User login
Promising new therapy for critical limb ischemia
CHICAGO– A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
CHICAGO– A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
CHICAGO– A single set of intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia showed safety as well as evidence of efficacy through 12 months of follow-up in the STOP-CLI trial.
“Patients treated with JVS-100 demonstrated dose-dependent improvement across multiple patient-centered outcomes, including pain, quality of life, and wound healing, with less change in macrovascular objective measures in this small study,” Dr. Melina R. Kibbe reported at the American Heart Association scientific sessions. JVS-100 is a nonviral plasmid encoding human stromal cell–derived factor-1 (SDF-1), a natural chemokine protein that promotes angiogenesis by recruiting endothelial progenitor cells from the bone marrow to ischemic sites, explained Dr. Kibbe, professor and vice chair of surgical research and deputy director of the Simpson Querrey Institute for BioNanotechnology at Northwestern University, Chicago.
STOP-CLI was an exploratory, phase IIa, double-blind, first-in-humans study involving 48 patients with Rutherford classification 4 or 5 critical climb ischemia (CLI). All had an ankle-brachial index of 0.4 or lower, an ankle systolic blood pressure of 70 mm Hg or less or a toe systolic blood pressure of 50 or less, and were poor candidates for surgical revascularization. None had Buerger’s disease.
Participants were randomized to one of four study arms, and within each study arm further randomized 3:1 to stromal cell–derived factor-1 (SDF-1) or placebo injections. The patients received either 8 or 16 injections, each containing either 0.5 or 1.0 mg of SDF-1 or placebo. The injections, given in a single session, were placed at least 0.5 cm apart throughout the ischemic area of the affected limb.
By chance, most patients randomized to the placebo group were Rutherford 4, a category of CLI defined by rest pain, while the majority in the active treatment arms were Rutherford 5, a more severe disease manifestation characterized by ulcers. As a consequence, the SDF-1 recipients also had far larger nonhealing wounds, with an average area of 6.4 cm2, compared with 1.5 cm2, in controls.
The SDF-1 injections proved safe and were well tolerated, with no treatment-related serious adverse events and no safety signals evident in the laboratory results.
Turning to efficacy endpoints, Dr. Kibbe said self-rated visual analog scale pain scores showed clear, dose-dependent improvement over time in the SDF-1 treatment cohorts and no change in controls.
Similarly, the active treatment groups showed improved quality of life scores on all domains of the Short Form-36: physical functioning, bodily pain, general health, social functioning, energy/fatigue, emotional well-being, and overall physical and mental health, the surgeon continued.
Wound area decreased significantly in the SDF-1-treated groups, with the biggest reduction – more than 8 cm2 – being noted in the three patients who received eight 1-mg injections. That was also the group with the largest wounds at baseline, with an average area of 11.4 cm2.
Of note, the major limb amputation rate was “remarkably low” for patients with such severe CLI, according to Dr. Kibbe. The rate was less than 10% over the course of 12 months, with one patient in each of the four active treatment arms having a major amputation at time intervals of 58-112 days post injection. No major limb amputations occurred in the control group.
There was a hint of improvement with SDF-1 therapy over placebo in ankle-brachial index and transcutaneous oxygen pressure, but the between-group differences were too narrow in this study to allow for any conclusions. That must await planned much larger phase III trials, according to Dr. Kibbe.
Audience members, citing the numerous failures of once-promising stem cell therapies for CLI at phase III testing over the last 10-15 years, wondered why Dr. Kibbe thinks SDF-1 will fare any better.
“This is much debated and discussed among all the people involved in these kinds of trials,” she replied. “I’d say, briefly, that a lot of it has to do with patient selection. I think when you have a mixed bag of patients in a trial, including patients with Buerger’s disease, treated in multiple different countries, using different definitions of when to amputate, all those things come into play and could account for why those phase III trials were not successful.”
“Having been involved in lots of the different gene- and cell-based therapy trials, I think one of the unique benefits of this therapy is that it kind of bridges between the two. SDF-1 basically homes your endothelial progenitor cells to the site of ischemic injury for enhanced vasculogenesis. But SDF-1 also has direct effects on endothelial cells, including stimulating proliferation and preventing apoptosis,” she added.
JVS-100 has also successfully completed a phase II clinical trial for the treatment of heart failure. In addition, the agent is being developed as a treatment for acute MI, chronic angina, and for muscle regeneration.
The STOP-CLI study was sponsored by Juventas Therapeutics. Dr. Kibbe reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Intramuscular injections of stromal cell–derived factor-1 in patients with critical limb ischemia demonstrated safety and efficacy; the therapy is moving forward to phase III testing.
Major finding: The major limb amputation rate was less than 10% during 12 months of follow-up after a single dose of the novel therapy.
Data source: The STOP-CLI trial was a phase IIa, 12-month, randomized, double-blind, placebo-controlled, six-center trial including 48 patients with critical limb ischemia.
Disclosures: The STOP-CLI trial was sponsored by Juventas Therapeutics. The presenter reported serving as a consultant to Johnson & Johnson/Cordis and Pluristem.
First expression of cardiovascular disease differs by gender
CHICAGO – Two out of three 55-year-old men and women will develop some form of cardiovascular disease during their remaining lifetime, but the first manifestation differs considerably by gender, according to a new analysis from The Rotterdam Study.
In men, the first manifestation of cardiovascular disease is most often a coronary heart disease event. Women are more likely to experience cerebrovascular disease or heart failure as their first event, Dr. Maarten J.G. Leening reported at the American Heart Association scientific sessions. Simultaneously with his presentation, the findings were published online in BMJ.
This new finding has important implications for primary prevention, noted Dr. Leening of Erasmus University, Rotterdam.
“Most of the attention of late in primary prevention has been drawn to the 2013 [AHA/ACC] lipid guidelines. But as we can see, for women it’s heart failure and cerebrovascular disease that are the major contributors to overall risk. And we know from the statin trials that there is no or only very limited reduction in heart failure and that the risk reduction for cerebrovascular disease is much smaller than for CHD. So our findings highlight the importance of blood pressure control and lifestyle factors, especially smoking, for primary prevention in women, since those are the best-established risk factors for cerebrovascular disease and heart failure,” he said.
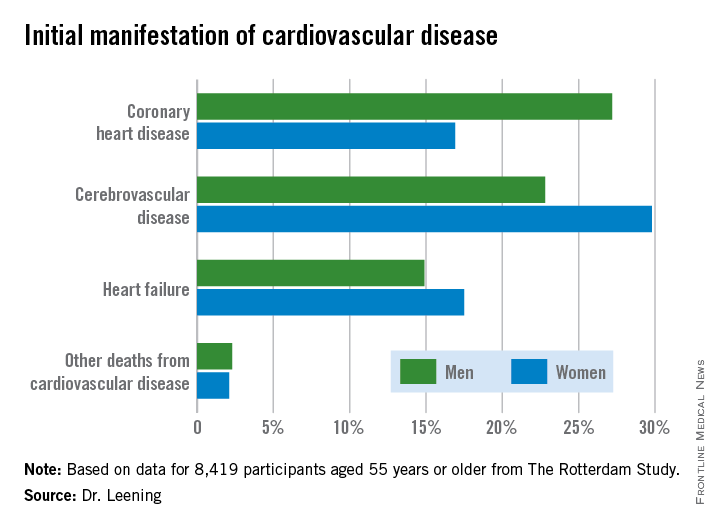
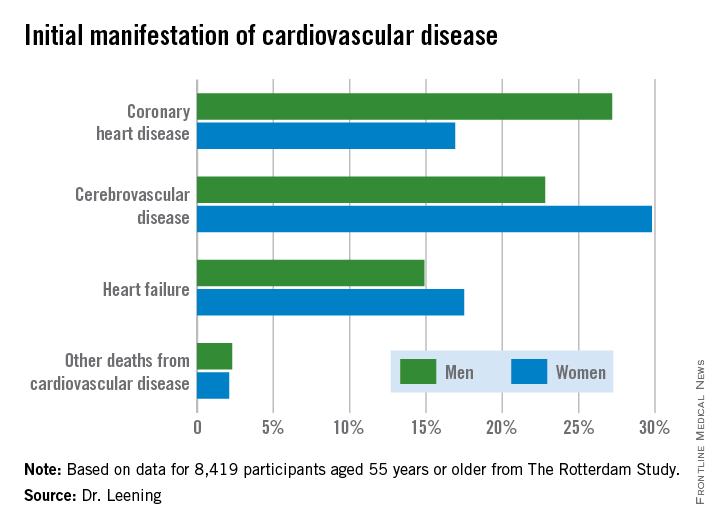
Dr. Leening reported on 8,419 participants of The Rotterdam Study aged 55 or older, all free from cardiovascular disease at baseline. During a median 13.5 years and up to 20 years of follow-up in this ongoing prospective population-based study, 2,888 subjects developed cardiovascular disease. Coronary heart disease accounted for 826 of these first events, cerebrovascular events accounted for another 1,198, heart failure 726, and there were 102 other cardiovascular deaths.
At age 55, the overall lifetime risk of developing cardiovascular disease was 67.1% in men and 66.4% in women, although these rates slightly underestimate the true risks because data on peripheral arterial disease, nonfatal aortic aneurysms, and angina managed exclusively medically weren’t collected.
This is the first study to scrutinize gender differences in the first manifestations of cardiovascular disease, broadly defined. For purposes of primary prevention, the first manifestation of cardiovascular disease is what’s most relevant, not the final fatal one, he noted.
From an absolute risk perspective, CHD as the first manifestation of cardiovascular disease clearly stood out as the major difference between men and women. In a comparison of 1,000 women with an equal number of men, there were 102 fewer cases of CHD in the women. This was offset by 70 more cases of cerebrovascular disease and 26 additional cases of heart failure in the women.
It’s particularly noteworthy that heart failure accounted for one-quarter of the first manifestations of cardiovascular disease in women, given that by definition none of the cases of heart failure recorded in this study were preceded by CHD. This underscores the importance of focusing of risk factors other than dyslipidemia, the physician said.
The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. Dr. Leening reported having no financial conflicts of interest.
CHICAGO – Two out of three 55-year-old men and women will develop some form of cardiovascular disease during their remaining lifetime, but the first manifestation differs considerably by gender, according to a new analysis from The Rotterdam Study.
In men, the first manifestation of cardiovascular disease is most often a coronary heart disease event. Women are more likely to experience cerebrovascular disease or heart failure as their first event, Dr. Maarten J.G. Leening reported at the American Heart Association scientific sessions. Simultaneously with his presentation, the findings were published online in BMJ.
This new finding has important implications for primary prevention, noted Dr. Leening of Erasmus University, Rotterdam.
“Most of the attention of late in primary prevention has been drawn to the 2013 [AHA/ACC] lipid guidelines. But as we can see, for women it’s heart failure and cerebrovascular disease that are the major contributors to overall risk. And we know from the statin trials that there is no or only very limited reduction in heart failure and that the risk reduction for cerebrovascular disease is much smaller than for CHD. So our findings highlight the importance of blood pressure control and lifestyle factors, especially smoking, for primary prevention in women, since those are the best-established risk factors for cerebrovascular disease and heart failure,” he said.

Dr. Leening reported on 8,419 participants of The Rotterdam Study aged 55 or older, all free from cardiovascular disease at baseline. During a median 13.5 years and up to 20 years of follow-up in this ongoing prospective population-based study, 2,888 subjects developed cardiovascular disease. Coronary heart disease accounted for 826 of these first events, cerebrovascular events accounted for another 1,198, heart failure 726, and there were 102 other cardiovascular deaths.
At age 55, the overall lifetime risk of developing cardiovascular disease was 67.1% in men and 66.4% in women, although these rates slightly underestimate the true risks because data on peripheral arterial disease, nonfatal aortic aneurysms, and angina managed exclusively medically weren’t collected.
This is the first study to scrutinize gender differences in the first manifestations of cardiovascular disease, broadly defined. For purposes of primary prevention, the first manifestation of cardiovascular disease is what’s most relevant, not the final fatal one, he noted.
From an absolute risk perspective, CHD as the first manifestation of cardiovascular disease clearly stood out as the major difference between men and women. In a comparison of 1,000 women with an equal number of men, there were 102 fewer cases of CHD in the women. This was offset by 70 more cases of cerebrovascular disease and 26 additional cases of heart failure in the women.
It’s particularly noteworthy that heart failure accounted for one-quarter of the first manifestations of cardiovascular disease in women, given that by definition none of the cases of heart failure recorded in this study were preceded by CHD. This underscores the importance of focusing of risk factors other than dyslipidemia, the physician said.
The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. Dr. Leening reported having no financial conflicts of interest.
CHICAGO – Two out of three 55-year-old men and women will develop some form of cardiovascular disease during their remaining lifetime, but the first manifestation differs considerably by gender, according to a new analysis from The Rotterdam Study.
In men, the first manifestation of cardiovascular disease is most often a coronary heart disease event. Women are more likely to experience cerebrovascular disease or heart failure as their first event, Dr. Maarten J.G. Leening reported at the American Heart Association scientific sessions. Simultaneously with his presentation, the findings were published online in BMJ.
This new finding has important implications for primary prevention, noted Dr. Leening of Erasmus University, Rotterdam.
“Most of the attention of late in primary prevention has been drawn to the 2013 [AHA/ACC] lipid guidelines. But as we can see, for women it’s heart failure and cerebrovascular disease that are the major contributors to overall risk. And we know from the statin trials that there is no or only very limited reduction in heart failure and that the risk reduction for cerebrovascular disease is much smaller than for CHD. So our findings highlight the importance of blood pressure control and lifestyle factors, especially smoking, for primary prevention in women, since those are the best-established risk factors for cerebrovascular disease and heart failure,” he said.

Dr. Leening reported on 8,419 participants of The Rotterdam Study aged 55 or older, all free from cardiovascular disease at baseline. During a median 13.5 years and up to 20 years of follow-up in this ongoing prospective population-based study, 2,888 subjects developed cardiovascular disease. Coronary heart disease accounted for 826 of these first events, cerebrovascular events accounted for another 1,198, heart failure 726, and there were 102 other cardiovascular deaths.
At age 55, the overall lifetime risk of developing cardiovascular disease was 67.1% in men and 66.4% in women, although these rates slightly underestimate the true risks because data on peripheral arterial disease, nonfatal aortic aneurysms, and angina managed exclusively medically weren’t collected.
This is the first study to scrutinize gender differences in the first manifestations of cardiovascular disease, broadly defined. For purposes of primary prevention, the first manifestation of cardiovascular disease is what’s most relevant, not the final fatal one, he noted.
From an absolute risk perspective, CHD as the first manifestation of cardiovascular disease clearly stood out as the major difference between men and women. In a comparison of 1,000 women with an equal number of men, there were 102 fewer cases of CHD in the women. This was offset by 70 more cases of cerebrovascular disease and 26 additional cases of heart failure in the women.
It’s particularly noteworthy that heart failure accounted for one-quarter of the first manifestations of cardiovascular disease in women, given that by definition none of the cases of heart failure recorded in this study were preceded by CHD. This underscores the importance of focusing of risk factors other than dyslipidemia, the physician said.
The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. Dr. Leening reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Women are more likely to have cerebrovascular disease or heart failure as their first cardiovascular disease event.
Major finding: Among 1,000 women free of cardiovascular disease at age 55, there were 102 fewer cases of coronary heart disease as the initial manifestation of cardiovascular disease during their remaining lifetime than in an equal number of 55-year-old men.
Data source: An analysis from the ongoing, prospective, population-based Rotterdam Study of 8,419 subjects.
Disclosures: The Netherlands Organization for Health Research and Development and a variety of Dutch foundations funded the study. The presenter reported having no financial conflicts.
Zero coronary calcium means very low 10-year event risk
CHICAGO – Absence of coronary artery calcium upon imaging results in an impressively low cardiovascular event rate over the next 10 years regardless of an individual’s level of standard risk factors, according to prospective data from the MESA study.
In contrast, a coronary artery calcium (CAC) score of 1-10, often described as minimal CAC, nearly doubles the 10-year risk, compared with a baseline CAC score of 0.
Prior to these new 10-year data, many cardiologists considered a CAC score of 1-10 as tantamount to no CAC. Not so, Dr. Parag H. Joshi said at the American Heart Association scientific sessions.
“A CAC of 0 is presumably identifying someone without any atherosclerosis. Just the presence of minimal calcium suggests that atherosclerosis is building up. Our data suggest that among individuals with a CAC of 1-10, current smoking, elevated non-HDL cholesterol, and particularly hypertension should be treated aggressively,” said Dr. Joshi, a clinical fellow in cardiovascular diseases and prevention at Johns Hopkins University, Baltimore.
Prior studies totaling more than 50,000 subjects with a CAC score of 0 have shown very low cardiovascular event rates over 4-5 years of follow-up. However, current cardiovascular risk estimates focus on 10-year risk. This new analysis from MESA (Multi-Ethnic Study of Atherosclerosis) is the first study to provide prospective, 10-year events data, and those data are highly reassuring, he added.
MESA is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline, when their CAC score was determined. At that time, 3,415 participants had a CAC score of 0 and 508 had a score of 1-10.
During a median 10.3 years of follow-up, 123 cardiovascular events occurred, roughly one-third of which were nonfatal acute MIs and half of which were nonfatal strokes; the remainder were cardiovascular deaths.
The event rate was 2.9/1,000 person-years in subjects with a CAC of 0 and significantly greater at 5.5/1,000 person-years with a score of 1-10. However, since the cardiovascular risk factor profile of the zero CAC group was generally more favorable, Dr. Joshi and coinvestigators carried out a Cox proportional hazards analysis factoring in demographics, standard cardiovascular risk factors, body mass index, C-reactive protein level, and carotid intima media thickness. The adjusted 10-year event risk in the group with a CAC score of 1-10 was 1.9-fold greater than with a CAC of 0.
The highest 10-year event rate was noted in subjects with at least three of the following four risk factors at baseline: hypertension, current smoking, diabetes, and hyperlipidemia. The rate was 6.5/1,000 person-years in such individuals if they had a CAC of 0 and doubled at 13.1/1,000 person-years with a score of 1-10.
In a multivariate Cox analysis, age, smoking, and hypertension proved to be significant predictors of cardiovascular events in the group with a CAC of 0 as well as in those with a CAC of 1-10. But there was one important difference between the two groups: While the hazard ratio for cardiovascular events associated with hypertension versus no hypertension was 2.1 in subjects with a CAC of 0, the presence of hypertension in individuals with a CAC of 1-10 increased their event risk by 10.2-fold, or nearly five times greater than the risk increase associated with hypertension in persons with a CAC of 0, Dr. Joshi observed.
Non–HDL cholesterol level was predictive of cardiovascular risk in subjects with a CAC of 1-10 but not in those with a score of 0.
When actual event rates were compared with those predicted by the atherosclerotic cardiovascular disease (ASCVD) risk estimator introduced in the 2013 AHA/American College of Cardiology cholesterol guidelines, the event rate in subjects with an ASCVD 10-year risk estimate of 7.5%-15% but a CAC of 0 was just 4.4%.
Audience members noted that CAC scores didn’t do a very good job of stratifying stroke risk in MESA. That’s not surprising, since the score reflects coronary but not carotid artery calcium. But it is a limitation of CAC as a predictive tool, especially in light of the fact that strokes accounted for half of all cardiovascular events in the study.
Asked where he and his coinvestigators plan to go from here, Dr. Joshi said a randomized, controlled trial would be ideal, but to date funding isn’t available. However, the observational data from MESA and other studies suggest such a trial may not even be needed.
“Certainly the guidelines do allow for CAC scoring to be used in clinical decision making,” he noted.
The MESA study is funded by the National Heart, Lung, and Blood Institute. Dr. Joshi reported having no financial conflicts.
CHICAGO – Absence of coronary artery calcium upon imaging results in an impressively low cardiovascular event rate over the next 10 years regardless of an individual’s level of standard risk factors, according to prospective data from the MESA study.
In contrast, a coronary artery calcium (CAC) score of 1-10, often described as minimal CAC, nearly doubles the 10-year risk, compared with a baseline CAC score of 0.
Prior to these new 10-year data, many cardiologists considered a CAC score of 1-10 as tantamount to no CAC. Not so, Dr. Parag H. Joshi said at the American Heart Association scientific sessions.
“A CAC of 0 is presumably identifying someone without any atherosclerosis. Just the presence of minimal calcium suggests that atherosclerosis is building up. Our data suggest that among individuals with a CAC of 1-10, current smoking, elevated non-HDL cholesterol, and particularly hypertension should be treated aggressively,” said Dr. Joshi, a clinical fellow in cardiovascular diseases and prevention at Johns Hopkins University, Baltimore.
Prior studies totaling more than 50,000 subjects with a CAC score of 0 have shown very low cardiovascular event rates over 4-5 years of follow-up. However, current cardiovascular risk estimates focus on 10-year risk. This new analysis from MESA (Multi-Ethnic Study of Atherosclerosis) is the first study to provide prospective, 10-year events data, and those data are highly reassuring, he added.
MESA is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline, when their CAC score was determined. At that time, 3,415 participants had a CAC score of 0 and 508 had a score of 1-10.
During a median 10.3 years of follow-up, 123 cardiovascular events occurred, roughly one-third of which were nonfatal acute MIs and half of which were nonfatal strokes; the remainder were cardiovascular deaths.
The event rate was 2.9/1,000 person-years in subjects with a CAC of 0 and significantly greater at 5.5/1,000 person-years with a score of 1-10. However, since the cardiovascular risk factor profile of the zero CAC group was generally more favorable, Dr. Joshi and coinvestigators carried out a Cox proportional hazards analysis factoring in demographics, standard cardiovascular risk factors, body mass index, C-reactive protein level, and carotid intima media thickness. The adjusted 10-year event risk in the group with a CAC score of 1-10 was 1.9-fold greater than with a CAC of 0.
The highest 10-year event rate was noted in subjects with at least three of the following four risk factors at baseline: hypertension, current smoking, diabetes, and hyperlipidemia. The rate was 6.5/1,000 person-years in such individuals if they had a CAC of 0 and doubled at 13.1/1,000 person-years with a score of 1-10.
In a multivariate Cox analysis, age, smoking, and hypertension proved to be significant predictors of cardiovascular events in the group with a CAC of 0 as well as in those with a CAC of 1-10. But there was one important difference between the two groups: While the hazard ratio for cardiovascular events associated with hypertension versus no hypertension was 2.1 in subjects with a CAC of 0, the presence of hypertension in individuals with a CAC of 1-10 increased their event risk by 10.2-fold, or nearly five times greater than the risk increase associated with hypertension in persons with a CAC of 0, Dr. Joshi observed.
Non–HDL cholesterol level was predictive of cardiovascular risk in subjects with a CAC of 1-10 but not in those with a score of 0.
When actual event rates were compared with those predicted by the atherosclerotic cardiovascular disease (ASCVD) risk estimator introduced in the 2013 AHA/American College of Cardiology cholesterol guidelines, the event rate in subjects with an ASCVD 10-year risk estimate of 7.5%-15% but a CAC of 0 was just 4.4%.
Audience members noted that CAC scores didn’t do a very good job of stratifying stroke risk in MESA. That’s not surprising, since the score reflects coronary but not carotid artery calcium. But it is a limitation of CAC as a predictive tool, especially in light of the fact that strokes accounted for half of all cardiovascular events in the study.
Asked where he and his coinvestigators plan to go from here, Dr. Joshi said a randomized, controlled trial would be ideal, but to date funding isn’t available. However, the observational data from MESA and other studies suggest such a trial may not even be needed.
“Certainly the guidelines do allow for CAC scoring to be used in clinical decision making,” he noted.
The MESA study is funded by the National Heart, Lung, and Blood Institute. Dr. Joshi reported having no financial conflicts.
CHICAGO – Absence of coronary artery calcium upon imaging results in an impressively low cardiovascular event rate over the next 10 years regardless of an individual’s level of standard risk factors, according to prospective data from the MESA study.
In contrast, a coronary artery calcium (CAC) score of 1-10, often described as minimal CAC, nearly doubles the 10-year risk, compared with a baseline CAC score of 0.
Prior to these new 10-year data, many cardiologists considered a CAC score of 1-10 as tantamount to no CAC. Not so, Dr. Parag H. Joshi said at the American Heart Association scientific sessions.
“A CAC of 0 is presumably identifying someone without any atherosclerosis. Just the presence of minimal calcium suggests that atherosclerosis is building up. Our data suggest that among individuals with a CAC of 1-10, current smoking, elevated non-HDL cholesterol, and particularly hypertension should be treated aggressively,” said Dr. Joshi, a clinical fellow in cardiovascular diseases and prevention at Johns Hopkins University, Baltimore.
Prior studies totaling more than 50,000 subjects with a CAC score of 0 have shown very low cardiovascular event rates over 4-5 years of follow-up. However, current cardiovascular risk estimates focus on 10-year risk. This new analysis from MESA (Multi-Ethnic Study of Atherosclerosis) is the first study to provide prospective, 10-year events data, and those data are highly reassuring, he added.
MESA is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline, when their CAC score was determined. At that time, 3,415 participants had a CAC score of 0 and 508 had a score of 1-10.
During a median 10.3 years of follow-up, 123 cardiovascular events occurred, roughly one-third of which were nonfatal acute MIs and half of which were nonfatal strokes; the remainder were cardiovascular deaths.
The event rate was 2.9/1,000 person-years in subjects with a CAC of 0 and significantly greater at 5.5/1,000 person-years with a score of 1-10. However, since the cardiovascular risk factor profile of the zero CAC group was generally more favorable, Dr. Joshi and coinvestigators carried out a Cox proportional hazards analysis factoring in demographics, standard cardiovascular risk factors, body mass index, C-reactive protein level, and carotid intima media thickness. The adjusted 10-year event risk in the group with a CAC score of 1-10 was 1.9-fold greater than with a CAC of 0.
The highest 10-year event rate was noted in subjects with at least three of the following four risk factors at baseline: hypertension, current smoking, diabetes, and hyperlipidemia. The rate was 6.5/1,000 person-years in such individuals if they had a CAC of 0 and doubled at 13.1/1,000 person-years with a score of 1-10.
In a multivariate Cox analysis, age, smoking, and hypertension proved to be significant predictors of cardiovascular events in the group with a CAC of 0 as well as in those with a CAC of 1-10. But there was one important difference between the two groups: While the hazard ratio for cardiovascular events associated with hypertension versus no hypertension was 2.1 in subjects with a CAC of 0, the presence of hypertension in individuals with a CAC of 1-10 increased their event risk by 10.2-fold, or nearly five times greater than the risk increase associated with hypertension in persons with a CAC of 0, Dr. Joshi observed.
Non–HDL cholesterol level was predictive of cardiovascular risk in subjects with a CAC of 1-10 but not in those with a score of 0.
When actual event rates were compared with those predicted by the atherosclerotic cardiovascular disease (ASCVD) risk estimator introduced in the 2013 AHA/American College of Cardiology cholesterol guidelines, the event rate in subjects with an ASCVD 10-year risk estimate of 7.5%-15% but a CAC of 0 was just 4.4%.
Audience members noted that CAC scores didn’t do a very good job of stratifying stroke risk in MESA. That’s not surprising, since the score reflects coronary but not carotid artery calcium. But it is a limitation of CAC as a predictive tool, especially in light of the fact that strokes accounted for half of all cardiovascular events in the study.
Asked where he and his coinvestigators plan to go from here, Dr. Joshi said a randomized, controlled trial would be ideal, but to date funding isn’t available. However, the observational data from MESA and other studies suggest such a trial may not even be needed.
“Certainly the guidelines do allow for CAC scoring to be used in clinical decision making,” he noted.
The MESA study is funded by the National Heart, Lung, and Blood Institute. Dr. Joshi reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: A coronary artery calcium score of 0 appears to trump the 10-year atherosclerotic cardiovascular disease risk estimator introduced in the 2013 AHA/ACC cholesterol guidelines.
Major finding: The actual 10-year cardiovascular event rate in subjects with a coronary artery calcium score of 0 was just 4.4% – below the guideline-recommended threshold for statin therapy– even though their predicted risk using the AHA/ACC risk estimator was 7.5%-15%.
Data source: The Multi-Ethnic Study of Atherosclerosis is a prospective, population-based cohort study. This analysis included 6,814 subjects aged 45-84 who were free of clinical cardiovascular disease at baseline.
Disclosures: The MESA study is funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
Big savings achievable with evidence-based radiotherapy for breast Ca
SAN ANTONIO – Hefty reductions in annual health care spending for breast cancer therapy would be achieved if physicians increased their use of evidence-based adjuvant radiation therapy following lumpectomy for early-stage breast cancer, according to Dr. Rachel A. Greenup.
The potential savings would amount to $164 million annually, based on the American College of Surgeons National Cancer Data Base for the year 2011. And since that database captures only about 70% of all newly diagnosed cancers in the United States, that savings figure is likely an underestimate, according to Dr. Greenup, a surgical oncologist at Duke University, Durham, N.C.
She and her coinvestigators identified 43,247 women in the national database with clinically node-negative, T1-T2 invasive breast cancer treated by lumpectomy during 2011. Their median age was 63 years, with a median tumor size of 1.2 cm. Only 26% of women received the least expensive adjuvant radiation therapy for which they were potentially eligible based upon current guidelines and best practices. Sixty-seven percent of patients received more costly regimens.
Overall, 22% of the women were deemed by investigators to be eligible for no radiotherapy because they fit the criteria laid out in the CALGB (Cancer and Leukemia Group B) 9493 study: age 70 years or older, with T1N0, estrogen receptor–positive disease. CALGB 9493 demonstrated at median follow-ups of 5 years (N. Engl. J. Med. 2004;351:971-7) and 12.6 years (J. Clin. Oncol. 2013;31:2382-7) that women who fit that profile could safely forego radiotherapy.
The investigators considered another 62% of patients to be eligible for hypofractionated whole breast irradiation (HF-WBI), defined as 15-24 fractions at 40-58 Gy, as their least costly appropriate radiotherapy option because they were at least 50 years old with T1-T2 NO invasive breast cancer. Sixteen percent of patients were deemed eligible for conventional fractionated whole breast irradiation (CF-WBI) as their least expensive option.
Treatment costs were determined using the Medicare Physician Fee Schedule payment data for 2011 and the average procedural codes billed per regimen. The cost per patient was $13,358.37 for CF-WBI and $8,327.98 for HF-WBI.
Of the 9,651 women considered eligible for no radiation therapy, only 36% actually did not undergo irradiation. Forty-four percent had CF-WBI, 18% had HF-WBI, and the rest had accelerated partial breast irradiation. The total cost of radiation therapy in this subgroup was $72.2 million.
Among the considerably larger group for whom HF-WBI was deemed the least costly radiation therapy option for which they were eligible, 68% had CF-WBI, 17% had no radiotherapy, and only 6.5% received HF-WBI. The total cost of radiation therapy in this group was $276.8 million in 2011. Had they undergone evidence-based therapy, the cost would have dropped to $185 million, Dr. Greenup said at the San Antonio Breast Cancer Symposium.
For the 6,685 women who were considered candidates for CF-WBI, 75% received that form of radiation therapy, 7% got HF-WBI, and 18% received no radiotherapy. Their total radiation therapy costs amounted to $71.2 million.
The total estimated cost of actual radiation treatment in the study population was $420.2 million, compared with $256.2 million, or 39% less, had the women received the least expensive regimen for which they were eligible.
An estimated $125 billion is spent annually on cancer treatment in the United States, with breast cancer therapy accounting for the largest proportion of that, Dr. Greenup noted.
She reported having no relevant financial conflicts.
SAN ANTONIO – Hefty reductions in annual health care spending for breast cancer therapy would be achieved if physicians increased their use of evidence-based adjuvant radiation therapy following lumpectomy for early-stage breast cancer, according to Dr. Rachel A. Greenup.
The potential savings would amount to $164 million annually, based on the American College of Surgeons National Cancer Data Base for the year 2011. And since that database captures only about 70% of all newly diagnosed cancers in the United States, that savings figure is likely an underestimate, according to Dr. Greenup, a surgical oncologist at Duke University, Durham, N.C.
She and her coinvestigators identified 43,247 women in the national database with clinically node-negative, T1-T2 invasive breast cancer treated by lumpectomy during 2011. Their median age was 63 years, with a median tumor size of 1.2 cm. Only 26% of women received the least expensive adjuvant radiation therapy for which they were potentially eligible based upon current guidelines and best practices. Sixty-seven percent of patients received more costly regimens.
Overall, 22% of the women were deemed by investigators to be eligible for no radiotherapy because they fit the criteria laid out in the CALGB (Cancer and Leukemia Group B) 9493 study: age 70 years or older, with T1N0, estrogen receptor–positive disease. CALGB 9493 demonstrated at median follow-ups of 5 years (N. Engl. J. Med. 2004;351:971-7) and 12.6 years (J. Clin. Oncol. 2013;31:2382-7) that women who fit that profile could safely forego radiotherapy.
The investigators considered another 62% of patients to be eligible for hypofractionated whole breast irradiation (HF-WBI), defined as 15-24 fractions at 40-58 Gy, as their least costly appropriate radiotherapy option because they were at least 50 years old with T1-T2 NO invasive breast cancer. Sixteen percent of patients were deemed eligible for conventional fractionated whole breast irradiation (CF-WBI) as their least expensive option.
Treatment costs were determined using the Medicare Physician Fee Schedule payment data for 2011 and the average procedural codes billed per regimen. The cost per patient was $13,358.37 for CF-WBI and $8,327.98 for HF-WBI.
Of the 9,651 women considered eligible for no radiation therapy, only 36% actually did not undergo irradiation. Forty-four percent had CF-WBI, 18% had HF-WBI, and the rest had accelerated partial breast irradiation. The total cost of radiation therapy in this subgroup was $72.2 million.
Among the considerably larger group for whom HF-WBI was deemed the least costly radiation therapy option for which they were eligible, 68% had CF-WBI, 17% had no radiotherapy, and only 6.5% received HF-WBI. The total cost of radiation therapy in this group was $276.8 million in 2011. Had they undergone evidence-based therapy, the cost would have dropped to $185 million, Dr. Greenup said at the San Antonio Breast Cancer Symposium.
For the 6,685 women who were considered candidates for CF-WBI, 75% received that form of radiation therapy, 7% got HF-WBI, and 18% received no radiotherapy. Their total radiation therapy costs amounted to $71.2 million.
The total estimated cost of actual radiation treatment in the study population was $420.2 million, compared with $256.2 million, or 39% less, had the women received the least expensive regimen for which they were eligible.
An estimated $125 billion is spent annually on cancer treatment in the United States, with breast cancer therapy accounting for the largest proportion of that, Dr. Greenup noted.
She reported having no relevant financial conflicts.
SAN ANTONIO – Hefty reductions in annual health care spending for breast cancer therapy would be achieved if physicians increased their use of evidence-based adjuvant radiation therapy following lumpectomy for early-stage breast cancer, according to Dr. Rachel A. Greenup.
The potential savings would amount to $164 million annually, based on the American College of Surgeons National Cancer Data Base for the year 2011. And since that database captures only about 70% of all newly diagnosed cancers in the United States, that savings figure is likely an underestimate, according to Dr. Greenup, a surgical oncologist at Duke University, Durham, N.C.
She and her coinvestigators identified 43,247 women in the national database with clinically node-negative, T1-T2 invasive breast cancer treated by lumpectomy during 2011. Their median age was 63 years, with a median tumor size of 1.2 cm. Only 26% of women received the least expensive adjuvant radiation therapy for which they were potentially eligible based upon current guidelines and best practices. Sixty-seven percent of patients received more costly regimens.
Overall, 22% of the women were deemed by investigators to be eligible for no radiotherapy because they fit the criteria laid out in the CALGB (Cancer and Leukemia Group B) 9493 study: age 70 years or older, with T1N0, estrogen receptor–positive disease. CALGB 9493 demonstrated at median follow-ups of 5 years (N. Engl. J. Med. 2004;351:971-7) and 12.6 years (J. Clin. Oncol. 2013;31:2382-7) that women who fit that profile could safely forego radiotherapy.
The investigators considered another 62% of patients to be eligible for hypofractionated whole breast irradiation (HF-WBI), defined as 15-24 fractions at 40-58 Gy, as their least costly appropriate radiotherapy option because they were at least 50 years old with T1-T2 NO invasive breast cancer. Sixteen percent of patients were deemed eligible for conventional fractionated whole breast irradiation (CF-WBI) as their least expensive option.
Treatment costs were determined using the Medicare Physician Fee Schedule payment data for 2011 and the average procedural codes billed per regimen. The cost per patient was $13,358.37 for CF-WBI and $8,327.98 for HF-WBI.
Of the 9,651 women considered eligible for no radiation therapy, only 36% actually did not undergo irradiation. Forty-four percent had CF-WBI, 18% had HF-WBI, and the rest had accelerated partial breast irradiation. The total cost of radiation therapy in this subgroup was $72.2 million.
Among the considerably larger group for whom HF-WBI was deemed the least costly radiation therapy option for which they were eligible, 68% had CF-WBI, 17% had no radiotherapy, and only 6.5% received HF-WBI. The total cost of radiation therapy in this group was $276.8 million in 2011. Had they undergone evidence-based therapy, the cost would have dropped to $185 million, Dr. Greenup said at the San Antonio Breast Cancer Symposium.
For the 6,685 women who were considered candidates for CF-WBI, 75% received that form of radiation therapy, 7% got HF-WBI, and 18% received no radiotherapy. Their total radiation therapy costs amounted to $71.2 million.
The total estimated cost of actual radiation treatment in the study population was $420.2 million, compared with $256.2 million, or 39% less, had the women received the least expensive regimen for which they were eligible.
An estimated $125 billion is spent annually on cancer treatment in the United States, with breast cancer therapy accounting for the largest proportion of that, Dr. Greenup noted.
She reported having no relevant financial conflicts.
AT SABCS 2014
Key clinical point: The national cost of radiation therapy for breast cancer is inflated by the widespread practice of utilizing costlier regimens than warranted.
Major finding: The annual cost of adjuvant radiotherapy after lumpectomy for early-stage breast cancer in the United States could be reduced by 39%, or $164 million, if physicians used the least expensive evidence-based regimens.
Data source: A retrospective analysis of the American College of Surgeons National Cancer Data Base, which included more than 43,000 women with clinically node-negative, T1-T2 invasive breast cancer treated with lumpectomy in 2011.
Disclosures: The presenter reported having no financial conflicts regarding this study.
Transfusion linked to bad outcomes in percutaneous peripheral vascular interventions
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
CHICAGO – Periprocedural blood transfusion rates vary greatly among hospitals performing similar percutaneous interventions for peripheral arterial disease, but these rates can be markedly reduced via a focused quality improvement program.
That’s been the lesson learned in Michigan, where blood transfusion rates dropped by 52% statewide at the 44 hospitals participating in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium Vascular Intervention Collaborative (BMC2 PCI-VIC), Dr. Peter K. Henke reported at the American Heart Association scientific sessions.
That’s good news because periprocedural blood transfusions in patients undergoing percutaneous interventions for peripheral arterial disease (PAD) are associated with startlingly high major morbidity and mortality rates. Indeed, among 18,127 patients undergoing nonhybrid percutaneous interventions for PAD in the BMC2 PCI-VIC registry, periprocedural blood transfusion was an independent predictor of a 25-fold increased risk of MI, a 12.7-fold increase in in-hospital mortality, a 6-fold increased risk of TIA/stroke, and a 49-fold increase in vascular access complications in a logistic regression analysis adjusted for patient demographics, comorbid disease states, and periprocedural medications, according to Dr. Henke, professor of vascular surgery at the University of Michigan, Ann Arbor.
That being said, he was quick to add that he believes these associations largely reflect correlation, not causality. Transfusion recipients were significantly older and sicker than nontransfused patients undergoing the same percutaneous peripheral vascular interventions. They were far more likely to have critical limb ischemia and undergo an urgent or emergent procedure. Of note, as statewide transfusion rates fell from about 6.6% to 3.2% in response to the quality improvement program, crude in-hospital mortality didn’t change significantly, again suggesting a noncausal relationship.
The quality improvement project was undertaken in response to the observation that periprocedural transfusion rates varied institutionally across the state from 0% to 14% for patients undergoing the same percutaneous interventions for PAD. That was a red flag indicating an opportunity for improved practice.
“The median nadir hemoglobin varied within a rather narrow range of 6.8-8.5 g/dL, yet the transfusion rates were quite wide ranging,” the surgeon observed.
Over a 2-year period, the BMC2 PCI-VIC quality improvement team made repeated site visits to the hospitals with the lowest transfusion rates. They performed detailed analysis of peripheral vascular procedure processes, protocols, and order sets in order to identify best practices. Those best practices were then shared at meetings with representatives of all the participating hospitals. Feedback was provided. And transfusion rates began dropping.
Analysis of the 18,000-plus patients enrolled in the registry led to identification of a specific set of risk factors for blood transfusions, most of which occurred after patients had left the catheterization lab. These risk factors included low creatinine clearance, preprocedural anemia, chronic obstructive pulmonary disease, use of warfarin, cerebrovascular disease, critical limb ischemia, and urgent or emergent procedures.
This was the largest-ever study focused on transfusion in patients undergoing endovascular procedures for PAD, according to Dr. Henke. He noted that the results are consistent with a recent report by other investigators regarding the implications of periprocedural blood transfusion in patients undergoing percutaneous coronary intervention. In more than 2.2 million patients who underwent PCI in 2009-2013, transfusion rates varied institutionally from 0% to 13%. Transfusion was associated with 4.6-fold in-hospital mortality, a 3.6-fold increase in acute MI, and a 7.7-fold increased risk of stroke (JAMA 2014;311:836-43).
Dr. Henke reported no financial conflicts of interest regarding the PAD transfusion study, which was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: High institutional blood transfusion rates in conjunction with percutaneous interventions for peripheral arterial disease can be sharply and safely lowered through a focused quality improvement program.
Major finding: The average periprocedural transfusion rate at 44 Michigan hospitals fell from 6.6% to 3.2% in response to the performance improvement program.
Data source: A retrospective analysis of prospectively gathered data on 18,127 Michigan patients who underwent nonhybrid percutaneous interventions for peripheral arterial disease.
Disclosures: The study was funded by Blue Cross Blue Shield of Michigan and the Blue Care Network. The presenter reported having no financial conflicts.
Adjuvant taxane benefit for breast cancer varies by subtype
SAN ANTONIO – Extended follow-up of the nearly 5,000 women enrolled in the phase III multicenter E1199 trial has provided a wealth of new findings – and additional leads – regarding optimal adjuvant chemotherapy in patients with axillary lymph node–positive or high-risk, lymph node–negative breast cancer.
“The relative effectiveness of weekly paclitaxel and every-3-week docetaxel may vary by subtype,” Dr. Joseph A. Sparano reported at the San Antonio Breast Cancer Symposium.
The E1199 trial, conducted in 1999-2001, included 4,950 breast cancer patients who first received four cycles of standard adjuvant chemotherapy consisting of intravenous doxorubicin and cyclophosphamide at 3-week intervals. They were then assigned in a 2x2 factorial design to intravenous paclitaxel (Abraxane) at the then-standard 3-week intervals for four cycles, to weekly paclitaxel for 12 weeks, or to docetaxel (Taxotere) given at either of the same two dosing schedules.
The goal was to determine the optimally effective adjuvant taxane and dosing regimen. And in the initial report based upon a median 5.3 years of follow-up (N. Engl. J. Med. 2008;358:1663-71), weekly paclitaxel was the clear winner. It was associated with a 27% improvement in the likelihood of disease-free survival (DFS) and a 32% gain in overall survival (OS), compared with paclitaxel given every 3 weeks. Docetaxel every 3 weeks also resulted in improved DFS, but not in OS.
Dr. Sparano’s newly updated analysis was based upon a median 12.1 years of follow-up. By this point the number of DFS events – defined as recurrence, contralateral breast cancer, or death – had climbed to 1,639, compared with 1,058 in the initial report. The all-cause death count had risen from 686 to 1,283.
With the extended follow-up, both weekly paclitaxel and docetaxel every 3 weeks still remained superior to every-3-weeks paclitaxel in terms of DFS, with relative risk reductions of 16% and 21% for DFS events, respectively. However, trends favoring these two regimens in terms of OS didn’t reach statistical significance, reported Dr. Sparano, professor of medicine and women’s health at Albert Einstein College of Medicine, New York.
Among the more interesting findings to emerge from the extended analysis was the differential treatment response based upon breast cancer subtype. Among the 1,025 women with triple-negative breast cancer (TNBC), weekly paclitaxel was associated with highly significant relative risk reductions of 31% both for DFS events and mortality. This regimen boosted the 10-year DFS rate from 59% with every-3-weeks paclitaxel to 69%, while improving 10-year overall survival from 66% to 75%.
“Our findings also show that there remains substantial room for improvement in this population, despite the improvement seen with weekly paclitaxel,” the medical oncologist observed.
This is the rationale for the pending NRG-BR003 trial, in which patients with TNBC are randomized to four cycles of adjuvant chemotherapy followed by weekly paclitaxel for 12 weeks, as in the most effective arm of E1199, or to the same regimen supplemented by carboplatin in an effort to enhance outcomes.
In E1199, TNBC was associated with a very high annual risk of cancer recurrence within the first 3 years, which declined substantially thereafter. In contrast, for the 2,785 participants with hormone receptor (HR)-positive/HER2-negative breast cancer, the recurrence risk remained persistently strong beyond 5 years.
Obesity, which was present in 35% of all E1199 participants, was associated with worse outcomes in the HR-positive/HER2-negative group, where in a multivariate analysis it was independently associated with a 23% reduction in OS and a recurrence risk peaking 3-8 years after diagnosis.
“I think an important finding from this study is that obesity tends to be associated with later relapse. So if dietary and lifestyle and other metabolic-type interventions are going to be successful, there may be an adequate window to implement those changes and have a real impact on outcomes,” according to Dr. Sparano.
Black race was also independently associated with worse outcomes in HR-positive/HER2-negative patients but not in those with TNBC. In nonobese women with HER-positive/HER2-negative disease, black race was associated with roughly 2.3-fold increased risks of DFS events and mortality.
“These findings indicate an effect of race independent of obesity. That’s an important finding given the fact that obesity rates were twofold higher in patients of black race,” Dr. Sparano said.
In women with HR-positive/HER2-negative breast cancer, the only study regimen that proved more effective than paclitaxel every 3 weeks was docetaxel every 3 weeks, which in the extended follow-up resulted in a 24% reduction in the risk of DFS events but no significant advantage in OS.
“This suggests that attention to extended adjuvant endocrine therapy may be more relevant in this subpopulation, which is at risk for late relapse, particularly at the time of this study, when extended adjuvant therapy was not common practice,” he noted.
Late relapse remains “a vexing problem” in HR-positive/HER2-negative patients, Dr. Sparano continued. A major effort is underway to address this via an ECOG-ACRIN North American Breast Cancer Intergroup project designed to establish a late-relapse biospecimen bank featuring blood and/or tumor samples from 10,000 women enrolled in the TAILORx (Hormone Therapy With or Without Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Negative Breast Cancer) and E5103 trials.
SAN ANTONIO – Extended follow-up of the nearly 5,000 women enrolled in the phase III multicenter E1199 trial has provided a wealth of new findings – and additional leads – regarding optimal adjuvant chemotherapy in patients with axillary lymph node–positive or high-risk, lymph node–negative breast cancer.
“The relative effectiveness of weekly paclitaxel and every-3-week docetaxel may vary by subtype,” Dr. Joseph A. Sparano reported at the San Antonio Breast Cancer Symposium.
The E1199 trial, conducted in 1999-2001, included 4,950 breast cancer patients who first received four cycles of standard adjuvant chemotherapy consisting of intravenous doxorubicin and cyclophosphamide at 3-week intervals. They were then assigned in a 2x2 factorial design to intravenous paclitaxel (Abraxane) at the then-standard 3-week intervals for four cycles, to weekly paclitaxel for 12 weeks, or to docetaxel (Taxotere) given at either of the same two dosing schedules.
The goal was to determine the optimally effective adjuvant taxane and dosing regimen. And in the initial report based upon a median 5.3 years of follow-up (N. Engl. J. Med. 2008;358:1663-71), weekly paclitaxel was the clear winner. It was associated with a 27% improvement in the likelihood of disease-free survival (DFS) and a 32% gain in overall survival (OS), compared with paclitaxel given every 3 weeks. Docetaxel every 3 weeks also resulted in improved DFS, but not in OS.
Dr. Sparano’s newly updated analysis was based upon a median 12.1 years of follow-up. By this point the number of DFS events – defined as recurrence, contralateral breast cancer, or death – had climbed to 1,639, compared with 1,058 in the initial report. The all-cause death count had risen from 686 to 1,283.
With the extended follow-up, both weekly paclitaxel and docetaxel every 3 weeks still remained superior to every-3-weeks paclitaxel in terms of DFS, with relative risk reductions of 16% and 21% for DFS events, respectively. However, trends favoring these two regimens in terms of OS didn’t reach statistical significance, reported Dr. Sparano, professor of medicine and women’s health at Albert Einstein College of Medicine, New York.
Among the more interesting findings to emerge from the extended analysis was the differential treatment response based upon breast cancer subtype. Among the 1,025 women with triple-negative breast cancer (TNBC), weekly paclitaxel was associated with highly significant relative risk reductions of 31% both for DFS events and mortality. This regimen boosted the 10-year DFS rate from 59% with every-3-weeks paclitaxel to 69%, while improving 10-year overall survival from 66% to 75%.
“Our findings also show that there remains substantial room for improvement in this population, despite the improvement seen with weekly paclitaxel,” the medical oncologist observed.
This is the rationale for the pending NRG-BR003 trial, in which patients with TNBC are randomized to four cycles of adjuvant chemotherapy followed by weekly paclitaxel for 12 weeks, as in the most effective arm of E1199, or to the same regimen supplemented by carboplatin in an effort to enhance outcomes.
In E1199, TNBC was associated with a very high annual risk of cancer recurrence within the first 3 years, which declined substantially thereafter. In contrast, for the 2,785 participants with hormone receptor (HR)-positive/HER2-negative breast cancer, the recurrence risk remained persistently strong beyond 5 years.
Obesity, which was present in 35% of all E1199 participants, was associated with worse outcomes in the HR-positive/HER2-negative group, where in a multivariate analysis it was independently associated with a 23% reduction in OS and a recurrence risk peaking 3-8 years after diagnosis.
“I think an important finding from this study is that obesity tends to be associated with later relapse. So if dietary and lifestyle and other metabolic-type interventions are going to be successful, there may be an adequate window to implement those changes and have a real impact on outcomes,” according to Dr. Sparano.
Black race was also independently associated with worse outcomes in HR-positive/HER2-negative patients but not in those with TNBC. In nonobese women with HER-positive/HER2-negative disease, black race was associated with roughly 2.3-fold increased risks of DFS events and mortality.
“These findings indicate an effect of race independent of obesity. That’s an important finding given the fact that obesity rates were twofold higher in patients of black race,” Dr. Sparano said.
In women with HR-positive/HER2-negative breast cancer, the only study regimen that proved more effective than paclitaxel every 3 weeks was docetaxel every 3 weeks, which in the extended follow-up resulted in a 24% reduction in the risk of DFS events but no significant advantage in OS.
“This suggests that attention to extended adjuvant endocrine therapy may be more relevant in this subpopulation, which is at risk for late relapse, particularly at the time of this study, when extended adjuvant therapy was not common practice,” he noted.
Late relapse remains “a vexing problem” in HR-positive/HER2-negative patients, Dr. Sparano continued. A major effort is underway to address this via an ECOG-ACRIN North American Breast Cancer Intergroup project designed to establish a late-relapse biospecimen bank featuring blood and/or tumor samples from 10,000 women enrolled in the TAILORx (Hormone Therapy With or Without Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Negative Breast Cancer) and E5103 trials.
SAN ANTONIO – Extended follow-up of the nearly 5,000 women enrolled in the phase III multicenter E1199 trial has provided a wealth of new findings – and additional leads – regarding optimal adjuvant chemotherapy in patients with axillary lymph node–positive or high-risk, lymph node–negative breast cancer.
“The relative effectiveness of weekly paclitaxel and every-3-week docetaxel may vary by subtype,” Dr. Joseph A. Sparano reported at the San Antonio Breast Cancer Symposium.
The E1199 trial, conducted in 1999-2001, included 4,950 breast cancer patients who first received four cycles of standard adjuvant chemotherapy consisting of intravenous doxorubicin and cyclophosphamide at 3-week intervals. They were then assigned in a 2x2 factorial design to intravenous paclitaxel (Abraxane) at the then-standard 3-week intervals for four cycles, to weekly paclitaxel for 12 weeks, or to docetaxel (Taxotere) given at either of the same two dosing schedules.
The goal was to determine the optimally effective adjuvant taxane and dosing regimen. And in the initial report based upon a median 5.3 years of follow-up (N. Engl. J. Med. 2008;358:1663-71), weekly paclitaxel was the clear winner. It was associated with a 27% improvement in the likelihood of disease-free survival (DFS) and a 32% gain in overall survival (OS), compared with paclitaxel given every 3 weeks. Docetaxel every 3 weeks also resulted in improved DFS, but not in OS.
Dr. Sparano’s newly updated analysis was based upon a median 12.1 years of follow-up. By this point the number of DFS events – defined as recurrence, contralateral breast cancer, or death – had climbed to 1,639, compared with 1,058 in the initial report. The all-cause death count had risen from 686 to 1,283.
With the extended follow-up, both weekly paclitaxel and docetaxel every 3 weeks still remained superior to every-3-weeks paclitaxel in terms of DFS, with relative risk reductions of 16% and 21% for DFS events, respectively. However, trends favoring these two regimens in terms of OS didn’t reach statistical significance, reported Dr. Sparano, professor of medicine and women’s health at Albert Einstein College of Medicine, New York.
Among the more interesting findings to emerge from the extended analysis was the differential treatment response based upon breast cancer subtype. Among the 1,025 women with triple-negative breast cancer (TNBC), weekly paclitaxel was associated with highly significant relative risk reductions of 31% both for DFS events and mortality. This regimen boosted the 10-year DFS rate from 59% with every-3-weeks paclitaxel to 69%, while improving 10-year overall survival from 66% to 75%.
“Our findings also show that there remains substantial room for improvement in this population, despite the improvement seen with weekly paclitaxel,” the medical oncologist observed.
This is the rationale for the pending NRG-BR003 trial, in which patients with TNBC are randomized to four cycles of adjuvant chemotherapy followed by weekly paclitaxel for 12 weeks, as in the most effective arm of E1199, or to the same regimen supplemented by carboplatin in an effort to enhance outcomes.
In E1199, TNBC was associated with a very high annual risk of cancer recurrence within the first 3 years, which declined substantially thereafter. In contrast, for the 2,785 participants with hormone receptor (HR)-positive/HER2-negative breast cancer, the recurrence risk remained persistently strong beyond 5 years.
Obesity, which was present in 35% of all E1199 participants, was associated with worse outcomes in the HR-positive/HER2-negative group, where in a multivariate analysis it was independently associated with a 23% reduction in OS and a recurrence risk peaking 3-8 years after diagnosis.
“I think an important finding from this study is that obesity tends to be associated with later relapse. So if dietary and lifestyle and other metabolic-type interventions are going to be successful, there may be an adequate window to implement those changes and have a real impact on outcomes,” according to Dr. Sparano.
Black race was also independently associated with worse outcomes in HR-positive/HER2-negative patients but not in those with TNBC. In nonobese women with HER-positive/HER2-negative disease, black race was associated with roughly 2.3-fold increased risks of DFS events and mortality.
“These findings indicate an effect of race independent of obesity. That’s an important finding given the fact that obesity rates were twofold higher in patients of black race,” Dr. Sparano said.
In women with HR-positive/HER2-negative breast cancer, the only study regimen that proved more effective than paclitaxel every 3 weeks was docetaxel every 3 weeks, which in the extended follow-up resulted in a 24% reduction in the risk of DFS events but no significant advantage in OS.
“This suggests that attention to extended adjuvant endocrine therapy may be more relevant in this subpopulation, which is at risk for late relapse, particularly at the time of this study, when extended adjuvant therapy was not common practice,” he noted.
Late relapse remains “a vexing problem” in HR-positive/HER2-negative patients, Dr. Sparano continued. A major effort is underway to address this via an ECOG-ACRIN North American Breast Cancer Intergroup project designed to establish a late-relapse biospecimen bank featuring blood and/or tumor samples from 10,000 women enrolled in the TAILORx (Hormone Therapy With or Without Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Negative Breast Cancer) and E5103 trials.
AT SABCS 2014
Key clinical point: The relative effectiveness of adjuvant taxane therapy given weekly or every 3 weeks appears to vary according to breast cancer subtype.
Major finding: At a median 12.1 years of follow-up, the most effective adjuvant taxane regimen for women with triple-negative breast cancer was weekly paclitaxel for 12 weeks.
Data source: The E1199 trial included 4,950 women with axillary lymph node-positive or high-risk, lymph node-negative breast cancer who were randomized to adjuvant docetaxel or paclitaxel, given either weekly or every 3 weeks.
Disclosures: The E1199 trial was funded by the National Cancer Institute and the Breast Cancer Research Foundation. The presenter reported having no financial conflicts.
Aromatase inhibitors linked to dental problems
SAN ANTONIO – Adjuvant aromatase inhibitor therapy in women with early-stage breast cancer was associated with worse oral health–related quality of life in a preliminary analysis from an ongoing prospective cohort study.
“Oral health is critical to overall health, and the findings of this study suggest the need to focus attention on dental health education for these patients related to improved home care regimens and optimizing intervals for dental evaluations,” Linda S. Taichman, Ph.D., said at the San Antonio Breast Cancer Symposium.
The study – the first ever to report on oral health issues in women with early breast cancer on aromatase inhibitors (AIs) – included 58 postmenopausal women. Half were on adjuvant AIs; the other half were recruited at the time of screening mammography, didn’t have breast cancer, and weren’t taking an AI. The two groups were similar in terms of demographics, frequency of daily brushing and flossing, and frequency of dental visits.
At entry into what is scheduled to be an 18-month study, participants completed the Oral Health Impact Profile–14 and the Michigan Oral Health–Related Quality of Life Scale, both of which measure self-reported dysfunction, discomfort, and disability on a self-rated 5-point scale.
The adjuvant AI users reported significantly lower oral health–related quality of life than controls in multiple domains: more pain and aching in the mouth; greater discomfort when eating; a need to limit the foods they eat; interruption of meals; a feeling of being self-conscious, tense, and embarrassed about problems in their mouth; and being irritable with others. The longer patients had been on an AI, the lower their oral health quality of life scores, Dr. Taichman of the University of Michigan School of Dentistry, Ann Arbor, reported.
Oral health quality of life was unrelated to whether a breast cancer patient had undergone chemotherapy.
Saliva flow rate as an indicator of oral health was measured at baseline. Women on AIs were less likely to be able to produce a 2-mL saliva sample.
The study sample size was insufficient to determine if there were differences between specific AIs in terms of patient oral health. Additional studies are ongoing, Dr. Taichman added.
She noted that both groups of women had room for improvement in terms of their oral care regimens. At baseline, only about 40% of subjects reported brushing every day, and less than half of the women flossed daily.
The study is funded by the Michigan Institute for Clinical Health Research. She reported having no relevant financial conflicts.
SAN ANTONIO – Adjuvant aromatase inhibitor therapy in women with early-stage breast cancer was associated with worse oral health–related quality of life in a preliminary analysis from an ongoing prospective cohort study.
“Oral health is critical to overall health, and the findings of this study suggest the need to focus attention on dental health education for these patients related to improved home care regimens and optimizing intervals for dental evaluations,” Linda S. Taichman, Ph.D., said at the San Antonio Breast Cancer Symposium.
The study – the first ever to report on oral health issues in women with early breast cancer on aromatase inhibitors (AIs) – included 58 postmenopausal women. Half were on adjuvant AIs; the other half were recruited at the time of screening mammography, didn’t have breast cancer, and weren’t taking an AI. The two groups were similar in terms of demographics, frequency of daily brushing and flossing, and frequency of dental visits.
At entry into what is scheduled to be an 18-month study, participants completed the Oral Health Impact Profile–14 and the Michigan Oral Health–Related Quality of Life Scale, both of which measure self-reported dysfunction, discomfort, and disability on a self-rated 5-point scale.
The adjuvant AI users reported significantly lower oral health–related quality of life than controls in multiple domains: more pain and aching in the mouth; greater discomfort when eating; a need to limit the foods they eat; interruption of meals; a feeling of being self-conscious, tense, and embarrassed about problems in their mouth; and being irritable with others. The longer patients had been on an AI, the lower their oral health quality of life scores, Dr. Taichman of the University of Michigan School of Dentistry, Ann Arbor, reported.
Oral health quality of life was unrelated to whether a breast cancer patient had undergone chemotherapy.
Saliva flow rate as an indicator of oral health was measured at baseline. Women on AIs were less likely to be able to produce a 2-mL saliva sample.
The study sample size was insufficient to determine if there were differences between specific AIs in terms of patient oral health. Additional studies are ongoing, Dr. Taichman added.
She noted that both groups of women had room for improvement in terms of their oral care regimens. At baseline, only about 40% of subjects reported brushing every day, and less than half of the women flossed daily.
The study is funded by the Michigan Institute for Clinical Health Research. She reported having no relevant financial conflicts.
SAN ANTONIO – Adjuvant aromatase inhibitor therapy in women with early-stage breast cancer was associated with worse oral health–related quality of life in a preliminary analysis from an ongoing prospective cohort study.
“Oral health is critical to overall health, and the findings of this study suggest the need to focus attention on dental health education for these patients related to improved home care regimens and optimizing intervals for dental evaluations,” Linda S. Taichman, Ph.D., said at the San Antonio Breast Cancer Symposium.
The study – the first ever to report on oral health issues in women with early breast cancer on aromatase inhibitors (AIs) – included 58 postmenopausal women. Half were on adjuvant AIs; the other half were recruited at the time of screening mammography, didn’t have breast cancer, and weren’t taking an AI. The two groups were similar in terms of demographics, frequency of daily brushing and flossing, and frequency of dental visits.
At entry into what is scheduled to be an 18-month study, participants completed the Oral Health Impact Profile–14 and the Michigan Oral Health–Related Quality of Life Scale, both of which measure self-reported dysfunction, discomfort, and disability on a self-rated 5-point scale.
The adjuvant AI users reported significantly lower oral health–related quality of life than controls in multiple domains: more pain and aching in the mouth; greater discomfort when eating; a need to limit the foods they eat; interruption of meals; a feeling of being self-conscious, tense, and embarrassed about problems in their mouth; and being irritable with others. The longer patients had been on an AI, the lower their oral health quality of life scores, Dr. Taichman of the University of Michigan School of Dentistry, Ann Arbor, reported.
Oral health quality of life was unrelated to whether a breast cancer patient had undergone chemotherapy.
Saliva flow rate as an indicator of oral health was measured at baseline. Women on AIs were less likely to be able to produce a 2-mL saliva sample.
The study sample size was insufficient to determine if there were differences between specific AIs in terms of patient oral health. Additional studies are ongoing, Dr. Taichman added.
She noted that both groups of women had room for improvement in terms of their oral care regimens. At baseline, only about 40% of subjects reported brushing every day, and less than half of the women flossed daily.
The study is funded by the Michigan Institute for Clinical Health Research. She reported having no relevant financial conflicts.
AT SABCS 2014
Key clinical point: Breast cancer patients on adjuvant aromatase inhibitor therapy have previously unappreciated dental health issues.
Major finding: Women who had been on adjuvant aromatase inhibitor therapy for a median of 5.7 months when they completed standardized instruments measuring oral health–related quality of life reported significantly more problems in the domains of physical pain and disability as well as psychological discomfort, compared with controls.
Data source: An ongoing prospective cohort study involving 58 postmenopausal women.
Disclosures: The study is funded by the Michigan Institute for Clinical Health Research. The presenter reported having no relevant financial conflicts.
Unrecognized MI Common With Impaired Fasting Glucose
CHICAGO– Impaired fasting glucose was associated with a doubled risk of asymptomatic or unrecognized myocardial infarction during prospective follow-up of subjects without overt cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis.
The clinical relevance of this finding lies in previous work establishing that asymptomatic, unrecognized MIs are associated with a prognosis as serious as for symptomatic MIs.
“Because of the high prevalence of impaired fasting glucose, the implications of this finding may have ramifications for a large proportion of the adult population,” Dr. Richard B. Stacey observed at the American Heart Association Scientific Sessions.
The magnitude of the risk of unrecognized MI was associated with duration of exposure to abnormal blood glucose levels in the landmark population-based MESA study. Subjects with impaired fasting glucose (IFG) noted on at least three of five biannual examinations conducted over a 10-year period had an adjusted 1.97-fold greater risk of experiencing an unrecognized MI during that time, compared with MESA participants who had normal blood glucose, added Dr. Stacey of Wake Forest University in Winston-Salem, N.C.
He presented two new analyses from the MESA study. One was cross-sectional: Among 4,955 study participants with normal fasting glucose and 930 with IFG upon study enrollment, a baseline 12-lead ECG revealed a prior unrecognized MI in 1.4% of those with normal fasting glucose and 3.2% of those with IFG, a 2.3-fold increase. MESA participants were on average in their mid-60s, and none had clinical cardiovascular disease.
In a multivariate logistic regression analysis fully adjusted for demographics and the standard cardiovascular risk factors as well as body mass index and the use of antihypertensive and lipid-lowering medications, IFG was associated with a 1.63-fold increased risk of prior asymptomatic or unrecognized MI (P < .001).
The other, prospective, longitudinal analysis, included 872 subjects with baseline normal fasting glucose and 545 with IFG, none of whom had evidence of a prior unrecognized MI at entry. This analysis excluded individuals who developed diabetes mellitus, had a clinically recognized MI, or underwent coronary revascularization during follow-up. During 10 years of follow-up, 14.9% of the group who continued to have normal fasting glucose experienced an unrecognized MI, as did 23.8% of those with IFG at entry. The unrecognized MIs were detected either by the presence of pathologic Q waves or minor Q waves with ST-T abnormalities on ECG or by an MRI showing late gadolinium enhancement indicative of myocardial scar.
The MESA study is funded by the National Institutes of Health. Dr. Stacey reported having no financial conflicts.
CHICAGO– Impaired fasting glucose was associated with a doubled risk of asymptomatic or unrecognized myocardial infarction during prospective follow-up of subjects without overt cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis.
The clinical relevance of this finding lies in previous work establishing that asymptomatic, unrecognized MIs are associated with a prognosis as serious as for symptomatic MIs.
“Because of the high prevalence of impaired fasting glucose, the implications of this finding may have ramifications for a large proportion of the adult population,” Dr. Richard B. Stacey observed at the American Heart Association Scientific Sessions.
The magnitude of the risk of unrecognized MI was associated with duration of exposure to abnormal blood glucose levels in the landmark population-based MESA study. Subjects with impaired fasting glucose (IFG) noted on at least three of five biannual examinations conducted over a 10-year period had an adjusted 1.97-fold greater risk of experiencing an unrecognized MI during that time, compared with MESA participants who had normal blood glucose, added Dr. Stacey of Wake Forest University in Winston-Salem, N.C.
He presented two new analyses from the MESA study. One was cross-sectional: Among 4,955 study participants with normal fasting glucose and 930 with IFG upon study enrollment, a baseline 12-lead ECG revealed a prior unrecognized MI in 1.4% of those with normal fasting glucose and 3.2% of those with IFG, a 2.3-fold increase. MESA participants were on average in their mid-60s, and none had clinical cardiovascular disease.
In a multivariate logistic regression analysis fully adjusted for demographics and the standard cardiovascular risk factors as well as body mass index and the use of antihypertensive and lipid-lowering medications, IFG was associated with a 1.63-fold increased risk of prior asymptomatic or unrecognized MI (P < .001).
The other, prospective, longitudinal analysis, included 872 subjects with baseline normal fasting glucose and 545 with IFG, none of whom had evidence of a prior unrecognized MI at entry. This analysis excluded individuals who developed diabetes mellitus, had a clinically recognized MI, or underwent coronary revascularization during follow-up. During 10 years of follow-up, 14.9% of the group who continued to have normal fasting glucose experienced an unrecognized MI, as did 23.8% of those with IFG at entry. The unrecognized MIs were detected either by the presence of pathologic Q waves or minor Q waves with ST-T abnormalities on ECG or by an MRI showing late gadolinium enhancement indicative of myocardial scar.
The MESA study is funded by the National Institutes of Health. Dr. Stacey reported having no financial conflicts.
CHICAGO– Impaired fasting glucose was associated with a doubled risk of asymptomatic or unrecognized myocardial infarction during prospective follow-up of subjects without overt cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis.
The clinical relevance of this finding lies in previous work establishing that asymptomatic, unrecognized MIs are associated with a prognosis as serious as for symptomatic MIs.
“Because of the high prevalence of impaired fasting glucose, the implications of this finding may have ramifications for a large proportion of the adult population,” Dr. Richard B. Stacey observed at the American Heart Association Scientific Sessions.
The magnitude of the risk of unrecognized MI was associated with duration of exposure to abnormal blood glucose levels in the landmark population-based MESA study. Subjects with impaired fasting glucose (IFG) noted on at least three of five biannual examinations conducted over a 10-year period had an adjusted 1.97-fold greater risk of experiencing an unrecognized MI during that time, compared with MESA participants who had normal blood glucose, added Dr. Stacey of Wake Forest University in Winston-Salem, N.C.
He presented two new analyses from the MESA study. One was cross-sectional: Among 4,955 study participants with normal fasting glucose and 930 with IFG upon study enrollment, a baseline 12-lead ECG revealed a prior unrecognized MI in 1.4% of those with normal fasting glucose and 3.2% of those with IFG, a 2.3-fold increase. MESA participants were on average in their mid-60s, and none had clinical cardiovascular disease.
In a multivariate logistic regression analysis fully adjusted for demographics and the standard cardiovascular risk factors as well as body mass index and the use of antihypertensive and lipid-lowering medications, IFG was associated with a 1.63-fold increased risk of prior asymptomatic or unrecognized MI (P < .001).
The other, prospective, longitudinal analysis, included 872 subjects with baseline normal fasting glucose and 545 with IFG, none of whom had evidence of a prior unrecognized MI at entry. This analysis excluded individuals who developed diabetes mellitus, had a clinically recognized MI, or underwent coronary revascularization during follow-up. During 10 years of follow-up, 14.9% of the group who continued to have normal fasting glucose experienced an unrecognized MI, as did 23.8% of those with IFG at entry. The unrecognized MIs were detected either by the presence of pathologic Q waves or minor Q waves with ST-T abnormalities on ECG or by an MRI showing late gadolinium enhancement indicative of myocardial scar.
The MESA study is funded by the National Institutes of Health. Dr. Stacey reported having no financial conflicts.
Breast cancer mortality down, but racial differences persist
SAN ANTONIO – Breast cancer mortality in American women under age 50 declined markedly among whites during a recent two-decade period, less so in blacks.
“The racial gaps in survival have not closed. Young black women continue to have a worse outcome than young whites,” Dr. Foluso O. Ademuyiwa said at the San Antonio Breast Cancer Symposium.

She presented an analysis of Surveillance, Epidemiology, and End Results (SEER) registry data that included 162,976 women diagnosed with invasive breast cancer at age 18-49 during 1990-2009. Whites accounted for 77.7% of the total, blacks 12.5%, and other races 9.8%.
Twenty percent of patients had died by the median followup of 85 months. Five- and 10-year overall survival rates were 87.9% and 79.5%, respectively, among white patients, 74.9% and 64.3% for blacks, and 88.5% and 80.7% for women of other races, reported Dr. Ademuyiwa of Washington University, St. Louis.
During all 5-year sections of the study period, the highest risk of breast cancer mortality was in the first 3 years following diagnosis. However, annual risk of mortality in the first 3 years declined for white women from 3.4% during 1990-1994 to 1.9% during 2005-2009, and from 6.4% during 1990-1994 to 4.5% during 2005-2009 for black women.
Young black breast cancer patients had a more aggressive tumor biology. Thirty-seven percent of them had estrogen receptor–negative tumors, compared with 24% of white patients. Their disease was also at a higher stage and grade at diagnosis. Moreover, their tumors were larger in size; only 34.5% of black patients’ tumors were less than 2 cm in size, compared with 47.3% for white patients and 45.3% for women of other races.
A greater proportion of the invasive breast cancer cases in young women were diagnosed in more recent years. Roughly 13% of all cases in the SEER registry for 1990-2009 were diagnosed in 1990-1994, 16% in 1995-1999, 35% in 2000-2004, and 36% during 2005-2009. The explanation for this trend may be more widespread use of screening, changes in the reproductive risk profile over time, or some combination of the two factors, according to Dr. Ademuyiwa.
She reported having no financial conflicts with regard to this study.
SAN ANTONIO – Breast cancer mortality in American women under age 50 declined markedly among whites during a recent two-decade period, less so in blacks.
“The racial gaps in survival have not closed. Young black women continue to have a worse outcome than young whites,” Dr. Foluso O. Ademuyiwa said at the San Antonio Breast Cancer Symposium.

She presented an analysis of Surveillance, Epidemiology, and End Results (SEER) registry data that included 162,976 women diagnosed with invasive breast cancer at age 18-49 during 1990-2009. Whites accounted for 77.7% of the total, blacks 12.5%, and other races 9.8%.
Twenty percent of patients had died by the median followup of 85 months. Five- and 10-year overall survival rates were 87.9% and 79.5%, respectively, among white patients, 74.9% and 64.3% for blacks, and 88.5% and 80.7% for women of other races, reported Dr. Ademuyiwa of Washington University, St. Louis.
During all 5-year sections of the study period, the highest risk of breast cancer mortality was in the first 3 years following diagnosis. However, annual risk of mortality in the first 3 years declined for white women from 3.4% during 1990-1994 to 1.9% during 2005-2009, and from 6.4% during 1990-1994 to 4.5% during 2005-2009 for black women.
Young black breast cancer patients had a more aggressive tumor biology. Thirty-seven percent of them had estrogen receptor–negative tumors, compared with 24% of white patients. Their disease was also at a higher stage and grade at diagnosis. Moreover, their tumors were larger in size; only 34.5% of black patients’ tumors were less than 2 cm in size, compared with 47.3% for white patients and 45.3% for women of other races.
A greater proportion of the invasive breast cancer cases in young women were diagnosed in more recent years. Roughly 13% of all cases in the SEER registry for 1990-2009 were diagnosed in 1990-1994, 16% in 1995-1999, 35% in 2000-2004, and 36% during 2005-2009. The explanation for this trend may be more widespread use of screening, changes in the reproductive risk profile over time, or some combination of the two factors, according to Dr. Ademuyiwa.
She reported having no financial conflicts with regard to this study.
SAN ANTONIO – Breast cancer mortality in American women under age 50 declined markedly among whites during a recent two-decade period, less so in blacks.
“The racial gaps in survival have not closed. Young black women continue to have a worse outcome than young whites,” Dr. Foluso O. Ademuyiwa said at the San Antonio Breast Cancer Symposium.

She presented an analysis of Surveillance, Epidemiology, and End Results (SEER) registry data that included 162,976 women diagnosed with invasive breast cancer at age 18-49 during 1990-2009. Whites accounted for 77.7% of the total, blacks 12.5%, and other races 9.8%.
Twenty percent of patients had died by the median followup of 85 months. Five- and 10-year overall survival rates were 87.9% and 79.5%, respectively, among white patients, 74.9% and 64.3% for blacks, and 88.5% and 80.7% for women of other races, reported Dr. Ademuyiwa of Washington University, St. Louis.
During all 5-year sections of the study period, the highest risk of breast cancer mortality was in the first 3 years following diagnosis. However, annual risk of mortality in the first 3 years declined for white women from 3.4% during 1990-1994 to 1.9% during 2005-2009, and from 6.4% during 1990-1994 to 4.5% during 2005-2009 for black women.
Young black breast cancer patients had a more aggressive tumor biology. Thirty-seven percent of them had estrogen receptor–negative tumors, compared with 24% of white patients. Their disease was also at a higher stage and grade at diagnosis. Moreover, their tumors were larger in size; only 34.5% of black patients’ tumors were less than 2 cm in size, compared with 47.3% for white patients and 45.3% for women of other races.
A greater proportion of the invasive breast cancer cases in young women were diagnosed in more recent years. Roughly 13% of all cases in the SEER registry for 1990-2009 were diagnosed in 1990-1994, 16% in 1995-1999, 35% in 2000-2004, and 36% during 2005-2009. The explanation for this trend may be more widespread use of screening, changes in the reproductive risk profile over time, or some combination of the two factors, according to Dr. Ademuyiwa.
She reported having no financial conflicts with regard to this study.
AT SABCS 2014
Key clinical point: Breast cancer mortality in patients under age 50 has declined in recent decades, but gains have been greater in white patients and the mortality gap between black and white women has not closed.
Major finding: During 2005-2009, the annual risk of breast cancer mortality in the first 3 years after diagnosis was 1.9% among young white patients compared with 4.5% in blacks.
Data source: A retrospective analysis of 162,976 women in the National Cancer Institute’s Surveillance, Epidemiology, and End Results registry who were diagnosed with invasive breast cancer at age 18-49 during 1990-2009.
Disclosures: Dr. Ademuyiwa reported having no financial conflicts.
How physicians are using ‘the power of zero’ in primary prevention
CHICAGO – Coronary artery calcium testing has established itself as a true “game changer” in primary cardiovascular prevention, proponents of the risk-stratification tool said at the American Heart Association scientific sessions.
Knowing a patient’s coronary artery calcium score facilitates a more informed physician-patient discussion and shared decision making regarding whether to go on decades-long statin therapy, according to Dr. Khurram Nasir of the center for prevention and wellness research at Baptist Health Medical Center in Miami Beach.
“In our view, a much underappreciated value of coronary artery calcium testing lies in the power of zero. Roughly half of adults have a coronary artery calcium score of 0, and this results in a very low cardiovascular event rate,” the cardiologist said.
He presented an analysis of 4,758 nondiabetic participants in the prospective, population-based MESA (Multi-Ethnic Study of Atherosclerosis) in which he examined how they fared in terms of cardiovascular events over a median 10.3 years of follow-up. All were free of known cardiovascular disease at baseline. With the risk estimator included in the 2013 AHA/ACC cholesterol management guidelines, 2,377 subjects would be recommended for high-intensity statin therapy at baseline on the basis of a 10-year atherosclerotic cardiovascular disease risk estimate of at least 7.5%. Another 589 participants were recommended for consideration of a moderate-intensity statin based on an estimated 10-year risk of 5%-7.4%.
Forty-one percent of MESA subjects recommended for a high-intensity statin according to the AHA/ACC risk estimator had a coronary artery calcium (CAC) score of 0, and their 10-year composite rate of MI, stroke, or cardiovascular death was just 4.9% – well below the 7.5% threshold recommended for statin therapy. In contrast, if any CAC was present, the event rate was 10.5%.
With a relative risk reduction with statin therapy of 30%, the number needed to treat for 5 years to prevent one cardiovascular event in the group with a CAC of 0 would be 128. In the presence of any CAC, the number needed to treat fell to a far more reasonable 56, Dr. Nasir said.
Similarly, among the group recommended for consideration of statin therapy on the basis of a 10-year risk of 5%-7.4%, the actual event rate in the 57% of subjects with a CAC of 0 was just 1.5%. If any CAC was present, the event rate shot up to 7.2%. The number needed to treat in this cohort was 445 among those with a CAC of 0 and 90 with any CAC present.
“I think coronary artery calcium is a game changer in primary prevention,” Dr. Michael J. Blaha commented. “It sufficiently moves the needle to make you think differently about a patient. I’m not sure some of the other tests have sufficient evidence to say, ‘I’m going to think about not treating you if it’s negative and treating you if it’s positive,’ but coronary artery calcium has that evidence.”
In his own cardiology practice at Johns Hopkins University, Baltimore, Dr. Blaha finds himself using CAC testing often, especially in his many statin-reluctant patients.
“I have a lot of patients who would fit under a recommendation for statin therapy under the 2013 AHA/ACC cholesterol management guidelines, but who really don’t want to take medications. I know you see these patients in your practices, too. This is lifelong therapy, and they want a really good reason to take it or not to take it. If a patient is reluctant to take a statin and has a CAC score of 0, I will sometimes emphasize lifestyle therapy. It certainly redoubles my interest in lifestyle therapy. But if the CAC score is elevated, then I can make a specific case that the number needed to treat is very favorable, compared to the number needed to harm,” explained Dr. Blaha, a coinvestigator with Dr. Nasir in the MESA study.
Other situations where he finds CAC testing useful in daily practice include uncertainty as to a patient’s true risk level because the individual’s situation isn’t adequately captured by the AHA/ACC risk estimator. A patient with rheumatologic disease would be one example; another would be an individual who is neither white nor African American. He said he also utilizes CAC testing in statin-intolerant patients, where the results are useful in deciding how many different statins to try before saying “enough.”
Audience members asked what it’s going to take to get insurers to cover CAC testing for risk stratification. Dr. Blaha replied that more long-term outcomes and cost-effectiveness data are coming. In the meantime, at an out-of-pocket cost of $75-$100, a lot of his statin-reluctant patients consider CAC testing a good buy.
“They say, ‘I’ll take this test to help me decide whether to take a pill for the rest of my life,’” according to Dr. Blaha.
Dr. Nasir said the evidence in support of CAC testing is now so strong that he believes physicians have an obligation to mention it as an option during the statin treatment decision discussion.
“At this moment, most patients are making their decision based on the guesstimate of their risk we are giving them using the risk calculator. If they have the ability through a $75-$100 test that costs about the same as 18 months of statin therapy to know that their true risk is not, say, 10%, but actually 5%, they’re less likely to choose therapy. Is it even ethical to withhold from our patients that there is a test out there that can reduce their estimated risk to a point that they can avoid statin therapy?” the cardiologist asked.
Dr. Nasir reported serving on an advisory board for Quest Diagnostics. Dr. Blaha reported having no financial conflicts.
CHICAGO – Coronary artery calcium testing has established itself as a true “game changer” in primary cardiovascular prevention, proponents of the risk-stratification tool said at the American Heart Association scientific sessions.
Knowing a patient’s coronary artery calcium score facilitates a more informed physician-patient discussion and shared decision making regarding whether to go on decades-long statin therapy, according to Dr. Khurram Nasir of the center for prevention and wellness research at Baptist Health Medical Center in Miami Beach.
“In our view, a much underappreciated value of coronary artery calcium testing lies in the power of zero. Roughly half of adults have a coronary artery calcium score of 0, and this results in a very low cardiovascular event rate,” the cardiologist said.
He presented an analysis of 4,758 nondiabetic participants in the prospective, population-based MESA (Multi-Ethnic Study of Atherosclerosis) in which he examined how they fared in terms of cardiovascular events over a median 10.3 years of follow-up. All were free of known cardiovascular disease at baseline. With the risk estimator included in the 2013 AHA/ACC cholesterol management guidelines, 2,377 subjects would be recommended for high-intensity statin therapy at baseline on the basis of a 10-year atherosclerotic cardiovascular disease risk estimate of at least 7.5%. Another 589 participants were recommended for consideration of a moderate-intensity statin based on an estimated 10-year risk of 5%-7.4%.
Forty-one percent of MESA subjects recommended for a high-intensity statin according to the AHA/ACC risk estimator had a coronary artery calcium (CAC) score of 0, and their 10-year composite rate of MI, stroke, or cardiovascular death was just 4.9% – well below the 7.5% threshold recommended for statin therapy. In contrast, if any CAC was present, the event rate was 10.5%.
With a relative risk reduction with statin therapy of 30%, the number needed to treat for 5 years to prevent one cardiovascular event in the group with a CAC of 0 would be 128. In the presence of any CAC, the number needed to treat fell to a far more reasonable 56, Dr. Nasir said.
Similarly, among the group recommended for consideration of statin therapy on the basis of a 10-year risk of 5%-7.4%, the actual event rate in the 57% of subjects with a CAC of 0 was just 1.5%. If any CAC was present, the event rate shot up to 7.2%. The number needed to treat in this cohort was 445 among those with a CAC of 0 and 90 with any CAC present.
“I think coronary artery calcium is a game changer in primary prevention,” Dr. Michael J. Blaha commented. “It sufficiently moves the needle to make you think differently about a patient. I’m not sure some of the other tests have sufficient evidence to say, ‘I’m going to think about not treating you if it’s negative and treating you if it’s positive,’ but coronary artery calcium has that evidence.”
In his own cardiology practice at Johns Hopkins University, Baltimore, Dr. Blaha finds himself using CAC testing often, especially in his many statin-reluctant patients.
“I have a lot of patients who would fit under a recommendation for statin therapy under the 2013 AHA/ACC cholesterol management guidelines, but who really don’t want to take medications. I know you see these patients in your practices, too. This is lifelong therapy, and they want a really good reason to take it or not to take it. If a patient is reluctant to take a statin and has a CAC score of 0, I will sometimes emphasize lifestyle therapy. It certainly redoubles my interest in lifestyle therapy. But if the CAC score is elevated, then I can make a specific case that the number needed to treat is very favorable, compared to the number needed to harm,” explained Dr. Blaha, a coinvestigator with Dr. Nasir in the MESA study.
Other situations where he finds CAC testing useful in daily practice include uncertainty as to a patient’s true risk level because the individual’s situation isn’t adequately captured by the AHA/ACC risk estimator. A patient with rheumatologic disease would be one example; another would be an individual who is neither white nor African American. He said he also utilizes CAC testing in statin-intolerant patients, where the results are useful in deciding how many different statins to try before saying “enough.”
Audience members asked what it’s going to take to get insurers to cover CAC testing for risk stratification. Dr. Blaha replied that more long-term outcomes and cost-effectiveness data are coming. In the meantime, at an out-of-pocket cost of $75-$100, a lot of his statin-reluctant patients consider CAC testing a good buy.
“They say, ‘I’ll take this test to help me decide whether to take a pill for the rest of my life,’” according to Dr. Blaha.
Dr. Nasir said the evidence in support of CAC testing is now so strong that he believes physicians have an obligation to mention it as an option during the statin treatment decision discussion.
“At this moment, most patients are making their decision based on the guesstimate of their risk we are giving them using the risk calculator. If they have the ability through a $75-$100 test that costs about the same as 18 months of statin therapy to know that their true risk is not, say, 10%, but actually 5%, they’re less likely to choose therapy. Is it even ethical to withhold from our patients that there is a test out there that can reduce their estimated risk to a point that they can avoid statin therapy?” the cardiologist asked.
Dr. Nasir reported serving on an advisory board for Quest Diagnostics. Dr. Blaha reported having no financial conflicts.
CHICAGO – Coronary artery calcium testing has established itself as a true “game changer” in primary cardiovascular prevention, proponents of the risk-stratification tool said at the American Heart Association scientific sessions.
Knowing a patient’s coronary artery calcium score facilitates a more informed physician-patient discussion and shared decision making regarding whether to go on decades-long statin therapy, according to Dr. Khurram Nasir of the center for prevention and wellness research at Baptist Health Medical Center in Miami Beach.
“In our view, a much underappreciated value of coronary artery calcium testing lies in the power of zero. Roughly half of adults have a coronary artery calcium score of 0, and this results in a very low cardiovascular event rate,” the cardiologist said.
He presented an analysis of 4,758 nondiabetic participants in the prospective, population-based MESA (Multi-Ethnic Study of Atherosclerosis) in which he examined how they fared in terms of cardiovascular events over a median 10.3 years of follow-up. All were free of known cardiovascular disease at baseline. With the risk estimator included in the 2013 AHA/ACC cholesterol management guidelines, 2,377 subjects would be recommended for high-intensity statin therapy at baseline on the basis of a 10-year atherosclerotic cardiovascular disease risk estimate of at least 7.5%. Another 589 participants were recommended for consideration of a moderate-intensity statin based on an estimated 10-year risk of 5%-7.4%.
Forty-one percent of MESA subjects recommended for a high-intensity statin according to the AHA/ACC risk estimator had a coronary artery calcium (CAC) score of 0, and their 10-year composite rate of MI, stroke, or cardiovascular death was just 4.9% – well below the 7.5% threshold recommended for statin therapy. In contrast, if any CAC was present, the event rate was 10.5%.
With a relative risk reduction with statin therapy of 30%, the number needed to treat for 5 years to prevent one cardiovascular event in the group with a CAC of 0 would be 128. In the presence of any CAC, the number needed to treat fell to a far more reasonable 56, Dr. Nasir said.
Similarly, among the group recommended for consideration of statin therapy on the basis of a 10-year risk of 5%-7.4%, the actual event rate in the 57% of subjects with a CAC of 0 was just 1.5%. If any CAC was present, the event rate shot up to 7.2%. The number needed to treat in this cohort was 445 among those with a CAC of 0 and 90 with any CAC present.
“I think coronary artery calcium is a game changer in primary prevention,” Dr. Michael J. Blaha commented. “It sufficiently moves the needle to make you think differently about a patient. I’m not sure some of the other tests have sufficient evidence to say, ‘I’m going to think about not treating you if it’s negative and treating you if it’s positive,’ but coronary artery calcium has that evidence.”
In his own cardiology practice at Johns Hopkins University, Baltimore, Dr. Blaha finds himself using CAC testing often, especially in his many statin-reluctant patients.
“I have a lot of patients who would fit under a recommendation for statin therapy under the 2013 AHA/ACC cholesterol management guidelines, but who really don’t want to take medications. I know you see these patients in your practices, too. This is lifelong therapy, and they want a really good reason to take it or not to take it. If a patient is reluctant to take a statin and has a CAC score of 0, I will sometimes emphasize lifestyle therapy. It certainly redoubles my interest in lifestyle therapy. But if the CAC score is elevated, then I can make a specific case that the number needed to treat is very favorable, compared to the number needed to harm,” explained Dr. Blaha, a coinvestigator with Dr. Nasir in the MESA study.
Other situations where he finds CAC testing useful in daily practice include uncertainty as to a patient’s true risk level because the individual’s situation isn’t adequately captured by the AHA/ACC risk estimator. A patient with rheumatologic disease would be one example; another would be an individual who is neither white nor African American. He said he also utilizes CAC testing in statin-intolerant patients, where the results are useful in deciding how many different statins to try before saying “enough.”
Audience members asked what it’s going to take to get insurers to cover CAC testing for risk stratification. Dr. Blaha replied that more long-term outcomes and cost-effectiveness data are coming. In the meantime, at an out-of-pocket cost of $75-$100, a lot of his statin-reluctant patients consider CAC testing a good buy.
“They say, ‘I’ll take this test to help me decide whether to take a pill for the rest of my life,’” according to Dr. Blaha.
Dr. Nasir said the evidence in support of CAC testing is now so strong that he believes physicians have an obligation to mention it as an option during the statin treatment decision discussion.
“At this moment, most patients are making their decision based on the guesstimate of their risk we are giving them using the risk calculator. If they have the ability through a $75-$100 test that costs about the same as 18 months of statin therapy to know that their true risk is not, say, 10%, but actually 5%, they’re less likely to choose therapy. Is it even ethical to withhold from our patients that there is a test out there that can reduce their estimated risk to a point that they can avoid statin therapy?” the cardiologist asked.
Dr. Nasir reported serving on an advisory board for Quest Diagnostics. Dr. Blaha reported having no financial conflicts.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS