User login
AAP report flags risks of prescribing codeine for children
The risks of using codeine to treat pain or cough in children may often outweigh the benefits, sometimes even leading to death, and call into question whether its widespread use should continue in pediatric patients, according to an American Academy of Pediatrics technical report.
“It is clear that one of the keys to improving analgesia and reducing opioid-related adverse effects is both provider and parental education regarding the effective use of nonopioid analgesics,” wrote Joseph D. Tobias, MD, and his colleagues from the AAP Committee on Drugs’ Section on Anesthesiology and Pain Medicine (Pediatrics 2016 Sept 19. doi: 10.1542/peds.2016-2396). “The answer may not lie in using more medication or different medications but merely using more effectively other options that are currently available.”
Individual patients respond differently to codeine because the conversion rates of the liver enzyme that metabolizes codeine into morphine, CYP2D6, vary greatly according to genetic differences. Some children experience no therapeutic effect at all while others have stopped breathing or died, particularly those who metabolize the drug extremely rapidly. Those with at least two copies of the CYP2D6 gene have a particularly elevated level of enzyme activity. Also at high risk for respiratory depression or death are children with obstructive sleep apnea.
Poor metabolizers, who therefore experience less effect from codeine, include disproportionately more individuals of Northern European descent. Ultrarapid metabolizers, on the other hand, comprise approximately 29% of patients of African/Ethiopian heritage and 21% from Middle Eastern countries. An estimated 3.4%-6.5% of African Americans and whites are ultrafast metabolizers. Genetic tests can identify those at higher risk, but even children with normal metabolism can experience severe adverse effects.
The World Health Organization removed codeine from its list of essential medications, the U.S. Food and Drug Administration added a black box warning to labels of codeine formulations used for tonsillectomy and/or adenoidectomy in children, and the European Medicines Agency recommended against using codeine in children under age 12 years and in those between 12 and 18 years who have breathing difficulties.
Yet research has shown that the use of codeine for pain relief in children remains very common; codeine is prescribed more than any other opioid in some studies. Otolaryngologists, dentists, pediatricians, and family practice physicians, respectively, prescribe it most often, likely because few safe, effective therapeutics exist for treating pain or cough in children. Oxycodone has been used as an alternative, but this drug also lacks adequate data on its use, and hydrocodone has similar concerns with rapid metabolizers.
Although most of the serious adverse events resulting in codeine use in children have followed adenotonsillectomy in children with disordered breathing, the authors warned that “physicians cannot assume such problems will occur only” after such procedures.
“Given the increasing prevalence of obesity in the United States, it is likely that some patients presenting for nonotolaryngologic procedures may have undiagnosed sleep-disordered breathing and may also be at risk if they require extended postoperative analgesia,” they wrote. They called for better parental education regarding pain relief and more formal restrictions for its use in pediatrics.
The report did not use external funding, and the authors reported no relevant financial disclosures.
Our scientific understanding of the underlying mechanism for respiratory suppression sometimes seen in children taking codeine is increasing, but these safety concerns aren’t new. The clinical report from Tobias et al. provides a timeline for our awareness of, and organizational response to, the reports of adverse events that goes back several years. Sadly, the investigators also provide evidence that codeine prescription patterns haven’t significantly changed, even among pediatric medical professionals.
Change is difficult in all aspects of life, and medical practice is no different. But as pediatric caregivers, the burden is on us to model safe and effective pain management. There is simply no excuse for our continued prescription of a drug with questionable benefit that, in many patients, has such an unfavorable risk-benefit ratio. And this concern is even greater when codeine is recommended for pediatric cough, an indication lacking solid evidence of benefit.
Unfortunately, there are limited pharmaceutical options for treating pediatric pain and cough, and we are often compelled to attempt to fit our square pegs into the round hole of adult medicine. The report’s authors point out that perhaps maximizing the effectiveness of drugs with proven track records in children should be the focus of our efforts. Although not mentioned in the report, benefits from the low-hanging fruit of science-based nonpharmaceutical approaches should be similarly prioritized.
These comments were provided by Clay Jones, M.D., a neonatal hospitalist at Wellesley (Mass.) Hospital. Dr. Jones had no relevant financial disclosures.
Our scientific understanding of the underlying mechanism for respiratory suppression sometimes seen in children taking codeine is increasing, but these safety concerns aren’t new. The clinical report from Tobias et al. provides a timeline for our awareness of, and organizational response to, the reports of adverse events that goes back several years. Sadly, the investigators also provide evidence that codeine prescription patterns haven’t significantly changed, even among pediatric medical professionals.
Change is difficult in all aspects of life, and medical practice is no different. But as pediatric caregivers, the burden is on us to model safe and effective pain management. There is simply no excuse for our continued prescription of a drug with questionable benefit that, in many patients, has such an unfavorable risk-benefit ratio. And this concern is even greater when codeine is recommended for pediatric cough, an indication lacking solid evidence of benefit.
Unfortunately, there are limited pharmaceutical options for treating pediatric pain and cough, and we are often compelled to attempt to fit our square pegs into the round hole of adult medicine. The report’s authors point out that perhaps maximizing the effectiveness of drugs with proven track records in children should be the focus of our efforts. Although not mentioned in the report, benefits from the low-hanging fruit of science-based nonpharmaceutical approaches should be similarly prioritized.
These comments were provided by Clay Jones, M.D., a neonatal hospitalist at Wellesley (Mass.) Hospital. Dr. Jones had no relevant financial disclosures.
Our scientific understanding of the underlying mechanism for respiratory suppression sometimes seen in children taking codeine is increasing, but these safety concerns aren’t new. The clinical report from Tobias et al. provides a timeline for our awareness of, and organizational response to, the reports of adverse events that goes back several years. Sadly, the investigators also provide evidence that codeine prescription patterns haven’t significantly changed, even among pediatric medical professionals.
Change is difficult in all aspects of life, and medical practice is no different. But as pediatric caregivers, the burden is on us to model safe and effective pain management. There is simply no excuse for our continued prescription of a drug with questionable benefit that, in many patients, has such an unfavorable risk-benefit ratio. And this concern is even greater when codeine is recommended for pediatric cough, an indication lacking solid evidence of benefit.
Unfortunately, there are limited pharmaceutical options for treating pediatric pain and cough, and we are often compelled to attempt to fit our square pegs into the round hole of adult medicine. The report’s authors point out that perhaps maximizing the effectiveness of drugs with proven track records in children should be the focus of our efforts. Although not mentioned in the report, benefits from the low-hanging fruit of science-based nonpharmaceutical approaches should be similarly prioritized.
These comments were provided by Clay Jones, M.D., a neonatal hospitalist at Wellesley (Mass.) Hospital. Dr. Jones had no relevant financial disclosures.
The risks of using codeine to treat pain or cough in children may often outweigh the benefits, sometimes even leading to death, and call into question whether its widespread use should continue in pediatric patients, according to an American Academy of Pediatrics technical report.
“It is clear that one of the keys to improving analgesia and reducing opioid-related adverse effects is both provider and parental education regarding the effective use of nonopioid analgesics,” wrote Joseph D. Tobias, MD, and his colleagues from the AAP Committee on Drugs’ Section on Anesthesiology and Pain Medicine (Pediatrics 2016 Sept 19. doi: 10.1542/peds.2016-2396). “The answer may not lie in using more medication or different medications but merely using more effectively other options that are currently available.”
Individual patients respond differently to codeine because the conversion rates of the liver enzyme that metabolizes codeine into morphine, CYP2D6, vary greatly according to genetic differences. Some children experience no therapeutic effect at all while others have stopped breathing or died, particularly those who metabolize the drug extremely rapidly. Those with at least two copies of the CYP2D6 gene have a particularly elevated level of enzyme activity. Also at high risk for respiratory depression or death are children with obstructive sleep apnea.
Poor metabolizers, who therefore experience less effect from codeine, include disproportionately more individuals of Northern European descent. Ultrarapid metabolizers, on the other hand, comprise approximately 29% of patients of African/Ethiopian heritage and 21% from Middle Eastern countries. An estimated 3.4%-6.5% of African Americans and whites are ultrafast metabolizers. Genetic tests can identify those at higher risk, but even children with normal metabolism can experience severe adverse effects.
The World Health Organization removed codeine from its list of essential medications, the U.S. Food and Drug Administration added a black box warning to labels of codeine formulations used for tonsillectomy and/or adenoidectomy in children, and the European Medicines Agency recommended against using codeine in children under age 12 years and in those between 12 and 18 years who have breathing difficulties.
Yet research has shown that the use of codeine for pain relief in children remains very common; codeine is prescribed more than any other opioid in some studies. Otolaryngologists, dentists, pediatricians, and family practice physicians, respectively, prescribe it most often, likely because few safe, effective therapeutics exist for treating pain or cough in children. Oxycodone has been used as an alternative, but this drug also lacks adequate data on its use, and hydrocodone has similar concerns with rapid metabolizers.
Although most of the serious adverse events resulting in codeine use in children have followed adenotonsillectomy in children with disordered breathing, the authors warned that “physicians cannot assume such problems will occur only” after such procedures.
“Given the increasing prevalence of obesity in the United States, it is likely that some patients presenting for nonotolaryngologic procedures may have undiagnosed sleep-disordered breathing and may also be at risk if they require extended postoperative analgesia,” they wrote. They called for better parental education regarding pain relief and more formal restrictions for its use in pediatrics.
The report did not use external funding, and the authors reported no relevant financial disclosures.
The risks of using codeine to treat pain or cough in children may often outweigh the benefits, sometimes even leading to death, and call into question whether its widespread use should continue in pediatric patients, according to an American Academy of Pediatrics technical report.
“It is clear that one of the keys to improving analgesia and reducing opioid-related adverse effects is both provider and parental education regarding the effective use of nonopioid analgesics,” wrote Joseph D. Tobias, MD, and his colleagues from the AAP Committee on Drugs’ Section on Anesthesiology and Pain Medicine (Pediatrics 2016 Sept 19. doi: 10.1542/peds.2016-2396). “The answer may not lie in using more medication or different medications but merely using more effectively other options that are currently available.”
Individual patients respond differently to codeine because the conversion rates of the liver enzyme that metabolizes codeine into morphine, CYP2D6, vary greatly according to genetic differences. Some children experience no therapeutic effect at all while others have stopped breathing or died, particularly those who metabolize the drug extremely rapidly. Those with at least two copies of the CYP2D6 gene have a particularly elevated level of enzyme activity. Also at high risk for respiratory depression or death are children with obstructive sleep apnea.
Poor metabolizers, who therefore experience less effect from codeine, include disproportionately more individuals of Northern European descent. Ultrarapid metabolizers, on the other hand, comprise approximately 29% of patients of African/Ethiopian heritage and 21% from Middle Eastern countries. An estimated 3.4%-6.5% of African Americans and whites are ultrafast metabolizers. Genetic tests can identify those at higher risk, but even children with normal metabolism can experience severe adverse effects.
The World Health Organization removed codeine from its list of essential medications, the U.S. Food and Drug Administration added a black box warning to labels of codeine formulations used for tonsillectomy and/or adenoidectomy in children, and the European Medicines Agency recommended against using codeine in children under age 12 years and in those between 12 and 18 years who have breathing difficulties.
Yet research has shown that the use of codeine for pain relief in children remains very common; codeine is prescribed more than any other opioid in some studies. Otolaryngologists, dentists, pediatricians, and family practice physicians, respectively, prescribe it most often, likely because few safe, effective therapeutics exist for treating pain or cough in children. Oxycodone has been used as an alternative, but this drug also lacks adequate data on its use, and hydrocodone has similar concerns with rapid metabolizers.
Although most of the serious adverse events resulting in codeine use in children have followed adenotonsillectomy in children with disordered breathing, the authors warned that “physicians cannot assume such problems will occur only” after such procedures.
“Given the increasing prevalence of obesity in the United States, it is likely that some patients presenting for nonotolaryngologic procedures may have undiagnosed sleep-disordered breathing and may also be at risk if they require extended postoperative analgesia,” they wrote. They called for better parental education regarding pain relief and more formal restrictions for its use in pediatrics.
The report did not use external funding, and the authors reported no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: Codeine use in children carries significant risks, such as breathing depression and death.
Major finding: Children with African/Ethiopian and Middle Eastern descent are more likely to be rapid metabolizers of codeine and at greater risk for serious adverse effects.
Data source: A review of the most current literature on the adverse effects of codeine use in pediatric patients and guidance issued by regulatory and professional medical organizations.
Disclosures: The report did not use external funding, and the authors reported no relevant financial disclosures.
AAP speaks out on dismissal of vaccine-refusing patients, vaccine hesitancy
For years, pediatricians have sought a blessing from the American Academy of Pediatrics that acknowledged it was valid for members to dismiss families from their practice if they refused to vaccinate despite all attempts to persuade them. Now, a new clinical report has essentially delivered just that.
The report does not represent an official policy change from the AAP, but it does for the first time acknowledge that “firing” patients who persistently refuse vaccination is “an acceptable option” (Pediatrics. 2016 Aug. doi: 10.1542/peds.2016-2146).
“A number of pediatricians feel so strongly that if they don’t agree on vaccines, which are so basic to the delivery of care and have made such a big difference in children’s lives, how will they agree on a number of other things they’ll need to discuss?” Kathryn M. Edwards, MD, director of the Vanderbilt Vaccine Research Program, Nashville, Tenn., and a coauthor of the report, explained in an interview.
The AAP has received pressure from its members over recent years as increasing numbers of pediatricians choose to dismiss some or all of their patients whose parents were resolved not vaccinate, coauthor Jesse M. Hackell, MD, a practicing pediatrician and managing partner at Pomona Pediatrics, an affiliate of Boston Children’s Health Physicians, said in an interview.
In fact, a new study has revealed that 12% of pediatricians reported dismissing vaccine-refusing families in 2013, up from 6% in 2006. At the same time, the proportion of families refusing vaccines has nearly doubled in the same time.
“There was a groundswell of opinion that enough is enough and we can’t provide quality care if we can’t provide something we know is so important,” Dr. Hackell said. “We felt the Academy needed to stop being so adamantly opposed to the possibility of dismissal – not to recommend dismissal but simply to state it is an acceptable option.”
The AAP responds to fellows’ concerns
While the AAP continues to recommend doctors attempt to persuade families as long as possible to vaccinate, the new report discusses dismissal as a viable option as long as it adheres to relevant state laws that prohibit abandonment of patients.
“The decision to dismiss a family who continues to refuse immunization is not one that should be made lightly, nor should it be made without considering and respecting the reasons for the parents’ point of view,” the report states. “Nevertheless, the individual pediatrician may consider dismissal of families who refuse vaccination as an acceptable option.”
The report does note that some practice settings, such as hospitals or large health care organizations, may not allow dismissal of patients, and that pediatricians “should carefully evaluate the availability of other qualified providers for the family” if they live in an area with limited access to pediatric care.
But the report finally acknowledges those pediatricians who are “just philosophically wired to not accept vaccine refusals,” Stuart A. Cohen, MD, an assistant professor of pediatrics at the University of California, San Diego, and chair of AAP District 9 in California, said in an interview.
“It really interferes with your physician-patient relationship,” Dr. Cohen said, who was not a coauthor of the report.
Now, if pediatricians feel it necessary to dismiss nonvaccinating patients, “then the Academy understands because of concerns for other patients, but it must be done in a way that’s respectful and tries to ensure patients understand the safety and necessity of vaccines,” Dr. Edwards said.
The report still includes the AAP recommendation that “pediatricians continue to engage with vaccine-hesitant parents, provide other health care services to their children, and attempt to modify their opposition to vaccines.” And a number of members of the AAP’s infectious diseases and bioethics committees were uncomfortable with dismissing patients, Dr. Edwards said, but “there were certain people who needed this, who needed some blessing that this was not inappropriate after all the other things the pediatrician had done.”
Vaccines undergo thorough testing for safety and effectiveness
But the report also aims to provide pediatricians with strategies for doing everything possible first.
“We needed to address enabling the clinician to have some very specific talking points to use and not get involved in a philosophical discussion that can take an hour,” Dr. Hackell said. “They need to make a clear statement that vaccines are important, and if you don’t get them, bad things like death can happen.”
The report therefore provides a comprehensive overview of vaccine development, from the initial identification of the need for a vaccine through the various phases of clinical testing and ongoing postlicensure monitoring. This background information can arm pediatricians with foundational knowledge that’s helpful in talking with patients.
“Vaccine development is a long and arduous process, often lasting many years and involving a combination of public and private partnerships,” the report states. “The current system for developing, testing and regulating vaccines requires that the vaccines demonstrate both safety and efficacy before licensure and that long-term safety is monitored.”
The report briefly explains the multiple mechanisms for continuing to track and study adverse events and other safety concerns:
• Vaccine Adverse Events Reporting System (VAERS). A voluntary passive reporting system used to identify potential safety signals.
• Vaccine Safety Datalink (VSD). A network of linked databases from health care systems across the United States involving millions of individuals
• Post-Licensure Rapid Immunization Safety Monitoring system (PRISM). A system which monitors vaccine safety using health insurance claims data from 107 million individuals .
• Clinical Immunization Safety Assessment Project (CISA). A system that answers individual health care providers’ specific questions on vaccine safety.
Vaccine hesitancy and vaccine exemptions
Opposition to vaccination is not new, the report states, describing it as dating back to Edward Jenner’s smallpox vaccine in the early 1800s.
“Although vaccine hesitancy is not a new phenomenon, it may have a greater effect on public health today,” the report states. “With the ease of global travel, vaccine-preventable diseases are spread more quickly and may unexpectedly appear in areas where health care professionals are unfamiliar with their clinical presentation.”
The historical presence of vaccination opposition has led to circumstances in the United States today in which parents can seek nonmedical exemptions from vaccines in 47 states, and their use has increased with their availability. Yet, the increase in use of exemptions and of “alternative” immunization schedules runs the risk of eroding the herd immunity that protects the community, the report notes.
“For these reasons, we believe the better approach is to work to eliminate all nonmedical exemptions for childhood vaccines,” the authors write. The American Medical Association and the Infectious Diseases Society of America espouse this position, and the AAP is developing a similar statement.
“Families should not have to fear going to school or the grocery store or a house of worship and worry about their kids getting sick,” said Dr. Cohen. “We now strongly say that we need to work with legislators, families, and other advocates for children at the state level to spread more laws like California’s SB 277 that would abolish philosophical exemptions.”
Dr. Cohen also emphasized the importance of communicating to parents that there are no valid “alternative schedules” for vaccination. There is the Centers for Disease Control and Prevention recommended schedule and anything else is a “nonrecommended vaccine schedule because it hasn’t been studied.” That change in terminology drives home the point that the CDC schedule is the only one fully tested for safety and effectiveness.
Meanwhile, however, pediatricians need the tools to address vaccine hesitancy, starting with understanding it. The report describes the pattern of disease incidence, vaccine uptake, disease reduction, adverse event increase and resulting vaccine hesitancy, punctuated by periodic outbreaks that restore eroded confidence in vaccines.
“When diseases are present, parents are worried and want a vaccine, and when they’re gone, they don’t,” Dr. Edwards said. “We need to remember that there is a dependence on the maintenance of herd immunity by immunization by your neighbors.”
Specific strategies to counter vaccine hesitancy
Just over half of physicians spend 10-19 minutes discussing vaccines with concerned parents, and 8% spend at least 20 minutes with such parents, found a study cited in the report. Other research has found these discussions take a toll on doctors’ job satisfaction.
Yet pediatricians remain the single biggest influence on parents’ vaccination decisions, cited by nearly 80% of parents in one large study.
“The pediatrician should appreciate that vaccine-hesitant parents are a heterogeneous group and that specific parental vaccine concerns should be individually identified and addressed,” the report states. “Although many techniques for working with vaccine-hesitant parents have been suggested, scant data are available to determine the efficacy of these methods.”
It goes on to recommend that physicians should discuss the development and safety testing of vaccines “in a nonconfrontational dialogue with the parents while listening to and acknowledging their concerns.”
Pediatricians should not, however, delay vaccines or limit the number per visit – thereby deviating from the CDC recommended and AAP-endorsed schedule – unless it’s the only way a parent agrees to vaccinate.
Another strategy is the presumptive approach: Present all vaccine recommendations as required immunizations that the provider expects a parent to agree to, although pediatricians should consider their experience and relationship with a family since this approach may not work well for some parents.
The report also emphasizes the potential effectiveness of personalizing vaccine conversations by having doctors share their own experience, such as the fact that they vaccinated themselves, their children, and/or their grandchildren.
“Parents often are more likely to be persuaded by stories and anecdotes about the successes of vaccines,” the authors write. “Personal examples of children who were sick with vaccine-preventable illnesses can be much more effective than simply reading the numbers of children infected with a disease each year.”
The report also offers several suggestions for reducing the pain from administering vaccinations: administering vaccines quickly without aspirating; saving the most painful injection for last; holding the child upright; providing tactile stimulation; breastfeeding or providing sweet solutions and topical anesthetics after administration; and using distraction, such as deep breathing, pinwheels, or toys to decrease children’s pain and anxiety.
But the bottom line is that pediatricians have one key message they must communicate to parents, the report states: “The clear message parents should hear is that vaccines are safe and effective, and serious disease can occur if your child and family are not immunized.”
For years, pediatricians have sought a blessing from the American Academy of Pediatrics that acknowledged it was valid for members to dismiss families from their practice if they refused to vaccinate despite all attempts to persuade them. Now, a new clinical report has essentially delivered just that.
The report does not represent an official policy change from the AAP, but it does for the first time acknowledge that “firing” patients who persistently refuse vaccination is “an acceptable option” (Pediatrics. 2016 Aug. doi: 10.1542/peds.2016-2146).
“A number of pediatricians feel so strongly that if they don’t agree on vaccines, which are so basic to the delivery of care and have made such a big difference in children’s lives, how will they agree on a number of other things they’ll need to discuss?” Kathryn M. Edwards, MD, director of the Vanderbilt Vaccine Research Program, Nashville, Tenn., and a coauthor of the report, explained in an interview.
The AAP has received pressure from its members over recent years as increasing numbers of pediatricians choose to dismiss some or all of their patients whose parents were resolved not vaccinate, coauthor Jesse M. Hackell, MD, a practicing pediatrician and managing partner at Pomona Pediatrics, an affiliate of Boston Children’s Health Physicians, said in an interview.
In fact, a new study has revealed that 12% of pediatricians reported dismissing vaccine-refusing families in 2013, up from 6% in 2006. At the same time, the proportion of families refusing vaccines has nearly doubled in the same time.
“There was a groundswell of opinion that enough is enough and we can’t provide quality care if we can’t provide something we know is so important,” Dr. Hackell said. “We felt the Academy needed to stop being so adamantly opposed to the possibility of dismissal – not to recommend dismissal but simply to state it is an acceptable option.”
The AAP responds to fellows’ concerns
While the AAP continues to recommend doctors attempt to persuade families as long as possible to vaccinate, the new report discusses dismissal as a viable option as long as it adheres to relevant state laws that prohibit abandonment of patients.
“The decision to dismiss a family who continues to refuse immunization is not one that should be made lightly, nor should it be made without considering and respecting the reasons for the parents’ point of view,” the report states. “Nevertheless, the individual pediatrician may consider dismissal of families who refuse vaccination as an acceptable option.”
The report does note that some practice settings, such as hospitals or large health care organizations, may not allow dismissal of patients, and that pediatricians “should carefully evaluate the availability of other qualified providers for the family” if they live in an area with limited access to pediatric care.
But the report finally acknowledges those pediatricians who are “just philosophically wired to not accept vaccine refusals,” Stuart A. Cohen, MD, an assistant professor of pediatrics at the University of California, San Diego, and chair of AAP District 9 in California, said in an interview.
“It really interferes with your physician-patient relationship,” Dr. Cohen said, who was not a coauthor of the report.
Now, if pediatricians feel it necessary to dismiss nonvaccinating patients, “then the Academy understands because of concerns for other patients, but it must be done in a way that’s respectful and tries to ensure patients understand the safety and necessity of vaccines,” Dr. Edwards said.
The report still includes the AAP recommendation that “pediatricians continue to engage with vaccine-hesitant parents, provide other health care services to their children, and attempt to modify their opposition to vaccines.” And a number of members of the AAP’s infectious diseases and bioethics committees were uncomfortable with dismissing patients, Dr. Edwards said, but “there were certain people who needed this, who needed some blessing that this was not inappropriate after all the other things the pediatrician had done.”
Vaccines undergo thorough testing for safety and effectiveness
But the report also aims to provide pediatricians with strategies for doing everything possible first.
“We needed to address enabling the clinician to have some very specific talking points to use and not get involved in a philosophical discussion that can take an hour,” Dr. Hackell said. “They need to make a clear statement that vaccines are important, and if you don’t get them, bad things like death can happen.”
The report therefore provides a comprehensive overview of vaccine development, from the initial identification of the need for a vaccine through the various phases of clinical testing and ongoing postlicensure monitoring. This background information can arm pediatricians with foundational knowledge that’s helpful in talking with patients.
“Vaccine development is a long and arduous process, often lasting many years and involving a combination of public and private partnerships,” the report states. “The current system for developing, testing and regulating vaccines requires that the vaccines demonstrate both safety and efficacy before licensure and that long-term safety is monitored.”
The report briefly explains the multiple mechanisms for continuing to track and study adverse events and other safety concerns:
• Vaccine Adverse Events Reporting System (VAERS). A voluntary passive reporting system used to identify potential safety signals.
• Vaccine Safety Datalink (VSD). A network of linked databases from health care systems across the United States involving millions of individuals
• Post-Licensure Rapid Immunization Safety Monitoring system (PRISM). A system which monitors vaccine safety using health insurance claims data from 107 million individuals .
• Clinical Immunization Safety Assessment Project (CISA). A system that answers individual health care providers’ specific questions on vaccine safety.
Vaccine hesitancy and vaccine exemptions
Opposition to vaccination is not new, the report states, describing it as dating back to Edward Jenner’s smallpox vaccine in the early 1800s.
“Although vaccine hesitancy is not a new phenomenon, it may have a greater effect on public health today,” the report states. “With the ease of global travel, vaccine-preventable diseases are spread more quickly and may unexpectedly appear in areas where health care professionals are unfamiliar with their clinical presentation.”
The historical presence of vaccination opposition has led to circumstances in the United States today in which parents can seek nonmedical exemptions from vaccines in 47 states, and their use has increased with their availability. Yet, the increase in use of exemptions and of “alternative” immunization schedules runs the risk of eroding the herd immunity that protects the community, the report notes.
“For these reasons, we believe the better approach is to work to eliminate all nonmedical exemptions for childhood vaccines,” the authors write. The American Medical Association and the Infectious Diseases Society of America espouse this position, and the AAP is developing a similar statement.
“Families should not have to fear going to school or the grocery store or a house of worship and worry about their kids getting sick,” said Dr. Cohen. “We now strongly say that we need to work with legislators, families, and other advocates for children at the state level to spread more laws like California’s SB 277 that would abolish philosophical exemptions.”
Dr. Cohen also emphasized the importance of communicating to parents that there are no valid “alternative schedules” for vaccination. There is the Centers for Disease Control and Prevention recommended schedule and anything else is a “nonrecommended vaccine schedule because it hasn’t been studied.” That change in terminology drives home the point that the CDC schedule is the only one fully tested for safety and effectiveness.
Meanwhile, however, pediatricians need the tools to address vaccine hesitancy, starting with understanding it. The report describes the pattern of disease incidence, vaccine uptake, disease reduction, adverse event increase and resulting vaccine hesitancy, punctuated by periodic outbreaks that restore eroded confidence in vaccines.
“When diseases are present, parents are worried and want a vaccine, and when they’re gone, they don’t,” Dr. Edwards said. “We need to remember that there is a dependence on the maintenance of herd immunity by immunization by your neighbors.”
Specific strategies to counter vaccine hesitancy
Just over half of physicians spend 10-19 minutes discussing vaccines with concerned parents, and 8% spend at least 20 minutes with such parents, found a study cited in the report. Other research has found these discussions take a toll on doctors’ job satisfaction.
Yet pediatricians remain the single biggest influence on parents’ vaccination decisions, cited by nearly 80% of parents in one large study.
“The pediatrician should appreciate that vaccine-hesitant parents are a heterogeneous group and that specific parental vaccine concerns should be individually identified and addressed,” the report states. “Although many techniques for working with vaccine-hesitant parents have been suggested, scant data are available to determine the efficacy of these methods.”
It goes on to recommend that physicians should discuss the development and safety testing of vaccines “in a nonconfrontational dialogue with the parents while listening to and acknowledging their concerns.”
Pediatricians should not, however, delay vaccines or limit the number per visit – thereby deviating from the CDC recommended and AAP-endorsed schedule – unless it’s the only way a parent agrees to vaccinate.
Another strategy is the presumptive approach: Present all vaccine recommendations as required immunizations that the provider expects a parent to agree to, although pediatricians should consider their experience and relationship with a family since this approach may not work well for some parents.
The report also emphasizes the potential effectiveness of personalizing vaccine conversations by having doctors share their own experience, such as the fact that they vaccinated themselves, their children, and/or their grandchildren.
“Parents often are more likely to be persuaded by stories and anecdotes about the successes of vaccines,” the authors write. “Personal examples of children who were sick with vaccine-preventable illnesses can be much more effective than simply reading the numbers of children infected with a disease each year.”
The report also offers several suggestions for reducing the pain from administering vaccinations: administering vaccines quickly without aspirating; saving the most painful injection for last; holding the child upright; providing tactile stimulation; breastfeeding or providing sweet solutions and topical anesthetics after administration; and using distraction, such as deep breathing, pinwheels, or toys to decrease children’s pain and anxiety.
But the bottom line is that pediatricians have one key message they must communicate to parents, the report states: “The clear message parents should hear is that vaccines are safe and effective, and serious disease can occur if your child and family are not immunized.”
For years, pediatricians have sought a blessing from the American Academy of Pediatrics that acknowledged it was valid for members to dismiss families from their practice if they refused to vaccinate despite all attempts to persuade them. Now, a new clinical report has essentially delivered just that.
The report does not represent an official policy change from the AAP, but it does for the first time acknowledge that “firing” patients who persistently refuse vaccination is “an acceptable option” (Pediatrics. 2016 Aug. doi: 10.1542/peds.2016-2146).
“A number of pediatricians feel so strongly that if they don’t agree on vaccines, which are so basic to the delivery of care and have made such a big difference in children’s lives, how will they agree on a number of other things they’ll need to discuss?” Kathryn M. Edwards, MD, director of the Vanderbilt Vaccine Research Program, Nashville, Tenn., and a coauthor of the report, explained in an interview.
The AAP has received pressure from its members over recent years as increasing numbers of pediatricians choose to dismiss some or all of their patients whose parents were resolved not vaccinate, coauthor Jesse M. Hackell, MD, a practicing pediatrician and managing partner at Pomona Pediatrics, an affiliate of Boston Children’s Health Physicians, said in an interview.
In fact, a new study has revealed that 12% of pediatricians reported dismissing vaccine-refusing families in 2013, up from 6% in 2006. At the same time, the proportion of families refusing vaccines has nearly doubled in the same time.
“There was a groundswell of opinion that enough is enough and we can’t provide quality care if we can’t provide something we know is so important,” Dr. Hackell said. “We felt the Academy needed to stop being so adamantly opposed to the possibility of dismissal – not to recommend dismissal but simply to state it is an acceptable option.”
The AAP responds to fellows’ concerns
While the AAP continues to recommend doctors attempt to persuade families as long as possible to vaccinate, the new report discusses dismissal as a viable option as long as it adheres to relevant state laws that prohibit abandonment of patients.
“The decision to dismiss a family who continues to refuse immunization is not one that should be made lightly, nor should it be made without considering and respecting the reasons for the parents’ point of view,” the report states. “Nevertheless, the individual pediatrician may consider dismissal of families who refuse vaccination as an acceptable option.”
The report does note that some practice settings, such as hospitals or large health care organizations, may not allow dismissal of patients, and that pediatricians “should carefully evaluate the availability of other qualified providers for the family” if they live in an area with limited access to pediatric care.
But the report finally acknowledges those pediatricians who are “just philosophically wired to not accept vaccine refusals,” Stuart A. Cohen, MD, an assistant professor of pediatrics at the University of California, San Diego, and chair of AAP District 9 in California, said in an interview.
“It really interferes with your physician-patient relationship,” Dr. Cohen said, who was not a coauthor of the report.
Now, if pediatricians feel it necessary to dismiss nonvaccinating patients, “then the Academy understands because of concerns for other patients, but it must be done in a way that’s respectful and tries to ensure patients understand the safety and necessity of vaccines,” Dr. Edwards said.
The report still includes the AAP recommendation that “pediatricians continue to engage with vaccine-hesitant parents, provide other health care services to their children, and attempt to modify their opposition to vaccines.” And a number of members of the AAP’s infectious diseases and bioethics committees were uncomfortable with dismissing patients, Dr. Edwards said, but “there were certain people who needed this, who needed some blessing that this was not inappropriate after all the other things the pediatrician had done.”
Vaccines undergo thorough testing for safety and effectiveness
But the report also aims to provide pediatricians with strategies for doing everything possible first.
“We needed to address enabling the clinician to have some very specific talking points to use and not get involved in a philosophical discussion that can take an hour,” Dr. Hackell said. “They need to make a clear statement that vaccines are important, and if you don’t get them, bad things like death can happen.”
The report therefore provides a comprehensive overview of vaccine development, from the initial identification of the need for a vaccine through the various phases of clinical testing and ongoing postlicensure monitoring. This background information can arm pediatricians with foundational knowledge that’s helpful in talking with patients.
“Vaccine development is a long and arduous process, often lasting many years and involving a combination of public and private partnerships,” the report states. “The current system for developing, testing and regulating vaccines requires that the vaccines demonstrate both safety and efficacy before licensure and that long-term safety is monitored.”
The report briefly explains the multiple mechanisms for continuing to track and study adverse events and other safety concerns:
• Vaccine Adverse Events Reporting System (VAERS). A voluntary passive reporting system used to identify potential safety signals.
• Vaccine Safety Datalink (VSD). A network of linked databases from health care systems across the United States involving millions of individuals
• Post-Licensure Rapid Immunization Safety Monitoring system (PRISM). A system which monitors vaccine safety using health insurance claims data from 107 million individuals .
• Clinical Immunization Safety Assessment Project (CISA). A system that answers individual health care providers’ specific questions on vaccine safety.
Vaccine hesitancy and vaccine exemptions
Opposition to vaccination is not new, the report states, describing it as dating back to Edward Jenner’s smallpox vaccine in the early 1800s.
“Although vaccine hesitancy is not a new phenomenon, it may have a greater effect on public health today,” the report states. “With the ease of global travel, vaccine-preventable diseases are spread more quickly and may unexpectedly appear in areas where health care professionals are unfamiliar with their clinical presentation.”
The historical presence of vaccination opposition has led to circumstances in the United States today in which parents can seek nonmedical exemptions from vaccines in 47 states, and their use has increased with their availability. Yet, the increase in use of exemptions and of “alternative” immunization schedules runs the risk of eroding the herd immunity that protects the community, the report notes.
“For these reasons, we believe the better approach is to work to eliminate all nonmedical exemptions for childhood vaccines,” the authors write. The American Medical Association and the Infectious Diseases Society of America espouse this position, and the AAP is developing a similar statement.
“Families should not have to fear going to school or the grocery store or a house of worship and worry about their kids getting sick,” said Dr. Cohen. “We now strongly say that we need to work with legislators, families, and other advocates for children at the state level to spread more laws like California’s SB 277 that would abolish philosophical exemptions.”
Dr. Cohen also emphasized the importance of communicating to parents that there are no valid “alternative schedules” for vaccination. There is the Centers for Disease Control and Prevention recommended schedule and anything else is a “nonrecommended vaccine schedule because it hasn’t been studied.” That change in terminology drives home the point that the CDC schedule is the only one fully tested for safety and effectiveness.
Meanwhile, however, pediatricians need the tools to address vaccine hesitancy, starting with understanding it. The report describes the pattern of disease incidence, vaccine uptake, disease reduction, adverse event increase and resulting vaccine hesitancy, punctuated by periodic outbreaks that restore eroded confidence in vaccines.
“When diseases are present, parents are worried and want a vaccine, and when they’re gone, they don’t,” Dr. Edwards said. “We need to remember that there is a dependence on the maintenance of herd immunity by immunization by your neighbors.”
Specific strategies to counter vaccine hesitancy
Just over half of physicians spend 10-19 minutes discussing vaccines with concerned parents, and 8% spend at least 20 minutes with such parents, found a study cited in the report. Other research has found these discussions take a toll on doctors’ job satisfaction.
Yet pediatricians remain the single biggest influence on parents’ vaccination decisions, cited by nearly 80% of parents in one large study.
“The pediatrician should appreciate that vaccine-hesitant parents are a heterogeneous group and that specific parental vaccine concerns should be individually identified and addressed,” the report states. “Although many techniques for working with vaccine-hesitant parents have been suggested, scant data are available to determine the efficacy of these methods.”
It goes on to recommend that physicians should discuss the development and safety testing of vaccines “in a nonconfrontational dialogue with the parents while listening to and acknowledging their concerns.”
Pediatricians should not, however, delay vaccines or limit the number per visit – thereby deviating from the CDC recommended and AAP-endorsed schedule – unless it’s the only way a parent agrees to vaccinate.
Another strategy is the presumptive approach: Present all vaccine recommendations as required immunizations that the provider expects a parent to agree to, although pediatricians should consider their experience and relationship with a family since this approach may not work well for some parents.
The report also emphasizes the potential effectiveness of personalizing vaccine conversations by having doctors share their own experience, such as the fact that they vaccinated themselves, their children, and/or their grandchildren.
“Parents often are more likely to be persuaded by stories and anecdotes about the successes of vaccines,” the authors write. “Personal examples of children who were sick with vaccine-preventable illnesses can be much more effective than simply reading the numbers of children infected with a disease each year.”
The report also offers several suggestions for reducing the pain from administering vaccinations: administering vaccines quickly without aspirating; saving the most painful injection for last; holding the child upright; providing tactile stimulation; breastfeeding or providing sweet solutions and topical anesthetics after administration; and using distraction, such as deep breathing, pinwheels, or toys to decrease children’s pain and anxiety.
But the bottom line is that pediatricians have one key message they must communicate to parents, the report states: “The clear message parents should hear is that vaccines are safe and effective, and serious disease can occur if your child and family are not immunized.”
FROM PEDIATRICS
Vaccine refusals and pediatrician dismissals increasing
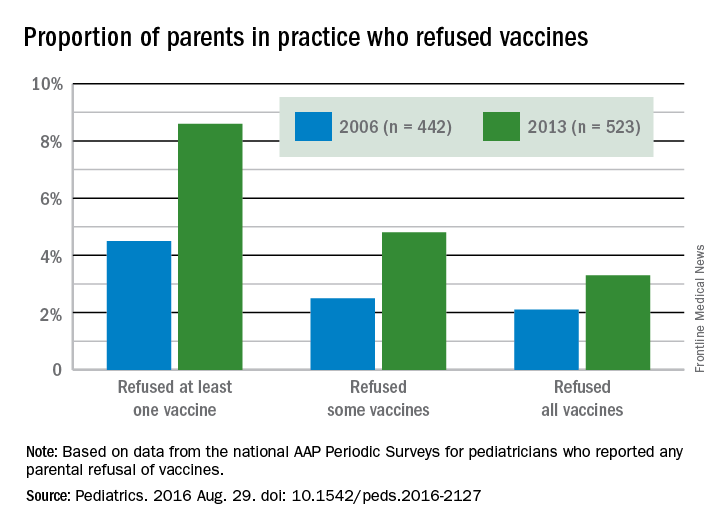
More parents have been refusing vaccines in recent years than a decade ago, according to surveys of pediatricians by the American Academy of Pediatrics published in Pediatrics Aug. 29.
“In a busy practice, vaccine refusals and delays occur daily (if not multiple times per day),” wrote Catherine Hough-Telford, MD, of the University of Alabama at Birmingham and her colleagues (Pediatrics. 2016 Aug. 29. doi: 10.1542/peds.2016-2127).

“From the perspective of the pediatricians, parents who delay vaccines may do so because of concern for their child’s discomfort and concern about immune system burden, whereas vaccine refusers are more likely to believe that vaccines are unnecessary,” the authors wrote. “Pediatricians report that they continue to provide education to vaccine-refusing and delaying parents at high rates.”
Dr. Hough-Telford’s team compared the national AAP Periodic Surveys of 2006 and 2013 that dealt exclusively with immunizations to learn how currently practicing pediatricians perceived three issues related to vaccination: the prevalence of vaccine refusals and delays, why parents refuse or delay vaccines, and the decision of doctors to dismiss families who refuse to vaccinate.
The researchers excluded pediatricians who did not routinely administer vaccines or otherwise adhere to the Centers for Disease Control and Prevention–recommended immunization schedule for their patients. The 2006 survey had a response rate of 52.6% and included 629 final respondents; the 2013 survey had a response rate of 52.7% and included 627 respondents.
The proportion of pediatricians reporting parental refusals in their practice increased from 74.5% in 2006 to 87% in 2013 (odds ratio, 2.29; P less than .001). These pediatricians estimated in 2013 that 8.6% of their patients refused at least one vaccine, compared with 4.5% in 2006. Similarly, 2.5% of parents refused some vaccines in 2006, the physicians reported, compared with 4.8% in 2013. Those refusing all vaccines increased from 2.1% in 2006 to 3.3% in 2013.
A perceived 73.1% of parents refused vaccines in 2013 because they regarded them as unnecessary, the pediatricians reported, compared with 63.4% in 2006. But parental concern over autism and/or thimerosal dropped from 74.2% of vaccine-refusing parents in 2006 to 64.3% in 2013. Further, the parents refusing vaccines because of safety/side effects concerns dropped from 73.7% in 2006 to 66.6% in 2013, and those worried about their children receiving too many shots more than halved from 42.1% in 2006 to 17% in 2013. Concern among parents about their baby being too small to receive vaccines also dropped.

Physicians estimated that 7.3% of their parents wanted to delay one vaccine, 7.1% wanted to delay multiple vaccines, and 4.3% wanted to delay all vaccines. Urban, inner-city pediatricians were less likely to have parents requesting delays than were parents in other areas, but requests for vaccine delays were geographically similar across different U.S. regions. Only the 2013 survey included questions on delaying vaccines.
Pediatricians reported that 75% of their patients wanted to delay vaccines to reduce discomfort to their child, and 72.5% wanted to delay because they perceived too many vaccines would overburden their child’s immune system.
The percentage of pediatricians who always dismiss patients who continue to refuse vaccines increased from 6.1% in 2006 to 11.7% in 2013; they cited a lack of trust between physician and patient as a major reason (87.4% in 2006; 79.9% in 2013). Further, 80.5% of pediatricians reported in 2013 (the only year asked) that they dismissed vaccine-refusing patients out of concern for their other patients.
Despite no notable geographic differences in dismissals in 2006, the 2013 survey revealed that pediatricians in the West had three to four times greater odds of dismissing patients than those in the Midwest and South. Suburban pediatricians had three times greater odds of dismissing patients than did urban physicians.
The research was funded by the AAP and the CDC Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
More parents have been refusing vaccines in recent years than a decade ago, according to surveys of pediatricians by the American Academy of Pediatrics published in Pediatrics Aug. 29.
“In a busy practice, vaccine refusals and delays occur daily (if not multiple times per day),” wrote Catherine Hough-Telford, MD, of the University of Alabama at Birmingham and her colleagues (Pediatrics. 2016 Aug. 29. doi: 10.1542/peds.2016-2127).

“From the perspective of the pediatricians, parents who delay vaccines may do so because of concern for their child’s discomfort and concern about immune system burden, whereas vaccine refusers are more likely to believe that vaccines are unnecessary,” the authors wrote. “Pediatricians report that they continue to provide education to vaccine-refusing and delaying parents at high rates.”
Dr. Hough-Telford’s team compared the national AAP Periodic Surveys of 2006 and 2013 that dealt exclusively with immunizations to learn how currently practicing pediatricians perceived three issues related to vaccination: the prevalence of vaccine refusals and delays, why parents refuse or delay vaccines, and the decision of doctors to dismiss families who refuse to vaccinate.
The researchers excluded pediatricians who did not routinely administer vaccines or otherwise adhere to the Centers for Disease Control and Prevention–recommended immunization schedule for their patients. The 2006 survey had a response rate of 52.6% and included 629 final respondents; the 2013 survey had a response rate of 52.7% and included 627 respondents.
The proportion of pediatricians reporting parental refusals in their practice increased from 74.5% in 2006 to 87% in 2013 (odds ratio, 2.29; P less than .001). These pediatricians estimated in 2013 that 8.6% of their patients refused at least one vaccine, compared with 4.5% in 2006. Similarly, 2.5% of parents refused some vaccines in 2006, the physicians reported, compared with 4.8% in 2013. Those refusing all vaccines increased from 2.1% in 2006 to 3.3% in 2013.
A perceived 73.1% of parents refused vaccines in 2013 because they regarded them as unnecessary, the pediatricians reported, compared with 63.4% in 2006. But parental concern over autism and/or thimerosal dropped from 74.2% of vaccine-refusing parents in 2006 to 64.3% in 2013. Further, the parents refusing vaccines because of safety/side effects concerns dropped from 73.7% in 2006 to 66.6% in 2013, and those worried about their children receiving too many shots more than halved from 42.1% in 2006 to 17% in 2013. Concern among parents about their baby being too small to receive vaccines also dropped.

Physicians estimated that 7.3% of their parents wanted to delay one vaccine, 7.1% wanted to delay multiple vaccines, and 4.3% wanted to delay all vaccines. Urban, inner-city pediatricians were less likely to have parents requesting delays than were parents in other areas, but requests for vaccine delays were geographically similar across different U.S. regions. Only the 2013 survey included questions on delaying vaccines.
Pediatricians reported that 75% of their patients wanted to delay vaccines to reduce discomfort to their child, and 72.5% wanted to delay because they perceived too many vaccines would overburden their child’s immune system.
The percentage of pediatricians who always dismiss patients who continue to refuse vaccines increased from 6.1% in 2006 to 11.7% in 2013; they cited a lack of trust between physician and patient as a major reason (87.4% in 2006; 79.9% in 2013). Further, 80.5% of pediatricians reported in 2013 (the only year asked) that they dismissed vaccine-refusing patients out of concern for their other patients.
Despite no notable geographic differences in dismissals in 2006, the 2013 survey revealed that pediatricians in the West had three to four times greater odds of dismissing patients than those in the Midwest and South. Suburban pediatricians had three times greater odds of dismissing patients than did urban physicians.
The research was funded by the AAP and the CDC Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
More parents have been refusing vaccines in recent years than a decade ago, according to surveys of pediatricians by the American Academy of Pediatrics published in Pediatrics Aug. 29.
“In a busy practice, vaccine refusals and delays occur daily (if not multiple times per day),” wrote Catherine Hough-Telford, MD, of the University of Alabama at Birmingham and her colleagues (Pediatrics. 2016 Aug. 29. doi: 10.1542/peds.2016-2127).

“From the perspective of the pediatricians, parents who delay vaccines may do so because of concern for their child’s discomfort and concern about immune system burden, whereas vaccine refusers are more likely to believe that vaccines are unnecessary,” the authors wrote. “Pediatricians report that they continue to provide education to vaccine-refusing and delaying parents at high rates.”
Dr. Hough-Telford’s team compared the national AAP Periodic Surveys of 2006 and 2013 that dealt exclusively with immunizations to learn how currently practicing pediatricians perceived three issues related to vaccination: the prevalence of vaccine refusals and delays, why parents refuse or delay vaccines, and the decision of doctors to dismiss families who refuse to vaccinate.
The researchers excluded pediatricians who did not routinely administer vaccines or otherwise adhere to the Centers for Disease Control and Prevention–recommended immunization schedule for their patients. The 2006 survey had a response rate of 52.6% and included 629 final respondents; the 2013 survey had a response rate of 52.7% and included 627 respondents.
The proportion of pediatricians reporting parental refusals in their practice increased from 74.5% in 2006 to 87% in 2013 (odds ratio, 2.29; P less than .001). These pediatricians estimated in 2013 that 8.6% of their patients refused at least one vaccine, compared with 4.5% in 2006. Similarly, 2.5% of parents refused some vaccines in 2006, the physicians reported, compared with 4.8% in 2013. Those refusing all vaccines increased from 2.1% in 2006 to 3.3% in 2013.
A perceived 73.1% of parents refused vaccines in 2013 because they regarded them as unnecessary, the pediatricians reported, compared with 63.4% in 2006. But parental concern over autism and/or thimerosal dropped from 74.2% of vaccine-refusing parents in 2006 to 64.3% in 2013. Further, the parents refusing vaccines because of safety/side effects concerns dropped from 73.7% in 2006 to 66.6% in 2013, and those worried about their children receiving too many shots more than halved from 42.1% in 2006 to 17% in 2013. Concern among parents about their baby being too small to receive vaccines also dropped.

Physicians estimated that 7.3% of their parents wanted to delay one vaccine, 7.1% wanted to delay multiple vaccines, and 4.3% wanted to delay all vaccines. Urban, inner-city pediatricians were less likely to have parents requesting delays than were parents in other areas, but requests for vaccine delays were geographically similar across different U.S. regions. Only the 2013 survey included questions on delaying vaccines.
Pediatricians reported that 75% of their patients wanted to delay vaccines to reduce discomfort to their child, and 72.5% wanted to delay because they perceived too many vaccines would overburden their child’s immune system.
The percentage of pediatricians who always dismiss patients who continue to refuse vaccines increased from 6.1% in 2006 to 11.7% in 2013; they cited a lack of trust between physician and patient as a major reason (87.4% in 2006; 79.9% in 2013). Further, 80.5% of pediatricians reported in 2013 (the only year asked) that they dismissed vaccine-refusing patients out of concern for their other patients.
Despite no notable geographic differences in dismissals in 2006, the 2013 survey revealed that pediatricians in the West had three to four times greater odds of dismissing patients than those in the Midwest and South. Suburban pediatricians had three times greater odds of dismissing patients than did urban physicians.
The research was funded by the AAP and the CDC Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
FROM PEDIATRICS
Key clinical point: More parents have been refusing vaccines, and more pediatricians have been dismissing vaccine-refusing patients, since 2006.
Major finding: 11.7% of pediatricians reported dismissing patients for refusing vaccines in 2013, compared with 6.1% in 2006, and 8.6% of parents refused at least one vaccine in 2013, compared with 4.5% in 2006.
Data source: The findings are based on surveys of practicing members of the American Academy of Pediatrics, with 629 pediatricians in 2006 and 627 pediatricians in 2013.
Disclosures: The research was funded by the AAP and the Centers for Disease Control and Prevention Childhood Immunization Support Program. Dr. Kimberlin was a site principal investigator for two multisite studies conducted by GlaxoSmithKline and Gilead.
Hepatitis B vaccine response suppressed by maternal antibodies
Maternal antibodies against hepatitis blunt the immune response to the hepatitis B vaccine in newborns, but the booster dose is unaffected by maternal antibodies, a study found.
Previous research also has identified a suppressed response to vaccination due to maternal antibodies with vaccines such as the measles, hepatitis A, mumps and tetanus vaccines.
“Maternal antibodies are a double-edged sword for infants,” wrote X. Chen of the Zhongnan Hospital of Wuhan (China) University, and colleagues (J Viral Hepat. 2016 Jul 29. doi: 10.1111/jvh.12572). “These neutralizing antibodies can protect neonates against most infectious diseases in early life; however, as shown in the study here, these antibodies can also suppress the immune response of infants to vaccines.”
The researchers first assessed transplacental transfer of antibodies by measuring anti–hepatitis B antibodies in 90 mothers and their newborns. The mothers were all positive for anti–hepatitis B antibodies with a median titer of 250. Before the infants had been vaccinated, 97% were positive for anti–hepatitis B antibodies. Among infants whose mothers had titers above 100 IU/L, 100% were positive for antibodies.
Then the researchers measured titers and rate of anti–hepatitis B positivity in 1,055 mothers and their 1,063 infants, aged 7-24 months, after the babies had received doses of the 10 mcg HBV vaccine at ages 0, 1 and 6 months, per the recommended schedule in China. In the United States, infants are recommended to receive the vaccine at birth, at 1-4 months, and then at 6-18 months.
Among the 405 mothers with antibodies of less than 10 IU/L, 89% of their newborns responded sufficiently to the vaccine to be positive for anti–hepatitis B antibodies. Among the 451 mothers with antibodies from 10-499 IU/L, 85% of the infants had positivity, and among the 207 mothers with antibodies of 500 IU/L and higher, 77% of the infants were positive for anti–hepatitis B antibodies.
Titers followed the same inverse pattern. The median titer was 169 in infants whose mothers had low titers, but the median titer was 79 in infants of mothers with high titers. When mothers with titers in the middle range, the infants’ median titer was 141.
Among 162 newborns who tested negative for anti–hepatitis B antibodies after receiving all three doses of the vaccine, 92% showed positivity for the antibodies after receiving a “catch-up” booster dose, without significant interference from maternal antibodies. Of another 11 infants still lacking positivity, 8 assessed 1 month later, achieved it after a second catch-up booster, suggesting “the suppression effects of the maternal anti-HBVs can be overcome.”
The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
Maternal antibodies against hepatitis blunt the immune response to the hepatitis B vaccine in newborns, but the booster dose is unaffected by maternal antibodies, a study found.
Previous research also has identified a suppressed response to vaccination due to maternal antibodies with vaccines such as the measles, hepatitis A, mumps and tetanus vaccines.
“Maternal antibodies are a double-edged sword for infants,” wrote X. Chen of the Zhongnan Hospital of Wuhan (China) University, and colleagues (J Viral Hepat. 2016 Jul 29. doi: 10.1111/jvh.12572). “These neutralizing antibodies can protect neonates against most infectious diseases in early life; however, as shown in the study here, these antibodies can also suppress the immune response of infants to vaccines.”
The researchers first assessed transplacental transfer of antibodies by measuring anti–hepatitis B antibodies in 90 mothers and their newborns. The mothers were all positive for anti–hepatitis B antibodies with a median titer of 250. Before the infants had been vaccinated, 97% were positive for anti–hepatitis B antibodies. Among infants whose mothers had titers above 100 IU/L, 100% were positive for antibodies.
Then the researchers measured titers and rate of anti–hepatitis B positivity in 1,055 mothers and their 1,063 infants, aged 7-24 months, after the babies had received doses of the 10 mcg HBV vaccine at ages 0, 1 and 6 months, per the recommended schedule in China. In the United States, infants are recommended to receive the vaccine at birth, at 1-4 months, and then at 6-18 months.
Among the 405 mothers with antibodies of less than 10 IU/L, 89% of their newborns responded sufficiently to the vaccine to be positive for anti–hepatitis B antibodies. Among the 451 mothers with antibodies from 10-499 IU/L, 85% of the infants had positivity, and among the 207 mothers with antibodies of 500 IU/L and higher, 77% of the infants were positive for anti–hepatitis B antibodies.
Titers followed the same inverse pattern. The median titer was 169 in infants whose mothers had low titers, but the median titer was 79 in infants of mothers with high titers. When mothers with titers in the middle range, the infants’ median titer was 141.
Among 162 newborns who tested negative for anti–hepatitis B antibodies after receiving all three doses of the vaccine, 92% showed positivity for the antibodies after receiving a “catch-up” booster dose, without significant interference from maternal antibodies. Of another 11 infants still lacking positivity, 8 assessed 1 month later, achieved it after a second catch-up booster, suggesting “the suppression effects of the maternal anti-HBVs can be overcome.”
The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
Maternal antibodies against hepatitis blunt the immune response to the hepatitis B vaccine in newborns, but the booster dose is unaffected by maternal antibodies, a study found.
Previous research also has identified a suppressed response to vaccination due to maternal antibodies with vaccines such as the measles, hepatitis A, mumps and tetanus vaccines.
“Maternal antibodies are a double-edged sword for infants,” wrote X. Chen of the Zhongnan Hospital of Wuhan (China) University, and colleagues (J Viral Hepat. 2016 Jul 29. doi: 10.1111/jvh.12572). “These neutralizing antibodies can protect neonates against most infectious diseases in early life; however, as shown in the study here, these antibodies can also suppress the immune response of infants to vaccines.”
The researchers first assessed transplacental transfer of antibodies by measuring anti–hepatitis B antibodies in 90 mothers and their newborns. The mothers were all positive for anti–hepatitis B antibodies with a median titer of 250. Before the infants had been vaccinated, 97% were positive for anti–hepatitis B antibodies. Among infants whose mothers had titers above 100 IU/L, 100% were positive for antibodies.
Then the researchers measured titers and rate of anti–hepatitis B positivity in 1,055 mothers and their 1,063 infants, aged 7-24 months, after the babies had received doses of the 10 mcg HBV vaccine at ages 0, 1 and 6 months, per the recommended schedule in China. In the United States, infants are recommended to receive the vaccine at birth, at 1-4 months, and then at 6-18 months.
Among the 405 mothers with antibodies of less than 10 IU/L, 89% of their newborns responded sufficiently to the vaccine to be positive for anti–hepatitis B antibodies. Among the 451 mothers with antibodies from 10-499 IU/L, 85% of the infants had positivity, and among the 207 mothers with antibodies of 500 IU/L and higher, 77% of the infants were positive for anti–hepatitis B antibodies.
Titers followed the same inverse pattern. The median titer was 169 in infants whose mothers had low titers, but the median titer was 79 in infants of mothers with high titers. When mothers with titers in the middle range, the infants’ median titer was 141.
Among 162 newborns who tested negative for anti–hepatitis B antibodies after receiving all three doses of the vaccine, 92% showed positivity for the antibodies after receiving a “catch-up” booster dose, without significant interference from maternal antibodies. Of another 11 infants still lacking positivity, 8 assessed 1 month later, achieved it after a second catch-up booster, suggesting “the suppression effects of the maternal anti-HBVs can be overcome.”
The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
FROM THE JOURNAL OF VIRAL HEPATITIS
Key clinical point: Maternal immunity to hepatitis B suppresses infant response to vaccination.
Major finding: 89%, 85%, and 77% of newborns tested positive for anti–hepatitis B antibodies after a standard three-dose regimen when born to mothers with low, medium, and high titers, respectively.
Data source: The findings are based on titers and antibody positivity against hepatitis B in 1,055 mothers and 1,063 newborns at multiple centers in China from March 2012 to November 2015 before and after infant immunization against hepatitis B.
Disclosures: The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
Autism follow-up screening by PCPs yields high accuracy
Primary care providers can effectively conduct the follow-up interview after a positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) without missing cases or flagging too many false positives, suggests a recent study.
“The online M-CHAT/F [M-CHAT Follow-up Interview] enabled PCPs [primary care providers] to clarify positive parent responses to M-CHAT items during well-child visits, rather than requiring another visit or call by a trained interviewer,” wrote Raymond Sturner, MD, of Johns Hopkins University, Baltimore, and his colleagues.
“This study found that the performance of the M-CHAT/F by a PCP was equivalent to one administered by trained Kennedy Krieger Institute Center for Autism and Related Disorders staff,” they wrote (Pediatrics. 2016 Aug 19. doi: 10.1542/peds.2015-3036). “This report is the first demonstrating feasibility of administration of the M-CHAT/F during the time of well-child visit in community practices.”
The authors recruited 47 primary care providers at 22 clinics in Maryland to complete an M-CHAT/F during children’s 18- and 24-month routine visits if their initial M-CHAT yielded a positive screening. Each family was then contacted again for an M-CHAT/F conducted by a trained research assistant from the Kennedy Krieger Institute Center for Autism and Related Disorders.
The PCPs volunteered for the study and primarily had suburban practices, with just 18% rural and 9% urban practices. Just under a third of children at the practices were insured by Medicaid, and the demographic breakdown included 39% white, 33% African-American, 16% Asian, and 8% Hispanic.
Of the 5,071 children screened (mean age, 23 months), 6.7% had a positive screen. Of the 197 M-CHAT/Fs the PCPs completed, 99 children then underwent a full autism spectrum disorder (ASD) diagnostic evaluation, including administration of the Autism Diagnostic Observation Schedule and the Mullen Scales of Early Learning.
PCPs and research assistants agreed 86.6% of the time on the result of the M-CHAT/F screening, with statistically equivalent positive predictive value (PPV), sensitivity, specificity, and overall accuracy. The research assistants’ PPV was 0.84, and the PCPs’ PPV was 0.88. The PPV for any developmental delay diagnosis was similarly equivalent between the research assistants and PCPs.
Dr. Sturner and his associates noted that the findings confirm “previous studies showing that most children with false-positive screens have developmental difficulties of a degree that would make them eligible for early intervention. Some children with false-positive screens had atypical features not meeting criteria for ASD.”
The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
Primary care providers can effectively conduct the follow-up interview after a positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) without missing cases or flagging too many false positives, suggests a recent study.
“The online M-CHAT/F [M-CHAT Follow-up Interview] enabled PCPs [primary care providers] to clarify positive parent responses to M-CHAT items during well-child visits, rather than requiring another visit or call by a trained interviewer,” wrote Raymond Sturner, MD, of Johns Hopkins University, Baltimore, and his colleagues.
“This study found that the performance of the M-CHAT/F by a PCP was equivalent to one administered by trained Kennedy Krieger Institute Center for Autism and Related Disorders staff,” they wrote (Pediatrics. 2016 Aug 19. doi: 10.1542/peds.2015-3036). “This report is the first demonstrating feasibility of administration of the M-CHAT/F during the time of well-child visit in community practices.”
The authors recruited 47 primary care providers at 22 clinics in Maryland to complete an M-CHAT/F during children’s 18- and 24-month routine visits if their initial M-CHAT yielded a positive screening. Each family was then contacted again for an M-CHAT/F conducted by a trained research assistant from the Kennedy Krieger Institute Center for Autism and Related Disorders.
The PCPs volunteered for the study and primarily had suburban practices, with just 18% rural and 9% urban practices. Just under a third of children at the practices were insured by Medicaid, and the demographic breakdown included 39% white, 33% African-American, 16% Asian, and 8% Hispanic.
Of the 5,071 children screened (mean age, 23 months), 6.7% had a positive screen. Of the 197 M-CHAT/Fs the PCPs completed, 99 children then underwent a full autism spectrum disorder (ASD) diagnostic evaluation, including administration of the Autism Diagnostic Observation Schedule and the Mullen Scales of Early Learning.
PCPs and research assistants agreed 86.6% of the time on the result of the M-CHAT/F screening, with statistically equivalent positive predictive value (PPV), sensitivity, specificity, and overall accuracy. The research assistants’ PPV was 0.84, and the PCPs’ PPV was 0.88. The PPV for any developmental delay diagnosis was similarly equivalent between the research assistants and PCPs.
Dr. Sturner and his associates noted that the findings confirm “previous studies showing that most children with false-positive screens have developmental difficulties of a degree that would make them eligible for early intervention. Some children with false-positive screens had atypical features not meeting criteria for ASD.”
The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
Primary care providers can effectively conduct the follow-up interview after a positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) without missing cases or flagging too many false positives, suggests a recent study.
“The online M-CHAT/F [M-CHAT Follow-up Interview] enabled PCPs [primary care providers] to clarify positive parent responses to M-CHAT items during well-child visits, rather than requiring another visit or call by a trained interviewer,” wrote Raymond Sturner, MD, of Johns Hopkins University, Baltimore, and his colleagues.
“This study found that the performance of the M-CHAT/F by a PCP was equivalent to one administered by trained Kennedy Krieger Institute Center for Autism and Related Disorders staff,” they wrote (Pediatrics. 2016 Aug 19. doi: 10.1542/peds.2015-3036). “This report is the first demonstrating feasibility of administration of the M-CHAT/F during the time of well-child visit in community practices.”
The authors recruited 47 primary care providers at 22 clinics in Maryland to complete an M-CHAT/F during children’s 18- and 24-month routine visits if their initial M-CHAT yielded a positive screening. Each family was then contacted again for an M-CHAT/F conducted by a trained research assistant from the Kennedy Krieger Institute Center for Autism and Related Disorders.
The PCPs volunteered for the study and primarily had suburban practices, with just 18% rural and 9% urban practices. Just under a third of children at the practices were insured by Medicaid, and the demographic breakdown included 39% white, 33% African-American, 16% Asian, and 8% Hispanic.
Of the 5,071 children screened (mean age, 23 months), 6.7% had a positive screen. Of the 197 M-CHAT/Fs the PCPs completed, 99 children then underwent a full autism spectrum disorder (ASD) diagnostic evaluation, including administration of the Autism Diagnostic Observation Schedule and the Mullen Scales of Early Learning.
PCPs and research assistants agreed 86.6% of the time on the result of the M-CHAT/F screening, with statistically equivalent positive predictive value (PPV), sensitivity, specificity, and overall accuracy. The research assistants’ PPV was 0.84, and the PCPs’ PPV was 0.88. The PPV for any developmental delay diagnosis was similarly equivalent between the research assistants and PCPs.
Dr. Sturner and his associates noted that the findings confirm “previous studies showing that most children with false-positive screens have developmental difficulties of a degree that would make them eligible for early intervention. Some children with false-positive screens had atypical features not meeting criteria for ASD.”
The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
FROM PEDIATRICS
Key clinical point: Primary care providers can conduct the M-CHAT/F following a positive M-CHAT screening for autism spectrum disorders.
Major finding: Primary care providers and trained interviewers agreed 86.6% of the time on the screening results of the M-CHAT/F for ASDs.
Data source: A cohort study of 5,071 children, mean age 23 months, screened with the M-CHAT, and a subsequent 197 children screened with the M-CHAT/F in 22 Maryland primary care practices.
Disclosures: The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
Clean-catch urine method effective in young infants
Urine samples from a noninvasive clean-catch method have no significantly greater contamination rate than do those from urethral catheterization, making clean catch a quick, effective option to attempt before catheterization, a recent study found.
The clean-catch method uses a standard bladder stimulation technique and was most successful in infants less than 90 days old.
“Because the use of urethral catheterization is an invasive method that could be associated with adverse events in up to 20% of children, our findings support the use of the clean-catch urine standardized stimulation technique as an alternative to invasive methods to obtain a urine specimen,” wrote Mélanie Labrosse, MD, PhD, and her associates at the University of Montreal (Pediatrics. 2016 Aug 19. doi: 10.1542/peds.2016-0573). “However, until further studies on proportion and predictive factors of contamination become available, it would be more cautious to perform invasive methods in children who appear ill, who have a positive urinalysis, or before beginning antibiotics.”
The clean-catch method involved providing the infants an opportunity to feed over 20 minutes, after which a practitioner cleaned the genitals and the parent then held the infant by the armpits. Female infants’ hips were flexed and male infants’ legs dangled.
“Examiners then alternated between bladder stimulation maneuvers, which consisted of gentle tapping in the suprapubic area at a frequency of 100 taps per minute for 30 seconds, and lumbar paravertebral massage maneuvers for 30 seconds,” the authors wrote. “These two stimulation maneuvers were repeated until micturition began or for a maximum of 300 seconds.”
The researchers attempted the clean-catch technique with 126 infants under 6 months old. About half were boys, a quarter of whom were circumcised, and the whole sample had a median age of 55 days. The procedure took a median 45 seconds and was effective in 49% of the children (at least 1 mL of urine collected within 5 minutes).
The procedure was more likely to be effective in infants under 3 months old, with three times greater odds of success for those aged 30-59 days and four times greater odds of success for those aged 0-29 days and those aged 60-89 days (odds ratio 3.2, 4.3, and 4.4, respectively). Only 26% of the children aged 91-180 days yielded a successful clean-catch sample, compared with 61% of infants under 30 days and 54% of infants under 90 days old. UTI was present in 11 (9%) children.
Likelihood of a successful clean-catch sample was not affected by infant sex, low oral intake, or recent urination (within an hour).
While 16% of the clean catches were contaminated, this rate was not statistically different from the 6% of contaminated samples among those undergoing the invasive method.
The authors suggested using the clean-catch technique as a first attempt in two situations: ruling out UTI in children aged 2-6 months and in children under 6 months who need a urinalysis in which urine typically would be obtained noninvasively.
“In addition, trying the CCU procedure instead of using a collection bag seems reasonable, considering the wait time associated with this technique and the logistics involved in changing the bag every 30 minutes,” the authors noted.
They reported having no disclosures. No external funding source was noted in the study.
Urine samples from a noninvasive clean-catch method have no significantly greater contamination rate than do those from urethral catheterization, making clean catch a quick, effective option to attempt before catheterization, a recent study found.
The clean-catch method uses a standard bladder stimulation technique and was most successful in infants less than 90 days old.
“Because the use of urethral catheterization is an invasive method that could be associated with adverse events in up to 20% of children, our findings support the use of the clean-catch urine standardized stimulation technique as an alternative to invasive methods to obtain a urine specimen,” wrote Mélanie Labrosse, MD, PhD, and her associates at the University of Montreal (Pediatrics. 2016 Aug 19. doi: 10.1542/peds.2016-0573). “However, until further studies on proportion and predictive factors of contamination become available, it would be more cautious to perform invasive methods in children who appear ill, who have a positive urinalysis, or before beginning antibiotics.”
The clean-catch method involved providing the infants an opportunity to feed over 20 minutes, after which a practitioner cleaned the genitals and the parent then held the infant by the armpits. Female infants’ hips were flexed and male infants’ legs dangled.
“Examiners then alternated between bladder stimulation maneuvers, which consisted of gentle tapping in the suprapubic area at a frequency of 100 taps per minute for 30 seconds, and lumbar paravertebral massage maneuvers for 30 seconds,” the authors wrote. “These two stimulation maneuvers were repeated until micturition began or for a maximum of 300 seconds.”
The researchers attempted the clean-catch technique with 126 infants under 6 months old. About half were boys, a quarter of whom were circumcised, and the whole sample had a median age of 55 days. The procedure took a median 45 seconds and was effective in 49% of the children (at least 1 mL of urine collected within 5 minutes).
The procedure was more likely to be effective in infants under 3 months old, with three times greater odds of success for those aged 30-59 days and four times greater odds of success for those aged 0-29 days and those aged 60-89 days (odds ratio 3.2, 4.3, and 4.4, respectively). Only 26% of the children aged 91-180 days yielded a successful clean-catch sample, compared with 61% of infants under 30 days and 54% of infants under 90 days old. UTI was present in 11 (9%) children.
Likelihood of a successful clean-catch sample was not affected by infant sex, low oral intake, or recent urination (within an hour).
While 16% of the clean catches were contaminated, this rate was not statistically different from the 6% of contaminated samples among those undergoing the invasive method.
The authors suggested using the clean-catch technique as a first attempt in two situations: ruling out UTI in children aged 2-6 months and in children under 6 months who need a urinalysis in which urine typically would be obtained noninvasively.
“In addition, trying the CCU procedure instead of using a collection bag seems reasonable, considering the wait time associated with this technique and the logistics involved in changing the bag every 30 minutes,” the authors noted.
They reported having no disclosures. No external funding source was noted in the study.
Urine samples from a noninvasive clean-catch method have no significantly greater contamination rate than do those from urethral catheterization, making clean catch a quick, effective option to attempt before catheterization, a recent study found.
The clean-catch method uses a standard bladder stimulation technique and was most successful in infants less than 90 days old.
“Because the use of urethral catheterization is an invasive method that could be associated with adverse events in up to 20% of children, our findings support the use of the clean-catch urine standardized stimulation technique as an alternative to invasive methods to obtain a urine specimen,” wrote Mélanie Labrosse, MD, PhD, and her associates at the University of Montreal (Pediatrics. 2016 Aug 19. doi: 10.1542/peds.2016-0573). “However, until further studies on proportion and predictive factors of contamination become available, it would be more cautious to perform invasive methods in children who appear ill, who have a positive urinalysis, or before beginning antibiotics.”
The clean-catch method involved providing the infants an opportunity to feed over 20 minutes, after which a practitioner cleaned the genitals and the parent then held the infant by the armpits. Female infants’ hips were flexed and male infants’ legs dangled.
“Examiners then alternated between bladder stimulation maneuvers, which consisted of gentle tapping in the suprapubic area at a frequency of 100 taps per minute for 30 seconds, and lumbar paravertebral massage maneuvers for 30 seconds,” the authors wrote. “These two stimulation maneuvers were repeated until micturition began or for a maximum of 300 seconds.”
The researchers attempted the clean-catch technique with 126 infants under 6 months old. About half were boys, a quarter of whom were circumcised, and the whole sample had a median age of 55 days. The procedure took a median 45 seconds and was effective in 49% of the children (at least 1 mL of urine collected within 5 minutes).
The procedure was more likely to be effective in infants under 3 months old, with three times greater odds of success for those aged 30-59 days and four times greater odds of success for those aged 0-29 days and those aged 60-89 days (odds ratio 3.2, 4.3, and 4.4, respectively). Only 26% of the children aged 91-180 days yielded a successful clean-catch sample, compared with 61% of infants under 30 days and 54% of infants under 90 days old. UTI was present in 11 (9%) children.
Likelihood of a successful clean-catch sample was not affected by infant sex, low oral intake, or recent urination (within an hour).
While 16% of the clean catches were contaminated, this rate was not statistically different from the 6% of contaminated samples among those undergoing the invasive method.
The authors suggested using the clean-catch technique as a first attempt in two situations: ruling out UTI in children aged 2-6 months and in children under 6 months who need a urinalysis in which urine typically would be obtained noninvasively.
“In addition, trying the CCU procedure instead of using a collection bag seems reasonable, considering the wait time associated with this technique and the logistics involved in changing the bag every 30 minutes,” the authors noted.
They reported having no disclosures. No external funding source was noted in the study.
FROM PEDIATRICS
Key clinical point: A noninvasive clean-catch urine sample method can be effective in infants under 90 days old.
Major finding: Forty-nine percent of infants under 6 months old produced a successful clean catch; odds of success were 3-4 times greater in infants under 90 days.
Data source: A prospective cohort study of 126 infants under 6 months old in a Montreal pediatric emergency department between May and October 2015.
Disclosures: The authors reported having no disclosures. No external funding source was noted in the study.
Lidocaine gel doesn’t relieve IUD insertion pain but cuts need for dilation
While vaginal lidocaine gel does not significantly reduce pain from intrauterine device (IUD) insertion, it does appear to reduce the pain from tenaculum placement in nulliparous women and the likelihood that women will need cervical dilation for insertion, according to the results of a randomized controlled trial.
Despite the effectiveness of IUDs in preventing pregnancy, some women don’t choose this contraception method because they fear the pain of insertion, past research has found. Pain is also more likely in women who have not given birth. In fact, just 5.9% of nulliparous women use IUDs, compared with 16.8% of women with one or two prior births, wrote Rachel B. Rapkin, MD, MPH, of the University of South Florida in Tampa, and her colleagues (Obstet Gynecol. 2016;128:621-8. doi: 10.1097/AOG.0000000000001596).
The researchers tested the effectiveness of self-administered lidocaine gel as pain relief with 59 nulliparous women aged 14-50 years from University of Pittsburgh Medical Center clinics between July 2012 and May 2013. All of the women requested an IUD. A total of 30 women were randomized to apply the 2% lidocaine vaginal gel 5 minutes before the IUD insertion. Another 29 women were randomized to apply a placebo gel. Nearly all the women reported that inserting the gel was somewhat or very easy and that they had no pain after insertion.
There was one unsuccessful IUD insertion in the placebo group. That woman had intolerable pain while attempting uterine sound so the procedure was aborted. She was included in the intention-to-treat analysis with all missing data set to 100 mm on a 100-mm visual analog scale.
In the intention-to-treat analysis, the median change in pain from baseline to IUD insertion was 61 mm for women who used the lidocaine gel. Women using placebo reported a median score of 69 mm, which was not significantly different from those using the lidocaine gel (P = .06). However, women using lidocaine did report significantly less pain when the tenaculum was placed: a median 32 mm on the pain scale, compared with 56 mm reported by those who used the placebo gel (P = .02).
Although 87% of the physicians reported that insertion was easy in the women with lidocaine, compared with 64% who said it was easy in women using the placebo gel, the difference was not significant (P = .07). However, significantly more women needed cervical dilation before IUD placement if they used the placebo (34.5%), compared with those using the lidocaine (3.3%, P = .002). The women also reported pain scores after speculum placement, uterine sounding, and 5 minutes after speculum removal, but none of these showed significant differences.
Overall, more than one-third of the women needed to take pain medication for at least 3 days after the IUD was inserted, but the majority of women (86%) would probably or definitely recommend an IUD to a friend, and 76% reported satisfaction with the insertion experience. Cramping and pain medication use after insertion did not vary between the two groups.
The generalizability of the trial may be limited because it enrolled participants who were largely white and college educated and it used clinicians with at least 4 years of IUD insertion experience.
The research was funded by the Society of Family Planning Research Fund. Dr. Rapkin reported having no financial disclosures. Her coauthors reported consulting work for Merck and research funding from Bayer Healthcare and Medicines 360 Inc. One of the coauthors is founder and president of the nonprofit Basic Health International.
While vaginal lidocaine gel does not significantly reduce pain from intrauterine device (IUD) insertion, it does appear to reduce the pain from tenaculum placement in nulliparous women and the likelihood that women will need cervical dilation for insertion, according to the results of a randomized controlled trial.
Despite the effectiveness of IUDs in preventing pregnancy, some women don’t choose this contraception method because they fear the pain of insertion, past research has found. Pain is also more likely in women who have not given birth. In fact, just 5.9% of nulliparous women use IUDs, compared with 16.8% of women with one or two prior births, wrote Rachel B. Rapkin, MD, MPH, of the University of South Florida in Tampa, and her colleagues (Obstet Gynecol. 2016;128:621-8. doi: 10.1097/AOG.0000000000001596).
The researchers tested the effectiveness of self-administered lidocaine gel as pain relief with 59 nulliparous women aged 14-50 years from University of Pittsburgh Medical Center clinics between July 2012 and May 2013. All of the women requested an IUD. A total of 30 women were randomized to apply the 2% lidocaine vaginal gel 5 minutes before the IUD insertion. Another 29 women were randomized to apply a placebo gel. Nearly all the women reported that inserting the gel was somewhat or very easy and that they had no pain after insertion.
There was one unsuccessful IUD insertion in the placebo group. That woman had intolerable pain while attempting uterine sound so the procedure was aborted. She was included in the intention-to-treat analysis with all missing data set to 100 mm on a 100-mm visual analog scale.
In the intention-to-treat analysis, the median change in pain from baseline to IUD insertion was 61 mm for women who used the lidocaine gel. Women using placebo reported a median score of 69 mm, which was not significantly different from those using the lidocaine gel (P = .06). However, women using lidocaine did report significantly less pain when the tenaculum was placed: a median 32 mm on the pain scale, compared with 56 mm reported by those who used the placebo gel (P = .02).
Although 87% of the physicians reported that insertion was easy in the women with lidocaine, compared with 64% who said it was easy in women using the placebo gel, the difference was not significant (P = .07). However, significantly more women needed cervical dilation before IUD placement if they used the placebo (34.5%), compared with those using the lidocaine (3.3%, P = .002). The women also reported pain scores after speculum placement, uterine sounding, and 5 minutes after speculum removal, but none of these showed significant differences.
Overall, more than one-third of the women needed to take pain medication for at least 3 days after the IUD was inserted, but the majority of women (86%) would probably or definitely recommend an IUD to a friend, and 76% reported satisfaction with the insertion experience. Cramping and pain medication use after insertion did not vary between the two groups.
The generalizability of the trial may be limited because it enrolled participants who were largely white and college educated and it used clinicians with at least 4 years of IUD insertion experience.
The research was funded by the Society of Family Planning Research Fund. Dr. Rapkin reported having no financial disclosures. Her coauthors reported consulting work for Merck and research funding from Bayer Healthcare and Medicines 360 Inc. One of the coauthors is founder and president of the nonprofit Basic Health International.
While vaginal lidocaine gel does not significantly reduce pain from intrauterine device (IUD) insertion, it does appear to reduce the pain from tenaculum placement in nulliparous women and the likelihood that women will need cervical dilation for insertion, according to the results of a randomized controlled trial.
Despite the effectiveness of IUDs in preventing pregnancy, some women don’t choose this contraception method because they fear the pain of insertion, past research has found. Pain is also more likely in women who have not given birth. In fact, just 5.9% of nulliparous women use IUDs, compared with 16.8% of women with one or two prior births, wrote Rachel B. Rapkin, MD, MPH, of the University of South Florida in Tampa, and her colleagues (Obstet Gynecol. 2016;128:621-8. doi: 10.1097/AOG.0000000000001596).
The researchers tested the effectiveness of self-administered lidocaine gel as pain relief with 59 nulliparous women aged 14-50 years from University of Pittsburgh Medical Center clinics between July 2012 and May 2013. All of the women requested an IUD. A total of 30 women were randomized to apply the 2% lidocaine vaginal gel 5 minutes before the IUD insertion. Another 29 women were randomized to apply a placebo gel. Nearly all the women reported that inserting the gel was somewhat or very easy and that they had no pain after insertion.
There was one unsuccessful IUD insertion in the placebo group. That woman had intolerable pain while attempting uterine sound so the procedure was aborted. She was included in the intention-to-treat analysis with all missing data set to 100 mm on a 100-mm visual analog scale.
In the intention-to-treat analysis, the median change in pain from baseline to IUD insertion was 61 mm for women who used the lidocaine gel. Women using placebo reported a median score of 69 mm, which was not significantly different from those using the lidocaine gel (P = .06). However, women using lidocaine did report significantly less pain when the tenaculum was placed: a median 32 mm on the pain scale, compared with 56 mm reported by those who used the placebo gel (P = .02).
Although 87% of the physicians reported that insertion was easy in the women with lidocaine, compared with 64% who said it was easy in women using the placebo gel, the difference was not significant (P = .07). However, significantly more women needed cervical dilation before IUD placement if they used the placebo (34.5%), compared with those using the lidocaine (3.3%, P = .002). The women also reported pain scores after speculum placement, uterine sounding, and 5 minutes after speculum removal, but none of these showed significant differences.
Overall, more than one-third of the women needed to take pain medication for at least 3 days after the IUD was inserted, but the majority of women (86%) would probably or definitely recommend an IUD to a friend, and 76% reported satisfaction with the insertion experience. Cramping and pain medication use after insertion did not vary between the two groups.
The generalizability of the trial may be limited because it enrolled participants who were largely white and college educated and it used clinicians with at least 4 years of IUD insertion experience.
The research was funded by the Society of Family Planning Research Fund. Dr. Rapkin reported having no financial disclosures. Her coauthors reported consulting work for Merck and research funding from Bayer Healthcare and Medicines 360 Inc. One of the coauthors is founder and president of the nonprofit Basic Health International.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Vaginal lidocaine gel does not decrease IUD insertion pain but decreases likelihood of needing cervical dilation.
Major finding: More than a third of women using placebo gel required cervical dilation before IUD placement, compared with 3.3% of women using lidocaine gel (P = .002).
Data source: The findings are based on a randomized, double-blind, placebo-controlled trial involving 59 nulliparous women between July 2012 and May 2013.
Disclosures: The research was funded by the Society of Family Planning Research Fund. Dr. Rapkin reported having no financial disclosures. Her coauthors reported consulting work for Merck and research funding from Bayer Healthcare and Medicines 360 Inc. One of the coauthors is founder and president of the nonprofit Basic Health International.
Meningococcal B vaccine less protective than expected during outbreak
One-third of the individuals who received a multicomponent meningococcal B vaccine during an outbreak did not show a protective response against the outbreak strain, though they also did not contract the disease, a study showed.
“This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain,” Nicole E. Basta, PhD, of the University of Minnesota, Minneapolis, and her associates wrote in the New England Journal of Medicine (2016;375:220-8. doi:10.1056/NEJMoa1514866).
“Our results indicate that knowledge of [human complement serum bactericidal antibodies] immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens,” the investigators added.
Amidst an outbreak of meningococcal B at Princeton (N.J.) University in the winter of 2013, the Food and Drug Administration allowed use of the meningococcal serogroup B (4CMenB) vaccine before its licensure, in an attempt to control the outbreak. Two strains of meningitis were used in developing this vaccine: 5/99 strain, which was not at all similar to the outbreak strain, and 44/76-SL, which was 96% genetically similar to the outbreak strain.
Among 535 people who completed the study, 499 individuals received two doses of 4CMenB 10 weeks apart, 17 received one dose of the vaccine, and 19 were unvaccinated. When the fully vaccinated individuals were tested for titers 8 weeks after their second dose, two-thirds (66.1%) had low-level seropositivity for the outbreak strain, with a geometric mean titer (GMT) of 7.6. One in five of the unvaccinated people (21.1%) had low seropositivity for the outbreak strain (GMT, 2.8), and 58.8% of the partly vaccinated individuals had seropositivity with a GMT of 5.4.
The researchers then assessed titers for the two strains used in developing 4CMenB in 61 fully vaccinated individuals, all randomly selected from the one-third of fully vaccinated people who did not have a detectable response to the outbreak strain. Most of them (86.9%) were seropositive for the 44/76-SL strain with a GMT of 17.4, and all of them were seropositive for the 5/99 strain, with a considerably larger GMT of 256.3.
Among those fully vaccinated who responded to the outbreak strain with titers above 8, all had seropositivity for 44/76-SL (GMT, 178.8), and 96.7% had seropositivity for the 5/99 strain (GMT, 214.2). Among the 18 unvaccinated individuals, just 1 had very low seropositivity to the 5/99 strain (GMT, 1.2), and 6 of them (33.3%) responded to the 44/76-SL strain (GMT, 3.2).
“These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease,” the authors wrote.
The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had financial disclosures.
Proving the clinical efficacy of Neisseria meningitidis serogroup B (MenB) vaccines has been difficult. There is substantial genetic (and corresponding antigenic) diversity, and serogroup B meningococcal disease is both uncommon and in decline in countries where the burden is well understood. The Advisory Committee on Immunization Practices reviewed available data and concluded that a public health recommendation for vaccination of all adolescents could not be supported but that sufficient evidence existed to support individual decision making by clinicians with regard to patients between 16 and 23 years of age. Vaccination of all adolescents would prevent 15-29 cases and five to nine deaths annually in the United States. The effect of vaccinating college students is correspondingly smaller – preventing perhaps nine cases and one death, at an estimated cost per quality-adjusted life-year of $9.4 million.
The Centers for Disease Control and Prevention notes that the MenB vaccines approved in the United States should provide “protection against most, but not all, serogroup B strains” and represent an important contribution to the prevention of serogroup B meningococcal disease. More than 60,000 persons have received at least one vaccination with 4CMenB in studies conducted worldwide, including studies at universities in the United States and Canada, and the safety profile for 4CMenB has been good. For a relatively uncommon but devastating infectious disease, the regulatory approval of a vaccine in the absence of ideal data may be necessary and appropriate if the vaccine is deployed in the context of a systematic public health response and if there is a commitment to the generation of additional information necessary for determining recommendations for use. The regulatory approval and clinical use of vaccines for pathogens that cause outbreaks will remain challenging.
Jerome H. Kim, MD, is with the International Vaccine Institute in Seoul, South Korea. He reported having no relevant financial disclosures. These comments were excerpted from the editorial accompanying the study (doi:10.1056/NEJMe1606015).
Proving the clinical efficacy of Neisseria meningitidis serogroup B (MenB) vaccines has been difficult. There is substantial genetic (and corresponding antigenic) diversity, and serogroup B meningococcal disease is both uncommon and in decline in countries where the burden is well understood. The Advisory Committee on Immunization Practices reviewed available data and concluded that a public health recommendation for vaccination of all adolescents could not be supported but that sufficient evidence existed to support individual decision making by clinicians with regard to patients between 16 and 23 years of age. Vaccination of all adolescents would prevent 15-29 cases and five to nine deaths annually in the United States. The effect of vaccinating college students is correspondingly smaller – preventing perhaps nine cases and one death, at an estimated cost per quality-adjusted life-year of $9.4 million.
The Centers for Disease Control and Prevention notes that the MenB vaccines approved in the United States should provide “protection against most, but not all, serogroup B strains” and represent an important contribution to the prevention of serogroup B meningococcal disease. More than 60,000 persons have received at least one vaccination with 4CMenB in studies conducted worldwide, including studies at universities in the United States and Canada, and the safety profile for 4CMenB has been good. For a relatively uncommon but devastating infectious disease, the regulatory approval of a vaccine in the absence of ideal data may be necessary and appropriate if the vaccine is deployed in the context of a systematic public health response and if there is a commitment to the generation of additional information necessary for determining recommendations for use. The regulatory approval and clinical use of vaccines for pathogens that cause outbreaks will remain challenging.
Jerome H. Kim, MD, is with the International Vaccine Institute in Seoul, South Korea. He reported having no relevant financial disclosures. These comments were excerpted from the editorial accompanying the study (doi:10.1056/NEJMe1606015).
Proving the clinical efficacy of Neisseria meningitidis serogroup B (MenB) vaccines has been difficult. There is substantial genetic (and corresponding antigenic) diversity, and serogroup B meningococcal disease is both uncommon and in decline in countries where the burden is well understood. The Advisory Committee on Immunization Practices reviewed available data and concluded that a public health recommendation for vaccination of all adolescents could not be supported but that sufficient evidence existed to support individual decision making by clinicians with regard to patients between 16 and 23 years of age. Vaccination of all adolescents would prevent 15-29 cases and five to nine deaths annually in the United States. The effect of vaccinating college students is correspondingly smaller – preventing perhaps nine cases and one death, at an estimated cost per quality-adjusted life-year of $9.4 million.
The Centers for Disease Control and Prevention notes that the MenB vaccines approved in the United States should provide “protection against most, but not all, serogroup B strains” and represent an important contribution to the prevention of serogroup B meningococcal disease. More than 60,000 persons have received at least one vaccination with 4CMenB in studies conducted worldwide, including studies at universities in the United States and Canada, and the safety profile for 4CMenB has been good. For a relatively uncommon but devastating infectious disease, the regulatory approval of a vaccine in the absence of ideal data may be necessary and appropriate if the vaccine is deployed in the context of a systematic public health response and if there is a commitment to the generation of additional information necessary for determining recommendations for use. The regulatory approval and clinical use of vaccines for pathogens that cause outbreaks will remain challenging.
Jerome H. Kim, MD, is with the International Vaccine Institute in Seoul, South Korea. He reported having no relevant financial disclosures. These comments were excerpted from the editorial accompanying the study (doi:10.1056/NEJMe1606015).
One-third of the individuals who received a multicomponent meningococcal B vaccine during an outbreak did not show a protective response against the outbreak strain, though they also did not contract the disease, a study showed.
“This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain,” Nicole E. Basta, PhD, of the University of Minnesota, Minneapolis, and her associates wrote in the New England Journal of Medicine (2016;375:220-8. doi:10.1056/NEJMoa1514866).
“Our results indicate that knowledge of [human complement serum bactericidal antibodies] immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens,” the investigators added.
Amidst an outbreak of meningococcal B at Princeton (N.J.) University in the winter of 2013, the Food and Drug Administration allowed use of the meningococcal serogroup B (4CMenB) vaccine before its licensure, in an attempt to control the outbreak. Two strains of meningitis were used in developing this vaccine: 5/99 strain, which was not at all similar to the outbreak strain, and 44/76-SL, which was 96% genetically similar to the outbreak strain.
Among 535 people who completed the study, 499 individuals received two doses of 4CMenB 10 weeks apart, 17 received one dose of the vaccine, and 19 were unvaccinated. When the fully vaccinated individuals were tested for titers 8 weeks after their second dose, two-thirds (66.1%) had low-level seropositivity for the outbreak strain, with a geometric mean titer (GMT) of 7.6. One in five of the unvaccinated people (21.1%) had low seropositivity for the outbreak strain (GMT, 2.8), and 58.8% of the partly vaccinated individuals had seropositivity with a GMT of 5.4.
The researchers then assessed titers for the two strains used in developing 4CMenB in 61 fully vaccinated individuals, all randomly selected from the one-third of fully vaccinated people who did not have a detectable response to the outbreak strain. Most of them (86.9%) were seropositive for the 44/76-SL strain with a GMT of 17.4, and all of them were seropositive for the 5/99 strain, with a considerably larger GMT of 256.3.
Among those fully vaccinated who responded to the outbreak strain with titers above 8, all had seropositivity for 44/76-SL (GMT, 178.8), and 96.7% had seropositivity for the 5/99 strain (GMT, 214.2). Among the 18 unvaccinated individuals, just 1 had very low seropositivity to the 5/99 strain (GMT, 1.2), and 6 of them (33.3%) responded to the 44/76-SL strain (GMT, 3.2).
“These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease,” the authors wrote.
The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had financial disclosures.
One-third of the individuals who received a multicomponent meningococcal B vaccine during an outbreak did not show a protective response against the outbreak strain, though they also did not contract the disease, a study showed.
“This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain,” Nicole E. Basta, PhD, of the University of Minnesota, Minneapolis, and her associates wrote in the New England Journal of Medicine (2016;375:220-8. doi:10.1056/NEJMoa1514866).
“Our results indicate that knowledge of [human complement serum bactericidal antibodies] immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens,” the investigators added.
Amidst an outbreak of meningococcal B at Princeton (N.J.) University in the winter of 2013, the Food and Drug Administration allowed use of the meningococcal serogroup B (4CMenB) vaccine before its licensure, in an attempt to control the outbreak. Two strains of meningitis were used in developing this vaccine: 5/99 strain, which was not at all similar to the outbreak strain, and 44/76-SL, which was 96% genetically similar to the outbreak strain.
Among 535 people who completed the study, 499 individuals received two doses of 4CMenB 10 weeks apart, 17 received one dose of the vaccine, and 19 were unvaccinated. When the fully vaccinated individuals were tested for titers 8 weeks after their second dose, two-thirds (66.1%) had low-level seropositivity for the outbreak strain, with a geometric mean titer (GMT) of 7.6. One in five of the unvaccinated people (21.1%) had low seropositivity for the outbreak strain (GMT, 2.8), and 58.8% of the partly vaccinated individuals had seropositivity with a GMT of 5.4.
The researchers then assessed titers for the two strains used in developing 4CMenB in 61 fully vaccinated individuals, all randomly selected from the one-third of fully vaccinated people who did not have a detectable response to the outbreak strain. Most of them (86.9%) were seropositive for the 44/76-SL strain with a GMT of 17.4, and all of them were seropositive for the 5/99 strain, with a considerably larger GMT of 256.3.
Among those fully vaccinated who responded to the outbreak strain with titers above 8, all had seropositivity for 44/76-SL (GMT, 178.8), and 96.7% had seropositivity for the 5/99 strain (GMT, 214.2). Among the 18 unvaccinated individuals, just 1 had very low seropositivity to the 5/99 strain (GMT, 1.2), and 6 of them (33.3%) responded to the 44/76-SL strain (GMT, 3.2).
“These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease,” the authors wrote.
The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The 4CMenB vaccine provided less protection than anticipated during an outbreak.
Major finding: A total of 66.1% of vaccinated individuals had seropositivity against the meningococcal B outbreak strain.
Data source: The findings are based on the immunogenicity of 4CMenB against a meningococcal B outbreak strain in 535 individuals during an outbreak at a New Jersey university in the winter of 2013.
Disclosures: The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had disclosures.
Screen teens for suicide risk, AAP advises
Screening teens for suicide risk is among the recommendations in a new clinical report from the American Academy of Pediatrics Committee on Adolescence.
Suicide is now the second-leading cause of death for teens aged 15-19 years, having surpassed homicide since 2007, when the last report on adolescent suicide was published by the AAP (Pediatrics. 2016 June 27 doi: 10.1542/peds.2016-1420). Excessive Internet use and cyberbullying are considered to be risk factors for suicide.
In addition to screening for suicide risk, the AAP advises screening for mood disorders, and for substance abuse and dependence. “Ask about emotional difficulties and use of drugs and alcohol, identify lack of developmental progress, and estimate level of distress, impairment of functioning, and level of danger to self and others,” the committee members wrote. The report offers several examples of questions to use for screening during the visit or interview.
“Self-report scales may be helpful, but they do not substitute for pediatricians asking teens directly (and sensitively) about mood disorders, substance use, suicidal and self-harmful thoughts, behaviors, stress, and distress,” Benjamin Shain, MD, PhD, the report’s author and a member of the AAP Committee on Adolescence, said in an interview. “Since ‘black box’ warning labels were added to antidepressants in 2004, new, population-based research has indicated that the benefits of these medications outweigh the risks for many patients. Physicians should not shy away from prescribing these medications when they are clinically indicated.”
Risk factors
A significant risk factor for suicide is involvement in bullying, whether the teen is the victim, the bully, or both, with the latter – bully victims – at the highest risk. The increased risk for suicidal thoughts or attempts extends to involvement with cyberbullying, in addition to physical, social, and verbal bullying, according to the committee members.
Results of a meta-analysis indicate girls who are victims or perpetrators of bullying are at higher risk for suicidal thoughts or attempts regardless of how common or rare the bullying is, whereas boys had an increased risk only when the bullying was frequent. Bullying as early as age 8 years was associated with attempted and completed suicides in adolescence, according to one study.
In a 2013 survey of U.S. high school students, 24% of girls and 16% of boys reported being bullied on school property in the past year. Cyberbullying occurred among 21% of the girls and 9% of the boys.
Teens reporting video game or Internet use for at least 5 hours a day were at higher risk for suicidal thoughts, suicide attempts, and depression, the committee noted. An additional wrinkle of Internet use is that teens may read about others’ suicides, which increases their own risk, or they may seek out pro-suicide websites.
“Suicide-related searches were found to be associated with completed suicides among young adults,” the report noted.
The good news is that major search engines prioritize institutional sites and mental health support sites in response to searches for “suicide,” Dr. Shain said.
“Despite the negatives, there is evidence that the Internet and electronic media, in general, provide a large amount of support, including keeping teens connected to their friends, family, teachers, and others, and also providing entertainment and valuable or interesting information,” Dr. Shain noted. “The Internet may be particularly useful for distressed teens.”
“More than 90% of adolescent suicide victims met criteria for a psychiatric disorder before their death,” the report noted. “Immediate risk factors include agitation, intoxication, and a recent stressful life event.”
Take action to help at-risk teens
The committee advises taking the following steps to assist at-risk teens:
In routine history taking, ask questions about mood disorders, use of drugs and alcohol, suicidal thoughts, bullying, sexual orientation, and other risk factors associated with suicide throughout adolescence.
Develop working relationships with emergency departments and colleagues in child and adolescent psychiatry, clinical psychology, and other mental health professions to optimally evaluate and manage the care of adolescents who are at risk for suicide.
Become familiar with local, state, and national resources that are concerned with treatment of psychopathology and suicide prevention in youth, including local hospitals with psychiatric units, mental health agencies, family and children’s services, crisis hotlines, and crisis intervention centers.
Consider additional training and ongoing education in diagnosing and managing adolescent mood disorders, especially if you practice in an underserved area.
During routine evaluations and where consistent with state law, ask whether firearms are kept in the home and discuss with parents the increased risk of adolescent suicide with the presence of firearms. Specifically for adolescents at risk for suicide, advise parents to remove guns and ammunition from the house and secure supplies of prescription and over-the-counter medications.
Learn about the benefits and risks of antidepressant medications, and how to monitor depressed patients. Educate the family regarding the following warning signs that warrant contacting you: new or more frequent thoughts of wanting to die; self-destructive behavior; signs of increased anxiety/panic, agitation, aggressiveness, impulsivity, insomnia or irritability; new or more involuntary restlessness (akathisia), such as pacing or fidgeting; extreme degree of elation or energy; fast, driven speech; or new onset of unrealistic plans or goals.
Risk factors for suicide
Fixed risk factors
• Family history of suicide or suicide attempts.
• History of adoption.
• Male gender.
• Parental mental health problems.
• Lesbian, gay, bisexual, or questioning sexual orientation.
• Transgender identification.
• A history of physical or sexual abuse.
• Previous suicide attempt.
Social/environmental risk factors
• Bullying.
• Impaired parent-child relationship.
• Living outside of the home (homeless or in a corrections facility or group home).
• Difficulties in school.
• Neither working nor attending school.
• Social isolation.
• Presence of stressful life events, such as legal or romantic difficulties or an argument with a parent.
• An unsupported social environment for LGBTQ adolescents.
Personal mental health problems
• Sleep disturbances.
• Depression.
• Bipolar disorder.
• Substance intoxication and substance use disorders.
• Psychosis.
• Posttraumatic stress disorder.
• Panic attacks.
• A history of aggression.
• Impulsivity.
• Severe anger.
• Pathologic Internet use.
Source: Pediatrics. 2016 June 27 doi: 10.1542/peds.2016-1420.
Screening teens for suicide risk is among the recommendations in a new clinical report from the American Academy of Pediatrics Committee on Adolescence.
Suicide is now the second-leading cause of death for teens aged 15-19 years, having surpassed homicide since 2007, when the last report on adolescent suicide was published by the AAP (Pediatrics. 2016 June 27 doi: 10.1542/peds.2016-1420). Excessive Internet use and cyberbullying are considered to be risk factors for suicide.
In addition to screening for suicide risk, the AAP advises screening for mood disorders, and for substance abuse and dependence. “Ask about emotional difficulties and use of drugs and alcohol, identify lack of developmental progress, and estimate level of distress, impairment of functioning, and level of danger to self and others,” the committee members wrote. The report offers several examples of questions to use for screening during the visit or interview.
“Self-report scales may be helpful, but they do not substitute for pediatricians asking teens directly (and sensitively) about mood disorders, substance use, suicidal and self-harmful thoughts, behaviors, stress, and distress,” Benjamin Shain, MD, PhD, the report’s author and a member of the AAP Committee on Adolescence, said in an interview. “Since ‘black box’ warning labels were added to antidepressants in 2004, new, population-based research has indicated that the benefits of these medications outweigh the risks for many patients. Physicians should not shy away from prescribing these medications when they are clinically indicated.”
Risk factors
A significant risk factor for suicide is involvement in bullying, whether the teen is the victim, the bully, or both, with the latter – bully victims – at the highest risk. The increased risk for suicidal thoughts or attempts extends to involvement with cyberbullying, in addition to physical, social, and verbal bullying, according to the committee members.
Results of a meta-analysis indicate girls who are victims or perpetrators of bullying are at higher risk for suicidal thoughts or attempts regardless of how common or rare the bullying is, whereas boys had an increased risk only when the bullying was frequent. Bullying as early as age 8 years was associated with attempted and completed suicides in adolescence, according to one study.
In a 2013 survey of U.S. high school students, 24% of girls and 16% of boys reported being bullied on school property in the past year. Cyberbullying occurred among 21% of the girls and 9% of the boys.
Teens reporting video game or Internet use for at least 5 hours a day were at higher risk for suicidal thoughts, suicide attempts, and depression, the committee noted. An additional wrinkle of Internet use is that teens may read about others’ suicides, which increases their own risk, or they may seek out pro-suicide websites.
“Suicide-related searches were found to be associated with completed suicides among young adults,” the report noted.
The good news is that major search engines prioritize institutional sites and mental health support sites in response to searches for “suicide,” Dr. Shain said.
“Despite the negatives, there is evidence that the Internet and electronic media, in general, provide a large amount of support, including keeping teens connected to their friends, family, teachers, and others, and also providing entertainment and valuable or interesting information,” Dr. Shain noted. “The Internet may be particularly useful for distressed teens.”
“More than 90% of adolescent suicide victims met criteria for a psychiatric disorder before their death,” the report noted. “Immediate risk factors include agitation, intoxication, and a recent stressful life event.”
Take action to help at-risk teens
The committee advises taking the following steps to assist at-risk teens:
In routine history taking, ask questions about mood disorders, use of drugs and alcohol, suicidal thoughts, bullying, sexual orientation, and other risk factors associated with suicide throughout adolescence.
Develop working relationships with emergency departments and colleagues in child and adolescent psychiatry, clinical psychology, and other mental health professions to optimally evaluate and manage the care of adolescents who are at risk for suicide.
Become familiar with local, state, and national resources that are concerned with treatment of psychopathology and suicide prevention in youth, including local hospitals with psychiatric units, mental health agencies, family and children’s services, crisis hotlines, and crisis intervention centers.
Consider additional training and ongoing education in diagnosing and managing adolescent mood disorders, especially if you practice in an underserved area.
During routine evaluations and where consistent with state law, ask whether firearms are kept in the home and discuss with parents the increased risk of adolescent suicide with the presence of firearms. Specifically for adolescents at risk for suicide, advise parents to remove guns and ammunition from the house and secure supplies of prescription and over-the-counter medications.
Learn about the benefits and risks of antidepressant medications, and how to monitor depressed patients. Educate the family regarding the following warning signs that warrant contacting you: new or more frequent thoughts of wanting to die; self-destructive behavior; signs of increased anxiety/panic, agitation, aggressiveness, impulsivity, insomnia or irritability; new or more involuntary restlessness (akathisia), such as pacing or fidgeting; extreme degree of elation or energy; fast, driven speech; or new onset of unrealistic plans or goals.
Risk factors for suicide
Fixed risk factors
• Family history of suicide or suicide attempts.
• History of adoption.
• Male gender.
• Parental mental health problems.
• Lesbian, gay, bisexual, or questioning sexual orientation.
• Transgender identification.
• A history of physical or sexual abuse.
• Previous suicide attempt.
Social/environmental risk factors
• Bullying.
• Impaired parent-child relationship.
• Living outside of the home (homeless or in a corrections facility or group home).
• Difficulties in school.
• Neither working nor attending school.
• Social isolation.
• Presence of stressful life events, such as legal or romantic difficulties or an argument with a parent.
• An unsupported social environment for LGBTQ adolescents.
Personal mental health problems
• Sleep disturbances.
• Depression.
• Bipolar disorder.
• Substance intoxication and substance use disorders.
• Psychosis.
• Posttraumatic stress disorder.
• Panic attacks.
• A history of aggression.
• Impulsivity.
• Severe anger.
• Pathologic Internet use.
Source: Pediatrics. 2016 June 27 doi: 10.1542/peds.2016-1420.
Screening teens for suicide risk is among the recommendations in a new clinical report from the American Academy of Pediatrics Committee on Adolescence.
Suicide is now the second-leading cause of death for teens aged 15-19 years, having surpassed homicide since 2007, when the last report on adolescent suicide was published by the AAP (Pediatrics. 2016 June 27 doi: 10.1542/peds.2016-1420). Excessive Internet use and cyberbullying are considered to be risk factors for suicide.
In addition to screening for suicide risk, the AAP advises screening for mood disorders, and for substance abuse and dependence. “Ask about emotional difficulties and use of drugs and alcohol, identify lack of developmental progress, and estimate level of distress, impairment of functioning, and level of danger to self and others,” the committee members wrote. The report offers several examples of questions to use for screening during the visit or interview.
“Self-report scales may be helpful, but they do not substitute for pediatricians asking teens directly (and sensitively) about mood disorders, substance use, suicidal and self-harmful thoughts, behaviors, stress, and distress,” Benjamin Shain, MD, PhD, the report’s author and a member of the AAP Committee on Adolescence, said in an interview. “Since ‘black box’ warning labels were added to antidepressants in 2004, new, population-based research has indicated that the benefits of these medications outweigh the risks for many patients. Physicians should not shy away from prescribing these medications when they are clinically indicated.”
Risk factors
A significant risk factor for suicide is involvement in bullying, whether the teen is the victim, the bully, or both, with the latter – bully victims – at the highest risk. The increased risk for suicidal thoughts or attempts extends to involvement with cyberbullying, in addition to physical, social, and verbal bullying, according to the committee members.
Results of a meta-analysis indicate girls who are victims or perpetrators of bullying are at higher risk for suicidal thoughts or attempts regardless of how common or rare the bullying is, whereas boys had an increased risk only when the bullying was frequent. Bullying as early as age 8 years was associated with attempted and completed suicides in adolescence, according to one study.
In a 2013 survey of U.S. high school students, 24% of girls and 16% of boys reported being bullied on school property in the past year. Cyberbullying occurred among 21% of the girls and 9% of the boys.
Teens reporting video game or Internet use for at least 5 hours a day were at higher risk for suicidal thoughts, suicide attempts, and depression, the committee noted. An additional wrinkle of Internet use is that teens may read about others’ suicides, which increases their own risk, or they may seek out pro-suicide websites.
“Suicide-related searches were found to be associated with completed suicides among young adults,” the report noted.
The good news is that major search engines prioritize institutional sites and mental health support sites in response to searches for “suicide,” Dr. Shain said.
“Despite the negatives, there is evidence that the Internet and electronic media, in general, provide a large amount of support, including keeping teens connected to their friends, family, teachers, and others, and also providing entertainment and valuable or interesting information,” Dr. Shain noted. “The Internet may be particularly useful for distressed teens.”
“More than 90% of adolescent suicide victims met criteria for a psychiatric disorder before their death,” the report noted. “Immediate risk factors include agitation, intoxication, and a recent stressful life event.”
Take action to help at-risk teens
The committee advises taking the following steps to assist at-risk teens:
In routine history taking, ask questions about mood disorders, use of drugs and alcohol, suicidal thoughts, bullying, sexual orientation, and other risk factors associated with suicide throughout adolescence.
Develop working relationships with emergency departments and colleagues in child and adolescent psychiatry, clinical psychology, and other mental health professions to optimally evaluate and manage the care of adolescents who are at risk for suicide.
Become familiar with local, state, and national resources that are concerned with treatment of psychopathology and suicide prevention in youth, including local hospitals with psychiatric units, mental health agencies, family and children’s services, crisis hotlines, and crisis intervention centers.
Consider additional training and ongoing education in diagnosing and managing adolescent mood disorders, especially if you practice in an underserved area.
During routine evaluations and where consistent with state law, ask whether firearms are kept in the home and discuss with parents the increased risk of adolescent suicide with the presence of firearms. Specifically for adolescents at risk for suicide, advise parents to remove guns and ammunition from the house and secure supplies of prescription and over-the-counter medications.
Learn about the benefits and risks of antidepressant medications, and how to monitor depressed patients. Educate the family regarding the following warning signs that warrant contacting you: new or more frequent thoughts of wanting to die; self-destructive behavior; signs of increased anxiety/panic, agitation, aggressiveness, impulsivity, insomnia or irritability; new or more involuntary restlessness (akathisia), such as pacing or fidgeting; extreme degree of elation or energy; fast, driven speech; or new onset of unrealistic plans or goals.
Risk factors for suicide
Fixed risk factors
• Family history of suicide or suicide attempts.
• History of adoption.
• Male gender.
• Parental mental health problems.
• Lesbian, gay, bisexual, or questioning sexual orientation.
• Transgender identification.
• A history of physical or sexual abuse.
• Previous suicide attempt.
Social/environmental risk factors
• Bullying.
• Impaired parent-child relationship.
• Living outside of the home (homeless or in a corrections facility or group home).
• Difficulties in school.
• Neither working nor attending school.
• Social isolation.
• Presence of stressful life events, such as legal or romantic difficulties or an argument with a parent.
• An unsupported social environment for LGBTQ adolescents.
Personal mental health problems
• Sleep disturbances.
• Depression.
• Bipolar disorder.
• Substance intoxication and substance use disorders.
• Psychosis.
• Posttraumatic stress disorder.
• Panic attacks.
• A history of aggression.
• Impulsivity.
• Severe anger.
• Pathologic Internet use.
Source: Pediatrics. 2016 June 27 doi: 10.1542/peds.2016-1420.
FROM PEDIATRICS
Infant bronchiolitis risk linked to gut flora composition
Infants with gut flora dominated by the genus Bacteroides have more than four times greater odds of developing bronchiolitis than those with microbiota dominated by Enterobacter and Veillonella combined, according to results of a recent study. Two other bacterial profiles, one dominated by Bifidobacterium and another by Escherichia, were not associated with any higher or lower risk of bronchiolitis.
“Our observations, in conjunction with earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection,” wrote Dr. Kohei Hasegawa of Harvard Medical School in Boston, and his associates (Pediatrics. 2016 June 27. doi: 10.1542/peds.2016-0218). “That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis.”
The researchers collected stool samples from 115 healthy infants from a Massachusetts General Hospital primary care group practice, and from 40 infants who were hospitalized with bronchiolitis from November 2013 through April 2014 at one of three children’s hospitals in Wilmington, Del.; Boston; and Louisville, Ky. The groups were age matched, and the infants overall were a median 3 months old. Just over half were male, and just over half were white. Of those with bronchiolitis, 65% had respiratory syncytial virus and 23% had rhinovirus.
Further, “compared with healthy controls, infants with bronchiolitis were [significantly] more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed,” the authors reported.
The researchers used a 16S rRNA gene-sequencing method similar to the one used by the Human Microbiome Project to identify the composition of the fecal samples’ microbiota. Four different bacterial profiles emerged. The most common was an Escherichia-dominant profile, occurring in 30% of the infants overall. Microbiota dominated by Bacteroides followed next, occurring in 28% of infants, while 22% of infants had a Enterobacter/Veillonella–dominant profile, and 21% had a Bifidobacterium-dominant profile. Those with a Bacteroides-dominant profile were older, more likely to be born vaginally, and more likely to be prenatally exposed to maternal smoking.
In infants with bronchiolitis, however, flora dominated by Bacteroides was most common, occurring in 44% of the ill infants. Enterobacter/Veillonella–dominant microbiota occurred in only 15% of the infants with bronchiolitis. Infants’ risk of bronchiolitis was not significantly different among those with Bifidobacterium-dominant or Escherichia-dominant profiles, compared with the Enterobacter/Veillonella–dominant profile.
Patients with Bacteroides-dominant microflora had 4.59 greater odds of severe bronchiolitis than those with Enterobacter/Veillonella–dominant microbiota (P = .008). These odds dropped only to 3.89 after adjustments for age, sex, prematurity, mode of birth, and a history of systemic antibiotic use (P = .03). Similarly, adjusting for age, sex, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment resulted in 4.12 greater odds of bronchiolitis in those with a Bacteroides-dominant profile (P = .02).
The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
This cross-sectional, case-control study raises multiple hypotheses about the relationship between different gut microbiota compositions and the presence of bronchiolitis while also exposing limitations in the study. For instance, polysaccharide A of Bacteroides suppresses T-cell responses to inflammatory stimuli. Inappropriate suppression of “cellular learning” in infancy may alter subsequent mucosal immunity upon infection, resulting in exacerbated inflammatory responses to environmental challenges. Thus, an increased abundance of enteric Bacteroides before a viral challenge may be hypothesized to increase the likelihood of reduced viral immunity and an inappropriate response to an infection.
However, in the study by Hasegawa et al., the gut microbiota was sampled only at the time of hospitalization for infection and once in age-matched controls. Any of the observed microbiota profiles may not reflect earlier states of the microbiota and critical windows of early immune priming. Therefore, prospective longitudinal studies will be essential to determine whether the observed microbiota profiles at the time of bronchiolitis preceded symptoms, were concurrent with the disease onset, or occurred after the disease was well under way. Only through these types of studies, coupled with preclinical mechanistic models of bronchiolitis, can causality be established.
The associations identified by Hasegawa et al., if upheld by the necessary prospective and causal studies, may yield new insights into the failures of antibiotic therapy and suggest alternative approaches to therapeutically modify the microbiota and thus reduce the risk and severity of viral bronchiolitis in infants.
Respiratory tract research has entered a new era. Through a combination of clinical and preclinical models, genomics, immunology, and metabolomics, investigations into the gut-lung axis are expected to drive a paradigm shift in which pulmonary health is viewed through a wider lens of multisystem interactions that includes the microbiota, and through which new preventive strategies, diagnostics and therapeutics may be envisioned for common respiratory diseases.
These comments were condensed from an editorial by Dr. Patrick C. Seed that was published in Pediatrics (doi: 10.1542/peds.2016-1377) alongside the original research. Dr. Seed is supported by the National Institutes of Health, and he reported having no disclosures.
This cross-sectional, case-control study raises multiple hypotheses about the relationship between different gut microbiota compositions and the presence of bronchiolitis while also exposing limitations in the study. For instance, polysaccharide A of Bacteroides suppresses T-cell responses to inflammatory stimuli. Inappropriate suppression of “cellular learning” in infancy may alter subsequent mucosal immunity upon infection, resulting in exacerbated inflammatory responses to environmental challenges. Thus, an increased abundance of enteric Bacteroides before a viral challenge may be hypothesized to increase the likelihood of reduced viral immunity and an inappropriate response to an infection.
However, in the study by Hasegawa et al., the gut microbiota was sampled only at the time of hospitalization for infection and once in age-matched controls. Any of the observed microbiota profiles may not reflect earlier states of the microbiota and critical windows of early immune priming. Therefore, prospective longitudinal studies will be essential to determine whether the observed microbiota profiles at the time of bronchiolitis preceded symptoms, were concurrent with the disease onset, or occurred after the disease was well under way. Only through these types of studies, coupled with preclinical mechanistic models of bronchiolitis, can causality be established.
The associations identified by Hasegawa et al., if upheld by the necessary prospective and causal studies, may yield new insights into the failures of antibiotic therapy and suggest alternative approaches to therapeutically modify the microbiota and thus reduce the risk and severity of viral bronchiolitis in infants.
Respiratory tract research has entered a new era. Through a combination of clinical and preclinical models, genomics, immunology, and metabolomics, investigations into the gut-lung axis are expected to drive a paradigm shift in which pulmonary health is viewed through a wider lens of multisystem interactions that includes the microbiota, and through which new preventive strategies, diagnostics and therapeutics may be envisioned for common respiratory diseases.
These comments were condensed from an editorial by Dr. Patrick C. Seed that was published in Pediatrics (doi: 10.1542/peds.2016-1377) alongside the original research. Dr. Seed is supported by the National Institutes of Health, and he reported having no disclosures.
This cross-sectional, case-control study raises multiple hypotheses about the relationship between different gut microbiota compositions and the presence of bronchiolitis while also exposing limitations in the study. For instance, polysaccharide A of Bacteroides suppresses T-cell responses to inflammatory stimuli. Inappropriate suppression of “cellular learning” in infancy may alter subsequent mucosal immunity upon infection, resulting in exacerbated inflammatory responses to environmental challenges. Thus, an increased abundance of enteric Bacteroides before a viral challenge may be hypothesized to increase the likelihood of reduced viral immunity and an inappropriate response to an infection.
However, in the study by Hasegawa et al., the gut microbiota was sampled only at the time of hospitalization for infection and once in age-matched controls. Any of the observed microbiota profiles may not reflect earlier states of the microbiota and critical windows of early immune priming. Therefore, prospective longitudinal studies will be essential to determine whether the observed microbiota profiles at the time of bronchiolitis preceded symptoms, were concurrent with the disease onset, or occurred after the disease was well under way. Only through these types of studies, coupled with preclinical mechanistic models of bronchiolitis, can causality be established.
The associations identified by Hasegawa et al., if upheld by the necessary prospective and causal studies, may yield new insights into the failures of antibiotic therapy and suggest alternative approaches to therapeutically modify the microbiota and thus reduce the risk and severity of viral bronchiolitis in infants.
Respiratory tract research has entered a new era. Through a combination of clinical and preclinical models, genomics, immunology, and metabolomics, investigations into the gut-lung axis are expected to drive a paradigm shift in which pulmonary health is viewed through a wider lens of multisystem interactions that includes the microbiota, and through which new preventive strategies, diagnostics and therapeutics may be envisioned for common respiratory diseases.
These comments were condensed from an editorial by Dr. Patrick C. Seed that was published in Pediatrics (doi: 10.1542/peds.2016-1377) alongside the original research. Dr. Seed is supported by the National Institutes of Health, and he reported having no disclosures.
Infants with gut flora dominated by the genus Bacteroides have more than four times greater odds of developing bronchiolitis than those with microbiota dominated by Enterobacter and Veillonella combined, according to results of a recent study. Two other bacterial profiles, one dominated by Bifidobacterium and another by Escherichia, were not associated with any higher or lower risk of bronchiolitis.
“Our observations, in conjunction with earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection,” wrote Dr. Kohei Hasegawa of Harvard Medical School in Boston, and his associates (Pediatrics. 2016 June 27. doi: 10.1542/peds.2016-0218). “That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis.”
The researchers collected stool samples from 115 healthy infants from a Massachusetts General Hospital primary care group practice, and from 40 infants who were hospitalized with bronchiolitis from November 2013 through April 2014 at one of three children’s hospitals in Wilmington, Del.; Boston; and Louisville, Ky. The groups were age matched, and the infants overall were a median 3 months old. Just over half were male, and just over half were white. Of those with bronchiolitis, 65% had respiratory syncytial virus and 23% had rhinovirus.
Further, “compared with healthy controls, infants with bronchiolitis were [significantly] more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed,” the authors reported.
The researchers used a 16S rRNA gene-sequencing method similar to the one used by the Human Microbiome Project to identify the composition of the fecal samples’ microbiota. Four different bacterial profiles emerged. The most common was an Escherichia-dominant profile, occurring in 30% of the infants overall. Microbiota dominated by Bacteroides followed next, occurring in 28% of infants, while 22% of infants had a Enterobacter/Veillonella–dominant profile, and 21% had a Bifidobacterium-dominant profile. Those with a Bacteroides-dominant profile were older, more likely to be born vaginally, and more likely to be prenatally exposed to maternal smoking.
In infants with bronchiolitis, however, flora dominated by Bacteroides was most common, occurring in 44% of the ill infants. Enterobacter/Veillonella–dominant microbiota occurred in only 15% of the infants with bronchiolitis. Infants’ risk of bronchiolitis was not significantly different among those with Bifidobacterium-dominant or Escherichia-dominant profiles, compared with the Enterobacter/Veillonella–dominant profile.
Patients with Bacteroides-dominant microflora had 4.59 greater odds of severe bronchiolitis than those with Enterobacter/Veillonella–dominant microbiota (P = .008). These odds dropped only to 3.89 after adjustments for age, sex, prematurity, mode of birth, and a history of systemic antibiotic use (P = .03). Similarly, adjusting for age, sex, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment resulted in 4.12 greater odds of bronchiolitis in those with a Bacteroides-dominant profile (P = .02).
The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
Infants with gut flora dominated by the genus Bacteroides have more than four times greater odds of developing bronchiolitis than those with microbiota dominated by Enterobacter and Veillonella combined, according to results of a recent study. Two other bacterial profiles, one dominated by Bifidobacterium and another by Escherichia, were not associated with any higher or lower risk of bronchiolitis.
“Our observations, in conjunction with earlier studies, suggest a causal pathway linking the gut microbiota in early infancy to the respiratory tract immune response against viral infection,” wrote Dr. Kohei Hasegawa of Harvard Medical School in Boston, and his associates (Pediatrics. 2016 June 27. doi: 10.1542/peds.2016-0218). “That is, the Bacteroides-dominant gut microbiota in early infancy attenuates the development of immune function in the respiratory tract and thereby leads to an increased susceptibility to bronchiolitis.”
The researchers collected stool samples from 115 healthy infants from a Massachusetts General Hospital primary care group practice, and from 40 infants who were hospitalized with bronchiolitis from November 2013 through April 2014 at one of three children’s hospitals in Wilmington, Del.; Boston; and Louisville, Ky. The groups were age matched, and the infants overall were a median 3 months old. Just over half were male, and just over half were white. Of those with bronchiolitis, 65% had respiratory syncytial virus and 23% had rhinovirus.
Further, “compared with healthy controls, infants with bronchiolitis were [significantly] more likely to have a parental history of asthma, maternal antibiotic use during pregnancy, a history of premature birth, a sibling at home, and corticosteroid use before the enrollment, but they were less likely to be breastfed,” the authors reported.
The researchers used a 16S rRNA gene-sequencing method similar to the one used by the Human Microbiome Project to identify the composition of the fecal samples’ microbiota. Four different bacterial profiles emerged. The most common was an Escherichia-dominant profile, occurring in 30% of the infants overall. Microbiota dominated by Bacteroides followed next, occurring in 28% of infants, while 22% of infants had a Enterobacter/Veillonella–dominant profile, and 21% had a Bifidobacterium-dominant profile. Those with a Bacteroides-dominant profile were older, more likely to be born vaginally, and more likely to be prenatally exposed to maternal smoking.
In infants with bronchiolitis, however, flora dominated by Bacteroides was most common, occurring in 44% of the ill infants. Enterobacter/Veillonella–dominant microbiota occurred in only 15% of the infants with bronchiolitis. Infants’ risk of bronchiolitis was not significantly different among those with Bifidobacterium-dominant or Escherichia-dominant profiles, compared with the Enterobacter/Veillonella–dominant profile.
Patients with Bacteroides-dominant microflora had 4.59 greater odds of severe bronchiolitis than those with Enterobacter/Veillonella–dominant microbiota (P = .008). These odds dropped only to 3.89 after adjustments for age, sex, prematurity, mode of birth, and a history of systemic antibiotic use (P = .03). Similarly, adjusting for age, sex, parental history of asthma, maternal antibiotic use during pregnancy, and systemic corticosteroid use before enrollment resulted in 4.12 greater odds of bronchiolitis in those with a Bacteroides-dominant profile (P = .02).
The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.
PEDIATRICS
Key clinical point: Infants with Bacteroides-dominant microbiota have the highest risk of bronchiolitis.
Major finding: 44% of infants with bronchiolitis had Bacteroides-dominant fecal microbiota, compared with 15% with Enterobacter/Veillonella–dominant microbiota.
Data source: The findings are based on a case-control study involving 155 infants from three children’s hospitals in Delaware, Massachusetts, and Kentucky, of whom 40 had bronchiolitis between November 2013 and April 2014.
Disclosures: The research was funded by the National Institutes of Health. Dr. Hasegawa reported having no disclosures. Two authors reported owning shares at the microbiome research company Diversigen, and one had consulted on bronchiolitis for Regeneron. The other authors reported having no disclosures.