User login
Who is liable when a surgical error occurs?
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
In this Article
- Medical error vs negligence
- Caring for the second victim
- 10 starting points for reducing medical errors
How gynecologic procedures and pharmacologic treatments can affect the uterus
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
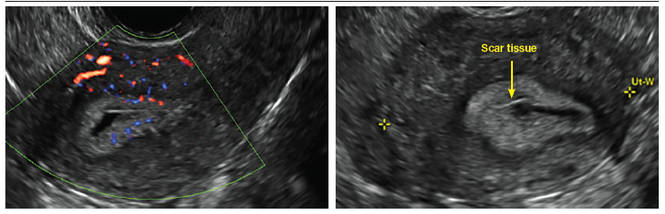
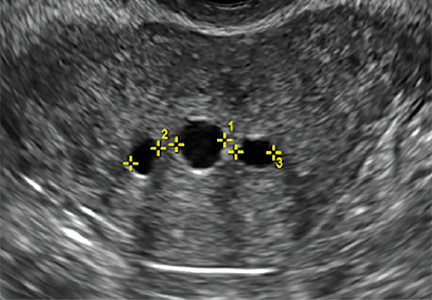
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
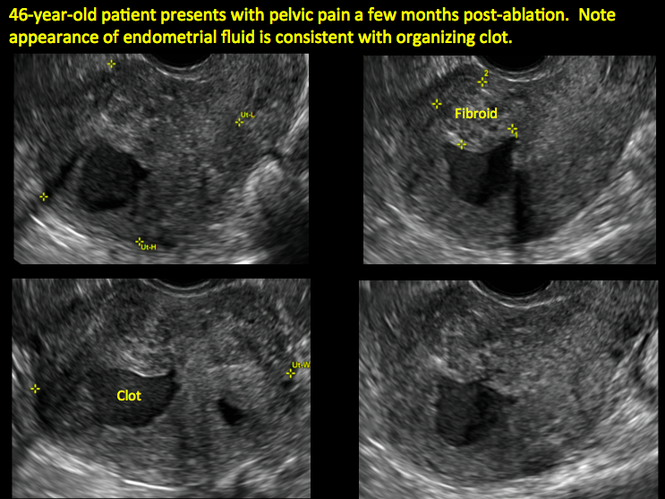
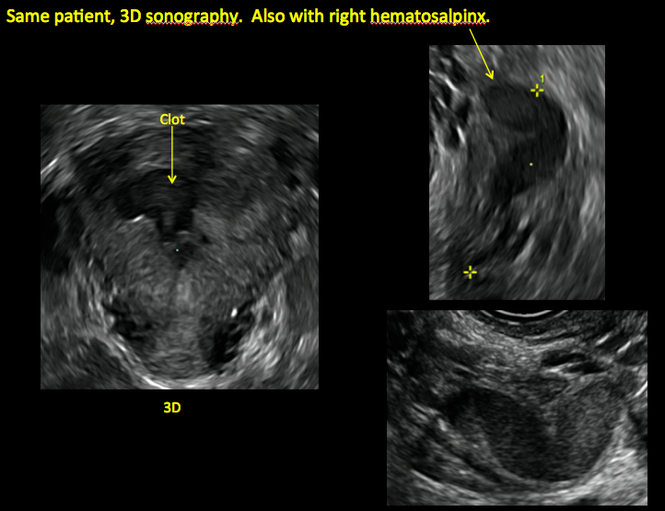
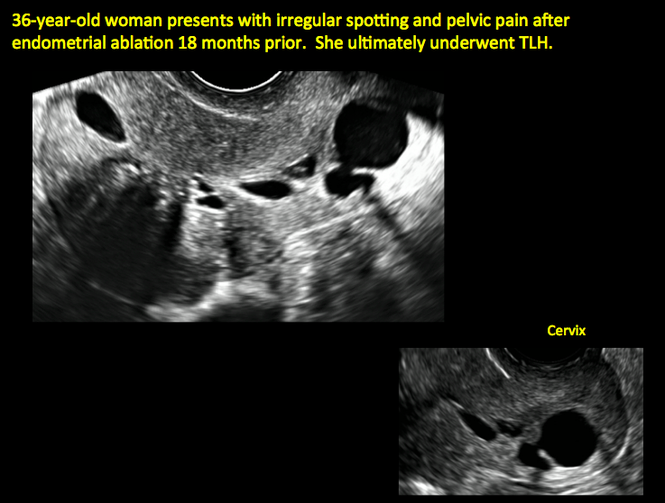
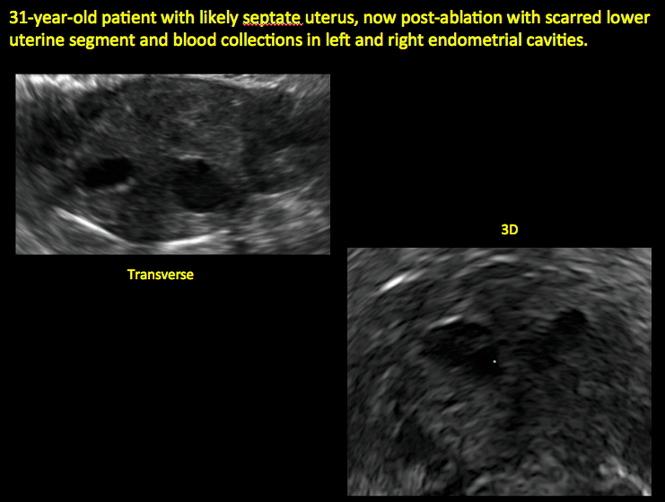
Postablation endometrial destruction
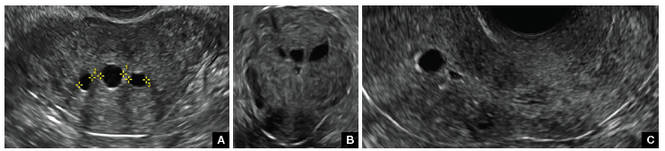
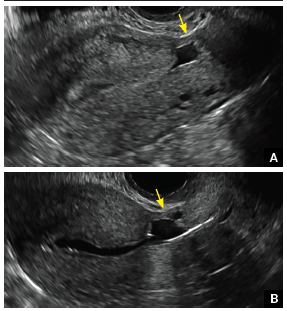
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
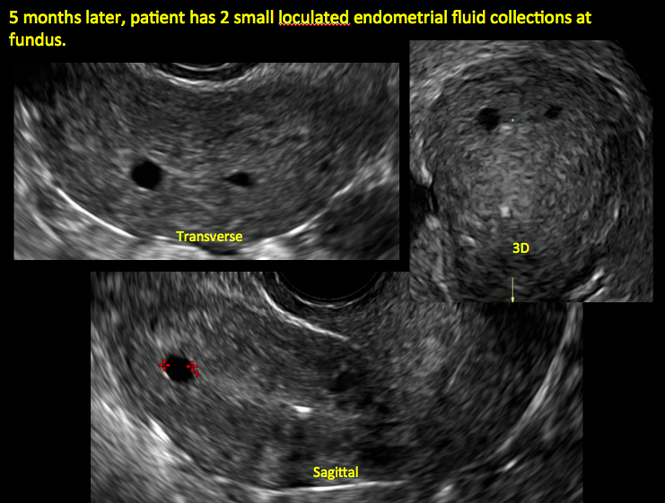
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
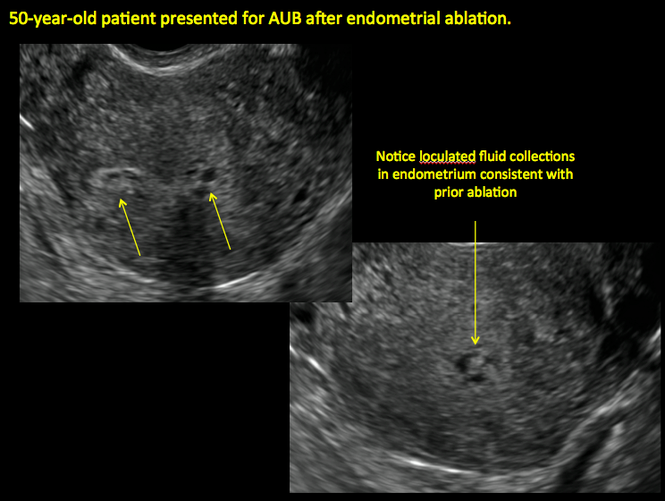
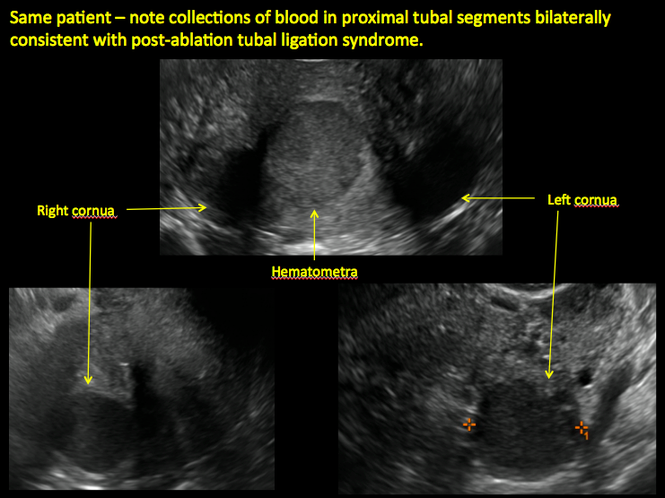
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
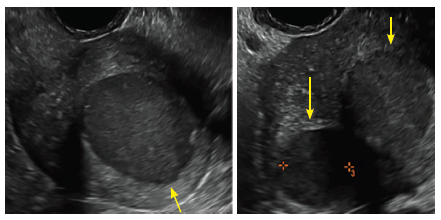
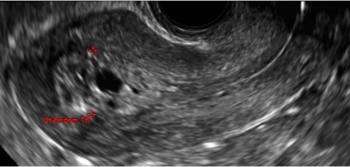
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
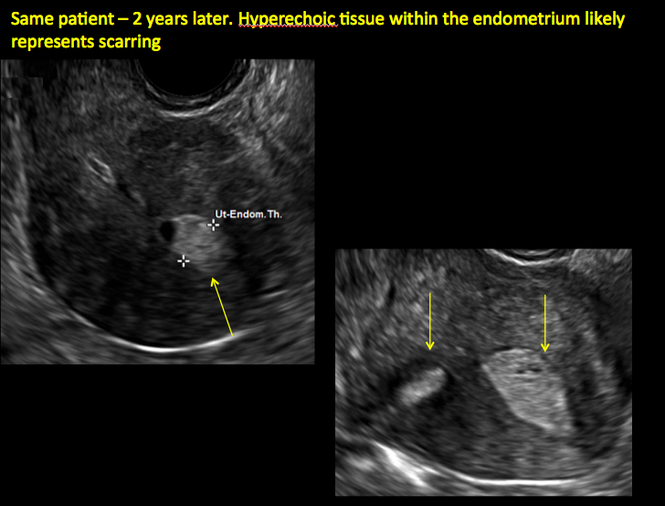
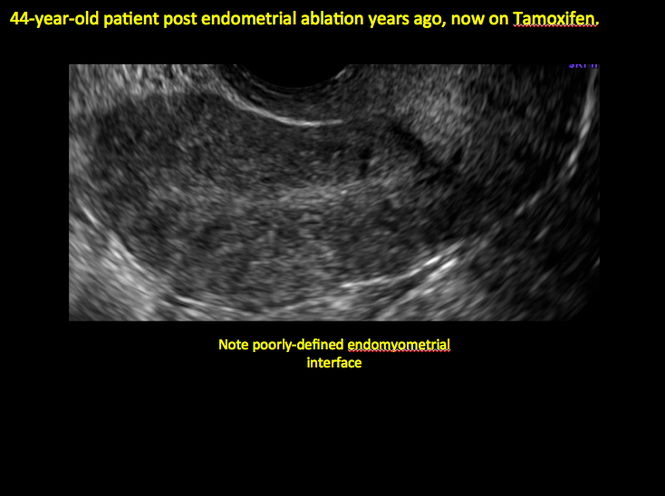
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
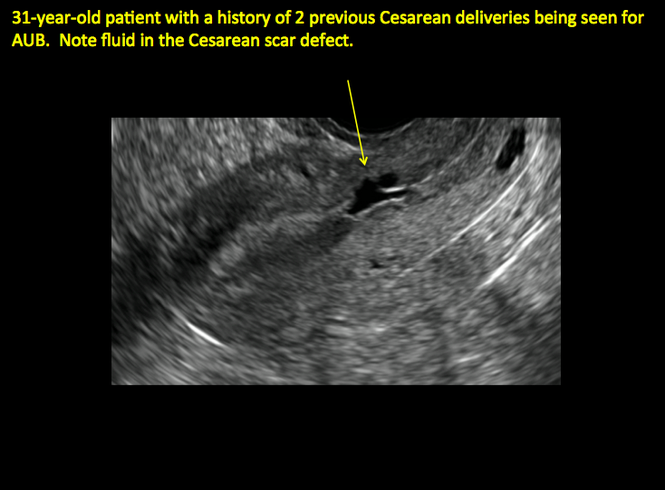
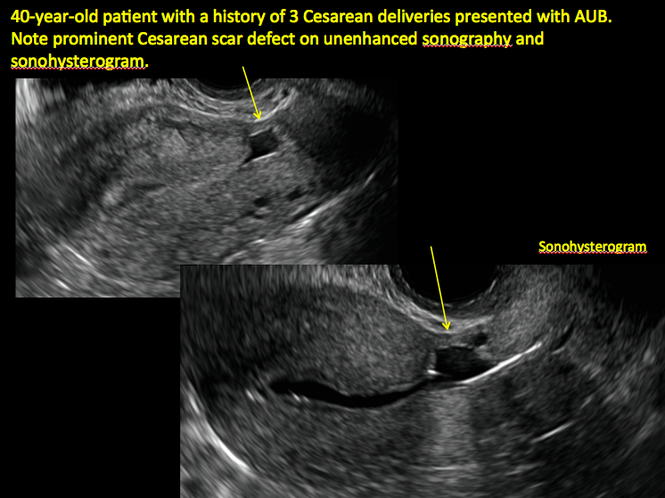
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
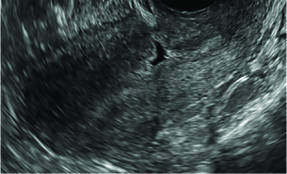
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 | 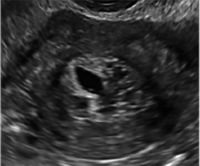 | 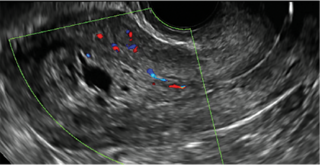 | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
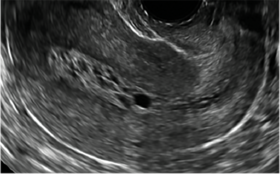 | 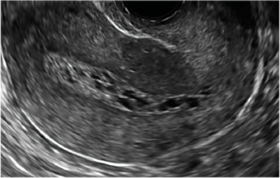 | 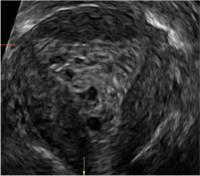 | ||
ADDITIONAL CASES - Postablation

ADDITIONAL CASES - Cesarean scar defect
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Transvaginal ultrasound: We are gaining a better understanding of its clinical applications

Steven R. Goldstein, MD
In my first book I coined the phrase "sonomicroscopy." We are seeing things with transvaginal ultrasonography (TVUS) that you could not see with your naked eye even if you could it hold it at arms length and squint at it. For instance, cardiac activity can be seen easily within an embryo of 4 mm at 47 days since the last menstrual period. If there were any possible way to hold this 4-mm embryo in your hand, you would not appreciate cardiac pulsations contained within it! This is one of the beauties, and yet potential foibles, of TVUS.
In this excellent pictorial article, Michelle Stalnaker Ozcan, MD, and Andrew M. Kaunitz, MD, have done an outstanding job of turning this low-power "sonomicroscope" into the uterus to better understand a number of unique yet important clinical applications of TVUS.
Tamoxifen is known to cause a slight but statistically significant increase in endometrial cancer. In 1994, I first described an unusual ultrasound appearance in the uterus of patients receiving tamoxifen, which was being misinterpreted as "endometrial thickening," and resulted in many unnecessary biopsies and dilation and curettage procedures.1 This type of uterine change has been seen in other selective estrogen-receptor modulators as well.2,3 In this article, Drs. Ozcan and Kaunitz correctly point out that such an ultrasound pattern does not necessitate any intervention in the absence of bleeding.
Another common question I am often asked is, "How do we handle the patient whose status is post-endometrial ablation and presents with staining?" The scarring shown in the figures that follow make any kind of meaningful evaluation extremely difficult.
There has been an epidemic of cesarean scar pregnancies when a subsequent gestation implants in the cesarean scar defect.4 Perhaps the time has come when all patients with a previous cesarean delivery should have their lower uterine segment scanned to look for such a defect as shown in the pictures that follow. If we are not yet ready for that, at least early TVUS scans in subsequent pregnancies, in my opinion, should be employed to make an early diagnosis of such cases that are the precursors of morbidly adherent placenta, a potentially life-threatening situation that appears to be increasing in frequency.
Finally, look to obgmanagement.com for next month's web-exclusive look at outstanding images of patients who have undergone transcervical sterilization.
Dr. Goldstein is Professor, Department of Obstetrics and Gynecology, New York University School of Medicine, Director, Gynecologic Ultrasound, and Co-Director, Bone Densitometry, New York University Medical Center. He also serves on the OBG Management Board of Editors.
Dr. Goldstein reports that he has an equipment loan from Philips, and is past President of the American Institute of Ultrasound in Medicine.
References
- Goldstein SR. Unusual ultrasonographic appearance of the uterus in patients receiving tamoxifen. Am J Obstet Gynecol. 1994;170(2):447–451.
- Goldstein SR, Neven P, Cummings S, et al. Postmenopausal evaluation and risk reduction with lasofoxifene (PEARL) trial: 5-year gynecological outcomes. Menopause. 2011;18(1):17–22.
- Goldstein SR, Nanavati N. Adverse events that are associated with the selective estrogen receptor modulator levormeloxifene in an aborted phase III osteoporosis treatment study. Am J Obstet Gynecol. 2002;187(3):521–527.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol. 2012;207(1):14–29.
New technology, minimally invasive surgical procedures, and medications continue to change how physicians manage specific medical issues. Many procedures and medications used by gynecologists can cause characteristic findings on sonography. These findings can guide subsequent counseling and management decisions and are important to accurately interpret on imaging. Among these conditions are Asherman syndrome, postendometrial ablation uterine damage, cesarean scar defect, and altered endometrium as a result of tamoxifen use. In this article, we provide 2 dimensional and 3 dimensional sono‑graphic images of uterine presentations of these 4 conditions.
Asherman syndromeCharacterized by variable scarring, or intrauterine adhesions, inside the uterine cavity following endometrial trauma due to surgical procedures, Asherman syndrome can cause menstrual changes and infertility. Should pregnancy occur in the setting of Asherman syndrome, placental abnormalities may result.1 Intrauterine adhesions can follow many surgical procedures, including curettage (diagnostic or for missed/elective abortion or retained products of conception), cesarean delivery, and hysteroscopic myomectomy. They may even occur after spontaneous abortion without curettage. Rates of Asherman syndrome are highest after procedures that tend to cause the most intrauterine inflammation, including2:
- curettage after septic abortion
- late curettage after retained products of conception
- hysteroscopy with multiple myomectomies.
In severe cases Asherman syndrome can result in complete obliteration of the uterine cavity.3
Clinicians should be cognizant of the appearance of Asherman syndrome on imaging because patients reporting menstrual abnormalities, pelvic pain (FIGURE 1), infertility, and other symptoms may exhibit intrauterine lesions on sonohysterography, or sometimes unenhanced sonography if endometrial fluid/blood is present. Depending on symptoms and patient reproductive plans, treatment may be indicated.2
| FIGURE 1 Asherman syndrome | ||||
|
Postablation endometrial destruction
Surgical destruction of the endometrium to the level of the basalis has been associated with the formation of intrauterine adhesions (FIGURE 2) as well as pockets of hematometra (FIGURE 3). In a large Cochrane systematic review, the reported rate of hematometra was 0.9% following non− resectoscopic ablation and 2.4% following resectoscopic ablation.4
FIGURE 2 Intrauterine changes postablation | ||||
| ||||
| Loculated fluid collections in the endometrium on transverse (A), sagittal (B), and 3 dimensional images (C) of a 41-year-old patient who presented with dysmenorrhea 3 years after an endometrial ablation procedure. The patient ultimately underwent transvaginal hysterectomy. | ||||
| ||||
| ||||
| 2 dimensional sonograms of a 40-year-old patient with a history of bilateral tubal ligation who presented for severe cyclic pelvic pain postablation. |
Postablation tubal sterilization syndrome—cyclic cramping with or without vaginal bleeding—occurs in up to 10% of previously sterilized women who undergo endometrial ablation.4 The syndrome is thought to be caused by bleeding from active endometrium trapped at the uterine cornua by intrauterine adhesions postablation.
FIGURE 4 Cesarean scar defect with 1 previous cesarean delivery | ||||
| ||||
| Unenhanced sonogram in a 41-year-old patient. Myometrial notch is seen at both the endometrial surface and the serosal surface. | ||||
| ||||
| ||||
| Unenhanced sonogram (A) and sonohysterogram (B) in a 40-year-old patient. |
In patients with postablation tubal sterilization syndrome, imaging can reveal loculated endometrial fluid collections, hyperechoic foci/scarring, and a poorly defined endomyometrial interface. See ADDITIONAL CASES-Postablation at the bottom of this article for additional imaging case presentations.
Cesarean scar defect on imaging
In 1961, Poidevin first described the lower uterine segment myometrial notch or “niche,” now known as cesarean scar defect, as a wedge-shaped defect in the myometrium of women who had undergone cesarean delivery. He noted that it appeared after a 6-month healing period.5
Using sonography with Doppler to view the defect, it appears relatively avascular and may decrease in size over time (FIGURES 4 and 5). Studies now are focusing on sonographic measurement of the cesarean scar defect as a clinical predictor of outcome for future pregnancies, as uterine rupture and abnormal placentation, including cesarean scar ectopics, can be associated with it.6-8
See ADDITIONAL CASES-Cesarean scar defect at the bottom of this article for 2 imaging case presentations.
Endometrial changes with tamoxifen use
Tamoxifen use causes changes in the endometrium that on sonography can appear concerning for endometrial cancer. These changes include endometrial thickening and hyperechogenicity as well as cystic and heterogenous areas.9
Despite this imaging presentation, endometrial changes on sonography in the setting of tamoxifen use have been shown to be a poor predictor of actual endometrial pathology. In a study by Gerber and colleagues, the endometrial thickness in patients taking tamoxifen increased from a mean of 3.5 mm pretreatment to a mean of 9.2 mm after 3-year treatment.9 Using a cutoff value of 10 mm for abnormal endometrial thickness, screening transvaginal ultrasonography (TVUS) resulted in a high false-positive rate and iatrogenic morbidity. Endometrial cancer was detected in only 0.4% of patients (1 case), atrophy in 73%, polyps in 4%, and hyperplasia in 2%.9
Thus, routine screening sonographic assessment of the endometrium in asymptomatic women taking tamoxifen is not recommended. For women presenting with abnormal bleeding or other concerns, however, TVUS is appropriate (CASES 1 and 2).
| CASE 1 Endometrial polyps identified with tamoxifen use | ||||
| A 56-year-old patient with a history of breast cancer presently taking tamoxifen presented with postmenopausal bleeding. Endometrial biopsy results revealed endometrial polyps. | ||||
 |  |  | ||
| CASE 2 Benign endometrial changes with tamoxifen use | ||||
| A 50-year-old patient with a history of breast cancer currently taking tamoxifen presented with abnormal uterine bleeding. Endometrial biopsy results indicated benign endometrial changes. | ||||
 |  |  | ||
ADDITIONAL CASES - Postablation










ADDITIONAL CASES - Cesarean scar defect


Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
- Engelbrechtsen L, Langhoff-Roos J, Kjer JJ, Istre O. Placenta accreta: adherent placenta due to Asherman syndrome. Clin Case Rep. 2015;3(3):175−178.
- Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118.
- Song D, Xia E, Xiao Y, Li TC, Huang X, Liu Y. Management of false passage created during hysteroscopic adhesiolysis for Asherman’s syndrome. J Obstet Gynaecol. 2016;36(1):87−92.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;4:CD001501.
- Poidevin LO. The value of hysterography in the prediction of cesarean section wound defects. Am J Obstet Gynecol. 1961;81:67−71.
- Naji O, Abdallah Y, Bij De Vaate AJ, et al. Standardized approach for imaging and measuring Cesarean section scars using ultrasonography. Ultrasound Obstet Gynecol. 2012;39(3):252−259.
- Kok N, Wiersma IC, Opmeer BC, et al. Sonographic measurement of lower uterine segment thickness to predict uterine rupture during a trial of labor in women with previous Cesarean section: a meta-analysis. Ultrasound Obstet Gynecol. 2013;42(2):132−139.
- Nezhat C, Grace L, Soliemannjad R, Razavi GM, Nezhat A. Cesarean scar defect: What is it and how should it be treated? OBG Manag. 2016;28(4):32, 34, 36, 38–39, 53.
- Gerber B, Krause A, Müller H, et al. Effects of adjuvant tamoxifen on the endometrium in postmenopausal women with breast cancer: a prospective long-term study using transvaginal ultrasound. J Clin Oncol. 2000; 18(20):3464–3667.
In this Article
- Foreword by Steven R. Goldstein, MD
- Uterine changes postablation
- Endometrial changes with tamoxifen use
Sodium fluorescein emerges as alternative to indigo carmine in cystoscopy
WASHINGTON – Sodium fluorescein is proving to be an excellent agent for helping to verify ureteral efflux during intraoperative cystoscopy, Dr. Jay Goldberg said at the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Goldberg said he first read about the use of the dye as an alternative to indigo carmine, which is no longer available, in a study published in 2015; the study reported good results with a 10% preparation of sodium fluorescein administered at 0.25-1 mL intravenously during intraoperative cystoscopy (Obstet Gynecol. 2015 Mar;125[3]:548-50).
Since then, he and his colleagues at Einstein Medical Center, Philadelphia, have been evaluating the use of 0.1 mL of 10% sodium fluorescein IV during cystoscopies performed at the end of gynecologic surgeries, measuring the time to visualization and the level of satisfaction with the dye.
Thus far, in more than 50 cases, the average time until colored ureteral jets were seen has been 4.3 minutes (a range of 2-6.8 minutes, consistent with the 2015 study). And according to questionnaires completed by each surgeon, the degree of certainty for visualizing the ureteral jets was improved with fluorescein (a rating of 5 on a 5-point scale, compared with 2.9 without the dye).
Compared with both indigo carmine and methylene blue, fluorescein was preferred, Dr. Goldberg reported, with surgeons citing quicker onset, better color contrast, cheaper cost, and fewer side effects.
“Given that it’s at least equivalent and probably better, and that it’s 50 times cheaper [than indigo carmine], even if indigo carmine comes back again, I’m certainly not going to be switching back,” Dr. Goldberg said during a seminar on cystoscopy after hysterectomy.
Intravenous sodium fluorescein is routinely used in ophthalmology in retinal angiography, at a dosage of 5 mL of 10% fluorescein, he said. The most common complications reported in the literature are nausea, vomiting, and flushing or rash (rates of 2.9%, 1.2%, and 0.5%, respectively, according to a 1991 report). None of their patients has experienced any complications, Dr. Goldberg said.
Methylene blue (50 mg IV over a period of 5 minutes) appears to have been a common go-to dye for gynecologic surgeons, along with pyridium (a 200-mg oral dose prior to surgery), ever since production of indigo carmine was discontinued because of a lack of raw material.
“But with methylene blue, it may take longer to see the blue urine efflux from the ureteral orifices,” Dr. Goldberg said. And with pyridium, “by the time cystoscopy is performed, the bladder will have already been stained orange, making it more difficult to see the same colored urine jets.”
“Sodium fluorescein is very quick in onset. I wait [to have it administered] until I am actually ready to insert the cystoscope,” he said.
And at 60 cents per dose, fluorescein is less expensive than methylene blue ($1.50) and significantly less expensive than indigo carmine ($30 a dose), he said.
Dr. Goldberg reported having no relevant financial conflicts.
WASHINGTON – Sodium fluorescein is proving to be an excellent agent for helping to verify ureteral efflux during intraoperative cystoscopy, Dr. Jay Goldberg said at the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Goldberg said he first read about the use of the dye as an alternative to indigo carmine, which is no longer available, in a study published in 2015; the study reported good results with a 10% preparation of sodium fluorescein administered at 0.25-1 mL intravenously during intraoperative cystoscopy (Obstet Gynecol. 2015 Mar;125[3]:548-50).
Since then, he and his colleagues at Einstein Medical Center, Philadelphia, have been evaluating the use of 0.1 mL of 10% sodium fluorescein IV during cystoscopies performed at the end of gynecologic surgeries, measuring the time to visualization and the level of satisfaction with the dye.
Thus far, in more than 50 cases, the average time until colored ureteral jets were seen has been 4.3 minutes (a range of 2-6.8 minutes, consistent with the 2015 study). And according to questionnaires completed by each surgeon, the degree of certainty for visualizing the ureteral jets was improved with fluorescein (a rating of 5 on a 5-point scale, compared with 2.9 without the dye).
Compared with both indigo carmine and methylene blue, fluorescein was preferred, Dr. Goldberg reported, with surgeons citing quicker onset, better color contrast, cheaper cost, and fewer side effects.
“Given that it’s at least equivalent and probably better, and that it’s 50 times cheaper [than indigo carmine], even if indigo carmine comes back again, I’m certainly not going to be switching back,” Dr. Goldberg said during a seminar on cystoscopy after hysterectomy.
Intravenous sodium fluorescein is routinely used in ophthalmology in retinal angiography, at a dosage of 5 mL of 10% fluorescein, he said. The most common complications reported in the literature are nausea, vomiting, and flushing or rash (rates of 2.9%, 1.2%, and 0.5%, respectively, according to a 1991 report). None of their patients has experienced any complications, Dr. Goldberg said.
Methylene blue (50 mg IV over a period of 5 minutes) appears to have been a common go-to dye for gynecologic surgeons, along with pyridium (a 200-mg oral dose prior to surgery), ever since production of indigo carmine was discontinued because of a lack of raw material.
“But with methylene blue, it may take longer to see the blue urine efflux from the ureteral orifices,” Dr. Goldberg said. And with pyridium, “by the time cystoscopy is performed, the bladder will have already been stained orange, making it more difficult to see the same colored urine jets.”
“Sodium fluorescein is very quick in onset. I wait [to have it administered] until I am actually ready to insert the cystoscope,” he said.
And at 60 cents per dose, fluorescein is less expensive than methylene blue ($1.50) and significantly less expensive than indigo carmine ($30 a dose), he said.
Dr. Goldberg reported having no relevant financial conflicts.
WASHINGTON – Sodium fluorescein is proving to be an excellent agent for helping to verify ureteral efflux during intraoperative cystoscopy, Dr. Jay Goldberg said at the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Goldberg said he first read about the use of the dye as an alternative to indigo carmine, which is no longer available, in a study published in 2015; the study reported good results with a 10% preparation of sodium fluorescein administered at 0.25-1 mL intravenously during intraoperative cystoscopy (Obstet Gynecol. 2015 Mar;125[3]:548-50).
Since then, he and his colleagues at Einstein Medical Center, Philadelphia, have been evaluating the use of 0.1 mL of 10% sodium fluorescein IV during cystoscopies performed at the end of gynecologic surgeries, measuring the time to visualization and the level of satisfaction with the dye.
Thus far, in more than 50 cases, the average time until colored ureteral jets were seen has been 4.3 minutes (a range of 2-6.8 minutes, consistent with the 2015 study). And according to questionnaires completed by each surgeon, the degree of certainty for visualizing the ureteral jets was improved with fluorescein (a rating of 5 on a 5-point scale, compared with 2.9 without the dye).
Compared with both indigo carmine and methylene blue, fluorescein was preferred, Dr. Goldberg reported, with surgeons citing quicker onset, better color contrast, cheaper cost, and fewer side effects.
“Given that it’s at least equivalent and probably better, and that it’s 50 times cheaper [than indigo carmine], even if indigo carmine comes back again, I’m certainly not going to be switching back,” Dr. Goldberg said during a seminar on cystoscopy after hysterectomy.
Intravenous sodium fluorescein is routinely used in ophthalmology in retinal angiography, at a dosage of 5 mL of 10% fluorescein, he said. The most common complications reported in the literature are nausea, vomiting, and flushing or rash (rates of 2.9%, 1.2%, and 0.5%, respectively, according to a 1991 report). None of their patients has experienced any complications, Dr. Goldberg said.
Methylene blue (50 mg IV over a period of 5 minutes) appears to have been a common go-to dye for gynecologic surgeons, along with pyridium (a 200-mg oral dose prior to surgery), ever since production of indigo carmine was discontinued because of a lack of raw material.
“But with methylene blue, it may take longer to see the blue urine efflux from the ureteral orifices,” Dr. Goldberg said. And with pyridium, “by the time cystoscopy is performed, the bladder will have already been stained orange, making it more difficult to see the same colored urine jets.”
“Sodium fluorescein is very quick in onset. I wait [to have it administered] until I am actually ready to insert the cystoscope,” he said.
And at 60 cents per dose, fluorescein is less expensive than methylene blue ($1.50) and significantly less expensive than indigo carmine ($30 a dose), he said.
Dr. Goldberg reported having no relevant financial conflicts.
AT ACOG 2016
Key clinical point: Sodium fluorescein is an effective, and possibly superior, alternative to the unavailable indigo carmine.Major finding: In more than 50 cases of intraoperative cystoscopy, the average time from intravenous administration of the dye to visualization of colored ureteral jets has been 4.3 minutes.
Data source: An observational study of more than 50 cystoscopies.
Disclosures: Dr. Goldberg and his colleagues reported having no relevant financial conflicts.
Cystoscopy after hysterectomy: Consider more frequent use
WASHINGTON – Universal cystoscopy at the time of hysterectomy – or at least more frequent use of the procedure – is worth considering since delayed diagnosis of urinary tract injury causes increased morbidity for patients, and in all likelihood increases litigation, Dr. Jay Goldberg and Dr. Cheung Kim suggested at the annual meeting of the American College of Obstetricians and Gynecologists.
“We’re often hesitant to do cystoscopy because we don’t want to add time,” said Dr. Kim. “But I always feel that no matter how much time it takes, I’ll be happier in the end if I do it. [And] if you have [experience], a routine, and readily available equipment, it can take as little as 10 minutes.”
Universal cystoscopy to confirm ureteral patency is “fairly straightforward, low risk, and more likely to detect most injuries [than visual inspection alone], particularly ureteral injuries,” Dr. Kim said. On the other hand, it adds to operating time and increases procedure cost, and there is some research suggesting it may be relatively “low yield” and lead to some false positives.
Dr. Kim and Dr. Goldberg both practice at the Einstein Healthcare Network in Philadelphia. Here are some of the findings they shared, and advice they gave, on the use of cystoscopy – universal or selective – after hysterectomy.
Conflicting findings
There is conflicting opinion as to whether universal or selective cystoscopy after hysterectomy is best, and “there’s data on both sides,” said Dr. Goldberg, vice chairman of ob.gyn. and director of the Philadelphia Fibroid Center at Einstein.
A prospective study done at Louisiana State University, New Orleans, to evaluate the impact of a universal approach, for instance, showed an incidence of urinary tract injury of 4.3% (2.9% bladder injury, 1.8% ureteral injury, plus cases of simultaneous injury) in 839 hysterectomies for benign disease. The injury detection rate using intraoperative cystoscopy was 97.4%, and the majority of injuries – 76% – were not suspected prior to cystoscopy being performed (Obstet Gynecol. 2009 Jan;113[1]:6-10).
But researchers in Boston who looked retrospectively at 1,982 hysterectomies performed for any gynecologic indication found a much lower incidence of complications, and reported that cystoscopy did not detect any of the bladder injuries (0.71%) or ureteral injuries (0.25%) incurred in the group. Cystoscopy was performed selectively, however, in 250 of the patients, and was either normal or omitted in the patients who had complications (Obstet Gynecol. 2012 Dec;120[6]:1363-70).
Cystoscopy failed to detect any of the bladder injuries, but “all five of the ureteral injuries occurred in patients who had not undergone cystoscopy,” said Dr. Kim, chairman of ob.gyn. at Einstein Medical Center Montgomery in East Norriton, Pa.
Possible false-positives
Cystoscopy may lead on occasion to an incorrect presumption of a ureteral injury in patients with a pre-existing nonfunctional kidney, Dr. Goldberg noted.
He relayed the case of a 42-year-old patient who underwent a total abdominal hysterectomy without apparent complication. Cystoscopy was then performed with indigo carmine. An efflux of dye was seen from the left ureteral orifice but not from the right orifice.
Urology was consulted and investigated the presumed ureteral injury with additional surgical exploration. An intraoperative intravenous pyelogram (IVP) was eventually performed and was unable to identify the right kidney. A CT then showed an atrophic right kidney with compensatory hypertrophy of the left kidney, probably due to congenital right multicystic dysplastic kidney.
An estimated 0.2% of the population – 1 in 500 – will have a unilateral nonfunctional kidney, the majority of which have not been previously diagnosed. Etiologies include multicystic dysplastic kidney, congenital unilateral renal agenesis, and vascular events. “As we do more and more cystoscopies, this scenario is going to come up every so often,” said Dr. Goldberg, who reported on two such cases last year (Obstet Gynecol. 2015 Sep;126[3]:635-7).
It is also possible, Dr. Kim noted, that a weak urine jet observed on cystoscopy may not necessarily reflect injury. In the LSU study evaluating a universal approach, there was no injury detected on further evaluation in each of the 21 cases of low, subnormal dye efflux from the ureteral orifices. “So it’s not a benign process to undergo cystoscopy in terms of what the ramifications might be,” Dr. Kim said.
Increasing use
Ob.gyn. residents are required by the Accreditation Council for Graduate Medical Education to have completed 15 cystoscopies by the time they graduate, and according to recent survey findings, residents are more likely to utilize universal cystoscopy at the time of hysterectomy than currently practicing gynecologic surgeons.
The survey of ob.gyn residents (n = 56) shows universal cystoscopy (defined as greater than 90%) was performed in only a minority of cases during residency: 27% of total laparoscopic hysterectomies (TLH), 14% of laparoscopically assisted vaginal hysterectomies (LAVH), 12% of vaginal hysterectomies (VH), 2% of total abdominal hysterectomies (TAH), and 0% of supracervical hysterectomies (SCH), for instance.
Yet for every hysterectomy type, residents planned to perform universal cystoscopy post-residency more often than they had during their training (49% TLH, 34% LAVH, 34% VH, 15% TAH, 12% SCH), and “residents familiar with the literature on cystoscopy were statistically more likely to plan to perform universal cystoscopy,” said Dr. Goldberg, the senior author of the paper (Womens Health (Lond Engl). 2015 Nov;11[6]:825-31).
Litigation possible
Failure to detect a urinary tract injury at the time of hysterectomy may result in the need for future additional surgeries. Litigation in the Philadelphia market suggests that “if you have an injury that’s missed, there’s a chance that litigation may result,” Dr. Kim said.
Plaintiff’s attorneys have argued that not recognizing ureteral injury during surgery is a deviation from acceptable practice, while defense attorneys have contended that unavoidable complications occur and that no evaluation is required, or supported by the medical literature, when injury is not intraoperatively suspected. Currently, as cystoscopy is performed less than 25% of the time for all types of hysterectomy, the standard of care does not require the procedure intraoperatively if no injury is suspected, Dr. Goldberg said.
Primary prevention of urinary tract injury is most important, both physicians emphasized. The best way to accomplish this is to meticulously identify the anatomy and know the path of the ureter, and to document that the ureter has been identified and viewed as outside of the operative area, they said.
Dr. Kim and Dr. Goldberg reported having no relevant financial disclosures.
WASHINGTON – Universal cystoscopy at the time of hysterectomy – or at least more frequent use of the procedure – is worth considering since delayed diagnosis of urinary tract injury causes increased morbidity for patients, and in all likelihood increases litigation, Dr. Jay Goldberg and Dr. Cheung Kim suggested at the annual meeting of the American College of Obstetricians and Gynecologists.
“We’re often hesitant to do cystoscopy because we don’t want to add time,” said Dr. Kim. “But I always feel that no matter how much time it takes, I’ll be happier in the end if I do it. [And] if you have [experience], a routine, and readily available equipment, it can take as little as 10 minutes.”
Universal cystoscopy to confirm ureteral patency is “fairly straightforward, low risk, and more likely to detect most injuries [than visual inspection alone], particularly ureteral injuries,” Dr. Kim said. On the other hand, it adds to operating time and increases procedure cost, and there is some research suggesting it may be relatively “low yield” and lead to some false positives.
Dr. Kim and Dr. Goldberg both practice at the Einstein Healthcare Network in Philadelphia. Here are some of the findings they shared, and advice they gave, on the use of cystoscopy – universal or selective – after hysterectomy.
Conflicting findings
There is conflicting opinion as to whether universal or selective cystoscopy after hysterectomy is best, and “there’s data on both sides,” said Dr. Goldberg, vice chairman of ob.gyn. and director of the Philadelphia Fibroid Center at Einstein.
A prospective study done at Louisiana State University, New Orleans, to evaluate the impact of a universal approach, for instance, showed an incidence of urinary tract injury of 4.3% (2.9% bladder injury, 1.8% ureteral injury, plus cases of simultaneous injury) in 839 hysterectomies for benign disease. The injury detection rate using intraoperative cystoscopy was 97.4%, and the majority of injuries – 76% – were not suspected prior to cystoscopy being performed (Obstet Gynecol. 2009 Jan;113[1]:6-10).
But researchers in Boston who looked retrospectively at 1,982 hysterectomies performed for any gynecologic indication found a much lower incidence of complications, and reported that cystoscopy did not detect any of the bladder injuries (0.71%) or ureteral injuries (0.25%) incurred in the group. Cystoscopy was performed selectively, however, in 250 of the patients, and was either normal or omitted in the patients who had complications (Obstet Gynecol. 2012 Dec;120[6]:1363-70).
Cystoscopy failed to detect any of the bladder injuries, but “all five of the ureteral injuries occurred in patients who had not undergone cystoscopy,” said Dr. Kim, chairman of ob.gyn. at Einstein Medical Center Montgomery in East Norriton, Pa.
Possible false-positives
Cystoscopy may lead on occasion to an incorrect presumption of a ureteral injury in patients with a pre-existing nonfunctional kidney, Dr. Goldberg noted.
He relayed the case of a 42-year-old patient who underwent a total abdominal hysterectomy without apparent complication. Cystoscopy was then performed with indigo carmine. An efflux of dye was seen from the left ureteral orifice but not from the right orifice.
Urology was consulted and investigated the presumed ureteral injury with additional surgical exploration. An intraoperative intravenous pyelogram (IVP) was eventually performed and was unable to identify the right kidney. A CT then showed an atrophic right kidney with compensatory hypertrophy of the left kidney, probably due to congenital right multicystic dysplastic kidney.
An estimated 0.2% of the population – 1 in 500 – will have a unilateral nonfunctional kidney, the majority of which have not been previously diagnosed. Etiologies include multicystic dysplastic kidney, congenital unilateral renal agenesis, and vascular events. “As we do more and more cystoscopies, this scenario is going to come up every so often,” said Dr. Goldberg, who reported on two such cases last year (Obstet Gynecol. 2015 Sep;126[3]:635-7).
It is also possible, Dr. Kim noted, that a weak urine jet observed on cystoscopy may not necessarily reflect injury. In the LSU study evaluating a universal approach, there was no injury detected on further evaluation in each of the 21 cases of low, subnormal dye efflux from the ureteral orifices. “So it’s not a benign process to undergo cystoscopy in terms of what the ramifications might be,” Dr. Kim said.
Increasing use
Ob.gyn. residents are required by the Accreditation Council for Graduate Medical Education to have completed 15 cystoscopies by the time they graduate, and according to recent survey findings, residents are more likely to utilize universal cystoscopy at the time of hysterectomy than currently practicing gynecologic surgeons.
The survey of ob.gyn residents (n = 56) shows universal cystoscopy (defined as greater than 90%) was performed in only a minority of cases during residency: 27% of total laparoscopic hysterectomies (TLH), 14% of laparoscopically assisted vaginal hysterectomies (LAVH), 12% of vaginal hysterectomies (VH), 2% of total abdominal hysterectomies (TAH), and 0% of supracervical hysterectomies (SCH), for instance.
Yet for every hysterectomy type, residents planned to perform universal cystoscopy post-residency more often than they had during their training (49% TLH, 34% LAVH, 34% VH, 15% TAH, 12% SCH), and “residents familiar with the literature on cystoscopy were statistically more likely to plan to perform universal cystoscopy,” said Dr. Goldberg, the senior author of the paper (Womens Health (Lond Engl). 2015 Nov;11[6]:825-31).
Litigation possible
Failure to detect a urinary tract injury at the time of hysterectomy may result in the need for future additional surgeries. Litigation in the Philadelphia market suggests that “if you have an injury that’s missed, there’s a chance that litigation may result,” Dr. Kim said.
Plaintiff’s attorneys have argued that not recognizing ureteral injury during surgery is a deviation from acceptable practice, while defense attorneys have contended that unavoidable complications occur and that no evaluation is required, or supported by the medical literature, when injury is not intraoperatively suspected. Currently, as cystoscopy is performed less than 25% of the time for all types of hysterectomy, the standard of care does not require the procedure intraoperatively if no injury is suspected, Dr. Goldberg said.
Primary prevention of urinary tract injury is most important, both physicians emphasized. The best way to accomplish this is to meticulously identify the anatomy and know the path of the ureter, and to document that the ureter has been identified and viewed as outside of the operative area, they said.
Dr. Kim and Dr. Goldberg reported having no relevant financial disclosures.
WASHINGTON – Universal cystoscopy at the time of hysterectomy – or at least more frequent use of the procedure – is worth considering since delayed diagnosis of urinary tract injury causes increased morbidity for patients, and in all likelihood increases litigation, Dr. Jay Goldberg and Dr. Cheung Kim suggested at the annual meeting of the American College of Obstetricians and Gynecologists.
“We’re often hesitant to do cystoscopy because we don’t want to add time,” said Dr. Kim. “But I always feel that no matter how much time it takes, I’ll be happier in the end if I do it. [And] if you have [experience], a routine, and readily available equipment, it can take as little as 10 minutes.”
Universal cystoscopy to confirm ureteral patency is “fairly straightforward, low risk, and more likely to detect most injuries [than visual inspection alone], particularly ureteral injuries,” Dr. Kim said. On the other hand, it adds to operating time and increases procedure cost, and there is some research suggesting it may be relatively “low yield” and lead to some false positives.
Dr. Kim and Dr. Goldberg both practice at the Einstein Healthcare Network in Philadelphia. Here are some of the findings they shared, and advice they gave, on the use of cystoscopy – universal or selective – after hysterectomy.
Conflicting findings
There is conflicting opinion as to whether universal or selective cystoscopy after hysterectomy is best, and “there’s data on both sides,” said Dr. Goldberg, vice chairman of ob.gyn. and director of the Philadelphia Fibroid Center at Einstein.
A prospective study done at Louisiana State University, New Orleans, to evaluate the impact of a universal approach, for instance, showed an incidence of urinary tract injury of 4.3% (2.9% bladder injury, 1.8% ureteral injury, plus cases of simultaneous injury) in 839 hysterectomies for benign disease. The injury detection rate using intraoperative cystoscopy was 97.4%, and the majority of injuries – 76% – were not suspected prior to cystoscopy being performed (Obstet Gynecol. 2009 Jan;113[1]:6-10).
But researchers in Boston who looked retrospectively at 1,982 hysterectomies performed for any gynecologic indication found a much lower incidence of complications, and reported that cystoscopy did not detect any of the bladder injuries (0.71%) or ureteral injuries (0.25%) incurred in the group. Cystoscopy was performed selectively, however, in 250 of the patients, and was either normal or omitted in the patients who had complications (Obstet Gynecol. 2012 Dec;120[6]:1363-70).
Cystoscopy failed to detect any of the bladder injuries, but “all five of the ureteral injuries occurred in patients who had not undergone cystoscopy,” said Dr. Kim, chairman of ob.gyn. at Einstein Medical Center Montgomery in East Norriton, Pa.
Possible false-positives
Cystoscopy may lead on occasion to an incorrect presumption of a ureteral injury in patients with a pre-existing nonfunctional kidney, Dr. Goldberg noted.
He relayed the case of a 42-year-old patient who underwent a total abdominal hysterectomy without apparent complication. Cystoscopy was then performed with indigo carmine. An efflux of dye was seen from the left ureteral orifice but not from the right orifice.
Urology was consulted and investigated the presumed ureteral injury with additional surgical exploration. An intraoperative intravenous pyelogram (IVP) was eventually performed and was unable to identify the right kidney. A CT then showed an atrophic right kidney with compensatory hypertrophy of the left kidney, probably due to congenital right multicystic dysplastic kidney.
An estimated 0.2% of the population – 1 in 500 – will have a unilateral nonfunctional kidney, the majority of which have not been previously diagnosed. Etiologies include multicystic dysplastic kidney, congenital unilateral renal agenesis, and vascular events. “As we do more and more cystoscopies, this scenario is going to come up every so often,” said Dr. Goldberg, who reported on two such cases last year (Obstet Gynecol. 2015 Sep;126[3]:635-7).
It is also possible, Dr. Kim noted, that a weak urine jet observed on cystoscopy may not necessarily reflect injury. In the LSU study evaluating a universal approach, there was no injury detected on further evaluation in each of the 21 cases of low, subnormal dye efflux from the ureteral orifices. “So it’s not a benign process to undergo cystoscopy in terms of what the ramifications might be,” Dr. Kim said.
Increasing use
Ob.gyn. residents are required by the Accreditation Council for Graduate Medical Education to have completed 15 cystoscopies by the time they graduate, and according to recent survey findings, residents are more likely to utilize universal cystoscopy at the time of hysterectomy than currently practicing gynecologic surgeons.
The survey of ob.gyn residents (n = 56) shows universal cystoscopy (defined as greater than 90%) was performed in only a minority of cases during residency: 27% of total laparoscopic hysterectomies (TLH), 14% of laparoscopically assisted vaginal hysterectomies (LAVH), 12% of vaginal hysterectomies (VH), 2% of total abdominal hysterectomies (TAH), and 0% of supracervical hysterectomies (SCH), for instance.
Yet for every hysterectomy type, residents planned to perform universal cystoscopy post-residency more often than they had during their training (49% TLH, 34% LAVH, 34% VH, 15% TAH, 12% SCH), and “residents familiar with the literature on cystoscopy were statistically more likely to plan to perform universal cystoscopy,” said Dr. Goldberg, the senior author of the paper (Womens Health (Lond Engl). 2015 Nov;11[6]:825-31).
Litigation possible
Failure to detect a urinary tract injury at the time of hysterectomy may result in the need for future additional surgeries. Litigation in the Philadelphia market suggests that “if you have an injury that’s missed, there’s a chance that litigation may result,” Dr. Kim said.
Plaintiff’s attorneys have argued that not recognizing ureteral injury during surgery is a deviation from acceptable practice, while defense attorneys have contended that unavoidable complications occur and that no evaluation is required, or supported by the medical literature, when injury is not intraoperatively suspected. Currently, as cystoscopy is performed less than 25% of the time for all types of hysterectomy, the standard of care does not require the procedure intraoperatively if no injury is suspected, Dr. Goldberg said.
Primary prevention of urinary tract injury is most important, both physicians emphasized. The best way to accomplish this is to meticulously identify the anatomy and know the path of the ureter, and to document that the ureter has been identified and viewed as outside of the operative area, they said.
Dr. Kim and Dr. Goldberg reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2016
Children’s Bone Development Linked to Mothers’ Placenta Size
A larger placenta during pregnancy may lead to larger bones in children, according to a study published in the Journal of Bone and Mineral Research.
Researchers studied 518 children who underwent bone scans at ages 9, 15, and 17. Measurements of thickness, volume, and weight also were taken from their mothers’ placenta. They found that a greater placenta size at birth was associated with larger bones at each age in childhood. This relationship remained robust even after adjusting for factors such as the child’s height, weight, and pubertal status.
Overall, larger bones in early life can lead to larger, stronger bones in older adulthood, thus reducing the risk of osteoporosis and broken bones in later life.
Suggested Reading
Holroyd CR, Osmond C, Barker D, et al. Placental size is associated differentially with postnatal bone size and density. J Bone Miner Res. 2016 Mar 21. [Epub ahead of print]
A larger placenta during pregnancy may lead to larger bones in children, according to a study published in the Journal of Bone and Mineral Research.
Researchers studied 518 children who underwent bone scans at ages 9, 15, and 17. Measurements of thickness, volume, and weight also were taken from their mothers’ placenta. They found that a greater placenta size at birth was associated with larger bones at each age in childhood. This relationship remained robust even after adjusting for factors such as the child’s height, weight, and pubertal status.
Overall, larger bones in early life can lead to larger, stronger bones in older adulthood, thus reducing the risk of osteoporosis and broken bones in later life.
A larger placenta during pregnancy may lead to larger bones in children, according to a study published in the Journal of Bone and Mineral Research.
Researchers studied 518 children who underwent bone scans at ages 9, 15, and 17. Measurements of thickness, volume, and weight also were taken from their mothers’ placenta. They found that a greater placenta size at birth was associated with larger bones at each age in childhood. This relationship remained robust even after adjusting for factors such as the child’s height, weight, and pubertal status.
Overall, larger bones in early life can lead to larger, stronger bones in older adulthood, thus reducing the risk of osteoporosis and broken bones in later life.
Suggested Reading
Holroyd CR, Osmond C, Barker D, et al. Placental size is associated differentially with postnatal bone size and density. J Bone Miner Res. 2016 Mar 21. [Epub ahead of print]
Suggested Reading
Holroyd CR, Osmond C, Barker D, et al. Placental size is associated differentially with postnatal bone size and density. J Bone Miner Res. 2016 Mar 21. [Epub ahead of print]
Oxandrolone, propranolol combo increases growth in severely burned children
CHICAGO – Combination therapy with oxandrolone and propranolol can attenuate burn-induced growth arrest and increase growth rate in severely burned children, according to findings from a prospective, randomized clinical trial.
Of 612 children with burns over at least 30% of their total body surface area (average of more than 50%), 103 were randomized to receive treatment with both oxandrolone and propranolol, 67 received oxandrolone alone, 194 received propranolol alone, and 248 served as controls. After a minimum of 1 year of treatment, the average growth rate was 5.9 cm in the control group and 7.6 cm in the group receiving combination therapy, Dr. David N. Herndon of the University of Texas Medical Branch at Galveston reported at the annual meeting of the American Surgical Association.
“The rate of growth with combination therapy was significantly greater than with either of the individual drugs alone,” Dr. Herndon said.
Further, the period of growth arrest was significantly shorter – by 84 days – among those in the combination-treatment group, compared with those in the control group.
Study subjects were children treated at Shriners Hospitals for Children – Galveston from 1997 to 2015. Boys aged 6 months to 14 years and girls aged 6 months to 12 years were included to eliminate the variable onset of postpubescent growth delay. About two-thirds in each group were boys, and the ages in the patient groups were similar. Mortality was low and was similar across the groups, as was hospital length of stay.
Dr. Herndon and his colleagues controlled for heterogeneous burn distribution between the groups in the course of their analyses, as well as age.
In children with severe, extensive burn injury, the hypercatabolic response is mediated by increased production of catecholamines and corticosteroids, coupled with decreased production of testosterone. This contributes to growth arrest and to decreased strength for up to 2 years after burn injury, he explained. Children with burns over 50% of their total body surface routinely survive acute hospitalization but, at 3 months post injury, are thin, have difficulty walking, and require occupational and physical therapy to help them perform even the simplest activities of daily living.
At 1 year, a raised inflammatory mass covers their wounds, they experience itching, and they have, in large part, stunted growth; there is severe loss of lean body mass and strength, and fracture risk is increased, Dr. Herndon said.
In previous work, he and his colleagues showed that administration of propranolol at an average dose of 4 mg/kg per day for 1 year decreased cardiac work and resting energy expenditure while increasing peripheral lean mass. Further, they found that the testosterone analog oxandrolone, given at 0.1 mg/kg twice per day for 1 year, improved lean body mass accretion and bone mineral content.
The current study was conducted to test the effects of administering both agents in combination.
“The combined use of oxandrolone and propranolol in severely burned children confers an additional benefit on growth over either treatment alone,” Dr. Herndon said, adding that the additive effects of combination therapy may be due to the effects of oxandrolone on bone growth and the anti-inflammatory effects of propranolol.
“The additional benefits point out mechanistic changes that may be eventful in the treatment of hypermetabolism generally and in inflammatory states,” he concluded.
Dr. Herndon reported having no disclosures.
CHICAGO – Combination therapy with oxandrolone and propranolol can attenuate burn-induced growth arrest and increase growth rate in severely burned children, according to findings from a prospective, randomized clinical trial.
Of 612 children with burns over at least 30% of their total body surface area (average of more than 50%), 103 were randomized to receive treatment with both oxandrolone and propranolol, 67 received oxandrolone alone, 194 received propranolol alone, and 248 served as controls. After a minimum of 1 year of treatment, the average growth rate was 5.9 cm in the control group and 7.6 cm in the group receiving combination therapy, Dr. David N. Herndon of the University of Texas Medical Branch at Galveston reported at the annual meeting of the American Surgical Association.
“The rate of growth with combination therapy was significantly greater than with either of the individual drugs alone,” Dr. Herndon said.
Further, the period of growth arrest was significantly shorter – by 84 days – among those in the combination-treatment group, compared with those in the control group.
Study subjects were children treated at Shriners Hospitals for Children – Galveston from 1997 to 2015. Boys aged 6 months to 14 years and girls aged 6 months to 12 years were included to eliminate the variable onset of postpubescent growth delay. About two-thirds in each group were boys, and the ages in the patient groups were similar. Mortality was low and was similar across the groups, as was hospital length of stay.
Dr. Herndon and his colleagues controlled for heterogeneous burn distribution between the groups in the course of their analyses, as well as age.
In children with severe, extensive burn injury, the hypercatabolic response is mediated by increased production of catecholamines and corticosteroids, coupled with decreased production of testosterone. This contributes to growth arrest and to decreased strength for up to 2 years after burn injury, he explained. Children with burns over 50% of their total body surface routinely survive acute hospitalization but, at 3 months post injury, are thin, have difficulty walking, and require occupational and physical therapy to help them perform even the simplest activities of daily living.
At 1 year, a raised inflammatory mass covers their wounds, they experience itching, and they have, in large part, stunted growth; there is severe loss of lean body mass and strength, and fracture risk is increased, Dr. Herndon said.
In previous work, he and his colleagues showed that administration of propranolol at an average dose of 4 mg/kg per day for 1 year decreased cardiac work and resting energy expenditure while increasing peripheral lean mass. Further, they found that the testosterone analog oxandrolone, given at 0.1 mg/kg twice per day for 1 year, improved lean body mass accretion and bone mineral content.
The current study was conducted to test the effects of administering both agents in combination.
“The combined use of oxandrolone and propranolol in severely burned children confers an additional benefit on growth over either treatment alone,” Dr. Herndon said, adding that the additive effects of combination therapy may be due to the effects of oxandrolone on bone growth and the anti-inflammatory effects of propranolol.
“The additional benefits point out mechanistic changes that may be eventful in the treatment of hypermetabolism generally and in inflammatory states,” he concluded.
Dr. Herndon reported having no disclosures.
CHICAGO – Combination therapy with oxandrolone and propranolol can attenuate burn-induced growth arrest and increase growth rate in severely burned children, according to findings from a prospective, randomized clinical trial.
Of 612 children with burns over at least 30% of their total body surface area (average of more than 50%), 103 were randomized to receive treatment with both oxandrolone and propranolol, 67 received oxandrolone alone, 194 received propranolol alone, and 248 served as controls. After a minimum of 1 year of treatment, the average growth rate was 5.9 cm in the control group and 7.6 cm in the group receiving combination therapy, Dr. David N. Herndon of the University of Texas Medical Branch at Galveston reported at the annual meeting of the American Surgical Association.
“The rate of growth with combination therapy was significantly greater than with either of the individual drugs alone,” Dr. Herndon said.
Further, the period of growth arrest was significantly shorter – by 84 days – among those in the combination-treatment group, compared with those in the control group.
Study subjects were children treated at Shriners Hospitals for Children – Galveston from 1997 to 2015. Boys aged 6 months to 14 years and girls aged 6 months to 12 years were included to eliminate the variable onset of postpubescent growth delay. About two-thirds in each group were boys, and the ages in the patient groups were similar. Mortality was low and was similar across the groups, as was hospital length of stay.
Dr. Herndon and his colleagues controlled for heterogeneous burn distribution between the groups in the course of their analyses, as well as age.
In children with severe, extensive burn injury, the hypercatabolic response is mediated by increased production of catecholamines and corticosteroids, coupled with decreased production of testosterone. This contributes to growth arrest and to decreased strength for up to 2 years after burn injury, he explained. Children with burns over 50% of their total body surface routinely survive acute hospitalization but, at 3 months post injury, are thin, have difficulty walking, and require occupational and physical therapy to help them perform even the simplest activities of daily living.
At 1 year, a raised inflammatory mass covers their wounds, they experience itching, and they have, in large part, stunted growth; there is severe loss of lean body mass and strength, and fracture risk is increased, Dr. Herndon said.
In previous work, he and his colleagues showed that administration of propranolol at an average dose of 4 mg/kg per day for 1 year decreased cardiac work and resting energy expenditure while increasing peripheral lean mass. Further, they found that the testosterone analog oxandrolone, given at 0.1 mg/kg twice per day for 1 year, improved lean body mass accretion and bone mineral content.
The current study was conducted to test the effects of administering both agents in combination.
“The combined use of oxandrolone and propranolol in severely burned children confers an additional benefit on growth over either treatment alone,” Dr. Herndon said, adding that the additive effects of combination therapy may be due to the effects of oxandrolone on bone growth and the anti-inflammatory effects of propranolol.
“The additional benefits point out mechanistic changes that may be eventful in the treatment of hypermetabolism generally and in inflammatory states,” he concluded.
Dr. Herndon reported having no disclosures.
AT THE ASA ANNUAL MEETING
Key clinical point: Combination therapy with oxandrolone and propranolol can attenuate burn-induced growth arrest and increase growth rate in severely burned children, according to findings from a prospective, randomized clinical trial.
Major finding: After a minimum of 1 year of treatment, the average growth rate was 5.9 cm in the control group and 7.6 cm in the group receiving combination therapy.
Data source: A prospective, randomized clinical trial involving 612 children.
Disclosures: Dr. Herndon reported having no disclosures.
Association of Frailty on One-Year Postoperative Mortality Following Major Elective Non-Cardiac Surgery
Clinical question: What is the association of preoperative frailty on one-year postoperative mortality?
Background: Frailty is an aggregate expression of susceptibility to poor outcomes owing to age and disease-related deficits that accumulate with multiple domains. Frailty in this study was defined by the Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnoses indicator. It is a binary variable that uses 12 clusters of frailty-defining diagnoses.
Study design: Population-based retrospective cohort study.
Setting: All hospital and physician services funded through the public health care system in Toronto.
Synopsis: The study had 202,980 patients who underwent major elective non-cardiac surgery. Frailty-defining diagnoses were present in 6,289 patients (3.1%). Mean age for the frail population was about 77 years. Joint replacements were the most common procedures for the frail and non-frail groups. Knee replacements were more prevalent in the non-frail group. One year after surgery, 855 frail patients (13.6%) and 9,433 non-frail patients (4.8%) died (unadjusted hazard ratio [HR], 2.98; 95% CI, 2.78–3.20). When adjusted for age, sex, neighborhood income quintile, and procedure, one-year mortality risk remained significantly higher in the frail group. One-year risk of death was significantly higher in frail patients for all surgical procedures, especially with total joint arthroplasty.
The relative hazard ratio of mortality in frail versus non-frail was extremely high in the early postoperative period, most notably at postoperative day three.
One major weakness of the study is that there is no universal definition of frailty, plus the results are difficult to generalize across populations.
Bottom line: Presence of preoperative frailty-defining diagnoses is associated with increased risk for one-year postoperative mortality; the risk appears to be very high in the early postoperative period.
Citation: McIsaac D, Bryson G, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study [published online ahead of print January 20, 2016]. JAMA Surg. doi:10.1001/jamasurg.2015.5085.
Short Take
Early Discharge Associated with Longer Length of Stay
Retrospective analysis showed early discharge before noon was associated with longer length of stay, especially among emergent admissions. However, multiple metrics should be used to measure true effectiveness of an early discharge program.
Citation: Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients [published online ahead of print December 30, 2015]. J Hosp Med. doi:10.1002/jhm.2529.
Clinical question: What is the association of preoperative frailty on one-year postoperative mortality?
Background: Frailty is an aggregate expression of susceptibility to poor outcomes owing to age and disease-related deficits that accumulate with multiple domains. Frailty in this study was defined by the Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnoses indicator. It is a binary variable that uses 12 clusters of frailty-defining diagnoses.
Study design: Population-based retrospective cohort study.
Setting: All hospital and physician services funded through the public health care system in Toronto.
Synopsis: The study had 202,980 patients who underwent major elective non-cardiac surgery. Frailty-defining diagnoses were present in 6,289 patients (3.1%). Mean age for the frail population was about 77 years. Joint replacements were the most common procedures for the frail and non-frail groups. Knee replacements were more prevalent in the non-frail group. One year after surgery, 855 frail patients (13.6%) and 9,433 non-frail patients (4.8%) died (unadjusted hazard ratio [HR], 2.98; 95% CI, 2.78–3.20). When adjusted for age, sex, neighborhood income quintile, and procedure, one-year mortality risk remained significantly higher in the frail group. One-year risk of death was significantly higher in frail patients for all surgical procedures, especially with total joint arthroplasty.
The relative hazard ratio of mortality in frail versus non-frail was extremely high in the early postoperative period, most notably at postoperative day three.
One major weakness of the study is that there is no universal definition of frailty, plus the results are difficult to generalize across populations.
Bottom line: Presence of preoperative frailty-defining diagnoses is associated with increased risk for one-year postoperative mortality; the risk appears to be very high in the early postoperative period.
Citation: McIsaac D, Bryson G, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study [published online ahead of print January 20, 2016]. JAMA Surg. doi:10.1001/jamasurg.2015.5085.
Short Take
Early Discharge Associated with Longer Length of Stay
Retrospective analysis showed early discharge before noon was associated with longer length of stay, especially among emergent admissions. However, multiple metrics should be used to measure true effectiveness of an early discharge program.
Citation: Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients [published online ahead of print December 30, 2015]. J Hosp Med. doi:10.1002/jhm.2529.
Clinical question: What is the association of preoperative frailty on one-year postoperative mortality?
Background: Frailty is an aggregate expression of susceptibility to poor outcomes owing to age and disease-related deficits that accumulate with multiple domains. Frailty in this study was defined by the Johns Hopkins Adjusted Clinical Groups (ACG) frailty-defining diagnoses indicator. It is a binary variable that uses 12 clusters of frailty-defining diagnoses.
Study design: Population-based retrospective cohort study.
Setting: All hospital and physician services funded through the public health care system in Toronto.
Synopsis: The study had 202,980 patients who underwent major elective non-cardiac surgery. Frailty-defining diagnoses were present in 6,289 patients (3.1%). Mean age for the frail population was about 77 years. Joint replacements were the most common procedures for the frail and non-frail groups. Knee replacements were more prevalent in the non-frail group. One year after surgery, 855 frail patients (13.6%) and 9,433 non-frail patients (4.8%) died (unadjusted hazard ratio [HR], 2.98; 95% CI, 2.78–3.20). When adjusted for age, sex, neighborhood income quintile, and procedure, one-year mortality risk remained significantly higher in the frail group. One-year risk of death was significantly higher in frail patients for all surgical procedures, especially with total joint arthroplasty.
The relative hazard ratio of mortality in frail versus non-frail was extremely high in the early postoperative period, most notably at postoperative day three.
One major weakness of the study is that there is no universal definition of frailty, plus the results are difficult to generalize across populations.
Bottom line: Presence of preoperative frailty-defining diagnoses is associated with increased risk for one-year postoperative mortality; the risk appears to be very high in the early postoperative period.
Citation: McIsaac D, Bryson G, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study [published online ahead of print January 20, 2016]. JAMA Surg. doi:10.1001/jamasurg.2015.5085.
Short Take
Early Discharge Associated with Longer Length of Stay
Retrospective analysis showed early discharge before noon was associated with longer length of stay, especially among emergent admissions. However, multiple metrics should be used to measure true effectiveness of an early discharge program.
Citation: Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients [published online ahead of print December 30, 2015]. J Hosp Med. doi:10.1002/jhm.2529.
Transgender surgery making inroads
The way Dr. Marci L. Bowers sees it, societal acceptance of transgender persons has come a long way, and the future of transgender surgery is bright.
“Who thought that we’d have our decathlon winner Bruce Jenner become Caitlyn?” Dr. Bowers said at the annual scientific meeting of the Society of Gynecologic Surgeons in Indian Wells, Calif. “Who thought that the brothers who created “The Matrix” movies [Larry and Andy Wachowski] would become sisters? All in this past year?”
As the first transgender surgeon to perform transgender surgery in North America, Dr. Bowers knows of what she speaks. In fact, she recently joined the faculty at Mount Sinai Beth Israel Medical Center in New York to help launch what she said will be the first U.S.-based surgical training program for transgender medicine in nearly 40 years.
“An academic institution doing these procedures is really revolutionary,” she said. “I think it’s going to really help how things are taught and described to practitioners.”
She said she also hopes the effort helps stem the “high percentage” of transgender teenagers who attempt or commit suicide. According to 7,261 transgender students in grades 6-12 who responded to the 2009 National School Climate Survey, 61% reported feeling unsafe at school because of their sexual orientation and 40% because of how they expressed their gender; 19% said they have been punched, kicked, or injured with a weapon on at least one occasion within the last year because of their sexual orientation and 13% because of their gender expression; and 53% reported cyberbullying because of their gender identity.
“We need to stop losing these people,” she said. “My kids are now in their early 20s. This generation is asking for honesty in the areas of sexuality and gender identity.”
Dr. Bowers, who graduated from the University of Minnesota Medical School in 1986 and did her ob.gyn. residency at the University of Washington, Seattle, characterized the notion of being “misgendered” as a biologic process. “If you look around nature, there is no single measure anywhere in biology that offers only two choices, besides gender,” said Dr. Bowers, who underwent male to female reassignment surgery at age 39. “So when you think about it, the world is represented by a spectrum; it’s represented by diversity. That’s what transgender is, the inner concept of maleness and femaleness. It can’t be just two choices. This is what’s coming to the surface as this movement takes hold.”
After practicing ob.gyn. in Seattle for 13 years, Dr. Bowers relocated to Trinidad, Colo., where she learned and began to practice transgender surgery under the tutelage of the late Dr. Stanley Biber, who performed more than 4,000 sex reassignment surgeries. After working there for 8 years, Dr. Bowers moved her practice to Burlingame, Calif., where she currently performs about 140 male to female operations each year and has a 3-year waiting list. During each 3-hour operation the testicles are removed, the glans penis becomes the clitoris, the scrotum becomes the labia majora, the urethra becomes the labia minora mucosa, the scrotum/penile skin becomes the vagina, and the Cowper’s glands and prostate are retained. Results are “rather convincing,” she said.
Most patients require a hospital stay of up to 3 days, and the most common complication is wound separation/dehiscence, which occurs in 3%-9% of cases. Out-of-pocket costs average about $25,000 per case, but a growing number of insurers now pay for the procedure.
“A dozen years ago, only one company in the Fortune 500 covered transgender surgery,” she said. “Now in our practice, nearly 90% of insurers do, about 70% of the Fortune 500 companies do, and 12 states mandate coverage for all of their citizens to be covered for transgender surgery. It’s really changed.”
In 2009 the American Medical Association passed a resolution supporting public and private insurance coverage for the treatment of gender identity disorder. According to Dr. Bowers, this came about in part because of a 2009 landmark study conducted by the National Center for Transgender Quality and the Gay and Lesbian Task Force that found that more than half of transgender and gender nonconforming people who were bullied, harassed, or assaulted in school because of their gender identity have attempted suicide. In 2011, ACOG’s Committee on Health Care for Underserved Women published an opinion on health care for transgender individuals. Part of its recommendation was that ob. gyns. “should be prepared to assist or refer transgender individuals for routine treatment and screening as well as hormonal and surgical therapies.” According to guidelines from the World Professional Association for Transgender Health (WPATH), individuals seeking transgender surgery should undergo a psychological evaluation, 1 year of cross-sex hormone therapy, and 1 year of desired gender role, and be at least 18 years of age before undergoing surgery itself.
“Gender identity is established early; this is not something that somebody wakes up with,” said Dr. Bowers, who has appeared on “The Oprah Winfrey Show,” “CBS Sunday Morning,” “Discovery Health,” and CNN, and was named one of Huffington Post’s 50 Transgender Icons. “Yes, they need to have a psychological evaluation. Yes, they need to live in their desired gender role, and yes, they need to be on hormones, but other than that, they rarely regret their decision to move forward medically and surgically. Almost never.”
During a presentation at the annual meeting of the American College of Physicians, Dr. Henry Ng noted that some transgender patients are ambivalent about undergoing gender reassignment surgery. “A lot of them don’t want surgery because it’s not a covered benefit under most health plans, it’s expensive, and it does require a lot of healing time because it’s a very invasive procedure,” said Dr. Ng of the departments of internal medicine and pediatrics at Case Western Reserve University, Cleveland. “Especially for certain procedures like phalloplasty, those procedures have not been developed to a point where we can avoid a lot of complications.”
Dr. Ng, who is also clinical director of the MetroHealth Pride Clinic in Cleveland, noted that general health screening guidelines for transgender patients can be found at www.transhealth.ucsf.edu/protocols. “The good news is that it’s really no different than that versus gender people: cardiovascular health; tobacco use; addressing issues of mood disorders/depression, in part related to the experience of microaggressions and macroaggressions from discrimination, violence, and hate-motivated violence on a day-to-day basis; thyroid disease; respiratory illnesses that may be associated with increased use of tobacco products; sexual health; and vaccinations,” he said. “These are all important to include in a general health screening for transgender people.” A free consultation service known as TransLine offers physicians clinical information about transgender issues and individualized case consultation. For information, visit www.project-health.org/transline.
According to the Human Rights Campaign’s Corporate Quality Index, scores of major employers in the United States, including 3M, Amazon.com, American Express, Boeing, General Motors, Johnson & Johnson, Morgan Stanley, Nike, Procter & Gamble, Starbucks, UnitedHealth Group, Visa, and Xerox, offer at least one transgender-inclusive health care coverage plan. A list of insurers who offer transgender health coverage can be found here. The Human Rights Campaign also notes that seven states that have both bans on insurance exclusions for transgender health care and provide transgender inclusive benefits for state employees: New York, Massachusetts, Connecticut, Rhode Island, California, Oregon, and Washington. The National Center for Transgender Quality notes that since May of 2014, Medicare coverage decisions for transition-related surgeries are “made individually on the basis of medical need and applicable standards of care, similar to other doctor or hospital services under Medicare.”
When a patient realizes that his or her employer has not opted in to cover transgender care as part of its health insurance offerings, “it’s heartbreaking to be the middle man,” Dr. Cecile Unger, a surgeon at the Cleveland Clinic center for female pelvic medicine and reconstructive surgery, said in an interview. “Some patients start calculating how much they need to save weekly or monthly [in order to pay out of pocket]. They figure out where they want to have surgery. We provide them with the exact self-pay numbers. They usually will shop around a bit to see what some of the other providers are offering. Some patients will try to get their names on the books a year-and-a-half or two ahead of time.”
The cost of procedures varies. For example, the price of a vaginoplasty at the Philadelphia Center for Transgender Surgery is $12,600, plus $7,150 in anesthesia, operating room, and hospital stay charges, for a total of $19,750. The center’s cost for female to male surgery at the center are slightly higher. For example, the price of a phalloplasty, scrotoplasty, testicular implants, glansplasty, and transposition of the clitoris is $15,500, plus $5,750 in anesthesia, OR, and hospital charges, for a total of $21,250.
Wound separation and wound-healing problems are the most common complications after gender reassignment surgery, Dr. Unger said, “but within 4-12 weeks usually those issues resolve themselves with a bit of conservative wound care, and don’t require more surgery. Infection is quite rare. Hematoma formation is not common in the first few days after surgery. In female to male procedures, there’s a bit of a risk for stricture of the urethra, which can lead to problems with voiding and fistula formation.”
Discussing realistic expectations with patients preoperatively is key, Dr. Unger said, especially in terms of scarring and cosmesis. “Most of the time you get a great result, but patients should also understand that everybody’s anatomy is different and everybody’s wound healing is different, so [they] have to be flexible and understand that secondary procedures are sometimes necessary to get the perfect outcomes,” she said.
Another procedure Dr. Bowers provides in her practice is functional clitoroplasty for females who have undergone genital mutation, a procedure that has impacted an estimated 140 million women worldwide, especially those in Indonesia. “These women often have never had orgasm in their life because all or part of the clitoris and the labia have been excised,” Dr. Bowers said. “It’s one of the great human tragedies.”
In a procedure that takes about an hour, Dr. Bowers restores refibulation and sensation for women who have been genitally mutilated – at no charge to them. “We 100% of the time find the clitoris when we do these operations,” she said. “We refibulate, we release the suspensory ligament, we anchor the clitoral body down, and that restores function. From the letters I receive, this is a miracle for these patients, to feel orgasm for the first time in your life. Imagine restoring sight to a blind person. It’s that profound.”
Dr. Bowers disclosed that she is a member of WPATH and that she serves on the board of directors of GLAAD and the Transgender Law Center. The meeting was jointly sponsored by the American College of Surgeons.
The way Dr. Marci L. Bowers sees it, societal acceptance of transgender persons has come a long way, and the future of transgender surgery is bright.
“Who thought that we’d have our decathlon winner Bruce Jenner become Caitlyn?” Dr. Bowers said at the annual scientific meeting of the Society of Gynecologic Surgeons in Indian Wells, Calif. “Who thought that the brothers who created “The Matrix” movies [Larry and Andy Wachowski] would become sisters? All in this past year?”
As the first transgender surgeon to perform transgender surgery in North America, Dr. Bowers knows of what she speaks. In fact, she recently joined the faculty at Mount Sinai Beth Israel Medical Center in New York to help launch what she said will be the first U.S.-based surgical training program for transgender medicine in nearly 40 years.
“An academic institution doing these procedures is really revolutionary,” she said. “I think it’s going to really help how things are taught and described to practitioners.”
She said she also hopes the effort helps stem the “high percentage” of transgender teenagers who attempt or commit suicide. According to 7,261 transgender students in grades 6-12 who responded to the 2009 National School Climate Survey, 61% reported feeling unsafe at school because of their sexual orientation and 40% because of how they expressed their gender; 19% said they have been punched, kicked, or injured with a weapon on at least one occasion within the last year because of their sexual orientation and 13% because of their gender expression; and 53% reported cyberbullying because of their gender identity.
“We need to stop losing these people,” she said. “My kids are now in their early 20s. This generation is asking for honesty in the areas of sexuality and gender identity.”
Dr. Bowers, who graduated from the University of Minnesota Medical School in 1986 and did her ob.gyn. residency at the University of Washington, Seattle, characterized the notion of being “misgendered” as a biologic process. “If you look around nature, there is no single measure anywhere in biology that offers only two choices, besides gender,” said Dr. Bowers, who underwent male to female reassignment surgery at age 39. “So when you think about it, the world is represented by a spectrum; it’s represented by diversity. That’s what transgender is, the inner concept of maleness and femaleness. It can’t be just two choices. This is what’s coming to the surface as this movement takes hold.”
After practicing ob.gyn. in Seattle for 13 years, Dr. Bowers relocated to Trinidad, Colo., where she learned and began to practice transgender surgery under the tutelage of the late Dr. Stanley Biber, who performed more than 4,000 sex reassignment surgeries. After working there for 8 years, Dr. Bowers moved her practice to Burlingame, Calif., where she currently performs about 140 male to female operations each year and has a 3-year waiting list. During each 3-hour operation the testicles are removed, the glans penis becomes the clitoris, the scrotum becomes the labia majora, the urethra becomes the labia minora mucosa, the scrotum/penile skin becomes the vagina, and the Cowper’s glands and prostate are retained. Results are “rather convincing,” she said.
Most patients require a hospital stay of up to 3 days, and the most common complication is wound separation/dehiscence, which occurs in 3%-9% of cases. Out-of-pocket costs average about $25,000 per case, but a growing number of insurers now pay for the procedure.
“A dozen years ago, only one company in the Fortune 500 covered transgender surgery,” she said. “Now in our practice, nearly 90% of insurers do, about 70% of the Fortune 500 companies do, and 12 states mandate coverage for all of their citizens to be covered for transgender surgery. It’s really changed.”
In 2009 the American Medical Association passed a resolution supporting public and private insurance coverage for the treatment of gender identity disorder. According to Dr. Bowers, this came about in part because of a 2009 landmark study conducted by the National Center for Transgender Quality and the Gay and Lesbian Task Force that found that more than half of transgender and gender nonconforming people who were bullied, harassed, or assaulted in school because of their gender identity have attempted suicide. In 2011, ACOG’s Committee on Health Care for Underserved Women published an opinion on health care for transgender individuals. Part of its recommendation was that ob. gyns. “should be prepared to assist or refer transgender individuals for routine treatment and screening as well as hormonal and surgical therapies.” According to guidelines from the World Professional Association for Transgender Health (WPATH), individuals seeking transgender surgery should undergo a psychological evaluation, 1 year of cross-sex hormone therapy, and 1 year of desired gender role, and be at least 18 years of age before undergoing surgery itself.
“Gender identity is established early; this is not something that somebody wakes up with,” said Dr. Bowers, who has appeared on “The Oprah Winfrey Show,” “CBS Sunday Morning,” “Discovery Health,” and CNN, and was named one of Huffington Post’s 50 Transgender Icons. “Yes, they need to have a psychological evaluation. Yes, they need to live in their desired gender role, and yes, they need to be on hormones, but other than that, they rarely regret their decision to move forward medically and surgically. Almost never.”
During a presentation at the annual meeting of the American College of Physicians, Dr. Henry Ng noted that some transgender patients are ambivalent about undergoing gender reassignment surgery. “A lot of them don’t want surgery because it’s not a covered benefit under most health plans, it’s expensive, and it does require a lot of healing time because it’s a very invasive procedure,” said Dr. Ng of the departments of internal medicine and pediatrics at Case Western Reserve University, Cleveland. “Especially for certain procedures like phalloplasty, those procedures have not been developed to a point where we can avoid a lot of complications.”
Dr. Ng, who is also clinical director of the MetroHealth Pride Clinic in Cleveland, noted that general health screening guidelines for transgender patients can be found at www.transhealth.ucsf.edu/protocols. “The good news is that it’s really no different than that versus gender people: cardiovascular health; tobacco use; addressing issues of mood disorders/depression, in part related to the experience of microaggressions and macroaggressions from discrimination, violence, and hate-motivated violence on a day-to-day basis; thyroid disease; respiratory illnesses that may be associated with increased use of tobacco products; sexual health; and vaccinations,” he said. “These are all important to include in a general health screening for transgender people.” A free consultation service known as TransLine offers physicians clinical information about transgender issues and individualized case consultation. For information, visit www.project-health.org/transline.
According to the Human Rights Campaign’s Corporate Quality Index, scores of major employers in the United States, including 3M, Amazon.com, American Express, Boeing, General Motors, Johnson & Johnson, Morgan Stanley, Nike, Procter & Gamble, Starbucks, UnitedHealth Group, Visa, and Xerox, offer at least one transgender-inclusive health care coverage plan. A list of insurers who offer transgender health coverage can be found here. The Human Rights Campaign also notes that seven states that have both bans on insurance exclusions for transgender health care and provide transgender inclusive benefits for state employees: New York, Massachusetts, Connecticut, Rhode Island, California, Oregon, and Washington. The National Center for Transgender Quality notes that since May of 2014, Medicare coverage decisions for transition-related surgeries are “made individually on the basis of medical need and applicable standards of care, similar to other doctor or hospital services under Medicare.”
When a patient realizes that his or her employer has not opted in to cover transgender care as part of its health insurance offerings, “it’s heartbreaking to be the middle man,” Dr. Cecile Unger, a surgeon at the Cleveland Clinic center for female pelvic medicine and reconstructive surgery, said in an interview. “Some patients start calculating how much they need to save weekly or monthly [in order to pay out of pocket]. They figure out where they want to have surgery. We provide them with the exact self-pay numbers. They usually will shop around a bit to see what some of the other providers are offering. Some patients will try to get their names on the books a year-and-a-half or two ahead of time.”
The cost of procedures varies. For example, the price of a vaginoplasty at the Philadelphia Center for Transgender Surgery is $12,600, plus $7,150 in anesthesia, operating room, and hospital stay charges, for a total of $19,750. The center’s cost for female to male surgery at the center are slightly higher. For example, the price of a phalloplasty, scrotoplasty, testicular implants, glansplasty, and transposition of the clitoris is $15,500, plus $5,750 in anesthesia, OR, and hospital charges, for a total of $21,250.
Wound separation and wound-healing problems are the most common complications after gender reassignment surgery, Dr. Unger said, “but within 4-12 weeks usually those issues resolve themselves with a bit of conservative wound care, and don’t require more surgery. Infection is quite rare. Hematoma formation is not common in the first few days after surgery. In female to male procedures, there’s a bit of a risk for stricture of the urethra, which can lead to problems with voiding and fistula formation.”
Discussing realistic expectations with patients preoperatively is key, Dr. Unger said, especially in terms of scarring and cosmesis. “Most of the time you get a great result, but patients should also understand that everybody’s anatomy is different and everybody’s wound healing is different, so [they] have to be flexible and understand that secondary procedures are sometimes necessary to get the perfect outcomes,” she said.
Another procedure Dr. Bowers provides in her practice is functional clitoroplasty for females who have undergone genital mutation, a procedure that has impacted an estimated 140 million women worldwide, especially those in Indonesia. “These women often have never had orgasm in their life because all or part of the clitoris and the labia have been excised,” Dr. Bowers said. “It’s one of the great human tragedies.”
In a procedure that takes about an hour, Dr. Bowers restores refibulation and sensation for women who have been genitally mutilated – at no charge to them. “We 100% of the time find the clitoris when we do these operations,” she said. “We refibulate, we release the suspensory ligament, we anchor the clitoral body down, and that restores function. From the letters I receive, this is a miracle for these patients, to feel orgasm for the first time in your life. Imagine restoring sight to a blind person. It’s that profound.”
Dr. Bowers disclosed that she is a member of WPATH and that she serves on the board of directors of GLAAD and the Transgender Law Center. The meeting was jointly sponsored by the American College of Surgeons.
The way Dr. Marci L. Bowers sees it, societal acceptance of transgender persons has come a long way, and the future of transgender surgery is bright.
“Who thought that we’d have our decathlon winner Bruce Jenner become Caitlyn?” Dr. Bowers said at the annual scientific meeting of the Society of Gynecologic Surgeons in Indian Wells, Calif. “Who thought that the brothers who created “The Matrix” movies [Larry and Andy Wachowski] would become sisters? All in this past year?”
As the first transgender surgeon to perform transgender surgery in North America, Dr. Bowers knows of what she speaks. In fact, she recently joined the faculty at Mount Sinai Beth Israel Medical Center in New York to help launch what she said will be the first U.S.-based surgical training program for transgender medicine in nearly 40 years.
“An academic institution doing these procedures is really revolutionary,” she said. “I think it’s going to really help how things are taught and described to practitioners.”
She said she also hopes the effort helps stem the “high percentage” of transgender teenagers who attempt or commit suicide. According to 7,261 transgender students in grades 6-12 who responded to the 2009 National School Climate Survey, 61% reported feeling unsafe at school because of their sexual orientation and 40% because of how they expressed their gender; 19% said they have been punched, kicked, or injured with a weapon on at least one occasion within the last year because of their sexual orientation and 13% because of their gender expression; and 53% reported cyberbullying because of their gender identity.
“We need to stop losing these people,” she said. “My kids are now in their early 20s. This generation is asking for honesty in the areas of sexuality and gender identity.”
Dr. Bowers, who graduated from the University of Minnesota Medical School in 1986 and did her ob.gyn. residency at the University of Washington, Seattle, characterized the notion of being “misgendered” as a biologic process. “If you look around nature, there is no single measure anywhere in biology that offers only two choices, besides gender,” said Dr. Bowers, who underwent male to female reassignment surgery at age 39. “So when you think about it, the world is represented by a spectrum; it’s represented by diversity. That’s what transgender is, the inner concept of maleness and femaleness. It can’t be just two choices. This is what’s coming to the surface as this movement takes hold.”
After practicing ob.gyn. in Seattle for 13 years, Dr. Bowers relocated to Trinidad, Colo., where she learned and began to practice transgender surgery under the tutelage of the late Dr. Stanley Biber, who performed more than 4,000 sex reassignment surgeries. After working there for 8 years, Dr. Bowers moved her practice to Burlingame, Calif., where she currently performs about 140 male to female operations each year and has a 3-year waiting list. During each 3-hour operation the testicles are removed, the glans penis becomes the clitoris, the scrotum becomes the labia majora, the urethra becomes the labia minora mucosa, the scrotum/penile skin becomes the vagina, and the Cowper’s glands and prostate are retained. Results are “rather convincing,” she said.
Most patients require a hospital stay of up to 3 days, and the most common complication is wound separation/dehiscence, which occurs in 3%-9% of cases. Out-of-pocket costs average about $25,000 per case, but a growing number of insurers now pay for the procedure.
“A dozen years ago, only one company in the Fortune 500 covered transgender surgery,” she said. “Now in our practice, nearly 90% of insurers do, about 70% of the Fortune 500 companies do, and 12 states mandate coverage for all of their citizens to be covered for transgender surgery. It’s really changed.”
In 2009 the American Medical Association passed a resolution supporting public and private insurance coverage for the treatment of gender identity disorder. According to Dr. Bowers, this came about in part because of a 2009 landmark study conducted by the National Center for Transgender Quality and the Gay and Lesbian Task Force that found that more than half of transgender and gender nonconforming people who were bullied, harassed, or assaulted in school because of their gender identity have attempted suicide. In 2011, ACOG’s Committee on Health Care for Underserved Women published an opinion on health care for transgender individuals. Part of its recommendation was that ob. gyns. “should be prepared to assist or refer transgender individuals for routine treatment and screening as well as hormonal and surgical therapies.” According to guidelines from the World Professional Association for Transgender Health (WPATH), individuals seeking transgender surgery should undergo a psychological evaluation, 1 year of cross-sex hormone therapy, and 1 year of desired gender role, and be at least 18 years of age before undergoing surgery itself.
“Gender identity is established early; this is not something that somebody wakes up with,” said Dr. Bowers, who has appeared on “The Oprah Winfrey Show,” “CBS Sunday Morning,” “Discovery Health,” and CNN, and was named one of Huffington Post’s 50 Transgender Icons. “Yes, they need to have a psychological evaluation. Yes, they need to live in their desired gender role, and yes, they need to be on hormones, but other than that, they rarely regret their decision to move forward medically and surgically. Almost never.”
During a presentation at the annual meeting of the American College of Physicians, Dr. Henry Ng noted that some transgender patients are ambivalent about undergoing gender reassignment surgery. “A lot of them don’t want surgery because it’s not a covered benefit under most health plans, it’s expensive, and it does require a lot of healing time because it’s a very invasive procedure,” said Dr. Ng of the departments of internal medicine and pediatrics at Case Western Reserve University, Cleveland. “Especially for certain procedures like phalloplasty, those procedures have not been developed to a point where we can avoid a lot of complications.”
Dr. Ng, who is also clinical director of the MetroHealth Pride Clinic in Cleveland, noted that general health screening guidelines for transgender patients can be found at www.transhealth.ucsf.edu/protocols. “The good news is that it’s really no different than that versus gender people: cardiovascular health; tobacco use; addressing issues of mood disorders/depression, in part related to the experience of microaggressions and macroaggressions from discrimination, violence, and hate-motivated violence on a day-to-day basis; thyroid disease; respiratory illnesses that may be associated with increased use of tobacco products; sexual health; and vaccinations,” he said. “These are all important to include in a general health screening for transgender people.” A free consultation service known as TransLine offers physicians clinical information about transgender issues and individualized case consultation. For information, visit www.project-health.org/transline.
According to the Human Rights Campaign’s Corporate Quality Index, scores of major employers in the United States, including 3M, Amazon.com, American Express, Boeing, General Motors, Johnson & Johnson, Morgan Stanley, Nike, Procter & Gamble, Starbucks, UnitedHealth Group, Visa, and Xerox, offer at least one transgender-inclusive health care coverage plan. A list of insurers who offer transgender health coverage can be found here. The Human Rights Campaign also notes that seven states that have both bans on insurance exclusions for transgender health care and provide transgender inclusive benefits for state employees: New York, Massachusetts, Connecticut, Rhode Island, California, Oregon, and Washington. The National Center for Transgender Quality notes that since May of 2014, Medicare coverage decisions for transition-related surgeries are “made individually on the basis of medical need and applicable standards of care, similar to other doctor or hospital services under Medicare.”
When a patient realizes that his or her employer has not opted in to cover transgender care as part of its health insurance offerings, “it’s heartbreaking to be the middle man,” Dr. Cecile Unger, a surgeon at the Cleveland Clinic center for female pelvic medicine and reconstructive surgery, said in an interview. “Some patients start calculating how much they need to save weekly or monthly [in order to pay out of pocket]. They figure out where they want to have surgery. We provide them with the exact self-pay numbers. They usually will shop around a bit to see what some of the other providers are offering. Some patients will try to get their names on the books a year-and-a-half or two ahead of time.”
The cost of procedures varies. For example, the price of a vaginoplasty at the Philadelphia Center for Transgender Surgery is $12,600, plus $7,150 in anesthesia, operating room, and hospital stay charges, for a total of $19,750. The center’s cost for female to male surgery at the center are slightly higher. For example, the price of a phalloplasty, scrotoplasty, testicular implants, glansplasty, and transposition of the clitoris is $15,500, plus $5,750 in anesthesia, OR, and hospital charges, for a total of $21,250.
Wound separation and wound-healing problems are the most common complications after gender reassignment surgery, Dr. Unger said, “but within 4-12 weeks usually those issues resolve themselves with a bit of conservative wound care, and don’t require more surgery. Infection is quite rare. Hematoma formation is not common in the first few days after surgery. In female to male procedures, there’s a bit of a risk for stricture of the urethra, which can lead to problems with voiding and fistula formation.”
Discussing realistic expectations with patients preoperatively is key, Dr. Unger said, especially in terms of scarring and cosmesis. “Most of the time you get a great result, but patients should also understand that everybody’s anatomy is different and everybody’s wound healing is different, so [they] have to be flexible and understand that secondary procedures are sometimes necessary to get the perfect outcomes,” she said.
Another procedure Dr. Bowers provides in her practice is functional clitoroplasty for females who have undergone genital mutation, a procedure that has impacted an estimated 140 million women worldwide, especially those in Indonesia. “These women often have never had orgasm in their life because all or part of the clitoris and the labia have been excised,” Dr. Bowers said. “It’s one of the great human tragedies.”
In a procedure that takes about an hour, Dr. Bowers restores refibulation and sensation for women who have been genitally mutilated – at no charge to them. “We 100% of the time find the clitoris when we do these operations,” she said. “We refibulate, we release the suspensory ligament, we anchor the clitoral body down, and that restores function. From the letters I receive, this is a miracle for these patients, to feel orgasm for the first time in your life. Imagine restoring sight to a blind person. It’s that profound.”
Dr. Bowers disclosed that she is a member of WPATH and that she serves on the board of directors of GLAAD and the Transgender Law Center. The meeting was jointly sponsored by the American College of Surgeons.
VIDEO: Is hysterectomy still best for complex atypical hyperplasia?
WASHINGTON – Hysterectomy has long been the first-line therapy for complex atypical endometrial hyperplasia in patients who don’t desire to preserve their fertility. Is it time to consider hormone treatment in a larger population of patients?
That’s the question that experts debated at the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Amanda Nickles Fader, associate professor and director of the Kelly Gynecologic Oncology Service* at the Johns Hopkins Hospital, Baltimore, said in an interview that changing patient demographics – particularly the growing number of overweight and obese women – are driving the need to consider the use of progestin in more cases. The obesity epidemic translates into younger women developing the condition, and it creates the potential for more complications in surgery, she said. Endometrial hyperplasia is very sensitive to hormone therapy, specifically progestin agents, with 75%-90% response rates with up-front treatment, Dr. Fader added.
But Dr. David Cohn, director of the division of gynecologic oncology at the Ohio State University, Columbus, said in an interview that surgery remains the standard of care because it is curative. Hormone treatment is appropriate in selected patients, but it is currently understudied and questions remain about the duration of treatment and about the type of hormones to use, he said.
Dr. Cohn and Dr. Fader both reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mschneider@frontlinemedcom.com
On Twitter @maryellenny
*Correction, 5/17/2016: An earlier version of this story misstated Dr. Fader's title.
WASHINGTON – Hysterectomy has long been the first-line therapy for complex atypical endometrial hyperplasia in patients who don’t desire to preserve their fertility. Is it time to consider hormone treatment in a larger population of patients?
That’s the question that experts debated at the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Amanda Nickles Fader, associate professor and director of the Kelly Gynecologic Oncology Service* at the Johns Hopkins Hospital, Baltimore, said in an interview that changing patient demographics – particularly the growing number of overweight and obese women – are driving the need to consider the use of progestin in more cases. The obesity epidemic translates into younger women developing the condition, and it creates the potential for more complications in surgery, she said. Endometrial hyperplasia is very sensitive to hormone therapy, specifically progestin agents, with 75%-90% response rates with up-front treatment, Dr. Fader added.
But Dr. David Cohn, director of the division of gynecologic oncology at the Ohio State University, Columbus, said in an interview that surgery remains the standard of care because it is curative. Hormone treatment is appropriate in selected patients, but it is currently understudied and questions remain about the duration of treatment and about the type of hormones to use, he said.
Dr. Cohn and Dr. Fader both reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mschneider@frontlinemedcom.com
On Twitter @maryellenny
*Correction, 5/17/2016: An earlier version of this story misstated Dr. Fader's title.
WASHINGTON – Hysterectomy has long been the first-line therapy for complex atypical endometrial hyperplasia in patients who don’t desire to preserve their fertility. Is it time to consider hormone treatment in a larger population of patients?
That’s the question that experts debated at the annual meeting of the American College of Obstetricians and Gynecologists.
Dr. Amanda Nickles Fader, associate professor and director of the Kelly Gynecologic Oncology Service* at the Johns Hopkins Hospital, Baltimore, said in an interview that changing patient demographics – particularly the growing number of overweight and obese women – are driving the need to consider the use of progestin in more cases. The obesity epidemic translates into younger women developing the condition, and it creates the potential for more complications in surgery, she said. Endometrial hyperplasia is very sensitive to hormone therapy, specifically progestin agents, with 75%-90% response rates with up-front treatment, Dr. Fader added.
But Dr. David Cohn, director of the division of gynecologic oncology at the Ohio State University, Columbus, said in an interview that surgery remains the standard of care because it is curative. Hormone treatment is appropriate in selected patients, but it is currently understudied and questions remain about the duration of treatment and about the type of hormones to use, he said.
Dr. Cohn and Dr. Fader both reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mschneider@frontlinemedcom.com
On Twitter @maryellenny
*Correction, 5/17/2016: An earlier version of this story misstated Dr. Fader's title.
EXPERT ANALYSIS FROM ACOG 2016
Primary small cell cancer of the anus rare, but devastating
LOS ANGELES – Primary small cell cancer of the anus is a rare but devastating condition and overall survival may not be improved with surgical treatment.
Those are key findings from what is believed to be the largest analysis of its kind to date.
“There are very limited data for patients with anal small cell cancers who need preoperative counseling and risk stratification,” study author Dr. Cornelius A. Thiels said in an interview at the annual meeting of the American Society of Colon and Rectal Surgeons. “There are also no data to guide treatment, so, until now, management was based on the treatment of small cell of the lung, and other anal cancers.”
Cancers of the anal canal are estimated to represent about 2.5% of all gastrointestinal neoplasms, while primary small cell cancer of the anus is believed to account for less than 1% of all anal neoplasms, according to Dr. Thiels, who is a third-year general surgery resident in the department of surgery and a surgical outcomes fellow in the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery at the Mayo Clinic, Rochester, Minn.
In an effort to evaluate the outcomes of patients with primary small cell cancer of the anus, the researchers reviewed their own institutional experience in treating nine patients with this condition between from 1994-2014, as well as National Cancer Data Base (NCDB) records of 174 patients from 1998-2014. The NCDB is maintained by collecting data prospectively from more than 1,500 facilities across the United States and is estimated to capture approximately 70% of all newly diagnosed cases of cancer annually. Institutional data allowed the researchers to identify details, including how these patients presented and what type of chemotherapy they received. However, analysis of a national database was necessary given the rarity of the diagnosis.
In the analysis of NCDB records, the mean patient age was 59 years and 74% were female. Most of the tumors (95%) were high grade and the majority of patients presented with advanced disease (50 with stage IV disease, 49 with stage III disease, 29 with stage II disease, 25 with stage I disease, and 21 with unknown stage). Overall survival was 66% at 12 months and 29% at 36 months. Among patients with stage I-III disease, survival was 72% at 12 months and 39% at 36 months.
Of the 103 patients with stage I-III disease, 95% received medical therapy, 70% underwent medical management alone, while 30% underwent surgery with curative intent. Patients who did not undergo surgery tended to have a higher stage of disease, compared with those who did (57% vs. 26%: P = .005). Overall survival at 36 months was similar between the two groups (33.9% in the surgery group vs. 35.8% in the no surgery group; P = .87).
“Unfortunately, it seems from our own experience and from national data that additional research is needed to determine how best to treat these patients and that surgery may not prolong survival in many of these patients,” Dr. Thiels said. “Although additional research is needed to optimize outcomes for these patients, harnessing the power of a national cancer database like the NCDB allows us to improve our understanding of these otherwise extremely rare, and difficult to study, tumors.”
Dr. Thiels reported having no financial disclosures.
LOS ANGELES – Primary small cell cancer of the anus is a rare but devastating condition and overall survival may not be improved with surgical treatment.
Those are key findings from what is believed to be the largest analysis of its kind to date.
“There are very limited data for patients with anal small cell cancers who need preoperative counseling and risk stratification,” study author Dr. Cornelius A. Thiels said in an interview at the annual meeting of the American Society of Colon and Rectal Surgeons. “There are also no data to guide treatment, so, until now, management was based on the treatment of small cell of the lung, and other anal cancers.”
Cancers of the anal canal are estimated to represent about 2.5% of all gastrointestinal neoplasms, while primary small cell cancer of the anus is believed to account for less than 1% of all anal neoplasms, according to Dr. Thiels, who is a third-year general surgery resident in the department of surgery and a surgical outcomes fellow in the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery at the Mayo Clinic, Rochester, Minn.
In an effort to evaluate the outcomes of patients with primary small cell cancer of the anus, the researchers reviewed their own institutional experience in treating nine patients with this condition between from 1994-2014, as well as National Cancer Data Base (NCDB) records of 174 patients from 1998-2014. The NCDB is maintained by collecting data prospectively from more than 1,500 facilities across the United States and is estimated to capture approximately 70% of all newly diagnosed cases of cancer annually. Institutional data allowed the researchers to identify details, including how these patients presented and what type of chemotherapy they received. However, analysis of a national database was necessary given the rarity of the diagnosis.
In the analysis of NCDB records, the mean patient age was 59 years and 74% were female. Most of the tumors (95%) were high grade and the majority of patients presented with advanced disease (50 with stage IV disease, 49 with stage III disease, 29 with stage II disease, 25 with stage I disease, and 21 with unknown stage). Overall survival was 66% at 12 months and 29% at 36 months. Among patients with stage I-III disease, survival was 72% at 12 months and 39% at 36 months.
Of the 103 patients with stage I-III disease, 95% received medical therapy, 70% underwent medical management alone, while 30% underwent surgery with curative intent. Patients who did not undergo surgery tended to have a higher stage of disease, compared with those who did (57% vs. 26%: P = .005). Overall survival at 36 months was similar between the two groups (33.9% in the surgery group vs. 35.8% in the no surgery group; P = .87).
“Unfortunately, it seems from our own experience and from national data that additional research is needed to determine how best to treat these patients and that surgery may not prolong survival in many of these patients,” Dr. Thiels said. “Although additional research is needed to optimize outcomes for these patients, harnessing the power of a national cancer database like the NCDB allows us to improve our understanding of these otherwise extremely rare, and difficult to study, tumors.”
Dr. Thiels reported having no financial disclosures.
LOS ANGELES – Primary small cell cancer of the anus is a rare but devastating condition and overall survival may not be improved with surgical treatment.
Those are key findings from what is believed to be the largest analysis of its kind to date.
“There are very limited data for patients with anal small cell cancers who need preoperative counseling and risk stratification,” study author Dr. Cornelius A. Thiels said in an interview at the annual meeting of the American Society of Colon and Rectal Surgeons. “There are also no data to guide treatment, so, until now, management was based on the treatment of small cell of the lung, and other anal cancers.”
Cancers of the anal canal are estimated to represent about 2.5% of all gastrointestinal neoplasms, while primary small cell cancer of the anus is believed to account for less than 1% of all anal neoplasms, according to Dr. Thiels, who is a third-year general surgery resident in the department of surgery and a surgical outcomes fellow in the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery at the Mayo Clinic, Rochester, Minn.
In an effort to evaluate the outcomes of patients with primary small cell cancer of the anus, the researchers reviewed their own institutional experience in treating nine patients with this condition between from 1994-2014, as well as National Cancer Data Base (NCDB) records of 174 patients from 1998-2014. The NCDB is maintained by collecting data prospectively from more than 1,500 facilities across the United States and is estimated to capture approximately 70% of all newly diagnosed cases of cancer annually. Institutional data allowed the researchers to identify details, including how these patients presented and what type of chemotherapy they received. However, analysis of a national database was necessary given the rarity of the diagnosis.
In the analysis of NCDB records, the mean patient age was 59 years and 74% were female. Most of the tumors (95%) were high grade and the majority of patients presented with advanced disease (50 with stage IV disease, 49 with stage III disease, 29 with stage II disease, 25 with stage I disease, and 21 with unknown stage). Overall survival was 66% at 12 months and 29% at 36 months. Among patients with stage I-III disease, survival was 72% at 12 months and 39% at 36 months.
Of the 103 patients with stage I-III disease, 95% received medical therapy, 70% underwent medical management alone, while 30% underwent surgery with curative intent. Patients who did not undergo surgery tended to have a higher stage of disease, compared with those who did (57% vs. 26%: P = .005). Overall survival at 36 months was similar between the two groups (33.9% in the surgery group vs. 35.8% in the no surgery group; P = .87).
“Unfortunately, it seems from our own experience and from national data that additional research is needed to determine how best to treat these patients and that surgery may not prolong survival in many of these patients,” Dr. Thiels said. “Although additional research is needed to optimize outcomes for these patients, harnessing the power of a national cancer database like the NCDB allows us to improve our understanding of these otherwise extremely rare, and difficult to study, tumors.”
Dr. Thiels reported having no financial disclosures.
AT THE ASCRS ANNUAL MEETING
Key clinical point: Among patients with primary small cell cancer of the anus, survival was 29% at 36 months.
Major finding: Overall survival among patients with primary small cell cancer of the anus was 66% at 12 months and 29% at 36 months.
Data source: A review of National Cancer Data Base records from 174 patients with primary cell cancer of the anus who were treated from 1998-2014.
Disclosures: Dr. Thiels reported having no financial disclosures.