User login
Study identifies predictors of bariatric surgery attrition
BALTIMORE – Even in a public health system like Canada’s, almost and researchers have identified patient characteristics that could be predictive of dropout risk that would potentially have implications in a nonuniversal system, such as that of the United States, according to a study of almost 18,000 patients reported at the annual meeting Society of American Gastrointestinal and Endoscopic Surgeons.
“Even in a universal health care system, clear disparities exist among patient populations having bariatric surgery,” said Aristithes Doumouras, MD, of McMaster University in Hamilton, Ont. “Extensive work-ups and long wait times can have an impact on the delivery of bariatric care.”
Dr. Doumouras reported on results of a retrospective, population-based study of 17,703 patients referred for surgery during 2009-2015 in the Ontario Bariatric Network, a province-wide network of 11 hospitals credentialed to perform bariatric surgery. The study found that 23.2% of patients referred for bariatric surgery did not go through with it and that overall average wait times between referral and the operation were just short of a year – 362.2 days to be precise.
The goal of the study was to identify any factors associated with attrition, Dr. Doumouras said.
“Predictors of interest included patient demographics – age, sex, income quintile, immigration status, employment status, smoking status – and comorbidities, such as diabetes, heart failure, hypertension, sleep apnea, and renal disease,” he said. “The study also evaluated health services factors, such as overall wait time to bariatric surgery, presence of centers of excellence, and health care utilization.”
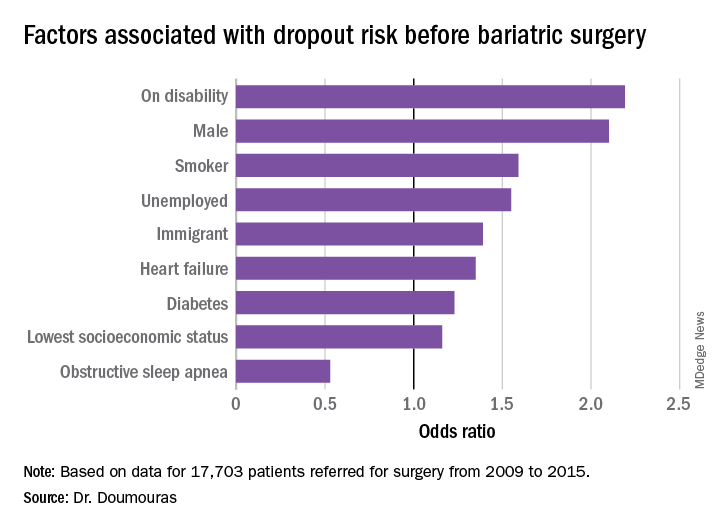
The study found that demographics with more than twice the odds of attrition were male gender and presence of a disability (P less than .01). Smokers were 60% more likely to drop out (P less than .01), he said. “To receive bariatric surgery in Ontario, smokers must go through a smoking cessation program.”
Unemployed individuals and immigrants also had higher rates of attrition, at 55% and 39%, respectively, and were more likely to not go through with the operation (P less than .01). Health factors associated with attrition, but to a lesser extent, were diabetes (odds ratio, 1.23) and heart failure (OR, 1.35; P less than .01).
“Low socioeconomic status actually had a very low impact in our system on attrition after adjustment for other demographic factors such as disability and unemployment,” Dr. Doumouras said, noting a 16% greater risk of attrition in this group (P = .02).
“Interestingly,” he noted, “there was one factor associated with less dropout – obstructive sleep apnea – probably because people hate using the CPAP machines every single night.” People with OSA were 47% less likely to drop out than were people without the disease (P less than .001).
When asked if the findings would be applicable in the United States, Dr. Doumouras said they would to an extent.
“I think we can say confidently that they would apply to most universal health care systems,” he said. “In nonuniversal health care systems, the interplay between insurance status, socioeconomic status, and the like makes it more of a complex relationship, but if you were to take any kind of health care system, even in the United States, you would probably see very similar trends in terms of who can get bariatric surgery.”
He added, “I think also the length of work-up matters. Only a 3- or 4-week work-up probably affects attrition as well. These are relatively universal things.”
Dr. Doumouras has no financial relationships to disclose.
SOURCE: Doumouras A et al. SAGES 2019, Abstract S118.
BALTIMORE – Even in a public health system like Canada’s, almost and researchers have identified patient characteristics that could be predictive of dropout risk that would potentially have implications in a nonuniversal system, such as that of the United States, according to a study of almost 18,000 patients reported at the annual meeting Society of American Gastrointestinal and Endoscopic Surgeons.

“Even in a universal health care system, clear disparities exist among patient populations having bariatric surgery,” said Aristithes Doumouras, MD, of McMaster University in Hamilton, Ont. “Extensive work-ups and long wait times can have an impact on the delivery of bariatric care.”
Dr. Doumouras reported on results of a retrospective, population-based study of 17,703 patients referred for surgery during 2009-2015 in the Ontario Bariatric Network, a province-wide network of 11 hospitals credentialed to perform bariatric surgery. The study found that 23.2% of patients referred for bariatric surgery did not go through with it and that overall average wait times between referral and the operation were just short of a year – 362.2 days to be precise.
The goal of the study was to identify any factors associated with attrition, Dr. Doumouras said.
“Predictors of interest included patient demographics – age, sex, income quintile, immigration status, employment status, smoking status – and comorbidities, such as diabetes, heart failure, hypertension, sleep apnea, and renal disease,” he said. “The study also evaluated health services factors, such as overall wait time to bariatric surgery, presence of centers of excellence, and health care utilization.”
The study found that demographics with more than twice the odds of attrition were male gender and presence of a disability (P less than .01). Smokers were 60% more likely to drop out (P less than .01), he said. “To receive bariatric surgery in Ontario, smokers must go through a smoking cessation program.”
Unemployed individuals and immigrants also had higher rates of attrition, at 55% and 39%, respectively, and were more likely to not go through with the operation (P less than .01). Health factors associated with attrition, but to a lesser extent, were diabetes (odds ratio, 1.23) and heart failure (OR, 1.35; P less than .01).
“Low socioeconomic status actually had a very low impact in our system on attrition after adjustment for other demographic factors such as disability and unemployment,” Dr. Doumouras said, noting a 16% greater risk of attrition in this group (P = .02).
“Interestingly,” he noted, “there was one factor associated with less dropout – obstructive sleep apnea – probably because people hate using the CPAP machines every single night.” People with OSA were 47% less likely to drop out than were people without the disease (P less than .001).
When asked if the findings would be applicable in the United States, Dr. Doumouras said they would to an extent.
“I think we can say confidently that they would apply to most universal health care systems,” he said. “In nonuniversal health care systems, the interplay between insurance status, socioeconomic status, and the like makes it more of a complex relationship, but if you were to take any kind of health care system, even in the United States, you would probably see very similar trends in terms of who can get bariatric surgery.”
He added, “I think also the length of work-up matters. Only a 3- or 4-week work-up probably affects attrition as well. These are relatively universal things.”
Dr. Doumouras has no financial relationships to disclose.
SOURCE: Doumouras A et al. SAGES 2019, Abstract S118.
BALTIMORE – Even in a public health system like Canada’s, almost and researchers have identified patient characteristics that could be predictive of dropout risk that would potentially have implications in a nonuniversal system, such as that of the United States, according to a study of almost 18,000 patients reported at the annual meeting Society of American Gastrointestinal and Endoscopic Surgeons.

“Even in a universal health care system, clear disparities exist among patient populations having bariatric surgery,” said Aristithes Doumouras, MD, of McMaster University in Hamilton, Ont. “Extensive work-ups and long wait times can have an impact on the delivery of bariatric care.”
Dr. Doumouras reported on results of a retrospective, population-based study of 17,703 patients referred for surgery during 2009-2015 in the Ontario Bariatric Network, a province-wide network of 11 hospitals credentialed to perform bariatric surgery. The study found that 23.2% of patients referred for bariatric surgery did not go through with it and that overall average wait times between referral and the operation were just short of a year – 362.2 days to be precise.
The goal of the study was to identify any factors associated with attrition, Dr. Doumouras said.
“Predictors of interest included patient demographics – age, sex, income quintile, immigration status, employment status, smoking status – and comorbidities, such as diabetes, heart failure, hypertension, sleep apnea, and renal disease,” he said. “The study also evaluated health services factors, such as overall wait time to bariatric surgery, presence of centers of excellence, and health care utilization.”
The study found that demographics with more than twice the odds of attrition were male gender and presence of a disability (P less than .01). Smokers were 60% more likely to drop out (P less than .01), he said. “To receive bariatric surgery in Ontario, smokers must go through a smoking cessation program.”
Unemployed individuals and immigrants also had higher rates of attrition, at 55% and 39%, respectively, and were more likely to not go through with the operation (P less than .01). Health factors associated with attrition, but to a lesser extent, were diabetes (odds ratio, 1.23) and heart failure (OR, 1.35; P less than .01).
“Low socioeconomic status actually had a very low impact in our system on attrition after adjustment for other demographic factors such as disability and unemployment,” Dr. Doumouras said, noting a 16% greater risk of attrition in this group (P = .02).
“Interestingly,” he noted, “there was one factor associated with less dropout – obstructive sleep apnea – probably because people hate using the CPAP machines every single night.” People with OSA were 47% less likely to drop out than were people without the disease (P less than .001).
When asked if the findings would be applicable in the United States, Dr. Doumouras said they would to an extent.
“I think we can say confidently that they would apply to most universal health care systems,” he said. “In nonuniversal health care systems, the interplay between insurance status, socioeconomic status, and the like makes it more of a complex relationship, but if you were to take any kind of health care system, even in the United States, you would probably see very similar trends in terms of who can get bariatric surgery.”
He added, “I think also the length of work-up matters. Only a 3- or 4-week work-up probably affects attrition as well. These are relatively universal things.”
Dr. Doumouras has no financial relationships to disclose.
SOURCE: Doumouras A et al. SAGES 2019, Abstract S118.
REPORTING FROM SAGES 2019
Severe OSA increases cardiovascular risk after surgery
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
FROM JAMA
Appendectomy linked to increased risk of subsequent Parkinson’s
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
REPORTING FROM DDW 2019
Key clinical point: Appendectomy appears to increase the risk of Parkinson’s disease.
Major finding: The overall relative risk of developing Parkinson’s disease in patients after appendectomy was 3.19 (95% CI, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure.
Study details: A population-based study of more than 62 million medical records from a national database.
Disclosures: The researchers reported having no financial disclosures.
Source: Sheriff MZ et al. DDW 2019, Abstract 739.
Managing 2nd trimester loss: Shared decision making, honor patient preference
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Time to embrace minimally invasive colorectal surgery?
LAS VEGAS – Two-thirds of colon resections in the United States are open procedures, but a colorectal surgeon told colleagues that evidence shows minimally invasive surgery deserves a wider place in his field.
Why? Because minimally invasive surgery – despite its limited utilization – is linked to multiple improved outcomes in colorectal surgery, said Matthew G. Mutch, MD, chief of colon and rectal surgery at Washington University, St. Louis, in a presentation at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“Our goal should be to offer minimally invasive surgery to as many patients as possible by as many different methods as needed,” Dr. Mutch said. “If you’re willing to take this on and do this over a regular basis, you’ll get over that learning curve and expand the number of patients you can offer laparoscopy to.”
According to Dr. Mutch, benefits of minimally invasive colorectal surgery include:
- Improved short-term outcomes – length of stay and return of bowel function, and morbidity and mortality. A 2012 retrospective study of 85,712 colon resections that found laparoscopic resections, when feasible, “had better outcomes than open colectomy in the immediate perioperative period.” (Ann Surg. 2012 Sep;256[3]462-8).
- Improved long-term outcomes: faster recovery, fewer hernias, and fewer bowel obstructions.
- Lower overall costs.
- Fewer complications in the elderly.
When it comes to laparoscopic colorectal surgery, Dr. Mutch cautioned that the robotic technology has unclear benefit in rectal cancer, and the cost in colorectal cancer is unclear.
Another alternative is to perform laparoscopic colorectal surgery through alternative extraction sites such as the rectum, vagina, stomach, and even a stoma site or perineal wound. Both transanal and transvaginal extraction are feasible and safe, he said, adding that transvaginal procedures are best performed in conjunction with a hysterectomy. One benefit of these procedures is that they avoid abdominal wall trauma. However, he cautioned that colorectal surgery is unique because a cancerous specimen cannot be morcellated and must instead be removed whole.
Dr. Mutch also discussed laparoendoscopic resection of colon polyps. Benefits include shorter length of stay and faster recovery, he said, but complications can include perforation and bleeding. And, he said, there’s currently no code for the procedure.
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Mutch has no relevant disclosures.
LAS VEGAS – Two-thirds of colon resections in the United States are open procedures, but a colorectal surgeon told colleagues that evidence shows minimally invasive surgery deserves a wider place in his field.
Why? Because minimally invasive surgery – despite its limited utilization – is linked to multiple improved outcomes in colorectal surgery, said Matthew G. Mutch, MD, chief of colon and rectal surgery at Washington University, St. Louis, in a presentation at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“Our goal should be to offer minimally invasive surgery to as many patients as possible by as many different methods as needed,” Dr. Mutch said. “If you’re willing to take this on and do this over a regular basis, you’ll get over that learning curve and expand the number of patients you can offer laparoscopy to.”
According to Dr. Mutch, benefits of minimally invasive colorectal surgery include:
- Improved short-term outcomes – length of stay and return of bowel function, and morbidity and mortality. A 2012 retrospective study of 85,712 colon resections that found laparoscopic resections, when feasible, “had better outcomes than open colectomy in the immediate perioperative period.” (Ann Surg. 2012 Sep;256[3]462-8).
- Improved long-term outcomes: faster recovery, fewer hernias, and fewer bowel obstructions.
- Lower overall costs.
- Fewer complications in the elderly.
When it comes to laparoscopic colorectal surgery, Dr. Mutch cautioned that the robotic technology has unclear benefit in rectal cancer, and the cost in colorectal cancer is unclear.
Another alternative is to perform laparoscopic colorectal surgery through alternative extraction sites such as the rectum, vagina, stomach, and even a stoma site or perineal wound. Both transanal and transvaginal extraction are feasible and safe, he said, adding that transvaginal procedures are best performed in conjunction with a hysterectomy. One benefit of these procedures is that they avoid abdominal wall trauma. However, he cautioned that colorectal surgery is unique because a cancerous specimen cannot be morcellated and must instead be removed whole.
Dr. Mutch also discussed laparoendoscopic resection of colon polyps. Benefits include shorter length of stay and faster recovery, he said, but complications can include perforation and bleeding. And, he said, there’s currently no code for the procedure.
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Mutch has no relevant disclosures.
LAS VEGAS – Two-thirds of colon resections in the United States are open procedures, but a colorectal surgeon told colleagues that evidence shows minimally invasive surgery deserves a wider place in his field.
Why? Because minimally invasive surgery – despite its limited utilization – is linked to multiple improved outcomes in colorectal surgery, said Matthew G. Mutch, MD, chief of colon and rectal surgery at Washington University, St. Louis, in a presentation at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
“Our goal should be to offer minimally invasive surgery to as many patients as possible by as many different methods as needed,” Dr. Mutch said. “If you’re willing to take this on and do this over a regular basis, you’ll get over that learning curve and expand the number of patients you can offer laparoscopy to.”
According to Dr. Mutch, benefits of minimally invasive colorectal surgery include:
- Improved short-term outcomes – length of stay and return of bowel function, and morbidity and mortality. A 2012 retrospective study of 85,712 colon resections that found laparoscopic resections, when feasible, “had better outcomes than open colectomy in the immediate perioperative period.” (Ann Surg. 2012 Sep;256[3]462-8).
- Improved long-term outcomes: faster recovery, fewer hernias, and fewer bowel obstructions.
- Lower overall costs.
- Fewer complications in the elderly.
When it comes to laparoscopic colorectal surgery, Dr. Mutch cautioned that the robotic technology has unclear benefit in rectal cancer, and the cost in colorectal cancer is unclear.
Another alternative is to perform laparoscopic colorectal surgery through alternative extraction sites such as the rectum, vagina, stomach, and even a stoma site or perineal wound. Both transanal and transvaginal extraction are feasible and safe, he said, adding that transvaginal procedures are best performed in conjunction with a hysterectomy. One benefit of these procedures is that they avoid abdominal wall trauma. However, he cautioned that colorectal surgery is unique because a cancerous specimen cannot be morcellated and must instead be removed whole.
Dr. Mutch also discussed laparoendoscopic resection of colon polyps. Benefits include shorter length of stay and faster recovery, he said, but complications can include perforation and bleeding. And, he said, there’s currently no code for the procedure.
Global Academy for Medical Education and this news organization are owned by the same parent company. Dr. Mutch has no relevant disclosures.
EXPERT ANALYSIS FROM MISS
Energy-based therapies in female genital cosmetic surgery: Hype, hope, and a way forward
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
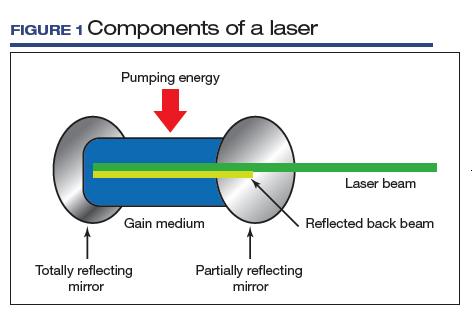
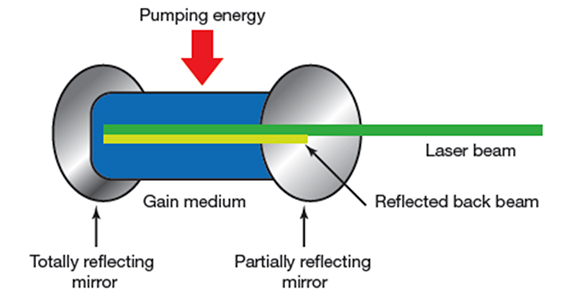
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
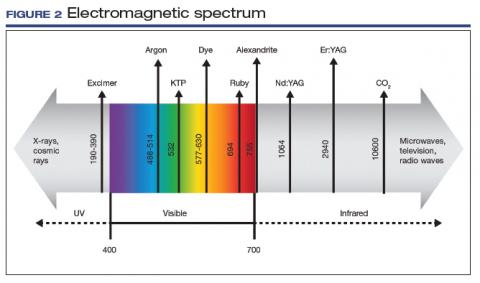
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
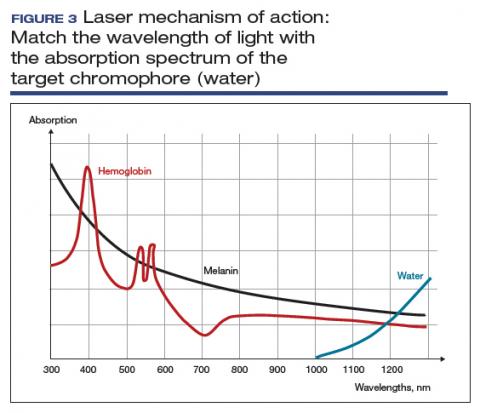
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
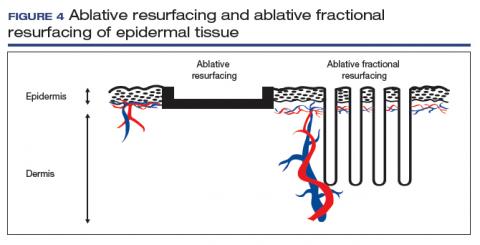
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
Energy-based therapy use in gynecology dates back to the early 1970s, when ablative carbon dioxide (C02) lasers were employed to treat cervical erosions.1 Soon after, reports were published on laser treatment for diethylstilbestrol-associated vaginal adenosis, laser laparoscopy for adhesiolysis, laser hysteroscopy, and laser genital wart ablation.2 Starting around 2011, the first articles were published on the use of fractional C02 laser treatment for vulvovaginal atrophy.3,4 Use of laser and light-based therapies to treat “vaginal rejuvenation” is now increasing at an annual rate of 26%. In a few years, North America is expected to be the largest market for vaginal laser rejuvenation. In 2016, more than 500,000 feminine rejuvenation procedures were performed in the United States, and it is estimated that more than 27,000 energy-based devices will be in operation by 2021.5
Clearly, there is considerable public interest and intrigue in office-based female genital cosmetic procedures. In 2018, the US Food and Drug Administration contacted 7 manufacturers of energy-based devices to request revision and clarification for marketing of these devices, since these technologies are neither cleared nor approved for cosmetic vulvovaginal conditions.6 The companies responded within 30 days.
In this article, we appraise the existing literature regarding the mechanism of action of energy-based therapies used in gynecology and review outcomes of their use in female genital cosmetic surgery.
Laser technology devices and how they work
LASER is an acronym for Light Amplification by Stimulated Emission of Radiation. Laser devices are composed of 1) an excitable medium (gas, liquid, solid) needed to emit light, 2) an energy source to excite the medium, 3) mirrors to bounce the light back and forth, and 4) a delivery and cooling system (FIGURE 1).
The electromagnetic spectrum is the range of all the wavelengths of light, including visible light, radio waves, infrared light, ultraviolet light, x-rays, and gamma rays (FIGURE 2). Most lasers used for the treatment of vulvovaginal disorders, typically C02 and erbium:yttrium aluminum garnet (Er:YAG) lasers, involve the infrared wavelengths.
The basic principle of laser treatment is to match the wavelength of the laser with the absorption spectrum of the desired target—a chromophore such as hemoglobin, melanin, or water (FIGURE 3). In essence, light is absorbed by the chromophore (which in vulvar and vaginal tissues is mostly water) and transformed into heat, leading to target destruction. In a fractionated (or fractional) laser beam, the laser is broken up into many smaller beams that treat only portions of the treatment area, with areas of intact epithelium in between the treated areas. At appropriately low thermal denaturation temperatures (45° to 50°C), tissue regeneration can occur through activation of heat shock proteins and tissue growth factors, creating neocollagenesis and neovascularization.
The concept of ablative resurfacing versus fractional resurfacing is borrowed from dermatology (FIGURE 4), understanding that tissue ablation and thermal denaturation occur at temperatures greater than 100°C, as occurs with carbonization of vulvar condylomata.
Continue to: In dermatology, fractionated lasers...
In dermatology, fractionated lasers have been used in the treatment of hair removal, vascular and pigmented lesions, scars, wound healing, tattoo removal, warts, and actinic keratoses. For these conditions, the targeted chromophores are water, hemoglobin, melanosomes, and tattoo ink. The laser pulses must be shorter than the target tissue thermal relaxation times in order to avoid excess heating and collateral tissue damage. Choosing appropriate settings is critical to achieve selective heating, or destruction, of the target tissue. These settings include appropriate wavelengths, pulse durations, and fluence, which is energy delivered per unit area (typically, joules per square centimeter).
For gynecologic conditions, the lasers used are most often CO2, Er:YAG, and hybrid (which include ablative and nonablative wavelengths) devices. In the epithelium of the vagina and vulva, these lasers generally have a very shallow depth of optical penetration, on the order of 10 to 200 µm.
Radiofrequency-based devices emit focused electromagnetic waves
Radiofrequency systems use a wand to deliver radiofrequency energy to create heat within the subepithelial layers of vulvar and vaginal tissues, while the surface remains cool. These devices can use monopolar or bipolar energy (current) to create a reverse thermal gradient designed to heat the deeper tissues transepithelially at a higher temperature while a coolant protects the surface epithelium. Some wand technologies require multiple treatments, while others require only a single treatment.
The TABLE lists currently available energy-based technologies.
Therapeutic uses for energy-based devices
Investigators have studied laser devices for treating various gynecologic conditions, including vulvovaginal atrophy, stress urinary incontinence (UI), vaginal laxity, lichen sclerosus, and vulvodynia.
Vulvovaginal atrophy
Genitourinary syndrome of menopause (GSM) includes symptoms of vulvovaginal irritation, burning, itching, discharge, dyspareunia, lower urinary tract symptoms such as frequency and urinary tract infections, and vaginal dryness or vulvovaginal atrophy.7 First-line treatment for vulvovaginal atrophy includes the use of nonhormonal lubricants for intercourse and vaginal moisturizers, which temporarily moisten the vaginal epithelium. Low-dose vaginal estrogen is a second-line therapy for symptomatic vulvovaginal atrophy; newer pharmacologic options include dehydroepiandrosterone (DHEA) suppositories (prasterone), solubilized estradiol capsules, and the selective estrogen receptor modulator (SERM) ospemifene.
Fractionated CO2, Erb:YAG, and hybrid lasers also have been used to treat women with symptomatic vulvovaginal atrophy and GSM through similar mechanisms described in dermatologic conditions with low-temperature laser activation of tissue proteins and growth factors creating new connective tissue and angiogenesis. A number of landmark studies have been published detailing patient outcomes with energy-based treatments for these symptoms.
Three-arm trial. Cruz and colleagues conducted a double-blind randomized trial to evaluate the efficacy of fractional CO2 laser vaginal treatment compared with local estriol therapy and the combination of laser plus estriol.8 The investigators randomly assigned 45 postmenopausal women to treatment with fractional CO2 laser with placebo vaginal cream, estriol with sham laser, or laser plus estriol. Treatment consisted of 2 sessions 4 weeks apart, with 20 consecutive weeks of estriol or placebo 3 times per week.
At weeks 8 and 20, the Vaginal Health Index (VHI) average score was significantly higher in all study arms. At week 20, the laser plus estriol group also showed incremental improvement in the VHI score (P = .01). The laser and the laser plus estriol groups had significant improvement in dyspareunia, burning, and dryness, while the estriol group improved only in dryness (P<.001). The laser plus estriol group had significant improvement in the total Female Sexual Function Index (FSFI) score (P = .02) and in the individual domains of pain, desire, and lubrication. Although the laser-alone group had significant worsening in the FSFI pain domain (P = .04), all treatment arms had comparable FSFI total scores at week 20. No adverse events were recorded during the study period.
Continue to: Retrospective study...
Retrospective study. To assess the efficacy of 3, 4, or 5 treatments with microablative fractional CO2 laser therapy for symptoms of GSM, Athanasiou and colleagues studied outcomes in 94 postmenopausal women.9 The intensity or bothersomeness of GSM symptoms as well as sexual function significantly improved in this cohort. The intensity of dyspareunia and dryness decreased from a median of 9 (minimum–maximum, 5–10) and 8 (0–10), respectively, at baseline to 0 (0–6) and 0 (0–8) at 1 month after the last laser therapy (P<.001 for all). The FSFI score and the frequency of sexual intercourse rose from 10.8 (2–26.9) and 1 (0–8) at baseline to 27.8 (15.2–35.4) and 4 (2–8) at 1 month after the last laser therapy (P<.001 for all).
The positive effects of laser therapy were unchanged throughout the 12 months of follow-up, and the pattern was the same for symptom-free rates. No adverse events were recorded during the study period.
The investigators noted that, based on short- and long-term follow-up, 4 or 5 laser treatments may be superior to 3 treatments for lowering the intensity of GSM symptoms. They found no differences in outcomes between 4 and 5 laser treatments.
Prospective comparative cohort study. Gaspar and colleagues recruited 50 postmenopausal women with GSM and assigned 25 participants to 2 weeks of pretreatment with estriol ovules 3 times per week (for epithelial hydration) followed by 3 sessions of Er:YAG nonablative laser treatments; 25 women in the active control group received treatment with estriol ovules over 8 weeks.10 Pre- and posttreatment biopsies, maturation index, maturation value, pH, and VAS symptom analysis were recorded up to 18 months after treatment.
Up to the 6-month follow-up, both treatment groups had a statistically significant reduction of all GSM symptoms. At all follow-ups, however, symptom relief was more prominent in the laser-treated group. In addition, the effects of the laser therapy remained statistically significant at the 12- and 18-month follow-ups, while the treatment effects of estriol were diminished at 12 months and, at 18 months, this group had some symptoms that were significantly worse than before treatment.
Overall, adverse effects were minimal and transient in both groups, affecting 4% of participants in the laser group, and 12% in the estriol group.
Long-term effectiveness evaluation. To assess the long-term efficacy and acceptability of vaginal laser treatment for the management of GSM, Gambacciani and colleagues treated 205 postmenopausal women with an Er:YAG laser for 3 applications every 30 days, with evaluations performed after 1, 3, 6, 12, 18, and 24 months from the last laser treatment.11 An active control group (n = 49) received 3 months of local treatment with either hormonal (estriol gel twice weekly) or nonhormonal (hyaluronic acid-based preparations or moisturizers and lubricants) agents.
Treatment with the ER:YAG laser induced a significant decrease (P<.01) in scores of the Visual Analog Scale (VAS) for vulvovaginal atrophy symptoms for vaginal dryness and dyspareunia and an increase in the VHI score (P<.01) up to 12 months after the last treatment. After 18 and 24 months, values returned to levels similar to those at baseline.
Women who also had stress UI (n = 114) received additional laser treatment of the anterior vaginal wall specifically designed for UI, with assessment based on the International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form (ICIQ-UI SF). Laser treatment induced a significant decrease (P<.05) in ICIQ-UI SF scores compared with baseline values, and scores remained lower than baseline values after 1, 2, 3, 6, and 12 months after the last laser treatment. Values measured after 18 and 24 months, however, did not differ significantly from baseline.
In the control group, the VAS score showed a similar decrease and comparable pattern during the treatment period. However, after the end of the treatment period, the control group’s VAS scores for vaginal dryness and dyspareunia showed a progressive increase, and after 6 months, the values were significantly different from corresponding values measured in the laser therapy group. The follow-up period in the control group ended after 6 months, because almost all patients started a new local or systemic treatment for their GSM symptoms. No adverse events related to treatment were recorded throughout the study period.
In an earlier pilot study by the same authors, 19 women with GSM who also had mild to moderate stress UI were treated with a vaginal Er:YAG laser.12 Compared with vaginal estriol treatment in the active control group, laser treatment was associated with a significant improvement (P<.01) in ICIQ-SF scores, with rapid and long-lasting effects that persisted up to week 24 of the observation period.
Continue to: Urinary incontinence...
Urinary incontinence
The cause of UI is considered to be multifactorial, including disruption in connective tissue supports of the urethrovesical junction leading to urethral hypermobility, pelvic floor muscle weakness, nerve damage to the urethral rhabdosphincter related to pudendal neuropathy or pelvic plexopathy, and atrophic changes of the urethra mucosa and submucosa. Purported mechanisms of action for energy-based therapies designed for treatment of UI relate to direct effects on connective tissue, blood vessels, and possibly nerves.
In 3 clinical trials designed specifically to treat UI with an Er:YAG laser, women showed subjective symptomatic improvement.
Ogrinc and colleagues followed 175 pre- and postmenopausal women with stress UI or mixed UI in a prospective nonrandomized study.13 They treated women with an Er:YAG laser for an average of 2.5 (0.5) procedures separated by a 2-month period and performed follow-up assessments at 2, 6, and 12 months after treatment.
After treatment, 77% of women with stress UI had significant improvement in symptoms based on the ICIQ SF and the Incontinence Severity Index (ISI), while only 34% of those with mixed UI had no symptoms at 1-year follow-up. No major adverse effects were noted in either group.
Okui compared the effects of Er:YAG laser treatment with those of tension-free vaginal tape (TVT) or transobturator tape (TOT) sling procedures (n = 50 in each group) in women with stress UI or mixed UI.14 At 12 months after treatment, all 3 treatments demonstrated comparable improvements in the women with stress UI. Some patients with mixed UI in the TVT and TOT groups showed exacerbation, while all women in the laser-treated group tended to have symptom improvement.
In another recent study, Blaganje and colleagues randomly assigned 114 premenopausal parous women with stress UI to an Er:YAG laser procedure or sham treatment.15 Three months after treatment, ICIQ-UI SF scores were significantly more improved (P<.001) in the laser-treated group than in the sham group. In addition, 21% of laser-treated patients were dry at follow-up compared with 4% of the sham-treated group.
Key takeaway. While these studies showed promising short-term results for laser treatment of UI, they need to be replicated in appropriately powered clinical trials that include critical subjective and objective outcomes as well as longer-term follow-up for both effectiveness and safety.
Vaginal laxity/pre-prolapse
Vaginal laxity is defined as the symptom of excessive vaginal looseness.16 Also referred to as “pre-prolapse,” this subjective symptom generally refers to a widened vaginal opening (genital hiatus) but with pelvic organ prolapse that is within the vagina or hymen.17 Notably, the definition is ambiguous, and rigorous clinical data based on validated outcomes and prolapse grading are lacking.
Krychman and colleagues conducted the first randomized controlled study comparing monopolar radiofrequency at the vaginal introitus with sham therapy for vaginal laxity in 174 premenopausal women, known as the VIVEVE I trial.18 The primary outcome, the proportion of women reporting no vaginal laxity at 6 months after treatment, was assessed using a vaginal laxity questionnaire, a 7-point rating scale for laxity or tightness ranging from very loose to very tight. With a single radiofrequency treatment, 43.5% of the active group and 19.6% (P = .002) of the sham group obtained the primary outcome.
There were also statistically significant improvements in overall sexual function and decreased sexual distress. The adjusted odds ratio (OR, 3.39; 95% confidence interval, 1.54–7.45) showed that the likelihood of no vaginal laxity at 6 months was more than 3 times greater for women who received the active treatment compared with those who received sham treatment. Adverse events were mild, resolved spontaneously, and were similar in the 2 groups.
Continue to: Outlook for energy-based...
Outlook for energy-based therapies: Cautiously optimistic
Preliminary outcome data on the use of energy-based therapies for female genital cosmetic surgery is largely positive for the treatment of vulvovaginal atrophy, but some case series suggest the potential for scarring, burning, and inefficacy. This prompted the FDA to send “It has come to our attention” letters to a number of device manufacturers in 2018.6
Supportive evidence is weak. Early data are encouraging regarding fractionated laser therapy for the treatment of vulvovaginal atrophy and stress UI and radiofrequency wand therapy for vaginal laxity and stress UI. Unfortunately, the level of evidence to support wide use of these technologies for all pelvic floor disorders is weak. A recent committee opinion from the International Urogynecology Association noted that only 8 studies (1 randomized trial and 7 observational studies) on these conditions fulfilled the criteria of good quality.19 The International Continence Society and the International Society for the Study of Vulvovaginal Disorders recently published a best practice consensus document declaring laser and energy-based treatments in gynecology and urology to be largely experimental.20
Questions persist. Knowledge gaps exist, and recommendations related to subspecialty training—who should perform these procedures (gynecologists, plastic surgeons, urologists, dermatologists, family practitioners) and the level of training needed to safely perform them—are lacking. Patient selection and physician knowledge and experience related to female genital anatomy, female sexual function and dysfunction, multidisciplinary treatment options for various pelvic support problems and UI, as well as psychologic screening for body dysmorphic disorders, need to be considered in terms of treating both the functional and aesthetic aspects related to cosmetic and reconstructive gynecologic surgery.
Special considerations. The use of energy-based therapies in special populations, such as survivors of breast cancer or other gynecologic cancers, as well as women undergoing chemotherapy, radiation therapy, and hormonal manipulation (particularly with antiestrogenic SERMs and aromatase inhibitors) has not been adequately evaluated. A discussion of the risks, benefits, alternatives, and limited long-term outcome data for energy-based therapies in cancer survivors, as for all patients, must be included for adequate informed consent prior to undertaking these treatments.
Guidelines for appropriate tissue priming, laser settings, and concomitant energy-based technology with local hormone treatment (also known as laser-augmented drug delivery) need to be developed. Comparative long-term studies are needed to determine the safety and effectiveness of these technologies.
Caution advised. Given the lack of long-term safety and effectiveness data on energy-based therapies for the vague indications of vaginal laxity, and even for the well-defined conditions of stress UI and vulvovaginal atrophy, clinicians should exercise caution before promoting treatment, which can be expensive and is not without potential complications, such as vaginal pain, adhesive agglutination, and persistent dryness and dyspareunia.21
Fortunately, many randomized trials on various energy-based devices for gynecologic indications (GSM, stress UI, vaginal laxity, lichen sclerosus) are underway, and results from these studies will help inform future clinical practice and guideline development.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
- Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO2 laser. Obstet Gynecol. 1973;41:795-796.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy-based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Gaspar A, Addamo G, Brandi H. Vaginal fractional CO2 laser: a minimally invasive option for vaginal rejuvenation. Am J Cosmetic Surg. 2011;28:156-162.
- Salvatore S, Leone Roberti Maggiore U, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015;22:845-849.
- Benedetto AV. What's new in cosmetic dermatology. Dermatol Clin. 2019;37:117-128.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal rejuvenation or vaginal cosmetic procedures: FDA safety communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed April 8, 2019.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Athanasiou S, Pitsouni E, Grigoradis T, et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019;26:248-255.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Russo E, et al. Long-term effects of vaginal erbium laser in the treatment of genitourinary syndrome of menopause. Climacteric. 2018;21:148-152.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Ogrinc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med. 2015;47:689-697.
- Okui N. Comparison between erbium-doped yttrium aluminum garnet laser therapy and sling procedures in the treatment of stress and mixed urinary incontinence. World J Urol. 2018. doi:10.1007/s00345-018-2445-x.
- Blaganje M, Scepanovic D, Zgur L, et al. Non-ablative Er:YAG laser therapy effect on stress urinary incontinence related to quality of life and sexual function: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:153-158.
- Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecologic J. 2016;27:165-194.
- Garcia B, Pardo J. Academic cosmetic gynecology and energy-based therapies: ambiguities, explorations, and the FDA advisories. Int Urogynecol J. 2019;30:1-2.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Shobeiri SA, Kerkhof MH, Minassian VA, et al; IUGA Research and Development Committee. IUGA committee opinion: laser-based vaginal devices for treatment of stress urinary incontinence, genitourinary syndrome of menopause, and vaginal laxity. Int Urogynecol J. 2019;30:371-376.
- Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. Neurourol Urodyn. 2019;38:1009-1023.
- Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause. 2019;26:423-427.
Assessing and treating sexual function after vaginal surgery
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
Novel strategies may help curb bariatric SSI
BALTIMORE – While rates of surgical site infections after bariatric surgery have been reported in the low single digits, SSIs have continued to be a persistent complication.
At the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons, researchers reported on two strategies to reduce SSI in bariatric surgery: a predictive tool that identifies risk factors for wound infection, allowing surgeons to employ protective measures before and during surgery, and a change in surgical practice leading to a 78% reduction in wound infection rates that resulted from a single-center study.
Jerry Dang, MD, of the University of Alberta, Edmonton, reported that the BariWound predictive tool designed to stratify patients into risk categories showed a high level of accuracy with an area under the curve of 0.73. Cynthia Weber, MD, of University Hospitals, Cleveland, reported that changing the method for performing circular-stapled gastrojejunostomy (GJ) from the transoral to the transabdominal approach along with more vigilant use of wound protection reduced wound infection rates from 6% to 1.3%.
Dr. Dang noted that SSI has been reported as the most common hospital-acquired complication in bariatric surgery, with reported rates of between 1% and 10%. A 2014 analysis of the American College of Surgeons National Surgical Quality Improvement Program database reported an SSI rate of 1.8% (Surg Endosc. 2014;28:3285-92). Although these rates are low, Dr. Dang explained that his group wanted to identify factors associated with SSI within 30 days of bariatric surgery. They analyzed outcomes data of 274,187 patients in the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database who had bariatric surgery in 2015 and 2016 (196,608 by laparoscopic sleeve gastrectomy [SG] and 77,579 laparoscopic Roux-en-Y gastric bypass [RYGB]). Their analysis determined an incisional SSI rate of 0.47% (n = 1,291). “Incisional SSI rates were four times higher for laparoscopic RYGB: 1.04% vs. 0.25%,” Dr. Dang said.
On multivariable logistic regression, the adjusted odds ratio of SSI after RYGB vs. SG was 3.13 (P less than .001). Other significant risk factors were chronic steroid or immunosuppressant use (odds ratio, 1.75; P = .001), female sex (OR, 1.48; P less than .001) and history of gastroesophageal reflux disease (OR, 1.45; P less than .001). Other factors with a 21%-31% greater risk of SSI were white race (P = .002), history of diabetes (P less than .001), hypertension (P less than .001), obstructive sleep apnea (P = .001), and longer operation times (P less than .001). Each single-digit increase in body mass index increased risk by 3%, and older age actually had a protective effect for unknown reasons, Dr. Dang noted.
The BariWound tool assigns points to each risk factor. Each hour of operation time and each 10 kg/m2 of weight carry a value of 1 point, with partial points allowed. RYGB equals 5 points, and chronic steroid/immunosuppressant use, 4 points. The tool assigns risk to four categories based on score and 30-day SSI rate:
- Low, less than 15 (1% risk of SSI).
- Moderate, 15-21.9 (1%-5%).
- High, 22-26.9 (5%-10%).
- Very high, greater than 27 (greater than 10%).
“The BariWound tool can help to inform clinical decision making so patients can know they’re at higher risk, and this could allow for us to target high-risk patients with preventive packages, such as the Cleveland Clinic Technique of wound protection, wound irrigation, and wound packing as a resource-saving measure,” Dr. Dang said. “Targeting high-risk populations can reduce cost and operating time.”
Dr. Weber reported on her institution’s study of SSIs using two different methods for circular stapling of GJ that involved two different surgeons who performed 333 RYGB procedures from January 2016 to March 2018. Surgeon “A” had traditionally used the transoral technique without wound protection to insert the anvil of the stapler; surgeon “B” used wound protection and the transabdominal technique for stapler insertion. Wound protection involves draping of the stapler with sterile plastic.
“In a quarterly review, we detected a higher than expected wound complication rate of 6%,” Dr. Weber said. “Of particular concern was the development of five recent wound infection cases, which all occurred in the transoral group for a rate of 8.9% in that cohort.”
That left the quality team questioning the safety profile of the transoral technique, Dr. Weber said. “We wanted to know why and whether or not the main contributor to the development of a wound infection was the technique for the anvil introduction or was it the difference between surgeons using wound protection.”
Halfway through the study period, surgeon A made two modifications: He adopted the transabdominal technique for a subset of patients; and because of the surgeon’s comfort level and expertise with the transoral approach, he continued using that approach but added wound protection. Surgeon B continued with the transabdominal approach with wound protection. The share of transabdominal insertions in the study population increased from 69.2% before the change to 75% after. Demographics between the pre- and postchange patient populations were similar, as were the rates of revision surgery between the two groups.
“We noticed a significant reduction in total wound complications from 6% to 1.3%, and we noticed a complete elimination of surgical site infections after adding wound protection to the transoral technique,” Dr. Weber said.
Dr. Weber noted a number of limitations with the study: its retrospective nature; the lack of control for other intraoperative factors that contribute to SSIs; relatively low incidence of SSI; and surgeon’s choice to determine the technique of anvil insertion.
“We found that our quality improvement intervention was efficacious and decided that it was not the technique of anvil insertion, but it was the wound protection that was key to preventing wound infections, as we saw complete elimination after we added wound protection to the transoral technique,” Dr. Weber said. “Using proper precautions with the circular stapler and anastomosis can be done using either technique for anvil insertion. Overall self-assessment of outcomes leads to best practice.”
Dr. Dang had no financial relationships to disclose. Dr. Weber’s coauthor Leena Khatian, MD, MPH, disclosed relationships with Torax Medical, Medtronic, and Gore.
SOURCES: Weber C et al. SAGES 2109, Presentation S049; Dang J et al. SAGES 2019, Presentation S050.
BALTIMORE – While rates of surgical site infections after bariatric surgery have been reported in the low single digits, SSIs have continued to be a persistent complication.
At the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons, researchers reported on two strategies to reduce SSI in bariatric surgery: a predictive tool that identifies risk factors for wound infection, allowing surgeons to employ protective measures before and during surgery, and a change in surgical practice leading to a 78% reduction in wound infection rates that resulted from a single-center study.
Jerry Dang, MD, of the University of Alberta, Edmonton, reported that the BariWound predictive tool designed to stratify patients into risk categories showed a high level of accuracy with an area under the curve of 0.73. Cynthia Weber, MD, of University Hospitals, Cleveland, reported that changing the method for performing circular-stapled gastrojejunostomy (GJ) from the transoral to the transabdominal approach along with more vigilant use of wound protection reduced wound infection rates from 6% to 1.3%.
Dr. Dang noted that SSI has been reported as the most common hospital-acquired complication in bariatric surgery, with reported rates of between 1% and 10%. A 2014 analysis of the American College of Surgeons National Surgical Quality Improvement Program database reported an SSI rate of 1.8% (Surg Endosc. 2014;28:3285-92). Although these rates are low, Dr. Dang explained that his group wanted to identify factors associated with SSI within 30 days of bariatric surgery. They analyzed outcomes data of 274,187 patients in the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database who had bariatric surgery in 2015 and 2016 (196,608 by laparoscopic sleeve gastrectomy [SG] and 77,579 laparoscopic Roux-en-Y gastric bypass [RYGB]). Their analysis determined an incisional SSI rate of 0.47% (n = 1,291). “Incisional SSI rates were four times higher for laparoscopic RYGB: 1.04% vs. 0.25%,” Dr. Dang said.
On multivariable logistic regression, the adjusted odds ratio of SSI after RYGB vs. SG was 3.13 (P less than .001). Other significant risk factors were chronic steroid or immunosuppressant use (odds ratio, 1.75; P = .001), female sex (OR, 1.48; P less than .001) and history of gastroesophageal reflux disease (OR, 1.45; P less than .001). Other factors with a 21%-31% greater risk of SSI were white race (P = .002), history of diabetes (P less than .001), hypertension (P less than .001), obstructive sleep apnea (P = .001), and longer operation times (P less than .001). Each single-digit increase in body mass index increased risk by 3%, and older age actually had a protective effect for unknown reasons, Dr. Dang noted.
The BariWound tool assigns points to each risk factor. Each hour of operation time and each 10 kg/m2 of weight carry a value of 1 point, with partial points allowed. RYGB equals 5 points, and chronic steroid/immunosuppressant use, 4 points. The tool assigns risk to four categories based on score and 30-day SSI rate:
- Low, less than 15 (1% risk of SSI).
- Moderate, 15-21.9 (1%-5%).
- High, 22-26.9 (5%-10%).
- Very high, greater than 27 (greater than 10%).
“The BariWound tool can help to inform clinical decision making so patients can know they’re at higher risk, and this could allow for us to target high-risk patients with preventive packages, such as the Cleveland Clinic Technique of wound protection, wound irrigation, and wound packing as a resource-saving measure,” Dr. Dang said. “Targeting high-risk populations can reduce cost and operating time.”
Dr. Weber reported on her institution’s study of SSIs using two different methods for circular stapling of GJ that involved two different surgeons who performed 333 RYGB procedures from January 2016 to March 2018. Surgeon “A” had traditionally used the transoral technique without wound protection to insert the anvil of the stapler; surgeon “B” used wound protection and the transabdominal technique for stapler insertion. Wound protection involves draping of the stapler with sterile plastic.
“In a quarterly review, we detected a higher than expected wound complication rate of 6%,” Dr. Weber said. “Of particular concern was the development of five recent wound infection cases, which all occurred in the transoral group for a rate of 8.9% in that cohort.”
That left the quality team questioning the safety profile of the transoral technique, Dr. Weber said. “We wanted to know why and whether or not the main contributor to the development of a wound infection was the technique for the anvil introduction or was it the difference between surgeons using wound protection.”
Halfway through the study period, surgeon A made two modifications: He adopted the transabdominal technique for a subset of patients; and because of the surgeon’s comfort level and expertise with the transoral approach, he continued using that approach but added wound protection. Surgeon B continued with the transabdominal approach with wound protection. The share of transabdominal insertions in the study population increased from 69.2% before the change to 75% after. Demographics between the pre- and postchange patient populations were similar, as were the rates of revision surgery between the two groups.
“We noticed a significant reduction in total wound complications from 6% to 1.3%, and we noticed a complete elimination of surgical site infections after adding wound protection to the transoral technique,” Dr. Weber said.
Dr. Weber noted a number of limitations with the study: its retrospective nature; the lack of control for other intraoperative factors that contribute to SSIs; relatively low incidence of SSI; and surgeon’s choice to determine the technique of anvil insertion.
“We found that our quality improvement intervention was efficacious and decided that it was not the technique of anvil insertion, but it was the wound protection that was key to preventing wound infections, as we saw complete elimination after we added wound protection to the transoral technique,” Dr. Weber said. “Using proper precautions with the circular stapler and anastomosis can be done using either technique for anvil insertion. Overall self-assessment of outcomes leads to best practice.”
Dr. Dang had no financial relationships to disclose. Dr. Weber’s coauthor Leena Khatian, MD, MPH, disclosed relationships with Torax Medical, Medtronic, and Gore.
SOURCES: Weber C et al. SAGES 2109, Presentation S049; Dang J et al. SAGES 2019, Presentation S050.
BALTIMORE – While rates of surgical site infections after bariatric surgery have been reported in the low single digits, SSIs have continued to be a persistent complication.
At the annual meeting of the Society of American Gastrointestinal Endoscopic Surgeons, researchers reported on two strategies to reduce SSI in bariatric surgery: a predictive tool that identifies risk factors for wound infection, allowing surgeons to employ protective measures before and during surgery, and a change in surgical practice leading to a 78% reduction in wound infection rates that resulted from a single-center study.
Jerry Dang, MD, of the University of Alberta, Edmonton, reported that the BariWound predictive tool designed to stratify patients into risk categories showed a high level of accuracy with an area under the curve of 0.73. Cynthia Weber, MD, of University Hospitals, Cleveland, reported that changing the method for performing circular-stapled gastrojejunostomy (GJ) from the transoral to the transabdominal approach along with more vigilant use of wound protection reduced wound infection rates from 6% to 1.3%.
Dr. Dang noted that SSI has been reported as the most common hospital-acquired complication in bariatric surgery, with reported rates of between 1% and 10%. A 2014 analysis of the American College of Surgeons National Surgical Quality Improvement Program database reported an SSI rate of 1.8% (Surg Endosc. 2014;28:3285-92). Although these rates are low, Dr. Dang explained that his group wanted to identify factors associated with SSI within 30 days of bariatric surgery. They analyzed outcomes data of 274,187 patients in the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database who had bariatric surgery in 2015 and 2016 (196,608 by laparoscopic sleeve gastrectomy [SG] and 77,579 laparoscopic Roux-en-Y gastric bypass [RYGB]). Their analysis determined an incisional SSI rate of 0.47% (n = 1,291). “Incisional SSI rates were four times higher for laparoscopic RYGB: 1.04% vs. 0.25%,” Dr. Dang said.
On multivariable logistic regression, the adjusted odds ratio of SSI after RYGB vs. SG was 3.13 (P less than .001). Other significant risk factors were chronic steroid or immunosuppressant use (odds ratio, 1.75; P = .001), female sex (OR, 1.48; P less than .001) and history of gastroesophageal reflux disease (OR, 1.45; P less than .001). Other factors with a 21%-31% greater risk of SSI were white race (P = .002), history of diabetes (P less than .001), hypertension (P less than .001), obstructive sleep apnea (P = .001), and longer operation times (P less than .001). Each single-digit increase in body mass index increased risk by 3%, and older age actually had a protective effect for unknown reasons, Dr. Dang noted.
The BariWound tool assigns points to each risk factor. Each hour of operation time and each 10 kg/m2 of weight carry a value of 1 point, with partial points allowed. RYGB equals 5 points, and chronic steroid/immunosuppressant use, 4 points. The tool assigns risk to four categories based on score and 30-day SSI rate:
- Low, less than 15 (1% risk of SSI).
- Moderate, 15-21.9 (1%-5%).
- High, 22-26.9 (5%-10%).
- Very high, greater than 27 (greater than 10%).
“The BariWound tool can help to inform clinical decision making so patients can know they’re at higher risk, and this could allow for us to target high-risk patients with preventive packages, such as the Cleveland Clinic Technique of wound protection, wound irrigation, and wound packing as a resource-saving measure,” Dr. Dang said. “Targeting high-risk populations can reduce cost and operating time.”
Dr. Weber reported on her institution’s study of SSIs using two different methods for circular stapling of GJ that involved two different surgeons who performed 333 RYGB procedures from January 2016 to March 2018. Surgeon “A” had traditionally used the transoral technique without wound protection to insert the anvil of the stapler; surgeon “B” used wound protection and the transabdominal technique for stapler insertion. Wound protection involves draping of the stapler with sterile plastic.
“In a quarterly review, we detected a higher than expected wound complication rate of 6%,” Dr. Weber said. “Of particular concern was the development of five recent wound infection cases, which all occurred in the transoral group for a rate of 8.9% in that cohort.”
That left the quality team questioning the safety profile of the transoral technique, Dr. Weber said. “We wanted to know why and whether or not the main contributor to the development of a wound infection was the technique for the anvil introduction or was it the difference between surgeons using wound protection.”
Halfway through the study period, surgeon A made two modifications: He adopted the transabdominal technique for a subset of patients; and because of the surgeon’s comfort level and expertise with the transoral approach, he continued using that approach but added wound protection. Surgeon B continued with the transabdominal approach with wound protection. The share of transabdominal insertions in the study population increased from 69.2% before the change to 75% after. Demographics between the pre- and postchange patient populations were similar, as were the rates of revision surgery between the two groups.
“We noticed a significant reduction in total wound complications from 6% to 1.3%, and we noticed a complete elimination of surgical site infections after adding wound protection to the transoral technique,” Dr. Weber said.
Dr. Weber noted a number of limitations with the study: its retrospective nature; the lack of control for other intraoperative factors that contribute to SSIs; relatively low incidence of SSI; and surgeon’s choice to determine the technique of anvil insertion.
“We found that our quality improvement intervention was efficacious and decided that it was not the technique of anvil insertion, but it was the wound protection that was key to preventing wound infections, as we saw complete elimination after we added wound protection to the transoral technique,” Dr. Weber said. “Using proper precautions with the circular stapler and anastomosis can be done using either technique for anvil insertion. Overall self-assessment of outcomes leads to best practice.”
Dr. Dang had no financial relationships to disclose. Dr. Weber’s coauthor Leena Khatian, MD, MPH, disclosed relationships with Torax Medical, Medtronic, and Gore.
SOURCES: Weber C et al. SAGES 2109, Presentation S049; Dang J et al. SAGES 2019, Presentation S050.
REPORTING FROM SAGES 2019
Key clinical point: .
Major findings: The BariWound predictive model had an accuracy of area under the curve of 0.73; wound infection rates decreased from 6% to 1.3% after the change in practice.
Study details: Analysis of 274,187 cases from the 2015 MBSAQIP database; and a retrospective analysis of 333 bariatric cases performed from January 2016 to March 2018 at a single center.
Disclosures: Dr. Dang has no relationships to disclose. Dr. Weber has no disclosures, although coauthor Leena Khatian, MD, MPH, disclosed relationships with Torax Medical, Medtronic, and Gore.
Sources: Weber C et al. SAGES 2109, Presentation S049; Dang J et al. SAGES 2019, Presentation S050.
The genesis of vaginal anomalies
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
Vaginal anomalies and their surgical correction
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.