lowering drugs other than a GLP-1.
During a median follow-up of 5-6 years, GLP-1 use was significantly associated with reduced colorectal cancer incidence compared with aspirin use (hazard ratio [HR], 0.64). The findings were similar among people considered to be at an increased colorectal cancer risk due to health or family history: In that group, GLP-1 users had a roughly 42% lower risk of the disease (HR, 0.58).
Overall, the risk reduction with GLP-1 use was seen regardless of obesity or diabetes status, but the association was strongest among people who began treatment before age 45.
When the researchers examined individual GLP-1 medications, only semaglutide (Ozempic), liraglutide (Saxenda/Victoza), and dulaglutide (Trulicity) were associated with significant risk reductions.
As for safety outcomes, aspirin users had slightly higher rates of gastrointestinal bleeding and gastric ulcers and were more likely to suffer acute kidney injury (2.8% vs 1.15% among GLP-1 users; HR, 0.37). GLP-1 users experienced more diarrhea (6.8% vs 5.4%) and abdominal pain (19% vs 16.3%) than aspirin users did.
Jones said that both the risk reduction and safety profile associated with GLP-1s “underscore a potential public health impact” and warrant prospective validation.
Study discussant Joel Saltzman, MD, an ASCO gastrointestinal cancer expert, called the findings “thought-provoking.”
Broadly, he said, the study raises important questions about how metabolic disease, obesity, and cancer risk are interconnected — and how prevention strategies might evolve as more data emerge.
“It will certainly be interesting over the upcoming years to see how [GLP-1s] fit into colorectal cancer prevention,” said Saltzman, of Taussig Cancer Center, Cleveland Clinic, Cleveland.
Improved CRC Outcomes?
Looking beyond prevention, Jones and his colleagues conducted a separate analysis of patients diagnosed with colorectal cancer, to see whether GLP-1 therapy was associated with outcomes.
In that analysis, also using the TriNetX database, they matched 5170 patients with colorectal cancer who were on GLP-1 therapy with the same number of patients who were not on a GLP-1 medication.
Over 10 years, GLP-1 use was associated with a 53% reduction in all-cause mortality compared with nonuse (HR, 0.47), corresponding to an absolute risk reduction of 5.6% and a number needed to treat of 18.
The survival benefit was consistent across age, diabetes status, BMI, cancer stage, and treatment subgroups. GLP-1 use was not associated with a statistically significant change in the risk for metastases (HR, 0.895).
Meanwhile, another study presented at the meeting, by researchers at Mayo Clinic, Jacksonville, Florida, yielded similar findings.
Researchers led by Yajur Arya, MD, focused specifically on patients with colon cancer and comorbid obesity comparing outcomes in nearly 2000 patients taking a GLP-1 with more than 16,000 matched patients who were not on a GLP-1 agent.
Over 5 years of follow-up, GLP-1 users had a lower risk for overall mortality (HR, 0.46). They also showed decreased risks for myocardial infarction (HR, 0.83), sepsis (risk difference, -3.48%), and need for mechanical ventilation (HR, 0.49).
Both Jones and Arya stressed, however, that the findings only serve to highlight possible benefits of GLP-1 use beyond diabetes and weight management. Prospective studies, they said, are needed to better understand why these associations exist, and to potentially guide practice in the future.
None of the studies had commercial funding. Jones, Arya, and Saltzman had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The GLP-1 drugs widely prescribed for diabetes and weight loss might also help reduce the risk for colorectal cancer and possibly improve outcomes in people who have the disease, according to a series of studies presented at ASCO Gastrointestinal Cancers Symposium 2026.
In one study, researchers observed a 36% lower risk for colorectal cancer among people who used GLP-1 receptor agonists vs those who used aspirin — a drug long investigated for colorectal cancer primary prevention.
While aspirin has shown “modest efficacy” in that regard, it also carries a bleeding risk that limits its use, Colton Jones, MD, a hematology and oncology fellow with The University of Texas San Antonio, told conference attendees.
Emerging evidence suggests that GLP-1s possess anti-inflammatory and anti-neoplastic properties, while some recent observational studies have linked the medications to reduced risks for certain cancers, particularly obesity-related types.
However, Jones said, research into a possible role for GLP-1s in cancer risk reduction is still in the early stages.
Prevention Potential
To conduct a “real-world” analysis, Jones and his colleagues turned to the TriNetX database, which contains electronic health records from about 150 million patients at more than 100 US healthcare organizations.
The researchers created two propensity score-matched cohorts of GLP-1 users and aspirin users, with 140,828 patients (average age, 58 years) in each. None had a history of colorectal cancer, and none were using anti-inflammatory medications other than aspirin or glucose-lowering drugs other than a GLP-1.
During a median follow-up of 5-6 years, GLP-1 use was significantly associated with reduced colorectal cancer incidence compared with aspirin use (hazard ratio [HR], 0.64). The findings were similar among people considered to be at an increased colorectal cancer risk due to health or family history: In that group, GLP-1 users had a roughly 42% lower risk of the disease (HR, 0.58).
Overall, the risk reduction with GLP-1 use was seen regardless of obesity or diabetes status, but the association was strongest among people who began treatment before age 45.
When the researchers examined individual GLP-1 medications, only semaglutide (Ozempic), liraglutide (Saxenda/Victoza), and dulaglutide (Trulicity) were associated with significant risk reductions.
As for safety outcomes, aspirin users had slightly higher rates of gastrointestinal bleeding and gastric ulcers and were more likely to suffer acute kidney injury (2.8% vs 1.15% among GLP-1 users; HR, 0.37). GLP-1 users experienced more diarrhea (6.8% vs 5.4%) and abdominal pain (19% vs 16.3%) than aspirin users did.
Jones said that both the risk reduction and safety profile associated with GLP-1s “underscore a potential public health impact” and warrant prospective validation.
Study discussant Joel Saltzman, MD, an ASCO gastrointestinal cancer expert, called the findings “thought-provoking.”
Broadly, he said, the study raises important questions about how metabolic disease, obesity, and cancer risk are interconnected — and how prevention strategies might evolve as more data emerge.
“It will certainly be interesting over the upcoming years to see how [GLP-1s] fit into colorectal cancer prevention,” said Saltzman, of Taussig Cancer Center, Cleveland Clinic, Cleveland.
Improved CRC Outcomes?
Looking beyond prevention, Jones and his colleagues conducted a separate analysis of patients diagnosed with colorectal cancer, to see whether GLP-1 therapy was associated with outcomes.
In that analysis, also using the TriNetX database, they matched 5170 patients with colorectal cancer who were on GLP-1 therapy with the same number of patients who were not on a GLP-1 medication.
Over 10 years, GLP-1 use was associated with a 53% reduction in all-cause mortality compared with nonuse (HR, 0.47), corresponding to an absolute risk reduction of 5.6% and a number needed to treat of 18.
The survival benefit was consistent across age, diabetes status, BMI, cancer stage, and treatment subgroups. GLP-1 use was not associated with a statistically significant change in the risk for metastases (HR, 0.895).
Meanwhile, another study presented at the meeting, by researchers at Mayo Clinic, Jacksonville, Florida, yielded similar findings.
Researchers led by Yajur Arya, MD, focused specifically on patients with colon cancer and comorbid obesity comparing outcomes in nearly 2000 patients taking a GLP-1 with more than 16,000 matched patients who were not on a GLP-1 agent.
Over 5 years of follow-up, GLP-1 users had a lower risk for overall mortality (HR, 0.46). They also showed decreased risks for myocardial infarction (HR, 0.83), sepsis (risk difference, -3.48%), and need for mechanical ventilation (HR, 0.49).
Both Jones and Arya stressed, however, that the findings only serve to highlight possible benefits of GLP-1 use beyond diabetes and weight management. Prospective studies, they said, are needed to better understand why these associations exist, and to potentially guide practice in the future.
None of the studies had commercial funding. Jones, Arya, and Saltzman had no relevant disclosures.
A version of this article first appeared on Medscape.com.
GLP-1 Drugs Tied to Lower CRC Risk and Better Outcomes
GLP-1 Drugs Tied to Lower CRC Risk and Better Outcomes
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Regular physical activity—especially walking—may improve fatigue and boost quality of life for people with nonmetastatic colorectal cancer during the first 2 years after diagnosis, according to research presented at ASCO Gastrointestinal Cancers Symposium 2026.
The study, which tracked over 1700 patients with colorectal cancer, found that those with nonmetastatic disease who walked for exercise 6-12 months after their diagnosis showed significant improvement in their fatigue scores over time. Their quality-of-life ratings rose in tandem.
The findings suggest that simple, sustained movement may play a meaningful role in long-term survivorship care, lead investigator Louisa Liu, MD, of Cedars-Sinai Medical Center in Los Angeles, said during a press briefing.
“Fatigue is one of the most common and debilitating symptoms our patients experience, often long after treatment ends,” Liu noted.
The new data, she said, show that an accessible form of exercise, especially when maintained over time, “can make a real difference in how patients feel and function during recovery.”
Joel Saltzman, MD, an ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic, Cleveland, agreed.
This is a “super-important study for all of us in the cancer community,” Saltzman told the briefing, especially in light of the CHALLENGE trial.
That study demonstrated that a structured exercise program can actually improve overall survival for patients with early-stage colon cancer who completed surgery and adjuvant chemotherapy.
“When you couple that with how patients feel, it really begs the question: Are we as a society doing enough cancer rehabilitation?” Saltzman said. “Everyone’s familiar with cardiac rehab, but oncologic rehabilitation is really something that really should be thought about in the future.”
Among long-term colorectal cancer survivors, nearly 40% continue to experience moderate-to-severe fatigue years after treatment — a challenge that affects functional recovery, daily activity, and quality of life.
“Yet,” Liu said, “our toolbox of effective interventions remains limited.”
Growing evidence supports physical activity as a nonpharmacologic approach for managing cancer-related fatigue. The mechanisms, Liu noted, may be multiple and include reductions in systemic inflammation, preserved muscle mass, better sleep quality and improvements in psychological stress.
In fact, current clinical guidelines recommend physical activity as part of survivorship care, but some key questions remain unanswered, Liu said.
“We still don’t fully understand when during recovery activity is most beneficial, what types of activity are best for different patients, or how these effects play out in real-world longitudinal settings, especially in colorectal cancer survivors,” she explained.
To address some of those gaps, Liu and colleagues analyzed data from 1718 patients with colorectal cancer (mean age, 67 years; 48% women) enrolled in the International ColoCare prospective cohort study. Nearly 1 in 5 had metastatic disease at diagnosis.
Physical activity was assessed at baseline and at 6, 12, and 24 months after diagnosis using a validated questionnaire. Participants’ total number of metabolic equivalent of task (MET) minutes per week — a measurement of energy spent during physical activity — were calculated for walking, moderate activities, and vigorous activities.
Total physical activity was categorized as low (fewer than 600 MET min/wk), moderate (600-3000 MET min/wk), or high (over 3000 MET min/wk).
Cancer-related fatigue and quality of life were measured using the European Organization for Research and Treatment of Cancer QLQ-C30 scale.
Overall, patients who were more physically active reported less fatigue and better quality of life as they moved further into recovery. And walking, Liu said, showed the “clearest and most consistent” association with these improved outcomes.
Among patients with nonmetastatic disease, those who reported regular walking 6-12 months after diagnosis showed significantly lower fatigue and higher quality-of-life scores over 2 years. Fatigue scores in this group improved steadily with time, from 32.5 at diagnosis to 29 at 12 months post-diagnosis and 26.8 at 24 months post-diagnosis.
Patients with metastatic disease also showed reductions in fatigue scores — from 40.7 at diagnosis to 37.1 at 12 months and 36.4 at 24 months — although those differences did not reach statistical significance.
Liu pointed out that patients with metastatic disease, not surprisingly, reported greater fatigue and poorer quality of life across all time points vs those with early-stage disease.
So, she said, “we don’t yet have strong evidence that physical activity changes the fatigue trajectory in the long run for metastatic patients. But this is an area where more targeted research is really needed.”
Looking at patterns of physical activity, the researchers found that activity levels at the time of diagnosis did not reliably predict long-term fatigue and quality-of-life outcomes. Instead, a patient’s activity level maintained between diagnosis and 1 year follow-up was a predictor of better outcomes.
“Short-term increases in physical activity didn’t seem to make a meaningful difference,” Liu said. “This suggests that when it comes to managing cancer-related fatigue, the key is to build steady, lasting habits that patients can stick with throughout their recovery.”
The study had no commercial funding. Liu and Saltzman had no disclosures.
A version of this article first appeared on Medscape.com.
Regular physical activity—especially walking—may improve fatigue and boost quality of life for people with nonmetastatic colorectal cancer during the first 2 years after diagnosis, according to research presented at ASCO Gastrointestinal Cancers Symposium 2026.
The study, which tracked over 1700 patients with colorectal cancer, found that those with nonmetastatic disease who walked for exercise 6-12 months after their diagnosis showed significant improvement in their fatigue scores over time. Their quality-of-life ratings rose in tandem.
The findings suggest that simple, sustained movement may play a meaningful role in long-term survivorship care, lead investigator Louisa Liu, MD, of Cedars-Sinai Medical Center in Los Angeles, said during a press briefing.
“Fatigue is one of the most common and debilitating symptoms our patients experience, often long after treatment ends,” Liu noted.
The new data, she said, show that an accessible form of exercise, especially when maintained over time, “can make a real difference in how patients feel and function during recovery.”
Joel Saltzman, MD, an ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic, Cleveland, agreed.
This is a “super-important study for all of us in the cancer community,” Saltzman told the briefing, especially in light of the CHALLENGE trial.
That study demonstrated that a structured exercise program can actually improve overall survival for patients with early-stage colon cancer who completed surgery and adjuvant chemotherapy.
“When you couple that with how patients feel, it really begs the question: Are we as a society doing enough cancer rehabilitation?” Saltzman said. “Everyone’s familiar with cardiac rehab, but oncologic rehabilitation is really something that really should be thought about in the future.”
Among long-term colorectal cancer survivors, nearly 40% continue to experience moderate-to-severe fatigue years after treatment — a challenge that affects functional recovery, daily activity, and quality of life.
“Yet,” Liu said, “our toolbox of effective interventions remains limited.”
Growing evidence supports physical activity as a nonpharmacologic approach for managing cancer-related fatigue. The mechanisms, Liu noted, may be multiple and include reductions in systemic inflammation, preserved muscle mass, better sleep quality and improvements in psychological stress.
In fact, current clinical guidelines recommend physical activity as part of survivorship care, but some key questions remain unanswered, Liu said.
“We still don’t fully understand when during recovery activity is most beneficial, what types of activity are best for different patients, or how these effects play out in real-world longitudinal settings, especially in colorectal cancer survivors,” she explained.
To address some of those gaps, Liu and colleagues analyzed data from 1718 patients with colorectal cancer (mean age, 67 years; 48% women) enrolled in the International ColoCare prospective cohort study. Nearly 1 in 5 had metastatic disease at diagnosis.
Physical activity was assessed at baseline and at 6, 12, and 24 months after diagnosis using a validated questionnaire. Participants’ total number of metabolic equivalent of task (MET) minutes per week — a measurement of energy spent during physical activity — were calculated for walking, moderate activities, and vigorous activities.
Total physical activity was categorized as low (fewer than 600 MET min/wk), moderate (600-3000 MET min/wk), or high (over 3000 MET min/wk).
Cancer-related fatigue and quality of life were measured using the European Organization for Research and Treatment of Cancer QLQ-C30 scale.
Overall, patients who were more physically active reported less fatigue and better quality of life as they moved further into recovery. And walking, Liu said, showed the “clearest and most consistent” association with these improved outcomes.
Among patients with nonmetastatic disease, those who reported regular walking 6-12 months after diagnosis showed significantly lower fatigue and higher quality-of-life scores over 2 years. Fatigue scores in this group improved steadily with time, from 32.5 at diagnosis to 29 at 12 months post-diagnosis and 26.8 at 24 months post-diagnosis.
Patients with metastatic disease also showed reductions in fatigue scores — from 40.7 at diagnosis to 37.1 at 12 months and 36.4 at 24 months — although those differences did not reach statistical significance.
Liu pointed out that patients with metastatic disease, not surprisingly, reported greater fatigue and poorer quality of life across all time points vs those with early-stage disease.
So, she said, “we don’t yet have strong evidence that physical activity changes the fatigue trajectory in the long run for metastatic patients. But this is an area where more targeted research is really needed.”
Looking at patterns of physical activity, the researchers found that activity levels at the time of diagnosis did not reliably predict long-term fatigue and quality-of-life outcomes. Instead, a patient’s activity level maintained between diagnosis and 1 year follow-up was a predictor of better outcomes.
“Short-term increases in physical activity didn’t seem to make a meaningful difference,” Liu said. “This suggests that when it comes to managing cancer-related fatigue, the key is to build steady, lasting habits that patients can stick with throughout their recovery.”
The study had no commercial funding. Liu and Saltzman had no disclosures.
A version of this article first appeared on Medscape.com.
Regular physical activity—especially walking—may improve fatigue and boost quality of life for people with nonmetastatic colorectal cancer during the first 2 years after diagnosis, according to research presented at ASCO Gastrointestinal Cancers Symposium 2026.
The study, which tracked over 1700 patients with colorectal cancer, found that those with nonmetastatic disease who walked for exercise 6-12 months after their diagnosis showed significant improvement in their fatigue scores over time. Their quality-of-life ratings rose in tandem.
The findings suggest that simple, sustained movement may play a meaningful role in long-term survivorship care, lead investigator Louisa Liu, MD, of Cedars-Sinai Medical Center in Los Angeles, said during a press briefing.
“Fatigue is one of the most common and debilitating symptoms our patients experience, often long after treatment ends,” Liu noted.
The new data, she said, show that an accessible form of exercise, especially when maintained over time, “can make a real difference in how patients feel and function during recovery.”
Joel Saltzman, MD, an ASCO expert in gastrointestinal cancers based at Taussig Cancer Center, Cleveland Clinic, Cleveland, agreed.
This is a “super-important study for all of us in the cancer community,” Saltzman told the briefing, especially in light of the CHALLENGE trial.
That study demonstrated that a structured exercise program can actually improve overall survival for patients with early-stage colon cancer who completed surgery and adjuvant chemotherapy.
“When you couple that with how patients feel, it really begs the question: Are we as a society doing enough cancer rehabilitation?” Saltzman said. “Everyone’s familiar with cardiac rehab, but oncologic rehabilitation is really something that really should be thought about in the future.”
Among long-term colorectal cancer survivors, nearly 40% continue to experience moderate-to-severe fatigue years after treatment — a challenge that affects functional recovery, daily activity, and quality of life.
“Yet,” Liu said, “our toolbox of effective interventions remains limited.”
Growing evidence supports physical activity as a nonpharmacologic approach for managing cancer-related fatigue. The mechanisms, Liu noted, may be multiple and include reductions in systemic inflammation, preserved muscle mass, better sleep quality and improvements in psychological stress.
In fact, current clinical guidelines recommend physical activity as part of survivorship care, but some key questions remain unanswered, Liu said.
“We still don’t fully understand when during recovery activity is most beneficial, what types of activity are best for different patients, or how these effects play out in real-world longitudinal settings, especially in colorectal cancer survivors,” she explained.
To address some of those gaps, Liu and colleagues analyzed data from 1718 patients with colorectal cancer (mean age, 67 years; 48% women) enrolled in the International ColoCare prospective cohort study. Nearly 1 in 5 had metastatic disease at diagnosis.
Physical activity was assessed at baseline and at 6, 12, and 24 months after diagnosis using a validated questionnaire. Participants’ total number of metabolic equivalent of task (MET) minutes per week — a measurement of energy spent during physical activity — were calculated for walking, moderate activities, and vigorous activities.
Total physical activity was categorized as low (fewer than 600 MET min/wk), moderate (600-3000 MET min/wk), or high (over 3000 MET min/wk).
Cancer-related fatigue and quality of life were measured using the European Organization for Research and Treatment of Cancer QLQ-C30 scale.
Overall, patients who were more physically active reported less fatigue and better quality of life as they moved further into recovery. And walking, Liu said, showed the “clearest and most consistent” association with these improved outcomes.
Among patients with nonmetastatic disease, those who reported regular walking 6-12 months after diagnosis showed significantly lower fatigue and higher quality-of-life scores over 2 years. Fatigue scores in this group improved steadily with time, from 32.5 at diagnosis to 29 at 12 months post-diagnosis and 26.8 at 24 months post-diagnosis.
Patients with metastatic disease also showed reductions in fatigue scores — from 40.7 at diagnosis to 37.1 at 12 months and 36.4 at 24 months — although those differences did not reach statistical significance.
Liu pointed out that patients with metastatic disease, not surprisingly, reported greater fatigue and poorer quality of life across all time points vs those with early-stage disease.
So, she said, “we don’t yet have strong evidence that physical activity changes the fatigue trajectory in the long run for metastatic patients. But this is an area where more targeted research is really needed.”
Looking at patterns of physical activity, the researchers found that activity levels at the time of diagnosis did not reliably predict long-term fatigue and quality-of-life outcomes. Instead, a patient’s activity level maintained between diagnosis and 1 year follow-up was a predictor of better outcomes.
“Short-term increases in physical activity didn’t seem to make a meaningful difference,” Liu said. “This suggests that when it comes to managing cancer-related fatigue, the key is to build steady, lasting habits that patients can stick with throughout their recovery.”
The study had no commercial funding. Liu and Saltzman had no disclosures.
A version of this article first appeared on Medscape.com.
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Simple Steps: Walking May Ease Colorectal Cancer Fatigue
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
TOPLINE:
Among patients with ulcerative colitis (UC) who develop colorectal cancer (CRC), greater background mucosal inflammation at the time of CRC diagnosis is associated with progressively worse survival outcomes, with tumors arising within the UC-involved segment having worse prognosis.
METHODOLOGY:
- Patients with UC are at an increased risk for CRC, with risk influenced by the extent and intensity of underlying mucosal inflammation.
- Researchers retrospectively reviewed medical records of patients with UC diagnosed with CRC between 1983 and 2020 at 43 institutions across Japan to determine whether inflammation at cancer diagnosis affected prognosis.
- After endoscopic assessment, tumors were classified as arising inside the UC‑involved segment at diagnosis (within‑area tumors) or outside that segment (outside‑area tumors).
- The Mayo endoscopic score (MES) was used to grade background mucosal inflammation in the within‑area group as inactive (MES 0), mild-moderate (MES 1-2), or severe (MES 3).
- The primary endpoint was 5-year recurrence-free survival, and the secondary endpoint was 5-year cancer-specific survival.
TAKEAWAY:
- Among 723 patients followed for a median of 51 months, 683 had within-area tumors (mean age at CRC diagnosis, 51.8 years; 61.9% male) and 40 had outside-area tumors (mean age at CRC diagnosis, 61.1 years; 60.0% male).
- The within-area group had lower rate of 5-year recurrence-free survival than the outside-area group (75.1% vs 87.6%; P = .022), and lower rate of 5-year cancer-specific survival (81.1% vs 94.3%; P = .038).
- Within-area tumor location independently predicted worse recurrence-free survival (adjusted hazard ratio, 2.99; P = .030).
- In the within‑area group, higher MES was associated with stepwise (although nonsignificant) declines in recurrence‑free survival (inactive, 84.4%; mild-moderate, 79.4%; severe, 73.8%; P = .150). Corresponding cancer‑specific survival rates in these groups declined significantly (89.0%, 84.8%, and 73.8%, respectively; P = .048).
IN PRACTICE:
“These findings shift the clinical focus from inflammation as a risk factor for carcinogenesis to inflammation as a prognostic determinant, highlighting a potential new role for systematic endoscopic assessment of the background mucosa at cancer diagnosis,” the authors wrote.
SOURCE:
This study was led by Akiyoshi Ikebata, Department of Surgery, Keio University School of Medicine, Tokyo, Japan. It was published online in December 2025, in the Journal of Crohn's and Colitis.
LIMITATIONS:
The retrospective design introduced potential for unmeasured confounding and selection bias. The MES was assigned by local physicians without central review, which may have introduced variability. The small size of the outside‑area tumor group increased the risk for baseline imbalances.
DISCLOSURES:
No specific funding source was reported. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with ulcerative colitis (UC) who develop colorectal cancer (CRC), greater background mucosal inflammation at the time of CRC diagnosis is associated with progressively worse survival outcomes, with tumors arising within the UC-involved segment having worse prognosis.
METHODOLOGY:
- Patients with UC are at an increased risk for CRC, with risk influenced by the extent and intensity of underlying mucosal inflammation.
- Researchers retrospectively reviewed medical records of patients with UC diagnosed with CRC between 1983 and 2020 at 43 institutions across Japan to determine whether inflammation at cancer diagnosis affected prognosis.
- After endoscopic assessment, tumors were classified as arising inside the UC‑involved segment at diagnosis (within‑area tumors) or outside that segment (outside‑area tumors).
- The Mayo endoscopic score (MES) was used to grade background mucosal inflammation in the within‑area group as inactive (MES 0), mild-moderate (MES 1-2), or severe (MES 3).
- The primary endpoint was 5-year recurrence-free survival, and the secondary endpoint was 5-year cancer-specific survival.
TAKEAWAY:
- Among 723 patients followed for a median of 51 months, 683 had within-area tumors (mean age at CRC diagnosis, 51.8 years; 61.9% male) and 40 had outside-area tumors (mean age at CRC diagnosis, 61.1 years; 60.0% male).
- The within-area group had lower rate of 5-year recurrence-free survival than the outside-area group (75.1% vs 87.6%; P = .022), and lower rate of 5-year cancer-specific survival (81.1% vs 94.3%; P = .038).
- Within-area tumor location independently predicted worse recurrence-free survival (adjusted hazard ratio, 2.99; P = .030).
- In the within‑area group, higher MES was associated with stepwise (although nonsignificant) declines in recurrence‑free survival (inactive, 84.4%; mild-moderate, 79.4%; severe, 73.8%; P = .150). Corresponding cancer‑specific survival rates in these groups declined significantly (89.0%, 84.8%, and 73.8%, respectively; P = .048).
IN PRACTICE:
“These findings shift the clinical focus from inflammation as a risk factor for carcinogenesis to inflammation as a prognostic determinant, highlighting a potential new role for systematic endoscopic assessment of the background mucosa at cancer diagnosis,” the authors wrote.
SOURCE:
This study was led by Akiyoshi Ikebata, Department of Surgery, Keio University School of Medicine, Tokyo, Japan. It was published online in December 2025, in the Journal of Crohn's and Colitis.
LIMITATIONS:
The retrospective design introduced potential for unmeasured confounding and selection bias. The MES was assigned by local physicians without central review, which may have introduced variability. The small size of the outside‑area tumor group increased the risk for baseline imbalances.
DISCLOSURES:
No specific funding source was reported. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with ulcerative colitis (UC) who develop colorectal cancer (CRC), greater background mucosal inflammation at the time of CRC diagnosis is associated with progressively worse survival outcomes, with tumors arising within the UC-involved segment having worse prognosis.
METHODOLOGY:
- Patients with UC are at an increased risk for CRC, with risk influenced by the extent and intensity of underlying mucosal inflammation.
- Researchers retrospectively reviewed medical records of patients with UC diagnosed with CRC between 1983 and 2020 at 43 institutions across Japan to determine whether inflammation at cancer diagnosis affected prognosis.
- After endoscopic assessment, tumors were classified as arising inside the UC‑involved segment at diagnosis (within‑area tumors) or outside that segment (outside‑area tumors).
- The Mayo endoscopic score (MES) was used to grade background mucosal inflammation in the within‑area group as inactive (MES 0), mild-moderate (MES 1-2), or severe (MES 3).
- The primary endpoint was 5-year recurrence-free survival, and the secondary endpoint was 5-year cancer-specific survival.
TAKEAWAY:
- Among 723 patients followed for a median of 51 months, 683 had within-area tumors (mean age at CRC diagnosis, 51.8 years; 61.9% male) and 40 had outside-area tumors (mean age at CRC diagnosis, 61.1 years; 60.0% male).
- The within-area group had lower rate of 5-year recurrence-free survival than the outside-area group (75.1% vs 87.6%; P = .022), and lower rate of 5-year cancer-specific survival (81.1% vs 94.3%; P = .038).
- Within-area tumor location independently predicted worse recurrence-free survival (adjusted hazard ratio, 2.99; P = .030).
- In the within‑area group, higher MES was associated with stepwise (although nonsignificant) declines in recurrence‑free survival (inactive, 84.4%; mild-moderate, 79.4%; severe, 73.8%; P = .150). Corresponding cancer‑specific survival rates in these groups declined significantly (89.0%, 84.8%, and 73.8%, respectively; P = .048).
IN PRACTICE:
“These findings shift the clinical focus from inflammation as a risk factor for carcinogenesis to inflammation as a prognostic determinant, highlighting a potential new role for systematic endoscopic assessment of the background mucosa at cancer diagnosis,” the authors wrote.
SOURCE:
This study was led by Akiyoshi Ikebata, Department of Surgery, Keio University School of Medicine, Tokyo, Japan. It was published online in December 2025, in the Journal of Crohn's and Colitis.
LIMITATIONS:
The retrospective design introduced potential for unmeasured confounding and selection bias. The MES was assigned by local physicians without central review, which may have introduced variability. The small size of the outside‑area tumor group increased the risk for baseline imbalances.
DISCLOSURES:
No specific funding source was reported. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
Ulcerative Colitis With Background Mucosal Inflammation Signals Poor Survival in Colorectal Cancer
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
TOPLINE:
Among patients with high-risk malignant colorectal polyps, 19% had residual disease, with rates of 25% in the immediate surgery group vs 9% in the nonoperative management group. The rate of rectum and sphincter preservation in the nonoperative surveillance group was over 90%, and all recurrences were successfully treated with salvage surgery or chemoradiotherapy.
METHODOLOGY:
- Although guidelines in the US recommend colorectal resection when a malignant colorectal polyp has high-risk features, some patients choose nonoperative management instead to avoid the associated averse effects and impact on quality of life. The safety of nonoperative management, however, remains unclear.
- A single-center cohort study conducted between 2015 and 2022 included 336 patients who underwent polypectomy in the colon (n = 226) or rectum (n = 110) and had at least one high-risk feature. High-risk features included positive margins, piecemeal resection with unclear margin, lymphovascular invasion, perineural invasion, poor differentiation, and tumor budding.
- The analysis compared rates of residual disease between those who had immediate surgery (62%) and nonoperative management (38%) following the removal of a malignant polyp, 15% of whom (n = 19) received systemic chemotherapy after polypectomy.
- Researchers also assessed the rates of distant metastasis between the two groups and the association between specific high-risk features and residual disease or post-treatment complications.
TAKEAWAY:
- In the overall population, 19% of patients had residual disease (63 of 336). Among the 208 patients who had immediate surgery, 25% (n = 51) had residual disease, including 9% (n = 19) with residual disease in the bowel wall and 19% (n = 39) in locoregional lymph nodes. Postoperative complications occurred in 12% of patients (n = 25) in the immediate surgery group, with 3% (n = 7) having complications considered grade 3 or higher.
- Among the 128 patients who received nonoperative surveillance, 9% (n = 12) developed recurrence during surveillance, 6% (n = 7) in the bowel wall and 4% (n = 5) in locoregional lymph nodes. All recurrences in the nonoperative surveillance group were successfully treated with either salvage surgery (n = 6) or chemoradiotherapy (n = 6).
- Among patients in the nonoperative group with a malignant polyp removed from the rectum, the rate of rectum preservation was 94% (74 of 79 patients); the sphincter preservation rate was 91% for tumors < 5 cm from the anal verge.
- Distant metastases occurred in 2% of all patients across both groups.
IN PRACTICE:
"The risk of residual disease after the removal of a malignant colorectal polyp with [high-risk features] is considerable, but nonoperative management offers the potential for organ preservation, with the availability of effective salvage options if rectal cancer is detected," the authors of the study concluded.
SOURCE:
The study, led by Thikhamporn Tawantanakorn, MD, and Martin R. Weiser, MD, of Memorial Sloan Kettering Cancer Center in New York City, was published online in JCO Oncology Advances.
LIMITATIONS:
The researchers noted several limitations, including variable follow-up among patients and challenges in assessing polypectomy histology, particularly after piecemeal resection, which limited evaluation of certain high-risk features such as tumor budding. Additionally, as the study was conducted at a specialized cancer center with dedicated gastrointestinal pathology and radiology services and readily available office endoscopy, the results may not be fully generalizable to less specialized centers.
DISCLOSURES:
Jinru Shia, MD, reported receiving consulting fees from Paige.AI and research funding through their institution. Andrea Cercek, MD, disclosed consulting roles with multiple pharmaceutical companies, including GlaxoSmithKline, Incyte, Merck, and others, as well as research funding from GlaxoSmithKline and Pfizer. Weiser reported receiving royalties as a section editor for UpToDate. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with high-risk malignant colorectal polyps, 19% had residual disease, with rates of 25% in the immediate surgery group vs 9% in the nonoperative management group. The rate of rectum and sphincter preservation in the nonoperative surveillance group was over 90%, and all recurrences were successfully treated with salvage surgery or chemoradiotherapy.
METHODOLOGY:
- Although guidelines in the US recommend colorectal resection when a malignant colorectal polyp has high-risk features, some patients choose nonoperative management instead to avoid the associated averse effects and impact on quality of life. The safety of nonoperative management, however, remains unclear.
- A single-center cohort study conducted between 2015 and 2022 included 336 patients who underwent polypectomy in the colon (n = 226) or rectum (n = 110) and had at least one high-risk feature. High-risk features included positive margins, piecemeal resection with unclear margin, lymphovascular invasion, perineural invasion, poor differentiation, and tumor budding.
- The analysis compared rates of residual disease between those who had immediate surgery (62%) and nonoperative management (38%) following the removal of a malignant polyp, 15% of whom (n = 19) received systemic chemotherapy after polypectomy.
- Researchers also assessed the rates of distant metastasis between the two groups and the association between specific high-risk features and residual disease or post-treatment complications.
TAKEAWAY:
- In the overall population, 19% of patients had residual disease (63 of 336). Among the 208 patients who had immediate surgery, 25% (n = 51) had residual disease, including 9% (n = 19) with residual disease in the bowel wall and 19% (n = 39) in locoregional lymph nodes. Postoperative complications occurred in 12% of patients (n = 25) in the immediate surgery group, with 3% (n = 7) having complications considered grade 3 or higher.
- Among the 128 patients who received nonoperative surveillance, 9% (n = 12) developed recurrence during surveillance, 6% (n = 7) in the bowel wall and 4% (n = 5) in locoregional lymph nodes. All recurrences in the nonoperative surveillance group were successfully treated with either salvage surgery (n = 6) or chemoradiotherapy (n = 6).
- Among patients in the nonoperative group with a malignant polyp removed from the rectum, the rate of rectum preservation was 94% (74 of 79 patients); the sphincter preservation rate was 91% for tumors < 5 cm from the anal verge.
- Distant metastases occurred in 2% of all patients across both groups.
IN PRACTICE:
"The risk of residual disease after the removal of a malignant colorectal polyp with [high-risk features] is considerable, but nonoperative management offers the potential for organ preservation, with the availability of effective salvage options if rectal cancer is detected," the authors of the study concluded.
SOURCE:
The study, led by Thikhamporn Tawantanakorn, MD, and Martin R. Weiser, MD, of Memorial Sloan Kettering Cancer Center in New York City, was published online in JCO Oncology Advances.
LIMITATIONS:
The researchers noted several limitations, including variable follow-up among patients and challenges in assessing polypectomy histology, particularly after piecemeal resection, which limited evaluation of certain high-risk features such as tumor budding. Additionally, as the study was conducted at a specialized cancer center with dedicated gastrointestinal pathology and radiology services and readily available office endoscopy, the results may not be fully generalizable to less specialized centers.
DISCLOSURES:
Jinru Shia, MD, reported receiving consulting fees from Paige.AI and research funding through their institution. Andrea Cercek, MD, disclosed consulting roles with multiple pharmaceutical companies, including GlaxoSmithKline, Incyte, Merck, and others, as well as research funding from GlaxoSmithKline and Pfizer. Weiser reported receiving royalties as a section editor for UpToDate. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with high-risk malignant colorectal polyps, 19% had residual disease, with rates of 25% in the immediate surgery group vs 9% in the nonoperative management group. The rate of rectum and sphincter preservation in the nonoperative surveillance group was over 90%, and all recurrences were successfully treated with salvage surgery or chemoradiotherapy.
METHODOLOGY:
- Although guidelines in the US recommend colorectal resection when a malignant colorectal polyp has high-risk features, some patients choose nonoperative management instead to avoid the associated averse effects and impact on quality of life. The safety of nonoperative management, however, remains unclear.
- A single-center cohort study conducted between 2015 and 2022 included 336 patients who underwent polypectomy in the colon (n = 226) or rectum (n = 110) and had at least one high-risk feature. High-risk features included positive margins, piecemeal resection with unclear margin, lymphovascular invasion, perineural invasion, poor differentiation, and tumor budding.
- The analysis compared rates of residual disease between those who had immediate surgery (62%) and nonoperative management (38%) following the removal of a malignant polyp, 15% of whom (n = 19) received systemic chemotherapy after polypectomy.
- Researchers also assessed the rates of distant metastasis between the two groups and the association between specific high-risk features and residual disease or post-treatment complications.
TAKEAWAY:
- In the overall population, 19% of patients had residual disease (63 of 336). Among the 208 patients who had immediate surgery, 25% (n = 51) had residual disease, including 9% (n = 19) with residual disease in the bowel wall and 19% (n = 39) in locoregional lymph nodes. Postoperative complications occurred in 12% of patients (n = 25) in the immediate surgery group, with 3% (n = 7) having complications considered grade 3 or higher.
- Among the 128 patients who received nonoperative surveillance, 9% (n = 12) developed recurrence during surveillance, 6% (n = 7) in the bowel wall and 4% (n = 5) in locoregional lymph nodes. All recurrences in the nonoperative surveillance group were successfully treated with either salvage surgery (n = 6) or chemoradiotherapy (n = 6).
- Among patients in the nonoperative group with a malignant polyp removed from the rectum, the rate of rectum preservation was 94% (74 of 79 patients); the sphincter preservation rate was 91% for tumors < 5 cm from the anal verge.
- Distant metastases occurred in 2% of all patients across both groups.
IN PRACTICE:
"The risk of residual disease after the removal of a malignant colorectal polyp with [high-risk features] is considerable, but nonoperative management offers the potential for organ preservation, with the availability of effective salvage options if rectal cancer is detected," the authors of the study concluded.
SOURCE:
The study, led by Thikhamporn Tawantanakorn, MD, and Martin R. Weiser, MD, of Memorial Sloan Kettering Cancer Center in New York City, was published online in JCO Oncology Advances.
LIMITATIONS:
The researchers noted several limitations, including variable follow-up among patients and challenges in assessing polypectomy histology, particularly after piecemeal resection, which limited evaluation of certain high-risk features such as tumor budding. Additionally, as the study was conducted at a specialized cancer center with dedicated gastrointestinal pathology and radiology services and readily available office endoscopy, the results may not be fully generalizable to less specialized centers.
DISCLOSURES:
Jinru Shia, MD, reported receiving consulting fees from Paige.AI and research funding through their institution. Andrea Cercek, MD, disclosed consulting roles with multiple pharmaceutical companies, including GlaxoSmithKline, Incyte, Merck, and others, as well as research funding from GlaxoSmithKline and Pfizer. Weiser reported receiving royalties as a section editor for UpToDate. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?
Is It Safe to Skip Surgery After Malignant Colorectal Polyp Removal?