User login
VIDEO: A case study in diagnosing depression or demoralization after retirement
Why is your geriatric patient whose life seemed fulfilling before retirement now talking about not feeling “right”? “Am I depressed, or is this normal,” your patient wants to know. What should be your reply, and what interventions can you take to help this patient in the context of a 15-minute appointment?
In this video, part of the Mental Health Consult series of roundtable discussions, our panel members discuss their recommendations for work-up and next steps for managing a 65-year-old recently retired man with a history of prostate cancer but no psychiatric disorders. He has some mild depressive symptoms, and he brings up suicide during the office visit.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to differentiate between the distress often inherent in life passages and mental illness, and how practice models drive treatment decisions and reimbursement.
On Twitter @whitneymcknight
Why is your geriatric patient whose life seemed fulfilling before retirement now talking about not feeling “right”? “Am I depressed, or is this normal,” your patient wants to know. What should be your reply, and what interventions can you take to help this patient in the context of a 15-minute appointment?
In this video, part of the Mental Health Consult series of roundtable discussions, our panel members discuss their recommendations for work-up and next steps for managing a 65-year-old recently retired man with a history of prostate cancer but no psychiatric disorders. He has some mild depressive symptoms, and he brings up suicide during the office visit.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to differentiate between the distress often inherent in life passages and mental illness, and how practice models drive treatment decisions and reimbursement.
On Twitter @whitneymcknight
Why is your geriatric patient whose life seemed fulfilling before retirement now talking about not feeling “right”? “Am I depressed, or is this normal,” your patient wants to know. What should be your reply, and what interventions can you take to help this patient in the context of a 15-minute appointment?
In this video, part of the Mental Health Consult series of roundtable discussions, our panel members discuss their recommendations for work-up and next steps for managing a 65-year-old recently retired man with a history of prostate cancer but no psychiatric disorders. He has some mild depressive symptoms, and he brings up suicide during the office visit.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to differentiate between the distress often inherent in life passages and mental illness, and how practice models drive treatment decisions and reimbursement.
On Twitter @whitneymcknight
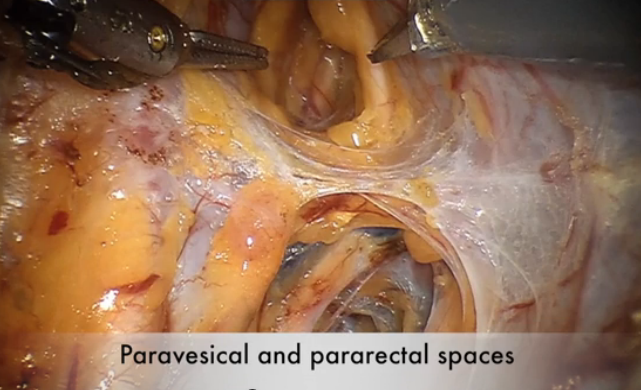
Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
This video is brought to you by ![]()
VIDEO: Telecardiology improves chronic care management, reduces cost
MINNEAPOLIS – The use of telecardiology can vastly improve outcomes, reduce hospitalizations, and lower health care costs, explained Michael Shen, MD, a cardiologist and chief medical officer at Duxlink Health in Sunrise, Fla.
Dr. Shen recently spoke at the American Telemedicine Association annual meeting about the impact of telecardiology on the practice of cardiology.
“This is at the very beginning of the technology, and it will be very good for cardiologists to be early adopters – to be the early users – so they can engage the technology as leaders, rather than followers,” explained Dr. Shen.
In a video interview at the meeting, he discussed how telecardiology has advanced over the years and how the technology can improve chronic care management. Dr. Shen also shared details about a telecardiology program implemented in his practice, and he discussed how the program has affected patient care and hospital readmissions.
Dr. Shen had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
MINNEAPOLIS – The use of telecardiology can vastly improve outcomes, reduce hospitalizations, and lower health care costs, explained Michael Shen, MD, a cardiologist and chief medical officer at Duxlink Health in Sunrise, Fla.
Dr. Shen recently spoke at the American Telemedicine Association annual meeting about the impact of telecardiology on the practice of cardiology.
“This is at the very beginning of the technology, and it will be very good for cardiologists to be early adopters – to be the early users – so they can engage the technology as leaders, rather than followers,” explained Dr. Shen.
In a video interview at the meeting, he discussed how telecardiology has advanced over the years and how the technology can improve chronic care management. Dr. Shen also shared details about a telecardiology program implemented in his practice, and he discussed how the program has affected patient care and hospital readmissions.
Dr. Shen had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
MINNEAPOLIS – The use of telecardiology can vastly improve outcomes, reduce hospitalizations, and lower health care costs, explained Michael Shen, MD, a cardiologist and chief medical officer at Duxlink Health in Sunrise, Fla.
Dr. Shen recently spoke at the American Telemedicine Association annual meeting about the impact of telecardiology on the practice of cardiology.
“This is at the very beginning of the technology, and it will be very good for cardiologists to be early adopters – to be the early users – so they can engage the technology as leaders, rather than followers,” explained Dr. Shen.
In a video interview at the meeting, he discussed how telecardiology has advanced over the years and how the technology can improve chronic care management. Dr. Shen also shared details about a telecardiology program implemented in his practice, and he discussed how the program has affected patient care and hospital readmissions.
Dr. Shen had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
VIDEO: Minimal disease activity criteria sought for axial spondyloarthritis
DENVER – U.S. rheumatologists plan to develop new criteria to define minimal disease activity in patients with axial spondyloarthritis to better gauge in routine clinical practice how well these patients respond to treatment.
A score on the Ankylosing Spondylitis Disease Activity Score (ASDAS) of less than 1.3 is the most commonly used measure today of minimal disease activity, but the ASDAS isn’t suitable for point-of-care assessment in routine practice because of the need for a C-reactive protein level or erythrocyte sedimentation rate.
“In the clinic, ASDAS is very difficult or next to impossible to do” because it needs CRP or ESR, which are “never available at the point of care,” Atul A. Deodhar, MD, said in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN). Another limitation of ASDAS is that it focuses exclusively on musculoskeletal measures and does not take into account extra-articular manifestations of axial spondyloarthritis such as those in the eye, gastrointestinal tract, or skin.
“We would like a clinical measurement that might tell us that a patient is appropriately treated and at minimal disease activity,” said Dr. Deodhar, professor of medicine and medical director of the rheumatology clinics at Oregon Health & Science University in Portland.
As outgoing chair of SPARTAN, Dr. Deodhar introduced a proposal that SPARTAN develop new minimal disease activity criteria for patients with axial spondyloarthritis, presenting the rationale for this project at the meeting along with the incoming chair Lianne S. Gensler, MD.
“We want a way to measure disease activity at a stage that is not full remission but with enough of a reduction in disease activity to make a difference,” said Dr. Gensler, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco. Another frequently used gauge of minimal disease activity in axial spondyloarthritis, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), is flawed by being totally based on subjective measurements without input from the attending rheumatologist, Dr. Gensler said.
The SPARTAN leadership will try to partner in this effort with the OMERACT (Outcome Measures in Rheumatology) program, the Assessment of Spondyloarthritis International Society (ASAS), or both, but if necessary SPARTAN will develop new minimal disease activity criteria on its own, Dr. Deodhar said.
Dr. Deodhar has received research support from 10 drug companies. Dr. Gensler has been a consultant to or has received research support from AbbVie, Amgen, Janssen, Novartis, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
DENVER – U.S. rheumatologists plan to develop new criteria to define minimal disease activity in patients with axial spondyloarthritis to better gauge in routine clinical practice how well these patients respond to treatment.
A score on the Ankylosing Spondylitis Disease Activity Score (ASDAS) of less than 1.3 is the most commonly used measure today of minimal disease activity, but the ASDAS isn’t suitable for point-of-care assessment in routine practice because of the need for a C-reactive protein level or erythrocyte sedimentation rate.
“In the clinic, ASDAS is very difficult or next to impossible to do” because it needs CRP or ESR, which are “never available at the point of care,” Atul A. Deodhar, MD, said in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN). Another limitation of ASDAS is that it focuses exclusively on musculoskeletal measures and does not take into account extra-articular manifestations of axial spondyloarthritis such as those in the eye, gastrointestinal tract, or skin.
“We would like a clinical measurement that might tell us that a patient is appropriately treated and at minimal disease activity,” said Dr. Deodhar, professor of medicine and medical director of the rheumatology clinics at Oregon Health & Science University in Portland.
As outgoing chair of SPARTAN, Dr. Deodhar introduced a proposal that SPARTAN develop new minimal disease activity criteria for patients with axial spondyloarthritis, presenting the rationale for this project at the meeting along with the incoming chair Lianne S. Gensler, MD.
“We want a way to measure disease activity at a stage that is not full remission but with enough of a reduction in disease activity to make a difference,” said Dr. Gensler, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco. Another frequently used gauge of minimal disease activity in axial spondyloarthritis, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), is flawed by being totally based on subjective measurements without input from the attending rheumatologist, Dr. Gensler said.
The SPARTAN leadership will try to partner in this effort with the OMERACT (Outcome Measures in Rheumatology) program, the Assessment of Spondyloarthritis International Society (ASAS), or both, but if necessary SPARTAN will develop new minimal disease activity criteria on its own, Dr. Deodhar said.
Dr. Deodhar has received research support from 10 drug companies. Dr. Gensler has been a consultant to or has received research support from AbbVie, Amgen, Janssen, Novartis, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
DENVER – U.S. rheumatologists plan to develop new criteria to define minimal disease activity in patients with axial spondyloarthritis to better gauge in routine clinical practice how well these patients respond to treatment.
A score on the Ankylosing Spondylitis Disease Activity Score (ASDAS) of less than 1.3 is the most commonly used measure today of minimal disease activity, but the ASDAS isn’t suitable for point-of-care assessment in routine practice because of the need for a C-reactive protein level or erythrocyte sedimentation rate.
“In the clinic, ASDAS is very difficult or next to impossible to do” because it needs CRP or ESR, which are “never available at the point of care,” Atul A. Deodhar, MD, said in an interview at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN). Another limitation of ASDAS is that it focuses exclusively on musculoskeletal measures and does not take into account extra-articular manifestations of axial spondyloarthritis such as those in the eye, gastrointestinal tract, or skin.
“We would like a clinical measurement that might tell us that a patient is appropriately treated and at minimal disease activity,” said Dr. Deodhar, professor of medicine and medical director of the rheumatology clinics at Oregon Health & Science University in Portland.
As outgoing chair of SPARTAN, Dr. Deodhar introduced a proposal that SPARTAN develop new minimal disease activity criteria for patients with axial spondyloarthritis, presenting the rationale for this project at the meeting along with the incoming chair Lianne S. Gensler, MD.
“We want a way to measure disease activity at a stage that is not full remission but with enough of a reduction in disease activity to make a difference,” said Dr. Gensler, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco. Another frequently used gauge of minimal disease activity in axial spondyloarthritis, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), is flawed by being totally based on subjective measurements without input from the attending rheumatologist, Dr. Gensler said.
The SPARTAN leadership will try to partner in this effort with the OMERACT (Outcome Measures in Rheumatology) program, the Assessment of Spondyloarthritis International Society (ASAS), or both, but if necessary SPARTAN will develop new minimal disease activity criteria on its own, Dr. Deodhar said.
Dr. Deodhar has received research support from 10 drug companies. Dr. Gensler has been a consultant to or has received research support from AbbVie, Amgen, Janssen, Novartis, and UCB.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE 2016 SPARTAN ANNUAL MEETING
Promoting your older patient’s healthy ‘brain aging’
VIDEO: Alzheimer’s anti-tau drug fails, but shows hint of effect when taken alone
TORONTO – A highly anticipated phase III trial of an anti-tau drug has posted negative topline results, conferring no cognitive or functional benefits when given in conjunction with standard-of-care Alzheimer’s disease medications.
The drug, LMTX (TauRx, Singapore), also did not slow the progression of brain atrophy on imaging in either of two doses tested, according to a company press release.
Although the study didn’t meet its clinical endpoints in the overall cohort of 891 patients with mild-moderate Alzheimer’s disease, TauRx promoted it as “promising,” based on a subgroup analysis of patients who took the drug as monotherapy. None of these patients were taking any standard-of-care Alzheimer’s medications; the press release did not say how many this group comprised. But it did imply that the group was small enough that the treatment effect was diluted in the pooled primary analysis.
In patients who took the drug as monotherapy, LMTX was associated with dose-dependent, statistically significant improvements in the Alzheimer’s Disease Assessment Scale measures of cognition (ADAS-cog) and Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL). The drug was also associated with a slowing of brain ventricular expansion, compared with controls, suggesting that it could be preserving brain mass.
ADAS-cog scores for patients taking LMTX monotherapy 75 mg twice a day declined 6.3 points less than did controls, indicating preserved cognition. Those taking LMTX monotherapy 125 mg twice a day declined 5.8 points less than did controls. On the ADCS-ADL, patients taking 75 mg twice a day scored 6.5 points higher than controls, indicating better function, and those taking 125 mg twice a day scored 6.9 points higher.
Lateral ventricular volume expansion on MRI was significantly less than that seen in controls. For those taking 75 mg twice a day, ventricular expansion was reduced by 38%, and for those taking 125 mg twice a day, expansion was reduced by 33%.
This indicates a decrease in the rate of brain atrophy, the press release said, and the finding was “confirmed by corresponding increases in the whole-brain volumes in the same patient groups.”
The press release also said that the imaging findings offer physiologic confirmation of the cognitive and functional findings. “This is the first treatment in which a clinical effect has been supported by evidence in delay of progression in brain atrophy shown by MRI scans.”
The missing number of patients who took LMTX monotherapy is key, however, in determining whether the positive effects in that group are real or a chance finding, according to Richard J. Caselli, MD, associate director and clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic, Scottsdale, Ariz.
“Were the monotherapy results a fluke that washed out with bigger numbers or a meaningful effect? That needs to be clarified,” he said when asked to comment on the study. “Smaller ‘N’ trials can have skewed results due to random chances that mean nothing, and that is my fear. It’s unproven at this point but the burden of proof will rest on the investigators to replicate the positive outcome.”
The finding of a positive signal in monotherapy only is puzzling and also demands an explanation, said Michael Wolfe, PhD, a professor of neurology at Harvard University, Boston.
LMTX is a purified form of the dye methylene blue. Its method of action in preventing or dissolving tau tangles is not fully elucidated. A 2013 paper in a German chemistry journal, Angewandte Chemie, suggested that it works through oxidation to maintain the tau protein as a monomer, which prevents aggregation into filaments. There is no reason to think this pathway could intersect or interfere with any of the standard-of-care Alzheimer’s medications.
“There’s nothing obvious that comes to mind regarding interaction,” between the drug classes, Dr. Wolfe said in an interview. “We can’t say anything about this mechanistically. Any explanation here is just hand waving, I think.”
Dean Hartley, PhD, director of science initiatives at the Alzheimer’s Association, commented on the trial in a video interview at the Alzheimer’s Association International Conference 2016.
Neither Dr. Caselli nor Dr. Wolfe had relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – A highly anticipated phase III trial of an anti-tau drug has posted negative topline results, conferring no cognitive or functional benefits when given in conjunction with standard-of-care Alzheimer’s disease medications.
The drug, LMTX (TauRx, Singapore), also did not slow the progression of brain atrophy on imaging in either of two doses tested, according to a company press release.
Although the study didn’t meet its clinical endpoints in the overall cohort of 891 patients with mild-moderate Alzheimer’s disease, TauRx promoted it as “promising,” based on a subgroup analysis of patients who took the drug as monotherapy. None of these patients were taking any standard-of-care Alzheimer’s medications; the press release did not say how many this group comprised. But it did imply that the group was small enough that the treatment effect was diluted in the pooled primary analysis.
In patients who took the drug as monotherapy, LMTX was associated with dose-dependent, statistically significant improvements in the Alzheimer’s Disease Assessment Scale measures of cognition (ADAS-cog) and Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL). The drug was also associated with a slowing of brain ventricular expansion, compared with controls, suggesting that it could be preserving brain mass.
ADAS-cog scores for patients taking LMTX monotherapy 75 mg twice a day declined 6.3 points less than did controls, indicating preserved cognition. Those taking LMTX monotherapy 125 mg twice a day declined 5.8 points less than did controls. On the ADCS-ADL, patients taking 75 mg twice a day scored 6.5 points higher than controls, indicating better function, and those taking 125 mg twice a day scored 6.9 points higher.
Lateral ventricular volume expansion on MRI was significantly less than that seen in controls. For those taking 75 mg twice a day, ventricular expansion was reduced by 38%, and for those taking 125 mg twice a day, expansion was reduced by 33%.
This indicates a decrease in the rate of brain atrophy, the press release said, and the finding was “confirmed by corresponding increases in the whole-brain volumes in the same patient groups.”
The press release also said that the imaging findings offer physiologic confirmation of the cognitive and functional findings. “This is the first treatment in which a clinical effect has been supported by evidence in delay of progression in brain atrophy shown by MRI scans.”
The missing number of patients who took LMTX monotherapy is key, however, in determining whether the positive effects in that group are real or a chance finding, according to Richard J. Caselli, MD, associate director and clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic, Scottsdale, Ariz.
“Were the monotherapy results a fluke that washed out with bigger numbers or a meaningful effect? That needs to be clarified,” he said when asked to comment on the study. “Smaller ‘N’ trials can have skewed results due to random chances that mean nothing, and that is my fear. It’s unproven at this point but the burden of proof will rest on the investigators to replicate the positive outcome.”
The finding of a positive signal in monotherapy only is puzzling and also demands an explanation, said Michael Wolfe, PhD, a professor of neurology at Harvard University, Boston.
LMTX is a purified form of the dye methylene blue. Its method of action in preventing or dissolving tau tangles is not fully elucidated. A 2013 paper in a German chemistry journal, Angewandte Chemie, suggested that it works through oxidation to maintain the tau protein as a monomer, which prevents aggregation into filaments. There is no reason to think this pathway could intersect or interfere with any of the standard-of-care Alzheimer’s medications.
“There’s nothing obvious that comes to mind regarding interaction,” between the drug classes, Dr. Wolfe said in an interview. “We can’t say anything about this mechanistically. Any explanation here is just hand waving, I think.”
Dean Hartley, PhD, director of science initiatives at the Alzheimer’s Association, commented on the trial in a video interview at the Alzheimer’s Association International Conference 2016.
Neither Dr. Caselli nor Dr. Wolfe had relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – A highly anticipated phase III trial of an anti-tau drug has posted negative topline results, conferring no cognitive or functional benefits when given in conjunction with standard-of-care Alzheimer’s disease medications.
The drug, LMTX (TauRx, Singapore), also did not slow the progression of brain atrophy on imaging in either of two doses tested, according to a company press release.
Although the study didn’t meet its clinical endpoints in the overall cohort of 891 patients with mild-moderate Alzheimer’s disease, TauRx promoted it as “promising,” based on a subgroup analysis of patients who took the drug as monotherapy. None of these patients were taking any standard-of-care Alzheimer’s medications; the press release did not say how many this group comprised. But it did imply that the group was small enough that the treatment effect was diluted in the pooled primary analysis.
In patients who took the drug as monotherapy, LMTX was associated with dose-dependent, statistically significant improvements in the Alzheimer’s Disease Assessment Scale measures of cognition (ADAS-cog) and Alzheimer’s Disease Cooperative Study Activities of Daily Living inventory (ADCS-ADL). The drug was also associated with a slowing of brain ventricular expansion, compared with controls, suggesting that it could be preserving brain mass.
ADAS-cog scores for patients taking LMTX monotherapy 75 mg twice a day declined 6.3 points less than did controls, indicating preserved cognition. Those taking LMTX monotherapy 125 mg twice a day declined 5.8 points less than did controls. On the ADCS-ADL, patients taking 75 mg twice a day scored 6.5 points higher than controls, indicating better function, and those taking 125 mg twice a day scored 6.9 points higher.
Lateral ventricular volume expansion on MRI was significantly less than that seen in controls. For those taking 75 mg twice a day, ventricular expansion was reduced by 38%, and for those taking 125 mg twice a day, expansion was reduced by 33%.
This indicates a decrease in the rate of brain atrophy, the press release said, and the finding was “confirmed by corresponding increases in the whole-brain volumes in the same patient groups.”
The press release also said that the imaging findings offer physiologic confirmation of the cognitive and functional findings. “This is the first treatment in which a clinical effect has been supported by evidence in delay of progression in brain atrophy shown by MRI scans.”
The missing number of patients who took LMTX monotherapy is key, however, in determining whether the positive effects in that group are real or a chance finding, according to Richard J. Caselli, MD, associate director and clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic, Scottsdale, Ariz.
“Were the monotherapy results a fluke that washed out with bigger numbers or a meaningful effect? That needs to be clarified,” he said when asked to comment on the study. “Smaller ‘N’ trials can have skewed results due to random chances that mean nothing, and that is my fear. It’s unproven at this point but the burden of proof will rest on the investigators to replicate the positive outcome.”
The finding of a positive signal in monotherapy only is puzzling and also demands an explanation, said Michael Wolfe, PhD, a professor of neurology at Harvard University, Boston.
LMTX is a purified form of the dye methylene blue. Its method of action in preventing or dissolving tau tangles is not fully elucidated. A 2013 paper in a German chemistry journal, Angewandte Chemie, suggested that it works through oxidation to maintain the tau protein as a monomer, which prevents aggregation into filaments. There is no reason to think this pathway could intersect or interfere with any of the standard-of-care Alzheimer’s medications.
“There’s nothing obvious that comes to mind regarding interaction,” between the drug classes, Dr. Wolfe said in an interview. “We can’t say anything about this mechanistically. Any explanation here is just hand waving, I think.”
Dean Hartley, PhD, director of science initiatives at the Alzheimer’s Association, commented on the trial in a video interview at the Alzheimer’s Association International Conference 2016.
Neither Dr. Caselli nor Dr. Wolfe had relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
AT AAIC 2016
Key clinical point: Negative primary outcomes from a phase III trial of the anti-tau drug LMTX for Alzheimer’s disease were tempered by intriguing positive subanalysis data.
Major finding: ADAS-cog scores for patients taking LMTX monotherapy 75 mg twice a day declined 6.3 points less than did controls, indicating preserved cognition. Those for patients taking LMTX monotherapy 125 mg twice a day declined 5.8 points less than did controls.
Data source: A phase III trial of the anti-tau drug LMTX in 891 patients with mild-moderate Alzheimer’s disease.
Disclosures: The trial was sponsored by TauRx. Neither Dr. Caselli nor Dr. Wolfe had relevant disclosures.
VIDEO: Proposed Alzheimer’s funding boost could mean wider research scope
TORONTO – In 2011, President Barack Obama signed into law the National Alzheimer’s Project Act, with the lofty goal of preventing or effectively treating Alzheimer’s disease by 2025. A year later, a panel of leading researchers translated that goal into harsh reality: Reaching it would cost at least $2 billion each year. That level of funding would put Alzheimer’s research on the same footing as research on cancer, heart disease, and HIV – areas that have experienced rapid and sustained clinical progress.
But securing federal funding support has been a slow go. At the end of 2016, even with historic funding increases, the total allocation still fell short of $1 billion. That’s about to change for the better. The proposed 2017 budget, if approved, will boost the allocation to $1.4 billion.
What will that new money mean to researchers who’ve struggled for years to get grants? And what could it mean to doctors, patients, and families who still face a devastating disease with no cure, no prevention, and no effective treatments? Robert J. Egge, chief public policy officer for the Alzheimer’s Association, breaks it down in this video interview at the Alzheimer’s Association International Conference 2016.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – In 2011, President Barack Obama signed into law the National Alzheimer’s Project Act, with the lofty goal of preventing or effectively treating Alzheimer’s disease by 2025. A year later, a panel of leading researchers translated that goal into harsh reality: Reaching it would cost at least $2 billion each year. That level of funding would put Alzheimer’s research on the same footing as research on cancer, heart disease, and HIV – areas that have experienced rapid and sustained clinical progress.
But securing federal funding support has been a slow go. At the end of 2016, even with historic funding increases, the total allocation still fell short of $1 billion. That’s about to change for the better. The proposed 2017 budget, if approved, will boost the allocation to $1.4 billion.
What will that new money mean to researchers who’ve struggled for years to get grants? And what could it mean to doctors, patients, and families who still face a devastating disease with no cure, no prevention, and no effective treatments? Robert J. Egge, chief public policy officer for the Alzheimer’s Association, breaks it down in this video interview at the Alzheimer’s Association International Conference 2016.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – In 2011, President Barack Obama signed into law the National Alzheimer’s Project Act, with the lofty goal of preventing or effectively treating Alzheimer’s disease by 2025. A year later, a panel of leading researchers translated that goal into harsh reality: Reaching it would cost at least $2 billion each year. That level of funding would put Alzheimer’s research on the same footing as research on cancer, heart disease, and HIV – areas that have experienced rapid and sustained clinical progress.
But securing federal funding support has been a slow go. At the end of 2016, even with historic funding increases, the total allocation still fell short of $1 billion. That’s about to change for the better. The proposed 2017 budget, if approved, will boost the allocation to $1.4 billion.
What will that new money mean to researchers who’ve struggled for years to get grants? And what could it mean to doctors, patients, and families who still face a devastating disease with no cure, no prevention, and no effective treatments? Robert J. Egge, chief public policy officer for the Alzheimer’s Association, breaks it down in this video interview at the Alzheimer’s Association International Conference 2016.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
AT AAIC 2016
VIDEO: Loss of function in Rab10 gene cuts Alzheimer’s risk by up to 40%
TORONTO – A rare loss of function in a gene that influences gamma secretase trafficking has been found to reduce the risk of Alzheimer’s by up to 40%, even in people who are homozygous carriers of the apolipoprotein E (apo E) epsilon 4 allele.
The gene – Rab10 – is a member of a family of about 60 genes involved in intracellular protein transport, Keoni Kauwe, PhD, said at the Alzheimer’s Association International Conference 2016. In addition to influencing endocytosis, ciliary transport, phagosome maturation, and insulin transport, Rab10 appears to assist in the transport of gamma secretase to the cell membrane.
By studying a population of elders who appear resistant to developing Alzheimer’s, Dr. Kauwe of Brigham Young University, Provo, Utah, and his associates determined that many of them share a natural inhibition of Rab10 – a unique characteristic that strongly contributes to their resistance to Alzheimer’s, he said. This knocked-down function would decrease the amount of gamma secretase to reach the cell membrane, Dr. Kauwe reasoned. Therefore, the proteolytic pathway that creates amyloid beta (Abeta) should be attenuated, allowing much less amyloid beta to be created and, presumably, be around to initiate the amyloid cascade that sparks Alzheimer’s disease.
His study cohort was drawn from two large data sets of cognitively normal elders, including many who were apo E epsilon 4 homozygotes. About 5% of the sample showed the loss-of-function Rab10 variant, which Dr. Kauwe said conferred a 20%-40% risk reduction for developing Alzheimer’s disease.
To understand the gene’s effects on amyloid beta production, he both overexpressed it and silenced it in cell lines. Overexpression resulted in a significant increase in the Abeta42/Abeta40 ratio (a riskier Alzheimer’s profile), while silencing it resulted in a significant decrease in the Abeta42/Abeta40 ratio (a more favorable profile).
Rab10 could be a therapeutic target, similar to the PCSK9 gene that influences low-density lipoprotein creation, Dr. Kauwe said. Monoclonal antibodies that block PCSK9 have recently been developed after a similar observation that naturally occurring loss of functions in the gene was associated with lower LDL levels.
“We’re going to be looking at the same thing with Rab10,” he said. “We do think that Rab10 inhibition could be a big story. It’s a high-risk venture, certainly, but it could also be high reward.”
Dr. Kauwe discussed his findings in a video interview at the conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – A rare loss of function in a gene that influences gamma secretase trafficking has been found to reduce the risk of Alzheimer’s by up to 40%, even in people who are homozygous carriers of the apolipoprotein E (apo E) epsilon 4 allele.
The gene – Rab10 – is a member of a family of about 60 genes involved in intracellular protein transport, Keoni Kauwe, PhD, said at the Alzheimer’s Association International Conference 2016. In addition to influencing endocytosis, ciliary transport, phagosome maturation, and insulin transport, Rab10 appears to assist in the transport of gamma secretase to the cell membrane.
By studying a population of elders who appear resistant to developing Alzheimer’s, Dr. Kauwe of Brigham Young University, Provo, Utah, and his associates determined that many of them share a natural inhibition of Rab10 – a unique characteristic that strongly contributes to their resistance to Alzheimer’s, he said. This knocked-down function would decrease the amount of gamma secretase to reach the cell membrane, Dr. Kauwe reasoned. Therefore, the proteolytic pathway that creates amyloid beta (Abeta) should be attenuated, allowing much less amyloid beta to be created and, presumably, be around to initiate the amyloid cascade that sparks Alzheimer’s disease.
His study cohort was drawn from two large data sets of cognitively normal elders, including many who were apo E epsilon 4 homozygotes. About 5% of the sample showed the loss-of-function Rab10 variant, which Dr. Kauwe said conferred a 20%-40% risk reduction for developing Alzheimer’s disease.
To understand the gene’s effects on amyloid beta production, he both overexpressed it and silenced it in cell lines. Overexpression resulted in a significant increase in the Abeta42/Abeta40 ratio (a riskier Alzheimer’s profile), while silencing it resulted in a significant decrease in the Abeta42/Abeta40 ratio (a more favorable profile).
Rab10 could be a therapeutic target, similar to the PCSK9 gene that influences low-density lipoprotein creation, Dr. Kauwe said. Monoclonal antibodies that block PCSK9 have recently been developed after a similar observation that naturally occurring loss of functions in the gene was associated with lower LDL levels.
“We’re going to be looking at the same thing with Rab10,” he said. “We do think that Rab10 inhibition could be a big story. It’s a high-risk venture, certainly, but it could also be high reward.”
Dr. Kauwe discussed his findings in a video interview at the conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
TORONTO – A rare loss of function in a gene that influences gamma secretase trafficking has been found to reduce the risk of Alzheimer’s by up to 40%, even in people who are homozygous carriers of the apolipoprotein E (apo E) epsilon 4 allele.
The gene – Rab10 – is a member of a family of about 60 genes involved in intracellular protein transport, Keoni Kauwe, PhD, said at the Alzheimer’s Association International Conference 2016. In addition to influencing endocytosis, ciliary transport, phagosome maturation, and insulin transport, Rab10 appears to assist in the transport of gamma secretase to the cell membrane.
By studying a population of elders who appear resistant to developing Alzheimer’s, Dr. Kauwe of Brigham Young University, Provo, Utah, and his associates determined that many of them share a natural inhibition of Rab10 – a unique characteristic that strongly contributes to their resistance to Alzheimer’s, he said. This knocked-down function would decrease the amount of gamma secretase to reach the cell membrane, Dr. Kauwe reasoned. Therefore, the proteolytic pathway that creates amyloid beta (Abeta) should be attenuated, allowing much less amyloid beta to be created and, presumably, be around to initiate the amyloid cascade that sparks Alzheimer’s disease.
His study cohort was drawn from two large data sets of cognitively normal elders, including many who were apo E epsilon 4 homozygotes. About 5% of the sample showed the loss-of-function Rab10 variant, which Dr. Kauwe said conferred a 20%-40% risk reduction for developing Alzheimer’s disease.
To understand the gene’s effects on amyloid beta production, he both overexpressed it and silenced it in cell lines. Overexpression resulted in a significant increase in the Abeta42/Abeta40 ratio (a riskier Alzheimer’s profile), while silencing it resulted in a significant decrease in the Abeta42/Abeta40 ratio (a more favorable profile).
Rab10 could be a therapeutic target, similar to the PCSK9 gene that influences low-density lipoprotein creation, Dr. Kauwe said. Monoclonal antibodies that block PCSK9 have recently been developed after a similar observation that naturally occurring loss of functions in the gene was associated with lower LDL levels.
“We’re going to be looking at the same thing with Rab10,” he said. “We do think that Rab10 inhibition could be a big story. It’s a high-risk venture, certainly, but it could also be high reward.”
Dr. Kauwe discussed his findings in a video interview at the conference.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @alz_gal
AT AAIC 2016
Webcast: Hormonal contraception and risk of venous thromboembolism

Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:

Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:

Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice: