User login
Hepatitis D Virus Classified as Carcinogenic: Implications
The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
Individuals with HBV-HDV coinfection face an elevated risk for liver cancer, highlighting the need for HBV vaccination, systematic screening, and early antiviral treatment to reduce the progression to cirrhosis and HCC.
About 12 million people globally have HBV-HDV coinfection, representing 5% of all chronic HBV cases. The prevalence of this condition varies regionally, with a likely underdiagnosis. True coinfection rates may reach 13%-14%, the highest in Europe’s Mediterranean region.
Virus Biology
HDV is an incomplete virus that infects hepatocytes and requires the envelope protein of hepatitis B surface antigen (HBsAg) for cell exit. Infection occurs only with chronic HBV infection, either as a superinfection or simultaneous acquisition. Humans are the only known natural host.
HDV coinfection worsens HBV-induced hepatic inflammation and prognosis, and up to 80% of patients develop cirrhosis. Triple infection with the HBV virus, HDV, and HIV further increases this risk, and the global prevalence is likely underestimated.
Cancer Risk
HDV infection significantly increases the risk for HCC compared with HBV infection alone. Many patients die from decompensated cirrhosis or HCC, reflecting the aggressive nature of coinfection.
The molecular mechanisms underlying HDV oncogenesis remain unclear. Research conducted over the past 15 years has provided insights that could inform the development of more effective treatments.
Early vaccination prophylaxis is critical for reducing the risk for HCC, despite limited options.
Treatment Options
Randomized controlled trials have demonstrated antiviral efficacy for:
- Pegylated interferon alpha (Peg-IFN) is approved for HBV and is active against HDV.
- Bulevirtide, a synthetic myristoylated lipopeptide entry inhibitor, is used alone or in combination with Peg-IFN.
Suppression of HBV remains central. Nucleoside and nucleotide analogs, such as entecavir, tenofovir alafenamide fumarate, and tenofovir disoproxil fumarate, significantly reduce HCC progression in treated patients compared with untreated patients at risk.
Promising therapeutics include lonafarnib, a farnesyltransferase inhibitor that blocks HDV particle formation, and nucleic acid polymers targeting the host chaperone DNAJB12 to inhibit HBV and HDV replication.
Guideline Updates
The 2023 addendum to the S3 guidelines covers the prophylaxis, diagnosis, and treatment of HBV, including HDV management.
IARC experts also re-evaluated the human cytomegalovirus and Merkel cell polyomavirus. Complete assessments are expected in the next edition of IARC Monographs.
HBV Vaccination
HBV vaccination is the only effective prophylaxis against HBV and HDV. Introduced in 1982 for high-risk groups, it reduced chronic infections, with the WHO expanding its recommendations from 1992 onward.
Infants and young children are at the highest risk of developing this disease. Acute HBV infection often resolves in adults, but infants face up to a 90% risk of developing chronic infection. Newborns of mothers with chronic or undiagnosed HBV infections are particularly vulnerable.
Routine infant immunization includes three doses, with the first dose administered within 12 hours of birth. In Germany, the Standing Committee on Vaccination (STIKO) recommends the administration of combination vaccines, with the hexavalent vaccine administered at 2, 4, and 11 months in a 2 + 1 schedule.
Timely vaccination is crucial because undetected chronic infections often lead to late-stage HCC diagnosis. Adults in high-risk groups should receive HBV vaccination counseling.
STIKO recommends vaccination for close contacts of individuals who are HBsAg-positive, individuals with high-risk sexual contacts, immunocompromised persons, and those with preexisting conditions that increase the risk for severe HBV infection.
Since 2021, insured adults aged 35 years or older in Germany have undergone one-time HBV and HCV screening. HDV testing is recommended for all HBsAg-positive patients. Current frameworks may miss cases, and additional or personalized screening could improve the detection of previously unrecognized infections.
This story was translated from Univadis Germany.
A version of this article appeared on Medscape.com.
The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
Individuals with HBV-HDV coinfection face an elevated risk for liver cancer, highlighting the need for HBV vaccination, systematic screening, and early antiviral treatment to reduce the progression to cirrhosis and HCC.
About 12 million people globally have HBV-HDV coinfection, representing 5% of all chronic HBV cases. The prevalence of this condition varies regionally, with a likely underdiagnosis. True coinfection rates may reach 13%-14%, the highest in Europe’s Mediterranean region.
Virus Biology
HDV is an incomplete virus that infects hepatocytes and requires the envelope protein of hepatitis B surface antigen (HBsAg) for cell exit. Infection occurs only with chronic HBV infection, either as a superinfection or simultaneous acquisition. Humans are the only known natural host.
HDV coinfection worsens HBV-induced hepatic inflammation and prognosis, and up to 80% of patients develop cirrhosis. Triple infection with the HBV virus, HDV, and HIV further increases this risk, and the global prevalence is likely underestimated.
Cancer Risk
HDV infection significantly increases the risk for HCC compared with HBV infection alone. Many patients die from decompensated cirrhosis or HCC, reflecting the aggressive nature of coinfection.
The molecular mechanisms underlying HDV oncogenesis remain unclear. Research conducted over the past 15 years has provided insights that could inform the development of more effective treatments.
Early vaccination prophylaxis is critical for reducing the risk for HCC, despite limited options.
Treatment Options
Randomized controlled trials have demonstrated antiviral efficacy for:
- Pegylated interferon alpha (Peg-IFN) is approved for HBV and is active against HDV.
- Bulevirtide, a synthetic myristoylated lipopeptide entry inhibitor, is used alone or in combination with Peg-IFN.
Suppression of HBV remains central. Nucleoside and nucleotide analogs, such as entecavir, tenofovir alafenamide fumarate, and tenofovir disoproxil fumarate, significantly reduce HCC progression in treated patients compared with untreated patients at risk.
Promising therapeutics include lonafarnib, a farnesyltransferase inhibitor that blocks HDV particle formation, and nucleic acid polymers targeting the host chaperone DNAJB12 to inhibit HBV and HDV replication.
Guideline Updates
The 2023 addendum to the S3 guidelines covers the prophylaxis, diagnosis, and treatment of HBV, including HDV management.
IARC experts also re-evaluated the human cytomegalovirus and Merkel cell polyomavirus. Complete assessments are expected in the next edition of IARC Monographs.
HBV Vaccination
HBV vaccination is the only effective prophylaxis against HBV and HDV. Introduced in 1982 for high-risk groups, it reduced chronic infections, with the WHO expanding its recommendations from 1992 onward.
Infants and young children are at the highest risk of developing this disease. Acute HBV infection often resolves in adults, but infants face up to a 90% risk of developing chronic infection. Newborns of mothers with chronic or undiagnosed HBV infections are particularly vulnerable.
Routine infant immunization includes three doses, with the first dose administered within 12 hours of birth. In Germany, the Standing Committee on Vaccination (STIKO) recommends the administration of combination vaccines, with the hexavalent vaccine administered at 2, 4, and 11 months in a 2 + 1 schedule.
Timely vaccination is crucial because undetected chronic infections often lead to late-stage HCC diagnosis. Adults in high-risk groups should receive HBV vaccination counseling.
STIKO recommends vaccination for close contacts of individuals who are HBsAg-positive, individuals with high-risk sexual contacts, immunocompromised persons, and those with preexisting conditions that increase the risk for severe HBV infection.
Since 2021, insured adults aged 35 years or older in Germany have undergone one-time HBV and HCV screening. HDV testing is recommended for all HBsAg-positive patients. Current frameworks may miss cases, and additional or personalized screening could improve the detection of previously unrecognized infections.
This story was translated from Univadis Germany.
A version of this article appeared on Medscape.com.
The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
Individuals with HBV-HDV coinfection face an elevated risk for liver cancer, highlighting the need for HBV vaccination, systematic screening, and early antiviral treatment to reduce the progression to cirrhosis and HCC.
About 12 million people globally have HBV-HDV coinfection, representing 5% of all chronic HBV cases. The prevalence of this condition varies regionally, with a likely underdiagnosis. True coinfection rates may reach 13%-14%, the highest in Europe’s Mediterranean region.
Virus Biology
HDV is an incomplete virus that infects hepatocytes and requires the envelope protein of hepatitis B surface antigen (HBsAg) for cell exit. Infection occurs only with chronic HBV infection, either as a superinfection or simultaneous acquisition. Humans are the only known natural host.
HDV coinfection worsens HBV-induced hepatic inflammation and prognosis, and up to 80% of patients develop cirrhosis. Triple infection with the HBV virus, HDV, and HIV further increases this risk, and the global prevalence is likely underestimated.
Cancer Risk
HDV infection significantly increases the risk for HCC compared with HBV infection alone. Many patients die from decompensated cirrhosis or HCC, reflecting the aggressive nature of coinfection.
The molecular mechanisms underlying HDV oncogenesis remain unclear. Research conducted over the past 15 years has provided insights that could inform the development of more effective treatments.
Early vaccination prophylaxis is critical for reducing the risk for HCC, despite limited options.
Treatment Options
Randomized controlled trials have demonstrated antiviral efficacy for:
- Pegylated interferon alpha (Peg-IFN) is approved for HBV and is active against HDV.
- Bulevirtide, a synthetic myristoylated lipopeptide entry inhibitor, is used alone or in combination with Peg-IFN.
Suppression of HBV remains central. Nucleoside and nucleotide analogs, such as entecavir, tenofovir alafenamide fumarate, and tenofovir disoproxil fumarate, significantly reduce HCC progression in treated patients compared with untreated patients at risk.
Promising therapeutics include lonafarnib, a farnesyltransferase inhibitor that blocks HDV particle formation, and nucleic acid polymers targeting the host chaperone DNAJB12 to inhibit HBV and HDV replication.
Guideline Updates
The 2023 addendum to the S3 guidelines covers the prophylaxis, diagnosis, and treatment of HBV, including HDV management.
IARC experts also re-evaluated the human cytomegalovirus and Merkel cell polyomavirus. Complete assessments are expected in the next edition of IARC Monographs.
HBV Vaccination
HBV vaccination is the only effective prophylaxis against HBV and HDV. Introduced in 1982 for high-risk groups, it reduced chronic infections, with the WHO expanding its recommendations from 1992 onward.
Infants and young children are at the highest risk of developing this disease. Acute HBV infection often resolves in adults, but infants face up to a 90% risk of developing chronic infection. Newborns of mothers with chronic or undiagnosed HBV infections are particularly vulnerable.
Routine infant immunization includes three doses, with the first dose administered within 12 hours of birth. In Germany, the Standing Committee on Vaccination (STIKO) recommends the administration of combination vaccines, with the hexavalent vaccine administered at 2, 4, and 11 months in a 2 + 1 schedule.
Timely vaccination is crucial because undetected chronic infections often lead to late-stage HCC diagnosis. Adults in high-risk groups should receive HBV vaccination counseling.
STIKO recommends vaccination for close contacts of individuals who are HBsAg-positive, individuals with high-risk sexual contacts, immunocompromised persons, and those with preexisting conditions that increase the risk for severe HBV infection.
Since 2021, insured adults aged 35 years or older in Germany have undergone one-time HBV and HCV screening. HDV testing is recommended for all HBsAg-positive patients. Current frameworks may miss cases, and additional or personalized screening could improve the detection of previously unrecognized infections.
This story was translated from Univadis Germany.
A version of this article appeared on Medscape.com.
A Rare Delayed Presentation of Immune-Related Hepatitis in a Patient Treated With Pembrolizumab
Background
Immune checkpoint inhibitors, including pembrolizumab, are associated with a spectrum of immune-related adverse events (irAEs), including immune- mediated hepatitis. Typically, this toxicity manifests within the first 14 weeks of therapy. Delayed presentations beyond one year are exceedingly rare and pose diagnostic challenges.
Case Presentation
We report an elderly patient (over 90 years old) with stage IVa squamous cell carcinoma of the lung and high microsatellite instability (MSI) who had been receiving pembrolizumab since 2023. In 2024—13 months into therapy—he presented with subjective fevers, weakness, and altered mental status. Laboratory evaluation revealed cholestatic jaundice with AST 310 U/L, ALT 291 U/L, alkaline phosphatase 860 U/L, and total bilirubin 5.7 mg/dL. Infectious workup was negative. Imaging via MRCP showed multiple scattered hepatic cysts and a small pancreatic cyst, without biliary obstruction.
Further evaluation, including serologies for hepatitis B and C, CMV, HSV, autoimmune hepatitis panel, iron studies, and ceruloplasmin, was unremarkable except for mildly elevated alpha-1 antitrypsin. Scattered liver cysts were seen on an MRI. The overall findings were most consistent with immune-related hepatitis, as pembrolizumab is known to cause both hepatocellular and cholestatic patterns of liver injury.
The patient was started on high-dose prednisone, resulting in rapid clinical and biochemical improvement. Two weeks post-discharge, liver function tests (LFTs) had markedly improved (bilirubin 1.3, AST 19, ALT 40, ALP 193). Given the severity of transaminitis and hyperbilirubinemia (AST >8x ULN, bilirubin >3x ULN), pembrolizumab was permanently discontinued. LFTs normalized after completion of the steroid taper.
Conclusions
This case highlights a rare instance of delayed immune-related hepatitis occurring over a year after initiation of pembrolizumab, far beyond the typical window of onset. Clinicians should maintain a high index of suspicion for irAEs even in late stages of immunotherapy, particularly when common etiologies are excluded. Prompt recognition and corticosteroid treatment can lead to favorable outcomes, even in older patients.
Background
Immune checkpoint inhibitors, including pembrolizumab, are associated with a spectrum of immune-related adverse events (irAEs), including immune- mediated hepatitis. Typically, this toxicity manifests within the first 14 weeks of therapy. Delayed presentations beyond one year are exceedingly rare and pose diagnostic challenges.
Case Presentation
We report an elderly patient (over 90 years old) with stage IVa squamous cell carcinoma of the lung and high microsatellite instability (MSI) who had been receiving pembrolizumab since 2023. In 2024—13 months into therapy—he presented with subjective fevers, weakness, and altered mental status. Laboratory evaluation revealed cholestatic jaundice with AST 310 U/L, ALT 291 U/L, alkaline phosphatase 860 U/L, and total bilirubin 5.7 mg/dL. Infectious workup was negative. Imaging via MRCP showed multiple scattered hepatic cysts and a small pancreatic cyst, without biliary obstruction.
Further evaluation, including serologies for hepatitis B and C, CMV, HSV, autoimmune hepatitis panel, iron studies, and ceruloplasmin, was unremarkable except for mildly elevated alpha-1 antitrypsin. Scattered liver cysts were seen on an MRI. The overall findings were most consistent with immune-related hepatitis, as pembrolizumab is known to cause both hepatocellular and cholestatic patterns of liver injury.
The patient was started on high-dose prednisone, resulting in rapid clinical and biochemical improvement. Two weeks post-discharge, liver function tests (LFTs) had markedly improved (bilirubin 1.3, AST 19, ALT 40, ALP 193). Given the severity of transaminitis and hyperbilirubinemia (AST >8x ULN, bilirubin >3x ULN), pembrolizumab was permanently discontinued. LFTs normalized after completion of the steroid taper.
Conclusions
This case highlights a rare instance of delayed immune-related hepatitis occurring over a year after initiation of pembrolizumab, far beyond the typical window of onset. Clinicians should maintain a high index of suspicion for irAEs even in late stages of immunotherapy, particularly when common etiologies are excluded. Prompt recognition and corticosteroid treatment can lead to favorable outcomes, even in older patients.
Background
Immune checkpoint inhibitors, including pembrolizumab, are associated with a spectrum of immune-related adverse events (irAEs), including immune- mediated hepatitis. Typically, this toxicity manifests within the first 14 weeks of therapy. Delayed presentations beyond one year are exceedingly rare and pose diagnostic challenges.
Case Presentation
We report an elderly patient (over 90 years old) with stage IVa squamous cell carcinoma of the lung and high microsatellite instability (MSI) who had been receiving pembrolizumab since 2023. In 2024—13 months into therapy—he presented with subjective fevers, weakness, and altered mental status. Laboratory evaluation revealed cholestatic jaundice with AST 310 U/L, ALT 291 U/L, alkaline phosphatase 860 U/L, and total bilirubin 5.7 mg/dL. Infectious workup was negative. Imaging via MRCP showed multiple scattered hepatic cysts and a small pancreatic cyst, without biliary obstruction.
Further evaluation, including serologies for hepatitis B and C, CMV, HSV, autoimmune hepatitis panel, iron studies, and ceruloplasmin, was unremarkable except for mildly elevated alpha-1 antitrypsin. Scattered liver cysts were seen on an MRI. The overall findings were most consistent with immune-related hepatitis, as pembrolizumab is known to cause both hepatocellular and cholestatic patterns of liver injury.
The patient was started on high-dose prednisone, resulting in rapid clinical and biochemical improvement. Two weeks post-discharge, liver function tests (LFTs) had markedly improved (bilirubin 1.3, AST 19, ALT 40, ALP 193). Given the severity of transaminitis and hyperbilirubinemia (AST >8x ULN, bilirubin >3x ULN), pembrolizumab was permanently discontinued. LFTs normalized after completion of the steroid taper.
Conclusions
This case highlights a rare instance of delayed immune-related hepatitis occurring over a year after initiation of pembrolizumab, far beyond the typical window of onset. Clinicians should maintain a high index of suspicion for irAEs even in late stages of immunotherapy, particularly when common etiologies are excluded. Prompt recognition and corticosteroid treatment can lead to favorable outcomes, even in older patients.
The Most Common Chronic Liver Disease in the World
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, what is MASLD?
Paul N. Williams, MD:
Watto: We talked about a really stripped-down way of testing people for MASLD. If we see mildly elevated liver enzymes, what should we be testing, and how does alcohol factor in?
Williams: Before you can make a definitive diagnosis of MASLD, you need to rule out other causes of liver inflammation — things that would cause a patient’s transaminases to increase. Alcohol is synergistic with everything that can harm the liver.
A great place to start is to gauge someone’s alcohol intake to make sure it isn’t causing hepatic inflammation. The phosphatidyl ethanol level is a serologic test to determine chronic, heavy alcohol use. It’s a new kid on the block. I’ve seen it mostly ordered by hepatologists. It is a way of determining whether someone has had fairly consistent alcohol use up to 4 weeks after the fact. The cutoff for a positive test is 20 ng/mL.
Dr Tapper frames the test this way. He isn’t using the test to catch someone in a lie about their alcohol use. He tells patients that he orders this test for all patients with liver inflammation, because alcohol is a common cause. The test helps him better understand the factors that might be affecting the patient’s liver function.
If the test comes back positive, you can have a conversation about that, and if it’s not positive, you move on to the next possible cause. Other fairly common causes of liver inflammation are relatively easy to address.
Watto: Instead of ordering ceruloplasmin or alpha-1 antitrypsin tests, for example, the first thing Dr Tapper recommends is checking for hepatitis B and C. We can cure hepatitis C. We can’t cure hepatitis B, but it’s important to know if the patient has it. Primary care physicians should be comfortable ordering these tests.
Really high ALT levels (eg, in the 200s) don’t usually happen from steatotic liver disease. In those cases, we would send an expanded panel that might include tests for autoimmune hepatitis-ANA, anti–smooth muscle antibody, and IgG levels. Otherwise, most of these patients don’t need much more testing.
What is a FIB4 score and how does that factor in?
Williams: The FIB4 score estimates the degree of fibrosis based on the ALT and AST levels, platelet count, and the patient’s age. These data are plugged into a formula. If the FIB4 score is low (meaning not much fibrosis is present), you can stop there and do your counseling about lifestyle changes and address the reversible factors.
If the FIB4 score is above a certain threshold (1.3 in young adults and 2.0 in older adults), you need to find a more concrete way to determine the degree of fibrosis, typically through imaging.
Elastography can be done either with ultrasound or MRI. Ultrasound is typically ordered, but Dr Tapper recommends doing MRI on patients with a BMI > 40. Those patients are probably better served by doing MRI to determine the degree of liver fibrosis.
Watto: Patients with low FIB4 scores probably don’t need elastography but those with high FIB4 scores do. For the interpretation of ultrasound-based elastography results, Dr Tapper gave us the “rule of 5s”.
Elastography results are reported in kilopascal (kPa) units. A finding of 5 kPa or less is normal. Forty percent of those with a result of 10 kPa might have advanced liver disease. Above 15 kPa, the likelihood of cirrhosis is high, becoming very likely at 25 kPa. Finally, with a result of > 25 kPa, portal hypertension is likely, and you might need to have a conversation about starting the patient on medicine to prevent variceal bleeding.
We are moving toward more noninvasive testing and avoiding biopsies. We have cutoff values for MRI-based elastography as well. Both of these tests can help stage the liver.
What can we tell people about diet?
Williams: Weight loss is helpful. You can reverse fibrosis with weight loss. You can truly help your liver and bring it closer to its healthy baseline with weight loss. A loss of 7.5% body weight can reduce steatohepatitis, and with around 10% of body weight loss, you can actually resolve fibrosis, which is remarkable.
We all know that weight loss can be very therapeutic for many conditions. It’s just very hard to achieve. As primary care doctors, we should use what we have in our armamentarium to achieve that goal. Often, that will include certain medications.
Watto: I like giving patients the 10% number because if they weigh 220 pounds, they need to lose 22 pounds. If they weigh 300 pounds, it’s 30 pounds. Most people who weigh 300 pounds think they need to lose 100 pounds to have any sort of health benefit, but it’s much less than that. So, I do find that helpful.
But now a new drug has been approved. It’s a thyroid memetic called resmetirom. It was from the MAESTRO-NASH trial. Without weight loss, it helped to reverse fibrosis.
This is going to be used more and more in the future. It’s still being worked out exactly where the place is for that drug, so much so that Dr Tapper, as a liver expert, hadn’t even had the chance to prescribe it yet. Of course, it was very recently approved.
Dr. Tapper is one of our most celebrated guests, so check out the full podcast here.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, what is MASLD?
Paul N. Williams, MD:
Watto: We talked about a really stripped-down way of testing people for MASLD. If we see mildly elevated liver enzymes, what should we be testing, and how does alcohol factor in?
Williams: Before you can make a definitive diagnosis of MASLD, you need to rule out other causes of liver inflammation — things that would cause a patient’s transaminases to increase. Alcohol is synergistic with everything that can harm the liver.
A great place to start is to gauge someone’s alcohol intake to make sure it isn’t causing hepatic inflammation. The phosphatidyl ethanol level is a serologic test to determine chronic, heavy alcohol use. It’s a new kid on the block. I’ve seen it mostly ordered by hepatologists. It is a way of determining whether someone has had fairly consistent alcohol use up to 4 weeks after the fact. The cutoff for a positive test is 20 ng/mL.
Dr Tapper frames the test this way. He isn’t using the test to catch someone in a lie about their alcohol use. He tells patients that he orders this test for all patients with liver inflammation, because alcohol is a common cause. The test helps him better understand the factors that might be affecting the patient’s liver function.
If the test comes back positive, you can have a conversation about that, and if it’s not positive, you move on to the next possible cause. Other fairly common causes of liver inflammation are relatively easy to address.
Watto: Instead of ordering ceruloplasmin or alpha-1 antitrypsin tests, for example, the first thing Dr Tapper recommends is checking for hepatitis B and C. We can cure hepatitis C. We can’t cure hepatitis B, but it’s important to know if the patient has it. Primary care physicians should be comfortable ordering these tests.
Really high ALT levels (eg, in the 200s) don’t usually happen from steatotic liver disease. In those cases, we would send an expanded panel that might include tests for autoimmune hepatitis-ANA, anti–smooth muscle antibody, and IgG levels. Otherwise, most of these patients don’t need much more testing.
What is a FIB4 score and how does that factor in?
Williams: The FIB4 score estimates the degree of fibrosis based on the ALT and AST levels, platelet count, and the patient’s age. These data are plugged into a formula. If the FIB4 score is low (meaning not much fibrosis is present), you can stop there and do your counseling about lifestyle changes and address the reversible factors.
If the FIB4 score is above a certain threshold (1.3 in young adults and 2.0 in older adults), you need to find a more concrete way to determine the degree of fibrosis, typically through imaging.
Elastography can be done either with ultrasound or MRI. Ultrasound is typically ordered, but Dr Tapper recommends doing MRI on patients with a BMI > 40. Those patients are probably better served by doing MRI to determine the degree of liver fibrosis.
Watto: Patients with low FIB4 scores probably don’t need elastography but those with high FIB4 scores do. For the interpretation of ultrasound-based elastography results, Dr Tapper gave us the “rule of 5s”.
Elastography results are reported in kilopascal (kPa) units. A finding of 5 kPa or less is normal. Forty percent of those with a result of 10 kPa might have advanced liver disease. Above 15 kPa, the likelihood of cirrhosis is high, becoming very likely at 25 kPa. Finally, with a result of > 25 kPa, portal hypertension is likely, and you might need to have a conversation about starting the patient on medicine to prevent variceal bleeding.
We are moving toward more noninvasive testing and avoiding biopsies. We have cutoff values for MRI-based elastography as well. Both of these tests can help stage the liver.
What can we tell people about diet?
Williams: Weight loss is helpful. You can reverse fibrosis with weight loss. You can truly help your liver and bring it closer to its healthy baseline with weight loss. A loss of 7.5% body weight can reduce steatohepatitis, and with around 10% of body weight loss, you can actually resolve fibrosis, which is remarkable.
We all know that weight loss can be very therapeutic for many conditions. It’s just very hard to achieve. As primary care doctors, we should use what we have in our armamentarium to achieve that goal. Often, that will include certain medications.
Watto: I like giving patients the 10% number because if they weigh 220 pounds, they need to lose 22 pounds. If they weigh 300 pounds, it’s 30 pounds. Most people who weigh 300 pounds think they need to lose 100 pounds to have any sort of health benefit, but it’s much less than that. So, I do find that helpful.
But now a new drug has been approved. It’s a thyroid memetic called resmetirom. It was from the MAESTRO-NASH trial. Without weight loss, it helped to reverse fibrosis.
This is going to be used more and more in the future. It’s still being worked out exactly where the place is for that drug, so much so that Dr Tapper, as a liver expert, hadn’t even had the chance to prescribe it yet. Of course, it was very recently approved.
Dr. Tapper is one of our most celebrated guests, so check out the full podcast here.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, what is MASLD?
Paul N. Williams, MD:
Watto: We talked about a really stripped-down way of testing people for MASLD. If we see mildly elevated liver enzymes, what should we be testing, and how does alcohol factor in?
Williams: Before you can make a definitive diagnosis of MASLD, you need to rule out other causes of liver inflammation — things that would cause a patient’s transaminases to increase. Alcohol is synergistic with everything that can harm the liver.
A great place to start is to gauge someone’s alcohol intake to make sure it isn’t causing hepatic inflammation. The phosphatidyl ethanol level is a serologic test to determine chronic, heavy alcohol use. It’s a new kid on the block. I’ve seen it mostly ordered by hepatologists. It is a way of determining whether someone has had fairly consistent alcohol use up to 4 weeks after the fact. The cutoff for a positive test is 20 ng/mL.
Dr Tapper frames the test this way. He isn’t using the test to catch someone in a lie about their alcohol use. He tells patients that he orders this test for all patients with liver inflammation, because alcohol is a common cause. The test helps him better understand the factors that might be affecting the patient’s liver function.
If the test comes back positive, you can have a conversation about that, and if it’s not positive, you move on to the next possible cause. Other fairly common causes of liver inflammation are relatively easy to address.
Watto: Instead of ordering ceruloplasmin or alpha-1 antitrypsin tests, for example, the first thing Dr Tapper recommends is checking for hepatitis B and C. We can cure hepatitis C. We can’t cure hepatitis B, but it’s important to know if the patient has it. Primary care physicians should be comfortable ordering these tests.
Really high ALT levels (eg, in the 200s) don’t usually happen from steatotic liver disease. In those cases, we would send an expanded panel that might include tests for autoimmune hepatitis-ANA, anti–smooth muscle antibody, and IgG levels. Otherwise, most of these patients don’t need much more testing.
What is a FIB4 score and how does that factor in?
Williams: The FIB4 score estimates the degree of fibrosis based on the ALT and AST levels, platelet count, and the patient’s age. These data are plugged into a formula. If the FIB4 score is low (meaning not much fibrosis is present), you can stop there and do your counseling about lifestyle changes and address the reversible factors.
If the FIB4 score is above a certain threshold (1.3 in young adults and 2.0 in older adults), you need to find a more concrete way to determine the degree of fibrosis, typically through imaging.
Elastography can be done either with ultrasound or MRI. Ultrasound is typically ordered, but Dr Tapper recommends doing MRI on patients with a BMI > 40. Those patients are probably better served by doing MRI to determine the degree of liver fibrosis.
Watto: Patients with low FIB4 scores probably don’t need elastography but those with high FIB4 scores do. For the interpretation of ultrasound-based elastography results, Dr Tapper gave us the “rule of 5s”.
Elastography results are reported in kilopascal (kPa) units. A finding of 5 kPa or less is normal. Forty percent of those with a result of 10 kPa might have advanced liver disease. Above 15 kPa, the likelihood of cirrhosis is high, becoming very likely at 25 kPa. Finally, with a result of > 25 kPa, portal hypertension is likely, and you might need to have a conversation about starting the patient on medicine to prevent variceal bleeding.
We are moving toward more noninvasive testing and avoiding biopsies. We have cutoff values for MRI-based elastography as well. Both of these tests can help stage the liver.
What can we tell people about diet?
Williams: Weight loss is helpful. You can reverse fibrosis with weight loss. You can truly help your liver and bring it closer to its healthy baseline with weight loss. A loss of 7.5% body weight can reduce steatohepatitis, and with around 10% of body weight loss, you can actually resolve fibrosis, which is remarkable.
We all know that weight loss can be very therapeutic for many conditions. It’s just very hard to achieve. As primary care doctors, we should use what we have in our armamentarium to achieve that goal. Often, that will include certain medications.
Watto: I like giving patients the 10% number because if they weigh 220 pounds, they need to lose 22 pounds. If they weigh 300 pounds, it’s 30 pounds. Most people who weigh 300 pounds think they need to lose 100 pounds to have any sort of health benefit, but it’s much less than that. So, I do find that helpful.
But now a new drug has been approved. It’s a thyroid memetic called resmetirom. It was from the MAESTRO-NASH trial. Without weight loss, it helped to reverse fibrosis.
This is going to be used more and more in the future. It’s still being worked out exactly where the place is for that drug, so much so that Dr Tapper, as a liver expert, hadn’t even had the chance to prescribe it yet. Of course, it was very recently approved.
Dr. Tapper is one of our most celebrated guests, so check out the full podcast here.
A version of this article appeared on Medscape.com.
Hepatitis Kills 3500 People Each Day, Says WHO
The number of deaths from viral hepatitis worldwide increased from 1.1 million in 2019 to 1.3 million in 2022. These figures equate to approximately 3500 deaths per day due to the disease, which is the second leading cause of mortality from infectious agents globally.
These data are part of the recently released Global Hepatitis Report 2024, which was published by the World Health Organization (WHO) during the World Hepatitis Summit in Lisbon, Portugal.
“This report paints a concerning picture: Despite global progress in preventing hepatitis infections, deaths are increasing because very few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
Hepatitis B significantly is associated with the highest mortality rate. It accounted for 83% of deaths from the disease in 2022. Meanwhile, hepatitis C was responsible for 17% of deaths. The mortality of other, less common types of hepatitis was not considered in the ranking.
The report also indicates that more than 6000 people worldwide are infected with viral hepatitis every day. The 2.2 million new cases in 2022 represent a slight decrease from 2.5 million in 2019, but the WHO considers the incidence high.
The organization’s updated statistics indicate that about 254 million people had hepatitis B in 2022, while 50 million had type C.
“Besides the deaths, the number of new cases every year is also striking. These are diseases that continue to spread. In the case of hepatitis C, the spread results from lack of access to disposable or properly sterilized sharp materials,” said Thor Dantas, MD, PhD, a physician and director of the Brazilian Society of Hepatology’s Viral Hepatitis Committee.
The situation of hepatitis B is particularly problematic, given that there is a safe and effective vaccine against it, said Dantas. “It’s remarkable that we continue to have so many new cases worldwide. This shows that we are failing in access to preventive measures for control and spread.”
Half of chronic hepatitis B and C cases occur in people between ages 30 and 54 years, while 12% affect children. There are more infections among men, who represent 58% of all cases.
The WHO also drew attention to the difficulty of accessing diagnosis and treatment. Only 13% of people with chronic hepatitis B infection were diagnosed, while only 3% — equivalent to 7 million people — received antiviral therapy by the end of 2022. This result is well below the WHO’s global target, which aims to treat 80% of cases by 2030.
Brazil has a higher diagnostic rate than the global average but is still below the target. According to the report, in 2022, the country diagnosed 34.2% of all hepatitis B infections. However, treatment coverage remains low: 3.6% of the total.
For hepatitis C, the scenario is somewhat different. During the same period, Brazil diagnosed 36% of total cases, with a treatment rate of 24%.
In 2022, Brazil had 2578 deaths from hepatitis B and 2977 from hepatitis C.
Because hepatitis is a silent disease, diagnosis often comes late, when the disease is already quite advanced, said Dr. Dantas. “Viral hepatitis evolves over the years essentially asymptomatically. Malaria shows symptoms, and tuberculosis shows symptoms. Viral hepatitis does not. They are only discovered through active searching.”
The WHO report shows significant regional differences in infection rates. Almost two thirds of cases are concentrated in the following 10 countries: China, India, Indonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, the Philippines, and Russia.
In terms of hepatitis C incidence, Brazil ranks 15th globally, with 536,000 cases in 2022, representing 1.1% of the global total. The list is led by Pakistan, with 8.8 million cases, equivalent to 17.8% of the total. Next are India, with 5.5 million (11.2%), and China, with 4 million (8.1%).
In addition to regional differences, the report also reveals profound disparities in the prices paid for major treatments.
“Price disparities between, and even within, WHO regions persist, with many countries paying above global reference values, including for nonpatented medications,” according to the report.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
The number of deaths from viral hepatitis worldwide increased from 1.1 million in 2019 to 1.3 million in 2022. These figures equate to approximately 3500 deaths per day due to the disease, which is the second leading cause of mortality from infectious agents globally.
These data are part of the recently released Global Hepatitis Report 2024, which was published by the World Health Organization (WHO) during the World Hepatitis Summit in Lisbon, Portugal.
“This report paints a concerning picture: Despite global progress in preventing hepatitis infections, deaths are increasing because very few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
Hepatitis B significantly is associated with the highest mortality rate. It accounted for 83% of deaths from the disease in 2022. Meanwhile, hepatitis C was responsible for 17% of deaths. The mortality of other, less common types of hepatitis was not considered in the ranking.
The report also indicates that more than 6000 people worldwide are infected with viral hepatitis every day. The 2.2 million new cases in 2022 represent a slight decrease from 2.5 million in 2019, but the WHO considers the incidence high.
The organization’s updated statistics indicate that about 254 million people had hepatitis B in 2022, while 50 million had type C.
“Besides the deaths, the number of new cases every year is also striking. These are diseases that continue to spread. In the case of hepatitis C, the spread results from lack of access to disposable or properly sterilized sharp materials,” said Thor Dantas, MD, PhD, a physician and director of the Brazilian Society of Hepatology’s Viral Hepatitis Committee.
The situation of hepatitis B is particularly problematic, given that there is a safe and effective vaccine against it, said Dantas. “It’s remarkable that we continue to have so many new cases worldwide. This shows that we are failing in access to preventive measures for control and spread.”
Half of chronic hepatitis B and C cases occur in people between ages 30 and 54 years, while 12% affect children. There are more infections among men, who represent 58% of all cases.
The WHO also drew attention to the difficulty of accessing diagnosis and treatment. Only 13% of people with chronic hepatitis B infection were diagnosed, while only 3% — equivalent to 7 million people — received antiviral therapy by the end of 2022. This result is well below the WHO’s global target, which aims to treat 80% of cases by 2030.
Brazil has a higher diagnostic rate than the global average but is still below the target. According to the report, in 2022, the country diagnosed 34.2% of all hepatitis B infections. However, treatment coverage remains low: 3.6% of the total.
For hepatitis C, the scenario is somewhat different. During the same period, Brazil diagnosed 36% of total cases, with a treatment rate of 24%.
In 2022, Brazil had 2578 deaths from hepatitis B and 2977 from hepatitis C.
Because hepatitis is a silent disease, diagnosis often comes late, when the disease is already quite advanced, said Dr. Dantas. “Viral hepatitis evolves over the years essentially asymptomatically. Malaria shows symptoms, and tuberculosis shows symptoms. Viral hepatitis does not. They are only discovered through active searching.”
The WHO report shows significant regional differences in infection rates. Almost two thirds of cases are concentrated in the following 10 countries: China, India, Indonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, the Philippines, and Russia.
In terms of hepatitis C incidence, Brazil ranks 15th globally, with 536,000 cases in 2022, representing 1.1% of the global total. The list is led by Pakistan, with 8.8 million cases, equivalent to 17.8% of the total. Next are India, with 5.5 million (11.2%), and China, with 4 million (8.1%).
In addition to regional differences, the report also reveals profound disparities in the prices paid for major treatments.
“Price disparities between, and even within, WHO regions persist, with many countries paying above global reference values, including for nonpatented medications,” according to the report.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
The number of deaths from viral hepatitis worldwide increased from 1.1 million in 2019 to 1.3 million in 2022. These figures equate to approximately 3500 deaths per day due to the disease, which is the second leading cause of mortality from infectious agents globally.
These data are part of the recently released Global Hepatitis Report 2024, which was published by the World Health Organization (WHO) during the World Hepatitis Summit in Lisbon, Portugal.
“This report paints a concerning picture: Despite global progress in preventing hepatitis infections, deaths are increasing because very few people with hepatitis are being diagnosed and treated,” said WHO Director-General Tedros Adhanom Ghebreyesus, PhD.
Hepatitis B significantly is associated with the highest mortality rate. It accounted for 83% of deaths from the disease in 2022. Meanwhile, hepatitis C was responsible for 17% of deaths. The mortality of other, less common types of hepatitis was not considered in the ranking.
The report also indicates that more than 6000 people worldwide are infected with viral hepatitis every day. The 2.2 million new cases in 2022 represent a slight decrease from 2.5 million in 2019, but the WHO considers the incidence high.
The organization’s updated statistics indicate that about 254 million people had hepatitis B in 2022, while 50 million had type C.
“Besides the deaths, the number of new cases every year is also striking. These are diseases that continue to spread. In the case of hepatitis C, the spread results from lack of access to disposable or properly sterilized sharp materials,” said Thor Dantas, MD, PhD, a physician and director of the Brazilian Society of Hepatology’s Viral Hepatitis Committee.
The situation of hepatitis B is particularly problematic, given that there is a safe and effective vaccine against it, said Dantas. “It’s remarkable that we continue to have so many new cases worldwide. This shows that we are failing in access to preventive measures for control and spread.”
Half of chronic hepatitis B and C cases occur in people between ages 30 and 54 years, while 12% affect children. There are more infections among men, who represent 58% of all cases.
The WHO also drew attention to the difficulty of accessing diagnosis and treatment. Only 13% of people with chronic hepatitis B infection were diagnosed, while only 3% — equivalent to 7 million people — received antiviral therapy by the end of 2022. This result is well below the WHO’s global target, which aims to treat 80% of cases by 2030.
Brazil has a higher diagnostic rate than the global average but is still below the target. According to the report, in 2022, the country diagnosed 34.2% of all hepatitis B infections. However, treatment coverage remains low: 3.6% of the total.
For hepatitis C, the scenario is somewhat different. During the same period, Brazil diagnosed 36% of total cases, with a treatment rate of 24%.
In 2022, Brazil had 2578 deaths from hepatitis B and 2977 from hepatitis C.
Because hepatitis is a silent disease, diagnosis often comes late, when the disease is already quite advanced, said Dr. Dantas. “Viral hepatitis evolves over the years essentially asymptomatically. Malaria shows symptoms, and tuberculosis shows symptoms. Viral hepatitis does not. They are only discovered through active searching.”
The WHO report shows significant regional differences in infection rates. Almost two thirds of cases are concentrated in the following 10 countries: China, India, Indonesia, Nigeria, Pakistan, Ethiopia, Bangladesh, Vietnam, the Philippines, and Russia.
In terms of hepatitis C incidence, Brazil ranks 15th globally, with 536,000 cases in 2022, representing 1.1% of the global total. The list is led by Pakistan, with 8.8 million cases, equivalent to 17.8% of the total. Next are India, with 5.5 million (11.2%), and China, with 4 million (8.1%).
In addition to regional differences, the report also reveals profound disparities in the prices paid for major treatments.
“Price disparities between, and even within, WHO regions persist, with many countries paying above global reference values, including for nonpatented medications,” according to the report.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.A version of this article appeared on Medscape.com.
Integrating Telemedicine for HCV With Opioid Use Disorder Treatment Works
People with opioid use disorder (OUD) who have hepatitis C virus (HCV) were twice as likely to be treated and cured of HCV if they received facilitated telemedicine treatment within their opioid treatment program than if they were referred for off-site treatment, the results of a new study showed.
In addition, among cured patients, illicit drug use fell significantly, and there were few reinfections, reported the researchers, led by Andrew Talal, MD, MPH, with the University at Buffalo, State University of New York, Buffalo.
The study was published online in JAMA.
HCV is a major public health concern, especially among people with OUD. Geographic and logistical barriers often prevent this underserved population from accessing treatment; however, telemedicine has the potential to overcome these obstacles.
In a prospective cluster randomized clinical trial, Dr. Talal and colleagues assessed the impact of embedding facilitated telemedicine for HCV care into 12 opioid treatment programs in New York State.
They studied 602 HCV-infected adults (61% male; 51% White) with OUD. Of these, 290 (mean age, 47.1 years) were enrolled in facilitated telemedicine programs onsite, and 312 (mean age, 48.9 years) received an off-site referral (usual care).
Telemedicine participants had an initial telemedicine encounter facilitated by study case managers onsite who also administered a blood test. The telemedicine clinician subsequently evaluated participants and ordered direct-acting antiviral (DAA) medication that was delivered to the opioid treatment program monthly (as refills required) and dispensed along with methadone.
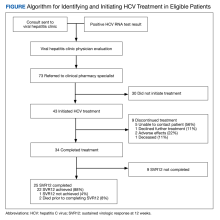
In the telemedicine group, 268 of 290 individuals (92.4%) initiated HCV treatment compared with 126 of 312 (40.4%) in the referral group.
Participants in the telemedicine group were also seen sooner and started treatment faster.
The interval between screening and initial appointments was 14 days with telemedicine vs 18 days with a referral (P = .04). The time between the initial visit and DAA initiation was 49.9 days with telemedicine vs 123.5 days with a referral (P < .001).
Intention-to-treat analysis showed significantly higher HCV cure rates with telemedicine than with referral (90.3% vs 39.4%, respectively). Similarly, the observed cure rates were also higher in the telemedicine group (84.8% vs 34.0%).
Sustained virologic response was durable, with only 13 reinfections (incidence, 2.5 per 100 person-years) occurring during the 2-year follow-up period, the researchers reported.
In addition, illicit drug use decreased significantly among cured patients in both the telemedicine group (P < .001) and the referral group (P = .001). Adults in both groups rated healthcare delivery satisfaction as high or very high.
“Our study demonstrates how telemedicine successfully integrates medical and behavioral treatment,” Dr. Talal said in a statement.
The intervention “builds patient-clinician trust across the screen, and significant decreases in substance use were observed in cured participants with minimal HCV reinfections,” the study team wrote.
Support for this research was provided by the Patient-Centered Outcomes Research Institute and by the Troup Fund of the Kaleida Health Foundation.
A version of this article appeared on Medscape.com .
People with opioid use disorder (OUD) who have hepatitis C virus (HCV) were twice as likely to be treated and cured of HCV if they received facilitated telemedicine treatment within their opioid treatment program than if they were referred for off-site treatment, the results of a new study showed.
In addition, among cured patients, illicit drug use fell significantly, and there were few reinfections, reported the researchers, led by Andrew Talal, MD, MPH, with the University at Buffalo, State University of New York, Buffalo.
The study was published online in JAMA.
HCV is a major public health concern, especially among people with OUD. Geographic and logistical barriers often prevent this underserved population from accessing treatment; however, telemedicine has the potential to overcome these obstacles.
In a prospective cluster randomized clinical trial, Dr. Talal and colleagues assessed the impact of embedding facilitated telemedicine for HCV care into 12 opioid treatment programs in New York State.
They studied 602 HCV-infected adults (61% male; 51% White) with OUD. Of these, 290 (mean age, 47.1 years) were enrolled in facilitated telemedicine programs onsite, and 312 (mean age, 48.9 years) received an off-site referral (usual care).
Telemedicine participants had an initial telemedicine encounter facilitated by study case managers onsite who also administered a blood test. The telemedicine clinician subsequently evaluated participants and ordered direct-acting antiviral (DAA) medication that was delivered to the opioid treatment program monthly (as refills required) and dispensed along with methadone.
In the telemedicine group, 268 of 290 individuals (92.4%) initiated HCV treatment compared with 126 of 312 (40.4%) in the referral group.
Participants in the telemedicine group were also seen sooner and started treatment faster.
The interval between screening and initial appointments was 14 days with telemedicine vs 18 days with a referral (P = .04). The time between the initial visit and DAA initiation was 49.9 days with telemedicine vs 123.5 days with a referral (P < .001).
Intention-to-treat analysis showed significantly higher HCV cure rates with telemedicine than with referral (90.3% vs 39.4%, respectively). Similarly, the observed cure rates were also higher in the telemedicine group (84.8% vs 34.0%).
Sustained virologic response was durable, with only 13 reinfections (incidence, 2.5 per 100 person-years) occurring during the 2-year follow-up period, the researchers reported.
In addition, illicit drug use decreased significantly among cured patients in both the telemedicine group (P < .001) and the referral group (P = .001). Adults in both groups rated healthcare delivery satisfaction as high or very high.
“Our study demonstrates how telemedicine successfully integrates medical and behavioral treatment,” Dr. Talal said in a statement.
The intervention “builds patient-clinician trust across the screen, and significant decreases in substance use were observed in cured participants with minimal HCV reinfections,” the study team wrote.
Support for this research was provided by the Patient-Centered Outcomes Research Institute and by the Troup Fund of the Kaleida Health Foundation.
A version of this article appeared on Medscape.com .
People with opioid use disorder (OUD) who have hepatitis C virus (HCV) were twice as likely to be treated and cured of HCV if they received facilitated telemedicine treatment within their opioid treatment program than if they were referred for off-site treatment, the results of a new study showed.
In addition, among cured patients, illicit drug use fell significantly, and there were few reinfections, reported the researchers, led by Andrew Talal, MD, MPH, with the University at Buffalo, State University of New York, Buffalo.
The study was published online in JAMA.
HCV is a major public health concern, especially among people with OUD. Geographic and logistical barriers often prevent this underserved population from accessing treatment; however, telemedicine has the potential to overcome these obstacles.
In a prospective cluster randomized clinical trial, Dr. Talal and colleagues assessed the impact of embedding facilitated telemedicine for HCV care into 12 opioid treatment programs in New York State.
They studied 602 HCV-infected adults (61% male; 51% White) with OUD. Of these, 290 (mean age, 47.1 years) were enrolled in facilitated telemedicine programs onsite, and 312 (mean age, 48.9 years) received an off-site referral (usual care).
Telemedicine participants had an initial telemedicine encounter facilitated by study case managers onsite who also administered a blood test. The telemedicine clinician subsequently evaluated participants and ordered direct-acting antiviral (DAA) medication that was delivered to the opioid treatment program monthly (as refills required) and dispensed along with methadone.
In the telemedicine group, 268 of 290 individuals (92.4%) initiated HCV treatment compared with 126 of 312 (40.4%) in the referral group.
Participants in the telemedicine group were also seen sooner and started treatment faster.
The interval between screening and initial appointments was 14 days with telemedicine vs 18 days with a referral (P = .04). The time between the initial visit and DAA initiation was 49.9 days with telemedicine vs 123.5 days with a referral (P < .001).
Intention-to-treat analysis showed significantly higher HCV cure rates with telemedicine than with referral (90.3% vs 39.4%, respectively). Similarly, the observed cure rates were also higher in the telemedicine group (84.8% vs 34.0%).
Sustained virologic response was durable, with only 13 reinfections (incidence, 2.5 per 100 person-years) occurring during the 2-year follow-up period, the researchers reported.
In addition, illicit drug use decreased significantly among cured patients in both the telemedicine group (P < .001) and the referral group (P = .001). Adults in both groups rated healthcare delivery satisfaction as high or very high.
“Our study demonstrates how telemedicine successfully integrates medical and behavioral treatment,” Dr. Talal said in a statement.
The intervention “builds patient-clinician trust across the screen, and significant decreases in substance use were observed in cured participants with minimal HCV reinfections,” the study team wrote.
Support for this research was provided by the Patient-Centered Outcomes Research Institute and by the Troup Fund of the Kaleida Health Foundation.
A version of this article appeared on Medscape.com .
FROM JAMA
Hepatitis E Vaccine Shows Long-Term Efficacy
The hepatitis E virus (HEV) is among the leading global causes of acute viral hepatitis. Molecular studies of HEV strains have identified four main genotypes. Genotypes 1 and 2 are limited to humans and are transmitted through contaminated water in resource-limited countries, mainly in Asia. Genotypes 3 and 4 are zoonotic, causing sporadic indigenous hepatitis E in nearly all countries.
Each year, approximately 20 million HEV infections occur worldwide, resulting in around 3.3 million symptomatic infections and 70,000 deaths. Despite this toll, HEV infection remains underestimated, and Western countries are likely not immune to the virus. To date, two recombinant vaccines against hepatitis E, based on genotype 1, have been developed and approved in China, but further studies are needed to determine the duration of vaccination protection.
Ten-Year Results
This study is an extension of a randomized, double-blind, placebo-controlled phase 3 clinical trial of the Hecolin hepatitis E vaccine that was conducted in Dongtai County, Jiangsu, China. In the initial trial, healthy adults aged 16-65 years were recruited, stratified by age and sex, and randomly assigned in a 1:1 ratio to receive three doses of intramuscular hepatitis E vaccine or placebo at months 0, 1, and 6.
A hepatitis E surveillance system, including 205 clinical sentinels covering the entire study region, was established before the study began and maintained for 10 years after vaccination to identify individuals with suspected hepatitis. In addition, an external control cohort was formed to assess vaccine efficacy. The primary endpoint was the vaccine’s efficacy in preventing confirmed hepatitis E occurring at least 30 days after the administration of the third vaccine dose.
Follow-up occurred every 3 months. Participants with hepatitis symptoms for 3 days or more underwent alanine aminotransferase (ALT) concentration measurement. Patients with ALT concentrations ≥ 2.5 times the upper limit of normal were considered to have acute hepatitis. A diagnosis of HEV-confirmed infection was made for patients with acute hepatitis presenting with at least two of the following markers: Presence of HEV RNA, presence of positive anti-HEV immunoglobulin (Ig) M antibodies, and at least fourfold increase in anti-HEV IgG concentrations.
For the efficacy analysis, a Poisson regression model was used to estimate the relative risk and its 95% CI of incidence between groups. Incidence was reported as the number of patients with hepatitis E per 10,000 person-years.
Immunogenicity persistence was assessed by measuring anti-HEV IgG in participants. Serum samples were collected at months 0, 7, 13, 19, 31, 43, 55, 79, and 103 for Qingdao district participants and at months 0, 7, 19, 31, 43, 67, and 91 for Anfeng district participants.
Efficacy and Duration
The follow-up period extended from 2007 to 2017. In total, 97,356 participants completed the three-dose regimen and were included in the per-protocol population (48,693 in the vaccine group and 48,663 in the placebo group), and 178,236 residents from the study region participated in the external control cohort. During the study period, 90 cases of hepatitis E were identified, with 13 in the vaccine group (0.2 per 10,000 person-years) and 77 in the placebo group (1.4 per 10,000 person-years). This indicated a vaccine efficacy of 86.6% in the per-protocol analysis.
In the subgroups evaluated for immunogenicity persistence, among those who were initially seronegative and received three doses of hepatitis E vaccine, 254 out of 291 vaccinated participants (87.3%) in Qingdao after 8.5 years and 1270 (73.0%) out of 1740 vaccinated participants in Anfeng after 7.5 years maintained detectable antibody concentrations.
The identification of infections despite vaccination is notable, especially with eight cases occurring beyond the fourth year following the last dose. This information is crucial for understanding potential immunity decline over time and highlights the importance of exploring various vaccination strategies to optimize protection.
An ongoing phase 4 clinical trial in Bangladesh, exploring different administration schedules and target populations, could help optimize vaccination strategies. The remarkable efficacy (100%) observed over a 30-month period for the two-dose schedule (doses are administered 1 month apart) is promising.
The observation of higher IgG antibody avidity in participants with infections despite vaccination underscores the importance of robust antibody responses to mitigate disease severity and duration. Several study limitations, such as lack of data on deaths and emigrations, a single-center study design, predominance of genotype 4 infections, and the risk for bias in the external control cohort, should be acknowledged.
In conclusion, this study provides compelling evidence of sustained protection of the hepatitis E vaccine over a decade. The observed persistence of induced antibodies for at least 8.5 years supports the long-term efficacy of the vaccine. Diverse global trials, further investigation into the impact of natural infections on vaccine-induced antibodies, and confirmation of inter-genotypic protection are needed.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The hepatitis E virus (HEV) is among the leading global causes of acute viral hepatitis. Molecular studies of HEV strains have identified four main genotypes. Genotypes 1 and 2 are limited to humans and are transmitted through contaminated water in resource-limited countries, mainly in Asia. Genotypes 3 and 4 are zoonotic, causing sporadic indigenous hepatitis E in nearly all countries.
Each year, approximately 20 million HEV infections occur worldwide, resulting in around 3.3 million symptomatic infections and 70,000 deaths. Despite this toll, HEV infection remains underestimated, and Western countries are likely not immune to the virus. To date, two recombinant vaccines against hepatitis E, based on genotype 1, have been developed and approved in China, but further studies are needed to determine the duration of vaccination protection.
Ten-Year Results
This study is an extension of a randomized, double-blind, placebo-controlled phase 3 clinical trial of the Hecolin hepatitis E vaccine that was conducted in Dongtai County, Jiangsu, China. In the initial trial, healthy adults aged 16-65 years were recruited, stratified by age and sex, and randomly assigned in a 1:1 ratio to receive three doses of intramuscular hepatitis E vaccine or placebo at months 0, 1, and 6.
A hepatitis E surveillance system, including 205 clinical sentinels covering the entire study region, was established before the study began and maintained for 10 years after vaccination to identify individuals with suspected hepatitis. In addition, an external control cohort was formed to assess vaccine efficacy. The primary endpoint was the vaccine’s efficacy in preventing confirmed hepatitis E occurring at least 30 days after the administration of the third vaccine dose.
Follow-up occurred every 3 months. Participants with hepatitis symptoms for 3 days or more underwent alanine aminotransferase (ALT) concentration measurement. Patients with ALT concentrations ≥ 2.5 times the upper limit of normal were considered to have acute hepatitis. A diagnosis of HEV-confirmed infection was made for patients with acute hepatitis presenting with at least two of the following markers: Presence of HEV RNA, presence of positive anti-HEV immunoglobulin (Ig) M antibodies, and at least fourfold increase in anti-HEV IgG concentrations.
For the efficacy analysis, a Poisson regression model was used to estimate the relative risk and its 95% CI of incidence between groups. Incidence was reported as the number of patients with hepatitis E per 10,000 person-years.
Immunogenicity persistence was assessed by measuring anti-HEV IgG in participants. Serum samples were collected at months 0, 7, 13, 19, 31, 43, 55, 79, and 103 for Qingdao district participants and at months 0, 7, 19, 31, 43, 67, and 91 for Anfeng district participants.
Efficacy and Duration
The follow-up period extended from 2007 to 2017. In total, 97,356 participants completed the three-dose regimen and were included in the per-protocol population (48,693 in the vaccine group and 48,663 in the placebo group), and 178,236 residents from the study region participated in the external control cohort. During the study period, 90 cases of hepatitis E were identified, with 13 in the vaccine group (0.2 per 10,000 person-years) and 77 in the placebo group (1.4 per 10,000 person-years). This indicated a vaccine efficacy of 86.6% in the per-protocol analysis.
In the subgroups evaluated for immunogenicity persistence, among those who were initially seronegative and received three doses of hepatitis E vaccine, 254 out of 291 vaccinated participants (87.3%) in Qingdao after 8.5 years and 1270 (73.0%) out of 1740 vaccinated participants in Anfeng after 7.5 years maintained detectable antibody concentrations.
The identification of infections despite vaccination is notable, especially with eight cases occurring beyond the fourth year following the last dose. This information is crucial for understanding potential immunity decline over time and highlights the importance of exploring various vaccination strategies to optimize protection.
An ongoing phase 4 clinical trial in Bangladesh, exploring different administration schedules and target populations, could help optimize vaccination strategies. The remarkable efficacy (100%) observed over a 30-month period for the two-dose schedule (doses are administered 1 month apart) is promising.
The observation of higher IgG antibody avidity in participants with infections despite vaccination underscores the importance of robust antibody responses to mitigate disease severity and duration. Several study limitations, such as lack of data on deaths and emigrations, a single-center study design, predominance of genotype 4 infections, and the risk for bias in the external control cohort, should be acknowledged.
In conclusion, this study provides compelling evidence of sustained protection of the hepatitis E vaccine over a decade. The observed persistence of induced antibodies for at least 8.5 years supports the long-term efficacy of the vaccine. Diverse global trials, further investigation into the impact of natural infections on vaccine-induced antibodies, and confirmation of inter-genotypic protection are needed.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The hepatitis E virus (HEV) is among the leading global causes of acute viral hepatitis. Molecular studies of HEV strains have identified four main genotypes. Genotypes 1 and 2 are limited to humans and are transmitted through contaminated water in resource-limited countries, mainly in Asia. Genotypes 3 and 4 are zoonotic, causing sporadic indigenous hepatitis E in nearly all countries.
Each year, approximately 20 million HEV infections occur worldwide, resulting in around 3.3 million symptomatic infections and 70,000 deaths. Despite this toll, HEV infection remains underestimated, and Western countries are likely not immune to the virus. To date, two recombinant vaccines against hepatitis E, based on genotype 1, have been developed and approved in China, but further studies are needed to determine the duration of vaccination protection.
Ten-Year Results
This study is an extension of a randomized, double-blind, placebo-controlled phase 3 clinical trial of the Hecolin hepatitis E vaccine that was conducted in Dongtai County, Jiangsu, China. In the initial trial, healthy adults aged 16-65 years were recruited, stratified by age and sex, and randomly assigned in a 1:1 ratio to receive three doses of intramuscular hepatitis E vaccine or placebo at months 0, 1, and 6.
A hepatitis E surveillance system, including 205 clinical sentinels covering the entire study region, was established before the study began and maintained for 10 years after vaccination to identify individuals with suspected hepatitis. In addition, an external control cohort was formed to assess vaccine efficacy. The primary endpoint was the vaccine’s efficacy in preventing confirmed hepatitis E occurring at least 30 days after the administration of the third vaccine dose.
Follow-up occurred every 3 months. Participants with hepatitis symptoms for 3 days or more underwent alanine aminotransferase (ALT) concentration measurement. Patients with ALT concentrations ≥ 2.5 times the upper limit of normal were considered to have acute hepatitis. A diagnosis of HEV-confirmed infection was made for patients with acute hepatitis presenting with at least two of the following markers: Presence of HEV RNA, presence of positive anti-HEV immunoglobulin (Ig) M antibodies, and at least fourfold increase in anti-HEV IgG concentrations.
For the efficacy analysis, a Poisson regression model was used to estimate the relative risk and its 95% CI of incidence between groups. Incidence was reported as the number of patients with hepatitis E per 10,000 person-years.
Immunogenicity persistence was assessed by measuring anti-HEV IgG in participants. Serum samples were collected at months 0, 7, 13, 19, 31, 43, 55, 79, and 103 for Qingdao district participants and at months 0, 7, 19, 31, 43, 67, and 91 for Anfeng district participants.
Efficacy and Duration
The follow-up period extended from 2007 to 2017. In total, 97,356 participants completed the three-dose regimen and were included in the per-protocol population (48,693 in the vaccine group and 48,663 in the placebo group), and 178,236 residents from the study region participated in the external control cohort. During the study period, 90 cases of hepatitis E were identified, with 13 in the vaccine group (0.2 per 10,000 person-years) and 77 in the placebo group (1.4 per 10,000 person-years). This indicated a vaccine efficacy of 86.6% in the per-protocol analysis.
In the subgroups evaluated for immunogenicity persistence, among those who were initially seronegative and received three doses of hepatitis E vaccine, 254 out of 291 vaccinated participants (87.3%) in Qingdao after 8.5 years and 1270 (73.0%) out of 1740 vaccinated participants in Anfeng after 7.5 years maintained detectable antibody concentrations.
The identification of infections despite vaccination is notable, especially with eight cases occurring beyond the fourth year following the last dose. This information is crucial for understanding potential immunity decline over time and highlights the importance of exploring various vaccination strategies to optimize protection.
An ongoing phase 4 clinical trial in Bangladesh, exploring different administration schedules and target populations, could help optimize vaccination strategies. The remarkable efficacy (100%) observed over a 30-month period for the two-dose schedule (doses are administered 1 month apart) is promising.
The observation of higher IgG antibody avidity in participants with infections despite vaccination underscores the importance of robust antibody responses to mitigate disease severity and duration. Several study limitations, such as lack of data on deaths and emigrations, a single-center study design, predominance of genotype 4 infections, and the risk for bias in the external control cohort, should be acknowledged.
In conclusion, this study provides compelling evidence of sustained protection of the hepatitis E vaccine over a decade. The observed persistence of induced antibodies for at least 8.5 years supports the long-term efficacy of the vaccine. Diverse global trials, further investigation into the impact of natural infections on vaccine-induced antibodies, and confirmation of inter-genotypic protection are needed.
This story was translated from JIM, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Outcomes and Barriers Associated with Telehealth-Based Hepatitis C Treatment During Early Phases of the COVID-19 Pandemic
Although 2.4 million adults in the United States have been diagnosed with hepatitis C virus (HCV) infection, it remains underdiagnosed and undertreated, particularly among difficult to reach populations, such as persons who inject drugs, marginally housed individuals, correctional populations, and pregnant women.1 Though the US Preventive Services Task Force (USPSTF) broadened HCV screening recommendations to include individuals aged 18 to 79 years, rates of new HCV prescriptions sharply declined during the COVID-19 pandemic.2,3
During the pandemic, many health care systems adopted virtual health care modalities. Within the Veteran Health Administration (VHA), there was an 11-fold increase in virtual encounters. However, veterans aged > 45 years, homeless, and had other insurance were less likely to utilize virtual care.4,5 As health care delivery continues to evolve, health systems must adapt and test innovative models for the treatment of HCV.
There is limited understanding of HCV treatments when exclusively conducted virtually. The aim of this study was to evaluate the effects of the HCV treatment program at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) during the early phase of the COVID-19 pandemic, when telehealth modalities and mail-order prescriptions were used for HCV diagnosis and treatment. The secondary aim of this study was to understand patient factors associated with treatment initiation and discontinuation for patients using telehealth.
Methods
The VHA is the largest provider of HCV care in the US.6 At VAGLAHS, veterans with HCV are referred for evaluation to a viral hepatitis clinic staffed by gastroenterologists and infectious disease specialists. Veterans with detectable HCV on an HCV RNA test have an additional workup ordered if necessary and are referred to an HCV-specialist pharmacist or physician’s assistant to start treatment. In March 2020, all HCV evaluations and treatment initiation in the viral hepatitis clinic started being conducted exclusively via telehealth. This was the primary modality of HCV evaluations and treatment initiation until COVID-19 restrictions were lifted to permit in-person evaluations. Prescriptions were delivered by mail to patients following treatment initiation appointments.
We retrospectively reviewed electronic health records of veterans referred to start treatment March 1, 2020, through September 30, 2020. The endpoint of the reviewed records was set because during this specific time frame, VAGLAHS used an exclusively telehealth-based model for HCV evaluation and treatment. Patients were followed until June 15, 2021. Due to evolving COVID-19 restrictions at the time, and despite requests received, treatment initiations by the pharmacy team were suspended in March 2020 but HCV treatments resumed in May. Data collected included baseline demographics (age, sex, race, ethnicity, housing status, distance to VAGLAHS), comorbidities (cirrhosis, hepatitis B virus coinfection, HIV coinfection), psychiatric conditions (mood or psychotic disorder, alcohol use disorder [AUD], opioid use disorder), and treatment characteristics (HCV genotype, HCV treatment regimen, baseline viral load). Distance from the patient’s home to VAGLAHS was calculated using CDXZipStream software. Comorbidities and psychiatric conditions were identified by the presence of the appropriate diagnosis via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in the health record and confirmed by review of clinician notes. Active AUD was defined as: (1) the presence of AUD diagnosis code; (2) AUD Identification Test-Consumption (AUDIT-C) score of high or severe risk based on established cutoffs; and (3) active alcohol use noted in the electronic health record. All patients had an AUDIT-C score completed within 1 year of initiating treatment. Opioid use disorder was defined by the presence of diagnostic codes for opioid dependence or opioid abuse.
The reasons for treatment noninitiation and discontinuation were each captured. We calculated descriptive statistics to analyze the frequency distributions of all variables. Independent t tests were used to analyze continuous data and Pearson χ2 test was used to analyze categorical data. Statistical significance was set as P < .05.
Results
From March 1, 2020, through September 30, 2020, 73 veterans were referred to the HCV clinical pharmacist for treatment (Figure). Forty-three veterans (59%) initiated HCV treatment and 34 (79%) completed the full treatment course (Table 1). Twenty-five patients (65%) had their sustained virologic response at 12 weeks (SVR12) testing and 22 patients achieved SVR12 (88%; 30% of total sample). One patient did not achieve SVR, and 2 patients died (variceal hemorrhage and progression of cerebral amyloidosis/function decline) before the completion of laboratory testing. From March 2020 to May 2020, HCV treatments requests were paused as new COVID-19 policies were being introduced; 33 patients were referred during this time and 21 initiated treatment.
Veterans that did not start HCV treatment had a significantly higher rate of active AUD when compared with those that initiated treatment: 30% vs 9% (P = .02). Of the patients who started and discontinued treatment, none had active AUD. Other baseline demographics, clinical characteristics, and treatment characteristics were similar between the groups. No patient demographic characteristics were significantly associated with HCV treatment discontinuation. We did not observe any major health disparities in initiation or discontinuation by sex, race, ethnicity, or geography. Eleven patients (37%) could not be contacted, which was the most common reason veterans did not initiate treatment (Table 2). Of the 9 patients that did not complete SVR12, 5 patients could not be contacted for follow-up, which was the most common reason veterans discontinued treatment.
Discussion
This study highlights the experience of treating patients with HCV with an exclusively telehealth model in the months following implementation of stay-at-home orders from March 19, 2020, to September 30, 2020, during the COVID-19 pandemic at VAGLAHS. We were able to successfully complete treatment for 34 veterans (47%) and achieved SVR rates of 88%. We found that AUD was associated with unsuccessful treatment initiation. There were no statistically significant patient characteristic findings for treatment discontinuation in our study (Table 3). Unhealthy alcohol use and AUD are highly prevalent among veterans with HCV and prior to the pandemic, studies have demonstrated AUD as a barrier to HCV treatment.7
Since worse hepatic outcomes have been observed in veterans with HCV and AUD and increased harmful patterns of drinking occurred during the pandemic, a renewed interest in treating AUD in these veterans during the era of telehealth is critical.8 While we were unable to ascertain whether alcohol misuse in our cohort increased during the pandemic or whether changes in drinking patterns affected HCV treatment outcomes before and after the pandemic, such an association should reinforce the need for clinicians to expeditiously link patients to substance use care. It should also stimulate further considerations of addressing social determinants of health not captured in this study.
During the pandemic, veterans with posttraumatic stress disorder, a history of serving in combat roles, and experiencing related financial stressors had higher risk of AUD.9,10 For veterans with AUD who initiated HCV treatment, none discontinued their therapy, aligning with other studies showed that patients with AUD were able to achieve high rates of SVR and emphasizing that veterans should be treated irrespective of an AUD diagnosis.11 However, more innovative engagement initiatives for veterans with AUD should be explored as we continue to adapt more telehealth-based care for HCV direct-acting antiviral treatments. A more in-depth understanding of how alcohol use relates to treatment noninitiation is warranted, as this may stem from behavioral patterns that could not be captured in the present study.
The inability to reach veterans by telephone was a major reason for noninitiation and discontinuation of treatment. While the expansion of telehealth services has been noted across the VHA, there is still room for improving methods of engaging veterans in health care postpandemic.12 Prior studies in veteran populations that were successful in increasing uptake of HCV treatment have employed telehealth strategies that further emphasizes its integral role in HCV elimination.13 Although our study did not show mental health comorbidities and housing status as statistically significant, it is important to note that 20% of patients referred for HCV treatment had an incomplete evaluation which can lead to potentially unobserved indicators not captured by our study such as quality of linkage to care. It is imperative to stress the best practices for HCV initiation by integrating a multidisciplinary team to address patients’ psychosocial comorbidities.14 Finally, we did not observe any major disparities in treating veterans with HCV during the pandemic. This observation is reassuring and consistent with other VHA data given the heightened recognition of health disparities seen in health care sectors across the country, especially evident during the COVID-19 pandemic and the current era of increased adaptation of telehealth.
Limitations
Limitations to this study include its retrospective nature, small sample size, and short study time frame as a proportion of veterans have yet to complete HCV treatment which can potentially explain how larger studies were able to find other statistically significant patient-related factors impacting treatment initiation compared to ours. Given the lack of universal standardized diagnostic criterion of AUD, this can limit how our study can be compared to others in similar populations. Additionally, this study was conducted at a single facility with a predominantly older male veteran population, which may not be generalizable to other populations.
Conclusions
Treating HCV during the COVID-19 pandemic with telehealth and mail-out medications was feasible and led to high SVR rates, but unhealthy alcohol use and an inability to contact veterans were predominant barriers to success. Future quality improvement efforts should focus on addressing these barriers and exploring the relationship between alcohol use and HCV treatment initiation.
1. Patel AA, Bui A, Prohl E, et al. Innovations in Hepatitis C Screening and Treatment. Hepatol Commun. 2020;5(3):371-386. Published 2020 Dec 7. doi:10.1002/hep4.1646
2. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970-975. doi:10.1001/jama.2020.1123
3. Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376. doi:10.1016/j.amepre.2021.03.011
4. Rosen CS, Morland LA, Glassman LH, et al. Virtual mental health care in the Veterans Health Administration’s immediate response to coronavirus disease-19. Am Psychol. 2021;76(1):26-38. doi:10.1037/amp0000751
5. Balut MD, Wyte-Lake T, Steers WN, et al. Expansion of telemedicine during COVID-19 at a VA specialty clinic. Healthc (Amst). 2022;10(1):100599. doi:10.1016/j.hjdsi.2021.100599
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
7. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992-1000. doi:10.1111/apt.14328
8. Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393-401. doi:10.1016/j.jhep.2017.10.019
9. Pedersen ER, Davis JP, Fitzke RE, Lee DS, Saba S. American Veterans in the Era of COVID-19: Reactions to the Pandemic, Posttraumatic Stress Disorder, and Substance Use Behaviors. Int J Ment Health Addict. 2023;21(2):767-782. doi:10.1007/s11469-021-00620-0
10. Na PJ, Norman SB, Nichter B, et al. Prevalence, risk and protective factors of alcohol use disorder during the COVID-19 pandemic in U.S. military veterans. Drug Alcohol Depend. 2021;225:108818. doi:10.1016/j.drugalcdep.2021.108818
11. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. doi:10.1016/j.drugalcdep.2016.10.021
12. Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
13. Fleming BS, Ifeachor AP, Andres AM, et al. Improving Veteran Access to Treatment for Hepatitis C Virus Infection: Addressing social issues and treatment barriers significantly increases access to HCV care, and many veterans successfully start therapy with the help of additional support staff. Fed Pract. 2017;34(Suppl 4):S24-S28.
14. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
Although 2.4 million adults in the United States have been diagnosed with hepatitis C virus (HCV) infection, it remains underdiagnosed and undertreated, particularly among difficult to reach populations, such as persons who inject drugs, marginally housed individuals, correctional populations, and pregnant women.1 Though the US Preventive Services Task Force (USPSTF) broadened HCV screening recommendations to include individuals aged 18 to 79 years, rates of new HCV prescriptions sharply declined during the COVID-19 pandemic.2,3
During the pandemic, many health care systems adopted virtual health care modalities. Within the Veteran Health Administration (VHA), there was an 11-fold increase in virtual encounters. However, veterans aged > 45 years, homeless, and had other insurance were less likely to utilize virtual care.4,5 As health care delivery continues to evolve, health systems must adapt and test innovative models for the treatment of HCV.
There is limited understanding of HCV treatments when exclusively conducted virtually. The aim of this study was to evaluate the effects of the HCV treatment program at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) during the early phase of the COVID-19 pandemic, when telehealth modalities and mail-order prescriptions were used for HCV diagnosis and treatment. The secondary aim of this study was to understand patient factors associated with treatment initiation and discontinuation for patients using telehealth.
Methods
The VHA is the largest provider of HCV care in the US.6 At VAGLAHS, veterans with HCV are referred for evaluation to a viral hepatitis clinic staffed by gastroenterologists and infectious disease specialists. Veterans with detectable HCV on an HCV RNA test have an additional workup ordered if necessary and are referred to an HCV-specialist pharmacist or physician’s assistant to start treatment. In March 2020, all HCV evaluations and treatment initiation in the viral hepatitis clinic started being conducted exclusively via telehealth. This was the primary modality of HCV evaluations and treatment initiation until COVID-19 restrictions were lifted to permit in-person evaluations. Prescriptions were delivered by mail to patients following treatment initiation appointments.
We retrospectively reviewed electronic health records of veterans referred to start treatment March 1, 2020, through September 30, 2020. The endpoint of the reviewed records was set because during this specific time frame, VAGLAHS used an exclusively telehealth-based model for HCV evaluation and treatment. Patients were followed until June 15, 2021. Due to evolving COVID-19 restrictions at the time, and despite requests received, treatment initiations by the pharmacy team were suspended in March 2020 but HCV treatments resumed in May. Data collected included baseline demographics (age, sex, race, ethnicity, housing status, distance to VAGLAHS), comorbidities (cirrhosis, hepatitis B virus coinfection, HIV coinfection), psychiatric conditions (mood or psychotic disorder, alcohol use disorder [AUD], opioid use disorder), and treatment characteristics (HCV genotype, HCV treatment regimen, baseline viral load). Distance from the patient’s home to VAGLAHS was calculated using CDXZipStream software. Comorbidities and psychiatric conditions were identified by the presence of the appropriate diagnosis via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in the health record and confirmed by review of clinician notes. Active AUD was defined as: (1) the presence of AUD diagnosis code; (2) AUD Identification Test-Consumption (AUDIT-C) score of high or severe risk based on established cutoffs; and (3) active alcohol use noted in the electronic health record. All patients had an AUDIT-C score completed within 1 year of initiating treatment. Opioid use disorder was defined by the presence of diagnostic codes for opioid dependence or opioid abuse.
The reasons for treatment noninitiation and discontinuation were each captured. We calculated descriptive statistics to analyze the frequency distributions of all variables. Independent t tests were used to analyze continuous data and Pearson χ2 test was used to analyze categorical data. Statistical significance was set as P < .05.
Results
From March 1, 2020, through September 30, 2020, 73 veterans were referred to the HCV clinical pharmacist for treatment (Figure). Forty-three veterans (59%) initiated HCV treatment and 34 (79%) completed the full treatment course (Table 1). Twenty-five patients (65%) had their sustained virologic response at 12 weeks (SVR12) testing and 22 patients achieved SVR12 (88%; 30% of total sample). One patient did not achieve SVR, and 2 patients died (variceal hemorrhage and progression of cerebral amyloidosis/function decline) before the completion of laboratory testing. From March 2020 to May 2020, HCV treatments requests were paused as new COVID-19 policies were being introduced; 33 patients were referred during this time and 21 initiated treatment.
Veterans that did not start HCV treatment had a significantly higher rate of active AUD when compared with those that initiated treatment: 30% vs 9% (P = .02). Of the patients who started and discontinued treatment, none had active AUD. Other baseline demographics, clinical characteristics, and treatment characteristics were similar between the groups. No patient demographic characteristics were significantly associated with HCV treatment discontinuation. We did not observe any major health disparities in initiation or discontinuation by sex, race, ethnicity, or geography. Eleven patients (37%) could not be contacted, which was the most common reason veterans did not initiate treatment (Table 2). Of the 9 patients that did not complete SVR12, 5 patients could not be contacted for follow-up, which was the most common reason veterans discontinued treatment.
Discussion
This study highlights the experience of treating patients with HCV with an exclusively telehealth model in the months following implementation of stay-at-home orders from March 19, 2020, to September 30, 2020, during the COVID-19 pandemic at VAGLAHS. We were able to successfully complete treatment for 34 veterans (47%) and achieved SVR rates of 88%. We found that AUD was associated with unsuccessful treatment initiation. There were no statistically significant patient characteristic findings for treatment discontinuation in our study (Table 3). Unhealthy alcohol use and AUD are highly prevalent among veterans with HCV and prior to the pandemic, studies have demonstrated AUD as a barrier to HCV treatment.7
Since worse hepatic outcomes have been observed in veterans with HCV and AUD and increased harmful patterns of drinking occurred during the pandemic, a renewed interest in treating AUD in these veterans during the era of telehealth is critical.8 While we were unable to ascertain whether alcohol misuse in our cohort increased during the pandemic or whether changes in drinking patterns affected HCV treatment outcomes before and after the pandemic, such an association should reinforce the need for clinicians to expeditiously link patients to substance use care. It should also stimulate further considerations of addressing social determinants of health not captured in this study.
During the pandemic, veterans with posttraumatic stress disorder, a history of serving in combat roles, and experiencing related financial stressors had higher risk of AUD.9,10 For veterans with AUD who initiated HCV treatment, none discontinued their therapy, aligning with other studies showed that patients with AUD were able to achieve high rates of SVR and emphasizing that veterans should be treated irrespective of an AUD diagnosis.11 However, more innovative engagement initiatives for veterans with AUD should be explored as we continue to adapt more telehealth-based care for HCV direct-acting antiviral treatments. A more in-depth understanding of how alcohol use relates to treatment noninitiation is warranted, as this may stem from behavioral patterns that could not be captured in the present study.
The inability to reach veterans by telephone was a major reason for noninitiation and discontinuation of treatment. While the expansion of telehealth services has been noted across the VHA, there is still room for improving methods of engaging veterans in health care postpandemic.12 Prior studies in veteran populations that were successful in increasing uptake of HCV treatment have employed telehealth strategies that further emphasizes its integral role in HCV elimination.13 Although our study did not show mental health comorbidities and housing status as statistically significant, it is important to note that 20% of patients referred for HCV treatment had an incomplete evaluation which can lead to potentially unobserved indicators not captured by our study such as quality of linkage to care. It is imperative to stress the best practices for HCV initiation by integrating a multidisciplinary team to address patients’ psychosocial comorbidities.14 Finally, we did not observe any major disparities in treating veterans with HCV during the pandemic. This observation is reassuring and consistent with other VHA data given the heightened recognition of health disparities seen in health care sectors across the country, especially evident during the COVID-19 pandemic and the current era of increased adaptation of telehealth.
Limitations
Limitations to this study include its retrospective nature, small sample size, and short study time frame as a proportion of veterans have yet to complete HCV treatment which can potentially explain how larger studies were able to find other statistically significant patient-related factors impacting treatment initiation compared to ours. Given the lack of universal standardized diagnostic criterion of AUD, this can limit how our study can be compared to others in similar populations. Additionally, this study was conducted at a single facility with a predominantly older male veteran population, which may not be generalizable to other populations.
Conclusions
Treating HCV during the COVID-19 pandemic with telehealth and mail-out medications was feasible and led to high SVR rates, but unhealthy alcohol use and an inability to contact veterans were predominant barriers to success. Future quality improvement efforts should focus on addressing these barriers and exploring the relationship between alcohol use and HCV treatment initiation.
Although 2.4 million adults in the United States have been diagnosed with hepatitis C virus (HCV) infection, it remains underdiagnosed and undertreated, particularly among difficult to reach populations, such as persons who inject drugs, marginally housed individuals, correctional populations, and pregnant women.1 Though the US Preventive Services Task Force (USPSTF) broadened HCV screening recommendations to include individuals aged 18 to 79 years, rates of new HCV prescriptions sharply declined during the COVID-19 pandemic.2,3
During the pandemic, many health care systems adopted virtual health care modalities. Within the Veteran Health Administration (VHA), there was an 11-fold increase in virtual encounters. However, veterans aged > 45 years, homeless, and had other insurance were less likely to utilize virtual care.4,5 As health care delivery continues to evolve, health systems must adapt and test innovative models for the treatment of HCV.
There is limited understanding of HCV treatments when exclusively conducted virtually. The aim of this study was to evaluate the effects of the HCV treatment program at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) during the early phase of the COVID-19 pandemic, when telehealth modalities and mail-order prescriptions were used for HCV diagnosis and treatment. The secondary aim of this study was to understand patient factors associated with treatment initiation and discontinuation for patients using telehealth.
Methods
The VHA is the largest provider of HCV care in the US.6 At VAGLAHS, veterans with HCV are referred for evaluation to a viral hepatitis clinic staffed by gastroenterologists and infectious disease specialists. Veterans with detectable HCV on an HCV RNA test have an additional workup ordered if necessary and are referred to an HCV-specialist pharmacist or physician’s assistant to start treatment. In March 2020, all HCV evaluations and treatment initiation in the viral hepatitis clinic started being conducted exclusively via telehealth. This was the primary modality of HCV evaluations and treatment initiation until COVID-19 restrictions were lifted to permit in-person evaluations. Prescriptions were delivered by mail to patients following treatment initiation appointments.
We retrospectively reviewed electronic health records of veterans referred to start treatment March 1, 2020, through September 30, 2020. The endpoint of the reviewed records was set because during this specific time frame, VAGLAHS used an exclusively telehealth-based model for HCV evaluation and treatment. Patients were followed until June 15, 2021. Due to evolving COVID-19 restrictions at the time, and despite requests received, treatment initiations by the pharmacy team were suspended in March 2020 but HCV treatments resumed in May. Data collected included baseline demographics (age, sex, race, ethnicity, housing status, distance to VAGLAHS), comorbidities (cirrhosis, hepatitis B virus coinfection, HIV coinfection), psychiatric conditions (mood or psychotic disorder, alcohol use disorder [AUD], opioid use disorder), and treatment characteristics (HCV genotype, HCV treatment regimen, baseline viral load). Distance from the patient’s home to VAGLAHS was calculated using CDXZipStream software. Comorbidities and psychiatric conditions were identified by the presence of the appropriate diagnosis via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in the health record and confirmed by review of clinician notes. Active AUD was defined as: (1) the presence of AUD diagnosis code; (2) AUD Identification Test-Consumption (AUDIT-C) score of high or severe risk based on established cutoffs; and (3) active alcohol use noted in the electronic health record. All patients had an AUDIT-C score completed within 1 year of initiating treatment. Opioid use disorder was defined by the presence of diagnostic codes for opioid dependence or opioid abuse.
The reasons for treatment noninitiation and discontinuation were each captured. We calculated descriptive statistics to analyze the frequency distributions of all variables. Independent t tests were used to analyze continuous data and Pearson χ2 test was used to analyze categorical data. Statistical significance was set as P < .05.
Results
From March 1, 2020, through September 30, 2020, 73 veterans were referred to the HCV clinical pharmacist for treatment (Figure). Forty-three veterans (59%) initiated HCV treatment and 34 (79%) completed the full treatment course (Table 1). Twenty-five patients (65%) had their sustained virologic response at 12 weeks (SVR12) testing and 22 patients achieved SVR12 (88%; 30% of total sample). One patient did not achieve SVR, and 2 patients died (variceal hemorrhage and progression of cerebral amyloidosis/function decline) before the completion of laboratory testing. From March 2020 to May 2020, HCV treatments requests were paused as new COVID-19 policies were being introduced; 33 patients were referred during this time and 21 initiated treatment.
Veterans that did not start HCV treatment had a significantly higher rate of active AUD when compared with those that initiated treatment: 30% vs 9% (P = .02). Of the patients who started and discontinued treatment, none had active AUD. Other baseline demographics, clinical characteristics, and treatment characteristics were similar between the groups. No patient demographic characteristics were significantly associated with HCV treatment discontinuation. We did not observe any major health disparities in initiation or discontinuation by sex, race, ethnicity, or geography. Eleven patients (37%) could not be contacted, which was the most common reason veterans did not initiate treatment (Table 2). Of the 9 patients that did not complete SVR12, 5 patients could not be contacted for follow-up, which was the most common reason veterans discontinued treatment.
Discussion
This study highlights the experience of treating patients with HCV with an exclusively telehealth model in the months following implementation of stay-at-home orders from March 19, 2020, to September 30, 2020, during the COVID-19 pandemic at VAGLAHS. We were able to successfully complete treatment for 34 veterans (47%) and achieved SVR rates of 88%. We found that AUD was associated with unsuccessful treatment initiation. There were no statistically significant patient characteristic findings for treatment discontinuation in our study (Table 3). Unhealthy alcohol use and AUD are highly prevalent among veterans with HCV and prior to the pandemic, studies have demonstrated AUD as a barrier to HCV treatment.7
Since worse hepatic outcomes have been observed in veterans with HCV and AUD and increased harmful patterns of drinking occurred during the pandemic, a renewed interest in treating AUD in these veterans during the era of telehealth is critical.8 While we were unable to ascertain whether alcohol misuse in our cohort increased during the pandemic or whether changes in drinking patterns affected HCV treatment outcomes before and after the pandemic, such an association should reinforce the need for clinicians to expeditiously link patients to substance use care. It should also stimulate further considerations of addressing social determinants of health not captured in this study.
During the pandemic, veterans with posttraumatic stress disorder, a history of serving in combat roles, and experiencing related financial stressors had higher risk of AUD.9,10 For veterans with AUD who initiated HCV treatment, none discontinued their therapy, aligning with other studies showed that patients with AUD were able to achieve high rates of SVR and emphasizing that veterans should be treated irrespective of an AUD diagnosis.11 However, more innovative engagement initiatives for veterans with AUD should be explored as we continue to adapt more telehealth-based care for HCV direct-acting antiviral treatments. A more in-depth understanding of how alcohol use relates to treatment noninitiation is warranted, as this may stem from behavioral patterns that could not be captured in the present study.
The inability to reach veterans by telephone was a major reason for noninitiation and discontinuation of treatment. While the expansion of telehealth services has been noted across the VHA, there is still room for improving methods of engaging veterans in health care postpandemic.12 Prior studies in veteran populations that were successful in increasing uptake of HCV treatment have employed telehealth strategies that further emphasizes its integral role in HCV elimination.13 Although our study did not show mental health comorbidities and housing status as statistically significant, it is important to note that 20% of patients referred for HCV treatment had an incomplete evaluation which can lead to potentially unobserved indicators not captured by our study such as quality of linkage to care. It is imperative to stress the best practices for HCV initiation by integrating a multidisciplinary team to address patients’ psychosocial comorbidities.14 Finally, we did not observe any major disparities in treating veterans with HCV during the pandemic. This observation is reassuring and consistent with other VHA data given the heightened recognition of health disparities seen in health care sectors across the country, especially evident during the COVID-19 pandemic and the current era of increased adaptation of telehealth.
Limitations
Limitations to this study include its retrospective nature, small sample size, and short study time frame as a proportion of veterans have yet to complete HCV treatment which can potentially explain how larger studies were able to find other statistically significant patient-related factors impacting treatment initiation compared to ours. Given the lack of universal standardized diagnostic criterion of AUD, this can limit how our study can be compared to others in similar populations. Additionally, this study was conducted at a single facility with a predominantly older male veteran population, which may not be generalizable to other populations.
Conclusions
Treating HCV during the COVID-19 pandemic with telehealth and mail-out medications was feasible and led to high SVR rates, but unhealthy alcohol use and an inability to contact veterans were predominant barriers to success. Future quality improvement efforts should focus on addressing these barriers and exploring the relationship between alcohol use and HCV treatment initiation.
1. Patel AA, Bui A, Prohl E, et al. Innovations in Hepatitis C Screening and Treatment. Hepatol Commun. 2020;5(3):371-386. Published 2020 Dec 7. doi:10.1002/hep4.1646
2. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970-975. doi:10.1001/jama.2020.1123
3. Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376. doi:10.1016/j.amepre.2021.03.011
4. Rosen CS, Morland LA, Glassman LH, et al. Virtual mental health care in the Veterans Health Administration’s immediate response to coronavirus disease-19. Am Psychol. 2021;76(1):26-38. doi:10.1037/amp0000751
5. Balut MD, Wyte-Lake T, Steers WN, et al. Expansion of telemedicine during COVID-19 at a VA specialty clinic. Healthc (Amst). 2022;10(1):100599. doi:10.1016/j.hjdsi.2021.100599
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
7. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992-1000. doi:10.1111/apt.14328
8. Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393-401. doi:10.1016/j.jhep.2017.10.019
9. Pedersen ER, Davis JP, Fitzke RE, Lee DS, Saba S. American Veterans in the Era of COVID-19: Reactions to the Pandemic, Posttraumatic Stress Disorder, and Substance Use Behaviors. Int J Ment Health Addict. 2023;21(2):767-782. doi:10.1007/s11469-021-00620-0
10. Na PJ, Norman SB, Nichter B, et al. Prevalence, risk and protective factors of alcohol use disorder during the COVID-19 pandemic in U.S. military veterans. Drug Alcohol Depend. 2021;225:108818. doi:10.1016/j.drugalcdep.2021.108818
11. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. doi:10.1016/j.drugalcdep.2016.10.021
12. Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
13. Fleming BS, Ifeachor AP, Andres AM, et al. Improving Veteran Access to Treatment for Hepatitis C Virus Infection: Addressing social issues and treatment barriers significantly increases access to HCV care, and many veterans successfully start therapy with the help of additional support staff. Fed Pract. 2017;34(Suppl 4):S24-S28.
14. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
1. Patel AA, Bui A, Prohl E, et al. Innovations in Hepatitis C Screening and Treatment. Hepatol Commun. 2020;5(3):371-386. Published 2020 Dec 7. doi:10.1002/hep4.1646
2. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970-975. doi:10.1001/jama.2020.1123
3. Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376. doi:10.1016/j.amepre.2021.03.011
4. Rosen CS, Morland LA, Glassman LH, et al. Virtual mental health care in the Veterans Health Administration’s immediate response to coronavirus disease-19. Am Psychol. 2021;76(1):26-38. doi:10.1037/amp0000751
5. Balut MD, Wyte-Lake T, Steers WN, et al. Expansion of telemedicine during COVID-19 at a VA specialty clinic. Healthc (Amst). 2022;10(1):100599. doi:10.1016/j.hjdsi.2021.100599
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
7. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992-1000. doi:10.1111/apt.14328
8. Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393-401. doi:10.1016/j.jhep.2017.10.019
9. Pedersen ER, Davis JP, Fitzke RE, Lee DS, Saba S. American Veterans in the Era of COVID-19: Reactions to the Pandemic, Posttraumatic Stress Disorder, and Substance Use Behaviors. Int J Ment Health Addict. 2023;21(2):767-782. doi:10.1007/s11469-021-00620-0
10. Na PJ, Norman SB, Nichter B, et al. Prevalence, risk and protective factors of alcohol use disorder during the COVID-19 pandemic in U.S. military veterans. Drug Alcohol Depend. 2021;225:108818. doi:10.1016/j.drugalcdep.2021.108818
11. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. doi:10.1016/j.drugalcdep.2016.10.021
12. Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
13. Fleming BS, Ifeachor AP, Andres AM, et al. Improving Veteran Access to Treatment for Hepatitis C Virus Infection: Addressing social issues and treatment barriers significantly increases access to HCV care, and many veterans successfully start therapy with the help of additional support staff. Fed Pract. 2017;34(Suppl 4):S24-S28.
14. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
The Breakthrough Drug Whose Full Promise Remains Unrealized
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Celebrating a Decade of Sofosbuvir for Hepatitis C
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
New KDIGO guideline encourages use of HCV-positive kidneys for HCV-negative recipients
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Patients exposed to HIV, hepatitis at Massachusetts hospital
The negligent administration of intravenous medications during endoscopy procedures performed between June 14, 2021, and April 19, 2023, at Salem Hospital, located about 20 miles northeast of Boston, has caused a “heightened risk of exposure to these harmful life-altering and life-threatening infections,” according to the lawsuit filed at Suffolk County Superior Court in Boston by Keches Law Group on behalf of plaintiff Melinda Cashman and others.
Although patients were notified in early November of their potential exposure, it could take months or even years to determine if infection has occurred. Attorneys for Ms. Cashman claim that the plaintiff “suffered and will continue to suffer severe emotional distress and anguish” as a result of the associated risks.
The lawyers argue that Ms. Cashman and others like her may “suffer permanent injuries,” along with “extreme anxiety and decreased quality of life.” They are seeking monetary damages to offset disruptions to relationships, increased medical bills, and any mental health therapy required.
Outreach to potentially affected patients began after the hospital was made aware, earlier this year, of an “isolated practice” that could have led to viral transmission, according to a statement from Mass General Brigham, but there is no evidence to date of any infections resulting from this incident. “We sincerely apologize to those who have been impacted and we remain committed to delivering high-quality, compassionate healthcare to our community.”
Hepatitis B and C are both treatable with antiviral mediations, and hepatitis C is curable in 95% of cases, according to the Centers for Disease Control and Prevention. HIV, although not curable, can be managed with antiretroviral therapy.
Mass General Brigham is working with the Massachusetts Department of Public Health, which will conduct an onsite investigation into quality-control practices. Affected patients can reach out to a clinician-staffed hotline with questions and receive free screening for the viruses, hospital officials report.
A version of this article appeared on Medscape.com.
The negligent administration of intravenous medications during endoscopy procedures performed between June 14, 2021, and April 19, 2023, at Salem Hospital, located about 20 miles northeast of Boston, has caused a “heightened risk of exposure to these harmful life-altering and life-threatening infections,” according to the lawsuit filed at Suffolk County Superior Court in Boston by Keches Law Group on behalf of plaintiff Melinda Cashman and others.
Although patients were notified in early November of their potential exposure, it could take months or even years to determine if infection has occurred. Attorneys for Ms. Cashman claim that the plaintiff “suffered and will continue to suffer severe emotional distress and anguish” as a result of the associated risks.
The lawyers argue that Ms. Cashman and others like her may “suffer permanent injuries,” along with “extreme anxiety and decreased quality of life.” They are seeking monetary damages to offset disruptions to relationships, increased medical bills, and any mental health therapy required.
Outreach to potentially affected patients began after the hospital was made aware, earlier this year, of an “isolated practice” that could have led to viral transmission, according to a statement from Mass General Brigham, but there is no evidence to date of any infections resulting from this incident. “We sincerely apologize to those who have been impacted and we remain committed to delivering high-quality, compassionate healthcare to our community.”
Hepatitis B and C are both treatable with antiviral mediations, and hepatitis C is curable in 95% of cases, according to the Centers for Disease Control and Prevention. HIV, although not curable, can be managed with antiretroviral therapy.
Mass General Brigham is working with the Massachusetts Department of Public Health, which will conduct an onsite investigation into quality-control practices. Affected patients can reach out to a clinician-staffed hotline with questions and receive free screening for the viruses, hospital officials report.
A version of this article appeared on Medscape.com.
The negligent administration of intravenous medications during endoscopy procedures performed between June 14, 2021, and April 19, 2023, at Salem Hospital, located about 20 miles northeast of Boston, has caused a “heightened risk of exposure to these harmful life-altering and life-threatening infections,” according to the lawsuit filed at Suffolk County Superior Court in Boston by Keches Law Group on behalf of plaintiff Melinda Cashman and others.
Although patients were notified in early November of their potential exposure, it could take months or even years to determine if infection has occurred. Attorneys for Ms. Cashman claim that the plaintiff “suffered and will continue to suffer severe emotional distress and anguish” as a result of the associated risks.
The lawyers argue that Ms. Cashman and others like her may “suffer permanent injuries,” along with “extreme anxiety and decreased quality of life.” They are seeking monetary damages to offset disruptions to relationships, increased medical bills, and any mental health therapy required.
Outreach to potentially affected patients began after the hospital was made aware, earlier this year, of an “isolated practice” that could have led to viral transmission, according to a statement from Mass General Brigham, but there is no evidence to date of any infections resulting from this incident. “We sincerely apologize to those who have been impacted and we remain committed to delivering high-quality, compassionate healthcare to our community.”
Hepatitis B and C are both treatable with antiviral mediations, and hepatitis C is curable in 95% of cases, according to the Centers for Disease Control and Prevention. HIV, although not curable, can be managed with antiretroviral therapy.
Mass General Brigham is working with the Massachusetts Department of Public Health, which will conduct an onsite investigation into quality-control practices. Affected patients can reach out to a clinician-staffed hotline with questions and receive free screening for the viruses, hospital officials report.
A version of this article appeared on Medscape.com.