User login
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
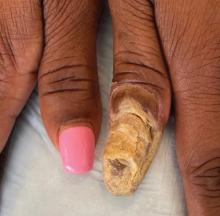
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
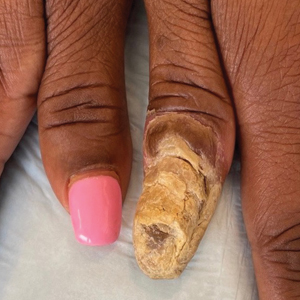
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
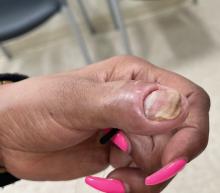
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
PRACTICE POINTS
- Nail psoriasis can masquerade as other dermatologic conditions, including squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
- Nail psoriasis can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.
- Deucravacitinib, an oral tyrosine kinase 2 inhibitor approved for the treatment of plaque psoriasis, has shown promise as an effective treatment for nail psoriasis in cases that are refractory to standard therapies.
Early liver transplantation for alcoholic hepatitis
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.