User login
Liver cancer deaths expected to increase again in 2018
Liver cancer mortality for 2018 is expected to be lowest in Utah and highest in Hawaii.
A total of 30,200 deaths from liver and intrahepatic bile duct cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That’s up from the 28,920 predicted by the ACS for 2017.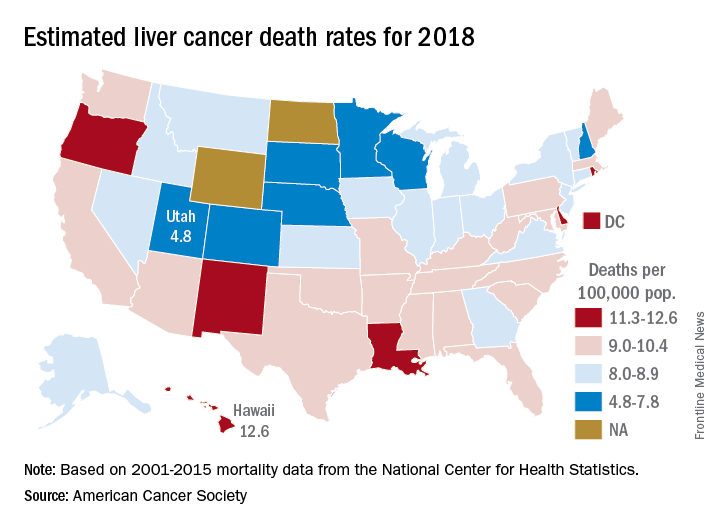
Mortality from liver cancer has been rising since the early 1980s, and in the last 10 years for which data are available (2006-2015), it increased by 2.5% per year. Over almost the same period (2005-2014), incidence rose by approximately 3% a year, and 42,220 new cases are expected in 2018, the ACS noted.
Over the most recent 5 years with available data (2011-2015), racial and ethnic disparities put American Indian/Alaska Natives at the highest mortality risk – 14.8 per 100,000 for men and 7.0 for women – followed by Asian/Pacific Islanders at 14.0 and 6.0, respectively. Non-Hispanic whites had the lowest rates: 8.2 for men and 3.4 for women, according to the ACS report.
Read more about the ACS’s research and estimates here.
Liver cancer mortality for 2018 is expected to be lowest in Utah and highest in Hawaii.
A total of 30,200 deaths from liver and intrahepatic bile duct cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That’s up from the 28,920 predicted by the ACS for 2017.
Mortality from liver cancer has been rising since the early 1980s, and in the last 10 years for which data are available (2006-2015), it increased by 2.5% per year. Over almost the same period (2005-2014), incidence rose by approximately 3% a year, and 42,220 new cases are expected in 2018, the ACS noted.
Over the most recent 5 years with available data (2011-2015), racial and ethnic disparities put American Indian/Alaska Natives at the highest mortality risk – 14.8 per 100,000 for men and 7.0 for women – followed by Asian/Pacific Islanders at 14.0 and 6.0, respectively. Non-Hispanic whites had the lowest rates: 8.2 for men and 3.4 for women, according to the ACS report.
Read more about the ACS’s research and estimates here.
Liver cancer mortality for 2018 is expected to be lowest in Utah and highest in Hawaii.
A total of 30,200 deaths from liver and intrahepatic bile duct cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That’s up from the 28,920 predicted by the ACS for 2017.
Mortality from liver cancer has been rising since the early 1980s, and in the last 10 years for which data are available (2006-2015), it increased by 2.5% per year. Over almost the same period (2005-2014), incidence rose by approximately 3% a year, and 42,220 new cases are expected in 2018, the ACS noted.
Over the most recent 5 years with available data (2011-2015), racial and ethnic disparities put American Indian/Alaska Natives at the highest mortality risk – 14.8 per 100,000 for men and 7.0 for women – followed by Asian/Pacific Islanders at 14.0 and 6.0, respectively. Non-Hispanic whites had the lowest rates: 8.2 for men and 3.4 for women, according to the ACS report.
Read more about the ACS’s research and estimates here.
ROBOT trial compares surgical approaches to esophagectomy
SAN FRANCISCO – Patients undergoing had less morbidity and pain and similarly good oncologic outcomes, when the surgery was performed by robot-assisted laparoscopy instead of by the open technique, a phase 3 clinical trial has found.
Investigators of the ROBOT (Robot-assisted Thoracolaparoscopic Esophagectomy vs. Open Transthoracic Esophagectomy) trial, led by Pieter C. van der Sluis, MD, a surgeon at the University Medical Center Utrecht, the Netherlands, randomized 112 patients with resectable esophageal cancer to open transthoracic esophagectomy – considered to be the gold standard – or robot-assisted minimally invasive thoracolaparoscopic esophagectomy.
“Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy improves postoperative outcome. There were no differences in oncologic outcomes, and our oncologic outcomes were in concordance with the highest standards nowadays,” Dr. van der Sluis summarized. “This trial provides evidence for the minimally invasive approach over the open approach, and especially the robot-assisted minimally invasive esophagectomy.”
The investigators will report a full cost comparison separately. “We see that costs are lower, though not significantly lower, with the robot,” he said, giving a preview. “We are going to show that the real costs of the operation are in the complications. When you have complications that involve the ICU and reoperations, some patients are in the hospital for months after the surgery. So by investing a little extra money in the surgical procedure, you might actually get it back by reducing the complications.”
When asked by an attendee why the trial did not compare robotic esophagectomy with thoracoscopic esophagectomy, Dr. van der Sluis noted that such comparison is complicated by many factors; for example, the challenge of finding surgeons skilled in both techniques, and the likelihood of small differences in outcomes, potentially requiring enrollment of thousands of patients to have adequate study power. “We concluded that such a trial might not be feasible,” he said.
Parsing the findings
“The complication rates [in this trial] are very high in the robotic and open groups, much higher than reported in some well-controlled prospective and retrospective studies,” commented session attendee Kenneth Meredith, MD, FACS, professor at Florida State University, Sarasota, and director of gastrointestinal oncology, Sarasota Memorial Institute for Cancer Care.
He wondered how extensive the investigators’ experience with robotics was and how many cases they had done on their learning curve. Data from his group suggest that surgeons must perform 29 cases of robotic esophagectomy before the complication rate drops (Dis Esophagus. 2017;30:1-7).
“That’s more then half of the patients in the robotic arm of their study,” he noted in an interview. “I find this needs to be explained. If the authors are past their learning curve, why were the complication rates so high?” Additionally, the 80% rate in the open group “is among the highest I’ve seen in many years.”
The lack of significant differences in complete resection rate and in lymph node harvest was also surprising, as he and other robotics users have found that this technique can improve these outcomes, Dr. Meredith added. This could likewise be a learning curve phenomenon.
Although ROBOT’s comparison of robotic with open esophagectomy is relevant, “it would have been more relevant to compare robotic to minimally invasive esophagectomy [MIE],” he maintained, as MIE has been shown to improve outcomes relative to open surgery (Lancet. 2012;379:1887-92).
“There are many high-volume centers in MIE but not necessarily robotics. The two are often mutually exclusive, and a multicenter trial in which each center performs high volumes of their respective technique, rather then mandating each center perform an operation they may not be facile in,” would be practical, Dr. Meredith concluded.
Study details
“The main objective in our trial was to reduce surgical trauma and reduce the percentage of complications,” Dr. van der Sluis told attendees of the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Results showed that compared with peers in the open surgery group, patients in the robotic-assisted surgery group specifically had a lower rate of pulmonary complications (32% vs. 58%, P = .005), largely due to a reduction in rate of pneumonia (28% vs. 55%, P = .005), and a lower rate of cardiac complications (22% vs. 47%, P = .006), almost entirely due to a reduction in rate of atrial fibrillation (22% vs. 46%, P = .01).
There was a trend toward fewer wound infections with robotics (4% vs. 14%, P = .09), with a large difference in thoracic wound infections (0% vs. 9%, P = .06).
The two groups were statistically indistinguishable on rates of anastomotic leakage (24% and 20%) and recurrent laryngeal nerve injury (9% and 11%). The fairly high rate of anastomotic leakage was likely due to the center’s use of cervical anastomosis at the time of the trial, according to Dr. van der Sluis; they have since started using thoracic anastomosis, and will report results with that technique soon.
There was also no significant difference between groups in the rate of in-hospital mortality (4% with robotic surgery and 2% with open surgery), median hospital length of stay (14 and 16 days), and ICU length of stay (1 day in each group).
Patients in the robotics group more commonly had functional recovery within 2 weeks (70% vs. 51%, P = .04). And on the Quality of Life Questionnaire Core 30, they had better scores for health-related quality of life at discharge (57.9 vs 44.6, P = .02) and at 6 weeks (68.7 vs. 57.6, P = .03), and for physical functioning at discharge (54.5 vs. 41.0, P = .03) and 6 weeks (69.3 vs. 58.6, P = .049).
The two groups were similar on rates of R0 resection (93% and 96%) and median number of lymph nodes retrieved (27 and 25), reported Dr. van der Sluis. Pain during the first 14 days after surgery was lower for the robotics group (P = .003).
With a median follow-up of 40 months, the robotics and open groups did not differ significantly on disease-free survival (median, 26 and 28 months) and overall survival (not reached in either group).
Dr. van der Sluis disclosed no relevant conflicts of interest.
SOURCE: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
SAN FRANCISCO – Patients undergoing had less morbidity and pain and similarly good oncologic outcomes, when the surgery was performed by robot-assisted laparoscopy instead of by the open technique, a phase 3 clinical trial has found.
Investigators of the ROBOT (Robot-assisted Thoracolaparoscopic Esophagectomy vs. Open Transthoracic Esophagectomy) trial, led by Pieter C. van der Sluis, MD, a surgeon at the University Medical Center Utrecht, the Netherlands, randomized 112 patients with resectable esophageal cancer to open transthoracic esophagectomy – considered to be the gold standard – or robot-assisted minimally invasive thoracolaparoscopic esophagectomy.
“Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy improves postoperative outcome. There were no differences in oncologic outcomes, and our oncologic outcomes were in concordance with the highest standards nowadays,” Dr. van der Sluis summarized. “This trial provides evidence for the minimally invasive approach over the open approach, and especially the robot-assisted minimally invasive esophagectomy.”
The investigators will report a full cost comparison separately. “We see that costs are lower, though not significantly lower, with the robot,” he said, giving a preview. “We are going to show that the real costs of the operation are in the complications. When you have complications that involve the ICU and reoperations, some patients are in the hospital for months after the surgery. So by investing a little extra money in the surgical procedure, you might actually get it back by reducing the complications.”
When asked by an attendee why the trial did not compare robotic esophagectomy with thoracoscopic esophagectomy, Dr. van der Sluis noted that such comparison is complicated by many factors; for example, the challenge of finding surgeons skilled in both techniques, and the likelihood of small differences in outcomes, potentially requiring enrollment of thousands of patients to have adequate study power. “We concluded that such a trial might not be feasible,” he said.
Parsing the findings
“The complication rates [in this trial] are very high in the robotic and open groups, much higher than reported in some well-controlled prospective and retrospective studies,” commented session attendee Kenneth Meredith, MD, FACS, professor at Florida State University, Sarasota, and director of gastrointestinal oncology, Sarasota Memorial Institute for Cancer Care.
He wondered how extensive the investigators’ experience with robotics was and how many cases they had done on their learning curve. Data from his group suggest that surgeons must perform 29 cases of robotic esophagectomy before the complication rate drops (Dis Esophagus. 2017;30:1-7).
“That’s more then half of the patients in the robotic arm of their study,” he noted in an interview. “I find this needs to be explained. If the authors are past their learning curve, why were the complication rates so high?” Additionally, the 80% rate in the open group “is among the highest I’ve seen in many years.”
The lack of significant differences in complete resection rate and in lymph node harvest was also surprising, as he and other robotics users have found that this technique can improve these outcomes, Dr. Meredith added. This could likewise be a learning curve phenomenon.
Although ROBOT’s comparison of robotic with open esophagectomy is relevant, “it would have been more relevant to compare robotic to minimally invasive esophagectomy [MIE],” he maintained, as MIE has been shown to improve outcomes relative to open surgery (Lancet. 2012;379:1887-92).
“There are many high-volume centers in MIE but not necessarily robotics. The two are often mutually exclusive, and a multicenter trial in which each center performs high volumes of their respective technique, rather then mandating each center perform an operation they may not be facile in,” would be practical, Dr. Meredith concluded.
Study details
“The main objective in our trial was to reduce surgical trauma and reduce the percentage of complications,” Dr. van der Sluis told attendees of the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Results showed that compared with peers in the open surgery group, patients in the robotic-assisted surgery group specifically had a lower rate of pulmonary complications (32% vs. 58%, P = .005), largely due to a reduction in rate of pneumonia (28% vs. 55%, P = .005), and a lower rate of cardiac complications (22% vs. 47%, P = .006), almost entirely due to a reduction in rate of atrial fibrillation (22% vs. 46%, P = .01).
There was a trend toward fewer wound infections with robotics (4% vs. 14%, P = .09), with a large difference in thoracic wound infections (0% vs. 9%, P = .06).
The two groups were statistically indistinguishable on rates of anastomotic leakage (24% and 20%) and recurrent laryngeal nerve injury (9% and 11%). The fairly high rate of anastomotic leakage was likely due to the center’s use of cervical anastomosis at the time of the trial, according to Dr. van der Sluis; they have since started using thoracic anastomosis, and will report results with that technique soon.
There was also no significant difference between groups in the rate of in-hospital mortality (4% with robotic surgery and 2% with open surgery), median hospital length of stay (14 and 16 days), and ICU length of stay (1 day in each group).
Patients in the robotics group more commonly had functional recovery within 2 weeks (70% vs. 51%, P = .04). And on the Quality of Life Questionnaire Core 30, they had better scores for health-related quality of life at discharge (57.9 vs 44.6, P = .02) and at 6 weeks (68.7 vs. 57.6, P = .03), and for physical functioning at discharge (54.5 vs. 41.0, P = .03) and 6 weeks (69.3 vs. 58.6, P = .049).
The two groups were similar on rates of R0 resection (93% and 96%) and median number of lymph nodes retrieved (27 and 25), reported Dr. van der Sluis. Pain during the first 14 days after surgery was lower for the robotics group (P = .003).
With a median follow-up of 40 months, the robotics and open groups did not differ significantly on disease-free survival (median, 26 and 28 months) and overall survival (not reached in either group).
Dr. van der Sluis disclosed no relevant conflicts of interest.
SOURCE: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
SAN FRANCISCO – Patients undergoing had less morbidity and pain and similarly good oncologic outcomes, when the surgery was performed by robot-assisted laparoscopy instead of by the open technique, a phase 3 clinical trial has found.
Investigators of the ROBOT (Robot-assisted Thoracolaparoscopic Esophagectomy vs. Open Transthoracic Esophagectomy) trial, led by Pieter C. van der Sluis, MD, a surgeon at the University Medical Center Utrecht, the Netherlands, randomized 112 patients with resectable esophageal cancer to open transthoracic esophagectomy – considered to be the gold standard – or robot-assisted minimally invasive thoracolaparoscopic esophagectomy.
“Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy improves postoperative outcome. There were no differences in oncologic outcomes, and our oncologic outcomes were in concordance with the highest standards nowadays,” Dr. van der Sluis summarized. “This trial provides evidence for the minimally invasive approach over the open approach, and especially the robot-assisted minimally invasive esophagectomy.”
The investigators will report a full cost comparison separately. “We see that costs are lower, though not significantly lower, with the robot,” he said, giving a preview. “We are going to show that the real costs of the operation are in the complications. When you have complications that involve the ICU and reoperations, some patients are in the hospital for months after the surgery. So by investing a little extra money in the surgical procedure, you might actually get it back by reducing the complications.”
When asked by an attendee why the trial did not compare robotic esophagectomy with thoracoscopic esophagectomy, Dr. van der Sluis noted that such comparison is complicated by many factors; for example, the challenge of finding surgeons skilled in both techniques, and the likelihood of small differences in outcomes, potentially requiring enrollment of thousands of patients to have adequate study power. “We concluded that such a trial might not be feasible,” he said.
Parsing the findings
“The complication rates [in this trial] are very high in the robotic and open groups, much higher than reported in some well-controlled prospective and retrospective studies,” commented session attendee Kenneth Meredith, MD, FACS, professor at Florida State University, Sarasota, and director of gastrointestinal oncology, Sarasota Memorial Institute for Cancer Care.
He wondered how extensive the investigators’ experience with robotics was and how many cases they had done on their learning curve. Data from his group suggest that surgeons must perform 29 cases of robotic esophagectomy before the complication rate drops (Dis Esophagus. 2017;30:1-7).
“That’s more then half of the patients in the robotic arm of their study,” he noted in an interview. “I find this needs to be explained. If the authors are past their learning curve, why were the complication rates so high?” Additionally, the 80% rate in the open group “is among the highest I’ve seen in many years.”
The lack of significant differences in complete resection rate and in lymph node harvest was also surprising, as he and other robotics users have found that this technique can improve these outcomes, Dr. Meredith added. This could likewise be a learning curve phenomenon.
Although ROBOT’s comparison of robotic with open esophagectomy is relevant, “it would have been more relevant to compare robotic to minimally invasive esophagectomy [MIE],” he maintained, as MIE has been shown to improve outcomes relative to open surgery (Lancet. 2012;379:1887-92).
“There are many high-volume centers in MIE but not necessarily robotics. The two are often mutually exclusive, and a multicenter trial in which each center performs high volumes of their respective technique, rather then mandating each center perform an operation they may not be facile in,” would be practical, Dr. Meredith concluded.
Study details
“The main objective in our trial was to reduce surgical trauma and reduce the percentage of complications,” Dr. van der Sluis told attendees of the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Results showed that compared with peers in the open surgery group, patients in the robotic-assisted surgery group specifically had a lower rate of pulmonary complications (32% vs. 58%, P = .005), largely due to a reduction in rate of pneumonia (28% vs. 55%, P = .005), and a lower rate of cardiac complications (22% vs. 47%, P = .006), almost entirely due to a reduction in rate of atrial fibrillation (22% vs. 46%, P = .01).
There was a trend toward fewer wound infections with robotics (4% vs. 14%, P = .09), with a large difference in thoracic wound infections (0% vs. 9%, P = .06).
The two groups were statistically indistinguishable on rates of anastomotic leakage (24% and 20%) and recurrent laryngeal nerve injury (9% and 11%). The fairly high rate of anastomotic leakage was likely due to the center’s use of cervical anastomosis at the time of the trial, according to Dr. van der Sluis; they have since started using thoracic anastomosis, and will report results with that technique soon.
There was also no significant difference between groups in the rate of in-hospital mortality (4% with robotic surgery and 2% with open surgery), median hospital length of stay (14 and 16 days), and ICU length of stay (1 day in each group).
Patients in the robotics group more commonly had functional recovery within 2 weeks (70% vs. 51%, P = .04). And on the Quality of Life Questionnaire Core 30, they had better scores for health-related quality of life at discharge (57.9 vs 44.6, P = .02) and at 6 weeks (68.7 vs. 57.6, P = .03), and for physical functioning at discharge (54.5 vs. 41.0, P = .03) and 6 weeks (69.3 vs. 58.6, P = .049).
The two groups were similar on rates of R0 resection (93% and 96%) and median number of lymph nodes retrieved (27 and 25), reported Dr. van der Sluis. Pain during the first 14 days after surgery was lower for the robotics group (P = .003).
With a median follow-up of 40 months, the robotics and open groups did not differ significantly on disease-free survival (median, 26 and 28 months) and overall survival (not reached in either group).
Dr. van der Sluis disclosed no relevant conflicts of interest.
SOURCE: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
REPORTING FROM THE 2018 GI CANCERS SYMPOSIUM
Key clinical point: Patients with esophageal cancer undergoing esophagectomy are less likely to experience complications when the surgery is performed robotically.
Major finding: Compared with open transthoracic esophagectomy, robot-assisted minimally invasive thoracolaparoscopic esophagectomy had a lower rate of MCDC grade 2 or higher surgery-related postoperative complications (59% vs. 80%).
Data source: A single-center phase 3 randomized controlled trial among 112 patients with resectable esophageal cancer.
Disclosures: Dr. van der Sluis disclosed no relevant conflicts of interest.
Source: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
Preoperative exercise lowers postoperative lung resection complications
with a systematic review suggesting it reduces postoperative complications and duration of hospital stay.
The review and meta-analysis, published in the February British Journal of Sports Medicine, looked at the impact of preoperative exercise in patients undergoing surgery for a range of cancers.
Their review of 13 interventional trials, involving 806 patients and six tumor types, found the postoperative benefits of exercise were evident only in patients undergoing lung resection.
Data from five randomized controlled trials and one quasirandomized trial in lung cancer patients showed a significant 48% reduction in postoperative complications, and a significant mean reduction of 2.86 days in hospital stay among patients undergoing lung resection, compared with controls.
“Postoperative complication is a major concern for patients undergoing oncological surgery,” wrote Dr. Daniel Steffens, from the Surgical Outcomes Research Centre at the Royal Prince Alfred Hospital, Sydney, and his coauthors. They suggested the benefits for patients undergoing lung resection were significant enough that exercise before surgery should be considered as standard preoperative care.
“Such findings may also [have impacts] on health care costs and on patients’ quality of life, and consequently, have important implications for patients, health care professionals and policy makers.”
The exercise regimens in the lung cancer studies mostly involved aerobic exercise, such as walking, and breathing exercises to train respiratory muscles, as well as use of an exercise bicycle. The exercises were undertaken in the 1-2 weeks before surgery, with a frequency ranging from three times a week to three times a day.
The authors noted that trials involving a higher frequency of exercise showed a larger effect size, which suggested there was a dose-response relationship.
There was little evidence of benefit in other tumor types. Two studies examined the benefits of preoperative pelvic floor muscle exercises in men undergoing radical prostatectomy and found significant benefits in quality of life, assessed using the International Continence Society Male Short form. However, the authors pointed out that the quality of evidence was very low.
One study investigated the effects of preoperative mouth-opening exercise training in patients undergoing surgery for oral cancer and found enhanced postoperative quality of life in these patients, but the researchers did not report estimates.
For patients undergoing surgery for colon cancer, colorectal liver metastases, and esophageal cancer, there was no benefit of exercise either in postoperative complications or duration of hospital stay. In all these studies, the authors rated the quality of evidence as “very low.”
“Despite the evidence suggesting that exercise improves physical and mental health in patients with cancer, there are only a limited number of trials investigating the effect of preoperative exercise on patients’ quality of life,” the authors wrote. “Therefore, the effect of preoperative exercise on quality of life at short-term and long-term postoperation should be explored in future trials.”
No conflicts of interest were declared.
chestphysician@frontlinemedcom.com
SOURCE: Steffens D et al. Br J Sports Med. 2018 Feb 1. doi: 10.1136/bjsports-2017-098032
with a systematic review suggesting it reduces postoperative complications and duration of hospital stay.
The review and meta-analysis, published in the February British Journal of Sports Medicine, looked at the impact of preoperative exercise in patients undergoing surgery for a range of cancers.
Their review of 13 interventional trials, involving 806 patients and six tumor types, found the postoperative benefits of exercise were evident only in patients undergoing lung resection.
Data from five randomized controlled trials and one quasirandomized trial in lung cancer patients showed a significant 48% reduction in postoperative complications, and a significant mean reduction of 2.86 days in hospital stay among patients undergoing lung resection, compared with controls.
“Postoperative complication is a major concern for patients undergoing oncological surgery,” wrote Dr. Daniel Steffens, from the Surgical Outcomes Research Centre at the Royal Prince Alfred Hospital, Sydney, and his coauthors. They suggested the benefits for patients undergoing lung resection were significant enough that exercise before surgery should be considered as standard preoperative care.
“Such findings may also [have impacts] on health care costs and on patients’ quality of life, and consequently, have important implications for patients, health care professionals and policy makers.”
The exercise regimens in the lung cancer studies mostly involved aerobic exercise, such as walking, and breathing exercises to train respiratory muscles, as well as use of an exercise bicycle. The exercises were undertaken in the 1-2 weeks before surgery, with a frequency ranging from three times a week to three times a day.
The authors noted that trials involving a higher frequency of exercise showed a larger effect size, which suggested there was a dose-response relationship.
There was little evidence of benefit in other tumor types. Two studies examined the benefits of preoperative pelvic floor muscle exercises in men undergoing radical prostatectomy and found significant benefits in quality of life, assessed using the International Continence Society Male Short form. However, the authors pointed out that the quality of evidence was very low.
One study investigated the effects of preoperative mouth-opening exercise training in patients undergoing surgery for oral cancer and found enhanced postoperative quality of life in these patients, but the researchers did not report estimates.
For patients undergoing surgery for colon cancer, colorectal liver metastases, and esophageal cancer, there was no benefit of exercise either in postoperative complications or duration of hospital stay. In all these studies, the authors rated the quality of evidence as “very low.”
“Despite the evidence suggesting that exercise improves physical and mental health in patients with cancer, there are only a limited number of trials investigating the effect of preoperative exercise on patients’ quality of life,” the authors wrote. “Therefore, the effect of preoperative exercise on quality of life at short-term and long-term postoperation should be explored in future trials.”
No conflicts of interest were declared.
chestphysician@frontlinemedcom.com
SOURCE: Steffens D et al. Br J Sports Med. 2018 Feb 1. doi: 10.1136/bjsports-2017-098032
with a systematic review suggesting it reduces postoperative complications and duration of hospital stay.
The review and meta-analysis, published in the February British Journal of Sports Medicine, looked at the impact of preoperative exercise in patients undergoing surgery for a range of cancers.
Their review of 13 interventional trials, involving 806 patients and six tumor types, found the postoperative benefits of exercise were evident only in patients undergoing lung resection.
Data from five randomized controlled trials and one quasirandomized trial in lung cancer patients showed a significant 48% reduction in postoperative complications, and a significant mean reduction of 2.86 days in hospital stay among patients undergoing lung resection, compared with controls.
“Postoperative complication is a major concern for patients undergoing oncological surgery,” wrote Dr. Daniel Steffens, from the Surgical Outcomes Research Centre at the Royal Prince Alfred Hospital, Sydney, and his coauthors. They suggested the benefits for patients undergoing lung resection were significant enough that exercise before surgery should be considered as standard preoperative care.
“Such findings may also [have impacts] on health care costs and on patients’ quality of life, and consequently, have important implications for patients, health care professionals and policy makers.”
The exercise regimens in the lung cancer studies mostly involved aerobic exercise, such as walking, and breathing exercises to train respiratory muscles, as well as use of an exercise bicycle. The exercises were undertaken in the 1-2 weeks before surgery, with a frequency ranging from three times a week to three times a day.
The authors noted that trials involving a higher frequency of exercise showed a larger effect size, which suggested there was a dose-response relationship.
There was little evidence of benefit in other tumor types. Two studies examined the benefits of preoperative pelvic floor muscle exercises in men undergoing radical prostatectomy and found significant benefits in quality of life, assessed using the International Continence Society Male Short form. However, the authors pointed out that the quality of evidence was very low.
One study investigated the effects of preoperative mouth-opening exercise training in patients undergoing surgery for oral cancer and found enhanced postoperative quality of life in these patients, but the researchers did not report estimates.
For patients undergoing surgery for colon cancer, colorectal liver metastases, and esophageal cancer, there was no benefit of exercise either in postoperative complications or duration of hospital stay. In all these studies, the authors rated the quality of evidence as “very low.”
“Despite the evidence suggesting that exercise improves physical and mental health in patients with cancer, there are only a limited number of trials investigating the effect of preoperative exercise on patients’ quality of life,” the authors wrote. “Therefore, the effect of preoperative exercise on quality of life at short-term and long-term postoperation should be explored in future trials.”
No conflicts of interest were declared.
chestphysician@frontlinemedcom.com
SOURCE: Steffens D et al. Br J Sports Med. 2018 Feb 1. doi: 10.1136/bjsports-2017-098032
FROM THE BRITISH JOURNAL OF SPORTS MEDICINE
Key clinical point: Exercising before oncologic surgery appears to lower the risk of postoperative complications and reduce hospital stay for lung cancer patients.
Major finding: Patients who participated in preoperative exercise before lung cancer surgery had a 48% reduction in postoperative complications, compared with controls.
Data source: Systematic review and meta-analysis of 13 interventional trials involving 806 patients.
Disclosures: No conflicts of interest were declared.
Source: Steffens D et al. Br J Sports Med. 2018, Feb 1. doi: 10.1136/bjsports-2017-098032
Retroperitoneal lymphadenectomy did not impact OS and DFS for high risk, nonmetastatic renal cell carcinoma
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Lymphadenectomy did not improve overall survival or disease-free survival in patients with high-risk, nonmetastatic renal cell carcinoma who received either adjuvant therapy or placebo.
Major finding: There was no overall survival benefit for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20).
Study details: Patients enrolled prospectively in the ASSURE trial.
Disclosures: The study was funded by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Although one author did report conflicts of interest with Pfizer, Novartis, and Argos, the rest reported no conflicts of interest.
Source: Ristau BT et al. J Urol. Jan 2018. doi: 10.1016/j.juro.2017.07.042.
HIPEC shows survival benefit for advanced ovarian cancer
Patients with newly diagnosed advanced-stage ovarian cancer who were referred to receive three cycles of neoadjuvant chemotherapy experienced statistically significant improved recurrence-free survival and overall survival from hyperthermic intraperitoneal chemotherapy (HIPEC) during interval cytoreductive surgery, results of a phase 3 trial showed.
After 4.7 years’ median follow-up, 89% of patients who received surgery with no HIPEC had disease recurrence or death, compared with 81% of patients treated with HIPEC (hazard ratio, 0.66; P = .003). Patients in the HIPEC cohort experienced recurrence-free survival a median of 3.5 months longer than patients who received surgery alone (10.7 months vs. 14.2 months), Willemien J. van Driel, MD, PhD, of the Netherlands Cancer Institute, Amsterdam, and her colleagues reported in the New England Journal of Medicine.
Dr. van Driel and her coauthors also reported a median 11.8 months increased overall survival (33.9 months vs. 45.7 months) for HIPEC, compared with surgery alone.
Both recurrence-free survival and overall survival remained consistently beneficial for patients in the HIPEC group across prespecified stratification factors and subgroups, including age, histology type, regional involvement, and previous surgery, according to the researchers.
They also reported that no significant differences between the two groups were noted in the incidence of adverse events of any grade. In total, grade 3 or 4 adverse events were reported by 32 patients (27%) who received HIPEC and 30 patients (25%) who received surgery (P = .76); the most common were abdominal pain, infection, and ileus.
Combination treatment with intravenous and intraperitoneal chemotherapy has been shown to prolong overall survival after primary cytoreductive surgery, according to the authors.
“Catheter-related problems, increased demands on the patient, and gastrointestinal and renal side effects have hampered the adoption of this approach in most countries,” the researchers wrote. “Hyperthermia increases the penetration of chemotherapy at the peritoneal surface and increases the sensitivity of the cancer to chemotherapy by impairing DNA repair [and] … can circumvent most of these drawbacks while maintaining its advantages.”
This research was supported by the Dutch Cancer Society. Dr. van Driel reported no relevant financial disclosures. Two other researchers reported funding from various pharmaceutical companies as well as the KFW–Dutch Cancer Foundation.
SOURCE: van Driel WJ et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMoa1708618.
Although the data reported by Dr. van Driel and her colleagues represent an important first step, the findings “should not drive changes in practice yet,” according to David R. Spriggs, MD, and Oliver Zivanovick, MD.
Dr. Spriggs and Dr. Zivanovic raised questions surrounding the efficacy of adding HIPEC to surgery and suggested that the benefit observed by Dr. van Driel and her coauthors could be attributed to several variables such as the route of intraperitoneal administration or the skill level of the attending surgeon.
Currently, certain patients with advanced ovarian cancer receive primary surgery instead of neoadjuvant chemotherapy. HIPEC does not change that approach, Dr. Spriggs and Dr. Zivanovic said.
They went on to note that further “well-designed” research could reveal other patient subgroups that warrant further investigation such as those who underwent an optimal cytoreductive procedure.
“These considerations will be important for clinical trial investigators as they focus on the positive effect of HIPEC as an intervention as compared with the effects of promising new agent combinations or immunotherapy treatments,” they wrote.
Dr. Spriggs is the associate director for clinical and translational research at Memorial Sloan Kettering Cancer Center in New York, and Dr. Zivanovic is a gynecologic oncologic surgeon at Sloan Kettering. These remarks were taken from their invited commentary on the report by Dr. van Driel and her associates. Dr. Spriggs reported that he is employed by the New England Journal of Medicine as an associate editor. Dr. Zivanovic reported no relevant financial disclosures.
SOURCE: Spriggs DR et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMe1714556.
Although the data reported by Dr. van Driel and her colleagues represent an important first step, the findings “should not drive changes in practice yet,” according to David R. Spriggs, MD, and Oliver Zivanovick, MD.
Dr. Spriggs and Dr. Zivanovic raised questions surrounding the efficacy of adding HIPEC to surgery and suggested that the benefit observed by Dr. van Driel and her coauthors could be attributed to several variables such as the route of intraperitoneal administration or the skill level of the attending surgeon.
Currently, certain patients with advanced ovarian cancer receive primary surgery instead of neoadjuvant chemotherapy. HIPEC does not change that approach, Dr. Spriggs and Dr. Zivanovic said.
They went on to note that further “well-designed” research could reveal other patient subgroups that warrant further investigation such as those who underwent an optimal cytoreductive procedure.
“These considerations will be important for clinical trial investigators as they focus on the positive effect of HIPEC as an intervention as compared with the effects of promising new agent combinations or immunotherapy treatments,” they wrote.
Dr. Spriggs is the associate director for clinical and translational research at Memorial Sloan Kettering Cancer Center in New York, and Dr. Zivanovic is a gynecologic oncologic surgeon at Sloan Kettering. These remarks were taken from their invited commentary on the report by Dr. van Driel and her associates. Dr. Spriggs reported that he is employed by the New England Journal of Medicine as an associate editor. Dr. Zivanovic reported no relevant financial disclosures.
SOURCE: Spriggs DR et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMe1714556.
Although the data reported by Dr. van Driel and her colleagues represent an important first step, the findings “should not drive changes in practice yet,” according to David R. Spriggs, MD, and Oliver Zivanovick, MD.
Dr. Spriggs and Dr. Zivanovic raised questions surrounding the efficacy of adding HIPEC to surgery and suggested that the benefit observed by Dr. van Driel and her coauthors could be attributed to several variables such as the route of intraperitoneal administration or the skill level of the attending surgeon.
Currently, certain patients with advanced ovarian cancer receive primary surgery instead of neoadjuvant chemotherapy. HIPEC does not change that approach, Dr. Spriggs and Dr. Zivanovic said.
They went on to note that further “well-designed” research could reveal other patient subgroups that warrant further investigation such as those who underwent an optimal cytoreductive procedure.
“These considerations will be important for clinical trial investigators as they focus on the positive effect of HIPEC as an intervention as compared with the effects of promising new agent combinations or immunotherapy treatments,” they wrote.
Dr. Spriggs is the associate director for clinical and translational research at Memorial Sloan Kettering Cancer Center in New York, and Dr. Zivanovic is a gynecologic oncologic surgeon at Sloan Kettering. These remarks were taken from their invited commentary on the report by Dr. van Driel and her associates. Dr. Spriggs reported that he is employed by the New England Journal of Medicine as an associate editor. Dr. Zivanovic reported no relevant financial disclosures.
SOURCE: Spriggs DR et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMe1714556.
Patients with newly diagnosed advanced-stage ovarian cancer who were referred to receive three cycles of neoadjuvant chemotherapy experienced statistically significant improved recurrence-free survival and overall survival from hyperthermic intraperitoneal chemotherapy (HIPEC) during interval cytoreductive surgery, results of a phase 3 trial showed.
After 4.7 years’ median follow-up, 89% of patients who received surgery with no HIPEC had disease recurrence or death, compared with 81% of patients treated with HIPEC (hazard ratio, 0.66; P = .003). Patients in the HIPEC cohort experienced recurrence-free survival a median of 3.5 months longer than patients who received surgery alone (10.7 months vs. 14.2 months), Willemien J. van Driel, MD, PhD, of the Netherlands Cancer Institute, Amsterdam, and her colleagues reported in the New England Journal of Medicine.
Dr. van Driel and her coauthors also reported a median 11.8 months increased overall survival (33.9 months vs. 45.7 months) for HIPEC, compared with surgery alone.
Both recurrence-free survival and overall survival remained consistently beneficial for patients in the HIPEC group across prespecified stratification factors and subgroups, including age, histology type, regional involvement, and previous surgery, according to the researchers.
They also reported that no significant differences between the two groups were noted in the incidence of adverse events of any grade. In total, grade 3 or 4 adverse events were reported by 32 patients (27%) who received HIPEC and 30 patients (25%) who received surgery (P = .76); the most common were abdominal pain, infection, and ileus.
Combination treatment with intravenous and intraperitoneal chemotherapy has been shown to prolong overall survival after primary cytoreductive surgery, according to the authors.
“Catheter-related problems, increased demands on the patient, and gastrointestinal and renal side effects have hampered the adoption of this approach in most countries,” the researchers wrote. “Hyperthermia increases the penetration of chemotherapy at the peritoneal surface and increases the sensitivity of the cancer to chemotherapy by impairing DNA repair [and] … can circumvent most of these drawbacks while maintaining its advantages.”
This research was supported by the Dutch Cancer Society. Dr. van Driel reported no relevant financial disclosures. Two other researchers reported funding from various pharmaceutical companies as well as the KFW–Dutch Cancer Foundation.
SOURCE: van Driel WJ et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMoa1708618.
Patients with newly diagnosed advanced-stage ovarian cancer who were referred to receive three cycles of neoadjuvant chemotherapy experienced statistically significant improved recurrence-free survival and overall survival from hyperthermic intraperitoneal chemotherapy (HIPEC) during interval cytoreductive surgery, results of a phase 3 trial showed.
After 4.7 years’ median follow-up, 89% of patients who received surgery with no HIPEC had disease recurrence or death, compared with 81% of patients treated with HIPEC (hazard ratio, 0.66; P = .003). Patients in the HIPEC cohort experienced recurrence-free survival a median of 3.5 months longer than patients who received surgery alone (10.7 months vs. 14.2 months), Willemien J. van Driel, MD, PhD, of the Netherlands Cancer Institute, Amsterdam, and her colleagues reported in the New England Journal of Medicine.
Dr. van Driel and her coauthors also reported a median 11.8 months increased overall survival (33.9 months vs. 45.7 months) for HIPEC, compared with surgery alone.
Both recurrence-free survival and overall survival remained consistently beneficial for patients in the HIPEC group across prespecified stratification factors and subgroups, including age, histology type, regional involvement, and previous surgery, according to the researchers.
They also reported that no significant differences between the two groups were noted in the incidence of adverse events of any grade. In total, grade 3 or 4 adverse events were reported by 32 patients (27%) who received HIPEC and 30 patients (25%) who received surgery (P = .76); the most common were abdominal pain, infection, and ileus.
Combination treatment with intravenous and intraperitoneal chemotherapy has been shown to prolong overall survival after primary cytoreductive surgery, according to the authors.
“Catheter-related problems, increased demands on the patient, and gastrointestinal and renal side effects have hampered the adoption of this approach in most countries,” the researchers wrote. “Hyperthermia increases the penetration of chemotherapy at the peritoneal surface and increases the sensitivity of the cancer to chemotherapy by impairing DNA repair [and] … can circumvent most of these drawbacks while maintaining its advantages.”
This research was supported by the Dutch Cancer Society. Dr. van Driel reported no relevant financial disclosures. Two other researchers reported funding from various pharmaceutical companies as well as the KFW–Dutch Cancer Foundation.
SOURCE: van Driel WJ et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMoa1708618.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Administering HIPEC during interval cytoreductive surgery lengthened survival without increasing safety risk for patients with advanced-stage ovarian cancer.
Major finding: Patients who received HIPEC experienced a median recurrence-free survival that was 3.5 months longer and overall survival that was 11.8 months longer than patients who received surgery alone.
Study details: A multicenter, open-label phase 3 trial that randomly assigned patients who had received neoadjuvant chemotherapy to receive either HIPEC or surgery alone with an endpoint of recurrence-free survival.
Disclosures: This research was supported by the Dutch Cancer Society. Dr. van Driel reported no relevant financial disclosures. Two other researchers reported funding from various pharmaceutical companies as well as the KFW–Dutch Cancer Foundation.
Source: van Driel WJ et al. N Engl J Med. 2018 Jan 18. doi: 10.1056/NEJMoa1708618.
Age at RRSO affects peritoneal cancer risk in BRCA mutation carriers
Carriers of the BRCA1/2 mutation who undergo risk-reducing salpingo-oophorectomy (RRSO) still face a risk of developing metachronous peritoneal carcinomatosis, according to new findings published in Cancer.
The risk was predominantly observed in BRCA1 mutation carriers, and disease development generally occurred within 5 years following RRSO. Women who underwent RRSO at an age older than that currently recommended also had a higher risk of developing peritoneal carcinoma.
Their median age was 52 years at the time they underwent surgery, and 60 years when they were diagnosed with peritoneal carcinomatosis. As compared with the control group, the patients were older at the time they underwent RRSO (P = .025).
In eight RRSO specimens that were obtained from women who subsequently developed peritoneal carcinomatosis, five (62.5%), had serous tubal intraepithelial carcinoma (STIC) and one had epithelial atypia.
“The findings of the current study can be used to refine gynecologic counseling for BRCA1/2 mutation carriers who consider risk-reducing surgery and to stress the importance of complete RRSO at the recommended ages,” wrote lead author Marline G. Harmsen, MD, PhD, of the Radboud University Medical Center, Nijmegen, the Netherlands.
BRCA1/2 mutation carriers face a higher risk of developing ovarian carcinoma, and RRSO can reduce that risk by 80%-96%. Surgery is recommended for carriers of BRCA1 mutations aged 35-40 years and for BRCA2 mutation carriers aged 40-45 years.
In this study, Dr. Harmsen and her colleagues conducted a literature search in order to collect and analyze clinical and pathological data from women with BRCA1/2 mutation who developed peritoneal carcinomatosis following RRSO. The cases that were identified were then compared with a cohort from a single institution.
Of the 36 cases that were identified, 86.1% were BRCA1 mutation carriers and the remaining 5 patients had a BRCA2 mutation. The median age at the time of surgery differed significantly between BRCA1 (51 years; range, 30-71 years) and BRCA2 mutation carriers (57 years; range, 56-65 years) (P = .006).
The majority of women had undergone salpingo-oophorectomy (31; 86.1%), and 16 (44.4%) had also had a hysterectomy.
The authors found that several statistically significant differences between the case studies and the control group: the median age surgery (52 vs. 46 years), percentage of BRCA1 mutation carriers (86.1% vs. 53.1%, P less than .001), and percentage of serous tubal intraepithelial carcinomas in the RRSO specimens (62.5% vs. 0%; P less than .001).
SOURCE: Hamsen MG et al. Cancer. 2018 Jan 9. doi: 10.1002/cncr.31211.
In an accompanying editorial, Christine S. Walsh, MD, of Cedars-Sinai Medical Center in Los Angeles, notes that the study authors have “done a commendable job in trying to shed light on a rare condition,” which occurs in about 1%-4% of women who undergo risk-reducing salpingo-oophorectomy (RRSO).
These findings can provide more information to clinicians, as they seek to guide and counsel women after they undergo RRSO, she wrote.
Dr. Walsh pointed out that National Comprehensive Cancer Network guidelines for genetic/familial high-risk assessment of breast and ovarian cancer specify the optimal ages when RRSO should be performed, but despite efforts to encourage this, occult gynecologic carcinomas still are found in approximately 4.5%-9% of women, with serous tubal intraepithelial carcinoma lesions developing in approximately 5%-8% of them.
“Ideally, the goal should be to intervene with a prophylactic surgery before the development of cancerous or precancerous pathology,” she wrote. Dr. Walsh had no disclosures.
In an accompanying editorial, Christine S. Walsh, MD, of Cedars-Sinai Medical Center in Los Angeles, notes that the study authors have “done a commendable job in trying to shed light on a rare condition,” which occurs in about 1%-4% of women who undergo risk-reducing salpingo-oophorectomy (RRSO).
These findings can provide more information to clinicians, as they seek to guide and counsel women after they undergo RRSO, she wrote.
Dr. Walsh pointed out that National Comprehensive Cancer Network guidelines for genetic/familial high-risk assessment of breast and ovarian cancer specify the optimal ages when RRSO should be performed, but despite efforts to encourage this, occult gynecologic carcinomas still are found in approximately 4.5%-9% of women, with serous tubal intraepithelial carcinoma lesions developing in approximately 5%-8% of them.
“Ideally, the goal should be to intervene with a prophylactic surgery before the development of cancerous or precancerous pathology,” she wrote. Dr. Walsh had no disclosures.
In an accompanying editorial, Christine S. Walsh, MD, of Cedars-Sinai Medical Center in Los Angeles, notes that the study authors have “done a commendable job in trying to shed light on a rare condition,” which occurs in about 1%-4% of women who undergo risk-reducing salpingo-oophorectomy (RRSO).
These findings can provide more information to clinicians, as they seek to guide and counsel women after they undergo RRSO, she wrote.
Dr. Walsh pointed out that National Comprehensive Cancer Network guidelines for genetic/familial high-risk assessment of breast and ovarian cancer specify the optimal ages when RRSO should be performed, but despite efforts to encourage this, occult gynecologic carcinomas still are found in approximately 4.5%-9% of women, with serous tubal intraepithelial carcinoma lesions developing in approximately 5%-8% of them.
“Ideally, the goal should be to intervene with a prophylactic surgery before the development of cancerous or precancerous pathology,” she wrote. Dr. Walsh had no disclosures.
Carriers of the BRCA1/2 mutation who undergo risk-reducing salpingo-oophorectomy (RRSO) still face a risk of developing metachronous peritoneal carcinomatosis, according to new findings published in Cancer.
The risk was predominantly observed in BRCA1 mutation carriers, and disease development generally occurred within 5 years following RRSO. Women who underwent RRSO at an age older than that currently recommended also had a higher risk of developing peritoneal carcinoma.
Their median age was 52 years at the time they underwent surgery, and 60 years when they were diagnosed with peritoneal carcinomatosis. As compared with the control group, the patients were older at the time they underwent RRSO (P = .025).
In eight RRSO specimens that were obtained from women who subsequently developed peritoneal carcinomatosis, five (62.5%), had serous tubal intraepithelial carcinoma (STIC) and one had epithelial atypia.
“The findings of the current study can be used to refine gynecologic counseling for BRCA1/2 mutation carriers who consider risk-reducing surgery and to stress the importance of complete RRSO at the recommended ages,” wrote lead author Marline G. Harmsen, MD, PhD, of the Radboud University Medical Center, Nijmegen, the Netherlands.
BRCA1/2 mutation carriers face a higher risk of developing ovarian carcinoma, and RRSO can reduce that risk by 80%-96%. Surgery is recommended for carriers of BRCA1 mutations aged 35-40 years and for BRCA2 mutation carriers aged 40-45 years.
In this study, Dr. Harmsen and her colleagues conducted a literature search in order to collect and analyze clinical and pathological data from women with BRCA1/2 mutation who developed peritoneal carcinomatosis following RRSO. The cases that were identified were then compared with a cohort from a single institution.
Of the 36 cases that were identified, 86.1% were BRCA1 mutation carriers and the remaining 5 patients had a BRCA2 mutation. The median age at the time of surgery differed significantly between BRCA1 (51 years; range, 30-71 years) and BRCA2 mutation carriers (57 years; range, 56-65 years) (P = .006).
The majority of women had undergone salpingo-oophorectomy (31; 86.1%), and 16 (44.4%) had also had a hysterectomy.
The authors found that several statistically significant differences between the case studies and the control group: the median age surgery (52 vs. 46 years), percentage of BRCA1 mutation carriers (86.1% vs. 53.1%, P less than .001), and percentage of serous tubal intraepithelial carcinomas in the RRSO specimens (62.5% vs. 0%; P less than .001).
SOURCE: Hamsen MG et al. Cancer. 2018 Jan 9. doi: 10.1002/cncr.31211.
Carriers of the BRCA1/2 mutation who undergo risk-reducing salpingo-oophorectomy (RRSO) still face a risk of developing metachronous peritoneal carcinomatosis, according to new findings published in Cancer.
The risk was predominantly observed in BRCA1 mutation carriers, and disease development generally occurred within 5 years following RRSO. Women who underwent RRSO at an age older than that currently recommended also had a higher risk of developing peritoneal carcinoma.
Their median age was 52 years at the time they underwent surgery, and 60 years when they were diagnosed with peritoneal carcinomatosis. As compared with the control group, the patients were older at the time they underwent RRSO (P = .025).
In eight RRSO specimens that were obtained from women who subsequently developed peritoneal carcinomatosis, five (62.5%), had serous tubal intraepithelial carcinoma (STIC) and one had epithelial atypia.
“The findings of the current study can be used to refine gynecologic counseling for BRCA1/2 mutation carriers who consider risk-reducing surgery and to stress the importance of complete RRSO at the recommended ages,” wrote lead author Marline G. Harmsen, MD, PhD, of the Radboud University Medical Center, Nijmegen, the Netherlands.
BRCA1/2 mutation carriers face a higher risk of developing ovarian carcinoma, and RRSO can reduce that risk by 80%-96%. Surgery is recommended for carriers of BRCA1 mutations aged 35-40 years and for BRCA2 mutation carriers aged 40-45 years.
In this study, Dr. Harmsen and her colleagues conducted a literature search in order to collect and analyze clinical and pathological data from women with BRCA1/2 mutation who developed peritoneal carcinomatosis following RRSO. The cases that were identified were then compared with a cohort from a single institution.
Of the 36 cases that were identified, 86.1% were BRCA1 mutation carriers and the remaining 5 patients had a BRCA2 mutation. The median age at the time of surgery differed significantly between BRCA1 (51 years; range, 30-71 years) and BRCA2 mutation carriers (57 years; range, 56-65 years) (P = .006).
The majority of women had undergone salpingo-oophorectomy (31; 86.1%), and 16 (44.4%) had also had a hysterectomy.
The authors found that several statistically significant differences between the case studies and the control group: the median age surgery (52 vs. 46 years), percentage of BRCA1 mutation carriers (86.1% vs. 53.1%, P less than .001), and percentage of serous tubal intraepithelial carcinomas in the RRSO specimens (62.5% vs. 0%; P less than .001).
SOURCE: Hamsen MG et al. Cancer. 2018 Jan 9. doi: 10.1002/cncr.31211.
FROM CANCER
Key clinical point: BRCA mutation carriers who undergo risk-reducing surgery at an older age have a higher risk of developing metachronous peritoneal carcinomatosis.
Major finding: Women with BRCA 1 were at a higher risk for developing peritoneal carcinomatosis, as were those who underwent surgery at an older age.
Data source: A literature search to identify patients with BRCA 1/2 mutations who had undergone risk-reducing surgery and developed peritoneal carcinomatosis; 36 cases were identified and compared with a control group from a single institution.
Disclosures: No specific funding was disclosed. The authors had no disclosures.
Source: Hamsen MG et al. Cancer. 2018 Jan 9. doi: 10.1002/cncr.31211.
Pain after breast surgery may not be caused by the operation
, according to a study of almost 2,000 women recruited from the Mastectomy Reconstructive Outcomes Consortium (MROC).
In the February issue of The Breast, investigators from the University of Michigan, Ann Arbor and Memorial Sloan Kettering Cancer Center, New York, wrote that almost half of the study subjects had some level of pain before their operations and that, at 2 years afterward, their pain had increased but not in a clinically meaningful way. This finding is consistent with earlier research, which investigators noted found that “one-fourth to one-half of women who undergo postmastectomy report persistent pain months and years after surgery.”
“Average clinical pain severity was strikingly similar for preoperative and postoperative assessments,” said lead author Randy S. Roth, PhD, of the University of Michigan, and his coauthors. “Postoperative levels of pain, acute postoperative pain and (marginally) level of depression held consistent relationship at 2-year follow-up with all outcome measures.”
The prospective, multicenter cohort study of 1,996 women was undertaken over 5 years. Most patients had immediate (92.7%) and bilateral (53.8%) reconstruction; 47.6% had sentinel lymph node biopsy and 25.9% had axillary lymph node dissection. Most had no adjuvant therapy: 70.3% received no radiation and 52.7% no chemotherapy.
At 2 years, the Numerical Pain Rating Scale (NPRS) measured what Dr. Roth and his coauthors called a “significant increase in pain intensity” – from an average rating of 1.1 to 1.2, an increase of 9%. However, the absolute change and standard deviation (1.7 for both intervals) “suggest that this was not a clinically meaningful change.” The researchers also recorded more complaints of bodily discomfort after 2 years, “but the statistical parameters again indicate little clinically meaningful differences from preoperative status.”
Pain ratings measured with the McGill Pain Questionnaire showed a significant decrease in the MPQ affective pain rating, from 1.6 preoperatively to 0.8 at 2 years (P less than .001), and virtually no change in the MPQ sensory rating, from 3.2 to 3.1.
The researchers drew some conclusions about demographic profiles and pain after breast reconstruction. Older age was associated with more severe pain on NPRS, and higher body mass index was linked with chronic postsurgical pain for the MPQ sensory rating, NPRS score, and body discomfort scores.
Treatment characteristics associated with chronic postsurgical pain (CPSP) include radiation therapy during or after reconstruction and chemotherapy before reconstruction. Chemotherapy during or after reconstruction was associated with higher MPQ affective rating scores at 2 years (P = .011), as was chemotherapy both before and during or after reconstruction (P = .001). The latter also was linked to higher NPRS scores (P = .0015).
The type of surgery also was a factor in CPSP, the researchers wrote. Both MPQ sensory and affective ratings were higher in women who had free transverse flap surgery, or deep or superficial inferior epigastric perforator surgery than in women who had tissue expander/implant reconstruction. Lymph node status and timing of surgery had no impact on chronic pain.
One noteworthy finding, Dr. Roth and his coauthors wrote, is that “careful examination of our data suggests that CPSP following breast reconstruction may be of less clinical concern as a direct consequence of breast reconstruction than suggested by previous investigations of major surgery, including mastectomy and breast reconstruction.” Future studies of chronic postsurgical pain in breast reconstruction “will require greater methodological rigor” to reach more sound conclusions to use in patient counseling.
Dr. Roth and his coauthors had no financial relationships to disclose.
SOURCE: Roth RS et al. Breast 2018;37:119-25.
, according to a study of almost 2,000 women recruited from the Mastectomy Reconstructive Outcomes Consortium (MROC).
In the February issue of The Breast, investigators from the University of Michigan, Ann Arbor and Memorial Sloan Kettering Cancer Center, New York, wrote that almost half of the study subjects had some level of pain before their operations and that, at 2 years afterward, their pain had increased but not in a clinically meaningful way. This finding is consistent with earlier research, which investigators noted found that “one-fourth to one-half of women who undergo postmastectomy report persistent pain months and years after surgery.”
“Average clinical pain severity was strikingly similar for preoperative and postoperative assessments,” said lead author Randy S. Roth, PhD, of the University of Michigan, and his coauthors. “Postoperative levels of pain, acute postoperative pain and (marginally) level of depression held consistent relationship at 2-year follow-up with all outcome measures.”
The prospective, multicenter cohort study of 1,996 women was undertaken over 5 years. Most patients had immediate (92.7%) and bilateral (53.8%) reconstruction; 47.6% had sentinel lymph node biopsy and 25.9% had axillary lymph node dissection. Most had no adjuvant therapy: 70.3% received no radiation and 52.7% no chemotherapy.
At 2 years, the Numerical Pain Rating Scale (NPRS) measured what Dr. Roth and his coauthors called a “significant increase in pain intensity” – from an average rating of 1.1 to 1.2, an increase of 9%. However, the absolute change and standard deviation (1.7 for both intervals) “suggest that this was not a clinically meaningful change.” The researchers also recorded more complaints of bodily discomfort after 2 years, “but the statistical parameters again indicate little clinically meaningful differences from preoperative status.”
Pain ratings measured with the McGill Pain Questionnaire showed a significant decrease in the MPQ affective pain rating, from 1.6 preoperatively to 0.8 at 2 years (P less than .001), and virtually no change in the MPQ sensory rating, from 3.2 to 3.1.
The researchers drew some conclusions about demographic profiles and pain after breast reconstruction. Older age was associated with more severe pain on NPRS, and higher body mass index was linked with chronic postsurgical pain for the MPQ sensory rating, NPRS score, and body discomfort scores.
Treatment characteristics associated with chronic postsurgical pain (CPSP) include radiation therapy during or after reconstruction and chemotherapy before reconstruction. Chemotherapy during or after reconstruction was associated with higher MPQ affective rating scores at 2 years (P = .011), as was chemotherapy both before and during or after reconstruction (P = .001). The latter also was linked to higher NPRS scores (P = .0015).
The type of surgery also was a factor in CPSP, the researchers wrote. Both MPQ sensory and affective ratings were higher in women who had free transverse flap surgery, or deep or superficial inferior epigastric perforator surgery than in women who had tissue expander/implant reconstruction. Lymph node status and timing of surgery had no impact on chronic pain.
One noteworthy finding, Dr. Roth and his coauthors wrote, is that “careful examination of our data suggests that CPSP following breast reconstruction may be of less clinical concern as a direct consequence of breast reconstruction than suggested by previous investigations of major surgery, including mastectomy and breast reconstruction.” Future studies of chronic postsurgical pain in breast reconstruction “will require greater methodological rigor” to reach more sound conclusions to use in patient counseling.
Dr. Roth and his coauthors had no financial relationships to disclose.
SOURCE: Roth RS et al. Breast 2018;37:119-25.
, according to a study of almost 2,000 women recruited from the Mastectomy Reconstructive Outcomes Consortium (MROC).
In the February issue of The Breast, investigators from the University of Michigan, Ann Arbor and Memorial Sloan Kettering Cancer Center, New York, wrote that almost half of the study subjects had some level of pain before their operations and that, at 2 years afterward, their pain had increased but not in a clinically meaningful way. This finding is consistent with earlier research, which investigators noted found that “one-fourth to one-half of women who undergo postmastectomy report persistent pain months and years after surgery.”
“Average clinical pain severity was strikingly similar for preoperative and postoperative assessments,” said lead author Randy S. Roth, PhD, of the University of Michigan, and his coauthors. “Postoperative levels of pain, acute postoperative pain and (marginally) level of depression held consistent relationship at 2-year follow-up with all outcome measures.”
The prospective, multicenter cohort study of 1,996 women was undertaken over 5 years. Most patients had immediate (92.7%) and bilateral (53.8%) reconstruction; 47.6% had sentinel lymph node biopsy and 25.9% had axillary lymph node dissection. Most had no adjuvant therapy: 70.3% received no radiation and 52.7% no chemotherapy.
At 2 years, the Numerical Pain Rating Scale (NPRS) measured what Dr. Roth and his coauthors called a “significant increase in pain intensity” – from an average rating of 1.1 to 1.2, an increase of 9%. However, the absolute change and standard deviation (1.7 for both intervals) “suggest that this was not a clinically meaningful change.” The researchers also recorded more complaints of bodily discomfort after 2 years, “but the statistical parameters again indicate little clinically meaningful differences from preoperative status.”
Pain ratings measured with the McGill Pain Questionnaire showed a significant decrease in the MPQ affective pain rating, from 1.6 preoperatively to 0.8 at 2 years (P less than .001), and virtually no change in the MPQ sensory rating, from 3.2 to 3.1.
The researchers drew some conclusions about demographic profiles and pain after breast reconstruction. Older age was associated with more severe pain on NPRS, and higher body mass index was linked with chronic postsurgical pain for the MPQ sensory rating, NPRS score, and body discomfort scores.
Treatment characteristics associated with chronic postsurgical pain (CPSP) include radiation therapy during or after reconstruction and chemotherapy before reconstruction. Chemotherapy during or after reconstruction was associated with higher MPQ affective rating scores at 2 years (P = .011), as was chemotherapy both before and during or after reconstruction (P = .001). The latter also was linked to higher NPRS scores (P = .0015).
The type of surgery also was a factor in CPSP, the researchers wrote. Both MPQ sensory and affective ratings were higher in women who had free transverse flap surgery, or deep or superficial inferior epigastric perforator surgery than in women who had tissue expander/implant reconstruction. Lymph node status and timing of surgery had no impact on chronic pain.
One noteworthy finding, Dr. Roth and his coauthors wrote, is that “careful examination of our data suggests that CPSP following breast reconstruction may be of less clinical concern as a direct consequence of breast reconstruction than suggested by previous investigations of major surgery, including mastectomy and breast reconstruction.” Future studies of chronic postsurgical pain in breast reconstruction “will require greater methodological rigor” to reach more sound conclusions to use in patient counseling.
Dr. Roth and his coauthors had no financial relationships to disclose.
SOURCE: Roth RS et al. Breast 2018;37:119-25.
FROM THE BREAST
Key clinical point: Breast reconstruction surgery may not be the cause of persistent pain afterward.
Major finding: McGill Pain Questionnaire affective pain rating decreased from 1.6 preoperatively to 0.8 at 2 years.
Data source: Five-year prospective multicenter cohort study of 1,966 women recruited for the Mastectomy Reconstruction Outcomes Consortium.
Disclosures: Dr. Roth and his coauthors reported having no financial disclosures.
Source: Roth RS et al. Breast 2018;37:119-25.
EndoPredict results reflected tumor response to neoadjuvant therapy
SAN ANTONIO – The results of the EndoPredict test appear to predict tumor response in patients with early hormone receptor–positive, HER2-negative breast cancer given neoadjuvant therapy, based on results of a study conducted by the Austrian Breast & Colorectal Cancer Study Group (ABCSG).
“Very good tumor shrinkage in estrogen receptor–positive, HER2-negative disease is going to happen only in a minority of patients, and biomarkers that would predict excellent tumor shrinkage are an unmet medical need,” commented lead investigator Peter Dubsky, MD, PhD, who is head of the Breast Center at Hirslanden Klinik St. Anna, Lucerne, Switzerland. “As a surgeon, that would help me to predict breast conservation at diagnosis, but as a surgical oncologist, I would also recognize that tumor response is an important component of future survival.”
The ABCSG findings suggest expanded utility for EndoPredict. The test’s molecular score is currently used along with tumor size and nodal status to predict the 10-year distant recurrence rate, and whether patients may safely forgo chemotherapy or are at high risk and may need adjuvant chemotherapy in addition to endocrine therapy.
Dr. Dubsky and his coinvestigators assessed performance of the EndoPredict test among 217 patients treated on ABCSG 34, a randomized phase 2 neoadjuvant trial. Findings showed that among patients given neoadjuvant endocrine therapy because they had less aggressive disease features, an EndoPredict high-risk result was associated with poor response (negative predictive value of 92%), defined as a residual cancer burden (RCB) of II or III, he reported at the San Antonio Breast Cancer Symposium.
On the other hand, among patients given neoadjuvant chemotherapy because they had more aggressive disease features, a low-risk result was associated with poor response (negative predictive value of 100%).
“Clinicians really gave us two distinct cohorts within ABCSG 34. In the luminal A–type patients who were treated with neoendocrine therapy, a high EndoPredict score predicted a low chance of tumor shrinkage. In the more aggressive ER-positive tumors, so-called luminal B type, treated with neoadjuvant chemotherapy, there was absolutely no excellent response in the low-risk group,” Dr. Dubsky summarized. “We believe that this molecular score may contribute to patient selection for biomarker-driven studies, especially in the neoadjuvant setting.”
Session attendee Steven Vogl, MD, a medical oncologist with the Montefiore Medical Center in New York, commented, “I have trouble correlating an RCB of 0 or I with what you as a surgeon do for the patient, because you are talking about pathologic complete response or just a few cells there. That’s not what determines how much breast you take off: It’s determined by the total size of the tumor and the size of the breast. So if it’s less than a few centimeters, I’m sure you can do a lumpectomy in every patient. Tell me why I should care that you are getting an RCB of 0 or I in these endocrine patients.”
“Because it’s more likely that these patients will have a smaller tumor and better tumor shrinkage,” Dr. Dubsky replied. “You are of course right, RCB 0 or I was not designed to help surgeons. But it helps me as a translational scientist to have a surrogate and an exact classification for good tumor shrinkage. That’s how I used it.”
C. Kent Osborne, MD, codirector of SABCS and director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston, asked, “We see it in the clinic, and I’m sure you have as well, patients whose tumor doesn’t shrink very much, but the Ki-67 really drops. And that may or may not be a better factor than the actual tumor shrinkage. So how many patients who had tumors that didn’t shrink, which was your endpoint, had a reduction in Ki-67 that was, say, 5%?”
“We haven’t looked at that specifically, but we will do so as we carry on with the follow-up of these patients. Then we can learn more about the prognosis,” Dr. Dubsky replied.
Study details
ABCSG 34 was a randomized phase 2 trial testing addition of the cancer vaccine tecemotide (Stimuvax) to neoadjuvant standard of care among patients with HER2-negative early breast cancer.
Dr. Dubsky and coinvestigators restricted analyses to patients with hormone receptor–positive disease who, depending on clinical and pathologic factors, received neoadjuvant chemotherapy (eight cycles of epirubicin-cyclophosphamide and docetaxel) or neoadjuvant endocrine therapy (6 months of letrozole [Femara]) as standard of care. They were then randomized to additionally receive tecemotide or not before undergoing surgery.
Overall, 25% of the 134 patients in the neoadjuvant chemotherapy group had a good tumor response, defined as pathologic complete response in both breast and nodes (RCB of 0) or minimal residual disease (RCB of I).
Higher EndoPredict score was associated with greater likelihood of good response to chemotherapy. EndoPredict risk group (high vs. low) had a negative predictive value of 100%, a positive predictive value of 26.4%, a true-positive rate of 100%, and a true-negative rate of 8.9% for predicting response (P = .112).
Area under the receiver operating characteristic curve was 0.736.
In a multivariate model, EndoPredict score as a continuous variable was not an independent predictor of response. “The good response was largely driven by covariates that included cell proliferation, and it was Ki-67 that was significant,” Dr. Dubsky noted.
Overall, 18% of the 83 patients in the neoadjuvant endocrine therapy group had a good tumor response (RCB of 0 or I). Here, lower EndoPredict score was associated with greater likelihood of good response. EndoPredict risk group (high vs. low) had a negative predictive value of 92.3%, a positive predictive value of 27.3%, a true-positive rate of 80.0%, and a true-negative rate of 52.9% for predicting response (P = .024). Area under the curve was 0.726.
In a multivariate model here, EndoPredict score as a continuous variable, its estrogen receptor–signaling/differentiation component, and Ki-67 did not independently predict response. “It was maybe a bit surprising that T stage was the strongest factor, possibly indicating that we should have simply treated those women longer than 6 months,” Dr. Dubsky commented. The EndoPredict proliferation component was also a significant predictor.
“Possibly, the very narrow distribution of Ki-67 [among patients given neoendocrine therapy] may have prevented this factor from playing a bigger role in this particular model,” he speculated.
Dr. Dubsky disclosed that he receives consulting fees from Myriad, the maker of EndoPredict, and from Cepheid, Nanostring, and Amgen.
SOURCE: Dubsky P et al. SABCS 2017 Abstract GS6-04.
SAN ANTONIO – The results of the EndoPredict test appear to predict tumor response in patients with early hormone receptor–positive, HER2-negative breast cancer given neoadjuvant therapy, based on results of a study conducted by the Austrian Breast & Colorectal Cancer Study Group (ABCSG).
“Very good tumor shrinkage in estrogen receptor–positive, HER2-negative disease is going to happen only in a minority of patients, and biomarkers that would predict excellent tumor shrinkage are an unmet medical need,” commented lead investigator Peter Dubsky, MD, PhD, who is head of the Breast Center at Hirslanden Klinik St. Anna, Lucerne, Switzerland. “As a surgeon, that would help me to predict breast conservation at diagnosis, but as a surgical oncologist, I would also recognize that tumor response is an important component of future survival.”
The ABCSG findings suggest expanded utility for EndoPredict. The test’s molecular score is currently used along with tumor size and nodal status to predict the 10-year distant recurrence rate, and whether patients may safely forgo chemotherapy or are at high risk and may need adjuvant chemotherapy in addition to endocrine therapy.
Dr. Dubsky and his coinvestigators assessed performance of the EndoPredict test among 217 patients treated on ABCSG 34, a randomized phase 2 neoadjuvant trial. Findings showed that among patients given neoadjuvant endocrine therapy because they had less aggressive disease features, an EndoPredict high-risk result was associated with poor response (negative predictive value of 92%), defined as a residual cancer burden (RCB) of II or III, he reported at the San Antonio Breast Cancer Symposium.
On the other hand, among patients given neoadjuvant chemotherapy because they had more aggressive disease features, a low-risk result was associated with poor response (negative predictive value of 100%).
“Clinicians really gave us two distinct cohorts within ABCSG 34. In the luminal A–type patients who were treated with neoendocrine therapy, a high EndoPredict score predicted a low chance of tumor shrinkage. In the more aggressive ER-positive tumors, so-called luminal B type, treated with neoadjuvant chemotherapy, there was absolutely no excellent response in the low-risk group,” Dr. Dubsky summarized. “We believe that this molecular score may contribute to patient selection for biomarker-driven studies, especially in the neoadjuvant setting.”
Session attendee Steven Vogl, MD, a medical oncologist with the Montefiore Medical Center in New York, commented, “I have trouble correlating an RCB of 0 or I with what you as a surgeon do for the patient, because you are talking about pathologic complete response or just a few cells there. That’s not what determines how much breast you take off: It’s determined by the total size of the tumor and the size of the breast. So if it’s less than a few centimeters, I’m sure you can do a lumpectomy in every patient. Tell me why I should care that you are getting an RCB of 0 or I in these endocrine patients.”
“Because it’s more likely that these patients will have a smaller tumor and better tumor shrinkage,” Dr. Dubsky replied. “You are of course right, RCB 0 or I was not designed to help surgeons. But it helps me as a translational scientist to have a surrogate and an exact classification for good tumor shrinkage. That’s how I used it.”
C. Kent Osborne, MD, codirector of SABCS and director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston, asked, “We see it in the clinic, and I’m sure you have as well, patients whose tumor doesn’t shrink very much, but the Ki-67 really drops. And that may or may not be a better factor than the actual tumor shrinkage. So how many patients who had tumors that didn’t shrink, which was your endpoint, had a reduction in Ki-67 that was, say, 5%?”
“We haven’t looked at that specifically, but we will do so as we carry on with the follow-up of these patients. Then we can learn more about the prognosis,” Dr. Dubsky replied.
Study details
ABCSG 34 was a randomized phase 2 trial testing addition of the cancer vaccine tecemotide (Stimuvax) to neoadjuvant standard of care among patients with HER2-negative early breast cancer.
Dr. Dubsky and coinvestigators restricted analyses to patients with hormone receptor–positive disease who, depending on clinical and pathologic factors, received neoadjuvant chemotherapy (eight cycles of epirubicin-cyclophosphamide and docetaxel) or neoadjuvant endocrine therapy (6 months of letrozole [Femara]) as standard of care. They were then randomized to additionally receive tecemotide or not before undergoing surgery.
Overall, 25% of the 134 patients in the neoadjuvant chemotherapy group had a good tumor response, defined as pathologic complete response in both breast and nodes (RCB of 0) or minimal residual disease (RCB of I).
Higher EndoPredict score was associated with greater likelihood of good response to chemotherapy. EndoPredict risk group (high vs. low) had a negative predictive value of 100%, a positive predictive value of 26.4%, a true-positive rate of 100%, and a true-negative rate of 8.9% for predicting response (P = .112).
Area under the receiver operating characteristic curve was 0.736.
In a multivariate model, EndoPredict score as a continuous variable was not an independent predictor of response. “The good response was largely driven by covariates that included cell proliferation, and it was Ki-67 that was significant,” Dr. Dubsky noted.
Overall, 18% of the 83 patients in the neoadjuvant endocrine therapy group had a good tumor response (RCB of 0 or I). Here, lower EndoPredict score was associated with greater likelihood of good response. EndoPredict risk group (high vs. low) had a negative predictive value of 92.3%, a positive predictive value of 27.3%, a true-positive rate of 80.0%, and a true-negative rate of 52.9% for predicting response (P = .024). Area under the curve was 0.726.
In a multivariate model here, EndoPredict score as a continuous variable, its estrogen receptor–signaling/differentiation component, and Ki-67 did not independently predict response. “It was maybe a bit surprising that T stage was the strongest factor, possibly indicating that we should have simply treated those women longer than 6 months,” Dr. Dubsky commented. The EndoPredict proliferation component was also a significant predictor.
“Possibly, the very narrow distribution of Ki-67 [among patients given neoendocrine therapy] may have prevented this factor from playing a bigger role in this particular model,” he speculated.
Dr. Dubsky disclosed that he receives consulting fees from Myriad, the maker of EndoPredict, and from Cepheid, Nanostring, and Amgen.
SOURCE: Dubsky P et al. SABCS 2017 Abstract GS6-04.
SAN ANTONIO – The results of the EndoPredict test appear to predict tumor response in patients with early hormone receptor–positive, HER2-negative breast cancer given neoadjuvant therapy, based on results of a study conducted by the Austrian Breast & Colorectal Cancer Study Group (ABCSG).
“Very good tumor shrinkage in estrogen receptor–positive, HER2-negative disease is going to happen only in a minority of patients, and biomarkers that would predict excellent tumor shrinkage are an unmet medical need,” commented lead investigator Peter Dubsky, MD, PhD, who is head of the Breast Center at Hirslanden Klinik St. Anna, Lucerne, Switzerland. “As a surgeon, that would help me to predict breast conservation at diagnosis, but as a surgical oncologist, I would also recognize that tumor response is an important component of future survival.”
The ABCSG findings suggest expanded utility for EndoPredict. The test’s molecular score is currently used along with tumor size and nodal status to predict the 10-year distant recurrence rate, and whether patients may safely forgo chemotherapy or are at high risk and may need adjuvant chemotherapy in addition to endocrine therapy.
Dr. Dubsky and his coinvestigators assessed performance of the EndoPredict test among 217 patients treated on ABCSG 34, a randomized phase 2 neoadjuvant trial. Findings showed that among patients given neoadjuvant endocrine therapy because they had less aggressive disease features, an EndoPredict high-risk result was associated with poor response (negative predictive value of 92%), defined as a residual cancer burden (RCB) of II or III, he reported at the San Antonio Breast Cancer Symposium.
On the other hand, among patients given neoadjuvant chemotherapy because they had more aggressive disease features, a low-risk result was associated with poor response (negative predictive value of 100%).
“Clinicians really gave us two distinct cohorts within ABCSG 34. In the luminal A–type patients who were treated with neoendocrine therapy, a high EndoPredict score predicted a low chance of tumor shrinkage. In the more aggressive ER-positive tumors, so-called luminal B type, treated with neoadjuvant chemotherapy, there was absolutely no excellent response in the low-risk group,” Dr. Dubsky summarized. “We believe that this molecular score may contribute to patient selection for biomarker-driven studies, especially in the neoadjuvant setting.”
Session attendee Steven Vogl, MD, a medical oncologist with the Montefiore Medical Center in New York, commented, “I have trouble correlating an RCB of 0 or I with what you as a surgeon do for the patient, because you are talking about pathologic complete response or just a few cells there. That’s not what determines how much breast you take off: It’s determined by the total size of the tumor and the size of the breast. So if it’s less than a few centimeters, I’m sure you can do a lumpectomy in every patient. Tell me why I should care that you are getting an RCB of 0 or I in these endocrine patients.”
“Because it’s more likely that these patients will have a smaller tumor and better tumor shrinkage,” Dr. Dubsky replied. “You are of course right, RCB 0 or I was not designed to help surgeons. But it helps me as a translational scientist to have a surrogate and an exact classification for good tumor shrinkage. That’s how I used it.”
C. Kent Osborne, MD, codirector of SABCS and director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston, asked, “We see it in the clinic, and I’m sure you have as well, patients whose tumor doesn’t shrink very much, but the Ki-67 really drops. And that may or may not be a better factor than the actual tumor shrinkage. So how many patients who had tumors that didn’t shrink, which was your endpoint, had a reduction in Ki-67 that was, say, 5%?”
“We haven’t looked at that specifically, but we will do so as we carry on with the follow-up of these patients. Then we can learn more about the prognosis,” Dr. Dubsky replied.
Study details
ABCSG 34 was a randomized phase 2 trial testing addition of the cancer vaccine tecemotide (Stimuvax) to neoadjuvant standard of care among patients with HER2-negative early breast cancer.
Dr. Dubsky and coinvestigators restricted analyses to patients with hormone receptor–positive disease who, depending on clinical and pathologic factors, received neoadjuvant chemotherapy (eight cycles of epirubicin-cyclophosphamide and docetaxel) or neoadjuvant endocrine therapy (6 months of letrozole [Femara]) as standard of care. They were then randomized to additionally receive tecemotide or not before undergoing surgery.
Overall, 25% of the 134 patients in the neoadjuvant chemotherapy group had a good tumor response, defined as pathologic complete response in both breast and nodes (RCB of 0) or minimal residual disease (RCB of I).
Higher EndoPredict score was associated with greater likelihood of good response to chemotherapy. EndoPredict risk group (high vs. low) had a negative predictive value of 100%, a positive predictive value of 26.4%, a true-positive rate of 100%, and a true-negative rate of 8.9% for predicting response (P = .112).
Area under the receiver operating characteristic curve was 0.736.
In a multivariate model, EndoPredict score as a continuous variable was not an independent predictor of response. “The good response was largely driven by covariates that included cell proliferation, and it was Ki-67 that was significant,” Dr. Dubsky noted.
Overall, 18% of the 83 patients in the neoadjuvant endocrine therapy group had a good tumor response (RCB of 0 or I). Here, lower EndoPredict score was associated with greater likelihood of good response. EndoPredict risk group (high vs. low) had a negative predictive value of 92.3%, a positive predictive value of 27.3%, a true-positive rate of 80.0%, and a true-negative rate of 52.9% for predicting response (P = .024). Area under the curve was 0.726.
In a multivariate model here, EndoPredict score as a continuous variable, its estrogen receptor–signaling/differentiation component, and Ki-67 did not independently predict response. “It was maybe a bit surprising that T stage was the strongest factor, possibly indicating that we should have simply treated those women longer than 6 months,” Dr. Dubsky commented. The EndoPredict proliferation component was also a significant predictor.
“Possibly, the very narrow distribution of Ki-67 [among patients given neoendocrine therapy] may have prevented this factor from playing a bigger role in this particular model,” he speculated.
Dr. Dubsky disclosed that he receives consulting fees from Myriad, the maker of EndoPredict, and from Cepheid, Nanostring, and Amgen.
SOURCE: Dubsky P et al. SABCS 2017 Abstract GS6-04.
REPORTING FROM SABCS 2017
Key clinical point:
Major finding: EndoPredict predicted poor tumor shrinkage in patients given neoadjuvant endocrine therapy (high-risk test result NPV, 92%) or neoadjuvant chemotherapy (low-risk test result NPV, 100%).
Data source: A cohort study of 217 patients with HR–positive, HER2-negative breast cancer enrolled in a phase 2 trial of neoadjuvant therapy (ABCSG 34).
Disclosures: Dr. Dubsky disclosed that he receives consulting fees from Cepheid, Myriad, Nanostring, and Amgen.
High rate of arm morbidity in young breast cancer survivors
SAN ANTONIO – as compared with having a sentinel lymph node biopsy (SLNB), according to new findings.
In a large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger, the incidence of arm swelling 1 year after diagnosis among women who underwent breast-conserving surgery was 6% for the SLNB group versus 24% for those who had ALND. Among patients who had a mastectomy, the rates were similar; 6% versus 23% for SLNB or ALND, respectively.
“Young breast cancer survivors report high rates of arm morbidity in the first year of follow-up,” said lead author Anne Kuijer, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston. “Axillary node dissection, increased BMI [body mass index] and socioeconomic status were all independently associated with an increased risk of arm swelling,” she said at the San Antonio Breast Cancer Symposium.
She noted that patients who received mastectomy with radiation therapy were twice as likely to have decreased range of motion at 1 year, compared with patients treated with breast-conserving treatment.
In this study, the authors evaluated the incidence of arm morbidity associated with both ALND and SLNB in patients who were enrolled in the Young Women’s Breast Cancer Study. This multicenter prospective cohort study was designed to explore biological, medical, and psychosocial issues experienced by young breast cancer patients.
Within this large cohort, 55% had undergone an SLNB only, and 41% an ALND. The remaining patients did not undergo either procedure.
The primary endpoint of this study was to examine the incidence of patient-reported arm swelling or decreased range of motion at 1 year after their breast cancer diagnosis. Patients used the Cancer Rehabilitation Evaluation System (CARES-SF) to measure their symptoms.
Overall, at 1 year, 13% of the cohort reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Several factors were associated with a higher risk of arm morbidity. Patients with a BMI of greater than 25 were more likely to report arm swelling vs. those with lower BMI (odds ratio, 1.7; P = .03) as well as have less range of motion (OR, 1.5; P = .05). Women who reported feeling financially comfortable were 40% less likely to report swelling (P = .02) and 90% less likely to report decreased range of motion (P = .67).
In addition, those who underwent ALND were 3.4 times more likely to report swelling, compared with women who had SLNB, but it was not associated with a reduction in range of motion.
One limitation of the study is that the cohort included patients who had received treatment at large cancer centers in the Northeast, suggesting that they may have been of higher socioeconomic status and may have led more active lifestyles, compared with the general population. Another limitation is that arm morbidity was self-reported and not objectively measured.
“I think our findings highlight opportunities for preoperative counseling, early referral of patients to physical therapy, and identification of resources for support of those at increased risk,” said Dr. Kuijer.
SOURCE: Kuijer et al. SABCS Abstract GS5-03
SAN ANTONIO – as compared with having a sentinel lymph node biopsy (SLNB), according to new findings.
In a large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger, the incidence of arm swelling 1 year after diagnosis among women who underwent breast-conserving surgery was 6% for the SLNB group versus 24% for those who had ALND. Among patients who had a mastectomy, the rates were similar; 6% versus 23% for SLNB or ALND, respectively.
“Young breast cancer survivors report high rates of arm morbidity in the first year of follow-up,” said lead author Anne Kuijer, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston. “Axillary node dissection, increased BMI [body mass index] and socioeconomic status were all independently associated with an increased risk of arm swelling,” she said at the San Antonio Breast Cancer Symposium.
She noted that patients who received mastectomy with radiation therapy were twice as likely to have decreased range of motion at 1 year, compared with patients treated with breast-conserving treatment.
In this study, the authors evaluated the incidence of arm morbidity associated with both ALND and SLNB in patients who were enrolled in the Young Women’s Breast Cancer Study. This multicenter prospective cohort study was designed to explore biological, medical, and psychosocial issues experienced by young breast cancer patients.
Within this large cohort, 55% had undergone an SLNB only, and 41% an ALND. The remaining patients did not undergo either procedure.
The primary endpoint of this study was to examine the incidence of patient-reported arm swelling or decreased range of motion at 1 year after their breast cancer diagnosis. Patients used the Cancer Rehabilitation Evaluation System (CARES-SF) to measure their symptoms.
Overall, at 1 year, 13% of the cohort reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Several factors were associated with a higher risk of arm morbidity. Patients with a BMI of greater than 25 were more likely to report arm swelling vs. those with lower BMI (odds ratio, 1.7; P = .03) as well as have less range of motion (OR, 1.5; P = .05). Women who reported feeling financially comfortable were 40% less likely to report swelling (P = .02) and 90% less likely to report decreased range of motion (P = .67).
In addition, those who underwent ALND were 3.4 times more likely to report swelling, compared with women who had SLNB, but it was not associated with a reduction in range of motion.
One limitation of the study is that the cohort included patients who had received treatment at large cancer centers in the Northeast, suggesting that they may have been of higher socioeconomic status and may have led more active lifestyles, compared with the general population. Another limitation is that arm morbidity was self-reported and not objectively measured.
“I think our findings highlight opportunities for preoperative counseling, early referral of patients to physical therapy, and identification of resources for support of those at increased risk,” said Dr. Kuijer.
SOURCE: Kuijer et al. SABCS Abstract GS5-03
SAN ANTONIO – as compared with having a sentinel lymph node biopsy (SLNB), according to new findings.
In a large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger, the incidence of arm swelling 1 year after diagnosis among women who underwent breast-conserving surgery was 6% for the SLNB group versus 24% for those who had ALND. Among patients who had a mastectomy, the rates were similar; 6% versus 23% for SLNB or ALND, respectively.
“Young breast cancer survivors report high rates of arm morbidity in the first year of follow-up,” said lead author Anne Kuijer, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston. “Axillary node dissection, increased BMI [body mass index] and socioeconomic status were all independently associated with an increased risk of arm swelling,” she said at the San Antonio Breast Cancer Symposium.
She noted that patients who received mastectomy with radiation therapy were twice as likely to have decreased range of motion at 1 year, compared with patients treated with breast-conserving treatment.
In this study, the authors evaluated the incidence of arm morbidity associated with both ALND and SLNB in patients who were enrolled in the Young Women’s Breast Cancer Study. This multicenter prospective cohort study was designed to explore biological, medical, and psychosocial issues experienced by young breast cancer patients.
Within this large cohort, 55% had undergone an SLNB only, and 41% an ALND. The remaining patients did not undergo either procedure.
The primary endpoint of this study was to examine the incidence of patient-reported arm swelling or decreased range of motion at 1 year after their breast cancer diagnosis. Patients used the Cancer Rehabilitation Evaluation System (CARES-SF) to measure their symptoms.
Overall, at 1 year, 13% of the cohort reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Several factors were associated with a higher risk of arm morbidity. Patients with a BMI of greater than 25 were more likely to report arm swelling vs. those with lower BMI (odds ratio, 1.7; P = .03) as well as have less range of motion (OR, 1.5; P = .05). Women who reported feeling financially comfortable were 40% less likely to report swelling (P = .02) and 90% less likely to report decreased range of motion (P = .67).
In addition, those who underwent ALND were 3.4 times more likely to report swelling, compared with women who had SLNB, but it was not associated with a reduction in range of motion.
One limitation of the study is that the cohort included patients who had received treatment at large cancer centers in the Northeast, suggesting that they may have been of higher socioeconomic status and may have led more active lifestyles, compared with the general population. Another limitation is that arm morbidity was self-reported and not objectively measured.
“I think our findings highlight opportunities for preoperative counseling, early referral of patients to physical therapy, and identification of resources for support of those at increased risk,” said Dr. Kuijer.
SOURCE: Kuijer et al. SABCS Abstract GS5-03
REPORTING FROM SABCS 2017
Key clinical point: A significant rate of arm swelling and decreased range of motion was seen in young breast cancer patients 1 year after undergoing surgery.
Major finding: At 1 year, 13% of a large cohort of breast cancer patients aged 40 years or younger reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Data source: Large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger.
Disclosures:. This study was funded by the National Institutes of Health, the Susan G. Komen Foundation, The Pink Agenda, and the Breast Cancer Research Foundation. Dr. Kuijer and her colleagues declare no conflicts of interest.
Source: Kuijer et al. SABCS 2017 Abstract GS5-03.
Shaping practice: Z1071 continues to redefine axillary management
SAN DIEGO – A 2013 breast cancer trial is changing the way lymph nodes are managed in women with node-positive disease who have an axillary pathologic complete response to neoadjuvant chemotherapy.
Emerging additional data support the initial theory of the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, said Judy C. Boughey, MD, FACS, at the American College of Surgeons Clinical Congress: Performing sentinel lymph node surgery after chemotherapy is an acceptable alternative for some women. This change in practice could bestow a profound long-term benefit on the approximately 40% of patients, who have an axillary pathologic complete response after neoadjuvant chemotherapy (NAC) – patients who otherwise might undergo an unnecessary axillary node exploration, which can lead to higher risk of lymphedema, said Dr. Boughey, head of surgical research at the Mayo Clinic, Rochester, Minn.
“About 20% of patients who are treated with chemotherapy for their breast cancer receive the chemotherapy prior to surgery. Of those who do receive neoadjuvant chemotherapy, probably half could benefit from this approach,” she said. “Lymphedema after axillary dissection is one of the situations patients are most concerned about. This approach is a great one when patients have a good chemotherapy response, and we want to reliably reassure ourselves that there’s no disease left in the axilla without automatically removing all the nodes. Of course, if there is any remaining disease in any of the lymph nodes, the current standard is still to remove all the nodes. This approach, however, optimizes management for patients who have the best response to chemotherapy.”
Neoadjuvant therapy success
Prechemotherapy nodal exploration was routine a decade or so ago and is what many surgeons were most comfortable with, Dr. Boughey said. “We know the false-negative rate, and chemotherapy doesn’t interfere with axillary staging. However, it means patients have to go through two surgeries, and, although the chemotherapy does not interfere with the procedure, if any of the sentinel nodes are positive and an axillary dissection is performed at the same setting, then systemic therapy will be delayed. However, most importantly, when the sentinel node is removed prior to chemotherapy, we lose the ability to assess axillary response to chemotherapy – which correlates with survival.”
The biggest drawback of axillary dissection is its potential for lifelong morbidity from lymphedema. “Women know about this. They worry about this, and they want to avoid it if at all possible,” Dr. Boughey said.
More effective, targeted chemotherapeutic agents have resulted in higher rates of eradication of disease with neoadjuvant treatment. So this leads to the question: Why not reassess nodes after treatment, when these drugs have had a chance to work? Doing so reduces the one-size-fits-all prescription of axillary dissection and, thus, the number of women with lasting adverse events.
Some early data supported this theory
In 2009, researchers at the MD Anderson Center reported that sentinel node surgery after chemotherapy in patients with node-negative breast cancer resulted in fewer positive sentinel nodes and decreased unnecessary axillary dissections. Node identification rates were about 98% whether the surgery came before or after treatment. The false-negative rate hovered around 5%. And there were significantly fewer axillary dissections with posttreatment surgery: 20% vs. 36% in women with T2 disease and 30% vs. 51% in those with T3 disease. Importantly, holding off on the surgery didn’t lead to higher local-regional failure rates or survival among the 3,746 women treated during 1994-2007.
The American College of Surgeons Oncology Group Z1071 trial was designed to explore this question in patients with node-positive breast cancer. The Z1071 trial enrolled 756 women who had clinical T0-T4, N1-N2, M0 breast cancer and received neoadjuvant chemotherapy. Patients underwent both sentinel lymph node surgery and axillary lymph node dissection following chemotherapy. The primary endpoint was the false-negative rate of sentinel lymph node surgery after chemotherapy in women who presented with cN1 disease and had at least two sentinel nodes resected; a rate of 10% lower was considered acceptable and would justify the approach.
Of the entire cohort, 40% had a complete pathologic nodal response rate. The sentinel node identification rate was nearly 93%. The false-negative rate among 525 women with two or more positive sentinel nodes, however, was 12.6% – short of the 10% rate investigators needed to deem the study a success, Dr. Boughey said.
But there were some positive findings in subgroup analyses. Among women who had nodes identified with a dual tracer (both dye and radioactive clipping), the false-negative rate dipped to 10.8%. It was just 9% in those who had more than two sentinel nodes identified.
A recent subanalysis of the Z1071 trial further refined these data. It looked at 170 of the patients with cN1 disease (32%) who had had a clip placed in the positive lymph node at the time of percutaneous biopsy and compared false-negative rates among them with rates in the 355 patients who were not clipped.
“When we looked at them, if the clipped node came out during the sentinel node surgery, then the false-negative rate dropped down to about 7%,” Dr. Boughey said. The comparator group pointed out the value of using a clip. The false-negative rate was 13% in patients who didn’t have a clip placed and 19% in the patients whose clip wasn’t retrieved until axillary dissection.
The results of Z1071 and its subanalyses have popularized nodal clipping, Dr. Boughey said. “When we ran Z1071, clipping wasn’t commonly being performed, but there has been a huge uptake in it now.”
Confirmatory data
Other recent studies confirm the feasibility of this approach in women who have clinically negative nodes after NAC.
In 2013, the German study SENTINA (sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy) explored the false-negative rate in women who had sentinel node biopsy before or after neoadjuvant chemotherapy. Overall, it found an unacceptably high false-negative rate of 14% in women with node positive disease who converted to clinically negative nodal status. However, when the analysis was limited to those cases with at least two sentinel nodes, the false-negative rate was less than 10%, once more suggesting a potential role for sentinel node surgery after neoadjuvant chemotherapy.
In 2015, the Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) study highlighted the potential effect of sentinel node surgery after NAC. The prospective study showed not only that the strategy was safe, with a false-negative rate around 8%, but also that it could have eliminated complete axillary dissection in about 30% of the cohort.
The study enrolled 153 women with biopsy-proven node-positive breast cancer (T0-3, N1-2) who underwent both sentinel node surgery and complete nodal dissection. Immunohistochemistry of the retrieved sentinel nodes was mandatory, and the presence of any tumor cells in the sentinel node rendered it positive.
The sentinel node retrieval rate was 88%, and the false-negative rate, 8.4%. The study also employed dual tracers of isotope and blue dye in a majority of patients; this was associated with a threefold decrease in the false-negative rate in those patients, dropping it to around 5%. “By using sentinel node biopsy after NAC, axillary node dissection could potentially be avoided in at least 30% of patients who present with node-positive breast cancer,” the study’s team concluded.
Long-term consequences?
It’s increasingly clear that for carefully selected patients, with robust NAC response, a postchemotherapy assessment can accurately assess nodal disease – especially if dual tracers are employed, several sentinel nodes examined, and the biopsy-proven positive node is resected. What isn’t clear yet is the long-term effect of this strategy, Dr. Boughey said.
“Five years ago, when Z1071 was first being reported, I would discuss it in terms of the controversy, and give the pros and cons,” she said. “But now that we have more information about this strategy under our belts, I feel much more confident. However, we still do not have information on patients with node-positive disease who have been treated with sentinel node only after neoadjuvant chemotherapy and followed for 5 or 10 years. That’s the piece we just can’t have, without time.”
Dr. Boughey had no relevant financial disclosures.
SOURCE: Boughey JC. Session PS108.
SAN DIEGO – A 2013 breast cancer trial is changing the way lymph nodes are managed in women with node-positive disease who have an axillary pathologic complete response to neoadjuvant chemotherapy.
Emerging additional data support the initial theory of the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, said Judy C. Boughey, MD, FACS, at the American College of Surgeons Clinical Congress: Performing sentinel lymph node surgery after chemotherapy is an acceptable alternative for some women. This change in practice could bestow a profound long-term benefit on the approximately 40% of patients, who have an axillary pathologic complete response after neoadjuvant chemotherapy (NAC) – patients who otherwise might undergo an unnecessary axillary node exploration, which can lead to higher risk of lymphedema, said Dr. Boughey, head of surgical research at the Mayo Clinic, Rochester, Minn.
“About 20% of patients who are treated with chemotherapy for their breast cancer receive the chemotherapy prior to surgery. Of those who do receive neoadjuvant chemotherapy, probably half could benefit from this approach,” she said. “Lymphedema after axillary dissection is one of the situations patients are most concerned about. This approach is a great one when patients have a good chemotherapy response, and we want to reliably reassure ourselves that there’s no disease left in the axilla without automatically removing all the nodes. Of course, if there is any remaining disease in any of the lymph nodes, the current standard is still to remove all the nodes. This approach, however, optimizes management for patients who have the best response to chemotherapy.”
Neoadjuvant therapy success
Prechemotherapy nodal exploration was routine a decade or so ago and is what many surgeons were most comfortable with, Dr. Boughey said. “We know the false-negative rate, and chemotherapy doesn’t interfere with axillary staging. However, it means patients have to go through two surgeries, and, although the chemotherapy does not interfere with the procedure, if any of the sentinel nodes are positive and an axillary dissection is performed at the same setting, then systemic therapy will be delayed. However, most importantly, when the sentinel node is removed prior to chemotherapy, we lose the ability to assess axillary response to chemotherapy – which correlates with survival.”
The biggest drawback of axillary dissection is its potential for lifelong morbidity from lymphedema. “Women know about this. They worry about this, and they want to avoid it if at all possible,” Dr. Boughey said.
More effective, targeted chemotherapeutic agents have resulted in higher rates of eradication of disease with neoadjuvant treatment. So this leads to the question: Why not reassess nodes after treatment, when these drugs have had a chance to work? Doing so reduces the one-size-fits-all prescription of axillary dissection and, thus, the number of women with lasting adverse events.
Some early data supported this theory
In 2009, researchers at the MD Anderson Center reported that sentinel node surgery after chemotherapy in patients with node-negative breast cancer resulted in fewer positive sentinel nodes and decreased unnecessary axillary dissections. Node identification rates were about 98% whether the surgery came before or after treatment. The false-negative rate hovered around 5%. And there were significantly fewer axillary dissections with posttreatment surgery: 20% vs. 36% in women with T2 disease and 30% vs. 51% in those with T3 disease. Importantly, holding off on the surgery didn’t lead to higher local-regional failure rates or survival among the 3,746 women treated during 1994-2007.
The American College of Surgeons Oncology Group Z1071 trial was designed to explore this question in patients with node-positive breast cancer. The Z1071 trial enrolled 756 women who had clinical T0-T4, N1-N2, M0 breast cancer and received neoadjuvant chemotherapy. Patients underwent both sentinel lymph node surgery and axillary lymph node dissection following chemotherapy. The primary endpoint was the false-negative rate of sentinel lymph node surgery after chemotherapy in women who presented with cN1 disease and had at least two sentinel nodes resected; a rate of 10% lower was considered acceptable and would justify the approach.
Of the entire cohort, 40% had a complete pathologic nodal response rate. The sentinel node identification rate was nearly 93%. The false-negative rate among 525 women with two or more positive sentinel nodes, however, was 12.6% – short of the 10% rate investigators needed to deem the study a success, Dr. Boughey said.
But there were some positive findings in subgroup analyses. Among women who had nodes identified with a dual tracer (both dye and radioactive clipping), the false-negative rate dipped to 10.8%. It was just 9% in those who had more than two sentinel nodes identified.
A recent subanalysis of the Z1071 trial further refined these data. It looked at 170 of the patients with cN1 disease (32%) who had had a clip placed in the positive lymph node at the time of percutaneous biopsy and compared false-negative rates among them with rates in the 355 patients who were not clipped.
“When we looked at them, if the clipped node came out during the sentinel node surgery, then the false-negative rate dropped down to about 7%,” Dr. Boughey said. The comparator group pointed out the value of using a clip. The false-negative rate was 13% in patients who didn’t have a clip placed and 19% in the patients whose clip wasn’t retrieved until axillary dissection.
The results of Z1071 and its subanalyses have popularized nodal clipping, Dr. Boughey said. “When we ran Z1071, clipping wasn’t commonly being performed, but there has been a huge uptake in it now.”
Confirmatory data
Other recent studies confirm the feasibility of this approach in women who have clinically negative nodes after NAC.
In 2013, the German study SENTINA (sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy) explored the false-negative rate in women who had sentinel node biopsy before or after neoadjuvant chemotherapy. Overall, it found an unacceptably high false-negative rate of 14% in women with node positive disease who converted to clinically negative nodal status. However, when the analysis was limited to those cases with at least two sentinel nodes, the false-negative rate was less than 10%, once more suggesting a potential role for sentinel node surgery after neoadjuvant chemotherapy.
In 2015, the Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) study highlighted the potential effect of sentinel node surgery after NAC. The prospective study showed not only that the strategy was safe, with a false-negative rate around 8%, but also that it could have eliminated complete axillary dissection in about 30% of the cohort.
The study enrolled 153 women with biopsy-proven node-positive breast cancer (T0-3, N1-2) who underwent both sentinel node surgery and complete nodal dissection. Immunohistochemistry of the retrieved sentinel nodes was mandatory, and the presence of any tumor cells in the sentinel node rendered it positive.
The sentinel node retrieval rate was 88%, and the false-negative rate, 8.4%. The study also employed dual tracers of isotope and blue dye in a majority of patients; this was associated with a threefold decrease in the false-negative rate in those patients, dropping it to around 5%. “By using sentinel node biopsy after NAC, axillary node dissection could potentially be avoided in at least 30% of patients who present with node-positive breast cancer,” the study’s team concluded.
Long-term consequences?
It’s increasingly clear that for carefully selected patients, with robust NAC response, a postchemotherapy assessment can accurately assess nodal disease – especially if dual tracers are employed, several sentinel nodes examined, and the biopsy-proven positive node is resected. What isn’t clear yet is the long-term effect of this strategy, Dr. Boughey said.
“Five years ago, when Z1071 was first being reported, I would discuss it in terms of the controversy, and give the pros and cons,” she said. “But now that we have more information about this strategy under our belts, I feel much more confident. However, we still do not have information on patients with node-positive disease who have been treated with sentinel node only after neoadjuvant chemotherapy and followed for 5 or 10 years. That’s the piece we just can’t have, without time.”
Dr. Boughey had no relevant financial disclosures.
SOURCE: Boughey JC. Session PS108.
SAN DIEGO – A 2013 breast cancer trial is changing the way lymph nodes are managed in women with node-positive disease who have an axillary pathologic complete response to neoadjuvant chemotherapy.
Emerging additional data support the initial theory of the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial, said Judy C. Boughey, MD, FACS, at the American College of Surgeons Clinical Congress: Performing sentinel lymph node surgery after chemotherapy is an acceptable alternative for some women. This change in practice could bestow a profound long-term benefit on the approximately 40% of patients, who have an axillary pathologic complete response after neoadjuvant chemotherapy (NAC) – patients who otherwise might undergo an unnecessary axillary node exploration, which can lead to higher risk of lymphedema, said Dr. Boughey, head of surgical research at the Mayo Clinic, Rochester, Minn.
“About 20% of patients who are treated with chemotherapy for their breast cancer receive the chemotherapy prior to surgery. Of those who do receive neoadjuvant chemotherapy, probably half could benefit from this approach,” she said. “Lymphedema after axillary dissection is one of the situations patients are most concerned about. This approach is a great one when patients have a good chemotherapy response, and we want to reliably reassure ourselves that there’s no disease left in the axilla without automatically removing all the nodes. Of course, if there is any remaining disease in any of the lymph nodes, the current standard is still to remove all the nodes. This approach, however, optimizes management for patients who have the best response to chemotherapy.”
Neoadjuvant therapy success
Prechemotherapy nodal exploration was routine a decade or so ago and is what many surgeons were most comfortable with, Dr. Boughey said. “We know the false-negative rate, and chemotherapy doesn’t interfere with axillary staging. However, it means patients have to go through two surgeries, and, although the chemotherapy does not interfere with the procedure, if any of the sentinel nodes are positive and an axillary dissection is performed at the same setting, then systemic therapy will be delayed. However, most importantly, when the sentinel node is removed prior to chemotherapy, we lose the ability to assess axillary response to chemotherapy – which correlates with survival.”
The biggest drawback of axillary dissection is its potential for lifelong morbidity from lymphedema. “Women know about this. They worry about this, and they want to avoid it if at all possible,” Dr. Boughey said.
More effective, targeted chemotherapeutic agents have resulted in higher rates of eradication of disease with neoadjuvant treatment. So this leads to the question: Why not reassess nodes after treatment, when these drugs have had a chance to work? Doing so reduces the one-size-fits-all prescription of axillary dissection and, thus, the number of women with lasting adverse events.
Some early data supported this theory
In 2009, researchers at the MD Anderson Center reported that sentinel node surgery after chemotherapy in patients with node-negative breast cancer resulted in fewer positive sentinel nodes and decreased unnecessary axillary dissections. Node identification rates were about 98% whether the surgery came before or after treatment. The false-negative rate hovered around 5%. And there were significantly fewer axillary dissections with posttreatment surgery: 20% vs. 36% in women with T2 disease and 30% vs. 51% in those with T3 disease. Importantly, holding off on the surgery didn’t lead to higher local-regional failure rates or survival among the 3,746 women treated during 1994-2007.
The American College of Surgeons Oncology Group Z1071 trial was designed to explore this question in patients with node-positive breast cancer. The Z1071 trial enrolled 756 women who had clinical T0-T4, N1-N2, M0 breast cancer and received neoadjuvant chemotherapy. Patients underwent both sentinel lymph node surgery and axillary lymph node dissection following chemotherapy. The primary endpoint was the false-negative rate of sentinel lymph node surgery after chemotherapy in women who presented with cN1 disease and had at least two sentinel nodes resected; a rate of 10% lower was considered acceptable and would justify the approach.
Of the entire cohort, 40% had a complete pathologic nodal response rate. The sentinel node identification rate was nearly 93%. The false-negative rate among 525 women with two or more positive sentinel nodes, however, was 12.6% – short of the 10% rate investigators needed to deem the study a success, Dr. Boughey said.
But there were some positive findings in subgroup analyses. Among women who had nodes identified with a dual tracer (both dye and radioactive clipping), the false-negative rate dipped to 10.8%. It was just 9% in those who had more than two sentinel nodes identified.
A recent subanalysis of the Z1071 trial further refined these data. It looked at 170 of the patients with cN1 disease (32%) who had had a clip placed in the positive lymph node at the time of percutaneous biopsy and compared false-negative rates among them with rates in the 355 patients who were not clipped.
“When we looked at them, if the clipped node came out during the sentinel node surgery, then the false-negative rate dropped down to about 7%,” Dr. Boughey said. The comparator group pointed out the value of using a clip. The false-negative rate was 13% in patients who didn’t have a clip placed and 19% in the patients whose clip wasn’t retrieved until axillary dissection.
The results of Z1071 and its subanalyses have popularized nodal clipping, Dr. Boughey said. “When we ran Z1071, clipping wasn’t commonly being performed, but there has been a huge uptake in it now.”
Confirmatory data
Other recent studies confirm the feasibility of this approach in women who have clinically negative nodes after NAC.
In 2013, the German study SENTINA (sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy) explored the false-negative rate in women who had sentinel node biopsy before or after neoadjuvant chemotherapy. Overall, it found an unacceptably high false-negative rate of 14% in women with node positive disease who converted to clinically negative nodal status. However, when the analysis was limited to those cases with at least two sentinel nodes, the false-negative rate was less than 10%, once more suggesting a potential role for sentinel node surgery after neoadjuvant chemotherapy.
In 2015, the Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) study highlighted the potential effect of sentinel node surgery after NAC. The prospective study showed not only that the strategy was safe, with a false-negative rate around 8%, but also that it could have eliminated complete axillary dissection in about 30% of the cohort.
The study enrolled 153 women with biopsy-proven node-positive breast cancer (T0-3, N1-2) who underwent both sentinel node surgery and complete nodal dissection. Immunohistochemistry of the retrieved sentinel nodes was mandatory, and the presence of any tumor cells in the sentinel node rendered it positive.
The sentinel node retrieval rate was 88%, and the false-negative rate, 8.4%. The study also employed dual tracers of isotope and blue dye in a majority of patients; this was associated with a threefold decrease in the false-negative rate in those patients, dropping it to around 5%. “By using sentinel node biopsy after NAC, axillary node dissection could potentially be avoided in at least 30% of patients who present with node-positive breast cancer,” the study’s team concluded.
Long-term consequences?
It’s increasingly clear that for carefully selected patients, with robust NAC response, a postchemotherapy assessment can accurately assess nodal disease – especially if dual tracers are employed, several sentinel nodes examined, and the biopsy-proven positive node is resected. What isn’t clear yet is the long-term effect of this strategy, Dr. Boughey said.
“Five years ago, when Z1071 was first being reported, I would discuss it in terms of the controversy, and give the pros and cons,” she said. “But now that we have more information about this strategy under our belts, I feel much more confident. However, we still do not have information on patients with node-positive disease who have been treated with sentinel node only after neoadjuvant chemotherapy and followed for 5 or 10 years. That’s the piece we just can’t have, without time.”
Dr. Boughey had no relevant financial disclosures.
SOURCE: Boughey JC. Session PS108.
EXPERT ANALYSIS FROM THE ACS CLINICAL CONGRESS





