User login
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
THE DIAGNOSIS: Cutaneous Cryptococcosis
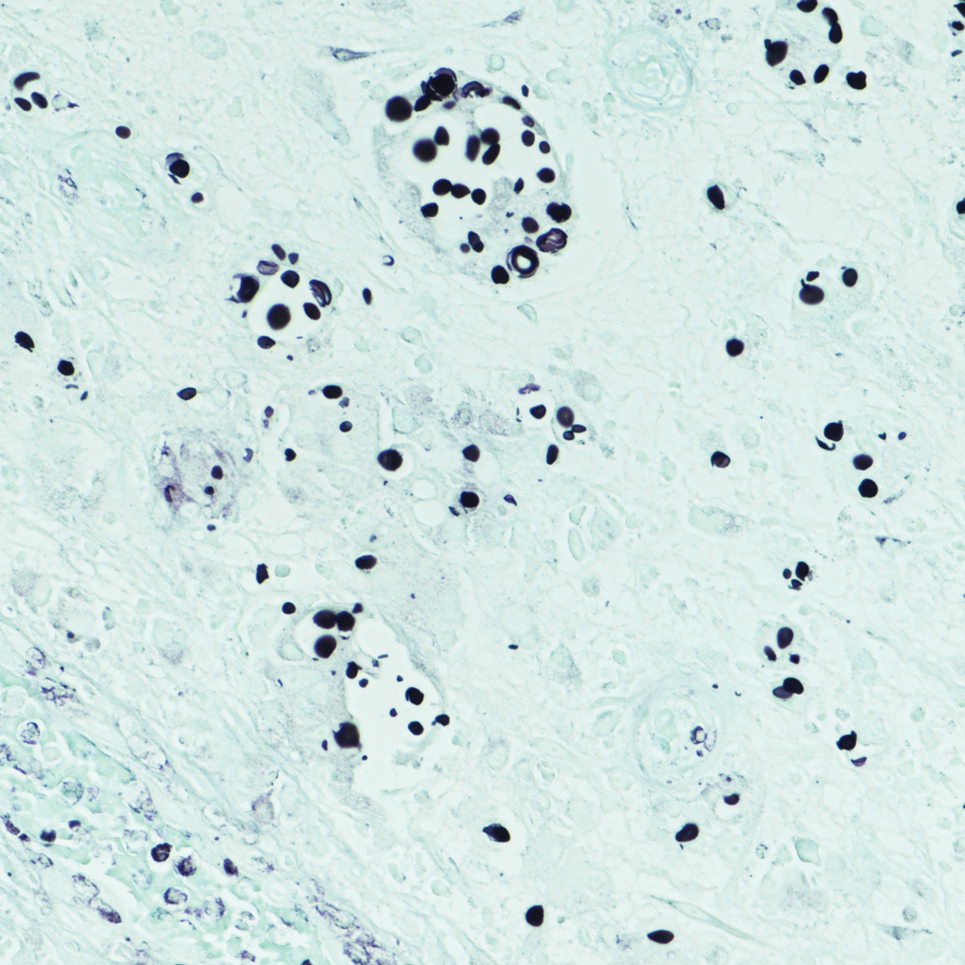
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
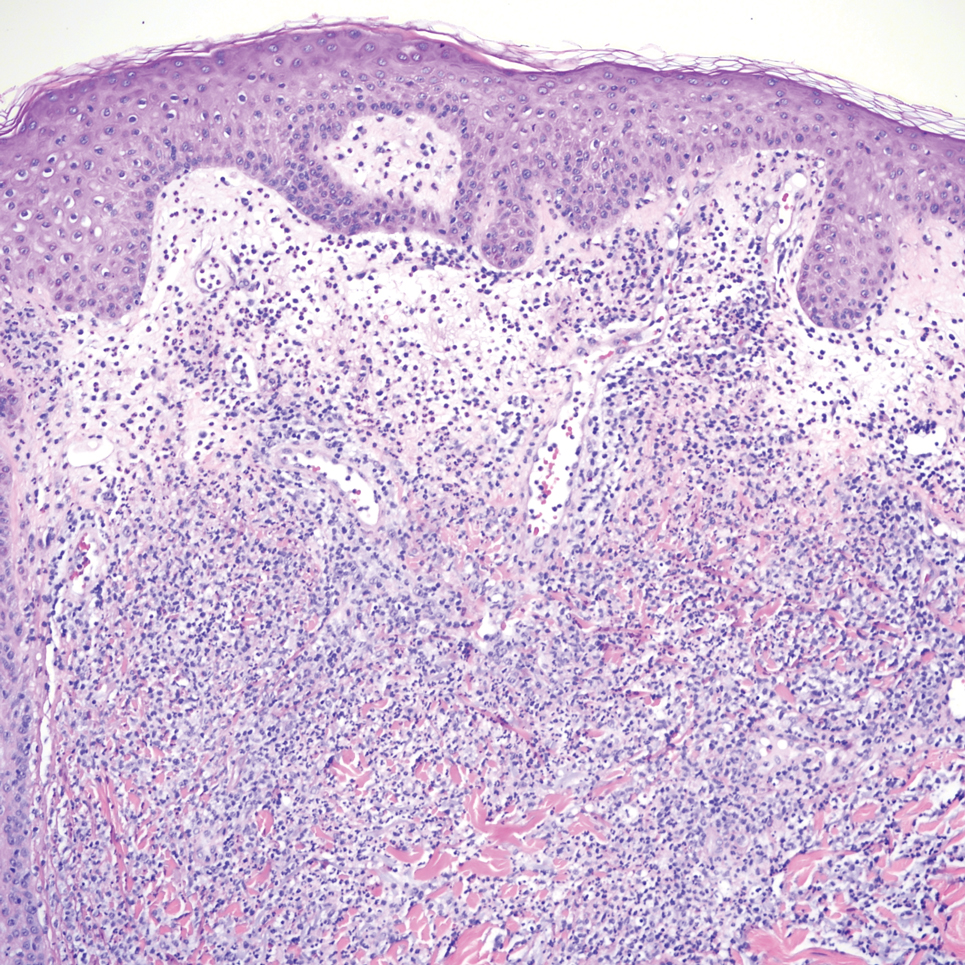
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
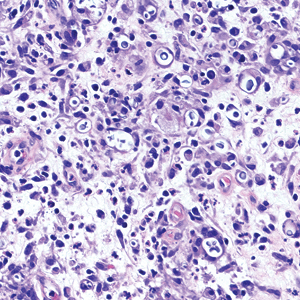
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
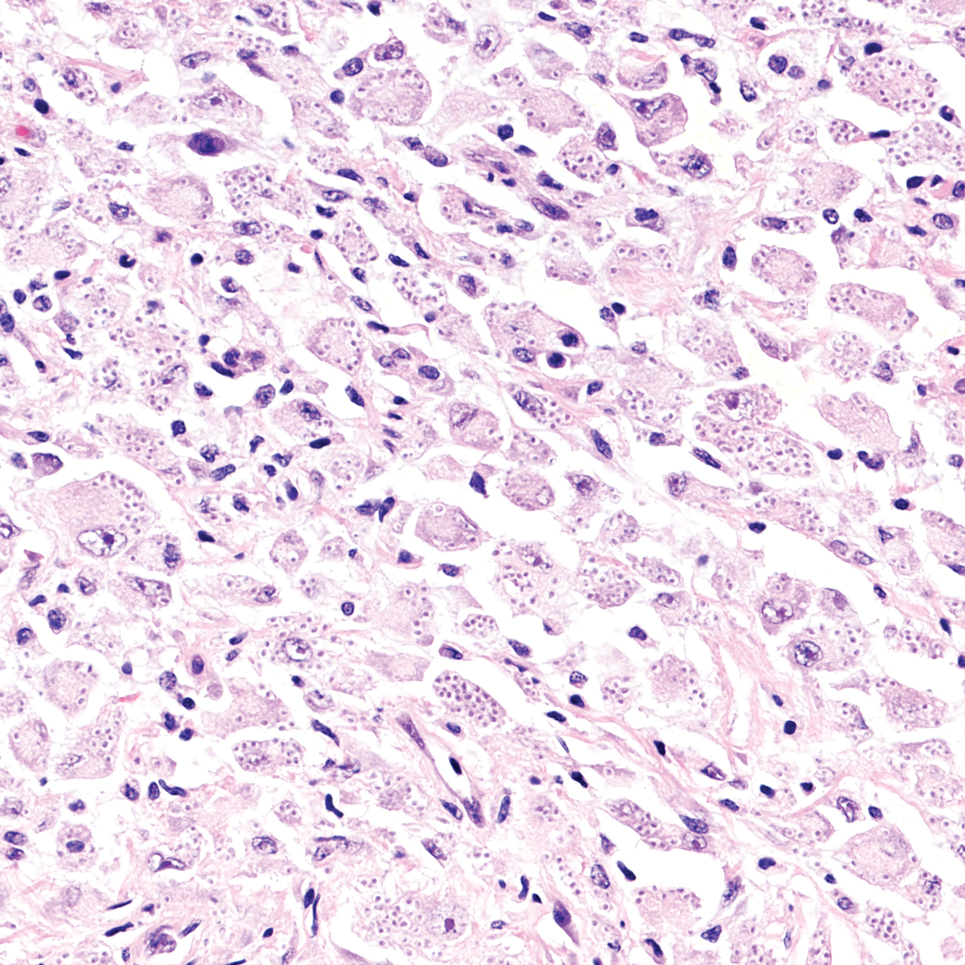
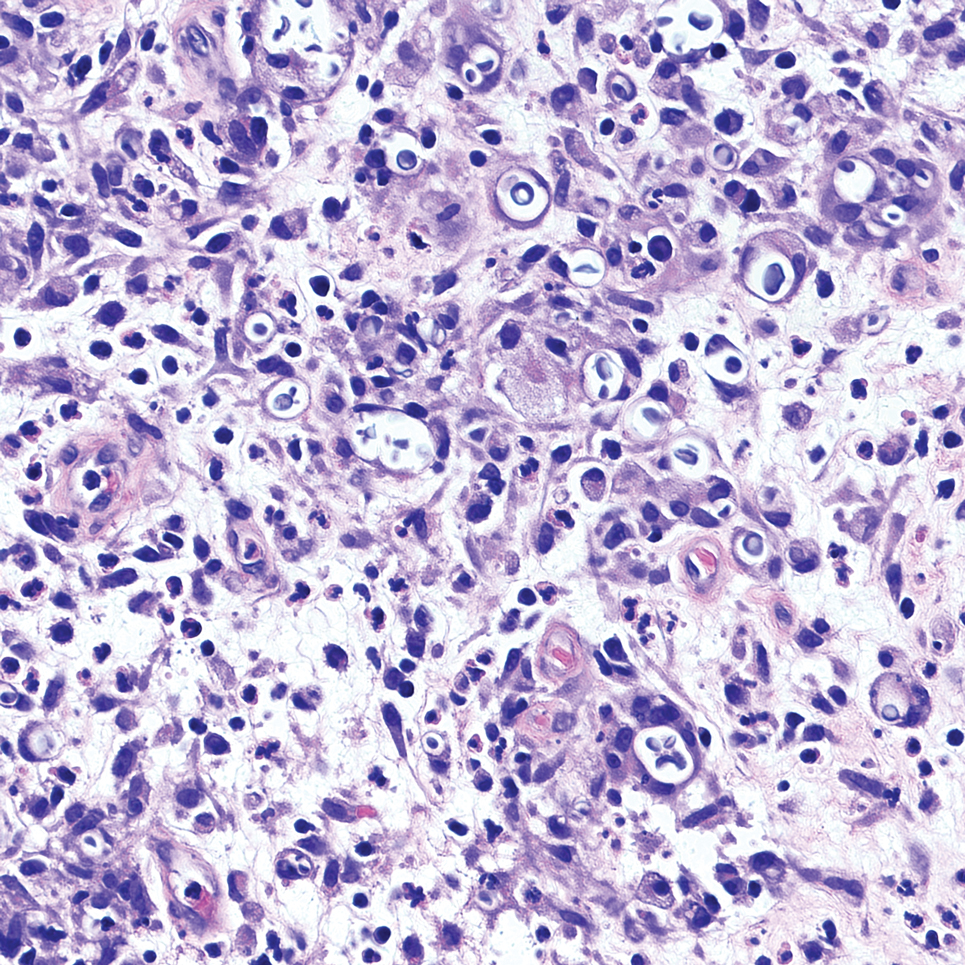
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13
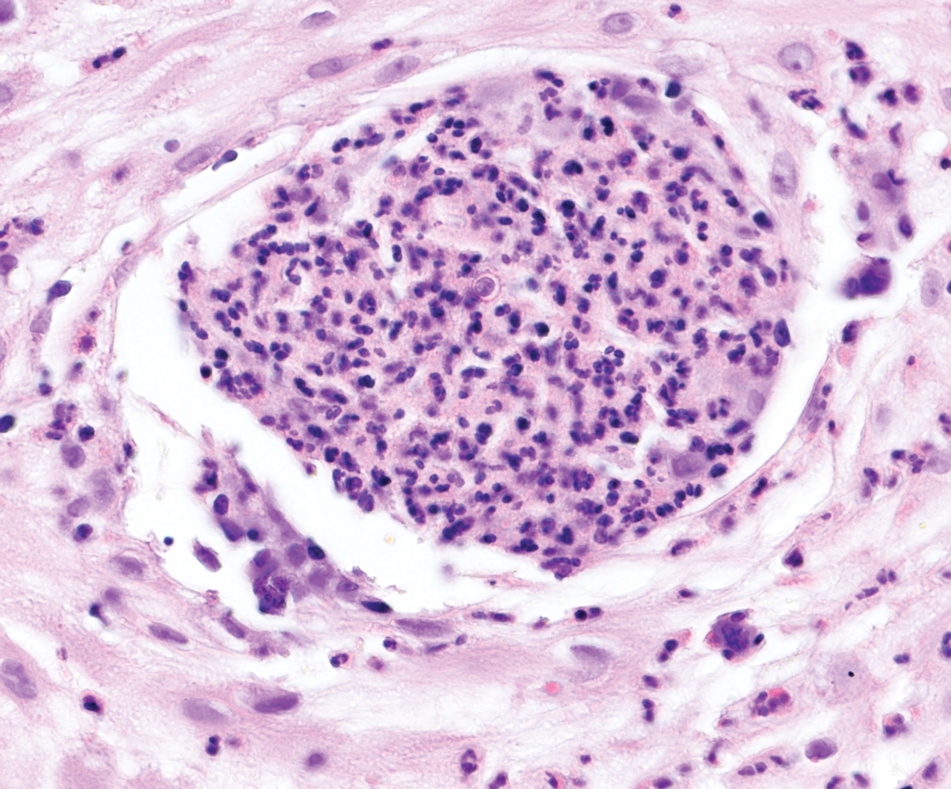
Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10
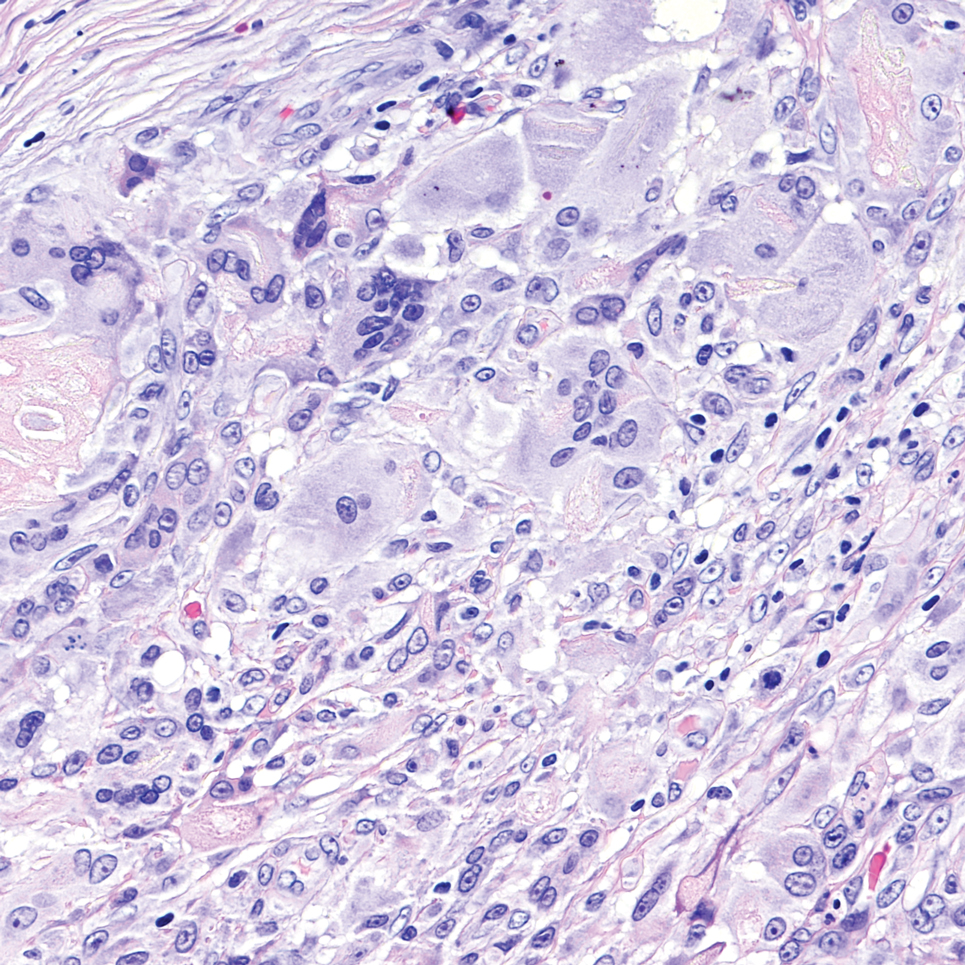
Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
A 51-year-old man with a history of multiple sclerosis treated with fingolimod presented to the dermatology department with an ulcerated lesion on the left forearm of 2 to 3 months’ duration. The patient reported that he recently presented to the emergency department for drainage of the lesion, which was unsuccessful. Shortly after, he traumatized the lesion at his construction job. At the current presentation, physical examination revealed a 1-cm, flesh-colored to faintly pink, ulcerated nodule on the left forearm. A biopsy was performed.
Blistering Disease During the Treatment of Chronic Hepatitis C With Ledipasvir/Sofosbuvir (FULL)
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
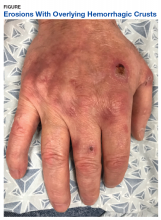
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
Porphyria cutanea tarda (PCT) is the most common type of porphyria. The accumulation of porphyrin in various organ systems results from a deficiency of uroporphyrinogen decarboxylase (UROD).1-3 Chronic hepatitis C virus (HCV) causes a hepatic decrease in hepcidin production, resulting in increased iron absorption. Iron loading and increased oxidative stress in the liver leads to nonporphyrin inhibition of UROD production and to oxidation of porphyrinogens to porphyrins.4 This in turn leads to accumulation of uroporphyrins and carboxylated metabolites that can be detected in urine.4
Signs of PCT include blisters, vesicles, and possibly milia developing on sun-exposed areas of the skin, such as the face, forearms, and dorsal hands.4 Case reports have demonstrated a resolution of PCT in patients with chronic HCV with treatment with direct-acting antivirals (DAAs), such as ledipasvir/sofosbuvir.1,3 However, here we present 2 cases of patients who developed blistering diseases during treatment of chronic HCV with ledipasvir/sofosbuvir. Neither demonstrated complete resolution of symptoms during the treatment regimen.
Cases
Patient 1
A 63-year-old white male with a history of chronic HCV (genotype 1a), bipolar disorder, hyperlipidemia, tobacco dependence, and cirrhosis (F4 by elastography) presented with minimally to moderately painful blisters on his bilateral dorsal hands that had developed around weeks 8 to 9 of treatment with ledipasvir/sofosbuvir. The patient reported that no new blisters had appeared following completion of 12 weeks of treatment and that his current blisters were in various stages of healing. He reported alcohol use of 1 to 2 twelve-ounce beers daily and no history of dioxin exposure. His medications included doxepin, hydralazine, hydrochlorothiazide, quetiapine, folic acid, and thiamine. His hepatitis C viral load was 440,000 IU/mL prior to treatment. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron level was 135 µg/dL, total iron-binding capacity (TIBC) was 323 µg/dL, and ferritin was 299.0 ng/mL. His HFE
A physical examination on presentation revealed erosions with overlying hemorrhagic crusts on the bilateral dorsal hands (Figure).
At the 4-month follow-up, the patient reported no new blister formations. A physical examination revealed well-healed scars and several clustered milia on bilateral dorsal hands with no active vesicles or bullae noted.
Patient 2
An African American male aged 63 years presented with a 1-month history of moderately painful blisters on his bilateral dorsal hands during treatment of chronic HCV (genotype 1a) with ledipasvir/sofosbuvir. His medical history included gout, tobacco and alcohol addiction, osteoarthritis, and hepatic fibrosis (F3 by elastography). The patient’s medications included allopurinol, lisinopril, and hydrochlorothiazide. He reported no history of dioxin exposure. On the day of presentation, he was on week 9 of the 12-week treatment ledipasvir/sofosbuvir regimen. Laboratory results included an initial HCV viral load of 1,618,605 IU/mL. Tests for hepatitis B surface antigen and HIV antibodies were negative. His iron was 191 µg/dL, TIBC 388 µg/dL, and ferritin 459.0 ng/mL. After 4 weeks of treatment, the patient’s hepatitis C viral load was undetectable.
A physical examination revealed several resolving erosions to his bilateral dorsal hands, some of which had overlying crusting along with one small hemorrhagic vesicle on the right dorsal hand. A punch biopsy of the hemorrhagic vesicle was performed and demonstrated a cell-poor subepidermal blister with festooning of the dermal papilla. A direct immunofluorescence study showed immunoglobulin (Ig) G fluorescence along the dermal-epidermal junction and within vessel walls in the superficial dermis. Weak IgM and C3 fluorescence also was noted within vessel walls in the superficial dermis. All of the patient findings and history were consistent with PCT, although pseudo-PCT also was a consideration. A 24-hour urine sample yielded negative results for porphobilinogen. Urine porphyrin test results were not available, leading to a presumptive histological diagnosis of PCT.
The patient completed 11 of the prescribed 12 weeks of ledipasvir/sofosbuvir. The blisters resolved shortly thereafter.
Discussion
PCT has a well-established association with chronic HCV infection.4 We present 2 cases of a blistering disease clinically and histologically compatible with PCT that developed in patients only after initiation of treatment for chronic HCV with ledipasvir/sofosbuvir. One case was confirmed as PCT on the basis of compatible histopathologic findings and a urine porphyrin assay that showed elevated levels of uroporphyrins and carboxylated metabolites. The second case was clinically and histologically suggestive of PCT but not confirmed by urine porphyrin testing. In both patients, after 8 to 9 weeks of a 12-week course of antiviral therapy, the blistering lesions were noted but appeared to be resolving, and no new lesions were noted after discontinuation of therapy. It appeared that the antiviral treatment temporally triggered the initiation of the blistering skin disease, and as the chronic HCV infection cleared after treatment, the blistering lesions also began to resolve.
Mechanistically, it is known that the virally-induced hepatic damage leads to inhibition of uroporphyrinogen decarboxylase, and the subsequent oxidation of porphyrinogens to porphyrins. Cofactors such as HIV infection also may contribute to development of PCT.5
De novo PCT has been documented during therapy using interferon and ribavirin.6 The hemolytic anemia and increased hepatic iron were implicated as potential etiologies.6 Patients with HCV and PCT treated with the newer direct-acting antiviral therapies have been described to have experienced improvement in PCT symptoms.3
Although there were rare reports of deterioration in renal and liver function,7 reactivation of HBV infection,8 and Stevens-Johnson syndrome9 with antiviral therapy, these complications were not observed in these patients. Both patients also had successful resolution of HCV infection, and by completion of the antiviral therapy, the blistering also resolved.
Conclusion
PCT is an extrahepatic manifestation of HCV infection. Health care providers should be aware of the association of chronic HCV infection with PCT. The findings of PCT should not result in the delay or discontinuation of antiviral therapy.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.
1. Combalia A, To-Figueras J, Laguno M, Martinez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184.
2. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150(7):1599-1608.
3. Tong Y, Song YK, Tyring S. Resolution of porphyria cutanea tarda in patients with hepatitis C following ledipasvir/sofosbuvir combination therapy. JAMA Dermatol. 2016;152(12):1393-1395.
4. Ryan Caballes F, Sendi H, Bonkovsky H. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
5. Quansah R, Cooper CJ, Said S, Bizet J, Paez D, Hernandez GT. Hepatitis C- and HIV-induced porphyria cutanea tarda. Am J Case Rep. 2014;15:35-40.
6. Azim J, McCurdy H, Moseley RH. Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C. World J Gastroenterol. 2008;14(38):5913-5915.
7. Ahmed M. Harvoni-induced deterioration of renal and liver function. Adv Res Gastroentero Hepatol. 2017;2(3):555588.
8. De Monte A, Courion J, Anty R, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27-30.
9. Verma N, Singh S, Sawatkar G, Singh V. Sofosbuvir induced Steven Johnson Syndrome in a patient with hepatitis C virus-related cirrhosis. Hepatol Commun. 2017;2(1):16-20.