User login
Current gout guidelines stress ‘treat to target’
SNOWMASS, COLO. – The current American College of Rheumatology gout guidelines contain a number of recommendations that may come as a surprise to rheumatologists and primary care physicians alike.
The guidelines state, for example, that urate-lowering therapy should be undertaken routinely in any patient with an established diagnosis of gout who has comorbid chronic kidney disease (CKD) that is stage 2 or worse, meaning an estimated glomerular filtration rate of 89 mL/minute per 1.73 m2 or less.
The rationale is that it’s particularly important to try to prevent acute gout attacks in such patients because their renal dysfunction makes it problematic to use colchicine and NSAIDs to quell attacks. Intriguing studies suggest that lowering serum urate may actually slow progression of CKD, Dr. Michael H. Pillinger said at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The guidelines name the other indications for urate lowering in gout patients as the presence of a tophus on clinical examination or an imaging study, a history of two or more gout attacks per year, or a history of kidney stones.
Traditionally, urate-lowering therapy has been initiated during quiescent periods, but the ACR guidelines state that it also can be started during an acute attack if effective anti-inflammatory management has been instituted.
"This goes against what I was taught," observed Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
The guidelines (Arthritis Care Res. 2012;64:1431-46 and 1447-61) emphasize the importance of a treat-to-target approach.
"The primary care physicians I talk to still don’t know this. The ACR recommends a minimum serum urate target of less than 6.0 mg/dL, but the guidelines are very clear that if 6 isn’t good enough, you keep going. You go below 5. When I see patients with tophaceous gout, my target is never 6. My target is 5 or 4. That’s what I teach my fellows," explained Dr. Pillinger, who served on an expert panel that advised the guideline-writing task force.
The ACR urate-lowering algorithm begins with either allopurinol or febuxostat (Uloric) as first-line therapy. The guideline committee, which expressly excluded cost as a consideration, offered no guidance as to which xanthine oxidase inhibitor is preferred. Dr. Pillinger noted that febuxostat is a more specific xanthine oxidase inhibitor, is simpler to dose, and is far less likely to cause hypersensitivity reactions than is allopurinol. It is also more effective, although not dramatically more so. And it is considerably more expensive.
Febuxostat is approved by the Food and Drug Administration specifically for use in patients with mild to moderate CKD. Allopurinol is not. However, the gout guidelines endorse the use of allopurinol in that setting.
When allopurinol is the initial drug, the guidelines recommend dosing it in a manner that is different from how most physicians have been using it, the rheumatologist said. The recommended starting dose is lower than has been customary: 100 mg/day, and 50 mg/day in patients with stage 4 or 5 CKD. The drug is to be titrated upward every 2-5 weeks as needed to achieve the target urate level. The maximum dose is 800 mg/day, even in patients with comorbid CKD. Although the guidelines don’t provide guidance as to the size of the stepwise dosing increases, Dr. Pillinger usually boosts the allopurinol dose by 100 mg at a time, or 50 mg in patients with CKD.
"Most patients don’t get to target at 300 mg/day. You’ve got to go higher," he said.
An important innovation in the current guidelines is the recommendation for testing for the HLA-B*5801 allele in patients of Korean, Thai, or Han Chinese ancestry who are being considered for allopurinol therapy. The presence of this allele confers a several hundred–fold increased risk of allopurinol hypersensitivity.
Probenecid is endorsed as the alternative first-line urate-lowering agent, but only if at least one xanthine oxidase inhibitor is contraindicated or not tolerated. No other agents get the nod as first-line therapy.
The guidelines state that if a patient’s serum urate is not at target despite maximum-dose therapy with a first-line xanthine oxidase inhibitor, it is not appropriate to switch to the other xanthine oxidase inhibitor. Instead, it is time to add a uricosuric agent: probenecid, losartan, or fenofibrate. If the urate level still is not at target and the patient is generally well, with few gout attacks, then that’s an acceptable result. However, if the patient has moderate tophaceous gout or chronic gouty arthropathy, it’s appropriate to place the patient on pegloticase (Krystexxa) while discontinuing all other urate-lowering agents.
The ACR guidelines stress that it is vital to always try to prevent gout attacks during initiation of urate-lowering therapy. The recommended first-line agents for prophylaxis are low-dose colchicine or a low-dose NSAID, with prednisone at a dose not to exceed 10 mg/day reserved as second-line therapy in the event the first-line agents are not tolerated or are ineffective.
Prophylaxis is supposed to continue as long as a patient has any evidence of disease activity. And once all symptoms and tophi have resolved, all measures needed to keep the serum urate below 6.0 mg/dL are to be continued indefinitely.
"For most patients," Dr. Pillinger concluded, "gout treatment is almost always forever."
He reported having received research grants from Takeda, which markets febuxostat in the United States, and Savient, which markets pegloticase.
SNOWMASS, COLO. – The current American College of Rheumatology gout guidelines contain a number of recommendations that may come as a surprise to rheumatologists and primary care physicians alike.
The guidelines state, for example, that urate-lowering therapy should be undertaken routinely in any patient with an established diagnosis of gout who has comorbid chronic kidney disease (CKD) that is stage 2 or worse, meaning an estimated glomerular filtration rate of 89 mL/minute per 1.73 m2 or less.
The rationale is that it’s particularly important to try to prevent acute gout attacks in such patients because their renal dysfunction makes it problematic to use colchicine and NSAIDs to quell attacks. Intriguing studies suggest that lowering serum urate may actually slow progression of CKD, Dr. Michael H. Pillinger said at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The guidelines name the other indications for urate lowering in gout patients as the presence of a tophus on clinical examination or an imaging study, a history of two or more gout attacks per year, or a history of kidney stones.
Traditionally, urate-lowering therapy has been initiated during quiescent periods, but the ACR guidelines state that it also can be started during an acute attack if effective anti-inflammatory management has been instituted.
"This goes against what I was taught," observed Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
The guidelines (Arthritis Care Res. 2012;64:1431-46 and 1447-61) emphasize the importance of a treat-to-target approach.
"The primary care physicians I talk to still don’t know this. The ACR recommends a minimum serum urate target of less than 6.0 mg/dL, but the guidelines are very clear that if 6 isn’t good enough, you keep going. You go below 5. When I see patients with tophaceous gout, my target is never 6. My target is 5 or 4. That’s what I teach my fellows," explained Dr. Pillinger, who served on an expert panel that advised the guideline-writing task force.
The ACR urate-lowering algorithm begins with either allopurinol or febuxostat (Uloric) as first-line therapy. The guideline committee, which expressly excluded cost as a consideration, offered no guidance as to which xanthine oxidase inhibitor is preferred. Dr. Pillinger noted that febuxostat is a more specific xanthine oxidase inhibitor, is simpler to dose, and is far less likely to cause hypersensitivity reactions than is allopurinol. It is also more effective, although not dramatically more so. And it is considerably more expensive.
Febuxostat is approved by the Food and Drug Administration specifically for use in patients with mild to moderate CKD. Allopurinol is not. However, the gout guidelines endorse the use of allopurinol in that setting.
When allopurinol is the initial drug, the guidelines recommend dosing it in a manner that is different from how most physicians have been using it, the rheumatologist said. The recommended starting dose is lower than has been customary: 100 mg/day, and 50 mg/day in patients with stage 4 or 5 CKD. The drug is to be titrated upward every 2-5 weeks as needed to achieve the target urate level. The maximum dose is 800 mg/day, even in patients with comorbid CKD. Although the guidelines don’t provide guidance as to the size of the stepwise dosing increases, Dr. Pillinger usually boosts the allopurinol dose by 100 mg at a time, or 50 mg in patients with CKD.
"Most patients don’t get to target at 300 mg/day. You’ve got to go higher," he said.
An important innovation in the current guidelines is the recommendation for testing for the HLA-B*5801 allele in patients of Korean, Thai, or Han Chinese ancestry who are being considered for allopurinol therapy. The presence of this allele confers a several hundred–fold increased risk of allopurinol hypersensitivity.
Probenecid is endorsed as the alternative first-line urate-lowering agent, but only if at least one xanthine oxidase inhibitor is contraindicated or not tolerated. No other agents get the nod as first-line therapy.
The guidelines state that if a patient’s serum urate is not at target despite maximum-dose therapy with a first-line xanthine oxidase inhibitor, it is not appropriate to switch to the other xanthine oxidase inhibitor. Instead, it is time to add a uricosuric agent: probenecid, losartan, or fenofibrate. If the urate level still is not at target and the patient is generally well, with few gout attacks, then that’s an acceptable result. However, if the patient has moderate tophaceous gout or chronic gouty arthropathy, it’s appropriate to place the patient on pegloticase (Krystexxa) while discontinuing all other urate-lowering agents.
The ACR guidelines stress that it is vital to always try to prevent gout attacks during initiation of urate-lowering therapy. The recommended first-line agents for prophylaxis are low-dose colchicine or a low-dose NSAID, with prednisone at a dose not to exceed 10 mg/day reserved as second-line therapy in the event the first-line agents are not tolerated or are ineffective.
Prophylaxis is supposed to continue as long as a patient has any evidence of disease activity. And once all symptoms and tophi have resolved, all measures needed to keep the serum urate below 6.0 mg/dL are to be continued indefinitely.
"For most patients," Dr. Pillinger concluded, "gout treatment is almost always forever."
He reported having received research grants from Takeda, which markets febuxostat in the United States, and Savient, which markets pegloticase.
SNOWMASS, COLO. – The current American College of Rheumatology gout guidelines contain a number of recommendations that may come as a surprise to rheumatologists and primary care physicians alike.
The guidelines state, for example, that urate-lowering therapy should be undertaken routinely in any patient with an established diagnosis of gout who has comorbid chronic kidney disease (CKD) that is stage 2 or worse, meaning an estimated glomerular filtration rate of 89 mL/minute per 1.73 m2 or less.
The rationale is that it’s particularly important to try to prevent acute gout attacks in such patients because their renal dysfunction makes it problematic to use colchicine and NSAIDs to quell attacks. Intriguing studies suggest that lowering serum urate may actually slow progression of CKD, Dr. Michael H. Pillinger said at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The guidelines name the other indications for urate lowering in gout patients as the presence of a tophus on clinical examination or an imaging study, a history of two or more gout attacks per year, or a history of kidney stones.
Traditionally, urate-lowering therapy has been initiated during quiescent periods, but the ACR guidelines state that it also can be started during an acute attack if effective anti-inflammatory management has been instituted.
"This goes against what I was taught," observed Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
The guidelines (Arthritis Care Res. 2012;64:1431-46 and 1447-61) emphasize the importance of a treat-to-target approach.
"The primary care physicians I talk to still don’t know this. The ACR recommends a minimum serum urate target of less than 6.0 mg/dL, but the guidelines are very clear that if 6 isn’t good enough, you keep going. You go below 5. When I see patients with tophaceous gout, my target is never 6. My target is 5 or 4. That’s what I teach my fellows," explained Dr. Pillinger, who served on an expert panel that advised the guideline-writing task force.
The ACR urate-lowering algorithm begins with either allopurinol or febuxostat (Uloric) as first-line therapy. The guideline committee, which expressly excluded cost as a consideration, offered no guidance as to which xanthine oxidase inhibitor is preferred. Dr. Pillinger noted that febuxostat is a more specific xanthine oxidase inhibitor, is simpler to dose, and is far less likely to cause hypersensitivity reactions than is allopurinol. It is also more effective, although not dramatically more so. And it is considerably more expensive.
Febuxostat is approved by the Food and Drug Administration specifically for use in patients with mild to moderate CKD. Allopurinol is not. However, the gout guidelines endorse the use of allopurinol in that setting.
When allopurinol is the initial drug, the guidelines recommend dosing it in a manner that is different from how most physicians have been using it, the rheumatologist said. The recommended starting dose is lower than has been customary: 100 mg/day, and 50 mg/day in patients with stage 4 or 5 CKD. The drug is to be titrated upward every 2-5 weeks as needed to achieve the target urate level. The maximum dose is 800 mg/day, even in patients with comorbid CKD. Although the guidelines don’t provide guidance as to the size of the stepwise dosing increases, Dr. Pillinger usually boosts the allopurinol dose by 100 mg at a time, or 50 mg in patients with CKD.
"Most patients don’t get to target at 300 mg/day. You’ve got to go higher," he said.
An important innovation in the current guidelines is the recommendation for testing for the HLA-B*5801 allele in patients of Korean, Thai, or Han Chinese ancestry who are being considered for allopurinol therapy. The presence of this allele confers a several hundred–fold increased risk of allopurinol hypersensitivity.
Probenecid is endorsed as the alternative first-line urate-lowering agent, but only if at least one xanthine oxidase inhibitor is contraindicated or not tolerated. No other agents get the nod as first-line therapy.
The guidelines state that if a patient’s serum urate is not at target despite maximum-dose therapy with a first-line xanthine oxidase inhibitor, it is not appropriate to switch to the other xanthine oxidase inhibitor. Instead, it is time to add a uricosuric agent: probenecid, losartan, or fenofibrate. If the urate level still is not at target and the patient is generally well, with few gout attacks, then that’s an acceptable result. However, if the patient has moderate tophaceous gout or chronic gouty arthropathy, it’s appropriate to place the patient on pegloticase (Krystexxa) while discontinuing all other urate-lowering agents.
The ACR guidelines stress that it is vital to always try to prevent gout attacks during initiation of urate-lowering therapy. The recommended first-line agents for prophylaxis are low-dose colchicine or a low-dose NSAID, with prednisone at a dose not to exceed 10 mg/day reserved as second-line therapy in the event the first-line agents are not tolerated or are ineffective.
Prophylaxis is supposed to continue as long as a patient has any evidence of disease activity. And once all symptoms and tophi have resolved, all measures needed to keep the serum urate below 6.0 mg/dL are to be continued indefinitely.
"For most patients," Dr. Pillinger concluded, "gout treatment is almost always forever."
He reported having received research grants from Takeda, which markets febuxostat in the United States, and Savient, which markets pegloticase.
EXPERT ANALYSIS FROM THE ACR WINTER RHEUMATOLOGY SYMPOSIUM
Skin cancer risk stands out in anti-TNF biologics use
SNOWMASS, COLO. – The use of anti–tumor necrosis factor biologics in patients with rheumatoid arthritis, psoriasis, and other serious autoimmune diseases is not associated with increased risk of most forms of cancer, with two highly visible and important exceptions: nonmelanoma skin cancer and malignant melanoma.
That’s the largely reassuring conclusion to be drawn from two large studies conducted by researchers at Stockholm’s Karolinska Institute, according to Dr. Jeffrey R. Curtis, director of the arthritis clinical intervention program at the University of Alabama, Birmingham.
The Swedish group conducted a comprehensive meta-analysis of all 74 pharmaceutical company–sponsored randomized controlled trials of anti-TNF biologics lasting at least 4 weeks. The investigators used individual patient data for 15,418 anti-TNF recipients and 7,486 subjects randomized to methotrexate and other comparators. About half of the randomized trials focused on rheumatoid arthritis (RA) patients; the other half involved patients with the other approved indications for anti-TNF therapy.
The use of individual patient data was a nuance of the meta-analysis that wowed Dr. Curtis.
"This is a really great paper. One of the reasons I was very impressed with it is that getting drug companies to give up any raw person-level data to anybody is difficult. The European Medicines Agency requested it, and that’s probably the only reason it happened," Dr. Curtis observed at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The overall relative risk for all forms of cancer, excluding nonmelanoma skin cancer, in patients on anti-TNF biologics was 0.99. Their cancer rate was 641 per 100,000 person-years (Pharmacoepidemiol. Drug Saf. 2011;20:119-30).
"You can tell your patients the risk for cancer is less than 1 in 100 – it’s about 6 per 1,000 – and it’s not increased compared to background therapy," the rheumatologist said.
The exception was nonmelanoma skin cancer. The relative risk for this malignancy among users of all anti-TNF biologics was increased twofold.
In a separate study, the same group used prospectively recorded data from Swedish national registries to investigate the association between anti-TNF therapy and melanoma. This analysis involved 10,878 RA patients treated with anti-TNF biologics, 42,198 others who were not, and 162,743 matched controls from the general population.
Compared with controls, RA patients not on anti-TNF biologics did not have a significantly increased risk of malignant melanoma. However, the 38 first invasive melanomas that occurred in RA patients taking a TNF antagonist signified a 1.5-fold increased risk of this malignancy compared to the general population. This 50% increase in relative risk was statistically significant. The absolute increase in risk was quite small: 20 additional cases per 100,000 person-years. The number needed to harm – that is, the number of patients who needed to be treated with an anti-TNF biologic to cause one additional case of melanoma – was 50,000 (BMJ 2013;346:f1939 [doi: 10.1136/bmj.f1939]).
Dr. Curtis noted that patients who have been urged to consider going on a TNF antagonist often return to the office waving a printout of the product labeling that warns of the possibility of malignancy.
"The bottom line: I tell patients that based on the data that there doesn’t appear to be an increased risk for all types of cancer, including hematologic, with the exceptions of nonmelanoma skin cancer and melanoma. Those are the two we need to advise patients about," he said.
Dr. Curtis reported receiving funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and 10 pharmaceutical companies.
SNOWMASS, COLO. – The use of anti–tumor necrosis factor biologics in patients with rheumatoid arthritis, psoriasis, and other serious autoimmune diseases is not associated with increased risk of most forms of cancer, with two highly visible and important exceptions: nonmelanoma skin cancer and malignant melanoma.
That’s the largely reassuring conclusion to be drawn from two large studies conducted by researchers at Stockholm’s Karolinska Institute, according to Dr. Jeffrey R. Curtis, director of the arthritis clinical intervention program at the University of Alabama, Birmingham.
The Swedish group conducted a comprehensive meta-analysis of all 74 pharmaceutical company–sponsored randomized controlled trials of anti-TNF biologics lasting at least 4 weeks. The investigators used individual patient data for 15,418 anti-TNF recipients and 7,486 subjects randomized to methotrexate and other comparators. About half of the randomized trials focused on rheumatoid arthritis (RA) patients; the other half involved patients with the other approved indications for anti-TNF therapy.
The use of individual patient data was a nuance of the meta-analysis that wowed Dr. Curtis.
"This is a really great paper. One of the reasons I was very impressed with it is that getting drug companies to give up any raw person-level data to anybody is difficult. The European Medicines Agency requested it, and that’s probably the only reason it happened," Dr. Curtis observed at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The overall relative risk for all forms of cancer, excluding nonmelanoma skin cancer, in patients on anti-TNF biologics was 0.99. Their cancer rate was 641 per 100,000 person-years (Pharmacoepidemiol. Drug Saf. 2011;20:119-30).
"You can tell your patients the risk for cancer is less than 1 in 100 – it’s about 6 per 1,000 – and it’s not increased compared to background therapy," the rheumatologist said.
The exception was nonmelanoma skin cancer. The relative risk for this malignancy among users of all anti-TNF biologics was increased twofold.
In a separate study, the same group used prospectively recorded data from Swedish national registries to investigate the association between anti-TNF therapy and melanoma. This analysis involved 10,878 RA patients treated with anti-TNF biologics, 42,198 others who were not, and 162,743 matched controls from the general population.
Compared with controls, RA patients not on anti-TNF biologics did not have a significantly increased risk of malignant melanoma. However, the 38 first invasive melanomas that occurred in RA patients taking a TNF antagonist signified a 1.5-fold increased risk of this malignancy compared to the general population. This 50% increase in relative risk was statistically significant. The absolute increase in risk was quite small: 20 additional cases per 100,000 person-years. The number needed to harm – that is, the number of patients who needed to be treated with an anti-TNF biologic to cause one additional case of melanoma – was 50,000 (BMJ 2013;346:f1939 [doi: 10.1136/bmj.f1939]).
Dr. Curtis noted that patients who have been urged to consider going on a TNF antagonist often return to the office waving a printout of the product labeling that warns of the possibility of malignancy.
"The bottom line: I tell patients that based on the data that there doesn’t appear to be an increased risk for all types of cancer, including hematologic, with the exceptions of nonmelanoma skin cancer and melanoma. Those are the two we need to advise patients about," he said.
Dr. Curtis reported receiving funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and 10 pharmaceutical companies.
SNOWMASS, COLO. – The use of anti–tumor necrosis factor biologics in patients with rheumatoid arthritis, psoriasis, and other serious autoimmune diseases is not associated with increased risk of most forms of cancer, with two highly visible and important exceptions: nonmelanoma skin cancer and malignant melanoma.
That’s the largely reassuring conclusion to be drawn from two large studies conducted by researchers at Stockholm’s Karolinska Institute, according to Dr. Jeffrey R. Curtis, director of the arthritis clinical intervention program at the University of Alabama, Birmingham.
The Swedish group conducted a comprehensive meta-analysis of all 74 pharmaceutical company–sponsored randomized controlled trials of anti-TNF biologics lasting at least 4 weeks. The investigators used individual patient data for 15,418 anti-TNF recipients and 7,486 subjects randomized to methotrexate and other comparators. About half of the randomized trials focused on rheumatoid arthritis (RA) patients; the other half involved patients with the other approved indications for anti-TNF therapy.
The use of individual patient data was a nuance of the meta-analysis that wowed Dr. Curtis.
"This is a really great paper. One of the reasons I was very impressed with it is that getting drug companies to give up any raw person-level data to anybody is difficult. The European Medicines Agency requested it, and that’s probably the only reason it happened," Dr. Curtis observed at the Winter Rheumatology symposium sponsored by the American College of Rheumatology.
The overall relative risk for all forms of cancer, excluding nonmelanoma skin cancer, in patients on anti-TNF biologics was 0.99. Their cancer rate was 641 per 100,000 person-years (Pharmacoepidemiol. Drug Saf. 2011;20:119-30).
"You can tell your patients the risk for cancer is less than 1 in 100 – it’s about 6 per 1,000 – and it’s not increased compared to background therapy," the rheumatologist said.
The exception was nonmelanoma skin cancer. The relative risk for this malignancy among users of all anti-TNF biologics was increased twofold.
In a separate study, the same group used prospectively recorded data from Swedish national registries to investigate the association between anti-TNF therapy and melanoma. This analysis involved 10,878 RA patients treated with anti-TNF biologics, 42,198 others who were not, and 162,743 matched controls from the general population.
Compared with controls, RA patients not on anti-TNF biologics did not have a significantly increased risk of malignant melanoma. However, the 38 first invasive melanomas that occurred in RA patients taking a TNF antagonist signified a 1.5-fold increased risk of this malignancy compared to the general population. This 50% increase in relative risk was statistically significant. The absolute increase in risk was quite small: 20 additional cases per 100,000 person-years. The number needed to harm – that is, the number of patients who needed to be treated with an anti-TNF biologic to cause one additional case of melanoma – was 50,000 (BMJ 2013;346:f1939 [doi: 10.1136/bmj.f1939]).
Dr. Curtis noted that patients who have been urged to consider going on a TNF antagonist often return to the office waving a printout of the product labeling that warns of the possibility of malignancy.
"The bottom line: I tell patients that based on the data that there doesn’t appear to be an increased risk for all types of cancer, including hematologic, with the exceptions of nonmelanoma skin cancer and melanoma. Those are the two we need to advise patients about," he said.
Dr. Curtis reported receiving funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and 10 pharmaceutical companies.
EXPERT ANALYSIS FROM THE ACR WINTER RHEUMATOLOGY SYMPOSIUM
Expert calls pegloticase a powerhouse gout drug not to be feared
SNOWMASS, COLO. – Pegloticase (Krystexxa) is a gout drug that’s expensive, inconveniently administered by intravenous infusion every 2 weeks, and saddled with a substantial rate of immunogenicity, with infusion reactions that can include anaphylaxis.
So why does gout authority Dr. Michael H. Pillinger call pegloticase "a greatly underestimated and underutilized drug"? And why do the current American College of Rheumatology gout guidelines recommend pegloticase for refractory gout?
"Nothing else we have will get rid of tophi the way this drug gets rid of tophi," Dr. Pillinger explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Why do we care about that? Because tophi are erosive, they’re damaging, and also because they’re much more extensive than we think they are," said Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
Besides, the safety concern regarding this powerhouse urate-lowering drug has been resolved. The infusion reactions are readily avoidable. Pegloticase is not a drug rheumatologists should be scared of, he emphasized.
Dr. Pillinger cited an eye-opening study that demonstrated just how much larger gout patients’ total body urate burden actually is compared with what’s apparent clinically. Investigators at the University of British Columbia, Vancouver, used dual-energy CT to assess urate deposits in 20 consecutive patients with tophaceous gout and 10 controls with other arthritic conditions. Physical evaluation of the gout patients turned up 111 areas of urate deposition; dual-energy CT revealed 440 such areas. The mean total urate volume was a hefty 40.2 cm3 (Ann. Rheum. Dis. 2009;68:1609-12).
"There’s a lot more tophi under the surface," Dr. Pillinger commented.
Pegloticase is a recombinant porcine uricase that’s modified with a baboon N-terminus. It’s pegylated to reduce immunogenicity to the uricase and increase stability and half-life. Paradoxically, the drug is still quite immunogenic because many patients develop antibodies to the polyethylene glycol used in pegylation.
The drug’s urate-lowering effect is unmatched. Within 12-24 hours of the first dose, the plasma uric acid level plummets to almost nothing.
"What happens is that very, very quickly these patients are going to split into two groups. For one group this is the greatest drug in the world; their uric acid remains almost undetectable – and that’s in our sickest refractory patients. And then there’s another group that starts to fail. It’s in the neighborhood of 30%-40% of patients, so it’s a real problem. We know they’re failing because their uric acid level starts rising. They’re making antibodies and inactivating the drug every time it’s given. And they’re the ones who get bad infusion reactions," the rheumatologist explained.
The solution to using pegloticase safely is to routinely measure serum urate 1-2 days before each infusion. If the serum urate climbs to 6 mg/dL on one or two occasions, it’s time to discontinue the drug.
"For everybody else, they’re going to do really, really well," Dr. Pillinger said.
A key point emphasized in the 2012 ACR gout guidelines (Arthritis Care Res. 2012;64:1431-46; 1447-61) is that when patients go on pegloticase, all other urate-lowering therapies must be stopped as a matter of safety. Otherwise, it’s impossible to use the pre-infusion plasma urate measurement to determine if pegloticase has stopped working.
The ACR guidelines recommend pegloticase as third-line urate-lowering therapy in patients who are not at target despite maximum-dose therapy with a xanthine oxidase inhibitor plus second-line therapy with probenecid, losartan, or fenofibrate.
Dr. Pillinger reported having received research grants from Savient, which markets pegloticase, and Takeda.
SNOWMASS, COLO. – Pegloticase (Krystexxa) is a gout drug that’s expensive, inconveniently administered by intravenous infusion every 2 weeks, and saddled with a substantial rate of immunogenicity, with infusion reactions that can include anaphylaxis.
So why does gout authority Dr. Michael H. Pillinger call pegloticase "a greatly underestimated and underutilized drug"? And why do the current American College of Rheumatology gout guidelines recommend pegloticase for refractory gout?
"Nothing else we have will get rid of tophi the way this drug gets rid of tophi," Dr. Pillinger explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Why do we care about that? Because tophi are erosive, they’re damaging, and also because they’re much more extensive than we think they are," said Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
Besides, the safety concern regarding this powerhouse urate-lowering drug has been resolved. The infusion reactions are readily avoidable. Pegloticase is not a drug rheumatologists should be scared of, he emphasized.
Dr. Pillinger cited an eye-opening study that demonstrated just how much larger gout patients’ total body urate burden actually is compared with what’s apparent clinically. Investigators at the University of British Columbia, Vancouver, used dual-energy CT to assess urate deposits in 20 consecutive patients with tophaceous gout and 10 controls with other arthritic conditions. Physical evaluation of the gout patients turned up 111 areas of urate deposition; dual-energy CT revealed 440 such areas. The mean total urate volume was a hefty 40.2 cm3 (Ann. Rheum. Dis. 2009;68:1609-12).
"There’s a lot more tophi under the surface," Dr. Pillinger commented.
Pegloticase is a recombinant porcine uricase that’s modified with a baboon N-terminus. It’s pegylated to reduce immunogenicity to the uricase and increase stability and half-life. Paradoxically, the drug is still quite immunogenic because many patients develop antibodies to the polyethylene glycol used in pegylation.
The drug’s urate-lowering effect is unmatched. Within 12-24 hours of the first dose, the plasma uric acid level plummets to almost nothing.
"What happens is that very, very quickly these patients are going to split into two groups. For one group this is the greatest drug in the world; their uric acid remains almost undetectable – and that’s in our sickest refractory patients. And then there’s another group that starts to fail. It’s in the neighborhood of 30%-40% of patients, so it’s a real problem. We know they’re failing because their uric acid level starts rising. They’re making antibodies and inactivating the drug every time it’s given. And they’re the ones who get bad infusion reactions," the rheumatologist explained.
The solution to using pegloticase safely is to routinely measure serum urate 1-2 days before each infusion. If the serum urate climbs to 6 mg/dL on one or two occasions, it’s time to discontinue the drug.
"For everybody else, they’re going to do really, really well," Dr. Pillinger said.
A key point emphasized in the 2012 ACR gout guidelines (Arthritis Care Res. 2012;64:1431-46; 1447-61) is that when patients go on pegloticase, all other urate-lowering therapies must be stopped as a matter of safety. Otherwise, it’s impossible to use the pre-infusion plasma urate measurement to determine if pegloticase has stopped working.
The ACR guidelines recommend pegloticase as third-line urate-lowering therapy in patients who are not at target despite maximum-dose therapy with a xanthine oxidase inhibitor plus second-line therapy with probenecid, losartan, or fenofibrate.
Dr. Pillinger reported having received research grants from Savient, which markets pegloticase, and Takeda.
SNOWMASS, COLO. – Pegloticase (Krystexxa) is a gout drug that’s expensive, inconveniently administered by intravenous infusion every 2 weeks, and saddled with a substantial rate of immunogenicity, with infusion reactions that can include anaphylaxis.
So why does gout authority Dr. Michael H. Pillinger call pegloticase "a greatly underestimated and underutilized drug"? And why do the current American College of Rheumatology gout guidelines recommend pegloticase for refractory gout?
"Nothing else we have will get rid of tophi the way this drug gets rid of tophi," Dr. Pillinger explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Why do we care about that? Because tophi are erosive, they’re damaging, and also because they’re much more extensive than we think they are," said Dr. Pillinger, a rheumatologist and director of the crystal diseases study group at New York University.
Besides, the safety concern regarding this powerhouse urate-lowering drug has been resolved. The infusion reactions are readily avoidable. Pegloticase is not a drug rheumatologists should be scared of, he emphasized.
Dr. Pillinger cited an eye-opening study that demonstrated just how much larger gout patients’ total body urate burden actually is compared with what’s apparent clinically. Investigators at the University of British Columbia, Vancouver, used dual-energy CT to assess urate deposits in 20 consecutive patients with tophaceous gout and 10 controls with other arthritic conditions. Physical evaluation of the gout patients turned up 111 areas of urate deposition; dual-energy CT revealed 440 such areas. The mean total urate volume was a hefty 40.2 cm3 (Ann. Rheum. Dis. 2009;68:1609-12).
"There’s a lot more tophi under the surface," Dr. Pillinger commented.
Pegloticase is a recombinant porcine uricase that’s modified with a baboon N-terminus. It’s pegylated to reduce immunogenicity to the uricase and increase stability and half-life. Paradoxically, the drug is still quite immunogenic because many patients develop antibodies to the polyethylene glycol used in pegylation.
The drug’s urate-lowering effect is unmatched. Within 12-24 hours of the first dose, the plasma uric acid level plummets to almost nothing.
"What happens is that very, very quickly these patients are going to split into two groups. For one group this is the greatest drug in the world; their uric acid remains almost undetectable – and that’s in our sickest refractory patients. And then there’s another group that starts to fail. It’s in the neighborhood of 30%-40% of patients, so it’s a real problem. We know they’re failing because their uric acid level starts rising. They’re making antibodies and inactivating the drug every time it’s given. And they’re the ones who get bad infusion reactions," the rheumatologist explained.
The solution to using pegloticase safely is to routinely measure serum urate 1-2 days before each infusion. If the serum urate climbs to 6 mg/dL on one or two occasions, it’s time to discontinue the drug.
"For everybody else, they’re going to do really, really well," Dr. Pillinger said.
A key point emphasized in the 2012 ACR gout guidelines (Arthritis Care Res. 2012;64:1431-46; 1447-61) is that when patients go on pegloticase, all other urate-lowering therapies must be stopped as a matter of safety. Otherwise, it’s impossible to use the pre-infusion plasma urate measurement to determine if pegloticase has stopped working.
The ACR guidelines recommend pegloticase as third-line urate-lowering therapy in patients who are not at target despite maximum-dose therapy with a xanthine oxidase inhibitor plus second-line therapy with probenecid, losartan, or fenofibrate.
Dr. Pillinger reported having received research grants from Savient, which markets pegloticase, and Takeda.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
New insights into aromatase inhibitor therapy nonpersistence
SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
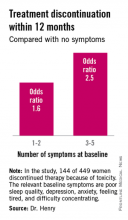
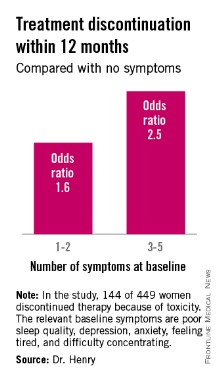
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
AT SABCS 2013
Major finding: Breast cancer patients with at least three of five self-reported symptoms prior to going on adjuvant aromatase inhibitor therapy were 2.5-fold more likely to discontinue treatment within the first 12 months because of toxicity.
Data source: The Exemestane and Letrozole Pharmacogenetics trial included 449 evaluable women with early-stage estrogen receptor–positive breast cancer who were prospectively evaluated for the relationship between a variety of baseline patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy.
Disclosures: The study was funded by the Damon Runyon Cancer Research Foundation. Dr. Norah L. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
Striking trends emerge in SLE joint replacement
SNOWMASS, COLO. – Total knee replacement surgery rates in patients with systemic lupus erythematosus jumped sixfold nationally during a recent 15-year span – and buried within this statistic is some very good news.
The sharp rise in total knee replacement (TKR) among systemic lupus erythematosus (SLE) patients has been driven by a hefty increase in operations performed for osteoarthritis, while TKR for active SLE in the knee has declined. These trends reflect the increased longevity of patients with SLE resulting from improved medical management. For the first time, large numbers of SLE patients are surviving to an age when they, like other Americans, are more vulnerable to osteoarthritis, Dr. Susan M. Goodman explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Lupus is no longer the highly mortal disease that it was," she observed. "Clearly, patients are surviving and – I don’t know how else to put it – they’ve become middle-aged women who are having knee replacements, which is kind of a success."
She presented highlights of a soon-to-be-published study involving analysis of 10 state databases including nearly 2.8 million arthroplasties performed during 1991-2005. The rate of TKR in SLE patients climbed sixfold from 0.03 per 100,000 population in 1991 to 0.18 per 100,000 in 2005. Meanwhile, total hip replacement (THR) in SLE patients showed a modest but statistically significant increase from 0.11 to 0.18 cases per 100,000.
The proportion of lupus patients undergoing arthroplasty for avascular necrosis fell from 53% in 1991 to 24% in 2005, while the proportion undergoing arthroplasty because they developed osteoarthritis went from 23% to 61%.
Virtually all of the increase in total arthroplasties among SLE patients occurred in women aged 45 years and older. Their rate more than tripled during the study years, going from 0.076 to 0.271 cases per 100,000. Rates in female SLE patients aged 44 years and younger actually took a significant drop from 0.073 to 0.067 per 100,000. Rates in males aged 44 and younger remained flat over time, while men aged 45 and up showed a modest increase from a low baseline rate of 0.009 cases per 100,000 in 1991 to 0.034 per 100,000 in 2005, according to Dr. Goodman, a rheumatologist at the Hospital for Special Surgery and Cornell University, New York.
The proportion of TKRs among all arthroplasties performed in SLE patients increased from 16% in 1991 to 48% in 2005. Meanwhile, THRs decreased from 66% of all arthroplasties to 40%. Other joint replacements didn’t change much over time.
A particularly striking finding in the study was that the mean age at the time of arthroplasty in SLE patients increased by nearly a decade – 47.3 years in 1991 to 56.8 years in 2005. In contrast, the mean age at arthroplasty for osteoarthritis patients without SLE decreased from 71.5 to 69.0 years.
Dr. Goodman turned to additional studies by her research group and other investigators to provide a picture of arthroplasty outcomes in SLE patients.
In-hospital postoperative mortality was found to be increased in SLE patients, compared with rheumatoid arthritis patients or controls undergoing TKR or THR in a study of more than 1.5 million TKRs and THRs included in the Nationwide Inpatient Sample for 1993-2006. The Nationwide Inpatient Sample is the largest all-payer inpatient health care database in the United States.
In a multivariate logistic regression analysis adjusted for comorbidities, hospital type, and other potential confounders, investigators at Stanford (Calif.) University found that SLE patients undergoing THR had a 3.5-fold increased risk of death, compared with controls. This was driven by a 4.9-fold increased death risk in SLE patients undergoing nonelective THR or TKR, typically because of a fracture. In contrast, the rate of in-hospital death following TKR or THR in rheumatoid arthritis patients wasn’t significantly different from controls. The one comorbidity present on admission that was associated with markedly increased postoperative mortality was renal disease in SLE patients (J. Rheumatol. 2010;37:1467-72).
"Baseline renal dysfunction really seems to be a marker in lupus patients for bad perioperative outcome," Dr. Goodman observed, adding that there is a need for "increased vigilance" regarding this comorbidity.
A study of 57 SLE patients and 107 age-matched osteoarthritis patients who underwent THR at the Hospital for Special Surgery demonstrated that the lupus patients had more baseline comorbidities as reflected in their mean Charlson Comorbidity Index of 1.9, compared with 0.3 in the osteoarthritis group. Seventy-nine percent of the SLE patients, but none of the osteoarthritis patients, were on immunosuppressant therapy. The lupus patients had a 19% incidence of postoperative major adverse events, including deep vein thrombosis, arrhythmia, acute renal insufficiency, or additional surgery, compared with a 6% rate in the osteoarthritis group. The 6-day mean length of stay in the SLE group was a full day longer than that of the osteoarthritis patients, according to Dr. Goodman.
Another Hospital for Special Surgery study included 56 SLE patients undergoing THR and 45 with TKR, as well as 108 age-matched controls undergoing THR and 89 with TKR. The SLE patients had significantly worse baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores than did the osteoarthritis patients. At 2 years of follow-up, however, all four groups ended up with very good pain and function outcomes, with WOMAC scores in the 80-92 range.
Yet despite these excellent outcomes, the SLE patients still felt more limited by their chronic disease. This was reflected in their lower health-related quality of life on the SF-36 physical component summary score at 2 years of follow-up: 39 in the SLE THR patients, compared with 50.1 in the osteoarthritis THR group, and 38 in the SLE TKR patients, compared with 48.4 in the osteoarthritis controls.
This and other studies paint a picture of contemporary SLE patients undergoing TKR as more closely resembling osteoarthritis patients with TKR than SLE patients undergoing THR. The average age of the SLE TKR patients, at 62.4 years, was 8 years older than the SLE THR group. The SLE TKR group’s mean body mass index of 31.5 kg/m2 was 5 kg/m2 greater than in the SLE THR group. And none of the SLE TKR patients had avascular necrosis, compared with one-third of those undergoing THR.
Dr. Goodman reported having no relevant financial relationships.
SNOWMASS, COLO. – Total knee replacement surgery rates in patients with systemic lupus erythematosus jumped sixfold nationally during a recent 15-year span – and buried within this statistic is some very good news.
The sharp rise in total knee replacement (TKR) among systemic lupus erythematosus (SLE) patients has been driven by a hefty increase in operations performed for osteoarthritis, while TKR for active SLE in the knee has declined. These trends reflect the increased longevity of patients with SLE resulting from improved medical management. For the first time, large numbers of SLE patients are surviving to an age when they, like other Americans, are more vulnerable to osteoarthritis, Dr. Susan M. Goodman explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Lupus is no longer the highly mortal disease that it was," she observed. "Clearly, patients are surviving and – I don’t know how else to put it – they’ve become middle-aged women who are having knee replacements, which is kind of a success."
She presented highlights of a soon-to-be-published study involving analysis of 10 state databases including nearly 2.8 million arthroplasties performed during 1991-2005. The rate of TKR in SLE patients climbed sixfold from 0.03 per 100,000 population in 1991 to 0.18 per 100,000 in 2005. Meanwhile, total hip replacement (THR) in SLE patients showed a modest but statistically significant increase from 0.11 to 0.18 cases per 100,000.
The proportion of lupus patients undergoing arthroplasty for avascular necrosis fell from 53% in 1991 to 24% in 2005, while the proportion undergoing arthroplasty because they developed osteoarthritis went from 23% to 61%.
Virtually all of the increase in total arthroplasties among SLE patients occurred in women aged 45 years and older. Their rate more than tripled during the study years, going from 0.076 to 0.271 cases per 100,000. Rates in female SLE patients aged 44 years and younger actually took a significant drop from 0.073 to 0.067 per 100,000. Rates in males aged 44 and younger remained flat over time, while men aged 45 and up showed a modest increase from a low baseline rate of 0.009 cases per 100,000 in 1991 to 0.034 per 100,000 in 2005, according to Dr. Goodman, a rheumatologist at the Hospital for Special Surgery and Cornell University, New York.
The proportion of TKRs among all arthroplasties performed in SLE patients increased from 16% in 1991 to 48% in 2005. Meanwhile, THRs decreased from 66% of all arthroplasties to 40%. Other joint replacements didn’t change much over time.
A particularly striking finding in the study was that the mean age at the time of arthroplasty in SLE patients increased by nearly a decade – 47.3 years in 1991 to 56.8 years in 2005. In contrast, the mean age at arthroplasty for osteoarthritis patients without SLE decreased from 71.5 to 69.0 years.
Dr. Goodman turned to additional studies by her research group and other investigators to provide a picture of arthroplasty outcomes in SLE patients.
In-hospital postoperative mortality was found to be increased in SLE patients, compared with rheumatoid arthritis patients or controls undergoing TKR or THR in a study of more than 1.5 million TKRs and THRs included in the Nationwide Inpatient Sample for 1993-2006. The Nationwide Inpatient Sample is the largest all-payer inpatient health care database in the United States.
In a multivariate logistic regression analysis adjusted for comorbidities, hospital type, and other potential confounders, investigators at Stanford (Calif.) University found that SLE patients undergoing THR had a 3.5-fold increased risk of death, compared with controls. This was driven by a 4.9-fold increased death risk in SLE patients undergoing nonelective THR or TKR, typically because of a fracture. In contrast, the rate of in-hospital death following TKR or THR in rheumatoid arthritis patients wasn’t significantly different from controls. The one comorbidity present on admission that was associated with markedly increased postoperative mortality was renal disease in SLE patients (J. Rheumatol. 2010;37:1467-72).
"Baseline renal dysfunction really seems to be a marker in lupus patients for bad perioperative outcome," Dr. Goodman observed, adding that there is a need for "increased vigilance" regarding this comorbidity.
A study of 57 SLE patients and 107 age-matched osteoarthritis patients who underwent THR at the Hospital for Special Surgery demonstrated that the lupus patients had more baseline comorbidities as reflected in their mean Charlson Comorbidity Index of 1.9, compared with 0.3 in the osteoarthritis group. Seventy-nine percent of the SLE patients, but none of the osteoarthritis patients, were on immunosuppressant therapy. The lupus patients had a 19% incidence of postoperative major adverse events, including deep vein thrombosis, arrhythmia, acute renal insufficiency, or additional surgery, compared with a 6% rate in the osteoarthritis group. The 6-day mean length of stay in the SLE group was a full day longer than that of the osteoarthritis patients, according to Dr. Goodman.
Another Hospital for Special Surgery study included 56 SLE patients undergoing THR and 45 with TKR, as well as 108 age-matched controls undergoing THR and 89 with TKR. The SLE patients had significantly worse baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores than did the osteoarthritis patients. At 2 years of follow-up, however, all four groups ended up with very good pain and function outcomes, with WOMAC scores in the 80-92 range.
Yet despite these excellent outcomes, the SLE patients still felt more limited by their chronic disease. This was reflected in their lower health-related quality of life on the SF-36 physical component summary score at 2 years of follow-up: 39 in the SLE THR patients, compared with 50.1 in the osteoarthritis THR group, and 38 in the SLE TKR patients, compared with 48.4 in the osteoarthritis controls.
This and other studies paint a picture of contemporary SLE patients undergoing TKR as more closely resembling osteoarthritis patients with TKR than SLE patients undergoing THR. The average age of the SLE TKR patients, at 62.4 years, was 8 years older than the SLE THR group. The SLE TKR group’s mean body mass index of 31.5 kg/m2 was 5 kg/m2 greater than in the SLE THR group. And none of the SLE TKR patients had avascular necrosis, compared with one-third of those undergoing THR.
Dr. Goodman reported having no relevant financial relationships.
SNOWMASS, COLO. – Total knee replacement surgery rates in patients with systemic lupus erythematosus jumped sixfold nationally during a recent 15-year span – and buried within this statistic is some very good news.
The sharp rise in total knee replacement (TKR) among systemic lupus erythematosus (SLE) patients has been driven by a hefty increase in operations performed for osteoarthritis, while TKR for active SLE in the knee has declined. These trends reflect the increased longevity of patients with SLE resulting from improved medical management. For the first time, large numbers of SLE patients are surviving to an age when they, like other Americans, are more vulnerable to osteoarthritis, Dr. Susan M. Goodman explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Lupus is no longer the highly mortal disease that it was," she observed. "Clearly, patients are surviving and – I don’t know how else to put it – they’ve become middle-aged women who are having knee replacements, which is kind of a success."
She presented highlights of a soon-to-be-published study involving analysis of 10 state databases including nearly 2.8 million arthroplasties performed during 1991-2005. The rate of TKR in SLE patients climbed sixfold from 0.03 per 100,000 population in 1991 to 0.18 per 100,000 in 2005. Meanwhile, total hip replacement (THR) in SLE patients showed a modest but statistically significant increase from 0.11 to 0.18 cases per 100,000.
The proportion of lupus patients undergoing arthroplasty for avascular necrosis fell from 53% in 1991 to 24% in 2005, while the proportion undergoing arthroplasty because they developed osteoarthritis went from 23% to 61%.
Virtually all of the increase in total arthroplasties among SLE patients occurred in women aged 45 years and older. Their rate more than tripled during the study years, going from 0.076 to 0.271 cases per 100,000. Rates in female SLE patients aged 44 years and younger actually took a significant drop from 0.073 to 0.067 per 100,000. Rates in males aged 44 and younger remained flat over time, while men aged 45 and up showed a modest increase from a low baseline rate of 0.009 cases per 100,000 in 1991 to 0.034 per 100,000 in 2005, according to Dr. Goodman, a rheumatologist at the Hospital for Special Surgery and Cornell University, New York.
The proportion of TKRs among all arthroplasties performed in SLE patients increased from 16% in 1991 to 48% in 2005. Meanwhile, THRs decreased from 66% of all arthroplasties to 40%. Other joint replacements didn’t change much over time.
A particularly striking finding in the study was that the mean age at the time of arthroplasty in SLE patients increased by nearly a decade – 47.3 years in 1991 to 56.8 years in 2005. In contrast, the mean age at arthroplasty for osteoarthritis patients without SLE decreased from 71.5 to 69.0 years.
Dr. Goodman turned to additional studies by her research group and other investigators to provide a picture of arthroplasty outcomes in SLE patients.
In-hospital postoperative mortality was found to be increased in SLE patients, compared with rheumatoid arthritis patients or controls undergoing TKR or THR in a study of more than 1.5 million TKRs and THRs included in the Nationwide Inpatient Sample for 1993-2006. The Nationwide Inpatient Sample is the largest all-payer inpatient health care database in the United States.
In a multivariate logistic regression analysis adjusted for comorbidities, hospital type, and other potential confounders, investigators at Stanford (Calif.) University found that SLE patients undergoing THR had a 3.5-fold increased risk of death, compared with controls. This was driven by a 4.9-fold increased death risk in SLE patients undergoing nonelective THR or TKR, typically because of a fracture. In contrast, the rate of in-hospital death following TKR or THR in rheumatoid arthritis patients wasn’t significantly different from controls. The one comorbidity present on admission that was associated with markedly increased postoperative mortality was renal disease in SLE patients (J. Rheumatol. 2010;37:1467-72).
"Baseline renal dysfunction really seems to be a marker in lupus patients for bad perioperative outcome," Dr. Goodman observed, adding that there is a need for "increased vigilance" regarding this comorbidity.
A study of 57 SLE patients and 107 age-matched osteoarthritis patients who underwent THR at the Hospital for Special Surgery demonstrated that the lupus patients had more baseline comorbidities as reflected in their mean Charlson Comorbidity Index of 1.9, compared with 0.3 in the osteoarthritis group. Seventy-nine percent of the SLE patients, but none of the osteoarthritis patients, were on immunosuppressant therapy. The lupus patients had a 19% incidence of postoperative major adverse events, including deep vein thrombosis, arrhythmia, acute renal insufficiency, or additional surgery, compared with a 6% rate in the osteoarthritis group. The 6-day mean length of stay in the SLE group was a full day longer than that of the osteoarthritis patients, according to Dr. Goodman.
Another Hospital for Special Surgery study included 56 SLE patients undergoing THR and 45 with TKR, as well as 108 age-matched controls undergoing THR and 89 with TKR. The SLE patients had significantly worse baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores than did the osteoarthritis patients. At 2 years of follow-up, however, all four groups ended up with very good pain and function outcomes, with WOMAC scores in the 80-92 range.
Yet despite these excellent outcomes, the SLE patients still felt more limited by their chronic disease. This was reflected in their lower health-related quality of life on the SF-36 physical component summary score at 2 years of follow-up: 39 in the SLE THR patients, compared with 50.1 in the osteoarthritis THR group, and 38 in the SLE TKR patients, compared with 48.4 in the osteoarthritis controls.
This and other studies paint a picture of contemporary SLE patients undergoing TKR as more closely resembling osteoarthritis patients with TKR than SLE patients undergoing THR. The average age of the SLE TKR patients, at 62.4 years, was 8 years older than the SLE THR group. The SLE TKR group’s mean body mass index of 31.5 kg/m2 was 5 kg/m2 greater than in the SLE THR group. And none of the SLE TKR patients had avascular necrosis, compared with one-third of those undergoing THR.
Dr. Goodman reported having no relevant financial relationships.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
WOEST results guide antithrombotic therapy in PCI
SNOWMASS, COLO. – A recent randomized trial provides physicians with important new guidance on how to manage antithrombotic therapy in patients requiring oral anticoagulation who develop an acute coronary syndrome and undergo percutaneous coronary revascularization with stent implantation.
"I think this is one of the most important trials in cardiology published last year. I think it has to rank in the top five," Dr. Patrick T. O’Gara commented at the Annual Cardiovascular Conference at Snowmass.
The trial is WOEST, a Dutch/Belgian multicenter, randomized, open-label study in which 573 such patients were assigned to triple antithrombotic therapy with clopidogrel and aspirin on top of their background warfarin, or to dual therapy with warfarin and clopidogrel.
The primary outcome was the rate of bleeding during the first year following stent implantation. The rate was 19.4% in patients on double therapy and 44.4% in those on triple therapy, for a highly significant 64% reduction in relative risk favoring the less aggressive antithrombotic strategy. At least one blood transfusion was required during the follow-up period by 3.9% of patients receiving dual therapy, compared with 9.5% of patients on triple therapy (Lancet 2013;381:1107-15).
"The more than twofold excess risk of bleeding in patients treated with triple versus double antithrombotic therapy is not a surprise. What was a surprise in this particular study was that a secondary endpoint of death/MI/stroke/target vessel revascularization/stent thrombosis was also higher in the triple-therapy group," said Dr. O’Gara, professor of medicine at Harvard Medical School and director of clinical cardiology at Brigham and Women’s Hospital, Boston.
Indeed, the rate of this composite endpoint was 17.6% with triple therapy, compared with 11.1% with double therapy, for a 40% relative risk reduction.
"This study implies that the use of clopidogrel and a vitamin K antagonist is not only safer but actually might be more efficacious than a strategy of triple antithrombotic therapy following stent deployment," observed Dr. O’Gara, the American College of Cardiology (ACC) president-elect.
"Obviously this will need to be validated in other groups, and the sample size here is relatively small at under 600 patients, but this study has set the standard against which we need to design future trials and begin to make some clinical decisions. I think this gives us a great deal of cover with the use of clopidogrel plus warfarin after PCI [percutaneous coronary intervention] in patients, particularly in those in whom you think the risk of recurrent stroke is relatively low," according to the cardiologist.
Dr. O’Gara, who chairs the ACC/American Heart Association STEMI Guideline Writing Committee, predicted the committee will take a close look at WOEST when it meets this spring to adjudicate revisions in the 2013 guidelines.
WOEST was funded by Dutch and Belgian research foundations. Dr. O’Gara reported having no financial conflicts.
SNOWMASS, COLO. – A recent randomized trial provides physicians with important new guidance on how to manage antithrombotic therapy in patients requiring oral anticoagulation who develop an acute coronary syndrome and undergo percutaneous coronary revascularization with stent implantation.
"I think this is one of the most important trials in cardiology published last year. I think it has to rank in the top five," Dr. Patrick T. O’Gara commented at the Annual Cardiovascular Conference at Snowmass.
The trial is WOEST, a Dutch/Belgian multicenter, randomized, open-label study in which 573 such patients were assigned to triple antithrombotic therapy with clopidogrel and aspirin on top of their background warfarin, or to dual therapy with warfarin and clopidogrel.
The primary outcome was the rate of bleeding during the first year following stent implantation. The rate was 19.4% in patients on double therapy and 44.4% in those on triple therapy, for a highly significant 64% reduction in relative risk favoring the less aggressive antithrombotic strategy. At least one blood transfusion was required during the follow-up period by 3.9% of patients receiving dual therapy, compared with 9.5% of patients on triple therapy (Lancet 2013;381:1107-15).
"The more than twofold excess risk of bleeding in patients treated with triple versus double antithrombotic therapy is not a surprise. What was a surprise in this particular study was that a secondary endpoint of death/MI/stroke/target vessel revascularization/stent thrombosis was also higher in the triple-therapy group," said Dr. O’Gara, professor of medicine at Harvard Medical School and director of clinical cardiology at Brigham and Women’s Hospital, Boston.
Indeed, the rate of this composite endpoint was 17.6% with triple therapy, compared with 11.1% with double therapy, for a 40% relative risk reduction.
"This study implies that the use of clopidogrel and a vitamin K antagonist is not only safer but actually might be more efficacious than a strategy of triple antithrombotic therapy following stent deployment," observed Dr. O’Gara, the American College of Cardiology (ACC) president-elect.
"Obviously this will need to be validated in other groups, and the sample size here is relatively small at under 600 patients, but this study has set the standard against which we need to design future trials and begin to make some clinical decisions. I think this gives us a great deal of cover with the use of clopidogrel plus warfarin after PCI [percutaneous coronary intervention] in patients, particularly in those in whom you think the risk of recurrent stroke is relatively low," according to the cardiologist.
Dr. O’Gara, who chairs the ACC/American Heart Association STEMI Guideline Writing Committee, predicted the committee will take a close look at WOEST when it meets this spring to adjudicate revisions in the 2013 guidelines.
WOEST was funded by Dutch and Belgian research foundations. Dr. O’Gara reported having no financial conflicts.
SNOWMASS, COLO. – A recent randomized trial provides physicians with important new guidance on how to manage antithrombotic therapy in patients requiring oral anticoagulation who develop an acute coronary syndrome and undergo percutaneous coronary revascularization with stent implantation.
"I think this is one of the most important trials in cardiology published last year. I think it has to rank in the top five," Dr. Patrick T. O’Gara commented at the Annual Cardiovascular Conference at Snowmass.
The trial is WOEST, a Dutch/Belgian multicenter, randomized, open-label study in which 573 such patients were assigned to triple antithrombotic therapy with clopidogrel and aspirin on top of their background warfarin, or to dual therapy with warfarin and clopidogrel.
The primary outcome was the rate of bleeding during the first year following stent implantation. The rate was 19.4% in patients on double therapy and 44.4% in those on triple therapy, for a highly significant 64% reduction in relative risk favoring the less aggressive antithrombotic strategy. At least one blood transfusion was required during the follow-up period by 3.9% of patients receiving dual therapy, compared with 9.5% of patients on triple therapy (Lancet 2013;381:1107-15).
"The more than twofold excess risk of bleeding in patients treated with triple versus double antithrombotic therapy is not a surprise. What was a surprise in this particular study was that a secondary endpoint of death/MI/stroke/target vessel revascularization/stent thrombosis was also higher in the triple-therapy group," said Dr. O’Gara, professor of medicine at Harvard Medical School and director of clinical cardiology at Brigham and Women’s Hospital, Boston.
Indeed, the rate of this composite endpoint was 17.6% with triple therapy, compared with 11.1% with double therapy, for a 40% relative risk reduction.
"This study implies that the use of clopidogrel and a vitamin K antagonist is not only safer but actually might be more efficacious than a strategy of triple antithrombotic therapy following stent deployment," observed Dr. O’Gara, the American College of Cardiology (ACC) president-elect.
"Obviously this will need to be validated in other groups, and the sample size here is relatively small at under 600 patients, but this study has set the standard against which we need to design future trials and begin to make some clinical decisions. I think this gives us a great deal of cover with the use of clopidogrel plus warfarin after PCI [percutaneous coronary intervention] in patients, particularly in those in whom you think the risk of recurrent stroke is relatively low," according to the cardiologist.
Dr. O’Gara, who chairs the ACC/American Heart Association STEMI Guideline Writing Committee, predicted the committee will take a close look at WOEST when it meets this spring to adjudicate revisions in the 2013 guidelines.
WOEST was funded by Dutch and Belgian research foundations. Dr. O’Gara reported having no financial conflicts.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Approaching ventricular arrhythmias in a structurally normal heart
SNOWMASS, COLO. – Two generally benign forms of ventricular arrhythmia identifiable by their characteristic signatures on an electrocardiogram – and readily curable by catheter ablation – are ventricular tachycardia originating in the right ventricular outflow tract and premature ventricular contraction–induced cardiomyopathy, Dr. Samuel J. Asirvatham said at the Annual Cardiovascular Conference at Snowmass.
"I think these are two patterns of the ECG that you have to commit to memory. These are two patterns of ventricular tachycardia that are readily treatable, generally benign, and found in structurally normal hearts," said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
• Right ventricular outflow tract (RVOT) ventricular tachycardia (VT): This arrhythmia arises in patients with no coronary artery disease and no valvular disease – in short, no structural heart disease at all. It is often exercise-provoked in males, and can be either exercise-provoked or hormonally cyclic in females. The common symptoms include palpitations and dizziness.
Dr. Asirvatham provided three ECG clues to the diagnosis. One is a negative V1 lead, indicative of delayed left ventricular activation, as seen in left bundle branch block. The second clue is a strong inferior axis, with the II and III aVF leads being positive. The third giveaway is negative leads at I aVR and II aVL, which indicates the impulse originated in the right ventricle and is simultaneously moving away from these two superior leads.
"You can sometimes treat RVOT VT with beta-blockers or calcium channel blockers, but very often ablation is a better idea for these patients in order to save them from lifelong medications and breakthroughs. Usually if a single source in the ventricle is identified, ablation is a curative procedure," the cardiologist explained.
A caveat: The presence of a little R wave in V1 is a clue that the anatomic location of that patient’s premature ventricular contractions (PVCs) is likely going to make ablation challenging. The little R wave in V1 suggests the presence of a deep-tissue focus that needs to be targeted for ablation. It means the patient doesn’t have a complete left bundle branch block, perhaps because the origin of the impulse lies in remnants of embryologic conduction tissue located in the outflow tract, or in a sleeve of myocardium extending up the pulmonary artery. Addressing such a focus successfully via catheter ablation requires an advanced skill set.
• PVC-induced cardiomyopathy: This condition features PVCs plus runs of nonsustained VT and a depressed left-ventricular ejection fraction (LVEF).
"We have a classic chicken versus egg situation here: We know that when the heart is bad you get ventricular arrhythmias, but now we also understand that when you have ventricular arrhythmias it can make the heart bad. It’s our job to tease out which one is the primary culprit," according to Dr. Asirvatham.
The distinction is critical. If the arrhythmias are secondary to a bad heart, the patient should be directed down the pathway of heart failure management and possibly eventual transplantation. But if the cardiomyopathy is due to PVCs, it’s a condition that’s curable through ablation. In making the distinction, Dr. Asirvatham leans heavily on several factors: PVC morphology and coupling interval on the ECG, and total PVC burden over the course of 24 hours on Holter monitoring.
If the PVC morphology looks exactly the same beat after beat, chances are that the impulses are coming from one potentially ablatable spot, and that the ventricular dysfunction followed from the frequent PVCs. In contrast, if the impulses are multimorphic and arise from numerous locations, the likelihood is that the culprit is primary heart disease.
A VT rate in excess of 200 bpm is generally malignant, as is a coupling interval of less than 200 ms between the VT and the preceding normal QRS. Those, along with poly- or multimorphic VT, are worrisome features. In their absence, the key factor in determining whether PVCs are the cause of the patient’s cardiomyopathy or vice versa is how many PVCs there are.
"There’s no definite number, but in general it’s unusual to get cardiomyopathy in someone who has less than 10,000 PVCs per day. It’s more common when you get beyond 20,000 per day, and certainly beyond 30,000. Those are good numbers to keep in mind. The more PVCs you have per day, the more likely you are to get ventricular dysfunction. The corollary is also true: If ventricular dysfunction has occurred with frequent PVCs, once you get rid of the PVCs, in the vast majority of patients the ventricular function improves," according to the electrophysiologist.
On occasion, and with careful informed consent, it’s helpful to turn to empiric amiodarone to help sort out if a patient truly has PVC-induced cardiomyopathy.
"No one likes to prescribe amiodarone in a young patient with cardiomyopathy because amiodarone can produce bad things even when used for 3-6 months. But amiodarone is like an eraser for PVCs, and if, once the PVCs are gone, the ventricular function is still bad, then don’t waste time and put the patient at risk for the possibility of complications by doing a complex ablation. On the other hand, if, after giving amiodarone and the PVCs are gone the ventricular function normalizes, that’s very good evidence that ablation is worth the risk," he explained.
The results of catheter ablation of PVC-induced cardiomyopathy are often spectacular. Dr. Asirvatham recounted the story of a young adult patient whose LVEF had dropped to as low as 6%-8%. After ablation, it’s now 65%-plus and the patient is now an active surgical resident.
Dr. Asirvatham reported serving as a consultant to close to a dozen pharmaceutical and medical device companies.
SNOWMASS, COLO. – Two generally benign forms of ventricular arrhythmia identifiable by their characteristic signatures on an electrocardiogram – and readily curable by catheter ablation – are ventricular tachycardia originating in the right ventricular outflow tract and premature ventricular contraction–induced cardiomyopathy, Dr. Samuel J. Asirvatham said at the Annual Cardiovascular Conference at Snowmass.
"I think these are two patterns of the ECG that you have to commit to memory. These are two patterns of ventricular tachycardia that are readily treatable, generally benign, and found in structurally normal hearts," said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
• Right ventricular outflow tract (RVOT) ventricular tachycardia (VT): This arrhythmia arises in patients with no coronary artery disease and no valvular disease – in short, no structural heart disease at all. It is often exercise-provoked in males, and can be either exercise-provoked or hormonally cyclic in females. The common symptoms include palpitations and dizziness.
Dr. Asirvatham provided three ECG clues to the diagnosis. One is a negative V1 lead, indicative of delayed left ventricular activation, as seen in left bundle branch block. The second clue is a strong inferior axis, with the II and III aVF leads being positive. The third giveaway is negative leads at I aVR and II aVL, which indicates the impulse originated in the right ventricle and is simultaneously moving away from these two superior leads.
"You can sometimes treat RVOT VT with beta-blockers or calcium channel blockers, but very often ablation is a better idea for these patients in order to save them from lifelong medications and breakthroughs. Usually if a single source in the ventricle is identified, ablation is a curative procedure," the cardiologist explained.
A caveat: The presence of a little R wave in V1 is a clue that the anatomic location of that patient’s premature ventricular contractions (PVCs) is likely going to make ablation challenging. The little R wave in V1 suggests the presence of a deep-tissue focus that needs to be targeted for ablation. It means the patient doesn’t have a complete left bundle branch block, perhaps because the origin of the impulse lies in remnants of embryologic conduction tissue located in the outflow tract, or in a sleeve of myocardium extending up the pulmonary artery. Addressing such a focus successfully via catheter ablation requires an advanced skill set.
• PVC-induced cardiomyopathy: This condition features PVCs plus runs of nonsustained VT and a depressed left-ventricular ejection fraction (LVEF).
"We have a classic chicken versus egg situation here: We know that when the heart is bad you get ventricular arrhythmias, but now we also understand that when you have ventricular arrhythmias it can make the heart bad. It’s our job to tease out which one is the primary culprit," according to Dr. Asirvatham.
The distinction is critical. If the arrhythmias are secondary to a bad heart, the patient should be directed down the pathway of heart failure management and possibly eventual transplantation. But if the cardiomyopathy is due to PVCs, it’s a condition that’s curable through ablation. In making the distinction, Dr. Asirvatham leans heavily on several factors: PVC morphology and coupling interval on the ECG, and total PVC burden over the course of 24 hours on Holter monitoring.
If the PVC morphology looks exactly the same beat after beat, chances are that the impulses are coming from one potentially ablatable spot, and that the ventricular dysfunction followed from the frequent PVCs. In contrast, if the impulses are multimorphic and arise from numerous locations, the likelihood is that the culprit is primary heart disease.
A VT rate in excess of 200 bpm is generally malignant, as is a coupling interval of less than 200 ms between the VT and the preceding normal QRS. Those, along with poly- or multimorphic VT, are worrisome features. In their absence, the key factor in determining whether PVCs are the cause of the patient’s cardiomyopathy or vice versa is how many PVCs there are.
"There’s no definite number, but in general it’s unusual to get cardiomyopathy in someone who has less than 10,000 PVCs per day. It’s more common when you get beyond 20,000 per day, and certainly beyond 30,000. Those are good numbers to keep in mind. The more PVCs you have per day, the more likely you are to get ventricular dysfunction. The corollary is also true: If ventricular dysfunction has occurred with frequent PVCs, once you get rid of the PVCs, in the vast majority of patients the ventricular function improves," according to the electrophysiologist.
On occasion, and with careful informed consent, it’s helpful to turn to empiric amiodarone to help sort out if a patient truly has PVC-induced cardiomyopathy.
"No one likes to prescribe amiodarone in a young patient with cardiomyopathy because amiodarone can produce bad things even when used for 3-6 months. But amiodarone is like an eraser for PVCs, and if, once the PVCs are gone, the ventricular function is still bad, then don’t waste time and put the patient at risk for the possibility of complications by doing a complex ablation. On the other hand, if, after giving amiodarone and the PVCs are gone the ventricular function normalizes, that’s very good evidence that ablation is worth the risk," he explained.
The results of catheter ablation of PVC-induced cardiomyopathy are often spectacular. Dr. Asirvatham recounted the story of a young adult patient whose LVEF had dropped to as low as 6%-8%. After ablation, it’s now 65%-plus and the patient is now an active surgical resident.
Dr. Asirvatham reported serving as a consultant to close to a dozen pharmaceutical and medical device companies.
SNOWMASS, COLO. – Two generally benign forms of ventricular arrhythmia identifiable by their characteristic signatures on an electrocardiogram – and readily curable by catheter ablation – are ventricular tachycardia originating in the right ventricular outflow tract and premature ventricular contraction–induced cardiomyopathy, Dr. Samuel J. Asirvatham said at the Annual Cardiovascular Conference at Snowmass.
"I think these are two patterns of the ECG that you have to commit to memory. These are two patterns of ventricular tachycardia that are readily treatable, generally benign, and found in structurally normal hearts," said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
• Right ventricular outflow tract (RVOT) ventricular tachycardia (VT): This arrhythmia arises in patients with no coronary artery disease and no valvular disease – in short, no structural heart disease at all. It is often exercise-provoked in males, and can be either exercise-provoked or hormonally cyclic in females. The common symptoms include palpitations and dizziness.
Dr. Asirvatham provided three ECG clues to the diagnosis. One is a negative V1 lead, indicative of delayed left ventricular activation, as seen in left bundle branch block. The second clue is a strong inferior axis, with the II and III aVF leads being positive. The third giveaway is negative leads at I aVR and II aVL, which indicates the impulse originated in the right ventricle and is simultaneously moving away from these two superior leads.
"You can sometimes treat RVOT VT with beta-blockers or calcium channel blockers, but very often ablation is a better idea for these patients in order to save them from lifelong medications and breakthroughs. Usually if a single source in the ventricle is identified, ablation is a curative procedure," the cardiologist explained.
A caveat: The presence of a little R wave in V1 is a clue that the anatomic location of that patient’s premature ventricular contractions (PVCs) is likely going to make ablation challenging. The little R wave in V1 suggests the presence of a deep-tissue focus that needs to be targeted for ablation. It means the patient doesn’t have a complete left bundle branch block, perhaps because the origin of the impulse lies in remnants of embryologic conduction tissue located in the outflow tract, or in a sleeve of myocardium extending up the pulmonary artery. Addressing such a focus successfully via catheter ablation requires an advanced skill set.
• PVC-induced cardiomyopathy: This condition features PVCs plus runs of nonsustained VT and a depressed left-ventricular ejection fraction (LVEF).
"We have a classic chicken versus egg situation here: We know that when the heart is bad you get ventricular arrhythmias, but now we also understand that when you have ventricular arrhythmias it can make the heart bad. It’s our job to tease out which one is the primary culprit," according to Dr. Asirvatham.
The distinction is critical. If the arrhythmias are secondary to a bad heart, the patient should be directed down the pathway of heart failure management and possibly eventual transplantation. But if the cardiomyopathy is due to PVCs, it’s a condition that’s curable through ablation. In making the distinction, Dr. Asirvatham leans heavily on several factors: PVC morphology and coupling interval on the ECG, and total PVC burden over the course of 24 hours on Holter monitoring.
If the PVC morphology looks exactly the same beat after beat, chances are that the impulses are coming from one potentially ablatable spot, and that the ventricular dysfunction followed from the frequent PVCs. In contrast, if the impulses are multimorphic and arise from numerous locations, the likelihood is that the culprit is primary heart disease.
A VT rate in excess of 200 bpm is generally malignant, as is a coupling interval of less than 200 ms between the VT and the preceding normal QRS. Those, along with poly- or multimorphic VT, are worrisome features. In their absence, the key factor in determining whether PVCs are the cause of the patient’s cardiomyopathy or vice versa is how many PVCs there are.
"There’s no definite number, but in general it’s unusual to get cardiomyopathy in someone who has less than 10,000 PVCs per day. It’s more common when you get beyond 20,000 per day, and certainly beyond 30,000. Those are good numbers to keep in mind. The more PVCs you have per day, the more likely you are to get ventricular dysfunction. The corollary is also true: If ventricular dysfunction has occurred with frequent PVCs, once you get rid of the PVCs, in the vast majority of patients the ventricular function improves," according to the electrophysiologist.
On occasion, and with careful informed consent, it’s helpful to turn to empiric amiodarone to help sort out if a patient truly has PVC-induced cardiomyopathy.
"No one likes to prescribe amiodarone in a young patient with cardiomyopathy because amiodarone can produce bad things even when used for 3-6 months. But amiodarone is like an eraser for PVCs, and if, once the PVCs are gone, the ventricular function is still bad, then don’t waste time and put the patient at risk for the possibility of complications by doing a complex ablation. On the other hand, if, after giving amiodarone and the PVCs are gone the ventricular function normalizes, that’s very good evidence that ablation is worth the risk," he explained.
The results of catheter ablation of PVC-induced cardiomyopathy are often spectacular. Dr. Asirvatham recounted the story of a young adult patient whose LVEF had dropped to as low as 6%-8%. After ablation, it’s now 65%-plus and the patient is now an active surgical resident.
Dr. Asirvatham reported serving as a consultant to close to a dozen pharmaceutical and medical device companies.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
No benefit seen for investigational antiangiogenic agent in metastatic breast cancer
SAN ANTONIO – Adding the investigational antiangiogenesis agent ramucirumab to first-line chemotherapy did not significantly benefit metastatic breast cancer patients in a large phase III trial, Dr. John R. Mackey said at the San Antonio Breast Cancer Symposium.
The ROSE/TRIO-12 trial included 1,144 metastatic breast cancer patients in 25 countries who were randomized 2:1 to first-line docetaxel plus ramucirumab, a recombinant human IgG1 monoclonal antibody that binds to vascular endothelial growth factor receptor-2, or to docetaxel and placebo. After a median follow-up of 16.2 months, investigator-assessed progression-free survival was 9.5 months in the ramucirumab group and was not significantly different at 8.2 months in controls. No clinically defined subgroup showed a benefit with combination therapy, reported Dr. Mackey, professor of oncology at the University of Alberta, Edmonton, and director of Translational Research in Oncology (TRIO), which conducted the trial.
Median overall survival was 27.3 months with ramucirumab and 27.2 months in controls. However, the overall response rate, disease control rate, and time to progression were greater in the combined therapy group.
"We’re hopeful that we can go back to the tissue samples and find predictive biomarkers because there is a signal here. We are seeing improvements in several endpoints and some patients clearly are benefiting, but we do not understand the biology sufficiently well to be able to pick them out of the general population," he said.
Dr. Mackey noted that ramucirumab is the focus of a broad phase III clinical trial program. Definitive phase III studies of the antiangiogenesis agent in lung and colon cancer are underway. And two phase III trials in gastric cancer – REGARD and RAINBOW – have been completed with positive results for overall survival. "I think we can say that at least in another disease setting, this agent has activity."
Despite the negative results in ROSE/TRIO-12 plus the negative outcomes for trials of bevacizumab (Avastin), this is not the end of the line for antiangiogenesis agents in breast cancer, said conference codirector Dr. Peter Ravdin of the University of Texas, San Antonio. "Breast cancer is a really diverse disease. Drugs that may on average have little value in breast cancer may be very effective in selected populations."
The ROSE study was funded by Eli Lilly. Dr. Mackey declared having no financial conflicts of interest.
SAN ANTONIO – Adding the investigational antiangiogenesis agent ramucirumab to first-line chemotherapy did not significantly benefit metastatic breast cancer patients in a large phase III trial, Dr. John R. Mackey said at the San Antonio Breast Cancer Symposium.
The ROSE/TRIO-12 trial included 1,144 metastatic breast cancer patients in 25 countries who were randomized 2:1 to first-line docetaxel plus ramucirumab, a recombinant human IgG1 monoclonal antibody that binds to vascular endothelial growth factor receptor-2, or to docetaxel and placebo. After a median follow-up of 16.2 months, investigator-assessed progression-free survival was 9.5 months in the ramucirumab group and was not significantly different at 8.2 months in controls. No clinically defined subgroup showed a benefit with combination therapy, reported Dr. Mackey, professor of oncology at the University of Alberta, Edmonton, and director of Translational Research in Oncology (TRIO), which conducted the trial.
Median overall survival was 27.3 months with ramucirumab and 27.2 months in controls. However, the overall response rate, disease control rate, and time to progression were greater in the combined therapy group.
"We’re hopeful that we can go back to the tissue samples and find predictive biomarkers because there is a signal here. We are seeing improvements in several endpoints and some patients clearly are benefiting, but we do not understand the biology sufficiently well to be able to pick them out of the general population," he said.
Dr. Mackey noted that ramucirumab is the focus of a broad phase III clinical trial program. Definitive phase III studies of the antiangiogenesis agent in lung and colon cancer are underway. And two phase III trials in gastric cancer – REGARD and RAINBOW – have been completed with positive results for overall survival. "I think we can say that at least in another disease setting, this agent has activity."
Despite the negative results in ROSE/TRIO-12 plus the negative outcomes for trials of bevacizumab (Avastin), this is not the end of the line for antiangiogenesis agents in breast cancer, said conference codirector Dr. Peter Ravdin of the University of Texas, San Antonio. "Breast cancer is a really diverse disease. Drugs that may on average have little value in breast cancer may be very effective in selected populations."
The ROSE study was funded by Eli Lilly. Dr. Mackey declared having no financial conflicts of interest.
SAN ANTONIO – Adding the investigational antiangiogenesis agent ramucirumab to first-line chemotherapy did not significantly benefit metastatic breast cancer patients in a large phase III trial, Dr. John R. Mackey said at the San Antonio Breast Cancer Symposium.
The ROSE/TRIO-12 trial included 1,144 metastatic breast cancer patients in 25 countries who were randomized 2:1 to first-line docetaxel plus ramucirumab, a recombinant human IgG1 monoclonal antibody that binds to vascular endothelial growth factor receptor-2, or to docetaxel and placebo. After a median follow-up of 16.2 months, investigator-assessed progression-free survival was 9.5 months in the ramucirumab group and was not significantly different at 8.2 months in controls. No clinically defined subgroup showed a benefit with combination therapy, reported Dr. Mackey, professor of oncology at the University of Alberta, Edmonton, and director of Translational Research in Oncology (TRIO), which conducted the trial.
Median overall survival was 27.3 months with ramucirumab and 27.2 months in controls. However, the overall response rate, disease control rate, and time to progression were greater in the combined therapy group.
"We’re hopeful that we can go back to the tissue samples and find predictive biomarkers because there is a signal here. We are seeing improvements in several endpoints and some patients clearly are benefiting, but we do not understand the biology sufficiently well to be able to pick them out of the general population," he said.
Dr. Mackey noted that ramucirumab is the focus of a broad phase III clinical trial program. Definitive phase III studies of the antiangiogenesis agent in lung and colon cancer are underway. And two phase III trials in gastric cancer – REGARD and RAINBOW – have been completed with positive results for overall survival. "I think we can say that at least in another disease setting, this agent has activity."
Despite the negative results in ROSE/TRIO-12 plus the negative outcomes for trials of bevacizumab (Avastin), this is not the end of the line for antiangiogenesis agents in breast cancer, said conference codirector Dr. Peter Ravdin of the University of Texas, San Antonio. "Breast cancer is a really diverse disease. Drugs that may on average have little value in breast cancer may be very effective in selected populations."
The ROSE study was funded by Eli Lilly. Dr. Mackey declared having no financial conflicts of interest.
AT SABCS 2013
Major finding: Metastatic breast cancer patients randomized to docetaxel plus ramucirumab had a median progression-free survival of 9.5 months, not significantly better than the 8.2 months with docetaxel plus placebo.
Data source: The ROSE/TRIO-12 trial included 1,444 patients with metastatic breast cancer who were randomized 2:1 to first-line docetaxel plus ramucirumab or placebo.
Disclosures: The ROSE trial was funded by Eli Lilly. The presenter reported having no financial conflicts.
CRT shown highly cost effective in mild heart failure
DALLAS – Cardiac resynchronization therapy demonstrated a robust cost-effectiveness of $18,275 per quality-adjusted life-year gained in an analysis from the landmark REVERSE trial.
REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) was the first large, international, randomized, double-blind clinical trial to establish that cardiac resynchronization therapy (CRT) is clinically beneficial in patients with mild heart failure (J. Am. Coll. Cardiol. 2008;52:1834-43). The incremental cost-effectiveness ratio (ICER) of $18,275 per quality-adjusted life-year (QALY) gained in the new secondary analysis is well under the figure of $50,000 per QALY gained generally accepted as the benchmark defining cost-effectiveness, Dr. Michael R. Gold noted at the American Heart Association scientific sessions.
He presented an analysis based on an economic model that utilized 5-year follow-up data from REVERSE to predict the impact of CRT on life-years and costs out to 10 years. REVERSE included 610 patients with New York Heart Association (NYHA) class I or II heart failure, a QRS interval of 120 milliseconds or more, and a left ventricular ejection fraction of 40% or less, all of whom were on optimal medical therapy. Participants were implanted with a CRT device, which was placed in either the on or off position for the first year in North American patients or the first 2 years in Europeans, after which everyone’s device was switched on for the remainder of follow-up.
The model projected that patients with CRT turned on would gain an extra 0.83 years of life compared with those without active CRT. The ICER ratio of $18,275 per QALY gained through CRT was determined by applying costs based on national Medicare rates to the reduced heart failure hospitalization rate resulting from the device therapy, explained Dr. Gold, professor of medicine and director of cardiology at the Medical University of South Carolina, Charleston.
CRT was found to be highly cost effective in all patient subgroups examined in the analysis. For example, the projected ICER with cardiac resynchronization therapy switched on in patients with heart failure of ischemic etiology was $15,648 per QALY. In those with left bundle branch block, it was $22,086 per QALY.
Significantly fewer patients were projected to progress to NYHA class III heart failure over the course of 10 years with CRT on than off, by a margin of 18.7% to 28.1%. From the 5-year follow-up data, it was estimated that at 10 years 31% of the CRT-on group would be in NYHA class I, compared with 22.3% of controls with CRT off.
A combined CRT/implantable cardioverter-defibrillator (CRT-D) with both functions switched on had an ICER of $13,050 per QALY gained compared with CRT only.
On the basis of the results of REVERSE and the Resynchronization/Defibrillation in Ambulatory Heart Failure Trial (RAFT, N. Engl. J. Med. 2010;363:2385-95), in 2012 the Food and Drug Administration expanded the indication for Medtronic’s CRT-D device to include the treatment of patients with NYHA class II heart failure with a left ventricular ejection fraction of 30% or less, left bundle branch block, and a QRS duration of at least 130 milliseconds. The new secondary analysis of REVERSE demonstrates that CRT is not only clinically effective in patients with mild heart failure, as recognized by the FDA, it is highly cost effective as well, according to Dr. Gold.
The REVERSE trial was sponsored by Medtronic. Dr. Gold is a consultant to Medtronic and Boston Scientific.
DALLAS – Cardiac resynchronization therapy demonstrated a robust cost-effectiveness of $18,275 per quality-adjusted life-year gained in an analysis from the landmark REVERSE trial.
REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) was the first large, international, randomized, double-blind clinical trial to establish that cardiac resynchronization therapy (CRT) is clinically beneficial in patients with mild heart failure (J. Am. Coll. Cardiol. 2008;52:1834-43). The incremental cost-effectiveness ratio (ICER) of $18,275 per quality-adjusted life-year (QALY) gained in the new secondary analysis is well under the figure of $50,000 per QALY gained generally accepted as the benchmark defining cost-effectiveness, Dr. Michael R. Gold noted at the American Heart Association scientific sessions.
He presented an analysis based on an economic model that utilized 5-year follow-up data from REVERSE to predict the impact of CRT on life-years and costs out to 10 years. REVERSE included 610 patients with New York Heart Association (NYHA) class I or II heart failure, a QRS interval of 120 milliseconds or more, and a left ventricular ejection fraction of 40% or less, all of whom were on optimal medical therapy. Participants were implanted with a CRT device, which was placed in either the on or off position for the first year in North American patients or the first 2 years in Europeans, after which everyone’s device was switched on for the remainder of follow-up.
The model projected that patients with CRT turned on would gain an extra 0.83 years of life compared with those without active CRT. The ICER ratio of $18,275 per QALY gained through CRT was determined by applying costs based on national Medicare rates to the reduced heart failure hospitalization rate resulting from the device therapy, explained Dr. Gold, professor of medicine and director of cardiology at the Medical University of South Carolina, Charleston.
CRT was found to be highly cost effective in all patient subgroups examined in the analysis. For example, the projected ICER with cardiac resynchronization therapy switched on in patients with heart failure of ischemic etiology was $15,648 per QALY. In those with left bundle branch block, it was $22,086 per QALY.
Significantly fewer patients were projected to progress to NYHA class III heart failure over the course of 10 years with CRT on than off, by a margin of 18.7% to 28.1%. From the 5-year follow-up data, it was estimated that at 10 years 31% of the CRT-on group would be in NYHA class I, compared with 22.3% of controls with CRT off.
A combined CRT/implantable cardioverter-defibrillator (CRT-D) with both functions switched on had an ICER of $13,050 per QALY gained compared with CRT only.
On the basis of the results of REVERSE and the Resynchronization/Defibrillation in Ambulatory Heart Failure Trial (RAFT, N. Engl. J. Med. 2010;363:2385-95), in 2012 the Food and Drug Administration expanded the indication for Medtronic’s CRT-D device to include the treatment of patients with NYHA class II heart failure with a left ventricular ejection fraction of 30% or less, left bundle branch block, and a QRS duration of at least 130 milliseconds. The new secondary analysis of REVERSE demonstrates that CRT is not only clinically effective in patients with mild heart failure, as recognized by the FDA, it is highly cost effective as well, according to Dr. Gold.
The REVERSE trial was sponsored by Medtronic. Dr. Gold is a consultant to Medtronic and Boston Scientific.
DALLAS – Cardiac resynchronization therapy demonstrated a robust cost-effectiveness of $18,275 per quality-adjusted life-year gained in an analysis from the landmark REVERSE trial.
REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) was the first large, international, randomized, double-blind clinical trial to establish that cardiac resynchronization therapy (CRT) is clinically beneficial in patients with mild heart failure (J. Am. Coll. Cardiol. 2008;52:1834-43). The incremental cost-effectiveness ratio (ICER) of $18,275 per quality-adjusted life-year (QALY) gained in the new secondary analysis is well under the figure of $50,000 per QALY gained generally accepted as the benchmark defining cost-effectiveness, Dr. Michael R. Gold noted at the American Heart Association scientific sessions.
He presented an analysis based on an economic model that utilized 5-year follow-up data from REVERSE to predict the impact of CRT on life-years and costs out to 10 years. REVERSE included 610 patients with New York Heart Association (NYHA) class I or II heart failure, a QRS interval of 120 milliseconds or more, and a left ventricular ejection fraction of 40% or less, all of whom were on optimal medical therapy. Participants were implanted with a CRT device, which was placed in either the on or off position for the first year in North American patients or the first 2 years in Europeans, after which everyone’s device was switched on for the remainder of follow-up.
The model projected that patients with CRT turned on would gain an extra 0.83 years of life compared with those without active CRT. The ICER ratio of $18,275 per QALY gained through CRT was determined by applying costs based on national Medicare rates to the reduced heart failure hospitalization rate resulting from the device therapy, explained Dr. Gold, professor of medicine and director of cardiology at the Medical University of South Carolina, Charleston.
CRT was found to be highly cost effective in all patient subgroups examined in the analysis. For example, the projected ICER with cardiac resynchronization therapy switched on in patients with heart failure of ischemic etiology was $15,648 per QALY. In those with left bundle branch block, it was $22,086 per QALY.
Significantly fewer patients were projected to progress to NYHA class III heart failure over the course of 10 years with CRT on than off, by a margin of 18.7% to 28.1%. From the 5-year follow-up data, it was estimated that at 10 years 31% of the CRT-on group would be in NYHA class I, compared with 22.3% of controls with CRT off.
A combined CRT/implantable cardioverter-defibrillator (CRT-D) with both functions switched on had an ICER of $13,050 per QALY gained compared with CRT only.
On the basis of the results of REVERSE and the Resynchronization/Defibrillation in Ambulatory Heart Failure Trial (RAFT, N. Engl. J. Med. 2010;363:2385-95), in 2012 the Food and Drug Administration expanded the indication for Medtronic’s CRT-D device to include the treatment of patients with NYHA class II heart failure with a left ventricular ejection fraction of 30% or less, left bundle branch block, and a QRS duration of at least 130 milliseconds. The new secondary analysis of REVERSE demonstrates that CRT is not only clinically effective in patients with mild heart failure, as recognized by the FDA, it is highly cost effective as well, according to Dr. Gold.
The REVERSE trial was sponsored by Medtronic. Dr. Gold is a consultant to Medtronic and Boston Scientific.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Implantation of a cardiac resynchronization therapy device in patients with mild heart failure on optimal medical therapy had a projected cost-effectiveness of $18,275 per quality-adjusted life-year gained, compared with controls on optimal medical therapy alone.
Data source: This cost-benefit analysis utilized 5-year follow-up data from the randomized, double-blind prospective REVERSE trial, in which 610 patients with mild heart failure were implanted with a cardiac resynchronization therapy device placed in the on or off position.
Disclosures: The study was sponsored by Medtronic. The presenter is a consultant to Medtronic and Boston Scientific.
Postop atrial fib has two peaks
DALLAS – New-onset postoperative atrial fibrillation following cardiac surgery occurs in two distinct phases, with different risk factors for each.
That’s the key finding in an analysis of the recent experience at the University of Alabama, Birmingham, where new-onset postoperative AF occurred in 27% of 1,583 consecutive patients who underwent coronary artery bypass graft and/or valve surgery.
The first peak in the onset of postoperative AF occurred in the first 3 hours after surgery. This was followed by a sharp decline in incidence over the next 24 hours. The second peak happened at postoperative day 2, followed by a more gradual tail off, Dr. Spencer J. Melby reported at the American Heart Association scientific sessions.
Ninety-six percent of all cases of postoperative AF in this retrospective chart review began within the first 7 days after surgery. Of the 423 patients who developed this common and vexing condition, 16% did so within the first 24 hours, 48% had their onset at 24-72 hours, and 36% did so after 72 hours, according to Dr. Melby, a cardiothoracic surgeon at the university.
In a multivariate analysis, the most potent risk factor for onset of postoperative AF during the first phase was mitral valve repair or replacement, which was associated with a 2.5-fold increased risk. The other significant risk factors for early-onset AF were older age – with 70-year-olds having a 1.6-fold greater risk than 50-year-olds – and longer ischemic time, with patients whose cross-clamp time was 70 minutes having a 1.3-fold greater risk compared with those who had a cross-clamp time of 35 minutes.
Advanced age was also a risk factor for late-phase onset of AF, but it was a stronger risk factor there than for early onset AF, with 70-year-olds being at threefold greater risk than 50-year-olds – nearly twice as great a risk as for early-onset postoperative AF. The other significant risk factors for late-phase postoperative AF in a multivariate analysis were white race, with a 1.9-fold increased risk, and heavier body weight, with patients weighing 100 kg being at 1.6-fold greater risk than those tipping the scales at 70 kg.
"We believe that these different risk factors at each time phase are indicative of change over time in the mechanisms that drive postoperative atrial fibrillation," the surgeon said.
The first peak may be the result of tissue trauma, which is typically greater with more complex operations having longer cross-clamp times. And mitral valve procedures often entail contact with the left atrium.
"We’ve found that in patients with onset in the second peak there’s a polymorphonuclear leukocyte riot going on in the pericardial space after surgery. Inflammation is high, oxidative stress is high. Troponin levels are extremely high in the pericardial fluid, so there are problems in the heart muscle itself. The body is trying to heal and may be overdoing it as reflected by the inflammation in that space," Dr. Melby explained.
Dr. Brendan M. Everett, session cochair, noted that postoperative day 2 is when a host of process-of-care issues potentially relevant to postoperative AF come into play.
"Postop day 2 is when pain control begins to slip a bit. Patients are mobilized, chest tubes are pulled. A lot of factors are going on that are very important overall to getting a patient up and out of the hospital but are serious stimuli – adrenergic and otherwise – that may force a patient to go into atrial fibrillation," observed Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
Audience members praised Dr. Melby’s study as having "tremendous implications" for clinical practice, especially with regard to patient management guidelines. Dr. Melby noted that postoperative AF is a costly matter: It increases hospital length of stay by 1-2 days at a cost of roughly $10,000 per episode. Nationally, postoperative AF costs $4 billion per year.
Dr. Melby reported having no financial conflicts regarding his study.
DALLAS – New-onset postoperative atrial fibrillation following cardiac surgery occurs in two distinct phases, with different risk factors for each.
That’s the key finding in an analysis of the recent experience at the University of Alabama, Birmingham, where new-onset postoperative AF occurred in 27% of 1,583 consecutive patients who underwent coronary artery bypass graft and/or valve surgery.
The first peak in the onset of postoperative AF occurred in the first 3 hours after surgery. This was followed by a sharp decline in incidence over the next 24 hours. The second peak happened at postoperative day 2, followed by a more gradual tail off, Dr. Spencer J. Melby reported at the American Heart Association scientific sessions.
Ninety-six percent of all cases of postoperative AF in this retrospective chart review began within the first 7 days after surgery. Of the 423 patients who developed this common and vexing condition, 16% did so within the first 24 hours, 48% had their onset at 24-72 hours, and 36% did so after 72 hours, according to Dr. Melby, a cardiothoracic surgeon at the university.
In a multivariate analysis, the most potent risk factor for onset of postoperative AF during the first phase was mitral valve repair or replacement, which was associated with a 2.5-fold increased risk. The other significant risk factors for early-onset AF were older age – with 70-year-olds having a 1.6-fold greater risk than 50-year-olds – and longer ischemic time, with patients whose cross-clamp time was 70 minutes having a 1.3-fold greater risk compared with those who had a cross-clamp time of 35 minutes.
Advanced age was also a risk factor for late-phase onset of AF, but it was a stronger risk factor there than for early onset AF, with 70-year-olds being at threefold greater risk than 50-year-olds – nearly twice as great a risk as for early-onset postoperative AF. The other significant risk factors for late-phase postoperative AF in a multivariate analysis were white race, with a 1.9-fold increased risk, and heavier body weight, with patients weighing 100 kg being at 1.6-fold greater risk than those tipping the scales at 70 kg.
"We believe that these different risk factors at each time phase are indicative of change over time in the mechanisms that drive postoperative atrial fibrillation," the surgeon said.
The first peak may be the result of tissue trauma, which is typically greater with more complex operations having longer cross-clamp times. And mitral valve procedures often entail contact with the left atrium.
"We’ve found that in patients with onset in the second peak there’s a polymorphonuclear leukocyte riot going on in the pericardial space after surgery. Inflammation is high, oxidative stress is high. Troponin levels are extremely high in the pericardial fluid, so there are problems in the heart muscle itself. The body is trying to heal and may be overdoing it as reflected by the inflammation in that space," Dr. Melby explained.
Dr. Brendan M. Everett, session cochair, noted that postoperative day 2 is when a host of process-of-care issues potentially relevant to postoperative AF come into play.
"Postop day 2 is when pain control begins to slip a bit. Patients are mobilized, chest tubes are pulled. A lot of factors are going on that are very important overall to getting a patient up and out of the hospital but are serious stimuli – adrenergic and otherwise – that may force a patient to go into atrial fibrillation," observed Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
Audience members praised Dr. Melby’s study as having "tremendous implications" for clinical practice, especially with regard to patient management guidelines. Dr. Melby noted that postoperative AF is a costly matter: It increases hospital length of stay by 1-2 days at a cost of roughly $10,000 per episode. Nationally, postoperative AF costs $4 billion per year.
Dr. Melby reported having no financial conflicts regarding his study.
DALLAS – New-onset postoperative atrial fibrillation following cardiac surgery occurs in two distinct phases, with different risk factors for each.
That’s the key finding in an analysis of the recent experience at the University of Alabama, Birmingham, where new-onset postoperative AF occurred in 27% of 1,583 consecutive patients who underwent coronary artery bypass graft and/or valve surgery.
The first peak in the onset of postoperative AF occurred in the first 3 hours after surgery. This was followed by a sharp decline in incidence over the next 24 hours. The second peak happened at postoperative day 2, followed by a more gradual tail off, Dr. Spencer J. Melby reported at the American Heart Association scientific sessions.
Ninety-six percent of all cases of postoperative AF in this retrospective chart review began within the first 7 days after surgery. Of the 423 patients who developed this common and vexing condition, 16% did so within the first 24 hours, 48% had their onset at 24-72 hours, and 36% did so after 72 hours, according to Dr. Melby, a cardiothoracic surgeon at the university.
In a multivariate analysis, the most potent risk factor for onset of postoperative AF during the first phase was mitral valve repair or replacement, which was associated with a 2.5-fold increased risk. The other significant risk factors for early-onset AF were older age – with 70-year-olds having a 1.6-fold greater risk than 50-year-olds – and longer ischemic time, with patients whose cross-clamp time was 70 minutes having a 1.3-fold greater risk compared with those who had a cross-clamp time of 35 minutes.
Advanced age was also a risk factor for late-phase onset of AF, but it was a stronger risk factor there than for early onset AF, with 70-year-olds being at threefold greater risk than 50-year-olds – nearly twice as great a risk as for early-onset postoperative AF. The other significant risk factors for late-phase postoperative AF in a multivariate analysis were white race, with a 1.9-fold increased risk, and heavier body weight, with patients weighing 100 kg being at 1.6-fold greater risk than those tipping the scales at 70 kg.
"We believe that these different risk factors at each time phase are indicative of change over time in the mechanisms that drive postoperative atrial fibrillation," the surgeon said.
The first peak may be the result of tissue trauma, which is typically greater with more complex operations having longer cross-clamp times. And mitral valve procedures often entail contact with the left atrium.
"We’ve found that in patients with onset in the second peak there’s a polymorphonuclear leukocyte riot going on in the pericardial space after surgery. Inflammation is high, oxidative stress is high. Troponin levels are extremely high in the pericardial fluid, so there are problems in the heart muscle itself. The body is trying to heal and may be overdoing it as reflected by the inflammation in that space," Dr. Melby explained.
Dr. Brendan M. Everett, session cochair, noted that postoperative day 2 is when a host of process-of-care issues potentially relevant to postoperative AF come into play.
"Postop day 2 is when pain control begins to slip a bit. Patients are mobilized, chest tubes are pulled. A lot of factors are going on that are very important overall to getting a patient up and out of the hospital but are serious stimuli – adrenergic and otherwise – that may force a patient to go into atrial fibrillation," observed Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
Audience members praised Dr. Melby’s study as having "tremendous implications" for clinical practice, especially with regard to patient management guidelines. Dr. Melby noted that postoperative AF is a costly matter: It increases hospital length of stay by 1-2 days at a cost of roughly $10,000 per episode. Nationally, postoperative AF costs $4 billion per year.
Dr. Melby reported having no financial conflicts regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: The risk of new-onset postoperative atrial fibrillation is not constant over time; it peaks within the first 3 hours after surgery, falls off, then peaks again at postop day 2.
Data source: A retrospective chart review of 1,583 cardiac surgery patients at a single institution.
Disclosures: The presenter reported having no relevant financial interests.