User login
Denosumab Shows Favorable Results in FREEDOM Extension
HOUSTON – Denosumab continued to achieve reduced fractures rates, progressively rising bone mineral density, and sustained reduction in bone turnover biomarkers through 6 years of continuous use in the ongoing 7-year extension of the landmark FREEDOM trial.
FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) was the pivotal phase III, double-blind, placebo-controlled, 3-year randomized trial that led to marketing approval of denosumab (Prolia) at 60 mg subcutaneously every 6 months for treatment of postmenopausal osteoporosis (N. Engl. J. Med. 2009;361:756-65).
The FREEDOM extension study is evaluating the long-term safety and efficacy of denosumab for an additional 7 years. Thus, participants who were in the active treatment arm of the original study will receive denosumab for a total of 10 years, whereas patients who crossed over to denosumab after 3 years on placebo will be on the novel RANKL (receptor-activated nuclear factor–kappaB ligand) inhibitor for 7 years.
At the conference, Dr. Henry G. Bone presented updated study results through 6 years. In all, 4,550 postmenopausal women from the original randomized trial were enrolled in the extension and have been followed for an additional 3 years.
In the original FREEDOM trial, 3 years of denosumab resulted in a 10.1% increase in BMD in the lumbar spine, compared with baseline. With an additional 3 years of open-label therapy, this figure has increased to 15.1%. The gain in the crossover group paralleled that seen with active therapy in the original trial; that is, after 6 years – the last 3 on open-label denosumab – those patients had a 9.4% increase over baseline in lumbar spine BMD, reported Dr. Bone, chief of the endocrinology department at St. John Hospital and Medical Center, Detroit, and director of the Michigan Bone and Mineral Clinic.
Total hip BMD rose from a 5.7% increase over baseline after 3 years of denosumab to a 7.5% increase after 6 years. The crossover group had a 4.8% increase at this site after 3 years of active therapy.
During the first 3 years of the FREEDOM trial, new vertebral fractures occurred in 2.3% of the denosumab group, compared with 7.2% of placebo-treated controls. During the subsequent 3 years of open-label therapy, the original denosumab cohort had a 3.5% incidence of new vertebral fractures. Because it would have been unethical to have a placebo arm during those second 3 years, statistical modeling using a method known as "virtual twins" was used to project outcomes had the original control group remained on placebo. Their expected rate of new vertebral fractures during the first 3 years of the extension study was 6.3%.
The incidence of nonvertebral fractures during the 3-year, double-blind phase of FREEDOM was 8.0% in the placebo arm and significantly lower, at 6.5%, in the denosumab group. During the second 3-year period of denosumab therapy, the incidence was 3.8%.
"It’s interesting that the nonvertebral fracture rate in the second 3 years is actually quite a bit lower than in the first 3 years, suggesting there may be an additional benefit of long treatment," Dr. Bone said.
The projected rate of new nonvertebral fractures during years 4-6 in the "virtual twins" was 7.5%.
Turning to safety issues, the endocrinologist said that there were two adjudicated cases of osteonecrosis of the jaw during 6 years of denosumab therapy, and two more in the control group that occurred after crossover to active treatment. There has been one documented case of atypical femoral fracture during 6 consecutive years on denosumab. There has been one serious skin infection in the control group after they were crossed to 3 years of denosumab, and several more during years 4-6 in the long-term extension group.
The FREEDOM study extension is sponsored by Amgen. The presenter is a consultant to and on the speakers bureau for the company.
Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months, Prolia, postmenopausal osteoporosis, RANKL, receptor-activated nuclear factor–kappaB ligand inhibitor, Dr. Henry G. Bone, postmenopausal women,
HOUSTON – Denosumab continued to achieve reduced fractures rates, progressively rising bone mineral density, and sustained reduction in bone turnover biomarkers through 6 years of continuous use in the ongoing 7-year extension of the landmark FREEDOM trial.
FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) was the pivotal phase III, double-blind, placebo-controlled, 3-year randomized trial that led to marketing approval of denosumab (Prolia) at 60 mg subcutaneously every 6 months for treatment of postmenopausal osteoporosis (N. Engl. J. Med. 2009;361:756-65).
The FREEDOM extension study is evaluating the long-term safety and efficacy of denosumab for an additional 7 years. Thus, participants who were in the active treatment arm of the original study will receive denosumab for a total of 10 years, whereas patients who crossed over to denosumab after 3 years on placebo will be on the novel RANKL (receptor-activated nuclear factor–kappaB ligand) inhibitor for 7 years.
At the conference, Dr. Henry G. Bone presented updated study results through 6 years. In all, 4,550 postmenopausal women from the original randomized trial were enrolled in the extension and have been followed for an additional 3 years.
In the original FREEDOM trial, 3 years of denosumab resulted in a 10.1% increase in BMD in the lumbar spine, compared with baseline. With an additional 3 years of open-label therapy, this figure has increased to 15.1%. The gain in the crossover group paralleled that seen with active therapy in the original trial; that is, after 6 years – the last 3 on open-label denosumab – those patients had a 9.4% increase over baseline in lumbar spine BMD, reported Dr. Bone, chief of the endocrinology department at St. John Hospital and Medical Center, Detroit, and director of the Michigan Bone and Mineral Clinic.
Total hip BMD rose from a 5.7% increase over baseline after 3 years of denosumab to a 7.5% increase after 6 years. The crossover group had a 4.8% increase at this site after 3 years of active therapy.
During the first 3 years of the FREEDOM trial, new vertebral fractures occurred in 2.3% of the denosumab group, compared with 7.2% of placebo-treated controls. During the subsequent 3 years of open-label therapy, the original denosumab cohort had a 3.5% incidence of new vertebral fractures. Because it would have been unethical to have a placebo arm during those second 3 years, statistical modeling using a method known as "virtual twins" was used to project outcomes had the original control group remained on placebo. Their expected rate of new vertebral fractures during the first 3 years of the extension study was 6.3%.
The incidence of nonvertebral fractures during the 3-year, double-blind phase of FREEDOM was 8.0% in the placebo arm and significantly lower, at 6.5%, in the denosumab group. During the second 3-year period of denosumab therapy, the incidence was 3.8%.
"It’s interesting that the nonvertebral fracture rate in the second 3 years is actually quite a bit lower than in the first 3 years, suggesting there may be an additional benefit of long treatment," Dr. Bone said.
The projected rate of new nonvertebral fractures during years 4-6 in the "virtual twins" was 7.5%.
Turning to safety issues, the endocrinologist said that there were two adjudicated cases of osteonecrosis of the jaw during 6 years of denosumab therapy, and two more in the control group that occurred after crossover to active treatment. There has been one documented case of atypical femoral fracture during 6 consecutive years on denosumab. There has been one serious skin infection in the control group after they were crossed to 3 years of denosumab, and several more during years 4-6 in the long-term extension group.
The FREEDOM study extension is sponsored by Amgen. The presenter is a consultant to and on the speakers bureau for the company.
HOUSTON – Denosumab continued to achieve reduced fractures rates, progressively rising bone mineral density, and sustained reduction in bone turnover biomarkers through 6 years of continuous use in the ongoing 7-year extension of the landmark FREEDOM trial.
FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) was the pivotal phase III, double-blind, placebo-controlled, 3-year randomized trial that led to marketing approval of denosumab (Prolia) at 60 mg subcutaneously every 6 months for treatment of postmenopausal osteoporosis (N. Engl. J. Med. 2009;361:756-65).
The FREEDOM extension study is evaluating the long-term safety and efficacy of denosumab for an additional 7 years. Thus, participants who were in the active treatment arm of the original study will receive denosumab for a total of 10 years, whereas patients who crossed over to denosumab after 3 years on placebo will be on the novel RANKL (receptor-activated nuclear factor–kappaB ligand) inhibitor for 7 years.
At the conference, Dr. Henry G. Bone presented updated study results through 6 years. In all, 4,550 postmenopausal women from the original randomized trial were enrolled in the extension and have been followed for an additional 3 years.
In the original FREEDOM trial, 3 years of denosumab resulted in a 10.1% increase in BMD in the lumbar spine, compared with baseline. With an additional 3 years of open-label therapy, this figure has increased to 15.1%. The gain in the crossover group paralleled that seen with active therapy in the original trial; that is, after 6 years – the last 3 on open-label denosumab – those patients had a 9.4% increase over baseline in lumbar spine BMD, reported Dr. Bone, chief of the endocrinology department at St. John Hospital and Medical Center, Detroit, and director of the Michigan Bone and Mineral Clinic.
Total hip BMD rose from a 5.7% increase over baseline after 3 years of denosumab to a 7.5% increase after 6 years. The crossover group had a 4.8% increase at this site after 3 years of active therapy.
During the first 3 years of the FREEDOM trial, new vertebral fractures occurred in 2.3% of the denosumab group, compared with 7.2% of placebo-treated controls. During the subsequent 3 years of open-label therapy, the original denosumab cohort had a 3.5% incidence of new vertebral fractures. Because it would have been unethical to have a placebo arm during those second 3 years, statistical modeling using a method known as "virtual twins" was used to project outcomes had the original control group remained on placebo. Their expected rate of new vertebral fractures during the first 3 years of the extension study was 6.3%.
The incidence of nonvertebral fractures during the 3-year, double-blind phase of FREEDOM was 8.0% in the placebo arm and significantly lower, at 6.5%, in the denosumab group. During the second 3-year period of denosumab therapy, the incidence was 3.8%.
"It’s interesting that the nonvertebral fracture rate in the second 3 years is actually quite a bit lower than in the first 3 years, suggesting there may be an additional benefit of long treatment," Dr. Bone said.
The projected rate of new nonvertebral fractures during years 4-6 in the "virtual twins" was 7.5%.
Turning to safety issues, the endocrinologist said that there were two adjudicated cases of osteonecrosis of the jaw during 6 years of denosumab therapy, and two more in the control group that occurred after crossover to active treatment. There has been one documented case of atypical femoral fracture during 6 consecutive years on denosumab. There has been one serious skin infection in the control group after they were crossed to 3 years of denosumab, and several more during years 4-6 in the long-term extension group.
The FREEDOM study extension is sponsored by Amgen. The presenter is a consultant to and on the speakers bureau for the company.
Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months, Prolia, postmenopausal osteoporosis, RANKL, receptor-activated nuclear factor–kappaB ligand inhibitor, Dr. Henry G. Bone, postmenopausal women,
Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months, Prolia, postmenopausal osteoporosis, RANKL, receptor-activated nuclear factor–kappaB ligand inhibitor, Dr. Henry G. Bone, postmenopausal women,
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: The incidence of nonvertebral fractures in a large group of postmenopausal women with osteoporosis was 6.5% during their first 3 years on denosumab and significantly lower at 3.8% during their next 3 years on the drug, which suggests additional benefit with longer-term therapy.
Data Source: These data come from an ongoing, 7-year, open-label extension of the original 7,868-patient, phase-III FREEDOM study.
Disclosures: The FREEDOM study extension is sponsored by Amgen. The presenter is a consultant to and on the speakers bureau for the company.
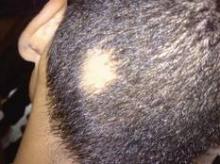
New Era in Alopecia Areata Therapy Beckons
RALEIGH, N.C. – Recent genetic insights into the roots of alopecia areata have fostered the development of new therapeutic targets, with clinical trials offering promising results.
The investigational drugs are, for the first time, addressing the underlying disease mechanism, Angela M. Christiano, Ph.D., said at the annual meeting of the Society for Investigative Dermatology.
Alopecia areata affects roughly 5.3 million Americans of all ages and ethnic groups. The dramatic hair loss exacts an underappreciated, often devastating toll in terms of patient self-esteem and quality of life. To date, treatment has been unsatisfactory, with intralesional injections of glucocorticoids being the mainstay. Wigs are big.
But all of that may be about to change. "Our translational studies offer us the beginning of a therapeutic arsenal," said Dr. Christiano, professor of dermatology and genetics at Columbia University in New York.
She and her colleagues have identified interleukin-15 as a novel and highly promising therapeutic target in alopecia areata.
Interleukin-15 is required for the growth and sustenance of the natural killer-type CD8+ cells that surround the growing end of the hair follicle during active disease. This dense infiltrate of activated T cells, known as the "swarm of bees," is the pathognomonic finding in alopecia areata.
Interleukin-15 is an attractive therapeutic target because it can be addressed along the IL-15 signaling pathway by using Janus kinase inhibitors. Dr. Christiano and her coworkers have found evidence that both the oral and compounded topical formulations of tofacitinib and ruxolitinib are effective for the prevention and treatment of alopecia areata in a mouse model of the disease.
Tofacitinib inhibits the Janus kinase 3 (JAK-3) enzyme located along the IL-15 signaling pathway. The investigational oral agent recently received a thumbs-up recommendation for the treatment of rheumatoid arthritis from a Food and Drug Administration advisory panel.
In Dr. Christiano’s mouse model of alopecia areata, when tofacitinib was administered when mice received diseased grafts, 100% of the animals retained their hair. The same phenomenon occurred when the mice were treated prophylactically with oral ruxolitinib (Jakafi), a JAK1/2 inhibitor marketed for the treatment of myelofibrosis and under investigation for the treatment of other diseases.
Dr. Christiano and her coworkers also formulated the two JAK inhibitors into a 0.5% cream for topical application. The topical agents prevented the development of alopecia areata in mice, and they triggered hair regrowth when applied to mice with established disease. At the molecular level, these drugs resulted in reversal of a pathologic interferon-gamma gene expression signature.
"The follicular expression of molecules changes back to an immune-privileged phenotype. We’re very encouraged by what we’ve seen so far with this," she said.
Alopecia has long been recognized as having a strong genetic component. The relative risk of the disease in first-degree relatives of affected individuals is increased 10-fold. Twin concordance studies have demonstrated that when one monozygotic twin has alopecia areata, there is more than a 50% chance that the other twin will be affected, whereas there is zero concordance in dizygotic twin pairs.
It’s also well recognized in the research community that the normal hair follicle is one of the few tissues in the human body that enjoys a state of immune privilege protecting it from autoimmune attack. Normal hair follicles are cloaked from immune recognition. The collapse of this immune privilege is what allows the "swarm of bees" leading to alopecia areata.
"Notably, the stem cell compartment is spared from the disease, which gives patients the ability, even after many years of longstanding disease, to experience spontaneous regrowth for reasons we really don’t understand," Dr. Christiano explained.
While the JAK inhibitors haven’t made it to clinical studies, another novel therapy in alopecia areata will be the focus of a small clinical trial slated to begin in July. Abatacept (Orencia) is a biologic agent approved for the treatment of rheumatoid arthritis. It is also in ongoing clinical trials for other autoimmune diseases, including type 1 diabetes. Abatacept is a soluble fusion protein that serves as a selective costimulation modulator and T-cell activation suppressant. Last summer, the intravenously administered formulation of abatacept was joined by a subcutaneous version permitting patient self-treatment.
"That makes it more appealing for the treatment of alopecia areata," she noted.
Dr. Christiano’s research is funded by the National Institutes of Health, American Skin Association, and National Alopecia Areata Foundation. She reported having no financial conflicts.
RALEIGH, N.C. – Recent genetic insights into the roots of alopecia areata have fostered the development of new therapeutic targets, with clinical trials offering promising results.
The investigational drugs are, for the first time, addressing the underlying disease mechanism, Angela M. Christiano, Ph.D., said at the annual meeting of the Society for Investigative Dermatology.
Alopecia areata affects roughly 5.3 million Americans of all ages and ethnic groups. The dramatic hair loss exacts an underappreciated, often devastating toll in terms of patient self-esteem and quality of life. To date, treatment has been unsatisfactory, with intralesional injections of glucocorticoids being the mainstay. Wigs are big.
But all of that may be about to change. "Our translational studies offer us the beginning of a therapeutic arsenal," said Dr. Christiano, professor of dermatology and genetics at Columbia University in New York.
She and her colleagues have identified interleukin-15 as a novel and highly promising therapeutic target in alopecia areata.
Interleukin-15 is required for the growth and sustenance of the natural killer-type CD8+ cells that surround the growing end of the hair follicle during active disease. This dense infiltrate of activated T cells, known as the "swarm of bees," is the pathognomonic finding in alopecia areata.
Interleukin-15 is an attractive therapeutic target because it can be addressed along the IL-15 signaling pathway by using Janus kinase inhibitors. Dr. Christiano and her coworkers have found evidence that both the oral and compounded topical formulations of tofacitinib and ruxolitinib are effective for the prevention and treatment of alopecia areata in a mouse model of the disease.
Tofacitinib inhibits the Janus kinase 3 (JAK-3) enzyme located along the IL-15 signaling pathway. The investigational oral agent recently received a thumbs-up recommendation for the treatment of rheumatoid arthritis from a Food and Drug Administration advisory panel.
In Dr. Christiano’s mouse model of alopecia areata, when tofacitinib was administered when mice received diseased grafts, 100% of the animals retained their hair. The same phenomenon occurred when the mice were treated prophylactically with oral ruxolitinib (Jakafi), a JAK1/2 inhibitor marketed for the treatment of myelofibrosis and under investigation for the treatment of other diseases.
Dr. Christiano and her coworkers also formulated the two JAK inhibitors into a 0.5% cream for topical application. The topical agents prevented the development of alopecia areata in mice, and they triggered hair regrowth when applied to mice with established disease. At the molecular level, these drugs resulted in reversal of a pathologic interferon-gamma gene expression signature.
"The follicular expression of molecules changes back to an immune-privileged phenotype. We’re very encouraged by what we’ve seen so far with this," she said.
Alopecia has long been recognized as having a strong genetic component. The relative risk of the disease in first-degree relatives of affected individuals is increased 10-fold. Twin concordance studies have demonstrated that when one monozygotic twin has alopecia areata, there is more than a 50% chance that the other twin will be affected, whereas there is zero concordance in dizygotic twin pairs.
It’s also well recognized in the research community that the normal hair follicle is one of the few tissues in the human body that enjoys a state of immune privilege protecting it from autoimmune attack. Normal hair follicles are cloaked from immune recognition. The collapse of this immune privilege is what allows the "swarm of bees" leading to alopecia areata.
"Notably, the stem cell compartment is spared from the disease, which gives patients the ability, even after many years of longstanding disease, to experience spontaneous regrowth for reasons we really don’t understand," Dr. Christiano explained.
While the JAK inhibitors haven’t made it to clinical studies, another novel therapy in alopecia areata will be the focus of a small clinical trial slated to begin in July. Abatacept (Orencia) is a biologic agent approved for the treatment of rheumatoid arthritis. It is also in ongoing clinical trials for other autoimmune diseases, including type 1 diabetes. Abatacept is a soluble fusion protein that serves as a selective costimulation modulator and T-cell activation suppressant. Last summer, the intravenously administered formulation of abatacept was joined by a subcutaneous version permitting patient self-treatment.
"That makes it more appealing for the treatment of alopecia areata," she noted.
Dr. Christiano’s research is funded by the National Institutes of Health, American Skin Association, and National Alopecia Areata Foundation. She reported having no financial conflicts.
RALEIGH, N.C. – Recent genetic insights into the roots of alopecia areata have fostered the development of new therapeutic targets, with clinical trials offering promising results.
The investigational drugs are, for the first time, addressing the underlying disease mechanism, Angela M. Christiano, Ph.D., said at the annual meeting of the Society for Investigative Dermatology.
Alopecia areata affects roughly 5.3 million Americans of all ages and ethnic groups. The dramatic hair loss exacts an underappreciated, often devastating toll in terms of patient self-esteem and quality of life. To date, treatment has been unsatisfactory, with intralesional injections of glucocorticoids being the mainstay. Wigs are big.
But all of that may be about to change. "Our translational studies offer us the beginning of a therapeutic arsenal," said Dr. Christiano, professor of dermatology and genetics at Columbia University in New York.
She and her colleagues have identified interleukin-15 as a novel and highly promising therapeutic target in alopecia areata.
Interleukin-15 is required for the growth and sustenance of the natural killer-type CD8+ cells that surround the growing end of the hair follicle during active disease. This dense infiltrate of activated T cells, known as the "swarm of bees," is the pathognomonic finding in alopecia areata.
Interleukin-15 is an attractive therapeutic target because it can be addressed along the IL-15 signaling pathway by using Janus kinase inhibitors. Dr. Christiano and her coworkers have found evidence that both the oral and compounded topical formulations of tofacitinib and ruxolitinib are effective for the prevention and treatment of alopecia areata in a mouse model of the disease.
Tofacitinib inhibits the Janus kinase 3 (JAK-3) enzyme located along the IL-15 signaling pathway. The investigational oral agent recently received a thumbs-up recommendation for the treatment of rheumatoid arthritis from a Food and Drug Administration advisory panel.
In Dr. Christiano’s mouse model of alopecia areata, when tofacitinib was administered when mice received diseased grafts, 100% of the animals retained their hair. The same phenomenon occurred when the mice were treated prophylactically with oral ruxolitinib (Jakafi), a JAK1/2 inhibitor marketed for the treatment of myelofibrosis and under investigation for the treatment of other diseases.
Dr. Christiano and her coworkers also formulated the two JAK inhibitors into a 0.5% cream for topical application. The topical agents prevented the development of alopecia areata in mice, and they triggered hair regrowth when applied to mice with established disease. At the molecular level, these drugs resulted in reversal of a pathologic interferon-gamma gene expression signature.
"The follicular expression of molecules changes back to an immune-privileged phenotype. We’re very encouraged by what we’ve seen so far with this," she said.
Alopecia has long been recognized as having a strong genetic component. The relative risk of the disease in first-degree relatives of affected individuals is increased 10-fold. Twin concordance studies have demonstrated that when one monozygotic twin has alopecia areata, there is more than a 50% chance that the other twin will be affected, whereas there is zero concordance in dizygotic twin pairs.
It’s also well recognized in the research community that the normal hair follicle is one of the few tissues in the human body that enjoys a state of immune privilege protecting it from autoimmune attack. Normal hair follicles are cloaked from immune recognition. The collapse of this immune privilege is what allows the "swarm of bees" leading to alopecia areata.
"Notably, the stem cell compartment is spared from the disease, which gives patients the ability, even after many years of longstanding disease, to experience spontaneous regrowth for reasons we really don’t understand," Dr. Christiano explained.
While the JAK inhibitors haven’t made it to clinical studies, another novel therapy in alopecia areata will be the focus of a small clinical trial slated to begin in July. Abatacept (Orencia) is a biologic agent approved for the treatment of rheumatoid arthritis. It is also in ongoing clinical trials for other autoimmune diseases, including type 1 diabetes. Abatacept is a soluble fusion protein that serves as a selective costimulation modulator and T-cell activation suppressant. Last summer, the intravenously administered formulation of abatacept was joined by a subcutaneous version permitting patient self-treatment.
"That makes it more appealing for the treatment of alopecia areata," she noted.
Dr. Christiano’s research is funded by the National Institutes of Health, American Skin Association, and National Alopecia Areata Foundation. She reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE SOCIETY FOR INVESTIGATIVE DERMATOLOGY
Collagenase Might Be a First-Line Treatment for Dupuytren's Contractures
BERLIN – Medical therapy might prove to be the preferred approach for treating patients with early-stage Dupuytren’s contracture, suggest the findings of a small retrospective study.
Injection of collagenase Clostridium histolyticum in patients with early-stage Dupuytren’s contracture resulted in significantly better clinical outcomes than those that were seen in patients with advanced disease. Collagenase C. histolyticum (Xiaflex) injections are generally given only after patients with Dupuytren’s contracture develop fixed-flexion contracture angles of 30 degrees or more.
The retrospective study suggests a new treatment paradigm: The early injection of palpable cords with lesser fixed-flexion contracture angles results in near normal correction, bringing improvement that’s significantly greater than that achieved with delayed therapy, according to Dr. Clayton A. Peimer, an orthopedic surgeon at Michigan State University, East Lansing.
This is off-label therapy. At present, Xiaflex is approved in the United States and Europe under more restrictive conditions (injection of only a single palpable cord at a time, with a 4-week hiatus before either a repeat injection or an injection of another affected cord).
The finding warrants confirmation in prospective longitudinal studies aimed at determining whether collagenase injections into early-stage joints slows progression of Dupuytren’s contracture and improves upon the high contracture recurrence rate that follows surgical release, he added.
In a separate Australian study presented at the annual European Congress of Rheumatology, Dr. David Gilpin of the Brisbane (Queensland) Hand and Upper Limb Clinic reported that concurrent injections of collagenase C. histolyticum into two affected palpable cords in the same hand proved safe, effective, and well tolerated.
The advantages of being able to safely treat multiple contractures at the same time include shortened recovery times, fewer office visits for patients, and more efficient use of physicians’ time, noted Dr. Gilpin.
His study included 12 patients with three or more fixed-flexion contractures of 20% or greater in the proximal interphalangeal (PIP) and/or metacarpophalangeal (MP) joints on the same hand. The participants initially received a single collagenase injection in one palpable cord, followed roughly 24 hours later by the standard finger extension procedure that breaks the now-weakened cord. After 30 days, the patients received two injections into two different palpable cords on the same hand, followed by finger extension procedures the next day.
The first injection resulted in a mean 81% reduction in the degree of fixed-flexion contracture in treated MP joints, and a 66% decrease in PIP joints. The subsequent two concurrent injections of other affected cords showed near identical effectiveness (mean reduction in fixed-flexion contractures, 80% in MP and 63% in PIP joints).
Blood samples that were obtained 24 hours after the dual injections showed no detectable systemic levels of the biologic agent.
Not surprisingly, several treatment-related adverse events were more common when patients received two injections rather than one. These included injection site pain or discomfort, skin discoloration, itching, and blood blisters.
Dr. Peimer reviewed the charts of 302 patients with Dupuytren’s contracture who were treated with collagenase injections at 10 U.S. community and academic practices. Of affected joints, 20% were treated at an early stage (defined as a fixed-flexion contracture angle of 30 degrees or less). Their mean initial fixed-flexion contracture angle was 25 degrees, improving to 3.8 degrees after treatment. In contrast, the mean baseline fixed-flexion contracture angle in patients with advanced disease was 58 degrees, with an improvement to 14 degrees following collagenase treatment. No serious treatment-related adverse events occurred.
Dupuytren’s contracture is the result of a usually painless progressive thickening and shortening of the palmar fascia. This leads to curling of the fingers and impaired hand function. The most common surgical treatment, limited fasciectomy, can result in complications including digital nerve injury, hematoma, and complex regional pain syndrome. Xiaflex was approved in the United States in 2010 as the first nonsurgical treatment for Dupuytren’s contracture. European approval followed the next year.
The studies were sponsored by Auxillium Pharmaceuticals, which markets Xiaflex. Dr. Peimer and Dr. Gilpin were paid by the company for their research, and Dr. Gilpin owns stock in Auxillium.
BERLIN – Medical therapy might prove to be the preferred approach for treating patients with early-stage Dupuytren’s contracture, suggest the findings of a small retrospective study.
Injection of collagenase Clostridium histolyticum in patients with early-stage Dupuytren’s contracture resulted in significantly better clinical outcomes than those that were seen in patients with advanced disease. Collagenase C. histolyticum (Xiaflex) injections are generally given only after patients with Dupuytren’s contracture develop fixed-flexion contracture angles of 30 degrees or more.
The retrospective study suggests a new treatment paradigm: The early injection of palpable cords with lesser fixed-flexion contracture angles results in near normal correction, bringing improvement that’s significantly greater than that achieved with delayed therapy, according to Dr. Clayton A. Peimer, an orthopedic surgeon at Michigan State University, East Lansing.
This is off-label therapy. At present, Xiaflex is approved in the United States and Europe under more restrictive conditions (injection of only a single palpable cord at a time, with a 4-week hiatus before either a repeat injection or an injection of another affected cord).
The finding warrants confirmation in prospective longitudinal studies aimed at determining whether collagenase injections into early-stage joints slows progression of Dupuytren’s contracture and improves upon the high contracture recurrence rate that follows surgical release, he added.
In a separate Australian study presented at the annual European Congress of Rheumatology, Dr. David Gilpin of the Brisbane (Queensland) Hand and Upper Limb Clinic reported that concurrent injections of collagenase C. histolyticum into two affected palpable cords in the same hand proved safe, effective, and well tolerated.
The advantages of being able to safely treat multiple contractures at the same time include shortened recovery times, fewer office visits for patients, and more efficient use of physicians’ time, noted Dr. Gilpin.
His study included 12 patients with three or more fixed-flexion contractures of 20% or greater in the proximal interphalangeal (PIP) and/or metacarpophalangeal (MP) joints on the same hand. The participants initially received a single collagenase injection in one palpable cord, followed roughly 24 hours later by the standard finger extension procedure that breaks the now-weakened cord. After 30 days, the patients received two injections into two different palpable cords on the same hand, followed by finger extension procedures the next day.
The first injection resulted in a mean 81% reduction in the degree of fixed-flexion contracture in treated MP joints, and a 66% decrease in PIP joints. The subsequent two concurrent injections of other affected cords showed near identical effectiveness (mean reduction in fixed-flexion contractures, 80% in MP and 63% in PIP joints).
Blood samples that were obtained 24 hours after the dual injections showed no detectable systemic levels of the biologic agent.
Not surprisingly, several treatment-related adverse events were more common when patients received two injections rather than one. These included injection site pain or discomfort, skin discoloration, itching, and blood blisters.
Dr. Peimer reviewed the charts of 302 patients with Dupuytren’s contracture who were treated with collagenase injections at 10 U.S. community and academic practices. Of affected joints, 20% were treated at an early stage (defined as a fixed-flexion contracture angle of 30 degrees or less). Their mean initial fixed-flexion contracture angle was 25 degrees, improving to 3.8 degrees after treatment. In contrast, the mean baseline fixed-flexion contracture angle in patients with advanced disease was 58 degrees, with an improvement to 14 degrees following collagenase treatment. No serious treatment-related adverse events occurred.
Dupuytren’s contracture is the result of a usually painless progressive thickening and shortening of the palmar fascia. This leads to curling of the fingers and impaired hand function. The most common surgical treatment, limited fasciectomy, can result in complications including digital nerve injury, hematoma, and complex regional pain syndrome. Xiaflex was approved in the United States in 2010 as the first nonsurgical treatment for Dupuytren’s contracture. European approval followed the next year.
The studies were sponsored by Auxillium Pharmaceuticals, which markets Xiaflex. Dr. Peimer and Dr. Gilpin were paid by the company for their research, and Dr. Gilpin owns stock in Auxillium.
BERLIN – Medical therapy might prove to be the preferred approach for treating patients with early-stage Dupuytren’s contracture, suggest the findings of a small retrospective study.
Injection of collagenase Clostridium histolyticum in patients with early-stage Dupuytren’s contracture resulted in significantly better clinical outcomes than those that were seen in patients with advanced disease. Collagenase C. histolyticum (Xiaflex) injections are generally given only after patients with Dupuytren’s contracture develop fixed-flexion contracture angles of 30 degrees or more.
The retrospective study suggests a new treatment paradigm: The early injection of palpable cords with lesser fixed-flexion contracture angles results in near normal correction, bringing improvement that’s significantly greater than that achieved with delayed therapy, according to Dr. Clayton A. Peimer, an orthopedic surgeon at Michigan State University, East Lansing.
This is off-label therapy. At present, Xiaflex is approved in the United States and Europe under more restrictive conditions (injection of only a single palpable cord at a time, with a 4-week hiatus before either a repeat injection or an injection of another affected cord).
The finding warrants confirmation in prospective longitudinal studies aimed at determining whether collagenase injections into early-stage joints slows progression of Dupuytren’s contracture and improves upon the high contracture recurrence rate that follows surgical release, he added.
In a separate Australian study presented at the annual European Congress of Rheumatology, Dr. David Gilpin of the Brisbane (Queensland) Hand and Upper Limb Clinic reported that concurrent injections of collagenase C. histolyticum into two affected palpable cords in the same hand proved safe, effective, and well tolerated.
The advantages of being able to safely treat multiple contractures at the same time include shortened recovery times, fewer office visits for patients, and more efficient use of physicians’ time, noted Dr. Gilpin.
His study included 12 patients with three or more fixed-flexion contractures of 20% or greater in the proximal interphalangeal (PIP) and/or metacarpophalangeal (MP) joints on the same hand. The participants initially received a single collagenase injection in one palpable cord, followed roughly 24 hours later by the standard finger extension procedure that breaks the now-weakened cord. After 30 days, the patients received two injections into two different palpable cords on the same hand, followed by finger extension procedures the next day.
The first injection resulted in a mean 81% reduction in the degree of fixed-flexion contracture in treated MP joints, and a 66% decrease in PIP joints. The subsequent two concurrent injections of other affected cords showed near identical effectiveness (mean reduction in fixed-flexion contractures, 80% in MP and 63% in PIP joints).
Blood samples that were obtained 24 hours after the dual injections showed no detectable systemic levels of the biologic agent.
Not surprisingly, several treatment-related adverse events were more common when patients received two injections rather than one. These included injection site pain or discomfort, skin discoloration, itching, and blood blisters.
Dr. Peimer reviewed the charts of 302 patients with Dupuytren’s contracture who were treated with collagenase injections at 10 U.S. community and academic practices. Of affected joints, 20% were treated at an early stage (defined as a fixed-flexion contracture angle of 30 degrees or less). Their mean initial fixed-flexion contracture angle was 25 degrees, improving to 3.8 degrees after treatment. In contrast, the mean baseline fixed-flexion contracture angle in patients with advanced disease was 58 degrees, with an improvement to 14 degrees following collagenase treatment. No serious treatment-related adverse events occurred.
Dupuytren’s contracture is the result of a usually painless progressive thickening and shortening of the palmar fascia. This leads to curling of the fingers and impaired hand function. The most common surgical treatment, limited fasciectomy, can result in complications including digital nerve injury, hematoma, and complex regional pain syndrome. Xiaflex was approved in the United States in 2010 as the first nonsurgical treatment for Dupuytren’s contracture. European approval followed the next year.
The studies were sponsored by Auxillium Pharmaceuticals, which markets Xiaflex. Dr. Peimer and Dr. Gilpin were paid by the company for their research, and Dr. Gilpin owns stock in Auxillium.
AT THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Major Finding: The first injection of collagenase C. histolyticum into palpable cords of Dupuytren’s contracture resulted in a mean 81% reduction in the degree of fixed-flexion contracture in treated metacarpophalangeal joints, and a 66% decrease in proximal interphalangeal joints.
Data Source: The multiple concurrent injections study involved 12 patients, whereas the early-stage treatment study included 302 treated patients.
Disclosures: The studies were sponsored by Auxillium Pharmaceuticals, which markets Xiaflex. Dr. Peimer and Dr. Gilpin were paid by the company for their research, and Dr. Gilpin owns stock in Auxillium.
New Stripped-Down Rituximab Retreatment Regimen May Be Effective
BERLIN – Rheumatoid arthritis patients who respond favorably to a first course of rituximab and later need retreatment do as well with a follow-up single 1-g intravenous infusion as with the approved dosing regimen consisting of two 1-g infusions given 2 weeks apart, according to a randomized trial presented at the annual European Congress of Rheumatology.
The study findings point the way to a simpler, less costly, and more patient friendly dosing regimen than the one now contained in the product labeling, noted Dr. Maxime Dougados, professor of rheumatology at René Descartes University, Paris.
He presented data from a 2-year, prospective, multicenter, randomized, controlled trial involving 224 patients with moderate to severe RA that was inadequately responsive to antitumor necrosis factor–alpha therapy. All the study subjects had received a first induction cycle of rituximab (Rituxan) at the approved dosage of two 1-g intravenous infusions delivered 2 weeks apart.
The good or moderate response rate at 6 months by EULAR (European League Against Rheumatism) criteria was 71%. At that point, 100 responders were randomized to either open-label retreatment with the licensed regimen of two 1-g infusions 2 weeks apart or the investigational dose consisting of a single 1-g infusion.
Participants were reassessed for disease activity every 8 weeks. Additional retreatment with the assigned regimen was carried out as warranted by return of active RA, defined as a DAS28 (disease activity score based on a 28-joint count) greater than 3.2), as long as at least 6 months had elapsed since the last course of treatment.
The primary study end point was the area under the curve for the DAS28-CRP (C-reactive protein) score at week 104. It averaged 2,761 in the single-infusion retreatment group and was similar at 2,666 in the standard retreatment group, demonstrating the noninferiority of the investigational retreatment regimen.
The median time to a second retreatment was 263 days in the single-infusion group and similar at 255 days in the two-infusion group.
The safety profiles were comparable. Serious adverse events occurred in 29% of single-infusion retreatment patients over the course of the 2-year study, compared with 37% of those who received the approved two-infusion regimen. In all, 2% of patients in the single-infusion group had an IgG level less than 6.82 g/L (the lower limit of normal) at the 2-year mark, compared with 11% in the twin-infusion arm.
The serious infection rate was 7.2 per 100 person-years in the single-infusion group and 1.5 per 100 person-years in those retreated with two 1-g infusions 2 weeks apart.
A caveat regarding this study is that the radiographic or structural effect of the two retreatment strategies wasn’t assessed, the rheumatologist observed.
This clinical trial was supported by Roche. The presenter reported having received research grant support from the sponsor to conduct the study.
BERLIN – Rheumatoid arthritis patients who respond favorably to a first course of rituximab and later need retreatment do as well with a follow-up single 1-g intravenous infusion as with the approved dosing regimen consisting of two 1-g infusions given 2 weeks apart, according to a randomized trial presented at the annual European Congress of Rheumatology.
The study findings point the way to a simpler, less costly, and more patient friendly dosing regimen than the one now contained in the product labeling, noted Dr. Maxime Dougados, professor of rheumatology at René Descartes University, Paris.
He presented data from a 2-year, prospective, multicenter, randomized, controlled trial involving 224 patients with moderate to severe RA that was inadequately responsive to antitumor necrosis factor–alpha therapy. All the study subjects had received a first induction cycle of rituximab (Rituxan) at the approved dosage of two 1-g intravenous infusions delivered 2 weeks apart.
The good or moderate response rate at 6 months by EULAR (European League Against Rheumatism) criteria was 71%. At that point, 100 responders were randomized to either open-label retreatment with the licensed regimen of two 1-g infusions 2 weeks apart or the investigational dose consisting of a single 1-g infusion.
Participants were reassessed for disease activity every 8 weeks. Additional retreatment with the assigned regimen was carried out as warranted by return of active RA, defined as a DAS28 (disease activity score based on a 28-joint count) greater than 3.2), as long as at least 6 months had elapsed since the last course of treatment.
The primary study end point was the area under the curve for the DAS28-CRP (C-reactive protein) score at week 104. It averaged 2,761 in the single-infusion retreatment group and was similar at 2,666 in the standard retreatment group, demonstrating the noninferiority of the investigational retreatment regimen.
The median time to a second retreatment was 263 days in the single-infusion group and similar at 255 days in the two-infusion group.
The safety profiles were comparable. Serious adverse events occurred in 29% of single-infusion retreatment patients over the course of the 2-year study, compared with 37% of those who received the approved two-infusion regimen. In all, 2% of patients in the single-infusion group had an IgG level less than 6.82 g/L (the lower limit of normal) at the 2-year mark, compared with 11% in the twin-infusion arm.
The serious infection rate was 7.2 per 100 person-years in the single-infusion group and 1.5 per 100 person-years in those retreated with two 1-g infusions 2 weeks apart.
A caveat regarding this study is that the radiographic or structural effect of the two retreatment strategies wasn’t assessed, the rheumatologist observed.
This clinical trial was supported by Roche. The presenter reported having received research grant support from the sponsor to conduct the study.
BERLIN – Rheumatoid arthritis patients who respond favorably to a first course of rituximab and later need retreatment do as well with a follow-up single 1-g intravenous infusion as with the approved dosing regimen consisting of two 1-g infusions given 2 weeks apart, according to a randomized trial presented at the annual European Congress of Rheumatology.
The study findings point the way to a simpler, less costly, and more patient friendly dosing regimen than the one now contained in the product labeling, noted Dr. Maxime Dougados, professor of rheumatology at René Descartes University, Paris.
He presented data from a 2-year, prospective, multicenter, randomized, controlled trial involving 224 patients with moderate to severe RA that was inadequately responsive to antitumor necrosis factor–alpha therapy. All the study subjects had received a first induction cycle of rituximab (Rituxan) at the approved dosage of two 1-g intravenous infusions delivered 2 weeks apart.
The good or moderate response rate at 6 months by EULAR (European League Against Rheumatism) criteria was 71%. At that point, 100 responders were randomized to either open-label retreatment with the licensed regimen of two 1-g infusions 2 weeks apart or the investigational dose consisting of a single 1-g infusion.
Participants were reassessed for disease activity every 8 weeks. Additional retreatment with the assigned regimen was carried out as warranted by return of active RA, defined as a DAS28 (disease activity score based on a 28-joint count) greater than 3.2), as long as at least 6 months had elapsed since the last course of treatment.
The primary study end point was the area under the curve for the DAS28-CRP (C-reactive protein) score at week 104. It averaged 2,761 in the single-infusion retreatment group and was similar at 2,666 in the standard retreatment group, demonstrating the noninferiority of the investigational retreatment regimen.
The median time to a second retreatment was 263 days in the single-infusion group and similar at 255 days in the two-infusion group.
The safety profiles were comparable. Serious adverse events occurred in 29% of single-infusion retreatment patients over the course of the 2-year study, compared with 37% of those who received the approved two-infusion regimen. In all, 2% of patients in the single-infusion group had an IgG level less than 6.82 g/L (the lower limit of normal) at the 2-year mark, compared with 11% in the twin-infusion arm.
The serious infection rate was 7.2 per 100 person-years in the single-infusion group and 1.5 per 100 person-years in those retreated with two 1-g infusions 2 weeks apart.
A caveat regarding this study is that the radiographic or structural effect of the two retreatment strategies wasn’t assessed, the rheumatologist observed.
This clinical trial was supported by Roche. The presenter reported having received research grant support from the sponsor to conduct the study.
AT THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Major Finding: Retreatment of RA patients who were responders to an initial course of rituximab provided similar clinical outcomes regardless of whether the follow-up course entailed a single 1-g infusion or the approved two 1-g infusions given 2 weeks apart.
Data Source: This was a randomized, controlled, prospective, French national study including 224 RA patients.
Disclosures: This clinical trial was supported by Roche. The presenter reported having received research grant support from the sponsor to conduct the study.
Weight Loss Through Dieting Increases Insulin Sensitivity
HOUSTON – Diet-induced weight loss with or without an accompanying exercise training program improved insulin sensitivity and other key cardiometabolic risk factors in an obese elderly population, a randomized trial showed.
However, exercise training alone in the absence of weight loss had no effect on insulin sensitivity in the 52-week-long clinical trial.
"This is actually a novel finding in this population of obese older adults, and it suggests a distinct complementary effect of regular exercise only in the setting of prerequisite weight loss," observed Dr. Matthew F. Bouchonville of the University of New Mexico, Albuquerque.
He reported on 107 obese subjects with a mean body mass index of 37 kg/m2, all of whom were at least 65 years old. Sixty percent were women. Participants were randomized to one of four groups: a 52-week supervised dietary intervention with the goal of a 10% loss in body weight at 6 months and maintenance of that slimmed-down weight during the following 6 months; an exercise program; a combined diet-and-exercise intervention; or a control group. Essentially the study compared the metabolic effects of weight loss without exercise, versus exercise without weight loss, versus both interventions.
The primary outcome was change in insulin sensitivity index (ISI) over 1 year as measured via a 75-g oral glucose tolerance test. At 6 months, there was nearly a 40% increase in ISI in both weight-loss interventions – that is, the diet-only and diet-plus-exercise groups – with no significant difference between the two. Moreover, the exercise-only intervention had no effect on ISI; it was identical to that in the control group. Thus, at that intermediate 6-month time point there didn’t seem to be any added value for an exercise program in terms of enhancement of insulin sensitivity, Dr. Bouchonville explained.
But that changed as patients were followed from the 6-month mark out to 12 months. During that period, insulin sensitivity continued to improve in the diet-only group such that at 1 year their ISI was 70% better than at baseline, while the ISI in the diet-plus-exercise group showed an even more robust improvement: an 86% gain over baseline. The exercise training–only group still showed no change in insulin sensitivity at 12 months.
"The combination of these interventions is associated with an even greater improvement in insulin sensitivity," Dr. Bouchonville said. "This lends support to the recommendation that lifestyle interventions directed at this population incorporate both diet-induced weight loss and also regular exercise."
That recommendation was made on the basis of an earlier analysis of the data showing that the combination of weight loss and exercise training provided greater improvement in physical function and reduction in physical frailty than did either intervention alone (N. Engl. J. Med. 2011;364:1218-29).
This new analysis of the randomized trial data focused on change in insulin sensitivity because insulin resistance has been implicated as the main driving force behind the metabolic syndrome, the physician continued.
The same pattern seen with regard to change in insulin sensitivity recurred for the other cardiometabolic risk factors serving as secondary outcomes in the study: that is, no effect for exercise alone, but significant improvements noted in both the diet and diet-plus-exercise groups. For example, abdominal adiposity as measured by MRI decreased by an average of nearly 800 cc and C-reactive protein dropped on average by nearly 2 mg/dL in the combined intervention group, while triglyceride levels declined by 25-30 mg/dL in both of the weight loss interventions. Blood pressure also improved significantly, but again, only in the weight-loss interventions, not with exercise alone.
Audience members expressed surprise that the exercise training program alone did not have a significant impact on insulin sensitivity or the other cardiometabolic risk factors. They wondered if the exercise was not sufficiently intensive. Dr. Bouchonville replied that all indications are that it was. The program entailed three 90-minute group workouts per week led by a physical therapist. Each session included aerobic exercise, resistance training, and balance and flexibility exercises. Participants gradually reached 70%-85% of their peak heart rate. Their peak oxygen consumption improved significantly over time, as did their physical function scores, although the improvements on these measures were even greater in the combined diet-plus-exercise arm.
The diet intervention was pretty intensive. Patients met weekly with a dietician who prescribed a balanced diet that included 1 g of high-quality protein per kilo of body weight while maintaining an energy deficit of 500-750 kcal per day. They kept food diaries, and there were weekly weigh-ins. Body weight fell by 10% in the diet-only group and 9% in the diet-plus-exercise group but did not decrease in the exercise or control groups.
Dr. Bouchonville explained that this study was undertaken because the appropriate treatment for obese older adults has been controversial. Until now, some have argued that it’s prohibitively difficult to achieve lasting weight loss in this population because of unhealthy diet habits and a sedentary lifestyle. It has also been asserted that weight loss in the elderly could worsen frailty by accelerating age-related loss of muscle mass.
It is essential to determine the best evidence-based approach to the treatment of obese older adults because it is a population growing by leaps and bounds, he stressed. The Census Bureau estimates that 20% of the U.S. population will be age 65 years or older by 2030, while the Centers for Disease Control and Prevention predicts that by then 42% of Americans will be obese.
The clinical trial was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
HOUSTON – Diet-induced weight loss with or without an accompanying exercise training program improved insulin sensitivity and other key cardiometabolic risk factors in an obese elderly population, a randomized trial showed.
However, exercise training alone in the absence of weight loss had no effect on insulin sensitivity in the 52-week-long clinical trial.
"This is actually a novel finding in this population of obese older adults, and it suggests a distinct complementary effect of regular exercise only in the setting of prerequisite weight loss," observed Dr. Matthew F. Bouchonville of the University of New Mexico, Albuquerque.
He reported on 107 obese subjects with a mean body mass index of 37 kg/m2, all of whom were at least 65 years old. Sixty percent were women. Participants were randomized to one of four groups: a 52-week supervised dietary intervention with the goal of a 10% loss in body weight at 6 months and maintenance of that slimmed-down weight during the following 6 months; an exercise program; a combined diet-and-exercise intervention; or a control group. Essentially the study compared the metabolic effects of weight loss without exercise, versus exercise without weight loss, versus both interventions.
The primary outcome was change in insulin sensitivity index (ISI) over 1 year as measured via a 75-g oral glucose tolerance test. At 6 months, there was nearly a 40% increase in ISI in both weight-loss interventions – that is, the diet-only and diet-plus-exercise groups – with no significant difference between the two. Moreover, the exercise-only intervention had no effect on ISI; it was identical to that in the control group. Thus, at that intermediate 6-month time point there didn’t seem to be any added value for an exercise program in terms of enhancement of insulin sensitivity, Dr. Bouchonville explained.
But that changed as patients were followed from the 6-month mark out to 12 months. During that period, insulin sensitivity continued to improve in the diet-only group such that at 1 year their ISI was 70% better than at baseline, while the ISI in the diet-plus-exercise group showed an even more robust improvement: an 86% gain over baseline. The exercise training–only group still showed no change in insulin sensitivity at 12 months.
"The combination of these interventions is associated with an even greater improvement in insulin sensitivity," Dr. Bouchonville said. "This lends support to the recommendation that lifestyle interventions directed at this population incorporate both diet-induced weight loss and also regular exercise."
That recommendation was made on the basis of an earlier analysis of the data showing that the combination of weight loss and exercise training provided greater improvement in physical function and reduction in physical frailty than did either intervention alone (N. Engl. J. Med. 2011;364:1218-29).
This new analysis of the randomized trial data focused on change in insulin sensitivity because insulin resistance has been implicated as the main driving force behind the metabolic syndrome, the physician continued.
The same pattern seen with regard to change in insulin sensitivity recurred for the other cardiometabolic risk factors serving as secondary outcomes in the study: that is, no effect for exercise alone, but significant improvements noted in both the diet and diet-plus-exercise groups. For example, abdominal adiposity as measured by MRI decreased by an average of nearly 800 cc and C-reactive protein dropped on average by nearly 2 mg/dL in the combined intervention group, while triglyceride levels declined by 25-30 mg/dL in both of the weight loss interventions. Blood pressure also improved significantly, but again, only in the weight-loss interventions, not with exercise alone.
Audience members expressed surprise that the exercise training program alone did not have a significant impact on insulin sensitivity or the other cardiometabolic risk factors. They wondered if the exercise was not sufficiently intensive. Dr. Bouchonville replied that all indications are that it was. The program entailed three 90-minute group workouts per week led by a physical therapist. Each session included aerobic exercise, resistance training, and balance and flexibility exercises. Participants gradually reached 70%-85% of their peak heart rate. Their peak oxygen consumption improved significantly over time, as did their physical function scores, although the improvements on these measures were even greater in the combined diet-plus-exercise arm.
The diet intervention was pretty intensive. Patients met weekly with a dietician who prescribed a balanced diet that included 1 g of high-quality protein per kilo of body weight while maintaining an energy deficit of 500-750 kcal per day. They kept food diaries, and there were weekly weigh-ins. Body weight fell by 10% in the diet-only group and 9% in the diet-plus-exercise group but did not decrease in the exercise or control groups.
Dr. Bouchonville explained that this study was undertaken because the appropriate treatment for obese older adults has been controversial. Until now, some have argued that it’s prohibitively difficult to achieve lasting weight loss in this population because of unhealthy diet habits and a sedentary lifestyle. It has also been asserted that weight loss in the elderly could worsen frailty by accelerating age-related loss of muscle mass.
It is essential to determine the best evidence-based approach to the treatment of obese older adults because it is a population growing by leaps and bounds, he stressed. The Census Bureau estimates that 20% of the U.S. population will be age 65 years or older by 2030, while the Centers for Disease Control and Prevention predicts that by then 42% of Americans will be obese.
The clinical trial was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
HOUSTON – Diet-induced weight loss with or without an accompanying exercise training program improved insulin sensitivity and other key cardiometabolic risk factors in an obese elderly population, a randomized trial showed.
However, exercise training alone in the absence of weight loss had no effect on insulin sensitivity in the 52-week-long clinical trial.
"This is actually a novel finding in this population of obese older adults, and it suggests a distinct complementary effect of regular exercise only in the setting of prerequisite weight loss," observed Dr. Matthew F. Bouchonville of the University of New Mexico, Albuquerque.
He reported on 107 obese subjects with a mean body mass index of 37 kg/m2, all of whom were at least 65 years old. Sixty percent were women. Participants were randomized to one of four groups: a 52-week supervised dietary intervention with the goal of a 10% loss in body weight at 6 months and maintenance of that slimmed-down weight during the following 6 months; an exercise program; a combined diet-and-exercise intervention; or a control group. Essentially the study compared the metabolic effects of weight loss without exercise, versus exercise without weight loss, versus both interventions.
The primary outcome was change in insulin sensitivity index (ISI) over 1 year as measured via a 75-g oral glucose tolerance test. At 6 months, there was nearly a 40% increase in ISI in both weight-loss interventions – that is, the diet-only and diet-plus-exercise groups – with no significant difference between the two. Moreover, the exercise-only intervention had no effect on ISI; it was identical to that in the control group. Thus, at that intermediate 6-month time point there didn’t seem to be any added value for an exercise program in terms of enhancement of insulin sensitivity, Dr. Bouchonville explained.
But that changed as patients were followed from the 6-month mark out to 12 months. During that period, insulin sensitivity continued to improve in the diet-only group such that at 1 year their ISI was 70% better than at baseline, while the ISI in the diet-plus-exercise group showed an even more robust improvement: an 86% gain over baseline. The exercise training–only group still showed no change in insulin sensitivity at 12 months.
"The combination of these interventions is associated with an even greater improvement in insulin sensitivity," Dr. Bouchonville said. "This lends support to the recommendation that lifestyle interventions directed at this population incorporate both diet-induced weight loss and also regular exercise."
That recommendation was made on the basis of an earlier analysis of the data showing that the combination of weight loss and exercise training provided greater improvement in physical function and reduction in physical frailty than did either intervention alone (N. Engl. J. Med. 2011;364:1218-29).
This new analysis of the randomized trial data focused on change in insulin sensitivity because insulin resistance has been implicated as the main driving force behind the metabolic syndrome, the physician continued.
The same pattern seen with regard to change in insulin sensitivity recurred for the other cardiometabolic risk factors serving as secondary outcomes in the study: that is, no effect for exercise alone, but significant improvements noted in both the diet and diet-plus-exercise groups. For example, abdominal adiposity as measured by MRI decreased by an average of nearly 800 cc and C-reactive protein dropped on average by nearly 2 mg/dL in the combined intervention group, while triglyceride levels declined by 25-30 mg/dL in both of the weight loss interventions. Blood pressure also improved significantly, but again, only in the weight-loss interventions, not with exercise alone.
Audience members expressed surprise that the exercise training program alone did not have a significant impact on insulin sensitivity or the other cardiometabolic risk factors. They wondered if the exercise was not sufficiently intensive. Dr. Bouchonville replied that all indications are that it was. The program entailed three 90-minute group workouts per week led by a physical therapist. Each session included aerobic exercise, resistance training, and balance and flexibility exercises. Participants gradually reached 70%-85% of their peak heart rate. Their peak oxygen consumption improved significantly over time, as did their physical function scores, although the improvements on these measures were even greater in the combined diet-plus-exercise arm.
The diet intervention was pretty intensive. Patients met weekly with a dietician who prescribed a balanced diet that included 1 g of high-quality protein per kilo of body weight while maintaining an energy deficit of 500-750 kcal per day. They kept food diaries, and there were weekly weigh-ins. Body weight fell by 10% in the diet-only group and 9% in the diet-plus-exercise group but did not decrease in the exercise or control groups.
Dr. Bouchonville explained that this study was undertaken because the appropriate treatment for obese older adults has been controversial. Until now, some have argued that it’s prohibitively difficult to achieve lasting weight loss in this population because of unhealthy diet habits and a sedentary lifestyle. It has also been asserted that weight loss in the elderly could worsen frailty by accelerating age-related loss of muscle mass.
It is essential to determine the best evidence-based approach to the treatment of obese older adults because it is a population growing by leaps and bounds, he stressed. The Census Bureau estimates that 20% of the U.S. population will be age 65 years or older by 2030, while the Centers for Disease Control and Prevention predicts that by then 42% of Americans will be obese.
The clinical trial was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Dietary weight loss plus an exercise training program boosted insulin sensitivity by 86% in 1 year and diet alone improved it by 70% in a group of obese elderly patients, but exercise training alone had no impact.
Data Source: This was a 12-month, randomized, clinical trial involving 107 obese subjects aged 65 or older who were assigned to a dietary weight loss intervention, 270 minutes per week of supervised exercise, both, or a control arm that got neither.
Disclosures: This clinical trial was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
Study Debunks 'Andropause' in Aging Men
HOUSTON – A decline in testosterone is not an inevitable result of aging in men, according to a large population-based, longitudinal study.
"There’s an often-spoken-about concept that there is a decrease in testosterone with increasing age in men. Some people have even gone so far as to talk about an ‘andropause,’ or sudden drop in testosterone at some particular critical point in time reached in middle age. But we showed there’s almost no change in testosterone with age. At baseline, the mean serum total testosterone was 16.2 nmol/L, and at 5 years of follow-up it was 15.6. So essentially there is no age effect," declared Dr. Gary A. Wittert, professor of medicine at the University of Adelaide (South Australia).
He is director of the ongoing longitudinal MAILES (Male Adelaide Inflammation Lifestyle Environment Study). For this analysis of testosterone trends over time, he reported on 1,382 community-dwelling MAILES participants who averaged 54 years of age at entry. None was on medications that were known to affect hormones.
Although there was no significant change in testosterone over the course of 5 years in the overall group, some men did experience a significant decline in levels. A multivariate linear regression analysis identified several potent predictors of a progressive fall in testosterone (unmarried status, being depressed at baseline and at follow-up, cardiovascular disease, obesity, and weight gain during follow-up).
"It is critical that doctors understand that declining testosterone levels are not a natural part of aging and that they are most likely due to health-related behaviors or health status itself – particularly the burden of chronic disease, obesity, and depression," he said.
Counsel patients that the most important thing they can do to maintain their manhood, "or their mojo, is to prevent obesity. I think the most important target for preventing the decline in testosterone and all its consequences is to deal with the rapidly increasing obesity epidemic and maintain the healthiest possible lifestyle," Dr. Wittert said.
Men who maintained a normal weight had no change over time in testosterone. Those who lost weight showed a modest increase in the hormone. And men who transitioned from normal weight at baseline to obesity at follow-up showed a reduction in testosterone with no compensatory increase in luteinizing hormone levels, suggesting a central failure at the hypothalamic/pituitary level, he continued.
Dr. Wittert is the principal investigator in a six-center Australian prospective randomized trial that’s looking at whether testosterone replacement therapy plus a lifestyle intervention prevents progression from prediabetes to diabetes in hypogonadal men older than age 50 who are at increased risk. The control arm in the study, which is just beginning enrollment, will receive placebo plus the lifestyle intervention.
One of the hypotheses being tested is whether testosterone replacement improves motivation to engage in lifestyle change, a benefit that Dr. Wittert and others have observed anecdotally. Secondary end points in the study include the effect of testosterone replacement on cardiovascular disease risk and on bone health.
Testosterone levels tended to decline in smokers who quit. That’s clearly not a valid reason to continue smoking. Yet this intriguing relationship between smoking and testosterone is worthy of further investigation, in Dr. Wittert’s view.
The study is being funded by the National Health and Medical Research Council of Australia.
HOUSTON – A decline in testosterone is not an inevitable result of aging in men, according to a large population-based, longitudinal study.
"There’s an often-spoken-about concept that there is a decrease in testosterone with increasing age in men. Some people have even gone so far as to talk about an ‘andropause,’ or sudden drop in testosterone at some particular critical point in time reached in middle age. But we showed there’s almost no change in testosterone with age. At baseline, the mean serum total testosterone was 16.2 nmol/L, and at 5 years of follow-up it was 15.6. So essentially there is no age effect," declared Dr. Gary A. Wittert, professor of medicine at the University of Adelaide (South Australia).
He is director of the ongoing longitudinal MAILES (Male Adelaide Inflammation Lifestyle Environment Study). For this analysis of testosterone trends over time, he reported on 1,382 community-dwelling MAILES participants who averaged 54 years of age at entry. None was on medications that were known to affect hormones.
Although there was no significant change in testosterone over the course of 5 years in the overall group, some men did experience a significant decline in levels. A multivariate linear regression analysis identified several potent predictors of a progressive fall in testosterone (unmarried status, being depressed at baseline and at follow-up, cardiovascular disease, obesity, and weight gain during follow-up).
"It is critical that doctors understand that declining testosterone levels are not a natural part of aging and that they are most likely due to health-related behaviors or health status itself – particularly the burden of chronic disease, obesity, and depression," he said.
Counsel patients that the most important thing they can do to maintain their manhood, "or their mojo, is to prevent obesity. I think the most important target for preventing the decline in testosterone and all its consequences is to deal with the rapidly increasing obesity epidemic and maintain the healthiest possible lifestyle," Dr. Wittert said.
Men who maintained a normal weight had no change over time in testosterone. Those who lost weight showed a modest increase in the hormone. And men who transitioned from normal weight at baseline to obesity at follow-up showed a reduction in testosterone with no compensatory increase in luteinizing hormone levels, suggesting a central failure at the hypothalamic/pituitary level, he continued.
Dr. Wittert is the principal investigator in a six-center Australian prospective randomized trial that’s looking at whether testosterone replacement therapy plus a lifestyle intervention prevents progression from prediabetes to diabetes in hypogonadal men older than age 50 who are at increased risk. The control arm in the study, which is just beginning enrollment, will receive placebo plus the lifestyle intervention.
One of the hypotheses being tested is whether testosterone replacement improves motivation to engage in lifestyle change, a benefit that Dr. Wittert and others have observed anecdotally. Secondary end points in the study include the effect of testosterone replacement on cardiovascular disease risk and on bone health.
Testosterone levels tended to decline in smokers who quit. That’s clearly not a valid reason to continue smoking. Yet this intriguing relationship between smoking and testosterone is worthy of further investigation, in Dr. Wittert’s view.
The study is being funded by the National Health and Medical Research Council of Australia.
HOUSTON – A decline in testosterone is not an inevitable result of aging in men, according to a large population-based, longitudinal study.
"There’s an often-spoken-about concept that there is a decrease in testosterone with increasing age in men. Some people have even gone so far as to talk about an ‘andropause,’ or sudden drop in testosterone at some particular critical point in time reached in middle age. But we showed there’s almost no change in testosterone with age. At baseline, the mean serum total testosterone was 16.2 nmol/L, and at 5 years of follow-up it was 15.6. So essentially there is no age effect," declared Dr. Gary A. Wittert, professor of medicine at the University of Adelaide (South Australia).
He is director of the ongoing longitudinal MAILES (Male Adelaide Inflammation Lifestyle Environment Study). For this analysis of testosterone trends over time, he reported on 1,382 community-dwelling MAILES participants who averaged 54 years of age at entry. None was on medications that were known to affect hormones.
Although there was no significant change in testosterone over the course of 5 years in the overall group, some men did experience a significant decline in levels. A multivariate linear regression analysis identified several potent predictors of a progressive fall in testosterone (unmarried status, being depressed at baseline and at follow-up, cardiovascular disease, obesity, and weight gain during follow-up).
"It is critical that doctors understand that declining testosterone levels are not a natural part of aging and that they are most likely due to health-related behaviors or health status itself – particularly the burden of chronic disease, obesity, and depression," he said.
Counsel patients that the most important thing they can do to maintain their manhood, "or their mojo, is to prevent obesity. I think the most important target for preventing the decline in testosterone and all its consequences is to deal with the rapidly increasing obesity epidemic and maintain the healthiest possible lifestyle," Dr. Wittert said.
Men who maintained a normal weight had no change over time in testosterone. Those who lost weight showed a modest increase in the hormone. And men who transitioned from normal weight at baseline to obesity at follow-up showed a reduction in testosterone with no compensatory increase in luteinizing hormone levels, suggesting a central failure at the hypothalamic/pituitary level, he continued.
Dr. Wittert is the principal investigator in a six-center Australian prospective randomized trial that’s looking at whether testosterone replacement therapy plus a lifestyle intervention prevents progression from prediabetes to diabetes in hypogonadal men older than age 50 who are at increased risk. The control arm in the study, which is just beginning enrollment, will receive placebo plus the lifestyle intervention.
One of the hypotheses being tested is whether testosterone replacement improves motivation to engage in lifestyle change, a benefit that Dr. Wittert and others have observed anecdotally. Secondary end points in the study include the effect of testosterone replacement on cardiovascular disease risk and on bone health.
Testosterone levels tended to decline in smokers who quit. That’s clearly not a valid reason to continue smoking. Yet this intriguing relationship between smoking and testosterone is worthy of further investigation, in Dr. Wittert’s view.
The study is being funded by the National Health and Medical Research Council of Australia.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Mean serum total testosterone showed no significant decline during 5 years of follow-up in a large group of middle-aged and elderly men. Obesity and depression were prominent factors in those who did experience a drop in the hormone level.
Data Source: The ongoing Male Adelaide Inflammation Lifestyle Environment Study is a longitudinal study of close to 2,000 Australian men.
Disclosures: The study is funded by the National Health and Medical Research Council of Australia.
Cognitive Impairment Seen in Childhood CNS Vasculitis
BERLIN – Patients with childhood primary angiitis of the central nervous system are at elevated risk for poor cognitive outcome, and the risk is highest by far in the subgroup with small-vessel disease presenting with seizures.
In the years since use of immunosuppressive therapy has become common, mortality among affected children has lessened. "Most children survive. However, in day-to-day clinical practice, it’s our observation that what matters most to parents of these children is their long-term cognitive outcome. Parents ask us, ‘Will our child attend a regular school? Will our child achieve the same levels of academic performance and social and vocational accomplishments as their siblings?’ " observed Dr. Peter Gowdie of the University of Toronto Hospital for Sick Children.
He and his coinvestigators sought answers to these questions in their single-center, retrospective, cohort study involving 63 patients with childhood primary angiitis of the CNS (cPACNS) without known premorbid cognitive deficits. Nineteen children had the small-vessel subtype, which is angiography negative and requires brain biopsy for diagnosis. Forty-four had large-vessel disease, which is identifiable on angiography and for which brain biopsy is therefore not indicated.
The median age at diagnosis was 8.1 years, with a median time to cognitive testing of 14.8 months.
Patients with large- and small-vessel cPACNS differed in several key ways in terms of clinical presentation, as previously noted in other studies.
Neurocognitive testing was carried out using the Wechsler Intelligence Scale for Children (WISC), a comprehensive battery of 10 subtests assessing a variety of domains.
Scores of 85-115 on the full scale IQ portion of the WISC are considered within average range. The majority of children with small-vessel cPACNS – 53% to be exact – scored below 85, which indicates global cognitive impairment. This was twice the rate seen in children with large-vessel disease. The mean full-scale IQ score in patients with small-vessel cPACNS was 82, compared with 97 in those with large-vessel disease.
The specific cognitive domains where patients with small-vessel disease were disadvantaged were verbal comprehension, with a mean score of 91 compared with 101 in youngsters with large-vessel disease; processing speed, where the difference in mean scores was 83 versus 96; and working memory, on which patients with small-vessel cPACNS had an average score of 81 compared with 96 in those with large-vessel disease.
"Neurocognitive testing is helpful in determining the cognitive burden of cPACNS. Characterization of the cognitive deficits may be helpful in tailoring early rehabilitation interventions," the rheumatologist said.
Patients with large-vessel cPACNS and no seizures had an average Full Scale IQ score of 99. IQ scores were slightly but not significantly lower in those with large-vessel disease who presented with seizures as well as in those with small-vessel disease and no seizures. However, the mean full-scale IQ score was 79 in patients with small-vessel cPACNS who presented with seizures.
Dr. Gowdie reported having no financial conflicts of interest.
BERLIN – Patients with childhood primary angiitis of the central nervous system are at elevated risk for poor cognitive outcome, and the risk is highest by far in the subgroup with small-vessel disease presenting with seizures.
In the years since use of immunosuppressive therapy has become common, mortality among affected children has lessened. "Most children survive. However, in day-to-day clinical practice, it’s our observation that what matters most to parents of these children is their long-term cognitive outcome. Parents ask us, ‘Will our child attend a regular school? Will our child achieve the same levels of academic performance and social and vocational accomplishments as their siblings?’ " observed Dr. Peter Gowdie of the University of Toronto Hospital for Sick Children.
He and his coinvestigators sought answers to these questions in their single-center, retrospective, cohort study involving 63 patients with childhood primary angiitis of the CNS (cPACNS) without known premorbid cognitive deficits. Nineteen children had the small-vessel subtype, which is angiography negative and requires brain biopsy for diagnosis. Forty-four had large-vessel disease, which is identifiable on angiography and for which brain biopsy is therefore not indicated.
The median age at diagnosis was 8.1 years, with a median time to cognitive testing of 14.8 months.
Patients with large- and small-vessel cPACNS differed in several key ways in terms of clinical presentation, as previously noted in other studies.
Neurocognitive testing was carried out using the Wechsler Intelligence Scale for Children (WISC), a comprehensive battery of 10 subtests assessing a variety of domains.
Scores of 85-115 on the full scale IQ portion of the WISC are considered within average range. The majority of children with small-vessel cPACNS – 53% to be exact – scored below 85, which indicates global cognitive impairment. This was twice the rate seen in children with large-vessel disease. The mean full-scale IQ score in patients with small-vessel cPACNS was 82, compared with 97 in those with large-vessel disease.
The specific cognitive domains where patients with small-vessel disease were disadvantaged were verbal comprehension, with a mean score of 91 compared with 101 in youngsters with large-vessel disease; processing speed, where the difference in mean scores was 83 versus 96; and working memory, on which patients with small-vessel cPACNS had an average score of 81 compared with 96 in those with large-vessel disease.
"Neurocognitive testing is helpful in determining the cognitive burden of cPACNS. Characterization of the cognitive deficits may be helpful in tailoring early rehabilitation interventions," the rheumatologist said.
Patients with large-vessel cPACNS and no seizures had an average Full Scale IQ score of 99. IQ scores were slightly but not significantly lower in those with large-vessel disease who presented with seizures as well as in those with small-vessel disease and no seizures. However, the mean full-scale IQ score was 79 in patients with small-vessel cPACNS who presented with seizures.
Dr. Gowdie reported having no financial conflicts of interest.
BERLIN – Patients with childhood primary angiitis of the central nervous system are at elevated risk for poor cognitive outcome, and the risk is highest by far in the subgroup with small-vessel disease presenting with seizures.
In the years since use of immunosuppressive therapy has become common, mortality among affected children has lessened. "Most children survive. However, in day-to-day clinical practice, it’s our observation that what matters most to parents of these children is their long-term cognitive outcome. Parents ask us, ‘Will our child attend a regular school? Will our child achieve the same levels of academic performance and social and vocational accomplishments as their siblings?’ " observed Dr. Peter Gowdie of the University of Toronto Hospital for Sick Children.
He and his coinvestigators sought answers to these questions in their single-center, retrospective, cohort study involving 63 patients with childhood primary angiitis of the CNS (cPACNS) without known premorbid cognitive deficits. Nineteen children had the small-vessel subtype, which is angiography negative and requires brain biopsy for diagnosis. Forty-four had large-vessel disease, which is identifiable on angiography and for which brain biopsy is therefore not indicated.
The median age at diagnosis was 8.1 years, with a median time to cognitive testing of 14.8 months.
Patients with large- and small-vessel cPACNS differed in several key ways in terms of clinical presentation, as previously noted in other studies.
Neurocognitive testing was carried out using the Wechsler Intelligence Scale for Children (WISC), a comprehensive battery of 10 subtests assessing a variety of domains.
Scores of 85-115 on the full scale IQ portion of the WISC are considered within average range. The majority of children with small-vessel cPACNS – 53% to be exact – scored below 85, which indicates global cognitive impairment. This was twice the rate seen in children with large-vessel disease. The mean full-scale IQ score in patients with small-vessel cPACNS was 82, compared with 97 in those with large-vessel disease.
The specific cognitive domains where patients with small-vessel disease were disadvantaged were verbal comprehension, with a mean score of 91 compared with 101 in youngsters with large-vessel disease; processing speed, where the difference in mean scores was 83 versus 96; and working memory, on which patients with small-vessel cPACNS had an average score of 81 compared with 96 in those with large-vessel disease.
"Neurocognitive testing is helpful in determining the cognitive burden of cPACNS. Characterization of the cognitive deficits may be helpful in tailoring early rehabilitation interventions," the rheumatologist said.
Patients with large-vessel cPACNS and no seizures had an average Full Scale IQ score of 99. IQ scores were slightly but not significantly lower in those with large-vessel disease who presented with seizures as well as in those with small-vessel disease and no seizures. However, the mean full-scale IQ score was 79 in patients with small-vessel cPACNS who presented with seizures.
Dr. Gowdie reported having no financial conflicts of interest.
AT THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Major Finding: Thirty-five percent of patients with childhood primary angiitis of the CNS had significantly impaired cognitive functioning when assessed by neurocognitive testing an average of 15 months after diagnosis.
Data Source: This was a single-center, retrospective, cohort study involving 63 patients.
Disclosures: The presenter reported having no financial disclosures.
Tooth Loss Predicts Rheumatoid Arthritis
BERLIN – Multiple tooth loss appears to be a marker for increased risk of subsequent rheumatoid arthritis – and the more missing teeth, the higher the arthritis disease activity and the worse the treatment response, according to studies presented at the annual European Congress of Rheumatology.
Tooth loss is considered a surrogate marker for periodontitis, a common chronic inflammatory process involving the gums and other tooth-supporting structures. Investigators are increasingly interested in exploring a possible causal relationship between this oral disease and chronic diseases elsewhere in the body having a prominent systemic inflammatory component, including coronary artery disease and rheumatoid arthritis (RA).
Dr. Gisela Westhoff reported on 540 patients with early arthritis participating in the ongoing observational CAPEA (Course and Prognosis of Early Arthritis) study. Patients averaged 56 years of age, with a mean symptom duration of 13 weeks at enrollment. A total of 59% were negative for rheumatoid factor and/or positive for anti–citrullinated protein antibody, 67% met current diagnostic criteria for RA, and 87% were on disease-modifying antirheumatic drugs.
At 6 months, 52% of the patients achieved a good response to treatment according to EULAR response criteria. Another 32% had a moderate response, and 16% had no response.
Patients had a mean of 19 teeth at enrollment. In all, 24% of subjects had 10 or fewer teeth, 15% had 11-20, and 22% had 28 or more teeth. Patients missing teeth tended to be older, heavier, and smokers. At 6 months of follow-up, those with 10 or fewer teeth had a significantly greater erythrocyte sedimentation rate, higher tender and swollen joint counts, and a higher Disease Activity Score-28 than did those with more than 10 teeth.
In a multivariate analysis adjusted for age, body mass index, and other potential confounders, patients with 10 or fewer teeth at baseline were 3.8 times as likely to have an insufficient treatment response as those with at least 28 teeth. The only other significant predictor of poor therapeutic response was smoking, which was associated with a 1.7-fold increased likelihood, noted Dr. Westhoff of the German Rheumatism Research Center in Berlin.
In a separate presentation, Dr. Axel Finckh of University Hospital, Geneva, reported that tooth loss was associated with swollen joints in a group of healthy individuals at increased risk of developing RA.
He presented data from an ongoing prospective observational study of 366 first-degree relatives of patients with RA. All were healthy at enrollment, at which time they had a mean of 28 teeth. Six percent had 20 or fewer teeth, 20% had 21-27, and 28% had a full complement of 32 teeth.
Subjects with one or more swollen joints on physical examination had an average of 26 teeth, compared with 29 teeth for those with no swollen joints. In a multivariate analysis, patients with less than 20 teeth were at 8.1-fold greater risk of having at least one swollen joint than those with 32 teeth. Individuals with 20-28 teeth were at 4.1-fold increased likelihood, while those with 28-31 teeth were at 3.6-fold increased risk.
Moreover, participants with less than 20 teeth had an adjusted 5.3-fold increased likelihood of having morning stiffness lasting more than 1 hour for at least 6 weeks than subjects with 32 teeth.
This study was sponsored by the Swiss League Against Rheumatism. Neither Dr. Finckh nor Dr. Westhoff reported having any financial conflicts.
BERLIN – Multiple tooth loss appears to be a marker for increased risk of subsequent rheumatoid arthritis – and the more missing teeth, the higher the arthritis disease activity and the worse the treatment response, according to studies presented at the annual European Congress of Rheumatology.
Tooth loss is considered a surrogate marker for periodontitis, a common chronic inflammatory process involving the gums and other tooth-supporting structures. Investigators are increasingly interested in exploring a possible causal relationship between this oral disease and chronic diseases elsewhere in the body having a prominent systemic inflammatory component, including coronary artery disease and rheumatoid arthritis (RA).
Dr. Gisela Westhoff reported on 540 patients with early arthritis participating in the ongoing observational CAPEA (Course and Prognosis of Early Arthritis) study. Patients averaged 56 years of age, with a mean symptom duration of 13 weeks at enrollment. A total of 59% were negative for rheumatoid factor and/or positive for anti–citrullinated protein antibody, 67% met current diagnostic criteria for RA, and 87% were on disease-modifying antirheumatic drugs.
At 6 months, 52% of the patients achieved a good response to treatment according to EULAR response criteria. Another 32% had a moderate response, and 16% had no response.
Patients had a mean of 19 teeth at enrollment. In all, 24% of subjects had 10 or fewer teeth, 15% had 11-20, and 22% had 28 or more teeth. Patients missing teeth tended to be older, heavier, and smokers. At 6 months of follow-up, those with 10 or fewer teeth had a significantly greater erythrocyte sedimentation rate, higher tender and swollen joint counts, and a higher Disease Activity Score-28 than did those with more than 10 teeth.
In a multivariate analysis adjusted for age, body mass index, and other potential confounders, patients with 10 or fewer teeth at baseline were 3.8 times as likely to have an insufficient treatment response as those with at least 28 teeth. The only other significant predictor of poor therapeutic response was smoking, which was associated with a 1.7-fold increased likelihood, noted Dr. Westhoff of the German Rheumatism Research Center in Berlin.
In a separate presentation, Dr. Axel Finckh of University Hospital, Geneva, reported that tooth loss was associated with swollen joints in a group of healthy individuals at increased risk of developing RA.
He presented data from an ongoing prospective observational study of 366 first-degree relatives of patients with RA. All were healthy at enrollment, at which time they had a mean of 28 teeth. Six percent had 20 or fewer teeth, 20% had 21-27, and 28% had a full complement of 32 teeth.
Subjects with one or more swollen joints on physical examination had an average of 26 teeth, compared with 29 teeth for those with no swollen joints. In a multivariate analysis, patients with less than 20 teeth were at 8.1-fold greater risk of having at least one swollen joint than those with 32 teeth. Individuals with 20-28 teeth were at 4.1-fold increased likelihood, while those with 28-31 teeth were at 3.6-fold increased risk.
Moreover, participants with less than 20 teeth had an adjusted 5.3-fold increased likelihood of having morning stiffness lasting more than 1 hour for at least 6 weeks than subjects with 32 teeth.
This study was sponsored by the Swiss League Against Rheumatism. Neither Dr. Finckh nor Dr. Westhoff reported having any financial conflicts.
BERLIN – Multiple tooth loss appears to be a marker for increased risk of subsequent rheumatoid arthritis – and the more missing teeth, the higher the arthritis disease activity and the worse the treatment response, according to studies presented at the annual European Congress of Rheumatology.
Tooth loss is considered a surrogate marker for periodontitis, a common chronic inflammatory process involving the gums and other tooth-supporting structures. Investigators are increasingly interested in exploring a possible causal relationship between this oral disease and chronic diseases elsewhere in the body having a prominent systemic inflammatory component, including coronary artery disease and rheumatoid arthritis (RA).
Dr. Gisela Westhoff reported on 540 patients with early arthritis participating in the ongoing observational CAPEA (Course and Prognosis of Early Arthritis) study. Patients averaged 56 years of age, with a mean symptom duration of 13 weeks at enrollment. A total of 59% were negative for rheumatoid factor and/or positive for anti–citrullinated protein antibody, 67% met current diagnostic criteria for RA, and 87% were on disease-modifying antirheumatic drugs.
At 6 months, 52% of the patients achieved a good response to treatment according to EULAR response criteria. Another 32% had a moderate response, and 16% had no response.
Patients had a mean of 19 teeth at enrollment. In all, 24% of subjects had 10 or fewer teeth, 15% had 11-20, and 22% had 28 or more teeth. Patients missing teeth tended to be older, heavier, and smokers. At 6 months of follow-up, those with 10 or fewer teeth had a significantly greater erythrocyte sedimentation rate, higher tender and swollen joint counts, and a higher Disease Activity Score-28 than did those with more than 10 teeth.
In a multivariate analysis adjusted for age, body mass index, and other potential confounders, patients with 10 or fewer teeth at baseline were 3.8 times as likely to have an insufficient treatment response as those with at least 28 teeth. The only other significant predictor of poor therapeutic response was smoking, which was associated with a 1.7-fold increased likelihood, noted Dr. Westhoff of the German Rheumatism Research Center in Berlin.
In a separate presentation, Dr. Axel Finckh of University Hospital, Geneva, reported that tooth loss was associated with swollen joints in a group of healthy individuals at increased risk of developing RA.
He presented data from an ongoing prospective observational study of 366 first-degree relatives of patients with RA. All were healthy at enrollment, at which time they had a mean of 28 teeth. Six percent had 20 or fewer teeth, 20% had 21-27, and 28% had a full complement of 32 teeth.
Subjects with one or more swollen joints on physical examination had an average of 26 teeth, compared with 29 teeth for those with no swollen joints. In a multivariate analysis, patients with less than 20 teeth were at 8.1-fold greater risk of having at least one swollen joint than those with 32 teeth. Individuals with 20-28 teeth were at 4.1-fold increased likelihood, while those with 28-31 teeth were at 3.6-fold increased risk.
Moreover, participants with less than 20 teeth had an adjusted 5.3-fold increased likelihood of having morning stiffness lasting more than 1 hour for at least 6 weeks than subjects with 32 teeth.
This study was sponsored by the Swiss League Against Rheumatism. Neither Dr. Finckh nor Dr. Westhoff reported having any financial conflicts.
AT THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Herbal Remedy Proved Effective in Ankle Sprains
BERLIN – Traumeel ointment and gel proved equal to diclofenac gel 1% in achieving pain reduction and improved joint function in patients with acute ankle sprain, according to a large randomized trial presented at the annual European Congress of Rheumatology.
The Traumeel Acute Ankle Sprain Study (TAASS) was a multicenter, double-blind clinical trial of 449 physically active patients aged 18-40 years with a grade 1 or 2 acute lateral ankle sprain. They were randomized to 2 g of Traumeel ointment, Traumeel gel, or diclofenac 1% gel applied three times daily for 2 weeks.
Traumeel is an over-the-counter homeopathic remedy containing a proprietary mix of 12 medicinal herbs, including arnica, calendula, hypericum, chamomile, witch hazel, belladonna, and monkshood, as well as two minerals. It is available in the United States and more than 60 other countries. Diclofenac gel 1% (Voltaren), in contrast, is a prescription medication that is approved for treating osteoarthritic joints.
Dr. Carlos G. de Vega explained that TAASS had two primary end points. One was change in self-assessed ankle pain between baseline and day 7 on a 0-100 visual analog scale. The Traumeel ointment group had a median 61% reduction from a baseline pain score of 53. Patients on Traumeel gel averaged a 71% reduction, while the diclofenac gel group had a median 69% reduction.
Total pain relief was reported on day 7 by 8.5% of the Traumeel ointment group, 5.0% of patients on Traumeel gel, and 5.9% on topical diclofenac.
The other primary end point in TAASS was change in the Activities of Daily Living 0-100 subscale of the Foot and Ankle Ability Measurement between baseline and day 7. The Traumeel ointment group improved by a median of 26.2 points from a baseline score of 51. The Traumeel gel group also improved by a median of 26.2 points, while the diclofenac gel group improved by 25 points, reported Dr. de Vega of the Medyr Clinic in Madrid.
At 6 weeks of follow-up, all participants reported total pain relief and normal functioning. The median time to normal activity was 19.1 days in the Traumeel ointment group and 19.4 days in each of the other two study arms.
All treatments were similarly well tolerated.
The clinical relevance of the TAASS findings lies in the fact that acute lateral ankle sprain is the most common ligamentous injury caused by sports and other physical activity. And a significant proportion of patients are more favorably disposed to a "natural" herbal therapy than to prescription medications, Dr. de Vega noted.
He reported receiving a research grant from Biologische Heilmittel Heel, which sponsored TAASS.
BERLIN – Traumeel ointment and gel proved equal to diclofenac gel 1% in achieving pain reduction and improved joint function in patients with acute ankle sprain, according to a large randomized trial presented at the annual European Congress of Rheumatology.
The Traumeel Acute Ankle Sprain Study (TAASS) was a multicenter, double-blind clinical trial of 449 physically active patients aged 18-40 years with a grade 1 or 2 acute lateral ankle sprain. They were randomized to 2 g of Traumeel ointment, Traumeel gel, or diclofenac 1% gel applied three times daily for 2 weeks.
Traumeel is an over-the-counter homeopathic remedy containing a proprietary mix of 12 medicinal herbs, including arnica, calendula, hypericum, chamomile, witch hazel, belladonna, and monkshood, as well as two minerals. It is available in the United States and more than 60 other countries. Diclofenac gel 1% (Voltaren), in contrast, is a prescription medication that is approved for treating osteoarthritic joints.
Dr. Carlos G. de Vega explained that TAASS had two primary end points. One was change in self-assessed ankle pain between baseline and day 7 on a 0-100 visual analog scale. The Traumeel ointment group had a median 61% reduction from a baseline pain score of 53. Patients on Traumeel gel averaged a 71% reduction, while the diclofenac gel group had a median 69% reduction.
Total pain relief was reported on day 7 by 8.5% of the Traumeel ointment group, 5.0% of patients on Traumeel gel, and 5.9% on topical diclofenac.
The other primary end point in TAASS was change in the Activities of Daily Living 0-100 subscale of the Foot and Ankle Ability Measurement between baseline and day 7. The Traumeel ointment group improved by a median of 26.2 points from a baseline score of 51. The Traumeel gel group also improved by a median of 26.2 points, while the diclofenac gel group improved by 25 points, reported Dr. de Vega of the Medyr Clinic in Madrid.
At 6 weeks of follow-up, all participants reported total pain relief and normal functioning. The median time to normal activity was 19.1 days in the Traumeel ointment group and 19.4 days in each of the other two study arms.
All treatments were similarly well tolerated.
The clinical relevance of the TAASS findings lies in the fact that acute lateral ankle sprain is the most common ligamentous injury caused by sports and other physical activity. And a significant proportion of patients are more favorably disposed to a "natural" herbal therapy than to prescription medications, Dr. de Vega noted.
He reported receiving a research grant from Biologische Heilmittel Heel, which sponsored TAASS.
BERLIN – Traumeel ointment and gel proved equal to diclofenac gel 1% in achieving pain reduction and improved joint function in patients with acute ankle sprain, according to a large randomized trial presented at the annual European Congress of Rheumatology.
The Traumeel Acute Ankle Sprain Study (TAASS) was a multicenter, double-blind clinical trial of 449 physically active patients aged 18-40 years with a grade 1 or 2 acute lateral ankle sprain. They were randomized to 2 g of Traumeel ointment, Traumeel gel, or diclofenac 1% gel applied three times daily for 2 weeks.
Traumeel is an over-the-counter homeopathic remedy containing a proprietary mix of 12 medicinal herbs, including arnica, calendula, hypericum, chamomile, witch hazel, belladonna, and monkshood, as well as two minerals. It is available in the United States and more than 60 other countries. Diclofenac gel 1% (Voltaren), in contrast, is a prescription medication that is approved for treating osteoarthritic joints.
Dr. Carlos G. de Vega explained that TAASS had two primary end points. One was change in self-assessed ankle pain between baseline and day 7 on a 0-100 visual analog scale. The Traumeel ointment group had a median 61% reduction from a baseline pain score of 53. Patients on Traumeel gel averaged a 71% reduction, while the diclofenac gel group had a median 69% reduction.
Total pain relief was reported on day 7 by 8.5% of the Traumeel ointment group, 5.0% of patients on Traumeel gel, and 5.9% on topical diclofenac.
The other primary end point in TAASS was change in the Activities of Daily Living 0-100 subscale of the Foot and Ankle Ability Measurement between baseline and day 7. The Traumeel ointment group improved by a median of 26.2 points from a baseline score of 51. The Traumeel gel group also improved by a median of 26.2 points, while the diclofenac gel group improved by 25 points, reported Dr. de Vega of the Medyr Clinic in Madrid.
At 6 weeks of follow-up, all participants reported total pain relief and normal functioning. The median time to normal activity was 19.1 days in the Traumeel ointment group and 19.4 days in each of the other two study arms.
All treatments were similarly well tolerated.
The clinical relevance of the TAASS findings lies in the fact that acute lateral ankle sprain is the most common ligamentous injury caused by sports and other physical activity. And a significant proportion of patients are more favorably disposed to a "natural" herbal therapy than to prescription medications, Dr. de Vega noted.
He reported receiving a research grant from Biologische Heilmittel Heel, which sponsored TAASS.
AT THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Chloroquine Eyed for Treating Metabolic Syndrome
HOUSTON – The antimalarial/immunomodulatory drug chloroquine might take on a new life as a treatment for patients with the metabolic syndrome.
In a randomized, double-blind trial involving 277 healthy Singapore volunteers assigned to low-dose chloroquine or placebo, mean triglyceride levels after 12 weeks were 77.9 mg/dL in the chloroquine group, an 11% reduction compared with the mean 87.7 mg/dL in controls.
Moreover, the total cholesterol–high-density lipoprotein ratio at the 12-week mark was 3.31 mg/dL in the chloroquine group, 7% better than the 3.51 mg/dL in controls, according to Dr. Lawrence Lee of the National University of Singapore.
"This cheap and safe treatment should be investigated further for reducing the risk of cardiovascular disease, especially in high-risk patients, such as those who are obese or diabetic, where the effect could be more clinically significant," he noted at the annual meeting of the Endocrine Society.
Twelve weeks of low-dose chloroquine had no impact on HDL, insulin levels, blood glucose, or the homeostatic model assessment (HOMA) insulin sensitivity index.
The chloroquine regimen consisted of 300 mg/day for the first 7 days followed by 300 mg once weekly for the next 11 weeks.
Previous animal studies by investigators at Washington University at St. Louis showed that daily doses of chloroquine or hydroxychloroquine reduced insulin resistance and improved lipid metabolism, most likely via activation of the ataxia telangiectasis mutated (ATM) gene (Cell Metab. 2006;4:377-89).
This work has led to ongoing large prospective clinical trials of daily chloroquine as a treatment for dyslipidemia and insulin resistance.
Dr. Lee and coworkers hypothesized that low-dose, once-weekly chloroquine could also be effective in metabolic syndrome, and with a more favorable safety profile than that of daily therapy. This study in healthy volunteers was a prelude to further studies in patients with metabolic syndrome.
The current study was actually a substudy of the Chloroquine for Influenza Prevention (CHIP) trial, in which 1,500 healthy volunteers were randomized to chloroquine or placebo, and which established that chloroquine does not prevent influenza (Lancet Infect. Dis. 2011; 677-83).
Dr. Lee reported having no financial conflicts. The CHIP trial was funded by the National Medical Research Council of Singapore.
HOUSTON – The antimalarial/immunomodulatory drug chloroquine might take on a new life as a treatment for patients with the metabolic syndrome.
In a randomized, double-blind trial involving 277 healthy Singapore volunteers assigned to low-dose chloroquine or placebo, mean triglyceride levels after 12 weeks were 77.9 mg/dL in the chloroquine group, an 11% reduction compared with the mean 87.7 mg/dL in controls.
Moreover, the total cholesterol–high-density lipoprotein ratio at the 12-week mark was 3.31 mg/dL in the chloroquine group, 7% better than the 3.51 mg/dL in controls, according to Dr. Lawrence Lee of the National University of Singapore.
"This cheap and safe treatment should be investigated further for reducing the risk of cardiovascular disease, especially in high-risk patients, such as those who are obese or diabetic, where the effect could be more clinically significant," he noted at the annual meeting of the Endocrine Society.
Twelve weeks of low-dose chloroquine had no impact on HDL, insulin levels, blood glucose, or the homeostatic model assessment (HOMA) insulin sensitivity index.
The chloroquine regimen consisted of 300 mg/day for the first 7 days followed by 300 mg once weekly for the next 11 weeks.
Previous animal studies by investigators at Washington University at St. Louis showed that daily doses of chloroquine or hydroxychloroquine reduced insulin resistance and improved lipid metabolism, most likely via activation of the ataxia telangiectasis mutated (ATM) gene (Cell Metab. 2006;4:377-89).
This work has led to ongoing large prospective clinical trials of daily chloroquine as a treatment for dyslipidemia and insulin resistance.
Dr. Lee and coworkers hypothesized that low-dose, once-weekly chloroquine could also be effective in metabolic syndrome, and with a more favorable safety profile than that of daily therapy. This study in healthy volunteers was a prelude to further studies in patients with metabolic syndrome.
The current study was actually a substudy of the Chloroquine for Influenza Prevention (CHIP) trial, in which 1,500 healthy volunteers were randomized to chloroquine or placebo, and which established that chloroquine does not prevent influenza (Lancet Infect. Dis. 2011; 677-83).
Dr. Lee reported having no financial conflicts. The CHIP trial was funded by the National Medical Research Council of Singapore.
HOUSTON – The antimalarial/immunomodulatory drug chloroquine might take on a new life as a treatment for patients with the metabolic syndrome.
In a randomized, double-blind trial involving 277 healthy Singapore volunteers assigned to low-dose chloroquine or placebo, mean triglyceride levels after 12 weeks were 77.9 mg/dL in the chloroquine group, an 11% reduction compared with the mean 87.7 mg/dL in controls.
Moreover, the total cholesterol–high-density lipoprotein ratio at the 12-week mark was 3.31 mg/dL in the chloroquine group, 7% better than the 3.51 mg/dL in controls, according to Dr. Lawrence Lee of the National University of Singapore.
"This cheap and safe treatment should be investigated further for reducing the risk of cardiovascular disease, especially in high-risk patients, such as those who are obese or diabetic, where the effect could be more clinically significant," he noted at the annual meeting of the Endocrine Society.
Twelve weeks of low-dose chloroquine had no impact on HDL, insulin levels, blood glucose, or the homeostatic model assessment (HOMA) insulin sensitivity index.
The chloroquine regimen consisted of 300 mg/day for the first 7 days followed by 300 mg once weekly for the next 11 weeks.
Previous animal studies by investigators at Washington University at St. Louis showed that daily doses of chloroquine or hydroxychloroquine reduced insulin resistance and improved lipid metabolism, most likely via activation of the ataxia telangiectasis mutated (ATM) gene (Cell Metab. 2006;4:377-89).
This work has led to ongoing large prospective clinical trials of daily chloroquine as a treatment for dyslipidemia and insulin resistance.
Dr. Lee and coworkers hypothesized that low-dose, once-weekly chloroquine could also be effective in metabolic syndrome, and with a more favorable safety profile than that of daily therapy. This study in healthy volunteers was a prelude to further studies in patients with metabolic syndrome.
The current study was actually a substudy of the Chloroquine for Influenza Prevention (CHIP) trial, in which 1,500 healthy volunteers were randomized to chloroquine or placebo, and which established that chloroquine does not prevent influenza (Lancet Infect. Dis. 2011; 677-83).
Dr. Lee reported having no financial conflicts. The CHIP trial was funded by the National Medical Research Council of Singapore.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: Once-weekly chloroquine resulted in an 11% reduction in triglycerides, a 7% decrease in the total cholesterol–HDL cholesterol ratio, and a nonsignificant 5% decrease in LDL cholesterol compared with placebo.
Data Source: This was a 12-week, randomized, double-blind clinical trial involving 277 healthy volunteers.
Disclosures: Dr. Lee reported having no financial conflicts. The CHIP trial was funded by the National Medical Research Council of Singapore.