User login
4 cases involving intraoperative injuries to adjacent organs
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar. Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM: The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE: Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT: An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Bowel perforation during myomectomy: $200,000 verdictA 44-year-old woman underwent hysteroscopic myomectomy. During the procedure the ObGyn realized that he had perforated the uterus. He switched to a laparoscopic procedure, found a 3-cm uterine tear, and converted to laparotomy to repair the injury. The postsurgical pathology report revealed multiple colon fragments.
Three days after surgery, the patient became ill and was found to have a bowel injury. She underwent bowel resection with colostomy and, a year later, colostomy reversal. She sustained abdominal scarring.
PATIENT'S CLAIM: The ObGyn was negligent in performing the myomectomy. He should have identified the bowel injury intraoperatively. When the pathology report indicated multiple colon fragments, he should have investigated rather than wait for the patient to develop symptoms.
PHYSICIAN'S DEFENSE: Uterine and colon injuries are known complications of the procedure and can occur within the standard of care. The ObGyn intraoperatively inspected the organs adjacent to the uterus but there was no evident injury. The patient’s postsurgical treatment was proper.
VERDICT: A $200,000 Illinois verdict was returned.
Injured ureter allegedly not treatedA 42-year-old woman underwent hysterectomy on December 6. Postoperatively, she reported increasing dysuria with pain and fever. On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient. The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM: The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE: Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT: The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar. Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM: The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE: Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT: An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Bowel perforation during myomectomy: $200,000 verdictA 44-year-old woman underwent hysteroscopic myomectomy. During the procedure the ObGyn realized that he had perforated the uterus. He switched to a laparoscopic procedure, found a 3-cm uterine tear, and converted to laparotomy to repair the injury. The postsurgical pathology report revealed multiple colon fragments.
Three days after surgery, the patient became ill and was found to have a bowel injury. She underwent bowel resection with colostomy and, a year later, colostomy reversal. She sustained abdominal scarring.
PATIENT'S CLAIM: The ObGyn was negligent in performing the myomectomy. He should have identified the bowel injury intraoperatively. When the pathology report indicated multiple colon fragments, he should have investigated rather than wait for the patient to develop symptoms.
PHYSICIAN'S DEFENSE: Uterine and colon injuries are known complications of the procedure and can occur within the standard of care. The ObGyn intraoperatively inspected the organs adjacent to the uterus but there was no evident injury. The patient’s postsurgical treatment was proper.
VERDICT: A $200,000 Illinois verdict was returned.
Injured ureter allegedly not treatedA 42-year-old woman underwent hysterectomy on December 6. Postoperatively, she reported increasing dysuria with pain and fever. On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient. The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM: The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE: Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT: The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar. Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM: The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE: Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT: An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Bowel perforation during myomectomy: $200,000 verdictA 44-year-old woman underwent hysteroscopic myomectomy. During the procedure the ObGyn realized that he had perforated the uterus. He switched to a laparoscopic procedure, found a 3-cm uterine tear, and converted to laparotomy to repair the injury. The postsurgical pathology report revealed multiple colon fragments.
Three days after surgery, the patient became ill and was found to have a bowel injury. She underwent bowel resection with colostomy and, a year later, colostomy reversal. She sustained abdominal scarring.
PATIENT'S CLAIM: The ObGyn was negligent in performing the myomectomy. He should have identified the bowel injury intraoperatively. When the pathology report indicated multiple colon fragments, he should have investigated rather than wait for the patient to develop symptoms.
PHYSICIAN'S DEFENSE: Uterine and colon injuries are known complications of the procedure and can occur within the standard of care. The ObGyn intraoperatively inspected the organs adjacent to the uterus but there was no evident injury. The patient’s postsurgical treatment was proper.
VERDICT: A $200,000 Illinois verdict was returned.
Injured ureter allegedly not treatedA 42-year-old woman underwent hysterectomy on December 6. Postoperatively, she reported increasing dysuria with pain and fever. On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient. The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM: The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE: Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT: The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
Syndecan-1 may predict kidney injury after ped heart surgery
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The biomarker syndecan-1 may aid in determining acute kidney injury risk for children having cardiac surgery.
Major finding: Children with elevated levels of syndecan-1 had a two- to ninefold greater risk of acute kidney injury.
Data source: Single-institution, prospective cohort study of 289 pediatric patients who had cardiac surgery from September 2013 to December 2014.
Disclosures: Dr. Cavalcante and coauthors had no financial relationships to disclose.
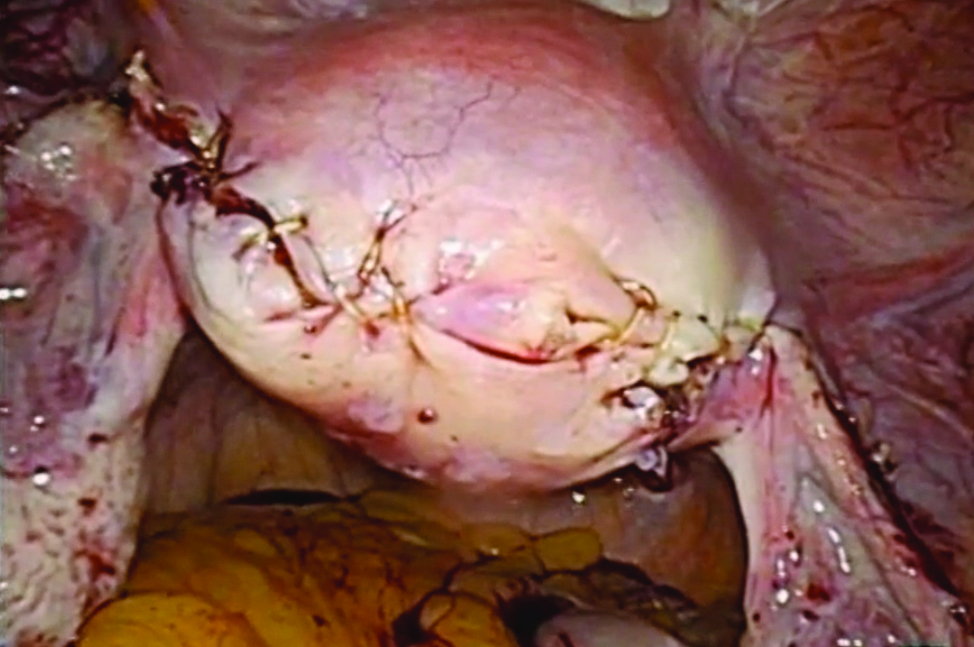
Preventing and managing vaginal cuff dehiscence
Vaginal cuff dehiscence, or separation of the vaginal incision, is a rare postoperative complication unique to hysterectomy. Morbidity related to evisceration of abdominal contents can be profound and prompt intervention is required.
A 10-year observational study of 11,000 patients described a 0.24% cumulative incidence after all modes of hysterectomy.1 Though data are varied, the mode of hysterectomy does have an impact on the risk of dehiscence.
Laparoscopic (0.64%-1.35%) and robotic (0.46%-1.5%) hysterectomy have a higher incidence than abdominal (0.15%-0.26%) and vaginal (0.08%-0.25%) approaches.2 The use of monopolar cautery for colpotomy and different closure techniques may account for these differences.
Cuff cellulitis, early sexual intercourse, cigarette smoking, poor nutrition, obesity, menopausal status, and corticosteroid use are all proposed risk factors that promote infection, pressure at the vaginal cuff, and poor wound healing. Although some are modifiable, the rarity of this complication has made establishing causality and promoting prevention challenging.
Prevention
• Preoperatively. Treating bacterial vaginosis, Trichomonas vaginalis, gonorrhea, and chlamydia can decrease the risk of cuff cellulitis and dehiscence.3
• Intraoperatively. Surgeons should ensure adequate vaginal margins (greater than 1 cm) with full-thickness cuff closures while avoiding excessive electrocautery.4 Retrospective data show that transvaginal cuff closure is associated with a decreased risk of dehiscence.5 However, given the lack of randomized data and the difficulty controlling for surgeon experience, gynecologists should use the approach that they are most comfortable with. Though the various laparoscopic cuff closure techniques have limited evidence regarding superiority, some experts propose using two-layer cuff closure and barbed sutures.6-8 Several retrospective studies have found an equivalent or a decreased incidence of cuff dehiscence with barbed sutures, compared with other methods (e.g., 0-Vicryl, Endo Stitch).9,10
• Postoperatively. Women should avoid intercourse and lifting more than 15 pounds for at least 6-8 weeks as the vaginal cuff gains tensile strength. Vaginal estrogen can promote healing in postmenopausal patients.11
Management
Patients with vaginal cuff dehiscence commonly present within the first several weeks to months after surgery with pelvic pain (60%-100%), vaginal bleeding (30%-60%), vaginal discharge (30%), or vaginal pressure/mass (30%).1,7 Posthysterectomy patients with these complaints warrant an urgent evaluation. The diagnosis is made during a pelvic exam.
Broad-spectrum antibiotics are necessary because all vaginal cuff separations or dehiscences expose the peritoneal cavity to vaginal flora. Nonsurgical management is reasonable for small separations – less than 25% of the cuff – if there is no evidence of evisceration.
However, surgically closing all recognized cuff dehiscences is reasonable, given the potential for further separation. A vaginal approach is preferred when possible. Women with vaginal cuff dehiscence, stable vital signs, and no evidence of bowel evisceration can be repaired vaginally without an abdominal survey.
In contrast, women with bowel evisceration have a surgical emergency because of the risk of peritonitis and bowel injury. If the eviscerated bowel is not reducible, it should be irrigated and wrapped in a warm moist towel or gauze in preparation for inspection and reduction in the operating room. If the bowel is reducible, the patient can be placed in Trendelenburg’s position. Her vagina should be packed to reduce the risk of re-evisceration as she moves toward operative cuff repair.
If the physician is concerned about bowel injury, inspection via laparoscopy or laparotomy would be reasonable. However, when bowel injury is not suspected, a vaginal technique for dehiscence repair has been described by Matthews et al.:12
1. Expose the cuff with a weighted speculum and Breisky-Navratil retractors.
2. Sharply debride the cuff edges back to viable tissue.
3. Dissect adherent bowel or omentum to allow for full-thickness closure.
4. Place full-thickness, interrupted delayed absorbable sutures to reapproximate the cuff edges.
Cuff dehiscence is a rare but potentially morbid complication of hysterectomy. Prevention, recognition, and appropriate management can avoid life-threatening sequelae.
References
1. Obstet Gynecol. 2011 Oct;118(4):794-801.
2. JSLS. 2012 Oct-Dec;16(4):530-6.
3. Am J Obstet Gynecol. 1990 Sep;163(3):1016-21; discussion 1021-3.
4. Obstet Gynecol. 2013 Mar;121(3):654-73.
5. Obstet Gynecol. 2012 Sep;120(3):516-23.
6. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):474-80.
7. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):134-8.
8. Obstet Gynecol. 2009 Aug;114(2 Pt 1):231-5.
9. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23.
10. Int J Surg. 2015 Jul;19:27-30.
11. Maturitas. 2006 Feb 20;53(3):282-98.
12. Obstet Gynecol. 2014 Oct;124(4):705-8.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Vaginal cuff dehiscence, or separation of the vaginal incision, is a rare postoperative complication unique to hysterectomy. Morbidity related to evisceration of abdominal contents can be profound and prompt intervention is required.
A 10-year observational study of 11,000 patients described a 0.24% cumulative incidence after all modes of hysterectomy.1 Though data are varied, the mode of hysterectomy does have an impact on the risk of dehiscence.
Laparoscopic (0.64%-1.35%) and robotic (0.46%-1.5%) hysterectomy have a higher incidence than abdominal (0.15%-0.26%) and vaginal (0.08%-0.25%) approaches.2 The use of monopolar cautery for colpotomy and different closure techniques may account for these differences.
Cuff cellulitis, early sexual intercourse, cigarette smoking, poor nutrition, obesity, menopausal status, and corticosteroid use are all proposed risk factors that promote infection, pressure at the vaginal cuff, and poor wound healing. Although some are modifiable, the rarity of this complication has made establishing causality and promoting prevention challenging.
Prevention
• Preoperatively. Treating bacterial vaginosis, Trichomonas vaginalis, gonorrhea, and chlamydia can decrease the risk of cuff cellulitis and dehiscence.3
• Intraoperatively. Surgeons should ensure adequate vaginal margins (greater than 1 cm) with full-thickness cuff closures while avoiding excessive electrocautery.4 Retrospective data show that transvaginal cuff closure is associated with a decreased risk of dehiscence.5 However, given the lack of randomized data and the difficulty controlling for surgeon experience, gynecologists should use the approach that they are most comfortable with. Though the various laparoscopic cuff closure techniques have limited evidence regarding superiority, some experts propose using two-layer cuff closure and barbed sutures.6-8 Several retrospective studies have found an equivalent or a decreased incidence of cuff dehiscence with barbed sutures, compared with other methods (e.g., 0-Vicryl, Endo Stitch).9,10
• Postoperatively. Women should avoid intercourse and lifting more than 15 pounds for at least 6-8 weeks as the vaginal cuff gains tensile strength. Vaginal estrogen can promote healing in postmenopausal patients.11
Management
Patients with vaginal cuff dehiscence commonly present within the first several weeks to months after surgery with pelvic pain (60%-100%), vaginal bleeding (30%-60%), vaginal discharge (30%), or vaginal pressure/mass (30%).1,7 Posthysterectomy patients with these complaints warrant an urgent evaluation. The diagnosis is made during a pelvic exam.
Broad-spectrum antibiotics are necessary because all vaginal cuff separations or dehiscences expose the peritoneal cavity to vaginal flora. Nonsurgical management is reasonable for small separations – less than 25% of the cuff – if there is no evidence of evisceration.
However, surgically closing all recognized cuff dehiscences is reasonable, given the potential for further separation. A vaginal approach is preferred when possible. Women with vaginal cuff dehiscence, stable vital signs, and no evidence of bowel evisceration can be repaired vaginally without an abdominal survey.
In contrast, women with bowel evisceration have a surgical emergency because of the risk of peritonitis and bowel injury. If the eviscerated bowel is not reducible, it should be irrigated and wrapped in a warm moist towel or gauze in preparation for inspection and reduction in the operating room. If the bowel is reducible, the patient can be placed in Trendelenburg’s position. Her vagina should be packed to reduce the risk of re-evisceration as she moves toward operative cuff repair.
If the physician is concerned about bowel injury, inspection via laparoscopy or laparotomy would be reasonable. However, when bowel injury is not suspected, a vaginal technique for dehiscence repair has been described by Matthews et al.:12
1. Expose the cuff with a weighted speculum and Breisky-Navratil retractors.
2. Sharply debride the cuff edges back to viable tissue.
3. Dissect adherent bowel or omentum to allow for full-thickness closure.
4. Place full-thickness, interrupted delayed absorbable sutures to reapproximate the cuff edges.
Cuff dehiscence is a rare but potentially morbid complication of hysterectomy. Prevention, recognition, and appropriate management can avoid life-threatening sequelae.
References
1. Obstet Gynecol. 2011 Oct;118(4):794-801.
2. JSLS. 2012 Oct-Dec;16(4):530-6.
3. Am J Obstet Gynecol. 1990 Sep;163(3):1016-21; discussion 1021-3.
4. Obstet Gynecol. 2013 Mar;121(3):654-73.
5. Obstet Gynecol. 2012 Sep;120(3):516-23.
6. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):474-80.
7. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):134-8.
8. Obstet Gynecol. 2009 Aug;114(2 Pt 1):231-5.
9. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23.
10. Int J Surg. 2015 Jul;19:27-30.
11. Maturitas. 2006 Feb 20;53(3):282-98.
12. Obstet Gynecol. 2014 Oct;124(4):705-8.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Vaginal cuff dehiscence, or separation of the vaginal incision, is a rare postoperative complication unique to hysterectomy. Morbidity related to evisceration of abdominal contents can be profound and prompt intervention is required.
A 10-year observational study of 11,000 patients described a 0.24% cumulative incidence after all modes of hysterectomy.1 Though data are varied, the mode of hysterectomy does have an impact on the risk of dehiscence.
Laparoscopic (0.64%-1.35%) and robotic (0.46%-1.5%) hysterectomy have a higher incidence than abdominal (0.15%-0.26%) and vaginal (0.08%-0.25%) approaches.2 The use of monopolar cautery for colpotomy and different closure techniques may account for these differences.
Cuff cellulitis, early sexual intercourse, cigarette smoking, poor nutrition, obesity, menopausal status, and corticosteroid use are all proposed risk factors that promote infection, pressure at the vaginal cuff, and poor wound healing. Although some are modifiable, the rarity of this complication has made establishing causality and promoting prevention challenging.
Prevention
• Preoperatively. Treating bacterial vaginosis, Trichomonas vaginalis, gonorrhea, and chlamydia can decrease the risk of cuff cellulitis and dehiscence.3
• Intraoperatively. Surgeons should ensure adequate vaginal margins (greater than 1 cm) with full-thickness cuff closures while avoiding excessive electrocautery.4 Retrospective data show that transvaginal cuff closure is associated with a decreased risk of dehiscence.5 However, given the lack of randomized data and the difficulty controlling for surgeon experience, gynecologists should use the approach that they are most comfortable with. Though the various laparoscopic cuff closure techniques have limited evidence regarding superiority, some experts propose using two-layer cuff closure and barbed sutures.6-8 Several retrospective studies have found an equivalent or a decreased incidence of cuff dehiscence with barbed sutures, compared with other methods (e.g., 0-Vicryl, Endo Stitch).9,10
• Postoperatively. Women should avoid intercourse and lifting more than 15 pounds for at least 6-8 weeks as the vaginal cuff gains tensile strength. Vaginal estrogen can promote healing in postmenopausal patients.11
Management
Patients with vaginal cuff dehiscence commonly present within the first several weeks to months after surgery with pelvic pain (60%-100%), vaginal bleeding (30%-60%), vaginal discharge (30%), or vaginal pressure/mass (30%).1,7 Posthysterectomy patients with these complaints warrant an urgent evaluation. The diagnosis is made during a pelvic exam.
Broad-spectrum antibiotics are necessary because all vaginal cuff separations or dehiscences expose the peritoneal cavity to vaginal flora. Nonsurgical management is reasonable for small separations – less than 25% of the cuff – if there is no evidence of evisceration.
However, surgically closing all recognized cuff dehiscences is reasonable, given the potential for further separation. A vaginal approach is preferred when possible. Women with vaginal cuff dehiscence, stable vital signs, and no evidence of bowel evisceration can be repaired vaginally without an abdominal survey.
In contrast, women with bowel evisceration have a surgical emergency because of the risk of peritonitis and bowel injury. If the eviscerated bowel is not reducible, it should be irrigated and wrapped in a warm moist towel or gauze in preparation for inspection and reduction in the operating room. If the bowel is reducible, the patient can be placed in Trendelenburg’s position. Her vagina should be packed to reduce the risk of re-evisceration as she moves toward operative cuff repair.
If the physician is concerned about bowel injury, inspection via laparoscopy or laparotomy would be reasonable. However, when bowel injury is not suspected, a vaginal technique for dehiscence repair has been described by Matthews et al.:12
1. Expose the cuff with a weighted speculum and Breisky-Navratil retractors.
2. Sharply debride the cuff edges back to viable tissue.
3. Dissect adherent bowel or omentum to allow for full-thickness closure.
4. Place full-thickness, interrupted delayed absorbable sutures to reapproximate the cuff edges.
Cuff dehiscence is a rare but potentially morbid complication of hysterectomy. Prevention, recognition, and appropriate management can avoid life-threatening sequelae.
References
1. Obstet Gynecol. 2011 Oct;118(4):794-801.
2. JSLS. 2012 Oct-Dec;16(4):530-6.
3. Am J Obstet Gynecol. 1990 Sep;163(3):1016-21; discussion 1021-3.
4. Obstet Gynecol. 2013 Mar;121(3):654-73.
5. Obstet Gynecol. 2012 Sep;120(3):516-23.
6. J Am Assoc Gynecol Laparosc. 2002 Nov;9(4):474-80.
7. Eur J Obstet Gynecol Reprod Biol. 2006 Mar 1;125(1):134-8.
8. Obstet Gynecol. 2009 Aug;114(2 Pt 1):231-5.
9. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):218-23.
10. Int J Surg. 2015 Jul;19:27-30.
11. Maturitas. 2006 Feb 20;53(3):282-98.
12. Obstet Gynecol. 2014 Oct;124(4):705-8.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
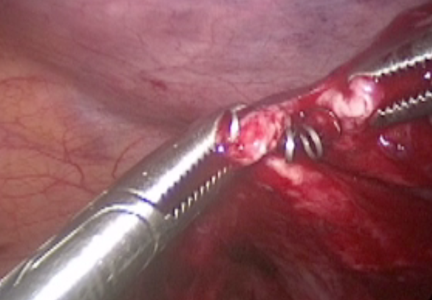
Laparoscopic salpingectomy and cornual resection repurposed
I am pleased to introduce this month’s video, from the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Penn State. Dr. Michelle Pacis addresses an increasingly important topic to MIGS surgeons: removal of previously hysteroscopically placed tubal occlusion devices. These devices offer a permanent alterative to laparoscopic sterilization. If women require their removal, however, whether desired or because of complications, gynecologic surgeons must be familiar with the steps involved.
The following video was produced in order to demonstrate a reproducible technique for laparoscopic removal of a device for women requesting this procedure. Key objectives of the video include:
- review of the techniques available to remove hysteroscopic tubal occlusion devices, as well as the contraindications and advantages/disadvantages of these approaches.
- discuss recommended imaging and materials required for the technique described and tips for their use.
- demonstrate salpingectomy and repair technique.
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com
I am pleased to introduce this month’s video, from the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Penn State. Dr. Michelle Pacis addresses an increasingly important topic to MIGS surgeons: removal of previously hysteroscopically placed tubal occlusion devices. These devices offer a permanent alterative to laparoscopic sterilization. If women require their removal, however, whether desired or because of complications, gynecologic surgeons must be familiar with the steps involved.
The following video was produced in order to demonstrate a reproducible technique for laparoscopic removal of a device for women requesting this procedure. Key objectives of the video include:
- review of the techniques available to remove hysteroscopic tubal occlusion devices, as well as the contraindications and advantages/disadvantages of these approaches.
- discuss recommended imaging and materials required for the technique described and tips for their use.
- demonstrate salpingectomy and repair technique.
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com
I am pleased to introduce this month’s video, from the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Penn State. Dr. Michelle Pacis addresses an increasingly important topic to MIGS surgeons: removal of previously hysteroscopically placed tubal occlusion devices. These devices offer a permanent alterative to laparoscopic sterilization. If women require their removal, however, whether desired or because of complications, gynecologic surgeons must be familiar with the steps involved.
The following video was produced in order to demonstrate a reproducible technique for laparoscopic removal of a device for women requesting this procedure. Key objectives of the video include:
- review of the techniques available to remove hysteroscopic tubal occlusion devices, as well as the contraindications and advantages/disadvantages of these approaches.
- discuss recommended imaging and materials required for the technique described and tips for their use.
- demonstrate salpingectomy and repair technique.
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com
Failure to convert to laparotomy: $6.25M settlement
Failure to convert to laparotomy: $6.25M settlement
A 67-year-old woman with urinary incontinence underwent robot-assisted laparoscopic prolapse surgery and hysterectomy. Complications arose, including an injury to the transverse colon. Postoperatively, the patient developed sepsis and had multiple surgeries. At the time of trial, she used a colostomy bag and had a malabsorption syndrome that required frequent intravenous treatment for dehydration.
Patient's claim:
The gynecologist deviated from the standard of care by failing to convert from a laparoscopic procedure to an open procedure when complications developed.
Defendants' defense:
The procedure was properly performed.
Verdict:
A $6.25 million New Jersey settlement was reached, paid jointly by the gynecologist and the medical center.
Circumcision requires revision
A day after birth, a baby underwent circumcision performed by the mother’s ObGyn. Revision surgery was performed 2.5 years later. When the boy was age 7 years, urethral stricture developed and was treated.
Parents' claim:
Circumcision was improperly performed. Once the child was able to talk, he said that his penis constantly hurt. Pain was only relieved by revision surgery.
Physician's defense:
There was no negligence. Redundant foreskin is often left following a circumcision.
Verdict:
A Michigan defense verdict was returned.
Mother with CP has child with CP
A pregnant woman with cerebral palsy (CP) reported a prior preterm delivery at 24 weeks’ gestation to a high-risk prenatal clinic. At that time, she was offered synthetic progesterone (170HP) injections, but she declined because of the cost. She declined 170HP several times. Nine weeks after her initial visit, she declined 170HP despite ultrasonography (US) showing a shortened cervical length (25 mm) for the gestational age. In 2 weeks, when the cervical length measured 9 mm, she was hospitalized to rule out preterm labor but, before tests began, she left the hospital. Five days later, when her cervical length was 11 mm, she received the first 170HP injection. In the next month she received 4 injections, but she failed to show for the fifth injection and US. The next day, she went to the hospital with cramping. She was given steroids and medication to stop labor. US results indicated that the baby was in breech position. The mother consented to cesarean delivery, but the baby was born vaginally an hour later. The child has mild brain damage, CP, developmental delays, and learning disabilities.
Parents' claim: The mother should have been offered vaginal progesterone, which is cheaper. Given the high risk of preterm birth, steroids administered earlier would have improved fetal development. Cesarean delivery should have been performed.
Defendants' defense: Vaginal progesterone was not available at the time. Starting steroids earlier would not have improved fetal outcome. A cesarean delivery was not possible because the baby was in the birth canal.
Verdict: A $3,500,000 Michigan settlement was reached.
Fallopian tubes grow back, pregnancy
A couple decided they did not want more children and sought counseling from the woman’s ObGyn, who recommended laparoscopic tubal ligation. Several months after surgery, the patient became pregnant and gave birth to a son.
Parents' claim:
The additional child placed an economic hardship on the family, now raising 4 children. The youngest child has language delays and learning disabilities.
Physician's defense:
Regrowth of the fallopian tubes resulting in unwanted pregnancy is a known complication of tubal ligation.
Verdict:
A $397,000 Maryland verdict was returned, including funds for the cost of raising the fourth child and to cope with the child’s special needs.
Challenges in managing labor

At 37 weeks' gestation, a woman was hospitalized in labor. At 1:15 pm, she was dilated 3 cm. At 1:30 pm, she was dilated 4−5 cm with increasing contractions and a reassuring fetal heart rate (FHR). The ObGyn covering for the mother’s ObGyn ordered oxytocin augmentation, which started at 2:45 pm. Shortly thereafter, contractions became more frequent and uterine tachysystole was observed. At 4:12 pm, FHR showed multiple deep decelerations with slow recovery. The baseline dropped to a 90-bpm range and remained that way for 17 minutes. At that point, the ObGyn stopped oxytocin and administered terbutaline; the FHR returned to baseline.
After vaginal delivery, the baby’s Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. Two days later, the baby had seizures and was transferred to the neonatal intensive care unit. An electroencephalogram confirmed seizure activity. Initial imaging results were normal. However, magnetic resonance imaging performed a week after delivery showed bilateral brain damage. The child has spastic displegia, is unable to ambulate, and is blind.
Parents' claim: A suit was filed against the hospital and both ObGyns. The hospital settled before trial. The case was discontinued against the primary ObGyn. The covering ObGyn allegedly made 4 departures from accepted medical practice that caused the child’s injury: ordering and administering oxytocin, failing to closely monitor the FHR, failing to timely administer terbutaline, and failing to timely respond to and correct tachysystole.
Physician's defense: The child’s injury occurred before or after labor. The pregnancy was complicated by multiple kidney infections. A week before delivery, US revealed a blood-flow abnormality. An intranatal hypoxic event did not cause the injury, proven by the fact that, after terbutaline was administered, the FHR promptly normalized.
Verdict: A $3 million New York settlement was reached with the hospital. A $134 million verdict was returned against the covering ObGyn.
Brachial plexus injury during delivery
At 37 weeks' gestation, a mother was admitted to the hospital for induction of labor. Increasing doses of oxytocin were administered. Near midnight, FHR monitoring indicated fetal distress. The ObGyn was called and he ordered cesarean delivery. Once he arrived and examined the mother, he found no fetal concerns and decided to proceed with the original birth plan. At 3:30 am, the patient was fully dilated and in active labor. The ObGyn used a vacuum extractor. Upon delivery of the baby’s head, the ObGyn encountered shoulder dystocia and called for assistance. The child was born with a near-total brachial plexus injury: avulsions of all 5 brachial plexus nerves with trauma to the cervical nerve roots at C5−C8 and T1. The child has undergone multiple nerve grafts and orthopedic operations.
Parents' claim: Fetal distress should have prompted the ObGyn to perform cesarean delivery. There was no reason to use vacuum extraction. Based on the severity of the outcome, the ObGyn must have applied excessive force and inappropriate traction during delivery maneuvers.
Physician's defense: The standard of care did not require a cesarean delivery. The vacuum extractor did not cause shoulder dystocia. The ObGyn did not apply excessive force or traction to complete the delivery. The extent of the outcome was partially due to a fetal anomaly and hypotonia.
Verdict: An Illinois defense verdict was returned.
HPV-positive pap tests results never reported
A single mother of 4 children underwent Papanicolaou (Pap) tests in 2004, 2005, and 2007 at a federally funded clinic. Each time, she tested positive for oncogenic human papillomaviruses. In 2011, the patient died of cervical cancer.
Estate's claim: The patient was never notified that the results of the 3 Pap tests were abnormal because all correspondence was sent to an outdated address although she had been treated at the same clinic for other issues during that period of time. Cervical dysplasia identified in 2004 progressed to cancer and metastasized, leading to her death 7 years later.
Defendants' defense: The case was settled during trial.
Verdict: A $4,950,000 Illinois settlement was reached.
Failure to convert to laparotomy: $6.25M settlement

A 67-year-old woman with urinary incontinence underwent robot-assisted laparoscopic prolapse surgery and hysterectomy. Complications arose, including an injury to the transverse colon. Postoperatively, the patient developed sepsis and had multiple surgeries. At the time of trial, she used a colostomy bag and had a malabsorption syndrome that required frequent intravenous treatment for dehydration.
Patient's claim:
The gynecologist deviated from the standard of care by failing to convert from a laparoscopic procedure to an open procedure when complications developed.
Defendants' defense:
The procedure was properly performed.
Verdict:
A $6.25 million New Jersey settlement was reached, paid jointly by the gynecologist and the medical center.
Circumcision requires revision
A day after birth, a baby underwent circumcision performed by the mother’s ObGyn. Revision surgery was performed 2.5 years later. When the boy was age 7 years, urethral stricture developed and was treated.
Parents' claim:
Circumcision was improperly performed. Once the child was able to talk, he said that his penis constantly hurt. Pain was only relieved by revision surgery.
Physician's defense:
There was no negligence. Redundant foreskin is often left following a circumcision.
Verdict:
A Michigan defense verdict was returned.
Mother with CP has child with CP
A pregnant woman with cerebral palsy (CP) reported a prior preterm delivery at 24 weeks’ gestation to a high-risk prenatal clinic. At that time, she was offered synthetic progesterone (170HP) injections, but she declined because of the cost. She declined 170HP several times. Nine weeks after her initial visit, she declined 170HP despite ultrasonography (US) showing a shortened cervical length (25 mm) for the gestational age. In 2 weeks, when the cervical length measured 9 mm, she was hospitalized to rule out preterm labor but, before tests began, she left the hospital. Five days later, when her cervical length was 11 mm, she received the first 170HP injection. In the next month she received 4 injections, but she failed to show for the fifth injection and US. The next day, she went to the hospital with cramping. She was given steroids and medication to stop labor. US results indicated that the baby was in breech position. The mother consented to cesarean delivery, but the baby was born vaginally an hour later. The child has mild brain damage, CP, developmental delays, and learning disabilities.
Parents' claim: The mother should have been offered vaginal progesterone, which is cheaper. Given the high risk of preterm birth, steroids administered earlier would have improved fetal development. Cesarean delivery should have been performed.
Defendants' defense: Vaginal progesterone was not available at the time. Starting steroids earlier would not have improved fetal outcome. A cesarean delivery was not possible because the baby was in the birth canal.
Verdict: A $3,500,000 Michigan settlement was reached.
Fallopian tubes grow back, pregnancy
A couple decided they did not want more children and sought counseling from the woman’s ObGyn, who recommended laparoscopic tubal ligation. Several months after surgery, the patient became pregnant and gave birth to a son.
Parents' claim:
The additional child placed an economic hardship on the family, now raising 4 children. The youngest child has language delays and learning disabilities.
Physician's defense:
Regrowth of the fallopian tubes resulting in unwanted pregnancy is a known complication of tubal ligation.
Verdict:
A $397,000 Maryland verdict was returned, including funds for the cost of raising the fourth child and to cope with the child’s special needs.
Challenges in managing labor

At 37 weeks' gestation, a woman was hospitalized in labor. At 1:15 pm, she was dilated 3 cm. At 1:30 pm, she was dilated 4−5 cm with increasing contractions and a reassuring fetal heart rate (FHR). The ObGyn covering for the mother’s ObGyn ordered oxytocin augmentation, which started at 2:45 pm. Shortly thereafter, contractions became more frequent and uterine tachysystole was observed. At 4:12 pm, FHR showed multiple deep decelerations with slow recovery. The baseline dropped to a 90-bpm range and remained that way for 17 minutes. At that point, the ObGyn stopped oxytocin and administered terbutaline; the FHR returned to baseline.
After vaginal delivery, the baby’s Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. Two days later, the baby had seizures and was transferred to the neonatal intensive care unit. An electroencephalogram confirmed seizure activity. Initial imaging results were normal. However, magnetic resonance imaging performed a week after delivery showed bilateral brain damage. The child has spastic displegia, is unable to ambulate, and is blind.
Parents' claim: A suit was filed against the hospital and both ObGyns. The hospital settled before trial. The case was discontinued against the primary ObGyn. The covering ObGyn allegedly made 4 departures from accepted medical practice that caused the child’s injury: ordering and administering oxytocin, failing to closely monitor the FHR, failing to timely administer terbutaline, and failing to timely respond to and correct tachysystole.
Physician's defense: The child’s injury occurred before or after labor. The pregnancy was complicated by multiple kidney infections. A week before delivery, US revealed a blood-flow abnormality. An intranatal hypoxic event did not cause the injury, proven by the fact that, after terbutaline was administered, the FHR promptly normalized.
Verdict: A $3 million New York settlement was reached with the hospital. A $134 million verdict was returned against the covering ObGyn.
Brachial plexus injury during delivery
At 37 weeks' gestation, a mother was admitted to the hospital for induction of labor. Increasing doses of oxytocin were administered. Near midnight, FHR monitoring indicated fetal distress. The ObGyn was called and he ordered cesarean delivery. Once he arrived and examined the mother, he found no fetal concerns and decided to proceed with the original birth plan. At 3:30 am, the patient was fully dilated and in active labor. The ObGyn used a vacuum extractor. Upon delivery of the baby’s head, the ObGyn encountered shoulder dystocia and called for assistance. The child was born with a near-total brachial plexus injury: avulsions of all 5 brachial plexus nerves with trauma to the cervical nerve roots at C5−C8 and T1. The child has undergone multiple nerve grafts and orthopedic operations.
Parents' claim: Fetal distress should have prompted the ObGyn to perform cesarean delivery. There was no reason to use vacuum extraction. Based on the severity of the outcome, the ObGyn must have applied excessive force and inappropriate traction during delivery maneuvers.
Physician's defense: The standard of care did not require a cesarean delivery. The vacuum extractor did not cause shoulder dystocia. The ObGyn did not apply excessive force or traction to complete the delivery. The extent of the outcome was partially due to a fetal anomaly and hypotonia.
Verdict: An Illinois defense verdict was returned.
HPV-positive pap tests results never reported
A single mother of 4 children underwent Papanicolaou (Pap) tests in 2004, 2005, and 2007 at a federally funded clinic. Each time, she tested positive for oncogenic human papillomaviruses. In 2011, the patient died of cervical cancer.
Estate's claim: The patient was never notified that the results of the 3 Pap tests were abnormal because all correspondence was sent to an outdated address although she had been treated at the same clinic for other issues during that period of time. Cervical dysplasia identified in 2004 progressed to cancer and metastasized, leading to her death 7 years later.
Defendants' defense: The case was settled during trial.
Verdict: A $4,950,000 Illinois settlement was reached.
Failure to convert to laparotomy: $6.25M settlement

A 67-year-old woman with urinary incontinence underwent robot-assisted laparoscopic prolapse surgery and hysterectomy. Complications arose, including an injury to the transverse colon. Postoperatively, the patient developed sepsis and had multiple surgeries. At the time of trial, she used a colostomy bag and had a malabsorption syndrome that required frequent intravenous treatment for dehydration.
Patient's claim:
The gynecologist deviated from the standard of care by failing to convert from a laparoscopic procedure to an open procedure when complications developed.
Defendants' defense:
The procedure was properly performed.
Verdict:
A $6.25 million New Jersey settlement was reached, paid jointly by the gynecologist and the medical center.
Circumcision requires revision
A day after birth, a baby underwent circumcision performed by the mother’s ObGyn. Revision surgery was performed 2.5 years later. When the boy was age 7 years, urethral stricture developed and was treated.
Parents' claim:
Circumcision was improperly performed. Once the child was able to talk, he said that his penis constantly hurt. Pain was only relieved by revision surgery.
Physician's defense:
There was no negligence. Redundant foreskin is often left following a circumcision.
Verdict:
A Michigan defense verdict was returned.
Mother with CP has child with CP
A pregnant woman with cerebral palsy (CP) reported a prior preterm delivery at 24 weeks’ gestation to a high-risk prenatal clinic. At that time, she was offered synthetic progesterone (170HP) injections, but she declined because of the cost. She declined 170HP several times. Nine weeks after her initial visit, she declined 170HP despite ultrasonography (US) showing a shortened cervical length (25 mm) for the gestational age. In 2 weeks, when the cervical length measured 9 mm, she was hospitalized to rule out preterm labor but, before tests began, she left the hospital. Five days later, when her cervical length was 11 mm, she received the first 170HP injection. In the next month she received 4 injections, but she failed to show for the fifth injection and US. The next day, she went to the hospital with cramping. She was given steroids and medication to stop labor. US results indicated that the baby was in breech position. The mother consented to cesarean delivery, but the baby was born vaginally an hour later. The child has mild brain damage, CP, developmental delays, and learning disabilities.
Parents' claim: The mother should have been offered vaginal progesterone, which is cheaper. Given the high risk of preterm birth, steroids administered earlier would have improved fetal development. Cesarean delivery should have been performed.
Defendants' defense: Vaginal progesterone was not available at the time. Starting steroids earlier would not have improved fetal outcome. A cesarean delivery was not possible because the baby was in the birth canal.
Verdict: A $3,500,000 Michigan settlement was reached.
Fallopian tubes grow back, pregnancy
A couple decided they did not want more children and sought counseling from the woman’s ObGyn, who recommended laparoscopic tubal ligation. Several months after surgery, the patient became pregnant and gave birth to a son.
Parents' claim:
The additional child placed an economic hardship on the family, now raising 4 children. The youngest child has language delays and learning disabilities.
Physician's defense:
Regrowth of the fallopian tubes resulting in unwanted pregnancy is a known complication of tubal ligation.
Verdict:
A $397,000 Maryland verdict was returned, including funds for the cost of raising the fourth child and to cope with the child’s special needs.
Challenges in managing labor

At 37 weeks' gestation, a woman was hospitalized in labor. At 1:15 pm, she was dilated 3 cm. At 1:30 pm, she was dilated 4−5 cm with increasing contractions and a reassuring fetal heart rate (FHR). The ObGyn covering for the mother’s ObGyn ordered oxytocin augmentation, which started at 2:45 pm. Shortly thereafter, contractions became more frequent and uterine tachysystole was observed. At 4:12 pm, FHR showed multiple deep decelerations with slow recovery. The baseline dropped to a 90-bpm range and remained that way for 17 minutes. At that point, the ObGyn stopped oxytocin and administered terbutaline; the FHR returned to baseline.
After vaginal delivery, the baby’s Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. Two days later, the baby had seizures and was transferred to the neonatal intensive care unit. An electroencephalogram confirmed seizure activity. Initial imaging results were normal. However, magnetic resonance imaging performed a week after delivery showed bilateral brain damage. The child has spastic displegia, is unable to ambulate, and is blind.
Parents' claim: A suit was filed against the hospital and both ObGyns. The hospital settled before trial. The case was discontinued against the primary ObGyn. The covering ObGyn allegedly made 4 departures from accepted medical practice that caused the child’s injury: ordering and administering oxytocin, failing to closely monitor the FHR, failing to timely administer terbutaline, and failing to timely respond to and correct tachysystole.
Physician's defense: The child’s injury occurred before or after labor. The pregnancy was complicated by multiple kidney infections. A week before delivery, US revealed a blood-flow abnormality. An intranatal hypoxic event did not cause the injury, proven by the fact that, after terbutaline was administered, the FHR promptly normalized.
Verdict: A $3 million New York settlement was reached with the hospital. A $134 million verdict was returned against the covering ObGyn.
Brachial plexus injury during delivery
At 37 weeks' gestation, a mother was admitted to the hospital for induction of labor. Increasing doses of oxytocin were administered. Near midnight, FHR monitoring indicated fetal distress. The ObGyn was called and he ordered cesarean delivery. Once he arrived and examined the mother, he found no fetal concerns and decided to proceed with the original birth plan. At 3:30 am, the patient was fully dilated and in active labor. The ObGyn used a vacuum extractor. Upon delivery of the baby’s head, the ObGyn encountered shoulder dystocia and called for assistance. The child was born with a near-total brachial plexus injury: avulsions of all 5 brachial plexus nerves with trauma to the cervical nerve roots at C5−C8 and T1. The child has undergone multiple nerve grafts and orthopedic operations.
Parents' claim: Fetal distress should have prompted the ObGyn to perform cesarean delivery. There was no reason to use vacuum extraction. Based on the severity of the outcome, the ObGyn must have applied excessive force and inappropriate traction during delivery maneuvers.
Physician's defense: The standard of care did not require a cesarean delivery. The vacuum extractor did not cause shoulder dystocia. The ObGyn did not apply excessive force or traction to complete the delivery. The extent of the outcome was partially due to a fetal anomaly and hypotonia.
Verdict: An Illinois defense verdict was returned.
HPV-positive pap tests results never reported
A single mother of 4 children underwent Papanicolaou (Pap) tests in 2004, 2005, and 2007 at a federally funded clinic. Each time, she tested positive for oncogenic human papillomaviruses. In 2011, the patient died of cervical cancer.
Estate's claim: The patient was never notified that the results of the 3 Pap tests were abnormal because all correspondence was sent to an outdated address although she had been treated at the same clinic for other issues during that period of time. Cervical dysplasia identified in 2004 progressed to cancer and metastasized, leading to her death 7 years later.
Defendants' defense: The case was settled during trial.
Verdict: A $4,950,000 Illinois settlement was reached.
Additional Medical Verdicts
• Circumcision requires revision
• Mother with CP has child with CP
• Fallopian tubes grow back, pregnancy
• Challenges in managing labor
• Brachial plexus injury during delivery
• HPV-positive Pap tests results never reported
Letters to the Editor: Determining fetal demise; SERMS in menopause; Aspirin for preeclampsia; Treating cesarean scar defect
Determining fetal demise
I appreciate and thank Drs. Esplin and Eller for their discussion of fetal monitoring pitfalls. I agree with their sentiment that this is an inexact science. After 40 years of looking at these strips, I am convinced there must be a better way. I look forward to some innovative approach to better determine fetal well-being in labor. This article raises a question I have asked, and sought the answer to, for years.
On occasion, I have diagnosed intrauterine fetal demise by detecting the maternal heart rate with an internal fetal scalp electrode. On one particular occasion, somewhere between the time of admission, spontaneous rupture of membranes, and applying the fetal scalp electrode, the fetus died. This case was similar to the one you describe in which early efforts with the external Doppler were unsatisfactory and fetal status was suspect. My question: “What is the time interval from the moment of fetal death and loss of fetal electrical activity until the fetus becomes an effective conduit for the conduction of the maternal cardiac signal? Is it minutes, hours, days? Clearly, this would be difficult to evaluate other than on animal models, but I have yet to find an answer.
Edward Hall, MD
Edgewood, Kentucky
Drs. Esplin and Eller respond
We are grateful for your interest in our article. Unfortunately the answer to your question about the timing between fetal demise and the appearance of maternal electrocardiac activity detected by a fetal scalp electrode after transmission through the fetal body is not clear. We are not aware of any data that would conclusively prove the time required for this to occur. It is likely that this type of information would require an animal model to elucidate. However, we are aware of at least 2 clinical cases in which fetal cardiac activity was convincingly documented at admission and for several hours intrapartum with subsequent episodic loss of signal and then delivery of a dead fetus wherein retrospective review confirmed that for a period of time the maternal heart rate was recorded and interpreted to be the fetal heart rate. From these experiences we conclude that this is possible shortly after the fetal demise, likely within minutes to hours.
Despite this uncertainty, we are confident that the information in our article will help clinicians identify and correct those instances when the maternal heart rate is being recorded instead of the fetal heart rate. Fortunately, this rarely involves a situation in which there has been an undiagnosed intrauterine fetal demise.
“SERMs” definition inaccurate
I disagree with Drs. Liu and Collins’ description of selective estrogen receptor modulators (SERMs) on page S18, in which they state, “Estrogens and SERMs are lipid-soluble steroid hormones that bind to 2 specific hormone receptors, estrogen receptor α and estrogen receptor β…” SERMs are not hormones, and they are defined improperly as such.
Gideon G. Panter, MD
New York, New York
Drs. Liu and Collins respond
Thank you for your interest in our article. SERMs are typically synthetic organic compounds that can activate estrogen receptors or modify activity of the estrogen receptor and, thus, can be considered hormones.
Stop aspirin in pregnancy?
Like many colleagues, I had been stopping low-dose aspirin prior to planned or expected delivery. Evidence suggests a bigger risk of rebound hypercoagulability than bleeding after stopping low-dose aspirin, according to an article on aspirin use in the perioperative period.1 Because of lack of benefit and increased risks of stopping aspirin, it may be time to change our practice and continue aspirin to minimize peridelivery thromboembolic risk.
Mark Jacobs, MD
Mill Valley, CA
Reference
- Gerstein NS, Schulman PM, Gerstein WH, Petersen TR, Tawil I. Should more patients continue aspirin therapy perioperatively?: clinical impact of aspirin withdrawal syndrome. Ann Surg. 2012;255:811–819.
Dr. Barbieri responds
I thank Dr. Jacobs for his advice to continue low-dose aspirin throughout pregnancy in women taking aspirin for prevention of preeclampsia. The review he references is focused on elderly patients taking aspirin for existing heart disease, which is a very different population than pregnant women. There are no high-quality data from clinical trials on whether to continue or stop low-dose aspirin in pregnant women as they approach their due date. I think obstetricians can use their best judgment in making the decision of whether to stop low-dose aspirin at 36 or 37 weeks or continue aspirin throughout the pregnancy.
Technique for preventing cesarean scar defect
I read with interest the proposed treatment options that Dr. Nezhat and colleagues suggested for cesarean scar defect. However, nowhere did I see mention of preventing this defect.
For 30 years I have been closing the hysterotomy in a fashion that I believe leaves no presence of an isthmocele and is a superior closure. I overlap the upper flap with the lower flap and, most importantly, close with chromic catgut. A cesarean scar “niche” occurs with involution of the uterus causing the suture line to bunch up. Chromic catgut has a shorter half-life and will “give;” a suture made of polypropylene will not stretch. I use a running interlocking line with sutures about 0.5 inches apart.
Donald M. Werner, MD
Binghamton, New York
Dr. Nezhat and colleagues respond
We thank Dr. Werner for his inquiry regarding the prevention of cesarean scar defects; as we all agree, the best treatment is prevention. As mentioned in our article, there are no definitive results from the studies published to date that show superiority of one surgical technique over another in regard to hysterotomy closure and prevention of cesarean scar defects. Possible risk factors for developing cesarean scar defects include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries. Although these factors may be associated with increased risk of cesarean scar defects, additional randomized controlled trials need to be performed prior to being able to offer a recommendation on a conclusive preventative measure. For additional information, I would direct you to references 3 and 4 in our article. We thank you for sharing your positive experience and eagerly await additional studies on the topic.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com
Determining fetal demise
I appreciate and thank Drs. Esplin and Eller for their discussion of fetal monitoring pitfalls. I agree with their sentiment that this is an inexact science. After 40 years of looking at these strips, I am convinced there must be a better way. I look forward to some innovative approach to better determine fetal well-being in labor. This article raises a question I have asked, and sought the answer to, for years.
On occasion, I have diagnosed intrauterine fetal demise by detecting the maternal heart rate with an internal fetal scalp electrode. On one particular occasion, somewhere between the time of admission, spontaneous rupture of membranes, and applying the fetal scalp electrode, the fetus died. This case was similar to the one you describe in which early efforts with the external Doppler were unsatisfactory and fetal status was suspect. My question: “What is the time interval from the moment of fetal death and loss of fetal electrical activity until the fetus becomes an effective conduit for the conduction of the maternal cardiac signal? Is it minutes, hours, days? Clearly, this would be difficult to evaluate other than on animal models, but I have yet to find an answer.
Edward Hall, MD
Edgewood, Kentucky
Drs. Esplin and Eller respond
We are grateful for your interest in our article. Unfortunately the answer to your question about the timing between fetal demise and the appearance of maternal electrocardiac activity detected by a fetal scalp electrode after transmission through the fetal body is not clear. We are not aware of any data that would conclusively prove the time required for this to occur. It is likely that this type of information would require an animal model to elucidate. However, we are aware of at least 2 clinical cases in which fetal cardiac activity was convincingly documented at admission and for several hours intrapartum with subsequent episodic loss of signal and then delivery of a dead fetus wherein retrospective review confirmed that for a period of time the maternal heart rate was recorded and interpreted to be the fetal heart rate. From these experiences we conclude that this is possible shortly after the fetal demise, likely within minutes to hours.
Despite this uncertainty, we are confident that the information in our article will help clinicians identify and correct those instances when the maternal heart rate is being recorded instead of the fetal heart rate. Fortunately, this rarely involves a situation in which there has been an undiagnosed intrauterine fetal demise.
“SERMs” definition inaccurate
I disagree with Drs. Liu and Collins’ description of selective estrogen receptor modulators (SERMs) on page S18, in which they state, “Estrogens and SERMs are lipid-soluble steroid hormones that bind to 2 specific hormone receptors, estrogen receptor α and estrogen receptor β…” SERMs are not hormones, and they are defined improperly as such.
Gideon G. Panter, MD
New York, New York
Drs. Liu and Collins respond
Thank you for your interest in our article. SERMs are typically synthetic organic compounds that can activate estrogen receptors or modify activity of the estrogen receptor and, thus, can be considered hormones.
Stop aspirin in pregnancy?
Like many colleagues, I had been stopping low-dose aspirin prior to planned or expected delivery. Evidence suggests a bigger risk of rebound hypercoagulability than bleeding after stopping low-dose aspirin, according to an article on aspirin use in the perioperative period.1 Because of lack of benefit and increased risks of stopping aspirin, it may be time to change our practice and continue aspirin to minimize peridelivery thromboembolic risk.
Mark Jacobs, MD
Mill Valley, CA
Reference
- Gerstein NS, Schulman PM, Gerstein WH, Petersen TR, Tawil I. Should more patients continue aspirin therapy perioperatively?: clinical impact of aspirin withdrawal syndrome. Ann Surg. 2012;255:811–819.
Dr. Barbieri responds
I thank Dr. Jacobs for his advice to continue low-dose aspirin throughout pregnancy in women taking aspirin for prevention of preeclampsia. The review he references is focused on elderly patients taking aspirin for existing heart disease, which is a very different population than pregnant women. There are no high-quality data from clinical trials on whether to continue or stop low-dose aspirin in pregnant women as they approach their due date. I think obstetricians can use their best judgment in making the decision of whether to stop low-dose aspirin at 36 or 37 weeks or continue aspirin throughout the pregnancy.
Technique for preventing cesarean scar defect
I read with interest the proposed treatment options that Dr. Nezhat and colleagues suggested for cesarean scar defect. However, nowhere did I see mention of preventing this defect.
For 30 years I have been closing the hysterotomy in a fashion that I believe leaves no presence of an isthmocele and is a superior closure. I overlap the upper flap with the lower flap and, most importantly, close with chromic catgut. A cesarean scar “niche” occurs with involution of the uterus causing the suture line to bunch up. Chromic catgut has a shorter half-life and will “give;” a suture made of polypropylene will not stretch. I use a running interlocking line with sutures about 0.5 inches apart.
Donald M. Werner, MD
Binghamton, New York
Dr. Nezhat and colleagues respond
We thank Dr. Werner for his inquiry regarding the prevention of cesarean scar defects; as we all agree, the best treatment is prevention. As mentioned in our article, there are no definitive results from the studies published to date that show superiority of one surgical technique over another in regard to hysterotomy closure and prevention of cesarean scar defects. Possible risk factors for developing cesarean scar defects include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries. Although these factors may be associated with increased risk of cesarean scar defects, additional randomized controlled trials need to be performed prior to being able to offer a recommendation on a conclusive preventative measure. For additional information, I would direct you to references 3 and 4 in our article. We thank you for sharing your positive experience and eagerly await additional studies on the topic.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com
Determining fetal demise
I appreciate and thank Drs. Esplin and Eller for their discussion of fetal monitoring pitfalls. I agree with their sentiment that this is an inexact science. After 40 years of looking at these strips, I am convinced there must be a better way. I look forward to some innovative approach to better determine fetal well-being in labor. This article raises a question I have asked, and sought the answer to, for years.
On occasion, I have diagnosed intrauterine fetal demise by detecting the maternal heart rate with an internal fetal scalp electrode. On one particular occasion, somewhere between the time of admission, spontaneous rupture of membranes, and applying the fetal scalp electrode, the fetus died. This case was similar to the one you describe in which early efforts with the external Doppler were unsatisfactory and fetal status was suspect. My question: “What is the time interval from the moment of fetal death and loss of fetal electrical activity until the fetus becomes an effective conduit for the conduction of the maternal cardiac signal? Is it minutes, hours, days? Clearly, this would be difficult to evaluate other than on animal models, but I have yet to find an answer.
Edward Hall, MD
Edgewood, Kentucky
Drs. Esplin and Eller respond
We are grateful for your interest in our article. Unfortunately the answer to your question about the timing between fetal demise and the appearance of maternal electrocardiac activity detected by a fetal scalp electrode after transmission through the fetal body is not clear. We are not aware of any data that would conclusively prove the time required for this to occur. It is likely that this type of information would require an animal model to elucidate. However, we are aware of at least 2 clinical cases in which fetal cardiac activity was convincingly documented at admission and for several hours intrapartum with subsequent episodic loss of signal and then delivery of a dead fetus wherein retrospective review confirmed that for a period of time the maternal heart rate was recorded and interpreted to be the fetal heart rate. From these experiences we conclude that this is possible shortly after the fetal demise, likely within minutes to hours.
Despite this uncertainty, we are confident that the information in our article will help clinicians identify and correct those instances when the maternal heart rate is being recorded instead of the fetal heart rate. Fortunately, this rarely involves a situation in which there has been an undiagnosed intrauterine fetal demise.
“SERMs” definition inaccurate
I disagree with Drs. Liu and Collins’ description of selective estrogen receptor modulators (SERMs) on page S18, in which they state, “Estrogens and SERMs are lipid-soluble steroid hormones that bind to 2 specific hormone receptors, estrogen receptor α and estrogen receptor β…” SERMs are not hormones, and they are defined improperly as such.
Gideon G. Panter, MD
New York, New York
Drs. Liu and Collins respond
Thank you for your interest in our article. SERMs are typically synthetic organic compounds that can activate estrogen receptors or modify activity of the estrogen receptor and, thus, can be considered hormones.
Stop aspirin in pregnancy?
Like many colleagues, I had been stopping low-dose aspirin prior to planned or expected delivery. Evidence suggests a bigger risk of rebound hypercoagulability than bleeding after stopping low-dose aspirin, according to an article on aspirin use in the perioperative period.1 Because of lack of benefit and increased risks of stopping aspirin, it may be time to change our practice and continue aspirin to minimize peridelivery thromboembolic risk.
Mark Jacobs, MD
Mill Valley, CA
Reference
- Gerstein NS, Schulman PM, Gerstein WH, Petersen TR, Tawil I. Should more patients continue aspirin therapy perioperatively?: clinical impact of aspirin withdrawal syndrome. Ann Surg. 2012;255:811–819.
Dr. Barbieri responds
I thank Dr. Jacobs for his advice to continue low-dose aspirin throughout pregnancy in women taking aspirin for prevention of preeclampsia. The review he references is focused on elderly patients taking aspirin for existing heart disease, which is a very different population than pregnant women. There are no high-quality data from clinical trials on whether to continue or stop low-dose aspirin in pregnant women as they approach their due date. I think obstetricians can use their best judgment in making the decision of whether to stop low-dose aspirin at 36 or 37 weeks or continue aspirin throughout the pregnancy.
Technique for preventing cesarean scar defect
I read with interest the proposed treatment options that Dr. Nezhat and colleagues suggested for cesarean scar defect. However, nowhere did I see mention of preventing this defect.
For 30 years I have been closing the hysterotomy in a fashion that I believe leaves no presence of an isthmocele and is a superior closure. I overlap the upper flap with the lower flap and, most importantly, close with chromic catgut. A cesarean scar “niche” occurs with involution of the uterus causing the suture line to bunch up. Chromic catgut has a shorter half-life and will “give;” a suture made of polypropylene will not stretch. I use a running interlocking line with sutures about 0.5 inches apart.
Donald M. Werner, MD
Binghamton, New York
Dr. Nezhat and colleagues respond
We thank Dr. Werner for his inquiry regarding the prevention of cesarean scar defects; as we all agree, the best treatment is prevention. As mentioned in our article, there are no definitive results from the studies published to date that show superiority of one surgical technique over another in regard to hysterotomy closure and prevention of cesarean scar defects. Possible risk factors for developing cesarean scar defects include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries. Although these factors may be associated with increased risk of cesarean scar defects, additional randomized controlled trials need to be performed prior to being able to offer a recommendation on a conclusive preventative measure. For additional information, I would direct you to references 3 and 4 in our article. We thank you for sharing your positive experience and eagerly await additional studies on the topic.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com
Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by ![]()
50 years of gynecologic surgery: A large dose of ingenuity, a small dose of controversy
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Gyn. oncologists are in demand for robotic hysterectomy
SAN DIEGO – The demand for gynecologic oncologists to perform robotic hysterectomies – even for benign indications – has increased to the point that additional fellowship training spots will be necessary to meet the need, Dr. Kayla M. Wishall said at the annual meeting of the Society of Gynecologic Oncology.
More and more patients want their hysterectomies performed robotically. They find the high-quality optics and minimally invasive nature of the robotic procedure appealing – smaller incisions, less blood loss, shorter hospital stay, and faster recovery. And gynecologic oncologists are getting an increasing number of referrals because of their special expertise in robotic surgery and extensive experience with higher-risk patients, explained Dr. Wishall, a gynecologic oncologist at Hahnemann University Hospital/Drexel University in Philadelphia.
“This trend will likely tax the limited resources of gynecologic oncologists,” she added.
Another possible reason for the growing demand for gynecologic oncologist–performed robotic hysterectomies is that these subspecialists achieve better outcomes than gynecologists who do robotic hysterectomies, at least according to the findings of a retrospective study performed by Dr. Wishall, which included all of the 468 robotic hysterectomies performed at a large academic medical center in a recent 5-year period.
Gynecologic oncologists performed 64 (16.5%) of the 387 robotic hysterectomies done for benign indications. All told, gynecologists did 254 of the robotic hysterectomies; gynecologic oncologists performed 214.
Even though patients referred to gynecologic oncologists for these procedures were older, heavier, more likely to have had previous abdominal surgery, more often members of racial minorities, and had a higher prevalence of cardiac comorbidities, they experienced significantly fewer intra- and postoperative complications than patients whose robotic hysterectomies were performed by gynecologists, Dr. Wishall reported.
The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists. But the rate of cardiac comorbidities, for instance, was 36.4% among patients seeing gynecologic oncologists, compared with 23.6% among those seeing gynecologists.
Moreover, gynecologists were about 10-fold more likely than gynecologic oncologists to call for an intraoperative consultation and sixfold more likely to convert their robotic hysterectomy to an open procedure. Their average operating room time was about 40% longer (244 minutes versus 171 minutes), too, in this single-center experience.
Dr. Wishall reported having no financial conflicts related to her study, which was conducted free of commercial support.
I read this article initially with amusement and then with outrage and disdain. This article summarizes the single-site, retrospective study by Dr. Kayla Wishall at the annual meeting of the Society of Gynecologic Oncology. Not only is this nonscience, but nonsensical science. As a single center retrospective study, conclusions must be suspect.
 |
Dr. Charles E. Miller |
The comparison numbers of the two groups are small. While confounders would appear to be greater in the oncology group, we know nothing about the difficulty of the surgeries themselves – size of uterus, adnexal disease, endometriosis, pelvic adhesions, etc. Oftentimes, gynecologic oncologists dealing with endometrial carcinoma are going to face a less difficult challenge than a generalist dealing with an 18-weeks–size uterus in a woman who has undergone three prior C-sections, an open myomectomy, or stage IV endometriosis.
We are also not privy to the experience of the surgeons involved; that is, the number of procedures performed by each surgeon in the compared groups. It is certainly well known that complications decrease with surgeon experience. In a multicenter analysis by Peter Lim et al., looking at robotic assisted hysterectomies performed by high-volume surgeons (60 or more prior procedures), the intraoperative complication rate was only 0.7% and the postoperative complication rate 6.3% (Int J Gynaecol Obstet. 2016 Jun;133[3]:359-64).
As a benign gynecologist who has been performing minimally invasive gynecologic surgery for 30 years and more recently, robotic surgery, I am shocked with the tenor of this study, as it would imply that unless someone is boarded in gynecologic oncology, he or she should not be performing robotic hysterectomies.
I would advise Dr. Wishall to reevaluate her surgeon population and look at the impact of experience as well as procedure difficultly. I am absolutely sure that she will find that many of the surgeons with excellent outcomes will be generalists, who are well experienced in robotic hysterectomy.
Dr. Charles E. Miller is a clinical associate professor at the University of Illinois at Chicago, and a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. He reported having no financial disclosures relevant to this article.
I read this article initially with amusement and then with outrage and disdain. This article summarizes the single-site, retrospective study by Dr. Kayla Wishall at the annual meeting of the Society of Gynecologic Oncology. Not only is this nonscience, but nonsensical science. As a single center retrospective study, conclusions must be suspect.
 |
Dr. Charles E. Miller |
The comparison numbers of the two groups are small. While confounders would appear to be greater in the oncology group, we know nothing about the difficulty of the surgeries themselves – size of uterus, adnexal disease, endometriosis, pelvic adhesions, etc. Oftentimes, gynecologic oncologists dealing with endometrial carcinoma are going to face a less difficult challenge than a generalist dealing with an 18-weeks–size uterus in a woman who has undergone three prior C-sections, an open myomectomy, or stage IV endometriosis.
We are also not privy to the experience of the surgeons involved; that is, the number of procedures performed by each surgeon in the compared groups. It is certainly well known that complications decrease with surgeon experience. In a multicenter analysis by Peter Lim et al., looking at robotic assisted hysterectomies performed by high-volume surgeons (60 or more prior procedures), the intraoperative complication rate was only 0.7% and the postoperative complication rate 6.3% (Int J Gynaecol Obstet. 2016 Jun;133[3]:359-64).
As a benign gynecologist who has been performing minimally invasive gynecologic surgery for 30 years and more recently, robotic surgery, I am shocked with the tenor of this study, as it would imply that unless someone is boarded in gynecologic oncology, he or she should not be performing robotic hysterectomies.
I would advise Dr. Wishall to reevaluate her surgeon population and look at the impact of experience as well as procedure difficultly. I am absolutely sure that she will find that many of the surgeons with excellent outcomes will be generalists, who are well experienced in robotic hysterectomy.
Dr. Charles E. Miller is a clinical associate professor at the University of Illinois at Chicago, and a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. He reported having no financial disclosures relevant to this article.
I read this article initially with amusement and then with outrage and disdain. This article summarizes the single-site, retrospective study by Dr. Kayla Wishall at the annual meeting of the Society of Gynecologic Oncology. Not only is this nonscience, but nonsensical science. As a single center retrospective study, conclusions must be suspect.
 |
Dr. Charles E. Miller |
The comparison numbers of the two groups are small. While confounders would appear to be greater in the oncology group, we know nothing about the difficulty of the surgeries themselves – size of uterus, adnexal disease, endometriosis, pelvic adhesions, etc. Oftentimes, gynecologic oncologists dealing with endometrial carcinoma are going to face a less difficult challenge than a generalist dealing with an 18-weeks–size uterus in a woman who has undergone three prior C-sections, an open myomectomy, or stage IV endometriosis.
We are also not privy to the experience of the surgeons involved; that is, the number of procedures performed by each surgeon in the compared groups. It is certainly well known that complications decrease with surgeon experience. In a multicenter analysis by Peter Lim et al., looking at robotic assisted hysterectomies performed by high-volume surgeons (60 or more prior procedures), the intraoperative complication rate was only 0.7% and the postoperative complication rate 6.3% (Int J Gynaecol Obstet. 2016 Jun;133[3]:359-64).
As a benign gynecologist who has been performing minimally invasive gynecologic surgery for 30 years and more recently, robotic surgery, I am shocked with the tenor of this study, as it would imply that unless someone is boarded in gynecologic oncology, he or she should not be performing robotic hysterectomies.
I would advise Dr. Wishall to reevaluate her surgeon population and look at the impact of experience as well as procedure difficultly. I am absolutely sure that she will find that many of the surgeons with excellent outcomes will be generalists, who are well experienced in robotic hysterectomy.
Dr. Charles E. Miller is a clinical associate professor at the University of Illinois at Chicago, and a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill. He reported having no financial disclosures relevant to this article.
SAN DIEGO – The demand for gynecologic oncologists to perform robotic hysterectomies – even for benign indications – has increased to the point that additional fellowship training spots will be necessary to meet the need, Dr. Kayla M. Wishall said at the annual meeting of the Society of Gynecologic Oncology.
More and more patients want their hysterectomies performed robotically. They find the high-quality optics and minimally invasive nature of the robotic procedure appealing – smaller incisions, less blood loss, shorter hospital stay, and faster recovery. And gynecologic oncologists are getting an increasing number of referrals because of their special expertise in robotic surgery and extensive experience with higher-risk patients, explained Dr. Wishall, a gynecologic oncologist at Hahnemann University Hospital/Drexel University in Philadelphia.
“This trend will likely tax the limited resources of gynecologic oncologists,” she added.
Another possible reason for the growing demand for gynecologic oncologist–performed robotic hysterectomies is that these subspecialists achieve better outcomes than gynecologists who do robotic hysterectomies, at least according to the findings of a retrospective study performed by Dr. Wishall, which included all of the 468 robotic hysterectomies performed at a large academic medical center in a recent 5-year period.
Gynecologic oncologists performed 64 (16.5%) of the 387 robotic hysterectomies done for benign indications. All told, gynecologists did 254 of the robotic hysterectomies; gynecologic oncologists performed 214.
Even though patients referred to gynecologic oncologists for these procedures were older, heavier, more likely to have had previous abdominal surgery, more often members of racial minorities, and had a higher prevalence of cardiac comorbidities, they experienced significantly fewer intra- and postoperative complications than patients whose robotic hysterectomies were performed by gynecologists, Dr. Wishall reported.
The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists. But the rate of cardiac comorbidities, for instance, was 36.4% among patients seeing gynecologic oncologists, compared with 23.6% among those seeing gynecologists.
Moreover, gynecologists were about 10-fold more likely than gynecologic oncologists to call for an intraoperative consultation and sixfold more likely to convert their robotic hysterectomy to an open procedure. Their average operating room time was about 40% longer (244 minutes versus 171 minutes), too, in this single-center experience.
Dr. Wishall reported having no financial conflicts related to her study, which was conducted free of commercial support.
SAN DIEGO – The demand for gynecologic oncologists to perform robotic hysterectomies – even for benign indications – has increased to the point that additional fellowship training spots will be necessary to meet the need, Dr. Kayla M. Wishall said at the annual meeting of the Society of Gynecologic Oncology.
More and more patients want their hysterectomies performed robotically. They find the high-quality optics and minimally invasive nature of the robotic procedure appealing – smaller incisions, less blood loss, shorter hospital stay, and faster recovery. And gynecologic oncologists are getting an increasing number of referrals because of their special expertise in robotic surgery and extensive experience with higher-risk patients, explained Dr. Wishall, a gynecologic oncologist at Hahnemann University Hospital/Drexel University in Philadelphia.
“This trend will likely tax the limited resources of gynecologic oncologists,” she added.
Another possible reason for the growing demand for gynecologic oncologist–performed robotic hysterectomies is that these subspecialists achieve better outcomes than gynecologists who do robotic hysterectomies, at least according to the findings of a retrospective study performed by Dr. Wishall, which included all of the 468 robotic hysterectomies performed at a large academic medical center in a recent 5-year period.
Gynecologic oncologists performed 64 (16.5%) of the 387 robotic hysterectomies done for benign indications. All told, gynecologists did 254 of the robotic hysterectomies; gynecologic oncologists performed 214.
Even though patients referred to gynecologic oncologists for these procedures were older, heavier, more likely to have had previous abdominal surgery, more often members of racial minorities, and had a higher prevalence of cardiac comorbidities, they experienced significantly fewer intra- and postoperative complications than patients whose robotic hysterectomies were performed by gynecologists, Dr. Wishall reported.
The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists. But the rate of cardiac comorbidities, for instance, was 36.4% among patients seeing gynecologic oncologists, compared with 23.6% among those seeing gynecologists.
Moreover, gynecologists were about 10-fold more likely than gynecologic oncologists to call for an intraoperative consultation and sixfold more likely to convert their robotic hysterectomy to an open procedure. Their average operating room time was about 40% longer (244 minutes versus 171 minutes), too, in this single-center experience.
Dr. Wishall reported having no financial conflicts related to her study, which was conducted free of commercial support.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Gynecologic oncologists achieved better robotic hysterectomy outcomes than gynecologists despite challenging referrals.
Major finding: The combined intraoperative and postoperative complication rate for robotic hysterectomies performed by gynecologic oncologists was 5.2%, compared with 16% for gynecologists.
Data source: A retrospective observational study conducted at a single center included 254 women whose robotic hysterectomies were performed by gynecologists and 214 done by gynecologic oncologists.
Disclosures: Dr. Wishall reported having no financial conflicts related to the study, which was conducted free of commercial support.
Tissue extraction: Can the pendulum change direction?
On April 17, 2014, the US Food and Drug Administration (FDA) released a safety communication that discouraged the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This recommendation was based mainly on the premise that fibroids may contain an underlying malignancy (in 1 in 350 women) that laparoscopic power morcellation could spread, thereby potentially worsening a cancer prognosis.1 Whether we liked it or not, minimally invasive gynecologic surgery as we knew it had changed forever on that fateful day.
During the last 2 years, the implications of that initial safety communication, along with its update in November of 2014,2 have been far reaching. From a health care industry perspective, the largest manufacturer of power morcellators, Johnson & Johnson, completely pulled its device out of the market within months of the initial FDA safety communication.3 Although several other companies remained in the market, health insurers such as Highmark and Aetna took the stance of not paying for the routine use of a power morcellator.4,5 Soon government officials became involved in the campaign against power morcellators and trial lawyers made this surgical device the latest “hot target.”6,7
The power morcellator’s absence impactFor clinicians, the narrative painted had moved the pendulum so far against power morcellation that it did not come as a surprise that practice patterns were significantly impacted. Harris and colleagues recently measured that impact by evaluating practice patterns and postoperative complications before and after the FDA safety communication on power morcellation. In their retrospective cohort study of patients within the Michigan Surgical Quality Collaborative, utilization of minimally invasive hysterectomy decreased and major surgical, nontransfusion complications and 30-day hospital readmission rates increased after release of the safety communication.8
Further support of this change in practice patterns was demonstrated in a recent survey conducted by the AAGL and American College of Obstetricians and Gynecologists Collaborative Ambulatory Research Network (ACOG CARN). In this survey study, Lum and colleagues were able to show that power morcellation use decreased among AAGL and ACOG CARN members after the FDA warnings, and rates of laparotomy increased.9
Critical to the discussion, yet apparently overlooked during the formulation of the initial FDA warning, was the potential clinical significance of the downstream impact of converting minimally invasive surgical cases to more invasive laparotomies. A decision-tree analysis published by Siedhoff and colleagues highlighted this point by predicting fewer overall deaths for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy.10 An ability to weigh the benefits and risks of procedure-related complications that are associated with laparotomy, including death, should have been part of the conversation from the beginning.
There is a silver lining emergingBased on this domino effect, it would seem that the damage done to minimally invasive gynecologic surgery over the past 2 years is irreparable. So is there any silver lining to the current state of affairs in tissue extraction? I would argue yes.
We have updated estimates for incidence of unsuspected leiomyosarcoma. First of all, discussions surrounding the FDA’s estimated 1 in 350 risk of encountering an unsuspected uterine sarcoma during the treatment of fibroids prompted a more critical evaluation of the scientific literature. In fact, Parker and colleagues, in a commentary published in Obstetrics & Gynecology, summarized very nicely the flawed methodology in the FDA’s determination of risk and in turn presented several updated calculations and studies that placed the prevalence somewhere in the range of 2 in 8,720 (0.023%) to 1 in 1,550 (0.064%).11
We are speaking with the FDA. Channels of communication with the FDA have since been developed and societies such as the AAGL were invited by the Center for Devices and Radiological Health (CDRH) at the FDA to serve in their Network of Experts. This Network of Experts is an FDA-vetted group of non−FDA affiliated scientists, clinicians, and engineers who provide the CDRH at the FDA with rapid access to scientific, engineering, and medical expertise when it is needed to supplement existing knowledge and expertise within the CDRH. By developing these lines of communication, the CDRH is able to broaden its exposure to scientific viewpoints without the influence of external, or non−FDA, policy advice or opinions.
We have been innovating our techniques. Clinicians also began to develop innovative techniques for tissue extraction that also included contained, in-bag power morcellation. Vargas and colleagues showed similar perioperative outcomes when comparing open power morcellation with contained power morcellation within an insufflated isolation bag. The mean operative time was prolonged by only 26 minutes with in-bag morcellation.12
Although the initial experience of surgeons involved various containment systems that were off label in their usage and not designed to be paired with a power morcellator, it allowed for the identification of potential limitations, such as the risk of leakage. Cohen and colleagues, in their published experience, demonstrated a 9.2% spillage in 76 patients who underwent contained power morcellation.13
Could a new FDA approval turn the tide?In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| FIGURE 1 PneumoLiner tissue containment device | FIGURE 2 PneumoLiner device placement | |
 |  |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
I do believe the pendulum can change directionAs surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed May 24, 2016.
- Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Updated April 7, 2014. Accessed May 24, 2016.
- Kamp J, Levitz J. Johnson & Johnson pulls hysterectomy device from hospitals. The Wall Street Journal. July 30, 2014. http://www.wsj.com/articles/johnson-johnson-to-call-for-voluntary-return-of-morcellators-1406754350. Accessed May 25, 2016.
- Levitz J. Health insurer to stop covering uterine procedure. The Wall Street Journal. August 2, 2014. http://www.wsj.com/articles/health-insurer-to-stop-covering-uterine-procedure-1406999176. Accessed May 25, 2016.
- Kamp J. Aetna to stop covering routine use of power morcellator. The Wall Street Journal. May 5, 2015. http://www.wsj.com/articles/aetna-to-stop-covering-routine-use-of-power-morcellator-1430838666. Accessed May 25, 2016.
- Kamp J. Senators want more companies to pull surgical device from market. The Wall Street Journal. August 19, 2014. http://www.wsj.com/articles/senators-want-more-companies-to-pull-surgical-device-from-market-1408405349. Accessed May 25, 2016.
- Silverman E. Power morcellators are the latest hot target among trial lawyers. The Wall Street Journal. December 2, 2014. http://blogs.wsj.com/pharmalot/2014/12/02/power-morcellators-are-the-latest-hot-target-among-trial-lawyers/. Accessed May 25, 2016.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after the US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1−e13.
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration warnings against power morcellation. J Minim Invasiv Gynecol. 2016;23(4):548−556.
- Siedhoff M, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
- Parker WH, Kaunitz AM, Pritts EA, et al; Leiomyoma Morcellation Review Group. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18−22.
- Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasiv Gynecol. 2015;22(3):433−438.
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257.e1−e6.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. April 7, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed May 24, 2016.
On April 17, 2014, the US Food and Drug Administration (FDA) released a safety communication that discouraged the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This recommendation was based mainly on the premise that fibroids may contain an underlying malignancy (in 1 in 350 women) that laparoscopic power morcellation could spread, thereby potentially worsening a cancer prognosis.1 Whether we liked it or not, minimally invasive gynecologic surgery as we knew it had changed forever on that fateful day.
During the last 2 years, the implications of that initial safety communication, along with its update in November of 2014,2 have been far reaching. From a health care industry perspective, the largest manufacturer of power morcellators, Johnson & Johnson, completely pulled its device out of the market within months of the initial FDA safety communication.3 Although several other companies remained in the market, health insurers such as Highmark and Aetna took the stance of not paying for the routine use of a power morcellator.4,5 Soon government officials became involved in the campaign against power morcellators and trial lawyers made this surgical device the latest “hot target.”6,7
The power morcellator’s absence impactFor clinicians, the narrative painted had moved the pendulum so far against power morcellation that it did not come as a surprise that practice patterns were significantly impacted. Harris and colleagues recently measured that impact by evaluating practice patterns and postoperative complications before and after the FDA safety communication on power morcellation. In their retrospective cohort study of patients within the Michigan Surgical Quality Collaborative, utilization of minimally invasive hysterectomy decreased and major surgical, nontransfusion complications and 30-day hospital readmission rates increased after release of the safety communication.8
Further support of this change in practice patterns was demonstrated in a recent survey conducted by the AAGL and American College of Obstetricians and Gynecologists Collaborative Ambulatory Research Network (ACOG CARN). In this survey study, Lum and colleagues were able to show that power morcellation use decreased among AAGL and ACOG CARN members after the FDA warnings, and rates of laparotomy increased.9
Critical to the discussion, yet apparently overlooked during the formulation of the initial FDA warning, was the potential clinical significance of the downstream impact of converting minimally invasive surgical cases to more invasive laparotomies. A decision-tree analysis published by Siedhoff and colleagues highlighted this point by predicting fewer overall deaths for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy.10 An ability to weigh the benefits and risks of procedure-related complications that are associated with laparotomy, including death, should have been part of the conversation from the beginning.
There is a silver lining emergingBased on this domino effect, it would seem that the damage done to minimally invasive gynecologic surgery over the past 2 years is irreparable. So is there any silver lining to the current state of affairs in tissue extraction? I would argue yes.
We have updated estimates for incidence of unsuspected leiomyosarcoma. First of all, discussions surrounding the FDA’s estimated 1 in 350 risk of encountering an unsuspected uterine sarcoma during the treatment of fibroids prompted a more critical evaluation of the scientific literature. In fact, Parker and colleagues, in a commentary published in Obstetrics & Gynecology, summarized very nicely the flawed methodology in the FDA’s determination of risk and in turn presented several updated calculations and studies that placed the prevalence somewhere in the range of 2 in 8,720 (0.023%) to 1 in 1,550 (0.064%).11
We are speaking with the FDA. Channels of communication with the FDA have since been developed and societies such as the AAGL were invited by the Center for Devices and Radiological Health (CDRH) at the FDA to serve in their Network of Experts. This Network of Experts is an FDA-vetted group of non−FDA affiliated scientists, clinicians, and engineers who provide the CDRH at the FDA with rapid access to scientific, engineering, and medical expertise when it is needed to supplement existing knowledge and expertise within the CDRH. By developing these lines of communication, the CDRH is able to broaden its exposure to scientific viewpoints without the influence of external, or non−FDA, policy advice or opinions.
We have been innovating our techniques. Clinicians also began to develop innovative techniques for tissue extraction that also included contained, in-bag power morcellation. Vargas and colleagues showed similar perioperative outcomes when comparing open power morcellation with contained power morcellation within an insufflated isolation bag. The mean operative time was prolonged by only 26 minutes with in-bag morcellation.12
Although the initial experience of surgeons involved various containment systems that were off label in their usage and not designed to be paired with a power morcellator, it allowed for the identification of potential limitations, such as the risk of leakage. Cohen and colleagues, in their published experience, demonstrated a 9.2% spillage in 76 patients who underwent contained power morcellation.13
Could a new FDA approval turn the tide?In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| FIGURE 1 PneumoLiner tissue containment device | FIGURE 2 PneumoLiner device placement | |
 |  |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
I do believe the pendulum can change directionAs surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
On April 17, 2014, the US Food and Drug Administration (FDA) released a safety communication that discouraged the use of laparoscopic power morcellation during hysterectomy or myomectomy for the treatment of women with uterine fibroids. This recommendation was based mainly on the premise that fibroids may contain an underlying malignancy (in 1 in 350 women) that laparoscopic power morcellation could spread, thereby potentially worsening a cancer prognosis.1 Whether we liked it or not, minimally invasive gynecologic surgery as we knew it had changed forever on that fateful day.
During the last 2 years, the implications of that initial safety communication, along with its update in November of 2014,2 have been far reaching. From a health care industry perspective, the largest manufacturer of power morcellators, Johnson & Johnson, completely pulled its device out of the market within months of the initial FDA safety communication.3 Although several other companies remained in the market, health insurers such as Highmark and Aetna took the stance of not paying for the routine use of a power morcellator.4,5 Soon government officials became involved in the campaign against power morcellators and trial lawyers made this surgical device the latest “hot target.”6,7
The power morcellator’s absence impactFor clinicians, the narrative painted had moved the pendulum so far against power morcellation that it did not come as a surprise that practice patterns were significantly impacted. Harris and colleagues recently measured that impact by evaluating practice patterns and postoperative complications before and after the FDA safety communication on power morcellation. In their retrospective cohort study of patients within the Michigan Surgical Quality Collaborative, utilization of minimally invasive hysterectomy decreased and major surgical, nontransfusion complications and 30-day hospital readmission rates increased after release of the safety communication.8
Further support of this change in practice patterns was demonstrated in a recent survey conducted by the AAGL and American College of Obstetricians and Gynecologists Collaborative Ambulatory Research Network (ACOG CARN). In this survey study, Lum and colleagues were able to show that power morcellation use decreased among AAGL and ACOG CARN members after the FDA warnings, and rates of laparotomy increased.9
Critical to the discussion, yet apparently overlooked during the formulation of the initial FDA warning, was the potential clinical significance of the downstream impact of converting minimally invasive surgical cases to more invasive laparotomies. A decision-tree analysis published by Siedhoff and colleagues highlighted this point by predicting fewer overall deaths for laparoscopic hysterectomy with morcellation compared with abdominal hysterectomy.10 An ability to weigh the benefits and risks of procedure-related complications that are associated with laparotomy, including death, should have been part of the conversation from the beginning.
There is a silver lining emergingBased on this domino effect, it would seem that the damage done to minimally invasive gynecologic surgery over the past 2 years is irreparable. So is there any silver lining to the current state of affairs in tissue extraction? I would argue yes.
We have updated estimates for incidence of unsuspected leiomyosarcoma. First of all, discussions surrounding the FDA’s estimated 1 in 350 risk of encountering an unsuspected uterine sarcoma during the treatment of fibroids prompted a more critical evaluation of the scientific literature. In fact, Parker and colleagues, in a commentary published in Obstetrics & Gynecology, summarized very nicely the flawed methodology in the FDA’s determination of risk and in turn presented several updated calculations and studies that placed the prevalence somewhere in the range of 2 in 8,720 (0.023%) to 1 in 1,550 (0.064%).11
We are speaking with the FDA. Channels of communication with the FDA have since been developed and societies such as the AAGL were invited by the Center for Devices and Radiological Health (CDRH) at the FDA to serve in their Network of Experts. This Network of Experts is an FDA-vetted group of non−FDA affiliated scientists, clinicians, and engineers who provide the CDRH at the FDA with rapid access to scientific, engineering, and medical expertise when it is needed to supplement existing knowledge and expertise within the CDRH. By developing these lines of communication, the CDRH is able to broaden its exposure to scientific viewpoints without the influence of external, or non−FDA, policy advice or opinions.
We have been innovating our techniques. Clinicians also began to develop innovative techniques for tissue extraction that also included contained, in-bag power morcellation. Vargas and colleagues showed similar perioperative outcomes when comparing open power morcellation with contained power morcellation within an insufflated isolation bag. The mean operative time was prolonged by only 26 minutes with in-bag morcellation.12
Although the initial experience of surgeons involved various containment systems that were off label in their usage and not designed to be paired with a power morcellator, it allowed for the identification of potential limitations, such as the risk of leakage. Cohen and colleagues, in their published experience, demonstrated a 9.2% spillage in 76 patients who underwent contained power morcellation.13
Could a new FDA approval turn the tide?In an interesting turn of events, the FDA, on April 7, 2016, nearly 2 years after their initial warning about power morcellation, gave de novo classification clearance to Advanced Surgical Concepts for its PneumoLiner device.14 This first-of-a-kind medical device was developed for the purpose of completely containing fluid, cells, and tissue fragments during laparoscopic power morcellation, isolating uterine tissue that is not suspected to contain cancer (FIGURES 1 and 2). It is important to note, however, that it has not been shown to reduce the risk of spreading cancer during this procedure. Although a surprise to some, the approval of such a product is in keeping with the FDA’s safety communication update in November 2014 that encouraged the development of containment systems designed specifically for gynecologic surgery.
| FIGURE 1 PneumoLiner tissue containment device | FIGURE 2 PneumoLiner device placement | |
 |  |
Currently, the PneumoLiner is not yet available for purchase until Olympus, who will be the exclusive distributor, finalizes its formal training program rollout for ensuring safe and effective use of the device by gynecologic surgeons. As part of the FDA approval process, a training protocol was validated in a laboratory setting by surgeons with varying levels of experience in order to demonstrate that adherence to this process showed no damage to any of the containment systems used during the study.
As I look forward to the opportunity to learn more about this new containment system and potentially incorporate it into my surgical armamentarium, several questions immediately come to mind.
- Will hospitals, many of which pulled morcellators off their shelves in the wake of the FDA safety communication, be willing to embrace such innovative new containment technology and make it available to their surgeons?
- Will the litigious landscape painted by trial lawyers prevent surgeons from offering this option to their patients?
- Can surgeons undo much of the misinformation being propagated by the media as it relates to tissue extraction and the risk of encountering a malignancy?
I hope the answer to all of these questions is yes.
I do believe the pendulum can change directionAs surgeons it is our duty to evaluate potentially innovative solutions to the surgical challenges we face in clinical practice while maintaining an evidence-based approach. Most importantly, all of this must be balanced by sound clinical judgment that no technology can replace. With these guiding principles in mind, I believe that the pendulum regarding tissue extraction can change direction.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed May 24, 2016.
- Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Updated April 7, 2014. Accessed May 24, 2016.
- Kamp J, Levitz J. Johnson & Johnson pulls hysterectomy device from hospitals. The Wall Street Journal. July 30, 2014. http://www.wsj.com/articles/johnson-johnson-to-call-for-voluntary-return-of-morcellators-1406754350. Accessed May 25, 2016.
- Levitz J. Health insurer to stop covering uterine procedure. The Wall Street Journal. August 2, 2014. http://www.wsj.com/articles/health-insurer-to-stop-covering-uterine-procedure-1406999176. Accessed May 25, 2016.
- Kamp J. Aetna to stop covering routine use of power morcellator. The Wall Street Journal. May 5, 2015. http://www.wsj.com/articles/aetna-to-stop-covering-routine-use-of-power-morcellator-1430838666. Accessed May 25, 2016.
- Kamp J. Senators want more companies to pull surgical device from market. The Wall Street Journal. August 19, 2014. http://www.wsj.com/articles/senators-want-more-companies-to-pull-surgical-device-from-market-1408405349. Accessed May 25, 2016.
- Silverman E. Power morcellators are the latest hot target among trial lawyers. The Wall Street Journal. December 2, 2014. http://blogs.wsj.com/pharmalot/2014/12/02/power-morcellators-are-the-latest-hot-target-among-trial-lawyers/. Accessed May 25, 2016.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after the US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1−e13.
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration warnings against power morcellation. J Minim Invasiv Gynecol. 2016;23(4):548−556.
- Siedhoff M, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
- Parker WH, Kaunitz AM, Pritts EA, et al; Leiomyoma Morcellation Review Group. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18−22.
- Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasiv Gynecol. 2015;22(3):433−438.
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257.e1−e6.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. April 7, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed May 24, 2016.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed May 24, 2016.
- Updated laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Updated April 7, 2014. Accessed May 24, 2016.
- Kamp J, Levitz J. Johnson & Johnson pulls hysterectomy device from hospitals. The Wall Street Journal. July 30, 2014. http://www.wsj.com/articles/johnson-johnson-to-call-for-voluntary-return-of-morcellators-1406754350. Accessed May 25, 2016.
- Levitz J. Health insurer to stop covering uterine procedure. The Wall Street Journal. August 2, 2014. http://www.wsj.com/articles/health-insurer-to-stop-covering-uterine-procedure-1406999176. Accessed May 25, 2016.
- Kamp J. Aetna to stop covering routine use of power morcellator. The Wall Street Journal. May 5, 2015. http://www.wsj.com/articles/aetna-to-stop-covering-routine-use-of-power-morcellator-1430838666. Accessed May 25, 2016.
- Kamp J. Senators want more companies to pull surgical device from market. The Wall Street Journal. August 19, 2014. http://www.wsj.com/articles/senators-want-more-companies-to-pull-surgical-device-from-market-1408405349. Accessed May 25, 2016.
- Silverman E. Power morcellators are the latest hot target among trial lawyers. The Wall Street Journal. December 2, 2014. http://blogs.wsj.com/pharmalot/2014/12/02/power-morcellators-are-the-latest-hot-target-among-trial-lawyers/. Accessed May 25, 2016.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after the US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1−e13.
- Lum DA, Sokol ER, Berek JS, et al. Impact of the 2014 Food and Drug Administration warnings against power morcellation. J Minim Invasiv Gynecol. 2016;23(4):548−556.
- Siedhoff M, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
- Parker WH, Kaunitz AM, Pritts EA, et al; Leiomyoma Morcellation Review Group. US Food and Drug Administration’s guidance regarding morcellation of leiomyomas: well-intentioned, but is it harmful for women? Obstet Gynecol. 2016;127(1):18−22.
- Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasiv Gynecol. 2015;22(3):433−438.
- Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257.e1−e6.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. April 7, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed May 24, 2016.