User login
Malpractice: Diagnostic errors top allegation involving children
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.
Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
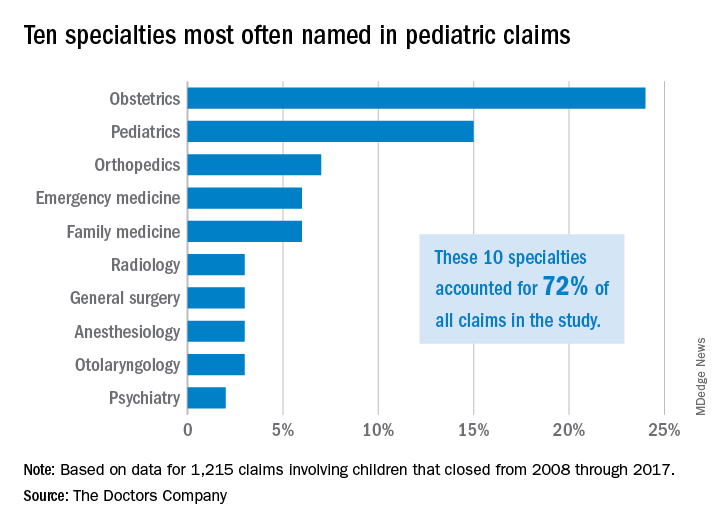
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.

Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
Diagnostic error is the most common allegation against pediatricians when sued by patients and their families, a study finds.

Investigators with The Doctors Company, a national medical liability insurer, examined 1,215 closed claims involving children from the company’s database between 2008 and 2017. Results showed that diagnostic mistakes, including delayed diagnosis, incorrect diagnosis, and failure to diagnose, were the most common accusations among claims that involved children ages 1 through 17. Poor medical treatment was the second most common allegation for claims that involved children aged 1-9, while surgical treatment-related error was the second most frequent accusation for children ages 10-17.
Pediatricians, orthopedic surgeons, and emergency medicine physicians were the most frequently named specialists in claims associated with children older than 1 month. Obstetricians were most frequently defendants in claims involving neonates. For these cases, errors during labor and delivery care were the most common complaints.
Of the 1,215 claims, obstetricians were named in 24% of the cases and pediatricians were named in 15% of the cases. The majority of claims were filed against physicians in the first 3 years following the medical incident alleged, according to the study, published by The Doctors Company.
The average patient payment in each case was $630,456, and the average expense to defend each claim was $157,502, according to the analysis. Claims that involved neonates had the highest average payment ($936,843) and the highest defense costs ($187,117), while claims involving children aged 10-17 years had the lowest average payment ($386,849) and cost the least to defend ($129,816).
For cases involving neonates, the type of therapy selected during labor and delivery and how it was managed were the most common factors contributing to the alleged injury, according to the analysis.
The most frequent factors contributing to patient harm for other age groups involved patient assessment issues and communication problems between the patient/family and the physician. Inadequate patient assessments were closely linked to incorrect diagnoses, while incomplete communication between patients/family members and providers impacted clinicians’ ability to make correct diagnoses, according to the study.
This analysis “shows that pediatric malpractice lawsuits impact nearly every area of medicine,” William F. Getman, MD, a pediatrician in Austin, Tex., said in an interview. “I was surprised to see that the most common age of a patient in a malpractice lawsuit was less than 1 month old. This age group also sustained the most severe injuries and had the highest indemnity paid.”
The study offers several key takeaways, including the importance of identifying system weaknesses in your medical practice and evaluating if improvements are needed, according to Darrell Ranum, vice president for patient safety and risk management for The Doctors Company.
Simple improvements, such as implementing tracking mechanisms for test results and referrals, can reduce the chance that important information falls through the cracks and delays diagnosis or treatment, Mr. Ranum said in an interview.
“When parents raise questions about their child’s complaints, this is the best opportunity to identify illnesses and conditions that represent a serious threat to children,” he said. “Prepare office staff members to know what complaints need to be evaluated by a clinician or require immediate care.”
In addition, the study findings point to the need to improve communication in all areas of the practice spectrum, Dr. Getman said.
“Many of the lawsuits could have been avoided by improvements in communication – doctor to patient, patient to doctor, doctor to nurse, doctor to doctor, nurse to patient, etc.,” he said. “Finding more effective and accurate ways to communicate will avoid mistakes, improve care, and improve outcomes. Examples of ways to improve communication include use of an interpreter when indicated, verbal and written explanations of instructions, and system improvements in tracking messages/labs/data. There are innumerable other ways to improve communication in health care.”
SOURCE: Ranum, D. The Doctor’s Advocate. First Quarter 2019.
FDA orders companies to cease all sales of transvaginal mesh for POP repair
The mandate came after Boston Scientific and Coloplast failed to provide adequate safety and efficacy information to the federal regulatory body in the wake of a 2016 reclassification to Class III (high-risk) devices, according to an FDA press statement. Both companies were required to submit a premarket approval application to continue marketing the mesh in the United States. Boston Scientific did file two PMAs, one for each of its transvaginal mesh products, but the FDA said the applications did not contain the required efficacy and safety data.
Both companies will have 10 days to submit their plan to withdraw these products from the market.
“In order for these mesh devices to stay on the market, we determined that we needed evidence that they worked better than surgery without the use of mesh to repair POP. That evidence was lacking in these premarket applications, and we couldn’t assure women that these devices were safe and effective long term,” said Jeffrey Shuren, MD, director of FDA’s Center for Devices and Radiological Health. “Patient safety is our highest priority, and women must have access to safe medical devices that provide relief from symptoms and better management of their medical conditions. The FDA has committed to taking forceful new actions to enhance device safety and encourage innovations that lead to safer medical devices, so that patients have access to safe and effective medical devices and the information they need to make informed decisions about their care.”
The deadline for submitting premarket approval applications for POP repair with transvaginal mesh was July 5, 2018. Manufacturers that did not file PMAs were required to pull their devices from the market. Those that did could keep selling the mesh while FDA reviewed their PMAs.
Boston Scientific submitted PMAs for its two devices, the Uphold LITE Vaginal Support System and the Xenform Soft Tissue Repair System. Coloplast filed a PMA for its device, Restorelle DirectFix Anterior. But in February, the FDA convened an advisory panel to discuss just how to evaluate the safety and efficacy of the products.
To prove efficacy, the panel concluded, transvaginal POP repair with mesh should be better than repair with native tissue at 36 months, and the safety should be superior to repair with native tissue repair. The FDA agreed. However, the submitted premarket approval application did not include these kinds of data. Therefore, the agency declined to approve the devices.
In addition to stopping U.S. sales, FDA has required Boston Scientific and Coloplast to continue safety and efficacy follow-up of all women included in their 522 studies.
Coloplast did not have a press or public statement on its website as of April 16. Boston Scientific did have one.
“Up to 50% of women in the U.S. will suffer from POP during their lives, and we believe these women should have access to safe and effective treatment options,” according to the statement. “As a global leader in the pelvic floor space, we remain steadfast in our commitment to helping women live better and healthier lives. We also remain confident in the benefits and safety of our treatments for POP, and we look forward to continuing to work with the FDA on our PMAs for the Uphold LITE Vaginal Support System and the Xenform Soft Tissue Repair Matrix, which are currently under review.”
The FDA statement also included advice to women who have had the mesh procedure for POP, and for their physicians
“Women who have had transvaginal mesh placed for the surgical repair of POP should continue with their annual and other routine check-ups and follow-up care. There is no need to take additional action if they are satisfied with their surgery and are not having complications or symptoms. Patients should notify their health care professionals if they have complications or symptoms, including persistent vaginal bleeding or discharge, pelvic or groin pain, or pain with sex. They should also let their health care professional know if they have surgical mesh, especially if they plan to have another surgery or other medical procedures. Women who were planning to have mesh placed transvaginally for the repair of POP should discuss other treatment options with their doctors.”
The Food and Drug Administration’s decision ordering manufacturers to remove mesh for transvaginal repair of prolapse from the market was based on the products’ effectiveness and safety profile, compared with vaginal native tissue repairs. Previous studies have shown that polypropylene mesh for anterior repair had similar or slightly higher success, compared with native tissue repairs. This was not a sufficient benefit considering the potential adverse events that include mesh exposure, and the pelvic pain and dyspareunia associated with using these products. There is no additional benefit of using polypropylene mesh in the posterior compartment.
It would be interesting to review the information provided by manufacturers as part of the premarket approval. What were the primary endpoints for efficacy that were used? What were the rates of complications for mesh exposure, pelvic pain, and dyspareunia? How did the rates of pelvic pain and dyspareunia compare with native tissue repair.
Gynecologic surgeons still have a number of options for treating vaginal prolapse, which include vaginal native tissue repairs, and laparoscopic and abdominal surgeries that involve native tissue or polypropylene mesh. It will be interesting to see how the FDA’s Medical Device Safety action plan will affect future innovations for treating vaginal prolapse, while at the same time providing women and their physicians with products that are safe and effective.
Jose S. Maceda, MD, is a urogynecologist at Axia Women’s Health in King of Prussia, Penn. Dr. Maceda, who was asked to comment on the FDA decision, has no relevant financial disclosures.
The Food and Drug Administration’s decision ordering manufacturers to remove mesh for transvaginal repair of prolapse from the market was based on the products’ effectiveness and safety profile, compared with vaginal native tissue repairs. Previous studies have shown that polypropylene mesh for anterior repair had similar or slightly higher success, compared with native tissue repairs. This was not a sufficient benefit considering the potential adverse events that include mesh exposure, and the pelvic pain and dyspareunia associated with using these products. There is no additional benefit of using polypropylene mesh in the posterior compartment.
It would be interesting to review the information provided by manufacturers as part of the premarket approval. What were the primary endpoints for efficacy that were used? What were the rates of complications for mesh exposure, pelvic pain, and dyspareunia? How did the rates of pelvic pain and dyspareunia compare with native tissue repair.
Gynecologic surgeons still have a number of options for treating vaginal prolapse, which include vaginal native tissue repairs, and laparoscopic and abdominal surgeries that involve native tissue or polypropylene mesh. It will be interesting to see how the FDA’s Medical Device Safety action plan will affect future innovations for treating vaginal prolapse, while at the same time providing women and their physicians with products that are safe and effective.
Jose S. Maceda, MD, is a urogynecologist at Axia Women’s Health in King of Prussia, Penn. Dr. Maceda, who was asked to comment on the FDA decision, has no relevant financial disclosures.
The Food and Drug Administration’s decision ordering manufacturers to remove mesh for transvaginal repair of prolapse from the market was based on the products’ effectiveness and safety profile, compared with vaginal native tissue repairs. Previous studies have shown that polypropylene mesh for anterior repair had similar or slightly higher success, compared with native tissue repairs. This was not a sufficient benefit considering the potential adverse events that include mesh exposure, and the pelvic pain and dyspareunia associated with using these products. There is no additional benefit of using polypropylene mesh in the posterior compartment.
It would be interesting to review the information provided by manufacturers as part of the premarket approval. What were the primary endpoints for efficacy that were used? What were the rates of complications for mesh exposure, pelvic pain, and dyspareunia? How did the rates of pelvic pain and dyspareunia compare with native tissue repair.
Gynecologic surgeons still have a number of options for treating vaginal prolapse, which include vaginal native tissue repairs, and laparoscopic and abdominal surgeries that involve native tissue or polypropylene mesh. It will be interesting to see how the FDA’s Medical Device Safety action plan will affect future innovations for treating vaginal prolapse, while at the same time providing women and their physicians with products that are safe and effective.
Jose S. Maceda, MD, is a urogynecologist at Axia Women’s Health in King of Prussia, Penn. Dr. Maceda, who was asked to comment on the FDA decision, has no relevant financial disclosures.
The mandate came after Boston Scientific and Coloplast failed to provide adequate safety and efficacy information to the federal regulatory body in the wake of a 2016 reclassification to Class III (high-risk) devices, according to an FDA press statement. Both companies were required to submit a premarket approval application to continue marketing the mesh in the United States. Boston Scientific did file two PMAs, one for each of its transvaginal mesh products, but the FDA said the applications did not contain the required efficacy and safety data.
Both companies will have 10 days to submit their plan to withdraw these products from the market.
“In order for these mesh devices to stay on the market, we determined that we needed evidence that they worked better than surgery without the use of mesh to repair POP. That evidence was lacking in these premarket applications, and we couldn’t assure women that these devices were safe and effective long term,” said Jeffrey Shuren, MD, director of FDA’s Center for Devices and Radiological Health. “Patient safety is our highest priority, and women must have access to safe medical devices that provide relief from symptoms and better management of their medical conditions. The FDA has committed to taking forceful new actions to enhance device safety and encourage innovations that lead to safer medical devices, so that patients have access to safe and effective medical devices and the information they need to make informed decisions about their care.”
The deadline for submitting premarket approval applications for POP repair with transvaginal mesh was July 5, 2018. Manufacturers that did not file PMAs were required to pull their devices from the market. Those that did could keep selling the mesh while FDA reviewed their PMAs.
Boston Scientific submitted PMAs for its two devices, the Uphold LITE Vaginal Support System and the Xenform Soft Tissue Repair System. Coloplast filed a PMA for its device, Restorelle DirectFix Anterior. But in February, the FDA convened an advisory panel to discuss just how to evaluate the safety and efficacy of the products.
To prove efficacy, the panel concluded, transvaginal POP repair with mesh should be better than repair with native tissue at 36 months, and the safety should be superior to repair with native tissue repair. The FDA agreed. However, the submitted premarket approval application did not include these kinds of data. Therefore, the agency declined to approve the devices.
In addition to stopping U.S. sales, FDA has required Boston Scientific and Coloplast to continue safety and efficacy follow-up of all women included in their 522 studies.
Coloplast did not have a press or public statement on its website as of April 16. Boston Scientific did have one.
“Up to 50% of women in the U.S. will suffer from POP during their lives, and we believe these women should have access to safe and effective treatment options,” according to the statement. “As a global leader in the pelvic floor space, we remain steadfast in our commitment to helping women live better and healthier lives. We also remain confident in the benefits and safety of our treatments for POP, and we look forward to continuing to work with the FDA on our PMAs for the Uphold LITE Vaginal Support System and the Xenform Soft Tissue Repair Matrix, which are currently under review.”
The FDA statement also included advice to women who have had the mesh procedure for POP, and for their physicians
“Women who have had transvaginal mesh placed for the surgical repair of POP should continue with their annual and other routine check-ups and follow-up care. There is no need to take additional action if they are satisfied with their surgery and are not having complications or symptoms. Patients should notify their health care professionals if they have complications or symptoms, including persistent vaginal bleeding or discharge, pelvic or groin pain, or pain with sex. They should also let their health care professional know if they have surgical mesh, especially if they plan to have another surgery or other medical procedures. Women who were planning to have mesh placed transvaginally for the repair of POP should discuss other treatment options with their doctors.”
The mandate came after Boston Scientific and Coloplast failed to provide adequate safety and efficacy information to the federal regulatory body in the wake of a 2016 reclassification to Class III (high-risk) devices, according to an FDA press statement. Both companies were required to submit a premarket approval application to continue marketing the mesh in the United States. Boston Scientific did file two PMAs, one for each of its transvaginal mesh products, but the FDA said the applications did not contain the required efficacy and safety data.
Both companies will have 10 days to submit their plan to withdraw these products from the market.
“In order for these mesh devices to stay on the market, we determined that we needed evidence that they worked better than surgery without the use of mesh to repair POP. That evidence was lacking in these premarket applications, and we couldn’t assure women that these devices were safe and effective long term,” said Jeffrey Shuren, MD, director of FDA’s Center for Devices and Radiological Health. “Patient safety is our highest priority, and women must have access to safe medical devices that provide relief from symptoms and better management of their medical conditions. The FDA has committed to taking forceful new actions to enhance device safety and encourage innovations that lead to safer medical devices, so that patients have access to safe and effective medical devices and the information they need to make informed decisions about their care.”
The deadline for submitting premarket approval applications for POP repair with transvaginal mesh was July 5, 2018. Manufacturers that did not file PMAs were required to pull their devices from the market. Those that did could keep selling the mesh while FDA reviewed their PMAs.
Boston Scientific submitted PMAs for its two devices, the Uphold LITE Vaginal Support System and the Xenform Soft Tissue Repair System. Coloplast filed a PMA for its device, Restorelle DirectFix Anterior. But in February, the FDA convened an advisory panel to discuss just how to evaluate the safety and efficacy of the products.
To prove efficacy, the panel concluded, transvaginal POP repair with mesh should be better than repair with native tissue at 36 months, and the safety should be superior to repair with native tissue repair. The FDA agreed. However, the submitted premarket approval application did not include these kinds of data. Therefore, the agency declined to approve the devices.
In addition to stopping U.S. sales, FDA has required Boston Scientific and Coloplast to continue safety and efficacy follow-up of all women included in their 522 studies.
Coloplast did not have a press or public statement on its website as of April 16. Boston Scientific did have one.
“Up to 50% of women in the U.S. will suffer from POP during their lives, and we believe these women should have access to safe and effective treatment options,” according to the statement. “As a global leader in the pelvic floor space, we remain steadfast in our commitment to helping women live better and healthier lives. We also remain confident in the benefits and safety of our treatments for POP, and we look forward to continuing to work with the FDA on our PMAs for the Uphold LITE Vaginal Support System and the Xenform Soft Tissue Repair Matrix, which are currently under review.”
The FDA statement also included advice to women who have had the mesh procedure for POP, and for their physicians
“Women who have had transvaginal mesh placed for the surgical repair of POP should continue with their annual and other routine check-ups and follow-up care. There is no need to take additional action if they are satisfied with their surgery and are not having complications or symptoms. Patients should notify their health care professionals if they have complications or symptoms, including persistent vaginal bleeding or discharge, pelvic or groin pain, or pain with sex. They should also let their health care professional know if they have surgical mesh, especially if they plan to have another surgery or other medical procedures. Women who were planning to have mesh placed transvaginally for the repair of POP should discuss other treatment options with their doctors.”
Enhanced recovery also enhances unplanned patient contact
TUCSON, ARIZ. – A retrospective study at the Mayo Clinic Arizona found a near doubling in the percentage of patients who had contact with the medical system in the 2 weeks following surgery.
“That’s a big change and a burden for the clinician, so they need to anticipate that,” Rachael Haverland, MD, a fellow at Mayo Clinic Arizona, Phoenix, said in an interview at the annual scientific meeting of the Society of Gynecologic Surgeons.
“A lot of research has gone into the safety profile of ERAS, the cost-effectiveness of ERAS, and the effects on the patient, but there has not been a lot of research on how it affects the clinician’s practice. I think it’s very important for physicians to know that so that they can plan ahead, maybe add more clinical staff to help with some of these phone calls, and maybe setting aside special clinic time for unscheduled visits to address some of these patient concerns,” she added.
The most common issues revolve around pain management – how to take medications and how to manage pain in the context of few restrictions – suggesting that preoperative counseling could help. “Setting expectations for pain, going through their medication regimen after surgery so they know what medications they can take, how to control their pain, and their restrictions. We don’t have many restrictions. We want them walking even the same day, and there are no dietary restrictions. Just reiterating some of those facts, because it’s still new, especially to patients. They’re not used to having limited restrictions,” Dr. Haverland said.
Other patient concerns included dysuria and frequency of urination following surgery. The least common questions were related to activity restrictions, according to Dr. Haverland.
The researchers are developing a preoperative video for ERAS that they hope will improve matters. It aims to anticipate patient questions before and after surgery, and they plan to track its impact on clinician burden. “I’m hoping that when we do our next set of data that it cuts down on some of those unscheduled patient hours,” she said.
The researchers examined data from 200 hysterectomy patients. A total of 90 underwent surgery in 2012, before ERAS was implemented, and 110 in 2014, 1 year after ERAS was begun. They looked at patient phone calls, ED visits, and unscheduled postoperative visits.
Before ERAS, in the 2 weeks after surgery, 42.2% of patients had any medical care. That rose to 74.5% after ERAS. The difference seemed to be driven by phone calls, which rose from 38.9% before ERAS to 68.2% after (P less than .0001). There also was a trend toward more in-person visits (12.2% vs. 21.8%; odds ratio, 2.00; P = .08) and unscheduled office visits (10.0% vs. 18.2%; P = .01).
Patients undergoing a concomitant sling procedure were more likely to seek in-person medical care within 2 weeks regardless of ERAS protocol (OR, 3.16; P = .04). The researchers found no significant differences in readmission rates, operative time, blood loss, or ED visits.
The study received no funding. Dr. Haverland reported no relevant financial disclosures.
SOURCE: Haverland R et al. SGS 2019, Oral Poster 05.
TUCSON, ARIZ. – A retrospective study at the Mayo Clinic Arizona found a near doubling in the percentage of patients who had contact with the medical system in the 2 weeks following surgery.
“That’s a big change and a burden for the clinician, so they need to anticipate that,” Rachael Haverland, MD, a fellow at Mayo Clinic Arizona, Phoenix, said in an interview at the annual scientific meeting of the Society of Gynecologic Surgeons.
“A lot of research has gone into the safety profile of ERAS, the cost-effectiveness of ERAS, and the effects on the patient, but there has not been a lot of research on how it affects the clinician’s practice. I think it’s very important for physicians to know that so that they can plan ahead, maybe add more clinical staff to help with some of these phone calls, and maybe setting aside special clinic time for unscheduled visits to address some of these patient concerns,” she added.
The most common issues revolve around pain management – how to take medications and how to manage pain in the context of few restrictions – suggesting that preoperative counseling could help. “Setting expectations for pain, going through their medication regimen after surgery so they know what medications they can take, how to control their pain, and their restrictions. We don’t have many restrictions. We want them walking even the same day, and there are no dietary restrictions. Just reiterating some of those facts, because it’s still new, especially to patients. They’re not used to having limited restrictions,” Dr. Haverland said.
Other patient concerns included dysuria and frequency of urination following surgery. The least common questions were related to activity restrictions, according to Dr. Haverland.
The researchers are developing a preoperative video for ERAS that they hope will improve matters. It aims to anticipate patient questions before and after surgery, and they plan to track its impact on clinician burden. “I’m hoping that when we do our next set of data that it cuts down on some of those unscheduled patient hours,” she said.
The researchers examined data from 200 hysterectomy patients. A total of 90 underwent surgery in 2012, before ERAS was implemented, and 110 in 2014, 1 year after ERAS was begun. They looked at patient phone calls, ED visits, and unscheduled postoperative visits.
Before ERAS, in the 2 weeks after surgery, 42.2% of patients had any medical care. That rose to 74.5% after ERAS. The difference seemed to be driven by phone calls, which rose from 38.9% before ERAS to 68.2% after (P less than .0001). There also was a trend toward more in-person visits (12.2% vs. 21.8%; odds ratio, 2.00; P = .08) and unscheduled office visits (10.0% vs. 18.2%; P = .01).
Patients undergoing a concomitant sling procedure were more likely to seek in-person medical care within 2 weeks regardless of ERAS protocol (OR, 3.16; P = .04). The researchers found no significant differences in readmission rates, operative time, blood loss, or ED visits.
The study received no funding. Dr. Haverland reported no relevant financial disclosures.
SOURCE: Haverland R et al. SGS 2019, Oral Poster 05.
TUCSON, ARIZ. – A retrospective study at the Mayo Clinic Arizona found a near doubling in the percentage of patients who had contact with the medical system in the 2 weeks following surgery.
“That’s a big change and a burden for the clinician, so they need to anticipate that,” Rachael Haverland, MD, a fellow at Mayo Clinic Arizona, Phoenix, said in an interview at the annual scientific meeting of the Society of Gynecologic Surgeons.
“A lot of research has gone into the safety profile of ERAS, the cost-effectiveness of ERAS, and the effects on the patient, but there has not been a lot of research on how it affects the clinician’s practice. I think it’s very important for physicians to know that so that they can plan ahead, maybe add more clinical staff to help with some of these phone calls, and maybe setting aside special clinic time for unscheduled visits to address some of these patient concerns,” she added.
The most common issues revolve around pain management – how to take medications and how to manage pain in the context of few restrictions – suggesting that preoperative counseling could help. “Setting expectations for pain, going through their medication regimen after surgery so they know what medications they can take, how to control their pain, and their restrictions. We don’t have many restrictions. We want them walking even the same day, and there are no dietary restrictions. Just reiterating some of those facts, because it’s still new, especially to patients. They’re not used to having limited restrictions,” Dr. Haverland said.
Other patient concerns included dysuria and frequency of urination following surgery. The least common questions were related to activity restrictions, according to Dr. Haverland.
The researchers are developing a preoperative video for ERAS that they hope will improve matters. It aims to anticipate patient questions before and after surgery, and they plan to track its impact on clinician burden. “I’m hoping that when we do our next set of data that it cuts down on some of those unscheduled patient hours,” she said.
The researchers examined data from 200 hysterectomy patients. A total of 90 underwent surgery in 2012, before ERAS was implemented, and 110 in 2014, 1 year after ERAS was begun. They looked at patient phone calls, ED visits, and unscheduled postoperative visits.
Before ERAS, in the 2 weeks after surgery, 42.2% of patients had any medical care. That rose to 74.5% after ERAS. The difference seemed to be driven by phone calls, which rose from 38.9% before ERAS to 68.2% after (P less than .0001). There also was a trend toward more in-person visits (12.2% vs. 21.8%; odds ratio, 2.00; P = .08) and unscheduled office visits (10.0% vs. 18.2%; P = .01).
Patients undergoing a concomitant sling procedure were more likely to seek in-person medical care within 2 weeks regardless of ERAS protocol (OR, 3.16; P = .04). The researchers found no significant differences in readmission rates, operative time, blood loss, or ED visits.
The study received no funding. Dr. Haverland reported no relevant financial disclosures.
SOURCE: Haverland R et al. SGS 2019, Oral Poster 05.
REPORTING FROM SGS 2019
Anxiety can impact patient satisfaction after GERD surgery
BALTIMORE – according to a study from the Ohio State University presented at the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons.
Carla Holcomb, MD, a minimally invasive surgery/bariatric fellow at the Ohio State’s Wexner Medical Center, Columbus, reported the upshot of the study findings. “Preoperative counseling is especially important regarding postoperative expectations in patients with anxiety,” she said.
The retrospective study evaluated 271 patients who had laparoscopic Nissen fundoplication (LNF) during 2011-2016 at the medical center, comparing outcomes in patients who were on serotonin-modulating medication for depression (n = 103), benzodiazepines for anxiety (n = 44), or neither (n = 124). The researchers evaluated a number of metrics – DeMeester score of esophageal acid exposure, pre- and postoperative health-related quality of life, and postoperative antacid use and need for endoscopic dilation – across all cohorts. While some scores among the anxiety cohort trended higher (DeMeester score of 43 vs. 38 for the no-anxiety patients) they were not statistically significant, Dr. Holcomb noted. Patients taking antidepressants reported similar subjective outcomes and satisfaction rates to those not taking antidepressants.
However, when patients were queried about their overall satisfaction after laparoscopic Nissen fundoplication 77%-87% in the no-depression, depression, and no-anxiety groups reported they were satisfied, while only 37% of those in the anxiety group did so. That is based on a response rate of 53% to a telephone inquiry 15 months after LNF.
“The patients who had anxiety looked vastly different from the rest of the population,” said Dr. Holcomb. “Patients taking antidepressants reported similar objective outcomes and high satisfaction rates, [compared with] patients not taking antidepressants after LNF, and although LNF does improve gastroesophageal reflux disease symptoms in patients taking anxiolytics, they rarely achieve satisfaction in long-term follow-up.”
Among the study limitations Dr. Holcomb acknowledged were the 53% long-term response rate and not knowing if an anatomical reason may explain the higher health-related quality of life scores in the anxiety group – 7 vs. 4 in the no-anxiety group – at long-term follow-up, although the overall score was low at 5.
Dr. Holcomb had no relevant financial disclosures.
SOURCE: Holcomb CN et al. SAGES 2019, Session SS04.
BALTIMORE – according to a study from the Ohio State University presented at the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons.
Carla Holcomb, MD, a minimally invasive surgery/bariatric fellow at the Ohio State’s Wexner Medical Center, Columbus, reported the upshot of the study findings. “Preoperative counseling is especially important regarding postoperative expectations in patients with anxiety,” she said.
The retrospective study evaluated 271 patients who had laparoscopic Nissen fundoplication (LNF) during 2011-2016 at the medical center, comparing outcomes in patients who were on serotonin-modulating medication for depression (n = 103), benzodiazepines for anxiety (n = 44), or neither (n = 124). The researchers evaluated a number of metrics – DeMeester score of esophageal acid exposure, pre- and postoperative health-related quality of life, and postoperative antacid use and need for endoscopic dilation – across all cohorts. While some scores among the anxiety cohort trended higher (DeMeester score of 43 vs. 38 for the no-anxiety patients) they were not statistically significant, Dr. Holcomb noted. Patients taking antidepressants reported similar subjective outcomes and satisfaction rates to those not taking antidepressants.
However, when patients were queried about their overall satisfaction after laparoscopic Nissen fundoplication 77%-87% in the no-depression, depression, and no-anxiety groups reported they were satisfied, while only 37% of those in the anxiety group did so. That is based on a response rate of 53% to a telephone inquiry 15 months after LNF.
“The patients who had anxiety looked vastly different from the rest of the population,” said Dr. Holcomb. “Patients taking antidepressants reported similar objective outcomes and high satisfaction rates, [compared with] patients not taking antidepressants after LNF, and although LNF does improve gastroesophageal reflux disease symptoms in patients taking anxiolytics, they rarely achieve satisfaction in long-term follow-up.”
Among the study limitations Dr. Holcomb acknowledged were the 53% long-term response rate and not knowing if an anatomical reason may explain the higher health-related quality of life scores in the anxiety group – 7 vs. 4 in the no-anxiety group – at long-term follow-up, although the overall score was low at 5.
Dr. Holcomb had no relevant financial disclosures.
SOURCE: Holcomb CN et al. SAGES 2019, Session SS04.
BALTIMORE – according to a study from the Ohio State University presented at the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons.
Carla Holcomb, MD, a minimally invasive surgery/bariatric fellow at the Ohio State’s Wexner Medical Center, Columbus, reported the upshot of the study findings. “Preoperative counseling is especially important regarding postoperative expectations in patients with anxiety,” she said.
The retrospective study evaluated 271 patients who had laparoscopic Nissen fundoplication (LNF) during 2011-2016 at the medical center, comparing outcomes in patients who were on serotonin-modulating medication for depression (n = 103), benzodiazepines for anxiety (n = 44), or neither (n = 124). The researchers evaluated a number of metrics – DeMeester score of esophageal acid exposure, pre- and postoperative health-related quality of life, and postoperative antacid use and need for endoscopic dilation – across all cohorts. While some scores among the anxiety cohort trended higher (DeMeester score of 43 vs. 38 for the no-anxiety patients) they were not statistically significant, Dr. Holcomb noted. Patients taking antidepressants reported similar subjective outcomes and satisfaction rates to those not taking antidepressants.
However, when patients were queried about their overall satisfaction after laparoscopic Nissen fundoplication 77%-87% in the no-depression, depression, and no-anxiety groups reported they were satisfied, while only 37% of those in the anxiety group did so. That is based on a response rate of 53% to a telephone inquiry 15 months after LNF.
“The patients who had anxiety looked vastly different from the rest of the population,” said Dr. Holcomb. “Patients taking antidepressants reported similar objective outcomes and high satisfaction rates, [compared with] patients not taking antidepressants after LNF, and although LNF does improve gastroesophageal reflux disease symptoms in patients taking anxiolytics, they rarely achieve satisfaction in long-term follow-up.”
Among the study limitations Dr. Holcomb acknowledged were the 53% long-term response rate and not knowing if an anatomical reason may explain the higher health-related quality of life scores in the anxiety group – 7 vs. 4 in the no-anxiety group – at long-term follow-up, although the overall score was low at 5.
Dr. Holcomb had no relevant financial disclosures.
SOURCE: Holcomb CN et al. SAGES 2019, Session SS04.
REPORTING FROM SAGES 2019
Key clinical point: Patients on anxiolytics for anxiety would benefit from counseling before laparoscopic Nissen fundoplication.
Major finding: Fewer than 40% of patients with anxiety reported satisfaction after LNF despite vast improvement in reflux symptoms.
Study details: Retrospective cohort, single-center study with a prospectively maintained database of 271 patients who had laparoscopic Nissen fundoplication during 2011-2016.
Disclosures: Dr. Holcomb had no financial relationships to disclose
Source: Holcomb CN et al. SAGES 2019, Session SS04.
Energy-based devices for vaginal rejuvenation described in FDA adverse event reports
The use of was implicated in nearly four dozen adverse event reports found in the agency’s medical device adverse event reporting database, researchers report.
The 45 unique event reports, submitted to the FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning, said the researchers, led by Jusleen Ahluwalia, MD, of the department of dermatology at the University of California, San Diego, and her coauthors. They included 31 that were reported by the patients, 8 reported by the manufacturer; 4 reported by the distributor, and 2 not specified.
These findings emphasize the need for clinical trials to evaluate the safety and efficacy of the lasers and radiofrequency devices that have been marketed and used for so-called vaginal rejuvenation procedures, they wrote in Lasers in Surgery and Medicine. The coauthors are Arisa Ortiz, MD, also with the University of California, San Diego, and Mathew M. Avram, MD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston. “Randomized studies are necessary to compare these therapies with standard modalities and to establish the safety of these devices,” they wrote.
In July 2018, the FDA issued a safety communication alerting patients and health care providers that the safety and effectiveness of energy-based devices has not been established for procedures described as “vaginal rejuvenation.” Scott Gottlieb, MD, FDA commissioner at the time, issued a statement decrying “deceptive health claims and significant risks” related to devices marketed for those medical procedures. In a November 2018 update, the FDA said they contacted some device manufacturers to express concerns that the devices were being marketed inappropriately and that manufacturers they had contacted so far “responded with adequate corrections.”
In their report, Dr. Ahluwalia and her associates noted that “vaginal rejuvenation” is an ill-defined term that may encompass a variety of procedures related to tightening; dyspareunia; dysuria; urinary incontinence; vulvar issues including irritation, dryness, and atrophy; and orgasmic dysfunction.
They found a total of 58 records in their review of the Manufacturer and User Facility Device Experience database, of which 25 were reported prior to the FDA’s July 2018 statement. Of 45 unique event descriptions found in those records, 39 were categorized as patient-related injuries, while 2 were operator-related injuries, 2 were device malfunctions, and 2 were not specified.
Pain was the most commonly adverse event, accounting for 19 reports in their analysis, while 11 patients reported numbness or burning.
Among the laser- and energy-based devices specifically described in the 39 patient-report injuries, the MonaLisa Touch had the highest number of adverse event reports (16), the data show. “However, this may be reflective of length of time bias as it is one of the first devices utilized to promote vaginal rejuvenation,” the authors pointed out.
In light of these findings, the authors advised clinicians to ask patients about their reasons for seeking vaginal rejuvenation procedures. “Normal variety of female genital appearances should also be reviewed when patients express cosmetic concerns,” they added. Concerns about related to genitourinary syndrome of menopause “or optimizing sexual function may be alleviated by exploring nonprocedural, conservative approaches, such as hormonal creams, if not contraindicated, and/or counseling,” they noted.
The authors provided conflict of interest disclosures related to Zalea, Inmode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Adverse events related to devices and drugs can be reported to the FDA’s Medwatch program.
SOURCE: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
The use of was implicated in nearly four dozen adverse event reports found in the agency’s medical device adverse event reporting database, researchers report.
The 45 unique event reports, submitted to the FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning, said the researchers, led by Jusleen Ahluwalia, MD, of the department of dermatology at the University of California, San Diego, and her coauthors. They included 31 that were reported by the patients, 8 reported by the manufacturer; 4 reported by the distributor, and 2 not specified.
These findings emphasize the need for clinical trials to evaluate the safety and efficacy of the lasers and radiofrequency devices that have been marketed and used for so-called vaginal rejuvenation procedures, they wrote in Lasers in Surgery and Medicine. The coauthors are Arisa Ortiz, MD, also with the University of California, San Diego, and Mathew M. Avram, MD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston. “Randomized studies are necessary to compare these therapies with standard modalities and to establish the safety of these devices,” they wrote.
In July 2018, the FDA issued a safety communication alerting patients and health care providers that the safety and effectiveness of energy-based devices has not been established for procedures described as “vaginal rejuvenation.” Scott Gottlieb, MD, FDA commissioner at the time, issued a statement decrying “deceptive health claims and significant risks” related to devices marketed for those medical procedures. In a November 2018 update, the FDA said they contacted some device manufacturers to express concerns that the devices were being marketed inappropriately and that manufacturers they had contacted so far “responded with adequate corrections.”
In their report, Dr. Ahluwalia and her associates noted that “vaginal rejuvenation” is an ill-defined term that may encompass a variety of procedures related to tightening; dyspareunia; dysuria; urinary incontinence; vulvar issues including irritation, dryness, and atrophy; and orgasmic dysfunction.
They found a total of 58 records in their review of the Manufacturer and User Facility Device Experience database, of which 25 were reported prior to the FDA’s July 2018 statement. Of 45 unique event descriptions found in those records, 39 were categorized as patient-related injuries, while 2 were operator-related injuries, 2 were device malfunctions, and 2 were not specified.
Pain was the most commonly adverse event, accounting for 19 reports in their analysis, while 11 patients reported numbness or burning.
Among the laser- and energy-based devices specifically described in the 39 patient-report injuries, the MonaLisa Touch had the highest number of adverse event reports (16), the data show. “However, this may be reflective of length of time bias as it is one of the first devices utilized to promote vaginal rejuvenation,” the authors pointed out.
In light of these findings, the authors advised clinicians to ask patients about their reasons for seeking vaginal rejuvenation procedures. “Normal variety of female genital appearances should also be reviewed when patients express cosmetic concerns,” they added. Concerns about related to genitourinary syndrome of menopause “or optimizing sexual function may be alleviated by exploring nonprocedural, conservative approaches, such as hormonal creams, if not contraindicated, and/or counseling,” they noted.
The authors provided conflict of interest disclosures related to Zalea, Inmode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Adverse events related to devices and drugs can be reported to the FDA’s Medwatch program.
SOURCE: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
The use of was implicated in nearly four dozen adverse event reports found in the agency’s medical device adverse event reporting database, researchers report.
The 45 unique event reports, submitted to the FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning, said the researchers, led by Jusleen Ahluwalia, MD, of the department of dermatology at the University of California, San Diego, and her coauthors. They included 31 that were reported by the patients, 8 reported by the manufacturer; 4 reported by the distributor, and 2 not specified.
These findings emphasize the need for clinical trials to evaluate the safety and efficacy of the lasers and radiofrequency devices that have been marketed and used for so-called vaginal rejuvenation procedures, they wrote in Lasers in Surgery and Medicine. The coauthors are Arisa Ortiz, MD, also with the University of California, San Diego, and Mathew M. Avram, MD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston. “Randomized studies are necessary to compare these therapies with standard modalities and to establish the safety of these devices,” they wrote.
In July 2018, the FDA issued a safety communication alerting patients and health care providers that the safety and effectiveness of energy-based devices has not been established for procedures described as “vaginal rejuvenation.” Scott Gottlieb, MD, FDA commissioner at the time, issued a statement decrying “deceptive health claims and significant risks” related to devices marketed for those medical procedures. In a November 2018 update, the FDA said they contacted some device manufacturers to express concerns that the devices were being marketed inappropriately and that manufacturers they had contacted so far “responded with adequate corrections.”
In their report, Dr. Ahluwalia and her associates noted that “vaginal rejuvenation” is an ill-defined term that may encompass a variety of procedures related to tightening; dyspareunia; dysuria; urinary incontinence; vulvar issues including irritation, dryness, and atrophy; and orgasmic dysfunction.
They found a total of 58 records in their review of the Manufacturer and User Facility Device Experience database, of which 25 were reported prior to the FDA’s July 2018 statement. Of 45 unique event descriptions found in those records, 39 were categorized as patient-related injuries, while 2 were operator-related injuries, 2 were device malfunctions, and 2 were not specified.
Pain was the most commonly adverse event, accounting for 19 reports in their analysis, while 11 patients reported numbness or burning.
Among the laser- and energy-based devices specifically described in the 39 patient-report injuries, the MonaLisa Touch had the highest number of adverse event reports (16), the data show. “However, this may be reflective of length of time bias as it is one of the first devices utilized to promote vaginal rejuvenation,” the authors pointed out.
In light of these findings, the authors advised clinicians to ask patients about their reasons for seeking vaginal rejuvenation procedures. “Normal variety of female genital appearances should also be reviewed when patients express cosmetic concerns,” they added. Concerns about related to genitourinary syndrome of menopause “or optimizing sexual function may be alleviated by exploring nonprocedural, conservative approaches, such as hormonal creams, if not contraindicated, and/or counseling,” they noted.
The authors provided conflict of interest disclosures related to Zalea, Inmode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Adverse events related to devices and drugs can be reported to the FDA’s Medwatch program.
SOURCE: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
FROM LASERS IN SURGERY AND MEDICINE
Key clinical point: Nearly four dozen distinct adverse event reports related to energy-based devices used for vaginal rejuvenation were found in an analysis of an FDA database.
Major finding: The 45 unique event reports, disclosed to FDA during October 2015–January 2019, described 46 patients in total, of whom 33 reported long-term effects including pain, numbness, and burning.
Study details: Cross-sectional analysis of records in the Manufacturer and User Facility Device Experience database entered during October 2015–January 2019.
Disclosures: Authors provided conflict of interest disclosures related to ZALEA, InMode, Cytrellis, Zeltiq Aesthetics, Soliton, Sciton, Allergan, and Sienna Biopharmaceuticals, among others.
Source: Ahluwalia J et al. Lasers Surg Med. 2019 Mar 29. doi: 10.1002/lsm.23084.
Furosemide speeds ureteral patency confirmation, but is time savings worth the risk?
TUCSON, ARIZ. – according to results from a new randomized, controlled trial.
“It does make a difference, but is that really a [meaningful] difference? Every medication has adverse effects, so is it worth that extra time [savings] to take on that potential for side effects? It highlights the importance of statistical significance versus clinical significance, and I think [the clinical significance] can just be answered by each individual physician,” Simon Patton, MD, said in an interview.
Dr. Patton is a urogynecologist at Ascension Via Christi Medical Group in Wichita, Kan. He presented the study, which was conducted during his time as a fellow at the University of South Florida, Tampa, at the annual scientific meeting of the Society of Gynecologic Surgeons. Dr. Patton isn’t sure just how often physicians use furosemide during routine cystoscopy. “It would be great to do a survey to find out how many people use it in their practice,” he said.
Cystoscopy is used to during a surgery to ensure that no injury has been done to the bladder or the urethra, and the American Urogynecological Society recommends that it be performed during any pelvic reconstructive surgery. A key element of the test is confirming that the ureters are open. By increasing urine flow, furosemide can reduce the time to confirmation. But after conferring with a colleague who used the procedure, Dr. Patton looked for some data to support the practice and couldn’t find any.
Although the cystoscopy itself generally is safe, furosemide can cause hypotension, change in renal function, and even dehydration at higher doses. During the question-and-answer period, one attendee noted these issues and pointed out that furosemide can potentiate renal failure, especially among patients taking cephalosporins. “If you’re going to do this sort of trial, you have to consider potential adverse events. A single dose is probably not going to [cause an issue], but in the context of a study you want to monitor the adverse events that have been reported,” this attendee said.
The researchers did not observe any of these adverse events during the study, but Dr. Patton noted that the study was not powered to detect them. “We felt that with the low-dose, single-time [exposure], it was appropriate to not worry too much about those side effects,” he replied.
Another potential concern is that the increased urine flow could mask a kink in the ureter by forcing it open.
In the study, his team randomized 145 patients with a planned cystoscopy as part of a procedure to receive 10-mg furosemide (1 cc) or saline (1 cc) during a cystoscopy performed by an attending or a fellow. The median time to confirmation of ureteral patency was 86.5 seconds in the furosemide group, compared with 165.0 seconds in the saline group (difference, 78.5 seconds; P less than .001). The time to the first ureteral jet was 59 seconds versus 74 seconds, respectively (P less than .006). A Kaplan-Meier survival curve analysis also showed a significant improvement in time to ureteral patency confirmation (log-rank P less than .001).
The study was not funded. Dr. Patton has no relevant financial disclosures.
SOURCE: Patton S et al. SGS 2019, Abstract 10.
TUCSON, ARIZ. – according to results from a new randomized, controlled trial.
“It does make a difference, but is that really a [meaningful] difference? Every medication has adverse effects, so is it worth that extra time [savings] to take on that potential for side effects? It highlights the importance of statistical significance versus clinical significance, and I think [the clinical significance] can just be answered by each individual physician,” Simon Patton, MD, said in an interview.
Dr. Patton is a urogynecologist at Ascension Via Christi Medical Group in Wichita, Kan. He presented the study, which was conducted during his time as a fellow at the University of South Florida, Tampa, at the annual scientific meeting of the Society of Gynecologic Surgeons. Dr. Patton isn’t sure just how often physicians use furosemide during routine cystoscopy. “It would be great to do a survey to find out how many people use it in their practice,” he said.
Cystoscopy is used to during a surgery to ensure that no injury has been done to the bladder or the urethra, and the American Urogynecological Society recommends that it be performed during any pelvic reconstructive surgery. A key element of the test is confirming that the ureters are open. By increasing urine flow, furosemide can reduce the time to confirmation. But after conferring with a colleague who used the procedure, Dr. Patton looked for some data to support the practice and couldn’t find any.
Although the cystoscopy itself generally is safe, furosemide can cause hypotension, change in renal function, and even dehydration at higher doses. During the question-and-answer period, one attendee noted these issues and pointed out that furosemide can potentiate renal failure, especially among patients taking cephalosporins. “If you’re going to do this sort of trial, you have to consider potential adverse events. A single dose is probably not going to [cause an issue], but in the context of a study you want to monitor the adverse events that have been reported,” this attendee said.
The researchers did not observe any of these adverse events during the study, but Dr. Patton noted that the study was not powered to detect them. “We felt that with the low-dose, single-time [exposure], it was appropriate to not worry too much about those side effects,” he replied.
Another potential concern is that the increased urine flow could mask a kink in the ureter by forcing it open.
In the study, his team randomized 145 patients with a planned cystoscopy as part of a procedure to receive 10-mg furosemide (1 cc) or saline (1 cc) during a cystoscopy performed by an attending or a fellow. The median time to confirmation of ureteral patency was 86.5 seconds in the furosemide group, compared with 165.0 seconds in the saline group (difference, 78.5 seconds; P less than .001). The time to the first ureteral jet was 59 seconds versus 74 seconds, respectively (P less than .006). A Kaplan-Meier survival curve analysis also showed a significant improvement in time to ureteral patency confirmation (log-rank P less than .001).
The study was not funded. Dr. Patton has no relevant financial disclosures.
SOURCE: Patton S et al. SGS 2019, Abstract 10.
TUCSON, ARIZ. – according to results from a new randomized, controlled trial.
“It does make a difference, but is that really a [meaningful] difference? Every medication has adverse effects, so is it worth that extra time [savings] to take on that potential for side effects? It highlights the importance of statistical significance versus clinical significance, and I think [the clinical significance] can just be answered by each individual physician,” Simon Patton, MD, said in an interview.
Dr. Patton is a urogynecologist at Ascension Via Christi Medical Group in Wichita, Kan. He presented the study, which was conducted during his time as a fellow at the University of South Florida, Tampa, at the annual scientific meeting of the Society of Gynecologic Surgeons. Dr. Patton isn’t sure just how often physicians use furosemide during routine cystoscopy. “It would be great to do a survey to find out how many people use it in their practice,” he said.
Cystoscopy is used to during a surgery to ensure that no injury has been done to the bladder or the urethra, and the American Urogynecological Society recommends that it be performed during any pelvic reconstructive surgery. A key element of the test is confirming that the ureters are open. By increasing urine flow, furosemide can reduce the time to confirmation. But after conferring with a colleague who used the procedure, Dr. Patton looked for some data to support the practice and couldn’t find any.
Although the cystoscopy itself generally is safe, furosemide can cause hypotension, change in renal function, and even dehydration at higher doses. During the question-and-answer period, one attendee noted these issues and pointed out that furosemide can potentiate renal failure, especially among patients taking cephalosporins. “If you’re going to do this sort of trial, you have to consider potential adverse events. A single dose is probably not going to [cause an issue], but in the context of a study you want to monitor the adverse events that have been reported,” this attendee said.
The researchers did not observe any of these adverse events during the study, but Dr. Patton noted that the study was not powered to detect them. “We felt that with the low-dose, single-time [exposure], it was appropriate to not worry too much about those side effects,” he replied.
Another potential concern is that the increased urine flow could mask a kink in the ureter by forcing it open.
In the study, his team randomized 145 patients with a planned cystoscopy as part of a procedure to receive 10-mg furosemide (1 cc) or saline (1 cc) during a cystoscopy performed by an attending or a fellow. The median time to confirmation of ureteral patency was 86.5 seconds in the furosemide group, compared with 165.0 seconds in the saline group (difference, 78.5 seconds; P less than .001). The time to the first ureteral jet was 59 seconds versus 74 seconds, respectively (P less than .006). A Kaplan-Meier survival curve analysis also showed a significant improvement in time to ureteral patency confirmation (log-rank P less than .001).
The study was not funded. Dr. Patton has no relevant financial disclosures.
SOURCE: Patton S et al. SGS 2019, Abstract 10.
REPORTING FROM SGS 2019
SUI cure definition may need updating
TUCSON, ARIZ. – The definition of a surgical cure for stress urinary incontinence (SUI) varies significantly from one clinical trial to another, but the best choice might be an International Consultation on Incontinence Questionnaire (ICIQ) score of 5 or less, according to a study that correlated a patient’s definition of success with various measures of success or failure.
Adoption of a standard definition could make clinical trial results easier to interpret, as well as improve consistency in clinical practice.
The study was a planned secondary analysis of a randomized, controlled trial that compared midurethral sling to Burch colpopexy in women undergoing abdominal sacrocolpopexy. The original study found no difference in outcomes between the two approaches with respect to stress-specific incontinence rates at 6 months, although the midurethral sling was associated with better secondary, patient-reported outcomes.
That incongruity between objective and subjective outcomes raised questions. “I would frequently have the nurse tell me that a patient didn’t do well [on the stress incontinence test], but you would talk to the patient, and she was happy as could be. She wasn’t using pads, she was perfectly dry. So I thought there was a little bit of a disconnect between the definitions we were using, and what the patients wanted from the procedure,” Emanuel Trabuco, MD, said in an interview.
Dr. Trabuco is a consultant and the chair of the division of urogynecology at Mayo Clinic in Rochester, Minn. He presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
because as things currently stand, different clinical trials use a range of different outcomes, and as the nurse’s experience shows, an objective outcome might not match patient perception. In fact, objective urinary incontinence tests may not be so objective at all.
“Urodynamics is inherently [challenging]. You can have women that come in with stress incontinence symptoms asking for treatment, and we do urodynamics and they don’t leak. It’s a false negative. Conversely, other women presenting with other issues like overactive bladder – you do urodynamics, and they leak. So that’s a false positive. We have this desire for objectivity, but the tests we have are neither sensitive nor specific,” said Dr. Trabuco.
The researchers examined 13 different methods of determining SUI cure, and then linked them to answers to two questions from 104 trial participants. The first question: “In your opinion, how successful has treatment for your urinary leakage been?” Responses ranged from 0 (not at all) to 10 (very successful). The second question: “Compared to how you were before your recent surgery, how are your urinary leakage symptoms now?” Responses ranged from 0 (much worse) to 10 (much better).
At 6 months, the largest Cohen’s d value for patient perception of symptom improvement was associated with ICIQ score greater than or equal to 5 (–13.5, mean ratings of 9.7 versus 4.6), which was better than definitions based on a negative cough stress test (–6.5) and the strict composite definition, which included a negative cough stress test, ICIQ = 0, and no retreatment (–6.4).
The researchers examined the correlation between each definition of SUI cure and the answers to the above questions, and found that the highest Cohen’s d values for agreement with patient’s perception of symptom improvement were: ICIQ score greater than or equal to 5 (Cohen’s d at 6 months, 12 months, and 24 months; –13.5; –13.0; –12.6, respectively); ICIQ score less than or equal to 5 with no (“not-at-all” or “somewhat”) SUI symptoms on Urinary Distress Inventory, Short Form (UDI-6) (–7.2; –7.2; and –8.1); and ICIQ score less than or equal to 5 with no SUI symptoms (never or rarely) on Medical, Epidemiologic, and Social aspects of Aging (MESA) urinary incontinence questionnaire (–7.0, –7.0, –6.4).
The results argue against the use of cough stress test, said Dr. Trabuco. “If you think about the time commitment that our patients give us to participate in a trial, we should make that participation as least onerous as we can. If the cough stress test doesn’t really add anything to patient perception of surgical success and improvement, why put the poor patient through a catheterization and a cough test and a prolonged visit? For all of those reasons, I hope this is something that others will look at and try to standardize,” said Dr. Trabuco.
Mayo Medical School, Rochester, Minn., funded the study. Dr. Trabuco has no relevant financial disclosures.
SOURCE: Trabuco E et al. SGS 2019, oral poster 14.
TUCSON, ARIZ. – The definition of a surgical cure for stress urinary incontinence (SUI) varies significantly from one clinical trial to another, but the best choice might be an International Consultation on Incontinence Questionnaire (ICIQ) score of 5 or less, according to a study that correlated a patient’s definition of success with various measures of success or failure.
Adoption of a standard definition could make clinical trial results easier to interpret, as well as improve consistency in clinical practice.
The study was a planned secondary analysis of a randomized, controlled trial that compared midurethral sling to Burch colpopexy in women undergoing abdominal sacrocolpopexy. The original study found no difference in outcomes between the two approaches with respect to stress-specific incontinence rates at 6 months, although the midurethral sling was associated with better secondary, patient-reported outcomes.
That incongruity between objective and subjective outcomes raised questions. “I would frequently have the nurse tell me that a patient didn’t do well [on the stress incontinence test], but you would talk to the patient, and she was happy as could be. She wasn’t using pads, she was perfectly dry. So I thought there was a little bit of a disconnect between the definitions we were using, and what the patients wanted from the procedure,” Emanuel Trabuco, MD, said in an interview.
Dr. Trabuco is a consultant and the chair of the division of urogynecology at Mayo Clinic in Rochester, Minn. He presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
because as things currently stand, different clinical trials use a range of different outcomes, and as the nurse’s experience shows, an objective outcome might not match patient perception. In fact, objective urinary incontinence tests may not be so objective at all.
“Urodynamics is inherently [challenging]. You can have women that come in with stress incontinence symptoms asking for treatment, and we do urodynamics and they don’t leak. It’s a false negative. Conversely, other women presenting with other issues like overactive bladder – you do urodynamics, and they leak. So that’s a false positive. We have this desire for objectivity, but the tests we have are neither sensitive nor specific,” said Dr. Trabuco.
The researchers examined 13 different methods of determining SUI cure, and then linked them to answers to two questions from 104 trial participants. The first question: “In your opinion, how successful has treatment for your urinary leakage been?” Responses ranged from 0 (not at all) to 10 (very successful). The second question: “Compared to how you were before your recent surgery, how are your urinary leakage symptoms now?” Responses ranged from 0 (much worse) to 10 (much better).
At 6 months, the largest Cohen’s d value for patient perception of symptom improvement was associated with ICIQ score greater than or equal to 5 (–13.5, mean ratings of 9.7 versus 4.6), which was better than definitions based on a negative cough stress test (–6.5) and the strict composite definition, which included a negative cough stress test, ICIQ = 0, and no retreatment (–6.4).
The researchers examined the correlation between each definition of SUI cure and the answers to the above questions, and found that the highest Cohen’s d values for agreement with patient’s perception of symptom improvement were: ICIQ score greater than or equal to 5 (Cohen’s d at 6 months, 12 months, and 24 months; –13.5; –13.0; –12.6, respectively); ICIQ score less than or equal to 5 with no (“not-at-all” or “somewhat”) SUI symptoms on Urinary Distress Inventory, Short Form (UDI-6) (–7.2; –7.2; and –8.1); and ICIQ score less than or equal to 5 with no SUI symptoms (never or rarely) on Medical, Epidemiologic, and Social aspects of Aging (MESA) urinary incontinence questionnaire (–7.0, –7.0, –6.4).
The results argue against the use of cough stress test, said Dr. Trabuco. “If you think about the time commitment that our patients give us to participate in a trial, we should make that participation as least onerous as we can. If the cough stress test doesn’t really add anything to patient perception of surgical success and improvement, why put the poor patient through a catheterization and a cough test and a prolonged visit? For all of those reasons, I hope this is something that others will look at and try to standardize,” said Dr. Trabuco.
Mayo Medical School, Rochester, Minn., funded the study. Dr. Trabuco has no relevant financial disclosures.
SOURCE: Trabuco E et al. SGS 2019, oral poster 14.
TUCSON, ARIZ. – The definition of a surgical cure for stress urinary incontinence (SUI) varies significantly from one clinical trial to another, but the best choice might be an International Consultation on Incontinence Questionnaire (ICIQ) score of 5 or less, according to a study that correlated a patient’s definition of success with various measures of success or failure.
Adoption of a standard definition could make clinical trial results easier to interpret, as well as improve consistency in clinical practice.
The study was a planned secondary analysis of a randomized, controlled trial that compared midurethral sling to Burch colpopexy in women undergoing abdominal sacrocolpopexy. The original study found no difference in outcomes between the two approaches with respect to stress-specific incontinence rates at 6 months, although the midurethral sling was associated with better secondary, patient-reported outcomes.
That incongruity between objective and subjective outcomes raised questions. “I would frequently have the nurse tell me that a patient didn’t do well [on the stress incontinence test], but you would talk to the patient, and she was happy as could be. She wasn’t using pads, she was perfectly dry. So I thought there was a little bit of a disconnect between the definitions we were using, and what the patients wanted from the procedure,” Emanuel Trabuco, MD, said in an interview.
Dr. Trabuco is a consultant and the chair of the division of urogynecology at Mayo Clinic in Rochester, Minn. He presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
because as things currently stand, different clinical trials use a range of different outcomes, and as the nurse’s experience shows, an objective outcome might not match patient perception. In fact, objective urinary incontinence tests may not be so objective at all.
“Urodynamics is inherently [challenging]. You can have women that come in with stress incontinence symptoms asking for treatment, and we do urodynamics and they don’t leak. It’s a false negative. Conversely, other women presenting with other issues like overactive bladder – you do urodynamics, and they leak. So that’s a false positive. We have this desire for objectivity, but the tests we have are neither sensitive nor specific,” said Dr. Trabuco.
The researchers examined 13 different methods of determining SUI cure, and then linked them to answers to two questions from 104 trial participants. The first question: “In your opinion, how successful has treatment for your urinary leakage been?” Responses ranged from 0 (not at all) to 10 (very successful). The second question: “Compared to how you were before your recent surgery, how are your urinary leakage symptoms now?” Responses ranged from 0 (much worse) to 10 (much better).
At 6 months, the largest Cohen’s d value for patient perception of symptom improvement was associated with ICIQ score greater than or equal to 5 (–13.5, mean ratings of 9.7 versus 4.6), which was better than definitions based on a negative cough stress test (–6.5) and the strict composite definition, which included a negative cough stress test, ICIQ = 0, and no retreatment (–6.4).
The researchers examined the correlation between each definition of SUI cure and the answers to the above questions, and found that the highest Cohen’s d values for agreement with patient’s perception of symptom improvement were: ICIQ score greater than or equal to 5 (Cohen’s d at 6 months, 12 months, and 24 months; –13.5; –13.0; –12.6, respectively); ICIQ score less than or equal to 5 with no (“not-at-all” or “somewhat”) SUI symptoms on Urinary Distress Inventory, Short Form (UDI-6) (–7.2; –7.2; and –8.1); and ICIQ score less than or equal to 5 with no SUI symptoms (never or rarely) on Medical, Epidemiologic, and Social aspects of Aging (MESA) urinary incontinence questionnaire (–7.0, –7.0, –6.4).
The results argue against the use of cough stress test, said Dr. Trabuco. “If you think about the time commitment that our patients give us to participate in a trial, we should make that participation as least onerous as we can. If the cough stress test doesn’t really add anything to patient perception of surgical success and improvement, why put the poor patient through a catheterization and a cough test and a prolonged visit? For all of those reasons, I hope this is something that others will look at and try to standardize,” said Dr. Trabuco.
Mayo Medical School, Rochester, Minn., funded the study. Dr. Trabuco has no relevant financial disclosures.
SOURCE: Trabuco E et al. SGS 2019, oral poster 14.
REPORTING FROM SGS 2019
In endometrial cancer and SUI, concomitant surgery improves outcomes
TUCSON, ARIZ. – according to a study examining the effects of an SUI screen among endometrial cancer patients.
An estimated 40%-80% of women with endometrial cancer experience SUI. The malignancy often is caught early enough to be treated with curative intent, and that is leading physicians and patients to think more about quality of life outcomes.
And yet, few patients receive concomitant surgery. Twenty percent of the women in the current study opted for concomitant surgeries, yet large database studies show the frequency of concomitant surgeries is about 2.5%. “There’s huge room for improvement in this area. The take-home message is that this is prevalent, this is doable, and this is something that could truly benefit this population,” Evelyn Hall, MD, said in an interview. Dr. Hall is a fellow in female pelvic medicine and reconstructive medicine at Brown University, Providence, R.I. She presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
It’s not entirely surprising that SUI tends to be overlooked in patients with endometrial cancer. After all, they are going through a life-changing medical diagnosis, and oncologists are laser focused on achieving a cure when possible. But a bigger picture view, especially in light of the high cure rate for endometrial cancer when detected early, should encourage physicians to think differently about patient management.
The biggest trick may be incorporating concomitant surgeries into the surgical work flow. “It can be challenging logistically. It requires surgical planning and coordination between the two surgeons,” said Dr. Hall. But she said the experience at Brown University showed that it was possible with some patience. “It took a while to get the balls rolling, but once we figured out [it] worked for our institution, we’ve seen a continued uptake,” she said.
An important remaining question is the safety of the concomitant surgeries. Dr. Hall did not report any between-group differences in her presentation, but analysis is ongoing. They found a statistically significant increase in the number of readmissions among the concomitant surgery group, but most were deemed unlikely to be related to concomitant surgery.
In the study, 1,322 endometrial surgical candidates were screened for SUI, and 53% tested positive. Of these, 556 patients were offered concomitant surgical or nonsurgical SUI treatment: 21% chose concomitant surgery, 19% chose nonsurgical SUI treatment, and 60% of patients opted for no SUI treatment.
At 6 months after surgery, the concomitant surgery group was more likely to have a Urinary Distress Inventory (UDI)–Stress score of 0 than were those who were treated nonsurgically (odds ratio, 2.8; P = .0001) and those in the no-treatment group (OR, 3.7; P less than .0001). The concomitant group also was more likely to have a surgical site infection (SSI) score of 0 than was the nonsurgical group (OR, 2.9; P = .0008) and the no-treatment group (OR, 2.7; P less than .0001). Severe/very severe SSI scores occurred in 57% of the concomitant group at baseline, and this frequency dropped to 14% at 6 weeks (P less than .0001).
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Hall has no relevant financial disclosures.
SOURCE: Hall E et al. SGS 2019, oral presentation 12.
TUCSON, ARIZ. – according to a study examining the effects of an SUI screen among endometrial cancer patients.
An estimated 40%-80% of women with endometrial cancer experience SUI. The malignancy often is caught early enough to be treated with curative intent, and that is leading physicians and patients to think more about quality of life outcomes.
And yet, few patients receive concomitant surgery. Twenty percent of the women in the current study opted for concomitant surgeries, yet large database studies show the frequency of concomitant surgeries is about 2.5%. “There’s huge room for improvement in this area. The take-home message is that this is prevalent, this is doable, and this is something that could truly benefit this population,” Evelyn Hall, MD, said in an interview. Dr. Hall is a fellow in female pelvic medicine and reconstructive medicine at Brown University, Providence, R.I. She presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
It’s not entirely surprising that SUI tends to be overlooked in patients with endometrial cancer. After all, they are going through a life-changing medical diagnosis, and oncologists are laser focused on achieving a cure when possible. But a bigger picture view, especially in light of the high cure rate for endometrial cancer when detected early, should encourage physicians to think differently about patient management.
The biggest trick may be incorporating concomitant surgeries into the surgical work flow. “It can be challenging logistically. It requires surgical planning and coordination between the two surgeons,” said Dr. Hall. But she said the experience at Brown University showed that it was possible with some patience. “It took a while to get the balls rolling, but once we figured out [it] worked for our institution, we’ve seen a continued uptake,” she said.
An important remaining question is the safety of the concomitant surgeries. Dr. Hall did not report any between-group differences in her presentation, but analysis is ongoing. They found a statistically significant increase in the number of readmissions among the concomitant surgery group, but most were deemed unlikely to be related to concomitant surgery.
In the study, 1,322 endometrial surgical candidates were screened for SUI, and 53% tested positive. Of these, 556 patients were offered concomitant surgical or nonsurgical SUI treatment: 21% chose concomitant surgery, 19% chose nonsurgical SUI treatment, and 60% of patients opted for no SUI treatment.
At 6 months after surgery, the concomitant surgery group was more likely to have a Urinary Distress Inventory (UDI)–Stress score of 0 than were those who were treated nonsurgically (odds ratio, 2.8; P = .0001) and those in the no-treatment group (OR, 3.7; P less than .0001). The concomitant group also was more likely to have a surgical site infection (SSI) score of 0 than was the nonsurgical group (OR, 2.9; P = .0008) and the no-treatment group (OR, 2.7; P less than .0001). Severe/very severe SSI scores occurred in 57% of the concomitant group at baseline, and this frequency dropped to 14% at 6 weeks (P less than .0001).
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Hall has no relevant financial disclosures.
SOURCE: Hall E et al. SGS 2019, oral presentation 12.
TUCSON, ARIZ. – according to a study examining the effects of an SUI screen among endometrial cancer patients.
An estimated 40%-80% of women with endometrial cancer experience SUI. The malignancy often is caught early enough to be treated with curative intent, and that is leading physicians and patients to think more about quality of life outcomes.
And yet, few patients receive concomitant surgery. Twenty percent of the women in the current study opted for concomitant surgeries, yet large database studies show the frequency of concomitant surgeries is about 2.5%. “There’s huge room for improvement in this area. The take-home message is that this is prevalent, this is doable, and this is something that could truly benefit this population,” Evelyn Hall, MD, said in an interview. Dr. Hall is a fellow in female pelvic medicine and reconstructive medicine at Brown University, Providence, R.I. She presented the study at the annual scientific meeting of the Society of Gynecologic Surgeons.
It’s not entirely surprising that SUI tends to be overlooked in patients with endometrial cancer. After all, they are going through a life-changing medical diagnosis, and oncologists are laser focused on achieving a cure when possible. But a bigger picture view, especially in light of the high cure rate for endometrial cancer when detected early, should encourage physicians to think differently about patient management.
The biggest trick may be incorporating concomitant surgeries into the surgical work flow. “It can be challenging logistically. It requires surgical planning and coordination between the two surgeons,” said Dr. Hall. But she said the experience at Brown University showed that it was possible with some patience. “It took a while to get the balls rolling, but once we figured out [it] worked for our institution, we’ve seen a continued uptake,” she said.
An important remaining question is the safety of the concomitant surgeries. Dr. Hall did not report any between-group differences in her presentation, but analysis is ongoing. They found a statistically significant increase in the number of readmissions among the concomitant surgery group, but most were deemed unlikely to be related to concomitant surgery.
In the study, 1,322 endometrial surgical candidates were screened for SUI, and 53% tested positive. Of these, 556 patients were offered concomitant surgical or nonsurgical SUI treatment: 21% chose concomitant surgery, 19% chose nonsurgical SUI treatment, and 60% of patients opted for no SUI treatment.
At 6 months after surgery, the concomitant surgery group was more likely to have a Urinary Distress Inventory (UDI)–Stress score of 0 than were those who were treated nonsurgically (odds ratio, 2.8; P = .0001) and those in the no-treatment group (OR, 3.7; P less than .0001). The concomitant group also was more likely to have a surgical site infection (SSI) score of 0 than was the nonsurgical group (OR, 2.9; P = .0008) and the no-treatment group (OR, 2.7; P less than .0001). Severe/very severe SSI scores occurred in 57% of the concomitant group at baseline, and this frequency dropped to 14% at 6 weeks (P less than .0001).
The study was funded by the Patient-Centered Outcomes Research Institute. Dr. Hall has no relevant financial disclosures.
SOURCE: Hall E et al. SGS 2019, oral presentation 12.
REPORTING FROM SGS 2019
Stress incontinence surgery improves sexual dysfunction
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
REPORTING FROM SGS 2019
HM19: Pediatric medical and surgical co-management
Anticipatory and prevention-heavy approach
Presenter
Erin Shaughnessy, MD, MSHCM
Session title
Reaching Across the Aisle: Pediatric Co-Management with Surgery and Subspecialists
Session summary
Dr. Shaughnessy articulated a balanced approach to the importance of careful selection of patients needing to be co-managed by pediatric hospitalists. She compared two personal and very different experiences.
She initially managed a well-developed surgical co-management service at a quaternary, academic, free-standing children’s hospital, in which surgeons and subspecialists also admitted and managed patients to their own services. Currently, Dr. Shaughnessy is a division chief at Phoenix Children’s Hospital, a free-standing children’s hospital with a community hospital background, in which hospitalists admit most, if not all the patients, while subspecialty services have been transitioning only recently to having their own admitting services and employing the ideas of limited co-management.
She reminded the HM19 audience of the essential principles of co-management: shared responsibility, authority and accountability for the care of a hospitalized patient, discussing the scenarios, both from literature and real life, in which the line could become blurry at times.
Many pediatric programs are moving away from a traditional consultation model, Dr. Shaughnessy said, in which a consult is called for a new or a persistent problem with a patient, and where a consulting team signs off upon the resolved issue.
The more modern co-management model infuses a need for anticipatory and prevention-heavy approach, intertwined with fiscally responsible ideas that must be palatable for all: administration, hospitalists, and patients.
Dr. Shaughnessy reviewed a number of articles from both adult and pediatric literature with varied results, some that have shown decreased length of stay, decreased number of medical complications, decreased readmissions, decreased number of tests, but some that have also shown an increase in median hospital costs, emphasizing perhaps the importance of context in which one practices.
Finally, she identified patient selection, collaborative relationships, clear roles delineation, and excellence in communication as four main factors deciding the faith of a co-management model.
Key takeaways for HM
1. Careful selection of patients to be co-managed is essential and can prevent potential increase in costs and negative outcomes.
2. Success in medical and surgical co-management relies on well-delineated roles, collaborative culture, and immaculate communication.
Dr. Giordano is a pediatric neurosurgery hospitalist and assistant professor in pediatrics at Columbia University Irving Medical Center in New York.
Anticipatory and prevention-heavy approach
Anticipatory and prevention-heavy approach
Presenter
Erin Shaughnessy, MD, MSHCM
Session title
Reaching Across the Aisle: Pediatric Co-Management with Surgery and Subspecialists
Session summary
Dr. Shaughnessy articulated a balanced approach to the importance of careful selection of patients needing to be co-managed by pediatric hospitalists. She compared two personal and very different experiences.
She initially managed a well-developed surgical co-management service at a quaternary, academic, free-standing children’s hospital, in which surgeons and subspecialists also admitted and managed patients to their own services. Currently, Dr. Shaughnessy is a division chief at Phoenix Children’s Hospital, a free-standing children’s hospital with a community hospital background, in which hospitalists admit most, if not all the patients, while subspecialty services have been transitioning only recently to having their own admitting services and employing the ideas of limited co-management.
She reminded the HM19 audience of the essential principles of co-management: shared responsibility, authority and accountability for the care of a hospitalized patient, discussing the scenarios, both from literature and real life, in which the line could become blurry at times.
Many pediatric programs are moving away from a traditional consultation model, Dr. Shaughnessy said, in which a consult is called for a new or a persistent problem with a patient, and where a consulting team signs off upon the resolved issue.
The more modern co-management model infuses a need for anticipatory and prevention-heavy approach, intertwined with fiscally responsible ideas that must be palatable for all: administration, hospitalists, and patients.
Dr. Shaughnessy reviewed a number of articles from both adult and pediatric literature with varied results, some that have shown decreased length of stay, decreased number of medical complications, decreased readmissions, decreased number of tests, but some that have also shown an increase in median hospital costs, emphasizing perhaps the importance of context in which one practices.
Finally, she identified patient selection, collaborative relationships, clear roles delineation, and excellence in communication as four main factors deciding the faith of a co-management model.
Key takeaways for HM
1. Careful selection of patients to be co-managed is essential and can prevent potential increase in costs and negative outcomes.
2. Success in medical and surgical co-management relies on well-delineated roles, collaborative culture, and immaculate communication.
Dr. Giordano is a pediatric neurosurgery hospitalist and assistant professor in pediatrics at Columbia University Irving Medical Center in New York.
Presenter
Erin Shaughnessy, MD, MSHCM
Session title
Reaching Across the Aisle: Pediatric Co-Management with Surgery and Subspecialists
Session summary
Dr. Shaughnessy articulated a balanced approach to the importance of careful selection of patients needing to be co-managed by pediatric hospitalists. She compared two personal and very different experiences.
She initially managed a well-developed surgical co-management service at a quaternary, academic, free-standing children’s hospital, in which surgeons and subspecialists also admitted and managed patients to their own services. Currently, Dr. Shaughnessy is a division chief at Phoenix Children’s Hospital, a free-standing children’s hospital with a community hospital background, in which hospitalists admit most, if not all the patients, while subspecialty services have been transitioning only recently to having their own admitting services and employing the ideas of limited co-management.
She reminded the HM19 audience of the essential principles of co-management: shared responsibility, authority and accountability for the care of a hospitalized patient, discussing the scenarios, both from literature and real life, in which the line could become blurry at times.
Many pediatric programs are moving away from a traditional consultation model, Dr. Shaughnessy said, in which a consult is called for a new or a persistent problem with a patient, and where a consulting team signs off upon the resolved issue.
The more modern co-management model infuses a need for anticipatory and prevention-heavy approach, intertwined with fiscally responsible ideas that must be palatable for all: administration, hospitalists, and patients.
Dr. Shaughnessy reviewed a number of articles from both adult and pediatric literature with varied results, some that have shown decreased length of stay, decreased number of medical complications, decreased readmissions, decreased number of tests, but some that have also shown an increase in median hospital costs, emphasizing perhaps the importance of context in which one practices.
Finally, she identified patient selection, collaborative relationships, clear roles delineation, and excellence in communication as four main factors deciding the faith of a co-management model.
Key takeaways for HM
1. Careful selection of patients to be co-managed is essential and can prevent potential increase in costs and negative outcomes.
2. Success in medical and surgical co-management relies on well-delineated roles, collaborative culture, and immaculate communication.
Dr. Giordano is a pediatric neurosurgery hospitalist and assistant professor in pediatrics at Columbia University Irving Medical Center in New York.